User login
Micronychia of the Index Finger
Congenital onychodysplasia of the index finger (COIF), or Iso-Kikuchi syndrome, is a rare disorder characterized by malformation of one or both nails of the index fingers. The various anomalies described are anonychia, micronychia, polyonychia, malalignment, or hemi-onychogryphosis. It may be associated with abnormalities of the underlying phalangeal bone, the most masked being bifurcation of the terminal phalange.1 Initially thought to be nonhereditary and nonfamilial,2 it is now known that COIF can be inherited in an autosomal-dominant fashion.3 Millman and Strier3 described a family of 9 patients with COIF. It rarely is described outside of Japan. Padmavathy et al4 described a case in an Indian patient with COIF that was associated with the absence of a ring finger in addition to anomalies of the metacarpal bones.
Congenital onychodysplasia of the index finger has a broad spectrum regarding its etiology and clinical features.5 The pathogenesis of COIF still is poorly understood. Deficient circulation in digital arteries is thought to be a putative mechanism for developing a deformed nail. The nail is affected on the radial side of the index finger, likely because of the smaller caliber of the artery on that side.5 Hereditary as well as nonhereditary sporadic cases have been reported. In addition to the various fingernail anomalies, skeletal abnormalities also have been reported. Baran and Stroud6 have reported deformed lunulae as a manifestation of COIF.
The Diagnosis: Congenital Onychodysplasia of the Index Finger
The differential diagnosis of COIF includes hidrotic ectodermal dysplasia, nail-patella syndrome, Poland syndrome, and DOOR syndrome. Hidrotic ectodermal dysplasia exhibits onychodystrophy, generalized hypotrichosis, palmoplantar keratoderma, and dental anomalies.7 Nail-patella syndrome presents with hypoplasia of the fingernails and toenails, triangular nail lunulae, absent or hypoplastic patellae, and elbow and iliac horn dysplasia. Poland syndrome is distinguished from COIF by the congenital absence of the pectoralis major muscle on the ipsilateral side of the involved digits. The DOOR syndrome tetrad is comprised of deafness, onychodystrophy, osteodystrophy, and mental retardation.8 Unlike these conditions, COIF does not involve systems other than the nails and phalanges.
Treatment of this condition is mainly conservative, as patients typically do not have symptoms.9 Surgical interventions can be considered for cosmetic concerns. Knowledge of this congenital entity and its clinical findings is essential to prevent unnecessary procedures and workup.
- De Berker AR, Baran R. Science of the nail apparatus. Diseases of the Nails and Their Management. In: Baran R, De Berker AR, Holzberg M, et al, eds. 4th ed. Willey-Blackwell; 2012:1-50.
- Kikuchi I, Horikawa S, Amano F. Congenital onychodysplasia of the index fingers. Arch Dermatol. 1974;110:743-746.
- Millman AJ, Strier RP. Congenital onychodysplasia of the index fingers: report of a family. J Am Acad Dermatol. 1982;7:57-65.
- Padmavathy L, Rao L, Ethirajan N, et al. Iso-Kikuchi syndrome with absence of ring fingers and metacarpal bone abnormality. Indian J Dermatol Venereol Leprol. 2008;74:513.
- Hadj-Rabia S, Juhlin L, Baran R. Hereditary and congenital nail disorders. In: Baran R, De Berker AR, Holzberg M, et al, eds. Diseases of the Nails and Their Management. 4th ed. Wiley-Blackwell; 2012:485-490.
- Baran R, Stroud JD. Congenital onychodysplasia of the index fingers: Iso and Kikuchi syndrome. Arch Dermatol. 1984;120:243-244.
- Valerio E, Favot F, Mattei I, et al. Congenital isolated Iso-Kikuchi syndrome in a newborn. Clin Case Rep. 2015;3:866.
- Danarti R, Rahmayani S, Wirohadidjojo YW, et al. Deafness, onychodystrophy, osteodystrophy, mental retardation, and seizures (DOORS) syndrome: a new case report from Indonesia and review of the literature. Eur J Dermatol. 2020;30:404-407.
- Milani-Nejad N, Mosser-Goldfarb J. Congenital onychodysplasia of index fingers: Iso-Kikuchi syndrome. J Pediatr. 2020;218:254.
Congenital onychodysplasia of the index finger (COIF), or Iso-Kikuchi syndrome, is a rare disorder characterized by malformation of one or both nails of the index fingers. The various anomalies described are anonychia, micronychia, polyonychia, malalignment, or hemi-onychogryphosis. It may be associated with abnormalities of the underlying phalangeal bone, the most masked being bifurcation of the terminal phalange.1 Initially thought to be nonhereditary and nonfamilial,2 it is now known that COIF can be inherited in an autosomal-dominant fashion.3 Millman and Strier3 described a family of 9 patients with COIF. It rarely is described outside of Japan. Padmavathy et al4 described a case in an Indian patient with COIF that was associated with the absence of a ring finger in addition to anomalies of the metacarpal bones.
Congenital onychodysplasia of the index finger has a broad spectrum regarding its etiology and clinical features.5 The pathogenesis of COIF still is poorly understood. Deficient circulation in digital arteries is thought to be a putative mechanism for developing a deformed nail. The nail is affected on the radial side of the index finger, likely because of the smaller caliber of the artery on that side.5 Hereditary as well as nonhereditary sporadic cases have been reported. In addition to the various fingernail anomalies, skeletal abnormalities also have been reported. Baran and Stroud6 have reported deformed lunulae as a manifestation of COIF.
The Diagnosis: Congenital Onychodysplasia of the Index Finger
The differential diagnosis of COIF includes hidrotic ectodermal dysplasia, nail-patella syndrome, Poland syndrome, and DOOR syndrome. Hidrotic ectodermal dysplasia exhibits onychodystrophy, generalized hypotrichosis, palmoplantar keratoderma, and dental anomalies.7 Nail-patella syndrome presents with hypoplasia of the fingernails and toenails, triangular nail lunulae, absent or hypoplastic patellae, and elbow and iliac horn dysplasia. Poland syndrome is distinguished from COIF by the congenital absence of the pectoralis major muscle on the ipsilateral side of the involved digits. The DOOR syndrome tetrad is comprised of deafness, onychodystrophy, osteodystrophy, and mental retardation.8 Unlike these conditions, COIF does not involve systems other than the nails and phalanges.
Treatment of this condition is mainly conservative, as patients typically do not have symptoms.9 Surgical interventions can be considered for cosmetic concerns. Knowledge of this congenital entity and its clinical findings is essential to prevent unnecessary procedures and workup.
Congenital onychodysplasia of the index finger (COIF), or Iso-Kikuchi syndrome, is a rare disorder characterized by malformation of one or both nails of the index fingers. The various anomalies described are anonychia, micronychia, polyonychia, malalignment, or hemi-onychogryphosis. It may be associated with abnormalities of the underlying phalangeal bone, the most masked being bifurcation of the terminal phalange.1 Initially thought to be nonhereditary and nonfamilial,2 it is now known that COIF can be inherited in an autosomal-dominant fashion.3 Millman and Strier3 described a family of 9 patients with COIF. It rarely is described outside of Japan. Padmavathy et al4 described a case in an Indian patient with COIF that was associated with the absence of a ring finger in addition to anomalies of the metacarpal bones.
Congenital onychodysplasia of the index finger has a broad spectrum regarding its etiology and clinical features.5 The pathogenesis of COIF still is poorly understood. Deficient circulation in digital arteries is thought to be a putative mechanism for developing a deformed nail. The nail is affected on the radial side of the index finger, likely because of the smaller caliber of the artery on that side.5 Hereditary as well as nonhereditary sporadic cases have been reported. In addition to the various fingernail anomalies, skeletal abnormalities also have been reported. Baran and Stroud6 have reported deformed lunulae as a manifestation of COIF.
The Diagnosis: Congenital Onychodysplasia of the Index Finger
The differential diagnosis of COIF includes hidrotic ectodermal dysplasia, nail-patella syndrome, Poland syndrome, and DOOR syndrome. Hidrotic ectodermal dysplasia exhibits onychodystrophy, generalized hypotrichosis, palmoplantar keratoderma, and dental anomalies.7 Nail-patella syndrome presents with hypoplasia of the fingernails and toenails, triangular nail lunulae, absent or hypoplastic patellae, and elbow and iliac horn dysplasia. Poland syndrome is distinguished from COIF by the congenital absence of the pectoralis major muscle on the ipsilateral side of the involved digits. The DOOR syndrome tetrad is comprised of deafness, onychodystrophy, osteodystrophy, and mental retardation.8 Unlike these conditions, COIF does not involve systems other than the nails and phalanges.
Treatment of this condition is mainly conservative, as patients typically do not have symptoms.9 Surgical interventions can be considered for cosmetic concerns. Knowledge of this congenital entity and its clinical findings is essential to prevent unnecessary procedures and workup.
- De Berker AR, Baran R. Science of the nail apparatus. Diseases of the Nails and Their Management. In: Baran R, De Berker AR, Holzberg M, et al, eds. 4th ed. Willey-Blackwell; 2012:1-50.
- Kikuchi I, Horikawa S, Amano F. Congenital onychodysplasia of the index fingers. Arch Dermatol. 1974;110:743-746.
- Millman AJ, Strier RP. Congenital onychodysplasia of the index fingers: report of a family. J Am Acad Dermatol. 1982;7:57-65.
- Padmavathy L, Rao L, Ethirajan N, et al. Iso-Kikuchi syndrome with absence of ring fingers and metacarpal bone abnormality. Indian J Dermatol Venereol Leprol. 2008;74:513.
- Hadj-Rabia S, Juhlin L, Baran R. Hereditary and congenital nail disorders. In: Baran R, De Berker AR, Holzberg M, et al, eds. Diseases of the Nails and Their Management. 4th ed. Wiley-Blackwell; 2012:485-490.
- Baran R, Stroud JD. Congenital onychodysplasia of the index fingers: Iso and Kikuchi syndrome. Arch Dermatol. 1984;120:243-244.
- Valerio E, Favot F, Mattei I, et al. Congenital isolated Iso-Kikuchi syndrome in a newborn. Clin Case Rep. 2015;3:866.
- Danarti R, Rahmayani S, Wirohadidjojo YW, et al. Deafness, onychodystrophy, osteodystrophy, mental retardation, and seizures (DOORS) syndrome: a new case report from Indonesia and review of the literature. Eur J Dermatol. 2020;30:404-407.
- Milani-Nejad N, Mosser-Goldfarb J. Congenital onychodysplasia of index fingers: Iso-Kikuchi syndrome. J Pediatr. 2020;218:254.
- De Berker AR, Baran R. Science of the nail apparatus. Diseases of the Nails and Their Management. In: Baran R, De Berker AR, Holzberg M, et al, eds. 4th ed. Willey-Blackwell; 2012:1-50.
- Kikuchi I, Horikawa S, Amano F. Congenital onychodysplasia of the index fingers. Arch Dermatol. 1974;110:743-746.
- Millman AJ, Strier RP. Congenital onychodysplasia of the index fingers: report of a family. J Am Acad Dermatol. 1982;7:57-65.
- Padmavathy L, Rao L, Ethirajan N, et al. Iso-Kikuchi syndrome with absence of ring fingers and metacarpal bone abnormality. Indian J Dermatol Venereol Leprol. 2008;74:513.
- Hadj-Rabia S, Juhlin L, Baran R. Hereditary and congenital nail disorders. In: Baran R, De Berker AR, Holzberg M, et al, eds. Diseases of the Nails and Their Management. 4th ed. Wiley-Blackwell; 2012:485-490.
- Baran R, Stroud JD. Congenital onychodysplasia of the index fingers: Iso and Kikuchi syndrome. Arch Dermatol. 1984;120:243-244.
- Valerio E, Favot F, Mattei I, et al. Congenital isolated Iso-Kikuchi syndrome in a newborn. Clin Case Rep. 2015;3:866.
- Danarti R, Rahmayani S, Wirohadidjojo YW, et al. Deafness, onychodystrophy, osteodystrophy, mental retardation, and seizures (DOORS) syndrome: a new case report from Indonesia and review of the literature. Eur J Dermatol. 2020;30:404-407.
- Milani-Nejad N, Mosser-Goldfarb J. Congenital onychodysplasia of index fingers: Iso-Kikuchi syndrome. J Pediatr. 2020;218:254.
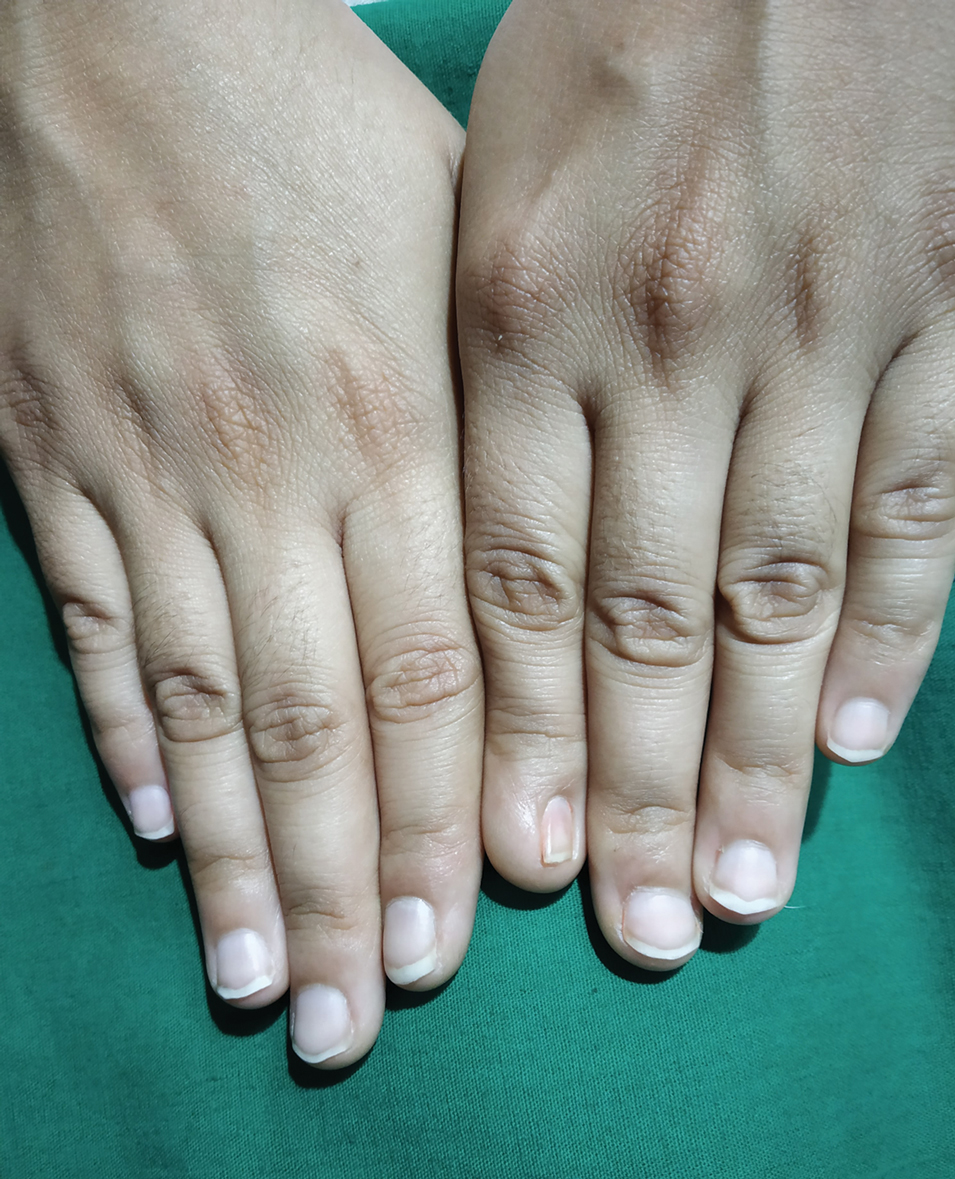
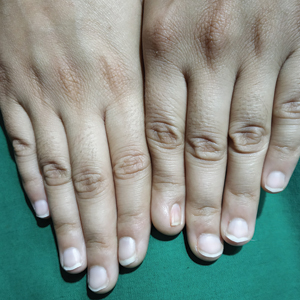
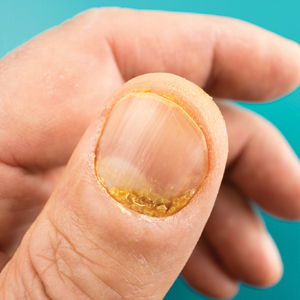
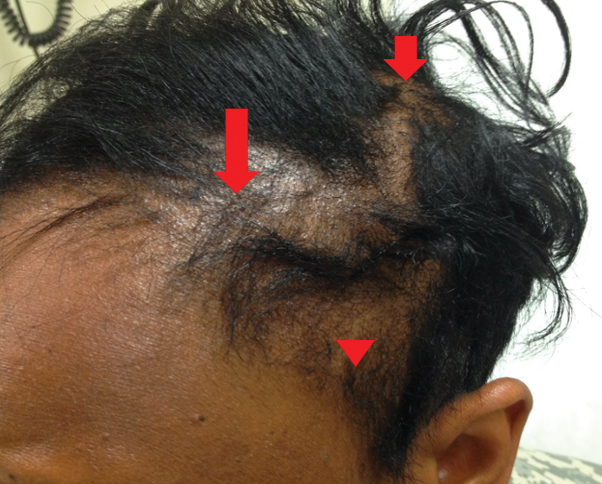
A 21-year-old Indian woman who was initially seeking dermatology consultation for acne also was noted to have micronychia of the nail of the left index finger. The affected nail was narrow and half as broad as the unaffected normal nail on the right index finger. The patient confirmed that this finding had been present since birth; she faced no cosmetic disability and had not sought medical care for diagnosis or treatment. There was no history of trauma, complications during pregnancy, family history of micronychia or similar eruptions, or any other inciting event. The teeth, hair, and skin as well as the patient’s height, weight, and physical and mental development were normal. Systemic examination revealed no abnormalities. Radiography of the hands did not reveal any apparent bony abnormalities.
From Buns to Braids and Ponytails: Entering a New Era of Female Military Hair-Grooming Standards
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
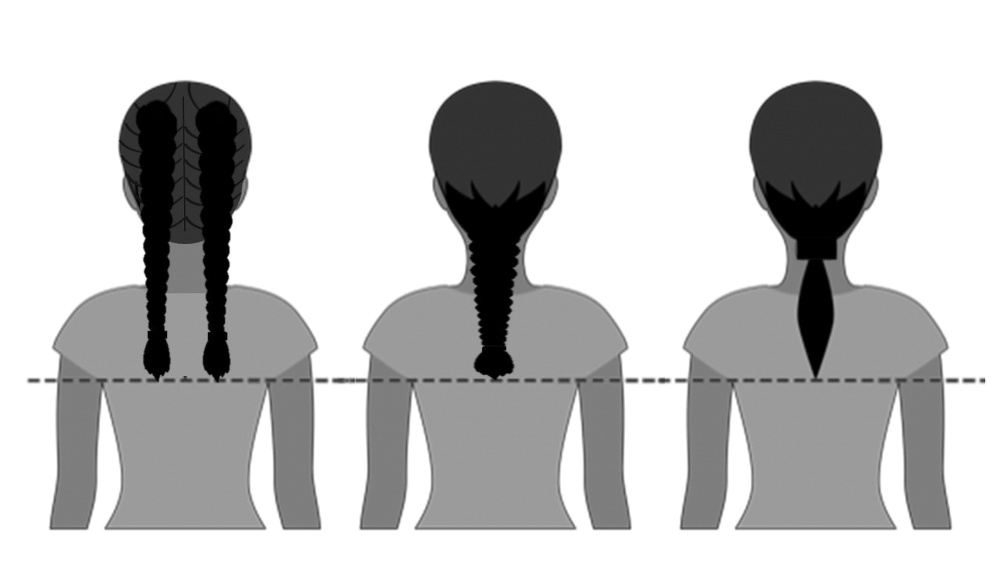
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.


Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18
Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.




Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18

Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
Professional appearance of servicemembers has been a long-standing custom in the US Military. Specific standards are determined by each branch. Initially, men dominated the military.1,2 As the number of women as well as racial diversity increased in the military, modifications to grooming standards were slow to change and resulted in female hair standards requiring a uniform tight and sleek style or short haircut. Clinicians can be attuned to these occupational standards and their implications on the diagnosis and management of common diseases of the hair and scalp.
History of Hairstyle Standards for Female Servicemembers
For half a century, female servicemembers had limited hairstyle choices. They were not authorized to have hair shorter than one-quarter inch in length. They could choose either short hair worn down or long hair with neatly secured loose ends in the form of a bun or a tucked braid—both of which could not extend past the bottom edge of the uniform collar.3-5 Female navy sailors and air force airmen with long hair were only allowed to wear ponytails during physical training; however, army soldiers previously were limited to wearing a bun.3,6,7 Cornrows and microbraids were authorized in the mid-1990s for the US Air Force, but policy stated that locs were prohibited due to their “unkempt” and “matted” nature. Furthermore, the size of hair bulk in the air force was restricted to no more than 3 inches and could not obstruct wear of the uniform cap.5 Based on these regulations, female servicemembers with longer hair had to utilize tight hairstyles that caused prolonged traction and pressure along the scalp, which contributed to headaches, a sore scalp, and alopecia over time. Normalization of these symptoms led to underreporting, as women lived with the consequences or turned to shorter hairstyles.
In the last decade alone, female servicemembers have witnessed the greatest number of changes in authorized hairstyles despite being part of the military for more than 50 years (Figure 1).1-11 In 2014, the language used in the air force instructions to describe locs was revised to remove ethnically offensive terms.4,5 This same year, the army allowed female soldiers to wear ponytails during physical training, a privilege that had been authorized by other services years prior.3,6,7 By the end of 2018, locs were authorized by all services, and female sailors could wear a ponytail in all navy uniforms as long as it did not extend 3 inches below the collar.3,4,6-8 In 2018, the air force increased authorized hair bulk up to 3.5 inches from the previous mandate of 3 inches and approved female buzz cuts6,9; in 2020, it allowed hair bulk up to 4 inches. As of 2021, female airmen can wear a ponytail and/or braid(s) as long as it starts below the crown of the head and the length does not extend below a horizontal line running between the top of each sleeve inseam at the underarm (Figures 2–4).6 In an ongoing effort to be more inclusive of hair density differences, female airmen will be authorized to wear a ponytail not exceeding a maximum width bulk of 1 ft starting June 25, 2021, so long as they can comply with the above regulations.11 The army now allows ponytails and braids across all uniforms, as long they do not extend past the bottom of the shoulder blades. This change came just months after authorizing the wearing of ponytails tucked under the uniform blouse with tactical headgear.10 These changes allow for a variety of hairstyles for members to practice while avoiding the physical consequences that develop from repetitive traction and pressure along the same areas of the hair and scalp.




Common Hair Disorders in Female Servicemembers
Herein, we discuss 3 of the most common hair and scalp disorders linked to grooming practices utilized by women to meet prior military regulations: trichorrhexis nodosa (TN), extracranial headaches, and traction alopecia (TA). It is essential that health care providers are able to promptly recognize these conditions, understand their risk factors, and be familiar with first-line treatment options. With these new standards, the hope is that the incidence of the following conditions decreases, thus improving servicemembers’ medical readiness and overall quality of life.
Trichorrhexis Nodosa
Acquired TN is a defect in the hair shaft that causes the hair to break easily secondary to chemical, thermal, or mechanical trauma. This can include but is not limited to chemical relaxers, blow-dryers, excessive brushing or styling, flat irons, and tightly packed hairstyles. The condition is characterized by a thickened hair diameter and splitting at the tip. Clinically, it may present as brittle, lusterless, broken hair with split ends, as well as a positive tug test.14 Management includes gentle hair care and avoidance of harsh hair care practices and treatments.
Extracranial Headaches
Headaches are a common concern among military servicemembers15 and generally are classified as primary or secondary. A less commonly discussed primary headache disorder includes external-pressure headaches, which result from either sustained compression or traction of the soft tissues of the scalp, usually from wearing headbands, helmets, or tight hairstyles.16 Additional at-risk groups include those who chronically wear surgical scrub caps or flight caps, especially if clipped or pinned to the hair. In our 38 years of combined military clinical experience, we can attest that these types of headaches are common among female servicemembers. The diagnostic criteria for an external-pressure headache, commonly referred to by patients as a “ponytail headache,” includes at least 2 headache episodes triggered within 1 hour of sustained traction on the scalp, maximal at the site of traction and resolving within 1 hour after relieving the traction.16 Management includes removal of the pressure-causing source, usually a tight ponytail or bun.
Traction Alopecia
Traction alopecia is hair loss caused by repetitive or prolonged tension on the hair secondary to tight hairstyles. It can be clinically classified into 2 types: marginal and nonmarginal patchy alopecia (Figure 5).13,17,18 Traction alopecia most commonly is found in individuals with ethnic hair, predominantly Black women. Hairstyles with the highest risk for causing TA include tight buns, ponytails, cornrows, weaves, and locs—all of which are utilized by female servicemembers to maintain a professional appearance and adhere to grooming regulations.13,18 Other groups at risk include athletes (eg, ballerinas, gymnasts) and those with chronic headwear use (eg, turbans, helmets, nurse caps, wigs).18 Early TA typically presents with perifollicular erythema followed by follicular-based papules or pustules.13,18 Marginal TA classically includes frontotemporal hair loss or thinning with or without a fringe sign.17,18 Nonmarginal TA includes patchy alopecia most commonly involving the parietal or occipital scalp, seen with chignons, buns, ponytails, or the use of clips, extensions, or bobby pins.18 The first line in management is avoidance of traction-causing hairstyles or headgear. Medical therapy may be warranted and consists of a single agent or combination regimen to include oral or topical antibiotics, topical or intralesional steroids, and topical minoxidil.13,18

