User login
Nine Seasons of a Bronchiolitis Observation Unit and Home Oxygen Therapy Protocol
Bronchiolitis is the leading cause of hospitalization in infants aged <1 year in the United States.1-3 Estimates suggest that 1.5% to 2.0% of US infants require hospitalization every year, with a median (interquartile range) length of stay of 2 days (1-4),3 incurring direct medical costs of $555 million annually.1 Evidence suggests that few interventions, aside from supportive care, are effective for bronchiolitis.4-7 Adherence to standardized clinical guidelines could improve outcomes and resource use by streamlining care and limiting ineffective interventions, thereby decreasing hospital length of stay, which is a major medical cost.8-13 For this reason, many hospitals have adopted bronchiolitis guidelines, although institutional practices vary.14,15
Two relatively unexplored methods to reduce the inpatient burden of bronchiolitis are the use of observation units (OU) and home oxygen therapy (HOT). Motivated by research demonstrating the safety and effectiveness of an emergency department (ED)–based HOT protocol,16 where 36 of 37 patients with mild hypoxemia discharged on HOT avoided hospital admission, our institution implemented an observation unit and home oxygen therapy (OU-HOT) protocol designed to return children with bronchiolitis home earlier from the hospital. In the first winter season of implementation (2010 to 2011), the OU-HOT protocol was associated with significant reductions in length of stay and substantial cost savings, without an increase in return visits to the ED or inpatient readmissions.17 The objectives of this study were to determine whether these encouraging initial findings persisted and to measure the long-term impact of the OU-HOT protocol.
METHODS
We conducted a retrospective cohort study of children hospitalized with bronchiolitis at Primary Children’s Hospital, a freestanding children’s hospital in Salt Lake City, Utah. Discharge diagnosis and procedures codes, as well as laboratory, imaging, pharmacy, and supply costs, were obtained from the Intermountain Healthcare enterprise data warehouse. A crosswalk available from the Centers for Medicare and Medicaid Services was used to convert International Classification of Diseases (ICD)-10 discharge diagnosis and procedure codes to ICD-9 equivalents.18 This study was approved by the University of Utah institutional review board (00110419).
Patients
Children aged 3 to 24 months who were discharged with a diagnosis of bronchiolitis (466.xx) during winter seasons from 2007 to 2019 were included. A winter season was defined as November 1 to April 30. Both observation and inpatient encounters were included in the cohort. We excluded patients with discharge diagnosis or procedure codes indicating tracheostomy (519.0-519.09, V44.0, V55.0, 31.1, 31.21, 31.41, 31.74, 97.23), ventilator dependence (V46.1x), chronic lung disease (518.83, 770.7), or pulmonary hypertension (416.xx). Patients with both bronchiolitis and a concurrent diagnosis, such as otitis media or pneumonia, were included unless exclusion criteria were met.
Intervention and Process Measures
Our institution implemented the OU-HOT protocol at the start of the 2010-2011 winter season.17 The aim of the OU-HOT protocol was to discharge children with bronchiolitis home sooner by increasing use of both an OU, with frequent assessment of discharge readiness, and HOT to help children become ready for discharge. Similar to most OUs, admission to our unit was limited to patients who met hospital admission criteria, and had a short anticipated length of stay (<48 hours). As a self-contained 20-bed unit providing 24-hour dedicated pediatrician/pediatric emergency medicine physician and nursing coverage, the OU actively monitored patients’ discharge readiness, with a goal to facilitate patient throughput more akin to an ED rather than a traditional inpatient unit. Patients who could not be discharged from the OU within 48 hours were transferred to the inpatient unit. Although the OU existed at the time of protocol implementation, its use for patients with bronchiolitis was not actively encouraged until implementation.
Hospitalized patients—in either inpatient or observation units—were eligible for discharge on HOT if they met the following criteria: hypoxemia was the only indication for continued hospitalization, the child’s oxygen requirement was <0.5 L/min for at least 6 hours (0.8 L/min for children aged >1 year), the child’s caregiver(s) were willing to manage oxygen at home, and the child had reliable access to primary care provider follow up. We used two process measures across winter seasons: (1) the percentage of patients discharged from the OU, and (2) the percentage of patients discharged with HOT. The percentage of patients discharged on HOT was estimated by a manual chart review and an electronic medical record (EMR) HOT flag that came into existence with our hospital system’s adoption of a new EMR (2017-2019). Chart review randomly sampled patients from 2007-2017, totaling 457 patients. To estimate the reliability of this method, we calculated the sensitivity, specificity, positive predictive value, and negative predictive value of the EMR HOT flag using chart review as the gold standard.
Outcome Measures
The main outcome measure was mean hospital length of stay. Balancing measures were revisit rates (stratified into ED visits and readmissions) and annual per-population bronchiolitis admission rates. Visits were considered revisits if they occurred within 7 days of initial hospital discharge, and included visits to Primary Children’s Hospital as well as 22 other Intermountain Healthcare hospitals. Population estimates from the Utah Department of Health were used to calculate the annual population-based rate of bronchiolitis admissions to Primary Children’s Hospital.19 Annual admission rates were calculated per 10,000 children aged 3 to 24 months who resided in Utah each year of the study period, and were evaluated to determine if patients were admitted more frequently after OU-HOT implementation. Secondary outcome measures included the percentage of patients discharged within 24 hours and mean inflation-adjusted cost per episode of care (in 2019 dollars). Hospitalization costs were determined using Intermountain Healthcare’s internal cost accounting system, an activity-based method that aggregates costs of individual resources according to date of service.20 Costs were adjusted to 2019 dollars and were defined as the total costs of a patient’s initial hospitalization as well as any 7-day revisit encounters.
Data Analysis
Demographic data were compared before and after OU-HOT protocol implementation using Pearson chi-square tests. Multivariable linear or logistic regression models were used to compare measures before and after OU-HOT protocol implementation via an interrupted time-series approach. The interrupted time-series analysis measured two types of changes after protocol implementation during the 2010-2011 winter season: (1) any immediate change in the level of an outcome (immediate effect) and (2) any change of an outcome going forward over time (change in slope).21 Covariates in the regression models included patient age, sex, race, ethnicity, and insurance type, as well as presence of an underlying complex chronic condition, mechanical ventilation use, and pediatric intensive care unit (PICU) admission during hospitalization. Data were analyzed in STATA 15 (StataCorp LLC).22
RESULTS
A total of 7,116 patients met inclusion criteria over the study period (2,061 pre-implementation, 5,055 post-implementation). A comparison of patient characteristics before and after HOT protocol implementation is presented in Table 1. Patients were similar in terms of age, sex, and insurance type. Patients in the postimplementation period were more likely to have a complex chronic condition, require admission to the PICU, and need mechanical ventilation (P < .01). Differences between cohorts with regard to race/ethnicity distribution largely were a result of improved capture of these data elements in the postimplementation period. For example, 30% of patients were classified as “race/ethnicity unknown” in the preimplementation cohort, compared with 4% of patients in the postimplementation period.
Process Measures
Figure 1 shows trends in OU and HOT use by winter season. The percentage of patients discharged from the OU increased immediately after OU-HOT protocol implementation (absolute 26.9% immediate increase; 95% CI, 21.9-42.2). The change in the proportion of OU use per season also increased (change in slope +3.9% per season; 95% CI, 3.4%-4.4%). The percentage of patients discharged with HOT increased immediately after OU-HOT protocol implementation (26.0% immediate change; 95% CI, 18.9%-33.1%); however, the immediate increase in HOT discharges was coupled with a declining rate of HOT discharges per season in the postprotocol period compared with the preprotocol period (change in slope –4.5% per season; 95% CI, –7.5% to –1.5%). Our chart review and EMR flag included 1,354 patients, or 19.0% of our cohort. Our EMR flag for HOT in the last two seasons of the study had a positive predictive value of 100% (5 of 5 identified by EMR flag as receiving HOT were confirmed by chart review) and negative predictive value of 89% (31 of 35 identified by EMR flag as not receiving HOT were confirmed by chart review). The specificity of the EMR flag was 100% (31 of 31 of those confirmed by chart review as not receiving HOT, who were correctly identified by EMR) and the sensitivity was 55% (5 of 9 of those confirmed by chart review as receiving HOT, who were correctly identified by EMR).

Primary and Secondary Outcomes
Trends in length of stay across winter seasons are presented in Figure 2. The OU-HOT protocol was associated with an immediate reduction of 30.6 hours in mean length of stay (95% CI, –37.1 to –24.2). The rate of change in length of stay postimplementation did not differ significantly from the rate of change preimplementation (change in slope –0.6 hours per season; 95% CI, –2.3 to 1.1 hours). The percentage of patients discharged within 24 hours of admission rose immediately after protocol implementation, by 23.8 absolute percentage points (95% CI, 11.7-28.8). Slopes of the preintervention and postintervention regression lines did not differ significantly (change in slope –0.1% per season; 95% CI, –1.4% to 1.1%). Immediate decreases in length of stay were accompanied by an immediate decrease in mean cost per episode of care (–$4,181; 95% CI, –$4,829 to –$3,533). Protocol implementation also was associated with a decreased slope in cost postimplementation (change in slope –$403 per season; 95% CI, –$543 to –$264). The total cost savings, estimated by the product of the average cost savings per episode of care and the number of bronchiolitis admissions included in the study after OU-HOT implementation, amounted to $21.1 million over the 9-year period, or $2.3 million per winter season.

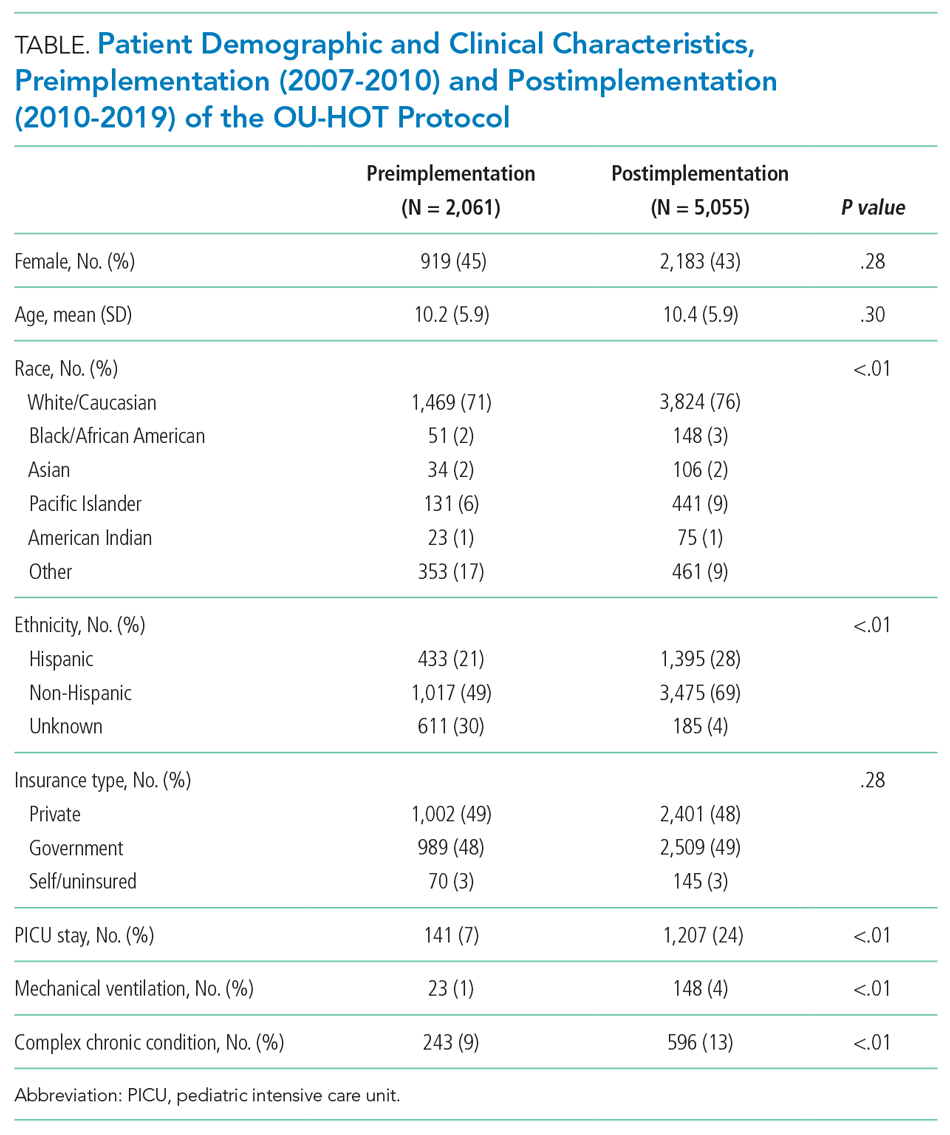
Balancing Measures
We observed an immediate reduction in 7-day hospital revisits (–1.1% immediate change; 95% CI, –1.8% to –0.4%), but an increasing slope in revisits after implementation (change in slope 0.4% per season; 95% CI, 0.1%-0.8%) (Figure 3). Stratifying revisits into ED visits and readmissions revealed that the revisit findings reflected changes in ED return visits, for which there was an immediate reduction at the time of implementation (–1.0% immediate change; 95% CI, –1.6% to –0.4%), but an increasing slope postimplementation (change in slope 0.5% per season; 95% CI, 0.2-0.8). Neither an immediate intervention effect (0.0% immediate change; 95% CI, –0.5% to 0.4%) nor a change in slope (change in slope 0.0% per season; 95% CI, –0.1% to 0.1%) were observed for inpatient readmissions alone. The annual rate of bronchiolitis admissions to Primary Children’s Hospital per 10,000 children who reside in Utah decreased after implementation of the OU-HOT protocol (immediate intervention effect –6.2 admissions; 95% CI, –10.8 to –1.6; change in slope –1.8 admissions per season; 95% CI, –2.8 to –0.69).
DISCUSSION
Our OU-HOT protocol was associated with immediate improvements in care delivered to children hospitalized for bronchiolitis, including decreased length of stay and cost savings. These improvements in outcomes largely have been sustained over a 9-year period. The OU-HOT protocol also appears to be safe as evidenced by a stable rate of readmissions over the study period and only a small increase in revisits to EDs across Intermountain Healthcare facilities, which see most children in the catchment area. Our OU-HOT protocol represents a combination of two interventions: (1) the creation of an OU focused on discharge within 24 to 48 hours of admission and (2) encouragement to discharge children with HOT. We found that use of the OU and a commitment to timely discharges has been sustained in recent years, while the commitment to HOT has appeared to wane.
Earlier investigations have evaluated the efficacy of HOT in the ED setting to prevent hospital admissions, finding high levels of caregiver comfort, estimating $1,300 per patient cost savings, and reporting readmission rates of approximately 5%.16,23-25 Our study is unique in addressing HOT among a population of patients already hospitalized with bronchiolitis. The cost reductions we observed with our OU-HOT protocol were similar to those noted in the ED-based HOT protocols. However, we recorded lower readmission rates, likely because of the additional time allotted to caregivers to better gauge illness trajectory in the inpatient setting vs the ED, as well as additional time for hospitalized patients to reach the plateau or convalescent phase of illness. The small increase in ED revisits that we measured in recent years might be related to the concurrent rise in patient acuity and complexity.
Considering that length of stay has remained low despite less commitment to HOT, our results suggest that the OU might be the more impactful of the two interventions, and these data support the use of such a unit for a subset of patients with bronchiolitis. However, it is important to note that while the EMR HOT flag demonstrated high specificity, positive predictive value, and negative predictive value, the sensitivity was low (56%). As a result, it is possible that we have underestimated HOT use in the 2017-2018 and 2018-2019 seasons, the final two years of the study. Alternatively, the discrepancy between sustained outcomes and lagging use of HOT could be explained by improved identification of patients who would experience the greatest benefit with oxygen in terms of length of stay reductions, with fewer patients discharged on HOT but greater per-patient benefit. Finally, in an era that encourages reduced monitor use and less aggressive response to transient mild desaturations,13,26,27 it is possible that fewer patients are identified with clinically actionable hypoxemia around the time they would be otherwise discharged.
Our OU-HOT model is not unprecedented. Increasingly, other formerly inpatient indications are being successfully managed in the observation, outpatient, and home setting, such as parenteral antibiotic treatment28,29 and chemotherapy administration.30 Considering the inpatient burden of bronchiolitis, similar strategies to expedite discharge are needed. Although outpatient intravenous antibiotic and chemotherapy administration have been widely adopted, we are aware of only one other pediatric health care system in the United States (Children’s Hospital Colorado) that routinely discharges inpatients with bronchiolitis on HOT.
This study has several limitations. First, although the interrupted time-series analysis is designed to account for trends that precede an intervention and covariates that differ before and after the intervention, it is possible that important unmeasured patient factors or changes in practice patterns differed between the pre- and post-intervention cohorts. There were no major changes to the OU-HOT protocol or discharge criteria after implementation, but individual practice management of bronchiolitis during the study period likely has evolved as new evidence emerges. Second, one could postulate that the increase in discharges within 24 hours and accompanying decreases in average length of stay and cost could be achieved by hospitalizing healthier patients over time, which the presence of an OU might incentivize. To the contrary, we found that population-based bronchiolitis admission rates have declined and disease severity appears to be increased since implementation of the OU-HOT protocol. The increase in medically complex children and PICU use in our postimplementation cohort aligns with recently published data suggesting these are national trends.3,31 Third, HOT use was estimated from a sample of the cohort using a chart review and a newly available EMR flag. A low sensitivity and a small sample for the positive predictive value are limitations of the EMR flag.
Additionally, there are almost certainly unmeasured ambulatory burdens of HOT not captured by this study. ED-based protocols have estimated that patients discharged with HOT have a median of two follow-up ambulatory visits before oxygen is discontinued32; however, the ambulatory burden associated with discharge on HOT after a hospitalization and the extent to which demographic factors affect that burden is unknown. Furthermore, one insurance company charged $94 for a month of HOT in 2019; paying even a portion of this charge represents a nontrivial financial burden for many families, even considering inpatient cost savings. Although the decision to discharge on oxygen or remain hospitalized until the child did not need oxygen was left to the parents, their posthospitalization perspectives were not assessed in this study. Although reports indicate that families largely feel positive about HOT after discharge from an ED setting, with 90% of caregivers preferring HOT use to inpatient admission and most reporting no difficulty with home management,23 it is uncertain whether this would also apply after inpatient hospitalization.
CONCLUSION
The OU-HOT bronchiolitis protocol was associated with decreases in inpatient length of stay and cost while appearing safe to implement. The sustained use of the OU combined with declining use of HOT suggests that the OU might be the more impactful intervention. As previously inpatient indications such as parenteral antibiotics and chemotherapy increasingly have been administered in observation and outpatient settings, bronchiolitis appears ideal for a similar strategy that allows patients to spend less time in the hospital. Studies are needed to understand the outpatient burden of HOT and the generalizability of our findings.
1. Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132(1):28-36. https://doi.org/10.1542/peds.2012-3877
2. Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122(1):58-64. https://doi.org/10.1542/peds.2007-2087
3. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
4. Schroeder AR, Mansbach JM. Recent evidence on the management of bronchiolitis. Curr Opin Pediatr. 2014;26(3):328-333. https://doi.org/10.1097/MOP.0000000000000090
5. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. https://doi.org/10.1542/peds.2006-2223
6. Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474. https://doi.org/10.1542/peds.2014-2742
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195
8. Perlstein PH, Kotagal UR, Bolling C, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics. 1999;104(6):1334-1341. https://doi.org/10.1542/peds.104.6.1334
9. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154(10):1001-1007. https://doi.org/10.1001/archpedi.154.10.1001
10. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17(5):195-199. https://doi.org/10.1177/106286060201700507
11. Barben J, Kuehni CE, Trachsel D, Hammer J; Swiss Paediatric Respiratory Research Group. Management of acute bronchiolitis: can evidence based guidelines alter clinical practice? Thorax. 2008;63(12):1103-1109. https://doi.org/10.1136/thx.2007.094706
12. Bryan MA, Desai AD, Wilson L, Wright DR, Mangione-Smith R. Association of bronchiolitis clinical pathway adherence with length of stay and costs. Pediatrics. 2017;139(3):e20163432. https://doi.org/10.1542/peds.2016-3432
13. Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. https://doi.org/10.1542/hpeds.2018-0023
14. Macias CG, Mansbach JM, Fisher ES, et al. Variability in inpatient management of children hospitalized with bronchiolitis. Acad Pediatr. 2015;15(1):69-76. https://doi.org/10.1016/j.acap.2014.07.005
15. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570-6.e3. https://doi.org/10.1016/j.jpeds.2014.05.021
16. Bajaj L, Turner CG, Bothner J. A randomized trial of home oxygen therapy from the emergency department for acute bronchiolitis. Pediatrics. 2006;117(3):633-640. https://doi.org/10.1542/peds.2005-1322
17. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
18. National Bureau of Economic Research. ICD-9-CM to and from ICD-10-CM and ICD-10-PCS crosswalk or general equivalence mappings. Accessed December 2, 2020. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
19. Utah Department of Health, Indicator-Based Information System for Public Health. Accessed February 15, 2020. https://ibis.health.utah.gov/ibisph-view
20. James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185-1191. https://doi.org/10.1377/hlthaff.2011.0358
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-44. https://doi.org/10.1016/j.acap.2013.08.002
22. StataCorp. Stata Statistical Software: Release 15. StataCorp LLC; 2017.
23. Freeman JF, Deakyne S, Bajaj L. Emergency department-initiated home oxygen for bronchiolitis: a prospective study of community follow-up, caregiver satisfaction, and outcomes. Acad Emerg Med. 2017;24(8):920-929. https://doi.org/10.1111/acem.13179
24. Freeman JF, Brou L, Mistry R. Feasibility and capacity for widespread use of emergency department-based home oxygen for bronchiolitis. Am J Emerg Med. 2017;35(9):1379-1381. https://doi.org/10.1016/j.ajem.2017.03.069
25. Halstead S, Roosevelt G, Deakyne S, Bajaj L. Discharged on supplemental oxygen from an emergency department in patients with bronchiolitis. Pediatrics. 2012;129(3):e605-610. https://doi.org/10.1542/peds.2011-0889
26. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
27. Burrows J, Berg K, McCulloh R. Intermittent pulse oximetry use and length of stay in bronchiolitis: bystander or primary Driver? Hosp Pediatr. 2019;9(2):142-143. https://doi.org/10.1542/hpeds.2018-0183
28. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. https://doi.org/10.1093/cid/ciy745
29. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. https://doi.org/10.1016/j.ijantimicag.2015.07.001
30. Beaty RS, Bernhardt MB, Berger AH, Hesselgrave JE, Russell HV, Okcu MF. Inpatient versus outpatient vincristine, dactinomycin, and cyclophosphamide for pediatric cancers: quality and cost implications. Pediatr Blood Cancer. 2015;62(11):1925-1928. https://doi.org/10.1002/pbc.25610
31. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
32. Freeman JF, Weng H-YC, Sandweiss D. Outpatient management of home oxygen for bronchiolitis. Clin Pediatr (Phila). 2015;54(1):62-66. https://doi.org/10.1177/0009922814547564
Bronchiolitis is the leading cause of hospitalization in infants aged <1 year in the United States.1-3 Estimates suggest that 1.5% to 2.0% of US infants require hospitalization every year, with a median (interquartile range) length of stay of 2 days (1-4),3 incurring direct medical costs of $555 million annually.1 Evidence suggests that few interventions, aside from supportive care, are effective for bronchiolitis.4-7 Adherence to standardized clinical guidelines could improve outcomes and resource use by streamlining care and limiting ineffective interventions, thereby decreasing hospital length of stay, which is a major medical cost.8-13 For this reason, many hospitals have adopted bronchiolitis guidelines, although institutional practices vary.14,15
Two relatively unexplored methods to reduce the inpatient burden of bronchiolitis are the use of observation units (OU) and home oxygen therapy (HOT). Motivated by research demonstrating the safety and effectiveness of an emergency department (ED)–based HOT protocol,16 where 36 of 37 patients with mild hypoxemia discharged on HOT avoided hospital admission, our institution implemented an observation unit and home oxygen therapy (OU-HOT) protocol designed to return children with bronchiolitis home earlier from the hospital. In the first winter season of implementation (2010 to 2011), the OU-HOT protocol was associated with significant reductions in length of stay and substantial cost savings, without an increase in return visits to the ED or inpatient readmissions.17 The objectives of this study were to determine whether these encouraging initial findings persisted and to measure the long-term impact of the OU-HOT protocol.
METHODS
We conducted a retrospective cohort study of children hospitalized with bronchiolitis at Primary Children’s Hospital, a freestanding children’s hospital in Salt Lake City, Utah. Discharge diagnosis and procedures codes, as well as laboratory, imaging, pharmacy, and supply costs, were obtained from the Intermountain Healthcare enterprise data warehouse. A crosswalk available from the Centers for Medicare and Medicaid Services was used to convert International Classification of Diseases (ICD)-10 discharge diagnosis and procedure codes to ICD-9 equivalents.18 This study was approved by the University of Utah institutional review board (00110419).
Patients
Children aged 3 to 24 months who were discharged with a diagnosis of bronchiolitis (466.xx) during winter seasons from 2007 to 2019 were included. A winter season was defined as November 1 to April 30. Both observation and inpatient encounters were included in the cohort. We excluded patients with discharge diagnosis or procedure codes indicating tracheostomy (519.0-519.09, V44.0, V55.0, 31.1, 31.21, 31.41, 31.74, 97.23), ventilator dependence (V46.1x), chronic lung disease (518.83, 770.7), or pulmonary hypertension (416.xx). Patients with both bronchiolitis and a concurrent diagnosis, such as otitis media or pneumonia, were included unless exclusion criteria were met.
Intervention and Process Measures
Our institution implemented the OU-HOT protocol at the start of the 2010-2011 winter season.17 The aim of the OU-HOT protocol was to discharge children with bronchiolitis home sooner by increasing use of both an OU, with frequent assessment of discharge readiness, and HOT to help children become ready for discharge. Similar to most OUs, admission to our unit was limited to patients who met hospital admission criteria, and had a short anticipated length of stay (<48 hours). As a self-contained 20-bed unit providing 24-hour dedicated pediatrician/pediatric emergency medicine physician and nursing coverage, the OU actively monitored patients’ discharge readiness, with a goal to facilitate patient throughput more akin to an ED rather than a traditional inpatient unit. Patients who could not be discharged from the OU within 48 hours were transferred to the inpatient unit. Although the OU existed at the time of protocol implementation, its use for patients with bronchiolitis was not actively encouraged until implementation.
Hospitalized patients—in either inpatient or observation units—were eligible for discharge on HOT if they met the following criteria: hypoxemia was the only indication for continued hospitalization, the child’s oxygen requirement was <0.5 L/min for at least 6 hours (0.8 L/min for children aged >1 year), the child’s caregiver(s) were willing to manage oxygen at home, and the child had reliable access to primary care provider follow up. We used two process measures across winter seasons: (1) the percentage of patients discharged from the OU, and (2) the percentage of patients discharged with HOT. The percentage of patients discharged on HOT was estimated by a manual chart review and an electronic medical record (EMR) HOT flag that came into existence with our hospital system’s adoption of a new EMR (2017-2019). Chart review randomly sampled patients from 2007-2017, totaling 457 patients. To estimate the reliability of this method, we calculated the sensitivity, specificity, positive predictive value, and negative predictive value of the EMR HOT flag using chart review as the gold standard.
Outcome Measures
The main outcome measure was mean hospital length of stay. Balancing measures were revisit rates (stratified into ED visits and readmissions) and annual per-population bronchiolitis admission rates. Visits were considered revisits if they occurred within 7 days of initial hospital discharge, and included visits to Primary Children’s Hospital as well as 22 other Intermountain Healthcare hospitals. Population estimates from the Utah Department of Health were used to calculate the annual population-based rate of bronchiolitis admissions to Primary Children’s Hospital.19 Annual admission rates were calculated per 10,000 children aged 3 to 24 months who resided in Utah each year of the study period, and were evaluated to determine if patients were admitted more frequently after OU-HOT implementation. Secondary outcome measures included the percentage of patients discharged within 24 hours and mean inflation-adjusted cost per episode of care (in 2019 dollars). Hospitalization costs were determined using Intermountain Healthcare’s internal cost accounting system, an activity-based method that aggregates costs of individual resources according to date of service.20 Costs were adjusted to 2019 dollars and were defined as the total costs of a patient’s initial hospitalization as well as any 7-day revisit encounters.
Data Analysis
Demographic data were compared before and after OU-HOT protocol implementation using Pearson chi-square tests. Multivariable linear or logistic regression models were used to compare measures before and after OU-HOT protocol implementation via an interrupted time-series approach. The interrupted time-series analysis measured two types of changes after protocol implementation during the 2010-2011 winter season: (1) any immediate change in the level of an outcome (immediate effect) and (2) any change of an outcome going forward over time (change in slope).21 Covariates in the regression models included patient age, sex, race, ethnicity, and insurance type, as well as presence of an underlying complex chronic condition, mechanical ventilation use, and pediatric intensive care unit (PICU) admission during hospitalization. Data were analyzed in STATA 15 (StataCorp LLC).22
RESULTS
A total of 7,116 patients met inclusion criteria over the study period (2,061 pre-implementation, 5,055 post-implementation). A comparison of patient characteristics before and after HOT protocol implementation is presented in Table 1. Patients were similar in terms of age, sex, and insurance type. Patients in the postimplementation period were more likely to have a complex chronic condition, require admission to the PICU, and need mechanical ventilation (P < .01). Differences between cohorts with regard to race/ethnicity distribution largely were a result of improved capture of these data elements in the postimplementation period. For example, 30% of patients were classified as “race/ethnicity unknown” in the preimplementation cohort, compared with 4% of patients in the postimplementation period.

Process Measures
Figure 1 shows trends in OU and HOT use by winter season. The percentage of patients discharged from the OU increased immediately after OU-HOT protocol implementation (absolute 26.9% immediate increase; 95% CI, 21.9-42.2). The change in the proportion of OU use per season also increased (change in slope +3.9% per season; 95% CI, 3.4%-4.4%). The percentage of patients discharged with HOT increased immediately after OU-HOT protocol implementation (26.0% immediate change; 95% CI, 18.9%-33.1%); however, the immediate increase in HOT discharges was coupled with a declining rate of HOT discharges per season in the postprotocol period compared with the preprotocol period (change in slope –4.5% per season; 95% CI, –7.5% to –1.5%). Our chart review and EMR flag included 1,354 patients, or 19.0% of our cohort. Our EMR flag for HOT in the last two seasons of the study had a positive predictive value of 100% (5 of 5 identified by EMR flag as receiving HOT were confirmed by chart review) and negative predictive value of 89% (31 of 35 identified by EMR flag as not receiving HOT were confirmed by chart review). The specificity of the EMR flag was 100% (31 of 31 of those confirmed by chart review as not receiving HOT, who were correctly identified by EMR) and the sensitivity was 55% (5 of 9 of those confirmed by chart review as receiving HOT, who were correctly identified by EMR).

Primary and Secondary Outcomes
Trends in length of stay across winter seasons are presented in Figure 2. The OU-HOT protocol was associated with an immediate reduction of 30.6 hours in mean length of stay (95% CI, –37.1 to –24.2). The rate of change in length of stay postimplementation did not differ significantly from the rate of change preimplementation (change in slope –0.6 hours per season; 95% CI, –2.3 to 1.1 hours). The percentage of patients discharged within 24 hours of admission rose immediately after protocol implementation, by 23.8 absolute percentage points (95% CI, 11.7-28.8). Slopes of the preintervention and postintervention regression lines did not differ significantly (change in slope –0.1% per season; 95% CI, –1.4% to 1.1%). Immediate decreases in length of stay were accompanied by an immediate decrease in mean cost per episode of care (–$4,181; 95% CI, –$4,829 to –$3,533). Protocol implementation also was associated with a decreased slope in cost postimplementation (change in slope –$403 per season; 95% CI, –$543 to –$264). The total cost savings, estimated by the product of the average cost savings per episode of care and the number of bronchiolitis admissions included in the study after OU-HOT implementation, amounted to $21.1 million over the 9-year period, or $2.3 million per winter season.

Balancing Measures
We observed an immediate reduction in 7-day hospital revisits (–1.1% immediate change; 95% CI, –1.8% to –0.4%), but an increasing slope in revisits after implementation (change in slope 0.4% per season; 95% CI, 0.1%-0.8%) (Figure 3). Stratifying revisits into ED visits and readmissions revealed that the revisit findings reflected changes in ED return visits, for which there was an immediate reduction at the time of implementation (–1.0% immediate change; 95% CI, –1.6% to –0.4%), but an increasing slope postimplementation (change in slope 0.5% per season; 95% CI, 0.2-0.8). Neither an immediate intervention effect (0.0% immediate change; 95% CI, –0.5% to 0.4%) nor a change in slope (change in slope 0.0% per season; 95% CI, –0.1% to 0.1%) were observed for inpatient readmissions alone. The annual rate of bronchiolitis admissions to Primary Children’s Hospital per 10,000 children who reside in Utah decreased after implementation of the OU-HOT protocol (immediate intervention effect –6.2 admissions; 95% CI, –10.8 to –1.6; change in slope –1.8 admissions per season; 95% CI, –2.8 to –0.69).

DISCUSSION
Our OU-HOT protocol was associated with immediate improvements in care delivered to children hospitalized for bronchiolitis, including decreased length of stay and cost savings. These improvements in outcomes largely have been sustained over a 9-year period. The OU-HOT protocol also appears to be safe as evidenced by a stable rate of readmissions over the study period and only a small increase in revisits to EDs across Intermountain Healthcare facilities, which see most children in the catchment area. Our OU-HOT protocol represents a combination of two interventions: (1) the creation of an OU focused on discharge within 24 to 48 hours of admission and (2) encouragement to discharge children with HOT. We found that use of the OU and a commitment to timely discharges has been sustained in recent years, while the commitment to HOT has appeared to wane.
Earlier investigations have evaluated the efficacy of HOT in the ED setting to prevent hospital admissions, finding high levels of caregiver comfort, estimating $1,300 per patient cost savings, and reporting readmission rates of approximately 5%.16,23-25 Our study is unique in addressing HOT among a population of patients already hospitalized with bronchiolitis. The cost reductions we observed with our OU-HOT protocol were similar to those noted in the ED-based HOT protocols. However, we recorded lower readmission rates, likely because of the additional time allotted to caregivers to better gauge illness trajectory in the inpatient setting vs the ED, as well as additional time for hospitalized patients to reach the plateau or convalescent phase of illness. The small increase in ED revisits that we measured in recent years might be related to the concurrent rise in patient acuity and complexity.
Considering that length of stay has remained low despite less commitment to HOT, our results suggest that the OU might be the more impactful of the two interventions, and these data support the use of such a unit for a subset of patients with bronchiolitis. However, it is important to note that while the EMR HOT flag demonstrated high specificity, positive predictive value, and negative predictive value, the sensitivity was low (56%). As a result, it is possible that we have underestimated HOT use in the 2017-2018 and 2018-2019 seasons, the final two years of the study. Alternatively, the discrepancy between sustained outcomes and lagging use of HOT could be explained by improved identification of patients who would experience the greatest benefit with oxygen in terms of length of stay reductions, with fewer patients discharged on HOT but greater per-patient benefit. Finally, in an era that encourages reduced monitor use and less aggressive response to transient mild desaturations,13,26,27 it is possible that fewer patients are identified with clinically actionable hypoxemia around the time they would be otherwise discharged.
Our OU-HOT model is not unprecedented. Increasingly, other formerly inpatient indications are being successfully managed in the observation, outpatient, and home setting, such as parenteral antibiotic treatment28,29 and chemotherapy administration.30 Considering the inpatient burden of bronchiolitis, similar strategies to expedite discharge are needed. Although outpatient intravenous antibiotic and chemotherapy administration have been widely adopted, we are aware of only one other pediatric health care system in the United States (Children’s Hospital Colorado) that routinely discharges inpatients with bronchiolitis on HOT.
This study has several limitations. First, although the interrupted time-series analysis is designed to account for trends that precede an intervention and covariates that differ before and after the intervention, it is possible that important unmeasured patient factors or changes in practice patterns differed between the pre- and post-intervention cohorts. There were no major changes to the OU-HOT protocol or discharge criteria after implementation, but individual practice management of bronchiolitis during the study period likely has evolved as new evidence emerges. Second, one could postulate that the increase in discharges within 24 hours and accompanying decreases in average length of stay and cost could be achieved by hospitalizing healthier patients over time, which the presence of an OU might incentivize. To the contrary, we found that population-based bronchiolitis admission rates have declined and disease severity appears to be increased since implementation of the OU-HOT protocol. The increase in medically complex children and PICU use in our postimplementation cohort aligns with recently published data suggesting these are national trends.3,31 Third, HOT use was estimated from a sample of the cohort using a chart review and a newly available EMR flag. A low sensitivity and a small sample for the positive predictive value are limitations of the EMR flag.
Additionally, there are almost certainly unmeasured ambulatory burdens of HOT not captured by this study. ED-based protocols have estimated that patients discharged with HOT have a median of two follow-up ambulatory visits before oxygen is discontinued32; however, the ambulatory burden associated with discharge on HOT after a hospitalization and the extent to which demographic factors affect that burden is unknown. Furthermore, one insurance company charged $94 for a month of HOT in 2019; paying even a portion of this charge represents a nontrivial financial burden for many families, even considering inpatient cost savings. Although the decision to discharge on oxygen or remain hospitalized until the child did not need oxygen was left to the parents, their posthospitalization perspectives were not assessed in this study. Although reports indicate that families largely feel positive about HOT after discharge from an ED setting, with 90% of caregivers preferring HOT use to inpatient admission and most reporting no difficulty with home management,23 it is uncertain whether this would also apply after inpatient hospitalization.
CONCLUSION
The OU-HOT bronchiolitis protocol was associated with decreases in inpatient length of stay and cost while appearing safe to implement. The sustained use of the OU combined with declining use of HOT suggests that the OU might be the more impactful intervention. As previously inpatient indications such as parenteral antibiotics and chemotherapy increasingly have been administered in observation and outpatient settings, bronchiolitis appears ideal for a similar strategy that allows patients to spend less time in the hospital. Studies are needed to understand the outpatient burden of HOT and the generalizability of our findings.
Bronchiolitis is the leading cause of hospitalization in infants aged <1 year in the United States.1-3 Estimates suggest that 1.5% to 2.0% of US infants require hospitalization every year, with a median (interquartile range) length of stay of 2 days (1-4),3 incurring direct medical costs of $555 million annually.1 Evidence suggests that few interventions, aside from supportive care, are effective for bronchiolitis.4-7 Adherence to standardized clinical guidelines could improve outcomes and resource use by streamlining care and limiting ineffective interventions, thereby decreasing hospital length of stay, which is a major medical cost.8-13 For this reason, many hospitals have adopted bronchiolitis guidelines, although institutional practices vary.14,15
Two relatively unexplored methods to reduce the inpatient burden of bronchiolitis are the use of observation units (OU) and home oxygen therapy (HOT). Motivated by research demonstrating the safety and effectiveness of an emergency department (ED)–based HOT protocol,16 where 36 of 37 patients with mild hypoxemia discharged on HOT avoided hospital admission, our institution implemented an observation unit and home oxygen therapy (OU-HOT) protocol designed to return children with bronchiolitis home earlier from the hospital. In the first winter season of implementation (2010 to 2011), the OU-HOT protocol was associated with significant reductions in length of stay and substantial cost savings, without an increase in return visits to the ED or inpatient readmissions.17 The objectives of this study were to determine whether these encouraging initial findings persisted and to measure the long-term impact of the OU-HOT protocol.
METHODS
We conducted a retrospective cohort study of children hospitalized with bronchiolitis at Primary Children’s Hospital, a freestanding children’s hospital in Salt Lake City, Utah. Discharge diagnosis and procedures codes, as well as laboratory, imaging, pharmacy, and supply costs, were obtained from the Intermountain Healthcare enterprise data warehouse. A crosswalk available from the Centers for Medicare and Medicaid Services was used to convert International Classification of Diseases (ICD)-10 discharge diagnosis and procedure codes to ICD-9 equivalents.18 This study was approved by the University of Utah institutional review board (00110419).
Patients
Children aged 3 to 24 months who were discharged with a diagnosis of bronchiolitis (466.xx) during winter seasons from 2007 to 2019 were included. A winter season was defined as November 1 to April 30. Both observation and inpatient encounters were included in the cohort. We excluded patients with discharge diagnosis or procedure codes indicating tracheostomy (519.0-519.09, V44.0, V55.0, 31.1, 31.21, 31.41, 31.74, 97.23), ventilator dependence (V46.1x), chronic lung disease (518.83, 770.7), or pulmonary hypertension (416.xx). Patients with both bronchiolitis and a concurrent diagnosis, such as otitis media or pneumonia, were included unless exclusion criteria were met.
Intervention and Process Measures
Our institution implemented the OU-HOT protocol at the start of the 2010-2011 winter season.17 The aim of the OU-HOT protocol was to discharge children with bronchiolitis home sooner by increasing use of both an OU, with frequent assessment of discharge readiness, and HOT to help children become ready for discharge. Similar to most OUs, admission to our unit was limited to patients who met hospital admission criteria, and had a short anticipated length of stay (<48 hours). As a self-contained 20-bed unit providing 24-hour dedicated pediatrician/pediatric emergency medicine physician and nursing coverage, the OU actively monitored patients’ discharge readiness, with a goal to facilitate patient throughput more akin to an ED rather than a traditional inpatient unit. Patients who could not be discharged from the OU within 48 hours were transferred to the inpatient unit. Although the OU existed at the time of protocol implementation, its use for patients with bronchiolitis was not actively encouraged until implementation.
Hospitalized patients—in either inpatient or observation units—were eligible for discharge on HOT if they met the following criteria: hypoxemia was the only indication for continued hospitalization, the child’s oxygen requirement was <0.5 L/min for at least 6 hours (0.8 L/min for children aged >1 year), the child’s caregiver(s) were willing to manage oxygen at home, and the child had reliable access to primary care provider follow up. We used two process measures across winter seasons: (1) the percentage of patients discharged from the OU, and (2) the percentage of patients discharged with HOT. The percentage of patients discharged on HOT was estimated by a manual chart review and an electronic medical record (EMR) HOT flag that came into existence with our hospital system’s adoption of a new EMR (2017-2019). Chart review randomly sampled patients from 2007-2017, totaling 457 patients. To estimate the reliability of this method, we calculated the sensitivity, specificity, positive predictive value, and negative predictive value of the EMR HOT flag using chart review as the gold standard.
Outcome Measures
The main outcome measure was mean hospital length of stay. Balancing measures were revisit rates (stratified into ED visits and readmissions) and annual per-population bronchiolitis admission rates. Visits were considered revisits if they occurred within 7 days of initial hospital discharge, and included visits to Primary Children’s Hospital as well as 22 other Intermountain Healthcare hospitals. Population estimates from the Utah Department of Health were used to calculate the annual population-based rate of bronchiolitis admissions to Primary Children’s Hospital.19 Annual admission rates were calculated per 10,000 children aged 3 to 24 months who resided in Utah each year of the study period, and were evaluated to determine if patients were admitted more frequently after OU-HOT implementation. Secondary outcome measures included the percentage of patients discharged within 24 hours and mean inflation-adjusted cost per episode of care (in 2019 dollars). Hospitalization costs were determined using Intermountain Healthcare’s internal cost accounting system, an activity-based method that aggregates costs of individual resources according to date of service.20 Costs were adjusted to 2019 dollars and were defined as the total costs of a patient’s initial hospitalization as well as any 7-day revisit encounters.
Data Analysis
Demographic data were compared before and after OU-HOT protocol implementation using Pearson chi-square tests. Multivariable linear or logistic regression models were used to compare measures before and after OU-HOT protocol implementation via an interrupted time-series approach. The interrupted time-series analysis measured two types of changes after protocol implementation during the 2010-2011 winter season: (1) any immediate change in the level of an outcome (immediate effect) and (2) any change of an outcome going forward over time (change in slope).21 Covariates in the regression models included patient age, sex, race, ethnicity, and insurance type, as well as presence of an underlying complex chronic condition, mechanical ventilation use, and pediatric intensive care unit (PICU) admission during hospitalization. Data were analyzed in STATA 15 (StataCorp LLC).22
RESULTS
A total of 7,116 patients met inclusion criteria over the study period (2,061 pre-implementation, 5,055 post-implementation). A comparison of patient characteristics before and after HOT protocol implementation is presented in Table 1. Patients were similar in terms of age, sex, and insurance type. Patients in the postimplementation period were more likely to have a complex chronic condition, require admission to the PICU, and need mechanical ventilation (P < .01). Differences between cohorts with regard to race/ethnicity distribution largely were a result of improved capture of these data elements in the postimplementation period. For example, 30% of patients were classified as “race/ethnicity unknown” in the preimplementation cohort, compared with 4% of patients in the postimplementation period.

Process Measures
Figure 1 shows trends in OU and HOT use by winter season. The percentage of patients discharged from the OU increased immediately after OU-HOT protocol implementation (absolute 26.9% immediate increase; 95% CI, 21.9-42.2). The change in the proportion of OU use per season also increased (change in slope +3.9% per season; 95% CI, 3.4%-4.4%). The percentage of patients discharged with HOT increased immediately after OU-HOT protocol implementation (26.0% immediate change; 95% CI, 18.9%-33.1%); however, the immediate increase in HOT discharges was coupled with a declining rate of HOT discharges per season in the postprotocol period compared with the preprotocol period (change in slope –4.5% per season; 95% CI, –7.5% to –1.5%). Our chart review and EMR flag included 1,354 patients, or 19.0% of our cohort. Our EMR flag for HOT in the last two seasons of the study had a positive predictive value of 100% (5 of 5 identified by EMR flag as receiving HOT were confirmed by chart review) and negative predictive value of 89% (31 of 35 identified by EMR flag as not receiving HOT were confirmed by chart review). The specificity of the EMR flag was 100% (31 of 31 of those confirmed by chart review as not receiving HOT, who were correctly identified by EMR) and the sensitivity was 55% (5 of 9 of those confirmed by chart review as receiving HOT, who were correctly identified by EMR).

Primary and Secondary Outcomes
Trends in length of stay across winter seasons are presented in Figure 2. The OU-HOT protocol was associated with an immediate reduction of 30.6 hours in mean length of stay (95% CI, –37.1 to –24.2). The rate of change in length of stay postimplementation did not differ significantly from the rate of change preimplementation (change in slope –0.6 hours per season; 95% CI, –2.3 to 1.1 hours). The percentage of patients discharged within 24 hours of admission rose immediately after protocol implementation, by 23.8 absolute percentage points (95% CI, 11.7-28.8). Slopes of the preintervention and postintervention regression lines did not differ significantly (change in slope –0.1% per season; 95% CI, –1.4% to 1.1%). Immediate decreases in length of stay were accompanied by an immediate decrease in mean cost per episode of care (–$4,181; 95% CI, –$4,829 to –$3,533). Protocol implementation also was associated with a decreased slope in cost postimplementation (change in slope –$403 per season; 95% CI, –$543 to –$264). The total cost savings, estimated by the product of the average cost savings per episode of care and the number of bronchiolitis admissions included in the study after OU-HOT implementation, amounted to $21.1 million over the 9-year period, or $2.3 million per winter season.

Balancing Measures
We observed an immediate reduction in 7-day hospital revisits (–1.1% immediate change; 95% CI, –1.8% to –0.4%), but an increasing slope in revisits after implementation (change in slope 0.4% per season; 95% CI, 0.1%-0.8%) (Figure 3). Stratifying revisits into ED visits and readmissions revealed that the revisit findings reflected changes in ED return visits, for which there was an immediate reduction at the time of implementation (–1.0% immediate change; 95% CI, –1.6% to –0.4%), but an increasing slope postimplementation (change in slope 0.5% per season; 95% CI, 0.2-0.8). Neither an immediate intervention effect (0.0% immediate change; 95% CI, –0.5% to 0.4%) nor a change in slope (change in slope 0.0% per season; 95% CI, –0.1% to 0.1%) were observed for inpatient readmissions alone. The annual rate of bronchiolitis admissions to Primary Children’s Hospital per 10,000 children who reside in Utah decreased after implementation of the OU-HOT protocol (immediate intervention effect –6.2 admissions; 95% CI, –10.8 to –1.6; change in slope –1.8 admissions per season; 95% CI, –2.8 to –0.69).

