User login
Female genital cutting: Caring for patients through the lens of health care equity
Female genital cutting (FGC), also known as female circumcision or female genital mutilation, is defined by the World Health Organization (WHO) as “the partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.”1 It is a culturally determined practice that is mainly concentrated in certain parts of Africa, the Middle East, and Asia and now is observed worldwide among migrants from those areas.1 Approximately 200 million women and girls alive today have undergone FGC in 31 countries, although encouragingly the practice’s prevalence seems to be declining, especially among younger women.2
Too often, FGC goes unrecognized in women who present for medical care, even in cases where a genitourinary exam is performed and documented.3,4 As a result, patients face delays in diagnosis and management of associated complications and symptoms. Female genital cutting is usually excluded from medical school or residency training curricula,5 and physicians often lack familiarity with the necessary clinical or surgical management of patients who have had the procedure.6 It is crucial, however, that ObGyns feel comfortable recognizing FGC and clinically caring for pregnant and nonpregnant patients who have undergone the procedure. The obstetric-gynecologic setting should be the clinical space in which FGC is correctly diagnosed and from where patients with complications can be referred for appropriate care.
FGC: Through the lens of inequity
Providing culturally competent and sensitive care to women who have undergone FGC is paramount to reducing health care inequities for these patients. Beyond the medical recommendations we review below, we suggest the following considerations when approaching care for these patients.
Acknowledge our biases. It is paramount for us, as providers, to acknowledge our own biases and how these might affect our relationship with the patient and how our care is received. This starts with our language and terminology: The term female genital mutilation can be judgmental or offensive to our patients, many of whom do not consider themselves to have been mutilated. This is why we prefer to use the term female genital cutting, or whichever word the patient uses, so as not to alienate a patient who might already face many other barriers and microaggressions in seeking health care.
Control our responses. Another way we must check our bias is by controlling our reactions during history taking or examining patients who have undergone FGC. Understandably, providers might be shocked to hear patients recount their childhood experiences of FGC or by examining an infibulated scar, but patients report noticing and experiencing hurt, distress, and shame when providers display judgment, horror, or disgust.7 Patients have reported that they are acutely aware that they might be viewed as “backward” and “primitive” in US health care settings.8 These kinds of feelings and experiences can further exacerbate patients’ distrust and avoidance of the health care system altogether. Therefore, providers should acknowledge their own biases regarding the issue as well as those of their staff and work to mitigate them.
Avoid stigmatization. While FGC can have long-term effects (discussed below), it is important to remember that many women who have undergone FGC do not experience symptoms that are bothersome or feel that FGC is central to their lives or lived experiences. While we must be thorough in our history taking to explore possible urinary, gynecologic, and sexual symptoms of concern and bother to the patient, we must avoid stigmatizing our patients by assuming that all who have undergone FGC are “sexually disabled,” which may lead a provider to recommend medically unindicated intervention, such as clitoral reconstruction.9
Continue to: Classifying FGC types...
Classifying FGC types
The WHO has classified FGC into 4 different types1:
- type 1, partial or total removal of the clitoris or prepuce
- type 2, partial or total removal of part of the clitoris and labia minora
- type 3 (also known as infibulation), the narrowing of the vaginal orifice by cutting, removing, and/or repositioning the labia, and
- type 4, all other procedures to the female genitalia for nonmedical reasons.
Long-term complications
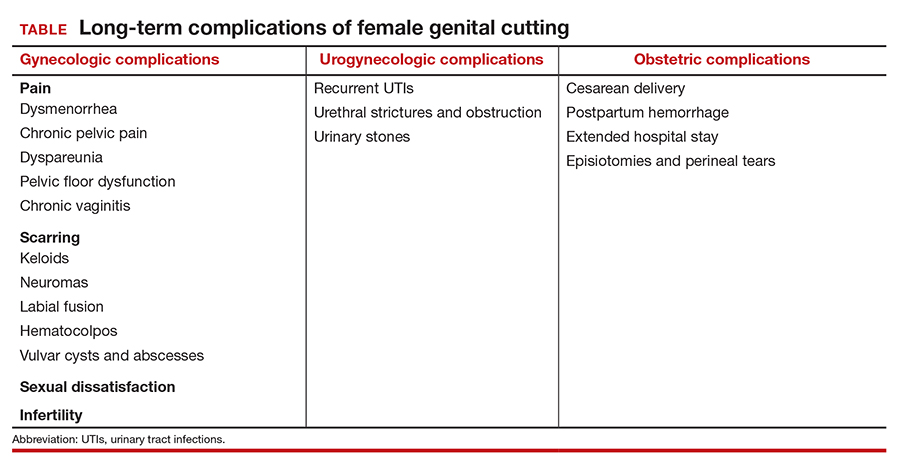
Female genital cutting, especially types 2 and 3, can lead to long-term obstetric and gynecologic complications that the ObGyn should be able to diagnose and manage (TABLE).
The most common long-term complications of FGC are dysmenorrhea, dyspareunia, recurrent vaginal and urinary tract infections, and sexual dysfunction/dissatisfaction.10 One recent cross-sectional study that used validated questionnaires on pelvic floor and psychosexual symptoms found that women with FGC had higher distress scores than women who had not undergone FGC, indicating various pelvic floor symptoms responsible for impact on their daily lives.11
Infertility can result from a combination of physical barriers (vaginal stenosis and an infibulated scar) and psychologic barriers secondary to dyspareunia, for example.12 Labor and delivery also presents a challenge to both patients and providers, especially in cases of infibulation. Studies show that patients who have undergone FGC are at increased risk of adverse obstetric outcomes, including postpartum hemorrhage, episiotomy, cesarean delivery, and extended hospital stay.13 Neonatal complications, including infant resuscitation and perinatal death, are more commonly reported in studies outside the United States.13
Clinical management recommendations
It is important to be aware of the WHO FCG classifications and be able to recognize evidence of the procedure on examination. The ObGyn should perform a detailed physical exam of the external genitalia as well as a pelvic floor exam of every patient. If the patient does not disclose a history of FGC but it is suspected based on the examination, the clinician should inquire sensitively if the patient is aware of having undergone any genital procedures.
Especially when a history of FGC has been confirmed, clinicians should ask patients sensitively about their urinary and sexual function and satisfaction. Validated tools, such as the Female Sexual Function Index, the Female Sexual Distress Scale, and the Pelvic Floor Disability Index, may be helpful in gathering an objective and detailed assessment of the patient’s symptoms and level of distress.14 Clinicians also should ask about the patient’s detailed obstetric history, particularly regarding the second stage, delivery, and postpartum complications. The clinician also should specifically inquire about a history of defibulation or additional genital procedures.
Patients with urethral strictures or stenosis may require an exam under anesthesia, cystoscopy, urethral dilation, or urethroplasty.12 Those with chronic urinary tract or vaginal infections may require chronic oral suppressive therapy or defibulation (described below). Defibulation also may be considered for relief of severe dysmenorrhea and menorrhagia that may be resulting from hematocolpos. The ObGyn also should make certain to evaluate for other common causes of these symptoms that may be unrelated to FGC, such as endometriosis.
Many women who have undergone FGC do not report dyspareunia or sexual dissatisfaction; however, infibulation especially has been associated with higher rates of these sequelae.12 In addition to defibulation, pelvic floor physical therapy with an experienced therapist may be helpful for patients with pelvic floor dysfunction, vaginismus, and/or dyspareunia.
The defibulation procedure
Defibulation (or deinfibulation) is a surgical reconstructive procedure that opens the infibulated scar of patients who have undergone type 3 FGC (infibulation), thus exposing the urethra and introitus, and in almost half of cases an intact clitoris.15 Defibulation may be specifically requested by a patient or it may be recommended by the ObGyn either for reducing complications of pregnancy or to address the patient’s gynecologic, sexual, or urogynecologic symptoms by allowing penetrative intercourse, urinary flow, physiologic delivery, and menstruation.16
Defibulation should be performed under regional or general anesthesia and can be performed during pregnancy (or even in labor). An anterior incision is made on the infibulated scar, creating a new labia major, and the edges are sutured separately. Postoperatively, patients should be instructed to perform sitz baths and to expect a change in their urinary voiding stream.12 The few studies that have evaluated defibulation have shown high rates of success in addressing preoperative symptoms; the complication rates of defibulation are low and the satisfaction rates are high.16
The ethical conundrum of reinfibulation
Reinfibulation is defined as the restitching or reapproximation of scar tissue or the labia after delivery or a gynecologic procedure, and it is often performed routinely after every delivery in patients’ countries of origin.17
Postpartum reinfibulation on patient request raises legal and ethical issues for the ObGyn. In the United Kingdom, reinfibulation is illegal, and some international organizations, including the International Federation of Gynecology and Obstetrics and the WHO, have recommended against the practice. In the United States, reinfibulation of an adult is legal, as it falls under the umbrella of elective female genital cosmetic surgery.18,19
The procedure could create or exacerbate long-term complications and should generally be discouraged. However, if despite extensive counseling (preferably in the prenatal period) a patient insists on having the procedure, the ObGyn may need to elevate the principle of patient autonomy and either comply or find a practitioner who is comfortable performing it. One retrospective review in Switzerland suggested that specific care and informative counseling prenatally with the inclusion of a patient’s partner in the discussion can improve the acceptability of defibulation without reinfibulation.20
Conclusion
It is important for ObGyns to be familiar with the practice of FGC and to be trained in its recognition on examination and care for the long-term complications that can result from the practice. At the same time, ObGyns should be especially conscious of their biases in order to provide culturally competent care and reduce health care stigmatization and inequities for these patients.
- World Health Organization. Female genital mutilation. February 3, 2020. https://www.who.int/news-room/fact-sheets/detail/female-genital-mutilation. Accessed February 22, 2021.
- UNICEF. Female genital mutilation (FGM). February 2020. https://data.unicef.org/topic/child-protection/female-genital-mutilation/. Accessed February 22, 2021.
- Stoklosa H, Nour NM. The eye cannot see what the mind does not know: female genital mutilation. Emerg Med J. 2018;35:585-586. doi: 10.1136/emermed-2018-207994.
- Abdulcadir J, Dugerdil A, Boulvain M, et al. Missed opportunities for diagnosis of female genital mutilation. Int J Gynaecol Obstet. 2014;125:256-260. doi: 10.1016/j.ijgo.2013.11.016.
- Jäger F, Schulze S, Hohlfeld P. Female genital mutilation in Switzerland: a survey among gynaecologists. Swiss Med Wkly. 2002;132:259-264.
- Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol. 2007;27:161-164. doi: 10.1080/01443610601124257.
- Chalmers B, Hashi KO. 432 Somali women’s birth experiences in Canada after earlier female genital mutilation. Birth. 2000;27:227-234. doi: 10.1046/j.1523-536x.2000.00227.x.
- Shahawy S, Amanuel H, Nour NM. Perspectives on female genital cutting among immigrant women and men in Boston. Soc Sci Med. 2019;220:331-339. doi: 10.1016/j.socscimed.2018.11.030.
- Sharif Mohamed F, Wild V, Earp BD, et al. Clitoral reconstruction after female genital mutilation/cutting: a review of surgical techniques and ethical debate. J Sex Med. 2020;17:531-542. doi: 10.1016/j.jsxm.2019.12.004.
- Nour NM. Female genital cutting: a persisting practice. Rev Obstet Gynecol. 2008 Summer;1(3):135-139.
- Binkova A, Uebelhart M, Dällenbach P, et al. A cross-sectional study on pelvic floor symptoms in women living with female genital mutilation/cutting. Reprod Health. 2021;18:39. doi: 10.1186/s12978-021-01097-9.
- Nour NM. Female genital cutting: clinical and cultural guidelines. Obstet Gynecol Surv. 2004;59:272-279. doi: 10.1097/01.ogx.0000118939.19371.af.
- WHO Study Group on Female Genital Mutilation and Obstetric Outcome; Banks E, Meirik O, Farley T, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367:1835-1841. doi: 10.1016/S0140-6736(06)68805-3.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007. doi: 10.1097/AOG.0b013e31821921ce.
- Nour NM, Michels KB, Bryant AE. Defibulation to treat female genital cutting: effect on symptoms and sexual function. Obstet Gynecol. 2006;108:55-60. doi: 10.1097/01.AOG.0000224613.72892.77.
- Johnson C, Nour NM. Surgical techniques: defibulation of type III female genital cutting. J Sex Med. 2007;4:1544-1547. doi: 10.1111/j.1743-6109.2007.00616.x.
- Serour GI. The issue of reinfibulation. Int J Gynaecol Obstet. 2010;109:93-96. doi: 10.1016/j.ijgo.2010.01.001.
