User login
One-week postsurgical interval for voiding trial increases pass rate
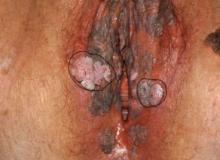
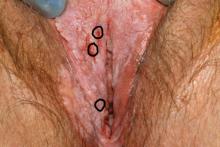
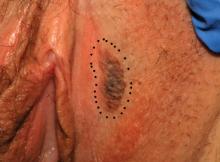
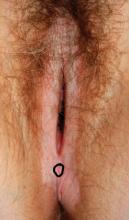
Women who underwent vaginal prolapse surgery and did not immediately have a successful voiding trial were seven times more likely to pass their second voiding trial if their follow-up was 7 days after surgery instead of 4 days, according to a study in the American Journal of Obstetrics and Gynecology.
“This information is useful for setting expectations and for counseling patients on when it might be best to repeat a voiding trial in those with transient incomplete bladder emptying on the day of surgery, especially for those who may not live close to their surgeon, or for those who have difficulty traveling to the office,” said Jeffrey S. Schachar, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., and colleagues. “Despite a higher rate of initial unsuccessful office voiding trials, however, the early group did have significantly fewer days with an indwelling transurethral catheter, as well as total catheterization days,” including self-catheterization.
The researchers note that rates of temporary use of catheters after surgery vary widely, from 12% to 83%, likely because no consensus exists on how long to wait for voiding trials and what constitutes a successful trial.
“It is critical to identify patients with incomplete bladder emptying in order to prevent pain, myogenic and neurogenic damage, ureteral reflux and bladder overdistension that may further impair voiding function,” the authors wrote. “However, extending bladder drainage beyond the necessary recovery period may be associated with higher rates of urinary tract infection (UTI) and patient bother.”
To learn more about the best duration for postoperative catheter use, the researchers enrolled 102 patients before they underwent vaginal prolapse surgery at Wake Forest Baptist Health and Cleveland Clinic Florida from February 2017 to November 2019. The 29 patients with a successful voiding trial within 6 hours after surgery left the study, and 5 others were excluded for needing longer vaginal packing.
The voiding trial involved helping the patient stand to drain the bladder via the catheter, backfilling the bladder with 300 mL of saline solution through the catheter, removing the catheter to give women 1 hour to urinate, and then measuring the postvoid residual with a catheter or ultrasound. At least 100 mL postvoid residual was considered persistent incomplete bladder emptying.
The 60 remaining patients who did not pass the initial voiding trial and opted to remain in the study received a transurethral indwelling catheter and were randomly assigned to return for a second voiding trial either 2-4 days after surgery (depending on day of the week) or 7 days after surgery. The groups were demographically and clinically similar, with predominantly white postmenopausal, non-smoking women with stage II or III multicompartment pelvic organ prolapse.
Women without successful trials could continue with the transurethral catheter or give themselves intermittent catheterizations with a follow-up schedule determined by their surgeon. The researchers then tracked the women for 6 weeks to determine the rate of unsuccessful repeat voiding trials.
Among the women who returned 2-4 days post surgery, 23% had unsuccessful follow-up voiding trials, compared with 3% in the group returning 7 days after surgery (relative risk = 7; P = .02). The researchers calculated that one case of persistent postoperative incomplete bladder emptying was prevented for every five patients who used a catheter for 7 days after surgery.
Kevin A. Ault, MD, professor of obstetrics and gynecology at the University of Kansas Medical Center in Kansas City, said the study was well done, although the findings were unsurprising. He said the clinical implication is straightforward – to wait a week before doing a second voiding trial.
“I suspect these findings match the clinical experience of many surgeons. It is always good to see a well-done clinical trial on a topic,” Dr Ault said in an interview. “The most notable finding is how this impacts patient counseling. Gynecologists should tell their patients that it will take a week with a catheter when this problem arises.”
“The main limitation is whether this finding can be extrapolated to other gynecological surgeries, such as hysterectomy,” said Dr. Ault, who was not involved in the study. “Urinary retention is likely less common after that surgery, but it is still bothersome to patients.”
Dr. Schachar and associates also reported that patients in the earlier group “used significantly more morphine dose equivalents within 24 hours of the office voiding trial than the late-voiding trial group, which was expected given the proximity to surgery” (3 vs. 0.38; P = .005). However, new postoperative pain medication prescriptions and refills were similar in both groups.
Secondary endpoints included UTI rates, total days with a catheter, and patient experience of discomfort with the catheter. The two groups of women reported similar levels of catheter bother, but there was a nonsignificant difference in UTI rates: 23% in the earlier group, compared with 7% in the later group (P = .07).
The early-voiding trial group had an average 5 days with an indwelling transurethral catheter, compared with a significantly different 7 days in the later group (P = .0007). The early group also had fewer total days with an indwelling transurethral catheter and self-catheterization (6 days), compared with the late group (7 days; P = .0013). No patients had persistent incomplete bladder emptying after 17 days post surgery.
“Being able to adequately predict which patients are more likely to have unsuccessful postoperative voiding trials allows surgeons to better counsel their patients and may guide clinical decisions,” Dr. Schachar and associates said. They acknowledged, however, that their study’s biggest weakness is the small enrollment, which led to larger confidence intervals related to relative risk differences between the groups.
The study did not use external funding. Four of the investigators received grant, research funding, or honoraria from one or many medical device or pharmaceutical companies. The remaining researchers had no disclosures. Dr. Ault said he had no relevant financial disclosures.
SOURCE: Schachar JS et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.001.
Women who underwent vaginal prolapse surgery and did not immediately have a successful voiding trial were seven times more likely to pass their second voiding trial if their follow-up was 7 days after surgery instead of 4 days, according to a study in the American Journal of Obstetrics and Gynecology.
“This information is useful for setting expectations and for counseling patients on when it might be best to repeat a voiding trial in those with transient incomplete bladder emptying on the day of surgery, especially for those who may not live close to their surgeon, or for those who have difficulty traveling to the office,” said Jeffrey S. Schachar, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., and colleagues. “Despite a higher rate of initial unsuccessful office voiding trials, however, the early group did have significantly fewer days with an indwelling transurethral catheter, as well as total catheterization days,” including self-catheterization.
The researchers note that rates of temporary use of catheters after surgery vary widely, from 12% to 83%, likely because no consensus exists on how long to wait for voiding trials and what constitutes a successful trial.
“It is critical to identify patients with incomplete bladder emptying in order to prevent pain, myogenic and neurogenic damage, ureteral reflux and bladder overdistension that may further impair voiding function,” the authors wrote. “However, extending bladder drainage beyond the necessary recovery period may be associated with higher rates of urinary tract infection (UTI) and patient bother.”
To learn more about the best duration for postoperative catheter use, the researchers enrolled 102 patients before they underwent vaginal prolapse surgery at Wake Forest Baptist Health and Cleveland Clinic Florida from February 2017 to November 2019. The 29 patients with a successful voiding trial within 6 hours after surgery left the study, and 5 others were excluded for needing longer vaginal packing.
The voiding trial involved helping the patient stand to drain the bladder via the catheter, backfilling the bladder with 300 mL of saline solution through the catheter, removing the catheter to give women 1 hour to urinate, and then measuring the postvoid residual with a catheter or ultrasound. At least 100 mL postvoid residual was considered persistent incomplete bladder emptying.
The 60 remaining patients who did not pass the initial voiding trial and opted to remain in the study received a transurethral indwelling catheter and were randomly assigned to return for a second voiding trial either 2-4 days after surgery (depending on day of the week) or 7 days after surgery. The groups were demographically and clinically similar, with predominantly white postmenopausal, non-smoking women with stage II or III multicompartment pelvic organ prolapse.
Women without successful trials could continue with the transurethral catheter or give themselves intermittent catheterizations with a follow-up schedule determined by their surgeon. The researchers then tracked the women for 6 weeks to determine the rate of unsuccessful repeat voiding trials.
Among the women who returned 2-4 days post surgery, 23% had unsuccessful follow-up voiding trials, compared with 3% in the group returning 7 days after surgery (relative risk = 7; P = .02). The researchers calculated that one case of persistent postoperative incomplete bladder emptying was prevented for every five patients who used a catheter for 7 days after surgery.
Kevin A. Ault, MD, professor of obstetrics and gynecology at the University of Kansas Medical Center in Kansas City, said the study was well done, although the findings were unsurprising. He said the clinical implication is straightforward – to wait a week before doing a second voiding trial.
“I suspect these findings match the clinical experience of many surgeons. It is always good to see a well-done clinical trial on a topic,” Dr Ault said in an interview. “The most notable finding is how this impacts patient counseling. Gynecologists should tell their patients that it will take a week with a catheter when this problem arises.”
“The main limitation is whether this finding can be extrapolated to other gynecological surgeries, such as hysterectomy,” said Dr. Ault, who was not involved in the study. “Urinary retention is likely less common after that surgery, but it is still bothersome to patients.”
Dr. Schachar and associates also reported that patients in the earlier group “used significantly more morphine dose equivalents within 24 hours of the office voiding trial than the late-voiding trial group, which was expected given the proximity to surgery” (3 vs. 0.38; P = .005). However, new postoperative pain medication prescriptions and refills were similar in both groups.
Secondary endpoints included UTI rates, total days with a catheter, and patient experience of discomfort with the catheter. The two groups of women reported similar levels of catheter bother, but there was a nonsignificant difference in UTI rates: 23% in the earlier group, compared with 7% in the later group (P = .07).
The early-voiding trial group had an average 5 days with an indwelling transurethral catheter, compared with a significantly different 7 days in the later group (P = .0007). The early group also had fewer total days with an indwelling transurethral catheter and self-catheterization (6 days), compared with the late group (7 days; P = .0013). No patients had persistent incomplete bladder emptying after 17 days post surgery.
“Being able to adequately predict which patients are more likely to have unsuccessful postoperative voiding trials allows surgeons to better counsel their patients and may guide clinical decisions,” Dr. Schachar and associates said. They acknowledged, however, that their study’s biggest weakness is the small enrollment, which led to larger confidence intervals related to relative risk differences between the groups.
The study did not use external funding. Four of the investigators received grant, research funding, or honoraria from one or many medical device or pharmaceutical companies. The remaining researchers had no disclosures. Dr. Ault said he had no relevant financial disclosures.
SOURCE: Schachar JS et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.001.
Women who underwent vaginal prolapse surgery and did not immediately have a successful voiding trial were seven times more likely to pass their second voiding trial if their follow-up was 7 days after surgery instead of 4 days, according to a study in the American Journal of Obstetrics and Gynecology.
“This information is useful for setting expectations and for counseling patients on when it might be best to repeat a voiding trial in those with transient incomplete bladder emptying on the day of surgery, especially for those who may not live close to their surgeon, or for those who have difficulty traveling to the office,” said Jeffrey S. Schachar, MD, of Wake Forest Baptist Health in Winston-Salem, N.C., and colleagues. “Despite a higher rate of initial unsuccessful office voiding trials, however, the early group did have significantly fewer days with an indwelling transurethral catheter, as well as total catheterization days,” including self-catheterization.
The researchers note that rates of temporary use of catheters after surgery vary widely, from 12% to 83%, likely because no consensus exists on how long to wait for voiding trials and what constitutes a successful trial.
“It is critical to identify patients with incomplete bladder emptying in order to prevent pain, myogenic and neurogenic damage, ureteral reflux and bladder overdistension that may further impair voiding function,” the authors wrote. “However, extending bladder drainage beyond the necessary recovery period may be associated with higher rates of urinary tract infection (UTI) and patient bother.”
To learn more about the best duration for postoperative catheter use, the researchers enrolled 102 patients before they underwent vaginal prolapse surgery at Wake Forest Baptist Health and Cleveland Clinic Florida from February 2017 to November 2019. The 29 patients with a successful voiding trial within 6 hours after surgery left the study, and 5 others were excluded for needing longer vaginal packing.
The voiding trial involved helping the patient stand to drain the bladder via the catheter, backfilling the bladder with 300 mL of saline solution through the catheter, removing the catheter to give women 1 hour to urinate, and then measuring the postvoid residual with a catheter or ultrasound. At least 100 mL postvoid residual was considered persistent incomplete bladder emptying.
The 60 remaining patients who did not pass the initial voiding trial and opted to remain in the study received a transurethral indwelling catheter and were randomly assigned to return for a second voiding trial either 2-4 days after surgery (depending on day of the week) or 7 days after surgery. The groups were demographically and clinically similar, with predominantly white postmenopausal, non-smoking women with stage II or III multicompartment pelvic organ prolapse.
Women without successful trials could continue with the transurethral catheter or give themselves intermittent catheterizations with a follow-up schedule determined by their surgeon. The researchers then tracked the women for 6 weeks to determine the rate of unsuccessful repeat voiding trials.
Among the women who returned 2-4 days post surgery, 23% had unsuccessful follow-up voiding trials, compared with 3% in the group returning 7 days after surgery (relative risk = 7; P = .02). The researchers calculated that one case of persistent postoperative incomplete bladder emptying was prevented for every five patients who used a catheter for 7 days after surgery.
Kevin A. Ault, MD, professor of obstetrics and gynecology at the University of Kansas Medical Center in Kansas City, said the study was well done, although the findings were unsurprising. He said the clinical implication is straightforward – to wait a week before doing a second voiding trial.
“I suspect these findings match the clinical experience of many surgeons. It is always good to see a well-done clinical trial on a topic,” Dr Ault said in an interview. “The most notable finding is how this impacts patient counseling. Gynecologists should tell their patients that it will take a week with a catheter when this problem arises.”
“The main limitation is whether this finding can be extrapolated to other gynecological surgeries, such as hysterectomy,” said Dr. Ault, who was not involved in the study. “Urinary retention is likely less common after that surgery, but it is still bothersome to patients.”
Dr. Schachar and associates also reported that patients in the earlier group “used significantly more morphine dose equivalents within 24 hours of the office voiding trial than the late-voiding trial group, which was expected given the proximity to surgery” (3 vs. 0.38; P = .005). However, new postoperative pain medication prescriptions and refills were similar in both groups.
Secondary endpoints included UTI rates, total days with a catheter, and patient experience of discomfort with the catheter. The two groups of women reported similar levels of catheter bother, but there was a nonsignificant difference in UTI rates: 23% in the earlier group, compared with 7% in the later group (P = .07).
The early-voiding trial group had an average 5 days with an indwelling transurethral catheter, compared with a significantly different 7 days in the later group (P = .0007). The early group also had fewer total days with an indwelling transurethral catheter and self-catheterization (6 days), compared with the late group (7 days; P = .0013). No patients had persistent incomplete bladder emptying after 17 days post surgery.
“Being able to adequately predict which patients are more likely to have unsuccessful postoperative voiding trials allows surgeons to better counsel their patients and may guide clinical decisions,” Dr. Schachar and associates said. They acknowledged, however, that their study’s biggest weakness is the small enrollment, which led to larger confidence intervals related to relative risk differences between the groups.
The study did not use external funding. Four of the investigators received grant, research funding, or honoraria from one or many medical device or pharmaceutical companies. The remaining researchers had no disclosures. Dr. Ault said he had no relevant financial disclosures.
SOURCE: Schachar JS et al. Am J Obstet Gynecol. 2020 Jun. doi: 10.1016/j.ajog.2020.06.001.
FROM AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Expert clarifies guidance on adolescent polycystic ovary syndrome
A trio of international expert recommendations mainly agree on essentials for the diagnosis and treatment of polycystic ovary syndrome in adolescents, but some confusion persists, according to Robert L. Rosenfield, MD, of the University of California, San Francisco.