DISCUSSION
Our OU-HOT protocol was associated with immediate improvements in care delivered to children hospitalized for bronchiolitis, including decreased length of stay and cost savings. These improvements in outcomes largely have been sustained over a 9-year period. The OU-HOT protocol also appears to be safe as evidenced by a stable rate of readmissions over the study period and only a small increase in revisits to EDs across Intermountain Healthcare facilities, which see most children in the catchment area. Our OU-HOT protocol represents a combination of two interventions: (1) the creation of an OU focused on discharge within 24 to 48 hours of admission and (2) encouragement to discharge children with HOT. We found that use of the OU and a commitment to timely discharges has been sustained in recent years, while the commitment to HOT has appeared to wane.
Earlier investigations have evaluated the efficacy of HOT in the ED setting to prevent hospital admissions, finding high levels of caregiver comfort, estimating $1,300 per patient cost savings, and reporting readmission rates of approximately 5%.16,23-25 Our study is unique in addressing HOT among a population of patients already hospitalized with bronchiolitis. The cost reductions we observed with our OU-HOT protocol were similar to those noted in the ED-based HOT protocols. However, we recorded lower readmission rates, likely because of the additional time allotted to caregivers to better gauge illness trajectory in the inpatient setting vs the ED, as well as additional time for hospitalized patients to reach the plateau or convalescent phase of illness. The small increase in ED revisits that we measured in recent years might be related to the concurrent rise in patient acuity and complexity.
Considering that length of stay has remained low despite less commitment to HOT, our results suggest that the OU might be the more impactful of the two interventions, and these data support the use of such a unit for a subset of patients with bronchiolitis. However, it is important to note that while the EMR HOT flag demonstrated high specificity, positive predictive value, and negative predictive value, the sensitivity was low (56%). As a result, it is possible that we have underestimated HOT use in the 2017-2018 and 2018-2019 seasons, the final two years of the study. Alternatively, the discrepancy between sustained outcomes and lagging use of HOT could be explained by improved identification of patients who would experience the greatest benefit with oxygen in terms of length of stay reductions, with fewer patients discharged on HOT but greater per-patient benefit. Finally, in an era that encourages reduced monitor use and less aggressive response to transient mild desaturations,13,26,27 it is possible that fewer patients are identified with clinically actionable hypoxemia around the time they would be otherwise discharged.
Our OU-HOT model is not unprecedented. Increasingly, other formerly inpatient indications are being successfully managed in the observation, outpatient, and home setting, such as parenteral antibiotic treatment28,29 and chemotherapy administration.30 Considering the inpatient burden of bronchiolitis, similar strategies to expedite discharge are needed. Although outpatient intravenous antibiotic and chemotherapy administration have been widely adopted, we are aware of only one other pediatric health care system in the United States (Children’s Hospital Colorado) that routinely discharges inpatients with bronchiolitis on HOT.
This study has several limitations. First, although the interrupted time-series analysis is designed to account for trends that precede an intervention and covariates that differ before and after the intervention, it is possible that important unmeasured patient factors or changes in practice patterns differed between the pre- and post-intervention cohorts. There were no major changes to the OU-HOT protocol or discharge criteria after implementation, but individual practice management of bronchiolitis during the study period likely has evolved as new evidence emerges. Second, one could postulate that the increase in discharges within 24 hours and accompanying decreases in average length of stay and cost could be achieved by hospitalizing healthier patients over time, which the presence of an OU might incentivize. To the contrary, we found that population-based bronchiolitis admission rates have declined and disease severity appears to be increased since implementation of the OU-HOT protocol. The increase in medically complex children and PICU use in our postimplementation cohort aligns with recently published data suggesting these are national trends.3,31 Third, HOT use was estimated from a sample of the cohort using a chart review and a newly available EMR flag. A low sensitivity and a small sample for the positive predictive value are limitations of the EMR flag.
Additionally, there are almost certainly unmeasured ambulatory burdens of HOT not captured by this study. ED-based protocols have estimated that patients discharged with HOT have a median of two follow-up ambulatory visits before oxygen is discontinued32; however, the ambulatory burden associated with discharge on HOT after a hospitalization and the extent to which demographic factors affect that burden is unknown. Furthermore, one insurance company charged $94 for a month of HOT in 2019; paying even a portion of this charge represents a nontrivial financial burden for many families, even considering inpatient cost savings. Although the decision to discharge on oxygen or remain hospitalized until the child did not need oxygen was left to the parents, their posthospitalization perspectives were not assessed in this study. Although reports indicate that families largely feel positive about HOT after discharge from an ED setting, with 90% of caregivers preferring HOT use to inpatient admission and most reporting no difficulty with home management,23 it is uncertain whether this would also apply after inpatient hospitalization.
CONCLUSION
The OU-HOT bronchiolitis protocol was associated with decreases in inpatient length of stay and cost while appearing safe to implement. The sustained use of the OU combined with declining use of HOT suggests that the OU might be the more impactful intervention. As previously inpatient indications such as parenteral antibiotics and chemotherapy increasingly have been administered in observation and outpatient settings, bronchiolitis appears ideal for a similar strategy that allows patients to spend less time in the hospital. Studies are needed to understand the outpatient burden of HOT and the generalizability of our findings.
1. Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132(1):28-36. https://doi.org/10.1542/peds.2012-3877
2. Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122(1):58-64. https://doi.org/10.1542/peds.2007-2087
3. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
4. Schroeder AR, Mansbach JM. Recent evidence on the management of bronchiolitis. Curr Opin Pediatr. 2014;26(3):328-333. https://doi.org/10.1097/MOP.0000000000000090
5. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. https://doi.org/10.1542/peds.2006-2223
6. Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474. https://doi.org/10.1542/peds.2014-2742
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195
8. Perlstein PH, Kotagal UR, Bolling C, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics. 1999;104(6):1334-1341. https://doi.org/10.1542/peds.104.6.1334
9. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154(10):1001-1007. https://doi.org/10.1001/archpedi.154.10.1001
10. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17(5):195-199. https://doi.org/10.1177/106286060201700507
11. Barben J, Kuehni CE, Trachsel D, Hammer J; Swiss Paediatric Respiratory Research Group. Management of acute bronchiolitis: can evidence based guidelines alter clinical practice? Thorax. 2008;63(12):1103-1109. https://doi.org/10.1136/thx.2007.094706
12. Bryan MA, Desai AD, Wilson L, Wright DR, Mangione-Smith R. Association of bronchiolitis clinical pathway adherence with length of stay and costs. Pediatrics. 2017;139(3):e20163432. https://doi.org/10.1542/peds.2016-3432
13. Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. https://doi.org/10.1542/hpeds.2018-0023
14. Macias CG, Mansbach JM, Fisher ES, et al. Variability in inpatient management of children hospitalized with bronchiolitis. Acad Pediatr. 2015;15(1):69-76. https://doi.org/10.1016/j.acap.2014.07.005
15. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570-6.e3. https://doi.org/10.1016/j.jpeds.2014.05.021
16. Bajaj L, Turner CG, Bothner J. A randomized trial of home oxygen therapy from the emergency department for acute bronchiolitis. Pediatrics. 2006;117(3):633-640. https://doi.org/10.1542/peds.2005-1322
17. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
18. National Bureau of Economic Research. ICD-9-CM to and from ICD-10-CM and ICD-10-PCS crosswalk or general equivalence mappings. Accessed December 2, 2020. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
19. Utah Department of Health, Indicator-Based Information System for Public Health. Accessed February 15, 2020. https://ibis.health.utah.gov/ibisph-view
20. James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185-1191. https://doi.org/10.1377/hlthaff.2011.0358
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-44. https://doi.org/10.1016/j.acap.2013.08.002
22. StataCorp. Stata Statistical Software: Release 15. StataCorp LLC; 2017.
23. Freeman JF, Deakyne S, Bajaj L. Emergency department-initiated home oxygen for bronchiolitis: a prospective study of community follow-up, caregiver satisfaction, and outcomes. Acad Emerg Med. 2017;24(8):920-929. https://doi.org/10.1111/acem.13179
24. Freeman JF, Brou L, Mistry R. Feasibility and capacity for widespread use of emergency department-based home oxygen for bronchiolitis. Am J Emerg Med. 2017;35(9):1379-1381. https://doi.org/10.1016/j.ajem.2017.03.069
25. Halstead S, Roosevelt G, Deakyne S, Bajaj L. Discharged on supplemental oxygen from an emergency department in patients with bronchiolitis. Pediatrics. 2012;129(3):e605-610. https://doi.org/10.1542/peds.2011-0889
26. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
27. Burrows J, Berg K, McCulloh R. Intermittent pulse oximetry use and length of stay in bronchiolitis: bystander or primary Driver? Hosp Pediatr. 2019;9(2):142-143. https://doi.org/10.1542/hpeds.2018-0183
28. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. https://doi.org/10.1093/cid/ciy745
29. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. https://doi.org/10.1016/j.ijantimicag.2015.07.001
30. Beaty RS, Bernhardt MB, Berger AH, Hesselgrave JE, Russell HV, Okcu MF. Inpatient versus outpatient vincristine, dactinomycin, and cyclophosphamide for pediatric cancers: quality and cost implications. Pediatr Blood Cancer. 2015;62(11):1925-1928. https://doi.org/10.1002/pbc.25610
31. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
32. Freeman JF, Weng H-YC, Sandweiss D. Outpatient management of home oxygen for bronchiolitis. Clin Pediatr (Phila). 2015;54(1):62-66. https://doi.org/10.1177/0009922814547564
1. Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132(1):28-36. https://doi.org/10.1542/peds.2012-3877
2. Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122(1):58-64. https://doi.org/10.1542/peds.2007-2087
3. Fujiogi M, Goto T, Yasunaga H, et al. Trends in bronchiolitis hospitalizations in the United States: 2000–2016. Pediatrics. 2019;144(6):e20192614. https://doi.org/10.1542/peds.2019-2614
4. Schroeder AR, Mansbach JM. Recent evidence on the management of bronchiolitis. Curr Opin Pediatr. 2014;26(3):328-333. https://doi.org/10.1097/MOP.0000000000000090
5. American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774-1793. https://doi.org/10.1542/peds.2006-2223
6. Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474. https://doi.org/10.1542/peds.2014-2742
7. Riese J, Porter T, Fierce J, Riese A, Richardson T, Alverson BK. Clinical outcomes of bronchiolitis after implementation of a general ward high flow nasal cannula guideline. Hosp Pediatr. 2017;7(4):197-203. https://doi.org/10.1542/hpeds.2016-0195
8. Perlstein PH, Kotagal UR, Bolling C, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics. 1999;104(6):1334-1341. https://doi.org/10.1542/peds.104.6.1334
9. Perlstein PH, Kotagal UR, Schoettker PJ, et al. Sustaining the implementation of an evidence-based guideline for bronchiolitis. Arch Pediatr Adolesc Med. 2000;154(10):1001-1007. https://doi.org/10.1001/archpedi.154.10.1001
10. Wilson SD, Dahl BB, Wells RD. An evidence-based clinical pathway for bronchiolitis safely reduces antibiotic overuse. Am J Med Qual. 2002;17(5):195-199. https://doi.org/10.1177/106286060201700507
11. Barben J, Kuehni CE, Trachsel D, Hammer J; Swiss Paediatric Respiratory Research Group. Management of acute bronchiolitis: can evidence based guidelines alter clinical practice? Thorax. 2008;63(12):1103-1109. https://doi.org/10.1136/thx.2007.094706
12. Bryan MA, Desai AD, Wilson L, Wright DR, Mangione-Smith R. Association of bronchiolitis clinical pathway adherence with length of stay and costs. Pediatrics. 2017;139(3):e20163432. https://doi.org/10.1542/peds.2016-3432
13. Mittal S, Marlowe L, Blakeslee S, et al. Successful use of quality improvement methodology to reduce inpatient length of stay in bronchiolitis through judicious use of intermittent pulse oximetry. Hosp Pediatr. 2019;9(2):73-78. https://doi.org/10.1542/hpeds.2018-0023
14. Macias CG, Mansbach JM, Fisher ES, et al. Variability in inpatient management of children hospitalized with bronchiolitis. Acad Pediatr. 2015;15(1):69-76. https://doi.org/10.1016/j.acap.2014.07.005
15. Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570-6.e3. https://doi.org/10.1016/j.jpeds.2014.05.021
16. Bajaj L, Turner CG, Bothner J. A randomized trial of home oxygen therapy from the emergency department for acute bronchiolitis. Pediatrics. 2006;117(3):633-640. https://doi.org/10.1542/peds.2005-1322
17. Sandweiss DR, Mundorff MB, Hill T, et al. Decreasing hospital length of stay for bronchiolitis by using an observation unit and home oxygen therapy. JAMA Pediatr. 2013;167(5):422-428. https://doi.org/10.1001/jamapediatrics.2013.1435
18. National Bureau of Economic Research. ICD-9-CM to and from ICD-10-CM and ICD-10-PCS crosswalk or general equivalence mappings. Accessed December 2, 2020. http://www.nber.org/data/icd9-icd-10-cm-and-pcs-crosswalk-general-equivalence-mapping.html
19. Utah Department of Health, Indicator-Based Information System for Public Health. Accessed February 15, 2020. https://ibis.health.utah.gov/ibisph-view
20. James BC, Savitz LA. How Intermountain trimmed health care costs through robust quality improvement efforts. Health Aff (Millwood). 2011;30(6):1185-1191. https://doi.org/10.1377/hlthaff.2011.0358
21. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-44. https://doi.org/10.1016/j.acap.2013.08.002
22. StataCorp. Stata Statistical Software: Release 15. StataCorp LLC; 2017.
23. Freeman JF, Deakyne S, Bajaj L. Emergency department-initiated home oxygen for bronchiolitis: a prospective study of community follow-up, caregiver satisfaction, and outcomes. Acad Emerg Med. 2017;24(8):920-929. https://doi.org/10.1111/acem.13179
24. Freeman JF, Brou L, Mistry R. Feasibility and capacity for widespread use of emergency department-based home oxygen for bronchiolitis. Am J Emerg Med. 2017;35(9):1379-1381. https://doi.org/10.1016/j.ajem.2017.03.069
25. Halstead S, Roosevelt G, Deakyne S, Bajaj L. Discharged on supplemental oxygen from an emergency department in patients with bronchiolitis. Pediatrics. 2012;129(3):e605-610. https://doi.org/10.1542/peds.2011-0889
26. Quinonez RA, Coon ER, Schroeder AR, Moyer VA. When technology creates uncertainty: pulse oximetry and overdiagnosis of hypoxaemia in bronchiolitis. BMJ. 2017;358:j3850. https://doi.org/10.1136/bmj.j3850
27. Burrows J, Berg K, McCulloh R. Intermittent pulse oximetry use and length of stay in bronchiolitis: bystander or primary Driver? Hosp Pediatr. 2019;9(2):142-143. https://doi.org/10.1542/hpeds.2018-0183
28. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. https://doi.org/10.1093/cid/ciy745
29. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. https://doi.org/10.1016/j.ijantimicag.2015.07.001
30. Beaty RS, Bernhardt MB, Berger AH, Hesselgrave JE, Russell HV, Okcu MF. Inpatient versus outpatient vincristine, dactinomycin, and cyclophosphamide for pediatric cancers: quality and cost implications. Pediatr Blood Cancer. 2015;62(11):1925-1928. https://doi.org/10.1002/pbc.25610
31. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high-flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
32. Freeman JF, Weng H-YC, Sandweiss D. Outpatient management of home oxygen for bronchiolitis. Clin Pediatr (Phila). 2015;54(1):62-66. https://doi.org/10.1177/0009922814547564
© 2021 Society of Hospital Medicine
Automating Measurement of Trainee Work Hours
Across the country, residents are bound to a set of rules from the Accreditation Council for Graduate Medical Education (ACGME) designed to mini mize fatigue, maintain quality of life, and reduce fatigue-related patient safety events. Adherence to work hours regulations is required to maintain accreditation. Among other guidelines, residents are required to work fewer than 80 hours per week on average over 4 consecutive weeks.1 When work hour violations occur, programs risk citation, penalties, and harm to the program’s reputation.
Residents self-report their adherence to program regulations in an annual survey conducted by the ACGME.2 To collect more frequent data, most training programs monitor resident work hours through self-report on an electronic tracking platform.3 These data generally are used internally to identify problems and opportunities for improvement. However, self-report approaches are subject to imperfect recall and incomplete reporting, and require time and effort to complete.4
The widespread adoption of electronic health records (EHRs) brings new opportunity to measure and promote adherence to work hours. EHR log data capture when users log in and out of the system, along with their location and specific actions. These data offer a compelling alternative to self-report because they are already being collected and can be analyzed almost immediately. Recent studies using EHR log data to approximate resident work hours in a pediatric hospital successfully approximated scheduled hours, but the approach was customized to their hospital’s workflows and might not generalize to other settings.5 Furthermore, earlier studies have not captured evening out-of-hospital work, which contributes to total work hours and is associated with physician burnout.6
We developed a computational method that sought to accurately capture work hours, including out-of-hospital work, which could be used as a screening tool to identify at-risk residents and rotations in near real-time. We estimated work hours, including EHR and non-EHR work, from these EHR data and compared these daily estimations to self-report. We then used a heuristic to estimate the frequency of exceeding the 80-hour workweek in a large internal medicine residency program.
METHODS
The population included 82 internal medicine interns (PGY-1) and 121 residents (PGY-2 = 60, PGY-3 = 61) who rotated through University of California, San Francisco Medical Center (UCSFMC) between July 1, 2018, and June 30, 2019, on inpatient rotations. In the UCSF internal medicine residency program, interns spend an average of 5 months per year and residents spend an average of 2 months per year on inpatient rotations at UCSFMC. Scheduled inpatient rotations generally are in 1-month blocks and include general medical wards, cardiology, liver transplant, night-float, and a procedures and jeopardy rotation where interns perform procedures at UCSFMC and serve as backup for their colleagues across sites. Although expected shift duration differs by rotation, types of shifts include regular length days, call days that are not overnight (but expected duration of work is into the late evening), 28-hour overnight call (PGY-2 and PGY-3), and night-float.
Data Source
This computational method was developed at UCSFMC. This study was approved by the University of California, San Francisco institutional review board. Using the UCSF Epic Clarity database, EHR access log data were obtained, including all Epic logins/logoffs, times, and access devices. Access devices identified included medical center computers, personal computers, and mobile devices.
Trainees self-report their work hours in MedHub, a widely used electronic tracking platform for self-report of resident work hours.7 Data were extracted from this database for interns and residents who matched the criteria above. The self-report data were considered the gold standard for comparison, because it is the best available despite its known limitations.
We used data collected from UCSF’s physician scheduling platform, AMiON, to identify interns and residents assigned to rotations at UCSF hospitals.8 AMiON also was used to capture half-days of off-site scheduled clinics and teaching, which count toward the workday but would not be associated with on-campus logins.
Developing a Computational Method to Measure Work Hours
We developed a heuristic to accomplish two goals: (1) infer the duration of continuous in-hospital work hours while providing clinical care and (2) measure “out-of-hospital” work. Logins from medical center computers were considered to be “on-campus” work. Logins from personal computers were considered to be “out-of-hospital.” “Out-of-hospital” login sessions were further subdivided into “out-of-hospital work” and “out-of-hospital study” based on activity during the session; if any work activities listed in Appendix Table 1 were performed, the session was attributed to work. If only chart review was performed, the session was attributed to study and did not count towards total hours worked. Logins from mobile devices also did not count towards total hours worked.
We inferred continuous in-hospital work by linking on-campus EHR sessions from the first on-campus login until the last on-campus logoff (Figure 1). 
If there was overlapping time measurement between on-campus work and personal computer logins (for example, a resident was inferred to be doing on-campus work based on frequent medical center computer logins but there were also logins from personal computers), we inferred this to indicate that a personal device had been brought on-campus and the time was only attributed to on-campus work and was not double counted as out-of-hospital work. Out-of-hospital work that did not overlap with inferred on-campus work time contributed to the total hours worked in a week, consistent with ACGME guidelines.
Our internal medicine residents work at three hospitals: UCSFMC and two affiliated teaching hospitals. Although this study measured work hours while the residents were on an inpatient rotation at UCSFMC, trainees also might have occasional half-day clinics or teaching activities at other sites not captured by these EHR log data. The allocated time for that scheduled activity (extracted from AMiON) was counted as work hours. If the trainee was assigned to a morning half-day of off-site work (eg, didactics), this was counted the same as an 8
Comparison of EHR-Derived Work Hours Heuristic to Self-Report
Because resident adherence with daily self-report is imperfect, we compared EHR-derived work to self-report on days when both were available. We generated scatter plots of EHR-derived work hours compared with self-report and calculated the mean absolute error of estimation. We fit a linear mixed-effect model for each PGY, modeling self-reported hours as a linear function of estimated hours (fixed effect) with a random intercept (random effect) for each trainee to account for variations among individuals. StatsModels, version 0.11.1, was used for statistical analyses.9
We reviewed detailed data from outlier clusters to understand situations where the heuristic might not perform optimally. To assess whether EHR-derived work hours reasonably overlapped with expected shifts, 20 8-day blocks from separate interns and residents were randomly selected for qualitative detail review in comparison with AMiON schedule data.
Estimating Hours Worked and Work Hours Violations
After validating against self-report on a daily basis, we used our heuristic to infer the average rate at which the 80-hour workweek was exceeded across all inpatient rotations at UCSFMC. This was determined both including “out-of-hospital” work as derived from logins on personal computers and excluding it. Using the estimated daily hours worked, we built a near real-time dashboard to assist program leadership with identifying at-risk trainees and trends across the program.
RESULTS
Data from 82 interns (PGY-1) and 121 internal medicine residents (PGY-2 and PGY-3) who rotated at UCSFMC between July 1, 2018, and June 30, 2019, were included in the study. Table 1 shows the number of days and rotations worked at UCSFMC as well as the frequency of self-report of work hours according to program year. 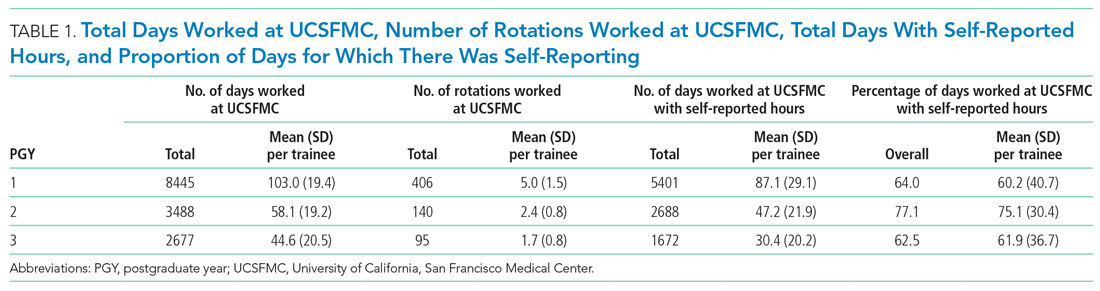
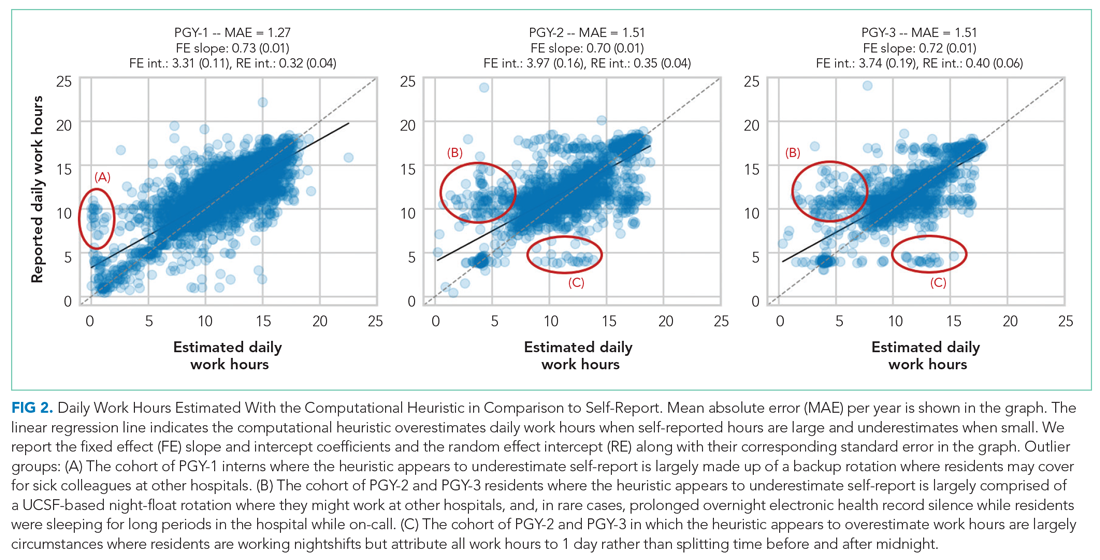
Qualitative review of EHR-derived data compared with schedule data showed that, although residents often reported homogenous daily work hours, EHR-derived work hours often varied as expected on a day-to-day basis according to the schedule (Appendix Table 2).
Because out-of-hospital EHR use does not count as work if done for educational purposes, we evaluated the proportion of out-of-hospital EHR use that is considered work and found that 67% of PGY-1, 50% of PGY-2, and 53% of PGY-3 out-of-hospital sessions included at least one work activity, as denoted in Appendix Table 1. Out-of-hospital work therefore represented 85% of PGY-1, 66% of PGY-2, and 73% of PGY-3 time spent in the EHR out-of-hospital. These sessions were counted towards work hours in accordance with ACGME rules and included 29% of PGY-1 workdays and 21% of PGY-2 and PGY-3 workdays. This amounted to a median of 1.0 hours per day (95% CI, 0.1-4.6 hours) of out-of-hospital work for PGY-1, 0.9 hours per day (95% CI, 0.1-4.1 hours) for PGY-2, and 0.8 hours per day (95% CI, 0.1-4.7 hours) for PGY-3 residents. Out-of-hospital logins that did not include work activities, as denoted in Appendix Table 1, were labeled out-of-hospital study and did not count towards work hours; this amounted to a median of 0.3 hours per day (95% CI, 0.02-1.6 hours) for PGY-1, 0.5 hours per day (95% CI, 0.04-0.25 hours) for PGY-2, and 0.3 hours per day (95% CI, 0.03-1.7 hours) for PGY-3. Mobile device logins also were not counted towards total work hours, with a median of 3 minutes per day for PGY-1, 6 minutes per day for PGY-2, and 5 minutes per day for PGY-3.
The percentage of rotation months where average hours worked exceeded 80 hours weekly is shown in Table 2. Inclusion of out-of-hospital work hours substantially increased the frequency at which the 80-hour workweek was exceeded. The frequency of individual residents working more than 80 hours weekly on average is shown in Appendix Figure 3. A narrow majority of PGY-1 and PGY-2 trainees and a larger majority of PGY-3 trainees never worked in excess of 80 hours per week when averaged over the course of a rotation, but several trainees did on several occasions.
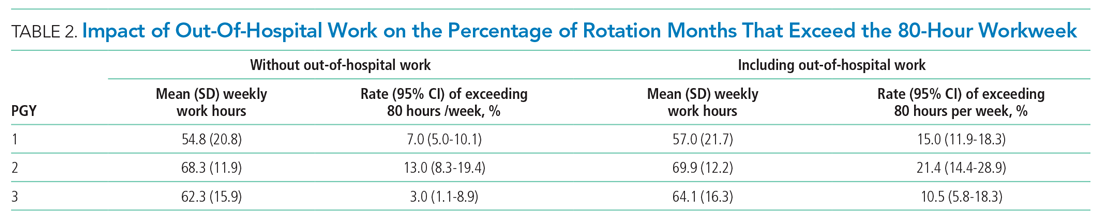
Estimations from the computational method were built into a dashboard for use as screening tool by residency program directors (Appendix Figure 4).
DISCUSSION
EHR log data can be used to automate measurement of trainee work hours, providing timely data to program directors for identifying residents at risk of exceeding work hours limits. We demonstrated this by developing a data-driven approach to link on-campus logins that can be replicated in other training programs. We further demonstrated that out-of-hospital work substantially contributed to resident work hours and the frequency with which they exceed the 80-hour workweek, making it a critical component of any work hour estimation approach. Inclusive of out-of-hospital work, our computational method found that residents exceeded the 80-hour workweek 10% to 21% of the time, depending on their year in residency, with a small majority of residents never exceeding the 80-hour workweek.
Historically, most ACGME residency programs have relied on resident self-report to determine work hours.3 The validity of this method has been extensively studied and results remain mixed; in some surveys, residents admit to underreporting their hours while other validation studies, including the use of clock-in and clock-out or time-stamped parking data, align with self-report relatively well.10-12 Regardless of the reliability of self-report, it is a cumbersome task that residents have difficulty adhering to, as shown in our study, where only slightly more than one-half of the days worked had associated self-report. By relying on resident self-report, we are adding to the burden of clerical work, which is associated with physician burnout.13 Furthermore, because self-report typically does not happen in real-time, it limits a program’s ability to intervene on recent or impending work-hour violations. Our computational method enabled us to build a dashboard that is updated daily and provides critical insight into resident work hours at any time, without waiting for retrospective self-report.
Our study builds on previous work by Dziorny et al using EHR log data to algorithmically measure in-hospital work.5 In their study, the authors isolated shifts with a login gap of 4 hours and then combined shifts according to a set of heuristics. However, their logic integrated an extensive workflow analysis of trainee shifts, which might limit generalizability.5 Our approach computationally derives the temporal threshold for linking EHR sessions, which in our data was 5 hours but might differ at other sites. Automated derivation of this threshold will support generalizability to other programs and sites, although programs will still need to manually account for off-site work such as didactics. In a subsequent study evaluating the 80-hour workweek, Dziorny et al evaluated shift duration and appropriate time-off between shifts and found systematic underreporting of work.14 In our study, we prioritized evaluation of the 80-hour workweek and found general alignment between self-report and EHR-derived work-hour estimates, with a tendency to underestimate at lower reported work hours and overestimate at higher reported work hours (potentially because of underreporting as illustrated by Dziorny et al). We included the important out-of-hospital logins as discrete work events because out-of-hospital work contributes to the total hours worked and to the number of workweeks that exceed the 80-hour workweek, and might contribute to burnout.15 The incidence of exceeding the 80-hour workweek increased by 7% to 8% across all residents when out-of-hospital work was included, demonstrating that tools such as ResQ (ResQ Medical) that rely primarily on geolocation data might not sufficiently capture the ways in which residents spend their time working.16
Our approach has limitations. We determined on-campus vs out-of-hospital locations based on whether the login device belonged to the medical center or was a personal computer. Consequently, if trainees exclusively used a personal computer while on-campus and never used a medical center computer, we would have captured this work done while logged into the EHR but would not have inferred on-campus work. Although nearly all trainees in our organization use medical center computers throughout the day, this might impact generalizability for programs where trainees use personal computers exclusively in the hospital. Our approach also assumes trainees will use the EHR at the beginning and end of their workdays, which could lead to underestimation of work hours in trainees who do not employ this practice. With regards to work done on personal computers, our heuristic required that at least one work activity (as denoted in Appendix Table 1) be included in the session in order for it to count as work. Although this approach allows us to exclude sessions where trainees might be reviewing charts exclusively for educational purposes, it is difficult to infer the true intent of chart review.
There might be periods of time where residents are doing in-hospital work but more than 5 hours elapsed between EHR user sessions. As we have started adapting this computational method for other residency programs, we have added logic that allows for long periods of time in the operating room to be considered part of a continuous workday. There also are limitations to assigning blocks of time to off-site clinics; clinics that are associated with after-hours work but use a different EHR would not be captured in total out-of-hospital work.
Although correlation with self-report was good, we identified clusters of inaccuracy. This likely resulted from our residency program covering three medical centers, two of which were not included in the data set. For example, if a resident had an off-site clinic that was not accounted for in AMiON, EHR-derived work hours might have been underestimated relative to self-report. Operationally leveraging an automated system for measuring work hours in the form of dashboards and other tools could provide the impetus to ensure accurate documentation of schedule anomalies.
CONCLUSION
Implementation of our EHR-derived work-hour model will allow ACGME residency programs to understand and act upon trainee work-hour violations closer to real time, as the data extraction is daily and automated. Automation will save busy residents a cumbersome task, provide more complete data than self-report, and empower residency programs to intervene quickly to support overworked trainees.
Acknowledgments
The authors thank Drs Bradley Monash, Larissa Thomas, and Rebecca Berman for providing residency program input.
1. Accreditation Council for Graduate Medical Education. Common program requirements. Accessed August 12, 2020. https://www.acgme.org/What-We-Do/Accreditation/Common-Program-Requirements
2. Accreditation Council for Graduate Medical Education. Resident/fellow and faculty surveys. Accessed August 12, 2020. https://www.acgme.org/Data-Collection-Systems/Resident-Fellow-and-Faculty-Surveys
3. Petre M, Geana R, Cipparrone N, et al. Comparing electronic and manual tracking systems for monitoring resident duty hours. Ochsner J. 2016;16(1):16-21.
4. Gonzalo JD, Yang JJ, Ngo L, Clark A, Reynolds EE, Herzig SJ. Accuracy of residents’ retrospective perceptions of 16-hour call admitting shift compliance and characteristics. Grad Med Educ. 2013;5(4):630-633. https://doi.org/10.4300/jgme-d-12-00311.1
5. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Automatic detection of front-line clinician hospital shifts: a novel use of electronic health record timestamp data. Appl Clin Inform. 2019;10(1):28-37. https://doi.org/10.1055/s-0038-1676819
6. Gardner RL, Cooper E, Haskell J, et al. Physician stress and burnout: the impact of health information technology. J Am Med Inform Assoc. 2019;26(2):106-114. https://doi.org/10.1093/jamia/ocy145
7. MedHub. Accessed April 7, 2021. https://www.medhub.com
8. AMiON. Accessed April 7, 2021. https://www.amion.com
9. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference. https://conference.scipy.org/proceedings/scipy2010/pdfs/seabold.pdf
10. Todd SR, Fahy BN, Paukert JL, Mersinger D, Johnson ML, Bass BL. How accurate are self-reported resident duty hours? J Surg Educ. 2010;67(2):103-107. https://doi.org/10.1016/j.jsurg.2009.08.004
11. Chadaga SR, Keniston A, Casey D, Albert RK. Correlation between self-reported resident duty hours and time-stamped parking data. J Grad Med Educ. 2012;4(2):254-256. https://doi.org/10.4300/JGME-D-11-00142.1
12. Drolet BC, Schwede M, Bishop KD, Fischer SA. Compliance and falsification of duty hours: reports from residents and program directors. J Grad Med Educ. 2013;5(3):368-373. https://doi.org/10.4300/JGME-D-12-00375.1
13. Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317(9):901. https://doi.org/10.1001/jama.2017.0076
14. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Pediatric trainees systematically under-report duty hour violations compared to electronic health record defined shifts. PLOS ONE. 2019;14(12):e0226493. https://doi.org/10.1371/journal.pone.0226493
15. Saag HS, Shah K, Jones SA, Testa PA, Horwitz LI. Pajama time: working after work in the electronic health record. J Gen Intern Med. 2019;34(9):1695-1696. https://doi.org/10.1007/s11606-019-05055-x
16. ResQ Medical. Accessed April 7, 2021. https://resqmedical.com
Across the country, residents are bound to a set of rules from the Accreditation Council for Graduate Medical Education (ACGME) designed to mini mize fatigue, maintain quality of life, and reduce fatigue-related patient safety events. Adherence to work hours regulations is required to maintain accreditation. Among other guidelines, residents are required to work fewer than 80 hours per week on average over 4 consecutive weeks.1 When work hour violations occur, programs risk citation, penalties, and harm to the program’s reputation.
Residents self-report their adherence to program regulations in an annual survey conducted by the ACGME.2 To collect more frequent data, most training programs monitor resident work hours through self-report on an electronic tracking platform.3 These data generally are used internally to identify problems and opportunities for improvement. However, self-report approaches are subject to imperfect recall and incomplete reporting, and require time and effort to complete.4
The widespread adoption of electronic health records (EHRs) brings new opportunity to measure and promote adherence to work hours. EHR log data capture when users log in and out of the system, along with their location and specific actions. These data offer a compelling alternative to self-report because they are already being collected and can be analyzed almost immediately. Recent studies using EHR log data to approximate resident work hours in a pediatric hospital successfully approximated scheduled hours, but the approach was customized to their hospital’s workflows and might not generalize to other settings.5 Furthermore, earlier studies have not captured evening out-of-hospital work, which contributes to total work hours and is associated with physician burnout.6
We developed a computational method that sought to accurately capture work hours, including out-of-hospital work, which could be used as a screening tool to identify at-risk residents and rotations in near real-time. We estimated work hours, including EHR and non-EHR work, from these EHR data and compared these daily estimations to self-report. We then used a heuristic to estimate the frequency of exceeding the 80-hour workweek in a large internal medicine residency program.
METHODS
The population included 82 internal medicine interns (PGY-1) and 121 residents (PGY-2 = 60, PGY-3 = 61) who rotated through University of California, San Francisco Medical Center (UCSFMC) between July 1, 2018, and June 30, 2019, on inpatient rotations. In the UCSF internal medicine residency program, interns spend an average of 5 months per year and residents spend an average of 2 months per year on inpatient rotations at UCSFMC. Scheduled inpatient rotations generally are in 1-month blocks and include general medical wards, cardiology, liver transplant, night-float, and a procedures and jeopardy rotation where interns perform procedures at UCSFMC and serve as backup for their colleagues across sites. Although expected shift duration differs by rotation, types of shifts include regular length days, call days that are not overnight (but expected duration of work is into the late evening), 28-hour overnight call (PGY-2 and PGY-3), and night-float.
Data Source
This computational method was developed at UCSFMC. This study was approved by the University of California, San Francisco institutional review board. Using the UCSF Epic Clarity database, EHR access log data were obtained, including all Epic logins/logoffs, times, and access devices. Access devices identified included medical center computers, personal computers, and mobile devices.
Trainees self-report their work hours in MedHub, a widely used electronic tracking platform for self-report of resident work hours.7 Data were extracted from this database for interns and residents who matched the criteria above. The self-report data were considered the gold standard for comparison, because it is the best available despite its known limitations.
We used data collected from UCSF’s physician scheduling platform, AMiON, to identify interns and residents assigned to rotations at UCSF hospitals.8 AMiON also was used to capture half-days of off-site scheduled clinics and teaching, which count toward the workday but would not be associated with on-campus logins.
Developing a Computational Method to Measure Work Hours
We developed a heuristic to accomplish two goals: (1) infer the duration of continuous in-hospital work hours while providing clinical care and (2) measure “out-of-hospital” work. Logins from medical center computers were considered to be “on-campus” work. Logins from personal computers were considered to be “out-of-hospital.” “Out-of-hospital” login sessions were further subdivided into “out-of-hospital work” and “out-of-hospital study” based on activity during the session; if any work activities listed in Appendix Table 1 were performed, the session was attributed to work. If only chart review was performed, the session was attributed to study and did not count towards total hours worked. Logins from mobile devices also did not count towards total hours worked.
We inferred continuous in-hospital work by linking on-campus EHR sessions from the first on-campus login until the last on-campus logoff (Figure 1). 
If there was overlapping time measurement between on-campus work and personal computer logins (for example, a resident was inferred to be doing on-campus work based on frequent medical center computer logins but there were also logins from personal computers), we inferred this to indicate that a personal device had been brought on-campus and the time was only attributed to on-campus work and was not double counted as out-of-hospital work. Out-of-hospital work that did not overlap with inferred on-campus work time contributed to the total hours worked in a week, consistent with ACGME guidelines.
Our internal medicine residents work at three hospitals: UCSFMC and two affiliated teaching hospitals. Although this study measured work hours while the residents were on an inpatient rotation at UCSFMC, trainees also might have occasional half-day clinics or teaching activities at other sites not captured by these EHR log data. The allocated time for that scheduled activity (extracted from AMiON) was counted as work hours. If the trainee was assigned to a morning half-day of off-site work (eg, didactics), this was counted the same as an 8
Comparison of EHR-Derived Work Hours Heuristic to Self-Report
Because resident adherence with daily self-report is imperfect, we compared EHR-derived work to self-report on days when both were available. We generated scatter plots of EHR-derived work hours compared with self-report and calculated the mean absolute error of estimation. We fit a linear mixed-effect model for each PGY, modeling self-reported hours as a linear function of estimated hours (fixed effect) with a random intercept (random effect) for each trainee to account for variations among individuals. StatsModels, version 0.11.1, was used for statistical analyses.9
We reviewed detailed data from outlier clusters to understand situations where the heuristic might not perform optimally. To assess whether EHR-derived work hours reasonably overlapped with expected shifts, 20 8-day blocks from separate interns and residents were randomly selected for qualitative detail review in comparison with AMiON schedule data.
Estimating Hours Worked and Work Hours Violations
After validating against self-report on a daily basis, we used our heuristic to infer the average rate at which the 80-hour workweek was exceeded across all inpatient rotations at UCSFMC. This was determined both including “out-of-hospital” work as derived from logins on personal computers and excluding it. Using the estimated daily hours worked, we built a near real-time dashboard to assist program leadership with identifying at-risk trainees and trends across the program.
RESULTS
Data from 82 interns (PGY-1) and 121 internal medicine residents (PGY-2 and PGY-3) who rotated at UCSFMC between July 1, 2018, and June 30, 2019, were included in the study. Table 1 shows the number of days and rotations worked at UCSFMC as well as the frequency of self-report of work hours according to program year. 

Qualitative review of EHR-derived data compared with schedule data showed that, although residents often reported homogenous daily work hours, EHR-derived work hours often varied as expected on a day-to-day basis according to the schedule (Appendix Table 2).
Because out-of-hospital EHR use does not count as work if done for educational purposes, we evaluated the proportion of out-of-hospital EHR use that is considered work and found that 67% of PGY-1, 50% of PGY-2, and 53% of PGY-3 out-of-hospital sessions included at least one work activity, as denoted in Appendix Table 1. Out-of-hospital work therefore represented 85% of PGY-1, 66% of PGY-2, and 73% of PGY-3 time spent in the EHR out-of-hospital. These sessions were counted towards work hours in accordance with ACGME rules and included 29% of PGY-1 workdays and 21% of PGY-2 and PGY-3 workdays. This amounted to a median of 1.0 hours per day (95% CI, 0.1-4.6 hours) of out-of-hospital work for PGY-1, 0.9 hours per day (95% CI, 0.1-4.1 hours) for PGY-2, and 0.8 hours per day (95% CI, 0.1-4.7 hours) for PGY-3 residents. Out-of-hospital logins that did not include work activities, as denoted in Appendix Table 1, were labeled out-of-hospital study and did not count towards work hours; this amounted to a median of 0.3 hours per day (95% CI, 0.02-1.6 hours) for PGY-1, 0.5 hours per day (95% CI, 0.04-0.25 hours) for PGY-2, and 0.3 hours per day (95% CI, 0.03-1.7 hours) for PGY-3. Mobile device logins also were not counted towards total work hours, with a median of 3 minutes per day for PGY-1, 6 minutes per day for PGY-2, and 5 minutes per day for PGY-3.
The percentage of rotation months where average hours worked exceeded 80 hours weekly is shown in Table 2. Inclusion of out-of-hospital work hours substantially increased the frequency at which the 80-hour workweek was exceeded. The frequency of individual residents working more than 80 hours weekly on average is shown in Appendix Figure 3. A narrow majority of PGY-1 and PGY-2 trainees and a larger majority of PGY-3 trainees never worked in excess of 80 hours per week when averaged over the course of a rotation, but several trainees did on several occasions.

Estimations from the computational method were built into a dashboard for use as screening tool by residency program directors (Appendix Figure 4).
DISCUSSION
EHR log data can be used to automate measurement of trainee work hours, providing timely data to program directors for identifying residents at risk of exceeding work hours limits. We demonstrated this by developing a data-driven approach to link on-campus logins that can be replicated in other training programs. We further demonstrated that out-of-hospital work substantially contributed to resident work hours and the frequency with which they exceed the 80-hour workweek, making it a critical component of any work hour estimation approach. Inclusive of out-of-hospital work, our computational method found that residents exceeded the 80-hour workweek 10% to 21% of the time, depending on their year in residency, with a small majority of residents never exceeding the 80-hour workweek.
Historically, most ACGME residency programs have relied on resident self-report to determine work hours.3 The validity of this method has been extensively studied and results remain mixed; in some surveys, residents admit to underreporting their hours while other validation studies, including the use of clock-in and clock-out or time-stamped parking data, align with self-report relatively well.10-12 Regardless of the reliability of self-report, it is a cumbersome task that residents have difficulty adhering to, as shown in our study, where only slightly more than one-half of the days worked had associated self-report. By relying on resident self-report, we are adding to the burden of clerical work, which is associated with physician burnout.13 Furthermore, because self-report typically does not happen in real-time, it limits a program’s ability to intervene on recent or impending work-hour violations. Our computational method enabled us to build a dashboard that is updated daily and provides critical insight into resident work hours at any time, without waiting for retrospective self-report.
Our study builds on previous work by Dziorny et al using EHR log data to algorithmically measure in-hospital work.5 In their study, the authors isolated shifts with a login gap of 4 hours and then combined shifts according to a set of heuristics. However, their logic integrated an extensive workflow analysis of trainee shifts, which might limit generalizability.5 Our approach computationally derives the temporal threshold for linking EHR sessions, which in our data was 5 hours but might differ at other sites. Automated derivation of this threshold will support generalizability to other programs and sites, although programs will still need to manually account for off-site work such as didactics. In a subsequent study evaluating the 80-hour workweek, Dziorny et al evaluated shift duration and appropriate time-off between shifts and found systematic underreporting of work.14 In our study, we prioritized evaluation of the 80-hour workweek and found general alignment between self-report and EHR-derived work-hour estimates, with a tendency to underestimate at lower reported work hours and overestimate at higher reported work hours (potentially because of underreporting as illustrated by Dziorny et al). We included the important out-of-hospital logins as discrete work events because out-of-hospital work contributes to the total hours worked and to the number of workweeks that exceed the 80-hour workweek, and might contribute to burnout.15 The incidence of exceeding the 80-hour workweek increased by 7% to 8% across all residents when out-of-hospital work was included, demonstrating that tools such as ResQ (ResQ Medical) that rely primarily on geolocation data might not sufficiently capture the ways in which residents spend their time working.16
Our approach has limitations. We determined on-campus vs out-of-hospital locations based on whether the login device belonged to the medical center or was a personal computer. Consequently, if trainees exclusively used a personal computer while on-campus and never used a medical center computer, we would have captured this work done while logged into the EHR but would not have inferred on-campus work. Although nearly all trainees in our organization use medical center computers throughout the day, this might impact generalizability for programs where trainees use personal computers exclusively in the hospital. Our approach also assumes trainees will use the EHR at the beginning and end of their workdays, which could lead to underestimation of work hours in trainees who do not employ this practice. With regards to work done on personal computers, our heuristic required that at least one work activity (as denoted in Appendix Table 1) be included in the session in order for it to count as work. Although this approach allows us to exclude sessions where trainees might be reviewing charts exclusively for educational purposes, it is difficult to infer the true intent of chart review.
There might be periods of time where residents are doing in-hospital work but more than 5 hours elapsed between EHR user sessions. As we have started adapting this computational method for other residency programs, we have added logic that allows for long periods of time in the operating room to be considered part of a continuous workday. There also are limitations to assigning blocks of time to off-site clinics; clinics that are associated with after-hours work but use a different EHR would not be captured in total out-of-hospital work.
Although correlation with self-report was good, we identified clusters of inaccuracy. This likely resulted from our residency program covering three medical centers, two of which were not included in the data set. For example, if a resident had an off-site clinic that was not accounted for in AMiON, EHR-derived work hours might have been underestimated relative to self-report. Operationally leveraging an automated system for measuring work hours in the form of dashboards and other tools could provide the impetus to ensure accurate documentation of schedule anomalies.
CONCLUSION
Implementation of our EHR-derived work-hour model will allow ACGME residency programs to understand and act upon trainee work-hour violations closer to real time, as the data extraction is daily and automated. Automation will save busy residents a cumbersome task, provide more complete data than self-report, and empower residency programs to intervene quickly to support overworked trainees.
Acknowledgments
The authors thank Drs Bradley Monash, Larissa Thomas, and Rebecca Berman for providing residency program input.
Across the country, residents are bound to a set of rules from the Accreditation Council for Graduate Medical Education (ACGME) designed to mini mize fatigue, maintain quality of life, and reduce fatigue-related patient safety events. Adherence to work hours regulations is required to maintain accreditation. Among other guidelines, residents are required to work fewer than 80 hours per week on average over 4 consecutive weeks.1 When work hour violations occur, programs risk citation, penalties, and harm to the program’s reputation.
Residents self-report their adherence to program regulations in an annual survey conducted by the ACGME.2 To collect more frequent data, most training programs monitor resident work hours through self-report on an electronic tracking platform.3 These data generally are used internally to identify problems and opportunities for improvement. However, self-report approaches are subject to imperfect recall and incomplete reporting, and require time and effort to complete.4
The widespread adoption of electronic health records (EHRs) brings new opportunity to measure and promote adherence to work hours. EHR log data capture when users log in and out of the system, along with their location and specific actions. These data offer a compelling alternative to self-report because they are already being collected and can be analyzed almost immediately. Recent studies using EHR log data to approximate resident work hours in a pediatric hospital successfully approximated scheduled hours, but the approach was customized to their hospital’s workflows and might not generalize to other settings.5 Furthermore, earlier studies have not captured evening out-of-hospital work, which contributes to total work hours and is associated with physician burnout.6
We developed a computational method that sought to accurately capture work hours, including out-of-hospital work, which could be used as a screening tool to identify at-risk residents and rotations in near real-time. We estimated work hours, including EHR and non-EHR work, from these EHR data and compared these daily estimations to self-report. We then used a heuristic to estimate the frequency of exceeding the 80-hour workweek in a large internal medicine residency program.
METHODS
The population included 82 internal medicine interns (PGY-1) and 121 residents (PGY-2 = 60, PGY-3 = 61) who rotated through University of California, San Francisco Medical Center (UCSFMC) between July 1, 2018, and June 30, 2019, on inpatient rotations. In the UCSF internal medicine residency program, interns spend an average of 5 months per year and residents spend an average of 2 months per year on inpatient rotations at UCSFMC. Scheduled inpatient rotations generally are in 1-month blocks and include general medical wards, cardiology, liver transplant, night-float, and a procedures and jeopardy rotation where interns perform procedures at UCSFMC and serve as backup for their colleagues across sites. Although expected shift duration differs by rotation, types of shifts include regular length days, call days that are not overnight (but expected duration of work is into the late evening), 28-hour overnight call (PGY-2 and PGY-3), and night-float.
Data Source
This computational method was developed at UCSFMC. This study was approved by the University of California, San Francisco institutional review board. Using the UCSF Epic Clarity database, EHR access log data were obtained, including all Epic logins/logoffs, times, and access devices. Access devices identified included medical center computers, personal computers, and mobile devices.
Trainees self-report their work hours in MedHub, a widely used electronic tracking platform for self-report of resident work hours.7 Data were extracted from this database for interns and residents who matched the criteria above. The self-report data were considered the gold standard for comparison, because it is the best available despite its known limitations.
We used data collected from UCSF’s physician scheduling platform, AMiON, to identify interns and residents assigned to rotations at UCSF hospitals.8 AMiON also was used to capture half-days of off-site scheduled clinics and teaching, which count toward the workday but would not be associated with on-campus logins.
Developing a Computational Method to Measure Work Hours
We developed a heuristic to accomplish two goals: (1) infer the duration of continuous in-hospital work hours while providing clinical care and (2) measure “out-of-hospital” work. Logins from medical center computers were considered to be “on-campus” work. Logins from personal computers were considered to be “out-of-hospital.” “Out-of-hospital” login sessions were further subdivided into “out-of-hospital work” and “out-of-hospital study” based on activity during the session; if any work activities listed in Appendix Table 1 were performed, the session was attributed to work. If only chart review was performed, the session was attributed to study and did not count towards total hours worked. Logins from mobile devices also did not count towards total hours worked.
We inferred continuous in-hospital work by linking on-campus EHR sessions from the first on-campus login until the last on-campus logoff (Figure 1). 
If there was overlapping time measurement between on-campus work and personal computer logins (for example, a resident was inferred to be doing on-campus work based on frequent medical center computer logins but there were also logins from personal computers), we inferred this to indicate that a personal device had been brought on-campus and the time was only attributed to on-campus work and was not double counted as out-of-hospital work. Out-of-hospital work that did not overlap with inferred on-campus work time contributed to the total hours worked in a week, consistent with ACGME guidelines.
Our internal medicine residents work at three hospitals: UCSFMC and two affiliated teaching hospitals. Although this study measured work hours while the residents were on an inpatient rotation at UCSFMC, trainees also might have occasional half-day clinics or teaching activities at other sites not captured by these EHR log data. The allocated time for that scheduled activity (extracted from AMiON) was counted as work hours. If the trainee was assigned to a morning half-day of off-site work (eg, didactics), this was counted the same as an 8
Comparison of EHR-Derived Work Hours Heuristic to Self-Report
Because resident adherence with daily self-report is imperfect, we compared EHR-derived work to self-report on days when both were available. We generated scatter plots of EHR-derived work hours compared with self-report and calculated the mean absolute error of estimation. We fit a linear mixed-effect model for each PGY, modeling self-reported hours as a linear function of estimated hours (fixed effect) with a random intercept (random effect) for each trainee to account for variations among individuals. StatsModels, version 0.11.1, was used for statistical analyses.9
We reviewed detailed data from outlier clusters to understand situations where the heuristic might not perform optimally. To assess whether EHR-derived work hours reasonably overlapped with expected shifts, 20 8-day blocks from separate interns and residents were randomly selected for qualitative detail review in comparison with AMiON schedule data.
Estimating Hours Worked and Work Hours Violations
After validating against self-report on a daily basis, we used our heuristic to infer the average rate at which the 80-hour workweek was exceeded across all inpatient rotations at UCSFMC. This was determined both including “out-of-hospital” work as derived from logins on personal computers and excluding it. Using the estimated daily hours worked, we built a near real-time dashboard to assist program leadership with identifying at-risk trainees and trends across the program.
RESULTS
Data from 82 interns (PGY-1) and 121 internal medicine residents (PGY-2 and PGY-3) who rotated at UCSFMC between July 1, 2018, and June 30, 2019, were included in the study. Table 1 shows the number of days and rotations worked at UCSFMC as well as the frequency of self-report of work hours according to program year. 

