User login
A Modified Anchor Taping Technique for Distal Onychocryptosis
Practice Gap
Onychocryptosis, colloquially known as an ingrown nail, most commonly affects the lateral folds of the toenails. It also can affect the fingernails and the distal aspect of the nail unit, though these presentations are not as well described in the literature. In onychocryptosis, the nail plate grows downward into the periungual skin, resulting in chronic pain and inflammation. Risk factors include overtrimming the nails with rounded edges, local trauma, nail surgery, wearing tight footwear, obesity, and onychomycosis.1
Although surgical intervention might be required for severe or refractory disease, conservative treatment options are first line and often curative. A variety of techniques have been designed to separate the ingrown portion of the nail plate from underlying skin, including placement of an intervening piece of dental floss, cotton, or plastic tubing.2
Anchor taping is another effective method of treating onychocryptosis; a strip of tape is used to gently pull and secure the affected nail fold away from the overlying nail plate. This technique has been well described for the treatment of onychocryptosis of the lateral toenail.3-5 In 2017, Arai and Haneke5 presented a modified technique for the treatment of distal disease.
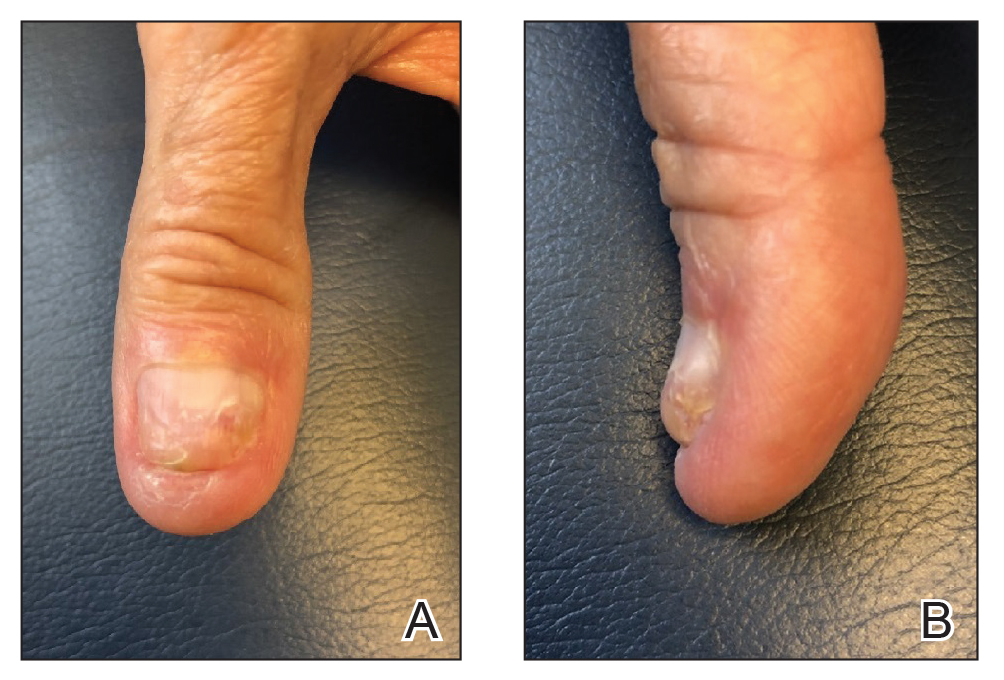
We present a simplified method that was used successfully in a case of distal onychocryptosis of the thumbnail that occurred approximately 4 months after complete nail avulsion with a nail matrix biopsy (Figure 1).
The Technique
A strongly adhesive, soft cotton, elastic tape that is 1-inch wide, such as Elastikon Elastic Tape (Johnson & Johnson), is used to pull and secure the hyponychium away from the overlying nail plate. When this technique is used for lateral onychocryptosis, a single strip of tape is secured to the affected lateral nail fold, pulled obliquely and proximally, and secured to the base of the digit.3-5 In the Arai and Haneke5 method for the treatment of distal disease, a piece of tape is first placed at the distal nail fold, pulled proximally, and secured to the ventral aspect of the digit. Then, 1 or 2 additional strips of tape are applied to the lateral nail folds, pulled obliquely, and adhered to the base of the digit, as in the classic technique for lateral onychocryptosis.5
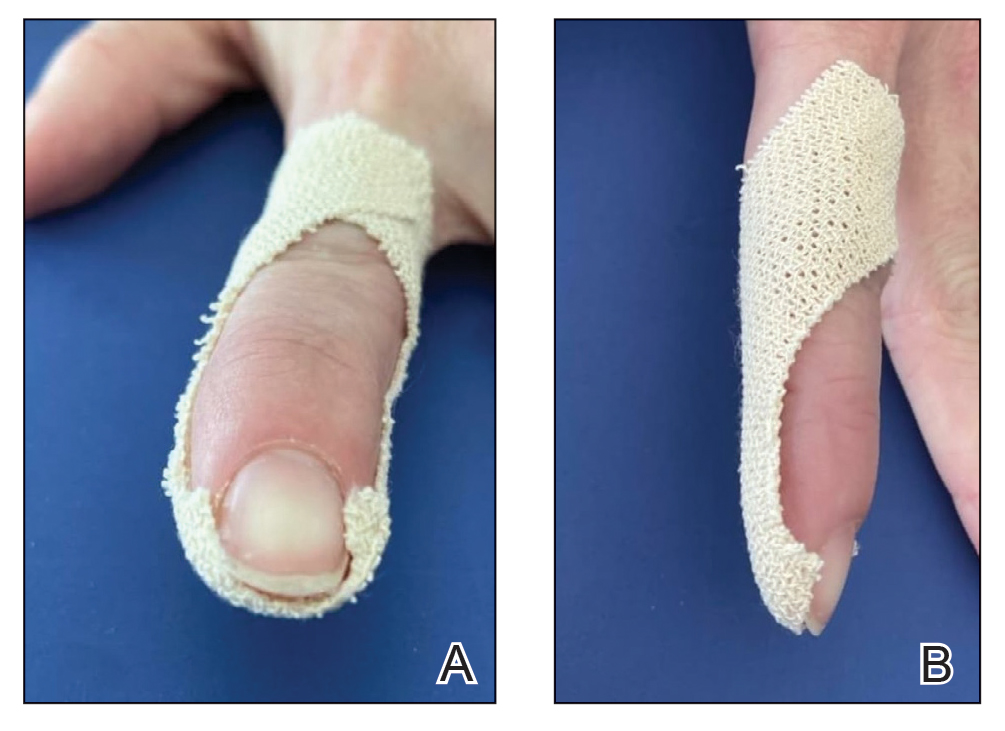
In our modification for the treatment of distal disease, only 2 strips of tape are required, each approximately 5-cm long. The first strip of tape is applied to the hyponychium parallel to the long axis of the finger, pulled away from the distal edge of the nail plate, and secured obliquely and proximally to the base of the finger on one side. The second strip of tape is applied to the hyponychium in the same manner, directly overlying the first strip, but is then pulled obliquely in the opposite direction and secured to the other side of the proximal finger (Figure 2). The 2 strips of tape are applied directly overlying each other at the distal nail fold but with opposing tension vectors to optimize pull on the distal nail fold. This modification eliminates the need to apply an initial strip of tape along the long axis of the digit, as described by Arai and Haneke.5
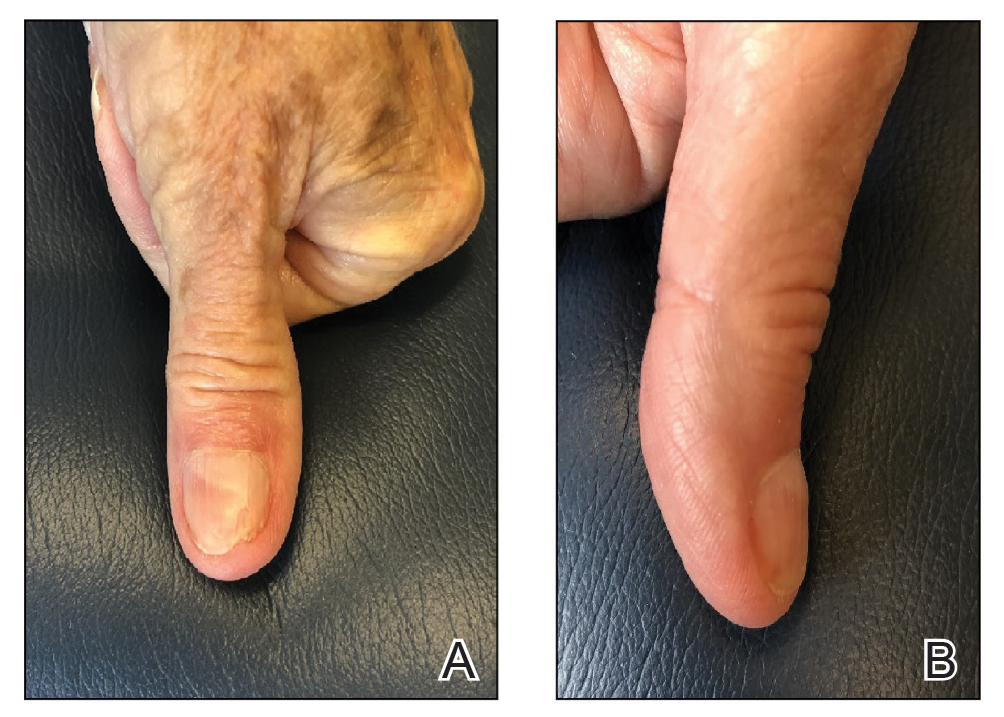
The patient is instructed on this method in the office and will change the tape at home daily for 2 to 6 weeks, until the nail plate has grown out over the hyponychium (Figure 3). This technique also can be combined with other modalities, such as dilute vinegar soaks performed daily after changing the tape to ease inflammation and prevent infection. Because strongly adhesive tape is used, it also is recommended that the patient soak the tape before removing it to prevent damage to underlying skin.
Practice Implications
Anchor taping is a common and effective treatment of onychocryptosis. Most techniques described in the literature are for lateral toenail cases, which often are managed by podiatry. A modification for the treatment of distal onychocryptosis has been previously described.5 We describe a similar modification using 2 tape strips pulled in opposite directions, which successfully resolved a case of distal onychocryptosis of the fingernail that developed following a nail procedure.
Because nail dystrophy is a relatively common complication of nail surgery, dermatologic surgeons should be aware of this simple, cost-effective, and noninvasive technique for the treatment of distal onychocryptosis.
- Geizhals S, Lipner SR. Review of onychocryptosis: epidemiology, pathogenesis, risk factors, diagnosis and treatment. Dermatol Online J. 2019;25:13030/qt9985w2n0
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown toenail management. Am Fam Physician. 2019;100:158-164.
- Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555. doi:10.1370/afm.1712
- Watabe A, Yamasaki K, Hashimoto A, et al. Retrospective evaluation of conservative treatment for 140 ingrown toenails with a novel taping procedure. Acta Derm Venereol. 2015;95:822-825. doi:10.2340/00015555-2065
- Arai H, Haneke E. Noninvasive treatment for ingrown nails: anchor taping, acrylic affixed gutter splint, sculptured nail, and others. In: Baran R, Hadj-Rabia S, Silverman R, eds. Pediatric Nail Disorders. CRC Press; 2017:252-274.
Practice Gap
Onychocryptosis, colloquially known as an ingrown nail, most commonly affects the lateral folds of the toenails. It also can affect the fingernails and the distal aspect of the nail unit, though these presentations are not as well described in the literature. In onychocryptosis, the nail plate grows downward into the periungual skin, resulting in chronic pain and inflammation. Risk factors include overtrimming the nails with rounded edges, local trauma, nail surgery, wearing tight footwear, obesity, and onychomycosis.1
Although surgical intervention might be required for severe or refractory disease, conservative treatment options are first line and often curative. A variety of techniques have been designed to separate the ingrown portion of the nail plate from underlying skin, including placement of an intervening piece of dental floss, cotton, or plastic tubing.2
Anchor taping is another effective method of treating onychocryptosis; a strip of tape is used to gently pull and secure the affected nail fold away from the overlying nail plate. This technique has been well described for the treatment of onychocryptosis of the lateral toenail.3-5 In 2017, Arai and Haneke5 presented a modified technique for the treatment of distal disease.
We present a simplified method that was used successfully in a case of distal onychocryptosis of the thumbnail that occurred approximately 4 months after complete nail avulsion with a nail matrix biopsy (Figure 1).

The Technique
A strongly adhesive, soft cotton, elastic tape that is 1-inch wide, such as Elastikon Elastic Tape (Johnson & Johnson), is used to pull and secure the hyponychium away from the overlying nail plate. When this technique is used for lateral onychocryptosis, a single strip of tape is secured to the affected lateral nail fold, pulled obliquely and proximally, and secured to the base of the digit.3-5 In the Arai and Haneke5 method for the treatment of distal disease, a piece of tape is first placed at the distal nail fold, pulled proximally, and secured to the ventral aspect of the digit. Then, 1 or 2 additional strips of tape are applied to the lateral nail folds, pulled obliquely, and adhered to the base of the digit, as in the classic technique for lateral onychocryptosis.5
In our modification for the treatment of distal disease, only 2 strips of tape are required, each approximately 5-cm long. The first strip of tape is applied to the hyponychium parallel to the long axis of the finger, pulled away from the distal edge of the nail plate, and secured obliquely and proximally to the base of the finger on one side. The second strip of tape is applied to the hyponychium in the same manner, directly overlying the first strip, but is then pulled obliquely in the opposite direction and secured to the other side of the proximal finger (Figure 2). The 2 strips of tape are applied directly overlying each other at the distal nail fold but with opposing tension vectors to optimize pull on the distal nail fold. This modification eliminates the need to apply an initial strip of tape along the long axis of the digit, as described by Arai and Haneke.5

The patient is instructed on this method in the office and will change the tape at home daily for 2 to 6 weeks, until the nail plate has grown out over the hyponychium (Figure 3). This technique also can be combined with other modalities, such as dilute vinegar soaks performed daily after changing the tape to ease inflammation and prevent infection. Because strongly adhesive tape is used, it also is recommended that the patient soak the tape before removing it to prevent damage to underlying skin.

Practice Implications
Anchor taping is a common and effective treatment of onychocryptosis. Most techniques described in the literature are for lateral toenail cases, which often are managed by podiatry. A modification for the treatment of distal onychocryptosis has been previously described.5 We describe a similar modification using 2 tape strips pulled in opposite directions, which successfully resolved a case of distal onychocryptosis of the fingernail that developed following a nail procedure.
Because nail dystrophy is a relatively common complication of nail surgery, dermatologic surgeons should be aware of this simple, cost-effective, and noninvasive technique for the treatment of distal onychocryptosis.
Practice Gap
Onychocryptosis, colloquially known as an ingrown nail, most commonly affects the lateral folds of the toenails. It also can affect the fingernails and the distal aspect of the nail unit, though these presentations are not as well described in the literature. In onychocryptosis, the nail plate grows downward into the periungual skin, resulting in chronic pain and inflammation. Risk factors include overtrimming the nails with rounded edges, local trauma, nail surgery, wearing tight footwear, obesity, and onychomycosis.1
Although surgical intervention might be required for severe or refractory disease, conservative treatment options are first line and often curative. A variety of techniques have been designed to separate the ingrown portion of the nail plate from underlying skin, including placement of an intervening piece of dental floss, cotton, or plastic tubing.2
Anchor taping is another effective method of treating onychocryptosis; a strip of tape is used to gently pull and secure the affected nail fold away from the overlying nail plate. This technique has been well described for the treatment of onychocryptosis of the lateral toenail.3-5 In 2017, Arai and Haneke5 presented a modified technique for the treatment of distal disease.
We present a simplified method that was used successfully in a case of distal onychocryptosis of the thumbnail that occurred approximately 4 months after complete nail avulsion with a nail matrix biopsy (Figure 1).

The Technique
A strongly adhesive, soft cotton, elastic tape that is 1-inch wide, such as Elastikon Elastic Tape (Johnson & Johnson), is used to pull and secure the hyponychium away from the overlying nail plate. When this technique is used for lateral onychocryptosis, a single strip of tape is secured to the affected lateral nail fold, pulled obliquely and proximally, and secured to the base of the digit.3-5 In the Arai and Haneke5 method for the treatment of distal disease, a piece of tape is first placed at the distal nail fold, pulled proximally, and secured to the ventral aspect of the digit. Then, 1 or 2 additional strips of tape are applied to the lateral nail folds, pulled obliquely, and adhered to the base of the digit, as in the classic technique for lateral onychocryptosis.5
In our modification for the treatment of distal disease, only 2 strips of tape are required, each approximately 5-cm long. The first strip of tape is applied to the hyponychium parallel to the long axis of the finger, pulled away from the distal edge of the nail plate, and secured obliquely and proximally to the base of the finger on one side. The second strip of tape is applied to the hyponychium in the same manner, directly overlying the first strip, but is then pulled obliquely in the opposite direction and secured to the other side of the proximal finger (Figure 2). The 2 strips of tape are applied directly overlying each other at the distal nail fold but with opposing tension vectors to optimize pull on the distal nail fold. This modification eliminates the need to apply an initial strip of tape along the long axis of the digit, as described by Arai and Haneke.5

The patient is instructed on this method in the office and will change the tape at home daily for 2 to 6 weeks, until the nail plate has grown out over the hyponychium (Figure 3). This technique also can be combined with other modalities, such as dilute vinegar soaks performed daily after changing the tape to ease inflammation and prevent infection. Because strongly adhesive tape is used, it also is recommended that the patient soak the tape before removing it to prevent damage to underlying skin.

Practice Implications
Anchor taping is a common and effective treatment of onychocryptosis. Most techniques described in the literature are for lateral toenail cases, which often are managed by podiatry. A modification for the treatment of distal onychocryptosis has been previously described.5 We describe a similar modification using 2 tape strips pulled in opposite directions, which successfully resolved a case of distal onychocryptosis of the fingernail that developed following a nail procedure.
Because nail dystrophy is a relatively common complication of nail surgery, dermatologic surgeons should be aware of this simple, cost-effective, and noninvasive technique for the treatment of distal onychocryptosis.
- Geizhals S, Lipner SR. Review of onychocryptosis: epidemiology, pathogenesis, risk factors, diagnosis and treatment. Dermatol Online J. 2019;25:13030/qt9985w2n0
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown toenail management. Am Fam Physician. 2019;100:158-164.
- Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555. doi:10.1370/afm.1712
- Watabe A, Yamasaki K, Hashimoto A, et al. Retrospective evaluation of conservative treatment for 140 ingrown toenails with a novel taping procedure. Acta Derm Venereol. 2015;95:822-825. doi:10.2340/00015555-2065
- Arai H, Haneke E. Noninvasive treatment for ingrown nails: anchor taping, acrylic affixed gutter splint, sculptured nail, and others. In: Baran R, Hadj-Rabia S, Silverman R, eds. Pediatric Nail Disorders. CRC Press; 2017:252-274.
- Geizhals S, Lipner SR. Review of onychocryptosis: epidemiology, pathogenesis, risk factors, diagnosis and treatment. Dermatol Online J. 2019;25:13030/qt9985w2n0
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown toenail management. Am Fam Physician. 2019;100:158-164.
- Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555. doi:10.1370/afm.1712
- Watabe A, Yamasaki K, Hashimoto A, et al. Retrospective evaluation of conservative treatment for 140 ingrown toenails with a novel taping procedure. Acta Derm Venereol. 2015;95:822-825. doi:10.2340/00015555-2065
- Arai H, Haneke E. Noninvasive treatment for ingrown nails: anchor taping, acrylic affixed gutter splint, sculptured nail, and others. In: Baran R, Hadj-Rabia S, Silverman R, eds. Pediatric Nail Disorders. CRC Press; 2017:252-274.
Review eyes nail unit toxicities secondary to targeted cancer therapy
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Microbiome: Gut dysbiosis linked to development of alopecia areata
presented at the annual meeting of the Society for Investigative Dermatology.
There have been reports of gut microbiome dysbiosis associated with autoimmune diseases such as rheumatoid arthritis, diabetes, and celiac disease. “It is now clear that these events not just shape the immune response in the gut, but also distant sites and immune-privileged organs,” Tanya Sezin, a doctor of natural science from the University of Lübeck (Germany) and Columbia University, New York, said in her presentation.
Whether the gut microbiome may also play a role as an environmental factor in alopecia areata, another T-cell–mediated autoimmune disease for which there are few available treatment options, is being evaluated at the Christiano Laboratory at Columbia University, Dr. Sezin noted. “Much of the difficulty underlying the lack of an effective treatment has been the incomplete understanding of the pathogenesis of AA.”
She also referred to several case reports describing hair growth in patients who received fecal microbiota transplantation (FMT), including a 20-year-old with alopecia universalis, who experienced hair growth after receiving FMT for Crohn’s disease.
Dr. Sezin and colleagues at the lab first performed a study in mice to test whether the gut microbiome was involved in the pathogenesis of AA. Mice given an antibiotic cocktail of ampicillin, neomycin, and vancomycin prior to or at the time of a skin graft taken from a mouse model of AA to induce AA were protected from hair loss, while mice given the antibiotic cocktail after skin grafting were not protected from hair loss.
“16S rRNA sequencing analysis of the gut microbiota revealed a significant shift in gut microbiome composition in animals treated with antibiotics and protected from hair loss, as reflected by significant changes in alpha and beta diversity,” Dr. Sezin explained. “In AA mice, we also observed differential abundance of families from the Bacteroidetes and Firmicutes phyla.” Specifically, Lactobacillus murinus and Muribaculum intestinale were overrepresented in mice with AA.
The investigators then performed 16S rRNA sequencing on 26 patients with AA, who stopped treatment for 30 days beforehand, and 9 participants who did not have AA as controls. “Though we did not observe difference in alpha and beta diversity, we see changes in the relative abundance of several families belonging to the Firmicutes phyla,” in patients with AA, Dr. Sezin said.
In another cohort of 30 patients with AA and 20 participants without AA, who stopped treatment before the study, Dr. Sezin and colleagues found “differences in the relative abundance of members of the Firmicutes and Bacteroides phyla,” including Bacteroides caccae, Prevotella copri, Syntrophomonas wolfei, Blautia wexlerae, and Eubacterium eligens, she said. “Consistent with our findings, there are previous reports in the literature showing gut dysbiosis in several other autoimmune diseases associated with differential regulation of some of the top species we have identified.”