In a commentary published in the Journal of Pediatric & Adolescent Gynecology, Dr. Rosenfield, who convened one of the three conferences at which guidance was developed, noted that the three recommendations – published by the Pediatric Endocrine Society, the International Consortium of Paediatric Endocrinology, and the International PCOS Network in 2015, 2017, and 2018, respectively – “are fairly dense” and reviews have suggested a lack of agreement. His comments offer perspective and practice suggestions that follow the consensus of the recommendations.
“All the documents agree on the core diagnostic criteria for adolescent PCOS: otherwise unexplained evidence of ovulatory dysfunction, as indicated by menstrual abnormalities based on stage-appropriate standards, and evidence of an androgen excess disorder,” Dr. Rosenfield said.
The main differences among the recommendations from the three groups reflect tension between the value of an early diagnosis and the liabilities of a mistaken diagnosis in the context of attitudes about adolescent contraception. “These are issues not likely to be resolved easily, yet they are matters for every physician to consider in management of each case,” he said.
Dr. Rosenfield emphasized that clinicians must consider PCOS “in the general context of all causes of adolescent menstrual disturbances,” when evaluating a girl within 1-2 years of menarche who presents with a menstrual abnormality, hirsutism, and/or acne that has been resistant to topical treatment.
A key point on which the recommendations differ is whether further assessment is needed if the menstrual abnormality has persisted for 1 year (the 2018 recommendations) or 2 years (the 2015 and 2017 recommendations), Dr. Rosenfield explained. “What the conferees struggled with is differentiating how long after menarche a menstrual abnormality should persist to avoid confusing PCOS with normal immaturity of the menstrual cycle,” known as physiologic adolescent anovulation (PAA). “The degree of certainty is improved only modestly by waiting 2 years rather than 1 year to make a diagnosis.”
However, the three documents agree that girls suspected of having PCOS within the first 1-2 years after menarche should be evaluated at that time, and followed with a diagnosis of “at risk for PCOS” if the early test results are consistent with a PCOS diagnosis, he said.
Another point of difference among the groups is the extent to which hirsutism and acne represent clinical evidence of hyperandrogenism that justifies testing for biochemical hyperandrogenism, Dr. Rosenfield said.
“All three sets of adolescent PCOS recommendations agree that investigation for biochemical hyperandrogenism be initiated by measuring serum total and/or free testosterone by specialty assays with well-defined reference ranges,” he said.
However, “documentation of biochemical hyperandrogenism has been problematic because standard platform assays of testosterone give grossly inaccurate results.”
As said Dr. Rosenfield. Guidelines in the United States favor estrogen-progestin combined oral contraceptives as first-line therapy, while the international guidelines support contraceptives if contraception also is desired; otherwise the 2017 guidelines recommend metformin as a first-line treatment.
“Agreement is uniform that healthy lifestyle management is first-line therapy for management of the associated obesity and metabolic disturbances, i.e., prior to and/or in conjunction with metformin therapy,” he noted.
In general, Dr. Rosenfield acknowledged that front-line clinicians cannot easily evaluate all early postmenarcheal girls for abnormal menstrual cycles. Instead, he advocated a “middle ground” approach between early diagnosis and potentially labeling a girl with a false positive diagnosis.
Postmenarcheal girls who are amenorrheic for 2 months could be assessed for signs of PCOS or pregnancy, and whether she is generally in good health, he said. “However, for example, if she remains amenorrheic for more than 90 days or if two successive periods are more than 2 months apart, laboratory screening would be reasonable.”
PCOS is “a diagnosis of exclusion for which referral to a specialist is advisable” to rule out other conditions such as non-classic congenital adrenal hyperplasia, hyperprolactinemia, endogenous Cushing syndrome, thyroid dysfunction, and virilizing tumors, said Dr. Rosenfield.
However, PCOS accounts for most cases of adolescent hyperandrogenism. The symptomatic treatment of early postmenarcheal girls at risk of PCOS is recommended to manage menstrual abnormality, hirsutism, acne, or obesity, and these girls should be reassessed by the time they finish high school after a 3-month treatment withdrawal period, he emphasized.
Dr. Rosenfield had no relevant financial conflicts to disclose.
SOURCE: Rosenfield RL. J Pediatr Adolesc Gynecol. 2020 June 29. doi: 10.1016/j.jpag.2020.06.017.
A trio of international expert recommendations mainly agree on essentials for the diagnosis and treatment of polycystic ovary syndrome in adolescents, but some confusion persists, according to Robert L. Rosenfield, MD, of the University of California, San Francisco.
In a commentary published in the Journal of Pediatric & Adolescent Gynecology, Dr. Rosenfield, who convened one of the three conferences at which guidance was developed, noted that the three recommendations – published by the Pediatric Endocrine Society, the International Consortium of Paediatric Endocrinology, and the International PCOS Network in 2015, 2017, and 2018, respectively – “are fairly dense” and reviews have suggested a lack of agreement. His comments offer perspective and practice suggestions that follow the consensus of the recommendations.
“All the documents agree on the core diagnostic criteria for adolescent PCOS: otherwise unexplained evidence of ovulatory dysfunction, as indicated by menstrual abnormalities based on stage-appropriate standards, and evidence of an androgen excess disorder,” Dr. Rosenfield said.
The main differences among the recommendations from the three groups reflect tension between the value of an early diagnosis and the liabilities of a mistaken diagnosis in the context of attitudes about adolescent contraception. “These are issues not likely to be resolved easily, yet they are matters for every physician to consider in management of each case,” he said.
Dr. Rosenfield emphasized that clinicians must consider PCOS “in the general context of all causes of adolescent menstrual disturbances,” when evaluating a girl within 1-2 years of menarche who presents with a menstrual abnormality, hirsutism, and/or acne that has been resistant to topical treatment.
A key point on which the recommendations differ is whether further assessment is needed if the menstrual abnormality has persisted for 1 year (the 2018 recommendations) or 2 years (the 2015 and 2017 recommendations), Dr. Rosenfield explained. “What the conferees struggled with is differentiating how long after menarche a menstrual abnormality should persist to avoid confusing PCOS with normal immaturity of the menstrual cycle,” known as physiologic adolescent anovulation (PAA). “The degree of certainty is improved only modestly by waiting 2 years rather than 1 year to make a diagnosis.”
However, the three documents agree that girls suspected of having PCOS within the first 1-2 years after menarche should be evaluated at that time, and followed with a diagnosis of “at risk for PCOS” if the early test results are consistent with a PCOS diagnosis, he said.
Another point of difference among the groups is the extent to which hirsutism and acne represent clinical evidence of hyperandrogenism that justifies testing for biochemical hyperandrogenism, Dr. Rosenfield said.
“All three sets of adolescent PCOS recommendations agree that investigation for biochemical hyperandrogenism be initiated by measuring serum total and/or free testosterone by specialty assays with well-defined reference ranges,” he said.
However, “documentation of biochemical hyperandrogenism has been problematic because standard platform assays of testosterone give grossly inaccurate results.”
As said Dr. Rosenfield. Guidelines in the United States favor estrogen-progestin combined oral contraceptives as first-line therapy, while the international guidelines support contraceptives if contraception also is desired; otherwise the 2017 guidelines recommend metformin as a first-line treatment.
“Agreement is uniform that healthy lifestyle management is first-line therapy for management of the associated obesity and metabolic disturbances, i.e., prior to and/or in conjunction with metformin therapy,” he noted.
In general, Dr. Rosenfield acknowledged that front-line clinicians cannot easily evaluate all early postmenarcheal girls for abnormal menstrual cycles. Instead, he advocated a “middle ground” approach between early diagnosis and potentially labeling a girl with a false positive diagnosis.
Postmenarcheal girls who are amenorrheic for 2 months could be assessed for signs of PCOS or pregnancy, and whether she is generally in good health, he said. “However, for example, if she remains amenorrheic for more than 90 days or if two successive periods are more than 2 months apart, laboratory screening would be reasonable.”
PCOS is “a diagnosis of exclusion for which referral to a specialist is advisable” to rule out other conditions such as non-classic congenital adrenal hyperplasia, hyperprolactinemia, endogenous Cushing syndrome, thyroid dysfunction, and virilizing tumors, said Dr. Rosenfield.
However, PCOS accounts for most cases of adolescent hyperandrogenism. The symptomatic treatment of early postmenarcheal girls at risk of PCOS is recommended to manage menstrual abnormality, hirsutism, acne, or obesity, and these girls should be reassessed by the time they finish high school after a 3-month treatment withdrawal period, he emphasized.
Dr. Rosenfield had no relevant financial conflicts to disclose.
SOURCE: Rosenfield RL. J Pediatr Adolesc Gynecol. 2020 June 29. doi: 10.1016/j.jpag.2020.06.017.
A trio of international expert recommendations mainly agree on essentials for the diagnosis and treatment of polycystic ovary syndrome in adolescents, but some confusion persists, according to Robert L. Rosenfield, MD, of the University of California, San Francisco.
In a commentary published in the Journal of Pediatric & Adolescent Gynecology, Dr. Rosenfield, who convened one of the three conferences at which guidance was developed, noted that the three recommendations – published by the Pediatric Endocrine Society, the International Consortium of Paediatric Endocrinology, and the International PCOS Network in 2015, 2017, and 2018, respectively – “are fairly dense” and reviews have suggested a lack of agreement. His comments offer perspective and practice suggestions that follow the consensus of the recommendations.
“All the documents agree on the core diagnostic criteria for adolescent PCOS: otherwise unexplained evidence of ovulatory dysfunction, as indicated by menstrual abnormalities based on stage-appropriate standards, and evidence of an androgen excess disorder,” Dr. Rosenfield said.
The main differences among the recommendations from the three groups reflect tension between the value of an early diagnosis and the liabilities of a mistaken diagnosis in the context of attitudes about adolescent contraception. “These are issues not likely to be resolved easily, yet they are matters for every physician to consider in management of each case,” he said.
Dr. Rosenfield emphasized that clinicians must consider PCOS “in the general context of all causes of adolescent menstrual disturbances,” when evaluating a girl within 1-2 years of menarche who presents with a menstrual abnormality, hirsutism, and/or acne that has been resistant to topical treatment.
A key point on which the recommendations differ is whether further assessment is needed if the menstrual abnormality has persisted for 1 year (the 2018 recommendations) or 2 years (the 2015 and 2017 recommendations), Dr. Rosenfield explained. “What the conferees struggled with is differentiating how long after menarche a menstrual abnormality should persist to avoid confusing PCOS with normal immaturity of the menstrual cycle,” known as physiologic adolescent anovulation (PAA). “The degree of certainty is improved only modestly by waiting 2 years rather than 1 year to make a diagnosis.”
However, the three documents agree that girls suspected of having PCOS within the first 1-2 years after menarche should be evaluated at that time, and followed with a diagnosis of “at risk for PCOS” if the early test results are consistent with a PCOS diagnosis, he said.
Another point of difference among the groups is the extent to which hirsutism and acne represent clinical evidence of hyperandrogenism that justifies testing for biochemical hyperandrogenism, Dr. Rosenfield said.
“All three sets of adolescent PCOS recommendations agree that investigation for biochemical hyperandrogenism be initiated by measuring serum total and/or free testosterone by specialty assays with well-defined reference ranges,” he said.
However, “documentation of biochemical hyperandrogenism has been problematic because standard platform assays of testosterone give grossly inaccurate results.”
As said Dr. Rosenfield. Guidelines in the United States favor estrogen-progestin combined oral contraceptives as first-line therapy, while the international guidelines support contraceptives if contraception also is desired; otherwise the 2017 guidelines recommend metformin as a first-line treatment.
“Agreement is uniform that healthy lifestyle management is first-line therapy for management of the associated obesity and metabolic disturbances, i.e., prior to and/or in conjunction with metformin therapy,” he noted.
In general, Dr. Rosenfield acknowledged that front-line clinicians cannot easily evaluate all early postmenarcheal girls for abnormal menstrual cycles. Instead, he advocated a “middle ground” approach between early diagnosis and potentially labeling a girl with a false positive diagnosis.
Postmenarcheal girls who are amenorrheic for 2 months could be assessed for signs of PCOS or pregnancy, and whether she is generally in good health, he said. “However, for example, if she remains amenorrheic for more than 90 days or if two successive periods are more than 2 months apart, laboratory screening would be reasonable.”
PCOS is “a diagnosis of exclusion for which referral to a specialist is advisable” to rule out other conditions such as non-classic congenital adrenal hyperplasia, hyperprolactinemia, endogenous Cushing syndrome, thyroid dysfunction, and virilizing tumors, said Dr. Rosenfield.
However, PCOS accounts for most cases of adolescent hyperandrogenism. The symptomatic treatment of early postmenarcheal girls at risk of PCOS is recommended to manage menstrual abnormality, hirsutism, acne, or obesity, and these girls should be reassessed by the time they finish high school after a 3-month treatment withdrawal period, he emphasized.
Dr. Rosenfield had no relevant financial conflicts to disclose.
SOURCE: Rosenfield RL. J Pediatr Adolesc Gynecol. 2020 June 29. doi: 10.1016/j.jpag.2020.06.017.
FROM THE JOURNAL OF PEDIATRIC AND ADOLESCENT GYNECOLOGY
Heavy menstrual bleeding difficult to control in young patients with inherited platelet disorders
Physician consensus and a broadly effective treatment for heavy menstrual bleeding was not found among young patients with inherited platelet function disorders, according to the results of a retrospective chart review reported in the Journal of Pediatric and Adolescent Gynecology.
Heavy menstrual bleeding (HMB) in girls with inherited platelet function disorders (IPFD) can be difficult to control despite ongoing follow-up and treatment changes, reported Christine M. Pennesi, MD, of the University of Michigan, Ann Arbor, and colleagues.
They assessed 34 young women and girls (ages 9-25 years) diagnosed with IPFDs referred to gynecology and/or hematology at a tertiary care hospital between 2006 and 2018.
Billing codes were used to determine hormonal or nonhormonal treatments, and outcomes over a 1- to 2-year period were collected. The initial treatment was defined as the first treatment prescribed after referral. The primary outcome was treatment failure, defined as a change in treatment method because of continued bleeding.
The majority (56%) of patients failed initial treatment (n = 19); among all 34 individuals followed in the study, an average of 2.7 total treatments were required.
Six patients (18%) remained uncontrolled despite numerous treatment changes (mean treatment changes, four; range, two to seven), and two patients (6%) remained uncontrolled because of noncompliance with treatment.
Overall, the researchers identified a 18% failure rate of successfully treatment of HMB in young women and girls with IPFDs over a 2-year follow-up period.
Of the 26 women who achieved control of HMB within 2-year follow-up, 54% (n = 14) were on hormonal treatments, 27% (n = 7) on nonhormonal treatments, 12% (n = 3) on combined treatments, and 8% (n = 2) on no treatment at time of control, the authors stated.
“The heterogeneity in treatments that were described in this study, clearly demonstrate that, in selecting treatment methods for HMB in young women, other considerations are often in play. This includes patient preference and need for contraception. Some patients or parents may have personal or religious objections to hormonal methods or worry about hormones in this young age group,” the researchers speculated.
“Appropriate counseling in these patients should include that it would not be unexpected for a patient to need more than one treatment before control of bleeding is achieved. This may help to alleviate the fear of teenagers when continued bleeding occurs after starting their initial treatment,” Dr. Pennesi and colleagues concluded.
