User login
Variation in COVID-19 Mortality Across 117 US Hospitals in High- and Low-Burden Settings
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). 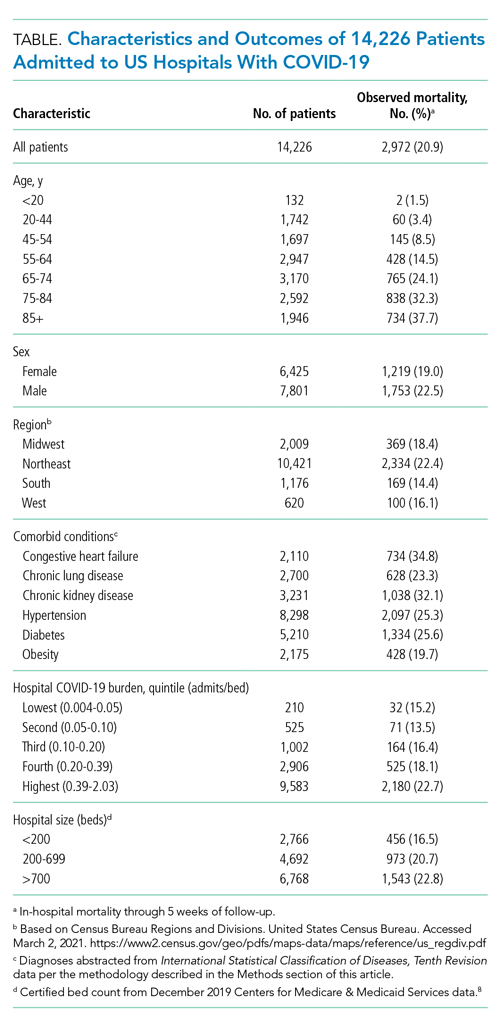

Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on interhospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
ACKNOWLEGMENTS
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
1. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Centers for Disease Control and Prevention. Updated November 2, 2020. Accessed December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2
3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1-12. https://doi.org/10.1001/jamainternmed.2020.4568
4. Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1-3. https://doi.org/10.1007/s11606-020-06084-7
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention. Accessed June 2, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
6. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
7. About the American Community Survey. United States Census Bureau. Updated January 4, 2021. Accessed March 2, 2021. https://www.census.gov/programs-surveys/acs/about.html
8. Provider of service files. Centers for Medicare & Medicaid Services. Revised January 15, 2020. Accessed March 2, 2021. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file
9. Ash AS, Fienberg SE, Louis TA, et al. Statistical issues in assessing hospital performance. Committee of Presidents of Statistical Societies white paper. January 2012. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf
10. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;12;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401
11. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33-41. https://doi.org/10.7326/M20-3905
12. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/NEJMsa2011686
13. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. https://doi.org/10.1056/NEJMoa2004500
14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
15. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). 

Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on interhospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
ACKNOWLEGMENTS
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
It is clear that certain patient-level factors, such as age, sex, and comorbidities, predict outcomes of SARS-CoV-2 infection.1,2 Less is known about whether hospital-level factors, including surges of patients with COVID-19, are associated with patient outcomes.
In a multicenter cohort study of 2,215 patients with COVID-19 in 65 intensive care units (ICU) across the United States, mortality rates varied widely (6.6%-80.8%), with improved survival for patients admitted to a hospital with more (>100) rather than fewer (<50) ICU beds.3 A different study found that at the state level, COVID-19 mortality increased with increasing COVID-19 admissions.4 Together, these studies suggest that surges in COVID-19 patient volume may be associated with excess mortality. However, the first study was restricted to the ICU population, limiting generalizability, and did not consider admission volume, only ICU bed count. Meanwhile, the second study considered both hospital capacity and patient volume, but it describes a relatively small sample, did not adjust for patient-level predictors of mortality, and does not report outcomes at the hospital level.
Here, we used a large dataset to compare in-hospital mortality rates for patients with COVID-19 across US hospitals, hypothesizing that mortality would be higher in hospitals with the highest burden of COVID-19 admissions. By adjusting for patient-level predictors of mortality and normalizing admission volume for hospital size, we are able to describe residual variability in mortality that may be attributable to differences in COVID-19 patient volume.
METHODS
We included patients with an International Statistical Classification of Diseases, Tenth Revision (ICD)-10 diagnosis of COVID-19 (U07.1) who were admitted to a US hospital that contracts with CarePort Health.5 CarePort is a platform for discharge planning and care coordination that contracts with hospitals in all US regions and auto-extracts data using interface feeds.
We restricted the population to patients admitted between April 1 and April 30, 2020, after a new ICD-10 code for confirmed COVID-19 infection became available, and to hospitals that provided real-time ICD-10 data and pertinent demographic information and could be linked to Centers for Medicare & Medicaid Services (CMS) data by National Provider Identifier. We assumed that the 145 patients (1.0%) who remained hospitalized at 5 weeks all survived. For the 5.9% of patients with multiple admissions during the study period, we included only the first admission with a diagnosis code for COVID-19.
We adjusted for patient age, sex, and the 31 comorbidities in the Elixhauser index, defined by ICD-10 codes. This set of comorbidities includes those previously associated with COVID-19 survival.1,2,6 Unfortunately, inconsistent reporting of vital signs and laboratory data precluded adjusting for acute illness severity. For those patients whose residence zip code was known, we report the racial breakdown (White vs non-White) and adjusted gross income (AGI), based on linked information from the 2018 American Community Survey.7
We defined COVID-19 burden as the quotient of COVID-19 admissions in April 2020 and each hospital’s certified bed count, as reported to the CMS.8 This allowed us to normalize COVID-19 patient volume for variation in hospital size, acknowledging that admitting 10 patients with COVID-19 to a 1,000-bed hospital is different from admitting 10 patients with COVID-19 to a 20-bed hospital. Certified bed count seemed the ideal denominator because it excludes beds not readily deployable to care for patients with COVID-19 (eg, radiology suites, labor and delivery rooms).
We computed hospital-specific adjusted mortality proportions and 95% confidence intervals based on hierarchical multivariable logistic regression, adjusting for age, sex, and comorbidities, and a random effect for each hospital.9,10 Hypothesizing that there may be a threshold of burden beyond which mortality begins to rise, we compared the in-hospital mortality rate at hospitals in the highest quintile of COVID-19 burden to all other hospitals.
We conducted eight post-hoc sensitivity analyses: (1) restricting the study population to patients aged 75 years and older; (2) restricting study hospitals to those with at least 100 beds and 20 COVID-19 admissions; (3) assuming that all patients who remained hospitalized at 5 weeks had died; (4) using each patient’s last admission during the month of April rather than the first; sequentially incorporating (5) zip code–level information on race (limited to White, non-White) and (6) AGI (treated as a continuous variable) into our model; (7) computing two burdens for each hospital (one for each half of April) and using whichever was higher; and (8) treating COVID-19 burden as a continuous predictor. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc) using the GLIMMIX procedure. This study was deemed exempt by the University of California, San Francisco Institutional Review Board.
RESULTS
The study population included 14,226 patients with COVID-19 (median age, 66 years [range, 0-110 years]; 45.2% women) at 117 US hospitals. Based on patients’ zip code of residence, we estimate that 47.0% of patients were White and 29.1% Black, and that the mean household AGI was $61,956. Most hospitals were nonprofit (56%) or private (39%), with approximately one quarter coming from each US census region (range, 25 hospitals [21%] in Midwest to 33 hospitals [28%] in Northeast). Nine hospitals (8%) had more than 700 beds, 40 (34%) had 300 to 700 beds, and 68 (58%) had fewer than 300 beds. Thirty-six hospitals (30.8%) admitted fewer than 20 patients with COVID-19, while six hospitals (5.1%) admitted more than 500 such patients. COVID burden ranged from 0.004 to 2.03 admissions per bed.
As of June 5, 2020, 78.1% of patients had been discharged alive, 20.9% had died, and 1.0% remained hospitalized. At the hospital level, the observed mortality ranged from 0% to 44.4%, was 17.1% among hospitals in COVID-19 burden quintiles one through four, and was 22.7% in the highest burden quintile (Table). 

Results were similar across multiple sensitivity analyses (see Appendix Table), although the relationship between COVID-19 burden and in-hospital mortality was attenuated and not significant when the sample was restricted to hospitals with at least 100 beds and 20 COVID-19 admissions, or in analyses adjusted for race and AGI.
DISCUSSION
In this study of 14,226 patients with COVID-19 across 117 US hospitals, those patients admitted to the most burdened hospitals had a higher odds of death. This relationship, which persisted after adjusting for age, sex, and comorbid conditions, suggests that a threshold exists at which patient surges may cause excess mortality.
Notably, in sensitivity analyses adjusting for race and AGI, COVID-19 burden was no longer associated with in-hospital mortality and the point estimate was attenuated. This raises the possibility that our primary results are confounded by these factors. However, prior studies of hospitalized patients have not found race to be predictive of mortality, after adjusting for other factors.11,12
We also note that the relationship between COVID-19 burden and mortality was not significant (P = .07) when the sample was restricted to larger hospitals with more than 20 COVID-19 admissions; again, the point estimate was attenuated. This suggests that larger hospitals may be more resilient in the face of patient surges. Whether this is due to increased availability of staff who can be redeployed to patient care (as with researchers at academic centers), increased experience managing severe respiratory failure, or other factors is uncertain.
Interestingly, in-hospital mortality varied widely across study hospitals, even among the most-burdened hospitals. The reasons for this residual variability—after adjusting for age, sex, and comorbidities and stratifying by COVID-19 burden—are uncertain. To the extent that this variability reflects differences in patient management, hospital staffing, or use of investigational or advanced therapies, it will be critical to identify and disseminate any replicable best practices from high-burden hospitals with low mortality rates.
Whereas other reports have often described single-center or regional experiences,13-15 leaving open the possibility that their results were highly influenced by the local nature of the pandemic in their respective settings, our report from a large sample of hospitals across the United States in high- and low-burden settings provides a more generalizable description of mortality rates for hospitalized patients. Additional study strengths include our adjustment for comorbidities known to be associated with COVID-19 survival, the reporting of definitive outcomes for 99% of patients, and the inclusion of multiple sensitivity analyses to assess the stability of findings.
Our principal limitation is the inability to adjust for severity of acute illness due to inconsistent reporting of laboratory and vital signs data from study hospitals and missing information on interhospital transfers. While our adjusted analyses clearly suggest an association between COVID-19 burden and patient outcomes, our results may still be confounded by differences in illness severity at study hospitals. Thus, our findings should be considered hypothesis-generating and will require confirmation in future studies that include adjustment for acute illness severity.
Other limitations of our study include overrepresentation of large urban hospitals in the Northeast, although this represents the geography of the US pandemic during the study period. Our adjustment for race/ethnicity and socioeconomic status was limited in that we only had zip code-of-residence level information, did not know the zip code of residence for one quarter of study patients, and had to bifurcate the population into White/non-White categories. Finally, our definition of burden does not account for hospital resources, including staffing, ICU capacity, and the availability of advanced or investigational therapies.
CONCLUSION
In this study of 14,226 patients with COVID-19 admitted to 1 of 117 US hospitals, we found that the odds of in-hospital mortality were higher in hospitals that had the highest burden of COVID-19 admissions. This relationship, which persisted after adjustment for age, sex, and comorbid conditions, suggests that patient surges may be an independent risk factor for in-hospital death among patients with COVID-19.
ACKNOWLEGMENTS
The authors thank Bocheng Jing, MS, Senior Statistician at the UCSF Pepper Center, for providing code to identify Elixhauser conditions from ICD-10 data; and Scott Kerber, BS, and Scott Magnoni, MS, both of CarePort Health, for assistance with data extraction. They were not compensated for this work beyond their regular salaries.
1. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Centers for Disease Control and Prevention. Updated November 2, 2020. Accessed December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2
3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1-12. https://doi.org/10.1001/jamainternmed.2020.4568
4. Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1-3. https://doi.org/10.1007/s11606-020-06084-7
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention. Accessed June 2, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
6. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
7. About the American Community Survey. United States Census Bureau. Updated January 4, 2021. Accessed March 2, 2021. https://www.census.gov/programs-surveys/acs/about.html
8. Provider of service files. Centers for Medicare & Medicaid Services. Revised January 15, 2020. Accessed March 2, 2021. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file
9. Ash AS, Fienberg SE, Louis TA, et al. Statistical issues in assessing hospital performance. Committee of Presidents of Statistical Societies white paper. January 2012. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf
10. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;12;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401
11. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33-41. https://doi.org/10.7326/M20-3905
12. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/NEJMsa2011686
13. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. https://doi.org/10.1056/NEJMoa2004500
14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
15. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
1. Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Centers for Disease Control and Prevention. Updated November 2, 2020. Accessed December 29, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
2. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763-1770. https://doi.org/10.1016/S0140-6736(20)31189-2
3. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1-12. https://doi.org/10.1001/jamainternmed.2020.4568
4. Karaca-Mandic P, Sen S, Georgiou A, Zhu Y, Basu A. Association of COVID-19-related hospital use and overall covid-19 mortality in the USA. J Gen Intern Med. 2020:1-3. https://doi.org/10.1007/s11606-020-06084-7
5. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. Centers for Disease Control and Prevention. Accessed June 2, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf
6. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. https://doi.org/10.1097/01.mlr.0000182534.19832.83
7. About the American Community Survey. United States Census Bureau. Updated January 4, 2021. Accessed March 2, 2021. https://www.census.gov/programs-surveys/acs/about.html
8. Provider of service files. Centers for Medicare & Medicaid Services. Revised January 15, 2020. Accessed March 2, 2021. https://www.cms.gov/research-statistics-data-systems/provider-services-current-files/2019-pos-file
9. Ash AS, Fienberg SE, Louis TA, et al. Statistical issues in assessing hospital performance. Committee of Presidents of Statistical Societies white paper. January 2012. Accessed March 1, 2021. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Downloads/Statistical-Issues-in-Assessing-Hospital-Performance.pdf
10. Bratzler DW, Normand SL, Wang Y, et al. An administrative claims model for profiling hospital 30-day mortality rates for pneumonia patients. PLoS One. 2011;12;6(4):e17401. https://doi.org/10.1371/journal.pone.0017401
11. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33-41. https://doi.org/10.7326/M20-3905
12. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. https://doi.org/10.1056/NEJMsa2011686
13. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022. https://doi.org/10.1056/NEJMoa2004500
14. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. https://doi.org/10.1016/S2213-2600(20)30079-5
15. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
© 2021 Society of Hospital Medicine
Supine-Related Pseudoanemia in Hospitalized Patients
The World Health Organization (WHO) defines anemia as a hemoglobin value less than 12 g/dL in women and less than 13 g/dL in men.1 Hospital-acquired anemia is loosely defined as normal hemoglobin levels on admission that, at their nadir during hospitalization or on discharge, are less than WHO sex-defined cutoffs. Hospital-acquired anemia or significant decreases in hemoglobin are often identified during hospitalization.2-6 Potential causes include blood loss from phlebotomy, occult gastrointestinal bleeding, hemolysis, anemia of inflammation, and hemodilution due to fluid resuscitation. Of these causes, some are dangerous to patients, some are iatrogenic, and some are due to laboratory error.7 Physicians often evaluate decreases in hemoglobin, which could otherwise be explained by laboratory error, hemodilution, or expected decrease in hemoglobin due to hospitalization, to identify causes that may lead to potential harm.
Jacob et al8 demonstrated the effect of posture on hemoglobin concentrations in healthy volunteers, showing an average 11% relative increase in hemoglobin when going from lying to standing. This increase was attributed to shifts in plasma volume to the vascular space with recumbence. They hypothesized that the initial hemoglobin on admission is measured when patients are upright or recently upright, whereas after admission, patients are more likely to be supine, resulting in lower hemoglobin concentrations. Others have also demonstrated similar effects of patient posture on hemoglobin concentration.9-13 However, these prior results are not readily generalizable to hospitalized patients. These prior studies enrolled healthy volunteers, and most examined postural changes from the supine and standing positions; blood is rarely obtained from hospitalized patients when they are standing.
The aim of this study was to investigate whether postural changes in hemoglobin can be demonstrated in positions that patients routinely encountered during in-hospital phlebotomy: upright in a chair or recumbent in a bed. Patient position, which is not standardized during blood draws, may contribute to lower measured hemoglobin concentrations in some patients, especially sicker individuals who are recumbent more frequently. We hypothesized that going from supine to upright in a chair would result in a relative increase in hemoglobin concentration of 5% to 6%, approximately half the value of going from supine to standing.8 To investigate this, we conducted a quasi-experimental study exploring the effect of position (supine or sitting in chair) on hemoglobin concentrations in medical inpatients.
METHODS
Participants
Patients were enrolled in this single-center study between October 2017 and August 2018. Patients aged 18 years or older who were hospitalized on the general internal medicine wards were screened to determine if they met the following inclusion criteria: hospitalized for <5 days, had blood work scheduled as part of routine care (in order to decrease phlebotomy required by this study), had baseline hemoglobin >8 g/dL, and were able to remain supine without interruption overnight and able to sit in a chair for at least 1 hour the following morning. Patients were excluded from the study if they had a hematologic malignancy, were at risk of >100 mL of blood loss (eg, admitted for gastrointestinal bleeding, planned surgery), had a transfusion requirement, or received intravascular modifiers such as fluid (>100 cc/h) or intravenous diuretics. The Johns Hopkins Institutional Review Board approved this study, and all patients provided written informed consent.
Study Design
Patients enrolled in this quasi-experimental study were asked to remain supine for at least 6 hours overnight. Adherence to the recumbent position was tracked by patient self-report and by corroboration with the patient’s nurse overnight. Any interruptions to supine positioning resulted in exclusion from the study. The following morning, a member of the study team performed phlebotomy while the patient remained supine. Patients were then asked to sit comfortably in a chair for at least 1 hour with their feet on the ground; the blood draw was then repeated. All blood samples were acquired by venipuncture. Prior to each blood draw, a tourniquet was placed over the upper arm below the axilla. An antecubital vein on either arm was visualized under ultrasound guidance, and a 23-G × 3/4” butterfly needle was used for venipuncture. The vials of blood were immediately inverted after blood collection. Hemoglobin assays were processed and analyzed using Sysmex XN-10 analyzer (Sysmex Corporation). The reference range for hemoglobin in our facility was 12.0 to 15.0 g/dL for women and 13.9 to 16.3 g/dL for men. Laboratory technicians were blinded to and uninvolved in the study.
We determined, a priori, that 33 enrolled patients would provide 80% power (alpha 0.05) to detect an average hemoglobin change of 4.1%, assuming that the standard deviation of the hemoglobin change was twice the mean (ie, SD = 8.2%). The Wilcoxon signed-rank test was used to test the significance of postural hemoglobin changes. Analyses were conducted using JMP Pro 13.0 (SAS) and GraphPad Prism 8 (GraphPad Software). Significance was defined at P < .05 for all analyses.
RESULTS
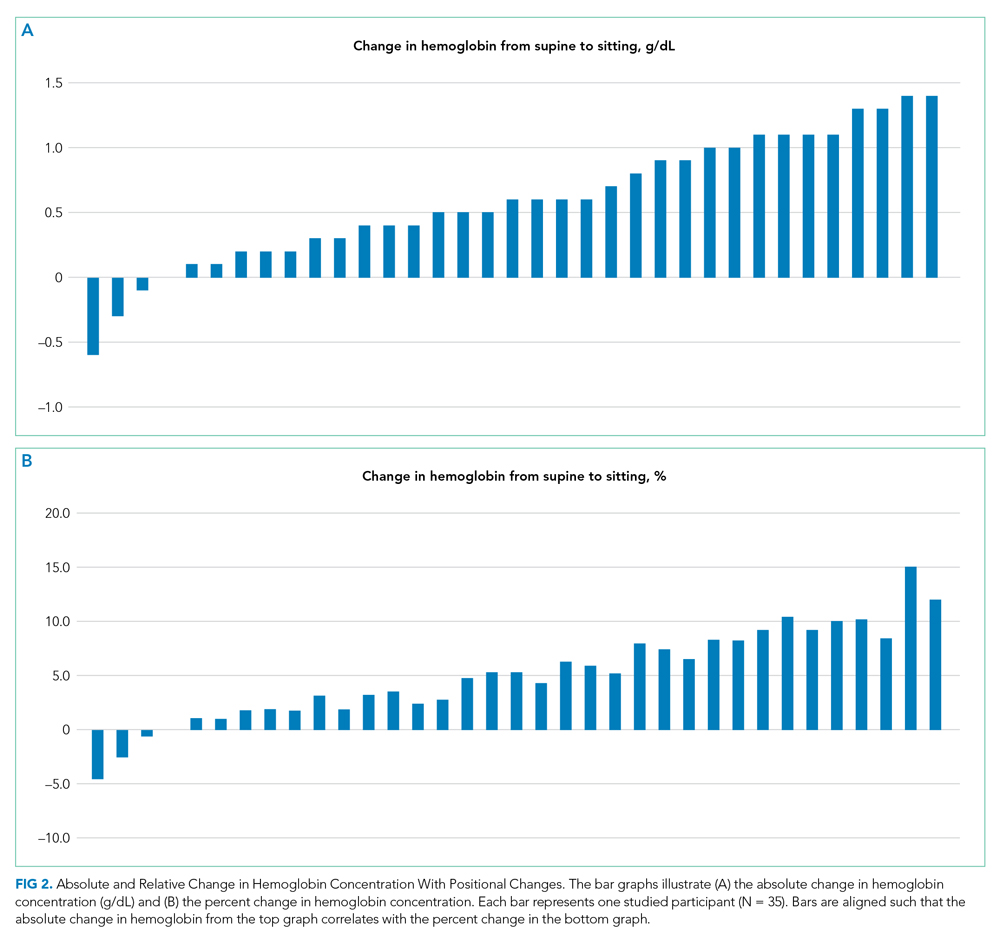
Thirty-nine patients were consented and enrolled in the study; four patients were excluded prior to blood draw (two patients because of interruption of supine time, two patients because of refusal in the morning). Of the 35 patients who completed the study, 13 were women (37%); median age was 49 years (range, 25-83 years). Median supine hemoglobin concentration in our sample was 11.7 g/dL (range, 9.3-18.1 g/dL), and median baseline creatinine level was 0.70 mg/dL (range, 0.5-2.5 mg/dL). Median supine hemoglobin levels were 11.7 g/dL (range, 9.6-13.2 g/dL) in women and 11.8 g/dL (range, 9.3-18.1 g/dL) in men. In aggregate, patients had a median increase in hemoglobin concentration of 0.60 g/dL (range, –0.6 to 1.4 g/dL) with sitting, a 5.2% (range, –4.5% to 15.1%) relative change (P < .001) (Figure 1). 
DISCUSSION
International blood collection guidelines acknowledge postural changes in laboratory values and recommend standardization of patient position to either sitting in a chair or lying flat in a bed, without changes in position for 15 minutes prior to blood draw.14 When these positional accommodations cannot be met, documenting positional disruptions is recommended so that laboratory values can be interpreted accordingly. To the best of our knowledge, no hospital in the United States has standardized patient position as part of phlebotomy procedure such that patient position is documented and can be made available to interpreting providers.
Relative increases in hemoglobin or hematocrit range from 7% to 12% when patients go from supine to standing.8,9,11 The reverse relationship has also been shown, where upright-to-supine position results in decreases in hemoglobin concentrations.10,13 We found that going from supine to a seated position resulted in significant increases in hemoglobin of 0.6 g/dL and in a more than 1 g/dL increase in 29% of the patients. Although four of the 35 patients experienced either no change or a slight decrease in their hemoglobin concentration when going from supine to upright and not all patients saw a uniform effect, providers should be aware that the patient’s position can contribute to changes in hemoglobin concentration in the hospitalized setting. Providers may be able to use this information to avoid an extensive diagnostic workup when anemia is identified in hospitalized patients, although more research is needed to identify patient subsets who are at higher risk for this effect.
Until hospitals implement protocols that require phlebotomists to report patient position during phlebotomy in a standardized fashion, providers should be alert to the fact that supine positioning may result in a hemoglobin level that is significantly lower than that when drawn in a sitting position, and in almost one-third of patients, this difference may be 1.0 g/dL or greater.
Given our study criteria requiring supine positions of at least 6 hours and a baseline hemoglobin concentration >8 g/dL, our sample of patients may have been younger and healthier than the average hospitalized patient on general internal medicine wards. Since greater relative changes in plasma volume shifts and hemoglobin might be seen in patients with lower baseline hemoglobin and lower baseline plasma protein, this selection bias may underestimate the effects of position on hemoglobin changes for the average inpatient population. Additionally, we intentionally sought to obtain sitting hemoglobin levels after the supine samples to avoid the possibility of incorrectly attributing dropping hemoglobin levels to progressive hospital-acquired anemia from phlebotomy or illness. Any concomitant trend of falling hemoglobin levels in our patients would be expected to lead to a systematic underestimation of the positional change in hemoglobin we observed. We did not objectively observe adherence to supine and upright position and instead relied on patient self-reporting, which is one possible contributor to the variable effects of position on hemoglobin concentration, with some patients having no change or decreases in hemoglobin concentrations.
CONCLUSION
Posture can significantly influence hemoglobin levels in hospitalized patients on general medicine wards. Further research can determine whether it would be cost and time effective to standardize patient positions prior to phlebotomy, or at least to report patient positioning with the laboratory testing results.
1. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302-316.
2. Martin ND, Scantling D. Hospital-acquired anemia. J Infus Nurs. 2015;38(5):330-338. https://doi.org/10.1097/NAN.0000000000000121
3. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
4. Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646-1653. https://doi.org/10.1001/archinternmed.2011.361
5. Languasco A, Cazap N, Marciano S, et al. Hemoglobin concentration variations over time in general medical inpatients. J Hosp Med. 2010;5(5):283-288. https://doi.org/10.1002/jhm.650
6. van der Bom JG, Cannegieter SC. Hospital-acquired anemia: the contribution of diagnostic blood loss. J Thromb Haemost. 2015;13(6):1157-1159. https://doi.org/10.1111/jth.12886
7. Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27(5):499-508. https://doi.org/10.1007/s10877-013-9456-3
8. Jacob G, Raj SR, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80(5):611-614. https://doi.org/10.4065/80.5.611
9. Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13(4):304-310. https://doi.org/10.1136/jcp.13.4.304
10. Tombridge TL. Effect of posture on hematology results. Am J ClinPathol. 1968;49(4):491-493. https://doi.org/10.1093/ajcp/49.4.491
11. Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414-417. https://doi.org/10.1152/jappl.1978.45.3.414
12. Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155(2):157-163. https://doi.org/10.1111/j.1748-1716.1995.tb09960.x
13. Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemoter. 2017;39(2):127-132. https://doi.org/10.1016/j.bjhh.2017.01.004
14. Simundic AM, Bölenius K, Cadamuro J, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. https://doi.org/10.1515/cclm-2018-0602
The World Health Organization (WHO) defines anemia as a hemoglobin value less than 12 g/dL in women and less than 13 g/dL in men.1 Hospital-acquired anemia is loosely defined as normal hemoglobin levels on admission that, at their nadir during hospitalization or on discharge, are less than WHO sex-defined cutoffs. Hospital-acquired anemia or significant decreases in hemoglobin are often identified during hospitalization.2-6 Potential causes include blood loss from phlebotomy, occult gastrointestinal bleeding, hemolysis, anemia of inflammation, and hemodilution due to fluid resuscitation. Of these causes, some are dangerous to patients, some are iatrogenic, and some are due to laboratory error.7 Physicians often evaluate decreases in hemoglobin, which could otherwise be explained by laboratory error, hemodilution, or expected decrease in hemoglobin due to hospitalization, to identify causes that may lead to potential harm.
Jacob et al8 demonstrated the effect of posture on hemoglobin concentrations in healthy volunteers, showing an average 11% relative increase in hemoglobin when going from lying to standing. This increase was attributed to shifts in plasma volume to the vascular space with recumbence. They hypothesized that the initial hemoglobin on admission is measured when patients are upright or recently upright, whereas after admission, patients are more likely to be supine, resulting in lower hemoglobin concentrations. Others have also demonstrated similar effects of patient posture on hemoglobin concentration.9-13 However, these prior results are not readily generalizable to hospitalized patients. These prior studies enrolled healthy volunteers, and most examined postural changes from the supine and standing positions; blood is rarely obtained from hospitalized patients when they are standing.
The aim of this study was to investigate whether postural changes in hemoglobin can be demonstrated in positions that patients routinely encountered during in-hospital phlebotomy: upright in a chair or recumbent in a bed. Patient position, which is not standardized during blood draws, may contribute to lower measured hemoglobin concentrations in some patients, especially sicker individuals who are recumbent more frequently. We hypothesized that going from supine to upright in a chair would result in a relative increase in hemoglobin concentration of 5% to 6%, approximately half the value of going from supine to standing.8 To investigate this, we conducted a quasi-experimental study exploring the effect of position (supine or sitting in chair) on hemoglobin concentrations in medical inpatients.
METHODS
Participants
Patients were enrolled in this single-center study between October 2017 and August 2018. Patients aged 18 years or older who were hospitalized on the general internal medicine wards were screened to determine if they met the following inclusion criteria: hospitalized for <5 days, had blood work scheduled as part of routine care (in order to decrease phlebotomy required by this study), had baseline hemoglobin >8 g/dL, and were able to remain supine without interruption overnight and able to sit in a chair for at least 1 hour the following morning. Patients were excluded from the study if they had a hematologic malignancy, were at risk of >100 mL of blood loss (eg, admitted for gastrointestinal bleeding, planned surgery), had a transfusion requirement, or received intravascular modifiers such as fluid (>100 cc/h) or intravenous diuretics. The Johns Hopkins Institutional Review Board approved this study, and all patients provided written informed consent.
Study Design
Patients enrolled in this quasi-experimental study were asked to remain supine for at least 6 hours overnight. Adherence to the recumbent position was tracked by patient self-report and by corroboration with the patient’s nurse overnight. Any interruptions to supine positioning resulted in exclusion from the study. The following morning, a member of the study team performed phlebotomy while the patient remained supine. Patients were then asked to sit comfortably in a chair for at least 1 hour with their feet on the ground; the blood draw was then repeated. All blood samples were acquired by venipuncture. Prior to each blood draw, a tourniquet was placed over the upper arm below the axilla. An antecubital vein on either arm was visualized under ultrasound guidance, and a 23-G × 3/4” butterfly needle was used for venipuncture. The vials of blood were immediately inverted after blood collection. Hemoglobin assays were processed and analyzed using Sysmex XN-10 analyzer (Sysmex Corporation). The reference range for hemoglobin in our facility was 12.0 to 15.0 g/dL for women and 13.9 to 16.3 g/dL for men. Laboratory technicians were blinded to and uninvolved in the study.
We determined, a priori, that 33 enrolled patients would provide 80% power (alpha 0.05) to detect an average hemoglobin change of 4.1%, assuming that the standard deviation of the hemoglobin change was twice the mean (ie, SD = 8.2%). The Wilcoxon signed-rank test was used to test the significance of postural hemoglobin changes. Analyses were conducted using JMP Pro 13.0 (SAS) and GraphPad Prism 8 (GraphPad Software). Significance was defined at P < .05 for all analyses.
RESULTS
Thirty-nine patients were consented and enrolled in the study; four patients were excluded prior to blood draw (two patients because of interruption of supine time, two patients because of refusal in the morning). Of the 35 patients who completed the study, 13 were women (37%); median age was 49 years (range, 25-83 years). Median supine hemoglobin concentration in our sample was 11.7 g/dL (range, 9.3-18.1 g/dL), and median baseline creatinine level was 0.70 mg/dL (range, 0.5-2.5 mg/dL). Median supine hemoglobin levels were 11.7 g/dL (range, 9.6-13.2 g/dL) in women and 11.8 g/dL (range, 9.3-18.1 g/dL) in men. In aggregate, patients had a median increase in hemoglobin concentration of 0.60 g/dL (range, –0.6 to 1.4 g/dL) with sitting, a 5.2% (range, –4.5% to 15.1%) relative change (P < .001) (Figure 1). 

DISCUSSION
International blood collection guidelines acknowledge postural changes in laboratory values and recommend standardization of patient position to either sitting in a chair or lying flat in a bed, without changes in position for 15 minutes prior to blood draw.14 When these positional accommodations cannot be met, documenting positional disruptions is recommended so that laboratory values can be interpreted accordingly. To the best of our knowledge, no hospital in the United States has standardized patient position as part of phlebotomy procedure such that patient position is documented and can be made available to interpreting providers.
Relative increases in hemoglobin or hematocrit range from 7% to 12% when patients go from supine to standing.8,9,11 The reverse relationship has also been shown, where upright-to-supine position results in decreases in hemoglobin concentrations.10,13 We found that going from supine to a seated position resulted in significant increases in hemoglobin of 0.6 g/dL and in a more than 1 g/dL increase in 29% of the patients. Although four of the 35 patients experienced either no change or a slight decrease in their hemoglobin concentration when going from supine to upright and not all patients saw a uniform effect, providers should be aware that the patient’s position can contribute to changes in hemoglobin concentration in the hospitalized setting. Providers may be able to use this information to avoid an extensive diagnostic workup when anemia is identified in hospitalized patients, although more research is needed to identify patient subsets who are at higher risk for this effect.
Until hospitals implement protocols that require phlebotomists to report patient position during phlebotomy in a standardized fashion, providers should be alert to the fact that supine positioning may result in a hemoglobin level that is significantly lower than that when drawn in a sitting position, and in almost one-third of patients, this difference may be 1.0 g/dL or greater.
Given our study criteria requiring supine positions of at least 6 hours and a baseline hemoglobin concentration >8 g/dL, our sample of patients may have been younger and healthier than the average hospitalized patient on general internal medicine wards. Since greater relative changes in plasma volume shifts and hemoglobin might be seen in patients with lower baseline hemoglobin and lower baseline plasma protein, this selection bias may underestimate the effects of position on hemoglobin changes for the average inpatient population. Additionally, we intentionally sought to obtain sitting hemoglobin levels after the supine samples to avoid the possibility of incorrectly attributing dropping hemoglobin levels to progressive hospital-acquired anemia from phlebotomy or illness. Any concomitant trend of falling hemoglobin levels in our patients would be expected to lead to a systematic underestimation of the positional change in hemoglobin we observed. We did not objectively observe adherence to supine and upright position and instead relied on patient self-reporting, which is one possible contributor to the variable effects of position on hemoglobin concentration, with some patients having no change or decreases in hemoglobin concentrations.
CONCLUSION
Posture can significantly influence hemoglobin levels in hospitalized patients on general medicine wards. Further research can determine whether it would be cost and time effective to standardize patient positions prior to phlebotomy, or at least to report patient positioning with the laboratory testing results.
The World Health Organization (WHO) defines anemia as a hemoglobin value less than 12 g/dL in women and less than 13 g/dL in men.1 Hospital-acquired anemia is loosely defined as normal hemoglobin levels on admission that, at their nadir during hospitalization or on discharge, are less than WHO sex-defined cutoffs. Hospital-acquired anemia or significant decreases in hemoglobin are often identified during hospitalization.2-6 Potential causes include blood loss from phlebotomy, occult gastrointestinal bleeding, hemolysis, anemia of inflammation, and hemodilution due to fluid resuscitation. Of these causes, some are dangerous to patients, some are iatrogenic, and some are due to laboratory error.7 Physicians often evaluate decreases in hemoglobin, which could otherwise be explained by laboratory error, hemodilution, or expected decrease in hemoglobin due to hospitalization, to identify causes that may lead to potential harm.
Jacob et al8 demonstrated the effect of posture on hemoglobin concentrations in healthy volunteers, showing an average 11% relative increase in hemoglobin when going from lying to standing. This increase was attributed to shifts in plasma volume to the vascular space with recumbence. They hypothesized that the initial hemoglobin on admission is measured when patients are upright or recently upright, whereas after admission, patients are more likely to be supine, resulting in lower hemoglobin concentrations. Others have also demonstrated similar effects of patient posture on hemoglobin concentration.9-13 However, these prior results are not readily generalizable to hospitalized patients. These prior studies enrolled healthy volunteers, and most examined postural changes from the supine and standing positions; blood is rarely obtained from hospitalized patients when they are standing.
The aim of this study was to investigate whether postural changes in hemoglobin can be demonstrated in positions that patients routinely encountered during in-hospital phlebotomy: upright in a chair or recumbent in a bed. Patient position, which is not standardized during blood draws, may contribute to lower measured hemoglobin concentrations in some patients, especially sicker individuals who are recumbent more frequently. We hypothesized that going from supine to upright in a chair would result in a relative increase in hemoglobin concentration of 5% to 6%, approximately half the value of going from supine to standing.8 To investigate this, we conducted a quasi-experimental study exploring the effect of position (supine or sitting in chair) on hemoglobin concentrations in medical inpatients.
METHODS
Participants
Patients were enrolled in this single-center study between October 2017 and August 2018. Patients aged 18 years or older who were hospitalized on the general internal medicine wards were screened to determine if they met the following inclusion criteria: hospitalized for <5 days, had blood work scheduled as part of routine care (in order to decrease phlebotomy required by this study), had baseline hemoglobin >8 g/dL, and were able to remain supine without interruption overnight and able to sit in a chair for at least 1 hour the following morning. Patients were excluded from the study if they had a hematologic malignancy, were at risk of >100 mL of blood loss (eg, admitted for gastrointestinal bleeding, planned surgery), had a transfusion requirement, or received intravascular modifiers such as fluid (>100 cc/h) or intravenous diuretics. The Johns Hopkins Institutional Review Board approved this study, and all patients provided written informed consent.
Study Design
Patients enrolled in this quasi-experimental study were asked to remain supine for at least 6 hours overnight. Adherence to the recumbent position was tracked by patient self-report and by corroboration with the patient’s nurse overnight. Any interruptions to supine positioning resulted in exclusion from the study. The following morning, a member of the study team performed phlebotomy while the patient remained supine. Patients were then asked to sit comfortably in a chair for at least 1 hour with their feet on the ground; the blood draw was then repeated. All blood samples were acquired by venipuncture. Prior to each blood draw, a tourniquet was placed over the upper arm below the axilla. An antecubital vein on either arm was visualized under ultrasound guidance, and a 23-G × 3/4” butterfly needle was used for venipuncture. The vials of blood were immediately inverted after blood collection. Hemoglobin assays were processed and analyzed using Sysmex XN-10 analyzer (Sysmex Corporation). The reference range for hemoglobin in our facility was 12.0 to 15.0 g/dL for women and 13.9 to 16.3 g/dL for men. Laboratory technicians were blinded to and uninvolved in the study.
We determined, a priori, that 33 enrolled patients would provide 80% power (alpha 0.05) to detect an average hemoglobin change of 4.1%, assuming that the standard deviation of the hemoglobin change was twice the mean (ie, SD = 8.2%). The Wilcoxon signed-rank test was used to test the significance of postural hemoglobin changes. Analyses were conducted using JMP Pro 13.0 (SAS) and GraphPad Prism 8 (GraphPad Software). Significance was defined at P < .05 for all analyses.
RESULTS
Thirty-nine patients were consented and enrolled in the study; four patients were excluded prior to blood draw (two patients because of interruption of supine time, two patients because of refusal in the morning). Of the 35 patients who completed the study, 13 were women (37%); median age was 49 years (range, 25-83 years). Median supine hemoglobin concentration in our sample was 11.7 g/dL (range, 9.3-18.1 g/dL), and median baseline creatinine level was 0.70 mg/dL (range, 0.5-2.5 mg/dL). Median supine hemoglobin levels were 11.7 g/dL (range, 9.6-13.2 g/dL) in women and 11.8 g/dL (range, 9.3-18.1 g/dL) in men. In aggregate, patients had a median increase in hemoglobin concentration of 0.60 g/dL (range, –0.6 to 1.4 g/dL) with sitting, a 5.2% (range, –4.5% to 15.1%) relative change (P < .001) (Figure 1). 

DISCUSSION
International blood collection guidelines acknowledge postural changes in laboratory values and recommend standardization of patient position to either sitting in a chair or lying flat in a bed, without changes in position for 15 minutes prior to blood draw.14 When these positional accommodations cannot be met, documenting positional disruptions is recommended so that laboratory values can be interpreted accordingly. To the best of our knowledge, no hospital in the United States has standardized patient position as part of phlebotomy procedure such that patient position is documented and can be made available to interpreting providers.
