User login
Cohort study finds a twofold greater psoriasis risk linked to a PCOS diagnosis
PCOS is characterized by androgen elevation that can lead to insulin resistance and metabolic syndrome, which have also been associated with an increased risk of psoriasis. Previous retrospective analyses have suggested an increased risk of psoriasis associated with PCOS, and psoriasis patients with PCOS have been reported to have more severe skin lesions, compared with those who do not have PCOS.
“The incidence of psoriasis is indeed higher in the PCOS group than in the control group, and the comorbidities related to metabolic syndrome did not modify the adjusted hazard ratio,” said Ming-Li Chen, during her presentation of the study results at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. Dr. Chen is at Chung Shan Medical University in Taiwan.
The researchers analyzed 1 million randomly selected records from Taiwan’s Longitudinal Health Insurance database, a subset of the country’s National Health Insurance Program. Between 2000 and 2012, they identified a case group with at least three outpatient diagnoses or one inpatient diagnosis of PCOS; they then compared each with four patients who did not have PCOS who were matched by age and index year. The mean age in both groups was about 27 years.
The mean follow-up times were 6.99 years for 4,707 cases and 6.94 years for 18,828 controls. Comorbidities were slightly higher in the PCOS group, including asthma (6.7% vs. 4.9%; P less than .001), chronic obstructive pulmonary disease (14% vs. 11%; P less than .001), chronic liver disease (8.0% vs. 5.0%; P less than .001), diabetes mellitus (3.0% vs. 1.4%; P less than .001), hypertension (2.4% vs. 1.5%; P less than .001), hyperlipidemia (5.4% vs. 2.5%; P less than .001), depression (5.4% vs. 3.9%; P less than .001), and sleep apnea (0.23% vs. 0.10%; P = .040).
There was a higher cumulative incidence of psoriasis in the PCOS group (adjusted hazard ratio, 2.07; 95% confidence interval, 1.25-3.44). Other factors associated with increased risk of psoriasis were advanced age (greater than 50 years old; aHR, 14.13; 95% CI, 1.8-110.7) and having a cancer diagnosis (aHR, 11.72; 95% CI, 2.87-47.9).
When PCOS patients were stratified by age, the researchers noted a higher risk of psoriasis among those 20 years or younger (aHR, 4.02; 95% CI, 1.16-13.9) than among those aged 20-50 years (aHR, 1.88; 95% CI, 1.07-3.29). Among those older than 50 years, there was no significantly increased risk, although the number of psoriasis diagnoses and population sizes were small in the latter category. Among patients with PCOS, a cancer diagnosis was not associated with a statistically significant increased risk of psoriasis.
The mechanisms underlying the association between PCOS and psoriasis should be studied further, she noted.
Following Dr. Chen’s prerecorded presentation, there was a live discussion session led by Alice Gottlieb, MD, PhD, medical director of Mount Sinai Beth Israel Dermatology, New York, and Ennio Lubrano, MD, associate professor of rheumatology at the University of Molise (Italy). Dr. Gottlieb noted that the study did not appear to account for weight in the association between PCOS and psoriasis, since heavier people are known to be at greater risk of developing psoriasis. Dr. Chen acknowledged that the study had no records of BMI or weight.
Dr. Gottlieb also wondered if treatment of PCOS led to any improvements in psoriasis in patients with the two diagnoses. “If we treat PCOS, does the psoriasis get better?” Again, the study did not address the question. “We didn’t follow up on therapies,” Dr. Chen said.
Dr. Chen reported no relevant financial disclosures. Dr. Gottlieb is a consultant, advisory board member and/or speaker for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Incyte, Janssen, Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB Pharma and Xbiotech. She has received research or educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis and Xbiotech.
PCOS is characterized by androgen elevation that can lead to insulin resistance and metabolic syndrome, which have also been associated with an increased risk of psoriasis. Previous retrospective analyses have suggested an increased risk of psoriasis associated with PCOS, and psoriasis patients with PCOS have been reported to have more severe skin lesions, compared with those who do not have PCOS.
“The incidence of psoriasis is indeed higher in the PCOS group than in the control group, and the comorbidities related to metabolic syndrome did not modify the adjusted hazard ratio,” said Ming-Li Chen, during her presentation of the study results at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. Dr. Chen is at Chung Shan Medical University in Taiwan.
The researchers analyzed 1 million randomly selected records from Taiwan’s Longitudinal Health Insurance database, a subset of the country’s National Health Insurance Program. Between 2000 and 2012, they identified a case group with at least three outpatient diagnoses or one inpatient diagnosis of PCOS; they then compared each with four patients who did not have PCOS who were matched by age and index year. The mean age in both groups was about 27 years.
The mean follow-up times were 6.99 years for 4,707 cases and 6.94 years for 18,828 controls. Comorbidities were slightly higher in the PCOS group, including asthma (6.7% vs. 4.9%; P less than .001), chronic obstructive pulmonary disease (14% vs. 11%; P less than .001), chronic liver disease (8.0% vs. 5.0%; P less than .001), diabetes mellitus (3.0% vs. 1.4%; P less than .001), hypertension (2.4% vs. 1.5%; P less than .001), hyperlipidemia (5.4% vs. 2.5%; P less than .001), depression (5.4% vs. 3.9%; P less than .001), and sleep apnea (0.23% vs. 0.10%; P = .040).
There was a higher cumulative incidence of psoriasis in the PCOS group (adjusted hazard ratio, 2.07; 95% confidence interval, 1.25-3.44). Other factors associated with increased risk of psoriasis were advanced age (greater than 50 years old; aHR, 14.13; 95% CI, 1.8-110.7) and having a cancer diagnosis (aHR, 11.72; 95% CI, 2.87-47.9).
When PCOS patients were stratified by age, the researchers noted a higher risk of psoriasis among those 20 years or younger (aHR, 4.02; 95% CI, 1.16-13.9) than among those aged 20-50 years (aHR, 1.88; 95% CI, 1.07-3.29). Among those older than 50 years, there was no significantly increased risk, although the number of psoriasis diagnoses and population sizes were small in the latter category. Among patients with PCOS, a cancer diagnosis was not associated with a statistically significant increased risk of psoriasis.
The mechanisms underlying the association between PCOS and psoriasis should be studied further, she noted.
Following Dr. Chen’s prerecorded presentation, there was a live discussion session led by Alice Gottlieb, MD, PhD, medical director of Mount Sinai Beth Israel Dermatology, New York, and Ennio Lubrano, MD, associate professor of rheumatology at the University of Molise (Italy). Dr. Gottlieb noted that the study did not appear to account for weight in the association between PCOS and psoriasis, since heavier people are known to be at greater risk of developing psoriasis. Dr. Chen acknowledged that the study had no records of BMI or weight.
Dr. Gottlieb also wondered if treatment of PCOS led to any improvements in psoriasis in patients with the two diagnoses. “If we treat PCOS, does the psoriasis get better?” Again, the study did not address the question. “We didn’t follow up on therapies,” Dr. Chen said.
Dr. Chen reported no relevant financial disclosures. Dr. Gottlieb is a consultant, advisory board member and/or speaker for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Incyte, Janssen, Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB Pharma and Xbiotech. She has received research or educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis and Xbiotech.
PCOS is characterized by androgen elevation that can lead to insulin resistance and metabolic syndrome, which have also been associated with an increased risk of psoriasis. Previous retrospective analyses have suggested an increased risk of psoriasis associated with PCOS, and psoriasis patients with PCOS have been reported to have more severe skin lesions, compared with those who do not have PCOS.
“The incidence of psoriasis is indeed higher in the PCOS group than in the control group, and the comorbidities related to metabolic syndrome did not modify the adjusted hazard ratio,” said Ming-Li Chen, during her presentation of the study results at the virtual annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. Dr. Chen is at Chung Shan Medical University in Taiwan.
The researchers analyzed 1 million randomly selected records from Taiwan’s Longitudinal Health Insurance database, a subset of the country’s National Health Insurance Program. Between 2000 and 2012, they identified a case group with at least three outpatient diagnoses or one inpatient diagnosis of PCOS; they then compared each with four patients who did not have PCOS who were matched by age and index year. The mean age in both groups was about 27 years.
The mean follow-up times were 6.99 years for 4,707 cases and 6.94 years for 18,828 controls. Comorbidities were slightly higher in the PCOS group, including asthma (6.7% vs. 4.9%; P less than .001), chronic obstructive pulmonary disease (14% vs. 11%; P less than .001), chronic liver disease (8.0% vs. 5.0%; P less than .001), diabetes mellitus (3.0% vs. 1.4%; P less than .001), hypertension (2.4% vs. 1.5%; P less than .001), hyperlipidemia (5.4% vs. 2.5%; P less than .001), depression (5.4% vs. 3.9%; P less than .001), and sleep apnea (0.23% vs. 0.10%; P = .040).
There was a higher cumulative incidence of psoriasis in the PCOS group (adjusted hazard ratio, 2.07; 95% confidence interval, 1.25-3.44). Other factors associated with increased risk of psoriasis were advanced age (greater than 50 years old; aHR, 14.13; 95% CI, 1.8-110.7) and having a cancer diagnosis (aHR, 11.72; 95% CI, 2.87-47.9).
When PCOS patients were stratified by age, the researchers noted a higher risk of psoriasis among those 20 years or younger (aHR, 4.02; 95% CI, 1.16-13.9) than among those aged 20-50 years (aHR, 1.88; 95% CI, 1.07-3.29). Among those older than 50 years, there was no significantly increased risk, although the number of psoriasis diagnoses and population sizes were small in the latter category. Among patients with PCOS, a cancer diagnosis was not associated with a statistically significant increased risk of psoriasis.
The mechanisms underlying the association between PCOS and psoriasis should be studied further, she noted.
Following Dr. Chen’s prerecorded presentation, there was a live discussion session led by Alice Gottlieb, MD, PhD, medical director of Mount Sinai Beth Israel Dermatology, New York, and Ennio Lubrano, MD, associate professor of rheumatology at the University of Molise (Italy). Dr. Gottlieb noted that the study did not appear to account for weight in the association between PCOS and psoriasis, since heavier people are known to be at greater risk of developing psoriasis. Dr. Chen acknowledged that the study had no records of BMI or weight.
Dr. Gottlieb also wondered if treatment of PCOS led to any improvements in psoriasis in patients with the two diagnoses. “If we treat PCOS, does the psoriasis get better?” Again, the study did not address the question. “We didn’t follow up on therapies,” Dr. Chen said.
Dr. Chen reported no relevant financial disclosures. Dr. Gottlieb is a consultant, advisory board member and/or speaker for AbbVie, Allergan, Avotres Therapeutics, Beiersdorf, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Incyte, Janssen, Leo, Novartis, Reddy Labs, Sun Pharmaceutical Industries, UCB Pharma and Xbiotech. She has received research or educational grants from Boehringer Ingelheim, Incyte, Janssen, Novartis and Xbiotech.
FROM GRAPPA 2020 VIRTUAL ANNUAL MEETING
Postpartum tubal ligation safe in obese women
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
FROM OBSTETRICS & GYNECOLOGY
Range of interventions reduces likelihood of infection after hysterectomy
Improving hand hygiene, optimizing antibiotic order sets, and removing catheters sooner were among the interventions associated with a decreased risk of infections after hysterectomy, according to research presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
“Implementation of said study author Shitanshu Uppal, MBBS, an ob.gyn. specializing in gynecologic oncology at the University of Michigan in Ann Arbor.
To assess the impact of quality improvement efforts on infectious morbidity after hysterectomy, Dr. Uppal and colleagues analyzed data from 1,867 hysterectomies performed between Oct. 8, 2015, and Oct. 7, 2018. Patients were at least 18 years old, younger than 90 years old, and underwent hysterectomy via any route at the University of Michigan Medical Center Hospital.
Interventions to reduce infections included the use of cefazolin as a preferred antibiotic, the addition of metronidazole to first-generation cephalosporins for antibiotic prophylaxis, subcuticular closure of open incisions, and earlier removal of Foley catheters. In addition, the institution evaluated and shared information about doctors’ hand hygiene, implemented enhanced recovery after surgery (ERAS) protocols, and held periodic meetings with doctors to discuss efforts to reduce infections. Most interventions were implemented by November 2017.
The primary outcome was overall infection rate in the 30 days after surgery for each of the 3 years included the study. Infections included superficial surgical site infections, deep infections, Clostridium difficile infections, and urinary tract infections, among others.
Patients’ baseline clinical characteristics did not differ during the 3 years studied. Length of stay decreased, which may be attributed to increased use of ERAS protocols and institution of same-day discharge in laparoscopic cases, Dr. Uppal said. The rate of malignancy on final pathology decreased from 29% in year 1 to 23% in year 3. The rate of laparoscopic surgery increased from 55% to 64%.
Infectious morbidity rates decreased from 7% in year 1 (47 infections per 644 cases) to 4% in year 3 (22 infections per 616 cases).
