User login
Adverse pregnancy outcomes and later cardiovascular disease
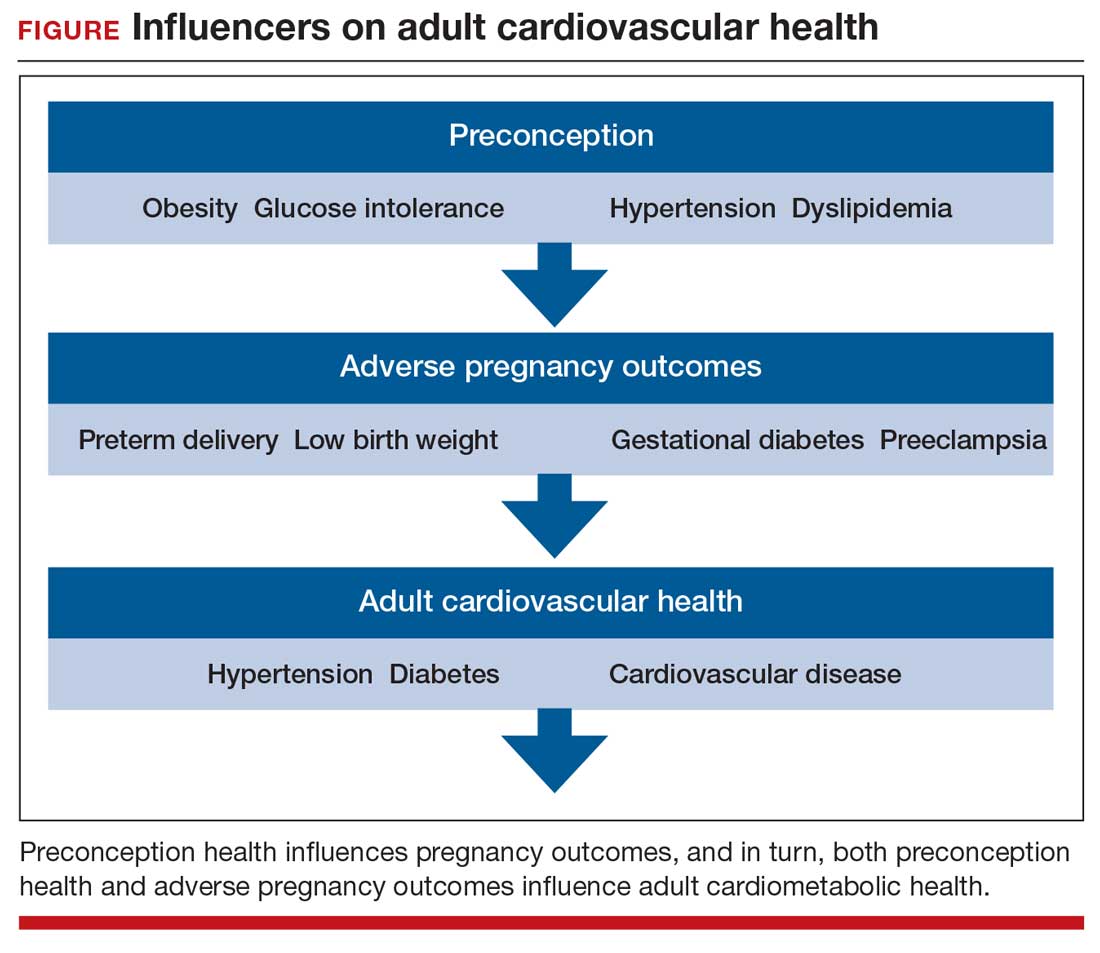
Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.
Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.

Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.

Preconception health influences pregnancy outcomes, and in turn, both preconception health and an APO influence adult cardiometabolic health (FIGURE). This editorial is focused on the link between APOs and later cardiometabolic morbidity and mortality, recognizing that preconception health greatly influences the risk of an APO and lifetime cardiometabolic disease.

Adverse pregnancy outcomes
Major APOs include miscarriage, preterm birth (birth <37 weeks’ gestation), low birth weight (birth weight ≤2,500 g; 5.5 lb), gestational diabetes (GDM), preeclampsia, and placental abruption. In the United States, among all births, reported rates of the following APOs are:1-3
- preterm birth, 10.2%
- low birth weight, 8.3%
- GDM, 6%
- preeclampsia, 5%
- placental abruption, 1%.
Miscarriage occurs in approximately 10% to 15% of pregnancies, influenced by both the age of the woman and the method used to diagnose pregnancy.4 Miscarriage, preterm birth, low birth weight, GDM, preeclampsia, and placental abruption have been reported to be associated with an increased risk of later cardiovascular morbidity and mortality.
APOs and cardiovascular disease
Cardiovascular disease (CVD) affects the majority of people past the age of 60 years and includes 4 major subcategories:
- coronary heart disease, including myocardial infarction, angina, and heart failure
- CVD, stroke, and transient ischemic attack
- peripheral artery disease
- atherosclerosis of the aorta leading to aortic aneurysm.
Multiple meta-analyses report that APOs are associated with CVD in later life. A comprehensive review reported that the risk of CVD was increased following a pregnancy with one of these APOs: severe preeclampsia (odds ratio [OR], 2.74), GDM (OR, 1.68), preterm birth (OR, 1.93), low birth weight (OR, 1.29), and placental abruption (OR, 1.82).5
The link between APOs and CVD may be explained in part by the association of APOs with multiple risk factors for CVD, including chronic hypertension, type 2 diabetes mellitus (T2DM), and dyslipidemia. A meta-analysis of 43 studies reported that, compared with controls, women with a history of preeclampsia have a 3.13 times greater risk of developing chronic hypertension.6 Among women with preeclampsia, approximately 20% will develop hypertension within 15 years.7 A meta-analysis of 20 studies reported that women with a history of GDM had a 9.51-times greater risk of developing T2DM than women without GDM.8 Among women with a history of GDM, over 16 years of follow-up, T2DM was diagnosed in 16.2%, compared with 1.9% of control women.8
CVD prevention—Breastfeeding: An antidote for APOs
Pregnancy stresses both the cardiovascular and metabolic systems. Breastfeeding is an antidote to the stresses imposed by pregnancy. Breastfeeding women have lower blood glucose9 and blood pressure.10
Breastfeeding reduces the risk of CVD. In a study of 100,864 parous Australian women, with a mean age of 60 years, ever breastfeeding was associated a lower risk of CVD hospitalization (adjusted hazard ratio [aHR], 0.86; 95% confidence interval [CI], 0.78–0.96; P = .005) and CVD mortality (aHR, 0.66; 95% CI, 0.49–0.89; P = .006).11
Continue to: CVD prevention—American Heart Association recommendations...
CVD prevention—American Heart Association recommendations
The American Heart Association12 recommends lifestyle interventions to reduce the risk of CVD, including:
- Eat a high-quality diet that includes vegetables, fruit, whole grains, beans, legumes, nuts, plant-based protein, lean animal protein, and fish.
- Limit intake of sugary drinks and foods, fatty or processed meats, full-fat dairy products, eggs, highly processed foods, and tropical oils.
- Exercise at least 150 minutes weekly at a moderate activity level, including muscle-strengthening activity.
- Reduce prolonged intervals of sitting.
- Live a tobacco- and nicotine-free life.
- Strive to maintain a normal body mass index.
- Consider using an activity tracker to monitor activity level.
- After 40 years of age calculate CVD risk using a validated calculator such as the American Cardiology Association risk calculator.13 This calculator uses age, gender, and lipid and blood pressure measurements to calculate the 10-year risk of atherosclerotic CVD, including coronary death, myocardial infarction, and stroke.
Medications to reduce CVD risk
Historically, ObGyns have not routinely prescribed medications to treat hypertension, dyslipidemia, or to prevent diabetes. The recent increase in the valuation of return ambulatory visits and a reduction in the valuation assigned to procedural care may provide ObGyn practices the additional resources needed to manage some chronic diseases. Physician assistants and nurse practitioners may help ObGyn practices to manage hypertension, dyslipidemia, and prediabetes.
Prior to initiating a medicine, counseling about healthy living, including smoking cessation, exercise, heart-healthy diet, and achieving an optimal body mass index is warranted.
For treatment of stage II hypertension, defined as blood pressure (BP) measurements with systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg, therapeutic lifestyle interventions include: optimizing weight, following the DASH diet, restricting dietary sodium, physical activity, and reducing alcohol consumption. Medication treatment for essential hypertension is guided by the magnitude of BP reduction needed to achieve normotension. For women with hypertension needing antihypertensive medication and planning another pregnancy in the near future, labetalol or extended-release nifedipine may be first-line medications. For women who have completed their families or who have no immediate plans for pregnancy, an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, or thiazide diuretic are commonly prescribed.14
For the treatment of elevated low-density lipoprotein (LDL) cholesterol in women who have not had a cardiovascular event, statin therapy is often warranted when both the LDL cholesterol is >100 mg/dL and the woman has a calculated 10-year risk of >10% for a cardiovascular event using the American Heart Association or American College of Cardiology calculator. Most women who meet these criteria will be older than age 40 years and many will be under the care of an internal medicine or family medicine specialist, limiting the role of the ObGyn.15-17
For prevention of diabetes in women with a history of GDM, both weight loss and metformin (1,750 mg daily) have been shown in clinical trials to reduce the risk of developing T2DM.18 Among 350 women with a history of GDM who were followed for 10 years, metformin 850 mg twice daily reduced the risk of developing T2DM by 40% compared with placebo.19 In the same study, lifestyle changes without metformin, including loss of 7% of body weight plus 150 minutes of exercise weekly was associated with a 35% reduction in the risk of developing T2DM.19 Metformin is one of the least expensive prescription medications and is cost-effective for the prevention of T2DM.18
Low-dose aspirin treatment for the prevention of CVD in women who have not had a cardiovascular event must balance a modest reduction in cardiovascular events with a small increased risk of bleeding events. The US Preventive Services Task Force (USPSTF) recommends low-dose aspirin for a limited group of women, those aged 50 to 59 years of age with a 10-year risk of a cardiovascular event >10% who are willing to take aspirin for 10 years. The USPSTF concluded that there is insufficient evidence to recommend low-dose aspirin prevention of CVD in women aged <50 years.20
Continue to: Beyond the fourth trimester...
Beyond the fourth trimester
The fourth trimester is the 12-week period following birth. At the comprehensive postpartum visit, the American College of Obstetricians and Gynecologists (ACOG) recommends that women with APOs be counseled about their increased lifetime risk of maternal cardiometabolic disease.21 In addition, ACOG recommends that at this visit the clinician who will assume primary responsibility for the woman’s ongoing medical care in her primary medical home be clarified. One option is to ensure a high-quality hand-off to an internal medicine or family medicine clinician. Another option is for a clinician in the ObGyn’s office practice, including a physician assistant, nurse practitioner, or office-based ObGyn, to assume some role in the primary care of the woman.
An APO is not only a pregnancy problem
An APO reverberates across a woman’s lifetime, increasing the risk of CVD and diabetes. In the United States the mean age at first birth is 27 years.1 The mean life expectancy of US women is 81 years.22 Following a birth complicated by an APO there are 5 decades of opportunity to improve health through lifestyle changes and medication treatment of obesity, hypertension, dyslipidemia, and hyperglycemia, thereby reducing the risk of CVD.

- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2019. Natl Vital Stat Rep. 2021;70:1-51.
- Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth—United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67:1201-1207. doi: 10.15585/mmwr.mm6743a2.
- Fingar KR, Mabry-Hernandez I, Ngo-Metzger Q, et al. Delivery hospitalizations involving preeclampsia and eclampsia, 2005–2014. Statistical brief #222. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Agency for Healthcare Research and Quality: Rockville, MD; April 2017.
- Magnus MC, Wilcox AJ, Morken NH, et al. Role of maternal age and pregnancy history in risk of miscarriage: prospective register-based study. BMJ. 2019;364:869.
- Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143:e902-e916. doi: 10.1161 /CIR.0000000000000961.
- Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1-19. doi: 10.1007/s10654-013- 9762-6.
- Groenfol TK, Zoet GA, Franx A, et al; on behalf of the PREVENT Group. Trajectory of cardiovascular risk factors after hypertensive disorders of pregnancy. Hypertension. 2019;73:171-178. doi: 10.1161/HYPERTENSIONAHA.118.11726.
- Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361.
- Tarrant M, Chooniedass R, Fan HSL, et al. Breastfeeding and postpartum glucose regulation among women with prior gestational diabetes: a systematic review. J Hum Lact. 2020;36:723-738. doi: 10.1177/0890334420950259.