Dr. Sezin said her group is recruiting patients for a clinical trial evaluating FMT in patients with AA. “We plan to study the association between changes in the gut microbiome and immune cell composition in AA patients undergoing FMT,” she said. “Additionally, functional studies in mice are also currently [being conducted] to further pinpoint the contribution of gut microbiome to the pathogenesis of AA.”
When asked during the discussion session if there was any relationship between the skin microbiome and AA, Dr. Sezin said there was no connection found in mice studies, which she and her colleagues are investigating further. “In the human samples, we are currently recruiting more patients and healthy controls to try to get a better understanding of whether we see differences in the skin microbiome,” she added.
Dr. Sezin explained that how the gut microbiota “is really remediated in alopecia areata” is not well understood. “We think that it is possible that we see intestinal permeability in the gut due to the gut dysbiosis that we see in alopecia areata patients, and this might lead to systemic distribution of bacteria, which might cross-react or present cross reactivity with the antigens” identified in AA, which is also being investigated, she said.
FMT not a ‘simple fix’ for AA
Leslie Castelo-Soccio, MD, PhD, a dermatologist at the Children’s Hospital of Philadelphia, who was not involved with the research, said in an interview that the findings presented by Dr. Sezin show how AA shares similarities with other autoimmune diseases. “It does highlight how important the gut microbiome is to human disease, and that differences in relative abundance of bacteria play one part as a trigger in a genetically susceptible person.”
However, while some autoimmune diseases have a big difference in alpha and beta diversity, for example, “this has not been seen in people with alopecia areata,” Dr. Castelo-Soccio pointed out. “The differences are more subtle in terms of amounts of certain bacteria,” she said, noting that, in this study, the biggest differences were seen in the studies of mice.
Dr. Castelo-Soccio also said there may be also be differences in the gut microbiome in children and adults. “The gut microbiome shifts in very early childhood from a very diverse microbiome to a more ‘adult microbiome’ around age 4, which is the age we see the first peak of many autoimmune diseases, including alopecia areata. I think microbiome work in humans needs to focus on this transition point.”
As for the clinical trial at Columbia that is evaluating FMT in patients with AA, Dr. Castelo-Soccio said she is excited. “There is much to learn about fecal transplant for all diseases and about the role of the gut microbiome and environment. Most of what we know for fecal transplant centers on its use for Clostridium difficile infections.”
Patients and their families have been asking about the potential for FMT in alopecia area, Dr. Castelo-Soccio said, but some believe it is a “simple fix” when the reality is much more complex.
“When I speak to patients and families about this, I explain that currently the ‘active ingredient’ in fecal transplants is not definitively established. In any one donor, the community of bacteria is highly variable and can be from batch to batch. While the short-term risks are relatively low – cramping, diarrhea, discomfort, mode of delivery – there are reports of transmission of infectious bacteria from donors like [Escherichia] coli, which have led to severe infections.”
Long-term safety and durability of effects are also unclear, “so we do not know if a patient receiving one [FMT] will need many in the future. We do not know how changing the microbiome could affect the transplant recipient in terms of noninfectious diseases/disorders. We are learning about the role of microbiome in obesity, insulin resistance, mood disorders. We could be ‘fixing’ one trigger of alopecia but setting up [the] patient for other noninfectious conditions,” Dr. Castelo-Soccio said.
Dr. Sezin and Dr. Castelo-Soccio reported no relevant financial disclosures.
presented at the annual meeting of the Society for Investigative Dermatology.
There have been reports of gut microbiome dysbiosis associated with autoimmune diseases such as rheumatoid arthritis, diabetes, and celiac disease. “It is now clear that these events not just shape the immune response in the gut, but also distant sites and immune-privileged organs,” Tanya Sezin, a doctor of natural science from the University of Lübeck (Germany) and Columbia University, New York, said in her presentation.
Whether the gut microbiome may also play a role as an environmental factor in alopecia areata, another T-cell–mediated autoimmune disease for which there are few available treatment options, is being evaluated at the Christiano Laboratory at Columbia University, Dr. Sezin noted. “Much of the difficulty underlying the lack of an effective treatment has been the incomplete understanding of the pathogenesis of AA.”
She also referred to several case reports describing hair growth in patients who received fecal microbiota transplantation (FMT), including a 20-year-old with alopecia universalis, who experienced hair growth after receiving FMT for Crohn’s disease.
Dr. Sezin and colleagues at the lab first performed a study in mice to test whether the gut microbiome was involved in the pathogenesis of AA. Mice given an antibiotic cocktail of ampicillin, neomycin, and vancomycin prior to or at the time of a skin graft taken from a mouse model of AA to induce AA were protected from hair loss, while mice given the antibiotic cocktail after skin grafting were not protected from hair loss.
“16S rRNA sequencing analysis of the gut microbiota revealed a significant shift in gut microbiome composition in animals treated with antibiotics and protected from hair loss, as reflected by significant changes in alpha and beta diversity,” Dr. Sezin explained. “In AA mice, we also observed differential abundance of families from the Bacteroidetes and Firmicutes phyla.” Specifically, Lactobacillus murinus and Muribaculum intestinale were overrepresented in mice with AA.
The investigators then performed 16S rRNA sequencing on 26 patients with AA, who stopped treatment for 30 days beforehand, and 9 participants who did not have AA as controls. “Though we did not observe difference in alpha and beta diversity, we see changes in the relative abundance of several families belonging to the Firmicutes phyla,” in patients with AA, Dr. Sezin said.
In another cohort of 30 patients with AA and 20 participants without AA, who stopped treatment before the study, Dr. Sezin and colleagues found “differences in the relative abundance of members of the Firmicutes and Bacteroides phyla,” including Bacteroides caccae, Prevotella copri, Syntrophomonas wolfei, Blautia wexlerae, and Eubacterium eligens, she said. “Consistent with our findings, there are previous reports in the literature showing gut dysbiosis in several other autoimmune diseases associated with differential regulation of some of the top species we have identified.”
Dr. Sezin said her group is recruiting patients for a clinical trial evaluating FMT in patients with AA. “We plan to study the association between changes in the gut microbiome and immune cell composition in AA patients undergoing FMT,” she said. “Additionally, functional studies in mice are also currently [being conducted] to further pinpoint the contribution of gut microbiome to the pathogenesis of AA.”
When asked during the discussion session if there was any relationship between the skin microbiome and AA, Dr. Sezin said there was no connection found in mice studies, which she and her colleagues are investigating further. “In the human samples, we are currently recruiting more patients and healthy controls to try to get a better understanding of whether we see differences in the skin microbiome,” she added.
Dr. Sezin explained that how the gut microbiota “is really remediated in alopecia areata” is not well understood. “We think that it is possible that we see intestinal permeability in the gut due to the gut dysbiosis that we see in alopecia areata patients, and this might lead to systemic distribution of bacteria, which might cross-react or present cross reactivity with the antigens” identified in AA, which is also being investigated, she said.
FMT not a ‘simple fix’ for AA
Leslie Castelo-Soccio, MD, PhD, a dermatologist at the Children’s Hospital of Philadelphia, who was not involved with the research, said in an interview that the findings presented by Dr. Sezin show how AA shares similarities with other autoimmune diseases. “It does highlight how important the gut microbiome is to human disease, and that differences in relative abundance of bacteria play one part as a trigger in a genetically susceptible person.”
However, while some autoimmune diseases have a big difference in alpha and beta diversity, for example, “this has not been seen in people with alopecia areata,” Dr. Castelo-Soccio pointed out. “The differences are more subtle in terms of amounts of certain bacteria,” she said, noting that, in this study, the biggest differences were seen in the studies of mice.
Dr. Castelo-Soccio also said there may be also be differences in the gut microbiome in children and adults. “The gut microbiome shifts in very early childhood from a very diverse microbiome to a more ‘adult microbiome’ around age 4, which is the age we see the first peak of many autoimmune diseases, including alopecia areata. I think microbiome work in humans needs to focus on this transition point.”
As for the clinical trial at Columbia that is evaluating FMT in patients with AA, Dr. Castelo-Soccio said she is excited. “There is much to learn about fecal transplant for all diseases and about the role of the gut microbiome and environment. Most of what we know for fecal transplant centers on its use for Clostridium difficile infections.”
Patients and their families have been asking about the potential for FMT in alopecia area, Dr. Castelo-Soccio said, but some believe it is a “simple fix” when the reality is much more complex.
“When I speak to patients and families about this, I explain that currently the ‘active ingredient’ in fecal transplants is not definitively established. In any one donor, the community of bacteria is highly variable and can be from batch to batch. While the short-term risks are relatively low – cramping, diarrhea, discomfort, mode of delivery – there are reports of transmission of infectious bacteria from donors like [Escherichia] coli, which have led to severe infections.”
Long-term safety and durability of effects are also unclear, “so we do not know if a patient receiving one [FMT] will need many in the future. We do not know how changing the microbiome could affect the transplant recipient in terms of noninfectious diseases/disorders. We are learning about the role of microbiome in obesity, insulin resistance, mood disorders. We could be ‘fixing’ one trigger of alopecia but setting up [the] patient for other noninfectious conditions,” Dr. Castelo-Soccio said.
Dr. Sezin and Dr. Castelo-Soccio reported no relevant financial disclosures.
presented at the annual meeting of the Society for Investigative Dermatology.
There have been reports of gut microbiome dysbiosis associated with autoimmune diseases such as rheumatoid arthritis, diabetes, and celiac disease. “It is now clear that these events not just shape the immune response in the gut, but also distant sites and immune-privileged organs,” Tanya Sezin, a doctor of natural science from the University of Lübeck (Germany) and Columbia University, New York, said in her presentation.
Whether the gut microbiome may also play a role as an environmental factor in alopecia areata, another T-cell–mediated autoimmune disease for which there are few available treatment options, is being evaluated at the Christiano Laboratory at Columbia University, Dr. Sezin noted. “Much of the difficulty underlying the lack of an effective treatment has been the incomplete understanding of the pathogenesis of AA.”
She also referred to several case reports describing hair growth in patients who received fecal microbiota transplantation (FMT), including a 20-year-old with alopecia universalis, who experienced hair growth after receiving FMT for Crohn’s disease.
Dr. Sezin and colleagues at the lab first performed a study in mice to test whether the gut microbiome was involved in the pathogenesis of AA. Mice given an antibiotic cocktail of ampicillin, neomycin, and vancomycin prior to or at the time of a skin graft taken from a mouse model of AA to induce AA were protected from hair loss, while mice given the antibiotic cocktail after skin grafting were not protected from hair loss.
“16S rRNA sequencing analysis of the gut microbiota revealed a significant shift in gut microbiome composition in animals treated with antibiotics and protected from hair loss, as reflected by significant changes in alpha and beta diversity,” Dr. Sezin explained. “In AA mice, we also observed differential abundance of families from the Bacteroidetes and Firmicutes phyla.” Specifically, Lactobacillus murinus and Muribaculum intestinale were overrepresented in mice with AA.
The investigators then performed 16S rRNA sequencing on 26 patients with AA, who stopped treatment for 30 days beforehand, and 9 participants who did not have AA as controls. “Though we did not observe difference in alpha and beta diversity, we see changes in the relative abundance of several families belonging to the Firmicutes phyla,” in patients with AA, Dr. Sezin said.
In another cohort of 30 patients with AA and 20 participants without AA, who stopped treatment before the study, Dr. Sezin and colleagues found “differences in the relative abundance of members of the Firmicutes and Bacteroides phyla,” including Bacteroides caccae, Prevotella copri, Syntrophomonas wolfei, Blautia wexlerae, and Eubacterium eligens, she said. “Consistent with our findings, there are previous reports in the literature showing gut dysbiosis in several other autoimmune diseases associated with differential regulation of some of the top species we have identified.”
Dr. Sezin said her group is recruiting patients for a clinical trial evaluating FMT in patients with AA. “We plan to study the association between changes in the gut microbiome and immune cell composition in AA patients undergoing FMT,” she said. “Additionally, functional studies in mice are also currently [being conducted] to further pinpoint the contribution of gut microbiome to the pathogenesis of AA.”
When asked during the discussion session if there was any relationship between the skin microbiome and AA, Dr. Sezin said there was no connection found in mice studies, which she and her colleagues are investigating further. “In the human samples, we are currently recruiting more patients and healthy controls to try to get a better understanding of whether we see differences in the skin microbiome,” she added.
Dr. Sezin explained that how the gut microbiota “is really remediated in alopecia areata” is not well understood. “We think that it is possible that we see intestinal permeability in the gut due to the gut dysbiosis that we see in alopecia areata patients, and this might lead to systemic distribution of bacteria, which might cross-react or present cross reactivity with the antigens” identified in AA, which is also being investigated, she said.
FMT not a ‘simple fix’ for AA
Leslie Castelo-Soccio, MD, PhD, a dermatologist at the Children’s Hospital of Philadelphia, who was not involved with the research, said in an interview that the findings presented by Dr. Sezin show how AA shares similarities with other autoimmune diseases. “It does highlight how important the gut microbiome is to human disease, and that differences in relative abundance of bacteria play one part as a trigger in a genetically susceptible person.”
However, while some autoimmune diseases have a big difference in alpha and beta diversity, for example, “this has not been seen in people with alopecia areata,” Dr. Castelo-Soccio pointed out. “The differences are more subtle in terms of amounts of certain bacteria,” she said, noting that, in this study, the biggest differences were seen in the studies of mice.
Dr. Castelo-Soccio also said there may be also be differences in the gut microbiome in children and adults. “The gut microbiome shifts in very early childhood from a very diverse microbiome to a more ‘adult microbiome’ around age 4, which is the age we see the first peak of many autoimmune diseases, including alopecia areata. I think microbiome work in humans needs to focus on this transition point.”
As for the clinical trial at Columbia that is evaluating FMT in patients with AA, Dr. Castelo-Soccio said she is excited. “There is much to learn about fecal transplant for all diseases and about the role of the gut microbiome and environment. Most of what we know for fecal transplant centers on its use for Clostridium difficile infections.”
Patients and their families have been asking about the potential for FMT in alopecia area, Dr. Castelo-Soccio said, but some believe it is a “simple fix” when the reality is much more complex.
“When I speak to patients and families about this, I explain that currently the ‘active ingredient’ in fecal transplants is not definitively established. In any one donor, the community of bacteria is highly variable and can be from batch to batch. While the short-term risks are relatively low – cramping, diarrhea, discomfort, mode of delivery – there are reports of transmission of infectious bacteria from donors like [Escherichia] coli, which have led to severe infections.”
Long-term safety and durability of effects are also unclear, “so we do not know if a patient receiving one [FMT] will need many in the future. We do not know how changing the microbiome could affect the transplant recipient in terms of noninfectious diseases/disorders. We are learning about the role of microbiome in obesity, insulin resistance, mood disorders. We could be ‘fixing’ one trigger of alopecia but setting up [the] patient for other noninfectious conditions,” Dr. Castelo-Soccio said.
Dr. Sezin and Dr. Castelo-Soccio reported no relevant financial disclosures.
FROM SID 2021
The Top 100 Most-Cited Articles on Nail Psoriasis: A Bibliometric Analysis
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
Use of Complementary Alternative Medicine and Supplementation for Skin Disease
Complementary alternative medicine (CAM) has been described by the National Center for Complementary and Integrative Medicine as “health care approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice.”1 Although this definition is broad, CAM encompasses therapies such as traditional Chinese medicine, herbal therapies, dietary supplements, and mind/body interventions. The use of CAM has grown, and according to a 2012 National Center for Complementary and Integrative Health survey, more than 30% of US adults and 12% of US children use health care approaches that are considered outside of conventional medical practice. In a survey study of US adults, at least 17.7% of respondents said they had taken a dietary supplement other than a vitamin or mineral in the last year.1 Data from the 2007 National Health Interview Survey showed that the prevalence of adults with skin conditions using CAM was 84.5% compared to 38.3% in the general population.2 In addition, 8.15 million US patients with dermatologic conditions reported using CAM over a 5-year period.3 Complementary alternative medicine has emerged as an alternative or adjunct to standard treatments, making it important for dermatologists to understand the existing literature on these therapies. Herein, we review the current evidence-based literature that exists on CAM for the treatment of atopic dermatitis (AD), psoriasis, and alopecia areata (AA).
Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition with considerable morbidity.4,5 The pathophysiology of AD is multifactorial and includes aspects of barrier dysfunction, IgE hypersensitivity, abnormal cell-mediated immune response, and environmental factors.6 Atopic dermatitis also is one of the most common inflammatory skin conditions in adults, affecting more than 7% of the US population and up to 20% of the total population in developed countries. Of those affected, 40% have moderate or severe symptoms that result in a substantial impact on quality of life.7 Despite advances in understanding disease pathology and treatment, a subset of patients opt to defer conventional treatments such as topical and systemic corticosteroids, antibiotics, nonsteroidal immunomodulators, and biologics. Patients may seek alternative therapies when typical treatments fail or when the perceived side effects outweigh the benefits.5,8 The use of CAM has been well described in patients with AD; however, the existing evidence supporting its use along with its safety profile have not been thoroughly explored. Herein, we will discuss some of the most well-studied supplements for treatment of AD, including evening primrose oil (EPO), fish oil, and probiotics.5
Oral supplementation with polyunsaturated fatty acids commonly is reported in patients with AD.5,8 The idea that a fatty acid deficiency could lead to atopic skin conditions has been around since 1937, when it was suggested that patients with AD had lower levels of blood unsaturated fatty acids.9 Conflicting evidence regarding oral fatty acid ingestion and AD disease severity has emerged.10,11 One unsaturated fatty acid, γ-linolenic acid (GLA), has demonstrated anti-inflammatory properties and involvement in barrier repair.12 It is converted to dihomo-GLA in the body, which acts on cyclooxygenase enzymes to produce the inflammatory mediator prostaglandin E1. The production of GLA is mediated by the enzyme delta-6 desaturase in the metabolization of linoleic acid.12 However, it has been reported that in a subset of patients with AD, a malfunction of delta-6 desaturase may play a role in disease progression and result in lower baseline levels of GLA.10,12 Evening primrose oil and borage oil contain high amounts of GLA (8%–10% and 23%, respectively); thus, supplementation with these oils has been studied in AD.13
EPO for AD
Studies investigating EPO (Oenothera biennis) and its association with AD severity have shown mixed results. A Cochrane review reported that oral borage oil and EPO were not effective treatments for AD,14 while another larger randomized controlled trial (RCT) found no statistically significant improvement in AD symptoms.15 However, multiple smaller studies have found that clinical symptoms of AD, such as erythema, xerosis, pruritus, and total body surface area involved, did improve with oral EPO supplementation when compared to placebo, and the results were statistically significant (P=.04).16,17 One study looked at different dosages of EPO and found that groups ingesting both 160 mg and 320 mg daily experienced reductions in eczema area and severity index score, with greater improvement noted with the higher dosage.17 Side effects associated with oral EPO include an anticoagulant effect and transient gastrointestinal tract upset.8,14 There currently is not enough evidence or safety data to recommend this supplement to AD patients.
Although topical use of fatty acids with high concentrations of GLA, such as EPO and borage oil, have demonstrated improvement in subjective symptom severity, most studies have not reached statistical significance.10,11 One study used a 10% EPO cream for 2 weeks compared to placebo and found statistically significant improvement in patient-reported AD symptoms (P=.045). However, this study only included 10 participants, and therefore larger studies are necessary to confirm this result.18 Some RCTs have shown that topical coconut oil, sunflower seed oil, and sandalwood album oil improve AD symptom severity, but again, large controlled trials are needed.5 Unfortunately, many essential oils, including EPO, can cause a secondary allergic contact dermatitis and potentially worsen AD.19
Fish Oil for AD
Fish oil is a commonly used supplement for AD due to its high content of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-3 fatty acids exert anti-inflammatory effects by displacing arachidonic acid, a proinflammatory omega-6 fatty acid thought to increase IgE, as well as helper T cell (TH2) cytokines and prostaglandin E2.8,20 A 2012 Cochrane review found that, while some studies revealed mild improvement in AD symptoms with oral fish oil supplementation, these RCTs were of poor methodological quality.21 Multiple smaller studies have shown a decrease in pruritus, severity, and physician-rated clinical scores with fish oil use.5,8,20,22 One study with 145 participants reported that 6 g of fish oil once daily compared to isoenergetic corn oil for 16 weeks identified no statistically significant differences between the treatment groups.20 No adverse events were identified in any of the reported trials. Further studies should be conducted to assess the utility and dosing of fish oil supplements in AD patients.
Probiotics for AD
Probiotics consist of live microorganisms that enhance the microflora of the gastrointestinal tract.8,20 They have been shown to influence food digestion and also have demonstrated potential influence on the skin-gut axis.23 The theory that intestinal dysbiosis plays a role in AD pathogenesis has been investigated in multiple studies.23-25 The central premise is that low-fiber and high-fat Western diets lead to fundamental changes in the gut microbiome, resulting in fewer anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs).23-25 These SCFAs are produced by microbes during the fermentation of dietary fiber and are known for their effect on epithelial barrier integrity and anti-inflammatory properties mediated through G protein–coupled receptor 43.25 Multiple studies have shown that the gut microbiome in patients with AD have higher proportions of Clostridium difficile, Escherichia coli, and Staphylococcus aureus and lower levels of Bifidobacterium, Bacteroidetes, and Bacteroides species compared to healthy controls.26,27 Metagenomic analysis of fecal samples from patients with AD have shown a reduction of Faecalibacterium prausnitzii species when compared to controls, along with a decreased SCFA production, leading to the hypothesis that the gut microbiome may play a role in epithelial barrier disruption.28,29 Systematic reviews and smaller studies have found that oral probiotic use does lead to AD symptom improvement.8,30,31 A systematic review of 25 RCTs with 1599 participants found that supplementation with oral probiotics significantly decreased the SCORAD (SCORing Atopic Dermatitis) index in adults and children older than 1 year with AD but had no effect on infants younger than 1 year (P<.001). They also found that supplementation with diverse microbes or Lactobacillus species showed greater benefit than Bifidobacterium species alone.30 Another study analyzed the effect of oral Lactobacillus fermentum (1×109 CFU twice daily) in 53 children with AD vs placebo for 16 weeks. This study found a statically significant decrease in SCORAD index between oral probiotics and placebo, with 92% (n=24) of participants supplementing with probiotics having a lower SCORAD index than baseline compared to 63% (n=17) in the placebo group (P=.01).31 However, the use of probiotics for AD treatment has remained controversial. Two recent systematic reviews, including 39 RCTs of 2599 randomized patients, found that the use of currently available oral probiotics made little or no difference in patient-rated AD symptoms, investigator-rated AD symptoms, or quality of life.32,33 No adverse effects were observed in the included studies. Unfortunately, the individual RCTs included were heterogeneous, and future studies with standardized probiotic supplementation should be undertaken before probiotics can be routinely recommended.
