User login
Molecular Diagnostic Testing for Hair Loss Currently Limited
When it comes to androgenetic alopecia, female pattern hair loss, and alopecia areata, the role of molecular genetic testing remains limited, but that’s not to say it won’t play a major role in the future, noted Dr. Pedram Yazdan.
In fact, molecular genetic testing will likely play a prominent role with respect to prediction and diagnosis of hair loss, disease severity, and expected response to therapy, he noted.
Genetic factors appear to play a significant role in hair-loss pathogenesis, but the remarkable advances in genomic discovery and molecular diagnostic testing seen in other areas of medicine haven’t quite made their way to this indication (Sem. Cut. Med. Surg. 2012;31:259-67).
"The current gold standard in diagnosis of these alopecias is by clinical history, examination, and, when necessary, scalp biopsy for histopathologic evaluation," wrote Dr. Yazdan of the department of dermatology at Northwestern University, Chicago.
An important role for molecular diagnostics likely lies in the small number of cases in which the diagnosis cannot be ascertained by the existing modalities – cases in which the clinical and histopathologic features of the condition are ambiguous and thus make a definitive diagnosis difficult, Dr. Yazdan noted.
Another role may relate to predicting the course and severity of hair loss, which is currently difficult to accomplish as "there are no reliable and validated clinical or histologic features that can provide patients with prognostic information," he wrote.
"It is conceivable that once the underlying genetic risk profiles of these forms of hair loss are more fully established, this information can potentially be used to aid in more definitively elucidating pathogenesis of the hair loss," which in turn, would aid in the development of diagnostic testing, he noted.
Molecular diagnostic testing for alopecia would also allow for risk stratification in terms of development and severity, and, importantly, would advance the field of pharmacogenetics for alopecia. Currently, treatment options are limited in both number and effectiveness.
Dr. Yazdan described a future in which both therapeutic and targeted preventive therapies, coupled with testing to determine treatment response potential, will allow for personalized treatment of these common and complex conditions, which cause patients substantial anxiety.
He reported having no conflicts of interest.
When it comes to androgenetic alopecia, female pattern hair loss, and alopecia areata, the role of molecular genetic testing remains limited, but that’s not to say it won’t play a major role in the future, noted Dr. Pedram Yazdan.
In fact, molecular genetic testing will likely play a prominent role with respect to prediction and diagnosis of hair loss, disease severity, and expected response to therapy, he noted.
Genetic factors appear to play a significant role in hair-loss pathogenesis, but the remarkable advances in genomic discovery and molecular diagnostic testing seen in other areas of medicine haven’t quite made their way to this indication (Sem. Cut. Med. Surg. 2012;31:259-67).
"The current gold standard in diagnosis of these alopecias is by clinical history, examination, and, when necessary, scalp biopsy for histopathologic evaluation," wrote Dr. Yazdan of the department of dermatology at Northwestern University, Chicago.
An important role for molecular diagnostics likely lies in the small number of cases in which the diagnosis cannot be ascertained by the existing modalities – cases in which the clinical and histopathologic features of the condition are ambiguous and thus make a definitive diagnosis difficult, Dr. Yazdan noted.
Another role may relate to predicting the course and severity of hair loss, which is currently difficult to accomplish as "there are no reliable and validated clinical or histologic features that can provide patients with prognostic information," he wrote.
"It is conceivable that once the underlying genetic risk profiles of these forms of hair loss are more fully established, this information can potentially be used to aid in more definitively elucidating pathogenesis of the hair loss," which in turn, would aid in the development of diagnostic testing, he noted.
Molecular diagnostic testing for alopecia would also allow for risk stratification in terms of development and severity, and, importantly, would advance the field of pharmacogenetics for alopecia. Currently, treatment options are limited in both number and effectiveness.
Dr. Yazdan described a future in which both therapeutic and targeted preventive therapies, coupled with testing to determine treatment response potential, will allow for personalized treatment of these common and complex conditions, which cause patients substantial anxiety.
He reported having no conflicts of interest.
When it comes to androgenetic alopecia, female pattern hair loss, and alopecia areata, the role of molecular genetic testing remains limited, but that’s not to say it won’t play a major role in the future, noted Dr. Pedram Yazdan.