- Park S, Choi NK. Breastfeeding and maternal hypertension. Am J Hypertens. 2018;31:615-621. doi: 10.1093/ajh/hpx219.
- Nguyen B, Gale J, Nassar N, et al. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian cohort study. J Am Heart Assoc. 2019;8:e011056. doi: 10.1161/JAHA.118.011056.
- Eight things you can do to prevent heart disease and stroke. American Heart Association website. https://www.heart.org/en/healthy-living /healthy-lifestyle/prevent-heart-disease-andstroke. Last Reviewed March 14, 2019. Accessed May 19, 2021.
- ASCVD risk estimator plus. American College of Cardiology website. https://tools.acc.org /ascvd-risk-estimator-plus/#!/calculate /estimate/. Accessed May 19, 2021.
- Ferdinand KC, Nasser SA. Management of essential hypertension. Cardiol Clin. 2017;35:231-246. doi: 10.1016/j.ccl.2016.12.005.
- Packard CJ. LDL cholesterol: how low to go? Trends Cardiovasc Med. 2018;28:348-354. doi: 10.1016/j.tcm.2017.12.011.
- Simons L. An updated review of lipid-modifying therapy. Med J Aust. 2019;211:87-92. doi: 10.5694 /mja2.50142.
- Chou R, Dana T, Blazina I, et al. Statins for the prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008. doi: 10.1001/jama.2015.15629.
- Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for type 2 diabetes mellitus prevention. Am J Prev Med. 2018;55:565-574. doi: 10.1016/j.amepre.2018.04.038.
- Aorda VR, Christophi CA, Edelstein SL, et al, for the Diabetes Prevention Program Research Group. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646- 1653. doi: 10.1210/jc.2014-3761.
- Bibbins-Domingo K, U.S. Preventive Services Task Force. Aspirin use of the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Int Med. 2016; 164: 836-845. doi: 10.7326/M16-0577.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi: 10.1097 /AOG.0000000000002633.
- National Center for Health Statistics. Health, United States, 2017: Table 015. Hyattsville, MD; 2021. https://www.cdc.gov/nchs/data /hus/2017/015.pdf. Accessed May 18, 2021.
Pilot study: Hybrid laser found effective for treating genitourinary syndrome of menopause
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
FROM ASLMS 2021
Air pollution linked to increased fibroid risk in Black women
Black women exposed to ozone air pollution have an increased risk of developing fibroids, according to new research published in Human Production.
Uterine fibroids are a common type of pelvic growth, affecting up to 80% of women by the time they reach age 50, according to the U.S. Department of Health and Human Services. Black women are hit hardest by fibroids; they are diagnosed two to three times the rate of White women and tend to have more severe symptoms.
Researchers are unclear on why exposure to ozone air pollution increases the risk of developing fibroids. However, they believe that when it comes to identifying causes of fibroids and explanations for racial disparities in fibroids, more research that focuses on environmental and neighborhood-level risk factors could help inform policy and interventions to improve gynecologic health.
“A large body of literature from the environmental justice field has documented that people of color, and Black people specifically, are inequitably exposed to air pollution,” study author Amelia K. Wesselink, PhD, assistant professor at Boston University School of Public Health, said in an interview. “And there is growing evidence that air pollution can influence gynecologic health and therefore may contribute to racial disparities in gynecologic outcomes.”
Dr. Wesselink and colleagues wanted to know the extent to which three air pollutants – particulate matter (PM2.5), nitrogen dioxide (NO2) and ozone (03) – were linked to the development of fibroids. To figure this out, they analyzed data on nearly 22,000 premenopausal Black women who lived in 56 metropolitan areas in the United States between 2007 and 2011. They assigned air pollution exposures to participants’ residential addresses collected at baseline and over follow-up and tried to capture long-term exposure to air pollutants.
During the study, nearly 30% of participants reported that they were diagnosed with fibroids. Researchers observed that the exposure to PM2.5 and NO2 was not associated with an increased risk of developing these fibroids.
Dr. Wesselink said the findings may have underestimated fibroid incidence, so they “need to be replicated in a prospective, ultrasound-based study that can identify all fibroid cases.”
“There has not been a lot of research on how air pollution influences fibroid risk, but the two studies that are out there show some evidence of an association,” said Dr. Wesselink. “The fact that our results were consistent with this is interesting. The surprising part of our findings was that we observed an association for ozone, but not for PM2.5 or NO2.”
Nathaniel DeNicola, MD, MSHP, FACOG, a Washington-based obstetrics and gynecology physician affiliated with John Hopkins Health System, applauded the methodology of the study and said the findings prove that patients and doctors should be talking about the environment and exposures to air pollutants.
“[Air pollution] has numerous components to it. And we should try to figure out exactly what components are most dangerous to human health and what doses and what times of life,” said Dr. DeNicola, an environmental health expert.
The increased risk of developing fibroids is a “historical observation” and air pollution may be part of a multifactorial cause of that, Dr. DeNicola said. He said he wouldn’t be surprised if future studies show that “higher exposure [to air pollution] – due to how city planning works, often communities of color are in the areas with the most dense air pollution – exacerbates some other mechanism already in place.
Although it’s unclear how ozone exposure increases fibroid risk, Dr. Wesselink said it may be through a mechanism that is unique to ozone.
“In other words, it might be that there is a factor related to ozone that we did not account for that explains our findings. Vitamin D is a factor that we were not able to account for in this study,” Dr. Wesselink said. “Future work on this topic should consider the role of vitamin D [exposure or deficiency].”
Dr. DeNicola said ozone’s impact may also be tied to its “known association” with hypertension. A 2017 study by Drew B. Day, PhD, of Duke University, Durham, N.C., and colleagues, found that ozone exposure has been linked to hypertension. Meanwhile, a 2015 study has found an association between hypertension and fibroids.
“[This study] does raise an important message. It shines a light where more research needs to be done,” Dr. DeNicola said. “The ozone connection to hypertension was probably most compelling as a true risk factor for uterine fibroids.”
Dr. Wesselink said future work on fibroid etiology should focus on environmental and neighborhood-level exposures to pollutants.
Black women exposed to ozone air pollution have an increased risk of developing fibroids, according to new research published in Human Production.
Uterine fibroids are a common type of pelvic growth, affecting up to 80% of women by the time they reach age 50, according to the U.S. Department of Health and Human Services. Black women are hit hardest by fibroids; they are diagnosed two to three times the rate of White women and tend to have more severe symptoms.
Researchers are unclear on why exposure to ozone air pollution increases the risk of developing fibroids. However, they believe that when it comes to identifying causes of fibroids and explanations for racial disparities in fibroids, more research that focuses on environmental and neighborhood-level risk factors could help inform policy and interventions to improve gynecologic health.
“A large body of literature from the environmental justice field has documented that people of color, and Black people specifically, are inequitably exposed to air pollution,” study author Amelia K. Wesselink, PhD, assistant professor at Boston University School of Public Health, said in an interview. “And there is growing evidence that air pollution can influence gynecologic health and therefore may contribute to racial disparities in gynecologic outcomes.”
Dr. Wesselink and colleagues wanted to know the extent to which three air pollutants – particulate matter (PM2.5), nitrogen dioxide (NO2) and ozone (03) – were linked to the development of fibroids. To figure this out, they analyzed data on nearly 22,000 premenopausal Black women who lived in 56 metropolitan areas in the United States between 2007 and 2011. They assigned air pollution exposures to participants’ residential addresses collected at baseline and over follow-up and tried to capture long-term exposure to air pollutants.
During the study, nearly 30% of participants reported that they were diagnosed with fibroids. Researchers observed that the exposure to PM2.5 and NO2 was not associated with an increased risk of developing these fibroids.
Dr. Wesselink said the findings may have underestimated fibroid incidence, so they “need to be replicated in a prospective, ultrasound-based study that can identify all fibroid cases.”
“There has not been a lot of research on how air pollution influences fibroid risk, but the two studies that are out there show some evidence of an association,” said Dr. Wesselink. “The fact that our results were consistent with this is interesting. The surprising part of our findings was that we observed an association for ozone, but not for PM2.5 or NO2.”
Nathaniel DeNicola, MD, MSHP, FACOG, a Washington-based obstetrics and gynecology physician affiliated with John Hopkins Health System, applauded the methodology of the study and said the findings prove that patients and doctors should be talking about the environment and exposures to air pollutants.
“[Air pollution] has numerous components to it. And we should try to figure out exactly what components are most dangerous to human health and what doses and what times of life,” said Dr. DeNicola, an environmental health expert.
The increased risk of developing fibroids is a “historical observation” and air pollution may be part of a multifactorial cause of that, Dr. DeNicola said. He said he wouldn’t be surprised if future studies show that “higher exposure [to air pollution] – due to how city planning works, often communities of color are in the areas with the most dense air pollution – exacerbates some other mechanism already in place.
Although it’s unclear how ozone exposure increases fibroid risk, Dr. Wesselink said it may be through a mechanism that is unique to ozone.
“In other words, it might be that there is a factor related to ozone that we did not account for that explains our findings. Vitamin D is a factor that we were not able to account for in this study,” Dr. Wesselink said. “Future work on this topic should consider the role of vitamin D [exposure or deficiency].”
Dr. DeNicola said ozone’s impact may also be tied to its “known association” with hypertension. A 2017 study by Drew B. Day, PhD, of Duke University, Durham, N.C., and colleagues, found that ozone exposure has been linked to hypertension. Meanwhile, a 2015 study has found an association between hypertension and fibroids.
“[This study] does raise an important message. It shines a light where more research needs to be done,” Dr. DeNicola said. “The ozone connection to hypertension was probably most compelling as a true risk factor for uterine fibroids.”
Dr. Wesselink said future work on fibroid etiology should focus on environmental and neighborhood-level exposures to pollutants.
Black women exposed to ozone air pollution have an increased risk of developing fibroids, according to new research published in Human Production.
Uterine fibroids are a common type of pelvic growth, affecting up to 80% of women by the time they reach age 50, according to the U.S. Department of Health and Human Services. Black women are hit hardest by fibroids; they are diagnosed two to three times the rate of White women and tend to have more severe symptoms.
Researchers are unclear on why exposure to ozone air pollution increases the risk of developing fibroids. However, they believe that when it comes to identifying causes of fibroids and explanations for racial disparities in fibroids, more research that focuses on environmental and neighborhood-level risk factors could help inform policy and interventions to improve gynecologic health.
“A large body of literature from the environmental justice field has documented that people of color, and Black people specifically, are inequitably exposed to air pollution,” study author Amelia K. Wesselink, PhD, assistant professor at Boston University School of Public Health, said in an interview. “And there is growing evidence that air pollution can influence gynecologic health and therefore may contribute to racial disparities in gynecologic outcomes.”
Dr. Wesselink and colleagues wanted to know the extent to which three air pollutants – particulate matter (PM2.5), nitrogen dioxide (NO2) and ozone (03) – were linked to the development of fibroids. To figure this out, they analyzed data on nearly 22,000 premenopausal Black women who lived in 56 metropolitan areas in the United States between 2007 and 2011. They assigned air pollution exposures to participants’ residential addresses collected at baseline and over follow-up and tried to capture long-term exposure to air pollutants.
During the study, nearly 30% of participants reported that they were diagnosed with fibroids. Researchers observed that the exposure to PM2.5 and NO2 was not associated with an increased risk of developing these fibroids.
Dr. Wesselink said the findings may have underestimated fibroid incidence, so they “need to be replicated in a prospective, ultrasound-based study that can identify all fibroid cases.”
“There has not been a lot of research on how air pollution influences fibroid risk, but the two studies that are out there show some evidence of an association,” said Dr. Wesselink. “The fact that our results were consistent with this is interesting. The surprising part of our findings was that we observed an association for ozone, but not for PM2.5 or NO2.”
Nathaniel DeNicola, MD, MSHP, FACOG, a Washington-based obstetrics and gynecology physician affiliated with John Hopkins Health System, applauded the methodology of the study and said the findings prove that patients and doctors should be talking about the environment and exposures to air pollutants.
“[Air pollution] has numerous components to it. And we should try to figure out exactly what components are most dangerous to human health and what doses and what times of life,” said Dr. DeNicola, an environmental health expert.
The increased risk of developing fibroids is a “historical observation” and air pollution may be part of a multifactorial cause of that, Dr. DeNicola said. He said he wouldn’t be surprised if future studies show that “higher exposure [to air pollution] – due to how city planning works, often communities of color are in the areas with the most dense air pollution – exacerbates some other mechanism already in place.
Although it’s unclear how ozone exposure increases fibroid risk, Dr. Wesselink said it may be through a mechanism that is unique to ozone.