Relative increases in hemoglobin or hematocrit range from 7% to 12% when patients go from supine to standing.8,9,11 The reverse relationship has also been shown, where upright-to-supine position results in decreases in hemoglobin concentrations.10,13 We found that going from supine to a seated position resulted in significant increases in hemoglobin of 0.6 g/dL and in a more than 1 g/dL increase in 29% of the patients. Although four of the 35 patients experienced either no change or a slight decrease in their hemoglobin concentration when going from supine to upright and not all patients saw a uniform effect, providers should be aware that the patient’s position can contribute to changes in hemoglobin concentration in the hospitalized setting. Providers may be able to use this information to avoid an extensive diagnostic workup when anemia is identified in hospitalized patients, although more research is needed to identify patient subsets who are at higher risk for this effect.
Until hospitals implement protocols that require phlebotomists to report patient position during phlebotomy in a standardized fashion, providers should be alert to the fact that supine positioning may result in a hemoglobin level that is significantly lower than that when drawn in a sitting position, and in almost one-third of patients, this difference may be 1.0 g/dL or greater.
Given our study criteria requiring supine positions of at least 6 hours and a baseline hemoglobin concentration >8 g/dL, our sample of patients may have been younger and healthier than the average hospitalized patient on general internal medicine wards. Since greater relative changes in plasma volume shifts and hemoglobin might be seen in patients with lower baseline hemoglobin and lower baseline plasma protein, this selection bias may underestimate the effects of position on hemoglobin changes for the average inpatient population. Additionally, we intentionally sought to obtain sitting hemoglobin levels after the supine samples to avoid the possibility of incorrectly attributing dropping hemoglobin levels to progressive hospital-acquired anemia from phlebotomy or illness. Any concomitant trend of falling hemoglobin levels in our patients would be expected to lead to a systematic underestimation of the positional change in hemoglobin we observed. We did not objectively observe adherence to supine and upright position and instead relied on patient self-reporting, which is one possible contributor to the variable effects of position on hemoglobin concentration, with some patients having no change or decreases in hemoglobin concentrations.
CONCLUSION
Posture can significantly influence hemoglobin levels in hospitalized patients on general medicine wards. Further research can determine whether it would be cost and time effective to standardize patient positions prior to phlebotomy, or at least to report patient positioning with the laboratory testing results.
1. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302-316.
2. Martin ND, Scantling D. Hospital-acquired anemia. J Infus Nurs. 2015;38(5):330-338. https://doi.org/10.1097/NAN.0000000000000121
3. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
4. Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646-1653. https://doi.org/10.1001/archinternmed.2011.361
5. Languasco A, Cazap N, Marciano S, et al. Hemoglobin concentration variations over time in general medical inpatients. J Hosp Med. 2010;5(5):283-288. https://doi.org/10.1002/jhm.650
6. van der Bom JG, Cannegieter SC. Hospital-acquired anemia: the contribution of diagnostic blood loss. J Thromb Haemost. 2015;13(6):1157-1159. https://doi.org/10.1111/jth.12886
7. Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27(5):499-508. https://doi.org/10.1007/s10877-013-9456-3
8. Jacob G, Raj SR, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80(5):611-614. https://doi.org/10.4065/80.5.611
9. Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13(4):304-310. https://doi.org/10.1136/jcp.13.4.304
10. Tombridge TL. Effect of posture on hematology results. Am J ClinPathol. 1968;49(4):491-493. https://doi.org/10.1093/ajcp/49.4.491
11. Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414-417. https://doi.org/10.1152/jappl.1978.45.3.414
12. Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155(2):157-163. https://doi.org/10.1111/j.1748-1716.1995.tb09960.x
13. Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemoter. 2017;39(2):127-132. https://doi.org/10.1016/j.bjhh.2017.01.004
14. Simundic AM, Bölenius K, Cadamuro J, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. https://doi.org/10.1515/cclm-2018-0602
1. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38(3):302-316.
2. Martin ND, Scantling D. Hospital-acquired anemia. J Infus Nurs. 2015;38(5):330-338. https://doi.org/10.1097/NAN.0000000000000121
3. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. https://doi.org/10.1111/j.1525-1497.2005.0094.x
4. Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171(18):1646-1653. https://doi.org/10.1001/archinternmed.2011.361
5. Languasco A, Cazap N, Marciano S, et al. Hemoglobin concentration variations over time in general medical inpatients. J Hosp Med. 2010;5(5):283-288. https://doi.org/10.1002/jhm.650
6. van der Bom JG, Cannegieter SC. Hospital-acquired anemia: the contribution of diagnostic blood loss. J Thromb Haemost. 2015;13(6):1157-1159. https://doi.org/10.1111/jth.12886
7. Berkow L. Factors affecting hemoglobin measurement. J Clin Monit Comput. 2013;27(5):499-508. https://doi.org/10.1007/s10877-013-9456-3
8. Jacob G, Raj SR, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80(5):611-614. https://doi.org/10.4065/80.5.611
9. Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13(4):304-310. https://doi.org/10.1136/jcp.13.4.304
10. Tombridge TL. Effect of posture on hematology results. Am J ClinPathol. 1968;49(4):491-493. https://doi.org/10.1093/ajcp/49.4.491
11. Hagan RD, Diaz FJ, Horvath SM. Plasma volume changes with movement to supine and standing positions. J Appl Physiol. 1978;45(3):414-417. https://doi.org/10.1152/jappl.1978.45.3.414
12. Maw GJ, Mackenzie IL, Taylor NA. Redistribution of body fluids during postural manipulations. Acta Physiol Scand. 1995;155(2):157-163. https://doi.org/10.1111/j.1748-1716.1995.tb09960.x
13. Lima-Oliveira G, Guidi GC, Salvagno GL, Danese E, Montagnana M, Lippi G. Patient posture for blood collection by venipuncture: recall for standardization after 28 years. Rev Bras Hematol Hemoter. 2017;39(2):127-132. https://doi.org/10.1016/j.bjhh.2017.01.004
14. Simundic AM, Bölenius K, Cadamuro J, et al. Working Group for Preanalytical Phase (WG-PRE), of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) and Latin American Working Group for Preanalytical Phase (WG-PRE-LATAM) of the Latin America Confederation of Clinical Biochemistry (COLABIOCLI). Joint EFLM-COLABIOCLI recommendation for venous blood sampling. Clin Chem Lab Med. 2018;56(12):2015-2038. https://doi.org/10.1515/cclm-2018-0602
© 2021 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Management of Acute and Chronic Pain in Sickle Cell Disease
Sickle cell disease (SCD) affects an estimated 100,000 people in the United States.1 Pain is the most common complication of SCD and the primary reason patients with SCD seek medical attention.2 In 2016, three-fourths of the approximately 130,000 SCD-related hospitalizations in the United States involved pain crises.3 When managing patients with SCD and chronic pain, an individualized and interdisciplinary approach is crucial. In 2020, the American Society of Hematology (ASH) developed guidelines reflecting the latest evidence in managing acute and chronic pain in adult and pediatric patients with SCD. The ASH guidelines provide 18 recommendations; here, we highlight the 8 recommendations most pertinent to the hospitalist.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Acute Pain
Acute pain in the guideline is defined as pain that results in an unplanned visit to an acute care center for treatment.
Recommendation 1. For adult and pediatric patients presenting to an acute care setting with SCD-related acute pain, the ASH guideline panel recommends rapid (ie, within 1 hour of arrival at the emergency department [ED]) assessment and administration of analgesia, with reassessments every 30-60 minutes to optimize pain control (Strong recommendation; low certainty in the evidence about effects).
Although the perceived benefits are unclear due to insufficient evidence, the panel agrees that delaying pain management results in undeniable harm to patients. Hence, this recommendation was deemed both acceptable and ethical. Rapid evaluation also allows for earlier identification and treatment of other potential SCD-related complications.
Recommendation 2. For adult and pediatric patients presenting to an acute care setting with SCD-associated pain for whom opioid therapy is indicated, the ASH guideline panel suggests tailored opioid dosing based on consideration of baseline opioid therapy and prior effective therapy. (For adults: conditional recommendation; moderate certainty in the evidence about effects. For children: conditional recommendation; low certainty in the evidence about effects).
One randomized controlled trial examined patient-specific opioid dosing (based on current chronic opioid therapy [COT] and previously known effective acute pain management) vs weight-based dosing in the ED and found that participants randomized into the patient-specific protocol had a greater reduction in pain and decreased rate of hospital admission.4
The panel acknowledges that intravenous patient-controlled opioid analgesia is generally the standard of care at most institutions. However, no clear data address whether continuous opioid infusion in addition to on-demand dosing is beneficial.
Recommendation 3. For adult and pediatric patients with acute pain related to SCD, the ASH guideline panel suggests a short course (5 to 7 days) of nonsteroidal anti-inflammatory drugs (NSAIDs) in addition to opioids (Conditional recommendation; very low certainty in the evidence about effects).
The use of NSAIDs for managing pain in hospitalized patients with SCD has been associated with a reduction in the use of opioids in the inpatient setting and decreased lengths of stay.5 The potential harms of NSAIDs, including renal and gastrointestinal toxicity, however, should be factored into the decision-making as the risks may outweigh the potential benefits.
Recommendation 4. For adult and pediatric patients with SCD hospitalized for acute pain, the ASH guideline panel suggests a subanesthetic (analgesic) infusion of ketamine as adjunctive treatment of pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects). The guideline panel also suggests regional anesthesia for localized pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects).
Studies have demonstrated reduced pain and opioid utilization in individuals who received adjuvant ketamine infusions6 or regional anesthesia (ie, epidural).7 Feasibility, however, is limited to centers that have the appropriate experience and expertise with these interventions.
Recommendation 5. For adult and pediatric patients who have recurrent acute pain associated with SCD, the ASH guideline panel suggests against chronic monthly transfusion therapy as a first-line strategy to prevent or reduce recurrent acute pain episodes (Conditional recommendation; low certainty in the evidence about effects). The evidence for monthly transfusions in preventing recurrent pain is limited. There is, however, a moderate risk of harm, including iron overload and transfusion reactions, in addition to substantial burden and costs.
Chronic Pain
Chronic pain in the guideline is defined as ongoing pain present on most days over the past 6 months.
Recommendation 6. For adult patients with SCD who have chronic pain from the SCD-related identifiable cause avascular necrosis (AVN) of the bone, the ASH guideline panel suggests the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) or NSAIDs in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). For patients with no identifiable cause beyond SCD, the guideline panel suggests SNRIs, tricyclic antidepressants, or gabapentinoids for pain management (Conditional recommendation; very low certainty in the evidence about effects). Given the lack of direct evidence, indirect evidence was used to formulate these recommendations. For pain associated with AVN, data were extrapolated from literature on osteoarthritis, a form of degenerative arthropathy. For pain without an identifiable cause, evidence was taken from studies on fibromyalgia, a condition the panel felt most closely aligned with chronic pain related to SCD.
No recommendations were made for pediatric patients as the indirect evidence base only addressed adult patients.
Recommendation 7. For adult and pediatric patients with SCD and emerging and/or recently developed chronic pain, the ASH guideline panel does not recommend initiating COT unless pain is refractory to multiple other treatment modalities (Conditional recommendation; very low certainty in the evidence about effects). For patients receiving COT who are functioning well and have perceived benefit, the ASH guideline panel suggests shared decision-making for continuation of COT (Conditional recommendation; very low certainty in the evidence about effects).
High-quality data on the benefit of long-term COT in individuals with chronic noncancer pain are lacking. The panel maintains that the decision to initiate or continue COT should be individualized after weighing appropriate risks and benefits.
Recommendation 8. For adult and pediatric patients with chronic pain related to SCD, the panel suggests cognitive and behavioral pain management strategies in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). Cognitive behavioral therapy may decrease overall pain intensity and improve coping skills.8 The panel agrees that medications alone may not be effective in reducing the burden of chronic pain in adult and pediatric patients with SCD.
CRITIQUE
The guidelines were created by a multidisciplinary panel that included physicians from hematology, pain medicine, psychiatry, and emergency medicine, a doctoral nurse practitioner, and two patient representatives. The Mayo Evidence-Based Practice Research Program supported the guideline-development process. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach was used to assess evidence and make recommendations.
High-quality data in treating acute and chronic pain in both adult and pediatric patients with SCD are limited. As such, the majority of recommendations in these guidelines are conditional. The panel included studies that were indirectly related to SCD based on consensus (eg, inferred data from disease processes thought to be similar to SCD). One panelist disclosed receiving direct payments from a company that could be affected by these guidelines; however, it was deemed that the conflict was unlikely to have influenced any recommendations.
AREAS IN NEED OF FUTURE STUDY
The panel acknowledges that further investigation is needed for both nonpharmacologic and pharmacologic modalities in treating acute and chronic pain related to SCD. Examples include evaluating the comparative-effectiveness of COT vs nonopioid pharmacotherapy, the benefits and harms of continuous opioid infusions in acute pain crises, and the impact of chronic transfusions on acute and chronic pain.
1. Data & statistics on sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/data.html
2. Complications and treatments of sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/treatments.html
3. Fingar KR, Owens PL, Reid LD, Mistry KB, Barrett ML. Characteristics of Inpatient Hospital Stays Involving Sickle Cell Disease, 2000-2016. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Statistical Brief 251. September 2019. Accessed August 23, 2020. www.hcup-us.ahrq.gov/reports/statbriefs/sb251-Sickle-Cell-Disease-Stays-2016.pdf
4. Tanabe P, Silva S, Bosworth HB, et al. A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD). Am J Hematol. 2018;93(2):159-168. https://doi.org/10.1002/ajh.24948
5. Perlin E, Finke H, Castro O, et al. Enhancement of pain control with ketorolac tromethamine in patients with sickle cell vaso-occlusive crisis. Am J Hematol. 1994;46(1):43-47. https://doi.org/10.1002/ajh.2830460108
6. Sheehy KA, Lippold C, Rice AL, et al. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res. 2017;10:787-795. https://doi.org/10.2147/jpr.s131156
7. New T, Venable C, Fraser L, et al. Management of refractory pain in hospitalized adolescents with sickle cell disease: changing from intravenous opioids to continuous infusion epidural analgesia. J Pediatr Hematol Oncol. 2014;36(6):e398-e402. https://doi.org/10.1097/mph.0000000000000026
8. Schatz J, Schlenz AM, McClellan CB, et al. Changes in coping, pain, and activity after cognitive-behavioral training: a randomized clinical trial for pediatric sickle cell disease using smartphones. Clin J Pain. 2015;31(6):536-547. https://doi.org/10.1097/ajp.0000000000000183
Sickle cell disease (SCD) affects an estimated 100,000 people in the United States.1 Pain is the most common complication of SCD and the primary reason patients with SCD seek medical attention.2 In 2016, three-fourths of the approximately 130,000 SCD-related hospitalizations in the United States involved pain crises.3 When managing patients with SCD and chronic pain, an individualized and interdisciplinary approach is crucial. In 2020, the American Society of Hematology (ASH) developed guidelines reflecting the latest evidence in managing acute and chronic pain in adult and pediatric patients with SCD. The ASH guidelines provide 18 recommendations; here, we highlight the 8 recommendations most pertinent to the hospitalist.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Acute Pain
Acute pain in the guideline is defined as pain that results in an unplanned visit to an acute care center for treatment.
Recommendation 1. For adult and pediatric patients presenting to an acute care setting with SCD-related acute pain, the ASH guideline panel recommends rapid (ie, within 1 hour of arrival at the emergency department [ED]) assessment and administration of analgesia, with reassessments every 30-60 minutes to optimize pain control (Strong recommendation; low certainty in the evidence about effects).
Although the perceived benefits are unclear due to insufficient evidence, the panel agrees that delaying pain management results in undeniable harm to patients. Hence, this recommendation was deemed both acceptable and ethical. Rapid evaluation also allows for earlier identification and treatment of other potential SCD-related complications.
Recommendation 2. For adult and pediatric patients presenting to an acute care setting with SCD-associated pain for whom opioid therapy is indicated, the ASH guideline panel suggests tailored opioid dosing based on consideration of baseline opioid therapy and prior effective therapy. (For adults: conditional recommendation; moderate certainty in the evidence about effects. For children: conditional recommendation; low certainty in the evidence about effects).
One randomized controlled trial examined patient-specific opioid dosing (based on current chronic opioid therapy [COT] and previously known effective acute pain management) vs weight-based dosing in the ED and found that participants randomized into the patient-specific protocol had a greater reduction in pain and decreased rate of hospital admission.4
The panel acknowledges that intravenous patient-controlled opioid analgesia is generally the standard of care at most institutions. However, no clear data address whether continuous opioid infusion in addition to on-demand dosing is beneficial.
Recommendation 3. For adult and pediatric patients with acute pain related to SCD, the ASH guideline panel suggests a short course (5 to 7 days) of nonsteroidal anti-inflammatory drugs (NSAIDs) in addition to opioids (Conditional recommendation; very low certainty in the evidence about effects).
The use of NSAIDs for managing pain in hospitalized patients with SCD has been associated with a reduction in the use of opioids in the inpatient setting and decreased lengths of stay.5 The potential harms of NSAIDs, including renal and gastrointestinal toxicity, however, should be factored into the decision-making as the risks may outweigh the potential benefits.
Recommendation 4. For adult and pediatric patients with SCD hospitalized for acute pain, the ASH guideline panel suggests a subanesthetic (analgesic) infusion of ketamine as adjunctive treatment of pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects). The guideline panel also suggests regional anesthesia for localized pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects).
Studies have demonstrated reduced pain and opioid utilization in individuals who received adjuvant ketamine infusions6 or regional anesthesia (ie, epidural).7 Feasibility, however, is limited to centers that have the appropriate experience and expertise with these interventions.
Recommendation 5. For adult and pediatric patients who have recurrent acute pain associated with SCD, the ASH guideline panel suggests against chronic monthly transfusion therapy as a first-line strategy to prevent or reduce recurrent acute pain episodes (Conditional recommendation; low certainty in the evidence about effects). The evidence for monthly transfusions in preventing recurrent pain is limited. There is, however, a moderate risk of harm, including iron overload and transfusion reactions, in addition to substantial burden and costs.
Chronic Pain
Chronic pain in the guideline is defined as ongoing pain present on most days over the past 6 months.
Recommendation 6. For adult patients with SCD who have chronic pain from the SCD-related identifiable cause avascular necrosis (AVN) of the bone, the ASH guideline panel suggests the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) or NSAIDs in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). For patients with no identifiable cause beyond SCD, the guideline panel suggests SNRIs, tricyclic antidepressants, or gabapentinoids for pain management (Conditional recommendation; very low certainty in the evidence about effects). Given the lack of direct evidence, indirect evidence was used to formulate these recommendations. For pain associated with AVN, data were extrapolated from literature on osteoarthritis, a form of degenerative arthropathy. For pain without an identifiable cause, evidence was taken from studies on fibromyalgia, a condition the panel felt most closely aligned with chronic pain related to SCD.
No recommendations were made for pediatric patients as the indirect evidence base only addressed adult patients.
Recommendation 7. For adult and pediatric patients with SCD and emerging and/or recently developed chronic pain, the ASH guideline panel does not recommend initiating COT unless pain is refractory to multiple other treatment modalities (Conditional recommendation; very low certainty in the evidence about effects). For patients receiving COT who are functioning well and have perceived benefit, the ASH guideline panel suggests shared decision-making for continuation of COT (Conditional recommendation; very low certainty in the evidence about effects).
High-quality data on the benefit of long-term COT in individuals with chronic noncancer pain are lacking. The panel maintains that the decision to initiate or continue COT should be individualized after weighing appropriate risks and benefits.
Recommendation 8. For adult and pediatric patients with chronic pain related to SCD, the panel suggests cognitive and behavioral pain management strategies in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). Cognitive behavioral therapy may decrease overall pain intensity and improve coping skills.8 The panel agrees that medications alone may not be effective in reducing the burden of chronic pain in adult and pediatric patients with SCD.
CRITIQUE
The guidelines were created by a multidisciplinary panel that included physicians from hematology, pain medicine, psychiatry, and emergency medicine, a doctoral nurse practitioner, and two patient representatives. The Mayo Evidence-Based Practice Research Program supported the guideline-development process. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach was used to assess evidence and make recommendations.
High-quality data in treating acute and chronic pain in both adult and pediatric patients with SCD are limited. As such, the majority of recommendations in these guidelines are conditional. The panel included studies that were indirectly related to SCD based on consensus (eg, inferred data from disease processes thought to be similar to SCD). One panelist disclosed receiving direct payments from a company that could be affected by these guidelines; however, it was deemed that the conflict was unlikely to have influenced any recommendations.
AREAS IN NEED OF FUTURE STUDY
The panel acknowledges that further investigation is needed for both nonpharmacologic and pharmacologic modalities in treating acute and chronic pain related to SCD. Examples include evaluating the comparative-effectiveness of COT vs nonopioid pharmacotherapy, the benefits and harms of continuous opioid infusions in acute pain crises, and the impact of chronic transfusions on acute and chronic pain.
Sickle cell disease (SCD) affects an estimated 100,000 people in the United States.1 Pain is the most common complication of SCD and the primary reason patients with SCD seek medical attention.2 In 2016, three-fourths of the approximately 130,000 SCD-related hospitalizations in the United States involved pain crises.3 When managing patients with SCD and chronic pain, an individualized and interdisciplinary approach is crucial. In 2020, the American Society of Hematology (ASH) developed guidelines reflecting the latest evidence in managing acute and chronic pain in adult and pediatric patients with SCD. The ASH guidelines provide 18 recommendations; here, we highlight the 8 recommendations most pertinent to the hospitalist.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Acute Pain
Acute pain in the guideline is defined as pain that results in an unplanned visit to an acute care center for treatment.
Recommendation 1. For adult and pediatric patients presenting to an acute care setting with SCD-related acute pain, the ASH guideline panel recommends rapid (ie, within 1 hour of arrival at the emergency department [ED]) assessment and administration of analgesia, with reassessments every 30-60 minutes to optimize pain control (Strong recommendation; low certainty in the evidence about effects).
Although the perceived benefits are unclear due to insufficient evidence, the panel agrees that delaying pain management results in undeniable harm to patients. Hence, this recommendation was deemed both acceptable and ethical. Rapid evaluation also allows for earlier identification and treatment of other potential SCD-related complications.
Recommendation 2. For adult and pediatric patients presenting to an acute care setting with SCD-associated pain for whom opioid therapy is indicated, the ASH guideline panel suggests tailored opioid dosing based on consideration of baseline opioid therapy and prior effective therapy. (For adults: conditional recommendation; moderate certainty in the evidence about effects. For children: conditional recommendation; low certainty in the evidence about effects).
One randomized controlled trial examined patient-specific opioid dosing (based on current chronic opioid therapy [COT] and previously known effective acute pain management) vs weight-based dosing in the ED and found that participants randomized into the patient-specific protocol had a greater reduction in pain and decreased rate of hospital admission.4
The panel acknowledges that intravenous patient-controlled opioid analgesia is generally the standard of care at most institutions. However, no clear data address whether continuous opioid infusion in addition to on-demand dosing is beneficial.
Recommendation 3. For adult and pediatric patients with acute pain related to SCD, the ASH guideline panel suggests a short course (5 to 7 days) of nonsteroidal anti-inflammatory drugs (NSAIDs) in addition to opioids (Conditional recommendation; very low certainty in the evidence about effects).
The use of NSAIDs for managing pain in hospitalized patients with SCD has been associated with a reduction in the use of opioids in the inpatient setting and decreased lengths of stay.5 The potential harms of NSAIDs, including renal and gastrointestinal toxicity, however, should be factored into the decision-making as the risks may outweigh the potential benefits.
Recommendation 4. For adult and pediatric patients with SCD hospitalized for acute pain, the ASH guideline panel suggests a subanesthetic (analgesic) infusion of ketamine as adjunctive treatment of pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects). The guideline panel also suggests regional anesthesia for localized pain refractory or not effectively treated with opioids alone (Conditional recommendation; very low certainty in the evidence about effects).
Studies have demonstrated reduced pain and opioid utilization in individuals who received adjuvant ketamine infusions6 or regional anesthesia (ie, epidural).7 Feasibility, however, is limited to centers that have the appropriate experience and expertise with these interventions.
Recommendation 5. For adult and pediatric patients who have recurrent acute pain associated with SCD, the ASH guideline panel suggests against chronic monthly transfusion therapy as a first-line strategy to prevent or reduce recurrent acute pain episodes (Conditional recommendation; low certainty in the evidence about effects). The evidence for monthly transfusions in preventing recurrent pain is limited. There is, however, a moderate risk of harm, including iron overload and transfusion reactions, in addition to substantial burden and costs.
Chronic Pain
Chronic pain in the guideline is defined as ongoing pain present on most days over the past 6 months.
Recommendation 6. For adult patients with SCD who have chronic pain from the SCD-related identifiable cause avascular necrosis (AVN) of the bone, the ASH guideline panel suggests the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) or NSAIDs in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). For patients with no identifiable cause beyond SCD, the guideline panel suggests SNRIs, tricyclic antidepressants, or gabapentinoids for pain management (Conditional recommendation; very low certainty in the evidence about effects). Given the lack of direct evidence, indirect evidence was used to formulate these recommendations. For pain associated with AVN, data were extrapolated from literature on osteoarthritis, a form of degenerative arthropathy. For pain without an identifiable cause, evidence was taken from studies on fibromyalgia, a condition the panel felt most closely aligned with chronic pain related to SCD.
No recommendations were made for pediatric patients as the indirect evidence base only addressed adult patients.
Recommendation 7. For adult and pediatric patients with SCD and emerging and/or recently developed chronic pain, the ASH guideline panel does not recommend initiating COT unless pain is refractory to multiple other treatment modalities (Conditional recommendation; very low certainty in the evidence about effects). For patients receiving COT who are functioning well and have perceived benefit, the ASH guideline panel suggests shared decision-making for continuation of COT (Conditional recommendation; very low certainty in the evidence about effects).
High-quality data on the benefit of long-term COT in individuals with chronic noncancer pain are lacking. The panel maintains that the decision to initiate or continue COT should be individualized after weighing appropriate risks and benefits.
Recommendation 8. For adult and pediatric patients with chronic pain related to SCD, the panel suggests cognitive and behavioral pain management strategies in the context of a comprehensive disease and pain management plan (Conditional recommendation; very low certainty in the evidence about effects). Cognitive behavioral therapy may decrease overall pain intensity and improve coping skills.8 The panel agrees that medications alone may not be effective in reducing the burden of chronic pain in adult and pediatric patients with SCD.
CRITIQUE
The guidelines were created by a multidisciplinary panel that included physicians from hematology, pain medicine, psychiatry, and emergency medicine, a doctoral nurse practitioner, and two patient representatives. The Mayo Evidence-Based Practice Research Program supported the guideline-development process. The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach was used to assess evidence and make recommendations.
High-quality data in treating acute and chronic pain in both adult and pediatric patients with SCD are limited. As such, the majority of recommendations in these guidelines are conditional. The panel included studies that were indirectly related to SCD based on consensus (eg, inferred data from disease processes thought to be similar to SCD). One panelist disclosed receiving direct payments from a company that could be affected by these guidelines; however, it was deemed that the conflict was unlikely to have influenced any recommendations.
AREAS IN NEED OF FUTURE STUDY
The panel acknowledges that further investigation is needed for both nonpharmacologic and pharmacologic modalities in treating acute and chronic pain related to SCD. Examples include evaluating the comparative-effectiveness of COT vs nonopioid pharmacotherapy, the benefits and harms of continuous opioid infusions in acute pain crises, and the impact of chronic transfusions on acute and chronic pain.
1. Data & statistics on sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/data.html
2. Complications and treatments of sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/treatments.html
3. Fingar KR, Owens PL, Reid LD, Mistry KB, Barrett ML. Characteristics of Inpatient Hospital Stays Involving Sickle Cell Disease, 2000-2016. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Statistical Brief 251. September 2019. Accessed August 23, 2020. www.hcup-us.ahrq.gov/reports/statbriefs/sb251-Sickle-Cell-Disease-Stays-2016.pdf
4. Tanabe P, Silva S, Bosworth HB, et al. A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD). Am J Hematol. 2018;93(2):159-168. https://doi.org/10.1002/ajh.24948
5. Perlin E, Finke H, Castro O, et al. Enhancement of pain control with ketorolac tromethamine in patients with sickle cell vaso-occlusive crisis. Am J Hematol. 1994;46(1):43-47. https://doi.org/10.1002/ajh.2830460108
6. Sheehy KA, Lippold C, Rice AL, et al. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res. 2017;10:787-795. https://doi.org/10.2147/jpr.s131156
7. New T, Venable C, Fraser L, et al. Management of refractory pain in hospitalized adolescents with sickle cell disease: changing from intravenous opioids to continuous infusion epidural analgesia. J Pediatr Hematol Oncol. 2014;36(6):e398-e402. https://doi.org/10.1097/mph.0000000000000026
8. Schatz J, Schlenz AM, McClellan CB, et al. Changes in coping, pain, and activity after cognitive-behavioral training: a randomized clinical trial for pediatric sickle cell disease using smartphones. Clin J Pain. 2015;31(6):536-547. https://doi.org/10.1097/ajp.0000000000000183
1. Data & statistics on sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/data.html
2. Complications and treatments of sickle cell disease. Centers for Disease Control and Prevention. Accessed August 23, 2020. https://www.cdc.gov/ncbddd/sicklecell/treatments.html
3. Fingar KR, Owens PL, Reid LD, Mistry KB, Barrett ML. Characteristics of Inpatient Hospital Stays Involving Sickle Cell Disease, 2000-2016. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Statistical Brief 251. September 2019. Accessed August 23, 2020. www.hcup-us.ahrq.gov/reports/statbriefs/sb251-Sickle-Cell-Disease-Stays-2016.pdf
4. Tanabe P, Silva S, Bosworth HB, et al. A randomized controlled trial comparing two vaso-occlusive episode (VOE) protocols in sickle cell disease (SCD). Am J Hematol. 2018;93(2):159-168. https://doi.org/10.1002/ajh.24948
5. Perlin E, Finke H, Castro O, et al. Enhancement of pain control with ketorolac tromethamine in patients with sickle cell vaso-occlusive crisis. Am J Hematol. 1994;46(1):43-47. https://doi.org/10.1002/ajh.2830460108
6. Sheehy KA, Lippold C, Rice AL, et al. Subanesthetic ketamine for pain management in hospitalized children, adolescents, and young adults: a single-center cohort study. J Pain Res. 2017;10:787-795. https://doi.org/10.2147/jpr.s131156
7. New T, Venable C, Fraser L, et al. Management of refractory pain in hospitalized adolescents with sickle cell disease: changing from intravenous opioids to continuous infusion epidural analgesia. J Pediatr Hematol Oncol. 2014;36(6):e398-e402. https://doi.org/10.1097/mph.0000000000000026
8. Schatz J, Schlenz AM, McClellan CB, et al. Changes in coping, pain, and activity after cognitive-behavioral training: a randomized clinical trial for pediatric sickle cell disease using smartphones. Clin J Pain. 2015;31(6):536-547. https://doi.org/10.1097/ajp.0000000000000183
© 2021 Society of Hospital Medicine
Procedural Competency Among Hospitalists: A Literature Review and Future Considerations
Over the past 20 years, hospitalists have served as the primary workforce for the clinical care of medical inpatients in the United States.1,2 Core competencies1 state that hospitalists should be able to perform the following bedside procedures: lumbar puncture, paracentesis, thoracentesis, arthrocentesis, and central venous catheter placement. More recently, standard of care has dictated that these procedures be performed under ultrasound guidance,3-6 and thus hospitalists are also expected to be adept at point-of-care ultrasound (POCUS).7
However, no current national standard exists for ensuring basic competency among hospitalists performing bedside procedures. In addition, hospitalists’ procedural volumes are declining,8,9 and standards for procedural training during internal medicine residency have been reduced.10 As a result, many residents who intend to become hospitalists are no longer prepared to perform these procedures.
The ramifications of the loss of procedural competency for hospitalists are manifold. Technical errors are a significant source of patient morbidity and mortality,11-15 and complications arising specifically from nonoperative procedures range from 0 to 19%,16 although these data do not distinguish technical errors from unpreventable adverse events nor the degree to which hospitalists contributed to these complications. Second, hospitalists in academic medical centers might be ill equipped to function as supervisors of trainees performing procedures, which could perpetuate a cycle of suboptimal technical skills.17 Finally, the discrepancy between consensus guidelines for hospitalists and their scope of practice represents a significant area of risk management for institutions that base their credentialing policies on published competencies.
There are many compelling reasons for why hospitalists should maintain—in fact reclaim—a primary role in bedside procedures.18 Hospitalists in community and rural settings might not have easy access to procedural specialists. In academic institutions, hospitalists are the primary instructors and supervisors of procedures performed by internal medicine residents. The increased availability of POCUS allows formally trained hospitalists to perform procedures more safely under imaging guidance.16
The literature on procedures performed by hospitalists, although limited, has focused on POCUS, systems innovations such as medical procedure services (MPS), and policy recommendations for procedural credentialing. Most studies on effective procedural instructional approaches have been conducted among trainees, who are procedural novices. This research does not sufficiently address the dilemma that hospitalists face as independent physicians for whom procedures are not a significant component of their practice, yet are expected to perform invasive procedures occasionally. The purpose of our literature review is to synthesize the available research to characterize contributors to hospitalists’ procedural competency. We conclude with considerations for hospital medicine practice.
METHODS
We performed a structured literature search for peer-reviewed articles related to hospitalists conducting procedures, being trained in procedures, or related to hospitalist-run MPS. We focused our search on the core hospitalist procedures with the highest potential morbidity (ie, lumbar puncture, abdominal paracentesis, thoracentesis, and central venous catheterization). We searched PubMed and Google Scholar for articles published since 1996 (when the term “hospitalists” was first coined) using keyword searches for [hospitalist OR hospital medicine] AND [procedur* OR medical procedur* OR medical procedure service] OR [(procedur* AND (train* OR educat* OR teach OR instruct*)] OR abdominal paracentes* OR thoracentes* OR lumbar puncture OR central venous catheter* OR ultrasound OR point-of-care. We included original research, brief research reports, perspectives, guidelines, and consensus statements. Exclusion criteria were articles that focused on nonhospitalists and conference abstracts. We used pearling to identify secondary sources from included articles’ bibliographies, without limits on year of publication.
RESULTS
Trends Towards Specialist Referrals
Between 1986 and 2007, the number and variety of procedures performed by internists decreased by half.19 Hospitalists still completed procedures in greater volume and variety than nonhospitalists,8 with approximately 50% of hospitalists performing lumbar punctures (50%), abdominal paracenteses (49%), and thoracenteses (44%) compared with less than 25% for all three procedures for nonhospitalists. Additionally, only 11% of surveyed hospitalists8 performed all nine core procedures, although these included procedures that are largely cognitive in nature (eg, electrocardiogram interpretation, chest X-ray interpretation) or procedures that have been relegated to other specialists (eg, endotracheal intubation, ventilator management, or joint injection/aspiration).
Surveys showed that, especially in larger cities and academic centers, procedural specialists have taken over a disproportionate share of procedures even as the number of procedures performed continued to rise.20 Between 1993 and 2008, the number of paracenteses and thoracenteses increased by 133% and decreased by 14%, respectively, but the share of procedures performed by radiologists increased by 964% and 358%, respectively, as evident in an analysis of Medicare billing data.20 A more recent study of Medicare claims from 2004 to 2016 similarly revealed that the percentage of paracenteses performed by radiologists compared with nonradiologists rose from 70% to 80% and thoracenteses from 47% to 66%, respectively.21 Comparable trends were apparent in claims data for lumbar punctures; between 1991 and 2011, the share of lumbar punctures performed by radiologists rose from 11% to 48%.22
In academic medical centers, hospitalists might have the opportunity to pursue other activities (eg, education, administration, research) as they progress in their careers, resulting in less clinical activity. Although hospitalists who are more clinically active in hospital care tended to perform more procedures,8 those with smaller clinical footprints reported lower levels of comfort with performing procedures8 and might have less available time to maintain procedural competency or train in new technologies such as POCUS.17
Additionally, hospitalists in both academic and community settings cited efficiency as a major reason for procedural referral. Hospitalists tended to perform more procedures if they had fixed salaries or if less than 50% of their income was based on clinical productivity, although this trend was not significant.8 Further, they also might be motivated by competing opportunity costs such as time lost caring for other patients or length of shift, which influences the amount of time spent at work.23
Notably, speculation that hospitalists referred more complex cases to specialists was not borne out by studies examining referral patterns.21,24,25
Procedural Outcomes for Hospitalists vs Nonhospitalists
No convincing data exist that procedures performed by specialists have better outcomes than those completed at the bedside by well-trained generalists, although studies were limited to the inpatient setting, to generalists who have some exposure to procedures, and to internal medicine residents on inpatient rotations. In one retrospective review, interventional radiology (IR) referrals were associated with more platelet or plasma transfusions and intensive care unit transfers than those performed at the bedside by internal medicine residents, findings that remained significant after accounting for complexity (eg, Model for End-stage Liver Disease score, need for dialysis, and platelet count).24 Similarly, a prospective audit of 529 bedside procedures did not show any differences in complication rates between generalists and pulmonologists, once generalists underwent standardized training and used pleural safety checklists and ultrasound guidance.26 An administrative database review of 130,000 inpatient thoracenteses across several university hospitals between 2010 and 2013 found that the risk of iatrogenic pneumothorax was similar among operators from IR, medicine, and pulmonary (2.8%, 2.9%, and 3.1%, respectively)27; these findings have been reproduced in other studies.28 Finally, the increasing adoption of procedural ultrasound permits procedures to be conducted more safely at the bedside, without the need to refer to radiology for imaging guidance.3-5
IR procedures also are associated with increased healthcare costs compared with bedside procedures. One study showed that hospital costs for admissions when paracenteses were performed by radiologists were higher than those in which the procedure was completed at the bedside by gastroenterologists or hepatologists.25 A chart review examining 399 paracenteses, thoracenteses, and lumbar punctures found that the average procedure cost increased by 38% for referred procedures and 56% for radiology-performed procedures, as compared with bedside procedures.29 Needing ancillary staffing in dedicated suites to perform procedures contributed to the excess cost.9 Moreover, referred procedures resulted in increased length of stay, which can incur additional costs. However, the data were conflicting; two studies did not show a statistical difference,25,28 while others found an increased length of stay,24,27,29 which might be due to the unavailability of specialists during off hours, thereby delaying nonemergent procedures.21 Detailed cost analyses have controlled for the use of procedural facilities and blood transfusions among IR specialists and simulation training among generalists, showing that total costs were $663 per patient undergoing IR procedures compared with $134 per patient undergoing bedside procedures.30
Lack of Standardized Procedural Training or Assessment
A robust body of primary studies and systematic reviews supports the use of simulation for procedural training to improve comfort and skill as well as reduce complication rates and costs.31,32 A systematic review that investigated the impact of four paradigms of procedural training found that MPS and quality improvement/patient safety approaches led to the most active learning compared with apprenticeship (ie, “see one, do one”) and approaches based on educational theories.33 Nevertheless, the vast majority of the research has been conducted in trainees,32,34 with sparse evidence among practicing physicians. One cohort study of attending physicians’ central venous catheter insertion skills on simulators found low and variable short-term performance, showing overall poor adherence to checklists.35 One article suggested that hospitalists’ procedural skills were below established thresholds of competency at baseline and that simulation-based training did not result in sustained skills, but the small sample size and high attrition limited meaningful conclusions.36 Although continuing medical education courses are available to hospitalists, there is no published evidence supporting their effectiveness.
Proxies for procedural skill have included comfort and experience, yet these markers have broadly been shown to be inadequate.34,36,37 Additionally, the natural decline of skill over time has invoked the need for periodic reassessment of proficiency.36,38 Credentialing has been equally inconstant; a survey of the Society of Hospital Medicine’s (SHM) POCUS task force revealed that only half of respondents reported their hospitals required a minimum number of procedures for initial credentialing and recredentialing.39 In short, periodic assessment of procedural skills among hospitalists has not been a routine process at many institutions.
Role of Hospitalist-Run Medical Procedure Services
It might not be necessary for all hospitalists to be proficient and credentialed in a given procedure,1 and a trend has emerged in the creation of MPS staffed by hospitalists as proceduralists. The primary aim of these MPS has been to recapture the procedures—and associated revenue—that would otherwise be referred to specialists. Moreover, concentrating procedures among a core group of hospitalists endeavors to support patient safety through several principles: (1) to increase technical proficiency through higher procedural volumes, (2) to facilitate rigorous training and assessment among dedicated individuals, and (3) to systematize best practices of operator performance, communication, and documentation.