Qualitative review of EHR-derived data compared with schedule data showed that, although residents often reported homogenous daily work hours, EHR-derived work hours often varied as expected on a day-to-day basis according to the schedule (Appendix Table 2).
Because out-of-hospital EHR use does not count as work if done for educational purposes, we evaluated the proportion of out-of-hospital EHR use that is considered work and found that 67% of PGY-1, 50% of PGY-2, and 53% of PGY-3 out-of-hospital sessions included at least one work activity, as denoted in Appendix Table 1. Out-of-hospital work therefore represented 85% of PGY-1, 66% of PGY-2, and 73% of PGY-3 time spent in the EHR out-of-hospital. These sessions were counted towards work hours in accordance with ACGME rules and included 29% of PGY-1 workdays and 21% of PGY-2 and PGY-3 workdays. This amounted to a median of 1.0 hours per day (95% CI, 0.1-4.6 hours) of out-of-hospital work for PGY-1, 0.9 hours per day (95% CI, 0.1-4.1 hours) for PGY-2, and 0.8 hours per day (95% CI, 0.1-4.7 hours) for PGY-3 residents. Out-of-hospital logins that did not include work activities, as denoted in Appendix Table 1, were labeled out-of-hospital study and did not count towards work hours; this amounted to a median of 0.3 hours per day (95% CI, 0.02-1.6 hours) for PGY-1, 0.5 hours per day (95% CI, 0.04-0.25 hours) for PGY-2, and 0.3 hours per day (95% CI, 0.03-1.7 hours) for PGY-3. Mobile device logins also were not counted towards total work hours, with a median of 3 minutes per day for PGY-1, 6 minutes per day for PGY-2, and 5 minutes per day for PGY-3.
The percentage of rotation months where average hours worked exceeded 80 hours weekly is shown in Table 2. Inclusion of out-of-hospital work hours substantially increased the frequency at which the 80-hour workweek was exceeded. The frequency of individual residents working more than 80 hours weekly on average is shown in Appendix Figure 3. A narrow majority of PGY-1 and PGY-2 trainees and a larger majority of PGY-3 trainees never worked in excess of 80 hours per week when averaged over the course of a rotation, but several trainees did on several occasions.

Estimations from the computational method were built into a dashboard for use as screening tool by residency program directors (Appendix Figure 4).
DISCUSSION
EHR log data can be used to automate measurement of trainee work hours, providing timely data to program directors for identifying residents at risk of exceeding work hours limits. We demonstrated this by developing a data-driven approach to link on-campus logins that can be replicated in other training programs. We further demonstrated that out-of-hospital work substantially contributed to resident work hours and the frequency with which they exceed the 80-hour workweek, making it a critical component of any work hour estimation approach. Inclusive of out-of-hospital work, our computational method found that residents exceeded the 80-hour workweek 10% to 21% of the time, depending on their year in residency, with a small majority of residents never exceeding the 80-hour workweek.
Historically, most ACGME residency programs have relied on resident self-report to determine work hours.3 The validity of this method has been extensively studied and results remain mixed; in some surveys, residents admit to underreporting their hours while other validation studies, including the use of clock-in and clock-out or time-stamped parking data, align with self-report relatively well.10-12 Regardless of the reliability of self-report, it is a cumbersome task that residents have difficulty adhering to, as shown in our study, where only slightly more than one-half of the days worked had associated self-report. By relying on resident self-report, we are adding to the burden of clerical work, which is associated with physician burnout.13 Furthermore, because self-report typically does not happen in real-time, it limits a program’s ability to intervene on recent or impending work-hour violations. Our computational method enabled us to build a dashboard that is updated daily and provides critical insight into resident work hours at any time, without waiting for retrospective self-report.
Our study builds on previous work by Dziorny et al using EHR log data to algorithmically measure in-hospital work.5 In their study, the authors isolated shifts with a login gap of 4 hours and then combined shifts according to a set of heuristics. However, their logic integrated an extensive workflow analysis of trainee shifts, which might limit generalizability.5 Our approach computationally derives the temporal threshold for linking EHR sessions, which in our data was 5 hours but might differ at other sites. Automated derivation of this threshold will support generalizability to other programs and sites, although programs will still need to manually account for off-site work such as didactics. In a subsequent study evaluating the 80-hour workweek, Dziorny et al evaluated shift duration and appropriate time-off between shifts and found systematic underreporting of work.14 In our study, we prioritized evaluation of the 80-hour workweek and found general alignment between self-report and EHR-derived work-hour estimates, with a tendency to underestimate at lower reported work hours and overestimate at higher reported work hours (potentially because of underreporting as illustrated by Dziorny et al). We included the important out-of-hospital logins as discrete work events because out-of-hospital work contributes to the total hours worked and to the number of workweeks that exceed the 80-hour workweek, and might contribute to burnout.15 The incidence of exceeding the 80-hour workweek increased by 7% to 8% across all residents when out-of-hospital work was included, demonstrating that tools such as ResQ (ResQ Medical) that rely primarily on geolocation data might not sufficiently capture the ways in which residents spend their time working.16
Our approach has limitations. We determined on-campus vs out-of-hospital locations based on whether the login device belonged to the medical center or was a personal computer. Consequently, if trainees exclusively used a personal computer while on-campus and never used a medical center computer, we would have captured this work done while logged into the EHR but would not have inferred on-campus work. Although nearly all trainees in our organization use medical center computers throughout the day, this might impact generalizability for programs where trainees use personal computers exclusively in the hospital. Our approach also assumes trainees will use the EHR at the beginning and end of their workdays, which could lead to underestimation of work hours in trainees who do not employ this practice. With regards to work done on personal computers, our heuristic required that at least one work activity (as denoted in Appendix Table 1) be included in the session in order for it to count as work. Although this approach allows us to exclude sessions where trainees might be reviewing charts exclusively for educational purposes, it is difficult to infer the true intent of chart review.
There might be periods of time where residents are doing in-hospital work but more than 5 hours elapsed between EHR user sessions. As we have started adapting this computational method for other residency programs, we have added logic that allows for long periods of time in the operating room to be considered part of a continuous workday. There also are limitations to assigning blocks of time to off-site clinics; clinics that are associated with after-hours work but use a different EHR would not be captured in total out-of-hospital work.
Although correlation with self-report was good, we identified clusters of inaccuracy. This likely resulted from our residency program covering three medical centers, two of which were not included in the data set. For example, if a resident had an off-site clinic that was not accounted for in AMiON, EHR-derived work hours might have been underestimated relative to self-report. Operationally leveraging an automated system for measuring work hours in the form of dashboards and other tools could provide the impetus to ensure accurate documentation of schedule anomalies.
CONCLUSION
Implementation of our EHR-derived work-hour model will allow ACGME residency programs to understand and act upon trainee work-hour violations closer to real time, as the data extraction is daily and automated. Automation will save busy residents a cumbersome task, provide more complete data than self-report, and empower residency programs to intervene quickly to support overworked trainees.
Acknowledgments
The authors thank Drs Bradley Monash, Larissa Thomas, and Rebecca Berman for providing residency program input.
1. Accreditation Council for Graduate Medical Education. Common program requirements. Accessed August 12, 2020. https://www.acgme.org/What-We-Do/Accreditation/Common-Program-Requirements
2. Accreditation Council for Graduate Medical Education. Resident/fellow and faculty surveys. Accessed August 12, 2020. https://www.acgme.org/Data-Collection-Systems/Resident-Fellow-and-Faculty-Surveys
3. Petre M, Geana R, Cipparrone N, et al. Comparing electronic and manual tracking systems for monitoring resident duty hours. Ochsner J. 2016;16(1):16-21.
4. Gonzalo JD, Yang JJ, Ngo L, Clark A, Reynolds EE, Herzig SJ. Accuracy of residents’ retrospective perceptions of 16-hour call admitting shift compliance and characteristics. Grad Med Educ. 2013;5(4):630-633. https://doi.org/10.4300/jgme-d-12-00311.1
5. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Automatic detection of front-line clinician hospital shifts: a novel use of electronic health record timestamp data. Appl Clin Inform. 2019;10(1):28-37. https://doi.org/10.1055/s-0038-1676819
6. Gardner RL, Cooper E, Haskell J, et al. Physician stress and burnout: the impact of health information technology. J Am Med Inform Assoc. 2019;26(2):106-114. https://doi.org/10.1093/jamia/ocy145
7. MedHub. Accessed April 7, 2021. https://www.medhub.com
8. AMiON. Accessed April 7, 2021. https://www.amion.com
9. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference. https://conference.scipy.org/proceedings/scipy2010/pdfs/seabold.pdf
10. Todd SR, Fahy BN, Paukert JL, Mersinger D, Johnson ML, Bass BL. How accurate are self-reported resident duty hours? J Surg Educ. 2010;67(2):103-107. https://doi.org/10.1016/j.jsurg.2009.08.004
11. Chadaga SR, Keniston A, Casey D, Albert RK. Correlation between self-reported resident duty hours and time-stamped parking data. J Grad Med Educ. 2012;4(2):254-256. https://doi.org/10.4300/JGME-D-11-00142.1
12. Drolet BC, Schwede M, Bishop KD, Fischer SA. Compliance and falsification of duty hours: reports from residents and program directors. J Grad Med Educ. 2013;5(3):368-373. https://doi.org/10.4300/JGME-D-12-00375.1
13. Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317(9):901. https://doi.org/10.1001/jama.2017.0076
14. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Pediatric trainees systematically under-report duty hour violations compared to electronic health record defined shifts. PLOS ONE. 2019;14(12):e0226493. https://doi.org/10.1371/journal.pone.0226493
15. Saag HS, Shah K, Jones SA, Testa PA, Horwitz LI. Pajama time: working after work in the electronic health record. J Gen Intern Med. 2019;34(9):1695-1696. https://doi.org/10.1007/s11606-019-05055-x
16. ResQ Medical. Accessed April 7, 2021. https://resqmedical.com
1. Accreditation Council for Graduate Medical Education. Common program requirements. Accessed August 12, 2020. https://www.acgme.org/What-We-Do/Accreditation/Common-Program-Requirements
2. Accreditation Council for Graduate Medical Education. Resident/fellow and faculty surveys. Accessed August 12, 2020. https://www.acgme.org/Data-Collection-Systems/Resident-Fellow-and-Faculty-Surveys
3. Petre M, Geana R, Cipparrone N, et al. Comparing electronic and manual tracking systems for monitoring resident duty hours. Ochsner J. 2016;16(1):16-21.
4. Gonzalo JD, Yang JJ, Ngo L, Clark A, Reynolds EE, Herzig SJ. Accuracy of residents’ retrospective perceptions of 16-hour call admitting shift compliance and characteristics. Grad Med Educ. 2013;5(4):630-633. https://doi.org/10.4300/jgme-d-12-00311.1
5. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Automatic detection of front-line clinician hospital shifts: a novel use of electronic health record timestamp data. Appl Clin Inform. 2019;10(1):28-37. https://doi.org/10.1055/s-0038-1676819
6. Gardner RL, Cooper E, Haskell J, et al. Physician stress and burnout: the impact of health information technology. J Am Med Inform Assoc. 2019;26(2):106-114. https://doi.org/10.1093/jamia/ocy145
7. MedHub. Accessed April 7, 2021. https://www.medhub.com
8. AMiON. Accessed April 7, 2021. https://www.amion.com
9. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference. https://conference.scipy.org/proceedings/scipy2010/pdfs/seabold.pdf
10. Todd SR, Fahy BN, Paukert JL, Mersinger D, Johnson ML, Bass BL. How accurate are self-reported resident duty hours? J Surg Educ. 2010;67(2):103-107. https://doi.org/10.1016/j.jsurg.2009.08.004
11. Chadaga SR, Keniston A, Casey D, Albert RK. Correlation between self-reported resident duty hours and time-stamped parking data. J Grad Med Educ. 2012;4(2):254-256. https://doi.org/10.4300/JGME-D-11-00142.1
12. Drolet BC, Schwede M, Bishop KD, Fischer SA. Compliance and falsification of duty hours: reports from residents and program directors. J Grad Med Educ. 2013;5(3):368-373. https://doi.org/10.4300/JGME-D-12-00375.1
13. Shanafelt TD, Dyrbye LN, West CP. Addressing physician burnout: the way forward. JAMA. 2017;317(9):901. https://doi.org/10.1001/jama.2017.0076
14. Dziorny AC, Orenstein EW, Lindell RB, Hames NA, Washington N, Desai B. Pediatric trainees systematically under-report duty hour violations compared to electronic health record defined shifts. PLOS ONE. 2019;14(12):e0226493. https://doi.org/10.1371/journal.pone.0226493
15. Saag HS, Shah K, Jones SA, Testa PA, Horwitz LI. Pajama time: working after work in the electronic health record. J Gen Intern Med. 2019;34(9):1695-1696. https://doi.org/10.1007/s11606-019-05055-x
16. ResQ Medical. Accessed April 7, 2021. https://resqmedical.com
© 2021 Society of Hospital Medicine
Implementation of a Pharmacist-Managed Transitions of Care Tool
Effective transitions of care (TOC) are essential to ensure quality continuity of care after hospital discharge. About 20 to 30% of patients experience an adverse event (AE) in the peridischarge period when discharged to the community.1 Additionally, about two-thirds of AEs are preventable.1 The Joint Commission has identified various breakdowns in care that are associated with poor outcomes, including a lack of standardized discharge procedures, limited time dedicated to discharge planning and processes, and patients who lack the necessary resources or skills to implement discharge care plans.2
Background
The most impactful TOC programs are those that target patients who are at high risk for readmission or adverse outcomes.3 Factors such as advanced age, polypharmacy, cognitive impairment, and lack of social support are patient characteristics that have been associated with unfavorable outcomes after discharge.4 To identify this subset of high-risk individuals, various risk assessment scores have been developed, ranging from those that are used locally at the facility level to those that are nationally validated. The LACE score (Length of hospital stay; Acuity of the admission; Comorbidities measured with the Charlson comorbidity index score; and Emergency department visits within the past 6 months) is a validated index scoring tool that is used to identify medical and surgical patients at risk for readmission or death within 30 days of hospital discharge. On a 19-point scale, a score of ≥ 10 is considered high risk.5 Specific to the US Department of Veterans Affairs (VA), the Care Assessment Needs (CAN) score was developed to risk stratify the veteran population. The CAN score is generated using information including patient demographics, medical conditions, VA health care utilization, vital signs, laboratory values, medications, and socioeconomic status. This score is expressed as a percentile that compares the probability of death or admission among veterans at 90 days and 1 year postdischarge. Veterans in the 99th percentile have a 74% risk for these adverse outcomes at 1 year.6
The Joint Commission states that a fundamental component to assuring safe and effective TOC is medication management, which includes the involvement of pharmacists.2 TOC programs with pharmacist involvement have shown significant improvements related to reduced 30-day hospital readmissions and health care costs in addition to significant medication-related interventions.7-9 While this body of evidence continues to grow and demonstrates that pharmacists are an integral component of the TOC process, there is no gold standard program. Brantley and colleagues noted that a weakness of many TOC programs is that they are one dimensional, meaning that they focus on only 1 element of care transitions or 1 specific patient population or disease.10
There is well-supported evidence of high-impact interventions for pharmacists involved early in the admission process, but data are less robust on the discharge process. 11,12 Therefore, the primary focus of this project was to develop a pharmacist-based TOC program and implement a process for communicating high-risk patients who are discharging from our hospital across the continuum of care.
Setting
The Richard L. Roudebush VA Medical Center (RLRVAMC) is a tertiary care referral center for veterans in Indiana and eastern Illinois. Acute care clinical pharmacists are fully integrated into the acute care teams and practice under a comprehensive care model. Pharmacists attend daily patient care rounds and conduct discharge medication reconciliation for all patients with additional bedside counseling for patients who are being discharged home.
Primary care services are provided by patient aligned care teams (PACTs), multidisciplinary teams composed of physicians, advanced practice nurses, pharmacists, mental health care providers, registered nurses, dieticians, and care coordinators. Ambulatory Care or PACT clinical pharmacists are established within each RLRVAMC PACT clinic and provide comprehensive care management through an independent scope of practice for several chronic diseases, including hypertension, type 2 diabetes mellitus (T2DM), dyslipidemia, hypothyroidism, and tobacco cessation. Prior to this project implementation, there was no formalized or standardized method for facilitating routine communication of patients between acute care and PACT pharmacists in the TOC process.
Pilot Study
In 2017, RLRVAMC implemented a TOC pharmacy program pilot. A pharmacy resident and both acute care and PACT clinical pharmacy specialists (CPSs) developed the service. The pilot program was conducted from September 1, 2017 to March 1, 2018. The initial phase consisted of the development of an electronic TOC tool to standardize communication between acute care and PACT pharmacists. The TOC tool was created on a secure site accessible only to pharmacy personnel and not part of the formal medical record. (Figure 1).
The acute care pharmacist identified high-risk patients through calculated CAN and LACE scores during the discharge process and offered PACT pharmacist follow-up to the patient during bedside discharge counseling. Information was then entered into the TOC tool, including patient identifiers and a message with specific information outlining the reason for referral. PACT pharmacists routinely reviewed the tool and attempted to phone each patient within 7 days of discharge. Follow-up included medication reconciliation and chronic disease management as warranted at the discretion of the PACT pharmacist. All postdischarge follow-up appointments were created and documented in the electronic health record. A retrospective chart review was completed on patients who were entered into the TOC tool.
Patients were eligible for referral if they were discharged during the study period with primary care established in one of the facility’s PACT clinics. Additionally, patients had to meet ≥ 1 of the following criteria, deeming them a high risk for readmission: LACE score ≥ 10, CAN score ≥ 90th percentile, or be considered high risk based on the discretion of the acute care pharmacist. Patients were included in the analysis if they met the CAN or LACE score requirement. Patients were excluded if they received primary care from a site other than a RLRVAMC PACT clinic. This included non-VA primary care, home-based primary care, or VA community-based outpatient clinics (CBOCs). Patients also were excluded if they required further institutional care postdischarge (ie, subacute rehabilitation, extended care facility, etc), discharged to hospice, or against medical advice.
The average referral rate per month during the pilot study was 19 patients, with 113 total referrals during the 6-month study period. Lower rates of index emergency department (ED) visits (5.3% vs 23.3%) and readmissions (1% vs 6.7%) were seen in the group of patients who received PACT pharmacist follow-up postdischarge compared with those who did not. Additionally, PACT pharmacists were able to make > 120 interventions, averaging 1.7 interventions per patient. Of note, these results were not statistically analyzed and were assessed as observational data to determine whether the program had the potential to be impactful. The results of the pilot study demonstrated positive outcomes associated with having a pharmacist-based TOC process and led to the desire for further development and implementation of the TOC program at the RLRVAMC. These positive results prompted a second phase project to address barriers, make improvements, and ensure sustainability.
Methods
Phase 2 was a quality improvement initiative; therefore, institutional review board approval was not needed. The aim of phase 2 was to improve, expand, and sustain the TOC program that was implemented in the pilot study. Barriers identified after discussion with acute care and PACT pharmacists included difficulty in making referrals due to required entry of cumbersome readmission risk factor calculations, limiting inclusion to patients who receive primary care at the main hospital facility, and the expansion of pharmacy staff with new pharmacists who were not knowledgeable of the referral process.
Design
To overcome barriers, 4 main targeted interventions were needed: streamlining the referral process, enhancing pharmacy staff education, updating the discharge note template, and expanding the criteria to include patients who receive care at VA CBOCs. The referral process was streamlined by removing required calculated readmission risk scores, allowing pharmacist judgement to take precedence for referrals. Focused face-to-face education was provided to acute care and PACT pharmacists about the referral process and inclusion criteria to increase awareness and provide guidance of who may benefit from entry into the tool. Unlike the first phase of the study, education was provided for outpatient staff pharmacists responsible for discharging patients on the weekends. Additionally, the pharmacists received a printed quick reference guide of the information covered during the education sessions (Figure 2). Referral prompts were embedded into the standard pharmacy discharge note template to serve as a reminder to discharging pharmacists to assess patients for inclusion into the tool and provided a direct link to the tool. Expansion to include VA CBOCs occurred postpilot study, allowing increased patient access to this TOC service. All other aspects of the program were continued from the pilot phase.
Patients were eligible if they were discharged from RLRVAMC between October 1, 2018 and February 28, 2019. Additionally, the patient had to be established in a PACT clinic for primary care and have been referred to the tool based on the discretion of an acute care pharmacist. Patients were excluded if they were discharged against medical advice or to any facility where the patient and/or caregiver would not be responsible for medication administration (eg, subacute rehabilitation, extended care facility), or if the patient refused pharmacy follow-up.
Outcomes
The primary outcomes assessed were all-cause and index ED visits and readmissions within 30 days of discharge. All-cause ED visits and readmissions were defined as a second visit to RLRVAMC , regardless of readmission diagnosis. Index ED visits and readmissions were defined as those that were related to the initial admission diagnosis. Additional data collected and analyzed included the number of patients referred by pharmacists, number and type of medication discrepancies, medication changes, counseling interventions, time to follow-up postdischarge, and number of patients added to the PACT pharmacist’s clinic schedule for further management. A discrepancy identified by a PACT pharmacist was defined as a difference between the discharge medication list and the patient-reported medication list at the time of follow-up. Patients who were referred to the TOC tool but were unable to be reached by telephone served as the control group for this study.
Data Collection
A retrospective chart review was completed on patients entered into the tool. Data were collected and kept in a secured Microsoft Excel workbook. Baseline characteristics were analyzed using either a χ2 for nominal data or Student t test for continuous data. The primary outcomes were analyzed using a χ2 test. All statistical tests were analyzed using MiniTab 19 Statistical Software.
Results
Pharmacists added 172 patients into the TOC tool; 139 patients met inclusion criteria. Of those excluded, most were because the PACT pharmacist did not attempt to contact the patient since they already had a primary care visit scheduled postdischarge (Table 1). Of the 139 patients who met the inclusion criteria, 99 were successfully contacted by a PACT pharmacist. Most patients were aged in their 60s, male, and white. Both groups had a similar quantity of outpatient medications on admission and medication changes made at discharge. Additionally, both groups had a similar number of patients with hospitalizations and/or ED visits in the 3 months before hospital admission that resulted in TOC tool referral (Table 2).
Hospital Readmission
Hospital 30-day readmission rates for patients who were successfully followed by pharmacy compared with those who were not were 5.1% vs 15.0% (P = .049) for index readmissions and 8.1% vs 27.5% (P = .03) for all-cause readmissions. No statistically significant difference existed between those patients with follow-up compared with those without follow-up for either index (10.1% vs 12.5%, respectively; P = .68) or for all-cause ED visit rates (15.2% vs 20.0%, respectively; P = .49).
Patient Encounters
The average time to follow-up was 8.8 days, which was above the predetermined goal of contact within 7 days. Additionally, this was a decline from the initial pilot study, which had an average time to reach of 4.7 days. All patients reached by a pharmacist received medication reconciliation, with ≥ 28% of patients having ≥ 1 discrepancy. There were 43 discrepancies among all patients. Of the discrepancies, 25 were reported as errors performed by the patient, and 18 were from an error during the discharge process. The discrepancies that resulted from patient error were primarily patients who took the wrong dose of prescribed medications. Other patient discrepancies included taking medications not as scheduled, omitting medications (both intentionally and mistakenly), continuing to take medications that had been discontinued by a health care provider and improper administration technique. Examples of provider errors that occurred during the discharge process included not ordering medications for patient to pick up at discharge, not discontinuing a medication from the patient’s profile, and failure to renew expired prescriptions.
Additional counseling was provided to 75% of patients: The most common reason for counseling was T2DM, hypertension, and dyslipidemia management. PACT pharmacists changed medication regimens for 27.3% of patients for improved control of chronic diseases or relief of medication AEs.
At the end of each visit, patients were assessed to determine whether they could benefit from additional pharmacy follow-up. Thirty-seven patients were added to the pharmacist schedules for disease management appointments. The most common conditions for these appointments were T2DM, hypertension, tobacco cessation, and hyperlipidemia. Among the 37 patients who had pharmacy follow-up, there were 137 additional pharmacy appointments within the study period.
Program Referrals
After expansion to include the VA CBOCs, elimination of the elevated LACE or CAN score requirement, and additional staff education, the rate of referrals per month increased during phase 2 in comparison to the pilot study (Figure 3). There were a mean (SD) of 34 (10) referrals per month. Although not statistically analyzed, it is an objective increase in comparison to a mean 19 referrals per month in the pilot study.
Discussion
The continued development and use of a pharmacist-driven TOC tool at RLRVAMC increased communication and follow-up of high-risk patients, demonstrated the ability of pharmacists to identify and intervene in medication-related issues postdischarge, and successfully reduce 30-day readmissions. This program emphasized pharmacist involvement during the discharge process and created a standardized mechanism for TOC follow-up, addressing multiple areas that were identified by The Joint Commission as being associated with poor outcomes. The advanced pharmacy practice model at RLRVAMC allowed for a multidimensional program, including prospective patient identification and multiple pharmacy touchpoints. This is unique in comparison to many of the one-dimensional programs described in the literature.
Polypharmacy has been identified as a major predictor of medication discrepancies postdischarge, and patients with ≥ 10 active medications have been found to be at highest risk.13,14 Patients in this study had a mean 13 active medications on admission, with a mean 5 medication changes at discharge. PACT pharmacists documented 28 of 99 patients with ≥ 1 medication-related discrepancy at postdischarge reconciliation. This 28% discrepancy rate is consistent with discrepancy rates previously reported in the literature, which ranged from 14 to 45% in large meta-analyses.14,15 The majority of these discrepancies (58%) were related to patients who took the wrong dose of a prescribed medication.
Targeted interventions to overcome barriers in the pilot study increased the referral rates to the TOC tool; however, the increase in referral rate was associated with increased time to follow up by ambulatory care pharmacists. The extended follow-up times were seen most often in the 2 busiest primary care clinics, one of which is considered a teaching clinic for medical residents. Pharmacists were required to integrate these calls into their normal work schedule and were not provided additional time for calling, allowing for an increased follow-up time. The increased follow-up time likely contributed to the increased number of patients excluded due to already having PACT follow-up, giving more time for the primary care provider to have an appointment with the patient. The ambulatory care pharmacist could then determine whether further intervention was needed. In the summer of 2018, a decrease in referral rates occurred for a short time, but this is likely explained by incoming new residents and staff within the pharmacy department and decreased awareness among the new staff. The enhanced staff education took place during September 2018 and lead to increased referral rates compared with those seen in months prior.
PACT pharmacists were not only able to identify discrepancies, but also provide timely intervention on a multitude of medication-related issues by using their scope of practice (SOP). Most interventions were related to medication or disease counseling, including lifestyle, device, and disease education. The independent SOP of our PACT pharmacists is a unique aspect of this program and allowed pharmacists to independently adjust many aspects of a patient’s medication regimen during follow-up visits.
The outcomes of 30-day index and all-cause readmissions, as well as index and all-cause ED visit rates, were lower in the subset of patients who received PACT pharmacist follow-up after discharge (Table 3). The difference was most pronounced in the all-cause readmission rates: Only 8.1% of patients who received PACT follow-up experienced a readmission compared with 27.5% of those who did not. The difference between the groups regarding ED visit rates were not as pronounced, but this may be attributed to a limited sample size. These data indicate that the role of the pharmacist in identifying discrepancies and performing interventions at follow-up may play a clinically significant part in reducing both ED visit rates and hospital readmissions.
Limitations
There are some limitations identified within this study. Although the referral criteria were relaxed from the pilot study and enhanced education was created, continued education regarding appropriate referral of TOC patients continues to be necessary given intermittent staff changeover, incorporation of pharmacy trainees, and modifications to clinic workflow. Patients who were discharged to facilities were not included. This ensured that appropriate and consistent PACT pharmacist follow-up would be available, but likely reduced our sample size.
Although performing this study in a closed health care system with pharmacists who have independent SOPs is a strength of our study, also it can limit generalizability. Not all facilities house both acute care and ambulatory care in one location with wide SOPs to allow for comprehensive and continued care. Last, this study used convenience sampling, potentially introducing selection bias, as patients unable to be reached by PACT pharmacists may inherently be at increased risk for hospital readmission. However, in the 3 months preceding the hospital admission that resulted in TOC tool referral, both groups had a similar number of patients with hospital admissions and ED visits.
The TOC tool has become fully integrated into the daily workflow for both acute care and PACT pharmacists. After the conclusion of the study period, the referral rates into the tool have been maintained at a steady level, even surpassing the rates seen during the study period. In comparison with the pilot study, PACT pharmacists reported a subjective increase in referrals placed for procedures such as medication reconciliation or adherence checks. This is likely because acute care pharmacists were able to use their clinical judgement rather than to rely solely on calculated readmission risk scores for TOC tool referral.
The success of the TOC program led to the expansion to other specialty areas. ED pharmacists now refer patients from the ED who were not admitted to the hospital but would benefit from PACT follow-up. Additionally, the option to refer hematology and oncology patients was added to allow these patients to be followed up by our hematology/oncology CPSs by phone appointments. Unique reasons for follow-up for this patient population include concerns about delayed chemotherapy cycles or chemotherapy-associated AEs.
Conclusions
This study outlines the creation and continued improvement of a pharmacist-based TOC program. The program was designed as a method of communication between acute care and PACT pharmacists about high-risk patients. The creation of this program allowed PACT pharmacists not only to identify discrepancies and make interventions on high-risk patients, but also demonstrate that having pharmacists involved in these programs may have a positive impact on readmissions and ED visits. The success of the TOC tool at the RLRVAMC has led to its expansion and is now an integral part of the daily workflow for both acute care and PACT pharmacists.
1. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse effects affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. doi:10.7326/0003-4819-138-3-200302040-00007
2. The Joint Commission. Transitions of care: the need for collaboration across entire care continuum. Published February 2013. Accessed February 25, 2021. http://www.jointcommission.org/assets/1/6/TOC_Hot_Topics.pdf
3. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. doi:10.1001/jamainternmed.2014.1608
4. Medicare Hospital Compare. Readmissions and deaths. Accessed February 25, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/VA-Data
5. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. doi:10.1503/cmaj.091117
6. US Department of Veteran Affairs. Care Assessment Needs (CAN) score report. Updated May 14, 2019. Accessed February 25, 2021. https://www.va.gov/HEALTHCAREEXCELLENCE/about/organization/examples/care-assessment-needs.asp
7. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565-571. doi:10.1001/archinte.166.5.565
8. Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and post-discharge call-backs. J Hosp Med. 2016;11(1):40-44. doi:10.1002/jhm.2493
9. Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449-1465. doi:10.1111/j.1475-6773.2004.00298.x
10. Brantley AF, Rossi DM, Barnes-Warren S, Francisco JC, Schatten I, Dave V. Bridging gaps in care: implementation of a pharmacist-led transitions of care program. Am J Health Syst Pharm. 2018;75(5)(suppl 1):S1-S5. doi:10.2146/ajhp160652
11. Scarsi KK, Fotis MA, Noskin GA. Pharmacist participation in medical rounds reduces medical errors. Am J Health Syst Pharm. 2002;59(21):2089-2092. doi:10.1093/ajhp/59.21.2089
12. Pevnick JM, Nguyen C, Jackevicius CA, et al. Improving admission medication reconciliation with pharmacists or pharmacy technicians in the emergency department: a randomised controlled trial. BMJ Qual Saf. 2018;27:512-520. doi:10.1136/bmjqs-2017-006761.
13. Kirwin J, Canales AE, Bentley ML, et al; American College of Clinical Pharmacy. Process indicators of quality clinical pharmacy services during transitions of care. Pharmacotherapy. 2012;32(11):e338-e347. doi:10.1002/phar.1214
14. Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5, part 2):397-403. doi:10.7326/0003-4819-158-5-201303051-00006
15. Stitt DM, Elliot DP, Thompson SN. Medication discrepancies identified at time of hospital discharge in a geriatric population. Am J Geriatr Pharmacother. 2011;9(4):234-240. doi:10.1016/j.amjopharm.2011.06.002
Effective transitions of care (TOC) are essential to ensure quality continuity of care after hospital discharge. About 20 to 30% of patients experience an adverse event (AE) in the peridischarge period when discharged to the community.1 Additionally, about two-thirds of AEs are preventable.1 The Joint Commission has identified various breakdowns in care that are associated with poor outcomes, including a lack of standardized discharge procedures, limited time dedicated to discharge planning and processes, and patients who lack the necessary resources or skills to implement discharge care plans.2
Background
The most impactful TOC programs are those that target patients who are at high risk for readmission or adverse outcomes.3 Factors such as advanced age, polypharmacy, cognitive impairment, and lack of social support are patient characteristics that have been associated with unfavorable outcomes after discharge.4 To identify this subset of high-risk individuals, various risk assessment scores have been developed, ranging from those that are used locally at the facility level to those that are nationally validated. The LACE score (Length of hospital stay; Acuity of the admission; Comorbidities measured with the Charlson comorbidity index score; and Emergency department visits within the past 6 months) is a validated index scoring tool that is used to identify medical and surgical patients at risk for readmission or death within 30 days of hospital discharge. On a 19-point scale, a score of ≥ 10 is considered high risk.5 Specific to the US Department of Veterans Affairs (VA), the Care Assessment Needs (CAN) score was developed to risk stratify the veteran population. The CAN score is generated using information including patient demographics, medical conditions, VA health care utilization, vital signs, laboratory values, medications, and socioeconomic status. This score is expressed as a percentile that compares the probability of death or admission among veterans at 90 days and 1 year postdischarge. Veterans in the 99th percentile have a 74% risk for these adverse outcomes at 1 year.6
The Joint Commission states that a fundamental component to assuring safe and effective TOC is medication management, which includes the involvement of pharmacists.2 TOC programs with pharmacist involvement have shown significant improvements related to reduced 30-day hospital readmissions and health care costs in addition to significant medication-related interventions.7-9 While this body of evidence continues to grow and demonstrates that pharmacists are an integral component of the TOC process, there is no gold standard program. Brantley and colleagues noted that a weakness of many TOC programs is that they are one dimensional, meaning that they focus on only 1 element of care transitions or 1 specific patient population or disease.10
There is well-supported evidence of high-impact interventions for pharmacists involved early in the admission process, but data are less robust on the discharge process. 11,12 Therefore, the primary focus of this project was to develop a pharmacist-based TOC program and implement a process for communicating high-risk patients who are discharging from our hospital across the continuum of care.
Setting
The Richard L. Roudebush VA Medical Center (RLRVAMC) is a tertiary care referral center for veterans in Indiana and eastern Illinois. Acute care clinical pharmacists are fully integrated into the acute care teams and practice under a comprehensive care model. Pharmacists attend daily patient care rounds and conduct discharge medication reconciliation for all patients with additional bedside counseling for patients who are being discharged home.
Primary care services are provided by patient aligned care teams (PACTs), multidisciplinary teams composed of physicians, advanced practice nurses, pharmacists, mental health care providers, registered nurses, dieticians, and care coordinators. Ambulatory Care or PACT clinical pharmacists are established within each RLRVAMC PACT clinic and provide comprehensive care management through an independent scope of practice for several chronic diseases, including hypertension, type 2 diabetes mellitus (T2DM), dyslipidemia, hypothyroidism, and tobacco cessation. Prior to this project implementation, there was no formalized or standardized method for facilitating routine communication of patients between acute care and PACT pharmacists in the TOC process.
Pilot Study
In 2017, RLRVAMC implemented a TOC pharmacy program pilot. A pharmacy resident and both acute care and PACT clinical pharmacy specialists (CPSs) developed the service. The pilot program was conducted from September 1, 2017 to March 1, 2018. The initial phase consisted of the development of an electronic TOC tool to standardize communication between acute care and PACT pharmacists. The TOC tool was created on a secure site accessible only to pharmacy personnel and not part of the formal medical record. (Figure 1).
The acute care pharmacist identified high-risk patients through calculated CAN and LACE scores during the discharge process and offered PACT pharmacist follow-up to the patient during bedside discharge counseling. Information was then entered into the TOC tool, including patient identifiers and a message with specific information outlining the reason for referral. PACT pharmacists routinely reviewed the tool and attempted to phone each patient within 7 days of discharge. Follow-up included medication reconciliation and chronic disease management as warranted at the discretion of the PACT pharmacist. All postdischarge follow-up appointments were created and documented in the electronic health record. A retrospective chart review was completed on patients who were entered into the TOC tool.
Patients were eligible for referral if they were discharged during the study period with primary care established in one of the facility’s PACT clinics. Additionally, patients had to meet ≥ 1 of the following criteria, deeming them a high risk for readmission: LACE score ≥ 10, CAN score ≥ 90th percentile, or be considered high risk based on the discretion of the acute care pharmacist. Patients were included in the analysis if they met the CAN or LACE score requirement. Patients were excluded if they received primary care from a site other than a RLRVAMC PACT clinic. This included non-VA primary care, home-based primary care, or VA community-based outpatient clinics (CBOCs). Patients also were excluded if they required further institutional care postdischarge (ie, subacute rehabilitation, extended care facility, etc), discharged to hospice, or against medical advice.
The average referral rate per month during the pilot study was 19 patients, with 113 total referrals during the 6-month study period. Lower rates of index emergency department (ED) visits (5.3% vs 23.3%) and readmissions (1% vs 6.7%) were seen in the group of patients who received PACT pharmacist follow-up postdischarge compared with those who did not. Additionally, PACT pharmacists were able to make > 120 interventions, averaging 1.7 interventions per patient. Of note, these results were not statistically analyzed and were assessed as observational data to determine whether the program had the potential to be impactful. The results of the pilot study demonstrated positive outcomes associated with having a pharmacist-based TOC process and led to the desire for further development and implementation of the TOC program at the RLRVAMC. These positive results prompted a second phase project to address barriers, make improvements, and ensure sustainability.
Methods
Phase 2 was a quality improvement initiative; therefore, institutional review board approval was not needed. The aim of phase 2 was to improve, expand, and sustain the TOC program that was implemented in the pilot study. Barriers identified after discussion with acute care and PACT pharmacists included difficulty in making referrals due to required entry of cumbersome readmission risk factor calculations, limiting inclusion to patients who receive primary care at the main hospital facility, and the expansion of pharmacy staff with new pharmacists who were not knowledgeable of the referral process.
Design
To overcome barriers, 4 main targeted interventions were needed: streamlining the referral process, enhancing pharmacy staff education, updating the discharge note template, and expanding the criteria to include patients who receive care at VA CBOCs. The referral process was streamlined by removing required calculated readmission risk scores, allowing pharmacist judgement to take precedence for referrals. Focused face-to-face education was provided to acute care and PACT pharmacists about the referral process and inclusion criteria to increase awareness and provide guidance of who may benefit from entry into the tool. Unlike the first phase of the study, education was provided for outpatient staff pharmacists responsible for discharging patients on the weekends. Additionally, the pharmacists received a printed quick reference guide of the information covered during the education sessions (Figure 2). Referral prompts were embedded into the standard pharmacy discharge note template to serve as a reminder to discharging pharmacists to assess patients for inclusion into the tool and provided a direct link to the tool. Expansion to include VA CBOCs occurred postpilot study, allowing increased patient access to this TOC service. All other aspects of the program were continued from the pilot phase.
Patients were eligible if they were discharged from RLRVAMC between October 1, 2018 and February 28, 2019. Additionally, the patient had to be established in a PACT clinic for primary care and have been referred to the tool based on the discretion of an acute care pharmacist. Patients were excluded if they were discharged against medical advice or to any facility where the patient and/or caregiver would not be responsible for medication administration (eg, subacute rehabilitation, extended care facility), or if the patient refused pharmacy follow-up.
Outcomes
The primary outcomes assessed were all-cause and index ED visits and readmissions within 30 days of discharge. All-cause ED visits and readmissions were defined as a second visit to RLRVAMC , regardless of readmission diagnosis. Index ED visits and readmissions were defined as those that were related to the initial admission diagnosis. Additional data collected and analyzed included the number of patients referred by pharmacists, number and type of medication discrepancies, medication changes, counseling interventions, time to follow-up postdischarge, and number of patients added to the PACT pharmacist’s clinic schedule for further management. A discrepancy identified by a PACT pharmacist was defined as a difference between the discharge medication list and the patient-reported medication list at the time of follow-up. Patients who were referred to the TOC tool but were unable to be reached by telephone served as the control group for this study.
Data Collection
A retrospective chart review was completed on patients entered into the tool. Data were collected and kept in a secured Microsoft Excel workbook. Baseline characteristics were analyzed using either a χ2 for nominal data or Student t test for continuous data. The primary outcomes were analyzed using a χ2 test. All statistical tests were analyzed using MiniTab 19 Statistical Software.
Results
Pharmacists added 172 patients into the TOC tool; 139 patients met inclusion criteria. Of those excluded, most were because the PACT pharmacist did not attempt to contact the patient since they already had a primary care visit scheduled postdischarge (Table 1). Of the 139 patients who met the inclusion criteria, 99 were successfully contacted by a PACT pharmacist. Most patients were aged in their 60s, male, and white. Both groups had a similar quantity of outpatient medications on admission and medication changes made at discharge. Additionally, both groups had a similar number of patients with hospitalizations and/or ED visits in the 3 months before hospital admission that resulted in TOC tool referral (Table 2).
Hospital Readmission
Hospital 30-day readmission rates for patients who were successfully followed by pharmacy compared with those who were not were 5.1% vs 15.0% (P = .049) for index readmissions and 8.1% vs 27.5% (P = .03) for all-cause readmissions. No statistically significant difference existed between those patients with follow-up compared with those without follow-up for either index (10.1% vs 12.5%, respectively; P = .68) or for all-cause ED visit rates (15.2% vs 20.0%, respectively; P = .49).
Patient Encounters
The average time to follow-up was 8.8 days, which was above the predetermined goal of contact within 7 days. Additionally, this was a decline from the initial pilot study, which had an average time to reach of 4.7 days. All patients reached by a pharmacist received medication reconciliation, with ≥ 28% of patients having ≥ 1 discrepancy. There were 43 discrepancies among all patients. Of the discrepancies, 25 were reported as errors performed by the patient, and 18 were from an error during the discharge process. The discrepancies that resulted from patient error were primarily patients who took the wrong dose of prescribed medications. Other patient discrepancies included taking medications not as scheduled, omitting medications (both intentionally and mistakenly), continuing to take medications that had been discontinued by a health care provider and improper administration technique. Examples of provider errors that occurred during the discharge process included not ordering medications for patient to pick up at discharge, not discontinuing a medication from the patient’s profile, and failure to renew expired prescriptions.
Additional counseling was provided to 75% of patients: The most common reason for counseling was T2DM, hypertension, and dyslipidemia management. PACT pharmacists changed medication regimens for 27.3% of patients for improved control of chronic diseases or relief of medication AEs.
At the end of each visit, patients were assessed to determine whether they could benefit from additional pharmacy follow-up. Thirty-seven patients were added to the pharmacist schedules for disease management appointments. The most common conditions for these appointments were T2DM, hypertension, tobacco cessation, and hyperlipidemia. Among the 37 patients who had pharmacy follow-up, there were 137 additional pharmacy appointments within the study period.
Program Referrals
After expansion to include the VA CBOCs, elimination of the elevated LACE or CAN score requirement, and additional staff education, the rate of referrals per month increased during phase 2 in comparison to the pilot study (Figure 3). There were a mean (SD) of 34 (10) referrals per month. Although not statistically analyzed, it is an objective increase in comparison to a mean 19 referrals per month in the pilot study.
Discussion
The continued development and use of a pharmacist-driven TOC tool at RLRVAMC increased communication and follow-up of high-risk patients, demonstrated the ability of pharmacists to identify and intervene in medication-related issues postdischarge, and successfully reduce 30-day readmissions. This program emphasized pharmacist involvement during the discharge process and created a standardized mechanism for TOC follow-up, addressing multiple areas that were identified by The Joint Commission as being associated with poor outcomes. The advanced pharmacy practice model at RLRVAMC allowed for a multidimensional program, including prospective patient identification and multiple pharmacy touchpoints. This is unique in comparison to many of the one-dimensional programs described in the literature.
Polypharmacy has been identified as a major predictor of medication discrepancies postdischarge, and patients with ≥ 10 active medications have been found to be at highest risk.13,14 Patients in this study had a mean 13 active medications on admission, with a mean 5 medication changes at discharge. PACT pharmacists documented 28 of 99 patients with ≥ 1 medication-related discrepancy at postdischarge reconciliation. This 28% discrepancy rate is consistent with discrepancy rates previously reported in the literature, which ranged from 14 to 45% in large meta-analyses.14,15 The majority of these discrepancies (58%) were related to patients who took the wrong dose of a prescribed medication.
Targeted interventions to overcome barriers in the pilot study increased the referral rates to the TOC tool; however, the increase in referral rate was associated with increased time to follow up by ambulatory care pharmacists. The extended follow-up times were seen most often in the 2 busiest primary care clinics, one of which is considered a teaching clinic for medical residents. Pharmacists were required to integrate these calls into their normal work schedule and were not provided additional time for calling, allowing for an increased follow-up time. The increased follow-up time likely contributed to the increased number of patients excluded due to already having PACT follow-up, giving more time for the primary care provider to have an appointment with the patient. The ambulatory care pharmacist could then determine whether further intervention was needed. In the summer of 2018, a decrease in referral rates occurred for a short time, but this is likely explained by incoming new residents and staff within the pharmacy department and decreased awareness among the new staff. The enhanced staff education took place during September 2018 and lead to increased referral rates compared with those seen in months prior.
PACT pharmacists were not only able to identify discrepancies, but also provide timely intervention on a multitude of medication-related issues by using their scope of practice (SOP). Most interventions were related to medication or disease counseling, including lifestyle, device, and disease education. The independent SOP of our PACT pharmacists is a unique aspect of this program and allowed pharmacists to independently adjust many aspects of a patient’s medication regimen during follow-up visits.
The outcomes of 30-day index and all-cause readmissions, as well as index and all-cause ED visit rates, were lower in the subset of patients who received PACT pharmacist follow-up after discharge (Table 3). The difference was most pronounced in the all-cause readmission rates: Only 8.1% of patients who received PACT follow-up experienced a readmission compared with 27.5% of those who did not. The difference between the groups regarding ED visit rates were not as pronounced, but this may be attributed to a limited sample size. These data indicate that the role of the pharmacist in identifying discrepancies and performing interventions at follow-up may play a clinically significant part in reducing both ED visit rates and hospital readmissions.
Limitations
There are some limitations identified within this study. Although the referral criteria were relaxed from the pilot study and enhanced education was created, continued education regarding appropriate referral of TOC patients continues to be necessary given intermittent staff changeover, incorporation of pharmacy trainees, and modifications to clinic workflow. Patients who were discharged to facilities were not included. This ensured that appropriate and consistent PACT pharmacist follow-up would be available, but likely reduced our sample size.
Although performing this study in a closed health care system with pharmacists who have independent SOPs is a strength of our study, also it can limit generalizability. Not all facilities house both acute care and ambulatory care in one location with wide SOPs to allow for comprehensive and continued care. Last, this study used convenience sampling, potentially introducing selection bias, as patients unable to be reached by PACT pharmacists may inherently be at increased risk for hospital readmission. However, in the 3 months preceding the hospital admission that resulted in TOC tool referral, both groups had a similar number of patients with hospital admissions and ED visits.
The TOC tool has become fully integrated into the daily workflow for both acute care and PACT pharmacists. After the conclusion of the study period, the referral rates into the tool have been maintained at a steady level, even surpassing the rates seen during the study period. In comparison with the pilot study, PACT pharmacists reported a subjective increase in referrals placed for procedures such as medication reconciliation or adherence checks. This is likely because acute care pharmacists were able to use their clinical judgement rather than to rely solely on calculated readmission risk scores for TOC tool referral.
The success of the TOC program led to the expansion to other specialty areas. ED pharmacists now refer patients from the ED who were not admitted to the hospital but would benefit from PACT follow-up. Additionally, the option to refer hematology and oncology patients was added to allow these patients to be followed up by our hematology/oncology CPSs by phone appointments. Unique reasons for follow-up for this patient population include concerns about delayed chemotherapy cycles or chemotherapy-associated AEs.
Conclusions
This study outlines the creation and continued improvement of a pharmacist-based TOC program. The program was designed as a method of communication between acute care and PACT pharmacists about high-risk patients. The creation of this program allowed PACT pharmacists not only to identify discrepancies and make interventions on high-risk patients, but also demonstrate that having pharmacists involved in these programs may have a positive impact on readmissions and ED visits. The success of the TOC tool at the RLRVAMC has led to its expansion and is now an integral part of the daily workflow for both acute care and PACT pharmacists.
Effective transitions of care (TOC) are essential to ensure quality continuity of care after hospital discharge. About 20 to 30% of patients experience an adverse event (AE) in the peridischarge period when discharged to the community.1 Additionally, about two-thirds of AEs are preventable.1 The Joint Commission has identified various breakdowns in care that are associated with poor outcomes, including a lack of standardized discharge procedures, limited time dedicated to discharge planning and processes, and patients who lack the necessary resources or skills to implement discharge care plans.2
Background
The most impactful TOC programs are those that target patients who are at high risk for readmission or adverse outcomes.3 Factors such as advanced age, polypharmacy, cognitive impairment, and lack of social support are patient characteristics that have been associated with unfavorable outcomes after discharge.4 To identify this subset of high-risk individuals, various risk assessment scores have been developed, ranging from those that are used locally at the facility level to those that are nationally validated. The LACE score (Length of hospital stay; Acuity of the admission; Comorbidities measured with the Charlson comorbidity index score; and Emergency department visits within the past 6 months) is a validated index scoring tool that is used to identify medical and surgical patients at risk for readmission or death within 30 days of hospital discharge. On a 19-point scale, a score of ≥ 10 is considered high risk.5 Specific to the US Department of Veterans Affairs (VA), the Care Assessment Needs (CAN) score was developed to risk stratify the veteran population. The CAN score is generated using information including patient demographics, medical conditions, VA health care utilization, vital signs, laboratory values, medications, and socioeconomic status. This score is expressed as a percentile that compares the probability of death or admission among veterans at 90 days and 1 year postdischarge. Veterans in the 99th percentile have a 74% risk for these adverse outcomes at 1 year.6
The Joint Commission states that a fundamental component to assuring safe and effective TOC is medication management, which includes the involvement of pharmacists.2 TOC programs with pharmacist involvement have shown significant improvements related to reduced 30-day hospital readmissions and health care costs in addition to significant medication-related interventions.7-9 While this body of evidence continues to grow and demonstrates that pharmacists are an integral component of the TOC process, there is no gold standard program. Brantley and colleagues noted that a weakness of many TOC programs is that they are one dimensional, meaning that they focus on only 1 element of care transitions or 1 specific patient population or disease.10
There is well-supported evidence of high-impact interventions for pharmacists involved early in the admission process, but data are less robust on the discharge process. 11,12 Therefore, the primary focus of this project was to develop a pharmacist-based TOC program and implement a process for communicating high-risk patients who are discharging from our hospital across the continuum of care.
Setting
The Richard L. Roudebush VA Medical Center (RLRVAMC) is a tertiary care referral center for veterans in Indiana and eastern Illinois. Acute care clinical pharmacists are fully integrated into the acute care teams and practice under a comprehensive care model. Pharmacists attend daily patient care rounds and conduct discharge medication reconciliation for all patients with additional bedside counseling for patients who are being discharged home.
Primary care services are provided by patient aligned care teams (PACTs), multidisciplinary teams composed of physicians, advanced practice nurses, pharmacists, mental health care providers, registered nurses, dieticians, and care coordinators. Ambulatory Care or PACT clinical pharmacists are established within each RLRVAMC PACT clinic and provide comprehensive care management through an independent scope of practice for several chronic diseases, including hypertension, type 2 diabetes mellitus (T2DM), dyslipidemia, hypothyroidism, and tobacco cessation. Prior to this project implementation, there was no formalized or standardized method for facilitating routine communication of patients between acute care and PACT pharmacists in the TOC process.
Pilot Study
In 2017, RLRVAMC implemented a TOC pharmacy program pilot. A pharmacy resident and both acute care and PACT clinical pharmacy specialists (CPSs) developed the service. The pilot program was conducted from September 1, 2017 to March 1, 2018. The initial phase consisted of the development of an electronic TOC tool to standardize communication between acute care and PACT pharmacists. The TOC tool was created on a secure site accessible only to pharmacy personnel and not part of the formal medical record. (Figure 1).
The acute care pharmacist identified high-risk patients through calculated CAN and LACE scores during the discharge process and offered PACT pharmacist follow-up to the patient during bedside discharge counseling. Information was then entered into the TOC tool, including patient identifiers and a message with specific information outlining the reason for referral. PACT pharmacists routinely reviewed the tool and attempted to phone each patient within 7 days of discharge. Follow-up included medication reconciliation and chronic disease management as warranted at the discretion of the PACT pharmacist. All postdischarge follow-up appointments were created and documented in the electronic health record. A retrospective chart review was completed on patients who were entered into the TOC tool.
Patients were eligible for referral if they were discharged during the study period with primary care established in one of the facility’s PACT clinics. Additionally, patients had to meet ≥ 1 of the following criteria, deeming them a high risk for readmission: LACE score ≥ 10, CAN score ≥ 90th percentile, or be considered high risk based on the discretion of the acute care pharmacist. Patients were included in the analysis if they met the CAN or LACE score requirement. Patients were excluded if they received primary care from a site other than a RLRVAMC PACT clinic. This included non-VA primary care, home-based primary care, or VA community-based outpatient clinics (CBOCs). Patients also were excluded if they required further institutional care postdischarge (ie, subacute rehabilitation, extended care facility, etc), discharged to hospice, or against medical advice.
The average referral rate per month during the pilot study was 19 patients, with 113 total referrals during the 6-month study period. Lower rates of index emergency department (ED) visits (5.3% vs 23.3%) and readmissions (1% vs 6.7%) were seen in the group of patients who received PACT pharmacist follow-up postdischarge compared with those who did not. Additionally, PACT pharmacists were able to make > 120 interventions, averaging 1.7 interventions per patient. Of note, these results were not statistically analyzed and were assessed as observational data to determine whether the program had the potential to be impactful. The results of the pilot study demonstrated positive outcomes associated with having a pharmacist-based TOC process and led to the desire for further development and implementation of the TOC program at the RLRVAMC. These positive results prompted a second phase project to address barriers, make improvements, and ensure sustainability.
Methods
Phase 2 was a quality improvement initiative; therefore, institutional review board approval was not needed. The aim of phase 2 was to improve, expand, and sustain the TOC program that was implemented in the pilot study. Barriers identified after discussion with acute care and PACT pharmacists included difficulty in making referrals due to required entry of cumbersome readmission risk factor calculations, limiting inclusion to patients who receive primary care at the main hospital facility, and the expansion of pharmacy staff with new pharmacists who were not knowledgeable of the referral process.
Design
To overcome barriers, 4 main targeted interventions were needed: streamlining the referral process, enhancing pharmacy staff education, updating the discharge note template, and expanding the criteria to include patients who receive care at VA CBOCs. The referral process was streamlined by removing required calculated readmission risk scores, allowing pharmacist judgement to take precedence for referrals. Focused face-to-face education was provided to acute care and PACT pharmacists about the referral process and inclusion criteria to increase awareness and provide guidance of who may benefit from entry into the tool. Unlike the first phase of the study, education was provided for outpatient staff pharmacists responsible for discharging patients on the weekends. Additionally, the pharmacists received a printed quick reference guide of the information covered during the education sessions (Figure 2). Referral prompts were embedded into the standard pharmacy discharge note template to serve as a reminder to discharging pharmacists to assess patients for inclusion into the tool and provided a direct link to the tool. Expansion to include VA CBOCs occurred postpilot study, allowing increased patient access to this TOC service. All other aspects of the program were continued from the pilot phase.
Patients were eligible if they were discharged from RLRVAMC between October 1, 2018 and February 28, 2019. Additionally, the patient had to be established in a PACT clinic for primary care and have been referred to the tool based on the discretion of an acute care pharmacist. Patients were excluded if they were discharged against medical advice or to any facility where the patient and/or caregiver would not be responsible for medication administration (eg, subacute rehabilitation, extended care facility), or if the patient refused pharmacy follow-up.
Outcomes
The primary outcomes assessed were all-cause and index ED visits and readmissions within 30 days of discharge. All-cause ED visits and readmissions were defined as a second visit to RLRVAMC , regardless of readmission diagnosis. Index ED visits and readmissions were defined as those that were related to the initial admission diagnosis. Additional data collected and analyzed included the number of patients referred by pharmacists, number and type of medication discrepancies, medication changes, counseling interventions, time to follow-up postdischarge, and number of patients added to the PACT pharmacist’s clinic schedule for further management. A discrepancy identified by a PACT pharmacist was defined as a difference between the discharge medication list and the patient-reported medication list at the time of follow-up. Patients who were referred to the TOC tool but were unable to be reached by telephone served as the control group for this study.
Data Collection
A retrospective chart review was completed on patients entered into the tool. Data were collected and kept in a secured Microsoft Excel workbook. Baseline characteristics were analyzed using either a χ2 for nominal data or Student t test for continuous data. The primary outcomes were analyzed using a χ2 test. All statistical tests were analyzed using MiniTab 19 Statistical Software.
Results
Pharmacists added 172 patients into the TOC tool; 139 patients met inclusion criteria. Of those excluded, most were because the PACT pharmacist did not attempt to contact the patient since they already had a primary care visit scheduled postdischarge (Table 1). Of the 139 patients who met the inclusion criteria, 99 were successfully contacted by a PACT pharmacist. Most patients were aged in their 60s, male, and white. Both groups had a similar quantity of outpatient medications on admission and medication changes made at discharge. Additionally, both groups had a similar number of patients with hospitalizations and/or ED visits in the 3 months before hospital admission that resulted in TOC tool referral (Table 2).
Hospital Readmission
Hospital 30-day readmission rates for patients who were successfully followed by pharmacy compared with those who were not were 5.1% vs 15.0% (P = .049) for index readmissions and 8.1% vs 27.5% (P = .03) for all-cause readmissions. No statistically significant difference existed between those patients with follow-up compared with those without follow-up for either index (10.1% vs 12.5%, respectively; P = .68) or for all-cause ED visit rates (15.2% vs 20.0%, respectively; P = .49).
Patient Encounters
The average time to follow-up was 8.8 days, which was above the predetermined goal of contact within 7 days. Additionally, this was a decline from the initial pilot study, which had an average time to reach of 4.7 days. All patients reached by a pharmacist received medication reconciliation, with ≥ 28% of patients having ≥ 1 discrepancy. There were 43 discrepancies among all patients. Of the discrepancies, 25 were reported as errors performed by the patient, and 18 were from an error during the discharge process. The discrepancies that resulted from patient error were primarily patients who took the wrong dose of prescribed medications. Other patient discrepancies included taking medications not as scheduled, omitting medications (both intentionally and mistakenly), continuing to take medications that had been discontinued by a health care provider and improper administration technique. Examples of provider errors that occurred during the discharge process included not ordering medications for patient to pick up at discharge, not discontinuing a medication from the patient’s profile, and failure to renew expired prescriptions.
Additional counseling was provided to 75% of patients: The most common reason for counseling was T2DM, hypertension, and dyslipidemia management. PACT pharmacists changed medication regimens for 27.3% of patients for improved control of chronic diseases or relief of medication AEs.
At the end of each visit, patients were assessed to determine whether they could benefit from additional pharmacy follow-up. Thirty-seven patients were added to the pharmacist schedules for disease management appointments. The most common conditions for these appointments were T2DM, hypertension, tobacco cessation, and hyperlipidemia. Among the 37 patients who had pharmacy follow-up, there were 137 additional pharmacy appointments within the study period.
Program Referrals
After expansion to include the VA CBOCs, elimination of the elevated LACE or CAN score requirement, and additional staff education, the rate of referrals per month increased during phase 2 in comparison to the pilot study (Figure 3). There were a mean (SD) of 34 (10) referrals per month. Although not statistically analyzed, it is an objective increase in comparison to a mean 19 referrals per month in the pilot study.
Discussion
The continued development and use of a pharmacist-driven TOC tool at RLRVAMC increased communication and follow-up of high-risk patients, demonstrated the ability of pharmacists to identify and intervene in medication-related issues postdischarge, and successfully reduce 30-day readmissions. This program emphasized pharmacist involvement during the discharge process and created a standardized mechanism for TOC follow-up, addressing multiple areas that were identified by The Joint Commission as being associated with poor outcomes. The advanced pharmacy practice model at RLRVAMC allowed for a multidimensional program, including prospective patient identification and multiple pharmacy touchpoints. This is unique in comparison to many of the one-dimensional programs described in the literature.
Polypharmacy has been identified as a major predictor of medication discrepancies postdischarge, and patients with ≥ 10 active medications have been found to be at highest risk.13,14 Patients in this study had a mean 13 active medications on admission, with a mean 5 medication changes at discharge. PACT pharmacists documented 28 of 99 patients with ≥ 1 medication-related discrepancy at postdischarge reconciliation. This 28% discrepancy rate is consistent with discrepancy rates previously reported in the literature, which ranged from 14 to 45% in large meta-analyses.14,15 The majority of these discrepancies (58%) were related to patients who took the wrong dose of a prescribed medication.
Targeted interventions to overcome barriers in the pilot study increased the referral rates to the TOC tool; however, the increase in referral rate was associated with increased time to follow up by ambulatory care pharmacists. The extended follow-up times were seen most often in the 2 busiest primary care clinics, one of which is considered a teaching clinic for medical residents. Pharmacists were required to integrate these calls into their normal work schedule and were not provided additional time for calling, allowing for an increased follow-up time. The increased follow-up time likely contributed to the increased number of patients excluded due to already having PACT follow-up, giving more time for the primary care provider to have an appointment with the patient. The ambulatory care pharmacist could then determine whether further intervention was needed. In the summer of 2018, a decrease in referral rates occurred for a short time, but this is likely explained by incoming new residents and staff within the pharmacy department and decreased awareness among the new staff. The enhanced staff education took place during September 2018 and lead to increased referral rates compared with those seen in months prior.
PACT pharmacists were not only able to identify discrepancies, but also provide timely intervention on a multitude of medication-related issues by using their scope of practice (SOP). Most interventions were related to medication or disease counseling, including lifestyle, device, and disease education. The independent SOP of our PACT pharmacists is a unique aspect of this program and allowed pharmacists to independently adjust many aspects of a patient’s medication regimen during follow-up visits.
The outcomes of 30-day index and all-cause readmissions, as well as index and all-cause ED visit rates, were lower in the subset of patients who received PACT pharmacist follow-up after discharge (Table 3). The difference was most pronounced in the all-cause readmission rates: Only 8.1% of patients who received PACT follow-up experienced a readmission compared with 27.5% of those who did not. The difference between the groups regarding ED visit rates were not as pronounced, but this may be attributed to a limited sample size. These data indicate that the role of the pharmacist in identifying discrepancies and performing interventions at follow-up may play a clinically significant part in reducing both ED visit rates and hospital readmissions.
Limitations
There are some limitations identified within this study. Although the referral criteria were relaxed from the pilot study and enhanced education was created, continued education regarding appropriate referral of TOC patients continues to be necessary given intermittent staff changeover, incorporation of pharmacy trainees, and modifications to clinic workflow. Patients who were discharged to facilities were not included. This ensured that appropriate and consistent PACT pharmacist follow-up would be available, but likely reduced our sample size.
Although performing this study in a closed health care system with pharmacists who have independent SOPs is a strength of our study, also it can limit generalizability. Not all facilities house both acute care and ambulatory care in one location with wide SOPs to allow for comprehensive and continued care. Last, this study used convenience sampling, potentially introducing selection bias, as patients unable to be reached by PACT pharmacists may inherently be at increased risk for hospital readmission. However, in the 3 months preceding the hospital admission that resulted in TOC tool referral, both groups had a similar number of patients with hospital admissions and ED visits.
The TOC tool has become fully integrated into the daily workflow for both acute care and PACT pharmacists. After the conclusion of the study period, the referral rates into the tool have been maintained at a steady level, even surpassing the rates seen during the study period. In comparison with the pilot study, PACT pharmacists reported a subjective increase in referrals placed for procedures such as medication reconciliation or adherence checks. This is likely because acute care pharmacists were able to use their clinical judgement rather than to rely solely on calculated readmission risk scores for TOC tool referral.
The success of the TOC program led to the expansion to other specialty areas. ED pharmacists now refer patients from the ED who were not admitted to the hospital but would benefit from PACT follow-up. Additionally, the option to refer hematology and oncology patients was added to allow these patients to be followed up by our hematology/oncology CPSs by phone appointments. Unique reasons for follow-up for this patient population include concerns about delayed chemotherapy cycles or chemotherapy-associated AEs.
Conclusions
This study outlines the creation and continued improvement of a pharmacist-based TOC program. The program was designed as a method of communication between acute care and PACT pharmacists about high-risk patients. The creation of this program allowed PACT pharmacists not only to identify discrepancies and make interventions on high-risk patients, but also demonstrate that having pharmacists involved in these programs may have a positive impact on readmissions and ED visits. The success of the TOC tool at the RLRVAMC has led to its expansion and is now an integral part of the daily workflow for both acute care and PACT pharmacists.
1. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse effects affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. doi:10.7326/0003-4819-138-3-200302040-00007
2. The Joint Commission. Transitions of care: the need for collaboration across entire care continuum. Published February 2013. Accessed February 25, 2021. http://www.jointcommission.org/assets/1/6/TOC_Hot_Topics.pdf
3. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. doi:10.1001/jamainternmed.2014.1608
4. Medicare Hospital Compare. Readmissions and deaths. Accessed February 25, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/VA-Data
5. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. doi:10.1503/cmaj.091117
6. US Department of Veteran Affairs. Care Assessment Needs (CAN) score report. Updated May 14, 2019. Accessed February 25, 2021. https://www.va.gov/HEALTHCAREEXCELLENCE/about/organization/examples/care-assessment-needs.asp
7. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565-571. doi:10.1001/archinte.166.5.565
8. Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and post-discharge call-backs. J Hosp Med. 2016;11(1):40-44. doi:10.1002/jhm.2493
9. Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449-1465. doi:10.1111/j.1475-6773.2004.00298.x
10. Brantley AF, Rossi DM, Barnes-Warren S, Francisco JC, Schatten I, Dave V. Bridging gaps in care: implementation of a pharmacist-led transitions of care program. Am J Health Syst Pharm. 2018;75(5)(suppl 1):S1-S5. doi:10.2146/ajhp160652
11. Scarsi KK, Fotis MA, Noskin GA. Pharmacist participation in medical rounds reduces medical errors. Am J Health Syst Pharm. 2002;59(21):2089-2092. doi:10.1093/ajhp/59.21.2089
12. Pevnick JM, Nguyen C, Jackevicius CA, et al. Improving admission medication reconciliation with pharmacists or pharmacy technicians in the emergency department: a randomised controlled trial. BMJ Qual Saf. 2018;27:512-520. doi:10.1136/bmjqs-2017-006761.
13. Kirwin J, Canales AE, Bentley ML, et al; American College of Clinical Pharmacy. Process indicators of quality clinical pharmacy services during transitions of care. Pharmacotherapy. 2012;32(11):e338-e347. doi:10.1002/phar.1214
14. Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5, part 2):397-403. doi:10.7326/0003-4819-158-5-201303051-00006
15. Stitt DM, Elliot DP, Thompson SN. Medication discrepancies identified at time of hospital discharge in a geriatric population. Am J Geriatr Pharmacother. 2011;9(4):234-240. doi:10.1016/j.amjopharm.2011.06.002
1. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse effects affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. doi:10.7326/0003-4819-138-3-200302040-00007
2. The Joint Commission. Transitions of care: the need for collaboration across entire care continuum. Published February 2013. Accessed February 25, 2021. http://www.jointcommission.org/assets/1/6/TOC_Hot_Topics.pdf
3. Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095-1107. doi:10.1001/jamainternmed.2014.1608
4. Medicare Hospital Compare. Readmissions and deaths. Accessed February 25, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/VA-Data
5. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. doi:10.1503/cmaj.091117
6. US Department of Veteran Affairs. Care Assessment Needs (CAN) score report. Updated May 14, 2019. Accessed February 25, 2021. https://www.va.gov/HEALTHCAREEXCELLENCE/about/organization/examples/care-assessment-needs.asp
7. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565-571. doi:10.1001/archinte.166.5.565
8. Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and post-discharge call-backs. J Hosp Med. 2016;11(1):40-44. doi:10.1002/jhm.2493
9. Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449-1465. doi:10.1111/j.1475-6773.2004.00298.x
10. Brantley AF, Rossi DM, Barnes-Warren S, Francisco JC, Schatten I, Dave V. Bridging gaps in care: implementation of a pharmacist-led transitions of care program. Am J Health Syst Pharm. 2018;75(5)(suppl 1):S1-S5. doi:10.2146/ajhp160652
11. Scarsi KK, Fotis MA, Noskin GA. Pharmacist participation in medical rounds reduces medical errors. Am J Health Syst Pharm. 2002;59(21):2089-2092. doi:10.1093/ajhp/59.21.2089
12. Pevnick JM, Nguyen C, Jackevicius CA, et al. Improving admission medication reconciliation with pharmacists or pharmacy technicians in the emergency department: a randomised controlled trial. BMJ Qual Saf. 2018;27:512-520. doi:10.1136/bmjqs-2017-006761.
13. Kirwin J, Canales AE, Bentley ML, et al; American College of Clinical Pharmacy. Process indicators of quality clinical pharmacy services during transitions of care. Pharmacotherapy. 2012;32(11):e338-e347. doi:10.1002/phar.1214
14. Kwan JL, Lo L, Sampson M, et al. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5, part 2):397-403. doi:10.7326/0003-4819-158-5-201303051-00006
15. Stitt DM, Elliot DP, Thompson SN. Medication discrepancies identified at time of hospital discharge in a geriatric population. Am J Geriatr Pharmacother. 2011;9(4):234-240. doi:10.1016/j.amjopharm.2011.06.002
VA Academic Affiliations Matter in the Era of Community Care: A Model From California
The Veterans Health Administration (VHA), 1 of 3 administrative branches in the US Department of Veterans Affairs (VA), is the largest integrated health care system in the United States.1 The VHA has 4 missions: providing health care to eligible veterans; supporting research to benefit veterans and the larger society; providing education for health care trainees; and supporting emergency response.1 In service of these goals, VA has academic affiliations with universities throughout the country, offering unique, extensive training and research opportunities. Both the VA and the affiliate benefit from these partnerships. For example, VA affiliations with University of California (UC) medical schools benefit veteran care while facilitating the UC academic mission. Through these affiliations, trainees who learn within the VHA’s highly effective integrated care model become health care professionals (HCPs) who are prepared to enter health care systems in California and meet the state’s demand for high-quality integrated care with an emphasis on primary care, mental health care, and care for aging populations.2,3
This report explores the history of the VHA, current veteran demographics and needs, VA academic affiliations, and the integrated care model of training in all VHA facilities. The VA and UC academic affiliation is described further with regard to shared research and educational functions. Finally, we identify potential risks to academic affiliations associated with increased VA reliance on community-based care following the implementation of recent legislation. We provide suggestions for VA academic affiliates to help assess and guide the potential impact of increased VA-managed community care.
VHA Resources
The VHA serves more than 9 million veterans through 170 medical centers and 1,074 outpatient care sites.1 In fiscal year 2017, the VA provided 109 million outpatient visits, and treated 615,000 inpatient medicine/surgical patients and 149,000 patients in inpatient mental health.4 The VHA focuses on the distinct concerns of veterans, which arise from military service as well as their broader health care needs. Veterans have higher rates of medical and mental health conditions than those of the general public; different cohorts in this population experience distinct medical and mental health concerns (Table 1).5
In addition, although veterans are disproportionately older men, the population is diversifying.6 For example, the number of female veterans is growing; furthermore, changes in the law now allow lesbian, gay, bisexual, and transgender (LGBT) individuals to serve openly, which has both reduced barriers for this population and allowed for LGBT veterans who were not eligible for VA care due to less than honorable discharges to have those discharges upgraded. As a result, care has been tailored to include the development of Women Veterans Program Managers and related services and LGBT and related identities resources such as LGBT Veteran Care Coordinators in every VA facility nationwide.7,8 The VA continues to adapt to serve all veterans; part of this adaptation is training HCPs to provide veteran-centered care for a growing and diversifying population.
VHA Resources in California
California has the largest population of veterans in the United States (Table 2).9,10 Of the 9,116,200 VA enrollees nationwide, 760,910 (8%) reside in California, and of those, 463,410 had at least 1 VA visit in the past year.3,10 The VHA is organized into 21 Veterans Integrated Service Networks (VISNs) that include multiple health care systems in the region associated with each VISN. California is part of VISN 21 (Northern California, Nevada, and Pacific Islands) and VISN 22 (Southern California, Nevada, and New Mexico). Among veterans who served in the recent Iraq and Afghanistan conflicts, 5.5% accessed care in VISN 21 and 9.3% accessed care in VISN 22.11 The VHA provides critical infrastructure for meeting complex veteran needs, as well as related specialized training, education, and research for HCPs. This specialization has been the basis for the broad system of affiliations between VA and academic systems.
The VA continues to be a high priority in the federal budget process.12 In 2017, slightly more than 9% of the VA health care budget, $6.4 billion, was spent on medical care in California.10 Consequently, California has a noteworthy portion of VA infrastructure (Table 3).13,14 California has 8 VA medical centers (VAMCs) with hospital service (Fresno, Loma Linda, Long Beach, Palo Alto, Sacramento, San Diego, San Francisco, West Los Angeles), 3 VAMCs without hospital service (2 locations in the Palo Alto system and Sepulveda), 1 stand-alone extended-care facility (Martinez Community Living Center), and 1 stand-alone residential care facility (San Diego Domiciliary).9 The vast VA infrastructure in California and large population of veterans creates a strong demand for HCPs in the state.
VA Education and Collaboration
VA has been training clinicians and scholars since 1946, when VA academic affiliations were established by Memorandum Number 2.15,16 Today, the VA is the largest educator of HCPs in the United States.17 In 2015, an estimated $10.3 to $12.5 billion was spent on mandatory Medicare graduate medical education (GME).18 In 2017, the VA spent $1.78 billion of discretionary funding on GME to fund 11,000 full-time equivalent (FTE) slots, leading to > 43,000 physician residents (> 30% of all physician residents) spending part of their training in a VHA facility.18,19
This training mission has multiple benefits. It provides the VA with access to new HCPs who have the necessary training in veteran-specific needs, while supporting the national need for HCPs. In 2018, 120,890 clinical trainees received some or all of their training in the VA system.20 Of the 152 US medical schools that are accredited by the Liaison Committee on Medical Education, 95% collaborate with the VA for training while 100% of the 34 doctor of osteopathic medicine programs have VA training collaborations.20 The VA currently has an additional 18 partnerships with nursing schools.21 Further, 1,800 college and universities, including Hispanic-serving institutions and historically black colleges and universities, have VHA affiliations that provide training for more than 40 clinical health profession education programs.17
This training model has been successful in supporting VA staffing, as health care providers who trained in the VA are more likely to work in the VA.22 Among current VA employees, > 80% of optometrists, > 70% of podiatrists and psychologists, and > 60% of physicians received some part of their training in the VA system.23 In combination with recent increased funding for staffing, the ability of the VA to directly hire trainees in identified professions, and the expansion of loan forgiveness to high-demand specialties (eg, psychiatry), the training partnership between the VA and affiliates has been critical in maintaining the needed VA workforce.22,24,25
The VA Office of Academic Affiliations is responsible for all graduate medical and dental education administration in the VA system, which makes up 85% of its total budget. For each trainee, the VA provides approximately $60,000 toward their stipend in exchange for training and patient care time at a VHA hospital (Kenneth R. Jones, PhD, email communication, August 27, 2018).
California Health Care Education
The UC public university system, founded in 1869, currently has 10 campuses with a combined student body of > 280,000 students, along with 227,000 faculty and staff members.26 For every research dollar provided by California, the UC secures $7 in federal and private funding.26 The UC has 6 medical centers (Davis, Irvine, Los Angeles, Riverside, San Diego, and San Francisco); each is affiliated with at least 1 local VAMC.27,28
California trains a substantial share of health care trainees. In 2016, there were 10,429 physician residents in training in California.29 In 2017/2018, the San Francisco VAMC trained 1,178 medical students/residents, 57 pharmacy students, 25 nurse practitioner students, 19 optometry interns/students/residents, 11 dental students/residents, and 3 physical therapy students.20 In total, 6,223 UC health professions students were trained in VHA facilities during the 2017/2018 training year (Table 4).20 As of 2016, there were 105,907 physicians in California, and of those, 57% completed their GME in California.29 In California in 2015, 74 GME-sponsoring institutions graduated 3,568 residents and fellows, an increase of 10% since 1997.30 Of these sponsoring institutions, 6 of the top 8 programs were UC schools that graduated 48.4% (1,727) of all California residents and fellows in 2015.30
Despite these resources, California faces a major shortage of HCPs, particularly in primary, behavioral health, and older adult care.3 Today, 7 million Californians live in counties with a federally designated shortage of primary, dental, and mental health care providers.3 Most of these Californians are Latino, African American, or Native American, and they live in fast-growing rural and urban regions, including Los Angeles; the San Joaquin Valley; and the Inland Empire (San Bernardino and Riverside Counties).3 Current recommendations to meet increasing demands as California’s population increases, grows older, and faces increased health care demands include expanding residency programs to yield 1,872 additional primary care physicians and 2,202 additional psychiatrists by 2030.3 To meet this shortage and prepare for future health care demands, health care education is paramount; in California, VA and UC affiliations are central to addressing these needs.
The VA plays a particularly important role in supporting GME, which is essential to meeting both VA and California’s unmet HCP needs, as GME determines the number of medical practitioners available per specialty.30 The VA was the second largest GME fund provider in California at $90,662,608 (Medicare provided $552,235,626) and the California government provided a small portion of GME funding.30 VA education funding is a direct result of the VA provision of clinical care in one of the most innovative and modern health care systems in the world.
These VA training opportunities benefit the UC system and California by helping train integrated care practitioners to meet the increasing demand. Integrated care—the coordination of mental health care, substance use disorder treatment, and primary care services—is designed to improve health outcomes by helping people with multiple and complex health care needs access care.31,32
As the largest integrated health care system in the country, the VA brings important clinical, research, and educational opportunities to academic affiliates. A systematic review examining cost and quality outcomes in integrated care systems found improved quality of care compared with nonintegrated care systems; thus, many US government agencies and the World Health Organization are establishing integrated care systems as a standard and universal approach.31,33,34 While cost savings as a result of integrated care are unclear, most studies in this review reported a decrease in utilization of services.33 The presumption of more efficient and higher quality care is also predicated on features such as system-wide accessibility of comprehensive medical records that provide more information to HCPs, promote collaboration, and measure and reward performance, all of which are possible using the VA electronic health record (EHR) system.35,36 The VA offers an excellent opportunity for training in integrated care as this model is required of all VAMCs and community-based outpatient clinics (CBOCs).37
Providing integrated care to the citizens of California is among the 10 priorities of the California Future Health Workforce Commission (a group of California health care leaders cochaired by the UC system president) for immediate action and guides their recommendations on developing and expanding the health care workforce; therefore, training in an integrated health care system is especially important for California HCPs.3 Nearly three-quarters of California’s population aged ≥ 65 years has a chronic health condition that could benefit from integrated care; however, the current supply of HCPs is insufficient to meet the growing demand for geriatric care.38,39
The VA has a robust training program to produce scholars and practitioners who specialize in geriatric care. This includes the Geriatric Scholars Program, which has the goal of integrating geriatrics into primary care through professional development. The Geriatric Scholars Program is a component of the VA Geriatric Research Education and Clinical Centers at urban VAMCs to help provide education and clinical resource connections with rural CBOCs where geriatrics expertise is lacking.
The California Future Health Workforce Commission is highlighting the need to prioritize workforce development in primary care, mental health care, and care for the aging.3 These priorities are shared as foundational services within the VHA.40 The alignment of these priorities creates an excellent rationale for increasing training and education of the UC health care workforce in the California VA system through academic affiliations.
VA Research Collaborations
The VA Office of Research and Development has existed for more than 90 years with a mission to improve veteran health and well-being via research and attract, train, and retain high-caliber researchers. VA provides a rich environment to conduct observational and interventional research due to its large, diverse veteran population, institutional support, and integrated information system with extensive EHR data.41 The success of the VA in facilitating research is evidenced by the fact that 3 VA investigators have been awarded Nobel prizes, and 7 have received Lasker Foundation Awards.42 The size of the VA allows for innovative large-scale research, such as the Million Veteran Program (MVP). The MVP study developed a mega-biobank of VA health records, questionnaires, and blood samples from nearly 1 million veterans to study genetic influences on health and disease and integrate genetic testing into health care delivery.43 In addition to producing high-quality, innovative research, more than 60% of VA investigators also provide direct patient care.42
VA research areas of focus include homelessness, polytrauma, traumatic brain injury, hearing and vision loss, spinal cord injury, mental health, pain management, precision medicine, prosthetics and amputation care, women’s health, and chronic diseases, such as Parkinson and Alzheimer diseases.44 The VA estimates that, in 2021, total VA research spending will include a request of $787 million in addition to $370 million from the National Institutes of Health, the Department of Defense, and the Centers for Disease Control and Prevention, and $170 million from other nonfederal sources, for a projected total of $1.3 billion. This budget will support 2,200 projects with direct research and reimbursable employment of 3,275 FTEs,which are key to supporting VA academic affiliations.45 These funds translate into substantial benefits to the UC system, including shared research and training resources, grant-funding opportunities for UC faculty, and the ability to recruit top researchers, educators, and clinicians to its institutions.
VA Reliance on Community Care
The current VHA model is an integrated health care system that provides comprehensive, wraparound services to enrolled veterans, which are cost-effective, high quality, and consistently found to have equal or superior quality of care compared with that in the community.6,46-50 Despite public criticism about wait times and access to care in the VA system, one study showed that VA wait-time statistics were comparable with or faster than those for community HCPs.51,52 However, VA care coordination has undergone several changes to address these public criticisms, namely, the Veterans Access, Choice and Accountability Act of 2014 (38 USC § 1703 VACAA) and the VA MISSION Act of 2018 (42 USC § 274). VACAA was designed to increase access to care for veterans who live ≥ 40 miles from VA health care facilities or who are unable to been seen within 30 days of their preferred or clinically appropriate date.53 More than 2 million veterans (almost 25% of VHA-enrolled veterans) have received community care since the inception of VACAA in 2014.54
Recently, the MISSION Act mandated developing additional VA-coordinated community-based care through the establishment of a Veterans Community Care Program, which was established using existing VA 2019 fiscal year funds and did not include additional appropriations despite expanded criteria for community care referrals.55 Without additional future appropriations, VA funds would be shifted from VA care into community care. While increasing access to community care has in some cases led to care that is faster and closer and that was previously inaccessible in local VA specialty care, these efforts could reduce veteran engagement with the VA system.56
The changes implemented in VACAA and the VA MISSION Act were driven by important and valid concerns, including evidence of VA staff and officials covering up service deficiencies.51 Veterans in rural areas often have limited access to VA resources, and long travel to VAMCs or clinics can be an impediment. Veterans who have chosen community care tended to be those who have poorer health status, who live further away from VA facilities, women, and those who identified as White or Hispanic.56,57 While VA health care is on average equivalent to or better than community resources, there is significant variability in quality within the VA system. Advocates have argued that providing competition and choice for veterans places pressure on the VA to improve care where it is not meeting expectations. Therefore, access to community care is an important resource for veterans and needs to be implemented effectively and efficiently to help veterans receive the care they need. However, expansion of community care access, depending on how it is implemented, also can have effects on academic partnerships and the education and research missions that should be incorporated into planning.
Each VA health care system receives funding through the Veterans Equitable Reimbursement Allocation (VERA), which provides funds largely based on the number of enrolled veterans and the complexity of the care they receive.58 One potential implication of the shift among veterans to community care is a reduction in patients enrolled in VA programs, thus decreasing funding given to the VA to allocate for training and research. By definition, increased VA-managed community care means less opportunity for integrated training that brings together primary, mental health, and substance use care to meet patient needs. The Center for Medicare and Medicaid Services has developed a national initiative to help states develop programs in integrated care, particularly for individuals who are eligible for both Medicare and Medicaid.59 For states to develop integrated care, they need trainees who function well in this model. Integrated care training is particularly vulnerable to disruption because any portion of a veteran’s care being transferred to the community can impede integration. In effect, training in integrated care, likely the most efficient and cost-effective approach to health care for reasons discussed earlier, could be reduced as providers and trainees are required to manage and coordinate patient care between separate institutions.35
Educational Impact
The shift in usage from VA to community care has potential implications for academic affiliates, particularly in education and research.60 If more people are served in community settings, potentially some VAMCs could be reduced, realigned, or closed. If this restructuring happens, academic partnerships could be impacted negatively. The VA is instituting an Infrastructure Review Commission with the task of examining current VA utilization. If a VA site with an academic affiliate was considered for realignment or closure, the reduction would eliminate the ability of the academic affiliate to provide education and research collaborations at that site.
In a less drastic manner, increasing care in the community may change opportunities for academic affiliates to partner with the VA. As noted, the UC system and California veterans benefit immensely from the VHA as an integrated health care system with dedicated missions of education and research. This partnership is a model in which the VA is the primary source of care for eligible enrolled veterans and provides integrated comprehensive services. If the VA moves to serving primarily as a coordinator of community HCPs rather than a direct provider of health care, academic affiliates would need to make major adjustments to both the education and training models. This change could particularly affect specialty training programs that rely on having adequate volumes of patients to provide an extensive experience to meet training needs. If fewer veterans receive care directly from the VA and are instead dispersed in the community, that will reduce the ability of academic faculty to participate in the education of medical and affiliated trainees and to participate in research in VA settings. It is unclear what other model could replace such a system and be as beneficial to the VA and the academic partners with which it is currently affiliated.
Given the needs that led to the VA increasing access to care and the potential implications discussed for the VA and partnerships with academic affiliates, VA health care systems and academic affiliate partners should consider several steps. These steps involve assessment, coordination, and promotion.
Both the VA and academic affiliates would benefit if the VA shared assessment data on the use of community care, particularly identifying changes that relate to key training and/or research missions. Such data sharing can be critical to determine whether any risks (or potential opportunities) need to be addressed. In addition, increasing research on the outcomes related to both VA care and community-based care is of high value to determine whether the current changes are achieving intended goals. The VA recently funded such work through its research service, and such work is critical for guiding future policy for the VA and for the affiliates.
Coordination among the VA, academic affiliates, and community partners is vital for change. The issue of community care expansion should be a standing item on coordination meetings and shared governance councils between the institutions. It may make sense to establish specific workgroups or committees to coordinate tracking and assessment of the effect of community care expansion on the shared academic mission. One way to address the potential effect of increased community care on the research and education missions would be to include community partners into the partnerships. This strategy could potentially take a number of different forms, from providing education and training to community HCPs, having VA trainees rotate to community settings, or inviting community settings to be research sites for clinical trials. Such partnerships could potentially improve patient care and support the other academic missions. Coordination could be meaningfully improved by having community HCPs access the VA EHR, thus easing communications. Funding is available for EHR access in the VA MISSION Act and should be a high priority as community care expands. The more that community partners can access and connect with the VA EHR the better they will be able to coordinate care.
Third, the VA and its academic partners need to promote and educate veterans, their families, and their advocates on the benefits that are available through VA care and that are enhanced through academic partnerships. While the VA has been the target of justified criticism, many of its strengths addressed here are not broadly recognized. The VA could promote its sharing of staff and resources with the top academic health care institutions in an area and that veterans often have access to resources that otherwise would not be available without the academic affiliate. Making sure veterans are aware of the benefits available can potentially mitigate the need for community care.
Conclusions
Given changes from VACAA and the VA MISSION Act, VA and academic affiliates should be active partners in planning for future health care by providing input and feedback on VA structure to help shape federal and state systems moving forward. Institutions can take steps to steer their futures and meet growing clinical, training, and research needs. The VA and its academic partners in health care research are well positioned to develop projects to assess the effects of these changes. Evaluation of key variables including patient care, education, and research productivity are warranted to guide policymakers as they assess whether these changes in the VA are achieving the expressed goals of improving veteran care. Other opportunities to collaborate in the wake of the MISSION Act remain to be discovered within each academic affiliation. By strengthening working relationships between VA and academic teams, these deeply important partnerships can continue to produce clinical, research, and education outcomes that meet the needs of our veterans, our federal and state health care systems, and our country.
Acknowledgments
Dr. Sells was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations VA Quality Scholars Advanced Fellowship Program.
1. US Department of Veterans Affairs, Veterans Health Administration. About VHA. Updated January 22, 2021. Accessed March 9, 2021. https://www.va.gov/health/aboutvha.asp
2. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee to Evaluate the Department of Veterans Affairs Mental Health Services. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed March 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK499502/
3. California Future Health Workforce Commission. Meeting the demand for health: final report of the California Future Health Workforce Commission. Published February 2019. Accessed March 9, 2021. https://futurehealthworkforce.org/wp-content/uploads/2019/03/MeetingDemandForHealthFinalReportCFHWC.pdf
4. US Department of Veterans Affairs. Veterans Health Administration fiscal year 2017 annual report. Published 2017. Accessed March 9, 2021. https://www.va.gov/HEALTH/docs/VHA_AnnualReport_FY2017.pdf
5. US Department of Veterans Affairs. Public health: health care use by Gulf War & OEF/OIF/OND veterans. Updated March 28, 2017. Accessed March 9, 2021. https://www.publichealth.va.gov/epidemiology/reports/health-care-use-gulfwar-oefoifond/index.asp
6. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13.
7. US Department of Veterans Affairs. Patient care services: veterans with lesbian, gay, bisexual and transgender (LGBT) and related identities. Updated August 31, 2020. Accessed March 9, 2021. https://www.patientcare.va.gov/LGBT/index.asp
8. US Department of Veterans Affairs. Women veterans health care: women veterans program managers. Updated March 28, 2017. Accessed March 9, 2021. https://www.womens health.va.gov/WOMENSHEALTH/programoverview/wvpm.asp
9. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA facilities by state. Published May 15, 2017. Accessed March 9, 2021. https://www.va.gov/vetdata/docs/SpecialReports/VA_Facilities_By_State.PDF
10. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. State summaries: California. Published September 2018. Accessed March 9, 2021. https://www.va.gov/vetdata/docs/SpecialReports/State_Summaries_California.pdf
11. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Care Services, Post-Deployment Health Group, Epidemiology Program. Analysis of VA health care utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. Published January 2017. Accessed March 9, 2021. https://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2015-qtr3.pdf
12. US Department of Veterans Affairs, Office of Budget. Annual budget submission, president’s budget request – fiscal year 2021. Updated February 10, 2020. Accessed March 9, 2021. https://www.va.gov/budget/products.asp
13. US Department of Veterans Affairs. Department of Veterans Affairs statistics at a glance. Updated February 2020. Accessed March 10, 2021. https://www.va.gov/vetdata/docs/Quickfacts/Stats_at_a_glance_4_6_20.PDF
14. US Department of Veterans Affairs VW. Locations, California. Updated October 12, 2018. Accessed March 10, 2021. https://www.va.gov/directory/guide/state.asp?dnum=ALL&STATE=CA
15. Baker, R. R., & Pickren, W. E. (2007). Psychology and the Department of Veterans Affairs: A historical analysis of training, research, practice, and advocacy. American Psychological Association. doi:10.1037/11544-000
16. Functions of Veterans Health Administration: health-care personnel education and training programs. 38 USC § 7302. Accessed March 16, 2021. https://www.govinfo.gov/app/details/USCODE-2011-title38/USCODE-2011-title38-partV-chap73-subchapI-sec7302
17. US Department of Veterans Affairs, Office of Academic Affiliations. Mission of the Office of Academic Affiliations. Published September 24, 2019. Accessed March 10, 2021. https://www.va.gov/oaa/oaa_mission.asp
18. Congressional Research Service. Federal support for graduate medical education: an overview. CRS report R44376. Updated December 27, 2018. Accessed March 10, 2021. https://fas.org/sgp/crs/misc/R44376.pdf
19. Association of American Medical Colleges. 2018 Report on residents. Table B3: number of active residents, by type of medical school, GME specialty, and sex. Accessed March 10, 2021. https://www.aamc.org/data-reports/students-residents/interactive-data/table-b3-number-active-residents-type-medical-school-gme-specialty-and-sex
20. US Department of Veterans Affairs, Office of Academic Affiliations. National summary trainees unique school list - academic year: 2017-2018.
21. US Department of Veterans Affairs, Office of Academic Affiliations. VA nursing academic partnerships. Updated December 12, 2018. Accessed March 10, 2021. https://www.va.gov/oaa/vanap/default.asp
22. Keitz SA, Aron DC, Brannen JL, et al. Impact of clinical training on recruiting graduating health professionals. Am J Manag Care. 2019;25(4):e111-e118. Published 2019 Apr 1.
23. US Department of Veterans Affairs, Office of Academic Affiliations. Health professions education: academic year 2019-2020. Published 2020. https://www.va.gov/OAA/docs/OAA_Statistics_2020.pdf
24. US Department of Veterans Affairs, Veterans Health Administration. Hiring programs and initiatives. Updated March 10, 2021. Accessed March 10, 2021. https://www.vacareers.va.gov/Benefits/HiringProgramsInitiatives/
25. US Department of Veterans Affairs, Veterans Health Administration. Students and trainees. Updated March 10, 2021. Accessed March 10, 2021. https://www.vacareers.va.gov/Careers/StudentsTrainees
26. The Regents of the University of California. The UC system. Accessed March 10, 2021. https://www.universityofcalifornia.edu/uc-system
27. The Regents of the University of California. The parts of UC. Accessed March 10, 2021. https://www.universityofcalifornia.edu/uc-system/parts-of-uc
28. US Department of Veterans Affairs. Locations: VISN 21: Sierra Pacific Network. Updated October 12, 2018. Accessed March 10, 2021. https://www.va.gov/directory/guide/region.asp?ID=1021
29. Association of American Medical Colleges. California physician workforce profile. Published 2017. Accessed March 10, 2021. https://www.aamc.org/system/files/2019-08/california2017.pdf
30. Rittenhouse D, Ament A, Grumbach K, Petterson S, Levin Z, Bazemore A. California Health Care Foundation: guide to graduate medical education funding in California. Published September 2018. Accessed March 10, 2021. https://www.chcf.org/wp-content/uploads/2018/08/GuideGraduateMedicalEducationFunding.pdf
31. US Department of Health and Human Services, Health Resources and Services Administration. Integrated behavioral health resource library. Accessed March 18, 2020. https://www.hrsa.gov/behavioral-health/library
32. US Department of Veterans Affairs. Patient care services: primary care - mental health integration (PC-MHI). Updated August 1, 2016. Accessed March 10, 2021. https://www.patientcare.va.gov/primarycare/PCMHI.asp

