User login
How can neurologists diagnose and treat menstrual migraine?
STOWE, VT. – , said Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center in Irvine, Calif. Compared with headaches associated with nonmenstrual migraine, headaches resulting from menstrual migraine last longer and are more difficult to treat. They tend to be associated with morning awakening and with nausea and vomiting. But in younger women with regular menses, menstrual migraine is predictable. The disorder offers “an incredible chance to be preemptive and think about short-term preventive strategies,” Dr. Hutchinson said at the annual meeting of the Headache Cooperative of New England.
What is menstrual migraine?
Menstrual migraine occurs during the perimenstrual window, which begins at 2 days before onset of bleeding and ends at 3 days of menses. Migraine that occurs during this window at least two-thirds of the time satisfies the criteria for menstrual migraine. A prospective headache diary is recommended, but not required, for making the diagnosis, said Dr. Hutchinson.
Most women with migraine have perimenstrual exacerbation of their headaches, as well as headaches at other times of the month. This phenotype is called menstrually related migraine. Pure menstrual migraine is migraine associated exclusively with menses. The International Classification of Headache Disorders-3 recognizes that menstrual migraine can be with or without aura. A headache diary can help distinguish between menstrual migraine and menstrually related migraine.
For pure menstrual migraine, it is appropriate to treat during the perimenstrual window. Preventive treatment may not be necessary throughout the month, said Dr. Hutchinson. Furthermore, hormonal treatment is the type of therapy most likely to be effective, she added. Menstrually related migraine requires a broader approach.
Gathering information during the visit
A 1972 study by Somerville and colleagues indicated that a decrease in estrogen is a powerful trigger of migraine. The investigators administered estrogen (i.e., intramuscular estradiol) or progesterone during the late luteal phase to women with menstrual migraine. Among women who received estrogen, migraine onset was postponed until the estrogen level decreased. The administration of progesterone postponed bleeding, but did not affect migraine. Progesterone treatment prevents migraine effectively on occasion, but estrogen treatment is much more likely to be a successful strategy, said Dr. Hutchinson.
Neurologists should ask certain questions of women with migraine, whether the patients are new or not, to gather information needed to make treatment decisions. For example, it is advisable to ask a woman whether she often has a headache with her period. “You may not want to use the word ‘migraine,’ because many women have been taught that headache is part of PMS,” said Dr. Hutchinson. Asking a woman how pregnancy, delivery, and breastfeeding affected her headaches can add further detail to her history and provide insight about the effects of hormonal changes. Asking what type of birth control the woman is taking can influence the choice of treatment, since some therapies are not appropriate during pregnancy.
Available treatments
NSAIDs are among the treatments that neurologists should consider for the short-term prevention of menstrually related migraine, said Dr. Hutchinson. A study of 35 patients by Sances et al. compared placebo with 550 mg of naproxen sodium given twice daily. Treatment began at 7 days before bleeding onset and continued until the 6th day of menses. Patients underwent treatment for three menstrual cycles. Naproxen sodium significantly reduced headache intensity, headache duration, and the number of headache days, compared with baseline. Treatment was superior to placebo at 3 months. Approximately 33% of patients in the active group were headache free, but no controls were.
Magnesium is another potentially effective option. Facchinetti et al. compared placebo with 360 mg/day of magnesium in a study of 20 patients. Treatment, which was given for two cycles, began at 15 days before menses and ended at the start of menses. Compared with placebo, magnesium reduced the number of headache days and the total pain index. Magnesium is inexpensive, but it causes diarrhea in some patients. “Some women choose to take magnesium all month long, other women start at around ovulation,” said Dr. Hutchinson.
Hormonal treatments are another possible option for the short-term prevention of menstrually related migraine. For women who do not plan to become pregnant, oral contraceptive pills can keep estrogen levels high enough to prevent menstrually related migraine. Gynecologists may suggest that a woman take the pill continuously, skipping the placebo, for an entire year, but Dr. Hutchinson recommends that a woman stop taking the pill for 4 days approximately every 3 months. This discontinuation allows for withdrawal bleeding, but is not likely to cause a prolonged enough decrease in estrogen to provoke migraine, she said. The continuous contraceptive ring, which is inserted vaginally, is an alternative to the pill.
For women who do not want or need contraception, an estrogen patch or gel may be appropriate. Two studies in the 1980s found that a gel containing 1.5 mg of estradiol per 2.5 g reduced migraine frequency, duration, and severity. These studies did not gather long-term safety data, however. A 2006 study by MacGregor et al. found that percutaneous estradiol was associated with a 22% reduction in the number of migraine days, as well as with decreases in headache severity and associated nausea. But the risk of migraine during the 5 days following treatment cessation was increased by 40%. This finding suggests that the treatment period should be extended, said Dr. Hutchinson.
In addition to the timing, the dose of treatment affects the outcome. Smite et al. found no benefit of a 50-mcg dose of estradiol, compared with placebo. Pradalier and colleagues found that a 100-mcg dose was associated with decreased use of rescue medication, compared with a 25-mcg dose. These studies did not gather long-term safety data.
Oral contraceptives and the risk of stroke
Combined oral contraceptives, however, are associated with increased risk of stroke in women with migraine with aura. The dose of estrogen in the contraceptive affects the level of risk, said Dr. Hutchinson. A systematic review by Sheikh et al. found that high-dose ethinyl estradiol (i.e., greater than 50 mcg) was associated with a higher risk of ischemic and hemorrhagic stroke than low-dose ethinyl estradiol (i.e., less than 50 mcg) was. A 20-mcg dose was associated with an odds ratio of stroke of 1.7. Furthermore, among women using combined hormonal contraception, the risk of stroke was higher in women with aura than in women without aura.
“I like to look at the big picture,” said Dr. Hutchinson. “There’s a big difference between a woman who has one or two auras a year that last for 10 minutes and a woman who has complicated aura. I’m going to approach [the latter] woman differently.”
No consensus guidelines for prescribing combined oral contraceptives to women with migraine and aura have been developed. The International Headache Society says that physicians may prescribe low-dose estrogen to women with simple visual aura. The American College of Obstetricians and Gynecologists recommends progestin-only intrauterine or barrier contraception for this population. The World Health Organization holds that estrogen-containing contraception is contraindicated in all women who have migraine with aura.
“If you have women who have migraine without aura, low–estrogen dose combined hormonal contraceptives can be quite appropriate,” said Dr. Hutchinson. “I would tend to go with a 10- or 20-mcg low dose. It could be an option for women with migraine with aura, but only if the benefits outweigh the risks.” In a study by Calhoun et al., the vaginal ring was associated with reduced aura frequency in women with migraine and aura.
Choosing preventive and rescue medications
Although no triptan has FDA approval for the short-term prevention of menstrual migraine, studies have suggested that they are effective. In a study by Sances and colleagues, a twice-daily 1-mg dose of naratriptan taken 6 days perimenstrually reduced the frequency of menstrual-related migraine. At least 50% of treated patients in the study had no menstrual-related migraine. Silberstein and colleagues found that 59% of women who took 2.5 mg of frovatriptan twice daily had no menstrual-related migraine during the 6-day perimenstrual period, compared with 33% of women who received placebo.
Patients with menstrual migraine sometimes need rescue medication. Sumatriptan, either as an injection or an inhaled therapy, is one option. Another injectable option is a 60-mg intramuscular dose of ketorolac. Finally, occipital or sphenopalatine nerve block may be effective as well.
Dr. Hutchinson reported consulting for or serving on the advisory board of Alder, Allergan, Amgen, Biohaven, electroCore, Lilly, Novartis, Supernus, Teva, Theranica, and Upsher-Smith. She has served on speakers bureaus for Allergan, Amgen, electroCore, Lilly, Novartis, Supernus, and Teva.
STOWE, VT. – , said Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center in Irvine, Calif. Compared with headaches associated with nonmenstrual migraine, headaches resulting from menstrual migraine last longer and are more difficult to treat. They tend to be associated with morning awakening and with nausea and vomiting. But in younger women with regular menses, menstrual migraine is predictable. The disorder offers “an incredible chance to be preemptive and think about short-term preventive strategies,” Dr. Hutchinson said at the annual meeting of the Headache Cooperative of New England.
What is menstrual migraine?
Menstrual migraine occurs during the perimenstrual window, which begins at 2 days before onset of bleeding and ends at 3 days of menses. Migraine that occurs during this window at least two-thirds of the time satisfies the criteria for menstrual migraine. A prospective headache diary is recommended, but not required, for making the diagnosis, said Dr. Hutchinson.
Most women with migraine have perimenstrual exacerbation of their headaches, as well as headaches at other times of the month. This phenotype is called menstrually related migraine. Pure menstrual migraine is migraine associated exclusively with menses. The International Classification of Headache Disorders-3 recognizes that menstrual migraine can be with or without aura. A headache diary can help distinguish between menstrual migraine and menstrually related migraine.
For pure menstrual migraine, it is appropriate to treat during the perimenstrual window. Preventive treatment may not be necessary throughout the month, said Dr. Hutchinson. Furthermore, hormonal treatment is the type of therapy most likely to be effective, she added. Menstrually related migraine requires a broader approach.
Gathering information during the visit
A 1972 study by Somerville and colleagues indicated that a decrease in estrogen is a powerful trigger of migraine. The investigators administered estrogen (i.e., intramuscular estradiol) or progesterone during the late luteal phase to women with menstrual migraine. Among women who received estrogen, migraine onset was postponed until the estrogen level decreased. The administration of progesterone postponed bleeding, but did not affect migraine. Progesterone treatment prevents migraine effectively on occasion, but estrogen treatment is much more likely to be a successful strategy, said Dr. Hutchinson.
Neurologists should ask certain questions of women with migraine, whether the patients are new or not, to gather information needed to make treatment decisions. For example, it is advisable to ask a woman whether she often has a headache with her period. “You may not want to use the word ‘migraine,’ because many women have been taught that headache is part of PMS,” said Dr. Hutchinson. Asking a woman how pregnancy, delivery, and breastfeeding affected her headaches can add further detail to her history and provide insight about the effects of hormonal changes. Asking what type of birth control the woman is taking can influence the choice of treatment, since some therapies are not appropriate during pregnancy.
Available treatments
NSAIDs are among the treatments that neurologists should consider for the short-term prevention of menstrually related migraine, said Dr. Hutchinson. A study of 35 patients by Sances et al. compared placebo with 550 mg of naproxen sodium given twice daily. Treatment began at 7 days before bleeding onset and continued until the 6th day of menses. Patients underwent treatment for three menstrual cycles. Naproxen sodium significantly reduced headache intensity, headache duration, and the number of headache days, compared with baseline. Treatment was superior to placebo at 3 months. Approximately 33% of patients in the active group were headache free, but no controls were.
Magnesium is another potentially effective option. Facchinetti et al. compared placebo with 360 mg/day of magnesium in a study of 20 patients. Treatment, which was given for two cycles, began at 15 days before menses and ended at the start of menses. Compared with placebo, magnesium reduced the number of headache days and the total pain index. Magnesium is inexpensive, but it causes diarrhea in some patients. “Some women choose to take magnesium all month long, other women start at around ovulation,” said Dr. Hutchinson.
Hormonal treatments are another possible option for the short-term prevention of menstrually related migraine. For women who do not plan to become pregnant, oral contraceptive pills can keep estrogen levels high enough to prevent menstrually related migraine. Gynecologists may suggest that a woman take the pill continuously, skipping the placebo, for an entire year, but Dr. Hutchinson recommends that a woman stop taking the pill for 4 days approximately every 3 months. This discontinuation allows for withdrawal bleeding, but is not likely to cause a prolonged enough decrease in estrogen to provoke migraine, she said. The continuous contraceptive ring, which is inserted vaginally, is an alternative to the pill.
For women who do not want or need contraception, an estrogen patch or gel may be appropriate. Two studies in the 1980s found that a gel containing 1.5 mg of estradiol per 2.5 g reduced migraine frequency, duration, and severity. These studies did not gather long-term safety data, however. A 2006 study by MacGregor et al. found that percutaneous estradiol was associated with a 22% reduction in the number of migraine days, as well as with decreases in headache severity and associated nausea. But the risk of migraine during the 5 days following treatment cessation was increased by 40%. This finding suggests that the treatment period should be extended, said Dr. Hutchinson.
In addition to the timing, the dose of treatment affects the outcome. Smite et al. found no benefit of a 50-mcg dose of estradiol, compared with placebo. Pradalier and colleagues found that a 100-mcg dose was associated with decreased use of rescue medication, compared with a 25-mcg dose. These studies did not gather long-term safety data.
Oral contraceptives and the risk of stroke
Combined oral contraceptives, however, are associated with increased risk of stroke in women with migraine with aura. The dose of estrogen in the contraceptive affects the level of risk, said Dr. Hutchinson. A systematic review by Sheikh et al. found that high-dose ethinyl estradiol (i.e., greater than 50 mcg) was associated with a higher risk of ischemic and hemorrhagic stroke than low-dose ethinyl estradiol (i.e., less than 50 mcg) was. A 20-mcg dose was associated with an odds ratio of stroke of 1.7. Furthermore, among women using combined hormonal contraception, the risk of stroke was higher in women with aura than in women without aura.
“I like to look at the big picture,” said Dr. Hutchinson. “There’s a big difference between a woman who has one or two auras a year that last for 10 minutes and a woman who has complicated aura. I’m going to approach [the latter] woman differently.”
No consensus guidelines for prescribing combined oral contraceptives to women with migraine and aura have been developed. The International Headache Society says that physicians may prescribe low-dose estrogen to women with simple visual aura. The American College of Obstetricians and Gynecologists recommends progestin-only intrauterine or barrier contraception for this population. The World Health Organization holds that estrogen-containing contraception is contraindicated in all women who have migraine with aura.
“If you have women who have migraine without aura, low–estrogen dose combined hormonal contraceptives can be quite appropriate,” said Dr. Hutchinson. “I would tend to go with a 10- or 20-mcg low dose. It could be an option for women with migraine with aura, but only if the benefits outweigh the risks.” In a study by Calhoun et al., the vaginal ring was associated with reduced aura frequency in women with migraine and aura.
Choosing preventive and rescue medications
Although no triptan has FDA approval for the short-term prevention of menstrual migraine, studies have suggested that they are effective. In a study by Sances and colleagues, a twice-daily 1-mg dose of naratriptan taken 6 days perimenstrually reduced the frequency of menstrual-related migraine. At least 50% of treated patients in the study had no menstrual-related migraine. Silberstein and colleagues found that 59% of women who took 2.5 mg of frovatriptan twice daily had no menstrual-related migraine during the 6-day perimenstrual period, compared with 33% of women who received placebo.
Patients with menstrual migraine sometimes need rescue medication. Sumatriptan, either as an injection or an inhaled therapy, is one option. Another injectable option is a 60-mg intramuscular dose of ketorolac. Finally, occipital or sphenopalatine nerve block may be effective as well.
Dr. Hutchinson reported consulting for or serving on the advisory board of Alder, Allergan, Amgen, Biohaven, electroCore, Lilly, Novartis, Supernus, Teva, Theranica, and Upsher-Smith. She has served on speakers bureaus for Allergan, Amgen, electroCore, Lilly, Novartis, Supernus, and Teva.
STOWE, VT. – , said Susan Hutchinson, MD, director of the Orange County Migraine and Headache Center in Irvine, Calif. Compared with headaches associated with nonmenstrual migraine, headaches resulting from menstrual migraine last longer and are more difficult to treat. They tend to be associated with morning awakening and with nausea and vomiting. But in younger women with regular menses, menstrual migraine is predictable. The disorder offers “an incredible chance to be preemptive and think about short-term preventive strategies,” Dr. Hutchinson said at the annual meeting of the Headache Cooperative of New England.
What is menstrual migraine?
Menstrual migraine occurs during the perimenstrual window, which begins at 2 days before onset of bleeding and ends at 3 days of menses. Migraine that occurs during this window at least two-thirds of the time satisfies the criteria for menstrual migraine. A prospective headache diary is recommended, but not required, for making the diagnosis, said Dr. Hutchinson.
Most women with migraine have perimenstrual exacerbation of their headaches, as well as headaches at other times of the month. This phenotype is called menstrually related migraine. Pure menstrual migraine is migraine associated exclusively with menses. The International Classification of Headache Disorders-3 recognizes that menstrual migraine can be with or without aura. A headache diary can help distinguish between menstrual migraine and menstrually related migraine.
For pure menstrual migraine, it is appropriate to treat during the perimenstrual window. Preventive treatment may not be necessary throughout the month, said Dr. Hutchinson. Furthermore, hormonal treatment is the type of therapy most likely to be effective, she added. Menstrually related migraine requires a broader approach.
Gathering information during the visit
A 1972 study by Somerville and colleagues indicated that a decrease in estrogen is a powerful trigger of migraine. The investigators administered estrogen (i.e., intramuscular estradiol) or progesterone during the late luteal phase to women with menstrual migraine. Among women who received estrogen, migraine onset was postponed until the estrogen level decreased. The administration of progesterone postponed bleeding, but did not affect migraine. Progesterone treatment prevents migraine effectively on occasion, but estrogen treatment is much more likely to be a successful strategy, said Dr. Hutchinson.