One of the authors participated in funded trials and received funding from several pharmaceutical companies. The others reported having no disclosures.
SOURCE: Pennesi CM et al. J Pediatr Adolesc Gynecol. 2020 Jun 22. doi: 10.1016/j.jpag.2020.06.019.
Physician consensus and a broadly effective treatment for heavy menstrual bleeding was not found among young patients with inherited platelet function disorders, according to the results of a retrospective chart review reported in the Journal of Pediatric and Adolescent Gynecology.
Heavy menstrual bleeding (HMB) in girls with inherited platelet function disorders (IPFD) can be difficult to control despite ongoing follow-up and treatment changes, reported Christine M. Pennesi, MD, of the University of Michigan, Ann Arbor, and colleagues.
They assessed 34 young women and girls (ages 9-25 years) diagnosed with IPFDs referred to gynecology and/or hematology at a tertiary care hospital between 2006 and 2018.
Billing codes were used to determine hormonal or nonhormonal treatments, and outcomes over a 1- to 2-year period were collected. The initial treatment was defined as the first treatment prescribed after referral. The primary outcome was treatment failure, defined as a change in treatment method because of continued bleeding.
The majority (56%) of patients failed initial treatment (n = 19); among all 34 individuals followed in the study, an average of 2.7 total treatments were required.
Six patients (18%) remained uncontrolled despite numerous treatment changes (mean treatment changes, four; range, two to seven), and two patients (6%) remained uncontrolled because of noncompliance with treatment.
Overall, the researchers identified a 18% failure rate of successfully treatment of HMB in young women and girls with IPFDs over a 2-year follow-up period.
Of the 26 women who achieved control of HMB within 2-year follow-up, 54% (n = 14) were on hormonal treatments, 27% (n = 7) on nonhormonal treatments, 12% (n = 3) on combined treatments, and 8% (n = 2) on no treatment at time of control, the authors stated.
“The heterogeneity in treatments that were described in this study, clearly demonstrate that, in selecting treatment methods for HMB in young women, other considerations are often in play. This includes patient preference and need for contraception. Some patients or parents may have personal or religious objections to hormonal methods or worry about hormones in this young age group,” the researchers speculated.
“Appropriate counseling in these patients should include that it would not be unexpected for a patient to need more than one treatment before control of bleeding is achieved. This may help to alleviate the fear of teenagers when continued bleeding occurs after starting their initial treatment,” Dr. Pennesi and colleagues concluded.
One of the authors participated in funded trials and received funding from several pharmaceutical companies. The others reported having no disclosures.
SOURCE: Pennesi CM et al. J Pediatr Adolesc Gynecol. 2020 Jun 22. doi: 10.1016/j.jpag.2020.06.019.
Physician consensus and a broadly effective treatment for heavy menstrual bleeding was not found among young patients with inherited platelet function disorders, according to the results of a retrospective chart review reported in the Journal of Pediatric and Adolescent Gynecology.
Heavy menstrual bleeding (HMB) in girls with inherited platelet function disorders (IPFD) can be difficult to control despite ongoing follow-up and treatment changes, reported Christine M. Pennesi, MD, of the University of Michigan, Ann Arbor, and colleagues.
They assessed 34 young women and girls (ages 9-25 years) diagnosed with IPFDs referred to gynecology and/or hematology at a tertiary care hospital between 2006 and 2018.
Billing codes were used to determine hormonal or nonhormonal treatments, and outcomes over a 1- to 2-year period were collected. The initial treatment was defined as the first treatment prescribed after referral. The primary outcome was treatment failure, defined as a change in treatment method because of continued bleeding.
The majority (56%) of patients failed initial treatment (n = 19); among all 34 individuals followed in the study, an average of 2.7 total treatments were required.
Six patients (18%) remained uncontrolled despite numerous treatment changes (mean treatment changes, four; range, two to seven), and two patients (6%) remained uncontrolled because of noncompliance with treatment.
Overall, the researchers identified a 18% failure rate of successfully treatment of HMB in young women and girls with IPFDs over a 2-year follow-up period.
Of the 26 women who achieved control of HMB within 2-year follow-up, 54% (n = 14) were on hormonal treatments, 27% (n = 7) on nonhormonal treatments, 12% (n = 3) on combined treatments, and 8% (n = 2) on no treatment at time of control, the authors stated.
“The heterogeneity in treatments that were described in this study, clearly demonstrate that, in selecting treatment methods for HMB in young women, other considerations are often in play. This includes patient preference and need for contraception. Some patients or parents may have personal or religious objections to hormonal methods or worry about hormones in this young age group,” the researchers speculated.
“Appropriate counseling in these patients should include that it would not be unexpected for a patient to need more than one treatment before control of bleeding is achieved. This may help to alleviate the fear of teenagers when continued bleeding occurs after starting their initial treatment,” Dr. Pennesi and colleagues concluded.
One of the authors participated in funded trials and received funding from several pharmaceutical companies. The others reported having no disclosures.
SOURCE: Pennesi CM et al. J Pediatr Adolesc Gynecol. 2020 Jun 22. doi: 10.1016/j.jpag.2020.06.019.
FROM THE JOURNAL OF PEDIATRIC AND ADOLESCENT GYNECOLOGY
Physician leadership: Racial disparities and racism. Where do we go from here?
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
The destructive toll COVID-19 has caused worldwide is devastating. In the United States, the disproportionate deaths of Black, Indigenous, and Latinx people due to structural racism, amplified by economic adversity, is unacceptable. Meanwhile, the continued murder of Black people by those sworn to protect the public is abhorrent and can no longer be ignored. Black lives matter. These crises have rightly gripped our attention, and should galvanize physicians individually and collectively to use our privileged voices and relative power for justice. We must strive for engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.
The COVID-19 pandemic has illuminated the vast inequities in our country. It has highlighted the continued poor outcomes our health and health care systems create for Black, Indigenous, and Latinx communities. It also has demonstrated clearly that we are all connected—one large community, interdependent yet rife with differential power, privilege, and oppression. We must address these racial disparities—not only in the name of justice and good health for all but also because it is a moral and ethical imperative for us as physicians—and SARS-CoV-2 clearly shows us that it is in the best interest of everyone to do so.
First step: A deep dive look at systemic racism
What is first needed is an examination and acknowledgement by medicine and health care at large of the deeply entrenched roots of systemic and institutional racism in our profession and care systems, and their disproportionate and unjust impact on the health and livelihood of communities of color. The COVID-19 pandemic is only a recent example that highlights the perpetuation of a system that harms people of color. Racism, sexism, gender discrimination, economic and social injustice, religious persecution, and violence against women and children are age-old. We have yet to see health care institutions implement system-wide intersectional and antiracist practices to address them. Mandatory implicit bias training, policies for inclusion and diversity, and position statements are necessary first steps; however, they are not a panacea. They are insufficient to create the bold changes we need. The time for words has long passed. It is time to listen, to hear the cries of anguish and outrage, to examine our privileged position, to embrace change and discomfort, and most importantly to act, and to lead in dismantling the structures around us that perpetuate racial inequity.
How can we, as physicians and leaders, join in action and make an impact?
Dr. Camara Jones, past president of the American Public Health Association, describes 3 levels of racism:
- structural or systemic
- individual or personally mediated
- internalized.
Interventions at each level are important if we are to promote equity in health and health care. This framework can help us think about the following strategic initiatives.
Continue to: 1. Commit to becoming an antiracist and engage in independent study...
1. Commit to becoming antiracist and engage in independent study. This is an important first step as it will form the foundations for interventions—one cannot facilitate change without understanding the matter at hand. This step also may be the most personally challenging step forcing all of us to wrestle with discomfort, sadness, fear, guilt, and a host of other emotional responses. Remember that great change has never been born out of comfort, and the discomfort physicians may experience while unlearning racism and learning antiracism pales in comparison to what communities of color experience daily. We must actively work to unlearn the racist and anti-Black culture that is so deeply woven into every aspect of our existence.
Learn the history that was not given to us as kids in school. Read the brilliant literary works of Black, Indigenous, and Latinx artists and scholars on dismantling racism. Expand our vocabulary and knowledge of core concepts in racism, racial justice, and equity. Examine and reflect on our day-to-day practices. Be vocal in our commitment to antiracism—the time has passed for staying silent. If you are white, facilitate conversations about race with your white colleagues; the inherent power of racism relegates it to an issue that can never be on the table, but it is time to dismantle that power. Learn what acts of meaningful and intentional alliances are and when we need to give up power or privilege to a person of color. We also need to recognize that we as physicians, while leaders in many spaces, are not leaders in the powerful racial justice grassroots movements. We should learn from these movements, follow their lead, and use our privilege to uplift racial justice in our settings.
2. Embrace the current complexities with empathy and humility, finding ways to exercise our civic responsibility to the public with compassion. During the COVID-19 pandemic we have seen the devastation that social isolation, job loss, and illness can create. Suddenly those who could never have imagined themselves without food are waiting hours in their cars for food bank donations or are finding empty shelves in stores. Those who were not safe at home were suddenly imprisoned indefinitely in unsafe situations. Those who were comfortable, well-insured, and healthy are facing an invisible health threat, insecurity, fear, anxiety, and loss. Additionally, our civic institutions are failing. Those of us who always took our right to vote for granted are being forced to stand in hours’-long lines to exercise that right; while those who have been systematically disenfranchised are enduring even greater threats to their constitutional right to exercise their political power, disallowing them to speak for their families and communities and to vote for the justice they deserve. This may be an opportunity to stop blaming victims and recognize the toll that structural and systemic contributions to inequity have created over generations.
3. Meaningfully engage with and advocate for patients. In health and health care, we must begin to engage with the communities we serve and truly listen to their needs, desires, and barriers to care, and respond accordingly. Policies that try to address the social determinants of health without that engagement, and without the acknowledgement of the structural issues that cause them, however well-intentioned, are unlikely to accomplish their goals. We need to advocate as physicians and leaders in our settings for every policy, practice, and procedure to be scrutinized using an antiracist lens. To execute this, we need to:
- ask why clinic and hospital practices are built the way they are and how to make them more reflexive and responsive to individual patient’s needs
- examine what the disproportionate impacts might be on different groups of patients from a systems-level
- be ready to dismantle and/or rebuild something that is exacerbating disparate outcomes and experiences
- advocate for change that is built upon the narratives of patients and their communities.
We should include patients in the creation of hospital policies and guidelines in order to shift power toward them and to be transparent about how the system operates in order to facilitate trust and collaboration that centers patients and communities in the systems created to serve them.
Continue to: 4. Intentionally repair and build trust...
4. Intentionally repair and build trust. To create a safe environment, we must repair what we have broken and earn the trust of communities by uplifting their voices and redistributing our power to them in changing the systems and structures that have, for generations, kept Black, Indigenous, and Latinx people oppressed. Building trust requires first owning our histories of colonization, genocide, and slavery—now turned mass incarceration, debasement, and exploitation—that has existed for centuries. We as physicians need to do an honest examination of how we have eroded the trust of the very communities we care for since our profession’s creation. We need to acknowledge, as a white-dominant profession, the medical experimentation on and exploitation of Black and Brown bodies, and how this formed the foundation for a very valid deep distrust and fear of the medical establishment. We need to recognize how our inherent racial biases continue to feed this distrust, like when we don’t treat patients’ pain adequately or make them feel like we believe and listen to their needs and concerns. We must acknowledge our complicity in perpetuating the racial inequities in health, again highlighted by the COVID-19 pandemic.
5. Increase Black, Indigenous, and Latinx representation in physician and other health care professions’ workforce. Racism impacts not only patients but also our colleagues of color. The lack of racial diversity is a symptom of racism and a representation of the continued exclusion and devaluing of physicians of color. We must recognize this legacy of exclusion and facilitate intentional recruitment, retention, inclusion, and belonging of people of color into our workforce. Tokenism, the act of symbolically including one or few people from underrepresented groups, has been a weapon used by our workforce against physicians of color, resulting in isolation, “othering,” demoralization, and other deleterious impacts. We need to reverse this history and diversify our training programs and workforce to ensure justice in our own community.
6. Design multifaceted interventions. Multilevel problems require multilevel solutions. Interventions targeted solely at one level, while helpful, are unlikely to result in the larger scale changes our society needs to implement if we are to eradicate the impact of racism on health. We have long known that it is not just “preexisting conditions” or “poor” individual behaviors that lead to negative and disparate health outcomes—these are impacted by social and structural determinants much larger and more deleterious than that. It is critically important that we allocate and redistribute resources to create safe and affordable housing; childcare and preschool facilities; healthy, available, and affordable food; equitable and affordable educational opportunities; and a clean environment to support the health of all communities—not only those with the highest tax base. It is imperative that we strive to understand the lives of our fellow human beings who have been subjected to intergenerational social injustices and oppressions that have continued to place them at the margins of society. We need to center the lived experiences of communities of color in the design of multilevel interventions, especially Black and Indigenous communities. While we as physicians cannot individually impact education, economic, or food/environment systems, we can use our power to advocate for providing resources for the patients we care for and can create strategies within the health care system to address these needs in order to achieve optimal health. Robust and equitable social structures are the foundations for health, and ensuring equitable access to them is critical to reducing disparities.
Commit to lead
We must commit to unlearning our internalized racism, rebuilding relationships with communities of color, and engaging in antiracist practices. As a profession dedicated to healing, we have an obligation to be leaders in advocating for these changes, and dismantling the inequitable structure of our health care system.
Our challenge now is to articulate solutions. While antiracism should be informed by the lived experiences of communities of color, the work of antiracism is not their responsibility. In fact, it is the responsibility of our white-dominated systems and institutions to change.
There are some solutions that are easier to enumerate because they have easily measurable outcomes or activities, such as:
- collecting data transparently
- identifying inequities in access, treatment, and care
- conducting rigorous root cause analysis of those barriers to care
- increasing diverse racial and gender representation on decision-making bodies, from board rooms to committees, from leadership teams to research participants
- redistribute power by paving the way for underrepresented colleagues to participate in clinical, administrative, educational, executive, and health policy spaces
- mentoring new leaders who come from marginalized communities.
Every patient deserves our expertise and access to high-quality care. We should review our patient panels to ensure we are taking steps personally to be just and eliminate disparities, and we should monitor the results of those efforts.
Continue to: Be open to solutions that may make us “uncomfortable”...
Be open to solutions that may make us “uncomfortable”
There are other solutions, perhaps those that would be more effective on a larger scale, which may be harder to measure using our traditional ways of inquiry or measurement. Solutions that may create discomfort, anger, or fear for those who have held their power or positions for a long time. We need to begin to engage in developing, cultivating, and valuing innovative strategies that produce equally valid knowledge, evidence, and solutions without engaging in a randomized controlled trial. We need to reinvent the way inquiry, investigation, and implementation are done, and utilize novel, justice-informed strategies that include real-world evidence to produce results that are applicable to all (not just those willing to participate in sponsored trials). Only then will we be able to provide equitable health outcomes for all.
We also must accept responsibility for the past and humbly ask communities to work with us as we struggle to eliminate racism and dehumanization of Black lives by calling out our actions or inaction, recognizing the impact of our privileged status, and stepping down or stepping aside to allow others to lead. Sometimes it is as simple as turning off the Zoom camera so others can talk. By redistributing power and focusing this work upon the narratives of marginalized communities, we can improve our system for everyone. We must lead with action within our practices and systems; become advocates within our communities, institutions, and profession; strategize and organize interventions at both structural and individual levels to first recognize and name—then change—the systems; and unlearn behaviors that perpetuate racism.
