User login
ASCCP guidelines for managing abnormal cervical cancer tests: What’s new?
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
The 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors Consensus Guidelines, which represent a consensus of nearly 20 professional organizations and patient advocates, are a culmination of almost 10 years of research.1 With the last version issued in 2012,2 these latest guidelines offer the most recent recommendations regarding safely triaging women with abnormal cervical cancer screening results.
According to the consensus, research has shown that risk-based management allows clinicians to better discriminate women who will likely develop precancer from those who can safely continue with routine screening. As you will hear from guidelines coauthor Dr. Warner Huh, one of the most important differences between these guidelines and the 2012 version is a new emphasis on the principle of “equal management for equal risk.” Essentially, this insures that all women who have the same amount of risk for progression to precancer or cancer are managed the same.
The guidelines were once again published in the Journal of Lower Genital Tract Disease, and the tables they reference are publicly available. Additionally, ASCCP is developing a new management guidelines app to facilitate the use of the guidelines on smartphones and computers. With the publicly available risk tables, and the ASCCP navigation app, the guidelines will more easily accommodate updates as new information and technology become available.
OBG Management : The latest ASCCP guidelines, published in April, represent a “paradigm shift” from results to risk-based guidelines. Can you explain what this means and why the shift was undertaken?
Warner K. Huh, MD: Yes, the shift occurred because we needed to focus less on algorithms and more on risk. We started promulgating a concept of “equal management for equal risk” back in 2012. What this means is that if we have a method to look up a risk score based on relevant test results and other pieces of information, then all patients with that score should be managed in the same manner. We wanted that to be the underlying principle.
Focusing on risk tables also makes it easier to incorporate any future technologies used for risk estimation without having to rebuild algorithms from scratch. ASCCP is developing a new management guidelines app to streamline navigation of the guidelines. This app makes them easier for clinicians to use; they simply plug in certain variables from the patient’s history and receive 1 of 5 outputs: treatment, colposcopy, or surveillance at 1, 3, or 5 years.
The only drawbacks, if you view them as such, are that the clinician must plug in all the variables, and then must sit back and trust in what we have done. Clinicians have to trust that the system works and will simplify the clinical decision making.
We spent a lot of time determining what the risk thresholds should be. Some may argue they are arbitrary, but the decisions were data-driven, and carefully, thoughtfully vetted; we deliberated about whether the cut points actually made sense clinically to a practicing clinician base. The clinical action thresholds refer to a specific percentage below which a woman falls into one bucket and above which she falls into another bucket.
The other element that is unique about the guidelines is that instead of looking at the patient’s current screening result in isolation, the user sees it along with the prior one because prior history dictates subsequent risk.
It’s important that clinicians understand why this system is so markedly different from what we have done previously, and why risk-based guidelines make infinitely more sense than algorithmic ones. It’s because: 1) they can be easier to use; 2) they incorporate new data more efficiently and effectively than algorithm-based guidelines; and 3) they can incorporate future technologies seamlessly rather than having to create yet another algorithm.
Continue to: OBG Management : What do clinicians need in order to execute the guidelines?...
OBG Management : What do clinicians need in order to execute the guidelines?
Dr. Huh: Nothing. All of the information needed—the guidelines article and risk tables—are publicly available. However, to make navigation of the guidelines easier, the plan is for the app that I mentioned. I have the app on my phone and am actively beta testing it now. We’re planning on creating a web-based application as well, that will allow users to access the Internet and their electronic health record system so that they can plug in information directly from patient charts. The web-based app will be similar to the web-based Breast Cancer Surveillance Consortium’s Risk Calculator (https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm). You will pull it up, plug in the requested information, including the patient’s age; their Pap smear and genotyping results; and their previous screening history.
OBG Management : When will the app be available for users?
Dr. Huh: It will be available for release on June 8.
OBG Management : Were HPV vaccination levels incorporated into the new guidelines?
OBG Management : Have recommendations regarding colposcopy changed?
Dr. Huh: Not really. About 3 years ago, we created basic colposcopy guidelines—the ASCCP Colposcopy Standards—so everything about colposcopy references back to those guidelines. Those colposcopy standards covered terminology and risk-based colposcopy, which actually aligns beautifully with these guidelines.
OBG Management : To narrow in on some changes from the prior guidelines, can colposcopy be deferred in certain patients?
Dr. Huh: Yes. Not everyone who has an abnormal screening test needs to come back for colposcopy.
OBG Management : How has guidance for expedited treatment or treatment without colposcopic biopsy changed?
Dr. Huh: This was heavily debated within not only the treatment group that I co-chaired with Richard Guido, MD, but also within the entire steering committee. The recommendation is that if the patient has an immediate risk of CIN 3 that is >60%, the patient should go straight to treatment without a colposcopic biopsy. The main reason for this is that you do not want to biopsy a patient and then lose them to follow-up.
When a woman has >60% immediate risk of CIN 3, we are fairly certain that colposcopy is not going to change management ultimately, so we recommend that patients receive treatment right away. We have already been doing this for 15 to 20 years, so this is not a new concept. It is just more formally codified here by assigning a percentage to the risk. Those who have between 25% and 60% immediate risk of CIN 3 should receive immediate colposcopy. We realize that not all clinicians have the ability to do this, so if clinicians can’t treat immediately, we recommend they do whatever they can to prevent losing the patient to follow-up.
OBG Management : How should a positive primary HPV screening test be managed?
Dr. Huh: If a woman has a positive primary HPV screening test, genotyping should be performed. If genotyping reveals HPV 16 or 18, then the patient should proceed to colposcopy. If genotyping reveals other forms of HPV, reflex cytology or a Pap smear should follow. ●
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
- Perkins RB, Guido RS, Castle PE, et al, for the 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2020;24(2):102-131.
- Massad LS, Einstein MH, Huh WK, et al, for the 2012 ASCCP Consensus Guidelines Conference. 2012 Updated Consensus Guidelines for the Management of Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J Low Genit Tract Dis. 2013;17(5):S1-S27.
In your practice, are you planning to have a chaperone present for all intimate examinations?
Although pelvic examinations may only last a few minutes, the examination is scary and uncomfortable for many patients. To help minimize fear and discomfort, the exam should take place in a comfortable and professional environment. The clinician should provide appropriate gowns, private facilities for undressing, sensitively use draping, and clearly explain the components of the examination. Trained professional chaperones play an important role in intimate physical examinations, including:
- providing reassurance to the patient of the professional integrity of the intimate examination
- supporting and educating the patient during the examination
- increasing the efficiency of the clinician during a procedure
- acting as a witness should a misunderstanding with the patient arise.
Major medical professional societies have issued guidance to clinicians on the use of a chaperone during intimate physical examinations. Professional society guidance ranges from endorsing joint decision-making between physician and patient on the presence of a chaperone to more proscriptive guidance that emphasizes the importance of a chaperone at every intimate physical examination.
Examples of professional societies’ guidance that supports joint decision-making between physician and patient about the presence of a chaperone include:
- American Medical Association: “Adopt a policy that patients are free to request a chaperone and ensure that the policy is communicated to patients. Always honor a patient’s request to have a chaperone.”1
- Society of Obstetricians and Gynaecologists of Canada: “It is a reasonable and acceptable practice to perform a physical examination, including breast and pelvic examination without the presence of a third person in the room unless the woman or health care provider indicates a desire for a third party to be present.” “If the health care provider chooses to have a third person present during all examinations, the health care provider should explain this policy to the woman.”2
- American College of Physicians: “Care and respect should guide the performance of the physical examination. The location and degree of privacy should be appropriate for the examination being performed, with chaperone services as an option. An appropriate setting and sufficient time should be allocated to encourage exploration of aspects of the patient’s life pertinent to health, including habits, relationships, sexuality, vocation, culture, religion, and spirituality.”3
By contrast, the following professional society guidance strongly recommends the presence of a chaperone for every intimate physical examination:
- United States Veterans Administration: “A female chaperone must be in the examination room during breast and pelvic exams…this includes procedures such as urodynamic testing or treatments such as pelvic floor physical therapy.”4
- Royal College of Obstetricians and Gynaecologists: “The presence of a chaperone is considered essential for every pelvic examination. Verbal consent should be obtained in the presence of the chaperone who is to be present during the examination and recorded in the notes. If the patient declines the presence of a chaperone, the doctor should explain that a chaperone is also required to help in many cases and then attempt to arrange for the chaperone to be standing nearby within earshot. The reasons for declining a chaperone and alternative arrangements offered should be documented. Consent should also be specific to whether the intended examination is vaginal, rectal or both. Communication skills are essential in conducting intimate examinations.”5
- American College Health Association (ACHA): “It is ACHA’s recommendation that, as part of institutional policy, a chaperone be provided for every sensitive medical examination and procedure.”6
Continue to: New guidance from ACOG on trained chaperones...
New guidance from ACOG on trained chaperones
The American College of Obstetricians and Gynecologists (ACOG) recently issued a committee opinion recommending “that a chaperone be present for all breast, genital, and rectal examinations. The need for a chaperone is irrespective of the sex or gender of the person performing the examination and applies to examinations performed in the outpatient and inpatient settings, including labor and delivery, as well as during diagnostic studies such as transvaginal ultrasonography and urodynamic testing.”7
This new proscriptive guidance will significantly change practice for the many obstetrician-gynecologists who do not routinely have a chaperone present during intimate examinations. The policy provides exceptions to the presence of a chaperone in cases of medical emergencies and if the patient declines a chaperone. ACOG recommends that when a patient declines a chaperone the clinician should educate the patient that a “chaperone is an integral part of the clinical team whose role includes assisting with the examination and protecting the patient and the physician. Any concerns the patient has regarding the presence of a chaperone should be elicited and addressed if feasible. If, after counseling, the patient refuses the chaperone, this decision should be respected and documented in the medical record.”7 ACOG discourages the use of family members, medical students, and residents as chaperones.
Sexual trauma is common and may cause lasting adverse effects, including poor health.1 When sexual trauma is reported, the experience may not be believed or taken seriously, compounding the injury. Sometimes sexual trauma contributes to risky behaviors including smoking cigarettes, excessive alcohol consumption, drug misuse, and risky sex as a means to cope with the mental distress of the trauma.
Trauma-informed medical care has four pillars:
1. Recognize that many people have experienced significant trauma(s), which adversely impacts their health.
2. Be aware of the signs and symptoms of trauma.
3. Integrate knowledge about trauma into medical encounters.
4. Avoid re-traumatizing the person.
Symptoms of psychological distress caused by past trauma include anxiety, fear, anger, irritability, mood swings, feeling disconnected, numbness, sadness, or hopelessness. Clinical actions that help to reduce distress among trauma survivors include:
• sensitively ask patients to share their traumatic experiences
• empower the patient by explicitly giving her control over all aspects of the examination, indicating that the exam will stop if the patient feels uncomfortable
• explain the steps in the exam and educate about the purpose of each step
• keep the patient’s body covered as much as possible
• use the smallest speculum that permits an adequate exam
• utilize a chaperone to help support the patient.
Clinicians can strengthen their empathic skills by reflecting on how their own personal experiences, traumas, cultural-biases, and gender influence their ap-proach to the care of patients.