Neurologists should ask certain questions of women with migraine, whether the patients are new or not, to gather information needed to make treatment decisions. For example, it is advisable to ask a woman whether she often has a headache with her period. “You may not want to use the word ‘migraine,’ because many women have been taught that headache is part of PMS,” said Dr. Hutchinson. Asking a woman how pregnancy, delivery, and breastfeeding affected her headaches can add further detail to her history and provide insight about the effects of hormonal changes. Asking what type of birth control the woman is taking can influence the choice of treatment, since some therapies are not appropriate during pregnancy.
Available treatments
NSAIDs are among the treatments that neurologists should consider for the short-term prevention of menstrually related migraine, said Dr. Hutchinson. A study of 35 patients by Sances et al. compared placebo with 550 mg of naproxen sodium given twice daily. Treatment began at 7 days before bleeding onset and continued until the 6th day of menses. Patients underwent treatment for three menstrual cycles. Naproxen sodium significantly reduced headache intensity, headache duration, and the number of headache days, compared with baseline. Treatment was superior to placebo at 3 months. Approximately 33% of patients in the active group were headache free, but no controls were.
Magnesium is another potentially effective option. Facchinetti et al. compared placebo with 360 mg/day of magnesium in a study of 20 patients. Treatment, which was given for two cycles, began at 15 days before menses and ended at the start of menses. Compared with placebo, magnesium reduced the number of headache days and the total pain index. Magnesium is inexpensive, but it causes diarrhea in some patients. “Some women choose to take magnesium all month long, other women start at around ovulation,” said Dr. Hutchinson.
Hormonal treatments are another possible option for the short-term prevention of menstrually related migraine. For women who do not plan to become pregnant, oral contraceptive pills can keep estrogen levels high enough to prevent menstrually related migraine. Gynecologists may suggest that a woman take the pill continuously, skipping the placebo, for an entire year, but Dr. Hutchinson recommends that a woman stop taking the pill for 4 days approximately every 3 months. This discontinuation allows for withdrawal bleeding, but is not likely to cause a prolonged enough decrease in estrogen to provoke migraine, she said. The continuous contraceptive ring, which is inserted vaginally, is an alternative to the pill.
For women who do not want or need contraception, an estrogen patch or gel may be appropriate. Two studies in the 1980s found that a gel containing 1.5 mg of estradiol per 2.5 g reduced migraine frequency, duration, and severity. These studies did not gather long-term safety data, however. A 2006 study by MacGregor et al. found that percutaneous estradiol was associated with a 22% reduction in the number of migraine days, as well as with decreases in headache severity and associated nausea. But the risk of migraine during the 5 days following treatment cessation was increased by 40%. This finding suggests that the treatment period should be extended, said Dr. Hutchinson.
In addition to the timing, the dose of treatment affects the outcome. Smite et al. found no benefit of a 50-mcg dose of estradiol, compared with placebo. Pradalier and colleagues found that a 100-mcg dose was associated with decreased use of rescue medication, compared with a 25-mcg dose. These studies did not gather long-term safety data.
Oral contraceptives and the risk of stroke
Combined oral contraceptives, however, are associated with increased risk of stroke in women with migraine with aura. The dose of estrogen in the contraceptive affects the level of risk, said Dr. Hutchinson. A systematic review by Sheikh et al. found that high-dose ethinyl estradiol (i.e., greater than 50 mcg) was associated with a higher risk of ischemic and hemorrhagic stroke than low-dose ethinyl estradiol (i.e., less than 50 mcg) was. A 20-mcg dose was associated with an odds ratio of stroke of 1.7. Furthermore, among women using combined hormonal contraception, the risk of stroke was higher in women with aura than in women without aura.
“I like to look at the big picture,” said Dr. Hutchinson. “There’s a big difference between a woman who has one or two auras a year that last for 10 minutes and a woman who has complicated aura. I’m going to approach [the latter] woman differently.”
No consensus guidelines for prescribing combined oral contraceptives to women with migraine and aura have been developed. The International Headache Society says that physicians may prescribe low-dose estrogen to women with simple visual aura. The American College of Obstetricians and Gynecologists recommends progestin-only intrauterine or barrier contraception for this population. The World Health Organization holds that estrogen-containing contraception is contraindicated in all women who have migraine with aura.
“If you have women who have migraine without aura, low–estrogen dose combined hormonal contraceptives can be quite appropriate,” said Dr. Hutchinson. “I would tend to go with a 10- or 20-mcg low dose. It could be an option for women with migraine with aura, but only if the benefits outweigh the risks.” In a study by Calhoun et al., the vaginal ring was associated with reduced aura frequency in women with migraine and aura.
Choosing preventive and rescue medications
Although no triptan has FDA approval for the short-term prevention of menstrual migraine, studies have suggested that they are effective. In a study by Sances and colleagues, a twice-daily 1-mg dose of naratriptan taken 6 days perimenstrually reduced the frequency of menstrual-related migraine. At least 50% of treated patients in the study had no menstrual-related migraine. Silberstein and colleagues found that 59% of women who took 2.5 mg of frovatriptan twice daily had no menstrual-related migraine during the 6-day perimenstrual period, compared with 33% of women who received placebo.
Patients with menstrual migraine sometimes need rescue medication. Sumatriptan, either as an injection or an inhaled therapy, is one option. Another injectable option is a 60-mg intramuscular dose of ketorolac. Finally, occipital or sphenopalatine nerve block may be effective as well.
Dr. Hutchinson reported consulting for or serving on the advisory board of Alder, Allergan, Amgen, Biohaven, electroCore, Lilly, Novartis, Supernus, Teva, Theranica, and Upsher-Smith. She has served on speakers bureaus for Allergan, Amgen, electroCore, Lilly, Novartis, Supernus, and Teva.
REPORTING FROM HCNE Stowe 2020
Can a drug FDA approved for endometriosis become a mainstay for nonsurgical treatment of HMB in women with fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
The STD epidemic: Why we need to care about this escalating problem
The sexually transmitted disease (STD) epidemic in the United States is intensifying, and it disproportionately impacts high-risk communities. In 2018, rates of reportable STDs, including syphilis and Neisseria gonorrhoeae and Chlamydia trachomatis infections, reached an all-time high.1 That year, there were 1.8 million cases of chlamydia (increased 19% since 2014), 583,405 cases of gonorrhea (increased 63% since 2014), and 35,063 cases of primary and secondary syphilis (71% increase from 2014).1
Cases of newborn syphilis have more than doubled in 4 years, with rates reaching a 20-year high.1
This surge has not received the attention it deserves given the broad-reaching impact of these infections on women’s health and maternal-child health.2 As ObGyns, we are on the front line, and we need to be engaged in evidence-based strategies and population-based health initiatives to expedite diagnoses and treatment and to reduce the ongoing spread of these infections.
Disparities exist and continue to fuel this epidemic
The STD burden is disproportionately high among reproductive-aged women, and half of all reported STDs occur in women aged 15 to 24 years. African American women have rates up to 12 times higher than white women.3,4 Substantial geographic variability also exists, with the South, Southeast, and West having some of the highest STD rates.
These disparities are fueled by inequalities in socioeconomic status (SES), including employment, insurance, education, incarceration, stress/trauma exposure, and discrimination.5-7 Those with lower SES often have trouble accessing and affording quality health care, including sexual health services. Access to quality health care, including STD prevention and treatment, that meets the needs of lower SES populations is key to reducing STD disparities in the United States; however, access likely will be insufficient unless the structural inequities that drive these disparities are addressed.
Clinical consequences for women, infants, and mothers
STDs are most prevalent among reproductive-aged women and can lead to pelvic inflammatory disease, infertility, ectopic pregnancy,4,8 and increased risk of acquiring human immunodeficiency virus (HIV). STDs during pregnancy present additional consequences. Congenital syphilis is perhaps the most salient, with neonates experiencing substantial disability or death.
In addition, STDs contribute to overall peripartum and long-term adverse health outcomes.4,9,10 Untreated chlamydia infection, for example, is associated with neonatal pneumonia, neonatal conjunctivitis, low birth weight, premature rupture of membranes, preterm labor, and postpartum endometritis.2,11 Untreated gonorrhea is linked to disseminated gonococcal infection in the newborn, neonatal conjunctivitis, low birth weight, miscarriage, premature rupture of membranes, preterm labor, and chorioamnionitis.2,12
As preterm birth is the leading cause of infant morbidity and mortality and disproportionately affects African American women and women in the southeastern United States,13 there is a critical public heath need to improve STD screening, treatment, and prevention of reinfection among high-risk pregnant women.
Quality clinical services for STDs: Areas for focus
More and more, STDs are being diagnosed in primary care settings. In January 2020, the Centers for Disease Control and Prevention (CDC) released a document, referred to as STD QCS (quality clinical services), that outlines recommendations for basic and specialty-level STD clinical services.14 ObGyns and other clinicians who provide primary care should meet the basic recommendations as a minimum.
The STD QCS outlines 8 recommendation areas: sexual history and physical examination, prevention, screening,
Continue to: Sexual history and physical examination...
Sexual history and physical examination
A complete sexual history and risk assessment should be performed at a complete initial or annual visit and as indicated. Routinely updating the sexual history and risk assessment is important to normalize these questions within the frame of the person’s overall health, and it may be valuable in reducing stigma. This routine approach may be important particularly for younger patients and others whose risk for STDs may change frequently and dramatically.
Creating a safe space that permits privacy and assurance of confidentiality may help build trust and set the stage for disclosure. The American College of Obstetricians and Gynecologists recommends that all young people have time alone without parents for confidential counseling and discussion.15 All states allow minors to consent for STD services themselves, although 11 states limit this to those beyond a certain age.16
The CDC recommends using the 5 P’s—partners, practices, protection, past history of STDs, and prevention of pregnancy—as a guide for discussion.14 ObGyns are more likely than other providers to perform this screening routinely. While a pelvic examination should be available for STD evaluation as needed, it is not required for routine screening.
Prevention
ObGyns should employ several recommendations for STD prevention. These include providing or referring patients for vaccination against hepatitis B and human papillomavirus and providing brief STD/HIV prevention counseling along with contraceptive counseling. ObGyns should be familiar with HIV pre-exposure prophylaxis (PrEP) and nonoccupational postexposure prophylaxis (nPEP) and provide risk assessment, education, and referral or link to HIV care. Providing these services would improve access to care and further remove barriers to care. ObGyns also could consider providing condoms in their offices.14
Screening
STD screening of women at risk is critical since more than 80% of infected women are asymptomatic.8 Because young people are disproportionately experiencing STDs, annual screening for chlamydia and gonorrhea is recommended for women younger than 25 years. For women older than 25, those at increased risk can be screened.
Risk factors for chlamydia infection include having new or multiple sex partners, sex partners with concurrent partners, or sex partners who have an STD. For gonorrhea, risk factors include living in a high-morbidity area, having a previous or coexisting STD, new or multiple sex partners, inconsistent condom use in people who are not in a mutually monogamous relationship, and exchanging sex for money or drugs. Screening for syphilis in nonpregnant women is recommended for those who have had any sexual activity with a person recently diagnosed with syphilis or those who personally display signs or symptoms of infection.17
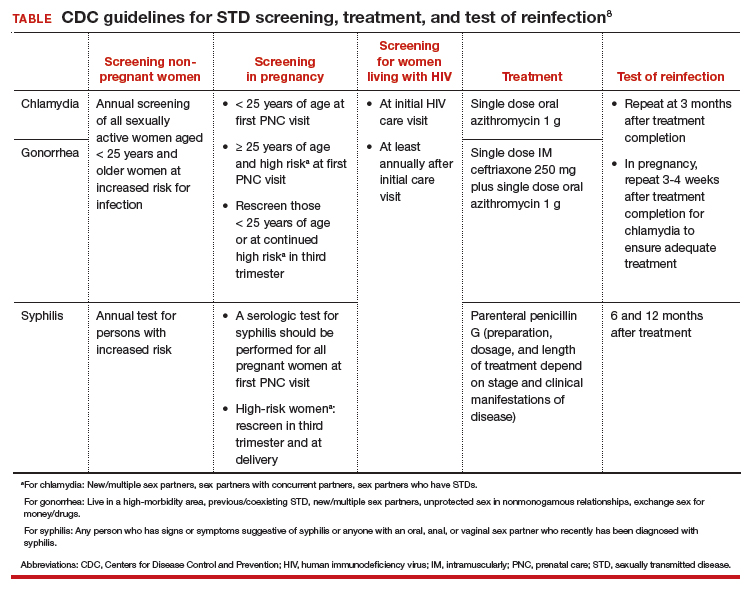
STD screening is especially important for pregnant women, and treatment of infections may improve pregnancy outcomes. The CDC recommends screening at the first prenatal care visit for chlamydia and gonorrhea in pregnant women younger than 25 years of age and in older pregnant women at increased risk; women younger than 25 years or at continued high risk should be rescreened in their third trimester. The CDC recommends screening all women for syphilis at their first prenatal care visit and rescreening those at high risk in the third trimester and at delivery (TABLE).18
Continue to: Partner services...
Partner services
Clearly outlined partner management services is paramount for preventing STD reinfection.14 Reinfection rates for chlamydia and gonorrhea among young women are high and vary by study population.19 At a minimum, ObGyns should counsel patients with an STD that their partner(s) should be notified and encouraged to seek services.
For states in which it is legal, expedited partner therapy (EPT)—the clinician provides medication for the partner without seeing the partner—should be provided for chlamydia or gonorrhea if the partner is unlikely to access timely care. EPT is legal in most states. (To check the legal status of EPT in your state, visit https://www.cdc.gov/std/ept/legal/default.htm.) Research is needed to evaluate optimal strategies for effective implementation of EPT services in different clinical settings.
Laboratory tests
ObGyns should be able to provide a wide range of laboratory evaluations (for example, a nucleic acid amplification test [NAAT] for genital chlamydia and gonorrhea, quantitative nontreponemal serologic test for syphilis, treponemal serologic test for syphilis) that can be ordered for screening or diagnostic purposes. To improve rates of recommended screening, consider having clinic-level policies that support screening, such as standing orders, express or walk-in screening appointments, lab panels, and reflex testing.
Further, having rapid results or point-of-care testing available would help decrease lags in time to treatment. Delays in treatment are particularly important in lower-resource communities; thus, point-of-care testing may be especially valuable with immediate access to treatment on site.
Treatment
Adequate and timely treatment of STDs is critical to decrease sequelae and the likelihood of transmission to others. Treatment is evolving, particularly for gonorrhea. Over the past several years, gonorrhea has become resistant to 6 previously recommended treatment options.20 Since 2015, the CDC recommends dual therapy for gonorrhea with an injection of ceftriaxone and oral azithromycin.
The first-line recommended treatments for bacterial STDs are listed in the TABLE. When possible, it is preferred to offer directly observed therapy at the time of the visit. This decreases the time to treatment and ensures that therapy is completed.
A call to action for ObGyns
Clinicians have multiple opportunities to address and reduce the surge of STDs in the United States. We play a critical role in screening, diagnosing, and treating patients, and it is thus imperative to be up-to-date on the recommended guidelines. Further, clinicians can advocate for more rapid testing modalities, with the goal of obtaining point-of-care testing results when possible and implementing strategies to improve partner treatment.
While a positive STD result may be associated with significant patient distress, it also may be an opportunity for enhancing the patient-provider relationship, coupling education with motivational approaches to help patients increase protective health behaviors.
It is critical to approach clinical care in a nonjudgmental manner to improve patients’ comfort in their relationship with the health care system. ●
- Be aware of up-to-date screening, treatment, and follow-up recommendations for STDs
- Develop strategies to maximize partner treatment, including expedited partner therapy
- Identify high-risk individuals for whom counseling on HIV and unintended pregnancy prevention strategies can be reinforced, including PrEP and contraception
- Create a clinical environment that normalizes STD testing and destigmatizes infection
- Integrate client-centered counseling to improve protective health behaviors
Abbreviations: HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
- Centers for Disease Control and Prevention. 2018 STD surveillance report. https://www.cdc.gov/nchhstp /newsroom/2019/2018-STD-surveillance-report.html. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDs): STDs during pregnancy—CDC fact sheet (detailed). www.cdc.gov/std/pregnancy/stdfact -sheet-pregnancy-detailed.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in racial and ethnic minorities 2017. https://www.cdc.gov/std/stats17 /minorities.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in women and infants. https://www.cdc.gov/std/stats17/womenandinf .htm. Accessed March 19, 2020.
- Semega JL, Fontenot KR, Kollar MA; US Census Bureau. Income and poverty in the United States: 2016. Washington, DC: US Government Printing Office; 2017. https://www.census.gov/content/dam/Census/library /publications/2017/demo/P60-259.pdf. Accessed March 19, 2020.
- Harling G, Subramanian S, Barnighausen T, et al. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40:575-581.
- Meyer PA, Penman-Aguilar A, Campbell VA, et al; Centers for Disease Control and Prevention. Conclusion and future directions: CDC Health Disparities and Inequalities Report— United States, 2013. MMWR Suppl. 2013;62(3):184-186.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03): 1-137.
- Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis. 1990;161:531-536.
- Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect. 2019;95:60-66.
- Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183:662-668.
- Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397-404.