Inaction is shirking our responsibility among the medical community
Benign inaction and unintentional acquiescence with “the way things are and have always been” abdicates our responsibility as physicians to improve the health of our patients and our communities. The modern Hippocratic Oath reminds us: “I will remember that I remain a member of society, with special obligations to all my fellow human beings, those sound of mind and body as well as the infirm.” We have a professional and ethical responsibility to ensure health equity, and thus racial equity. As physicians, as healers, as leaders we must address racial inequities at all levels as we commit to improving the health of our nation. We can no longer stand silent in the face of the violence, brutality, and injustices our patients, friends, family, neighbors, communities, and society as a whole live through daily. It is unjust and inhumane to do so.
To be silent is to be complicit. As Gandhi said so long ago, we must “be the change we wish to see in the world.” And as Ijeoma Olua teaches us, “Anti-racism is the commitment to fight racism wherever you find it, including in yourself. And it’s the only way forward.”
- “So You Want to Talk about Race” Ijeoma Oluo
- “How to Be an Antiracist” Ibram X. Kendi
- “Between the World and Me” Ta-Nehisi Coates
- A conversation on race and privilege (Angela Davis and Jane Elliot) https://www.youtube.com/watch?reload=9&v=S0jf8D5WHoo
- Uncomfortable conversations with a Black man (Emmanuel Acho) https://www.youtube.com/watch?v=h8jUA7JBkF4
Antiracism – defined as the work of actively opposing racism by advocating for changes in political, economic, and social life. Antiracism tends to be an individualized approach, and set up in opposition to individual racist behaviors and impacts
Black Lives Matter – a political movement to address systemic and state violence against African Americans. Per the Black Lives Matter organizers: “In 2013, three radical Black organizers—Alicia Garza, Patrisse Cullors, and Opal Tometi—created a Black-centered political will and movement building project called BlackLivesMatter. It was in response to the acquittal of Trayvon Martin’s murderer, George Zimmerman. The project is now a member-led global network of more than 40 chapters. Members organize and build local power to intervene in violence inflicted on Black communities by the state and vigilantes. Black Lives Matter is an ideological and political intervention in a world where Black lives are systematically and intentionally targeted for demise. It is an affirmation of Black folks’ humanity, our contributions to this society, and our resilience in the face of deadly oppression.”
Implicit bias – also known as unconscious or hidden bias, implicit biases are negative associations that people unknowingly hold. They are expressed automatically, without conscious awareness. Many studies have indicated that implicit biases affect individuals’ attitudes and actions, thus creating real-world implications, even though individuals may not even be aware that those biases exist within themselves. Notably, implicit biases have been shown to trump individuals stated commitments to equality and fairness, thereby producing behavior that diverges from the explicit attitudes that many people profess.
Othering – view or treat (a person or group of people) as intrinsically different from and alien to oneself. (From https://lexico.com.)
For a full glossary of terms, visit RacialEquityTools.org (https://www.racialequitytools.org/glossary#anti-black)
2020 Update on abnormal uterine bleeding
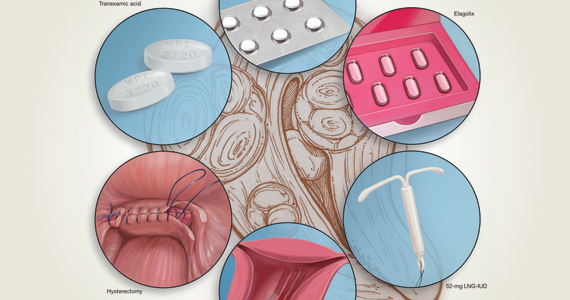
Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.

Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.

Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
How effective is elagolix treatment in women with fibroids and HMB?
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
Telemedicine: Navigating legal issues
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
In the first 2 articles of this series, “Telemedicine: A primer for today’s ObGyn” and “Telemedicine: Common hurdles and proper coding for ObGyns,” which appeared in the May and June issues of
Legal issues surrounding telemedicine
There are numerous legal, regulatory, and compliance issues that existed before the pandemic that likely will continue to be of concern postpandemic. Although the recent 1135 waiver (allowing Medicare to pay for office, hospital, and other visits furnished via telehealth)1 and other regulations are now in place for almost every aspect of telemedicine, virtual medicine is not a free-for-all (even though it may seem like it). Practicing ethical telemedicine entails abiding by numerous federal and state-specific laws and requirements. It is important to be aware of the laws in each state in which your patients are located and to practice according to the requirements of these laws. This often requires consultation with an experienced health care attorney who is knowledgeable about the use of telemedicine and who can help you with issues surrounding:
- Malpractice insurance. It is an important first step to contact your practice’s malpractice insurance carrier and confirm coverage for telemedicine visits. Telemedicine visits are considered the same as in-person visits when determining scope of practice and malpractice liability. Nevertheless, a best practice is to have written verification from your malpractice carrier about the types of telemedicine services and claims for which your ObGyn practice is covered. Additionally, if you care for patients virtually who live in a state in which you are not licensed, check with your carrier to determine if potential claims will be covered.
- Corporate practice laws. These laws require that your practice be governed by a health care professional and not someone with a nonmedical background. This becomes important if you are looking to create a virtual practice in another state. States that prohibit the corporate practice of medicine have state-specific mandates that require strict adherence. Consult with a health care attorney before entering into a business arrangement with a nonphysician or corporate entity.
- Delegation agreement requirements. These laws require physician collaboration and/or supervision of allied health care workers such as nurse practitioners (NPs) and physician assistants (PAs) and may limit the number of allied health care providers that a physician may supervise. Many states are allowing allied health care workers to practice at the top of their license, but this is still state specific. Thus, it is an important issue to consider, especially for practices that rely heavily on the services of advanced practice registered nurses (APRNs), for example, who have a broad scope of practice and who may be qualified to care for many common ObGyn problems.
- Informed consent requirements. Some states have no requirements regarding consent for a virtual visit. Others require either written or verbal consent. In states that do not require informed consent, it is best practice to nevertheless obtain either written or oral consent and to document in the patient’s record that consent was obtained before initiating a virtual visit. The consent should follow state-mandated disclosures, as well as the practice’s policies regarding billing, scheduling, and cancellations of telemedicine visits.
- Interstate licensing laws. Because of the COVID-19 pandemic, federal and state licensure waivers are in place to allow physicians to care for patients outside the physician’s home state, but these waivers likely will be lifted postpandemic. Once waivers are lifted, physicians will need to be licensed not only in the state in which they practice but also in the state where the patient is located at the time of treatment. Even physicians who practice in states that belong to the Interstate Medical Licensure Compact2 must apply for and obtain a license to practice within Compact member states. Membership in the Interstate Medical Licensure Compact expedites the licensure process, but does not alleviate the need to obtain a license to practice in each member state. To ensure compliance with interstate licensure laws, seek advice from a health care attorney specializing in telemedicine.
- Drug monitoring laws. The Ryan Haight Online Pharmacy Consumer Protection Act of 20083 implemented a requirement that physicians have at least one in-person, face-to-face visit with patients before prescribing a controlled substance for the first time. Because state laws may vary, we suggest consulting with a health care attorney to understand your state’s requirements for prescribing controlled substances to new patients and when using telemedicine (see “Prescription drugs” at https://www.cdc.gov/phlp/publications/topic/prescription.html for more information).
- Data privacy and security. From a content perspective, health care data and personally identifiable information are extremely rich, which makes electronic health records (EHRs), or the digital form of patients’ medical histories and other data, particularly tempting targets for hackers and cyber criminals. We caution that services such as Facetime and Skype are not encrypted; they have been granted waivers for telemedicine use, but these waivers are probably not going to be permanent once the COVID-19 crisis passes.
- HIPAA compliance. Generally—and certainly under normal circumstances—telemedicine is subject to the same rules governing protected health information (PHI) as any other technology and process used in physician practices. The Health Insurance Portability and Accountability Act (HIPAA) Security Rule includes guidelines on telemedicine and stipulates that only authorized users should have access to ePHI, that a system of secure communication must be established to protect the security of ePHI, and that a system to monitor communications must be maintained, among other requirements.4 Third parties that provide telemedicine, data storage, and other services, with a few exceptions, must have a business associate agreement (BAA) with a covered entity. Covered entities include health care providers, health plans, and health and health care clearinghouses. Such an agreement should include specific language that ensures that HIPAA requirements will be met and that governs permitted and required uses of PHI, strictly limits other uses of PHI, and establishes appropriate safeguards and steps that must be taken in the event of a breach or disallowed disclosure of PHI. Best practice requires that providers establish robust protocols, policies, and processes for handling sensitive information.
During the COVID-19 pandemic, however, certain HIPAA restrictions relating to telemedicine have been temporarily waived by the US Department of Health and Human Services (HHS). More specifically, HHS Secretary Alex Azar has exercised his authority to waive sanctions against covered hospitals for noncompliance with requirements: to obtain a patient’s consent to speak with family members or friends involved in the patient’s care, to distribute a notice of privacy practices, to request privacy restrictions, to request confidential communications, and the use of nonpublic facing audio and video communications products, among others.5 These are temporary measures only; once the national public health emergency has passed or at the HHS Secretary’s discretion based on new developments, this position on discretionary nonenforcement may end.
Continue to: Crisis creates opportunity: The future of telemedicine...
Crisis creates opportunity: The future of telemedicine
It was just a few years ago when the use of telemedicine was relegated to treating patients in only rural areas or those located a great distance from brick and mortar practices. But the pandemic, along with the coincident relaxation of the Centers for Medicare and Medicaid Services’ (CMS) requirements for conducting telemedicine visits has made the technology highly attractive to ObGyns who can now treat many patients 24/7 from their homes using laptops and even mobile devices. In addition, the pandemic has prompted an expansion of current procedural terminology (CPT) codes that makes it possible to bill patients for telemedicine services and be appropriately compensated.
Thus, as awful as COVID-19 is, we can conclude that it has provided us with opportunities. We predict that when the crisis has abated, although the current relaxation of HIPAA guidelines will probably be rescinded, restrictions will not likely return to precoronavirus status; changes will certainly be made, and telemedicine will likely become part and parcel of caring for ObGyn patients.
Telemedicine has been used successfully for years to improve patient access to medical care while reducing health care costs. In 2016, an estimated 61% of US health care institutions and 40% to 50% of US hospitals used telemedicine.6 And according to the results of a survey of America’s physicians conducted in April 2020, almost half (48%) are treating patients through telemedicine, which is up from just 18% 2 years ago.7
Letting loose the genie in the bottle
Widespread use of telemedicine traditionally has been limited by low reimbursement rates and interstate licensing and practice issues, but we predict that the use of telemedicine is going to significantly increase in the future. Here’s why:8 Disruptive innovation was defined by Professor Clayton Christensen of the Harvard Business School in 1997.9 Disruptive innovation explains the process by which a disruptive force spurs the development of simple, convenient, and affordable solutions that then replace processes that are expensive and complicated. According to Christensen, a critical element of the process is a technology that makes a product or service more accessible to a larger number of people while reducing cost and increasing ease of use. For example, innovations making equipment for dialysis cheaper and simpler helped make it possible to administer the treatment in neighborhood clinics, rather than in centralized hospitals, thus disrupting the hospital’s share of the dialysis business.
The concept of telemedicine and the technology for its implementation have been available for more than 15 years. However, it was the coronavirus that released the genie from the bottle, serving as the disruptive force to release the innovation. Telemedicine has demonstrated that the technology offers solutions that address patients’ urgent, unmet needs for access to care at an affordable price and that enhances the productivity of the ObGyn. The result is simple, convenient, and affordable; patients can readily access the medical care they need to effectively maintain their health or manage conditions that arise.
Telemedicine has reached a level of critical mass. Data suggest that patients, especially younger ones, have accepted and appreciate the use of this technology.10 It gives patients more opportunities to receive health care in their homes or at work where they feel more comfortable and less anxious than they do in physicians’ offices.
Several other health care issues may be altered by telemedicine.
The physician shortage. If the data are to be believed, there will be a significant shortage of physicians—and perhaps ObGyns—in the near future.11 Telemedicine can help the problem by making it possible to provide medical care not only in rural areas where there are no ObGyns but also in urban areas where a shortage may be looming.
Continuing medical education (CME). CME is moving from large, expensive, in-person conferences to virtual conferences and online learning.
The American health care budget is bloated with expenses exceeding $3 trillion.12 Telemedicine can help reduce health care costs by facilitating patient appointments that do not require office staff or many of the overhead expenses associated with brick and mortar operations. Telemedicine reduces the financial impact of patient no-shows. Because patients are keen on participating, the use of telemedicine likely will improve patient engagement and clinical outcomes. Telemedicine already has a reputation of reducing unnecessary office and emergency room visits and hospital admissions.13
Clinical trials. One of the obstacles to overcome in the early stages of a clinical trial is finding participants. Telemedicine will make patient recruitment more straightforward. And because telemedicine makes distance from the office a nonissue, recruiters will be less restricted by geographic boundaries.
In addition, telemedicine allows for the participants of the trial to stay in their homes most of the time while wearing remote monitoring devices. Such devices would enable trial researchers to spot deviations from patients’ baseline readings.
The bottom line
COVID-19 has provided the opportunity for us to see how telemedicine can contribute to reducing the spread of infectious diseases by protecting physicians, their staff, and patients themselves. Once the COVID-19 crisis has passed, it is likely that telemedicine will continue to move health care delivery from the hospital or clinic into the home. The growth and integration of information and communication technologies into health care delivery holds great potential for patients, providers, and payers in health systems of the future. ●
CVS is using telemedicine to complement the company’s retail “Minute Clinic,” which offers routine preventive and clinical services, such as vaccine administration, disease screenings, treatment for minor illnesses and injuries, and monitoring of chronic conditions—services that traditionally were provided in physician’s offices only. These clinics are open 7 days per week, providing services on a walk-in basis at an affordable price—about $60 per visit compared with an average of $150 for an uninsured patient to see a primary care physician in his/her office.1 While this seems to be fulfilling an unmet need for patients, the service may prove disruptive to traditional health care delivery by removing a lucrative source of income from physicians.
Reference
1. CVS Health. CVS Health’s MinuteClinic introduces new virtual care offering. August 8, 2018. https://cvshealth.com/newsroom/press-releases/cvs-healths-minuteclinic-introduces-new-virtual-care-offering. Accessed June 16, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
- CMS.gov. 1135 Waiver – At A Glance.https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertEmergPrep/Downloads/1135-Waivers-At-A-Glance.pdf. Accessed June 16, 2020.
- Interstate Medical Licensure Compact. https://www.imlcc.org/. Accessed June 16, 2020.
- American Psychiatric Association. The Ryan Haight OnlinePharmacy Consumer Protection Act of 2008. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/toolkit/ryan-haight-act. Accessed June 16, 2020.
- American Medical Association. HIPAA security rule and riskanalysis. https://www.ama-assn.org/practice-management/hipaa/hipaa-security-rule-risk-analysis#:~:text=The%20HIPAA%20Security%20Rule%20requires,and%20security%20of%20this%20information. Accessed June 16, 2020.
- HHS.gov. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Content last reviewed on March 30, 2020.https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Accessed June 16, 2020.
- Mahar J, Rosencrance J, Rasmussen P. The Future of Telemedicine (And What’s in the Way). Consult QD. March 1,2019. https://consultqd.clevelandclinic.org/the-future-of-telemedicine-and-whats-in-the-way. Accessed June 23, 2020.