MPS have been implemented around the country and have demonstrated several advantages. In one institution, medical firms that were offered the use of an MPS had 48% more procedural attempts by nonspecialists, without significant differences in the proportions of successful attempts or complications compared with the firms who more often referred to specialists.40 A retrospective study analyzed outcomes of 1,707 bedside procedures, of which 548 were performed by an MPS, and found that procedures done by the MPS were more likely to result in lower rates of unsuccessful procedures and to use best-practice safety processes (ie, to involve attending physicians, to use ultrasound guidance, and to avoid femoral sites for catheterization).12 Satisfaction was high among patients who underwent bedside procedures performed by a hospitalist-supervised, intern-based procedure service with a focus on bedside communication.41 From a workforce perspective, MPS have also allowed surgical or radiological subspecialties to focus on more complex cases with higher reimbursement rates,18,42 for proceduralists to expand beyond core procedures (eg, bone marrow biopsies43), and to train advanced practice providers.44 Although studies have not shown that the outcomes of procedures completed by an MPS are better than the outcomes of procedures performed by other specialists,45 one can potentially extrapolate from earlier data that procedures done at the bedside by nonradiologists would have comparable outcomes.
DISCUSSION
A myriad of factors is influencing hospitalists’ scope of practice with respect to bedside procedures. Some evidence suggests that procedures performed by specialists are not superior to those done by generalists and might be associated with increased costs. The most promising developments in the past few decades include simulation-based training, which has demonstrated effectiveness across an array of clinical outcomes but has not been sufficiently evaluated in hospitalists to draw conclusions, and hospitalist-led MPS, which promote safe and productive procedural clinical practices. However, decreasing procedural volume, increasing referrals to specialists, dwindling hospitalist interest and/or confidence, time constraints, limited training opportunities, nonuniform credentialing policies, and lack of standardized assessment are cumulatively contributing to a loss of procedural competency among hospitalists.
Taken together, these forces should compel hospital medicine groups that expect their hospitalists to perform their own procedures to identify necessary steps for ensuring the safety of hospitalized patients under their care. The following considerations derive from the available—albeit modest—evidence on procedural performance in hospital medicine (Table).
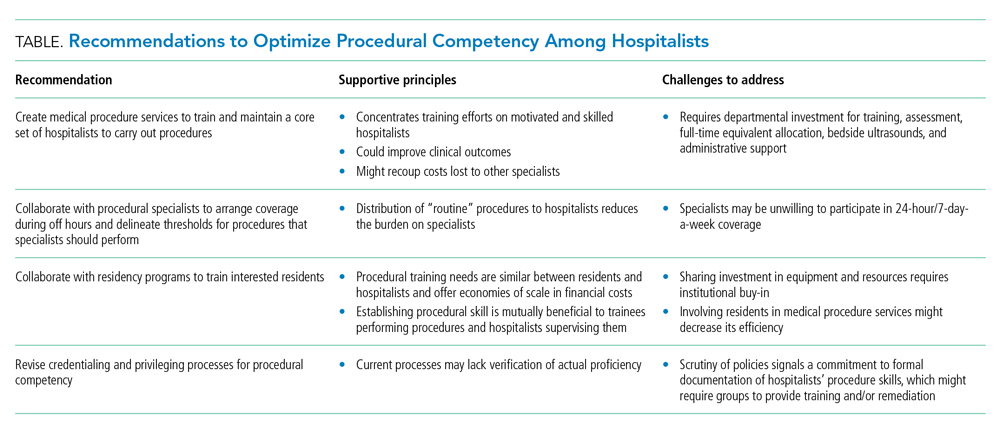
1. Create MPS to establish a core set of hospitalists to perform procedures and train them using evidence-based practices. Creation of an MPS places the responsibility of core bedside procedures in the hands of a select group of proceduralists. This strategy streamlines training and assessment of individual procedural competency to meet standards set by SHM36,46 and improves educational outcomes.47-49 MPS could improve clinical outcomes,12,42,50-52 including length of stay and cost, while maintaining patient satisfaction,41 as well as recoup lost revenue from referrals by increasing the volume of procedures done by generalists,40,49 although no robust data supporting the latter point exists. Implementing an MPS requires full-time equivalent (FTE) support for proceduralists and administrative support for data collection and tracking complications. Furthermore, a well-functioning MPS will require investment in portable ultrasound machines and training in POCUS, which has been shown to decrease complications and increase success of invasive bedside procedures.3-7 Hospital medicine groups should be aware that staffing an MPS can divert hospitalist labor and resources from other needed clinical areas, especially during the initial, low-volume phases of implementation. Strategies to offset relative value unit (RVU) loss include combining the MPS with existing clinical roles such as medical consults, code triage, and rapid response teams; or with services with lower patient caps, which might work particularly well in community hospitals. In many institutions, hospitalists can bill for procedural consults in addition to the procedures when the consult involves nonmedical patients, which is relevant when the procedure ultimately cannot be performed (eg, too little ascites to safely perform a paracentesis). Further research should establish best practices of MPS to ensure maximum procedural productivity and safety, because there are no rigorous prospective studies that evaluate strategies to create this service. Such strategies include determining the optimal ratio of proceduralists to general hospitalists, hospital characteristics that benefit most from MPS (eg, referral centers, urban-based settings), volume and type of procedures performed, and the proportion and type of referrals that are most cost-effective.
2. Establish policies with procedural specialists to arrange coverage for off-hours procedures and delineate thresholds for procedures that specialists should perform. Expanding hospitalists’ capabilities in performing procedures should trigger reconsideration of the medical center’s approach to procedural safety. A goal would be to have hospital medicine groups work collaboratively with specialists and other disciplines (eg, surgery, emergency medicine, anesthesia, or radiology) to ensure 24-hour, 7-day a week coverage of urgent bedside procedures. The potential to decrease length of stay and improve off-hour procedural quality might be a compelling rationale for hospital administration, whether or not an MPS is used. That said, we recognize that other services might be unable or unwilling to provide such coverage and that specialist off-hour coverage would incur increased costs and could reduce exposure opportunities for internal medicine residents.
A hospital-level procedures committee might be required to support an institutional imperative for procedural safety and to oversee the implementation of approaches that are practical, financially sustainable, and equitable for all service lines, especially because hospitalist groups might bear the early costs of training and retraining.
3. Hospitalist–proceduralists should collaborate with internal medicine residency programs to offer intensive procedural training experiences to residents who want these skills to be part of their future practice. Robust procedural training for trainees promotes better outcomes for the current workforce and helps to populate the future workforce with procedurally competent practitioners. Simulation-based training is a well-established procedural instruction method that is safe, authentic, and effective in terms of clinical outcomes.34 As the primary teachers of residents in many institutions, hospitalists often are the ones who impart procedural skills to residents, despite uneven skill sets. It is in the interest of internal medicine residency program directors to advocate for a core group of hospitalist–proceduralists, as MPS offer an infrastructure for training that has been shown to increase procedural volume and improve skills.47,48,50 Program directors could therefore be incentivized to sponsor some of these procedural roles with teaching and administration funds, as a trade-off for securing higher-quality procedural training and closer supervision for their trainees. The dual necessity of teaching procedural skills to residents and attending physicians alike offers economies of scale for the use of facilities, personnel, and equipment, and gives faculty an opportunity to extend their clinical teaching skills into the domain of procedural supervision.
4. Hospital medicine groups should re-evaluate credentialing and privileging to ensure procedural competency. Given the lack of published data that characterizes how many hospital medicine groups credential hospitalists to perform procedures and what practices they use to assess competency, hospital medicine groups might be signing off on procedures without verifying hospitalists’ proficiency in core procedures. SHM’s position statement on credentialing for ultrasound-guided procedures46 describes standards that could be applied to other procedures. It proposes that credentialing processes should be grounded in simulation- and patient-based assessments of cognitive and psychomotor skills, using published checklists and global ratings for feedback. Simulation training could support provisional certification, but hospitalists should reach minimum thresholds of supervised patient-based experience before initial credentialing, with continuous reassessment of competency to mitigate skill decay. Prospectively tracking procedural metrics, such as procedural volume and complication rates, also will support systematic skill assessment. Finally, similar to any other medical error, near misses and complications should trigger periprocedural safety reviews.
Limitations
The modest body of research on hospitalists and procedures is the central limitation of our synthesis. Much of the literature consisted of consensus statements, retrospective studies, and small prospective educational studies. As a result, we did not adopt all strategies considered standard in a scoping or systematic review. The literature on MPS specifically was insufficient to draw conclusions about their operational and financial impact or effects on procedure quality. Our primary recommendation to implement MPS requires significant fiscal investment and infrastructure. It also entails risks that must be proactively addressed, including the potential for net financial loss and decreased educational opportunities for residents.
CONCLUSIONS
Hospitalists regularly face the predicament of being expected to independently perform procedures, with little access to training, minimal experience, and no ongoing assessment to ensure their proficiency or the safety of their patients. Past assumptions about hospitalists’ responsibility do not reflect realities in practice patterns and have not translated to widespread adoption of procedural training, monitoring, and assessment mechanisms. Our work summarizes a body of literature that, although limited in empiric studies of hospitalists themselves, offers insights with recommendations for hospital medicine groups wishing to uphold procedural skills as part of their providers’ professional identity.
1. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: Development and methodology. J Hosp Med. 2006;1(1):48-56. https://doi.org/10.1002/jhm.6
2. Wachter RM, Goldman L. Zero to 50,000 — The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E7-E15. https://doi.org/10.12788/jhm.3095
4. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3197
5. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940
6. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287
7. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079
8. Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25(5):448-452. https://doi.org/10.1007/s11606-010-1284-2
9. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. https://doi.org/10.1002/jhm.602
10. American Board of Internal Medicine. Policies and procedures for certification. Accessed December 3, 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf
11. Myers LC. Toward preventing medical malpractice claims related to chest procedures. Ann Am Thorac Soc. 2020;17(6):776-779. https://doi.org/10.1513/AnnalsATS.201912-863RL
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604
14. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324(6):377-384. https://doi.org/10.1056/NEJM199102073240605
15. Myers LC, Gartland RM, Skillings J, et al. An examination of medical malpractice claims involving physician trainees. Acad Med. 2020;95(8):1215-1222. https://doi.org/10.1097/ACM.0000000000003117
16. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
17. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. https://doi.org/10.1097/ACM.0000000000001327
18. Nelson B. Hospitalists try to reclaim lead role in bedside procedures. The Hospitalist. March 2015. Accessed June 27, 2020. https://www.the-hospitalist.org/hospitalist/article/122571/hospitalists-try-reclaim-lead-role-bedside-procedures
19. Wigton RS, Alguire P; American College of Physicians. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355-360. https://doi.org/10.7326/0003-4819-146-5-200703060-00007
20. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. https://doi.org/10.1016/j.jacr.2010.04.013
21. Gottumukkala RV, Prabhakar AM, Hemingway J, Hughes DR, Duszak R Jr. Disparities over time in volume, day of the week, and patient complexity between paracentesis and thoracentesis procedures performed by radiologists versus those performed by nonradiologists. J Vasc Interv Radiol. 2019;30(11):1769-1778.e1. https://doi.org/10.1016/j.jvir.2019.04.015
22. Kroll H, Duszak R Jr, Nsiah E, Hughes DR, Sumer S, Wintermark M. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. Am J Roentgenol. 2014;204(1):15-19. https://doi.org/10.2214/AJR.14.12622
23. Jensen T, Lai A, Mourad M. Can lessons from systems-based mastery learning for thoracentesis be translated to hospitalists? J Hosp Med. 2016;11(11):811-812. https://doi.org/10.1002/jhm.2655
24. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
25. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. https://doi.org/10.1002/jhm.2153
26. See KC, Ong V, Teoh CM, et al. Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology. 2014;19(3):396-402. https://doi.org/10.1111/resp.12244
27. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. https://doi.org/10.1016/S1553-7250(16)42004-0
28. Berger MS, Divilov V, Paredes H, Sun E. Abdominal paracentesis: safety and efficacy comparing medicine resident bedside paracentesis vs. paracentesis performed by interventional radiology. J Clin Gastroenterol Hepatol. 2018;2(4). https://doi.org/10.21767/2575-7733.1000050
29. Kay C, Wozniak EM, Szabo A, Jackson JL. Examining invasive bedside procedure performance at an academic medical center. South Med J. 2016;109(7):402-407. https://doi.org/10.14423/SMJ.0000000000000485
30. Barsuk JH, Cohen ER, Feinglass J, et al. Cost savings of performing paracentesis procedures at the bedside after simulation-based education. Simul Healthc. 2014;9(5):312-318. https://doi.org/10.1097/SIH.0000000000000040
31. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93(5):729-735. https://doi.org/10.1097/ACM.0000000000001965
32. Huang GC, McSparron JI, Balk EM, et al. Procedural instruction in invasive bedside procedures: a systematic review and meta-analysis of effective teaching approaches. BMJ Qual Saf. 2016;25(4):281-294. https://doi.org/10.1136/bmjqs-2014-003518
33. Brydges R, Stroud L, Wong BM, Holmboe ES, Imrie K, Hatala R. Core competencies or a competent core? a scoping review and realist synthesis of invasive bedside procedural skills training in internal medicine. Acad Med. 2017;92(11):1632-1643. https://doi.org/10.1097/ACM.0000000000001726
34. Brydges R, Hatala R, Zendejas B, Erwin PJ, Cook DA. Linking simulation-based educational assessments and patient-related outcomes: a systematic review and meta-analysis. Acad Med. 2015;90(2):246-256. https://doi.org/10.1097/ACM.0000000000000549
35. Barsuk JH, Cohen ER, Nguyen D, et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44(10):1871-1881. https://doi.org/10.1097/CCM.0000000000001831
36. Crocker JT, Hale CP, Vanka A, Ricotta DN, McSparron JI, Huang GC. Raising the bar for procedural competency among hospitalists. Ann Intern Med. 2019;170(9):654-655. https://doi.org/10.7326/M18-3007
37. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201-208. https://doi.org/10.4300/JGME-D-16-00426.1
38. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. https://doi.org/10.1097/ACM.0000000000000734
39. Jensen T, Soni N, Tierney D, Lucas B. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837
40. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. https://doi.org/10.1002/jhm.159
41. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: Is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
42. Ault MJ, Rosen BT. Proceduralists — leading patient-safety initiatives. N Engl J Med. 2007;356(17):1789-1790. https://doi.org/10.1056/NEJMc063239
43. Obasi JU, Umpierrez De Reguero AP. Safety profile of bone marrow aspiration and biopsies performed by the hospitalist procedure service at an academic center: an observational study. Blood. 2019;134(suppl 1): 5848. https://doi.org/10.1182/blood-2019-121444
44. Gisondi MA, Regan L, Branzetti J, Hopson LR. More learners, finite resources, and the changing landscape of procedural training at the bedside. Acad Med. 2018;93(5):699-704. https://doi.org/10.1097/ACM.0000000000002062
45. McCormack J. The new proceduralists: Have they found their niche? American Medical News. September 17, 2007. Accessed August 30, 2020. https://amednews.com/article/20070917/business/309179994/4/
46. Lucas BP, Tierney DM, Jensen TP, et al; Society of Hospital Medicine Point-of-Care Ultrasound Task Force. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917
47. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
48. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1
49. Montuno A, Hunt BR, Lee MM. Potential impact of a bedside procedure service on training procedurally competent hospitalists in a community-based residency program. J Community Hosp Intern Med Perspect. 2016;6(3):31054. https://doi.org/10.3402/jchimp.v6.31054
50. Smith CC, Gordon CE, Feller‐Kopman D, et al. Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency. J Gen Intern Med. 2004;19(5p2):510-513. https://doi.org/10.1111/j.1525-1497.2004.30161.x
51. Mourad M. Capsule commentary on Tukey et al., the impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):518. https://doi.org/10.1007/s11606-013-2740-6
52. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care: a systematic review and methodologic critique of the literature. 2002. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews . Accessed June 26, 2020. https://www.ncbi.nlm.nih.gov/books/NBK69189/
Over the past 20 years, hospitalists have served as the primary workforce for the clinical care of medical inpatients in the United States.1,2 Core competencies1 state that hospitalists should be able to perform the following bedside procedures: lumbar puncture, paracentesis, thoracentesis, arthrocentesis, and central venous catheter placement. More recently, standard of care has dictated that these procedures be performed under ultrasound guidance,3-6 and thus hospitalists are also expected to be adept at point-of-care ultrasound (POCUS).7
However, no current national standard exists for ensuring basic competency among hospitalists performing bedside procedures. In addition, hospitalists’ procedural volumes are declining,8,9 and standards for procedural training during internal medicine residency have been reduced.10 As a result, many residents who intend to become hospitalists are no longer prepared to perform these procedures.
The ramifications of the loss of procedural competency for hospitalists are manifold. Technical errors are a significant source of patient morbidity and mortality,11-15 and complications arising specifically from nonoperative procedures range from 0 to 19%,16 although these data do not distinguish technical errors from unpreventable adverse events nor the degree to which hospitalists contributed to these complications. Second, hospitalists in academic medical centers might be ill equipped to function as supervisors of trainees performing procedures, which could perpetuate a cycle of suboptimal technical skills.17 Finally, the discrepancy between consensus guidelines for hospitalists and their scope of practice represents a significant area of risk management for institutions that base their credentialing policies on published competencies.
There are many compelling reasons for why hospitalists should maintain—in fact reclaim—a primary role in bedside procedures.18 Hospitalists in community and rural settings might not have easy access to procedural specialists. In academic institutions, hospitalists are the primary instructors and supervisors of procedures performed by internal medicine residents. The increased availability of POCUS allows formally trained hospitalists to perform procedures more safely under imaging guidance.16
The literature on procedures performed by hospitalists, although limited, has focused on POCUS, systems innovations such as medical procedure services (MPS), and policy recommendations for procedural credentialing. Most studies on effective procedural instructional approaches have been conducted among trainees, who are procedural novices. This research does not sufficiently address the dilemma that hospitalists face as independent physicians for whom procedures are not a significant component of their practice, yet are expected to perform invasive procedures occasionally. The purpose of our literature review is to synthesize the available research to characterize contributors to hospitalists’ procedural competency. We conclude with considerations for hospital medicine practice.
METHODS
We performed a structured literature search for peer-reviewed articles related to hospitalists conducting procedures, being trained in procedures, or related to hospitalist-run MPS. We focused our search on the core hospitalist procedures with the highest potential morbidity (ie, lumbar puncture, abdominal paracentesis, thoracentesis, and central venous catheterization). We searched PubMed and Google Scholar for articles published since 1996 (when the term “hospitalists” was first coined) using keyword searches for [hospitalist OR hospital medicine] AND [procedur* OR medical procedur* OR medical procedure service] OR [(procedur* AND (train* OR educat* OR teach OR instruct*)] OR abdominal paracentes* OR thoracentes* OR lumbar puncture OR central venous catheter* OR ultrasound OR point-of-care. We included original research, brief research reports, perspectives, guidelines, and consensus statements. Exclusion criteria were articles that focused on nonhospitalists and conference abstracts. We used pearling to identify secondary sources from included articles’ bibliographies, without limits on year of publication.
RESULTS
Trends Towards Specialist Referrals
Between 1986 and 2007, the number and variety of procedures performed by internists decreased by half.19 Hospitalists still completed procedures in greater volume and variety than nonhospitalists,8 with approximately 50% of hospitalists performing lumbar punctures (50%), abdominal paracenteses (49%), and thoracenteses (44%) compared with less than 25% for all three procedures for nonhospitalists. Additionally, only 11% of surveyed hospitalists8 performed all nine core procedures, although these included procedures that are largely cognitive in nature (eg, electrocardiogram interpretation, chest X-ray interpretation) or procedures that have been relegated to other specialists (eg, endotracheal intubation, ventilator management, or joint injection/aspiration).
Surveys showed that, especially in larger cities and academic centers, procedural specialists have taken over a disproportionate share of procedures even as the number of procedures performed continued to rise.20 Between 1993 and 2008, the number of paracenteses and thoracenteses increased by 133% and decreased by 14%, respectively, but the share of procedures performed by radiologists increased by 964% and 358%, respectively, as evident in an analysis of Medicare billing data.20 A more recent study of Medicare claims from 2004 to 2016 similarly revealed that the percentage of paracenteses performed by radiologists compared with nonradiologists rose from 70% to 80% and thoracenteses from 47% to 66%, respectively.21 Comparable trends were apparent in claims data for lumbar punctures; between 1991 and 2011, the share of lumbar punctures performed by radiologists rose from 11% to 48%.22
In academic medical centers, hospitalists might have the opportunity to pursue other activities (eg, education, administration, research) as they progress in their careers, resulting in less clinical activity. Although hospitalists who are more clinically active in hospital care tended to perform more procedures,8 those with smaller clinical footprints reported lower levels of comfort with performing procedures8 and might have less available time to maintain procedural competency or train in new technologies such as POCUS.17
Additionally, hospitalists in both academic and community settings cited efficiency as a major reason for procedural referral. Hospitalists tended to perform more procedures if they had fixed salaries or if less than 50% of their income was based on clinical productivity, although this trend was not significant.8 Further, they also might be motivated by competing opportunity costs such as time lost caring for other patients or length of shift, which influences the amount of time spent at work.23
Notably, speculation that hospitalists referred more complex cases to specialists was not borne out by studies examining referral patterns.21,24,25
Procedural Outcomes for Hospitalists vs Nonhospitalists
No convincing data exist that procedures performed by specialists have better outcomes than those completed at the bedside by well-trained generalists, although studies were limited to the inpatient setting, to generalists who have some exposure to procedures, and to internal medicine residents on inpatient rotations. In one retrospective review, interventional radiology (IR) referrals were associated with more platelet or plasma transfusions and intensive care unit transfers than those performed at the bedside by internal medicine residents, findings that remained significant after accounting for complexity (eg, Model for End-stage Liver Disease score, need for dialysis, and platelet count).24 Similarly, a prospective audit of 529 bedside procedures did not show any differences in complication rates between generalists and pulmonologists, once generalists underwent standardized training and used pleural safety checklists and ultrasound guidance.26 An administrative database review of 130,000 inpatient thoracenteses across several university hospitals between 2010 and 2013 found that the risk of iatrogenic pneumothorax was similar among operators from IR, medicine, and pulmonary (2.8%, 2.9%, and 3.1%, respectively)27; these findings have been reproduced in other studies.28 Finally, the increasing adoption of procedural ultrasound permits procedures to be conducted more safely at the bedside, without the need to refer to radiology for imaging guidance.3-5
IR procedures also are associated with increased healthcare costs compared with bedside procedures. One study showed that hospital costs for admissions when paracenteses were performed by radiologists were higher than those in which the procedure was completed at the bedside by gastroenterologists or hepatologists.25 A chart review examining 399 paracenteses, thoracenteses, and lumbar punctures found that the average procedure cost increased by 38% for referred procedures and 56% for radiology-performed procedures, as compared with bedside procedures.29 Needing ancillary staffing in dedicated suites to perform procedures contributed to the excess cost.9 Moreover, referred procedures resulted in increased length of stay, which can incur additional costs. However, the data were conflicting; two studies did not show a statistical difference,25,28 while others found an increased length of stay,24,27,29 which might be due to the unavailability of specialists during off hours, thereby delaying nonemergent procedures.21 Detailed cost analyses have controlled for the use of procedural facilities and blood transfusions among IR specialists and simulation training among generalists, showing that total costs were $663 per patient undergoing IR procedures compared with $134 per patient undergoing bedside procedures.30
Lack of Standardized Procedural Training or Assessment
A robust body of primary studies and systematic reviews supports the use of simulation for procedural training to improve comfort and skill as well as reduce complication rates and costs.31,32 A systematic review that investigated the impact of four paradigms of procedural training found that MPS and quality improvement/patient safety approaches led to the most active learning compared with apprenticeship (ie, “see one, do one”) and approaches based on educational theories.33 Nevertheless, the vast majority of the research has been conducted in trainees,32,34 with sparse evidence among practicing physicians. One cohort study of attending physicians’ central venous catheter insertion skills on simulators found low and variable short-term performance, showing overall poor adherence to checklists.35 One article suggested that hospitalists’ procedural skills were below established thresholds of competency at baseline and that simulation-based training did not result in sustained skills, but the small sample size and high attrition limited meaningful conclusions.36 Although continuing medical education courses are available to hospitalists, there is no published evidence supporting their effectiveness.
Proxies for procedural skill have included comfort and experience, yet these markers have broadly been shown to be inadequate.34,36,37 Additionally, the natural decline of skill over time has invoked the need for periodic reassessment of proficiency.36,38 Credentialing has been equally inconstant; a survey of the Society of Hospital Medicine’s (SHM) POCUS task force revealed that only half of respondents reported their hospitals required a minimum number of procedures for initial credentialing and recredentialing.39 In short, periodic assessment of procedural skills among hospitalists has not been a routine process at many institutions.
Role of Hospitalist-Run Medical Procedure Services
It might not be necessary for all hospitalists to be proficient and credentialed in a given procedure,1 and a trend has emerged in the creation of MPS staffed by hospitalists as proceduralists. The primary aim of these MPS has been to recapture the procedures—and associated revenue—that would otherwise be referred to specialists. Moreover, concentrating procedures among a core group of hospitalists endeavors to support patient safety through several principles: (1) to increase technical proficiency through higher procedural volumes, (2) to facilitate rigorous training and assessment among dedicated individuals, and (3) to systematize best practices of operator performance, communication, and documentation.
MPS have been implemented around the country and have demonstrated several advantages. In one institution, medical firms that were offered the use of an MPS had 48% more procedural attempts by nonspecialists, without significant differences in the proportions of successful attempts or complications compared with the firms who more often referred to specialists.40 A retrospective study analyzed outcomes of 1,707 bedside procedures, of which 548 were performed by an MPS, and found that procedures done by the MPS were more likely to result in lower rates of unsuccessful procedures and to use best-practice safety processes (ie, to involve attending physicians, to use ultrasound guidance, and to avoid femoral sites for catheterization).12 Satisfaction was high among patients who underwent bedside procedures performed by a hospitalist-supervised, intern-based procedure service with a focus on bedside communication.41 From a workforce perspective, MPS have also allowed surgical or radiological subspecialties to focus on more complex cases with higher reimbursement rates,18,42 for proceduralists to expand beyond core procedures (eg, bone marrow biopsies43), and to train advanced practice providers.44 Although studies have not shown that the outcomes of procedures completed by an MPS are better than the outcomes of procedures performed by other specialists,45 one can potentially extrapolate from earlier data that procedures done at the bedside by nonradiologists would have comparable outcomes.
DISCUSSION
A myriad of factors is influencing hospitalists’ scope of practice with respect to bedside procedures. Some evidence suggests that procedures performed by specialists are not superior to those done by generalists and might be associated with increased costs. The most promising developments in the past few decades include simulation-based training, which has demonstrated effectiveness across an array of clinical outcomes but has not been sufficiently evaluated in hospitalists to draw conclusions, and hospitalist-led MPS, which promote safe and productive procedural clinical practices. However, decreasing procedural volume, increasing referrals to specialists, dwindling hospitalist interest and/or confidence, time constraints, limited training opportunities, nonuniform credentialing policies, and lack of standardized assessment are cumulatively contributing to a loss of procedural competency among hospitalists.
Taken together, these forces should compel hospital medicine groups that expect their hospitalists to perform their own procedures to identify necessary steps for ensuring the safety of hospitalized patients under their care. The following considerations derive from the available—albeit modest—evidence on procedural performance in hospital medicine (Table).

1. Create MPS to establish a core set of hospitalists to perform procedures and train them using evidence-based practices. Creation of an MPS places the responsibility of core bedside procedures in the hands of a select group of proceduralists. This strategy streamlines training and assessment of individual procedural competency to meet standards set by SHM36,46 and improves educational outcomes.47-49 MPS could improve clinical outcomes,12,42,50-52 including length of stay and cost, while maintaining patient satisfaction,41 as well as recoup lost revenue from referrals by increasing the volume of procedures done by generalists,40,49 although no robust data supporting the latter point exists. Implementing an MPS requires full-time equivalent (FTE) support for proceduralists and administrative support for data collection and tracking complications. Furthermore, a well-functioning MPS will require investment in portable ultrasound machines and training in POCUS, which has been shown to decrease complications and increase success of invasive bedside procedures.3-7 Hospital medicine groups should be aware that staffing an MPS can divert hospitalist labor and resources from other needed clinical areas, especially during the initial, low-volume phases of implementation. Strategies to offset relative value unit (RVU) loss include combining the MPS with existing clinical roles such as medical consults, code triage, and rapid response teams; or with services with lower patient caps, which might work particularly well in community hospitals. In many institutions, hospitalists can bill for procedural consults in addition to the procedures when the consult involves nonmedical patients, which is relevant when the procedure ultimately cannot be performed (eg, too little ascites to safely perform a paracentesis). Further research should establish best practices of MPS to ensure maximum procedural productivity and safety, because there are no rigorous prospective studies that evaluate strategies to create this service. Such strategies include determining the optimal ratio of proceduralists to general hospitalists, hospital characteristics that benefit most from MPS (eg, referral centers, urban-based settings), volume and type of procedures performed, and the proportion and type of referrals that are most cost-effective.
2. Establish policies with procedural specialists to arrange coverage for off-hours procedures and delineate thresholds for procedures that specialists should perform. Expanding hospitalists’ capabilities in performing procedures should trigger reconsideration of the medical center’s approach to procedural safety. A goal would be to have hospital medicine groups work collaboratively with specialists and other disciplines (eg, surgery, emergency medicine, anesthesia, or radiology) to ensure 24-hour, 7-day a week coverage of urgent bedside procedures. The potential to decrease length of stay and improve off-hour procedural quality might be a compelling rationale for hospital administration, whether or not an MPS is used. That said, we recognize that other services might be unable or unwilling to provide such coverage and that specialist off-hour coverage would incur increased costs and could reduce exposure opportunities for internal medicine residents.
A hospital-level procedures committee might be required to support an institutional imperative for procedural safety and to oversee the implementation of approaches that are practical, financially sustainable, and equitable for all service lines, especially because hospitalist groups might bear the early costs of training and retraining.
3. Hospitalist–proceduralists should collaborate with internal medicine residency programs to offer intensive procedural training experiences to residents who want these skills to be part of their future practice. Robust procedural training for trainees promotes better outcomes for the current workforce and helps to populate the future workforce with procedurally competent practitioners. Simulation-based training is a well-established procedural instruction method that is safe, authentic, and effective in terms of clinical outcomes.34 As the primary teachers of residents in many institutions, hospitalists often are the ones who impart procedural skills to residents, despite uneven skill sets. It is in the interest of internal medicine residency program directors to advocate for a core group of hospitalist–proceduralists, as MPS offer an infrastructure for training that has been shown to increase procedural volume and improve skills.47,48,50 Program directors could therefore be incentivized to sponsor some of these procedural roles with teaching and administration funds, as a trade-off for securing higher-quality procedural training and closer supervision for their trainees. The dual necessity of teaching procedural skills to residents and attending physicians alike offers economies of scale for the use of facilities, personnel, and equipment, and gives faculty an opportunity to extend their clinical teaching skills into the domain of procedural supervision.
4. Hospital medicine groups should re-evaluate credentialing and privileging to ensure procedural competency. Given the lack of published data that characterizes how many hospital medicine groups credential hospitalists to perform procedures and what practices they use to assess competency, hospital medicine groups might be signing off on procedures without verifying hospitalists’ proficiency in core procedures. SHM’s position statement on credentialing for ultrasound-guided procedures46 describes standards that could be applied to other procedures. It proposes that credentialing processes should be grounded in simulation- and patient-based assessments of cognitive and psychomotor skills, using published checklists and global ratings for feedback. Simulation training could support provisional certification, but hospitalists should reach minimum thresholds of supervised patient-based experience before initial credentialing, with continuous reassessment of competency to mitigate skill decay. Prospectively tracking procedural metrics, such as procedural volume and complication rates, also will support systematic skill assessment. Finally, similar to any other medical error, near misses and complications should trigger periprocedural safety reviews.
Limitations
The modest body of research on hospitalists and procedures is the central limitation of our synthesis. Much of the literature consisted of consensus statements, retrospective studies, and small prospective educational studies. As a result, we did not adopt all strategies considered standard in a scoping or systematic review. The literature on MPS specifically was insufficient to draw conclusions about their operational and financial impact or effects on procedure quality. Our primary recommendation to implement MPS requires significant fiscal investment and infrastructure. It also entails risks that must be proactively addressed, including the potential for net financial loss and decreased educational opportunities for residents.
CONCLUSIONS
Hospitalists regularly face the predicament of being expected to independently perform procedures, with little access to training, minimal experience, and no ongoing assessment to ensure their proficiency or the safety of their patients. Past assumptions about hospitalists’ responsibility do not reflect realities in practice patterns and have not translated to widespread adoption of procedural training, monitoring, and assessment mechanisms. Our work summarizes a body of literature that, although limited in empiric studies of hospitalists themselves, offers insights with recommendations for hospital medicine groups wishing to uphold procedural skills as part of their providers’ professional identity.
Over the past 20 years, hospitalists have served as the primary workforce for the clinical care of medical inpatients in the United States.1,2 Core competencies1 state that hospitalists should be able to perform the following bedside procedures: lumbar puncture, paracentesis, thoracentesis, arthrocentesis, and central venous catheter placement. More recently, standard of care has dictated that these procedures be performed under ultrasound guidance,3-6 and thus hospitalists are also expected to be adept at point-of-care ultrasound (POCUS).7
However, no current national standard exists for ensuring basic competency among hospitalists performing bedside procedures. In addition, hospitalists’ procedural volumes are declining,8,9 and standards for procedural training during internal medicine residency have been reduced.10 As a result, many residents who intend to become hospitalists are no longer prepared to perform these procedures.
The ramifications of the loss of procedural competency for hospitalists are manifold. Technical errors are a significant source of patient morbidity and mortality,11-15 and complications arising specifically from nonoperative procedures range from 0 to 19%,16 although these data do not distinguish technical errors from unpreventable adverse events nor the degree to which hospitalists contributed to these complications. Second, hospitalists in academic medical centers might be ill equipped to function as supervisors of trainees performing procedures, which could perpetuate a cycle of suboptimal technical skills.17 Finally, the discrepancy between consensus guidelines for hospitalists and their scope of practice represents a significant area of risk management for institutions that base their credentialing policies on published competencies.
There are many compelling reasons for why hospitalists should maintain—in fact reclaim—a primary role in bedside procedures.18 Hospitalists in community and rural settings might not have easy access to procedural specialists. In academic institutions, hospitalists are the primary instructors and supervisors of procedures performed by internal medicine residents. The increased availability of POCUS allows formally trained hospitalists to perform procedures more safely under imaging guidance.16
The literature on procedures performed by hospitalists, although limited, has focused on POCUS, systems innovations such as medical procedure services (MPS), and policy recommendations for procedural credentialing. Most studies on effective procedural instructional approaches have been conducted among trainees, who are procedural novices. This research does not sufficiently address the dilemma that hospitalists face as independent physicians for whom procedures are not a significant component of their practice, yet are expected to perform invasive procedures occasionally. The purpose of our literature review is to synthesize the available research to characterize contributors to hospitalists’ procedural competency. We conclude with considerations for hospital medicine practice.
METHODS
We performed a structured literature search for peer-reviewed articles related to hospitalists conducting procedures, being trained in procedures, or related to hospitalist-run MPS. We focused our search on the core hospitalist procedures with the highest potential morbidity (ie, lumbar puncture, abdominal paracentesis, thoracentesis, and central venous catheterization). We searched PubMed and Google Scholar for articles published since 1996 (when the term “hospitalists” was first coined) using keyword searches for [hospitalist OR hospital medicine] AND [procedur* OR medical procedur* OR medical procedure service] OR [(procedur* AND (train* OR educat* OR teach OR instruct*)] OR abdominal paracentes* OR thoracentes* OR lumbar puncture OR central venous catheter* OR ultrasound OR point-of-care. We included original research, brief research reports, perspectives, guidelines, and consensus statements. Exclusion criteria were articles that focused on nonhospitalists and conference abstracts. We used pearling to identify secondary sources from included articles’ bibliographies, without limits on year of publication.
RESULTS
Trends Towards Specialist Referrals
Between 1986 and 2007, the number and variety of procedures performed by internists decreased by half.19 Hospitalists still completed procedures in greater volume and variety than nonhospitalists,8 with approximately 50% of hospitalists performing lumbar punctures (50%), abdominal paracenteses (49%), and thoracenteses (44%) compared with less than 25% for all three procedures for nonhospitalists. Additionally, only 11% of surveyed hospitalists8 performed all nine core procedures, although these included procedures that are largely cognitive in nature (eg, electrocardiogram interpretation, chest X-ray interpretation) or procedures that have been relegated to other specialists (eg, endotracheal intubation, ventilator management, or joint injection/aspiration).
Surveys showed that, especially in larger cities and academic centers, procedural specialists have taken over a disproportionate share of procedures even as the number of procedures performed continued to rise.20 Between 1993 and 2008, the number of paracenteses and thoracenteses increased by 133% and decreased by 14%, respectively, but the share of procedures performed by radiologists increased by 964% and 358%, respectively, as evident in an analysis of Medicare billing data.20 A more recent study of Medicare claims from 2004 to 2016 similarly revealed that the percentage of paracenteses performed by radiologists compared with nonradiologists rose from 70% to 80% and thoracenteses from 47% to 66%, respectively.21 Comparable trends were apparent in claims data for lumbar punctures; between 1991 and 2011, the share of lumbar punctures performed by radiologists rose from 11% to 48%.22
In academic medical centers, hospitalists might have the opportunity to pursue other activities (eg, education, administration, research) as they progress in their careers, resulting in less clinical activity. Although hospitalists who are more clinically active in hospital care tended to perform more procedures,8 those with smaller clinical footprints reported lower levels of comfort with performing procedures8 and might have less available time to maintain procedural competency or train in new technologies such as POCUS.17
Additionally, hospitalists in both academic and community settings cited efficiency as a major reason for procedural referral. Hospitalists tended to perform more procedures if they had fixed salaries or if less than 50% of their income was based on clinical productivity, although this trend was not significant.8 Further, they also might be motivated by competing opportunity costs such as time lost caring for other patients or length of shift, which influences the amount of time spent at work.23
Notably, speculation that hospitalists referred more complex cases to specialists was not borne out by studies examining referral patterns.21,24,25
Procedural Outcomes for Hospitalists vs Nonhospitalists
No convincing data exist that procedures performed by specialists have better outcomes than those completed at the bedside by well-trained generalists, although studies were limited to the inpatient setting, to generalists who have some exposure to procedures, and to internal medicine residents on inpatient rotations. In one retrospective review, interventional radiology (IR) referrals were associated with more platelet or plasma transfusions and intensive care unit transfers than those performed at the bedside by internal medicine residents, findings that remained significant after accounting for complexity (eg, Model for End-stage Liver Disease score, need for dialysis, and platelet count).24 Similarly, a prospective audit of 529 bedside procedures did not show any differences in complication rates between generalists and pulmonologists, once generalists underwent standardized training and used pleural safety checklists and ultrasound guidance.26 An administrative database review of 130,000 inpatient thoracenteses across several university hospitals between 2010 and 2013 found that the risk of iatrogenic pneumothorax was similar among operators from IR, medicine, and pulmonary (2.8%, 2.9%, and 3.1%, respectively)27; these findings have been reproduced in other studies.28 Finally, the increasing adoption of procedural ultrasound permits procedures to be conducted more safely at the bedside, without the need to refer to radiology for imaging guidance.3-5
IR procedures also are associated with increased healthcare costs compared with bedside procedures. One study showed that hospital costs for admissions when paracenteses were performed by radiologists were higher than those in which the procedure was completed at the bedside by gastroenterologists or hepatologists.25 A chart review examining 399 paracenteses, thoracenteses, and lumbar punctures found that the average procedure cost increased by 38% for referred procedures and 56% for radiology-performed procedures, as compared with bedside procedures.29 Needing ancillary staffing in dedicated suites to perform procedures contributed to the excess cost.9 Moreover, referred procedures resulted in increased length of stay, which can incur additional costs. However, the data were conflicting; two studies did not show a statistical difference,25,28 while others found an increased length of stay,24,27,29 which might be due to the unavailability of specialists during off hours, thereby delaying nonemergent procedures.21 Detailed cost analyses have controlled for the use of procedural facilities and blood transfusions among IR specialists and simulation training among generalists, showing that total costs were $663 per patient undergoing IR procedures compared with $134 per patient undergoing bedside procedures.30
Lack of Standardized Procedural Training or Assessment
A robust body of primary studies and systematic reviews supports the use of simulation for procedural training to improve comfort and skill as well as reduce complication rates and costs.31,32 A systematic review that investigated the impact of four paradigms of procedural training found that MPS and quality improvement/patient safety approaches led to the most active learning compared with apprenticeship (ie, “see one, do one”) and approaches based on educational theories.33 Nevertheless, the vast majority of the research has been conducted in trainees,32,34 with sparse evidence among practicing physicians. One cohort study of attending physicians’ central venous catheter insertion skills on simulators found low and variable short-term performance, showing overall poor adherence to checklists.35 One article suggested that hospitalists’ procedural skills were below established thresholds of competency at baseline and that simulation-based training did not result in sustained skills, but the small sample size and high attrition limited meaningful conclusions.36 Although continuing medical education courses are available to hospitalists, there is no published evidence supporting their effectiveness.
