User login
Justices appear split over birth control mandate case
U.S. Supreme Court justices appear divided over whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraception mandate.
During oral arguments on May 6, the court expressed differing perspectives about the administration’s authority to allow for more exemptions under the health law’s birth control mandate and whether the expansions were reasonable. Justices heard the consolidated cases – Little Sisters of the Poor v. Pennsylvania and Trump v. Pennsylvania – by teleconference because of the COVID-19 pandemic. They are expected to make a decision by the summer.
Associate justice Ruth Bader Ginsburg, who participated in the telephone conference call from a hospital where she was recovering from a gallbladder condition, said the exemptions ignored the intent of Congress to provide women with comprehensive coverage through the ACA.
“The glaring feature of what the government has done in expanding this exemption is to toss to the winds entirely Congress’s instruction that women need and shall have seamless, no-cost, comprehensive coverage,” she said during oral arguments. “This leaves the women to hunt for other government programs that might cover them, and for those who are not covered by Medicaid or one of the other government programs, they can get contraceptive coverage only from paying out of their own pocket, which is exactly what Congress didn’t want to happen.”
Associate Justice Samuel Alito Jr., meanwhile, indicated that a lower court opinion that had blocked the exemptions from going forward conflicts with the Supreme Court’s ruling in a related case, Burwell v. Hobby Lobby.
“Explain to me why the Third Circuit’s analysis of the question of substantial burden is not squarely inconsistent with our reasoning in Hobby Lobby,” Associate Justice Alito said during oral arguments. “Hobby Lobby held that, if a person sincerely believes that it is immoral to perform an act that has the effect of enabling another person to commit an immoral act, a federal court does not have the right to say that this person is wrong on the question of moral complicity. That’s precisely the situation here. Reading the Third Circuit’s discussion of the substantial burden question, I wondered whether they had read that part of the Hobby Lobby decision.”
The dispute surrounding the ACA’s birth control mandate and the extent of exemptions afforded has gone on for a decade and has led to numerous legal challenges. The ACA initially required all employers to cover birth control for employees with no copayments, but exempted group health plans of religious employers. Those religious employers were primarily churches and other houses of worship. After a number of complaints and lawsuits, the Obama administration created a workaround for nonprofit religious employers not included in that exemption to opt out of the mandate. However, critics argued the process itself was a violation of their religious freedom.
The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved however, and in May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
In 2018, the Trump administration announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of “sincerely held religious beliefs.” A second rule allowed nonprofit organizations and small businesses that had nonreligious moral convictions against the mandate to opt out.
Thirteen states and the District of Columbia then sued the Trump administration over the rules, as well as Pennsylvania and New Jersey in a separate case. Little Sisters of the Poor, a religious nonprofit operating a home in Pittsburgh, intervened in the case as an aggrieved party. An appeal court temporarily barred the regulations from moving forward.
During oral arguments, Solicitor General for the Department of Justice Noel J. Francisco said the exemptions are lawful because they are authorized under a provision of the ACA as well as the Religious Freedom Restoration Act (RFRA).
“RFRA at the very least authorizes the religious exemption,” Mr. Francisco said during oral arguments.
Chief Deputy Attorney General for Pennsylvania Michael J. Fischer argued that the Trump administration’s moral and religious exemption rules rest on overly broad assertions of agency authority.
“First, the agencies twist a narrow delegation that allows the Health Resources and Services Administration to decide which preventive services insurers must cover under the Women’s Health Amendment into a grant of authority so broad it allows them to permit virtually any employer or college to opt out of providing contraceptive coverage entirely, including for reasons as amorphous as vaguely defined moral beliefs,” he said during oral arguments. “Second, the agencies claim that RFRA, a statute that limits government action, affirmatively authorizes them to permit employers to deny women their rights to contraceptive coverage even in the absence of a RFRA violation in the first place.”
U.S. Supreme Court justices appear divided over whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraception mandate.
During oral arguments on May 6, the court expressed differing perspectives about the administration’s authority to allow for more exemptions under the health law’s birth control mandate and whether the expansions were reasonable. Justices heard the consolidated cases – Little Sisters of the Poor v. Pennsylvania and Trump v. Pennsylvania – by teleconference because of the COVID-19 pandemic. They are expected to make a decision by the summer.
Associate justice Ruth Bader Ginsburg, who participated in the telephone conference call from a hospital where she was recovering from a gallbladder condition, said the exemptions ignored the intent of Congress to provide women with comprehensive coverage through the ACA.
“The glaring feature of what the government has done in expanding this exemption is to toss to the winds entirely Congress’s instruction that women need and shall have seamless, no-cost, comprehensive coverage,” she said during oral arguments. “This leaves the women to hunt for other government programs that might cover them, and for those who are not covered by Medicaid or one of the other government programs, they can get contraceptive coverage only from paying out of their own pocket, which is exactly what Congress didn’t want to happen.”
Associate Justice Samuel Alito Jr., meanwhile, indicated that a lower court opinion that had blocked the exemptions from going forward conflicts with the Supreme Court’s ruling in a related case, Burwell v. Hobby Lobby.
“Explain to me why the Third Circuit’s analysis of the question of substantial burden is not squarely inconsistent with our reasoning in Hobby Lobby,” Associate Justice Alito said during oral arguments. “Hobby Lobby held that, if a person sincerely believes that it is immoral to perform an act that has the effect of enabling another person to commit an immoral act, a federal court does not have the right to say that this person is wrong on the question of moral complicity. That’s precisely the situation here. Reading the Third Circuit’s discussion of the substantial burden question, I wondered whether they had read that part of the Hobby Lobby decision.”
The dispute surrounding the ACA’s birth control mandate and the extent of exemptions afforded has gone on for a decade and has led to numerous legal challenges. The ACA initially required all employers to cover birth control for employees with no copayments, but exempted group health plans of religious employers. Those religious employers were primarily churches and other houses of worship. After a number of complaints and lawsuits, the Obama administration created a workaround for nonprofit religious employers not included in that exemption to opt out of the mandate. However, critics argued the process itself was a violation of their religious freedom.
The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved however, and in May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
In 2018, the Trump administration announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of “sincerely held religious beliefs.” A second rule allowed nonprofit organizations and small businesses that had nonreligious moral convictions against the mandate to opt out.
Thirteen states and the District of Columbia then sued the Trump administration over the rules, as well as Pennsylvania and New Jersey in a separate case. Little Sisters of the Poor, a religious nonprofit operating a home in Pittsburgh, intervened in the case as an aggrieved party. An appeal court temporarily barred the regulations from moving forward.
During oral arguments, Solicitor General for the Department of Justice Noel J. Francisco said the exemptions are lawful because they are authorized under a provision of the ACA as well as the Religious Freedom Restoration Act (RFRA).
“RFRA at the very least authorizes the religious exemption,” Mr. Francisco said during oral arguments.
Chief Deputy Attorney General for Pennsylvania Michael J. Fischer argued that the Trump administration’s moral and religious exemption rules rest on overly broad assertions of agency authority.
“First, the agencies twist a narrow delegation that allows the Health Resources and Services Administration to decide which preventive services insurers must cover under the Women’s Health Amendment into a grant of authority so broad it allows them to permit virtually any employer or college to opt out of providing contraceptive coverage entirely, including for reasons as amorphous as vaguely defined moral beliefs,” he said during oral arguments. “Second, the agencies claim that RFRA, a statute that limits government action, affirmatively authorizes them to permit employers to deny women their rights to contraceptive coverage even in the absence of a RFRA violation in the first place.”
U.S. Supreme Court justices appear divided over whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraception mandate.
During oral arguments on May 6, the court expressed differing perspectives about the administration’s authority to allow for more exemptions under the health law’s birth control mandate and whether the expansions were reasonable. Justices heard the consolidated cases – Little Sisters of the Poor v. Pennsylvania and Trump v. Pennsylvania – by teleconference because of the COVID-19 pandemic. They are expected to make a decision by the summer.
Associate justice Ruth Bader Ginsburg, who participated in the telephone conference call from a hospital where she was recovering from a gallbladder condition, said the exemptions ignored the intent of Congress to provide women with comprehensive coverage through the ACA.