“In other words, it might be that there is a factor related to ozone that we did not account for that explains our findings. Vitamin D is a factor that we were not able to account for in this study,” Dr. Wesselink said. “Future work on this topic should consider the role of vitamin D [exposure or deficiency].”
Dr. DeNicola said ozone’s impact may also be tied to its “known association” with hypertension. A 2017 study by Drew B. Day, PhD, of Duke University, Durham, N.C., and colleagues, found that ozone exposure has been linked to hypertension. Meanwhile, a 2015 study has found an association between hypertension and fibroids.
“[This study] does raise an important message. It shines a light where more research needs to be done,” Dr. DeNicola said. “The ozone connection to hypertension was probably most compelling as a true risk factor for uterine fibroids.”
Dr. Wesselink said future work on fibroid etiology should focus on environmental and neighborhood-level exposures to pollutants.
FDA approves ibrexafungerp for vaginal yeast infection
Ibrexafungerp is the first drug approved in a new antifungal class for vulvovaginal candidiasis (VVC) in more than 20 years, the drug’s manufacturer Scynexis said in a press release. It becomes the first and only nonazole treatment for vaginal yeast infections.
The biotechnology company said approval came after positive results from two phase 3 studies in which oral ibrexafungerp demonstrated efficacy and tolerability. The most common reactions observed in clinical trials were diarrhea, nausea, abdominal pain, dizziness, and vomiting.
There are few other treatments for vaginal yeast infections, which is the second most common cause of vaginitis. Those previously approved agents include several topical azole antifungals and oral fluconazole (Diflucan), which, Scynexis said, is the only other orally administered antifungal approved for the treatment of VVC in the United States and has accounted for over more than 90% of prescriptions written for the condition each year.
However, the company noted, oral fluconazole reports a 55% therapeutic cure rate on its label, which now also includes warnings of potential fetal harm, demonstrating the need for new oral options.
The new drug may not fill that need for pregnant women, however, as the company noted that ibrexafungerp should not be used during pregnancy, and administration during pregnancy “may cause fetal harm based on animal studies.”
Because of possible teratogenic effects, the company advised clinicians to verify pregnancy status in females of reproductive potential before prescribing ibrexafungerp and advises effective contraception during treatment.
VVC can come with substantial morbidity, including genital pain, itching and burning, reduced sexual pleasure, and psychological distress.
David Angulo, MD, chief medical officer for Scynexis, said in a statement the tablets brings new benefits.
Dr. Angulo said the drug “has a differentiated fungicidal mechanism of action that kills a broad range of Candida species, including azole-resistant strains. We are working on completing our CANDLE study investigating ibrexafungerp for the prevention of recurrent VVC and expect we will be submitting a supplemental NDA [new drug application] in the first half of 2022.”
Scynexis said it partnered with Amplity Health, a Pennsylvania-based pharmaceutical company, to support U.S. marketing of the drug. The commercial launch will follow the approval.
Ibrexafungerp was granted approval through both the FDA’s Qualified Infectious Disease Product and Fast Track designations. It is expected to be marketed exclusively in the United States for 10 years.
A version of this article first appeared on Medscape.com.
Ibrexafungerp is the first drug approved in a new antifungal class for vulvovaginal candidiasis (VVC) in more than 20 years, the drug’s manufacturer Scynexis said in a press release. It becomes the first and only nonazole treatment for vaginal yeast infections.
The biotechnology company said approval came after positive results from two phase 3 studies in which oral ibrexafungerp demonstrated efficacy and tolerability. The most common reactions observed in clinical trials were diarrhea, nausea, abdominal pain, dizziness, and vomiting.
There are few other treatments for vaginal yeast infections, which is the second most common cause of vaginitis. Those previously approved agents include several topical azole antifungals and oral fluconazole (Diflucan), which, Scynexis said, is the only other orally administered antifungal approved for the treatment of VVC in the United States and has accounted for over more than 90% of prescriptions written for the condition each year.
However, the company noted, oral fluconazole reports a 55% therapeutic cure rate on its label, which now also includes warnings of potential fetal harm, demonstrating the need for new oral options.
The new drug may not fill that need for pregnant women, however, as the company noted that ibrexafungerp should not be used during pregnancy, and administration during pregnancy “may cause fetal harm based on animal studies.”
Because of possible teratogenic effects, the company advised clinicians to verify pregnancy status in females of reproductive potential before prescribing ibrexafungerp and advises effective contraception during treatment.
VVC can come with substantial morbidity, including genital pain, itching and burning, reduced sexual pleasure, and psychological distress.
David Angulo, MD, chief medical officer for Scynexis, said in a statement the tablets brings new benefits.
Dr. Angulo said the drug “has a differentiated fungicidal mechanism of action that kills a broad range of Candida species, including azole-resistant strains. We are working on completing our CANDLE study investigating ibrexafungerp for the prevention of recurrent VVC and expect we will be submitting a supplemental NDA [new drug application] in the first half of 2022.”
Scynexis said it partnered with Amplity Health, a Pennsylvania-based pharmaceutical company, to support U.S. marketing of the drug. The commercial launch will follow the approval.
Ibrexafungerp was granted approval through both the FDA’s Qualified Infectious Disease Product and Fast Track designations. It is expected to be marketed exclusively in the United States for 10 years.
A version of this article first appeared on Medscape.com.
Ibrexafungerp is the first drug approved in a new antifungal class for vulvovaginal candidiasis (VVC) in more than 20 years, the drug’s manufacturer Scynexis said in a press release. It becomes the first and only nonazole treatment for vaginal yeast infections.
The biotechnology company said approval came after positive results from two phase 3 studies in which oral ibrexafungerp demonstrated efficacy and tolerability. The most common reactions observed in clinical trials were diarrhea, nausea, abdominal pain, dizziness, and vomiting.
There are few other treatments for vaginal yeast infections, which is the second most common cause of vaginitis. Those previously approved agents include several topical azole antifungals and oral fluconazole (Diflucan), which, Scynexis said, is the only other orally administered antifungal approved for the treatment of VVC in the United States and has accounted for over more than 90% of prescriptions written for the condition each year.
However, the company noted, oral fluconazole reports a 55% therapeutic cure rate on its label, which now also includes warnings of potential fetal harm, demonstrating the need for new oral options.
The new drug may not fill that need for pregnant women, however, as the company noted that ibrexafungerp should not be used during pregnancy, and administration during pregnancy “may cause fetal harm based on animal studies.”
Because of possible teratogenic effects, the company advised clinicians to verify pregnancy status in females of reproductive potential before prescribing ibrexafungerp and advises effective contraception during treatment.
VVC can come with substantial morbidity, including genital pain, itching and burning, reduced sexual pleasure, and psychological distress.
David Angulo, MD, chief medical officer for Scynexis, said in a statement the tablets brings new benefits.
Dr. Angulo said the drug “has a differentiated fungicidal mechanism of action that kills a broad range of Candida species, including azole-resistant strains. We are working on completing our CANDLE study investigating ibrexafungerp for the prevention of recurrent VVC and expect we will be submitting a supplemental NDA [new drug application] in the first half of 2022.”
Scynexis said it partnered with Amplity Health, a Pennsylvania-based pharmaceutical company, to support U.S. marketing of the drug. The commercial launch will follow the approval.
Ibrexafungerp was granted approval through both the FDA’s Qualified Infectious Disease Product and Fast Track designations. It is expected to be marketed exclusively in the United States for 10 years.
A version of this article first appeared on Medscape.com.
Addressing an uncharted front in the war on COVID-19: Vaccination during pregnancy
In December 2020, the US Food and Drug Administration’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.1 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.2 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.3 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.4,5
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- The presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes.
- Potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- The ability to take precautions (social distancing, wearing a mask, etc)
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
In December 2020, the US Food and Drug Administration’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.1 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.2 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.3 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.4,5
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- The presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes.
- Potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- The ability to take precautions (social distancing, wearing a mask, etc)
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
In December 2020, the US Food and Drug Administration’s Emergency Use Authorization of the first COVID-19 vaccine presented us with a new tactic in the war against SARS-COV-2—and a new dilemma for obstetricians. What we had learned about COVID-19 infection in pregnancy by that point was alarming. While the vast majority (>90%) of pregnant women who contract COVID-19 recover without requiring hospitalization, pregnant women are at increased risk for severe illness and mechanical ventilation when compared with their nonpregnant counterparts.1 Vertical transmission to the fetus is a rare event, but the increased risk of preterm birth, miscarriage, and preeclampsia makes the fetus a second victim in many cases.2 Moreover, much is still unknown about the long-term impact of severe illness on maternal and fetal health.
Gaining vaccine approval
The COVID-19 vaccine, with its high efficacy rates in the nonpregnant adult population, presents an opportunity to reduce maternal morbidity related to this devastating illness. But unlike other vaccines, such as the flu shot and TDAP, results from prospective studies on COVID-19 vaccination of expectant women are pending. Under the best of circumstances, gaining acceptance of any vaccine during pregnancy faces barriers such as vaccine hesitancy and a general concern from pregnant women about the effect of medical interventions on the fetus. There is no reason to expect that either the mRNA vaccines or the replication-incompetent adenovirus recombinant vector vaccine could cause harm to the developing fetus, but the fact that currently available COVID-19 vaccines use newer technologies complicates the decision for many women.
Nevertheless, what we do know now is much more than we did in December, particularly when it comes to the mRNA vaccines. To date, observational studies of women who received the mRNA vaccine in pregnancy have shown no increased risk of adverse maternal, fetal, or obstetric outcomes.3 Emerging data also indicate that antibodies to the SARS-CoV-2 spike protein—the target of all 3 vaccines—is present in cord blood, potentially protecting the infant in the first months of life from contracting COVID-19 if the mother receives the vaccine during pregnancy.4,5
Our approach to counseling
How can we best help our patients navigate the risks and benefits of the COVID-19 vaccine? First, by acknowledging the obvious: We are in the midst of a pandemic with high rates of community spread, which makes COVID-19 different from any other vaccine-preventable disease at this time. Providing patients with a structure for making an educated decision is essential, taking into account (1) what we know about COVID-19 infection during pregnancy, (2) what we know about vaccine efficacy and safety to date, and (3) individual factors such as:
- The presence of comorbidities such as obesity, heart disease, respiratory disease, and diabetes.
- Potential exposures—“Do you have children in school or daycare? Do childcare providers or other workers come to your home? What is your occupation?”
- The ability to take precautions (social distancing, wearing a mask, etc)
All things considered, the decision to accept the COVID-19 vaccine or not ultimately belongs to the patient. Given disease prevalence and the latest information on vaccine safety in pregnancy, I have been advising my patients in the second trimester or beyond to receive the vaccine with the caveat that delaying the vaccine until the postpartum period is a completely valid alternative. The most important gift we can offer our patients is to arm them with the necessary information so that they can make the choice best for them and their family as we continue to fight this war on COVID-19.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320.
- Soheili M, Moradi G, Baradaran HR, et al. Clinical manifestation and maternal complications and neonatal outcomes in pregnant women with COVID-19: a comprehensive evidence synthesis and meta-analysis. J Matern Fetal Neonatal Med. February 18, 2021. doi: 10.1080/14767058.2021.1888923.
- Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. April 21, 2021. doi: 10.1056/NEJMoa2104983.
- Mithal LB, Otero S, Shanes ED, et al. Cord blood antibodies following maternal COVID-19 vaccination during pregnancy. Am J Obstet Gynecol. 2021;S0002-9378(21)00215-5. doi: 10.1016/j.ajog.2021.03.035.
- Rottenstreich A, Zarbiv G, Oiknine-Djian E, et al. Efficient maternofetal transplacental transfer of anti- SARS-CoV-2 spike antibodies after antenatal SARS-CoV-2 BNT162b2 mRNA vaccination. Clin Infect Dis. 2021;ciab266. doi: 10.1093/cid/ciab266.
2021 Update on cervical disease
Infection with high-risk human papillomavirus (hrHPV) is an essential step in the development of cervical cancer and its precursors, as well as in several other cancers, including oropharyngeal, vulvar, vaginal, anal, and penile cancers. At least 13 HPV strains, known collectively as hrHPV, have been associated with cervical cancer, in addition to more than 150 low-risk HPV types that have not been associated with cancer (for example, HPV 6 and 11).1 Up to 80% of women (and most men, although men are not tested routinely) will become infected with at least one of the high-risk HPV types throughout their lives, although in most cases these infections will be transient and have no clinical impact for the patient. Patients who test positive consecutively over time for hrHPV, and especially those who test positive for one of the most virulent HPV types (HPV 16 or 18), have a higher risk of developing cervical cancer or precancer. In addition, many patients who acquire HPV at a young age may “clear” the infection, which usually means that the virus becomes inactive; however, often, for unknown reasons, the virus can be reactivated in some women later in life.
