User login
Atypical Fibroxanthoma Arising Within Erosive Pustular Dermatosis of the Scalp
Atypical fibroxanthoma (AFX) is a low-grade dermal malignancy comprised of atypical spindle cells.1 Classified as a superficial fibrohistiocytic tumor with intermediate malignant potential, AFX has an incidence of approximately 0.24% worldwide.2 The tumor appears mainly on the head and neck in sun-exposed areas but can occur less frequently on the trunk and limbs in non–sun-exposed areas. There is a 70% to 80% predominance in men aged 69 to 77 years, with lesions primarily occurring in sun-exposed areas of the head and neck.3 A median period of 4 months between time of onset and time of diagnosis has been previously established.4
When AFX does occur in non–sun-exposed areas, it tends to be in a younger patient population. Clinically, it presents as a rather nondescript, firm, erythematous papule or nodule less than 2 cm in diameter. Atypical fibroxanthoma most often presents asymptomatically, but the tumor may ulcerate and bleed, though pain and pruritus are uncommon.5 Findings are nonspecific, and the diagnosis must be confirmed with biopsy, as it can resemble other common dermatological lesions. The pathogenesis of AFX has been controversial. Two different studies looked at AFX using electron microscopy and concluded that the tumor most closely resembled a myofibroblast,6,7 which is consistent with current thinking today.
Atypical fibroxanthoma is believed to be associated with p53 mutation and is closely linked with exposure to UV radiation due to its predominance in sun-exposed areas. Other predisposing factors may include prior exposure to UV radiation, history of organ transplantation, immunosuppression, advanced age in men, and xeroderma pigmentosum. The differential diagnosis for AFX encompasses basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, adnexal tumor, and pyogenic granuloma.
Case Report
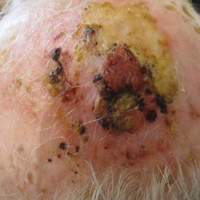
On physical examination, the lesions appeared erosive with crusting and granulation tissue (Figure 1A). The presentation was consistent with erosive pustular dermatosis of the scalp. Biopsy revealed granulation tissue. The patient underwent PDT and prednisone treatment with improvement. Additional biopsies revealed AKs. His condition improved with 2 PDT sessions but never fully cleared. During the PDT sessions, the patient reported intense unilateral headaches without visual changes. The headaches were intermittent and not apparently related to the treatments. He was referred for a temporal artery biopsy and rebiopsy of the remaining lesion on the scalp. The temporal artery biopsy was negative. The lesion that remained was a large nodule on the vertex scalp, and biopsy revealed AFX.
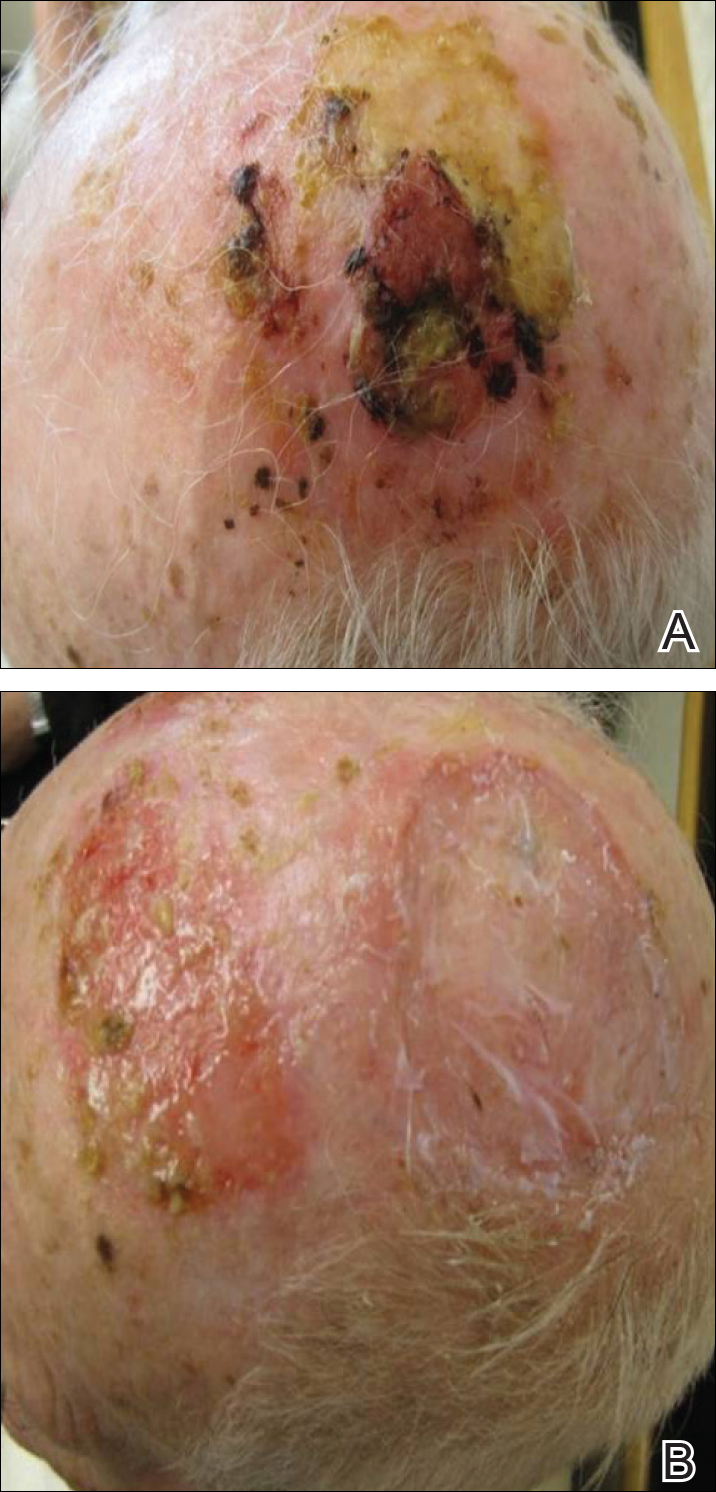
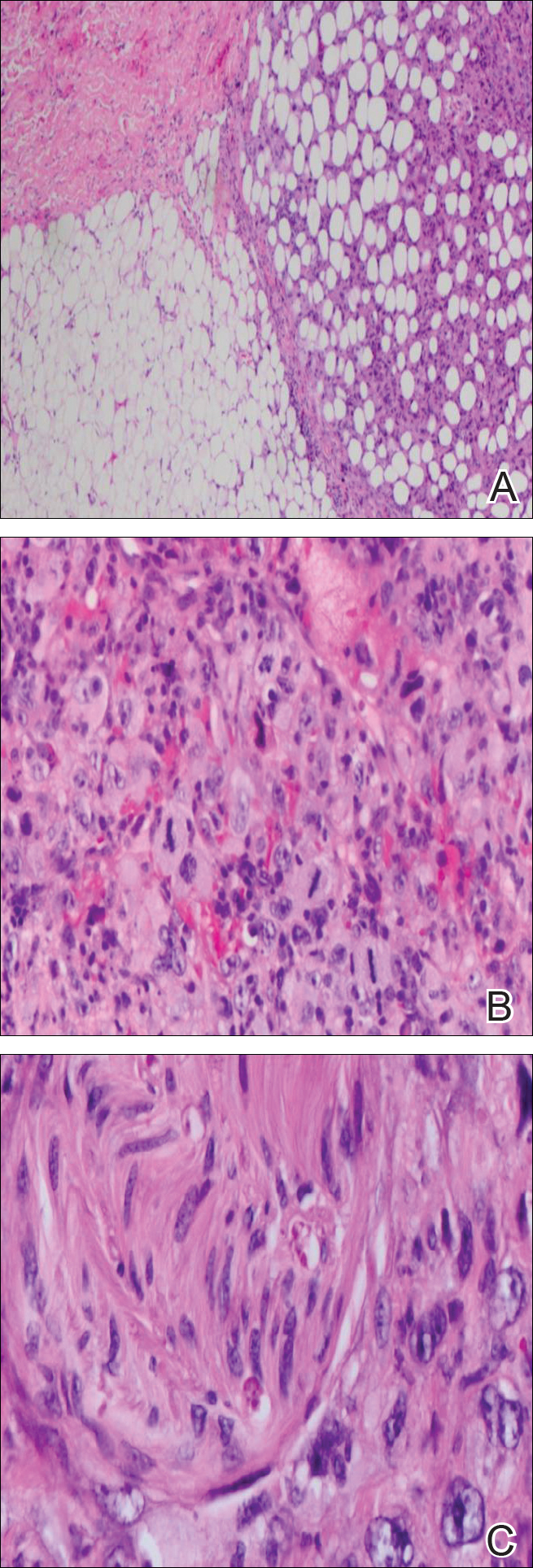
Immunohistochemical marker studies for S-100 and cytokeratin were negative. Invasion into subcutaneous fat was encountered (Figure 2A). Highly atypical spindle cells and mitoses were present (Figure 2B). Neoplastic cells were noted adjacent to nerve (Figure 2C). Excision of the lesion was curative, and his symptoms of pain and erosive pustular dermatosis resolved weeks thereafter (Figure 1B). The area of erosive pustular dermatosis was not excised, but symptoms resolved weeks following excision of the AFX.
Comment
Our case of AFX is unique due to the patient’s atypical presentation of severe pain. Because AFX usually presents asymptomatically, pain is an uncommon symptom. Based on the histologic findings in our case, we suspected that neural involvement of the tumor most likely explained the intense pain that our patient experienced.
The presence of erosive pustular dermatosis of the scalp also is interesting in our case. This elderly man had an extensive history of actinic damage and had reported pustules, scaling, itching, and scabbing of the scalp. It is possible that erosive pustular dermatosis was superimposed over the tumor and could have been the reason that multiple biopsies were needed to eventually arrive at a diagnosis. The coexistence of the 2 entities suggests that the chronic actinic damage played a role in the etiology of both.
Classification
There is a question regarding nomenclature when discussing AFX. Atypical fibroxanthoma has been referred to as a variant of undifferentiated pleomorphic sarcoma, which is a type of soft tissue sarcoma. Atypical fibroxanthoma can be referred to as undifferentiated pleomorphic sarcoma if it is more than 2 cm in diameter, if it involves the fascia or subcutaneous tissue, or if there is evidence of necrosis.3 Atypical fibroxanthoma generally is confined to the head and neck region and usually is less than 2 cm in diameter. In this patient, the presentation was consistent with AFX, as there was evidence of necrosis and invasion into the subcutaneous fat. The fact that the lesion also appeared on the scalp further supported the diagnosis of AFX.
Pathology
Biopsy of AFX typically reveals a spindle cell proliferation that usually arises in the setting of profound actinic damage. The epidermis may or may not be ulcerated, and in most cases, it is seen in close proximity to the overlying epidermis but not arising from it.8 Classic AFX is composed of highly atypical histiocytelike (epithelioid) cells admixed with pleomorphic spindle cells and giant cells, all showing frequent mitoses including atypical ones.9 Several histologic subtypes of AFX have been described, including clear cell, granular cell, pigmented cell, chondroid, osteoid, osteoclastic, and the most common spindle cell subtype.9 Features that indicate potential aggressive behavior include infiltration into the subcutaneous tissue, vascular invasion, and presence of necrosis. A diagnosis of AFX is made by exclusion of other malignant neoplasms with similar morphology, namely spindle cell squamous cell carcinoma, spindle cell melanoma, and leiomyoscarcoma.9 As such, immunohistochemistry plays a critical role in distinguishing these lesions, as they arise as part of the differential diagnosis. A panel of immunohistochemical stains is helpful for diagnosis and commonly includes but is not limited to S-100, Melan-A, smooth muscle actin, desmin, and cytokeratin.
Sampling error is an inherent flaw in any biopsy specimen. The eventual diagnosis of AFX in our case supports the argument for multiple biopsies of an unknown lesion, seeing as the affected area was interpreted as both granulation tissue and AK prior to the eventual diagnosis. Repeat biopsies, especially if a lesion is nonhealing, often can help clinicians arrive at a definitive diagnosis.
Treatment
Different treatment options have been used to manage AFX. Mohs micrographic surgery is most often used because of its tissue-sparing potential, often giving the most cosmetically appealing result. Wide local excision is another surgical technique utilized, generally with fixed margins of at least 1 cm.10 Radiation at the tumor site is used as a treatment method but most often during cases of reoccurrence. Cryotherapy as well as electrodesiccation and curettage are possible treatment options but are not the standard of care.
- Helwig EB. Atypical fibroxanthoma, in tumor seminar. proceedings of 18th Annual Seminar of San Antonio Society of Pathologists, 1961. Tex State J Med. 1963;59:664-667.
- Anderson HL, Joseph AK. A pilot feasibility study of a rare skin tumor database. Dermatol Surg. 2007;33:693-696.
- Iorizzo LJ 3rd, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
- Fretzin DF, Helwig EB. Atypical fibroxanthoma of the skin. a clinicopathologic study of 140 cases. Cancer. 1973;31:1541-1552.
- Vandergriff TW, Reed JA, Orengo IF. An unusual presentation of atypical fibroxanthoma. Dermatol Online J. 2008;14:6.
- Weedon D, Kerr JF. Atypical fibroxanthoma of skin: an electron microscope study. Pathology. 1975;7:173-177.
- Woyke S, Domagala W, Olszewski W, et al. Pseudosarcoma of the skin. an electron microscopic study and comparison with the fine structure of spindle-cell variant of squamous carcinoma. Cancer. 1974;33:970-980.
- Edward S, Yung A. Essential Dermatopathology. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
- Luzar B, Calonje E. Morphologic and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
- González-García R, Nam-Cha SH, Muñoz-Guerra MF, et al. Atypical fibroxanthoma of the head and neck: report of 5 cases. J Oral Maxillofac Surg. 2007;65:526-531.
Atypical fibroxanthoma (AFX) is a low-grade dermal malignancy comprised of atypical spindle cells.1 Classified as a superficial fibrohistiocytic tumor with intermediate malignant potential, AFX has an incidence of approximately 0.24% worldwide.2 The tumor appears mainly on the head and neck in sun-exposed areas but can occur less frequently on the trunk and limbs in non–sun-exposed areas. There is a 70% to 80% predominance in men aged 69 to 77 years, with lesions primarily occurring in sun-exposed areas of the head and neck.3 A median period of 4 months between time of onset and time of diagnosis has been previously established.4
When AFX does occur in non–sun-exposed areas, it tends to be in a younger patient population. Clinically, it presents as a rather nondescript, firm, erythematous papule or nodule less than 2 cm in diameter. Atypical fibroxanthoma most often presents asymptomatically, but the tumor may ulcerate and bleed, though pain and pruritus are uncommon.5 Findings are nonspecific, and the diagnosis must be confirmed with biopsy, as it can resemble other common dermatological lesions. The pathogenesis of AFX has been controversial. Two different studies looked at AFX using electron microscopy and concluded that the tumor most closely resembled a myofibroblast,6,7 which is consistent with current thinking today.
Atypical fibroxanthoma is believed to be associated with p53 mutation and is closely linked with exposure to UV radiation due to its predominance in sun-exposed areas. Other predisposing factors may include prior exposure to UV radiation, history of organ transplantation, immunosuppression, advanced age in men, and xeroderma pigmentosum. The differential diagnosis for AFX encompasses basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, adnexal tumor, and pyogenic granuloma.
Case Report
On physical examination, the lesions appeared erosive with crusting and granulation tissue (Figure 1A). The presentation was consistent with erosive pustular dermatosis of the scalp. Biopsy revealed granulation tissue. The patient underwent PDT and prednisone treatment with improvement. Additional biopsies revealed AKs. His condition improved with 2 PDT sessions but never fully cleared. During the PDT sessions, the patient reported intense unilateral headaches without visual changes. The headaches were intermittent and not apparently related to the treatments. He was referred for a temporal artery biopsy and rebiopsy of the remaining lesion on the scalp. The temporal artery biopsy was negative. The lesion that remained was a large nodule on the vertex scalp, and biopsy revealed AFX.


Immunohistochemical marker studies for S-100 and cytokeratin were negative. Invasion into subcutaneous fat was encountered (Figure 2A). Highly atypical spindle cells and mitoses were present (Figure 2B). Neoplastic cells were noted adjacent to nerve (Figure 2C). Excision of the lesion was curative, and his symptoms of pain and erosive pustular dermatosis resolved weeks thereafter (Figure 1B). The area of erosive pustular dermatosis was not excised, but symptoms resolved weeks following excision of the AFX.
Comment
Our case of AFX is unique due to the patient’s atypical presentation of severe pain. Because AFX usually presents asymptomatically, pain is an uncommon symptom. Based on the histologic findings in our case, we suspected that neural involvement of the tumor most likely explained the intense pain that our patient experienced.
The presence of erosive pustular dermatosis of the scalp also is interesting in our case. This elderly man had an extensive history of actinic damage and had reported pustules, scaling, itching, and scabbing of the scalp. It is possible that erosive pustular dermatosis was superimposed over the tumor and could have been the reason that multiple biopsies were needed to eventually arrive at a diagnosis. The coexistence of the 2 entities suggests that the chronic actinic damage played a role in the etiology of both.