- Merritt Hawkins. Survey: Physician Practice Patterns Changing As A Result Of COVID-19. April 22, 2020.https://www.merritthawkins.com/news-and-insights/media-room/press/-Physician-Practice-Patterns-Changing-as-a-Result-of-COVID-19/. Accessed June 17, 2020.
- The Medical Futurist. COVID-19 and the rise of telemedicine.March 31, 2020. https://medicalfuturist.com/covid-19-was-needed-for-telemedicine-to-finally-go-mainstream/. Accessed June 16, 2020.
- Christensen C, Euchner J. Managing disruption: an interview with Clayton Christensen. Research-Technology Management. 2011;54:1, 11-17.
- Wordstream. 4 major trends for post-COVID-19 world. Last updated May 1, 2020. https://www.wordstream.com/blog/ws/2020/03/23/covid-19-business-trends. Accessed June16, 2020.
- Rosenberg J. Physician shortage likely to impact ob/gyn workforce in coming years. AJMC. September 21, 2019. https://www.ajmc.com/newsroom/physician-shortage-likely-to-impact-obgyn-workforce-in-coming-years. Accessed June 16, 2020.
- CMS.gov. National Health Expenditure Data: Historical. Page last modified December 17, 2019. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical. Accessed June 17, 2020.
- Cohen JK. Study: Telehealth program reduces unnecessary ED visits by 6.7%. Hospital Review. February 27, 2017.https://www.beckershospitalreview.com/telehealth/study-telehealth-program-reduces-unnecessary-ed-visits-by-6-7.html. Accessed June 23, 2020.
Pubovaginal sling during urethral diverticulectomy reduces stress incontinence
a large retrospective cohort study has found.
However, in 66% of cases in which the diverticulectomy alone was performed, women also saw their stress urinary incontinence (SUI) resolve.
For a study published online in the American Journal of Obstetrics and Gynecology, Sarah E. Bradley, MD, of Georgetown University in Washington and colleagues analyzed records for 485 urethral diverticulectomies performed at 11 institutions over a 16-year period. One-fifth of patients had an autologous fascial pubovaginal sling (PVS) placed at the time of surgery.
The concomitant sling was associated with a significantly greater reduction of SUI after adjustment for prior diverticulectomy, prior incontinence surgery, age, race, and parity (adjusted odds ratio, 2.27; 95% confidence interval, 1.02-5.03; P = .043).
However, 10% of women in the sling-treated group had recurrent UTI from 6 weeks after surgery, compared with 3% of those in the diverticulectomy-only group (P = .001). Even after adjustment for higher rates of UTI before surgery in the sling group, the odds of recurrent UTI still were higher with the concomitant sling. Women within the sling group also were more likely to experience urinary retention at more than 6 weeks after surgery (8% vs. 1%; P equal to .0001).
Dr. Bradley and her colleagues noted that theirs was the largest study to date evaluating postoperative SUI in patients undergoing diverticulectomy with and without a PVS, noting that many surgeons do not routinely offer the sling at the time of diverticulectomy.
They also acknowledged a selection bias in their study. “With the previously thought theoretical increased risk of the addition of PVS, it is likely that most providers would prefer only to offer this concomitant procedure to those with significantly bothersome SUI. Additionally, the majority of women that underwent PVS (83%) came from two of the 11 participating institutions,” the researchers wrote.
In an interview, Catherine A. Mathews, MD, of Wake Forest University in Winston Salem, N.C., argued for a different interpretation of the study’s results.
“The study was beautifully done and it’s an ideal subject for a review, but in some respect the authors missed the opportunity to highlight that there was a spontaneous resolution of stress incontinence symptoms in 66% of women who received diverticulectomy alone,” Dr. Matthews said, adding that this has important implications for medical decision-making and patient choice.
“Morbidity associated with the pubovaginal sling was very low in this study, probably because it was being done by very proficient surgeons, but in many centers it is higher,” Dr. Matthews said. Even with the overall low morbidity seen in the study, “there was still a significant price to pay” for some women in the pubovaginal sling–treated group. “Recurrent UTI can be challenging to manage in the long term, with antibiotic morbidity and significant symptom bother. For the patients with urinary retention, having to manage it with a catheter is a really awful.”
Dr. Matthews said that the study made a case for interval, rather than concomitant, sling placement in women undergoing urethral diverticulectomy. “If you have a patient who insists on addressing symptoms concomitantly, this study provides good information about the long-term likelihood of two complications: urinary retention and recurrent UTI,” she said. “The vast majority of patients that I’m counseling would choose not to have the sling because of these complications.” And while avoiding reoperation may seem a good reason to opt for the PVS during diverticulectomy, the sling was not associated with a decrease in reoperations, compared with diverticulectomy alone, she noted.
“As we can see in the study, diverticulectomy itself has a high impact on stress incontinence,” Dr. Matthews continued. “If you restore the urethral anatomy and wait for the urethra to heal, you have a very good chance that the incontinence resolves.” For those women who do not see resolution and whose symptoms are still severe enough to bother them, “you’d have the flexibility postoperatively to offer not only a pubovaginal sling, but a synthetic mesh sling or a urethral bulking procedure.”
Dr. Bradley and her colleagues reported no relevant financial disclosures. Dr. Matthews disclosed financial support from Boston Scientific and serving as an expert witness for Johnson & Johnson.
SOURCE: Bradley SE et al. Am J Obstet Gynecol. 2020. doi: 10.1016/j.ajog.2020.06.002.
a large retrospective cohort study has found.
However, in 66% of cases in which the diverticulectomy alone was performed, women also saw their stress urinary incontinence (SUI) resolve.
For a study published online in the American Journal of Obstetrics and Gynecology, Sarah E. Bradley, MD, of Georgetown University in Washington and colleagues analyzed records for 485 urethral diverticulectomies performed at 11 institutions over a 16-year period. One-fifth of patients had an autologous fascial pubovaginal sling (PVS) placed at the time of surgery.
The concomitant sling was associated with a significantly greater reduction of SUI after adjustment for prior diverticulectomy, prior incontinence surgery, age, race, and parity (adjusted odds ratio, 2.27; 95% confidence interval, 1.02-5.03; P = .043).
However, 10% of women in the sling-treated group had recurrent UTI from 6 weeks after surgery, compared with 3% of those in the diverticulectomy-only group (P = .001). Even after adjustment for higher rates of UTI before surgery in the sling group, the odds of recurrent UTI still were higher with the concomitant sling. Women within the sling group also were more likely to experience urinary retention at more than 6 weeks after surgery (8% vs. 1%; P equal to .0001).
Dr. Bradley and her colleagues noted that theirs was the largest study to date evaluating postoperative SUI in patients undergoing diverticulectomy with and without a PVS, noting that many surgeons do not routinely offer the sling at the time of diverticulectomy.
They also acknowledged a selection bias in their study. “With the previously thought theoretical increased risk of the addition of PVS, it is likely that most providers would prefer only to offer this concomitant procedure to those with significantly bothersome SUI. Additionally, the majority of women that underwent PVS (83%) came from two of the 11 participating institutions,” the researchers wrote.
In an interview, Catherine A. Mathews, MD, of Wake Forest University in Winston Salem, N.C., argued for a different interpretation of the study’s results.
“The study was beautifully done and it’s an ideal subject for a review, but in some respect the authors missed the opportunity to highlight that there was a spontaneous resolution of stress incontinence symptoms in 66% of women who received diverticulectomy alone,” Dr. Matthews said, adding that this has important implications for medical decision-making and patient choice.
“Morbidity associated with the pubovaginal sling was very low in this study, probably because it was being done by very proficient surgeons, but in many centers it is higher,” Dr. Matthews said. Even with the overall low morbidity seen in the study, “there was still a significant price to pay” for some women in the pubovaginal sling–treated group. “Recurrent UTI can be challenging to manage in the long term, with antibiotic morbidity and significant symptom bother. For the patients with urinary retention, having to manage it with a catheter is a really awful.”
Dr. Matthews said that the study made a case for interval, rather than concomitant, sling placement in women undergoing urethral diverticulectomy. “If you have a patient who insists on addressing symptoms concomitantly, this study provides good information about the long-term likelihood of two complications: urinary retention and recurrent UTI,” she said. “The vast majority of patients that I’m counseling would choose not to have the sling because of these complications.” And while avoiding reoperation may seem a good reason to opt for the PVS during diverticulectomy, the sling was not associated with a decrease in reoperations, compared with diverticulectomy alone, she noted.
“As we can see in the study, diverticulectomy itself has a high impact on stress incontinence,” Dr. Matthews continued. “If you restore the urethral anatomy and wait for the urethra to heal, you have a very good chance that the incontinence resolves.” For those women who do not see resolution and whose symptoms are still severe enough to bother them, “you’d have the flexibility postoperatively to offer not only a pubovaginal sling, but a synthetic mesh sling or a urethral bulking procedure.”
Dr. Bradley and her colleagues reported no relevant financial disclosures. Dr. Matthews disclosed financial support from Boston Scientific and serving as an expert witness for Johnson & Johnson.
SOURCE: Bradley SE et al. Am J Obstet Gynecol. 2020. doi: 10.1016/j.ajog.2020.06.002.
a large retrospective cohort study has found.
However, in 66% of cases in which the diverticulectomy alone was performed, women also saw their stress urinary incontinence (SUI) resolve.
For a study published online in the American Journal of Obstetrics and Gynecology, Sarah E. Bradley, MD, of Georgetown University in Washington and colleagues analyzed records for 485 urethral diverticulectomies performed at 11 institutions over a 16-year period. One-fifth of patients had an autologous fascial pubovaginal sling (PVS) placed at the time of surgery.
The concomitant sling was associated with a significantly greater reduction of SUI after adjustment for prior diverticulectomy, prior incontinence surgery, age, race, and parity (adjusted odds ratio, 2.27; 95% confidence interval, 1.02-5.03; P = .043).
However, 10% of women in the sling-treated group had recurrent UTI from 6 weeks after surgery, compared with 3% of those in the diverticulectomy-only group (P = .001). Even after adjustment for higher rates of UTI before surgery in the sling group, the odds of recurrent UTI still were higher with the concomitant sling. Women within the sling group also were more likely to experience urinary retention at more than 6 weeks after surgery (8% vs. 1%; P equal to .0001).
Dr. Bradley and her colleagues noted that theirs was the largest study to date evaluating postoperative SUI in patients undergoing diverticulectomy with and without a PVS, noting that many surgeons do not routinely offer the sling at the time of diverticulectomy.
They also acknowledged a selection bias in their study. “With the previously thought theoretical increased risk of the addition of PVS, it is likely that most providers would prefer only to offer this concomitant procedure to those with significantly bothersome SUI. Additionally, the majority of women that underwent PVS (83%) came from two of the 11 participating institutions,” the researchers wrote.
In an interview, Catherine A. Mathews, MD, of Wake Forest University in Winston Salem, N.C., argued for a different interpretation of the study’s results.
“The study was beautifully done and it’s an ideal subject for a review, but in some respect the authors missed the opportunity to highlight that there was a spontaneous resolution of stress incontinence symptoms in 66% of women who received diverticulectomy alone,” Dr. Matthews said, adding that this has important implications for medical decision-making and patient choice.
“Morbidity associated with the pubovaginal sling was very low in this study, probably because it was being done by very proficient surgeons, but in many centers it is higher,” Dr. Matthews said. Even with the overall low morbidity seen in the study, “there was still a significant price to pay” for some women in the pubovaginal sling–treated group. “Recurrent UTI can be challenging to manage in the long term, with antibiotic morbidity and significant symptom bother. For the patients with urinary retention, having to manage it with a catheter is a really awful.”
Dr. Matthews said that the study made a case for interval, rather than concomitant, sling placement in women undergoing urethral diverticulectomy. “If you have a patient who insists on addressing symptoms concomitantly, this study provides good information about the long-term likelihood of two complications: urinary retention and recurrent UTI,” she said. “The vast majority of patients that I’m counseling would choose not to have the sling because of these complications.” And while avoiding reoperation may seem a good reason to opt for the PVS during diverticulectomy, the sling was not associated with a decrease in reoperations, compared with diverticulectomy alone, she noted.
“As we can see in the study, diverticulectomy itself has a high impact on stress incontinence,” Dr. Matthews continued. “If you restore the urethral anatomy and wait for the urethra to heal, you have a very good chance that the incontinence resolves.” For those women who do not see resolution and whose symptoms are still severe enough to bother them, “you’d have the flexibility postoperatively to offer not only a pubovaginal sling, but a synthetic mesh sling or a urethral bulking procedure.”
Dr. Bradley and her colleagues reported no relevant financial disclosures. Dr. Matthews disclosed financial support from Boston Scientific and serving as an expert witness for Johnson & Johnson.
SOURCE: Bradley SE et al. Am J Obstet Gynecol. 2020. doi: 10.1016/j.ajog.2020.06.002.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Telemedicine: Common hurdles and proper coding for ObGyns
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
Since the COVID-19 pandemic began, many significant changes have occurred that have made the implementation of telemedicine easier and more attractive for gynecologic practices. In the first article in this series, we discussed the benefits of telemedicine to physicians and patients, how to get started using telemedicine, and implementing a workflow. This article will discuss the common hurdles in the process and the proper coding to use to insure reimbursement for services rendered.
Barriers to implementing telemedicine
Incorrect assumptions
Latecomers to telemedicine often assume that patients prefer face-to-face visits when, in fact, many may prefer the convenience of virtual visits. More than 50% of patients who are surveyed about their experience with telemedicine say that online tools have helped improve their relationship with their providers.1 Telemedicine has grown astronomically during the COVID-19 pandemic to the point where many patients now expect their health care providers to be able to conduct virtual visits. Practices that do not offer telemedicine may find their patients seeking services elsewhere. Nearly two-thirds of health care professionals expect their commitment to telemedicine to increase significantly in the next 3 years.2 Of those providers who have not yet adopted the practice, nearly 85% expect to implement telemedicine in the near future.3 COVID-19 has motivated the increased use of telemedicine to enhance the communication with patients, making it possible for patients to have enhanced access to health care during this pandemic while minimizing infectious transmission of COVID-19 to physicians and their staff.4
Admittedly, telemedicine is not appropriate for all patients. In general, situations that do not lend themselves to telemedicine are those for which an in-person visit is required to evaluate the patient via a physical examination, to perform a protocol-driven procedure, or provide an aggressive intervention. Additional patients for whom telemedicine may be inappropriate include those with cognitive disorders, those with language barriers, those with emergency situations that warrant an office visit or a visit to the emergency department, and patients who do not have access to the technology to conduct a virtual visit.
Cost and complexity
The process of implementing electronic health records (EHRs) left a bitter taste in the mouths of many health care professionals. But EHRs are complicated and expensive. Implementation often resulted in lost productivity. Because the learning curve was so steep, many physicians had to decrease the number of patients they saw before becoming comfortable with the conversion from paper charts to an EHR.
Telemedicine implementation is much less onerous and expensive. Telemedicine is available as a cloud-based platform, which requires less information technology (IT) support and less hardware and software. The technology required for patients to participate in telemedicine is nearly ubiquitous. According to the Pew Research Center, 96% of Americans own a cell phone (81% have a smart phone), and more than half (52%) own a tablet, so the basic equipment to connect patients to providers is already in place.5
On the provider side, the basic equipment required for a telemedicine program is a computer with video and audio capabilities and a broadband connection that is fast enough to show video in real time and to provide high-quality viewing of any images to be reviewed.
