User login
Glucocorticoid-induced diabetes and adrenal suppression
To the Editor: We found the article by Drs. Lansang and Kramer1 on glucocorticoid-induced diabetes and adrenal suppression in the November 2011 issue to be a useful and clinically oriented review. However, we strongly believe there is an issue that should be addressed.
It is well accepted that the short cosyntropin (Cortrosyn) stimulation test is the best screening maneuver for assessing adrenocortical insufficiency. The authors state, however, that 250 μg is preferable to lower doses (10 μg or 1 μg), since these are not yet widely accepted, and refer to an article by Axelrod from 1976.2
Based on studies showing that 250 μg of cosyntropin is a pharmacologic rather than a physiologic stimulus that may overstimulate partially atrophied or mildly dysfunctional adrenal glands, multiple studies in the last 20 years have shown that the low-dose test has an equal or better result than the classic 250-μg dose test.3 Dorin et al,4 in a meta-analysis of the diagnosis of adrenocortical insufficiency that included more than 30 studies, found similar sensitivity and specificity in primary and secondary adrenal insufficiency comparing the 250-μg dose vs the low dose. In cases of mild primary adrenal failure, the low-dose test has better performance. A previous investigation in our research center contrasting 250 μg vs 10 μg proved that 10 μg had a better sensitivity than the standard dose, with excellent reproducibility and interchangeability.5 Similar findings have been shown by other authors contrasting 1 μg vs 250 μg of cosyntropin.6
We believe that the limited use of the low-dose cosyntropin test is not a matter of acceptance or performance but a consequence of the lack of vials containing lower doses of cosyntropin (1 to 10 μg), which makes this test technically challenging.2,4 The steps needed for one-dose testing and the preservation time of the preparation are strong limitations to its wide use in clinical practice and endocrine laboratories.
- Lansang MC, Hustak LK. Glucocorticoid-induced diabetes and adrenal suppression: how to detect and manage them. Cleve Clin J Med 2011; 78:748–756.
- Axelrod L. Glucocorticoid therapy. Medicine (Baltimore) 1976; 55:39–65.
- Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab 1991; 72:773–778.
- Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med 2003; 139:194–204.
- González-González JG, De la Garza-Hernández NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Pérez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol 1998; 159:275–280.
- Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamopituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab 1999; 84:838–843.
To the Editor: We found the article by Drs. Lansang and Kramer1 on glucocorticoid-induced diabetes and adrenal suppression in the November 2011 issue to be a useful and clinically oriented review. However, we strongly believe there is an issue that should be addressed.
It is well accepted that the short cosyntropin (Cortrosyn) stimulation test is the best screening maneuver for assessing adrenocortical insufficiency. The authors state, however, that 250 μg is preferable to lower doses (10 μg or 1 μg), since these are not yet widely accepted, and refer to an article by Axelrod from 1976.2
Based on studies showing that 250 μg of cosyntropin is a pharmacologic rather than a physiologic stimulus that may overstimulate partially atrophied or mildly dysfunctional adrenal glands, multiple studies in the last 20 years have shown that the low-dose test has an equal or better result than the classic 250-μg dose test.3 Dorin et al,4 in a meta-analysis of the diagnosis of adrenocortical insufficiency that included more than 30 studies, found similar sensitivity and specificity in primary and secondary adrenal insufficiency comparing the 250-μg dose vs the low dose. In cases of mild primary adrenal failure, the low-dose test has better performance. A previous investigation in our research center contrasting 250 μg vs 10 μg proved that 10 μg had a better sensitivity than the standard dose, with excellent reproducibility and interchangeability.5 Similar findings have been shown by other authors contrasting 1 μg vs 250 μg of cosyntropin.6
We believe that the limited use of the low-dose cosyntropin test is not a matter of acceptance or performance but a consequence of the lack of vials containing lower doses of cosyntropin (1 to 10 μg), which makes this test technically challenging.2,4 The steps needed for one-dose testing and the preservation time of the preparation are strong limitations to its wide use in clinical practice and endocrine laboratories.
To the Editor: We found the article by Drs. Lansang and Kramer1 on glucocorticoid-induced diabetes and adrenal suppression in the November 2011 issue to be a useful and clinically oriented review. However, we strongly believe there is an issue that should be addressed.
It is well accepted that the short cosyntropin (Cortrosyn) stimulation test is the best screening maneuver for assessing adrenocortical insufficiency. The authors state, however, that 250 μg is preferable to lower doses (10 μg or 1 μg), since these are not yet widely accepted, and refer to an article by Axelrod from 1976.2
Based on studies showing that 250 μg of cosyntropin is a pharmacologic rather than a physiologic stimulus that may overstimulate partially atrophied or mildly dysfunctional adrenal glands, multiple studies in the last 20 years have shown that the low-dose test has an equal or better result than the classic 250-μg dose test.3 Dorin et al,4 in a meta-analysis of the diagnosis of adrenocortical insufficiency that included more than 30 studies, found similar sensitivity and specificity in primary and secondary adrenal insufficiency comparing the 250-μg dose vs the low dose. In cases of mild primary adrenal failure, the low-dose test has better performance. A previous investigation in our research center contrasting 250 μg vs 10 μg proved that 10 μg had a better sensitivity than the standard dose, with excellent reproducibility and interchangeability.5 Similar findings have been shown by other authors contrasting 1 μg vs 250 μg of cosyntropin.6
We believe that the limited use of the low-dose cosyntropin test is not a matter of acceptance or performance but a consequence of the lack of vials containing lower doses of cosyntropin (1 to 10 μg), which makes this test technically challenging.2,4 The steps needed for one-dose testing and the preservation time of the preparation are strong limitations to its wide use in clinical practice and endocrine laboratories.
- Lansang MC, Hustak LK. Glucocorticoid-induced diabetes and adrenal suppression: how to detect and manage them. Cleve Clin J Med 2011; 78:748–756.
- Axelrod L. Glucocorticoid therapy. Medicine (Baltimore) 1976; 55:39–65.
- Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab 1991; 72:773–778.
- Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med 2003; 139:194–204.
- González-González JG, De la Garza-Hernández NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Pérez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol 1998; 159:275–280.
- Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamopituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab 1999; 84:838–843.
- Lansang MC, Hustak LK. Glucocorticoid-induced diabetes and adrenal suppression: how to detect and manage them. Cleve Clin J Med 2011; 78:748–756.
- Axelrod L. Glucocorticoid therapy. Medicine (Baltimore) 1976; 55:39–65.
- Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab 1991; 72:773–778.
- Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med 2003; 139:194–204.
- González-González JG, De la Garza-Hernández NE, Mancillas-Adame LG, Montes-Villarreal J, Villarreal-Pérez JZ. A high-sensitivity test in the assessment of adrenocortical insufficiency: 10 microg vs 250 microg cosyntropin dose assessment of adrenocortical insufficiency. J Endocrinol 1998; 159:275–280.
- Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamopituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab 1999; 84:838–843.
Protease inhibitors: Silver bullets for chronic hepatitis C infection?
The treatment of hepatitis c virus (HCV) infection is on the brink of major changes with the recent approval of the first direct-acting antiviral agents, the protease inhibitors boceprevir (Victrelis) and telaprevir (Incivek).
Both drugs were approved by the US Food and Drug Administration (FDA) Advisory Panel for Chronic Hepatitis C in May 2011 and are believed to significantly improve treatment outcomes for patients with HCV genotype 1 infection.
A MAJOR PUBLIC HEALTH PROBLEM
HCV infection is a major public health problem. Nearly 4 million people in the United States are infected.6,7 Most patients with acute HCV infection become chronically infected, and up to 25% eventually develop cirrhosis and its complications, making HCV infection the leading indication for liver transplantation.8–10
Chronic HCV infection has a large global impact, with 180 million people affected across all economic and social groups.11 The highest prevalence of HCV has been reported in Egypt (14%), in part due to the use of inadequately sterilized needles in mass programs to treat endemic schistosomiasis. In developed countries, hepatocellular carcinoma associated with HCV has the fastest growing cancer-related death rate.12
CURRENTLY, FEWER THAN 50% OF PATIENTS ARE CURED
The goal of HCV treatment is to eradicate the virus. However, most infected patients (especially in the United States and Europe) are infected with HCV genotype 1, which is the most difficult genotype to treat.
Successful treatment of HCV is defined as achieving a sustained virologic response—ie, the absence of detectable HCV RNA in the serum 24 weeks after completion of therapy. Once a sustained virologic response is achieved, lifetime “cure” of HCV infection is expected in more than 99% of patients.13
The current standard therapy for HCV, pegylated interferon plus ribavirin for 48 weeks, is effective in only 40% to 50% of patients with genotype 1 infection.14 Therefore, assessing predictors of response before starting treatment can help select patients who are most likely to benefit from therapy.
Viral factors associated with a sustained virologic response include HCV genotypes other than genotype 1 and a low baseline viral load.
Beneficial patient-related factors include younger age, nonblack ethnicity, low body weight (≤ 75 kg), low body mass index, absence of insulin resistance, and absence of advanced fibrosis or cirrhosis.
More recently, a single-nucleotide polymorphism near the interleukin 28B (IL28B) gene, coding for interferon lambda 3, was found to be associated with a twofold difference in the rates of sustained virologic response: patients with the favorable genotype CC were two times more likely to achieve a sustained virologic response than patients with the CT or TT genotypes.15–17
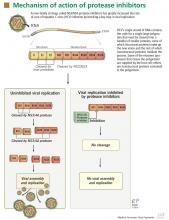
PROTEASE INHIBITORS: MECHANISM OF ACTION
NS3/4A protease inhibitors rely on the principle of end-product inhibition, in which the cleavage product of the protease (a peptide) acts to inhibit the enzyme activity; this is why they are called peptidomimetics. The active site of the NS3/4A protease is a shallow groove composed of three highly conserved amino acid residues, which may explain why protease inhibitors display high antiviral efficacy but pose a low barrier to the development of resistance.20
Protease inhibitors are prone to resistance
The development of viral resistance to protease inhibitors has been a major drawback to their use in patients with chronic HCV infection.21
HCV is a highly variable virus with many genetically distinct but closely related quasispecies circulating in the blood at any given time. Drug-resistant, mutated variants preexist within the patient’s quasispecies, but only in small quantities because of their lesser replication fitness compared with the wild-type virus.22 When direct-acting antiviral therapy is started, the quantity of the wild-type virus decreases and the mutated virus gains replication fitness. Using protease inhibitors as monotherapy selects resistant viral populations rapidly within a few days or weeks.
HCV subtypes 1a and 1b may have different resistance profiles. With genotype 1a, some resistance-associated amino acid substitutions require only one nucleotide change, but with genotype 1b, two nucleotide changes are needed, making resistance less frequent in patients with HCV genotype 1b.23
BOCEPREVIR
Boceprevir is a specific inhibitor of the HCV viral protease NS3/4A.
In phase 3 clinical trials, boceprevir 800 mg three times a day was used with pegylated interferon alfa-2b (PegIntron) 1.5 μg/kg/week and ribavirin (Rebetol) 600 to 1,400 mg daily according to body weight.
Before patients started taking boceprevir, they went through a 4-week lead-in phase, during which they received pegylated interferon and ribavirin. This schedule appeared to reduce the incidence of viral breakthrough in phase 2 trials, and it produced higher rates of sustained virologic response and lower relapse rates compared with triple therapy without a lead-in phase.
Rapid virologic response was defined as undetectable HCV RNA at week 4 of boceprevir therapy (week 8 of the whole regimen).
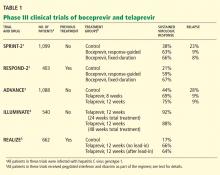
Boceprevir in previously untreated patients with HCV genotype 1: The SPRINT-2 trial
The Serine Protease Inhibitor Therapy 2 (SPRINT-2) trial1 included more than 1,000 previously untreated adults with HCV genotype 1 infection (938 nonblack patients and 159 black patients; two other nonblack patients did not receive any study drug and were not included in the analysis). In this double-blind trial, patients were randomized into three groups:
- The control group received the standard of care with pegylated interferon and ribavirin for 48 weeks
- The response-guided therapy group received boceprevir plus pegylated interferon and ribavirin for 24 weeks after the 4-week lead-in phase; if HCV RNA was undetectable from week 8 to week 24, treatment was considered complete, but if HCV RNA was detectable at any point from week 8 to week 24, pegylated interferon and ribavirin were continued for a total of 48 weeks.
- The fixed-duration therapy group received boceprevir, pegylated interferon, and ribavirin for 44 weeks after the lead-in period.
The rate of relapse was 8% and 9% in the boceprevir groups vs 23% in the control group. Patients in the boceprevir groups who had a decrease in HCV RNA of less than 1 log10 during the lead-in phase were found to have a significantly higher rate of boceprevirresistant variants than those who achieved a decrease of HCV RNA of 1 log10 or more.
Boceprevir in previously treated patients with HCV genotype 1: The RESPOND-2 trial
The Retreatment With HCV Serine Protease Inhibitor Boceprevir and PegIntron/Rebetol 2) (RESPOND-2) trial2 was designed to assess the efficacy of combined boceprevir, pegylated interferon, and ribavirin for repeat treatment of patients with HCV genotype 1. These patients had previously undergone standard treatment and had a reduction of 2 log10 or more in HCV RNA after 12 weeks of therapy but with detectable HCV RNA during the therapy period or had had a relapse (defined as undetectable HCV RNA at the end of a previous course of therapy with HCV RNA positivity thereafter). Importantly, null-responders (those who had a reduction of less than 2 log10 in HCV RNA after 12 weeks of therapy) were excluded from this trial.
After a lead-in period of interferon-ribavirin treatment for 4 weeks, 403 patients were assigned to one of three treatment groups:
- Pegylated interferon and ribavirin for 44 weeks (the control group)
- Boceprevir, pegylated interferon, and ribavirin in a response-guided regimen
- Boceprevir, pegylated interferon, and ribavirin for 44 weeks (the fixed-duration group).
Sustained virologic response was achieved in only 21% of patients in the control group. Adding boceprevir increased the rate to 59% in the response-guided therapy group and to 67% in the fixed-duration group. Previous relapsers had better rates than partial responders (69%–75% vs 40%–52%).
Importantly, patients who had a poor response to pegylated interferon and ribavirin during the lead-in phase (defined as having less than a 1-log decrease in the virus before starting boceprevir) had significantly lower rates of sustained virologic response and higher rates of resistance-associated virus variants.
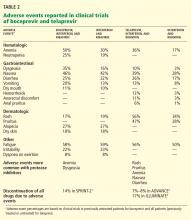
Side effects of boceprevir
Overall, boceprevir is well tolerated. The most common side effects of triple therapy are those usually seen with pegylated interferon and ribavirin, such as flulike symptoms and fatigue (Table 2). However, anemia was more frequent in the boceprevir groups in both SPRINT-2 and RESPOND-2 (45%–50% compared with 20%–29% in the control groups). Erythropoietin was allowed in these studies and was used in about 40% of patients.
The other common side effect associated with boceprevir was dysgeusia (alteration of taste). Dysgeusia was reported by approximately 40% of patients; however, most dysgeusia events were mild to moderate in intensity and did not lead to treatment cessation.
In the SPRINT-2 trial,1 the study drugs had to be discontinued in 12% to 16% of patients in the boceprevir groups because of adverse events, which was similar to the rate (16%) in the control group. Erythropoietin was allowed in this trial, and it was used in 43% of patients in the boceprevir groups compared with 24% in the control group, with discontinuation owing to anemia occurring in 2% and 1% of cases, respectively.
TELAPREVIR
Telaprevir, the other protease NS3/4A inhibitor, has also shown efficacy over current standard therapy in phase 3 clinical trials. It was used in a dose of 750 mg three times a day with pegylated interferon alfa-2a (Pegasys) 180 μg per week and ribavirin (Copegus) 1,000 to 1,200 mg daily according to body weight. A lead-in phase with pegylated interferon and ribavirin was not applied with telaprevir, as it was in the boceprevir trials. Extended rapid virologic response was defined as an undetectable HCV RNA at weeks 4 and 12 of therapy.
Telaprevir in previously untreated patients with HCV genotype 1
The ADVANCE study3 was a double-blind randomized trial assessing the efficacy and safety of telaprevir in combination with pegylated interferon and ribavirin in more than 1,000 previously untreated patients. The three treatment groups received:
- Telaprevir, pegylated interferon, and ribavirin for 8 weeks, followed by pegylated interferon and ribavirin alone for 16 weeks in patients who achieved an extended rapid virologic response (total duration of 24 weeks) or 40 weeks in patients who did not (total duration of 48 weeks)
- Telaprevir, pegylated interferon, and ribavirin for 12 weeks, followed by pegylated interferon-ribavirin alone for 12 (total of 24 weeks) or 36 weeks (total of 48 weeks) according to extended rapid virologic response
- Standard care with pegylated interferon and ribavirin for 48 weeks.
The rate of sustained virologic response was 69% in the group that received telaprevir for 8 weeks and 75% in the group that received it for 12 weeks compared with 44% in the control group (P < .0001 for both) (Table 2). Patients infected with HCV genotype 1b had a higher sustained virologic response rate (79%) than those infected with HCV genotype 1a (71%).
Sustained virologic response rates were lower in black patients and patients with bridging fibrosis or cirrhosis, but were still significantly higher in the telaprevir groups than in the control group. The results of this subset analysis were limited by small numbers of patients in each category.
In total, 57% of those who received telaprevir for 8 weeks and 58% of those who received it for 12 weeks achieved an extended rapid virologic response and were able to cut the duration of their therapy in half (from 48 weeks to 24 weeks).
The relapse rates were 9% in the telaprevir groups and 28% in the control group.
The rate of virologic failure was lower in patients who received triple therapy than in those who received interferon-ribavirin alone (8% in the group that got telaprevir for 12 weeks and 13% in the group that got it for 8 weeks, vs 32% in the control group). The failure rate was also lower in patients with HCV genotype 1b infection than in those with genotype 1a.
The ILLUMINATE study4 (Illustrating the Effects of Combination Therapy With Telaprevir) investigated whether longer duration of treatment than that given in the ADVANCE trial increased the rate of sustained virologic response. Previously untreated patients received telaprevir, interferon, and ribavirin for 12 weeks, and those who achieved an extended rapid virologic response were randomized at week 20 to continue interferonribavirin treatment for 24 or 48 weeks of total treatment.
The sustained virologic response rates in patients who achieved an extended rapid virologic response were 92% in the group that received pegylated interferon and ribavirin for 12 weeks, and 88% in those who received it for 48 weeks. Thus, the results of this study support the use of response-guided therapy for telaprevir-based regimens.
Telaprevir in previously treated patients with HCV genotype 1: The REALIZE trial
In this phase 3 placebo-controlled trial,5 622 patients with prior relapse, partial response, or null response were randomly allocated into one of three groups:
- Telaprevir for 12 weeks plus pegylated interferon and ribavirin for 48 weeks
- Lead-in for 4 weeks followed by 12 weeks of triple therapy and another 32 weeks of pegylated interferon and ribavirin
- Pegylated interferon and ribavirin for 48 weeks (the control group).
The overall sustained virologic response rates were 66% and 64%, respectively, in the telaprevir groups vs 17% in the control group (P < .0001). The sustained virologic response rates in the telaprevir groups were 83% to 88% in prior relapsers, 54% to 59% in partial responders, and 29% to 33% in null-responders. Of note, patients did not benefit from the lead-in phase.
This was the only trial to investigate the response to triple therapy in null-responders, a group in which treatment has been considered hopeless. A response rate of approximately 31% was encouraging, especially if we compare it with the 5% response rate achieved with the current standard of care with pegylated interferon and ribavirin.
Telaprevir side effects
As with boceprevir-based triple therapy, the most common adverse events were related to pegylated interferon (Table 2).
Nearly 50% of patients who receive telaprevir develop a skin rash that is primarily eczematous, can be managed with topical steroids, and usually resolves when telaprevir is discontinued. Severe rashes occurred in 3% to 6% of patients in the ADVANCE trial,3 and three suspected cases of Stevens-Johnson syndrome have been reported to the FDA.
Other side effects that were more frequent with telaprevir included pruritus, nausea, diarrhea, and anemia. On average, the hemoglobin level decreased by an additional 1 g/dL in the telaprevir treatment groups compared with the groups that received only pegylated interferon-ribavirin. Erythropoietin use was not allowed in the phase 3 telaprevir studies, and anemia was managed by ribavirin dose reduction.
In the ADVANCE trial,3 study drugs were discontinued owing to adverse events in 7% to 8% of the patients in the telaprevir groups compared with 4% in the control group. In the ILLUMINATE trial,4 17% of patients had to permanently discontinue all study drugs due to adverse events.
FDA-APPROVED TREATMENT REGIMENS FOR BOCEPREVIR AND TELAPREVIR
For treatment algorithms, see the eFigures that accompany this article online.
Boceprevir in previously untreated patients
- Week 0—Start pegylated interferon and ribavirin
- Week 4—Add boceprevir
- Week 8—Measure HCV RNA
- Week 12—Measure HCV RNA; stop treatment if it is more than 100 IU/mL
- Week 24—Measure HCV RNA; stop treatment if it is detectable
- Week 28—Stop all treatment if HCV RNA was undetectable at weeks 8 and 24
- Week 36—Measure HCV RNA; stop boceprevir
- Week 48—Stop all treatment (eFigure 1).
Boceprevir in previously treated patients
- Week 0—Start pegylated interferon and ribavirin
- Week 4—Add boceprevir
- Week 8—Measure HCV RNA
- Week 12—Measure HCV RNA; stop treatment if it is more than 100 IU/mL
- Week 24—Measure HCV RNA; stop treatment if it is detectable
- Week 36—if HCV RNA was not detectable at week 8, stop all treatment now; if HCV RNA was detectable at week 8, stop boceprevir now but continue pegylated interferon and ribavirin
- Week 48—Stop all treatment (eFigure 2).
Telaprevir in previously untreated patients and prior relapsers
- Week 0—start telaprevir, pegylated interferon, and ribavirin
- Week 4—measure HCV RNA; stop all treatment if it is more than 1,000 IU/mL
- Week 12—Stop telaprevir; measure HCV RNA; stop all treatment if HCV RNA is more than 1,000 IU/mL
- Week 24—Stop pegylated interferon and ribavirin if HCV RNA was undetectable at week 12; measure HCV RNA and stop treatment if it is detectable; otherwise, continue pegylated interferon and ribavirin
- Week 48—Stop all treatment (eFigure 3).
Telaprevir in patients who previously achieved a partial or null response
- Week 0—Start telaprevir, pegylated interferon, and ribavirin
- Week 4—Measure HCV RNA; stop treatment if it is more than 1,000 IU/mL
- Week 12—Measure HCV RNA; stop all treatment if it is more than 1,000 IU/mL; if less than 1,000 IU/mL then stop telaprevir but continue pegylated interferon and ribavirin
- Week 24—Measure HCV RNA; stop treatment if HCV RNA is detectable
- Week 48—Stop all treatment (eFigure 4).
Drug interactions with boceprevir and telaprevir
Both boceprevir and telaprevir inhibit cytochrome P450 3A (CYP3A) and thus are contraindicated in combination with drugs highly dependent on CYP3A for clearance and with drugs for which elevated plasma concentrations are associated with serious adverse events, such as atorvastatin (Lipitor), simvastatin (Zocor), sildenafil (Viagra), midazolam (Versed), and St. John’s wort. Giving potent inducers of CYP3A with boceprevir or telaprevir may lead to lower exposure and loss of efficacy of both protease inhibitors.
EMERGING THERAPIES FOR HCV
Thanks to a better understanding of the biology of HCV infection, the effort to develop new therapeutic agents started to focus on targeting specific steps of the viral life cycle, including attachment, entry into cells, replication, and release.24
Currently, more than 50 clinical trials are evaluating new direct-acting antivirals to treat HCV infection.25 Monoclonal and polyclonal antibodies that target the molecular process involved in HCV attachment and entry are being developed.26 The nonstructural protein NS5B (RNA polymerase) is intimately involved in viral replication and represents a promising target.27 Several nucleosides and nonnucleoside protease inhibitors have already entered clinical trials.
The low fidelity of the HCV replication machinery leads to a very high mutation rate, thus enabling the virus to quickly develop mutations that resist agents targeting viral enzymes.28 Therefore, a novel approach is to target host cofactors that are essential for HCV replication. An intriguing study by Lanford et al29 demonstrated that antagonizing microRNA-122 (the most abundant microRNA in the liver and an essential cofactor for viral RNA replication) by the oligonucleotide SPC3649 caused marked and prolonged reduction of HCV viremia in chronically infected chimpanzees.29
Although we are still in the early stages of drug development, the future holds great promise for newer drugs to improve the sustained virologic response, shorten the duration of treatment, improve tolerability with interferon-sparing regimens, and decrease viral resistance.
FUTURE PERSPECTIVES
With the introduction of the first direct-acting antiviral medications for HCV (boceprevir and telaprevir), 2011 will be marked as the year that changed hepatitis C treatment for the better. Triple therapy with pegylated interferon, ribavirin, and either boceprevir or telaprevir has the potential for increasing the rate of sustained virologic response to around 70% in previously untreated patients and 65% in previously treated patients who are infected with HCV genotype 1. The IL28B polymorphisms appear to play a role in the rate of sustained virologic response achieved with triple therapy, with preliminary data showing a better response rate in patients who have the CC genotype.17
These drugs will add up to $50,000 to the cost of treating hepatitis C virus infection, depending on the drug used and the length of treatment. However, they may be well worth it if they prevent liver failure and the need for transplantation.
Many questions remain, such as how to use these new regimens to treat special patient populations—for example, those with a recurrence of HCV infection after liver transplantation, those co-infected with HCV and human immunodeficiency virus, and those infected with HCV genotypes other than genotype 1.
Other direct-acting antiviral agents that specifically target the replication cycle of HCV are currently in clinical development. In fact, the future has already started with the release of the Interferon-Free Regimen for the Management of HCV (INFORM-1) study results.30 This was the first trial to evaluate an interferon-free regimen for patients with chronic HCV infection using two direct-acting antiviral drugs (the protease inhibitor danoprevir and the polymerase inhibitor RG7128), with promising results.
- Poordad F, McCone J, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1195–1206.
- Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1207–1217.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; for the ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
- Sherman KE, Flamm SL, Afdhal NH, et al; for the ILLUMINATE Study Team. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med 2011; 365:1014–1024.
- Zeuzem S, Andreone P, Pol S, et al; for the REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med 2011; 364:2417–2428.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714.
- Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology 2010; 51:729–733.
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36:S30–S34.
- Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology 2002; 36:S47–S56.
- Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002; 36:S35–S46.
- Lavanchy D. The global burden of hepatitis C. Liver Int 2009; 29(suppl 1):74–81.
- National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002—June 10–12, 2002. Hepatology 2002; 36:S3–S20.
- Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis 2011; 52:889–900.
- Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med 2006; 355:2444–2451.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461:399–401.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009; 41:1100–1104.
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010; 139:120–129.e118.
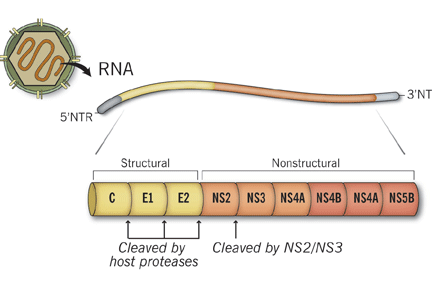
- Nielsen SU, Bassendine MF, Burt AD, Bevitt DJ, Toms GL. Characterization of the genome and structural proteins of hepatitis C virus resolved from infected human liver. J Gen Virol 2004; 85:1497–1507.
- Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology 2004; 39:5–19.
- Nelson DR. The role of triple therapy with protease inhibitors in hepatitis C virus genotype 1 naive patients. Liver Int 2011; 31(suppl 1):53–57.
- Pawlotsky JM. Treatment failure and resistance with direct-acting antiviral drugs against hepatitis C virus. Hepatology 2011; 53:1742–1751.
- Monto A, Schooley RT, Lai JC, et al. Lessons from HIV therapy applied to viral hepatitis therapy: summary of a workshop. Am J Gastroenterol 2010; 105:989–1004.
- McCown MF, Rajyaguru S, Kular S, Cammack N, Najera I. GT-1a or GT-1b subtype-specific resistance profiles for hepatitis C virus inhibitors telaprevir and HCV-796. Antimicrob Agents Chemother 2009; 53:2129–2132.
- Cholongitas E, Papatheodoridis GV. Review article: novel therapeutic options for chronic hepatitis C. Aliment Pharmacol Ther 2008; 27:866–884.
- Naggie S, Patel K, McHutchison J. Hepatitis C virus directly acting antivirals: current developments with NS3/4A HCV serine protease inhibitors. J Antimicrob Chemother 2010; 65:2063–2069.
- Mir HM, Birerdinc A, Younossi ZM. Monoclonal and polyclonal antibodies against the HCV envelope proteins. Clin Liver Dis 2009; 13:477–486.
- Birerdinc A, Younossi ZM. Emerging therapies for hepatitis C virus. Expert Opin Emerg Drugs 2010; 15:535–544.
- Khattab MA. Targeting host factors: a novel rationale for the management of hepatitis C virus. World J Gastroenterol 2009; 15:3472–3479.
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 2010; 327:198–201.
- Gane EJ, Roberts SK, Stedman CA, et al. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 2010; 376:1467–1475.
The treatment of hepatitis c virus (HCV) infection is on the brink of major changes with the recent approval of the first direct-acting antiviral agents, the protease inhibitors boceprevir (Victrelis) and telaprevir (Incivek).
Both drugs were approved by the US Food and Drug Administration (FDA) Advisory Panel for Chronic Hepatitis C in May 2011 and are believed to significantly improve treatment outcomes for patients with HCV genotype 1 infection.
A MAJOR PUBLIC HEALTH PROBLEM
HCV infection is a major public health problem. Nearly 4 million people in the United States are infected.6,7 Most patients with acute HCV infection become chronically infected, and up to 25% eventually develop cirrhosis and its complications, making HCV infection the leading indication for liver transplantation.8–10
Chronic HCV infection has a large global impact, with 180 million people affected across all economic and social groups.11 The highest prevalence of HCV has been reported in Egypt (14%), in part due to the use of inadequately sterilized needles in mass programs to treat endemic schistosomiasis. In developed countries, hepatocellular carcinoma associated with HCV has the fastest growing cancer-related death rate.12
CURRENTLY, FEWER THAN 50% OF PATIENTS ARE CURED
The goal of HCV treatment is to eradicate the virus. However, most infected patients (especially in the United States and Europe) are infected with HCV genotype 1, which is the most difficult genotype to treat.
Successful treatment of HCV is defined as achieving a sustained virologic response—ie, the absence of detectable HCV RNA in the serum 24 weeks after completion of therapy. Once a sustained virologic response is achieved, lifetime “cure” of HCV infection is expected in more than 99% of patients.13
The current standard therapy for HCV, pegylated interferon plus ribavirin for 48 weeks, is effective in only 40% to 50% of patients with genotype 1 infection.14 Therefore, assessing predictors of response before starting treatment can help select patients who are most likely to benefit from therapy.
Viral factors associated with a sustained virologic response include HCV genotypes other than genotype 1 and a low baseline viral load.
Beneficial patient-related factors include younger age, nonblack ethnicity, low body weight (≤ 75 kg), low body mass index, absence of insulin resistance, and absence of advanced fibrosis or cirrhosis.
More recently, a single-nucleotide polymorphism near the interleukin 28B (IL28B) gene, coding for interferon lambda 3, was found to be associated with a twofold difference in the rates of sustained virologic response: patients with the favorable genotype CC were two times more likely to achieve a sustained virologic response than patients with the CT or TT genotypes.15–17
PROTEASE INHIBITORS: MECHANISM OF ACTION
NS3/4A protease inhibitors rely on the principle of end-product inhibition, in which the cleavage product of the protease (a peptide) acts to inhibit the enzyme activity; this is why they are called peptidomimetics. The active site of the NS3/4A protease is a shallow groove composed of three highly conserved amino acid residues, which may explain why protease inhibitors display high antiviral efficacy but pose a low barrier to the development of resistance.20
Protease inhibitors are prone to resistance
The development of viral resistance to protease inhibitors has been a major drawback to their use in patients with chronic HCV infection.21
HCV is a highly variable virus with many genetically distinct but closely related quasispecies circulating in the blood at any given time. Drug-resistant, mutated variants preexist within the patient’s quasispecies, but only in small quantities because of their lesser replication fitness compared with the wild-type virus.22 When direct-acting antiviral therapy is started, the quantity of the wild-type virus decreases and the mutated virus gains replication fitness. Using protease inhibitors as monotherapy selects resistant viral populations rapidly within a few days or weeks.
HCV subtypes 1a and 1b may have different resistance profiles. With genotype 1a, some resistance-associated amino acid substitutions require only one nucleotide change, but with genotype 1b, two nucleotide changes are needed, making resistance less frequent in patients with HCV genotype 1b.23
BOCEPREVIR
Boceprevir is a specific inhibitor of the HCV viral protease NS3/4A.
In phase 3 clinical trials, boceprevir 800 mg three times a day was used with pegylated interferon alfa-2b (PegIntron) 1.5 μg/kg/week and ribavirin (Rebetol) 600 to 1,400 mg daily according to body weight.
Before patients started taking boceprevir, they went through a 4-week lead-in phase, during which they received pegylated interferon and ribavirin. This schedule appeared to reduce the incidence of viral breakthrough in phase 2 trials, and it produced higher rates of sustained virologic response and lower relapse rates compared with triple therapy without a lead-in phase.
Rapid virologic response was defined as undetectable HCV RNA at week 4 of boceprevir therapy (week 8 of the whole regimen).
Boceprevir in previously untreated patients with HCV genotype 1: The SPRINT-2 trial
The Serine Protease Inhibitor Therapy 2 (SPRINT-2) trial1 included more than 1,000 previously untreated adults with HCV genotype 1 infection (938 nonblack patients and 159 black patients; two other nonblack patients did not receive any study drug and were not included in the analysis). In this double-blind trial, patients were randomized into three groups:
- The control group received the standard of care with pegylated interferon and ribavirin for 48 weeks
- The response-guided therapy group received boceprevir plus pegylated interferon and ribavirin for 24 weeks after the 4-week lead-in phase; if HCV RNA was undetectable from week 8 to week 24, treatment was considered complete, but if HCV RNA was detectable at any point from week 8 to week 24, pegylated interferon and ribavirin were continued for a total of 48 weeks.
- The fixed-duration therapy group received boceprevir, pegylated interferon, and ribavirin for 44 weeks after the lead-in period.
The rate of relapse was 8% and 9% in the boceprevir groups vs 23% in the control group. Patients in the boceprevir groups who had a decrease in HCV RNA of less than 1 log10 during the lead-in phase were found to have a significantly higher rate of boceprevirresistant variants than those who achieved a decrease of HCV RNA of 1 log10 or more.
Boceprevir in previously treated patients with HCV genotype 1: The RESPOND-2 trial
The Retreatment With HCV Serine Protease Inhibitor Boceprevir and PegIntron/Rebetol 2) (RESPOND-2) trial2 was designed to assess the efficacy of combined boceprevir, pegylated interferon, and ribavirin for repeat treatment of patients with HCV genotype 1. These patients had previously undergone standard treatment and had a reduction of 2 log10 or more in HCV RNA after 12 weeks of therapy but with detectable HCV RNA during the therapy period or had had a relapse (defined as undetectable HCV RNA at the end of a previous course of therapy with HCV RNA positivity thereafter). Importantly, null-responders (those who had a reduction of less than 2 log10 in HCV RNA after 12 weeks of therapy) were excluded from this trial.
After a lead-in period of interferon-ribavirin treatment for 4 weeks, 403 patients were assigned to one of three treatment groups:
- Pegylated interferon and ribavirin for 44 weeks (the control group)
- Boceprevir, pegylated interferon, and ribavirin in a response-guided regimen
- Boceprevir, pegylated interferon, and ribavirin for 44 weeks (the fixed-duration group).
Sustained virologic response was achieved in only 21% of patients in the control group. Adding boceprevir increased the rate to 59% in the response-guided therapy group and to 67% in the fixed-duration group. Previous relapsers had better rates than partial responders (69%–75% vs 40%–52%).
Importantly, patients who had a poor response to pegylated interferon and ribavirin during the lead-in phase (defined as having less than a 1-log decrease in the virus before starting boceprevir) had significantly lower rates of sustained virologic response and higher rates of resistance-associated virus variants.
Side effects of boceprevir
Overall, boceprevir is well tolerated. The most common side effects of triple therapy are those usually seen with pegylated interferon and ribavirin, such as flulike symptoms and fatigue (Table 2). However, anemia was more frequent in the boceprevir groups in both SPRINT-2 and RESPOND-2 (45%–50% compared with 20%–29% in the control groups). Erythropoietin was allowed in these studies and was used in about 40% of patients.
The other common side effect associated with boceprevir was dysgeusia (alteration of taste). Dysgeusia was reported by approximately 40% of patients; however, most dysgeusia events were mild to moderate in intensity and did not lead to treatment cessation.
In the SPRINT-2 trial,1 the study drugs had to be discontinued in 12% to 16% of patients in the boceprevir groups because of adverse events, which was similar to the rate (16%) in the control group. Erythropoietin was allowed in this trial, and it was used in 43% of patients in the boceprevir groups compared with 24% in the control group, with discontinuation owing to anemia occurring in 2% and 1% of cases, respectively.
TELAPREVIR
Telaprevir, the other protease NS3/4A inhibitor, has also shown efficacy over current standard therapy in phase 3 clinical trials. It was used in a dose of 750 mg three times a day with pegylated interferon alfa-2a (Pegasys) 180 μg per week and ribavirin (Copegus) 1,000 to 1,200 mg daily according to body weight. A lead-in phase with pegylated interferon and ribavirin was not applied with telaprevir, as it was in the boceprevir trials. Extended rapid virologic response was defined as an undetectable HCV RNA at weeks 4 and 12 of therapy.
Telaprevir in previously untreated patients with HCV genotype 1
The ADVANCE study3 was a double-blind randomized trial assessing the efficacy and safety of telaprevir in combination with pegylated interferon and ribavirin in more than 1,000 previously untreated patients. The three treatment groups received:
- Telaprevir, pegylated interferon, and ribavirin for 8 weeks, followed by pegylated interferon and ribavirin alone for 16 weeks in patients who achieved an extended rapid virologic response (total duration of 24 weeks) or 40 weeks in patients who did not (total duration of 48 weeks)
- Telaprevir, pegylated interferon, and ribavirin for 12 weeks, followed by pegylated interferon-ribavirin alone for 12 (total of 24 weeks) or 36 weeks (total of 48 weeks) according to extended rapid virologic response
- Standard care with pegylated interferon and ribavirin for 48 weeks.
The rate of sustained virologic response was 69% in the group that received telaprevir for 8 weeks and 75% in the group that received it for 12 weeks compared with 44% in the control group (P < .0001 for both) (Table 2). Patients infected with HCV genotype 1b had a higher sustained virologic response rate (79%) than those infected with HCV genotype 1a (71%).
Sustained virologic response rates were lower in black patients and patients with bridging fibrosis or cirrhosis, but were still significantly higher in the telaprevir groups than in the control group. The results of this subset analysis were limited by small numbers of patients in each category.
In total, 57% of those who received telaprevir for 8 weeks and 58% of those who received it for 12 weeks achieved an extended rapid virologic response and were able to cut the duration of their therapy in half (from 48 weeks to 24 weeks).
The relapse rates were 9% in the telaprevir groups and 28% in the control group.
The rate of virologic failure was lower in patients who received triple therapy than in those who received interferon-ribavirin alone (8% in the group that got telaprevir for 12 weeks and 13% in the group that got it for 8 weeks, vs 32% in the control group). The failure rate was also lower in patients with HCV genotype 1b infection than in those with genotype 1a.
The ILLUMINATE study4 (Illustrating the Effects of Combination Therapy With Telaprevir) investigated whether longer duration of treatment than that given in the ADVANCE trial increased the rate of sustained virologic response. Previously untreated patients received telaprevir, interferon, and ribavirin for 12 weeks, and those who achieved an extended rapid virologic response were randomized at week 20 to continue interferonribavirin treatment for 24 or 48 weeks of total treatment.