“The glaring feature of what the government has done in expanding this exemption is to toss to the winds entirely Congress’s instruction that women need and shall have seamless, no-cost, comprehensive coverage,” she said during oral arguments. “This leaves the women to hunt for other government programs that might cover them, and for those who are not covered by Medicaid or one of the other government programs, they can get contraceptive coverage only from paying out of their own pocket, which is exactly what Congress didn’t want to happen.”
Associate Justice Samuel Alito Jr., meanwhile, indicated that a lower court opinion that had blocked the exemptions from going forward conflicts with the Supreme Court’s ruling in a related case, Burwell v. Hobby Lobby.
“Explain to me why the Third Circuit’s analysis of the question of substantial burden is not squarely inconsistent with our reasoning in Hobby Lobby,” Associate Justice Alito said during oral arguments. “Hobby Lobby held that, if a person sincerely believes that it is immoral to perform an act that has the effect of enabling another person to commit an immoral act, a federal court does not have the right to say that this person is wrong on the question of moral complicity. That’s precisely the situation here. Reading the Third Circuit’s discussion of the substantial burden question, I wondered whether they had read that part of the Hobby Lobby decision.”
The dispute surrounding the ACA’s birth control mandate and the extent of exemptions afforded has gone on for a decade and has led to numerous legal challenges. The ACA initially required all employers to cover birth control for employees with no copayments, but exempted group health plans of religious employers. Those religious employers were primarily churches and other houses of worship. After a number of complaints and lawsuits, the Obama administration created a workaround for nonprofit religious employers not included in that exemption to opt out of the mandate. However, critics argued the process itself was a violation of their religious freedom.
The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved however, and in May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
In 2018, the Trump administration announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of “sincerely held religious beliefs.” A second rule allowed nonprofit organizations and small businesses that had nonreligious moral convictions against the mandate to opt out.
Thirteen states and the District of Columbia then sued the Trump administration over the rules, as well as Pennsylvania and New Jersey in a separate case. Little Sisters of the Poor, a religious nonprofit operating a home in Pittsburgh, intervened in the case as an aggrieved party. An appeal court temporarily barred the regulations from moving forward.
During oral arguments, Solicitor General for the Department of Justice Noel J. Francisco said the exemptions are lawful because they are authorized under a provision of the ACA as well as the Religious Freedom Restoration Act (RFRA).
“RFRA at the very least authorizes the religious exemption,” Mr. Francisco said during oral arguments.
Chief Deputy Attorney General for Pennsylvania Michael J. Fischer argued that the Trump administration’s moral and religious exemption rules rest on overly broad assertions of agency authority.
“First, the agencies twist a narrow delegation that allows the Health Resources and Services Administration to decide which preventive services insurers must cover under the Women’s Health Amendment into a grant of authority so broad it allows them to permit virtually any employer or college to opt out of providing contraceptive coverage entirely, including for reasons as amorphous as vaguely defined moral beliefs,” he said during oral arguments. “Second, the agencies claim that RFRA, a statute that limits government action, affirmatively authorizes them to permit employers to deny women their rights to contraceptive coverage even in the absence of a RFRA violation in the first place.”
Do women treated with ceftriaxone and doxycycline for PID benefit from added metronidazole to broaden anaerobic coverage?
Wiesenfeld HC, Meyn LA, Darville T, et al. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis. February 13, 2020. doi:10.1093/cid/ciaa101.
EXPERT COMMENTARY
Pelvic inflammatory disease remains prevalent among young women and is commonly diagnosed in emergency departments and sexually transmitted disease (STD) clinics. This tubal infection is associated with significant reproductive sequelae, including tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. In addition, these women remain at risk for recurrent PID.
Bacterial vaginosis is present in more than half of women with PID. Not surprisingly, anaerobic microorganisms are more commonly isolated from the upper genital tract of patients with acute PID than either Neisseria gonorrhoeae or Chlamydia trachomatis, yet recommended antimicrobial regimens do not necessarily include antibiotics with an excellent antianaerobic spectrum.
Details of the study
In a randomized, double-blind, placebo-controlled trial, Wiesenfeld and colleagues enrolled women from hospital emergency departments or an STD clinic with symptoms of lower abdominal or pelvic pain associated with pelvic organ tenderness. The 233 study participants were randomly assigned to 2 treatment arms: ceftriaxone, doxycycline, and placebo (n = 117) or ceftriaxone, doxycycline, and metronidazole (n = 116).
Findings. Women treated with metronidazole were less likely to have pelvic organ tenderness a month after enrollment compared with the placebo group (9% vs 20%, respectively). Although the clinical cure rates at 30 days were statistically similar in both arms of the study, those receiving metronidazole had a 97% clinical cure rate while those not treated with metronidazole had a 90% clinical cure rate
(P = .38).
Moreover, the concurrent disorders of bacterial vaginosis and trichomonas vaginitis were more effectively treated in the metronidazole group, and fewer women had positive follow-up endometrial cultures for anaerobic bacteria compared with the placebo group (8% vs 21%, respectively).
The anticipated gastrointestinal adverse effects of a combination doxycycline-and-metronidazole regimen was a significant concern; however, combination therapy was no more likely to cause gastrointestinal adverse effects than doxycycline alone.
Continue to: Study strengths and limitations...
Study strengths and limitations
This well-designed randomized, double-blinded clinical trial was performed by clinical investigators experienced in the clinical diagnosis of PID. The demography of the population and their history of C trachomatis, N gonorrhoeae, plus the concurrent diagnosis of bacterial vaginosis make the diagnosis believable and real world, and these factors contribute to the generalizability of the study results.
However, PID is an imprecise clinical diagnosis (specificity averages 65%) when held to the gold standard of diagnostic laparoscopy to confirm the presence of acute salpingitis. Given the reticence of investigators and clinicians to embark on such an invasive procedure to confirm this diagnosis, endometrial biopsy showing evidence of histologic acute endometritis has been offered as an alternative gold standard. Confirmation of acute endometritis in the trial participants would have enhanced the validity of this study.
This study challenges a long held, but never proven, belief that the combination of doxycycline and metronidazole would be poorly tolerated as a combination antimicrobial regimen. It also further solidifies the role of anaerobic bacteria as major players in the microbial etiology of acute PID. In addition, it appears that treating bacterial vaginosis concurrently may lessen the likelihood of endometrial recolonization with anaerobic bacteria. ●
Metronidazole should be added routinely to the standard antibiotic regimen of ceftriaxone and doxycycline for the treatment of women with PID.
DAVID E. SOPER, MD
Wiesenfeld HC, Meyn LA, Darville T, et al. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis. February 13, 2020. doi:10.1093/cid/ciaa101.
EXPERT COMMENTARY
Pelvic inflammatory disease remains prevalent among young women and is commonly diagnosed in emergency departments and sexually transmitted disease (STD) clinics. This tubal infection is associated with significant reproductive sequelae, including tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. In addition, these women remain at risk for recurrent PID.
Bacterial vaginosis is present in more than half of women with PID. Not surprisingly, anaerobic microorganisms are more commonly isolated from the upper genital tract of patients with acute PID than either Neisseria gonorrhoeae or Chlamydia trachomatis, yet recommended antimicrobial regimens do not necessarily include antibiotics with an excellent antianaerobic spectrum.
Details of the study
In a randomized, double-blind, placebo-controlled trial, Wiesenfeld and colleagues enrolled women from hospital emergency departments or an STD clinic with symptoms of lower abdominal or pelvic pain associated with pelvic organ tenderness. The 233 study participants were randomly assigned to 2 treatment arms: ceftriaxone, doxycycline, and placebo (n = 117) or ceftriaxone, doxycycline, and metronidazole (n = 116).
Findings. Women treated with metronidazole were less likely to have pelvic organ tenderness a month after enrollment compared with the placebo group (9% vs 20%, respectively). Although the clinical cure rates at 30 days were statistically similar in both arms of the study, those receiving metronidazole had a 97% clinical cure rate while those not treated with metronidazole had a 90% clinical cure rate
(P = .38).
Moreover, the concurrent disorders of bacterial vaginosis and trichomonas vaginitis were more effectively treated in the metronidazole group, and fewer women had positive follow-up endometrial cultures for anaerobic bacteria compared with the placebo group (8% vs 21%, respectively).
The anticipated gastrointestinal adverse effects of a combination doxycycline-and-metronidazole regimen was a significant concern; however, combination therapy was no more likely to cause gastrointestinal adverse effects than doxycycline alone.