- March of Dimes. Maternal, infant, and child health in the United States, 2016. https://www.marchofdimes.org /materials/March-of-Dimes-2016-Databook.pdf. Accessed March 19, 2020.
- Barrow RY, Ahmed F, Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(5):1-20.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 598: The initial reproductive health visit. May 2014. https:// www.acog.org/-/media /project/acog/acogorg/clinical/files/committee-opinion /articles/2014/05/the-initial-reproductive-health-visit.pdf. Accessed March 31, 2020.
- Guttmacher Institute. An overview of consent to reproductive health services by young people. March 1, 2020. https://www .guttmacher.org/state-policy/explore/overview-minors -consent-law. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Pocket guide for providers: Syphilis: a provider’s guide to treatment and prevention. 2017. https://www.cdc.gov/std/syphilis /Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines: syphilis during pregnancy. https://www.cdc.gov/std/tg2015/syphilis -pregnancy.htm. Accessed March 19, 2020.
- Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-489.
- Bodie M, Gale-Rowe M, Alexandre S, et al. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: there is no time like the present. Can Commun Dis Rep. 2019;45:54-62.
The sexually transmitted disease (STD) epidemic in the United States is intensifying, and it disproportionately impacts high-risk communities. In 2018, rates of reportable STDs, including syphilis and Neisseria gonorrhoeae and Chlamydia trachomatis infections, reached an all-time high.1 That year, there were 1.8 million cases of chlamydia (increased 19% since 2014), 583,405 cases of gonorrhea (increased 63% since 2014), and 35,063 cases of primary and secondary syphilis (71% increase from 2014).1
Cases of newborn syphilis have more than doubled in 4 years, with rates reaching a 20-year high.1
This surge has not received the attention it deserves given the broad-reaching impact of these infections on women’s health and maternal-child health.2 As ObGyns, we are on the front line, and we need to be engaged in evidence-based strategies and population-based health initiatives to expedite diagnoses and treatment and to reduce the ongoing spread of these infections.

Disparities exist and continue to fuel this epidemic
The STD burden is disproportionately high among reproductive-aged women, and half of all reported STDs occur in women aged 15 to 24 years. African American women have rates up to 12 times higher than white women.3,4 Substantial geographic variability also exists, with the South, Southeast, and West having some of the highest STD rates.
These disparities are fueled by inequalities in socioeconomic status (SES), including employment, insurance, education, incarceration, stress/trauma exposure, and discrimination.5-7 Those with lower SES often have trouble accessing and affording quality health care, including sexual health services. Access to quality health care, including STD prevention and treatment, that meets the needs of lower SES populations is key to reducing STD disparities in the United States; however, access likely will be insufficient unless the structural inequities that drive these disparities are addressed.
Clinical consequences for women, infants, and mothers
STDs are most prevalent among reproductive-aged women and can lead to pelvic inflammatory disease, infertility, ectopic pregnancy,4,8 and increased risk of acquiring human immunodeficiency virus (HIV). STDs during pregnancy present additional consequences. Congenital syphilis is perhaps the most salient, with neonates experiencing substantial disability or death.
In addition, STDs contribute to overall peripartum and long-term adverse health outcomes.4,9,10 Untreated chlamydia infection, for example, is associated with neonatal pneumonia, neonatal conjunctivitis, low birth weight, premature rupture of membranes, preterm labor, and postpartum endometritis.2,11 Untreated gonorrhea is linked to disseminated gonococcal infection in the newborn, neonatal conjunctivitis, low birth weight, miscarriage, premature rupture of membranes, preterm labor, and chorioamnionitis.2,12
As preterm birth is the leading cause of infant morbidity and mortality and disproportionately affects African American women and women in the southeastern United States,13 there is a critical public heath need to improve STD screening, treatment, and prevention of reinfection among high-risk pregnant women.
Quality clinical services for STDs: Areas for focus
More and more, STDs are being diagnosed in primary care settings. In January 2020, the Centers for Disease Control and Prevention (CDC) released a document, referred to as STD QCS (quality clinical services), that outlines recommendations for basic and specialty-level STD clinical services.14 ObGyns and other clinicians who provide primary care should meet the basic recommendations as a minimum.
The STD QCS outlines 8 recommendation areas: sexual history and physical examination, prevention, screening,
Continue to: Sexual history and physical examination...
Sexual history and physical examination
A complete sexual history and risk assessment should be performed at a complete initial or annual visit and as indicated. Routinely updating the sexual history and risk assessment is important to normalize these questions within the frame of the person’s overall health, and it may be valuable in reducing stigma. This routine approach may be important particularly for younger patients and others whose risk for STDs may change frequently and dramatically.
Creating a safe space that permits privacy and assurance of confidentiality may help build trust and set the stage for disclosure. The American College of Obstetricians and Gynecologists recommends that all young people have time alone without parents for confidential counseling and discussion.15 All states allow minors to consent for STD services themselves, although 11 states limit this to those beyond a certain age.16
The CDC recommends using the 5 P’s—partners, practices, protection, past history of STDs, and prevention of pregnancy—as a guide for discussion.14 ObGyns are more likely than other providers to perform this screening routinely. While a pelvic examination should be available for STD evaluation as needed, it is not required for routine screening.
Prevention
ObGyns should employ several recommendations for STD prevention. These include providing or referring patients for vaccination against hepatitis B and human papillomavirus and providing brief STD/HIV prevention counseling along with contraceptive counseling. ObGyns should be familiar with HIV pre-exposure prophylaxis (PrEP) and nonoccupational postexposure prophylaxis (nPEP) and provide risk assessment, education, and referral or link to HIV care. Providing these services would improve access to care and further remove barriers to care. ObGyns also could consider providing condoms in their offices.14
Screening
STD screening of women at risk is critical since more than 80% of infected women are asymptomatic.8 Because young people are disproportionately experiencing STDs, annual screening for chlamydia and gonorrhea is recommended for women younger than 25 years. For women older than 25, those at increased risk can be screened.
Risk factors for chlamydia infection include having new or multiple sex partners, sex partners with concurrent partners, or sex partners who have an STD. For gonorrhea, risk factors include living in a high-morbidity area, having a previous or coexisting STD, new or multiple sex partners, inconsistent condom use in people who are not in a mutually monogamous relationship, and exchanging sex for money or drugs. Screening for syphilis in nonpregnant women is recommended for those who have had any sexual activity with a person recently diagnosed with syphilis or those who personally display signs or symptoms of infection.17
STD screening is especially important for pregnant women, and treatment of infections may improve pregnancy outcomes. The CDC recommends screening at the first prenatal care visit for chlamydia and gonorrhea in pregnant women younger than 25 years of age and in older pregnant women at increased risk; women younger than 25 years or at continued high risk should be rescreened in their third trimester. The CDC recommends screening all women for syphilis at their first prenatal care visit and rescreening those at high risk in the third trimester and at delivery (TABLE).18

Continue to: Partner services...
Partner services
Clearly outlined partner management services is paramount for preventing STD reinfection.14 Reinfection rates for chlamydia and gonorrhea among young women are high and vary by study population.19 At a minimum, ObGyns should counsel patients with an STD that their partner(s) should be notified and encouraged to seek services.
For states in which it is legal, expedited partner therapy (EPT)—the clinician provides medication for the partner without seeing the partner—should be provided for chlamydia or gonorrhea if the partner is unlikely to access timely care. EPT is legal in most states. (To check the legal status of EPT in your state, visit https://www.cdc.gov/std/ept/legal/default.htm.) Research is needed to evaluate optimal strategies for effective implementation of EPT services in different clinical settings.
Laboratory tests
ObGyns should be able to provide a wide range of laboratory evaluations (for example, a nucleic acid amplification test [NAAT] for genital chlamydia and gonorrhea, quantitative nontreponemal serologic test for syphilis, treponemal serologic test for syphilis) that can be ordered for screening or diagnostic purposes. To improve rates of recommended screening, consider having clinic-level policies that support screening, such as standing orders, express or walk-in screening appointments, lab panels, and reflex testing.
Further, having rapid results or point-of-care testing available would help decrease lags in time to treatment. Delays in treatment are particularly important in lower-resource communities; thus, point-of-care testing may be especially valuable with immediate access to treatment on site.
Treatment
Adequate and timely treatment of STDs is critical to decrease sequelae and the likelihood of transmission to others. Treatment is evolving, particularly for gonorrhea. Over the past several years, gonorrhea has become resistant to 6 previously recommended treatment options.20 Since 2015, the CDC recommends dual therapy for gonorrhea with an injection of ceftriaxone and oral azithromycin.
The first-line recommended treatments for bacterial STDs are listed in the TABLE. When possible, it is preferred to offer directly observed therapy at the time of the visit. This decreases the time to treatment and ensures that therapy is completed.
A call to action for ObGyns
Clinicians have multiple opportunities to address and reduce the surge of STDs in the United States. We play a critical role in screening, diagnosing, and treating patients, and it is thus imperative to be up-to-date on the recommended guidelines. Further, clinicians can advocate for more rapid testing modalities, with the goal of obtaining point-of-care testing results when possible and implementing strategies to improve partner treatment.
While a positive STD result may be associated with significant patient distress, it also may be an opportunity for enhancing the patient-provider relationship, coupling education with motivational approaches to help patients increase protective health behaviors.
It is critical to approach clinical care in a nonjudgmental manner to improve patients’ comfort in their relationship with the health care system. ●
- Be aware of up-to-date screening, treatment, and follow-up recommendations for STDs
- Develop strategies to maximize partner treatment, including expedited partner therapy
- Identify high-risk individuals for whom counseling on HIV and unintended pregnancy prevention strategies can be reinforced, including PrEP and contraception
- Create a clinical environment that normalizes STD testing and destigmatizes infection
- Integrate client-centered counseling to improve protective health behaviors
Abbreviations: HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
The sexually transmitted disease (STD) epidemic in the United States is intensifying, and it disproportionately impacts high-risk communities. In 2018, rates of reportable STDs, including syphilis and Neisseria gonorrhoeae and Chlamydia trachomatis infections, reached an all-time high.1 That year, there were 1.8 million cases of chlamydia (increased 19% since 2014), 583,405 cases of gonorrhea (increased 63% since 2014), and 35,063 cases of primary and secondary syphilis (71% increase from 2014).1
Cases of newborn syphilis have more than doubled in 4 years, with rates reaching a 20-year high.1
This surge has not received the attention it deserves given the broad-reaching impact of these infections on women’s health and maternal-child health.2 As ObGyns, we are on the front line, and we need to be engaged in evidence-based strategies and population-based health initiatives to expedite diagnoses and treatment and to reduce the ongoing spread of these infections.

Disparities exist and continue to fuel this epidemic
The STD burden is disproportionately high among reproductive-aged women, and half of all reported STDs occur in women aged 15 to 24 years. African American women have rates up to 12 times higher than white women.3,4 Substantial geographic variability also exists, with the South, Southeast, and West having some of the highest STD rates.
These disparities are fueled by inequalities in socioeconomic status (SES), including employment, insurance, education, incarceration, stress/trauma exposure, and discrimination.5-7 Those with lower SES often have trouble accessing and affording quality health care, including sexual health services. Access to quality health care, including STD prevention and treatment, that meets the needs of lower SES populations is key to reducing STD disparities in the United States; however, access likely will be insufficient unless the structural inequities that drive these disparities are addressed.
Clinical consequences for women, infants, and mothers
STDs are most prevalent among reproductive-aged women and can lead to pelvic inflammatory disease, infertility, ectopic pregnancy,4,8 and increased risk of acquiring human immunodeficiency virus (HIV). STDs during pregnancy present additional consequences. Congenital syphilis is perhaps the most salient, with neonates experiencing substantial disability or death.
In addition, STDs contribute to overall peripartum and long-term adverse health outcomes.4,9,10 Untreated chlamydia infection, for example, is associated with neonatal pneumonia, neonatal conjunctivitis, low birth weight, premature rupture of membranes, preterm labor, and postpartum endometritis.2,11 Untreated gonorrhea is linked to disseminated gonococcal infection in the newborn, neonatal conjunctivitis, low birth weight, miscarriage, premature rupture of membranes, preterm labor, and chorioamnionitis.2,12
As preterm birth is the leading cause of infant morbidity and mortality and disproportionately affects African American women and women in the southeastern United States,13 there is a critical public heath need to improve STD screening, treatment, and prevention of reinfection among high-risk pregnant women.
Quality clinical services for STDs: Areas for focus
More and more, STDs are being diagnosed in primary care settings. In January 2020, the Centers for Disease Control and Prevention (CDC) released a document, referred to as STD QCS (quality clinical services), that outlines recommendations for basic and specialty-level STD clinical services.14 ObGyns and other clinicians who provide primary care should meet the basic recommendations as a minimum.
The STD QCS outlines 8 recommendation areas: sexual history and physical examination, prevention, screening,
Continue to: Sexual history and physical examination...
Sexual history and physical examination
A complete sexual history and risk assessment should be performed at a complete initial or annual visit and as indicated. Routinely updating the sexual history and risk assessment is important to normalize these questions within the frame of the person’s overall health, and it may be valuable in reducing stigma. This routine approach may be important particularly for younger patients and others whose risk for STDs may change frequently and dramatically.
Creating a safe space that permits privacy and assurance of confidentiality may help build trust and set the stage for disclosure. The American College of Obstetricians and Gynecologists recommends that all young people have time alone without parents for confidential counseling and discussion.15 All states allow minors to consent for STD services themselves, although 11 states limit this to those beyond a certain age.16
The CDC recommends using the 5 P’s—partners, practices, protection, past history of STDs, and prevention of pregnancy—as a guide for discussion.14 ObGyns are more likely than other providers to perform this screening routinely. While a pelvic examination should be available for STD evaluation as needed, it is not required for routine screening.
Prevention
ObGyns should employ several recommendations for STD prevention. These include providing or referring patients for vaccination against hepatitis B and human papillomavirus and providing brief STD/HIV prevention counseling along with contraceptive counseling. ObGyns should be familiar with HIV pre-exposure prophylaxis (PrEP) and nonoccupational postexposure prophylaxis (nPEP) and provide risk assessment, education, and referral or link to HIV care. Providing these services would improve access to care and further remove barriers to care. ObGyns also could consider providing condoms in their offices.14
Screening
STD screening of women at risk is critical since more than 80% of infected women are asymptomatic.8 Because young people are disproportionately experiencing STDs, annual screening for chlamydia and gonorrhea is recommended for women younger than 25 years. For women older than 25, those at increased risk can be screened.
Risk factors for chlamydia infection include having new or multiple sex partners, sex partners with concurrent partners, or sex partners who have an STD. For gonorrhea, risk factors include living in a high-morbidity area, having a previous or coexisting STD, new or multiple sex partners, inconsistent condom use in people who are not in a mutually monogamous relationship, and exchanging sex for money or drugs. Screening for syphilis in nonpregnant women is recommended for those who have had any sexual activity with a person recently diagnosed with syphilis or those who personally display signs or symptoms of infection.17
STD screening is especially important for pregnant women, and treatment of infections may improve pregnancy outcomes. The CDC recommends screening at the first prenatal care visit for chlamydia and gonorrhea in pregnant women younger than 25 years of age and in older pregnant women at increased risk; women younger than 25 years or at continued high risk should be rescreened in their third trimester. The CDC recommends screening all women for syphilis at their first prenatal care visit and rescreening those at high risk in the third trimester and at delivery (TABLE).18

Continue to: Partner services...
Partner services
Clearly outlined partner management services is paramount for preventing STD reinfection.14 Reinfection rates for chlamydia and gonorrhea among young women are high and vary by study population.19 At a minimum, ObGyns should counsel patients with an STD that their partner(s) should be notified and encouraged to seek services.
For states in which it is legal, expedited partner therapy (EPT)—the clinician provides medication for the partner without seeing the partner—should be provided for chlamydia or gonorrhea if the partner is unlikely to access timely care. EPT is legal in most states. (To check the legal status of EPT in your state, visit https://www.cdc.gov/std/ept/legal/default.htm.) Research is needed to evaluate optimal strategies for effective implementation of EPT services in different clinical settings.
Laboratory tests
ObGyns should be able to provide a wide range of laboratory evaluations (for example, a nucleic acid amplification test [NAAT] for genital chlamydia and gonorrhea, quantitative nontreponemal serologic test for syphilis, treponemal serologic test for syphilis) that can be ordered for screening or diagnostic purposes. To improve rates of recommended screening, consider having clinic-level policies that support screening, such as standing orders, express or walk-in screening appointments, lab panels, and reflex testing.
Further, having rapid results or point-of-care testing available would help decrease lags in time to treatment. Delays in treatment are particularly important in lower-resource communities; thus, point-of-care testing may be especially valuable with immediate access to treatment on site.
Treatment
Adequate and timely treatment of STDs is critical to decrease sequelae and the likelihood of transmission to others. Treatment is evolving, particularly for gonorrhea. Over the past several years, gonorrhea has become resistant to 6 previously recommended treatment options.20 Since 2015, the CDC recommends dual therapy for gonorrhea with an injection of ceftriaxone and oral azithromycin.
The first-line recommended treatments for bacterial STDs are listed in the TABLE. When possible, it is preferred to offer directly observed therapy at the time of the visit. This decreases the time to treatment and ensures that therapy is completed.