The sustained virologic response rates in patients who achieved an extended rapid virologic response were 92% in the group that received pegylated interferon and ribavirin for 12 weeks, and 88% in those who received it for 48 weeks. Thus, the results of this study support the use of response-guided therapy for telaprevir-based regimens.
Telaprevir in previously treated patients with HCV genotype 1: The REALIZE trial
In this phase 3 placebo-controlled trial,5 622 patients with prior relapse, partial response, or null response were randomly allocated into one of three groups:
- Telaprevir for 12 weeks plus pegylated interferon and ribavirin for 48 weeks
- Lead-in for 4 weeks followed by 12 weeks of triple therapy and another 32 weeks of pegylated interferon and ribavirin
- Pegylated interferon and ribavirin for 48 weeks (the control group).
The overall sustained virologic response rates were 66% and 64%, respectively, in the telaprevir groups vs 17% in the control group (P < .0001). The sustained virologic response rates in the telaprevir groups were 83% to 88% in prior relapsers, 54% to 59% in partial responders, and 29% to 33% in null-responders. Of note, patients did not benefit from the lead-in phase.
This was the only trial to investigate the response to triple therapy in null-responders, a group in which treatment has been considered hopeless. A response rate of approximately 31% was encouraging, especially if we compare it with the 5% response rate achieved with the current standard of care with pegylated interferon and ribavirin.
Telaprevir side effects
As with boceprevir-based triple therapy, the most common adverse events were related to pegylated interferon (Table 2).
Nearly 50% of patients who receive telaprevir develop a skin rash that is primarily eczematous, can be managed with topical steroids, and usually resolves when telaprevir is discontinued. Severe rashes occurred in 3% to 6% of patients in the ADVANCE trial,3 and three suspected cases of Stevens-Johnson syndrome have been reported to the FDA.
Other side effects that were more frequent with telaprevir included pruritus, nausea, diarrhea, and anemia. On average, the hemoglobin level decreased by an additional 1 g/dL in the telaprevir treatment groups compared with the groups that received only pegylated interferon-ribavirin. Erythropoietin use was not allowed in the phase 3 telaprevir studies, and anemia was managed by ribavirin dose reduction.
In the ADVANCE trial,3 study drugs were discontinued owing to adverse events in 7% to 8% of the patients in the telaprevir groups compared with 4% in the control group. In the ILLUMINATE trial,4 17% of patients had to permanently discontinue all study drugs due to adverse events.
FDA-APPROVED TREATMENT REGIMENS FOR BOCEPREVIR AND TELAPREVIR
For treatment algorithms, see the eFigures that accompany this article online.
Boceprevir in previously untreated patients
- Week 0—Start pegylated interferon and ribavirin
- Week 4—Add boceprevir
- Week 8—Measure HCV RNA
- Week 12—Measure HCV RNA; stop treatment if it is more than 100 IU/mL
- Week 24—Measure HCV RNA; stop treatment if it is detectable
- Week 28—Stop all treatment if HCV RNA was undetectable at weeks 8 and 24
- Week 36—Measure HCV RNA; stop boceprevir
- Week 48—Stop all treatment (eFigure 1).
Boceprevir in previously treated patients
- Week 0—Start pegylated interferon and ribavirin
- Week 4—Add boceprevir
- Week 8—Measure HCV RNA
- Week 12—Measure HCV RNA; stop treatment if it is more than 100 IU/mL
- Week 24—Measure HCV RNA; stop treatment if it is detectable
- Week 36—if HCV RNA was not detectable at week 8, stop all treatment now; if HCV RNA was detectable at week 8, stop boceprevir now but continue pegylated interferon and ribavirin
- Week 48—Stop all treatment (eFigure 2).
Telaprevir in previously untreated patients and prior relapsers
- Week 0—start telaprevir, pegylated interferon, and ribavirin
- Week 4—measure HCV RNA; stop all treatment if it is more than 1,000 IU/mL
- Week 12—Stop telaprevir; measure HCV RNA; stop all treatment if HCV RNA is more than 1,000 IU/mL
- Week 24—Stop pegylated interferon and ribavirin if HCV RNA was undetectable at week 12; measure HCV RNA and stop treatment if it is detectable; otherwise, continue pegylated interferon and ribavirin
- Week 48—Stop all treatment (eFigure 3).
Telaprevir in patients who previously achieved a partial or null response
- Week 0—Start telaprevir, pegylated interferon, and ribavirin
- Week 4—Measure HCV RNA; stop treatment if it is more than 1,000 IU/mL
- Week 12—Measure HCV RNA; stop all treatment if it is more than 1,000 IU/mL; if less than 1,000 IU/mL then stop telaprevir but continue pegylated interferon and ribavirin
- Week 24—Measure HCV RNA; stop treatment if HCV RNA is detectable
- Week 48—Stop all treatment (eFigure 4).
Drug interactions with boceprevir and telaprevir
Both boceprevir and telaprevir inhibit cytochrome P450 3A (CYP3A) and thus are contraindicated in combination with drugs highly dependent on CYP3A for clearance and with drugs for which elevated plasma concentrations are associated with serious adverse events, such as atorvastatin (Lipitor), simvastatin (Zocor), sildenafil (Viagra), midazolam (Versed), and St. John’s wort. Giving potent inducers of CYP3A with boceprevir or telaprevir may lead to lower exposure and loss of efficacy of both protease inhibitors.
EMERGING THERAPIES FOR HCV
Thanks to a better understanding of the biology of HCV infection, the effort to develop new therapeutic agents started to focus on targeting specific steps of the viral life cycle, including attachment, entry into cells, replication, and release.24
Currently, more than 50 clinical trials are evaluating new direct-acting antivirals to treat HCV infection.25 Monoclonal and polyclonal antibodies that target the molecular process involved in HCV attachment and entry are being developed.26 The nonstructural protein NS5B (RNA polymerase) is intimately involved in viral replication and represents a promising target.27 Several nucleosides and nonnucleoside protease inhibitors have already entered clinical trials.
The low fidelity of the HCV replication machinery leads to a very high mutation rate, thus enabling the virus to quickly develop mutations that resist agents targeting viral enzymes.28 Therefore, a novel approach is to target host cofactors that are essential for HCV replication. An intriguing study by Lanford et al29 demonstrated that antagonizing microRNA-122 (the most abundant microRNA in the liver and an essential cofactor for viral RNA replication) by the oligonucleotide SPC3649 caused marked and prolonged reduction of HCV viremia in chronically infected chimpanzees.29
Although we are still in the early stages of drug development, the future holds great promise for newer drugs to improve the sustained virologic response, shorten the duration of treatment, improve tolerability with interferon-sparing regimens, and decrease viral resistance.
FUTURE PERSPECTIVES
With the introduction of the first direct-acting antiviral medications for HCV (boceprevir and telaprevir), 2011 will be marked as the year that changed hepatitis C treatment for the better. Triple therapy with pegylated interferon, ribavirin, and either boceprevir or telaprevir has the potential for increasing the rate of sustained virologic response to around 70% in previously untreated patients and 65% in previously treated patients who are infected with HCV genotype 1. The IL28B polymorphisms appear to play a role in the rate of sustained virologic response achieved with triple therapy, with preliminary data showing a better response rate in patients who have the CC genotype.17
These drugs will add up to $50,000 to the cost of treating hepatitis C virus infection, depending on the drug used and the length of treatment. However, they may be well worth it if they prevent liver failure and the need for transplantation.
Many questions remain, such as how to use these new regimens to treat special patient populations—for example, those with a recurrence of HCV infection after liver transplantation, those co-infected with HCV and human immunodeficiency virus, and those infected with HCV genotypes other than genotype 1.
Other direct-acting antiviral agents that specifically target the replication cycle of HCV are currently in clinical development. In fact, the future has already started with the release of the Interferon-Free Regimen for the Management of HCV (INFORM-1) study results.30 This was the first trial to evaluate an interferon-free regimen for patients with chronic HCV infection using two direct-acting antiviral drugs (the protease inhibitor danoprevir and the polymerase inhibitor RG7128), with promising results.
The treatment of hepatitis c virus (HCV) infection is on the brink of major changes with the recent approval of the first direct-acting antiviral agents, the protease inhibitors boceprevir (Victrelis) and telaprevir (Incivek).
Both drugs were approved by the US Food and Drug Administration (FDA) Advisory Panel for Chronic Hepatitis C in May 2011 and are believed to significantly improve treatment outcomes for patients with HCV genotype 1 infection.
A MAJOR PUBLIC HEALTH PROBLEM
HCV infection is a major public health problem. Nearly 4 million people in the United States are infected.6,7 Most patients with acute HCV infection become chronically infected, and up to 25% eventually develop cirrhosis and its complications, making HCV infection the leading indication for liver transplantation.8–10
Chronic HCV infection has a large global impact, with 180 million people affected across all economic and social groups.11 The highest prevalence of HCV has been reported in Egypt (14%), in part due to the use of inadequately sterilized needles in mass programs to treat endemic schistosomiasis. In developed countries, hepatocellular carcinoma associated with HCV has the fastest growing cancer-related death rate.12
CURRENTLY, FEWER THAN 50% OF PATIENTS ARE CURED
The goal of HCV treatment is to eradicate the virus. However, most infected patients (especially in the United States and Europe) are infected with HCV genotype 1, which is the most difficult genotype to treat.
Successful treatment of HCV is defined as achieving a sustained virologic response—ie, the absence of detectable HCV RNA in the serum 24 weeks after completion of therapy. Once a sustained virologic response is achieved, lifetime “cure” of HCV infection is expected in more than 99% of patients.13
The current standard therapy for HCV, pegylated interferon plus ribavirin for 48 weeks, is effective in only 40% to 50% of patients with genotype 1 infection.14 Therefore, assessing predictors of response before starting treatment can help select patients who are most likely to benefit from therapy.
Viral factors associated with a sustained virologic response include HCV genotypes other than genotype 1 and a low baseline viral load.
Beneficial patient-related factors include younger age, nonblack ethnicity, low body weight (≤ 75 kg), low body mass index, absence of insulin resistance, and absence of advanced fibrosis or cirrhosis.
More recently, a single-nucleotide polymorphism near the interleukin 28B (IL28B) gene, coding for interferon lambda 3, was found to be associated with a twofold difference in the rates of sustained virologic response: patients with the favorable genotype CC were two times more likely to achieve a sustained virologic response than patients with the CT or TT genotypes.15–17
PROTEASE INHIBITORS: MECHANISM OF ACTION
NS3/4A protease inhibitors rely on the principle of end-product inhibition, in which the cleavage product of the protease (a peptide) acts to inhibit the enzyme activity; this is why they are called peptidomimetics. The active site of the NS3/4A protease is a shallow groove composed of three highly conserved amino acid residues, which may explain why protease inhibitors display high antiviral efficacy but pose a low barrier to the development of resistance.20
Protease inhibitors are prone to resistance
The development of viral resistance to protease inhibitors has been a major drawback to their use in patients with chronic HCV infection.21
HCV is a highly variable virus with many genetically distinct but closely related quasispecies circulating in the blood at any given time. Drug-resistant, mutated variants preexist within the patient’s quasispecies, but only in small quantities because of their lesser replication fitness compared with the wild-type virus.22 When direct-acting antiviral therapy is started, the quantity of the wild-type virus decreases and the mutated virus gains replication fitness. Using protease inhibitors as monotherapy selects resistant viral populations rapidly within a few days or weeks.
HCV subtypes 1a and 1b may have different resistance profiles. With genotype 1a, some resistance-associated amino acid substitutions require only one nucleotide change, but with genotype 1b, two nucleotide changes are needed, making resistance less frequent in patients with HCV genotype 1b.23
BOCEPREVIR
Boceprevir is a specific inhibitor of the HCV viral protease NS3/4A.
In phase 3 clinical trials, boceprevir 800 mg three times a day was used with pegylated interferon alfa-2b (PegIntron) 1.5 μg/kg/week and ribavirin (Rebetol) 600 to 1,400 mg daily according to body weight.
Before patients started taking boceprevir, they went through a 4-week lead-in phase, during which they received pegylated interferon and ribavirin. This schedule appeared to reduce the incidence of viral breakthrough in phase 2 trials, and it produced higher rates of sustained virologic response and lower relapse rates compared with triple therapy without a lead-in phase.
Rapid virologic response was defined as undetectable HCV RNA at week 4 of boceprevir therapy (week 8 of the whole regimen).
Boceprevir in previously untreated patients with HCV genotype 1: The SPRINT-2 trial
The Serine Protease Inhibitor Therapy 2 (SPRINT-2) trial1 included more than 1,000 previously untreated adults with HCV genotype 1 infection (938 nonblack patients and 159 black patients; two other nonblack patients did not receive any study drug and were not included in the analysis). In this double-blind trial, patients were randomized into three groups:
- The control group received the standard of care with pegylated interferon and ribavirin for 48 weeks
- The response-guided therapy group received boceprevir plus pegylated interferon and ribavirin for 24 weeks after the 4-week lead-in phase; if HCV RNA was undetectable from week 8 to week 24, treatment was considered complete, but if HCV RNA was detectable at any point from week 8 to week 24, pegylated interferon and ribavirin were continued for a total of 48 weeks.
- The fixed-duration therapy group received boceprevir, pegylated interferon, and ribavirin for 44 weeks after the lead-in period.
The rate of relapse was 8% and 9% in the boceprevir groups vs 23% in the control group. Patients in the boceprevir groups who had a decrease in HCV RNA of less than 1 log10 during the lead-in phase were found to have a significantly higher rate of boceprevirresistant variants than those who achieved a decrease of HCV RNA of 1 log10 or more.
Boceprevir in previously treated patients with HCV genotype 1: The RESPOND-2 trial
The Retreatment With HCV Serine Protease Inhibitor Boceprevir and PegIntron/Rebetol 2) (RESPOND-2) trial2 was designed to assess the efficacy of combined boceprevir, pegylated interferon, and ribavirin for repeat treatment of patients with HCV genotype 1. These patients had previously undergone standard treatment and had a reduction of 2 log10 or more in HCV RNA after 12 weeks of therapy but with detectable HCV RNA during the therapy period or had had a relapse (defined as undetectable HCV RNA at the end of a previous course of therapy with HCV RNA positivity thereafter). Importantly, null-responders (those who had a reduction of less than 2 log10 in HCV RNA after 12 weeks of therapy) were excluded from this trial.
After a lead-in period of interferon-ribavirin treatment for 4 weeks, 403 patients were assigned to one of three treatment groups:
- Pegylated interferon and ribavirin for 44 weeks (the control group)
- Boceprevir, pegylated interferon, and ribavirin in a response-guided regimen
- Boceprevir, pegylated interferon, and ribavirin for 44 weeks (the fixed-duration group).
Sustained virologic response was achieved in only 21% of patients in the control group. Adding boceprevir increased the rate to 59% in the response-guided therapy group and to 67% in the fixed-duration group. Previous relapsers had better rates than partial responders (69%–75% vs 40%–52%).
Importantly, patients who had a poor response to pegylated interferon and ribavirin during the lead-in phase (defined as having less than a 1-log decrease in the virus before starting boceprevir) had significantly lower rates of sustained virologic response and higher rates of resistance-associated virus variants.
Side effects of boceprevir
Overall, boceprevir is well tolerated. The most common side effects of triple therapy are those usually seen with pegylated interferon and ribavirin, such as flulike symptoms and fatigue (Table 2). However, anemia was more frequent in the boceprevir groups in both SPRINT-2 and RESPOND-2 (45%–50% compared with 20%–29% in the control groups). Erythropoietin was allowed in these studies and was used in about 40% of patients.
The other common side effect associated with boceprevir was dysgeusia (alteration of taste). Dysgeusia was reported by approximately 40% of patients; however, most dysgeusia events were mild to moderate in intensity and did not lead to treatment cessation.
In the SPRINT-2 trial,1 the study drugs had to be discontinued in 12% to 16% of patients in the boceprevir groups because of adverse events, which was similar to the rate (16%) in the control group. Erythropoietin was allowed in this trial, and it was used in 43% of patients in the boceprevir groups compared with 24% in the control group, with discontinuation owing to anemia occurring in 2% and 1% of cases, respectively.
TELAPREVIR
Telaprevir, the other protease NS3/4A inhibitor, has also shown efficacy over current standard therapy in phase 3 clinical trials. It was used in a dose of 750 mg three times a day with pegylated interferon alfa-2a (Pegasys) 180 μg per week and ribavirin (Copegus) 1,000 to 1,200 mg daily according to body weight. A lead-in phase with pegylated interferon and ribavirin was not applied with telaprevir, as it was in the boceprevir trials. Extended rapid virologic response was defined as an undetectable HCV RNA at weeks 4 and 12 of therapy.
Telaprevir in previously untreated patients with HCV genotype 1
The ADVANCE study3 was a double-blind randomized trial assessing the efficacy and safety of telaprevir in combination with pegylated interferon and ribavirin in more than 1,000 previously untreated patients. The three treatment groups received:
- Telaprevir, pegylated interferon, and ribavirin for 8 weeks, followed by pegylated interferon and ribavirin alone for 16 weeks in patients who achieved an extended rapid virologic response (total duration of 24 weeks) or 40 weeks in patients who did not (total duration of 48 weeks)
- Telaprevir, pegylated interferon, and ribavirin for 12 weeks, followed by pegylated interferon-ribavirin alone for 12 (total of 24 weeks) or 36 weeks (total of 48 weeks) according to extended rapid virologic response
- Standard care with pegylated interferon and ribavirin for 48 weeks.
The rate of sustained virologic response was 69% in the group that received telaprevir for 8 weeks and 75% in the group that received it for 12 weeks compared with 44% in the control group (P < .0001 for both) (Table 2). Patients infected with HCV genotype 1b had a higher sustained virologic response rate (79%) than those infected with HCV genotype 1a (71%).
Sustained virologic response rates were lower in black patients and patients with bridging fibrosis or cirrhosis, but were still significantly higher in the telaprevir groups than in the control group. The results of this subset analysis were limited by small numbers of patients in each category.
In total, 57% of those who received telaprevir for 8 weeks and 58% of those who received it for 12 weeks achieved an extended rapid virologic response and were able to cut the duration of their therapy in half (from 48 weeks to 24 weeks).
The relapse rates were 9% in the telaprevir groups and 28% in the control group.
The rate of virologic failure was lower in patients who received triple therapy than in those who received interferon-ribavirin alone (8% in the group that got telaprevir for 12 weeks and 13% in the group that got it for 8 weeks, vs 32% in the control group). The failure rate was also lower in patients with HCV genotype 1b infection than in those with genotype 1a.
The ILLUMINATE study4 (Illustrating the Effects of Combination Therapy With Telaprevir) investigated whether longer duration of treatment than that given in the ADVANCE trial increased the rate of sustained virologic response. Previously untreated patients received telaprevir, interferon, and ribavirin for 12 weeks, and those who achieved an extended rapid virologic response were randomized at week 20 to continue interferonribavirin treatment for 24 or 48 weeks of total treatment.
The sustained virologic response rates in patients who achieved an extended rapid virologic response were 92% in the group that received pegylated interferon and ribavirin for 12 weeks, and 88% in those who received it for 48 weeks. Thus, the results of this study support the use of response-guided therapy for telaprevir-based regimens.
Telaprevir in previously treated patients with HCV genotype 1: The REALIZE trial
In this phase 3 placebo-controlled trial,5 622 patients with prior relapse, partial response, or null response were randomly allocated into one of three groups:
- Telaprevir for 12 weeks plus pegylated interferon and ribavirin for 48 weeks
- Lead-in for 4 weeks followed by 12 weeks of triple therapy and another 32 weeks of pegylated interferon and ribavirin
- Pegylated interferon and ribavirin for 48 weeks (the control group).
The overall sustained virologic response rates were 66% and 64%, respectively, in the telaprevir groups vs 17% in the control group (P < .0001). The sustained virologic response rates in the telaprevir groups were 83% to 88% in prior relapsers, 54% to 59% in partial responders, and 29% to 33% in null-responders. Of note, patients did not benefit from the lead-in phase.
This was the only trial to investigate the response to triple therapy in null-responders, a group in which treatment has been considered hopeless. A response rate of approximately 31% was encouraging, especially if we compare it with the 5% response rate achieved with the current standard of care with pegylated interferon and ribavirin.
Telaprevir side effects
As with boceprevir-based triple therapy, the most common adverse events were related to pegylated interferon (Table 2).
Nearly 50% of patients who receive telaprevir develop a skin rash that is primarily eczematous, can be managed with topical steroids, and usually resolves when telaprevir is discontinued. Severe rashes occurred in 3% to 6% of patients in the ADVANCE trial,3 and three suspected cases of Stevens-Johnson syndrome have been reported to the FDA.
Other side effects that were more frequent with telaprevir included pruritus, nausea, diarrhea, and anemia. On average, the hemoglobin level decreased by an additional 1 g/dL in the telaprevir treatment groups compared with the groups that received only pegylated interferon-ribavirin. Erythropoietin use was not allowed in the phase 3 telaprevir studies, and anemia was managed by ribavirin dose reduction.
In the ADVANCE trial,3 study drugs were discontinued owing to adverse events in 7% to 8% of the patients in the telaprevir groups compared with 4% in the control group. In the ILLUMINATE trial,4 17% of patients had to permanently discontinue all study drugs due to adverse events.
FDA-APPROVED TREATMENT REGIMENS FOR BOCEPREVIR AND TELAPREVIR
For treatment algorithms, see the eFigures that accompany this article online.
Boceprevir in previously untreated patients
- Week 0—Start pegylated interferon and ribavirin
- Week 4—Add boceprevir
- Week 8—Measure HCV RNA
- Week 12—Measure HCV RNA; stop treatment if it is more than 100 IU/mL
- Week 24—Measure HCV RNA; stop treatment if it is detectable
- Week 28—Stop all treatment if HCV RNA was undetectable at weeks 8 and 24
- Week 36—Measure HCV RNA; stop boceprevir
- Week 48—Stop all treatment (eFigure 1).
Boceprevir in previously treated patients
- Week 0—Start pegylated interferon and ribavirin
- Week 4—Add boceprevir
- Week 8—Measure HCV RNA
- Week 12—Measure HCV RNA; stop treatment if it is more than 100 IU/mL
- Week 24—Measure HCV RNA; stop treatment if it is detectable
- Week 36—if HCV RNA was not detectable at week 8, stop all treatment now; if HCV RNA was detectable at week 8, stop boceprevir now but continue pegylated interferon and ribavirin
- Week 48—Stop all treatment (eFigure 2).
Telaprevir in previously untreated patients and prior relapsers
- Week 0—start telaprevir, pegylated interferon, and ribavirin
- Week 4—measure HCV RNA; stop all treatment if it is more than 1,000 IU/mL
- Week 12—Stop telaprevir; measure HCV RNA; stop all treatment if HCV RNA is more than 1,000 IU/mL
- Week 24—Stop pegylated interferon and ribavirin if HCV RNA was undetectable at week 12; measure HCV RNA and stop treatment if it is detectable; otherwise, continue pegylated interferon and ribavirin
- Week 48—Stop all treatment (eFigure 3).
Telaprevir in patients who previously achieved a partial or null response
- Week 0—Start telaprevir, pegylated interferon, and ribavirin
- Week 4—Measure HCV RNA; stop treatment if it is more than 1,000 IU/mL
- Week 12—Measure HCV RNA; stop all treatment if it is more than 1,000 IU/mL; if less than 1,000 IU/mL then stop telaprevir but continue pegylated interferon and ribavirin
- Week 24—Measure HCV RNA; stop treatment if HCV RNA is detectable
- Week 48—Stop all treatment (eFigure 4).
Drug interactions with boceprevir and telaprevir
Both boceprevir and telaprevir inhibit cytochrome P450 3A (CYP3A) and thus are contraindicated in combination with drugs highly dependent on CYP3A for clearance and with drugs for which elevated plasma concentrations are associated with serious adverse events, such as atorvastatin (Lipitor), simvastatin (Zocor), sildenafil (Viagra), midazolam (Versed), and St. John’s wort. Giving potent inducers of CYP3A with boceprevir or telaprevir may lead to lower exposure and loss of efficacy of both protease inhibitors.
EMERGING THERAPIES FOR HCV
Thanks to a better understanding of the biology of HCV infection, the effort to develop new therapeutic agents started to focus on targeting specific steps of the viral life cycle, including attachment, entry into cells, replication, and release.24
Currently, more than 50 clinical trials are evaluating new direct-acting antivirals to treat HCV infection.25 Monoclonal and polyclonal antibodies that target the molecular process involved in HCV attachment and entry are being developed.26 The nonstructural protein NS5B (RNA polymerase) is intimately involved in viral replication and represents a promising target.27 Several nucleosides and nonnucleoside protease inhibitors have already entered clinical trials.
The low fidelity of the HCV replication machinery leads to a very high mutation rate, thus enabling the virus to quickly develop mutations that resist agents targeting viral enzymes.28 Therefore, a novel approach is to target host cofactors that are essential for HCV replication. An intriguing study by Lanford et al29 demonstrated that antagonizing microRNA-122 (the most abundant microRNA in the liver and an essential cofactor for viral RNA replication) by the oligonucleotide SPC3649 caused marked and prolonged reduction of HCV viremia in chronically infected chimpanzees.29
Although we are still in the early stages of drug development, the future holds great promise for newer drugs to improve the sustained virologic response, shorten the duration of treatment, improve tolerability with interferon-sparing regimens, and decrease viral resistance.
FUTURE PERSPECTIVES
With the introduction of the first direct-acting antiviral medications for HCV (boceprevir and telaprevir), 2011 will be marked as the year that changed hepatitis C treatment for the better. Triple therapy with pegylated interferon, ribavirin, and either boceprevir or telaprevir has the potential for increasing the rate of sustained virologic response to around 70% in previously untreated patients and 65% in previously treated patients who are infected with HCV genotype 1. The IL28B polymorphisms appear to play a role in the rate of sustained virologic response achieved with triple therapy, with preliminary data showing a better response rate in patients who have the CC genotype.17
These drugs will add up to $50,000 to the cost of treating hepatitis C virus infection, depending on the drug used and the length of treatment. However, they may be well worth it if they prevent liver failure and the need for transplantation.
Many questions remain, such as how to use these new regimens to treat special patient populations—for example, those with a recurrence of HCV infection after liver transplantation, those co-infected with HCV and human immunodeficiency virus, and those infected with HCV genotypes other than genotype 1.
Other direct-acting antiviral agents that specifically target the replication cycle of HCV are currently in clinical development. In fact, the future has already started with the release of the Interferon-Free Regimen for the Management of HCV (INFORM-1) study results.30 This was the first trial to evaluate an interferon-free regimen for patients with chronic HCV infection using two direct-acting antiviral drugs (the protease inhibitor danoprevir and the polymerase inhibitor RG7128), with promising results.
- Poordad F, McCone J, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1195–1206.
- Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1207–1217.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; for the ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
- Sherman KE, Flamm SL, Afdhal NH, et al; for the ILLUMINATE Study Team. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med 2011; 365:1014–1024.
- Zeuzem S, Andreone P, Pol S, et al; for the REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med 2011; 364:2417–2428.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714.
- Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology 2010; 51:729–733.
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36:S30–S34.
- Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology 2002; 36:S47–S56.
- Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002; 36:S35–S46.
- Lavanchy D. The global burden of hepatitis C. Liver Int 2009; 29(suppl 1):74–81.
- National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002—June 10–12, 2002. Hepatology 2002; 36:S3–S20.
- Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis 2011; 52:889–900.
- Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med 2006; 355:2444–2451.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461:399–401.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009; 41:1100–1104.
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010; 139:120–129.e118.
- Nielsen SU, Bassendine MF, Burt AD, Bevitt DJ, Toms GL. Characterization of the genome and structural proteins of hepatitis C virus resolved from infected human liver. J Gen Virol 2004; 85:1497–1507.
- Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology 2004; 39:5–19.
- Nelson DR. The role of triple therapy with protease inhibitors in hepatitis C virus genotype 1 naive patients. Liver Int 2011; 31(suppl 1):53–57.
- Pawlotsky JM. Treatment failure and resistance with direct-acting antiviral drugs against hepatitis C virus. Hepatology 2011; 53:1742–1751.
- Monto A, Schooley RT, Lai JC, et al. Lessons from HIV therapy applied to viral hepatitis therapy: summary of a workshop. Am J Gastroenterol 2010; 105:989–1004.
- McCown MF, Rajyaguru S, Kular S, Cammack N, Najera I. GT-1a or GT-1b subtype-specific resistance profiles for hepatitis C virus inhibitors telaprevir and HCV-796. Antimicrob Agents Chemother 2009; 53:2129–2132.
- Cholongitas E, Papatheodoridis GV. Review article: novel therapeutic options for chronic hepatitis C. Aliment Pharmacol Ther 2008; 27:866–884.
- Naggie S, Patel K, McHutchison J. Hepatitis C virus directly acting antivirals: current developments with NS3/4A HCV serine protease inhibitors. J Antimicrob Chemother 2010; 65:2063–2069.
- Mir HM, Birerdinc A, Younossi ZM. Monoclonal and polyclonal antibodies against the HCV envelope proteins. Clin Liver Dis 2009; 13:477–486.
- Birerdinc A, Younossi ZM. Emerging therapies for hepatitis C virus. Expert Opin Emerg Drugs 2010; 15:535–544.
- Khattab MA. Targeting host factors: a novel rationale for the management of hepatitis C virus. World J Gastroenterol 2009; 15:3472–3479.
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 2010; 327:198–201.
- Gane EJ, Roberts SK, Stedman CA, et al. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 2010; 376:1467–1475.
- Poordad F, McCone J, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1195–1206.
- Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011; 364:1207–1217.
- Jacobson IM, McHutchison JG, Dusheiko G, et al; for the ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364:2405–2416.
- Sherman KE, Flamm SL, Afdhal NH, et al; for the ILLUMINATE Study Team. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med 2011; 365:1014–1024.
- Zeuzem S, Andreone P, Pol S, et al; for the REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med 2011; 364:2417–2428.
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–714.
- Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology 2010; 51:729–733.
- Kim WR. The burden of hepatitis C in the United States. Hepatology 2002; 36:S30–S34.
- Marcellin P, Asselah T, Boyer N. Fibrosis and disease progression in hepatitis C. Hepatology 2002; 36:S47–S56.
- Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002; 36:S35–S46.
- Lavanchy D. The global burden of hepatitis C. Liver Int 2009; 29(suppl 1):74–81.
- National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002—June 10–12, 2002. Hepatology 2002; 36:S3–S20.
- Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis 2011; 52:889–900.
- Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med 2006; 355:2444–2451.
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009; 461:399–401.
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009; 41:1100–1104.
- Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010; 139:120–129.e118.
- Nielsen SU, Bassendine MF, Burt AD, Bevitt DJ, Toms GL. Characterization of the genome and structural proteins of hepatitis C virus resolved from infected human liver. J Gen Virol 2004; 85:1497–1507.
- Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology 2004; 39:5–19.
- Nelson DR. The role of triple therapy with protease inhibitors in hepatitis C virus genotype 1 naive patients. Liver Int 2011; 31(suppl 1):53–57.
- Pawlotsky JM. Treatment failure and resistance with direct-acting antiviral drugs against hepatitis C virus. Hepatology 2011; 53:1742–1751.
- Monto A, Schooley RT, Lai JC, et al. Lessons from HIV therapy applied to viral hepatitis therapy: summary of a workshop. Am J Gastroenterol 2010; 105:989–1004.
- McCown MF, Rajyaguru S, Kular S, Cammack N, Najera I. GT-1a or GT-1b subtype-specific resistance profiles for hepatitis C virus inhibitors telaprevir and HCV-796. Antimicrob Agents Chemother 2009; 53:2129–2132.
- Cholongitas E, Papatheodoridis GV. Review article: novel therapeutic options for chronic hepatitis C. Aliment Pharmacol Ther 2008; 27:866–884.
- Naggie S, Patel K, McHutchison J. Hepatitis C virus directly acting antivirals: current developments with NS3/4A HCV serine protease inhibitors. J Antimicrob Chemother 2010; 65:2063–2069.
- Mir HM, Birerdinc A, Younossi ZM. Monoclonal and polyclonal antibodies against the HCV envelope proteins. Clin Liver Dis 2009; 13:477–486.
- Birerdinc A, Younossi ZM. Emerging therapies for hepatitis C virus. Expert Opin Emerg Drugs 2010; 15:535–544.
- Khattab MA. Targeting host factors: a novel rationale for the management of hepatitis C virus. World J Gastroenterol 2009; 15:3472–3479.
- Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 2010; 327:198–201.
- Gane EJ, Roberts SK, Stedman CA, et al. Oral combination therapy with a nucleoside polymerase inhibitor (RG7128) and danoprevir for chronic hepatitis C genotype 1 infection (INFORM-1): a randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 2010; 376:1467–1475.
KEY POINTS
- Standard care with the combination of pegylated interferon and ribavirin produces a sustained virologic response in about 40% of patients infected with HCV genotype 1, the most prevalent genotype in North America.
- New phase 3 trials showed that the addition of an oral protease inhibitor (boceprevir or telaprevir) increased the sustained virologic response rates to 70% in patients infected with HCV genotype 1.
- Boceprevir and telaprevir must be used in combination with pegylated interferon and ribavirin; they should not be used as monotherapy because of concern about the development of drug-resistant mutations.
- The main side effects of boceprevir were anemia and dysgeusia. Adverse events associated with telaprevir included rash, pruritus, anemia, and diarrhea.
Update on contraceptive options: A case-based discussion
Contraceptive counseling is both an art and a science. The role of the health care provider is to determine the patient’s medical eligibility and match her preferences and lifestyle to an appropriate method for both contraceptive and potentially noncontraceptive benefits, while minimizing the risk of unintended pregnancy.
Women throughout the range of reproductive years need appropriate counseling and education on hormones, the menstrual cycle, and the efficacy of contraception as part of their routine gynecologic evaluation. Issues of access to birth control, cost, possible side effects, and actual effectiveness of methods are important to discuss.
In this paper we will discuss common clinical practice case scenarios to illustrate contraceptive counseling and management, including:
- Perimenopausal women
- Women with thrombophilia
- Women who contemplate becoming pregnant in the future
- Women with psychiatric illness
- Women with hypertension.
HALF OF ALL PREGNANCIES ARE UNPLANNED
Although many contraceptive options are available, 48% of all pregnancies in the United States are unintended.1 In 2009, the national teen birth rate was 39.1 births per 1,000 girls and women age 15 to 19 years, which was 37% lower than in 1991.2 Still, African American and Hispanic teenagers living in southern states have disproportionately higher rates.
The rate of unintended pregnancy is a little lower at the older end of the reproductive age range, but still high: 35% of all pregnancies in women over 40 years old are also unintended.2
To find out why these numbers are so high, in 2007 the US Centers for Disease Control and Prevention (CDC) conducted a survey3 that included 8,000 women reporting unintended pregnancy who had not used contraception. Of these, 39% were married. Surprisingly, more than one-third of women said they did not know they could get pregnant when they did.3
WHAT’S NEW IN CONTRACEPTION?
The “pill” was approved by the US Food and Drug Administration (FDA) more than 50 years ago, and it is still the most commonly used contraceptive method (followed by surgical sterilization). Enovid, the pill formulated by Dr. John Rock and Dr. Gregory Pincus in the 1950s, contained 150 μg of mestranol (equivalent to 90 μg of ethinyl estradiol) and 9.85 mg of norethynodrel, a very potent progestin. Our current oral contraceptive pills contain much lower hormone doses and have fewer androgenic side effects.4
In May 2010, the CDC and the World Health Organization (WHO) updated their safety guidelines for all hormonal contraceptives and the use of these agents in patients with various medical and family histories. They ranked contraceptive methods from those with no restriction to those with unacceptable risk to their use. This document can be accessed at www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm.5
New developments in oral contraceptives are notably in the 19-nortestosterone derivatives, the family that includes the second-generation progestogens already available such as norgestimate (contained in Ortho-Cyclen) and norethindrone (contained in Loestrin). A newer progestin, dienogest, is available in a preparation that also contains estradiol valerate (Natazia). Drospirenone, which is similar to spironolactone, is contained in Yaz, Yasmin, and newer products that also contain levomefolate calcium (Beyaz, Safyral).
LoLoestrin Fe, which contains active pills containing 10 μg of ethinyl estradiol and 1 mg of norethindrone and placebo pills with 75 mg of ferrous fumarate, was recently approved by the FDA and offers an ultra-low dose of estrogen.
Depot medroxyprogesterone acetate now comes in a 104-mg suspension for subcutaneous injection every 3 months; it is called depo-subQ provera 104. Standard medroxyprogesterone acetate 150 mg for intramuscular injection every 3 months (Depo-Provera) is still available and has gone generic. The newer product offers the advantages of lower dose and less weight-gain. Also, it allows capable and willing patients to self-administer their contraceptives. However, it is more expensive—$ 104 per injection for a patient without insurance at Cleveland Clinic, compared with $46 for Depo-Provera and $10 for the generic intramuscular preparation for a patient with insurance.
A new option for emergency contraception, ulipristal (ella) is a progesterone antagonist-agonist available only by prescription. Taken in a single oral dose of 30 μg, it is effective for up to 120 hours after unprotected intercourse. It joins Plan B (levonorgestrel 1.5 mg in a single dose) and Next Choice (two doses of levonorgestrel 0.75 mg each), which are available over-the-counter for women age 17 years or older, and by prescription for those 16 years and younger, for use up to 72 hours after unprotected intercourse.
CASE 1: CONTRACEPTION IN PERIMENOPAUSE
A 48-year-old attorney who has had two children complains of irregular menstrual cycles and of occasional hot flashes at night that wake her from sleep. She keeps a menstrual calendar; it shows her last menstrual period was 3 months ago. She took oral contraceptives for 15 years before she had her first child. She is using condoms intermittently for contraception. Her body mass index is normal at 24 kg/m2, and she does not smoke. How do you counsel her?
A variety of hormonal options
This healthy perimenopausal woman has a variety of hormonal contraception options that would have the added benefit of regulating her menstrual cycle or suppressing it altogether. These include the levonorgestrel intrauterine system (Mirena IUS), various injectable products (such as Depo-Provera or the newer depo-subQ provera 104), contraceptive pills, the Ortho Evra contraceptive patch, and the vaginal contraceptive ring (NuvaRing). Of these, low-dose birth control pills may be the best option, as they would help with cycle control, offer contraception, and better regulate hormonal fluctuations to reduce her hot flashes.
Hormonal contraception can safely be used in women in their 30s and 40s, and often until menopause if the benefit outweighs the risk.
An estradiol valerate-dienogest oral contraceptive with a quadriphasic dosing schedule (Natazia) has been studied in women up to age 50. Although it was approved in 2010 in the United States, this pill has been used in Europe since the 1990s. The 26 active pills contain tapering doses of the active drugs, with the aim of mimicking the natural menstrual cycle, similar to triphasic pills. Estradiol valerate is a bioidentical estrogen, as it is rapidly metabolized to estradiol (E2), which is identical to 17-beta estradiol and estrone (E3) produced by the ovary. A dose of 2 mg of estradiol valerate is equivalent to 10 μg of ethinyl estradiol, which is the estrogen component in most other oral contraceptives. Low-dose pills by definition contain less than 50 μg of ethinyl estradiol. Dienogest, the progesterone component, has a 17-cyanomethyl group that accounts for its strongly progestogenic and weakly antiandrogenic properties.
All oral hormonal contraceptives can increase triglycerides by inducing the CYP450 system in the liver. However, in clinical trials, estradiol valerate-dienogest also caused other changes in lipid metabolism, such as a nonsignificant increase in high-density lipoprotein cholesterol and a slight reduction in low-density lipoprotein cholesterol and lipoprotein(a) compared with ethinyl estradiol-levonorgestrel preparations.6
It is important to advise patients that, compared with users of other oral contraceptives, estradiol valerate-dienogest users may experience fewer days of menstrual bleeding and more cycles without withdrawal bleeding. This product can therefore be an effective alternative for women with menorrhagia.