Proxies for procedural skill have included comfort and experience, yet these markers have broadly been shown to be inadequate.34,36,37 Additionally, the natural decline of skill over time has invoked the need for periodic reassessment of proficiency.36,38 Credentialing has been equally inconstant; a survey of the Society of Hospital Medicine’s (SHM) POCUS task force revealed that only half of respondents reported their hospitals required a minimum number of procedures for initial credentialing and recredentialing.39 In short, periodic assessment of procedural skills among hospitalists has not been a routine process at many institutions.
Role of Hospitalist-Run Medical Procedure Services
It might not be necessary for all hospitalists to be proficient and credentialed in a given procedure,1 and a trend has emerged in the creation of MPS staffed by hospitalists as proceduralists. The primary aim of these MPS has been to recapture the procedures—and associated revenue—that would otherwise be referred to specialists. Moreover, concentrating procedures among a core group of hospitalists endeavors to support patient safety through several principles: (1) to increase technical proficiency through higher procedural volumes, (2) to facilitate rigorous training and assessment among dedicated individuals, and (3) to systematize best practices of operator performance, communication, and documentation.
MPS have been implemented around the country and have demonstrated several advantages. In one institution, medical firms that were offered the use of an MPS had 48% more procedural attempts by nonspecialists, without significant differences in the proportions of successful attempts or complications compared with the firms who more often referred to specialists.40 A retrospective study analyzed outcomes of 1,707 bedside procedures, of which 548 were performed by an MPS, and found that procedures done by the MPS were more likely to result in lower rates of unsuccessful procedures and to use best-practice safety processes (ie, to involve attending physicians, to use ultrasound guidance, and to avoid femoral sites for catheterization).12 Satisfaction was high among patients who underwent bedside procedures performed by a hospitalist-supervised, intern-based procedure service with a focus on bedside communication.41 From a workforce perspective, MPS have also allowed surgical or radiological subspecialties to focus on more complex cases with higher reimbursement rates,18,42 for proceduralists to expand beyond core procedures (eg, bone marrow biopsies43), and to train advanced practice providers.44 Although studies have not shown that the outcomes of procedures completed by an MPS are better than the outcomes of procedures performed by other specialists,45 one can potentially extrapolate from earlier data that procedures done at the bedside by nonradiologists would have comparable outcomes.
DISCUSSION
A myriad of factors is influencing hospitalists’ scope of practice with respect to bedside procedures. Some evidence suggests that procedures performed by specialists are not superior to those done by generalists and might be associated with increased costs. The most promising developments in the past few decades include simulation-based training, which has demonstrated effectiveness across an array of clinical outcomes but has not been sufficiently evaluated in hospitalists to draw conclusions, and hospitalist-led MPS, which promote safe and productive procedural clinical practices. However, decreasing procedural volume, increasing referrals to specialists, dwindling hospitalist interest and/or confidence, time constraints, limited training opportunities, nonuniform credentialing policies, and lack of standardized assessment are cumulatively contributing to a loss of procedural competency among hospitalists.
Taken together, these forces should compel hospital medicine groups that expect their hospitalists to perform their own procedures to identify necessary steps for ensuring the safety of hospitalized patients under their care. The following considerations derive from the available—albeit modest—evidence on procedural performance in hospital medicine (Table).

1. Create MPS to establish a core set of hospitalists to perform procedures and train them using evidence-based practices. Creation of an MPS places the responsibility of core bedside procedures in the hands of a select group of proceduralists. This strategy streamlines training and assessment of individual procedural competency to meet standards set by SHM36,46 and improves educational outcomes.47-49 MPS could improve clinical outcomes,12,42,50-52 including length of stay and cost, while maintaining patient satisfaction,41 as well as recoup lost revenue from referrals by increasing the volume of procedures done by generalists,40,49 although no robust data supporting the latter point exists. Implementing an MPS requires full-time equivalent (FTE) support for proceduralists and administrative support for data collection and tracking complications. Furthermore, a well-functioning MPS will require investment in portable ultrasound machines and training in POCUS, which has been shown to decrease complications and increase success of invasive bedside procedures.3-7 Hospital medicine groups should be aware that staffing an MPS can divert hospitalist labor and resources from other needed clinical areas, especially during the initial, low-volume phases of implementation. Strategies to offset relative value unit (RVU) loss include combining the MPS with existing clinical roles such as medical consults, code triage, and rapid response teams; or with services with lower patient caps, which might work particularly well in community hospitals. In many institutions, hospitalists can bill for procedural consults in addition to the procedures when the consult involves nonmedical patients, which is relevant when the procedure ultimately cannot be performed (eg, too little ascites to safely perform a paracentesis). Further research should establish best practices of MPS to ensure maximum procedural productivity and safety, because there are no rigorous prospective studies that evaluate strategies to create this service. Such strategies include determining the optimal ratio of proceduralists to general hospitalists, hospital characteristics that benefit most from MPS (eg, referral centers, urban-based settings), volume and type of procedures performed, and the proportion and type of referrals that are most cost-effective.
2. Establish policies with procedural specialists to arrange coverage for off-hours procedures and delineate thresholds for procedures that specialists should perform. Expanding hospitalists’ capabilities in performing procedures should trigger reconsideration of the medical center’s approach to procedural safety. A goal would be to have hospital medicine groups work collaboratively with specialists and other disciplines (eg, surgery, emergency medicine, anesthesia, or radiology) to ensure 24-hour, 7-day a week coverage of urgent bedside procedures. The potential to decrease length of stay and improve off-hour procedural quality might be a compelling rationale for hospital administration, whether or not an MPS is used. That said, we recognize that other services might be unable or unwilling to provide such coverage and that specialist off-hour coverage would incur increased costs and could reduce exposure opportunities for internal medicine residents.
A hospital-level procedures committee might be required to support an institutional imperative for procedural safety and to oversee the implementation of approaches that are practical, financially sustainable, and equitable for all service lines, especially because hospitalist groups might bear the early costs of training and retraining.
3. Hospitalist–proceduralists should collaborate with internal medicine residency programs to offer intensive procedural training experiences to residents who want these skills to be part of their future practice. Robust procedural training for trainees promotes better outcomes for the current workforce and helps to populate the future workforce with procedurally competent practitioners. Simulation-based training is a well-established procedural instruction method that is safe, authentic, and effective in terms of clinical outcomes.34 As the primary teachers of residents in many institutions, hospitalists often are the ones who impart procedural skills to residents, despite uneven skill sets. It is in the interest of internal medicine residency program directors to advocate for a core group of hospitalist–proceduralists, as MPS offer an infrastructure for training that has been shown to increase procedural volume and improve skills.47,48,50 Program directors could therefore be incentivized to sponsor some of these procedural roles with teaching and administration funds, as a trade-off for securing higher-quality procedural training and closer supervision for their trainees. The dual necessity of teaching procedural skills to residents and attending physicians alike offers economies of scale for the use of facilities, personnel, and equipment, and gives faculty an opportunity to extend their clinical teaching skills into the domain of procedural supervision.
4. Hospital medicine groups should re-evaluate credentialing and privileging to ensure procedural competency. Given the lack of published data that characterizes how many hospital medicine groups credential hospitalists to perform procedures and what practices they use to assess competency, hospital medicine groups might be signing off on procedures without verifying hospitalists’ proficiency in core procedures. SHM’s position statement on credentialing for ultrasound-guided procedures46 describes standards that could be applied to other procedures. It proposes that credentialing processes should be grounded in simulation- and patient-based assessments of cognitive and psychomotor skills, using published checklists and global ratings for feedback. Simulation training could support provisional certification, but hospitalists should reach minimum thresholds of supervised patient-based experience before initial credentialing, with continuous reassessment of competency to mitigate skill decay. Prospectively tracking procedural metrics, such as procedural volume and complication rates, also will support systematic skill assessment. Finally, similar to any other medical error, near misses and complications should trigger periprocedural safety reviews.
Limitations
The modest body of research on hospitalists and procedures is the central limitation of our synthesis. Much of the literature consisted of consensus statements, retrospective studies, and small prospective educational studies. As a result, we did not adopt all strategies considered standard in a scoping or systematic review. The literature on MPS specifically was insufficient to draw conclusions about their operational and financial impact or effects on procedure quality. Our primary recommendation to implement MPS requires significant fiscal investment and infrastructure. It also entails risks that must be proactively addressed, including the potential for net financial loss and decreased educational opportunities for residents.
CONCLUSIONS
Hospitalists regularly face the predicament of being expected to independently perform procedures, with little access to training, minimal experience, and no ongoing assessment to ensure their proficiency or the safety of their patients. Past assumptions about hospitalists’ responsibility do not reflect realities in practice patterns and have not translated to widespread adoption of procedural training, monitoring, and assessment mechanisms. Our work summarizes a body of literature that, although limited in empiric studies of hospitalists themselves, offers insights with recommendations for hospital medicine groups wishing to uphold procedural skills as part of their providers’ professional identity.
1. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: Development and methodology. J Hosp Med. 2006;1(1):48-56. https://doi.org/10.1002/jhm.6
2. Wachter RM, Goldman L. Zero to 50,000 — The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E7-E15. https://doi.org/10.12788/jhm.3095
4. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3197
5. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940
6. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287
7. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079
8. Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25(5):448-452. https://doi.org/10.1007/s11606-010-1284-2
9. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. https://doi.org/10.1002/jhm.602
10. American Board of Internal Medicine. Policies and procedures for certification. Accessed December 3, 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf
11. Myers LC. Toward preventing medical malpractice claims related to chest procedures. Ann Am Thorac Soc. 2020;17(6):776-779. https://doi.org/10.1513/AnnalsATS.201912-863RL
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604
14. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324(6):377-384. https://doi.org/10.1056/NEJM199102073240605
15. Myers LC, Gartland RM, Skillings J, et al. An examination of medical malpractice claims involving physician trainees. Acad Med. 2020;95(8):1215-1222. https://doi.org/10.1097/ACM.0000000000003117
16. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
17. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. https://doi.org/10.1097/ACM.0000000000001327
18. Nelson B. Hospitalists try to reclaim lead role in bedside procedures. The Hospitalist. March 2015. Accessed June 27, 2020. https://www.the-hospitalist.org/hospitalist/article/122571/hospitalists-try-reclaim-lead-role-bedside-procedures
19. Wigton RS, Alguire P; American College of Physicians. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355-360. https://doi.org/10.7326/0003-4819-146-5-200703060-00007
20. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. https://doi.org/10.1016/j.jacr.2010.04.013
21. Gottumukkala RV, Prabhakar AM, Hemingway J, Hughes DR, Duszak R Jr. Disparities over time in volume, day of the week, and patient complexity between paracentesis and thoracentesis procedures performed by radiologists versus those performed by nonradiologists. J Vasc Interv Radiol. 2019;30(11):1769-1778.e1. https://doi.org/10.1016/j.jvir.2019.04.015
22. Kroll H, Duszak R Jr, Nsiah E, Hughes DR, Sumer S, Wintermark M. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. Am J Roentgenol. 2014;204(1):15-19. https://doi.org/10.2214/AJR.14.12622
23. Jensen T, Lai A, Mourad M. Can lessons from systems-based mastery learning for thoracentesis be translated to hospitalists? J Hosp Med. 2016;11(11):811-812. https://doi.org/10.1002/jhm.2655
24. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
25. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. https://doi.org/10.1002/jhm.2153
26. See KC, Ong V, Teoh CM, et al. Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology. 2014;19(3):396-402. https://doi.org/10.1111/resp.12244
27. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. https://doi.org/10.1016/S1553-7250(16)42004-0
28. Berger MS, Divilov V, Paredes H, Sun E. Abdominal paracentesis: safety and efficacy comparing medicine resident bedside paracentesis vs. paracentesis performed by interventional radiology. J Clin Gastroenterol Hepatol. 2018;2(4). https://doi.org/10.21767/2575-7733.1000050
29. Kay C, Wozniak EM, Szabo A, Jackson JL. Examining invasive bedside procedure performance at an academic medical center. South Med J. 2016;109(7):402-407. https://doi.org/10.14423/SMJ.0000000000000485
30. Barsuk JH, Cohen ER, Feinglass J, et al. Cost savings of performing paracentesis procedures at the bedside after simulation-based education. Simul Healthc. 2014;9(5):312-318. https://doi.org/10.1097/SIH.0000000000000040
31. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93(5):729-735. https://doi.org/10.1097/ACM.0000000000001965
32. Huang GC, McSparron JI, Balk EM, et al. Procedural instruction in invasive bedside procedures: a systematic review and meta-analysis of effective teaching approaches. BMJ Qual Saf. 2016;25(4):281-294. https://doi.org/10.1136/bmjqs-2014-003518
33. Brydges R, Stroud L, Wong BM, Holmboe ES, Imrie K, Hatala R. Core competencies or a competent core? a scoping review and realist synthesis of invasive bedside procedural skills training in internal medicine. Acad Med. 2017;92(11):1632-1643. https://doi.org/10.1097/ACM.0000000000001726
34. Brydges R, Hatala R, Zendejas B, Erwin PJ, Cook DA. Linking simulation-based educational assessments and patient-related outcomes: a systematic review and meta-analysis. Acad Med. 2015;90(2):246-256. https://doi.org/10.1097/ACM.0000000000000549
35. Barsuk JH, Cohen ER, Nguyen D, et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44(10):1871-1881. https://doi.org/10.1097/CCM.0000000000001831
36. Crocker JT, Hale CP, Vanka A, Ricotta DN, McSparron JI, Huang GC. Raising the bar for procedural competency among hospitalists. Ann Intern Med. 2019;170(9):654-655. https://doi.org/10.7326/M18-3007
37. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201-208. https://doi.org/10.4300/JGME-D-16-00426.1
38. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. https://doi.org/10.1097/ACM.0000000000000734
39. Jensen T, Soni N, Tierney D, Lucas B. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837
40. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. https://doi.org/10.1002/jhm.159
41. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: Is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
42. Ault MJ, Rosen BT. Proceduralists — leading patient-safety initiatives. N Engl J Med. 2007;356(17):1789-1790. https://doi.org/10.1056/NEJMc063239
43. Obasi JU, Umpierrez De Reguero AP. Safety profile of bone marrow aspiration and biopsies performed by the hospitalist procedure service at an academic center: an observational study. Blood. 2019;134(suppl 1): 5848. https://doi.org/10.1182/blood-2019-121444
44. Gisondi MA, Regan L, Branzetti J, Hopson LR. More learners, finite resources, and the changing landscape of procedural training at the bedside. Acad Med. 2018;93(5):699-704. https://doi.org/10.1097/ACM.0000000000002062
45. McCormack J. The new proceduralists: Have they found their niche? American Medical News. September 17, 2007. Accessed August 30, 2020. https://amednews.com/article/20070917/business/309179994/4/
46. Lucas BP, Tierney DM, Jensen TP, et al; Society of Hospital Medicine Point-of-Care Ultrasound Task Force. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917
47. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
48. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1
49. Montuno A, Hunt BR, Lee MM. Potential impact of a bedside procedure service on training procedurally competent hospitalists in a community-based residency program. J Community Hosp Intern Med Perspect. 2016;6(3):31054. https://doi.org/10.3402/jchimp.v6.31054
50. Smith CC, Gordon CE, Feller‐Kopman D, et al. Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency. J Gen Intern Med. 2004;19(5p2):510-513. https://doi.org/10.1111/j.1525-1497.2004.30161.x
51. Mourad M. Capsule commentary on Tukey et al., the impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):518. https://doi.org/10.1007/s11606-013-2740-6
52. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care: a systematic review and methodologic critique of the literature. 2002. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews . Accessed June 26, 2020. https://www.ncbi.nlm.nih.gov/books/NBK69189/
1. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: Development and methodology. J Hosp Med. 2006;1(1):48-56. https://doi.org/10.1002/jhm.6
2. Wachter RM, Goldman L. Zero to 50,000 — The 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
3. Cho J, Jensen TP, Reierson K, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E7-E15. https://doi.org/10.12788/jhm.3095
4. Soni NJ, Franco-Sadud R, Kobaidze K, et al. Recommendations on the use of ultrasound guidance for adult lumbar puncture: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14(10):591-601. https://doi.org/10.12788/jhm.3197
5. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):126-135. https://doi.org/10.12788/jhm.2940
6. Franco-Sadud R, Schnobrich D, Mathews BK, et al. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287
7. Soni NJ, Schnobrich D, Mathews BK, et al. Point-of-care ultrasound for hospitalists: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E6. https://doi.org/10.12788/jhm.3079
8. Thakkar R, Wright SM, Alguire P, Wigton RS, Boonyasai RT. Procedures performed by hospitalist and non-hospitalist general internists. J Gen Intern Med. 2010;25(5):448-452. https://doi.org/10.1007/s11606-010-1284-2
9. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. https://doi.org/10.1002/jhm.602
10. American Board of Internal Medicine. Policies and procedures for certification. Accessed December 3, 2020. https://www.abim.org/~/media/ABIM%20Public/Files/pdf/publications/certification-guides/policies-and-procedures.pdf
11. Myers LC. Toward preventing medical malpractice claims related to chest procedures. Ann Am Thorac Soc. 2020;17(6):776-779. https://doi.org/10.1513/AnnalsATS.201912-863RL
12. Tukey MH, Wiener RS. The impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):485-490. https://doi.org/10.1007/s11606-013-2709-5
13. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604
14. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. N Engl J Med. 1991;324(6):377-384. https://doi.org/10.1056/NEJM199102073240605
15. Myers LC, Gartland RM, Skillings J, et al. An examination of medical malpractice claims involving physician trainees. Acad Med. 2020;95(8):1215-1222. https://doi.org/10.1097/ACM.0000000000003117
16. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
17. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. https://doi.org/10.1097/ACM.0000000000001327
18. Nelson B. Hospitalists try to reclaim lead role in bedside procedures. The Hospitalist. March 2015. Accessed June 27, 2020. https://www.the-hospitalist.org/hospitalist/article/122571/hospitalists-try-reclaim-lead-role-bedside-procedures
19. Wigton RS, Alguire P; American College of Physicians. The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians. Ann Intern Med. 2007;146(5):355-360. https://doi.org/10.7326/0003-4819-146-5-200703060-00007
20. Duszak R Jr, Chatterjee AR, Schneider DA. National fluid shifts: fifteen-year trends in paracentesis and thoracentesis procedures. J Am Coll Radiol. 2010;7(11):859-864. https://doi.org/10.1016/j.jacr.2010.04.013
21. Gottumukkala RV, Prabhakar AM, Hemingway J, Hughes DR, Duszak R Jr. Disparities over time in volume, day of the week, and patient complexity between paracentesis and thoracentesis procedures performed by radiologists versus those performed by nonradiologists. J Vasc Interv Radiol. 2019;30(11):1769-1778.e1. https://doi.org/10.1016/j.jvir.2019.04.015
22. Kroll H, Duszak R Jr, Nsiah E, Hughes DR, Sumer S, Wintermark M. Trends in lumbar puncture over 2 decades: a dramatic shift to radiology. Am J Roentgenol. 2014;204(1):15-19. https://doi.org/10.2214/AJR.14.12622
23. Jensen T, Lai A, Mourad M. Can lessons from systems-based mastery learning for thoracentesis be translated to hospitalists? J Hosp Med. 2016;11(11):811-812. https://doi.org/10.1002/jhm.2655
24. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Clinical outcomes after bedside and interventional radiology paracentesis procedures. Am J Med. 2013;126(4):349-356. https://doi.org/10.1016/j.amjmed.2012.09.016
25. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties performing paracentesis procedures at university hospitals: implications for training and certification. J Hosp Med. 2014;9(3):162-168. https://doi.org/10.1002/jhm.2153
26. See KC, Ong V, Teoh CM, et al. Bedside pleural procedures by pulmonologists and non-pulmonologists: a 3-year safety audit. Respirology. 2014;19(3):396-402. https://doi.org/10.1111/resp.12244
27. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. https://doi.org/10.1016/S1553-7250(16)42004-0
28. Berger MS, Divilov V, Paredes H, Sun E. Abdominal paracentesis: safety and efficacy comparing medicine resident bedside paracentesis vs. paracentesis performed by interventional radiology. J Clin Gastroenterol Hepatol. 2018;2(4). https://doi.org/10.21767/2575-7733.1000050
29. Kay C, Wozniak EM, Szabo A, Jackson JL. Examining invasive bedside procedure performance at an academic medical center. South Med J. 2016;109(7):402-407. https://doi.org/10.14423/SMJ.0000000000000485
30. Barsuk JH, Cohen ER, Feinglass J, et al. Cost savings of performing paracentesis procedures at the bedside after simulation-based education. Simul Healthc. 2014;9(5):312-318. https://doi.org/10.1097/SIH.0000000000000040
31. Barsuk JH, Cohen ER, Williams MV, et al. Simulation-based mastery learning for thoracentesis skills improves patient outcomes: a randomized trial. Acad Med. 2018;93(5):729-735. https://doi.org/10.1097/ACM.0000000000001965
32. Huang GC, McSparron JI, Balk EM, et al. Procedural instruction in invasive bedside procedures: a systematic review and meta-analysis of effective teaching approaches. BMJ Qual Saf. 2016;25(4):281-294. https://doi.org/10.1136/bmjqs-2014-003518
33. Brydges R, Stroud L, Wong BM, Holmboe ES, Imrie K, Hatala R. Core competencies or a competent core? a scoping review and realist synthesis of invasive bedside procedural skills training in internal medicine. Acad Med. 2017;92(11):1632-1643. https://doi.org/10.1097/ACM.0000000000001726
34. Brydges R, Hatala R, Zendejas B, Erwin PJ, Cook DA. Linking simulation-based educational assessments and patient-related outcomes: a systematic review and meta-analysis. Acad Med. 2015;90(2):246-256. https://doi.org/10.1097/ACM.0000000000000549
35. Barsuk JH, Cohen ER, Nguyen D, et al. Attending physician adherence to a 29-component central venous catheter bundle checklist during simulated procedures. Crit Care Med. 2016;44(10):1871-1881. https://doi.org/10.1097/CCM.0000000000001831
36. Crocker JT, Hale CP, Vanka A, Ricotta DN, McSparron JI, Huang GC. Raising the bar for procedural competency among hospitalists. Ann Intern Med. 2019;170(9):654-655. https://doi.org/10.7326/M18-3007
37. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ procedural experience does not ensure competence: a research synthesis. J Grad Med Educ. 2017;9(2):201-208. https://doi.org/10.4300/JGME-D-16-00426.1
38. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. https://doi.org/10.1097/ACM.0000000000000734
39. Jensen T, Soni N, Tierney D, Lucas B. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. https://doi.org/10.12788/jhm.2837
40. Lucas BP, Asbury JK, Wang Y, et al. Impact of a bedside procedure service on general medicine inpatients: a firm-based trial. J Hosp Med. 2007;2(3):143-149. https://doi.org/10.1002/jhm.159
41. Mourad M, Auerbach AD, Maselli J, Sliwka D. Patient satisfaction with a hospitalist procedure service: Is bedside procedure teaching reassuring to patients? J Hosp Med. 2011;6(4):219-224. https://doi.org/10.1002/jhm.856
42. Ault MJ, Rosen BT. Proceduralists — leading patient-safety initiatives. N Engl J Med. 2007;356(17):1789-1790. https://doi.org/10.1056/NEJMc063239
43. Obasi JU, Umpierrez De Reguero AP. Safety profile of bone marrow aspiration and biopsies performed by the hospitalist procedure service at an academic center: an observational study. Blood. 2019;134(suppl 1): 5848. https://doi.org/10.1182/blood-2019-121444
44. Gisondi MA, Regan L, Branzetti J, Hopson LR. More learners, finite resources, and the changing landscape of procedural training at the bedside. Acad Med. 2018;93(5):699-704. https://doi.org/10.1097/ACM.0000000000002062
45. McCormack J. The new proceduralists: Have they found their niche? American Medical News. September 17, 2007. Accessed August 30, 2020. https://amednews.com/article/20070917/business/309179994/4/
46. Lucas BP, Tierney DM, Jensen TP, et al; Society of Hospital Medicine Point-of-Care Ultrasound Task Force. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2):117-125. https://doi.org/10.12788/jhm.2917
47. Lenhard A, Moallem M, Marrie RA, Becker J, Garland A. An intervention to improve procedure education for internal medicine residents. J Gen Intern Med. 2008;23(3):288-293. https://doi.org/10.1007/s11606-008-0513-4
48. Mourad M, Ranji S, Sliwka D. A randomized controlled trial of the impact of a teaching procedure service on the training of internal medicine residents. J Grad Med Educ. 2012;4(2):170-175. https://doi.org/10.4300/JGME-D-11-00136.1
49. Montuno A, Hunt BR, Lee MM. Potential impact of a bedside procedure service on training procedurally competent hospitalists in a community-based residency program. J Community Hosp Intern Med Perspect. 2016;6(3):31054. https://doi.org/10.3402/jchimp.v6.31054
50. Smith CC, Gordon CE, Feller‐Kopman D, et al. Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency. J Gen Intern Med. 2004;19(5p2):510-513. https://doi.org/10.1111/j.1525-1497.2004.30161.x
51. Mourad M. Capsule commentary on Tukey et al., the impact of a medical procedure service on patient safety, procedure quality and resident training opportunities. J Gen Intern Med. 2014;29(3):518. https://doi.org/10.1007/s11606-013-2740-6
52. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care: a systematic review and methodologic critique of the literature. 2002. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews . Accessed June 26, 2020. https://www.ncbi.nlm.nih.gov/books/NBK69189/
© 2021 Society of Hospital Medicine
Things We Do for No Reason™: Ova and Parasite Testing in Patients With Acute Diarrhea Arising During Hospitalization
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.
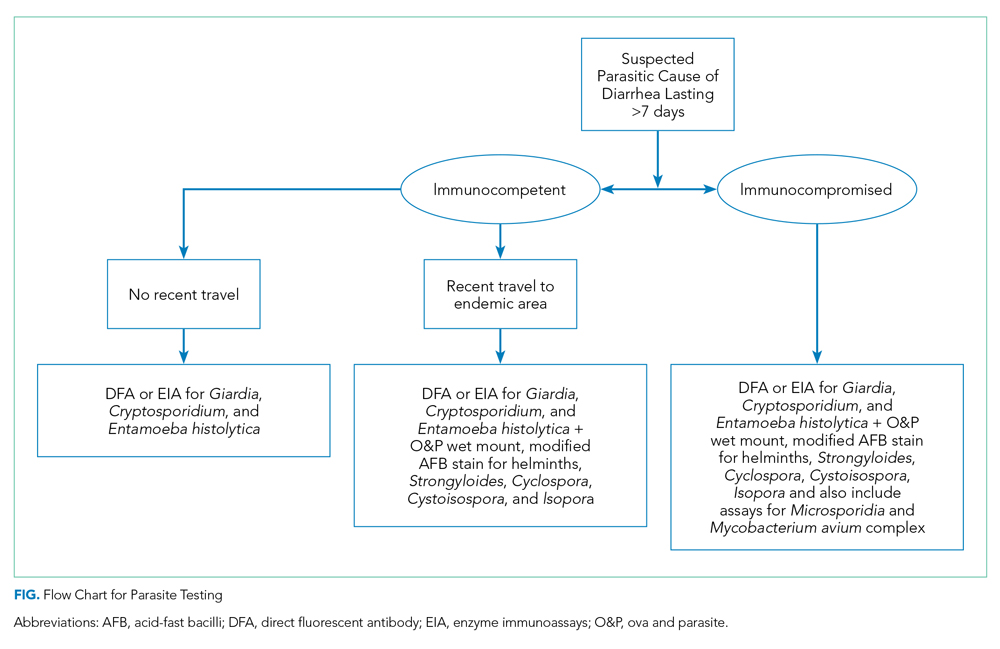
RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected] .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.

RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected] .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 54-year-old immunocompetent man admitted to the hospital for non–ST-segment elevation myocardial infarction develops profuse watery diarrhea after his third day of admission. He denies prior episodes of diarrhea. He does not have any fevers, blood in the stool, recent travel, or antibiotic use. Vital signs include a blood pressure of 128/82 mm Hg, heart rate of 120 beats per minute, respiratory rate of 16 breaths per min, oxygen saturation of 100% on room air, and temperature of 36.9 °C. His physical examination is normal, without signs of abdominal tenderness, rebound, or guarding. Complete blood count is normal, without eosinophilia. The comprehensive metabolic panel shows mild hypokalemia of 3.3 mmol/L. The hospitalist resuscitates him with normal saline, provides oral potassium repletion, and orders a stool culture, Clostridioides difficile test, and an ova and parasite (O&P) test. Loperamide and time resolve his symptoms in 2 days. Results of his stool culture, C difficile, and O&P tests return negative in 3 days.
BACKGROUND
Acute diarrhea is a common complaint in both inpatient and outpatient settings. It is defined as the passage of three or more liquid or poorly formed stools in a 24-hour period lasting less than 14 days. Persistent diarrhea lasts from 14 to 29 days, while chronic diarrhea lasts longer than 30 days. There are 47.8 million cases of acute diarrhea per year in the United States, costing $150 million in US health expenditures.1 Viral pathogens remain the most common cause of acute diarrhea in the United States.1,2 Standard O&P testing consists of applying a stool sample to a slide with either saline or iodine (wet mount) and evaluating the specimen with a microscope.
WHY YOU MIGHT THINK O&P TESTING IS HELPFUL
Giardia and Cryptosporidium remain the most commonly implicated parasitic pathogens in acute diarrheal episodes in the United States.3Cryptosporidium cases in the United States range from 2.2 to 3.9 per 100,000 persons,4 and Giardia cases in the United States range from 5.8 to 6.4 per 100,000 persons.5 To avoid missing potentially treatable causes, providers often order O&P tests reflexively as part of a standard workup for acute diarrhea. From 2001 to 2007, Associated Regional and University Pathologists Laboratories experienced a 379% increase in O&P testing.6 Many providers ordering these tests assume that standard O&P testing covers most, if not all, parasites and that a negative test will rule out a parasitic cause of disease. Furthermore, providers are unaware that more sensitive tests to detect certain parasites have replaced standard O&P microscopy.3
WHY O&P TESTING IS USUALLY UNNECESSARY
Most hospitalized patients do not have a parasitic infection
In a review of 5,681 completed O&P tests from a tertiary care medical center in Canada over a 5-year period, only 1.4% of tests were positive.7 In a 3-year retrospective analysis of stool samples obtained after 3 days of hospitalization, positive results were found in only 1 of 191 stool cultures and in 0 of 90 O&P samples.8 Current practice guidelines suggest not testing patients with stool studies in cases of acute diarrhea lasting less than 7 days in the absence of significant risk factors for parasitic disease because it has been shown to be a rare event and most cases will self-resolve with supportive care only.1,9
The stool O&P test has low sensitivity
Classically ordered stool O&P tests have low sensitivity for the detection of Giardia and Cryptosporidium, the two most common parasites in the United States.6,10,11 O&P studies detect Giardia in only 66% to 79% of specimen samples and Cryptosporidium in less than 5% of specimens. Diagnostic yields can be improved with the use of special stains such as modified acid-fast stain (MAF).6 Despite use of MAF staining, though, sensitivity for Cryptosporidium detection has remained at only 55%.12 Additionally, several studies have shown that physicians are generally unaware of the test characteristics of stool O&P tests and they do not know to order the newer more sensitive enzyme immunoassays (EIA) or direct fluorescent antibody (DFA) tests even in situations when testing for a parasitic infection is appropriate.10,11,13,14 As stated earlier, a parasitic infection without significant risk factors is a rare event. A negative test with low or moderate sensitivity is not additive to such a low clinical suspicion because it does not significantly change posttest probability.
Testing can have adverse consequences
In addition to the low yield, O&P testing is technically complex, is time intensive, and requires an experienced technician’s interpretation. Inappropriate testing increases the cost of care and staff workload without much benefit.6 As such, some institutions have opted to send the O&P tests to labs with experienced technicians. Other institutions have adopted a restrictive stool O&P testing approach that reduces healthcare time and costs and improves the rate of positive tests.13,15 A study at a single tertiary care medical center demonstrated an estimated cost savings of $21,931 annually by implementing a computer-based algorithm to restrict testing for stool cultures and O&P tests to patients with higher probabilities of infection.15 The algorithm directed clinicians to provide further information when attempting to order stool culture, O&P, or other specific stool tests. For patients hospitalized for more than 3 days, the system did not allow certain testing. For patients with worrisome features like severe symptoms or an immunocompromised state, the algorithm directed the clinician to place an infectious disease or gastroenterology consult rather than order stool tests. Decreased laboratory costs of all stool studies (including O&P) in adult inpatient locations led to the cost savings. Additionally, the study authors felt that they likely underestimated the cost savings because they did not account for other expenses in the analysis, such as nursing workload and supplies.15
WHAT YOU SHOULD DO INSTEAD
Clinicians should evaluate patients on a case-by-case basis and determine the need to test based on the presence of high-risk features (Table). 
When performing parasitic testing in patients without a recent travel history but with other high-risk features, test for the most prevalent parasites in the United States (ie, Giardia, Cryptosporidium, and Entamoeba histolytica) with DFA or EIA tests.3 DFA testing for Giardia is 99% sensitive.12 In patients with symptoms lasting more than 7 days and recent travel, in addition to the above DFA/EIA tests, perform O&P testing with wet mount, modified acid-fast bacilli stain to detect rare parasites such as helminths, Strongyloides, Cyclospora, and Cystoisospora.3 In patients who live or travel to endemic areas (about 10% of traveler’s diarrhea is caused by parasitic infections), have unexplained eosinophilia, or are part of a community outbreak (eg, childcare institutions or drinking water/food outbreaks), test for Giardia, Cryptosporidium, Cyclospora, Cystoisospora, Entamoeba histolytica, and Isospora belli.9 In addition, among patients with AIDS or immunosuppression, testing should include assays for Microsporidia, Strongyloides, and Mycobacterium avium complex (Figure).9,16 Newer tests, such as the multiplex real-time polymerase chain reaction assay, can also simultaneously detect Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum. For more information on parasitic testing, we suggest reading the review article “Beyond O&P times three.”3 It is important to familiarize yourself with the parasitic tests available at your respective clinics/hospital so the optimal test can be used.

RECOMMENDATIONS
- Prescribe a trial of “wait and see” for patients without high-risk features for parasitic disease.
- Test patients with high-risk features for parasitic disease by utilizing targeted testing.
- For patients with high-risk features but no travel history, first perform DFA, EIA, or multiplex real-time polymerase chain reaction testing to evaluate for Giardia, Cryptosporidium, and Entamoeba histolytica.
- If DFA/EIA testing is negative, obtain O&P tests with and without stains, such as acid-fast bacilli, for detection of other rare parasites.
CONCLUSION
Hospitalists should risk-stratify patients to determine when O&P testing is appropriate. Employ more targeted testing, especially use of DFA/EIA tests when evaluating for parasites. Avoid parasitic testing if symptoms have lasted less than 7 days and the patient has no other high-risk features. Become familiar with the tests available at your institution and their sensitivities. As in our clinical scenario, most acute cases of diarrhea resolve without intervention and should be managed and treated conservatively.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing [email protected] .
Acknowledgments
The authors thank Dr Lenny Feldman for his assistance with editing the manuscript.
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
1. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111(5):602-622. https://doi.org/10.1038/ajg.2016.126
2. DuPont HL. Acute infectious diarrhea in immunocompetent adults. N Engl J Med. 2014;370(16):1532-1540. https://doi.org/10.1056/nejmra1301069
3. Mohapatra S, Singh DP, Alcid D, Pitchumoni CS. Beyond O&P times three. Am J Gastroenterol. 2018;113(6):805-818. https://doi.org/10.1038/s41395-018-0083-y
4. Painter JE, Hlavsa MC, Collier SA, Xiao L, Yoder JS. Cryptosporidiosis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):1-14.
5. Painter JE, Gargano JW, Collier SA, Yoder JS. Giardiasis surveillance -- United States, 2011-2012. MMWR Suppl. 2015;64(3):15-25.
6. Polage CR, Stoddard GJ, Rolfs RT, Petti CA. Physician use of parasite tests in the United States from 1997 to 2006 and in a Utah Cryptosporidium outbreak in 2007. J Clin Microbiol. 2011;49(2):591-596. https://doi.org/10.1128/jcm.01806-10
7. Mosli M, Gregor J, Chande N, Lannigan R. Nonutility of routine testing of stool for ova and parasites in a tertiary care Canadian centre. Can J Microbiol. 2012;58(5):653-659. https://doi.org/10.1139/w2012-039
8. Siegel DL, Edelstein PH, Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979-982.
9. Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65(12):e45-e80. https://doi.org/10.1093/cid/cix669
10. Hennessy TW, Marcus R, Deneen V, et al. Survey of physician diagnostic practices for patients with acute diarrhea: clinical and public health implications. Clin Infect Dis. 2004;38 (Suppl 3):S203-S211. https://doi.org/10.1086/381588
11. Morin CA, Roberts CL, Mshar PA, Addiss DG, Hadler JL. What do physicians know about cryptosporidiosis? a survey of Connecticut physicians. Arch Intern Med. 1997;157(9):1017-1022.
12. McHardy IH, Wu M, Shimizu-Cohen R, Couturier MR, Humphries RM. Detection of intestinal protozoa in the clinical laboratory. J Clin Microbiol. 2014;52(3):712-720. https://doi.org/10.1128/jcm.02877-13
13. Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120(2):206-211.