Reference
1. Hall KS, Moreau C, Trussell J. Young women’s perceived health and lifetime sexual experience: results from the national survey of family growth. J Sex Med. 2012;9:1382-1391. doi: 10.1111/j.1743-6109.2012.02686.x.
Training of chaperones
Chaperones are health care professionals who should be trained for their specific role. Chaperones need to protect patient privacy and the confidentiality of health information. Chaperones should be trained to recognize the components of a professional intimate examination and to identify variances from standard practice. In many ambulatory practices, medical assistants perform the role of chaperone. The American Association of Medical Assistants (AAMA) offers national certification for medical assistants through an examination developed by the National Board of Medical Examiners. To be eligible for AAMA certification an individual must complete at least two semesters of medical assisting education that includes courses in anatomy, physiology, pharmacology, and relevant mathematics.
Reporting variances that occur during an intimate examination
Best practices are evolving on how to deal with the rare event of a chaperone witnessing a physician perform an intimate examination that is outside of standard professional practice. Chaperones may be reluctant to report a variance because physicians are in a powerful position, and the accuracy of their report will be challenged, threatening the chaperone’s employment. Processes for encouraging all team members to report concerns must be clearly explained to the chaperone and other members of the health care team. Clinicians should be aware that deviations from standard practice will be reported and investigated. Medical practices must develop a reporting system that ensures the reporting individual will be protected from retaliation.
In addition, the chaperone needs to know to whom they should report a variance. In large multispecialty medical practices, chaperones often can report concerns to nursing leaders or human resources. In small ambulatory practices, chaperones may be advised to report concerns about a physician to the practice manager or medical director. Regardless, every practice should have the best process for reporting a concern. In turn, the practice leaders who are responsible for investigating reports of concerning behavior should have a defined process for confidentially interviewing the chaperone, clinician, and patient.
Even when a chaperone is present for intimate examinations, problems can arise if the chaperone is not trained to recognize variances from standard practice or does not have a clear means for reporting variances and when the practice does not have a process for investigating reported variances.
Sadly, misconduct has been documented among priests, ministers, sports coaches, professors, scout masters, and clinicians. Trusted professionals are in positions of power in relation to their clients, patients, and students. Physicians and nurses are held in high esteem and trust by patients. To preserve the trust of the public we must treat all people with dignity and respect their autonomy. The presence of a chaperone during intimate examinations may help us fulfill Hippocrates’ edict, “First, do no harm.” ●
Ronee A. Skornik, MSW, MD
As a female obstetrician-gynecologist trained in psychiatric social work, I have found that some of my patients who have known me over a long period of time find the presence of a chaperone not only unnecessary but also uncomfortable both in terms of physical exposure and in what they may want to tell me during the examination. Personally, I strongly favor a chaperone for all intimate examinations, to safeguard both the patient and the clinician. However, I do understand why some patients prefer to see me without the presence of a chaperone, and I want to honor their wishes. If a chaperone is responsive to the patient’s requests, including where the chaperone stands and his or her role during the exam, the reluctant patient may be more willing to have a chaperone. A chaperone who develops a relationship with the patient and honors the patient’s preferences is a valuable member of the care team.
- American Medical Association. Code of Medical Ethics Opinion 1.2.4. https://www.ama-assn.org/delivering-care/ethics/use-chaperones. Accessed May 26, 2020.
- Society of Obstetricians and Gynaecologists of Canada. No. 266—The presence of a third party during breast and pelvic examinations. J Obstet Gynaecol Can. 2017;39:e496-e497. doi: 10.1016/j.jogc.2017.09.005.
- American College of Physicians. ACP Policy Com-pendium Summer 2016. https://www.acponline.org/system/files/documents/advocacy/acp_policy_compendium_summer_2016.pdf. Accessed May 26, 2020.
- Department of Veterans Affairs. VHA Directive 1330.01(2). Healthcare Services for Women Veterans. February 15, 2017. Amended July 24, 2018. http://www.va.gov/ vhapublications/ viewpublication.asp?pub_id=5332. Accessed May 26, 2020.
- Royal College of Obstetricians and Gynaecologists. Obtaining valid consent: clinical governance advice no. 6. January 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/clinical-governance-advice/cga6.pdf. Accessed May 26, 2020.
- American College Health Association Guidelines. Best practices for sensitive exams. October 2019. https://www.acha.org/documents/resources/guidelines/ACHA_Best_Practices_for_Sensitive_Exams_October2019.pdf. Accessed May 26, 2020.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50
Although pelvic examinations may only last a few minutes, the examination is scary and uncomfortable for many patients. To help minimize fear and discomfort, the exam should take place in a comfortable and professional environment. The clinician should provide appropriate gowns, private facilities for undressing, sensitively use draping, and clearly explain the components of the examination. Trained professional chaperones play an important role in intimate physical examinations, including:
- providing reassurance to the patient of the professional integrity of the intimate examination
- supporting and educating the patient during the examination
- increasing the efficiency of the clinician during a procedure
- acting as a witness should a misunderstanding with the patient arise.
Major medical professional societies have issued guidance to clinicians on the use of a chaperone during intimate physical examinations. Professional society guidance ranges from endorsing joint decision-making between physician and patient on the presence of a chaperone to more proscriptive guidance that emphasizes the importance of a chaperone at every intimate physical examination.
Examples of professional societies’ guidance that supports joint decision-making between physician and patient about the presence of a chaperone include:
- American Medical Association: “Adopt a policy that patients are free to request a chaperone and ensure that the policy is communicated to patients. Always honor a patient’s request to have a chaperone.”1
- Society of Obstetricians and Gynaecologists of Canada: “It is a reasonable and acceptable practice to perform a physical examination, including breast and pelvic examination without the presence of a third person in the room unless the woman or health care provider indicates a desire for a third party to be present.” “If the health care provider chooses to have a third person present during all examinations, the health care provider should explain this policy to the woman.”2
- American College of Physicians: “Care and respect should guide the performance of the physical examination. The location and degree of privacy should be appropriate for the examination being performed, with chaperone services as an option. An appropriate setting and sufficient time should be allocated to encourage exploration of aspects of the patient’s life pertinent to health, including habits, relationships, sexuality, vocation, culture, religion, and spirituality.”3
By contrast, the following professional society guidance strongly recommends the presence of a chaperone for every intimate physical examination:
- United States Veterans Administration: “A female chaperone must be in the examination room during breast and pelvic exams…this includes procedures such as urodynamic testing or treatments such as pelvic floor physical therapy.”4
- Royal College of Obstetricians and Gynaecologists: “The presence of a chaperone is considered essential for every pelvic examination. Verbal consent should be obtained in the presence of the chaperone who is to be present during the examination and recorded in the notes. If the patient declines the presence of a chaperone, the doctor should explain that a chaperone is also required to help in many cases and then attempt to arrange for the chaperone to be standing nearby within earshot. The reasons for declining a chaperone and alternative arrangements offered should be documented. Consent should also be specific to whether the intended examination is vaginal, rectal or both. Communication skills are essential in conducting intimate examinations.”5
- American College Health Association (ACHA): “It is ACHA’s recommendation that, as part of institutional policy, a chaperone be provided for every sensitive medical examination and procedure.”6
Continue to: New guidance from ACOG on trained chaperones...
New guidance from ACOG on trained chaperones
The American College of Obstetricians and Gynecologists (ACOG) recently issued a committee opinion recommending “that a chaperone be present for all breast, genital, and rectal examinations. The need for a chaperone is irrespective of the sex or gender of the person performing the examination and applies to examinations performed in the outpatient and inpatient settings, including labor and delivery, as well as during diagnostic studies such as transvaginal ultrasonography and urodynamic testing.”7
This new proscriptive guidance will significantly change practice for the many obstetrician-gynecologists who do not routinely have a chaperone present during intimate examinations. The policy provides exceptions to the presence of a chaperone in cases of medical emergencies and if the patient declines a chaperone. ACOG recommends that when a patient declines a chaperone the clinician should educate the patient that a “chaperone is an integral part of the clinical team whose role includes assisting with the examination and protecting the patient and the physician. Any concerns the patient has regarding the presence of a chaperone should be elicited and addressed if feasible. If, after counseling, the patient refuses the chaperone, this decision should be respected and documented in the medical record.”7 ACOG discourages the use of family members, medical students, and residents as chaperones.
Sexual trauma is common and may cause lasting adverse effects, including poor health.1 When sexual trauma is reported, the experience may not be believed or taken seriously, compounding the injury. Sometimes sexual trauma contributes to risky behaviors including smoking cigarettes, excessive alcohol consumption, drug misuse, and risky sex as a means to cope with the mental distress of the trauma.
Trauma-informed medical care has four pillars:
1. Recognize that many people have experienced significant trauma(s), which adversely impacts their health.
2. Be aware of the signs and symptoms of trauma.
3. Integrate knowledge about trauma into medical encounters.
4. Avoid re-traumatizing the person.
Symptoms of psychological distress caused by past trauma include anxiety, fear, anger, irritability, mood swings, feeling disconnected, numbness, sadness, or hopelessness. Clinical actions that help to reduce distress among trauma survivors include:
• sensitively ask patients to share their traumatic experiences
• empower the patient by explicitly giving her control over all aspects of the examination, indicating that the exam will stop if the patient feels uncomfortable
• explain the steps in the exam and educate about the purpose of each step
• keep the patient’s body covered as much as possible
• use the smallest speculum that permits an adequate exam
• utilize a chaperone to help support the patient.
Clinicians can strengthen their empathic skills by reflecting on how their own personal experiences, traumas, cultural-biases, and gender influence their ap-proach to the care of patients.
Reference
1. Hall KS, Moreau C, Trussell J. Young women’s perceived health and lifetime sexual experience: results from the national survey of family growth. J Sex Med. 2012;9:1382-1391. doi: 10.1111/j.1743-6109.2012.02686.x.
Training of chaperones
Chaperones are health care professionals who should be trained for their specific role. Chaperones need to protect patient privacy and the confidentiality of health information. Chaperones should be trained to recognize the components of a professional intimate examination and to identify variances from standard practice. In many ambulatory practices, medical assistants perform the role of chaperone. The American Association of Medical Assistants (AAMA) offers national certification for medical assistants through an examination developed by the National Board of Medical Examiners. To be eligible for AAMA certification an individual must complete at least two semesters of medical assisting education that includes courses in anatomy, physiology, pharmacology, and relevant mathematics.
Reporting variances that occur during an intimate examination
Best practices are evolving on how to deal with the rare event of a chaperone witnessing a physician perform an intimate examination that is outside of standard professional practice. Chaperones may be reluctant to report a variance because physicians are in a powerful position, and the accuracy of their report will be challenged, threatening the chaperone’s employment. Processes for encouraging all team members to report concerns must be clearly explained to the chaperone and other members of the health care team. Clinicians should be aware that deviations from standard practice will be reported and investigated. Medical practices must develop a reporting system that ensures the reporting individual will be protected from retaliation.
In addition, the chaperone needs to know to whom they should report a variance. In large multispecialty medical practices, chaperones often can report concerns to nursing leaders or human resources. In small ambulatory practices, chaperones may be advised to report concerns about a physician to the practice manager or medical director. Regardless, every practice should have the best process for reporting a concern. In turn, the practice leaders who are responsible for investigating reports of concerning behavior should have a defined process for confidentially interviewing the chaperone, clinician, and patient.