Continue to: Study strengths and limitations...
Study strengths and limitations
This well-designed randomized, double-blinded clinical trial was performed by clinical investigators experienced in the clinical diagnosis of PID. The demography of the population and their history of C trachomatis, N gonorrhoeae, plus the concurrent diagnosis of bacterial vaginosis make the diagnosis believable and real world, and these factors contribute to the generalizability of the study results.
However, PID is an imprecise clinical diagnosis (specificity averages 65%) when held to the gold standard of diagnostic laparoscopy to confirm the presence of acute salpingitis. Given the reticence of investigators and clinicians to embark on such an invasive procedure to confirm this diagnosis, endometrial biopsy showing evidence of histologic acute endometritis has been offered as an alternative gold standard. Confirmation of acute endometritis in the trial participants would have enhanced the validity of this study.
This study challenges a long held, but never proven, belief that the combination of doxycycline and metronidazole would be poorly tolerated as a combination antimicrobial regimen. It also further solidifies the role of anaerobic bacteria as major players in the microbial etiology of acute PID. In addition, it appears that treating bacterial vaginosis concurrently may lessen the likelihood of endometrial recolonization with anaerobic bacteria. ●
Metronidazole should be added routinely to the standard antibiotic regimen of ceftriaxone and doxycycline for the treatment of women with PID.
DAVID E. SOPER, MD
Wiesenfeld HC, Meyn LA, Darville T, et al. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis. February 13, 2020. doi:10.1093/cid/ciaa101.
EXPERT COMMENTARY
Pelvic inflammatory disease remains prevalent among young women and is commonly diagnosed in emergency departments and sexually transmitted disease (STD) clinics. This tubal infection is associated with significant reproductive sequelae, including tubal factor infertility, ectopic pregnancy, and chronic pelvic pain. In addition, these women remain at risk for recurrent PID.
Bacterial vaginosis is present in more than half of women with PID. Not surprisingly, anaerobic microorganisms are more commonly isolated from the upper genital tract of patients with acute PID than either Neisseria gonorrhoeae or Chlamydia trachomatis, yet recommended antimicrobial regimens do not necessarily include antibiotics with an excellent antianaerobic spectrum.
Details of the study
In a randomized, double-blind, placebo-controlled trial, Wiesenfeld and colleagues enrolled women from hospital emergency departments or an STD clinic with symptoms of lower abdominal or pelvic pain associated with pelvic organ tenderness. The 233 study participants were randomly assigned to 2 treatment arms: ceftriaxone, doxycycline, and placebo (n = 117) or ceftriaxone, doxycycline, and metronidazole (n = 116).
Findings. Women treated with metronidazole were less likely to have pelvic organ tenderness a month after enrollment compared with the placebo group (9% vs 20%, respectively). Although the clinical cure rates at 30 days were statistically similar in both arms of the study, those receiving metronidazole had a 97% clinical cure rate while those not treated with metronidazole had a 90% clinical cure rate
(P = .38).
Moreover, the concurrent disorders of bacterial vaginosis and trichomonas vaginitis were more effectively treated in the metronidazole group, and fewer women had positive follow-up endometrial cultures for anaerobic bacteria compared with the placebo group (8% vs 21%, respectively).
The anticipated gastrointestinal adverse effects of a combination doxycycline-and-metronidazole regimen was a significant concern; however, combination therapy was no more likely to cause gastrointestinal adverse effects than doxycycline alone.
Continue to: Study strengths and limitations...
Study strengths and limitations
This well-designed randomized, double-blinded clinical trial was performed by clinical investigators experienced in the clinical diagnosis of PID. The demography of the population and their history of C trachomatis, N gonorrhoeae, plus the concurrent diagnosis of bacterial vaginosis make the diagnosis believable and real world, and these factors contribute to the generalizability of the study results.
However, PID is an imprecise clinical diagnosis (specificity averages 65%) when held to the gold standard of diagnostic laparoscopy to confirm the presence of acute salpingitis. Given the reticence of investigators and clinicians to embark on such an invasive procedure to confirm this diagnosis, endometrial biopsy showing evidence of histologic acute endometritis has been offered as an alternative gold standard. Confirmation of acute endometritis in the trial participants would have enhanced the validity of this study.
This study challenges a long held, but never proven, belief that the combination of doxycycline and metronidazole would be poorly tolerated as a combination antimicrobial regimen. It also further solidifies the role of anaerobic bacteria as major players in the microbial etiology of acute PID. In addition, it appears that treating bacterial vaginosis concurrently may lessen the likelihood of endometrial recolonization with anaerobic bacteria. ●
Metronidazole should be added routinely to the standard antibiotic regimen of ceftriaxone and doxycycline for the treatment of women with PID.
DAVID E. SOPER, MD
Telemedicine: A primer for today’s ObGyn
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
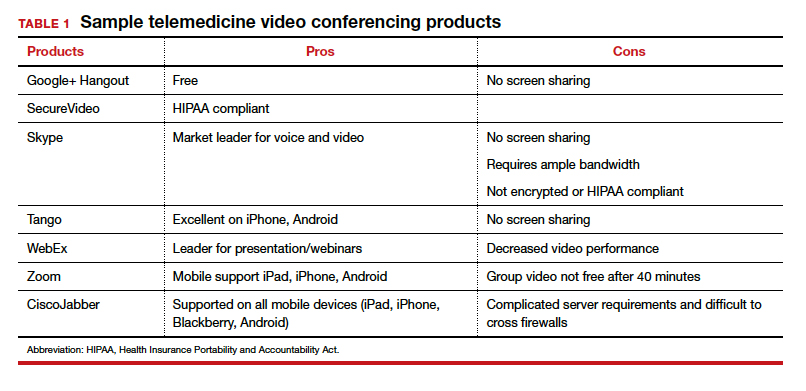
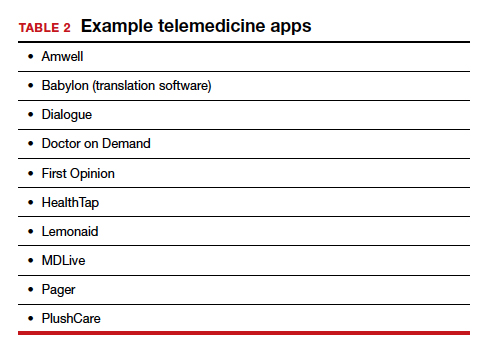
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.
Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
If telemedicine had not yet begun to play a significant role in your ObGyn practice, it is almost certain to now as the COVID-19 pandemic demands new ways of caring for our patients while keeping others safe from disease. According to the American College of Obstetricians and Gynecologists (ACOG), the term “telemedicine” refers to delivering traditional clinical diagnosis and monitoring via technology (see “ACOG weighs in on telehealth”).1
Whether they realize it or not, most ObGyns have practiced a simple form of telemedicine when they take phone calls from patients who are seeking medication refills. In these cases, physicians either can call the pharmacy to refill the medication or suggest patients make an office appointment to receive a new prescription (much to the chagrin of many patients—especially millennials). Physicians who acquiesce to patients’ phone requests to have prescriptions filled or to others seeking free medical advice are not compensated for these services, yet are legally responsible for their actions and advice—a situation that does not make for good medicine.
This is where telemedicine can be an important addition to an ObGyn practice. Telemedicine saves the patient the time and effort of coming to the office, while providing compensation to the physician for his/her time and advice and providing a record of the interaction, all of which makes for far better medicine. This article—the first of 3 on the subject—discusses the process of integrating telemedicine into a practice with minimal time, energy, and expense.
Telemedicine and the ObGyn practice
Many ObGyn patients do not require an in-person visit in order to receive effective care. There is even the potential to provide prenatal care via telemedicine by replacing some of the many prenatal well-care office visits with at-home care for pregnant women with low-risk pregnancies. A typical virtual visit for a low-risk pregnancy includes utilizing home monitoring equipment to track fetal heart rate, maternal blood pressure, and fundal height.2
Practices typically use telemedicine platforms to manage one or both of the following types of encounters: 1) walk-in visits through the practice’s web site; for most of these, patients tend not to care which physicians they see; their priority is usually the first available provider; and 2) appointment-based consultations, where patients schedule video chats in advance, usually with a specific provider.