Final Thoughts
Military hair-grooming standards have evolved over time. Recent changes show that the US Department of Defense is seriously evaluating policies that may be inherently exclusive. Prior grooming standards resulted in the widespread use of tight hairstyles and harsh hair treatments among female servicemembers with long hair. These practices resulted in TN, extracranial headaches, and TA, among other hair and scalp disorders. These occupational-related hair conditions impact female servicemembers’ mental and physical well-being and thus impact military readiness. Physicians should recognize that these conditions can be related to occupational grooming standards that may impact hair care practices.
The challenge that remains is a lack of standardized documentation for hair and scalp symptoms in the medical record. Due to a paucity in reporting and documentation, limited objective data exist to guide future recommendations for military grooming standards. Another obstacle is the lack of knowledge of hair diseases among primary care providers and patients, especially due to the underrepresentation of ethnic hair in medical textbooks.19 As a result, women frequently accept their hair symptoms as normal and either suffer through them, cut their hair short, or wear wigs before considering a visit to the doctor. Furthermore, hair-grooming standards can expose racial disparities, which are the driving force behind the current policy changes. Clinicians can strive to ask about hair and scalp symptoms and document the following in relation to hair and scalp disorders: occupational grooming requirements; skin and hair type; location, number, and size of scalp lesion(s); onset; duration; current and prior hair care practices; history of treatment; and clinical course accompanied with photographic documentation. Ultimately, improved awareness in patients, collaboration between physicians, and consistent clinical documentation can help create positive change and continued improvement in hair-grooming standards within the military. Improved reporting and documentation will facilitate further study into the effectiveness of the updated hair-grooming standards in female servicemembers.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
- United States Air Force Statistical Digest FY 1999. United States Air Force; 2000. Accessed June 8, 2021. https://media.defense.gov/2011/Apr/14/2001330240/-1/-1/0/AFD-110414-048.pdf
- Air Force demographics. Air Force Personnel Center website. Accessed June 8, 2021. https://www.afpc.af.mil/About/Air-Force-Demographics/
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Department of the Army; 2021. Accessed June 8, 2021. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN30302-AR_670-1-000-WEB-1.pdf
- Losey S. Loc hairstyles, off-duty earrings for men ok’d in new dress regs. Air Force Times. Published July 16, 2018. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-air-force/2018/07/16/loc-hairstyles-off-duty-earrings-for-men-okd-in-new-dress-regs/
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2011. Accessed June 8, 2021. https://www.uc.edu/content/dam/uc/afrotc/docs/Documents/AFI36-2903.pdf
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Department of the Air Force; 2021. Accessed June 8, 2021. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf
- U.S. Navy uniform regulations: summary of changes (26 February 2020). Navy Personnel Command website. Accessed June 8, 2021. https://www.mynavyhr.navy.mil/Portals/55/Navy%20Uniforms/Uniform%20Regulations/Documents/SOC_2020_02_26.pdf?ver=y8Wd0ykVXgISfFpOy8qHkg%3d%3d
- US Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. United States Marine Corps, 2018. Accessed June 8, 2021. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- Secretary of the Air Force Public Affairs. Air Force to allow longer braids, ponytails, bangs for women. United States Air Force website. Published January 21, 2021. Accessed June 8, 2021. https://www.af.mil/News/Article-Display/Article/2478173/air-force-to-allow-longer-braids-ponytails-bangs-for-women/
- Britzky H. The Army will now allow women to wear ponytails in all uniforms. Task & Purpose. Published May 6, 2021. Accessed June 8, 2021. https://taskandpurpose.com/news/army-women-ponytails-all-uniforms/
- Secretary of the Air Force Public Affairs. Air Force readdresses women’s hair standard after feedback. US Air Force website. Published June 11, 2021. Accessed June 27, 2021. https://www.af.mil/News/Article-Display/Article/2654774/air-force-readdresses-womens-hair-standard-after-feedback/
- Myers M. Esper direct services to review racial bias in grooming standards, training and more. Air Force Times. Published July 15, 2020. Accessed June 8, 2021. https://www.airforcetimes.com/news/your-military/2020/07/15/esper-directs-services-to-review-racial-bias-in-grooming-standards-training-and-more/
- Madu P, Kundu RV. Follicular and scarring disorders in skin of color: presentation and management. Am J Clin Dermatol. 2014;15:307-321.
- Quaresma M, Martinez Velasco M, Tosti A. Hair breakage in patients of African descent: role of dermoscopy. Skin Appendage Disord. 2015;1:99-104.
- Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21-34.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77.
- Sperling L, Cowper S, Knopp E. An Atlas of Hair Pathology with Clinical Correlations. CRC Press; 2012:67-68.
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159.
- Adelekun A, Onyekaba G, Lipoff JB. Skin color in dermatology textbooks: an updated evaluation and analysis. J Am Acad Dermatol. 2021;84:194-196.
Practice Points
- Military hair-grooming standards have undergone considerable changes to foster inclusivity and acknowledge racial diversity in hair and skin types.
- The chronic wearing of tight hairstyles can lead to hair breakage, headaches, and traction alopecia.
- A deliberate focus on diversity and inclusivity has started to drive policy change that eliminates racial and gender bias.
Gray hair goes away and squids go to space
Goodbye stress, goodbye gray hair
Last year was a doozy, so it wouldn’t be too surprising if we all had a few new gray strands in our hair. But what if we told you that you don’t need to start dying them or plucking them out? What if they could magically go back to the way they were? Well, it may be possible, sans magic and sans stress.
Investigators recently discovered that the age-old belief that stress will permanently turn your hair gray may not be true after all. There’s a strong possibility that it could turn back to its original color once the stressful agent is eliminated.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” said senior author Martin Picard, PhD, of Columbia University, New York.
For the study, 14 volunteers were asked to keep a stress diary and review their levels of stress throughout the week. The researchers used a new method of viewing and capturing the images of tiny parts of the hairs to see how much graying took place in each part of the strand. And what they found – some strands naturally turning back to the original color – had never been documented before.
How did it happen? Our good friend the mitochondria. We haven’t really heard that word since eighth-grade biology, but it’s actually the key link between stress hormones and hair pigmentation. Think of them as little radars picking up all different kinds of signals in your body, like mental/emotional stress. They get a big enough alert and they’re going to react, thus gray hair.
So that’s all it takes? Cut the stress and a full head of gray can go back to brown? Not exactly. The researchers said there may be a “threshold because of biological age and other factors.” They believe middle age is near that threshold and it could easily be pushed over due to stress and could potentially go back. But if you’ve been rocking the salt and pepper or silver fox for a number of years and are looking for change, you might want to just eliminate the stress and pick up a bottle of dye.
One small step for squid
Space does a number on the human body. Forget the obvious like going for a walk outside without a spacesuit, or even the well-known risks like the degradation of bone in microgravity; there are numerous smaller but still important changes to the body during spaceflight, like the disruption of the symbiotic relationship between gut bacteria and the human body. This causes the immune system to lose the ability to recognize threats, and illnesses spread more easily.
Naturally, if astronauts are going to undertake years-long journeys to Mars and beyond, a thorough understanding of this disturbance is necessary, and that’s why NASA has sent a bunch of squid to the International Space Station.
When it comes to animal studies, squid aren’t the usual culprits, but there’s a reason NASA chose calamari over the alternatives: The Hawaiian bobtail squid has a symbiotic relationship with bacteria that regulate their bioluminescence in much the same way that we have a symbiotic relationship with our gut bacteria, but the squid is a much simpler animal. If the bioluminescence-regulating bacteria are disturbed during their time in space, it will be much easier to figure out what’s going wrong.
The experiment is ongoing, but we should salute the brave squid who have taken a giant leap for squidkind. Though if NASA didn’t send them up in a giant bubble, we’re going to be very disappointed.
Less plastic, more vanilla
Have you been racked by guilt over the number of plastic water bottles you use? What about the amount of ice cream you eat? Well, this one’s for you.
Plastic isn’t the first thing you think about when you open up a pint of vanilla ice cream and catch the sweet, spicy vanilla scent, or when you smell those fresh vanilla scones coming out of the oven at the coffee shop, but a new study shows that the flavor of vanilla can come from water bottles.
Here’s the deal. A compound called vanillin is responsible for the scent of vanilla, and it can come naturally from the bean or it can be made synthetically. Believe it or not, 85% of vanillin is made synthetically from fossil fuels!
We’ve definitely grown accustomed to our favorite vanilla scents, foods, and cosmetics. In 2018, the global demand for vanillin was about 40,800 tons and is expected to grow to 65,000 tons by 2025, which far exceeds the supply of natural vanilla.
So what can we do? Well, we can use genetically engineered bacteria to turn plastic water bottles into vanillin, according to a study published in the journal Green Chemistry.
The plastic can be broken down into terephthalic acid, which is very similar, chemically speaking, to vanillin. Similar enough that a bit of bioengineering produced Escherichia coli that could convert the acid into the tasty treat, according to researchers at the University of Edinburgh.
A perfect solution? Decreasing plastic waste while producing a valued food product? The thought of consuming plastic isn’t appetizing, so just eat your ice cream and try to forget about it.
No withdrawals from this bank
Into each life, some milestones must fall: High school graduation, birth of a child, first house, 50th wedding anniversary, COVID-19. One LOTME staffer got really excited – way too excited, actually – when his Nissan Sentra reached 300,000 miles.
Well, there are milestones, and then there are milestones. “1,000 Reasons for Hope” is a report celebrating the first 1,000 brains donated to the VA-BU-CLF Brain Bank. For those of you keeping score at home, that would be the Department of Veterans Affairs, Boston University, and the Concussion Legacy Foundation.
The Brain Bank, created in 2008 to study concussions and chronic traumatic encephalopathy, is the brainchild – yes, we went there – of Chris Nowinski, PhD, a former professional wrestler, and Ann McKee, MD, an expert on neurogenerative disease. “Our discoveries have already inspired changes to sports that will prevent many future cases of CTE in the next generation of athletes,” Dr. Nowinski, the CEO of CLF, said in a written statement.
Data from the first thousand brains show that 706 men, including 305 former NFL players, had football as their primary exposure to head impacts. Women were underrepresented, making up only 2.8% of brain donations, so recruiting females is a priority. Anyone interested in pledging can go to PledgeMyBrain.org or call 617-992-0615 for the 24-hour emergency donation pager.
LOTME wanted to help, so we called the Brain Bank to find out about donating. They asked a few questions and we told them what we do for a living. “Oh, you’re with LOTME? Yeah, we’ve … um, seen that before. It’s, um … funny. Can we put you on hold?” We’re starting to get a little sick of the on-hold music by now.
Goodbye stress, goodbye gray hair
Last year was a doozy, so it wouldn’t be too surprising if we all had a few new gray strands in our hair. But what if we told you that you don’t need to start dying them or plucking them out? What if they could magically go back to the way they were? Well, it may be possible, sans magic and sans stress.
Investigators recently discovered that the age-old belief that stress will permanently turn your hair gray may not be true after all. There’s a strong possibility that it could turn back to its original color once the stressful agent is eliminated.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” said senior author Martin Picard, PhD, of Columbia University, New York.
For the study, 14 volunteers were asked to keep a stress diary and review their levels of stress throughout the week. The researchers used a new method of viewing and capturing the images of tiny parts of the hairs to see how much graying took place in each part of the strand. And what they found – some strands naturally turning back to the original color – had never been documented before.
How did it happen? Our good friend the mitochondria. We haven’t really heard that word since eighth-grade biology, but it’s actually the key link between stress hormones and hair pigmentation. Think of them as little radars picking up all different kinds of signals in your body, like mental/emotional stress. They get a big enough alert and they’re going to react, thus gray hair.
So that’s all it takes? Cut the stress and a full head of gray can go back to brown? Not exactly. The researchers said there may be a “threshold because of biological age and other factors.” They believe middle age is near that threshold and it could easily be pushed over due to stress and could potentially go back. But if you’ve been rocking the salt and pepper or silver fox for a number of years and are looking for change, you might want to just eliminate the stress and pick up a bottle of dye.
One small step for squid
Space does a number on the human body. Forget the obvious like going for a walk outside without a spacesuit, or even the well-known risks like the degradation of bone in microgravity; there are numerous smaller but still important changes to the body during spaceflight, like the disruption of the symbiotic relationship between gut bacteria and the human body. This causes the immune system to lose the ability to recognize threats, and illnesses spread more easily.
Naturally, if astronauts are going to undertake years-long journeys to Mars and beyond, a thorough understanding of this disturbance is necessary, and that’s why NASA has sent a bunch of squid to the International Space Station.
When it comes to animal studies, squid aren’t the usual culprits, but there’s a reason NASA chose calamari over the alternatives: The Hawaiian bobtail squid has a symbiotic relationship with bacteria that regulate their bioluminescence in much the same way that we have a symbiotic relationship with our gut bacteria, but the squid is a much simpler animal. If the bioluminescence-regulating bacteria are disturbed during their time in space, it will be much easier to figure out what’s going wrong.
The experiment is ongoing, but we should salute the brave squid who have taken a giant leap for squidkind. Though if NASA didn’t send them up in a giant bubble, we’re going to be very disappointed.
Less plastic, more vanilla
Have you been racked by guilt over the number of plastic water bottles you use? What about the amount of ice cream you eat? Well, this one’s for you.
Plastic isn’t the first thing you think about when you open up a pint of vanilla ice cream and catch the sweet, spicy vanilla scent, or when you smell those fresh vanilla scones coming out of the oven at the coffee shop, but a new study shows that the flavor of vanilla can come from water bottles.
Here’s the deal. A compound called vanillin is responsible for the scent of vanilla, and it can come naturally from the bean or it can be made synthetically. Believe it or not, 85% of vanillin is made synthetically from fossil fuels!
We’ve definitely grown accustomed to our favorite vanilla scents, foods, and cosmetics. In 2018, the global demand for vanillin was about 40,800 tons and is expected to grow to 65,000 tons by 2025, which far exceeds the supply of natural vanilla.
So what can we do? Well, we can use genetically engineered bacteria to turn plastic water bottles into vanillin, according to a study published in the journal Green Chemistry.
The plastic can be broken down into terephthalic acid, which is very similar, chemically speaking, to vanillin. Similar enough that a bit of bioengineering produced Escherichia coli that could convert the acid into the tasty treat, according to researchers at the University of Edinburgh.
A perfect solution? Decreasing plastic waste while producing a valued food product? The thought of consuming plastic isn’t appetizing, so just eat your ice cream and try to forget about it.
No withdrawals from this bank
Into each life, some milestones must fall: High school graduation, birth of a child, first house, 50th wedding anniversary, COVID-19. One LOTME staffer got really excited – way too excited, actually – when his Nissan Sentra reached 300,000 miles.
Well, there are milestones, and then there are milestones. “1,000 Reasons for Hope” is a report celebrating the first 1,000 brains donated to the VA-BU-CLF Brain Bank. For those of you keeping score at home, that would be the Department of Veterans Affairs, Boston University, and the Concussion Legacy Foundation.
The Brain Bank, created in 2008 to study concussions and chronic traumatic encephalopathy, is the brainchild – yes, we went there – of Chris Nowinski, PhD, a former professional wrestler, and Ann McKee, MD, an expert on neurogenerative disease. “Our discoveries have already inspired changes to sports that will prevent many future cases of CTE in the next generation of athletes,” Dr. Nowinski, the CEO of CLF, said in a written statement.
Data from the first thousand brains show that 706 men, including 305 former NFL players, had football as their primary exposure to head impacts. Women were underrepresented, making up only 2.8% of brain donations, so recruiting females is a priority. Anyone interested in pledging can go to PledgeMyBrain.org or call 617-992-0615 for the 24-hour emergency donation pager.
LOTME wanted to help, so we called the Brain Bank to find out about donating. They asked a few questions and we told them what we do for a living. “Oh, you’re with LOTME? Yeah, we’ve … um, seen that before. It’s, um … funny. Can we put you on hold?” We’re starting to get a little sick of the on-hold music by now.
Goodbye stress, goodbye gray hair
Last year was a doozy, so it wouldn’t be too surprising if we all had a few new gray strands in our hair. But what if we told you that you don’t need to start dying them or plucking them out? What if they could magically go back to the way they were? Well, it may be possible, sans magic and sans stress.
Investigators recently discovered that the age-old belief that stress will permanently turn your hair gray may not be true after all. There’s a strong possibility that it could turn back to its original color once the stressful agent is eliminated.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” said senior author Martin Picard, PhD, of Columbia University, New York.
For the study, 14 volunteers were asked to keep a stress diary and review their levels of stress throughout the week. The researchers used a new method of viewing and capturing the images of tiny parts of the hairs to see how much graying took place in each part of the strand. And what they found – some strands naturally turning back to the original color – had never been documented before.
How did it happen? Our good friend the mitochondria. We haven’t really heard that word since eighth-grade biology, but it’s actually the key link between stress hormones and hair pigmentation. Think of them as little radars picking up all different kinds of signals in your body, like mental/emotional stress. They get a big enough alert and they’re going to react, thus gray hair.
So that’s all it takes? Cut the stress and a full head of gray can go back to brown? Not exactly. The researchers said there may be a “threshold because of biological age and other factors.” They believe middle age is near that threshold and it could easily be pushed over due to stress and could potentially go back. But if you’ve been rocking the salt and pepper or silver fox for a number of years and are looking for change, you might want to just eliminate the stress and pick up a bottle of dye.
One small step for squid
Space does a number on the human body. Forget the obvious like going for a walk outside without a spacesuit, or even the well-known risks like the degradation of bone in microgravity; there are numerous smaller but still important changes to the body during spaceflight, like the disruption of the symbiotic relationship between gut bacteria and the human body. This causes the immune system to lose the ability to recognize threats, and illnesses spread more easily.
Naturally, if astronauts are going to undertake years-long journeys to Mars and beyond, a thorough understanding of this disturbance is necessary, and that’s why NASA has sent a bunch of squid to the International Space Station.
When it comes to animal studies, squid aren’t the usual culprits, but there’s a reason NASA chose calamari over the alternatives: The Hawaiian bobtail squid has a symbiotic relationship with bacteria that regulate their bioluminescence in much the same way that we have a symbiotic relationship with our gut bacteria, but the squid is a much simpler animal. If the bioluminescence-regulating bacteria are disturbed during their time in space, it will be much easier to figure out what’s going wrong.
The experiment is ongoing, but we should salute the brave squid who have taken a giant leap for squidkind. Though if NASA didn’t send them up in a giant bubble, we’re going to be very disappointed.
Less plastic, more vanilla
Have you been racked by guilt over the number of plastic water bottles you use? What about the amount of ice cream you eat? Well, this one’s for you.
Plastic isn’t the first thing you think about when you open up a pint of vanilla ice cream and catch the sweet, spicy vanilla scent, or when you smell those fresh vanilla scones coming out of the oven at the coffee shop, but a new study shows that the flavor of vanilla can come from water bottles.
Here’s the deal. A compound called vanillin is responsible for the scent of vanilla, and it can come naturally from the bean or it can be made synthetically. Believe it or not, 85% of vanillin is made synthetically from fossil fuels!
We’ve definitely grown accustomed to our favorite vanilla scents, foods, and cosmetics. In 2018, the global demand for vanillin was about 40,800 tons and is expected to grow to 65,000 tons by 2025, which far exceeds the supply of natural vanilla.
So what can we do? Well, we can use genetically engineered bacteria to turn plastic water bottles into vanillin, according to a study published in the journal Green Chemistry.
The plastic can be broken down into terephthalic acid, which is very similar, chemically speaking, to vanillin. Similar enough that a bit of bioengineering produced Escherichia coli that could convert the acid into the tasty treat, according to researchers at the University of Edinburgh.
A perfect solution? Decreasing plastic waste while producing a valued food product? The thought of consuming plastic isn’t appetizing, so just eat your ice cream and try to forget about it.
No withdrawals from this bank
Into each life, some milestones must fall: High school graduation, birth of a child, first house, 50th wedding anniversary, COVID-19. One LOTME staffer got really excited – way too excited, actually – when his Nissan Sentra reached 300,000 miles.
Well, there are milestones, and then there are milestones. “1,000 Reasons for Hope” is a report celebrating the first 1,000 brains donated to the VA-BU-CLF Brain Bank. For those of you keeping score at home, that would be the Department of Veterans Affairs, Boston University, and the Concussion Legacy Foundation.
The Brain Bank, created in 2008 to study concussions and chronic traumatic encephalopathy, is the brainchild – yes, we went there – of Chris Nowinski, PhD, a former professional wrestler, and Ann McKee, MD, an expert on neurogenerative disease. “Our discoveries have already inspired changes to sports that will prevent many future cases of CTE in the next generation of athletes,” Dr. Nowinski, the CEO of CLF, said in a written statement.
Data from the first thousand brains show that 706 men, including 305 former NFL players, had football as their primary exposure to head impacts. Women were underrepresented, making up only 2.8% of brain donations, so recruiting females is a priority. Anyone interested in pledging can go to PledgeMyBrain.org or call 617-992-0615 for the 24-hour emergency donation pager.
LOTME wanted to help, so we called the Brain Bank to find out about donating. They asked a few questions and we told them what we do for a living. “Oh, you’re with LOTME? Yeah, we’ve … um, seen that before. It’s, um … funny. Can we put you on hold?” We’re starting to get a little sick of the on-hold music by now.
Argyria From a Topical Home Remedy
To the Editor:
Argyria is a rare disease caused by chronic exposure to products with high silver content (eg, oral ingestion, inhalation, percutaneous absorption). With time, the blood levels of silver surpass the body’s renal and hepatic excretory capacities that lead to silver granules being deposited in the skin and internal organs, including the liver, spleen, adrenal glands, and bone marrow.1 The cutaneous deposition results in a blue or blue-gray pigmentation of the skin, mucous membranes, and nails. Intervals of exposure that span from 8 months to 5 years prior to symptom onset have been described in the literature.2 The discoloration that results often is permanent, with no established way of effectively removing silver deposits from the tissue.3
A 22-year-old autistic man, who was completely dependent on his mother’s care, presented to the emergency department with a primary concern of abdominal pain. The mother reported that he was indicating abdominal pain by motioning to his stomach for the last 5 days. The mother also reported he did not have a bowel movement during this time, and she noticed his hands were shaking. Prior to presentation, the mother had given him 2 enemas and had him on a 3-day strict liquid fast consisting of water, lemon juice, cayenne pepper, honey, and orange juice. Notably, the mother had a strong history of using naturopathic remedies for treatment of her son’s ailments.
On admission, the patient was stable. There was a 2-point decrease in the patient’s body mass index over the last month. Initial serum electrolytes were highly abnormal with a serum sodium level of 124 mEq/L (reference range, 135–145 mEq/L), blood urea nitrogen of 3 mg/dL (reference range, 7–20 mg/dL), creatinine of 0.77 mg/dL (reference range, 0.74–1.35 mg/dL), and lactic acid of 2.1 mEq/L (reference range, 0.5–1 mEq/L). Serum osmolality was 272 mOsm/kg (reference range, 275–295 mOsm/kg). Urine osmolality was 114 mOsm/kg (reference range, 500–850 mOsm/kg) with a low-normal urine sodium level of 41 mmol/24 hr (reference range, 40–220 mmol/24 hr). Abnormalities were felt to be secondary to malnutrition from the strict liquid diet (blood urea nitrogen and creatinine ratio of 3:1 suggestive of notable protein calorie malnutrition). The patient was given 1 L of normal saline in the emergency department, with further fluids held so as not to increase serum sodium level too rapidly. A regular diet was started.
Physical examination revealed dry mucosal membranes but otherwise was unremarkable. Active bowel sounds were noted, as well as a soft, nontender, and nondistended abdomen; however, when examining the patient’s hands for reported shaking, a distinct abnormality of the nails was noticed. The patient had slate blue discoloration of the lunula, along with hyperpigmented violaceous discoloration of the proximal nail bed on all 10 fingernails (Figure 1). No abnormalities were seen on the toenails. The mother had a distinct bluish gray discoloration of the face as well as similar nail findings (Figure 2), strongly suggestive of colloidal silver use. An urgent serum silver level was ordered on the patient as well as a heavy metal panel. The mother was found applying numerous “natural remedies” to the patient’s skin while in the hospital, including a liquid spray and lotion, both in unmarked bottles. At that time, the mother was informed that no external supplements should be applied to her son. The serum silver level was elevated substantially at 94.3 ng/mL (reference range, <1.0 ng/mL). When the mother was confronted, she initially denied use of silver but later admitted to notable silver content in the cream she was applying to her son’s skin. The mother reported that she read online that colloidal silver had been historically used to cure numerous ailments and she was ordering products from an online company. She was counseled on the dangers of both topical application and ingestion of silver, and all supplements were removed from the home.
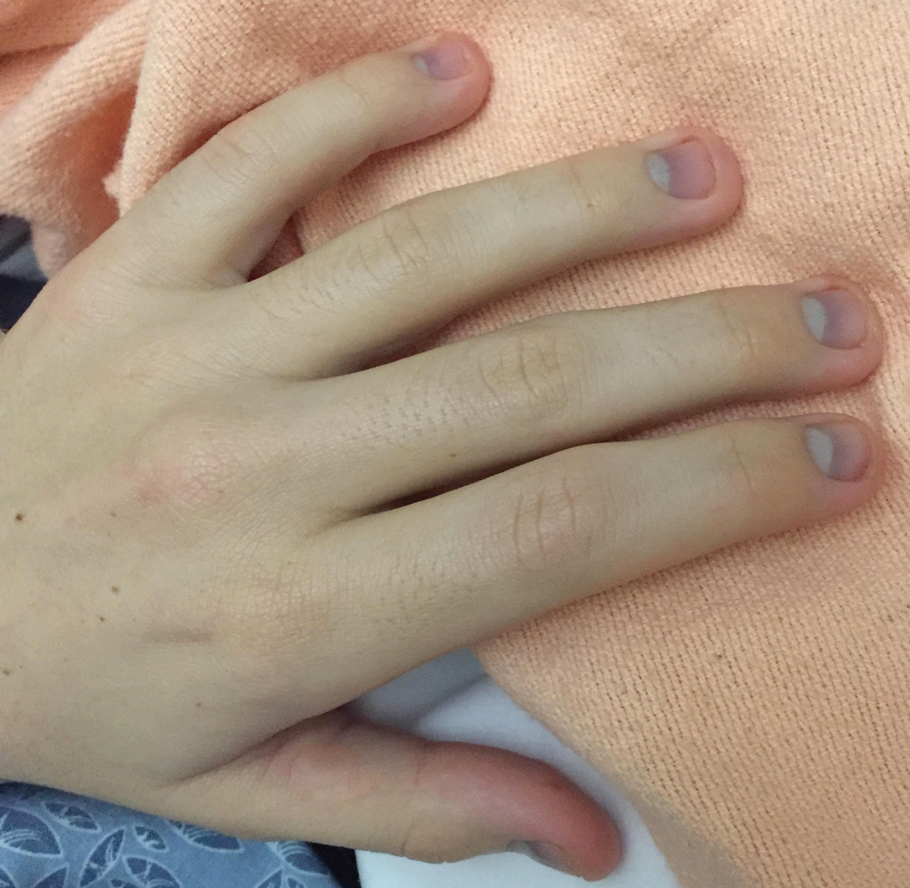
Argyria is a rare condition caused by chronic exposure to silver and is characterized by a blue-gray pigmentation in the skin and appendages, mucous membranes, and internal organs.4 Clinically, argyria is classified as generalized or localized. Generalized argyria results from ingestion or inhalation of silver compounds, where granules deposit preferentially in sun-exposed areas of skin as well as internal organs, with the highest concentration in the liver, spleen, and adrenal glands; discoloration often is permanent.5 On the contrary, localized argyria results from direct external contact with silver and granules deposited in the hands, eyes, and mucosa.5 Although the exact mechanism of penetration from topical silver remains unknown, it is thought to enter via the eccrine sweat ducts, as histopathology reveals silver granules found in highest concentration surrounding sweat glands in the dermis.6
Initial differential diagnoses for altered nail pigmentation include drug-induced causes, systemic diseases, cyanosis, and exposure to metals.7 The most commonly indicated medications resulting in blue nail pigment changes include antimalarials, minocycline, zidovudine, and phenothiazine. Systemic diseases that may cause blue nail color change include Wilson disease, hemochromatosis, Addison disease, methemoglobinemia, and alkaptonuria.7 Metals include gold, mercury, arsenic, bismuth, lead, and silver.4 After a thorough review of the patient’s medications and lack of support for any underlying disease process, contact with metals, particularly silver, was ranked highly on our differential list. In support of this theory, the mother’s bluish gray facial skin led to high clinical suspicion that she was ingesting colloidal silver and also was exposing her son to silver.
Treatment of argyria is challenging but first and foremost involves discontinuation of the source of chronic silver exposure. Unfortunately, the discoloration of generalized argyria often is permanent. Sunscreen can be used to help prevent any further darkening of pigment. The pigment in localized argyria has been reported to slowly fade with time, and there also have been reports of successful treatment using a low-fluence Q-switched 1064-nm Nd:YAG laser.8
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Lencastre A, Lobo M, João A. Argyria—case report. An Bras Dermatol. 2013;88:413-416.
- Park S-W, Kim J-H, Shin H-T, et al. An effective modality for argyria treatment: Q-switched 1,064-nm Nd:YAG laser. Ann Dermatol. 2013;25:511-512.
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Garcias-Ladaria J, Hernandez-Bel P, Torregrosa-Calatayud JL, et al. Localized cutaneous argyria: a report of 2 cases. Actas Dermosifiliogr. 2013;104:253-254.
- Kapur N, Landon G, Yu RC. Localized argyria in an antique restorer. Br J Dermatol. 2001;144:191-192.
- Kubba A, Kubba R, Batrani M, Pal T. Argyria an unrecognized cause of cutaneous pigmentation in Indian patients: a case series and review of the literature. Indian J Dermatol Venereol Leprol. 2013;79:805-811.
- Han TY, Chang HS, Lee HK, et al. Successful treatment of argyria using a low-fluence Q-switched 1064-nm Nd:YAG laser. Int J Dermatol. 2011;50:751-753.
To the Editor:
Argyria is a rare disease caused by chronic exposure to products with high silver content (eg, oral ingestion, inhalation, percutaneous absorption). With time, the blood levels of silver surpass the body’s renal and hepatic excretory capacities that lead to silver granules being deposited in the skin and internal organs, including the liver, spleen, adrenal glands, and bone marrow.1 The cutaneous deposition results in a blue or blue-gray pigmentation of the skin, mucous membranes, and nails. Intervals of exposure that span from 8 months to 5 years prior to symptom onset have been described in the literature.2 The discoloration that results often is permanent, with no established way of effectively removing silver deposits from the tissue.3
A 22-year-old autistic man, who was completely dependent on his mother’s care, presented to the emergency department with a primary concern of abdominal pain. The mother reported that he was indicating abdominal pain by motioning to his stomach for the last 5 days. The mother also reported he did not have a bowel movement during this time, and she noticed his hands were shaking. Prior to presentation, the mother had given him 2 enemas and had him on a 3-day strict liquid fast consisting of water, lemon juice, cayenne pepper, honey, and orange juice. Notably, the mother had a strong history of using naturopathic remedies for treatment of her son’s ailments.
On admission, the patient was stable. There was a 2-point decrease in the patient’s body mass index over the last month. Initial serum electrolytes were highly abnormal with a serum sodium level of 124 mEq/L (reference range, 135–145 mEq/L), blood urea nitrogen of 3 mg/dL (reference range, 7–20 mg/dL), creatinine of 0.77 mg/dL (reference range, 0.74–1.35 mg/dL), and lactic acid of 2.1 mEq/L (reference range, 0.5–1 mEq/L). Serum osmolality was 272 mOsm/kg (reference range, 275–295 mOsm/kg). Urine osmolality was 114 mOsm/kg (reference range, 500–850 mOsm/kg) with a low-normal urine sodium level of 41 mmol/24 hr (reference range, 40–220 mmol/24 hr). Abnormalities were felt to be secondary to malnutrition from the strict liquid diet (blood urea nitrogen and creatinine ratio of 3:1 suggestive of notable protein calorie malnutrition). The patient was given 1 L of normal saline in the emergency department, with further fluids held so as not to increase serum sodium level too rapidly. A regular diet was started.
Physical examination revealed dry mucosal membranes but otherwise was unremarkable. Active bowel sounds were noted, as well as a soft, nontender, and nondistended abdomen; however, when examining the patient’s hands for reported shaking, a distinct abnormality of the nails was noticed. The patient had slate blue discoloration of the lunula, along with hyperpigmented violaceous discoloration of the proximal nail bed on all 10 fingernails (Figure 1). No abnormalities were seen on the toenails. The mother had a distinct bluish gray discoloration of the face as well as similar nail findings (Figure 2), strongly suggestive of colloidal silver use. An urgent serum silver level was ordered on the patient as well as a heavy metal panel. The mother was found applying numerous “natural remedies” to the patient’s skin while in the hospital, including a liquid spray and lotion, both in unmarked bottles. At that time, the mother was informed that no external supplements should be applied to her son. The serum silver level was elevated substantially at 94.3 ng/mL (reference range, <1.0 ng/mL). When the mother was confronted, she initially denied use of silver but later admitted to notable silver content in the cream she was applying to her son’s skin. The mother reported that she read online that colloidal silver had been historically used to cure numerous ailments and she was ordering products from an online company. She was counseled on the dangers of both topical application and ingestion of silver, and all supplements were removed from the home.


Argyria is a rare condition caused by chronic exposure to silver and is characterized by a blue-gray pigmentation in the skin and appendages, mucous membranes, and internal organs.4 Clinically, argyria is classified as generalized or localized. Generalized argyria results from ingestion or inhalation of silver compounds, where granules deposit preferentially in sun-exposed areas of skin as well as internal organs, with the highest concentration in the liver, spleen, and adrenal glands; discoloration often is permanent.5 On the contrary, localized argyria results from direct external contact with silver and granules deposited in the hands, eyes, and mucosa.5 Although the exact mechanism of penetration from topical silver remains unknown, it is thought to enter via the eccrine sweat ducts, as histopathology reveals silver granules found in highest concentration surrounding sweat glands in the dermis.6
Initial differential diagnoses for altered nail pigmentation include drug-induced causes, systemic diseases, cyanosis, and exposure to metals.7 The most commonly indicated medications resulting in blue nail pigment changes include antimalarials, minocycline, zidovudine, and phenothiazine. Systemic diseases that may cause blue nail color change include Wilson disease, hemochromatosis, Addison disease, methemoglobinemia, and alkaptonuria.7 Metals include gold, mercury, arsenic, bismuth, lead, and silver.4 After a thorough review of the patient’s medications and lack of support for any underlying disease process, contact with metals, particularly silver, was ranked highly on our differential list. In support of this theory, the mother’s bluish gray facial skin led to high clinical suspicion that she was ingesting colloidal silver and also was exposing her son to silver.
Treatment of argyria is challenging but first and foremost involves discontinuation of the source of chronic silver exposure. Unfortunately, the discoloration of generalized argyria often is permanent. Sunscreen can be used to help prevent any further darkening of pigment. The pigment in localized argyria has been reported to slowly fade with time, and there also have been reports of successful treatment using a low-fluence Q-switched 1064-nm Nd:YAG laser.8
To the Editor:
Argyria is a rare disease caused by chronic exposure to products with high silver content (eg, oral ingestion, inhalation, percutaneous absorption). With time, the blood levels of silver surpass the body’s renal and hepatic excretory capacities that lead to silver granules being deposited in the skin and internal organs, including the liver, spleen, adrenal glands, and bone marrow.1 The cutaneous deposition results in a blue or blue-gray pigmentation of the skin, mucous membranes, and nails. Intervals of exposure that span from 8 months to 5 years prior to symptom onset have been described in the literature.2 The discoloration that results often is permanent, with no established way of effectively removing silver deposits from the tissue.3
A 22-year-old autistic man, who was completely dependent on his mother’s care, presented to the emergency department with a primary concern of abdominal pain. The mother reported that he was indicating abdominal pain by motioning to his stomach for the last 5 days. The mother also reported he did not have a bowel movement during this time, and she noticed his hands were shaking. Prior to presentation, the mother had given him 2 enemas and had him on a 3-day strict liquid fast consisting of water, lemon juice, cayenne pepper, honey, and orange juice. Notably, the mother had a strong history of using naturopathic remedies for treatment of her son’s ailments.
On admission, the patient was stable. There was a 2-point decrease in the patient’s body mass index over the last month. Initial serum electrolytes were highly abnormal with a serum sodium level of 124 mEq/L (reference range, 135–145 mEq/L), blood urea nitrogen of 3 mg/dL (reference range, 7–20 mg/dL), creatinine of 0.77 mg/dL (reference range, 0.74–1.35 mg/dL), and lactic acid of 2.1 mEq/L (reference range, 0.5–1 mEq/L). Serum osmolality was 272 mOsm/kg (reference range, 275–295 mOsm/kg). Urine osmolality was 114 mOsm/kg (reference range, 500–850 mOsm/kg) with a low-normal urine sodium level of 41 mmol/24 hr (reference range, 40–220 mmol/24 hr). Abnormalities were felt to be secondary to malnutrition from the strict liquid diet (blood urea nitrogen and creatinine ratio of 3:1 suggestive of notable protein calorie malnutrition). The patient was given 1 L of normal saline in the emergency department, with further fluids held so as not to increase serum sodium level too rapidly. A regular diet was started.
Physical examination revealed dry mucosal membranes but otherwise was unremarkable. Active bowel sounds were noted, as well as a soft, nontender, and nondistended abdomen; however, when examining the patient’s hands for reported shaking, a distinct abnormality of the nails was noticed. The patient had slate blue discoloration of the lunula, along with hyperpigmented violaceous discoloration of the proximal nail bed on all 10 fingernails (Figure 1). No abnormalities were seen on the toenails. The mother had a distinct bluish gray discoloration of the face as well as similar nail findings (Figure 2), strongly suggestive of colloidal silver use. An urgent serum silver level was ordered on the patient as well as a heavy metal panel. The mother was found applying numerous “natural remedies” to the patient’s skin while in the hospital, including a liquid spray and lotion, both in unmarked bottles. At that time, the mother was informed that no external supplements should be applied to her son. The serum silver level was elevated substantially at 94.3 ng/mL (reference range, <1.0 ng/mL). When the mother was confronted, she initially denied use of silver but later admitted to notable silver content in the cream she was applying to her son’s skin. The mother reported that she read online that colloidal silver had been historically used to cure numerous ailments and she was ordering products from an online company. She was counseled on the dangers of both topical application and ingestion of silver, and all supplements were removed from the home.