Even when a chaperone is present for intimate examinations, problems can arise if the chaperone is not trained to recognize variances from standard practice or does not have a clear means for reporting variances and when the practice does not have a process for investigating reported variances.
Sadly, misconduct has been documented among priests, ministers, sports coaches, professors, scout masters, and clinicians. Trusted professionals are in positions of power in relation to their clients, patients, and students. Physicians and nurses are held in high esteem and trust by patients. To preserve the trust of the public we must treat all people with dignity and respect their autonomy. The presence of a chaperone during intimate examinations may help us fulfill Hippocrates’ edict, “First, do no harm.” ●
Ronee A. Skornik, MSW, MD
As a female obstetrician-gynecologist trained in psychiatric social work, I have found that some of my patients who have known me over a long period of time find the presence of a chaperone not only unnecessary but also uncomfortable both in terms of physical exposure and in what they may want to tell me during the examination. Personally, I strongly favor a chaperone for all intimate examinations, to safeguard both the patient and the clinician. However, I do understand why some patients prefer to see me without the presence of a chaperone, and I want to honor their wishes. If a chaperone is responsive to the patient’s requests, including where the chaperone stands and his or her role during the exam, the reluctant patient may be more willing to have a chaperone. A chaperone who develops a relationship with the patient and honors the patient’s preferences is a valuable member of the care team.
Although pelvic examinations may only last a few minutes, the examination is scary and uncomfortable for many patients. To help minimize fear and discomfort, the exam should take place in a comfortable and professional environment. The clinician should provide appropriate gowns, private facilities for undressing, sensitively use draping, and clearly explain the components of the examination. Trained professional chaperones play an important role in intimate physical examinations, including:
- providing reassurance to the patient of the professional integrity of the intimate examination
- supporting and educating the patient during the examination
- increasing the efficiency of the clinician during a procedure
- acting as a witness should a misunderstanding with the patient arise.
Major medical professional societies have issued guidance to clinicians on the use of a chaperone during intimate physical examinations. Professional society guidance ranges from endorsing joint decision-making between physician and patient on the presence of a chaperone to more proscriptive guidance that emphasizes the importance of a chaperone at every intimate physical examination.
Examples of professional societies’ guidance that supports joint decision-making between physician and patient about the presence of a chaperone include:
- American Medical Association: “Adopt a policy that patients are free to request a chaperone and ensure that the policy is communicated to patients. Always honor a patient’s request to have a chaperone.”1
- Society of Obstetricians and Gynaecologists of Canada: “It is a reasonable and acceptable practice to perform a physical examination, including breast and pelvic examination without the presence of a third person in the room unless the woman or health care provider indicates a desire for a third party to be present.” “If the health care provider chooses to have a third person present during all examinations, the health care provider should explain this policy to the woman.”2
- American College of Physicians: “Care and respect should guide the performance of the physical examination. The location and degree of privacy should be appropriate for the examination being performed, with chaperone services as an option. An appropriate setting and sufficient time should be allocated to encourage exploration of aspects of the patient’s life pertinent to health, including habits, relationships, sexuality, vocation, culture, religion, and spirituality.”3
By contrast, the following professional society guidance strongly recommends the presence of a chaperone for every intimate physical examination:
- United States Veterans Administration: “A female chaperone must be in the examination room during breast and pelvic exams…this includes procedures such as urodynamic testing or treatments such as pelvic floor physical therapy.”4
- Royal College of Obstetricians and Gynaecologists: “The presence of a chaperone is considered essential for every pelvic examination. Verbal consent should be obtained in the presence of the chaperone who is to be present during the examination and recorded in the notes. If the patient declines the presence of a chaperone, the doctor should explain that a chaperone is also required to help in many cases and then attempt to arrange for the chaperone to be standing nearby within earshot. The reasons for declining a chaperone and alternative arrangements offered should be documented. Consent should also be specific to whether the intended examination is vaginal, rectal or both. Communication skills are essential in conducting intimate examinations.”5
- American College Health Association (ACHA): “It is ACHA’s recommendation that, as part of institutional policy, a chaperone be provided for every sensitive medical examination and procedure.”6
Continue to: New guidance from ACOG on trained chaperones...
New guidance from ACOG on trained chaperones
The American College of Obstetricians and Gynecologists (ACOG) recently issued a committee opinion recommending “that a chaperone be present for all breast, genital, and rectal examinations. The need for a chaperone is irrespective of the sex or gender of the person performing the examination and applies to examinations performed in the outpatient and inpatient settings, including labor and delivery, as well as during diagnostic studies such as transvaginal ultrasonography and urodynamic testing.”7
This new proscriptive guidance will significantly change practice for the many obstetrician-gynecologists who do not routinely have a chaperone present during intimate examinations. The policy provides exceptions to the presence of a chaperone in cases of medical emergencies and if the patient declines a chaperone. ACOG recommends that when a patient declines a chaperone the clinician should educate the patient that a “chaperone is an integral part of the clinical team whose role includes assisting with the examination and protecting the patient and the physician. Any concerns the patient has regarding the presence of a chaperone should be elicited and addressed if feasible. If, after counseling, the patient refuses the chaperone, this decision should be respected and documented in the medical record.”7 ACOG discourages the use of family members, medical students, and residents as chaperones.
Sexual trauma is common and may cause lasting adverse effects, including poor health.1 When sexual trauma is reported, the experience may not be believed or taken seriously, compounding the injury. Sometimes sexual trauma contributes to risky behaviors including smoking cigarettes, excessive alcohol consumption, drug misuse, and risky sex as a means to cope with the mental distress of the trauma.
Trauma-informed medical care has four pillars:
1. Recognize that many people have experienced significant trauma(s), which adversely impacts their health.
2. Be aware of the signs and symptoms of trauma.
3. Integrate knowledge about trauma into medical encounters.
4. Avoid re-traumatizing the person.
Symptoms of psychological distress caused by past trauma include anxiety, fear, anger, irritability, mood swings, feeling disconnected, numbness, sadness, or hopelessness. Clinical actions that help to reduce distress among trauma survivors include:
• sensitively ask patients to share their traumatic experiences
• empower the patient by explicitly giving her control over all aspects of the examination, indicating that the exam will stop if the patient feels uncomfortable
• explain the steps in the exam and educate about the purpose of each step
• keep the patient’s body covered as much as possible
• use the smallest speculum that permits an adequate exam
• utilize a chaperone to help support the patient.
Clinicians can strengthen their empathic skills by reflecting on how their own personal experiences, traumas, cultural-biases, and gender influence their ap-proach to the care of patients.
Reference
1. Hall KS, Moreau C, Trussell J. Young women’s perceived health and lifetime sexual experience: results from the national survey of family growth. J Sex Med. 2012;9:1382-1391. doi: 10.1111/j.1743-6109.2012.02686.x.
Training of chaperones
Chaperones are health care professionals who should be trained for their specific role. Chaperones need to protect patient privacy and the confidentiality of health information. Chaperones should be trained to recognize the components of a professional intimate examination and to identify variances from standard practice. In many ambulatory practices, medical assistants perform the role of chaperone. The American Association of Medical Assistants (AAMA) offers national certification for medical assistants through an examination developed by the National Board of Medical Examiners. To be eligible for AAMA certification an individual must complete at least two semesters of medical assisting education that includes courses in anatomy, physiology, pharmacology, and relevant mathematics.
Reporting variances that occur during an intimate examination
Best practices are evolving on how to deal with the rare event of a chaperone witnessing a physician perform an intimate examination that is outside of standard professional practice. Chaperones may be reluctant to report a variance because physicians are in a powerful position, and the accuracy of their report will be challenged, threatening the chaperone’s employment. Processes for encouraging all team members to report concerns must be clearly explained to the chaperone and other members of the health care team. Clinicians should be aware that deviations from standard practice will be reported and investigated. Medical practices must develop a reporting system that ensures the reporting individual will be protected from retaliation.
In addition, the chaperone needs to know to whom they should report a variance. In large multispecialty medical practices, chaperones often can report concerns to nursing leaders or human resources. In small ambulatory practices, chaperones may be advised to report concerns about a physician to the practice manager or medical director. Regardless, every practice should have the best process for reporting a concern. In turn, the practice leaders who are responsible for investigating reports of concerning behavior should have a defined process for confidentially interviewing the chaperone, clinician, and patient.
Even when a chaperone is present for intimate examinations, problems can arise if the chaperone is not trained to recognize variances from standard practice or does not have a clear means for reporting variances and when the practice does not have a process for investigating reported variances.
Sadly, misconduct has been documented among priests, ministers, sports coaches, professors, scout masters, and clinicians. Trusted professionals are in positions of power in relation to their clients, patients, and students. Physicians and nurses are held in high esteem and trust by patients. To preserve the trust of the public we must treat all people with dignity and respect their autonomy. The presence of a chaperone during intimate examinations may help us fulfill Hippocrates’ edict, “First, do no harm.” ●
Ronee A. Skornik, MSW, MD
As a female obstetrician-gynecologist trained in psychiatric social work, I have found that some of my patients who have known me over a long period of time find the presence of a chaperone not only unnecessary but also uncomfortable both in terms of physical exposure and in what they may want to tell me during the examination. Personally, I strongly favor a chaperone for all intimate examinations, to safeguard both the patient and the clinician. However, I do understand why some patients prefer to see me without the presence of a chaperone, and I want to honor their wishes. If a chaperone is responsive to the patient’s requests, including where the chaperone stands and his or her role during the exam, the reluctant patient may be more willing to have a chaperone. A chaperone who develops a relationship with the patient and honors the patient’s preferences is a valuable member of the care team.
- American Medical Association. Code of Medical Ethics Opinion 1.2.4. https://www.ama-assn.org/delivering-care/ethics/use-chaperones. Accessed May 26, 2020.
- Society of Obstetricians and Gynaecologists of Canada. No. 266—The presence of a third party during breast and pelvic examinations. J Obstet Gynaecol Can. 2017;39:e496-e497. doi: 10.1016/j.jogc.2017.09.005.
- American College of Physicians. ACP Policy Com-pendium Summer 2016. https://www.acponline.org/system/files/documents/advocacy/acp_policy_compendium_summer_2016.pdf. Accessed May 26, 2020.
- Department of Veterans Affairs. VHA Directive 1330.01(2). Healthcare Services for Women Veterans. February 15, 2017. Amended July 24, 2018. http://www.va.gov/ vhapublications/ viewpublication.asp?pub_id=5332. Accessed May 26, 2020.
- Royal College of Obstetricians and Gynaecologists. Obtaining valid consent: clinical governance advice no. 6. January 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/clinical-governance-advice/cga6.pdf. Accessed May 26, 2020.
- American College Health Association Guidelines. Best practices for sensitive exams. October 2019. https://www.acha.org/documents/resources/guidelines/ACHA_Best_Practices_for_Sensitive_Exams_October2019.pdf. Accessed May 26, 2020.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50
- American Medical Association. Code of Medical Ethics Opinion 1.2.4. https://www.ama-assn.org/delivering-care/ethics/use-chaperones. Accessed May 26, 2020.