- Shahawy S, Deshpande NA, Nour NM. Cross-cultural obstetric and gynecologic care of Muslim patients. Obstet Gynecol. 2015;126:969-973. doi: 10.1097/AOG.0000000000001112.
- American College of Obstetricians and Gynecologists. Elective female genital cosmetic surgery: ACOG committee opinion summary, number 795. Obstet Gynecol. 2020;135:249-250. doi: 10.1097/AOG.0000000000003617.
- Abdulcadir J, McLaren S, Boulvain M, et al. Health education and clinical care of immigrant women with female genital mutilation/cutting who request postpartum reinfibulation. Int J Gynaecol Obstet. 2016;135:69-72. doi: 10.1016/j.ijgo.2016.03.027.
Female genital cutting (FGC), also known as female circumcision or female genital mutilation, is defined by the World Health Organization (WHO) as “the partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.”1 It is a culturally determined practice that is mainly concentrated in certain parts of Africa, the Middle East, and Asia and now is observed worldwide among migrants from those areas.1 Approximately 200 million women and girls alive today have undergone FGC in 31 countries, although encouragingly the practice’s prevalence seems to be declining, especially among younger women.2
Too often, FGC goes unrecognized in women who present for medical care, even in cases where a genitourinary exam is performed and documented.3,4 As a result, patients face delays in diagnosis and management of associated complications and symptoms. Female genital cutting is usually excluded from medical school or residency training curricula,5 and physicians often lack familiarity with the necessary clinical or surgical management of patients who have had the procedure.6 It is crucial, however, that ObGyns feel comfortable recognizing FGC and clinically caring for pregnant and nonpregnant patients who have undergone the procedure. The obstetric-gynecologic setting should be the clinical space in which FGC is correctly diagnosed and from where patients with complications can be referred for appropriate care.
FGC: Through the lens of inequity
Providing culturally competent and sensitive care to women who have undergone FGC is paramount to reducing health care inequities for these patients. Beyond the medical recommendations we review below, we suggest the following considerations when approaching care for these patients.
Acknowledge our biases. It is paramount for us, as providers, to acknowledge our own biases and how these might affect our relationship with the patient and how our care is received. This starts with our language and terminology: The term female genital mutilation can be judgmental or offensive to our patients, many of whom do not consider themselves to have been mutilated. This is why we prefer to use the term female genital cutting, or whichever word the patient uses, so as not to alienate a patient who might already face many other barriers and microaggressions in seeking health care.
Control our responses. Another way we must check our bias is by controlling our reactions during history taking or examining patients who have undergone FGC. Understandably, providers might be shocked to hear patients recount their childhood experiences of FGC or by examining an infibulated scar, but patients report noticing and experiencing hurt, distress, and shame when providers display judgment, horror, or disgust.7 Patients have reported that they are acutely aware that they might be viewed as “backward” and “primitive” in US health care settings.8 These kinds of feelings and experiences can further exacerbate patients’ distrust and avoidance of the health care system altogether. Therefore, providers should acknowledge their own biases regarding the issue as well as those of their staff and work to mitigate them.
Avoid stigmatization. While FGC can have long-term effects (discussed below), it is important to remember that many women who have undergone FGC do not experience symptoms that are bothersome or feel that FGC is central to their lives or lived experiences. While we must be thorough in our history taking to explore possible urinary, gynecologic, and sexual symptoms of concern and bother to the patient, we must avoid stigmatizing our patients by assuming that all who have undergone FGC are “sexually disabled,” which may lead a provider to recommend medically unindicated intervention, such as clitoral reconstruction.9
Continue to: Classifying FGC types...
Classifying FGC types
The WHO has classified FGC into 4 different types1:
- type 1, partial or total removal of the clitoris or prepuce
- type 2, partial or total removal of part of the clitoris and labia minora
- type 3 (also known as infibulation), the narrowing of the vaginal orifice by cutting, removing, and/or repositioning the labia, and
- type 4, all other procedures to the female genitalia for nonmedical reasons.
Long-term complications
Female genital cutting, especially types 2 and 3, can lead to long-term obstetric and gynecologic complications that the ObGyn should be able to diagnose and manage (TABLE).
The most common long-term complications of FGC are dysmenorrhea, dyspareunia, recurrent vaginal and urinary tract infections, and sexual dysfunction/dissatisfaction.10 One recent cross-sectional study that used validated questionnaires on pelvic floor and psychosexual symptoms found that women with FGC had higher distress scores than women who had not undergone FGC, indicating various pelvic floor symptoms responsible for impact on their daily lives.11
Infertility can result from a combination of physical barriers (vaginal stenosis and an infibulated scar) and psychologic barriers secondary to dyspareunia, for example.12 Labor and delivery also presents a challenge to both patients and providers, especially in cases of infibulation. Studies show that patients who have undergone FGC are at increased risk of adverse obstetric outcomes, including postpartum hemorrhage, episiotomy, cesarean delivery, and extended hospital stay.13 Neonatal complications, including infant resuscitation and perinatal death, are more commonly reported in studies outside the United States.13

Clinical management recommendations
It is important to be aware of the WHO FCG classifications and be able to recognize evidence of the procedure on examination. The ObGyn should perform a detailed physical exam of the external genitalia as well as a pelvic floor exam of every patient. If the patient does not disclose a history of FGC but it is suspected based on the examination, the clinician should inquire sensitively if the patient is aware of having undergone any genital procedures.
Especially when a history of FGC has been confirmed, clinicians should ask patients sensitively about their urinary and sexual function and satisfaction. Validated tools, such as the Female Sexual Function Index, the Female Sexual Distress Scale, and the Pelvic Floor Disability Index, may be helpful in gathering an objective and detailed assessment of the patient’s symptoms and level of distress.14 Clinicians also should ask about the patient’s detailed obstetric history, particularly regarding the second stage, delivery, and postpartum complications. The clinician also should specifically inquire about a history of defibulation or additional genital procedures.
Patients with urethral strictures or stenosis may require an exam under anesthesia, cystoscopy, urethral dilation, or urethroplasty.12 Those with chronic urinary tract or vaginal infections may require chronic oral suppressive therapy or defibulation (described below). Defibulation also may be considered for relief of severe dysmenorrhea and menorrhagia that may be resulting from hematocolpos. The ObGyn also should make certain to evaluate for other common causes of these symptoms that may be unrelated to FGC, such as endometriosis.
Many women who have undergone FGC do not report dyspareunia or sexual dissatisfaction; however, infibulation especially has been associated with higher rates of these sequelae.12 In addition to defibulation, pelvic floor physical therapy with an experienced therapist may be helpful for patients with pelvic floor dysfunction, vaginismus, and/or dyspareunia.
The defibulation procedure
Defibulation (or deinfibulation) is a surgical reconstructive procedure that opens the infibulated scar of patients who have undergone type 3 FGC (infibulation), thus exposing the urethra and introitus, and in almost half of cases an intact clitoris.15 Defibulation may be specifically requested by a patient or it may be recommended by the ObGyn either for reducing complications of pregnancy or to address the patient’s gynecologic, sexual, or urogynecologic symptoms by allowing penetrative intercourse, urinary flow, physiologic delivery, and menstruation.16
Defibulation should be performed under regional or general anesthesia and can be performed during pregnancy (or even in labor). An anterior incision is made on the infibulated scar, creating a new labia major, and the edges are sutured separately. Postoperatively, patients should be instructed to perform sitz baths and to expect a change in their urinary voiding stream.12 The few studies that have evaluated defibulation have shown high rates of success in addressing preoperative symptoms; the complication rates of defibulation are low and the satisfaction rates are high.16
The ethical conundrum of reinfibulation
Reinfibulation is defined as the restitching or reapproximation of scar tissue or the labia after delivery or a gynecologic procedure, and it is often performed routinely after every delivery in patients’ countries of origin.17
Postpartum reinfibulation on patient request raises legal and ethical issues for the ObGyn. In the United Kingdom, reinfibulation is illegal, and some international organizations, including the International Federation of Gynecology and Obstetrics and the WHO, have recommended against the practice. In the United States, reinfibulation of an adult is legal, as it falls under the umbrella of elective female genital cosmetic surgery.18,19
The procedure could create or exacerbate long-term complications and should generally be discouraged. However, if despite extensive counseling (preferably in the prenatal period) a patient insists on having the procedure, the ObGyn may need to elevate the principle of patient autonomy and either comply or find a practitioner who is comfortable performing it. One retrospective review in Switzerland suggested that specific care and informative counseling prenatally with the inclusion of a patient’s partner in the discussion can improve the acceptability of defibulation without reinfibulation.20
Conclusion
It is important for ObGyns to be familiar with the practice of FGC and to be trained in its recognition on examination and care for the long-term complications that can result from the practice. At the same time, ObGyns should be especially conscious of their biases in order to provide culturally competent care and reduce health care stigmatization and inequities for these patients.
Female genital cutting (FGC), also known as female circumcision or female genital mutilation, is defined by the World Health Organization (WHO) as “the partial or total removal of the external female genitalia, or other injury to the female genital organs for non-medical reasons.”1 It is a culturally determined practice that is mainly concentrated in certain parts of Africa, the Middle East, and Asia and now is observed worldwide among migrants from those areas.1 Approximately 200 million women and girls alive today have undergone FGC in 31 countries, although encouragingly the practice’s prevalence seems to be declining, especially among younger women.2
Too often, FGC goes unrecognized in women who present for medical care, even in cases where a genitourinary exam is performed and documented.3,4 As a result, patients face delays in diagnosis and management of associated complications and symptoms. Female genital cutting is usually excluded from medical school or residency training curricula,5 and physicians often lack familiarity with the necessary clinical or surgical management of patients who have had the procedure.6 It is crucial, however, that ObGyns feel comfortable recognizing FGC and clinically caring for pregnant and nonpregnant patients who have undergone the procedure. The obstetric-gynecologic setting should be the clinical space in which FGC is correctly diagnosed and from where patients with complications can be referred for appropriate care.
FGC: Through the lens of inequity
Providing culturally competent and sensitive care to women who have undergone FGC is paramount to reducing health care inequities for these patients. Beyond the medical recommendations we review below, we suggest the following considerations when approaching care for these patients.
Acknowledge our biases. It is paramount for us, as providers, to acknowledge our own biases and how these might affect our relationship with the patient and how our care is received. This starts with our language and terminology: The term female genital mutilation can be judgmental or offensive to our patients, many of whom do not consider themselves to have been mutilated. This is why we prefer to use the term female genital cutting, or whichever word the patient uses, so as not to alienate a patient who might already face many other barriers and microaggressions in seeking health care.
Control our responses. Another way we must check our bias is by controlling our reactions during history taking or examining patients who have undergone FGC. Understandably, providers might be shocked to hear patients recount their childhood experiences of FGC or by examining an infibulated scar, but patients report noticing and experiencing hurt, distress, and shame when providers display judgment, horror, or disgust.7 Patients have reported that they are acutely aware that they might be viewed as “backward” and “primitive” in US health care settings.8 These kinds of feelings and experiences can further exacerbate patients’ distrust and avoidance of the health care system altogether. Therefore, providers should acknowledge their own biases regarding the issue as well as those of their staff and work to mitigate them.
Avoid stigmatization. While FGC can have long-term effects (discussed below), it is important to remember that many women who have undergone FGC do not experience symptoms that are bothersome or feel that FGC is central to their lives or lived experiences. While we must be thorough in our history taking to explore possible urinary, gynecologic, and sexual symptoms of concern and bother to the patient, we must avoid stigmatizing our patients by assuming that all who have undergone FGC are “sexually disabled,” which may lead a provider to recommend medically unindicated intervention, such as clitoral reconstruction.9
Continue to: Classifying FGC types...
Classifying FGC types
The WHO has classified FGC into 4 different types1:
- type 1, partial or total removal of the clitoris or prepuce
- type 2, partial or total removal of part of the clitoris and labia minora
- type 3 (also known as infibulation), the narrowing of the vaginal orifice by cutting, removing, and/or repositioning the labia, and
- type 4, all other procedures to the female genitalia for nonmedical reasons.
Long-term complications
Female genital cutting, especially types 2 and 3, can lead to long-term obstetric and gynecologic complications that the ObGyn should be able to diagnose and manage (TABLE).