“We saw a reduction in infection rate in all categories,” said Dr. Uppal. “However, the reduction in urinary tract infection as well as superficial surgical site infection was most pronounced.”
After adjustment for the route of surgery, body mass index, age, malignancy on final pathology, modality of surgery, and comorbidities, performance of hysterectomy in year 3 was independently predictive of lower rates of infectious morbidity by 56%.
In addition, the standardized incidence ratio for surgical site infection as reported by the Centers for Medicare & Medicaid Services decreased. In December 2016, Michigan Medicine’s ratio was 1.057. At the end of 2018, the projected ratio was 0.243.
The interventions started on different dates, and “we are unable to conclude, from all the implemented interventions, which one worked,” Dr. Uppal noted.
“I hope the elements from this quality improvement initiative will become the standard of care in order to benefit our patients,” said Amy Park, MD, section head of female pelvic medicine and reconstructive surgery at Cleveland Clinic.
In addition, the study “pertains to one of CMS’s major programs to reduce and prevent health care-associated infections,” Dr. Park said at the virtual meeting. Several CMS quality indicators in 2020 relate to gynecologic surgery, including postoperative wound dehiscence rate, catheter-related UTI rate, and surgical site infection related to hysterectomy. The CMS will reduce hospital payments to institutions in the worst performing quartile for hospital-acquired infection scores by 1%.
Dr. Uppal advised that hospitals need four things to achieve similar results: a commitment from the team and leadership to reduce infections; patience; making it easy for doctors to implement the new interventions; and periodic feedback.
The process may be tedious but worth it in the end, he said.
Dr. Uppal disclosed salary support from Blue Cross Blue Shield of Michigan. A coauthor disclosed salary support from the company plus royalties from UpToDate. Dr. Park disclosed speaking for Allergan and royalties from UpToDate.
SOURCE: Uppal S et al. SGS 2020, Abstract 16.
Improving hand hygiene, optimizing antibiotic order sets, and removing catheters sooner were among the interventions associated with a decreased risk of infections after hysterectomy, according to research presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
“Implementation of said study author Shitanshu Uppal, MBBS, an ob.gyn. specializing in gynecologic oncology at the University of Michigan in Ann Arbor.
To assess the impact of quality improvement efforts on infectious morbidity after hysterectomy, Dr. Uppal and colleagues analyzed data from 1,867 hysterectomies performed between Oct. 8, 2015, and Oct. 7, 2018. Patients were at least 18 years old, younger than 90 years old, and underwent hysterectomy via any route at the University of Michigan Medical Center Hospital.
Interventions to reduce infections included the use of cefazolin as a preferred antibiotic, the addition of metronidazole to first-generation cephalosporins for antibiotic prophylaxis, subcuticular closure of open incisions, and earlier removal of Foley catheters. In addition, the institution evaluated and shared information about doctors’ hand hygiene, implemented enhanced recovery after surgery (ERAS) protocols, and held periodic meetings with doctors to discuss efforts to reduce infections. Most interventions were implemented by November 2017.
The primary outcome was overall infection rate in the 30 days after surgery for each of the 3 years included the study. Infections included superficial surgical site infections, deep infections, Clostridium difficile infections, and urinary tract infections, among others.
Patients’ baseline clinical characteristics did not differ during the 3 years studied. Length of stay decreased, which may be attributed to increased use of ERAS protocols and institution of same-day discharge in laparoscopic cases, Dr. Uppal said. The rate of malignancy on final pathology decreased from 29% in year 1 to 23% in year 3. The rate of laparoscopic surgery increased from 55% to 64%.
Infectious morbidity rates decreased from 7% in year 1 (47 infections per 644 cases) to 4% in year 3 (22 infections per 616 cases).
“We saw a reduction in infection rate in all categories,” said Dr. Uppal. “However, the reduction in urinary tract infection as well as superficial surgical site infection was most pronounced.”
After adjustment for the route of surgery, body mass index, age, malignancy on final pathology, modality of surgery, and comorbidities, performance of hysterectomy in year 3 was independently predictive of lower rates of infectious morbidity by 56%.
In addition, the standardized incidence ratio for surgical site infection as reported by the Centers for Medicare & Medicaid Services decreased. In December 2016, Michigan Medicine’s ratio was 1.057. At the end of 2018, the projected ratio was 0.243.
The interventions started on different dates, and “we are unable to conclude, from all the implemented interventions, which one worked,” Dr. Uppal noted.
“I hope the elements from this quality improvement initiative will become the standard of care in order to benefit our patients,” said Amy Park, MD, section head of female pelvic medicine and reconstructive surgery at Cleveland Clinic.
In addition, the study “pertains to one of CMS’s major programs to reduce and prevent health care-associated infections,” Dr. Park said at the virtual meeting. Several CMS quality indicators in 2020 relate to gynecologic surgery, including postoperative wound dehiscence rate, catheter-related UTI rate, and surgical site infection related to hysterectomy. The CMS will reduce hospital payments to institutions in the worst performing quartile for hospital-acquired infection scores by 1%.
Dr. Uppal advised that hospitals need four things to achieve similar results: a commitment from the team and leadership to reduce infections; patience; making it easy for doctors to implement the new interventions; and periodic feedback.
The process may be tedious but worth it in the end, he said.
Dr. Uppal disclosed salary support from Blue Cross Blue Shield of Michigan. A coauthor disclosed salary support from the company plus royalties from UpToDate. Dr. Park disclosed speaking for Allergan and royalties from UpToDate.
SOURCE: Uppal S et al. SGS 2020, Abstract 16.
Improving hand hygiene, optimizing antibiotic order sets, and removing catheters sooner were among the interventions associated with a decreased risk of infections after hysterectomy, according to research presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
“Implementation of said study author Shitanshu Uppal, MBBS, an ob.gyn. specializing in gynecologic oncology at the University of Michigan in Ann Arbor.
To assess the impact of quality improvement efforts on infectious morbidity after hysterectomy, Dr. Uppal and colleagues analyzed data from 1,867 hysterectomies performed between Oct. 8, 2015, and Oct. 7, 2018. Patients were at least 18 years old, younger than 90 years old, and underwent hysterectomy via any route at the University of Michigan Medical Center Hospital.
Interventions to reduce infections included the use of cefazolin as a preferred antibiotic, the addition of metronidazole to first-generation cephalosporins for antibiotic prophylaxis, subcuticular closure of open incisions, and earlier removal of Foley catheters. In addition, the institution evaluated and shared information about doctors’ hand hygiene, implemented enhanced recovery after surgery (ERAS) protocols, and held periodic meetings with doctors to discuss efforts to reduce infections. Most interventions were implemented by November 2017.
The primary outcome was overall infection rate in the 30 days after surgery for each of the 3 years included the study. Infections included superficial surgical site infections, deep infections, Clostridium difficile infections, and urinary tract infections, among others.
Patients’ baseline clinical characteristics did not differ during the 3 years studied. Length of stay decreased, which may be attributed to increased use of ERAS protocols and institution of same-day discharge in laparoscopic cases, Dr. Uppal said. The rate of malignancy on final pathology decreased from 29% in year 1 to 23% in year 3. The rate of laparoscopic surgery increased from 55% to 64%.
Infectious morbidity rates decreased from 7% in year 1 (47 infections per 644 cases) to 4% in year 3 (22 infections per 616 cases).
“We saw a reduction in infection rate in all categories,” said Dr. Uppal. “However, the reduction in urinary tract infection as well as superficial surgical site infection was most pronounced.”
After adjustment for the route of surgery, body mass index, age, malignancy on final pathology, modality of surgery, and comorbidities, performance of hysterectomy in year 3 was independently predictive of lower rates of infectious morbidity by 56%.
In addition, the standardized incidence ratio for surgical site infection as reported by the Centers for Medicare & Medicaid Services decreased. In December 2016, Michigan Medicine’s ratio was 1.057. At the end of 2018, the projected ratio was 0.243.
The interventions started on different dates, and “we are unable to conclude, from all the implemented interventions, which one worked,” Dr. Uppal noted.
“I hope the elements from this quality improvement initiative will become the standard of care in order to benefit our patients,” said Amy Park, MD, section head of female pelvic medicine and reconstructive surgery at Cleveland Clinic.
In addition, the study “pertains to one of CMS’s major programs to reduce and prevent health care-associated infections,” Dr. Park said at the virtual meeting. Several CMS quality indicators in 2020 relate to gynecologic surgery, including postoperative wound dehiscence rate, catheter-related UTI rate, and surgical site infection related to hysterectomy. The CMS will reduce hospital payments to institutions in the worst performing quartile for hospital-acquired infection scores by 1%.
Dr. Uppal advised that hospitals need four things to achieve similar results: a commitment from the team and leadership to reduce infections; patience; making it easy for doctors to implement the new interventions; and periodic feedback.
The process may be tedious but worth it in the end, he said.
Dr. Uppal disclosed salary support from Blue Cross Blue Shield of Michigan. A coauthor disclosed salary support from the company plus royalties from UpToDate. Dr. Park disclosed speaking for Allergan and royalties from UpToDate.
SOURCE: Uppal S et al. SGS 2020, Abstract 16.
FROM SGS 2020
Tamoxifen can reduce bleeding in women with contraceptive implants
A study has found that
“Our data support the use of tamoxifen as an effective option that offers the benefit of a shorter duration of treatment than other approaches such as combined oral contraceptives,” wrote Alison B. Edelman, MD, MPH, of Oregon Health & Science University, Portland, and coauthors. The report is in Obstetrics & Gynecology.
To determine if a short course of tamoxifen – typically used to treat breast cancer – could prove beneficial in reducing bothersome bleeding, the researchers launched a 90-day, double-blind randomized trial of women between the ages of 15 and 45 years who had been using the etonogestrel 68-mg subdermal contraceptive implant for at least 30 days. All participants suffered from frequent or prolonged bleeding or spotting during the previous month; their mean age was 24, and most (n = 62) identified as White.
Of the initial 112 participants, 107 began treatment and were split into two groups: 10 mg of tamoxifen twice a day for 7 days (n = 55) or placebo (n = 52). One hundred and four patients completed treatment one, and 88 completed 90 days. After the first treatment, women in the tamoxifen group experienced 9.8 more consecutive days of amenorrhea (95% confidence interval, 4.6-15.0) compared with the placebo group, as well as more total days of no bleeding in the first 90 days (median 73.5 [24-89] versus 68 [11-81], P = .001).
Afterward, both groups underwent a 90-day, open-label study where all participants took tamoxifen. The differences between the groups mostly disappeared, as they both experienced more amenorrhea days (median 56 [6-81] for tamoxifen and 67.5 [7-83] for placebo) and fewer bleeding days (median 12 [0-63] for tamoxifen and 12 [0-82] for placebo) compared with the placebo group during the initial 90 days. Although no serious adverse events occurred, more women taking tamoxifen reported fluid retention (12 versus 1), headache (19 versus 1), and mood changes (13 versus 2).
“This is a very promising drug for this purpose,” Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview, adding that it is “a bit unconventional because tamoxifen is traditionally used for cancer or precancer.”
As such, she recognized that young people of reproductive age might be a little wary of the drug. That said, having an effective treatment for troublesome bleeding beyond estrogen-based products should ultimately prove beneficial for clinicians and patients alike.
“Unfortunately, we don’t have long-term data so it’s unclear what the safety outcomes are,” she said, “but having another option to address bothersome bleeding can help women stay on birth control longer. The alternative would be pregnancy, with its own associated risks.”
The authors acknowledged their study’s limitations, including a lack of Black patients and the likelihood that their volunteer cohort “may not reflect the general population of implant users who present for discontinuation owing to bleeding problems.” They also enrolled a small but notable number of women who had been using the implant for less than 3 months, noting that bleeding patterns often change from the first 90 days and so “some of these women would likely experience better (or worse) bleeding irrespective of treatment.”
The study was supported by a Merck Women’s Health Investigator Initiated Studies Program and the Oregon Clinical and Translational Research Institute. Four of the authors acknowledged receiving consulting fees and research support from various organizations and pharmaceutical companies. The remaining three had no relevant financial disclosures. Dr. Cansino is a member of the Ob.Gyn. News editorial advisory board. She said she had no relevant financial disclosures.
SOURCE: Edelman AB et al. Obstet Gynecol. 2020 Jul 9. doi: 10.1097/AOG.0000000000003896.
A study has found that
“Our data support the use of tamoxifen as an effective option that offers the benefit of a shorter duration of treatment than other approaches such as combined oral contraceptives,” wrote Alison B. Edelman, MD, MPH, of Oregon Health & Science University, Portland, and coauthors. The report is in Obstetrics & Gynecology.
To determine if a short course of tamoxifen – typically used to treat breast cancer – could prove beneficial in reducing bothersome bleeding, the researchers launched a 90-day, double-blind randomized trial of women between the ages of 15 and 45 years who had been using the etonogestrel 68-mg subdermal contraceptive implant for at least 30 days. All participants suffered from frequent or prolonged bleeding or spotting during the previous month; their mean age was 24, and most (n = 62) identified as White.