The use of topical probiotics in AD also has recently emerged. Multiple studies have shown that patients with AD have higher levels of colonization with S aureus, which is associated with T-cell dysfunction, more severe allergic skin reactions, and disruptions in barrier function.34,35 Therefore, altering the skin microbiota through topical probiotics could theoretically reduce AD symptoms and flares. Multiple RCTs and smaller studies have shown that topical probiotics can alter the skin microbiota, improve erythema, and decrease scaling and pruritus in AD patients.35-38 One study used a heat-treated Lactobacillus johnsonii 0.3% lotion twice daily for 3 weeks vs placebo in patients with AD with positive S aureus skin cultures. The S aureus load decreased in patients using the topical probiotic lotion, which correlated with lower SCORAD index that was statistically significant compared to placebo (P=.012).36 More robust studies are needed to determine if topical probiotics should routinely be recommended in AD.
Psoriasis
Psoriasis vulgaris is a chronic inflammatory skin condition characterized by pruritic, hyperkeratotic, scaly plaques.39,40 Keratinocyte hyperproliferation is central to psoriasis pathogenesis and is thought to be a T-cell–driven reaction to antigens or trauma in genetically predisposed individuals. Standard treatments for psoriasis currently include topical corticosteroids and anti-inflammatories, oral immunomodulatory therapy, biologic agents, and phototherapy.40 The use of CAM is highly prevalent among patients with psoriasis, with one study reporting that 51% (n=162) of psoriatic patients interviewed had used CAM.41 The most common reasons for CAM use included dissatisfaction with current treatment, adverse side effects of standard therapy, and patient-reported attempts at “trying everything to heal disease.”42 Herein, we will discuss some of the most frequently used supplements for treatment of psoriatic disease.39
Fish Oil for Psoriasis
One of the most common supplements used by patients with psoriasis is fish oil due to its purported anti-inflammatory qualities.20,39 The consensus on fish oil supplementation for psoriasis is mixed.43-45 Multiple RCTs have reported reductions in psoriasis area and severity index (PASI) scores or symptomatic improvement with variable doses of fish oil.44,46 One RCT found that using EPA 1.8 g once daily and DHA 1.2 g once daily for 12 weeks resulted in significant improvement in pruritus, scaling, and erythema (P<.05).44 Another study reported a significant decrease in erythema (P=.02) and total body surface area affected (P=.0001) with EPA 3.6 g once daily and DHA 2.4 g once daily supplementation compared to olive oil supplementation for 15 weeks.46 Alternatively, multiple studies have failed to show statistically significant improvement in psoriatic symptoms with fish oil supplementation at variable doses and time frames (14–216 mg daily EPA, 9–80 mg daily DHA, from 2 weeks to 9 months).40,47,48 Fish oil may impart anticoagulant properties and should not be started without the guidance of a physician. Currently, there are no data to make specific recommendations on the use of fish oil as an adjunct psoriatic treatment.
Curcumin for Psoriasis
Another supplement routinely utilized in patients with psoriasis is curcumin,40,49,50 a yellow phytochemical that is a major component of the spice turmeric. Curcumin has been shown to inhibit certain proinflammatory cytokines including IL-17, IL-6, IFN-γ, and tumor necrosis factor α and has been regarded as having immune-modulating, anti-inflammatory, and antibacterial properties.40,50 Curcumin also has been reported to suppress phosphorylase kinase, an enzyme that has increased activity in psoriatic plaques that correlates with markers of psoriatic hyperproliferation.50,51 When applied topically, turmeric microgel 0.5% has been reported to decrease scaling, erythema, and psoriatic plaque thickness over the course of 9 weeks.50 In a nonrandomized trial with 10 participants, researchers found that phosphorylase kinase activity levels in psoriatic skin biopsies of patients applying topical curcumin 1% were lower than placebo and topical calcipotriol applied in combination. The lower phosphorylase kinase levels correlated with level of disease severity, and topical curcumin 1% showed a superior outcome when compared to topical calcipotriol.40,49 Although these preliminary results are interesting, there still are not enough data at this time to recommend topical curcumin as a treatment of psoriasis. No known adverse events have been reported with the use of topical curcumin to date.
Oral curcumin has poor oral bioavailability, and 40% to 90% of oral doses are excreted, making supplementation a challenge.40 In one RCT, oral curcumin 2 g daily (using a lecithin-based delivery system to increase bioavailability) was administered in combination with topical methylprednisolone aceponate 0.1%, resulting in significant improvement in psoriatic symptoms and lower IL-22 compared to placebo and topical methylprednisolone aceponate (P<.05).52 Other studies also have reported decreased PASI scores with oral curcumin supplementation.53,54 Adverse effects reported with oral curcumin included gastrointestinal tract upset and hot flashes.53 Although there is early evidence that may support the use of oral curcumin supplementation for psoriasis, more data are needed before recommending this therapy.
Indigo Naturalis for Psoriasis
Topical indigo naturalis (IN) also has been reported to improve psoriasis symptoms.39,53,55 The antipsoriatic effects are thought to occur through the active ingredient in IN (indirubin), which is responsible for inhibition of keratinocyte proliferation.40 One study reported that topical IN 1.4% containing indirubin 0.16% with a petroleum ointment vehicle applied to psoriatic plaques over 12 weeks resulted in a significant decrease in PASI scores from 18.9 at baseline to 6.3 after IN treatment (P<.001).56 Another study found that over 8 weeks, topical application of IN 2.83% containing indirubin 0.24% to psoriatic plaques vs petroleum jelly resulted in 56.3% (n=9) of the treatment group achieving PASI 75 compared to 0% in the placebo group (n=24).55 One deterrent in topical IN treatment is the dark blue pigment it contains; however, no other adverse outcomes were found with topical IN treatment.56 Larger clinical trials are necessary to further explore IN as a potential adjunct treatment in patients with mild psoriatic disease. When taken orally, IN has caused gastrointestinal tract disturbance and elevated liver enzyme levels.57
Herbal Toxicities
It is important to consider that oral supplements including curcumin and IN are widely available over-the-counter and online without oversight by the US Food and Drug Administration.40 Herbal supplements typically are compounded with other ingredients and have been associated with hepatotoxicity as well as drug-supplement interactions, including abnormal bleeding and clotting.58 There exists a lack of general surveillance data, making the true burden of herbal toxicities more difficult to accurately discern. Although some supplements have been associated with anti-inflammatory qualities and disease improvement, other herbal supplements have been shown to possess immunostimulatory characteristics. Herbal supplements such as spirulina, chlorella, Aphanizomenon flos-aquae, and echinacea have been shown to upregulate inflammatory pathways in a variety of autoimmune skin conditions.59
Probiotics for Psoriasis
Data on probiotic use in patients with psoriasis are limited.23 A distinct pattern of dysbiosis has been identified in psoriatic patients, as there is thought to be depletion of beneficial bacteria such as Bifidobacterium, lactobacilli, and F prausnitzii and increased colonization with pathogenic organisms such as Salmonella, E coli, Heliobacter, Campylobacter, and Alcaligenes in psoriasis patients.23,59,60 Early mouse studies have supported this hypothesis, as mice fed with Lactobacillus pentosus have developed milder forms of imiquimod-induced psoriasis compared to placebo,55 and mice receiving probiotic supplementation have lower levels of psoriasis-related proinflammatory markers such as TH17-associated cytokines.61 Another study in humans found that daily oral Bifidobacterium infantis supplementation for 8 weeks in psoriatic patients resulted in lower C-reactive protein and tumor necrosis factor α levels compared to placebo.62 Studies on the use of topical probiotics in psoriasis have been limited, and more research is needed to explore this relationship.38 At this time, no specific recommendations can be made on the use of probiotics in psoriatic patients.
Alopecia Areata
Alopecia areata is nonscarring hair loss that can affect the scalp, face, or body.63,64 The pathophysiology of AA involves the attack of the hair follicle matrix epithelium by inflammatory cells without hair follicle stem cell destruction. The precise events that precipitate these episodes are unknown, but triggers such as emotional or physical stress, vaccines, or viral infections have been reported.65 There is no cure for AA, and current treatments such as topical minoxidil and corticosteroids (topical, intralesional, or oral) vary widely in efficacy.64 Although Janus kinase inhibitors recently have shown promising results in the treatment of AA, the need for prolonged therapy may be frustrating to patients.66 Severity of AA also can vary, with 30% of patients experiencing extensive hair loss.67 The use of CAM has been widely reported in AA due to high levels of dissatisfaction with existing therapies.68 Herein, we discuss the most studied alternative treatments used in AA
Garlic and Onion for Alopecia
One alternative treatment that has shown promising initial results is application of topical garlic and onion extracts to affected areas.64,69,70 Both garlic and onion belong to the Allium genus and are high in sulfur and phenolic compounds.70 They have been reported to possess bactericidal and vasodilatory activity,71 and it has been hypothesized that onion and garlic extracts may induce therapeutic effects through induction of a mild contact dermatitis.70 One single-blinded, controlled trial using topical crude onion juice reported that 86.9% (n=20) of patients had full regrowth of hair compared to 13.3% (n=2) of patients treated with a tap water placebo at 8 weeks (P<.0001). This study also noted that patients using onion juice had a higher rate of erythema at application site; unfortunately, the study was small with only 38 patients.70 Another double-blind RCT using garlic gel 5% with betamethasone valerate cream 0.1% compared to betamethasone valerate cream alone found that after 3 months, patients in the garlic gel group had increased terminal hairs and smaller patch sizes compared to the betamethasone valerate cream group.69 More studies are needed to confirm these results.
Aromatherapy With Essential Oils for Alopecia
Another alternative treatment in AA that has demonstrated positive results is aromatherapy skin massage with essential oils to patches of alopecia.72 Although certain essential oils, such as tea tree oil, have been reported to have specific antibacterial or anti-inflammatory properties, essential oils have been reported to cause allergic contact dermatitis and should be used with caution.73,74 For example, tea tree oil is a well-known cause of allergic contact dermatitis, and positive patch testing has ranged from 0.1% to 3.5% in studies assessing topical tea tree oil 5% application.75 Overall, there have been nearly 80 essential oils implicated in contact dermatitis, with high-concentration products being one of the highest risk factors for an allergic contact reaction.76 One RCT compared daily scalp massage with essential oils (rosemary, lavender, thyme, and cedarwood in a carrier oil) to daily scalp massage with a placebo carrier oil in AA patients. The results showed that at 7 months of treatment, 44% (n=19) of the aromatherapy group showed improvement compared to 15% (n=6) in the control group.77 Another study used a similar group of essential oils (thyme, rosemary, atlas cedar, lavender, and EPO in a carrier oil) with daily scalp massage and reported similar improvement of AA symptoms compared to control; the investigators also reported irritation at application site in 1 patient.78 There currently are not enough data to recommend aromatherapy skin massage for the treatment of AA, and this practice may cause harm to the patient by induction of allergic contact dermatitis.
There have been a few studies to suggest that the use of total glucosides of peony with compound glycyrrhizin and oral Korean red ginseng may have beneficial effects on AA treatment, but efficacy and safety data are lacking, and these therapies should not be recommended without more information.64,79,80
Final Thoughts
Dermatologic patients frequently are opting for CAM,2 and although some therapies may show promising initial results, alternative medicines also can drive adverse events.19,30 The lack of oversight from the US Food and Drug Administration on the products leads to many unknowns for true health risks with over-the-counter CAM supplements.40 As the use of CAM becomes increasingly common among dermatologic patients, it is important for dermatologists to understand the benefits and risks, especially for commonly treated conditions. More data is needed before CAM can be routinely recommended.
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Integrative Health website. Updated April 2021. Accessed April 25, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- Fuhrmann T, Smith N, Tausk F. Use of complementary and alternative medicine among adults with skin disease: updated results from a national survey. J Am Acad Dermatol. 2010;63:1000-1005.
- Landis ET, Davis SA, Feldman SR, et al. Complementary and alternative medicine use in dermatology in the United States. J Altern Complement Med. 2014;20:392-398.
- Solman L, Lloyd‐Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2016. part 1: treatment and prevention. Clin Exp Dermatol. 2019;44:363-369.
- Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol. 2016;17:557-581.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. In: Fortson EA, Feldman SR, Strowd LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:21-37.
- Atopic dermatitis in America. Asthma and Allergy Foundation of America website. Accessed July 30, 2021. https://www.aafa.org/atopic-dermatitis-in-america
- Schlichte MJ, Vandersall A, Katta R. Diet and eczema: a review of dietary supplements for the treatment of atopic dermatitis. Dermatol Pract Concept. 2016;6:23-29.
- Brown WR, Hansen AE. Arachidonic and linolic acid of the serum in normal and eczematous human subjects. Proc Soc Exp Bio Med. 1937;36:113-117.
- Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411-424.
- Ferreira MJ, Fiadeiro T, Silva M, et al. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998;7:213-216.
- Simon D, Eng PA, Borelli S, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014;31:180-188.
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411-1414.
- Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;4:CD004416.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ. 2003;327:2.
- Schalin-Karrila M, Mattila L, Jansen CT, et al. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11-19.
- Chung BY, Park SY, Jung MJ, et al. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409-416.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatolog Treat. 1990;1:199-201.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. Dermatitis. 2016;27:39-42.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371-1376.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema [published online February 15, 2012]. Cochrane Database Syst Rev. Accessed July 22, 2021. doi:10.1002/14651858.CD005205.pub3
- Balic´ A, Vlašic´ D, Žužul K, et al. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
- Salem I, Ramser A, Isham N, et al. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Pathogenesis Manage Atopic Dermatitis. 2011;41:112-124.
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286.
- Lee E, Lee S-Y, Kang M-J, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91-92.e1.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241-244.
- Kim H-J, Kim HY, Lee S-Y, et al. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376.
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860.
- Kim S-O, Ah Y-M, Yu YM, et al. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217-226.
- Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.

- Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135.
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15-21.
- Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222-228.
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:249-257.
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine. 2021;27:700-709.
- França K. Topical probiotics in dermatological therapy and skincare: a concise review. Dermatol Ther (Heidelb). 2020;11:71-77.
- Talbott W, Duffy N. Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. Am J Clin Dermatol. 2015;16:147-165.
- Gamret AC, Price A, Fertig RM, et al. Complementary and alternative medicine therapies for psoriasis: a systematic review. JAMA Dermatol. 2018;154:1330-1337.
- Fleischer AB, Feldman SR, Rapp SR, et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216-220.
- Ben-Arye E, Ziv M, Frenkel M, et al. Complementary medicine and psoriasis: linking the patient’s outlook with evidence-based medicine. Dermatology. 2003;207:302-307.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a Systematic review. JAMA Dermatol. 2018;154:934-950.
- Gupta AK, Ellis CN, Tellner DC, et al. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120:801-807.
- Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol. 2018;47:27-36.
- Søyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Heng MCY, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
- Sarafian G, Afshar M, Mansouri P, et al. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14:865-876.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Letters. 1994;341:19-22.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:283634.
- Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625-631.
- Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis. Eur J Dermatol. 2015;25:240-246.
- Cheng H-M, Wu Y-C, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17:439.
- Lin Y-K, Chang C-J, Chang Y-C, et al. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch Dermatol. 2008;144:1457-1464.
- Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019;54:891-896.
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacol Ther. 2013;37:3-17.
- Bax CE, Chakka S, Concha JSS, et al. The effects of immunostimulatory herbal supplements on autoimmune skin diseases. J Am Acad Dermatol. 2021;84:1051-1058.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes an altered gut microbiota in psoriatic arthritis and resembles dysbiosis of inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139.
- Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017;25:559-566.
- Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-339.
- Hosking A-M, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89.
- Tkachenko E, Okhovat J-P, Manjaly P, et al. Complementary & alternative medicine for alopecia areata: a systematic review [published online December 20, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.027
- Lepe K, Zito PM. Alopecia areata. In: StatPearls. StatPearls Publishing; 2021. Accessed July 22, 2021. https://pubmed.ncbi.nlm.nih.gov/30725685/
- Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata—a promising new dawn? Expert Rev Clin Pharmacol. 2020;13:43-51. doi:10.1080/17512433.2020.1702878
- van den Biggelaar FJHM, Smolders J, Jansen JFA. Complementary and alternative medicine in alopecia areata. AM J Clin Dermatol. 2010;11:11-20.
- Hussain ST, Mostaghimi A, Barr PJ, et al. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichology. 2017;9:160-164.
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32.
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346.
- Burian JP, Sacramento LVS, Carlos IZ. Fungal infection control by garlic extracts (Allium sativum L.) and modulation of peritoneal macrophages activity in murine model of sporotrichosis. Braz J Biol. 2017;77:848-855.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Lakshmi C, Srinivas CR. Allergic contact dermatitis following aromatherapy with valiya narayana thailam—an ayurvedic oil presenting as exfoliative dermatitis. Contact Dermatitis. 2009;61:297-298.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Groot AC de, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. dermatitis. 2016;27:39-42.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Ozmen I, Caliskan E, Arca E, et al. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Med J. 2015;57:233.
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391-395.
- Yang D-Q, You L-P, Song P-H, et al. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18:621-625.
Complementary alternative medicine (CAM) has been described by the National Center for Complementary and Integrative Medicine as “health care approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice.”1 Although this definition is broad, CAM encompasses therapies such as traditional Chinese medicine, herbal therapies, dietary supplements, and mind/body interventions. The use of CAM has grown, and according to a 2012 National Center for Complementary and Integrative Health survey, more than 30% of US adults and 12% of US children use health care approaches that are considered outside of conventional medical practice. In a survey study of US adults, at least 17.7% of respondents said they had taken a dietary supplement other than a vitamin or mineral in the last year.1 Data from the 2007 National Health Interview Survey showed that the prevalence of adults with skin conditions using CAM was 84.5% compared to 38.3% in the general population.2 In addition, 8.15 million US patients with dermatologic conditions reported using CAM over a 5-year period.3 Complementary alternative medicine has emerged as an alternative or adjunct to standard treatments, making it important for dermatologists to understand the existing literature on these therapies. Herein, we review the current evidence-based literature that exists on CAM for the treatment of atopic dermatitis (AD), psoriasis, and alopecia areata (AA).
Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition with considerable morbidity.4,5 The pathophysiology of AD is multifactorial and includes aspects of barrier dysfunction, IgE hypersensitivity, abnormal cell-mediated immune response, and environmental factors.6 Atopic dermatitis also is one of the most common inflammatory skin conditions in adults, affecting more than 7% of the US population and up to 20% of the total population in developed countries. Of those affected, 40% have moderate or severe symptoms that result in a substantial impact on quality of life.7 Despite advances in understanding disease pathology and treatment, a subset of patients opt to defer conventional treatments such as topical and systemic corticosteroids, antibiotics, nonsteroidal immunomodulators, and biologics. Patients may seek alternative therapies when typical treatments fail or when the perceived side effects outweigh the benefits.5,8 The use of CAM has been well described in patients with AD; however, the existing evidence supporting its use along with its safety profile have not been thoroughly explored. Herein, we will discuss some of the most well-studied supplements for treatment of AD, including evening primrose oil (EPO), fish oil, and probiotics.5
Oral supplementation with polyunsaturated fatty acids commonly is reported in patients with AD.5,8 The idea that a fatty acid deficiency could lead to atopic skin conditions has been around since 1937, when it was suggested that patients with AD had lower levels of blood unsaturated fatty acids.9 Conflicting evidence regarding oral fatty acid ingestion and AD disease severity has emerged.10,11 One unsaturated fatty acid, γ-linolenic acid (GLA), has demonstrated anti-inflammatory properties and involvement in barrier repair.12 It is converted to dihomo-GLA in the body, which acts on cyclooxygenase enzymes to produce the inflammatory mediator prostaglandin E1. The production of GLA is mediated by the enzyme delta-6 desaturase in the metabolization of linoleic acid.12 However, it has been reported that in a subset of patients with AD, a malfunction of delta-6 desaturase may play a role in disease progression and result in lower baseline levels of GLA.10,12 Evening primrose oil and borage oil contain high amounts of GLA (8%–10% and 23%, respectively); thus, supplementation with these oils has been studied in AD.13
EPO for AD
Studies investigating EPO (Oenothera biennis) and its association with AD severity have shown mixed results. A Cochrane review reported that oral borage oil and EPO were not effective treatments for AD,14 while another larger randomized controlled trial (RCT) found no statistically significant improvement in AD symptoms.15 However, multiple smaller studies have found that clinical symptoms of AD, such as erythema, xerosis, pruritus, and total body surface area involved, did improve with oral EPO supplementation when compared to placebo, and the results were statistically significant (P=.04).16,17 One study looked at different dosages of EPO and found that groups ingesting both 160 mg and 320 mg daily experienced reductions in eczema area and severity index score, with greater improvement noted with the higher dosage.17 Side effects associated with oral EPO include an anticoagulant effect and transient gastrointestinal tract upset.8,14 There currently is not enough evidence or safety data to recommend this supplement to AD patients.