- Society of Obstetricians and Gynaecologists of Canada. No. 266—The presence of a third party during breast and pelvic examinations. J Obstet Gynaecol Can. 2017;39:e496-e497. doi: 10.1016/j.jogc.2017.09.005.
- American College of Physicians. ACP Policy Com-pendium Summer 2016. https://www.acponline.org/system/files/documents/advocacy/acp_policy_compendium_summer_2016.pdf. Accessed May 26, 2020.
- Department of Veterans Affairs. VHA Directive 1330.01(2). Healthcare Services for Women Veterans. February 15, 2017. Amended July 24, 2018. http://www.va.gov/ vhapublications/ viewpublication.asp?pub_id=5332. Accessed May 26, 2020.
- Royal College of Obstetricians and Gynaecologists. Obtaining valid consent: clinical governance advice no. 6. January 2015. https://www.rcog.org.uk/globalassets/documents/guidelines/clinical-governance-advice/cga6.pdf. Accessed May 26, 2020.
- American College Health Association Guidelines. Best practices for sensitive exams. October 2019. https://www.acha.org/documents/resources/guidelines/ACHA_Best_Practices_for_Sensitive_Exams_October2019.pdf. Accessed May 26, 2020.
- American College of Obstetricians and Gynecologists Committee on Ethics. Sexual misconduct: ACOG Committee Opinion No. 796. Obstet Gynecol. 2020;135:e43-e50
FDA approves medication to treat heavy menstrual bleeding related to fibroids
The medication, marketed as Oriahnn, is an estrogen and progestin combination product that consists of elagolix, estradiol, and norethindrone acetate capsules packaged together for oral use, according to an FDA announcement.
“Uterine fibroids are the most common benign tumors affecting premenopausal women, and one of the most common symptoms from fibroids is heavy menstrual bleeding,” Christine P. Nguyen, MD, acting director of the division of urology, obstetrics, and gynecology in the FDA’s Center for Drug Evaluation and Research, said in a news release. “Although surgical treatments, such as hysterectomy, are available, patients may not qualify for surgery or want the procedure. Various nonsurgical therapies are used to treat fibroid-related heavy menstrual bleeding, but none have been FDA approved specifically for this use. Today’s approval provides an FDA-approved medical treatment option for these patients.”
Fibroids, which occur most commonly in women aged 35-49 years, typically resolve after menopause but are a leading reason for hysterectomy in the United States, according to the release.
Researchers established the efficacy of the treatment in two clinical trials that included 591 premenopausal women with heavy menstrual bleeding. Participants received the drug or placebo for 6 months. The investigators defined heavy menstrual bleeding as at least two menstrual cycles with greater than 80 mL of menstrual blood loss. The primary endpoint was the proportion of women who achieved menstrual blood loss less than 80 mL at the final month and 50% or greater reduction in menstrual blood loss volume from baseline to the final month. In one trial, 69% of patients who received Oriahnn met this endpoint, compared with 9% of patients who received placebo. In the second study, 77% of patients who received the drug achieved this endpoint, compared with 11% of patients who received placebo.
Oriahnn may cause bone loss that may not be completely recovered after stopping treatment, so women should not take the medication for more than 24 months, according to the FDA announcement. Health care professionals may recommend bone density scans before and during treatment.
The most common side effects included hot flushes, headache, fatigue, and irregular vaginal bleeding. The drug’s label includes a boxed warning about a risk of strokes and blood clots, especially in women at increased risk for these events. Contraindications include osteoporosis, a history of breast cancer or other hormonally sensitive cancer, liver disease, and abnormal uterine bleeding. Oriahnn does not prevent pregnancy and may increase blood pressure, according to the press release. AbbVie markets the drug.
The medication, marketed as Oriahnn, is an estrogen and progestin combination product that consists of elagolix, estradiol, and norethindrone acetate capsules packaged together for oral use, according to an FDA announcement.
“Uterine fibroids are the most common benign tumors affecting premenopausal women, and one of the most common symptoms from fibroids is heavy menstrual bleeding,” Christine P. Nguyen, MD, acting director of the division of urology, obstetrics, and gynecology in the FDA’s Center for Drug Evaluation and Research, said in a news release. “Although surgical treatments, such as hysterectomy, are available, patients may not qualify for surgery or want the procedure. Various nonsurgical therapies are used to treat fibroid-related heavy menstrual bleeding, but none have been FDA approved specifically for this use. Today’s approval provides an FDA-approved medical treatment option for these patients.”
Fibroids, which occur most commonly in women aged 35-49 years, typically resolve after menopause but are a leading reason for hysterectomy in the United States, according to the release.
Researchers established the efficacy of the treatment in two clinical trials that included 591 premenopausal women with heavy menstrual bleeding. Participants received the drug or placebo for 6 months. The investigators defined heavy menstrual bleeding as at least two menstrual cycles with greater than 80 mL of menstrual blood loss. The primary endpoint was the proportion of women who achieved menstrual blood loss less than 80 mL at the final month and 50% or greater reduction in menstrual blood loss volume from baseline to the final month. In one trial, 69% of patients who received Oriahnn met this endpoint, compared with 9% of patients who received placebo. In the second study, 77% of patients who received the drug achieved this endpoint, compared with 11% of patients who received placebo.
Oriahnn may cause bone loss that may not be completely recovered after stopping treatment, so women should not take the medication for more than 24 months, according to the FDA announcement. Health care professionals may recommend bone density scans before and during treatment.
The most common side effects included hot flushes, headache, fatigue, and irregular vaginal bleeding. The drug’s label includes a boxed warning about a risk of strokes and blood clots, especially in women at increased risk for these events. Contraindications include osteoporosis, a history of breast cancer or other hormonally sensitive cancer, liver disease, and abnormal uterine bleeding. Oriahnn does not prevent pregnancy and may increase blood pressure, according to the press release. AbbVie markets the drug.
The medication, marketed as Oriahnn, is an estrogen and progestin combination product that consists of elagolix, estradiol, and norethindrone acetate capsules packaged together for oral use, according to an FDA announcement.
“Uterine fibroids are the most common benign tumors affecting premenopausal women, and one of the most common symptoms from fibroids is heavy menstrual bleeding,” Christine P. Nguyen, MD, acting director of the division of urology, obstetrics, and gynecology in the FDA’s Center for Drug Evaluation and Research, said in a news release. “Although surgical treatments, such as hysterectomy, are available, patients may not qualify for surgery or want the procedure. Various nonsurgical therapies are used to treat fibroid-related heavy menstrual bleeding, but none have been FDA approved specifically for this use. Today’s approval provides an FDA-approved medical treatment option for these patients.”
Fibroids, which occur most commonly in women aged 35-49 years, typically resolve after menopause but are a leading reason for hysterectomy in the United States, according to the release.
Researchers established the efficacy of the treatment in two clinical trials that included 591 premenopausal women with heavy menstrual bleeding. Participants received the drug or placebo for 6 months. The investigators defined heavy menstrual bleeding as at least two menstrual cycles with greater than 80 mL of menstrual blood loss. The primary endpoint was the proportion of women who achieved menstrual blood loss less than 80 mL at the final month and 50% or greater reduction in menstrual blood loss volume from baseline to the final month. In one trial, 69% of patients who received Oriahnn met this endpoint, compared with 9% of patients who received placebo. In the second study, 77% of patients who received the drug achieved this endpoint, compared with 11% of patients who received placebo.
Oriahnn may cause bone loss that may not be completely recovered after stopping treatment, so women should not take the medication for more than 24 months, according to the FDA announcement. Health care professionals may recommend bone density scans before and during treatment.
The most common side effects included hot flushes, headache, fatigue, and irregular vaginal bleeding. The drug’s label includes a boxed warning about a risk of strokes and blood clots, especially in women at increased risk for these events. Contraindications include osteoporosis, a history of breast cancer or other hormonally sensitive cancer, liver disease, and abnormal uterine bleeding. Oriahnn does not prevent pregnancy and may increase blood pressure, according to the press release. AbbVie markets the drug.
Should all patients with advanced ovarian cancer receive frontline maintenance therapy?
The current standard frontline therapy for advanced epithelial ovarian, fallopian tube, and primary peritoneal cancer includes a combination of surgical cytoreduction and at least six cycles of platinum-based chemotherapy. While this achieves a complete clinical response (“remission”) in most, 85% of patients will recur and eventually succumb to the disease. This suggests that treatments are good at inducing remission, but poor at eradicating the disease altogether. This has motivated the consideration of maintenance therapy: extended treatment beyond completion of chemotherapy during the period of time when patients are clinically disease free.
Maintenance therapy is an appealing concept for clinicians who desperately want to “hold” their patients in a disease-free state for longer periods. It is also a profitable way to administer therapy as there is more compensation to the pharmaceutical industry from chronic, long-term drug administration rather than episodic treatment courses. However, the following question must be asked: Is this extended therapy worthwhile for all patients, and is it good value?
In the past 12 months, three major industry-sponsored clinical trials have been published (PRIMA, PAOLA-1, and VELIA)which suggest a benefit for all patients with advanced epithelial ovarian cancer in receiving prolonged poly (ADP-ribose) polymerase inhibitor (PARPi) therapy after primary chemotherapy.1-3 This has resulted in Food and Drug Administration approval for some of these agents as maintenance therapy. Despite differences in the drugs tested and the timing of therapy, these studies observed that treatment of advanced ovarian cancer with the addition of a PARPi during and/or after carboplatin and paclitaxel chemotherapy for up to an additional 3 years resulted in a longer progression-free survival (PFS) of approximately 6 months. PFS is defined as the time to measurable recurrence or death. However, this positive effect was not equally distributed across the whole population; rather, it appeared to be created by a substantial response in a smaller subgroup.
PARP inhibitor therapies such as olaparib, niraparib, veliparib, and rucaparib target a family of enzymes that repair DNA and stabilize the human genome through the repair of single-stranded DNA breaks. Inhibiting these enzymes facilitates the accumulation of single-stranded breaks, allowing the development of double-strand breaks, which in turn cannot be repaired if the cell has deficient homologous recombination (HRD) such as through a germline or somatic BRCA mutation, or alternative relevant mutation that confers a similar effect. The opportunistic pairing of a drug interaction with a pathway specific to the cancer is an example of a targeted therapy.
In order to improve the value of cancer drug therapy, there has been emphasis by cooperative research groups, such as the Gynecologic Oncology Group, to study the efficacy of targeted therapies, such as PARPi, in patients identified by biomarkers such as tumors that possess germline or somatic HRD in whom they are most likely to work. This approach makes good common sense and promises to deliver a large magnitude of clinical benefit in a smaller focused population. Therefore, even if drug costs are high, the treatment may still have value. Consistent with that principle, the recently published VELIA, PRIMA, and PAOLA-1 trials all showed impressive benefit in PFS (on average 11-12 months) for the subgroup of patients with HRD. However, these studies were designed and funded by the pharmaceutical industry, and abandoned the principle of biomarker-driven targeted therapy. They did not limit their studies to the HRD-positive population most likely to benefit, but instead included and reported on the impact on all-comers (patients with both HRD and HR-proficient tumors). Subsequently their final conclusions could be extrapolated to the general population of ovarian cancer patients, and in doing so, a larger share of the marketplace.