Although incorporating telemedicine into a practice may seem overwhelming, it requires minimal additional equipment, interfaces easily with a practice’s web site and electronic medical record (EMR) system, increases productivity, and improves workflow. And patients generally appreciate the option of not having to travel to the office for an appointment.
Most patients and physicians are already comfortable with their mobile phones, tablets, social media, and wearable technology, such as Fitbits. Telemedicine is a logical next step. And given the current situation with COVID-19, it is really not a matter of “if,” but rather “when” to incorporate telemedicine as a communication and practice tool, and the sooner the better.
Continue to: Getting started...
Getting started
Physicians and their colleagues and staff first need to become comfortable with telemedicine technology. Physicians can begin by using video communication for other purposes, such as for conducting staff meetings. They should practice starting and ending calls and adjusting audio volume and video quality to ensure good reception.
Selecting a video platform
TABLE 1 provides a list of the most popular video providers and the advantages and disadvantages of each, and TABLE 2 shows a list of free video chat apps. Apps are available that can:
- share and mark up lab tests, magnetic resonance images, and other medical documents without exposing the entire desktop
- securely send documents over a Health Insurance Portability and Accountability Act (HIPAA)-compliant video
- stream digital device images live while still seeing patients’ faces.


Physicians should make sure their implementation team has the necessary equipment, including webcams, microphones, and speakers, and they should take the time to do research and test out a few programs before selecting one for their practice. Consider appointing a telemedicine point person who is knowledgeable about the technology and can patiently explain it to others. And keep in mind that video chatting is dependent upon a fast, strong Internet connection that has sufficient bandwidth to transport a large amount of data. If your practice has connectivity problems, consider consulting with an information technology (IT) expert.
Testing it out and obtaining feedback
Once a team is comfortable using video within the practice, it is time to test it out with a few patients and perhaps a few payers. Most patients are eager to start using video for their medical encounters. Even senior patients are often willing to try consults via video. According to a recent survey, 64% of patients are willing to see a physician over video.3 And among those who were comfortable accepting an invitation to participate in a video encounter, increasing age was actually associated with a higher likelihood to accept an invite.
Physician colleagues, medical assistants, and nurse practitioners will need some basic telemedicine skills, and physicians and staff should be prepared to make video connections seamless for patients. Usually, patients need some guidance and encouragement, such as telling them to check their spam folder for their invites if the invites fail to arrive in their email inbox, adjusting audio settings, or setting up a webcam. In the beginning, ObGyns should make sure they build in plenty of buffer time for the unexpected, as there will certainly be some “bugs” that need to be worked out.
ObGyns should encourage and collect patient feedback to such questions as:
- What kinds of devices (laptop, mobile) do they prefer using?
- What kind of networks are they using (3G, corporate, home)?
- What features do they like? What features do they have a hard time finding?
- What do they like or not like about the video experience?
- Keep track of the types of questions patients ask, and be patient as patients become acclimated to the video consultation experience.
Continue to: Streamlining online workflow...
Streamlining online workflow
Armed with feedback from patients, it is time to start streamlining online workflow. Most ObGyns want to be able to manage video visits in a way that is similar to the way they manage face-to-face visits with patients. This may mean experimenting with a virtual waiting room. A virtual waiting room is a simple web page or link that can be sent to patients. On that page, patients sign in with minimal demographic information and select one of the time slots when the physician is available. Typically, these programs are designed to alert the physicians and/or staff when a patient enters the virtual waiting room. Patients have access to the online patient queue and can start a chat or video call when both parties are ready. Such a waiting room model serves as a stepping stone for new practices to familiarize themselves with video conferencing. This approach is also perfect for practices that already have a practice management system and just want to add a video component.
Influences on practice workflow
With good time management, telemedicine can improve the efficiency and productivity of your practice. Your daily schedule and management of patients will need some minor changes, but significant alterations to your existing schedule and workflow are generally unnecessary. One of the advantages of telemedicine is the convenience of prompt care and the easy access patients have to your practice. This decreases visits to the emergency department and to urgent care centers.
Consider scheduling telemedicine appointments at the end of the day when your staff has left the office, as no staff members are required for a telemedicine visit. Ideally, you should offer a set time to communicate with patients, as this avoids having to make multiple calls to reach a patient. Another advantage of telemedicine is that you can provide care in the evenings and on weekends if you want. Whereas before you might have been fielding calls from patients during these times and not being compensated, with telemedicine you can conduct a virtual visit from any location and any computer or mobile phone and receive remuneration for your care.
And while access to care has been a problem in many ObGyn practices, many additional patients can be accommodated into a busy ObGyn practice by using telemedicine.
Telemedicine and the coronavirus
The current health care crisis makes implementing telemedicine essential. Patients who think they may have COVID-19 or who have been diagnosed need to be quarantined. Such patients can be helped safely in the comfort of their own homes without endangering others. Patients can be triaged virtually. All those who are febrile or have respiratory symptoms can continue to avail themselves of virtual visits.
According to reports in the media, COVID-19 is stretching the health care workforce to its limits and creating a shortage, both because of the sheer number of cases and because health care workers are getting sick themselves. Physicians who test positive do not have to be completely removed from the workforce if they have the ability to care for patients remotely from their homes. And not incidentally the new environment has prompted the Centers for Medicaid and Medicare Services (CMS) and private payers to initiate national payment policies that create parity between office and telemedicine visits.4
Continue to: Bottom line...
Bottom line
Patient-driven care is the future, and telemedicine is part of that. Patients want to have ready access to their health care providers without having to devote hours to a medical encounter that could be completed in a matter of minutes via telemedicine.
In the next article in this series, we will review the proper coding for a telemedicine visit so that appropriate compensation is gleaned. We will also review the barriers to implementing telemedicine visits. The third article is written with the assistance of 2 health care attorneys, Anjali Dooley and Nadia de la Houssaye, who are experts in telemedicine and who have helped dozens of practices and hospitals implement the technology. They provide legal guidelines for ObGyns who are considering adding telemedicine to their practice. ●
The American College of Obstetricians and Gynecologists (ACOG) encourages all practices and facilities without telemedicine capabilities “to strategize about how telehealth could be integrated into their services as appropriate.”1 In doing so, they also encourage consideration of ways to care for those who may not have access to such technology or who do not know how to use it. They also explain that a number of federal telehealth policy changes have been made in response to the COVID-19 pandemic, and that most private health insurers are following suit.2 Such changes include:
- covering all telehealth visits for all traditional Medicare beneficiaries regardless of geographic location or originating site
- not requiring physicians to have a pre-existing relationship with a patient to provide a telehealth visit
- permitting the use of FaceTime, Skype, and other everyday communication technologies to provide telehealth visits.
A summary of the major telehealth policy changes, as well as information on how to code and bill for telehealth visits can be found at https://www.acog.org/clinical-information/physician-faqs/~/link .aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z.
References
- American College of Obstetricians and Gynecologists. COVID-19 FAQs for obstetriciangynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19faqs-for-ob-gyns-gynecology. Accessed April 8, 2020.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org/clinicalinformation/physician-faqs/~/link.aspx?_id=3803296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
- Implementing telehealth in practice. ACOG Committee Opinion. February 2020. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2020/02 /implementing-telehealth-in-practice. Accessed April 6, 2020.
- de Mooij MJM, Hodny RL, O’Neil DA, et al. OB nest: reimagining low-risk prenatal care. Mayo Clin Proc. 2018;93:458-466.
- Gardner MR, Jenkins SM, O’Neil DA, et al. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21:281-285.
- American College of Obstetricians and Gynecologists. Managing patients remotely: billing for digital and telehealth services. Updated April 2, 2020. https://www.acog.org /clinical-information/physician-faqs/~/link.aspx?_id=380 3296EAAD940C69525D4DD2679A00E&_z=z. Accessed April 8, 2020.
COVID-19 apps for the ObGyn health care provider

In the midst of the coronavirus disease 2019 (COVID-19) pandemic, health care providers, including ObGyns, need up-to-date information to keep pace with the ever-changing health care crisis. Literature regarding obstetric populations is emerging in journals.1,2 General guidance in the management of COVID-19–positive patients may also be helpful to the ObGyn provider. Although scientific journals are now publishing COVID-19 research at warp speed, those same journals tend to be too specialized for general readers.3 Mobile apps may make the information more accessible.