In fact, molecular genetic testing will likely play a prominent role with respect to prediction and diagnosis of hair loss, disease severity, and expected response to therapy, he noted.
Genetic factors appear to play a significant role in hair-loss pathogenesis, but the remarkable advances in genomic discovery and molecular diagnostic testing seen in other areas of medicine haven’t quite made their way to this indication (Sem. Cut. Med. Surg. 2012;31:259-67).
"The current gold standard in diagnosis of these alopecias is by clinical history, examination, and, when necessary, scalp biopsy for histopathologic evaluation," wrote Dr. Yazdan of the department of dermatology at Northwestern University, Chicago.
An important role for molecular diagnostics likely lies in the small number of cases in which the diagnosis cannot be ascertained by the existing modalities – cases in which the clinical and histopathologic features of the condition are ambiguous and thus make a definitive diagnosis difficult, Dr. Yazdan noted.
Another role may relate to predicting the course and severity of hair loss, which is currently difficult to accomplish as "there are no reliable and validated clinical or histologic features that can provide patients with prognostic information," he wrote.
"It is conceivable that once the underlying genetic risk profiles of these forms of hair loss are more fully established, this information can potentially be used to aid in more definitively elucidating pathogenesis of the hair loss," which in turn, would aid in the development of diagnostic testing, he noted.
Molecular diagnostic testing for alopecia would also allow for risk stratification in terms of development and severity, and, importantly, would advance the field of pharmacogenetics for alopecia. Currently, treatment options are limited in both number and effectiveness.
Dr. Yazdan described a future in which both therapeutic and targeted preventive therapies, coupled with testing to determine treatment response potential, will allow for personalized treatment of these common and complex conditions, which cause patients substantial anxiety.
He reported having no conflicts of interest.
Hair Transplantation
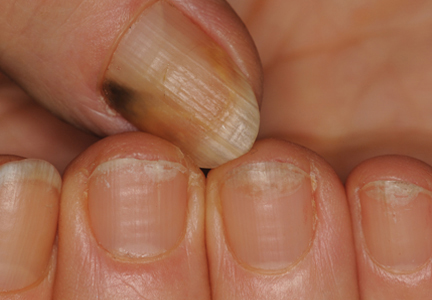
Dots and Lines: A Dermoscopic Sign of Regression of Longitudinal Melanonychia in Children
Subungual Exostosis
Prevalence of Scalp Disorders and Hair Loss in Children
What Is Your Diagnosis? Pressure Alopecia
High-Dose Finasteride Halts Hair Loss in Women
PRAGUE – Off-label use of oral finasteride at 5 mg/day proved safe and effective for the treatment of female pattern hair loss in 43 premenopausal women in an 18-month study.
Treatment effectiveness was assessed in two ways: patient satisfaction scores and two blinded investigators’ evaluation of photographs. As a precondition for study participation, patients needed to have normal serum androgen levels, no clinical signs of hyperandrogenism, and no wish to become pregnant ever again. They also had to go on drospirenone/ethinyl estradiol for oral contraception.
At the 6-month mark, 25 patients (58%) characterized their improvement as "huge" and 14 (33%) as moderate; 4 reported no improvement. These results were stable across time, with the women reporting the same results at 12 and 18 months of follow-up.
The investigators’ blinded assessments of patient photos were less generous: They characterized 19 patients (44%) as very improved, 17 as somewhat improved, and 7 as unimproved.
Diminished libido was reported by 8 patients; 4 had transient nausea or headaches, and 4 reported transient metrorrhagia. One patient had elevated liver function test results and was dropped from the study, Dr. Rui Oliveira-Soares said at the annual congress of the European Academy of Dermatology and Venereology.
"None of us are very pleased with the results we’re having with other drugs," Dr. Oliveira-Soares said. "Sometimes they are unsuccessful or have unacceptable adverse effects. Sometimes there is progression of disease despite every drug we use."
It has been known for more than 15 years that finasteride at 1 mg/day is an effective treatment for male pattern hair loss. It is approved for that indication, as well as for benign prostatic hypertrophy at 5 mg/day.