Of the initial 112 participants, 107 began treatment and were split into two groups: 10 mg of tamoxifen twice a day for 7 days (n = 55) or placebo (n = 52). One hundred and four patients completed treatment one, and 88 completed 90 days. After the first treatment, women in the tamoxifen group experienced 9.8 more consecutive days of amenorrhea (95% confidence interval, 4.6-15.0) compared with the placebo group, as well as more total days of no bleeding in the first 90 days (median 73.5 [24-89] versus 68 [11-81], P = .001).
Afterward, both groups underwent a 90-day, open-label study where all participants took tamoxifen. The differences between the groups mostly disappeared, as they both experienced more amenorrhea days (median 56 [6-81] for tamoxifen and 67.5 [7-83] for placebo) and fewer bleeding days (median 12 [0-63] for tamoxifen and 12 [0-82] for placebo) compared with the placebo group during the initial 90 days. Although no serious adverse events occurred, more women taking tamoxifen reported fluid retention (12 versus 1), headache (19 versus 1), and mood changes (13 versus 2).
“This is a very promising drug for this purpose,” Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview, adding that it is “a bit unconventional because tamoxifen is traditionally used for cancer or precancer.”
As such, she recognized that young people of reproductive age might be a little wary of the drug. That said, having an effective treatment for troublesome bleeding beyond estrogen-based products should ultimately prove beneficial for clinicians and patients alike.
“Unfortunately, we don’t have long-term data so it’s unclear what the safety outcomes are,” she said, “but having another option to address bothersome bleeding can help women stay on birth control longer. The alternative would be pregnancy, with its own associated risks.”
The authors acknowledged their study’s limitations, including a lack of Black patients and the likelihood that their volunteer cohort “may not reflect the general population of implant users who present for discontinuation owing to bleeding problems.” They also enrolled a small but notable number of women who had been using the implant for less than 3 months, noting that bleeding patterns often change from the first 90 days and so “some of these women would likely experience better (or worse) bleeding irrespective of treatment.”
The study was supported by a Merck Women’s Health Investigator Initiated Studies Program and the Oregon Clinical and Translational Research Institute. Four of the authors acknowledged receiving consulting fees and research support from various organizations and pharmaceutical companies. The remaining three had no relevant financial disclosures. Dr. Cansino is a member of the Ob.Gyn. News editorial advisory board. She said she had no relevant financial disclosures.
SOURCE: Edelman AB et al. Obstet Gynecol. 2020 Jul 9. doi: 10.1097/AOG.0000000000003896.
A study has found that
“Our data support the use of tamoxifen as an effective option that offers the benefit of a shorter duration of treatment than other approaches such as combined oral contraceptives,” wrote Alison B. Edelman, MD, MPH, of Oregon Health & Science University, Portland, and coauthors. The report is in Obstetrics & Gynecology.
To determine if a short course of tamoxifen – typically used to treat breast cancer – could prove beneficial in reducing bothersome bleeding, the researchers launched a 90-day, double-blind randomized trial of women between the ages of 15 and 45 years who had been using the etonogestrel 68-mg subdermal contraceptive implant for at least 30 days. All participants suffered from frequent or prolonged bleeding or spotting during the previous month; their mean age was 24, and most (n = 62) identified as White.
Of the initial 112 participants, 107 began treatment and were split into two groups: 10 mg of tamoxifen twice a day for 7 days (n = 55) or placebo (n = 52). One hundred and four patients completed treatment one, and 88 completed 90 days. After the first treatment, women in the tamoxifen group experienced 9.8 more consecutive days of amenorrhea (95% confidence interval, 4.6-15.0) compared with the placebo group, as well as more total days of no bleeding in the first 90 days (median 73.5 [24-89] versus 68 [11-81], P = .001).
Afterward, both groups underwent a 90-day, open-label study where all participants took tamoxifen. The differences between the groups mostly disappeared, as they both experienced more amenorrhea days (median 56 [6-81] for tamoxifen and 67.5 [7-83] for placebo) and fewer bleeding days (median 12 [0-63] for tamoxifen and 12 [0-82] for placebo) compared with the placebo group during the initial 90 days. Although no serious adverse events occurred, more women taking tamoxifen reported fluid retention (12 versus 1), headache (19 versus 1), and mood changes (13 versus 2).
“This is a very promising drug for this purpose,” Catherine Cansino, MD, MPH, of the University of California, Davis, said in an interview, adding that it is “a bit unconventional because tamoxifen is traditionally used for cancer or precancer.”
As such, she recognized that young people of reproductive age might be a little wary of the drug. That said, having an effective treatment for troublesome bleeding beyond estrogen-based products should ultimately prove beneficial for clinicians and patients alike.
“Unfortunately, we don’t have long-term data so it’s unclear what the safety outcomes are,” she said, “but having another option to address bothersome bleeding can help women stay on birth control longer. The alternative would be pregnancy, with its own associated risks.”
The authors acknowledged their study’s limitations, including a lack of Black patients and the likelihood that their volunteer cohort “may not reflect the general population of implant users who present for discontinuation owing to bleeding problems.” They also enrolled a small but notable number of women who had been using the implant for less than 3 months, noting that bleeding patterns often change from the first 90 days and so “some of these women would likely experience better (or worse) bleeding irrespective of treatment.”
The study was supported by a Merck Women’s Health Investigator Initiated Studies Program and the Oregon Clinical and Translational Research Institute. Four of the authors acknowledged receiving consulting fees and research support from various organizations and pharmaceutical companies. The remaining three had no relevant financial disclosures. Dr. Cansino is a member of the Ob.Gyn. News editorial advisory board. She said she had no relevant financial disclosures.
SOURCE: Edelman AB et al. Obstet Gynecol. 2020 Jul 9. doi: 10.1097/AOG.0000000000003896.
FROM OBSTETRICS & GYNECOLOGY
Treating VIN while preventing recurrence
Vulvar intraepithelial neoplasia (VIN) is a distressing condition that may require painful and disfiguring treatments. It is particularly problematic because more than a quarter of patients will experience recurrence of their disease after primary therapy. In this column we will explore the risk factors for recurrence, recommendations for early detection, and options to minimize its incidence.
VIN was traditionally characterized in three stages (I, II, III). However, as it became better understood that the previously named VIN I was not, in fact, a precursor for malignancy, but rather a benign manifestation of low-risk human papillomavirus (HPV) infection, it was removed from consideration as VIN. Furthermore, our understanding of VIN grew to recognize that there were two developmental pathways to vulvar neoplasia and malignancy. The first was via high-risk HPV infection, often with tobacco exposure as an accelerating factor, and typically among younger women. This has been named “usual type VIN” (uVIN). The second arises in the background of lichen sclerosus in older women and is named “differentiated type VIN” (dVIN). This type carries with it a higher risk for progression to cancer, coexisting in approximately 80% of cases of invasive squamous cell carcinoma. In addition, the progression to cancer appears to occur more quickly for dVIN lesions (22 months compared with 41 months in uVIN).1
While observation of VIN can be considered for young, asymptomatic women, it is not universally recommended because the risk of progression to cancer is approximately 8% (5% for uVIN and 33% for dVIN).1,2 Both subtypes of VIN can be treated with similar interventions including surgical excision (typically a wide local excision), ablative therapies (such as CO2 laser) or topical medical therapy such as imiquimod or 5-fluorouracil. (false-negative biopsies), and adequacy of margin status. However, given the proximity of this disease to vital structures such as the clitoris, urethral meatus, and anal verge, as well as issues with wound healing, and difficulty with reapproximation of vulvar tissues – particularly when large or multifocal disease is present – sometimes multimodal treatments or medical therapies are preferred to spare disfigurement or sexual, bladder, or bowel dysfunction.
Excision of VIN need not be deeper than the epidermis, although including a limited degree of dermis protects against incomplete resection of occult, coexisting early invasive disease. However, wide margins should ideally be at least 10 mm. This can prove to be a challenging goal for multiple reasons. First, while there are visual stigmata of VIN, its true extent can be determined only microscopically. In addition, the disease may be multifocal. Furthermore, particularly where it encroaches upon the anus, clitoris, or urethral meatus, resection margins may be limited because of the desire to preserve function of adjacent structures. The application of 2%-5% acetic acid in the operating room prior to marking the planned borders of excision can optimize the likelihood that the incisions will encompass the microscopic extent of VIN. As it does with cervical dysplasia, acetic acid is thought to cause reversible coagulation of nuclear proteins and cytokeratins, which are more abundant in dysplastic lesions, thus appearing white to the surgeon’s eye.
However, even with the surgeon’s best attempts to excise all disease, approximately half of VIN excisions will have positive margins. Fortunately, not all of these patients will go on to develop recurrent dysplasia. In fact, less than half of women with positive margins on excision will develop recurrent VIN disease.2 This incomplete incidence of recurrence may be in part due to an ablative effect of inflammation at the cut skin edges. Therefore, provided that there is no macroscopic disease remaining, close observation, rather than immediate reexcision, is recommended.
Positive excisional margins are a major risk factor for recurrence, carrying an eightfold increased risk, and also are associated with a more rapid onset of recurrence than for those with negative margins. Other predisposing risk factors for recurrence include advancing age, coexistence of dysplasia at other lower genital sites (including vaginal and cervical), immunosuppressive conditions or therapies (especially steroid use), HPV exposure, and the presence of lichen sclerosus.2 Continued tobacco use is a modifiable risk factor that has been shown to be associated with an increased recurrence risk of VIN. We should take the opportunity in the postoperative and surveillance period to educate our patients regarding the importance of smoking cessation in modifying their risk for recurrent or new disease.
HPV infection may not be a modifiable risk factor, but certainly can be prevented by encouraging the adoption of HPV vaccination.
Topical steroids used to treat lichen sclerosus can improve symptoms of this vulvar dystrophy as well as decrease the incidence of recurrent dVIN and invasive vulvar cancer. Treatment should continue until the skin has normalized its appearance and texture. This may involve chronic long-term therapy.3
Recognizing that more than a quarter of patients will recur, the recommended posttreatment follow-up for VIN is at 6 months, 12 months, and then annually. It should include close inspection of the vulva with consideration of application of topical 2%-5% acetic acid (I typically apply this with a soaked gauze sponge) and vulvar colposcopy (a hand-held magnification glass works well for this purpose). Patients should be counseled regarding their high risk for recurrence, informed of typical symptoms, and encouraged to perform regular vulva self-inspection (with use of a hand mirror).
For patients at the highest risk for recurrence (older patients, patients with positive excisional margins, HPV coinfection, lichen sclerosus, tobacco use, and immunosuppression), I recommend 6 monthly follow-up surveillance for 5 years. Most (75%) of recurrences will occur with the first 43 months after diagnosis with half occurring in the first 18 months.2 Patients who have had positive margins on their excisional specimen are at the highest risk for an earlier recurrence.
VIN is an insidious disease with a high recurrence rate. It is challenging to completely resect with negative margins. Patients with a history of VIN should receive close observation in the years following their excision, particularly if resection margins were positive, and clinicians should attempt to modify risk factors wherever possible, paying particularly close attention to older postmenopausal women with a history of lichen sclerosus as progression to malignancy is highest for these women.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Pathology. 2016 Jun 1;48(4)291-302.
2. Gynecol Oncol. 2018 Jan;148(1):126-31.
3. JAMA Dermatol. 2015 Oct;151(10):1061-7.
Vulvar intraepithelial neoplasia (VIN) is a distressing condition that may require painful and disfiguring treatments. It is particularly problematic because more than a quarter of patients will experience recurrence of their disease after primary therapy. In this column we will explore the risk factors for recurrence, recommendations for early detection, and options to minimize its incidence.
VIN was traditionally characterized in three stages (I, II, III). However, as it became better understood that the previously named VIN I was not, in fact, a precursor for malignancy, but rather a benign manifestation of low-risk human papillomavirus (HPV) infection, it was removed from consideration as VIN. Furthermore, our understanding of VIN grew to recognize that there were two developmental pathways to vulvar neoplasia and malignancy. The first was via high-risk HPV infection, often with tobacco exposure as an accelerating factor, and typically among younger women. This has been named “usual type VIN” (uVIN). The second arises in the background of lichen sclerosus in older women and is named “differentiated type VIN” (dVIN). This type carries with it a higher risk for progression to cancer, coexisting in approximately 80% of cases of invasive squamous cell carcinoma. In addition, the progression to cancer appears to occur more quickly for dVIN lesions (22 months compared with 41 months in uVIN).1
While observation of VIN can be considered for young, asymptomatic women, it is not universally recommended because the risk of progression to cancer is approximately 8% (5% for uVIN and 33% for dVIN).1,2 Both subtypes of VIN can be treated with similar interventions including surgical excision (typically a wide local excision), ablative therapies (such as CO2 laser) or topical medical therapy such as imiquimod or 5-fluorouracil. (false-negative biopsies), and adequacy of margin status. However, given the proximity of this disease to vital structures such as the clitoris, urethral meatus, and anal verge, as well as issues with wound healing, and difficulty with reapproximation of vulvar tissues – particularly when large or multifocal disease is present – sometimes multimodal treatments or medical therapies are preferred to spare disfigurement or sexual, bladder, or bowel dysfunction.