33. Hwang W, Chang J, Laclair M, Paz H. Effects of integrated delivery system on cost and quality. Am J Manag Care. 2013;19(5):e175-e184.
34. World Health Organization, World Organization of Family Doctors (Wonca). Integrating mental health into primary care: a global perspective. Published October 2008. Accessed March 10, 2021. https://www.who.int/mental_health/policy/Integratingmhintoprimarycare2008_lastversion.pdf
35. Congressional Budget Office. Comparing the costs of the veterans’ health care system with private-sector costs. Published December 10, 2014. Accessed March 10, 2021. https://www.cbo.gov/publication/49763
36. Souden M. Overview of VA data, information systems, national databases and research uses. Published October 2, 2017. Accessed March 10, 2021. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/2376-notes.pdf
37. US Department of Veterans Affairs, Veterans Health Administration. Uniform mental health services in VA medical centers and clinics. VHA handbook 1160.01. Published September 11, 2008. Recertified September 30, 2013. Amended November 16, 2015. Published September 11, 2008. Accessed March 10, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1762
38. Coffman JM, Fix M, Ko M. California physician supply and distribution: headed for a drought? Published June 25, 2018. Accessed March 10, 2021. https://www.chcf.org/publication/californias-physicians-headed-drought
39. Meng YY, Ahman T, Pickett M. California Health Care Foundation: 2015 Edition—Californians with the top chronic conditions: 11 million and counting. Published April 23, 2015. Accessed March 10, 2021. https://www.chcf.org/publication/2015-edition-californians-top-chronic-conditions-11-million-counting
40. US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Updated May 31, 2019. Accessed March 10, 2021. https://www.va.gov/oei/docs/va2018-2024strategicplan.pdf
41. Justice AC, Erdos J, Brandt C, Conigliaro J, Tierney W, Bryant K. The Veterans Affairs healthcare system: a unique laboratory for observational and interventional research. Med Care. 2006;44(8)(suppl 2):S7-S12. doi:10.1097/01.mlr.0000228027.80012.c5
42. US Department of Veterans Affairs, Office of Research and Development: About the Office of Research & Development. Published Updated March 4, 2021. Accessed March 10, 2021. https://www.research.va.gov/about/default.cfm
43. Gaziano JM, Concato J, Brophy M, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214-223. doi:10.1016/j.jclinepi.2015.09.016
44. US Department of Veterans Affairs. VA research program overview. Accessed March 12, 2021. https://www.research.va.gov/pubs/docs/va-research-overview-brochure.pdf
45. US Department of Veterans Affairs. FY 2021 budget submission: medical programs and information technology programs. Volume 2 of 4. Published February 2020. Accessed March 12, 2021. https://www.va.gov/budget/docs/summary/fy2021VAbudgetVolumeIImedicalProgramsAndInformationTechnology.pdf
46. Trivedi AN, Matula S, Miake-Lye I, Glassman PA, Shekelle P, Asch S. Systematic review: comparison of the quality of medical care in Veterans Affairs and non-Veterans Affairs settings. Med Care. 2011;49(1):76-88. doi:10.1097/MLR.0b013e3181f53575
47. Nugent GN, Hendricks A, Nugent L, Render ML. Value for taxpayers’ dollars: what VA care would cost at Medicare prices. Med Care Res Rev. 2004;61(4):495-508. doi:10.1177/1077558704269795
48. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631-1638. doi:10.1007/s11606-018-4433-7
49. O’Hanlon C, Huang C, Sloss E, et al. Comparing VA and non-VA quality of care: a systematic review. J Gen Intern Med. 2017;32(1):105-121. doi:10.1007/s11606-016-3775-2
50. Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
51. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix health care system. Published May 28, 2014. Accessed March 12, 2021. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf
52. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096. doi:10.1001/jamanetworkopen.2018.7096
53. US Department of Veterans Affairs. Fact sheet: Veterans Access, Choice and Accountability Act of 2014 (“Choice Act”). Accessed March 12, 2021. https://www.va.gov/opa/choiceact/documents/choice-act-summary.pdf
54. Mattocks KM, Cunningham K, Elwy AR, et al. Recommendations for the evaluation of cross-system care coordination from the VA State-of-the-art Working Group on VA/Non-VA Care. J Gen Intern Med. 2019;34(Suppl 1):18-23. doi:10.1007/s11606-019-04972-1
55. US Department of Veterans Affairs. Fact sheet: VA MISSION Act and new veterans community care program. Published June 15, 2018. Accessed March 12, 2021. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/FactSheet_20-13.pdf
56. Stroupe KT, Martinez R, Hogan TP, et al. Experiences with the veterans’ choice program. J Gen Intern Med. 2019;34(10):2141-2149. doi:10.1007/s11606-019-05224-y
57. Yoon J, Leung LB, Rubenstein LV, et al. Use of the veterans’ choice program and attrition from Veterans Health Administration primary care. Med Care. 2020;58(12):1091-1097. doi:10.1097/MLR.0000000000001401
58. US Department of Veterans Affairs. Veterans Equitable Resource Allocation (VERA). Updated March 9, 2021. Accessed March 12, 2021. https://catalog.data.gov/dataset/veterans-equitable-resource-allocation-vera
59. Integrated Care Resource Center. About us. Accessed March 12, 2021. https://www.integratedcareresourcecenter.com/about-us
60. Duhaney T. How veteran utilization of the Veterans Health Administration could impact privatization. Public Policy Aging Rep. 2020;30(1):29-35. doi:10.1093/ppar/prz032
The Veterans Health Administration (VHA), 1 of 3 administrative branches in the US Department of Veterans Affairs (VA), is the largest integrated health care system in the United States.1 The VHA has 4 missions: providing health care to eligible veterans; supporting research to benefit veterans and the larger society; providing education for health care trainees; and supporting emergency response.1 In service of these goals, VA has academic affiliations with universities throughout the country, offering unique, extensive training and research opportunities. Both the VA and the affiliate benefit from these partnerships. For example, VA affiliations with University of California (UC) medical schools benefit veteran care while facilitating the UC academic mission. Through these affiliations, trainees who learn within the VHA’s highly effective integrated care model become health care professionals (HCPs) who are prepared to enter health care systems in California and meet the state’s demand for high-quality integrated care with an emphasis on primary care, mental health care, and care for aging populations.2,3
This report explores the history of the VHA, current veteran demographics and needs, VA academic affiliations, and the integrated care model of training in all VHA facilities. The VA and UC academic affiliation is described further with regard to shared research and educational functions. Finally, we identify potential risks to academic affiliations associated with increased VA reliance on community-based care following the implementation of recent legislation. We provide suggestions for VA academic affiliates to help assess and guide the potential impact of increased VA-managed community care.
VHA Resources
The VHA serves more than 9 million veterans through 170 medical centers and 1,074 outpatient care sites.1 In fiscal year 2017, the VA provided 109 million outpatient visits, and treated 615,000 inpatient medicine/surgical patients and 149,000 patients in inpatient mental health.4 The VHA focuses on the distinct concerns of veterans, which arise from military service as well as their broader health care needs. Veterans have higher rates of medical and mental health conditions than those of the general public; different cohorts in this population experience distinct medical and mental health concerns (Table 1).5
In addition, although veterans are disproportionately older men, the population is diversifying.6 For example, the number of female veterans is growing; furthermore, changes in the law now allow lesbian, gay, bisexual, and transgender (LGBT) individuals to serve openly, which has both reduced barriers for this population and allowed for LGBT veterans who were not eligible for VA care due to less than honorable discharges to have those discharges upgraded. As a result, care has been tailored to include the development of Women Veterans Program Managers and related services and LGBT and related identities resources such as LGBT Veteran Care Coordinators in every VA facility nationwide.7,8 The VA continues to adapt to serve all veterans; part of this adaptation is training HCPs to provide veteran-centered care for a growing and diversifying population.
VHA Resources in California
California has the largest population of veterans in the United States (Table 2).9,10 Of the 9,116,200 VA enrollees nationwide, 760,910 (8%) reside in California, and of those, 463,410 had at least 1 VA visit in the past year.3,10 The VHA is organized into 21 Veterans Integrated Service Networks (VISNs) that include multiple health care systems in the region associated with each VISN. California is part of VISN 21 (Northern California, Nevada, and Pacific Islands) and VISN 22 (Southern California, Nevada, and New Mexico). Among veterans who served in the recent Iraq and Afghanistan conflicts, 5.5% accessed care in VISN 21 and 9.3% accessed care in VISN 22.11 The VHA provides critical infrastructure for meeting complex veteran needs, as well as related specialized training, education, and research for HCPs. This specialization has been the basis for the broad system of affiliations between VA and academic systems.
The VA continues to be a high priority in the federal budget process.12 In 2017, slightly more than 9% of the VA health care budget, $6.4 billion, was spent on medical care in California.10 Consequently, California has a noteworthy portion of VA infrastructure (Table 3).13,14 California has 8 VA medical centers (VAMCs) with hospital service (Fresno, Loma Linda, Long Beach, Palo Alto, Sacramento, San Diego, San Francisco, West Los Angeles), 3 VAMCs without hospital service (2 locations in the Palo Alto system and Sepulveda), 1 stand-alone extended-care facility (Martinez Community Living Center), and 1 stand-alone residential care facility (San Diego Domiciliary).9 The vast VA infrastructure in California and large population of veterans creates a strong demand for HCPs in the state.
VA Education and Collaboration
VA has been training clinicians and scholars since 1946, when VA academic affiliations were established by Memorandum Number 2.15,16 Today, the VA is the largest educator of HCPs in the United States.17 In 2015, an estimated $10.3 to $12.5 billion was spent on mandatory Medicare graduate medical education (GME).18 In 2017, the VA spent $1.78 billion of discretionary funding on GME to fund 11,000 full-time equivalent (FTE) slots, leading to > 43,000 physician residents (> 30% of all physician residents) spending part of their training in a VHA facility.18,19
This training mission has multiple benefits. It provides the VA with access to new HCPs who have the necessary training in veteran-specific needs, while supporting the national need for HCPs. In 2018, 120,890 clinical trainees received some or all of their training in the VA system.20 Of the 152 US medical schools that are accredited by the Liaison Committee on Medical Education, 95% collaborate with the VA for training while 100% of the 34 doctor of osteopathic medicine programs have VA training collaborations.20 The VA currently has an additional 18 partnerships with nursing schools.21 Further, 1,800 college and universities, including Hispanic-serving institutions and historically black colleges and universities, have VHA affiliations that provide training for more than 40 clinical health profession education programs.17
This training model has been successful in supporting VA staffing, as health care providers who trained in the VA are more likely to work in the VA.22 Among current VA employees, > 80% of optometrists, > 70% of podiatrists and psychologists, and > 60% of physicians received some part of their training in the VA system.23 In combination with recent increased funding for staffing, the ability of the VA to directly hire trainees in identified professions, and the expansion of loan forgiveness to high-demand specialties (eg, psychiatry), the training partnership between the VA and affiliates has been critical in maintaining the needed VA workforce.22,24,25
The VA Office of Academic Affiliations is responsible for all graduate medical and dental education administration in the VA system, which makes up 85% of its total budget. For each trainee, the VA provides approximately $60,000 toward their stipend in exchange for training and patient care time at a VHA hospital (Kenneth R. Jones, PhD, email communication, August 27, 2018).
California Health Care Education
The UC public university system, founded in 1869, currently has 10 campuses with a combined student body of > 280,000 students, along with 227,000 faculty and staff members.26 For every research dollar provided by California, the UC secures $7 in federal and private funding.26 The UC has 6 medical centers (Davis, Irvine, Los Angeles, Riverside, San Diego, and San Francisco); each is affiliated with at least 1 local VAMC.27,28
California trains a substantial share of health care trainees. In 2016, there were 10,429 physician residents in training in California.29 In 2017/2018, the San Francisco VAMC trained 1,178 medical students/residents, 57 pharmacy students, 25 nurse practitioner students, 19 optometry interns/students/residents, 11 dental students/residents, and 3 physical therapy students.20 In total, 6,223 UC health professions students were trained in VHA facilities during the 2017/2018 training year (Table 4).20 As of 2016, there were 105,907 physicians in California, and of those, 57% completed their GME in California.29 In California in 2015, 74 GME-sponsoring institutions graduated 3,568 residents and fellows, an increase of 10% since 1997.30 Of these sponsoring institutions, 6 of the top 8 programs were UC schools that graduated 48.4% (1,727) of all California residents and fellows in 2015.30
Despite these resources, California faces a major shortage of HCPs, particularly in primary, behavioral health, and older adult care.3 Today, 7 million Californians live in counties with a federally designated shortage of primary, dental, and mental health care providers.3 Most of these Californians are Latino, African American, or Native American, and they live in fast-growing rural and urban regions, including Los Angeles; the San Joaquin Valley; and the Inland Empire (San Bernardino and Riverside Counties).3 Current recommendations to meet increasing demands as California’s population increases, grows older, and faces increased health care demands include expanding residency programs to yield 1,872 additional primary care physicians and 2,202 additional psychiatrists by 2030.3 To meet this shortage and prepare for future health care demands, health care education is paramount; in California, VA and UC affiliations are central to addressing these needs.
The VA plays a particularly important role in supporting GME, which is essential to meeting both VA and California’s unmet HCP needs, as GME determines the number of medical practitioners available per specialty.30 The VA was the second largest GME fund provider in California at $90,662,608 (Medicare provided $552,235,626) and the California government provided a small portion of GME funding.30 VA education funding is a direct result of the VA provision of clinical care in one of the most innovative and modern health care systems in the world.
These VA training opportunities benefit the UC system and California by helping train integrated care practitioners to meet the increasing demand. Integrated care—the coordination of mental health care, substance use disorder treatment, and primary care services—is designed to improve health outcomes by helping people with multiple and complex health care needs access care.31,32
As the largest integrated health care system in the country, the VA brings important clinical, research, and educational opportunities to academic affiliates. A systematic review examining cost and quality outcomes in integrated care systems found improved quality of care compared with nonintegrated care systems; thus, many US government agencies and the World Health Organization are establishing integrated care systems as a standard and universal approach.31,33,34 While cost savings as a result of integrated care are unclear, most studies in this review reported a decrease in utilization of services.33 The presumption of more efficient and higher quality care is also predicated on features such as system-wide accessibility of comprehensive medical records that provide more information to HCPs, promote collaboration, and measure and reward performance, all of which are possible using the VA electronic health record (EHR) system.35,36 The VA offers an excellent opportunity for training in integrated care as this model is required of all VAMCs and community-based outpatient clinics (CBOCs).37
Providing integrated care to the citizens of California is among the 10 priorities of the California Future Health Workforce Commission (a group of California health care leaders cochaired by the UC system president) for immediate action and guides their recommendations on developing and expanding the health care workforce; therefore, training in an integrated health care system is especially important for California HCPs.3 Nearly three-quarters of California’s population aged ≥ 65 years has a chronic health condition that could benefit from integrated care; however, the current supply of HCPs is insufficient to meet the growing demand for geriatric care.38,39
The VA has a robust training program to produce scholars and practitioners who specialize in geriatric care. This includes the Geriatric Scholars Program, which has the goal of integrating geriatrics into primary care through professional development. The Geriatric Scholars Program is a component of the VA Geriatric Research Education and Clinical Centers at urban VAMCs to help provide education and clinical resource connections with rural CBOCs where geriatrics expertise is lacking.
The California Future Health Workforce Commission is highlighting the need to prioritize workforce development in primary care, mental health care, and care for the aging.3 These priorities are shared as foundational services within the VHA.40 The alignment of these priorities creates an excellent rationale for increasing training and education of the UC health care workforce in the California VA system through academic affiliations.
VA Research Collaborations
The VA Office of Research and Development has existed for more than 90 years with a mission to improve veteran health and well-being via research and attract, train, and retain high-caliber researchers. VA provides a rich environment to conduct observational and interventional research due to its large, diverse veteran population, institutional support, and integrated information system with extensive EHR data.41 The success of the VA in facilitating research is evidenced by the fact that 3 VA investigators have been awarded Nobel prizes, and 7 have received Lasker Foundation Awards.42 The size of the VA allows for innovative large-scale research, such as the Million Veteran Program (MVP). The MVP study developed a mega-biobank of VA health records, questionnaires, and blood samples from nearly 1 million veterans to study genetic influences on health and disease and integrate genetic testing into health care delivery.43 In addition to producing high-quality, innovative research, more than 60% of VA investigators also provide direct patient care.42
VA research areas of focus include homelessness, polytrauma, traumatic brain injury, hearing and vision loss, spinal cord injury, mental health, pain management, precision medicine, prosthetics and amputation care, women’s health, and chronic diseases, such as Parkinson and Alzheimer diseases.44 The VA estimates that, in 2021, total VA research spending will include a request of $787 million in addition to $370 million from the National Institutes of Health, the Department of Defense, and the Centers for Disease Control and Prevention, and $170 million from other nonfederal sources, for a projected total of $1.3 billion. This budget will support 2,200 projects with direct research and reimbursable employment of 3,275 FTEs,which are key to supporting VA academic affiliations.45 These funds translate into substantial benefits to the UC system, including shared research and training resources, grant-funding opportunities for UC faculty, and the ability to recruit top researchers, educators, and clinicians to its institutions.
VA Reliance on Community Care
The current VHA model is an integrated health care system that provides comprehensive, wraparound services to enrolled veterans, which are cost-effective, high quality, and consistently found to have equal or superior quality of care compared with that in the community.6,46-50 Despite public criticism about wait times and access to care in the VA system, one study showed that VA wait-time statistics were comparable with or faster than those for community HCPs.51,52 However, VA care coordination has undergone several changes to address these public criticisms, namely, the Veterans Access, Choice and Accountability Act of 2014 (38 USC § 1703 VACAA) and the VA MISSION Act of 2018 (42 USC § 274). VACAA was designed to increase access to care for veterans who live ≥ 40 miles from VA health care facilities or who are unable to been seen within 30 days of their preferred or clinically appropriate date.53 More than 2 million veterans (almost 25% of VHA-enrolled veterans) have received community care since the inception of VACAA in 2014.54
Recently, the MISSION Act mandated developing additional VA-coordinated community-based care through the establishment of a Veterans Community Care Program, which was established using existing VA 2019 fiscal year funds and did not include additional appropriations despite expanded criteria for community care referrals.55 Without additional future appropriations, VA funds would be shifted from VA care into community care. While increasing access to community care has in some cases led to care that is faster and closer and that was previously inaccessible in local VA specialty care, these efforts could reduce veteran engagement with the VA system.56
The changes implemented in VACAA and the VA MISSION Act were driven by important and valid concerns, including evidence of VA staff and officials covering up service deficiencies.51 Veterans in rural areas often have limited access to VA resources, and long travel to VAMCs or clinics can be an impediment. Veterans who have chosen community care tended to be those who have poorer health status, who live further away from VA facilities, women, and those who identified as White or Hispanic.56,57 While VA health care is on average equivalent to or better than community resources, there is significant variability in quality within the VA system. Advocates have argued that providing competition and choice for veterans places pressure on the VA to improve care where it is not meeting expectations. Therefore, access to community care is an important resource for veterans and needs to be implemented effectively and efficiently to help veterans receive the care they need. However, expansion of community care access, depending on how it is implemented, also can have effects on academic partnerships and the education and research missions that should be incorporated into planning.
Each VA health care system receives funding through the Veterans Equitable Reimbursement Allocation (VERA), which provides funds largely based on the number of enrolled veterans and the complexity of the care they receive.58 One potential implication of the shift among veterans to community care is a reduction in patients enrolled in VA programs, thus decreasing funding given to the VA to allocate for training and research. By definition, increased VA-managed community care means less opportunity for integrated training that brings together primary, mental health, and substance use care to meet patient needs. The Center for Medicare and Medicaid Services has developed a national initiative to help states develop programs in integrated care, particularly for individuals who are eligible for both Medicare and Medicaid.59 For states to develop integrated care, they need trainees who function well in this model. Integrated care training is particularly vulnerable to disruption because any portion of a veteran’s care being transferred to the community can impede integration. In effect, training in integrated care, likely the most efficient and cost-effective approach to health care for reasons discussed earlier, could be reduced as providers and trainees are required to manage and coordinate patient care between separate institutions.35
Educational Impact
The shift in usage from VA to community care has potential implications for academic affiliates, particularly in education and research.60 If more people are served in community settings, potentially some VAMCs could be reduced, realigned, or closed. If this restructuring happens, academic partnerships could be impacted negatively. The VA is instituting an Infrastructure Review Commission with the task of examining current VA utilization. If a VA site with an academic affiliate was considered for realignment or closure, the reduction would eliminate the ability of the academic affiliate to provide education and research collaborations at that site.
In a less drastic manner, increasing care in the community may change opportunities for academic affiliates to partner with the VA. As noted, the UC system and California veterans benefit immensely from the VHA as an integrated health care system with dedicated missions of education and research. This partnership is a model in which the VA is the primary source of care for eligible enrolled veterans and provides integrated comprehensive services. If the VA moves to serving primarily as a coordinator of community HCPs rather than a direct provider of health care, academic affiliates would need to make major adjustments to both the education and training models. This change could particularly affect specialty training programs that rely on having adequate volumes of patients to provide an extensive experience to meet training needs. If fewer veterans receive care directly from the VA and are instead dispersed in the community, that will reduce the ability of academic faculty to participate in the education of medical and affiliated trainees and to participate in research in VA settings. It is unclear what other model could replace such a system and be as beneficial to the VA and the academic partners with which it is currently affiliated.
Given the needs that led to the VA increasing access to care and the potential implications discussed for the VA and partnerships with academic affiliates, VA health care systems and academic affiliate partners should consider several steps. These steps involve assessment, coordination, and promotion.
Both the VA and academic affiliates would benefit if the VA shared assessment data on the use of community care, particularly identifying changes that relate to key training and/or research missions. Such data sharing can be critical to determine whether any risks (or potential opportunities) need to be addressed. In addition, increasing research on the outcomes related to both VA care and community-based care is of high value to determine whether the current changes are achieving intended goals. The VA recently funded such work through its research service, and such work is critical for guiding future policy for the VA and for the affiliates.
Coordination among the VA, academic affiliates, and community partners is vital for change. The issue of community care expansion should be a standing item on coordination meetings and shared governance councils between the institutions. It may make sense to establish specific workgroups or committees to coordinate tracking and assessment of the effect of community care expansion on the shared academic mission. One way to address the potential effect of increased community care on the research and education missions would be to include community partners into the partnerships. This strategy could potentially take a number of different forms, from providing education and training to community HCPs, having VA trainees rotate to community settings, or inviting community settings to be research sites for clinical trials. Such partnerships could potentially improve patient care and support the other academic missions. Coordination could be meaningfully improved by having community HCPs access the VA EHR, thus easing communications. Funding is available for EHR access in the VA MISSION Act and should be a high priority as community care expands. The more that community partners can access and connect with the VA EHR the better they will be able to coordinate care.
Third, the VA and its academic partners need to promote and educate veterans, their families, and their advocates on the benefits that are available through VA care and that are enhanced through academic partnerships. While the VA has been the target of justified criticism, many of its strengths addressed here are not broadly recognized. The VA could promote its sharing of staff and resources with the top academic health care institutions in an area and that veterans often have access to resources that otherwise would not be available without the academic affiliate. Making sure veterans are aware of the benefits available can potentially mitigate the need for community care.
Conclusions
Given changes from VACAA and the VA MISSION Act, VA and academic affiliates should be active partners in planning for future health care by providing input and feedback on VA structure to help shape federal and state systems moving forward. Institutions can take steps to steer their futures and meet growing clinical, training, and research needs. The VA and its academic partners in health care research are well positioned to develop projects to assess the effects of these changes. Evaluation of key variables including patient care, education, and research productivity are warranted to guide policymakers as they assess whether these changes in the VA are achieving the expressed goals of improving veteran care. Other opportunities to collaborate in the wake of the MISSION Act remain to be discovered within each academic affiliation. By strengthening working relationships between VA and academic teams, these deeply important partnerships can continue to produce clinical, research, and education outcomes that meet the needs of our veterans, our federal and state health care systems, and our country.
Acknowledgments
Dr. Sells was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations VA Quality Scholars Advanced Fellowship Program.
The Veterans Health Administration (VHA), 1 of 3 administrative branches in the US Department of Veterans Affairs (VA), is the largest integrated health care system in the United States.1 The VHA has 4 missions: providing health care to eligible veterans; supporting research to benefit veterans and the larger society; providing education for health care trainees; and supporting emergency response.1 In service of these goals, VA has academic affiliations with universities throughout the country, offering unique, extensive training and research opportunities. Both the VA and the affiliate benefit from these partnerships. For example, VA affiliations with University of California (UC) medical schools benefit veteran care while facilitating the UC academic mission. Through these affiliations, trainees who learn within the VHA’s highly effective integrated care model become health care professionals (HCPs) who are prepared to enter health care systems in California and meet the state’s demand for high-quality integrated care with an emphasis on primary care, mental health care, and care for aging populations.2,3
This report explores the history of the VHA, current veteran demographics and needs, VA academic affiliations, and the integrated care model of training in all VHA facilities. The VA and UC academic affiliation is described further with regard to shared research and educational functions. Finally, we identify potential risks to academic affiliations associated with increased VA reliance on community-based care following the implementation of recent legislation. We provide suggestions for VA academic affiliates to help assess and guide the potential impact of increased VA-managed community care.
VHA Resources
The VHA serves more than 9 million veterans through 170 medical centers and 1,074 outpatient care sites.1 In fiscal year 2017, the VA provided 109 million outpatient visits, and treated 615,000 inpatient medicine/surgical patients and 149,000 patients in inpatient mental health.4 The VHA focuses on the distinct concerns of veterans, which arise from military service as well as their broader health care needs. Veterans have higher rates of medical and mental health conditions than those of the general public; different cohorts in this population experience distinct medical and mental health concerns (Table 1).5
In addition, although veterans are disproportionately older men, the population is diversifying.6 For example, the number of female veterans is growing; furthermore, changes in the law now allow lesbian, gay, bisexual, and transgender (LGBT) individuals to serve openly, which has both reduced barriers for this population and allowed for LGBT veterans who were not eligible for VA care due to less than honorable discharges to have those discharges upgraded. As a result, care has been tailored to include the development of Women Veterans Program Managers and related services and LGBT and related identities resources such as LGBT Veteran Care Coordinators in every VA facility nationwide.7,8 The VA continues to adapt to serve all veterans; part of this adaptation is training HCPs to provide veteran-centered care for a growing and diversifying population.
VHA Resources in California
California has the largest population of veterans in the United States (Table 2).9,10 Of the 9,116,200 VA enrollees nationwide, 760,910 (8%) reside in California, and of those, 463,410 had at least 1 VA visit in the past year.3,10 The VHA is organized into 21 Veterans Integrated Service Networks (VISNs) that include multiple health care systems in the region associated with each VISN. California is part of VISN 21 (Northern California, Nevada, and Pacific Islands) and VISN 22 (Southern California, Nevada, and New Mexico). Among veterans who served in the recent Iraq and Afghanistan conflicts, 5.5% accessed care in VISN 21 and 9.3% accessed care in VISN 22.11 The VHA provides critical infrastructure for meeting complex veteran needs, as well as related specialized training, education, and research for HCPs. This specialization has been the basis for the broad system of affiliations between VA and academic systems.
The VA continues to be a high priority in the federal budget process.12 In 2017, slightly more than 9% of the VA health care budget, $6.4 billion, was spent on medical care in California.10 Consequently, California has a noteworthy portion of VA infrastructure (Table 3).13,14 California has 8 VA medical centers (VAMCs) with hospital service (Fresno, Loma Linda, Long Beach, Palo Alto, Sacramento, San Diego, San Francisco, West Los Angeles), 3 VAMCs without hospital service (2 locations in the Palo Alto system and Sepulveda), 1 stand-alone extended-care facility (Martinez Community Living Center), and 1 stand-alone residential care facility (San Diego Domiciliary).9 The vast VA infrastructure in California and large population of veterans creates a strong demand for HCPs in the state.
VA Education and Collaboration
VA has been training clinicians and scholars since 1946, when VA academic affiliations were established by Memorandum Number 2.15,16 Today, the VA is the largest educator of HCPs in the United States.17 In 2015, an estimated $10.3 to $12.5 billion was spent on mandatory Medicare graduate medical education (GME).18 In 2017, the VA spent $1.78 billion of discretionary funding on GME to fund 11,000 full-time equivalent (FTE) slots, leading to > 43,000 physician residents (> 30% of all physician residents) spending part of their training in a VHA facility.18,19
This training mission has multiple benefits. It provides the VA with access to new HCPs who have the necessary training in veteran-specific needs, while supporting the national need for HCPs. In 2018, 120,890 clinical trainees received some or all of their training in the VA system.20 Of the 152 US medical schools that are accredited by the Liaison Committee on Medical Education, 95% collaborate with the VA for training while 100% of the 34 doctor of osteopathic medicine programs have VA training collaborations.20 The VA currently has an additional 18 partnerships with nursing schools.21 Further, 1,800 college and universities, including Hispanic-serving institutions and historically black colleges and universities, have VHA affiliations that provide training for more than 40 clinical health profession education programs.17
This training model has been successful in supporting VA staffing, as health care providers who trained in the VA are more likely to work in the VA.22 Among current VA employees, > 80% of optometrists, > 70% of podiatrists and psychologists, and > 60% of physicians received some part of their training in the VA system.23 In combination with recent increased funding for staffing, the ability of the VA to directly hire trainees in identified professions, and the expansion of loan forgiveness to high-demand specialties (eg, psychiatry), the training partnership between the VA and affiliates has been critical in maintaining the needed VA workforce.22,24,25
The VA Office of Academic Affiliations is responsible for all graduate medical and dental education administration in the VA system, which makes up 85% of its total budget. For each trainee, the VA provides approximately $60,000 toward their stipend in exchange for training and patient care time at a VHA hospital (Kenneth R. Jones, PhD, email communication, August 27, 2018).
California Health Care Education
The UC public university system, founded in 1869, currently has 10 campuses with a combined student body of > 280,000 students, along with 227,000 faculty and staff members.26 For every research dollar provided by California, the UC secures $7 in federal and private funding.26 The UC has 6 medical centers (Davis, Irvine, Los Angeles, Riverside, San Diego, and San Francisco); each is affiliated with at least 1 local VAMC.27,28
California trains a substantial share of health care trainees. In 2016, there were 10,429 physician residents in training in California.29 In 2017/2018, the San Francisco VAMC trained 1,178 medical students/residents, 57 pharmacy students, 25 nurse practitioner students, 19 optometry interns/students/residents, 11 dental students/residents, and 3 physical therapy students.20 In total, 6,223 UC health professions students were trained in VHA facilities during the 2017/2018 training year (Table 4).20 As of 2016, there were 105,907 physicians in California, and of those, 57% completed their GME in California.29 In California in 2015, 74 GME-sponsoring institutions graduated 3,568 residents and fellows, an increase of 10% since 1997.30 Of these sponsoring institutions, 6 of the top 8 programs were UC schools that graduated 48.4% (1,727) of all California residents and fellows in 2015.30
Despite these resources, California faces a major shortage of HCPs, particularly in primary, behavioral health, and older adult care.3 Today, 7 million Californians live in counties with a federally designated shortage of primary, dental, and mental health care providers.3 Most of these Californians are Latino, African American, or Native American, and they live in fast-growing rural and urban regions, including Los Angeles; the San Joaquin Valley; and the Inland Empire (San Bernardino and Riverside Counties).3 Current recommendations to meet increasing demands as California’s population increases, grows older, and faces increased health care demands include expanding residency programs to yield 1,872 additional primary care physicians and 2,202 additional psychiatrists by 2030.3 To meet this shortage and prepare for future health care demands, health care education is paramount; in California, VA and UC affiliations are central to addressing these needs.
The VA plays a particularly important role in supporting GME, which is essential to meeting both VA and California’s unmet HCP needs, as GME determines the number of medical practitioners available per specialty.30 The VA was the second largest GME fund provider in California at $90,662,608 (Medicare provided $552,235,626) and the California government provided a small portion of GME funding.30 VA education funding is a direct result of the VA provision of clinical care in one of the most innovative and modern health care systems in the world.
These VA training opportunities benefit the UC system and California by helping train integrated care practitioners to meet the increasing demand. Integrated care—the coordination of mental health care, substance use disorder treatment, and primary care services—is designed to improve health outcomes by helping people with multiple and complex health care needs access care.31,32
As the largest integrated health care system in the country, the VA brings important clinical, research, and educational opportunities to academic affiliates. A systematic review examining cost and quality outcomes in integrated care systems found improved quality of care compared with nonintegrated care systems; thus, many US government agencies and the World Health Organization are establishing integrated care systems as a standard and universal approach.31,33,34 While cost savings as a result of integrated care are unclear, most studies in this review reported a decrease in utilization of services.33 The presumption of more efficient and higher quality care is also predicated on features such as system-wide accessibility of comprehensive medical records that provide more information to HCPs, promote collaboration, and measure and reward performance, all of which are possible using the VA electronic health record (EHR) system.35,36 The VA offers an excellent opportunity for training in integrated care as this model is required of all VAMCs and community-based outpatient clinics (CBOCs).37
Providing integrated care to the citizens of California is among the 10 priorities of the California Future Health Workforce Commission (a group of California health care leaders cochaired by the UC system president) for immediate action and guides their recommendations on developing and expanding the health care workforce; therefore, training in an integrated health care system is especially important for California HCPs.3 Nearly three-quarters of California’s population aged ≥ 65 years has a chronic health condition that could benefit from integrated care; however, the current supply of HCPs is insufficient to meet the growing demand for geriatric care.38,39
The VA has a robust training program to produce scholars and practitioners who specialize in geriatric care. This includes the Geriatric Scholars Program, which has the goal of integrating geriatrics into primary care through professional development. The Geriatric Scholars Program is a component of the VA Geriatric Research Education and Clinical Centers at urban VAMCs to help provide education and clinical resource connections with rural CBOCs where geriatrics expertise is lacking.
The California Future Health Workforce Commission is highlighting the need to prioritize workforce development in primary care, mental health care, and care for the aging.3 These priorities are shared as foundational services within the VHA.40 The alignment of these priorities creates an excellent rationale for increasing training and education of the UC health care workforce in the California VA system through academic affiliations.
VA Research Collaborations
The VA Office of Research and Development has existed for more than 90 years with a mission to improve veteran health and well-being via research and attract, train, and retain high-caliber researchers. VA provides a rich environment to conduct observational and interventional research due to its large, diverse veteran population, institutional support, and integrated information system with extensive EHR data.41 The success of the VA in facilitating research is evidenced by the fact that 3 VA investigators have been awarded Nobel prizes, and 7 have received Lasker Foundation Awards.42 The size of the VA allows for innovative large-scale research, such as the Million Veteran Program (MVP). The MVP study developed a mega-biobank of VA health records, questionnaires, and blood samples from nearly 1 million veterans to study genetic influences on health and disease and integrate genetic testing into health care delivery.43 In addition to producing high-quality, innovative research, more than 60% of VA investigators also provide direct patient care.42
VA research areas of focus include homelessness, polytrauma, traumatic brain injury, hearing and vision loss, spinal cord injury, mental health, pain management, precision medicine, prosthetics and amputation care, women’s health, and chronic diseases, such as Parkinson and Alzheimer diseases.44 The VA estimates that, in 2021, total VA research spending will include a request of $787 million in addition to $370 million from the National Institutes of Health, the Department of Defense, and the Centers for Disease Control and Prevention, and $170 million from other nonfederal sources, for a projected total of $1.3 billion. This budget will support 2,200 projects with direct research and reimbursable employment of 3,275 FTEs,which are key to supporting VA academic affiliations.45 These funds translate into substantial benefits to the UC system, including shared research and training resources, grant-funding opportunities for UC faculty, and the ability to recruit top researchers, educators, and clinicians to its institutions.
VA Reliance on Community Care
The current VHA model is an integrated health care system that provides comprehensive, wraparound services to enrolled veterans, which are cost-effective, high quality, and consistently found to have equal or superior quality of care compared with that in the community.6,46-50 Despite public criticism about wait times and access to care in the VA system, one study showed that VA wait-time statistics were comparable with or faster than those for community HCPs.51,52 However, VA care coordination has undergone several changes to address these public criticisms, namely, the Veterans Access, Choice and Accountability Act of 2014 (38 USC § 1703 VACAA) and the VA MISSION Act of 2018 (42 USC § 274). VACAA was designed to increase access to care for veterans who live ≥ 40 miles from VA health care facilities or who are unable to been seen within 30 days of their preferred or clinically appropriate date.53 More than 2 million veterans (almost 25% of VHA-enrolled veterans) have received community care since the inception of VACAA in 2014.54
Recently, the MISSION Act mandated developing additional VA-coordinated community-based care through the establishment of a Veterans Community Care Program, which was established using existing VA 2019 fiscal year funds and did not include additional appropriations despite expanded criteria for community care referrals.55 Without additional future appropriations, VA funds would be shifted from VA care into community care. While increasing access to community care has in some cases led to care that is faster and closer and that was previously inaccessible in local VA specialty care, these efforts could reduce veteran engagement with the VA system.56
The changes implemented in VACAA and the VA MISSION Act were driven by important and valid concerns, including evidence of VA staff and officials covering up service deficiencies.51 Veterans in rural areas often have limited access to VA resources, and long travel to VAMCs or clinics can be an impediment. Veterans who have chosen community care tended to be those who have poorer health status, who live further away from VA facilities, women, and those who identified as White or Hispanic.56,57 While VA health care is on average equivalent to or better than community resources, there is significant variability in quality within the VA system. Advocates have argued that providing competition and choice for veterans places pressure on the VA to improve care where it is not meeting expectations. Therefore, access to community care is an important resource for veterans and needs to be implemented effectively and efficiently to help veterans receive the care they need. However, expansion of community care access, depending on how it is implemented, also can have effects on academic partnerships and the education and research missions that should be incorporated into planning.
Each VA health care system receives funding through the Veterans Equitable Reimbursement Allocation (VERA), which provides funds largely based on the number of enrolled veterans and the complexity of the care they receive.58 One potential implication of the shift among veterans to community care is a reduction in patients enrolled in VA programs, thus decreasing funding given to the VA to allocate for training and research. By definition, increased VA-managed community care means less opportunity for integrated training that brings together primary, mental health, and substance use care to meet patient needs. The Center for Medicare and Medicaid Services has developed a national initiative to help states develop programs in integrated care, particularly for individuals who are eligible for both Medicare and Medicaid.59 For states to develop integrated care, they need trainees who function well in this model. Integrated care training is particularly vulnerable to disruption because any portion of a veteran’s care being transferred to the community can impede integration. In effect, training in integrated care, likely the most efficient and cost-effective approach to health care for reasons discussed earlier, could be reduced as providers and trainees are required to manage and coordinate patient care between separate institutions.35
Educational Impact
The shift in usage from VA to community care has potential implications for academic affiliates, particularly in education and research.60 If more people are served in community settings, potentially some VAMCs could be reduced, realigned, or closed. If this restructuring happens, academic partnerships could be impacted negatively. The VA is instituting an Infrastructure Review Commission with the task of examining current VA utilization. If a VA site with an academic affiliate was considered for realignment or closure, the reduction would eliminate the ability of the academic affiliate to provide education and research collaborations at that site.
In a less drastic manner, increasing care in the community may change opportunities for academic affiliates to partner with the VA. As noted, the UC system and California veterans benefit immensely from the VHA as an integrated health care system with dedicated missions of education and research. This partnership is a model in which the VA is the primary source of care for eligible enrolled veterans and provides integrated comprehensive services. If the VA moves to serving primarily as a coordinator of community HCPs rather than a direct provider of health care, academic affiliates would need to make major adjustments to both the education and training models. This change could particularly affect specialty training programs that rely on having adequate volumes of patients to provide an extensive experience to meet training needs. If fewer veterans receive care directly from the VA and are instead dispersed in the community, that will reduce the ability of academic faculty to participate in the education of medical and affiliated trainees and to participate in research in VA settings. It is unclear what other model could replace such a system and be as beneficial to the VA and the academic partners with which it is currently affiliated.
Given the needs that led to the VA increasing access to care and the potential implications discussed for the VA and partnerships with academic affiliates, VA health care systems and academic affiliate partners should consider several steps. These steps involve assessment, coordination, and promotion.
Both the VA and academic affiliates would benefit if the VA shared assessment data on the use of community care, particularly identifying changes that relate to key training and/or research missions. Such data sharing can be critical to determine whether any risks (or potential opportunities) need to be addressed. In addition, increasing research on the outcomes related to both VA care and community-based care is of high value to determine whether the current changes are achieving intended goals. The VA recently funded such work through its research service, and such work is critical for guiding future policy for the VA and for the affiliates.
Coordination among the VA, academic affiliates, and community partners is vital for change. The issue of community care expansion should be a standing item on coordination meetings and shared governance councils between the institutions. It may make sense to establish specific workgroups or committees to coordinate tracking and assessment of the effect of community care expansion on the shared academic mission. One way to address the potential effect of increased community care on the research and education missions would be to include community partners into the partnerships. This strategy could potentially take a number of different forms, from providing education and training to community HCPs, having VA trainees rotate to community settings, or inviting community settings to be research sites for clinical trials. Such partnerships could potentially improve patient care and support the other academic missions. Coordination could be meaningfully improved by having community HCPs access the VA EHR, thus easing communications. Funding is available for EHR access in the VA MISSION Act and should be a high priority as community care expands. The more that community partners can access and connect with the VA EHR the better they will be able to coordinate care.
Third, the VA and its academic partners need to promote and educate veterans, their families, and their advocates on the benefits that are available through VA care and that are enhanced through academic partnerships. While the VA has been the target of justified criticism, many of its strengths addressed here are not broadly recognized. The VA could promote its sharing of staff and resources with the top academic health care institutions in an area and that veterans often have access to resources that otherwise would not be available without the academic affiliate. Making sure veterans are aware of the benefits available can potentially mitigate the need for community care.
Conclusions
Given changes from VACAA and the VA MISSION Act, VA and academic affiliates should be active partners in planning for future health care by providing input and feedback on VA structure to help shape federal and state systems moving forward. Institutions can take steps to steer their futures and meet growing clinical, training, and research needs. The VA and its academic partners in health care research are well positioned to develop projects to assess the effects of these changes. Evaluation of key variables including patient care, education, and research productivity are warranted to guide policymakers as they assess whether these changes in the VA are achieving the expressed goals of improving veteran care. Other opportunities to collaborate in the wake of the MISSION Act remain to be discovered within each academic affiliation. By strengthening working relationships between VA and academic teams, these deeply important partnerships can continue to produce clinical, research, and education outcomes that meet the needs of our veterans, our federal and state health care systems, and our country.
Acknowledgments
Dr. Sells was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations VA Quality Scholars Advanced Fellowship Program.
1. US Department of Veterans Affairs, Veterans Health Administration. About VHA. Updated January 22, 2021. Accessed March 9, 2021. https://www.va.gov/health/aboutvha.asp
2. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee to Evaluate the Department of Veterans Affairs Mental Health Services. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed March 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK499502/
3. California Future Health Workforce Commission. Meeting the demand for health: final report of the California Future Health Workforce Commission. Published February 2019. Accessed March 9, 2021. https://futurehealthworkforce.org/wp-content/uploads/2019/03/MeetingDemandForHealthFinalReportCFHWC.pdf
4. US Department of Veterans Affairs. Veterans Health Administration fiscal year 2017 annual report. Published 2017. Accessed March 9, 2021. https://www.va.gov/HEALTH/docs/VHA_AnnualReport_FY2017.pdf
5. US Department of Veterans Affairs. Public health: health care use by Gulf War & OEF/OIF/OND veterans. Updated March 28, 2017. Accessed March 9, 2021. https://www.publichealth.va.gov/epidemiology/reports/health-care-use-gulfwar-oefoifond/index.asp
6. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13.
7. US Department of Veterans Affairs. Patient care services: veterans with lesbian, gay, bisexual and transgender (LGBT) and related identities. Updated August 31, 2020. Accessed March 9, 2021. https://www.patientcare.va.gov/LGBT/index.asp
8. US Department of Veterans Affairs. Women veterans health care: women veterans program managers. Updated March 28, 2017. Accessed March 9, 2021. https://www.womens health.va.gov/WOMENSHEALTH/programoverview/wvpm.asp
9. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA facilities by state. Published May 15, 2017. Accessed March 9, 2021. https://www.va.gov/vetdata/docs/SpecialReports/VA_Facilities_By_State.PDF
10. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. State summaries: California. Published September 2018. Accessed March 9, 2021. https://www.va.gov/vetdata/docs/SpecialReports/State_Summaries_California.pdf
11. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Care Services, Post-Deployment Health Group, Epidemiology Program. Analysis of VA health care utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. Published January 2017. Accessed March 9, 2021. https://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2015-qtr3.pdf
12. US Department of Veterans Affairs, Office of Budget. Annual budget submission, president’s budget request – fiscal year 2021. Updated February 10, 2020. Accessed March 9, 2021. https://www.va.gov/budget/products.asp
13. US Department of Veterans Affairs. Department of Veterans Affairs statistics at a glance. Updated February 2020. Accessed March 10, 2021. https://www.va.gov/vetdata/docs/Quickfacts/Stats_at_a_glance_4_6_20.PDF
14. US Department of Veterans Affairs VW. Locations, California. Updated October 12, 2018. Accessed March 10, 2021. https://www.va.gov/directory/guide/state.asp?dnum=ALL&STATE=CA
15. Baker, R. R., & Pickren, W. E. (2007). Psychology and the Department of Veterans Affairs: A historical analysis of training, research, practice, and advocacy. American Psychological Association. doi:10.1037/11544-000
16. Functions of Veterans Health Administration: health-care personnel education and training programs. 38 USC § 7302. Accessed March 16, 2021. https://www.govinfo.gov/app/details/USCODE-2011-title38/USCODE-2011-title38-partV-chap73-subchapI-sec7302
17. US Department of Veterans Affairs, Office of Academic Affiliations. Mission of the Office of Academic Affiliations. Published September 24, 2019. Accessed March 10, 2021. https://www.va.gov/oaa/oaa_mission.asp
18. Congressional Research Service. Federal support for graduate medical education: an overview. CRS report R44376. Updated December 27, 2018. Accessed March 10, 2021. https://fas.org/sgp/crs/misc/R44376.pdf
19. Association of American Medical Colleges. 2018 Report on residents. Table B3: number of active residents, by type of medical school, GME specialty, and sex. Accessed March 10, 2021. https://www.aamc.org/data-reports/students-residents/interactive-data/table-b3-number-active-residents-type-medical-school-gme-specialty-and-sex
20. US Department of Veterans Affairs, Office of Academic Affiliations. National summary trainees unique school list - academic year: 2017-2018.
21. US Department of Veterans Affairs, Office of Academic Affiliations. VA nursing academic partnerships. Updated December 12, 2018. Accessed March 10, 2021. https://www.va.gov/oaa/vanap/default.asp
22. Keitz SA, Aron DC, Brannen JL, et al. Impact of clinical training on recruiting graduating health professionals. Am J Manag Care. 2019;25(4):e111-e118. Published 2019 Apr 1.
23. US Department of Veterans Affairs, Office of Academic Affiliations. Health professions education: academic year 2019-2020. Published 2020. https://www.va.gov/OAA/docs/OAA_Statistics_2020.pdf
24. US Department of Veterans Affairs, Veterans Health Administration. Hiring programs and initiatives. Updated March 10, 2021. Accessed March 10, 2021. https://www.vacareers.va.gov/Benefits/HiringProgramsInitiatives/
25. US Department of Veterans Affairs, Veterans Health Administration. Students and trainees. Updated March 10, 2021. Accessed March 10, 2021. https://www.vacareers.va.gov/Careers/StudentsTrainees
26. The Regents of the University of California. The UC system. Accessed March 10, 2021. https://www.universityofcalifornia.edu/uc-system
27. The Regents of the University of California. The parts of UC. Accessed March 10, 2021. https://www.universityofcalifornia.edu/uc-system/parts-of-uc
28. US Department of Veterans Affairs. Locations: VISN 21: Sierra Pacific Network. Updated October 12, 2018. Accessed March 10, 2021. https://www.va.gov/directory/guide/region.asp?ID=1021
29. Association of American Medical Colleges. California physician workforce profile. Published 2017. Accessed March 10, 2021. https://www.aamc.org/system/files/2019-08/california2017.pdf
30. Rittenhouse D, Ament A, Grumbach K, Petterson S, Levin Z, Bazemore A. California Health Care Foundation: guide to graduate medical education funding in California. Published September 2018. Accessed March 10, 2021. https://www.chcf.org/wp-content/uploads/2018/08/GuideGraduateMedicalEducationFunding.pdf
31. US Department of Health and Human Services, Health Resources and Services Administration. Integrated behavioral health resource library. Accessed March 18, 2020. https://www.hrsa.gov/behavioral-health/library
32. US Department of Veterans Affairs. Patient care services: primary care - mental health integration (PC-MHI). Updated August 1, 2016. Accessed March 10, 2021. https://www.patientcare.va.gov/primarycare/PCMHI.asp