However, investigators found 12 years ago that finasteride at 1 mg/day is ineffective for female pattern hair loss (J. Am. Acad. Dermatol. 2000;43:768-76). And there have been conflicting reports as to whether the therapy is effective in female androgenetic alopecia at 2.5 mg/day, noted Dr. Oliveira-Soares of Hospital Cuf Descobertas in Lisbon.
Having recently shown in an as-yet-unpublished study that finasteride at a dosage of 5 mg/day was beneficial in postmenopausal women with androgenetic alopecia, Dr. Oliveira-Soares said he sought to learn whether this regimen was safe and effective in premenopausal women affected by the disorder. He reported on 43 patients treated with finasteride at 5 mg/day for 18 months, with formal outcome assessments conducted every 6 months.
Future studies should focus on how to identify likely nonresponders. Also, an 18-month study is not sufficient to draw solid conclusions about the possible long-term risks of extended therapy. An increased risk of breast cancer is a theoretic concern, although there are no clinical data to suggest it is an issue, he said.
The problem in conducting larger, longer-term studies of finasteride at 5 mg/day for female pattern hair loss is that because the drug is available as a relatively inexpensive generic, there is no industry interest in funding such research, he added.
Topical 2% minoxidil is the standard treatment for female pattern hair loss. Among the other drugs used are flutamide and spironolactone, which can have hepatic toxicity, and cyproterone acetate, which can have cardiovascular side effects.
Dr. Oliveira-Soares’ study was supported by hospital research funds. He reported having no relevant financial conflicts.
PRAGUE – Off-label use of oral finasteride at 5 mg/day proved safe and effective for the treatment of female pattern hair loss in 43 premenopausal women in an 18-month study.
Treatment effectiveness was assessed in two ways: patient satisfaction scores and two blinded investigators’ evaluation of photographs. As a precondition for study participation, patients needed to have normal serum androgen levels, no clinical signs of hyperandrogenism, and no wish to become pregnant ever again. They also had to go on drospirenone/ethinyl estradiol for oral contraception.
At the 6-month mark, 25 patients (58%) characterized their improvement as "huge" and 14 (33%) as moderate; 4 reported no improvement. These results were stable across time, with the women reporting the same results at 12 and 18 months of follow-up.
The investigators’ blinded assessments of patient photos were less generous: They characterized 19 patients (44%) as very improved, 17 as somewhat improved, and 7 as unimproved.
Diminished libido was reported by 8 patients; 4 had transient nausea or headaches, and 4 reported transient metrorrhagia. One patient had elevated liver function test results and was dropped from the study, Dr. Rui Oliveira-Soares said at the annual congress of the European Academy of Dermatology and Venereology.
"None of us are very pleased with the results we’re having with other drugs," Dr. Oliveira-Soares said. "Sometimes they are unsuccessful or have unacceptable adverse effects. Sometimes there is progression of disease despite every drug we use."
It has been known for more than 15 years that finasteride at 1 mg/day is an effective treatment for male pattern hair loss. It is approved for that indication, as well as for benign prostatic hypertrophy at 5 mg/day.
However, investigators found 12 years ago that finasteride at 1 mg/day is ineffective for female pattern hair loss (J. Am. Acad. Dermatol. 2000;43:768-76). And there have been conflicting reports as to whether the therapy is effective in female androgenetic alopecia at 2.5 mg/day, noted Dr. Oliveira-Soares of Hospital Cuf Descobertas in Lisbon.
Having recently shown in an as-yet-unpublished study that finasteride at a dosage of 5 mg/day was beneficial in postmenopausal women with androgenetic alopecia, Dr. Oliveira-Soares said he sought to learn whether this regimen was safe and effective in premenopausal women affected by the disorder. He reported on 43 patients treated with finasteride at 5 mg/day for 18 months, with formal outcome assessments conducted every 6 months.
Future studies should focus on how to identify likely nonresponders. Also, an 18-month study is not sufficient to draw solid conclusions about the possible long-term risks of extended therapy. An increased risk of breast cancer is a theoretic concern, although there are no clinical data to suggest it is an issue, he said.
The problem in conducting larger, longer-term studies of finasteride at 5 mg/day for female pattern hair loss is that because the drug is available as a relatively inexpensive generic, there is no industry interest in funding such research, he added.