This app review focuses on 3 apps that provide information about the ongoing COVID-19 pandemic and detail general guidance for treatment of COVID-19–positive patients. An initial search in early April 2020 of major national health care organizations and ObGyn-specific organizational apps yielded the Centers for Disease Control and Prevention (CDC) app. A subsequent search in the app stores using the term “COVID” yielded 2 additional apps: the Osler COVID Learning Centre app and the Relief Central app.
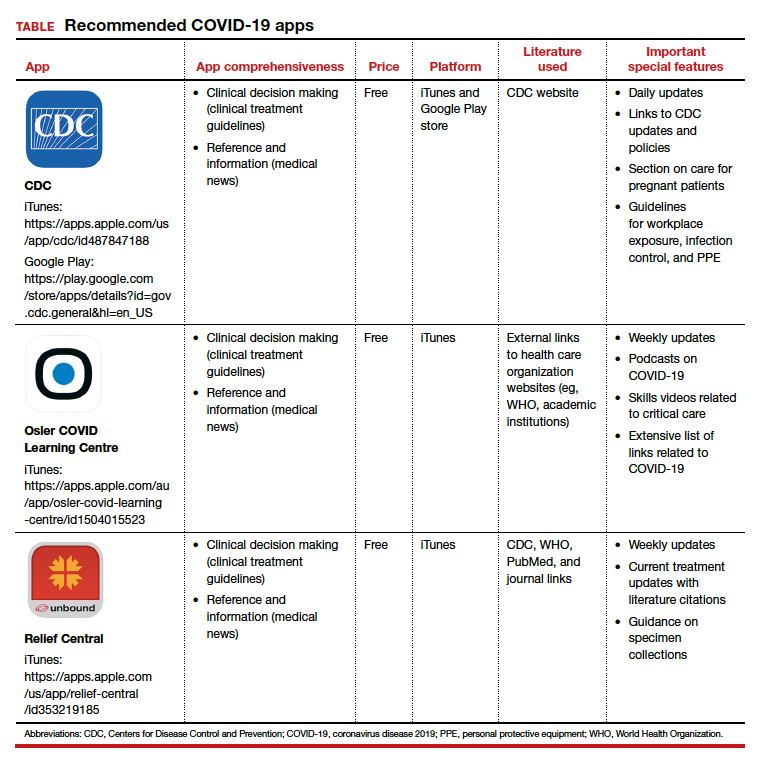
The CDC app contains a COVID-19-specific section that highlights pertinent information for health care providers as well as a section on caring for the obstetric patient. The Osler app includes podcasts and videos on critical care for noncritical care providers. Finally, the Relief Central app contains updated information on screening and treatment for COVID-19. The TABLE features details of the 3 apps.
Each app is evaluated based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 ●
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi:10.1016/j.ajog.2020.02.017.
- Dashraath P, Jing Lin Jeslyn W, Mei Xian Karen L, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. March 23, 2020. doi:10.1016/j.ajog.2020.03.021.
- Tingley K. Coronavirus is forcing medical research to speed up. New York Times Magazine. April 26, 2020:16-18.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.

In the midst of the coronavirus disease 2019 (COVID-19) pandemic, health care providers, including ObGyns, need up-to-date information to keep pace with the ever-changing health care crisis. Literature regarding obstetric populations is emerging in journals.1,2 General guidance in the management of COVID-19–positive patients may also be helpful to the ObGyn provider. Although scientific journals are now publishing COVID-19 research at warp speed, those same journals tend to be too specialized for general readers.3 Mobile apps may make the information more accessible.
This app review focuses on 3 apps that provide information about the ongoing COVID-19 pandemic and detail general guidance for treatment of COVID-19–positive patients. An initial search in early April 2020 of major national health care organizations and ObGyn-specific organizational apps yielded the Centers for Disease Control and Prevention (CDC) app. A subsequent search in the app stores using the term “COVID” yielded 2 additional apps: the Osler COVID Learning Centre app and the Relief Central app.
The CDC app contains a COVID-19-specific section that highlights pertinent information for health care providers as well as a section on caring for the obstetric patient. The Osler app includes podcasts and videos on critical care for noncritical care providers. Finally, the Relief Central app contains updated information on screening and treatment for COVID-19. The TABLE features details of the 3 apps.

Each app is evaluated based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 ●

In the midst of the coronavirus disease 2019 (COVID-19) pandemic, health care providers, including ObGyns, need up-to-date information to keep pace with the ever-changing health care crisis. Literature regarding obstetric populations is emerging in journals.1,2 General guidance in the management of COVID-19–positive patients may also be helpful to the ObGyn provider. Although scientific journals are now publishing COVID-19 research at warp speed, those same journals tend to be too specialized for general readers.3 Mobile apps may make the information more accessible.
This app review focuses on 3 apps that provide information about the ongoing COVID-19 pandemic and detail general guidance for treatment of COVID-19–positive patients. An initial search in early April 2020 of major national health care organizations and ObGyn-specific organizational apps yielded the Centers for Disease Control and Prevention (CDC) app. A subsequent search in the app stores using the term “COVID” yielded 2 additional apps: the Osler COVID Learning Centre app and the Relief Central app.
The CDC app contains a COVID-19-specific section that highlights pertinent information for health care providers as well as a section on caring for the obstetric patient. The Osler app includes podcasts and videos on critical care for noncritical care providers. Finally, the Relief Central app contains updated information on screening and treatment for COVID-19. The TABLE features details of the 3 apps.

Each app is evaluated based on a shortened version of the APPLICATIONS scoring system, APPLI (app comprehensiveness, price, platform, literature use, and important special features).4 ●
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi:10.1016/j.ajog.2020.02.017.
- Dashraath P, Jing Lin Jeslyn W, Mei Xian Karen L, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. March 23, 2020. doi:10.1016/j.ajog.2020.03.021.
- Tingley K. Coronavirus is forcing medical research to speed up. New York Times Magazine. April 26, 2020:16-18.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi:10.1016/j.ajog.2020.02.017.
- Dashraath P, Jing Lin Jeslyn W, Mei Xian Karen L, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. March 23, 2020. doi:10.1016/j.ajog.2020.03.021.
- Tingley K. Coronavirus is forcing medical research to speed up. New York Times Magazine. April 26, 2020:16-18.
- Chyjek K, Farag S, Chen KT. Rating pregnancy wheel applications using the APPLICATIONS scoring system. Obstet Gynecol. 2015;125:1478-1483.
COVID-19 and pregnancy: Is miscarriage a risk?

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020
Ob.gyns., peds, other PCPs seeking COVID-19 financial relief from feds
A handful of specialties – including family medicine, obstetrics/gynecology, pediatrics, and other primary care specialties – are calling for targeted and urgent relief payments from the federal government, saying that they have been left out of distributions aimed at alleviating the financial fallout associated with the novel coronavirus.
The federal government has already distributed about $150 billion – through direct payments and advances on reimbursement – to clinicians, but, to date, the money has only been given to providers who bill Medicare, and not even all of those individuals have received payments.
“It is critical that frontline physicians who may not participate in Medicare fee-for-service, in whole or in part, including obstetrician/gynecologists, pediatricians, and family physicians, have the resources they need to continue providing essential health care to patients amid the pandemic and in the months to come,” said the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists in a letter to Health & Human Services (Secretary Alex Azar.
In particular, the organizations are concerned that no money has been distributed or earmarked for clinicians who serve Medicaid recipients.
“The organizations that signed that letter are the primary providers of care to the Medicaid population,” Shawn Martin, senior VP for the AAFP, said in an interview. That’s true even for family physicians.
“Typically, in an average family medicine practice, their Medicaid panel size is equal to if not greater than the Medicare panel size,” he said.
On April 23, Mr. Azar said HHS was working on a distribution plan for providers who only take Medicaid, as well as for dentists and skilled nursing facilities. An HHS spokesperson confirmed that the agency still intends to provide money to those groups of providers and that the agency is committed to distributing funds quickly and with transparency.
Mr. Azar had also announced that the government would soon start distributing $20 billion in payments to Medicare providers, on top of the $30 billion that had already been handed out to clinicians on April 10 and 17.