The most common long-term complications of FGC are dysmenorrhea, dyspareunia, recurrent vaginal and urinary tract infections, and sexual dysfunction/dissatisfaction.10 One recent cross-sectional study that used validated questionnaires on pelvic floor and psychosexual symptoms found that women with FGC had higher distress scores than women who had not undergone FGC, indicating various pelvic floor symptoms responsible for impact on their daily lives.11
Infertility can result from a combination of physical barriers (vaginal stenosis and an infibulated scar) and psychologic barriers secondary to dyspareunia, for example.12 Labor and delivery also presents a challenge to both patients and providers, especially in cases of infibulation. Studies show that patients who have undergone FGC are at increased risk of adverse obstetric outcomes, including postpartum hemorrhage, episiotomy, cesarean delivery, and extended hospital stay.13 Neonatal complications, including infant resuscitation and perinatal death, are more commonly reported in studies outside the United States.13

Clinical management recommendations
It is important to be aware of the WHO FCG classifications and be able to recognize evidence of the procedure on examination. The ObGyn should perform a detailed physical exam of the external genitalia as well as a pelvic floor exam of every patient. If the patient does not disclose a history of FGC but it is suspected based on the examination, the clinician should inquire sensitively if the patient is aware of having undergone any genital procedures.
Especially when a history of FGC has been confirmed, clinicians should ask patients sensitively about their urinary and sexual function and satisfaction. Validated tools, such as the Female Sexual Function Index, the Female Sexual Distress Scale, and the Pelvic Floor Disability Index, may be helpful in gathering an objective and detailed assessment of the patient’s symptoms and level of distress.14 Clinicians also should ask about the patient’s detailed obstetric history, particularly regarding the second stage, delivery, and postpartum complications. The clinician also should specifically inquire about a history of defibulation or additional genital procedures.
Patients with urethral strictures or stenosis may require an exam under anesthesia, cystoscopy, urethral dilation, or urethroplasty.12 Those with chronic urinary tract or vaginal infections may require chronic oral suppressive therapy or defibulation (described below). Defibulation also may be considered for relief of severe dysmenorrhea and menorrhagia that may be resulting from hematocolpos. The ObGyn also should make certain to evaluate for other common causes of these symptoms that may be unrelated to FGC, such as endometriosis.
Many women who have undergone FGC do not report dyspareunia or sexual dissatisfaction; however, infibulation especially has been associated with higher rates of these sequelae.12 In addition to defibulation, pelvic floor physical therapy with an experienced therapist may be helpful for patients with pelvic floor dysfunction, vaginismus, and/or dyspareunia.
The defibulation procedure
Defibulation (or deinfibulation) is a surgical reconstructive procedure that opens the infibulated scar of patients who have undergone type 3 FGC (infibulation), thus exposing the urethra and introitus, and in almost half of cases an intact clitoris.15 Defibulation may be specifically requested by a patient or it may be recommended by the ObGyn either for reducing complications of pregnancy or to address the patient’s gynecologic, sexual, or urogynecologic symptoms by allowing penetrative intercourse, urinary flow, physiologic delivery, and menstruation.16
Defibulation should be performed under regional or general anesthesia and can be performed during pregnancy (or even in labor). An anterior incision is made on the infibulated scar, creating a new labia major, and the edges are sutured separately. Postoperatively, patients should be instructed to perform sitz baths and to expect a change in their urinary voiding stream.12 The few studies that have evaluated defibulation have shown high rates of success in addressing preoperative symptoms; the complication rates of defibulation are low and the satisfaction rates are high.16
The ethical conundrum of reinfibulation
Reinfibulation is defined as the restitching or reapproximation of scar tissue or the labia after delivery or a gynecologic procedure, and it is often performed routinely after every delivery in patients’ countries of origin.17
Postpartum reinfibulation on patient request raises legal and ethical issues for the ObGyn. In the United Kingdom, reinfibulation is illegal, and some international organizations, including the International Federation of Gynecology and Obstetrics and the WHO, have recommended against the practice. In the United States, reinfibulation of an adult is legal, as it falls under the umbrella of elective female genital cosmetic surgery.18,19
The procedure could create or exacerbate long-term complications and should generally be discouraged. However, if despite extensive counseling (preferably in the prenatal period) a patient insists on having the procedure, the ObGyn may need to elevate the principle of patient autonomy and either comply or find a practitioner who is comfortable performing it. One retrospective review in Switzerland suggested that specific care and informative counseling prenatally with the inclusion of a patient’s partner in the discussion can improve the acceptability of defibulation without reinfibulation.20
Conclusion
It is important for ObGyns to be familiar with the practice of FGC and to be trained in its recognition on examination and care for the long-term complications that can result from the practice. At the same time, ObGyns should be especially conscious of their biases in order to provide culturally competent care and reduce health care stigmatization and inequities for these patients.
- World Health Organization. Female genital mutilation. February 3, 2020. https://www.who.int/news-room/fact-sheets/detail/female-genital-mutilation. Accessed February 22, 2021.
- UNICEF. Female genital mutilation (FGM). February 2020. https://data.unicef.org/topic/child-protection/female-genital-mutilation/. Accessed February 22, 2021.
- Stoklosa H, Nour NM. The eye cannot see what the mind does not know: female genital mutilation. Emerg Med J. 2018;35:585-586. doi: 10.1136/emermed-2018-207994.
- Abdulcadir J, Dugerdil A, Boulvain M, et al. Missed opportunities for diagnosis of female genital mutilation. Int J Gynaecol Obstet. 2014;125:256-260. doi: 10.1016/j.ijgo.2013.11.016.
- Jäger F, Schulze S, Hohlfeld P. Female genital mutilation in Switzerland: a survey among gynaecologists. Swiss Med Wkly. 2002;132:259-264.
- Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol. 2007;27:161-164. doi: 10.1080/01443610601124257.
- Chalmers B, Hashi KO. 432 Somali women’s birth experiences in Canada after earlier female genital mutilation. Birth. 2000;27:227-234. doi: 10.1046/j.1523-536x.2000.00227.x.
- Shahawy S, Amanuel H, Nour NM. Perspectives on female genital cutting among immigrant women and men in Boston. Soc Sci Med. 2019;220:331-339. doi: 10.1016/j.socscimed.2018.11.030.
- Sharif Mohamed F, Wild V, Earp BD, et al. Clitoral reconstruction after female genital mutilation/cutting: a review of surgical techniques and ethical debate. J Sex Med. 2020;17:531-542. doi: 10.1016/j.jsxm.2019.12.004.
- Nour NM. Female genital cutting: a persisting practice. Rev Obstet Gynecol. 2008 Summer;1(3):135-139.
- Binkova A, Uebelhart M, Dällenbach P, et al. A cross-sectional study on pelvic floor symptoms in women living with female genital mutilation/cutting. Reprod Health. 2021;18:39. doi: 10.1186/s12978-021-01097-9.
- Nour NM. Female genital cutting: clinical and cultural guidelines. Obstet Gynecol Surv. 2004;59:272-279. doi: 10.1097/01.ogx.0000118939.19371.af.
- WHO Study Group on Female Genital Mutilation and Obstetric Outcome; Banks E, Meirik O, Farley T, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367:1835-1841. doi: 10.1016/S0140-6736(06)68805-3.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007. doi: 10.1097/AOG.0b013e31821921ce.
- Nour NM, Michels KB, Bryant AE. Defibulation to treat female genital cutting: effect on symptoms and sexual function. Obstet Gynecol. 2006;108:55-60. doi: 10.1097/01.AOG.0000224613.72892.77.
- Johnson C, Nour NM. Surgical techniques: defibulation of type III female genital cutting. J Sex Med. 2007;4:1544-1547. doi: 10.1111/j.1743-6109.2007.00616.x.
- Serour GI. The issue of reinfibulation. Int J Gynaecol Obstet. 2010;109:93-96. doi: 10.1016/j.ijgo.2010.01.001.
- Shahawy S, Deshpande NA, Nour NM. Cross-cultural obstetric and gynecologic care of Muslim patients. Obstet Gynecol. 2015;126:969-973. doi: 10.1097/AOG.0000000000001112.
- American College of Obstetricians and Gynecologists. Elective female genital cosmetic surgery: ACOG committee opinion summary, number 795. Obstet Gynecol. 2020;135:249-250. doi: 10.1097/AOG.0000000000003617.
- Abdulcadir J, McLaren S, Boulvain M, et al. Health education and clinical care of immigrant women with female genital mutilation/cutting who request postpartum reinfibulation. Int J Gynaecol Obstet. 2016;135:69-72. doi: 10.1016/j.ijgo.2016.03.027.
- World Health Organization. Female genital mutilation. February 3, 2020. https://www.who.int/news-room/fact-sheets/detail/female-genital-mutilation. Accessed February 22, 2021.
- UNICEF. Female genital mutilation (FGM). February 2020. https://data.unicef.org/topic/child-protection/female-genital-mutilation/. Accessed February 22, 2021.
- Stoklosa H, Nour NM. The eye cannot see what the mind does not know: female genital mutilation. Emerg Med J. 2018;35:585-586. doi: 10.1136/emermed-2018-207994.
- Abdulcadir J, Dugerdil A, Boulvain M, et al. Missed opportunities for diagnosis of female genital mutilation. Int J Gynaecol Obstet. 2014;125:256-260. doi: 10.1016/j.ijgo.2013.11.016.
- Jäger F, Schulze S, Hohlfeld P. Female genital mutilation in Switzerland: a survey among gynaecologists. Swiss Med Wkly. 2002;132:259-264.
- Zaidi N, Khalil A, Roberts C, et al. Knowledge of female genital mutilation among healthcare professionals. J Obstet Gynaecol. 2007;27:161-164. doi: 10.1080/01443610601124257.
- Chalmers B, Hashi KO. 432 Somali women’s birth experiences in Canada after earlier female genital mutilation. Birth. 2000;27:227-234. doi: 10.1046/j.1523-536x.2000.00227.x.
- Shahawy S, Amanuel H, Nour NM. Perspectives on female genital cutting among immigrant women and men in Boston. Soc Sci Med. 2019;220:331-339. doi: 10.1016/j.socscimed.2018.11.030.
- Sharif Mohamed F, Wild V, Earp BD, et al. Clitoral reconstruction after female genital mutilation/cutting: a review of surgical techniques and ethical debate. J Sex Med. 2020;17:531-542. doi: 10.1016/j.jsxm.2019.12.004.
- Nour NM. Female genital cutting: a persisting practice. Rev Obstet Gynecol. 2008 Summer;1(3):135-139.
- Binkova A, Uebelhart M, Dällenbach P, et al. A cross-sectional study on pelvic floor symptoms in women living with female genital mutilation/cutting. Reprod Health. 2021;18:39. doi: 10.1186/s12978-021-01097-9.
- Nour NM. Female genital cutting: clinical and cultural guidelines. Obstet Gynecol Surv. 2004;59:272-279. doi: 10.1097/01.ogx.0000118939.19371.af.
- WHO Study Group on Female Genital Mutilation and Obstetric Outcome; Banks E, Meirik O, Farley T, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367:1835-1841. doi: 10.1016/S0140-6736(06)68805-3.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 119: female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007. doi: 10.1097/AOG.0b013e31821921ce.
- Nour NM, Michels KB, Bryant AE. Defibulation to treat female genital cutting: effect on symptoms and sexual function. Obstet Gynecol. 2006;108:55-60. doi: 10.1097/01.AOG.0000224613.72892.77.
- Johnson C, Nour NM. Surgical techniques: defibulation of type III female genital cutting. J Sex Med. 2007;4:1544-1547. doi: 10.1111/j.1743-6109.2007.00616.x.
- Serour GI. The issue of reinfibulation. Int J Gynaecol Obstet. 2010;109:93-96. doi: 10.1016/j.ijgo.2010.01.001.
- Shahawy S, Deshpande NA, Nour NM. Cross-cultural obstetric and gynecologic care of Muslim patients. Obstet Gynecol. 2015;126:969-973. doi: 10.1097/AOG.0000000000001112.
- American College of Obstetricians and Gynecologists. Elective female genital cosmetic surgery: ACOG committee opinion summary, number 795. Obstet Gynecol. 2020;135:249-250. doi: 10.1097/AOG.0000000000003617.
- Abdulcadir J, McLaren S, Boulvain M, et al. Health education and clinical care of immigrant women with female genital mutilation/cutting who request postpartum reinfibulation. Int J Gynaecol Obstet. 2016;135:69-72. doi: 10.1016/j.ijgo.2016.03.027.
Do ObGyns plan on getting a 9vHPV vaccine?