Classification
There is a question regarding nomenclature when discussing AFX. Atypical fibroxanthoma has been referred to as a variant of undifferentiated pleomorphic sarcoma, which is a type of soft tissue sarcoma. Atypical fibroxanthoma can be referred to as undifferentiated pleomorphic sarcoma if it is more than 2 cm in diameter, if it involves the fascia or subcutaneous tissue, or if there is evidence of necrosis.3 Atypical fibroxanthoma generally is confined to the head and neck region and usually is less than 2 cm in diameter. In this patient, the presentation was consistent with AFX, as there was evidence of necrosis and invasion into the subcutaneous fat. The fact that the lesion also appeared on the scalp further supported the diagnosis of AFX.
Pathology
Biopsy of AFX typically reveals a spindle cell proliferation that usually arises in the setting of profound actinic damage. The epidermis may or may not be ulcerated, and in most cases, it is seen in close proximity to the overlying epidermis but not arising from it.8 Classic AFX is composed of highly atypical histiocytelike (epithelioid) cells admixed with pleomorphic spindle cells and giant cells, all showing frequent mitoses including atypical ones.9 Several histologic subtypes of AFX have been described, including clear cell, granular cell, pigmented cell, chondroid, osteoid, osteoclastic, and the most common spindle cell subtype.9 Features that indicate potential aggressive behavior include infiltration into the subcutaneous tissue, vascular invasion, and presence of necrosis. A diagnosis of AFX is made by exclusion of other malignant neoplasms with similar morphology, namely spindle cell squamous cell carcinoma, spindle cell melanoma, and leiomyoscarcoma.9 As such, immunohistochemistry plays a critical role in distinguishing these lesions, as they arise as part of the differential diagnosis. A panel of immunohistochemical stains is helpful for diagnosis and commonly includes but is not limited to S-100, Melan-A, smooth muscle actin, desmin, and cytokeratin.
Sampling error is an inherent flaw in any biopsy specimen. The eventual diagnosis of AFX in our case supports the argument for multiple biopsies of an unknown lesion, seeing as the affected area was interpreted as both granulation tissue and AK prior to the eventual diagnosis. Repeat biopsies, especially if a lesion is nonhealing, often can help clinicians arrive at a definitive diagnosis.
Treatment
Different treatment options have been used to manage AFX. Mohs micrographic surgery is most often used because of its tissue-sparing potential, often giving the most cosmetically appealing result. Wide local excision is another surgical technique utilized, generally with fixed margins of at least 1 cm.10 Radiation at the tumor site is used as a treatment method but most often during cases of reoccurrence. Cryotherapy as well as electrodesiccation and curettage are possible treatment options but are not the standard of care.
Atypical fibroxanthoma (AFX) is a low-grade dermal malignancy comprised of atypical spindle cells.1 Classified as a superficial fibrohistiocytic tumor with intermediate malignant potential, AFX has an incidence of approximately 0.24% worldwide.2 The tumor appears mainly on the head and neck in sun-exposed areas but can occur less frequently on the trunk and limbs in non–sun-exposed areas. There is a 70% to 80% predominance in men aged 69 to 77 years, with lesions primarily occurring in sun-exposed areas of the head and neck.3 A median period of 4 months between time of onset and time of diagnosis has been previously established.4
When AFX does occur in non–sun-exposed areas, it tends to be in a younger patient population. Clinically, it presents as a rather nondescript, firm, erythematous papule or nodule less than 2 cm in diameter. Atypical fibroxanthoma most often presents asymptomatically, but the tumor may ulcerate and bleed, though pain and pruritus are uncommon.5 Findings are nonspecific, and the diagnosis must be confirmed with biopsy, as it can resemble other common dermatological lesions. The pathogenesis of AFX has been controversial. Two different studies looked at AFX using electron microscopy and concluded that the tumor most closely resembled a myofibroblast,6,7 which is consistent with current thinking today.
Atypical fibroxanthoma is believed to be associated with p53 mutation and is closely linked with exposure to UV radiation due to its predominance in sun-exposed areas. Other predisposing factors may include prior exposure to UV radiation, history of organ transplantation, immunosuppression, advanced age in men, and xeroderma pigmentosum. The differential diagnosis for AFX encompasses basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, adnexal tumor, and pyogenic granuloma.
Case Report
On physical examination, the lesions appeared erosive with crusting and granulation tissue (Figure 1A). The presentation was consistent with erosive pustular dermatosis of the scalp. Biopsy revealed granulation tissue. The patient underwent PDT and prednisone treatment with improvement. Additional biopsies revealed AKs. His condition improved with 2 PDT sessions but never fully cleared. During the PDT sessions, the patient reported intense unilateral headaches without visual changes. The headaches were intermittent and not apparently related to the treatments. He was referred for a temporal artery biopsy and rebiopsy of the remaining lesion on the scalp. The temporal artery biopsy was negative. The lesion that remained was a large nodule on the vertex scalp, and biopsy revealed AFX.


Immunohistochemical marker studies for S-100 and cytokeratin were negative. Invasion into subcutaneous fat was encountered (Figure 2A). Highly atypical spindle cells and mitoses were present (Figure 2B). Neoplastic cells were noted adjacent to nerve (Figure 2C). Excision of the lesion was curative, and his symptoms of pain and erosive pustular dermatosis resolved weeks thereafter (Figure 1B). The area of erosive pustular dermatosis was not excised, but symptoms resolved weeks following excision of the AFX.
Comment
Our case of AFX is unique due to the patient’s atypical presentation of severe pain. Because AFX usually presents asymptomatically, pain is an uncommon symptom. Based on the histologic findings in our case, we suspected that neural involvement of the tumor most likely explained the intense pain that our patient experienced.
The presence of erosive pustular dermatosis of the scalp also is interesting in our case. This elderly man had an extensive history of actinic damage and had reported pustules, scaling, itching, and scabbing of the scalp. It is possible that erosive pustular dermatosis was superimposed over the tumor and could have been the reason that multiple biopsies were needed to eventually arrive at a diagnosis. The coexistence of the 2 entities suggests that the chronic actinic damage played a role in the etiology of both.
Classification
There is a question regarding nomenclature when discussing AFX. Atypical fibroxanthoma has been referred to as a variant of undifferentiated pleomorphic sarcoma, which is a type of soft tissue sarcoma. Atypical fibroxanthoma can be referred to as undifferentiated pleomorphic sarcoma if it is more than 2 cm in diameter, if it involves the fascia or subcutaneous tissue, or if there is evidence of necrosis.3 Atypical fibroxanthoma generally is confined to the head and neck region and usually is less than 2 cm in diameter. In this patient, the presentation was consistent with AFX, as there was evidence of necrosis and invasion into the subcutaneous fat. The fact that the lesion also appeared on the scalp further supported the diagnosis of AFX.
Pathology
Biopsy of AFX typically reveals a spindle cell proliferation that usually arises in the setting of profound actinic damage. The epidermis may or may not be ulcerated, and in most cases, it is seen in close proximity to the overlying epidermis but not arising from it.8 Classic AFX is composed of highly atypical histiocytelike (epithelioid) cells admixed with pleomorphic spindle cells and giant cells, all showing frequent mitoses including atypical ones.9 Several histologic subtypes of AFX have been described, including clear cell, granular cell, pigmented cell, chondroid, osteoid, osteoclastic, and the most common spindle cell subtype.9 Features that indicate potential aggressive behavior include infiltration into the subcutaneous tissue, vascular invasion, and presence of necrosis. A diagnosis of AFX is made by exclusion of other malignant neoplasms with similar morphology, namely spindle cell squamous cell carcinoma, spindle cell melanoma, and leiomyoscarcoma.9 As such, immunohistochemistry plays a critical role in distinguishing these lesions, as they arise as part of the differential diagnosis. A panel of immunohistochemical stains is helpful for diagnosis and commonly includes but is not limited to S-100, Melan-A, smooth muscle actin, desmin, and cytokeratin.
Sampling error is an inherent flaw in any biopsy specimen. The eventual diagnosis of AFX in our case supports the argument for multiple biopsies of an unknown lesion, seeing as the affected area was interpreted as both granulation tissue and AK prior to the eventual diagnosis. Repeat biopsies, especially if a lesion is nonhealing, often can help clinicians arrive at a definitive diagnosis.
Treatment
Different treatment options have been used to manage AFX. Mohs micrographic surgery is most often used because of its tissue-sparing potential, often giving the most cosmetically appealing result. Wide local excision is another surgical technique utilized, generally with fixed margins of at least 1 cm.10 Radiation at the tumor site is used as a treatment method but most often during cases of reoccurrence. Cryotherapy as well as electrodesiccation and curettage are possible treatment options but are not the standard of care.
- Helwig EB. Atypical fibroxanthoma, in tumor seminar. proceedings of 18th Annual Seminar of San Antonio Society of Pathologists, 1961. Tex State J Med. 1963;59:664-667.
- Anderson HL, Joseph AK. A pilot feasibility study of a rare skin tumor database. Dermatol Surg. 2007;33:693-696.
- Iorizzo LJ 3rd, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
- Fretzin DF, Helwig EB. Atypical fibroxanthoma of the skin. a clinicopathologic study of 140 cases. Cancer. 1973;31:1541-1552.
- Vandergriff TW, Reed JA, Orengo IF. An unusual presentation of atypical fibroxanthoma. Dermatol Online J. 2008;14:6.
- Weedon D, Kerr JF. Atypical fibroxanthoma of skin: an electron microscope study. Pathology. 1975;7:173-177.
- Woyke S, Domagala W, Olszewski W, et al. Pseudosarcoma of the skin. an electron microscopic study and comparison with the fine structure of spindle-cell variant of squamous carcinoma. Cancer. 1974;33:970-980.
- Edward S, Yung A. Essential Dermatopathology. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
- Luzar B, Calonje E. Morphologic and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
- González-García R, Nam-Cha SH, Muñoz-Guerra MF, et al. Atypical fibroxanthoma of the head and neck: report of 5 cases. J Oral Maxillofac Surg. 2007;65:526-531.
- Helwig EB. Atypical fibroxanthoma, in tumor seminar. proceedings of 18th Annual Seminar of San Antonio Society of Pathologists, 1961. Tex State J Med. 1963;59:664-667.
- Anderson HL, Joseph AK. A pilot feasibility study of a rare skin tumor database. Dermatol Surg. 2007;33:693-696.
- Iorizzo LJ 3rd, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
- Fretzin DF, Helwig EB. Atypical fibroxanthoma of the skin. a clinicopathologic study of 140 cases. Cancer. 1973;31:1541-1552.
- Vandergriff TW, Reed JA, Orengo IF. An unusual presentation of atypical fibroxanthoma. Dermatol Online J. 2008;14:6.
- Weedon D, Kerr JF. Atypical fibroxanthoma of skin: an electron microscope study. Pathology. 1975;7:173-177.
- Woyke S, Domagala W, Olszewski W, et al. Pseudosarcoma of the skin. an electron microscopic study and comparison with the fine structure of spindle-cell variant of squamous carcinoma. Cancer. 1974;33:970-980.
- Edward S, Yung A. Essential Dermatopathology. Philadelphia, PA: Lippincott Williams & Wilkins; 2012.
- Luzar B, Calonje E. Morphologic and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
- González-García R, Nam-Cha SH, Muñoz-Guerra MF, et al. Atypical fibroxanthoma of the head and neck: report of 5 cases. J Oral Maxillofac Surg. 2007;65:526-531.
Practice Points
- Atypical fibroxanthoma predominantly occurs in older men on the head and neck.
- Erosive pustular dermatosis may be a benign entity, but if it does not resolve, continue to rebiopsy, as rare tumors may mimic this condition.
Platelet-rich plasma treatment for hair loss continues to be refined
SAN DIEGO – There is currently no standard protocol for injecting autologous platelet-rich plasma to stimulate hair growth, but the technique appears to be about 50% effective, according to Marc R. Avram, MD.
“I tell patients that this is not FDA [Food and Drug Administration] approved, but we think it to be safe,” said Dr. Avram, clinical professor of dermatology at the Cornell University, New York, said at the annual Masters of Aesthetics Symposium. “We don’t know how well it’s going to work. There are a lot of published data on it, but none of [them are] randomized or controlled long-term.”
In Dr. Avram’s experience, he has found that PRP is a good option for patients with difficult hair loss, such as those who had extensive hair loss after chemotherapy but the hair never grew back in the same fashion, or patients who have failed treatment with finasteride and minoxidil.
Currently, there is no standard protocol for using PRP to stimulate hair growth, but the approach Dr. Avram follows is modeled on his experience of injecting thousands of patients with triamcinolone acetonide (Kenalog) for hair loss every 4-6 weeks. After drawing 20 ccs-30 ccs of blood from the patient, the vial is placed in a centrifuge for 10 minutes, a process that separates PRP from red blood cells. Next, the clinician injects PRP into the deep dermis/superficial subcutaneous tissue of the desired treatment area. An average of 4 ccs-8 ccs is injected during each session.
After three monthly treatments, patients follow up at 3 and 6 months after the last treatment to evaluate efficacy. “All patients are told if there is regrowth or thickening of terminal hair, maintenance treatments will be needed every 6-9 months,” he said.
Published clinical trials of PRP include a follow-up period of 3-12 months and most demonstrate an efficacy in the range of 50%-70%. “It seems to be more effective for earlier stages of hair loss, and there are no known side effects to date,” said Dr. Avram, who has authored five textbooks on hair and cosmetic dermatology. “I had one patient call up to say he thought he had an increase in hair loss 2-3 weeks after treatment, but that’s one patient in a couple hundred. This may be similar to the effect minoxidil has on some patients. I’ve had no other issues with side effects.”
In his opinion, future challenges in the use of PRP for restoring hair loss include better defining optimal candidates for the procedure and establishing a better treatment protocol. “How often should maintenance be done?” he asked. “Is this going to be helpful for alopecia areata and scarring alopecia? Also, we need to determine if finasteride, minoxidil, low-level light laser therapy, or any other medications can enhance PRP efficacy in combination. What’s the optimal combination for patients? We don’t know yet. But I think in the future we will.”
Dr. Avram disclosed that he is a consultant for Restoration Robotics.
SAN DIEGO – There is currently no standard protocol for injecting autologous platelet-rich plasma to stimulate hair growth, but the technique appears to be about 50% effective, according to Marc R. Avram, MD.
“I tell patients that this is not FDA [Food and Drug Administration] approved, but we think it to be safe,” said Dr. Avram, clinical professor of dermatology at the Cornell University, New York, said at the annual Masters of Aesthetics Symposium. “We don’t know how well it’s going to work. There are a lot of published data on it, but none of [them are] randomized or controlled long-term.”
In Dr. Avram’s experience, he has found that PRP is a good option for patients with difficult hair loss, such as those who had extensive hair loss after chemotherapy but the hair never grew back in the same fashion, or patients who have failed treatment with finasteride and minoxidil.
Currently, there is no standard protocol for using PRP to stimulate hair growth, but the approach Dr. Avram follows is modeled on his experience of injecting thousands of patients with triamcinolone acetonide (Kenalog) for hair loss every 4-6 weeks. After drawing 20 ccs-30 ccs of blood from the patient, the vial is placed in a centrifuge for 10 minutes, a process that separates PRP from red blood cells. Next, the clinician injects PRP into the deep dermis/superficial subcutaneous tissue of the desired treatment area. An average of 4 ccs-8 ccs is injected during each session.
After three monthly treatments, patients follow up at 3 and 6 months after the last treatment to evaluate efficacy. “All patients are told if there is regrowth or thickening of terminal hair, maintenance treatments will be needed every 6-9 months,” he said.
Published clinical trials of PRP include a follow-up period of 3-12 months and most demonstrate an efficacy in the range of 50%-70%. “It seems to be more effective for earlier stages of hair loss, and there are no known side effects to date,” said Dr. Avram, who has authored five textbooks on hair and cosmetic dermatology. “I had one patient call up to say he thought he had an increase in hair loss 2-3 weeks after treatment, but that’s one patient in a couple hundred. This may be similar to the effect minoxidil has on some patients. I’ve had no other issues with side effects.”
In his opinion, future challenges in the use of PRP for restoring hair loss include better defining optimal candidates for the procedure and establishing a better treatment protocol. “How often should maintenance be done?” he asked. “Is this going to be helpful for alopecia areata and scarring alopecia? Also, we need to determine if finasteride, minoxidil, low-level light laser therapy, or any other medications can enhance PRP efficacy in combination. What’s the optimal combination for patients? We don’t know yet. But I think in the future we will.”
Dr. Avram disclosed that he is a consultant for Restoration Robotics.
SAN DIEGO – There is currently no standard protocol for injecting autologous platelet-rich plasma to stimulate hair growth, but the technique appears to be about 50% effective, according to Marc R. Avram, MD.
“I tell patients that this is not FDA [Food and Drug Administration] approved, but we think it to be safe,” said Dr. Avram, clinical professor of dermatology at the Cornell University, New York, said at the annual Masters of Aesthetics Symposium. “We don’t know how well it’s going to work. There are a lot of published data on it, but none of [them are] randomized or controlled long-term.”
In Dr. Avram’s experience, he has found that PRP is a good option for patients with difficult hair loss, such as those who had extensive hair loss after chemotherapy but the hair never grew back in the same fashion, or patients who have failed treatment with finasteride and minoxidil.
Currently, there is no standard protocol for using PRP to stimulate hair growth, but the approach Dr. Avram follows is modeled on his experience of injecting thousands of patients with triamcinolone acetonide (Kenalog) for hair loss every 4-6 weeks. After drawing 20 ccs-30 ccs of blood from the patient, the vial is placed in a centrifuge for 10 minutes, a process that separates PRP from red blood cells. Next, the clinician injects PRP into the deep dermis/superficial subcutaneous tissue of the desired treatment area. An average of 4 ccs-8 ccs is injected during each session.