Excision of VIN need not be deeper than the epidermis, although including a limited degree of dermis protects against incomplete resection of occult, coexisting early invasive disease. However, wide margins should ideally be at least 10 mm. This can prove to be a challenging goal for multiple reasons. First, while there are visual stigmata of VIN, its true extent can be determined only microscopically. In addition, the disease may be multifocal. Furthermore, particularly where it encroaches upon the anus, clitoris, or urethral meatus, resection margins may be limited because of the desire to preserve function of adjacent structures. The application of 2%-5% acetic acid in the operating room prior to marking the planned borders of excision can optimize the likelihood that the incisions will encompass the microscopic extent of VIN. As it does with cervical dysplasia, acetic acid is thought to cause reversible coagulation of nuclear proteins and cytokeratins, which are more abundant in dysplastic lesions, thus appearing white to the surgeon’s eye.
However, even with the surgeon’s best attempts to excise all disease, approximately half of VIN excisions will have positive margins. Fortunately, not all of these patients will go on to develop recurrent dysplasia. In fact, less than half of women with positive margins on excision will develop recurrent VIN disease.2 This incomplete incidence of recurrence may be in part due to an ablative effect of inflammation at the cut skin edges. Therefore, provided that there is no macroscopic disease remaining, close observation, rather than immediate reexcision, is recommended.
Positive excisional margins are a major risk factor for recurrence, carrying an eightfold increased risk, and also are associated with a more rapid onset of recurrence than for those with negative margins. Other predisposing risk factors for recurrence include advancing age, coexistence of dysplasia at other lower genital sites (including vaginal and cervical), immunosuppressive conditions or therapies (especially steroid use), HPV exposure, and the presence of lichen sclerosus.2 Continued tobacco use is a modifiable risk factor that has been shown to be associated with an increased recurrence risk of VIN. We should take the opportunity in the postoperative and surveillance period to educate our patients regarding the importance of smoking cessation in modifying their risk for recurrent or new disease.
HPV infection may not be a modifiable risk factor, but certainly can be prevented by encouraging the adoption of HPV vaccination.
Topical steroids used to treat lichen sclerosus can improve symptoms of this vulvar dystrophy as well as decrease the incidence of recurrent dVIN and invasive vulvar cancer. Treatment should continue until the skin has normalized its appearance and texture. This may involve chronic long-term therapy.3
Recognizing that more than a quarter of patients will recur, the recommended posttreatment follow-up for VIN is at 6 months, 12 months, and then annually. It should include close inspection of the vulva with consideration of application of topical 2%-5% acetic acid (I typically apply this with a soaked gauze sponge) and vulvar colposcopy (a hand-held magnification glass works well for this purpose). Patients should be counseled regarding their high risk for recurrence, informed of typical symptoms, and encouraged to perform regular vulva self-inspection (with use of a hand mirror).
For patients at the highest risk for recurrence (older patients, patients with positive excisional margins, HPV coinfection, lichen sclerosus, tobacco use, and immunosuppression), I recommend 6 monthly follow-up surveillance for 5 years. Most (75%) of recurrences will occur with the first 43 months after diagnosis with half occurring in the first 18 months.2 Patients who have had positive margins on their excisional specimen are at the highest risk for an earlier recurrence.
VIN is an insidious disease with a high recurrence rate. It is challenging to completely resect with negative margins. Patients with a history of VIN should receive close observation in the years following their excision, particularly if resection margins were positive, and clinicians should attempt to modify risk factors wherever possible, paying particularly close attention to older postmenopausal women with a history of lichen sclerosus as progression to malignancy is highest for these women.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Pathology. 2016 Jun 1;48(4)291-302.
2. Gynecol Oncol. 2018 Jan;148(1):126-31.
3. JAMA Dermatol. 2015 Oct;151(10):1061-7.
Vulvar intraepithelial neoplasia (VIN) is a distressing condition that may require painful and disfiguring treatments. It is particularly problematic because more than a quarter of patients will experience recurrence of their disease after primary therapy. In this column we will explore the risk factors for recurrence, recommendations for early detection, and options to minimize its incidence.
VIN was traditionally characterized in three stages (I, II, III). However, as it became better understood that the previously named VIN I was not, in fact, a precursor for malignancy, but rather a benign manifestation of low-risk human papillomavirus (HPV) infection, it was removed from consideration as VIN. Furthermore, our understanding of VIN grew to recognize that there were two developmental pathways to vulvar neoplasia and malignancy. The first was via high-risk HPV infection, often with tobacco exposure as an accelerating factor, and typically among younger women. This has been named “usual type VIN” (uVIN). The second arises in the background of lichen sclerosus in older women and is named “differentiated type VIN” (dVIN). This type carries with it a higher risk for progression to cancer, coexisting in approximately 80% of cases of invasive squamous cell carcinoma. In addition, the progression to cancer appears to occur more quickly for dVIN lesions (22 months compared with 41 months in uVIN).1
While observation of VIN can be considered for young, asymptomatic women, it is not universally recommended because the risk of progression to cancer is approximately 8% (5% for uVIN and 33% for dVIN).1,2 Both subtypes of VIN can be treated with similar interventions including surgical excision (typically a wide local excision), ablative therapies (such as CO2 laser) or topical medical therapy such as imiquimod or 5-fluorouracil. (false-negative biopsies), and adequacy of margin status. However, given the proximity of this disease to vital structures such as the clitoris, urethral meatus, and anal verge, as well as issues with wound healing, and difficulty with reapproximation of vulvar tissues – particularly when large or multifocal disease is present – sometimes multimodal treatments or medical therapies are preferred to spare disfigurement or sexual, bladder, or bowel dysfunction.
Excision of VIN need not be deeper than the epidermis, although including a limited degree of dermis protects against incomplete resection of occult, coexisting early invasive disease. However, wide margins should ideally be at least 10 mm. This can prove to be a challenging goal for multiple reasons. First, while there are visual stigmata of VIN, its true extent can be determined only microscopically. In addition, the disease may be multifocal. Furthermore, particularly where it encroaches upon the anus, clitoris, or urethral meatus, resection margins may be limited because of the desire to preserve function of adjacent structures. The application of 2%-5% acetic acid in the operating room prior to marking the planned borders of excision can optimize the likelihood that the incisions will encompass the microscopic extent of VIN. As it does with cervical dysplasia, acetic acid is thought to cause reversible coagulation of nuclear proteins and cytokeratins, which are more abundant in dysplastic lesions, thus appearing white to the surgeon’s eye.
However, even with the surgeon’s best attempts to excise all disease, approximately half of VIN excisions will have positive margins. Fortunately, not all of these patients will go on to develop recurrent dysplasia. In fact, less than half of women with positive margins on excision will develop recurrent VIN disease.2 This incomplete incidence of recurrence may be in part due to an ablative effect of inflammation at the cut skin edges. Therefore, provided that there is no macroscopic disease remaining, close observation, rather than immediate reexcision, is recommended.
Positive excisional margins are a major risk factor for recurrence, carrying an eightfold increased risk, and also are associated with a more rapid onset of recurrence than for those with negative margins. Other predisposing risk factors for recurrence include advancing age, coexistence of dysplasia at other lower genital sites (including vaginal and cervical), immunosuppressive conditions or therapies (especially steroid use), HPV exposure, and the presence of lichen sclerosus.2 Continued tobacco use is a modifiable risk factor that has been shown to be associated with an increased recurrence risk of VIN. We should take the opportunity in the postoperative and surveillance period to educate our patients regarding the importance of smoking cessation in modifying their risk for recurrent or new disease.
HPV infection may not be a modifiable risk factor, but certainly can be prevented by encouraging the adoption of HPV vaccination.
Topical steroids used to treat lichen sclerosus can improve symptoms of this vulvar dystrophy as well as decrease the incidence of recurrent dVIN and invasive vulvar cancer. Treatment should continue until the skin has normalized its appearance and texture. This may involve chronic long-term therapy.3
Recognizing that more than a quarter of patients will recur, the recommended posttreatment follow-up for VIN is at 6 months, 12 months, and then annually. It should include close inspection of the vulva with consideration of application of topical 2%-5% acetic acid (I typically apply this with a soaked gauze sponge) and vulvar colposcopy (a hand-held magnification glass works well for this purpose). Patients should be counseled regarding their high risk for recurrence, informed of typical symptoms, and encouraged to perform regular vulva self-inspection (with use of a hand mirror).
For patients at the highest risk for recurrence (older patients, patients with positive excisional margins, HPV coinfection, lichen sclerosus, tobacco use, and immunosuppression), I recommend 6 monthly follow-up surveillance for 5 years. Most (75%) of recurrences will occur with the first 43 months after diagnosis with half occurring in the first 18 months.2 Patients who have had positive margins on their excisional specimen are at the highest risk for an earlier recurrence.
VIN is an insidious disease with a high recurrence rate. It is challenging to completely resect with negative margins. Patients with a history of VIN should receive close observation in the years following their excision, particularly if resection margins were positive, and clinicians should attempt to modify risk factors wherever possible, paying particularly close attention to older postmenopausal women with a history of lichen sclerosus as progression to malignancy is highest for these women.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Pathology. 2016 Jun 1;48(4)291-302.
2. Gynecol Oncol. 2018 Jan;148(1):126-31.
3. JAMA Dermatol. 2015 Oct;151(10):1061-7.
FDA allows qualified claims for UTI risk reduction with cranberry products
The Food and Drug Administration will not object to qualified health claims that consumption of certain cranberry juice products and cranberry supplement products may reduce the risk of recurrent urinary tract infections in otherwise healthy women.
In a letter of enforcement discretion issued on July 21, the FDA responded to a health claim petition submitted by Ocean Spray Cranberries. “A health claim characterizes the relationship between a substance and a disease or health-related condition,” according to the FDA. Ocean Spray Cranberries asked the FDA for an authorized health claim regarding the relationship between the consumption of cranberry beverages and supplements and a reduction in the risk of recurrent urinary tract infections (UTIs) in healthy women.
After reviewing the evidence, the FDA determined that the existing science did not support an authorized health claim, but did allow for a qualified health claim for certain cranberry juice beverages and supplements. A qualified health claim does not constitute an FDA approval; the FDA instead issues a Letter of Enforcement Discretion that includes language reflecting the level of scientific evidence for the claim.
The currently available scientific evidence for a relationship between cranberry and recurrent UTIs includes five intervention studies, according to the FDA letter. Two of these were high-quality, randomized, controlled trials in which daily consumption of a cranberry juice beverage was significantly associated with a reduced risk of recurrent UTIs. Another randomized, controlled trial yielded mixed results, and two other intervention studies that were moderate-quality, randomized, controlled trials showed no effect of cranberry juice consumption on UTI risk reduction.
The FDA’s letter of enforcement discretion states that, with regard to cranberry juice beverages, “Limited and inconsistent scientific evidence shows that by consuming one serving (8 oz) each day of a cranberry juice beverage, healthy women who have had a urinary tract infection may reduce their risk of recurrent UTI.”
Similarly, for cranberry dietary supplements, the FDA states that “Limited scientific evidence shows that, by consuming 500 mg each day of cranberry dietary supplement, healthy women who have had a urinary tract infection may reduce their risk of recurrent UTI.”
The qualified health claims apply specifically to cranberry juice beverages that contain at least 27% cranberry juice, and cranberry dietary supplements containing at least 500 mg of cranberry fruit powder. “The claims do not include other conventional foods or food products made from or containing cranberries, such as dried cranberries or cranberry sauce,” according to the FDA statement.
“With recurrent UTI, a major concern is the frequent use of antibiotics,” Constance Bohon, MD, an ob.gyn. in private practice in Washington and an assistant clinical professor at George Washington University, Washington, said in an interview.
“The challenge is to identify habits and/or nonantibiotic treatment to prevent recurrent UTI and decrease the need for antibiotics,” she said. “The regular use of cranberry can decrease the frequency of UTI in some, but not all, people.
“It does not appear to mask the symptoms of a UTI, so if it is not effective to prevent the infection, the presumptive diagnosis can be made based on the common symptoms,” she explained.
Dr. Bohon said that she has recommended the use of cranberry to some of her patients who have recurrent UTIs and has had success with many of them.
“I think it is important to make it clear that cranberry can be beneficial for some patients to decrease the frequency of UTI. It will not be effective for everyone who has frequent UTI, but for those who use it and have fewer UTIs, there will be less frequent exposure to antibiotics,” she emphasized. “What we need to know is who benefits the most from cranberry to prevent recurrent UTIs; whether age, race, coexisting health problems [such as diabetes], and use of hormonal contraception or menopause impact on its success.”