This knowledge of the natural history of HPV has led to improved approaches to cervical cancer prevention, which relies on a combined strategy that includes vaccinating as many children and young adults as possible against hrHPV, screening and triaging approaches that use HPV-based tests, and applying risk-based evaluation for abnormal screening results. New guidelines and information address the best approaches to each of these aspects of cervical cancer prevention, which we review here.
HPV vaccination: Recommendations and effect on cervical cancer rates
Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383;1340-1348.
Vaccination at ages 27 to 45, although approved by the US Food and Drug Administration, is recommended only in a shared decision-making capacity by ACIP and the American College of Obstetricians and Gynecologists (ACOG) due to the vaccine’s minimal effect on cancer prevention in this age group. The ACIP and ACOG do not recommend catch-up vaccination for adults aged 27 to 45 years, but they recognize that some who are not adequately vaccinated might be at risk for new HPV infection and thus may benefit from vaccination.4
In contrast, the American Cancer Society (ACS) does not endorse the 2019 ACIP recommendation for shared clinical decision making in 27- to 45-year-olds because of the low effectiveness and low cancer prevention potential of vaccination in this age group, the burden of decision making on patients and clinicians, and the lack of sufficient guidance on selecting individuals who might benefit.5
Decline in HPV infections
A study in the United States between 2003 and 2014 showed a 71% decline in vaccine-type HPV infections among girls and women aged 14 to 19 in the post–vaccine available era as compared with the prevaccine era, and a lesser but still reasonable decline among women in the 20- to 24-year-old age group.6 Overall, vaccine-type HPV infections decreased 89% for vaccinated girls and 34% for unvaccinated girls, demonstrating some herd immunity.6 Ideally, the vaccine is given before the onset of skin-to-skin genital sexual activity. Many studies have found the vaccine to be safe and that immunogenicity is maintained for at least 9 years.7-11
Decrease in invasive cervical cancer
Recently, Lei and colleagues published a study in the New England Journal of Medicine that reviewed outcomes for more than 1.6 million girls and women vaccinated against HPV in Sweden between 2006 and 2017.12 Among girls who were vaccinated at younger than 17 years of age, there were only 2 cases of cancer, in contrast to 17 cases among those vaccinated at age 17 to 30 and 538 cases among those not vaccinated.
This is the first study to show definitively the preventive effect of HPV vaccination on the development of invasive cancer and the tremendous advantage of vaccinating at a young age. Nonetheless, the advantage conferred by catch-up vaccination (that is, vaccinating those at ages 17–30) also was significant.
Despite the well-established benefits of HPV vaccination, only 57% of women and 52% of men in the recommended age groups have received all recommended doses.13 Based on these findings, we need to advocate to our patients to vaccinate all children as early as recommended or possible and to continue catch-up vaccination for those in their 20s, even if they have hrHPV, given the efficacy of the current nonvalent vaccine against at least 7 oncogenic types. It is not at all clear that there is a benefit to vaccinating older women to prevent cancer, and we should currently focus on vaccinating younger people and continue to screen older women as newer research indicates that cervical cancer is increasing among women older than age 65.14
Continue to: Updated guidance on cervical cancer screening for average-risk women...
Updated guidance on cervical cancer screening for average-risk women
US Preventive Services Task Force; Curry SJ, Frist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
As more is understood about the natural history of HPV and its role in the development of cervical cancer and its precursors, refinements and updates have been made to our approaches for screening people at risk. There is much evidence and experience available on recommending Pap testing and HPV cotesting (testing for HPV along with cytology even if the cytology result is normal) among women aged 30 to 65 years, as that has been an option since the 2012 guidelines were published.15
We know also that HPV testing is more sensitive for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or greater at 5 years and that a negative HPV test is more reassuring than a negative Pap test.16
Primary HPV tests
HPV tests can be used in conjunction with cytology (that is, cotesting) or as a primary screening that if positive, can reflex either to cytology or to testing for the most oncogenic subtypes. Currently, only 2 FDA-approved primary screening tests are available, the cobas 4800 HPV test system (Roche Diagnostics) and the BD Onclarity HPV assay (Becton, Dickinson and Company).17 Most laboratories in the United States do not yet have the technology for primary testing, and so instead they offer one of the remaining tests (Hybrid Capture 2 [Qiagen] and Cervista and Aptima [Hologic]), which do not necessarily have the same positive and negative predictive value as the tests specifically approved for primary testing. Thus, many clinicians and patients do not yet have access to primary HPV testing.
In addition, due to slow uptake of the HPV vaccine in many parts of the United States,13 there is concern that adding HPV testing in nonvaccinated women under age 30 would result in a surge of unnecessary colposcopy procedures for women with transient infections. Thus, several large expert organizations differ in opinion regarding screening among certain populations and by which test.
Screening guidance from national organizations
The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) differ in their recommendations for screening women in their 20s for cervical cancer.18,19 The USPSTF guidelines, which were published first, focus not only on the best test but also on what is feasible and likely to benefit public health, given our current testing capacity and vaccine coverage. The USPSTF recommends starting screening at age 21 with cytology and, if all results are normal, continuing every 3 years until age 30, at which point they recommend cytology every 3 years or cotesting every 5 years or primary HPV testing alone every 5 years (if all results are normal in each case).
In contrast, the ACS published "aspirational” guidelines, with the best evidence-based recommendations, but they acknowledge that due to availability of different testing options, some patients still need to be screened with existing modalities. The ACS recommends the onset of screening at age 25 with either primary HPV testing every 5 years (preferred) or cotesting every 5 years or cytology every 3 years.
Both the USPSTF and ACS guidelines state that if using cytology alone, the screening frequency should be every 3 years, and if using an HPV-based test, the screening interval (if all results are normal) can be extended to every 5 years.
Notably, the newest guidelines for cervical cancer screening essentially limit “screening” to low-risk women who are immunocompetent and who have never had an abnormal result, specifically high-grade dysplasia (that is, CIN 2 or CIN 3). Guidelines for higher-risk groups, including the immunosuppressed, and surveillance among women with prior abnormal results can be accessed (as can all the US guidelines) at the American Society for Colposcopy and Cervical Pathology (ASCCP) website (http://www.asccp.org/).
Continue to: New ASCCP management guidelines focus on individualized risk assessment...
New ASCCP management guidelines focus on individualized risk assessment
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
The ASCCP risk-based management guidelines introduce a paradigm shift from managing a specific cervical cancer screening result to using a clinical action threshold based on risk estimates that use both current and past test results to determine frequency and urgency of testing, management, and surveillance (FIGURE).20 The individualized risk estimate helps to target prevention for those at highest risk while minimizing overtesting and overtreatment.
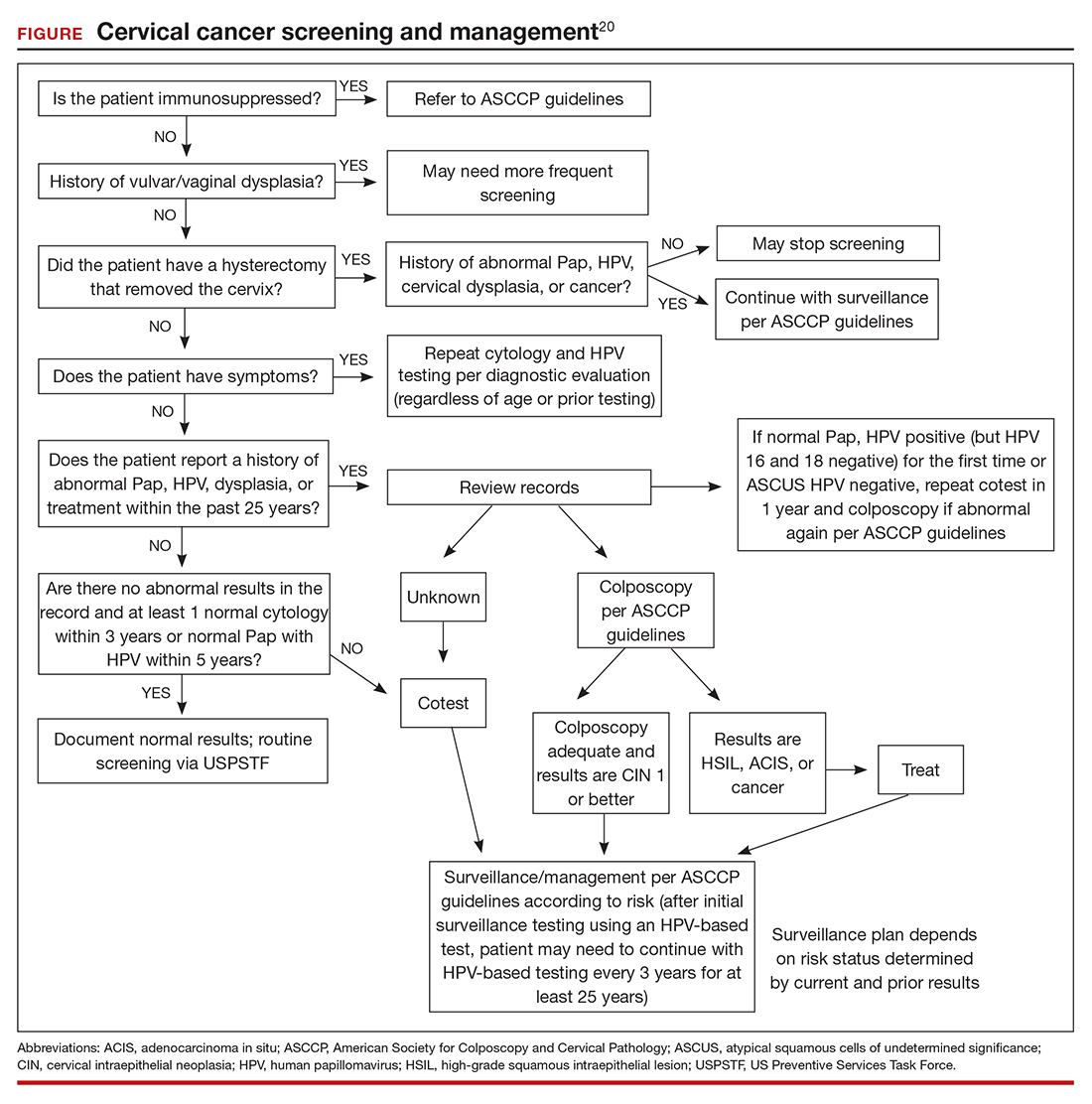
Estimating risk and determining management
The new risk-based management consensus guidelines use risk and clinical action thresholds to determine the appropriate management course for cervical screening abnormalities.20 New data indicate that a patient’s risk of developing cervical precancer or cancer can be estimated using current screening results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression.20 For each combination of current test results and screening history (including unknown history), the immediate and 5-year risk of CIN 3+ is estimated.
With respect to risk, the following concepts underlie the changes from the 2012 guidelines:
- Negative HPV tests reduce risk.
- Colposcopy performed for low-grade abnormalities, which confirms the absence of CIN 2+, reduces risk.
- A history of HPV-positive results increases risk.
- Prior treatment for CIN 2 or CIN 3 increases risk, and women with this history need to be followed closely for at least 25 years, regardless of age.
Once an individual’s risk is estimated, it is compared with 1 of the 6 proposed “clinical action thresholds”: treatment, optional treatment or colposcopy/biopsy, colposcopy/ biopsy, 1-year surveillance, 3-year surveillance, or 5-year return to regular screening (<0.15% 5-year CIN 3+ risk).
Key takeaways
Increasing knowledge of the natural history of HPV has led to improved approaches to prevention, including the nonvalent HPV vaccine, which protects against 7 high-risk and 2 low-risk HPV types; specific screening guidelines that take into consideration age, immune status, and prior abnormality; and risk-based management guidelines that use both current and prior results as well as age to recommend the best approach for managing an abnormal result and providing surveillance after an abnormal result. ●
Using the ASCCP risk thresholds, most patients with a history of an abnormal result, especially CIN 2+, likely will need more frequent surveillance testing for the foreseeable future. As increasing cohorts are vaccinated and as new biomarkers emerge that can help triage patients into more precise categories, the current risk categories likely will evolve. Hopefully, women at high risk will be appropriately managed, and those at low risk will avoid overtreatment.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68;698-702.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
- American College of Obstetricians and Gynecologists. Human papillomavirus vaccination: ACOG committee opinion no. 809. Obstet Gynecol. 2020;136:e15-e21.