Although topical use of fatty acids with high concentrations of GLA, such as EPO and borage oil, have demonstrated improvement in subjective symptom severity, most studies have not reached statistical significance.10,11 One study used a 10% EPO cream for 2 weeks compared to placebo and found statistically significant improvement in patient-reported AD symptoms (P=.045). However, this study only included 10 participants, and therefore larger studies are necessary to confirm this result.18 Some RCTs have shown that topical coconut oil, sunflower seed oil, and sandalwood album oil improve AD symptom severity, but again, large controlled trials are needed.5 Unfortunately, many essential oils, including EPO, can cause a secondary allergic contact dermatitis and potentially worsen AD.19
Fish Oil for AD
Fish oil is a commonly used supplement for AD due to its high content of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-3 fatty acids exert anti-inflammatory effects by displacing arachidonic acid, a proinflammatory omega-6 fatty acid thought to increase IgE, as well as helper T cell (TH2) cytokines and prostaglandin E2.8,20 A 2012 Cochrane review found that, while some studies revealed mild improvement in AD symptoms with oral fish oil supplementation, these RCTs were of poor methodological quality.21 Multiple smaller studies have shown a decrease in pruritus, severity, and physician-rated clinical scores with fish oil use.5,8,20,22 One study with 145 participants reported that 6 g of fish oil once daily compared to isoenergetic corn oil for 16 weeks identified no statistically significant differences between the treatment groups.20 No adverse events were identified in any of the reported trials. Further studies should be conducted to assess the utility and dosing of fish oil supplements in AD patients.
Probiotics for AD
Probiotics consist of live microorganisms that enhance the microflora of the gastrointestinal tract.8,20 They have been shown to influence food digestion and also have demonstrated potential influence on the skin-gut axis.23 The theory that intestinal dysbiosis plays a role in AD pathogenesis has been investigated in multiple studies.23-25 The central premise is that low-fiber and high-fat Western diets lead to fundamental changes in the gut microbiome, resulting in fewer anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs).23-25 These SCFAs are produced by microbes during the fermentation of dietary fiber and are known for their effect on epithelial barrier integrity and anti-inflammatory properties mediated through G protein–coupled receptor 43.25 Multiple studies have shown that the gut microbiome in patients with AD have higher proportions of Clostridium difficile, Escherichia coli, and Staphylococcus aureus and lower levels of Bifidobacterium, Bacteroidetes, and Bacteroides species compared to healthy controls.26,27 Metagenomic analysis of fecal samples from patients with AD have shown a reduction of Faecalibacterium prausnitzii species when compared to controls, along with a decreased SCFA production, leading to the hypothesis that the gut microbiome may play a role in epithelial barrier disruption.28,29 Systematic reviews and smaller studies have found that oral probiotic use does lead to AD symptom improvement.8,30,31 A systematic review of 25 RCTs with 1599 participants found that supplementation with oral probiotics significantly decreased the SCORAD (SCORing Atopic Dermatitis) index in adults and children older than 1 year with AD but had no effect on infants younger than 1 year (P<.001). They also found that supplementation with diverse microbes or Lactobacillus species showed greater benefit than Bifidobacterium species alone.30 Another study analyzed the effect of oral Lactobacillus fermentum (1×109 CFU twice daily) in 53 children with AD vs placebo for 16 weeks. This study found a statically significant decrease in SCORAD index between oral probiotics and placebo, with 92% (n=24) of participants supplementing with probiotics having a lower SCORAD index than baseline compared to 63% (n=17) in the placebo group (P=.01).31 However, the use of probiotics for AD treatment has remained controversial. Two recent systematic reviews, including 39 RCTs of 2599 randomized patients, found that the use of currently available oral probiotics made little or no difference in patient-rated AD symptoms, investigator-rated AD symptoms, or quality of life.32,33 No adverse effects were observed in the included studies. Unfortunately, the individual RCTs included were heterogeneous, and future studies with standardized probiotic supplementation should be undertaken before probiotics can be routinely recommended.
The use of topical probiotics in AD also has recently emerged. Multiple studies have shown that patients with AD have higher levels of colonization with S aureus, which is associated with T-cell dysfunction, more severe allergic skin reactions, and disruptions in barrier function.34,35 Therefore, altering the skin microbiota through topical probiotics could theoretically reduce AD symptoms and flares. Multiple RCTs and smaller studies have shown that topical probiotics can alter the skin microbiota, improve erythema, and decrease scaling and pruritus in AD patients.35-38 One study used a heat-treated Lactobacillus johnsonii 0.3% lotion twice daily for 3 weeks vs placebo in patients with AD with positive S aureus skin cultures. The S aureus load decreased in patients using the topical probiotic lotion, which correlated with lower SCORAD index that was statistically significant compared to placebo (P=.012).36 More robust studies are needed to determine if topical probiotics should routinely be recommended in AD.
Psoriasis
Psoriasis vulgaris is a chronic inflammatory skin condition characterized by pruritic, hyperkeratotic, scaly plaques.39,40 Keratinocyte hyperproliferation is central to psoriasis pathogenesis and is thought to be a T-cell–driven reaction to antigens or trauma in genetically predisposed individuals. Standard treatments for psoriasis currently include topical corticosteroids and anti-inflammatories, oral immunomodulatory therapy, biologic agents, and phototherapy.40 The use of CAM is highly prevalent among patients with psoriasis, with one study reporting that 51% (n=162) of psoriatic patients interviewed had used CAM.41 The most common reasons for CAM use included dissatisfaction with current treatment, adverse side effects of standard therapy, and patient-reported attempts at “trying everything to heal disease.”42 Herein, we will discuss some of the most frequently used supplements for treatment of psoriatic disease.39
Fish Oil for Psoriasis
One of the most common supplements used by patients with psoriasis is fish oil due to its purported anti-inflammatory qualities.20,39 The consensus on fish oil supplementation for psoriasis is mixed.43-45 Multiple RCTs have reported reductions in psoriasis area and severity index (PASI) scores or symptomatic improvement with variable doses of fish oil.44,46 One RCT found that using EPA 1.8 g once daily and DHA 1.2 g once daily for 12 weeks resulted in significant improvement in pruritus, scaling, and erythema (P<.05).44 Another study reported a significant decrease in erythema (P=.02) and total body surface area affected (P=.0001) with EPA 3.6 g once daily and DHA 2.4 g once daily supplementation compared to olive oil supplementation for 15 weeks.46 Alternatively, multiple studies have failed to show statistically significant improvement in psoriatic symptoms with fish oil supplementation at variable doses and time frames (14–216 mg daily EPA, 9–80 mg daily DHA, from 2 weeks to 9 months).40,47,48 Fish oil may impart anticoagulant properties and should not be started without the guidance of a physician. Currently, there are no data to make specific recommendations on the use of fish oil as an adjunct psoriatic treatment.
Curcumin for Psoriasis
Another supplement routinely utilized in patients with psoriasis is curcumin,40,49,50 a yellow phytochemical that is a major component of the spice turmeric. Curcumin has been shown to inhibit certain proinflammatory cytokines including IL-17, IL-6, IFN-γ, and tumor necrosis factor α and has been regarded as having immune-modulating, anti-inflammatory, and antibacterial properties.40,50 Curcumin also has been reported to suppress phosphorylase kinase, an enzyme that has increased activity in psoriatic plaques that correlates with markers of psoriatic hyperproliferation.50,51 When applied topically, turmeric microgel 0.5% has been reported to decrease scaling, erythema, and psoriatic plaque thickness over the course of 9 weeks.50 In a nonrandomized trial with 10 participants, researchers found that phosphorylase kinase activity levels in psoriatic skin biopsies of patients applying topical curcumin 1% were lower than placebo and topical calcipotriol applied in combination. The lower phosphorylase kinase levels correlated with level of disease severity, and topical curcumin 1% showed a superior outcome when compared to topical calcipotriol.40,49 Although these preliminary results are interesting, there still are not enough data at this time to recommend topical curcumin as a treatment of psoriasis. No known adverse events have been reported with the use of topical curcumin to date.
Oral curcumin has poor oral bioavailability, and 40% to 90% of oral doses are excreted, making supplementation a challenge.40 In one RCT, oral curcumin 2 g daily (using a lecithin-based delivery system to increase bioavailability) was administered in combination with topical methylprednisolone aceponate 0.1%, resulting in significant improvement in psoriatic symptoms and lower IL-22 compared to placebo and topical methylprednisolone aceponate (P<.05).52 Other studies also have reported decreased PASI scores with oral curcumin supplementation.53,54 Adverse effects reported with oral curcumin included gastrointestinal tract upset and hot flashes.53 Although there is early evidence that may support the use of oral curcumin supplementation for psoriasis, more data are needed before recommending this therapy.
Indigo Naturalis for Psoriasis
Topical indigo naturalis (IN) also has been reported to improve psoriasis symptoms.39,53,55 The antipsoriatic effects are thought to occur through the active ingredient in IN (indirubin), which is responsible for inhibition of keratinocyte proliferation.40 One study reported that topical IN 1.4% containing indirubin 0.16% with a petroleum ointment vehicle applied to psoriatic plaques over 12 weeks resulted in a significant decrease in PASI scores from 18.9 at baseline to 6.3 after IN treatment (P<.001).56 Another study found that over 8 weeks, topical application of IN 2.83% containing indirubin 0.24% to psoriatic plaques vs petroleum jelly resulted in 56.3% (n=9) of the treatment group achieving PASI 75 compared to 0% in the placebo group (n=24).55 One deterrent in topical IN treatment is the dark blue pigment it contains; however, no other adverse outcomes were found with topical IN treatment.56 Larger clinical trials are necessary to further explore IN as a potential adjunct treatment in patients with mild psoriatic disease. When taken orally, IN has caused gastrointestinal tract disturbance and elevated liver enzyme levels.57
Herbal Toxicities
It is important to consider that oral supplements including curcumin and IN are widely available over-the-counter and online without oversight by the US Food and Drug Administration.40 Herbal supplements typically are compounded with other ingredients and have been associated with hepatotoxicity as well as drug-supplement interactions, including abnormal bleeding and clotting.58 There exists a lack of general surveillance data, making the true burden of herbal toxicities more difficult to accurately discern. Although some supplements have been associated with anti-inflammatory qualities and disease improvement, other herbal supplements have been shown to possess immunostimulatory characteristics. Herbal supplements such as spirulina, chlorella, Aphanizomenon flos-aquae, and echinacea have been shown to upregulate inflammatory pathways in a variety of autoimmune skin conditions.59
Probiotics for Psoriasis
Data on probiotic use in patients with psoriasis are limited.23 A distinct pattern of dysbiosis has been identified in psoriatic patients, as there is thought to be depletion of beneficial bacteria such as Bifidobacterium, lactobacilli, and F prausnitzii and increased colonization with pathogenic organisms such as Salmonella, E coli, Heliobacter, Campylobacter, and Alcaligenes in psoriasis patients.23,59,60 Early mouse studies have supported this hypothesis, as mice fed with Lactobacillus pentosus have developed milder forms of imiquimod-induced psoriasis compared to placebo,55 and mice receiving probiotic supplementation have lower levels of psoriasis-related proinflammatory markers such as TH17-associated cytokines.61 Another study in humans found that daily oral Bifidobacterium infantis supplementation for 8 weeks in psoriatic patients resulted in lower C-reactive protein and tumor necrosis factor α levels compared to placebo.62 Studies on the use of topical probiotics in psoriasis have been limited, and more research is needed to explore this relationship.38 At this time, no specific recommendations can be made on the use of probiotics in psoriatic patients.
Alopecia Areata
Alopecia areata is nonscarring hair loss that can affect the scalp, face, or body.63,64 The pathophysiology of AA involves the attack of the hair follicle matrix epithelium by inflammatory cells without hair follicle stem cell destruction. The precise events that precipitate these episodes are unknown, but triggers such as emotional or physical stress, vaccines, or viral infections have been reported.65 There is no cure for AA, and current treatments such as topical minoxidil and corticosteroids (topical, intralesional, or oral) vary widely in efficacy.64 Although Janus kinase inhibitors recently have shown promising results in the treatment of AA, the need for prolonged therapy may be frustrating to patients.66 Severity of AA also can vary, with 30% of patients experiencing extensive hair loss.67 The use of CAM has been widely reported in AA due to high levels of dissatisfaction with existing therapies.68 Herein, we discuss the most studied alternative treatments used in AA
Garlic and Onion for Alopecia
One alternative treatment that has shown promising initial results is application of topical garlic and onion extracts to affected areas.64,69,70 Both garlic and onion belong to the Allium genus and are high in sulfur and phenolic compounds.70 They have been reported to possess bactericidal and vasodilatory activity,71 and it has been hypothesized that onion and garlic extracts may induce therapeutic effects through induction of a mild contact dermatitis.70 One single-blinded, controlled trial using topical crude onion juice reported that 86.9% (n=20) of patients had full regrowth of hair compared to 13.3% (n=2) of patients treated with a tap water placebo at 8 weeks (P<.0001). This study also noted that patients using onion juice had a higher rate of erythema at application site; unfortunately, the study was small with only 38 patients.70 Another double-blind RCT using garlic gel 5% with betamethasone valerate cream 0.1% compared to betamethasone valerate cream alone found that after 3 months, patients in the garlic gel group had increased terminal hairs and smaller patch sizes compared to the betamethasone valerate cream group.69 More studies are needed to confirm these results.
Aromatherapy With Essential Oils for Alopecia
Another alternative treatment in AA that has demonstrated positive results is aromatherapy skin massage with essential oils to patches of alopecia.72 Although certain essential oils, such as tea tree oil, have been reported to have specific antibacterial or anti-inflammatory properties, essential oils have been reported to cause allergic contact dermatitis and should be used with caution.73,74 For example, tea tree oil is a well-known cause of allergic contact dermatitis, and positive patch testing has ranged from 0.1% to 3.5% in studies assessing topical tea tree oil 5% application.75 Overall, there have been nearly 80 essential oils implicated in contact dermatitis, with high-concentration products being one of the highest risk factors for an allergic contact reaction.76 One RCT compared daily scalp massage with essential oils (rosemary, lavender, thyme, and cedarwood in a carrier oil) to daily scalp massage with a placebo carrier oil in AA patients. The results showed that at 7 months of treatment, 44% (n=19) of the aromatherapy group showed improvement compared to 15% (n=6) in the control group.77 Another study used a similar group of essential oils (thyme, rosemary, atlas cedar, lavender, and EPO in a carrier oil) with daily scalp massage and reported similar improvement of AA symptoms compared to control; the investigators also reported irritation at application site in 1 patient.78 There currently are not enough data to recommend aromatherapy skin massage for the treatment of AA, and this practice may cause harm to the patient by induction of allergic contact dermatitis.
There have been a few studies to suggest that the use of total glucosides of peony with compound glycyrrhizin and oral Korean red ginseng may have beneficial effects on AA treatment, but efficacy and safety data are lacking, and these therapies should not be recommended without more information.64,79,80
Final Thoughts
Dermatologic patients frequently are opting for CAM,2 and although some therapies may show promising initial results, alternative medicines also can drive adverse events.19,30 The lack of oversight from the US Food and Drug Administration on the products leads to many unknowns for true health risks with over-the-counter CAM supplements.40 As the use of CAM becomes increasingly common among dermatologic patients, it is important for dermatologists to understand the benefits and risks, especially for commonly treated conditions. More data is needed before CAM can be routinely recommended.
Complementary alternative medicine (CAM) has been described by the National Center for Complementary and Integrative Medicine as “health care approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice.”1 Although this definition is broad, CAM encompasses therapies such as traditional Chinese medicine, herbal therapies, dietary supplements, and mind/body interventions. The use of CAM has grown, and according to a 2012 National Center for Complementary and Integrative Health survey, more than 30% of US adults and 12% of US children use health care approaches that are considered outside of conventional medical practice. In a survey study of US adults, at least 17.7% of respondents said they had taken a dietary supplement other than a vitamin or mineral in the last year.1 Data from the 2007 National Health Interview Survey showed that the prevalence of adults with skin conditions using CAM was 84.5% compared to 38.3% in the general population.2 In addition, 8.15 million US patients with dermatologic conditions reported using CAM over a 5-year period.3 Complementary alternative medicine has emerged as an alternative or adjunct to standard treatments, making it important for dermatologists to understand the existing literature on these therapies. Herein, we review the current evidence-based literature that exists on CAM for the treatment of atopic dermatitis (AD), psoriasis, and alopecia areata (AA).
Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition with considerable morbidity.4,5 The pathophysiology of AD is multifactorial and includes aspects of barrier dysfunction, IgE hypersensitivity, abnormal cell-mediated immune response, and environmental factors.6 Atopic dermatitis also is one of the most common inflammatory skin conditions in adults, affecting more than 7% of the US population and up to 20% of the total population in developed countries. Of those affected, 40% have moderate or severe symptoms that result in a substantial impact on quality of life.7 Despite advances in understanding disease pathology and treatment, a subset of patients opt to defer conventional treatments such as topical and systemic corticosteroids, antibiotics, nonsteroidal immunomodulators, and biologics. Patients may seek alternative therapies when typical treatments fail or when the perceived side effects outweigh the benefits.5,8 The use of CAM has been well described in patients with AD; however, the existing evidence supporting its use along with its safety profile have not been thoroughly explored. Herein, we will discuss some of the most well-studied supplements for treatment of AD, including evening primrose oil (EPO), fish oil, and probiotics.5
Oral supplementation with polyunsaturated fatty acids commonly is reported in patients with AD.5,8 The idea that a fatty acid deficiency could lead to atopic skin conditions has been around since 1937, when it was suggested that patients with AD had lower levels of blood unsaturated fatty acids.9 Conflicting evidence regarding oral fatty acid ingestion and AD disease severity has emerged.10,11 One unsaturated fatty acid, γ-linolenic acid (GLA), has demonstrated anti-inflammatory properties and involvement in barrier repair.12 It is converted to dihomo-GLA in the body, which acts on cyclooxygenase enzymes to produce the inflammatory mediator prostaglandin E1. The production of GLA is mediated by the enzyme delta-6 desaturase in the metabolization of linoleic acid.12 However, it has been reported that in a subset of patients with AD, a malfunction of delta-6 desaturase may play a role in disease progression and result in lower baseline levels of GLA.10,12 Evening primrose oil and borage oil contain high amounts of GLA (8%–10% and 23%, respectively); thus, supplementation with these oils has been studied in AD.13
EPO for AD
Studies investigating EPO (Oenothera biennis) and its association with AD severity have shown mixed results. A Cochrane review reported that oral borage oil and EPO were not effective treatments for AD,14 while another larger randomized controlled trial (RCT) found no statistically significant improvement in AD symptoms.15 However, multiple smaller studies have found that clinical symptoms of AD, such as erythema, xerosis, pruritus, and total body surface area involved, did improve with oral EPO supplementation when compared to placebo, and the results were statistically significant (P=.04).16,17 One study looked at different dosages of EPO and found that groups ingesting both 160 mg and 320 mg daily experienced reductions in eczema area and severity index score, with greater improvement noted with the higher dosage.17 Side effects associated with oral EPO include an anticoagulant effect and transient gastrointestinal tract upset.8,14 There currently is not enough evidence or safety data to recommend this supplement to AD patients.
Although topical use of fatty acids with high concentrations of GLA, such as EPO and borage oil, have demonstrated improvement in subjective symptom severity, most studies have not reached statistical significance.10,11 One study used a 10% EPO cream for 2 weeks compared to placebo and found statistically significant improvement in patient-reported AD symptoms (P=.045). However, this study only included 10 participants, and therefore larger studies are necessary to confirm this result.18 Some RCTs have shown that topical coconut oil, sunflower seed oil, and sandalwood album oil improve AD symptom severity, but again, large controlled trials are needed.5 Unfortunately, many essential oils, including EPO, can cause a secondary allergic contact dermatitis and potentially worsen AD.19
Fish Oil for AD
Fish oil is a commonly used supplement for AD due to its high content of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-3 fatty acids exert anti-inflammatory effects by displacing arachidonic acid, a proinflammatory omega-6 fatty acid thought to increase IgE, as well as helper T cell (TH2) cytokines and prostaglandin E2.8,20 A 2012 Cochrane review found that, while some studies revealed mild improvement in AD symptoms with oral fish oil supplementation, these RCTs were of poor methodological quality.21 Multiple smaller studies have shown a decrease in pruritus, severity, and physician-rated clinical scores with fish oil use.5,8,20,22 One study with 145 participants reported that 6 g of fish oil once daily compared to isoenergetic corn oil for 16 weeks identified no statistically significant differences between the treatment groups.20 No adverse events were identified in any of the reported trials. Further studies should be conducted to assess the utility and dosing of fish oil supplements in AD patients.
Probiotics for AD
Probiotics consist of live microorganisms that enhance the microflora of the gastrointestinal tract.8,20 They have been shown to influence food digestion and also have demonstrated potential influence on the skin-gut axis.23 The theory that intestinal dysbiosis plays a role in AD pathogenesis has been investigated in multiple studies.23-25 The central premise is that low-fiber and high-fat Western diets lead to fundamental changes in the gut microbiome, resulting in fewer anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs).23-25 These SCFAs are produced by microbes during the fermentation of dietary fiber and are known for their effect on epithelial barrier integrity and anti-inflammatory properties mediated through G protein–coupled receptor 43.25 Multiple studies have shown that the gut microbiome in patients with AD have higher proportions of Clostridium difficile, Escherichia coli, and Staphylococcus aureus and lower levels of Bifidobacterium, Bacteroidetes, and Bacteroides species compared to healthy controls.26,27 Metagenomic analysis of fecal samples from patients with AD have shown a reduction of Faecalibacterium prausnitzii species when compared to controls, along with a decreased SCFA production, leading to the hypothesis that the gut microbiome may play a role in epithelial barrier disruption.28,29 Systematic reviews and smaller studies have found that oral probiotic use does lead to AD symptom improvement.8,30,31 A systematic review of 25 RCTs with 1599 participants found that supplementation with oral probiotics significantly decreased the SCORAD (SCORing Atopic Dermatitis) index in adults and children older than 1 year with AD but had no effect on infants younger than 1 year (P<.001). They also found that supplementation with diverse microbes or Lactobacillus species showed greater benefit than Bifidobacterium species alone.30 Another study analyzed the effect of oral Lactobacillus fermentum (1×109 CFU twice daily) in 53 children with AD vs placebo for 16 weeks. This study found a statically significant decrease in SCORAD index between oral probiotics and placebo, with 92% (n=24) of participants supplementing with probiotics having a lower SCORAD index than baseline compared to 63% (n=17) in the placebo group (P=.01).31 However, the use of probiotics for AD treatment has remained controversial. Two recent systematic reviews, including 39 RCTs of 2599 randomized patients, found that the use of currently available oral probiotics made little or no difference in patient-rated AD symptoms, investigator-rated AD symptoms, or quality of life.32,33 No adverse effects were observed in the included studies. Unfortunately, the individual RCTs included were heterogeneous, and future studies with standardized probiotic supplementation should be undertaken before probiotics can be routinely recommended.