That $50 billion came from the COVID-19–related $100 billion Provider Relief Fund, which was part of the Coronavirus Aid, Relief, and Economic Security Act, signed into law on March 27.
Additionally, the Centers for Medicare & Medicaid Services had distributed some $100 billion to providers who participated in Medicare Part A or B through the Medicare Advance Payment program, which is a deferred loan. The agency brought that program to a halt on April 27.
An additional $75 billion will now be available through the Public Health and Social Services Emergency Fund (PHSSEF) as part of the third congressional COVID relief package, signed into law on April 24.
Mr. Martin said that the AAFP and other physician organizations have been talking with HHS about how to distribute money from that new pool of funds. “There’s been a lot of progress, but there hasn’t been any action,” he said, adding that the purpose of the joint letter to HHS “is to say it’s time for action.”
COVID-19 damage
AAFP, AAP, and ACOG noted in the letter the damage that’s being inflicted by COVID-19. They cited data that show a 50% decline in measles, mumps, and rubella shots, a 42% drop in diphtheria and whooping cough vaccinations, and a 73% decline in human papillomavirus shots. The groups also noted a rise in child abuse injuries that are being seen in EDs and the potential for a worsening of the maternal mortality crisis in the United States.
Primary care physicians are also the go-to doctors for upper respiratory infections, noted the groups in the letter.
“Put simply, our physician members need to be able to keep their doors open and continue treating patients,” said the groups.
A study by Harvard University and Phreesia, a health care technology company, found that ambulatory practice visits had declined by at least half since early February, with a 71% drop in visits by 7- to 17-year-olds and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Research conducted by the Physicians Foundation and Merritt Hawkins shows that 21% of 842 physicians who responded to an early April survey said they’d been furloughed or been given a pay cut. That number rose to 30% among doctors who are not treating COVID-19 patients.
Although the majority in the survey (66%) said they planned to keep practicing in the same manner during the pandemic, 32% said they planned to change practices, opt out of patient care roles, close their practices temporarily, or retire. The survey has a margin of error of ±3.5%.
Internists seek consideration, too
The American College of Physicians also has urged HHS to give special consideration to its members. The group wrote to Mr. Azar on April 28, recommending that payments from the new $75 billion PHSSEF be prioritized for primary care, as well as for smaller practices, those that provide care in underserved areas, and internal medicine subspecialty practices.
“Internal medicine specialists and other primary care physicians have an essential role in delivering primary, preventive, and comprehensive care not only to patients with symptoms or diagnoses of COVID-19, but also to patients with other underlying medical conditions, including conditions like heart disease and diabetes that put them at greater risk of mortality from COVID-19,” wrote ACP President Jacqueline Fincher, MD, MACP.
ACP said the government could pay physicians on the basis of the amount of additional expenses incurred that were related to COVID-19, such as extra staffing or temporary relocation of their place of residence to prevent exposing family members to the virus. Pay should also be based on the percentage of revenue losses from all payers, including Medicare, Medicaid, and commercial insurers, Dr. Fincher said in the letter.
AAFP, AAP, and ACOG also had a suggestion for distributing payments to non-Medicare providers. “Given that most women’s health, pediatric, and family practices have received less financial relief to date, we recommend that HHS provide these practices with a larger proportion of funds relative to their reported revenue than is provided on average across specialties,” they wrote.
A version of this article originally appeared on Medscape.com.
A handful of specialties – including family medicine, obstetrics/gynecology, pediatrics, and other primary care specialties – are calling for targeted and urgent relief payments from the federal government, saying that they have been left out of distributions aimed at alleviating the financial fallout associated with the novel coronavirus.
The federal government has already distributed about $150 billion – through direct payments and advances on reimbursement – to clinicians, but, to date, the money has only been given to providers who bill Medicare, and not even all of those individuals have received payments.
“It is critical that frontline physicians who may not participate in Medicare fee-for-service, in whole or in part, including obstetrician/gynecologists, pediatricians, and family physicians, have the resources they need to continue providing essential health care to patients amid the pandemic and in the months to come,” said the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists in a letter to Health & Human Services (Secretary Alex Azar.
In particular, the organizations are concerned that no money has been distributed or earmarked for clinicians who serve Medicaid recipients.
“The organizations that signed that letter are the primary providers of care to the Medicaid population,” Shawn Martin, senior VP for the AAFP, said in an interview. That’s true even for family physicians.
“Typically, in an average family medicine practice, their Medicaid panel size is equal to if not greater than the Medicare panel size,” he said.
On April 23, Mr. Azar said HHS was working on a distribution plan for providers who only take Medicaid, as well as for dentists and skilled nursing facilities. An HHS spokesperson confirmed that the agency still intends to provide money to those groups of providers and that the agency is committed to distributing funds quickly and with transparency.
Mr. Azar had also announced that the government would soon start distributing $20 billion in payments to Medicare providers, on top of the $30 billion that had already been handed out to clinicians on April 10 and 17.
That $50 billion came from the COVID-19–related $100 billion Provider Relief Fund, which was part of the Coronavirus Aid, Relief, and Economic Security Act, signed into law on March 27.
Additionally, the Centers for Medicare & Medicaid Services had distributed some $100 billion to providers who participated in Medicare Part A or B through the Medicare Advance Payment program, which is a deferred loan. The agency brought that program to a halt on April 27.
An additional $75 billion will now be available through the Public Health and Social Services Emergency Fund (PHSSEF) as part of the third congressional COVID relief package, signed into law on April 24.
Mr. Martin said that the AAFP and other physician organizations have been talking with HHS about how to distribute money from that new pool of funds. “There’s been a lot of progress, but there hasn’t been any action,” he said, adding that the purpose of the joint letter to HHS “is to say it’s time for action.”
COVID-19 damage
AAFP, AAP, and ACOG noted in the letter the damage that’s being inflicted by COVID-19. They cited data that show a 50% decline in measles, mumps, and rubella shots, a 42% drop in diphtheria and whooping cough vaccinations, and a 73% decline in human papillomavirus shots. The groups also noted a rise in child abuse injuries that are being seen in EDs and the potential for a worsening of the maternal mortality crisis in the United States.
Primary care physicians are also the go-to doctors for upper respiratory infections, noted the groups in the letter.
“Put simply, our physician members need to be able to keep their doors open and continue treating patients,” said the groups.
A study by Harvard University and Phreesia, a health care technology company, found that ambulatory practice visits had declined by at least half since early February, with a 71% drop in visits by 7- to 17-year-olds and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Research conducted by the Physicians Foundation and Merritt Hawkins shows that 21% of 842 physicians who responded to an early April survey said they’d been furloughed or been given a pay cut. That number rose to 30% among doctors who are not treating COVID-19 patients.
Although the majority in the survey (66%) said they planned to keep practicing in the same manner during the pandemic, 32% said they planned to change practices, opt out of patient care roles, close their practices temporarily, or retire. The survey has a margin of error of ±3.5%.
Internists seek consideration, too
The American College of Physicians also has urged HHS to give special consideration to its members. The group wrote to Mr. Azar on April 28, recommending that payments from the new $75 billion PHSSEF be prioritized for primary care, as well as for smaller practices, those that provide care in underserved areas, and internal medicine subspecialty practices.
“Internal medicine specialists and other primary care physicians have an essential role in delivering primary, preventive, and comprehensive care not only to patients with symptoms or diagnoses of COVID-19, but also to patients with other underlying medical conditions, including conditions like heart disease and diabetes that put them at greater risk of mortality from COVID-19,” wrote ACP President Jacqueline Fincher, MD, MACP.
ACP said the government could pay physicians on the basis of the amount of additional expenses incurred that were related to COVID-19, such as extra staffing or temporary relocation of their place of residence to prevent exposing family members to the virus. Pay should also be based on the percentage of revenue losses from all payers, including Medicare, Medicaid, and commercial insurers, Dr. Fincher said in the letter.
AAFP, AAP, and ACOG also had a suggestion for distributing payments to non-Medicare providers. “Given that most women’s health, pediatric, and family practices have received less financial relief to date, we recommend that HHS provide these practices with a larger proportion of funds relative to their reported revenue than is provided on average across specialties,” they wrote.
A version of this article originally appeared on Medscape.com.
A handful of specialties – including family medicine, obstetrics/gynecology, pediatrics, and other primary care specialties – are calling for targeted and urgent relief payments from the federal government, saying that they have been left out of distributions aimed at alleviating the financial fallout associated with the novel coronavirus.