- Saslow D, Andrews KS, Manassaram-Baptiste D, et al; American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274-280.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216:594-603.
- Gee J, Weinbaum C, Sukumaran L, et al. Quadrivalent HPV vaccine safety review and safety monitoring plans for ninevalent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12:1406-1417.
- Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452-457.
- Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193- 203.
- Suragh TA, Lewis P, Arana J, et al. Safety of bivalent human papillomavirus vaccine in the US Vaccine Adverse Event Reporting System (VAERS), 2009–2017. Br J Clin Pharmacol. 2018;84:2928-2932.
- Pinto LA, Dillner J, Beddows S, et al. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799.
- Lei J, Ploner A, Elfstrom KM et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340- 1348.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109-1116.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Lower Genit Tract Dis. 2018;22: 314-317.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28-35.
- Salazar KL, Duhon DJ, Olsen R, et al. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019;8:284-292.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer Clin. 2020;70:321-346.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
Infection with high-risk human papillomavirus (hrHPV) is an essential step in the development of cervical cancer and its precursors, as well as in several other cancers, including oropharyngeal, vulvar, vaginal, anal, and penile cancers. At least 13 HPV strains, known collectively as hrHPV, have been associated with cervical cancer, in addition to more than 150 low-risk HPV types that have not been associated with cancer (for example, HPV 6 and 11).1 Up to 80% of women (and most men, although men are not tested routinely) will become infected with at least one of the high-risk HPV types throughout their lives, although in most cases these infections will be transient and have no clinical impact for the patient. Patients who test positive consecutively over time for hrHPV, and especially those who test positive for one of the most virulent HPV types (HPV 16 or 18), have a higher risk of developing cervical cancer or precancer. In addition, many patients who acquire HPV at a young age may “clear” the infection, which usually means that the virus becomes inactive; however, often, for unknown reasons, the virus can be reactivated in some women later in life.
This knowledge of the natural history of HPV has led to improved approaches to cervical cancer prevention, which relies on a combined strategy that includes vaccinating as many children and young adults as possible against hrHPV, screening and triaging approaches that use HPV-based tests, and applying risk-based evaluation for abnormal screening results. New guidelines and information address the best approaches to each of these aspects of cervical cancer prevention, which we review here.
HPV vaccination: Recommendations and effect on cervical cancer rates
Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383;1340-1348.

Vaccination at ages 27 to 45, although approved by the US Food and Drug Administration, is recommended only in a shared decision-making capacity by ACIP and the American College of Obstetricians and Gynecologists (ACOG) due to the vaccine’s minimal effect on cancer prevention in this age group. The ACIP and ACOG do not recommend catch-up vaccination for adults aged 27 to 45 years, but they recognize that some who are not adequately vaccinated might be at risk for new HPV infection and thus may benefit from vaccination.4
In contrast, the American Cancer Society (ACS) does not endorse the 2019 ACIP recommendation for shared clinical decision making in 27- to 45-year-olds because of the low effectiveness and low cancer prevention potential of vaccination in this age group, the burden of decision making on patients and clinicians, and the lack of sufficient guidance on selecting individuals who might benefit.5
Decline in HPV infections
A study in the United States between 2003 and 2014 showed a 71% decline in vaccine-type HPV infections among girls and women aged 14 to 19 in the post–vaccine available era as compared with the prevaccine era, and a lesser but still reasonable decline among women in the 20- to 24-year-old age group.6 Overall, vaccine-type HPV infections decreased 89% for vaccinated girls and 34% for unvaccinated girls, demonstrating some herd immunity.6 Ideally, the vaccine is given before the onset of skin-to-skin genital sexual activity. Many studies have found the vaccine to be safe and that immunogenicity is maintained for at least 9 years.7-11
Decrease in invasive cervical cancer
Recently, Lei and colleagues published a study in the New England Journal of Medicine that reviewed outcomes for more than 1.6 million girls and women vaccinated against HPV in Sweden between 2006 and 2017.12 Among girls who were vaccinated at younger than 17 years of age, there were only 2 cases of cancer, in contrast to 17 cases among those vaccinated at age 17 to 30 and 538 cases among those not vaccinated.
This is the first study to show definitively the preventive effect of HPV vaccination on the development of invasive cancer and the tremendous advantage of vaccinating at a young age. Nonetheless, the advantage conferred by catch-up vaccination (that is, vaccinating those at ages 17–30) also was significant.
Despite the well-established benefits of HPV vaccination, only 57% of women and 52% of men in the recommended age groups have received all recommended doses.13 Based on these findings, we need to advocate to our patients to vaccinate all children as early as recommended or possible and to continue catch-up vaccination for those in their 20s, even if they have hrHPV, given the efficacy of the current nonvalent vaccine against at least 7 oncogenic types. It is not at all clear that there is a benefit to vaccinating older women to prevent cancer, and we should currently focus on vaccinating younger people and continue to screen older women as newer research indicates that cervical cancer is increasing among women older than age 65.14
Continue to: Updated guidance on cervical cancer screening for average-risk women...
Updated guidance on cervical cancer screening for average-risk women
US Preventive Services Task Force; Curry SJ, Frist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
As more is understood about the natural history of HPV and its role in the development of cervical cancer and its precursors, refinements and updates have been made to our approaches for screening people at risk. There is much evidence and experience available on recommending Pap testing and HPV cotesting (testing for HPV along with cytology even if the cytology result is normal) among women aged 30 to 65 years, as that has been an option since the 2012 guidelines were published.15
We know also that HPV testing is more sensitive for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or greater at 5 years and that a negative HPV test is more reassuring than a negative Pap test.16
Primary HPV tests
HPV tests can be used in conjunction with cytology (that is, cotesting) or as a primary screening that if positive, can reflex either to cytology or to testing for the most oncogenic subtypes. Currently, only 2 FDA-approved primary screening tests are available, the cobas 4800 HPV test system (Roche Diagnostics) and the BD Onclarity HPV assay (Becton, Dickinson and Company).17 Most laboratories in the United States do not yet have the technology for primary testing, and so instead they offer one of the remaining tests (Hybrid Capture 2 [Qiagen] and Cervista and Aptima [Hologic]), which do not necessarily have the same positive and negative predictive value as the tests specifically approved for primary testing. Thus, many clinicians and patients do not yet have access to primary HPV testing.
In addition, due to slow uptake of the HPV vaccine in many parts of the United States,13 there is concern that adding HPV testing in nonvaccinated women under age 30 would result in a surge of unnecessary colposcopy procedures for women with transient infections. Thus, several large expert organizations differ in opinion regarding screening among certain populations and by which test.
Screening guidance from national organizations
The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) differ in their recommendations for screening women in their 20s for cervical cancer.18,19 The USPSTF guidelines, which were published first, focus not only on the best test but also on what is feasible and likely to benefit public health, given our current testing capacity and vaccine coverage. The USPSTF recommends starting screening at age 21 with cytology and, if all results are normal, continuing every 3 years until age 30, at which point they recommend cytology every 3 years or cotesting every 5 years or primary HPV testing alone every 5 years (if all results are normal in each case).
In contrast, the ACS published "aspirational” guidelines, with the best evidence-based recommendations, but they acknowledge that due to availability of different testing options, some patients still need to be screened with existing modalities. The ACS recommends the onset of screening at age 25 with either primary HPV testing every 5 years (preferred) or cotesting every 5 years or cytology every 3 years.
Both the USPSTF and ACS guidelines state that if using cytology alone, the screening frequency should be every 3 years, and if using an HPV-based test, the screening interval (if all results are normal) can be extended to every 5 years.
Notably, the newest guidelines for cervical cancer screening essentially limit “screening” to low-risk women who are immunocompetent and who have never had an abnormal result, specifically high-grade dysplasia (that is, CIN 2 or CIN 3). Guidelines for higher-risk groups, including the immunosuppressed, and surveillance among women with prior abnormal results can be accessed (as can all the US guidelines) at the American Society for Colposcopy and Cervical Pathology (ASCCP) website (http://www.asccp.org/).
Continue to: New ASCCP management guidelines focus on individualized risk assessment...
New ASCCP management guidelines focus on individualized risk assessment
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
The ASCCP risk-based management guidelines introduce a paradigm shift from managing a specific cervical cancer screening result to using a clinical action threshold based on risk estimates that use both current and past test results to determine frequency and urgency of testing, management, and surveillance (FIGURE).20 The individualized risk estimate helps to target prevention for those at highest risk while minimizing overtesting and overtreatment.

Estimating risk and determining management
The new risk-based management consensus guidelines use risk and clinical action thresholds to determine the appropriate management course for cervical screening abnormalities.20 New data indicate that a patient’s risk of developing cervical precancer or cancer can be estimated using current screening results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression.20 For each combination of current test results and screening history (including unknown history), the immediate and 5-year risk of CIN 3+ is estimated.
With respect to risk, the following concepts underlie the changes from the 2012 guidelines:
- Negative HPV tests reduce risk.
- Colposcopy performed for low-grade abnormalities, which confirms the absence of CIN 2+, reduces risk.
- A history of HPV-positive results increases risk.
- Prior treatment for CIN 2 or CIN 3 increases risk, and women with this history need to be followed closely for at least 25 years, regardless of age.
Once an individual’s risk is estimated, it is compared with 1 of the 6 proposed “clinical action thresholds”: treatment, optional treatment or colposcopy/biopsy, colposcopy/ biopsy, 1-year surveillance, 3-year surveillance, or 5-year return to regular screening (<0.15% 5-year CIN 3+ risk).
Key takeaways
Increasing knowledge of the natural history of HPV has led to improved approaches to prevention, including the nonvalent HPV vaccine, which protects against 7 high-risk and 2 low-risk HPV types; specific screening guidelines that take into consideration age, immune status, and prior abnormality; and risk-based management guidelines that use both current and prior results as well as age to recommend the best approach for managing an abnormal result and providing surveillance after an abnormal result. ●
Using the ASCCP risk thresholds, most patients with a history of an abnormal result, especially CIN 2+, likely will need more frequent surveillance testing for the foreseeable future. As increasing cohorts are vaccinated and as new biomarkers emerge that can help triage patients into more precise categories, the current risk categories likely will evolve. Hopefully, women at high risk will be appropriately managed, and those at low risk will avoid overtreatment.
Infection with high-risk human papillomavirus (hrHPV) is an essential step in the development of cervical cancer and its precursors, as well as in several other cancers, including oropharyngeal, vulvar, vaginal, anal, and penile cancers. At least 13 HPV strains, known collectively as hrHPV, have been associated with cervical cancer, in addition to more than 150 low-risk HPV types that have not been associated with cancer (for example, HPV 6 and 11).1 Up to 80% of women (and most men, although men are not tested routinely) will become infected with at least one of the high-risk HPV types throughout their lives, although in most cases these infections will be transient and have no clinical impact for the patient. Patients who test positive consecutively over time for hrHPV, and especially those who test positive for one of the most virulent HPV types (HPV 16 or 18), have a higher risk of developing cervical cancer or precancer. In addition, many patients who acquire HPV at a young age may “clear” the infection, which usually means that the virus becomes inactive; however, often, for unknown reasons, the virus can be reactivated in some women later in life.
This knowledge of the natural history of HPV has led to improved approaches to cervical cancer prevention, which relies on a combined strategy that includes vaccinating as many children and young adults as possible against hrHPV, screening and triaging approaches that use HPV-based tests, and applying risk-based evaluation for abnormal screening results. New guidelines and information address the best approaches to each of these aspects of cervical cancer prevention, which we review here.
HPV vaccination: Recommendations and effect on cervical cancer rates
Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68:698-702.
Lei J, Ploner A, Elfstrom KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383;1340-1348.