The growth in telemedicine means that telemedicine options are now more diverse, with many more affordable solutions. However, most telemedicine programs do require the purchase and set-up of new technology and equipment and the training of staff—some of which may be outside the budgets of health care providers in smaller independent practices. Many gynecologists have technology budgets that are already stretched thin. And for patients who do not have access to a smartphone or computer with Internet access, real-time telemedicine may be out of reach.
But with new guidelines put forth by the Centers for Medicare and Medicaid Services (CMS) in March 2020, connectivity can take place inexpensively using free platforms such as Google Hangouts, Skype, Facetime, and Facebook Messenger. If a non‒HIPAA-compliant platform is used initially, conversion to a HIPAA-compliant platform is recommended.6 These platforms do not require the purchase of, or subscription to, any expensive hardware or software. The disadvantages of these programs are the lack of documentation, the failure to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, and the lack of encryption; however, these disadvantages are no longer an issue after the new CMS guidelines.
Depending on the magnitude of the program, IT assistance may be needed to get started. It is imperative that the telemedicine program is interoperable with the EHR and the billing program. Otherwise, double and triple entry will erase the efficiency provided by conducting a virtual visit.
Continue to: Licensing...
Licensing
Another concern or barrier is a license to participate in telemedicine. The March 15, 2020, approval of telemedicine states that physicians who are licensed in the state where the patient is located do not require any additional license or permission to conduct virtual visits.7 CMS has temporarily waived the requirement that out-of-state providers be licensed in the state where they are providing services when they are licensed in another state. For questions regarding licensure, contact your State Board of Medicine or Department of Health for information on requirements for licenses across state lines (see “Resources,” at the end of the article).
Informed consent
Just like with any other aspect of providing care for patients, obtaining informed consent is paramount. Not only is getting informed patient consent a recommended best practice of the American Telemedicine Association (ATA), but it is actually a legal requirement in many states and could be a condition of getting paid, depending on the payer. To check the requirements regarding patient consent in your state, look at The National Telehealth Policy Resource Center’s state map (see “Resources.")
Some states do not have any requirements regarding consent for a virtual visit. Others require verbal consent. Even if it is not a legal requirement in your state, consider making it a part of your practice’s policy to obtain written or verbal consent and to document in the patient’s record that consent was obtained prior to the virtual visit so that you are protected when using this new technology.
Because telemedicine is a new way of receiving care for many patients, it is important to let them know how it works including how patient confidentiality and privacy are handled, what technical equipment is required, and what they should expect in terms of scheduling, cancellations, and billing policies. A sample consent form for telemedicine use is shown in FIGURE 1.


Liability insurance
Another hurdle that must be considered is liability insurance for conducting virtual visits with patients. Gynecologists who are going to offer telemedicine care to patients should request proof in writing that their liability insurance policy covers telemedicine malpractice and that the coverage extends to other states should the patient be in another state from the state in which the gynecologist holds a license. Additionally, gynecologists who provide telemedicine care should check with liability insurers regarding any requirements or limitations to conducting a virtual visit with their patients and should document them. For example, the policy may require that the physician keep a written or recorded record of the visit in the EHR. If that is the case, then using Skype, Facebook, or Google for the virtual visit, which do not include documentation, would be less desirable.
Privacy
Certainly, there is concern about privacy, and HIPAA compliance is critical to telemedicine success. Because of the COVID-19 emergency, as of March 1, 2020, physicians may now communicate with patients, and provide telehealth services, through remote communications without penalties.8 With these changes in the HIPAA requirements, physicians may use applications that allow for video chats, including Apple FaceTime, Facebook Messenger video chat, Google Hangouts video, and Skype, to provide telehealth without risk that the Office for Civil Rights will impose a penalty for noncompliance with HIPAA rules. The consent for patients should mention that these “public” applications potentially introduce privacy risks. This is a motivation for gynecologists to consider one of the programs that promises encryption, privacy, and HIPAA compliance, such as Updox, Doxy.me, and Amazon Chime. It is also important to recognize that a virtual visit could result in colleagues (if the patient is in an office setting) or family members (if the patient is in the home environment) overhearing conversations between the health care professional and the patient. Therefore, we suggest that patients conduct virtual visits in locations in which they feel assured of some semblance of privacy.
Continue to: Compensation for telemedicine...
Compensation for telemedicine
Perhaps the biggest barrier to virtual health adoption has been compensation for telemedicine visits. Both commercial payers and CMS have been slow to enact formal policies for telemedicine reimbursement. Because of this, the common misconceptions (that providers cannot be reimbursed for telemedicine appointments or that compensation occurs at a reduced rate) have persisted, making telemedicine economically unappealing.
The good news is that this is changing; legislation in most states is quickly embracing virtual health visits as a result of the COVID-19 pandemic.9 In fact, as of January 1, 2020, telemedicine services are no longer considered “optional” coverage in Medicare Advantage plans.10 Nor are they required to have an additional fee. Instead, CMS now allows telemedicine as a standard, covered benefit in all plans, enabling beneficiaries to seek care from their homes rather than requiring them to go to a health care facility.11 In the past, telemedicine was restricted for use in rural areas or when patients resided a great distance from their health care providers. Starting March 6, 2020, and for the duration of the COVID-19 public health emergency, Medicare will make payment for professional services furnished to beneficiaries in all areas of the country in all settings regardless of location or distance between the patient and the health care provider.12
In addition, since March 15, 2020, CMS has expanded access to telemedicine services for all Medicare beneficiaries—not just those who have been diagnosed with COVID-19.13 The expanded access also applies to pre-COVID-19 coverage from physician offices, skilled nursing facilities, and hospitals. This means that Medicare will now make payments to physicians for telemedicine services provided in any health care facility or in a patient’s home, so that patients do not need to go to the physician’s office.
The facts are that there are parity laws and that commercial payers and CMS are required by state law to reimburse for telemedicine—often at the same rate as that for a comparable in-person visit. On the commercial side, there has been an increase in commercial parity legislation that requires health plans to cover virtual visits in the same way they cover face-to-face services. With the new guidelines for reimbursement, every state and Washington DC has parity laws in place. (To stay abreast of state-by-state changes in virtual health reimbursement, the Center for Connected Health Policy and the Advisory Board Primer are valuable resources. See “Resources.”) As long as the provider performs and documents the elements of history and decision-making, including the time spent counseling, and documents the visit as if a face-to-face visit occurred, then clinicians have a billable evaluation and management (E&M) visit.
Continue to: Virtual services for Medicare patients...
Virtual services for Medicare patients
There are 3 main types of virtual services gynecologists can provide to Medicare patients: Medicare telehealth visits, virtual check-ins, and e-visits.
Medicare telehealth visits. Largely because of the COVID-19 pandemic, Medicare patients may now use telecommunication technology for any services that previously occurred in an in-person communication. The gynecologist must use an interactive audio and video telecommunications system that permits real-time communication between the physician and the patient, and the patient should have a prior established relationship with the gynecologist with whom the telemedicine visit is taking place. The new guidelines indicate that the US Department of Health and Human Services (HHS) will not conduct audits to ensure that such a prior relationship exists for claims submitted during this public health emergency.14
The Current Procedural Terminology (CPT) codes for virtual visits using synchronous audio/visual communication are:
- 99201-99295, Office visit for a new patient
- 99211-99215, Office visit for an established patient.
Important modifiers for telemedicine visits include:
- modifier 02 for POS (place of service) for telehealth Medicare
- modifier 95 for commercial payers.
(A list of all available CPT codes for telehealth services from CMS can be found in “Resources.”)
Virtual check-ins. Established Medicare patients may have a brief communication with gynecologists the traditional way using a telephone or via live video. These brief virtual services, usually 5 to 10 minutes in duration, are initiated by the patient. The purpose of the virtual check-in is to determine if an office visit or a test or procedure is indicated.
Medicare pays for these “virtual check-ins” (or brief communication technology-based services) for patients to communicate with their physicians and avoid unnecessary trips to the office. These brief virtual check-ins are only for established patients. If an existing patient contacts the gynecologist’s office to ask a question or determine if an office visit is necessary, the gynecologist may bill for it using code G2012.
E-visits. Established Medicare patients may have non–face-to-face patient-initiated communications with their gynecologists without going to the physician’s office. These services can be billed only when the physician has an established relationship with the patient. The services may be billed using CPT codes 99421 to 99423. Coding for these visits is determined by the length of time the gynecologist spends online with the patient:
- 99421: Online digital evaluation and management service, for an established patient 5 to 10 minutes spent on the virtual visit
- 99422: 11 to 20 minutes
- 99423: ≥ 21 minutes.
Many clinicians want to immediately start the communication process with their patients. Many will avail themselves of the free video communication offered by Google Hangouts, Skype, Facetime, and Facebook Messenger. Since the March 15, 2020, relaxation of the HIPAA restrictions for telemedicine, it is now possible to have a virtual visit with a patient using one of the free, non–HIPAA-compliant connections. This type of visit is no different than a telephone call but with an added video component. Using these free technologies, a gynecologist can have an asynchronous visit with a patient (referred to as the store and forward method of sending information or medical images), which means that the service takes place in one direction with no opportunity for interaction with the patient. Asynchronous visits are akin to video text messages left for the patient. By contrast, a synchronous or real-time video visit with a patient is a 2-way communication that provides medical care without examining the patient.
Using triangulation
There are some downsides to telemedicine visits. First, virtual visits on Skype, FaceTime, and other non–HIPAA-compliant methods are not conducted on an encrypted website. Second, no documentation is created for the doctor-patient encounter. Finally, unless the physician keeps a record of these virtual visits and submits the interactions to the practice coders, there will be no billing and no reimbursement for the visits. In this scenario, physicians are legally responsible for their decision-making, prescription writing, and medical advice, but do not receive compensation for their efforts.
This can be remedied by using “triangulation,” which involves: 1. the physician, 2. the patient, and 3. a scribe or medical assistant who will record the visit. Before initiating the virtual visit using triangulation, it is imperative to ask the patient for permission if your medical assistant (or any other person in the office who functions as a scribe) will be listening to the conversation. It is important to explain that the person is there to take accurate notes and ascertain that the notes are entered into the EHR. Also, the scribe or assistant will record the time, date, and duration of the visit, which is a requirement for billing purposes. The scribe may also ascertain that the visit is properly coded and entered into the practice management system, and that a bill is submitted to the insurance company. By using triangulation, you have documentation that consent was obtained, that the visit took place, that notes were taken, and that the patient’s insurance company will be billed for the visit (see FIGURE 2 for a sample documentation form).

Continue to: Which CPT codes should I use?...
Which CPT codes should I use?
The answer depends on a number of factors, but a good rule of thumb is to use the same codes that you would use for an in-person appointment (CPT codes 99211-99215 for an established patient visit and 99201-99205 for a new patient visit). These are the most common CPT codes for outpatient gynecologic office visits whether they take place face-to-face or as a synchronous virtual visit (via a real-time interactive audio and video telecommunications system).
For example, the reimbursement for code 99213 has a range from $73 to $100. You may wonder how you can achieve the complexity requirements for a level-3 office visit without a physical examination. Whether as a face-to-face or virtual visit, documentation for these encounters requires 2 of 3 of the following components:
- expanded problem-focused history
- expanded problem-focused exam (not accomplished with telemedicine)
- low-complexity medical decision-making OR
- at least 15 minutes spent face to face with the patient if coding is based on time.
If a gynecologist reviews the results of a recent lab test for an estrogen-deficient patient and adjusts the estrogen dosage, writes a prescription, and spends 15 minutes communicating with the patient, he/she has met the complexity requirements for a code 99213. Because Level 3 and 4 visits (99214 and 99215) require a comprehensive physical examination, it is necessary to document the time spent with the patient (code 99214 requires 25 to 39 minutes of consultation and code 99215 requires ≥ 40 minutes).
Some final billing and coding advice
Always confirm telemedicine billing guidelines before beginning to conduct telemedicine visits. Consider starting a phone call to a payer armed with the fact that the payer is required by law to offer parity between telemedicine and face-to-face visits. Then ask which specific billing codes should be used.
Until you and your practice become comfortable with the process of, and the coding and billing for, telemedicine, consider using a telemedicine platform that has a built-in rules engine that offers recommendations for each telemedicine visit based on past claims data. These systems help gynecologists determine which CPT code to use and which modifiers are appropriate for the various insurance companies. In other words, the rules engine helps you submit a clean claim that is less likely to be denied and very likely to be paid. There are some vendors who are so confident that their rules engine will match the service with the proper CPT code and modifier that they guarantee full private payer reimbursement for telemedicine visits, or the vendor will reimburse the claim.
Watch for the third and final installment in this series, which was written with the assistance of 2 attorneys. It will review the legal guidelines for implementing telemedicine in a gynecologic practice and discuss the future of the technology. ●
- COVID-19 and Telehealth Coding Options as of March 20, 2020. https://www.ismanet.org/pdf/COVID-19andTelehealthcodes3-20-2020Updates.pdf.
- Federation of State Medical Boards. US States and Territories Modifying Licensure Requirements for Physicians in Response to COVID-19. Last updated May 26, 2020. https://www.fsmb.org/siteassets/advocacy/pdf/state-emergency-declarations-licensures-requirementscovid-19.pdf.
- Center for Connected Health Policy. Current State Laws and Reimbursement Policies https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies.
- Centers for Medicare and Medicaid Services. List of Telehealth Services. Updated April 30, 2020. https://www.cms.gov/Medicare/Medicare-General-Information/Telehealth/Telehealth-Codes.
- American Medical Association. AMA quick guide to telemedicinein practice. Updated May 22, 2020. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
- Eddy N. Patients increasingly trusting of remote care technology. Healthcare IT News. October 22, 2019. https://www.healthcareitnews.com/news/patients-increasingly-trusting-remote-care-technology-says-new-report. Accessed May 26, 2020.
- Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Tsai JM, Cheng MJ, Tsai HH, et al. Acceptance and resistance of telehealth: the perspective of dual-factor concepts in technology adoption. Int J Inform Manag. 2019;49:34-44.
- Hollander J, Carr BG. Virtually perfect? Telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
- Pew Research Center. Internet and Technology. Mobile Fact Sheet. June 12, 2019. https://www.pewresearch.org /internet/fact-sheet/mobile/. Accessed May 18, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/ practice-management/digital/ama-quick-guide-telemedicine- practice. Accessed March 20, 2020.
- Center for Connected Health Policy. Federal and state regulation updates. https://www.cchpca.org. Accessed March 20, 2020.
- The White House. Proclamation on declaring a national emergency concerning the novel coronavirus disease (Covid-19) outbreak. March 13, 2020. https://www.whitehouse.gov/presidential-actions/proclamation-declaring-national-emergency-concerning-novel-coronavirus-disease-covid-19-outbreak/. Accessed May 18, 2020.
- Center for Connected Health Policy. Quick glance state telehealth actions in response to COVID-19. https://www.cchpca.org/sites/default/files/2020-05/STATE%20TELEHEALTH%20ACTIONS%20IN%20RESPONSE%20TO%20COVID%20
OVERVIEW%205.5.2020_0.pdf. AccessedMay 13, 2020. - Medicare.gov. https://www.medicare.gov/sign-up-change -plans/types-of-medicare-health-plans/medicare-advantage-plans/how-do-medicare-advantage-plans-work. Accessed May 13, 2020.