The use of topical probiotics in AD also has recently emerged. Multiple studies have shown that patients with AD have higher levels of colonization with S aureus, which is associated with T-cell dysfunction, more severe allergic skin reactions, and disruptions in barrier function.34,35 Therefore, altering the skin microbiota through topical probiotics could theoretically reduce AD symptoms and flares. Multiple RCTs and smaller studies have shown that topical probiotics can alter the skin microbiota, improve erythema, and decrease scaling and pruritus in AD patients.35-38 One study used a heat-treated Lactobacillus johnsonii 0.3% lotion twice daily for 3 weeks vs placebo in patients with AD with positive S aureus skin cultures. The S aureus load decreased in patients using the topical probiotic lotion, which correlated with lower SCORAD index that was statistically significant compared to placebo (P=.012).36 More robust studies are needed to determine if topical probiotics should routinely be recommended in AD.
Psoriasis
Psoriasis vulgaris is a chronic inflammatory skin condition characterized by pruritic, hyperkeratotic, scaly plaques.39,40 Keratinocyte hyperproliferation is central to psoriasis pathogenesis and is thought to be a T-cell–driven reaction to antigens or trauma in genetically predisposed individuals. Standard treatments for psoriasis currently include topical corticosteroids and anti-inflammatories, oral immunomodulatory therapy, biologic agents, and phototherapy.40 The use of CAM is highly prevalent among patients with psoriasis, with one study reporting that 51% (n=162) of psoriatic patients interviewed had used CAM.41 The most common reasons for CAM use included dissatisfaction with current treatment, adverse side effects of standard therapy, and patient-reported attempts at “trying everything to heal disease.”42 Herein, we will discuss some of the most frequently used supplements for treatment of psoriatic disease.39
Fish Oil for Psoriasis
One of the most common supplements used by patients with psoriasis is fish oil due to its purported anti-inflammatory qualities.20,39 The consensus on fish oil supplementation for psoriasis is mixed.43-45 Multiple RCTs have reported reductions in psoriasis area and severity index (PASI) scores or symptomatic improvement with variable doses of fish oil.44,46 One RCT found that using EPA 1.8 g once daily and DHA 1.2 g once daily for 12 weeks resulted in significant improvement in pruritus, scaling, and erythema (P<.05).44 Another study reported a significant decrease in erythema (P=.02) and total body surface area affected (P=.0001) with EPA 3.6 g once daily and DHA 2.4 g once daily supplementation compared to olive oil supplementation for 15 weeks.46 Alternatively, multiple studies have failed to show statistically significant improvement in psoriatic symptoms with fish oil supplementation at variable doses and time frames (14–216 mg daily EPA, 9–80 mg daily DHA, from 2 weeks to 9 months).40,47,48 Fish oil may impart anticoagulant properties and should not be started without the guidance of a physician. Currently, there are no data to make specific recommendations on the use of fish oil as an adjunct psoriatic treatment.
Curcumin for Psoriasis
Another supplement routinely utilized in patients with psoriasis is curcumin,40,49,50 a yellow phytochemical that is a major component of the spice turmeric. Curcumin has been shown to inhibit certain proinflammatory cytokines including IL-17, IL-6, IFN-γ, and tumor necrosis factor α and has been regarded as having immune-modulating, anti-inflammatory, and antibacterial properties.40,50 Curcumin also has been reported to suppress phosphorylase kinase, an enzyme that has increased activity in psoriatic plaques that correlates with markers of psoriatic hyperproliferation.50,51 When applied topically, turmeric microgel 0.5% has been reported to decrease scaling, erythema, and psoriatic plaque thickness over the course of 9 weeks.50 In a nonrandomized trial with 10 participants, researchers found that phosphorylase kinase activity levels in psoriatic skin biopsies of patients applying topical curcumin 1% were lower than placebo and topical calcipotriol applied in combination. The lower phosphorylase kinase levels correlated with level of disease severity, and topical curcumin 1% showed a superior outcome when compared to topical calcipotriol.40,49 Although these preliminary results are interesting, there still are not enough data at this time to recommend topical curcumin as a treatment of psoriasis. No known adverse events have been reported with the use of topical curcumin to date.
Oral curcumin has poor oral bioavailability, and 40% to 90% of oral doses are excreted, making supplementation a challenge.40 In one RCT, oral curcumin 2 g daily (using a lecithin-based delivery system to increase bioavailability) was administered in combination with topical methylprednisolone aceponate 0.1%, resulting in significant improvement in psoriatic symptoms and lower IL-22 compared to placebo and topical methylprednisolone aceponate (P<.05).52 Other studies also have reported decreased PASI scores with oral curcumin supplementation.53,54 Adverse effects reported with oral curcumin included gastrointestinal tract upset and hot flashes.53 Although there is early evidence that may support the use of oral curcumin supplementation for psoriasis, more data are needed before recommending this therapy.
Indigo Naturalis for Psoriasis
Topical indigo naturalis (IN) also has been reported to improve psoriasis symptoms.39,53,55 The antipsoriatic effects are thought to occur through the active ingredient in IN (indirubin), which is responsible for inhibition of keratinocyte proliferation.40 One study reported that topical IN 1.4% containing indirubin 0.16% with a petroleum ointment vehicle applied to psoriatic plaques over 12 weeks resulted in a significant decrease in PASI scores from 18.9 at baseline to 6.3 after IN treatment (P<.001).56 Another study found that over 8 weeks, topical application of IN 2.83% containing indirubin 0.24% to psoriatic plaques vs petroleum jelly resulted in 56.3% (n=9) of the treatment group achieving PASI 75 compared to 0% in the placebo group (n=24).55 One deterrent in topical IN treatment is the dark blue pigment it contains; however, no other adverse outcomes were found with topical IN treatment.56 Larger clinical trials are necessary to further explore IN as a potential adjunct treatment in patients with mild psoriatic disease. When taken orally, IN has caused gastrointestinal tract disturbance and elevated liver enzyme levels.57
Herbal Toxicities
It is important to consider that oral supplements including curcumin and IN are widely available over-the-counter and online without oversight by the US Food and Drug Administration.40 Herbal supplements typically are compounded with other ingredients and have been associated with hepatotoxicity as well as drug-supplement interactions, including abnormal bleeding and clotting.58 There exists a lack of general surveillance data, making the true burden of herbal toxicities more difficult to accurately discern. Although some supplements have been associated with anti-inflammatory qualities and disease improvement, other herbal supplements have been shown to possess immunostimulatory characteristics. Herbal supplements such as spirulina, chlorella, Aphanizomenon flos-aquae, and echinacea have been shown to upregulate inflammatory pathways in a variety of autoimmune skin conditions.59
Probiotics for Psoriasis
Data on probiotic use in patients with psoriasis are limited.23 A distinct pattern of dysbiosis has been identified in psoriatic patients, as there is thought to be depletion of beneficial bacteria such as Bifidobacterium, lactobacilli, and F prausnitzii and increased colonization with pathogenic organisms such as Salmonella, E coli, Heliobacter, Campylobacter, and Alcaligenes in psoriasis patients.23,59,60 Early mouse studies have supported this hypothesis, as mice fed with Lactobacillus pentosus have developed milder forms of imiquimod-induced psoriasis compared to placebo,55 and mice receiving probiotic supplementation have lower levels of psoriasis-related proinflammatory markers such as TH17-associated cytokines.61 Another study in humans found that daily oral Bifidobacterium infantis supplementation for 8 weeks in psoriatic patients resulted in lower C-reactive protein and tumor necrosis factor α levels compared to placebo.62 Studies on the use of topical probiotics in psoriasis have been limited, and more research is needed to explore this relationship.38 At this time, no specific recommendations can be made on the use of probiotics in psoriatic patients.
Alopecia Areata
Alopecia areata is nonscarring hair loss that can affect the scalp, face, or body.63,64 The pathophysiology of AA involves the attack of the hair follicle matrix epithelium by inflammatory cells without hair follicle stem cell destruction. The precise events that precipitate these episodes are unknown, but triggers such as emotional or physical stress, vaccines, or viral infections have been reported.65 There is no cure for AA, and current treatments such as topical minoxidil and corticosteroids (topical, intralesional, or oral) vary widely in efficacy.64 Although Janus kinase inhibitors recently have shown promising results in the treatment of AA, the need for prolonged therapy may be frustrating to patients.66 Severity of AA also can vary, with 30% of patients experiencing extensive hair loss.67 The use of CAM has been widely reported in AA due to high levels of dissatisfaction with existing therapies.68 Herein, we discuss the most studied alternative treatments used in AA
Garlic and Onion for Alopecia
One alternative treatment that has shown promising initial results is application of topical garlic and onion extracts to affected areas.64,69,70 Both garlic and onion belong to the Allium genus and are high in sulfur and phenolic compounds.70 They have been reported to possess bactericidal and vasodilatory activity,71 and it has been hypothesized that onion and garlic extracts may induce therapeutic effects through induction of a mild contact dermatitis.70 One single-blinded, controlled trial using topical crude onion juice reported that 86.9% (n=20) of patients had full regrowth of hair compared to 13.3% (n=2) of patients treated with a tap water placebo at 8 weeks (P<.0001). This study also noted that patients using onion juice had a higher rate of erythema at application site; unfortunately, the study was small with only 38 patients.70 Another double-blind RCT using garlic gel 5% with betamethasone valerate cream 0.1% compared to betamethasone valerate cream alone found that after 3 months, patients in the garlic gel group had increased terminal hairs and smaller patch sizes compared to the betamethasone valerate cream group.69 More studies are needed to confirm these results.
Aromatherapy With Essential Oils for Alopecia
Another alternative treatment in AA that has demonstrated positive results is aromatherapy skin massage with essential oils to patches of alopecia.72 Although certain essential oils, such as tea tree oil, have been reported to have specific antibacterial or anti-inflammatory properties, essential oils have been reported to cause allergic contact dermatitis and should be used with caution.73,74 For example, tea tree oil is a well-known cause of allergic contact dermatitis, and positive patch testing has ranged from 0.1% to 3.5% in studies assessing topical tea tree oil 5% application.75 Overall, there have been nearly 80 essential oils implicated in contact dermatitis, with high-concentration products being one of the highest risk factors for an allergic contact reaction.76 One RCT compared daily scalp massage with essential oils (rosemary, lavender, thyme, and cedarwood in a carrier oil) to daily scalp massage with a placebo carrier oil in AA patients. The results showed that at 7 months of treatment, 44% (n=19) of the aromatherapy group showed improvement compared to 15% (n=6) in the control group.77 Another study used a similar group of essential oils (thyme, rosemary, atlas cedar, lavender, and EPO in a carrier oil) with daily scalp massage and reported similar improvement of AA symptoms compared to control; the investigators also reported irritation at application site in 1 patient.78 There currently are not enough data to recommend aromatherapy skin massage for the treatment of AA, and this practice may cause harm to the patient by induction of allergic contact dermatitis.
There have been a few studies to suggest that the use of total glucosides of peony with compound glycyrrhizin and oral Korean red ginseng may have beneficial effects on AA treatment, but efficacy and safety data are lacking, and these therapies should not be recommended without more information.64,79,80
Final Thoughts
Dermatologic patients frequently are opting for CAM,2 and although some therapies may show promising initial results, alternative medicines also can drive adverse events.19,30 The lack of oversight from the US Food and Drug Administration on the products leads to many unknowns for true health risks with over-the-counter CAM supplements.40 As the use of CAM becomes increasingly common among dermatologic patients, it is important for dermatologists to understand the benefits and risks, especially for commonly treated conditions. More data is needed before CAM can be routinely recommended.
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Integrative Health website. Updated April 2021. Accessed April 25, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- Fuhrmann T, Smith N, Tausk F. Use of complementary and alternative medicine among adults with skin disease: updated results from a national survey. J Am Acad Dermatol. 2010;63:1000-1005.
- Landis ET, Davis SA, Feldman SR, et al. Complementary and alternative medicine use in dermatology in the United States. J Altern Complement Med. 2014;20:392-398.
- Solman L, Lloyd‐Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2016. part 1: treatment and prevention. Clin Exp Dermatol. 2019;44:363-369.
- Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol. 2016;17:557-581.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. In: Fortson EA, Feldman SR, Strowd LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:21-37.
- Atopic dermatitis in America. Asthma and Allergy Foundation of America website. Accessed July 30, 2021. https://www.aafa.org/atopic-dermatitis-in-america
- Schlichte MJ, Vandersall A, Katta R. Diet and eczema: a review of dietary supplements for the treatment of atopic dermatitis. Dermatol Pract Concept. 2016;6:23-29.
- Brown WR, Hansen AE. Arachidonic and linolic acid of the serum in normal and eczematous human subjects. Proc Soc Exp Bio Med. 1937;36:113-117.
- Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411-424.
- Ferreira MJ, Fiadeiro T, Silva M, et al. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998;7:213-216.
- Simon D, Eng PA, Borelli S, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014;31:180-188.
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411-1414.
- Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;4:CD004416.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ. 2003;327:2.
- Schalin-Karrila M, Mattila L, Jansen CT, et al. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11-19.
- Chung BY, Park SY, Jung MJ, et al. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409-416.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatolog Treat. 1990;1:199-201.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. Dermatitis. 2016;27:39-42.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371-1376.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema [published online February 15, 2012]. Cochrane Database Syst Rev. Accessed July 22, 2021. doi:10.1002/14651858.CD005205.pub3
- Balic´ A, Vlašic´ D, Žužul K, et al. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
- Salem I, Ramser A, Isham N, et al. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Pathogenesis Manage Atopic Dermatitis. 2011;41:112-124.
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286.
- Lee E, Lee S-Y, Kang M-J, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91-92.e1.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241-244.
- Kim H-J, Kim HY, Lee S-Y, et al. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376.
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860.
- Kim S-O, Ah Y-M, Yu YM, et al. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217-226.
- Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.

- Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135.
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15-21.
- Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222-228.
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:249-257.
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine. 2021;27:700-709.
- França K. Topical probiotics in dermatological therapy and skincare: a concise review. Dermatol Ther (Heidelb). 2020;11:71-77.
- Talbott W, Duffy N. Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. Am J Clin Dermatol. 2015;16:147-165.
- Gamret AC, Price A, Fertig RM, et al. Complementary and alternative medicine therapies for psoriasis: a systematic review. JAMA Dermatol. 2018;154:1330-1337.
- Fleischer AB, Feldman SR, Rapp SR, et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216-220.
- Ben-Arye E, Ziv M, Frenkel M, et al. Complementary medicine and psoriasis: linking the patient’s outlook with evidence-based medicine. Dermatology. 2003;207:302-307.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a Systematic review. JAMA Dermatol. 2018;154:934-950.
- Gupta AK, Ellis CN, Tellner DC, et al. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120:801-807.
- Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol. 2018;47:27-36.
- Søyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Heng MCY, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
- Sarafian G, Afshar M, Mansouri P, et al. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14:865-876.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Letters. 1994;341:19-22.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:283634.
- Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625-631.
- Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis. Eur J Dermatol. 2015;25:240-246.
- Cheng H-M, Wu Y-C, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17:439.
- Lin Y-K, Chang C-J, Chang Y-C, et al. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch Dermatol. 2008;144:1457-1464.
- Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019;54:891-896.
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacol Ther. 2013;37:3-17.
- Bax CE, Chakka S, Concha JSS, et al. The effects of immunostimulatory herbal supplements on autoimmune skin diseases. J Am Acad Dermatol. 2021;84:1051-1058.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes an altered gut microbiota in psoriatic arthritis and resembles dysbiosis of inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139.
- Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017;25:559-566.
- Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-339.
- Hosking A-M, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89.
- Tkachenko E, Okhovat J-P, Manjaly P, et al. Complementary & alternative medicine for alopecia areata: a systematic review [published online December 20, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.027
- Lepe K, Zito PM. Alopecia areata. In: StatPearls. StatPearls Publishing; 2021. Accessed July 22, 2021. https://pubmed.ncbi.nlm.nih.gov/30725685/
- Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata—a promising new dawn? Expert Rev Clin Pharmacol. 2020;13:43-51. doi:10.1080/17512433.2020.1702878
- van den Biggelaar FJHM, Smolders J, Jansen JFA. Complementary and alternative medicine in alopecia areata. AM J Clin Dermatol. 2010;11:11-20.
- Hussain ST, Mostaghimi A, Barr PJ, et al. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichology. 2017;9:160-164.
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32.
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346.
- Burian JP, Sacramento LVS, Carlos IZ. Fungal infection control by garlic extracts (Allium sativum L.) and modulation of peritoneal macrophages activity in murine model of sporotrichosis. Braz J Biol. 2017;77:848-855.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Lakshmi C, Srinivas CR. Allergic contact dermatitis following aromatherapy with valiya narayana thailam—an ayurvedic oil presenting as exfoliative dermatitis. Contact Dermatitis. 2009;61:297-298.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Groot AC de, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. dermatitis. 2016;27:39-42.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Ozmen I, Caliskan E, Arca E, et al. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Med J. 2015;57:233.
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391-395.
- Yang D-Q, You L-P, Song P-H, et al. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18:621-625.
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Integrative Health website. Updated April 2021. Accessed April 25, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- Fuhrmann T, Smith N, Tausk F. Use of complementary and alternative medicine among adults with skin disease: updated results from a national survey. J Am Acad Dermatol. 2010;63:1000-1005.
- Landis ET, Davis SA, Feldman SR, et al. Complementary and alternative medicine use in dermatology in the United States. J Altern Complement Med. 2014;20:392-398.
- Solman L, Lloyd‐Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2016. part 1: treatment and prevention. Clin Exp Dermatol. 2019;44:363-369.
- Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol. 2016;17:557-581.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. In: Fortson EA, Feldman SR, Strowd LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:21-37.
- Atopic dermatitis in America. Asthma and Allergy Foundation of America website. Accessed July 30, 2021. https://www.aafa.org/atopic-dermatitis-in-america
- Schlichte MJ, Vandersall A, Katta R. Diet and eczema: a review of dietary supplements for the treatment of atopic dermatitis. Dermatol Pract Concept. 2016;6:23-29.
- Brown WR, Hansen AE. Arachidonic and linolic acid of the serum in normal and eczematous human subjects. Proc Soc Exp Bio Med. 1937;36:113-117.
- Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411-424.
- Ferreira MJ, Fiadeiro T, Silva M, et al. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998;7:213-216.
- Simon D, Eng PA, Borelli S, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014;31:180-188.
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411-1414.
- Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;4:CD004416.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ. 2003;327:2.
- Schalin-Karrila M, Mattila L, Jansen CT, et al. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11-19.
- Chung BY, Park SY, Jung MJ, et al. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409-416.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatolog Treat. 1990;1:199-201.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. Dermatitis. 2016;27:39-42.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371-1376.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema [published online February 15, 2012]. Cochrane Database Syst Rev. Accessed July 22, 2021. doi:10.1002/14651858.CD005205.pub3
- Balic´ A, Vlašic´ D, Žužul K, et al. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
- Salem I, Ramser A, Isham N, et al. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Pathogenesis Manage Atopic Dermatitis. 2011;41:112-124.
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286.
- Lee E, Lee S-Y, Kang M-J, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91-92.e1.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241-244.
- Kim H-J, Kim HY, Lee S-Y, et al. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376.
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860.
- Kim S-O, Ah Y-M, Yu YM, et al. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217-226.
- Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.

- Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135.
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15-21.
- Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222-228.
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:249-257.
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine. 2021;27:700-709.
- França K. Topical probiotics in dermatological therapy and skincare: a concise review. Dermatol Ther (Heidelb). 2020;11:71-77.
- Talbott W, Duffy N. Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. Am J Clin Dermatol. 2015;16:147-165.
- Gamret AC, Price A, Fertig RM, et al. Complementary and alternative medicine therapies for psoriasis: a systematic review. JAMA Dermatol. 2018;154:1330-1337.
- Fleischer AB, Feldman SR, Rapp SR, et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216-220.
- Ben-Arye E, Ziv M, Frenkel M, et al. Complementary medicine and psoriasis: linking the patient’s outlook with evidence-based medicine. Dermatology. 2003;207:302-307.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a Systematic review. JAMA Dermatol. 2018;154:934-950.
- Gupta AK, Ellis CN, Tellner DC, et al. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120:801-807.
- Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol. 2018;47:27-36.
- Søyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Heng MCY, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
- Sarafian G, Afshar M, Mansouri P, et al. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14:865-876.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Letters. 1994;341:19-22.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:283634.
- Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625-631.
- Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis. Eur J Dermatol. 2015;25:240-246.
- Cheng H-M, Wu Y-C, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17:439.
- Lin Y-K, Chang C-J, Chang Y-C, et al. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch Dermatol. 2008;144:1457-1464.
- Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019;54:891-896.
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacol Ther. 2013;37:3-17.
- Bax CE, Chakka S, Concha JSS, et al. The effects of immunostimulatory herbal supplements on autoimmune skin diseases. J Am Acad Dermatol. 2021;84:1051-1058.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes an altered gut microbiota in psoriatic arthritis and resembles dysbiosis of inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139.
- Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017;25:559-566.
- Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-339.
- Hosking A-M, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89.
- Tkachenko E, Okhovat J-P, Manjaly P, et al. Complementary & alternative medicine for alopecia areata: a systematic review [published online December 20, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.027
- Lepe K, Zito PM. Alopecia areata. In: StatPearls. StatPearls Publishing; 2021. Accessed July 22, 2021. https://pubmed.ncbi.nlm.nih.gov/30725685/
- Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata—a promising new dawn? Expert Rev Clin Pharmacol. 2020;13:43-51. doi:10.1080/17512433.2020.1702878
- van den Biggelaar FJHM, Smolders J, Jansen JFA. Complementary and alternative medicine in alopecia areata. AM J Clin Dermatol. 2010;11:11-20.
- Hussain ST, Mostaghimi A, Barr PJ, et al. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichology. 2017;9:160-164.
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32.
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346.