The federal government has already distributed about $150 billion – through direct payments and advances on reimbursement – to clinicians, but, to date, the money has only been given to providers who bill Medicare, and not even all of those individuals have received payments.
“It is critical that frontline physicians who may not participate in Medicare fee-for-service, in whole or in part, including obstetrician/gynecologists, pediatricians, and family physicians, have the resources they need to continue providing essential health care to patients amid the pandemic and in the months to come,” said the American Academy of Family Physicians, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists in a letter to Health & Human Services (Secretary Alex Azar.
In particular, the organizations are concerned that no money has been distributed or earmarked for clinicians who serve Medicaid recipients.
“The organizations that signed that letter are the primary providers of care to the Medicaid population,” Shawn Martin, senior VP for the AAFP, said in an interview. That’s true even for family physicians.
“Typically, in an average family medicine practice, their Medicaid panel size is equal to if not greater than the Medicare panel size,” he said.
On April 23, Mr. Azar said HHS was working on a distribution plan for providers who only take Medicaid, as well as for dentists and skilled nursing facilities. An HHS spokesperson confirmed that the agency still intends to provide money to those groups of providers and that the agency is committed to distributing funds quickly and with transparency.
Mr. Azar had also announced that the government would soon start distributing $20 billion in payments to Medicare providers, on top of the $30 billion that had already been handed out to clinicians on April 10 and 17.
That $50 billion came from the COVID-19–related $100 billion Provider Relief Fund, which was part of the Coronavirus Aid, Relief, and Economic Security Act, signed into law on March 27.
Additionally, the Centers for Medicare & Medicaid Services had distributed some $100 billion to providers who participated in Medicare Part A or B through the Medicare Advance Payment program, which is a deferred loan. The agency brought that program to a halt on April 27.
An additional $75 billion will now be available through the Public Health and Social Services Emergency Fund (PHSSEF) as part of the third congressional COVID relief package, signed into law on April 24.
Mr. Martin said that the AAFP and other physician organizations have been talking with HHS about how to distribute money from that new pool of funds. “There’s been a lot of progress, but there hasn’t been any action,” he said, adding that the purpose of the joint letter to HHS “is to say it’s time for action.”
COVID-19 damage
AAFP, AAP, and ACOG noted in the letter the damage that’s being inflicted by COVID-19. They cited data that show a 50% decline in measles, mumps, and rubella shots, a 42% drop in diphtheria and whooping cough vaccinations, and a 73% decline in human papillomavirus shots. The groups also noted a rise in child abuse injuries that are being seen in EDs and the potential for a worsening of the maternal mortality crisis in the United States.
Primary care physicians are also the go-to doctors for upper respiratory infections, noted the groups in the letter.
“Put simply, our physician members need to be able to keep their doors open and continue treating patients,” said the groups.
A study by Harvard University and Phreesia, a health care technology company, found that ambulatory practice visits had declined by at least half since early February, with a 71% drop in visits by 7- to 17-year-olds and a 59% decline in visits by neonates, infants, and toddlers (up to age 6). Overall, pediatric practices experienced a 62% drop-off in visits.
Research conducted by the Physicians Foundation and Merritt Hawkins shows that 21% of 842 physicians who responded to an early April survey said they’d been furloughed or been given a pay cut. That number rose to 30% among doctors who are not treating COVID-19 patients.
Although the majority in the survey (66%) said they planned to keep practicing in the same manner during the pandemic, 32% said they planned to change practices, opt out of patient care roles, close their practices temporarily, or retire. The survey has a margin of error of ±3.5%.
Internists seek consideration, too
The American College of Physicians also has urged HHS to give special consideration to its members. The group wrote to Mr. Azar on April 28, recommending that payments from the new $75 billion PHSSEF be prioritized for primary care, as well as for smaller practices, those that provide care in underserved areas, and internal medicine subspecialty practices.
“Internal medicine specialists and other primary care physicians have an essential role in delivering primary, preventive, and comprehensive care not only to patients with symptoms or diagnoses of COVID-19, but also to patients with other underlying medical conditions, including conditions like heart disease and diabetes that put them at greater risk of mortality from COVID-19,” wrote ACP President Jacqueline Fincher, MD, MACP.
ACP said the government could pay physicians on the basis of the amount of additional expenses incurred that were related to COVID-19, such as extra staffing or temporary relocation of their place of residence to prevent exposing family members to the virus. Pay should also be based on the percentage of revenue losses from all payers, including Medicare, Medicaid, and commercial insurers, Dr. Fincher said in the letter.
AAFP, AAP, and ACOG also had a suggestion for distributing payments to non-Medicare providers. “Given that most women’s health, pediatric, and family practices have received less financial relief to date, we recommend that HHS provide these practices with a larger proportion of funds relative to their reported revenue than is provided on average across specialties,” they wrote.
A version of this article originally appeared on Medscape.com.
Menstrual cup use with copper IUDs linked to higher expulsion rates
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
FROM ACOG 2020
Do ObGyns think hormonal contraception should be offered over the counter?
In their advocacy column, “OTC hormonal contraception: An important goal in the fight for reproductive justice” (January 2020), Abby L. Schultz, MD, and Megan L. Evans, MD, MPH, discussed a recent committee opinion from the American College of Obstetricians and Gynecologists (ACOG) focused on improving contraception access by offering oral contraceptive pills, progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate over the counter (OTC). The authors agreed with ACOG’s stance and offered several reasons why.
OBG Management polled readers to see their thoughts on the question of whether or not hormonal contraception should be offered OTC.

In their advocacy column, “OTC hormonal contraception: An important goal in the fight for reproductive justice” (January 2020), Abby L. Schultz, MD, and Megan L. Evans, MD, MPH, discussed a recent committee opinion from the American College of Obstetricians and Gynecologists (ACOG) focused on improving contraception access by offering oral contraceptive pills, progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate over the counter (OTC). The authors agreed with ACOG’s stance and offered several reasons why.
OBG Management polled readers to see their thoughts on the question of whether or not hormonal contraception should be offered OTC.


In their advocacy column, “OTC hormonal contraception: An important goal in the fight for reproductive justice” (January 2020), Abby L. Schultz, MD, and Megan L. Evans, MD, MPH, discussed a recent committee opinion from the American College of Obstetricians and Gynecologists (ACOG) focused on improving contraception access by offering oral contraceptive pills, progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate over the counter (OTC). The authors agreed with ACOG’s stance and offered several reasons why.
OBG Management polled readers to see their thoughts on the question of whether or not hormonal contraception should be offered OTC.


Learning to live with COVID-19: Postpandemic life will be reflected in how effectively we leverage this crisis
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
While often compared with the Spanish influenza contagion of 1918, the current COVID-19 pandemic is arguably unprecedented in scale and scope, global reach, and the rate at which it has spread across the world.
Unprecedented times
The United States now has the greatest burden of COVID-19 disease worldwide.1 Although Boston has thus far been spared the full force of the disease’s impact, it is likely only a matter of time before it reaches here. To prepare for the imminent surge, we at Tufts Medical Center defined 4 short-term strategic imperatives to help guide our COVID-19 preparedness. Having a single unified strategy across our organization has helped to maintain focus and consistency in the messaging amidst all of the uncertainty. Our focus areas are outlined below.
1 Flatten the curve
This term refers to the use of “social distancing” and community isolation measures to keep the number of disease cases at a manageable level. COVID-19 is spread almost exclusively through contact with contaminated respiratory droplets. While several categories of risk have been described, the US Centers for Disease Control and Prevention (CDC) defines disease “exposure” as face-to-face contact within 6 feet of an infected individual for more than 15 minutes without wearing a mask.2 Intervening at all 3 of these touchpoints effectively reduces transmission. Interventions include limiting in-person meetings, increasing the space between individuals (both providers and patients), and routinely using personal protective equipment (PPE).
Another effective strategy is to divide frontline providers into smaller units or teams to limit cross-contamination: the inpatient team versus the outpatient team, the day team versus the night team, the “on” team versus the “off” team. If the infection lays one team low, other providers can step in until they recover and return to work.