33. Hwang W, Chang J, Laclair M, Paz H. Effects of integrated delivery system on cost and quality. Am J Manag Care. 2013;19(5):e175-e184.
34. World Health Organization, World Organization of Family Doctors (Wonca). Integrating mental health into primary care: a global perspective. Published October 2008. Accessed March 10, 2021. https://www.who.int/mental_health/policy/Integratingmhintoprimarycare2008_lastversion.pdf
35. Congressional Budget Office. Comparing the costs of the veterans’ health care system with private-sector costs. Published December 10, 2014. Accessed March 10, 2021. https://www.cbo.gov/publication/49763
36. Souden M. Overview of VA data, information systems, national databases and research uses. Published October 2, 2017. Accessed March 10, 2021. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/2376-notes.pdf
37. US Department of Veterans Affairs, Veterans Health Administration. Uniform mental health services in VA medical centers and clinics. VHA handbook 1160.01. Published September 11, 2008. Recertified September 30, 2013. Amended November 16, 2015. Published September 11, 2008. Accessed March 10, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1762
38. Coffman JM, Fix M, Ko M. California physician supply and distribution: headed for a drought? Published June 25, 2018. Accessed March 10, 2021. https://www.chcf.org/publication/californias-physicians-headed-drought
39. Meng YY, Ahman T, Pickett M. California Health Care Foundation: 2015 Edition—Californians with the top chronic conditions: 11 million and counting. Published April 23, 2015. Accessed March 10, 2021. https://www.chcf.org/publication/2015-edition-californians-top-chronic-conditions-11-million-counting
40. US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Updated May 31, 2019. Accessed March 10, 2021. https://www.va.gov/oei/docs/va2018-2024strategicplan.pdf
41. Justice AC, Erdos J, Brandt C, Conigliaro J, Tierney W, Bryant K. The Veterans Affairs healthcare system: a unique laboratory for observational and interventional research. Med Care. 2006;44(8)(suppl 2):S7-S12. doi:10.1097/01.mlr.0000228027.80012.c5
42. US Department of Veterans Affairs, Office of Research and Development: About the Office of Research & Development. Published Updated March 4, 2021. Accessed March 10, 2021. https://www.research.va.gov/about/default.cfm
43. Gaziano JM, Concato J, Brophy M, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214-223. doi:10.1016/j.jclinepi.2015.09.016
44. US Department of Veterans Affairs. VA research program overview. Accessed March 12, 2021. https://www.research.va.gov/pubs/docs/va-research-overview-brochure.pdf
45. US Department of Veterans Affairs. FY 2021 budget submission: medical programs and information technology programs. Volume 2 of 4. Published February 2020. Accessed March 12, 2021. https://www.va.gov/budget/docs/summary/fy2021VAbudgetVolumeIImedicalProgramsAndInformationTechnology.pdf
46. Trivedi AN, Matula S, Miake-Lye I, Glassman PA, Shekelle P, Asch S. Systematic review: comparison of the quality of medical care in Veterans Affairs and non-Veterans Affairs settings. Med Care. 2011;49(1):76-88. doi:10.1097/MLR.0b013e3181f53575
47. Nugent GN, Hendricks A, Nugent L, Render ML. Value for taxpayers’ dollars: what VA care would cost at Medicare prices. Med Care Res Rev. 2004;61(4):495-508. doi:10.1177/1077558704269795
48. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631-1638. doi:10.1007/s11606-018-4433-7
49. O’Hanlon C, Huang C, Sloss E, et al. Comparing VA and non-VA quality of care: a systematic review. J Gen Intern Med. 2017;32(1):105-121. doi:10.1007/s11606-016-3775-2
50. Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
51. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix health care system. Published May 28, 2014. Accessed March 12, 2021. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf
52. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096. doi:10.1001/jamanetworkopen.2018.7096
53. US Department of Veterans Affairs. Fact sheet: Veterans Access, Choice and Accountability Act of 2014 (“Choice Act”). Accessed March 12, 2021. https://www.va.gov/opa/choiceact/documents/choice-act-summary.pdf
54. Mattocks KM, Cunningham K, Elwy AR, et al. Recommendations for the evaluation of cross-system care coordination from the VA State-of-the-art Working Group on VA/Non-VA Care. J Gen Intern Med. 2019;34(Suppl 1):18-23. doi:10.1007/s11606-019-04972-1
55. US Department of Veterans Affairs. Fact sheet: VA MISSION Act and new veterans community care program. Published June 15, 2018. Accessed March 12, 2021. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/FactSheet_20-13.pdf
56. Stroupe KT, Martinez R, Hogan TP, et al. Experiences with the veterans’ choice program. J Gen Intern Med. 2019;34(10):2141-2149. doi:10.1007/s11606-019-05224-y
57. Yoon J, Leung LB, Rubenstein LV, et al. Use of the veterans’ choice program and attrition from Veterans Health Administration primary care. Med Care. 2020;58(12):1091-1097. doi:10.1097/MLR.0000000000001401
58. US Department of Veterans Affairs. Veterans Equitable Resource Allocation (VERA). Updated March 9, 2021. Accessed March 12, 2021. https://catalog.data.gov/dataset/veterans-equitable-resource-allocation-vera
59. Integrated Care Resource Center. About us. Accessed March 12, 2021. https://www.integratedcareresourcecenter.com/about-us
60. Duhaney T. How veteran utilization of the Veterans Health Administration could impact privatization. Public Policy Aging Rep. 2020;30(1):29-35. doi:10.1093/ppar/prz032
1. US Department of Veterans Affairs, Veterans Health Administration. About VHA. Updated January 22, 2021. Accessed March 9, 2021. https://www.va.gov/health/aboutvha.asp
2. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee to Evaluate the Department of Veterans Affairs Mental Health Services. Evaluation of the Department of Veterans Affairs Mental Health Services. National Academies Press; 2018. Accessed March 9, 2021. https://www.ncbi.nlm.nih.gov/books/NBK499502/
3. California Future Health Workforce Commission. Meeting the demand for health: final report of the California Future Health Workforce Commission. Published February 2019. Accessed March 9, 2021. https://futurehealthworkforce.org/wp-content/uploads/2019/03/MeetingDemandForHealthFinalReportCFHWC.pdf
4. US Department of Veterans Affairs. Veterans Health Administration fiscal year 2017 annual report. Published 2017. Accessed March 9, 2021. https://www.va.gov/HEALTH/docs/VHA_AnnualReport_FY2017.pdf
5. US Department of Veterans Affairs. Public health: health care use by Gulf War & OEF/OIF/OND veterans. Updated March 28, 2017. Accessed March 9, 2021. https://www.publichealth.va.gov/epidemiology/reports/health-care-use-gulfwar-oefoifond/index.asp
6. Eibner C, Krull H, Brown KM, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand Health Q. 2016;5(4):13.
7. US Department of Veterans Affairs. Patient care services: veterans with lesbian, gay, bisexual and transgender (LGBT) and related identities. Updated August 31, 2020. Accessed March 9, 2021. https://www.patientcare.va.gov/LGBT/index.asp
8. US Department of Veterans Affairs. Women veterans health care: women veterans program managers. Updated March 28, 2017. Accessed March 9, 2021. https://www.womens health.va.gov/WOMENSHEALTH/programoverview/wvpm.asp
9. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. VA facilities by state. Published May 15, 2017. Accessed March 9, 2021. https://www.va.gov/vetdata/docs/SpecialReports/VA_Facilities_By_State.PDF
10. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. State summaries: California. Published September 2018. Accessed March 9, 2021. https://www.va.gov/vetdata/docs/SpecialReports/State_Summaries_California.pdf
11. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Care Services, Post-Deployment Health Group, Epidemiology Program. Analysis of VA health care utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. Published January 2017. Accessed March 9, 2021. https://www.publichealth.va.gov/docs/epidemiology/healthcare-utilization-report-fy2015-qtr3.pdf
12. US Department of Veterans Affairs, Office of Budget. Annual budget submission, president’s budget request – fiscal year 2021. Updated February 10, 2020. Accessed March 9, 2021. https://www.va.gov/budget/products.asp
13. US Department of Veterans Affairs. Department of Veterans Affairs statistics at a glance. Updated February 2020. Accessed March 10, 2021. https://www.va.gov/vetdata/docs/Quickfacts/Stats_at_a_glance_4_6_20.PDF
14. US Department of Veterans Affairs VW. Locations, California. Updated October 12, 2018. Accessed March 10, 2021. https://www.va.gov/directory/guide/state.asp?dnum=ALL&STATE=CA
15. Baker, R. R., & Pickren, W. E. (2007). Psychology and the Department of Veterans Affairs: A historical analysis of training, research, practice, and advocacy. American Psychological Association. doi:10.1037/11544-000
16. Functions of Veterans Health Administration: health-care personnel education and training programs. 38 USC § 7302. Accessed March 16, 2021. https://www.govinfo.gov/app/details/USCODE-2011-title38/USCODE-2011-title38-partV-chap73-subchapI-sec7302
17. US Department of Veterans Affairs, Office of Academic Affiliations. Mission of the Office of Academic Affiliations. Published September 24, 2019. Accessed March 10, 2021. https://www.va.gov/oaa/oaa_mission.asp
18. Congressional Research Service. Federal support for graduate medical education: an overview. CRS report R44376. Updated December 27, 2018. Accessed March 10, 2021. https://fas.org/sgp/crs/misc/R44376.pdf
19. Association of American Medical Colleges. 2018 Report on residents. Table B3: number of active residents, by type of medical school, GME specialty, and sex. Accessed March 10, 2021. https://www.aamc.org/data-reports/students-residents/interactive-data/table-b3-number-active-residents-type-medical-school-gme-specialty-and-sex
20. US Department of Veterans Affairs, Office of Academic Affiliations. National summary trainees unique school list - academic year: 2017-2018.
21. US Department of Veterans Affairs, Office of Academic Affiliations. VA nursing academic partnerships. Updated December 12, 2018. Accessed March 10, 2021. https://www.va.gov/oaa/vanap/default.asp
22. Keitz SA, Aron DC, Brannen JL, et al. Impact of clinical training on recruiting graduating health professionals. Am J Manag Care. 2019;25(4):e111-e118. Published 2019 Apr 1.
23. US Department of Veterans Affairs, Office of Academic Affiliations. Health professions education: academic year 2019-2020. Published 2020. https://www.va.gov/OAA/docs/OAA_Statistics_2020.pdf
24. US Department of Veterans Affairs, Veterans Health Administration. Hiring programs and initiatives. Updated March 10, 2021. Accessed March 10, 2021. https://www.vacareers.va.gov/Benefits/HiringProgramsInitiatives/
25. US Department of Veterans Affairs, Veterans Health Administration. Students and trainees. Updated March 10, 2021. Accessed March 10, 2021. https://www.vacareers.va.gov/Careers/StudentsTrainees
26. The Regents of the University of California. The UC system. Accessed March 10, 2021. https://www.universityofcalifornia.edu/uc-system
27. The Regents of the University of California. The parts of UC. Accessed March 10, 2021. https://www.universityofcalifornia.edu/uc-system/parts-of-uc
28. US Department of Veterans Affairs. Locations: VISN 21: Sierra Pacific Network. Updated October 12, 2018. Accessed March 10, 2021. https://www.va.gov/directory/guide/region.asp?ID=1021
29. Association of American Medical Colleges. California physician workforce profile. Published 2017. Accessed March 10, 2021. https://www.aamc.org/system/files/2019-08/california2017.pdf
30. Rittenhouse D, Ament A, Grumbach K, Petterson S, Levin Z, Bazemore A. California Health Care Foundation: guide to graduate medical education funding in California. Published September 2018. Accessed March 10, 2021. https://www.chcf.org/wp-content/uploads/2018/08/GuideGraduateMedicalEducationFunding.pdf
31. US Department of Health and Human Services, Health Resources and Services Administration. Integrated behavioral health resource library. Accessed March 18, 2020. https://www.hrsa.gov/behavioral-health/library
32. US Department of Veterans Affairs. Patient care services: primary care - mental health integration (PC-MHI). Updated August 1, 2016. Accessed March 10, 2021. https://www.patientcare.va.gov/primarycare/PCMHI.asp