14. Jones JL, Lopez A, Wahlquist SP, Nadle J, Wilson M; Emerging Infections Program FoodNet Working Group. Survey of clinical laboratory practices for parasitic diseases. Clin Infect Dis. 2004;38(Suppl 3):S198-S202. https://doi.org/10.1086/381587
15. Tewell CE, Talbot TR, Nelson GE, et al. Reducing inappropriate testing for the evaluation of diarrhea among hospitalized patients. Am J Med. 2018;131(2):193-199.e1. https://doi.org/10.1016/j.amjmed.2017.10.006
16. Thielman NM, Guerrant RL. Clinical practice. acute infectious diarrhea. N Engl J Med. 2004;350(1):38-47. https://doi.org/10.1056/nejmcp031534
17. Marti H, Koella JC. Multiple stool examinations for ova and parasites and rate of false-negative results. J Clin Microbiol. 1993;31(11):3044-3045. https://doi.org/10.1128/jcm.31.11.3044-3045.1993
© 2021 Society of Hospital Medicine
Limiting Patient Autonomy: Mandatory COVID-19 Diagnostic Testing
Despite the important clinical and public health implications of a COVID-19 diagnosis, respect for autonomy allows patients to decline testing without explanation and with impunity. Whether physicians believe a test is indicated for clinical care of an individual patient, prevention of nosocomial transmission, or the greater public health, patients may refuse. Such refusals may be increasing due to quarantine requirements, concerns regarding contact tracing, and the persistent absence of a curative treatment.1,2 Mass screening of all healthcare workers (HCWs) is being considered to prevent hospital transmission,3 and universal screening in nursing homes has thwarted outbreaks while providing data to facilitate resource allocation.4 Given these circumstances, patients’ absolute right to refuse a noninvasive test with the potential for multifaceted downstream benefit is worthy of reconsideration, in favor of mandatory testing. Mandatory testing confers numerous benefits, including mitigating risk to other patients and HCWs, who play a central role in pandemic response. Because infected HCWs may transmit the virus to patients, they also should undergo mandatory testing,3 particularly in the presence of symptoms, since nasal secretions increase the diagnostic yield of testing.5 Although pretest probability (as an estimate of disease prevalence) typically determines the testing strategy for admitted patients, model-based analyses suggest that testing every 3 days for HCWs or continuously hospitalized patients would nearly eliminate infectivity.6
Tools for assisting frustrated HCWs navigating patients’ right to refuse testing have been developed that incorporate education, clear communication, and conflict resolution.7 Such approaches are, however, only moderately successful, making the use of personal protective equipment (PPE) based on a default assumption of COVID-19 positivity common.8 The burden and disheartening waste created by low-yield PPE use among patients unwilling to be tested becomes particularly evident in the context of shortages. Such vexing, stressful shortages, as well as the dual responsibilities of hospitals as stewards of both individual patient and population health, serve as reminders that efficient allocation of resources must be valued alongside the autonomous rights of patients.9 Moreover, recent reports suggest that test avoidance is a growing problem.1,2 Refusal to accept testing may be rooted in anxiety, concerns about the consequences of a positive result (eg, inability to attend school or work), or a desire for self-determination.1,2 The hesitancy that leads to refusal may also arise from misinformation, poor public health messaging, distrust in the establishment, and unproductive considerations related to conscientious objection without foundation.2 Concepts of individual liberty that often underlie steadfast adherence to the principles of self-determination created opposition to masks that antagonized public health efforts to limit the spread of COVID-19. Although influencing inpatients’ behavior to benefit both the public and HCWs may be distinct from community settings, the attitudes that lead to test refusal and defiance of mask-related ordinances likely have substantial commonalities.
THE PATIENT ROLE IN HEALTHCARE DECISIONS
As a pillar of ethical
ANALOGOUS EXPERIENCES: ETHICAL LESSONS AND PRACTICAL IMPLICATIONS
In non-healthcare settings, the controversies surrounding vaccination and access to schools for unvaccinated children are perhaps the public and professional debate most analogous to COVID-19 testing refusal.12 Although policymakers may distinguish between testing and vaccination, these interventions similarly hold the potential to limit disease incidence and mitigate health impact. To preserve public health, most states prevent (with varied exemptions) unvaccinated children from attending schools. COVID-19 testing may in the future become a requirement for participation in group social activities, athletic competitions, or physical presence in the workplace to facilitate quarantining and/or targeted use of PPE for transmission risk reduction. Given the dramatic mitigation benefits accruable on college campuses,13 required testing for in-person learning has become common.
There are also parallels, and therefore lessons, to be drawn from experience in testing for HIV, although HIV-related stigma and devalued status of the marginalized populations initially infected impacted the broader societal view of HIV compared with COVID-19. For example, antenatal HIV screening of pregnant women is strongly recommended to facilitate interventions that reduce the chance of vertical transmission.14 The limitations of purely elective testing are one justification for the current standard of opt-out screening. However, in this case, the health complications of refusal are largely the burden of the fetus, over whose future the mother holds a great deal of choice and responsibility, irrespective of HIV status. The public health implications of HIV test refusal are far less immediate than for COVID-19 infection because there is no effective curative therapy for COVID-19 and spread occurs through nonintimate, unintentional, and unpredictable exposure.
Translating societal attitudes and practices into the healthcare setting to consider mandated COVID-19 testing requires additional considerations related to both patients and providers: (1) HCWs have committed to a set of values and professional obligations that include tasks requiring risks15; (2) the public expects HCWs to perform their duties according to a social contract that has few restrictions16; (3) limiting patient access to hospital care due to COVID-19 testing refusal would contradict and create conflicts related to professional conceptions of hospitals and physicians as patient agents15; and (4) patients who conscientiously object to testing may seek healthcare less diligently, which may lead to health decrements. The associated postponement of essential care may unduly burden the healthcare system, particularly in situations such as ambulatory care–sensitive conditions.
HEALTHCARE WORKER PROTECTION, PATIENT ACCESS, AND THE VALUE OF PARSIMONY
The extent to which the public health justification for mandatory testing extends to hospitalized patients to protect HCWs is ambiguous. HCWs are of enormous instrumental value and are therefore essential for the pandemic response and health of the broader population. Their protection may therefore justify curtailing informed consent for diagnostic testing. Downstream effects on the supply of frontline HCWs may be realized. Poor control over working conditions may negatively impact motivation among HCWs. In addition, they may feel disenfranchised while obligatorily taking personal risks in caring for patients unwilling to commit to the common good through diagnostic test consent. Hospitalized patients who refuse testing may remain patients under investigation (PUIs), thus requiring special respiratory precautions (SRP) throughout their hospitalization, thereby placing a persistent burden on those with responsibilities requiring patient contact.17 Repeatedly donning and doffing PPE may remind at-risk HCWs that a myriad of benefits may accrue from frequent, ubiquitous testing. Their motivation may be tempered by the demoralizing requirement to care for patients who will not consent to a simple test, knowing that an opportunity to diminish the burdens of this communicable disease that has taken the lives of many HCWs is being relinquished.
Although HCWs could use SRP universally, their selective application in rooms of known COVID-19–positive patients and those with temporary PUI status has several advantages.17 First, we learned that HIV testing on patients was helpful in enabling surgeons to selectively implement special precautions among infected patients rather than universally applied intensive precautions. Even in the setting of high rates of HIV infection and educational interventions, HCWs do not reliably apply protective measures included in universal precautions.18 In keeping with these experiences, limiting the number of patients on SRP will minimize the “precautions fatigue” that drives nonadherent behavior among HCWs.17 As a result, minimizing the proportion of patients on SRP through testing (and liberation from unnecessary precautions in most cases) will improve uptake of crucial hand hygiene practices and adoption of vigilant PPE use. Second, definitive knowledge of COVID-19 status will increase patient access to care because, whether by personal choice or policy, many HCWs limit in-person contact with patients who are or may be COVID-19 positive. For example, many inpatient dialysis units do not accept patients without a negative COVID-19 nasal swab. Physical therapists may delay or avoid seeing a PUI, which will pose challenges for efficient determination of discharge disposition. Third, selective use of SRP will limit the environmental impact of disposed PPE, which is neither recyclable nor biodegradable. Infectious or regulated biomedical waste products are a significant source of environmental pollution, and the World Health Organization has recommended parsimonious, selective use of PPE to minimize the adverse environmental consequences of biomedical waste products.
CONCLUSION
In summary, there are substantial justifications for mandatory testing for COVID-19 in the hospital for HCWs and patients, as has been successfully piloted in selected long-term care facilities. Patients who refuse to allow testing may have to accept that their care may be compromised. For preservation of HCW supply and maintenance of HCW morale, hospital policies should make explicit, without punishment or coercion, that HCWs may modify the care they provide to patients who refuse to consent to COVID-19 testing.
1. Morris NP. Refusing testing during a pandemic. Am J Public Health. 2020;110(9):1354-1355. https://doi.org/10.2105/AJPH.2020.305810
2. Rubin R. First it was masks; now some refuse testing for SARS-CoV-2. JAMA. 2020;324(20):2015-2016. https://doi.org/10.1001/jama.2020.22003
3. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/S0140-6736(20)30917-X
4. McBee SM, Thomasson ED, Scott MA, et al. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21–May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1177-1179. http://dx.doi.org/10.15585/mmwr.mm6934a4
5. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72(2):323-326. https://doi.org/10.1093/cid/ciaa722
6. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. https://advances.sciencemag.org/content/7/1/eabd5393
7. Lu AC, Burgart AM. Elective surgery and COVID-19: a framework for the untested patient. Ann Surg. 2020;272(6):e291-e295. https://doi.org/10.1097/SLA.0000000000004474
8. Podboy A, Cholankeril G, Cianfichi L, Guzman E Jr, Ahmed A, Banerjee S. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159(4):1586-1588. https://doi.org/10.1053/j.gastro.2020.06.022
9. O’Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4
10. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part II: the autonomy model. Chest. 2011;139(6):1491-1497. https://doi.org/10.1378/chest.11-0516
11. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part I: the beneficence model. Chest. 2011;139(3):669-673. https://doi.org/10.1378/chest.10-2532
12. Hendrix KS, Sturm LA, Zimet GD, Meslin EM. Ethics and childhood vaccination policy in the United States. Am J Public Health. 2016;106(2):273-278. https://doi.org/10.2105/AJPH.2015.302952
13. Losina E, Leifer V, Millham L, et al. College campuses and COVID-19 mitigation: clinical and economic value. Ann Intern Med. Published online December 21, 2020. https://doi.org/10.7326/M20-6558
14. Selph SS, Bougatsos C, Dana T, Grusing S, Chou R. Screening for HIV infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(23):2349-2360. https://doi.org/10.1001/jama.2019.2593
15. Dranove D, White WD. Agency and the organization of health care delivery. Inquiry. 1987;24(4):405-415.
16. Huber SJ, Wynia MK. When pestilence prevails...physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-W11. https://www.tandfonline.com/doi/abs/10.1162/152651604773067497
17. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
18. Freeman SW, Chambers CV. Compliance with universal precautions in a medical practice with a high rate of HIV infection. J Am Board Fam Pract. 1992;5(3):313-318.
Despite the important clinical and public health implications of a COVID-19 diagnosis, respect for autonomy allows patients to decline testing without explanation and with impunity. Whether physicians believe a test is indicated for clinical care of an individual patient, prevention of nosocomial transmission, or the greater public health, patients may refuse. Such refusals may be increasing due to quarantine requirements, concerns regarding contact tracing, and the persistent absence of a curative treatment.1,2 Mass screening of all healthcare workers (HCWs) is being considered to prevent hospital transmission,3 and universal screening in nursing homes has thwarted outbreaks while providing data to facilitate resource allocation.4 Given these circumstances, patients’ absolute right to refuse a noninvasive test with the potential for multifaceted downstream benefit is worthy of reconsideration, in favor of mandatory testing. Mandatory testing confers numerous benefits, including mitigating risk to other patients and HCWs, who play a central role in pandemic response. Because infected HCWs may transmit the virus to patients, they also should undergo mandatory testing,3 particularly in the presence of symptoms, since nasal secretions increase the diagnostic yield of testing.5 Although pretest probability (as an estimate of disease prevalence) typically determines the testing strategy for admitted patients, model-based analyses suggest that testing every 3 days for HCWs or continuously hospitalized patients would nearly eliminate infectivity.6
Tools for assisting frustrated HCWs navigating patients’ right to refuse testing have been developed that incorporate education, clear communication, and conflict resolution.7 Such approaches are, however, only moderately successful, making the use of personal protective equipment (PPE) based on a default assumption of COVID-19 positivity common.8 The burden and disheartening waste created by low-yield PPE use among patients unwilling to be tested becomes particularly evident in the context of shortages. Such vexing, stressful shortages, as well as the dual responsibilities of hospitals as stewards of both individual patient and population health, serve as reminders that efficient allocation of resources must be valued alongside the autonomous rights of patients.9 Moreover, recent reports suggest that test avoidance is a growing problem.1,2 Refusal to accept testing may be rooted in anxiety, concerns about the consequences of a positive result (eg, inability to attend school or work), or a desire for self-determination.1,2 The hesitancy that leads to refusal may also arise from misinformation, poor public health messaging, distrust in the establishment, and unproductive considerations related to conscientious objection without foundation.2 Concepts of individual liberty that often underlie steadfast adherence to the principles of self-determination created opposition to masks that antagonized public health efforts to limit the spread of COVID-19. Although influencing inpatients’ behavior to benefit both the public and HCWs may be distinct from community settings, the attitudes that lead to test refusal and defiance of mask-related ordinances likely have substantial commonalities.
THE PATIENT ROLE IN HEALTHCARE DECISIONS
As a pillar of ethical
ANALOGOUS EXPERIENCES: ETHICAL LESSONS AND PRACTICAL IMPLICATIONS
In non-healthcare settings, the controversies surrounding vaccination and access to schools for unvaccinated children are perhaps the public and professional debate most analogous to COVID-19 testing refusal.12 Although policymakers may distinguish between testing and vaccination, these interventions similarly hold the potential to limit disease incidence and mitigate health impact. To preserve public health, most states prevent (with varied exemptions) unvaccinated children from attending schools. COVID-19 testing may in the future become a requirement for participation in group social activities, athletic competitions, or physical presence in the workplace to facilitate quarantining and/or targeted use of PPE for transmission risk reduction. Given the dramatic mitigation benefits accruable on college campuses,13 required testing for in-person learning has become common.
There are also parallels, and therefore lessons, to be drawn from experience in testing for HIV, although HIV-related stigma and devalued status of the marginalized populations initially infected impacted the broader societal view of HIV compared with COVID-19. For example, antenatal HIV screening of pregnant women is strongly recommended to facilitate interventions that reduce the chance of vertical transmission.14 The limitations of purely elective testing are one justification for the current standard of opt-out screening. However, in this case, the health complications of refusal are largely the burden of the fetus, over whose future the mother holds a great deal of choice and responsibility, irrespective of HIV status. The public health implications of HIV test refusal are far less immediate than for COVID-19 infection because there is no effective curative therapy for COVID-19 and spread occurs through nonintimate, unintentional, and unpredictable exposure.
Translating societal attitudes and practices into the healthcare setting to consider mandated COVID-19 testing requires additional considerations related to both patients and providers: (1) HCWs have committed to a set of values and professional obligations that include tasks requiring risks15; (2) the public expects HCWs to perform their duties according to a social contract that has few restrictions16; (3) limiting patient access to hospital care due to COVID-19 testing refusal would contradict and create conflicts related to professional conceptions of hospitals and physicians as patient agents15; and (4) patients who conscientiously object to testing may seek healthcare less diligently, which may lead to health decrements. The associated postponement of essential care may unduly burden the healthcare system, particularly in situations such as ambulatory care–sensitive conditions.
HEALTHCARE WORKER PROTECTION, PATIENT ACCESS, AND THE VALUE OF PARSIMONY
The extent to which the public health justification for mandatory testing extends to hospitalized patients to protect HCWs is ambiguous. HCWs are of enormous instrumental value and are therefore essential for the pandemic response and health of the broader population. Their protection may therefore justify curtailing informed consent for diagnostic testing. Downstream effects on the supply of frontline HCWs may be realized. Poor control over working conditions may negatively impact motivation among HCWs. In addition, they may feel disenfranchised while obligatorily taking personal risks in caring for patients unwilling to commit to the common good through diagnostic test consent. Hospitalized patients who refuse testing may remain patients under investigation (PUIs), thus requiring special respiratory precautions (SRP) throughout their hospitalization, thereby placing a persistent burden on those with responsibilities requiring patient contact.17 Repeatedly donning and doffing PPE may remind at-risk HCWs that a myriad of benefits may accrue from frequent, ubiquitous testing. Their motivation may be tempered by the demoralizing requirement to care for patients who will not consent to a simple test, knowing that an opportunity to diminish the burdens of this communicable disease that has taken the lives of many HCWs is being relinquished.
Although HCWs could use SRP universally, their selective application in rooms of known COVID-19–positive patients and those with temporary PUI status has several advantages.17 First, we learned that HIV testing on patients was helpful in enabling surgeons to selectively implement special precautions among infected patients rather than universally applied intensive precautions. Even in the setting of high rates of HIV infection and educational interventions, HCWs do not reliably apply protective measures included in universal precautions.18 In keeping with these experiences, limiting the number of patients on SRP will minimize the “precautions fatigue” that drives nonadherent behavior among HCWs.17 As a result, minimizing the proportion of patients on SRP through testing (and liberation from unnecessary precautions in most cases) will improve uptake of crucial hand hygiene practices and adoption of vigilant PPE use. Second, definitive knowledge of COVID-19 status will increase patient access to care because, whether by personal choice or policy, many HCWs limit in-person contact with patients who are or may be COVID-19 positive. For example, many inpatient dialysis units do not accept patients without a negative COVID-19 nasal swab. Physical therapists may delay or avoid seeing a PUI, which will pose challenges for efficient determination of discharge disposition. Third, selective use of SRP will limit the environmental impact of disposed PPE, which is neither recyclable nor biodegradable. Infectious or regulated biomedical waste products are a significant source of environmental pollution, and the World Health Organization has recommended parsimonious, selective use of PPE to minimize the adverse environmental consequences of biomedical waste products.
CONCLUSION
In summary, there are substantial justifications for mandatory testing for COVID-19 in the hospital for HCWs and patients, as has been successfully piloted in selected long-term care facilities. Patients who refuse to allow testing may have to accept that their care may be compromised. For preservation of HCW supply and maintenance of HCW morale, hospital policies should make explicit, without punishment or coercion, that HCWs may modify the care they provide to patients who refuse to consent to COVID-19 testing.
Despite the important clinical and public health implications of a COVID-19 diagnosis, respect for autonomy allows patients to decline testing without explanation and with impunity. Whether physicians believe a test is indicated for clinical care of an individual patient, prevention of nosocomial transmission, or the greater public health, patients may refuse. Such refusals may be increasing due to quarantine requirements, concerns regarding contact tracing, and the persistent absence of a curative treatment.1,2 Mass screening of all healthcare workers (HCWs) is being considered to prevent hospital transmission,3 and universal screening in nursing homes has thwarted outbreaks while providing data to facilitate resource allocation.4 Given these circumstances, patients’ absolute right to refuse a noninvasive test with the potential for multifaceted downstream benefit is worthy of reconsideration, in favor of mandatory testing. Mandatory testing confers numerous benefits, including mitigating risk to other patients and HCWs, who play a central role in pandemic response. Because infected HCWs may transmit the virus to patients, they also should undergo mandatory testing,3 particularly in the presence of symptoms, since nasal secretions increase the diagnostic yield of testing.5 Although pretest probability (as an estimate of disease prevalence) typically determines the testing strategy for admitted patients, model-based analyses suggest that testing every 3 days for HCWs or continuously hospitalized patients would nearly eliminate infectivity.6
Tools for assisting frustrated HCWs navigating patients’ right to refuse testing have been developed that incorporate education, clear communication, and conflict resolution.7 Such approaches are, however, only moderately successful, making the use of personal protective equipment (PPE) based on a default assumption of COVID-19 positivity common.8 The burden and disheartening waste created by low-yield PPE use among patients unwilling to be tested becomes particularly evident in the context of shortages. Such vexing, stressful shortages, as well as the dual responsibilities of hospitals as stewards of both individual patient and population health, serve as reminders that efficient allocation of resources must be valued alongside the autonomous rights of patients.9 Moreover, recent reports suggest that test avoidance is a growing problem.1,2 Refusal to accept testing may be rooted in anxiety, concerns about the consequences of a positive result (eg, inability to attend school or work), or a desire for self-determination.1,2 The hesitancy that leads to refusal may also arise from misinformation, poor public health messaging, distrust in the establishment, and unproductive considerations related to conscientious objection without foundation.2 Concepts of individual liberty that often underlie steadfast adherence to the principles of self-determination created opposition to masks that antagonized public health efforts to limit the spread of COVID-19. Although influencing inpatients’ behavior to benefit both the public and HCWs may be distinct from community settings, the attitudes that lead to test refusal and defiance of mask-related ordinances likely have substantial commonalities.
THE PATIENT ROLE IN HEALTHCARE DECISIONS
As a pillar of ethical
ANALOGOUS EXPERIENCES: ETHICAL LESSONS AND PRACTICAL IMPLICATIONS
In non-healthcare settings, the controversies surrounding vaccination and access to schools for unvaccinated children are perhaps the public and professional debate most analogous to COVID-19 testing refusal.12 Although policymakers may distinguish between testing and vaccination, these interventions similarly hold the potential to limit disease incidence and mitigate health impact. To preserve public health, most states prevent (with varied exemptions) unvaccinated children from attending schools. COVID-19 testing may in the future become a requirement for participation in group social activities, athletic competitions, or physical presence in the workplace to facilitate quarantining and/or targeted use of PPE for transmission risk reduction. Given the dramatic mitigation benefits accruable on college campuses,13 required testing for in-person learning has become common.
There are also parallels, and therefore lessons, to be drawn from experience in testing for HIV, although HIV-related stigma and devalued status of the marginalized populations initially infected impacted the broader societal view of HIV compared with COVID-19. For example, antenatal HIV screening of pregnant women is strongly recommended to facilitate interventions that reduce the chance of vertical transmission.14 The limitations of purely elective testing are one justification for the current standard of opt-out screening. However, in this case, the health complications of refusal are largely the burden of the fetus, over whose future the mother holds a great deal of choice and responsibility, irrespective of HIV status. The public health implications of HIV test refusal are far less immediate than for COVID-19 infection because there is no effective curative therapy for COVID-19 and spread occurs through nonintimate, unintentional, and unpredictable exposure.
Translating societal attitudes and practices into the healthcare setting to consider mandated COVID-19 testing requires additional considerations related to both patients and providers: (1) HCWs have committed to a set of values and professional obligations that include tasks requiring risks15; (2) the public expects HCWs to perform their duties according to a social contract that has few restrictions16; (3) limiting patient access to hospital care due to COVID-19 testing refusal would contradict and create conflicts related to professional conceptions of hospitals and physicians as patient agents15; and (4) patients who conscientiously object to testing may seek healthcare less diligently, which may lead to health decrements. The associated postponement of essential care may unduly burden the healthcare system, particularly in situations such as ambulatory care–sensitive conditions.
HEALTHCARE WORKER PROTECTION, PATIENT ACCESS, AND THE VALUE OF PARSIMONY
The extent to which the public health justification for mandatory testing extends to hospitalized patients to protect HCWs is ambiguous. HCWs are of enormous instrumental value and are therefore essential for the pandemic response and health of the broader population. Their protection may therefore justify curtailing informed consent for diagnostic testing. Downstream effects on the supply of frontline HCWs may be realized. Poor control over working conditions may negatively impact motivation among HCWs. In addition, they may feel disenfranchised while obligatorily taking personal risks in caring for patients unwilling to commit to the common good through diagnostic test consent. Hospitalized patients who refuse testing may remain patients under investigation (PUIs), thus requiring special respiratory precautions (SRP) throughout their hospitalization, thereby placing a persistent burden on those with responsibilities requiring patient contact.17 Repeatedly donning and doffing PPE may remind at-risk HCWs that a myriad of benefits may accrue from frequent, ubiquitous testing. Their motivation may be tempered by the demoralizing requirement to care for patients who will not consent to a simple test, knowing that an opportunity to diminish the burdens of this communicable disease that has taken the lives of many HCWs is being relinquished.
Although HCWs could use SRP universally, their selective application in rooms of known COVID-19–positive patients and those with temporary PUI status has several advantages.17 First, we learned that HIV testing on patients was helpful in enabling surgeons to selectively implement special precautions among infected patients rather than universally applied intensive precautions. Even in the setting of high rates of HIV infection and educational interventions, HCWs do not reliably apply protective measures included in universal precautions.18 In keeping with these experiences, limiting the number of patients on SRP will minimize the “precautions fatigue” that drives nonadherent behavior among HCWs.17 As a result, minimizing the proportion of patients on SRP through testing (and liberation from unnecessary precautions in most cases) will improve uptake of crucial hand hygiene practices and adoption of vigilant PPE use. Second, definitive knowledge of COVID-19 status will increase patient access to care because, whether by personal choice or policy, many HCWs limit in-person contact with patients who are or may be COVID-19 positive. For example, many inpatient dialysis units do not accept patients without a negative COVID-19 nasal swab. Physical therapists may delay or avoid seeing a PUI, which will pose challenges for efficient determination of discharge disposition. Third, selective use of SRP will limit the environmental impact of disposed PPE, which is neither recyclable nor biodegradable. Infectious or regulated biomedical waste products are a significant source of environmental pollution, and the World Health Organization has recommended parsimonious, selective use of PPE to minimize the adverse environmental consequences of biomedical waste products.
CONCLUSION
In summary, there are substantial justifications for mandatory testing for COVID-19 in the hospital for HCWs and patients, as has been successfully piloted in selected long-term care facilities. Patients who refuse to allow testing may have to accept that their care may be compromised. For preservation of HCW supply and maintenance of HCW morale, hospital policies should make explicit, without punishment or coercion, that HCWs may modify the care they provide to patients who refuse to consent to COVID-19 testing.
1. Morris NP. Refusing testing during a pandemic. Am J Public Health. 2020;110(9):1354-1355. https://doi.org/10.2105/AJPH.2020.305810
2. Rubin R. First it was masks; now some refuse testing for SARS-CoV-2. JAMA. 2020;324(20):2015-2016. https://doi.org/10.1001/jama.2020.22003
3. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/S0140-6736(20)30917-X
4. McBee SM, Thomasson ED, Scott MA, et al. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21–May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1177-1179. http://dx.doi.org/10.15585/mmwr.mm6934a4
5. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72(2):323-326. https://doi.org/10.1093/cid/ciaa722
6. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. https://advances.sciencemag.org/content/7/1/eabd5393
7. Lu AC, Burgart AM. Elective surgery and COVID-19: a framework for the untested patient. Ann Surg. 2020;272(6):e291-e295. https://doi.org/10.1097/SLA.0000000000004474
8. Podboy A, Cholankeril G, Cianfichi L, Guzman E Jr, Ahmed A, Banerjee S. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159(4):1586-1588. https://doi.org/10.1053/j.gastro.2020.06.022
9. O’Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4
10. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part II: the autonomy model. Chest. 2011;139(6):1491-1497. https://doi.org/10.1378/chest.11-0516
11. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part I: the beneficence model. Chest. 2011;139(3):669-673. https://doi.org/10.1378/chest.10-2532
12. Hendrix KS, Sturm LA, Zimet GD, Meslin EM. Ethics and childhood vaccination policy in the United States. Am J Public Health. 2016;106(2):273-278. https://doi.org/10.2105/AJPH.2015.302952
13. Losina E, Leifer V, Millham L, et al. College campuses and COVID-19 mitigation: clinical and economic value. Ann Intern Med. Published online December 21, 2020. https://doi.org/10.7326/M20-6558
14. Selph SS, Bougatsos C, Dana T, Grusing S, Chou R. Screening for HIV infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(23):2349-2360. https://doi.org/10.1001/jama.2019.2593
15. Dranove D, White WD. Agency and the organization of health care delivery. Inquiry. 1987;24(4):405-415.
16. Huber SJ, Wynia MK. When pestilence prevails...physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-W11. https://www.tandfonline.com/doi/abs/10.1162/152651604773067497
17. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
18. Freeman SW, Chambers CV. Compliance with universal precautions in a medical practice with a high rate of HIV infection. J Am Board Fam Pract. 1992;5(3):313-318.
1. Morris NP. Refusing testing during a pandemic. Am J Public Health. 2020;110(9):1354-1355. https://doi.org/10.2105/AJPH.2020.305810
2. Rubin R. First it was masks; now some refuse testing for SARS-CoV-2. JAMA. 2020;324(20):2015-2016. https://doi.org/10.1001/jama.2020.22003
3. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/S0140-6736(20)30917-X
4. McBee SM, Thomasson ED, Scott MA, et al. Notes from the field: universal statewide laboratory testing for SARS-CoV-2 in nursing homes—West Virginia, April 21–May 8, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(34):1177-1179. http://dx.doi.org/10.15585/mmwr.mm6934a4
5. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72(2):323-326. https://doi.org/10.1093/cid/ciaa722
6. Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. https://advances.sciencemag.org/content/7/1/eabd5393
7. Lu AC, Burgart AM. Elective surgery and COVID-19: a framework for the untested patient. Ann Surg. 2020;272(6):e291-e295. https://doi.org/10.1097/SLA.0000000000004474
8. Podboy A, Cholankeril G, Cianfichi L, Guzman E Jr, Ahmed A, Banerjee S. Implementation and impact of universal preprocedure testing of patients for COVID-19 before endoscopy. Gastroenterology. 2020;159(4):1586-1588. https://doi.org/10.1053/j.gastro.2020.06.022
9. O’Neill O. Some limits of informed consent. J Med Ethics. 2003;29(1):4-7. https://doi.org/10.1136/jme.29.1.4
10. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part II: the autonomy model. Chest. 2011;139(6):1491-1497. https://doi.org/10.1378/chest.11-0516
11. Will JF. A brief historical and theoretical perspective on patient autonomy and medical decision making: part I: the beneficence model. Chest. 2011;139(3):669-673. https://doi.org/10.1378/chest.10-2532
12. Hendrix KS, Sturm LA, Zimet GD, Meslin EM. Ethics and childhood vaccination policy in the United States. Am J Public Health. 2016;106(2):273-278. https://doi.org/10.2105/AJPH.2015.302952
13. Losina E, Leifer V, Millham L, et al. College campuses and COVID-19 mitigation: clinical and economic value. Ann Intern Med. Published online December 21, 2020. https://doi.org/10.7326/M20-6558
14. Selph SS, Bougatsos C, Dana T, Grusing S, Chou R. Screening for HIV infection in pregnant women: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321(23):2349-2360. https://doi.org/10.1001/jama.2019.2593
15. Dranove D, White WD. Agency and the organization of health care delivery. Inquiry. 1987;24(4):405-415.
16. Huber SJ, Wynia MK. When pestilence prevails...physician responsibilities in epidemics. Am J Bioeth. 2004;4(1):W5-W11. https://www.tandfonline.com/doi/abs/10.1162/152651604773067497
17. Ruhnke GW. COVID-19 diagnostic testing and the psychology of precautions fatigue. Cleve Clin J Med. 2020;88(1):19-21. https://doi.org/10.3949/ccjm.88a.20086
18. Freeman SW, Chambers CV. Compliance with universal precautions in a medical practice with a high rate of HIV infection. J Am Board Fam Pract. 1992;5(3):313-318.
© 2021 Society of Hospital Medicine
Striking While the Iron Is Hot: Using the Updated PHM Competencies in Time-Variable Training
In July 2020, the revision of The Pediatric Hospital Medicine Core Competencies was published, bringing to fruition three years of meticulous work.1 The working group produced 66 chapters outlining the knowledge, skills, and attitudes needed for competent pediatric hospitalist practice. The arrival of these competencies is especially prescient given pediatric hospital medicine’s (PHM’s) relatively new standing as an American Board of Medical Specialties certified subspecialty, as the competencies can serve as a guide for improvement of fellowship curricula, assessment systems, and faculty development. The competencies also represent an opportunity for PHM to take a bold step forward in the world of graduate medical education (GME) by realizing a key tenet of competency-based medical education (CBME)—competency-based, time-variable training (CBTVT), in which learners train until competence is achieved rather than for a predetermined duration.2,3 In this perspective, we describe how medical education in the United States adopted a time-based training paradigm (in which time-in-training is a surrogate for competence), how CBME has brought time-variable training to the fore, and how PHM has an opportunity to be on the leading edge of education innovation.
TIME-BASED TRAINING IN THE UNITED STATES
In the 1800s, during the time of the “Wild West,” medical education in the United States matched this moniker. There was little standardization across the multiple training pathways to become a practicing physician, including apprenticeships, lecture series, and university courses.4 Predictably, this led to significant heterogeneity in the quality of medical care that a patient of the day received. This problem became clearer as Americans traveled to Europe and witnessed more structured and rigorous training programs, only to return to the comparatively poor state of medical education back home.5 There was a clear need for curricular standardization.
In 1876, the American Medical College Association (which later became the Association of American Medical Colleges [AAMC]) was founded to meet this need, and in 1905 the Association adopted a set of minimum standards for medical training that included the now-familiar two years of basic sciences and two years of clinical training.6 Two subsequent national surveys in the United States were commissioned to evaluate whether medical schools met this new standard, with both surveys finding that roughly half of existing programs passed muster.7,8 As a result, nearly half of US medical schools had closed by 1920 in a crusade to standardize curricula and produce competent physicians. By the time the American Medical Association established initial standards for internship (an archetype of GME),4 time-based medical training was the dominant paradigm. This historical perspective highlights the rationale for standardization of education processes and curricula, particularly in terms of accountability to the American public. But heralded by the 1978 landmark paper by McGaghie et al,9 the paradigm began to shift in the late twentieth century from a focus on the process of physician training to outcomes.
CBME AND TIME VARIABILITY
In contrast to the process-focused model of the early 1900s, CBME starts by identifying patient and healthcare system needs, defining competencies required to meet those needs, and then designing curricular and assessment processes to help learners achieve those competencies.2 This outcomes-based approach grew as a response to calls for greater accountability to the public due to evidence that some graduates were unprepared for unsupervised practice, raising concerns that strictly time-based training was no longer defensible.10 CBME aims to mitigate these concerns by starting with desired outcomes of training and working backward to ensure those outcomes are met.
While many programs have attempted to implement CBME, most still rely heavily on time-in-training to determine competence. Learners participate in structured curricula and, unless they are extreme outliers, are deemed ready for unsupervised practice after a predetermined duration. This model presumes that competence and time are related in a fixed, predictable manner and that learners gain competence at a uniform rate. However, learners do not, in fact, progress uniformly. A study by Schumacher et al11 involving 23 pediatric residency programs showed significant interlearner variability in rates of entrustment (used as a surrogate for competence), leading the authors to call for time-variable training in GME. Significant interlearner variation in rates of competence attainment have been shown in other specialties as well.12 As more CBME studies on training outcomes emerge, the evidence is mounting that not all learners need the same duration of training to become competent providers. Time-in-training and competence attainment are not related in a fixed manner. As Dr Jason13 wrote in 1969, “By making time a constant, we make achievement a variable.” Variable achievement (competence, outcomes) was the very driver for medical education’s shift to a competency-based approach. If variable competence was not acceptable then, why should it be now? The goal of CBTVT is not shorter training, but rather flexible, individualized training both in terms of content and duration. While this also means some learners may need to extend their training, this should already be part of GME programs that are required to have remediation policies for learners who are not progressing as expected.
AN OPPORTUNITY FOR PHM
Time variability is an oft-cited tenet of CBME,2,3 but one that is being piloted by relatively few programs in the United States, mostly in undergraduate medical education (UME).14-16 The Education in Pediatrics Across the Continuum (EPAC), a consortium consisting of four institutions piloting CBTVT in UME,14 has shown early evidence of feasibility17 and that UME graduates from CBTVT programs enter residency with levels of competence similar to those of graduates of traditional time-based programs.18 We believe that PHM can take a step toward truly realizing CBME by implementing CBTVT in fellowship programs.
There are multiple reasons why this is an opportune time for PHM fellowships to consider CBTVT. First, PHM is a relatively new board-certified subspecialty with a recently revised set of core competencies1 that are likely to catalyze programmatic innovation. A key step in change management is building on previous efforts to generate more change.19 Programs can leverage the momentum from current and impending change initiatives to innovate and implement CBTVT. Second, the revised PHM competencies provide the first crucial step in implementing a CBME program by defining desired training outcomes necessary to deliver high-quality patient care. With PHM competencies now well defined, programs can focus on developing programs of assessment and corresponding faculty development, which can help deliver valid, defensible decisions about fellow competence.
Finally, PHM has a workforce that can support CBTVT. A major barrier to time-variable training in GME is the need for trainees-as-workforce. In many GME programs, residents and fellows provide a relatively inexpensive, renewable workforce. Trainees’ clinical rotations are often scheduled up to 1 year in advance to ensure care teams are fully staffed, particularly in the inpatient setting, creating a system where flexibility in training is impossible without creating gaps in clinical coverage. However, many PHM fellowships do not completely rely on fellows to cover clinical service lines. PHM fellows spend 32 weeks over 2 years in core clinical rotations with faculty supervision, in accordance with the Accreditation Council for Graduate Medical Education program requirements, both for 2- and 3-year programs. Some CBME experts estimate (based on previous and ongoing CBTVT pilots) that training duration is likely to vary by roughly 20% from current time-based practices when CBTVT is initially implemented.20 Thus, only a small number of clinical service weeks are likely to be affected. If a fellow were deemed ready for unsupervised practice before finishing 2 years of fellowship in a CBTVT program, the corresponding faculty supervisor could use the time previously assigned for supervision to pursue other priorities, such as education, scholarship, or quality improvement. Why provide supervision if a clinical competency committee has deemed a fellow ready for unsupervised practice? Some level of observation and formative feedback could continue, but full supervision would be redundant and unnecessary. CBTVT would allow for some fellows to experience the uncertainty that comes with unsupervised decision-making while still in an environment with trusted fellowship mentors and advisors.
STEPS TOWARD CHANGE
PHM fellowship programs likely cannot flip a switch to “turn on” CBTVT immediately, but they can take steps toward making the transition. Validity, or defensibility of decisions, will be crucial for assessment in CBTVT systems. Programs will need to develop robust assessment systems that collect myriad data to answer the question, “When is this learner competent to deliver high-quality care without supervision?” Programs can align assessment instruments, faculty-development initiatives, and clinical competency committee (CCC) processes with the 2020 PHM competencies to provide a defensible answer. Program leaders should then seek validity evidence, either in existing literature or through novel scholarly initiatives, to support these summative decisions. Engaging all fellowship stakeholders in transitions to CBTVT will be important and should include fellows, program directors, CCC members, clinical leadership, and members from accrediting and credentialing bodies.
CONCLUSION
As fellowship programs review and revise curricula and assessment systems around the updated PHM core competencies, they should also consider what changes are necessary to implement CBTVT. Time variability is not a novelty but, rather, is a corollary to the outcomes-based approach of CBME. PHM fellowships should strike while the iron is hot and build on current change initiatives prompted by the growth of our specialty to be leaders in CBTVT.
1. Maniscalco J, Gage S, Sofia Teferi M, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
2. Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach. 2010;32(8):638-645. https://doi.org/10.3109/0142159X.2010.501190
3. Lucey CR, Thibault GE, Ten Cate O. Competency-based, tme-variable education in the health professions: crossroads. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S1-S5. https://doi.org/10.1097/ACM.0000000000002080
4. Custers EJFM, Ten Cate O. The history of medical education in Europe and the United States, with respect to time and proficiency. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S49-S54. https://doi.org/10.1097/ACM.0000000000002079
5. Barr DA. Revolution or evolution? Putting the Flexner Report in context. Med Educ. 2011;45(1):17-22. https://doi.org/10.1111/j.1365-2923.2010.03850.x
6. Association of American Medical Colleges. Minutes of the Fifteenth Annual Meeting. April 10, 1905; Chicago, IL.
7. Bevan A. Council on Medical Education of the American Medical Association. JAMA. 1907;48(20):1701-1707.
8. Flexner A. Medical education in the United States and Canada. From the Carnegie Foundation for the Advancement of Teaching, Bulletin Number Four, 1910. Bull World Health Organ. 2002;80(7):594-602.
9. McGaghie WC, Sajid AW, Miller GE, et al. Competency-based curriculum development in medical education: an introduction. Public Health Pap. 1978;(68):11-91.
10. Frank JR, Snell L, Englander R, Holmboe ES, ICBME Collaborators. Implementing competency-based medical education: moving forward. Med Teach. 2017;39(6):568-573. https://doi.org/10.1080/0142159X.2017.1315069
11. Schumacher DJ, West DC, Schwartz A, et al. Longitudinal assessment of resident performance using entrustable professional activities. JAMA Netw Open. 2020;3(1):e1919316. https://doi.org/10.1001/jamanetworkopen.2019.19316
12. Warm EJ, Held J, Hellman M, et al. Entrusting observable practice activities and milestones over the 36 months of an internal medicine residency. Acad Med. 2016;91(10):1398-1405. https://doi.org/10.1097/ACM.0000000000001292
13. Jason H. Effective medical instruction: requirements and possibilities. In: Proceedings of a 1969 International Symposium on Medical Education. Medica; 1970:5-8.