After three monthly treatments, patients follow up at 3 and 6 months after the last treatment to evaluate efficacy. “All patients are told if there is regrowth or thickening of terminal hair, maintenance treatments will be needed every 6-9 months,” he said.
Published clinical trials of PRP include a follow-up period of 3-12 months and most demonstrate an efficacy in the range of 50%-70%. “It seems to be more effective for earlier stages of hair loss, and there are no known side effects to date,” said Dr. Avram, who has authored five textbooks on hair and cosmetic dermatology. “I had one patient call up to say he thought he had an increase in hair loss 2-3 weeks after treatment, but that’s one patient in a couple hundred. This may be similar to the effect minoxidil has on some patients. I’ve had no other issues with side effects.”
In his opinion, future challenges in the use of PRP for restoring hair loss include better defining optimal candidates for the procedure and establishing a better treatment protocol. “How often should maintenance be done?” he asked. “Is this going to be helpful for alopecia areata and scarring alopecia? Also, we need to determine if finasteride, minoxidil, low-level light laser therapy, or any other medications can enhance PRP efficacy in combination. What’s the optimal combination for patients? We don’t know yet. But I think in the future we will.”
Dr. Avram disclosed that he is a consultant for Restoration Robotics.
AT MOAS 2017
Alopecia patients share their struggles
SILVER SPRING, MD. – Alopecia areata patients struggle as much, if not more so, with the social and emotional challenges of the disease as with the physical challenges, according to patients and others who spoke at a public meeting on alopecia areata patient-focused drug development.
Alopecia areata affects as many as 6.8 million individuals in the United States, according to the National Alopecia Areata Foundation (NAAF). However, the particulars of alopecia can vary widely from one person to another; some patients experience total hair loss (alopecia universalis), while others retain eyebrows, eyelashes, or some body hair.
The FDA meeting, held on Sept. 11, is part of the agency’s patient-focused drug development initiative. “We wanted to hear the broader patient’s voice,” Theresa M. Mullin, PhD, director of the FDA’s Office of Strategic Programs, said in her opening remarks. Gary Sherwood, communications director for NAAF, said that the meeting was the culmination of a 5-year effort, begun in 2012 when alopecia areata was named as one of 39 disease categories under consideration for such a meeting. “It is too early to know what the exact results will be … but if the past is any indication, they may be significant. The meeting held with psoriasis yielded FDA approval of a treatment previously denied,” he added in an interview.
Two panel presentations featured patients who discussed their experiences with alopecia; each was followed by a discussion period where patients and family members in the audience were invited to share their experiences.
The “Health Effects and Daily Impacts” panel allowed several patients and their family members the opportunity to identify specific issues that may surprise clinicians.
“One thing I learned was how much the patients are bothered by sweating of the scalp; this can affect what type of head covering, hair piece, or hat/helmet they are able to wear, and thus limits activities,” Dr. Marathe continued. “This is not something I had focused on previously. I will be more inclined to ask about sweating and offer treatments, such as scalp botulinum toxin or aluminum chloride now that I have been alerted to this concern. Also, the challenges of facial makeup such as pencil for eyebrows was another thing that the FDA session brought home for me; I’m more inclined to suggest things such as microblading for eyebrows, or to try treatments like latanoprost for eyebrows/lashes.”
The second panel, “Current Approaches to Treatment,” included a different group of patients who shared stories of treatments that had been successful and those that had not. “The patients at the FDA meeting expressed very eloquently what our patients feel – different treatments may work temporarily and then stop working, which leads to a roller coaster of emotions of hope and disappointment,” A. Yasmine Kirkorian, MD, also a dermatologist at Children’s National Health System, said in an interview. “Patients and physicians would be interested in a treatment option with a track record for predictable efficacy with durable and sustained hair regrowth and minimal side effects.”
Dr. Marathe noted that in her experience, those who develop alopecia totalis or universalis at a younger age tend to have more recalcitrant disease. “It is still very hard for me to predict which children will regrow their hair spontaneously, or with topical therapies, versus those with more resistant disease. I hope that continued study will allow us to offer a more realistic prognosis for these patients,” she said.
Discussion after the treatment panel included testimonials from patients who reported successful treatment with tofacitinib (Xeljanz), a Janus kinase inhibitor approved for rheumatoid arthritis, which is not approved for treatment of alopecia.
“I absolutely agree with the focus on JAK inhibitors and increasing our understanding of how they work, as well as what some of the long-term effects are,” said Dr. Marathe. “The better we are able to target the pathogenesis of this condition, the more easily we can treat in a more focused fashion and reduce side effects,” but more clinical trials are needed to determine safety and efficacy for children and teens, she noted.
One of her hesitations in prescribing tofacitinib to her patients is that she cannot provide them with a sense of how long they will need to be on the treatment. “Current data show that the hair growth on the medication is usually lost upon stopping it; the question I still struggle with is whether it is realistic to put a 4- or 5-year-old on a medication that has no estimated or anticipated stop date,” she said.
As for what she offers patients in terms of resources for emotional support, Dr. Kirkorian said the psychosocial aspects of alopecia areata are always discussed at patient visits. “Psychosocial needs vary based on age, personality, and personal philosophy. We offer the gamut of outside resources from local support groups, the National Alopecia Areata Foundation, referral to psychology/psychiatry and, very importantly, referral to Camp Discovery. Children have told us across the board how important and meaningful it was to them to be able to just be themselves around other children who look like them.”
Dr. Marathe and Dr. Kirkorian were attendees at the meeting; they had no relevant disclosures. They are members of the Dermatology News Editorial Advisory Board.
SILVER SPRING, MD. – Alopecia areata patients struggle as much, if not more so, with the social and emotional challenges of the disease as with the physical challenges, according to patients and others who spoke at a public meeting on alopecia areata patient-focused drug development.
Alopecia areata affects as many as 6.8 million individuals in the United States, according to the National Alopecia Areata Foundation (NAAF). However, the particulars of alopecia can vary widely from one person to another; some patients experience total hair loss (alopecia universalis), while others retain eyebrows, eyelashes, or some body hair.
The FDA meeting, held on Sept. 11, is part of the agency’s patient-focused drug development initiative. “We wanted to hear the broader patient’s voice,” Theresa M. Mullin, PhD, director of the FDA’s Office of Strategic Programs, said in her opening remarks. Gary Sherwood, communications director for NAAF, said that the meeting was the culmination of a 5-year effort, begun in 2012 when alopecia areata was named as one of 39 disease categories under consideration for such a meeting. “It is too early to know what the exact results will be … but if the past is any indication, they may be significant. The meeting held with psoriasis yielded FDA approval of a treatment previously denied,” he added in an interview.
Two panel presentations featured patients who discussed their experiences with alopecia; each was followed by a discussion period where patients and family members in the audience were invited to share their experiences.
The “Health Effects and Daily Impacts” panel allowed several patients and their family members the opportunity to identify specific issues that may surprise clinicians.
“One thing I learned was how much the patients are bothered by sweating of the scalp; this can affect what type of head covering, hair piece, or hat/helmet they are able to wear, and thus limits activities,” Dr. Marathe continued. “This is not something I had focused on previously. I will be more inclined to ask about sweating and offer treatments, such as scalp botulinum toxin or aluminum chloride now that I have been alerted to this concern. Also, the challenges of facial makeup such as pencil for eyebrows was another thing that the FDA session brought home for me; I’m more inclined to suggest things such as microblading for eyebrows, or to try treatments like latanoprost for eyebrows/lashes.”
The second panel, “Current Approaches to Treatment,” included a different group of patients who shared stories of treatments that had been successful and those that had not. “The patients at the FDA meeting expressed very eloquently what our patients feel – different treatments may work temporarily and then stop working, which leads to a roller coaster of emotions of hope and disappointment,” A. Yasmine Kirkorian, MD, also a dermatologist at Children’s National Health System, said in an interview. “Patients and physicians would be interested in a treatment option with a track record for predictable efficacy with durable and sustained hair regrowth and minimal side effects.”
Dr. Marathe noted that in her experience, those who develop alopecia totalis or universalis at a younger age tend to have more recalcitrant disease. “It is still very hard for me to predict which children will regrow their hair spontaneously, or with topical therapies, versus those with more resistant disease. I hope that continued study will allow us to offer a more realistic prognosis for these patients,” she said.
Discussion after the treatment panel included testimonials from patients who reported successful treatment with tofacitinib (Xeljanz), a Janus kinase inhibitor approved for rheumatoid arthritis, which is not approved for treatment of alopecia.
“I absolutely agree with the focus on JAK inhibitors and increasing our understanding of how they work, as well as what some of the long-term effects are,” said Dr. Marathe. “The better we are able to target the pathogenesis of this condition, the more easily we can treat in a more focused fashion and reduce side effects,” but more clinical trials are needed to determine safety and efficacy for children and teens, she noted.
One of her hesitations in prescribing tofacitinib to her patients is that she cannot provide them with a sense of how long they will need to be on the treatment. “Current data show that the hair growth on the medication is usually lost upon stopping it; the question I still struggle with is whether it is realistic to put a 4- or 5-year-old on a medication that has no estimated or anticipated stop date,” she said.
As for what she offers patients in terms of resources for emotional support, Dr. Kirkorian said the psychosocial aspects of alopecia areata are always discussed at patient visits. “Psychosocial needs vary based on age, personality, and personal philosophy. We offer the gamut of outside resources from local support groups, the National Alopecia Areata Foundation, referral to psychology/psychiatry and, very importantly, referral to Camp Discovery. Children have told us across the board how important and meaningful it was to them to be able to just be themselves around other children who look like them.”
Dr. Marathe and Dr. Kirkorian were attendees at the meeting; they had no relevant disclosures. They are members of the Dermatology News Editorial Advisory Board.
SILVER SPRING, MD. – Alopecia areata patients struggle as much, if not more so, with the social and emotional challenges of the disease as with the physical challenges, according to patients and others who spoke at a public meeting on alopecia areata patient-focused drug development.
Alopecia areata affects as many as 6.8 million individuals in the United States, according to the National Alopecia Areata Foundation (NAAF). However, the particulars of alopecia can vary widely from one person to another; some patients experience total hair loss (alopecia universalis), while others retain eyebrows, eyelashes, or some body hair.
The FDA meeting, held on Sept. 11, is part of the agency’s patient-focused drug development initiative. “We wanted to hear the broader patient’s voice,” Theresa M. Mullin, PhD, director of the FDA’s Office of Strategic Programs, said in her opening remarks. Gary Sherwood, communications director for NAAF, said that the meeting was the culmination of a 5-year effort, begun in 2012 when alopecia areata was named as one of 39 disease categories under consideration for such a meeting. “It is too early to know what the exact results will be … but if the past is any indication, they may be significant. The meeting held with psoriasis yielded FDA approval of a treatment previously denied,” he added in an interview.
Two panel presentations featured patients who discussed their experiences with alopecia; each was followed by a discussion period where patients and family members in the audience were invited to share their experiences.
The “Health Effects and Daily Impacts” panel allowed several patients and their family members the opportunity to identify specific issues that may surprise clinicians.
“One thing I learned was how much the patients are bothered by sweating of the scalp; this can affect what type of head covering, hair piece, or hat/helmet they are able to wear, and thus limits activities,” Dr. Marathe continued. “This is not something I had focused on previously. I will be more inclined to ask about sweating and offer treatments, such as scalp botulinum toxin or aluminum chloride now that I have been alerted to this concern. Also, the challenges of facial makeup such as pencil for eyebrows was another thing that the FDA session brought home for me; I’m more inclined to suggest things such as microblading for eyebrows, or to try treatments like latanoprost for eyebrows/lashes.”
The second panel, “Current Approaches to Treatment,” included a different group of patients who shared stories of treatments that had been successful and those that had not. “The patients at the FDA meeting expressed very eloquently what our patients feel – different treatments may work temporarily and then stop working, which leads to a roller coaster of emotions of hope and disappointment,” A. Yasmine Kirkorian, MD, also a dermatologist at Children’s National Health System, said in an interview. “Patients and physicians would be interested in a treatment option with a track record for predictable efficacy with durable and sustained hair regrowth and minimal side effects.”
Dr. Marathe noted that in her experience, those who develop alopecia totalis or universalis at a younger age tend to have more recalcitrant disease. “It is still very hard for me to predict which children will regrow their hair spontaneously, or with topical therapies, versus those with more resistant disease. I hope that continued study will allow us to offer a more realistic prognosis for these patients,” she said.
Discussion after the treatment panel included testimonials from patients who reported successful treatment with tofacitinib (Xeljanz), a Janus kinase inhibitor approved for rheumatoid arthritis, which is not approved for treatment of alopecia.
“I absolutely agree with the focus on JAK inhibitors and increasing our understanding of how they work, as well as what some of the long-term effects are,” said Dr. Marathe. “The better we are able to target the pathogenesis of this condition, the more easily we can treat in a more focused fashion and reduce side effects,” but more clinical trials are needed to determine safety and efficacy for children and teens, she noted.
One of her hesitations in prescribing tofacitinib to her patients is that she cannot provide them with a sense of how long they will need to be on the treatment. “Current data show that the hair growth on the medication is usually lost upon stopping it; the question I still struggle with is whether it is realistic to put a 4- or 5-year-old on a medication that has no estimated or anticipated stop date,” she said.
As for what she offers patients in terms of resources for emotional support, Dr. Kirkorian said the psychosocial aspects of alopecia areata are always discussed at patient visits. “Psychosocial needs vary based on age, personality, and personal philosophy. We offer the gamut of outside resources from local support groups, the National Alopecia Areata Foundation, referral to psychology/psychiatry and, very importantly, referral to Camp Discovery. Children have told us across the board how important and meaningful it was to them to be able to just be themselves around other children who look like them.”
Dr. Marathe and Dr. Kirkorian were attendees at the meeting; they had no relevant disclosures. They are members of the Dermatology News Editorial Advisory Board.
AT AN FDA PUBLIC MEETING
VIDEO: Alopecia areata patients seek emotional support
SILVER SPRING, MD. – The emotional challenges facing alopecia areata patients are as tough, or tougher, than the physical challenges, according to many patients participating in a public meeting on alopecia areata patient-focused drug development.
A panel of patients shared their experiences of living with alopecia areata, including Elizabeth DeCarlo of Wilmington, Delaware. In a video interview at the meeting, held at FDA headquarters on Sept. 11, Ms. DeCarlo elaborated on what she would like clinicians to understand about alopecia patients that might surprise them, and what matters to her as a patient.
“I would tell them to be more compassionate,” Ms. DeCarlo said. “It’s very emotional.” She also emphasized the value of giving alopecia patients information about local support groups, as well as national organizations such as the National Alopecia Areata Foundation.
Ms. DeCarlo had no financial conflicts to disclose.
SILVER SPRING, MD. – The emotional challenges facing alopecia areata patients are as tough, or tougher, than the physical challenges, according to many patients participating in a public meeting on alopecia areata patient-focused drug development.
A panel of patients shared their experiences of living with alopecia areata, including Elizabeth DeCarlo of Wilmington, Delaware. In a video interview at the meeting, held at FDA headquarters on Sept. 11, Ms. DeCarlo elaborated on what she would like clinicians to understand about alopecia patients that might surprise them, and what matters to her as a patient.
“I would tell them to be more compassionate,” Ms. DeCarlo said. “It’s very emotional.” She also emphasized the value of giving alopecia patients information about local support groups, as well as national organizations such as the National Alopecia Areata Foundation.
Ms. DeCarlo had no financial conflicts to disclose.
SILVER SPRING, MD. – The emotional challenges facing alopecia areata patients are as tough, or tougher, than the physical challenges, according to many patients participating in a public meeting on alopecia areata patient-focused drug development.
A panel of patients shared their experiences of living with alopecia areata, including Elizabeth DeCarlo of Wilmington, Delaware. In a video interview at the meeting, held at FDA headquarters on Sept. 11, Ms. DeCarlo elaborated on what she would like clinicians to understand about alopecia patients that might surprise them, and what matters to her as a patient.
“I would tell them to be more compassionate,” Ms. DeCarlo said. “It’s very emotional.” She also emphasized the value of giving alopecia patients information about local support groups, as well as national organizations such as the National Alopecia Areata Foundation.
Ms. DeCarlo had no financial conflicts to disclose.
AT AN FDA PUBLIC MEETING
Maintenance therapy typically required after laser hair removal
REPORTING FROM MOAS 2017
SAN DIEGO – Hair removal ranks as the most popular laser procedure performed in the United States, but patients with blond, red, or gray hairs are out of luck, since those threadlike strands lack a chromophore for the laser to respond to.
“For now, I recommend that these patients get electrolysis or use eflornithine cream,” Arisa Ortiz, MD, said at the annual Masters of Aesthetics Symposium.
Future treatment options for patients with light-colored hair look promising, however. One emerging technology combines laser hair removal with the insertion of a silver nanoparticle into the unpigmented hair follicle. “These are currently in pivotal trials, so we should be seeing them on the market very soon,” she said.
According to Dr. Ortiz, director of laser and cosmetic dermatology in the department of dermatology at the University of California, San Diego, there is still a place for nonlaser hair removal, including shaving, waxing, threading, and electrolysis, but laser hair removal is safe, effective in skilled hands, and permanent. Key factors in optimizing treatment include understanding laser safety and laser-tissue interaction, proper patient selection, preoperative preparation, parameter selection, and recognizing complications.