Only 30% of the general population of ovarian, fallopian tube and primary peritoneal cancer patients carry a germline or somatic BRCA mutation and less than half carry this or alternative mutations which confer HRD. The remaining majority are HR-proficient tumors. However, the three study populations in the aforementioned trials were enriched for HRD tumors with 50%-60% subjects carrying germline or somatic HRD. Therefore, it is likely that the observed benefits in the “intent-to-treat” group were larger than what a clinician would observe in their patient population. Additionally, the large (11-12 month) gains in the HRD-positive group may have been so significant that they compensated for the subtle impact in the HR-proficient population (less than 3 months), resulting in an average total effect that, while being statistically significant for “all comers,” was actually only clinically significant for the HRD group. The positive impact for HRD tumors effectively boosted the results for the group as a whole.
The use of PFS as a primary endpoint raises another significant concern with the design of these PARPi maintenance trials. Much has been written about the importance of PFS as an endpoint for ovarian cancer because of confounding effects of subsequent therapy and to minimize the costs and duration of clinical trials.4 PFS is a quicker, less expensive endpoint to capture than overall survival. It usually correlates with overall survival, but typically only when there is a large magnitude of benefit in PFS. These arguments are fair when considering episodic drug therapies in the setting of measurable, active disease. However, maintenance therapy is given during a period of what patients think of as remission. Remission is valued by patients because it is a gateway to cure, and also because it is a time devoid of symptoms of disease, toxicity (therapeutic and financial), and the burden of frequent medical visits and interventions. While PFS is a measure of the length of remission, it is not a measure of cure. We should ask: What does it mean to a patient if she has a longer remission but needs to be on drug therapy (with its associated burdens and toxicities) in order to maintain that remission? We know that an increase in PFS with maintenance therapy does not always result in a commensurate increase in survival. One does not always precede the other. An example of this is the use of maintenance bevacizumab following upfront chemotherapy which improves PFS by 4 months, but is not associated with an increase in survival.5
When considering the value and ethics of maintenance therapy, it should be associated with a proven survival benefit or an improvement in quality of life. With respect to PARPi maintenance, we lack the data regarding the former, and have contrary evidence regarding the latter. In these three trials, PARPi maintenance was associated with significantly more toxicity than placebo including the commonly observed nausea and fatigue. Most of us would not like to be on a drug therapy for 3 years that made us feel nauseated or fatigued if it didn’t also increase our chance of cure or a longer life. While the significant PFS benefit of maintenance PARPi that is consistently observed in HRD-positive ovarian cancers suggests there will also likely be a clinically significant improvement in survival and cure in that specific subpopulation, this is less likely true for the majority of women with HR-proficient ovarian cancers. Time will tell this story, but as yet, we don’t know.
The use of maintenance PARPi therapy during and/or after primary cytotoxic chemotherapy for advanced epithelial ovarian, primary peritoneal, and fallopian tube cancer is associated with a substantial benefit in time to recurrence in a population with HRD tumors and a small benefit among the majority who don’t. However, it comes at the cost of toxicity at a time when patients would otherwise be free of disease and treatment. I propose that, until a survival benefit for all women has been observed, we should consider a targeted and biomarker-driven approach to maintenance PARPi prescription, favoring prescription for those with germline or somatic HRD mutations.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. González-Martín A et al. N Engl J Med. 2019 Dec 19;381(25):2391-402.
2. Ray-Coquard I et al. N Engl J Med. 2019 Dec 19;381(25):2416-28.
3. Coleman RL et al. N Engl J Med. 2019 Dec 19;381(25):2403-15.
4. Herzog TJ et al. Gynecol Oncol. 2014 Jan;132(1):8-17.
5. Tewari KS et al. J Clin Oncol. 2019 Sep 10;37(26):2317-28.
The current standard frontline therapy for advanced epithelial ovarian, fallopian tube, and primary peritoneal cancer includes a combination of surgical cytoreduction and at least six cycles of platinum-based chemotherapy. While this achieves a complete clinical response (“remission”) in most, 85% of patients will recur and eventually succumb to the disease. This suggests that treatments are good at inducing remission, but poor at eradicating the disease altogether. This has motivated the consideration of maintenance therapy: extended treatment beyond completion of chemotherapy during the period of time when patients are clinically disease free.
Maintenance therapy is an appealing concept for clinicians who desperately want to “hold” their patients in a disease-free state for longer periods. It is also a profitable way to administer therapy as there is more compensation to the pharmaceutical industry from chronic, long-term drug administration rather than episodic treatment courses. However, the following question must be asked: Is this extended therapy worthwhile for all patients, and is it good value?
In the past 12 months, three major industry-sponsored clinical trials have been published (PRIMA, PAOLA-1, and VELIA)which suggest a benefit for all patients with advanced epithelial ovarian cancer in receiving prolonged poly (ADP-ribose) polymerase inhibitor (PARPi) therapy after primary chemotherapy.1-3 This has resulted in Food and Drug Administration approval for some of these agents as maintenance therapy. Despite differences in the drugs tested and the timing of therapy, these studies observed that treatment of advanced ovarian cancer with the addition of a PARPi during and/or after carboplatin and paclitaxel chemotherapy for up to an additional 3 years resulted in a longer progression-free survival (PFS) of approximately 6 months. PFS is defined as the time to measurable recurrence or death. However, this positive effect was not equally distributed across the whole population; rather, it appeared to be created by a substantial response in a smaller subgroup.
PARP inhibitor therapies such as olaparib, niraparib, veliparib, and rucaparib target a family of enzymes that repair DNA and stabilize the human genome through the repair of single-stranded DNA breaks. Inhibiting these enzymes facilitates the accumulation of single-stranded breaks, allowing the development of double-strand breaks, which in turn cannot be repaired if the cell has deficient homologous recombination (HRD) such as through a germline or somatic BRCA mutation, or alternative relevant mutation that confers a similar effect. The opportunistic pairing of a drug interaction with a pathway specific to the cancer is an example of a targeted therapy.
In order to improve the value of cancer drug therapy, there has been emphasis by cooperative research groups, such as the Gynecologic Oncology Group, to study the efficacy of targeted therapies, such as PARPi, in patients identified by biomarkers such as tumors that possess germline or somatic HRD in whom they are most likely to work. This approach makes good common sense and promises to deliver a large magnitude of clinical benefit in a smaller focused population. Therefore, even if drug costs are high, the treatment may still have value. Consistent with that principle, the recently published VELIA, PRIMA, and PAOLA-1 trials all showed impressive benefit in PFS (on average 11-12 months) for the subgroup of patients with HRD. However, these studies were designed and funded by the pharmaceutical industry, and abandoned the principle of biomarker-driven targeted therapy. They did not limit their studies to the HRD-positive population most likely to benefit, but instead included and reported on the impact on all-comers (patients with both HRD and HR-proficient tumors). Subsequently their final conclusions could be extrapolated to the general population of ovarian cancer patients, and in doing so, a larger share of the marketplace.
Only 30% of the general population of ovarian, fallopian tube and primary peritoneal cancer patients carry a germline or somatic BRCA mutation and less than half carry this or alternative mutations which confer HRD. The remaining majority are HR-proficient tumors. However, the three study populations in the aforementioned trials were enriched for HRD tumors with 50%-60% subjects carrying germline or somatic HRD. Therefore, it is likely that the observed benefits in the “intent-to-treat” group were larger than what a clinician would observe in their patient population. Additionally, the large (11-12 month) gains in the HRD-positive group may have been so significant that they compensated for the subtle impact in the HR-proficient population (less than 3 months), resulting in an average total effect that, while being statistically significant for “all comers,” was actually only clinically significant for the HRD group. The positive impact for HRD tumors effectively boosted the results for the group as a whole.
The use of PFS as a primary endpoint raises another significant concern with the design of these PARPi maintenance trials. Much has been written about the importance of PFS as an endpoint for ovarian cancer because of confounding effects of subsequent therapy and to minimize the costs and duration of clinical trials.4 PFS is a quicker, less expensive endpoint to capture than overall survival. It usually correlates with overall survival, but typically only when there is a large magnitude of benefit in PFS. These arguments are fair when considering episodic drug therapies in the setting of measurable, active disease. However, maintenance therapy is given during a period of what patients think of as remission. Remission is valued by patients because it is a gateway to cure, and also because it is a time devoid of symptoms of disease, toxicity (therapeutic and financial), and the burden of frequent medical visits and interventions. While PFS is a measure of the length of remission, it is not a measure of cure. We should ask: What does it mean to a patient if she has a longer remission but needs to be on drug therapy (with its associated burdens and toxicities) in order to maintain that remission? We know that an increase in PFS with maintenance therapy does not always result in a commensurate increase in survival. One does not always precede the other. An example of this is the use of maintenance bevacizumab following upfront chemotherapy which improves PFS by 4 months, but is not associated with an increase in survival.5
When considering the value and ethics of maintenance therapy, it should be associated with a proven survival benefit or an improvement in quality of life. With respect to PARPi maintenance, we lack the data regarding the former, and have contrary evidence regarding the latter. In these three trials, PARPi maintenance was associated with significantly more toxicity than placebo including the commonly observed nausea and fatigue. Most of us would not like to be on a drug therapy for 3 years that made us feel nauseated or fatigued if it didn’t also increase our chance of cure or a longer life. While the significant PFS benefit of maintenance PARPi that is consistently observed in HRD-positive ovarian cancers suggests there will also likely be a clinically significant improvement in survival and cure in that specific subpopulation, this is less likely true for the majority of women with HR-proficient ovarian cancers. Time will tell this story, but as yet, we don’t know.
The use of maintenance PARPi therapy during and/or after primary cytotoxic chemotherapy for advanced epithelial ovarian, primary peritoneal, and fallopian tube cancer is associated with a substantial benefit in time to recurrence in a population with HRD tumors and a small benefit among the majority who don’t. However, it comes at the cost of toxicity at a time when patients would otherwise be free of disease and treatment. I propose that, until a survival benefit for all women has been observed, we should consider a targeted and biomarker-driven approach to maintenance PARPi prescription, favoring prescription for those with germline or somatic HRD mutations.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. González-Martín A et al. N Engl J Med. 2019 Dec 19;381(25):2391-402.
2. Ray-Coquard I et al. N Engl J Med. 2019 Dec 19;381(25):2416-28.
3. Coleman RL et al. N Engl J Med. 2019 Dec 19;381(25):2403-15.
4. Herzog TJ et al. Gynecol Oncol. 2014 Jan;132(1):8-17.
5. Tewari KS et al. J Clin Oncol. 2019 Sep 10;37(26):2317-28.
The current standard frontline therapy for advanced epithelial ovarian, fallopian tube, and primary peritoneal cancer includes a combination of surgical cytoreduction and at least six cycles of platinum-based chemotherapy. While this achieves a complete clinical response (“remission”) in most, 85% of patients will recur and eventually succumb to the disease. This suggests that treatments are good at inducing remission, but poor at eradicating the disease altogether. This has motivated the consideration of maintenance therapy: extended treatment beyond completion of chemotherapy during the period of time when patients are clinically disease free.