- Burian JP, Sacramento LVS, Carlos IZ. Fungal infection control by garlic extracts (Allium sativum L.) and modulation of peritoneal macrophages activity in murine model of sporotrichosis. Braz J Biol. 2017;77:848-855.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Lakshmi C, Srinivas CR. Allergic contact dermatitis following aromatherapy with valiya narayana thailam—an ayurvedic oil presenting as exfoliative dermatitis. Contact Dermatitis. 2009;61:297-298.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Groot AC de, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. dermatitis. 2016;27:39-42.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Ozmen I, Caliskan E, Arca E, et al. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Med J. 2015;57:233.
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391-395.
- Yang D-Q, You L-P, Song P-H, et al. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18:621-625.
Practice Points
- Dermatologic patients are increasingly opting for alternative treatments in addition to or instead of standard therapies for many common skin conditions.
- Dermatologists should be aware of the emerging evidence regarding the risks and benefits of some of the most popular alternative treatments in common skin disorders.
- Counseling patients on the side effects that accompany many supplements and the lack of data to support others is a crucial component of patient care.
Androgenetic alopecia fuels negative emotions and poor quality of life
and meta-analysis of 41 studies.
“Hair loss affects self-image, causes trichodynia, and plays a role in emotions and social activity, which may be associated with psychiatric problems and impaired health-related quality of life,” wrote Chun-Hsien Huang, MD, of Chang Gung Memorial Hospital, Linkou, Taiwan, and colleagues. However, systematic reviews of the associations between androgenetic alopecia (AGA) and health-related quality of life (HRQOL) are lacking, they said.
In a study published in JAMA Dermatology, the researchers reviewed data from a total of 7,995 AGA patients in 41 studies. The studies included 11 tools for HRQOL assessment and 29 tools for psychological assessment. Of these, the Dermatology Life Quality Index (DLQI) and the Hair-Specific Skindex-29 were used to assess quality of life, and the Center for Epidemiologic Studies Depression Scale (CES-D) was used for psychological assessment in the meta-analysis.
Overall, 27 studies identified 18 factors associated with HRQOL; those with an inverse effect were higher self-rated hair loss severity, lower VAS score, and higher educational level. Of note, neither physician-rated hair loss severity nor treatment response were factors in HRQOL, the researchers said.
The pooled DLQI score across studies was 8.16, and subgroup analysis showed no differences in HRQOL between men and women or between patients from European vs. Asian countries. However, five studies showed significant differences in HRQOL between men and women when different assessment tools were used, which emphasized the need for more studies to examine the association of AGA with HRQOL by sex, the researchers said.
The meta-analysis of the Hair-Specific Skindex-29 scores showed pooled averages of 21.95 for symptom dimension, 18.52 in function dimension, and 29.22 in emotion dimension. Of these, the emotion dimension scores indicated moderate emotional impairment.
The average pooled score on the CES-D in the meta-analysis was 14.98, indicating no association between AGA and depression, the researchers said. However, “depression accounts for only a part of the emotion dimension,” they said. “Therefore, emotion dimension could be impaired even if no depressive symptoms were noted.”
The pooled DLQI scores for AGA (8.16) were higher than scores for other skin conditions including alopecia areata (6.3), contact dermatitis (7.35), and acne vulgaris (7.45), but lower than the pooled scores for vitiligo (9.11), urticaria (9.8), psoriasis (10.53), and atopic dermatitis (11.2), the researchers noted. “However, additional head-to-head studies are needed for direct comparisons of HRQOL in patients with various dermatoses,” they said.
The study findings were limited by the cross-sectional design of many of the included studies, and the limited number of assessment tools included in the analysis, the researchers noted. Other limitations were the lack of specific domain scores and the inclusion of only three studies from China, they said.
However, the results are consistent with findings from previous studies, and suggest that patients with AGA may benefit from psychological and psychosocial support, the researchers said.
Quality of life issues deserve attention
“Studies of the quality-of-life impact of various conditions are becoming more common in the medical literature,” Jamie B. MacKelfresh, MD, associate professor of dermatology, Emory University, Atlanta, said in an interview.
“Androgenetic alopecia is the most common type of hair loss in men and women,” she noted. “Hair loss can be labeled as a cosmetic concern, so it is important that providers understand the significant quality-of-life impact androgenetic alopecia has on the many people with this diagnosis,” she emphasized.
Dr. MacKelfresh, who was asked to comment on the study, said she was surprised that the subgroup analysis of the DLQI showed no significant difference between men and women. “This surprised me because a number of past studies have highlighted the relatively greater quality-of-life impact of hair loss on women compared to men,” she noted.
However, she added, “I was not surprised to see that androgenetic alopecia has a significant quality-of-life impact on many patients, and that physician objective assessments of the hair loss do not always correlate with the amount of quality-of-life impact,” said Dr. MacKelfresh. “In the patients I see, I find hair loss very often has a significant quality-of-life impact on patients, regardless of gender, and the amount of quality-of-life impact definitely does not always correlate with the objective amount of hair loss,” she noted.
A takeaway message for clinicians is to be aware that androgenetic alopecia frequently has a significant impact on patients, “particularly in the emotional dimension,” and can affect both men and women, Dr. MacKelfresh said. “Objective assessments of hair loss severity by providers may not accurately predict the degree of quality-of-life impact a patient may experience; therefore providers should include quality-of-life questions as part of their standard evaluation of patients with androgenetic alopecia,” she said. In addition to treating the hair loss, providers can help these patients by guiding them to psychological support resources, she emphasized.
More research is needed to assess the impact of androgenetic alopecia on “men, women, and the non-binary gender population,” as well as the relationship between self-esteem and hair loss, she said. “Finally, it would be helpful to understand what interventions can best help improve androgenetic alopecia patients’ quality of life,” she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. MacKelfresh had no financial conflicts to disclose.
and meta-analysis of 41 studies.
“Hair loss affects self-image, causes trichodynia, and plays a role in emotions and social activity, which may be associated with psychiatric problems and impaired health-related quality of life,” wrote Chun-Hsien Huang, MD, of Chang Gung Memorial Hospital, Linkou, Taiwan, and colleagues. However, systematic reviews of the associations between androgenetic alopecia (AGA) and health-related quality of life (HRQOL) are lacking, they said.
In a study published in JAMA Dermatology, the researchers reviewed data from a total of 7,995 AGA patients in 41 studies. The studies included 11 tools for HRQOL assessment and 29 tools for psychological assessment. Of these, the Dermatology Life Quality Index (DLQI) and the Hair-Specific Skindex-29 were used to assess quality of life, and the Center for Epidemiologic Studies Depression Scale (CES-D) was used for psychological assessment in the meta-analysis.
Overall, 27 studies identified 18 factors associated with HRQOL; those with an inverse effect were higher self-rated hair loss severity, lower VAS score, and higher educational level. Of note, neither physician-rated hair loss severity nor treatment response were factors in HRQOL, the researchers said.
The pooled DLQI score across studies was 8.16, and subgroup analysis showed no differences in HRQOL between men and women or between patients from European vs. Asian countries. However, five studies showed significant differences in HRQOL between men and women when different assessment tools were used, which emphasized the need for more studies to examine the association of AGA with HRQOL by sex, the researchers said.
The meta-analysis of the Hair-Specific Skindex-29 scores showed pooled averages of 21.95 for symptom dimension, 18.52 in function dimension, and 29.22 in emotion dimension. Of these, the emotion dimension scores indicated moderate emotional impairment.
The average pooled score on the CES-D in the meta-analysis was 14.98, indicating no association between AGA and depression, the researchers said. However, “depression accounts for only a part of the emotion dimension,” they said. “Therefore, emotion dimension could be impaired even if no depressive symptoms were noted.”
The pooled DLQI scores for AGA (8.16) were higher than scores for other skin conditions including alopecia areata (6.3), contact dermatitis (7.35), and acne vulgaris (7.45), but lower than the pooled scores for vitiligo (9.11), urticaria (9.8), psoriasis (10.53), and atopic dermatitis (11.2), the researchers noted. “However, additional head-to-head studies are needed for direct comparisons of HRQOL in patients with various dermatoses,” they said.
The study findings were limited by the cross-sectional design of many of the included studies, and the limited number of assessment tools included in the analysis, the researchers noted. Other limitations were the lack of specific domain scores and the inclusion of only three studies from China, they said.
However, the results are consistent with findings from previous studies, and suggest that patients with AGA may benefit from psychological and psychosocial support, the researchers said.
Quality of life issues deserve attention
“Studies of the quality-of-life impact of various conditions are becoming more common in the medical literature,” Jamie B. MacKelfresh, MD, associate professor of dermatology, Emory University, Atlanta, said in an interview.
“Androgenetic alopecia is the most common type of hair loss in men and women,” she noted. “Hair loss can be labeled as a cosmetic concern, so it is important that providers understand the significant quality-of-life impact androgenetic alopecia has on the many people with this diagnosis,” she emphasized.
Dr. MacKelfresh, who was asked to comment on the study, said she was surprised that the subgroup analysis of the DLQI showed no significant difference between men and women. “This surprised me because a number of past studies have highlighted the relatively greater quality-of-life impact of hair loss on women compared to men,” she noted.
However, she added, “I was not surprised to see that androgenetic alopecia has a significant quality-of-life impact on many patients, and that physician objective assessments of the hair loss do not always correlate with the amount of quality-of-life impact,” said Dr. MacKelfresh. “In the patients I see, I find hair loss very often has a significant quality-of-life impact on patients, regardless of gender, and the amount of quality-of-life impact definitely does not always correlate with the objective amount of hair loss,” she noted.
A takeaway message for clinicians is to be aware that androgenetic alopecia frequently has a significant impact on patients, “particularly in the emotional dimension,” and can affect both men and women, Dr. MacKelfresh said. “Objective assessments of hair loss severity by providers may not accurately predict the degree of quality-of-life impact a patient may experience; therefore providers should include quality-of-life questions as part of their standard evaluation of patients with androgenetic alopecia,” she said. In addition to treating the hair loss, providers can help these patients by guiding them to psychological support resources, she emphasized.
More research is needed to assess the impact of androgenetic alopecia on “men, women, and the non-binary gender population,” as well as the relationship between self-esteem and hair loss, she said. “Finally, it would be helpful to understand what interventions can best help improve androgenetic alopecia patients’ quality of life,” she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. MacKelfresh had no financial conflicts to disclose.
and meta-analysis of 41 studies.
“Hair loss affects self-image, causes trichodynia, and plays a role in emotions and social activity, which may be associated with psychiatric problems and impaired health-related quality of life,” wrote Chun-Hsien Huang, MD, of Chang Gung Memorial Hospital, Linkou, Taiwan, and colleagues. However, systematic reviews of the associations between androgenetic alopecia (AGA) and health-related quality of life (HRQOL) are lacking, they said.
In a study published in JAMA Dermatology, the researchers reviewed data from a total of 7,995 AGA patients in 41 studies. The studies included 11 tools for HRQOL assessment and 29 tools for psychological assessment. Of these, the Dermatology Life Quality Index (DLQI) and the Hair-Specific Skindex-29 were used to assess quality of life, and the Center for Epidemiologic Studies Depression Scale (CES-D) was used for psychological assessment in the meta-analysis.
Overall, 27 studies identified 18 factors associated with HRQOL; those with an inverse effect were higher self-rated hair loss severity, lower VAS score, and higher educational level. Of note, neither physician-rated hair loss severity nor treatment response were factors in HRQOL, the researchers said.
The pooled DLQI score across studies was 8.16, and subgroup analysis showed no differences in HRQOL between men and women or between patients from European vs. Asian countries. However, five studies showed significant differences in HRQOL between men and women when different assessment tools were used, which emphasized the need for more studies to examine the association of AGA with HRQOL by sex, the researchers said.
The meta-analysis of the Hair-Specific Skindex-29 scores showed pooled averages of 21.95 for symptom dimension, 18.52 in function dimension, and 29.22 in emotion dimension. Of these, the emotion dimension scores indicated moderate emotional impairment.
The average pooled score on the CES-D in the meta-analysis was 14.98, indicating no association between AGA and depression, the researchers said. However, “depression accounts for only a part of the emotion dimension,” they said. “Therefore, emotion dimension could be impaired even if no depressive symptoms were noted.”
The pooled DLQI scores for AGA (8.16) were higher than scores for other skin conditions including alopecia areata (6.3), contact dermatitis (7.35), and acne vulgaris (7.45), but lower than the pooled scores for vitiligo (9.11), urticaria (9.8), psoriasis (10.53), and atopic dermatitis (11.2), the researchers noted. “However, additional head-to-head studies are needed for direct comparisons of HRQOL in patients with various dermatoses,” they said.
The study findings were limited by the cross-sectional design of many of the included studies, and the limited number of assessment tools included in the analysis, the researchers noted. Other limitations were the lack of specific domain scores and the inclusion of only three studies from China, they said.
However, the results are consistent with findings from previous studies, and suggest that patients with AGA may benefit from psychological and psychosocial support, the researchers said.
Quality of life issues deserve attention
“Studies of the quality-of-life impact of various conditions are becoming more common in the medical literature,” Jamie B. MacKelfresh, MD, associate professor of dermatology, Emory University, Atlanta, said in an interview.
“Androgenetic alopecia is the most common type of hair loss in men and women,” she noted. “Hair loss can be labeled as a cosmetic concern, so it is important that providers understand the significant quality-of-life impact androgenetic alopecia has on the many people with this diagnosis,” she emphasized.
Dr. MacKelfresh, who was asked to comment on the study, said she was surprised that the subgroup analysis of the DLQI showed no significant difference between men and women. “This surprised me because a number of past studies have highlighted the relatively greater quality-of-life impact of hair loss on women compared to men,” she noted.
However, she added, “I was not surprised to see that androgenetic alopecia has a significant quality-of-life impact on many patients, and that physician objective assessments of the hair loss do not always correlate with the amount of quality-of-life impact,” said Dr. MacKelfresh. “In the patients I see, I find hair loss very often has a significant quality-of-life impact on patients, regardless of gender, and the amount of quality-of-life impact definitely does not always correlate with the objective amount of hair loss,” she noted.
A takeaway message for clinicians is to be aware that androgenetic alopecia frequently has a significant impact on patients, “particularly in the emotional dimension,” and can affect both men and women, Dr. MacKelfresh said. “Objective assessments of hair loss severity by providers may not accurately predict the degree of quality-of-life impact a patient may experience; therefore providers should include quality-of-life questions as part of their standard evaluation of patients with androgenetic alopecia,” she said. In addition to treating the hair loss, providers can help these patients by guiding them to psychological support resources, she emphasized.
More research is needed to assess the impact of androgenetic alopecia on “men, women, and the non-binary gender population,” as well as the relationship between self-esteem and hair loss, she said. “Finally, it would be helpful to understand what interventions can best help improve androgenetic alopecia patients’ quality of life,” she noted.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. MacKelfresh had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
Graying of hair: Could it be reversed?
as hair pigment goes through its natural progression of senescence.
However, the recent publication that is a collaboration between the department of psychiatry at Columbia University, New York; and the departments of dermatology at the University College Dublin, University of Miami, and the University of Manchester (England); and the Monasterium Laboratory in Münster, Germany, demonstrates a quantitative mapping of human hair graying – and its reversal – in relation to stress.
In the study, hair color of single strands of hair from seven healthy females and seven healthy males, whose mean age was 35 years (range, 9-65 years), were analyzed. In addition to hair pigment analysis, study subjects documented the stress they were experiencing each week in diaries. Using either high resolution image scanners, electron microscopy, and/or hair shaft proteomics, the investigators were able to evaluate loss of pigment within fragments small enough to have grown over one hour.
When changes in hair color were noted, variations in up to 300 proteins were documented, including an up-regulation of the fatty acid synthesis and metabolism machinery in graying. Recent studies also corroborate that fatty acid synthesis by fatty acid synthase and “transport by CPT1A ... are sufficient drivers of cell senescence, and that fatty acid metabolism regulates melanocyte aging biology” the authors wrote.
Molecularly, the investigators found that gray hairs up-regulate proteins associated with energy metabolism, mitochondria, and antioxidant defenses. The graying correlated with stress was also reversible, “at least temporarily,” based on their retrospective analysis and analysis over the 2.5-year recruitment period, the investigators wrote. Specifically, they found that graying hair “may be acutely triggered by stressful life experiences, the removal of which can trigger reversal.” From the data, they also developed a mathematical model to predict what might happen to human hair over time.
Through this study, proof-of-concept evidence is provided indicating that biobehavioral factors are linked to human hair graying dynamics. Future analysis with larger sample sizes and incorporating neuroendocrine markers may further support these correlations. This is an interesting study that elucidates the mechanisms responsible for how stress and other life exposures manifest in human biology, and, if we as human beings effectively manage that stress, how it may both reverse the negative impact and outcomes affecting our body and health.
The study was supported by the Wharton Fund and grants from the National Institutes of Health.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
as hair pigment goes through its natural progression of senescence.
However, the recent publication that is a collaboration between the department of psychiatry at Columbia University, New York; and the departments of dermatology at the University College Dublin, University of Miami, and the University of Manchester (England); and the Monasterium Laboratory in Münster, Germany, demonstrates a quantitative mapping of human hair graying – and its reversal – in relation to stress.
In the study, hair color of single strands of hair from seven healthy females and seven healthy males, whose mean age was 35 years (range, 9-65 years), were analyzed. In addition to hair pigment analysis, study subjects documented the stress they were experiencing each week in diaries. Using either high resolution image scanners, electron microscopy, and/or hair shaft proteomics, the investigators were able to evaluate loss of pigment within fragments small enough to have grown over one hour.
When changes in hair color were noted, variations in up to 300 proteins were documented, including an up-regulation of the fatty acid synthesis and metabolism machinery in graying. Recent studies also corroborate that fatty acid synthesis by fatty acid synthase and “transport by CPT1A ... are sufficient drivers of cell senescence, and that fatty acid metabolism regulates melanocyte aging biology” the authors wrote.
Molecularly, the investigators found that gray hairs up-regulate proteins associated with energy metabolism, mitochondria, and antioxidant defenses. The graying correlated with stress was also reversible, “at least temporarily,” based on their retrospective analysis and analysis over the 2.5-year recruitment period, the investigators wrote. Specifically, they found that graying hair “may be acutely triggered by stressful life experiences, the removal of which can trigger reversal.” From the data, they also developed a mathematical model to predict what might happen to human hair over time.
Through this study, proof-of-concept evidence is provided indicating that biobehavioral factors are linked to human hair graying dynamics. Future analysis with larger sample sizes and incorporating neuroendocrine markers may further support these correlations. This is an interesting study that elucidates the mechanisms responsible for how stress and other life exposures manifest in human biology, and, if we as human beings effectively manage that stress, how it may both reverse the negative impact and outcomes affecting our body and health.
The study was supported by the Wharton Fund and grants from the National Institutes of Health.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
as hair pigment goes through its natural progression of senescence.
However, the recent publication that is a collaboration between the department of psychiatry at Columbia University, New York; and the departments of dermatology at the University College Dublin, University of Miami, and the University of Manchester (England); and the Monasterium Laboratory in Münster, Germany, demonstrates a quantitative mapping of human hair graying – and its reversal – in relation to stress.
In the study, hair color of single strands of hair from seven healthy females and seven healthy males, whose mean age was 35 years (range, 9-65 years), were analyzed. In addition to hair pigment analysis, study subjects documented the stress they were experiencing each week in diaries. Using either high resolution image scanners, electron microscopy, and/or hair shaft proteomics, the investigators were able to evaluate loss of pigment within fragments small enough to have grown over one hour.
When changes in hair color were noted, variations in up to 300 proteins were documented, including an up-regulation of the fatty acid synthesis and metabolism machinery in graying. Recent studies also corroborate that fatty acid synthesis by fatty acid synthase and “transport by CPT1A ... are sufficient drivers of cell senescence, and that fatty acid metabolism regulates melanocyte aging biology” the authors wrote.
Molecularly, the investigators found that gray hairs up-regulate proteins associated with energy metabolism, mitochondria, and antioxidant defenses. The graying correlated with stress was also reversible, “at least temporarily,” based on their retrospective analysis and analysis over the 2.5-year recruitment period, the investigators wrote. Specifically, they found that graying hair “may be acutely triggered by stressful life experiences, the removal of which can trigger reversal.” From the data, they also developed a mathematical model to predict what might happen to human hair over time.
Through this study, proof-of-concept evidence is provided indicating that biobehavioral factors are linked to human hair graying dynamics. Future analysis with larger sample sizes and incorporating neuroendocrine markers may further support these correlations. This is an interesting study that elucidates the mechanisms responsible for how stress and other life exposures manifest in human biology, and, if we as human beings effectively manage that stress, how it may both reverse the negative impact and outcomes affecting our body and health.
The study was supported by the Wharton Fund and grants from the National Institutes of Health.
Dr. Wesley and Dr. Lily Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
Counseling About Traction Alopecia: A "Compliment, Discuss, and Suggest" Method
Traction alopecia (TA)--one of the most common types of hair loss in Black women (although not exclusive to Black women)--is reversible when early corrective measures are taken; if chronic tension continues, however, permanent scarring alopecia ensues. Dermatologists can prevent worsening of this distressing hair loss. Due to a dearth of training among dermatologists in conditions occurring in patients with tightly coiled hair, it is imperative to add practical methods to the body of dermatology literature, with the goal of enhancing cultural humility.
Hairstyling among Black women often is a lengthy process and often results in relationship bonding with the hair care giver, in turn imparting hair care traditions to the next generation. Therefore, a well-received discussion about TA prevention not only has an impact on the patient but potentially on a multigenerational family of women and friends. We present a memory aid for discussing TA, with a focus on cultural humility and patient-centered communication.
Factors contributing to the risk of TA are hairstyles and hair care practices commonly used in Black individuals, including braids, locs, weaves, wigs, and chemical straightening.1 These styles often are worn to increase hair manageability or as a creative expression of beauty.