Argyria is a rare condition caused by chronic exposure to silver and is characterized by a blue-gray pigmentation in the skin and appendages, mucous membranes, and internal organs.4 Clinically, argyria is classified as generalized or localized. Generalized argyria results from ingestion or inhalation of silver compounds, where granules deposit preferentially in sun-exposed areas of skin as well as internal organs, with the highest concentration in the liver, spleen, and adrenal glands; discoloration often is permanent.5 On the contrary, localized argyria results from direct external contact with silver and granules deposited in the hands, eyes, and mucosa.5 Although the exact mechanism of penetration from topical silver remains unknown, it is thought to enter via the eccrine sweat ducts, as histopathology reveals silver granules found in highest concentration surrounding sweat glands in the dermis.6
Initial differential diagnoses for altered nail pigmentation include drug-induced causes, systemic diseases, cyanosis, and exposure to metals.7 The most commonly indicated medications resulting in blue nail pigment changes include antimalarials, minocycline, zidovudine, and phenothiazine. Systemic diseases that may cause blue nail color change include Wilson disease, hemochromatosis, Addison disease, methemoglobinemia, and alkaptonuria.7 Metals include gold, mercury, arsenic, bismuth, lead, and silver.4 After a thorough review of the patient’s medications and lack of support for any underlying disease process, contact with metals, particularly silver, was ranked highly on our differential list. In support of this theory, the mother’s bluish gray facial skin led to high clinical suspicion that she was ingesting colloidal silver and also was exposing her son to silver.
Treatment of argyria is challenging but first and foremost involves discontinuation of the source of chronic silver exposure. Unfortunately, the discoloration of generalized argyria often is permanent. Sunscreen can be used to help prevent any further darkening of pigment. The pigment in localized argyria has been reported to slowly fade with time, and there also have been reports of successful treatment using a low-fluence Q-switched 1064-nm Nd:YAG laser.8
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Lencastre A, Lobo M, João A. Argyria—case report. An Bras Dermatol. 2013;88:413-416.
- Park S-W, Kim J-H, Shin H-T, et al. An effective modality for argyria treatment: Q-switched 1,064-nm Nd:YAG laser. Ann Dermatol. 2013;25:511-512.
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Garcias-Ladaria J, Hernandez-Bel P, Torregrosa-Calatayud JL, et al. Localized cutaneous argyria: a report of 2 cases. Actas Dermosifiliogr. 2013;104:253-254.
- Kapur N, Landon G, Yu RC. Localized argyria in an antique restorer. Br J Dermatol. 2001;144:191-192.
- Kubba A, Kubba R, Batrani M, Pal T. Argyria an unrecognized cause of cutaneous pigmentation in Indian patients: a case series and review of the literature. Indian J Dermatol Venereol Leprol. 2013;79:805-811.
- Han TY, Chang HS, Lee HK, et al. Successful treatment of argyria using a low-fluence Q-switched 1064-nm Nd:YAG laser. Int J Dermatol. 2011;50:751-753.
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Lencastre A, Lobo M, João A. Argyria—case report. An Bras Dermatol. 2013;88:413-416.
- Park S-W, Kim J-H, Shin H-T, et al. An effective modality for argyria treatment: Q-switched 1,064-nm Nd:YAG laser. Ann Dermatol. 2013;25:511-512.
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Garcias-Ladaria J, Hernandez-Bel P, Torregrosa-Calatayud JL, et al. Localized cutaneous argyria: a report of 2 cases. Actas Dermosifiliogr. 2013;104:253-254.
- Kapur N, Landon G, Yu RC. Localized argyria in an antique restorer. Br J Dermatol. 2001;144:191-192.
- Kubba A, Kubba R, Batrani M, Pal T. Argyria an unrecognized cause of cutaneous pigmentation in Indian patients: a case series and review of the literature. Indian J Dermatol Venereol Leprol. 2013;79:805-811.
- Han TY, Chang HS, Lee HK, et al. Successful treatment of argyria using a low-fluence Q-switched 1064-nm Nd:YAG laser. Int J Dermatol. 2011;50:751-753.
Practice Points
- Argyria results from chronic exposure to products with a high silver content and may result in abnormalities of the skin and internal organs.
- Examination of the fingernails can provide important clues to underlying systemic conditions or external exposures.
A Practical Guide to Treatment of Hair Loss Beyond Standard Therapy
When I was a medical student rotating in dermatology, a patient with extensive alopecia looked at my long thick hair and said tearfully, “I just wish I could have hair like yours.”
I smiled, removed my wig, and replied, “You can have hair like mine.”
Determination and Perseverance
I was 2 years old when I was given a diagnosis of alopecia areata. Bald spots on my scalp would come and go for years but were not overly burdensome until I turned 12. At that point, my hair loss escalated despite frequent intralesional injections of triamcinolone; every 2 steps forward were followed by 3 steps backward.
As a freshman in high school, I finally took control of my condition and emotions, shaved my head, and purchased a wig—actions that confronted my hair loss and awoke a determination and perseverance that I did not think I would ever gain while living with this condition. As McGettigan1 wrote in the Journal of the American Academy of Dermatology in 2004, “Being diagnosed with [alopecia areata] does not mean one cannot have a full and meaningful life. By choosing to confront the condition and turn its negative aspects into positive actions, one can succeed in life.”1
As a Provider, Another Perspective
Now, as a dermatology resident, I have the distinct perspective of being patient and provider. Patients often want to know, “Why is this happening?”, “Is my hair going to grow back?”, and “What treatments are available?”
They want to feel supported, understood, and heard.
As health care providers, we must understand that hair loss can result in overwhelming fear, hopelessness, and loss of self-esteem. Although we can give good news and offer helpful treatment options to some patients, there are those for whom medical treatment fails, and we can offer no more than a supportive hand and warm smile.
But can we do even more than that? The answer is: “Yes.”
Management Options
I recommend that all patients with hair loss should receive a copy of the aforementioned McGettigan1 article, “Ahead With No Hair,” which is geared toward patients with alopecia areata but offers inspiring words to any patient struggling to cope with hair loss. Dermatologists also can offer management options for patients with hair loss, including camouflage, wigs, and cosmetic replacement of eyelashes and eyebrows. Of note, several companies offer wigs and brow replacement options for men and children.
Camouflage
We can offer creative and readily available camouflage options for patients with hair loss. For small bald spots and thinning hair on the scalp, keratin hair-building fibers can be extremely useful. This over-the-counter product comes in a variety of natural hair colors, conceals the underlying skin, and adds fullness to hair. The keratin fibers have an innate static charge that allows them to adhere to the hair shaft. Daily application typically is necessary; duration can be maximized if hair spray or other brand-specific bonding spray is used following application of the fibers. A simple online search using the term keratin hair building fibers will reveal many online and in-store options with 4- or 5-star reviews. Most negative reviews pertain to sweating or moisture that causes clumping, but overall this is an easy and affordable option for mild hair loss.
Wigs
For patients hoping to mask moderate or severe hair loss, I recommend wigs, which can be made from synthetic fibers or human hair. In order to effectively guide patients, it is helpful for providers to have some knowledge about the 2 types of wigs. Synthetic wigs are of variable quality, ranging from costume-grade to top-quality products that look and feel like human hair. They are more affordable and often are easier to maintain than human-hair wigs, and hairstyles hold up better after washing. Many synthetic wigs cannot withstand heat from a hot iron and have a slightly shorter lifespan (6–12 months) than human-hair wigs (1–2 years).
Human hair wigs are made of real human hair, so they look and feel natural. These wigs can be made from European, African, Indian, Malaysian, Chinese, or other ethnic hair. Patients can choose the texture of the hair, including silky (smooth), kinky (mimicking natural blow-dried Black hair), and yaki (mimicking relaxed Black hair), as well as the curl pattern (straight, wavy, or curly), length, color, density, and cap construction.
The cap of a wig is what the hair is tied to. The construction of wig caps varies to allow for realistic hair lines as well as security for active use or up-dos. Among the many cap-construction options, the most realistic-appearing are hand-tied monofilament, lace-front, and full-lace wigs, all of which may require tape or glue to keep them in place. Some wig companies offer nonslip so-called “alopecia caps” for patients with no scalp hair. Patients who find their wig irritating to the scalp should consider wearing a nylon wig cap or liner.
Wigs can be purchased in store or online and can be pre-made or custom-built to be tailored to the patient’s specific desires and expectations. The cost depends on the type and quality of hair, cap construction, and length; prices can range from less than $100 to more than $5000.
When choosing a wig, which option—synthetic or human hair—is better for a given patient? Synthetic wigs are rather inexpensive and easy to care for, making them great for new users and those who want to try different styles and colors. Human-hair wigs can be custom-made to match the patient’s natural hair; however, they require extra care to maintain their longevity. Both types of wigs have pros and cons depending on the patient’s budget, time required for maintenance and styling, and needs (Table 1). I encourage patients to have fun with all wig options: Now is the time, I tell them, to try out the cute or daring hair style they have always wanted. The great thing is that if the patient does not like their wig, they can readily change it.
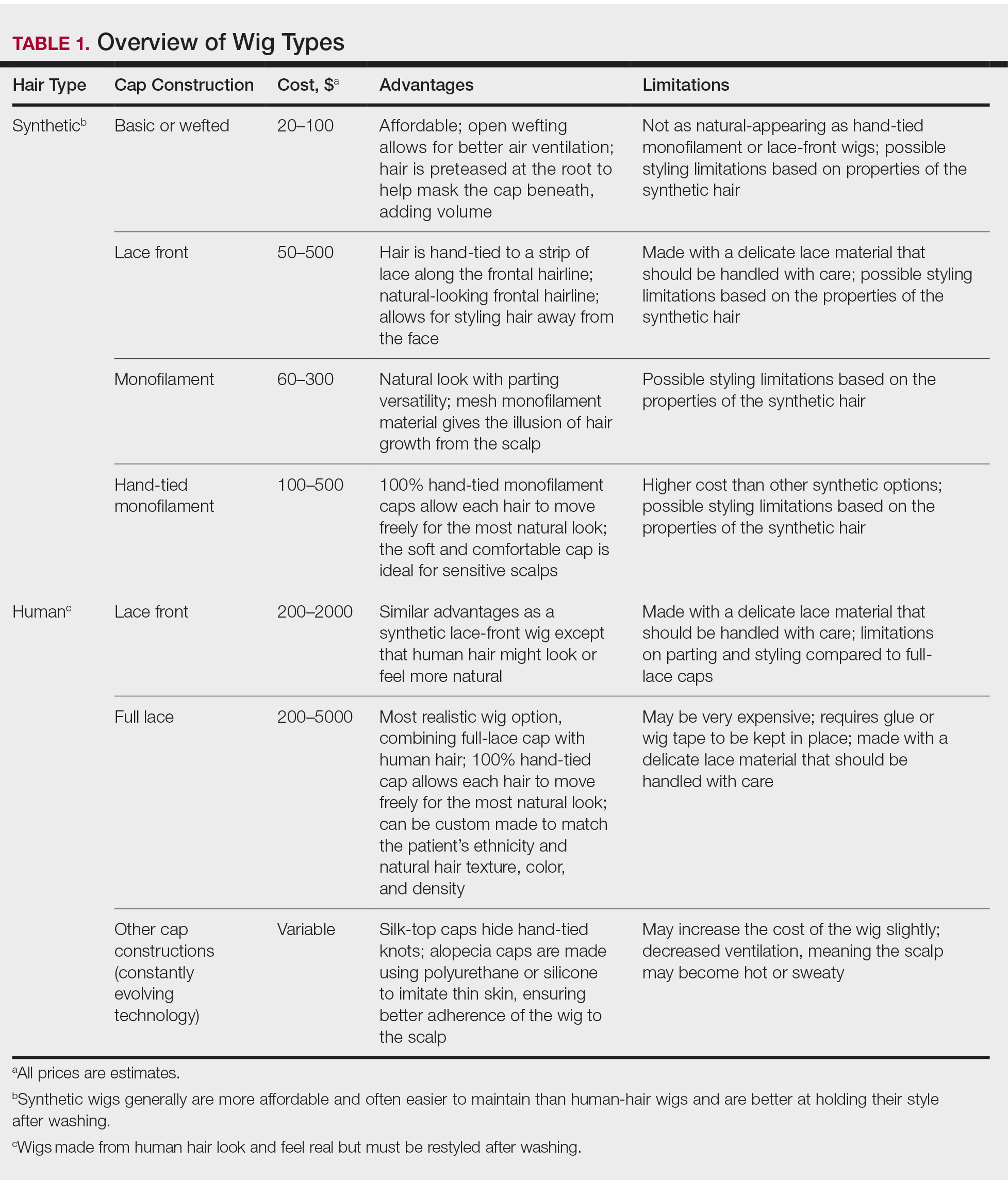
Good-quality wigs are expensive but sometimes are necessary to regain self-confidence and improve one’s quality of life. Advise patients to call their health insurance company to find out if a cranial or scalp prosthesis is covered by their policy. Coverage might require a written prescription for a cranial prosthesis, listing the diagnosis, diagnosis code, and letter of medical necessity. Patients can then purchase the wig online or through a certified distributor depending on their insurance requirements and obtain reimbursement (partial or full coverage). If a wig is not covered by insurance, a cranial prosthesis might be a flexible spending account–eligible expense. For guidance on the reimbursability of wigs, visit the National Alopecia Areata Foundation (NAAF) website (www.naaf.org/AccessHealthcare).
Eyelashes and Eyebrows
Cosmetic replacement of eyelashes (Table 2) and eyebrows (Table 3) is another treatment option that physicians can offer to hair-loss patients. For patients who desire false eyelashes, strip lashes that are glued to the eyelid margin are easiest to apply (but with caution—do not get glue in the eyes!). There are magnetic lashes, but these require natural lashes on which to adhere them. Eyebrows can be hand-drawn using brow pencils or powders with or without a stencil to maintain symmetry. There are even brow wigs and temporary brow tattoos that can last 1 to several days. Semi-permanent tattooing, including microblading, is an option that has amazing results but can be painful and expensive, often requiring touch-ups every 6 to 18 months.
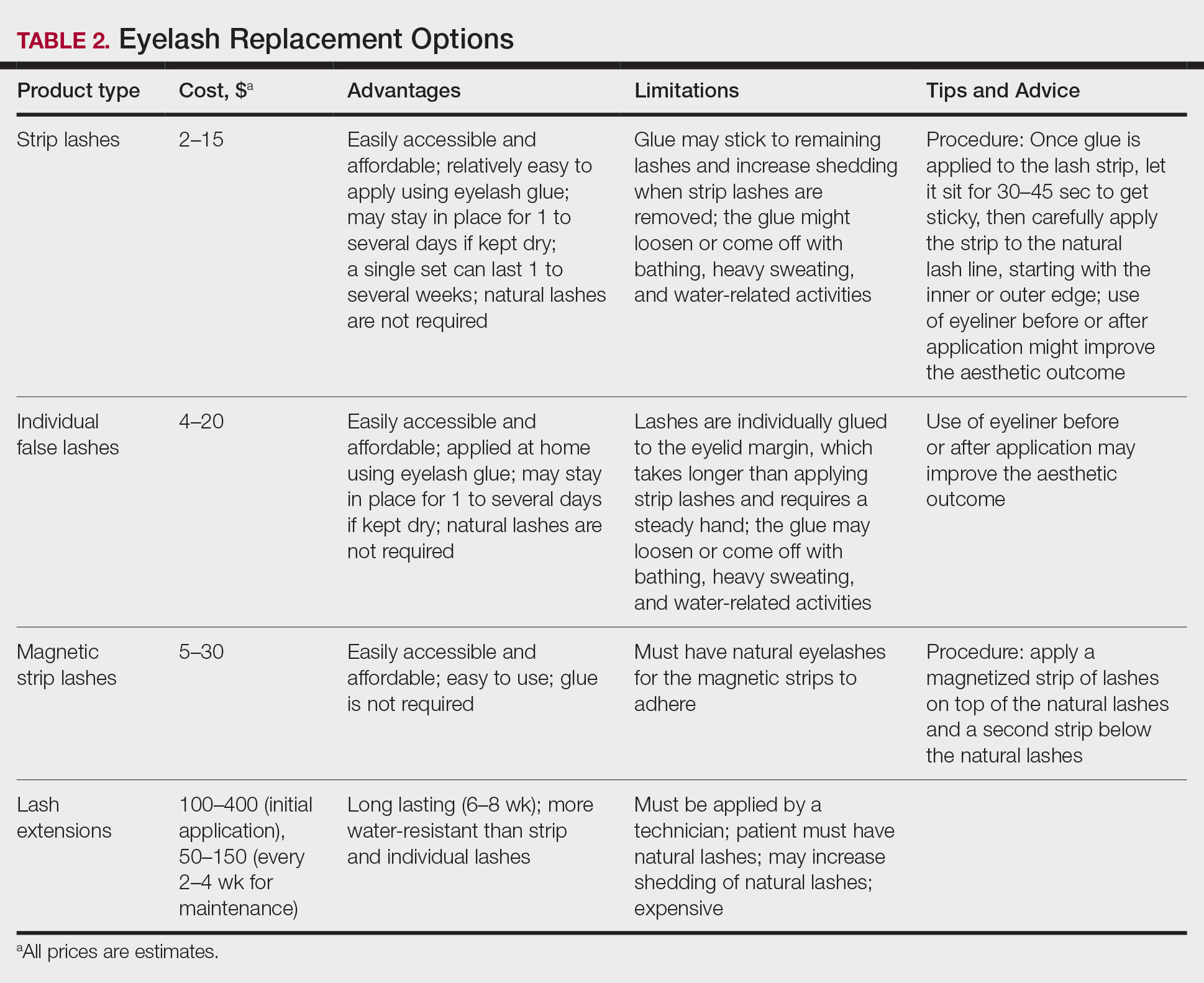
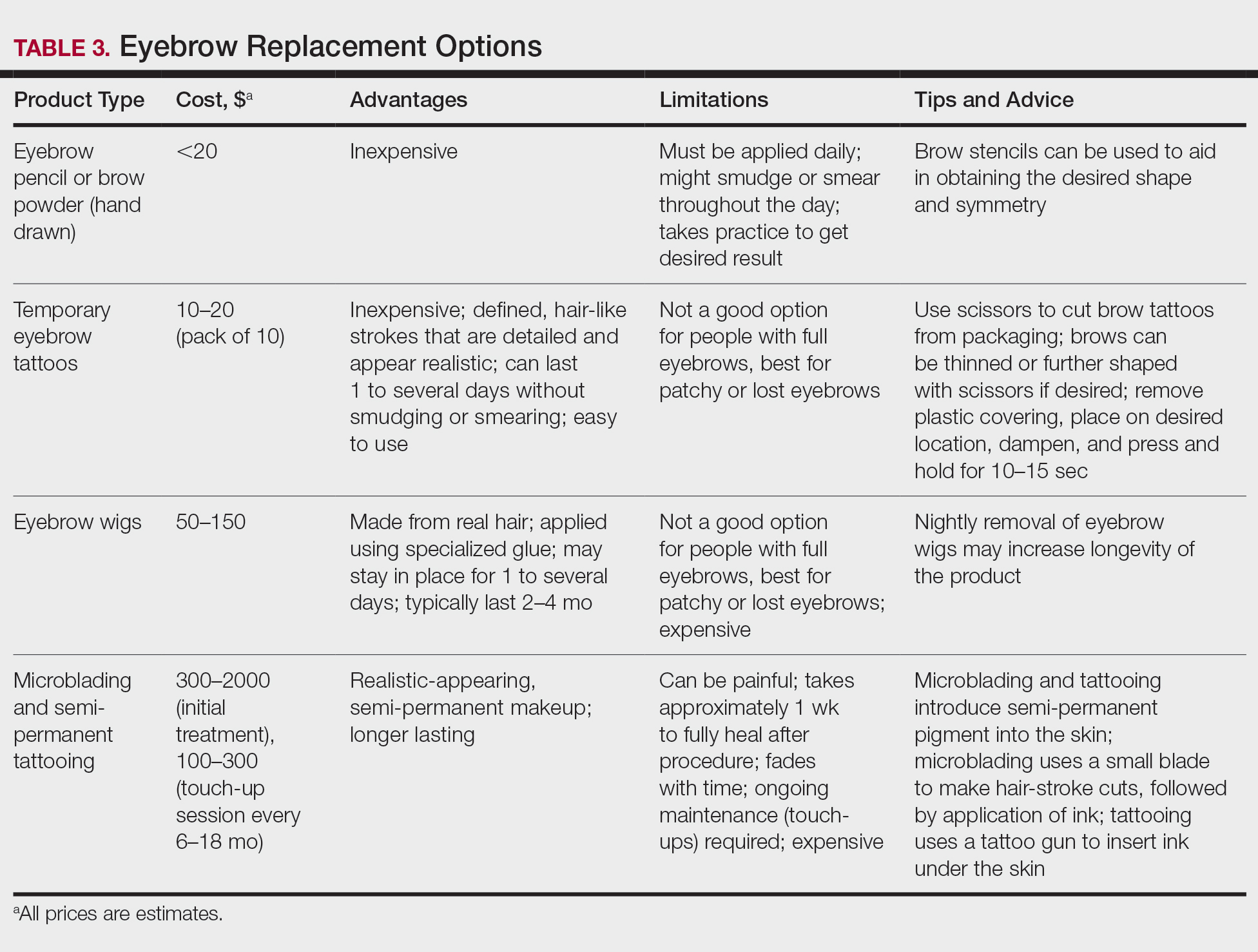
Resources Abound
Experiencing and treating hair loss can be overwhelming, but there are countless resources available for patients. The NAAF has utility beyond the concerns of alopecia areata patients; there also is useful information on YouTube and social media, and support groups exist for hair-loss patients. I recommend starting with the NAAF website, which offers many helpful resources and support groups for patients and their families, including tips on applying for insurance reimbursement and drafting an appeal letter. Lastly, several nonprofit organizations serve the hair-replacement needs of children and adults with hair loss (Table 4).
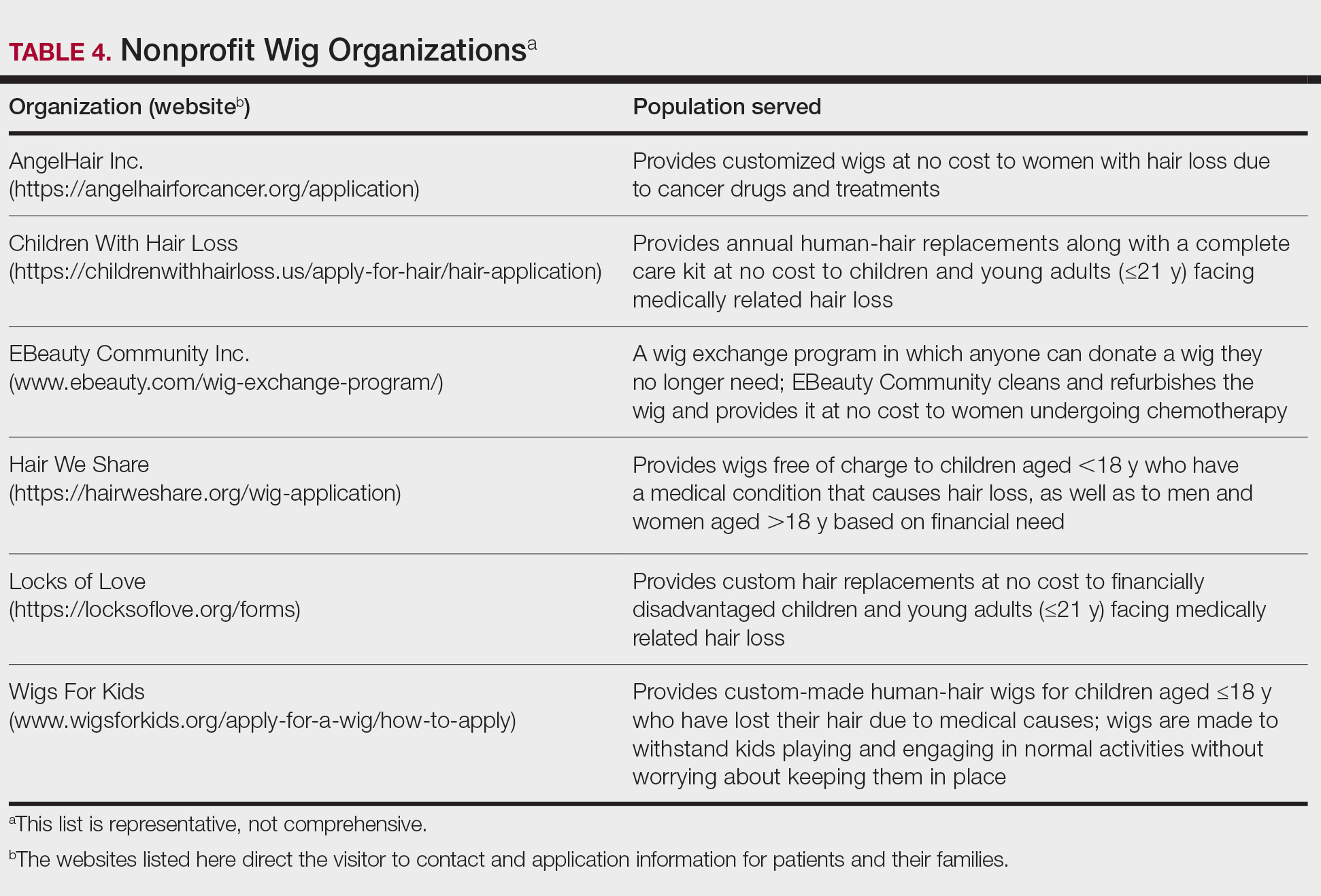
Final Thoughts
My experience as a patient with alopecia has been long and initially was challenging; however, I found the silver lining after choosing to confront my literal and figurative “losses” and move forward—to grow, so to speak. With the use of custom-made human-hair wigs, false strip eyelashes, and a mix of eyebrow replacement options, I have been able to regain my confidence and self-esteem. Now, my goal as a physician—a goal that I hope you will share—is to be knowledgeable about hair-replacement options and provide information and resources to patients to help them feel empowered, brave, and beautiful.
- McGettigan ML. Ahead with no hair. J Am Acad Dermatol. 2004;51(1 suppl):18-19.
When I was a medical student rotating in dermatology, a patient with extensive alopecia looked at my long thick hair and said tearfully, “I just wish I could have hair like yours.”
I smiled, removed my wig, and replied, “You can have hair like mine.”
Determination and Perseverance
I was 2 years old when I was given a diagnosis of alopecia areata. Bald spots on my scalp would come and go for years but were not overly burdensome until I turned 12. At that point, my hair loss escalated despite frequent intralesional injections of triamcinolone; every 2 steps forward were followed by 3 steps backward.
As a freshman in high school, I finally took control of my condition and emotions, shaved my head, and purchased a wig—actions that confronted my hair loss and awoke a determination and perseverance that I did not think I would ever gain while living with this condition. As McGettigan1 wrote in the Journal of the American Academy of Dermatology in 2004, “Being diagnosed with [alopecia areata] does not mean one cannot have a full and meaningful life. By choosing to confront the condition and turn its negative aspects into positive actions, one can succeed in life.”1
As a Provider, Another Perspective
Now, as a dermatology resident, I have the distinct perspective of being patient and provider. Patients often want to know, “Why is this happening?”, “Is my hair going to grow back?”, and “What treatments are available?”
They want to feel supported, understood, and heard.
As health care providers, we must understand that hair loss can result in overwhelming fear, hopelessness, and loss of self-esteem. Although we can give good news and offer helpful treatment options to some patients, there are those for whom medical treatment fails, and we can offer no more than a supportive hand and warm smile.
But can we do even more than that? The answer is: “Yes.”
Management Options
I recommend that all patients with hair loss should receive a copy of the aforementioned McGettigan1 article, “Ahead With No Hair,” which is geared toward patients with alopecia areata but offers inspiring words to any patient struggling to cope with hair loss. Dermatologists also can offer management options for patients with hair loss, including camouflage, wigs, and cosmetic replacement of eyelashes and eyebrows. Of note, several companies offer wigs and brow replacement options for men and children.
Camouflage
We can offer creative and readily available camouflage options for patients with hair loss. For small bald spots and thinning hair on the scalp, keratin hair-building fibers can be extremely useful. This over-the-counter product comes in a variety of natural hair colors, conceals the underlying skin, and adds fullness to hair. The keratin fibers have an innate static charge that allows them to adhere to the hair shaft. Daily application typically is necessary; duration can be maximized if hair spray or other brand-specific bonding spray is used following application of the fibers. A simple online search using the term keratin hair building fibers will reveal many online and in-store options with 4- or 5-star reviews. Most negative reviews pertain to sweating or moisture that causes clumping, but overall this is an easy and affordable option for mild hair loss.
Wigs
For patients hoping to mask moderate or severe hair loss, I recommend wigs, which can be made from synthetic fibers or human hair. In order to effectively guide patients, it is helpful for providers to have some knowledge about the 2 types of wigs. Synthetic wigs are of variable quality, ranging from costume-grade to top-quality products that look and feel like human hair. They are more affordable and often are easier to maintain than human-hair wigs, and hairstyles hold up better after washing. Many synthetic wigs cannot withstand heat from a hot iron and have a slightly shorter lifespan (6–12 months) than human-hair wigs (1–2 years).
Human hair wigs are made of real human hair, so they look and feel natural. These wigs can be made from European, African, Indian, Malaysian, Chinese, or other ethnic hair. Patients can choose the texture of the hair, including silky (smooth), kinky (mimicking natural blow-dried Black hair), and yaki (mimicking relaxed Black hair), as well as the curl pattern (straight, wavy, or curly), length, color, density, and cap construction.
The cap of a wig is what the hair is tied to. The construction of wig caps varies to allow for realistic hair lines as well as security for active use or up-dos. Among the many cap-construction options, the most realistic-appearing are hand-tied monofilament, lace-front, and full-lace wigs, all of which may require tape or glue to keep them in place. Some wig companies offer nonslip so-called “alopecia caps” for patients with no scalp hair. Patients who find their wig irritating to the scalp should consider wearing a nylon wig cap or liner.
Wigs can be purchased in store or online and can be pre-made or custom-built to be tailored to the patient’s specific desires and expectations. The cost depends on the type and quality of hair, cap construction, and length; prices can range from less than $100 to more than $5000.
When choosing a wig, which option—synthetic or human hair—is better for a given patient? Synthetic wigs are rather inexpensive and easy to care for, making them great for new users and those who want to try different styles and colors. Human-hair wigs can be custom-made to match the patient’s natural hair; however, they require extra care to maintain their longevity. Both types of wigs have pros and cons depending on the patient’s budget, time required for maintenance and styling, and needs (Table 1). I encourage patients to have fun with all wig options: Now is the time, I tell them, to try out the cute or daring hair style they have always wanted. The great thing is that if the patient does not like their wig, they can readily change it.