Vaccination at ages 27 to 45, although approved by the US Food and Drug Administration, is recommended only in a shared decision-making capacity by ACIP and the American College of Obstetricians and Gynecologists (ACOG) due to the vaccine’s minimal effect on cancer prevention in this age group. The ACIP and ACOG do not recommend catch-up vaccination for adults aged 27 to 45 years, but they recognize that some who are not adequately vaccinated might be at risk for new HPV infection and thus may benefit from vaccination.4
In contrast, the American Cancer Society (ACS) does not endorse the 2019 ACIP recommendation for shared clinical decision making in 27- to 45-year-olds because of the low effectiveness and low cancer prevention potential of vaccination in this age group, the burden of decision making on patients and clinicians, and the lack of sufficient guidance on selecting individuals who might benefit.5
Decline in HPV infections
A study in the United States between 2003 and 2014 showed a 71% decline in vaccine-type HPV infections among girls and women aged 14 to 19 in the post–vaccine available era as compared with the prevaccine era, and a lesser but still reasonable decline among women in the 20- to 24-year-old age group.6 Overall, vaccine-type HPV infections decreased 89% for vaccinated girls and 34% for unvaccinated girls, demonstrating some herd immunity.6 Ideally, the vaccine is given before the onset of skin-to-skin genital sexual activity. Many studies have found the vaccine to be safe and that immunogenicity is maintained for at least 9 years.7-11
Decrease in invasive cervical cancer
Recently, Lei and colleagues published a study in the New England Journal of Medicine that reviewed outcomes for more than 1.6 million girls and women vaccinated against HPV in Sweden between 2006 and 2017.12 Among girls who were vaccinated at younger than 17 years of age, there were only 2 cases of cancer, in contrast to 17 cases among those vaccinated at age 17 to 30 and 538 cases among those not vaccinated.
This is the first study to show definitively the preventive effect of HPV vaccination on the development of invasive cancer and the tremendous advantage of vaccinating at a young age. Nonetheless, the advantage conferred by catch-up vaccination (that is, vaccinating those at ages 17–30) also was significant.
Despite the well-established benefits of HPV vaccination, only 57% of women and 52% of men in the recommended age groups have received all recommended doses.13 Based on these findings, we need to advocate to our patients to vaccinate all children as early as recommended or possible and to continue catch-up vaccination for those in their 20s, even if they have hrHPV, given the efficacy of the current nonvalent vaccine against at least 7 oncogenic types. It is not at all clear that there is a benefit to vaccinating older women to prevent cancer, and we should currently focus on vaccinating younger people and continue to screen older women as newer research indicates that cervical cancer is increasing among women older than age 65.14
Continue to: Updated guidance on cervical cancer screening for average-risk women...
Updated guidance on cervical cancer screening for average-risk women
US Preventive Services Task Force; Curry SJ, Frist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
As more is understood about the natural history of HPV and its role in the development of cervical cancer and its precursors, refinements and updates have been made to our approaches for screening people at risk. There is much evidence and experience available on recommending Pap testing and HPV cotesting (testing for HPV along with cytology even if the cytology result is normal) among women aged 30 to 65 years, as that has been an option since the 2012 guidelines were published.15
We know also that HPV testing is more sensitive for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or greater at 5 years and that a negative HPV test is more reassuring than a negative Pap test.16
Primary HPV tests
HPV tests can be used in conjunction with cytology (that is, cotesting) or as a primary screening that if positive, can reflex either to cytology or to testing for the most oncogenic subtypes. Currently, only 2 FDA-approved primary screening tests are available, the cobas 4800 HPV test system (Roche Diagnostics) and the BD Onclarity HPV assay (Becton, Dickinson and Company).17 Most laboratories in the United States do not yet have the technology for primary testing, and so instead they offer one of the remaining tests (Hybrid Capture 2 [Qiagen] and Cervista and Aptima [Hologic]), which do not necessarily have the same positive and negative predictive value as the tests specifically approved for primary testing. Thus, many clinicians and patients do not yet have access to primary HPV testing.
In addition, due to slow uptake of the HPV vaccine in many parts of the United States,13 there is concern that adding HPV testing in nonvaccinated women under age 30 would result in a surge of unnecessary colposcopy procedures for women with transient infections. Thus, several large expert organizations differ in opinion regarding screening among certain populations and by which test.
Screening guidance from national organizations
The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) differ in their recommendations for screening women in their 20s for cervical cancer.18,19 The USPSTF guidelines, which were published first, focus not only on the best test but also on what is feasible and likely to benefit public health, given our current testing capacity and vaccine coverage. The USPSTF recommends starting screening at age 21 with cytology and, if all results are normal, continuing every 3 years until age 30, at which point they recommend cytology every 3 years or cotesting every 5 years or primary HPV testing alone every 5 years (if all results are normal in each case).
In contrast, the ACS published "aspirational” guidelines, with the best evidence-based recommendations, but they acknowledge that due to availability of different testing options, some patients still need to be screened with existing modalities. The ACS recommends the onset of screening at age 25 with either primary HPV testing every 5 years (preferred) or cotesting every 5 years or cytology every 3 years.
Both the USPSTF and ACS guidelines state that if using cytology alone, the screening frequency should be every 3 years, and if using an HPV-based test, the screening interval (if all results are normal) can be extended to every 5 years.
Notably, the newest guidelines for cervical cancer screening essentially limit “screening” to low-risk women who are immunocompetent and who have never had an abnormal result, specifically high-grade dysplasia (that is, CIN 2 or CIN 3). Guidelines for higher-risk groups, including the immunosuppressed, and surveillance among women with prior abnormal results can be accessed (as can all the US guidelines) at the American Society for Colposcopy and Cervical Pathology (ASCCP) website (http://www.asccp.org/).
Continue to: New ASCCP management guidelines focus on individualized risk assessment...
New ASCCP management guidelines focus on individualized risk assessment
Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
The ASCCP risk-based management guidelines introduce a paradigm shift from managing a specific cervical cancer screening result to using a clinical action threshold based on risk estimates that use both current and past test results to determine frequency and urgency of testing, management, and surveillance (FIGURE).20 The individualized risk estimate helps to target prevention for those at highest risk while minimizing overtesting and overtreatment.

Estimating risk and determining management
The new risk-based management consensus guidelines use risk and clinical action thresholds to determine the appropriate management course for cervical screening abnormalities.20 New data indicate that a patient’s risk of developing cervical precancer or cancer can be estimated using current screening results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression.20 For each combination of current test results and screening history (including unknown history), the immediate and 5-year risk of CIN 3+ is estimated.
With respect to risk, the following concepts underlie the changes from the 2012 guidelines:
- Negative HPV tests reduce risk.
- Colposcopy performed for low-grade abnormalities, which confirms the absence of CIN 2+, reduces risk.
- A history of HPV-positive results increases risk.
- Prior treatment for CIN 2 or CIN 3 increases risk, and women with this history need to be followed closely for at least 25 years, regardless of age.
Once an individual’s risk is estimated, it is compared with 1 of the 6 proposed “clinical action thresholds”: treatment, optional treatment or colposcopy/biopsy, colposcopy/ biopsy, 1-year surveillance, 3-year surveillance, or 5-year return to regular screening (<0.15% 5-year CIN 3+ risk).
Key takeaways
Increasing knowledge of the natural history of HPV has led to improved approaches to prevention, including the nonvalent HPV vaccine, which protects against 7 high-risk and 2 low-risk HPV types; specific screening guidelines that take into consideration age, immune status, and prior abnormality; and risk-based management guidelines that use both current and prior results as well as age to recommend the best approach for managing an abnormal result and providing surveillance after an abnormal result. ●
Using the ASCCP risk thresholds, most patients with a history of an abnormal result, especially CIN 2+, likely will need more frequent surveillance testing for the foreseeable future. As increasing cohorts are vaccinated and as new biomarkers emerge that can help triage patients into more precise categories, the current risk categories likely will evolve. Hopefully, women at high risk will be appropriately managed, and those at low risk will avoid overtreatment.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68;698-702.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
- American College of Obstetricians and Gynecologists. Human papillomavirus vaccination: ACOG committee opinion no. 809. Obstet Gynecol. 2020;136:e15-e21.
- Saslow D, Andrews KS, Manassaram-Baptiste D, et al; American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274-280.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216:594-603.
- Gee J, Weinbaum C, Sukumaran L, et al. Quadrivalent HPV vaccine safety review and safety monitoring plans for ninevalent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12:1406-1417.
- Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452-457.
- Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193- 203.
- Suragh TA, Lewis P, Arana J, et al. Safety of bivalent human papillomavirus vaccine in the US Vaccine Adverse Event Reporting System (VAERS), 2009–2017. Br J Clin Pharmacol. 2018;84:2928-2932.
- Pinto LA, Dillner J, Beddows S, et al. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799.
- Lei J, Ploner A, Elfstrom KM et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340- 1348.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109-1116.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Lower Genit Tract Dis. 2018;22: 314-317.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28-35.
- Salazar KL, Duhon DJ, Olsen R, et al. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019;8:284-292.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer Clin. 2020;70:321-346.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
- Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1-17.
- Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68;698-702.
- Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination—updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405-1408.
- American College of Obstetricians and Gynecologists. Human papillomavirus vaccination: ACOG committee opinion no. 809. Obstet Gynecol. 2020;136:e15-e21.
- Saslow D, Andrews KS, Manassaram-Baptiste D, et al; American Cancer Society Guideline Development Group. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J Clin. 2020;70:274-280.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction— National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis. 2017;216:594-603.
- Gee J, Weinbaum C, Sukumaran L, et al. Quadrivalent HPV vaccine safety review and safety monitoring plans for ninevalent HPV vaccine in the United States. Hum Vaccin Immunother. 2016;12:1406-1417.
- Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46:452-457.
- Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193- 203.
- Suragh TA, Lewis P, Arana J, et al. Safety of bivalent human papillomavirus vaccine in the US Vaccine Adverse Event Reporting System (VAERS), 2009–2017. Br J Clin Pharmacol. 2018;84:2928-2932.
- Pinto LA, Dillner J, Beddows S, et al. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799.
- Lei J, Ploner A, Elfstrom KM et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340- 1348.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109-1116.
- Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in Massachusetts compared with younger women. J Lower Genit Tract Dis. 2018;22: 314-317.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147-172.
- Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(5 suppl 1):S28-35.
- Salazar KL, Duhon DJ, Olsen R, et al. A review of the FDA-approved molecular testing platforms for human papillomavirus. J Am Soc Cytopathol. 2019;8:284-292.
- US Preventive Services Task Force; Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer Clin. 2020;70:321-346.
- Perkins RB, Guido RS, Castle PE, et al; 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-131.
Stop checking routine lipid panels every year
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
CASE 34-year-old woman with lipid panel results from 1 year ago
A woman with no chronic medical conditions was seen by her gynecologist for a routine well-woman examination. She does not see another primary care provider. She is age 34 years and has a levonorgestrel intrauterine device that was placed after the birth of her second child 2 years prior. She does not take any other medications. She has never smoked and drinks a glass of wine with dinner a couple of times each week. She finds it challenging with her full-time job and her parental responsibilities with 2 young children to get regular exercise but otherwise is active. She does not have a family history of premature cardiovascular disease. Last year, during her prior well-woman examination, she had a fasting lipid panel: her low-density lipoprotein (LDL) was 102 mg/dL (reference range, ≤160 mg/dL), high-density lipoprotein (HDL) 52 mg/dL (reference range, ≥40 mg/dL), triglycerides 140 mg/dL (reference range, <160 mg/dL), and total cholesterol 182 mg/dL (reference range, <200 mg/dL).
During this visit, the patient’s vitals are normal (blood pressure 116/58) and her physical examination is unremarkable. Her physician orders routine labs to be checked, including a fasting lipid panel. She has to figure out when she will be able to get these labs drawn, as she needs to coordinate with her work and childcare schedules. A week later, she leaves work at 4:00 PM and picks up her young children (aged 2 and 4 years) from childcare, bringing them to the laboratory to have her blood drawn. Not only are her children cranky in the waiting room, but she is feeling tired as she hasn’t eaten all day because her physician told her she is supposed to be fasting. She has to pay for parking at the lot for the laboratory since it is connected to the medical center.
Was this lipid panel high value?
When and how often should we be checking lipid panels?
Do patients need to fast for these tests?
The potential benefits and costs of routine lipid panel screening
Hyperlipidemia is relatively prevalent, usually asymptomatic, and has been linked to cardiovascular outcomes. Thus, screening for lipid abnormalities is recommended to identify patients that would benefit from various interventions aimed at reducing cardiovascular disease risk, including lipid-lowering therapy.1 High levels of LDL cholesterol and low levels of HDL cholesterol are important risk factors for coronary heart disease.
Lipid panels are widely available blood tests with modest monetary costs, generally ranging from about $10 to $100 in the outpatient setting. Of note, a 2014 study examining inpatient charges for this common laboratory test found that hospital charges in California ranged from about $10 to $10,000 for a lipid panel.2 Despite the relatively low cost of each individual lipid panel, the aggregate costs to the health system of these frequently and widely performed tests are large. In fact, low-cost, high-volume health services, such as repeat cholesterol testing, account for the majority of unnecessary health spending in the United States, contributing nearly twice as much unnecessary cost as high-priced low-value services.3
To the patient, the cost is not only monetary. Some patients will need to take an additional hour or two off work as well as consider childcare, transportation, parking, and other mundane logistics to sit in a laboratory waiting room—a cost that may not be considered modest at all by the patient.4,5
Therefore, like most services in health care, the answer to whether or not a lipid panel is high-value care is: it depends.5 In the correct circumstances, the test generally is regarded as high value due to well-documented potential benefits and low monetary costs. However, when performed unnecessarily—either in patient groups that are unlikely to benefit or at intervals that are too soon to add helpful information—then all that is left are the financial and psychosocial costs, which make this a low-value test in these scenarios. For this patient, this test contributed to inconvenience and mild hardships with essentially no benefit, thus would be considered low-value care.