Discussing TA can be distressing for physicians and patients, especially in the setting of hair texture discordance. In a study that surveyed Black patients' perception of their dermatologic care both in and outside of a skin of color clinic, 71% of respondents (12/17) said that they prefer a race-concordant dermatologist. Some respondents reported that non-skin of color clinic dermatologists examined their hair with the end of a pencil or not at all; patients interpreted these interactions as disrespectful and racially insensitive.2 Another study found that only 30.2% (19/63) of dermatology chief residents and 12.2% (5/41) of program directors reported a specific rotation during which residents gained experience treating skin of color patients.3
Due to a paucity of training in diagnosing and treating patients with tightly coiled hair who experience hair loss, some physicians might feel uncomfortable caring for patients who have tightly coiled hair. Although many Black patients prefer to see a race-concordant dermatologist because of their perceived cultural competence and shared experience, there is a paucity of Black dermatologists to see all patients who have tightly coiled hair.4 Therefore, all dermatologists should become skilled and comfortable discussing and treating TA in patients with all hair types.
METHOD FOR COUNSELING
The following scenarios are a guide to begin closing the competency gap in counseling about TA, using a "compliment, discuss, and suggest" method.
Scenario 1
A Black woman presents with a concern of "thinning edges" (a popular term on social media for TA). A hair-discordant dermatologist tells her, first, that she has TA caused by wearing tight hairstyles and, second, that the treatment is to stop wearing tight braids and weaves and to discontinue chemical relaxers. The dermatologist then leaves the room.
The Patient's Perspective
It is not uncommon for the patient to have feelings of frustration about how they will style their hair, especially if they are unfamiliar with caring for their hair in its natural state.5 Also, they might have feelings of dismay that the loving childhood hair care giver, often their mother or grandmother, unintentionally harmed them with a tight style. They also might feel betrayed by their hairstylist, who might not have encouraged them to see a dermatologist, or who continued to oblige their request for a high-risk hairstyle. The patient might feel uncomfortable communicating the dermatologist's new recommendations to their hair care team, who also are part of her emotional support system. The patient also might think that the hair-discordant dermatologist has no idea what they "go through" with their hair.
"Compliment, Discuss, and Suggest" Counseling
Traction alopecia is caused by tight hairstyles that often hurt when they are put in as tight braids, weaves, and ponytails.6 Risk increases if tight styles are applied to chemically straightened hair.1 Braids, sew-in weaves, and wigs with adhesive sometimes are referred to as protective styles. However, these styles can still lead to TA due to excessive tension.
- Compliment: "Your hair looks great. I know that you get many compliments."
- Discuss: "However, some of the styles might be increasing your risk for hair loss. Our goal is to preserve as many of your follicles as possible."
- Suggest: "Let's start by loosening the hairstyle if it is painful when being applied. Pain means inflammation, which can lead to scarring of hair follicles and worsening of hair loss."
Using pronouns such as we, us, and our is intentional. Doing so signals that the dermatologist is a partner with the patient in the treatment of TA. Starting with a simple initial recommendation gives the patient time to process the common thoughts highlighted in The Patient's Perspective section.6
Scenario 2
A Black child (we'll call her "Janet") is accompanied by her mother for follow-up of mild atopic dermatitis on the body and scalp. When the dermatologist examines the patient's scalp, they note that she has the fringe sign--retained short hairs along the frontal hairline--that is consistent with TA. Janet's hair is adorned with 2 tight ponytails in the front with colorful decorative balls on ponytail ties, barrettes, and 6 cornrow braids in the back with plastic beads on the ends. The dermatologist counsels about the atopic dermatitis and leaves the room.
"Compliment, Discuss, and Suggest" Counseling
The use of tight decorative balls on ponytail ties and numerous plastic beads increases the amount of tension and weight on the hair, which may lead to a higher risk for developing traction alopecia.6 It is quite common for children of African descent to wear hair adornments. Proper counseling regarding their use and possible implications is essential.
- Compliment: "You're doing a great job controlling the atopic dermatitis, which can cause Janet's scalp to be dry. Also, her hair is beautiful--it looks like you spent a lot of time on her hair. And Janet, I like the color of your barrettes."
- Discuss: "Mom, I just noticed that a few areas look tight. Let's look together." (The dermatologist points out areas where the scalp is tented upward due to traction, follicular pustules or papules, or the frontal fringe sign.) "I'm on a mission to #savetheedges because we want Janet to grow up with full edges." (Again, loss of "edges" refers to TA.)
- Suggest: "When you do Janet's hair, it's OK if every hair is not in place. In fact, making styles look and feel 1 or 2 weeks old will lessen tension on the scalp. Remove Janet's hair ties to release tension when she is at home and while she's sleeping, if possible. Every minute that the hair is loose really does help."6
The Parent's Perspective
All parents take pride in their children. In some Black communities, mothers are judged by how well they manage and style their children's hair. Some people might even suggest that parents of children with nonstyled, tightly coiled hair are not fit parents. Anthropologist Sylvia Boone, PhD, found that among the Mende tribe in Sierra Leone, "unkempt, 'neglected,' or 'messy' hair implied that a woman either had loose morals or was insane."7
Braids are commonly worn by people of African heritage for a variety of reasons, including ease of manageability, to decrease daily hairstyling time, and as an expression of creativity. Intricate neat hairstyles, despite the risk of pain and TA, are perceived as a sign that the child is cared for and loved.6
FINAL THOUGHTS
Patient-centered communication is associated with the patient trusting the physician, which is especially important in race-discordant physician-patient relationships. A study found that patient-physician race discordance led to shorter visits, a lower rating of patient affect, and less shared decision-making.8 Moreover, in a study of primary care clinicians, implicit bias was found to affect communication patterns and social interactions, impacting patient outcomes. Downstream effects of racial bias resulted in less speaking, smiling, and social comments when interacting with Black patients.9
These findings highlight the need to address interpersonal barriers to effective communication in race-discordant patient-physician dyads. A history of segregated neighborhoods and schools might contribute to structural barriers, resulting in lack of familiarity with cultural norms outside one's culture, which might globally perpetuate poor communication and patient outcomes.
The "compliment, discuss, and suggest" method might lead to more positive physician-patient encounters by having the dermatologist focus on empathetically understanding the patient's perspective.10 Effective communication, understanding cultural hair care practices, and a thorough scalp examination are paramount for patients with tightly coiled hair.11 Early intervention in TA is crucial and involves partnering with patients and parents to amend high-risk hairstyling routines with cultural humility.
Traction alopecia (TA)--one of the most common types of hair loss in Black women (although not exclusive to Black women)--is reversible when early corrective measures are taken; if chronic tension continues, however, permanent scarring alopecia ensues. Dermatologists can prevent worsening of this distressing hair loss. Due to a dearth of training among dermatologists in conditions occurring in patients with tightly coiled hair, it is imperative to add practical methods to the body of dermatology literature, with the goal of enhancing cultural humility.
Hairstyling among Black women often is a lengthy process and often results in relationship bonding with the hair care giver, in turn imparting hair care traditions to the next generation. Therefore, a well-received discussion about TA prevention not only has an impact on the patient but potentially on a multigenerational family of women and friends. We present a memory aid for discussing TA, with a focus on cultural humility and patient-centered communication.
Factors contributing to the risk of TA are hairstyles and hair care practices commonly used in Black individuals, including braids, locs, weaves, wigs, and chemical straightening.1 These styles often are worn to increase hair manageability or as a creative expression of beauty.
Discussing TA can be distressing for physicians and patients, especially in the setting of hair texture discordance. In a study that surveyed Black patients' perception of their dermatologic care both in and outside of a skin of color clinic, 71% of respondents (12/17) said that they prefer a race-concordant dermatologist. Some respondents reported that non-skin of color clinic dermatologists examined their hair with the end of a pencil or not at all; patients interpreted these interactions as disrespectful and racially insensitive.2 Another study found that only 30.2% (19/63) of dermatology chief residents and 12.2% (5/41) of program directors reported a specific rotation during which residents gained experience treating skin of color patients.3
Due to a paucity of training in diagnosing and treating patients with tightly coiled hair who experience hair loss, some physicians might feel uncomfortable caring for patients who have tightly coiled hair. Although many Black patients prefer to see a race-concordant dermatologist because of their perceived cultural competence and shared experience, there is a paucity of Black dermatologists to see all patients who have tightly coiled hair.4 Therefore, all dermatologists should become skilled and comfortable discussing and treating TA in patients with all hair types.
METHOD FOR COUNSELING
The following scenarios are a guide to begin closing the competency gap in counseling about TA, using a "compliment, discuss, and suggest" method.
Scenario 1
A Black woman presents with a concern of "thinning edges" (a popular term on social media for TA). A hair-discordant dermatologist tells her, first, that she has TA caused by wearing tight hairstyles and, second, that the treatment is to stop wearing tight braids and weaves and to discontinue chemical relaxers. The dermatologist then leaves the room.
The Patient's Perspective
It is not uncommon for the patient to have feelings of frustration about how they will style their hair, especially if they are unfamiliar with caring for their hair in its natural state.5 Also, they might have feelings of dismay that the loving childhood hair care giver, often their mother or grandmother, unintentionally harmed them with a tight style. They also might feel betrayed by their hairstylist, who might not have encouraged them to see a dermatologist, or who continued to oblige their request for a high-risk hairstyle. The patient might feel uncomfortable communicating the dermatologist's new recommendations to their hair care team, who also are part of her emotional support system. The patient also might think that the hair-discordant dermatologist has no idea what they "go through" with their hair.
"Compliment, Discuss, and Suggest" Counseling
Traction alopecia is caused by tight hairstyles that often hurt when they are put in as tight braids, weaves, and ponytails.6 Risk increases if tight styles are applied to chemically straightened hair.1 Braids, sew-in weaves, and wigs with adhesive sometimes are referred to as protective styles. However, these styles can still lead to TA due to excessive tension.
- Compliment: "Your hair looks great. I know that you get many compliments."
- Discuss: "However, some of the styles might be increasing your risk for hair loss. Our goal is to preserve as many of your follicles as possible."
- Suggest: "Let's start by loosening the hairstyle if it is painful when being applied. Pain means inflammation, which can lead to scarring of hair follicles and worsening of hair loss."
Using pronouns such as we, us, and our is intentional. Doing so signals that the dermatologist is a partner with the patient in the treatment of TA. Starting with a simple initial recommendation gives the patient time to process the common thoughts highlighted in The Patient's Perspective section.6
Scenario 2
A Black child (we'll call her "Janet") is accompanied by her mother for follow-up of mild atopic dermatitis on the body and scalp. When the dermatologist examines the patient's scalp, they note that she has the fringe sign--retained short hairs along the frontal hairline--that is consistent with TA. Janet's hair is adorned with 2 tight ponytails in the front with colorful decorative balls on ponytail ties, barrettes, and 6 cornrow braids in the back with plastic beads on the ends. The dermatologist counsels about the atopic dermatitis and leaves the room.
"Compliment, Discuss, and Suggest" Counseling
The use of tight decorative balls on ponytail ties and numerous plastic beads increases the amount of tension and weight on the hair, which may lead to a higher risk for developing traction alopecia.6 It is quite common for children of African descent to wear hair adornments. Proper counseling regarding their use and possible implications is essential.
- Compliment: "You're doing a great job controlling the atopic dermatitis, which can cause Janet's scalp to be dry. Also, her hair is beautiful--it looks like you spent a lot of time on her hair. And Janet, I like the color of your barrettes."
- Discuss: "Mom, I just noticed that a few areas look tight. Let's look together." (The dermatologist points out areas where the scalp is tented upward due to traction, follicular pustules or papules, or the frontal fringe sign.) "I'm on a mission to #savetheedges because we want Janet to grow up with full edges." (Again, loss of "edges" refers to TA.)
- Suggest: "When you do Janet's hair, it's OK if every hair is not in place. In fact, making styles look and feel 1 or 2 weeks old will lessen tension on the scalp. Remove Janet's hair ties to release tension when she is at home and while she's sleeping, if possible. Every minute that the hair is loose really does help."6
The Parent's Perspective
All parents take pride in their children. In some Black communities, mothers are judged by how well they manage and style their children's hair. Some people might even suggest that parents of children with nonstyled, tightly coiled hair are not fit parents. Anthropologist Sylvia Boone, PhD, found that among the Mende tribe in Sierra Leone, "unkempt, 'neglected,' or 'messy' hair implied that a woman either had loose morals or was insane."7
Braids are commonly worn by people of African heritage for a variety of reasons, including ease of manageability, to decrease daily hairstyling time, and as an expression of creativity. Intricate neat hairstyles, despite the risk of pain and TA, are perceived as a sign that the child is cared for and loved.6
FINAL THOUGHTS
Patient-centered communication is associated with the patient trusting the physician, which is especially important in race-discordant physician-patient relationships. A study found that patient-physician race discordance led to shorter visits, a lower rating of patient affect, and less shared decision-making.8 Moreover, in a study of primary care clinicians, implicit bias was found to affect communication patterns and social interactions, impacting patient outcomes. Downstream effects of racial bias resulted in less speaking, smiling, and social comments when interacting with Black patients.9
These findings highlight the need to address interpersonal barriers to effective communication in race-discordant patient-physician dyads. A history of segregated neighborhoods and schools might contribute to structural barriers, resulting in lack of familiarity with cultural norms outside one's culture, which might globally perpetuate poor communication and patient outcomes.
The "compliment, discuss, and suggest" method might lead to more positive physician-patient encounters by having the dermatologist focus on empathetically understanding the patient's perspective.10 Effective communication, understanding cultural hair care practices, and a thorough scalp examination are paramount for patients with tightly coiled hair.11 Early intervention in TA is crucial and involves partnering with patients and parents to amend high-risk hairstyling routines with cultural humility.
Traction alopecia (TA)--one of the most common types of hair loss in Black women (although not exclusive to Black women)--is reversible when early corrective measures are taken; if chronic tension continues, however, permanent scarring alopecia ensues. Dermatologists can prevent worsening of this distressing hair loss. Due to a dearth of training among dermatologists in conditions occurring in patients with tightly coiled hair, it is imperative to add practical methods to the body of dermatology literature, with the goal of enhancing cultural humility.
Hairstyling among Black women often is a lengthy process and often results in relationship bonding with the hair care giver, in turn imparting hair care traditions to the next generation. Therefore, a well-received discussion about TA prevention not only has an impact on the patient but potentially on a multigenerational family of women and friends. We present a memory aid for discussing TA, with a focus on cultural humility and patient-centered communication.
Factors contributing to the risk of TA are hairstyles and hair care practices commonly used in Black individuals, including braids, locs, weaves, wigs, and chemical straightening.1 These styles often are worn to increase hair manageability or as a creative expression of beauty.
Discussing TA can be distressing for physicians and patients, especially in the setting of hair texture discordance. In a study that surveyed Black patients' perception of their dermatologic care both in and outside of a skin of color clinic, 71% of respondents (12/17) said that they prefer a race-concordant dermatologist. Some respondents reported that non-skin of color clinic dermatologists examined their hair with the end of a pencil or not at all; patients interpreted these interactions as disrespectful and racially insensitive.2 Another study found that only 30.2% (19/63) of dermatology chief residents and 12.2% (5/41) of program directors reported a specific rotation during which residents gained experience treating skin of color patients.3
Due to a paucity of training in diagnosing and treating patients with tightly coiled hair who experience hair loss, some physicians might feel uncomfortable caring for patients who have tightly coiled hair. Although many Black patients prefer to see a race-concordant dermatologist because of their perceived cultural competence and shared experience, there is a paucity of Black dermatologists to see all patients who have tightly coiled hair.4 Therefore, all dermatologists should become skilled and comfortable discussing and treating TA in patients with all hair types.
METHOD FOR COUNSELING
The following scenarios are a guide to begin closing the competency gap in counseling about TA, using a "compliment, discuss, and suggest" method.
Scenario 1
A Black woman presents with a concern of "thinning edges" (a popular term on social media for TA). A hair-discordant dermatologist tells her, first, that she has TA caused by wearing tight hairstyles and, second, that the treatment is to stop wearing tight braids and weaves and to discontinue chemical relaxers. The dermatologist then leaves the room.
The Patient's Perspective
It is not uncommon for the patient to have feelings of frustration about how they will style their hair, especially if they are unfamiliar with caring for their hair in its natural state.5 Also, they might have feelings of dismay that the loving childhood hair care giver, often their mother or grandmother, unintentionally harmed them with a tight style. They also might feel betrayed by their hairstylist, who might not have encouraged them to see a dermatologist, or who continued to oblige their request for a high-risk hairstyle. The patient might feel uncomfortable communicating the dermatologist's new recommendations to their hair care team, who also are part of her emotional support system. The patient also might think that the hair-discordant dermatologist has no idea what they "go through" with their hair.
"Compliment, Discuss, and Suggest" Counseling
Traction alopecia is caused by tight hairstyles that often hurt when they are put in as tight braids, weaves, and ponytails.6 Risk increases if tight styles are applied to chemically straightened hair.1 Braids, sew-in weaves, and wigs with adhesive sometimes are referred to as protective styles. However, these styles can still lead to TA due to excessive tension.
- Compliment: "Your hair looks great. I know that you get many compliments."
- Discuss: "However, some of the styles might be increasing your risk for hair loss. Our goal is to preserve as many of your follicles as possible."
- Suggest: "Let's start by loosening the hairstyle if it is painful when being applied. Pain means inflammation, which can lead to scarring of hair follicles and worsening of hair loss."
Using pronouns such as we, us, and our is intentional. Doing so signals that the dermatologist is a partner with the patient in the treatment of TA. Starting with a simple initial recommendation gives the patient time to process the common thoughts highlighted in The Patient's Perspective section.6
Scenario 2
A Black child (we'll call her "Janet") is accompanied by her mother for follow-up of mild atopic dermatitis on the body and scalp. When the dermatologist examines the patient's scalp, they note that she has the fringe sign--retained short hairs along the frontal hairline--that is consistent with TA. Janet's hair is adorned with 2 tight ponytails in the front with colorful decorative balls on ponytail ties, barrettes, and 6 cornrow braids in the back with plastic beads on the ends. The dermatologist counsels about the atopic dermatitis and leaves the room.
"Compliment, Discuss, and Suggest" Counseling
The use of tight decorative balls on ponytail ties and numerous plastic beads increases the amount of tension and weight on the hair, which may lead to a higher risk for developing traction alopecia.6 It is quite common for children of African descent to wear hair adornments. Proper counseling regarding their use and possible implications is essential.
- Compliment: "You're doing a great job controlling the atopic dermatitis, which can cause Janet's scalp to be dry. Also, her hair is beautiful--it looks like you spent a lot of time on her hair. And Janet, I like the color of your barrettes."
- Discuss: "Mom, I just noticed that a few areas look tight. Let's look together." (The dermatologist points out areas where the scalp is tented upward due to traction, follicular pustules or papules, or the frontal fringe sign.) "I'm on a mission to #savetheedges because we want Janet to grow up with full edges." (Again, loss of "edges" refers to TA.)
- Suggest: "When you do Janet's hair, it's OK if every hair is not in place. In fact, making styles look and feel 1 or 2 weeks old will lessen tension on the scalp. Remove Janet's hair ties to release tension when she is at home and while she's sleeping, if possible. Every minute that the hair is loose really does help."6
The Parent's Perspective
All parents take pride in their children. In some Black communities, mothers are judged by how well they manage and style their children's hair. Some people might even suggest that parents of children with nonstyled, tightly coiled hair are not fit parents. Anthropologist Sylvia Boone, PhD, found that among the Mende tribe in Sierra Leone, "unkempt, 'neglected,' or 'messy' hair implied that a woman either had loose morals or was insane."7
Braids are commonly worn by people of African heritage for a variety of reasons, including ease of manageability, to decrease daily hairstyling time, and as an expression of creativity. Intricate neat hairstyles, despite the risk of pain and TA, are perceived as a sign that the child is cared for and loved.6
FINAL THOUGHTS
Patient-centered communication is associated with the patient trusting the physician, which is especially important in race-discordant physician-patient relationships. A study found that patient-physician race discordance led to shorter visits, a lower rating of patient affect, and less shared decision-making.8 Moreover, in a study of primary care clinicians, implicit bias was found to affect communication patterns and social interactions, impacting patient outcomes. Downstream effects of racial bias resulted in less speaking, smiling, and social comments when interacting with Black patients.9
These findings highlight the need to address interpersonal barriers to effective communication in race-discordant patient-physician dyads. A history of segregated neighborhoods and schools might contribute to structural barriers, resulting in lack of familiarity with cultural norms outside one's culture, which might globally perpetuate poor communication and patient outcomes.
The "compliment, discuss, and suggest" method might lead to more positive physician-patient encounters by having the dermatologist focus on empathetically understanding the patient's perspective.10 Effective communication, understanding cultural hair care practices, and a thorough scalp examination are paramount for patients with tightly coiled hair.11 Early intervention in TA is crucial and involves partnering with patients and parents to amend high-risk hairstyling routines with cultural humility.
Practice Points
- When communicating with patients regarding traction alopecia (TA), it is crucial to display cultural humility and empathy.
- Understanding the patient’s hair care goals and perspective allows dermatologists to take a more individualized approach to counseling about TA.
- The “compliment, discuss, and suggest” method is an empathetic and culturally sensitive method for discussing TA with patients.
Pediatric alopecia areata in the U.S. has increased twofold since 2009, study finds
according to results from the largest study to date on the topic.
“Alopecia areata is a relatively common cause of nonscarring hair loss in children,” Paige McKenzie said during the annual meeting of the Society for Pediatric Dermatology. “The only two epidemiologic studies that have been performed in children have been based on registry or survey data which is inherently at risk for bias,” she added, referring to studies published in 2017 and 2018. “Additionally, epidemiologic descriptions of alopecia areata in adults are limited and overall estimates have varied from 0.2% to 2%. Current understanding is also largely based on population studies in Olmsted County, Minnesota, an area with mostly White racial demographics, so it’s not representative of the U.S. population as a whole.”
To identify the incidence and prevalence of pediatric AA over time, and across age, race/ethnicity, and sex, Ms. McKenzie and colleagues conducted a retrospective cohort study from 2009 to 2020 using PEDSnet, a network of seven U.S. pediatric health institutions with a database of more than 6.5 million children. “PEDSnet is unique because it uses a common data model to standardize EHR data across different health systems and uses SNOMED [Systematized Nomenclature of Medicine]–Clinical Terms to identify specific patient populations,” said Ms. McKenzie, who was a clinical research fellow in the section of dermatology at the Children’s Hospital of Philadelphia during the 2020-2021 academic year.