Dr. Bohon had no relevant financial conflicts to disclose.
The Food and Drug Administration will not object to qualified health claims that consumption of certain cranberry juice products and cranberry supplement products may reduce the risk of recurrent urinary tract infections in otherwise healthy women.
In a letter of enforcement discretion issued on July 21, the FDA responded to a health claim petition submitted by Ocean Spray Cranberries. “A health claim characterizes the relationship between a substance and a disease or health-related condition,” according to the FDA. Ocean Spray Cranberries asked the FDA for an authorized health claim regarding the relationship between the consumption of cranberry beverages and supplements and a reduction in the risk of recurrent urinary tract infections (UTIs) in healthy women.
After reviewing the evidence, the FDA determined that the existing science did not support an authorized health claim, but did allow for a qualified health claim for certain cranberry juice beverages and supplements. A qualified health claim does not constitute an FDA approval; the FDA instead issues a Letter of Enforcement Discretion that includes language reflecting the level of scientific evidence for the claim.
The currently available scientific evidence for a relationship between cranberry and recurrent UTIs includes five intervention studies, according to the FDA letter. Two of these were high-quality, randomized, controlled trials in which daily consumption of a cranberry juice beverage was significantly associated with a reduced risk of recurrent UTIs. Another randomized, controlled trial yielded mixed results, and two other intervention studies that were moderate-quality, randomized, controlled trials showed no effect of cranberry juice consumption on UTI risk reduction.
The FDA’s letter of enforcement discretion states that, with regard to cranberry juice beverages, “Limited and inconsistent scientific evidence shows that by consuming one serving (8 oz) each day of a cranberry juice beverage, healthy women who have had a urinary tract infection may reduce their risk of recurrent UTI.”
Similarly, for cranberry dietary supplements, the FDA states that “Limited scientific evidence shows that, by consuming 500 mg each day of cranberry dietary supplement, healthy women who have had a urinary tract infection may reduce their risk of recurrent UTI.”
The qualified health claims apply specifically to cranberry juice beverages that contain at least 27% cranberry juice, and cranberry dietary supplements containing at least 500 mg of cranberry fruit powder. “The claims do not include other conventional foods or food products made from or containing cranberries, such as dried cranberries or cranberry sauce,” according to the FDA statement.
“With recurrent UTI, a major concern is the frequent use of antibiotics,” Constance Bohon, MD, an ob.gyn. in private practice in Washington and an assistant clinical professor at George Washington University, Washington, said in an interview.
“The challenge is to identify habits and/or nonantibiotic treatment to prevent recurrent UTI and decrease the need for antibiotics,” she said. “The regular use of cranberry can decrease the frequency of UTI in some, but not all, people.
“It does not appear to mask the symptoms of a UTI, so if it is not effective to prevent the infection, the presumptive diagnosis can be made based on the common symptoms,” she explained.
Dr. Bohon said that she has recommended the use of cranberry to some of her patients who have recurrent UTIs and has had success with many of them.
“I think it is important to make it clear that cranberry can be beneficial for some patients to decrease the frequency of UTI. It will not be effective for everyone who has frequent UTI, but for those who use it and have fewer UTIs, there will be less frequent exposure to antibiotics,” she emphasized. “What we need to know is who benefits the most from cranberry to prevent recurrent UTIs; whether age, race, coexisting health problems [such as diabetes], and use of hormonal contraception or menopause impact on its success.”
Dr. Bohon had no relevant financial conflicts to disclose.
The Food and Drug Administration will not object to qualified health claims that consumption of certain cranberry juice products and cranberry supplement products may reduce the risk of recurrent urinary tract infections in otherwise healthy women.
In a letter of enforcement discretion issued on July 21, the FDA responded to a health claim petition submitted by Ocean Spray Cranberries. “A health claim characterizes the relationship between a substance and a disease or health-related condition,” according to the FDA. Ocean Spray Cranberries asked the FDA for an authorized health claim regarding the relationship between the consumption of cranberry beverages and supplements and a reduction in the risk of recurrent urinary tract infections (UTIs) in healthy women.
After reviewing the evidence, the FDA determined that the existing science did not support an authorized health claim, but did allow for a qualified health claim for certain cranberry juice beverages and supplements. A qualified health claim does not constitute an FDA approval; the FDA instead issues a Letter of Enforcement Discretion that includes language reflecting the level of scientific evidence for the claim.
The currently available scientific evidence for a relationship between cranberry and recurrent UTIs includes five intervention studies, according to the FDA letter. Two of these were high-quality, randomized, controlled trials in which daily consumption of a cranberry juice beverage was significantly associated with a reduced risk of recurrent UTIs. Another randomized, controlled trial yielded mixed results, and two other intervention studies that were moderate-quality, randomized, controlled trials showed no effect of cranberry juice consumption on UTI risk reduction.
The FDA’s letter of enforcement discretion states that, with regard to cranberry juice beverages, “Limited and inconsistent scientific evidence shows that by consuming one serving (8 oz) each day of a cranberry juice beverage, healthy women who have had a urinary tract infection may reduce their risk of recurrent UTI.”
Similarly, for cranberry dietary supplements, the FDA states that “Limited scientific evidence shows that, by consuming 500 mg each day of cranberry dietary supplement, healthy women who have had a urinary tract infection may reduce their risk of recurrent UTI.”
The qualified health claims apply specifically to cranberry juice beverages that contain at least 27% cranberry juice, and cranberry dietary supplements containing at least 500 mg of cranberry fruit powder. “The claims do not include other conventional foods or food products made from or containing cranberries, such as dried cranberries or cranberry sauce,” according to the FDA statement.
“With recurrent UTI, a major concern is the frequent use of antibiotics,” Constance Bohon, MD, an ob.gyn. in private practice in Washington and an assistant clinical professor at George Washington University, Washington, said in an interview.
“The challenge is to identify habits and/or nonantibiotic treatment to prevent recurrent UTI and decrease the need for antibiotics,” she said. “The regular use of cranberry can decrease the frequency of UTI in some, but not all, people.
“It does not appear to mask the symptoms of a UTI, so if it is not effective to prevent the infection, the presumptive diagnosis can be made based on the common symptoms,” she explained.
Dr. Bohon said that she has recommended the use of cranberry to some of her patients who have recurrent UTIs and has had success with many of them.
“I think it is important to make it clear that cranberry can be beneficial for some patients to decrease the frequency of UTI. It will not be effective for everyone who has frequent UTI, but for those who use it and have fewer UTIs, there will be less frequent exposure to antibiotics,” she emphasized. “What we need to know is who benefits the most from cranberry to prevent recurrent UTIs; whether age, race, coexisting health problems [such as diabetes], and use of hormonal contraception or menopause impact on its success.”
Dr. Bohon had no relevant financial conflicts to disclose.
Local analgesia before prolapse surgery may not be needed to reduce postop pain
Preoperative pelvic floor muscle injections and pudendal nerve blocks with bupivacaine and dexamethasone do not significantly improve pain control after vaginal apical prolapse repair, compared with placebo, according to a study.
In a randomized trial, patients generally reported mild postoperative pain and low dosages of narcotic use. “The majority reported that they returned to their baseline activity by 2 weeks after surgery, which should be reassuring to similar urogynecology patient populations,” said Lauren Giugale, MD.
Although many gynecologic surgeries increasingly are performed as outpatient procedures, patients may have inadequate pain control and persistently use narcotics after surgery. In an effort to reduce postoperative pain, doctors have tried preemptive analgesia with various local anesthetic techniques. These approaches have had mixed results, however, and there is “no consensus on the ideal local anesthetic technique to reduce postoperative pain after vaginal reconstructive surgery,” said Dr. Giugale, of the University of Pittsburgh.
To evaluate whether preoperative pelvic floor muscle injections and pudendal nerve blocks with bupivacaine and dexamethasone improve postoperative pain control after vaginal apical prolapse repairs, Dr. Giugale and colleagues conducted a three-arm, double-blind trial that included 75 patients. Patients received placebo (normal saline), bupivacaine alone, or bupivacaine combined with 4 mg of dexamethasone at four injection sites.
Dr. Giugale presented the study results at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
A range of procedures
Participants received bilateral levator ani muscle injections via a transobturator approach and pudendal nerve blocks via a transvaginal approach. They received the injections – 5 mL at each site – after the administration of general anesthesia but before the start of surgery. “Anecdotally, we have had good success” with the transobturator approach to treating chronic pelvic pain, which was part of the rationale for the trial, said Dr. Giugale.
The study included women 18 years or older who were scheduled for a vaginal native tissue repair with apical support. Participants had to be able to tolerate general anesthesia with a standardized enhanced recovery after surgery (ERAS) protocol. The investigators excluded women undergoing mesh-augmented prolapse repairs or abdominal surgery and those with chronic pelvic pain or immunosuppression.
Each treatment arm had 25 patients. Patients had an average age of 69 years and an average body mass index of 27.5 kg/m2. Most patients were white, and demographic variables did not significantly differ among the groups.
“The distribution of prolapse procedures was similar among study groups, with colpocleisis being the most common, followed by uterosacral ligament suspension, levator myorrhaphy, and sacrospinous ligament fixation,” said Dr. Giugale. Rates of concomitant hysterectomy were similar for each group.
Before surgery, patients completed pain, nausea, and activities assessments. At 6 hours after surgery, they completed pain and nausea assessments. During postoperative days 1 through 3, patients documented pain scores and analgesic use. One week after surgery, patients completed pain and activities assessments. And at postoperative weeks 2, 6, and 12, they completed additional activities assessments. The assessments included validated handouts that patients completed at home, and no additional office visits were required.
The numeric rating scale pain score on the day after surgery was the primary outcome, and the median pain score did not significantly differ among the groups (3.75 in the placebo group, 4 in the bupivacaine group, and 3 in the bupivacaine plus dexamethasone group). Between-group differences in pain scores at other time points also were not significant.
Activities assessments, nausea and vomiting scores, the percentage of patients with same-day discharge, urinary retention, postoperative narcotic use as measured by oral morphine equivalents, and adverse events also did not significantly differ among the groups.
“One week after surgery, 52% of women reported that they were at or better than their baseline preoperative activity level, which increased to 70% at 2 weeks, 84% at 6 weeks, and 94% at 12 weeks,” Dr. Giugale said.
In all, 57% of patients used narcotic medicine the day after surgery, which decreased to 44% on day 3. The dosage was low, with a median oral morphine equivalent of 5 mg of oxycodone or less per day, she said.
Early postoperative pain may be influenced by procedure type, according to an exploratory analysis. Through the first postoperative day, “there was a trend toward more pain with uterosacral ligament suspension,” Dr. Giugale said. By day 3, sacrospinous ligament fixation was associated with significantly more postoperative pain.
The role of ERAS protocols
The heterogeneity of surgical procedures among the treatment groups and the use of a predefined ERAS protocol may have confounded the results. In addition, the researchers did not measure patient satisfaction, and the findings may not apply to different patient populations, Dr. Giugale noted.
“As more and more gynecologic surgery patients have surgery under these enhanced recovery protocols, maybe additional preemptive local analgesia for vaginal reconstructive surgery is not all that beneficial,” she said. “Maybe we are getting enough benefit from the enhanced [recovery] protocols themselves.”
The investigators studied a novel idea – dual local therapy for pain in patients undergoing pelvic floor surgery – and described a novel transobturator technique for levator injection, commented Sunil Balgobin, MD, associate director of the female pelvic medicine and reconstructive surgery fellowship at University of Texas Southwestern Medical Center, Dallas.
“For the current opioid problem, development of alternative pain control strategies is extremely important to reduce narcotic use and improve patient outcomes,” Dr. Balgobin said. The study “addresses an important gap in the literature, is relevant to surgeons performing vaginal apical procedures, and aims to advance research in this area for the potential benefit of ... patients.”
Interpretation of the results for individual procedure types may be limited by the smaller sample sizes, he added.
The researchers and Dr. Balgobin had no relevant financial disclosures.
SOURCE: Giugale L et al. SGS 2020, Abstract 10.
Preoperative pelvic floor muscle injections and pudendal nerve blocks with bupivacaine and dexamethasone do not significantly improve pain control after vaginal apical prolapse repair, compared with placebo, according to a study.
In a randomized trial, patients generally reported mild postoperative pain and low dosages of narcotic use. “The majority reported that they returned to their baseline activity by 2 weeks after surgery, which should be reassuring to similar urogynecology patient populations,” said Lauren Giugale, MD.
Although many gynecologic surgeries increasingly are performed as outpatient procedures, patients may have inadequate pain control and persistently use narcotics after surgery. In an effort to reduce postoperative pain, doctors have tried preemptive analgesia with various local anesthetic techniques. These approaches have had mixed results, however, and there is “no consensus on the ideal local anesthetic technique to reduce postoperative pain after vaginal reconstructive surgery,” said Dr. Giugale, of the University of Pittsburgh.