33. Hwang W, Chang J, Laclair M, Paz H. Effects of integrated delivery system on cost and quality. Am J Manag Care. 2013;19(5):e175-e184.
34. World Health Organization, World Organization of Family Doctors (Wonca). Integrating mental health into primary care: a global perspective. Published October 2008. Accessed March 10, 2021. https://www.who.int/mental_health/policy/Integratingmhintoprimarycare2008_lastversion.pdf
35. Congressional Budget Office. Comparing the costs of the veterans’ health care system with private-sector costs. Published December 10, 2014. Accessed March 10, 2021. https://www.cbo.gov/publication/49763
36. Souden M. Overview of VA data, information systems, national databases and research uses. Published October 2, 2017. Accessed March 10, 2021. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/2376-notes.pdf
37. US Department of Veterans Affairs, Veterans Health Administration. Uniform mental health services in VA medical centers and clinics. VHA handbook 1160.01. Published September 11, 2008. Recertified September 30, 2013. Amended November 16, 2015. Published September 11, 2008. Accessed March 10, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=1762
38. Coffman JM, Fix M, Ko M. California physician supply and distribution: headed for a drought? Published June 25, 2018. Accessed March 10, 2021. https://www.chcf.org/publication/californias-physicians-headed-drought
39. Meng YY, Ahman T, Pickett M. California Health Care Foundation: 2015 Edition—Californians with the top chronic conditions: 11 million and counting. Published April 23, 2015. Accessed March 10, 2021. https://www.chcf.org/publication/2015-edition-californians-top-chronic-conditions-11-million-counting
40. US Department of Veterans Affairs. Department of Veterans Affairs FY 2018-2024 strategic plan. Updated May 31, 2019. Accessed March 10, 2021. https://www.va.gov/oei/docs/va2018-2024strategicplan.pdf
41. Justice AC, Erdos J, Brandt C, Conigliaro J, Tierney W, Bryant K. The Veterans Affairs healthcare system: a unique laboratory for observational and interventional research. Med Care. 2006;44(8)(suppl 2):S7-S12. doi:10.1097/01.mlr.0000228027.80012.c5
42. US Department of Veterans Affairs, Office of Research and Development: About the Office of Research & Development. Published Updated March 4, 2021. Accessed March 10, 2021. https://www.research.va.gov/about/default.cfm
43. Gaziano JM, Concato J, Brophy M, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214-223. doi:10.1016/j.jclinepi.2015.09.016
44. US Department of Veterans Affairs. VA research program overview. Accessed March 12, 2021. https://www.research.va.gov/pubs/docs/va-research-overview-brochure.pdf
45. US Department of Veterans Affairs. FY 2021 budget submission: medical programs and information technology programs. Volume 2 of 4. Published February 2020. Accessed March 12, 2021. https://www.va.gov/budget/docs/summary/fy2021VAbudgetVolumeIImedicalProgramsAndInformationTechnology.pdf
46. Trivedi AN, Matula S, Miake-Lye I, Glassman PA, Shekelle P, Asch S. Systematic review: comparison of the quality of medical care in Veterans Affairs and non-Veterans Affairs settings. Med Care. 2011;49(1):76-88. doi:10.1097/MLR.0b013e3181f53575
47. Nugent GN, Hendricks A, Nugent L, Render ML. Value for taxpayers’ dollars: what VA care would cost at Medicare prices. Med Care Res Rev. 2004;61(4):495-508. doi:10.1177/1077558704269795
48. Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing quality of care in Veterans Affairs and non-Veterans Affairs settings. J Gen Intern Med. 2018;33(10):1631-1638. doi:10.1007/s11606-018-4433-7
49. O’Hanlon C, Huang C, Sloss E, et al. Comparing VA and non-VA quality of care: a systematic review. J Gen Intern Med. 2017;32(1):105-121. doi:10.1007/s11606-016-3775-2
50. Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
51. US Department of Veterans Affairs, Office of Inspector General. Veterans Health Administration interim report: review of patient wait times, scheduling practices, and alleged patient deaths at the Phoenix health care system. Published May 28, 2014. Accessed March 12, 2021. https://www.va.gov/oig/pubs/VAOIG-14-02603-178.pdf
52. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096. doi:10.1001/jamanetworkopen.2018.7096
53. US Department of Veterans Affairs. Fact sheet: Veterans Access, Choice and Accountability Act of 2014 (“Choice Act”). Accessed March 12, 2021. https://www.va.gov/opa/choiceact/documents/choice-act-summary.pdf
54. Mattocks KM, Cunningham K, Elwy AR, et al. Recommendations for the evaluation of cross-system care coordination from the VA State-of-the-art Working Group on VA/Non-VA Care. J Gen Intern Med. 2019;34(Suppl 1):18-23. doi:10.1007/s11606-019-04972-1
55. US Department of Veterans Affairs. Fact sheet: VA MISSION Act and new veterans community care program. Published June 15, 2018. Accessed March 12, 2021. https://www.va.gov/COMMUNITYCARE/docs/pubfiles/factsheets/FactSheet_20-13.pdf
56. Stroupe KT, Martinez R, Hogan TP, et al. Experiences with the veterans’ choice program. J Gen Intern Med. 2019;34(10):2141-2149. doi:10.1007/s11606-019-05224-y
57. Yoon J, Leung LB, Rubenstein LV, et al. Use of the veterans’ choice program and attrition from Veterans Health Administration primary care. Med Care. 2020;58(12):1091-1097. doi:10.1097/MLR.0000000000001401
58. US Department of Veterans Affairs. Veterans Equitable Resource Allocation (VERA). Updated March 9, 2021. Accessed March 12, 2021. https://catalog.data.gov/dataset/veterans-equitable-resource-allocation-vera
59. Integrated Care Resource Center. About us. Accessed March 12, 2021. https://www.integratedcareresourcecenter.com/about-us
60. Duhaney T. How veteran utilization of the Veterans Health Administration could impact privatization. Public Policy Aging Rep. 2020;30(1):29-35. doi:10.1093/ppar/prz032
A Rising Tide: No Hospital Is an Island Unto Itself in the Era of COVID-19
The early phase of the COVID-19 pandemic was an extraordinarily uncertain, yet innovative, time.1 Few data describe site-level effects of the many adaptations made to deal with surging case numbers, but studies of larger hospital referral regions (HRR) provide important clues.
In this issue of the Journal of Hospital Medicine, Janke et al2 describe how availability of hospital resources in a region relate to COVID-19 mortality between March and June 2020.The authors’ findings suggest that, at least for early periods of the pandemic, having more intensive care unit (ICU), hospital bed, or nursing capacity per COVID-19 case was associated with lower mortality, while physician availability was not. Moreover, months later there were no associations between service or physician availability and HRR COVID-19 mortality. The authors observed variations in mortality rates in places commonly thought to have been overwhelmed early in the pandemic (April 2020), as well as in cities (Boston, Philadelphia, Hartford, Detroit, and Camden, New Jersey) that had a less prominent place in the news at that time.
Larger hospitals tend to have the resources necessary to make wholesale changes when preparing for a pandemic wave. Thus, Janke et al’s results may not have fully captured the pandemic’s potential impact in settings with fewer resources or in smaller hospitals, which are currently being overwhelmed.3
The number of cases and hospitalizations in this third wave of COVID-19 continues to rise, and the strain on healthcare resources has been felt across entire regions, making the results of this study even more salient. Hospital outcomes for COVID-19 are sensitive to limitations in physical locations (number of beds, ICU capacity) and nursing capacity. Nurses more often are assigned specifically to a bed or unit, and the number of patients per nurse is limited by state or local statute. Innovations such as COVID-19 field hospitals or redeploying existing beds (eg, converting postanesthesia care units to ICUs) offset physically constrained resources.4 On the other hand, lower acuity in this phase of the pandemic (eg, fewer ICU admissions) and shorter lengths of stay may produce higher turnover, producing more workforce stress, regardless of bed availability.
Early work of our COVID-19 collaborative5 suggests that the focus on localizing patients to geographic units or teams has given way to strategies that utilize more flexible team and bed-finding approaches. Clinical care has evolved to focus on more aggressive discharge strategies, with remote monitoring and hospital-at-home capabilities. Overall, the pandemic is providing fodder for future studies examining interaction between case volumes, physician and nurse availability, and evolution in clinical care practices. Most critically, it provides an opportunity to study health system flexibility and robustness with a lens that incorporates a view of the hospital and its surroundings as tightly related parts of care delivery. Because if there is one thing the pandemic is teaching us, it is that, more than ever, no hospital can be an island unto itself, and each hospital is part of a larger ecosystem where rising tides are felt throughout.
1. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214.
3. Achenbach J, Brulliard K, Shammas B, Dupree J. Hospitals in nearly every region report a flood of covid-19 patients. Washington Post. October 26, 2020. Accessed March 4, 2021. https://www.washingtonpost.com/health/covid-hospitals-record-patients/2020/10/26/0bc362cc-17b2-11eb-befb-8864259bd2d8_story.html
4. Chaudhary MJ, Howell E, Ficke JR, et al. Caring for patients at a COVID-19 field hospital. J Hosp Med. 2021;16(2):117-119. https://doi.org/10.12788/jhm.3551
5. Welcome to the COVID-19 response working team knowledge base. HOMERun Hospital Medicine Reengineering Network COVID-19 Collaboration. Accessed March 4, 2021. https://www.hospitalinnovate.org/covid19/
The early phase of the COVID-19 pandemic was an extraordinarily uncertain, yet innovative, time.1 Few data describe site-level effects of the many adaptations made to deal with surging case numbers, but studies of larger hospital referral regions (HRR) provide important clues.
In this issue of the Journal of Hospital Medicine, Janke et al2 describe how availability of hospital resources in a region relate to COVID-19 mortality between March and June 2020.The authors’ findings suggest that, at least for early periods of the pandemic, having more intensive care unit (ICU), hospital bed, or nursing capacity per COVID-19 case was associated with lower mortality, while physician availability was not. Moreover, months later there were no associations between service or physician availability and HRR COVID-19 mortality. The authors observed variations in mortality rates in places commonly thought to have been overwhelmed early in the pandemic (April 2020), as well as in cities (Boston, Philadelphia, Hartford, Detroit, and Camden, New Jersey) that had a less prominent place in the news at that time.
Larger hospitals tend to have the resources necessary to make wholesale changes when preparing for a pandemic wave. Thus, Janke et al’s results may not have fully captured the pandemic’s potential impact in settings with fewer resources or in smaller hospitals, which are currently being overwhelmed.3
The number of cases and hospitalizations in this third wave of COVID-19 continues to rise, and the strain on healthcare resources has been felt across entire regions, making the results of this study even more salient. Hospital outcomes for COVID-19 are sensitive to limitations in physical locations (number of beds, ICU capacity) and nursing capacity. Nurses more often are assigned specifically to a bed or unit, and the number of patients per nurse is limited by state or local statute. Innovations such as COVID-19 field hospitals or redeploying existing beds (eg, converting postanesthesia care units to ICUs) offset physically constrained resources.4 On the other hand, lower acuity in this phase of the pandemic (eg, fewer ICU admissions) and shorter lengths of stay may produce higher turnover, producing more workforce stress, regardless of bed availability.
Early work of our COVID-19 collaborative5 suggests that the focus on localizing patients to geographic units or teams has given way to strategies that utilize more flexible team and bed-finding approaches. Clinical care has evolved to focus on more aggressive discharge strategies, with remote monitoring and hospital-at-home capabilities. Overall, the pandemic is providing fodder for future studies examining interaction between case volumes, physician and nurse availability, and evolution in clinical care practices. Most critically, it provides an opportunity to study health system flexibility and robustness with a lens that incorporates a view of the hospital and its surroundings as tightly related parts of care delivery. Because if there is one thing the pandemic is teaching us, it is that, more than ever, no hospital can be an island unto itself, and each hospital is part of a larger ecosystem where rising tides are felt throughout.
The early phase of the COVID-19 pandemic was an extraordinarily uncertain, yet innovative, time.1 Few data describe site-level effects of the many adaptations made to deal with surging case numbers, but studies of larger hospital referral regions (HRR) provide important clues.
In this issue of the Journal of Hospital Medicine, Janke et al2 describe how availability of hospital resources in a region relate to COVID-19 mortality between March and June 2020.The authors’ findings suggest that, at least for early periods of the pandemic, having more intensive care unit (ICU), hospital bed, or nursing capacity per COVID-19 case was associated with lower mortality, while physician availability was not. Moreover, months later there were no associations between service or physician availability and HRR COVID-19 mortality. The authors observed variations in mortality rates in places commonly thought to have been overwhelmed early in the pandemic (April 2020), as well as in cities (Boston, Philadelphia, Hartford, Detroit, and Camden, New Jersey) that had a less prominent place in the news at that time.
Larger hospitals tend to have the resources necessary to make wholesale changes when preparing for a pandemic wave. Thus, Janke et al’s results may not have fully captured the pandemic’s potential impact in settings with fewer resources or in smaller hospitals, which are currently being overwhelmed.3
The number of cases and hospitalizations in this third wave of COVID-19 continues to rise, and the strain on healthcare resources has been felt across entire regions, making the results of this study even more salient. Hospital outcomes for COVID-19 are sensitive to limitations in physical locations (number of beds, ICU capacity) and nursing capacity. Nurses more often are assigned specifically to a bed or unit, and the number of patients per nurse is limited by state or local statute. Innovations such as COVID-19 field hospitals or redeploying existing beds (eg, converting postanesthesia care units to ICUs) offset physically constrained resources.4 On the other hand, lower acuity in this phase of the pandemic (eg, fewer ICU admissions) and shorter lengths of stay may produce higher turnover, producing more workforce stress, regardless of bed availability.
Early work of our COVID-19 collaborative5 suggests that the focus on localizing patients to geographic units or teams has given way to strategies that utilize more flexible team and bed-finding approaches. Clinical care has evolved to focus on more aggressive discharge strategies, with remote monitoring and hospital-at-home capabilities. Overall, the pandemic is providing fodder for future studies examining interaction between case volumes, physician and nurse availability, and evolution in clinical care practices. Most critically, it provides an opportunity to study health system flexibility and robustness with a lens that incorporates a view of the hospital and its surroundings as tightly related parts of care delivery. Because if there is one thing the pandemic is teaching us, it is that, more than ever, no hospital can be an island unto itself, and each hospital is part of a larger ecosystem where rising tides are felt throughout.
1. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214.
3. Achenbach J, Brulliard K, Shammas B, Dupree J. Hospitals in nearly every region report a flood of covid-19 patients. Washington Post. October 26, 2020. Accessed March 4, 2021. https://www.washingtonpost.com/health/covid-hospitals-record-patients/2020/10/26/0bc362cc-17b2-11eb-befb-8864259bd2d8_story.html
4. Chaudhary MJ, Howell E, Ficke JR, et al. Caring for patients at a COVID-19 field hospital. J Hosp Med. 2021;16(2):117-119. https://doi.org/10.12788/jhm.3551
5. Welcome to the COVID-19 response working team knowledge base. HOMERun Hospital Medicine Reengineering Network COVID-19 Collaboration. Accessed March 4, 2021. https://www.hospitalinnovate.org/covid19/
1. Auerbach A, O’Leary KJ, Greysen SR, et al; HOMERuN COVID-19 Collaborative Group. Hospital ward adaptation during the COVID-19 pandemic: a national survey of academic medical centers. J Hosp Med. 2020;15(8):483-488. https://doi.org/10.12788/jhm.3476
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214.
3. Achenbach J, Brulliard K, Shammas B, Dupree J. Hospitals in nearly every region report a flood of covid-19 patients. Washington Post. October 26, 2020. Accessed March 4, 2021. https://www.washingtonpost.com/health/covid-hospitals-record-patients/2020/10/26/0bc362cc-17b2-11eb-befb-8864259bd2d8_story.html
4. Chaudhary MJ, Howell E, Ficke JR, et al. Caring for patients at a COVID-19 field hospital. J Hosp Med. 2021;16(2):117-119. https://doi.org/10.12788/jhm.3551
5. Welcome to the COVID-19 response working team knowledge base. HOMERun Hospital Medicine Reengineering Network COVID-19 Collaboration. Accessed March 4, 2021. https://www.hospitalinnovate.org/covid19/
© 2021 Society of Hospital Medicine
Hospital-Level Variability in Outcomes of Patients With COVID-19
Several studies have examined variation in outcomes of patients with COVID-19, with emphasis on hospital-level factors such as geographic location, workforce and resource availability, and COVID-19 community prevalence.1,2 Block et al1 examine variation in COVID-19 mortality across 11
Block et al1 also found variation within quintiles of COVID-19 burden, suggesting that other hospital-level factors are influencing their performance. In response to the initial surge of COVID-19 in the United States, hospitals and healthcare systems made rapid, often major, adjustments to provide care. Four interdependent components contribute to an effective surge response: system, space, staff, and supplies. Although all four components are important, effective systems are critical. Systems domains include command, or the creation of leadership teams throughout the organization; control, or management, of infrastructure; communication of rapid, comprehensible messages internally and externally; coordination of resources across departments and professions; and continuity of operations.3 Little is known about how well hospitals have implemented these systems components throughout the pandemic, and while Janke et al2 examined the association of resources with outcomes, neither their study nor Block et al’s was able to account for other organizational or systems-based aspects of surge response.
Studies that help us understand the organizational factors and care-delivery adaptations associated with better outcomes for patients with COVID-19 are sorely needed, and could provide important insights for organizational adaptation and change more generally. Janke et al2 and, in their accompanying editorial, Auerbach and Greysen,4 call for “innovative protocols” and “flexibility” to meet the needs of high-demand, novel situations. However, organizations’ ability to innovate and adapt relies on their relationships and teamwork capability.
The relational infrastructure within an organization provides the basis for effective teamwork, facilitating other aspects of an organization’s surge response and ability to adapt. Relationships characterized by trust and mindfulness create a context of psychological safety that encourages sharing new ideas, and enable teams to rapidly make sense of new situations and create shared understandings that facilitate effective action: improvising, adapting, and learning. Trust and psychological safety are especially important during crises, as decision-making tends to evolve toward top-down processes in times of crisis.
Hospitals currently collect few data that speak to relationships and teamwork, limiting our ability to study these vital organizational characteristics and their role in the larger COVID-19 response. Surveys related to patient safety culture or provider wellness and burnout are likely the only data regularly collected by hospitals. Expanding these data to include measures of relational infrastructure will create more robust data not only to conduct research regarding organizational factors that are associated with patient outcomes, but also to allow health systems to improve relationships and teaming as a means of improving outcomes. Examples include relational coordination,5 relationships,6and learning scales.7
The hospitals to which patients are admitted make a difference in patient survival. The study by Block et al1 highlights the importance of assessing the factors that enable health systems to adapt and innovate so that we can better understand hospital-level variation in outcomes.
1. Block B, Boscardin J, Covinsky K, Mourad M, Hu L, Smith A. Variation in COVID-19 mortality across 117 US hospitals in high and how-burden settings. J Hosp Med. 2021;16(4):215-218. https://doi.org/10.12788/jhm.3612
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214. https://doi.org/10.12788/jhm.3539
3. Watson SK, Rudge JW, Coker R. Health systems’ “surge capacity”: state of the art and priorities for future research. Milbank Q. 2013;91(1):78-122. https://doi.org/10.1111/milq.12003
4. Auerbach AD, Greysen SR. A rising tide: no hospital is an island unto itself in the era of COVID-19. J Hosp Med. 2021;16(4):254. https://doi.org/10.12788/jhm.3592
5. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Applied Behavioral Science. Published February 15, 2021. https://doi.org/10.1177/0021886321991597
6. Finley EP, Pugh JA, Lanham HJ, et al. Relationship quality and patient-assessed quality of care in VA primary care clinics: development and validation of the work relationships scale. Ann Fam Med. 2015; 11(6):543-549. https://doi.org/10.1370/afm.1554
7. Leykum LK, Palmer R, Lanham HJ, et al. Reciprocal learning and chronic care model implementation in primary care: results from a new scale of learning in primary care. BMC Health Serv Res. 2011;11:44. https://doi.org/10.1186/1472-6963-11-44
Several studies have examined variation in outcomes of patients with COVID-19, with emphasis on hospital-level factors such as geographic location, workforce and resource availability, and COVID-19 community prevalence.1,2 Block et al1 examine variation in COVID-19 mortality across 11
Block et al1 also found variation within quintiles of COVID-19 burden, suggesting that other hospital-level factors are influencing their performance. In response to the initial surge of COVID-19 in the United States, hospitals and healthcare systems made rapid, often major, adjustments to provide care. Four interdependent components contribute to an effective surge response: system, space, staff, and supplies. Although all four components are important, effective systems are critical. Systems domains include command, or the creation of leadership teams throughout the organization; control, or management, of infrastructure; communication of rapid, comprehensible messages internally and externally; coordination of resources across departments and professions; and continuity of operations.3 Little is known about how well hospitals have implemented these systems components throughout the pandemic, and while Janke et al2 examined the association of resources with outcomes, neither their study nor Block et al’s was able to account for other organizational or systems-based aspects of surge response.
Studies that help us understand the organizational factors and care-delivery adaptations associated with better outcomes for patients with COVID-19 are sorely needed, and could provide important insights for organizational adaptation and change more generally. Janke et al2 and, in their accompanying editorial, Auerbach and Greysen,4 call for “innovative protocols” and “flexibility” to meet the needs of high-demand, novel situations. However, organizations’ ability to innovate and adapt relies on their relationships and teamwork capability.
The relational infrastructure within an organization provides the basis for effective teamwork, facilitating other aspects of an organization’s surge response and ability to adapt. Relationships characterized by trust and mindfulness create a context of psychological safety that encourages sharing new ideas, and enable teams to rapidly make sense of new situations and create shared understandings that facilitate effective action: improvising, adapting, and learning. Trust and psychological safety are especially important during crises, as decision-making tends to evolve toward top-down processes in times of crisis.
Hospitals currently collect few data that speak to relationships and teamwork, limiting our ability to study these vital organizational characteristics and their role in the larger COVID-19 response. Surveys related to patient safety culture or provider wellness and burnout are likely the only data regularly collected by hospitals. Expanding these data to include measures of relational infrastructure will create more robust data not only to conduct research regarding organizational factors that are associated with patient outcomes, but also to allow health systems to improve relationships and teaming as a means of improving outcomes. Examples include relational coordination,5 relationships,6and learning scales.7
The hospitals to which patients are admitted make a difference in patient survival. The study by Block et al1 highlights the importance of assessing the factors that enable health systems to adapt and innovate so that we can better understand hospital-level variation in outcomes.
Several studies have examined variation in outcomes of patients with COVID-19, with emphasis on hospital-level factors such as geographic location, workforce and resource availability, and COVID-19 community prevalence.1,2 Block et al1 examine variation in COVID-19 mortality across 11
Block et al1 also found variation within quintiles of COVID-19 burden, suggesting that other hospital-level factors are influencing their performance. In response to the initial surge of COVID-19 in the United States, hospitals and healthcare systems made rapid, often major, adjustments to provide care. Four interdependent components contribute to an effective surge response: system, space, staff, and supplies. Although all four components are important, effective systems are critical. Systems domains include command, or the creation of leadership teams throughout the organization; control, or management, of infrastructure; communication of rapid, comprehensible messages internally and externally; coordination of resources across departments and professions; and continuity of operations.3 Little is known about how well hospitals have implemented these systems components throughout the pandemic, and while Janke et al2 examined the association of resources with outcomes, neither their study nor Block et al’s was able to account for other organizational or systems-based aspects of surge response.
Studies that help us understand the organizational factors and care-delivery adaptations associated with better outcomes for patients with COVID-19 are sorely needed, and could provide important insights for organizational adaptation and change more generally. Janke et al2 and, in their accompanying editorial, Auerbach and Greysen,4 call for “innovative protocols” and “flexibility” to meet the needs of high-demand, novel situations. However, organizations’ ability to innovate and adapt relies on their relationships and teamwork capability.
The relational infrastructure within an organization provides the basis for effective teamwork, facilitating other aspects of an organization’s surge response and ability to adapt. Relationships characterized by trust and mindfulness create a context of psychological safety that encourages sharing new ideas, and enable teams to rapidly make sense of new situations and create shared understandings that facilitate effective action: improvising, adapting, and learning. Trust and psychological safety are especially important during crises, as decision-making tends to evolve toward top-down processes in times of crisis.
Hospitals currently collect few data that speak to relationships and teamwork, limiting our ability to study these vital organizational characteristics and their role in the larger COVID-19 response. Surveys related to patient safety culture or provider wellness and burnout are likely the only data regularly collected by hospitals. Expanding these data to include measures of relational infrastructure will create more robust data not only to conduct research regarding organizational factors that are associated with patient outcomes, but also to allow health systems to improve relationships and teaming as a means of improving outcomes. Examples include relational coordination,5 relationships,6and learning scales.7
The hospitals to which patients are admitted make a difference in patient survival. The study by Block et al1 highlights the importance of assessing the factors that enable health systems to adapt and innovate so that we can better understand hospital-level variation in outcomes.
1. Block B, Boscardin J, Covinsky K, Mourad M, Hu L, Smith A. Variation in COVID-19 mortality across 117 US hospitals in high and how-burden settings. J Hosp Med. 2021;16(4):215-218. https://doi.org/10.12788/jhm.3612
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214. https://doi.org/10.12788/jhm.3539
3. Watson SK, Rudge JW, Coker R. Health systems’ “surge capacity”: state of the art and priorities for future research. Milbank Q. 2013;91(1):78-122. https://doi.org/10.1111/milq.12003
4. Auerbach AD, Greysen SR. A rising tide: no hospital is an island unto itself in the era of COVID-19. J Hosp Med. 2021;16(4):254. https://doi.org/10.12788/jhm.3592
5. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Applied Behavioral Science. Published February 15, 2021. https://doi.org/10.1177/0021886321991597
6. Finley EP, Pugh JA, Lanham HJ, et al. Relationship quality and patient-assessed quality of care in VA primary care clinics: development and validation of the work relationships scale. Ann Fam Med. 2015; 11(6):543-549. https://doi.org/10.1370/afm.1554
7. Leykum LK, Palmer R, Lanham HJ, et al. Reciprocal learning and chronic care model implementation in primary care: results from a new scale of learning in primary care. BMC Health Serv Res. 2011;11:44. https://doi.org/10.1186/1472-6963-11-44
1. Block B, Boscardin J, Covinsky K, Mourad M, Hu L, Smith A. Variation in COVID-19 mortality across 117 US hospitals in high and how-burden settings. J Hosp Med. 2021;16(4):215-218. https://doi.org/10.12788/jhm.3612
2. Janke AT, Mei H, Rothenberg C, Becher RD, Lin Z, Venkatesh AK. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. 2021;16(4):211-214. https://doi.org/10.12788/jhm.3539
3. Watson SK, Rudge JW, Coker R. Health systems’ “surge capacity”: state of the art and priorities for future research. Milbank Q. 2013;91(1):78-122. https://doi.org/10.1111/milq.12003
4. Auerbach AD, Greysen SR. A rising tide: no hospital is an island unto itself in the era of COVID-19. J Hosp Med. 2021;16(4):254. https://doi.org/10.12788/jhm.3592
5. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Applied Behavioral Science. Published February 15, 2021. https://doi.org/10.1177/0021886321991597
6. Finley EP, Pugh JA, Lanham HJ, et al. Relationship quality and patient-assessed quality of care in VA primary care clinics: development and validation of the work relationships scale. Ann Fam Med. 2015; 11(6):543-549. https://doi.org/10.1370/afm.1554
7. Leykum LK, Palmer R, Lanham HJ, et al. Reciprocal learning and chronic care model implementation in primary care: results from a new scale of learning in primary care. BMC Health Serv Res. 2011;11:44. https://doi.org/10.1186/1472-6963-11-44
© 2021 Society of Hospital Medicine
Preserving Margins to Promote Missions: COVID-19’s Toll on US Children’s Hospitals
Since the onset of the COVID-19 pandemic, the proclivity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for adults and its relative sparing of pediatric populations has been well characterized. Accordingly, policymakers have devoted significant attention to SARS-CoV-2’s impact on adult hospitals. Less consideration, however, has been given to children’s hospitals, which responded to the pandemic by suspending noncritical care encounters, conserving personal protective equipment, and implementing alternative care models.1 While important, these strategic decisions may threaten the financial health of children’s hospitals.
In this issue of the Journal of Hospital Medicine, Synhorst et al1 describe the impact of COVID-19 on US children’s hospitals.The authors utilized the Children’s Hospital Association’s PROSPECT database to compare year-over-year trends in healthcare encounters and hospital charges before and during the COVID-19 pandemic at 26 tertiary hospitals. The analysis focused on the first wave of COVID-19 in the United States from February through June 2020.
The results are staggering. Compared with 2019, the authors found significant decreases in healthcare encounters for all children’s hospitals beginning in March 2020, with a nadir in mid-April (corresponding to the first peak in adult hospitalizations). Inpatient bed days, emergency department (ED) visits, and surgeries decreased by a median of 36%, 65%, and 77%, respectively, per hospital during the nadir. Charges from February 1 to June 30, 2020, decreased by a median 24% per children’s hospital as compared to 2019—corresponding to a median $276 million decrease in charges per hospital. A quarter of hospitals faced more than $400 million in lost charges.1
Why do these trends matter? Large decreases in utilization and associated charges likely represent significant unmet demand for child healthcare for both acute and chronic disease management. For example, with limited in-person evaluation available at the onset of illness, caregivers are presenting to EDs with sicker children.2 With a shift to virtual care, clinicians may miss signs of child abuse from violence in the home—which can escalate during isolation.3 Children with chronic conditions may also be left without surveillance mechanisms, which may partly explain the autumn 2020 surge in acute mental health-related ED presentations.4 Furthermore, telemedicine may exacerbate care inequities for vulnerable populations lacking resources and/or English proficiency.
There is also a larger policy perspective to consider in evaluating these data: Because children’s hospitals largely operate in a fee-for-service reimbursement model, they often rely on marginal revenues to support mission-driven programming. In other words, revenue streams from profitable care segments (eg, elective surgeries) often help sustain institutional platforms operating at a loss, such as community safety net programs. Consequently, threats to marginal revenues can place mission-driven programming in jeopardy of being reduced or terminated.
The Synhorst et al1 study was limited to hospital charges, which likely overestimate revenue losses based on actual reimbursements. Yet, this is the first study to quantify COVID-19’s financial toll on children’s hospitals, and charges offer a reasonable proxy for balance sheet trends. Thus, it is safe to assume that most hospitals incurred substantial losses during the 2020 fiscal year. Unfortunately, as the authors highlight, these losses differentially impacted hospitals based on existing resources1—so some hospitals were likely forced to cut programs or reduce staff in an effort to return to profitability. In this way, COVID-19 has exposed the fragility of the fee-for-service model that children’s hospitals rely on for both patients and staff.
Children’s hospitals and the services they provide are essential to the health and well-being of children. The critical losses sustained by children’s hospitals due to COVID-19 threaten their ability to promote child health in the near and long term, with the greatest risk to vulnerable populations. Policymakers must act now to preserve these essential services for children.
1. Synhorst D, Hall M, Thurm C, et al. Healthcare encounter and financial impact of COVID-19 on children’s hospitals. J Hosp Med. 2021;16(4):223-226. https://doi.org/10.12788/jhm.3572
2. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
3. Humphreys KL, Myint MT, Zeanah CH. Increased risk for family violence during the COVID-19 pandemic. Pediatrics. 2020;146(1):e20200982. https://doi.org/10.1542/peds.2020-0982
4. Leeb RT, Bitsko RH, Radhakrishnan L, Martinez P, Njai R, Holland KM. Mental health-related emergency department visits among children aged <18 years during the COVID-19 pandemic—United States, January 1–October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1675-1680. https://doi.org/10.15585/mmwr.mm6945a3
Since the onset of the COVID-19 pandemic, the proclivity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for adults and its relative sparing of pediatric populations has been well characterized. Accordingly, policymakers have devoted significant attention to SARS-CoV-2’s impact on adult hospitals. Less consideration, however, has been given to children’s hospitals, which responded to the pandemic by suspending noncritical care encounters, conserving personal protective equipment, and implementing alternative care models.1 While important, these strategic decisions may threaten the financial health of children’s hospitals.
In this issue of the Journal of Hospital Medicine, Synhorst et al1 describe the impact of COVID-19 on US children’s hospitals.The authors utilized the Children’s Hospital Association’s PROSPECT database to compare year-over-year trends in healthcare encounters and hospital charges before and during the COVID-19 pandemic at 26 tertiary hospitals. The analysis focused on the first wave of COVID-19 in the United States from February through June 2020.
The results are staggering. Compared with 2019, the authors found significant decreases in healthcare encounters for all children’s hospitals beginning in March 2020, with a nadir in mid-April (corresponding to the first peak in adult hospitalizations). Inpatient bed days, emergency department (ED) visits, and surgeries decreased by a median of 36%, 65%, and 77%, respectively, per hospital during the nadir. Charges from February 1 to June 30, 2020, decreased by a median 24% per children’s hospital as compared to 2019—corresponding to a median $276 million decrease in charges per hospital. A quarter of hospitals faced more than $400 million in lost charges.1
Why do these trends matter? Large decreases in utilization and associated charges likely represent significant unmet demand for child healthcare for both acute and chronic disease management. For example, with limited in-person evaluation available at the onset of illness, caregivers are presenting to EDs with sicker children.2 With a shift to virtual care, clinicians may miss signs of child abuse from violence in the home—which can escalate during isolation.3 Children with chronic conditions may also be left without surveillance mechanisms, which may partly explain the autumn 2020 surge in acute mental health-related ED presentations.4 Furthermore, telemedicine may exacerbate care inequities for vulnerable populations lacking resources and/or English proficiency.
There is also a larger policy perspective to consider in evaluating these data: Because children’s hospitals largely operate in a fee-for-service reimbursement model, they often rely on marginal revenues to support mission-driven programming. In other words, revenue streams from profitable care segments (eg, elective surgeries) often help sustain institutional platforms operating at a loss, such as community safety net programs. Consequently, threats to marginal revenues can place mission-driven programming in jeopardy of being reduced or terminated.
The Synhorst et al1 study was limited to hospital charges, which likely overestimate revenue losses based on actual reimbursements. Yet, this is the first study to quantify COVID-19’s financial toll on children’s hospitals, and charges offer a reasonable proxy for balance sheet trends. Thus, it is safe to assume that most hospitals incurred substantial losses during the 2020 fiscal year. Unfortunately, as the authors highlight, these losses differentially impacted hospitals based on existing resources1—so some hospitals were likely forced to cut programs or reduce staff in an effort to return to profitability. In this way, COVID-19 has exposed the fragility of the fee-for-service model that children’s hospitals rely on for both patients and staff.
Children’s hospitals and the services they provide are essential to the health and well-being of children. The critical losses sustained by children’s hospitals due to COVID-19 threaten their ability to promote child health in the near and long term, with the greatest risk to vulnerable populations. Policymakers must act now to preserve these essential services for children.
Since the onset of the COVID-19 pandemic, the proclivity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for adults and its relative sparing of pediatric populations has been well characterized. Accordingly, policymakers have devoted significant attention to SARS-CoV-2’s impact on adult hospitals. Less consideration, however, has been given to children’s hospitals, which responded to the pandemic by suspending noncritical care encounters, conserving personal protective equipment, and implementing alternative care models.1 While important, these strategic decisions may threaten the financial health of children’s hospitals.
In this issue of the Journal of Hospital Medicine, Synhorst et al1 describe the impact of COVID-19 on US children’s hospitals.The authors utilized the Children’s Hospital Association’s PROSPECT database to compare year-over-year trends in healthcare encounters and hospital charges before and during the COVID-19 pandemic at 26 tertiary hospitals. The analysis focused on the first wave of COVID-19 in the United States from February through June 2020.
The results are staggering. Compared with 2019, the authors found significant decreases in healthcare encounters for all children’s hospitals beginning in March 2020, with a nadir in mid-April (corresponding to the first peak in adult hospitalizations). Inpatient bed days, emergency department (ED) visits, and surgeries decreased by a median of 36%, 65%, and 77%, respectively, per hospital during the nadir. Charges from February 1 to June 30, 2020, decreased by a median 24% per children’s hospital as compared to 2019—corresponding to a median $276 million decrease in charges per hospital. A quarter of hospitals faced more than $400 million in lost charges.1
Why do these trends matter? Large decreases in utilization and associated charges likely represent significant unmet demand for child healthcare for both acute and chronic disease management. For example, with limited in-person evaluation available at the onset of illness, caregivers are presenting to EDs with sicker children.2 With a shift to virtual care, clinicians may miss signs of child abuse from violence in the home—which can escalate during isolation.3 Children with chronic conditions may also be left without surveillance mechanisms, which may partly explain the autumn 2020 surge in acute mental health-related ED presentations.4 Furthermore, telemedicine may exacerbate care inequities for vulnerable populations lacking resources and/or English proficiency.
There is also a larger policy perspective to consider in evaluating these data: Because children’s hospitals largely operate in a fee-for-service reimbursement model, they often rely on marginal revenues to support mission-driven programming. In other words, revenue streams from profitable care segments (eg, elective surgeries) often help sustain institutional platforms operating at a loss, such as community safety net programs. Consequently, threats to marginal revenues can place mission-driven programming in jeopardy of being reduced or terminated.
The Synhorst et al1 study was limited to hospital charges, which likely overestimate revenue losses based on actual reimbursements. Yet, this is the first study to quantify COVID-19’s financial toll on children’s hospitals, and charges offer a reasonable proxy for balance sheet trends. Thus, it is safe to assume that most hospitals incurred substantial losses during the 2020 fiscal year. Unfortunately, as the authors highlight, these losses differentially impacted hospitals based on existing resources1—so some hospitals were likely forced to cut programs or reduce staff in an effort to return to profitability. In this way, COVID-19 has exposed the fragility of the fee-for-service model that children’s hospitals rely on for both patients and staff.
Children’s hospitals and the services they provide are essential to the health and well-being of children. The critical losses sustained by children’s hospitals due to COVID-19 threaten their ability to promote child health in the near and long term, with the greatest risk to vulnerable populations. Policymakers must act now to preserve these essential services for children.
1. Synhorst D, Hall M, Thurm C, et al. Healthcare encounter and financial impact of COVID-19 on children’s hospitals. J Hosp Med. 2021;16(4):223-226. https://doi.org/10.12788/jhm.3572
2. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
3. Humphreys KL, Myint MT, Zeanah CH. Increased risk for family violence during the COVID-19 pandemic. Pediatrics. 2020;146(1):e20200982. https://doi.org/10.1542/peds.2020-0982
4. Leeb RT, Bitsko RH, Radhakrishnan L, Martinez P, Njai R, Holland KM. Mental health-related emergency department visits among children aged <18 years during the COVID-19 pandemic—United States, January 1–October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1675-1680. https://doi.org/10.15585/mmwr.mm6945a3
1. Synhorst D, Hall M, Thurm C, et al. Healthcare encounter and financial impact of COVID-19 on children’s hospitals. J Hosp Med. 2021;16(4):223-226. https://doi.org/10.12788/jhm.3572
2. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
3. Humphreys KL, Myint MT, Zeanah CH. Increased risk for family violence during the COVID-19 pandemic. Pediatrics. 2020;146(1):e20200982. https://doi.org/10.1542/peds.2020-0982
4. Leeb RT, Bitsko RH, Radhakrishnan L, Martinez P, Njai R, Holland KM. Mental health-related emergency department visits among children aged <18 years during the COVID-19 pandemic—United States, January 1–October 17, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1675-1680. https://doi.org/10.15585/mmwr.mm6945a3
© 2021 Society of Hospital Medicine
Lenvatinib Plus Pembrolizumab Improves Outcomes in Previously Untreated Advanced Clear Cell Renal Cell Carcinoma
Study Overview
Objective. To evaluate the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab compared with sunitinib alone for the treatment of newly diagnosed advanced clear cell renal cell carcinoma (ccRCC).
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 1:1:1 ratio to receive treatment with 1 of 3 regimens: lenvatinib 20 mg daily plus pembrolizumab 200 mg on day 1 of each 21-day cycle; lenvatinib 18 mg daily plus everolimus 5 mg once daily for each 21-day cycle; or sunitinib 50 mg daily for 4 weeks followed by 2 weeks off. Patients were stratified according to geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group.
Setting and participants. A total of 1417 patients were screened, and 1069 patients underwent randomization between October 2016 and July 2019: 355 patients were randomized to the lenvatinib plus pembrolizumab group, 357 were randomized to the lenvatinib plus everolimus group, and 357 were randomized to the sunitinib alone group. The patients must have had a diagnosis of previously untreated advanced renal cell carcinoma with a clear-cell component. All the patients need to have a Karnofsky performance status of at least 70, adequate renal function, and controlled blood pressure with or without antihypertensive medications.
Main outcome measures. The primary endpoint assessed the progression-free survival (PFS) as evaluated by independent review committee using RECIST, version 1.1. Imaging was performed at the time of screening and every 8 weeks thereafter. Secondary endpoints were safety, overall survival (OS), and objective response rate as well as investigator-assessed PFS. Also, they assessed the duration of response. During the treatment period, the safety and adverse events were assessed up to 30 days from the last dose of the trial drug.
Main results. The baseline characteristics were well balanced between the treatment groups. More than 70% of enrolled participants were male. Approximately 60% of participants were MSKCC intermediate risk, 27% were favorable risk, and 9% were poor risk. Patients with a PD-L1 combined positive score of 1% or more represented 30% of the population. The remainder had a PD-L1 combined positive score of <1% (30%) or such data were not available (38%). Liver metastases were present in 17% of patients at baseline in each group, and 70% of patients had a prior nephrectomy. The data cutoff occurred in August 2020 for PFS and the median follow-up for OS was 26.6 months. Around 40% of the participants in the lenvatinib plus pembrolizumab group, 18.8% in the sunitinib group, and 31% in the lenvatinib plus everolimus group were still receiving trial treatment at data cutoff. The leading cause for discontinuing therapy was disease progression. Approximately 50% of patients in the lenvatinib/everolimus group and sunitinib group received subsequent checkpoint inhibitor therapy after progression.
The median PFS in the lenvatinib plus pembrolizumab group was significantly longer than in the sunitinib group, 23.9 months vs 9.2 months (hazard ratio [HR], 0.39; 95% CI, 0.32-0.49; P < 0.001). The median PFS was also significantly longer in the lenvatinib plus everolimus group compared with sunitinib, 14.7 vs 9.2 months (HR 0.65; 95% CI 0.53-0.80; P < 0.001). The PFS benefit favored the lenvatinib combination groups over sunitinib in all subgroups, including the MSKCC prognostic risk groups. The median OS was not reached with any treatment, with 79% of patients in the lenvatinib plus pembrolizumab group, 66% of patients in the lenvatinib plus everolimus group, and 70% in the sunitinib group still alive at 24 months. Survival was significantly longer in the lenvatinib plus pembrolizumab group compared with sunitinib (HR, 0.66; 95% CI, 0.49-0.88; P = 0.005). The OS favored lenvatinib/pembrolizumab over sunitinib in the PD-L1 positive or negative groups. The median duration of response in the lenvatinib plus pembrolizumab group was 25.8 months compared to 16.6 months and 14.6 months in the lenvatinib plus everolimus and sunitinib groups, respectively. Complete response rates were higher in the lenvatinib plus pembrolizumab group (16%) compared with lenvatinib/everolimus (9.8%) or sunitinib (4.2%). The median time to response was around 1.9 months in all 3 groups.
The most frequent adverse events seen in all groups were diarrhea, hypertension, fatigue, and nausea. Hypothyroidism was seen more frequently in the lenvatinib plus pembrolizumab group (47%). Grade 3 adverse events were seen in approximately 80% of patients in all groups. The most common grade 3 or higher adverse event was hypertension in all 3 groups. The median time for discontinuing treatment due to side effects was 8.97 months in the lenvatinib plus pembrolizumab arm, 5.49 months in the lenvatinib plus everolimus group, and 4.57 months in the sunitinib group. In the lenvatinib plus pembrolizumab group, 15 patients had grade 5 adverse events; 11 participants had fatal events not related to disease progression. In the lenvatinib plus everolimus group, there were 22 patients with grade 5 events, with 10 fatal events not related to disease progression. In the sunitinib group, 11 patients had grade 5 events, and only 2 fatal events were not linked to disease progression.
Conclusion. The combination of lenvatinib plus pembrolizumab significantly prolongs PFS and OS compared with sunitinib in patients with previously untreated and advanced ccRCC. The median OS has not yet been reached.
Commentary
The results of the current phase 3 CLEAR trial highlight the efficacy and safety of lenvatinib plus pembrolizumab as a first-line treatment in advanced ccRCC. This trial adds to the rapidly growing body of literature supporting the notion that the combination of anti-PD-1 based therapy with either CTLA-4 antibodies or VEGF receptor tyrosine kinase inhibitors (TKI) improves outcomes in previously untreated patients with advanced ccRCC. Previously presented data from Keynote-426 (pembrolizumab plus axitinib), Checkmate-214 (nivolumab plus ipilimumab), and Javelin Renal 101 (Avelumab plus axitinib) have also shown improved outcomes with combination therapy in the frontline setting.1-4 While the landscape of therapeutic options in the frontline setting continues to grow, there remains lack of clarity as to how to tailor our therapeutic decisions for specific patient populations. The exception would be nivolumab and ipilimumab, which are currently indicated for IMDC intermediate- or poor-risk patients.
The combination of VEGFR TKI therapy and PD-1 antibodies provides rapid disease control, with a median time to response in the current study of 1.9 months, and, generally speaking, a low risk of progression in the first 6 months of therapy. While cross-trial comparisons are always problematic, the PFS reported in this study and others with VEGFR TKI and PD-1 antibody combinations is quite impressive and surpasses that noted in Checkmate 214.3 While the median OS survival has not yet been reached, the long duration of PFS and complete response rate of 16% in this study certainly make this an attractive frontline option for newly diagnosed patients with advanced ccRCC. Longer follow-up is needed to confirm the survival benefit noted.
Applications for Clinical Practice
The current data support the use VEGFR TKI and anti-PD1 therapy in the frontline setting. How to choose between such combination regimens or combination immunotherapy remains unclear, and further biomarker-based assessments are needed to help guide therapeutic decisions for our patients.
1. Motzer, R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma [published online ahead of print, 2021 Feb 13]. N Engl J Med. 2021;10.1056/NEJMoa2035716. doi:10.1056/NEJMoa2035716
2. Rini, BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
3. Motzer, RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
4. Motzer, RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115.
Study Overview
Objective. To evaluate the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab compared with sunitinib alone for the treatment of newly diagnosed advanced clear cell renal cell carcinoma (ccRCC).
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 1:1:1 ratio to receive treatment with 1 of 3 regimens: lenvatinib 20 mg daily plus pembrolizumab 200 mg on day 1 of each 21-day cycle; lenvatinib 18 mg daily plus everolimus 5 mg once daily for each 21-day cycle; or sunitinib 50 mg daily for 4 weeks followed by 2 weeks off. Patients were stratified according to geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group.
Setting and participants. A total of 1417 patients were screened, and 1069 patients underwent randomization between October 2016 and July 2019: 355 patients were randomized to the lenvatinib plus pembrolizumab group, 357 were randomized to the lenvatinib plus everolimus group, and 357 were randomized to the sunitinib alone group. The patients must have had a diagnosis of previously untreated advanced renal cell carcinoma with a clear-cell component. All the patients need to have a Karnofsky performance status of at least 70, adequate renal function, and controlled blood pressure with or without antihypertensive medications.
Main outcome measures. The primary endpoint assessed the progression-free survival (PFS) as evaluated by independent review committee using RECIST, version 1.1. Imaging was performed at the time of screening and every 8 weeks thereafter. Secondary endpoints were safety, overall survival (OS), and objective response rate as well as investigator-assessed PFS. Also, they assessed the duration of response. During the treatment period, the safety and adverse events were assessed up to 30 days from the last dose of the trial drug.
Main results. The baseline characteristics were well balanced between the treatment groups. More than 70% of enrolled participants were male. Approximately 60% of participants were MSKCC intermediate risk, 27% were favorable risk, and 9% were poor risk. Patients with a PD-L1 combined positive score of 1% or more represented 30% of the population. The remainder had a PD-L1 combined positive score of <1% (30%) or such data were not available (38%). Liver metastases were present in 17% of patients at baseline in each group, and 70% of patients had a prior nephrectomy. The data cutoff occurred in August 2020 for PFS and the median follow-up for OS was 26.6 months. Around 40% of the participants in the lenvatinib plus pembrolizumab group, 18.8% in the sunitinib group, and 31% in the lenvatinib plus everolimus group were still receiving trial treatment at data cutoff. The leading cause for discontinuing therapy was disease progression. Approximately 50% of patients in the lenvatinib/everolimus group and sunitinib group received subsequent checkpoint inhibitor therapy after progression.
The median PFS in the lenvatinib plus pembrolizumab group was significantly longer than in the sunitinib group, 23.9 months vs 9.2 months (hazard ratio [HR], 0.39; 95% CI, 0.32-0.49; P < 0.001). The median PFS was also significantly longer in the lenvatinib plus everolimus group compared with sunitinib, 14.7 vs 9.2 months (HR 0.65; 95% CI 0.53-0.80; P < 0.001). The PFS benefit favored the lenvatinib combination groups over sunitinib in all subgroups, including the MSKCC prognostic risk groups. The median OS was not reached with any treatment, with 79% of patients in the lenvatinib plus pembrolizumab group, 66% of patients in the lenvatinib plus everolimus group, and 70% in the sunitinib group still alive at 24 months. Survival was significantly longer in the lenvatinib plus pembrolizumab group compared with sunitinib (HR, 0.66; 95% CI, 0.49-0.88; P = 0.005). The OS favored lenvatinib/pembrolizumab over sunitinib in the PD-L1 positive or negative groups. The median duration of response in the lenvatinib plus pembrolizumab group was 25.8 months compared to 16.6 months and 14.6 months in the lenvatinib plus everolimus and sunitinib groups, respectively. Complete response rates were higher in the lenvatinib plus pembrolizumab group (16%) compared with lenvatinib/everolimus (9.8%) or sunitinib (4.2%). The median time to response was around 1.9 months in all 3 groups.
The most frequent adverse events seen in all groups were diarrhea, hypertension, fatigue, and nausea. Hypothyroidism was seen more frequently in the lenvatinib plus pembrolizumab group (47%). Grade 3 adverse events were seen in approximately 80% of patients in all groups. The most common grade 3 or higher adverse event was hypertension in all 3 groups. The median time for discontinuing treatment due to side effects was 8.97 months in the lenvatinib plus pembrolizumab arm, 5.49 months in the lenvatinib plus everolimus group, and 4.57 months in the sunitinib group. In the lenvatinib plus pembrolizumab group, 15 patients had grade 5 adverse events; 11 participants had fatal events not related to disease progression. In the lenvatinib plus everolimus group, there were 22 patients with grade 5 events, with 10 fatal events not related to disease progression. In the sunitinib group, 11 patients had grade 5 events, and only 2 fatal events were not linked to disease progression.
Conclusion. The combination of lenvatinib plus pembrolizumab significantly prolongs PFS and OS compared with sunitinib in patients with previously untreated and advanced ccRCC. The median OS has not yet been reached.
Commentary
The results of the current phase 3 CLEAR trial highlight the efficacy and safety of lenvatinib plus pembrolizumab as a first-line treatment in advanced ccRCC. This trial adds to the rapidly growing body of literature supporting the notion that the combination of anti-PD-1 based therapy with either CTLA-4 antibodies or VEGF receptor tyrosine kinase inhibitors (TKI) improves outcomes in previously untreated patients with advanced ccRCC. Previously presented data from Keynote-426 (pembrolizumab plus axitinib), Checkmate-214 (nivolumab plus ipilimumab), and Javelin Renal 101 (Avelumab plus axitinib) have also shown improved outcomes with combination therapy in the frontline setting.1-4 While the landscape of therapeutic options in the frontline setting continues to grow, there remains lack of clarity as to how to tailor our therapeutic decisions for specific patient populations. The exception would be nivolumab and ipilimumab, which are currently indicated for IMDC intermediate- or poor-risk patients.
The combination of VEGFR TKI therapy and PD-1 antibodies provides rapid disease control, with a median time to response in the current study of 1.9 months, and, generally speaking, a low risk of progression in the first 6 months of therapy. While cross-trial comparisons are always problematic, the PFS reported in this study and others with VEGFR TKI and PD-1 antibody combinations is quite impressive and surpasses that noted in Checkmate 214.3 While the median OS survival has not yet been reached, the long duration of PFS and complete response rate of 16% in this study certainly make this an attractive frontline option for newly diagnosed patients with advanced ccRCC. Longer follow-up is needed to confirm the survival benefit noted.
Applications for Clinical Practice
The current data support the use VEGFR TKI and anti-PD1 therapy in the frontline setting. How to choose between such combination regimens or combination immunotherapy remains unclear, and further biomarker-based assessments are needed to help guide therapeutic decisions for our patients.
Study Overview
Objective. To evaluate the efficacy and safety of lenvatinib in combination with everolimus or pembrolizumab compared with sunitinib alone for the treatment of newly diagnosed advanced clear cell renal cell carcinoma (ccRCC).
Design. Global, multicenter, randomized, open-label, phase 3 trial.
Intervention. Patients were randomized in a 1:1:1 ratio to receive treatment with 1 of 3 regimens: lenvatinib 20 mg daily plus pembrolizumab 200 mg on day 1 of each 21-day cycle; lenvatinib 18 mg daily plus everolimus 5 mg once daily for each 21-day cycle; or sunitinib 50 mg daily for 4 weeks followed by 2 weeks off. Patients were stratified according to geographic region and Memorial Sloan Kettering Cancer Center (MSKCC) prognostic risk group.
Setting and participants. A total of 1417 patients were screened, and 1069 patients underwent randomization between October 2016 and July 2019: 355 patients were randomized to the lenvatinib plus pembrolizumab group, 357 were randomized to the lenvatinib plus everolimus group, and 357 were randomized to the sunitinib alone group. The patients must have had a diagnosis of previously untreated advanced renal cell carcinoma with a clear-cell component. All the patients need to have a Karnofsky performance status of at least 70, adequate renal function, and controlled blood pressure with or without antihypertensive medications.
Main outcome measures. The primary endpoint assessed the progression-free survival (PFS) as evaluated by independent review committee using RECIST, version 1.1. Imaging was performed at the time of screening and every 8 weeks thereafter. Secondary endpoints were safety, overall survival (OS), and objective response rate as well as investigator-assessed PFS. Also, they assessed the duration of response. During the treatment period, the safety and adverse events were assessed up to 30 days from the last dose of the trial drug.
Main results. The baseline characteristics were well balanced between the treatment groups. More than 70% of enrolled participants were male. Approximately 60% of participants were MSKCC intermediate risk, 27% were favorable risk, and 9% were poor risk. Patients with a PD-L1 combined positive score of 1% or more represented 30% of the population. The remainder had a PD-L1 combined positive score of <1% (30%) or such data were not available (38%). Liver metastases were present in 17% of patients at baseline in each group, and 70% of patients had a prior nephrectomy. The data cutoff occurred in August 2020 for PFS and the median follow-up for OS was 26.6 months. Around 40% of the participants in the lenvatinib plus pembrolizumab group, 18.8% in the sunitinib group, and 31% in the lenvatinib plus everolimus group were still receiving trial treatment at data cutoff. The leading cause for discontinuing therapy was disease progression. Approximately 50% of patients in the lenvatinib/everolimus group and sunitinib group received subsequent checkpoint inhibitor therapy after progression.
The median PFS in the lenvatinib plus pembrolizumab group was significantly longer than in the sunitinib group, 23.9 months vs 9.2 months (hazard ratio [HR], 0.39; 95% CI, 0.32-0.49; P < 0.001). The median PFS was also significantly longer in the lenvatinib plus everolimus group compared with sunitinib, 14.7 vs 9.2 months (HR 0.65; 95% CI 0.53-0.80; P < 0.001). The PFS benefit favored the lenvatinib combination groups over sunitinib in all subgroups, including the MSKCC prognostic risk groups. The median OS was not reached with any treatment, with 79% of patients in the lenvatinib plus pembrolizumab group, 66% of patients in the lenvatinib plus everolimus group, and 70% in the sunitinib group still alive at 24 months. Survival was significantly longer in the lenvatinib plus pembrolizumab group compared with sunitinib (HR, 0.66; 95% CI, 0.49-0.88; P = 0.005). The OS favored lenvatinib/pembrolizumab over sunitinib in the PD-L1 positive or negative groups. The median duration of response in the lenvatinib plus pembrolizumab group was 25.8 months compared to 16.6 months and 14.6 months in the lenvatinib plus everolimus and sunitinib groups, respectively. Complete response rates were higher in the lenvatinib plus pembrolizumab group (16%) compared with lenvatinib/everolimus (9.8%) or sunitinib (4.2%). The median time to response was around 1.9 months in all 3 groups.
The most frequent adverse events seen in all groups were diarrhea, hypertension, fatigue, and nausea. Hypothyroidism was seen more frequently in the lenvatinib plus pembrolizumab group (47%). Grade 3 adverse events were seen in approximately 80% of patients in all groups. The most common grade 3 or higher adverse event was hypertension in all 3 groups. The median time for discontinuing treatment due to side effects was 8.97 months in the lenvatinib plus pembrolizumab arm, 5.49 months in the lenvatinib plus everolimus group, and 4.57 months in the sunitinib group. In the lenvatinib plus pembrolizumab group, 15 patients had grade 5 adverse events; 11 participants had fatal events not related to disease progression. In the lenvatinib plus everolimus group, there were 22 patients with grade 5 events, with 10 fatal events not related to disease progression. In the sunitinib group, 11 patients had grade 5 events, and only 2 fatal events were not linked to disease progression.
Conclusion. The combination of lenvatinib plus pembrolizumab significantly prolongs PFS and OS compared with sunitinib in patients with previously untreated and advanced ccRCC. The median OS has not yet been reached.
Commentary
The results of the current phase 3 CLEAR trial highlight the efficacy and safety of lenvatinib plus pembrolizumab as a first-line treatment in advanced ccRCC. This trial adds to the rapidly growing body of literature supporting the notion that the combination of anti-PD-1 based therapy with either CTLA-4 antibodies or VEGF receptor tyrosine kinase inhibitors (TKI) improves outcomes in previously untreated patients with advanced ccRCC. Previously presented data from Keynote-426 (pembrolizumab plus axitinib), Checkmate-214 (nivolumab plus ipilimumab), and Javelin Renal 101 (Avelumab plus axitinib) have also shown improved outcomes with combination therapy in the frontline setting.1-4 While the landscape of therapeutic options in the frontline setting continues to grow, there remains lack of clarity as to how to tailor our therapeutic decisions for specific patient populations. The exception would be nivolumab and ipilimumab, which are currently indicated for IMDC intermediate- or poor-risk patients.
The combination of VEGFR TKI therapy and PD-1 antibodies provides rapid disease control, with a median time to response in the current study of 1.9 months, and, generally speaking, a low risk of progression in the first 6 months of therapy. While cross-trial comparisons are always problematic, the PFS reported in this study and others with VEGFR TKI and PD-1 antibody combinations is quite impressive and surpasses that noted in Checkmate 214.3 While the median OS survival has not yet been reached, the long duration of PFS and complete response rate of 16% in this study certainly make this an attractive frontline option for newly diagnosed patients with advanced ccRCC. Longer follow-up is needed to confirm the survival benefit noted.
Applications for Clinical Practice
The current data support the use VEGFR TKI and anti-PD1 therapy in the frontline setting. How to choose between such combination regimens or combination immunotherapy remains unclear, and further biomarker-based assessments are needed to help guide therapeutic decisions for our patients.
1. Motzer, R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma [published online ahead of print, 2021 Feb 13]. N Engl J Med. 2021;10.1056/NEJMoa2035716. doi:10.1056/NEJMoa2035716
2. Rini, BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
3. Motzer, RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
4. Motzer, RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115.
1. Motzer, R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma [published online ahead of print, 2021 Feb 13]. N Engl J Med. 2021;10.1056/NEJMoa2035716. doi:10.1056/NEJMoa2035716
2. Rini, BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127.
3. Motzer, RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290.
4. Motzer, RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103-1115.
“Thank You for Not Letting Me Crash and Burn”: The Imperative of Quality Physician Onboarding to Foster Job Satisfaction, Strengthen Workplace Culture, and Advance the Quadruple Aim
From The Ohio State University College of Medicine Department of Family and Community Medicine, Columbus, OH (Candy Magaña, Jná Báez, Christine Junk, Drs. Ahmad, Conroy, and Olayiwola); The Ohio State University College of Medicine Center for Primary Care Innovation and Transformation (Candy Magaña, Jná Báez, and Dr. Olayiwola); and The Ohio State University Wexner Medical Center (Christine Harsh, Erica Esposito).
Much has been discussed about the growing crisis of professional dissatisfaction among physicians, with increasing efforts being made to incorporate physician wellness into health system strategies that move from the Triple to the Quadruple Aim.1 For many years, our health care system has been focused on improving the health of populations, optimizing the patient experience, and reducing the cost of care (Triple Aim). The inclusion of the fourth aim, improving the experience of the teams that deliver care, has become paramount in achieving the other aims.
An area often overlooked in this focus on wellness, however, is the importance of the earliest days of employment to shape and predict long-term career contentment. This is a missed opportunity, as data suggest that organizations with standardized onboarding programs boast a 62% increased productivity rate and a 50% greater retention rate among new hires.2,3 Moreover, a study by the International Institute for Management Development found that businesses lose an estimated $37 billion annually because employees do not fully understand their jobs.4 The report ties losses to “actions taken by employees who have misunderstood or misinterpreted company policies, business processes, job function, or a combination of the three.” Additionally, onboarding programs that focus strictly on technical or functional orientation tasks miss important opportunities for culture integration during the onboarding process.5 It is therefore imperative to look to effective models of employee onboarding to develop systems that position physicians and practices for success.
Challenges With Traditional Physician Onboarding
In recent years, the Department of Family and Community Medicine at The Ohio State University College of Medicine has experienced rapid organizational change. Like many primary care systems nationwide responding to disruption in health care and changing demands on the clinical workforce, the department has hired new leadership, revised strategic priorities, and witnessed an influx of faculty and staff. It has also planned an expansion of ambulatory services that will more than double the clinical workforce over the next 3 years. While an exciting time, there has been a growing need to align strategy, culture, and human capital during these changes.
As we entered this phase of transformation, we recognized that our highly individualized, ad hoc orientation system presented shortcomings. During the act of revamping our physician recruitment process, stakeholder workgroup members specifically noted that improvement efforts were needed regarding new physician orientation, as no consistent structures were previously in place. New physician orientation had been a major gap for years, resulting in dissatisfaction in the first few months of physician practice, early physician turnover, and staff frustration. For physicians, we continued to learn about their frustration and unanswered questions regarding expectations, norms, structures, and processes.
Many new hires were left with a kind of “trial by fire” entry into their roles. On the first day of clinic, a new physician would most likely need to simultaneously see patients, learn the nuances of the electronic health record (EHR), figure out where the break room was located, and quickly learn population health issues for the patients they were serving. Opportunities to meet key clinic site leadership would be at random, and new physicians might not have the opportunity to meet leadership or staff until months into their tenure; this did not allow for a sense of belonging or understanding of the many resources available to them. We learned that the quality of these ad hoc orientations also varied based on the experience and priorities of each practice’s clinic and administrative leaders, who themselves felt ill-equipped to provide a consistent, robust, and confidence-building experience. In addition, practice site management was rarely given advance time to prepare for the arrival of new physicians, which resulted in physicians perceiving practices to be unwelcoming and disorganized. Their first days were often spent with patients in clinic with no structured orientation and without understanding workflows or having systems practice knowledge.
Institutionally, the interview process satisfied some transfer of knowledge, but we were unclear of what was being consistently shared and understood in the multiple ambulatory locations where our physicians enter practice. More importantly, we knew we were missing a critical opportunity to use orientation to imbue other values of diversity and inclusion, health equity, and operational excellence into the workforce. Based on anecdotal insights from employees and our own review of successful onboarding approaches from other industries, we also knew a more structured welcoming process would predict greater long-term career satisfaction for physicians and create a foundation for providing optimal care for patients when clinical encounters began.
Reengineering Physician Onboarding
In 2019, our department developed a multipronged approach to physician onboarding, which is already paying dividends in easing acculturation and fostering team cohesion. The department tapped its Center for Primary Care Innovation and Transformation (PCIT) to direct this effort, based on its expertise in practice transformation, clinical transformation and adaptations, and workflow efficiency through process and quality improvement. The PCIT team provides support to the department and the entire health system focused on technology and innovation, health equity, and health care efficiency.6 They applied many of the tools used in the Clinical Transformation in Technology approach to lead this initiative.7
The PCIT team began identifying key stakeholders (department, clinical and ambulatory leadership, clinicians and clinical staff, community partners, human resources, and resident physicians), and then engaging those individuals in dialogue surrounding orientation needs. During scheduled in-person and virtual work sessions, stakeholders were asked to provide input on pain points for new physicians and clinic leadership and were then empowered to create an onboarding program. Applying health care quality improvement techniques, we leveraged workflow mapping, current and future state planning, and goal setting, led by the skilled process improvement and clinical transformation specialists. We coordinated a multidisciplinary process improvement team that included clinic administrators, medical directors, human resources, administrative staff, ambulatory and resident leadership, clinical leadership, and recruitment liaisons. This diverse group of leadership and staff was brought together to address these critical identified gaps and weaknesses in new physician onboarding.
Through a series of learning sessions, the workgroup provided input that was used to form an itemized physician onboarding schedule, which was then leveraged to develop Plan-Do-Study-Act (PDSA) cycles, collecting feedback in real time. Some issues that seem small can cause major distress for new physicians. For example, in our inaugural orientation implementation, a physician provided feedback that they wanted to obtain information on setting up their work email on their personal devices and was having considerable trouble figuring out how to do so. This particular topic was not initially included in the first iteration of the Department’s orientation program. We rapidly sought out different ways to embed that into the onboarding experience. The first PDSA involved integrating the university information technology team (IT) into the process but was not successful because it required extra work for the new physician and reliance on the IT schedule. The next attempt was to have IT train a department staff member, but again, this still required that the physician find time to connect with that staff member. Finally, we decided to obtain a useful tip sheet that clearly outlined the process and could be included in orientation materials. This gave the new physicians control over how and when they would work on this issue. Based on these learnings, this was incorporated as a standing agenda item and resource for incoming physicians.
Essential Elements of Effective Onboarding
The new physician onboarding program consists of 5 key elements: (1) 2-week acclimation period; (2) peer learning and connection; (3) training before beginning patient care; (4) standardization, transparency, and accountability in all processes; (5) ongoing feedback for continued program improvement with individual support (Figure).
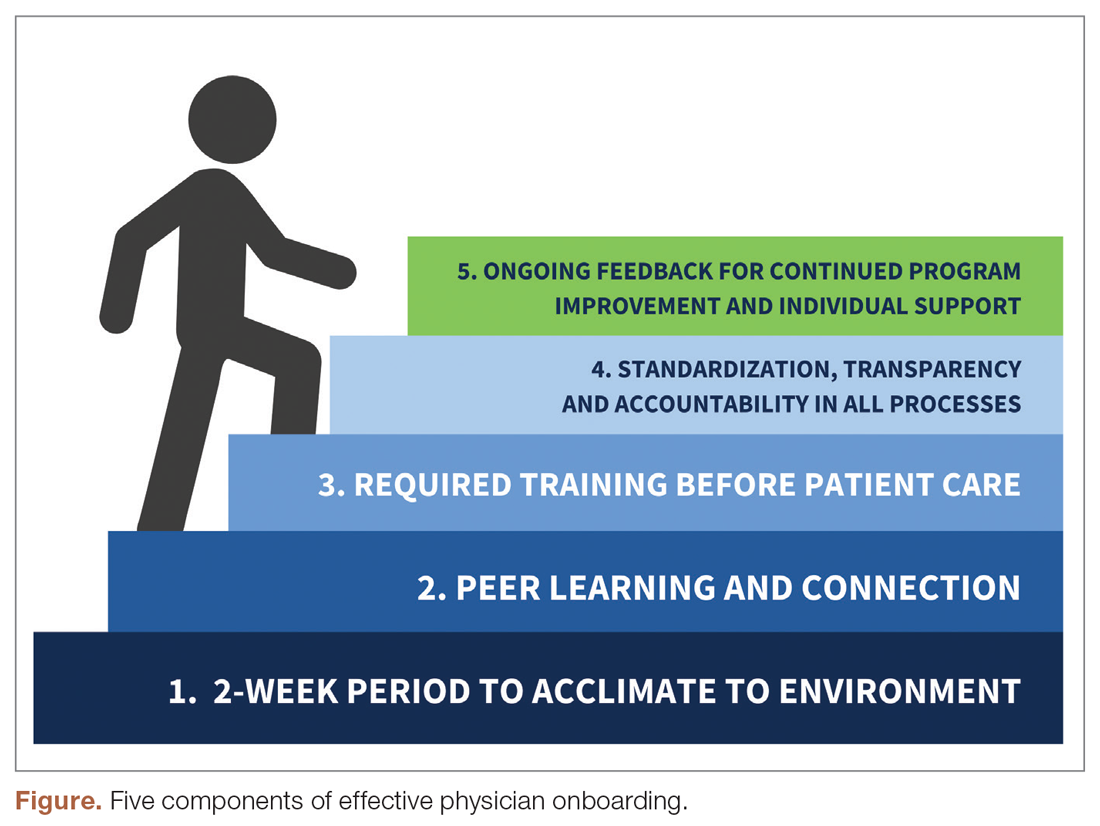
The program begins with a 2-week period of intentional investment in individual success, during which time no patients are scheduled. In week 1, we work with new hires to set expectations for performance, understand departmental norms, and introduce culture. Physicians meet formally and informally with department and institutional leadership, as well as attend team meetings and trainings that include a range of administrative and compliance requirements, such as quality standards and expectations, compliance, billing and coding specific to family medicine, EHR management, and institutionally mandated orientations. We are also adding implicit bias and antiracism training during this period, which are essential to creating a culture of unity and belonging.
During week 2, we focus on clinic-level orientation, assigning new hires an orientation buddy and a department sponsor, such as a physician lead or medical director. Physicians spend time with leadership at their clinic as they nurture relationships important for mentorship, sponsorship, and peer support. They also meet care team members, including front desk associates, medical assistants, behavioral health clinicians, nutritionists, social workers, pharmacists, and other key colleagues and care team members. This introduces the physician to the clinical environment and physical space as well as acclimates the physician to workflows and feedback loops for regular interaction.
When physicians ultimately begin patient care, they begin with an expected productivity rate of 50%, followed by an expected productivity rate of 75%, and then an expected productivity rate of 100%. This steady increase occurs over 3 to 4 weeks depending on the physician’s comfort level. They are also provided monthly reports on work relative value unit performance so that they can track and adapt practice patterns as necessary.More details on the program can be found in Appendix 1.
Takeaways From the Implementation of the New Program
Give time for new physicians to focus on acclimating to the role and environment.
The initial 2-week period of transition—without direct patient care—ensures that physicians feel comfortable in their new ecosystem. This also supports personal transitions, as many new hires are managing relocation and acclimating themselves and their families to new settings. Even residents from our training program who returned as attending physicians found this flexibility and slow reentry essential. This also gives the clinic time to orient to an additional provider, nurture them into the team culture, and develop relationships with the care team.
Cultivate spaces for shared learning, problem-solving, and peer connection.
Orientation is delivered primarily through group learning sessions with cohorts of new physicians, thus developing spaces for networking, fostering psychological safety, encouraging personal and professional rapport, emphasizing interactive learning, and reinforcing scheduling blocks at the departmental level. New hires also participate in peer shadowing to develop clinical competencies and are assigned a workplace buddy to foster a sense of belonging and create opportunities for additional knowledge sharing and cross-training.
Strengthen physician knowledge base, confidence, and comfort in the workplace before beginning direct patient care.
Without fluency in the workflows, culture, and operations of a practice, the urgency to have physicians begin clinical care can result in frustration for the physician, patients, and clinical and administrative staff. Therefore, we complete essential training prior to seeing any patients. This includes clinical workflows, referral processes, use of alternate modalities of care (eg, telehealth, eConsults), billing protocols, population health training, patient resources, office resources, and other essential daily processes and tools. This creates efficiency in administrative management, increased productivity, and better understanding of resources available for patients’ medical, social, and behavioral needs when patient care begins.
Embrace standardization, transparency, and accountability in as many processes as possible.
Standardized knowledge-sharing and checklists are mandated at every step of the orientation process, requiring sign off from the physician lead, practice manager, and new physicians upon completion. This offers all parties the opportunity to play a role in the delivery of and accountability for skills transfer and empowers new hires to press pause if they feel unsure about any domain in the training. It is also essential in guaranteeing that all physicians—regardless of which ambulatory location they practice in—receive consistent information and expectations. A sample checklist can be found in Appendix 2.
Commit to collecting and acting on feedback for continued program improvement and individual support.
As physicians complete the program, it is necessary to create structures to measure and enhance its impact, as well as evaluate how physicians are faring following the program. Each physician completes surveys at the end of the orientation program, attends a 90-day post-program check-in with the department chair, and receives follow-up trainings on advanced topics as they become more deeply embedded in the organization.
Lessons Learned
Feedback from surveys and 90-day check-ins with leadership and physicians reflect a high degree of clarity on job roles and duties, a sense of team camaraderie, easier system navigation, and a strong sense of support. We do recognize that sustaining change takes time and our study is limited by data demonstrating the impact of these efforts. We look forward to sharing more robust data from surveys and qualitative interviews with physicians, clinical leadership, and staff in the future. Our team will conduct interviews at 90-day and 180-day checkpoints with new physicians who have gone through this program, followed by a check-in after 1 year. Additionally, new physicians as well as key stakeholders, such as physician leads, practice managers, and members of the recruitment team, have started to participate in short surveys. These are designed to better understand their experiences, what worked well, what can be improved, and the overall satisfaction of the physician and other members of the extended care team.
What follows are some comments made by the initial group of physicians that went through this program and participated in follow-up interviews:
“I really feel like part of a bigger team.”
“I knew exactly what do to when I walked into the exam room on clinic Day 1.”
“It was great to make deep connections during the early process of joining.”
“Having a buddy to direct questions and ideas to is amazing and empowering.”
“Even though the orientation was long, I felt that I learned so much that I would not have otherwise.”
“Thank you for not letting me crash and burn!”
“Great culture! I love understanding our values of health equity, diversity, and inclusion.”
In the months since our endeavor began, we have learned just how essential it is to fully and effectively integrate new hires into the organization for their own satisfaction and success—and ours. Indeed, we cannot expect to achieve the Quadruple Aim without investing in the kind of transparent and intentional orientation process that defines expectations, aligns cultural values, mitigates costly and stressful operational misunderstandings, and communicates to physicians that, not only do they belong, but their sense of belonging is our priority. While we have yet to understand the impact of this program on the fourth aim of the Quadruple Aim, we are hopeful that the benefits will be far-reaching.
It is our ultimate hope that programs like this: (1) give physicians the confidence needed to create impactful patient-centered experiences; (2) enable physicians to become more cost-effective and efficient in care delivery; (3) allow physicians to understand the populations they are serving and access tools available to mitigate health disparities and other barriers; and (4) improve the collective experience of every member of the care team, practice leadership, and clinician-patient partnership.
Corresponding author: J. Nwando Olayiwola, MD, MPH, FAAFP, The Ohio State University College of Medicine, Department of Family and Community Medicine, 2231 N High St, Ste 250, Columbus, OH 43210; [email protected].
Financial disclosures: None.
Keywords: physician onboarding; Quadruple Aim; leadership; clinician satisfaction; care team satisfaction.
1. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6): 573-576.
2. Maurer R. Onboarding key to retaining, engaging talent. Society for Human Resource Management. April 16, 2015. Accessed January 8, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/onboarding-key-retaining-engaging-talent.aspx
3. Boston AG. New hire onboarding standardization and automation powers productivity gains. GlobeNewswire. March 8, 2011. Accessed January 8, 2021. http://www.globenewswire.com/news-release/2011/03/08/994239/0/en/New-Hire-Onboarding-Standardization-and-Automation-Powers-Productivity-Gains.html
4. $37 billion – US and UK business count the cost of employee misunderstanding. HR.com – Maximizing Human Potential. June 18, 2008. Accessed March 10, 2021. https://www.hr.com/en/communities/staffing_and_recruitment/37-billion---us-and-uk-businesses-count-the-cost-o_fhnduq4d.html
5. Employers risk driving new hires away with poor onboarding. Society for Human Resource Management. February 23, 2018. Accessed March 10, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/employers-new-hires-poor-onboarding.aspx
6. Center for Primary Care Innovation and Transformation. The Ohio State University College of Medicine. Accessed January 8, 2021. https://wexnermedical.osu.edu/departments/family-medicine/pcit
7. Olayiwola, J.N. and Magaña, C. Clinical transformation in technology: a fresh change management approach for primary care. Harvard Health Policy Review. February 2, 2019. Accessed March 10, 2021. http://www.hhpronline.org/articles/2019/2/2/clinical-transformation-in-technology-a-fresh-change-management-approach-for-primary-care
From The Ohio State University College of Medicine Department of Family and Community Medicine, Columbus, OH (Candy Magaña, Jná Báez, Christine Junk, Drs. Ahmad, Conroy, and Olayiwola); The Ohio State University College of Medicine Center for Primary Care Innovation and Transformation (Candy Magaña, Jná Báez, and Dr. Olayiwola); and The Ohio State University Wexner Medical Center (Christine Harsh, Erica Esposito).
Much has been discussed about the growing crisis of professional dissatisfaction among physicians, with increasing efforts being made to incorporate physician wellness into health system strategies that move from the Triple to the Quadruple Aim.1 For many years, our health care system has been focused on improving the health of populations, optimizing the patient experience, and reducing the cost of care (Triple Aim). The inclusion of the fourth aim, improving the experience of the teams that deliver care, has become paramount in achieving the other aims.
An area often overlooked in this focus on wellness, however, is the importance of the earliest days of employment to shape and predict long-term career contentment. This is a missed opportunity, as data suggest that organizations with standardized onboarding programs boast a 62% increased productivity rate and a 50% greater retention rate among new hires.2,3 Moreover, a study by the International Institute for Management Development found that businesses lose an estimated $37 billion annually because employees do not fully understand their jobs.4 The report ties losses to “actions taken by employees who have misunderstood or misinterpreted company policies, business processes, job function, or a combination of the three.” Additionally, onboarding programs that focus strictly on technical or functional orientation tasks miss important opportunities for culture integration during the onboarding process.5 It is therefore imperative to look to effective models of employee onboarding to develop systems that position physicians and practices for success.
Challenges With Traditional Physician Onboarding
In recent years, the Department of Family and Community Medicine at The Ohio State University College of Medicine has experienced rapid organizational change. Like many primary care systems nationwide responding to disruption in health care and changing demands on the clinical workforce, the department has hired new leadership, revised strategic priorities, and witnessed an influx of faculty and staff. It has also planned an expansion of ambulatory services that will more than double the clinical workforce over the next 3 years. While an exciting time, there has been a growing need to align strategy, culture, and human capital during these changes.
As we entered this phase of transformation, we recognized that our highly individualized, ad hoc orientation system presented shortcomings. During the act of revamping our physician recruitment process, stakeholder workgroup members specifically noted that improvement efforts were needed regarding new physician orientation, as no consistent structures were previously in place. New physician orientation had been a major gap for years, resulting in dissatisfaction in the first few months of physician practice, early physician turnover, and staff frustration. For physicians, we continued to learn about their frustration and unanswered questions regarding expectations, norms, structures, and processes.
Many new hires were left with a kind of “trial by fire” entry into their roles. On the first day of clinic, a new physician would most likely need to simultaneously see patients, learn the nuances of the electronic health record (EHR), figure out where the break room was located, and quickly learn population health issues for the patients they were serving. Opportunities to meet key clinic site leadership would be at random, and new physicians might not have the opportunity to meet leadership or staff until months into their tenure; this did not allow for a sense of belonging or understanding of the many resources available to them. We learned that the quality of these ad hoc orientations also varied based on the experience and priorities of each practice’s clinic and administrative leaders, who themselves felt ill-equipped to provide a consistent, robust, and confidence-building experience. In addition, practice site management was rarely given advance time to prepare for the arrival of new physicians, which resulted in physicians perceiving practices to be unwelcoming and disorganized. Their first days were often spent with patients in clinic with no structured orientation and without understanding workflows or having systems practice knowledge.
Institutionally, the interview process satisfied some transfer of knowledge, but we were unclear of what was being consistently shared and understood in the multiple ambulatory locations where our physicians enter practice. More importantly, we knew we were missing a critical opportunity to use orientation to imbue other values of diversity and inclusion, health equity, and operational excellence into the workforce. Based on anecdotal insights from employees and our own review of successful onboarding approaches from other industries, we also knew a more structured welcoming process would predict greater long-term career satisfaction for physicians and create a foundation for providing optimal care for patients when clinical encounters began.
Reengineering Physician Onboarding
In 2019, our department developed a multipronged approach to physician onboarding, which is already paying dividends in easing acculturation and fostering team cohesion. The department tapped its Center for Primary Care Innovation and Transformation (PCIT) to direct this effort, based on its expertise in practice transformation, clinical transformation and adaptations, and workflow efficiency through process and quality improvement. The PCIT team provides support to the department and the entire health system focused on technology and innovation, health equity, and health care efficiency.6 They applied many of the tools used in the Clinical Transformation in Technology approach to lead this initiative.7
The PCIT team began identifying key stakeholders (department, clinical and ambulatory leadership, clinicians and clinical staff, community partners, human resources, and resident physicians), and then engaging those individuals in dialogue surrounding orientation needs. During scheduled in-person and virtual work sessions, stakeholders were asked to provide input on pain points for new physicians and clinic leadership and were then empowered to create an onboarding program. Applying health care quality improvement techniques, we leveraged workflow mapping, current and future state planning, and goal setting, led by the skilled process improvement and clinical transformation specialists. We coordinated a multidisciplinary process improvement team that included clinic administrators, medical directors, human resources, administrative staff, ambulatory and resident leadership, clinical leadership, and recruitment liaisons. This diverse group of leadership and staff was brought together to address these critical identified gaps and weaknesses in new physician onboarding.
Through a series of learning sessions, the workgroup provided input that was used to form an itemized physician onboarding schedule, which was then leveraged to develop Plan-Do-Study-Act (PDSA) cycles, collecting feedback in real time. Some issues that seem small can cause major distress for new physicians. For example, in our inaugural orientation implementation, a physician provided feedback that they wanted to obtain information on setting up their work email on their personal devices and was having considerable trouble figuring out how to do so. This particular topic was not initially included in the first iteration of the Department’s orientation program. We rapidly sought out different ways to embed that into the onboarding experience. The first PDSA involved integrating the university information technology team (IT) into the process but was not successful because it required extra work for the new physician and reliance on the IT schedule. The next attempt was to have IT train a department staff member, but again, this still required that the physician find time to connect with that staff member. Finally, we decided to obtain a useful tip sheet that clearly outlined the process and could be included in orientation materials. This gave the new physicians control over how and when they would work on this issue. Based on these learnings, this was incorporated as a standing agenda item and resource for incoming physicians.
Essential Elements of Effective Onboarding
The new physician onboarding program consists of 5 key elements: (1) 2-week acclimation period; (2) peer learning and connection; (3) training before beginning patient care; (4) standardization, transparency, and accountability in all processes; (5) ongoing feedback for continued program improvement with individual support (Figure).