A call to action for ObGyns
Clinicians have multiple opportunities to address and reduce the surge of STDs in the United States. We play a critical role in screening, diagnosing, and treating patients, and it is thus imperative to be up-to-date on the recommended guidelines. Further, clinicians can advocate for more rapid testing modalities, with the goal of obtaining point-of-care testing results when possible and implementing strategies to improve partner treatment.
While a positive STD result may be associated with significant patient distress, it also may be an opportunity for enhancing the patient-provider relationship, coupling education with motivational approaches to help patients increase protective health behaviors.
It is critical to approach clinical care in a nonjudgmental manner to improve patients’ comfort in their relationship with the health care system. ●
- Be aware of up-to-date screening, treatment, and follow-up recommendations for STDs
- Develop strategies to maximize partner treatment, including expedited partner therapy
- Identify high-risk individuals for whom counseling on HIV and unintended pregnancy prevention strategies can be reinforced, including PrEP and contraception
- Create a clinical environment that normalizes STD testing and destigmatizes infection
- Integrate client-centered counseling to improve protective health behaviors
Abbreviations: HIV, human immunodeficiency virus; PrEP, pre-exposure prophylaxis; STD, sexually transmitted disease.
- Centers for Disease Control and Prevention. 2018 STD surveillance report. https://www.cdc.gov/nchhstp /newsroom/2019/2018-STD-surveillance-report.html. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDs): STDs during pregnancy—CDC fact sheet (detailed). www.cdc.gov/std/pregnancy/stdfact -sheet-pregnancy-detailed.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in racial and ethnic minorities 2017. https://www.cdc.gov/std/stats17 /minorities.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in women and infants. https://www.cdc.gov/std/stats17/womenandinf .htm. Accessed March 19, 2020.
- Semega JL, Fontenot KR, Kollar MA; US Census Bureau. Income and poverty in the United States: 2016. Washington, DC: US Government Printing Office; 2017. https://www.census.gov/content/dam/Census/library /publications/2017/demo/P60-259.pdf. Accessed March 19, 2020.
- Harling G, Subramanian S, Barnighausen T, et al. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40:575-581.
- Meyer PA, Penman-Aguilar A, Campbell VA, et al; Centers for Disease Control and Prevention. Conclusion and future directions: CDC Health Disparities and Inequalities Report— United States, 2013. MMWR Suppl. 2013;62(3):184-186.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03): 1-137.
- Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis. 1990;161:531-536.
- Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect. 2019;95:60-66.
- Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183:662-668.
- Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397-404.
- March of Dimes. Maternal, infant, and child health in the United States, 2016. https://www.marchofdimes.org /materials/March-of-Dimes-2016-Databook.pdf. Accessed March 19, 2020.
- Barrow RY, Ahmed F, Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(5):1-20.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 598: The initial reproductive health visit. May 2014. https:// www.acog.org/-/media /project/acog/acogorg/clinical/files/committee-opinion /articles/2014/05/the-initial-reproductive-health-visit.pdf. Accessed March 31, 2020.
- Guttmacher Institute. An overview of consent to reproductive health services by young people. March 1, 2020. https://www .guttmacher.org/state-policy/explore/overview-minors -consent-law. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Pocket guide for providers: Syphilis: a provider’s guide to treatment and prevention. 2017. https://www.cdc.gov/std/syphilis /Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines: syphilis during pregnancy. https://www.cdc.gov/std/tg2015/syphilis -pregnancy.htm. Accessed March 19, 2020.
- Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-489.
- Bodie M, Gale-Rowe M, Alexandre S, et al. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: there is no time like the present. Can Commun Dis Rep. 2019;45:54-62.
- Centers for Disease Control and Prevention. 2018 STD surveillance report. https://www.cdc.gov/nchhstp /newsroom/2019/2018-STD-surveillance-report.html. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted diseases (STDs): STDs during pregnancy—CDC fact sheet (detailed). www.cdc.gov/std/pregnancy/stdfact -sheet-pregnancy-detailed.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in racial and ethnic minorities 2017. https://www.cdc.gov/std/stats17 /minorities.htm. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2017: STDs in women and infants. https://www.cdc.gov/std/stats17/womenandinf .htm. Accessed March 19, 2020.
- Semega JL, Fontenot KR, Kollar MA; US Census Bureau. Income and poverty in the United States: 2016. Washington, DC: US Government Printing Office; 2017. https://www.census.gov/content/dam/Census/library /publications/2017/demo/P60-259.pdf. Accessed March 19, 2020.
- Harling G, Subramanian S, Barnighausen T, et al. Socioeconomic disparities in sexually transmitted infections among young adults in the United States: examining the interaction between income and race/ethnicity. Sex Transm Dis. 2013;40:575-581.
- Meyer PA, Penman-Aguilar A, Campbell VA, et al; Centers for Disease Control and Prevention. Conclusion and future directions: CDC Health Disparities and Inequalities Report— United States, 2013. MMWR Suppl. 2013;62(3):184-186.
- Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03): 1-137.
- Elliott B, Brunham RC, Laga M, et al. Maternal gonococcal infection as a preventable risk factor for low birth weight. J Infect Dis. 1990;161:531-536.
- Warr AJ, Pintye J, Kinuthia J, et al. Sexually transmitted infections during pregnancy and subsequent risk of stillbirth and infant mortality in Kenya: a prospective study. Sex Transm Infect. 2019;95:60-66.
- Andrews WW, Goldenberg RL, Mercer B, et al. The Preterm Prediction Study: association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am J Obstet Gynecol. 2000;183:662-668.
- Alger LS, Lovchik JC, Hebel JR, et al. The association of Chlamydia trachomatis, Neisseria gonorrhoeae, and group B streptococci with preterm rupture of the membranes and pregnancy outcome. Am J Obstet Gynecol. 1988;159:397-404.
- March of Dimes. Maternal, infant, and child health in the United States, 2016. https://www.marchofdimes.org /materials/March-of-Dimes-2016-Databook.pdf. Accessed March 19, 2020.
- Barrow RY, Ahmed F, Bolan GA, et al. Recommendations for providing quality sexually transmitted diseases clinical services, 2020. MMWR Recomm Rep. 2020;68(5):1-20.
- American College of Obstetricians and Gynecologists. ACOG committee opinion No. 598: The initial reproductive health visit. May 2014. https:// www.acog.org/-/media /project/acog/acogorg/clinical/files/committee-opinion /articles/2014/05/the-initial-reproductive-health-visit.pdf. Accessed March 31, 2020.
- Guttmacher Institute. An overview of consent to reproductive health services by young people. March 1, 2020. https://www .guttmacher.org/state-policy/explore/overview-minors -consent-law. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. Pocket guide for providers: Syphilis: a provider’s guide to treatment and prevention. 2017. https://www.cdc.gov/std/syphilis /Syphilis-Pocket-Guide-FINAL-508.pdf. Accessed March 19, 2020.
- Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines: syphilis during pregnancy. https://www.cdc.gov/std/tg2015/syphilis -pregnancy.htm. Accessed March 19, 2020.
- Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36:478-489.
- Bodie M, Gale-Rowe M, Alexandre S, et al. Addressing the rising rates of gonorrhea and drug-resistant gonorrhea: there is no time like the present. Can Commun Dis Rep. 2019;45:54-62.
FDA removes pregnancy category C warning from certain MS medications
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
COVID-19 experiences from the ob.gyn. front line
As the COVID-19 pandemic continues to spread across the United States, several members of the Ob.Gyn. News Editorial Advisory Board shared their experiences.
Catherine Cansino, MD, MPH, who is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, discussed the changes COVID-19 has had on local and regional practice in Sacramento and northern California.
There has been a dramatic increase in telehealth, using video, phone, and apps such as Zoom. Although ob.gyns. at the university are limiting outpatient appointments to essential visits only, we are continuing to offer telehealth to a few nonessential visits. This will be readdressed when the COVID-19 cases peak, Dr. Cansino said.
All patients admitted to labor & delivery undergo COVID-19 testing regardless of symptoms. For patients in the clinic who are expected to be induced or scheduled for cesarean delivery, we are screening them within 72 hours before admission.
In gynecology, only essential or urgent surgeries at UC Davis are being performed and include indications such as cancer, serious benign conditions unresponsive to conservative treatment (e.g., tubo-ovarian abscess, large symptomatic adnexal mass), and pregnancy termination. We are preserving access to abortion and reproductive health services since these are essential services.
We limit the number of providers involved in direct contact with inpatients to one or two, including a physician, nurse, and/or resident, Dr. Cansino said in an interview. Based on recent Liaison Committee on Medical Education policies related to concerns about educational experience during the pandemic, no medical students are allowed at the hospital at present. We also severely restrict the number of visitors in the inpatient and outpatient settings, including only two attendants (partner, doula, and such) during labor and delivery, and consider the impact on patients’ well-being when we restrict their visitors.
We are following University of California guidelines regarding face mask use, which have been in evolution over the last month. Face masks are used for patients and the health care providers primarily when patients either have known COVID-19 infection or are considered as patients under investigation or if the employee had a high-risk exposure. The use of face masks is becoming more permissive, rather than mandatory, to conserve personal protective equipment (PPE) for when the surge arrives.
Education is ongoing about caring for our families and ourselves if we get infected and need to isolate within our own homes. The department and health system is trying to balance the challenges of urgent patient care needs against the wellness concerns for the faculty, staff, and residents. Many physicians are also struggling with childcare problems, which add to our personal stress. There is anxiety among many physicians about exposure to asymptomatic carriers, including themselves, patients, and their families, Dr. Cansino said.
David Forstein, DO, dean and professor of obstetrics and gynecology at Touro College of Osteopathic Medicine, New York, said in an interview that the COVID-19 pandemic has “totally disrupted medical education. At almost all medical schools, didactics have moved completely online – ZOOM sessions abound, but labs become demonstrations, if at all, during the preclinical years. The clinical years have been put on hold, as well as student rotations suspended, out of caution for the students because hospitals needed to conserve PPE for the essential personnel and because administrators knew there would be less time for teaching. After initially requesting a pause, many hospitals now are asking students to come back because so many physicians, nurses, and residents have become ill with COVID-19 and either are quarantined or are patients in the hospital themselves.
“There has been a state-by-state call to consider graduating health professions students early, and press them into service, before their residencies actually begin. Some locations are looking for these new graduates to volunteer; some are willing to pay them a resident’s salary level. Medical schools are auditing their student records now to see which students would qualify to graduate early,” Dr. Forstein noted.
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology at the Einstein Health Care Network in Philadelphia, described in an email interview how COVID-19 has changed practice.
To minimize the number of providers on the front line, we have developed a Monday to Friday rotating schedule of three teams of five members, he explained. There will be a hospital-based team, an office-based team, and a telehealth-based team who will provide their services from home. On-call responsibilities remain the same.
The hospital team, working 7 a.m. to 5 p.m., will rotate through assignments each day:
- One person will cover labor and delivery.
- One person will cover triage and help on labor and delivery.
- One person will be assigned to the resident office.
- One person will be assigned to cover the team of the post call attending (Sunday through Thursday call).
- One person will be assigned to gynecology coverage, consults, and postpartum rounds.
To further minimize the patient interactions, when possible, each patient should be seen by the attending physician with the resident. This is a change from usual practice, where the patient is first seen by the resident, who reports back to the attending, and then both physicians see the patient together.
The network’s offices now open from 9 a.m. (many offices had been offering early-morning hours starting at 7 a.m.), and the physicians and advanced practice providers will work through the last scheduled patient appointment, Dr. Jaspan explained. “The office-based team will preferentially see in-person visits.”
Several offices have been closed so that ob.gyns. and staff can be reassigned to telehealth. The remaining five offices generally have one attending physician and one advanced practice provider.
The remaining team of ob.gyns. provides telehealth with the help of staff members. This involves an initial call to the patient by staff letting them know the doctor will be calling, checking them in, verifying insurance, and collecting payment, followed by the actual telehealth visit. If follow-up is needed, the staff member schedules the follow-up.
Dr. Jaspan called the new approach to prenatal care because of COVID-19 a “cataclysmic change in how we care for our patients. We have decided to further limit our obstetrical in-person visits. It is our feeling that these changes will enable patients to remain outside of the office and in the safety of their homes, provide appropriate social distancing, and diminish potential exposures to the office staff providers and patients.”
In-person visits will occur at: the initial visit, between 24 and 28 weeks, at 32 weeks, and at 36 or 37 weeks; if the patient at 36/37 has a blood pressure cuff, they will not have additional scheduled in-patient visits. We have partnered with the insurance companies to provide more than 88% of obstetrical patients with home blood pressure cuffs.
Obstetrical visits via telehealth will continue at our standard intervals: monthly until 26 weeks; twice monthly during 26-36 weeks; and weekly from 37 weeks to delivery. These visits should use a video component such as Zoom, Doxy.me, or FaceTime.
“If the patient has concerns or problems, we will see them at any time. However, the new standard will be telehealth visits and the exception will be the in-person visit,” Dr. Jaspan said.
In addition, we have worked our division of maternal-fetal medicine to adjust the antenatal testing schedules, and we have curtailed the frequency of ultrasound, he noted.
He emphasized the importance of documenting telehealth interactions with obstetrical patients, in addition to “providing adequate teaching and education for patients regarding kick counts to ensure fetal well-being.” It also is key to “properly document conversations with patients regarding bleeding, rupture of membranes, fetal movement, headache, visual changes, fevers, cough, nausea and vomiting, diarrhea, fatigue, muscle aches, etc.”
The residents’ schedule also has been modified to diminish their exposure. Within our new paradigm, we have scheduled video conferences to enable our program to maintain our commitment to academics.
It is imperative that we keep our patients safe, and it is critical to protect our staff members. Those who provide women’s health cannot be replaced by other nurses or physicians.
Mark P. Trolice, MD, is director of Fertility CARE: the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He related in an email interview that, on March 17, 2020, the American Society for Reproductive Medicine (ASRM) released “Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic.” This document serves as guidance on fertility care during the current crisis. Specifically, the recommendations include the following:
- Suspend initiation of new treatment cycles, including ovulation induction, intrauterine inseminations, in vitro fertilization including retrievals and frozen embryo transfers, and nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
- Minimize in-person interactions and increase utilization of telehealth.
As a member of ASRM for more than 2 decades and a participant of several of their committees, my practice immediately ceased treatment cycles to comply with this guidance.
Then on March 20, 2020, the Florida governor’s executive order 20-72 was released, stating, “All hospitals, ambulatory surgical centers, office surgery centers, dental, orthodontic and endodontic offices, and other health care practitioners’ offices in the State of Florida are prohibited from providing any medically unnecessary, nonurgent or nonemergency procedure or surgery which, if delayed, does not place a patient’s immediate health, safety, or well-being at risk, or will, if delayed, not contribute to the worsening of a serious or life-threatening medical condition.”
As a result, my practice has been limited to telemedicine consultations. While the ASRM guidance and the gubernatorial executive order pose a significant financial hardship on my center and all applicable medical clinics in my state, resulting in expected layoffs, salary reductions, and requests for government stimulus loans, the greater good takes priority and we pray for all the victims of this devastating pandemic.
The governor’s current executive order is set to expire on May 9, 2020, unless it is extended.
ASRM released an update of their guidance on March 30, 2020, offering no change from their prior recommendations. The organization plans to reevaluate the guidance at 2-week intervals.
Sangeeta Sinha, MD, an ob.gyn. in private practice at Stone Springs Hospital Center, Dulles, Va. said in an interview, “COVID 19 has put fear in all aspects of our daily activities which we are attempting to cope with.”
She related several changes made to her office and hospital environments. “In our office, we are now wearing a mask at all times, gloves to examine every patient. We have staggered physicians in the office to take televisits and in-office patients. We are screening all new patients on the phone to determine if they are sick, have traveled to high-risk, hot spot areas of the country, or have had contact with someone who tested positive for COVID-19. We are only seeing our pregnant women and have also pushed out their return appointments to 4 weeks if possible. There are several staff who are not working due to fear or are in self quarantine so we have shortage of staff in the office. At the hospital as well we are wearing a mask at all times, using personal protective equipment for deliveries and C-sections.
“We have had several scares, including a new transfer of an 18-year-old pregnant patient at 30 weeks with cough and sore throat, who later reported that her roommate is very sick and he works with someone who has tested positive for COVID-19. Thankfully she is healthy and well. We learned several lessons from this one.”
As the COVID-19 pandemic continues to spread across the United States, several members of the Ob.Gyn. News Editorial Advisory Board shared their experiences.
Catherine Cansino, MD, MPH, who is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, discussed the changes COVID-19 has had on local and regional practice in Sacramento and northern California.
There has been a dramatic increase in telehealth, using video, phone, and apps such as Zoom. Although ob.gyns. at the university are limiting outpatient appointments to essential visits only, we are continuing to offer telehealth to a few nonessential visits. This will be readdressed when the COVID-19 cases peak, Dr. Cansino said.
All patients admitted to labor & delivery undergo COVID-19 testing regardless of symptoms. For patients in the clinic who are expected to be induced or scheduled for cesarean delivery, we are screening them within 72 hours before admission.