To evaluate whether preoperative pelvic floor muscle injections and pudendal nerve blocks with bupivacaine and dexamethasone improve postoperative pain control after vaginal apical prolapse repairs, Dr. Giugale and colleagues conducted a three-arm, double-blind trial that included 75 patients. Patients received placebo (normal saline), bupivacaine alone, or bupivacaine combined with 4 mg of dexamethasone at four injection sites.
Dr. Giugale presented the study results at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
A range of procedures
Participants received bilateral levator ani muscle injections via a transobturator approach and pudendal nerve blocks via a transvaginal approach. They received the injections – 5 mL at each site – after the administration of general anesthesia but before the start of surgery. “Anecdotally, we have had good success” with the transobturator approach to treating chronic pelvic pain, which was part of the rationale for the trial, said Dr. Giugale.
The study included women 18 years or older who were scheduled for a vaginal native tissue repair with apical support. Participants had to be able to tolerate general anesthesia with a standardized enhanced recovery after surgery (ERAS) protocol. The investigators excluded women undergoing mesh-augmented prolapse repairs or abdominal surgery and those with chronic pelvic pain or immunosuppression.
Each treatment arm had 25 patients. Patients had an average age of 69 years and an average body mass index of 27.5 kg/m2. Most patients were white, and demographic variables did not significantly differ among the groups.
“The distribution of prolapse procedures was similar among study groups, with colpocleisis being the most common, followed by uterosacral ligament suspension, levator myorrhaphy, and sacrospinous ligament fixation,” said Dr. Giugale. Rates of concomitant hysterectomy were similar for each group.
Before surgery, patients completed pain, nausea, and activities assessments. At 6 hours after surgery, they completed pain and nausea assessments. During postoperative days 1 through 3, patients documented pain scores and analgesic use. One week after surgery, patients completed pain and activities assessments. And at postoperative weeks 2, 6, and 12, they completed additional activities assessments. The assessments included validated handouts that patients completed at home, and no additional office visits were required.
The numeric rating scale pain score on the day after surgery was the primary outcome, and the median pain score did not significantly differ among the groups (3.75 in the placebo group, 4 in the bupivacaine group, and 3 in the bupivacaine plus dexamethasone group). Between-group differences in pain scores at other time points also were not significant.
Activities assessments, nausea and vomiting scores, the percentage of patients with same-day discharge, urinary retention, postoperative narcotic use as measured by oral morphine equivalents, and adverse events also did not significantly differ among the groups.
“One week after surgery, 52% of women reported that they were at or better than their baseline preoperative activity level, which increased to 70% at 2 weeks, 84% at 6 weeks, and 94% at 12 weeks,” Dr. Giugale said.
In all, 57% of patients used narcotic medicine the day after surgery, which decreased to 44% on day 3. The dosage was low, with a median oral morphine equivalent of 5 mg of oxycodone or less per day, she said.
Early postoperative pain may be influenced by procedure type, according to an exploratory analysis. Through the first postoperative day, “there was a trend toward more pain with uterosacral ligament suspension,” Dr. Giugale said. By day 3, sacrospinous ligament fixation was associated with significantly more postoperative pain.
The role of ERAS protocols
The heterogeneity of surgical procedures among the treatment groups and the use of a predefined ERAS protocol may have confounded the results. In addition, the researchers did not measure patient satisfaction, and the findings may not apply to different patient populations, Dr. Giugale noted.
“As more and more gynecologic surgery patients have surgery under these enhanced recovery protocols, maybe additional preemptive local analgesia for vaginal reconstructive surgery is not all that beneficial,” she said. “Maybe we are getting enough benefit from the enhanced [recovery] protocols themselves.”
The investigators studied a novel idea – dual local therapy for pain in patients undergoing pelvic floor surgery – and described a novel transobturator technique for levator injection, commented Sunil Balgobin, MD, associate director of the female pelvic medicine and reconstructive surgery fellowship at University of Texas Southwestern Medical Center, Dallas.
“For the current opioid problem, development of alternative pain control strategies is extremely important to reduce narcotic use and improve patient outcomes,” Dr. Balgobin said. The study “addresses an important gap in the literature, is relevant to surgeons performing vaginal apical procedures, and aims to advance research in this area for the potential benefit of ... patients.”
Interpretation of the results for individual procedure types may be limited by the smaller sample sizes, he added.
The researchers and Dr. Balgobin had no relevant financial disclosures.
SOURCE: Giugale L et al. SGS 2020, Abstract 10.
Preoperative pelvic floor muscle injections and pudendal nerve blocks with bupivacaine and dexamethasone do not significantly improve pain control after vaginal apical prolapse repair, compared with placebo, according to a study.
In a randomized trial, patients generally reported mild postoperative pain and low dosages of narcotic use. “The majority reported that they returned to their baseline activity by 2 weeks after surgery, which should be reassuring to similar urogynecology patient populations,” said Lauren Giugale, MD.
Although many gynecologic surgeries increasingly are performed as outpatient procedures, patients may have inadequate pain control and persistently use narcotics after surgery. In an effort to reduce postoperative pain, doctors have tried preemptive analgesia with various local anesthetic techniques. These approaches have had mixed results, however, and there is “no consensus on the ideal local anesthetic technique to reduce postoperative pain after vaginal reconstructive surgery,” said Dr. Giugale, of the University of Pittsburgh.
To evaluate whether preoperative pelvic floor muscle injections and pudendal nerve blocks with bupivacaine and dexamethasone improve postoperative pain control after vaginal apical prolapse repairs, Dr. Giugale and colleagues conducted a three-arm, double-blind trial that included 75 patients. Patients received placebo (normal saline), bupivacaine alone, or bupivacaine combined with 4 mg of dexamethasone at four injection sites.
Dr. Giugale presented the study results at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
A range of procedures
Participants received bilateral levator ani muscle injections via a transobturator approach and pudendal nerve blocks via a transvaginal approach. They received the injections – 5 mL at each site – after the administration of general anesthesia but before the start of surgery. “Anecdotally, we have had good success” with the transobturator approach to treating chronic pelvic pain, which was part of the rationale for the trial, said Dr. Giugale.
The study included women 18 years or older who were scheduled for a vaginal native tissue repair with apical support. Participants had to be able to tolerate general anesthesia with a standardized enhanced recovery after surgery (ERAS) protocol. The investigators excluded women undergoing mesh-augmented prolapse repairs or abdominal surgery and those with chronic pelvic pain or immunosuppression.
Each treatment arm had 25 patients. Patients had an average age of 69 years and an average body mass index of 27.5 kg/m2. Most patients were white, and demographic variables did not significantly differ among the groups.
“The distribution of prolapse procedures was similar among study groups, with colpocleisis being the most common, followed by uterosacral ligament suspension, levator myorrhaphy, and sacrospinous ligament fixation,” said Dr. Giugale. Rates of concomitant hysterectomy were similar for each group.
Before surgery, patients completed pain, nausea, and activities assessments. At 6 hours after surgery, they completed pain and nausea assessments. During postoperative days 1 through 3, patients documented pain scores and analgesic use. One week after surgery, patients completed pain and activities assessments. And at postoperative weeks 2, 6, and 12, they completed additional activities assessments. The assessments included validated handouts that patients completed at home, and no additional office visits were required.
The numeric rating scale pain score on the day after surgery was the primary outcome, and the median pain score did not significantly differ among the groups (3.75 in the placebo group, 4 in the bupivacaine group, and 3 in the bupivacaine plus dexamethasone group). Between-group differences in pain scores at other time points also were not significant.
Activities assessments, nausea and vomiting scores, the percentage of patients with same-day discharge, urinary retention, postoperative narcotic use as measured by oral morphine equivalents, and adverse events also did not significantly differ among the groups.
“One week after surgery, 52% of women reported that they were at or better than their baseline preoperative activity level, which increased to 70% at 2 weeks, 84% at 6 weeks, and 94% at 12 weeks,” Dr. Giugale said.
In all, 57% of patients used narcotic medicine the day after surgery, which decreased to 44% on day 3. The dosage was low, with a median oral morphine equivalent of 5 mg of oxycodone or less per day, she said.
Early postoperative pain may be influenced by procedure type, according to an exploratory analysis. Through the first postoperative day, “there was a trend toward more pain with uterosacral ligament suspension,” Dr. Giugale said. By day 3, sacrospinous ligament fixation was associated with significantly more postoperative pain.
The role of ERAS protocols
The heterogeneity of surgical procedures among the treatment groups and the use of a predefined ERAS protocol may have confounded the results. In addition, the researchers did not measure patient satisfaction, and the findings may not apply to different patient populations, Dr. Giugale noted.
“As more and more gynecologic surgery patients have surgery under these enhanced recovery protocols, maybe additional preemptive local analgesia for vaginal reconstructive surgery is not all that beneficial,” she said. “Maybe we are getting enough benefit from the enhanced [recovery] protocols themselves.”
The investigators studied a novel idea – dual local therapy for pain in patients undergoing pelvic floor surgery – and described a novel transobturator technique for levator injection, commented Sunil Balgobin, MD, associate director of the female pelvic medicine and reconstructive surgery fellowship at University of Texas Southwestern Medical Center, Dallas.
“For the current opioid problem, development of alternative pain control strategies is extremely important to reduce narcotic use and improve patient outcomes,” Dr. Balgobin said. The study “addresses an important gap in the literature, is relevant to surgeons performing vaginal apical procedures, and aims to advance research in this area for the potential benefit of ... patients.”
Interpretation of the results for individual procedure types may be limited by the smaller sample sizes, he added.
The researchers and Dr. Balgobin had no relevant financial disclosures.
SOURCE: Giugale L et al. SGS 2020, Abstract 10.
FROM SGS 2020
Are laser treatments better than steroids for lichen sclerosus?
Laser treatment for lichen sclerosus was noninferior to steroid therapy after 6 months and may lead to better outcomes on various patient- and physician-reported measures, according to trial results presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
Patients with lichen sclerosus often present with itching, burning, and dysuria. Untreated, the vulvar dystrophy can cause architectural changes and is associated with an increased risk of vulvar malignancies.
Topical steroids are the standard treatment. To assess whether fractional CO2 laser treatment is noninferior to clobetasol propionate at 6 months, Linda Burkett, MD, and colleagues conducted a randomized controlled trial. Dr. Burkett is affiliated with MedStar Washington Hospital Center and Georgetown University in Washington and UPMC Magee-Womens Hospital in Pittsburgh.
The researchers enrolled 52 postmenopausal women with biopsy-proven lichen sclerosus. Patients had to have significant symptoms reflected by a score of at least 21 on the Skindex-29.
Twenty-seven women were assigned to receive laser therapy, and 25 were assigned to receive steroids. One patient in the steroid arm was lost to follow-up. About half of the patients in each group had prior clobetasol propionate exposure.
Patients in the steroid arm were started on 0.05% clobetasol propionate used nightly for 4 weeks, then three times per week for 8 weeks, and then as needed. They had a phone call follow-up at 2 weeks to confirm compliance and an optional in-person appointment at 3 months.
Patients in the laser arm received three laser treatments 4-6 weeks apart.
At 6 months, all patients returned for repeat assessments. The primary outcome was the Skindex-29, a dermatologic questionnaire. Secondary outcomes included a patient visual analog scale of bothersome vulvar symptoms, a provider visual assessment score, the Vaginal Health Index, the Vulvovaginal Symptom Questionnaire, the Patient Global Impression of Improvement, and the Patient Global Impression of Satisfaction.
Average Skindex-29 scores from baseline to 6 months improved more in the laser treatment group, compared with the steroid group, for all health-related quality of life categories: overall, emotional, functional, and symptoms. “At 6 months across all scores, patients reported very little bother,” Dr. Burkett said.
Differences between the groups were statistically significant for all but the functional subscore.
Average scores on subjective secondary outcomes improved more in the laser treatment group, compared with the steroid treatment group. The between-group differences were statistically significant for irritation and the Vulvovaginal Symptom Questionnaire.
For provider-based scores, patients in the laser group had greater improvement on all measures except perianal involvement, relative to patients in the steroid group. In addition, fusion of the labia minora and phimosis worsened in the steroid group.
Differences between the groups were statistically significant for phimosis, erosion, and the Vaginal Health Index.
Significantly more patients in the laser group than in the steroid group were satisfied or very satisfied with the results at 6 months (81% vs. 41%). Patients in the laser group were more likely to report that they were better or much better (89% vs. 62%), though the difference was not statistically significant.
There were no major adverse events.
The trial – the first randomized controlled study of energy-based treatment for lichen sclerosus – was conducted at a single center, and treatment was not blinded, Dr. Burkett noted.
“The treatment effect was pretty significant in favor of laser therapy,” said Cecile A. Ferrando, MD, MPH, of the Center for Urogynecology and Pelvic Reconstructive Surgery at Cleveland Clinic, commenting on the research.