Good-quality wigs are expensive but sometimes are necessary to regain self-confidence and improve one’s quality of life. Advise patients to call their health insurance company to find out if a cranial or scalp prosthesis is covered by their policy. Coverage might require a written prescription for a cranial prosthesis, listing the diagnosis, diagnosis code, and letter of medical necessity. Patients can then purchase the wig online or through a certified distributor depending on their insurance requirements and obtain reimbursement (partial or full coverage). If a wig is not covered by insurance, a cranial prosthesis might be a flexible spending account–eligible expense. For guidance on the reimbursability of wigs, visit the National Alopecia Areata Foundation (NAAF) website (www.naaf.org/AccessHealthcare).
Eyelashes and Eyebrows
Cosmetic replacement of eyelashes (Table 2) and eyebrows (Table 3) is another treatment option that physicians can offer to hair-loss patients. For patients who desire false eyelashes, strip lashes that are glued to the eyelid margin are easiest to apply (but with caution—do not get glue in the eyes!). There are magnetic lashes, but these require natural lashes on which to adhere them. Eyebrows can be hand-drawn using brow pencils or powders with or without a stencil to maintain symmetry. There are even brow wigs and temporary brow tattoos that can last 1 to several days. Semi-permanent tattooing, including microblading, is an option that has amazing results but can be painful and expensive, often requiring touch-ups every 6 to 18 months.


Resources Abound
Experiencing and treating hair loss can be overwhelming, but there are countless resources available for patients. The NAAF has utility beyond the concerns of alopecia areata patients; there also is useful information on YouTube and social media, and support groups exist for hair-loss patients. I recommend starting with the NAAF website, which offers many helpful resources and support groups for patients and their families, including tips on applying for insurance reimbursement and drafting an appeal letter. Lastly, several nonprofit organizations serve the hair-replacement needs of children and adults with hair loss (Table 4).

Final Thoughts
My experience as a patient with alopecia has been long and initially was challenging; however, I found the silver lining after choosing to confront my literal and figurative “losses” and move forward—to grow, so to speak. With the use of custom-made human-hair wigs, false strip eyelashes, and a mix of eyebrow replacement options, I have been able to regain my confidence and self-esteem. Now, my goal as a physician—a goal that I hope you will share—is to be knowledgeable about hair-replacement options and provide information and resources to patients to help them feel empowered, brave, and beautiful.
When I was a medical student rotating in dermatology, a patient with extensive alopecia looked at my long thick hair and said tearfully, “I just wish I could have hair like yours.”
I smiled, removed my wig, and replied, “You can have hair like mine.”
Determination and Perseverance
I was 2 years old when I was given a diagnosis of alopecia areata. Bald spots on my scalp would come and go for years but were not overly burdensome until I turned 12. At that point, my hair loss escalated despite frequent intralesional injections of triamcinolone; every 2 steps forward were followed by 3 steps backward.
As a freshman in high school, I finally took control of my condition and emotions, shaved my head, and purchased a wig—actions that confronted my hair loss and awoke a determination and perseverance that I did not think I would ever gain while living with this condition. As McGettigan1 wrote in the Journal of the American Academy of Dermatology in 2004, “Being diagnosed with [alopecia areata] does not mean one cannot have a full and meaningful life. By choosing to confront the condition and turn its negative aspects into positive actions, one can succeed in life.”1
As a Provider, Another Perspective
Now, as a dermatology resident, I have the distinct perspective of being patient and provider. Patients often want to know, “Why is this happening?”, “Is my hair going to grow back?”, and “What treatments are available?”
They want to feel supported, understood, and heard.
As health care providers, we must understand that hair loss can result in overwhelming fear, hopelessness, and loss of self-esteem. Although we can give good news and offer helpful treatment options to some patients, there are those for whom medical treatment fails, and we can offer no more than a supportive hand and warm smile.
But can we do even more than that? The answer is: “Yes.”
Management Options
I recommend that all patients with hair loss should receive a copy of the aforementioned McGettigan1 article, “Ahead With No Hair,” which is geared toward patients with alopecia areata but offers inspiring words to any patient struggling to cope with hair loss. Dermatologists also can offer management options for patients with hair loss, including camouflage, wigs, and cosmetic replacement of eyelashes and eyebrows. Of note, several companies offer wigs and brow replacement options for men and children.
Camouflage
We can offer creative and readily available camouflage options for patients with hair loss. For small bald spots and thinning hair on the scalp, keratin hair-building fibers can be extremely useful. This over-the-counter product comes in a variety of natural hair colors, conceals the underlying skin, and adds fullness to hair. The keratin fibers have an innate static charge that allows them to adhere to the hair shaft. Daily application typically is necessary; duration can be maximized if hair spray or other brand-specific bonding spray is used following application of the fibers. A simple online search using the term keratin hair building fibers will reveal many online and in-store options with 4- or 5-star reviews. Most negative reviews pertain to sweating or moisture that causes clumping, but overall this is an easy and affordable option for mild hair loss.
Wigs
For patients hoping to mask moderate or severe hair loss, I recommend wigs, which can be made from synthetic fibers or human hair. In order to effectively guide patients, it is helpful for providers to have some knowledge about the 2 types of wigs. Synthetic wigs are of variable quality, ranging from costume-grade to top-quality products that look and feel like human hair. They are more affordable and often are easier to maintain than human-hair wigs, and hairstyles hold up better after washing. Many synthetic wigs cannot withstand heat from a hot iron and have a slightly shorter lifespan (6–12 months) than human-hair wigs (1–2 years).
Human hair wigs are made of real human hair, so they look and feel natural. These wigs can be made from European, African, Indian, Malaysian, Chinese, or other ethnic hair. Patients can choose the texture of the hair, including silky (smooth), kinky (mimicking natural blow-dried Black hair), and yaki (mimicking relaxed Black hair), as well as the curl pattern (straight, wavy, or curly), length, color, density, and cap construction.
The cap of a wig is what the hair is tied to. The construction of wig caps varies to allow for realistic hair lines as well as security for active use or up-dos. Among the many cap-construction options, the most realistic-appearing are hand-tied monofilament, lace-front, and full-lace wigs, all of which may require tape or glue to keep them in place. Some wig companies offer nonslip so-called “alopecia caps” for patients with no scalp hair. Patients who find their wig irritating to the scalp should consider wearing a nylon wig cap or liner.
Wigs can be purchased in store or online and can be pre-made or custom-built to be tailored to the patient’s specific desires and expectations. The cost depends on the type and quality of hair, cap construction, and length; prices can range from less than $100 to more than $5000.
When choosing a wig, which option—synthetic or human hair—is better for a given patient? Synthetic wigs are rather inexpensive and easy to care for, making them great for new users and those who want to try different styles and colors. Human-hair wigs can be custom-made to match the patient’s natural hair; however, they require extra care to maintain their longevity. Both types of wigs have pros and cons depending on the patient’s budget, time required for maintenance and styling, and needs (Table 1). I encourage patients to have fun with all wig options: Now is the time, I tell them, to try out the cute or daring hair style they have always wanted. The great thing is that if the patient does not like their wig, they can readily change it.

Good-quality wigs are expensive but sometimes are necessary to regain self-confidence and improve one’s quality of life. Advise patients to call their health insurance company to find out if a cranial or scalp prosthesis is covered by their policy. Coverage might require a written prescription for a cranial prosthesis, listing the diagnosis, diagnosis code, and letter of medical necessity. Patients can then purchase the wig online or through a certified distributor depending on their insurance requirements and obtain reimbursement (partial or full coverage). If a wig is not covered by insurance, a cranial prosthesis might be a flexible spending account–eligible expense. For guidance on the reimbursability of wigs, visit the National Alopecia Areata Foundation (NAAF) website (www.naaf.org/AccessHealthcare).
Eyelashes and Eyebrows
Cosmetic replacement of eyelashes (Table 2) and eyebrows (Table 3) is another treatment option that physicians can offer to hair-loss patients. For patients who desire false eyelashes, strip lashes that are glued to the eyelid margin are easiest to apply (but with caution—do not get glue in the eyes!). There are magnetic lashes, but these require natural lashes on which to adhere them. Eyebrows can be hand-drawn using brow pencils or powders with or without a stencil to maintain symmetry. There are even brow wigs and temporary brow tattoos that can last 1 to several days. Semi-permanent tattooing, including microblading, is an option that has amazing results but can be painful and expensive, often requiring touch-ups every 6 to 18 months.


Resources Abound
Experiencing and treating hair loss can be overwhelming, but there are countless resources available for patients. The NAAF has utility beyond the concerns of alopecia areata patients; there also is useful information on YouTube and social media, and support groups exist for hair-loss patients. I recommend starting with the NAAF website, which offers many helpful resources and support groups for patients and their families, including tips on applying for insurance reimbursement and drafting an appeal letter. Lastly, several nonprofit organizations serve the hair-replacement needs of children and adults with hair loss (Table 4).