14. Andrews JS, Bale JF Jr, Soep JB, et al. Education in Pediatrics Across the Continuum (EPAC): first steps toward realizing the dream of competency-based education. Acad Med. 2018;93(3):414-420. https://doi.org/10.1097/ACM.0000000000002020
15. Mejicano GC, Bumsted TN. Describing the journey and lessons learned implementing a competency-based, time-variable undergraduate medical education curriculum. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S42-S48. https://doi.org/10.1097/ACM.0000000000002068
16. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
17. Murray KE, Lane JL, Carraccio C, et al. Crossing the gap: using competency-based assessment to determine whether learners are ready for the undergraduate-to-graduate transition. Acad Med. 2019;94(3):338-345. https://doi.org/10.1097/ACM.0000000000002535
18. Schwartz A, Balmer DF, Borman-Shoap E, et al. Shared mental models among clinical competency committees in the context of time-variable, competency-based advancement to residency. Acad Med. 2020;95(11S Association of American Medical Colleges Learn Serve Lead: Proceedings of the 59th Annual Research in Medical Education Presentations):S95-S102. https://doi.org/10.1097/ACM.0000000000003638
19. Kotter JP. Leading change: why transformation efforts fail. Harvard Business Review. May-June 1995. Accessed March 1, 2021. https://hbr.org/1995/05/leading-change-why-transformation-efforts-fail-2
20. Schumacher DJ, Caretta-Weyer H, Busari J, et al. Competency-based time-variable training internationally: ensuring practical next steps. Med Teach. Forthcoming.
In July 2020, the revision of The Pediatric Hospital Medicine Core Competencies was published, bringing to fruition three years of meticulous work.1 The working group produced 66 chapters outlining the knowledge, skills, and attitudes needed for competent pediatric hospitalist practice. The arrival of these competencies is especially prescient given pediatric hospital medicine’s (PHM’s) relatively new standing as an American Board of Medical Specialties certified subspecialty, as the competencies can serve as a guide for improvement of fellowship curricula, assessment systems, and faculty development. The competencies also represent an opportunity for PHM to take a bold step forward in the world of graduate medical education (GME) by realizing a key tenet of competency-based medical education (CBME)—competency-based, time-variable training (CBTVT), in which learners train until competence is achieved rather than for a predetermined duration.2,3 In this perspective, we describe how medical education in the United States adopted a time-based training paradigm (in which time-in-training is a surrogate for competence), how CBME has brought time-variable training to the fore, and how PHM has an opportunity to be on the leading edge of education innovation.
TIME-BASED TRAINING IN THE UNITED STATES
In the 1800s, during the time of the “Wild West,” medical education in the United States matched this moniker. There was little standardization across the multiple training pathways to become a practicing physician, including apprenticeships, lecture series, and university courses.4 Predictably, this led to significant heterogeneity in the quality of medical care that a patient of the day received. This problem became clearer as Americans traveled to Europe and witnessed more structured and rigorous training programs, only to return to the comparatively poor state of medical education back home.5 There was a clear need for curricular standardization.
In 1876, the American Medical College Association (which later became the Association of American Medical Colleges [AAMC]) was founded to meet this need, and in 1905 the Association adopted a set of minimum standards for medical training that included the now-familiar two years of basic sciences and two years of clinical training.6 Two subsequent national surveys in the United States were commissioned to evaluate whether medical schools met this new standard, with both surveys finding that roughly half of existing programs passed muster.7,8 As a result, nearly half of US medical schools had closed by 1920 in a crusade to standardize curricula and produce competent physicians. By the time the American Medical Association established initial standards for internship (an archetype of GME),4 time-based medical training was the dominant paradigm. This historical perspective highlights the rationale for standardization of education processes and curricula, particularly in terms of accountability to the American public. But heralded by the 1978 landmark paper by McGaghie et al,9 the paradigm began to shift in the late twentieth century from a focus on the process of physician training to outcomes.
CBME AND TIME VARIABILITY
In contrast to the process-focused model of the early 1900s, CBME starts by identifying patient and healthcare system needs, defining competencies required to meet those needs, and then designing curricular and assessment processes to help learners achieve those competencies.2 This outcomes-based approach grew as a response to calls for greater accountability to the public due to evidence that some graduates were unprepared for unsupervised practice, raising concerns that strictly time-based training was no longer defensible.10 CBME aims to mitigate these concerns by starting with desired outcomes of training and working backward to ensure those outcomes are met.
While many programs have attempted to implement CBME, most still rely heavily on time-in-training to determine competence. Learners participate in structured curricula and, unless they are extreme outliers, are deemed ready for unsupervised practice after a predetermined duration. This model presumes that competence and time are related in a fixed, predictable manner and that learners gain competence at a uniform rate. However, learners do not, in fact, progress uniformly. A study by Schumacher et al11 involving 23 pediatric residency programs showed significant interlearner variability in rates of entrustment (used as a surrogate for competence), leading the authors to call for time-variable training in GME. Significant interlearner variation in rates of competence attainment have been shown in other specialties as well.12 As more CBME studies on training outcomes emerge, the evidence is mounting that not all learners need the same duration of training to become competent providers. Time-in-training and competence attainment are not related in a fixed manner. As Dr Jason13 wrote in 1969, “By making time a constant, we make achievement a variable.” Variable achievement (competence, outcomes) was the very driver for medical education’s shift to a competency-based approach. If variable competence was not acceptable then, why should it be now? The goal of CBTVT is not shorter training, but rather flexible, individualized training both in terms of content and duration. While this also means some learners may need to extend their training, this should already be part of GME programs that are required to have remediation policies for learners who are not progressing as expected.
AN OPPORTUNITY FOR PHM
Time variability is an oft-cited tenet of CBME,2,3 but one that is being piloted by relatively few programs in the United States, mostly in undergraduate medical education (UME).14-16 The Education in Pediatrics Across the Continuum (EPAC), a consortium consisting of four institutions piloting CBTVT in UME,14 has shown early evidence of feasibility17 and that UME graduates from CBTVT programs enter residency with levels of competence similar to those of graduates of traditional time-based programs.18 We believe that PHM can take a step toward truly realizing CBME by implementing CBTVT in fellowship programs.
There are multiple reasons why this is an opportune time for PHM fellowships to consider CBTVT. First, PHM is a relatively new board-certified subspecialty with a recently revised set of core competencies1 that are likely to catalyze programmatic innovation. A key step in change management is building on previous efforts to generate more change.19 Programs can leverage the momentum from current and impending change initiatives to innovate and implement CBTVT. Second, the revised PHM competencies provide the first crucial step in implementing a CBME program by defining desired training outcomes necessary to deliver high-quality patient care. With PHM competencies now well defined, programs can focus on developing programs of assessment and corresponding faculty development, which can help deliver valid, defensible decisions about fellow competence.
Finally, PHM has a workforce that can support CBTVT. A major barrier to time-variable training in GME is the need for trainees-as-workforce. In many GME programs, residents and fellows provide a relatively inexpensive, renewable workforce. Trainees’ clinical rotations are often scheduled up to 1 year in advance to ensure care teams are fully staffed, particularly in the inpatient setting, creating a system where flexibility in training is impossible without creating gaps in clinical coverage. However, many PHM fellowships do not completely rely on fellows to cover clinical service lines. PHM fellows spend 32 weeks over 2 years in core clinical rotations with faculty supervision, in accordance with the Accreditation Council for Graduate Medical Education program requirements, both for 2- and 3-year programs. Some CBME experts estimate (based on previous and ongoing CBTVT pilots) that training duration is likely to vary by roughly 20% from current time-based practices when CBTVT is initially implemented.20 Thus, only a small number of clinical service weeks are likely to be affected. If a fellow were deemed ready for unsupervised practice before finishing 2 years of fellowship in a CBTVT program, the corresponding faculty supervisor could use the time previously assigned for supervision to pursue other priorities, such as education, scholarship, or quality improvement. Why provide supervision if a clinical competency committee has deemed a fellow ready for unsupervised practice? Some level of observation and formative feedback could continue, but full supervision would be redundant and unnecessary. CBTVT would allow for some fellows to experience the uncertainty that comes with unsupervised decision-making while still in an environment with trusted fellowship mentors and advisors.
STEPS TOWARD CHANGE
PHM fellowship programs likely cannot flip a switch to “turn on” CBTVT immediately, but they can take steps toward making the transition. Validity, or defensibility of decisions, will be crucial for assessment in CBTVT systems. Programs will need to develop robust assessment systems that collect myriad data to answer the question, “When is this learner competent to deliver high-quality care without supervision?” Programs can align assessment instruments, faculty-development initiatives, and clinical competency committee (CCC) processes with the 2020 PHM competencies to provide a defensible answer. Program leaders should then seek validity evidence, either in existing literature or through novel scholarly initiatives, to support these summative decisions. Engaging all fellowship stakeholders in transitions to CBTVT will be important and should include fellows, program directors, CCC members, clinical leadership, and members from accrediting and credentialing bodies.
CONCLUSION
As fellowship programs review and revise curricula and assessment systems around the updated PHM core competencies, they should also consider what changes are necessary to implement CBTVT. Time variability is not a novelty but, rather, is a corollary to the outcomes-based approach of CBME. PHM fellowships should strike while the iron is hot and build on current change initiatives prompted by the growth of our specialty to be leaders in CBTVT.
In July 2020, the revision of The Pediatric Hospital Medicine Core Competencies was published, bringing to fruition three years of meticulous work.1 The working group produced 66 chapters outlining the knowledge, skills, and attitudes needed for competent pediatric hospitalist practice. The arrival of these competencies is especially prescient given pediatric hospital medicine’s (PHM’s) relatively new standing as an American Board of Medical Specialties certified subspecialty, as the competencies can serve as a guide for improvement of fellowship curricula, assessment systems, and faculty development. The competencies also represent an opportunity for PHM to take a bold step forward in the world of graduate medical education (GME) by realizing a key tenet of competency-based medical education (CBME)—competency-based, time-variable training (CBTVT), in which learners train until competence is achieved rather than for a predetermined duration.2,3 In this perspective, we describe how medical education in the United States adopted a time-based training paradigm (in which time-in-training is a surrogate for competence), how CBME has brought time-variable training to the fore, and how PHM has an opportunity to be on the leading edge of education innovation.
TIME-BASED TRAINING IN THE UNITED STATES
In the 1800s, during the time of the “Wild West,” medical education in the United States matched this moniker. There was little standardization across the multiple training pathways to become a practicing physician, including apprenticeships, lecture series, and university courses.4 Predictably, this led to significant heterogeneity in the quality of medical care that a patient of the day received. This problem became clearer as Americans traveled to Europe and witnessed more structured and rigorous training programs, only to return to the comparatively poor state of medical education back home.5 There was a clear need for curricular standardization.
In 1876, the American Medical College Association (which later became the Association of American Medical Colleges [AAMC]) was founded to meet this need, and in 1905 the Association adopted a set of minimum standards for medical training that included the now-familiar two years of basic sciences and two years of clinical training.6 Two subsequent national surveys in the United States were commissioned to evaluate whether medical schools met this new standard, with both surveys finding that roughly half of existing programs passed muster.7,8 As a result, nearly half of US medical schools had closed by 1920 in a crusade to standardize curricula and produce competent physicians. By the time the American Medical Association established initial standards for internship (an archetype of GME),4 time-based medical training was the dominant paradigm. This historical perspective highlights the rationale for standardization of education processes and curricula, particularly in terms of accountability to the American public. But heralded by the 1978 landmark paper by McGaghie et al,9 the paradigm began to shift in the late twentieth century from a focus on the process of physician training to outcomes.
CBME AND TIME VARIABILITY
In contrast to the process-focused model of the early 1900s, CBME starts by identifying patient and healthcare system needs, defining competencies required to meet those needs, and then designing curricular and assessment processes to help learners achieve those competencies.2 This outcomes-based approach grew as a response to calls for greater accountability to the public due to evidence that some graduates were unprepared for unsupervised practice, raising concerns that strictly time-based training was no longer defensible.10 CBME aims to mitigate these concerns by starting with desired outcomes of training and working backward to ensure those outcomes are met.
While many programs have attempted to implement CBME, most still rely heavily on time-in-training to determine competence. Learners participate in structured curricula and, unless they are extreme outliers, are deemed ready for unsupervised practice after a predetermined duration. This model presumes that competence and time are related in a fixed, predictable manner and that learners gain competence at a uniform rate. However, learners do not, in fact, progress uniformly. A study by Schumacher et al11 involving 23 pediatric residency programs showed significant interlearner variability in rates of entrustment (used as a surrogate for competence), leading the authors to call for time-variable training in GME. Significant interlearner variation in rates of competence attainment have been shown in other specialties as well.12 As more CBME studies on training outcomes emerge, the evidence is mounting that not all learners need the same duration of training to become competent providers. Time-in-training and competence attainment are not related in a fixed manner. As Dr Jason13 wrote in 1969, “By making time a constant, we make achievement a variable.” Variable achievement (competence, outcomes) was the very driver for medical education’s shift to a competency-based approach. If variable competence was not acceptable then, why should it be now? The goal of CBTVT is not shorter training, but rather flexible, individualized training both in terms of content and duration. While this also means some learners may need to extend their training, this should already be part of GME programs that are required to have remediation policies for learners who are not progressing as expected.
AN OPPORTUNITY FOR PHM
Time variability is an oft-cited tenet of CBME,2,3 but one that is being piloted by relatively few programs in the United States, mostly in undergraduate medical education (UME).14-16 The Education in Pediatrics Across the Continuum (EPAC), a consortium consisting of four institutions piloting CBTVT in UME,14 has shown early evidence of feasibility17 and that UME graduates from CBTVT programs enter residency with levels of competence similar to those of graduates of traditional time-based programs.18 We believe that PHM can take a step toward truly realizing CBME by implementing CBTVT in fellowship programs.
There are multiple reasons why this is an opportune time for PHM fellowships to consider CBTVT. First, PHM is a relatively new board-certified subspecialty with a recently revised set of core competencies1 that are likely to catalyze programmatic innovation. A key step in change management is building on previous efforts to generate more change.19 Programs can leverage the momentum from current and impending change initiatives to innovate and implement CBTVT. Second, the revised PHM competencies provide the first crucial step in implementing a CBME program by defining desired training outcomes necessary to deliver high-quality patient care. With PHM competencies now well defined, programs can focus on developing programs of assessment and corresponding faculty development, which can help deliver valid, defensible decisions about fellow competence.
Finally, PHM has a workforce that can support CBTVT. A major barrier to time-variable training in GME is the need for trainees-as-workforce. In many GME programs, residents and fellows provide a relatively inexpensive, renewable workforce. Trainees’ clinical rotations are often scheduled up to 1 year in advance to ensure care teams are fully staffed, particularly in the inpatient setting, creating a system where flexibility in training is impossible without creating gaps in clinical coverage. However, many PHM fellowships do not completely rely on fellows to cover clinical service lines. PHM fellows spend 32 weeks over 2 years in core clinical rotations with faculty supervision, in accordance with the Accreditation Council for Graduate Medical Education program requirements, both for 2- and 3-year programs. Some CBME experts estimate (based on previous and ongoing CBTVT pilots) that training duration is likely to vary by roughly 20% from current time-based practices when CBTVT is initially implemented.20 Thus, only a small number of clinical service weeks are likely to be affected. If a fellow were deemed ready for unsupervised practice before finishing 2 years of fellowship in a CBTVT program, the corresponding faculty supervisor could use the time previously assigned for supervision to pursue other priorities, such as education, scholarship, or quality improvement. Why provide supervision if a clinical competency committee has deemed a fellow ready for unsupervised practice? Some level of observation and formative feedback could continue, but full supervision would be redundant and unnecessary. CBTVT would allow for some fellows to experience the uncertainty that comes with unsupervised decision-making while still in an environment with trusted fellowship mentors and advisors.
STEPS TOWARD CHANGE
PHM fellowship programs likely cannot flip a switch to “turn on” CBTVT immediately, but they can take steps toward making the transition. Validity, or defensibility of decisions, will be crucial for assessment in CBTVT systems. Programs will need to develop robust assessment systems that collect myriad data to answer the question, “When is this learner competent to deliver high-quality care without supervision?” Programs can align assessment instruments, faculty-development initiatives, and clinical competency committee (CCC) processes with the 2020 PHM competencies to provide a defensible answer. Program leaders should then seek validity evidence, either in existing literature or through novel scholarly initiatives, to support these summative decisions. Engaging all fellowship stakeholders in transitions to CBTVT will be important and should include fellows, program directors, CCC members, clinical leadership, and members from accrediting and credentialing bodies.
CONCLUSION
As fellowship programs review and revise curricula and assessment systems around the updated PHM core competencies, they should also consider what changes are necessary to implement CBTVT. Time variability is not a novelty but, rather, is a corollary to the outcomes-based approach of CBME. PHM fellowships should strike while the iron is hot and build on current change initiatives prompted by the growth of our specialty to be leaders in CBTVT.
1. Maniscalco J, Gage S, Sofia Teferi M, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
2. Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach. 2010;32(8):638-645. https://doi.org/10.3109/0142159X.2010.501190
3. Lucey CR, Thibault GE, Ten Cate O. Competency-based, tme-variable education in the health professions: crossroads. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S1-S5. https://doi.org/10.1097/ACM.0000000000002080
4. Custers EJFM, Ten Cate O. The history of medical education in Europe and the United States, with respect to time and proficiency. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S49-S54. https://doi.org/10.1097/ACM.0000000000002079
5. Barr DA. Revolution or evolution? Putting the Flexner Report in context. Med Educ. 2011;45(1):17-22. https://doi.org/10.1111/j.1365-2923.2010.03850.x
6. Association of American Medical Colleges. Minutes of the Fifteenth Annual Meeting. April 10, 1905; Chicago, IL.
7. Bevan A. Council on Medical Education of the American Medical Association. JAMA. 1907;48(20):1701-1707.
8. Flexner A. Medical education in the United States and Canada. From the Carnegie Foundation for the Advancement of Teaching, Bulletin Number Four, 1910. Bull World Health Organ. 2002;80(7):594-602.
9. McGaghie WC, Sajid AW, Miller GE, et al. Competency-based curriculum development in medical education: an introduction. Public Health Pap. 1978;(68):11-91.
10. Frank JR, Snell L, Englander R, Holmboe ES, ICBME Collaborators. Implementing competency-based medical education: moving forward. Med Teach. 2017;39(6):568-573. https://doi.org/10.1080/0142159X.2017.1315069
11. Schumacher DJ, West DC, Schwartz A, et al. Longitudinal assessment of resident performance using entrustable professional activities. JAMA Netw Open. 2020;3(1):e1919316. https://doi.org/10.1001/jamanetworkopen.2019.19316
12. Warm EJ, Held J, Hellman M, et al. Entrusting observable practice activities and milestones over the 36 months of an internal medicine residency. Acad Med. 2016;91(10):1398-1405. https://doi.org/10.1097/ACM.0000000000001292
13. Jason H. Effective medical instruction: requirements and possibilities. In: Proceedings of a 1969 International Symposium on Medical Education. Medica; 1970:5-8.
14. Andrews JS, Bale JF Jr, Soep JB, et al. Education in Pediatrics Across the Continuum (EPAC): first steps toward realizing the dream of competency-based education. Acad Med. 2018;93(3):414-420. https://doi.org/10.1097/ACM.0000000000002020
15. Mejicano GC, Bumsted TN. Describing the journey and lessons learned implementing a competency-based, time-variable undergraduate medical education curriculum. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S42-S48. https://doi.org/10.1097/ACM.0000000000002068
16. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
17. Murray KE, Lane JL, Carraccio C, et al. Crossing the gap: using competency-based assessment to determine whether learners are ready for the undergraduate-to-graduate transition. Acad Med. 2019;94(3):338-345. https://doi.org/10.1097/ACM.0000000000002535
18. Schwartz A, Balmer DF, Borman-Shoap E, et al. Shared mental models among clinical competency committees in the context of time-variable, competency-based advancement to residency. Acad Med. 2020;95(11S Association of American Medical Colleges Learn Serve Lead: Proceedings of the 59th Annual Research in Medical Education Presentations):S95-S102. https://doi.org/10.1097/ACM.0000000000003638
19. Kotter JP. Leading change: why transformation efforts fail. Harvard Business Review. May-June 1995. Accessed March 1, 2021. https://hbr.org/1995/05/leading-change-why-transformation-efforts-fail-2
20. Schumacher DJ, Caretta-Weyer H, Busari J, et al. Competency-based time-variable training internationally: ensuring practical next steps. Med Teach. Forthcoming.
1. Maniscalco J, Gage S, Sofia Teferi M, Fisher ES. The Pediatric Hospital Medicine Core Competencies: 2020 Revision. J Hosp Med. 2020;15(7):389-394. https://doi.org/10.12788/jhm.3391
2. Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach. 2010;32(8):638-645. https://doi.org/10.3109/0142159X.2010.501190
3. Lucey CR, Thibault GE, Ten Cate O. Competency-based, tme-variable education in the health professions: crossroads. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S1-S5. https://doi.org/10.1097/ACM.0000000000002080
4. Custers EJFM, Ten Cate O. The history of medical education in Europe and the United States, with respect to time and proficiency. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S49-S54. https://doi.org/10.1097/ACM.0000000000002079
5. Barr DA. Revolution or evolution? Putting the Flexner Report in context. Med Educ. 2011;45(1):17-22. https://doi.org/10.1111/j.1365-2923.2010.03850.x
6. Association of American Medical Colleges. Minutes of the Fifteenth Annual Meeting. April 10, 1905; Chicago, IL.
7. Bevan A. Council on Medical Education of the American Medical Association. JAMA. 1907;48(20):1701-1707.
8. Flexner A. Medical education in the United States and Canada. From the Carnegie Foundation for the Advancement of Teaching, Bulletin Number Four, 1910. Bull World Health Organ. 2002;80(7):594-602.
9. McGaghie WC, Sajid AW, Miller GE, et al. Competency-based curriculum development in medical education: an introduction. Public Health Pap. 1978;(68):11-91.
10. Frank JR, Snell L, Englander R, Holmboe ES, ICBME Collaborators. Implementing competency-based medical education: moving forward. Med Teach. 2017;39(6):568-573. https://doi.org/10.1080/0142159X.2017.1315069
11. Schumacher DJ, West DC, Schwartz A, et al. Longitudinal assessment of resident performance using entrustable professional activities. JAMA Netw Open. 2020;3(1):e1919316. https://doi.org/10.1001/jamanetworkopen.2019.19316
12. Warm EJ, Held J, Hellman M, et al. Entrusting observable practice activities and milestones over the 36 months of an internal medicine residency. Acad Med. 2016;91(10):1398-1405. https://doi.org/10.1097/ACM.0000000000001292
13. Jason H. Effective medical instruction: requirements and possibilities. In: Proceedings of a 1969 International Symposium on Medical Education. Medica; 1970:5-8.
14. Andrews JS, Bale JF Jr, Soep JB, et al. Education in Pediatrics Across the Continuum (EPAC): first steps toward realizing the dream of competency-based education. Acad Med. 2018;93(3):414-420. https://doi.org/10.1097/ACM.0000000000002020
15. Mejicano GC, Bumsted TN. Describing the journey and lessons learned implementing a competency-based, time-variable undergraduate medical education curriculum. Acad Med. 2018;93(3S Competency-Based, Time-Variable Education in the Health Professions):S42-S48. https://doi.org/10.1097/ACM.0000000000002068
16. Goldhamer MEJ, Pusic MV, Co JPT, Weinstein DF. Can COVID catalyze an educational transformation? Competency-based advancement in a crisis. N Engl J Med. 2020;383(11):1003-1005. https://doi.org/10.1056/NEJMp2018570
17. Murray KE, Lane JL, Carraccio C, et al. Crossing the gap: using competency-based assessment to determine whether learners are ready for the undergraduate-to-graduate transition. Acad Med. 2019;94(3):338-345. https://doi.org/10.1097/ACM.0000000000002535
18. Schwartz A, Balmer DF, Borman-Shoap E, et al. Shared mental models among clinical competency committees in the context of time-variable, competency-based advancement to residency. Acad Med. 2020;95(11S Association of American Medical Colleges Learn Serve Lead: Proceedings of the 59th Annual Research in Medical Education Presentations):S95-S102. https://doi.org/10.1097/ACM.0000000000003638
19. Kotter JP. Leading change: why transformation efforts fail. Harvard Business Review. May-June 1995. Accessed March 1, 2021. https://hbr.org/1995/05/leading-change-why-transformation-efforts-fail-2
20. Schumacher DJ, Caretta-Weyer H, Busari J, et al. Competency-based time-variable training internationally: ensuring practical next steps. Med Teach. Forthcoming.
© 2021 Society of Hospital Medicine
Development and Evolution of Hospital Medicine in Korea
The healthcare system in South Korea (Korea) is evolving. Korea began developing a national health insurance system for the entire population in 1989, and implementation took 12 years. The healthcare insurance premium was set in 1989 when the Korean gross domestic product (GDP) per capita was less than $5,000 USD. Since then, the incremental rise in healthcare insurance premiums, approximately 6% of the typical Korean income, has been relatively small considering the economic growth of Korea.1-3 The success and rapid adoption of national health insurance in Korea revealed unanticipated problems in three specific areas: low individual contributions relative to costs of care, low levels of reimbursement to providers, and incomplete coverage of medical services, which then require out-of-pocket payments (typically 20% of the inpatient care fee).4 Additionally, while little attention has been paid to quality and safety in the past, the nation has come to recognize the importance of these considerations,5 particularly with regard to patient expectations for and consumption of medical care and the consequent demand for better services from the medical community and government.6
Healthcare constitutes about 7.5% of Korea’s GDP. In contrast, healthcare spending accounts for about 17.7% of US GDP and about 8.8% of the GDP of member nations of the Organisation for Economic Co-operation and Development (OECD) in aggregate. Additionally, Korea has a longer length of hospital stay (16.5 days vs 7.3 days for OECD countries) and fewer practicing physicians (1.1 per 1,000 persons vs 1.9 per 1,000 for OECD countries). Therefore, the average cumulative annual patient hospital days per physician is 2,394 days in Korea, three times higher than that in the OECD countries.7 Furthermore, the number of physicians providing hospital care in Korea has remained relatively low.8
Despite recent growth in the total number of practicing physicians, the number of hospital-based physicians remains insufficient to cover admitted patients. The pressure for hospital-based physicians to serve large numbers of patients to generate sufficient revenue has deterred growth of more physicians focused on inpatient care.6 In order to operate the hospital, doctors must see as many patients as possible because physicians are reimbursed primarily by an inpatient care fee rather than by type or extent of care provided. Therefore, this low fee schedule makes it difficult to provide good quality inpatient care while simultaneously trying to increase accessibility. The costs of testing and treatment are reimbursed through a separate fee schedule.
THE NEED FOR A HOSPITALIST SYSTEM IN KOREA
The Korean government enacted two new policies regarding medical school graduates and residents that led to implementation of a hospitalist system. The first policy created a quota of medical residents to match the total number of medical school graduates.9 Before the new regulation, the number of medical resident positions exceeded the number of medical school graduates by 20%, which led to a shortage of resident applicants in “unpopular” medical specialties or departments. These specialties (eg, general surgery, obstetrics and gynecology, urology) have a lower fee schedule, which leads to lower wages while having very high workloads. The decrease in the number of available medical resident positions results in greater workloads for physicians providing inpatient care. These changes, including the shortage of a resident workforce, led to a burgeoning hospital-based attending physician workforce to care for hospitalized patients.
The other policy, created in December 2015 and known as the Act on the Improvement of Training Conditions and Status of Medical Residents, was enacted to improve working conditions for residents by limiting their working hours to 80 or fewer per week.10 These work-hour restrictions made it nearly impossible to provide 24-hour inpatient care depending solely on residents.10-12 This policy increased the need for hospitalists in Korea, similar to that of the US Accreditation Council for Graduate Medical Education in 2003, which limited resident work hours. Because of these changes, the hospitalist model was introduced to manage the growing volume of inpatients at teaching hospitals.13
The institutional implementation of the Korean hospitalist system was catalyzed by the need to solve current problems such as patient safety, healthcare quality, and residents’ well-being.8,14 Therefore, the hospitalist system was designed and implemented to meet the needs of patients, medical professionals, and hospitals.6,8,14 Furthermore, under the universal health insurance system in Korea, institutional implementation also meant creating a new set of fee schedules for hospitalists. The Korean hospitalist system reflects the nuances and challenges faced by the Korean healthcare system.5
DESIGN, IMPLEMENTATION, AND EVALUATION
A council was formed to implement a hospitalist model suitable for Korea. The council was composed of five groups: the Korean Association of Internal Medicine, Korean Surgical Society, Korean Medical Association, Korean Hospital Association, and Korean Academy of Medical Sciences. The Korean Association of Internal Medicine and the Korean Surgical Society were involved in creating a new medical discipline because their members provided a disproportionate amount of inpatient care and were most often responsible for hospital patient safety and quality care. Along with the support of the council, the Ministry of Health and Welfare began to realize the high demand on inpatient care and requested an official proposal to broaden the hospitalist model, with two conditions. First, the government requested a unified proposal from the medical community that reflected the collective voices of individual stakeholders, with an expectation that the new system would focus on patient safety and healthcare quality across all specialties. Second, the proposal had to be detailed and include a specific fee schedule for hospitalist services.
Before implementing a national hospitalist system, we conducted a pilot study supported by funding from the council. This first study, the Korean Hospitalist System Operation and Evaluation Research (September 2015 to August 2016)14, was designed to (a) determine hospitalist needs (eg, rotation schedule, salary, working conditions) for this new profession, (b) determine the necessary number of hospitalists and appropriate fee schedules to cover salary and benefits, and (c) provide Korean hospitalist models with operational methods to facilitate implementation at individual institutions. The council and research team selected four hospitals for the privately funded pilot study (Table). These hospitals already had an inpatient care system managed by specialists prior to the pilot study, so major changes in patient care and hospital operations were not required.
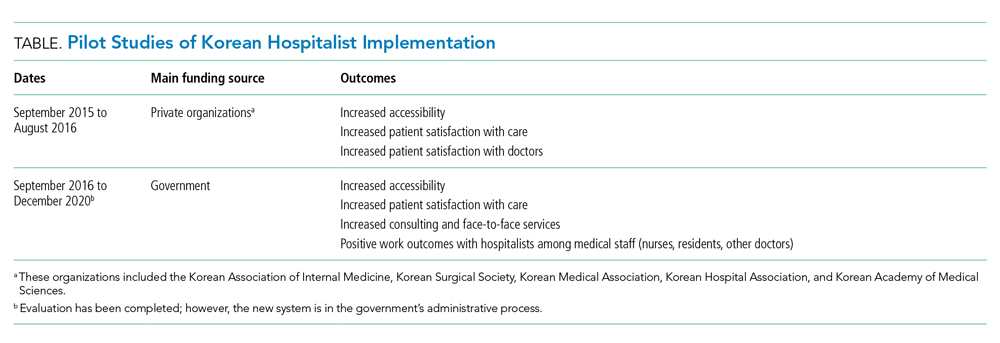
The pilot study demonstrated improvements in patient satisfaction, medical service quality, and patient safety.8,14,15 As a result, fees billed from hospitalist services were now covered by national health insurance; previously, they were not covered by any inpatient care fee or bundled service fee. The next study (phase 2) was then conducted to evaluate the national implementation of the hospitalist system and its outcomes. The first phase defined criteria for monitoring and evaluating the institutionalized hospitalist system in Korea. The second phase, initiated in September 2016, evaluated how the implemented system could be expanded nationwide. The government was actively involved in the study; 31 of 344 (9%) general hospitals were selected and able to apply the hospitalist fee schedule for care of patients who were admitted to the hospitalist ward.
To investigate the effect of the hospitalist system, we measured the satisfaction of patients and medical providers (eg, specialists, residents, and nurses). The outcomes were number of doctors’ calls to hospitalists and to other specialties, duration of time required to address medical problems, number of contacts with the hospitalist (residents or other physicians in the control group), and time spent with hospitalized patients. In addition, we analyzed claims costs and the operating costs for implementing the system within each hospital (Table).
The new set of fee schedules was created specifically for hospitalist care services so hospitals could now claim separate inpatient care fees and hospitalist fees. The standard hospitalist fee schedule is applied only to patients who are on hospitalist-led wards. The fee schedule has two criteria: The hospitalist ward must have 50 beds which are assigned to hospitalists only, and each hospitalist ward has a team of five hospitalists. A maximum of 25 patients could be assigned to an individual hospitalist. However, fees can be adjusted based on individual hospital operation and management after government approval.
Under the national insurance system, the fee schedule is essential to obtain reimbursement for provided services. As a new medical profession, it is important to have the institutional implementation of the hospitalist system. In contrast to those in the United States, inpatients in Korea pay inpatient care fees only, which are charged per day. Therefore, reducing the length of stay without increasing patient volume does not financially benefit the hospital.
DEFINITION OF A KOREAN HOSPITALIST AND SCOPE OF PRACTICE
The definition of a Korean hospitalist was carefully developed, and a reimbursement system for hospitalists was created within the Korean healthcare system. All hospitalists are medical specialists who have completed advanced education and clinical training in their specialty area (internal medicine or surgery). Creating a definition of a hospitalist and a standard fee schedule was necessary for national incorporation of this new approach. The model must also be accepted by related parties, including healthcare professionals such as hospital executives, nonhospitalist doctors, and nurses. Therefore, the system has a minimum of two requirements to operate at the hospital level, and the remaining factors can be modified by individual hospitals to protect the new discipline within the healthcare system. First, hospitalist services are provided only to hospitalist patients. To establish the new system, limiting the patient range to a specific group was necessary to prevent abuse of human-based resources within the hospital. Second, hospitalists must be stationed near the hospitalist ward to enhance accessibility, patient safety, and healthcare quality. In other words, a hospitalist provides services to hospitalized patients who paid the hospitalist fee, and the hospitalist does not provide care beyond the hospitalist ward (eg, they cannot provide outpatient consultation or provide care on other wards).
INPATIENT CARE REIMBURSEMENT BEFORE AND AFTER IMPLEMENTATION
Korean national health insurance is the universal health insurance under a single insurer (ie, the government). Therefore, all Korean citizens have national health insurance. Individuals can choose to have additional health insurance (private insurance) if they wish to pay an out-of-pocket fee with their additional private insurance. In Korea, the inpatient care fee includes all fees to provide care for the patient during hospital stays, such as the physician fee, facility fee, and consultation fee. The inpatient care fee schedule is charged per day including all the inpatient care composition. The claim and reimbursement system is different in Korea in that the hospital as a whole submits the claim for reimbursement. There is no separate claim from physicians, lab technicians, or facilities. Doctors provide care to patients and are then paid for the services in terms of salaries. Since the original inpatient care fee schedule was low and insufficient, it was difficult to provide high-quality care. Also, resources for spending on inpatient care management was limited. So a new system to improve inpatient care was needed. Implementing the hospitalist system in Korea led to the creation of a new fee schedule specifically for hospitalists so that hospitals could claim hospitalist fees on top of inpatient care fees when the patient was cared for by the hospitalist. The additional fees in the hospitalist fee schedule allow safe and high-quality care to be provided for patients during hospital stays.
KOREAN HOSPITAL MEDICINE TODAY
The Korean hospitalist system has been implemented for 3 years with the government’s active involvement, with approximately 250 specialists working as hospitalists as of August 2020. Importantly, Korean hospitalists are not limited to internal medicine specialists; in fact, several different specialties practice as hospitalists, including surgeons and other medical specialists, who account for 20% and 30% of the hospitalist workforce, respectively. Since the system’s implementation, all inpatient care and management is now transferred to specialists from residents in hospitalist wards. This change has increased patient safety and care quality. At the beginning of the pilot study, the concept of a “hospitalist” was new in Korea and the public did not know who a hospitalist was nor what a hospitalist did. However, after 3 years, patients now seek hospitalist care.
CONCLUSION
National implementation of the hospitalist model represents a key paradigm shift in the Korean healthcare system. The Korean hospitalist system is the result of the hospitals’, doctors’, and patients’ desire for higher-quality care. We expect to see growth of hospitalists in Korea and provision of better, safer, and more efficient inpatient care across specialties, payers, and government. Since we are at the early stage of the system, further efforts to support implementation are required. We hope our implementation process of a new medical system could serve as a model for other countries who are seeking to adopt hospitalist systems within their current healthcare paradigms.
Acknowledgments
The authors thank the medical professionals, government officers, and other professionals who put great effort into the implementation of the Korean Hospitalist System.
1. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24(1):63-71. https://doi.org/10.1093/heapol/czn037
2. Song YJ. The South Korean health care system. JMAJ. 2009;52(3):206-209.
3. Park YH, Park E-C. Healthcare policy. In: Preventive Medicine and Public Health. Vol 3.Gyechuk; 2017:821-833.
4. Park E-C, Lee TJ, Jun BY, Jung SH, Jeong HS. Health security. In: Preventive Medicine and Public Health. Vol 3. Gyechuk; 2017:888-897.
5. Jang S-I, Jang S-y, Park E-C. Trends of US hospitalist and suggestions for introduction of Korean hospitalist. Korean J Med. 2015;89(1):1-5. http://doi.org/10.3904/kjm.2015.89.1.1
6. Jang S-I. Korean hospitalist system implementation and development strategies based on pilot studies. J Korean Med Assoc. 2019;62(11):558-563. http://doi.org/10.5124/jkma.2019.62.11.558
7. OECD. Health at a Glance 2017: OECD indicators. OECD iLibrary. 2017. Accessed September 27, 2019. https://doi.org/10.1787/health_glance-2017-en
8. Jang S-I, Park E-C, Nam JM, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 2). Institute of Health Services Research, Yonsei University; 2018.
9. Koh DY. Political strategies to enhance medical residents. Health Policy. Vol 37. Seoul National University Hospital; 2014.
10. Act on the Improvement of Training Conditions and Status of Medical Residents. Vol No. 16260: Ministry of Health and Welfare; 2015.
11. Eom JS. Operating the hospitalist system. J Korean Med Assoc. 2016;59(5):342-344. http://doi.org/10.5124/jkma.2016.59.5.342
12. Kim S-S. Working conditions of interns/residents and patient safety: Painful training might not be authentic. J Korean Med Assoc. 2016;59(2):82-84. http://dx.doi.org/10.5124/jkma.2016.59.2.82
13. Oshimura J, Sperring J, Bauer BD, Rauch DA. Inpatient staffing within pediatric residency programs: work hour restrictions and the evolving role of the pediatric hospitalist. J Hosp Med. 2012;7(4):299-303. https://doi.org/10.1002/jhm.952
14. Park E-C, Lee SG, Kim T-H, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 1). Institute of Health Services Research, Yonsei University; 2016.
15. Jang S-I, Jung E-J, Park SK, Chae W, Kim Y-K. A study on the feasibility of the Korean hospitalist system and cost estimation. Ministry of Health and Welfare, Institute of Health Service Research Yonsei University; 2019.
The healthcare system in South Korea (Korea) is evolving. Korea began developing a national health insurance system for the entire population in 1989, and implementation took 12 years. The healthcare insurance premium was set in 1989 when the Korean gross domestic product (GDP) per capita was less than $5,000 USD. Since then, the incremental rise in healthcare insurance premiums, approximately 6% of the typical Korean income, has been relatively small considering the economic growth of Korea.1-3 The success and rapid adoption of national health insurance in Korea revealed unanticipated problems in three specific areas: low individual contributions relative to costs of care, low levels of reimbursement to providers, and incomplete coverage of medical services, which then require out-of-pocket payments (typically 20% of the inpatient care fee).4 Additionally, while little attention has been paid to quality and safety in the past, the nation has come to recognize the importance of these considerations,5 particularly with regard to patient expectations for and consumption of medical care and the consequent demand for better services from the medical community and government.6
Healthcare constitutes about 7.5% of Korea’s GDP. In contrast, healthcare spending accounts for about 17.7% of US GDP and about 8.8% of the GDP of member nations of the Organisation for Economic Co-operation and Development (OECD) in aggregate. Additionally, Korea has a longer length of hospital stay (16.5 days vs 7.3 days for OECD countries) and fewer practicing physicians (1.1 per 1,000 persons vs 1.9 per 1,000 for OECD countries). Therefore, the average cumulative annual patient hospital days per physician is 2,394 days in Korea, three times higher than that in the OECD countries.7 Furthermore, the number of physicians providing hospital care in Korea has remained relatively low.8
Despite recent growth in the total number of practicing physicians, the number of hospital-based physicians remains insufficient to cover admitted patients. The pressure for hospital-based physicians to serve large numbers of patients to generate sufficient revenue has deterred growth of more physicians focused on inpatient care.6 In order to operate the hospital, doctors must see as many patients as possible because physicians are reimbursed primarily by an inpatient care fee rather than by type or extent of care provided. Therefore, this low fee schedule makes it difficult to provide good quality inpatient care while simultaneously trying to increase accessibility. The costs of testing and treatment are reimbursed through a separate fee schedule.