All classes of hormonal contraception carry a similar risk of side effects, such as headache, breast tenderness, nausea, irregular bleeding, and mood changes. Some women have no side effects.
CASE 2: THROMBOPHILIA
A 39-year-old woman with a body mass index of 31 kg/m2 (obese) has a history of protein S deficiency with active lower-extremity deep vein thrombosis, for which she is taking warfarin (Coumadin). She experiences menorrhagia and dysmenorrhea due to intramural fibroids and possible adenomyosis seen on transvaginal ultrasonography and confirmed by magnetic resonance imaging. Hysteroscopy reveals no polyps or submucosal fibroids. An endometrial biopsy is negative for malignancy.
She desires contraception. How do you counsel her?
Estrogens are contraindicated—except, perhaps, in select cases
This patient has many reasons for heavy bleeding. She is on warfarin, which effectively inhibits synthesis of vitamin K-dependent coagulation factor. She also has fibroids and adenomyosis. The latter is a difficult condition to control, as the location of the intramuscular glands makes treatments such as ablation, dilation and curettage, and oral agents ineffective.
All estrogen-containing formulations (pills, ring, patch) are contraindicated in women with acute venous thromboembolism (VTE) and known thrombophilia. A newer agent approved for treating menorrhagia (not for contraception), tranexamic acid (Lysteda), also carries a contraindication for patients with thrombophilia or history of VTE; however, the evidence for the latter is controversial.7
The updated CDC guidelines for the use of hormonal contraceptives state that patients who receive anticoagulation for at least 3 months and who have no history of VTE or a low risk of recurrent VTE (no evidence of active cancer, no known thrombophilia) may use estrogen-containing contraceptives in select cases (category 3—theoretical risk outweighs benefits, but not an absolute contraindication).5 Although this is not common clinical practice, select patients may benefit from menstrual cycle control while receiving anticoagulation. However, other contraceptive alternatives are preferred if possible.
Progestin-only treatments such as the Mirena IUS (if the fibroids do not distort the uterine cavity) and the etonogestrel implant (Implanon) are nonsurgical options that may reduce menorrhagia and are safer alternatives for patients with thrombophilia.
The Paragard (copper) intrauterine device would provide nonhormonal contraception without diminishing menorrhagia. Obviously, barrier methods (which are less effective than hormonal contraception) can be suggested for contraception alone. A viable option for women finished with childbearing is hysterectomy, which provides contraceptive benefit and definitive treatment of menorrhagia due to adenomyosis.
Laboratory screening for VTE is not required before starting estrogen-containing contraceptives. However, one should take a detailed history and inquire about VTE events or a family history of recurrent VTE.
VTE rates among reproductive-age women are 4 to 5 per 10,000 women per year.8 The rate of VTE in oral contraceptive users is estimated as 9 to 10 per 10,000 women per year.9 However, rates of VTE associated with pregnancy and postpartum states are exponentially greater. Although recent studies have shown some discrepancy in rates of VTE across different classes of progestins,10,11 the absolute risk of VTE with hormonal contraceptives is very low.
In December 2011, an FDA panel voted 15 to 11 that the benefits of drospirenone-containing contraceptives (eg, Yaz, Yasmin, Beyaz, Safyral), such as preventing pregnancy, outweigh the potential risk. However, product labeling may change in the future to more accurately reflect the risk-benefit ratio. Stay tuned for better-designed trials to further assess VTE risk across progestins.
Health care providers should engage patients in an informed discussion about all risks and benefits of hormonal contraceptives and note this risk of VTE is higher in gravid women.
CASE 3: FUTURE FERTILITY
A 30-year-old surgical resident who has never been pregnant comes for her annual examination. She currently desires birth control but would like to be pregnant 1 to 2 years from now. She has no history of significant medical illness. Her body mass index is 23 kg/m2, and she takes no medications. How do you counsel her?
Many options; also consider folic acid
Effective counseling leads to patient-centered decision-making for all treatments and procedures. Contraceptive counseling should elicit the patient’s perspective about hormonal methods and educate her on efficacy, proper use, and common adverse effects.
Contraception should fit the patient’s lifestyle. Questions as simple as “Are you a good pill-taker?” or “Are you comfortable with injections?” will help you and the patient assess what will work effectively and will maintain good adherence.
Deciding on a contraceptive option that is cost-effective is crucial, particularly for many young women or adolescents. Many oral contraceptives are widely available as generic formulations for less than $10 per month. Although generic drugs are not required to be 100% bioequivalent to their brand-name counterparts, they can provide a more economical option. For a complete guide to different hormonal contraceptive formulations, we suggest Choosing a birth control method, available on the Web site of the Association of Reproductive Health Professionals at www.arhp.org/upload-Docs/choosingqrg.pdf.12
As discussed earlier, half of all pregnancies are unplanned, and so women of childbearing age should be ingesting 400 μg of folic acid daily. Debate exists as to whether Americans who eat a balanced diet need a multivitamin.13 However, there is no debate about folic acid, which is proven to prevent neural tube defects. Newer formulations of ethinyl estradiol-drospirenone (Beyaz, Safyral) now contain an active form of folic acid (levomefolate calcium 451 mg in each pill). For the above patient who needs contraception and is willing to take birth control, the addition of folic acid provides an essential element in preconception counseling.
Regardless of the current contraceptive choice, patients who actively desire pregnancy should take a prenatal vitamin that contains folic acid and iron.
In addition to combined oral contraceptives, other options for this patient include medroxyprogesterone acetate (intramuscular or subcutaneous), NuvaRing, or intrauterine devices. The Ortho Evra patch is also an option for this patient. However, since 2008 the patch has carried an FDA warning that the risk of VTE is twice as high with this product than with oral contraceptives that contain 30 μg of ethinyl estradiol plus levonorgestrel.14 Postmarketing data did not show any higher risk of VTE in patch users compared with oral contraceptive users less than 40 years of age, however.15
CASE 4: PSYCHIATRIC ILLNESS
A 21-year-old woman who has bipolar II disorder comes to your office for her annual gynecologic evaluation. She has one sexual partner and desires oral contraceptive pills. Lithium treatment has failed for her, but her condition is stable on carbamazepine (Tegretol). She asks if it is true that women can still get pregnant while on the birth control pill. How do you counsel her?
Possible interactions with psychiatric drugs
Like the woman in case 3, this patient has many options, including estrogen-containing pills, the vaginal ring, the patch, injectable contraceptives, and intrauterine devices.
Certain antiepileptic, antipsychotic, or headache medications such as carbamezapine, phenytoin (Dilantin), oxcarbazepine (Trileptal), and topiramate (Topamax) decrease levels of hormonal contraceptives by induction of the CYP450 enzymes. Conversely, it is suggested that lamotrigine (Lamictal) levels decrease by up to 49% while patients concomitantly take oral contraceptive pills, which can induce seizure activity.16 Also, antibiotics such as rifampin (Rifadin) and even herbs such as St. John’s Wort can decrease the effectiveness of hormonal contraceptives by increasing their metabolism.
On the positive side, depot medroxyprogesterone acetate raises the seizure threshold by a mechanism attributed to high levels of progestins and is a better option for epileptic patients. A bulletin of the American College of Gynecologists addresses the paucity of data on hormonal treatments in depressed patients. However, some evidence points to slight improvement of depressive symptoms after 1 year in patients who took Depo-Provera compared with those who discontinued the drug.17
The Pearl index, a measure of contraceptive efficacy
We refer to the Pearl index when answering our patients’ questions about contraceptive efficacy. The Pearl index is defined as the number of unintended pregnancies per 100 women per year. The typical (or actual) effectiveness for each contraceptive method is quoted rather than the theoretical (perfect-use) efficacy.
We suggest simplifying this discussion with patients. For example, for every 100 women using male condoms for contraception, 15 women have unintended pregnancies per year. With hormonal contraceptives (pill, patch, or ring), for every 100 women there are 8 per year with unintended pregnancy, 3 of 100 with Depo-Provera, and less than 1 in 100 using intrauterine devices or female or male sterilization.18
Efficacy decreases (and the failure rate increases) with frequency of intercourse, irregular menstrual cycles, missed pills, improper dosing, and drug-drug interactions as described above.
CASE 5: HYPERTENSION
A 33-year-old woman who has been pregnant twice experienced preeclampsia in her last pregnancy, and now her blood pressure is consistently approximately 140/90 mm Hg on multiple office visits and ambulatory monitoring. She desires contraception. How do you counsel her?
Avoid estrogen-containing products
According to the WHO and CDC guidelines,5 women with controlled or uncontrolled hypertension should not be offered combined oral contraceptives, the patch, or the ring (category 3—theoretical or proven risks outweigh the benefits, and category 4 for systolic blood pressure greater than 160 mm Hg or diastolic blood pressure greater than 100 mm Hg).
The progesterone-only pill (“mini pill”), medroxyprogesterone acetate (intramuscular or subcutaneous), Mirena IUS, the copper intrauterine device, and the etonogestrel implant are all safer options.
A small subset of patients develop elevated blood pressure after starting hormonal contraceptives. Estrogen-containing hormones can increase the liver’s output of angiotensinogen, which is a renin substrate that activates the renin-angiotensin-aldosterone system. If this becomes clinically apparent, these patients should refrain from estrogen-containing products and use progestin-only formulations as a safer alternative.
Patients with isolated elevated hypertriglyceridemia should avoid oral contraceptives. However, the patch, the ring, and progestin-only methods may be acceptable.
Diabetic patients with microvascular complications of retinopathy or nephropathy and any patient with macrovascular disease (stroke, cardiovascular disease) should not be offered estrogen-containing contraception.
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006; 38:90–96.
- Centers for Disease Control and Prevention. Vital signs: teenage pregnancy—United States 1991–2009. MMWR 2011; 60:414–420. www.cdc.gov/mmwr/preview/mmwrhtml/mm6013a5.htm. Accessed January 10, 2012.
- Nettleman MD, Chung H, Brewer J, Ayoola A, Reed PL. Reasons for unprotected intercourse: analysis of the PRAMS survey. Contraception 2007; 75:361–366.
- Nelson A. New low-dose, extended-cycle pills with levonorgestrel and ethinyl estradiol: an evolutionary step on birth control. Int J Womens Health 2010; 2:99–106.
- US Centers for Disease Control and Prevention. Appendix L. Summary of classifications for hormonal contraceptive methods and intrauterine devices. MMWR 2010; 59( RR04):76–81. www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm. Accessed January 10, 2012.
- Wiegratz L, Lee JH, Kutchera E, et al. Effect of dienogest-containing oral contraceptives in lipid metabolism. Contraception 2002; 65:223–229.
- Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case-control study using the General Practice Research Database. Br J Obstet Gynecol 2009; 116:91–97.
- Heinemann L, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception 2007; 75:328–336.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception 2007; 75:344–354.
- Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ 2011; 342:d2139.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ 2011; 342:d2151.
- Association of Reproductive Health Professionals (ARHP). Choosing a birth control method. www.arhp.org/uploadDocs/choosingqrg.pdf. Accessed January 10, 2012.
- Caballero B. Should healthy people take a multivitamin? Cleve Clin J Med 2010; 77:656–657.
- US Food and Drug Administration. Ortho Evra questions and answers (1/18/2008). www.fda.gov/Drugs/DrugSafety/Postmarket-DrugSafetyInformationforPatientsandProviders/ucm110403.htm. Accessed January 10, 2012.
- Jick SS, Hagberg KW, Hernandez RK, Kaye JA. Postmarketing study of ORTHO EVRA and levonorgestrel oral contraceptives containing hormonal contraceptives with 30 mcg of ethinyl estradiol in relation to nonfatal venous thromboembolism. Contraception 2010; 81:16–21.
- Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology 2003; 61:570–571.
- Westhoff C, Truman C, Kalmuss D, et al; Depressive symptoms and Depo-Provera. Contraception 1998; 57:237–240.
- Trusell Wynn LL. Reducing unintended pregnancy in the United States. Contraception 2008; 77:1–5.
Contraceptive counseling is both an art and a science. The role of the health care provider is to determine the patient’s medical eligibility and match her preferences and lifestyle to an appropriate method for both contraceptive and potentially noncontraceptive benefits, while minimizing the risk of unintended pregnancy.
Women throughout the range of reproductive years need appropriate counseling and education on hormones, the menstrual cycle, and the efficacy of contraception as part of their routine gynecologic evaluation. Issues of access to birth control, cost, possible side effects, and actual effectiveness of methods are important to discuss.
In this paper we will discuss common clinical practice case scenarios to illustrate contraceptive counseling and management, including:
- Perimenopausal women
- Women with thrombophilia
- Women who contemplate becoming pregnant in the future
- Women with psychiatric illness
- Women with hypertension.
HALF OF ALL PREGNANCIES ARE UNPLANNED
Although many contraceptive options are available, 48% of all pregnancies in the United States are unintended.1 In 2009, the national teen birth rate was 39.1 births per 1,000 girls and women age 15 to 19 years, which was 37% lower than in 1991.2 Still, African American and Hispanic teenagers living in southern states have disproportionately higher rates.
The rate of unintended pregnancy is a little lower at the older end of the reproductive age range, but still high: 35% of all pregnancies in women over 40 years old are also unintended.2
To find out why these numbers are so high, in 2007 the US Centers for Disease Control and Prevention (CDC) conducted a survey3 that included 8,000 women reporting unintended pregnancy who had not used contraception. Of these, 39% were married. Surprisingly, more than one-third of women said they did not know they could get pregnant when they did.3
WHAT’S NEW IN CONTRACEPTION?
The “pill” was approved by the US Food and Drug Administration (FDA) more than 50 years ago, and it is still the most commonly used contraceptive method (followed by surgical sterilization). Enovid, the pill formulated by Dr. John Rock and Dr. Gregory Pincus in the 1950s, contained 150 μg of mestranol (equivalent to 90 μg of ethinyl estradiol) and 9.85 mg of norethynodrel, a very potent progestin. Our current oral contraceptive pills contain much lower hormone doses and have fewer androgenic side effects.4
In May 2010, the CDC and the World Health Organization (WHO) updated their safety guidelines for all hormonal contraceptives and the use of these agents in patients with various medical and family histories. They ranked contraceptive methods from those with no restriction to those with unacceptable risk to their use. This document can be accessed at www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm.5
New developments in oral contraceptives are notably in the 19-nortestosterone derivatives, the family that includes the second-generation progestogens already available such as norgestimate (contained in Ortho-Cyclen) and norethindrone (contained in Loestrin). A newer progestin, dienogest, is available in a preparation that also contains estradiol valerate (Natazia). Drospirenone, which is similar to spironolactone, is contained in Yaz, Yasmin, and newer products that also contain levomefolate calcium (Beyaz, Safyral).
LoLoestrin Fe, which contains active pills containing 10 μg of ethinyl estradiol and 1 mg of norethindrone and placebo pills with 75 mg of ferrous fumarate, was recently approved by the FDA and offers an ultra-low dose of estrogen.
Depot medroxyprogesterone acetate now comes in a 104-mg suspension for subcutaneous injection every 3 months; it is called depo-subQ provera 104. Standard medroxyprogesterone acetate 150 mg for intramuscular injection every 3 months (Depo-Provera) is still available and has gone generic. The newer product offers the advantages of lower dose and less weight-gain. Also, it allows capable and willing patients to self-administer their contraceptives. However, it is more expensive—$ 104 per injection for a patient without insurance at Cleveland Clinic, compared with $46 for Depo-Provera and $10 for the generic intramuscular preparation for a patient with insurance.
A new option for emergency contraception, ulipristal (ella) is a progesterone antagonist-agonist available only by prescription. Taken in a single oral dose of 30 μg, it is effective for up to 120 hours after unprotected intercourse. It joins Plan B (levonorgestrel 1.5 mg in a single dose) and Next Choice (two doses of levonorgestrel 0.75 mg each), which are available over-the-counter for women age 17 years or older, and by prescription for those 16 years and younger, for use up to 72 hours after unprotected intercourse.
CASE 1: CONTRACEPTION IN PERIMENOPAUSE
A 48-year-old attorney who has had two children complains of irregular menstrual cycles and of occasional hot flashes at night that wake her from sleep. She keeps a menstrual calendar; it shows her last menstrual period was 3 months ago. She took oral contraceptives for 15 years before she had her first child. She is using condoms intermittently for contraception. Her body mass index is normal at 24 kg/m2, and she does not smoke. How do you counsel her?
A variety of hormonal options
This healthy perimenopausal woman has a variety of hormonal contraception options that would have the added benefit of regulating her menstrual cycle or suppressing it altogether. These include the levonorgestrel intrauterine system (Mirena IUS), various injectable products (such as Depo-Provera or the newer depo-subQ provera 104), contraceptive pills, the Ortho Evra contraceptive patch, and the vaginal contraceptive ring (NuvaRing). Of these, low-dose birth control pills may be the best option, as they would help with cycle control, offer contraception, and better regulate hormonal fluctuations to reduce her hot flashes.
Hormonal contraception can safely be used in women in their 30s and 40s, and often until menopause if the benefit outweighs the risk.
An estradiol valerate-dienogest oral contraceptive with a quadriphasic dosing schedule (Natazia) has been studied in women up to age 50. Although it was approved in 2010 in the United States, this pill has been used in Europe since the 1990s. The 26 active pills contain tapering doses of the active drugs, with the aim of mimicking the natural menstrual cycle, similar to triphasic pills. Estradiol valerate is a bioidentical estrogen, as it is rapidly metabolized to estradiol (E2), which is identical to 17-beta estradiol and estrone (E3) produced by the ovary. A dose of 2 mg of estradiol valerate is equivalent to 10 μg of ethinyl estradiol, which is the estrogen component in most other oral contraceptives. Low-dose pills by definition contain less than 50 μg of ethinyl estradiol. Dienogest, the progesterone component, has a 17-cyanomethyl group that accounts for its strongly progestogenic and weakly antiandrogenic properties.
All oral hormonal contraceptives can increase triglycerides by inducing the CYP450 system in the liver. However, in clinical trials, estradiol valerate-dienogest also caused other changes in lipid metabolism, such as a nonsignificant increase in high-density lipoprotein cholesterol and a slight reduction in low-density lipoprotein cholesterol and lipoprotein(a) compared with ethinyl estradiol-levonorgestrel preparations.6
It is important to advise patients that, compared with users of other oral contraceptives, estradiol valerate-dienogest users may experience fewer days of menstrual bleeding and more cycles without withdrawal bleeding. This product can therefore be an effective alternative for women with menorrhagia.
All classes of hormonal contraception carry a similar risk of side effects, such as headache, breast tenderness, nausea, irregular bleeding, and mood changes. Some women have no side effects.
CASE 2: THROMBOPHILIA
A 39-year-old woman with a body mass index of 31 kg/m2 (obese) has a history of protein S deficiency with active lower-extremity deep vein thrombosis, for which she is taking warfarin (Coumadin). She experiences menorrhagia and dysmenorrhea due to intramural fibroids and possible adenomyosis seen on transvaginal ultrasonography and confirmed by magnetic resonance imaging. Hysteroscopy reveals no polyps or submucosal fibroids. An endometrial biopsy is negative for malignancy.
She desires contraception. How do you counsel her?
Estrogens are contraindicated—except, perhaps, in select cases
This patient has many reasons for heavy bleeding. She is on warfarin, which effectively inhibits synthesis of vitamin K-dependent coagulation factor. She also has fibroids and adenomyosis. The latter is a difficult condition to control, as the location of the intramuscular glands makes treatments such as ablation, dilation and curettage, and oral agents ineffective.
All estrogen-containing formulations (pills, ring, patch) are contraindicated in women with acute venous thromboembolism (VTE) and known thrombophilia. A newer agent approved for treating menorrhagia (not for contraception), tranexamic acid (Lysteda), also carries a contraindication for patients with thrombophilia or history of VTE; however, the evidence for the latter is controversial.7
The updated CDC guidelines for the use of hormonal contraceptives state that patients who receive anticoagulation for at least 3 months and who have no history of VTE or a low risk of recurrent VTE (no evidence of active cancer, no known thrombophilia) may use estrogen-containing contraceptives in select cases (category 3—theoretical risk outweighs benefits, but not an absolute contraindication).5 Although this is not common clinical practice, select patients may benefit from menstrual cycle control while receiving anticoagulation. However, other contraceptive alternatives are preferred if possible.
Progestin-only treatments such as the Mirena IUS (if the fibroids do not distort the uterine cavity) and the etonogestrel implant (Implanon) are nonsurgical options that may reduce menorrhagia and are safer alternatives for patients with thrombophilia.
The Paragard (copper) intrauterine device would provide nonhormonal contraception without diminishing menorrhagia. Obviously, barrier methods (which are less effective than hormonal contraception) can be suggested for contraception alone. A viable option for women finished with childbearing is hysterectomy, which provides contraceptive benefit and definitive treatment of menorrhagia due to adenomyosis.
Laboratory screening for VTE is not required before starting estrogen-containing contraceptives. However, one should take a detailed history and inquire about VTE events or a family history of recurrent VTE.
VTE rates among reproductive-age women are 4 to 5 per 10,000 women per year.8 The rate of VTE in oral contraceptive users is estimated as 9 to 10 per 10,000 women per year.9 However, rates of VTE associated with pregnancy and postpartum states are exponentially greater. Although recent studies have shown some discrepancy in rates of VTE across different classes of progestins,10,11 the absolute risk of VTE with hormonal contraceptives is very low.
In December 2011, an FDA panel voted 15 to 11 that the benefits of drospirenone-containing contraceptives (eg, Yaz, Yasmin, Beyaz, Safyral), such as preventing pregnancy, outweigh the potential risk. However, product labeling may change in the future to more accurately reflect the risk-benefit ratio. Stay tuned for better-designed trials to further assess VTE risk across progestins.
Health care providers should engage patients in an informed discussion about all risks and benefits of hormonal contraceptives and note this risk of VTE is higher in gravid women.
CASE 3: FUTURE FERTILITY
A 30-year-old surgical resident who has never been pregnant comes for her annual examination. She currently desires birth control but would like to be pregnant 1 to 2 years from now. She has no history of significant medical illness. Her body mass index is 23 kg/m2, and she takes no medications. How do you counsel her?
Many options; also consider folic acid
Effective counseling leads to patient-centered decision-making for all treatments and procedures. Contraceptive counseling should elicit the patient’s perspective about hormonal methods and educate her on efficacy, proper use, and common adverse effects.
Contraception should fit the patient’s lifestyle. Questions as simple as “Are you a good pill-taker?” or “Are you comfortable with injections?” will help you and the patient assess what will work effectively and will maintain good adherence.
Deciding on a contraceptive option that is cost-effective is crucial, particularly for many young women or adolescents. Many oral contraceptives are widely available as generic formulations for less than $10 per month. Although generic drugs are not required to be 100% bioequivalent to their brand-name counterparts, they can provide a more economical option. For a complete guide to different hormonal contraceptive formulations, we suggest Choosing a birth control method, available on the Web site of the Association of Reproductive Health Professionals at www.arhp.org/upload-Docs/choosingqrg.pdf.12
As discussed earlier, half of all pregnancies are unplanned, and so women of childbearing age should be ingesting 400 μg of folic acid daily. Debate exists as to whether Americans who eat a balanced diet need a multivitamin.13 However, there is no debate about folic acid, which is proven to prevent neural tube defects. Newer formulations of ethinyl estradiol-drospirenone (Beyaz, Safyral) now contain an active form of folic acid (levomefolate calcium 451 mg in each pill). For the above patient who needs contraception and is willing to take birth control, the addition of folic acid provides an essential element in preconception counseling.
Regardless of the current contraceptive choice, patients who actively desire pregnancy should take a prenatal vitamin that contains folic acid and iron.
In addition to combined oral contraceptives, other options for this patient include medroxyprogesterone acetate (intramuscular or subcutaneous), NuvaRing, or intrauterine devices. The Ortho Evra patch is also an option for this patient. However, since 2008 the patch has carried an FDA warning that the risk of VTE is twice as high with this product than with oral contraceptives that contain 30 μg of ethinyl estradiol plus levonorgestrel.14 Postmarketing data did not show any higher risk of VTE in patch users compared with oral contraceptive users less than 40 years of age, however.15
CASE 4: PSYCHIATRIC ILLNESS
A 21-year-old woman who has bipolar II disorder comes to your office for her annual gynecologic evaluation. She has one sexual partner and desires oral contraceptive pills. Lithium treatment has failed for her, but her condition is stable on carbamazepine (Tegretol). She asks if it is true that women can still get pregnant while on the birth control pill. How do you counsel her?
Possible interactions with psychiatric drugs
Like the woman in case 3, this patient has many options, including estrogen-containing pills, the vaginal ring, the patch, injectable contraceptives, and intrauterine devices.
Certain antiepileptic, antipsychotic, or headache medications such as carbamezapine, phenytoin (Dilantin), oxcarbazepine (Trileptal), and topiramate (Topamax) decrease levels of hormonal contraceptives by induction of the CYP450 enzymes. Conversely, it is suggested that lamotrigine (Lamictal) levels decrease by up to 49% while patients concomitantly take oral contraceptive pills, which can induce seizure activity.16 Also, antibiotics such as rifampin (Rifadin) and even herbs such as St. John’s Wort can decrease the effectiveness of hormonal contraceptives by increasing their metabolism.
On the positive side, depot medroxyprogesterone acetate raises the seizure threshold by a mechanism attributed to high levels of progestins and is a better option for epileptic patients. A bulletin of the American College of Gynecologists addresses the paucity of data on hormonal treatments in depressed patients. However, some evidence points to slight improvement of depressive symptoms after 1 year in patients who took Depo-Provera compared with those who discontinued the drug.17
The Pearl index, a measure of contraceptive efficacy
We refer to the Pearl index when answering our patients’ questions about contraceptive efficacy. The Pearl index is defined as the number of unintended pregnancies per 100 women per year. The typical (or actual) effectiveness for each contraceptive method is quoted rather than the theoretical (perfect-use) efficacy.
We suggest simplifying this discussion with patients. For example, for every 100 women using male condoms for contraception, 15 women have unintended pregnancies per year. With hormonal contraceptives (pill, patch, or ring), for every 100 women there are 8 per year with unintended pregnancy, 3 of 100 with Depo-Provera, and less than 1 in 100 using intrauterine devices or female or male sterilization.18
Efficacy decreases (and the failure rate increases) with frequency of intercourse, irregular menstrual cycles, missed pills, improper dosing, and drug-drug interactions as described above.
CASE 5: HYPERTENSION
A 33-year-old woman who has been pregnant twice experienced preeclampsia in her last pregnancy, and now her blood pressure is consistently approximately 140/90 mm Hg on multiple office visits and ambulatory monitoring. She desires contraception. How do you counsel her?
Avoid estrogen-containing products
According to the WHO and CDC guidelines,5 women with controlled or uncontrolled hypertension should not be offered combined oral contraceptives, the patch, or the ring (category 3—theoretical or proven risks outweigh the benefits, and category 4 for systolic blood pressure greater than 160 mm Hg or diastolic blood pressure greater than 100 mm Hg).
The progesterone-only pill (“mini pill”), medroxyprogesterone acetate (intramuscular or subcutaneous), Mirena IUS, the copper intrauterine device, and the etonogestrel implant are all safer options.
A small subset of patients develop elevated blood pressure after starting hormonal contraceptives. Estrogen-containing hormones can increase the liver’s output of angiotensinogen, which is a renin substrate that activates the renin-angiotensin-aldosterone system. If this becomes clinically apparent, these patients should refrain from estrogen-containing products and use progestin-only formulations as a safer alternative.
Patients with isolated elevated hypertriglyceridemia should avoid oral contraceptives. However, the patch, the ring, and progestin-only methods may be acceptable.
Diabetic patients with microvascular complications of retinopathy or nephropathy and any patient with macrovascular disease (stroke, cardiovascular disease) should not be offered estrogen-containing contraception.
Contraceptive counseling is both an art and a science. The role of the health care provider is to determine the patient’s medical eligibility and match her preferences and lifestyle to an appropriate method for both contraceptive and potentially noncontraceptive benefits, while minimizing the risk of unintended pregnancy.
Women throughout the range of reproductive years need appropriate counseling and education on hormones, the menstrual cycle, and the efficacy of contraception as part of their routine gynecologic evaluation. Issues of access to birth control, cost, possible side effects, and actual effectiveness of methods are important to discuss.
In this paper we will discuss common clinical practice case scenarios to illustrate contraceptive counseling and management, including:
- Perimenopausal women
- Women with thrombophilia
- Women who contemplate becoming pregnant in the future
- Women with psychiatric illness
- Women with hypertension.
HALF OF ALL PREGNANCIES ARE UNPLANNED
Although many contraceptive options are available, 48% of all pregnancies in the United States are unintended.1 In 2009, the national teen birth rate was 39.1 births per 1,000 girls and women age 15 to 19 years, which was 37% lower than in 1991.2 Still, African American and Hispanic teenagers living in southern states have disproportionately higher rates.
The rate of unintended pregnancy is a little lower at the older end of the reproductive age range, but still high: 35% of all pregnancies in women over 40 years old are also unintended.2
To find out why these numbers are so high, in 2007 the US Centers for Disease Control and Prevention (CDC) conducted a survey3 that included 8,000 women reporting unintended pregnancy who had not used contraception. Of these, 39% were married. Surprisingly, more than one-third of women said they did not know they could get pregnant when they did.3
WHAT’S NEW IN CONTRACEPTION?
The “pill” was approved by the US Food and Drug Administration (FDA) more than 50 years ago, and it is still the most commonly used contraceptive method (followed by surgical sterilization). Enovid, the pill formulated by Dr. John Rock and Dr. Gregory Pincus in the 1950s, contained 150 μg of mestranol (equivalent to 90 μg of ethinyl estradiol) and 9.85 mg of norethynodrel, a very potent progestin. Our current oral contraceptive pills contain much lower hormone doses and have fewer androgenic side effects.4
In May 2010, the CDC and the World Health Organization (WHO) updated their safety guidelines for all hormonal contraceptives and the use of these agents in patients with various medical and family histories. They ranked contraceptive methods from those with no restriction to those with unacceptable risk to their use. This document can be accessed at www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm.5
New developments in oral contraceptives are notably in the 19-nortestosterone derivatives, the family that includes the second-generation progestogens already available such as norgestimate (contained in Ortho-Cyclen) and norethindrone (contained in Loestrin). A newer progestin, dienogest, is available in a preparation that also contains estradiol valerate (Natazia). Drospirenone, which is similar to spironolactone, is contained in Yaz, Yasmin, and newer products that also contain levomefolate calcium (Beyaz, Safyral).
LoLoestrin Fe, which contains active pills containing 10 μg of ethinyl estradiol and 1 mg of norethindrone and placebo pills with 75 mg of ferrous fumarate, was recently approved by the FDA and offers an ultra-low dose of estrogen.
Depot medroxyprogesterone acetate now comes in a 104-mg suspension for subcutaneous injection every 3 months; it is called depo-subQ provera 104. Standard medroxyprogesterone acetate 150 mg for intramuscular injection every 3 months (Depo-Provera) is still available and has gone generic. The newer product offers the advantages of lower dose and less weight-gain. Also, it allows capable and willing patients to self-administer their contraceptives. However, it is more expensive—$ 104 per injection for a patient without insurance at Cleveland Clinic, compared with $46 for Depo-Provera and $10 for the generic intramuscular preparation for a patient with insurance.
A new option for emergency contraception, ulipristal (ella) is a progesterone antagonist-agonist available only by prescription. Taken in a single oral dose of 30 μg, it is effective for up to 120 hours after unprotected intercourse. It joins Plan B (levonorgestrel 1.5 mg in a single dose) and Next Choice (two doses of levonorgestrel 0.75 mg each), which are available over-the-counter for women age 17 years or older, and by prescription for those 16 years and younger, for use up to 72 hours after unprotected intercourse.
CASE 1: CONTRACEPTION IN PERIMENOPAUSE
A 48-year-old attorney who has had two children complains of irregular menstrual cycles and of occasional hot flashes at night that wake her from sleep. She keeps a menstrual calendar; it shows her last menstrual period was 3 months ago. She took oral contraceptives for 15 years before she had her first child. She is using condoms intermittently for contraception. Her body mass index is normal at 24 kg/m2, and she does not smoke. How do you counsel her?
A variety of hormonal options
This healthy perimenopausal woman has a variety of hormonal contraception options that would have the added benefit of regulating her menstrual cycle or suppressing it altogether. These include the levonorgestrel intrauterine system (Mirena IUS), various injectable products (such as Depo-Provera or the newer depo-subQ provera 104), contraceptive pills, the Ortho Evra contraceptive patch, and the vaginal contraceptive ring (NuvaRing). Of these, low-dose birth control pills may be the best option, as they would help with cycle control, offer contraception, and better regulate hormonal fluctuations to reduce her hot flashes.
Hormonal contraception can safely be used in women in their 30s and 40s, and often until menopause if the benefit outweighs the risk.
An estradiol valerate-dienogest oral contraceptive with a quadriphasic dosing schedule (Natazia) has been studied in women up to age 50. Although it was approved in 2010 in the United States, this pill has been used in Europe since the 1990s. The 26 active pills contain tapering doses of the active drugs, with the aim of mimicking the natural menstrual cycle, similar to triphasic pills. Estradiol valerate is a bioidentical estrogen, as it is rapidly metabolized to estradiol (E2), which is identical to 17-beta estradiol and estrone (E3) produced by the ovary. A dose of 2 mg of estradiol valerate is equivalent to 10 μg of ethinyl estradiol, which is the estrogen component in most other oral contraceptives. Low-dose pills by definition contain less than 50 μg of ethinyl estradiol. Dienogest, the progesterone component, has a 17-cyanomethyl group that accounts for its strongly progestogenic and weakly antiandrogenic properties.
All oral hormonal contraceptives can increase triglycerides by inducing the CYP450 system in the liver. However, in clinical trials, estradiol valerate-dienogest also caused other changes in lipid metabolism, such as a nonsignificant increase in high-density lipoprotein cholesterol and a slight reduction in low-density lipoprotein cholesterol and lipoprotein(a) compared with ethinyl estradiol-levonorgestrel preparations.6
It is important to advise patients that, compared with users of other oral contraceptives, estradiol valerate-dienogest users may experience fewer days of menstrual bleeding and more cycles without withdrawal bleeding. This product can therefore be an effective alternative for women with menorrhagia.
All classes of hormonal contraception carry a similar risk of side effects, such as headache, breast tenderness, nausea, irregular bleeding, and mood changes. Some women have no side effects.
CASE 2: THROMBOPHILIA
A 39-year-old woman with a body mass index of 31 kg/m2 (obese) has a history of protein S deficiency with active lower-extremity deep vein thrombosis, for which she is taking warfarin (Coumadin). She experiences menorrhagia and dysmenorrhea due to intramural fibroids and possible adenomyosis seen on transvaginal ultrasonography and confirmed by magnetic resonance imaging. Hysteroscopy reveals no polyps or submucosal fibroids. An endometrial biopsy is negative for malignancy.
She desires contraception. How do you counsel her?
Estrogens are contraindicated—except, perhaps, in select cases
This patient has many reasons for heavy bleeding. She is on warfarin, which effectively inhibits synthesis of vitamin K-dependent coagulation factor. She also has fibroids and adenomyosis. The latter is a difficult condition to control, as the location of the intramuscular glands makes treatments such as ablation, dilation and curettage, and oral agents ineffective.
All estrogen-containing formulations (pills, ring, patch) are contraindicated in women with acute venous thromboembolism (VTE) and known thrombophilia. A newer agent approved for treating menorrhagia (not for contraception), tranexamic acid (Lysteda), also carries a contraindication for patients with thrombophilia or history of VTE; however, the evidence for the latter is controversial.7
The updated CDC guidelines for the use of hormonal contraceptives state that patients who receive anticoagulation for at least 3 months and who have no history of VTE or a low risk of recurrent VTE (no evidence of active cancer, no known thrombophilia) may use estrogen-containing contraceptives in select cases (category 3—theoretical risk outweighs benefits, but not an absolute contraindication).5 Although this is not common clinical practice, select patients may benefit from menstrual cycle control while receiving anticoagulation. However, other contraceptive alternatives are preferred if possible.
Progestin-only treatments such as the Mirena IUS (if the fibroids do not distort the uterine cavity) and the etonogestrel implant (Implanon) are nonsurgical options that may reduce menorrhagia and are safer alternatives for patients with thrombophilia.
The Paragard (copper) intrauterine device would provide nonhormonal contraception without diminishing menorrhagia. Obviously, barrier methods (which are less effective than hormonal contraception) can be suggested for contraception alone. A viable option for women finished with childbearing is hysterectomy, which provides contraceptive benefit and definitive treatment of menorrhagia due to adenomyosis.
Laboratory screening for VTE is not required before starting estrogen-containing contraceptives. However, one should take a detailed history and inquire about VTE events or a family history of recurrent VTE.
VTE rates among reproductive-age women are 4 to 5 per 10,000 women per year.8 The rate of VTE in oral contraceptive users is estimated as 9 to 10 per 10,000 women per year.9 However, rates of VTE associated with pregnancy and postpartum states are exponentially greater. Although recent studies have shown some discrepancy in rates of VTE across different classes of progestins,10,11 the absolute risk of VTE with hormonal contraceptives is very low.
In December 2011, an FDA panel voted 15 to 11 that the benefits of drospirenone-containing contraceptives (eg, Yaz, Yasmin, Beyaz, Safyral), such as preventing pregnancy, outweigh the potential risk. However, product labeling may change in the future to more accurately reflect the risk-benefit ratio. Stay tuned for better-designed trials to further assess VTE risk across progestins.
Health care providers should engage patients in an informed discussion about all risks and benefits of hormonal contraceptives and note this risk of VTE is higher in gravid women.
CASE 3: FUTURE FERTILITY
A 30-year-old surgical resident who has never been pregnant comes for her annual examination. She currently desires birth control but would like to be pregnant 1 to 2 years from now. She has no history of significant medical illness. Her body mass index is 23 kg/m2, and she takes no medications. How do you counsel her?
Many options; also consider folic acid
Effective counseling leads to patient-centered decision-making for all treatments and procedures. Contraceptive counseling should elicit the patient’s perspective about hormonal methods and educate her on efficacy, proper use, and common adverse effects.
Contraception should fit the patient’s lifestyle. Questions as simple as “Are you a good pill-taker?” or “Are you comfortable with injections?” will help you and the patient assess what will work effectively and will maintain good adherence.
Deciding on a contraceptive option that is cost-effective is crucial, particularly for many young women or adolescents. Many oral contraceptives are widely available as generic formulations for less than $10 per month. Although generic drugs are not required to be 100% bioequivalent to their brand-name counterparts, they can provide a more economical option. For a complete guide to different hormonal contraceptive formulations, we suggest Choosing a birth control method, available on the Web site of the Association of Reproductive Health Professionals at www.arhp.org/upload-Docs/choosingqrg.pdf.12
As discussed earlier, half of all pregnancies are unplanned, and so women of childbearing age should be ingesting 400 μg of folic acid daily. Debate exists as to whether Americans who eat a balanced diet need a multivitamin.13 However, there is no debate about folic acid, which is proven to prevent neural tube defects. Newer formulations of ethinyl estradiol-drospirenone (Beyaz, Safyral) now contain an active form of folic acid (levomefolate calcium 451 mg in each pill). For the above patient who needs contraception and is willing to take birth control, the addition of folic acid provides an essential element in preconception counseling.
Regardless of the current contraceptive choice, patients who actively desire pregnancy should take a prenatal vitamin that contains folic acid and iron.
In addition to combined oral contraceptives, other options for this patient include medroxyprogesterone acetate (intramuscular or subcutaneous), NuvaRing, or intrauterine devices. The Ortho Evra patch is also an option for this patient. However, since 2008 the patch has carried an FDA warning that the risk of VTE is twice as high with this product than with oral contraceptives that contain 30 μg of ethinyl estradiol plus levonorgestrel.14 Postmarketing data did not show any higher risk of VTE in patch users compared with oral contraceptive users less than 40 years of age, however.15
CASE 4: PSYCHIATRIC ILLNESS
A 21-year-old woman who has bipolar II disorder comes to your office for her annual gynecologic evaluation. She has one sexual partner and desires oral contraceptive pills. Lithium treatment has failed for her, but her condition is stable on carbamazepine (Tegretol). She asks if it is true that women can still get pregnant while on the birth control pill. How do you counsel her?