Final Thoughts
My experience as a patient with alopecia has been long and initially was challenging; however, I found the silver lining after choosing to confront my literal and figurative “losses” and move forward—to grow, so to speak. With the use of custom-made human-hair wigs, false strip eyelashes, and a mix of eyebrow replacement options, I have been able to regain my confidence and self-esteem. Now, my goal as a physician—a goal that I hope you will share—is to be knowledgeable about hair-replacement options and provide information and resources to patients to help them feel empowered, brave, and beautiful.
- McGettigan ML. Ahead with no hair. J Am Acad Dermatol. 2004;51(1 suppl):18-19.
- McGettigan ML. Ahead with no hair. J Am Acad Dermatol. 2004;51(1 suppl):18-19.
Practice Points
- Keratin hair-building fibers can help thinning hair appear thick and full.
- Wigs are useful in masking moderate to severe hair loss.
- False eyelashes, eyebrow wigs, temporary eyebrow tattoos, microblading, and other semipermanent makeup can disguise the loss of eyelashes and eyebrows.
Seaweed and other marine-derived products in skin care, part 1: Current indications
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Baricitinib hits mark for severe alopecia areata
in the phase 2/3 BRAVE-AA1 randomized trial, Brett King, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
The results with the 4-mg/day dose of the Janus kinase (JAK) 1 and -2 inhibitor were even more impressive. However, this higher dose, while approved in Europe and elsewhere for the treatment of rheumatoid arthritis, was rejected by the Food and Drug Administration because of safety concerns and is not available in the United States. The 2-mg dose of baricitinib is approved for RA in the United States.
There are currently no FDA-approved treatments for alopecia areata, noted Dr. King, a dermatologist at Yale University, New Haven, Conn.
He reported on 110 adults with severe alopecia areata as defined by a baseline Severity of Alopecia Tool (SALT) score of 87, meaning they averaged 87% scalp hair loss. They averaged a 16-year history of the autoimmune disease. The duration of the current episode was at least 4 years in more than one-third of participants. Clinicians rated more than three-quarters of patients as having no eyebrow or eyelash hair, or significant gaps and uneven distribution.
The primary outcome in this interim analysis was achievement of a SALT score of 20 or less at week 36, meaning hair loss had shrunk to 20% or less of the scalp. Fifty-two percent of patients on baricitinib 4 mg achieved this outcome, as did 33% of those randomized to baricitinib 2 mg and 4% of placebo-treated controls.
In addition, 60% of patients on the higher dose of the JAK inhibitor and 40% on the lower dose rated themselves as having either full eyebrows and eyelashes on both eyes at 36 weeks, or only minimal gaps with even distribution. None of the controls reported comparable improvement, Dr. King said at the conference, which was sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
There were no serious adverse events in this relatively small study. Six cases of herpes simplex and two of herpes zoster occurred in baricitinib-treated patients; there were none in controls.
Session moderator Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey, said that she was very impressed that baricitinib could achieve substantial hair regrowth in patients with a median duration of hair loss of about 16 years.
“It’s very interesting,” agreed comoderator Ashfaq A. Marghoob, MD, director of clinical dermatology at Memorial Sloan Kettering Cancer Center in Hauppauge, N.Y. “Having this kind of hair regrowth goes against what we learned in our residency, that the longer you’ve gone with hair loss, the less likely it is to ever come back.”
Separately, Eli Lilly issued a press release announcing that both the 2- and 4-mg doses of baricitinib had met the primary endpoint in the phase 3 BRAVE-AA2 trial, showing significantly greater hair regrowth compared with placebo in the 546-patient study. However, the company provided no data, instead stating that the full results will be presented at an upcoming medical conference.
in the phase 2/3 BRAVE-AA1 randomized trial, Brett King, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
The results with the 4-mg/day dose of the Janus kinase (JAK) 1 and -2 inhibitor were even more impressive. However, this higher dose, while approved in Europe and elsewhere for the treatment of rheumatoid arthritis, was rejected by the Food and Drug Administration because of safety concerns and is not available in the United States. The 2-mg dose of baricitinib is approved for RA in the United States.
There are currently no FDA-approved treatments for alopecia areata, noted Dr. King, a dermatologist at Yale University, New Haven, Conn.
He reported on 110 adults with severe alopecia areata as defined by a baseline Severity of Alopecia Tool (SALT) score of 87, meaning they averaged 87% scalp hair loss. They averaged a 16-year history of the autoimmune disease. The duration of the current episode was at least 4 years in more than one-third of participants. Clinicians rated more than three-quarters of patients as having no eyebrow or eyelash hair, or significant gaps and uneven distribution.
The primary outcome in this interim analysis was achievement of a SALT score of 20 or less at week 36, meaning hair loss had shrunk to 20% or less of the scalp. Fifty-two percent of patients on baricitinib 4 mg achieved this outcome, as did 33% of those randomized to baricitinib 2 mg and 4% of placebo-treated controls.
In addition, 60% of patients on the higher dose of the JAK inhibitor and 40% on the lower dose rated themselves as having either full eyebrows and eyelashes on both eyes at 36 weeks, or only minimal gaps with even distribution. None of the controls reported comparable improvement, Dr. King said at the conference, which was sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
There were no serious adverse events in this relatively small study. Six cases of herpes simplex and two of herpes zoster occurred in baricitinib-treated patients; there were none in controls.
Session moderator Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey, said that she was very impressed that baricitinib could achieve substantial hair regrowth in patients with a median duration of hair loss of about 16 years.
“It’s very interesting,” agreed comoderator Ashfaq A. Marghoob, MD, director of clinical dermatology at Memorial Sloan Kettering Cancer Center in Hauppauge, N.Y. “Having this kind of hair regrowth goes against what we learned in our residency, that the longer you’ve gone with hair loss, the less likely it is to ever come back.”
Separately, Eli Lilly issued a press release announcing that both the 2- and 4-mg doses of baricitinib had met the primary endpoint in the phase 3 BRAVE-AA2 trial, showing significantly greater hair regrowth compared with placebo in the 546-patient study. However, the company provided no data, instead stating that the full results will be presented at an upcoming medical conference.
in the phase 2/3 BRAVE-AA1 randomized trial, Brett King, MD, PhD, reported at Innovations in Dermatology: Virtual Spring Conference 2021.
The results with the 4-mg/day dose of the Janus kinase (JAK) 1 and -2 inhibitor were even more impressive. However, this higher dose, while approved in Europe and elsewhere for the treatment of rheumatoid arthritis, was rejected by the Food and Drug Administration because of safety concerns and is not available in the United States. The 2-mg dose of baricitinib is approved for RA in the United States.
There are currently no FDA-approved treatments for alopecia areata, noted Dr. King, a dermatologist at Yale University, New Haven, Conn.
He reported on 110 adults with severe alopecia areata as defined by a baseline Severity of Alopecia Tool (SALT) score of 87, meaning they averaged 87% scalp hair loss. They averaged a 16-year history of the autoimmune disease. The duration of the current episode was at least 4 years in more than one-third of participants. Clinicians rated more than three-quarters of patients as having no eyebrow or eyelash hair, or significant gaps and uneven distribution.
The primary outcome in this interim analysis was achievement of a SALT score of 20 or less at week 36, meaning hair loss had shrunk to 20% or less of the scalp. Fifty-two percent of patients on baricitinib 4 mg achieved this outcome, as did 33% of those randomized to baricitinib 2 mg and 4% of placebo-treated controls.
In addition, 60% of patients on the higher dose of the JAK inhibitor and 40% on the lower dose rated themselves as having either full eyebrows and eyelashes on both eyes at 36 weeks, or only minimal gaps with even distribution. None of the controls reported comparable improvement, Dr. King said at the conference, which was sponsored by MedscapeLIVE! and the producers of the Hawaii Dermatology Seminar and Caribbean Dermatology Symposium.
There were no serious adverse events in this relatively small study. Six cases of herpes simplex and two of herpes zoster occurred in baricitinib-treated patients; there were none in controls.
Session moderator Andrea L. Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey, said that she was very impressed that baricitinib could achieve substantial hair regrowth in patients with a median duration of hair loss of about 16 years.
“It’s very interesting,” agreed comoderator Ashfaq A. Marghoob, MD, director of clinical dermatology at Memorial Sloan Kettering Cancer Center in Hauppauge, N.Y. “Having this kind of hair regrowth goes against what we learned in our residency, that the longer you’ve gone with hair loss, the less likely it is to ever come back.”
Separately, Eli Lilly issued a press release announcing that both the 2- and 4-mg doses of baricitinib had met the primary endpoint in the phase 3 BRAVE-AA2 trial, showing significantly greater hair regrowth compared with placebo in the 546-patient study. However, the company provided no data, instead stating that the full results will be presented at an upcoming medical conference.
FROM INNOVATIONS IN DERMATOLOGY
Permanent Alopecia in Breast Cancer Patients: Role of Taxanes and Endocrine Therapies
Anagen effluvium during chemotherapy is common, typically beginning within 1 month of treatment onset and resolving by 6 months after the final course.1 Permanent chemotherapy-induced alopecia (PCIA), in which hair loss persists beyond 6 months after chemotherapy without recovery to original density, was first reported in patients following high-dose chemotherapy regimens for allogeneic bone marrow transplantation.2 There are now increasing reports of PCIA in patients with breast cancer; at least 400 such cases have been documented.3-16 In addition to chemotherapy, patients often receive adjuvant endocrine therapy with selective estrogen receptor modulators, aromatase inhibitors, or gonadotropin-releasing hormone agonists.5-16 Endocrine therapies also can lead to alopecia, but their role in PCIA has not been well defined.15,16 We describe 3 patients with breast cancer who experienced PCIA following chemotherapy with taxanes with or without endocrine therapies. We also review the literature on non–bone marrow transplantation PCIA to better characterize this entity and explore the role of endocrine therapies in PCIA.
Case Reports
Patient 1
A 62-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 5 years after completing chemotherapy. She underwent 6 cycles of docetaxel and carboplatin along with radiation therapy as well as 1 year of trastuzumab and did not receive endocrine therapy. At the current presentation, she reported patchy hair regrowth that gradually filled in but failed to return to full density. Physical examination revealed the hair was diffusely thin, especially bitemporally (Figures 1A and 1B), and she did not experience any loss of body hair. She had no family history of hair loss. Her medical history was notable for hypertension, chronic obstructive bronchitis, osteopenia, and depression. Her thyroid stimulating hormone (TSH) level was within reference range. Medications included lisinopril, metoprolol, escitalopram, and trazodone. A biopsy from the occipital scalp showed nonscarring alopecia with variation of hair follicle size, a decreased number of hair follicles, and a decreased anagen to telogen ratio (Figure 1C). She was treated with clobetasol solution and minoxidil solution 5% for 1 year with mild improvement. She experienced no further hair loss but did not regain original hair density.
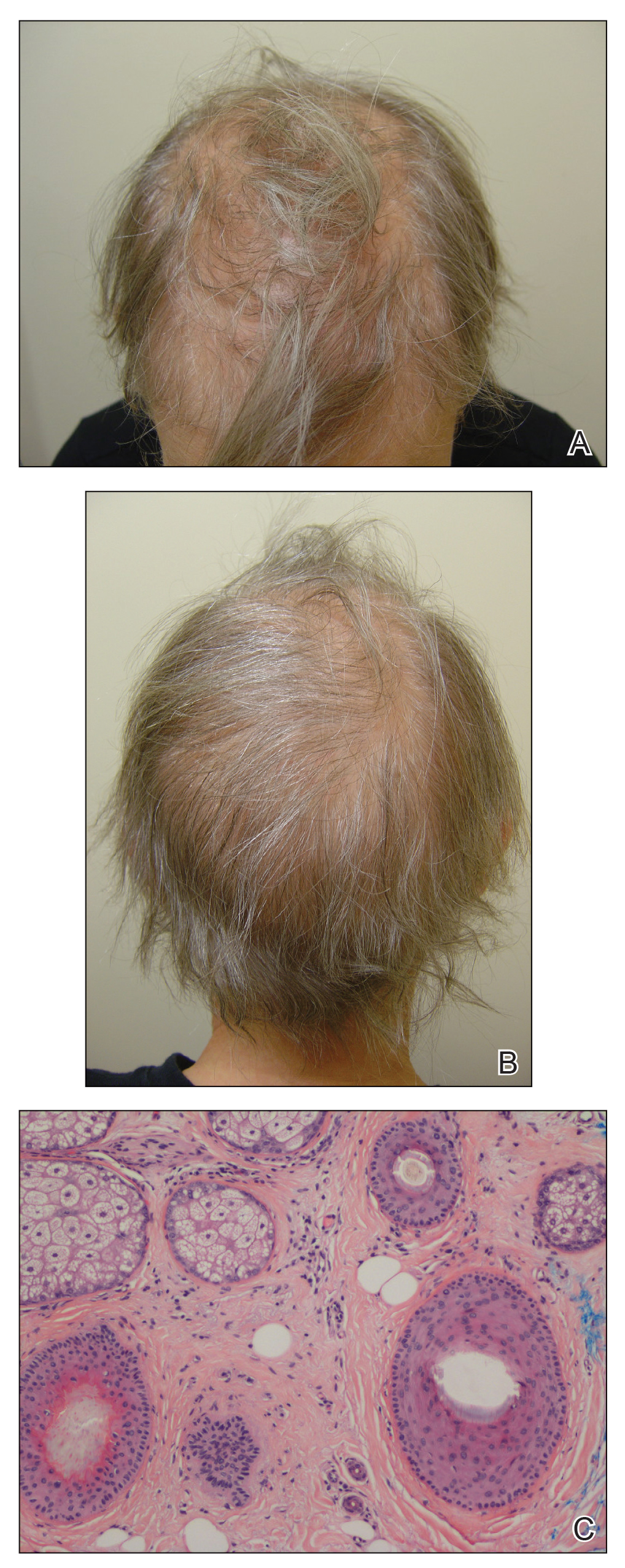
Patient 2
A 35-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 10 months after chemotherapy. She underwent 4 cycles of doxorubicin and cyclophosphamide followed by 4 cycles of paclitaxel and was started on trastuzumab. Tamoxifen was initiated 1 month after completing chemotherapy. She received radiation therapy the following month and continued trastuzumab for 1 year. At the current presentation, the patient noted that hair regrowth had started 1 month after the last course of chemotherapy but had progressed slowly. She denied body hair loss. Physical examination revealed diffuse thinning, especially over the crown, with scattered broken hairs throughout the scalp and several miniaturized hairs over the crown. She was evaluated as grade 3 on the Sinclair clinical grading scale used to evaluate female pattern hair loss (FPHL).17 Her family history was remarkable for FPHL in her maternal grandmother. She had no notable medical history, her TSH was normal, and she was taking tamoxifen and trastuzumab. Biopsy was not performed. The patient was started on minoxidil solution 2% and had mild improvement with no further broken-off hairs after 10 months. At that point, she was evaluated as grade 2 to 3 on the Sinclair scale.17
Patient 3
A 51-year-old woman with a history of papillary carcinoma and extensive ductal carcinoma in situ presented with persistent hair loss for 3.5 years following chemotherapy for recurrent breast cancer. After her initial diagnosis in the left breast, she received cyclophosphamide, methotrexate, and 5-fluorouracil but did not receive endocrine therapy. Her hair thinned during chemotherapy but returned to normal density within 1 year. She had a recurrence of the cancer in the right breast 14 years later and received 6 cycles of chemotherapy with cyclophosphamide and docetaxel followed by radiation therapy. After this course, her hair loss incompletely recovered. One year after chemotherapy, she underwent bilateral salpingo-oophorectomy and started anastrozole. Three months later, she noticed increased shedding and progressive thinning of the hair. Physical examination revealed diffuse thinning that was most pronounced over the crown. She also experienced lateral thinning of the eyebrows, decreased eyelashes, and dystrophic fingernails. Fluocinonide solution was discontinued by the patient due to scalp burning. She had a brother with bitemporal recession. Her medical history was notable for Hashimoto thyroiditis, vitamin D deficiency, and peripheral neuropathy. Her TSH occasionally was elevated, and she was intermittently on levothyroxine; however, her free T4 was maintained within reference range on all records. Her medications at the time of evaluation were anastrozole and gabapentin. Biopsies taken from the right and left temporal scalp revealed decreased follicle density with a majority of follicles in anagen, scattered miniaturized follicles, and a mild perivascular and perifollicular lymphoid infiltrate. Mild dermal fibrosis was present without evidence of frank scarring (Figure 2). She declined treatment, and there was no change in her condition over 3 years of follow-up.
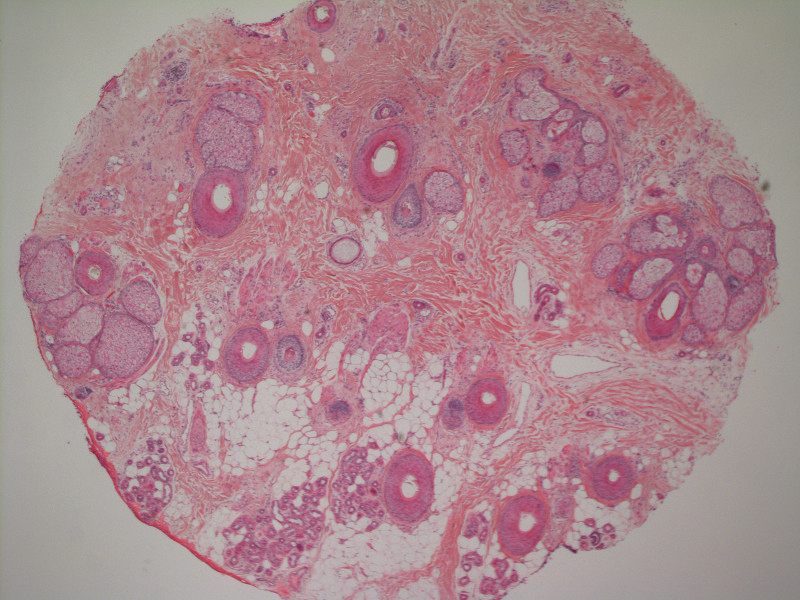
Comment
Classification of Chemotherapy-Induced Hair Loss
Chemotherapy-induced alopecia is typically an anagen effluvium that is reversed within 6 months following the final course of chemotherapy. When incomplete regrowth persists, the patient is considered to have PCIA.1 The pathophysiology of PCIA is unclear.
Traditional grading for chemotherapy-induced alopecia does not account for the patterns of loss seen in PCIA, of which the most common appears to be a female pattern with accentuated hair loss in androgen-dependent regions of the scalp.18 Other patterns include a diffuse type with body hair loss, patchy alopecia, and complete alopecia with or without body hair loss (Table).3-8 Whether these patterns all can be attributed to chemotherapy remains to be explored.
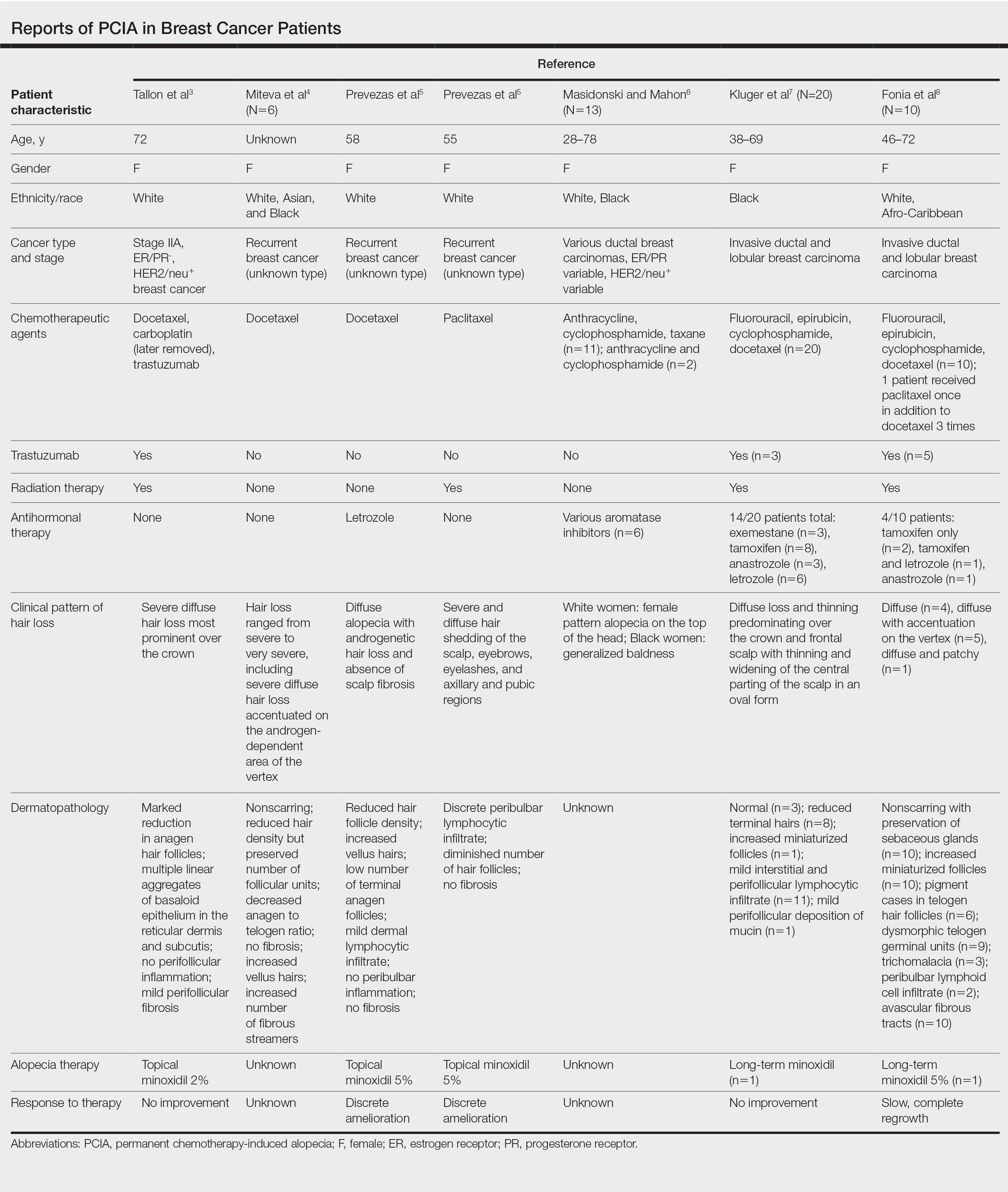
Breast Cancer Therapies Causing PCIA
The main agents thought to be responsible for PCIA in breast cancer patients are taxanes. The role of endocrine therapies has not been well explored. Trastuzumab lacks several of the common side effects of chemotherapy due to its specificity for the HER2/neu receptor and has not been found to increase the rate of hair loss when combined with standard chemotherapy.19,20 Although radiation therapy has the potential to damage hair follicles, and a dose-dependent relationship has been described for temporary and permanent alopecia at irradiated sites, permanent alopecia predominantly has been reported with cranial radiation used in the treatment of intracranial malignancies.21 The role of radiation therapy of the breasts in PCIA is unclear, as its inclusion in therapy has not been consistently reported in the literature.
Docetaxel is known to cause chemotherapy-induced alopecia, with an 83.4% incidence in phase 2 trials; however, it also appears to be related to PCIA.20 A PubMed search of articles indexed for MEDLINE was performed using the terms permanent chemotherapy induced alopecia, chemotherapy, docetaxel, endocrine therapies, hair loss, alopecia, and breast cancer. More than 400 cases of PCIA related to chemotherapy in breast cancer patients have been reported in the literature from a combination of case reports/series, retrospective surveys, and at least one prospective study. Data from some of the more detailed reports (n=52) are summarized in the Table. In the single-center, 3-year prospective study of women given adjuvant taxane-based or non–taxane-based chemotherapy, those who received taxane therapy were more likely to develop PCIA (odds ratio, 8.01).9
All 3 of our patients received taxanes. Interestingly, patient 3 underwent 2 rounds of chemotherapy 14 years apart and experienced full regrowth of the hair after the first course of taxane-free chemotherapy but experienced persistent hair loss following docetaxel treatment. Adjuvant endocrine therapies also may contribute to PCIA. A review of the side effects of endocrine therapies revealed an incidence of alopecia that was higher than expected; tamoxifen was the greatest offender. Additionally, using endocrine treatments in combination was found to have a synergistic effect on alopecia.18 Adjuvant endocrine therapy was used in patients 2 and 3. Although endocrine therapies appear to have a milder effect on hair loss compared to chemotherapy, these medications are continued for a longer duration, potentially contributing to the severity of hair loss and prolonging the time to regrowth.
Furthermore, endocrine therapies used in breast cancer treatment decrease estrogen levels or antagonize estrogen receptors, creating an environment of relative hyperandrogenism that may contribute to FPHL in genetically susceptible women.18 Although taxanes may cause irreversible hair loss in these patients, the action of endocrine therapies on the remaining hair follicles may affect the typical female pattern seen clinically. Patients 2 and 3 who presented with FPHL received adjuvant endocrine therapies and had positive family history, while patient 1 did not. Of note, patient 3 experienced worsening hair loss following the addition of anastrozole, which suggests a contribution of endocrine therapy to her PCIA. Our limited cases do not allow for evaluation of a worsened outcome with the combination of taxanes and endocrine therapies; however, we suggest further evaluation for a synergistic effect that may be contributing to PCIA.
Conclusion
Permanent alopecia in breast cancer patients appears to be a true potential adverse effect of taxanes and endocrine therapies, and it is important to characterize it appropriately so that its mechanism can be understood and appropriate treatment and counseling can take place. Although it may not influence clinical decision-making, patients should be informed that hair loss with chemotherapy can be permanent. Treatment with scalp cooling can reduce the risk for severe chemotherapy-induced alopecia, but it is unclear if it reduces risk for PCIA.12,15 Topical or oral minoxidil may be helpful in the treatment of PCIA once it has developed.7,8,15,22 Better characterization of these cases may elucidate risk factors for developing permanent alopecia, allowing for more appropriate risk stratification, counseling, and treatment.
- Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562-570.
- Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979-982.
- Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333-336.
- Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345-350.
- Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883-885.
- Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13-14.
- Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879-2884.
- Fonia A, Cota C, Setterfield JF, et al. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76:948-957.
- Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study [published online August 17, 2018]. Oncologist. 2019;24:414-420.
- Chan J, Adderley H, Alameddine M, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres [published online December 22, 2020]. Eur J Cancer Care (Engl). doi:10.1111/ecc.13395
- Bourgeois H, Denis F, Kerbrat P, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69(24 suppl). doi:10.1158/0008-5472.SABCS-09-3174
- Bertrand M, Mailliez A, Vercambre S, et al. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: long term follow up. Cancer Res. 2013;73(24 suppl). doi:10.1158/0008-5472.SABCS13-P3-09-15
- Kim S, Park HS, Kim JY, et al. Irreversible chemotherapy-induced alopecia in breast cancer patient. Cancer Res. 2016;76(4 suppl). doi:10.1158/1538-7445.SABCS15-P1-15-04
- Thorp NJ, Swift F, Arundell D, et al. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Res. 2015;75(9 suppl). doi:10.1158/1538-7445.SABCS14-P5-17-04
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80:1199-1213.
- Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155:724-728.
- Sinclair R, Jolley D, Mallari R, et al. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189-199.
- Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126-1134.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Baselga J. Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol. 2000;27(5 suppl 9):20-26.
- Lawenda BD, Gagne HM, Gierga DP, et al. Permanent alopecia after cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys. 2004;60:879-887.
- Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil [published online May 13, 2015]. Australas J Dermatol. 2016;57:E130-E132.
Anagen effluvium during chemotherapy is common, typically beginning within 1 month of treatment onset and resolving by 6 months after the final course.1 Permanent chemotherapy-induced alopecia (PCIA), in which hair loss persists beyond 6 months after chemotherapy without recovery to original density, was first reported in patients following high-dose chemotherapy regimens for allogeneic bone marrow transplantation.2 There are now increasing reports of PCIA in patients with breast cancer; at least 400 such cases have been documented.3-16 In addition to chemotherapy, patients often receive adjuvant endocrine therapy with selective estrogen receptor modulators, aromatase inhibitors, or gonadotropin-releasing hormone agonists.5-16 Endocrine therapies also can lead to alopecia, but their role in PCIA has not been well defined.15,16 We describe 3 patients with breast cancer who experienced PCIA following chemotherapy with taxanes with or without endocrine therapies. We also review the literature on non–bone marrow transplantation PCIA to better characterize this entity and explore the role of endocrine therapies in PCIA.
Case Reports
Patient 1
A 62-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 5 years after completing chemotherapy. She underwent 6 cycles of docetaxel and carboplatin along with radiation therapy as well as 1 year of trastuzumab and did not receive endocrine therapy. At the current presentation, she reported patchy hair regrowth that gradually filled in but failed to return to full density. Physical examination revealed the hair was diffusely thin, especially bitemporally (Figures 1A and 1B), and she did not experience any loss of body hair. She had no family history of hair loss. Her medical history was notable for hypertension, chronic obstructive bronchitis, osteopenia, and depression. Her thyroid stimulating hormone (TSH) level was within reference range. Medications included lisinopril, metoprolol, escitalopram, and trazodone. A biopsy from the occipital scalp showed nonscarring alopecia with variation of hair follicle size, a decreased number of hair follicles, and a decreased anagen to telogen ratio (Figure 1C). She was treated with clobetasol solution and minoxidil solution 5% for 1 year with mild improvement. She experienced no further hair loss but did not regain original hair density.