Maintenance therapy is an appealing concept for clinicians who desperately want to “hold” their patients in a disease-free state for longer periods. It is also a profitable way to administer therapy as there is more compensation to the pharmaceutical industry from chronic, long-term drug administration rather than episodic treatment courses. However, the following question must be asked: Is this extended therapy worthwhile for all patients, and is it good value?
In the past 12 months, three major industry-sponsored clinical trials have been published (PRIMA, PAOLA-1, and VELIA)which suggest a benefit for all patients with advanced epithelial ovarian cancer in receiving prolonged poly (ADP-ribose) polymerase inhibitor (PARPi) therapy after primary chemotherapy.1-3 This has resulted in Food and Drug Administration approval for some of these agents as maintenance therapy. Despite differences in the drugs tested and the timing of therapy, these studies observed that treatment of advanced ovarian cancer with the addition of a PARPi during and/or after carboplatin and paclitaxel chemotherapy for up to an additional 3 years resulted in a longer progression-free survival (PFS) of approximately 6 months. PFS is defined as the time to measurable recurrence or death. However, this positive effect was not equally distributed across the whole population; rather, it appeared to be created by a substantial response in a smaller subgroup.
PARP inhibitor therapies such as olaparib, niraparib, veliparib, and rucaparib target a family of enzymes that repair DNA and stabilize the human genome through the repair of single-stranded DNA breaks. Inhibiting these enzymes facilitates the accumulation of single-stranded breaks, allowing the development of double-strand breaks, which in turn cannot be repaired if the cell has deficient homologous recombination (HRD) such as through a germline or somatic BRCA mutation, or alternative relevant mutation that confers a similar effect. The opportunistic pairing of a drug interaction with a pathway specific to the cancer is an example of a targeted therapy.
In order to improve the value of cancer drug therapy, there has been emphasis by cooperative research groups, such as the Gynecologic Oncology Group, to study the efficacy of targeted therapies, such as PARPi, in patients identified by biomarkers such as tumors that possess germline or somatic HRD in whom they are most likely to work. This approach makes good common sense and promises to deliver a large magnitude of clinical benefit in a smaller focused population. Therefore, even if drug costs are high, the treatment may still have value. Consistent with that principle, the recently published VELIA, PRIMA, and PAOLA-1 trials all showed impressive benefit in PFS (on average 11-12 months) for the subgroup of patients with HRD. However, these studies were designed and funded by the pharmaceutical industry, and abandoned the principle of biomarker-driven targeted therapy. They did not limit their studies to the HRD-positive population most likely to benefit, but instead included and reported on the impact on all-comers (patients with both HRD and HR-proficient tumors). Subsequently their final conclusions could be extrapolated to the general population of ovarian cancer patients, and in doing so, a larger share of the marketplace.
Only 30% of the general population of ovarian, fallopian tube and primary peritoneal cancer patients carry a germline or somatic BRCA mutation and less than half carry this or alternative mutations which confer HRD. The remaining majority are HR-proficient tumors. However, the three study populations in the aforementioned trials were enriched for HRD tumors with 50%-60% subjects carrying germline or somatic HRD. Therefore, it is likely that the observed benefits in the “intent-to-treat” group were larger than what a clinician would observe in their patient population. Additionally, the large (11-12 month) gains in the HRD-positive group may have been so significant that they compensated for the subtle impact in the HR-proficient population (less than 3 months), resulting in an average total effect that, while being statistically significant for “all comers,” was actually only clinically significant for the HRD group. The positive impact for HRD tumors effectively boosted the results for the group as a whole.
The use of PFS as a primary endpoint raises another significant concern with the design of these PARPi maintenance trials. Much has been written about the importance of PFS as an endpoint for ovarian cancer because of confounding effects of subsequent therapy and to minimize the costs and duration of clinical trials.4 PFS is a quicker, less expensive endpoint to capture than overall survival. It usually correlates with overall survival, but typically only when there is a large magnitude of benefit in PFS. These arguments are fair when considering episodic drug therapies in the setting of measurable, active disease. However, maintenance therapy is given during a period of what patients think of as remission. Remission is valued by patients because it is a gateway to cure, and also because it is a time devoid of symptoms of disease, toxicity (therapeutic and financial), and the burden of frequent medical visits and interventions. While PFS is a measure of the length of remission, it is not a measure of cure. We should ask: What does it mean to a patient if she has a longer remission but needs to be on drug therapy (with its associated burdens and toxicities) in order to maintain that remission? We know that an increase in PFS with maintenance therapy does not always result in a commensurate increase in survival. One does not always precede the other. An example of this is the use of maintenance bevacizumab following upfront chemotherapy which improves PFS by 4 months, but is not associated with an increase in survival.5
When considering the value and ethics of maintenance therapy, it should be associated with a proven survival benefit or an improvement in quality of life. With respect to PARPi maintenance, we lack the data regarding the former, and have contrary evidence regarding the latter. In these three trials, PARPi maintenance was associated with significantly more toxicity than placebo including the commonly observed nausea and fatigue. Most of us would not like to be on a drug therapy for 3 years that made us feel nauseated or fatigued if it didn’t also increase our chance of cure or a longer life. While the significant PFS benefit of maintenance PARPi that is consistently observed in HRD-positive ovarian cancers suggests there will also likely be a clinically significant improvement in survival and cure in that specific subpopulation, this is less likely true for the majority of women with HR-proficient ovarian cancers. Time will tell this story, but as yet, we don’t know.
The use of maintenance PARPi therapy during and/or after primary cytotoxic chemotherapy for advanced epithelial ovarian, primary peritoneal, and fallopian tube cancer is associated with a substantial benefit in time to recurrence in a population with HRD tumors and a small benefit among the majority who don’t. However, it comes at the cost of toxicity at a time when patients would otherwise be free of disease and treatment. I propose that, until a survival benefit for all women has been observed, we should consider a targeted and biomarker-driven approach to maintenance PARPi prescription, favoring prescription for those with germline or somatic HRD mutations.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. González-Martín A et al. N Engl J Med. 2019 Dec 19;381(25):2391-402.
2. Ray-Coquard I et al. N Engl J Med. 2019 Dec 19;381(25):2416-28.
3. Coleman RL et al. N Engl J Med. 2019 Dec 19;381(25):2403-15.
4. Herzog TJ et al. Gynecol Oncol. 2014 Jan;132(1):8-17.
5. Tewari KS et al. J Clin Oncol. 2019 Sep 10;37(26):2317-28.
Patient-focused precautions, testing help blunt pandemic effects on heme-onc unit
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Most patients with lichen sclerosus receive appropriate treatment
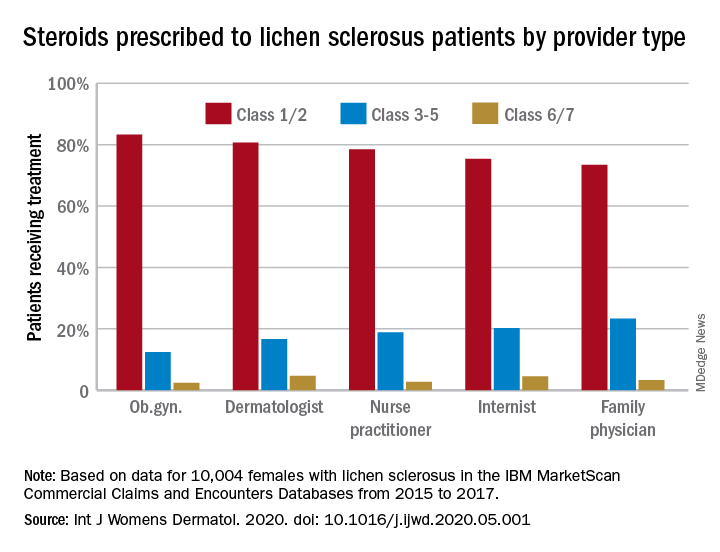
The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.

The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.

The claims-based prevalence of 0.05% found in the study is lower than previously reported, and only 16% of the diagnoses were in women aged 18-44 years, Laura E. Melnick, MD, and associates wrote after identifying 10,004 females aged 0-65 years with lichen sclerosus in the IBM MarketScan Commercial Claims and Encounters Databases from 2015 to 2017. The majority (79%) of those diagnosed were aged 45-65 years (average, 50.8 years).
In pediatric patients (up to age 17 years), the low prevalence (0.01%) “may be attributable to several factors including relative rarity, as well as variability in pediatric clinicians’ familiarity with [lichen sclerosus] and in patients’ clinical symptoms,” said Dr. Melnick and associates in the department of dermatology at New York University.
Just over half of all diagnoses (52.4%) were made by ob.gyns., with dermatologists next at 14.5%, followed by family physicians (6.5%), nurse practitioners (2.5%), and internists (0.4%), they reported in the International Journal of Women’s Dermatology.
Treatment for lichen sclerosus, in the form of high-potency topical corticosteroids, was mostly appropriate. Ob.gyns. prescribed class 1/2 steroids to 83% of their patients, tops among all clinicians. Dermatologists were just over 80%, and the other clinician categories were all over 70%, the investigators said.
“Understanding the current management of [lichen sclerosus] is important given that un- or undertreated disease can significantly impact patients’ quality of life, lead to increased lower urinary tract symptoms and irreversible architectural changes, and predispose women to squamous cell carcinoma,” they wrote.
SOURCE: Melnick LE et al. Int J Womens Dermatol. 2020. doi: 10.1016/j.ijwd.2020.05.001.
FROM THE INTERNATIONAL JOURNAL OF WOMEN’S DERMATOLOGY
Out of the pipeline: Remdesivir
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1
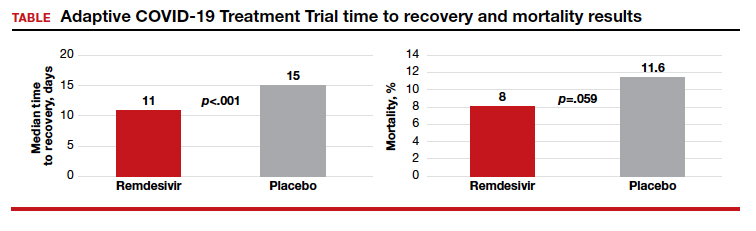
5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: [email protected]
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: [email protected]
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
Although the US Food and Drug Administration (FDA) has granted emergency use authorization of remdesivir (Gilead Sciences, Inc., Foster City, California) to treat COVID-19, the disease caused by SARS-CoV-2, the drug is considered an investigational agent, not yet formally approved by the FDA and whose efficacy and safety has not yet been fully characterized. Remdesivir has been shown to be effective in reducing the time to recovery of people with COVID-19 disease. It has not been tested in a large controlled clinical trial of pregnant women with COVID-19; however, remdesivir has been given to pregnant women infected with COVID-19 in a compassionate use protocol. For pregnant women, the drug should only be used if the potential benefit justifies the potential risk to the mother and fetus.1
Pharmacology. Remdesivir is a nucleoside RNA polymerase inhibitor. It has a molecular formula of
C27H35N6O8P and a molecular weight of 602.6 g/mol.1
Mechanism of action. From FDA’s fact sheet: “Remdesivir is an adenosine nucleotide prodrug that distributes into cells where it is metabolized to form the pharmacologically active nucleoside triphosphate metabolite. Metabolism of remdesivir to remdesivir triphosphate has been demonstrated in multiple cell types. Remdesivir triphosphate acts as an analog of adenosine triphosphate (ATP) and competes with the natural ATP substrate for incorporation into nascent RNA chains by the SARS-CoV-2 RNA-dependent RNA polymerase, which results in chain termination during replication of the viral RNA. Remdesivir triphosphate is a weak inhibitor of mammalian DNA and RNA polymerases with low potential for mitochondrial toxicity.”1
Treatment protocols
Remdesivir is authorized for treatment of hospitalized patients with severe COVID-19 disease, defined as patients with an oxygen saturation ≤ 94% on room air or requiring supplemental oxygen or requiring mechanical ventilation or requiring extracorporeal membrane oxygenation (ECMO). The optimal dose and duration of treatment of COVID-19 with remdesivir is unknown.1
Prior to initiating treatment, the estimated glomerular filtration rate should be documented to be ≥ 30 mL/min. An excipient used in the remdesivir formulation—sulfobutylether-β-cylcodextrin sodium salt—is renally cleared and accumulates in patients with decreased renal function.