Possible interactions with psychiatric drugs
Like the woman in case 3, this patient has many options, including estrogen-containing pills, the vaginal ring, the patch, injectable contraceptives, and intrauterine devices.
Certain antiepileptic, antipsychotic, or headache medications such as carbamezapine, phenytoin (Dilantin), oxcarbazepine (Trileptal), and topiramate (Topamax) decrease levels of hormonal contraceptives by induction of the CYP450 enzymes. Conversely, it is suggested that lamotrigine (Lamictal) levels decrease by up to 49% while patients concomitantly take oral contraceptive pills, which can induce seizure activity.16 Also, antibiotics such as rifampin (Rifadin) and even herbs such as St. John’s Wort can decrease the effectiveness of hormonal contraceptives by increasing their metabolism.
On the positive side, depot medroxyprogesterone acetate raises the seizure threshold by a mechanism attributed to high levels of progestins and is a better option for epileptic patients. A bulletin of the American College of Gynecologists addresses the paucity of data on hormonal treatments in depressed patients. However, some evidence points to slight improvement of depressive symptoms after 1 year in patients who took Depo-Provera compared with those who discontinued the drug.17
The Pearl index, a measure of contraceptive efficacy
We refer to the Pearl index when answering our patients’ questions about contraceptive efficacy. The Pearl index is defined as the number of unintended pregnancies per 100 women per year. The typical (or actual) effectiveness for each contraceptive method is quoted rather than the theoretical (perfect-use) efficacy.
We suggest simplifying this discussion with patients. For example, for every 100 women using male condoms for contraception, 15 women have unintended pregnancies per year. With hormonal contraceptives (pill, patch, or ring), for every 100 women there are 8 per year with unintended pregnancy, 3 of 100 with Depo-Provera, and less than 1 in 100 using intrauterine devices or female or male sterilization.18
Efficacy decreases (and the failure rate increases) with frequency of intercourse, irregular menstrual cycles, missed pills, improper dosing, and drug-drug interactions as described above.
CASE 5: HYPERTENSION
A 33-year-old woman who has been pregnant twice experienced preeclampsia in her last pregnancy, and now her blood pressure is consistently approximately 140/90 mm Hg on multiple office visits and ambulatory monitoring. She desires contraception. How do you counsel her?
Avoid estrogen-containing products
According to the WHO and CDC guidelines,5 women with controlled or uncontrolled hypertension should not be offered combined oral contraceptives, the patch, or the ring (category 3—theoretical or proven risks outweigh the benefits, and category 4 for systolic blood pressure greater than 160 mm Hg or diastolic blood pressure greater than 100 mm Hg).
The progesterone-only pill (“mini pill”), medroxyprogesterone acetate (intramuscular or subcutaneous), Mirena IUS, the copper intrauterine device, and the etonogestrel implant are all safer options.
A small subset of patients develop elevated blood pressure after starting hormonal contraceptives. Estrogen-containing hormones can increase the liver’s output of angiotensinogen, which is a renin substrate that activates the renin-angiotensin-aldosterone system. If this becomes clinically apparent, these patients should refrain from estrogen-containing products and use progestin-only formulations as a safer alternative.
Patients with isolated elevated hypertriglyceridemia should avoid oral contraceptives. However, the patch, the ring, and progestin-only methods may be acceptable.
Diabetic patients with microvascular complications of retinopathy or nephropathy and any patient with macrovascular disease (stroke, cardiovascular disease) should not be offered estrogen-containing contraception.
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006; 38:90–96.
- Centers for Disease Control and Prevention. Vital signs: teenage pregnancy—United States 1991–2009. MMWR 2011; 60:414–420. www.cdc.gov/mmwr/preview/mmwrhtml/mm6013a5.htm. Accessed January 10, 2012.
- Nettleman MD, Chung H, Brewer J, Ayoola A, Reed PL. Reasons for unprotected intercourse: analysis of the PRAMS survey. Contraception 2007; 75:361–366.
- Nelson A. New low-dose, extended-cycle pills with levonorgestrel and ethinyl estradiol: an evolutionary step on birth control. Int J Womens Health 2010; 2:99–106.
- US Centers for Disease Control and Prevention. Appendix L. Summary of classifications for hormonal contraceptive methods and intrauterine devices. MMWR 2010; 59( RR04):76–81. www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm. Accessed January 10, 2012.
- Wiegratz L, Lee JH, Kutchera E, et al. Effect of dienogest-containing oral contraceptives in lipid metabolism. Contraception 2002; 65:223–229.
- Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case-control study using the General Practice Research Database. Br J Obstet Gynecol 2009; 116:91–97.
- Heinemann L, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception 2007; 75:328–336.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception 2007; 75:344–354.
- Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ 2011; 342:d2139.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ 2011; 342:d2151.
- Association of Reproductive Health Professionals (ARHP). Choosing a birth control method. www.arhp.org/uploadDocs/choosingqrg.pdf. Accessed January 10, 2012.
- Caballero B. Should healthy people take a multivitamin? Cleve Clin J Med 2010; 77:656–657.
- US Food and Drug Administration. Ortho Evra questions and answers (1/18/2008). www.fda.gov/Drugs/DrugSafety/Postmarket-DrugSafetyInformationforPatientsandProviders/ucm110403.htm. Accessed January 10, 2012.
- Jick SS, Hagberg KW, Hernandez RK, Kaye JA. Postmarketing study of ORTHO EVRA and levonorgestrel oral contraceptives containing hormonal contraceptives with 30 mcg of ethinyl estradiol in relation to nonfatal venous thromboembolism. Contraception 2010; 81:16–21.
- Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology 2003; 61:570–571.
- Westhoff C, Truman C, Kalmuss D, et al; Depressive symptoms and Depo-Provera. Contraception 1998; 57:237–240.
- Trusell Wynn LL. Reducing unintended pregnancy in the United States. Contraception 2008; 77:1–5.
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health 2006; 38:90–96.
- Centers for Disease Control and Prevention. Vital signs: teenage pregnancy—United States 1991–2009. MMWR 2011; 60:414–420. www.cdc.gov/mmwr/preview/mmwrhtml/mm6013a5.htm. Accessed January 10, 2012.
- Nettleman MD, Chung H, Brewer J, Ayoola A, Reed PL. Reasons for unprotected intercourse: analysis of the PRAMS survey. Contraception 2007; 75:361–366.
- Nelson A. New low-dose, extended-cycle pills with levonorgestrel and ethinyl estradiol: an evolutionary step on birth control. Int J Womens Health 2010; 2:99–106.
- US Centers for Disease Control and Prevention. Appendix L. Summary of classifications for hormonal contraceptive methods and intrauterine devices. MMWR 2010; 59( RR04):76–81. www.cdc.gov/mmwr/preview/mmwrhtml/rr5904a13.htm. Accessed January 10, 2012.
- Wiegratz L, Lee JH, Kutchera E, et al. Effect of dienogest-containing oral contraceptives in lipid metabolism. Contraception 2002; 65:223–229.
- Sundström A, Seaman H, Kieler H, Alfredsson L. The risk of venous thromboembolism associated with the use of tranexamic acid and other drugs used to treat menorrhagia: a case-control study using the General Practice Research Database. Br J Obstet Gynecol 2009; 116:91–97.
- Heinemann L, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception 2007; 75:328–336.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception 2007; 75:344–354.
- Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ 2011; 342:d2139.
- Jick SS, Hernandez RK. Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: case-control study using United States claims data. BMJ 2011; 342:d2151.
- Association of Reproductive Health Professionals (ARHP). Choosing a birth control method. www.arhp.org/uploadDocs/choosingqrg.pdf. Accessed January 10, 2012.
- Caballero B. Should healthy people take a multivitamin? Cleve Clin J Med 2010; 77:656–657.
- US Food and Drug Administration. Ortho Evra questions and answers (1/18/2008). www.fda.gov/Drugs/DrugSafety/Postmarket-DrugSafetyInformationforPatientsandProviders/ucm110403.htm. Accessed January 10, 2012.
- Jick SS, Hagberg KW, Hernandez RK, Kaye JA. Postmarketing study of ORTHO EVRA and levonorgestrel oral contraceptives containing hormonal contraceptives with 30 mcg of ethinyl estradiol in relation to nonfatal venous thromboembolism. Contraception 2010; 81:16–21.
- Sabers A, Ohman I, Christensen J, Tomson T. Oral contraceptives reduce lamotrigine plasma levels. Neurology 2003; 61:570–571.
- Westhoff C, Truman C, Kalmuss D, et al; Depressive symptoms and Depo-Provera. Contraception 1998; 57:237–240.
- Trusell Wynn LL. Reducing unintended pregnancy in the United States. Contraception 2008; 77:1–5.
KEY POINTS
- Hormonal contraceptives have a number of noncontraceptive benefits, such as regulating the menstrual cycle.
- The Pearl index is the number of unintended pregnancies per 100 women per year. Rates are 15% using male condoms, 8% with oral contraceptives, 3% with depot medroxyprogesterone acetate (Depo-Provera) injections, and less than 1% with intrauterine devices or female or male sterilization.
- Estrogen-containing products should be avoided in patients with hypertension or who are at risk of venous thromboembolism.
A 37-year-old man with a chronic cough
A 37-year-old man presented to the emergency department with an 8-week history of a mildly productive cough and shortness of breath accompanied by high fevers, chills, and night sweats. He also had some nausea but no vomiting.
Four days earlier, he had been evaluated by his primary care physician, who prescribed a 14-day course of one double-strength trimethoprim-sulfamethoxazole tablet (Bactrim DS) every 12 hours for presumed acute bronchitis, but his symptoms did not improve.
He was unemployed, living in Arizona, married with children. He denied any use of tobacco, alcohol, or injection drugs. On further questioning, he disclosed that he had unintentionally lost 30 pounds over the past 2 to 3 months and had been feeling tired.
When asked about his medical history, he revealed that he had been diagnosed with human immunodeficiency virus (HIV) infection in 2008 and that recently he had not been taking his antiretroviral medication, a once-daily combination pill containing efavirenz, emtricitabine, and tenofovir (Atripla). He had no other significant medical history, and the only medication he was currently taking was the trimethoprim-sulfamethoxazole.
On examination, his temperature was 38.7°C (101.7°F), blood pressure 109/68 mm Hg, heart rate 60 beats per minute, respiratory rate 18 breaths per minute, and oxygen saturation 100% while breathing supplemental oxygen via nasal cannula at 2 L/min. He did not appear seriously ill.
Initial blood tests (Table 1) showed a normal white blood cell count, normal results on a complete metabolic panel, and a lactate dehydrogenase level of 539 IU/L (reference range 313–618). His serum lactate level was within normal limits.
HIV-specific tests performed on the second day of hospitalization showed extreme immunosuppression, with a CD4 count of 5 cells/μL (normal 326–1,404 cells/μL).
WHICH ORGANISM IS CAUSING HIS LUNG INFECTION?
1. Which of the following organisms is the least likely to be associated with this patient’s condition?
- Mycobacterium tuberculosis
- Pneumocystis jirovecii
- Coccidioides immitis
- Candida albicans
- Streptococcus pneumoniae
- Cytomegalovirus
Bacterial, fungal, and viral lung infections are common in HIV-infected patients, especially if they are not on antiretroviral therapy and their CD4 lymphocyte counts are low. Clues to the cause can be derived from the history, physical examination, and general laboratory studies. For instance, knowing where the patient lives and where he has travelled recently provides insight into exposure to endemic infectious agents.
The complete blood cell count with differential white blood cell count can help narrow the differential diagnosis but rarely helps exclude a possibility. Neutrophilia is common in bacterial infections. Lymphocytosis can be seen in tuberculosis, in fungal and viral infections, and, rarely, in hematologic malignancies. Eosinophilia can be seen in acute retroviral syndrome, fungal and helminthic infections, adrenal insufficiency, autoimmune disease, and lymphoma.
A caveat to these clues is that in severely immunocompromised hosts, like this man, diagnoses should not be excluded without firm evidence. This patient has severe, active immunosuppression, and only one of the six answer choices above is not a possible causative agent: C albicans rarely causes lung infection, even in immunocompromised people.
Mycobacterium tuberculosis
Tuberculosis can be the first manifestation of HIV infection. It can occur at any CD4 count, but as the count decreases, the risk of dissemination increases.1 Classic symptoms are fever, night sweats, hemoptysis, and weight loss.
The CD4 count also affects the radiographic presentation. If the count is higher than 350 cells/μL, then infiltration of the upper lobe is likely; if it is lower than 200 cells/μL, then middle, lower, miliary, and extrapulmonary manifestations are likely.1,2 Cavitation is less common in HIV-infected patients, but mediastinal adenopathy is more common.1
Definitive diagnosis is via sputum examination, blood culture, nucleic acid amplification, or microscopic study of biopsy specimens of affected tissues to look for acid-fast bacilli.1
Interferon-gamma-release assays such as the QuantiFERON test (Cellestis, Valencia, CA) or a tuberculin skin test can be used to check for latent tuberculosis infection. These tests can also provide evidence of active infection in the appropriate clinical context.3
Interferon-gamma-release assays have several advantages over skin testing: they are more sensitive (76% to 80%) and specific (97%); they do not give false-positive results in people who previously received bacille Calmette-Guérin vaccine; they react only minimally to previous exposure to nontuberculous mycobacteria; and interpretation is not subject to interreader variability.4,5 However, concordance between skin testing and interferon-gamma-release assays is low. Therefore, either or both tests can be used if tuberculosis is strongly suspected, and a positive result on either test should prompt further workup.6,7
Of note, both tests may be affected by immunosuppression, making both susceptible to false-negative results as the CD4 count declines.3
In any case, a positive acid-fast bacillus smear, radiographic evidence of latent infection, or pulmonary symptoms should be presumed to represent active tuberculosis. In such a situation, directly observed treatment with the typical four-drug regimen—rifampin (Rifadin), isoniazid, pyrazinamide, and ethambutol (Myambutol)—is recommended while awaiting definitive results from culture or polymerase chain reaction (PCR) testing.1
Pneumocystis jirovecii
P jirovecii was previously known as P carinii, and P jirovecii pneumonia is an AIDS-defining illness. Most cases occur when the CD4 count falls below 200 cells/μL.1 Symptoms, including a nonproductive cough, develop insidiously over days to weeks.
Physical examination may reveal inspiratory crackles; however, half of the time the physical examination is nondiagnostic. Oral candidiasis is a common coinfection. The lactate dehydrogenase level may be elevated.1,8 Radiographs show bilateral interstitial infiltrates, and in 10% to 20% of patients lung cysts develop—hence the name of the organism.1 Pneumothorax in a patient with HIV should prompt a workup for P jirovecii pneumonia.9,10
No consensus exists for the diagnosis. However, if sputum examination is unrevealing but suspicion is high, then bronchoalveolar lavage can help.11–13
Trimethoprim-sulfamethoxazole for 21 days is the first-line treatment, with glucocorticoids added if the Pao2 is less than 70 mm Hg or if the alveolar-arterial oxygen gradient is greater than 35 mm Hg.1
Coccidioides species
Coccidioides infection is typically due to either C immitis or C posadasii.14 People living in or travelling to areas where it is endemic, such as the southwestern United States, Mexico, and Central and South America, are at higher risk.14
Typical signs and symptoms of this fungal infection include an influenza-like illness with fever, cough, adenopathy, and wasting, and when combined with erythema nodosum, erythema multiforme, arthralgia, or ocular involvement, this constellation is colloquially termed “valley fever.”15 Most HIV-infected patients who have CD4 counts higher than 250 cells/μL present with focal pneumonia, while lower counts predispose to disseminated disease.1,2,16
Findings on examination are nonspecific and depend on the various pulmonary manifestations, which include acute, chronic progressive, or diffuse pneumonia, nodules, or cavities.14 Eosinophilia may accompany the infection.15
The diagnosis can be made by finding the organisms on direct microscopic examination of involved tissues or secretions or on culture of clinical specimens.1,2,14 Serologic tests, antigen detection tests, or culture can be helpful if positive, but negative results do not rule out the diagnosis.1,2,14
A caveat about testing: if the pretest probability of infection is low, positive tests for immunoglobulin M (IgM) do not necessarily equal infection, and the IgM test should be followed up with confirmatory testing. Along the same lines, a high pretest probability should not be ignored if initial tests are negative, and patients in this situation should also undergo further evaluation.17
Therapy with an azole drug such as fluconazole (Diflucan) or one of the amphotericin B preparations should be started, depending on the severity of the disease.1,2,14,18
Candida albicans
C albicans is a rare cause of lung infection.19,20 It is, however, a common inhabitant of the upper airway tract, and pulmonary infection is usually the result of aspiration or hematogenous spread from either the gastrointestinal tract or an infected central venous catheter.20
The presentation is relatively nonspecific. Fever despite broad-spectrum antibacterial therapy is a major clue. Radiographic abnormalities usually are due to other causes, such as superimposed infections or pulmonary hemorrhage.21 Sputum culture is unreliable because of colonization. The definitive diagnosis is based on lung biopsy demonstrating organisms within the tissue.19,20,22
Therapy with a systemic antifungal agent is recommended.
Streptococcus pneumoniae
S pneumoniae is one of the most common bacterial causes of community-acquired pneumonia in people with or without HIV.23–25 Moreover, two or more episodes of bacterial pneumonia in 12 months can be an AIDS-defining condition in patients with a positive serologic test for HIV.16 Therefore, in patients with fever, cough, and pulmonary infiltrates on chest radiography, S pneumoniae must always be considered.
Urinary antigen testing has a relatively high positive predictive value (> 89%) and specificity (96%) for diagnosing S pneumoniae pneumonia.26 Blood and sputum cultures should be done not only to confirm the diagnosis, but also because the rates of bacteremia and drug resistance are higher with S pneumoniae infection in the HIV-infected.1
A combination of a beta-lactam and a macrolide or respiratory fluoroquinolone is the treatment of choice.1
Cytomegalovirus
Although influenza is the most common cause of viral pneumonia in HIV-infected people, cytomegalovirus is an opportunistic cause.2 This is usually a reactivation of latent infection rather than new infection.27 Typically, infections occur at CD4 counts lower than 50 cells/μL, with cough, dyspnea, and fever that last for 2 to 4 weeks.2
Crackles may be heard on lung examination. The lactate dehydrogenase level can be elevated, as in P jirovecii pneumonia.2 Radiography can show a wide range of nonspecific findings, from reticular and ground-glass opacities to alveolar or interstitial infiltrates to nodules.
The diagnosis of cytomegalovirus pneumonia is not always clear. Since HIV-infected patients typically shed the virus in their airways, bronchoalveolar lavage is not adequate because a positive finding does not necessarily mean the patient has active viral pneumonitis.27 For this reason, infection should be confirmed by biopsy demonstrating characteristic cytomegalovirus inclusions in lung tissue.2
Importantly, once cytomegalovirus pneumonia is confirmed, the patient should be screened for cytomegalovirus retinitis even if he or she has no visual symptoms, as cytomegalovirus pneumonitis is typically a part of a disseminated infection.1
Treatment with intravenous ganciclovir (Cytovene) is required.1
CASE CONTINUED: POSITIVE TESTS FOR COCCIDIOIDES
Our patient began empiric treatment for community-acquired pneumonia with intravenous ceftriaxone (Rocephin) and azithromycin (Zithromax).
On the basis of these findings, the patient was immediately placed in negative pressure respiratory isolation and underwent induced sputum examinations for tuberculosis. Further tests for S pneumoniae, S aureus, Mycoplasma, Legionella, influenza, Pneumocystis, Cryptococcus, Histoplasma, and Coccidioides species were performed.
QuantiFERON testing was negative, and blood cultures were sterile. The first induced sputum examination was negative for acid-fast bacilli. PCR testing for mycobacterial DNA in the sputum was also negative.
Both silver and direct fluorescent antibody staining of the sputum were negative for Pneumocystis. On the basis of these findings and the patient’s lack of clinical improvement with trimethoprim-sulfamethoxazole, Pneumocystis infection was excluded.
THE PATIENT BEGINS TREATMENT
2. Which treatment is most appropriate for this patient?
- Posaconazole (Noxafil)
- Caspofungin (Cancidas) and surgery
- Fluconazole
- Voriconazole (Vfend) and surgery
- Amphotericin B
Solitary pulmonary cavities tend to be asymptomatic and do not require treatment, even if coccidioidal infection is microbiologically confirmed.
However, if there is pain, hemoptysis, or bacterial superinfection, antifungal therapy may result in improvement but not closure of the cavity.18 Therefore, in all cases of symptomatic coccidioidal pulmonary cavities, surgical resection is the only definitive treatment.
Coccidioidal cavities may rupture and cause pyopneumothorax, but this is an infrequent complication, and antifungal therapy combined with surgical decortication is the treatment of choice.18
Commonly prescribed antifungals include fluconazole and amphotericin B, the latter usually reserved for patients with significant hypoxia or rapid clinical deterioration.18 At this time, there are not enough clinical data to show that voriconazole or posaconazole is effective, and thus neither is approved for the treatment of coccidioidomycosis. Likewise, there have been no human trials of the efficacy of caspofungin against Coccidioides infection, although it has been shown to be active in mouse models.18
Our patient was started on oral fluconazole and observed for clinical improvement or, conversely, for signs of dissemination. After 2 days, he had markedly improved, and within 1 week he was almost back to his baseline level of health. Testing for all other infectious etiologies was unrevealing, and he was removed from negative pressure isolation.
However, as we mentioned above, his CD4 count was 5 cells/μL. We discussed the issue with the patient, and he said he was willing to comply with his treatment for both his Coccidioides and his HIV infection. After much deliberation, he said he was also willing to start and comply with prophylactic treatment for opportunistic infections.
PREVENTING OPPORTUNISTIC INFECTIONS IN HIV PATIENTS
3. Which of the following prophylactic regimens is most appropriate for this patient?
- Trimethoprim-sulfamethoxazole, atovaquone (Mepron), and azithromycin
- Trimethoprim-sulfamethoxazole and azithromycin
- Pentamidine (Nebupent), dapsone, and clarithromycin (Biaxin)
- Dapsone and clarithromycin
- Trimethoprim-sulfamethoxazole by itself
According to guidelines for the prevention of opportunistic diseases in patients with HIV, he needs primary prophylaxis against the following organisms: P jirovecii, Toxoplasma gondii, and Mycobacterium avium complex.1
The CD4 count dictates the appropriate time to start therapy. If the count is lower than 200 cells/μL or if the patient has oropharyngeal candidiasis regardless of the CD4 count, trimethoprim-sulfamethoxazole is indicated to prevent P jirovecii pneumonia. In those who cannot tolerate trimethoprim-sulfamethoxazole or who are allergic to it, dapsone, pentamidine, or atovaquone can be substituted.1
In patients seropositive for T gondii, a CD4 count lower than 100/μL indicates the need for prophylaxis.1 Prophylactic measures are similar to those for Pneumocystis. However, if the patient cannot tolerate trimethoprim-sulfamethoxazole, the recommended alternative is dapsone-pyrimethamine with leucovorin, which is also effective against Pneumocystis.1
Finally, if the CD4 count is lower than 50 cells/μL, prophylaxis against M avium complex is mandatory, with either azithromycin weekly or clarithromycin daily.1
Given our patient’s degree of immunosuppression, trimethoprim-sulfamethoxazole plus azithromycin is his most appropriate option.
Trimethoprim-sulfamethoxazole and azithromycin were added to his antimicrobial regimen before he was discharged. Two weeks later, he noted no side effects from any of the medications, he had no new symptoms, he was feeling well, and his cough had improved greatly. He did not have any signs of dissemination of his coccidioidal infection, and we concluded that the primary and only infection was located in the lungs.
DISSEMINATED COCCIDIOIDOMYCOSIS
4. Which of the following extrapulmonary sites is Coccidioides least likely to infect?
- Brain
- Skin
- Meninges
- Lymph nodes
- Bones
- Joints
Extrapulmonary coccidioidomycosis can involve almost any site. However, the most common sites of dissemination are the skin, lymph nodes, bones, and joints.14 The least likely site is the brain.
Central nervous system involvement
In the central nervous system, involvement is typically with the meninges, rather than frank involvement of the brain parenchyma.18,28,29 Although patients with HIV or those who are otherwise severely immunocompromised are at higher risk for coccidioidal meningitis, it is rare even in this population.30,31 Meningitis most commonly presents as headache, vomiting, meningismus, confusion, or diplopia.32,33
If neurologic findings are absent, experts do not generally recommend lumbar puncture because the incidence of meningeal involvement is low. When cerebrospinal fluid is obtained in an active case of coccidioidal meningitis, fluid analysis typically finds elevated protein, low glucose, and lymphocytic pleocytosis.1,32
Meningeal enhancement on CT or magnetic resonance imaging is common.34 The diagnosis is established by culture or serologic testing of cerebrospinal fluid (IgM titer, IgG titer, immunodiffusion, or complement fixation).14
Of note, cerebral infarction and hydrocephalus are feared complications and pose a serious risk of death in any patient.32,35 In these cases, treatment with antifungals is lifelong, regardless of immune system status.18
Skin involvement
Skin involvement is variable, consisting of nodules, verrucae, abscesses, or ulcerations.15,16 Hemorrhage from the skin is relatively common.36 From the skin, the infection can spread to the lymph nodes, leading to regional lymphadenopathy.14,15 Nodes can ulcerate, drain, or even become necrotic.
Bone and joint involvement
Once integrity of the blood vessels is disrupted, Coccidioides can spread via the blood to the bones or joints,14,15 causing osteomyelitis, septic arthritis, or synovitis. Subcutaneous abscesses or sinus tracts may subsequently develop.14,15
HOW LONG MUST HE BE TREATED?
On follow-up, the patient asked how long he needed to continue his antifungal regimen and if any other testing for his coccidioidal infection was necessary, since he was feeling better.
5. Which is the most appropriate response to the patient’s question?
- He can discontinue his antifungal drugs; no further testing is necessary
- He needs 14 more days of antifungal therapy and periodic serologic tests
- He needs 2.5 more months of antifungal therapy and monthly blood cultures
- He needs lifelong antifungal therapy and periodic urinary antigen levels
- He needs 5.5 more months of antifungal therapy; bronchoscopy with bronchoalveolar lavage at 1 year
How long to treat and how to monitor for coccidioidomycosis vary by patient.
Duration of therapy depends on symptoms and immune status
The severity of infection (Table 2) and the immune status are important factors that must be considered when tailoring a therapeutic regimen.
Immunocompetent patients without symptoms or with mild symptoms usually do not need therapy and are followed periodically for signs of improvement.14,18,29
Immunocompetent patients with severe symptoms typically receive 3 to 6 months of antifungal therapy.18
Immunocompromised patients (especially HIV-infected patients with CD4 counts < 250 cells/μL) need antifungal treatment, regardless of the severity of infection.14,18,29 In many cases, the type of infection will dictate the duration of therapy.
Diffuse pneumonia or extrapulmonary dissemination typically requires treatment for at least 1 year regardless of immune status.14,18 For those with HIV and diffuse pneumonia, dissemination, or meningitis, guidelines dictate that secondary prophylaxis be started after at least 1 year of therapy and improvement in clinical status; it should be continued indefinitely to prevent reactivation of latent infection.18
The guidelines say that in patients with higher CD4 counts (presumably > 250 cells/μL) and nonmeningeal coccidioidomycosis, providers may consider discontinuing secondary prophylaxis, as long as there is clinical evidence of improvement and control of the primary infection.18 However, many experts advocate continuing secondary prophylaxis regardless of the CD4 count, as the rates of relapse and dissemination are high.1,16,37
Monitoring
Regardless of the therapy chosen, disease monitoring every 2 to 4 months with clinical history and examination, radiography, and coccidioidal-specific testing is recommended for at least 1 year, and perhaps longer, to ensure complete resolution and to monitor for signs of dissemination.14,18
Which test to use is not clear. Serologic testing identifies antibodies (IgM or IgG) to coccidioidal antigens. IgM appears during the acute infection, and tests include immunodiffusion, latex agglutination, and enzymelinked immunoassays. The last two are highly sensitive but have a significant false-positive rate, and should be confirmed with the former if found to be positive.17,18 IgG appears weeks after the acute infection and can be evaluated with immunodiffusion or enzyme-linked immunoassay as well.
Keep in mind that these tests provide only qualitative results on the presence of these antibodies, not quantitative information. Furthermore, enzyme-linked immunoassay is not as accurate as immunodiffusion, which has a sensitivity in immunocompromised patients of only approximately 50%.38,39
For that reason, complement fixation titers are extremely helpful because they reflect the severity of infection, can be used to monitor the response to treatment, and can even provide insight into the prognosis.18 The sensitivity of this test in immunocompromised hosts is 60% to 70%.38 Titers can be checked to confirm the diagnosis and can be periodically monitored throughout the treatment course to ensure efficacy of therapy and to watch for reactivation of the infection.1 In fact, an initial complement fixation titer of 1:2 or 1:4 is associated with favorable outcomes, while a titer greater than 1:16 portends dissemination.18
The caveat to any serologic test (immunodiffusion, enzyme-linked immunoassay, and complement fixation) is that severely immunocompromised patients (as in our case) may not mount an immune response and may have falsely low titers even in the face of a severe infection, and therefore these tests may not be reliable.38 In these situations, urinary coccidioidal antigen detection assay (sensitivity 71%) or nucleic acid amplification of coccidioidal DNA (sensitivity 75%) may be of more help.40,41
Therefore, in the setting of HIV infection, an asymptomatic pulmonary cavity, and diffuse pulmonary involvement secondary to coccidioidal infection, lifelong antibiotics (treatment plus secondary prophylaxis) with periodic testing of urinary coccidioidal antigen levels is the best response to the patient’s question, given that his complement fixation titers were initially negative and antigen levels were positive.
CASE CONCLUDED
The patient continues to be followed for his HIV infection. He is undergoing serologic and urinary antigen testing for Coccidioides infection every 3 months in addition to his maintenance HIV testing. He is on chronic suppressive therapy with fluconazole. He has not had a recurrence of his Coccidioides infection, nor have there been any signs of dissemination.
CAVITARY LUNG LESIONS IN HIV PATIENTS
In patients with HIV, cavitary lung lesions on chest radiography can be due to a wide variety of etiologies that range from infection to malignancy. Historical clues, including environmental exposure, occupation, geographic residence, sick contacts, travel, or animal contact can be helpful in ordering subsequent confirmatory testing, especially in the case of infection.
Tuberculosis should be suspected, and appropriate isolation precautions should be taken until it is ruled out.
Laboratory testing, including the complete blood cell count with differential and CD4 count, provide ancillary data to narrow the differential diagnosis. For example, if the CD4 count is greater than 200 cells/μL, mycobacterial infection should be strongly suspected; however, lower CD4 counts should also prompt a search for opportunistic infections. In the appropriate clinical scenario, malignancies including Kaposi sarcoma, non-Hodgkin lymphoma, and bronchogenic carcinoma can be seen and should also be considered.
Nevertheless, the evaluation hinges on the sputum examination and CT scan of the chest to further characterize the cavity, surrounding lung parenchyma, lymph nodes, and potential fluid collections. Usually, further serologic tests and even bronchoscopy with bronchoalveolar lavage and transbronchial biopsy are required. Treatment should begin once the most likely diagnosis is established.
Coccidioidal pneumonia should be considered in all patients with immunodeficiency, including HIV patients, transplant recipients, those undergoing chemotherapy, and those with intrinsic immune system defects, especially if they have a history of exposure or if they are from an endemic region. Antifungal therapy should be initiated early, and dissemination must be ruled out. Suppressive therapy is mandatory for those with a severely compromised immune system, and serologic testing to ensure remission of the infection is needed. Patients who were previously exposed to Coccidioides or who vacationed or live in the southwestern United States (where it is prevalent) are at risk and may present with any number of symptoms.
- Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H; Centers for Disease Control and Prevention (CDC). Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009; 58:1–207.
- Huang L, Crothers K. HIV-associated opportunistic pneumonias. Respirology 2009; 14:474–485.
- Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005; 54:49–55.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Nahid P, Pai M, Hopewell PC. Advances in the diagnosis and treatment of tuberculosis. Proc Am Thorac Soc 2006; 3:103–110.
- Chapman AL, Munkanta M, Wilkinson KA, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS 2002; 16:2285–2293.
- Luetkemeyer AF, Charlebois ED, Flores LL, et al. Comparison of an interferon-gamma release assay with tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med 2007; 175:737–742.
- Zaman MK, White DA. Serum lactate dehydrogenase levels and Pneumocystis carinii pneumonia. Diagnostic and prognostic significance. Am Rev Respir Dis 1988; 137:796–800.
- Metersky ML, Colt HG, Olson LK, Shanks TG. AIDS-related spontaneous pneumothorax. Risk factors and treatment. Chest 1995; 108:946–951.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Baughman RP, Dohn MN, Frame PT. The continuing utility of bronchoalveolar lavage to diagnose opportunistic infection in AIDS patients. Am J Med 1994; 97:515–522.
- Kovacs JA, Ng VL, Masur H, et al. Diagnosis of Pneumocystis carinii pneumonia: improved detection in sputum with use of monoclonal antibodies. N Engl J Med 1988; 318:589–593.
- Stover DE, Zaman MB, Hajdu SI, Lange M, Gold J, Armstrong D. Bronchoalveolar lavage in the diagnosis of diffuse pulmonary infiltrates in the immunosuppressed host. Ann Intern Med 1984; 101:1–7.
- Parish JM, Blair JE. Coccidioidomycosis. Mayo Clin Proc 2008; 83:343–348.
- Drutz DJ, Catanzaro A. Coccidioidomycosis. Part I. Am Rev Respir Dis 1978; 117:559–585.
- Bartlett JG, Gallant JE, Pham PA. Medical Management of HIV Infection. Durham, NC: Knowledge Source Solutions, LLC; 2009.
- Kuberski T, Herrig J, Pappagianis D. False-positive IgM serology in coccidioidomycosis. J Clin Microbiol 2010; 48:2047–2049.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Kontoyiannis DP, Reddy BT, Torres HA, et al. Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis 2002; 34:400–403.
- Pappas PG, Kauffman CA, Andes D, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–535.
- Connolly JE, McAdams HP, Erasmus JJ, Rosado-de-Christenson ML. Opportunistic fungal pneumonia. J Thorac Imaging 1999; 14:51–62.
- Meersseman W, Lagrou K, Spriet I, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med 2009; 35:1526–1531.
- Miller RF, Foley NM, Kessel D, Jeffrey AA. Community acquired lobar pneumonia in patients with HIV infection and AIDS. Thorax 1994; 49:367–368.
- Polsky B, Gold JW, Whimbey E, et al. Bacterial pneumonia in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1986; 104:38–41.
- Rimland D, Navin TR, Lennox JL, et al; Pulmonary Opportunistic Infection Study Group. Prospective study of etiologic agents of community-acquired pneumonia in patients with HIV infection. AIDS 2002; 16:85–95.
- Boulware DR, Daley CL, Merrifield C, Hopewell PC, Janoff EN. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect 2007; 55:300–309.
- Salomon N, Perlman DC. Cytomegalovirus pneumonia. Semin Respir Infect 1999; 14:353–358.
- Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am 2003; 17:41–57.
- Drutz DJ, Catanzaro A. Coccidioidomycosis. Part II. Am Rev Respir Dis 1978; 117:727–771.
- Fish DG, Ampel NM, Galgiani JN, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine (Baltimore) 1990; 69:384–391.
- Mischel PS, Vinters HV. Coccidioidomycosis of the central nervous system: neuropathological and vasculopathic manifestations and clinical correlates. Clin Infect Dis 1995; 20:400–405.
- Johnson RH, Einstein HE. Coccidioidal meningitis. Clin Infect Dis 2006; 42:103–107.
- Vincent T, Galgiani JN, Huppert M, Salkin D. The natural history of coccidioidal meningitis: VA-Armed Forces cooperative studies, 1955–1958. Clin Infect Dis 1993; 16:247–254.
- Erly WK, Bellon RJ, Seeger JF, Carmody RF. MR imaging of acute coccidioidal meningitis. AJNR Am J Neuroradiol 1999; 20:509–514.
- Arsura EL, Johnson R, Penrose J, et al. Neuroimaging as a guide to predict outcomes for patients with coccidioidal meningitis. Clin Infect Dis 2005; 40:624–627.
- Tappero JW, Perkins BA, Wenger JD, Berger TG. Cutaneous manifestations of opportunistic infections in patients infected with human immunodeficiency virus. Clin Microbiol Rev 1995; 8:440–450.
- Catanzaro A, Galgiani JN, Levine BE, et al. Fluconazole in the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis. NIAID Mycoses Study Group. Am J Med 1995; 98:249–256.
- Blair JE, Coakley B, Santelli AC, Hentz JG, Wengenack NL. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia 2006; 162:317–324.
- Martins TB, Jaskowski TD, Mouritsen CL, Hill HR. Comparison of commercially available enzyme immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. J Clin Microbiol 1995; 33:940–943.
- Vucicevic D, Blair JE, Binnicker MJ, et al. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia 2010; 170:345–351.
- Durkin M, Connolly P, Kuberski T, et al. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin Infect Dis 2008; 47:e69–e73.
A 37-year-old man presented to the emergency department with an 8-week history of a mildly productive cough and shortness of breath accompanied by high fevers, chills, and night sweats. He also had some nausea but no vomiting.
Four days earlier, he had been evaluated by his primary care physician, who prescribed a 14-day course of one double-strength trimethoprim-sulfamethoxazole tablet (Bactrim DS) every 12 hours for presumed acute bronchitis, but his symptoms did not improve.
He was unemployed, living in Arizona, married with children. He denied any use of tobacco, alcohol, or injection drugs. On further questioning, he disclosed that he had unintentionally lost 30 pounds over the past 2 to 3 months and had been feeling tired.
When asked about his medical history, he revealed that he had been diagnosed with human immunodeficiency virus (HIV) infection in 2008 and that recently he had not been taking his antiretroviral medication, a once-daily combination pill containing efavirenz, emtricitabine, and tenofovir (Atripla). He had no other significant medical history, and the only medication he was currently taking was the trimethoprim-sulfamethoxazole.
On examination, his temperature was 38.7°C (101.7°F), blood pressure 109/68 mm Hg, heart rate 60 beats per minute, respiratory rate 18 breaths per minute, and oxygen saturation 100% while breathing supplemental oxygen via nasal cannula at 2 L/min. He did not appear seriously ill.
Initial blood tests (Table 1) showed a normal white blood cell count, normal results on a complete metabolic panel, and a lactate dehydrogenase level of 539 IU/L (reference range 313–618). His serum lactate level was within normal limits.
HIV-specific tests performed on the second day of hospitalization showed extreme immunosuppression, with a CD4 count of 5 cells/μL (normal 326–1,404 cells/μL).
WHICH ORGANISM IS CAUSING HIS LUNG INFECTION?
1. Which of the following organisms is the least likely to be associated with this patient’s condition?
- Mycobacterium tuberculosis
- Pneumocystis jirovecii
- Coccidioides immitis
- Candida albicans
- Streptococcus pneumoniae
- Cytomegalovirus
Bacterial, fungal, and viral lung infections are common in HIV-infected patients, especially if they are not on antiretroviral therapy and their CD4 lymphocyte counts are low. Clues to the cause can be derived from the history, physical examination, and general laboratory studies. For instance, knowing where the patient lives and where he has travelled recently provides insight into exposure to endemic infectious agents.
The complete blood cell count with differential white blood cell count can help narrow the differential diagnosis but rarely helps exclude a possibility. Neutrophilia is common in bacterial infections. Lymphocytosis can be seen in tuberculosis, in fungal and viral infections, and, rarely, in hematologic malignancies. Eosinophilia can be seen in acute retroviral syndrome, fungal and helminthic infections, adrenal insufficiency, autoimmune disease, and lymphoma.
A caveat to these clues is that in severely immunocompromised hosts, like this man, diagnoses should not be excluded without firm evidence. This patient has severe, active immunosuppression, and only one of the six answer choices above is not a possible causative agent: C albicans rarely causes lung infection, even in immunocompromised people.