In gynecology, only essential or urgent surgeries at UC Davis are being performed and include indications such as cancer, serious benign conditions unresponsive to conservative treatment (e.g., tubo-ovarian abscess, large symptomatic adnexal mass), and pregnancy termination. We are preserving access to abortion and reproductive health services since these are essential services.
We limit the number of providers involved in direct contact with inpatients to one or two, including a physician, nurse, and/or resident, Dr. Cansino said in an interview. Based on recent Liaison Committee on Medical Education policies related to concerns about educational experience during the pandemic, no medical students are allowed at the hospital at present. We also severely restrict the number of visitors in the inpatient and outpatient settings, including only two attendants (partner, doula, and such) during labor and delivery, and consider the impact on patients’ well-being when we restrict their visitors.
We are following University of California guidelines regarding face mask use, which have been in evolution over the last month. Face masks are used for patients and the health care providers primarily when patients either have known COVID-19 infection or are considered as patients under investigation or if the employee had a high-risk exposure. The use of face masks is becoming more permissive, rather than mandatory, to conserve personal protective equipment (PPE) for when the surge arrives.
Education is ongoing about caring for our families and ourselves if we get infected and need to isolate within our own homes. The department and health system is trying to balance the challenges of urgent patient care needs against the wellness concerns for the faculty, staff, and residents. Many physicians are also struggling with childcare problems, which add to our personal stress. There is anxiety among many physicians about exposure to asymptomatic carriers, including themselves, patients, and their families, Dr. Cansino said.
David Forstein, DO, dean and professor of obstetrics and gynecology at Touro College of Osteopathic Medicine, New York, said in an interview that the COVID-19 pandemic has “totally disrupted medical education. At almost all medical schools, didactics have moved completely online – ZOOM sessions abound, but labs become demonstrations, if at all, during the preclinical years. The clinical years have been put on hold, as well as student rotations suspended, out of caution for the students because hospitals needed to conserve PPE for the essential personnel and because administrators knew there would be less time for teaching. After initially requesting a pause, many hospitals now are asking students to come back because so many physicians, nurses, and residents have become ill with COVID-19 and either are quarantined or are patients in the hospital themselves.
“There has been a state-by-state call to consider graduating health professions students early, and press them into service, before their residencies actually begin. Some locations are looking for these new graduates to volunteer; some are willing to pay them a resident’s salary level. Medical schools are auditing their student records now to see which students would qualify to graduate early,” Dr. Forstein noted.
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology at the Einstein Health Care Network in Philadelphia, described in an email interview how COVID-19 has changed practice.
To minimize the number of providers on the front line, we have developed a Monday to Friday rotating schedule of three teams of five members, he explained. There will be a hospital-based team, an office-based team, and a telehealth-based team who will provide their services from home. On-call responsibilities remain the same.
The hospital team, working 7 a.m. to 5 p.m., will rotate through assignments each day:
- One person will cover labor and delivery.
- One person will cover triage and help on labor and delivery.
- One person will be assigned to the resident office.
- One person will be assigned to cover the team of the post call attending (Sunday through Thursday call).
- One person will be assigned to gynecology coverage, consults, and postpartum rounds.
To further minimize the patient interactions, when possible, each patient should be seen by the attending physician with the resident. This is a change from usual practice, where the patient is first seen by the resident, who reports back to the attending, and then both physicians see the patient together.
The network’s offices now open from 9 a.m. (many offices had been offering early-morning hours starting at 7 a.m.), and the physicians and advanced practice providers will work through the last scheduled patient appointment, Dr. Jaspan explained. “The office-based team will preferentially see in-person visits.”
Several offices have been closed so that ob.gyns. and staff can be reassigned to telehealth. The remaining five offices generally have one attending physician and one advanced practice provider.
The remaining team of ob.gyns. provides telehealth with the help of staff members. This involves an initial call to the patient by staff letting them know the doctor will be calling, checking them in, verifying insurance, and collecting payment, followed by the actual telehealth visit. If follow-up is needed, the staff member schedules the follow-up.
Dr. Jaspan called the new approach to prenatal care because of COVID-19 a “cataclysmic change in how we care for our patients. We have decided to further limit our obstetrical in-person visits. It is our feeling that these changes will enable patients to remain outside of the office and in the safety of their homes, provide appropriate social distancing, and diminish potential exposures to the office staff providers and patients.”
In-person visits will occur at: the initial visit, between 24 and 28 weeks, at 32 weeks, and at 36 or 37 weeks; if the patient at 36/37 has a blood pressure cuff, they will not have additional scheduled in-patient visits. We have partnered with the insurance companies to provide more than 88% of obstetrical patients with home blood pressure cuffs.
Obstetrical visits via telehealth will continue at our standard intervals: monthly until 26 weeks; twice monthly during 26-36 weeks; and weekly from 37 weeks to delivery. These visits should use a video component such as Zoom, Doxy.me, or FaceTime.
“If the patient has concerns or problems, we will see them at any time. However, the new standard will be telehealth visits and the exception will be the in-person visit,” Dr. Jaspan said.
In addition, we have worked our division of maternal-fetal medicine to adjust the antenatal testing schedules, and we have curtailed the frequency of ultrasound, he noted.
He emphasized the importance of documenting telehealth interactions with obstetrical patients, in addition to “providing adequate teaching and education for patients regarding kick counts to ensure fetal well-being.” It also is key to “properly document conversations with patients regarding bleeding, rupture of membranes, fetal movement, headache, visual changes, fevers, cough, nausea and vomiting, diarrhea, fatigue, muscle aches, etc.”
The residents’ schedule also has been modified to diminish their exposure. Within our new paradigm, we have scheduled video conferences to enable our program to maintain our commitment to academics.
It is imperative that we keep our patients safe, and it is critical to protect our staff members. Those who provide women’s health cannot be replaced by other nurses or physicians.
Mark P. Trolice, MD, is director of Fertility CARE: the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He related in an email interview that, on March 17, 2020, the American Society for Reproductive Medicine (ASRM) released “Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic.” This document serves as guidance on fertility care during the current crisis. Specifically, the recommendations include the following:
- Suspend initiation of new treatment cycles, including ovulation induction, intrauterine inseminations, in vitro fertilization including retrievals and frozen embryo transfers, and nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
- Minimize in-person interactions and increase utilization of telehealth.
As a member of ASRM for more than 2 decades and a participant of several of their committees, my practice immediately ceased treatment cycles to comply with this guidance.
Then on March 20, 2020, the Florida governor’s executive order 20-72 was released, stating, “All hospitals, ambulatory surgical centers, office surgery centers, dental, orthodontic and endodontic offices, and other health care practitioners’ offices in the State of Florida are prohibited from providing any medically unnecessary, nonurgent or nonemergency procedure or surgery which, if delayed, does not place a patient’s immediate health, safety, or well-being at risk, or will, if delayed, not contribute to the worsening of a serious or life-threatening medical condition.”
As a result, my practice has been limited to telemedicine consultations. While the ASRM guidance and the gubernatorial executive order pose a significant financial hardship on my center and all applicable medical clinics in my state, resulting in expected layoffs, salary reductions, and requests for government stimulus loans, the greater good takes priority and we pray for all the victims of this devastating pandemic.
The governor’s current executive order is set to expire on May 9, 2020, unless it is extended.
ASRM released an update of their guidance on March 30, 2020, offering no change from their prior recommendations. The organization plans to reevaluate the guidance at 2-week intervals.
Sangeeta Sinha, MD, an ob.gyn. in private practice at Stone Springs Hospital Center, Dulles, Va. said in an interview, “COVID 19 has put fear in all aspects of our daily activities which we are attempting to cope with.”
She related several changes made to her office and hospital environments. “In our office, we are now wearing a mask at all times, gloves to examine every patient. We have staggered physicians in the office to take televisits and in-office patients. We are screening all new patients on the phone to determine if they are sick, have traveled to high-risk, hot spot areas of the country, or have had contact with someone who tested positive for COVID-19. We are only seeing our pregnant women and have also pushed out their return appointments to 4 weeks if possible. There are several staff who are not working due to fear or are in self quarantine so we have shortage of staff in the office. At the hospital as well we are wearing a mask at all times, using personal protective equipment for deliveries and C-sections.
“We have had several scares, including a new transfer of an 18-year-old pregnant patient at 30 weeks with cough and sore throat, who later reported that her roommate is very sick and he works with someone who has tested positive for COVID-19. Thankfully she is healthy and well. We learned several lessons from this one.”
As the COVID-19 pandemic continues to spread across the United States, several members of the Ob.Gyn. News Editorial Advisory Board shared their experiences.
Catherine Cansino, MD, MPH, who is an associate clinical professor in the department of obstetrics and gynecology at the University of California, Davis, discussed the changes COVID-19 has had on local and regional practice in Sacramento and northern California.
There has been a dramatic increase in telehealth, using video, phone, and apps such as Zoom. Although ob.gyns. at the university are limiting outpatient appointments to essential visits only, we are continuing to offer telehealth to a few nonessential visits. This will be readdressed when the COVID-19 cases peak, Dr. Cansino said.
All patients admitted to labor & delivery undergo COVID-19 testing regardless of symptoms. For patients in the clinic who are expected to be induced or scheduled for cesarean delivery, we are screening them within 72 hours before admission.
In gynecology, only essential or urgent surgeries at UC Davis are being performed and include indications such as cancer, serious benign conditions unresponsive to conservative treatment (e.g., tubo-ovarian abscess, large symptomatic adnexal mass), and pregnancy termination. We are preserving access to abortion and reproductive health services since these are essential services.
We limit the number of providers involved in direct contact with inpatients to one or two, including a physician, nurse, and/or resident, Dr. Cansino said in an interview. Based on recent Liaison Committee on Medical Education policies related to concerns about educational experience during the pandemic, no medical students are allowed at the hospital at present. We also severely restrict the number of visitors in the inpatient and outpatient settings, including only two attendants (partner, doula, and such) during labor and delivery, and consider the impact on patients’ well-being when we restrict their visitors.
We are following University of California guidelines regarding face mask use, which have been in evolution over the last month. Face masks are used for patients and the health care providers primarily when patients either have known COVID-19 infection or are considered as patients under investigation or if the employee had a high-risk exposure. The use of face masks is becoming more permissive, rather than mandatory, to conserve personal protective equipment (PPE) for when the surge arrives.
Education is ongoing about caring for our families and ourselves if we get infected and need to isolate within our own homes. The department and health system is trying to balance the challenges of urgent patient care needs against the wellness concerns for the faculty, staff, and residents. Many physicians are also struggling with childcare problems, which add to our personal stress. There is anxiety among many physicians about exposure to asymptomatic carriers, including themselves, patients, and their families, Dr. Cansino said.
David Forstein, DO, dean and professor of obstetrics and gynecology at Touro College of Osteopathic Medicine, New York, said in an interview that the COVID-19 pandemic has “totally disrupted medical education. At almost all medical schools, didactics have moved completely online – ZOOM sessions abound, but labs become demonstrations, if at all, during the preclinical years. The clinical years have been put on hold, as well as student rotations suspended, out of caution for the students because hospitals needed to conserve PPE for the essential personnel and because administrators knew there would be less time for teaching. After initially requesting a pause, many hospitals now are asking students to come back because so many physicians, nurses, and residents have become ill with COVID-19 and either are quarantined or are patients in the hospital themselves.
“There has been a state-by-state call to consider graduating health professions students early, and press them into service, before their residencies actually begin. Some locations are looking for these new graduates to volunteer; some are willing to pay them a resident’s salary level. Medical schools are auditing their student records now to see which students would qualify to graduate early,” Dr. Forstein noted.
David M. Jaspan, DO, chairman of the department of obstetrics and gynecology at the Einstein Health Care Network in Philadelphia, described in an email interview how COVID-19 has changed practice.
To minimize the number of providers on the front line, we have developed a Monday to Friday rotating schedule of three teams of five members, he explained. There will be a hospital-based team, an office-based team, and a telehealth-based team who will provide their services from home. On-call responsibilities remain the same.
The hospital team, working 7 a.m. to 5 p.m., will rotate through assignments each day:
- One person will cover labor and delivery.
- One person will cover triage and help on labor and delivery.
- One person will be assigned to the resident office.
- One person will be assigned to cover the team of the post call attending (Sunday through Thursday call).
- One person will be assigned to gynecology coverage, consults, and postpartum rounds.
To further minimize the patient interactions, when possible, each patient should be seen by the attending physician with the resident. This is a change from usual practice, where the patient is first seen by the resident, who reports back to the attending, and then both physicians see the patient together.
The network’s offices now open from 9 a.m. (many offices had been offering early-morning hours starting at 7 a.m.), and the physicians and advanced practice providers will work through the last scheduled patient appointment, Dr. Jaspan explained. “The office-based team will preferentially see in-person visits.”
Several offices have been closed so that ob.gyns. and staff can be reassigned to telehealth. The remaining five offices generally have one attending physician and one advanced practice provider.
The remaining team of ob.gyns. provides telehealth with the help of staff members. This involves an initial call to the patient by staff letting them know the doctor will be calling, checking them in, verifying insurance, and collecting payment, followed by the actual telehealth visit. If follow-up is needed, the staff member schedules the follow-up.
Dr. Jaspan called the new approach to prenatal care because of COVID-19 a “cataclysmic change in how we care for our patients. We have decided to further limit our obstetrical in-person visits. It is our feeling that these changes will enable patients to remain outside of the office and in the safety of their homes, provide appropriate social distancing, and diminish potential exposures to the office staff providers and patients.”
In-person visits will occur at: the initial visit, between 24 and 28 weeks, at 32 weeks, and at 36 or 37 weeks; if the patient at 36/37 has a blood pressure cuff, they will not have additional scheduled in-patient visits. We have partnered with the insurance companies to provide more than 88% of obstetrical patients with home blood pressure cuffs.
Obstetrical visits via telehealth will continue at our standard intervals: monthly until 26 weeks; twice monthly during 26-36 weeks; and weekly from 37 weeks to delivery. These visits should use a video component such as Zoom, Doxy.me, or FaceTime.
“If the patient has concerns or problems, we will see them at any time. However, the new standard will be telehealth visits and the exception will be the in-person visit,” Dr. Jaspan said.
In addition, we have worked our division of maternal-fetal medicine to adjust the antenatal testing schedules, and we have curtailed the frequency of ultrasound, he noted.
He emphasized the importance of documenting telehealth interactions with obstetrical patients, in addition to “providing adequate teaching and education for patients regarding kick counts to ensure fetal well-being.” It also is key to “properly document conversations with patients regarding bleeding, rupture of membranes, fetal movement, headache, visual changes, fevers, cough, nausea and vomiting, diarrhea, fatigue, muscle aches, etc.”
The residents’ schedule also has been modified to diminish their exposure. Within our new paradigm, we have scheduled video conferences to enable our program to maintain our commitment to academics.
It is imperative that we keep our patients safe, and it is critical to protect our staff members. Those who provide women’s health cannot be replaced by other nurses or physicians.
Mark P. Trolice, MD, is director of Fertility CARE: the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He related in an email interview that, on March 17, 2020, the American Society for Reproductive Medicine (ASRM) released “Patient Management and Clinical Recommendations During the Coronavirus (COVID-19) Pandemic.” This document serves as guidance on fertility care during the current crisis. Specifically, the recommendations include the following:
- Suspend initiation of new treatment cycles, including ovulation induction, intrauterine inseminations, in vitro fertilization including retrievals and frozen embryo transfers, and nonurgent gamete cryopreservation.
- Strongly consider cancellation of all embryo transfers, whether fresh or frozen.
- Continue to care for patients who are currently “in cycle” or who require urgent stimulation and cryopreservation.
- Suspend elective surgeries and nonurgent diagnostic procedures.
- Minimize in-person interactions and increase utilization of telehealth.
As a member of ASRM for more than 2 decades and a participant of several of their committees, my practice immediately ceased treatment cycles to comply with this guidance.
Then on March 20, 2020, the Florida governor’s executive order 20-72 was released, stating, “All hospitals, ambulatory surgical centers, office surgery centers, dental, orthodontic and endodontic offices, and other health care practitioners’ offices in the State of Florida are prohibited from providing any medically unnecessary, nonurgent or nonemergency procedure or surgery which, if delayed, does not place a patient’s immediate health, safety, or well-being at risk, or will, if delayed, not contribute to the worsening of a serious or life-threatening medical condition.”
As a result, my practice has been limited to telemedicine consultations. While the ASRM guidance and the gubernatorial executive order pose a significant financial hardship on my center and all applicable medical clinics in my state, resulting in expected layoffs, salary reductions, and requests for government stimulus loans, the greater good takes priority and we pray for all the victims of this devastating pandemic.
The governor’s current executive order is set to expire on May 9, 2020, unless it is extended.
ASRM released an update of their guidance on March 30, 2020, offering no change from their prior recommendations. The organization plans to reevaluate the guidance at 2-week intervals.
Sangeeta Sinha, MD, an ob.gyn. in private practice at Stone Springs Hospital Center, Dulles, Va. said in an interview, “COVID 19 has put fear in all aspects of our daily activities which we are attempting to cope with.”