Patient 2
A 35-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 10 months after chemotherapy. She underwent 4 cycles of doxorubicin and cyclophosphamide followed by 4 cycles of paclitaxel and was started on trastuzumab. Tamoxifen was initiated 1 month after completing chemotherapy. She received radiation therapy the following month and continued trastuzumab for 1 year. At the current presentation, the patient noted that hair regrowth had started 1 month after the last course of chemotherapy but had progressed slowly. She denied body hair loss. Physical examination revealed diffuse thinning, especially over the crown, with scattered broken hairs throughout the scalp and several miniaturized hairs over the crown. She was evaluated as grade 3 on the Sinclair clinical grading scale used to evaluate female pattern hair loss (FPHL).17 Her family history was remarkable for FPHL in her maternal grandmother. She had no notable medical history, her TSH was normal, and she was taking tamoxifen and trastuzumab. Biopsy was not performed. The patient was started on minoxidil solution 2% and had mild improvement with no further broken-off hairs after 10 months. At that point, she was evaluated as grade 2 to 3 on the Sinclair scale.17
Patient 3
A 51-year-old woman with a history of papillary carcinoma and extensive ductal carcinoma in situ presented with persistent hair loss for 3.5 years following chemotherapy for recurrent breast cancer. After her initial diagnosis in the left breast, she received cyclophosphamide, methotrexate, and 5-fluorouracil but did not receive endocrine therapy. Her hair thinned during chemotherapy but returned to normal density within 1 year. She had a recurrence of the cancer in the right breast 14 years later and received 6 cycles of chemotherapy with cyclophosphamide and docetaxel followed by radiation therapy. After this course, her hair loss incompletely recovered. One year after chemotherapy, she underwent bilateral salpingo-oophorectomy and started anastrozole. Three months later, she noticed increased shedding and progressive thinning of the hair. Physical examination revealed diffuse thinning that was most pronounced over the crown. She also experienced lateral thinning of the eyebrows, decreased eyelashes, and dystrophic fingernails. Fluocinonide solution was discontinued by the patient due to scalp burning. She had a brother with bitemporal recession. Her medical history was notable for Hashimoto thyroiditis, vitamin D deficiency, and peripheral neuropathy. Her TSH occasionally was elevated, and she was intermittently on levothyroxine; however, her free T4 was maintained within reference range on all records. Her medications at the time of evaluation were anastrozole and gabapentin. Biopsies taken from the right and left temporal scalp revealed decreased follicle density with a majority of follicles in anagen, scattered miniaturized follicles, and a mild perivascular and perifollicular lymphoid infiltrate. Mild dermal fibrosis was present without evidence of frank scarring (Figure 2). She declined treatment, and there was no change in her condition over 3 years of follow-up.

Comment
Classification of Chemotherapy-Induced Hair Loss
Chemotherapy-induced alopecia is typically an anagen effluvium that is reversed within 6 months following the final course of chemotherapy. When incomplete regrowth persists, the patient is considered to have PCIA.1 The pathophysiology of PCIA is unclear.
Traditional grading for chemotherapy-induced alopecia does not account for the patterns of loss seen in PCIA, of which the most common appears to be a female pattern with accentuated hair loss in androgen-dependent regions of the scalp.18 Other patterns include a diffuse type with body hair loss, patchy alopecia, and complete alopecia with or without body hair loss (Table).3-8 Whether these patterns all can be attributed to chemotherapy remains to be explored.

Breast Cancer Therapies Causing PCIA
The main agents thought to be responsible for PCIA in breast cancer patients are taxanes. The role of endocrine therapies has not been well explored. Trastuzumab lacks several of the common side effects of chemotherapy due to its specificity for the HER2/neu receptor and has not been found to increase the rate of hair loss when combined with standard chemotherapy.19,20 Although radiation therapy has the potential to damage hair follicles, and a dose-dependent relationship has been described for temporary and permanent alopecia at irradiated sites, permanent alopecia predominantly has been reported with cranial radiation used in the treatment of intracranial malignancies.21 The role of radiation therapy of the breasts in PCIA is unclear, as its inclusion in therapy has not been consistently reported in the literature.
Docetaxel is known to cause chemotherapy-induced alopecia, with an 83.4% incidence in phase 2 trials; however, it also appears to be related to PCIA.20 A PubMed search of articles indexed for MEDLINE was performed using the terms permanent chemotherapy induced alopecia, chemotherapy, docetaxel, endocrine therapies, hair loss, alopecia, and breast cancer. More than 400 cases of PCIA related to chemotherapy in breast cancer patients have been reported in the literature from a combination of case reports/series, retrospective surveys, and at least one prospective study. Data from some of the more detailed reports (n=52) are summarized in the Table. In the single-center, 3-year prospective study of women given adjuvant taxane-based or non–taxane-based chemotherapy, those who received taxane therapy were more likely to develop PCIA (odds ratio, 8.01).9
All 3 of our patients received taxanes. Interestingly, patient 3 underwent 2 rounds of chemotherapy 14 years apart and experienced full regrowth of the hair after the first course of taxane-free chemotherapy but experienced persistent hair loss following docetaxel treatment. Adjuvant endocrine therapies also may contribute to PCIA. A review of the side effects of endocrine therapies revealed an incidence of alopecia that was higher than expected; tamoxifen was the greatest offender. Additionally, using endocrine treatments in combination was found to have a synergistic effect on alopecia.18 Adjuvant endocrine therapy was used in patients 2 and 3. Although endocrine therapies appear to have a milder effect on hair loss compared to chemotherapy, these medications are continued for a longer duration, potentially contributing to the severity of hair loss and prolonging the time to regrowth.
Furthermore, endocrine therapies used in breast cancer treatment decrease estrogen levels or antagonize estrogen receptors, creating an environment of relative hyperandrogenism that may contribute to FPHL in genetically susceptible women.18 Although taxanes may cause irreversible hair loss in these patients, the action of endocrine therapies on the remaining hair follicles may affect the typical female pattern seen clinically. Patients 2 and 3 who presented with FPHL received adjuvant endocrine therapies and had positive family history, while patient 1 did not. Of note, patient 3 experienced worsening hair loss following the addition of anastrozole, which suggests a contribution of endocrine therapy to her PCIA. Our limited cases do not allow for evaluation of a worsened outcome with the combination of taxanes and endocrine therapies; however, we suggest further evaluation for a synergistic effect that may be contributing to PCIA.
Conclusion
Permanent alopecia in breast cancer patients appears to be a true potential adverse effect of taxanes and endocrine therapies, and it is important to characterize it appropriately so that its mechanism can be understood and appropriate treatment and counseling can take place. Although it may not influence clinical decision-making, patients should be informed that hair loss with chemotherapy can be permanent. Treatment with scalp cooling can reduce the risk for severe chemotherapy-induced alopecia, but it is unclear if it reduces risk for PCIA.12,15 Topical or oral minoxidil may be helpful in the treatment of PCIA once it has developed.7,8,15,22 Better characterization of these cases may elucidate risk factors for developing permanent alopecia, allowing for more appropriate risk stratification, counseling, and treatment.
Anagen effluvium during chemotherapy is common, typically beginning within 1 month of treatment onset and resolving by 6 months after the final course.1 Permanent chemotherapy-induced alopecia (PCIA), in which hair loss persists beyond 6 months after chemotherapy without recovery to original density, was first reported in patients following high-dose chemotherapy regimens for allogeneic bone marrow transplantation.2 There are now increasing reports of PCIA in patients with breast cancer; at least 400 such cases have been documented.3-16 In addition to chemotherapy, patients often receive adjuvant endocrine therapy with selective estrogen receptor modulators, aromatase inhibitors, or gonadotropin-releasing hormone agonists.5-16 Endocrine therapies also can lead to alopecia, but their role in PCIA has not been well defined.15,16 We describe 3 patients with breast cancer who experienced PCIA following chemotherapy with taxanes with or without endocrine therapies. We also review the literature on non–bone marrow transplantation PCIA to better characterize this entity and explore the role of endocrine therapies in PCIA.
Case Reports
Patient 1
A 62-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 5 years after completing chemotherapy. She underwent 6 cycles of docetaxel and carboplatin along with radiation therapy as well as 1 year of trastuzumab and did not receive endocrine therapy. At the current presentation, she reported patchy hair regrowth that gradually filled in but failed to return to full density. Physical examination revealed the hair was diffusely thin, especially bitemporally (Figures 1A and 1B), and she did not experience any loss of body hair. She had no family history of hair loss. Her medical history was notable for hypertension, chronic obstructive bronchitis, osteopenia, and depression. Her thyroid stimulating hormone (TSH) level was within reference range. Medications included lisinopril, metoprolol, escitalopram, and trazodone. A biopsy from the occipital scalp showed nonscarring alopecia with variation of hair follicle size, a decreased number of hair follicles, and a decreased anagen to telogen ratio (Figure 1C). She was treated with clobetasol solution and minoxidil solution 5% for 1 year with mild improvement. She experienced no further hair loss but did not regain original hair density.

Patient 2
A 35-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 10 months after chemotherapy. She underwent 4 cycles of doxorubicin and cyclophosphamide followed by 4 cycles of paclitaxel and was started on trastuzumab. Tamoxifen was initiated 1 month after completing chemotherapy. She received radiation therapy the following month and continued trastuzumab for 1 year. At the current presentation, the patient noted that hair regrowth had started 1 month after the last course of chemotherapy but had progressed slowly. She denied body hair loss. Physical examination revealed diffuse thinning, especially over the crown, with scattered broken hairs throughout the scalp and several miniaturized hairs over the crown. She was evaluated as grade 3 on the Sinclair clinical grading scale used to evaluate female pattern hair loss (FPHL).17 Her family history was remarkable for FPHL in her maternal grandmother. She had no notable medical history, her TSH was normal, and she was taking tamoxifen and trastuzumab. Biopsy was not performed. The patient was started on minoxidil solution 2% and had mild improvement with no further broken-off hairs after 10 months. At that point, she was evaluated as grade 2 to 3 on the Sinclair scale.17
Patient 3
A 51-year-old woman with a history of papillary carcinoma and extensive ductal carcinoma in situ presented with persistent hair loss for 3.5 years following chemotherapy for recurrent breast cancer. After her initial diagnosis in the left breast, she received cyclophosphamide, methotrexate, and 5-fluorouracil but did not receive endocrine therapy. Her hair thinned during chemotherapy but returned to normal density within 1 year. She had a recurrence of the cancer in the right breast 14 years later and received 6 cycles of chemotherapy with cyclophosphamide and docetaxel followed by radiation therapy. After this course, her hair loss incompletely recovered. One year after chemotherapy, she underwent bilateral salpingo-oophorectomy and started anastrozole. Three months later, she noticed increased shedding and progressive thinning of the hair. Physical examination revealed diffuse thinning that was most pronounced over the crown. She also experienced lateral thinning of the eyebrows, decreased eyelashes, and dystrophic fingernails. Fluocinonide solution was discontinued by the patient due to scalp burning. She had a brother with bitemporal recession. Her medical history was notable for Hashimoto thyroiditis, vitamin D deficiency, and peripheral neuropathy. Her TSH occasionally was elevated, and she was intermittently on levothyroxine; however, her free T4 was maintained within reference range on all records. Her medications at the time of evaluation were anastrozole and gabapentin. Biopsies taken from the right and left temporal scalp revealed decreased follicle density with a majority of follicles in anagen, scattered miniaturized follicles, and a mild perivascular and perifollicular lymphoid infiltrate. Mild dermal fibrosis was present without evidence of frank scarring (Figure 2). She declined treatment, and there was no change in her condition over 3 years of follow-up.

Comment
Classification of Chemotherapy-Induced Hair Loss
Chemotherapy-induced alopecia is typically an anagen effluvium that is reversed within 6 months following the final course of chemotherapy. When incomplete regrowth persists, the patient is considered to have PCIA.1 The pathophysiology of PCIA is unclear.
Traditional grading for chemotherapy-induced alopecia does not account for the patterns of loss seen in PCIA, of which the most common appears to be a female pattern with accentuated hair loss in androgen-dependent regions of the scalp.18 Other patterns include a diffuse type with body hair loss, patchy alopecia, and complete alopecia with or without body hair loss (Table).3-8 Whether these patterns all can be attributed to chemotherapy remains to be explored.