Mycobacterium tuberculosis
Tuberculosis can be the first manifestation of HIV infection. It can occur at any CD4 count, but as the count decreases, the risk of dissemination increases.1 Classic symptoms are fever, night sweats, hemoptysis, and weight loss.
The CD4 count also affects the radiographic presentation. If the count is higher than 350 cells/μL, then infiltration of the upper lobe is likely; if it is lower than 200 cells/μL, then middle, lower, miliary, and extrapulmonary manifestations are likely.1,2 Cavitation is less common in HIV-infected patients, but mediastinal adenopathy is more common.1
Definitive diagnosis is via sputum examination, blood culture, nucleic acid amplification, or microscopic study of biopsy specimens of affected tissues to look for acid-fast bacilli.1
Interferon-gamma-release assays such as the QuantiFERON test (Cellestis, Valencia, CA) or a tuberculin skin test can be used to check for latent tuberculosis infection. These tests can also provide evidence of active infection in the appropriate clinical context.3
Interferon-gamma-release assays have several advantages over skin testing: they are more sensitive (76% to 80%) and specific (97%); they do not give false-positive results in people who previously received bacille Calmette-Guérin vaccine; they react only minimally to previous exposure to nontuberculous mycobacteria; and interpretation is not subject to interreader variability.4,5 However, concordance between skin testing and interferon-gamma-release assays is low. Therefore, either or both tests can be used if tuberculosis is strongly suspected, and a positive result on either test should prompt further workup.6,7
Of note, both tests may be affected by immunosuppression, making both susceptible to false-negative results as the CD4 count declines.3
In any case, a positive acid-fast bacillus smear, radiographic evidence of latent infection, or pulmonary symptoms should be presumed to represent active tuberculosis. In such a situation, directly observed treatment with the typical four-drug regimen—rifampin (Rifadin), isoniazid, pyrazinamide, and ethambutol (Myambutol)—is recommended while awaiting definitive results from culture or polymerase chain reaction (PCR) testing.1
Pneumocystis jirovecii
P jirovecii was previously known as P carinii, and P jirovecii pneumonia is an AIDS-defining illness. Most cases occur when the CD4 count falls below 200 cells/μL.1 Symptoms, including a nonproductive cough, develop insidiously over days to weeks.
Physical examination may reveal inspiratory crackles; however, half of the time the physical examination is nondiagnostic. Oral candidiasis is a common coinfection. The lactate dehydrogenase level may be elevated.1,8 Radiographs show bilateral interstitial infiltrates, and in 10% to 20% of patients lung cysts develop—hence the name of the organism.1 Pneumothorax in a patient with HIV should prompt a workup for P jirovecii pneumonia.9,10
No consensus exists for the diagnosis. However, if sputum examination is unrevealing but suspicion is high, then bronchoalveolar lavage can help.11–13
Trimethoprim-sulfamethoxazole for 21 days is the first-line treatment, with glucocorticoids added if the Pao2 is less than 70 mm Hg or if the alveolar-arterial oxygen gradient is greater than 35 mm Hg.1
Coccidioides species
Coccidioides infection is typically due to either C immitis or C posadasii.14 People living in or travelling to areas where it is endemic, such as the southwestern United States, Mexico, and Central and South America, are at higher risk.14
Typical signs and symptoms of this fungal infection include an influenza-like illness with fever, cough, adenopathy, and wasting, and when combined with erythema nodosum, erythema multiforme, arthralgia, or ocular involvement, this constellation is colloquially termed “valley fever.”15 Most HIV-infected patients who have CD4 counts higher than 250 cells/μL present with focal pneumonia, while lower counts predispose to disseminated disease.1,2,16
Findings on examination are nonspecific and depend on the various pulmonary manifestations, which include acute, chronic progressive, or diffuse pneumonia, nodules, or cavities.14 Eosinophilia may accompany the infection.15
The diagnosis can be made by finding the organisms on direct microscopic examination of involved tissues or secretions or on culture of clinical specimens.1,2,14 Serologic tests, antigen detection tests, or culture can be helpful if positive, but negative results do not rule out the diagnosis.1,2,14
A caveat about testing: if the pretest probability of infection is low, positive tests for immunoglobulin M (IgM) do not necessarily equal infection, and the IgM test should be followed up with confirmatory testing. Along the same lines, a high pretest probability should not be ignored if initial tests are negative, and patients in this situation should also undergo further evaluation.17
Therapy with an azole drug such as fluconazole (Diflucan) or one of the amphotericin B preparations should be started, depending on the severity of the disease.1,2,14,18
Candida albicans
C albicans is a rare cause of lung infection.19,20 It is, however, a common inhabitant of the upper airway tract, and pulmonary infection is usually the result of aspiration or hematogenous spread from either the gastrointestinal tract or an infected central venous catheter.20
The presentation is relatively nonspecific. Fever despite broad-spectrum antibacterial therapy is a major clue. Radiographic abnormalities usually are due to other causes, such as superimposed infections or pulmonary hemorrhage.21 Sputum culture is unreliable because of colonization. The definitive diagnosis is based on lung biopsy demonstrating organisms within the tissue.19,20,22
Therapy with a systemic antifungal agent is recommended.
Streptococcus pneumoniae
S pneumoniae is one of the most common bacterial causes of community-acquired pneumonia in people with or without HIV.23–25 Moreover, two or more episodes of bacterial pneumonia in 12 months can be an AIDS-defining condition in patients with a positive serologic test for HIV.16 Therefore, in patients with fever, cough, and pulmonary infiltrates on chest radiography, S pneumoniae must always be considered.
Urinary antigen testing has a relatively high positive predictive value (> 89%) and specificity (96%) for diagnosing S pneumoniae pneumonia.26 Blood and sputum cultures should be done not only to confirm the diagnosis, but also because the rates of bacteremia and drug resistance are higher with S pneumoniae infection in the HIV-infected.1
A combination of a beta-lactam and a macrolide or respiratory fluoroquinolone is the treatment of choice.1
Cytomegalovirus
Although influenza is the most common cause of viral pneumonia in HIV-infected people, cytomegalovirus is an opportunistic cause.2 This is usually a reactivation of latent infection rather than new infection.27 Typically, infections occur at CD4 counts lower than 50 cells/μL, with cough, dyspnea, and fever that last for 2 to 4 weeks.2
Crackles may be heard on lung examination. The lactate dehydrogenase level can be elevated, as in P jirovecii pneumonia.2 Radiography can show a wide range of nonspecific findings, from reticular and ground-glass opacities to alveolar or interstitial infiltrates to nodules.
The diagnosis of cytomegalovirus pneumonia is not always clear. Since HIV-infected patients typically shed the virus in their airways, bronchoalveolar lavage is not adequate because a positive finding does not necessarily mean the patient has active viral pneumonitis.27 For this reason, infection should be confirmed by biopsy demonstrating characteristic cytomegalovirus inclusions in lung tissue.2
Importantly, once cytomegalovirus pneumonia is confirmed, the patient should be screened for cytomegalovirus retinitis even if he or she has no visual symptoms, as cytomegalovirus pneumonitis is typically a part of a disseminated infection.1
Treatment with intravenous ganciclovir (Cytovene) is required.1
CASE CONTINUED: POSITIVE TESTS FOR COCCIDIOIDES
Our patient began empiric treatment for community-acquired pneumonia with intravenous ceftriaxone (Rocephin) and azithromycin (Zithromax).
On the basis of these findings, the patient was immediately placed in negative pressure respiratory isolation and underwent induced sputum examinations for tuberculosis. Further tests for S pneumoniae, S aureus, Mycoplasma, Legionella, influenza, Pneumocystis, Cryptococcus, Histoplasma, and Coccidioides species were performed.
QuantiFERON testing was negative, and blood cultures were sterile. The first induced sputum examination was negative for acid-fast bacilli. PCR testing for mycobacterial DNA in the sputum was also negative.
Both silver and direct fluorescent antibody staining of the sputum were negative for Pneumocystis. On the basis of these findings and the patient’s lack of clinical improvement with trimethoprim-sulfamethoxazole, Pneumocystis infection was excluded.
THE PATIENT BEGINS TREATMENT
2. Which treatment is most appropriate for this patient?
- Posaconazole (Noxafil)
- Caspofungin (Cancidas) and surgery
- Fluconazole
- Voriconazole (Vfend) and surgery
- Amphotericin B
Solitary pulmonary cavities tend to be asymptomatic and do not require treatment, even if coccidioidal infection is microbiologically confirmed.
However, if there is pain, hemoptysis, or bacterial superinfection, antifungal therapy may result in improvement but not closure of the cavity.18 Therefore, in all cases of symptomatic coccidioidal pulmonary cavities, surgical resection is the only definitive treatment.
Coccidioidal cavities may rupture and cause pyopneumothorax, but this is an infrequent complication, and antifungal therapy combined with surgical decortication is the treatment of choice.18
Commonly prescribed antifungals include fluconazole and amphotericin B, the latter usually reserved for patients with significant hypoxia or rapid clinical deterioration.18 At this time, there are not enough clinical data to show that voriconazole or posaconazole is effective, and thus neither is approved for the treatment of coccidioidomycosis. Likewise, there have been no human trials of the efficacy of caspofungin against Coccidioides infection, although it has been shown to be active in mouse models.18
Our patient was started on oral fluconazole and observed for clinical improvement or, conversely, for signs of dissemination. After 2 days, he had markedly improved, and within 1 week he was almost back to his baseline level of health. Testing for all other infectious etiologies was unrevealing, and he was removed from negative pressure isolation.
However, as we mentioned above, his CD4 count was 5 cells/μL. We discussed the issue with the patient, and he said he was willing to comply with his treatment for both his Coccidioides and his HIV infection. After much deliberation, he said he was also willing to start and comply with prophylactic treatment for opportunistic infections.
PREVENTING OPPORTUNISTIC INFECTIONS IN HIV PATIENTS
3. Which of the following prophylactic regimens is most appropriate for this patient?
- Trimethoprim-sulfamethoxazole, atovaquone (Mepron), and azithromycin
- Trimethoprim-sulfamethoxazole and azithromycin
- Pentamidine (Nebupent), dapsone, and clarithromycin (Biaxin)
- Dapsone and clarithromycin
- Trimethoprim-sulfamethoxazole by itself
According to guidelines for the prevention of opportunistic diseases in patients with HIV, he needs primary prophylaxis against the following organisms: P jirovecii, Toxoplasma gondii, and Mycobacterium avium complex.1
The CD4 count dictates the appropriate time to start therapy. If the count is lower than 200 cells/μL or if the patient has oropharyngeal candidiasis regardless of the CD4 count, trimethoprim-sulfamethoxazole is indicated to prevent P jirovecii pneumonia. In those who cannot tolerate trimethoprim-sulfamethoxazole or who are allergic to it, dapsone, pentamidine, or atovaquone can be substituted.1
In patients seropositive for T gondii, a CD4 count lower than 100/μL indicates the need for prophylaxis.1 Prophylactic measures are similar to those for Pneumocystis. However, if the patient cannot tolerate trimethoprim-sulfamethoxazole, the recommended alternative is dapsone-pyrimethamine with leucovorin, which is also effective against Pneumocystis.1
Finally, if the CD4 count is lower than 50 cells/μL, prophylaxis against M avium complex is mandatory, with either azithromycin weekly or clarithromycin daily.1
Given our patient’s degree of immunosuppression, trimethoprim-sulfamethoxazole plus azithromycin is his most appropriate option.
Trimethoprim-sulfamethoxazole and azithromycin were added to his antimicrobial regimen before he was discharged. Two weeks later, he noted no side effects from any of the medications, he had no new symptoms, he was feeling well, and his cough had improved greatly. He did not have any signs of dissemination of his coccidioidal infection, and we concluded that the primary and only infection was located in the lungs.
DISSEMINATED COCCIDIOIDOMYCOSIS
4. Which of the following extrapulmonary sites is Coccidioides least likely to infect?
- Brain
- Skin
- Meninges
- Lymph nodes
- Bones
- Joints
Extrapulmonary coccidioidomycosis can involve almost any site. However, the most common sites of dissemination are the skin, lymph nodes, bones, and joints.14 The least likely site is the brain.
Central nervous system involvement
In the central nervous system, involvement is typically with the meninges, rather than frank involvement of the brain parenchyma.18,28,29 Although patients with HIV or those who are otherwise severely immunocompromised are at higher risk for coccidioidal meningitis, it is rare even in this population.30,31 Meningitis most commonly presents as headache, vomiting, meningismus, confusion, or diplopia.32,33
If neurologic findings are absent, experts do not generally recommend lumbar puncture because the incidence of meningeal involvement is low. When cerebrospinal fluid is obtained in an active case of coccidioidal meningitis, fluid analysis typically finds elevated protein, low glucose, and lymphocytic pleocytosis.1,32
Meningeal enhancement on CT or magnetic resonance imaging is common.34 The diagnosis is established by culture or serologic testing of cerebrospinal fluid (IgM titer, IgG titer, immunodiffusion, or complement fixation).14
Of note, cerebral infarction and hydrocephalus are feared complications and pose a serious risk of death in any patient.32,35 In these cases, treatment with antifungals is lifelong, regardless of immune system status.18
Skin involvement
Skin involvement is variable, consisting of nodules, verrucae, abscesses, or ulcerations.15,16 Hemorrhage from the skin is relatively common.36 From the skin, the infection can spread to the lymph nodes, leading to regional lymphadenopathy.14,15 Nodes can ulcerate, drain, or even become necrotic.
Bone and joint involvement
Once integrity of the blood vessels is disrupted, Coccidioides can spread via the blood to the bones or joints,14,15 causing osteomyelitis, septic arthritis, or synovitis. Subcutaneous abscesses or sinus tracts may subsequently develop.14,15
HOW LONG MUST HE BE TREATED?
On follow-up, the patient asked how long he needed to continue his antifungal regimen and if any other testing for his coccidioidal infection was necessary, since he was feeling better.
5. Which is the most appropriate response to the patient’s question?
- He can discontinue his antifungal drugs; no further testing is necessary
- He needs 14 more days of antifungal therapy and periodic serologic tests
- He needs 2.5 more months of antifungal therapy and monthly blood cultures
- He needs lifelong antifungal therapy and periodic urinary antigen levels
- He needs 5.5 more months of antifungal therapy; bronchoscopy with bronchoalveolar lavage at 1 year
How long to treat and how to monitor for coccidioidomycosis vary by patient.
Duration of therapy depends on symptoms and immune status
The severity of infection (Table 2) and the immune status are important factors that must be considered when tailoring a therapeutic regimen.
Immunocompetent patients without symptoms or with mild symptoms usually do not need therapy and are followed periodically for signs of improvement.14,18,29
Immunocompetent patients with severe symptoms typically receive 3 to 6 months of antifungal therapy.18
Immunocompromised patients (especially HIV-infected patients with CD4 counts < 250 cells/μL) need antifungal treatment, regardless of the severity of infection.14,18,29 In many cases, the type of infection will dictate the duration of therapy.
Diffuse pneumonia or extrapulmonary dissemination typically requires treatment for at least 1 year regardless of immune status.14,18 For those with HIV and diffuse pneumonia, dissemination, or meningitis, guidelines dictate that secondary prophylaxis be started after at least 1 year of therapy and improvement in clinical status; it should be continued indefinitely to prevent reactivation of latent infection.18
The guidelines say that in patients with higher CD4 counts (presumably > 250 cells/μL) and nonmeningeal coccidioidomycosis, providers may consider discontinuing secondary prophylaxis, as long as there is clinical evidence of improvement and control of the primary infection.18 However, many experts advocate continuing secondary prophylaxis regardless of the CD4 count, as the rates of relapse and dissemination are high.1,16,37
Monitoring
Regardless of the therapy chosen, disease monitoring every 2 to 4 months with clinical history and examination, radiography, and coccidioidal-specific testing is recommended for at least 1 year, and perhaps longer, to ensure complete resolution and to monitor for signs of dissemination.14,18
Which test to use is not clear. Serologic testing identifies antibodies (IgM or IgG) to coccidioidal antigens. IgM appears during the acute infection, and tests include immunodiffusion, latex agglutination, and enzymelinked immunoassays. The last two are highly sensitive but have a significant false-positive rate, and should be confirmed with the former if found to be positive.17,18 IgG appears weeks after the acute infection and can be evaluated with immunodiffusion or enzyme-linked immunoassay as well.
Keep in mind that these tests provide only qualitative results on the presence of these antibodies, not quantitative information. Furthermore, enzyme-linked immunoassay is not as accurate as immunodiffusion, which has a sensitivity in immunocompromised patients of only approximately 50%.38,39
For that reason, complement fixation titers are extremely helpful because they reflect the severity of infection, can be used to monitor the response to treatment, and can even provide insight into the prognosis.18 The sensitivity of this test in immunocompromised hosts is 60% to 70%.38 Titers can be checked to confirm the diagnosis and can be periodically monitored throughout the treatment course to ensure efficacy of therapy and to watch for reactivation of the infection.1 In fact, an initial complement fixation titer of 1:2 or 1:4 is associated with favorable outcomes, while a titer greater than 1:16 portends dissemination.18
The caveat to any serologic test (immunodiffusion, enzyme-linked immunoassay, and complement fixation) is that severely immunocompromised patients (as in our case) may not mount an immune response and may have falsely low titers even in the face of a severe infection, and therefore these tests may not be reliable.38 In these situations, urinary coccidioidal antigen detection assay (sensitivity 71%) or nucleic acid amplification of coccidioidal DNA (sensitivity 75%) may be of more help.40,41
Therefore, in the setting of HIV infection, an asymptomatic pulmonary cavity, and diffuse pulmonary involvement secondary to coccidioidal infection, lifelong antibiotics (treatment plus secondary prophylaxis) with periodic testing of urinary coccidioidal antigen levels is the best response to the patient’s question, given that his complement fixation titers were initially negative and antigen levels were positive.
CASE CONCLUDED
The patient continues to be followed for his HIV infection. He is undergoing serologic and urinary antigen testing for Coccidioides infection every 3 months in addition to his maintenance HIV testing. He is on chronic suppressive therapy with fluconazole. He has not had a recurrence of his Coccidioides infection, nor have there been any signs of dissemination.
CAVITARY LUNG LESIONS IN HIV PATIENTS
In patients with HIV, cavitary lung lesions on chest radiography can be due to a wide variety of etiologies that range from infection to malignancy. Historical clues, including environmental exposure, occupation, geographic residence, sick contacts, travel, or animal contact can be helpful in ordering subsequent confirmatory testing, especially in the case of infection.
Tuberculosis should be suspected, and appropriate isolation precautions should be taken until it is ruled out.
Laboratory testing, including the complete blood cell count with differential and CD4 count, provide ancillary data to narrow the differential diagnosis. For example, if the CD4 count is greater than 200 cells/μL, mycobacterial infection should be strongly suspected; however, lower CD4 counts should also prompt a search for opportunistic infections. In the appropriate clinical scenario, malignancies including Kaposi sarcoma, non-Hodgkin lymphoma, and bronchogenic carcinoma can be seen and should also be considered.
Nevertheless, the evaluation hinges on the sputum examination and CT scan of the chest to further characterize the cavity, surrounding lung parenchyma, lymph nodes, and potential fluid collections. Usually, further serologic tests and even bronchoscopy with bronchoalveolar lavage and transbronchial biopsy are required. Treatment should begin once the most likely diagnosis is established.
Coccidioidal pneumonia should be considered in all patients with immunodeficiency, including HIV patients, transplant recipients, those undergoing chemotherapy, and those with intrinsic immune system defects, especially if they have a history of exposure or if they are from an endemic region. Antifungal therapy should be initiated early, and dissemination must be ruled out. Suppressive therapy is mandatory for those with a severely compromised immune system, and serologic testing to ensure remission of the infection is needed. Patients who were previously exposed to Coccidioides or who vacationed or live in the southwestern United States (where it is prevalent) are at risk and may present with any number of symptoms.
A 37-year-old man presented to the emergency department with an 8-week history of a mildly productive cough and shortness of breath accompanied by high fevers, chills, and night sweats. He also had some nausea but no vomiting.
Four days earlier, he had been evaluated by his primary care physician, who prescribed a 14-day course of one double-strength trimethoprim-sulfamethoxazole tablet (Bactrim DS) every 12 hours for presumed acute bronchitis, but his symptoms did not improve.
He was unemployed, living in Arizona, married with children. He denied any use of tobacco, alcohol, or injection drugs. On further questioning, he disclosed that he had unintentionally lost 30 pounds over the past 2 to 3 months and had been feeling tired.
When asked about his medical history, he revealed that he had been diagnosed with human immunodeficiency virus (HIV) infection in 2008 and that recently he had not been taking his antiretroviral medication, a once-daily combination pill containing efavirenz, emtricitabine, and tenofovir (Atripla). He had no other significant medical history, and the only medication he was currently taking was the trimethoprim-sulfamethoxazole.
On examination, his temperature was 38.7°C (101.7°F), blood pressure 109/68 mm Hg, heart rate 60 beats per minute, respiratory rate 18 breaths per minute, and oxygen saturation 100% while breathing supplemental oxygen via nasal cannula at 2 L/min. He did not appear seriously ill.
Initial blood tests (Table 1) showed a normal white blood cell count, normal results on a complete metabolic panel, and a lactate dehydrogenase level of 539 IU/L (reference range 313–618). His serum lactate level was within normal limits.
HIV-specific tests performed on the second day of hospitalization showed extreme immunosuppression, with a CD4 count of 5 cells/μL (normal 326–1,404 cells/μL).
WHICH ORGANISM IS CAUSING HIS LUNG INFECTION?
1. Which of the following organisms is the least likely to be associated with this patient’s condition?
- Mycobacterium tuberculosis
- Pneumocystis jirovecii
- Coccidioides immitis
- Candida albicans
- Streptococcus pneumoniae
- Cytomegalovirus
Bacterial, fungal, and viral lung infections are common in HIV-infected patients, especially if they are not on antiretroviral therapy and their CD4 lymphocyte counts are low. Clues to the cause can be derived from the history, physical examination, and general laboratory studies. For instance, knowing where the patient lives and where he has travelled recently provides insight into exposure to endemic infectious agents.
The complete blood cell count with differential white blood cell count can help narrow the differential diagnosis but rarely helps exclude a possibility. Neutrophilia is common in bacterial infections. Lymphocytosis can be seen in tuberculosis, in fungal and viral infections, and, rarely, in hematologic malignancies. Eosinophilia can be seen in acute retroviral syndrome, fungal and helminthic infections, adrenal insufficiency, autoimmune disease, and lymphoma.
A caveat to these clues is that in severely immunocompromised hosts, like this man, diagnoses should not be excluded without firm evidence. This patient has severe, active immunosuppression, and only one of the six answer choices above is not a possible causative agent: C albicans rarely causes lung infection, even in immunocompromised people.
Mycobacterium tuberculosis
Tuberculosis can be the first manifestation of HIV infection. It can occur at any CD4 count, but as the count decreases, the risk of dissemination increases.1 Classic symptoms are fever, night sweats, hemoptysis, and weight loss.
The CD4 count also affects the radiographic presentation. If the count is higher than 350 cells/μL, then infiltration of the upper lobe is likely; if it is lower than 200 cells/μL, then middle, lower, miliary, and extrapulmonary manifestations are likely.1,2 Cavitation is less common in HIV-infected patients, but mediastinal adenopathy is more common.1
Definitive diagnosis is via sputum examination, blood culture, nucleic acid amplification, or microscopic study of biopsy specimens of affected tissues to look for acid-fast bacilli.1
Interferon-gamma-release assays such as the QuantiFERON test (Cellestis, Valencia, CA) or a tuberculin skin test can be used to check for latent tuberculosis infection. These tests can also provide evidence of active infection in the appropriate clinical context.3
Interferon-gamma-release assays have several advantages over skin testing: they are more sensitive (76% to 80%) and specific (97%); they do not give false-positive results in people who previously received bacille Calmette-Guérin vaccine; they react only minimally to previous exposure to nontuberculous mycobacteria; and interpretation is not subject to interreader variability.4,5 However, concordance between skin testing and interferon-gamma-release assays is low. Therefore, either or both tests can be used if tuberculosis is strongly suspected, and a positive result on either test should prompt further workup.6,7
Of note, both tests may be affected by immunosuppression, making both susceptible to false-negative results as the CD4 count declines.3
In any case, a positive acid-fast bacillus smear, radiographic evidence of latent infection, or pulmonary symptoms should be presumed to represent active tuberculosis. In such a situation, directly observed treatment with the typical four-drug regimen—rifampin (Rifadin), isoniazid, pyrazinamide, and ethambutol (Myambutol)—is recommended while awaiting definitive results from culture or polymerase chain reaction (PCR) testing.1
Pneumocystis jirovecii
P jirovecii was previously known as P carinii, and P jirovecii pneumonia is an AIDS-defining illness. Most cases occur when the CD4 count falls below 200 cells/μL.1 Symptoms, including a nonproductive cough, develop insidiously over days to weeks.
Physical examination may reveal inspiratory crackles; however, half of the time the physical examination is nondiagnostic. Oral candidiasis is a common coinfection. The lactate dehydrogenase level may be elevated.1,8 Radiographs show bilateral interstitial infiltrates, and in 10% to 20% of patients lung cysts develop—hence the name of the organism.1 Pneumothorax in a patient with HIV should prompt a workup for P jirovecii pneumonia.9,10
No consensus exists for the diagnosis. However, if sputum examination is unrevealing but suspicion is high, then bronchoalveolar lavage can help.11–13
Trimethoprim-sulfamethoxazole for 21 days is the first-line treatment, with glucocorticoids added if the Pao2 is less than 70 mm Hg or if the alveolar-arterial oxygen gradient is greater than 35 mm Hg.1
Coccidioides species
Coccidioides infection is typically due to either C immitis or C posadasii.14 People living in or travelling to areas where it is endemic, such as the southwestern United States, Mexico, and Central and South America, are at higher risk.14
Typical signs and symptoms of this fungal infection include an influenza-like illness with fever, cough, adenopathy, and wasting, and when combined with erythema nodosum, erythema multiforme, arthralgia, or ocular involvement, this constellation is colloquially termed “valley fever.”15 Most HIV-infected patients who have CD4 counts higher than 250 cells/μL present with focal pneumonia, while lower counts predispose to disseminated disease.1,2,16
Findings on examination are nonspecific and depend on the various pulmonary manifestations, which include acute, chronic progressive, or diffuse pneumonia, nodules, or cavities.14 Eosinophilia may accompany the infection.15
The diagnosis can be made by finding the organisms on direct microscopic examination of involved tissues or secretions or on culture of clinical specimens.1,2,14 Serologic tests, antigen detection tests, or culture can be helpful if positive, but negative results do not rule out the diagnosis.1,2,14
A caveat about testing: if the pretest probability of infection is low, positive tests for immunoglobulin M (IgM) do not necessarily equal infection, and the IgM test should be followed up with confirmatory testing. Along the same lines, a high pretest probability should not be ignored if initial tests are negative, and patients in this situation should also undergo further evaluation.17
Therapy with an azole drug such as fluconazole (Diflucan) or one of the amphotericin B preparations should be started, depending on the severity of the disease.1,2,14,18
Candida albicans
C albicans is a rare cause of lung infection.19,20 It is, however, a common inhabitant of the upper airway tract, and pulmonary infection is usually the result of aspiration or hematogenous spread from either the gastrointestinal tract or an infected central venous catheter.20
The presentation is relatively nonspecific. Fever despite broad-spectrum antibacterial therapy is a major clue. Radiographic abnormalities usually are due to other causes, such as superimposed infections or pulmonary hemorrhage.21 Sputum culture is unreliable because of colonization. The definitive diagnosis is based on lung biopsy demonstrating organisms within the tissue.19,20,22
Therapy with a systemic antifungal agent is recommended.
Streptococcus pneumoniae
S pneumoniae is one of the most common bacterial causes of community-acquired pneumonia in people with or without HIV.23–25 Moreover, two or more episodes of bacterial pneumonia in 12 months can be an AIDS-defining condition in patients with a positive serologic test for HIV.16 Therefore, in patients with fever, cough, and pulmonary infiltrates on chest radiography, S pneumoniae must always be considered.
Urinary antigen testing has a relatively high positive predictive value (> 89%) and specificity (96%) for diagnosing S pneumoniae pneumonia.26 Blood and sputum cultures should be done not only to confirm the diagnosis, but also because the rates of bacteremia and drug resistance are higher with S pneumoniae infection in the HIV-infected.1
A combination of a beta-lactam and a macrolide or respiratory fluoroquinolone is the treatment of choice.1
Cytomegalovirus
Although influenza is the most common cause of viral pneumonia in HIV-infected people, cytomegalovirus is an opportunistic cause.2 This is usually a reactivation of latent infection rather than new infection.27 Typically, infections occur at CD4 counts lower than 50 cells/μL, with cough, dyspnea, and fever that last for 2 to 4 weeks.2
Crackles may be heard on lung examination. The lactate dehydrogenase level can be elevated, as in P jirovecii pneumonia.2 Radiography can show a wide range of nonspecific findings, from reticular and ground-glass opacities to alveolar or interstitial infiltrates to nodules.
The diagnosis of cytomegalovirus pneumonia is not always clear. Since HIV-infected patients typically shed the virus in their airways, bronchoalveolar lavage is not adequate because a positive finding does not necessarily mean the patient has active viral pneumonitis.27 For this reason, infection should be confirmed by biopsy demonstrating characteristic cytomegalovirus inclusions in lung tissue.2
Importantly, once cytomegalovirus pneumonia is confirmed, the patient should be screened for cytomegalovirus retinitis even if he or she has no visual symptoms, as cytomegalovirus pneumonitis is typically a part of a disseminated infection.1
Treatment with intravenous ganciclovir (Cytovene) is required.1
CASE CONTINUED: POSITIVE TESTS FOR COCCIDIOIDES
Our patient began empiric treatment for community-acquired pneumonia with intravenous ceftriaxone (Rocephin) and azithromycin (Zithromax).
On the basis of these findings, the patient was immediately placed in negative pressure respiratory isolation and underwent induced sputum examinations for tuberculosis. Further tests for S pneumoniae, S aureus, Mycoplasma, Legionella, influenza, Pneumocystis, Cryptococcus, Histoplasma, and Coccidioides species were performed.
QuantiFERON testing was negative, and blood cultures were sterile. The first induced sputum examination was negative for acid-fast bacilli. PCR testing for mycobacterial DNA in the sputum was also negative.
Both silver and direct fluorescent antibody staining of the sputum were negative for Pneumocystis. On the basis of these findings and the patient’s lack of clinical improvement with trimethoprim-sulfamethoxazole, Pneumocystis infection was excluded.
THE PATIENT BEGINS TREATMENT
2. Which treatment is most appropriate for this patient?
- Posaconazole (Noxafil)
- Caspofungin (Cancidas) and surgery
- Fluconazole
- Voriconazole (Vfend) and surgery
- Amphotericin B
Solitary pulmonary cavities tend to be asymptomatic and do not require treatment, even if coccidioidal infection is microbiologically confirmed.
However, if there is pain, hemoptysis, or bacterial superinfection, antifungal therapy may result in improvement but not closure of the cavity.18 Therefore, in all cases of symptomatic coccidioidal pulmonary cavities, surgical resection is the only definitive treatment.
Coccidioidal cavities may rupture and cause pyopneumothorax, but this is an infrequent complication, and antifungal therapy combined with surgical decortication is the treatment of choice.18
Commonly prescribed antifungals include fluconazole and amphotericin B, the latter usually reserved for patients with significant hypoxia or rapid clinical deterioration.18 At this time, there are not enough clinical data to show that voriconazole or posaconazole is effective, and thus neither is approved for the treatment of coccidioidomycosis. Likewise, there have been no human trials of the efficacy of caspofungin against Coccidioides infection, although it has been shown to be active in mouse models.18
Our patient was started on oral fluconazole and observed for clinical improvement or, conversely, for signs of dissemination. After 2 days, he had markedly improved, and within 1 week he was almost back to his baseline level of health. Testing for all other infectious etiologies was unrevealing, and he was removed from negative pressure isolation.
However, as we mentioned above, his CD4 count was 5 cells/μL. We discussed the issue with the patient, and he said he was willing to comply with his treatment for both his Coccidioides and his HIV infection. After much deliberation, he said he was also willing to start and comply with prophylactic treatment for opportunistic infections.
PREVENTING OPPORTUNISTIC INFECTIONS IN HIV PATIENTS
3. Which of the following prophylactic regimens is most appropriate for this patient?
- Trimethoprim-sulfamethoxazole, atovaquone (Mepron), and azithromycin
- Trimethoprim-sulfamethoxazole and azithromycin
- Pentamidine (Nebupent), dapsone, and clarithromycin (Biaxin)
- Dapsone and clarithromycin
- Trimethoprim-sulfamethoxazole by itself
According to guidelines for the prevention of opportunistic diseases in patients with HIV, he needs primary prophylaxis against the following organisms: P jirovecii, Toxoplasma gondii, and Mycobacterium avium complex.1
The CD4 count dictates the appropriate time to start therapy. If the count is lower than 200 cells/μL or if the patient has oropharyngeal candidiasis regardless of the CD4 count, trimethoprim-sulfamethoxazole is indicated to prevent P jirovecii pneumonia. In those who cannot tolerate trimethoprim-sulfamethoxazole or who are allergic to it, dapsone, pentamidine, or atovaquone can be substituted.1
In patients seropositive for T gondii, a CD4 count lower than 100/μL indicates the need for prophylaxis.1 Prophylactic measures are similar to those for Pneumocystis. However, if the patient cannot tolerate trimethoprim-sulfamethoxazole, the recommended alternative is dapsone-pyrimethamine with leucovorin, which is also effective against Pneumocystis.1
Finally, if the CD4 count is lower than 50 cells/μL, prophylaxis against M avium complex is mandatory, with either azithromycin weekly or clarithromycin daily.1
Given our patient’s degree of immunosuppression, trimethoprim-sulfamethoxazole plus azithromycin is his most appropriate option.
Trimethoprim-sulfamethoxazole and azithromycin were added to his antimicrobial regimen before he was discharged. Two weeks later, he noted no side effects from any of the medications, he had no new symptoms, he was feeling well, and his cough had improved greatly. He did not have any signs of dissemination of his coccidioidal infection, and we concluded that the primary and only infection was located in the lungs.
DISSEMINATED COCCIDIOIDOMYCOSIS
4. Which of the following extrapulmonary sites is Coccidioides least likely to infect?
- Brain
- Skin
- Meninges
- Lymph nodes
- Bones
- Joints
Extrapulmonary coccidioidomycosis can involve almost any site. However, the most common sites of dissemination are the skin, lymph nodes, bones, and joints.14 The least likely site is the brain.
Central nervous system involvement
In the central nervous system, involvement is typically with the meninges, rather than frank involvement of the brain parenchyma.18,28,29 Although patients with HIV or those who are otherwise severely immunocompromised are at higher risk for coccidioidal meningitis, it is rare even in this population.30,31 Meningitis most commonly presents as headache, vomiting, meningismus, confusion, or diplopia.32,33
If neurologic findings are absent, experts do not generally recommend lumbar puncture because the incidence of meningeal involvement is low. When cerebrospinal fluid is obtained in an active case of coccidioidal meningitis, fluid analysis typically finds elevated protein, low glucose, and lymphocytic pleocytosis.1,32
Meningeal enhancement on CT or magnetic resonance imaging is common.34 The diagnosis is established by culture or serologic testing of cerebrospinal fluid (IgM titer, IgG titer, immunodiffusion, or complement fixation).14
Of note, cerebral infarction and hydrocephalus are feared complications and pose a serious risk of death in any patient.32,35 In these cases, treatment with antifungals is lifelong, regardless of immune system status.18
Skin involvement
Skin involvement is variable, consisting of nodules, verrucae, abscesses, or ulcerations.15,16 Hemorrhage from the skin is relatively common.36 From the skin, the infection can spread to the lymph nodes, leading to regional lymphadenopathy.14,15 Nodes can ulcerate, drain, or even become necrotic.
Bone and joint involvement
Once integrity of the blood vessels is disrupted, Coccidioides can spread via the blood to the bones or joints,14,15 causing osteomyelitis, septic arthritis, or synovitis. Subcutaneous abscesses or sinus tracts may subsequently develop.14,15
HOW LONG MUST HE BE TREATED?
On follow-up, the patient asked how long he needed to continue his antifungal regimen and if any other testing for his coccidioidal infection was necessary, since he was feeling better.
5. Which is the most appropriate response to the patient’s question?
- He can discontinue his antifungal drugs; no further testing is necessary
- He needs 14 more days of antifungal therapy and periodic serologic tests
- He needs 2.5 more months of antifungal therapy and monthly blood cultures
- He needs lifelong antifungal therapy and periodic urinary antigen levels
- He needs 5.5 more months of antifungal therapy; bronchoscopy with bronchoalveolar lavage at 1 year
How long to treat and how to monitor for coccidioidomycosis vary by patient.
Duration of therapy depends on symptoms and immune status
The severity of infection (Table 2) and the immune status are important factors that must be considered when tailoring a therapeutic regimen.
Immunocompetent patients without symptoms or with mild symptoms usually do not need therapy and are followed periodically for signs of improvement.14,18,29
Immunocompetent patients with severe symptoms typically receive 3 to 6 months of antifungal therapy.18
Immunocompromised patients (especially HIV-infected patients with CD4 counts < 250 cells/μL) need antifungal treatment, regardless of the severity of infection.14,18,29 In many cases, the type of infection will dictate the duration of therapy.
Diffuse pneumonia or extrapulmonary dissemination typically requires treatment for at least 1 year regardless of immune status.14,18 For those with HIV and diffuse pneumonia, dissemination, or meningitis, guidelines dictate that secondary prophylaxis be started after at least 1 year of therapy and improvement in clinical status; it should be continued indefinitely to prevent reactivation of latent infection.18
The guidelines say that in patients with higher CD4 counts (presumably > 250 cells/μL) and nonmeningeal coccidioidomycosis, providers may consider discontinuing secondary prophylaxis, as long as there is clinical evidence of improvement and control of the primary infection.18 However, many experts advocate continuing secondary prophylaxis regardless of the CD4 count, as the rates of relapse and dissemination are high.1,16,37
Monitoring
Regardless of the therapy chosen, disease monitoring every 2 to 4 months with clinical history and examination, radiography, and coccidioidal-specific testing is recommended for at least 1 year, and perhaps longer, to ensure complete resolution and to monitor for signs of dissemination.14,18
Which test to use is not clear. Serologic testing identifies antibodies (IgM or IgG) to coccidioidal antigens. IgM appears during the acute infection, and tests include immunodiffusion, latex agglutination, and enzymelinked immunoassays. The last two are highly sensitive but have a significant false-positive rate, and should be confirmed with the former if found to be positive.17,18 IgG appears weeks after the acute infection and can be evaluated with immunodiffusion or enzyme-linked immunoassay as well.
Keep in mind that these tests provide only qualitative results on the presence of these antibodies, not quantitative information. Furthermore, enzyme-linked immunoassay is not as accurate as immunodiffusion, which has a sensitivity in immunocompromised patients of only approximately 50%.38,39
For that reason, complement fixation titers are extremely helpful because they reflect the severity of infection, can be used to monitor the response to treatment, and can even provide insight into the prognosis.18 The sensitivity of this test in immunocompromised hosts is 60% to 70%.38 Titers can be checked to confirm the diagnosis and can be periodically monitored throughout the treatment course to ensure efficacy of therapy and to watch for reactivation of the infection.1 In fact, an initial complement fixation titer of 1:2 or 1:4 is associated with favorable outcomes, while a titer greater than 1:16 portends dissemination.18
The caveat to any serologic test (immunodiffusion, enzyme-linked immunoassay, and complement fixation) is that severely immunocompromised patients (as in our case) may not mount an immune response and may have falsely low titers even in the face of a severe infection, and therefore these tests may not be reliable.38 In these situations, urinary coccidioidal antigen detection assay (sensitivity 71%) or nucleic acid amplification of coccidioidal DNA (sensitivity 75%) may be of more help.40,41
Therefore, in the setting of HIV infection, an asymptomatic pulmonary cavity, and diffuse pulmonary involvement secondary to coccidioidal infection, lifelong antibiotics (treatment plus secondary prophylaxis) with periodic testing of urinary coccidioidal antigen levels is the best response to the patient’s question, given that his complement fixation titers were initially negative and antigen levels were positive.