She related several changes made to her office and hospital environments. “In our office, we are now wearing a mask at all times, gloves to examine every patient. We have staggered physicians in the office to take televisits and in-office patients. We are screening all new patients on the phone to determine if they are sick, have traveled to high-risk, hot spot areas of the country, or have had contact with someone who tested positive for COVID-19. We are only seeing our pregnant women and have also pushed out their return appointments to 4 weeks if possible. There are several staff who are not working due to fear or are in self quarantine so we have shortage of staff in the office. At the hospital as well we are wearing a mask at all times, using personal protective equipment for deliveries and C-sections.
“We have had several scares, including a new transfer of an 18-year-old pregnant patient at 30 weeks with cough and sore throat, who later reported that her roommate is very sick and he works with someone who has tested positive for COVID-19. Thankfully she is healthy and well. We learned several lessons from this one.”
Barriers to clinical trial participation revealed by gynecologic cancer patients
A survey of gynecologic cancer survivors has revealed why some of these patients don’t participate in clinical trials.
Half of survey respondents with no history of trial participation said their medical team never mentioned the possibility of a trial. About 27% of respondents who never enrolled in a trial said they were interested in trial participation but didn’t qualify, the trial they wanted wasn’t available, their insurance didn’t cover participation, or the trial site was too far away.
Annie Ellis and Mary (Dicey) Jackson Scroggins, who are both ovarian cancer survivors and patient advocates, reported these findings in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“We thought it was important to hear and learn directly from gynecologic cancer survivors,” Ms. Ellis said in an interview. “So we decided to conduct a survey that would expand knowledge about clinical trial participation from a gynecologic cancer patient–specific perspective.”
Ms. Ellis and Ms. Scroggins used survivor networks and social media to distribute a 26-question survey on trial participation. The survey was completed by 189 survivors of gynecologic cancers, 49.19% of whom experienced recurrent disease. The most common diagnoses were ovarian cancer (69.84%) and endometrial or uterine cancer (23.28%).
Perspectives of nonparticipants
Most respondents (65.61%) had never participated in a clinical trial. The most common reason was that the patient’s doctor or medical team never discussed trial participation (50.40%).
There were patients who were interested in trial participation but couldn’t enroll because they didn’t qualify (14.40%), the location was too far away (7.20%), the trial they wanted wasn’t available (4.00%), or their insurance didn’t cover trial participation (1.60%).
Patients who were not interested in trial participation said they didn’t want to receive a placebo (11.20%), they weren’t interested in experimental therapies (3.20%), or they didn’t want to be randomized (2.40%). One patient (1.60%) said she does not trust the medical system.
“Given the frequent conversations about distrust in the medical system, we were surprised that only 1 of the 189 respondents indicated distrust in the medical system as a reason for not participating in a clinical trial,” Ms. Ellis said.
Perspectives of trial participants
Roughly a third of respondents (34.39%) had participated in a clinical trial. Most (86.15%) said they learned about the trial from their doctor. Other sources included the patient’s own research (13.85%), a trial matching service (3.08%), a family member or friend (3.08%), and a support group (1.54%).
The most common reasons patients participated in trials were: “my doctor recommended it,” “to help women in the future,” “to expand my treatment options,” and “to have a chance to benefit personally.”
Additional responses indicated that patients viewed their trial participation in a positive light.
“We were surprised to find that 100% of the respondents who had participated in a clinical trial indicated either that they would participate again (84.62%) or that they were not sure about future participation (15.38%),” Ms. Ellis said. “No respondent indicated that she would not consider another trial. From open comments in the survey, it was clear that even if they did not obtain the result they hoped for or if the experience wasn’t optimal, they maintained the option of participating again.”
Implications and next steps
The survey results suggest there is a need for more discussions about clinical trials with patients who have gynecologic cancers, according to Ms. Ellis and Ms. Scroggins.
“We feel that conversations about clinical trials, with health care team members, should be included at every care decision point, even if – or perhaps especially if – the patient belongs to a group perceived to be unlikely to agree to participate in a trial,” Ms. Ellis said.
“These conversations are necessary with all patients-survivors,” she said, “but they are particularly important and necessary with patients from populations underrepresented in the clinical trial system if we want more representative trial populations, more generalizable results, and the potential for better outcomes for all.”
For their part, Ms. Ellis and Ms. Scroggins plan to conduct more research on this topic to gain additional insights.
“We’d like to conduct a larger survey looking deeper into barriers to and reasons for participation, and to work with medical professionals to develop models of communication to encourage consideration of clinical trials,” Ms. Ellis said. “Additionally, we will work to have a more diverse respondent pool across many dimensions.”
Ms. Ellis is a research advocate on the scientific advisory committee of the Ovarian Cancer National Alliance in Washington. Ms. Scroggins is the director of global outreach and engagement at the International Gynecologic Cancer Society in Louisville, Ken. They have no conflicts of interest.
SOURCE: Ellis A and Scroggins MJ. SGO 2020, Abstract 540.
A survey of gynecologic cancer survivors has revealed why some of these patients don’t participate in clinical trials.
Half of survey respondents with no history of trial participation said their medical team never mentioned the possibility of a trial. About 27% of respondents who never enrolled in a trial said they were interested in trial participation but didn’t qualify, the trial they wanted wasn’t available, their insurance didn’t cover participation, or the trial site was too far away.
Annie Ellis and Mary (Dicey) Jackson Scroggins, who are both ovarian cancer survivors and patient advocates, reported these findings in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“We thought it was important to hear and learn directly from gynecologic cancer survivors,” Ms. Ellis said in an interview. “So we decided to conduct a survey that would expand knowledge about clinical trial participation from a gynecologic cancer patient–specific perspective.”
Ms. Ellis and Ms. Scroggins used survivor networks and social media to distribute a 26-question survey on trial participation. The survey was completed by 189 survivors of gynecologic cancers, 49.19% of whom experienced recurrent disease. The most common diagnoses were ovarian cancer (69.84%) and endometrial or uterine cancer (23.28%).
Perspectives of nonparticipants
Most respondents (65.61%) had never participated in a clinical trial. The most common reason was that the patient’s doctor or medical team never discussed trial participation (50.40%).
There were patients who were interested in trial participation but couldn’t enroll because they didn’t qualify (14.40%), the location was too far away (7.20%), the trial they wanted wasn’t available (4.00%), or their insurance didn’t cover trial participation (1.60%).
Patients who were not interested in trial participation said they didn’t want to receive a placebo (11.20%), they weren’t interested in experimental therapies (3.20%), or they didn’t want to be randomized (2.40%). One patient (1.60%) said she does not trust the medical system.
“Given the frequent conversations about distrust in the medical system, we were surprised that only 1 of the 189 respondents indicated distrust in the medical system as a reason for not participating in a clinical trial,” Ms. Ellis said.
Perspectives of trial participants
Roughly a third of respondents (34.39%) had participated in a clinical trial. Most (86.15%) said they learned about the trial from their doctor. Other sources included the patient’s own research (13.85%), a trial matching service (3.08%), a family member or friend (3.08%), and a support group (1.54%).
The most common reasons patients participated in trials were: “my doctor recommended it,” “to help women in the future,” “to expand my treatment options,” and “to have a chance to benefit personally.”
Additional responses indicated that patients viewed their trial participation in a positive light.
“We were surprised to find that 100% of the respondents who had participated in a clinical trial indicated either that they would participate again (84.62%) or that they were not sure about future participation (15.38%),” Ms. Ellis said. “No respondent indicated that she would not consider another trial. From open comments in the survey, it was clear that even if they did not obtain the result they hoped for or if the experience wasn’t optimal, they maintained the option of participating again.”
Implications and next steps
The survey results suggest there is a need for more discussions about clinical trials with patients who have gynecologic cancers, according to Ms. Ellis and Ms. Scroggins.
“We feel that conversations about clinical trials, with health care team members, should be included at every care decision point, even if – or perhaps especially if – the patient belongs to a group perceived to be unlikely to agree to participate in a trial,” Ms. Ellis said.
“These conversations are necessary with all patients-survivors,” she said, “but they are particularly important and necessary with patients from populations underrepresented in the clinical trial system if we want more representative trial populations, more generalizable results, and the potential for better outcomes for all.”
For their part, Ms. Ellis and Ms. Scroggins plan to conduct more research on this topic to gain additional insights.
“We’d like to conduct a larger survey looking deeper into barriers to and reasons for participation, and to work with medical professionals to develop models of communication to encourage consideration of clinical trials,” Ms. Ellis said. “Additionally, we will work to have a more diverse respondent pool across many dimensions.”
Ms. Ellis is a research advocate on the scientific advisory committee of the Ovarian Cancer National Alliance in Washington. Ms. Scroggins is the director of global outreach and engagement at the International Gynecologic Cancer Society in Louisville, Ken. They have no conflicts of interest.
SOURCE: Ellis A and Scroggins MJ. SGO 2020, Abstract 540.
A survey of gynecologic cancer survivors has revealed why some of these patients don’t participate in clinical trials.
Half of survey respondents with no history of trial participation said their medical team never mentioned the possibility of a trial. About 27% of respondents who never enrolled in a trial said they were interested in trial participation but didn’t qualify, the trial they wanted wasn’t available, their insurance didn’t cover participation, or the trial site was too far away.
Annie Ellis and Mary (Dicey) Jackson Scroggins, who are both ovarian cancer survivors and patient advocates, reported these findings in an abstract that had been slated for presentation at the Society of Gynecologic Oncology’s Annual Meeting on Women’s Cancer. The meeting was canceled because of the COVID-19 pandemic.
“We thought it was important to hear and learn directly from gynecologic cancer survivors,” Ms. Ellis said in an interview. “So we decided to conduct a survey that would expand knowledge about clinical trial participation from a gynecologic cancer patient–specific perspective.”
Ms. Ellis and Ms. Scroggins used survivor networks and social media to distribute a 26-question survey on trial participation. The survey was completed by 189 survivors of gynecologic cancers, 49.19% of whom experienced recurrent disease. The most common diagnoses were ovarian cancer (69.84%) and endometrial or uterine cancer (23.28%).
Perspectives of nonparticipants
Most respondents (65.61%) had never participated in a clinical trial. The most common reason was that the patient’s doctor or medical team never discussed trial participation (50.40%).
There were patients who were interested in trial participation but couldn’t enroll because they didn’t qualify (14.40%), the location was too far away (7.20%), the trial they wanted wasn’t available (4.00%), or their insurance didn’t cover trial participation (1.60%).
Patients who were not interested in trial participation said they didn’t want to receive a placebo (11.20%), they weren’t interested in experimental therapies (3.20%), or they didn’t want to be randomized (2.40%). One patient (1.60%) said she does not trust the medical system.
“Given the frequent conversations about distrust in the medical system, we were surprised that only 1 of the 189 respondents indicated distrust in the medical system as a reason for not participating in a clinical trial,” Ms. Ellis said.
Perspectives of trial participants
Roughly a third of respondents (34.39%) had participated in a clinical trial. Most (86.15%) said they learned about the trial from their doctor. Other sources included the patient’s own research (13.85%), a trial matching service (3.08%), a family member or friend (3.08%), and a support group (1.54%).
The most common reasons patients participated in trials were: “my doctor recommended it,” “to help women in the future,” “to expand my treatment options,” and “to have a chance to benefit personally.”
Additional responses indicated that patients viewed their trial participation in a positive light.
“We were surprised to find that 100% of the respondents who had participated in a clinical trial indicated either that they would participate again (84.62%) or that they were not sure about future participation (15.38%),” Ms. Ellis said. “No respondent indicated that she would not consider another trial. From open comments in the survey, it was clear that even if they did not obtain the result they hoped for or if the experience wasn’t optimal, they maintained the option of participating again.”
Implications and next steps
The survey results suggest there is a need for more discussions about clinical trials with patients who have gynecologic cancers, according to Ms. Ellis and Ms. Scroggins.
“We feel that conversations about clinical trials, with health care team members, should be included at every care decision point, even if – or perhaps especially if – the patient belongs to a group perceived to be unlikely to agree to participate in a trial,” Ms. Ellis said.
“These conversations are necessary with all patients-survivors,” she said, “but they are particularly important and necessary with patients from populations underrepresented in the clinical trial system if we want more representative trial populations, more generalizable results, and the potential for better outcomes for all.”
For their part, Ms. Ellis and Ms. Scroggins plan to conduct more research on this topic to gain additional insights.
“We’d like to conduct a larger survey looking deeper into barriers to and reasons for participation, and to work with medical professionals to develop models of communication to encourage consideration of clinical trials,” Ms. Ellis said. “Additionally, we will work to have a more diverse respondent pool across many dimensions.”
Ms. Ellis is a research advocate on the scientific advisory committee of the Ovarian Cancer National Alliance in Washington. Ms. Scroggins is the director of global outreach and engagement at the International Gynecologic Cancer Society in Louisville, Ken. They have no conflicts of interest.
SOURCE: Ellis A and Scroggins MJ. SGO 2020, Abstract 540.
FROM SGO 2020
How long is it safe to delay gynecologic cancer surgery?
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
As I write this column, there are more than 25,000 current cases of COVID-19 in the United States with an expected exponential rise in these numbers. Hospitals are issuing directives to cancel or postpone “elective” surgery to preserve the finite essential personal protective equipment (PPE), encourage social distancing, prevent exposure of at-risk patients within the hospital, and ensure bed and ventilator capacity for the impending surge in COVID-19 patients.
Many health systems have defined which surgeries they consider permissible, typically by using time parameters such as would not cause patient harm if not performed within 4 weeks, or 7 days, or 24 hours. This leaves surgeons in the unfamiliar position of rationing health care, a role with which, over the coming months, we may have to become increasingly comfortable. This is an enormous responsibility, the shift of resources between one population in need and another, and decisions should be based on data, not bias or hunch. We know that untreated cancer is life threatening, but there is a difference between untreated and delayed. What is a safe time to wait for gynecologic cancer surgery after diagnosis without negatively affecting survival from that cancer?
As I looked through my own upcoming surgical schedule, I sought guidance from the American College of Surgeons’ website, updated on March 17, 2020. In this site they tabulate an “Elective Surgery Acuity Scale” in which “most cancers” fit into tier 3a, which corresponds to high acuity surgery – “do not postpone.” This definition is fairly generalized and blunt; it does not account for the differences in cancers and occasional voluntary needs to postpone a patient’s cancer surgery for health optimization. There are limited data that measure the impact of surgical wait times on survival from gynecologic cancer. Most of this research is observational, and therefore, is influenced by confounders causing delay in surgery (e.g., comorbid conditions or socioeconomic factors that limit access to care). However, the current enforced delays are involuntary; driven by the system, not the patient; and access is universally restricted.
Endometrial cancer
Most data regarding outcomes and gynecologic cancer delay come from endometrial cancer. In 2016, Shalowitz et al. evaluated 182,000 endometrial cancer cases documented within the National Cancer Database (NCDB), which captures approximately 70% of cancer surgeries in the United States.1 They separated these patients into groups of low-grade (grade 1 and 2 endometrioid) and high-grade (grade 3 endometrioid and nonendometrioid) cancers, and evaluated the groups for their overall survival, stratified by the time period between diagnosis and surgery. Interestingly, those whose surgery was performed under 2 weeks from diagnosis had worse perioperative mortality and long-term survival. This seems to be a function of lack of medical optimization; low-volume, nonspecialized centers having less wait time; and the presentation of more advanced and symptomatic disease demanding a more urgent surgery. After those initial 2 weeks of worse outcomes, there was a period of stable outcomes and safety in waiting that extended up to 8 weeks for patients with low-grade cancers and up to 18 weeks for patients with high-grade cancers.
It may be counterintuitive to think that surgical delay affects patients with high-grade endometrial cancers less. These are more aggressive cancers, and there is patient and provider concern for metastatic spread with time elapsed. But an expedited surgery does not appear to be necessary for this group. The Shalowitz study demonstrated no risk for upstaging with surgical delay, meaning that advanced stage was not more likely to be identified in patients whose surgery was delayed, compared with those performed earlier. This observation suggests that the survival from high-grade endometrial cancers is largely determined by factors that cannot be controlled by the surgeon such as the stage at diagnosis, occult spread, and decreased responsiveness of the tumor to adjuvant therapy. In other words, fast-tracking these patients to surgery has limited influence on the outcomes for high-grade endometrial cancers.
For low-grade cancers, adverse outcomes were seen with a surgical delay of more than 8 weeks. But this may not have been caused by progression of disease (low-grade cancers also were not upstaged with delays), but rather may reflect that, in normal times, elective delays of more than 8 weeks are a function of necessary complex medical optimization of comorbidities (such as obesity-related disease). The survival that is measured by NCDB is not disease specific, and patients with comorbidities will be more likely to have impaired overall survival.