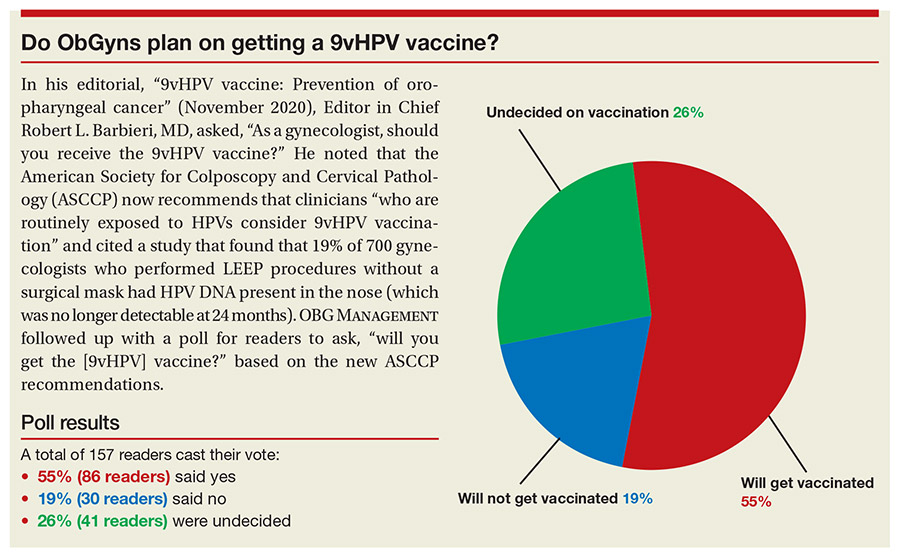
In his editorial, "9vHPV vaccine: Prevention of oropharyngeal cancer" (November 2020), Editor in Chief Robert L. Barbieri, MD, asked, "As a gynecologist, should you receive the 9vHPV vaccine?" He noted that the American Society for Colposcopy and Cervical Pathology (ASCCP) now recommends that clinicians "who are routinely exposed to HPVs consider 9vHPV vaccination" and cited a study that found that 19% of 700 gynecologists who performed LEEP procedures without a surgical mask had HPV DNA present in the nose (which was no longer detectable at 24 months). OBG MANAGEMENT followed up with a poll for readers to ask, "will you get the [9vHPV] vaccine?" based on the new ASCCP recommendations.
A total of 157 readers cast their vote:
55% (86 readers) said yes
19% (30 readers) said no
26% (41 readers) were undecided
In his editorial, "9vHPV vaccine: Prevention of oropharyngeal cancer" (November 2020), Editor in Chief Robert L. Barbieri, MD, asked, "As a gynecologist, should you receive the 9vHPV vaccine?" He noted that the American Society for Colposcopy and Cervical Pathology (ASCCP) now recommends that clinicians "who are routinely exposed to HPVs consider 9vHPV vaccination" and cited a study that found that 19% of 700 gynecologists who performed LEEP procedures without a surgical mask had HPV DNA present in the nose (which was no longer detectable at 24 months). OBG MANAGEMENT followed up with a poll for readers to ask, "will you get the [9vHPV] vaccine?" based on the new ASCCP recommendations.
A total of 157 readers cast their vote:
55% (86 readers) said yes
19% (30 readers) said no
26% (41 readers) were undecided

In his editorial, "9vHPV vaccine: Prevention of oropharyngeal cancer" (November 2020), Editor in Chief Robert L. Barbieri, MD, asked, "As a gynecologist, should you receive the 9vHPV vaccine?" He noted that the American Society for Colposcopy and Cervical Pathology (ASCCP) now recommends that clinicians "who are routinely exposed to HPVs consider 9vHPV vaccination" and cited a study that found that 19% of 700 gynecologists who performed LEEP procedures without a surgical mask had HPV DNA present in the nose (which was no longer detectable at 24 months). OBG MANAGEMENT followed up with a poll for readers to ask, "will you get the [9vHPV] vaccine?" based on the new ASCCP recommendations.
A total of 157 readers cast their vote:
55% (86 readers) said yes
19% (30 readers) said no
26% (41 readers) were undecided

HHS proposes overturning Title X ‘gag’ rule
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
FDA lifts in-person dispensing requirement for mifepristone
The Food and Drug Administration has lifted in-person dispensing requirements for mifepristone when used for medical termination of early pregnancy.
In an April 12, 2021, letter to the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, acting commissioner of food and drugs Janet Woodcock stated that the FDA would exercise discretion to permit the dispensing of mifepristone through the mail when done by or under the supervision of a certified prescriber; or through a mail-order pharmacy under the supervision of a certified prescriber.
The decision follows a trial period of suspension of the in-person dispensing requirement in response to safety concerns for patients as well as providers associated with in-person clinic visits during the COVID-19 pandemic. The Center for Drug Evaluation and Research reviewed safety and clinical outcomes data on mifepristone use when prescriptions were handled by mail or mail-order pharmacy and found that "the small number of adverse events reported to FDA during the COVID-19 public health emergency [PHE] provide no indication that any program deviation or noncompliance with the mifepristone [Risk Evaluation and Mitigation Strategy] program contributed to the reported adverse events," according to the letter. The analysis covers Mifeprex and the approved generic, mifepristone tablets, both 200-mg doses.
As long as other mifepristone REMS criteria are met, the FDA will continue to permit mail and mail-order prescriptions, according to the letter.
"By halting enforcement of the in-person dispensing requirement during the COVID-19 pandemic, the FDA is recognizing and responding to the available evidence - which has clearly and definitively demonstrated that the in-person dispensing requirement for mifepristone is unnecessary and restrictive," Maureen G. Phipps, MD, MPH, CEO of ACOG, said in a statement in response to the FDA decision.
ACOG petitioned the FDA to suspend the in-person requirement to reduce the risk of transmission in the wake of the COVID-19 pandemic, given safety concerns and the potential impact on hard-hit communities, particularly communities of color, Dr. Phipps emphasized. Data from a review period with a suspension of the in-person requirement yielded no additional safety concerns with mifepristone use, and contributed to the FDA decision to lift the requirement.
"Thanks to the FDA's intent to exercise discretion in enforcing the in-person dispensing requirement, those in need of an abortion or miscarriage management will be able to do so safety and effectively by acquiring mifepristone though the mail - just as they would any other medication with a similarly strong safety profile," said Dr. Phipps. "We are pleased to see mifepristone regulated on the basis of the scientific evidence during the pandemic, rather than political bias against comprehensive reproductive health care, and we look forward to working with policy makers to ensure this principle governs postpandemic care."
CDER is communicating the decision to all approved application holders subject to the mifepristone REMS program, according to the letter.
[email protected]
The Food and Drug Administration has lifted in-person dispensing requirements for mifepristone when used for medical termination of early pregnancy.
In an April 12, 2021, letter to the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, acting commissioner of food and drugs Janet Woodcock stated that the FDA would exercise discretion to permit the dispensing of mifepristone through the mail when done by or under the supervision of a certified prescriber; or through a mail-order pharmacy under the supervision of a certified prescriber.
The decision follows a trial period of suspension of the in-person dispensing requirement in response to safety concerns for patients as well as providers associated with in-person clinic visits during the COVID-19 pandemic. The Center for Drug Evaluation and Research reviewed safety and clinical outcomes data on mifepristone use when prescriptions were handled by mail or mail-order pharmacy and found that "the small number of adverse events reported to FDA during the COVID-19 public health emergency [PHE] provide no indication that any program deviation or noncompliance with the mifepristone [Risk Evaluation and Mitigation Strategy] program contributed to the reported adverse events," according to the letter. The analysis covers Mifeprex and the approved generic, mifepristone tablets, both 200-mg doses.
As long as other mifepristone REMS criteria are met, the FDA will continue to permit mail and mail-order prescriptions, according to the letter.
"By halting enforcement of the in-person dispensing requirement during the COVID-19 pandemic, the FDA is recognizing and responding to the available evidence - which has clearly and definitively demonstrated that the in-person dispensing requirement for mifepristone is unnecessary and restrictive," Maureen G. Phipps, MD, MPH, CEO of ACOG, said in a statement in response to the FDA decision.
ACOG petitioned the FDA to suspend the in-person requirement to reduce the risk of transmission in the wake of the COVID-19 pandemic, given safety concerns and the potential impact on hard-hit communities, particularly communities of color, Dr. Phipps emphasized. Data from a review period with a suspension of the in-person requirement yielded no additional safety concerns with mifepristone use, and contributed to the FDA decision to lift the requirement.
"Thanks to the FDA's intent to exercise discretion in enforcing the in-person dispensing requirement, those in need of an abortion or miscarriage management will be able to do so safety and effectively by acquiring mifepristone though the mail - just as they would any other medication with a similarly strong safety profile," said Dr. Phipps. "We are pleased to see mifepristone regulated on the basis of the scientific evidence during the pandemic, rather than political bias against comprehensive reproductive health care, and we look forward to working with policy makers to ensure this principle governs postpandemic care."
CDER is communicating the decision to all approved application holders subject to the mifepristone REMS program, according to the letter.
[email protected]
The Food and Drug Administration has lifted in-person dispensing requirements for mifepristone when used for medical termination of early pregnancy.
In an April 12, 2021, letter to the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, acting commissioner of food and drugs Janet Woodcock stated that the FDA would exercise discretion to permit the dispensing of mifepristone through the mail when done by or under the supervision of a certified prescriber; or through a mail-order pharmacy under the supervision of a certified prescriber.
The decision follows a trial period of suspension of the in-person dispensing requirement in response to safety concerns for patients as well as providers associated with in-person clinic visits during the COVID-19 pandemic. The Center for Drug Evaluation and Research reviewed safety and clinical outcomes data on mifepristone use when prescriptions were handled by mail or mail-order pharmacy and found that "the small number of adverse events reported to FDA during the COVID-19 public health emergency [PHE] provide no indication that any program deviation or noncompliance with the mifepristone [Risk Evaluation and Mitigation Strategy] program contributed to the reported adverse events," according to the letter. The analysis covers Mifeprex and the approved generic, mifepristone tablets, both 200-mg doses.
As long as other mifepristone REMS criteria are met, the FDA will continue to permit mail and mail-order prescriptions, according to the letter.
"By halting enforcement of the in-person dispensing requirement during the COVID-19 pandemic, the FDA is recognizing and responding to the available evidence - which has clearly and definitively demonstrated that the in-person dispensing requirement for mifepristone is unnecessary and restrictive," Maureen G. Phipps, MD, MPH, CEO of ACOG, said in a statement in response to the FDA decision.
ACOG petitioned the FDA to suspend the in-person requirement to reduce the risk of transmission in the wake of the COVID-19 pandemic, given safety concerns and the potential impact on hard-hit communities, particularly communities of color, Dr. Phipps emphasized. Data from a review period with a suspension of the in-person requirement yielded no additional safety concerns with mifepristone use, and contributed to the FDA decision to lift the requirement.
"Thanks to the FDA's intent to exercise discretion in enforcing the in-person dispensing requirement, those in need of an abortion or miscarriage management will be able to do so safety and effectively by acquiring mifepristone though the mail - just as they would any other medication with a similarly strong safety profile," said Dr. Phipps. "We are pleased to see mifepristone regulated on the basis of the scientific evidence during the pandemic, rather than political bias against comprehensive reproductive health care, and we look forward to working with policy makers to ensure this principle governs postpandemic care."
CDER is communicating the decision to all approved application holders subject to the mifepristone REMS program, according to the letter.
[email protected]
Patient-centered contraceptive care for medically complex patients
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
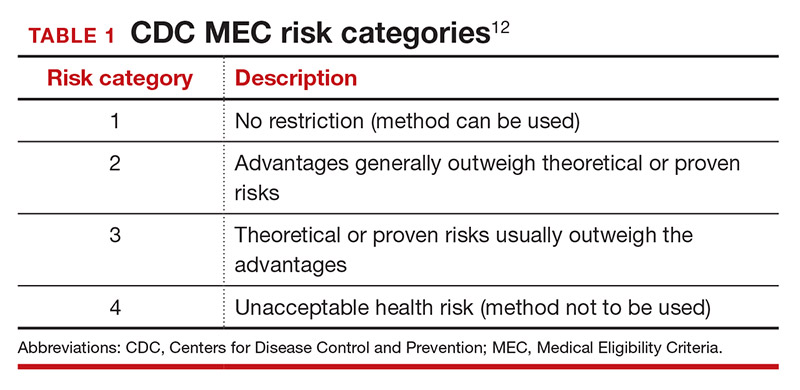
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).