CASE CONCLUDED
The patient continues to be followed for his HIV infection. He is undergoing serologic and urinary antigen testing for Coccidioides infection every 3 months in addition to his maintenance HIV testing. He is on chronic suppressive therapy with fluconazole. He has not had a recurrence of his Coccidioides infection, nor have there been any signs of dissemination.
CAVITARY LUNG LESIONS IN HIV PATIENTS
In patients with HIV, cavitary lung lesions on chest radiography can be due to a wide variety of etiologies that range from infection to malignancy. Historical clues, including environmental exposure, occupation, geographic residence, sick contacts, travel, or animal contact can be helpful in ordering subsequent confirmatory testing, especially in the case of infection.
Tuberculosis should be suspected, and appropriate isolation precautions should be taken until it is ruled out.
Laboratory testing, including the complete blood cell count with differential and CD4 count, provide ancillary data to narrow the differential diagnosis. For example, if the CD4 count is greater than 200 cells/μL, mycobacterial infection should be strongly suspected; however, lower CD4 counts should also prompt a search for opportunistic infections. In the appropriate clinical scenario, malignancies including Kaposi sarcoma, non-Hodgkin lymphoma, and bronchogenic carcinoma can be seen and should also be considered.
Nevertheless, the evaluation hinges on the sputum examination and CT scan of the chest to further characterize the cavity, surrounding lung parenchyma, lymph nodes, and potential fluid collections. Usually, further serologic tests and even bronchoscopy with bronchoalveolar lavage and transbronchial biopsy are required. Treatment should begin once the most likely diagnosis is established.
Coccidioidal pneumonia should be considered in all patients with immunodeficiency, including HIV patients, transplant recipients, those undergoing chemotherapy, and those with intrinsic immune system defects, especially if they have a history of exposure or if they are from an endemic region. Antifungal therapy should be initiated early, and dissemination must be ruled out. Suppressive therapy is mandatory for those with a severely compromised immune system, and serologic testing to ensure remission of the infection is needed. Patients who were previously exposed to Coccidioides or who vacationed or live in the southwestern United States (where it is prevalent) are at risk and may present with any number of symptoms.
- Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H; Centers for Disease Control and Prevention (CDC). Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009; 58:1–207.
- Huang L, Crothers K. HIV-associated opportunistic pneumonias. Respirology 2009; 14:474–485.
- Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005; 54:49–55.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Nahid P, Pai M, Hopewell PC. Advances in the diagnosis and treatment of tuberculosis. Proc Am Thorac Soc 2006; 3:103–110.
- Chapman AL, Munkanta M, Wilkinson KA, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS 2002; 16:2285–2293.
- Luetkemeyer AF, Charlebois ED, Flores LL, et al. Comparison of an interferon-gamma release assay with tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med 2007; 175:737–742.
- Zaman MK, White DA. Serum lactate dehydrogenase levels and Pneumocystis carinii pneumonia. Diagnostic and prognostic significance. Am Rev Respir Dis 1988; 137:796–800.
- Metersky ML, Colt HG, Olson LK, Shanks TG. AIDS-related spontaneous pneumothorax. Risk factors and treatment. Chest 1995; 108:946–951.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Baughman RP, Dohn MN, Frame PT. The continuing utility of bronchoalveolar lavage to diagnose opportunistic infection in AIDS patients. Am J Med 1994; 97:515–522.
- Kovacs JA, Ng VL, Masur H, et al. Diagnosis of Pneumocystis carinii pneumonia: improved detection in sputum with use of monoclonal antibodies. N Engl J Med 1988; 318:589–593.
- Stover DE, Zaman MB, Hajdu SI, Lange M, Gold J, Armstrong D. Bronchoalveolar lavage in the diagnosis of diffuse pulmonary infiltrates in the immunosuppressed host. Ann Intern Med 1984; 101:1–7.
- Parish JM, Blair JE. Coccidioidomycosis. Mayo Clin Proc 2008; 83:343–348.
- Drutz DJ, Catanzaro A. Coccidioidomycosis. Part I. Am Rev Respir Dis 1978; 117:559–585.
- Bartlett JG, Gallant JE, Pham PA. Medical Management of HIV Infection. Durham, NC: Knowledge Source Solutions, LLC; 2009.
- Kuberski T, Herrig J, Pappagianis D. False-positive IgM serology in coccidioidomycosis. J Clin Microbiol 2010; 48:2047–2049.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Kontoyiannis DP, Reddy BT, Torres HA, et al. Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis 2002; 34:400–403.
- Pappas PG, Kauffman CA, Andes D, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–535.
- Connolly JE, McAdams HP, Erasmus JJ, Rosado-de-Christenson ML. Opportunistic fungal pneumonia. J Thorac Imaging 1999; 14:51–62.
- Meersseman W, Lagrou K, Spriet I, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med 2009; 35:1526–1531.
- Miller RF, Foley NM, Kessel D, Jeffrey AA. Community acquired lobar pneumonia in patients with HIV infection and AIDS. Thorax 1994; 49:367–368.
- Polsky B, Gold JW, Whimbey E, et al. Bacterial pneumonia in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1986; 104:38–41.
- Rimland D, Navin TR, Lennox JL, et al; Pulmonary Opportunistic Infection Study Group. Prospective study of etiologic agents of community-acquired pneumonia in patients with HIV infection. AIDS 2002; 16:85–95.
- Boulware DR, Daley CL, Merrifield C, Hopewell PC, Janoff EN. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect 2007; 55:300–309.
- Salomon N, Perlman DC. Cytomegalovirus pneumonia. Semin Respir Infect 1999; 14:353–358.
- Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am 2003; 17:41–57.
- Drutz DJ, Catanzaro A. Coccidioidomycosis. Part II. Am Rev Respir Dis 1978; 117:727–771.
- Fish DG, Ampel NM, Galgiani JN, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine (Baltimore) 1990; 69:384–391.
- Mischel PS, Vinters HV. Coccidioidomycosis of the central nervous system: neuropathological and vasculopathic manifestations and clinical correlates. Clin Infect Dis 1995; 20:400–405.
- Johnson RH, Einstein HE. Coccidioidal meningitis. Clin Infect Dis 2006; 42:103–107.
- Vincent T, Galgiani JN, Huppert M, Salkin D. The natural history of coccidioidal meningitis: VA-Armed Forces cooperative studies, 1955–1958. Clin Infect Dis 1993; 16:247–254.
- Erly WK, Bellon RJ, Seeger JF, Carmody RF. MR imaging of acute coccidioidal meningitis. AJNR Am J Neuroradiol 1999; 20:509–514.
- Arsura EL, Johnson R, Penrose J, et al. Neuroimaging as a guide to predict outcomes for patients with coccidioidal meningitis. Clin Infect Dis 2005; 40:624–627.
- Tappero JW, Perkins BA, Wenger JD, Berger TG. Cutaneous manifestations of opportunistic infections in patients infected with human immunodeficiency virus. Clin Microbiol Rev 1995; 8:440–450.
- Catanzaro A, Galgiani JN, Levine BE, et al. Fluconazole in the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis. NIAID Mycoses Study Group. Am J Med 1995; 98:249–256.
- Blair JE, Coakley B, Santelli AC, Hentz JG, Wengenack NL. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia 2006; 162:317–324.
- Martins TB, Jaskowski TD, Mouritsen CL, Hill HR. Comparison of commercially available enzyme immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. J Clin Microbiol 1995; 33:940–943.
- Vucicevic D, Blair JE, Binnicker MJ, et al. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia 2010; 170:345–351.
- Durkin M, Connolly P, Kuberski T, et al. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin Infect Dis 2008; 47:e69–e73.
- Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H; Centers for Disease Control and Prevention (CDC). Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009; 58:1–207.
- Huang L, Crothers K. HIV-associated opportunistic pneumonias. Respirology 2009; 14:474–485.
- Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A; Division of Tuberculosis Elimination, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention (CDC). Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005; 54:49–55.
- Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146:340–354.
- Nahid P, Pai M, Hopewell PC. Advances in the diagnosis and treatment of tuberculosis. Proc Am Thorac Soc 2006; 3:103–110.
- Chapman AL, Munkanta M, Wilkinson KA, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS 2002; 16:2285–2293.
- Luetkemeyer AF, Charlebois ED, Flores LL, et al. Comparison of an interferon-gamma release assay with tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med 2007; 175:737–742.
- Zaman MK, White DA. Serum lactate dehydrogenase levels and Pneumocystis carinii pneumonia. Diagnostic and prognostic significance. Am Rev Respir Dis 1988; 137:796–800.
- Metersky ML, Colt HG, Olson LK, Shanks TG. AIDS-related spontaneous pneumothorax. Risk factors and treatment. Chest 1995; 108:946–951.
- Sepkowitz KA, Telzak EE, Gold JW, et al. Pneumothorax in AIDS. Ann Intern Med 1991; 114:455–459.
- Baughman RP, Dohn MN, Frame PT. The continuing utility of bronchoalveolar lavage to diagnose opportunistic infection in AIDS patients. Am J Med 1994; 97:515–522.
- Kovacs JA, Ng VL, Masur H, et al. Diagnosis of Pneumocystis carinii pneumonia: improved detection in sputum with use of monoclonal antibodies. N Engl J Med 1988; 318:589–593.
- Stover DE, Zaman MB, Hajdu SI, Lange M, Gold J, Armstrong D. Bronchoalveolar lavage in the diagnosis of diffuse pulmonary infiltrates in the immunosuppressed host. Ann Intern Med 1984; 101:1–7.
- Parish JM, Blair JE. Coccidioidomycosis. Mayo Clin Proc 2008; 83:343–348.
- Drutz DJ, Catanzaro A. Coccidioidomycosis. Part I. Am Rev Respir Dis 1978; 117:559–585.
- Bartlett JG, Gallant JE, Pham PA. Medical Management of HIV Infection. Durham, NC: Knowledge Source Solutions, LLC; 2009.
- Kuberski T, Herrig J, Pappagianis D. False-positive IgM serology in coccidioidomycosis. J Clin Microbiol 2010; 48:2047–2049.
- Galgiani JN, Ampel NM, Blair JE, et al; Infectious Diseases Society of America. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217–1223.
- Kontoyiannis DP, Reddy BT, Torres HA, et al. Pulmonary candidiasis in patients with cancer: an autopsy study. Clin Infect Dis 2002; 34:400–403.
- Pappas PG, Kauffman CA, Andes D, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:503–535.
- Connolly JE, McAdams HP, Erasmus JJ, Rosado-de-Christenson ML. Opportunistic fungal pneumonia. J Thorac Imaging 1999; 14:51–62.
- Meersseman W, Lagrou K, Spriet I, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med 2009; 35:1526–1531.
- Miller RF, Foley NM, Kessel D, Jeffrey AA. Community acquired lobar pneumonia in patients with HIV infection and AIDS. Thorax 1994; 49:367–368.
- Polsky B, Gold JW, Whimbey E, et al. Bacterial pneumonia in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1986; 104:38–41.
- Rimland D, Navin TR, Lennox JL, et al; Pulmonary Opportunistic Infection Study Group. Prospective study of etiologic agents of community-acquired pneumonia in patients with HIV infection. AIDS 2002; 16:85–95.
- Boulware DR, Daley CL, Merrifield C, Hopewell PC, Janoff EN. Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect 2007; 55:300–309.
- Salomon N, Perlman DC. Cytomegalovirus pneumonia. Semin Respir Infect 1999; 14:353–358.
- Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am 2003; 17:41–57.
- Drutz DJ, Catanzaro A. Coccidioidomycosis. Part II. Am Rev Respir Dis 1978; 117:727–771.
- Fish DG, Ampel NM, Galgiani JN, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine (Baltimore) 1990; 69:384–391.
- Mischel PS, Vinters HV. Coccidioidomycosis of the central nervous system: neuropathological and vasculopathic manifestations and clinical correlates. Clin Infect Dis 1995; 20:400–405.
- Johnson RH, Einstein HE. Coccidioidal meningitis. Clin Infect Dis 2006; 42:103–107.
- Vincent T, Galgiani JN, Huppert M, Salkin D. The natural history of coccidioidal meningitis: VA-Armed Forces cooperative studies, 1955–1958. Clin Infect Dis 1993; 16:247–254.
- Erly WK, Bellon RJ, Seeger JF, Carmody RF. MR imaging of acute coccidioidal meningitis. AJNR Am J Neuroradiol 1999; 20:509–514.
- Arsura EL, Johnson R, Penrose J, et al. Neuroimaging as a guide to predict outcomes for patients with coccidioidal meningitis. Clin Infect Dis 2005; 40:624–627.
- Tappero JW, Perkins BA, Wenger JD, Berger TG. Cutaneous manifestations of opportunistic infections in patients infected with human immunodeficiency virus. Clin Microbiol Rev 1995; 8:440–450.
- Catanzaro A, Galgiani JN, Levine BE, et al. Fluconazole in the treatment of chronic pulmonary and nonmeningeal disseminated coccidioidomycosis. NIAID Mycoses Study Group. Am J Med 1995; 98:249–256.
- Blair JE, Coakley B, Santelli AC, Hentz JG, Wengenack NL. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia 2006; 162:317–324.
- Martins TB, Jaskowski TD, Mouritsen CL, Hill HR. Comparison of commercially available enzyme immunoassay with traditional serological tests for detection of antibodies to Coccidioides immitis. J Clin Microbiol 1995; 33:940–943.
- Vucicevic D, Blair JE, Binnicker MJ, et al. The utility of Coccidioides polymerase chain reaction testing in the clinical setting. Mycopathologia 2010; 170:345–351.
- Durkin M, Connolly P, Kuberski T, et al. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin Infect Dis 2008; 47:e69–e73.
New and future therapies for lupus nephritis
Treatment for lupus nephritis has changed dramatically in recent years. Only 10 years ago, rheumatologists and nephrologists, whether specializing in adult or pediatric medicine, treated lupus nephritis with a similar regimen of monthly intravenous cyclophosphamide (Cytoxan) and glucocorticoids. Although the regimen is effective, side effects such as infection, hair loss, and infertility were extremely common.
Effective but very toxic therapy is common in autoimmune diseases. In the last decade, clinical trials have shown that less toxic drugs are as effective for treating lupus nephritis. This article will review new developments in therapy for lupus nephritis, which can be viewed as a prototype for other fields of medicine.
DEMOGRAPHICS ARE IMPORTANT
Although numerous factors have prognostic value in lupus nephritis (eg, serum creatinine, proteinuria, renal biopsy findings), the most important to consider when designing and interpreting studies are race and socioeconomic variables.
A retrospective study in Miami, FL,1 evaluated 213 patients with lupus nephritis, of whom 47% were Hispanic, 44% African American, and 20% white. At baseline, African Americans had higher blood pressure, higher serum creatinine levels, and lower household income. After 6 years, African Americans fared the worst in terms of doubling of serum creatinine, developing end-stage renal disease, and death; whites had the best outcomes, and Hispanics were in between. Low income was found to be a significant risk factor, independent of racial background.
In a similar retrospective study in New York City in 128 patients (43% white, 40% Hispanic, and 17% African American) with proliferative lupus nephritis,2 disease was much more likely to progress to renal failure over 10 years in patients living in a poor neighborhood, even after adjustment for race.
We need to keep in mind that racial and socioeconomic factors correlate with disease severity when we design and interpret studies of lupus nephritis. Study groups must be carefully balanced with patients of similar racial and socioeconomic profiles. Study findings must be interpreted with caution; for example, whether results from a study from China are applicable to an African American with lupus nephritis in New York City is unclear.
OLDER STANDARD THERAPY: EFFECTIVE BUT TOXIC
The last large National Institutes of Health study that involved only cyclophosphamide and a glucocorticoid was published in 2001,3 with 21 patients receiving cyclophosphamide alone and 20 patients receiving cyclophosphamide plus methylprednisolone. Although lupus nephritis improved, serious side effects occurred in one-third to one-half of patients in each group and included hypertension, hyperlipidemia, valvular heart disease, avascular necrosis, premature menopause, and major infections, including herpes zoster.
Less cyclophosphamide works just as well
The multicenter, prospective Euro-Lupus Nephritis Trial4 randomized 90 patients with proliferative lupus nephritis to receive either standard high-dose intravenous (IV) cyclophosphamide therapy (six monthly pulses and two quarterly pulses, with doses increasing according to the white blood cell count) or low-dose IV cyclophosphamide therapy (six pulses every 2 weeks at a fixed dose of 500 mg). Both regimens were followed by azathioprine (Imuran).
At 4 years, the two treatment groups were not significantly different in terms of treatment failure, remission rates, serum creatinine levels, 24-hour proteinuria, and freedom from renal flares. However, the rates of side effects were significantly different, with more patients in the low-dosage group free of severe infection.
One problem with this study is whether it is applicable to an American lupus nephritis population, since 84% of the patients were white. Since this study, others indicate that this regimen is probably also safe and effective for different racial groups in the United States.
At 10-year follow-up,5 both treatment groups still had identical excellent rates of freedom from end-stage renal disease. Serum creatinine and 24-hour proteinuria were also at excellent levels and identical in both groups. Nearly three quarters of patients still needed glucocorticoid therapy and more than half still needed immunosuppressive therapy, but the rates were not statistically significantly different between the treatment groups.
The cumulative dose of cyclophosphamide was 9.5 g in the standard-treatment group and 5.5 g in the low-dose group. This difference in exposure could make a tremendous difference to patients, not only for immediate side effects such as early menopause and infections, but for the risk of cancer in later decades.
This study showed clearly that low-dose cyclophosphamide is an option for induction therapy. Drawbacks of the study were that the population was mostly white and that patients had only moderately severe disease.
Low-dose cyclophosphamide has largely replaced the older National Institutes of Health regimen, although during the last decade drug therapy has undergone more changes.
MYCOPHENOLATE AND AZATHIOPRINE: ALTERNATIVES TO CYCLOPHOSPHAMIDE
In a Chinese study, mycophenolate was better than cyclophosphamide for induction
In a study in Hong Kong, Chan et al6 randomized 42 patients with severe lupus nephritis to receive either mycophenolate mofetil (available in the United States as CellCept; 2 g/day for 6 months, then 1 g/day for 6 months) or oral cyclophosphamide (2.5 mg/kg per day for 6 months) followed by azathioprine (1.5–2.0 mg/kg per day) for 6 months. Both groups also received prednisolone during the year.
At the end of the first year, the two groups were not significantly different in their rates of complete remission, partial remission, and relapse. The rate of infection, although not significantly different, was higher in the cyclophosphamide group (33% vs 19%). Two patients (10%) died in the cyclophosphamide group, but the difference in mortality rates was not statistically significant.
Nearly 5 years later,7 rates of chronic renal failure and relapse were still statistically the same in the two groups. Infections were fewer in the mycophenolate group (13% vs 40%, P = .013). The rate of amenorrhea was 36% in the cyclophosphamide group and only 4% in the mycophenolate group (P = .004). Four patients in the cyclophosphamide group and none in the mycophenolate group reached the composite end point of end-stage renal failure or death (P = .062).
This study appeared to offer a new option with equal efficacy and fewer side effects than standard therapy. However, its applicability to non-Chinese populations remained to be shown.
In a US study, mycophenolate or azathioprine was better than cyclophosphamide as maintenance
In a study in Miami,8 59 patients with lupus nephritis were given standard induction therapy with IV cyclophosphamide plus glucocorticoids for 6 months, then randomly assigned to one of three maintenance therapies for 1 to 3 years: IV injections of cyclophosphamide every 3 months (standard therapy), oral azathioprine, or oral mycophenolate. The population was 93% female, their average age was 33 years, and nearly half were African American, with many of the others being Hispanic. Patients tended to have severe disease, with nearly two-thirds having nephrotic syndrome.
After 6 years, there had been more deaths in the cyclophosphamide group than in the azathioprine group (P = .02) and in the mycophenolate group, although the latter difference was not statistically significant (P = .11). The combined rate of death and chronic renal failure was significantly higher with cyclophosphamide than with either of the oral agents. The cyclophosphamide group also had the highest relapse rate during the maintenance phase.
The differences in side effects were even more dramatic. Amenorrhea affected 32% of patients in the cyclophosphamide group, and only 7% and 6% in the azathioprine and mycophenolate groups, respectively. Rates of infections were 68% in the cyclophosphamide group and 28% and 21% in the azathioprine and mycophenolate groups, respectively. Patients given cyclophosphamide had 13 hospital days per patient per year, while the other groups each had only 1.
This study showed that maintenance therapy with oral azathioprine or mycophenolate was more effective and had fewer adverse effects than standard IV cyclophosphamide therapy. As a result of this study, oral agents for maintenance therapy became the new standard, but the question remained whether oral agents could safely be used for induction.
In a US study, mycophenolate was better than cyclophosphamide for induction
In a noninferiority study, Ginzler et al9 randomized 140 patients with severe lupus nephritis to receive either monthly IV cyclophosphamide or oral mycophenolate as induction therapy for 6 months. Adjunctive care with glucocorticoids was given in both groups. The study population was from 18 US academic centers and was predominantly female, and more than half were African American.
After 24 weeks, 22.5% of the mycophenolate patients were in complete remission by very strict criteria vs only 4% of those given cyclophosphamide (P = .005). The trend for partial remissions was also in favor of mycophenolate, although the difference was not statistically significant. The rate of complete and partial remissions, a prespecified end point, was significantly higher in the mycophenolate group. Although the study was trying to evaluate equivalency, it actually showed superiority for mycophenolate induction therapy.
Serum creatinine levels declined in both groups, but more in the mycophenolate group by 24 weeks. Urinary protein levels fell the same amount in both groups. At 3 years, the groups were statistically equivalent in terms of renal flares, renal failures, and deaths. However, the study groups were small, and the mycophenolate group did have a better trend for both renal failure (N = 4 vs 7) and deaths (N = 4 vs 8).
Mycophenolate also had fewer side effects, including infection, although again the numbers were too small to show statistical significance. The exception was diarrhea (N = 15 in the mycophenolate group vs 2 in the cyclophosphamide group).
A drawback of the study is that it was designed as a crossover study: a patient for whom therapy was failing after 3 months could switch to the other group, introducing potential confounding. Other problems involved the small population size and the question of whether results from patients in the United States were applicable to others worldwide.
In a worldwide study, mycophenolate was at least equivalent to cyclophosphamide for induction
The Aspreva Lupus Management Study (ALMS)10 used a similar design with 370 patients worldwide (United States, China, South America, and Europe) in one of the largest trials ever conducted in lupus nephritis. Patients were randomized to 6 months of induction therapy with either IV cyclophosphamide or oral mycophenolate but could not cross over.
At 6 months, response rates were identical between the two groups, with response defined as a combination of specific improvement in proteinuria, serum creatinine, and hematuria (50%–55%). In terms of individual renal and nonrenal variables, both groups appeared identical.
However, the side effect profiles differed between the two groups. As expected for mycophenolate, diarrhea was the most common side effect (occurring in 28% vs 12% in the cyclophosphamide group). Nausea and vomiting were more common with cyclophosphamide (45% and 37% respectively vs 14% and 13% in the mycophenolate group). Cyclophosphamide also caused hair loss in 35%, vs 10% in the mycophenolate group.
There were 14 deaths overall, which is a very low number considering the patients’ severity of illness, and it indicates the better results now achieved with therapy. The mortality rate was higher in the mycophenolate group (5% vs 3%), but the difference was not statistically significant. Six of the nine deaths with mycophenolate were from the same center in China, and none were from Europe or the United States. In summary, the study did not show that mycophenolate was superior to IV cyclophosphamide for induction therapy, but that they were equivalent in efficacy with different side effect profiles.
Membranous nephropathy: Mycophenolate vs cyclophosphamide
Less evidence is available about treatment for membranous disease, which is characterized by heavy proteinuria and the nephrotic syndrome but usually does not progress to renal failure. Radhakrishnan et al11 combined data from the trial by Ginzler et al9 and the ALMS trial10 and found 84 patients with pure membranous lupus, who were equally divided between the treatment groups receiving IV cyclophosphamide and mycophenolate. Consistent with the larger group’s data, mycophenolate and cyclophosphamide performed similarly in terms of efficacy, but there was a slightly higher rate of side effects with cyclophosphamide.
Maintenance therapy: Mycophenolate superior to azathioprine
The ALMS Maintenance Trial12 evaluated maintenance therapy in the same worldwide population that was studied for induction therapy. Of the 370 patients involved in the induction phase that compared IV cyclophosphamide and oral mycophenolate, 227 responded sufficiently to be rerandomized in a controlled, double-blinded trial of 36 months of maintenance therapy with corticosteroids and either mycophenolate (1 g twice daily) or azathioprine (2 mg/kg per day).
In intention-to-treat analysis, the time to treatment failure (ie, doubling of the serum creatinine level, progressing to renal failure, or death) was significantly shorter in the azathioprine group (P = .003). Every individual end point—end-stage renal disease, renal flares, doubling of serum creatinine, rescue immunosuppression required—was in favor of mycophenolate maintenance. At 3 years, the completion rate was 63% with mycophenolate and 49% with azathioprine. Serious adverse events and withdrawals because of adverse events were more common in the azathioprine group.
In summary, mycophenolate was superior to azathioprine in maintaining renal response and in preventing relapse in patients with active lupus nephritis who responded to induction therapy with either mycophenolate or IV cyclophosphamide. Mycophenolate was found to be superior regardless of initial induction treatment, race, or region and was confirmed by all key secondary end points.
Only one of the 227 patients died during the 3 years—from an auto accident. Again, this indicates the dramatically improved survival today compared with a decade ago.
RITUXIMAB: PROMISING BUT UNPROVEN
Rituximab (Rituxan) was originally approved to treat tumors, then rheumatoid arthritis, and most recently vasculitis. Evidence thus far is mixed regarding its use as a treatment for lupus nephritis. Although randomized clinical trials have not found it to be superior to standard regimens, there are many signs that it may be effective.
Rituximab in uncontrolled studies
Terrier et al13 analyzed prospective data from 136 patients with systemic lupus erythematosus, most of whom had renal disease, from the French Autoimmunity and Rituximab registry. Response occurred in 71% of patients using rituximab, with no difference found between patients receiving rituximab monotherapy and those concomitantly receiving immunosuppressive agents.
Melander et al14 retrospectively studied 19 women and 1 man who had been treated with rituximab for severe lupus nephritis and followed for at least 1 year. Three patients had concurrent therapy with cyclophosphamide, and 10 patients continued rituximab as maintenance therapy; 12 patients had lupus nephritis that had been refractory to standard treatment, and 6 had relapsing disease.
At a median follow-up of 22 months, 12 patients (60%) had achieved complete or partial renal remission.
Condon et al15 treated 21 patients who had severe lupus nephritis with two doses of rituximab and IV methylprednisolone 2 weeks apart, then maintenance therapy with mycophenolate without any oral steroids. At a mean follow-up of 35 months ( ± 14 months), 16 (76%) were in complete remission, with a mean time to remission of 12 months. Two (9.5%) achieved partial remission. The rate of toxicity was low.
Thus, rituximab appears promising in uncontrolled studies.
Placebo-controlled trials fail to prove rituximab effective
LUNAR trial. On the other hand, the largest placebo-controlled trial to evaluate rituximab in patients with proliferative lupus nephritis, the Lupus Nephritis Assessment With Rituximab (LUNAR) trial16 found differences in favor of rituximab, but none reached statistical significance. The trial randomized 140 patients to receive either mycophenolate plus periodic rituximab infusions or mycophenolate plus placebo infusions for 1 year. All patients received the same dosage of glucocorticoids, which was tapered over the year.
At the end of 1 year, the groups were not statistically different in terms of complete renal response and partial renal response. Rituximab appeared less likely to produce no response, but the difference was not statistically significant.
African Americans appeared to have a higher response rate to rituximab (70% in the rituximab group achieved a response vs 45% in the control group), but again, the difference did not reach statistical significance, and the total study population of African Americans was only 40.
Rituximab did have a statistically significant positive effect on two serologic markers at 1 year: levels of anti-dsDNA fell faster and complement rose faster. In addition, rates of adverse and serious adverse events were similar between the two groups, with no new or unexpected “safety signals.”
This study can be interpreted in a number of ways. The number of patients may have been too small to show significance and the follow-up may have been too short. On the other hand, it may simply not be effective to add rituximab to a full dose of mycophenolate and steroids, an already good treatment.
EXPLORER trial. Similarly, for patients with lupus without nephritis, the Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial17 also tested rituximab against a background of an effective therapeutic regimen and found no additional benefit. This study had design problems similar to those of the LUNAR trial.
Rituximab as rescue therapy
The evidence so far indicates that rituximab may have a role as rescue therapy for refractory or relapsing disease. Rituximab must be used with other therapies, but maintenance corticosteroid therapy is not necessary. Its role as a first-line agent in induction therapy for lupus nephritis remains unclear, although it may have an important role for nonwhites. In general, it has been well tolerated. Until a large randomized trial indicates otherwise, it should not be used as a first-line therapy.
The US Food and Drug Administration (FDA) sent out a warning about the danger of progressive multifocal leukoencephalopathy as an adverse effect of rituximab and of mycophenolate, but this does not appear to be a major concern for most patients and is only likely to occur in those who have been over-immunosuppressed for many years.
MULTITARGET THERAPY
The concept of using multiple drugs simultaneously—such as mycophenolate, steroids, and rituximab—is increasingly being tried. Multi-target therapy appears to offer the advantages of combining different modes of action with better results, and it offers fewer side effects because dosages of each individual drug can be lower when combined with other immunosuppressives.
Bao et al18 in China randomly assigned 40 patients with diffuse proliferative and membranous nephritis to 6 to 9 months of induction treatment with either multitarget therapy (mycophenolate, tacrolimus [Prograf], and glucocorticoids) or IV cyclophosphamide. More complete remissions occurred in the multitarget therapy group, both at 6 months (50% vs 5%) and at 9 months (65% vs 15%). Most adverse events were less frequent in the multitarget therapy group, although three patients (15%) in the multitarget therapy group developed new-onset hypertension vs none in the cyclophosphamide group.
NEW MEDICATIONS
Entirely new classes of drugs are being developed with immunomodulatory effects, including tolerance molecules, cytokine blockers, inhibitors of human B lymphocyte stimulator, and costimulatory blockers.
Belimumab offers small improvement for lupus
Belimumab (Benlysta) is a human monoclonal antibody that inhibits the biologic activity of human B lymphocyte stimulator; it has recently been approved by the FDA for lupus nephritis. In a worldwide study,19 867 patients with systemic lupus erythematosus were randomized to receive either belimumab (1 mg/kg or 10 mg/kg) or placebo.
The primary end point was the reduction of disease activity by a scoring system (SELENA-SLEDAI) that incorporated multiple features of lupus, including arthritis, vasculitis, proteinuria, rash, and others. Patients in the belimumab group had better outcomes, but the results were not dramatic. Because the drug is so expensive (about $25,000 per year) and the improvement offered is only incremental, this drug will not likely change the treatment of lupus very much.
Moreover, patients with lupus nephritis were not included in the study, but a new study is being planned to do so. Improvement is harder to demonstrate in lupus nephritis than in rheumatoid arthritis and systemic lupus erythematosus: significant changes in creatinine levels and 24-hour urinary protein must be achieved, rather than more qualitative signs and symptoms of joint pain, rash, and feeling better. Although belimumab is still unproven for lupus nephritis, it might be worth trying for patients failing other therapy.
Laquinimod: A promising experimental drug
Laquinimod is an oral immunomodulatory drug with a number of effects, including down-regulating major histocompatability complex II, chemokines, and adhesion-related molecules related to inflammation. It has been studied in more than 2,500 patients with multiple sclerosis. Pilot studies are now being done for its use for lupus nephritis. If it shows promise, a large randomized, controlled trial will be conducted.
Abatacept is in clinical trials
Abatacept (Orencia), a costimulation blocker, is undergoing clinical trials in lupus nephritis. Results should be available shortly.
INDIVIDUALIZE THERAPY
This past decade has seen such an increase in options to treat lupus nephritis that therapy can now be individualized.
Choosing IV cyclophosphamide vs mycophenolate
As a result of recent trials, doctors in the United States are increasingly using mycophenolate as the first-line drug for lupus nephritis. In Europe, however, many are choosing the shorter regimen of IV cyclophosphamide because of the results of the Euro-Lupus study.
Nowadays, I tend to use IV cyclophosphamide as the first-line drug only for patients with severe crescenteric glomerulonephritis or a very high serum creatinine level. In such cases, there is more experience with cyclophosphamide, and such severe disease does not lend itself to the luxury of trying out different therapies sequentially. If such a severely ill patient insists that a future pregnancy is very important, an alternative therapy of mycophenolate plus rituximab should be considered. I prefer mycophenolate for induction and maintenance therapy in most patients.
Dosing and formulation considerations for mycophenolate
Large dosages of mycophenolate are much better tolerated when broken up throughout the day. A patient who cannot tolerate 1 g twice daily may be able to tolerate 500 mg four times a day. The formulation can also make a difference. Some patients tolerate sustained-release mycophenolate (Myfortic) better than CellCept, and vice versa.
For patients who cannot tolerate mycophenolate, azathioprine is an acceptable alternative. In addition, for a patient who is already doing well on azathioprine, there is no need to change to mycophenolate.
Long maintenance therapy now acceptable
The ALMS Maintenance Trial12 found 3 years of maintenance therapy to be safe and effective. Such a long maintenance period is increasingly viewed as important, especially for patients in their teens and 20s, as it allows them to live a normal life, ie, to finish their education, get married, and become settled socially. Whether 5 years of maintenance therapy or even 10 years is advisable is still unknown.
Treatment during pregnancy
Neither mycophenolate nor azathioprine is recommended during pregnancy, although their effects are unknown. Because we have much more renal transplant experience with azathioprine during pregnancy, I recommend either switching from mycophenolate to azathioprine or trying to stop medication altogether if the patient has been well controlled.
- Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006; 69:1846–1851.
- Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 2003; 18:2039–2046.
- Illei GG, Austin HA, Crane M, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med 2001; 135:248–257.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002; 46:2121–2131.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010; 69:61–64.
- Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong King-Guangzhou Nephrology Study Group. N Engl J Med 2000; 343:1156–1162.
- Chan TM, Tse KC, Tang CS, Mok MY, Li FK; Hong Kong Nephrology Study Group. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 2005; 16:1076–1084.
- Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med 2004; 350:971–980.
- Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 2005; 353:2219–2228.
- Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009; 20:1103–1112.
- Radhakrishnan J, Moutzouris DA, Ginzler EM, Solomons N, Siempos II, Appel GB. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int 2010; 77:152–160.
- Dooley MA, Jayne D, Ginzler EM, et al; for the ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 2011; 365:1886–1895.
- Terrier B, Amoura Z, Ravaud P, et al; Club Rhumatismes et Inflammation. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010; 62:2458–2466.
- Melander C, Sallée M, Troillet P, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol 2009; 4:579–587.
- Condon MB, Griffith M, Cook HT, Levy J, Lightstone L, Cairns T. Treatment of class IV lupus nephritis with rituximab & mycophenolate mofetil (MMF) with no oral steroids is effective and safe (abstract). J Am Soc Nephrol 2010; 21(suppl):625A–626A.
- Furie RA, Looney RJ, Rovin E, et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study (abstract). Arthritis Rheum 2009; 60(suppl 1):S429.
- Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62:222–233.
- Bao H, Liu ZH, Zie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol 2008; 19:2001–2010.
- Navarra SV, Guzmán RM, Gallacher AE, et al; BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:721–731.
Treatment for lupus nephritis has changed dramatically in recent years. Only 10 years ago, rheumatologists and nephrologists, whether specializing in adult or pediatric medicine, treated lupus nephritis with a similar regimen of monthly intravenous cyclophosphamide (Cytoxan) and glucocorticoids. Although the regimen is effective, side effects such as infection, hair loss, and infertility were extremely common.
Effective but very toxic therapy is common in autoimmune diseases. In the last decade, clinical trials have shown that less toxic drugs are as effective for treating lupus nephritis. This article will review new developments in therapy for lupus nephritis, which can be viewed as a prototype for other fields of medicine.
DEMOGRAPHICS ARE IMPORTANT
Although numerous factors have prognostic value in lupus nephritis (eg, serum creatinine, proteinuria, renal biopsy findings), the most important to consider when designing and interpreting studies are race and socioeconomic variables.
A retrospective study in Miami, FL,1 evaluated 213 patients with lupus nephritis, of whom 47% were Hispanic, 44% African American, and 20% white. At baseline, African Americans had higher blood pressure, higher serum creatinine levels, and lower household income. After 6 years, African Americans fared the worst in terms of doubling of serum creatinine, developing end-stage renal disease, and death; whites had the best outcomes, and Hispanics were in between. Low income was found to be a significant risk factor, independent of racial background.
In a similar retrospective study in New York City in 128 patients (43% white, 40% Hispanic, and 17% African American) with proliferative lupus nephritis,2 disease was much more likely to progress to renal failure over 10 years in patients living in a poor neighborhood, even after adjustment for race.
We need to keep in mind that racial and socioeconomic factors correlate with disease severity when we design and interpret studies of lupus nephritis. Study groups must be carefully balanced with patients of similar racial and socioeconomic profiles. Study findings must be interpreted with caution; for example, whether results from a study from China are applicable to an African American with lupus nephritis in New York City is unclear.
OLDER STANDARD THERAPY: EFFECTIVE BUT TOXIC
The last large National Institutes of Health study that involved only cyclophosphamide and a glucocorticoid was published in 2001,3 with 21 patients receiving cyclophosphamide alone and 20 patients receiving cyclophosphamide plus methylprednisolone. Although lupus nephritis improved, serious side effects occurred in one-third to one-half of patients in each group and included hypertension, hyperlipidemia, valvular heart disease, avascular necrosis, premature menopause, and major infections, including herpes zoster.
Less cyclophosphamide works just as well
The multicenter, prospective Euro-Lupus Nephritis Trial4 randomized 90 patients with proliferative lupus nephritis to receive either standard high-dose intravenous (IV) cyclophosphamide therapy (six monthly pulses and two quarterly pulses, with doses increasing according to the white blood cell count) or low-dose IV cyclophosphamide therapy (six pulses every 2 weeks at a fixed dose of 500 mg). Both regimens were followed by azathioprine (Imuran).
At 4 years, the two treatment groups were not significantly different in terms of treatment failure, remission rates, serum creatinine levels, 24-hour proteinuria, and freedom from renal flares. However, the rates of side effects were significantly different, with more patients in the low-dosage group free of severe infection.
One problem with this study is whether it is applicable to an American lupus nephritis population, since 84% of the patients were white. Since this study, others indicate that this regimen is probably also safe and effective for different racial groups in the United States.
At 10-year follow-up,5 both treatment groups still had identical excellent rates of freedom from end-stage renal disease. Serum creatinine and 24-hour proteinuria were also at excellent levels and identical in both groups. Nearly three quarters of patients still needed glucocorticoid therapy and more than half still needed immunosuppressive therapy, but the rates were not statistically significantly different between the treatment groups.
The cumulative dose of cyclophosphamide was 9.5 g in the standard-treatment group and 5.5 g in the low-dose group. This difference in exposure could make a tremendous difference to patients, not only for immediate side effects such as early menopause and infections, but for the risk of cancer in later decades.
This study showed clearly that low-dose cyclophosphamide is an option for induction therapy. Drawbacks of the study were that the population was mostly white and that patients had only moderately severe disease.
Low-dose cyclophosphamide has largely replaced the older National Institutes of Health regimen, although during the last decade drug therapy has undergone more changes.
MYCOPHENOLATE AND AZATHIOPRINE: ALTERNATIVES TO CYCLOPHOSPHAMIDE
In a Chinese study, mycophenolate was better than cyclophosphamide for induction
In a study in Hong Kong, Chan et al6 randomized 42 patients with severe lupus nephritis to receive either mycophenolate mofetil (available in the United States as CellCept; 2 g/day for 6 months, then 1 g/day for 6 months) or oral cyclophosphamide (2.5 mg/kg per day for 6 months) followed by azathioprine (1.5–2.0 mg/kg per day) for 6 months. Both groups also received prednisolone during the year.