Baseline liver function tests should be performed prior to treatment and daily during the course of treatment. Remdesivir should not be initiated in patients with an alanine aminotransferase (ALT) level ≥ 5 times the upper limit of normal at baseline. Remdesivir should be discontinued in patients who develop an ALT level ≥ 5 times the upper limit of normal or in patients who develop elevated ALT levels and have increased bilirubin, alkaline phosphatase, or international normalized ratio.1
In one open-label study (GS-US-540-5773), remdesivir treatment was discontinued due to an adverse event in 5% of patients on a 5-day regimen and in 10% of patients on a 10-day regimen.1
Under the emergency use authorization, two treatment protocols have been proposed depending on the clinical severity of the COVID-19 infection1:
- Protocol 1: For people with COVID-19 requiring mechanical ventilation and/or ECMO, the duration of therapy is 10 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 9 days.
- Protocol 2: For people with COVID-19 disease not requiring mechanical ventilation and/or ECMO, the duration of therapy is 5 days, beginning with a loading dose of remdesivir 200 mg infused intravenously for 30 to 120 minutes on day 1 followed by a once-daily dose of 100 mg for 4 days. If the patient does not show clinical improvement, treatment may be extended for an additional 5 days.
Continue to: Randomized placebo-controlled trial results...
Randomized placebo-controlled trial results
The Adaptive COVID-19 Treatment Trial (ACTT), sponsored by the National Institute of Allergy and Infectious Diseases, is a randomized, double-blind, placebo-controlled trial conducted by Gilead Sciences. The study began in February and evaluated up to 10 days of remdesivir treatment—200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days in hospitalized adult patients with COVID-19. Patients were enrolled in a 1:1 manner to remdesivir or placebo, and time to recovery within 28 days after randomization was the trial’s endpoint. According to preliminary analysis of 606 recovered patients, recovery took a median of 11 days in the remdesivir group and 15 days in the placebo group (hazard ratio, 1.31; 95% confidence interval (CI), 1.12‒1.54; P<.001). Mortality rates were 8.0% and 11.6% in the remdesivir and placebo groups, respectively (P=.059).1

5 vs 10 days of remdesivir treatment
The Gilead Sciences‒sponsored study GS-US-540-5773 was a randomized, open-label multicenter trial of patients with severe COVID-19. A total of 197 adult patients received 10-day remdesivir treatment (200 mg IV once daily for 1 day followed by 100 mg IV once daily for 9 days). An additional 200 adult patients received 5-day remdesivir treatment (200 mg IV once daily followed by 100 mg IV for 4 days). Both groups also received standard of care. Results suggested that patients receiving 10 days of remdesivir had similar improvement in clinical status compared with those receiving a 5-day treatment course (10-to-5 day odds ratio, 0.76; 95% CI, 0.51‒1.13] on day 14).1 Improvement in clinical status was defined as an improvement of 2 or more points from baseline on a predefined 7-point scale that ranged from hospital discharge to increasing levels of oxygen support to death. Clinical recovery was achieved if patients ceased the need for oxygen support or were discharged.1
The time to clinical improvement for 50% of patients was similar in each treatment group (10 days in the 5-day group versus 11 days in the 10-day group). By day 14, observed clinical improvement rates were 65% and 54% in the 5- and 10-day treatment groups, respectively. Clinical recovery rates were 70% and 59% in the 5- and 10-day treatment groups and mortality rates were 8% and 11%.1
Adverse events
The use of remdesivir is contraindicated in patients who are hypersensitive to the drug. Its infusion may cause hypotension, nausea, vomiting, diaphoresis, and shivering. If signs of a clinically significant infusion reaction are observed the infusion should be discontinued. As noted above, elevation in ALT levels occurs with remdesivir treatment.1
Reporting serious adverse events. If a serious and unexpected adverse event occurs and appears to be associated with the use of remdesivir, the prescribing health care provider and/or the provider’s designee should complete and submit a MedWatch form to the FDA using one of the following methods1:
- Complete and submit the report online: www.fda.gov/medwatch/report.htm
- Return form FDA 3500 (available at http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM163919.pdf) to the FDA by mail (MedWatch, 5600 Fishers Lane, Rockville, MD 20852-9787) or fax (1-800-FDA-0178)
- Gilead requests that all FDA MedWatch forms also be returned to Gilead Pharmacovigilance and Epidemiology: fax: 1-650-522-5477 726; e-mail: [email protected]
Continue to: Drug interactions...
Drug interactions
Remdesivir has not been evaluated for drug-drug interactions in humans. The clinical relevance of in vitro drug interactions also has not been established. According to the FDA, remdesivir is a substrate for the drug metabolizing enzymes CYP2C8, CYP2D6, and CYP3A4, and is a substrate for organic anion transporting polypeptides 1B1 (OAPT1B1) and P-glycoprotein (P-gp) transporters. In vitro, remdesivir inhibits CYP3A4, OATP1B1, OATP1B3, BSEP, MRP4, and NTCP.1
Pregnancy risk summary
Remdesivir has not been studied adequately in pregnant women and only should be used during pregnancy if the potential benefit of the drug justifies the potential risk to both mother and fetus.
Nonclinical animal studies that included systemic exposure of the predominant circulating metabolite of remdesivir in pregnant rats and rabbits (at 4 times the recommended dose of human exposure) demonstrated no adverse effect on embryofetal development.1
Breastfeeding
The only information regarding breastfeeding and remdesivir comes from animal studies. The drug and its metabolites were detected in the plasma of nursing rat pups whose mothers given intravenous remdesivir daily from gestation day 6 to lactation day 20. Measured on lactation day 10, remdesivir exposure in the pups was about 1% that of maternal exposure.1
“Because of the potential for viral transmission to SARS-CoV-2-negative infants and adverse reactions from the drug in breastfeeding infants, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for remdesivir and any potential adverse effects on the breastfed child from remdesivir or from the underlying maternal condition.”1
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
- US Food and Drug Administration. Fact Sheet for Health Care Providers Emergency Use Authorization (UA) of Remdesivir (GS-5734)TM. https://www.fda.gov/media/137566/download. Accessed May 19, 2020.
BMD preserved with investigational drug for uterine fibroid bleeding
Combination therapy with relugolix, an investigational oral gonadotropin-releasing hormone antagonist, estradiol, and norethindrone acetate effectively preserved bone mineral density (BMD) in two replicate phase 3 studies enrolling women with heavy menstrual bleeding associated with uterine fibroids.
The BMD findings, released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, build upon previously reported positive primary endpoint data from the LIBERTY 1 and LIBERTY 2 studies. ACOG canceled the meeting and released abstracts for press coverage.
The developer of the drug, Myovant Sciences, plans to submit a new drug application to the Food and Drug Administration for approval of the single-tablet combination therapy for women with uterine fibroids, according to Albert Liao, the company’s director of corporate communications.
The two multinational LIBERTY studies randomized women who had a monthly menstrual blood loss volume of at least 80 mL in two consecutive cycles (or 160 mL in one cycle) in a 1:1:1 ratio to one of three groups: relugolix combination therapy for 24 weeks (once-daily relugolix 40 mg plus estradiol 1.0 mg plus norethindrone acetate 0.5 mg); relugolix alone (40 mg once daily) for 12 weeks followed by relugolix combination therapy for 12 weeks; or placebo for 24 weeks.
In October 2019 at the American Society for Reproductive Medicine Scientific Congress, investigators reported that 73% of women receiving combination therapy in the LIBERTY 1 trial achieved a menstrual blood loss of less than 80 mL and a 50% or greater reduction from baseline over the last 35 days of treatment, compared with 19% in the placebo group. The mean percent reduction in menstrual blood loss from baseline at week 24 was 84% for combination therapy and 23% for placebo.
Earlier in 2019, Myovant Sciences announced that, in the LIBERTY 2 study, 71% of women receiving combination therapy met the primary endpoints, compared with 15% in the placebo group. The reduction in menstrual blood loss in this study’s combination therapy arm was also 84%, according to a company press release from June 2019.
Each of the two clinical trials enrolled upwards of 380 women.
The new abstract released for press coverage by ACOG and published in Obstetrics & Gynecology reports that women receiving relugolix combination therapy in the LIBERTY 1 and LIBERTY 2 studies had a mean change in lumbar spine BMD of –0.36% and –0.13%, respectively, from baseline to 24 weeks. Percent change in lumbar spine BMD in the delayed combination therapy groups (12 initial weeks of relugolix monotherapy) was –1.82% and –2.12%. In the placebo groups, the change was 0.05% and 0.32%.
Michael R. McClung, MD, who is the lead author of the abstract and was scheduled to present the findings at the ACOG meeting, said in an interview that the slight decreases in lumbar spine BMD with combination therapy were noted largely at week 12 and are “clinically insignificant in my opinion.” BMD by dual-energy x-ray absorptiometry was assessed at weeks 12 and 24.
“There was no further increase [after week 12] and [in some patients] there was even a return to baseline,” said Dr. McClung, of the Oregon Osteoporosis Center in Portland.
The safety and efficacy of longer-term treatment with relugolix combination therapy has been investigated thus far through an open-label extension study that brought the treatment period to 52 weeks. The 1-year data has been positive and will be presented or published soon, said Mr. Liao. In addition, a “second, 52-week randomized withdrawal study has been designed to provide 2-year safety and efficacy data … and to evaluate the need for maintenance therapy.”
It’s important, Dr. McClung said, “for clinicians to be confident that BMD loss is prevented or minimized with longer-term relugolix combination therapy since treatment for uterine fibroids is not a short-term proposition. Given the stability of BMD values between weeks 12 and 24 in the LIBERTY studies, I’d anticipate that we will see stable values with longer-term treatment.”
Dr. McClung disclosed that he has served as a consultant/advisory board member and speaker for Amgen and a consultant/advisory board member for Myovant. Several of his coauthors disclosed employment and ownerships interests in Myovant.
SOURCE: McClung MR et al. Obstet Gynecol. 2020 May. doi: 10.1097/01.AOG.0000662944.34860.b4.