In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
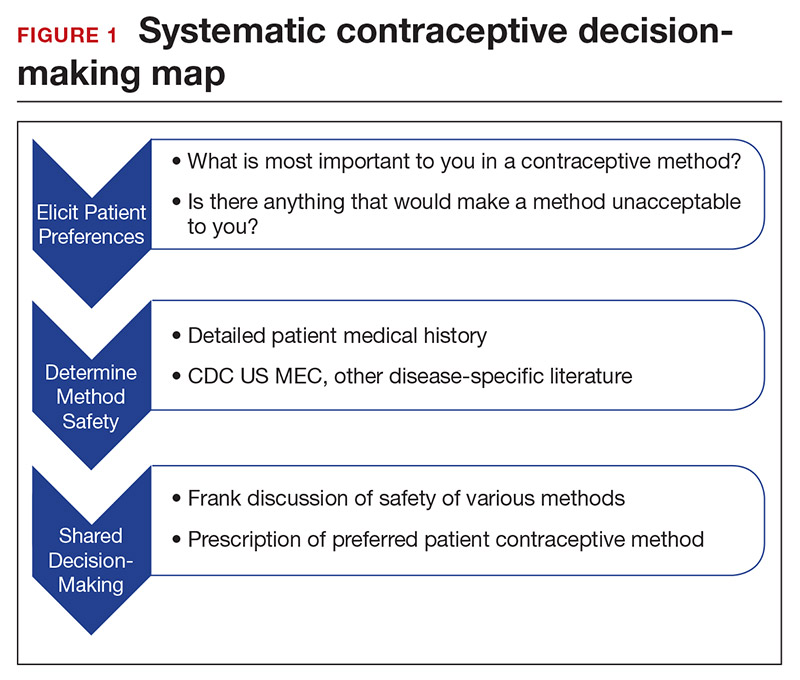
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.
CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12
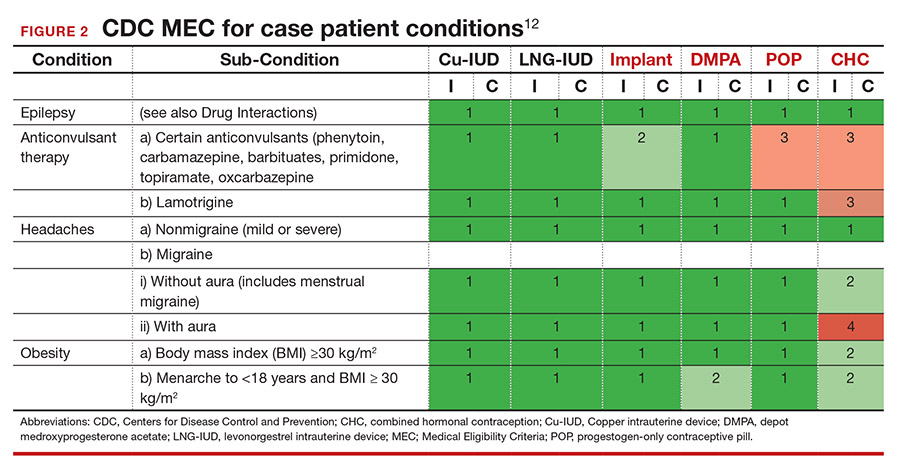
Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).


In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.

CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12

Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
CASE Patient-centered counseling for contraception
A 19-year-old woman (G0) with moderately well-controlled seizure disorder while taking levetiracetam, who reports migraines, and has a BMI of 32 kg/m2 presents to your office seeking contraception. She is currently sexually active with her second lifetime partner and uses condoms inconsistently. She is otherwise healthy and has no problems to report. Her last menstrual period (LMP) was 1 week ago, and a pregnancy test today is negative. How do you approach counseling for this patient?
The modern contraceptive patient
Our patients are becoming increasingly medically and socially complicated. Meeting the contraceptive needs of patients with multiple comorbidities can be a daunting task. Doing so in a patient-centered way that also recognizes the social contexts and intimacy inherent to contraceptive care can feel overwhelming. However, by employing a systematic approach to each patient, we can provide safe, effective, individualized care to our medically complex patients. Having a few “go-to tools” can streamline the process.
Medically complex patients are often told that they need to avoid pregnancy or optimize their health conditions prior to becoming pregnant, but they may not receive medically-appropriate contraception.1-3 Additionally, obesity rates in women of reproductive age in the United States are increasing, along with related medical complexities.4 Disparities in contraceptive access and use of particular methods exist by socioeconomic status, body mass index (BMI), age, and geography. 5,6 Evidence-based, shared decision making can improve contraceptive satisfaction.7
Clinicians need to stay attuned to all options. Staying current on available contraceptive methods can broaden clinicians’ thinking and allow patients more choices that are compatible with their medical needs. In the last 2 years alone, a 1-year combined estrogen-progestin vaginal ring, a drospirinone-only pill, and a nonhormonal spermicide have become available for prescription.8-10 Both 52 mg levonorgestrel-containing intrauterine devices (IUDs) are now US Food and Drug Administration (FDA)-approved for 6 years, and there is excellent data for off-label use to 7 years.11
Tools are available for use. To ensure patient safety, we must evaluate the relative risks of each method given their specific medical history. The Centers for Disease Control and Prevention (CDC) Medical Eligibility Criteria (MEC) provides a comprehensive reference for using each contraceptive method category with preexisting medical conditions on a scale from 1 (no restrictions) to 4 (unacceptable health risk) (TABLE 1).12 It is important to remember that pregnancy often poses a larger risk even than category 4 methods. With proper counseling and documentation, a category 3 method may be appropriate in some circumstances. The CDC MEC can serve as an excellent counseling tool and is available as a free smartphone app. The app can be downloaded via https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html (TABLE 2).


In a shared decision-making model, we contribute our medical knowledge, and the patient provides expertise on her own values and social context.13 By starting the contraceptive conversation with open-ended questions, we invite the patient to lead the discussion. We partner with them in finding a safe, effective method that is compatible with both the medical history and stated preferences. Bedsider.org has an interactive tool that allows patients to explore different contraceptive methods and compare their various characteristics. While tiered efficacy models may help us to organize our thinking as clinicians, it is important to recognize that patients may consider side effect profiles, nonreliance on clinicians for discontinuation, or other priorities above effectiveness.
Continue to: How to craft your approach...
How to craft your approach
Developing a systematic approach to the medically complex patient seeking contraception can help to change an initially daunting task into a fulfilling experience (FIGURE 1). Begin by eliciting patient priorities. Then frame the discussion around them, rather than around efficacy. Although anecdotal reasoning can initially be frustrating (“My best friend’s IUD was really painful and I don’t want anything like that inside me!”), learning about these experiences prior to counseling can be incredibly informative. Ask detailed questions about medical comorbidities, as these subtleties may change the relative safety of each method. Finally, engage the patient in a frank discussion of the relative merits, safety, and use of all medically appropriate contraceptive methods. The right method is the method that the patient will use.

CASE Continued: Applying our counseling method
Upon open-ended questioning, the patient tells you that she absolutely cannot be on a contraceptive method that will make her gain weight. She has several friends who told her that they gained weight on “the shot” and “the implant.” She wants to avoid these at all costs and thinks she might want to take “the pill.” She also tells you that she is in college and that her daily routine varies significantly between weekdays and weekends. She definitely does not want to get pregnant until she has completed her education, which will be at least 3 years from now.
To best counsel this patient and arrive at the most appropriate contraceptive option for her, clarify her medical history and employ shared decision-making for her chosen method.
Probe her seizure history
She tells you that she has had seizures since she was a child, and the last one occurred 4 months ago when she ran out of her anticonvulsant medication. Her seizures have never been associated with her menses. This is an important piece of information. The frequency of catamenial seizures can be decreased with use of any method that suppresses ovulation, such as depot-medroxyprogesterone (DMPA) injections, continuous combined hormonal contraceptive (CHC) pills or ring, or the implant. Noncatamenial seizures also can be suppressed by DMPA, which increases the seizure threshold.14 Many anticonvulsants are metabolized through cytochrome P450 in the liver and, therefore, interact with all oral contraceptive formulations. However, levetiracetam is not among them and may be safely taken with progestin-only pills. At this point, all contraceptive methods remain CDC MEC category 1 (FIGURE 2).12

Ask migraine specifics
It is important to clarify whether or not the patient experiences aura with her migraines. She says that she always knows when a migraine is coming on because she sees floaters in her vision for about 30 minutes prior to the onset of excruciating headache. One tool that may aid in the diagnosis of aura is the Visual Aura Rating Scale (VARS).15 The presence of aura renders all CHCs category 4 by the CDC MEC.12 (See FIGURE 2.)
Discuss contraceptive pros and cons
Have a frank discussion about the relative risks and benefits of each method. For instance, although DMPA may improve the patient’s seizures, she has expressed a desire to avoid weight gain, and DMPA is the only method consistently shown in studies to do so.16 Her seizures are not associated with menses, so menstrual suppression is neither beneficial nor deleterious. Although her current medication levetiracetam does not influence the metabolism of contraceptive methods, many anticonvulsants do. Offer anticipatory guidance around seeking gynecologic consultation with any future seizure medication changes.
Allow for shared decision-making on a final choice
The patient indicated that she had been considering “the pill” when she made this appointment, but you have explained that CHCs are contraindicated for her. She is concerned that she will not be able to stick to the strict dosing schedule of a progestin-only pill. Although you inform her that the drospirinone-only pill has a more forgiving window, the patient decides that she wants a “set it and forget it” method and opts for an IUD.
CASE Resolved
Following recommendations from the American College of Obstetricians and Gynecologists (ACOG), you provide for same-day insertion of a 52-mg levonorgestrel IUD.17 You use a paracervical block in addition to ibuprofen for pain control.18 The patient undergoes same-day testing for gonorrhea and chlamydia, and she understands that if a test is found to be positive, she can be treated without removing the IUD. You provide instruction on the importance of dual contraceptive use with barrier methods for the prevention of STIs. The patient is instructed on self-string checks, and she acknowledges that she will call if she has any concerns; no routine follow-up is required. She leaves her visit satisfied with her preferred, safe, effective contraceptive method in situ. ●
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
- Lauring JR, Lehman EB, Deimling TA, et al. Combined hormonal contraception use in reproductive-age women with contraindications to estrogen use. Am J Obstet Gynecol. 2016;215:330.e1-e7.
- Mendel A, Bernatsky S, Pineau CA, et al. Use of combined hormonal contraceptives among women with systemic lupus erythematosus with and without medical contraindications to oestrogen. Rheumatology (Oxford). 2019;58:1259-1267.
- Judge CP, Zhao X, Sileanu FE, et al. Medical contraindications to estrogen and contraceptive use among women veterans. Am J Obstet Gynecol. 2018;218:234.e1-234.e9.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1-8.
- Guttmacher Institute. Contraceptive use in the United States. April 2020. . Accessed March 22, 2021.
- Mosher WD, Lantos H, Burke AE. Obesity and contraceptive use among women 20–44 years of age in the United States: results from the 2011–15 National Survey of Family Growth (NSFG). Contraception. 2018:97:392-398.
- Dehlendorf C, Grumbach K, Schmittdiel JA, et al. Shared decision making in contraceptive counseling. Contraception. 2017;95:452-455.
- Annovera [package insert]. Boca Raton, FL: TherapeuticsMD, Inc; 2020.
- Slynd [package insert]. Florham Park, NJ: Exeltis; 2019.
- Phexxi [package insert]. San Diego, CA: Evofem; 2020.
- , et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016. . Accessed March 23, 2021.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681-692.
- Dutton C, Foldvary‐Schaefer N. Contraception in women with epilepsy: pharmacokinetic interactions, contraceptive options, and management. Int Rev Neurobiol. 83;2008:113-134.
- Eriksen MK, Thomsen LL, Olesen J. The visual aura rating scale (VARS) for migraine aura diagnosis. Cephalalgia. 2005;25:801-810.
- ME, , , et al. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81:30-34.
- Espey E, Hofler L. Long-acting reversible contraception: implants and intrauterine devices. Practice bulletin 186. Obstet Gynecol. 2017;130:e251-269.
- Akers AY, Steinway C, Sonalkar S, et al. Reducing pain during intrauterine device insertion: a randomized controlled trial in adolescents and young women. Obstet Gynecol. 2017;130:795-802.
Can a once-daily oral formulation treat symptoms of uterine fibroids without causing hot flashes or bone loss?