At the end of the first year, the two groups were not significantly different in their rates of complete remission, partial remission, and relapse. The rate of infection, although not significantly different, was higher in the cyclophosphamide group (33% vs 19%). Two patients (10%) died in the cyclophosphamide group, but the difference in mortality rates was not statistically significant.
Nearly 5 years later,7 rates of chronic renal failure and relapse were still statistically the same in the two groups. Infections were fewer in the mycophenolate group (13% vs 40%, P = .013). The rate of amenorrhea was 36% in the cyclophosphamide group and only 4% in the mycophenolate group (P = .004). Four patients in the cyclophosphamide group and none in the mycophenolate group reached the composite end point of end-stage renal failure or death (P = .062).
This study appeared to offer a new option with equal efficacy and fewer side effects than standard therapy. However, its applicability to non-Chinese populations remained to be shown.
In a US study, mycophenolate or azathioprine was better than cyclophosphamide as maintenance
In a study in Miami,8 59 patients with lupus nephritis were given standard induction therapy with IV cyclophosphamide plus glucocorticoids for 6 months, then randomly assigned to one of three maintenance therapies for 1 to 3 years: IV injections of cyclophosphamide every 3 months (standard therapy), oral azathioprine, or oral mycophenolate. The population was 93% female, their average age was 33 years, and nearly half were African American, with many of the others being Hispanic. Patients tended to have severe disease, with nearly two-thirds having nephrotic syndrome.
After 6 years, there had been more deaths in the cyclophosphamide group than in the azathioprine group (P = .02) and in the mycophenolate group, although the latter difference was not statistically significant (P = .11). The combined rate of death and chronic renal failure was significantly higher with cyclophosphamide than with either of the oral agents. The cyclophosphamide group also had the highest relapse rate during the maintenance phase.
The differences in side effects were even more dramatic. Amenorrhea affected 32% of patients in the cyclophosphamide group, and only 7% and 6% in the azathioprine and mycophenolate groups, respectively. Rates of infections were 68% in the cyclophosphamide group and 28% and 21% in the azathioprine and mycophenolate groups, respectively. Patients given cyclophosphamide had 13 hospital days per patient per year, while the other groups each had only 1.
This study showed that maintenance therapy with oral azathioprine or mycophenolate was more effective and had fewer adverse effects than standard IV cyclophosphamide therapy. As a result of this study, oral agents for maintenance therapy became the new standard, but the question remained whether oral agents could safely be used for induction.
In a US study, mycophenolate was better than cyclophosphamide for induction
In a noninferiority study, Ginzler et al9 randomized 140 patients with severe lupus nephritis to receive either monthly IV cyclophosphamide or oral mycophenolate as induction therapy for 6 months. Adjunctive care with glucocorticoids was given in both groups. The study population was from 18 US academic centers and was predominantly female, and more than half were African American.
After 24 weeks, 22.5% of the mycophenolate patients were in complete remission by very strict criteria vs only 4% of those given cyclophosphamide (P = .005). The trend for partial remissions was also in favor of mycophenolate, although the difference was not statistically significant. The rate of complete and partial remissions, a prespecified end point, was significantly higher in the mycophenolate group. Although the study was trying to evaluate equivalency, it actually showed superiority for mycophenolate induction therapy.
Serum creatinine levels declined in both groups, but more in the mycophenolate group by 24 weeks. Urinary protein levels fell the same amount in both groups. At 3 years, the groups were statistically equivalent in terms of renal flares, renal failures, and deaths. However, the study groups were small, and the mycophenolate group did have a better trend for both renal failure (N = 4 vs 7) and deaths (N = 4 vs 8).
Mycophenolate also had fewer side effects, including infection, although again the numbers were too small to show statistical significance. The exception was diarrhea (N = 15 in the mycophenolate group vs 2 in the cyclophosphamide group).
A drawback of the study is that it was designed as a crossover study: a patient for whom therapy was failing after 3 months could switch to the other group, introducing potential confounding. Other problems involved the small population size and the question of whether results from patients in the United States were applicable to others worldwide.
In a worldwide study, mycophenolate was at least equivalent to cyclophosphamide for induction
The Aspreva Lupus Management Study (ALMS)10 used a similar design with 370 patients worldwide (United States, China, South America, and Europe) in one of the largest trials ever conducted in lupus nephritis. Patients were randomized to 6 months of induction therapy with either IV cyclophosphamide or oral mycophenolate but could not cross over.
At 6 months, response rates were identical between the two groups, with response defined as a combination of specific improvement in proteinuria, serum creatinine, and hematuria (50%–55%). In terms of individual renal and nonrenal variables, both groups appeared identical.
However, the side effect profiles differed between the two groups. As expected for mycophenolate, diarrhea was the most common side effect (occurring in 28% vs 12% in the cyclophosphamide group). Nausea and vomiting were more common with cyclophosphamide (45% and 37% respectively vs 14% and 13% in the mycophenolate group). Cyclophosphamide also caused hair loss in 35%, vs 10% in the mycophenolate group.
There were 14 deaths overall, which is a very low number considering the patients’ severity of illness, and it indicates the better results now achieved with therapy. The mortality rate was higher in the mycophenolate group (5% vs 3%), but the difference was not statistically significant. Six of the nine deaths with mycophenolate were from the same center in China, and none were from Europe or the United States. In summary, the study did not show that mycophenolate was superior to IV cyclophosphamide for induction therapy, but that they were equivalent in efficacy with different side effect profiles.
Membranous nephropathy: Mycophenolate vs cyclophosphamide
Less evidence is available about treatment for membranous disease, which is characterized by heavy proteinuria and the nephrotic syndrome but usually does not progress to renal failure. Radhakrishnan et al11 combined data from the trial by Ginzler et al9 and the ALMS trial10 and found 84 patients with pure membranous lupus, who were equally divided between the treatment groups receiving IV cyclophosphamide and mycophenolate. Consistent with the larger group’s data, mycophenolate and cyclophosphamide performed similarly in terms of efficacy, but there was a slightly higher rate of side effects with cyclophosphamide.
Maintenance therapy: Mycophenolate superior to azathioprine
The ALMS Maintenance Trial12 evaluated maintenance therapy in the same worldwide population that was studied for induction therapy. Of the 370 patients involved in the induction phase that compared IV cyclophosphamide and oral mycophenolate, 227 responded sufficiently to be rerandomized in a controlled, double-blinded trial of 36 months of maintenance therapy with corticosteroids and either mycophenolate (1 g twice daily) or azathioprine (2 mg/kg per day).
In intention-to-treat analysis, the time to treatment failure (ie, doubling of the serum creatinine level, progressing to renal failure, or death) was significantly shorter in the azathioprine group (P = .003). Every individual end point—end-stage renal disease, renal flares, doubling of serum creatinine, rescue immunosuppression required—was in favor of mycophenolate maintenance. At 3 years, the completion rate was 63% with mycophenolate and 49% with azathioprine. Serious adverse events and withdrawals because of adverse events were more common in the azathioprine group.
In summary, mycophenolate was superior to azathioprine in maintaining renal response and in preventing relapse in patients with active lupus nephritis who responded to induction therapy with either mycophenolate or IV cyclophosphamide. Mycophenolate was found to be superior regardless of initial induction treatment, race, or region and was confirmed by all key secondary end points.
Only one of the 227 patients died during the 3 years—from an auto accident. Again, this indicates the dramatically improved survival today compared with a decade ago.
RITUXIMAB: PROMISING BUT UNPROVEN
Rituximab (Rituxan) was originally approved to treat tumors, then rheumatoid arthritis, and most recently vasculitis. Evidence thus far is mixed regarding its use as a treatment for lupus nephritis. Although randomized clinical trials have not found it to be superior to standard regimens, there are many signs that it may be effective.
Rituximab in uncontrolled studies
Terrier et al13 analyzed prospective data from 136 patients with systemic lupus erythematosus, most of whom had renal disease, from the French Autoimmunity and Rituximab registry. Response occurred in 71% of patients using rituximab, with no difference found between patients receiving rituximab monotherapy and those concomitantly receiving immunosuppressive agents.
Melander et al14 retrospectively studied 19 women and 1 man who had been treated with rituximab for severe lupus nephritis and followed for at least 1 year. Three patients had concurrent therapy with cyclophosphamide, and 10 patients continued rituximab as maintenance therapy; 12 patients had lupus nephritis that had been refractory to standard treatment, and 6 had relapsing disease.
At a median follow-up of 22 months, 12 patients (60%) had achieved complete or partial renal remission.
Condon et al15 treated 21 patients who had severe lupus nephritis with two doses of rituximab and IV methylprednisolone 2 weeks apart, then maintenance therapy with mycophenolate without any oral steroids. At a mean follow-up of 35 months ( ± 14 months), 16 (76%) were in complete remission, with a mean time to remission of 12 months. Two (9.5%) achieved partial remission. The rate of toxicity was low.
Thus, rituximab appears promising in uncontrolled studies.
Placebo-controlled trials fail to prove rituximab effective
LUNAR trial. On the other hand, the largest placebo-controlled trial to evaluate rituximab in patients with proliferative lupus nephritis, the Lupus Nephritis Assessment With Rituximab (LUNAR) trial16 found differences in favor of rituximab, but none reached statistical significance. The trial randomized 140 patients to receive either mycophenolate plus periodic rituximab infusions or mycophenolate plus placebo infusions for 1 year. All patients received the same dosage of glucocorticoids, which was tapered over the year.
At the end of 1 year, the groups were not statistically different in terms of complete renal response and partial renal response. Rituximab appeared less likely to produce no response, but the difference was not statistically significant.
African Americans appeared to have a higher response rate to rituximab (70% in the rituximab group achieved a response vs 45% in the control group), but again, the difference did not reach statistical significance, and the total study population of African Americans was only 40.
Rituximab did have a statistically significant positive effect on two serologic markers at 1 year: levels of anti-dsDNA fell faster and complement rose faster. In addition, rates of adverse and serious adverse events were similar between the two groups, with no new or unexpected “safety signals.”
This study can be interpreted in a number of ways. The number of patients may have been too small to show significance and the follow-up may have been too short. On the other hand, it may simply not be effective to add rituximab to a full dose of mycophenolate and steroids, an already good treatment.
EXPLORER trial. Similarly, for patients with lupus without nephritis, the Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial17 also tested rituximab against a background of an effective therapeutic regimen and found no additional benefit. This study had design problems similar to those of the LUNAR trial.
Rituximab as rescue therapy
The evidence so far indicates that rituximab may have a role as rescue therapy for refractory or relapsing disease. Rituximab must be used with other therapies, but maintenance corticosteroid therapy is not necessary. Its role as a first-line agent in induction therapy for lupus nephritis remains unclear, although it may have an important role for nonwhites. In general, it has been well tolerated. Until a large randomized trial indicates otherwise, it should not be used as a first-line therapy.
The US Food and Drug Administration (FDA) sent out a warning about the danger of progressive multifocal leukoencephalopathy as an adverse effect of rituximab and of mycophenolate, but this does not appear to be a major concern for most patients and is only likely to occur in those who have been over-immunosuppressed for many years.
MULTITARGET THERAPY
The concept of using multiple drugs simultaneously—such as mycophenolate, steroids, and rituximab—is increasingly being tried. Multi-target therapy appears to offer the advantages of combining different modes of action with better results, and it offers fewer side effects because dosages of each individual drug can be lower when combined with other immunosuppressives.
Bao et al18 in China randomly assigned 40 patients with diffuse proliferative and membranous nephritis to 6 to 9 months of induction treatment with either multitarget therapy (mycophenolate, tacrolimus [Prograf], and glucocorticoids) or IV cyclophosphamide. More complete remissions occurred in the multitarget therapy group, both at 6 months (50% vs 5%) and at 9 months (65% vs 15%). Most adverse events were less frequent in the multitarget therapy group, although three patients (15%) in the multitarget therapy group developed new-onset hypertension vs none in the cyclophosphamide group.
NEW MEDICATIONS
Entirely new classes of drugs are being developed with immunomodulatory effects, including tolerance molecules, cytokine blockers, inhibitors of human B lymphocyte stimulator, and costimulatory blockers.
Belimumab offers small improvement for lupus
Belimumab (Benlysta) is a human monoclonal antibody that inhibits the biologic activity of human B lymphocyte stimulator; it has recently been approved by the FDA for lupus nephritis. In a worldwide study,19 867 patients with systemic lupus erythematosus were randomized to receive either belimumab (1 mg/kg or 10 mg/kg) or placebo.
The primary end point was the reduction of disease activity by a scoring system (SELENA-SLEDAI) that incorporated multiple features of lupus, including arthritis, vasculitis, proteinuria, rash, and others. Patients in the belimumab group had better outcomes, but the results were not dramatic. Because the drug is so expensive (about $25,000 per year) and the improvement offered is only incremental, this drug will not likely change the treatment of lupus very much.
Moreover, patients with lupus nephritis were not included in the study, but a new study is being planned to do so. Improvement is harder to demonstrate in lupus nephritis than in rheumatoid arthritis and systemic lupus erythematosus: significant changes in creatinine levels and 24-hour urinary protein must be achieved, rather than more qualitative signs and symptoms of joint pain, rash, and feeling better. Although belimumab is still unproven for lupus nephritis, it might be worth trying for patients failing other therapy.
Laquinimod: A promising experimental drug
Laquinimod is an oral immunomodulatory drug with a number of effects, including down-regulating major histocompatability complex II, chemokines, and adhesion-related molecules related to inflammation. It has been studied in more than 2,500 patients with multiple sclerosis. Pilot studies are now being done for its use for lupus nephritis. If it shows promise, a large randomized, controlled trial will be conducted.
Abatacept is in clinical trials
Abatacept (Orencia), a costimulation blocker, is undergoing clinical trials in lupus nephritis. Results should be available shortly.
INDIVIDUALIZE THERAPY
This past decade has seen such an increase in options to treat lupus nephritis that therapy can now be individualized.
Choosing IV cyclophosphamide vs mycophenolate
As a result of recent trials, doctors in the United States are increasingly using mycophenolate as the first-line drug for lupus nephritis. In Europe, however, many are choosing the shorter regimen of IV cyclophosphamide because of the results of the Euro-Lupus study.
Nowadays, I tend to use IV cyclophosphamide as the first-line drug only for patients with severe crescenteric glomerulonephritis or a very high serum creatinine level. In such cases, there is more experience with cyclophosphamide, and such severe disease does not lend itself to the luxury of trying out different therapies sequentially. If such a severely ill patient insists that a future pregnancy is very important, an alternative therapy of mycophenolate plus rituximab should be considered. I prefer mycophenolate for induction and maintenance therapy in most patients.
Dosing and formulation considerations for mycophenolate
Large dosages of mycophenolate are much better tolerated when broken up throughout the day. A patient who cannot tolerate 1 g twice daily may be able to tolerate 500 mg four times a day. The formulation can also make a difference. Some patients tolerate sustained-release mycophenolate (Myfortic) better than CellCept, and vice versa.
For patients who cannot tolerate mycophenolate, azathioprine is an acceptable alternative. In addition, for a patient who is already doing well on azathioprine, there is no need to change to mycophenolate.
Long maintenance therapy now acceptable
The ALMS Maintenance Trial12 found 3 years of maintenance therapy to be safe and effective. Such a long maintenance period is increasingly viewed as important, especially for patients in their teens and 20s, as it allows them to live a normal life, ie, to finish their education, get married, and become settled socially. Whether 5 years of maintenance therapy or even 10 years is advisable is still unknown.
Treatment during pregnancy
Neither mycophenolate nor azathioprine is recommended during pregnancy, although their effects are unknown. Because we have much more renal transplant experience with azathioprine during pregnancy, I recommend either switching from mycophenolate to azathioprine or trying to stop medication altogether if the patient has been well controlled.
Treatment for lupus nephritis has changed dramatically in recent years. Only 10 years ago, rheumatologists and nephrologists, whether specializing in adult or pediatric medicine, treated lupus nephritis with a similar regimen of monthly intravenous cyclophosphamide (Cytoxan) and glucocorticoids. Although the regimen is effective, side effects such as infection, hair loss, and infertility were extremely common.
Effective but very toxic therapy is common in autoimmune diseases. In the last decade, clinical trials have shown that less toxic drugs are as effective for treating lupus nephritis. This article will review new developments in therapy for lupus nephritis, which can be viewed as a prototype for other fields of medicine.
DEMOGRAPHICS ARE IMPORTANT
Although numerous factors have prognostic value in lupus nephritis (eg, serum creatinine, proteinuria, renal biopsy findings), the most important to consider when designing and interpreting studies are race and socioeconomic variables.
A retrospective study in Miami, FL,1 evaluated 213 patients with lupus nephritis, of whom 47% were Hispanic, 44% African American, and 20% white. At baseline, African Americans had higher blood pressure, higher serum creatinine levels, and lower household income. After 6 years, African Americans fared the worst in terms of doubling of serum creatinine, developing end-stage renal disease, and death; whites had the best outcomes, and Hispanics were in between. Low income was found to be a significant risk factor, independent of racial background.
In a similar retrospective study in New York City in 128 patients (43% white, 40% Hispanic, and 17% African American) with proliferative lupus nephritis,2 disease was much more likely to progress to renal failure over 10 years in patients living in a poor neighborhood, even after adjustment for race.
We need to keep in mind that racial and socioeconomic factors correlate with disease severity when we design and interpret studies of lupus nephritis. Study groups must be carefully balanced with patients of similar racial and socioeconomic profiles. Study findings must be interpreted with caution; for example, whether results from a study from China are applicable to an African American with lupus nephritis in New York City is unclear.
OLDER STANDARD THERAPY: EFFECTIVE BUT TOXIC
The last large National Institutes of Health study that involved only cyclophosphamide and a glucocorticoid was published in 2001,3 with 21 patients receiving cyclophosphamide alone and 20 patients receiving cyclophosphamide plus methylprednisolone. Although lupus nephritis improved, serious side effects occurred in one-third to one-half of patients in each group and included hypertension, hyperlipidemia, valvular heart disease, avascular necrosis, premature menopause, and major infections, including herpes zoster.
Less cyclophosphamide works just as well
The multicenter, prospective Euro-Lupus Nephritis Trial4 randomized 90 patients with proliferative lupus nephritis to receive either standard high-dose intravenous (IV) cyclophosphamide therapy (six monthly pulses and two quarterly pulses, with doses increasing according to the white blood cell count) or low-dose IV cyclophosphamide therapy (six pulses every 2 weeks at a fixed dose of 500 mg). Both regimens were followed by azathioprine (Imuran).
At 4 years, the two treatment groups were not significantly different in terms of treatment failure, remission rates, serum creatinine levels, 24-hour proteinuria, and freedom from renal flares. However, the rates of side effects were significantly different, with more patients in the low-dosage group free of severe infection.
One problem with this study is whether it is applicable to an American lupus nephritis population, since 84% of the patients were white. Since this study, others indicate that this regimen is probably also safe and effective for different racial groups in the United States.
At 10-year follow-up,5 both treatment groups still had identical excellent rates of freedom from end-stage renal disease. Serum creatinine and 24-hour proteinuria were also at excellent levels and identical in both groups. Nearly three quarters of patients still needed glucocorticoid therapy and more than half still needed immunosuppressive therapy, but the rates were not statistically significantly different between the treatment groups.
The cumulative dose of cyclophosphamide was 9.5 g in the standard-treatment group and 5.5 g in the low-dose group. This difference in exposure could make a tremendous difference to patients, not only for immediate side effects such as early menopause and infections, but for the risk of cancer in later decades.
This study showed clearly that low-dose cyclophosphamide is an option for induction therapy. Drawbacks of the study were that the population was mostly white and that patients had only moderately severe disease.
Low-dose cyclophosphamide has largely replaced the older National Institutes of Health regimen, although during the last decade drug therapy has undergone more changes.
MYCOPHENOLATE AND AZATHIOPRINE: ALTERNATIVES TO CYCLOPHOSPHAMIDE
In a Chinese study, mycophenolate was better than cyclophosphamide for induction
In a study in Hong Kong, Chan et al6 randomized 42 patients with severe lupus nephritis to receive either mycophenolate mofetil (available in the United States as CellCept; 2 g/day for 6 months, then 1 g/day for 6 months) or oral cyclophosphamide (2.5 mg/kg per day for 6 months) followed by azathioprine (1.5–2.0 mg/kg per day) for 6 months. Both groups also received prednisolone during the year.
At the end of the first year, the two groups were not significantly different in their rates of complete remission, partial remission, and relapse. The rate of infection, although not significantly different, was higher in the cyclophosphamide group (33% vs 19%). Two patients (10%) died in the cyclophosphamide group, but the difference in mortality rates was not statistically significant.
Nearly 5 years later,7 rates of chronic renal failure and relapse were still statistically the same in the two groups. Infections were fewer in the mycophenolate group (13% vs 40%, P = .013). The rate of amenorrhea was 36% in the cyclophosphamide group and only 4% in the mycophenolate group (P = .004). Four patients in the cyclophosphamide group and none in the mycophenolate group reached the composite end point of end-stage renal failure or death (P = .062).
This study appeared to offer a new option with equal efficacy and fewer side effects than standard therapy. However, its applicability to non-Chinese populations remained to be shown.
In a US study, mycophenolate or azathioprine was better than cyclophosphamide as maintenance
In a study in Miami,8 59 patients with lupus nephritis were given standard induction therapy with IV cyclophosphamide plus glucocorticoids for 6 months, then randomly assigned to one of three maintenance therapies for 1 to 3 years: IV injections of cyclophosphamide every 3 months (standard therapy), oral azathioprine, or oral mycophenolate. The population was 93% female, their average age was 33 years, and nearly half were African American, with many of the others being Hispanic. Patients tended to have severe disease, with nearly two-thirds having nephrotic syndrome.
After 6 years, there had been more deaths in the cyclophosphamide group than in the azathioprine group (P = .02) and in the mycophenolate group, although the latter difference was not statistically significant (P = .11). The combined rate of death and chronic renal failure was significantly higher with cyclophosphamide than with either of the oral agents. The cyclophosphamide group also had the highest relapse rate during the maintenance phase.
The differences in side effects were even more dramatic. Amenorrhea affected 32% of patients in the cyclophosphamide group, and only 7% and 6% in the azathioprine and mycophenolate groups, respectively. Rates of infections were 68% in the cyclophosphamide group and 28% and 21% in the azathioprine and mycophenolate groups, respectively. Patients given cyclophosphamide had 13 hospital days per patient per year, while the other groups each had only 1.
This study showed that maintenance therapy with oral azathioprine or mycophenolate was more effective and had fewer adverse effects than standard IV cyclophosphamide therapy. As a result of this study, oral agents for maintenance therapy became the new standard, but the question remained whether oral agents could safely be used for induction.
In a US study, mycophenolate was better than cyclophosphamide for induction
In a noninferiority study, Ginzler et al9 randomized 140 patients with severe lupus nephritis to receive either monthly IV cyclophosphamide or oral mycophenolate as induction therapy for 6 months. Adjunctive care with glucocorticoids was given in both groups. The study population was from 18 US academic centers and was predominantly female, and more than half were African American.
After 24 weeks, 22.5% of the mycophenolate patients were in complete remission by very strict criteria vs only 4% of those given cyclophosphamide (P = .005). The trend for partial remissions was also in favor of mycophenolate, although the difference was not statistically significant. The rate of complete and partial remissions, a prespecified end point, was significantly higher in the mycophenolate group. Although the study was trying to evaluate equivalency, it actually showed superiority for mycophenolate induction therapy.
Serum creatinine levels declined in both groups, but more in the mycophenolate group by 24 weeks. Urinary protein levels fell the same amount in both groups. At 3 years, the groups were statistically equivalent in terms of renal flares, renal failures, and deaths. However, the study groups were small, and the mycophenolate group did have a better trend for both renal failure (N = 4 vs 7) and deaths (N = 4 vs 8).
Mycophenolate also had fewer side effects, including infection, although again the numbers were too small to show statistical significance. The exception was diarrhea (N = 15 in the mycophenolate group vs 2 in the cyclophosphamide group).
A drawback of the study is that it was designed as a crossover study: a patient for whom therapy was failing after 3 months could switch to the other group, introducing potential confounding. Other problems involved the small population size and the question of whether results from patients in the United States were applicable to others worldwide.
In a worldwide study, mycophenolate was at least equivalent to cyclophosphamide for induction
The Aspreva Lupus Management Study (ALMS)10 used a similar design with 370 patients worldwide (United States, China, South America, and Europe) in one of the largest trials ever conducted in lupus nephritis. Patients were randomized to 6 months of induction therapy with either IV cyclophosphamide or oral mycophenolate but could not cross over.
At 6 months, response rates were identical between the two groups, with response defined as a combination of specific improvement in proteinuria, serum creatinine, and hematuria (50%–55%). In terms of individual renal and nonrenal variables, both groups appeared identical.
However, the side effect profiles differed between the two groups. As expected for mycophenolate, diarrhea was the most common side effect (occurring in 28% vs 12% in the cyclophosphamide group). Nausea and vomiting were more common with cyclophosphamide (45% and 37% respectively vs 14% and 13% in the mycophenolate group). Cyclophosphamide also caused hair loss in 35%, vs 10% in the mycophenolate group.
There were 14 deaths overall, which is a very low number considering the patients’ severity of illness, and it indicates the better results now achieved with therapy. The mortality rate was higher in the mycophenolate group (5% vs 3%), but the difference was not statistically significant. Six of the nine deaths with mycophenolate were from the same center in China, and none were from Europe or the United States. In summary, the study did not show that mycophenolate was superior to IV cyclophosphamide for induction therapy, but that they were equivalent in efficacy with different side effect profiles.
Membranous nephropathy: Mycophenolate vs cyclophosphamide
Less evidence is available about treatment for membranous disease, which is characterized by heavy proteinuria and the nephrotic syndrome but usually does not progress to renal failure. Radhakrishnan et al11 combined data from the trial by Ginzler et al9 and the ALMS trial10 and found 84 patients with pure membranous lupus, who were equally divided between the treatment groups receiving IV cyclophosphamide and mycophenolate. Consistent with the larger group’s data, mycophenolate and cyclophosphamide performed similarly in terms of efficacy, but there was a slightly higher rate of side effects with cyclophosphamide.
Maintenance therapy: Mycophenolate superior to azathioprine
The ALMS Maintenance Trial12 evaluated maintenance therapy in the same worldwide population that was studied for induction therapy. Of the 370 patients involved in the induction phase that compared IV cyclophosphamide and oral mycophenolate, 227 responded sufficiently to be rerandomized in a controlled, double-blinded trial of 36 months of maintenance therapy with corticosteroids and either mycophenolate (1 g twice daily) or azathioprine (2 mg/kg per day).
In intention-to-treat analysis, the time to treatment failure (ie, doubling of the serum creatinine level, progressing to renal failure, or death) was significantly shorter in the azathioprine group (P = .003). Every individual end point—end-stage renal disease, renal flares, doubling of serum creatinine, rescue immunosuppression required—was in favor of mycophenolate maintenance. At 3 years, the completion rate was 63% with mycophenolate and 49% with azathioprine. Serious adverse events and withdrawals because of adverse events were more common in the azathioprine group.
In summary, mycophenolate was superior to azathioprine in maintaining renal response and in preventing relapse in patients with active lupus nephritis who responded to induction therapy with either mycophenolate or IV cyclophosphamide. Mycophenolate was found to be superior regardless of initial induction treatment, race, or region and was confirmed by all key secondary end points.
Only one of the 227 patients died during the 3 years—from an auto accident. Again, this indicates the dramatically improved survival today compared with a decade ago.
RITUXIMAB: PROMISING BUT UNPROVEN
Rituximab (Rituxan) was originally approved to treat tumors, then rheumatoid arthritis, and most recently vasculitis. Evidence thus far is mixed regarding its use as a treatment for lupus nephritis. Although randomized clinical trials have not found it to be superior to standard regimens, there are many signs that it may be effective.
Rituximab in uncontrolled studies
Terrier et al13 analyzed prospective data from 136 patients with systemic lupus erythematosus, most of whom had renal disease, from the French Autoimmunity and Rituximab registry. Response occurred in 71% of patients using rituximab, with no difference found between patients receiving rituximab monotherapy and those concomitantly receiving immunosuppressive agents.
Melander et al14 retrospectively studied 19 women and 1 man who had been treated with rituximab for severe lupus nephritis and followed for at least 1 year. Three patients had concurrent therapy with cyclophosphamide, and 10 patients continued rituximab as maintenance therapy; 12 patients had lupus nephritis that had been refractory to standard treatment, and 6 had relapsing disease.
At a median follow-up of 22 months, 12 patients (60%) had achieved complete or partial renal remission.
Condon et al15 treated 21 patients who had severe lupus nephritis with two doses of rituximab and IV methylprednisolone 2 weeks apart, then maintenance therapy with mycophenolate without any oral steroids. At a mean follow-up of 35 months ( ± 14 months), 16 (76%) were in complete remission, with a mean time to remission of 12 months. Two (9.5%) achieved partial remission. The rate of toxicity was low.
Thus, rituximab appears promising in uncontrolled studies.
Placebo-controlled trials fail to prove rituximab effective
LUNAR trial. On the other hand, the largest placebo-controlled trial to evaluate rituximab in patients with proliferative lupus nephritis, the Lupus Nephritis Assessment With Rituximab (LUNAR) trial16 found differences in favor of rituximab, but none reached statistical significance. The trial randomized 140 patients to receive either mycophenolate plus periodic rituximab infusions or mycophenolate plus placebo infusions for 1 year. All patients received the same dosage of glucocorticoids, which was tapered over the year.
At the end of 1 year, the groups were not statistically different in terms of complete renal response and partial renal response. Rituximab appeared less likely to produce no response, but the difference was not statistically significant.
African Americans appeared to have a higher response rate to rituximab (70% in the rituximab group achieved a response vs 45% in the control group), but again, the difference did not reach statistical significance, and the total study population of African Americans was only 40.
Rituximab did have a statistically significant positive effect on two serologic markers at 1 year: levels of anti-dsDNA fell faster and complement rose faster. In addition, rates of adverse and serious adverse events were similar between the two groups, with no new or unexpected “safety signals.”
This study can be interpreted in a number of ways. The number of patients may have been too small to show significance and the follow-up may have been too short. On the other hand, it may simply not be effective to add rituximab to a full dose of mycophenolate and steroids, an already good treatment.
EXPLORER trial. Similarly, for patients with lupus without nephritis, the Exploratory Phase II/III SLE Evaluation of Rituximab (EXPLORER) trial17 also tested rituximab against a background of an effective therapeutic regimen and found no additional benefit. This study had design problems similar to those of the LUNAR trial.
Rituximab as rescue therapy
The evidence so far indicates that rituximab may have a role as rescue therapy for refractory or relapsing disease. Rituximab must be used with other therapies, but maintenance corticosteroid therapy is not necessary. Its role as a first-line agent in induction therapy for lupus nephritis remains unclear, although it may have an important role for nonwhites. In general, it has been well tolerated. Until a large randomized trial indicates otherwise, it should not be used as a first-line therapy.
The US Food and Drug Administration (FDA) sent out a warning about the danger of progressive multifocal leukoencephalopathy as an adverse effect of rituximab and of mycophenolate, but this does not appear to be a major concern for most patients and is only likely to occur in those who have been over-immunosuppressed for many years.
MULTITARGET THERAPY
The concept of using multiple drugs simultaneously—such as mycophenolate, steroids, and rituximab—is increasingly being tried. Multi-target therapy appears to offer the advantages of combining different modes of action with better results, and it offers fewer side effects because dosages of each individual drug can be lower when combined with other immunosuppressives.
Bao et al18 in China randomly assigned 40 patients with diffuse proliferative and membranous nephritis to 6 to 9 months of induction treatment with either multitarget therapy (mycophenolate, tacrolimus [Prograf], and glucocorticoids) or IV cyclophosphamide. More complete remissions occurred in the multitarget therapy group, both at 6 months (50% vs 5%) and at 9 months (65% vs 15%). Most adverse events were less frequent in the multitarget therapy group, although three patients (15%) in the multitarget therapy group developed new-onset hypertension vs none in the cyclophosphamide group.
NEW MEDICATIONS
Entirely new classes of drugs are being developed with immunomodulatory effects, including tolerance molecules, cytokine blockers, inhibitors of human B lymphocyte stimulator, and costimulatory blockers.
Belimumab offers small improvement for lupus
Belimumab (Benlysta) is a human monoclonal antibody that inhibits the biologic activity of human B lymphocyte stimulator; it has recently been approved by the FDA for lupus nephritis. In a worldwide study,19 867 patients with systemic lupus erythematosus were randomized to receive either belimumab (1 mg/kg or 10 mg/kg) or placebo.
The primary end point was the reduction of disease activity by a scoring system (SELENA-SLEDAI) that incorporated multiple features of lupus, including arthritis, vasculitis, proteinuria, rash, and others. Patients in the belimumab group had better outcomes, but the results were not dramatic. Because the drug is so expensive (about $25,000 per year) and the improvement offered is only incremental, this drug will not likely change the treatment of lupus very much.
Moreover, patients with lupus nephritis were not included in the study, but a new study is being planned to do so. Improvement is harder to demonstrate in lupus nephritis than in rheumatoid arthritis and systemic lupus erythematosus: significant changes in creatinine levels and 24-hour urinary protein must be achieved, rather than more qualitative signs and symptoms of joint pain, rash, and feeling better. Although belimumab is still unproven for lupus nephritis, it might be worth trying for patients failing other therapy.
Laquinimod: A promising experimental drug
Laquinimod is an oral immunomodulatory drug with a number of effects, including down-regulating major histocompatability complex II, chemokines, and adhesion-related molecules related to inflammation. It has been studied in more than 2,500 patients with multiple sclerosis. Pilot studies are now being done for its use for lupus nephritis. If it shows promise, a large randomized, controlled trial will be conducted.
Abatacept is in clinical trials
Abatacept (Orencia), a costimulation blocker, is undergoing clinical trials in lupus nephritis. Results should be available shortly.
INDIVIDUALIZE THERAPY
This past decade has seen such an increase in options to treat lupus nephritis that therapy can now be individualized.
Choosing IV cyclophosphamide vs mycophenolate
As a result of recent trials, doctors in the United States are increasingly using mycophenolate as the first-line drug for lupus nephritis. In Europe, however, many are choosing the shorter regimen of IV cyclophosphamide because of the results of the Euro-Lupus study.
Nowadays, I tend to use IV cyclophosphamide as the first-line drug only for patients with severe crescenteric glomerulonephritis or a very high serum creatinine level. In such cases, there is more experience with cyclophosphamide, and such severe disease does not lend itself to the luxury of trying out different therapies sequentially. If such a severely ill patient insists that a future pregnancy is very important, an alternative therapy of mycophenolate plus rituximab should be considered. I prefer mycophenolate for induction and maintenance therapy in most patients.
Dosing and formulation considerations for mycophenolate
Large dosages of mycophenolate are much better tolerated when broken up throughout the day. A patient who cannot tolerate 1 g twice daily may be able to tolerate 500 mg four times a day. The formulation can also make a difference. Some patients tolerate sustained-release mycophenolate (Myfortic) better than CellCept, and vice versa.
For patients who cannot tolerate mycophenolate, azathioprine is an acceptable alternative. In addition, for a patient who is already doing well on azathioprine, there is no need to change to mycophenolate.
Long maintenance therapy now acceptable
The ALMS Maintenance Trial12 found 3 years of maintenance therapy to be safe and effective. Such a long maintenance period is increasingly viewed as important, especially for patients in their teens and 20s, as it allows them to live a normal life, ie, to finish their education, get married, and become settled socially. Whether 5 years of maintenance therapy or even 10 years is advisable is still unknown.
Treatment during pregnancy
Neither mycophenolate nor azathioprine is recommended during pregnancy, although their effects are unknown. Because we have much more renal transplant experience with azathioprine during pregnancy, I recommend either switching from mycophenolate to azathioprine or trying to stop medication altogether if the patient has been well controlled.
- Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006; 69:1846–1851.
- Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 2003; 18:2039–2046.
- Illei GG, Austin HA, Crane M, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med 2001; 135:248–257.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002; 46:2121–2131.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010; 69:61–64.
- Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong King-Guangzhou Nephrology Study Group. N Engl J Med 2000; 343:1156–1162.
- Chan TM, Tse KC, Tang CS, Mok MY, Li FK; Hong Kong Nephrology Study Group. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 2005; 16:1076–1084.
- Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med 2004; 350:971–980.
- Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 2005; 353:2219–2228.
- Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009; 20:1103–1112.
- Radhakrishnan J, Moutzouris DA, Ginzler EM, Solomons N, Siempos II, Appel GB. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int 2010; 77:152–160.
- Dooley MA, Jayne D, Ginzler EM, et al; for the ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 2011; 365:1886–1895.
- Terrier B, Amoura Z, Ravaud P, et al; Club Rhumatismes et Inflammation. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010; 62:2458–2466.
- Melander C, Sallée M, Troillet P, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol 2009; 4:579–587.
- Condon MB, Griffith M, Cook HT, Levy J, Lightstone L, Cairns T. Treatment of class IV lupus nephritis with rituximab & mycophenolate mofetil (MMF) with no oral steroids is effective and safe (abstract). J Am Soc Nephrol 2010; 21(suppl):625A–626A.
- Furie RA, Looney RJ, Rovin E, et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study (abstract). Arthritis Rheum 2009; 60(suppl 1):S429.
- Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62:222–233.
- Bao H, Liu ZH, Zie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol 2008; 19:2001–2010.
- Navarra SV, Guzmán RM, Gallacher AE, et al; BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:721–731.
- Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006; 69:1846–1851.
- Barr RG, Seliger S, Appel GB, et al. Prognosis in proliferative lupus nephritis: the role of socio-economic status and race/ethnicity. Nephrol Dial Transplant 2003; 18:2039–2046.
- Illei GG, Austin HA, Crane M, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med 2001; 135:248–257.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002; 46:2121–2131.
- Houssiau FA, Vasconcelos C, D’Cruz D, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis 2010; 69:61–64.
- Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong King-Guangzhou Nephrology Study Group. N Engl J Med 2000; 343:1156–1162.
- Chan TM, Tse KC, Tang CS, Mok MY, Li FK; Hong Kong Nephrology Study Group. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 2005; 16:1076–1084.
- Contreras G, Pardo V, Leclercq B, et al. Sequential therapies for proliferative lupus nephritis. N Engl J Med 2004; 350:971–980.
- Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med 2005; 353:2219–2228.
- Appel GB, Contreras G, Dooley MA, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol 2009; 20:1103–1112.
- Radhakrishnan J, Moutzouris DA, Ginzler EM, Solomons N, Siempos II, Appel GB. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int 2010; 77:152–160.
- Dooley MA, Jayne D, Ginzler EM, et al; for the ALMS Group. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N Engl J Med 2011; 365:1886–1895.
- Terrier B, Amoura Z, Ravaud P, et al; Club Rhumatismes et Inflammation. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum 2010; 62:2458–2466.
- Melander C, Sallée M, Troillet P, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol 2009; 4:579–587.
- Condon MB, Griffith M, Cook HT, Levy J, Lightstone L, Cairns T. Treatment of class IV lupus nephritis with rituximab & mycophenolate mofetil (MMF) with no oral steroids is effective and safe (abstract). J Am Soc Nephrol 2010; 21(suppl):625A–626A.
- Furie RA, Looney RJ, Rovin E, et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study (abstract). Arthritis Rheum 2009; 60(suppl 1):S429.
- Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62:222–233.
- Bao H, Liu ZH, Zie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol 2008; 19:2001–2010.
- Navarra SV, Guzmán RM, Gallacher AE, et al; BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:721–731.
KEY POINTS
- Mycophenolate is at least equivalent to intravenous cyclophosphamide for induction and maintenance treatment of severe lupus nephritis.
- The role of rituximab is unclear, and for now it should only be used in relapsing patients or patients whose disease is resistant to standard therapy.
- Using combination therapies for induction treatment and maintenance is becoming increasingly common.
- Three-year maintenance therapy is now considered advisable in most patients.