The first-degree target in laser hair removal is eumelanin contained in the bulb of hair follicles, she said, but the heat must diffuse to a secondary target – follicular stem cells in the bulge of the outer root sheath. “Pulse duration is important,” she said. “The thermal relaxation time of a terminal hair follicle is roughly 100 milliseconds. Longer pulse widths are going to be safer for darker skin types, and you want shorter pulse durations for fine hair, and longer pulse durations for thicker hair. Spot size is also important. Larger spot sizes are faster and create less pain and less epidermal damage.”
Indications include unwanted hair, hypertrichosis, and hirsutism/polycystic ovary syndrome (PCOS). “You want to counsel patients with PCOS properly, because they will require multiple treatments as they tend to make new hair follicles,” she said. Other indications include ingrown hairs, pseudofolliculitis barbae, and pilonidal cysts.
The best candidates for laser hair removal are patients who have a light skin color and dark hair, and those who have thick, coarse hair. “Be cautious when treating tanned patients, and adjust your setting to a longer pulse duration and a lower fluence,” she continued. “I tell (patients) they’ll likely need at least six treatments. You want to treat them every 6 to 8 weeks. If you do treatments sooner than that, it’s probably not cost effective for the patient, because of the way hair follicles cycle. It’s also important that they avoid the sun.”
Clinicians can achieve temporary hair removal with Q-switched lasers, which may be suitable for patients with pseudofolliculitis barbae but who may not want permanent hair removal. “This will just vaporize the actual hair follicle, but that heat is not extending to the stem cells, so it’s temporary hair removal, because the hair follicle transitions into the telogen phase,” Dr. Ortiz explained. “The hair will then grow back after a few months.”
Endpoints are the most important factor for laser hair removal. You want to see perifollicular erythema, perifollicular edema, or hair singeing. “Then you know you have an effective treatment setting,” she said. “Sometimes, however, it takes time for this erythema or edema to develop, so you don’t want to keep increasing your fluences to see this end point. If you’re not comfortable with the laser you’re using, I recommend waiting a few minutes after treatment, and looking for the end point. You could always go higher during the next treatment, if you need to.”
Higher fluences have been correlated with greater permanent hair removal, but also with more side effects. “The recommended treatment settings are going to be the highest possible tolerated fluence that yields the desired endpoint without any adverse effects,” Dr. Ortiz said.
The first hair removal laser to hit the market was the Ruby 694-nm laser, which is safe for Fitzpatrick skin types I-III. A long-term follow-up of the seminal study showed permanent posttreatment efficacy of up to 2 years (Arch Dermatol. 1998;134[7]:837-42). The Alexandrite 755-nm laser, meanwhile, penetrates deeper because it’s a longer wavelength, so there’s less melanin absorption, and it’s safer for darker skin types. “With a device like this, you want to make sure that you’re always holding the laser perpendicular to the skin surface so that your cryogen spray is firing at the same area as the laser. [That way] you don’t get a burn injury,” she said.
The diode at 800 nm and 810 nm penetrates even deeper, which results in less melanin absorption. “Originally these devices had smaller spot sizes, but now some of the newer devices have larger hand pieces and use contact cooling,” she said. “Some of the diode lasers cause singeing and char. The carbon actually sticks onto the sapphire window of the device, so you want to make sure you swipe the window after every few pulses so that you’re not putting the char onto the epidermis and causing an epidermal burn,” Dr. Ortiz advised.
She described the Nd:YAG 1,064-nm laser as the safest for skin types V and VI. It has the deepest penetration but the least melanin absorption. Intense pulsed light (IPL) can also be used for hair removal. IPLs “have a larger spot size, and you can use various cutoff filters to make them safer for darker skin types,” she said. “However, in head-to-head studies, usually laser hair removal does better than IPL.”
Potential complications from laser hair removal include paradoxical hypertrichosis; pigmentary alterations such as hyperpigmentation or hypopigmentation; infections/folliculitis, scarring, and eye injury. Dr. Ortiz underscored the importance of counseling patients about the need for maintenance treatments prior to initiating their first hair removal session. Laser hair removal removes about 85-90% of hairs permanently “so that leaves a significant number that remain, and new hairs may grow over time,” she said.
Authors of a recent study found that the plume release during laser hair removal should be considered a potential biohazard that warrants the use of smoke evacuators and good room ventilation (JAMA Dermatol. 2016;152[12]:1320-26). “We are learning that we should be more careful to evacuate the plume from laser hair removal or wear laser protective masks as the plume may contain harmful chemicals that we breathe in on a daily basis,” said Dr. Ortiz, who was not affiliated with the analysis.
She disclosed serving as a consultant to, receiving equipment from, and/or being a member of the scientific board of several device companies, including Alastin, Allergan, BTL, Cutera, InMode, Merz, Revance, Rodan and Fields, Sciton, and Sienna Biopharmaceuticals.
-[email protected]
REPORTING FROM MOAS 2017
SAN DIEGO – Hair removal ranks as the most popular laser procedure performed in the United States, but patients with blond, red, or gray hairs are out of luck, since those threadlike strands lack a chromophore for the laser to respond to.
“For now, I recommend that these patients get electrolysis or use eflornithine cream,” Arisa Ortiz, MD, said at the annual Masters of Aesthetics Symposium.
Future treatment options for patients with light-colored hair look promising, however. One emerging technology combines laser hair removal with the insertion of a silver nanoparticle into the unpigmented hair follicle. “These are currently in pivotal trials, so we should be seeing them on the market very soon,” she said.
According to Dr. Ortiz, director of laser and cosmetic dermatology in the department of dermatology at the University of California, San Diego, there is still a place for nonlaser hair removal, including shaving, waxing, threading, and electrolysis, but laser hair removal is safe, effective in skilled hands, and permanent. Key factors in optimizing treatment include understanding laser safety and laser-tissue interaction, proper patient selection, preoperative preparation, parameter selection, and recognizing complications.
The first-degree target in laser hair removal is eumelanin contained in the bulb of hair follicles, she said, but the heat must diffuse to a secondary target – follicular stem cells in the bulge of the outer root sheath. “Pulse duration is important,” she said. “The thermal relaxation time of a terminal hair follicle is roughly 100 milliseconds. Longer pulse widths are going to be safer for darker skin types, and you want shorter pulse durations for fine hair, and longer pulse durations for thicker hair. Spot size is also important. Larger spot sizes are faster and create less pain and less epidermal damage.”
Indications include unwanted hair, hypertrichosis, and hirsutism/polycystic ovary syndrome (PCOS). “You want to counsel patients with PCOS properly, because they will require multiple treatments as they tend to make new hair follicles,” she said. Other indications include ingrown hairs, pseudofolliculitis barbae, and pilonidal cysts.
The best candidates for laser hair removal are patients who have a light skin color and dark hair, and those who have thick, coarse hair. “Be cautious when treating tanned patients, and adjust your setting to a longer pulse duration and a lower fluence,” she continued. “I tell (patients) they’ll likely need at least six treatments. You want to treat them every 6 to 8 weeks. If you do treatments sooner than that, it’s probably not cost effective for the patient, because of the way hair follicles cycle. It’s also important that they avoid the sun.”
Clinicians can achieve temporary hair removal with Q-switched lasers, which may be suitable for patients with pseudofolliculitis barbae but who may not want permanent hair removal. “This will just vaporize the actual hair follicle, but that heat is not extending to the stem cells, so it’s temporary hair removal, because the hair follicle transitions into the telogen phase,” Dr. Ortiz explained. “The hair will then grow back after a few months.”
Endpoints are the most important factor for laser hair removal. You want to see perifollicular erythema, perifollicular edema, or hair singeing. “Then you know you have an effective treatment setting,” she said. “Sometimes, however, it takes time for this erythema or edema to develop, so you don’t want to keep increasing your fluences to see this end point. If you’re not comfortable with the laser you’re using, I recommend waiting a few minutes after treatment, and looking for the end point. You could always go higher during the next treatment, if you need to.”
Higher fluences have been correlated with greater permanent hair removal, but also with more side effects. “The recommended treatment settings are going to be the highest possible tolerated fluence that yields the desired endpoint without any adverse effects,” Dr. Ortiz said.
The first hair removal laser to hit the market was the Ruby 694-nm laser, which is safe for Fitzpatrick skin types I-III. A long-term follow-up of the seminal study showed permanent posttreatment efficacy of up to 2 years (Arch Dermatol. 1998;134[7]:837-42). The Alexandrite 755-nm laser, meanwhile, penetrates deeper because it’s a longer wavelength, so there’s less melanin absorption, and it’s safer for darker skin types. “With a device like this, you want to make sure that you’re always holding the laser perpendicular to the skin surface so that your cryogen spray is firing at the same area as the laser. [That way] you don’t get a burn injury,” she said.
The diode at 800 nm and 810 nm penetrates even deeper, which results in less melanin absorption. “Originally these devices had smaller spot sizes, but now some of the newer devices have larger hand pieces and use contact cooling,” she said. “Some of the diode lasers cause singeing and char. The carbon actually sticks onto the sapphire window of the device, so you want to make sure you swipe the window after every few pulses so that you’re not putting the char onto the epidermis and causing an epidermal burn,” Dr. Ortiz advised.
She described the Nd:YAG 1,064-nm laser as the safest for skin types V and VI. It has the deepest penetration but the least melanin absorption. Intense pulsed light (IPL) can also be used for hair removal. IPLs “have a larger spot size, and you can use various cutoff filters to make them safer for darker skin types,” she said. “However, in head-to-head studies, usually laser hair removal does better than IPL.”
Potential complications from laser hair removal include paradoxical hypertrichosis; pigmentary alterations such as hyperpigmentation or hypopigmentation; infections/folliculitis, scarring, and eye injury. Dr. Ortiz underscored the importance of counseling patients about the need for maintenance treatments prior to initiating their first hair removal session. Laser hair removal removes about 85-90% of hairs permanently “so that leaves a significant number that remain, and new hairs may grow over time,” she said.
Authors of a recent study found that the plume release during laser hair removal should be considered a potential biohazard that warrants the use of smoke evacuators and good room ventilation (JAMA Dermatol. 2016;152[12]:1320-26). “We are learning that we should be more careful to evacuate the plume from laser hair removal or wear laser protective masks as the plume may contain harmful chemicals that we breathe in on a daily basis,” said Dr. Ortiz, who was not affiliated with the analysis.
She disclosed serving as a consultant to, receiving equipment from, and/or being a member of the scientific board of several device companies, including Alastin, Allergan, BTL, Cutera, InMode, Merz, Revance, Rodan and Fields, Sciton, and Sienna Biopharmaceuticals.
-[email protected]
REPORTING FROM MOAS 2017
SAN DIEGO – Hair removal ranks as the most popular laser procedure performed in the United States, but patients with blond, red, or gray hairs are out of luck, since those threadlike strands lack a chromophore for the laser to respond to.
“For now, I recommend that these patients get electrolysis or use eflornithine cream,” Arisa Ortiz, MD, said at the annual Masters of Aesthetics Symposium.
Future treatment options for patients with light-colored hair look promising, however. One emerging technology combines laser hair removal with the insertion of a silver nanoparticle into the unpigmented hair follicle. “These are currently in pivotal trials, so we should be seeing them on the market very soon,” she said.
According to Dr. Ortiz, director of laser and cosmetic dermatology in the department of dermatology at the University of California, San Diego, there is still a place for nonlaser hair removal, including shaving, waxing, threading, and electrolysis, but laser hair removal is safe, effective in skilled hands, and permanent. Key factors in optimizing treatment include understanding laser safety and laser-tissue interaction, proper patient selection, preoperative preparation, parameter selection, and recognizing complications.
The first-degree target in laser hair removal is eumelanin contained in the bulb of hair follicles, she said, but the heat must diffuse to a secondary target – follicular stem cells in the bulge of the outer root sheath. “Pulse duration is important,” she said. “The thermal relaxation time of a terminal hair follicle is roughly 100 milliseconds. Longer pulse widths are going to be safer for darker skin types, and you want shorter pulse durations for fine hair, and longer pulse durations for thicker hair. Spot size is also important. Larger spot sizes are faster and create less pain and less epidermal damage.”
Indications include unwanted hair, hypertrichosis, and hirsutism/polycystic ovary syndrome (PCOS). “You want to counsel patients with PCOS properly, because they will require multiple treatments as they tend to make new hair follicles,” she said. Other indications include ingrown hairs, pseudofolliculitis barbae, and pilonidal cysts.
The best candidates for laser hair removal are patients who have a light skin color and dark hair, and those who have thick, coarse hair. “Be cautious when treating tanned patients, and adjust your setting to a longer pulse duration and a lower fluence,” she continued. “I tell (patients) they’ll likely need at least six treatments. You want to treat them every 6 to 8 weeks. If you do treatments sooner than that, it’s probably not cost effective for the patient, because of the way hair follicles cycle. It’s also important that they avoid the sun.”
Clinicians can achieve temporary hair removal with Q-switched lasers, which may be suitable for patients with pseudofolliculitis barbae but who may not want permanent hair removal. “This will just vaporize the actual hair follicle, but that heat is not extending to the stem cells, so it’s temporary hair removal, because the hair follicle transitions into the telogen phase,” Dr. Ortiz explained. “The hair will then grow back after a few months.”
Endpoints are the most important factor for laser hair removal. You want to see perifollicular erythema, perifollicular edema, or hair singeing. “Then you know you have an effective treatment setting,” she said. “Sometimes, however, it takes time for this erythema or edema to develop, so you don’t want to keep increasing your fluences to see this end point. If you’re not comfortable with the laser you’re using, I recommend waiting a few minutes after treatment, and looking for the end point. You could always go higher during the next treatment, if you need to.”
Higher fluences have been correlated with greater permanent hair removal, but also with more side effects. “The recommended treatment settings are going to be the highest possible tolerated fluence that yields the desired endpoint without any adverse effects,” Dr. Ortiz said.
The first hair removal laser to hit the market was the Ruby 694-nm laser, which is safe for Fitzpatrick skin types I-III. A long-term follow-up of the seminal study showed permanent posttreatment efficacy of up to 2 years (Arch Dermatol. 1998;134[7]:837-42). The Alexandrite 755-nm laser, meanwhile, penetrates deeper because it’s a longer wavelength, so there’s less melanin absorption, and it’s safer for darker skin types. “With a device like this, you want to make sure that you’re always holding the laser perpendicular to the skin surface so that your cryogen spray is firing at the same area as the laser. [That way] you don’t get a burn injury,” she said.
The diode at 800 nm and 810 nm penetrates even deeper, which results in less melanin absorption. “Originally these devices had smaller spot sizes, but now some of the newer devices have larger hand pieces and use contact cooling,” she said. “Some of the diode lasers cause singeing and char. The carbon actually sticks onto the sapphire window of the device, so you want to make sure you swipe the window after every few pulses so that you’re not putting the char onto the epidermis and causing an epidermal burn,” Dr. Ortiz advised.
She described the Nd:YAG 1,064-nm laser as the safest for skin types V and VI. It has the deepest penetration but the least melanin absorption. Intense pulsed light (IPL) can also be used for hair removal. IPLs “have a larger spot size, and you can use various cutoff filters to make them safer for darker skin types,” she said. “However, in head-to-head studies, usually laser hair removal does better than IPL.”
Potential complications from laser hair removal include paradoxical hypertrichosis; pigmentary alterations such as hyperpigmentation or hypopigmentation; infections/folliculitis, scarring, and eye injury. Dr. Ortiz underscored the importance of counseling patients about the need for maintenance treatments prior to initiating their first hair removal session. Laser hair removal removes about 85-90% of hairs permanently “so that leaves a significant number that remain, and new hairs may grow over time,” she said.
Authors of a recent study found that the plume release during laser hair removal should be considered a potential biohazard that warrants the use of smoke evacuators and good room ventilation (JAMA Dermatol. 2016;152[12]:1320-26). “We are learning that we should be more careful to evacuate the plume from laser hair removal or wear laser protective masks as the plume may contain harmful chemicals that we breathe in on a daily basis,” said Dr. Ortiz, who was not affiliated with the analysis.
She disclosed serving as a consultant to, receiving equipment from, and/or being a member of the scientific board of several device companies, including Alastin, Allergan, BTL, Cutera, InMode, Merz, Revance, Rodan and Fields, Sciton, and Sienna Biopharmaceuticals.