Visitor policies should be developed and strictly implemented. Many institutions do allow one support person in labor and delivery (L&D) regardless of the patient’s COVID-19 status, although that person should not be symptomatic or COVID-19 positive. Whether to test all patients and support persons for COVID-19 on arrival at L&D remains controversial.3 At a minimum, these individuals should be screened for symptoms. Although it was a major focus of initial preventative efforts, taking a travel and exposure history is no longer informative as the virus is now endemic and community spread is common.
Initial preventative efforts focused also on high-risk patients, but routine use of PPE for all encounters clearly is more effective because of the high rate of asymptomatic shedding. The virus can survive suspended in the air for up to 2 hours following an aerosol-generating procedure (AGP) and on surfaces for several hours or even days. Practices such as regular handwashing, cleaning of exposed work surfaces, and avoiding face touching should by now be part of our everyday routine.
Institutions throughout the United States have established inpatient COVID-19 units—so-called “dirty” units—with mixed success. As the pandemic spreads and the number of patients with asymptomatic shedding increases, it is harder to determine who is and who is not infected. Cross-contamination has rendered this approach largely ineffective. Whether this will change with the introduction of rapid point-of-care testing remains to be seen.
Continue to: 2 Preserve PPE...
2 Preserve PPE
PPE use is effective in reducing transmission. This includes tier 1 PPE with or without enhanced droplet precaution (surgical mask, eye protection, gloves, yellow gown) and tier 2 PPE (tier 1 plus N95 respirators or powered air-purifying respirators [PAPR]). Given the acute PPE shortage in many parts of the country, appropriate use of PPE is critical to maintain an adequate supply. For example, tier 2 PPE is required only in the setting of an AGP. This includes intubation and, in our determination, the second stage of labor for COVID-19–positive patients and patients under investigation (PUIs); we do not employ tier 2 PPE for all patients in the second stage of labor, although some hospitals endorse this practice.
Creative solutions to the impending PPE shortage abound, such as the use of 3D printers to make face shields and novel techniques to sterilize and reuse N95 respirators.
3 Create capacity
In the absence of effective treatment for COVID-19 and with a vaccine still many months away, supportive care is critical. The pulmonary sequelae with cytokine storm and acute hypoxemia can come on quickly, require urgent mechanical ventilatory support, and take several weeks to resolve.
Our ability to create inpatient capacity to accommodate ill patients, monitor them closely, and intubate early will likely be the most critical driver of the case fatality rate. This requires deferring outpatient visits (or doing them via telemedicine), expanding intensive care unit capabilities (especially ventilator beds), and canceling elective surgeries. What constitutes “elective surgery” is not always clear. Our institution, for example, regards abortion services as essential and not elective, but this is not the case throughout the United States.
Creating capacity also refers to staffing. Where necessary, providers should be retrained and redeployed. This may require emergency credentialing of providers in areas outside their usual clinical practice and permission may be needed from the Accreditation Council for Graduate Medical Education to engage trainees outside their usual duty hours.
4 Support and protect your workforce
Everyone is anxious, and people convey their anxiety in different ways. I have found it helpful to acknowledge those feelings and provide a forum for staff to express and share their anxieties. That said, hospitals are not a democracy. While staff members should be encouraged to ask questions and voice their opinions, everyone is expected to follow protocol regarding patient care.
Celebrating small successes and finding creative ways to alleviate the stress and inject humor can help. Most institutions are using electronic conferencing platforms (such as Zoom or Microsoft Teams) to stay in touch and to continue education initiatives through interactive didactic sessions, grand rounds, morbidity and mortality conferences, and e-journal clubs. These are also a great platform for social events, such as w(h)ine and book clubs and virtual karaoke.
Since many ObGyn providers are women, the closure of day-care centers and schools is particularly challenging. Share best practices among your staff on how to address this problem, such as alternating on-call shifts or matching providers needing day care with ‘furloughed’ college students who are looking to keep busy and make a little money.
Continue to: Avoid overcommunicating...
Avoid overcommunicating
Clear, concise, and timely communication is key. This can be challenging given the rapidly evolving science of COVID-19 and the daily barrage of information from both reliable and unreliable sources. Setting up regular online meetings with your faculty 2 or 3 times per week can keep people informed, promote engagement, and boost morale.
If an urgent e-mail announcement is needed, keep the message focused. Highlight only updated information and changes to existing policies and guidelines. And consider adding a brief anecdote to illustrate the staff’s creativity and resilience: a “best catch” story, for example, or a staff member who started a “commit to sit” program (spending time in the room with patients who want company but are not able to have their family in attendance).
Look to the future
COVID-19 will pass. Herd immunity will inevitably develop. The question is how quickly and at what cost. Children delivered today are being born into a society already profoundly altered by COVID-19. Some have started to call them Generation C.
Exactly what life will look like at the back end of this pandemic depends on how effectively we leverage this crisis. There are numerous opportunities to change the way we think about health care and educate the next generation of providers. These include increasing the use of telehealth and remote education, redesigning our traditional prenatal care paradigms, and reinforcing the importance of preventive medicine. This is an opportunity to put the “health” back into “health care.”
Look after yourself
Amid all the chaos and uncertainty, do not forget to take care of yourself and your family. Be calm, be kind, and be flexible. Stay safe.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
- Kommenda N, Gutierrez P, Adolphe J. Coronavirus world map: which countries have the most cases and deaths? The Guardian. April 1, 2020. https://www.theguardian.com/world/2020/mar/31/coronavirus-mapped-which-countries-have-the-most-cases-and-deaths. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19).Interim US guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html. Accessed April 1, 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Evaluating and testing persons for coronavirus disease 2020 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html. Accessed April 1, 2020.
ACOG offers guidance on optimizing patient care in the midst of COVID-19
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
The American College of Obstetricians and Gynecologists (ACOG) posted a useful resource on its website on March 30 for clinicians practicing ambulatory gynecology. The guidance, “COVID-19 FAQs for Obstetrician–Gynecologists, Gynecology” (https://www.acog.org/), is based on expert opinion and is intended to supplement guidance from the Centers for Disease Control and Prevention as well as previously issued ACOG guidance.1
Which patients need to be seen, and when
The ACOG guidance provides examples of patients needing in-person appointments, video or telephone visits, or for whom deferral of a visit until after the COVID-19 outbreak would be appropriate. Highlights include:
In-person appointments
- suspected ectopic pregnancy
- profuse vaginal bleeding
Video or telephone visits
- contraceptive counseling and prescribing
- management of menopausal symptoms
Deferral of a visit until after the COVID-19 outbreak
- routine well-woman visits for average-risk patients.
Cervical screening
With respect to patients with abnormal cervical cancer screening results, ACOG recommends the ASCCP’s guidance that2:
- for patients with low-grade test results, colposcopy/cervical biopsies be deferred up to 6 to 12 months
- for patients with high-grade results, colposcopy/cervical biopsies be performed within 3 months.
Contraception
Regarding contraceptive services, the ACOG guidance suggests that placement of intrauterine devices (IUDs) and contraceptive implants should continue “where possible.” If initiation of long-acting reversible contraception (LARC) is not feasible, the guidance recommends that use of self-administered contraceptives (including subcutaneous injections, oral, transdermal patch, and vaginal ring contraception) be encouraged as a bridge to later initiation of LARC.
The guidance suggests that removal of IUDs and implants be postponed when possible.
Finally, the guidance suggests that patients with an existing IUD or implant who seek removal and replacement of their contraceptives be counseled regarding extended use of these devices.
Individualize your approach
ACOG emphasizes that no single solution applies to all situations and that each practice or clinic should evaluate the individual situation, including the availability of local and regional resources, staffing, and personal protective equipment; the prevalence of COVID-19 in the region; and the type of practice.
A roadmap for care
This guidance from ACOG should help clinicians caring for women during the COVID-19 outbreak to counsel and guide patients in a prudent manner.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.
- American College of Obstetricians and Gynecologists website. COVID-19 FAQs for obstetrician-gynecologists, gynecology. https://www.acog.org/clinical-information/physician-faqs/covid19-faqs-for-ob-gyns-gynecology. Accessed April 3, 2020.
- ASCCP website. ASCCP interim guidance for timing of diagnostic and treatment procedures for patients with abnormal cervical screening tests. https://www.asccp.org/covid-19. Accessed April 3, 2020.