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
Al-Hendy A, Lukes AS, Poindexter AN 3rd, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283
Expert Commentary
By age 50, approximately 70% of White women and 80% of Black women will have uterine fibroids.1 Of these, about 25% will have symptoms—most often including heavy menstrual bleeding,2 and associated pain the second most common symptom.3 First-line treatment has traditionally been hormonal contraceptives. Injectable gonadotropin-releasing hormone (GnRH) antagonist like leuprolide acetate have been commonly employed, although their actual approved indication is “for concomitant use with iron therapy for preoperative hematologic improvement of patients with anemia caused by uterine leiomyomata (fibroids).”4 Recently, an oral GnRH antagonist, elagolix, combined with estrogen and progestogen, was approved for treatment of uterine fibroids for up to 24 months. However, it is dosed twice per day because of its short half-life and results in a loss of bone mineral density at 1 year.5,6
Details of the studies
Al-Hendy and colleagues report on two double-blind 24-week phase 3 trials involving women with heavy menstrual bleeding associated with fibroids. There were just under 400 women in each trial. There was a 1:1:1 randomization to: placebo, once-daily oral relugolix 40 mg with 1 mg estradiol and 0.5 mg norethindrone acetate, or oral relugolix by itself for 12 weeks followed by the combination for 12 weeks (referred to as the “delayed relugolix combination therapy” arm).
Results. The primary end point was the percentage of patients who had a volume of menstrual blood loss less than 80 mL and a ≥50% reduction in blood loss volume as measured by the alkaline hematin method. The baseline blood loss in these studies ranged from approximately 210–250 mL. Secondary end points included amenorrhea, volume of menstrual blood loss, distress from bleeding and pelvic discomfort, anemia, pain, uterine volume, and the largest fibroid volume.
In trials one and two, 73% and 71% of patients in the relugolix combination groups, respectively, achieved the primary endpoint, compared with 19% and 15% in the placebo groups (P <.001). In addition, all secondary endpoints except largest fibroid volume were significantly improved versus placebo. Adverse events, including any change in bone mineral density, were no different between the combination and placebo groups. The delayed combination groups did have more hot flashes and diminished bone density compared with both the placebo and combination groups.
Strengths and weaknesses
The studies appropriately enrolled women with a mean age of 41–42 years and a mean BMI >30 kg/m2, and more than 50% were African American. Thus, the samples are adequately representative of the type of population most likely to have fibroids and associated symptoms. The results showed the advantages of built-in “add back therapy” with estrogen plus progestogen, as the vasomotor symptoms and bone loss that treatment with a GnRH antagonist alone produces were reduced.
Although the trials were only conducted for 24 weeks, efficacy was seen as early as 4 weeks, and was clearly maintained throughout the full trials—and there is no scientific reason to assume it would not be maintained indefinitely. However, one cannot make a similar assumption about long-term safety. As another GnRH antagonist, with a shorter half-life requiring twice-daily-dosing with add back therapy, has been approved for use for 2 years, it is likely that the once-daily formulation of combination relugolix will be approved for this timeframe as well. Still, with patients’ mean age of 41–42 years, what will clinicians do after 2-year treatment? Clearly, study of long-term safety would be valuable. ●
Fibroids are extremely common in clinical practice, with their associated symptoms depending greatly on size and location. In many patients, symptoms are serious enough to be the most common indication for hysterectomy. In the past, combination oral contraceptives, injectable leuprolide acetate, and more recently, a GnRH antagonist given twice daily with estrogen/progestogen add-back have been utilized. The formulation described in Al-Hendy and colleagues’ study, which is dosed once per day and appears to not increase vasomotor symptoms or diminish bone mass, may provide a very nice “tool” in the clinician’s toolbox to either avoid any surgery in some patients (likely those aged closer to menopause) or optimize other patients preoperatively in terms of reversing anemia and reducing uterine volume, thus making any planned surgical procedure safer.
STEVEN R. GOLDSTEIN, MD, NCMP, CCD
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
- Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol. 2016;59:2-24.
- Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209:319.e1-319.e20.
- David M, Pitz CM, Mihaylova A, et al. Myoma-associated pain frequency and intensity: a retrospective evaluation of 1548 myoma patients. Eur J Obstet Gynecol Reprod Biol. 2016;199:137-140.
- Lupron Depot [package insert]. North Chicago, IL: AbbVie Inc.; 2018.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
- Oriahnn [package insert]. North Chicago, IL: AbbVie Inc.; 2020.
Optimize your treatment of endometriosis by using an FDA-approved hormonal medication
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
How physicians can provide better care to transgender patients
People who identify as transgender experience many health disparities, in addition to lack of access to quality care. The most commonly cited barrier is the lack of providers who are knowledgeable about transgender health care, according to past surveys.
Even those who do seek care often have unpleasant experiences. A 2015 survey conducted by the National Center for Transgender Equality found that 33% of those who saw a health care provider reported at least one unfavorable experience related to being transgender, such as being verbally harassed or refused treatment because of their gender identity. In fact, 23% of those surveyed say they did not seek health care they needed in the past year because of fear of being mistreated as a transgender person.
This interview has been edited for length and clarity.
Question: Surveys have shown that many people who identify as transgender will seek only transition care, not primary or preventive care. Why is that?
Dr. Brandt: My answer is multifactorial. Transgender patients do seek primary care – just not as readily. There’s a lot of misconceptions about health care needs for the LGBT community in general. For example, lesbian or bisexual women may be not as well informed about the need for Pap smears compared with their heterosexual counterparts. These misconceptions are further exacerbated in the transgender community.
The fact that a lot of patients seek only transition-related care, but not preventive services, such as primary care and gynecologic care, is also related to fears of discrimination and lack of education of providers. These patients are afraid when they walk into an office that they will be misgendered or their physician won’t be familiar with their health care needs.
What can clinics and clinicians do to create a safe and welcoming environment?
Dr. Brandt: It starts with educating office staff about terminology and gender identities.
A key feature of our EHR is the sexual orientation and gender identity platform, which asks questions about a patient’s gender identity, sexual orientation, sex assigned at birth, and organ inventory. These data are then found in the patient information tab and are just as relevant as their insurance status, age, and date of birth.
There are many ways a doctor’s office can signal to patients that they are inclusive. They can hang LGBTQ-friendly flags or symbols or a sign saying, “We have an anti-discrimination policy” in the waiting room. A welcoming environment can also be achieved by revising patient questionnaires or forms so that they aren’t gender-specific or binary.
Given that the patient may have limited contact with a primary care clinician, how do you prioritize what you address during the visit?
Dr. Brandt: Similar to cisgender patients, it depends initially on the age of the patient and the reason for the visit. The priorities of an otherwise healthy transgender patient in their 20s are going to be largely the same as for a cisgender patient of the same age. As patients age in the primary care world, you’re addressing more issues, such as colorectal screening, lipid disorders, and mammograms, and that doesn’t change. For the most part, the problems that you address should be specific for that age group.
It becomes more complicated when you add in factors such as hormone therapy and whether patients have had any type of gender-affirming surgery. Those things can change the usual recommendations for screening or risk assessment. We try to figure out what routine health maintenance and cancer screening a patient needs based on age and risk factors, in addition to hormone status and surgical state.
Do you think that many physicians are educated about the care of underserved populations such as transgender patients?
Dr. Brandt: Yes and no. We are definitely getting better at it. For example, the American College of Obstetricians and Gynecologists published a committee opinion highlighting transgender care. So organizations are starting to prioritize these populations and recognize that they are, in fact, underserved and they have special health care needs.
However, the knowledge gaps are still pretty big. I get calls daily from providers asking questions about how to manage patients on hormones, or how to examine a patient who has undergone a vaginoplasty. I hear a lot of horror stories from transgender patients who had their hormones stopped for absurd and medically misinformed reasons.
But I definitely think it’s getting better and it’s being addressed at all levels – the medical school level, the residency level, and the attending level. It just takes time to inform people and for people to get used to the health care needs of these patients.
What should physicians keep in mind when treating patients who identify as transgender?
Dr. Brandt: First and foremost, understanding the terminology and the difference between gender identity, sex, and sexual orientation. Being familiar with that language and being able to speak that language very comfortably and not being awkward about it is a really important thing for primary care physicians and indeed any physician who treats transgender patients.
Physicians should also be aware that any underserved population has higher rates of mental health issues, such as depression and anxiety. Obviously, that goes along with being underserved and the stigma and the disparities that exist for these patients. Having providers educate themselves about what those disparities are and how they impact a patient’s daily life and health is paramount to knowing how to treat patients.
What are your top health concerns for these patients and how do you address them?
Dr. Brandt: I think mental health and safety is probably the number one for me. About 41% of transgender adults have attempted suicide. That number is roughly 51% in transgender youth. That is an astonishing number. These patients have much higher rates of domestic violence, intimate partner violence, and sexual assault, especially trans women and trans women of color. So understanding those statistics is huge.
Obesity, smoking, and substance abuse are my next three. Again, those are things that should be addressed at any visit, regardless of the gender identity or sexual orientation of the patient, but those rates are particularly high in this population.
Fertility and long-term care for patients should be addressed. Many patients who identify as transgender are told they can’t have a family. As a primary care physician, you may see a patient before they are seen by an ob.gyn. or surgeon. Talking about what a patient’s long-term life goals are with fertility and family planning, and what that looks like for them, is a big thing for me. Other providers may not feel that’s a concern, but I believe it should be discussed before initiation of hormone therapy, which can significantly impact fertility in some patients.
Are there nuances to the physical examination that primary care physicians should be aware of when dealing with transmasculine patients vs. transfeminine patients?
Dr. Brandt: Absolutely. And this interview can’t cover the scope of those nuances. An example that comes to mind is the genital exam. For transgender women who have undergone a vaginoplasty, the pelvic exam can be very affirming. Whereas for transgender men, a gynecologic exam can significantly exacerbate dysphoria and there are ways to conduct the exam to limit this discomfort and avoid creating a traumatic experience for the patient. It’s important to be aware that the genital exam, or any type of genitourinary exam, can be either affirming or not affirming.
Sexually transmitted infections are up in the general population, and the trans population is at even higher risk. What should physicians think about when they assess this risk?
Dr. Brandt: It’s really important for primary care clinicians and for gynecologists to learn to be comfortable talking about sexual practices, because what people do behind closed doors is really a key to how to counsel patients about safe sex.
People are well aware of the need to have safe sex. However, depending on the type of sex that you’re having, what body parts go where, what is truly safe can vary and people may not know, for example, to wear a condom when sex toys are involved or that a transgender male on testosterone can become pregnant during penile-vaginal intercourse. Providers really should be very educated on the array of sexual practices that people have and how to counsel them about those. They should know how to ask patients the gender identity of their sexual partners, the sexual orientation of their partners, and what parts go where during sex.
Providers should also talk to patients about PrEP [pre-exposure prophylaxis], whether they identify as cisgender or transgender. My trans patients tend to be a lot more educated about PrEP than other patients. It’s something that many of the residents, even in a standard gynecologic clinic, for example, don’t talk to cisgender patients about because of the stigma surrounding HIV. Many providers still think that the only people who are at risk for HIV are men who have sex with men. And while those rates are higher in some populations, depending on sexual practices, those aren’t the only patients who qualify for PrEP.
Overall, in order to counsel patients about STIs and safe sexual practices, providers should learn to be comfortable talking about sex.
Do you have any strategies on how to make the appointment more successful in addressing those issues?
Dr. Brandt: Bedside manner is a hard thing to teach, and comfort in talking about sex, gender identity, and sexual orientation can vary – but there are a lot of continuing medical education courses that physicians can utilize through the World Professional Association for Transgender Health.
If providers start to notice an influx of patients who identify as transgender or if they want to start seeing transgender patients, it’s really important for them to have that training before they start interacting with patients. In all of medicine, we sort of learn as we go, but this patient population has been subjected to discrimination, violence, error, and misgendering. They have dealt with providers who didn’t understand their health care needs. While this field is evolving, knowing how to appropriately address a patient (using their correct name, pronouns, etc.) is an absolute must.
That needs to be part of a provider’s routine vernacular and not something that they sort of stumble through. You can scare a patient away as soon as they walk into the office with an uneducated front desk staff and things that are seen in the office. Seeking out those educational tools, being aware of your own deficits as a provider and the educational needs of your office, and addressing those needs is really key.
A version of this article first appeared on Medscape.com.
People who identify as transgender experience many health disparities, in addition to lack of access to quality care. The most commonly cited barrier is the lack of providers who are knowledgeable about transgender health care, according to past surveys.
Even those who do seek care often have unpleasant experiences. A 2015 survey conducted by the National Center for Transgender Equality found that 33% of those who saw a health care provider reported at least one unfavorable experience related to being transgender, such as being verbally harassed or refused treatment because of their gender identity. In fact, 23% of those surveyed say they did not seek health care they needed in the past year because of fear of being mistreated as a transgender person.