-[email protected]
Cosmetic Corner: Dermatologists Weigh in on Men’s Products
To improve patient care and outcomes, leading dermatologists offered their recommendations on men’s products. Consideration must be given to:
- bareMinerals SPF 30 Natural Sunscreen
Bare Escentuals Beauty, Inc
“I recommend this product to my male patients when they are not wearing a hat. It protects the scalp from UV damage without a heavy greasy finish.”—Shari Lipner, MD, PhD, New York, New York
- Ducray Alopexy 5% For Men
Pierre Fabre Laboratories
“This dermatologist-dispensed product for men addresses chronic hair loss as well as thinning hair. It contains an optimal level of minoxidil 5% in an elegant unscented formulation and is designed to spray on smoothly and evenly.”—Jeannette Graf, MD, Great Neck, New York
- Facial Fuel Energizing Scrub
Kiehl’s
“This product is great for oily skin and enlarged pores. The particles in the product allow one to get a deep-clean feeling.”—Gary Goldenberg, MD, New York, New York
- Physical Matte UV Defense SPF 50
SkinCeuticals
“For men I like to keep things simple. I recommend what I use with the single most important thing being daily sun protection. SkinCeuticals Physical Matte UV Defense SPF 50 is my favorite and I use it after I shave. It goes on smoothly and has a natural tint along with a high SPF.”—Jerome Potozkin, MD, Danville, California
- Ultimate Brushless Shave Cream
Kiehl’s
“I recommend this product for men with frequent irritation from shaving. This cream-based product helps to provide a close shave without as much irritation from other gel-based products. A small amount goes a long way!”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments and cleansing devices will be featured in upcoming editions of Cosmetic Corner. Please email your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on men’s products. Consideration must be given to:
- bareMinerals SPF 30 Natural Sunscreen
Bare Escentuals Beauty, Inc
“I recommend this product to my male patients when they are not wearing a hat. It protects the scalp from UV damage without a heavy greasy finish.”—Shari Lipner, MD, PhD, New York, New York
- Ducray Alopexy 5% For Men
Pierre Fabre Laboratories
“This dermatologist-dispensed product for men addresses chronic hair loss as well as thinning hair. It contains an optimal level of minoxidil 5% in an elegant unscented formulation and is designed to spray on smoothly and evenly.”—Jeannette Graf, MD, Great Neck, New York
- Facial Fuel Energizing Scrub
Kiehl’s
“This product is great for oily skin and enlarged pores. The particles in the product allow one to get a deep-clean feeling.”—Gary Goldenberg, MD, New York, New York
- Physical Matte UV Defense SPF 50
SkinCeuticals
“For men I like to keep things simple. I recommend what I use with the single most important thing being daily sun protection. SkinCeuticals Physical Matte UV Defense SPF 50 is my favorite and I use it after I shave. It goes on smoothly and has a natural tint along with a high SPF.”—Jerome Potozkin, MD, Danville, California
- Ultimate Brushless Shave Cream
Kiehl’s
“I recommend this product for men with frequent irritation from shaving. This cream-based product helps to provide a close shave without as much irritation from other gel-based products. A small amount goes a long way!”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments and cleansing devices will be featured in upcoming editions of Cosmetic Corner. Please email your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on men’s products. Consideration must be given to:
- bareMinerals SPF 30 Natural Sunscreen
Bare Escentuals Beauty, Inc
“I recommend this product to my male patients when they are not wearing a hat. It protects the scalp from UV damage without a heavy greasy finish.”—Shari Lipner, MD, PhD, New York, New York
- Ducray Alopexy 5% For Men
Pierre Fabre Laboratories
“This dermatologist-dispensed product for men addresses chronic hair loss as well as thinning hair. It contains an optimal level of minoxidil 5% in an elegant unscented formulation and is designed to spray on smoothly and evenly.”—Jeannette Graf, MD, Great Neck, New York
- Facial Fuel Energizing Scrub
Kiehl’s
“This product is great for oily skin and enlarged pores. The particles in the product allow one to get a deep-clean feeling.”—Gary Goldenberg, MD, New York, New York
- Physical Matte UV Defense SPF 50
SkinCeuticals
“For men I like to keep things simple. I recommend what I use with the single most important thing being daily sun protection. SkinCeuticals Physical Matte UV Defense SPF 50 is my favorite and I use it after I shave. It goes on smoothly and has a natural tint along with a high SPF.”—Jerome Potozkin, MD, Danville, California
- Ultimate Brushless Shave Cream
Kiehl’s
“I recommend this product for men with frequent irritation from shaving. This cream-based product helps to provide a close shave without as much irritation from other gel-based products. A small amount goes a long way!”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments and cleansing devices will be featured in upcoming editions of Cosmetic Corner. Please email your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Solitary Nodule With White Hairs
The Diagnosis: Trichofolliculoma
Microscopic examination revealed a dilated cystic follicle that communicated with the skin surface (Figure). The follicle was lined with squamous epithelium and surrounded by numerous secondary follicles, many of which contained a hair shaft. A diagnosis of trichofolliculoma was made.
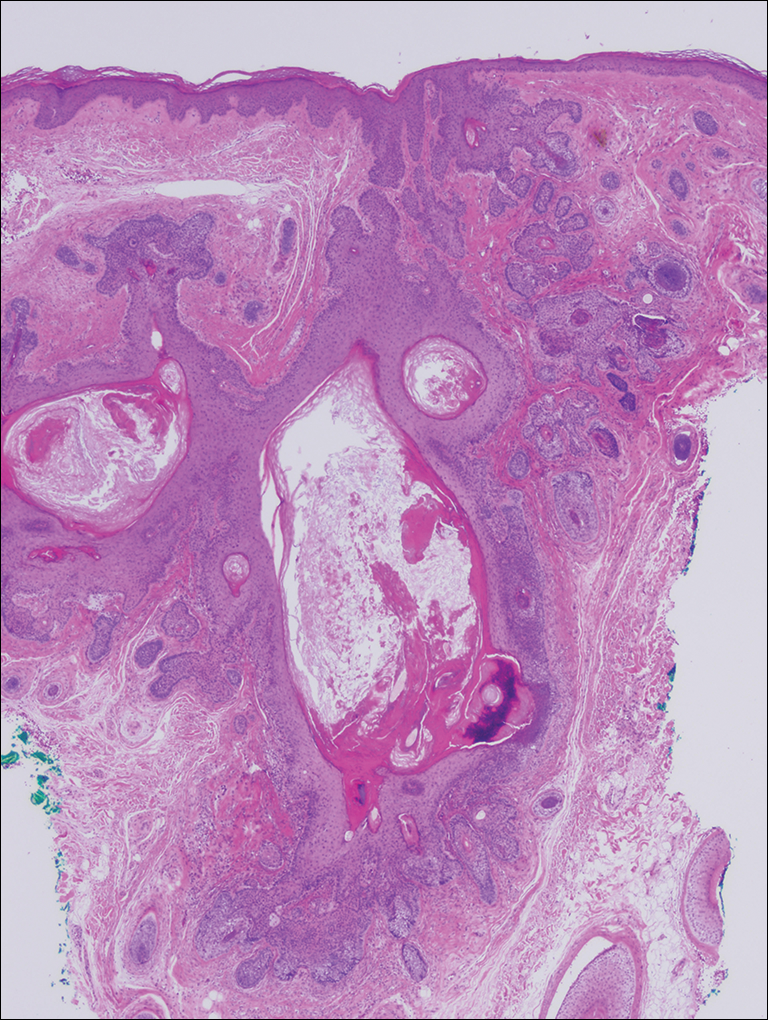
Clinically, the differential diagnosis of a flesh-colored papule on the scalp with prominent follicle includes dilated pore of Winer, epidermoid cyst, pilar sheath acanthoma, and trichoepithelioma.1,2 Multiple hair shafts present in a single follicle may be seen in pili multigemini, tufted folliculitis, trichostasis spinulosa, and trichofolliculoma. On histopathologic examination, a dilated central follicle surrounded with smaller secondary follicles was identified, consistent with trichofolliculoma.
Trichofolliculoma is a rare follicular hamartoma typically occurring on the face, scalp, or trunk as a solitary papule or nodule due to the proliferation of abnormal hair follicle stem cells.3,4 It may present as a flesh-colored nodule with a central pore that may drain sebum or contain white vellus hairs. Trichofolliculoma is considered a benign entity, despite one case report of malignant transformation.5 Biopsy is diagnostic and no further treatment is needed. Recurrence rarely occurs at the primary site after surgical excision, which may be performed for cosmetic purposes or to alleviate functional impairment.
- Ghosh SK, Bandyopadhyay D, Barma KD. Perifollicular nodule on the face of a young man. Indian J Dermatol Venereol Leprol. 2011;77:531-533.
- Gokalp H, Gurer MA, Alan S. Trichofolliculoma: a rare variant of hair follicle hamartoma. Dermatol Online J. 2013;19:19264.
- Choi CM, Lew BL, Sim WY. Multiple trichofolliculomas on unusual sites: a case report and review of the literature. Int J Dermatol. 2013;52:87-89.
- Misago N, Kimura T, Toda S, et al. A revaluation of trichofolliculoma: the histopathological and immunohistochemical features. Am J Dermatopathol. 2010;32:35-43.
- Stem JB, Stout DA. Trichofolliculoma showing perineural invasion. trichofolliculocarcinoma? Arch Dermatol. 1979;115:1003-1004.
The Diagnosis: Trichofolliculoma
Microscopic examination revealed a dilated cystic follicle that communicated with the skin surface (Figure). The follicle was lined with squamous epithelium and surrounded by numerous secondary follicles, many of which contained a hair shaft. A diagnosis of trichofolliculoma was made.

Clinically, the differential diagnosis of a flesh-colored papule on the scalp with prominent follicle includes dilated pore of Winer, epidermoid cyst, pilar sheath acanthoma, and trichoepithelioma.1,2 Multiple hair shafts present in a single follicle may be seen in pili multigemini, tufted folliculitis, trichostasis spinulosa, and trichofolliculoma. On histopathologic examination, a dilated central follicle surrounded with smaller secondary follicles was identified, consistent with trichofolliculoma.
Trichofolliculoma is a rare follicular hamartoma typically occurring on the face, scalp, or trunk as a solitary papule or nodule due to the proliferation of abnormal hair follicle stem cells.3,4 It may present as a flesh-colored nodule with a central pore that may drain sebum or contain white vellus hairs. Trichofolliculoma is considered a benign entity, despite one case report of malignant transformation.5 Biopsy is diagnostic and no further treatment is needed. Recurrence rarely occurs at the primary site after surgical excision, which may be performed for cosmetic purposes or to alleviate functional impairment.
The Diagnosis: Trichofolliculoma
Microscopic examination revealed a dilated cystic follicle that communicated with the skin surface (Figure). The follicle was lined with squamous epithelium and surrounded by numerous secondary follicles, many of which contained a hair shaft. A diagnosis of trichofolliculoma was made.

Clinically, the differential diagnosis of a flesh-colored papule on the scalp with prominent follicle includes dilated pore of Winer, epidermoid cyst, pilar sheath acanthoma, and trichoepithelioma.1,2 Multiple hair shafts present in a single follicle may be seen in pili multigemini, tufted folliculitis, trichostasis spinulosa, and trichofolliculoma. On histopathologic examination, a dilated central follicle surrounded with smaller secondary follicles was identified, consistent with trichofolliculoma.
Trichofolliculoma is a rare follicular hamartoma typically occurring on the face, scalp, or trunk as a solitary papule or nodule due to the proliferation of abnormal hair follicle stem cells.3,4 It may present as a flesh-colored nodule with a central pore that may drain sebum or contain white vellus hairs. Trichofolliculoma is considered a benign entity, despite one case report of malignant transformation.5 Biopsy is diagnostic and no further treatment is needed. Recurrence rarely occurs at the primary site after surgical excision, which may be performed for cosmetic purposes or to alleviate functional impairment.
- Ghosh SK, Bandyopadhyay D, Barma KD. Perifollicular nodule on the face of a young man. Indian J Dermatol Venereol Leprol. 2011;77:531-533.
- Gokalp H, Gurer MA, Alan S. Trichofolliculoma: a rare variant of hair follicle hamartoma. Dermatol Online J. 2013;19:19264.
- Choi CM, Lew BL, Sim WY. Multiple trichofolliculomas on unusual sites: a case report and review of the literature. Int J Dermatol. 2013;52:87-89.
- Misago N, Kimura T, Toda S, et al. A revaluation of trichofolliculoma: the histopathological and immunohistochemical features. Am J Dermatopathol. 2010;32:35-43.
- Stem JB, Stout DA. Trichofolliculoma showing perineural invasion. trichofolliculocarcinoma? Arch Dermatol. 1979;115:1003-1004.
- Ghosh SK, Bandyopadhyay D, Barma KD. Perifollicular nodule on the face of a young man. Indian J Dermatol Venereol Leprol. 2011;77:531-533.
- Gokalp H, Gurer MA, Alan S. Trichofolliculoma: a rare variant of hair follicle hamartoma. Dermatol Online J. 2013;19:19264.
- Choi CM, Lew BL, Sim WY. Multiple trichofolliculomas on unusual sites: a case report and review of the literature. Int J Dermatol. 2013;52:87-89.
- Misago N, Kimura T, Toda S, et al. A revaluation of trichofolliculoma: the histopathological and immunohistochemical features. Am J Dermatopathol. 2010;32:35-43.
- Stem JB, Stout DA. Trichofolliculoma showing perineural invasion. trichofolliculocarcinoma? Arch Dermatol. 1979;115:1003-1004.
A 72-year-old man presented with a new asymptomatic 0.7-cm flesh-colored papule with a central tuft of white hairs on the posterior scalp. The remainder of the physical examination was unremarkable. Biopsy for histopathologic examination was performed to confirm diagnosis.
Alopecia may be permanent in one in four pediatric HSCT patients
CHICAGO – Late dermatologic manifestations in children who have received hematopoietic stem cell transplants may be more common than previously thought, according to results of a new study.
Johanna Song, MD, and her collaborators reported that, in their prospective pediatric study, 25% of patients had permanent alopecia and 16% had psoriasis, noting that late nonmalignant skin effects of hematopoietic stem cell transplantation (HSCT) have been studied primarily retrospectively, and in adults. Vitiligo and nail changes were also seen.
In a poster presentation at the World Congress of Dermatology, Dr. Song and her colleagues noted that these figures are higher than the previously reported pediatric rates of 1.7% for vitiligo and 15.6% for permanent alopecia. “Early recognition of these late effects can facilitate prompt and appropriate treatment, if desired,” they said.
The single-center, cross-sectional cohort study tracked pediatric patients over an 18-month period and included patients who were at least 1 year post allogeneic HSCT and had not relapsed. Patients who were not English speaking were excluded.
The median age of the 85 patients enrolled in the study was 13.8 years, and participants were a median of 3.6 years post transplant at the time of enrollment. The study’s analysis attempted to determine which patient, transplant, and disease factors might be associated with the late nonmalignant skin changes, according to Dr. Song, a resident dermatologist at Harvard University, Boston, and her colleagues.
Most – 52– of the patients (61.2%) had hematologic malignancies; 12 patients (14.1%) received their transplant for bone marrow failure, and 11 patients (12.5%) had immunodeficiency. Three patients (3.5%) received HSCT for other malignancies, and seven (8.2%) for nonmalignant diseases.
Diffuse hair thinning was seen in 13 (62%) of the 21 patients who had alopecia, while 11 (52%) of the patients with alopecia had an androgenetic hair loss pattern. Chronic graft versus host disease (GVHD), skin chronic GVHD, a HSCT regimen that included busulfan conditioning, and a family history of early male-pattern alopecia were all significantly associated with post-HSCT permanent alopecia (P less than .05 for all).
The patients with androgenetic alopecia may be experiencing an accelerated time course of a condition to which they are already genetically disposed, noted Dr. Song and her colleagues.
Psoriasis was commonly seen on the scalp, affecting 11 of the 14 patients with psoriasis (79%), and involved the face in five of the patients (36%). Just one patient had psoriasis elsewhere on the body. There was a nonsignificant trend towards human leukocyte antigen mismatch among patients who had psoriasis. Although “psoriasis may be a marker of persistent immune dysregulation,” the investigators said, they did not identify any associated risk factors that would point toward this mechanism in their analysis.
Twelve patients (14%) had vitiligo, with halo nevi seen in four of these patients. Children who were younger than age 10 years and those who received their transplant for primary immunodeficiency were significantly more likely to have vitiligo (P less than .05 for both). Specific possible mechanisms triggering vitiligo could include thymic dysfunction resulting in loss of self-tolerance, and donor alloreactivity against the patient’s host antigens, according to the authors.
Nail changes such as pterygium and nail pitting, ridging, or thickening were seen in just five patients, all of whom had chronic GVHD of the skin. “Nail changes are likely a result of persistent inflammation and immune dysregulation from chronic GVHD,” said Dr. Song.
These late effects “can significantly impact patients’ quality of life,” according to the authors, who called for larger studies that follow pediatric HSCT patients longitudinally, beginning before the transplant – and for more investigation into the pathogenesis of specific late effects.
Dr. Song reported no relevant conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Late dermatologic manifestations in children who have received hematopoietic stem cell transplants may be more common than previously thought, according to results of a new study.
Johanna Song, MD, and her collaborators reported that, in their prospective pediatric study, 25% of patients had permanent alopecia and 16% had psoriasis, noting that late nonmalignant skin effects of hematopoietic stem cell transplantation (HSCT) have been studied primarily retrospectively, and in adults. Vitiligo and nail changes were also seen.
In a poster presentation at the World Congress of Dermatology, Dr. Song and her colleagues noted that these figures are higher than the previously reported pediatric rates of 1.7% for vitiligo and 15.6% for permanent alopecia. “Early recognition of these late effects can facilitate prompt and appropriate treatment, if desired,” they said.
The single-center, cross-sectional cohort study tracked pediatric patients over an 18-month period and included patients who were at least 1 year post allogeneic HSCT and had not relapsed. Patients who were not English speaking were excluded.
The median age of the 85 patients enrolled in the study was 13.8 years, and participants were a median of 3.6 years post transplant at the time of enrollment. The study’s analysis attempted to determine which patient, transplant, and disease factors might be associated with the late nonmalignant skin changes, according to Dr. Song, a resident dermatologist at Harvard University, Boston, and her colleagues.