THE NEED FOR A HOSPITALIST SYSTEM IN KOREA
The Korean government enacted two new policies regarding medical school graduates and residents that led to implementation of a hospitalist system. The first policy created a quota of medical residents to match the total number of medical school graduates.9 Before the new regulation, the number of medical resident positions exceeded the number of medical school graduates by 20%, which led to a shortage of resident applicants in “unpopular” medical specialties or departments. These specialties (eg, general surgery, obstetrics and gynecology, urology) have a lower fee schedule, which leads to lower wages while having very high workloads. The decrease in the number of available medical resident positions results in greater workloads for physicians providing inpatient care. These changes, including the shortage of a resident workforce, led to a burgeoning hospital-based attending physician workforce to care for hospitalized patients.
The other policy, created in December 2015 and known as the Act on the Improvement of Training Conditions and Status of Medical Residents, was enacted to improve working conditions for residents by limiting their working hours to 80 or fewer per week.10 These work-hour restrictions made it nearly impossible to provide 24-hour inpatient care depending solely on residents.10-12 This policy increased the need for hospitalists in Korea, similar to that of the US Accreditation Council for Graduate Medical Education in 2003, which limited resident work hours. Because of these changes, the hospitalist model was introduced to manage the growing volume of inpatients at teaching hospitals.13
The institutional implementation of the Korean hospitalist system was catalyzed by the need to solve current problems such as patient safety, healthcare quality, and residents’ well-being.8,14 Therefore, the hospitalist system was designed and implemented to meet the needs of patients, medical professionals, and hospitals.6,8,14 Furthermore, under the universal health insurance system in Korea, institutional implementation also meant creating a new set of fee schedules for hospitalists. The Korean hospitalist system reflects the nuances and challenges faced by the Korean healthcare system.5
DESIGN, IMPLEMENTATION, AND EVALUATION
A council was formed to implement a hospitalist model suitable for Korea. The council was composed of five groups: the Korean Association of Internal Medicine, Korean Surgical Society, Korean Medical Association, Korean Hospital Association, and Korean Academy of Medical Sciences. The Korean Association of Internal Medicine and the Korean Surgical Society were involved in creating a new medical discipline because their members provided a disproportionate amount of inpatient care and were most often responsible for hospital patient safety and quality care. Along with the support of the council, the Ministry of Health and Welfare began to realize the high demand on inpatient care and requested an official proposal to broaden the hospitalist model, with two conditions. First, the government requested a unified proposal from the medical community that reflected the collective voices of individual stakeholders, with an expectation that the new system would focus on patient safety and healthcare quality across all specialties. Second, the proposal had to be detailed and include a specific fee schedule for hospitalist services.
Before implementing a national hospitalist system, we conducted a pilot study supported by funding from the council. This first study, the Korean Hospitalist System Operation and Evaluation Research (September 2015 to August 2016)14, was designed to (a) determine hospitalist needs (eg, rotation schedule, salary, working conditions) for this new profession, (b) determine the necessary number of hospitalists and appropriate fee schedules to cover salary and benefits, and (c) provide Korean hospitalist models with operational methods to facilitate implementation at individual institutions. The council and research team selected four hospitals for the privately funded pilot study (Table). These hospitals already had an inpatient care system managed by specialists prior to the pilot study, so major changes in patient care and hospital operations were not required.

The pilot study demonstrated improvements in patient satisfaction, medical service quality, and patient safety.8,14,15 As a result, fees billed from hospitalist services were now covered by national health insurance; previously, they were not covered by any inpatient care fee or bundled service fee. The next study (phase 2) was then conducted to evaluate the national implementation of the hospitalist system and its outcomes. The first phase defined criteria for monitoring and evaluating the institutionalized hospitalist system in Korea. The second phase, initiated in September 2016, evaluated how the implemented system could be expanded nationwide. The government was actively involved in the study; 31 of 344 (9%) general hospitals were selected and able to apply the hospitalist fee schedule for care of patients who were admitted to the hospitalist ward.
To investigate the effect of the hospitalist system, we measured the satisfaction of patients and medical providers (eg, specialists, residents, and nurses). The outcomes were number of doctors’ calls to hospitalists and to other specialties, duration of time required to address medical problems, number of contacts with the hospitalist (residents or other physicians in the control group), and time spent with hospitalized patients. In addition, we analyzed claims costs and the operating costs for implementing the system within each hospital (Table).
The new set of fee schedules was created specifically for hospitalist care services so hospitals could now claim separate inpatient care fees and hospitalist fees. The standard hospitalist fee schedule is applied only to patients who are on hospitalist-led wards. The fee schedule has two criteria: The hospitalist ward must have 50 beds which are assigned to hospitalists only, and each hospitalist ward has a team of five hospitalists. A maximum of 25 patients could be assigned to an individual hospitalist. However, fees can be adjusted based on individual hospital operation and management after government approval.
Under the national insurance system, the fee schedule is essential to obtain reimbursement for provided services. As a new medical profession, it is important to have the institutional implementation of the hospitalist system. In contrast to those in the United States, inpatients in Korea pay inpatient care fees only, which are charged per day. Therefore, reducing the length of stay without increasing patient volume does not financially benefit the hospital.
DEFINITION OF A KOREAN HOSPITALIST AND SCOPE OF PRACTICE
The definition of a Korean hospitalist was carefully developed, and a reimbursement system for hospitalists was created within the Korean healthcare system. All hospitalists are medical specialists who have completed advanced education and clinical training in their specialty area (internal medicine or surgery). Creating a definition of a hospitalist and a standard fee schedule was necessary for national incorporation of this new approach. The model must also be accepted by related parties, including healthcare professionals such as hospital executives, nonhospitalist doctors, and nurses. Therefore, the system has a minimum of two requirements to operate at the hospital level, and the remaining factors can be modified by individual hospitals to protect the new discipline within the healthcare system. First, hospitalist services are provided only to hospitalist patients. To establish the new system, limiting the patient range to a specific group was necessary to prevent abuse of human-based resources within the hospital. Second, hospitalists must be stationed near the hospitalist ward to enhance accessibility, patient safety, and healthcare quality. In other words, a hospitalist provides services to hospitalized patients who paid the hospitalist fee, and the hospitalist does not provide care beyond the hospitalist ward (eg, they cannot provide outpatient consultation or provide care on other wards).
INPATIENT CARE REIMBURSEMENT BEFORE AND AFTER IMPLEMENTATION
Korean national health insurance is the universal health insurance under a single insurer (ie, the government). Therefore, all Korean citizens have national health insurance. Individuals can choose to have additional health insurance (private insurance) if they wish to pay an out-of-pocket fee with their additional private insurance. In Korea, the inpatient care fee includes all fees to provide care for the patient during hospital stays, such as the physician fee, facility fee, and consultation fee. The inpatient care fee schedule is charged per day including all the inpatient care composition. The claim and reimbursement system is different in Korea in that the hospital as a whole submits the claim for reimbursement. There is no separate claim from physicians, lab technicians, or facilities. Doctors provide care to patients and are then paid for the services in terms of salaries. Since the original inpatient care fee schedule was low and insufficient, it was difficult to provide high-quality care. Also, resources for spending on inpatient care management was limited. So a new system to improve inpatient care was needed. Implementing the hospitalist system in Korea led to the creation of a new fee schedule specifically for hospitalists so that hospitals could claim hospitalist fees on top of inpatient care fees when the patient was cared for by the hospitalist. The additional fees in the hospitalist fee schedule allow safe and high-quality care to be provided for patients during hospital stays.
KOREAN HOSPITAL MEDICINE TODAY
The Korean hospitalist system has been implemented for 3 years with the government’s active involvement, with approximately 250 specialists working as hospitalists as of August 2020. Importantly, Korean hospitalists are not limited to internal medicine specialists; in fact, several different specialties practice as hospitalists, including surgeons and other medical specialists, who account for 20% and 30% of the hospitalist workforce, respectively. Since the system’s implementation, all inpatient care and management is now transferred to specialists from residents in hospitalist wards. This change has increased patient safety and care quality. At the beginning of the pilot study, the concept of a “hospitalist” was new in Korea and the public did not know who a hospitalist was nor what a hospitalist did. However, after 3 years, patients now seek hospitalist care.
CONCLUSION
National implementation of the hospitalist model represents a key paradigm shift in the Korean healthcare system. The Korean hospitalist system is the result of the hospitals’, doctors’, and patients’ desire for higher-quality care. We expect to see growth of hospitalists in Korea and provision of better, safer, and more efficient inpatient care across specialties, payers, and government. Since we are at the early stage of the system, further efforts to support implementation are required. We hope our implementation process of a new medical system could serve as a model for other countries who are seeking to adopt hospitalist systems within their current healthcare paradigms.
Acknowledgments
The authors thank the medical professionals, government officers, and other professionals who put great effort into the implementation of the Korean Hospitalist System.
The healthcare system in South Korea (Korea) is evolving. Korea began developing a national health insurance system for the entire population in 1989, and implementation took 12 years. The healthcare insurance premium was set in 1989 when the Korean gross domestic product (GDP) per capita was less than $5,000 USD. Since then, the incremental rise in healthcare insurance premiums, approximately 6% of the typical Korean income, has been relatively small considering the economic growth of Korea.1-3 The success and rapid adoption of national health insurance in Korea revealed unanticipated problems in three specific areas: low individual contributions relative to costs of care, low levels of reimbursement to providers, and incomplete coverage of medical services, which then require out-of-pocket payments (typically 20% of the inpatient care fee).4 Additionally, while little attention has been paid to quality and safety in the past, the nation has come to recognize the importance of these considerations,5 particularly with regard to patient expectations for and consumption of medical care and the consequent demand for better services from the medical community and government.6
Healthcare constitutes about 7.5% of Korea’s GDP. In contrast, healthcare spending accounts for about 17.7% of US GDP and about 8.8% of the GDP of member nations of the Organisation for Economic Co-operation and Development (OECD) in aggregate. Additionally, Korea has a longer length of hospital stay (16.5 days vs 7.3 days for OECD countries) and fewer practicing physicians (1.1 per 1,000 persons vs 1.9 per 1,000 for OECD countries). Therefore, the average cumulative annual patient hospital days per physician is 2,394 days in Korea, three times higher than that in the OECD countries.7 Furthermore, the number of physicians providing hospital care in Korea has remained relatively low.8
Despite recent growth in the total number of practicing physicians, the number of hospital-based physicians remains insufficient to cover admitted patients. The pressure for hospital-based physicians to serve large numbers of patients to generate sufficient revenue has deterred growth of more physicians focused on inpatient care.6 In order to operate the hospital, doctors must see as many patients as possible because physicians are reimbursed primarily by an inpatient care fee rather than by type or extent of care provided. Therefore, this low fee schedule makes it difficult to provide good quality inpatient care while simultaneously trying to increase accessibility. The costs of testing and treatment are reimbursed through a separate fee schedule.
THE NEED FOR A HOSPITALIST SYSTEM IN KOREA
The Korean government enacted two new policies regarding medical school graduates and residents that led to implementation of a hospitalist system. The first policy created a quota of medical residents to match the total number of medical school graduates.9 Before the new regulation, the number of medical resident positions exceeded the number of medical school graduates by 20%, which led to a shortage of resident applicants in “unpopular” medical specialties or departments. These specialties (eg, general surgery, obstetrics and gynecology, urology) have a lower fee schedule, which leads to lower wages while having very high workloads. The decrease in the number of available medical resident positions results in greater workloads for physicians providing inpatient care. These changes, including the shortage of a resident workforce, led to a burgeoning hospital-based attending physician workforce to care for hospitalized patients.
The other policy, created in December 2015 and known as the Act on the Improvement of Training Conditions and Status of Medical Residents, was enacted to improve working conditions for residents by limiting their working hours to 80 or fewer per week.10 These work-hour restrictions made it nearly impossible to provide 24-hour inpatient care depending solely on residents.10-12 This policy increased the need for hospitalists in Korea, similar to that of the US Accreditation Council for Graduate Medical Education in 2003, which limited resident work hours. Because of these changes, the hospitalist model was introduced to manage the growing volume of inpatients at teaching hospitals.13
The institutional implementation of the Korean hospitalist system was catalyzed by the need to solve current problems such as patient safety, healthcare quality, and residents’ well-being.8,14 Therefore, the hospitalist system was designed and implemented to meet the needs of patients, medical professionals, and hospitals.6,8,14 Furthermore, under the universal health insurance system in Korea, institutional implementation also meant creating a new set of fee schedules for hospitalists. The Korean hospitalist system reflects the nuances and challenges faced by the Korean healthcare system.5
DESIGN, IMPLEMENTATION, AND EVALUATION
A council was formed to implement a hospitalist model suitable for Korea. The council was composed of five groups: the Korean Association of Internal Medicine, Korean Surgical Society, Korean Medical Association, Korean Hospital Association, and Korean Academy of Medical Sciences. The Korean Association of Internal Medicine and the Korean Surgical Society were involved in creating a new medical discipline because their members provided a disproportionate amount of inpatient care and were most often responsible for hospital patient safety and quality care. Along with the support of the council, the Ministry of Health and Welfare began to realize the high demand on inpatient care and requested an official proposal to broaden the hospitalist model, with two conditions. First, the government requested a unified proposal from the medical community that reflected the collective voices of individual stakeholders, with an expectation that the new system would focus on patient safety and healthcare quality across all specialties. Second, the proposal had to be detailed and include a specific fee schedule for hospitalist services.
Before implementing a national hospitalist system, we conducted a pilot study supported by funding from the council. This first study, the Korean Hospitalist System Operation and Evaluation Research (September 2015 to August 2016)14, was designed to (a) determine hospitalist needs (eg, rotation schedule, salary, working conditions) for this new profession, (b) determine the necessary number of hospitalists and appropriate fee schedules to cover salary and benefits, and (c) provide Korean hospitalist models with operational methods to facilitate implementation at individual institutions. The council and research team selected four hospitals for the privately funded pilot study (Table). These hospitals already had an inpatient care system managed by specialists prior to the pilot study, so major changes in patient care and hospital operations were not required.

The pilot study demonstrated improvements in patient satisfaction, medical service quality, and patient safety.8,14,15 As a result, fees billed from hospitalist services were now covered by national health insurance; previously, they were not covered by any inpatient care fee or bundled service fee. The next study (phase 2) was then conducted to evaluate the national implementation of the hospitalist system and its outcomes. The first phase defined criteria for monitoring and evaluating the institutionalized hospitalist system in Korea. The second phase, initiated in September 2016, evaluated how the implemented system could be expanded nationwide. The government was actively involved in the study; 31 of 344 (9%) general hospitals were selected and able to apply the hospitalist fee schedule for care of patients who were admitted to the hospitalist ward.
To investigate the effect of the hospitalist system, we measured the satisfaction of patients and medical providers (eg, specialists, residents, and nurses). The outcomes were number of doctors’ calls to hospitalists and to other specialties, duration of time required to address medical problems, number of contacts with the hospitalist (residents or other physicians in the control group), and time spent with hospitalized patients. In addition, we analyzed claims costs and the operating costs for implementing the system within each hospital (Table).
The new set of fee schedules was created specifically for hospitalist care services so hospitals could now claim separate inpatient care fees and hospitalist fees. The standard hospitalist fee schedule is applied only to patients who are on hospitalist-led wards. The fee schedule has two criteria: The hospitalist ward must have 50 beds which are assigned to hospitalists only, and each hospitalist ward has a team of five hospitalists. A maximum of 25 patients could be assigned to an individual hospitalist. However, fees can be adjusted based on individual hospital operation and management after government approval.
Under the national insurance system, the fee schedule is essential to obtain reimbursement for provided services. As a new medical profession, it is important to have the institutional implementation of the hospitalist system. In contrast to those in the United States, inpatients in Korea pay inpatient care fees only, which are charged per day. Therefore, reducing the length of stay without increasing patient volume does not financially benefit the hospital.
DEFINITION OF A KOREAN HOSPITALIST AND SCOPE OF PRACTICE
The definition of a Korean hospitalist was carefully developed, and a reimbursement system for hospitalists was created within the Korean healthcare system. All hospitalists are medical specialists who have completed advanced education and clinical training in their specialty area (internal medicine or surgery). Creating a definition of a hospitalist and a standard fee schedule was necessary for national incorporation of this new approach. The model must also be accepted by related parties, including healthcare professionals such as hospital executives, nonhospitalist doctors, and nurses. Therefore, the system has a minimum of two requirements to operate at the hospital level, and the remaining factors can be modified by individual hospitals to protect the new discipline within the healthcare system. First, hospitalist services are provided only to hospitalist patients. To establish the new system, limiting the patient range to a specific group was necessary to prevent abuse of human-based resources within the hospital. Second, hospitalists must be stationed near the hospitalist ward to enhance accessibility, patient safety, and healthcare quality. In other words, a hospitalist provides services to hospitalized patients who paid the hospitalist fee, and the hospitalist does not provide care beyond the hospitalist ward (eg, they cannot provide outpatient consultation or provide care on other wards).
INPATIENT CARE REIMBURSEMENT BEFORE AND AFTER IMPLEMENTATION
Korean national health insurance is the universal health insurance under a single insurer (ie, the government). Therefore, all Korean citizens have national health insurance. Individuals can choose to have additional health insurance (private insurance) if they wish to pay an out-of-pocket fee with their additional private insurance. In Korea, the inpatient care fee includes all fees to provide care for the patient during hospital stays, such as the physician fee, facility fee, and consultation fee. The inpatient care fee schedule is charged per day including all the inpatient care composition. The claim and reimbursement system is different in Korea in that the hospital as a whole submits the claim for reimbursement. There is no separate claim from physicians, lab technicians, or facilities. Doctors provide care to patients and are then paid for the services in terms of salaries. Since the original inpatient care fee schedule was low and insufficient, it was difficult to provide high-quality care. Also, resources for spending on inpatient care management was limited. So a new system to improve inpatient care was needed. Implementing the hospitalist system in Korea led to the creation of a new fee schedule specifically for hospitalists so that hospitals could claim hospitalist fees on top of inpatient care fees when the patient was cared for by the hospitalist. The additional fees in the hospitalist fee schedule allow safe and high-quality care to be provided for patients during hospital stays.
KOREAN HOSPITAL MEDICINE TODAY
The Korean hospitalist system has been implemented for 3 years with the government’s active involvement, with approximately 250 specialists working as hospitalists as of August 2020. Importantly, Korean hospitalists are not limited to internal medicine specialists; in fact, several different specialties practice as hospitalists, including surgeons and other medical specialists, who account for 20% and 30% of the hospitalist workforce, respectively. Since the system’s implementation, all inpatient care and management is now transferred to specialists from residents in hospitalist wards. This change has increased patient safety and care quality. At the beginning of the pilot study, the concept of a “hospitalist” was new in Korea and the public did not know who a hospitalist was nor what a hospitalist did. However, after 3 years, patients now seek hospitalist care.
CONCLUSION
National implementation of the hospitalist model represents a key paradigm shift in the Korean healthcare system. The Korean hospitalist system is the result of the hospitals’, doctors’, and patients’ desire for higher-quality care. We expect to see growth of hospitalists in Korea and provision of better, safer, and more efficient inpatient care across specialties, payers, and government. Since we are at the early stage of the system, further efforts to support implementation are required. We hope our implementation process of a new medical system could serve as a model for other countries who are seeking to adopt hospitalist systems within their current healthcare paradigms.
Acknowledgments
The authors thank the medical professionals, government officers, and other professionals who put great effort into the implementation of the Korean Hospitalist System.
1. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24(1):63-71. https://doi.org/10.1093/heapol/czn037
2. Song YJ. The South Korean health care system. JMAJ. 2009;52(3):206-209.
3. Park YH, Park E-C. Healthcare policy. In: Preventive Medicine and Public Health. Vol 3.Gyechuk; 2017:821-833.
4. Park E-C, Lee TJ, Jun BY, Jung SH, Jeong HS. Health security. In: Preventive Medicine and Public Health. Vol 3. Gyechuk; 2017:888-897.
5. Jang S-I, Jang S-y, Park E-C. Trends of US hospitalist and suggestions for introduction of Korean hospitalist. Korean J Med. 2015;89(1):1-5. http://doi.org/10.3904/kjm.2015.89.1.1
6. Jang S-I. Korean hospitalist system implementation and development strategies based on pilot studies. J Korean Med Assoc. 2019;62(11):558-563. http://doi.org/10.5124/jkma.2019.62.11.558
7. OECD. Health at a Glance 2017: OECD indicators. OECD iLibrary. 2017. Accessed September 27, 2019. https://doi.org/10.1787/health_glance-2017-en
8. Jang S-I, Park E-C, Nam JM, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 2). Institute of Health Services Research, Yonsei University; 2018.
9. Koh DY. Political strategies to enhance medical residents. Health Policy. Vol 37. Seoul National University Hospital; 2014.
10. Act on the Improvement of Training Conditions and Status of Medical Residents. Vol No. 16260: Ministry of Health and Welfare; 2015.
11. Eom JS. Operating the hospitalist system. J Korean Med Assoc. 2016;59(5):342-344. http://doi.org/10.5124/jkma.2016.59.5.342
12. Kim S-S. Working conditions of interns/residents and patient safety: Painful training might not be authentic. J Korean Med Assoc. 2016;59(2):82-84. http://dx.doi.org/10.5124/jkma.2016.59.2.82
13. Oshimura J, Sperring J, Bauer BD, Rauch DA. Inpatient staffing within pediatric residency programs: work hour restrictions and the evolving role of the pediatric hospitalist. J Hosp Med. 2012;7(4):299-303. https://doi.org/10.1002/jhm.952
14. Park E-C, Lee SG, Kim T-H, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 1). Institute of Health Services Research, Yonsei University; 2016.
15. Jang S-I, Jung E-J, Park SK, Chae W, Kim Y-K. A study on the feasibility of the Korean hospitalist system and cost estimation. Ministry of Health and Welfare, Institute of Health Service Research Yonsei University; 2019.
1. Kwon S. Thirty years of national health insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 2009;24(1):63-71. https://doi.org/10.1093/heapol/czn037
2. Song YJ. The South Korean health care system. JMAJ. 2009;52(3):206-209.
3. Park YH, Park E-C. Healthcare policy. In: Preventive Medicine and Public Health. Vol 3.Gyechuk; 2017:821-833.
4. Park E-C, Lee TJ, Jun BY, Jung SH, Jeong HS. Health security. In: Preventive Medicine and Public Health. Vol 3. Gyechuk; 2017:888-897.
5. Jang S-I, Jang S-y, Park E-C. Trends of US hospitalist and suggestions for introduction of Korean hospitalist. Korean J Med. 2015;89(1):1-5. http://doi.org/10.3904/kjm.2015.89.1.1
6. Jang S-I. Korean hospitalist system implementation and development strategies based on pilot studies. J Korean Med Assoc. 2019;62(11):558-563. http://doi.org/10.5124/jkma.2019.62.11.558
7. OECD. Health at a Glance 2017: OECD indicators. OECD iLibrary. 2017. Accessed September 27, 2019. https://doi.org/10.1787/health_glance-2017-en
8. Jang S-I, Park E-C, Nam JM, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 2). Institute of Health Services Research, Yonsei University; 2018.
9. Koh DY. Political strategies to enhance medical residents. Health Policy. Vol 37. Seoul National University Hospital; 2014.
10. Act on the Improvement of Training Conditions and Status of Medical Residents. Vol No. 16260: Ministry of Health and Welfare; 2015.
11. Eom JS. Operating the hospitalist system. J Korean Med Assoc. 2016;59(5):342-344. http://doi.org/10.5124/jkma.2016.59.5.342
12. Kim S-S. Working conditions of interns/residents and patient safety: Painful training might not be authentic. J Korean Med Assoc. 2016;59(2):82-84. http://dx.doi.org/10.5124/jkma.2016.59.2.82
13. Oshimura J, Sperring J, Bauer BD, Rauch DA. Inpatient staffing within pediatric residency programs: work hour restrictions and the evolving role of the pediatric hospitalist. J Hosp Med. 2012;7(4):299-303. https://doi.org/10.1002/jhm.952
14. Park E-C, Lee SG, Kim T-H, et al. A study on the implementation and the evaluation of Korean Hospitalist System to improve the quality of hospitalization (Phase 1). Institute of Health Services Research, Yonsei University; 2016.
15. Jang S-I, Jung E-J, Park SK, Chae W, Kim Y-K. A study on the feasibility of the Korean hospitalist system and cost estimation. Ministry of Health and Welfare, Institute of Health Service Research Yonsei University; 2019.
© 2021 Society of Hospital Medicine
Physician Responsiveness to Positive Blood Culture Results at the Minneapolis Veterans Affairs Hospital—Is Anyone Paying Attention?
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
The US Department of Veterans Affairs (VA) is the largest health care organization in the US, staffing more than 1,200 facilities and servicing about 9 million veterans.1 Identifying VA practices that promote effective health care delivery has the potential to impact thousands of patients every day. The Surgical service at the Minneapolis VA Medical Center (MVAMC) in Minnesota often questioned colleagues whether many of the ordered tests, including blood cultures for patients with suspected infections, were clinically necessary. Despite recommendations for utilizing culture-driven results in choosing appropriate antimicrobials, it was debated whether these additional tests were simply drawn and ignored resulting only in increased costs and venipuncture discomfort for the patient. Thus, the purpose of this quality improvement study was to determine whether positive blood culture results actually influence clinical management at MVAMC.
Background
Accepted best practice when responding to positive blood culture results entails empiric treatment with broad-spectrum antibiotics that subsequently narrows in breadth of coverage once the pathogen has been identified.2-4 This strategy has been labeled deescalation. Despite the acceptance of these standards, surveys of clinician attitudes towards antibiotics showed that 90% of physicians and residents stated they wanted more education on antimicrobials and 80% desired better schooling on antibiotic choices.5,6 Additionally, in an online survey 18% of 402 inpatient and emergency department providers, including residents, fellows, intensive care unit (ICU) and emergency department attending physicians, hospitalists, physician assistants, and nurse practitioners, described a lack of confidence when deescalating antibiotic therapy and 45% reported that they had received training on antimicrobial prescribing that was not fully adequate.7
These surveys hint at a potential gap in provider education or confidence, which may serve as a barrier to ideal care, further confounding other individualized considerations taken into account when deescalating care. These considerations include patient renal toxicity profiles, the potential for missed pathogens not identified in culture results, unknown sources of infection, and the mindset of many providers to remain on broad therapy if the patient’s condition is improving.8-10 A specific barrier to deescalation within the VA is the variance in antimicrobial stewardship practices between facilities. In a recent widespread survey of VA practices, Chou and colleagues identified that only 29 of 130 (22.3%) responding facilities had a formal policy to establish an antimicrobial stewardship program.11
Overcoming these barriers to deescalation through effective stewardship practices can help to promote improved clinical outcomes. Most studies have demonstrated that outcomes of deescalation strategies have equivalent or improved mortalityand equivalent or even decreased length of ICU stay.12-26 Although a 2014 study by Leone and colleagues reported longer overall ICU stay in deescalation treatment groups with equivalent mortality outcomes, newer data do not support these findings.16,20,22
Furthermore, antibiotics can be expensive. Deescalation, particularly in response to positive blood culture results, has been associated with reduced antibiotic cost due to both a decrease in overall antibiotic usage and the utilization of less expensive choices.22,24,26,27 The findings of these individual studies were corroborated in 2013 by a meta-analysis, including 89 additional studies.28 Besides the direct costs of the drugs, the development of regional antibiotic resistance has been labeled as one of the most pressing concerns in public health, and major initiatives have been undertaken to stem its spread.29,30 The majority of clinicians believe that deescalation of antibiotics would reduce antibiotic resistance. Thus, deescalation is widely cited as one of the primary goals in the management of resistance development.5,24,26,28,31,32
Due to the proposed benefits and challenges of implementation, MVAMC instituted a program where the electronic health records (EHR) for all patients with positive blood culture results were reviewed by the on-call infectious disease attending physician to advise the primary care team on antibiotic administration. The MVAMC system for notification of positive blood culture results has 2 components. The first is phone notification to the on-call resident when the positive result of the pathogen identification is noted by the microbiology laboratory staff. Notably, this protocol of phone notification is only performed when identifying the pathogen and not for the subsequent sensitivity profile. The second component occurs each morning when the on-call infectious disease attending physician reviews all positive blood culture results and the current therapy. If the infectious disease attending physician feels some alterations in management are warranted, the physician calls the primary service. Additionally, the primary service may always request a formal consult with the infectious disease team. This quality improvement study was initiated to examine the success of this deescalation/stewardship program to determine whether positive blood culture results influenced clinical management.
Methods
From July 1, 2015 to June 30, 2016, 212 positive blood cultures at the MVAMC were analyzed. Four cases that did not have an antibiotic spectrum score were excluded, leaving 208 cases reviewed. Duplicate blood cultures were excluded from analysis. The microbiology laboratory used the BD Bactec automated blood culture system using the Plus aerobic and Lytic anaerobic media (Becton, Dickinson and Company).
Antibiotic alterations in response to culture results were classified as either deescalation or escalation, using a spectrum score developed by Madaras-Kelly and colleagues.33 These investigators performed a 3-round modified Delphi survey of infectious disease staff of physicians and pharmacists. The resulting consensus spectrum score for each respective antibiotic reflected the relative susceptibilities of various pathogens to antibiotics and the intrinsic resistance of the pathogens. It is a nonlinear scale from 0 to 60 with a score of 0 indicating no antibacterial activity and a score of 60 indicating complete coverage of all critically identified pathogens. For example, a narrow-spectrum antibiotic such as metronidazole received a spectrum score of 4.0 and a broad-spectrum antibiotic such as piperacillin/tazobactam received a 42.3 score.
Any decrease in the spectrum score when antibiotics were changed was described as deescalation and an increase was labeled escalation. In cases where multiple antibiotics were used during empiric therapy, the cessation of ≥ 1 antibiotics was classified as a deescalation while the addition of ≥ 1 antibiotics was classified as an escalation.
Madaras-Kelly and colleagues calculated changes in spectrum score and compared them with Delphi participants’ judgments on deescalation with 20 antibiotic regimen vignettes and with non-Delphi steward judgments on deescalation of 300 pneumonia regimen vignettes. Antibiotic spectrum scores were assigned a value for the width of empiric treatment that was compared with the antibiotic spectrum score value derived through antibiotic changes made based on culture results. In the Madaras-Kelly cases, the change in breadth of antibiotic coverage was in agreement with expert classification in 96% of these VA patient cases using VA infectious disease specialists. This margin was noted as being superior to the inter-rater variability between the individual infectious disease specialists.
Data Recording and Analysis
Charts for review were flagged based on positive blood culture results from the microbiology laboratory. EHRs were manually reviewed to determine when antibiotics were started/stopped and when a member of the primary care team, usually a resident, was notified of culture results as documented by the microbiology laboratory personnel. Any alteration in antibiotics that fit the criteria of deescalation or escalation that occurred within 24 hours of notification of either critical laboratory value was recorded. The identity of infectious pathogens and the primary site of infection were not recorded as these data were not within the scope of the purpose of this study. We did not control for possible contaminants within positive blood cultures.
There were 3 time frames considered when determining culture driven alterations to the antibiotic regimen. The first 2 were changes within the 24 hours after notification of either (1) pathogen identification or (2) pathogen sensitivity. These were defined as culture-driven alterations in response to those particular laboratory findings. The third—whole case time frame—spanned from pathogen identification to 24 hours after sensitivity information was recorded. In cases where ≥ 1 antibiotic alteration was noted within a respective time frame, a classification of deescalation or escalation was still assigned. This was done by summing each change in spectrum score that occurred from antibiotic regimen alterations within the time frame, and classifying the net effect on the spectrum of coverage as either deescalation or escalation. Data were recorded in spreadsheet. RStudio 3.5.3 was used for statistical analysis.
Results
Of 208 cases assigned a spectrum score, 47 (22.6%) had the breadth of antibiotic coverage deescalated by the primary care team within 24 hours of pathogen identification with a mean (SD) physician response time of 8.0 (7.3) hours. Fourteen cases (6.7%) had the breadth of antibiotic coverage escalated from pathogen identification with a mean (SD) response time of 8.0 (7.4) hours. When taken together, within 24 hours of pathogen identification from positive blood cultures 61 cases (29.3%) had altered antibiotics, leaving 70.7% of cases unaltered (Tables 1 and 2). In this nonquantitative spectrum score method, deescalations typically involved larger changes in spectrum score than escalations.
Physician notification of pathogen sensitivities resulted in deescalation in 69 cases (33.2%) within 24 hours, with a mean (SD) response time of 10.4 (7) hours. The mean time to deescalation in response to pathogen identification was significantly shorter than the mean time to deescalation in response to sensitivities (P = .049). Broadening of coverage based on sensitivity information was reported for 17 cases (8.2%) within 24 hours, with a mean (SD) response time of 7.6 (6) hours (Table 3). In response to pathogen sensitivity results from positive blood cultures, 58.6% of cases had no antibiotic alterations. Deescalations involved notably larger changes in spectrum score than escalations.
More than half (58.6%) of cases resulted in an antibiotic alteration from empiric treatment when considering the time frame from empiric antibiotics to 24 hours after receiving sensitivity information. These were deemed the whole-case, culture-driven results. In addition to antibiotic alterations that occurred within 24 hours of either pathogen identification or sensitivity information, the whole-case category also considered antibiotic alterations that occurred more than 24 hours after pathogen identification was known and before sensitivity information was available, although this was rare. Some of these patients may have had their antibiotics altered twice, first after pathogen identification and later once sensitivities became available with the net effect recorded as the whole-case administration. Of those that had their antibiotics modified in response to laboratory results, by a ratio of 6.4:1, the change was a deescalation rather than an escalation.
Discussion
The strategy of the infectious disease team at MVAMC is one of deescalation. One challenge of quantifying deescalation was to make a reliable and agreed-upon definition of just what deescalation entails. In 2003, the pharmaceutical company Merck was granted a trademark for the phrase “De-Escalation Therapy” under the international class code 41 for educational and entertainment services. This seemed to correspond to marketing efforts for the antibiotic imipenem/cilastatin. Although the company trademarked the term, it was never defined. The usage of the phrase evolved from a reduction of the dosage of a specific antibiotic to a reduction in the number of antibiotics prescribed to that of monotherapy. The phrase continues to evolve and has now become associated with a change from combination therapy or broad-spectrum antibiotics to monotherapy, switching to an antibiotic that covers fewer pathogens, or even shortening the duration of antibiotic therapy.34 The trademark expired at about the same time the imipenem/cilastatin patent expired. Notably, this drug had initially been marketed for use in empiric antibiotic therapy.35
Barriers
The goal of the stewardship program was not to see a narrowing of the antibiotic spectrum in all patients. Some diseases such as diverticulitis or diabetic foot infections are usually associated with multiple pathogens where relatively broad-spectrum antibiotics seem to be preferred.36,37 Heenen and colleagues reported that infectious disease specialists recommended deescalation in < 50% of cases they examined.38
Comparing different institutions’ deescalation rates can be confusing due to varying definitions, differing patient populations, and health care provider behavior. Thus, the published rates of deescalation range widely from 10 to 70%.2,39,40 In addition to the varied definitions of deescalation, it is challenging to directly compare the rate of deescalation between studies due to institutional variation in empirical broad-spectrum antibiotic usage. A hospital that uses broad-spectrum antibiotics at a higher rate than another has the potential to deescalate more often than one that has low rates of empirical broad-spectrum antibiotic use. Some studies use a conservative definition of deescalation such as narrowing the spectrum of coverage, while others use a more general definition, including both the narrowing of spectrum and/or the discontinuation of antibiotics from empirical therapy.41-45 The more specific and validated definition of deescalation used in this study may allow for standardized comparisons. Another unique feature of this study is that all positive blood cultures were followed, not only those of a particular disease.
One issue that comes up in all research performed within the VA is how applicable these results are to the general public. Nevertheless, the stewardship program as it is structured at the MVAMC could be applied to other non-VA institutions. We recognize, however, that some smaller hospitals may not have infectious diseases specialists on staff. Despite limited in-house staff, the same daily monitoring can be performed off-site through review of the EHR, thus making this a viable system to more remote VA locations.
While deescalation remains the standard of care, there are many complexities that explain low deescalation rates. Individual considerations that can cause physicians to continue the empirically initiated broad-spectrum coverage include differing renal toxicities, suspecting additional pathogens beyond those documented in testing results, and differential Clostridium difficile risk.46,47 A major concern is the mind-set of many prescribers that streamlining to a different antibiotic or removing antibiotics while the patient is clinically improving on broad empiric therapy represents an unnecessary risk.48,49 These thoughts seem to stem from the old adage, “If it ain’t broke, don’t fix it.”
Due to the challenges in defining deescalation, we elected to use a well-accepted and validated methodology of Madaras-Kelly.33 We recognize the limitations of the methodology, including somewhat differing opinions as to what may constitute breadth and narrowing among clinicians and the somewhat arbitrary assignment of numerical values. This tool was developed to recognize only relative changes in antibiotic spectrum and is not quantitative. A spectrum score of piperacillin/tazobactam of 42.3 could not be construed as 3 times as broad as that of vancomycin at 13. Thus, we did not perform statistical analysis of the magnitude of changes because such analysis would be inconsistent with the intended purpose of the spectrum score method. Additionally, while this method demonstrated reliable classification of appropriate deescalation and escalation in previous studies, a case-by-case review determining appropriateness of antibiotic changes was not performed.
Clinical Response
This quality improvement study was initiated to determine whether positive blood culture results actually affect clinical management at MVAMC. The answer seems to be yes, with blood culture results altering antibiotic administration in about 60% of cases with the predominant change being deescalation. This overall rate of deescalation is toward the higher end of previously documented rates and coincides with the upper bound of the clinically advised deescalation rate described by Heenen and colleagues.38
As noted, the spectrum score is not quantitative. Still, one may be able to contend that the values may provide some insight into the magnitude of the changes in antibiotic selection. Deescalations were on average much larger changes in spectrum than escalations. The larger magnitude of deescalations reflects that when already starting with a very broad spectrum of coverage, it is much easier to get narrower than even broader. Stated another way, when starting therapy using piperacillin/tazobactam at a spectrum score of 42.3 on a 60-point scale, there is much more room for deescalation to 0 than escalation to 60. Additionally, escalations were more likely with much smaller of a spectrum change due to accurate empirical judgment of the suspected pathogens with new findings only necessitating a minor expansion of the spectrum of coverage.
Another finding within this investigation was the statistically significantly shorter response mean (SD) time when deescalating in response to pathogen identification (8 [7.3] h) than to sensitivity profile (10.4 [7] h). Overall when deescalating, the time of each individual response to antibiotic changes was highly irregular. There was no noticeable time point where a change was more likely to occur within the 24 hours after notification of a culture result. This erratic distribution further exemplifies the complexity of deescalation as it underscores the unique nature of each case. The timing of the dosage of previous antibiotics, the health status of the patient, and the individual physician attitudes about the progression and severity of the infection all likely played into this distribution.
Due to the lack of a regular or even skewed distribution, a Wilcoxon nonparametric rank sum test was performed (P = .049). Although this result was statistically significant, the 2.5-hour time difference is likely clinically irrelevant as both times represent fairly prompt physician responsiveness.50 Nonetheless, it suggests that it was more important to rapidly escalate the breadth of coverage for a patient with a positive blood culture than to deescalate as identified pathogens may have been left untreated with the prescribed antibiotic.
Future Study
Similar studies designed using the spectrum score methodology would allow for more meaningful interinstitutional comparison of antibiotic administration through the use of a unified definition of deescalation and escalation. Comparison of deescalation and escalation rates between hospital systems with similar patient populations with and without prompt infectious disease review and phone notification of blood culture results could further verify the value of such a protocol. It could also help determine which empiric antibiotics may be most effective in individual patient morbidity and mortality outcomes, length of stay, costs, and the development of antibiotic resistance. Chou and colleagues found that only 49 of 130 responding VA facilities had antimicrobial stewardship teams in place with even fewer (29) having a formal policy to establish an antimicrobial stewardship program.11 This significant variation in the practices of VA facilities across the nation underscores the benefit to be gained from implementation of value-added protocols such as daily infectious disease case monitoring and microbiology laboratory phone notification of positive blood culture results as it occurs at MVAMC.
They also noted that systems of patient-level antibiotic review, and the presence of at least one full-time infectious disease physician were both associated with a statistically significant decrease in the use of antimicrobials, corroborating the results of this analysis.11 Adapting the current system of infectious disease specialist review of positive blood culture results to use remote monitoring through the EHR could help to defer some of the cost of needing an in-house specialist while retaining the benefit of the oversite.