Continue to: When should we perform lipid screening in low-risk women?
When should we perform lipid screening in low-risk women?
There are conflicting guidelines and opinions about at what age lipid screening should be routinely performed in adults. The United States Preventive Services Task Force (USPSTF) 2016 guidelines found “insufficient evidence that screening for dyslipidemia before age 40 years has an effect on either short- or longer-term cardiovascular outcomes.”6 Therefore, the USPSTF “recommends neither for nor against screening for dyslipidemia in this age group,” and instead encourages “clinicians to use their clinical judgment for [these] patients.”6
A common practice is to obtain a baseline lipid profile at the time of initiation of care with an adult primary care practitioner, if the patient was not previously screened, and to then determine subsequent testing based on these results and the patient’s risk factors for cardiovascular disease. For patients with normal lipid screening results and lower cardiovascular risk factors (no hypertension, diabetes mellitus, cigarette smoking, family history of premature coronary heart disease), experts suggest follow-up lipid screening be performed in men at age 35 and in women at age 45.7 Therefore, for this patient who had essentially a normal lipid panel a year prior, she should not have required repeat lipid testing until she is age 45.
As for how frequently subsequent lipid testing should be performed, the Centers for Disease Control and Prevention states, “most healthy adults should have their cholesterol checked every 4 to 6 years.”8 Those taking lipid-lowering medications or those with risk factors such as heart disease, diabetes, or concerning family history should have their cholesterol checked more frequently. If patients are near a threshold for treatment, some experts suggest repeating measurements every 3 years, but even in these settings, annual testing would be considered excessive.7
A standard lipid panel screen includes total cholesterol, LDL, HDL, and triglycerides. While a variety of assays have been developed that subfractionate lipoprotein particles based on size, density, or charge, these tests do not add value for low-risk patient screening and should only be used on an individualized basis for selected intermediate to high-risk patients. The American Society for Clinical Pathology released a Choosing Wisely recommendation that advises, “Do not routinely order expanded lipid panels (particle sizing, nuclear magnetic resonance) as screening tests for cardiovascular disease.”9

Do lipid panels need to be fasting?
For adults who are not taking lipid-lowering therapy, measurement of either a fasting or a nonfasting plasma lipid profile is effective for documenting baseline LDL and estimating cardiovascular risk.1 In other words, nonfasting lipid testing is appropriate for most low-risk screening. Nonfasting testing generally is more convenient for patients; however, nonfasting lipid panels could result in elevated triglyceride levels. If an initial nonfasting lipid profile reveals a triglyceride level of 400 mg/dL or higher, then a repeat lipid profile in the fasting state should be performed for assessment of fasting triglyceride levels and baseline LDL.1 Some patients may prefer to simply get a fasting lipid panel initially so that they do not run the risk of having to return for a second test, especially if they are at increased risk for high triglyceride levels (ie, if they are obese, have diabetes, or are taking medications such as steroids, which can increase triglyceride levels).
The bottom line
Some patients receive primary care directly from their gynecologist, and thus it is important for women’s health clinicians to be aware of appropriate cholesterol screening practices. While lipid panels may commonly be ordered routinely as part of annual health check-ups, the evidence suggests that this is an unnecessary practice that contributes to wasteful health spending at both individual and system levels; it also is an avoidable inconvenience for patients. It is unclear when lipid screening should be initiated for adult patients, but it seems reasonable to check baseline levels for a new patient who has not previously been screened. In low-risk patients with normal lipid panel levels, experts recommend initiating retesting at age 45 for women and obtaining repeat lipid levels no more than every 4 to 6 years. For most patients, nonfasting lipid levels will suffice for screening. Avoiding common unnecessary testing is an effective way to improve value for patients. ●
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Hsia RY, Akosa Antwi Y, Nath JB, et al. Variation in charges for 10 common blood tests in California hospitals: a cross-sectional analysis. BMJ Open. 2014;4:E005482.
- Mafi JN, Russell K, Bortz BA, et al. Low-cost, high-volume health services contribute the most to unnecessary health spending. Health Aff. 2017;36:1701-1704.
- Covinsky KE. The problem of overuse. JAMA Intern Med. 2013;173:1446.
- Moriates C, Arora V, Shah N. Understanding Value-Based Healthcare. McGraw-Hill; 2015.
- Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008.
- Vijan S. Screening for lipid disorders in adults. UpToDate website. Updated February 28, 2020. Accessed April 9, 2021. https://www.uptodate.com/contents/screening-for-lipid-disorders-in-adults
- Getting your cholesterol checked. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed April 9, 2021. https://www.cdc.gov/cholesterol/cholesterol_screening.htm
- American Society for Clinical Pathology. Choosing Wisely website. Published September 14, 2016. Accessed April 9, 2021. https://www.choosingwisely.org/clinician-lists/american-society-clinical-pathology-expanded-lipid-panels-to-screen-for-cardiovascular-disease
FDA and power morcellation, gel for vaginal odor, and an intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
For cervical cancer screening, any strategy is acceptable
Cytology testing every 3 years, cytology/human papillomavirus cotesting every 5 years, and primary HPV testing every 5 years are similarly effective at reducing cervical cancer risk, said Rachel P. Brook, MD, of the University of California, Los Angeles Health Iris Cantor Women’s Health Center, during a presentation at the annual meeting of the American College of Physicians.
“The most important thing a primary care provider can do is to screen with whatever test is most accessible,” Dr. Brook said in an interview. She also noted that access to screening remains a pressing concern, particularly among underrepresented groups and women in rural areas. Even when women can access testing, follow-up after abnormal results can be inadequate, leading to increased risk of cervical cancer mortality.
To address some of these shortcomings, Dr. Brook provided an overview of current guidelines and appropriate responses to abnormal test results.
First, during her presentation, she noted that guideline recommendations do not apply to patients with additional risk factors, including a compromised immune system, HIV infection, previous treatment of cervical cancer or a high-grade cancerous lesion, or in utero exposure to diethylstilbestrol.
“This is very important,” Dr. Brook said during her presentation. “They should receive individualized care due to their above average risk of cervical cancer.”
Among women with average risk, both the USPSTF 2018 guideline and the ACS 2020 guideline recommend against screening women aged less than 21 years.
In a major change to the most recent ACS guideline, screening women aged 21-24 years is no longer recommended, in contrast with the USPSTF guideline, which still calls for cytology every 3 years for this age group. This recommendation by the USPSTF extends to women aged 25-29 years, a group for which the ACS recommends primary HPV testing every 5 years, cytology/HPV cotesting every 5 years, or cytology testing every 3 years. For both organizations, any of these three testing methods is recommended for women aged 30-65 years, followed by discontinuation of testing after 65 years, given adequate prior screening.
“For all these recommendations and guidelines, they’re pertinent to patients regardless of HPV vaccination status,” Dr. Brook said. But she added that increased rates of HPV vaccination may affect future screening guidelines, as vaccinated patients are more likely to have false positive cytology results because of low-risk HPV strains. This trend may steer future recommendations toward primary HPV testing, Dr. Brook said.
Presently, for applicable age groups, the ACS guideline favors HPV testing alone over cytology alone or cotesting, whereas the USPSTF guideline offers no preference between the three testing strategies.
Primary HPV vs. cytology testing
Dr. Brook said a single negative HPV test provides more than 95% assurance that a patient will not develop cervical cancer or a cancer precursor within the next 5 years. One negative HPV test offers similar reliability to about 3 negative cytology tests.
Switching to a 5-year testing cycle may be unsettling for patients who are used to getting a Pap test every year, but having a conversation about test accuracy can help assuage patient concerns, she said.
Still, Dr. Brook emphasized that any of the three testing strategies is ultimately acceptable.
“The take-home message here is – truly – that any of the recommended screening options will greatly reduce cervical cancer risk,” Dr. Brook said. “So, screen. And if there is any confusion or concern with your patients about which [screening strategy to use], just help them decide on any of the three. But please screen.”
Self-swabbing could improve screening in certain groups
To improve screening rates, particularly for women with poor access and those averse to a speculum exam, Dr. Brook highlighted self-swabbing primary HPV tests, which may soon be available. While no self-swabbing HPV tests are yet approved by the Food and Drug Administration, they offer a 76% sensitivity rate for cervical intraepithelial neoplasia grade 2, and a rate of 85% for CIN3, compared with 91% for physician-collected samples.
Regardless of the exact HPV test, Dr. Brook advised appropriate reflex testing.
“We need to make sure all primary HPV screening tests positive for types other than HPV-16 or -18 will require additional reflex triage testing with cytology,” Dr. Brook said in interview. “If not – if a woman has a primary HPV screening test that is positive and I cannot perform reflex cytology – I have to bring her back for an additional test and speculum exam to get cytology, which is an unnecessary burden to the patient, and also increases testing.”
Kathy L. MacLaughlin, MD, associate professor of family medicine at Mayo Clinic, Rochester, Minn., said this is one drawback to self-swabbing tests in an interview.
“If there is a positive HPV result [with a self-swabbing test], the patient will need to have a clinic appointment for Pap collection [if one of the ‘other’ 12 HPV types are identified], or be referred for a colposcopy [if HPV types 16 or 18 are identified],” Dr. MacLaughlin said. “There need to be plans in place for access to those services.”
Incidentally, it may be women who face barriers to access that need self-swabbing HPV tests the most, according to Dr. MacLaughlin.
“I think there is significant potential to improve screening rates among never-screened and underscreened women and those are the groups for whom this makes the most sense,” she said. “I don’t think anyone is suggesting that women who have the means and interest in scheduling a face-to-face visit for clinician-collected screening switch to self-screening, but it is a promising option [once FDA approved] for reaching other women and reducing disparities in screening rates.”
Dr. MacLaughlin suggested that self-screening programs could operate outside of normal business hours in a variety of settings, such as homes, community centers, and churches.
Until self-screening is an option, Dr. MacLaughlin agreed with Dr. Brook that any of the three testing strategies is suitable for screening, and recommended that primary care providers seize the opportunities presented to them.
“Individual primary care providers can improve screening rates by offering to update cervical cancer screening at a clinic appointment even if that was not the primary indication for the visit, especially for women who are long overdue,” Dr. MacLaughlin said. “If there is just no time to fit in the screening or the patient declines, then order a return visit and have the patient stop at the appointment desk as they leave.”
“I recognize we are asked to fit in more and more in less time, but I’ve found this to be effective when I have capacity in the clinic day to offer it,” she added.
Dr. Brook and Dr. MacLaughlin reported no conflicts of interest.
Cytology testing every 3 years, cytology/human papillomavirus cotesting every 5 years, and primary HPV testing every 5 years are similarly effective at reducing cervical cancer risk, said Rachel P. Brook, MD, of the University of California, Los Angeles Health Iris Cantor Women’s Health Center, during a presentation at the annual meeting of the American College of Physicians.
“The most important thing a primary care provider can do is to screen with whatever test is most accessible,” Dr. Brook said in an interview. She also noted that access to screening remains a pressing concern, particularly among underrepresented groups and women in rural areas. Even when women can access testing, follow-up after abnormal results can be inadequate, leading to increased risk of cervical cancer mortality.
To address some of these shortcomings, Dr. Brook provided an overview of current guidelines and appropriate responses to abnormal test results.
First, during her presentation, she noted that guideline recommendations do not apply to patients with additional risk factors, including a compromised immune system, HIV infection, previous treatment of cervical cancer or a high-grade cancerous lesion, or in utero exposure to diethylstilbestrol.
“This is very important,” Dr. Brook said during her presentation. “They should receive individualized care due to their above average risk of cervical cancer.”
Among women with average risk, both the USPSTF 2018 guideline and the ACS 2020 guideline recommend against screening women aged less than 21 years.
In a major change to the most recent ACS guideline, screening women aged 21-24 years is no longer recommended, in contrast with the USPSTF guideline, which still calls for cytology every 3 years for this age group. This recommendation by the USPSTF extends to women aged 25-29 years, a group for which the ACS recommends primary HPV testing every 5 years, cytology/HPV cotesting every 5 years, or cytology testing every 3 years. For both organizations, any of these three testing methods is recommended for women aged 30-65 years, followed by discontinuation of testing after 65 years, given adequate prior screening.