This interview has been edited for length and clarity.
Question: Surveys have shown that many people who identify as transgender will seek only transition care, not primary or preventive care. Why is that?
Dr. Brandt: My answer is multifactorial. Transgender patients do seek primary care – just not as readily. There’s a lot of misconceptions about health care needs for the LGBT community in general. For example, lesbian or bisexual women may be not as well informed about the need for Pap smears compared with their heterosexual counterparts. These misconceptions are further exacerbated in the transgender community.
The fact that a lot of patients seek only transition-related care, but not preventive services, such as primary care and gynecologic care, is also related to fears of discrimination and lack of education of providers. These patients are afraid when they walk into an office that they will be misgendered or their physician won’t be familiar with their health care needs.
What can clinics and clinicians do to create a safe and welcoming environment?
Dr. Brandt: It starts with educating office staff about terminology and gender identities.
A key feature of our EHR is the sexual orientation and gender identity platform, which asks questions about a patient’s gender identity, sexual orientation, sex assigned at birth, and organ inventory. These data are then found in the patient information tab and are just as relevant as their insurance status, age, and date of birth.
There are many ways a doctor’s office can signal to patients that they are inclusive. They can hang LGBTQ-friendly flags or symbols or a sign saying, “We have an anti-discrimination policy” in the waiting room. A welcoming environment can also be achieved by revising patient questionnaires or forms so that they aren’t gender-specific or binary.
Given that the patient may have limited contact with a primary care clinician, how do you prioritize what you address during the visit?
Dr. Brandt: Similar to cisgender patients, it depends initially on the age of the patient and the reason for the visit. The priorities of an otherwise healthy transgender patient in their 20s are going to be largely the same as for a cisgender patient of the same age. As patients age in the primary care world, you’re addressing more issues, such as colorectal screening, lipid disorders, and mammograms, and that doesn’t change. For the most part, the problems that you address should be specific for that age group.
It becomes more complicated when you add in factors such as hormone therapy and whether patients have had any type of gender-affirming surgery. Those things can change the usual recommendations for screening or risk assessment. We try to figure out what routine health maintenance and cancer screening a patient needs based on age and risk factors, in addition to hormone status and surgical state.
Do you think that many physicians are educated about the care of underserved populations such as transgender patients?
Dr. Brandt: Yes and no. We are definitely getting better at it. For example, the American College of Obstetricians and Gynecologists published a committee opinion highlighting transgender care. So organizations are starting to prioritize these populations and recognize that they are, in fact, underserved and they have special health care needs.
However, the knowledge gaps are still pretty big. I get calls daily from providers asking questions about how to manage patients on hormones, or how to examine a patient who has undergone a vaginoplasty. I hear a lot of horror stories from transgender patients who had their hormones stopped for absurd and medically misinformed reasons.
But I definitely think it’s getting better and it’s being addressed at all levels – the medical school level, the residency level, and the attending level. It just takes time to inform people and for people to get used to the health care needs of these patients.
What should physicians keep in mind when treating patients who identify as transgender?
Dr. Brandt: First and foremost, understanding the terminology and the difference between gender identity, sex, and sexual orientation. Being familiar with that language and being able to speak that language very comfortably and not being awkward about it is a really important thing for primary care physicians and indeed any physician who treats transgender patients.
Physicians should also be aware that any underserved population has higher rates of mental health issues, such as depression and anxiety. Obviously, that goes along with being underserved and the stigma and the disparities that exist for these patients. Having providers educate themselves about what those disparities are and how they impact a patient’s daily life and health is paramount to knowing how to treat patients.
What are your top health concerns for these patients and how do you address them?
Dr. Brandt: I think mental health and safety is probably the number one for me. About 41% of transgender adults have attempted suicide. That number is roughly 51% in transgender youth. That is an astonishing number. These patients have much higher rates of domestic violence, intimate partner violence, and sexual assault, especially trans women and trans women of color. So understanding those statistics is huge.
Obesity, smoking, and substance abuse are my next three. Again, those are things that should be addressed at any visit, regardless of the gender identity or sexual orientation of the patient, but those rates are particularly high in this population.
Fertility and long-term care for patients should be addressed. Many patients who identify as transgender are told they can’t have a family. As a primary care physician, you may see a patient before they are seen by an ob.gyn. or surgeon. Talking about what a patient’s long-term life goals are with fertility and family planning, and what that looks like for them, is a big thing for me. Other providers may not feel that’s a concern, but I believe it should be discussed before initiation of hormone therapy, which can significantly impact fertility in some patients.
Are there nuances to the physical examination that primary care physicians should be aware of when dealing with transmasculine patients vs. transfeminine patients?
Dr. Brandt: Absolutely. And this interview can’t cover the scope of those nuances. An example that comes to mind is the genital exam. For transgender women who have undergone a vaginoplasty, the pelvic exam can be very affirming. Whereas for transgender men, a gynecologic exam can significantly exacerbate dysphoria and there are ways to conduct the exam to limit this discomfort and avoid creating a traumatic experience for the patient. It’s important to be aware that the genital exam, or any type of genitourinary exam, can be either affirming or not affirming.
Sexually transmitted infections are up in the general population, and the trans population is at even higher risk. What should physicians think about when they assess this risk?
Dr. Brandt: It’s really important for primary care clinicians and for gynecologists to learn to be comfortable talking about sexual practices, because what people do behind closed doors is really a key to how to counsel patients about safe sex.
People are well aware of the need to have safe sex. However, depending on the type of sex that you’re having, what body parts go where, what is truly safe can vary and people may not know, for example, to wear a condom when sex toys are involved or that a transgender male on testosterone can become pregnant during penile-vaginal intercourse. Providers really should be very educated on the array of sexual practices that people have and how to counsel them about those. They should know how to ask patients the gender identity of their sexual partners, the sexual orientation of their partners, and what parts go where during sex.
Providers should also talk to patients about PrEP [pre-exposure prophylaxis], whether they identify as cisgender or transgender. My trans patients tend to be a lot more educated about PrEP than other patients. It’s something that many of the residents, even in a standard gynecologic clinic, for example, don’t talk to cisgender patients about because of the stigma surrounding HIV. Many providers still think that the only people who are at risk for HIV are men who have sex with men. And while those rates are higher in some populations, depending on sexual practices, those aren’t the only patients who qualify for PrEP.
Overall, in order to counsel patients about STIs and safe sexual practices, providers should learn to be comfortable talking about sex.
Do you have any strategies on how to make the appointment more successful in addressing those issues?
Dr. Brandt: Bedside manner is a hard thing to teach, and comfort in talking about sex, gender identity, and sexual orientation can vary – but there are a lot of continuing medical education courses that physicians can utilize through the World Professional Association for Transgender Health.
If providers start to notice an influx of patients who identify as transgender or if they want to start seeing transgender patients, it’s really important for them to have that training before they start interacting with patients. In all of medicine, we sort of learn as we go, but this patient population has been subjected to discrimination, violence, error, and misgendering. They have dealt with providers who didn’t understand their health care needs. While this field is evolving, knowing how to appropriately address a patient (using their correct name, pronouns, etc.) is an absolute must.
That needs to be part of a provider’s routine vernacular and not something that they sort of stumble through. You can scare a patient away as soon as they walk into the office with an uneducated front desk staff and things that are seen in the office. Seeking out those educational tools, being aware of your own deficits as a provider and the educational needs of your office, and addressing those needs is really key.
A version of this article first appeared on Medscape.com.
People who identify as transgender experience many health disparities, in addition to lack of access to quality care. The most commonly cited barrier is the lack of providers who are knowledgeable about transgender health care, according to past surveys.
Even those who do seek care often have unpleasant experiences. A 2015 survey conducted by the National Center for Transgender Equality found that 33% of those who saw a health care provider reported at least one unfavorable experience related to being transgender, such as being verbally harassed or refused treatment because of their gender identity. In fact, 23% of those surveyed say they did not seek health care they needed in the past year because of fear of being mistreated as a transgender person.
This interview has been edited for length and clarity.
Question: Surveys have shown that many people who identify as transgender will seek only transition care, not primary or preventive care. Why is that?
Dr. Brandt: My answer is multifactorial. Transgender patients do seek primary care – just not as readily. There’s a lot of misconceptions about health care needs for the LGBT community in general. For example, lesbian or bisexual women may be not as well informed about the need for Pap smears compared with their heterosexual counterparts. These misconceptions are further exacerbated in the transgender community.
The fact that a lot of patients seek only transition-related care, but not preventive services, such as primary care and gynecologic care, is also related to fears of discrimination and lack of education of providers. These patients are afraid when they walk into an office that they will be misgendered or their physician won’t be familiar with their health care needs.
What can clinics and clinicians do to create a safe and welcoming environment?
Dr. Brandt: It starts with educating office staff about terminology and gender identities.
A key feature of our EHR is the sexual orientation and gender identity platform, which asks questions about a patient’s gender identity, sexual orientation, sex assigned at birth, and organ inventory. These data are then found in the patient information tab and are just as relevant as their insurance status, age, and date of birth.
There are many ways a doctor’s office can signal to patients that they are inclusive. They can hang LGBTQ-friendly flags or symbols or a sign saying, “We have an anti-discrimination policy” in the waiting room. A welcoming environment can also be achieved by revising patient questionnaires or forms so that they aren’t gender-specific or binary.
Given that the patient may have limited contact with a primary care clinician, how do you prioritize what you address during the visit?
Dr. Brandt: Similar to cisgender patients, it depends initially on the age of the patient and the reason for the visit. The priorities of an otherwise healthy transgender patient in their 20s are going to be largely the same as for a cisgender patient of the same age. As patients age in the primary care world, you’re addressing more issues, such as colorectal screening, lipid disorders, and mammograms, and that doesn’t change. For the most part, the problems that you address should be specific for that age group.
It becomes more complicated when you add in factors such as hormone therapy and whether patients have had any type of gender-affirming surgery. Those things can change the usual recommendations for screening or risk assessment. We try to figure out what routine health maintenance and cancer screening a patient needs based on age and risk factors, in addition to hormone status and surgical state.
Do you think that many physicians are educated about the care of underserved populations such as transgender patients?
Dr. Brandt: Yes and no. We are definitely getting better at it. For example, the American College of Obstetricians and Gynecologists published a committee opinion highlighting transgender care. So organizations are starting to prioritize these populations and recognize that they are, in fact, underserved and they have special health care needs.
However, the knowledge gaps are still pretty big. I get calls daily from providers asking questions about how to manage patients on hormones, or how to examine a patient who has undergone a vaginoplasty. I hear a lot of horror stories from transgender patients who had their hormones stopped for absurd and medically misinformed reasons.
But I definitely think it’s getting better and it’s being addressed at all levels – the medical school level, the residency level, and the attending level. It just takes time to inform people and for people to get used to the health care needs of these patients.
What should physicians keep in mind when treating patients who identify as transgender?
Dr. Brandt: First and foremost, understanding the terminology and the difference between gender identity, sex, and sexual orientation. Being familiar with that language and being able to speak that language very comfortably and not being awkward about it is a really important thing for primary care physicians and indeed any physician who treats transgender patients.
Physicians should also be aware that any underserved population has higher rates of mental health issues, such as depression and anxiety. Obviously, that goes along with being underserved and the stigma and the disparities that exist for these patients. Having providers educate themselves about what those disparities are and how they impact a patient’s daily life and health is paramount to knowing how to treat patients.
What are your top health concerns for these patients and how do you address them?
Dr. Brandt: I think mental health and safety is probably the number one for me. About 41% of transgender adults have attempted suicide. That number is roughly 51% in transgender youth. That is an astonishing number. These patients have much higher rates of domestic violence, intimate partner violence, and sexual assault, especially trans women and trans women of color. So understanding those statistics is huge.
Obesity, smoking, and substance abuse are my next three. Again, those are things that should be addressed at any visit, regardless of the gender identity or sexual orientation of the patient, but those rates are particularly high in this population.
Fertility and long-term care for patients should be addressed. Many patients who identify as transgender are told they can’t have a family. As a primary care physician, you may see a patient before they are seen by an ob.gyn. or surgeon. Talking about what a patient’s long-term life goals are with fertility and family planning, and what that looks like for them, is a big thing for me. Other providers may not feel that’s a concern, but I believe it should be discussed before initiation of hormone therapy, which can significantly impact fertility in some patients.
Are there nuances to the physical examination that primary care physicians should be aware of when dealing with transmasculine patients vs. transfeminine patients?