“Compliance issues with clobetasol aside,” the findings raise the question of whether laser therapy should be offered as first-line treatment, Dr. Ferrando said.
The study might have been more robust had it excluded patients with previous clobetasol propionate exposure, Dr. Ferrando added.
Dr. Burkett noted that future studies may incorporate multiple centers, histology measures, and sham laser treatments and include only women who have not previously received clobetasol propionate.
The researchers had no relevant financial disclosures. Dr. Ferrando disclosed authorship royalties from UpToDate.
SOURCE: Burkett L et al. SGS 2020, Abstract 09.
Laser treatment for lichen sclerosus was noninferior to steroid therapy after 6 months and may lead to better outcomes on various patient- and physician-reported measures, according to trial results presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
Patients with lichen sclerosus often present with itching, burning, and dysuria. Untreated, the vulvar dystrophy can cause architectural changes and is associated with an increased risk of vulvar malignancies.
Topical steroids are the standard treatment. To assess whether fractional CO2 laser treatment is noninferior to clobetasol propionate at 6 months, Linda Burkett, MD, and colleagues conducted a randomized controlled trial. Dr. Burkett is affiliated with MedStar Washington Hospital Center and Georgetown University in Washington and UPMC Magee-Womens Hospital in Pittsburgh.
The researchers enrolled 52 postmenopausal women with biopsy-proven lichen sclerosus. Patients had to have significant symptoms reflected by a score of at least 21 on the Skindex-29.
Twenty-seven women were assigned to receive laser therapy, and 25 were assigned to receive steroids. One patient in the steroid arm was lost to follow-up. About half of the patients in each group had prior clobetasol propionate exposure.
Patients in the steroid arm were started on 0.05% clobetasol propionate used nightly for 4 weeks, then three times per week for 8 weeks, and then as needed. They had a phone call follow-up at 2 weeks to confirm compliance and an optional in-person appointment at 3 months.
Patients in the laser arm received three laser treatments 4-6 weeks apart.
At 6 months, all patients returned for repeat assessments. The primary outcome was the Skindex-29, a dermatologic questionnaire. Secondary outcomes included a patient visual analog scale of bothersome vulvar symptoms, a provider visual assessment score, the Vaginal Health Index, the Vulvovaginal Symptom Questionnaire, the Patient Global Impression of Improvement, and the Patient Global Impression of Satisfaction.
Average Skindex-29 scores from baseline to 6 months improved more in the laser treatment group, compared with the steroid group, for all health-related quality of life categories: overall, emotional, functional, and symptoms. “At 6 months across all scores, patients reported very little bother,” Dr. Burkett said.
Differences between the groups were statistically significant for all but the functional subscore.
Average scores on subjective secondary outcomes improved more in the laser treatment group, compared with the steroid treatment group. The between-group differences were statistically significant for irritation and the Vulvovaginal Symptom Questionnaire.
For provider-based scores, patients in the laser group had greater improvement on all measures except perianal involvement, relative to patients in the steroid group. In addition, fusion of the labia minora and phimosis worsened in the steroid group.
Differences between the groups were statistically significant for phimosis, erosion, and the Vaginal Health Index.
Significantly more patients in the laser group than in the steroid group were satisfied or very satisfied with the results at 6 months (81% vs. 41%). Patients in the laser group were more likely to report that they were better or much better (89% vs. 62%), though the difference was not statistically significant.
There were no major adverse events.
The trial – the first randomized controlled study of energy-based treatment for lichen sclerosus – was conducted at a single center, and treatment was not blinded, Dr. Burkett noted.
“The treatment effect was pretty significant in favor of laser therapy,” said Cecile A. Ferrando, MD, MPH, of the Center for Urogynecology and Pelvic Reconstructive Surgery at Cleveland Clinic, commenting on the research.
“Compliance issues with clobetasol aside,” the findings raise the question of whether laser therapy should be offered as first-line treatment, Dr. Ferrando said.
The study might have been more robust had it excluded patients with previous clobetasol propionate exposure, Dr. Ferrando added.
Dr. Burkett noted that future studies may incorporate multiple centers, histology measures, and sham laser treatments and include only women who have not previously received clobetasol propionate.
The researchers had no relevant financial disclosures. Dr. Ferrando disclosed authorship royalties from UpToDate.
SOURCE: Burkett L et al. SGS 2020, Abstract 09.
Laser treatment for lichen sclerosus was noninferior to steroid therapy after 6 months and may lead to better outcomes on various patient- and physician-reported measures, according to trial results presented at the virtual annual scientific meeting of the Society of Gynecologic Surgeons.
Patients with lichen sclerosus often present with itching, burning, and dysuria. Untreated, the vulvar dystrophy can cause architectural changes and is associated with an increased risk of vulvar malignancies.
Topical steroids are the standard treatment. To assess whether fractional CO2 laser treatment is noninferior to clobetasol propionate at 6 months, Linda Burkett, MD, and colleagues conducted a randomized controlled trial. Dr. Burkett is affiliated with MedStar Washington Hospital Center and Georgetown University in Washington and UPMC Magee-Womens Hospital in Pittsburgh.
The researchers enrolled 52 postmenopausal women with biopsy-proven lichen sclerosus. Patients had to have significant symptoms reflected by a score of at least 21 on the Skindex-29.
Twenty-seven women were assigned to receive laser therapy, and 25 were assigned to receive steroids. One patient in the steroid arm was lost to follow-up. About half of the patients in each group had prior clobetasol propionate exposure.
Patients in the steroid arm were started on 0.05% clobetasol propionate used nightly for 4 weeks, then three times per week for 8 weeks, and then as needed. They had a phone call follow-up at 2 weeks to confirm compliance and an optional in-person appointment at 3 months.
Patients in the laser arm received three laser treatments 4-6 weeks apart.
At 6 months, all patients returned for repeat assessments. The primary outcome was the Skindex-29, a dermatologic questionnaire. Secondary outcomes included a patient visual analog scale of bothersome vulvar symptoms, a provider visual assessment score, the Vaginal Health Index, the Vulvovaginal Symptom Questionnaire, the Patient Global Impression of Improvement, and the Patient Global Impression of Satisfaction.
Average Skindex-29 scores from baseline to 6 months improved more in the laser treatment group, compared with the steroid group, for all health-related quality of life categories: overall, emotional, functional, and symptoms. “At 6 months across all scores, patients reported very little bother,” Dr. Burkett said.
Differences between the groups were statistically significant for all but the functional subscore.
Average scores on subjective secondary outcomes improved more in the laser treatment group, compared with the steroid treatment group. The between-group differences were statistically significant for irritation and the Vulvovaginal Symptom Questionnaire.
For provider-based scores, patients in the laser group had greater improvement on all measures except perianal involvement, relative to patients in the steroid group. In addition, fusion of the labia minora and phimosis worsened in the steroid group.
Differences between the groups were statistically significant for phimosis, erosion, and the Vaginal Health Index.
Significantly more patients in the laser group than in the steroid group were satisfied or very satisfied with the results at 6 months (81% vs. 41%). Patients in the laser group were more likely to report that they were better or much better (89% vs. 62%), though the difference was not statistically significant.
There were no major adverse events.
The trial – the first randomized controlled study of energy-based treatment for lichen sclerosus – was conducted at a single center, and treatment was not blinded, Dr. Burkett noted.
“The treatment effect was pretty significant in favor of laser therapy,” said Cecile A. Ferrando, MD, MPH, of the Center for Urogynecology and Pelvic Reconstructive Surgery at Cleveland Clinic, commenting on the research.
“Compliance issues with clobetasol aside,” the findings raise the question of whether laser therapy should be offered as first-line treatment, Dr. Ferrando said.
The study might have been more robust had it excluded patients with previous clobetasol propionate exposure, Dr. Ferrando added.
Dr. Burkett noted that future studies may incorporate multiple centers, histology measures, and sham laser treatments and include only women who have not previously received clobetasol propionate.
The researchers had no relevant financial disclosures. Dr. Ferrando disclosed authorship royalties from UpToDate.
SOURCE: Burkett L et al. SGS 2020, Abstract 09.
FROM SGS 2020
Confronting the epidemic of racism in ObGyn practice
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
CASE Black woman in stable labor expresses fear
A 29-year-old Black woman (G1) at 39 0/7 weeks’ gestation presents to your labor and delivery unit reporting leaking fluid and contractions. She is found to have ruptured membranes and reassuring fetal testing. Her cervix is 4 cm dilated, and you recommend admission for expectant management of labor. She is otherwise healthy and has no significant medical history.
As you are finishing admitting this patient, you ask if she has any remaining questions. She asks quietly, “Am I going to die today?”
You provide reassurance of her stable clinical picture, then pause and ask the patient about her fears. She looks at you and says, “They didn’t believe Serena Williams, so why would they believe me?”
Your patient is referencing Serena Williams’ harrowing and public postpartum course, complicated by a pulmonary embolism and several reoperations.1 While many of us in the medical field may read this account as a story of challenges with an ultimate triumph, many expectant Black mothers hold Serena’s experience as a cautionary tale about deep-rooted inequities in our health care system that lead to potentially dangerous outcomes.
Disparities in care
They are right to be concerned. In the United States, Black mothers are 4 times more likely to die during or after pregnancy, mostly from preventable causes,2 and nearly 50% more likely to have a preterm delivery.3 These disparities extend beyond the delivery room to all aspects of ObGyn care. Black women are 2 to 3 times more likely to die from cervical cancer, and they are more likely to be diagnosed at a later stage, thus rendering treatment less effective.4 Black patients also have a higher burden of obesity, diabetes, and cardiac disease, and when they present to the hospital, receive evidence-based treatment at lower rates compared with White patients.5
Mourning the deaths of Ahmaud Arbery, Breonna Taylor, and George Floyd, amongst the many other Black lives taken unjustly in the United States, has highlighted egregious practices against people of color embedded within the systems meant to protect and serve our communities. We as ObGyn physicians must take professional onus to recognize a devastating but humbling truth—systemic racism has long pervaded our health care practices and systems, and now more than ever, we must do more to stand by and for our patients.
As ObGyns, we help support patients through some of the happiest, most vulnerable, and potentially most dire moments of their lives. We help patients through the birth of their children, reproductive struggles, gynecologic concerns, and cancer diagnoses. Many of us chose this field for the privilege of caring for patients at these critical moments in their lives, but we have often neglected the racism present in our practices, our hospital settings, and the medical system itself. We often fail to acknowledge our own implicit bias and the role that we play in contributing to acts and experiences of racism that our patients and our colleagues face on a daily basis.
Racism in our origins
The history of obstetrics and gynecology shows us a long record of physicians perpetrating injustices that target marginalized communities of color. Dr. James Sims, often given the title of “father of modern gynecology,” performed numerous experiments on unanesthetized Black female slaves to develop procedures for fistulae repair and other surgical techniques.6 Throughout the twentieth century, dating as recent as 1979, state laws written in the name of public safety forcibly sterilized women of color to control an “undesirable population.”7 When a patient of color declines a method of long-acting reversible contraception, birth control pills, or tubal ligation, do you take the time to reflect on the potential context of the patient’s decision?
It is critical to recognize the legacy that these acts have on our patients today, leading to a higher burden of disease and an understandable distrust of the medical system. The uncovering of the unethical practices of the National Institutions of Health‒funded Tuskegee syphilis study, in which hundreds of Black men with latent syphilis were passively monitored despite the knowledge of a proven treatment, has attributed to a measurable decrease in life expectancy among Black males.8 Even as we face the COVID-19 pandemic, the undercurrent of racism continues to do harm. Black patients are 5 times more likely to be hospitalized with COVID-19 than their White counterparts. This disparity, in part, is a product of a higher burden of comorbidities and the privilege associated with shelter-in-place policies, which disproportionately strain communities of color.9
We as a medical community need to do better for our patients. No matter how difficult to confront, each of us must acknowledge our own biases and our duty to combat persistent and perpetual racism in our medical system. We need to commit to amplifying the voices of our Black patients and colleagues. It is not enough to celebrate diversity for performance sake—it is time to recognize that diversity saves lives.
We have a responsibility to rectify these traditions of injustice and work toward a safer, more equitable, healthy future for our patients and their families. While this pledge may seem daunting, changes at individual and systems levels can make a difference for all patients that come through our doors. In addition, to honor our oath to “do no harm,” we must act; Black lives matter, and we are charged as medical providers to help our patients thrive, especially those from historically oppressed communities and who continue to suffer inexcusable injustices in health care and beyond.
Take action
Here is a collection of ways to institute an antiracist environment and more equitable care for your patients.
Self-reflect and educate
- Learn about the role racism plays in ObGyn and modern medicine. One place to start: read “Medical Bondage: Race, Gender and the Origins of American Gynecology” by Deidre Cooper Owens. Also check out articles and key readings curated by the Black Mamas Matter Alliance.
- Introduce and sustain antiracism training for all staff in your clinic or hospital system. To start, consider taking these free and quick implicit bias tests at a staff or department meeting.