“For all these recommendations and guidelines, they’re pertinent to patients regardless of HPV vaccination status,” Dr. Brook said. But she added that increased rates of HPV vaccination may affect future screening guidelines, as vaccinated patients are more likely to have false positive cytology results because of low-risk HPV strains. This trend may steer future recommendations toward primary HPV testing, Dr. Brook said.
Presently, for applicable age groups, the ACS guideline favors HPV testing alone over cytology alone or cotesting, whereas the USPSTF guideline offers no preference between the three testing strategies.
Primary HPV vs. cytology testing
Dr. Brook said a single negative HPV test provides more than 95% assurance that a patient will not develop cervical cancer or a cancer precursor within the next 5 years. One negative HPV test offers similar reliability to about 3 negative cytology tests.
Switching to a 5-year testing cycle may be unsettling for patients who are used to getting a Pap test every year, but having a conversation about test accuracy can help assuage patient concerns, she said.
Still, Dr. Brook emphasized that any of the three testing strategies is ultimately acceptable.
“The take-home message here is – truly – that any of the recommended screening options will greatly reduce cervical cancer risk,” Dr. Brook said. “So, screen. And if there is any confusion or concern with your patients about which [screening strategy to use], just help them decide on any of the three. But please screen.”
Self-swabbing could improve screening in certain groups
To improve screening rates, particularly for women with poor access and those averse to a speculum exam, Dr. Brook highlighted self-swabbing primary HPV tests, which may soon be available. While no self-swabbing HPV tests are yet approved by the Food and Drug Administration, they offer a 76% sensitivity rate for cervical intraepithelial neoplasia grade 2, and a rate of 85% for CIN3, compared with 91% for physician-collected samples.
Regardless of the exact HPV test, Dr. Brook advised appropriate reflex testing.
“We need to make sure all primary HPV screening tests positive for types other than HPV-16 or -18 will require additional reflex triage testing with cytology,” Dr. Brook said in interview. “If not – if a woman has a primary HPV screening test that is positive and I cannot perform reflex cytology – I have to bring her back for an additional test and speculum exam to get cytology, which is an unnecessary burden to the patient, and also increases testing.”
Kathy L. MacLaughlin, MD, associate professor of family medicine at Mayo Clinic, Rochester, Minn., said this is one drawback to self-swabbing tests in an interview.
“If there is a positive HPV result [with a self-swabbing test], the patient will need to have a clinic appointment for Pap collection [if one of the ‘other’ 12 HPV types are identified], or be referred for a colposcopy [if HPV types 16 or 18 are identified],” Dr. MacLaughlin said. “There need to be plans in place for access to those services.”
Incidentally, it may be women who face barriers to access that need self-swabbing HPV tests the most, according to Dr. MacLaughlin.
“I think there is significant potential to improve screening rates among never-screened and underscreened women and those are the groups for whom this makes the most sense,” she said. “I don’t think anyone is suggesting that women who have the means and interest in scheduling a face-to-face visit for clinician-collected screening switch to self-screening, but it is a promising option [once FDA approved] for reaching other women and reducing disparities in screening rates.”
Dr. MacLaughlin suggested that self-screening programs could operate outside of normal business hours in a variety of settings, such as homes, community centers, and churches.
Until self-screening is an option, Dr. MacLaughlin agreed with Dr. Brook that any of the three testing strategies is suitable for screening, and recommended that primary care providers seize the opportunities presented to them.
“Individual primary care providers can improve screening rates by offering to update cervical cancer screening at a clinic appointment even if that was not the primary indication for the visit, especially for women who are long overdue,” Dr. MacLaughlin said. “If there is just no time to fit in the screening or the patient declines, then order a return visit and have the patient stop at the appointment desk as they leave.”
“I recognize we are asked to fit in more and more in less time, but I’ve found this to be effective when I have capacity in the clinic day to offer it,” she added.
Dr. Brook and Dr. MacLaughlin reported no conflicts of interest.
Cytology testing every 3 years, cytology/human papillomavirus cotesting every 5 years, and primary HPV testing every 5 years are similarly effective at reducing cervical cancer risk, said Rachel P. Brook, MD, of the University of California, Los Angeles Health Iris Cantor Women’s Health Center, during a presentation at the annual meeting of the American College of Physicians.
“The most important thing a primary care provider can do is to screen with whatever test is most accessible,” Dr. Brook said in an interview. She also noted that access to screening remains a pressing concern, particularly among underrepresented groups and women in rural areas. Even when women can access testing, follow-up after abnormal results can be inadequate, leading to increased risk of cervical cancer mortality.
To address some of these shortcomings, Dr. Brook provided an overview of current guidelines and appropriate responses to abnormal test results.
First, during her presentation, she noted that guideline recommendations do not apply to patients with additional risk factors, including a compromised immune system, HIV infection, previous treatment of cervical cancer or a high-grade cancerous lesion, or in utero exposure to diethylstilbestrol.
“This is very important,” Dr. Brook said during her presentation. “They should receive individualized care due to their above average risk of cervical cancer.”
Among women with average risk, both the USPSTF 2018 guideline and the ACS 2020 guideline recommend against screening women aged less than 21 years.
In a major change to the most recent ACS guideline, screening women aged 21-24 years is no longer recommended, in contrast with the USPSTF guideline, which still calls for cytology every 3 years for this age group. This recommendation by the USPSTF extends to women aged 25-29 years, a group for which the ACS recommends primary HPV testing every 5 years, cytology/HPV cotesting every 5 years, or cytology testing every 3 years. For both organizations, any of these three testing methods is recommended for women aged 30-65 years, followed by discontinuation of testing after 65 years, given adequate prior screening.
“For all these recommendations and guidelines, they’re pertinent to patients regardless of HPV vaccination status,” Dr. Brook said. But she added that increased rates of HPV vaccination may affect future screening guidelines, as vaccinated patients are more likely to have false positive cytology results because of low-risk HPV strains. This trend may steer future recommendations toward primary HPV testing, Dr. Brook said.
Presently, for applicable age groups, the ACS guideline favors HPV testing alone over cytology alone or cotesting, whereas the USPSTF guideline offers no preference between the three testing strategies.
Primary HPV vs. cytology testing
Dr. Brook said a single negative HPV test provides more than 95% assurance that a patient will not develop cervical cancer or a cancer precursor within the next 5 years. One negative HPV test offers similar reliability to about 3 negative cytology tests.
Switching to a 5-year testing cycle may be unsettling for patients who are used to getting a Pap test every year, but having a conversation about test accuracy can help assuage patient concerns, she said.
Still, Dr. Brook emphasized that any of the three testing strategies is ultimately acceptable.
“The take-home message here is – truly – that any of the recommended screening options will greatly reduce cervical cancer risk,” Dr. Brook said. “So, screen. And if there is any confusion or concern with your patients about which [screening strategy to use], just help them decide on any of the three. But please screen.”
Self-swabbing could improve screening in certain groups
To improve screening rates, particularly for women with poor access and those averse to a speculum exam, Dr. Brook highlighted self-swabbing primary HPV tests, which may soon be available. While no self-swabbing HPV tests are yet approved by the Food and Drug Administration, they offer a 76% sensitivity rate for cervical intraepithelial neoplasia grade 2, and a rate of 85% for CIN3, compared with 91% for physician-collected samples.
Regardless of the exact HPV test, Dr. Brook advised appropriate reflex testing.
“We need to make sure all primary HPV screening tests positive for types other than HPV-16 or -18 will require additional reflex triage testing with cytology,” Dr. Brook said in interview. “If not – if a woman has a primary HPV screening test that is positive and I cannot perform reflex cytology – I have to bring her back for an additional test and speculum exam to get cytology, which is an unnecessary burden to the patient, and also increases testing.”
Kathy L. MacLaughlin, MD, associate professor of family medicine at Mayo Clinic, Rochester, Minn., said this is one drawback to self-swabbing tests in an interview.
“If there is a positive HPV result [with a self-swabbing test], the patient will need to have a clinic appointment for Pap collection [if one of the ‘other’ 12 HPV types are identified], or be referred for a colposcopy [if HPV types 16 or 18 are identified],” Dr. MacLaughlin said. “There need to be plans in place for access to those services.”
Incidentally, it may be women who face barriers to access that need self-swabbing HPV tests the most, according to Dr. MacLaughlin.
“I think there is significant potential to improve screening rates among never-screened and underscreened women and those are the groups for whom this makes the most sense,” she said. “I don’t think anyone is suggesting that women who have the means and interest in scheduling a face-to-face visit for clinician-collected screening switch to self-screening, but it is a promising option [once FDA approved] for reaching other women and reducing disparities in screening rates.”
Dr. MacLaughlin suggested that self-screening programs could operate outside of normal business hours in a variety of settings, such as homes, community centers, and churches.
Until self-screening is an option, Dr. MacLaughlin agreed with Dr. Brook that any of the three testing strategies is suitable for screening, and recommended that primary care providers seize the opportunities presented to them.
“Individual primary care providers can improve screening rates by offering to update cervical cancer screening at a clinic appointment even if that was not the primary indication for the visit, especially for women who are long overdue,” Dr. MacLaughlin said. “If there is just no time to fit in the screening or the patient declines, then order a return visit and have the patient stop at the appointment desk as they leave.”
“I recognize we are asked to fit in more and more in less time, but I’ve found this to be effective when I have capacity in the clinic day to offer it,” she added.
Dr. Brook and Dr. MacLaughlin reported no conflicts of interest.
FROM INTERNAL MEDICINE 2021
Pregnancy increases risk for symptomatic kidney stones
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnancy increases the risk for first-time symptomatic kidney stone formation which peaks close to the time of delivery but can persist even a year later, a population-based, case-controlled study suggests.
“We suspected the risk of a kidney stone event would be high during pregnancy, but we were surprised that the risk remained high for up to a year after delivery,” senior author Andrew Rule, MD, a nephrologist at Mayo Clinic, Rochester, Minn, said in a statement from his institution.
“[So] while most kidney stones that form during pregnancy are detected early by painful passage, some may remain stable in the kidney undetected for a longer period before dislodging and [again] resulting in a painful passage,” he added.
The study was published online April 15, 2021, in the American Journal of Kidney Diseases by Charat Thongprayoon, MD, also of the Mayo Clinic, and colleagues.
“The results of this study indicate that prenatal counseling regarding kidney stones may be warranted, especially for women with other risk factors for kidney stones, such as obesity,” he noted.
First-time stone formers
The observational study included 945 first-time symptomatic kidney stone formers aged between 15 and 45 years who were compared with 1,890 age-matched female controls from the Rochester Epidemiology Project. The latter is a medical record linkage system for almost all medical care administered in Olmsted County in Minnesota.
Compared with nonpregnant women, the odds of a symptomatic kidney stone forming in a pregnant woman was similar in the first trimester (odds ratio, 0.92; P = .8), began to increase during the second trimester (OR, 2.00; P = .007), further increased during the third trimester (OR, 2.69; P = .001), and peaked at 0-3 months after delivery (OR, 3.53; P < .001). The risk returned to baseline by 1 year after delivery.
These associations persisted after adjustment for age and race or for diabetes, hypertension, and obesity. These results did not significantly differ by age, race, time period, or number of prior pregnancies.
The risk of a pregnant woman developing a symptomatic kidney stone was higher in women with obesity, compared with those of normal weight (P = .01).
And compared with women who had not been pregnant before, one prior pregnancy also increased the risk of having a symptomatic kidney stone by approximately 30% (OR, 1.29; P = .03), although two or more prior pregnancies did not significantly increase symptomatic kidney stone risk.
Thus, “it can be inferred that the odds of a symptomatic kidney stone peak around the time of delivery,” the authors emphasized. “The odds of a first-time symptomatic kidney stone then decreased over time and were fully attenuated and no longer statistically significant by 12 months after delivery.”
Dr. Thongprayoon said there are several physiologic reasons why pregnancy might contribute to kidney stone formation.
During pregnancy, ureteral compression and ureteral relaxation caused by elevated progesterone levels can cause urinary stasis.
Furthermore, increased urinary calcium excretion and elevated urine pH during pregnancy can promote calcium phosphate stone formation. It is noteworthy that almost all pregnant, first-time stone formers had calcium phosphate stones.
“During pregnancy, a kidney stone may contribute to serious complications,” Dr. Thongprayoon explained.
General dietary recommendations for preventing kidney stones include drinking abundant fluids and consuming a low-salt diet.
The study was supported by the Mayo Clinic O’Brien Urology Research Center and a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.