Topical 2% minoxidil is the standard treatment for female pattern hair loss. Among the other drugs used are flutamide and spironolactone, which can have hepatic toxicity, and cyproterone acetate, which can have cardiovascular side effects.
Dr. Oliveira-Soares’ study was supported by hospital research funds. He reported having no relevant financial conflicts.
PRAGUE – Off-label use of oral finasteride at 5 mg/day proved safe and effective for the treatment of female pattern hair loss in 43 premenopausal women in an 18-month study.
Treatment effectiveness was assessed in two ways: patient satisfaction scores and two blinded investigators’ evaluation of photographs. As a precondition for study participation, patients needed to have normal serum androgen levels, no clinical signs of hyperandrogenism, and no wish to become pregnant ever again. They also had to go on drospirenone/ethinyl estradiol for oral contraception.
At the 6-month mark, 25 patients (58%) characterized their improvement as "huge" and 14 (33%) as moderate; 4 reported no improvement. These results were stable across time, with the women reporting the same results at 12 and 18 months of follow-up.
The investigators’ blinded assessments of patient photos were less generous: They characterized 19 patients (44%) as very improved, 17 as somewhat improved, and 7 as unimproved.
Diminished libido was reported by 8 patients; 4 had transient nausea or headaches, and 4 reported transient metrorrhagia. One patient had elevated liver function test results and was dropped from the study, Dr. Rui Oliveira-Soares said at the annual congress of the European Academy of Dermatology and Venereology.
"None of us are very pleased with the results we’re having with other drugs," Dr. Oliveira-Soares said. "Sometimes they are unsuccessful or have unacceptable adverse effects. Sometimes there is progression of disease despite every drug we use."
It has been known for more than 15 years that finasteride at 1 mg/day is an effective treatment for male pattern hair loss. It is approved for that indication, as well as for benign prostatic hypertrophy at 5 mg/day.
However, investigators found 12 years ago that finasteride at 1 mg/day is ineffective for female pattern hair loss (J. Am. Acad. Dermatol. 2000;43:768-76). And there have been conflicting reports as to whether the therapy is effective in female androgenetic alopecia at 2.5 mg/day, noted Dr. Oliveira-Soares of Hospital Cuf Descobertas in Lisbon.
Having recently shown in an as-yet-unpublished study that finasteride at a dosage of 5 mg/day was beneficial in postmenopausal women with androgenetic alopecia, Dr. Oliveira-Soares said he sought to learn whether this regimen was safe and effective in premenopausal women affected by the disorder. He reported on 43 patients treated with finasteride at 5 mg/day for 18 months, with formal outcome assessments conducted every 6 months.
Future studies should focus on how to identify likely nonresponders. Also, an 18-month study is not sufficient to draw solid conclusions about the possible long-term risks of extended therapy. An increased risk of breast cancer is a theoretic concern, although there are no clinical data to suggest it is an issue, he said.
The problem in conducting larger, longer-term studies of finasteride at 5 mg/day for female pattern hair loss is that because the drug is available as a relatively inexpensive generic, there is no industry interest in funding such research, he added.
Topical 2% minoxidil is the standard treatment for female pattern hair loss. Among the other drugs used are flutamide and spironolactone, which can have hepatic toxicity, and cyproterone acetate, which can have cardiovascular side effects.
Dr. Oliveira-Soares’ study was supported by hospital research funds. He reported having no relevant financial conflicts.
AT THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Of premenopausal women with androgenetic alopecia, 58% reported major improvement in response to oral finasteride at 5 mg/day, and another 33% reported moderate improvement.
Data Source: This was an open-label study of 43 premenopausal women whose hair loss was assessed at 6-month intervals during 18 months of treatment.
Disclosures: Dr. Oliveira-Soares’ study was supported by hospital research funds. He reported having no relevant financial conflicts.
Menkes Syndrome Presenting as Possible Child Abuse
Argan Oil for Dry Hair
We were recently asked by a reader if there is any scientific evidence on the benefits of using argan oil to treat dry hair and scalp.
Argan oil is native to Morocco and has been used for centuries in foods and topical preparations. It is a plant oil produced from the argan tree (Argania Spinosa L). Studies have found that the oil has cardioprotective and anti-thrombotic effects when ingested.