Breast Cancer Therapies Causing PCIA
The main agents thought to be responsible for PCIA in breast cancer patients are taxanes. The role of endocrine therapies has not been well explored. Trastuzumab lacks several of the common side effects of chemotherapy due to its specificity for the HER2/neu receptor and has not been found to increase the rate of hair loss when combined with standard chemotherapy.19,20 Although radiation therapy has the potential to damage hair follicles, and a dose-dependent relationship has been described for temporary and permanent alopecia at irradiated sites, permanent alopecia predominantly has been reported with cranial radiation used in the treatment of intracranial malignancies.21 The role of radiation therapy of the breasts in PCIA is unclear, as its inclusion in therapy has not been consistently reported in the literature.
Docetaxel is known to cause chemotherapy-induced alopecia, with an 83.4% incidence in phase 2 trials; however, it also appears to be related to PCIA.20 A PubMed search of articles indexed for MEDLINE was performed using the terms permanent chemotherapy induced alopecia, chemotherapy, docetaxel, endocrine therapies, hair loss, alopecia, and breast cancer. More than 400 cases of PCIA related to chemotherapy in breast cancer patients have been reported in the literature from a combination of case reports/series, retrospective surveys, and at least one prospective study. Data from some of the more detailed reports (n=52) are summarized in the Table. In the single-center, 3-year prospective study of women given adjuvant taxane-based or non–taxane-based chemotherapy, those who received taxane therapy were more likely to develop PCIA (odds ratio, 8.01).9
All 3 of our patients received taxanes. Interestingly, patient 3 underwent 2 rounds of chemotherapy 14 years apart and experienced full regrowth of the hair after the first course of taxane-free chemotherapy but experienced persistent hair loss following docetaxel treatment. Adjuvant endocrine therapies also may contribute to PCIA. A review of the side effects of endocrine therapies revealed an incidence of alopecia that was higher than expected; tamoxifen was the greatest offender. Additionally, using endocrine treatments in combination was found to have a synergistic effect on alopecia.18 Adjuvant endocrine therapy was used in patients 2 and 3. Although endocrine therapies appear to have a milder effect on hair loss compared to chemotherapy, these medications are continued for a longer duration, potentially contributing to the severity of hair loss and prolonging the time to regrowth.
Furthermore, endocrine therapies used in breast cancer treatment decrease estrogen levels or antagonize estrogen receptors, creating an environment of relative hyperandrogenism that may contribute to FPHL in genetically susceptible women.18 Although taxanes may cause irreversible hair loss in these patients, the action of endocrine therapies on the remaining hair follicles may affect the typical female pattern seen clinically. Patients 2 and 3 who presented with FPHL received adjuvant endocrine therapies and had positive family history, while patient 1 did not. Of note, patient 3 experienced worsening hair loss following the addition of anastrozole, which suggests a contribution of endocrine therapy to her PCIA. Our limited cases do not allow for evaluation of a worsened outcome with the combination of taxanes and endocrine therapies; however, we suggest further evaluation for a synergistic effect that may be contributing to PCIA.
Conclusion
Permanent alopecia in breast cancer patients appears to be a true potential adverse effect of taxanes and endocrine therapies, and it is important to characterize it appropriately so that its mechanism can be understood and appropriate treatment and counseling can take place. Although it may not influence clinical decision-making, patients should be informed that hair loss with chemotherapy can be permanent. Treatment with scalp cooling can reduce the risk for severe chemotherapy-induced alopecia, but it is unclear if it reduces risk for PCIA.12,15 Topical or oral minoxidil may be helpful in the treatment of PCIA once it has developed.7,8,15,22 Better characterization of these cases may elucidate risk factors for developing permanent alopecia, allowing for more appropriate risk stratification, counseling, and treatment.
- Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562-570.
- Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979-982.
- Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333-336.
- Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345-350.
- Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883-885.
- Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13-14.
- Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879-2884.
- Fonia A, Cota C, Setterfield JF, et al. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76:948-957.
- Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study [published online August 17, 2018]. Oncologist. 2019;24:414-420.
- Chan J, Adderley H, Alameddine M, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres [published online December 22, 2020]. Eur J Cancer Care (Engl). doi:10.1111/ecc.13395
- Bourgeois H, Denis F, Kerbrat P, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69(24 suppl). doi:10.1158/0008-5472.SABCS-09-3174
- Bertrand M, Mailliez A, Vercambre S, et al. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: long term follow up. Cancer Res. 2013;73(24 suppl). doi:10.1158/0008-5472.SABCS13-P3-09-15
- Kim S, Park HS, Kim JY, et al. Irreversible chemotherapy-induced alopecia in breast cancer patient. Cancer Res. 2016;76(4 suppl). doi:10.1158/1538-7445.SABCS15-P1-15-04
- Thorp NJ, Swift F, Arundell D, et al. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Res. 2015;75(9 suppl). doi:10.1158/1538-7445.SABCS14-P5-17-04
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80:1199-1213.
- Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155:724-728.
- Sinclair R, Jolley D, Mallari R, et al. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189-199.
- Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126-1134.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Baselga J. Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol. 2000;27(5 suppl 9):20-26.
- Lawenda BD, Gagne HM, Gierga DP, et al. Permanent alopecia after cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys. 2004;60:879-887.
- Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil [published online May 13, 2015]. Australas J Dermatol. 2016;57:E130-E132.
- Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562-570.
- Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979-982.
- Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333-336.
- Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345-350.
- Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883-885.
- Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13-14.
- Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879-2884.
- Fonia A, Cota C, Setterfield JF, et al. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76:948-957.
- Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study [published online August 17, 2018]. Oncologist. 2019;24:414-420.
- Chan J, Adderley H, Alameddine M, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres [published online December 22, 2020]. Eur J Cancer Care (Engl). doi:10.1111/ecc.13395
- Bourgeois H, Denis F, Kerbrat P, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69(24 suppl). doi:10.1158/0008-5472.SABCS-09-3174
- Bertrand M, Mailliez A, Vercambre S, et al. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: long term follow up. Cancer Res. 2013;73(24 suppl). doi:10.1158/0008-5472.SABCS13-P3-09-15
- Kim S, Park HS, Kim JY, et al. Irreversible chemotherapy-induced alopecia in breast cancer patient. Cancer Res. 2016;76(4 suppl). doi:10.1158/1538-7445.SABCS15-P1-15-04
- Thorp NJ, Swift F, Arundell D, et al. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Res. 2015;75(9 suppl). doi:10.1158/1538-7445.SABCS14-P5-17-04
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80:1199-1213.
- Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155:724-728.
- Sinclair R, Jolley D, Mallari R, et al. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189-199.
- Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126-1134.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Baselga J. Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol. 2000;27(5 suppl 9):20-26.
- Lawenda BD, Gagne HM, Gierga DP, et al. Permanent alopecia after cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys. 2004;60:879-887.
- Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil [published online May 13, 2015]. Australas J Dermatol. 2016;57:E130-E132.
Practice Points
- Permanent chemotherapy-induced alopecia (PCIA) is defined as hair loss that persists beyond 6 months after treatment with chemotherapy. It may be complicated by the addition of endocrine therapies.
- Patients and clinicians should be aware that PCIA can occur and appears to be a higher risk with taxane therapy.
The cutaneous benefits of bee venom, Part II: Acupuncture, wound healing, and various potential indications
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
Are There Mobile Applications Related to Nail Disorders?
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.
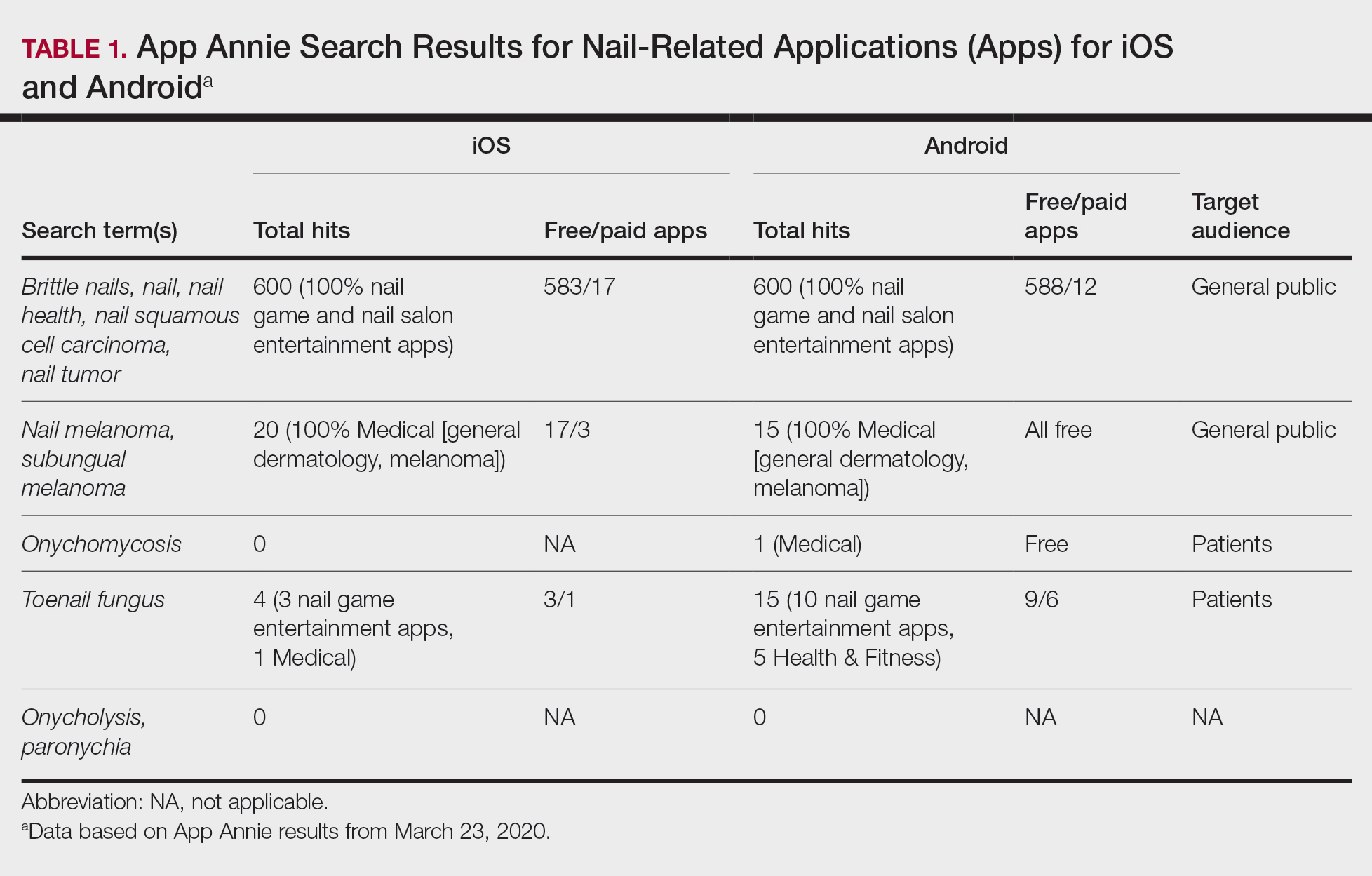
The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for
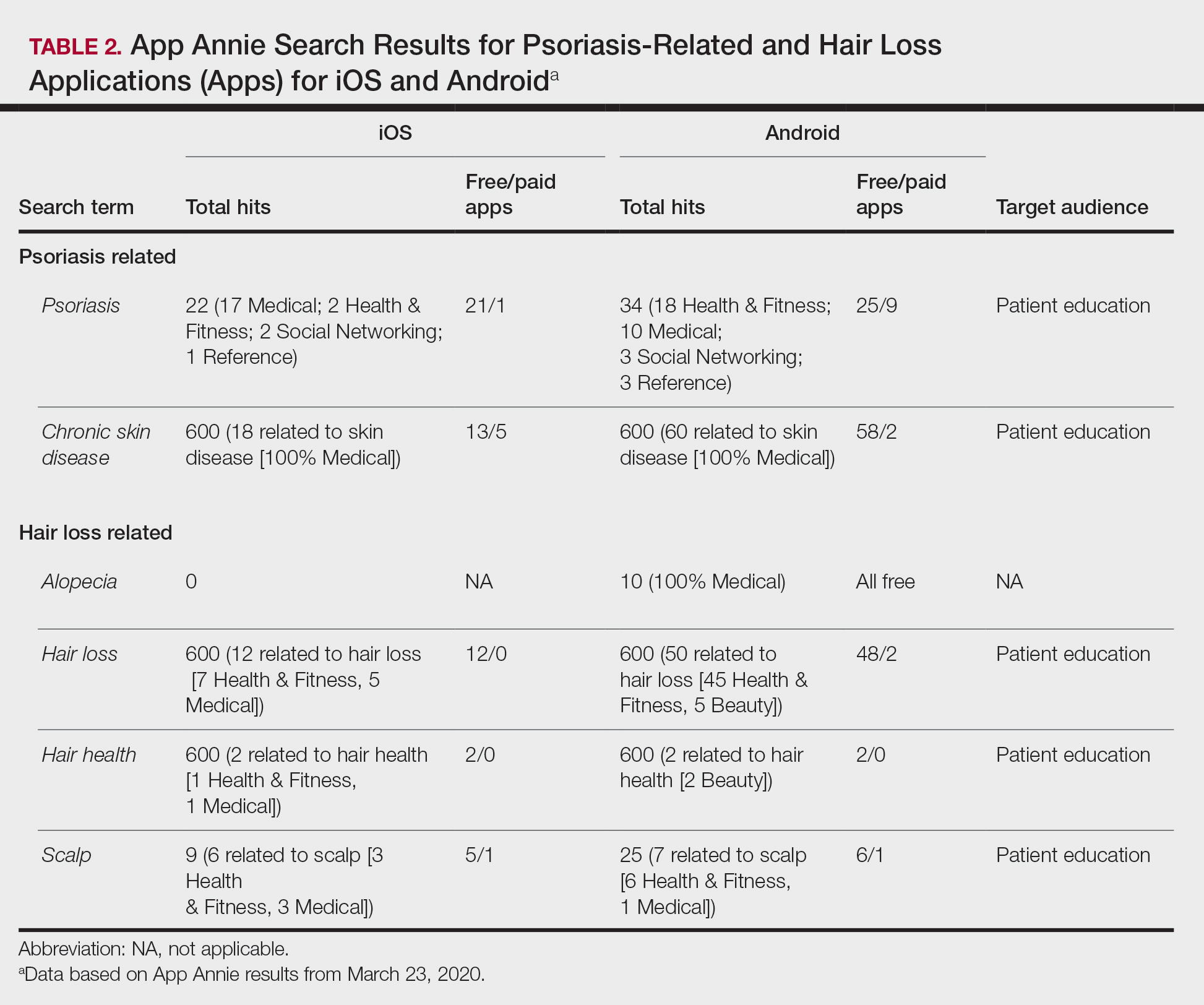
Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.

The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for

Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
The use of mobile devices in health care settings has enhanced clinical practice through real-time communication and direct patient monitoring.1 With advancements in technology, improving the accessibility and quality of patient care using mobile devices is a hot topic. In 2018, 261.34 million people worldwide used smartphones compared to 280.54 million in 2021—a 7.3% increase.2 Revenue from sales of mobile applications (apps) is projected to reach $693 billion in 2021.3
A range of apps targeted to patients is available for acne, melanoma, and teledermatology.4-6 Nail disorders are a common concern, representing 21.1 million outpatient visits in 2007 to 2016,7 but, to date, the availability of apps related to nail disorders has not been explored. In this study, we investigated iOS (Apple’s iPhone Operating System) and Android apps to determine the types of nail health apps that are available, using psoriasis and hair loss apps as comparator groups.
Methods
A standard app analytics and market data tool (App Annie; https://www.appannie.com/en/) was utilized to search for iOS and Android nail mobile apps.4,5 The analysis was performed on a single day (March 23, 2020), given that app searches can change on a daily basis. Our search included the following keywords:
Results
Nail-Related Apps
Using keywords for nail-related terms on iOS and Android platforms, our search returned few specific and informative apps related to nail disorders (Table 1). When the terms brittle nails, nail, nail health, nail squamous cell carcinoma, and nail tumor were searched, all available nail apps were either nail games or virtual nail salons for entertainment purposes. For the terms nail melanoma and subungual melanoma, there were no specific nail apps that appeared in the search results; rather, the App Annie search yielded only general dermatology and melanoma apps. The terms onycholysis and paronychia both yielded 0 hits for iOS and Android.

The only search terms that returned specific nail apps were onychomycosis and toenail fungus. Initially, when onychomycosis was searched, only 1 Google Play Medical category app was found: “Nail fungal infection (model onychomycosis).” Although this app recently was removed from the app store, it previously allowed the user to upload a nail photograph, with which a computing algorithm assessed whether the presentation was a fungal nail infection. Toenail fungus returned 1 iOS Medical category app and 5 Android Health & Fitness category apps with reference material for patients. Neither of the 2 medical apps for onychomycosis and toenail fungus referenced a physician involved in the app development.
Psoriasis Comparator
On the contrary, a search for

Hair Loss Comparator
Search terms related to hair conditions—specifically, alopecia—yielded 0 apps for iOS and 10 for Android platforms (Table 2). Using the search term hair loss, 12 apps for iOS and 50 apps for Android were found within the Health & Fitness, Medical, and Beauty categories. The search terms hair health and hair loss resulted in 2 and 12 apps in both iOS and Android, respectively. In addition, the search term scalp was associated with 6 related apps in iOS and 7 in Android, both in the Health & Fitness and Medical categories.
Other Findings
Most apps for psoriasis and hair health were identified as patient focused. Although iOS and Android are different operating systems, some health apps overlapped: subungual melanoma and toenail fungus had a 20% overlap; psoriasis, 19%; chronic skin disease, 2%; alopecia, 0%; hair loss and hair health, 10%; and scalp, 18%. iOS and Android nail entertainment games had approximately a 30% overlap. Tables 1 and 2 also compare the number of free and paid apps; most available apps were free.
Comment
With continued growth in mobile device ownership and app development has been parallel growth in the creation and use of apps to enhance medical care.1 In a study analyzing the most popular dermatology apps, 62% (18/29) and 38% (11/29) of apps targeted patients and physicians, respectively.6 Our study showed that (1) there are few nail disorder apps available for patient education and (2) there is no evidence that a physician was consulted for content input. Because patients who can effectively communicate their health concerns before and after seeing a physician have better self-reported clinical outcomes,9 it is important to have nail disorder apps available to patients for referencing. The nail health app options differ notably from psoriasis and hair loss apps, with apps for the latter 2 topics found in Medical and Health & Fitness categories—targeting patients who seek immediate access to health care and education.
Although there are several general dermatology apps that contain reference information for patients pertaining to nail conditions,6 using any of those apps would require a patient to have prior knowledge that dermatologists specialize in nail disorders and necessitate several steps to find nail-relevant information. For example, the patient would have to search dermatology in the iOS and Android app stores, select the available app (eg, Dermatology Database), and then search within that app for nail disorders. Therefore, a patient who is concerned about a possible subungual melanoma would not be able to easily find clinical images and explanations using an app.
Study Limitations
This study was subject to several limitations. Android and iOS app stores have undisclosed computing algorithms that might have filtered apps based on specific word inquiry. Also, our queries were based on specific relevant keywords for nail conditions, psoriasis, and hair loss; use of different keywords might have yielded different results. Additionally, app options change on a daily basis, so a search today (ie, any given day) might yield slightly different results than it did on March 23, 2020.
Conclusion
Specific nail disorder apps available for patient reference are limited. App developers should consider accessibility (ie, clear language, ease of use, cost-effectiveness, usability on iOS- and Android-operated devices) and content (accurate medical information from experts) when considering new apps. A solution to this problem is for established medical organizations to create nail disorder apps specifically for patients.10 For example, the American Academy of Dermatology has iOS and Android apps that are relevant to physicians (MyDermPath+, Dialogues in Dermatology, Mohs Surgery Appropriate Use Criteria) but no comparable apps for patients; patient-appropriate nail apps are necessary.11 In addition, it would be beneficial to patients if established app companies consulted with dermatologists on pertinent nail content.
In sum, we found few available nail health apps on the iOS or Android platforms that provided accessible and timely information to patients regarding nail disorders. There is an immediate need to produce apps related to nail health for appropriate patient education.
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
- Wallace S, Clark M, White J. ‘It’s on my iPhone’: attitudes to the use of mobile computing devices in medical education, a mixed-methods study. BMJ Open. 2012;2:e001099.
- O’Dea S. Number of smartphone users in the United States from 2018 to 2024 (in millions). Statista website. April 21, 2020. Accessed February 19, 2021. https://www.statista.com/statistics/201182/forecast-of-smartphone-users-in-the-us/
- Clement J. Worldwide mobile app revenues in 2014 to 2023. Statista website. Published February 4, 2021. Accessed February 19, 2021.https://www.statista.com/statistics/269025/worldwide-mobile-app-revenue-forecast/
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center Washington DC. Published June 19, 2018. Accessed February 19, 2021. https://www.pewresearch.org/global/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018 February;24:1-4. Accessed February 19, 2021. https://escholarship.org/uc/item/3hs7n9z6
- Tongdee E, Markowitz O. Mobile app rankings in dermatology. Cutis. 2018;102:252-256.
- Lipner SR, Hancock J, Fleischer AB. The ambulatory care burden of nail conditions in the United States [published online October 21, 2019]. J Dermatol Treat. doi:10.1080/09546634.2019
- Gu L, Lipner SR. Analysis of education on nail conditions at the American Academy of Dermatology annual meetings. Cutis. 2020;105:259-260.
- King A, Hoppe RB. “Best practice” for patient-centered communication: a narrative review. J Grad Med Educ. 2013;3:385-393.
- Larson RS. A path to better-quality mHealth apps. JMIR Mhealth Uhealth. 2018;6:E10414.
- Academy apps. American Academy of Dermatology website. Accessed February 19, 2021. https://www.aad.org/member/publications/apps
Practice Points
- Patient-targeted mobile applications (apps) might aid with clinical referencing and education.
- There are patient-directed psoriasis and hair loss apps on iOS and Android platforms, but informative apps related to nail disorders are limited.
- There is a need to develop apps related to nail health for patient education.