- Centers for Medicare and Medicaid Services. CMS finalizes policies to bring innovative telehealth benefit to Medicare Advantage. April 5, 2019. https://www.cms.gov/newsroom /press-releases/cms-finalizes-policies-bring-innovative-telehealth-benefit-medicare-advantage. Accessed May 18,2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed May 30, 2020.
- Centers for Medicare & Medicaid Services. Medicare telehealth frequently asked questions. https://www.cms.gov/files/document/medicare-telehealth-frequently-asked-questions-faqs-31720.pdf.
- American Hospital Association. Coronavirus update: CMS broadens access to telehealth during Covid-19 public health emergency. https://www.aha.org/advisory/2020-03-17-coronavirus-update-cms-broadens-access-telehealth-during-covid-19-public-health. Accessed May 18, 2020.
How to perform a vulvar biopsy
Many benign, premalignant, and malignant lesions can occur on the vulva. These can be challenging to differentiate by examination alone. A vulvar biopsy often is needed to appropriately diagnose—and ultimately treat—these various conditions.
In this article, we review vulvar biopsy procedures, describe how to prepare tissue specimens for the pathologist, and provide some brief case examples in which biopsy established the diagnosis.
Ask questions first
Prior to examining a patient with a vulvar lesion, obtain a detailed history. Asking specific questions may aid in making the correct diagnosis, such as:
- How long has the lesion been present? Has it changed? What color is it?
- Was any trigger, or trauma, associated with onset of the lesion?
- Does the lesion itch, burn, or cause pain? Is there any associated bleeding or discharge?
- Are other lesions present in the vagina, anus, or mouth, or are other skin lesions present?
- Are any systemic symptoms present, such as fever, lymphadenopathy, weight loss, or joint pain?
- What is the patient’s previous treatment history, including over-the-counter medications and prescribed medications?
- Has there been any incontinence of urine or stool? Does the patient use a pad?
- Is the patient scratching? Is there any nighttime scratching? It also can be useful to ask her partner, if she has one, about nighttime scratching.
- Is there a family history of vulvar conditions?
- Has there been any change in her use of products like soap, lotions, cleansing wipes, sprays, lubricants, or laundry detergent?
- Has the patient had any new partners or significant travel history?
Preprocedure counseling points
Prior to proceeding with a vulvar biopsy, review with the patient the risks, benefits, and alternatives and obtain patient consent for the procedure. Vulvar biopsy risks include pain, bleeding, infection, injury to surrounding tissue, and the need for further surgery. Make patients aware that some biopsies are nondiagnostic. We recommend that clinicians perform a time-out verification to ensure that the patient’s identity and planned procedure are correct.
Assess the biopsy site
A wide variety of lesions may require a biopsy for diagnosis. While it can be challenging to know where to biopsy, taking the time to determine the proper biopsy site may enhance pathology results.
When considering colored lesions, depth is the important factor, and a punch biopsy often is sufficient. A tumor should be biopsied in the thickest area. Lesions that are concerning for malignancy may require multiple biopsies. An erosion or ulcer is best biopsied on the edge, including a small amount of surrounding tissue. For most patients, biopsy of normal-appearing tissue is of low diagnostic yield. Lastly, we try to avoid biopsies directly on the midline to facilitate better healing.1
A photograph of the vulva prior to biopsy may be helpful for the pathologist to see the tissue. Some electronic medical records have the capability to include photographs. Due to the sensitive nature of these photographs, we prefer that a separate written patient consent be obtained prior to taking photographs. We find also that photos are a useful reference for progression of disease at follow-up in a shared care team.
Continue to: Anesthesia procedure and instrument kit...
Anesthesia procedure and instrument kit
Some patients may benefit from the application of topical lidocaine 4% cream (L.M.X.4) prior to the injection of a local anesthetic for tissue biopsy. Ideally, topical lidocaine should be placed on the vulva and covered with a dressing such as Tegaderm or cellophane up to 30 minutes before the anticipated biopsy procedure. The anesthetic effect generally lasts for about 60 minutes. Many patients report stinging for several seconds upon application. Due to clinic time restrictions, we tend to reserve this method for a limited subset of patients. If planning a return visit for a biopsy, the patient can place the topical anesthetic herself.
For the anesthetic injection, we recommend lidocaine 1% or 2% with epinephrine in all areas of the vulva except for the glans clitoris. For a punch biopsy, we draw up 1 to 3 mL in a 3-mL syringe and inject with a 21- to 30-gauge needle, using a lower gauge for thicker tissue. We have not found buffering the anesthetic with sodium bicarbonate to be of particular use. For the glans clitoris, lidocaine without epinephrine should be utilized.
Equipment. Depending on your office setting, having a premade instrument kit may be preferred to peel-pack equipment. We prefer a premade tray that contains sterile gauze, a hemostat, iris scissors, a needle driver, a scalpel handle, and Adson forceps (FIGURE 1).

Types of biopsy procedures
Punch biopsy. We recommend a 4-mm Keyes biopsy punch. As mentioned, we use a biopsy kit to facilitate the procedure. After the tissue is properly anesthetized and prepped, we test the area via gentle touch to the skin with the hemostat or Adson forceps. To perform the punch biopsy, gentle, consistent pressure in a clockwise-counterclockwise fashion yields the best results. The goal is to obtain a 5-mm depth for hair-bearing skin and a 3-mm depth for all other tissue.2 The tissue should then be excised at the base with scissors, taking care not to crush the specimen with forceps.
Punch biopsy permits sampling of the epidermis, dermis, and subcutaneous tissue. Hemostasis is maintained with either silver nitrate, Monsel’s solution (ferric sulfate), or a dissolvable suture such as 4-0 Monocryl (poliglecaprone 25) or Vicryl Rapide (polyglactin 910).
Stitch biopsy. We find the stitch biopsy to be very useful given the architecture of the vulva. A modification of the shave biopsy, the stitch biopsy is depicted in FIGURE 2. A 3-0 or 4-0 dissolvable suture is placed through the intended area of biopsy. Iris scissors are used to undermine the tissue while the suture is held on tension. The goal is to remove the suture with the specimen. Separate sutures are used for hemostasis. The stitch does not cause the crushing artifacts on prepared specimens. Depending on the proceduralist’s comfort, a relatively large sample can be obtained in this fashion. If the suture held on tension is inadvertently cut, a second pass can be made with suture; alternatively, care can be used to remove remaining tissue with forceps and scissors, again avoiding crush injury to the tissue.

Excisional biopsy. Often, a larger area or margins are desired. We find that with adequate preparation, patients tolerate excisions in the office quite well. The planned area for excision can be marked with ink to ensure margins. Adequate anesthesia is instilled. A No. 15 blade scalpel is often the best size used to excise vulvar tissue in an elliptical fashion. Depending on depth of incision, the tissue may need to be approximated in layers for cosmesis and healing.
When planning an excisional biopsy, place a stitch on the excised tissue to mark orientation or pin out the entire specimen to a foam board to help your pathologist interpret tissue orientation.
The box "Vulvar biopsy established the diagnosis" at the end of this discussion provides 6 case examples of vulvar lesions and the respective diagnoses confirmed by biopsy.
Continue to: Preparing tissue for the pathologist...
Preparing tissue for the pathologist
Here are 5 tips for preparing the biopsied specimen for pathology:
- Include a question for the pathologist, such as “rule out lichen sclerosus or lichen simplex chronicus.” The majority of specimens should be sent in formalin. At times, frozen sections are done in the operating room.
- Double-check that the proper paperwork is included with every specimen and be very specific regarding the exact location of the lesion on the vulva. Include photographs whenever possible.
- Request that a dermatopathologist or a gynecologic pathologist with a special interest in vulvar dermatology, when feasible, review the tissue.
- Check your laboratory’s protocol for sending biopsies from areas around ulcerated tissue. Often, special medium is required for immunohistochemistry stains.
- Call your pathologist with questions about results; he or she often is happy to clarify, and together you may be able to arrive at a diagnosis to better serve your patient.3
Complications and how to avoid them
Bleeding. Any procedure has bleeding risks. To avoid bleeding, review the patient’s medication list and medical history prior to biopsy, as certain medications, such as blood thinners, increase risk for bleeding. Counseling a patient on applying direct pressure to the biopsy site for 2 minutes is generally sufficient for any bleeding that may occur once she is discharged from the clinic.
Infection. With aseptic technique, infection of a biopsy site is rare. We use nonsterile gloves for biopsy procedures. This does not increase the risk of infection.4 If a patient has iodine allergy, dilute chlorhexidine is a reasonable alternative for skin cleansing. Instruct the patient to keep the site clean and dry; if the biopsy proximity is close to the urethra or anus, use of a peri-bottle may be preferred after toileting. Instruct patients not to pull sutures. While instructions are specific for each patient, we generally advise that patients wait 4 to 7 days before resuming use of topical medications.
Scarring or tattooing. Avoid using dyed suture on skin surfaces and counsel the patient that silver nitrate can permanently stain tissue. Usually, small biopsies heal well but a small scar is possible.
Key points to keep in mind
- Counsel patients on biopsy risks, benefits, and alternatives. Counsel regarding possible inconclusive results.
- Take time in choosing the biopsy site and consider multiple biopsies.
- Have all anticipated equipment available; consider using premade biopsy kits.
- Consider performing a stitch biopsy to avoid crush injury.
- Take photographs of the area to be biopsied and communicate with your pathologist to facilitate diagnosis.
Case 1
Biopsies were obtained of the areas highlighted in the photo. Pathology shows dVIN.
Image courtesy of Hope Haefner, MD.
Case 2
The examination is consistent with condylomata acuminata and biopsy is recommended with a 4-mm punch. Biopsy results are consistent with condylomata acuminata.
Image courtesy of Hope Haefner, MD.
Case 3
The final pathology shows high-grade squamous intraepithelial lesions (HSIL) of the vulva.
Image courtesy of Hope Haefner, MD.
Case 4
This presentation is an excellent opportunity for an excisional biopsy of the vulva. A marking pen is used to draw margins. A No. 15 blade is used to outline and then undermine the lesion, removing it in its entirety.
Final pathology shows a compound nevus of the vulva.
Image courtesy of Hope Haefner, MD.
Case 5
A 4-mm punch biopsy result is consistent with a diagnosis of lichen sclerosus.
Image courtesy of Hope Haefner, MD.
Case 6
A 4-mm punch biopsy result reveals that the pathology is significant for squamous cell carcinoma.
Image courtesy of Hope Haefner, MD.
- Edwards L, Lynch PJ. Genital Dermatology Atlas and Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2018.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 93: Diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111:1243-1253.
- Heller DS. Areas of confusion in pathologist-clinician communication as it relates to understanding the vulvar pathology report. J Low Genit Tract Dis. 2017;21:327-328.
- Rietz A, Barzin A, Jones K, et al. Sterile or non-sterile gloves for minor skin excisions? J Fam Pract. 2015;64:723-727.
Many benign, premalignant, and malignant lesions can occur on the vulva. These can be challenging to differentiate by examination alone. A vulvar biopsy often is needed to appropriately diagnose—and ultimately treat—these various conditions.
In this article, we review vulvar biopsy procedures, describe how to prepare tissue specimens for the pathologist, and provide some brief case examples in which biopsy established the diagnosis.
Ask questions first
Prior to examining a patient with a vulvar lesion, obtain a detailed history. Asking specific questions may aid in making the correct diagnosis, such as:
- How long has the lesion been present? Has it changed? What color is it?
- Was any trigger, or trauma, associated with onset of the lesion?
- Does the lesion itch, burn, or cause pain? Is there any associated bleeding or discharge?
- Are other lesions present in the vagina, anus, or mouth, or are other skin lesions present?
- Are any systemic symptoms present, such as fever, lymphadenopathy, weight loss, or joint pain?
- What is the patient’s previous treatment history, including over-the-counter medications and prescribed medications?
- Has there been any incontinence of urine or stool? Does the patient use a pad?
- Is the patient scratching? Is there any nighttime scratching? It also can be useful to ask her partner, if she has one, about nighttime scratching.
- Is there a family history of vulvar conditions?
- Has there been any change in her use of products like soap, lotions, cleansing wipes, sprays, lubricants, or laundry detergent?
- Has the patient had any new partners or significant travel history?
Preprocedure counseling points
Prior to proceeding with a vulvar biopsy, review with the patient the risks, benefits, and alternatives and obtain patient consent for the procedure. Vulvar biopsy risks include pain, bleeding, infection, injury to surrounding tissue, and the need for further surgery. Make patients aware that some biopsies are nondiagnostic. We recommend that clinicians perform a time-out verification to ensure that the patient’s identity and planned procedure are correct.
Assess the biopsy site
A wide variety of lesions may require a biopsy for diagnosis. While it can be challenging to know where to biopsy, taking the time to determine the proper biopsy site may enhance pathology results.
When considering colored lesions, depth is the important factor, and a punch biopsy often is sufficient. A tumor should be biopsied in the thickest area. Lesions that are concerning for malignancy may require multiple biopsies. An erosion or ulcer is best biopsied on the edge, including a small amount of surrounding tissue. For most patients, biopsy of normal-appearing tissue is of low diagnostic yield. Lastly, we try to avoid biopsies directly on the midline to facilitate better healing.1
A photograph of the vulva prior to biopsy may be helpful for the pathologist to see the tissue. Some electronic medical records have the capability to include photographs. Due to the sensitive nature of these photographs, we prefer that a separate written patient consent be obtained prior to taking photographs. We find also that photos are a useful reference for progression of disease at follow-up in a shared care team.
Continue to: Anesthesia procedure and instrument kit...
Anesthesia procedure and instrument kit
Some patients may benefit from the application of topical lidocaine 4% cream (L.M.X.4) prior to the injection of a local anesthetic for tissue biopsy. Ideally, topical lidocaine should be placed on the vulva and covered with a dressing such as Tegaderm or cellophane up to 30 minutes before the anticipated biopsy procedure. The anesthetic effect generally lasts for about 60 minutes. Many patients report stinging for several seconds upon application. Due to clinic time restrictions, we tend to reserve this method for a limited subset of patients. If planning a return visit for a biopsy, the patient can place the topical anesthetic herself.
For the anesthetic injection, we recommend lidocaine 1% or 2% with epinephrine in all areas of the vulva except for the glans clitoris. For a punch biopsy, we draw up 1 to 3 mL in a 3-mL syringe and inject with a 21- to 30-gauge needle, using a lower gauge for thicker tissue. We have not found buffering the anesthetic with sodium bicarbonate to be of particular use. For the glans clitoris, lidocaine without epinephrine should be utilized.
Equipment. Depending on your office setting, having a premade instrument kit may be preferred to peel-pack equipment. We prefer a premade tray that contains sterile gauze, a hemostat, iris scissors, a needle driver, a scalpel handle, and Adson forceps (FIGURE 1).

Types of biopsy procedures
Punch biopsy. We recommend a 4-mm Keyes biopsy punch. As mentioned, we use a biopsy kit to facilitate the procedure. After the tissue is properly anesthetized and prepped, we test the area via gentle touch to the skin with the hemostat or Adson forceps. To perform the punch biopsy, gentle, consistent pressure in a clockwise-counterclockwise fashion yields the best results. The goal is to obtain a 5-mm depth for hair-bearing skin and a 3-mm depth for all other tissue.2 The tissue should then be excised at the base with scissors, taking care not to crush the specimen with forceps.