Over the past several years, it has become popular in hair care products. While the benefits of consumption of argan oil have been well-studied, its use for hair has not been documented in peer-reviewed literature.
Argan oil may be used on any hair type. It is available in shampoos, conditioners, and leave-in products. I have found that argan oil is beneficial for patients with curly hair, particularly those of African or African-American descent, because it helps to reduce frizz and adds shine. A small amount may be applied to the scalp if dry.
In patients with fine hair, too much oil can be greasy and may weigh curls down. In those cases, small amounts of the oil may be more beneficial. If too much product is used, clarifying shampoos may help remove excess oil.
The number of personal care products on the U.S. market with argan oil as an ingredient increased from just 2 in 2007 to over 100 in 2011. There are many hair care brands that contain argan oil including Moroccanoil, DermOrganic, Josie Maran, One 'N Only, and Organix, among others.
There has been one report of anaphylaxis to argan oil in the literature (Allergy 2010;65:662–3). Studies must be done to assess its actual efficacy for dermatologic scalp conditions and use for ethnic hair.
- Naissan Wesley, M.D.
Do you have questions about treating patients with darker skin? If so, send them to [email protected].
We were recently asked by a reader if there is any scientific evidence on the benefits of using argan oil to treat dry hair and scalp.
Argan oil is native to Morocco and has been used for centuries in foods and topical preparations. It is a plant oil produced from the argan tree (Argania Spinosa L). Studies have found that the oil has cardioprotective and anti-thrombotic effects when ingested.
Over the past several years, it has become popular in hair care products. While the benefits of consumption of argan oil have been well-studied, its use for hair has not been documented in peer-reviewed literature.
Argan oil may be used on any hair type. It is available in shampoos, conditioners, and leave-in products. I have found that argan oil is beneficial for patients with curly hair, particularly those of African or African-American descent, because it helps to reduce frizz and adds shine. A small amount may be applied to the scalp if dry.
In patients with fine hair, too much oil can be greasy and may weigh curls down. In those cases, small amounts of the oil may be more beneficial. If too much product is used, clarifying shampoos may help remove excess oil.
The number of personal care products on the U.S. market with argan oil as an ingredient increased from just 2 in 2007 to over 100 in 2011. There are many hair care brands that contain argan oil including Moroccanoil, DermOrganic, Josie Maran, One 'N Only, and Organix, among others.
There has been one report of anaphylaxis to argan oil in the literature (Allergy 2010;65:662–3). Studies must be done to assess its actual efficacy for dermatologic scalp conditions and use for ethnic hair.
- Naissan Wesley, M.D.
Do you have questions about treating patients with darker skin? If so, send them to [email protected].
We were recently asked by a reader if there is any scientific evidence on the benefits of using argan oil to treat dry hair and scalp.
Argan oil is native to Morocco and has been used for centuries in foods and topical preparations. It is a plant oil produced from the argan tree (Argania Spinosa L). Studies have found that the oil has cardioprotective and anti-thrombotic effects when ingested.
Over the past several years, it has become popular in hair care products. While the benefits of consumption of argan oil have been well-studied, its use for hair has not been documented in peer-reviewed literature.
Argan oil may be used on any hair type. It is available in shampoos, conditioners, and leave-in products. I have found that argan oil is beneficial for patients with curly hair, particularly those of African or African-American descent, because it helps to reduce frizz and adds shine. A small amount may be applied to the scalp if dry.
In patients with fine hair, too much oil can be greasy and may weigh curls down. In those cases, small amounts of the oil may be more beneficial. If too much product is used, clarifying shampoos may help remove excess oil.
The number of personal care products on the U.S. market with argan oil as an ingredient increased from just 2 in 2007 to over 100 in 2011. There are many hair care brands that contain argan oil including Moroccanoil, DermOrganic, Josie Maran, One 'N Only, and Organix, among others.
There has been one report of anaphylaxis to argan oil in the literature (Allergy 2010;65:662–3). Studies must be done to assess its actual efficacy for dermatologic scalp conditions and use for ethnic hair.
- Naissan Wesley, M.D.
Do you have questions about treating patients with darker skin? If so, send them to [email protected].