Combination therapy with relugolix, an investigational oral gonadotropin-releasing hormone antagonist, estradiol, and norethindrone acetate effectively preserved bone mineral density (BMD) in two replicate phase 3 studies enrolling women with heavy menstrual bleeding associated with uterine fibroids.
The BMD findings, released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, build upon previously reported positive primary endpoint data from the LIBERTY 1 and LIBERTY 2 studies. ACOG canceled the meeting and released abstracts for press coverage.
The developer of the drug, Myovant Sciences, plans to submit a new drug application to the Food and Drug Administration for approval of the single-tablet combination therapy for women with uterine fibroids, according to Albert Liao, the company’s director of corporate communications.
The two multinational LIBERTY studies randomized women who had a monthly menstrual blood loss volume of at least 80 mL in two consecutive cycles (or 160 mL in one cycle) in a 1:1:1 ratio to one of three groups: relugolix combination therapy for 24 weeks (once-daily relugolix 40 mg plus estradiol 1.0 mg plus norethindrone acetate 0.5 mg); relugolix alone (40 mg once daily) for 12 weeks followed by relugolix combination therapy for 12 weeks; or placebo for 24 weeks.
In October 2019 at the American Society for Reproductive Medicine Scientific Congress, investigators reported that 73% of women receiving combination therapy in the LIBERTY 1 trial achieved a menstrual blood loss of less than 80 mL and a 50% or greater reduction from baseline over the last 35 days of treatment, compared with 19% in the placebo group. The mean percent reduction in menstrual blood loss from baseline at week 24 was 84% for combination therapy and 23% for placebo.
Earlier in 2019, Myovant Sciences announced that, in the LIBERTY 2 study, 71% of women receiving combination therapy met the primary endpoints, compared with 15% in the placebo group. The reduction in menstrual blood loss in this study’s combination therapy arm was also 84%, according to a company press release from June 2019.
Each of the two clinical trials enrolled upwards of 380 women.
The new abstract released for press coverage by ACOG and published in Obstetrics & Gynecology reports that women receiving relugolix combination therapy in the LIBERTY 1 and LIBERTY 2 studies had a mean change in lumbar spine BMD of –0.36% and –0.13%, respectively, from baseline to 24 weeks. Percent change in lumbar spine BMD in the delayed combination therapy groups (12 initial weeks of relugolix monotherapy) was –1.82% and –2.12%. In the placebo groups, the change was 0.05% and 0.32%.
Michael R. McClung, MD, who is the lead author of the abstract and was scheduled to present the findings at the ACOG meeting, said in an interview that the slight decreases in lumbar spine BMD with combination therapy were noted largely at week 12 and are “clinically insignificant in my opinion.” BMD by dual-energy x-ray absorptiometry was assessed at weeks 12 and 24.
“There was no further increase [after week 12] and [in some patients] there was even a return to baseline,” said Dr. McClung, of the Oregon Osteoporosis Center in Portland.
The safety and efficacy of longer-term treatment with relugolix combination therapy has been investigated thus far through an open-label extension study that brought the treatment period to 52 weeks. The 1-year data has been positive and will be presented or published soon, said Mr. Liao. In addition, a “second, 52-week randomized withdrawal study has been designed to provide 2-year safety and efficacy data … and to evaluate the need for maintenance therapy.”
It’s important, Dr. McClung said, “for clinicians to be confident that BMD loss is prevented or minimized with longer-term relugolix combination therapy since treatment for uterine fibroids is not a short-term proposition. Given the stability of BMD values between weeks 12 and 24 in the LIBERTY studies, I’d anticipate that we will see stable values with longer-term treatment.”
Dr. McClung disclosed that he has served as a consultant/advisory board member and speaker for Amgen and a consultant/advisory board member for Myovant. Several of his coauthors disclosed employment and ownerships interests in Myovant.
SOURCE: McClung MR et al. Obstet Gynecol. 2020 May. doi: 10.1097/01.AOG.0000662944.34860.b4.
Combination therapy with relugolix, an investigational oral gonadotropin-releasing hormone antagonist, estradiol, and norethindrone acetate effectively preserved bone mineral density (BMD) in two replicate phase 3 studies enrolling women with heavy menstrual bleeding associated with uterine fibroids.
The BMD findings, released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, build upon previously reported positive primary endpoint data from the LIBERTY 1 and LIBERTY 2 studies. ACOG canceled the meeting and released abstracts for press coverage.
The developer of the drug, Myovant Sciences, plans to submit a new drug application to the Food and Drug Administration for approval of the single-tablet combination therapy for women with uterine fibroids, according to Albert Liao, the company’s director of corporate communications.
The two multinational LIBERTY studies randomized women who had a monthly menstrual blood loss volume of at least 80 mL in two consecutive cycles (or 160 mL in one cycle) in a 1:1:1 ratio to one of three groups: relugolix combination therapy for 24 weeks (once-daily relugolix 40 mg plus estradiol 1.0 mg plus norethindrone acetate 0.5 mg); relugolix alone (40 mg once daily) for 12 weeks followed by relugolix combination therapy for 12 weeks; or placebo for 24 weeks.
In October 2019 at the American Society for Reproductive Medicine Scientific Congress, investigators reported that 73% of women receiving combination therapy in the LIBERTY 1 trial achieved a menstrual blood loss of less than 80 mL and a 50% or greater reduction from baseline over the last 35 days of treatment, compared with 19% in the placebo group. The mean percent reduction in menstrual blood loss from baseline at week 24 was 84% for combination therapy and 23% for placebo.
Earlier in 2019, Myovant Sciences announced that, in the LIBERTY 2 study, 71% of women receiving combination therapy met the primary endpoints, compared with 15% in the placebo group. The reduction in menstrual blood loss in this study’s combination therapy arm was also 84%, according to a company press release from June 2019.
Each of the two clinical trials enrolled upwards of 380 women.
The new abstract released for press coverage by ACOG and published in Obstetrics & Gynecology reports that women receiving relugolix combination therapy in the LIBERTY 1 and LIBERTY 2 studies had a mean change in lumbar spine BMD of –0.36% and –0.13%, respectively, from baseline to 24 weeks. Percent change in lumbar spine BMD in the delayed combination therapy groups (12 initial weeks of relugolix monotherapy) was –1.82% and –2.12%. In the placebo groups, the change was 0.05% and 0.32%.
Michael R. McClung, MD, who is the lead author of the abstract and was scheduled to present the findings at the ACOG meeting, said in an interview that the slight decreases in lumbar spine BMD with combination therapy were noted largely at week 12 and are “clinically insignificant in my opinion.” BMD by dual-energy x-ray absorptiometry was assessed at weeks 12 and 24.
“There was no further increase [after week 12] and [in some patients] there was even a return to baseline,” said Dr. McClung, of the Oregon Osteoporosis Center in Portland.
The safety and efficacy of longer-term treatment with relugolix combination therapy has been investigated thus far through an open-label extension study that brought the treatment period to 52 weeks. The 1-year data has been positive and will be presented or published soon, said Mr. Liao. In addition, a “second, 52-week randomized withdrawal study has been designed to provide 2-year safety and efficacy data … and to evaluate the need for maintenance therapy.”
It’s important, Dr. McClung said, “for clinicians to be confident that BMD loss is prevented or minimized with longer-term relugolix combination therapy since treatment for uterine fibroids is not a short-term proposition. Given the stability of BMD values between weeks 12 and 24 in the LIBERTY studies, I’d anticipate that we will see stable values with longer-term treatment.”
Dr. McClung disclosed that he has served as a consultant/advisory board member and speaker for Amgen and a consultant/advisory board member for Myovant. Several of his coauthors disclosed employment and ownerships interests in Myovant.
SOURCE: McClung MR et al. Obstet Gynecol. 2020 May. doi: 10.1097/01.AOG.0000662944.34860.b4.
FROM ACOG 2020
Respiratory particles generated by speech can remain airborne for up to 14 minutes
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
Stadnytskyi and colleagues explored the size of droplets created by speech using a highly sensitive laser system. They reported in PNAS that speaking resulted in the generation of a high number of medium-sized droplets (10- to 100-µm in diameter). Under the conditions of their experiment (27% humidity and 23° C) they reported that speech probably generates droplets that originate at a size of 12 to 21 µm in diameter and quickly dehydrate to an estimated diameter of 4 µm. The 4 µm-sized particles had a falling rate of only 0.06 cm·s−1 and remained airborne for 8 to 14 minutes.1
As reported by Hamner and colleagues, on March 10, 2020, 61 persons attended a 2.5-hour choir practice. One choir member had symptoms of an upper respiratory infection that began on March 7. Eventually that choir member tested positive for SARS-CoV-2. Of the 60 remaining persons, 52 (86.7%) eventually developed an upper respiratory illness. In total, 33 cases of SARS-CoV-2 were confirmed by nucleic acid testing and 20 probable cases were diagnosed (these individuals declined testing). The choir attendees developed symptoms at a median of 3 days following the practice, with a range of 1 to 12 days. Three of the 53 ill people were hospitalized, and two died.2
The Stadnytskyi study suggests that speech generates large respiratory droplets that dehydrate into very small droplets that may remain in the air for an extended period of time. If the SARS-CoV-2 virus were in the original large droplet, the rapid dehydration of the droplet would result in prolonged airborne presence of the virus and enhance its infectivity.
The Hamner study highlights the importance of vocalization and respiratory particles in transmitting the SARS-CoV-2 virus. For clinicians and patients, both studies support many recommendations to reduce viral transmission, including:
- all clinicians and patients need to wear face masks
- all clinicians and patients should avoid face-to-face contact if alternative approaches to communication are possible
- all clinicians and patients should avoid gathering in large groups or crowded public spaces and need to maintain physical distancing.
The COVID pandemic has dramatically changed how we practice medicine and socialize.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
- Stadnytskyi V, Bax CE, Bax A, et al. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. PNAS. May 13, 2020. https://doi.org/10.1073/pnas.2006874117.
- Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at choir practice—Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606-610. Early release, May 12, 2020.
Managing Trichomonas vaginalis infections
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18
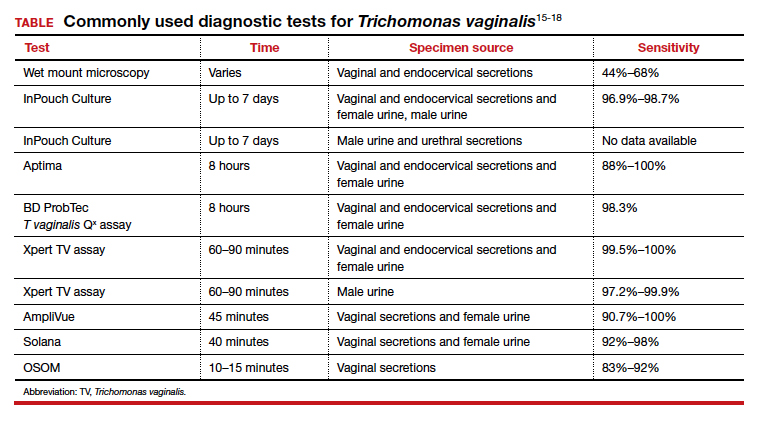
Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.