Another option for study would be a before and after design to determine whether the program of infectious disease specialist review led to increased use of deescalation strategies similar to studies investigating the efficacy of antimicrobial subcommittee implementation.13,20,23,24,26
Conclusions
This analysis of empiric antibiotic use at the MVAMC indicates promising rates of deescalation. The results indicate that the medical service may be right and that positive blood culture results appear to affect clinical decision making in an appropriate and timely fashion. The VA is the largest health care organization in the US. Thus, identifying and propagating effective stewardship practices on a widespread basis can have a significant effect on the public health of the nation.
These data suggest that the program implemented at the MVAMC of phone notification to the primary care team along with daily infectious disease staff monitoring of blood culture information should be widely adopted at sister institutions using either in-house or remote specialist review.
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
1. US Department of Veterans Affairs, Veterans Health Administration-About VHA. Updated January 22, 2021. Accessed February 19, 2021. https://www.va.gov/health/aboutvha.asp.
2. Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27(1):149-162. doi:10.1016/j.ccc.2010.09.009
3. Garnacho-Montero J, Gutiérrez-Pizarraya A, Escoresca-Ortega A, et al. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014;40(1):32-40. doi:10.1007/s00134-013-3077-7
4. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
5. Srinivasan A, Song X, Richards A, Sinkowitz-Cochran R, Cardo D, Rand C. A survey of knowledge, attitudes, and beliefs of house staff physicians from various specialties concerning antimicrobial use and resistance. Arch Intern Med. 2004;164(13):1451-1456. doi:10.1001/archinte.164.13.1451
6. Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. doi:10.1093/jpids/pis045
7. Salsgiver E, Bernstein D, Simon MS, et al. Knowledge, attitudes, and practices regarding antimicrobial use and stewardship among prescribers at acute-care hospitals. Infect Control Hosp Epidemiol. 2018;39(3):316-322. doi:10.1017/ice.2017.317
8. Bamgbola O. Review of vancomycin-induced renal toxicity: an update. Ther Adv Endocrinol Metab. 2016;7(3):136-147. doi:10.1177/2042018816638223
9. Kunni CM, Finland M. Restrictions imposed on antibiotic therapy by renal failure. Arch Intern Med. 1959;104:1030-1050. doi:10.1001/archinte.1959.00270120186021
10. Sartelli M, Catena F, Abu-Zidan FM, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg. 2017;12:22. Published 2017 May 4. doi:10.1186/s13017-017-0132-7
11. Chou AF, Graber CJ, Jones M, et al. Characteristics of antimicrobial stewardship programs at Veterans Affairs hospitals: results of a nationwide survey. Infect Control Hosp Epidemiol. 2016;37(6):647-654. doi:10.1017/ice.2016.26
12. Giantsou E, Liratzopoulos N, Efraimidou E, et al. De-escalation therapy rates are significantly higher by bronchoalveolar lavage than by tracheal aspirate. Intensive Care Med. 2007;33(9):1533-1540. doi:10.1007/s00134-007-0619-x
13. Malani AN, Richards PG, Kapila S, Otto MH, Czerwinski J, Singal B. Clinical and economic outcomes from a community hospital’s antimicrobial stewardship program. Am J Infect Control. 2013;41(2):145-148. doi:10.1016/j.ajic.2012.02.021
14. Souza-Oliveira AC, Cunha TM, Passos LB da S, Lopes GC, Gomes FA, Röder DVD de B. Ventilator-associated pneumonia: the influence of bacterial resistance, prescription errors, and de-escalation of antimicrobial therapy on mortality rates. Brazilian J Infect Dis. 2016;20(5):437-443. doi:10.1016/j.bjid.2016.06.006
15. Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care. 2012;16(1):R28. Published 2012 Feb 15. doi:10.1186/cc11197
16. Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial [published correction appears in Intensive Care Med. 2014 Nov;40(11):1794]. Intensive Care Med. 2014;40(10):1399-1408. doi:10.1007/s00134-014-3411-8
17. Gonzalez L, Cravoisy A, Barraud D, et al. Factors influencing the implementation of antibiotic de-escalation and impact of this strategy in critically ill patients. Crit Care. 2013;17(4):R140. Published 2013 Jul 12. doi:10.1186/cc12819
18. Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4(8):519-527. doi:10.1016/S1473-3099(04)01108-9
19. Peña C, Suarez C, Ocampo-Sosa A, et al. Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57(2):208-216. doi:10.1093/cid/cit223
20. Campion M, Scully G. Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. J Intensive Care Med. 2018;33(12):647-655. doi:10.1177/0885066618762747
21. Mokart D, Slehofer G, Lambert J, et al. De-escalation of antimicrobial treatment in neutropenic patients with severe sepsis: results from an observational study. Intensive Care Med. 2014;40(1):41-49. doi:10.1007/s00134-013-3148-9
22. Li H, Yang CH, Huang LO, et al. Antibiotics de-escalation in the treatment of ventilator-associated pneumonia in trauma patients: a retrospective study on propensity score matching method. Chin Med J (Engl). 2018;131(10):1151-1157. doi:10.4103/0366-6999.231529
23. Lindsay PJ, Rohailla S, Taggart LR, et al. Antimicrobial stewardship and intensive care unit mortality: a systematic review. Clin Infect Dis. 2019;68(5):748-756. doi:10.1093/cid/ciy550
24. Perez KK, Olsen RJ, Musick WL, et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect. 2014;69(3):216-225. doi:10.1016/j.jinf.2014.05.005
25. Ikai H, Morimoto T, Shimbo T, Imanaka Y, Koike K. Impact of postgraduate education on physician practice for community-acquired pneumonia. J Eval Clin Pract. 2012;18(2):389-395. doi:10.1111/j.1365-2753.2010.01594.x
26. Ruiz J, Ramirez P, Gordon M, et al. Antimicrobial stewardship programme in critical care medicine: A prospective interventional study. Med Intensiva. 2018;42(5):266-273. doi:10.1016/j.medin.2017.07.002
27. Berild D, Mohseni A, Diep LM, Jensenius M, Ringertz SH. Adjustment of antibiotic treatment according to the results of blood cultures leads to decreased antibiotic use and costs. J Antimicrob Chemother. 2006;57(2):326-330. doi:10.1093/jac/dki463
28. Davey P, Brown E, Charani E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;(4):CD003543. Published 2013 Apr 30. doi:10.1002/14651858.CD003543.pub3
29. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Revised December 2019. Accessed March 2, 2021. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
30. O’Neill J. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Published December 2014. Accessed February 19, 2021. https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf
31. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
32. De Waele JJ, Akova M, Antonelli M, et al. Antimicrobial resistance and antibiotic stewardship programs in the ICU: insistence and persistence in the fight against resistance. A position statement from ESICM/ESCMID/WAAAR round table on multi-drug resistance. Intensive Care Med. 2018;44(2):189-196. doi:10.1007/s00134-017-5036-1
33. Madaras-Kelly K, Jones M, Remington R, Hill N, Huttner B, Samore M. Development of an antibiotic spectrum score based on veterans affairs culture and susceptibility data for the purpose of measuring antibiotic de-escalation: a modified Delphi approach. Infect Control Hosp Epidemiol. 2014;35(9):1103-1113. doi:10.1086/677633
34. Tabah A, Cotta MO, Garnacho-Montero J, et al. A systematic review of the definitions, determinants, and clinical outcomes of antimicrobial de-escalation in the intensive care unit. Clin Infect Dis. 2016;62(8):1009-1017. doi:10.1093/cid/civ1199
35. Primaxin IV. Prescribing information. Merck & Co, Inc; 2001. Accessed February 23, 2021. https://www.merck.com/product/usa/pi_circulars/p/primaxin/primaxin_iv_pi.pdf
36. Coccolini F, Trevisan M, Montori G, et al. Mortality rate and antibiotic resistance in complicated diverticulitis: report of 272 consecutive patients worldwide: a prospective cohort study. Surg Infect (Larchmt). 2017;18(6):716-721. doi:10.1089/sur.2016.283
37. Selva Olid A, Solà I, Barajas-Nava LA, Gianneo OD, Bonfill Cosp X, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. Published 2015 Sep 4. doi:10.1002/14651858.CD009061.pub2
38. Heenen S, Jacobs F, Vincent JL. Antibiotic strategies in severe nosocomial sepsis: why do we not de-escalate more often?. Crit Care Med. 2012;40(5):1404-1409. doi:10.1097/CCM.0b013e3182416ecf
39. Morel J, Casoetto J, Jospé R, et al. De-escalation as part of a global strategy of empiric antibiotherapy management. A retrospective study in a medico-surgical intensive care unit. Crit Care. 2010;14(6):R225. doi:10.1186/cc9373
40. Moraes RB, Guillén JA, Zabaleta WJ, Borges FK. De-escalation, adequacy of antibiotic therapy and culture positivity in septic patients: an observational study. Descalonamento, adequação antimicrobiana e positividade de culturas em pacientes sépticos: estudo observacional. Rev Bras Ter Intensiva. 2016;28(3):315-322. doi:10.5935/0103-507X.20160044
41. Khasawneh FA, Karim A, Mahmood T, et al. Antibiotic de-escalation in bacteremic urinary tract infections: potential opportunities and effect on outcome. Infection. 2014;42(5):829-834. doi:10.1007/s15010-014-0639-8
42. Alshareef H, Alfahad W, Albaadani A, Alyazid H, Talib RB. Impact of antibiotic de-escalation on hospitalized patients with urinary tract infections: A retrospective cohort single center study. J Infect Public Health. 2020;13(7):985-990. doi:10.1016/j.jiph.2020.03.004
43. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions-a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. doi:10.1007/s00134-019-05871-z
44. Eachempati SR, Hydo LJ, Shou J, Barie PS. Does de-escalation of antibiotic therapy for ventilator-associated pneumonia affect the likelihood of recurrent pneumonia or mortality in critically ill surgical patients?. J Trauma. 2009;66(5):1343-1348. doi:10.1097/TA.0b013e31819dca4e
45. Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia [published correction appears in Chest. 2006 Jul;130(1):308]. Chest. 2006;129(5):1210-1218. doi:10.1378/chest.129.5.1210
46. Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J Jr. Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477. doi:10.1086/648363
47. Pépin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254-1260. doi:10.1086/496986
48. Seddon MM, Bookstaver PB, Justo JA, et al. Role of Early De-escalation of Antimicrobial Therapy on Risk of Clostridioides difficile Infection Following Enterobacteriaceae Bloodstream Infections. Clin Infect Dis. 2019;69(3):414-420. doi:10.1093/cid/ciy863
49. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol. 2015;36(9):1065-1072. doi:10.1017/ice.2015.136
50. Liu P, Ohl C, Johnson J, Williamson J, Beardsley J, Luther V. Frequency of empiric antibiotic de-escalation in an acute care hospital with an established antimicrobial stewardship program. BMC Infect Dis. 2016;16(1):751. Published 2016 Dec 12. doi:10.1186/s12879-016-2080-3
The COVID-19 Pandemic and Changes in Healthcare Utilization for Pediatric Respiratory and Nonrespiratory Illnesses in the United States
In the United States, respiratory illnesses are the most common cause of emergency department (ED) visits and hospitalizations in children.1 In response to the ongoing COVID-19 pandemic, several public health interventions, including school and business closures, stay-at-home orders, and mask mandates, were implemented to limit transmission of SARS-CoV-2.2,3 Studies have shown that children can contribute to the spread of SARS-CoV-2 infections, especially within households.4-6 Recent data suggest that COVID-19, and the associated public health measures enacted to slow its spread, may have affected the transmission of other respiratory pathogens.7 Similarly, the pandemic has likely affected healthcare utilization for nonrespiratory illnesses through adoption of social distancing recommendations, suspension and delays in nonemergent elective care, avoidance of healthcare settings, and the effect of decreased respiratory disease on exacerbation of chronic illness.8 The objective of this study was to examine associations between the COVID-19 pandemic and healthcare utilization for pediatric respiratory and nonrespiratory illnesses at US pediatric hospitals.
METHODS
Study Design
This is a multicenter, cross-sectional study of encounters at 44 pediatric hospitals that reported data to the Pediatric Health Information System (PHIS) database maintained by the Children’s Hospital Association (Lenexa, Kansas).
Study Population
Children 2 months to 18 years of age discharged from ED or inpatient settings with a nonsurgical diagnosis from January 1 to September 30 over a 4-year period (2017-2020) were included.
Exposure
The primary exposure was the 2020 COVID-19 pandemic time, divided into three periods: pre-COVID-19 (January-February 2020, the period prior to the pandemic in the United States), early COVID-19 (March-April 2020, coinciding with the first reported US pediatric case of COVID-19 on March 2, 2020), and COVID-19 (May-September 2020, marked by the implementation of at least two of the following containment measures in every US state: stay-at-home/shelter orders, school closures, nonessential business closures, restaurant closures, or prohibition of gatherings of more than 10 people).2
Outcomes
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and compared using chi-square tests. Continuous variables were summarized as median and interquartile range (IQR) and compared using Wilcoxon rank sum tests. Weekly observed-to-expected (O:E) ratios were calculated for each hospital by dividing the number of observed respiratory illness and nonrespiratory illness encounters in a given week in 2020 (observed) by the average number of encounters for that same week during 2017-2019 (expected). O:E ratios were then aggregated over the three COVID-19 study periods, and 95% confidence intervals were established around mean O:E ratios across individual hospitals. Outcomes were then stratified by respiratory illness subgroups, geographic region, and age. Additional details can be found in the Supplemental Methods in the Appendix.
RESULTS
Study Population
A total of 9,051,980 encounters were included in the study, 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. Median age was 5 years (IQR, 1-11 years), and 52.7% of the population was male (Appendix Table 2 and Appendix Table 3).
Respiratory vs Nonrespiratory Illness During the COVID-19 Pandemic
Over the study period, fewer respiratory and nonrespiratory illness encounters were observed than expected, with a larger decrease in respiratory illness encounters (Table, Appendix Table 4). 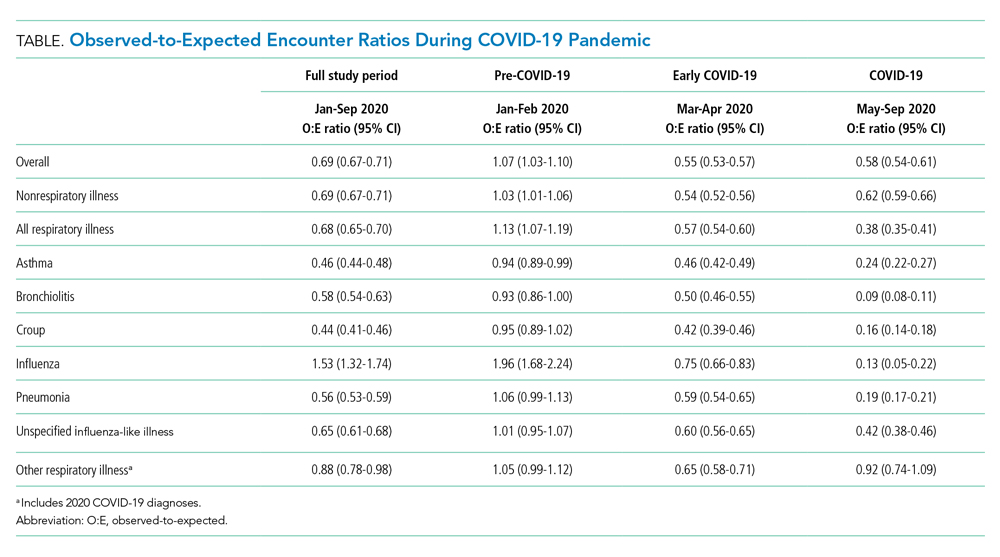

Respiratory Subgroup Analyses
The O:E ratio decreased for all respiratory subgroups over the study period (Table, Appendix Table 4). There were significant differences in specific respiratory subgroups, including asthma, bronchiolitis, croup, influenza, and pneumonia (Appendix Figure 1A). Temporal trends in respiratory encounters were consistent across hospital settings, ages, and geographic regions (Appendix Figure 1B-D). When comparing the with and without COVID-19 subgroups in the “other respiratory illnesses” cohort, other respiratory illness without COVID-19 decreased and remained lower than expected over the rest of the study period, while other respiratory illness with COVID-19 increased markedly during the summer months and declined thereafter (Appendix Figure 2).
All age groups had reductions in respiratory illness encounters during the early COVID-19 and COVID-19 periods, although the decline was less pronounced in the 12- to 17-year-old group (Appendix Figure 1B). Similarly, while all age groups experienced increases in encounters for respiratory illnesses during the summer months, only children in the 12- to 17-year-old group experienced increases beyond pre-COVID-19 levels. Importantly, this increase in respiratory encounters was largely driven by COVID-19 diagnoses (Appendix Figure 3). The trend in nonrespiratory illness encounters stratified by age is shown in Appendix Figure 4.
When patients were stratified by hospital setting, there were no differences between those hospitalized and those discharged from the ED (Appendix Figure 1C). Patterns in respiratory illnesses by geographic location were qualitatively similar until the beginning of the summer 2020, after which geographical variation became more evident (Appendix Figure 1D).
DISCUSSION
In this large, multicenter study evaluating ED visits and hospitalizations for respiratory and nonrespiratory illnesses at US pediatric hospitals during the 2020 COVID-19 pandemic, we found a significant and substantial decrease in healthcare encounters for respiratory illnesses. A rapid and marked decline in encounters for respiratory illness in a relatively short period of time (March 12-April 2) was observed across all hospitals and US regions. Declines were consistent across common respiratory illnesses. More modest, yet still substantial, declines were also observed for nonrespiratory illnesses.
There are likely multiple underlying reasons for the observed reductions. Social distancing measures almost certainly played an important role in interrupting respiratory infection transmission. Rapid reduction in influenza transmission during the early COVID-19 period has been attributed to social distancing measures,3 and influenza transmission in children decreases with school closures.9 It is also possible that some families delayed seeking care at hospitals due to COVID-19, leading to less frequent encounters but more severe illness. The similar decrease in O:E ratio for ED visits and hospitalizations, however, is inconsistent with this explanation.
We also found relative differences in changes in encounters for respiratory illness by age. Adolescents’ levels of respiratory healthcare use declined less and recovered at a faster rate than those of younger children, returning to pre-COVID-19 levels by the end of the study period. The reason for this age differential is likely multifaceted. Infections, such as bronchiolitis and pneumonia, are more likely to be a source of respiratory illness in younger than in older children. It is also likely that disproportionate relaxation of social distancing measures among adolescents, who are known to have a stronger pattern of social interaction, contributed to the faster rise in respiratory illness–related encounters in this age group.11 Adolescents have been reported to be more susceptible to, and more likely to transmit, SARS-CoV-2 compared to younger age groups.12 More modest, albeit similar, age-based changes were observed in encounters for nonrespiratory illnesses
Emerging evidence suggests that school-age children may play an important role in SARS-CoV-2 transmission in the community.4,14 Our finding that, compared to younger children, adolescents had significantly fewer reductions in respiratory illness encounters is concerning. These findings suggest that community-based efforts to help prevent respiratory illnesses, especially COVID-19, should focus on adolescents, who are most likely to maintain social interactions and transmit respiratory infections in the school setting and their households.
This study is limited by the inclusion of only tertiary care children’s hospitals, which may not be nationally representative, and the inability to assess the precise timing of when specific public health interventions were introduced. Moreover, previous studies suggest that social distancing behaviors may have changed even before formal recommendations were enacted.15 Future studies should investigate the local impact of state- and municipality-specific mandates on the burden of COVID-19 and other respiratory illnesses.
The COVID-19 pandemic was associated with substantial reductions in encounters for respiratory diseases, and also with more modest but still sizable reductions in encounters for nonrespiratory diseases. These reductions varied by age. Encounters among adolescents declined less and returned to previous levels faster compared with those of younger children.
ACKNOWLEDGMENT
This publication is dedicated to the memory of our coauthor, Dr. Michael Bendel-Stenzel. Dr. Bendel-Stenzel was dedicated to bettering the lives of children and advancing our knowledge of pediatrics through his research.
1. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
2. Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324(9):859-870. https://doi.org/10.1001/jama.2020.14348
3. Wiese AD, Everson J, Grijalva CG. Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis. Published online June 20, 2020. https://doi.org/10.1093/cid/ciaa834
4. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. https://doi.org/10.15585/mmwr.mm6944e1
5. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10-16. https://doi.org/10.1016/j.epidem.2015.04.003
6. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. Published online January 8, 2021. https://doi.org/10.1542/peds.2020-048090
7. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. https://doi.org/10.1542/peds.2020-006460
8. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
9. Luca G, Kerckhove KV, Coletti P, et al. The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. https://doi.org/10.1186/s12879-017-2934-3
10. Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378-3387.e11. https://doi.org/10.1016/j.jaip.2020.07.057
11. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. https://doi.org/10.3201/eid2610.201315
12. Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. https://doi.org/10.1038/s41591-020-0962-9
13. Hill RM, Rufino K, Kurian S, Saxena J, Saxena K, Williams L. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. Published online December 16, 2020. https://doi.org/10.1542/peds.2020-029280
14. Flasche S, Edmunds WJ. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. Published online December 8, 2020. https://doi.org/10.1016/S1473-3099(20)30927-0
15. Sehra ST, George M, Wiebe DJ, Fundin S, Baker JF. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US. JAMA Intern Med. Published online August 31, 2020. https://doi.org/10.1001/jamainternmed.2020.4288
In the United States, respiratory illnesses are the most common cause of emergency department (ED) visits and hospitalizations in children.1 In response to the ongoing COVID-19 pandemic, several public health interventions, including school and business closures, stay-at-home orders, and mask mandates, were implemented to limit transmission of SARS-CoV-2.2,3 Studies have shown that children can contribute to the spread of SARS-CoV-2 infections, especially within households.4-6 Recent data suggest that COVID-19, and the associated public health measures enacted to slow its spread, may have affected the transmission of other respiratory pathogens.7 Similarly, the pandemic has likely affected healthcare utilization for nonrespiratory illnesses through adoption of social distancing recommendations, suspension and delays in nonemergent elective care, avoidance of healthcare settings, and the effect of decreased respiratory disease on exacerbation of chronic illness.8 The objective of this study was to examine associations between the COVID-19 pandemic and healthcare utilization for pediatric respiratory and nonrespiratory illnesses at US pediatric hospitals.
METHODS
Study Design
This is a multicenter, cross-sectional study of encounters at 44 pediatric hospitals that reported data to the Pediatric Health Information System (PHIS) database maintained by the Children’s Hospital Association (Lenexa, Kansas).
Study Population
Children 2 months to 18 years of age discharged from ED or inpatient settings with a nonsurgical diagnosis from January 1 to September 30 over a 4-year period (2017-2020) were included.
Exposure
The primary exposure was the 2020 COVID-19 pandemic time, divided into three periods: pre-COVID-19 (January-February 2020, the period prior to the pandemic in the United States), early COVID-19 (March-April 2020, coinciding with the first reported US pediatric case of COVID-19 on March 2, 2020), and COVID-19 (May-September 2020, marked by the implementation of at least two of the following containment measures in every US state: stay-at-home/shelter orders, school closures, nonessential business closures, restaurant closures, or prohibition of gatherings of more than 10 people).2
Outcomes
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and compared using chi-square tests. Continuous variables were summarized as median and interquartile range (IQR) and compared using Wilcoxon rank sum tests. Weekly observed-to-expected (O:E) ratios were calculated for each hospital by dividing the number of observed respiratory illness and nonrespiratory illness encounters in a given week in 2020 (observed) by the average number of encounters for that same week during 2017-2019 (expected). O:E ratios were then aggregated over the three COVID-19 study periods, and 95% confidence intervals were established around mean O:E ratios across individual hospitals. Outcomes were then stratified by respiratory illness subgroups, geographic region, and age. Additional details can be found in the Supplemental Methods in the Appendix.
RESULTS
Study Population
A total of 9,051,980 encounters were included in the study, 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. Median age was 5 years (IQR, 1-11 years), and 52.7% of the population was male (Appendix Table 2 and Appendix Table 3).
Respiratory vs Nonrespiratory Illness During the COVID-19 Pandemic
Over the study period, fewer respiratory and nonrespiratory illness encounters were observed than expected, with a larger decrease in respiratory illness encounters (Table, Appendix Table 4). 

Respiratory Subgroup Analyses
The O:E ratio decreased for all respiratory subgroups over the study period (Table, Appendix Table 4). There were significant differences in specific respiratory subgroups, including asthma, bronchiolitis, croup, influenza, and pneumonia (Appendix Figure 1A). Temporal trends in respiratory encounters were consistent across hospital settings, ages, and geographic regions (Appendix Figure 1B-D). When comparing the with and without COVID-19 subgroups in the “other respiratory illnesses” cohort, other respiratory illness without COVID-19 decreased and remained lower than expected over the rest of the study period, while other respiratory illness with COVID-19 increased markedly during the summer months and declined thereafter (Appendix Figure 2).
All age groups had reductions in respiratory illness encounters during the early COVID-19 and COVID-19 periods, although the decline was less pronounced in the 12- to 17-year-old group (Appendix Figure 1B). Similarly, while all age groups experienced increases in encounters for respiratory illnesses during the summer months, only children in the 12- to 17-year-old group experienced increases beyond pre-COVID-19 levels. Importantly, this increase in respiratory encounters was largely driven by COVID-19 diagnoses (Appendix Figure 3). The trend in nonrespiratory illness encounters stratified by age is shown in Appendix Figure 4.
When patients were stratified by hospital setting, there were no differences between those hospitalized and those discharged from the ED (Appendix Figure 1C). Patterns in respiratory illnesses by geographic location were qualitatively similar until the beginning of the summer 2020, after which geographical variation became more evident (Appendix Figure 1D).
DISCUSSION
In this large, multicenter study evaluating ED visits and hospitalizations for respiratory and nonrespiratory illnesses at US pediatric hospitals during the 2020 COVID-19 pandemic, we found a significant and substantial decrease in healthcare encounters for respiratory illnesses. A rapid and marked decline in encounters for respiratory illness in a relatively short period of time (March 12-April 2) was observed across all hospitals and US regions. Declines were consistent across common respiratory illnesses. More modest, yet still substantial, declines were also observed for nonrespiratory illnesses.
There are likely multiple underlying reasons for the observed reductions. Social distancing measures almost certainly played an important role in interrupting respiratory infection transmission. Rapid reduction in influenza transmission during the early COVID-19 period has been attributed to social distancing measures,3 and influenza transmission in children decreases with school closures.9 It is also possible that some families delayed seeking care at hospitals due to COVID-19, leading to less frequent encounters but more severe illness. The similar decrease in O:E ratio for ED visits and hospitalizations, however, is inconsistent with this explanation.
We also found relative differences in changes in encounters for respiratory illness by age. Adolescents’ levels of respiratory healthcare use declined less and recovered at a faster rate than those of younger children, returning to pre-COVID-19 levels by the end of the study period. The reason for this age differential is likely multifaceted. Infections, such as bronchiolitis and pneumonia, are more likely to be a source of respiratory illness in younger than in older children. It is also likely that disproportionate relaxation of social distancing measures among adolescents, who are known to have a stronger pattern of social interaction, contributed to the faster rise in respiratory illness–related encounters in this age group.11 Adolescents have been reported to be more susceptible to, and more likely to transmit, SARS-CoV-2 compared to younger age groups.12 More modest, albeit similar, age-based changes were observed in encounters for nonrespiratory illnesses
Emerging evidence suggests that school-age children may play an important role in SARS-CoV-2 transmission in the community.4,14 Our finding that, compared to younger children, adolescents had significantly fewer reductions in respiratory illness encounters is concerning. These findings suggest that community-based efforts to help prevent respiratory illnesses, especially COVID-19, should focus on adolescents, who are most likely to maintain social interactions and transmit respiratory infections in the school setting and their households.
This study is limited by the inclusion of only tertiary care children’s hospitals, which may not be nationally representative, and the inability to assess the precise timing of when specific public health interventions were introduced. Moreover, previous studies suggest that social distancing behaviors may have changed even before formal recommendations were enacted.15 Future studies should investigate the local impact of state- and municipality-specific mandates on the burden of COVID-19 and other respiratory illnesses.
The COVID-19 pandemic was associated with substantial reductions in encounters for respiratory diseases, and also with more modest but still sizable reductions in encounters for nonrespiratory diseases. These reductions varied by age. Encounters among adolescents declined less and returned to previous levels faster compared with those of younger children.
ACKNOWLEDGMENT
This publication is dedicated to the memory of our coauthor, Dr. Michael Bendel-Stenzel. Dr. Bendel-Stenzel was dedicated to bettering the lives of children and advancing our knowledge of pediatrics through his research.
In the United States, respiratory illnesses are the most common cause of emergency department (ED) visits and hospitalizations in children.1 In response to the ongoing COVID-19 pandemic, several public health interventions, including school and business closures, stay-at-home orders, and mask mandates, were implemented to limit transmission of SARS-CoV-2.2,3 Studies have shown that children can contribute to the spread of SARS-CoV-2 infections, especially within households.4-6 Recent data suggest that COVID-19, and the associated public health measures enacted to slow its spread, may have affected the transmission of other respiratory pathogens.7 Similarly, the pandemic has likely affected healthcare utilization for nonrespiratory illnesses through adoption of social distancing recommendations, suspension and delays in nonemergent elective care, avoidance of healthcare settings, and the effect of decreased respiratory disease on exacerbation of chronic illness.8 The objective of this study was to examine associations between the COVID-19 pandemic and healthcare utilization for pediatric respiratory and nonrespiratory illnesses at US pediatric hospitals.
METHODS
Study Design
This is a multicenter, cross-sectional study of encounters at 44 pediatric hospitals that reported data to the Pediatric Health Information System (PHIS) database maintained by the Children’s Hospital Association (Lenexa, Kansas).
Study Population
Children 2 months to 18 years of age discharged from ED or inpatient settings with a nonsurgical diagnosis from January 1 to September 30 over a 4-year period (2017-2020) were included.
Exposure
The primary exposure was the 2020 COVID-19 pandemic time, divided into three periods: pre-COVID-19 (January-February 2020, the period prior to the pandemic in the United States), early COVID-19 (March-April 2020, coinciding with the first reported US pediatric case of COVID-19 on March 2, 2020), and COVID-19 (May-September 2020, marked by the implementation of at least two of the following containment measures in every US state: stay-at-home/shelter orders, school closures, nonessential business closures, restaurant closures, or prohibition of gatherings of more than 10 people).2
Outcomes
Statistical Analysis
Categorical variables were summarized using frequencies and percentages and compared using chi-square tests. Continuous variables were summarized as median and interquartile range (IQR) and compared using Wilcoxon rank sum tests. Weekly observed-to-expected (O:E) ratios were calculated for each hospital by dividing the number of observed respiratory illness and nonrespiratory illness encounters in a given week in 2020 (observed) by the average number of encounters for that same week during 2017-2019 (expected). O:E ratios were then aggregated over the three COVID-19 study periods, and 95% confidence intervals were established around mean O:E ratios across individual hospitals. Outcomes were then stratified by respiratory illness subgroups, geographic region, and age. Additional details can be found in the Supplemental Methods in the Appendix.
RESULTS
Study Population
A total of 9,051,980 encounters were included in the study, 6,811,799 with nonrespiratory illnesses and 2,240,181 with respiratory illnesses. Median age was 5 years (IQR, 1-11 years), and 52.7% of the population was male (Appendix Table 2 and Appendix Table 3).
Respiratory vs Nonrespiratory Illness During the COVID-19 Pandemic
Over the study period, fewer respiratory and nonrespiratory illness encounters were observed than expected, with a larger decrease in respiratory illness encounters (Table, Appendix Table 4). 

Respiratory Subgroup Analyses
The O:E ratio decreased for all respiratory subgroups over the study period (Table, Appendix Table 4). There were significant differences in specific respiratory subgroups, including asthma, bronchiolitis, croup, influenza, and pneumonia (Appendix Figure 1A). Temporal trends in respiratory encounters were consistent across hospital settings, ages, and geographic regions (Appendix Figure 1B-D). When comparing the with and without COVID-19 subgroups in the “other respiratory illnesses” cohort, other respiratory illness without COVID-19 decreased and remained lower than expected over the rest of the study period, while other respiratory illness with COVID-19 increased markedly during the summer months and declined thereafter (Appendix Figure 2).
All age groups had reductions in respiratory illness encounters during the early COVID-19 and COVID-19 periods, although the decline was less pronounced in the 12- to 17-year-old group (Appendix Figure 1B). Similarly, while all age groups experienced increases in encounters for respiratory illnesses during the summer months, only children in the 12- to 17-year-old group experienced increases beyond pre-COVID-19 levels. Importantly, this increase in respiratory encounters was largely driven by COVID-19 diagnoses (Appendix Figure 3). The trend in nonrespiratory illness encounters stratified by age is shown in Appendix Figure 4.
When patients were stratified by hospital setting, there were no differences between those hospitalized and those discharged from the ED (Appendix Figure 1C). Patterns in respiratory illnesses by geographic location were qualitatively similar until the beginning of the summer 2020, after which geographical variation became more evident (Appendix Figure 1D).
DISCUSSION
In this large, multicenter study evaluating ED visits and hospitalizations for respiratory and nonrespiratory illnesses at US pediatric hospitals during the 2020 COVID-19 pandemic, we found a significant and substantial decrease in healthcare encounters for respiratory illnesses. A rapid and marked decline in encounters for respiratory illness in a relatively short period of time (March 12-April 2) was observed across all hospitals and US regions. Declines were consistent across common respiratory illnesses. More modest, yet still substantial, declines were also observed for nonrespiratory illnesses.
There are likely multiple underlying reasons for the observed reductions. Social distancing measures almost certainly played an important role in interrupting respiratory infection transmission. Rapid reduction in influenza transmission during the early COVID-19 period has been attributed to social distancing measures,3 and influenza transmission in children decreases with school closures.9 It is also possible that some families delayed seeking care at hospitals due to COVID-19, leading to less frequent encounters but more severe illness. The similar decrease in O:E ratio for ED visits and hospitalizations, however, is inconsistent with this explanation.
We also found relative differences in changes in encounters for respiratory illness by age. Adolescents’ levels of respiratory healthcare use declined less and recovered at a faster rate than those of younger children, returning to pre-COVID-19 levels by the end of the study period. The reason for this age differential is likely multifaceted. Infections, such as bronchiolitis and pneumonia, are more likely to be a source of respiratory illness in younger than in older children. It is also likely that disproportionate relaxation of social distancing measures among adolescents, who are known to have a stronger pattern of social interaction, contributed to the faster rise in respiratory illness–related encounters in this age group.11 Adolescents have been reported to be more susceptible to, and more likely to transmit, SARS-CoV-2 compared to younger age groups.12 More modest, albeit similar, age-based changes were observed in encounters for nonrespiratory illnesses
Emerging evidence suggests that school-age children may play an important role in SARS-CoV-2 transmission in the community.4,14 Our finding that, compared to younger children, adolescents had significantly fewer reductions in respiratory illness encounters is concerning. These findings suggest that community-based efforts to help prevent respiratory illnesses, especially COVID-19, should focus on adolescents, who are most likely to maintain social interactions and transmit respiratory infections in the school setting and their households.
This study is limited by the inclusion of only tertiary care children’s hospitals, which may not be nationally representative, and the inability to assess the precise timing of when specific public health interventions were introduced. Moreover, previous studies suggest that social distancing behaviors may have changed even before formal recommendations were enacted.15 Future studies should investigate the local impact of state- and municipality-specific mandates on the burden of COVID-19 and other respiratory illnesses.
The COVID-19 pandemic was associated with substantial reductions in encounters for respiratory diseases, and also with more modest but still sizable reductions in encounters for nonrespiratory diseases. These reductions varied by age. Encounters among adolescents declined less and returned to previous levels faster compared with those of younger children.
ACKNOWLEDGMENT
This publication is dedicated to the memory of our coauthor, Dr. Michael Bendel-Stenzel. Dr. Bendel-Stenzel was dedicated to bettering the lives of children and advancing our knowledge of pediatrics through his research.
1. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
2. Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324(9):859-870. https://doi.org/10.1001/jama.2020.14348
3. Wiese AD, Everson J, Grijalva CG. Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis. Published online June 20, 2020. https://doi.org/10.1093/cid/ciaa834
4. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. https://doi.org/10.15585/mmwr.mm6944e1
5. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10-16. https://doi.org/10.1016/j.epidem.2015.04.003
6. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. Published online January 8, 2021. https://doi.org/10.1542/peds.2020-048090
7. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. https://doi.org/10.1542/peds.2020-006460
8. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
9. Luca G, Kerckhove KV, Coletti P, et al. The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. https://doi.org/10.1186/s12879-017-2934-3
10. Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378-3387.e11. https://doi.org/10.1016/j.jaip.2020.07.057
11. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. https://doi.org/10.3201/eid2610.201315
12. Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. https://doi.org/10.1038/s41591-020-0962-9
13. Hill RM, Rufino K, Kurian S, Saxena J, Saxena K, Williams L. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. Published online December 16, 2020. https://doi.org/10.1542/peds.2020-029280
14. Flasche S, Edmunds WJ. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. Published online December 8, 2020. https://doi.org/10.1016/S1473-3099(20)30927-0
15. Sehra ST, George M, Wiebe DJ, Fundin S, Baker JF. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US. JAMA Intern Med. Published online August 31, 2020. https://doi.org/10.1001/jamainternmed.2020.4288
1. Leyenaar JK, Ralston SL, Shieh MS, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-749. https://doi.org/10.1002/jhm.2624
2. Auger KA, Shah SS, Richardson T, et al. Association between statewide school closure and COVID-19 incidence and mortality in the US. JAMA. 2020;324(9):859-870. https://doi.org/10.1001/jama.2020.14348
3. Wiese AD, Everson J, Grijalva CG. Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis. Published online June 20, 2020. https://doi.org/10.1093/cid/ciaa834
4. Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. https://doi.org/10.15585/mmwr.mm6944e1
5. Worby CJ, Chaves SS, Wallinga J, Lipsitch M, Finelli L, Goldstein E. On the relative role of different age groups in influenza epidemics. Epidemics. 2015;13:10-16. https://doi.org/10.1016/j.epidem.2015.04.003
6. Zimmerman KO, Akinboyo IC, Brookhart MA, et al. Incidence and secondary transmission of SARS-CoV-2 infections in schools. Pediatrics. Published online January 8, 2021. https://doi.org/10.1542/peds.2020-048090
7. Hatoun J, Correa ET, Donahue SMA, Vernacchio L. Social distancing for COVID-19 and diagnoses of other infectious diseases in children. Pediatrics. 2020;146(4):e2020006460. https://doi.org/10.1542/peds.2020-006460
8. Chaiyachati BH, Agawu A, Zorc JJ, Balamuth F. Trends in pediatric emergency department utilization after institution of coronavirus disease-19 mandatory social distancing. J Pediatr. 2020;226:274-277.e1. https://doi.org/10.1016/j.jpeds.2020.07.048
9. Luca G, Kerckhove KV, Coletti P, et al. The impact of regular school closure on seasonal influenza epidemics: a data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):29. https://doi.org/10.1186/s12879-017-2934-3
10. Taquechel K, Diwadkar AR, Sayed S, et al. Pediatric asthma healthcare utilization, viral testing, and air pollution changes during the COVID-19 pandemic. J Allergy Clin Immunol Pract. 2020;8(10):3378-3387.e11. https://doi.org/10.1016/j.jaip.2020.07.057
11. Park YJ, Choe YJ, Park O, et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. https://doi.org/10.3201/eid2610.201315
12. Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. https://doi.org/10.1038/s41591-020-0962-9
13. Hill RM, Rufino K, Kurian S, Saxena J, Saxena K, Williams L. Suicide ideation and attempts in a pediatric emergency department before and during COVID-19. Pediatrics. Published online December 16, 2020. https://doi.org/10.1542/peds.2020-029280
14. Flasche S, Edmunds WJ. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. Published online December 8, 2020. https://doi.org/10.1016/S1473-3099(20)30927-0
15. Sehra ST, George M, Wiebe DJ, Fundin S, Baker JF. Cell phone activity in categories of places and associations with growth in cases of COVID-19 in the US. JAMA Intern Med. Published online August 31, 2020. https://doi.org/10.1001/jamainternmed.2020.4288
© 2021 Society of Hospital Medicine