A systematic review of all papers that looked at endometrial cancer outcomes associated with surgical delay determined that it is reasonable to delay surgery for up to 8 weeks.2
Ovarian cancer
The data for ovarian cancer surgery is more limited. Most literature discusses the impact of delay in the time between surgery and the receipt of adjuvant chemotherapy, but there are limited data exploring how a delay in primary debulking negatively affects patients. This is perhaps because advanced ovarian cancer surgery rarely is delayed because of symptoms and apparent advanced stage at diagnosis. When a patient’s surgery does need to be voluntarily delayed, for example for medical optimization, there is the option of neoadjuvant chemotherapy (NACT) in which surgery is performed after three or more cycles of chemotherapy. NACT has been shown in multiple studies to have noninferior cancer outcomes, compared with primary debulking surgery.3,4
Perhaps in this current environment in which access to operating rooms and supplies is rationed, we should consider offering more, or all, patients NACT? Hospital stays after primary cytoreductive surgeries are typically 3-7 days in length, and these patients are at a higher risk, compared with other gynecologic cancer surgeries, of ICU admission and blood transfusions, both limited resources in this current environment. The disadvantage of this approach is that, while chemotherapy can keep patients out of the hospital so that they can practice social distancing, this particular therapy adds to the immunocompromised population. However, even patients who undergo primary surgical cytoreductive surgery will need to rapidly transition to immunosuppressive cytotoxic therapy; therefore it is unlikely that this can be avoided entirely during this time.
Lower genital tract cancers
Surgery for patients with lower genital tract cancers – such as cervical and vulvar cancer – also can probably be safely delayed for a 4-week period, and possibly longer. A Canadian retrospective study looked collectively at cervical, vaginal, and vulvar cancers evaluating for disease progression associated with delay to surgery, using 28 days as a benchmark for delayed surgery.5 They found no significant increased progression associated with surgical delay greater than 28 days. This study evaluated progression of cancer and did not measure cancer survival, although it is unlikely we would see impaired survival without a significant increase in disease progression.
We also can look to outcomes from delayed radical hysterectomy for stage I cervical cancer in pregnancy to provided us with some data. A retrospective cohort study observed no difference in survival when 28 women with early-stage cervical cancer who were diagnosed in pregnancy (average wait time 20 weeks from diagnosis to treatment) were compared with the outcomes of 52 matched nonpregnant control patients (average wait time 8 weeks). Their survival was 89% versus 94% respectively (P = .08).6
Summary
Synthesizing this data, it appears that, in an environment of competing needs and resources, it is reasonable and safe to delay surgery for patients with gynecologic cancers for 4-6 weeks and potentially longer. This includes patients with high-grade endometrial cancers. Clearly, these decisions should be individualized to patients and different health systems. For example, a patient who presents with a cancer-associated life-threatening bowel obstruction or hemorrhage may need an immediate intervention, and communities minimally affected by the coronavirus pandemic may have more allowances for surgery. With respect to patient anxiety, most patients with cancer are keen to have surgery promptly, and breaking the news to them that their surgery may be delayed because of institutional and public health needs will be difficult. However, the data support that this is likely safe.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email Dr. Rossi at [email protected].
References
1. Am J Obstet Gynecol 2017;216(3):268 e1-68 e18.
2. Eur J Obstet Gynecol Reprod Biol 2020;246:1-6. doi: 10.1016/j.ejogrb.2020.01.004.
3. N Engl J Med 2010;363(10):943-53.
4. Lancet 2015;386(9990):249-57.
5. J Obstet Gynaecol Can 2015;37(4):338-44.
6. Am J Obstet Gynecol 2017;216(3):276 e1-76 e6. doi: 10.1016/j.ajog.2016.10.034.
Chlamydia trachomatis infections
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
CASE Pregnant woman with symptoms of genital infection
A 23-year-old primigravid woman at 15 weeks and 2 days’ gestation reported having a 2-week history of increased urinary frequency and vaginal discharge. She said she experienced similar symptoms 6 weeks previously that resolved within a week. The patient has had 3 sexual partners in the past year. Her current partner was experiencing a yellow urethral discharge and dysuria. On the patient’s speculum examination, the clinician noted a yellow-green discharge emanating from the cervix as well as cervical motion tenderness.
What is the most likely diagnosis, and how would you treat this patient?
The culprit was chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium that does not stain with Gram staining. A rigid cell wall encloses its intracellular component. C trachomatis infection begins when the chlamydial elementary body enters a susceptible host cell.
Once ingested, the organism’s surface antigens (major outer membrane protein and lipopolysaccharide antigens) provide intracellular sanctuary for the bacterium by inhibiting phagolysosomal fusion. Subsequently, the elementary body morphs into a reticular body, which replicates through adenosine triphosphate (ATP)–dependent binary fission. After approximately 48 hours of replication, the organism again morphs into an elementary body and is released to infect additional cells and acquire new ATP stores for further replication.
Chlamydia can be transmitted horizontally during oral, vaginal, or anal intercourse or vertically to the infant during vaginal delivery.
The US’s most common notifiable disease
According to the Centers for Disease Control and Prevention (CDC), the incidence of chlamydia infection in the United States increased considerably in recent years: from 976,455 cases in 2005 to 1,758,668 cases in 2018.1 In 2018, rates of chlamydia infection in women were nearly double the rates in men, with an incidence of 688.2 versus 377.5 per 100,000 cases, and a prevalence of 1,150,672 versus 612,020.1
Young adults have a higher frequency of chlamydia infection than any other age group. From 2017 to 2018, reported cases in women aged 15–19 years increased by 1.3%, to 3,306.8 per 100,000; in women aged 20–24 years, cases increased by 0.8%, to 4,064.6 per 100,000. In young men in the same age ranges, reported cases increased by 3.7%, to 959.0 cases per 100,000, and by 3.3%, to 1,784.5 per 100,000 cases, respectively.1
Both the incidence and prevalence of chlamydia infection are higher in African Americans than in whites, while Asians have the lowest rates.1 The prevalence of infection also is increased with incarceration, lower socioeconomic status, and residence in the southern United States.
The prevalence of chlamydia infection in pregnant women is approximately 2% to 3%, but it may be as high as 30% in high-risk populations, such as women who are unmarried, have multiple sex partners, are coinfected with another sexually transmitted disease (STD), have partners with nongonococcal urethritis, have mucopurulent discharge, have acute urethral syndrome, and have late or no prenatal care.2 Since chlamydia infection often is asymptomatic and some infections resolve spontaneously, the true prevalence of infection probably is underreported.
Continue to: Chlamydia infection can cause serious clinical manifestations...
Chlamydia infection can cause serious clinical manifestations
The 15 serotypes of C trachomatis are grouped into 3 categories according to clinical manifestations:
- Serotypes A, B, Ba, and C cause endemic trachoma, characterized by bilateral irritation of the eyelids that progresses to eyelid thickening and scarring, eventually leading to corneal abrasion and blindness.
- Serotypes D–K manifest as conjunctivitis and pneumonia in newborns, proctitis in men (especially in men who have sex with men), and genitourinary infections in women. Reactive arthritis and inclusion conjunctivitis also can occur with D–K serotypes.
- Serotypes L1–L3 cause lymphogranuloma venereum.
About 70% of women with chlamydia infection are asymptomatic. Those who have symptoms often present with endocervicitis or acute urethral syndrome (acute urethritis). Manifestations of these 2 conditions include a frothy yellow-green vaginal and/or urethral discharge, dysuria, and frequency. Women who engage in rectal intercourse also may notice a purulent discharge from the anus. Untreated, C trachomatis organisms may ascend the reproductive tract, causing both endometritis and pelvic inflammatory disease (PID).
While a single episode of PID increases tubal infertility risk by 10%, a second episode increases the risk by 40%.3 Over time, recurrent and/or chronic PID causes scarring and adhesion formation, which may result in chronic pelvic pain. In addition, chronic infection is the single most important risk factor for ectopic pregnancy. Finally, chlamydia infection is a risk factor for Fitz-Hugh-Cutis syndrome (perihepatitis). In this condition, organisms ascend from the site of pelvic infection along the pericolic gutter to ultimately infect the liver capsule.
Specific complications in pregnancy
Chlamydia infection in pregnant women is associated with preterm delivery and preterm premature rupture of membranes. Infants born to mothers with untreated chlamydia infection are at risk for pneumonia, conjunctivitis, and even perinatal death.2 Acquisition of infection occurs at the time of delivery rather than in the antepartum period.
The significant morbidity associated with chlamydia infection underscores the importance of regular screening, especially in pregnant women. The current United States Preventive Service Task Force guidelines recommend annual screening of all sexually active women who are 24 years of age or younger, as well as of older, high-risk women.
The CDC recommends routine screening of all pregnant women for chlamydia at the first prenatal visit. Repeat screening is recommended in the third trimester for all pregnant women younger than 25 years, those at increased risk, and those infected within the past 3 to 6 months or during the first trimester. Those who test positive should be retested 3 weeks after completion of treatment.1
Chlamydia screening strategies
Historically, a chlamydia diagnosis was made by isolating the organisms in tissue culture. In the 1990s, however, that extremely time-consuming and resource-intensive procedure was replaced by nucleic acid amplification testing (NAAT).
NAAT methodology. NAAT is the gold standard for diagnosing C trachomatis infection; this methodology utilizes various assays, including polymerase chain reaction, ligase chain reaction, and transcription-mediated amplification.
Continue to: Compared with previous culture and antigen detection techniques...
Compared with previous culture and antigen detection techniques, NAAT’s advantages include excellent sensitivity and specificity (>90% and ≥99%, respectively), enabling detection of a low inoculum of organisms in a sample obtained by noninvasive methods, such as first-void urine collection or vaginal swab.2,4,5 Furthermore, NAAT does not impose any specific storage regulations on collected specimens, is cost effective, and can jointly test for Neisseria gonorrhoeae, which commonly co-infects with C trachomatis.6
Screening in pregnancy. In 2012, Blatt and colleagues examined testing patterns in nearly 1.3 million obstetric patients and found that only 59% (761,315) of women were tested for chlamydia at least once in pregnancy.7 Only 1 in 3 women were tested during the first prenatal visit, as CDC guidelines recommend. Testing rates declined with increasing age. Of women screened, 3.5% tested positive for chlamydia.7 Of these, 3 of 4 were retested at least once, with almost 20% having at least 1 subsequent positive result.7
Of note, in a study of women who reported receptive anal intercourse (n = 2,818), 292 women tested positive for chlamydia; 10.4% tested positive in genital-only sites, 58.6% in genital and rectal sites, and 20.5% at the rectal site only.8
It is alarming that only 59% of pregnant women are screened for chlamydia given the significant perinatal complications associated with this infection. Barriers to screening pregnant women may include clinician discomfort in discussing STDs and patient refusal of screening. Furthermore, clinicians should routinely ask women about receptive anal sex. Women who report this risk factor should be tested for chlamydia in both the endocervix and rectum.
Retesting and follow-up. After the initial diagnosis of chlamydia, a test of cure 3 weeks after treatment is an important aspect of care. Thus, identifying and overcoming barriers to retesting is important. Clinicians should educate patients about the importance of follow-up. Also consider incorporating the use of home-based, self-obtained vaginal swabs for retesting. Results from 2 randomized trials showed that eliminating a patient’s transportation barriers and providing a home-based alternative to a follow-up visit significantly increased rescreening rates by 33% in STD clinic patients and by 59.2% in family planning clinic patients.9
Reinfection risk. The rate of venereal chlamydia transmission in heterosexual partners is 70%. Since sexually active chlamydia-positive patients are at risk for reinfection by their partner after treatment completion, clinicians should refer the sex partners for evaluation. If the sex partners are reluctant to have testing, it is reasonable to provide empiric antibiotic treatment to decrease the risk of re-infection in the patient.7 Before doing so, however, make certain that state law permits this practice, and be sure to document the prescribed treatment in the patient’s record.
Treatment options
Prompt treatment of C trachomatis infection is essential to decrease the risk of disease sequelae. Nonpregnant adults can be treated with oral doxycycline 100 mg twice daily for 7 days.
In a head-to-head study performed in a controlled environment that ensured treatment adherence, 97% efficacy was achieved with one oral dose of azithromycin (1 g) compared with 100% efficacy with doxycycline.10 However, in the real-world setting, imperfect adherence to the multi-day doxycycline regimen is associated with treatment failures. Thus, a single dose of azithromycin is preferable for patients with questionable compliance.11
In obstetric patients, azithromycin and amoxicillin are preferred as first-line agents for treatment of C trachomatis due to their improved safety profile in this demographic. Amoxicillin 500 mg orally 3 times daily for 7 days has 95% efficacy.2
Women allergic to these agents may be treated with an alternative regimen of erythromycin base, 500 mg orally 4 times daily for 7 days, or erythromycin ethylsuccinate, 800 mg orally 4 times daily for 7 days. Erythromycin should be reserved for second-line therapy because of its lower efficacy (64%) and frequent gastrointestinal adverse effects.2 Doxycycline is contraindicated in pregnancy because of possible teratogenic effects on the teeth and bone of the fetus.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
- Centers for Disease Control and Prevention Division of STD Prevention. Sexually transmitted disease surveillance 2018. October 2019. https://www.cdc.gov/std/stats18/default.htm. 2019. Accessed January 4, 2020.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams JD, et al, eds. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier Saunders; 2019:869.
- Ljubin-Sternak S, Meštrović T. Chlamydia trachomatis and genital mycoplasmas: pathogens with an impact on human reproductive health. J Pathog. 2014. doi: 10.1155/2014/183167.
- Meyer T. Diagnostic procedures to detect Chlamydia trachomatis infections. Microorganisms. 2016:4(3).
- Centers for Disease Control and Prevention.. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae. MMWR Recomm Rep. 2014;63:1-19.
- Wiesenfeld HC. Screening for Chlamydia trachomatis infections in women. N Engl J Med. 2017;376:765-773.
- Blatt AJ, Lieberman JM, Hoover DR, et al. Chlamydial and gonococcal testing during pregnancy in the United States. Am J Obstet Gynecol. 2012;207:55.e1-8.
- Llata E, Braxton J, Asbel L, et al. Rectal Chlamydia trachomatis and Neisseria gonorrhoeae infections among women reporting anal intercourse. Obstet Gynecol. 2018;132:692-697.
- Xu F, Stoner BP, Taylor SN, et al. Use of home-obtained vaginal swabs to facilitate rescreening for Chlamydia trachomatis infections: two randomized controlled trials. Obstet Gynecol. 2011;118(2 pt 1):231-239.
- Geisler WM, Uniyal A, Lee JY, et al. Azithromycin versus doxycycline for urogenital Chlamydia trachomatis infection. N Engl J Med. 2015;373:2512-2521.
- Quinn TC, Gaydos CA. Treatment for chlamydia infection—doxycycline versus azithromycin. N Engl J Med. 2015;373:2573-2575.
COVID-19 during pregnancy: How would you proceed in this case of a novel and ominous emerging pathogen?
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).
Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

CASE Pregnant patient with fever who has travel history to Italy
A 28-year-old primigravid woman at 12 weeks’ gestation just returned from a 2-week vacation in Italy. She requests medical evaluation because of malaise; fever; chills; rhinorrhea; mild dyspnea; a dry, nonproductive cough; and diarrhea. On physical examination, her temperature is 38.6° C (101.5° F), pulse 104 bpm, respirations 22/minute, and blood pressure 100/70 mm Hg. Auscultation of the lungs demonstrates scattered rales, rhonchi, and expiratory wheezes in both posterior lung fields. The fetal heart rate is 168 bpm. What are the most likely diagnoses? What diagnostic tests are indicated? And what clinical treatment is indicated?
In the presented case scenario, the patient’s symptoms are consistent with a viral influenza. Her recent travel history certainly makes coronavirus disease 2019 (COVID-19) the most likely diagnosis.
COVID-19, caused by a novel new coronavirus, has evolved with lightning speed since it was first identified in early December 2019.1 The disease originated in Wuhan, China. Its epicenter is now in Europe, and over 100 countries and regions have reported cases. New cases in the United States are being identified daily, and there is no clear end to the outbreak. Several areas of the United States have been particularly hard hit by this disease: Seattle, New Orleans, and New York City.
COVID-19 has provoked widespread unsettledness in many populations and an extraordinary response from public health officials, large corporations, professional organizations, and financial markets. We are learning more about somewhat unfamiliar public health concepts such as quarantine, containment, mitigation, reproduction number (R), and “flattening the curve.” Disneyland and Walt Disney World are now temporarily closed. Professional and collegiate sports organizations have cancelled or suspended games and tournaments. Scientific and trade association meetings have been postponed or cancelled. Broadway, Carnegie Hall, and the Metropolitan Museum of Art have now “turned out the lights.” The Centers for Disease Control and Prevention has recommended that everyone avoid gatherings that include more than 10 other persons.
This article will review the evolving epidemiology of COVID-19, describe the usual clinical manifestations of the disease, highlight the key diagnostic tests, and present guidelines for treatment. It will review the limited information currently available about the impact of COVID-19 in pregnant women. The review will conclude by describing measures that individuals can employ to prevent acquisition or transmission of infection and then by highlighting key “unanswered questions” about this new and ominous pathogen (TABLE).

Continue to: What we know about epidemiology...