Dr. Brandt: Absolutely. And this interview can’t cover the scope of those nuances. An example that comes to mind is the genital exam. For transgender women who have undergone a vaginoplasty, the pelvic exam can be very affirming. Whereas for transgender men, a gynecologic exam can significantly exacerbate dysphoria and there are ways to conduct the exam to limit this discomfort and avoid creating a traumatic experience for the patient. It’s important to be aware that the genital exam, or any type of genitourinary exam, can be either affirming or not affirming.
Sexually transmitted infections are up in the general population, and the trans population is at even higher risk. What should physicians think about when they assess this risk?
Dr. Brandt: It’s really important for primary care clinicians and for gynecologists to learn to be comfortable talking about sexual practices, because what people do behind closed doors is really a key to how to counsel patients about safe sex.
People are well aware of the need to have safe sex. However, depending on the type of sex that you’re having, what body parts go where, what is truly safe can vary and people may not know, for example, to wear a condom when sex toys are involved or that a transgender male on testosterone can become pregnant during penile-vaginal intercourse. Providers really should be very educated on the array of sexual practices that people have and how to counsel them about those. They should know how to ask patients the gender identity of their sexual partners, the sexual orientation of their partners, and what parts go where during sex.
Providers should also talk to patients about PrEP [pre-exposure prophylaxis], whether they identify as cisgender or transgender. My trans patients tend to be a lot more educated about PrEP than other patients. It’s something that many of the residents, even in a standard gynecologic clinic, for example, don’t talk to cisgender patients about because of the stigma surrounding HIV. Many providers still think that the only people who are at risk for HIV are men who have sex with men. And while those rates are higher in some populations, depending on sexual practices, those aren’t the only patients who qualify for PrEP.
Overall, in order to counsel patients about STIs and safe sexual practices, providers should learn to be comfortable talking about sex.
Do you have any strategies on how to make the appointment more successful in addressing those issues?
Dr. Brandt: Bedside manner is a hard thing to teach, and comfort in talking about sex, gender identity, and sexual orientation can vary – but there are a lot of continuing medical education courses that physicians can utilize through the World Professional Association for Transgender Health.
If providers start to notice an influx of patients who identify as transgender or if they want to start seeing transgender patients, it’s really important for them to have that training before they start interacting with patients. In all of medicine, we sort of learn as we go, but this patient population has been subjected to discrimination, violence, error, and misgendering. They have dealt with providers who didn’t understand their health care needs. While this field is evolving, knowing how to appropriately address a patient (using their correct name, pronouns, etc.) is an absolute must.
That needs to be part of a provider’s routine vernacular and not something that they sort of stumble through. You can scare a patient away as soon as they walk into the office with an uneducated front desk staff and things that are seen in the office. Seeking out those educational tools, being aware of your own deficits as a provider and the educational needs of your office, and addressing those needs is really key.
A version of this article first appeared on Medscape.com.
Antimicrobial, pH-modulating gel shows promise in preventing common STIs
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
An investigational vaginal gel significantly reduced urogenital chlamydia and gonorrhea in women at high risk for infection, compared with placebo, opening up new possibilities for an on-demand prevention option. Investigators of a randomized trial reported these findings in the American Journal of Obstetrics and Gynecology.
Rates of Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (GC) are on the rise in the United States, despite wide availability of male and female condoms to prevent sexually transmitted infections. This suggests that women need a more discrete method that they can better control. Other vaginal microbicides developed over the last few decades haven’t performed well in protecting against STIs or HIV in clinical trials.
The slightly alkaline nature of human semen has the potential to neutralize vaginal pH after intercourse, creating a more vulnerable environment for STIs. EVO100 is an investigational antimicrobial, bioadhesive vaginal gel that contains L-lactic acid, citric acid, and potassium bitartrate. In preclinical studies, it was highly effective at buffering the alkaline properties of human semen and maintaining vaginal pH levels. Patients generally tolerated it well, aside from some reports of vaginal itching and burning.
In the AMPREVENCE study, a double-blinded, placebo-controlled, randomized, phase 2b/3 trial, Todd Chappell, MD, of Adams Patterson Gynecology & Obstetrics, Memphis, and colleagues tested the efficacy and safety of EVO100 to prevent chlamydia and gonorrhea.
Investigators randomized 1:1,860 healthy, sexually active women to receive either EVO100 (n = 426) or placebo (n = 434). Participants had either been diagnosed or treated for these STIs up to 16 weeks prior to enrollment. Among those enrolled, 335 women in the EVO100 arm and 335 women in the placebo arm completed the study.
From this cohort, 764 women (EVO100: n = 376; placebo: n = 388) reported any use of either product. These women represented the “safety analysis population,” a predefined population for statistical analysis.
Participants averaged nearly 28 years of age, had a median body mass index of 28.9 kg/m2, and represented several racial/ethnic groups: White (54.3% [467/860]), African American (41.6% [358/860]), and non-Hispanic/Latinx ethnicity (67.1% [577/860]).
The women were instructed to apply the drug within 1 hour of initiating sexual intercourse. Investigators scheduled follow-up visits every 4 weeks during the 16-week study period, to obtain repeat CT/GC assessments, review diary entries, and to collect information about adverse effects and use of concomitant medications. During enrollment, participants consented to return to the clinic at each study visit. If a woman missed a visit, the study site would follow-up by telephone after the missed assessment visit.
Participants reported a mean number of 16 coital events (EVO100, 15.7 [13.5]; placebo, 16.3 [15.8]). EVO100 significantly reduced STI incidence for both types of STIs. CT infection rates among EVO100 users was 4.8% (14/289), half of what it was in placebo users (9.7% [28/290]) (P = .0256). The investigational method was even more successful in GC-analysis–eligible women: infection rates averaged 0.7% (2/280), compared with 3.2% (9/277) in the placebo group, a relative risk reduction of 78% (P = .0316).
Examining electronic diary entries of the participants, investigators reported similar adherence rates among the two treatment arms. However, additional sensitivity analyses in CT-eligible and GC-eligible populations on adherence yielded notably different results.
EVO100 users in the CT population who used the product as directed 100% of the time were significantly less likely to become infected, compared with the placebo group (2.3% vs. 16.9%, P = .0012). However, investigators found no significant differences in infection rates among women with poorer adherence rates in the two groups. Comparatively, they found no major differences in GC infection rates between the control and EVO100 groups, regardless of adherence rates, likely because of the small number of GC infections reported. Observed adverse events correlated with the drug’s known safety profile.
Most of the participants said they would likely recommend EVO100 to other women and continue using this preventive treatment.
A small GC subgroup caused by fewer infection cases and reliance on participant self-reporting of coital incidents may have limited the study’s results. “While use of the electronic diaries is helpful for collection of study data, it may encourage compliance and efficacy that may be higher in the ‘real-world’ population outside of the setting of a clinical trial,” noted Dr. Chappell and colleagues.
According to the investigators, this is the first prospective, randomized trial to study the use of an antimicrobial bioadhesive vaginal gel for preventing CT and GC infection. “EVO100 has the potential of fulfilling an unmet need in women’s sexual health as a new on-demand, woman-controlled option that reduces the risk of urogenital CT and GC infections,” the authors concluded.
The Food and Drug Administration has already approved EVO100 as a contraceptive option (Phexxi), Dr. Chappell said in an interview. Next steps are to conduct a phase 3 trial, which is currently underway. “If the findings are positive, we will submit to the FDA for review and approval of EVO100” for preventing these STIs.
These are promising results, Catherine Cansino, MD, MPH, an associate clinical professor with the department of obstetrics and gynecology at the University of California, Davis, said in an interview. It’s always helpful to look at effective treatments, “especially those that aren’t traditional antibiotics in order to decrease the risk of antibiotic resistance,” said Dr. Cansino, who was not part of the study. This is why EVO100 is such an attractive option.
Future studies should look at a broader population, she continued. “The population this study looked at is not the general population – these women had an infection at some point, previously,” which means they are potentially at higher risk for reinfection. “Looking at what their likelihood is of getting infected again, it’s hard to know if this would be the same or different from the general population.” If the drug appears to cause a decrease in new infections, the relative risk reduction is actually greater than what’s reported. If the reinfection rate for this population is lower because people who’ve had infections are practicing safer sex, the relative risk reduction would be lower, explained Dr. Cansino.
Dr. Chappell and several coauthors received research funding from Evofem Biosciences.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
The pandemic is making periods unbearable for some women
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Stories of how the pandemic has disrupted women’s periods reverberated across the Internet. Here’s what docs can do to help.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.
Following a recent article in the Guardian, the Internet has erupted with tales of periods gone awry. The stress and loss of normalcy over the last year appears to have altered cycles and amplified the premenstrual syndrome (PMS) symptoms many women experience. And after the piece published, many responded on social media with the same sentiment: “So, it’s not just me?”
Women have experienced the loss of their period, excessive and prolonged bleeding, severe mood swings, and irritability, according to the Guardian article. London-based gynecologist Anita Mitra, MBChB, PhD, took an informal survey and found that 65% of 5,677 respondents had noticed a change in their menstrual cycle, the Guardian reported. Another survey, which was posted on medRxiv but hasn’t been peer reviewed yet, found 53% of the 749 respondents had noticed a change in their menstrual cycle, including increased cycle length.
“The pandemic in itself has made more stress for women,” said Karen Carlson, MD, obstetrician and gynecologist at Nebraska Medicine. There’s preliminary evidence that the cycling progesterone and estrogen experienced by reproductive age women actually offers a protective effect against COVID-19, which is good news. But Dr. Carlson said that because they are less likely than men and the elderly to become seriously ill, many women have taken on a lot of the additional responsibilities brought on by the pandemic. They often juggle homeschooling and elder care in addition to the ubiquitous stressors of isolation and concerns around personal health.
“Abnormal bleeding is the most common reason people present to the gynecologist,” Dr. Carlson said in an interview. But in recent months, Dr. Carlson said she’s seen a slight uptick in these issues, and there might have been even more women presenting to their physicians if the pandemic hadn’t also suppressed access to care.
Stress, or rather the cortisol it causes the body to produce, is the culprit for disrupted cycles. It can suppress pituitary hormones that stimulate ovulation. “Some women don’t feel right because they are stuck in the one phase of the cycle,” Dr. Carlson said. They may go months without a period and when they do eventually shed their uterine lining the bleeding goes on for a while.
Some irregularity in a person’s cycle is a normal response to stress and even likely, given the last year. However, bleeding for more than 2 weeks or irregularity for more than 3 months could point to something more serious like an infection or cancer, Dr. Carlson said. Getting a clear history so you know when you need to do blood and hormone workups is critical.
Anxiety and depression amplified
For some women it’s not bleeding that’s a problem, rather their PMS has become crippling. And some of their significant others have noticed drastic changes in their mood. In the Guardian article, one woman said she’d gone from feeling withdrawn during her period to being totally unreachable and experiencing intense anxiety.
Maureen Whelihan, MD, a gynecologist in Palm Beach, Fla., said that, for the majority of her patients under 39 years of age, these feelings aren’t a hormone issue, but a stress and neuroreceptor issue. She says she’s seen approximately a 30% increase in mood disorders since the start of the pandemic. Even though many of her patients are cycling relatively normally, their anxiety and depression have been amplified.
Caroline Gurvich, PhD, a neuroscientist at Monash University in Melbourne, attributes this to the loss of typical coping mechanisms. “Having changes to the support system and routine and things that would keep them mentally healthy can exacerbate PMS,” she said in an interview. Dr. Gurvich’s advice is to build routines into the pandemic lifestyle. Normal wake and sleep times, healthy eating, and practices that bring happiness can be “crucial to keeping those PMS systems as controlled as possible.”
Telehealth has made it much easier to access some patients struggling with PMS and offer them the medication or counseling they need, Dr. Carlson said. But that approach doesn’t work for everyone. “I feel like there are a lot of silent sufferers,” she said.
This is where screening practices like the Patient Health Questionnaire-9 are so critical, according to Dr. Whelihan, who screens every patient as part of their routine iPad check-in process. Even in a normal year, “I think one-third of gynecology is psychiatry,” she said in an interview. She finds many of the patients struggling with excessive PMS symptoms, both during the pandemic and before, benefit from a child-sized dose of antidepressant. This may allow them to get to a place where they can make impactful routine decisions about exercise or sleep, and then taper off the antidepressant.
It may also be important for clinicians to help patients make the initial connection between their worsening mood or cognitive function and their period. Knowing their feelings of stress, irritability, fogginess, or being withdrawn are linked to their hormone cycle and possibly worsened by the stress of the pandemic can be helpful, Dr. Gurvich said. “If they become conscious of how they are feeling it can be helpful for management of these stressful symptoms,” she said.