She and her coauthors limited their analysis to children younger than age 18 who were assigned a SNOMED code for AA during at least one dermatology physician visit or at least two nondermatology physician visits. They also identified an incidence cohort that was a subset of the study cohort who had at least 12 months of follow-up. “To determine the accuracy of AA patient identification, we also reviewed 100 cases at random from one institution with a threshold of greater than 95% accuracy,” said Ms. McKenzie, who is now a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas.
Of 5,409,919 children included in the study, 5,801 had AA, for an overall prevalence of 0.11%. The prevalence doubled from 0.04% in 2009 to 0.08% in 2019. “It fell in 2020, which we believe is a result of the COVID-19 pandemic’s effects on health care utilization,” she said. AA prevalence peaked at 9 years of age and was higher among females, compared with males (0.12% vs. 0.09%, respectively). The prevalence was highest among Hispanic children (0.23%), followed by Asian children (0.17%), Black children (0.12%), and White children (0.08%).
The incidence cohort consisted of 2,896,241 children. Of these, 2,398 had AA between 2009-2020, for an overall incidence of 13.6 cases per 100,000 patient-years. The incidence rate of AA by age was normally distributed and peaked at 6 years of age. Rates were 22.8% higher in female patients than in male patients. In addition, incidence rates were highest among Hispanics (31.5/100,000 person-years), followed by Asians (23.1/100,000 person-years), Blacks (17.0/100,000 person-years), and Whites (8.8/100,000).
Logistic regression analysis showed general agreement with the unadjusted incidence data. Males were less likely to be diagnosed with AA, compared with females (adjusted odds ratio, 0.80; P < .001). Analysis across race/ethnicity revealed significantly increased rates among children from minority backgrounds when compared with white children. Hispanic children had the greatest risk of developing AA (aOR, 3.07), followed by Asian children (aOR, 2.02), and Black children (aOR, 1.73) (P < .001 for all associations). Patients with atopic dermatitis, thyroid disease, psoriasis, vitiligo, and trisomy 21 prior to AA diagnosis all had a significantly higher risk of developing AA, compared with those without those diagnoses.
“This is the largest description of pediatric AA to date,” Ms. McKenzie said. “The prevalence has increased steadily, with a twofold increase over the last 10 years, which mirrors other autoimmune disorders. Children who identify as Hispanic, Asian, and Black have significantly higher incidence rates of alopecia areata compared to those who identify as White.”
Moving forward, she added, “efforts should focus on increasing education and awareness of AA in diverse communities and in community pediatricians so that patients can be diagnosed correctly early on. We can also use this data to ensure that representative populations are included in clinical trials for patients with AA.”
Asked to comment on the results Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said that the study “is a great contribution to our understanding of the epidemiology of pediatric alopecia areata and also highlights how common alopecia areata is in children.” In an interview, she said that it would be interesting to see if this is a worldwide phenomenon or unique to the United States.
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized the work as being “very informative. Looking at a large cohort of pediatric patients with alopecia areata diagnosed by a dermatologist or two or more nondermatologists, the authors found a higher incidence and prevalence in nonwhite children here in the United States. I am worried in fact, the true incidence could be even higher than noted in the searched database because nonwhite children can often come from underserved and undercared for areas.”
The other authors were Christopher B. Forrest, MD, PhD, Mitchell Maltenfort, PhD, and Leslie Castelo-Soccio, MD, PhD, of Children’s Hospital of Philadelphia. Dr. Castelo-Soccio is a consultant for Pfizer; the other authors reported having no financial disclosures. Dr. Hordinsky disclosed receiving grant support for clinical research work on hair diseases from Pfizer, Eli Lilly, Concert Pharmaceuticals, and Target Derm and grant support from the National Alopecia Areata Foundation; and is on an advisory panel for Cassiopea. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
*This story was updated on 7/19/21.
according to results from the largest study to date on the topic.
“Alopecia areata is a relatively common cause of nonscarring hair loss in children,” Paige McKenzie said during the annual meeting of the Society for Pediatric Dermatology. “The only two epidemiologic studies that have been performed in children have been based on registry or survey data which is inherently at risk for bias,” she added, referring to studies published in 2017 and 2018. “Additionally, epidemiologic descriptions of alopecia areata in adults are limited and overall estimates have varied from 0.2% to 2%. Current understanding is also largely based on population studies in Olmsted County, Minnesota, an area with mostly White racial demographics, so it’s not representative of the U.S. population as a whole.”
To identify the incidence and prevalence of pediatric AA over time, and across age, race/ethnicity, and sex, Ms. McKenzie and colleagues conducted a retrospective cohort study from 2009 to 2020 using PEDSnet, a network of seven U.S. pediatric health institutions with a database of more than 6.5 million children. “PEDSnet is unique because it uses a common data model to standardize EHR data across different health systems and uses SNOMED [Systematized Nomenclature of Medicine]–Clinical Terms to identify specific patient populations,” said Ms. McKenzie, who was a clinical research fellow in the section of dermatology at the Children’s Hospital of Philadelphia during the 2020-2021 academic year.
She and her coauthors limited their analysis to children younger than age 18 who were assigned a SNOMED code for AA during at least one dermatology physician visit or at least two nondermatology physician visits. They also identified an incidence cohort that was a subset of the study cohort who had at least 12 months of follow-up. “To determine the accuracy of AA patient identification, we also reviewed 100 cases at random from one institution with a threshold of greater than 95% accuracy,” said Ms. McKenzie, who is now a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas.
Of 5,409,919 children included in the study, 5,801 had AA, for an overall prevalence of 0.11%. The prevalence doubled from 0.04% in 2009 to 0.08% in 2019. “It fell in 2020, which we believe is a result of the COVID-19 pandemic’s effects on health care utilization,” she said. AA prevalence peaked at 9 years of age and was higher among females, compared with males (0.12% vs. 0.09%, respectively). The prevalence was highest among Hispanic children (0.23%), followed by Asian children (0.17%), Black children (0.12%), and White children (0.08%).
The incidence cohort consisted of 2,896,241 children. Of these, 2,398 had AA between 2009-2020, for an overall incidence of 13.6 cases per 100,000 patient-years. The incidence rate of AA by age was normally distributed and peaked at 6 years of age. Rates were 22.8% higher in female patients than in male patients. In addition, incidence rates were highest among Hispanics (31.5/100,000 person-years), followed by Asians (23.1/100,000 person-years), Blacks (17.0/100,000 person-years), and Whites (8.8/100,000).
Logistic regression analysis showed general agreement with the unadjusted incidence data. Males were less likely to be diagnosed with AA, compared with females (adjusted odds ratio, 0.80; P < .001). Analysis across race/ethnicity revealed significantly increased rates among children from minority backgrounds when compared with white children. Hispanic children had the greatest risk of developing AA (aOR, 3.07), followed by Asian children (aOR, 2.02), and Black children (aOR, 1.73) (P < .001 for all associations). Patients with atopic dermatitis, thyroid disease, psoriasis, vitiligo, and trisomy 21 prior to AA diagnosis all had a significantly higher risk of developing AA, compared with those without those diagnoses.
“This is the largest description of pediatric AA to date,” Ms. McKenzie said. “The prevalence has increased steadily, with a twofold increase over the last 10 years, which mirrors other autoimmune disorders. Children who identify as Hispanic, Asian, and Black have significantly higher incidence rates of alopecia areata compared to those who identify as White.”
Moving forward, she added, “efforts should focus on increasing education and awareness of AA in diverse communities and in community pediatricians so that patients can be diagnosed correctly early on. We can also use this data to ensure that representative populations are included in clinical trials for patients with AA.”
Asked to comment on the results Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said that the study “is a great contribution to our understanding of the epidemiology of pediatric alopecia areata and also highlights how common alopecia areata is in children.” In an interview, she said that it would be interesting to see if this is a worldwide phenomenon or unique to the United States.
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized the work as being “very informative. Looking at a large cohort of pediatric patients with alopecia areata diagnosed by a dermatologist or two or more nondermatologists, the authors found a higher incidence and prevalence in nonwhite children here in the United States. I am worried in fact, the true incidence could be even higher than noted in the searched database because nonwhite children can often come from underserved and undercared for areas.”
The other authors were Christopher B. Forrest, MD, PhD, Mitchell Maltenfort, PhD, and Leslie Castelo-Soccio, MD, PhD, of Children’s Hospital of Philadelphia. Dr. Castelo-Soccio is a consultant for Pfizer; the other authors reported having no financial disclosures. Dr. Hordinsky disclosed receiving grant support for clinical research work on hair diseases from Pfizer, Eli Lilly, Concert Pharmaceuticals, and Target Derm and grant support from the National Alopecia Areata Foundation; and is on an advisory panel for Cassiopea. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
*This story was updated on 7/19/21.
according to results from the largest study to date on the topic.
“Alopecia areata is a relatively common cause of nonscarring hair loss in children,” Paige McKenzie said during the annual meeting of the Society for Pediatric Dermatology. “The only two epidemiologic studies that have been performed in children have been based on registry or survey data which is inherently at risk for bias,” she added, referring to studies published in 2017 and 2018. “Additionally, epidemiologic descriptions of alopecia areata in adults are limited and overall estimates have varied from 0.2% to 2%. Current understanding is also largely based on population studies in Olmsted County, Minnesota, an area with mostly White racial demographics, so it’s not representative of the U.S. population as a whole.”
To identify the incidence and prevalence of pediatric AA over time, and across age, race/ethnicity, and sex, Ms. McKenzie and colleagues conducted a retrospective cohort study from 2009 to 2020 using PEDSnet, a network of seven U.S. pediatric health institutions with a database of more than 6.5 million children. “PEDSnet is unique because it uses a common data model to standardize EHR data across different health systems and uses SNOMED [Systematized Nomenclature of Medicine]–Clinical Terms to identify specific patient populations,” said Ms. McKenzie, who was a clinical research fellow in the section of dermatology at the Children’s Hospital of Philadelphia during the 2020-2021 academic year.
She and her coauthors limited their analysis to children younger than age 18 who were assigned a SNOMED code for AA during at least one dermatology physician visit or at least two nondermatology physician visits. They also identified an incidence cohort that was a subset of the study cohort who had at least 12 months of follow-up. “To determine the accuracy of AA patient identification, we also reviewed 100 cases at random from one institution with a threshold of greater than 95% accuracy,” said Ms. McKenzie, who is now a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas.
Of 5,409,919 children included in the study, 5,801 had AA, for an overall prevalence of 0.11%. The prevalence doubled from 0.04% in 2009 to 0.08% in 2019. “It fell in 2020, which we believe is a result of the COVID-19 pandemic’s effects on health care utilization,” she said. AA prevalence peaked at 9 years of age and was higher among females, compared with males (0.12% vs. 0.09%, respectively). The prevalence was highest among Hispanic children (0.23%), followed by Asian children (0.17%), Black children (0.12%), and White children (0.08%).
The incidence cohort consisted of 2,896,241 children. Of these, 2,398 had AA between 2009-2020, for an overall incidence of 13.6 cases per 100,000 patient-years. The incidence rate of AA by age was normally distributed and peaked at 6 years of age. Rates were 22.8% higher in female patients than in male patients. In addition, incidence rates were highest among Hispanics (31.5/100,000 person-years), followed by Asians (23.1/100,000 person-years), Blacks (17.0/100,000 person-years), and Whites (8.8/100,000).
Logistic regression analysis showed general agreement with the unadjusted incidence data. Males were less likely to be diagnosed with AA, compared with females (adjusted odds ratio, 0.80; P < .001). Analysis across race/ethnicity revealed significantly increased rates among children from minority backgrounds when compared with white children. Hispanic children had the greatest risk of developing AA (aOR, 3.07), followed by Asian children (aOR, 2.02), and Black children (aOR, 1.73) (P < .001 for all associations). Patients with atopic dermatitis, thyroid disease, psoriasis, vitiligo, and trisomy 21 prior to AA diagnosis all had a significantly higher risk of developing AA, compared with those without those diagnoses.
“This is the largest description of pediatric AA to date,” Ms. McKenzie said. “The prevalence has increased steadily, with a twofold increase over the last 10 years, which mirrors other autoimmune disorders. Children who identify as Hispanic, Asian, and Black have significantly higher incidence rates of alopecia areata compared to those who identify as White.”
Moving forward, she added, “efforts should focus on increasing education and awareness of AA in diverse communities and in community pediatricians so that patients can be diagnosed correctly early on. We can also use this data to ensure that representative populations are included in clinical trials for patients with AA.”
Asked to comment on the results Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said that the study “is a great contribution to our understanding of the epidemiology of pediatric alopecia areata and also highlights how common alopecia areata is in children.” In an interview, she said that it would be interesting to see if this is a worldwide phenomenon or unique to the United States.
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study, characterized the work as being “very informative. Looking at a large cohort of pediatric patients with alopecia areata diagnosed by a dermatologist or two or more nondermatologists, the authors found a higher incidence and prevalence in nonwhite children here in the United States. I am worried in fact, the true incidence could be even higher than noted in the searched database because nonwhite children can often come from underserved and undercared for areas.”
The other authors were Christopher B. Forrest, MD, PhD, Mitchell Maltenfort, PhD, and Leslie Castelo-Soccio, MD, PhD, of Children’s Hospital of Philadelphia. Dr. Castelo-Soccio is a consultant for Pfizer; the other authors reported having no financial disclosures. Dr. Hordinsky disclosed receiving grant support for clinical research work on hair diseases from Pfizer, Eli Lilly, Concert Pharmaceuticals, and Target Derm and grant support from the National Alopecia Areata Foundation; and is on an advisory panel for Cassiopea. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
*This story was updated on 7/19/21.
FROM SPD 2021
Utilizing a Sleep Mask to Reduce Patient Anxiety During Nail Surgery
Practice Gap
Perioperative anxiety is common in patients undergoing nail surgery. Patients might worry about seeing blood; about the procedure itself, including nail avulsion; and about associated pain and disfigurement. Nail surgery causes a high level of anxiety that correlates positively with postoperative pain1 and overall patient dissatisfaction. Furthermore, surgery-related anxiety is a predictor of increased postoperative analgesic use2 and delayed recovery.3
Therefore, implementing strategies that reduce perioperative anxiety may help minimize postoperative pain. Squeezing a stress ball, hand-holding, virtual reality, and music are tools that have been studied to reduce anxiety in the context of Mohs micrographic surgery; these strategies have not been studied for nail surgery.
The Technique
Using a sleep mask is a practical solution to reduce patient anxiety during nail surgery. A minority of patients will choose to watch their surgical procedure; most become unnerved observing their nail surgery. Using a sleep mask diverts visual attention from the surgical field without physically interfering with the nail surgeon. Utilizing a sleep mask is cost-effective, with disposable sleep masks available online for less than $0.30 each. Patients can bring their own mask, or a mask can be offered prior to surgery.
If desired, patients are instructed to wear the sleep mask during the entirety of the procedure, starting from anesthetic infiltration until wound closure and dressing application. Any adjustments can be made with the patient’s free hand. The sleep mask can be offered to patients of all ages undergoing nail surgery under local anesthesia, except babies and young children, who require general anesthesia.
Practical Implications
Distraction is an important strategy to reduce anxiety and pain in patients undergoing surgical procedures. In an observational study of 3087 surgical patients, 36% reported that self-distraction was the most helpful strategy for coping with preoperative anxiety.4 In a randomized, open-label clinical trial of 72 patients undergoing peripheral venous catheterization, asking the patients simple questions during the procedure was more effective than local anesthesia in reducing the perception of pain.5
It is crucial to implement strategies to reduce anxiety in patients undergoing nail surgery. Using a sleep mask impedes direct visualization of the surgical field, thus distracting the patient’s sight and attention from the procedure. Furthermore, this technique is safe and cost-effective.
Controlled clinical trials are necessary to assess the efficacy of this method in reducing nail surgery–related anxiety in comparison to other techniques.
- Navarro-Gastón D, Munuera-Martínez PV. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: a cross-sectional study. Int J Environ Res Public Health. 2020;17:4481. doi:10.3390/ijerph17124481
- Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Aust H, Rüsch D, Schuster M, et al. Coping strategies in anxious surgical patients. BMC Health Serv Res. 2016;16:250. doi:10.1186/s12913-016-1492-5
- Balanyuk I, Ledonne G, Provenzano M, et al. Distraction technique for pain reduction in peripheral venous catheterization: randomized, controlled trial. Acta Biomed. 2018;89(suppl 4):55-63. doi:10.23750/abmv89i4-S.7115
Practice Gap
Perioperative anxiety is common in patients undergoing nail surgery. Patients might worry about seeing blood; about the procedure itself, including nail avulsion; and about associated pain and disfigurement. Nail surgery causes a high level of anxiety that correlates positively with postoperative pain1 and overall patient dissatisfaction. Furthermore, surgery-related anxiety is a predictor of increased postoperative analgesic use2 and delayed recovery.3
Therefore, implementing strategies that reduce perioperative anxiety may help minimize postoperative pain. Squeezing a stress ball, hand-holding, virtual reality, and music are tools that have been studied to reduce anxiety in the context of Mohs micrographic surgery; these strategies have not been studied for nail surgery.
The Technique
Using a sleep mask is a practical solution to reduce patient anxiety during nail surgery. A minority of patients will choose to watch their surgical procedure; most become unnerved observing their nail surgery. Using a sleep mask diverts visual attention from the surgical field without physically interfering with the nail surgeon. Utilizing a sleep mask is cost-effective, with disposable sleep masks available online for less than $0.30 each. Patients can bring their own mask, or a mask can be offered prior to surgery.
If desired, patients are instructed to wear the sleep mask during the entirety of the procedure, starting from anesthetic infiltration until wound closure and dressing application. Any adjustments can be made with the patient’s free hand. The sleep mask can be offered to patients of all ages undergoing nail surgery under local anesthesia, except babies and young children, who require general anesthesia.
Practical Implications
Distraction is an important strategy to reduce anxiety and pain in patients undergoing surgical procedures. In an observational study of 3087 surgical patients, 36% reported that self-distraction was the most helpful strategy for coping with preoperative anxiety.4 In a randomized, open-label clinical trial of 72 patients undergoing peripheral venous catheterization, asking the patients simple questions during the procedure was more effective than local anesthesia in reducing the perception of pain.5
It is crucial to implement strategies to reduce anxiety in patients undergoing nail surgery. Using a sleep mask impedes direct visualization of the surgical field, thus distracting the patient’s sight and attention from the procedure. Furthermore, this technique is safe and cost-effective.
Controlled clinical trials are necessary to assess the efficacy of this method in reducing nail surgery–related anxiety in comparison to other techniques.
Practice Gap
Perioperative anxiety is common in patients undergoing nail surgery. Patients might worry about seeing blood; about the procedure itself, including nail avulsion; and about associated pain and disfigurement. Nail surgery causes a high level of anxiety that correlates positively with postoperative pain1 and overall patient dissatisfaction. Furthermore, surgery-related anxiety is a predictor of increased postoperative analgesic use2 and delayed recovery.3
Therefore, implementing strategies that reduce perioperative anxiety may help minimize postoperative pain. Squeezing a stress ball, hand-holding, virtual reality, and music are tools that have been studied to reduce anxiety in the context of Mohs micrographic surgery; these strategies have not been studied for nail surgery.
The Technique
Using a sleep mask is a practical solution to reduce patient anxiety during nail surgery. A minority of patients will choose to watch their surgical procedure; most become unnerved observing their nail surgery. Using a sleep mask diverts visual attention from the surgical field without physically interfering with the nail surgeon. Utilizing a sleep mask is cost-effective, with disposable sleep masks available online for less than $0.30 each. Patients can bring their own mask, or a mask can be offered prior to surgery.
If desired, patients are instructed to wear the sleep mask during the entirety of the procedure, starting from anesthetic infiltration until wound closure and dressing application. Any adjustments can be made with the patient’s free hand. The sleep mask can be offered to patients of all ages undergoing nail surgery under local anesthesia, except babies and young children, who require general anesthesia.
Practical Implications
Distraction is an important strategy to reduce anxiety and pain in patients undergoing surgical procedures. In an observational study of 3087 surgical patients, 36% reported that self-distraction was the most helpful strategy for coping with preoperative anxiety.4 In a randomized, open-label clinical trial of 72 patients undergoing peripheral venous catheterization, asking the patients simple questions during the procedure was more effective than local anesthesia in reducing the perception of pain.5
It is crucial to implement strategies to reduce anxiety in patients undergoing nail surgery. Using a sleep mask impedes direct visualization of the surgical field, thus distracting the patient’s sight and attention from the procedure. Furthermore, this technique is safe and cost-effective.
Controlled clinical trials are necessary to assess the efficacy of this method in reducing nail surgery–related anxiety in comparison to other techniques.
- Navarro-Gastón D, Munuera-Martínez PV. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: a cross-sectional study. Int J Environ Res Public Health. 2020;17:4481. doi:10.3390/ijerph17124481
- Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Aust H, Rüsch D, Schuster M, et al. Coping strategies in anxious surgical patients. BMC Health Serv Res. 2016;16:250. doi:10.1186/s12913-016-1492-5
- Balanyuk I, Ledonne G, Provenzano M, et al. Distraction technique for pain reduction in peripheral venous catheterization: randomized, controlled trial. Acta Biomed. 2018;89(suppl 4):55-63. doi:10.23750/abmv89i4-S.7115
- Navarro-Gastón D, Munuera-Martínez PV. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: a cross-sectional study. Int J Environ Res Public Health. 2020;17:4481. doi:10.3390/ijerph17124481
- Ip HYV, Abrishami A, Peng PWH, et al. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657-677. doi:10.1097/ALN.0b013e3181aae87a
- Mavros MN, Athanasiou S, Gkegkes ID, et al. Do psychological variables affect early surgical recovery? PLoS One. 2011;6:E20306. doi:10.1371/journal.pone.0020306
- Aust H, Rüsch D, Schuster M, et al. Coping strategies in anxious surgical patients. BMC Health Serv Res. 2016;16:250. doi:10.1186/s12913-016-1492-5
- Balanyuk I, Ledonne G, Provenzano M, et al. Distraction technique for pain reduction in peripheral venous catheterization: randomized, controlled trial. Acta Biomed. 2018;89(suppl 4):55-63. doi:10.23750/abmv89i4-S.7115