Most – 52– of the patients (61.2%) had hematologic malignancies; 12 patients (14.1%) received their transplant for bone marrow failure, and 11 patients (12.5%) had immunodeficiency. Three patients (3.5%) received HSCT for other malignancies, and seven (8.2%) for nonmalignant diseases.
Diffuse hair thinning was seen in 13 (62%) of the 21 patients who had alopecia, while 11 (52%) of the patients with alopecia had an androgenetic hair loss pattern. Chronic graft versus host disease (GVHD), skin chronic GVHD, a HSCT regimen that included busulfan conditioning, and a family history of early male-pattern alopecia were all significantly associated with post-HSCT permanent alopecia (P less than .05 for all).
The patients with androgenetic alopecia may be experiencing an accelerated time course of a condition to which they are already genetically disposed, noted Dr. Song and her colleagues.
Psoriasis was commonly seen on the scalp, affecting 11 of the 14 patients with psoriasis (79%), and involved the face in five of the patients (36%). Just one patient had psoriasis elsewhere on the body. There was a nonsignificant trend towards human leukocyte antigen mismatch among patients who had psoriasis. Although “psoriasis may be a marker of persistent immune dysregulation,” the investigators said, they did not identify any associated risk factors that would point toward this mechanism in their analysis.
Twelve patients (14%) had vitiligo, with halo nevi seen in four of these patients. Children who were younger than age 10 years and those who received their transplant for primary immunodeficiency were significantly more likely to have vitiligo (P less than .05 for both). Specific possible mechanisms triggering vitiligo could include thymic dysfunction resulting in loss of self-tolerance, and donor alloreactivity against the patient’s host antigens, according to the authors.
Nail changes such as pterygium and nail pitting, ridging, or thickening were seen in just five patients, all of whom had chronic GVHD of the skin. “Nail changes are likely a result of persistent inflammation and immune dysregulation from chronic GVHD,” said Dr. Song.
These late effects “can significantly impact patients’ quality of life,” according to the authors, who called for larger studies that follow pediatric HSCT patients longitudinally, beginning before the transplant – and for more investigation into the pathogenesis of specific late effects.
Dr. Song reported no relevant conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Late dermatologic manifestations in children who have received hematopoietic stem cell transplants may be more common than previously thought, according to results of a new study.
Johanna Song, MD, and her collaborators reported that, in their prospective pediatric study, 25% of patients had permanent alopecia and 16% had psoriasis, noting that late nonmalignant skin effects of hematopoietic stem cell transplantation (HSCT) have been studied primarily retrospectively, and in adults. Vitiligo and nail changes were also seen.
In a poster presentation at the World Congress of Dermatology, Dr. Song and her colleagues noted that these figures are higher than the previously reported pediatric rates of 1.7% for vitiligo and 15.6% for permanent alopecia. “Early recognition of these late effects can facilitate prompt and appropriate treatment, if desired,” they said.
The single-center, cross-sectional cohort study tracked pediatric patients over an 18-month period and included patients who were at least 1 year post allogeneic HSCT and had not relapsed. Patients who were not English speaking were excluded.
The median age of the 85 patients enrolled in the study was 13.8 years, and participants were a median of 3.6 years post transplant at the time of enrollment. The study’s analysis attempted to determine which patient, transplant, and disease factors might be associated with the late nonmalignant skin changes, according to Dr. Song, a resident dermatologist at Harvard University, Boston, and her colleagues.
Most – 52– of the patients (61.2%) had hematologic malignancies; 12 patients (14.1%) received their transplant for bone marrow failure, and 11 patients (12.5%) had immunodeficiency. Three patients (3.5%) received HSCT for other malignancies, and seven (8.2%) for nonmalignant diseases.
Diffuse hair thinning was seen in 13 (62%) of the 21 patients who had alopecia, while 11 (52%) of the patients with alopecia had an androgenetic hair loss pattern. Chronic graft versus host disease (GVHD), skin chronic GVHD, a HSCT regimen that included busulfan conditioning, and a family history of early male-pattern alopecia were all significantly associated with post-HSCT permanent alopecia (P less than .05 for all).
The patients with androgenetic alopecia may be experiencing an accelerated time course of a condition to which they are already genetically disposed, noted Dr. Song and her colleagues.
Psoriasis was commonly seen on the scalp, affecting 11 of the 14 patients with psoriasis (79%), and involved the face in five of the patients (36%). Just one patient had psoriasis elsewhere on the body. There was a nonsignificant trend towards human leukocyte antigen mismatch among patients who had psoriasis. Although “psoriasis may be a marker of persistent immune dysregulation,” the investigators said, they did not identify any associated risk factors that would point toward this mechanism in their analysis.
Twelve patients (14%) had vitiligo, with halo nevi seen in four of these patients. Children who were younger than age 10 years and those who received their transplant for primary immunodeficiency were significantly more likely to have vitiligo (P less than .05 for both). Specific possible mechanisms triggering vitiligo could include thymic dysfunction resulting in loss of self-tolerance, and donor alloreactivity against the patient’s host antigens, according to the authors.
Nail changes such as pterygium and nail pitting, ridging, or thickening were seen in just five patients, all of whom had chronic GVHD of the skin. “Nail changes are likely a result of persistent inflammation and immune dysregulation from chronic GVHD,” said Dr. Song.
These late effects “can significantly impact patients’ quality of life,” according to the authors, who called for larger studies that follow pediatric HSCT patients longitudinally, beginning before the transplant – and for more investigation into the pathogenesis of specific late effects.
Dr. Song reported no relevant conflicts of interest.
[email protected]
On Twitter @karioakes
AT WCPD 2017
Key clinical point:
Major finding: Permanent alopecia was seen in 25% of patients, and 16% had psoriasis a median of 3.6 years after transplant.
Data source: A single-center prospective study of 85 children in routine post-HSCT follow-up.
Disclosures: Dr. Song reported no conflicts of interest.
Successive Potassium Hydroxide Testing for Improved Diagnosis of Tinea Pedis
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).
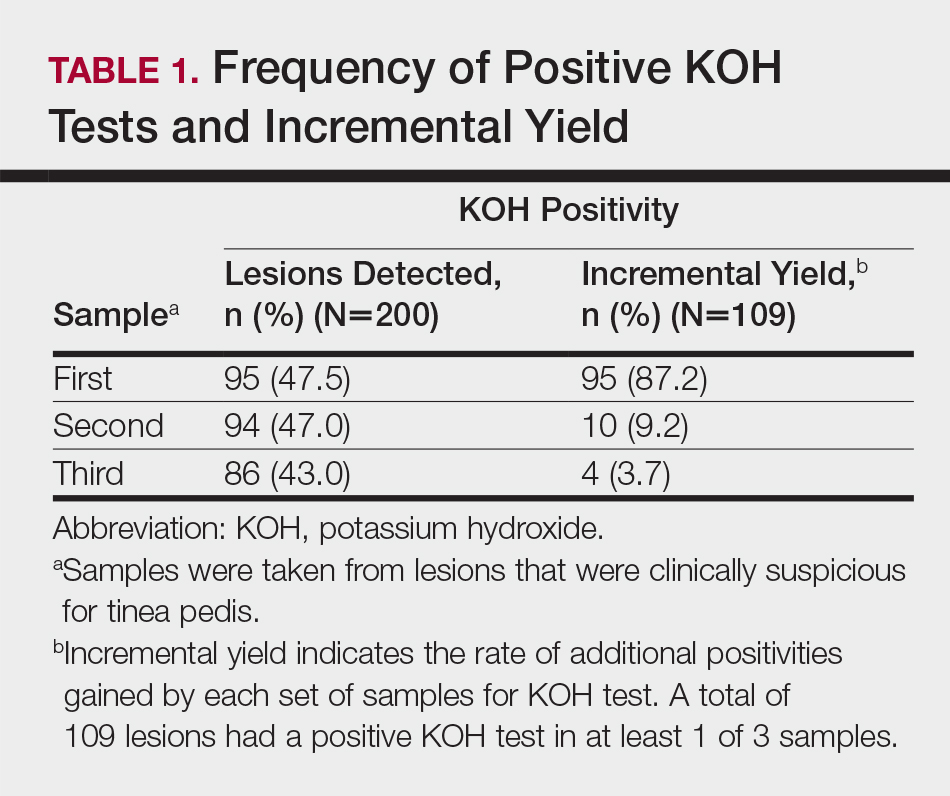
According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).
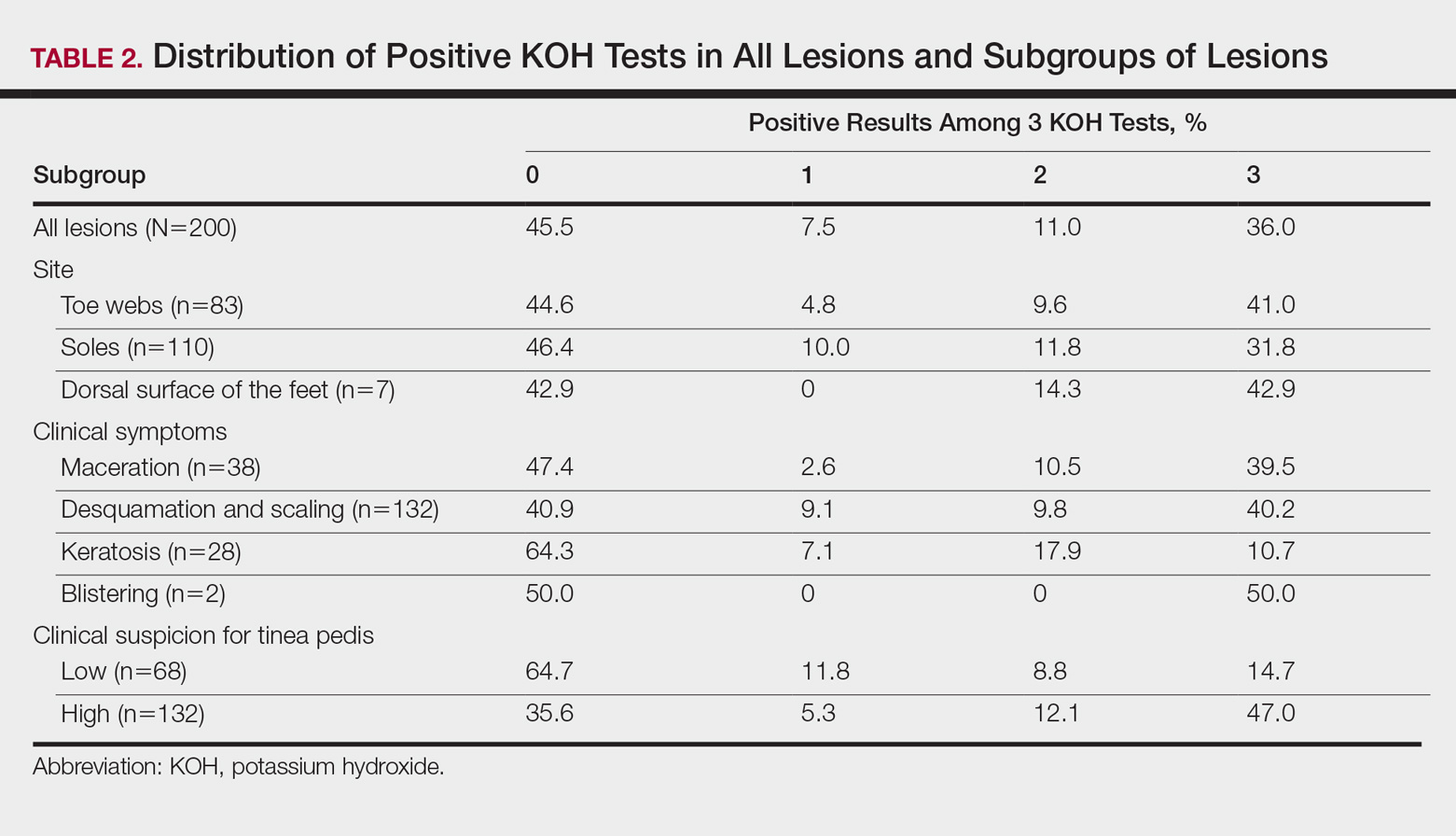
For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.
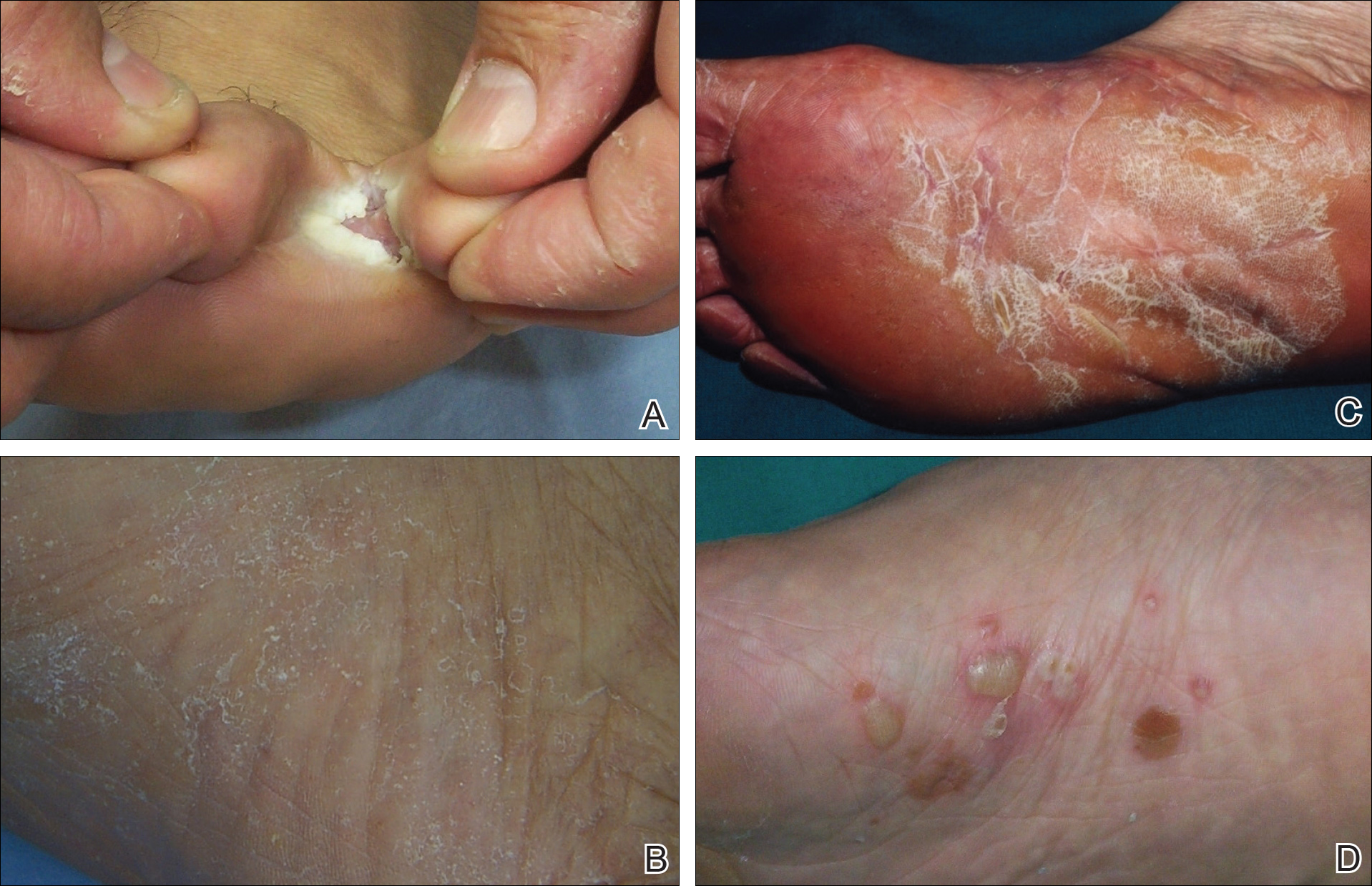
If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).

According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).

For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.

If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
The gold standard for diagnosing dermatophytosis is the use of direct microscopic examination together with fungal culture.1 However, in the last 2 decades, molecular techniques that currently are available worldwide have improved the diagnosis procedure.2,3 In the practice of dermatology, potassium hydroxide (KOH) testing is a commonly used method for the diagnosis of superficial fungal infections.4 The sensitivity and specificity of KOH testing in patients with tinea pedis have been reported as 73.3% and 42.5%, respectively.5 Repetition of this test after an initial negative test result is recommended if the clinical picture strongly suggests a fungal infection.6,7 Alternatively, several repetitions of direct microscopic examinations also have been proposed for detecting other microorganisms. For example, 3 negative sputum smears traditionally are recommended to exclude a diagnosis of pulmonary tuberculosis.8 However, after numerous investigations in various regions of the world, the World Health Organization reduced the recommended number of these specimens from 3 to 2 in 2007.9
The literature suggests that successive mycological tests, both with direct microscopy and fungal cultures, improve the diagnosis of onychomycosis.1,10,11 Therefore, if such investigations are increased in number, recommendations for successive mycological tests may be more reliable. In the current study, we aimed to investigate the value of successive KOH testing in the management of patients with clinically suspected tinea pedis.