What we know about epidemiology
COVID-19 is caused by a novel new coronavirus that shares some genetic overlap with the viruses that caused Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).2 The first case of COVID-19 was reported on December 1, 2019, from Wuhan, China.1 Within a very short period of time the disease has spread throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the infection to be a true pandemic. The countries with the highest prevalence of COVID-19 include China, South Korea, Iran, Italy, France, Spain, and the United States. However, more than 100 other countries and regions have reported cases. As of the first week of April, approximately 1 million persons in the world have been diagnosed with COVID-19. Of those infected, slightly more than 50,000 deaths have occurred. At the time of this writing, 234,483 cases have been documented in the United States, and current estimates indicate that approximately 7% of the population in the country could become infected.1,3,4
The virus responsible for COVID-19 is a single-stranded, enveloped RNA virus. Like its counterparts that caused SARS and MERS, this virus originates in animals, primarily bats. The early cases seem to have resulted from patient contact with exotic animals displayed in the Huanan Seafood Wholesale Market.1
The virus is transmitted directly by respiratory droplets and by close surface-to-hand contact with infected respiratory secretions. The virus appears to remain viable on environmental surfaces for 1 to 3 days, although the degree of infectivity over time is not well delineated. With direct exposure to respiratory droplets, the infectivity is relatively high; approximately 2 to 3 individuals become infected as the result of contact with an infected patient. By contrast, the “reproduction number (R)” for influenza is closer to 1.2,5
Certain persons appear to be at increased risk for developing infection and becoming seriously ill2,6:
- persons older than age 60
- persons with underlying medical illness
- persons who are immunosuppressed.
The reported range in the case fatality rate (CFR) varies from 1% to 13%, with the higher rates concentrated in older patients with comorbidities.3 These initial reports of high CFRs may be misleading because in the initial phases of this pandemic many patients with mild or no symptoms were not tested, and, thus, the overall prevalence of infection is not clear. By way of comparison, the CRF for influenza A and B is about 0.1%.2
Of note, the number of reported cases in the pediatric population is low, and the outcomes in these individuals are much better than in the older population.2,3,6 At present, there are only two reports of COVID-19 in pregnancy; these two studies include 18 women and 19 infants.7,8 The frequency of preterm delivery was 50% in these reports. Sixteen of the 18 patients were delivered by cesarean delivery; at least 6 of these procedures were performed for a non-reassuring fetal heart rate tracing. No maternal deaths were identified, and no cases of vertical transmission occurred.
We must remember that the number of patients described in these two reports is very small. Although the initial reports are favorable, in other influenza epidemics, pregnant women have not fared so well and have experienced disproportionately higher rates of morbidity and mortality.2
Reported clinical manifestations
The incubation period of COVID-19 ranges from 2 to 14 days; the median is 5.2 days. Many patients with proven COVID-19 infection are asymptomatic. When clinical findings are present, they usually are relatively mild and include low-grade fever, myalgias, arthralgias, sore throat, mild dyspnea, and a dry nonproductive cough. Some patients also may experience diarrhea. Of course, these findings are also consistent with influenza A or B or atypical pneumonia. One key to differentiation is the patient’s history of recent travel to an area of high COVID-19 prevalence or contact with a person who has been in one of these areas and who is clinically ill.2,3,9,10
In some patients, notably those who are older than 65 years of age and/or who have underlying medical illnesses, the respiratory manifestations are more prominent.6 These patients may develop severe dyspnea, pneumonia, adult respiratory distress syndrome (ARDS), multiorgan failure, and septic shock. Interestingly, the more severe manifestations tend to occur during the second week of the illness. In this group of more severely ill patients requiring hospitalization, 17% to 29% develop ARDS, and 23% to 32% require admission to the intensive care unit.2,6
Pregnant patients who become severely ill may be at risk for spontaneous miscarriage and preterm labor. With profound maternal hypoxia, fetal heart rate abnormalities may become apparent. To date, no clearly proven cases of vertical transmission of infection to the newborn have been identified. However, as noted above, current reports only include 18 pregnancies and 19 infants.2,3,7,8,11
Continue to: Diagnostic testing...
Diagnostic testing
Infected patients may have a decreased peripheral white blood cell count, with a specific decrease in the number of lymphocytes. Thrombocytopenia may be present, as well as an elevation in the hepatic transaminase enzymes (ALT, AST).2
X-ray, chest CT, and RT-PCR. The three most important diagnostic tests are chest x-ray, chest computed tomography (CT) scan, and real-time PCR (RT-PCR) or nucleic acid amplification test (NAAT).2,6 Specimens for RT-PCR or NAAT should be obtained from the oropharynx and nasopharynx using a synthetic-tipped applicator with an aluminum shaft. Patients who are intubated should have specimens obtained by broncho-alveolar lavage. The virus also has been recovered from blood and stool, but not yet from urine, amniotic fluid, placenta, cord blood, or breast milk.2
CT and chest x-ray show characteristic ground-glass opacities in both lung fields, combined with multiple areas of consolidation. Chest imaging is particularly helpful when the patient has all the major clinical manifestations, but the initial RT-PCR or NAAT is negative.
Treatment
Fortunately, most infected persons can be treated as outpatients. Because this condition may be confused with influenza A or B, initial treatment with a drug such as oseltamivir 75 mg orally twice daily for five days is very reasonable.9 Supportive therapy is critically important in this clinical setting. Acetaminophen, up to 3,000 mg/d in divided doses, or ibuprofen, up to 2,400 mg/d in divided doses, can be used to reduce fever and relieve myalgias and arthralgias. The latter drug, of course, should not be used in pregnant women. The patient should be encouraged to rest and to stay well hydrated. Loperamide can be used to treat diarrhea, 4 mg orally initially, then 2 mg orally after each loose stool up to a maximum of 16 mg/d. Pregnant patients should be cautioned to watch for signs of preterm labor.9,12 Patients should remain in relative isolation at home until they are free of signs of illness and they test negative for COVID-19.
For patients who are more severely ill at initial evaluation or who deteriorate while undergoing outpatient management, hospitalization is indicated.2,6 Patients should be placed in rooms that provide protection against aerosolized infection. They should receive supplemental oxygen and be observed closely for signs of superimposed bacterial infection. Depending upon the suspected bacterial pathogen, appropriate antibiotics may include ceftriaxone, which targets Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis; azithromycin, which targets mycoplasmas; and vancomycin, which specifically covers Staphylococcus aureus. Health care workers should wear appropriate personal protective equipment when interacting with these patients, including cap, N95 mask, face shield, gloves, gown, and shoe covers. If a woman with COVID-19 has delivered, and the pediatrician permits rooming in, the isolette should be positioned at least 6 feet away from the mother. The mother should use a mechanical breast pump to obtain milk and then have another family member feed the baby until the mother tests negative for the virus. The breast pump needs to be cleaned meticulously after each use. The number of visitors to the mother’s room should be strictly limited.3,9
At the present time, there is no specific antiviral drug approved by the US Food and Drug Administration for treatment of COVID-19. The National Institutes of Health is currently conducting a trial of remdesivir for affected patients.13 The drug is also available from the manufacturer outside of this trial on a “compassionate use” basis. Another treatment regimen receiving extensive publicity is the combination of azithromycin and hydroxychloroquine. Its effectiveness has not been confirmed in a properly designed randomized trial.
Prevention hinges on commonsense precautions
Although vaccine trials are underway, public health authorities estimate that a vaccine will not be commercially available for at least 12 to 18 months. Therefore, independent of “community/organizational” mitigation programs, individuals should observe the following commonsense precautions to minimize their risk of contracting or transmitting COVID-192,3,5,14:
- Eliminate any nonessential travel, particularly by plane or cruise ship.
- Avoid events that draw large crowds, such as concerts, theater performances, movies, and even religious services.
- When out in public, try to maintain a distance of 6 feet from others
- Remain at home if you feel ill, particularly if you have respiratory symptoms.
- Cough or sneeze into your sleeve rather than your bare hand.
- Avoid handshakes.
- Wash your hands frequently in warm soapy water for at least 20 seconds, particularly after touching environmental surfaces such as counter tops and handrails.
- If you use hand sanitizers, they should have an alcohol content of at least 60%.
- Clean environmental surfaces frequently with a dilute bleach solution.
CASE Resolved
The clinical manifestations displayed by this patient are consistent with viral influenza. The recent travel history to one of the European epicenters makes COVID-19 the most likely diagnosis. The patient should have a chest CT scan and a RT-PCR or NAAT to confirm the diagnosis. If the diagnosis is confirmed, she and her close contacts should be self-quarantined at home for 14 days. She should receive appropriate supportive care with anti-pyretics, analgesics, and anti-diarrhea agents. If she develops signs of serious respiratory compromise, she should be admitted to an isolation room in the hospital for intensive respiratory therapy and close observation for superimposed bacterial pneumonia.

- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
- Holshue ML, DeBolt C, Lindquist S, et al; Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929-936.
- Rasmussen SA, Smulian JC, Lednicky JA, et al. Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. February 24, 2020. doi: 10.1016/j.ajog.2020.02.017.
- Rasmussen SA, Jamieson DJ. Coronavirus disease 2019 (COVID-19) and pregnancy: responding to a rapidly evolving situation [in press]. Obstet Gynecol. 2020.
- Centers for Disease Control and Prevention. Coronavirus disease 2019: Cases in US. CDC website. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed March 18, 2020.
- Wang H, Wang Z, Dong Y, et al. Phase-adjusted estimation of the number of Coronavirus Disease 2019 cases in Wuhan, China. Cell Discov. 2020;6:10.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733.
- Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395;809-815.
- Lei D, Wang C, Li C, et al. Clinical characteristics of pregnancy with the 2019 novel coronavirus disease (COVID-19) infection. Chin J Perinat Med. 2020:23.
- Dotters-Katz S, Hughes BL. Coronavirus (COVID-19) and pregnancy: what maternal-fetal medicine subspecialists need to know. Society for Maternal-Fetal Medicine. March 17, 2020. https://s3.amazonaws.com/cdn.smfm.org/media/2267/COVID19-_updated_3-17-20_PDF.pdf. Accessed March 17, 2020.
- Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382:760-762.
- Yang H, Wang C, Poon LC. Novel coronavirus infection and pregnancy. Ultrasound Obstet Gynecol. March 5, 2020. doi:10.1002/uog.22006.
- American College of Obstetricians and Gynecologists. Practice Advisory: novel coronavirus 2019 (COVID-19). March 13, 2020. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Novel-Coronavirus2019?IsMobileSet=false. Accessed March 17, 2020.
- National Institutes of Health. NIH clinical trial of remdesivir to treat COVID-19 begins. February 25, 2020. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins. Accessed March 17, 2020.
- Munster VJ, Koopmans M, Van Doremalen N, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382:692-694.
Nearly half of STI events go without HIV testing
according to Danielle Petsis, MPH, of the Children’s Hospital of Philadelphia, and associates.
In a study published in Pediatrics, the investigators conducted a retrospective analysis of 1,816 acute STI events from 1,313 patients aged 13-24 years admitted between July 2014 and Dec. 2017 at two urban health care clinics. The most common STIs in the analysis were Chlamydia, gonorrhea, trichomoniasis, and syphilis; the mean age at diagnosis was 17 years, 71% of episodes occurred in females, and 97% occurred in African American patients.
Of the 1,816 events, HIV testing was completed within 90 days of the STI diagnosis for only 55%; there was 1 confirmed HIV diagnosis among the completed tests. When HIV testing did occur, in 38% of cases it was completed concurrently with STI testing or HIV testing was performed in 35% of the 872 follow-up cases. Of the 815 events where HIV testing was not performed, 27% had a test ordered by the provider but not completed by the patient; the patient leaving the laboratory before the test could be performed was the most common reason for test noncompletion (67%), followed by not showing up at all (18%) and errors in the medical record or laboratory (5%); the remaining patients gave as reasons for test noncompletion: declining an HIV test, a closed lab, or no reason.
Logistic regression showed that participants who were female and those with a previous history of STIs had significantly lower adjusted odds of HIV test completion, compared with males and those with no previous history of STIs, respectively, the investigators said. In addition, having insurance and having a family planning visit were associated with decreased odds of HIV testing, compared with not having insurance or a family planning visit.
“As we enter the fourth decade of the HIV epidemic, it remains clear that missed opportunities for diagnosis have the potential to delay HIV diagnosis and linkage to antiretroviral therapy or PrEP and prevention services, thus increasing the population risk of HIV transmission. Our data underscore the need for improved HIV testing education for providers of all levels of training and the need for public health agencies to clearly communicate the need for testing at the time of STI infection to reduce the number of missed opportunities for testing,” Ms. Petsis and colleagues concluded.
The study was supported by the National Institutes of Mental Health and the Children’s Hospital of Philadelphia Research Institute K-Readiness Award. One coauthor reported receiving funding from Bayer Healthcare, the Templeton Foundation, the National Institutes of Health, and Janssen Biotech. She also serves on expert advisory boards for Mylan Pharmaceuticals and Merck. The other authors have no relevant financial disclosures.
SOURCE: Wood S et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2019-2265.
according to Danielle Petsis, MPH, of the Children’s Hospital of Philadelphia, and associates.
In a study published in Pediatrics, the investigators conducted a retrospective analysis of 1,816 acute STI events from 1,313 patients aged 13-24 years admitted between July 2014 and Dec. 2017 at two urban health care clinics. The most common STIs in the analysis were Chlamydia, gonorrhea, trichomoniasis, and syphilis; the mean age at diagnosis was 17 years, 71% of episodes occurred in females, and 97% occurred in African American patients.
Of the 1,816 events, HIV testing was completed within 90 days of the STI diagnosis for only 55%; there was 1 confirmed HIV diagnosis among the completed tests. When HIV testing did occur, in 38% of cases it was completed concurrently with STI testing or HIV testing was performed in 35% of the 872 follow-up cases. Of the 815 events where HIV testing was not performed, 27% had a test ordered by the provider but not completed by the patient; the patient leaving the laboratory before the test could be performed was the most common reason for test noncompletion (67%), followed by not showing up at all (18%) and errors in the medical record or laboratory (5%); the remaining patients gave as reasons for test noncompletion: declining an HIV test, a closed lab, or no reason.
Logistic regression showed that participants who were female and those with a previous history of STIs had significantly lower adjusted odds of HIV test completion, compared with males and those with no previous history of STIs, respectively, the investigators said. In addition, having insurance and having a family planning visit were associated with decreased odds of HIV testing, compared with not having insurance or a family planning visit.
“As we enter the fourth decade of the HIV epidemic, it remains clear that missed opportunities for diagnosis have the potential to delay HIV diagnosis and linkage to antiretroviral therapy or PrEP and prevention services, thus increasing the population risk of HIV transmission. Our data underscore the need for improved HIV testing education for providers of all levels of training and the need for public health agencies to clearly communicate the need for testing at the time of STI infection to reduce the number of missed opportunities for testing,” Ms. Petsis and colleagues concluded.
The study was supported by the National Institutes of Mental Health and the Children’s Hospital of Philadelphia Research Institute K-Readiness Award. One coauthor reported receiving funding from Bayer Healthcare, the Templeton Foundation, the National Institutes of Health, and Janssen Biotech. She also serves on expert advisory boards for Mylan Pharmaceuticals and Merck. The other authors have no relevant financial disclosures.
SOURCE: Wood S et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2019-2265.
according to Danielle Petsis, MPH, of the Children’s Hospital of Philadelphia, and associates.
In a study published in Pediatrics, the investigators conducted a retrospective analysis of 1,816 acute STI events from 1,313 patients aged 13-24 years admitted between July 2014 and Dec. 2017 at two urban health care clinics. The most common STIs in the analysis were Chlamydia, gonorrhea, trichomoniasis, and syphilis; the mean age at diagnosis was 17 years, 71% of episodes occurred in females, and 97% occurred in African American patients.
Of the 1,816 events, HIV testing was completed within 90 days of the STI diagnosis for only 55%; there was 1 confirmed HIV diagnosis among the completed tests. When HIV testing did occur, in 38% of cases it was completed concurrently with STI testing or HIV testing was performed in 35% of the 872 follow-up cases. Of the 815 events where HIV testing was not performed, 27% had a test ordered by the provider but not completed by the patient; the patient leaving the laboratory before the test could be performed was the most common reason for test noncompletion (67%), followed by not showing up at all (18%) and errors in the medical record or laboratory (5%); the remaining patients gave as reasons for test noncompletion: declining an HIV test, a closed lab, or no reason.
Logistic regression showed that participants who were female and those with a previous history of STIs had significantly lower adjusted odds of HIV test completion, compared with males and those with no previous history of STIs, respectively, the investigators said. In addition, having insurance and having a family planning visit were associated with decreased odds of HIV testing, compared with not having insurance or a family planning visit.
“As we enter the fourth decade of the HIV epidemic, it remains clear that missed opportunities for diagnosis have the potential to delay HIV diagnosis and linkage to antiretroviral therapy or PrEP and prevention services, thus increasing the population risk of HIV transmission. Our data underscore the need for improved HIV testing education for providers of all levels of training and the need for public health agencies to clearly communicate the need for testing at the time of STI infection to reduce the number of missed opportunities for testing,” Ms. Petsis and colleagues concluded.
The study was supported by the National Institutes of Mental Health and the Children’s Hospital of Philadelphia Research Institute K-Readiness Award. One coauthor reported receiving funding from Bayer Healthcare, the Templeton Foundation, the National Institutes of Health, and Janssen Biotech. She also serves on expert advisory boards for Mylan Pharmaceuticals and Merck. The other authors have no relevant financial disclosures.
SOURCE: Wood S et al. Pediatrics. 2020 Mar 16. doi: 10.1542/peds.2019-2265.
FROM PEDIATRICS