The program begins with a 2-week period of intentional investment in individual success, during which time no patients are scheduled. In week 1, we work with new hires to set expectations for performance, understand departmental norms, and introduce culture. Physicians meet formally and informally with department and institutional leadership, as well as attend team meetings and trainings that include a range of administrative and compliance requirements, such as quality standards and expectations, compliance, billing and coding specific to family medicine, EHR management, and institutionally mandated orientations. We are also adding implicit bias and antiracism training during this period, which are essential to creating a culture of unity and belonging.
During week 2, we focus on clinic-level orientation, assigning new hires an orientation buddy and a department sponsor, such as a physician lead or medical director. Physicians spend time with leadership at their clinic as they nurture relationships important for mentorship, sponsorship, and peer support. They also meet care team members, including front desk associates, medical assistants, behavioral health clinicians, nutritionists, social workers, pharmacists, and other key colleagues and care team members. This introduces the physician to the clinical environment and physical space as well as acclimates the physician to workflows and feedback loops for regular interaction.
When physicians ultimately begin patient care, they begin with an expected productivity rate of 50%, followed by an expected productivity rate of 75%, and then an expected productivity rate of 100%. This steady increase occurs over 3 to 4 weeks depending on the physician’s comfort level. They are also provided monthly reports on work relative value unit performance so that they can track and adapt practice patterns as necessary.More details on the program can be found in Appendix 1.
Takeaways From the Implementation of the New Program
Give time for new physicians to focus on acclimating to the role and environment.
The initial 2-week period of transition—without direct patient care—ensures that physicians feel comfortable in their new ecosystem. This also supports personal transitions, as many new hires are managing relocation and acclimating themselves and their families to new settings. Even residents from our training program who returned as attending physicians found this flexibility and slow reentry essential. This also gives the clinic time to orient to an additional provider, nurture them into the team culture, and develop relationships with the care team.
Cultivate spaces for shared learning, problem-solving, and peer connection.
Orientation is delivered primarily through group learning sessions with cohorts of new physicians, thus developing spaces for networking, fostering psychological safety, encouraging personal and professional rapport, emphasizing interactive learning, and reinforcing scheduling blocks at the departmental level. New hires also participate in peer shadowing to develop clinical competencies and are assigned a workplace buddy to foster a sense of belonging and create opportunities for additional knowledge sharing and cross-training.
Strengthen physician knowledge base, confidence, and comfort in the workplace before beginning direct patient care.
Without fluency in the workflows, culture, and operations of a practice, the urgency to have physicians begin clinical care can result in frustration for the physician, patients, and clinical and administrative staff. Therefore, we complete essential training prior to seeing any patients. This includes clinical workflows, referral processes, use of alternate modalities of care (eg, telehealth, eConsults), billing protocols, population health training, patient resources, office resources, and other essential daily processes and tools. This creates efficiency in administrative management, increased productivity, and better understanding of resources available for patients’ medical, social, and behavioral needs when patient care begins.
Embrace standardization, transparency, and accountability in as many processes as possible.
Standardized knowledge-sharing and checklists are mandated at every step of the orientation process, requiring sign off from the physician lead, practice manager, and new physicians upon completion. This offers all parties the opportunity to play a role in the delivery of and accountability for skills transfer and empowers new hires to press pause if they feel unsure about any domain in the training. It is also essential in guaranteeing that all physicians—regardless of which ambulatory location they practice in—receive consistent information and expectations. A sample checklist can be found in Appendix 2.
Commit to collecting and acting on feedback for continued program improvement and individual support.
As physicians complete the program, it is necessary to create structures to measure and enhance its impact, as well as evaluate how physicians are faring following the program. Each physician completes surveys at the end of the orientation program, attends a 90-day post-program check-in with the department chair, and receives follow-up trainings on advanced topics as they become more deeply embedded in the organization.
Lessons Learned
Feedback from surveys and 90-day check-ins with leadership and physicians reflect a high degree of clarity on job roles and duties, a sense of team camaraderie, easier system navigation, and a strong sense of support. We do recognize that sustaining change takes time and our study is limited by data demonstrating the impact of these efforts. We look forward to sharing more robust data from surveys and qualitative interviews with physicians, clinical leadership, and staff in the future. Our team will conduct interviews at 90-day and 180-day checkpoints with new physicians who have gone through this program, followed by a check-in after 1 year. Additionally, new physicians as well as key stakeholders, such as physician leads, practice managers, and members of the recruitment team, have started to participate in short surveys. These are designed to better understand their experiences, what worked well, what can be improved, and the overall satisfaction of the physician and other members of the extended care team.
What follows are some comments made by the initial group of physicians that went through this program and participated in follow-up interviews:
“I really feel like part of a bigger team.”
“I knew exactly what do to when I walked into the exam room on clinic Day 1.”
“It was great to make deep connections during the early process of joining.”
“Having a buddy to direct questions and ideas to is amazing and empowering.”
“Even though the orientation was long, I felt that I learned so much that I would not have otherwise.”
“Thank you for not letting me crash and burn!”
“Great culture! I love understanding our values of health equity, diversity, and inclusion.”
In the months since our endeavor began, we have learned just how essential it is to fully and effectively integrate new hires into the organization for their own satisfaction and success—and ours. Indeed, we cannot expect to achieve the Quadruple Aim without investing in the kind of transparent and intentional orientation process that defines expectations, aligns cultural values, mitigates costly and stressful operational misunderstandings, and communicates to physicians that, not only do they belong, but their sense of belonging is our priority. While we have yet to understand the impact of this program on the fourth aim of the Quadruple Aim, we are hopeful that the benefits will be far-reaching.
It is our ultimate hope that programs like this: (1) give physicians the confidence needed to create impactful patient-centered experiences; (2) enable physicians to become more cost-effective and efficient in care delivery; (3) allow physicians to understand the populations they are serving and access tools available to mitigate health disparities and other barriers; and (4) improve the collective experience of every member of the care team, practice leadership, and clinician-patient partnership.
Corresponding author: J. Nwando Olayiwola, MD, MPH, FAAFP, The Ohio State University College of Medicine, Department of Family and Community Medicine, 2231 N High St, Ste 250, Columbus, OH 43210; [email protected].
Financial disclosures: None.
Keywords: physician onboarding; Quadruple Aim; leadership; clinician satisfaction; care team satisfaction.
From The Ohio State University College of Medicine Department of Family and Community Medicine, Columbus, OH (Candy Magaña, Jná Báez, Christine Junk, Drs. Ahmad, Conroy, and Olayiwola); The Ohio State University College of Medicine Center for Primary Care Innovation and Transformation (Candy Magaña, Jná Báez, and Dr. Olayiwola); and The Ohio State University Wexner Medical Center (Christine Harsh, Erica Esposito).
Much has been discussed about the growing crisis of professional dissatisfaction among physicians, with increasing efforts being made to incorporate physician wellness into health system strategies that move from the Triple to the Quadruple Aim.1 For many years, our health care system has been focused on improving the health of populations, optimizing the patient experience, and reducing the cost of care (Triple Aim). The inclusion of the fourth aim, improving the experience of the teams that deliver care, has become paramount in achieving the other aims.
An area often overlooked in this focus on wellness, however, is the importance of the earliest days of employment to shape and predict long-term career contentment. This is a missed opportunity, as data suggest that organizations with standardized onboarding programs boast a 62% increased productivity rate and a 50% greater retention rate among new hires.2,3 Moreover, a study by the International Institute for Management Development found that businesses lose an estimated $37 billion annually because employees do not fully understand their jobs.4 The report ties losses to “actions taken by employees who have misunderstood or misinterpreted company policies, business processes, job function, or a combination of the three.” Additionally, onboarding programs that focus strictly on technical or functional orientation tasks miss important opportunities for culture integration during the onboarding process.5 It is therefore imperative to look to effective models of employee onboarding to develop systems that position physicians and practices for success.
Challenges With Traditional Physician Onboarding
In recent years, the Department of Family and Community Medicine at The Ohio State University College of Medicine has experienced rapid organizational change. Like many primary care systems nationwide responding to disruption in health care and changing demands on the clinical workforce, the department has hired new leadership, revised strategic priorities, and witnessed an influx of faculty and staff. It has also planned an expansion of ambulatory services that will more than double the clinical workforce over the next 3 years. While an exciting time, there has been a growing need to align strategy, culture, and human capital during these changes.
As we entered this phase of transformation, we recognized that our highly individualized, ad hoc orientation system presented shortcomings. During the act of revamping our physician recruitment process, stakeholder workgroup members specifically noted that improvement efforts were needed regarding new physician orientation, as no consistent structures were previously in place. New physician orientation had been a major gap for years, resulting in dissatisfaction in the first few months of physician practice, early physician turnover, and staff frustration. For physicians, we continued to learn about their frustration and unanswered questions regarding expectations, norms, structures, and processes.
Many new hires were left with a kind of “trial by fire” entry into their roles. On the first day of clinic, a new physician would most likely need to simultaneously see patients, learn the nuances of the electronic health record (EHR), figure out where the break room was located, and quickly learn population health issues for the patients they were serving. Opportunities to meet key clinic site leadership would be at random, and new physicians might not have the opportunity to meet leadership or staff until months into their tenure; this did not allow for a sense of belonging or understanding of the many resources available to them. We learned that the quality of these ad hoc orientations also varied based on the experience and priorities of each practice’s clinic and administrative leaders, who themselves felt ill-equipped to provide a consistent, robust, and confidence-building experience. In addition, practice site management was rarely given advance time to prepare for the arrival of new physicians, which resulted in physicians perceiving practices to be unwelcoming and disorganized. Their first days were often spent with patients in clinic with no structured orientation and without understanding workflows or having systems practice knowledge.
Institutionally, the interview process satisfied some transfer of knowledge, but we were unclear of what was being consistently shared and understood in the multiple ambulatory locations where our physicians enter practice. More importantly, we knew we were missing a critical opportunity to use orientation to imbue other values of diversity and inclusion, health equity, and operational excellence into the workforce. Based on anecdotal insights from employees and our own review of successful onboarding approaches from other industries, we also knew a more structured welcoming process would predict greater long-term career satisfaction for physicians and create a foundation for providing optimal care for patients when clinical encounters began.
Reengineering Physician Onboarding
In 2019, our department developed a multipronged approach to physician onboarding, which is already paying dividends in easing acculturation and fostering team cohesion. The department tapped its Center for Primary Care Innovation and Transformation (PCIT) to direct this effort, based on its expertise in practice transformation, clinical transformation and adaptations, and workflow efficiency through process and quality improvement. The PCIT team provides support to the department and the entire health system focused on technology and innovation, health equity, and health care efficiency.6 They applied many of the tools used in the Clinical Transformation in Technology approach to lead this initiative.7
The PCIT team began identifying key stakeholders (department, clinical and ambulatory leadership, clinicians and clinical staff, community partners, human resources, and resident physicians), and then engaging those individuals in dialogue surrounding orientation needs. During scheduled in-person and virtual work sessions, stakeholders were asked to provide input on pain points for new physicians and clinic leadership and were then empowered to create an onboarding program. Applying health care quality improvement techniques, we leveraged workflow mapping, current and future state planning, and goal setting, led by the skilled process improvement and clinical transformation specialists. We coordinated a multidisciplinary process improvement team that included clinic administrators, medical directors, human resources, administrative staff, ambulatory and resident leadership, clinical leadership, and recruitment liaisons. This diverse group of leadership and staff was brought together to address these critical identified gaps and weaknesses in new physician onboarding.
Through a series of learning sessions, the workgroup provided input that was used to form an itemized physician onboarding schedule, which was then leveraged to develop Plan-Do-Study-Act (PDSA) cycles, collecting feedback in real time. Some issues that seem small can cause major distress for new physicians. For example, in our inaugural orientation implementation, a physician provided feedback that they wanted to obtain information on setting up their work email on their personal devices and was having considerable trouble figuring out how to do so. This particular topic was not initially included in the first iteration of the Department’s orientation program. We rapidly sought out different ways to embed that into the onboarding experience. The first PDSA involved integrating the university information technology team (IT) into the process but was not successful because it required extra work for the new physician and reliance on the IT schedule. The next attempt was to have IT train a department staff member, but again, this still required that the physician find time to connect with that staff member. Finally, we decided to obtain a useful tip sheet that clearly outlined the process and could be included in orientation materials. This gave the new physicians control over how and when they would work on this issue. Based on these learnings, this was incorporated as a standing agenda item and resource for incoming physicians.
Essential Elements of Effective Onboarding
The new physician onboarding program consists of 5 key elements: (1) 2-week acclimation period; (2) peer learning and connection; (3) training before beginning patient care; (4) standardization, transparency, and accountability in all processes; (5) ongoing feedback for continued program improvement with individual support (Figure).