Punch biopsy permits sampling of the epidermis, dermis, and subcutaneous tissue. Hemostasis is maintained with either silver nitrate, Monsel’s solution (ferric sulfate), or a dissolvable suture such as 4-0 Monocryl (poliglecaprone 25) or Vicryl Rapide (polyglactin 910).
Stitch biopsy. We find the stitch biopsy to be very useful given the architecture of the vulva. A modification of the shave biopsy, the stitch biopsy is depicted in FIGURE 2. A 3-0 or 4-0 dissolvable suture is placed through the intended area of biopsy. Iris scissors are used to undermine the tissue while the suture is held on tension. The goal is to remove the suture with the specimen. Separate sutures are used for hemostasis. The stitch does not cause the crushing artifacts on prepared specimens. Depending on the proceduralist’s comfort, a relatively large sample can be obtained in this fashion. If the suture held on tension is inadvertently cut, a second pass can be made with suture; alternatively, care can be used to remove remaining tissue with forceps and scissors, again avoiding crush injury to the tissue.

Excisional biopsy. Often, a larger area or margins are desired. We find that with adequate preparation, patients tolerate excisions in the office quite well. The planned area for excision can be marked with ink to ensure margins. Adequate anesthesia is instilled. A No. 15 blade scalpel is often the best size used to excise vulvar tissue in an elliptical fashion. Depending on depth of incision, the tissue may need to be approximated in layers for cosmesis and healing.
When planning an excisional biopsy, place a stitch on the excised tissue to mark orientation or pin out the entire specimen to a foam board to help your pathologist interpret tissue orientation.
The box "Vulvar biopsy established the diagnosis" at the end of this discussion provides 6 case examples of vulvar lesions and the respective diagnoses confirmed by biopsy.
Continue to: Preparing tissue for the pathologist...
Preparing tissue for the pathologist
Here are 5 tips for preparing the biopsied specimen for pathology:
- Include a question for the pathologist, such as “rule out lichen sclerosus or lichen simplex chronicus.” The majority of specimens should be sent in formalin. At times, frozen sections are done in the operating room.
- Double-check that the proper paperwork is included with every specimen and be very specific regarding the exact location of the lesion on the vulva. Include photographs whenever possible.
- Request that a dermatopathologist or a gynecologic pathologist with a special interest in vulvar dermatology, when feasible, review the tissue.
- Check your laboratory’s protocol for sending biopsies from areas around ulcerated tissue. Often, special medium is required for immunohistochemistry stains.
- Call your pathologist with questions about results; he or she often is happy to clarify, and together you may be able to arrive at a diagnosis to better serve your patient.3
Complications and how to avoid them
Bleeding. Any procedure has bleeding risks. To avoid bleeding, review the patient’s medication list and medical history prior to biopsy, as certain medications, such as blood thinners, increase risk for bleeding. Counseling a patient on applying direct pressure to the biopsy site for 2 minutes is generally sufficient for any bleeding that may occur once she is discharged from the clinic.
Infection. With aseptic technique, infection of a biopsy site is rare. We use nonsterile gloves for biopsy procedures. This does not increase the risk of infection.4 If a patient has iodine allergy, dilute chlorhexidine is a reasonable alternative for skin cleansing. Instruct the patient to keep the site clean and dry; if the biopsy proximity is close to the urethra or anus, use of a peri-bottle may be preferred after toileting. Instruct patients not to pull sutures. While instructions are specific for each patient, we generally advise that patients wait 4 to 7 days before resuming use of topical medications.
Scarring or tattooing. Avoid using dyed suture on skin surfaces and counsel the patient that silver nitrate can permanently stain tissue. Usually, small biopsies heal well but a small scar is possible.
Key points to keep in mind
- Counsel patients on biopsy risks, benefits, and alternatives. Counsel regarding possible inconclusive results.
- Take time in choosing the biopsy site and consider multiple biopsies.
- Have all anticipated equipment available; consider using premade biopsy kits.
- Consider performing a stitch biopsy to avoid crush injury.
- Take photographs of the area to be biopsied and communicate with your pathologist to facilitate diagnosis.
Case 1
Biopsies were obtained of the areas highlighted in the photo. Pathology shows dVIN.
Image courtesy of Hope Haefner, MD.
Case 2
The examination is consistent with condylomata acuminata and biopsy is recommended with a 4-mm punch. Biopsy results are consistent with condylomata acuminata.
Image courtesy of Hope Haefner, MD.
Case 3
The final pathology shows high-grade squamous intraepithelial lesions (HSIL) of the vulva.
Image courtesy of Hope Haefner, MD.
Case 4
This presentation is an excellent opportunity for an excisional biopsy of the vulva. A marking pen is used to draw margins. A No. 15 blade is used to outline and then undermine the lesion, removing it in its entirety.
Final pathology shows a compound nevus of the vulva.
Image courtesy of Hope Haefner, MD.
Case 5
A 4-mm punch biopsy result is consistent with a diagnosis of lichen sclerosus.
Image courtesy of Hope Haefner, MD.
Case 6
A 4-mm punch biopsy result reveals that the pathology is significant for squamous cell carcinoma.
Image courtesy of Hope Haefner, MD.
Many benign, premalignant, and malignant lesions can occur on the vulva. These can be challenging to differentiate by examination alone. A vulvar biopsy often is needed to appropriately diagnose—and ultimately treat—these various conditions.
In this article, we review vulvar biopsy procedures, describe how to prepare tissue specimens for the pathologist, and provide some brief case examples in which biopsy established the diagnosis.
Ask questions first
Prior to examining a patient with a vulvar lesion, obtain a detailed history. Asking specific questions may aid in making the correct diagnosis, such as:
- How long has the lesion been present? Has it changed? What color is it?
- Was any trigger, or trauma, associated with onset of the lesion?
- Does the lesion itch, burn, or cause pain? Is there any associated bleeding or discharge?
- Are other lesions present in the vagina, anus, or mouth, or are other skin lesions present?
- Are any systemic symptoms present, such as fever, lymphadenopathy, weight loss, or joint pain?
- What is the patient’s previous treatment history, including over-the-counter medications and prescribed medications?
- Has there been any incontinence of urine or stool? Does the patient use a pad?
- Is the patient scratching? Is there any nighttime scratching? It also can be useful to ask her partner, if she has one, about nighttime scratching.
- Is there a family history of vulvar conditions?
- Has there been any change in her use of products like soap, lotions, cleansing wipes, sprays, lubricants, or laundry detergent?
- Has the patient had any new partners or significant travel history?
Preprocedure counseling points
Prior to proceeding with a vulvar biopsy, review with the patient the risks, benefits, and alternatives and obtain patient consent for the procedure. Vulvar biopsy risks include pain, bleeding, infection, injury to surrounding tissue, and the need for further surgery. Make patients aware that some biopsies are nondiagnostic. We recommend that clinicians perform a time-out verification to ensure that the patient’s identity and planned procedure are correct.
Assess the biopsy site
A wide variety of lesions may require a biopsy for diagnosis. While it can be challenging to know where to biopsy, taking the time to determine the proper biopsy site may enhance pathology results.
When considering colored lesions, depth is the important factor, and a punch biopsy often is sufficient. A tumor should be biopsied in the thickest area. Lesions that are concerning for malignancy may require multiple biopsies. An erosion or ulcer is best biopsied on the edge, including a small amount of surrounding tissue. For most patients, biopsy of normal-appearing tissue is of low diagnostic yield. Lastly, we try to avoid biopsies directly on the midline to facilitate better healing.1
A photograph of the vulva prior to biopsy may be helpful for the pathologist to see the tissue. Some electronic medical records have the capability to include photographs. Due to the sensitive nature of these photographs, we prefer that a separate written patient consent be obtained prior to taking photographs. We find also that photos are a useful reference for progression of disease at follow-up in a shared care team.
Continue to: Anesthesia procedure and instrument kit...
Anesthesia procedure and instrument kit
Some patients may benefit from the application of topical lidocaine 4% cream (L.M.X.4) prior to the injection of a local anesthetic for tissue biopsy. Ideally, topical lidocaine should be placed on the vulva and covered with a dressing such as Tegaderm or cellophane up to 30 minutes before the anticipated biopsy procedure. The anesthetic effect generally lasts for about 60 minutes. Many patients report stinging for several seconds upon application. Due to clinic time restrictions, we tend to reserve this method for a limited subset of patients. If planning a return visit for a biopsy, the patient can place the topical anesthetic herself.
For the anesthetic injection, we recommend lidocaine 1% or 2% with epinephrine in all areas of the vulva except for the glans clitoris. For a punch biopsy, we draw up 1 to 3 mL in a 3-mL syringe and inject with a 21- to 30-gauge needle, using a lower gauge for thicker tissue. We have not found buffering the anesthetic with sodium bicarbonate to be of particular use. For the glans clitoris, lidocaine without epinephrine should be utilized.
Equipment. Depending on your office setting, having a premade instrument kit may be preferred to peel-pack equipment. We prefer a premade tray that contains sterile gauze, a hemostat, iris scissors, a needle driver, a scalpel handle, and Adson forceps (FIGURE 1).

Types of biopsy procedures
Punch biopsy. We recommend a 4-mm Keyes biopsy punch. As mentioned, we use a biopsy kit to facilitate the procedure. After the tissue is properly anesthetized and prepped, we test the area via gentle touch to the skin with the hemostat or Adson forceps. To perform the punch biopsy, gentle, consistent pressure in a clockwise-counterclockwise fashion yields the best results. The goal is to obtain a 5-mm depth for hair-bearing skin and a 3-mm depth for all other tissue.2 The tissue should then be excised at the base with scissors, taking care not to crush the specimen with forceps.
Punch biopsy permits sampling of the epidermis, dermis, and subcutaneous tissue. Hemostasis is maintained with either silver nitrate, Monsel’s solution (ferric sulfate), or a dissolvable suture such as 4-0 Monocryl (poliglecaprone 25) or Vicryl Rapide (polyglactin 910).
Stitch biopsy. We find the stitch biopsy to be very useful given the architecture of the vulva. A modification of the shave biopsy, the stitch biopsy is depicted in FIGURE 2. A 3-0 or 4-0 dissolvable suture is placed through the intended area of biopsy. Iris scissors are used to undermine the tissue while the suture is held on tension. The goal is to remove the suture with the specimen. Separate sutures are used for hemostasis. The stitch does not cause the crushing artifacts on prepared specimens. Depending on the proceduralist’s comfort, a relatively large sample can be obtained in this fashion. If the suture held on tension is inadvertently cut, a second pass can be made with suture; alternatively, care can be used to remove remaining tissue with forceps and scissors, again avoiding crush injury to the tissue.

Excisional biopsy. Often, a larger area or margins are desired. We find that with adequate preparation, patients tolerate excisions in the office quite well. The planned area for excision can be marked with ink to ensure margins. Adequate anesthesia is instilled. A No. 15 blade scalpel is often the best size used to excise vulvar tissue in an elliptical fashion. Depending on depth of incision, the tissue may need to be approximated in layers for cosmesis and healing.
When planning an excisional biopsy, place a stitch on the excised tissue to mark orientation or pin out the entire specimen to a foam board to help your pathologist interpret tissue orientation.
The box "Vulvar biopsy established the diagnosis" at the end of this discussion provides 6 case examples of vulvar lesions and the respective diagnoses confirmed by biopsy.
Continue to: Preparing tissue for the pathologist...
Preparing tissue for the pathologist
Here are 5 tips for preparing the biopsied specimen for pathology:
- Include a question for the pathologist, such as “rule out lichen sclerosus or lichen simplex chronicus.” The majority of specimens should be sent in formalin. At times, frozen sections are done in the operating room.
- Double-check that the proper paperwork is included with every specimen and be very specific regarding the exact location of the lesion on the vulva. Include photographs whenever possible.
- Request that a dermatopathologist or a gynecologic pathologist with a special interest in vulvar dermatology, when feasible, review the tissue.
- Check your laboratory’s protocol for sending biopsies from areas around ulcerated tissue. Often, special medium is required for immunohistochemistry stains.
- Call your pathologist with questions about results; he or she often is happy to clarify, and together you may be able to arrive at a diagnosis to better serve your patient.3
Complications and how to avoid them
Bleeding. Any procedure has bleeding risks. To avoid bleeding, review the patient’s medication list and medical history prior to biopsy, as certain medications, such as blood thinners, increase risk for bleeding. Counseling a patient on applying direct pressure to the biopsy site for 2 minutes is generally sufficient for any bleeding that may occur once she is discharged from the clinic.
Infection. With aseptic technique, infection of a biopsy site is rare. We use nonsterile gloves for biopsy procedures. This does not increase the risk of infection.4 If a patient has iodine allergy, dilute chlorhexidine is a reasonable alternative for skin cleansing. Instruct the patient to keep the site clean and dry; if the biopsy proximity is close to the urethra or anus, use of a peri-bottle may be preferred after toileting. Instruct patients not to pull sutures. While instructions are specific for each patient, we generally advise that patients wait 4 to 7 days before resuming use of topical medications.
Scarring or tattooing. Avoid using dyed suture on skin surfaces and counsel the patient that silver nitrate can permanently stain tissue. Usually, small biopsies heal well but a small scar is possible.
Key points to keep in mind
- Counsel patients on biopsy risks, benefits, and alternatives. Counsel regarding possible inconclusive results.
- Take time in choosing the biopsy site and consider multiple biopsies.
- Have all anticipated equipment available; consider using premade biopsy kits.
- Consider performing a stitch biopsy to avoid crush injury.
- Take photographs of the area to be biopsied and communicate with your pathologist to facilitate diagnosis.
Case 1
Biopsies were obtained of the areas highlighted in the photo. Pathology shows dVIN.
Image courtesy of Hope Haefner, MD.
Case 2
The examination is consistent with condylomata acuminata and biopsy is recommended with a 4-mm punch. Biopsy results are consistent with condylomata acuminata.
Image courtesy of Hope Haefner, MD.
Case 3
The final pathology shows high-grade squamous intraepithelial lesions (HSIL) of the vulva.
Image courtesy of Hope Haefner, MD.
Case 4
This presentation is an excellent opportunity for an excisional biopsy of the vulva. A marking pen is used to draw margins. A No. 15 blade is used to outline and then undermine the lesion, removing it in its entirety.
Final pathology shows a compound nevus of the vulva.
Image courtesy of Hope Haefner, MD.
Case 5
A 4-mm punch biopsy result is consistent with a diagnosis of lichen sclerosus.
Image courtesy of Hope Haefner, MD.
Case 6
A 4-mm punch biopsy result reveals that the pathology is significant for squamous cell carcinoma.
Image courtesy of Hope Haefner, MD.
- Edwards L, Lynch PJ. Genital Dermatology Atlas and Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2018.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 93: Diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111:1243-1253.
- Heller DS. Areas of confusion in pathologist-clinician communication as it relates to understanding the vulvar pathology report. J Low Genit Tract Dis. 2017;21:327-328.
- Rietz A, Barzin A, Jones K, et al. Sterile or non-sterile gloves for minor skin excisions? J Fam Pract. 2015;64:723-727.
- Edwards L, Lynch PJ. Genital Dermatology Atlas and Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2018.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 93: Diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111:1243-1253.
- Heller DS. Areas of confusion in pathologist-clinician communication as it relates to understanding the vulvar pathology report. J Low Genit Tract Dis. 2017;21:327-328.
- Rietz A, Barzin A, Jones K, et al. Sterile or non-sterile gloves for minor skin excisions? J Fam Pract. 2015;64:723-727.