Methods
Patients and Clinical Evaluation
One hundred thirty-five consecutive patients (63 male; 72 female) with clinical symptoms suggestive of intertriginous, vesiculobullous, and/or moccasin-type tinea pedis were enrolled in this prospective study. The mean age (SD) of patients was 45.9 (14.7) years (range, 11–77 years). Almost exclusively, the clinical symptoms suggestive of tinea pedis were desquamation or maceration in the toe webs, blistering lesions on the soles, and diffuse or patchy scaling or keratosis on the soles. A single dermatologist (B.F.K.) clinically evaluated the patients and found only 1 region showing different patterns suggestive of tinea pedis in 72 patients, 2 regions in 61 patients, and 3 regions in 2 patients. Therefore, 200 lesions from the 135 patients were chosen for the KOH test. The dermatologist recorded her level of suspicion for a fungal infection as low or high for each lesion, depending on the absence or presence of signs (eg, unilateral involvement, a well-defined border). None of the patients had used topical or systemic antifungal therapy for at least 1 month prior to the study.12
Clinical Sampling and Direct Microscopic Examination
The dermatologist took 3 samples of skin scrapings from each of the 200 lesions. All 3 samples from a given lesion were obtained from sites with the same clinical symptoms in a single session. Special attention was paid to samples from the active advancing borders of the lesions and the roofs of blisters if they were present.13 Upon completion of every 15 samples from every 5 lesions, the dermatologist randomized the order of the samples (https://www.random.org/). She then gave the samples, without the identities of the patients or any clinical information, to an experienced laboratory technician for direct microscopic examination. The technician prepared and examined the samples as described elsewhere5,7,14 and recorded the results as positive if hyphal elements were present or negative if they were not. The study was reviewed and approved by the Çukurova University Faculty of Medicine Ethics Committee (Adana, Turkey). Informed consent was obtained from each patient or from his/her guardian(s) prior to initiating the study.
Statistical Analysis
Statistical analysis was conducted using the χ2 test in the SPSS software version 20.0. McNemar test was used for analysis of the paired data.
Results
Among the 135 patients, lesions were suggestive of the intertriginous type of tinea pedis in 24 patients, moccasin type in 50 patients, and both intertriginous and moccasin type in 58 patients. Among the remaining 3 patients, 1 had lesions suggestive of the vesiculobullous type, and another patient had both the vesiculobullous and intertriginous types; the last patient demonstrated lesions that were inconsistent with any of these 3 subtypes of tinea pedis, and a well-defined eczematous plaque was observed on the dorsal surface of the patient’s left foot.
Among the 200 lesions from which skin scrapings were taken for KOH testing, 83 were in the toe webs, 110 were on the soles, and 7 were on the dorsal surfaces of the feet. Of these 7 dorsal lesions, 6 were extensions from lesions on the toe webs or soles and 1 was inconsistent with the 3 subtypes of tinea pedis. Among the 200 lesions, the main clinical symptom was maceration in 38 lesions, desquamation or scaling in 132 lesions, keratosis in 28 lesions, and blistering in 2 lesions. The dermatologist recorded the level of suspicion for tinea pedis as low in 68 lesions and high in 132.
According to the order in which the dermatologist took the 3 samples from each lesion, the KOH test was positive in 95 of the first set of 200 samples, 94 of the second set, and 86 of the third set; however, from the second set, the incremental yield (ie, the number of lesions in which the first KOH test was negative and the second was positive) was 10. The number of lesions in which the first and the second tests were negative and the third was positive was only 4. Therefore, the number of lesions with a positive KOH test was significantly increased from 95 to 105 by performing the second KOH test (P=.002). This number again increased from 105 to 109 when a third test was performed; however, this increase was not statistically significant (P=.125)(Table 1).

According to an evaluation that was not stratified by the dermatologist’s order of sampling, 72 lesions (36.0%) showed KOH test positivity in all 3 samples, 22 (11.0%) were positive in 2 samples, 15 (7.5%) were positive in only 1 sample, and 91 (45.5%) were positive in none of the samples (Table 2). When the data were subdivided based on the sites of the lesions, the toe web lesions (n=83) showed rates of 41.0%, 9.6%, and 4.8% for 3, 2, and 1 positive KOH tests, respectively. For the sole lesions (n=110), the rates were somewhat different at 31.8%, 11.8%, and 10.0%, respectively, but the difference was not statistically significant (P=.395).

For the subgroups based on the main clinical symptoms, the percentage of lesions having at least 1 positive KOH test from the 3 samples was 35.7% for the keratotic lesions (n=28). This rate was lower than macerated lesions (n=38) and desquamating or scaling lesions (n=132), which were 52.6% and 59.1%, respectively (Table 2). On the other hand, the percentage of lesions that produced only 1 or 2 positive KOH tests from the 3 samples was 25.0% for the keratotic lesions, which was higher than the rates for the macerated lesions and the desquamating or scaling lesions (13.1% and 18.9%, respectively). In particular, the difference between the keratotic lesions and the desquamating or scaling lesions in the distribution of the rates of 0, 1, 2, and 3 positive KOH tests was statistically significant (P=.019). The macerated, desquamating or scaling, keratotic, and blistering lesions are presented in the Figure.

If the dermatologist indicated a high suspicion of fungal infection, it was more likely that at least 1 of 3 KOH test results was positive. The rate of at least 1 positive test was 64.4% for the highly suspicious lesions (n=132) and 35.3% for the lesions with low suspicion of a fungal infection (n=68)(Table 2). The difference was statistically significant (P<.001). Conversely, if the suspicion was low, it was more likely that only 1 or 2 KOH tests were positive. The percentages of lesions having 3, 2, or 1 positive KOH tests were 14.7%, 8.8%, and 11.8%, respectively, for the low-suspicion lesions and 47.0%, 12.1%, and 5.3%, respectively, for the high-suspicion lesions. The difference was statistically significant (P<.001).
Comment
In the current study, we aimed to investigate if successive KOH tests provide an incremental diagnostic yield in the management of patients with clinically suspected tinea pedis and if these results differ among the subgroups of patients. Both in the evaluation taking into account the order of sampling and in the evaluation disregarding this order, we found that the second sample was necessary for all subgroups, and even the third sample was necessary for patients with keratotic lesions. The main limitation of the study was that we lacked a gold-standard technique (eg, a molecular-based technique); therefore, we are unable to comment on the false-negative and false-positive results of the successive KOH testing.
Summerbell et al11 found in their study that in initial specimens of toenails with apparent lesions taken from 473 patients, the KOH test was 73.8% sensitive for dermatophytes, and this rate was only somewhat higher for cultures (74.6%). Arabatzis et al2 investigated 92 skin, nail, and hair specimens from 67 patients with suspected dermatophytosis and found that the KOH test was superior to culture for the detection of dermatophytes (43% vs 33%). Moreover and more importantly, they noted that a real-time polymerase chain reaction (PCR) assay yielded a higher detection rate (51%).2 In another study, Wisselink et al3 examined 1437 clinical samples and demonstrated a great increase in the detection of dermatophytes using a real-time PCR assay (48.5%) compared to culture (26.9%). However, PCR may not reflect active disease and could lead to false-positive results.2,3 Therefore, the aforementioned weakness of our study will be overcome in further studies investigating the benefit of successive KOH testing compared to a molecular-based assay, such as the real-time PCR assay.
In this study, repeating the KOH test provided better results for achieving the diagnosis of tinea pedis in a large number of samples from clinically suspected lesions. Additionally, the distribution of 3, 2, or 1 positive results on the 3 KOH tests was different among the subgroups of lesions. Overall, positivity was less frequent in the keratotic lesions compared to the macerated or desquamating or scaling lesions. Moreover, positivity on all 3 tests also was less frequent in the keratotic lesions. Inversely, the frequency of samples with only 1 or 2 positive results was higher in this subgroup. The necessity for the second, even the third, tests was greater in this subgroup.
Our findings were consistent with the results of the studies performed with successive mycological tests on the nail specimens. Meireles et al1 repeated 156 mycological nail tests 3 times and found the rate of positivity in the first test to be 19.9%. When the results of the first and second tests were combined, this rate increased to 28.2%, and when the results of all 3 tests were combined, it increased to 37.8%.1 Gupta10 demonstrated that even a fourth culture provided an incremental diagnostic yield in the diagnosis of onychomycosis, yet 4 cultures may not be clinically practical. Furthermore, periodic acid–Schiff staining is a more effective measure of positivity in onychomycosis.15
Although the overall rate of positivity on the 3 tests in our study was unsurprisingly higher in lesions rated highly suspicious for a fungal infection, the rate of only 1 or 2 positive tests was surprisingly somewhat higher in low-suspicion lesions, which suggested that repeating the KOH test would be beneficial, even if the clinical suspicion for tinea pedis was low. The novel contribution of this study includes the finding that mycological information was markedly improved in highly suspicious tinea pedis lesions regardless of the infection site (Table 1) by using 3 successive KOH tests; the percentage of lesions with 1, 2, or 3 positive KOH tests was 5.3%, 12.1%, and 47.0%, respectively (Table 2). A single physician from a single geographical location introduces a limitation to the study for a variety of reasons, including bias in the cases chosen and possible overrepresentation of the causative organism due to region-specific incidence. It is unknown how different causative organisms affect KOH results. The lack of fungal culture results limits the value of this information.
Conclusion
In this study, we investigated the benefit of successive KOH testing in the laboratory diagnosis of tinea pedis and found that the use of second samples in particular provided a substantial increase in diagnostic yield. In other words, the utilization of successive KOH testing remarkably improved the diagnosis of tinea pedis. Therefore, we suggest that at least 2 samples of skin scrapings should be taken for the diagnosis of tinea pedis and that the number of samples should be at least 3 for keratotic lesions. However, further study by using a gold-standard method such as a molecular-based assay as well as taking the samples in daily or weekly intervals is recommended to achieve a more reliable result.
Acknowledgment
The authors would like to thank Gökçen Şahin (Adana, Turkey) for providing technical support in direct microscopic examination.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
- Meireles TE, Rocha MF, Brilhante RS, et al. Successive mycological nail tests for onychomycosis: a strategy to improve diagnosis efficiency. Braz J Infect Dis. 2008;2:333-337.
- Arabatzis M, Bruijnesteijn van Coppenraet LE, Kuijper EJ, et al. Diagnosis of dermatophyte infection by a novel multiplex real-time polymerase chain reaction detection/identification scheme. Br J Dermatol. 2007;157:681-689.
- Wisselink GJ, van Zanten E, Kooistra-Smid AM. Trapped in keratin; a comparison of dermatophyte detection in nail, skin and hair samples directly from clinical samples using culture and real-time PCR. J Microbiol Methods. 2011;85:62-66.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72:238-241.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis [published online June 22, 2010]. Dermatol Res Pract. 2010;2010:764843.
- Brodell RT, Helms SE, Snelson ME. Office dermatologic testing: the KOH preparation. Am Fam Physicin. 1991;43:2061-2065.
- McKay M. Office techniques for dermatologic diagnosis. In: Walkers HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston, MA: Butterworths; 1990:540-543.
- Wilmer A, Bryce E, Grant J. The role of the third acid-fast bacillus smear in tuberculosis screening for infection control purposes: a controversial topic revisited. Can J Infect Dis Med Microbiol. 2011;22:E1-E3.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy: WHO policy statement. http://www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Published 2011. Accessed July 24, 2017.
- Gupta A. The incremental diagnostic yield of successive re-cultures in patients with a clinical diagnosis of onychomycosis. J Am Acad Dermatol. 2005;52:P129.
- Summerbell RC, Cooper E, Bunn U, et al. Onychomycosis: a critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med Mycol. 2005;43:39-59.
- Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol. 1993;129:510-511.
- Ilkit M, Durdu M. Tinea pedis: the etiology and global epidemiology of a common fungal infection. Crit Rev Microbiol. 2015;41:374-388.
- McGinnis MR. Laboratory Handbook of Medical Mycology. New York, NY: Academic Press, Inc; 1980.
- Jeelani S, Ahmed QM, Lanker AM, et al. Histopathological examination of nail clippings using PAS staining (HPE-PAS): gold-standard in diagnosis of onychomycosis. Mycoses. 2015;58:27-32.
Practice Points
- At least 2 samples should be taken for potassium hydroxide examination when tinea pedis is sus-pected clinically.
- The number of samples should be at least 3 if keratotic lesions are present.
Hereditary Hypotrichosis Simplex of the Scalp
To the Editor:
Hereditary hypotrichosis simplex (HHS)(Online Mendelian Inheritance in Man [OMIM] 146520) is a rare form of hypotrichosis that typically presents in school-aged children as worsening hair loss localized to the scalp.1 Most patients are unaffected at birth and otherwise healthy without abnormalities of the nails, teeth, or perspiration. Examination of the scalp reveals normal follicular ostia and absence of scale and erythema; however, decreased follicular density may be noted.1 The histopathologic findings of HHS reveal velluslike hair follicles without associated fibrosis or inflammation.2 Examination of hair follicles with light microscopy is unremarkable.3,4 Historically, this condition has been largely regarded as autosomal dominant, with variable severity also described within families. Herein, we report a case of this rare disease in a child, with 2 family members displaying a less severe phenotype.
A 7-year-old girl presented with gradual thinning of the scalp hair of 3 to 4 years’ duration. Her mother reported the patient had normal hair density at birth. Over the next several years, she was noted to have an inability to grow lengthy hair. At approximately 3 years of age, thinning of scalp hair was identified. There was no prior history of increased shedding, hypohidrosis, or tooth or nail abnormalities. Family history revealed fine hair in her older sister and fine thin hair at the frontal scalp in her mother. Her mother reported similar inability to grow lengthy hair. Physical examination of the patient demonstrated short blonde hair with diffuse thinning of the crown (Figure 1). The longest hair was approximately 10 cm in length. Follicular ostia were without erythema or scale but notably fewer in number on the crown. Eyebrows, eyelashes, teeth, and fingernails were without abnormalities. A hair pull test was negative and hair mount revealed normal bulb and shaft. Microscopy of hair shafts under polarized light was unremarkable.
Two punch biopsies were obtained and submitted for vertical and horizontal sectioning. Sections demonstrated an intact epidermis, decreased follicle number, and small follicles with hypoplastic velluslike appearance (Figure 2). Fibrosis and inflammation were not seen; there was no increase in catagen or telogen hairs. Clinical and histopathological findings were consistent with HHS.
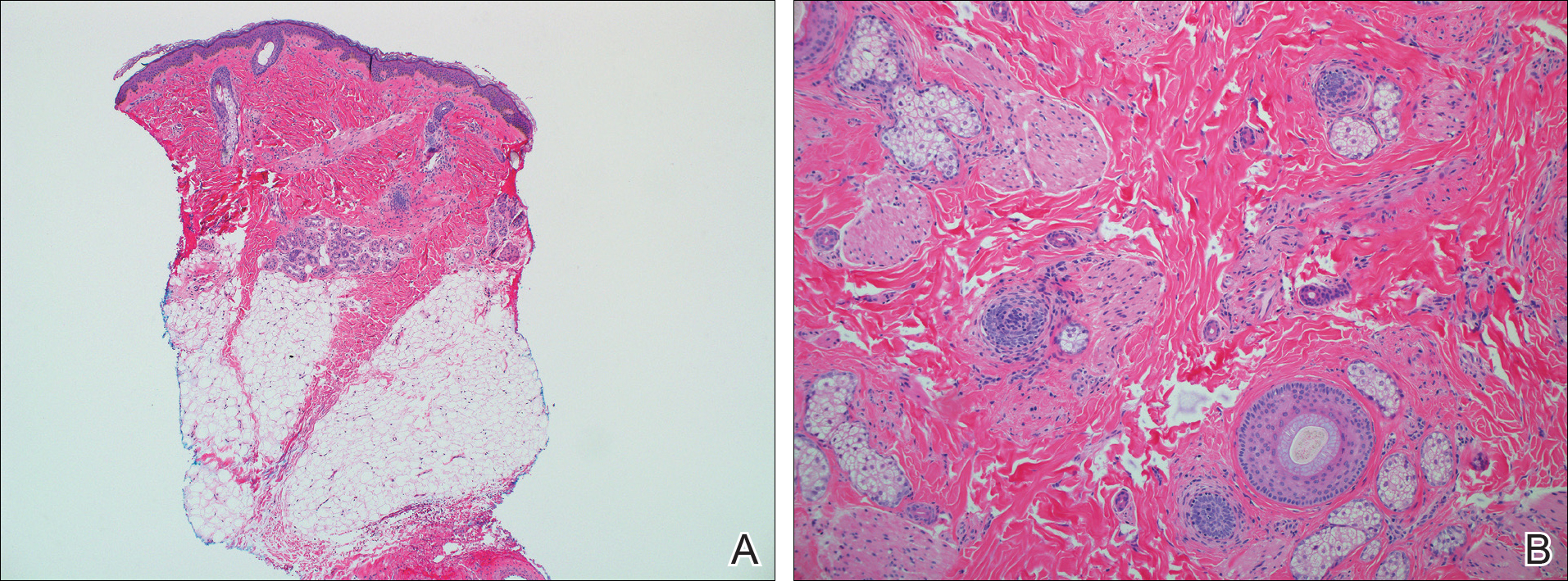
Hereditary hypotrichosis localized to the scalp was first described by Toribio and Quinones5 in 1974 in a large Spanish family presenting with normal scalp hair at birth followed by gradual diffuse hair loss. Hair loss that usually began in school-aged children with subsequent few fine hairs remaining on the scalp by the third decade of life was identified in these individuals.Eyelashes, eyebrows, pubic, axillary, and other truncal hairs were normal.5 Several similar cases of HHS localized to the scalp have since been reported.2,6 Hereditary hypotrichosis simplex is inherited in an autosomal-dominant fashion, with the exception of 1 reported sporadic case.3
Research on HHS has primarily focused on genetic analyses of several affected families. Betz et al7 mapped the gene for HHS to band 6p21.3 in 2 families of Danish origin and in the Spanish family initially described by Toribio and Quinones.5 Three years later, a nonsense mutation in the CDSN gene encoding corneodesmosin was described.8 Despite these genetic advances, the pathogenesis of HHS and the role that corneodesmosin may play remain unclear.