The program begins with a 2-week period of intentional investment in individual success, during which time no patients are scheduled. In week 1, we work with new hires to set expectations for performance, understand departmental norms, and introduce culture. Physicians meet formally and informally with department and institutional leadership, as well as attend team meetings and trainings that include a range of administrative and compliance requirements, such as quality standards and expectations, compliance, billing and coding specific to family medicine, EHR management, and institutionally mandated orientations. We are also adding implicit bias and antiracism training during this period, which are essential to creating a culture of unity and belonging.
During week 2, we focus on clinic-level orientation, assigning new hires an orientation buddy and a department sponsor, such as a physician lead or medical director. Physicians spend time with leadership at their clinic as they nurture relationships important for mentorship, sponsorship, and peer support. They also meet care team members, including front desk associates, medical assistants, behavioral health clinicians, nutritionists, social workers, pharmacists, and other key colleagues and care team members. This introduces the physician to the clinical environment and physical space as well as acclimates the physician to workflows and feedback loops for regular interaction.
When physicians ultimately begin patient care, they begin with an expected productivity rate of 50%, followed by an expected productivity rate of 75%, and then an expected productivity rate of 100%. This steady increase occurs over 3 to 4 weeks depending on the physician’s comfort level. They are also provided monthly reports on work relative value unit performance so that they can track and adapt practice patterns as necessary.More details on the program can be found in Appendix 1.
Takeaways From the Implementation of the New Program
Give time for new physicians to focus on acclimating to the role and environment.
The initial 2-week period of transition—without direct patient care—ensures that physicians feel comfortable in their new ecosystem. This also supports personal transitions, as many new hires are managing relocation and acclimating themselves and their families to new settings. Even residents from our training program who returned as attending physicians found this flexibility and slow reentry essential. This also gives the clinic time to orient to an additional provider, nurture them into the team culture, and develop relationships with the care team.
Cultivate spaces for shared learning, problem-solving, and peer connection.
Orientation is delivered primarily through group learning sessions with cohorts of new physicians, thus developing spaces for networking, fostering psychological safety, encouraging personal and professional rapport, emphasizing interactive learning, and reinforcing scheduling blocks at the departmental level. New hires also participate in peer shadowing to develop clinical competencies and are assigned a workplace buddy to foster a sense of belonging and create opportunities for additional knowledge sharing and cross-training.
Strengthen physician knowledge base, confidence, and comfort in the workplace before beginning direct patient care.
Without fluency in the workflows, culture, and operations of a practice, the urgency to have physicians begin clinical care can result in frustration for the physician, patients, and clinical and administrative staff. Therefore, we complete essential training prior to seeing any patients. This includes clinical workflows, referral processes, use of alternate modalities of care (eg, telehealth, eConsults), billing protocols, population health training, patient resources, office resources, and other essential daily processes and tools. This creates efficiency in administrative management, increased productivity, and better understanding of resources available for patients’ medical, social, and behavioral needs when patient care begins.
Embrace standardization, transparency, and accountability in as many processes as possible.
Standardized knowledge-sharing and checklists are mandated at every step of the orientation process, requiring sign off from the physician lead, practice manager, and new physicians upon completion. This offers all parties the opportunity to play a role in the delivery of and accountability for skills transfer and empowers new hires to press pause if they feel unsure about any domain in the training. It is also essential in guaranteeing that all physicians—regardless of which ambulatory location they practice in—receive consistent information and expectations. A sample checklist can be found in Appendix 2.
Commit to collecting and acting on feedback for continued program improvement and individual support.
As physicians complete the program, it is necessary to create structures to measure and enhance its impact, as well as evaluate how physicians are faring following the program. Each physician completes surveys at the end of the orientation program, attends a 90-day post-program check-in with the department chair, and receives follow-up trainings on advanced topics as they become more deeply embedded in the organization.
Lessons Learned
Feedback from surveys and 90-day check-ins with leadership and physicians reflect a high degree of clarity on job roles and duties, a sense of team camaraderie, easier system navigation, and a strong sense of support. We do recognize that sustaining change takes time and our study is limited by data demonstrating the impact of these efforts. We look forward to sharing more robust data from surveys and qualitative interviews with physicians, clinical leadership, and staff in the future. Our team will conduct interviews at 90-day and 180-day checkpoints with new physicians who have gone through this program, followed by a check-in after 1 year. Additionally, new physicians as well as key stakeholders, such as physician leads, practice managers, and members of the recruitment team, have started to participate in short surveys. These are designed to better understand their experiences, what worked well, what can be improved, and the overall satisfaction of the physician and other members of the extended care team.
What follows are some comments made by the initial group of physicians that went through this program and participated in follow-up interviews:
“I really feel like part of a bigger team.”
“I knew exactly what do to when I walked into the exam room on clinic Day 1.”
“It was great to make deep connections during the early process of joining.”
“Having a buddy to direct questions and ideas to is amazing and empowering.”
“Even though the orientation was long, I felt that I learned so much that I would not have otherwise.”
“Thank you for not letting me crash and burn!”
“Great culture! I love understanding our values of health equity, diversity, and inclusion.”
In the months since our endeavor began, we have learned just how essential it is to fully and effectively integrate new hires into the organization for their own satisfaction and success—and ours. Indeed, we cannot expect to achieve the Quadruple Aim without investing in the kind of transparent and intentional orientation process that defines expectations, aligns cultural values, mitigates costly and stressful operational misunderstandings, and communicates to physicians that, not only do they belong, but their sense of belonging is our priority. While we have yet to understand the impact of this program on the fourth aim of the Quadruple Aim, we are hopeful that the benefits will be far-reaching.
It is our ultimate hope that programs like this: (1) give physicians the confidence needed to create impactful patient-centered experiences; (2) enable physicians to become more cost-effective and efficient in care delivery; (3) allow physicians to understand the populations they are serving and access tools available to mitigate health disparities and other barriers; and (4) improve the collective experience of every member of the care team, practice leadership, and clinician-patient partnership.
Corresponding author: J. Nwando Olayiwola, MD, MPH, FAAFP, The Ohio State University College of Medicine, Department of Family and Community Medicine, 2231 N High St, Ste 250, Columbus, OH 43210; [email protected].
Financial disclosures: None.
Keywords: physician onboarding; Quadruple Aim; leadership; clinician satisfaction; care team satisfaction.
1. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6): 573-576.
2. Maurer R. Onboarding key to retaining, engaging talent. Society for Human Resource Management. April 16, 2015. Accessed January 8, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/onboarding-key-retaining-engaging-talent.aspx
3. Boston AG. New hire onboarding standardization and automation powers productivity gains. GlobeNewswire. March 8, 2011. Accessed January 8, 2021. http://www.globenewswire.com/news-release/2011/03/08/994239/0/en/New-Hire-Onboarding-Standardization-and-Automation-Powers-Productivity-Gains.html
4. $37 billion – US and UK business count the cost of employee misunderstanding. HR.com – Maximizing Human Potential. June 18, 2008. Accessed March 10, 2021. https://www.hr.com/en/communities/staffing_and_recruitment/37-billion---us-and-uk-businesses-count-the-cost-o_fhnduq4d.html
5. Employers risk driving new hires away with poor onboarding. Society for Human Resource Management. February 23, 2018. Accessed March 10, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/employers-new-hires-poor-onboarding.aspx
6. Center for Primary Care Innovation and Transformation. The Ohio State University College of Medicine. Accessed January 8, 2021. https://wexnermedical.osu.edu/departments/family-medicine/pcit
7. Olayiwola, J.N. and Magaña, C. Clinical transformation in technology: a fresh change management approach for primary care. Harvard Health Policy Review. February 2, 2019. Accessed March 10, 2021. http://www.hhpronline.org/articles/2019/2/2/clinical-transformation-in-technology-a-fresh-change-management-approach-for-primary-care
1. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6): 573-576.
2. Maurer R. Onboarding key to retaining, engaging talent. Society for Human Resource Management. April 16, 2015. Accessed January 8, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/onboarding-key-retaining-engaging-talent.aspx
3. Boston AG. New hire onboarding standardization and automation powers productivity gains. GlobeNewswire. March 8, 2011. Accessed January 8, 2021. http://www.globenewswire.com/news-release/2011/03/08/994239/0/en/New-Hire-Onboarding-Standardization-and-Automation-Powers-Productivity-Gains.html
4. $37 billion – US and UK business count the cost of employee misunderstanding. HR.com – Maximizing Human Potential. June 18, 2008. Accessed March 10, 2021. https://www.hr.com/en/communities/staffing_and_recruitment/37-billion---us-and-uk-businesses-count-the-cost-o_fhnduq4d.html
5. Employers risk driving new hires away with poor onboarding. Society for Human Resource Management. February 23, 2018. Accessed March 10, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/employers-new-hires-poor-onboarding.aspx
6. Center for Primary Care Innovation and Transformation. The Ohio State University College of Medicine. Accessed January 8, 2021. https://wexnermedical.osu.edu/departments/family-medicine/pcit
7. Olayiwola, J.N. and Magaña, C. Clinical transformation in technology: a fresh change management approach for primary care. Harvard Health Policy Review. February 2, 2019. Accessed March 10, 2021. http://www.hhpronline.org/articles/2019/2/2/clinical-transformation-in-technology-a-fresh-change-management-approach-for-primary-care
An Analysis of the Involvement and Attitudes of Resident Physicians in Reporting Errors in Patient Care
From Adelante Healthcare, Mesa, AZ (Dr. Chin), University Hospitals of Cleveland, Cleveland, OH (Drs. Delozier, Bascug, Levine, Bejanishvili, and Wynbrandt and Janet C. Peachey, Rachel M. Cerminara, and Sharon M. Darkovich), and Houston Methodist Hospitals, Houston, TX (Dr. Bhakta).
Abstract
Background: Resident physicians play an active role in the reporting of errors that occur in patient care. Previous studies indicate that residents significantly underreport errors in patient care.
Methods: Fifty-four of 80 eligible residents enrolled at University Hospitals–Regional Hospitals (UH-RH) during the 2018-2019 academic year completed a survey assessing their knowledge and experience in completing Patient Advocacy and Shared Stories (PASS) reports, which serve as incident reports in the UH health system in reporting errors in patient care. A series of interventions aimed at educating residents about the PASS report system were then conducted. The 54 residents who completed the first survey received it again 4 months later.
Results: Residents demonstrated greater understanding of when filing PASS reports was appropriate after the intervention, as significantly more residents reported having been involved in a situation where they should have filed a PASS report but did not (P = 0.036).
Conclusion: In this study, residents often did not report errors in patient care because they simply did not know the process for doing so. In addition, many residents often felt that the reporting of patient errors could be used as a form of retaliation.
Keywords: resident physicians; quality improvement; high-value care; medical errors; patient safety.
Resident physicians play a critical role in patient care. Residents undergo extensive supervised training in order to one day be able to practice medicine in an unsupervised setting, with the goal of providing the highest quality of care possible. One study reported that primary care provided by residents in a training program is of similar or higher quality than that provided by attending physicians.1
Besides providing high-quality care, it is important that residents play an active role in the reporting of errors that occur regarding patient care as well as in identifying events that may compromise patient safety and quality.2 In fact, increased reporting of patient errors has been shown to decrease liability-related costs for hospitals.3 Unfortunately, physicians, and residents in particular, have historically been poor reporters of errors in patient care.4 This is especially true when comparing physicians to other health professionals, such as nurses, in error reporting.5
Several studies have examined the involvement of residents in reporting errors in patient care. One recent study showed that a graduate medical education financial incentive program significantly increased the number of patient safety events reported by residents and fellows.6 This study, along with several others, supports the concept of using incentives to help improve the reporting of errors in patient care for physicians in training.7-10 Another study used Quality Improvement Knowledge Assessment Tool (QIKAT) scores to assess quality improvement (QI) knowledge. The study demonstrated that self-assessment scores of QI skills using QIKAT scores improved following a targeted intervention.11 Because further information on the involvement and attitudes of residents in reporting errors in patient care is needed, University Hospitals of Cleveland (UH) designed and implemented a QI study during the 2018-2019 academic year. This prospective study used anonymous surveys to objectively examine the involvement and attitudes of residents in reporting errors in patient care.
Methods
The UH health system uses Patient Advocacy and Shared Stories (PASS) reports as incident reports to not only disclose errors in patient care but also to identify any events that may compromise patient safety and quality. Based on preliminary review, nurses, ancillary staff, and administrators file the majority of PASS reports.
The study group consisted of residents at University Hospitals–Regional Hospitals (UH-RH), which is comprised of 2 hospitals: University Hospitals–Richmond Medical Center (UH-RMC) and University Hospitals –Bedford Medical Center (UH-BMC). UH-RMC and UH-BMC are 2 medium-sized university-affiliated community hospitals located in the Cleveland metropolitan area in Northeast Ohio. Both serve as clinical training sites for Case Western Reserve University School of Medicine and Lake Erie College of Osteopathic Medicine, the latter of which helped fund this study. The study was submitted to the Institutional Review Board (IRB) of University Hospitals of Cleveland and granted “not human subjects research” status as a QI study.
Surveys
UH-RH offers residency programs in dermatology, emergency medicine, family medicine, internal medicine, orthopedic surgery, and physical medicine and rehabilitation, along with a 1-year transitional/preliminary year. A total of 80 residents enrolled at UH-RH during the 2018-2019 academic year. All 80 residents at UH-RH received an email in December 2018 asking them to complete an anonymous survey regarding the PASS report system. The survey was administered using the REDCap software system and consisted of 15 multiple-choice questions. As an incentive for completing the survey, residents were offered a $10 Amazon gift card. The gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey. At the end of the week, 54 of 80 residents completed the first survey.
Following the first survey, efforts were undertaken by the study authors, in conjunction with the quality improvement department at UH-RH, to educate residents about the PASS report system. These interventions included giving a lecture on the PASS report system during resident didactic sessions, sending an email to all residents about the PASS report system, and providing residents an opportunity to complete an optional online training course regarding the PASS report system. As an incentive for completing the online training course, residents were offered a $10 Amazon gift card. As before, the gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine.
A second survey was administered in April 2019, 4 months after the first survey. To determine whether the intervention made an impact on the involvement and attitudes of residents in the reporting errors in patient care, only residents who completed the first survey were sent the second survey. The second survey consisted of the same questions as the first survey and was also administered using the REDCap software system. As an incentive for completing the survey, residents were offered another $10 Amazon gift card, again were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey.
Analysis
Chi-square analyses were utilized to examine differences between preintervention and postintervention responses across categories. All analyses were conducted using R statistical software, version 3.6.1 (R Foundation for Statistical Computing).
Results
A total of 54 of 80 eligible residents responded to the first survey (Table). Twenty-nine of 54 eligible residents responded to the second survey. Postintervention, significantly more residents indicated being involved in a situation where they should have filed a PASS report but did not (58.6% vs 53.7%; P = 0.036). Improvement was seen in PASS knowledge postintervention, where fewer residents reported not knowing how to file a PASS report (31.5% vs 55.2%; P = 0.059). No other improvements were significant, nor were there significant differences in responses between any other categories pre- and postintervention.
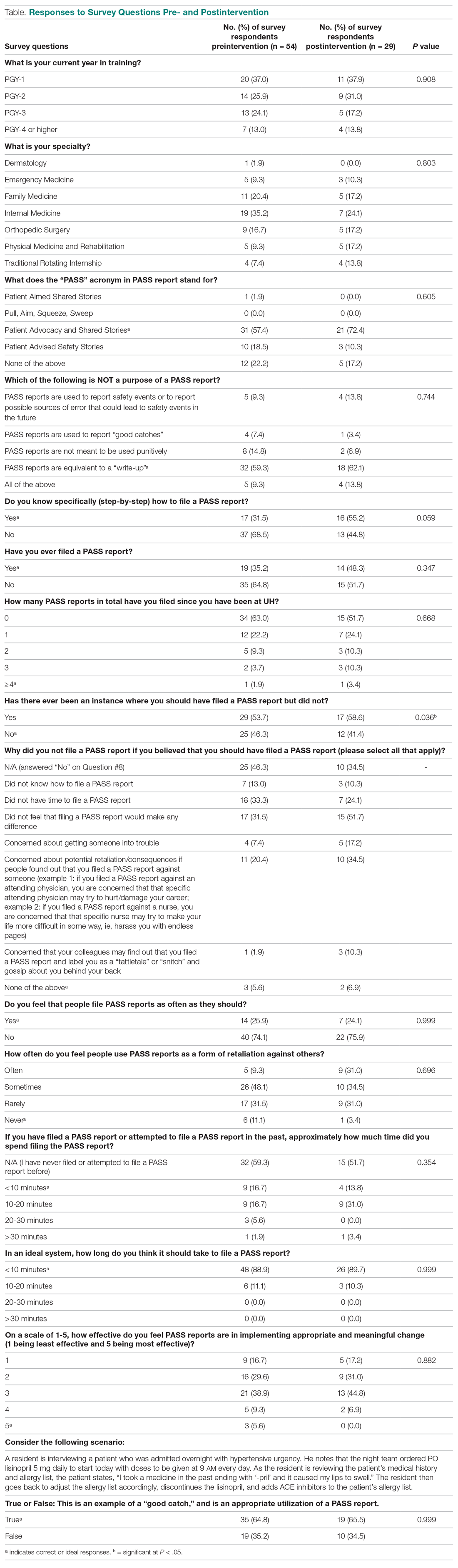
Discussion
Errors in patient care are a common occurrence in the hospital setting. Reporting errors when they happen is important for hospitals to gain data and better care for patients, but studies show that patient errors are usually underreported. This is concerning, as data on errors and other aspects of patient care are needed to inform quality improvement programs.
This study measured residents’ attitudes and knowledge regarding the filing of a PASS report. It also aimed to increase both the frequency of and knowledge about filing a PASS report with interventions. The results from each survey indicated a statistically significant increase in knowledge of when to file a PASS report. In the first survey, 53.7% of residents responded they they were involved in an instance where they should have filed a PASS report but did not. In the second survey, 58.5% of residents reported being involved in an instance where they should have filed a PASS report but did not. This difference was statistically significant (P = 0.036), sugesting that the intervention was successful at increasing residents’ knowledge regarding PASS reports and the appropriate times to file a PASS report.
The survey results also showed a trend toward increasing aggregate knowledge level of how to file PASS reports on the first survey and second surveys (from 31.5% vs 55.2%. This demonstrates an increase in knowledge of how to file a PASS report among residents at our hospital after the intervention. It should be noted that the intervention that was performed in this study was simple, easy to perform, and can be completed at any hospital system that uses a similar system for reporting patient errors.
Another important trend indicating the effectiveness of the intervention was a 15% increase in knowledge of what the PASS report acronym stands for, along with a 13.1% aggregate increase in the number of residents who filed a PASS report. This indicated that residents may have wanted to file a PASS report previously but simply did not know how to until the intervention. In addition, there was also a decrease in the aggregate percentages of residents who had never filed a PASS report and an increase in how many PASS reports were filed.
While PASS reports are a great way for hospitals to gain data and insight into problems at their sites, there was also a negative view of PASS reports. For example, a large percentage of residents indicated that filing a PASS report would not make any difference and that PASS reports are often used as a form of retaliation, either against themselves as the submitter or the person(s) mentioned in the PASS report. More specifically, more than 50% of residents felt that PASS reports were sometimes or often used as a form of retaliation against others. While many residents correctly identified in the survey that PASS reports are not equivalent to a “write-up,” it is concerning that they still feel there is a strong potential for retaliation when filing a PASS report. This finding is unfortunate but matches the results of a multicenter study that found that 44.6% of residents felt uncomfortable reporting patient errors, possibly secondary to fear of retaliation, along with issues with the reporting system.12
It is interesting to note that a minority of residents indicated that they feel that PASS reports are filed as often as they should be (25.9% on first survey and 24.1% on second survey). This is concerning, as the data gathered through PASS reports is used to improve patient care. However, the percentage reported in our study, although low, is higher than that reported in a similar study involving patients with Medicare insurance, which showed that only 14% of patient safety events were reported.13 These results demonstrate that further interventions are necessary in order to ensure that a PASS report is filed each time a patient safety event occurs.
Another finding of note is that the majority of residents also feel that the process of filing a PASS report is too time consuming. The majority of residents who have completed a PASS report stated that it took them between 10 and 20 minutes to complete a PASS report, but those same individuals also feel that it should take < 10 minutes to complete a PASS report. This is an important issue for hospital systems to address. Reducing the time it takes to file a PASS report may facilitate an increase in the amount of PASS reports filed.
We administered our surveys using email outreach to residents asking them to complete an anonymous online survey regarding the PASS report system using the REDCap software system. Researchers have various ways of administering surveys, ranging from paper surveys, emails, and even mobile apps. One study showed that online surveys tend to have higher response rates compared to non-online surveys, such as paper surveys and telephone surveys, which is likely due to the ease of use of online surveys.14 At the same time, unsolicited email surveys have been shown to have a negative influence on response rates. Mobile apps are a new way of administering surveys. However, research has not found any significant difference in the time required to complete the survey using mobile apps compared to other forms of administering surveys. In addition, surveys using mobile apps did not have increased response rates compared to other forms of administering surveys.15
To increase the response rate of our surveys, we offered gift cards to the study population for completing the survey. Studies have shown that surveys that offer incentives tend to have higher response rates than surveys that do not.16 Also, in addition to serving as a method for gathering data from our study population, we used our surveys as an intervention to increase awareness of PASS reporting, as reported in other studies. For example, another study used the HABITS questionnaire to not only gather information about children’s diet, but also to promote behavioral change towards healthy eating habits.17
This study had several limitations. First, the study was conducted using an anonymous online survey, which means we could not clarify questions that residents found confusing or needed further explanation. For example, 17 residents indicated in the first survey that they knew how to PASS report, but 19 residents indicated in the same survey that they have filed a PASS report in the past.
A second limitation of the study was that fewer residents completed the second survey (29 of 54 eligible residents) compared to the first survey (54 of 80 eligible residents). This may have impacted the results of the analysis, as certain findings were not statistically significant, despite trends in the data.
A third limitation of the study is that not all of the residents that completed the first and second surveys completed the entire intervention. For example, some residents did not attend the didactic lecture discussing PASS reports, and as such may not have received the appropriate training prior to completing the second survey.
The findings from this study can be used by the residency programs at UH-RH and by residency programs across the country to improve the involvement and attitudes of residents in reporting errors in patient care. Hospital staff need to be encouraged and educated on how to better report patient errors and the importance of reporting these errors. It would benefit hospital systems to provide continued and targeted training to familiarize physicians with the process of reporting patient errors, and take steps to reduce the time it takes to report patient errors. By increasing the reporting of errors, hospitals will be able to improve patient care through initiatives aimed at preventing errors.
Conclusion
Residents play an important role in providing high-quality care for patients. Part of providing high-quality care is the reporting of errors in patient care when they occur. Physicians, and in particular, residents, have historically underreported errors in patient care. Part of this underreporting results from residents not knowing or understanding the process of filing a report and feeling that the reports could be used as a form of retaliation. For hospital systems to continue to improve patient care, it is important for residents to not only know how to report errors in patient care but to feel comfortable doing so.
Corresponding author: Andrew J. Chin, DO, MS, MPH, Department of Internal Medicine, Adelante Healthcare, 1705 W Main St, Mesa, AZ 85201; [email protected].
Financial disclosures: None.
Funding: This study was funded by a research grant provided by Lake Eric College of Osteopathic Medicine to Andrew J. Chin and Anish Bhakta.
1. Zallman L, Ma J, Xiao L, Lasser KE. Quality of US primary care delivered by resident and staff physicians. J Gen Intern Med. 2010;25(11):1193-1197.
2. Bagain JP. The future of graduate medical education: a systems-based approach to ensure patient safety. Acad Med. 2015;90(9):1199-1202.
3. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical disclosure program. Ann Intern Med. 2010;153(4):213-221.
4. Kaldjian LC, Jones EW, Wu BJ, et al. Reporting medical errors to improve patient safety: a survey of physicians in teaching hospitals. Arch Intern Med. 2008;168(1):40-46.
5. Rowin EJ, Lucier D, Pauker SG, et al. Does error and adverse event reporting by physicians and nurses differ? Jt Comm J Qual Patient Saf. 2008;34(9):537-545.
6. Turner DA, Bae J, Cheely G, et al. Improving resident and fellow engagement in patient safety through a graduate medical education incentive program. J Grad Med Educ. 2018;10(6):671-675.
7. Macht R, Balen A, McAneny D, Hess D. A multifaceted intervention to increase surgery resident engagement in reporting adverse events. J Surg Educ. 2015;72(6):e117-e122.
8. Scott DR, Weimer M, English C, et al. A novel approach to increase residents’ involvement in reporting adverse events. Acad Med. 2011;86(6):742-746.
9. Stewart DA, Junn J, Adams MA, et al. House staff participation in patient safety reporting: identification of predominant barriers and implementation of a pilot program. South Med J. 2016;109(7):395-400.
10. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468.
11. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252.
12. Wijesekera TP, Sanders L, Windish DM. Education and reporting of diagnostic errors among physicians in internal medicine training programs. JAMA Intern Med. 2018;178(11):1548-1549.
13. Levinson DR. Hospital incident reporting systems do not capture most patient harm. Washington, D.C.: U.S. Department of Health and Human Services Office of the Inspector General. January 2012. Report No. OEI-06-09-00091.
14. Evans JR, Mathur A. The value of online surveys. Internet Research. 2005;15(2):192-219.
15. Marcano Belisario JS, Jamsek J, Huckvale K, et al. Comparison of self‐administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database of Syst Rev. 2015;7:MR000042.
16. Manfreda KL, Batagelj Z, Vehovar V. Design of web survey questionnaires: three basic experiments. J Comput Mediat Commun. 2002;7(3):JCMC731.
17. Wright ND, Groisman‐Perelstein AE, Wylie‐Rosett J, et al. A lifestyle assessment and intervention tool for pediatric weight management: the HABITS questionnaire. J Hum Nutr Diet. 2011;24(1):96-100.
From Adelante Healthcare, Mesa, AZ (Dr. Chin), University Hospitals of Cleveland, Cleveland, OH (Drs. Delozier, Bascug, Levine, Bejanishvili, and Wynbrandt and Janet C. Peachey, Rachel M. Cerminara, and Sharon M. Darkovich), and Houston Methodist Hospitals, Houston, TX (Dr. Bhakta).
Abstract
Background: Resident physicians play an active role in the reporting of errors that occur in patient care. Previous studies indicate that residents significantly underreport errors in patient care.
Methods: Fifty-four of 80 eligible residents enrolled at University Hospitals–Regional Hospitals (UH-RH) during the 2018-2019 academic year completed a survey assessing their knowledge and experience in completing Patient Advocacy and Shared Stories (PASS) reports, which serve as incident reports in the UH health system in reporting errors in patient care. A series of interventions aimed at educating residents about the PASS report system were then conducted. The 54 residents who completed the first survey received it again 4 months later.
Results: Residents demonstrated greater understanding of when filing PASS reports was appropriate after the intervention, as significantly more residents reported having been involved in a situation where they should have filed a PASS report but did not (P = 0.036).
Conclusion: In this study, residents often did not report errors in patient care because they simply did not know the process for doing so. In addition, many residents often felt that the reporting of patient errors could be used as a form of retaliation.
Keywords: resident physicians; quality improvement; high-value care; medical errors; patient safety.
Resident physicians play a critical role in patient care. Residents undergo extensive supervised training in order to one day be able to practice medicine in an unsupervised setting, with the goal of providing the highest quality of care possible. One study reported that primary care provided by residents in a training program is of similar or higher quality than that provided by attending physicians.1
Besides providing high-quality care, it is important that residents play an active role in the reporting of errors that occur regarding patient care as well as in identifying events that may compromise patient safety and quality.2 In fact, increased reporting of patient errors has been shown to decrease liability-related costs for hospitals.3 Unfortunately, physicians, and residents in particular, have historically been poor reporters of errors in patient care.4 This is especially true when comparing physicians to other health professionals, such as nurses, in error reporting.5
Several studies have examined the involvement of residents in reporting errors in patient care. One recent study showed that a graduate medical education financial incentive program significantly increased the number of patient safety events reported by residents and fellows.6 This study, along with several others, supports the concept of using incentives to help improve the reporting of errors in patient care for physicians in training.7-10 Another study used Quality Improvement Knowledge Assessment Tool (QIKAT) scores to assess quality improvement (QI) knowledge. The study demonstrated that self-assessment scores of QI skills using QIKAT scores improved following a targeted intervention.11 Because further information on the involvement and attitudes of residents in reporting errors in patient care is needed, University Hospitals of Cleveland (UH) designed and implemented a QI study during the 2018-2019 academic year. This prospective study used anonymous surveys to objectively examine the involvement and attitudes of residents in reporting errors in patient care.
Methods
The UH health system uses Patient Advocacy and Shared Stories (PASS) reports as incident reports to not only disclose errors in patient care but also to identify any events that may compromise patient safety and quality. Based on preliminary review, nurses, ancillary staff, and administrators file the majority of PASS reports.
The study group consisted of residents at University Hospitals–Regional Hospitals (UH-RH), which is comprised of 2 hospitals: University Hospitals–Richmond Medical Center (UH-RMC) and University Hospitals –Bedford Medical Center (UH-BMC). UH-RMC and UH-BMC are 2 medium-sized university-affiliated community hospitals located in the Cleveland metropolitan area in Northeast Ohio. Both serve as clinical training sites for Case Western Reserve University School of Medicine and Lake Erie College of Osteopathic Medicine, the latter of which helped fund this study. The study was submitted to the Institutional Review Board (IRB) of University Hospitals of Cleveland and granted “not human subjects research” status as a QI study.
Surveys
UH-RH offers residency programs in dermatology, emergency medicine, family medicine, internal medicine, orthopedic surgery, and physical medicine and rehabilitation, along with a 1-year transitional/preliminary year. A total of 80 residents enrolled at UH-RH during the 2018-2019 academic year. All 80 residents at UH-RH received an email in December 2018 asking them to complete an anonymous survey regarding the PASS report system. The survey was administered using the REDCap software system and consisted of 15 multiple-choice questions. As an incentive for completing the survey, residents were offered a $10 Amazon gift card. The gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey. At the end of the week, 54 of 80 residents completed the first survey.
Following the first survey, efforts were undertaken by the study authors, in conjunction with the quality improvement department at UH-RH, to educate residents about the PASS report system. These interventions included giving a lecture on the PASS report system during resident didactic sessions, sending an email to all residents about the PASS report system, and providing residents an opportunity to complete an optional online training course regarding the PASS report system. As an incentive for completing the online training course, residents were offered a $10 Amazon gift card. As before, the gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine.
A second survey was administered in April 2019, 4 months after the first survey. To determine whether the intervention made an impact on the involvement and attitudes of residents in the reporting errors in patient care, only residents who completed the first survey were sent the second survey. The second survey consisted of the same questions as the first survey and was also administered using the REDCap software system. As an incentive for completing the survey, residents were offered another $10 Amazon gift card, again were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey.
Analysis
Chi-square analyses were utilized to examine differences between preintervention and postintervention responses across categories. All analyses were conducted using R statistical software, version 3.6.1 (R Foundation for Statistical Computing).
Results
A total of 54 of 80 eligible residents responded to the first survey (Table). Twenty-nine of 54 eligible residents responded to the second survey. Postintervention, significantly more residents indicated being involved in a situation where they should have filed a PASS report but did not (58.6% vs 53.7%; P = 0.036). Improvement was seen in PASS knowledge postintervention, where fewer residents reported not knowing how to file a PASS report (31.5% vs 55.2%; P = 0.059). No other improvements were significant, nor were there significant differences in responses between any other categories pre- and postintervention.

Discussion
Errors in patient care are a common occurrence in the hospital setting. Reporting errors when they happen is important for hospitals to gain data and better care for patients, but studies show that patient errors are usually underreported. This is concerning, as data on errors and other aspects of patient care are needed to inform quality improvement programs.
This study measured residents’ attitudes and knowledge regarding the filing of a PASS report. It also aimed to increase both the frequency of and knowledge about filing a PASS report with interventions. The results from each survey indicated a statistically significant increase in knowledge of when to file a PASS report. In the first survey, 53.7% of residents responded they they were involved in an instance where they should have filed a PASS report but did not. In the second survey, 58.5% of residents reported being involved in an instance where they should have filed a PASS report but did not. This difference was statistically significant (P = 0.036), sugesting that the intervention was successful at increasing residents’ knowledge regarding PASS reports and the appropriate times to file a PASS report.
The survey results also showed a trend toward increasing aggregate knowledge level of how to file PASS reports on the first survey and second surveys (from 31.5% vs 55.2%. This demonstrates an increase in knowledge of how to file a PASS report among residents at our hospital after the intervention. It should be noted that the intervention that was performed in this study was simple, easy to perform, and can be completed at any hospital system that uses a similar system for reporting patient errors.
Another important trend indicating the effectiveness of the intervention was a 15% increase in knowledge of what the PASS report acronym stands for, along with a 13.1% aggregate increase in the number of residents who filed a PASS report. This indicated that residents may have wanted to file a PASS report previously but simply did not know how to until the intervention. In addition, there was also a decrease in the aggregate percentages of residents who had never filed a PASS report and an increase in how many PASS reports were filed.
While PASS reports are a great way for hospitals to gain data and insight into problems at their sites, there was also a negative view of PASS reports. For example, a large percentage of residents indicated that filing a PASS report would not make any difference and that PASS reports are often used as a form of retaliation, either against themselves as the submitter or the person(s) mentioned in the PASS report. More specifically, more than 50% of residents felt that PASS reports were sometimes or often used as a form of retaliation against others. While many residents correctly identified in the survey that PASS reports are not equivalent to a “write-up,” it is concerning that they still feel there is a strong potential for retaliation when filing a PASS report. This finding is unfortunate but matches the results of a multicenter study that found that 44.6% of residents felt uncomfortable reporting patient errors, possibly secondary to fear of retaliation, along with issues with the reporting system.12
It is interesting to note that a minority of residents indicated that they feel that PASS reports are filed as often as they should be (25.9% on first survey and 24.1% on second survey). This is concerning, as the data gathered through PASS reports is used to improve patient care. However, the percentage reported in our study, although low, is higher than that reported in a similar study involving patients with Medicare insurance, which showed that only 14% of patient safety events were reported.13 These results demonstrate that further interventions are necessary in order to ensure that a PASS report is filed each time a patient safety event occurs.
Another finding of note is that the majority of residents also feel that the process of filing a PASS report is too time consuming. The majority of residents who have completed a PASS report stated that it took them between 10 and 20 minutes to complete a PASS report, but those same individuals also feel that it should take < 10 minutes to complete a PASS report. This is an important issue for hospital systems to address. Reducing the time it takes to file a PASS report may facilitate an increase in the amount of PASS reports filed.
We administered our surveys using email outreach to residents asking them to complete an anonymous online survey regarding the PASS report system using the REDCap software system. Researchers have various ways of administering surveys, ranging from paper surveys, emails, and even mobile apps. One study showed that online surveys tend to have higher response rates compared to non-online surveys, such as paper surveys and telephone surveys, which is likely due to the ease of use of online surveys.14 At the same time, unsolicited email surveys have been shown to have a negative influence on response rates. Mobile apps are a new way of administering surveys. However, research has not found any significant difference in the time required to complete the survey using mobile apps compared to other forms of administering surveys. In addition, surveys using mobile apps did not have increased response rates compared to other forms of administering surveys.15
To increase the response rate of our surveys, we offered gift cards to the study population for completing the survey. Studies have shown that surveys that offer incentives tend to have higher response rates than surveys that do not.16 Also, in addition to serving as a method for gathering data from our study population, we used our surveys as an intervention to increase awareness of PASS reporting, as reported in other studies. For example, another study used the HABITS questionnaire to not only gather information about children’s diet, but also to promote behavioral change towards healthy eating habits.17
This study had several limitations. First, the study was conducted using an anonymous online survey, which means we could not clarify questions that residents found confusing or needed further explanation. For example, 17 residents indicated in the first survey that they knew how to PASS report, but 19 residents indicated in the same survey that they have filed a PASS report in the past.
A second limitation of the study was that fewer residents completed the second survey (29 of 54 eligible residents) compared to the first survey (54 of 80 eligible residents). This may have impacted the results of the analysis, as certain findings were not statistically significant, despite trends in the data.
A third limitation of the study is that not all of the residents that completed the first and second surveys completed the entire intervention. For example, some residents did not attend the didactic lecture discussing PASS reports, and as such may not have received the appropriate training prior to completing the second survey.
The findings from this study can be used by the residency programs at UH-RH and by residency programs across the country to improve the involvement and attitudes of residents in reporting errors in patient care. Hospital staff need to be encouraged and educated on how to better report patient errors and the importance of reporting these errors. It would benefit hospital systems to provide continued and targeted training to familiarize physicians with the process of reporting patient errors, and take steps to reduce the time it takes to report patient errors. By increasing the reporting of errors, hospitals will be able to improve patient care through initiatives aimed at preventing errors.
Conclusion
Residents play an important role in providing high-quality care for patients. Part of providing high-quality care is the reporting of errors in patient care when they occur. Physicians, and in particular, residents, have historically underreported errors in patient care. Part of this underreporting results from residents not knowing or understanding the process of filing a report and feeling that the reports could be used as a form of retaliation. For hospital systems to continue to improve patient care, it is important for residents to not only know how to report errors in patient care but to feel comfortable doing so.
Corresponding author: Andrew J. Chin, DO, MS, MPH, Department of Internal Medicine, Adelante Healthcare, 1705 W Main St, Mesa, AZ 85201; [email protected].
Financial disclosures: None.
Funding: This study was funded by a research grant provided by Lake Eric College of Osteopathic Medicine to Andrew J. Chin and Anish Bhakta.
From Adelante Healthcare, Mesa, AZ (Dr. Chin), University Hospitals of Cleveland, Cleveland, OH (Drs. Delozier, Bascug, Levine, Bejanishvili, and Wynbrandt and Janet C. Peachey, Rachel M. Cerminara, and Sharon M. Darkovich), and Houston Methodist Hospitals, Houston, TX (Dr. Bhakta).
Abstract
Background: Resident physicians play an active role in the reporting of errors that occur in patient care. Previous studies indicate that residents significantly underreport errors in patient care.
Methods: Fifty-four of 80 eligible residents enrolled at University Hospitals–Regional Hospitals (UH-RH) during the 2018-2019 academic year completed a survey assessing their knowledge and experience in completing Patient Advocacy and Shared Stories (PASS) reports, which serve as incident reports in the UH health system in reporting errors in patient care. A series of interventions aimed at educating residents about the PASS report system were then conducted. The 54 residents who completed the first survey received it again 4 months later.
Results: Residents demonstrated greater understanding of when filing PASS reports was appropriate after the intervention, as significantly more residents reported having been involved in a situation where they should have filed a PASS report but did not (P = 0.036).
Conclusion: In this study, residents often did not report errors in patient care because they simply did not know the process for doing so. In addition, many residents often felt that the reporting of patient errors could be used as a form of retaliation.
Keywords: resident physicians; quality improvement; high-value care; medical errors; patient safety.
Resident physicians play a critical role in patient care. Residents undergo extensive supervised training in order to one day be able to practice medicine in an unsupervised setting, with the goal of providing the highest quality of care possible. One study reported that primary care provided by residents in a training program is of similar or higher quality than that provided by attending physicians.1
Besides providing high-quality care, it is important that residents play an active role in the reporting of errors that occur regarding patient care as well as in identifying events that may compromise patient safety and quality.2 In fact, increased reporting of patient errors has been shown to decrease liability-related costs for hospitals.3 Unfortunately, physicians, and residents in particular, have historically been poor reporters of errors in patient care.4 This is especially true when comparing physicians to other health professionals, such as nurses, in error reporting.5
Several studies have examined the involvement of residents in reporting errors in patient care. One recent study showed that a graduate medical education financial incentive program significantly increased the number of patient safety events reported by residents and fellows.6 This study, along with several others, supports the concept of using incentives to help improve the reporting of errors in patient care for physicians in training.7-10 Another study used Quality Improvement Knowledge Assessment Tool (QIKAT) scores to assess quality improvement (QI) knowledge. The study demonstrated that self-assessment scores of QI skills using QIKAT scores improved following a targeted intervention.11 Because further information on the involvement and attitudes of residents in reporting errors in patient care is needed, University Hospitals of Cleveland (UH) designed and implemented a QI study during the 2018-2019 academic year. This prospective study used anonymous surveys to objectively examine the involvement and attitudes of residents in reporting errors in patient care.
Methods
The UH health system uses Patient Advocacy and Shared Stories (PASS) reports as incident reports to not only disclose errors in patient care but also to identify any events that may compromise patient safety and quality. Based on preliminary review, nurses, ancillary staff, and administrators file the majority of PASS reports.
The study group consisted of residents at University Hospitals–Regional Hospitals (UH-RH), which is comprised of 2 hospitals: University Hospitals–Richmond Medical Center (UH-RMC) and University Hospitals –Bedford Medical Center (UH-BMC). UH-RMC and UH-BMC are 2 medium-sized university-affiliated community hospitals located in the Cleveland metropolitan area in Northeast Ohio. Both serve as clinical training sites for Case Western Reserve University School of Medicine and Lake Erie College of Osteopathic Medicine, the latter of which helped fund this study. The study was submitted to the Institutional Review Board (IRB) of University Hospitals of Cleveland and granted “not human subjects research” status as a QI study.
Surveys
UH-RH offers residency programs in dermatology, emergency medicine, family medicine, internal medicine, orthopedic surgery, and physical medicine and rehabilitation, along with a 1-year transitional/preliminary year. A total of 80 residents enrolled at UH-RH during the 2018-2019 academic year. All 80 residents at UH-RH received an email in December 2018 asking them to complete an anonymous survey regarding the PASS report system. The survey was administered using the REDCap software system and consisted of 15 multiple-choice questions. As an incentive for completing the survey, residents were offered a $10 Amazon gift card. The gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey. At the end of the week, 54 of 80 residents completed the first survey.
Following the first survey, efforts were undertaken by the study authors, in conjunction with the quality improvement department at UH-RH, to educate residents about the PASS report system. These interventions included giving a lecture on the PASS report system during resident didactic sessions, sending an email to all residents about the PASS report system, and providing residents an opportunity to complete an optional online training course regarding the PASS report system. As an incentive for completing the online training course, residents were offered a $10 Amazon gift card. As before, the gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine.
A second survey was administered in April 2019, 4 months after the first survey. To determine whether the intervention made an impact on the involvement and attitudes of residents in the reporting errors in patient care, only residents who completed the first survey were sent the second survey. The second survey consisted of the same questions as the first survey and was also administered using the REDCap software system. As an incentive for completing the survey, residents were offered another $10 Amazon gift card, again were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey.
Analysis
Chi-square analyses were utilized to examine differences between preintervention and postintervention responses across categories. All analyses were conducted using R statistical software, version 3.6.1 (R Foundation for Statistical Computing).
Results
A total of 54 of 80 eligible residents responded to the first survey (Table). Twenty-nine of 54 eligible residents responded to the second survey. Postintervention, significantly more residents indicated being involved in a situation where they should have filed a PASS report but did not (58.6% vs 53.7%; P = 0.036). Improvement was seen in PASS knowledge postintervention, where fewer residents reported not knowing how to file a PASS report (31.5% vs 55.2%; P = 0.059). No other improvements were significant, nor were there significant differences in responses between any other categories pre- and postintervention.

Discussion
Errors in patient care are a common occurrence in the hospital setting. Reporting errors when they happen is important for hospitals to gain data and better care for patients, but studies show that patient errors are usually underreported. This is concerning, as data on errors and other aspects of patient care are needed to inform quality improvement programs.
This study measured residents’ attitudes and knowledge regarding the filing of a PASS report. It also aimed to increase both the frequency of and knowledge about filing a PASS report with interventions. The results from each survey indicated a statistically significant increase in knowledge of when to file a PASS report. In the first survey, 53.7% of residents responded they they were involved in an instance where they should have filed a PASS report but did not. In the second survey, 58.5% of residents reported being involved in an instance where they should have filed a PASS report but did not. This difference was statistically significant (P = 0.036), sugesting that the intervention was successful at increasing residents’ knowledge regarding PASS reports and the appropriate times to file a PASS report.
The survey results also showed a trend toward increasing aggregate knowledge level of how to file PASS reports on the first survey and second surveys (from 31.5% vs 55.2%. This demonstrates an increase in knowledge of how to file a PASS report among residents at our hospital after the intervention. It should be noted that the intervention that was performed in this study was simple, easy to perform, and can be completed at any hospital system that uses a similar system for reporting patient errors.
Another important trend indicating the effectiveness of the intervention was a 15% increase in knowledge of what the PASS report acronym stands for, along with a 13.1% aggregate increase in the number of residents who filed a PASS report. This indicated that residents may have wanted to file a PASS report previously but simply did not know how to until the intervention. In addition, there was also a decrease in the aggregate percentages of residents who had never filed a PASS report and an increase in how many PASS reports were filed.
While PASS reports are a great way for hospitals to gain data and insight into problems at their sites, there was also a negative view of PASS reports. For example, a large percentage of residents indicated that filing a PASS report would not make any difference and that PASS reports are often used as a form of retaliation, either against themselves as the submitter or the person(s) mentioned in the PASS report. More specifically, more than 50% of residents felt that PASS reports were sometimes or often used as a form of retaliation against others. While many residents correctly identified in the survey that PASS reports are not equivalent to a “write-up,” it is concerning that they still feel there is a strong potential for retaliation when filing a PASS report. This finding is unfortunate but matches the results of a multicenter study that found that 44.6% of residents felt uncomfortable reporting patient errors, possibly secondary to fear of retaliation, along with issues with the reporting system.12
It is interesting to note that a minority of residents indicated that they feel that PASS reports are filed as often as they should be (25.9% on first survey and 24.1% on second survey). This is concerning, as the data gathered through PASS reports is used to improve patient care. However, the percentage reported in our study, although low, is higher than that reported in a similar study involving patients with Medicare insurance, which showed that only 14% of patient safety events were reported.13 These results demonstrate that further interventions are necessary in order to ensure that a PASS report is filed each time a patient safety event occurs.
Another finding of note is that the majority of residents also feel that the process of filing a PASS report is too time consuming. The majority of residents who have completed a PASS report stated that it took them between 10 and 20 minutes to complete a PASS report, but those same individuals also feel that it should take < 10 minutes to complete a PASS report. This is an important issue for hospital systems to address. Reducing the time it takes to file a PASS report may facilitate an increase in the amount of PASS reports filed.
We administered our surveys using email outreach to residents asking them to complete an anonymous online survey regarding the PASS report system using the REDCap software system. Researchers have various ways of administering surveys, ranging from paper surveys, emails, and even mobile apps. One study showed that online surveys tend to have higher response rates compared to non-online surveys, such as paper surveys and telephone surveys, which is likely due to the ease of use of online surveys.14 At the same time, unsolicited email surveys have been shown to have a negative influence on response rates. Mobile apps are a new way of administering surveys. However, research has not found any significant difference in the time required to complete the survey using mobile apps compared to other forms of administering surveys. In addition, surveys using mobile apps did not have increased response rates compared to other forms of administering surveys.15
To increase the response rate of our surveys, we offered gift cards to the study population for completing the survey. Studies have shown that surveys that offer incentives tend to have higher response rates than surveys that do not.16 Also, in addition to serving as a method for gathering data from our study population, we used our surveys as an intervention to increase awareness of PASS reporting, as reported in other studies. For example, another study used the HABITS questionnaire to not only gather information about children’s diet, but also to promote behavioral change towards healthy eating habits.17
This study had several limitations. First, the study was conducted using an anonymous online survey, which means we could not clarify questions that residents found confusing or needed further explanation. For example, 17 residents indicated in the first survey that they knew how to PASS report, but 19 residents indicated in the same survey that they have filed a PASS report in the past.
A second limitation of the study was that fewer residents completed the second survey (29 of 54 eligible residents) compared to the first survey (54 of 80 eligible residents). This may have impacted the results of the analysis, as certain findings were not statistically significant, despite trends in the data.
A third limitation of the study is that not all of the residents that completed the first and second surveys completed the entire intervention. For example, some residents did not attend the didactic lecture discussing PASS reports, and as such may not have received the appropriate training prior to completing the second survey.
The findings from this study can be used by the residency programs at UH-RH and by residency programs across the country to improve the involvement and attitudes of residents in reporting errors in patient care. Hospital staff need to be encouraged and educated on how to better report patient errors and the importance of reporting these errors. It would benefit hospital systems to provide continued and targeted training to familiarize physicians with the process of reporting patient errors, and take steps to reduce the time it takes to report patient errors. By increasing the reporting of errors, hospitals will be able to improve patient care through initiatives aimed at preventing errors.
Conclusion
Residents play an important role in providing high-quality care for patients. Part of providing high-quality care is the reporting of errors in patient care when they occur. Physicians, and in particular, residents, have historically underreported errors in patient care. Part of this underreporting results from residents not knowing or understanding the process of filing a report and feeling that the reports could be used as a form of retaliation. For hospital systems to continue to improve patient care, it is important for residents to not only know how to report errors in patient care but to feel comfortable doing so.
Corresponding author: Andrew J. Chin, DO, MS, MPH, Department of Internal Medicine, Adelante Healthcare, 1705 W Main St, Mesa, AZ 85201; [email protected].
Financial disclosures: None.
Funding: This study was funded by a research grant provided by Lake Eric College of Osteopathic Medicine to Andrew J. Chin and Anish Bhakta.
1. Zallman L, Ma J, Xiao L, Lasser KE. Quality of US primary care delivered by resident and staff physicians. J Gen Intern Med. 2010;25(11):1193-1197.
2. Bagain JP. The future of graduate medical education: a systems-based approach to ensure patient safety. Acad Med. 2015;90(9):1199-1202.
3. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical disclosure program. Ann Intern Med. 2010;153(4):213-221.
4. Kaldjian LC, Jones EW, Wu BJ, et al. Reporting medical errors to improve patient safety: a survey of physicians in teaching hospitals. Arch Intern Med. 2008;168(1):40-46.
5. Rowin EJ, Lucier D, Pauker SG, et al. Does error and adverse event reporting by physicians and nurses differ? Jt Comm J Qual Patient Saf. 2008;34(9):537-545.
6. Turner DA, Bae J, Cheely G, et al. Improving resident and fellow engagement in patient safety through a graduate medical education incentive program. J Grad Med Educ. 2018;10(6):671-675.
7. Macht R, Balen A, McAneny D, Hess D. A multifaceted intervention to increase surgery resident engagement in reporting adverse events. J Surg Educ. 2015;72(6):e117-e122.
8. Scott DR, Weimer M, English C, et al. A novel approach to increase residents’ involvement in reporting adverse events. Acad Med. 2011;86(6):742-746.
9. Stewart DA, Junn J, Adams MA, et al. House staff participation in patient safety reporting: identification of predominant barriers and implementation of a pilot program. South Med J. 2016;109(7):395-400.
10. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468.
11. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252.
12. Wijesekera TP, Sanders L, Windish DM. Education and reporting of diagnostic errors among physicians in internal medicine training programs. JAMA Intern Med. 2018;178(11):1548-1549.
13. Levinson DR. Hospital incident reporting systems do not capture most patient harm. Washington, D.C.: U.S. Department of Health and Human Services Office of the Inspector General. January 2012. Report No. OEI-06-09-00091.
14. Evans JR, Mathur A. The value of online surveys. Internet Research. 2005;15(2):192-219.
15. Marcano Belisario JS, Jamsek J, Huckvale K, et al. Comparison of self‐administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database of Syst Rev. 2015;7:MR000042.
16. Manfreda KL, Batagelj Z, Vehovar V. Design of web survey questionnaires: three basic experiments. J Comput Mediat Commun. 2002;7(3):JCMC731.
17. Wright ND, Groisman‐Perelstein AE, Wylie‐Rosett J, et al. A lifestyle assessment and intervention tool for pediatric weight management: the HABITS questionnaire. J Hum Nutr Diet. 2011;24(1):96-100.
1. Zallman L, Ma J, Xiao L, Lasser KE. Quality of US primary care delivered by resident and staff physicians. J Gen Intern Med. 2010;25(11):1193-1197.
2. Bagain JP. The future of graduate medical education: a systems-based approach to ensure patient safety. Acad Med. 2015;90(9):1199-1202.
3. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical disclosure program. Ann Intern Med. 2010;153(4):213-221.
4. Kaldjian LC, Jones EW, Wu BJ, et al. Reporting medical errors to improve patient safety: a survey of physicians in teaching hospitals. Arch Intern Med. 2008;168(1):40-46.
5. Rowin EJ, Lucier D, Pauker SG, et al. Does error and adverse event reporting by physicians and nurses differ? Jt Comm J Qual Patient Saf. 2008;34(9):537-545.
6. Turner DA, Bae J, Cheely G, et al. Improving resident and fellow engagement in patient safety through a graduate medical education incentive program. J Grad Med Educ. 2018;10(6):671-675.
7. Macht R, Balen A, McAneny D, Hess D. A multifaceted intervention to increase surgery resident engagement in reporting adverse events. J Surg Educ. 2015;72(6):e117-e122.
8. Scott DR, Weimer M, English C, et al. A novel approach to increase residents’ involvement in reporting adverse events. Acad Med. 2011;86(6):742-746.
9. Stewart DA, Junn J, Adams MA, et al. House staff participation in patient safety reporting: identification of predominant barriers and implementation of a pilot program. South Med J. 2016;109(7):395-400.
10. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468.
11. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252.
12. Wijesekera TP, Sanders L, Windish DM. Education and reporting of diagnostic errors among physicians in internal medicine training programs. JAMA Intern Med. 2018;178(11):1548-1549.
13. Levinson DR. Hospital incident reporting systems do not capture most patient harm. Washington, D.C.: U.S. Department of Health and Human Services Office of the Inspector General. January 2012. Report No. OEI-06-09-00091.
14. Evans JR, Mathur A. The value of online surveys. Internet Research. 2005;15(2):192-219.
15. Marcano Belisario JS, Jamsek J, Huckvale K, et al. Comparison of self‐administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database of Syst Rev. 2015;7:MR000042.
16. Manfreda KL, Batagelj Z, Vehovar V. Design of web survey questionnaires: three basic experiments. J Comput Mediat Commun. 2002;7(3):JCMC731.
17. Wright ND, Groisman‐Perelstein AE, Wylie‐Rosett J, et al. A lifestyle assessment and intervention tool for pediatric weight management: the HABITS questionnaire. J Hum Nutr Diet. 2011;24(1):96-100.