- Familiarize yourself and your colleagues with facets of reproductive justice—the human right to have children, to not have children, and to nurture children in a safe and healthy environment—and incorporate these values in your practice. Request trainings in reproductive justice from community groups like Sister Song.
- Sign up for updates for state and national bills addressing health inequity and access to reproductive health services. Show your support by calling your congress-people, testifying, or donating to a cause that promotes these bills. You can stay up to date on national issues with government affairs newsletters from the American College of Obstetricians and Gynecologists. Sign up here.
- Continue the conversation and re-evaluate your personal and institution’s efforts to combat racism and social and reproductive injustices.
Provide access to high-quality reproductive health care
- Ask your patients what barriers they faced to come to your clinic and receive the care they needed. Consider incorporating the following screening tools regarding social determinants of health: PRAPARE screening tool, AAFP screening tool.
- Promote access to insurance and support programs, including nutrition, exercise and wellness, and safe home and school environments. Look up resources available to your patients by their zip codes using AAFP’s Neighborhood Navigator.
- Help patients access their medications at affordable prices in their neighborhoods by using free apps. Use the GoodRx app to identify discounts for prescriptions at various pharmacies, and search the Bedsider app to find out how your patients can get their birth control for free and delivered to their homes.
- Expand access to language services for patients who do not speak English as their first language. If working in a resource-limited setting, use the Google Translate app. Print out these free handouts for birth control fact sheets in different languages.
- Establish standardized protocols for common treatment paradigms to reduce the influence of bias in clinical scenarios. For example, institute a protocol for managing postoperative pain to ensure equal access to treatment.
- Institute the AIM (Alliance for Innovation on Maternal Health) patient safety bundle on the Reduction of Peripartum Racial/Ethnic Disparities. Learn more about AIM’s maternal safety and quality improvement initiative to reduce maternal morbidity and mortality here.
Support a diverse workforce
- Designate and/or hire a Diversity and Inclusion Officer at your institution to ensure that hiring practices actively achieve a diverse workforce and that employees feel supported in the work environment. Consider coalition-building between hospitals, like the UPHS-CHOP Alliance of Minority Physicians.
- Recruit diverse applicants by advertising positions to groups that focus on the advancement of underrepresented minorities in medicine. Engage with your local chapter of the National Medical Association and American Medical Women’s Association.
- Have a system in place for anonymous reporting of incidents involving bias or discrimination against staff, and develop a protocol to ensure action is taken in case of such incidents.
- Institute a recurring conference or Grand Rounds across disciplines to discuss the impacts of bias and discrimination on patients and providers at your institution. View examples of these conferences here.
- Ensure invited speakers and other educational opportunities are comprised of diverse representation.
- Create a work environment with safe spaces for the discussion of racism, discrimination, and bias.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
- Haskell R. Serena Williams on motherhood, marriage, and making her comeback. January 10, 2018. https://www.vogue.com/article/serena-williams-vogue-cover-interview-february-2018. Accessed July 1, 2020.
- Louis JM, Menard MK, Gee RE. Racial and ethnic disparities in maternal morbidity and mortality. Obstet Gynecol. 2015;125:690-694.
- Sigurdson K, Mitchell B, Liu J, et al. Racial/ethnic disparities in neonatal intensive care: a systematic review. Pediatrics. 2019;144:e20183114.
- Garner EI. Cervical cancer: disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s-247s.
- Arora S, Stouffer GA, Kucharska‐Newton A, et al. Fifteen‐year trends in management and outcomes of non–ST‐segment–elevation myocardial infarction among black and white patients: the ARIC community surveillance study, 2000–2014. J Am Heart Assoc. 2018;7:e010203.
- Zellars R. Black subjectivity and the origins of American gynecology. May 31, 2018. https://www.aaihs.org/black-subjectivity-and-the-origins-of-american-gynecology/. Accessed June 28, 2020.
- Ko K. Unwanted sterilization and eugenics programs in the United States. January 29, 2016. https://www.pbs.org/independentlens/blog/unwanted-sterilization-and-eugenics-programs-in-the-united-states/. Accessed June 28, 2020.
- Alsan M, Wanamaker M. Tuskegee and the health of black men. Q J Econ. 2018;133:407-455.
- Hooper MW, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020 May 11. doi: 10.1001/jama.2020.8598.
Candidiasis: The essentials of diagnosis and treatment
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8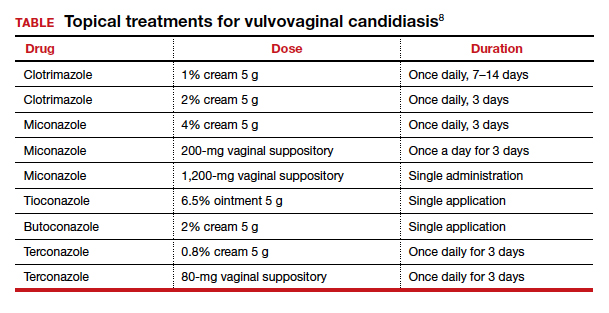
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
CASE Woman with vulvar itch and white vaginal discharge
A 26-year-old sexually active nulligravid woman requests evaluation for moderately intense “itching in the vagina and on the vulva.” She uses combination oral contraceptives and has 2 current sexual partners. On physical examination, you note a thick, white, curd-like discharge that is adherent to the vaginal epithelium. The vulva is erythematous, and small “satellite lesions” are evident in the intertriginous folds.
- What is the most likely diagnosis?
- How should you treat this patient?
Approximately 75% of all women will have at least 1 episode of vulvovaginal candidiasis (VVC) in their lifetime.1 Candida albicans, the most commonly identified organism in these infections, colonizes the vagina of many individuals commensally; higher rates of colonization occur in women with diabetes, obesity, recent use of broad-spectrum antibiotics, steroid use and immunosuppression, and in women who are pregnant. Of special interest, pregnant women have an increased risk of symptomatic infection, and they respond less favorably to conventional treatment regimens.1
Deconstructing C albicans and other species
Historically, in more than 90% of cases, C albicans is the principal cause of VVC. While it remains the most prevalent Candida species in the United States, over the last 15 years studies have demonstrated that in some countries, such as India and Nigeria, C albicans constitutes less than half of the cultured species in women with VVC. This observation may be due to the widespread availability and use of common antifungal medications, which leads to resistance and selection for resistant species.1,2
In asymptomatic women, vaginal colonies of C albicans grow in the yeast form. This condition is usually well tolerated by the host and does not cause a major immune response. In periods of stress for the host micro- and mycobiomes, however (dysbiosis, immune suppression, trauma), C albicans is induced into morphogenesis, proliferating and forming hyphae that are thought to activate the host immune response. The vaginal epithelium becomes sensitized to the presence of C albicans and recruits large numbers of neutrophils that, in turn, drive the pathophysiology of VVC.3
There is a theory that the separation of the urethra and anus by the vagina has exerted evolutionary pressure to maintain the presence of commensal C albicans yeast colonies in the vagina. C albicans exerts an antagonistic effect on many bacteria and, therefore, may act as a “microbiologic barrier” between the anus and the urethra to prevent urinary tract infections that, before the modern antibiotic era, may have caused serious morbidity and even mortality.3
Other organisms that cause VVC include C glabrata, C parapsilosis, and C tropicalis. Ex vivo experiments have shown that co-infection of C albicans with C glabrata enhances the ability of C glabrata to invade tissue.2 C glabrata is more frequently resistant to commonly used antifungal compounds than C albicans,2,4 which suggests that identifying the specific fungal pathogen is becoming increasingly important in planning targeted therapy.
Continue to: A common infection...
A common infection
While three-quarters of women will experience VVC at least once in their lifetime, between 40% and 45% will experience it more than once, and 5% to 8% will develop recurrent VVC. Among pregnant women, 15% will develop symptomatic VVC.1,2
However, because VVC is not a reportable disease and antifungal medication is available over the counter without physician consultation, these numbers likely underestimate the true incidence of the infection.4
Complications in pregnancy
Vaginal infections, including VVC, bacterial vaginosis (BV), and trichomoniasis, may be associated with 40% of preterm deliveries.5 The high concentrations of estrogen and progesterone during pregnancy create a uniquely glycogen-rich vaginal environment in which Candida species can flourish.2,4 Even asymptomatic colonization of the vagina with Candida species has been associated with preterm labor, preterm birth, and low birth weight.1,6 This association appears to have more severe consequences if VVC occurs in the second trimester compared with the first trimester.6
Additionally, congenital candidiasis of the newborn may result from intrauterine Candida infection or heavy maternal vaginal colonization at delivery, and the infection is evident within 24 hours of birth. It presents typically as oropharyngeal candidiasis (thrush) of the newborn.1
Clinical manifestations of infection
The classic manifestations of Candida infection are similar in both the pregnant and nonpregnant patient: acute vaginal and vulvar pruritus and thick, white, malodorous “cottage cheese” vaginal discharge.1,4 Exercise caution, however, in treating presumptively based on these symptoms alone, especially in pregnancy, because they are not specific to candidiasis.4 Vaginal discharge is not always present, and it may vary in appearance and odor. Pruritus is the most specific symptom of Candida infection, but studies show that it is an accurate predictor in only 38% of cases.7
Other common signs and symptoms include the sensation of burning, dysuria, dyspareunia, fissures, excoriations, and pruritus ani. Physical examination demonstrates erythema and swelling of labial, vulvar, and vaginal structures, with a normal cervix and an adherent white or off-white discharge. When the discharge is removed from the vaginal wall, small bleeding points may appear.1,4
Making the diagnosis
As mentioned, history alone is not sufficient to make a definitive diagnosis of candidiasis. The diagnosis should be made by examining vaginal secretions under a microscope or by culture.4 A wet mount and KOH (potassium hydroxide) prep help differentiate VVC, BV, and trichomoniasis. Culture is particularly valuable in identifying less common fungal organisms, such as C glabrata and C tropicalis.
Vaginal pH testing is not conclusive for Candida because vaginal pH is normal in VVC. However, pH assessment can rule in other causative organisms if the value is abnormal (that is, elevated pH of 4.5 or greater with BV and trichomoniasis).1
Treatment options
Acute infection. A pregnant woman who tests positive for VVC may safely be treated in any trimester with a 7-day course of a topical azole.8 If the patient prefers the convenience of oral therapy, after the first trimester, oral fluconazole, 150 mg on day 1 and day 3, may be used for treatment. Note that fluconazole has been associated with an increased risk of spontaneous abortion and cardiac septal defects when used in the first trimester.1
The Centers for Disease Control and Prevention recommends a number of topical treatments for VVC (TABLE).8 Several of these drugs are available over the counter without a prescription. Topical azoles are more effective than nystatin in treating VVC, and posttreatment cultures are negative in up to 90% of treated patients.8
Recurrent infections. Recurrent VVC is defined as 4 or more episodes of symptomatic VVC within 12 months.8 Typical first-line treatment of recurrent infections in nonpregnant patients is a 6-month course of fluconazole, 150 mg weekly.9,10 As noted, however, fluconazole should not be used in the first trimester of pregnancy. It is acceptable therapy thereafter for patients who have troublesome recurrent or persistent infections.
Continue to: Strategies for preventing recurrence...
Strategies for preventing recurrence
While it is logical to consider antimycotic prophylaxis in women with a history of recurring VVC and/or a significant number of known risk factors, data suggest that extended prophylaxis with an azole does not consistently achieve long-term elimination of vaginal Candida organisms after cessation of the azole.9
At-risk women should be counseled to make lifestyle adjustments, such as wearing breathable cotton clothing, particularly undergarments; promptly changing out of damp clothing; and forgoing the use of commercial intravaginal feminine hygiene products.
Recent research has shown that the use of Saccharomyces cerevisiae–based probiotics has promise for controlling the burden of C albicans in women receiving antifungal drugs for VVC and also for preventing recurrence; however, this approach has undergone limited testing in humans, and its efficacy and safety in pregnancy is unknown.11 ●
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.
- Duff P. Maternal and fetal infection. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:862.
- Goncalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927.
- Hall RA, Noverr MC. Fungal interactions with the human host: exploring the spectrum of symbiosis. Curr Opin Microbiol. 2017;40:58-64.
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971.
- Holzer I, Farr A, Kiss H; et al. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295:891-895.
- Farr A, Kiss H, Holzer I, et al. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94:989-996.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379.
- Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351:876-883.
- US Food and Drug Administration. FDA Drug Safety Communication: Use of long-term, high-dose Diflucan (fluconazole) during pregnancy may be associated with birth defects in infants. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communicationuse-long-term-high-dose-diflucan-fluconazole-during-pregnancy-may-be#. Updated August 4, 2017. Accessed July 6, 2020.
- Gaziano R, Sabbatini S, Roselletti E, et al. Saccharomyces cerevisiae-based probiotics as novel antimicrobial agents to prevent and treat vaginal infections. Front Microbiol. 2020;11:718.