Generalized forms of hypotrichosis (OMIM #605389) have long been reported and described as loss of scalp hair with involvement of eyebrows, eyelashes, and other body hair.9 Genetic studies have allowed for genome-wide linkage analysis, linking 3 families with this more generalized HHS phenotype to chromosome 18; specifically, an Italian family with sparse scalp and body hair but normal eyelashes and eyebrows,4 and 2 Pakistani families with thinning scalp hair and sparse truncal hair.10 A mutation in the APC downregulated 1 gene, APCDD1, also has been identified in these families.10 These genetic findings indicate that the generalized form of HHS is a distinct syndrome.
The differential diagnosis of HHS includes Marie-Unna hereditary hypotrichosis, loose anagen hair syndrome, trichothiodystrophy, and androgenetic alopecia. Marie-Unna hereditary hypotrichosis usually presents as near-complete absence of scalp hair at birth, development of wiry twisted hair in childhood, and progressive alopecia.3 Loose anagen hair syndrome usually demonstrates a ruffled cuticle on hair pull test and remits in late childhood. Polarization of the hair shaft can identify patients with trichothiodystrophy. Follicular miniaturization may lead one to consider early-onset androgenetic alopecia in some patients.
There is no effective treatment of HHS. Due to potential phenotypic variation, patients should be counseled that they may experience progressive or possible total loss of scalp hair by the third decade of life.2,3,5 As with other hair loss disorders, wigs or additional over-the-counter cosmetic options may be considered.3 Currently, there are no known patient resources specific for HHS. Therefore, our patient’s family was referred to the National Alopecia Areata Foundation website (https://naaf.org/) for resources on discussing alopecia with school-aged children. The psychological impact of alopecia should not be overlooked and psychiatric referral should be provided, if needed. Examination of family members along with clinical monitoring are recommended. Genetic counseling also may be offered.3
- Rodríguez Díaz E, Fernández Blasco G, Martín Pascual A, et al. Heredity hypotrichosis simplex of the scalp. Dermatology. 1995;191:139-141.
- Ibsen HH, Clemmensen OJ, Brandrup F. Familial hypotrichosis of the scalp. autosomal dominant inheritance in four generations. Acta Derm Venereol. 1991;71:349-351.
- Cambiaghi S, Barbareschi M. A sporadic case of congenital hypotrichosis simplex of the scalp: difficulties in diagnosis and classification. Pediatr Dermatol. 1999;16:301-304.
- Baumer A, Belli S, Trueb RM, et al. An autosomal dominant form of hereditary hypotrichosis simple maps to 18p11.32-p11.23 in an Italian family. Eur J Hum Genet. 2000;8:443-448.
- Toribio J, Quinones PA. Heredity hypotrichosis simplex of the scalp. evidence for autosomal dominant inheritance. Br J Dermatol. 1974;91:687-696.
- Kohn G, Metzker A. Hereditary hypotrichosis simplex of the scalp. Clin Genet. 1987;32:120-124.
- Betz RC, Lee YA, Bygum A, et al. A gene for hypotrichosis simplex of the scalp maps to chromosome 6p21.3. Am J Hum Genet. 2000;66:1979-1983.
- Levy-Nissenbaum E, Betz R, Frydman M, et al. Hypotrichosis of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat Genet. 2003;34:151-153.
- Just M, Ribera M, Fuente MJ, et al. Hereditary hypotrichosis simplex. Dermatology. 1998;196:339-342.
- Shimomura Y, Agalliu D, Vonica A, et al. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature. 2011;44:1043-1047.
To the Editor:
Hereditary hypotrichosis simplex (HHS)(Online Mendelian Inheritance in Man [OMIM] 146520) is a rare form of hypotrichosis that typically presents in school-aged children as worsening hair loss localized to the scalp.1 Most patients are unaffected at birth and otherwise healthy without abnormalities of the nails, teeth, or perspiration. Examination of the scalp reveals normal follicular ostia and absence of scale and erythema; however, decreased follicular density may be noted.1 The histopathologic findings of HHS reveal velluslike hair follicles without associated fibrosis or inflammation.2 Examination of hair follicles with light microscopy is unremarkable.3,4 Historically, this condition has been largely regarded as autosomal dominant, with variable severity also described within families. Herein, we report a case of this rare disease in a child, with 2 family members displaying a less severe phenotype.
A 7-year-old girl presented with gradual thinning of the scalp hair of 3 to 4 years’ duration. Her mother reported the patient had normal hair density at birth. Over the next several years, she was noted to have an inability to grow lengthy hair. At approximately 3 years of age, thinning of scalp hair was identified. There was no prior history of increased shedding, hypohidrosis, or tooth or nail abnormalities. Family history revealed fine hair in her older sister and fine thin hair at the frontal scalp in her mother. Her mother reported similar inability to grow lengthy hair. Physical examination of the patient demonstrated short blonde hair with diffuse thinning of the crown (Figure 1). The longest hair was approximately 10 cm in length. Follicular ostia were without erythema or scale but notably fewer in number on the crown. Eyebrows, eyelashes, teeth, and fingernails were without abnormalities. A hair pull test was negative and hair mount revealed normal bulb and shaft. Microscopy of hair shafts under polarized light was unremarkable.

Two punch biopsies were obtained and submitted for vertical and horizontal sectioning. Sections demonstrated an intact epidermis, decreased follicle number, and small follicles with hypoplastic velluslike appearance (Figure 2). Fibrosis and inflammation were not seen; there was no increase in catagen or telogen hairs. Clinical and histopathological findings were consistent with HHS.

Hereditary hypotrichosis localized to the scalp was first described by Toribio and Quinones5 in 1974 in a large Spanish family presenting with normal scalp hair at birth followed by gradual diffuse hair loss. Hair loss that usually began in school-aged children with subsequent few fine hairs remaining on the scalp by the third decade of life was identified in these individuals.Eyelashes, eyebrows, pubic, axillary, and other truncal hairs were normal.5 Several similar cases of HHS localized to the scalp have since been reported.2,6 Hereditary hypotrichosis simplex is inherited in an autosomal-dominant fashion, with the exception of 1 reported sporadic case.3
Research on HHS has primarily focused on genetic analyses of several affected families. Betz et al7 mapped the gene for HHS to band 6p21.3 in 2 families of Danish origin and in the Spanish family initially described by Toribio and Quinones.5 Three years later, a nonsense mutation in the CDSN gene encoding corneodesmosin was described.8 Despite these genetic advances, the pathogenesis of HHS and the role that corneodesmosin may play remain unclear.
Generalized forms of hypotrichosis (OMIM #605389) have long been reported and described as loss of scalp hair with involvement of eyebrows, eyelashes, and other body hair.9 Genetic studies have allowed for genome-wide linkage analysis, linking 3 families with this more generalized HHS phenotype to chromosome 18; specifically, an Italian family with sparse scalp and body hair but normal eyelashes and eyebrows,4 and 2 Pakistani families with thinning scalp hair and sparse truncal hair.10 A mutation in the APC downregulated 1 gene, APCDD1, also has been identified in these families.10 These genetic findings indicate that the generalized form of HHS is a distinct syndrome.
The differential diagnosis of HHS includes Marie-Unna hereditary hypotrichosis, loose anagen hair syndrome, trichothiodystrophy, and androgenetic alopecia. Marie-Unna hereditary hypotrichosis usually presents as near-complete absence of scalp hair at birth, development of wiry twisted hair in childhood, and progressive alopecia.3 Loose anagen hair syndrome usually demonstrates a ruffled cuticle on hair pull test and remits in late childhood. Polarization of the hair shaft can identify patients with trichothiodystrophy. Follicular miniaturization may lead one to consider early-onset androgenetic alopecia in some patients.
There is no effective treatment of HHS. Due to potential phenotypic variation, patients should be counseled that they may experience progressive or possible total loss of scalp hair by the third decade of life.2,3,5 As with other hair loss disorders, wigs or additional over-the-counter cosmetic options may be considered.3 Currently, there are no known patient resources specific for HHS. Therefore, our patient’s family was referred to the National Alopecia Areata Foundation website (https://naaf.org/) for resources on discussing alopecia with school-aged children. The psychological impact of alopecia should not be overlooked and psychiatric referral should be provided, if needed. Examination of family members along with clinical monitoring are recommended. Genetic counseling also may be offered.3
To the Editor:
Hereditary hypotrichosis simplex (HHS)(Online Mendelian Inheritance in Man [OMIM] 146520) is a rare form of hypotrichosis that typically presents in school-aged children as worsening hair loss localized to the scalp.1 Most patients are unaffected at birth and otherwise healthy without abnormalities of the nails, teeth, or perspiration. Examination of the scalp reveals normal follicular ostia and absence of scale and erythema; however, decreased follicular density may be noted.1 The histopathologic findings of HHS reveal velluslike hair follicles without associated fibrosis or inflammation.2 Examination of hair follicles with light microscopy is unremarkable.3,4 Historically, this condition has been largely regarded as autosomal dominant, with variable severity also described within families. Herein, we report a case of this rare disease in a child, with 2 family members displaying a less severe phenotype.
A 7-year-old girl presented with gradual thinning of the scalp hair of 3 to 4 years’ duration. Her mother reported the patient had normal hair density at birth. Over the next several years, she was noted to have an inability to grow lengthy hair. At approximately 3 years of age, thinning of scalp hair was identified. There was no prior history of increased shedding, hypohidrosis, or tooth or nail abnormalities. Family history revealed fine hair in her older sister and fine thin hair at the frontal scalp in her mother. Her mother reported similar inability to grow lengthy hair. Physical examination of the patient demonstrated short blonde hair with diffuse thinning of the crown (Figure 1). The longest hair was approximately 10 cm in length. Follicular ostia were without erythema or scale but notably fewer in number on the crown. Eyebrows, eyelashes, teeth, and fingernails were without abnormalities. A hair pull test was negative and hair mount revealed normal bulb and shaft. Microscopy of hair shafts under polarized light was unremarkable.

Two punch biopsies were obtained and submitted for vertical and horizontal sectioning. Sections demonstrated an intact epidermis, decreased follicle number, and small follicles with hypoplastic velluslike appearance (Figure 2). Fibrosis and inflammation were not seen; there was no increase in catagen or telogen hairs. Clinical and histopathological findings were consistent with HHS.

Hereditary hypotrichosis localized to the scalp was first described by Toribio and Quinones5 in 1974 in a large Spanish family presenting with normal scalp hair at birth followed by gradual diffuse hair loss. Hair loss that usually began in school-aged children with subsequent few fine hairs remaining on the scalp by the third decade of life was identified in these individuals.Eyelashes, eyebrows, pubic, axillary, and other truncal hairs were normal.5 Several similar cases of HHS localized to the scalp have since been reported.2,6 Hereditary hypotrichosis simplex is inherited in an autosomal-dominant fashion, with the exception of 1 reported sporadic case.3
Research on HHS has primarily focused on genetic analyses of several affected families. Betz et al7 mapped the gene for HHS to band 6p21.3 in 2 families of Danish origin and in the Spanish family initially described by Toribio and Quinones.5 Three years later, a nonsense mutation in the CDSN gene encoding corneodesmosin was described.8 Despite these genetic advances, the pathogenesis of HHS and the role that corneodesmosin may play remain unclear.
Generalized forms of hypotrichosis (OMIM #605389) have long been reported and described as loss of scalp hair with involvement of eyebrows, eyelashes, and other body hair.9 Genetic studies have allowed for genome-wide linkage analysis, linking 3 families with this more generalized HHS phenotype to chromosome 18; specifically, an Italian family with sparse scalp and body hair but normal eyelashes and eyebrows,4 and 2 Pakistani families with thinning scalp hair and sparse truncal hair.10 A mutation in the APC downregulated 1 gene, APCDD1, also has been identified in these families.10 These genetic findings indicate that the generalized form of HHS is a distinct syndrome.
The differential diagnosis of HHS includes Marie-Unna hereditary hypotrichosis, loose anagen hair syndrome, trichothiodystrophy, and androgenetic alopecia. Marie-Unna hereditary hypotrichosis usually presents as near-complete absence of scalp hair at birth, development of wiry twisted hair in childhood, and progressive alopecia.3 Loose anagen hair syndrome usually demonstrates a ruffled cuticle on hair pull test and remits in late childhood. Polarization of the hair shaft can identify patients with trichothiodystrophy. Follicular miniaturization may lead one to consider early-onset androgenetic alopecia in some patients.
There is no effective treatment of HHS. Due to potential phenotypic variation, patients should be counseled that they may experience progressive or possible total loss of scalp hair by the third decade of life.2,3,5 As with other hair loss disorders, wigs or additional over-the-counter cosmetic options may be considered.3 Currently, there are no known patient resources specific for HHS. Therefore, our patient’s family was referred to the National Alopecia Areata Foundation website (https://naaf.org/) for resources on discussing alopecia with school-aged children. The psychological impact of alopecia should not be overlooked and psychiatric referral should be provided, if needed. Examination of family members along with clinical monitoring are recommended. Genetic counseling also may be offered.3
- Rodríguez Díaz E, Fernández Blasco G, Martín Pascual A, et al. Heredity hypotrichosis simplex of the scalp. Dermatology. 1995;191:139-141.
- Ibsen HH, Clemmensen OJ, Brandrup F. Familial hypotrichosis of the scalp. autosomal dominant inheritance in four generations. Acta Derm Venereol. 1991;71:349-351.
- Cambiaghi S, Barbareschi M. A sporadic case of congenital hypotrichosis simplex of the scalp: difficulties in diagnosis and classification. Pediatr Dermatol. 1999;16:301-304.
- Baumer A, Belli S, Trueb RM, et al. An autosomal dominant form of hereditary hypotrichosis simple maps to 18p11.32-p11.23 in an Italian family. Eur J Hum Genet. 2000;8:443-448.
- Toribio J, Quinones PA. Heredity hypotrichosis simplex of the scalp. evidence for autosomal dominant inheritance. Br J Dermatol. 1974;91:687-696.
- Kohn G, Metzker A. Hereditary hypotrichosis simplex of the scalp. Clin Genet. 1987;32:120-124.
- Betz RC, Lee YA, Bygum A, et al. A gene for hypotrichosis simplex of the scalp maps to chromosome 6p21.3. Am J Hum Genet. 2000;66:1979-1983.
- Levy-Nissenbaum E, Betz R, Frydman M, et al. Hypotrichosis of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat Genet. 2003;34:151-153.
- Just M, Ribera M, Fuente MJ, et al. Hereditary hypotrichosis simplex. Dermatology. 1998;196:339-342.
- Shimomura Y, Agalliu D, Vonica A, et al. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature. 2011;44:1043-1047.
- Rodríguez Díaz E, Fernández Blasco G, Martín Pascual A, et al. Heredity hypotrichosis simplex of the scalp. Dermatology. 1995;191:139-141.
- Ibsen HH, Clemmensen OJ, Brandrup F. Familial hypotrichosis of the scalp. autosomal dominant inheritance in four generations. Acta Derm Venereol. 1991;71:349-351.
- Cambiaghi S, Barbareschi M. A sporadic case of congenital hypotrichosis simplex of the scalp: difficulties in diagnosis and classification. Pediatr Dermatol. 1999;16:301-304.
- Baumer A, Belli S, Trueb RM, et al. An autosomal dominant form of hereditary hypotrichosis simple maps to 18p11.32-p11.23 in an Italian family. Eur J Hum Genet. 2000;8:443-448.
- Toribio J, Quinones PA. Heredity hypotrichosis simplex of the scalp. evidence for autosomal dominant inheritance. Br J Dermatol. 1974;91:687-696.
- Kohn G, Metzker A. Hereditary hypotrichosis simplex of the scalp. Clin Genet. 1987;32:120-124.
- Betz RC, Lee YA, Bygum A, et al. A gene for hypotrichosis simplex of the scalp maps to chromosome 6p21.3. Am J Hum Genet. 2000;66:1979-1983.
- Levy-Nissenbaum E, Betz R, Frydman M, et al. Hypotrichosis of the scalp is associated with nonsense mutations in CDSN encoding corneodesmosin. Nat Genet. 2003;34:151-153.
- Just M, Ribera M, Fuente MJ, et al. Hereditary hypotrichosis simplex. Dermatology. 1998;196:339-342.
- Shimomura Y, Agalliu D, Vonica A, et al. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature. 2011;44:1043-1047.
Practice Points
- Hereditary hypotrichosis simplex (HHS) is a rare form of hypotrichosis that typically presents in school-aged children as worsening hair loss localized to the scalp.
- Historically, HHS has been largely regarded as autosomal dominant, with variable severity also described within families.
- There is no effective treatment of HHS. Due to potential phenotypic variation, patients should be counseled that they may experience progressive or possible total loss of scalp hair by the third decade of life.