- Entirely new drugs under study include costimulatory blockers, inhibitors of human B lymphocyte stimulator, tolerance molecules, and cytokine blockers.
Managing community-acquired pneumonia during flu season
General internists need to be able to recognize community-acquired pneumonia (CAP) so that diagnostic and therapeutic interventions can be initiated promptly. It is also important to understand the most likely and possible causes of CAP so that appropriate initial antimicrobial therapy can be chosen. Especially during flu season, influenza can present as CAP and should be included in the differential diagnosis.
When managing a patient with CAP, the internist must decide which level of care, diagnostic tests, antimicrobial agents, and follow-up plans are needed. These topics will be reviewed in this article.
TWO TERMS TO REMEMBER
- CAP refers to pneumonia acquired outside a health care facility. It can be either bacterial or viral.
- CABP (community-acquired bacterial pneumonia) refers only to those cases caused by bacterial pathogens.
NUMBERS AND TRENDS
In the United States, CAP is the number-one cause of death from infection and the sixth leading cause of death overall.1 Each year, it is responsible for about 4.2 million outpatient visits, more than 60,000 deaths, and more than $17 billion in health care expenses.2
Community-acquired bacterial pneumonia: Common, serious
In a population-based US study in 1991, the incidence of CABP requiring hospitalization was 266.8 per 100,000 people.3
Estimates of overall mortality in CABP vary depending on the severity of illness and comorbid conditions. A meta-analysis published in 1996 found the overall mortality rate to be 13.7%, with a range of 5.1% to 36.5% depending on severity.4
In hospitalized patients, mortality rates and length of hospital stay appear to be declining over time. Between 1993 and 2005, the age-adjusted mortality rate decreased from 8.9% to 4.1%, and the average length of stay decreased from 7.5 to 5.7 days, with an overall reduction in hospital cost.5
CABP is more prevalent in older people than in the general population, and it increases with age from 18.2 cases per 1,000 patient-years in patients 60 to 69 years to 52.3 cases per 1,000 patient-years in those older than 85 years.6 Risk factors for pneumonia in the elderly include heart disease, chronic lung disease, immunosuppressive drugs, alcoholism, and increasing age.7 Similar to the trend in the general population, the mortality rate in elderly CABP patients appears to be decreasing over time, possibly thanks to rising rates of pneumococcal and influenza vaccination.8
Among the general population, risk factors for developing CABP also include smoking, occupational dust exposure, history of childhood pneumonia, unemployment, and single marital status.9 The incidence of CABP does not appear to be higher among pregnant women, although it is the most frequent cause of nonobstetric death in this population.10
The use of proton pump inhibitors may be an emerging risk factor for CABP.11 Also, use of nonsteroidal anti-inflammatory drugs among patients with CABP is associated with a blunted inflammatory response as well as a higher risk of pleuropulmonary complications and a delay in presentation.12
Influenza is also common, potentially severe
Influenza is also very common and potentially severe. It can cause a spectrum of disease, from mild upper respiratory tract symptoms to severe viral pneumonia that can be life-threatening and complicated by respiratory failure and the acute respiratory distress syndrome (ARDS).
Influenza infection can also be complicated by subsequent bacterial pneumonia. However, the epidemiology of influenza infection differs from that of CABP in that influenza occurs seasonally.
In the United States, seasonal influenza causes 36,000 deaths and 200,000 hospitalizations annually.13,14 As with CABP, the risk of death from influenza increases with age: it is 16 times greater in people age 85 and older than in those ages 65 to 69.13
During yearly seasonal epidemics, those at the highest risk of hospitalization and death are at the extremes of age. Risk factors for complicated influenza include heart disease, lung disease, diabetes, renal failure, rheumatologic conditions, dementia, and neurologic disease.15,16 During the 2009 H1N1 influenza pandemic, unexpected severity was seen in previously healthy young adults as well as those with obesity, neurodegenerative disease, pregnancy, and asthma.17
PATHOGENS: TYPICAL, ATYPICAL, VIRAL
Identifying the etiologic organism in CAP is confounded by limitations in the available diagnostic tests and also by poor-quality specimens that often are contaminated with bacteria that colonize the upper airways. Given these caveats, the primary pathogens responsible for CAP broadly include typical bacterial pathogens, atypical bacterial pathogens, and viruses.
Typical bacterial pathogens include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis, and, less commonly, a variety of aerobic and anaerobic gram-negative rods including Pseudomonas aeruginosa, Acinetobacter species, and Klebsiella pneumoniae.
Atypical bacterial pathogens include Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella species.18
Viruses implicated in adult CAP include influenza A and B, parainfluenza viruses, respiratory syncytial virus, and adenovirus.19 More recently, human metapneumovirus has been described as a cause of adult CAP.20
Clues to uncommon microbes
Specific historic features or coexisting conditions that may suggest an uncommon microbiologic diagnosis include21:
- Recent travel to the southwestern United States or Southeast Asia
- Ill contacts
- Exposure to birds, bats, rabbits, or farm animals
- Alcoholism
- Chronic obstructive pulmonary disease
- Human immunodeficiency virus infection
- Structural lung disease
- Prolonged cough with whoop or posttussive vomiting
- Aspiration
- Bioterrorism.
In cases in which one or more of these conditions exist, CAP may also be caused by other agents not listed above, including Mycobacterium tuberculosis, oral anaerobes, atypical mycobacteria, Histoplasma capsulatum, Chlamydophila psittaci, Francisella tularensis, Coxiella burnettii, Pneumocystis jiroveci, Cryptococcus, Aspergillus, Coccidioides, Hantavirus, avian influenza, Burkholderia pseudomallei, severe acute respiratory syndrome virus, Bordetella pertussis, Bacillus anthracis, and Yersinia pestis.
HOW BACTERIA INVADE THE LUNGS
The pathophysiology of CABP involves both host defense and microbial virulence factors.
The airways are most commonly exposed to microbes by microaspiration of upper airway flora, although hematogenous seeding of the lungs in a bacteremic patient or contiguous spread of infection from an adjacent site can also occur.
Mucociliary clearance and the cough reflex are important initial defenses against infection and can be inhibited by neurologic diseases and conditions that impair the mucociliary mechanism. Mucosal immune cells, including macrophages and neutrophils, recognize invading pathogens and generate an antibody response.
Regulation of the host inflammatory response to infection depends on a complex interaction between immune cells, inflammatory cytokines (eg, tumor necrosis factor alpha, interleukin 1-beta, and interleukin 6), and anti-inflammatory cytokines such as interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor type I.22
The interaction and timing of the inflammatory and anti-inflammatory response are essential in manifesting an appropriate host response to infection. An inadequate inflammatory response can lead to sepsis and death, but an excessive, late anti-inflammatory response can lead to a systemic inflammatory response such as ARDS. Polymorphisms within the genes coding for these factors may explain the variation in severity of illness among patients with CABP.23
HOW INFLUENZA DOES ITS DAMAGE
There are three types of influenza virus: A, B, and C. Type A causes most human infections. The influenza A virus envelope comprises a lipid bilayer that contains the projecting glycoproteins hemagglutinin and neuraminidase. Influenza viruses are named on the basis of these proteins and are designated with an H and an N, respectively, each followed by a number referring to the subtype.
Influenza infection begins when the virus makes contact with the epithelium. Hemagglutinin binds to the host cell and allows viral entry, where it begins replication. Neuraminidase prevents viral aggregation and facilitates the release of virus from infected cells.24
Mature virions are released and spread to neighboring host cells; this process is associated with desquamation and inflammation of the airways, causing cough, rhinorrhea, and sore throat. Systemic symptoms are associated with the induction of interferon, which causes fever and myalgia.25
Recovery and immunity to influenza infection occurs through both humoral and cell-mediated immunity, with antibodies directed against the specific hemagglutinin and neuraminidase antigens of the infecting virus. Immunity wanes over time and with antigenic drift of circulating viruses, making the host susceptible to recurrent influenza infection.24
Influenza is often complicated by bacterial superinfection
The influenza virus acts synergistically with certain bacteria to increase infectivity, and this may explain why influenza is often complicated by bacterial superinfection.
Mechanisms leading to bacterial superinfection include increased binding and invasion of bacteria, increased viral replication, and modification of the host inflammatory response. Some S aureus strains produce a protease that directly activates influenza virus hemagglutinin; other bacteria can activate plasminogen to promote influenza replication. The resulting increase in proteases in host tissues promotes activation of influenza through cleavage of hemagglutinin.26
The influenza virus also causes damage to the airway epithelial layer, leading to increased exposure of the binding sites necessary for adherence of S pneumoniae.27
CLINICAL PRESENTATION OF COMMUNITY-ACQUIRED PNEUMONIA
Although CAP is common, agreement on its essential clinical signs and symptoms is surprisingly limited, due in part to heterogeneous patient presentations and in part to interobserver variability. The reader is referred to two excellent reviews on this topic.28,29
The diagnosis of CAP is made on clinical grounds, based on a combination of signs and symptoms. Symptoms of pneumonia can include cough, fever, chills, sputum production, dyspnea, and pleuritic pain. Physical findings can include tachypnea, tachycardia, hypoxemia, and consolidation or rales on auscultation. Laboratory data may show leukocytosis or elevated C-reactive protein, and radiographic studies may show evidence of a new infiltrate.21,30,31
Clinical presentation of influenza
Seasonal influenza as a cause of CAP is difficult to distinguish from bacterial causes. The clinical presentation of seasonal influenza most commonly includes fever or subjective feverishness, cough, myalgia, and weakness.32 In a recent multivariate analysis, five clinical features were shown to be predictive of pandemic H1N1 influenza pneumonia rather than CABP: age younger than 65 years, absence of confusion, white blood cell count less than 12 × 109/L, temperature higher than 38°C (100.4°F), and bilateral opacities on radiography.32,33
Complicated influenza infection can be either primary viral pneumonia or bacterial superinfection.
During the 1918 influenza pandemic, which predated the ability to isolate viruses, two clinical syndromes emerged: an ARDS associated with the rapid onset of cyanosis, delirium, and frothy blood-tinged sputum; and an acute bronchopneumonia characterized by necrosis, hemorrhage, edema, and vasculitis.34,35 The first syndrome has subsequently been shown to be associated with primary viral pneumonia, while the second is caused by bacterial superinfection. Modern reexamination of 1918 data has shown that bacterial superinfection was likely the reason for the distinctly fulminant presentation of that pandemic.36,37
The 2009 H1N1 influenza pandemic caused relatively mild disease in most patients. However, those with severe pneumonia more commonly developed ARDS from primary influenza pneumonia than from bacterial superinfection.17
A third influenza-associated infection is secondary bacterial pneumonia, which follows influenza infection and mimics the presentation of CABP. A typical patient presents with a recent history of influenza-like illness, followed 4 to 14 days later by a recurrence of fever, dyspnea, productive cough, and consolidation on chest radiographs.38 Leukocytosis with an increased number of immature neutrophil forms, prolonged duration of fever, and elevated erythrocyte sedimentation rate are more likely in patients with secondary bacterial pneumonia.39 Isolates from sputum samples commonly include S pneumoniae, S aureus, H influenzae, and other gram-negative rods.40
In recent flu seasons, methicillin-resistant S aureus (MRSA) has emerged as a cause of severe secondary pneumonia. Most of these isolates carry genes for the toxin Panton-Valentine leukocidin; the associated mortality rate is as high as 33%.41,42 Although community-acquired MRSA pneumonia has only been reported in case series, distinct clinical features that have been described include severe pneumonia with high fever, hypotension, shock, respiratory failure, leukopenia, and multilobar and cavitary infiltrates.43
WHEN TO SUSPECT INFLUENZA
The triad of fever, cough, and abrupt onset are the best predictors of influenza, but no single combination of signs and symptoms predict influenza infection with 100% certainty. Therefore, an understanding of local epidemiologic data regarding circulating influenza is essential to maintain a high index of suspicion.44
It is appropriate to suspect influenza in:
- Anyone who is epidemiologically linked to a known outbreak of influenza
- Children, adults, and health care workers who have fever and abrupt onset of respiratory symptoms
- Patients with fever plus exacerbation of underlying pulmonary disease
- Severely ill patients with fever or hypothermia, especially during influenza season.45
DIAGNOSTIC TESTING
Once the diagnosis of pulmonary infection is suggested by clinical features, the initial evaluation should include measurement of vital signs, physical examination, and radiographic imaging of the chest. Additional diagnostic measures to consider include viral testing, blood culture, sputum culture, urinary antigen testing for Legionella and for S pneumoniae, fungal culture, and mycobacterial smear and culture.
Chest radiography (with posterior-anterior and lateral films) is the study that usually demonstrates the presence of a pulmonary infiltrate. If initial chest radiographs do not show an infiltrate, imaging can be repeated after treatment is started if the patient’s clinical presentation still suggests pneumonia. Chest radiographs are of limited value in predicting the pathogen, but they help to determine the extent of pneumonia and to detect parapneumonic effusion.46
A caveat: anterior-posterior, posterior-anterior, and lateral views can miss more than 10% of effusions large enough to warrant thoracentesis, especially when there is lower-lobe consolidation.47
Blood cultures are recommended for patients admitted to the intensive care unit and for those with cavitary infiltrates, leukopenia, alcohol abuse, severe liver disease, asplenia, positive pneumococcal urinary antigen testing, or a pleural effusion.21 However, blood cultures are positive in only 3% to 14% of hospitalized patients with CABP, and the impact of a positive blood culture on management decisions in CABP appears to be quite small.48–50
For the highest yield, blood culture results should be obtained before antibiotics are given. Not only is this good practice, but obtaining blood culture results before starting antibiotics is one of the quality measures evaluated by the Center for Medicare and Medicaid Services.51
Sputum culture is considered optional for outpatients and patients with less-severe pneumonia.21 While it can provide a rapid diagnosis in certain cases, a good-quality sputum sample is obtained in only 39% to 54% of patients with CABP, yields a predominant morphotype in only 45% of cases, and provides a useful microbiologic diagnosis in only 14.4%.52,53 Fungal and mycobacterial cultures are only indicated in certain situations such as cavitary infiltrates or immunosuppression.
Urinary antigen testing for Legionella and S pneumoniae should be done in patients with more severe illness and in those for whom outpatient therapy has failed.21S pneumoniae testing has been shown to allow early diagnosis of pneumococcal pneumonia in 26% more patients than with Gram staining, but it fails to identify 22% of the rapid diagnoses initially identified by Gram staining.54 Thus, a sequential approach is reasonable, with urinary antigen testing for patients at high risk without useful results from sputum Gram staining. Also, recent data suggest that the pneumococcal urinary antigen test may allow optimization of antimicrobial therapy with good clinical outcomes.55
Endotracheal tests. If the patient is intubated, collection of endotracheal aspirates, bronchoscopy, or nonbronchoscopic bronchial lavage (sometimes called “mini-BAL”) should be performed.
Thoracentesis and pleural fluid cultures should be done if a pleural effusion is found. Empyema, large or loculated effusions, and parapneumonic effusions with a pH lower than 7.20, glucose levels less than 3.4 mmol/L (60 mg/dL), or positive results on microbial staining or culture should be drained by chest tube or surgically.56
Testing for influenza should be done if it will change the clinical management, such as the choice of antibiotic or infection control practices. Specimens should be obtained with either a nasopharyngeal swab or aspirate and tested with reverse transcriptase polymerase chain reaction, immunofluorescent staining, or rapid antigen detection, depending on local availability.45
Inflammatory biomarkers such as C-reactive protein and procalcitonin have been receiving interest as ways to predict the etiology and prognosis of CAP and to guide therapy. Several studies have shown that C-reactive protein can help distinguish between CAP and bronchitis, with higher values suggesting more severe pneumonia and pneumonia caused by S pneumoniae or L pneumophila.57 Procalcitonin may help discriminate between severe lower respiratory tract infections of bacterial and 2009 H1N1 origin, although less effectively than C-reactive protein. Low procalcitonin values, particularly when combined with low C-reactive protein levels, suggest that bacterial infection is unlikely.58
RISK STRATIFICATION AND SITE-OF-CARE DECISIONS
Following a presumptive diagnosis of CAP, it is important to decide not only what treatment the patient will receive but whether he or she should be hospitalized. If the patient is to be admitted to the hospital, the clinician must also decide if his or her condition warrants intensive care.
Severity-of-illness scores
A recent meta-analysis compared the performance characteristics of the PSI and CURB-65 scores for predicting mortality in CAP and found no significant differences in overall test performance.61
Another meta-analysis found that the PSI was more sensitive than the CURB-65 and had a low false-negative rate, and so was better at showing which patients do not need to be hospitalized. Conversely, the CURB-65 was more specific and had a higher positive predictive value, and thus was more likely to correctly classify high-risk patients.62
Other scoring systems that aid in deciding about hospital admission and level of care include the CRB-6563 (which can be used instead of the CURB-65 if laboratory values are not available), SMART-COP,64 and SCAP.,65
Guidelines on when to admit to the intensive care unit
Guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) also provide guidance on when intensive care admission is advised,21 and their criteria were recently validated.66 The guidelines advocate direct admission to the intensive care unit for patients requiring vasopressors or mechanical ventilation, and intensive care unit or high-level monitoring for patients with three of the following criteria for severe CAP21:
- Respiratory rate ≥ 30
- Pao2/Fio2 ratio ≤ 250
- Multilobar infiltrates
- Confusion or disorientation
- Uremia (blood urea nitrogen ≥ 20 mg/dL)
- Leukopenia (white blood cell count < 4.0 × 109/L)
- Thrombocytopenia (platelet count < 100 × 109/L)
- Hypothermia (core temperature < 36.0°C [96.8°F])
- Hypotension requiring aggressive fluid resuscitation.
None of these scoring systems or criteria is meant to replace clinical judgment. A recent study has suggested that an oxygen saturation of less than 92% is an appropriate threshold for hospital admission, in view of higher rates of adverse events in outpatients with saturations below this value.67
TREATMENT
Multiple studies have shown that treatment of CAP in accordance with guidelines has led to improved clinical outcomes.21,68–70
How fast must antibiotics be started?
Based on studies that showed a lower mortality rate when antibiotics were started sooner, Medicare and Medicaid adopted a quality measure calling for starting antibiotics within 4 hours in patients being admitted to the hospital.50,71 However, several subsequent studies showed that the diagnosis of pneumonia is often incorrect and that rapid administration of antibiotics could lead to misdiagnosis, overuse of antibiotics, and a higher risk of Clostridium difficile infection.72,73
The current IDSA/ATS guidelines21 recommend that the first antibiotic dose be given while the patient is still in the emergency department, but do not give a specific time within which it should be given. Medicare and Medicaid later updated their quality measure to antibiotic administration within 6 hours.
Which antibiotics should be used?
The selection of antimicrobial agent depends upon the patient’s severity of illness and comorbid conditions.
Although most studies of combination antibiotic therapy have been retrospective and observational, they suggest that a macrolide (ie, one of the “mycins”) added to a beta-lactam antibiotic is beneficial, possibly by covering atypical organisms or via anti-inflammatory action.74–76 The choice of one antibiotic over another appears to be less important, and a recent Cochrane review concluded that there was no significant difference in efficacy among five antibiotic pairs studied.77
Empiric outpatient treatment of a previously healthy patient with CAP and no risk factors for drug-resistant S pneumoniae should include either a macrolide (azithromycin [Zithromax], clarithromycin [Biaxin], or erythromycin) or doxycycline. If the patient has a chronic comorbid condition such as heart, lung, liver, or renal disease, diabetes mellitus, alcoholism, malignancy, asplenia, or immunosuppression or has received antimicrobials within the preceding 3 months, then treatment should include either a respiratory fluoroquinolone (moxifloxacin [Avelox] or levofloxacin [Levaquin]) or a beta-lactam plus a macrolide.21
Overall, published data suggest that the survival rate is about the same with fluoroquinolone monotherapy as with beta-lactam plus macrolide combination therapy, and better than with beta-lactam monotherapy.78
Selection of antibiotics for inpatient treatment of CAP is influenced by severity of illness. Inpatients who do not require intensive care should be treated with either a respiratory fluoroquinolone or combination therapy with a beta-lactam (cefotaxime [Claforan], ceftriaxone [Rocephin], ampicillin, or ertapenem [Invanz]) plus a macrolide or doxycycline.21,76,79
If a specific microbiologic diagnosis is made, then treatment can be narrowed. However in certain cases, such as invasive pneumococcal infection, combination therapy may still be superior.80,81 For patients who need intensive care, treatment should always include a beta-lactam plus either azithromycin or a respiratory fluoroquinolone.21 In certain situations, additional antibiotics may be added as well, such as agents to treat Pseudomonas, community-acquired MRSA, or both.
Switching to oral therapy; short-course therapy
In the interest of avoiding unnecessary antibiotics, numerous studies have addressed the issue of an “early switch” to oral antibiotics and “short-course” therapy for CAP. In general, once clinically stable, patients with CAP, including bacteremic S pneumoniae pneumonia, can be safely switched to oral antibiotics.82
The issue of short-course therapy is more complicated, and the appropriate length of therapy for CAP is not well established. However, 5 days of levofloxacin 750 mg was shown to be as successful as 7 to 10 days of levofloxacin 500 mg.83 In another study, in patients who improved after 3 days of intravenous therapy for CAP, there was no difference in clinical outcome between those who were changed to oral therapy for 5 more days and those who received an oral placebo.84
Most patients who achieve clinical stability in the first week do not need prolonged antibiotic therapy. However, certain conditions, such as S aureus bacteremic pneumonia, complicated pneumonia, and pneumonia due to unusual organisms, may require prolonged treatment.
Other therapies
Additional therapies studied in patients with pneumonia include early mobilization, adjunctive corticosteroids, and statin drugs.
Early mobilization was shown in one study to decrease hospital length of stay without increasing adverse effects.85
Corticosteroids are not supported as a standard of care for patients with severe CAP according to current available studies.86,87 Furthermore, a randomized, controlled trial showed that prednisolone daily for a week did not improve outcomes in hospitalized patients with CAP, and it was associated with increased late failure.88
Statin trials under way. Several observational studies have suggested that statins might be beneficial in managing sepsis through their effects on endothelial cell function, antioxidant effects, anti-inflammatory effects, and immunomodulatory effects.89 However, a recent large prospective multicenter cohort study of hospitalized patients with CAP did not find evidence of a protective effect of statins on clinically meaningful outcomes in CAP or significant differences in circulating biomarkers.90 Several randomized trials of statin therapy in patients with both ventilator-associated pneumonia and CAP are under way.
INFLUENZA TREATMENT: MOST EFFECTIVE WITHIN 48 HOURS
Treatment with antiviral drugs is most effective if started within 48 hours after symptom onset, although some patients with confirmed influenza who are either not improving or who are critically ill may still benefit from treatment started later.
Treatment should be considered in patients with laboratory-confirmed or suspected influenza who are at risk of developing complicated influenza and in otherwise healthy patients who wish to reduce the duration of illness or who have close contact with patients who are at high risk of complications.
Antiviral medications are oseltamivir (Tamiflu), zanamivir (Relenza), and the adamantines amantadine (Symmetrel) and rimantadine (Flumadine).
Due to evolving viral resistance patterns, the choice of antiviral drug depends on the strain. Seasonal H1N1 is best treated with zanamivir or an adamantine, while pandemic 2009 H1N1 and H3N2 are best treated with zanamivir or oseltamivir. When strain typing is not available, empiric therapy should be with either zanamivir monotherapy or a combination of oseltamivir plus rimantadine. Influenza B viruses are resistant to adamantines and should be treated only with either zanamivir or oseltamivir.45
FOLLOW-UP AND PREVENTION
Patients with CAP can generally be expected to improve within 3 to 7 days.91 However, it may be several weeks before they return to baseline.92
Follow-up plans may be guided by the time to clinical stability. For patients who do not achieve clinical stability until more than 72 hours after admission, more aggressive follow-up on discharge is indicated, since they are more likely to experience early readmission and death.93
Pneumococcal vaccination. Because S pneumoniae remains the most common cause of CAP, efforts should be made to vaccinate patients appropriately. The Advisory Committee on Immunization Practices (ACIP) and the US Centers for Disease Control and Prevention recommend that the pneumococcal polysaccharide vaccine (Pneumovax 23; PPSV23) be given to those over age 65. Those who were vaccinated before age 65 should receive another dose at age 65 or later if at least 5 years have passed since their previous dose. Those who receive it at or after age 65 should receive only a single dose. A second dose is recommended 5 years after the first dose for people age 19 to 64 years with functional or anatomic asplenia and for those who are immunocompromised.
Influenza vaccination for all. Of note, the ACIP updated its guidelines on influenza vaccination beginning with the 2010–2011 influenza season. It no longer advocates a risk-stratified approach. Instead, it recommends universal influenza vaccination for everybody more than 6 months old.94
Smoking cessation should be addressed. Smoking cessation is a Medicare and Medicaid quality measure and should be encouraged after an episode of CAP because quitting smoking reduces the risk of pneumococcal disease by approximately 14% each year thereafter.95
- Mortensen EM, Kapoor WN, Chang CC, Fine MJ. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis 2003; 37:1617–1624.
- File TM, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010; 122:130–141.
- Marston BJ, Plouffe JF, File TM, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med 1997; 157:1709–1718.
- Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. JAMA 1996; 275:134–141.
- Ruhnke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Trends in mortality and medical spending in patients hospitalized for community-acquired pneumonia: 1993–2005. Med Care 2010; 48:1111–1116.
- Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis 2004; 39:1642–1650.
- Koivula I, Sten M, Mäkelä PH. Risk factors for pneumonia in the elderly. Am J Med 1994; 96:313–320.
- Ruhnke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Marked reduction in 30-day mortality among elderly patients with community-acquired pneumonia. Am J Med 2011; 124:171–178.
- Farr BM, Bartlett CL, Wadsworth J, Miller DL. Risk factors for community-acquired pneumonia diagnosed upon hospital admission. British Thoracic Society Pneumonia Study Group. Respir Med 2000; 94:954–963.
- Graves CR. Pneumonia in pregnancy. Clin Obstet Gynecol 2010; 53:329–336.
- Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ 2011; 183:310–319.
- Voiriot G, Dury S, Parrot A, Mayaud C, Fartoukh M. Nonsteroidal antiinflammatory drugs may affect the presentation and course of community-acquired pneumonia. Chest 2011; 139:387–394.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Glezen WP, Decker M, Perrotta DM. Survey of underlying conditions of persons hospitalized with acute respiratory disease during influenza epidemics in Houston, 1978–1981. Am Rev Respir Dis 1987; 136:550–555.
- Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232–239.
- Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 2010; 38(suppl 4):e91–e97.
- Apisarnthanarak A, Mundy LM. Etiology of community-acquired pneumonia. Clin Chest Med 2005; 26:47–55.
- de Roux A, Marcos MA, Garcia E, et al. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest 2004; 125:1343–1351.
- Hamelin ME, Côté S, Laforge J, et al. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis 2005; 41:498–502.
- Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–S72.
- Kolling UK, Hansen F, Braun J, Rink L, Katus HA, Dalhoff K. Leucocyte response and anti-inflammatory cytokines in community acquired pneumonia. Thorax 2001; 56:121–125.
- Wunderink RG, Waterer GW. Community-acquired pneumonia: pathophysiology and host factors with focus on possible new approaches to management of lower respiratory tract infections. Infect Dis Clin North Am 2004; 18:743–759.
- Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine 2002; 20:3068–3087.
- Bender BS, Small PA. Influenza: pathogenesis and host defense. Semin Respir Infect 1992; 7:38–45.
- Scheiblauer H, Reinacher M, Tashiro M, Rott R. Interactions between bacteria and influenza A virus in the development of influenza pneumonia. J Infect Dis 1992; 166:783–791.
- McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19:571–582.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Benbassat J, Baumal R. Narrative review: should teaching of the respiratory physical examination be restricted only to signs with proven reliability and validity? J Gen Intern Med 2010; 25:865–872.
- Kolsuz M, Erginel S, Alatas O, et al. Acute phase reactants and cytokine levels in unilateral community-acquired pneumonia. Respiration 2003; 70:615–622.
- Alves DW, Kennedy MT. Community-acquired pneumonia in casualty: etiology, clinical features, diagnosis, and management (or a look at the “new” in pneumonia since 2002). Curr Opin Pulm Med 2004; 10:166–170.
- Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000; 160:3243–3247.
- Bewick T, Myles P, Greenwood S, et al; Influenza Clinical Information Network. Clinical and laboratory features distinguishing pandemic H1N1 influenza-related pneumonia from interpandemic community-acquired pneumonia in adults. Thorax 2011; 66:247–252.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis 2007; 195:1018–1028.
- Starr I. Influenza in 1918: recollections of the epidemic in Philadelphia. 1976. Ann Intern Med 2006; 145:138–140.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg Infect Dis 2008; 14:1193–1199.
- Treanor J. Influenza virus. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, PA: Elsevier/Churchill Livingstone; 2005:2060–2085.
- Jarstrand C, Tunevall G. The influence of bacterial superinfection on the clinical course of influenza. Studies from the influenza epidemics in Stockholm during the winters 1969–70 and 1971–72. Scand J Infect Dis 1975; 7:243–247.
- Schwarzmann SW, Adler JL, Sullivan RJ, Marine WM. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968–1969. Arch Intern Med 1971; 127:1037–1041.
- Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis 2006; 12:894–899.
- Centers for Disease Control and Prevention (CDC). Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006–January 2007. MMWR Morb Mortal Wkly Rep 2007; 56:325–329.
- Hidron AI, Low CE, Honig EG, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis 2009; 9:384–392.
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005; 293:987–997.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Boersma WG, Daniels JM, Löwenberg A, Boeve WJ, van de Jagt EJ. Reliability of radiographic findings and the relation to etiologic agents in community-acquired pneumonia. Respir Med 2006; 100:926–932.
- Brixey AG, Luo Y, Skouras V, Awdankiewicz A, Light RW. The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology 2011; 16:1000–1004.
- Campbell SG, Marrie TJ, Anstey R, Dickinson G, Ackroyd-Stolarz S. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest 2003; 123:1142–1150.
- Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med 2001; 95:78–82.
- Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med 2004; 164:637–644.
- Information & Quality Healthcare. http://www.IQH.org/attachments/219_CoreMHelpBookletpg4_11_3.pdf. Accessed November 14, 2011.
- Rosón B, Carratalà J, Verdaguer R, Dorca J, Manresa F, Gudiol F. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis 2000; 31:869–874.
- García-Vázquez E, Marcos MA, Mensa J, et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med 2004; 164:1807–1811.
- Rosón B, Fernández-Sabé N, Carratalà J, et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis 2004; 38:222–226.
- Sordé R, Falcó V, Lowak M, et al. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Intern Med 2011; 171:166–172.
- Koegelenberg CFN, Diacon AH, Bolliger CT. Parapneumonic pleural effusion and empyema. Respiration 2008; 75:241–250.
- Almirall J, Bolíbar I, Toran P, et al; Community-Acquired Pneumonia Maresme Study Group. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest 2004; 125:1335–1342.
- Ingram PR, Inglis T, Moxon D, Speers D. Procalcitonin and C-reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med 2010; 36:528–532.
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–250.
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–382.
- Chalmers JD, Singanayagam A, Akram AR, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 2010; 65:878–883.
- Loke YK, Kwok CS, Niruban A, Myint PK. Value of severity scales in predicting mortality from community-acquired pneumonia: systematic review and meta-analysis. Thorax 2010; 65:884–890.
- Capelastegui A, España PP, Quintana JM, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J 2006; 27:151–157.
- Charles PG, Wolfe R, Whitby M, et al; Australian Community-Acquired Pneumonia Study Collaboration. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008; 47:375–384.
- España PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med 2006; 174:1249–1256.
- Chalmers JD, Taylor JK, Mandal P, et al. Validation of the Infectious Diseases Society of America/American Thoracic Society minor criteria for intensive care unit admission in community-acquired pneumonia patients without major criteria or contraindications to intensive care unit care. Clin Infect Dis 2011; 53:503–511.
- Majumdar SR, Eurich DT, Gamble JM, Senthilselvan A, Marrie TJ. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: a population-based cohort study. Clin Infect Dis 2011; 52:325–331.
- Nathwani D, Rubinstein E, Barlow G, Davey P. Do guidelines for community-acquired pneumonia improve the cost-effectiveness of hospital care? Clin Infect Dis 2001; 32:728–741.
- Dean NC, Silver MP, Bateman KA, James B, Hadlock CJ, Hale D. Decreased mortality after implementation of a treatment guideline for community-acquired pneumonia. Am J Med 2001; 110:451–457.
- Capelastegui A, España PP, Quintana JM, et al. Improvement of process-of-care and outcomes after implementing a guideline for the management of community-acquired pneumonia: a controlled before-and-after design study. Clin Infect Dis 2004; 39:955–963.
- Silber SH, Garrett C, Singh R, et al. Early administration of antibiotics does not shorten time to clinical stability in patients with moderate-to-severe community-acquired pneumonia. Chest 2003; 124:1798–1804.
- Welker JA, Huston M, McCue JD. Antibiotic timing and errors in diagnosing pneumonia. Arch Intern Med 2008; 168:351–356.
- Polgreen PM, Chen YY, Cavanaugh JE, et al. An outbreak of severe Clostridium difficile-associated disease possibly related to inappropriate antimicrobial therapy for community-acquired pneumonia. Infect Control Hosp Epidemiol 2007; 28:212–214.
- Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med 2001; 161:1837–1842.
- Lodise TP, Kwa A, Cosler L, Gupta R, Smith RP. Comparison of beta-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother 2007; 51:3977–3982.
- Waterer GW, Rello J, Wunderink RG. Management of community-acquired pneumonia in adults. Am J Respir Crit Care Med 2011; 183:157–164.
- Bjerre LM, Verheij TJ, Kochen MM. Antibiotics for community acquired pneumonia in adult outpatients. Cochrane Database Syst Rev 2009; (4):CD002109.
- Frei CR, Labreche MJ, Attridge RT. Fluoroquinolones in community-acquired pneumonia: guide to selection and appropriate use. Drugs 2011; 71:757–770.
- Weiss K, Tillotson GS. The controversy of combination vs monotherapy in the treatment of hospitalized community-acquired pneumonia. Chest 2005; 128:940–946.
- Martínez JA, Horcajada JP, Almela M, et al. Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis 2003; 36:389–395.
- Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med 2001; 161:1837–1842.
- Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med 2001; 161:848–850.
- Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis 2003; 37:752–760.
- el Moussaoui R, de Borgie CA, van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ 2006; 332:1355.
- Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest 2003; 124:883–889.
- Salluh JI, Póvoa P, Soares M, Castro-Faria-Neto HC, Bozza FA, Bozza PT. The role of corticosteroids in severe community-acquired pneumonia: a systematic review. Crit Care 2008; 12:R76.
- Mikami K, Suzuki M, Kitagawa H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung 2007; 185:249–255.
- Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med 2010; 181:975–982.
- Chopra V, Flanders SA. Does statin use improve pneumonia outcomes? Chest 2009; 136:1381–1388.
- Yende S, Milbrandt EB, Kellum JA, et al. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med 2011; 39:1871–1878.
- Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998; 279:1452–1457.
- Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Feagan BG. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin Infect Dis 2000; 31:1362–1367.
- Aliberti S, Peyrani P, Filardo G, et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community-acquired pneumonia. Chest 2011; 140:482–488.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000; 342:681–689.
General internists need to be able to recognize community-acquired pneumonia (CAP) so that diagnostic and therapeutic interventions can be initiated promptly. It is also important to understand the most likely and possible causes of CAP so that appropriate initial antimicrobial therapy can be chosen. Especially during flu season, influenza can present as CAP and should be included in the differential diagnosis.
When managing a patient with CAP, the internist must decide which level of care, diagnostic tests, antimicrobial agents, and follow-up plans are needed. These topics will be reviewed in this article.
TWO TERMS TO REMEMBER
- CAP refers to pneumonia acquired outside a health care facility. It can be either bacterial or viral.
- CABP (community-acquired bacterial pneumonia) refers only to those cases caused by bacterial pathogens.
NUMBERS AND TRENDS
In the United States, CAP is the number-one cause of death from infection and the sixth leading cause of death overall.1 Each year, it is responsible for about 4.2 million outpatient visits, more than 60,000 deaths, and more than $17 billion in health care expenses.2
Community-acquired bacterial pneumonia: Common, serious
In a population-based US study in 1991, the incidence of CABP requiring hospitalization was 266.8 per 100,000 people.3
Estimates of overall mortality in CABP vary depending on the severity of illness and comorbid conditions. A meta-analysis published in 1996 found the overall mortality rate to be 13.7%, with a range of 5.1% to 36.5% depending on severity.4
In hospitalized patients, mortality rates and length of hospital stay appear to be declining over time. Between 1993 and 2005, the age-adjusted mortality rate decreased from 8.9% to 4.1%, and the average length of stay decreased from 7.5 to 5.7 days, with an overall reduction in hospital cost.5
CABP is more prevalent in older people than in the general population, and it increases with age from 18.2 cases per 1,000 patient-years in patients 60 to 69 years to 52.3 cases per 1,000 patient-years in those older than 85 years.6 Risk factors for pneumonia in the elderly include heart disease, chronic lung disease, immunosuppressive drugs, alcoholism, and increasing age.7 Similar to the trend in the general population, the mortality rate in elderly CABP patients appears to be decreasing over time, possibly thanks to rising rates of pneumococcal and influenza vaccination.8
Among the general population, risk factors for developing CABP also include smoking, occupational dust exposure, history of childhood pneumonia, unemployment, and single marital status.9 The incidence of CABP does not appear to be higher among pregnant women, although it is the most frequent cause of nonobstetric death in this population.10
The use of proton pump inhibitors may be an emerging risk factor for CABP.11 Also, use of nonsteroidal anti-inflammatory drugs among patients with CABP is associated with a blunted inflammatory response as well as a higher risk of pleuropulmonary complications and a delay in presentation.12
Influenza is also common, potentially severe
Influenza is also very common and potentially severe. It can cause a spectrum of disease, from mild upper respiratory tract symptoms to severe viral pneumonia that can be life-threatening and complicated by respiratory failure and the acute respiratory distress syndrome (ARDS).
Influenza infection can also be complicated by subsequent bacterial pneumonia. However, the epidemiology of influenza infection differs from that of CABP in that influenza occurs seasonally.
In the United States, seasonal influenza causes 36,000 deaths and 200,000 hospitalizations annually.13,14 As with CABP, the risk of death from influenza increases with age: it is 16 times greater in people age 85 and older than in those ages 65 to 69.13
During yearly seasonal epidemics, those at the highest risk of hospitalization and death are at the extremes of age. Risk factors for complicated influenza include heart disease, lung disease, diabetes, renal failure, rheumatologic conditions, dementia, and neurologic disease.15,16 During the 2009 H1N1 influenza pandemic, unexpected severity was seen in previously healthy young adults as well as those with obesity, neurodegenerative disease, pregnancy, and asthma.17
PATHOGENS: TYPICAL, ATYPICAL, VIRAL
Identifying the etiologic organism in CAP is confounded by limitations in the available diagnostic tests and also by poor-quality specimens that often are contaminated with bacteria that colonize the upper airways. Given these caveats, the primary pathogens responsible for CAP broadly include typical bacterial pathogens, atypical bacterial pathogens, and viruses.
Typical bacterial pathogens include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis, and, less commonly, a variety of aerobic and anaerobic gram-negative rods including Pseudomonas aeruginosa, Acinetobacter species, and Klebsiella pneumoniae.
Atypical bacterial pathogens include Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella species.18
Viruses implicated in adult CAP include influenza A and B, parainfluenza viruses, respiratory syncytial virus, and adenovirus.19 More recently, human metapneumovirus has been described as a cause of adult CAP.20
Clues to uncommon microbes
Specific historic features or coexisting conditions that may suggest an uncommon microbiologic diagnosis include21:
- Recent travel to the southwestern United States or Southeast Asia
- Ill contacts
- Exposure to birds, bats, rabbits, or farm animals
- Alcoholism
- Chronic obstructive pulmonary disease
- Human immunodeficiency virus infection
- Structural lung disease
- Prolonged cough with whoop or posttussive vomiting
- Aspiration
- Bioterrorism.
In cases in which one or more of these conditions exist, CAP may also be caused by other agents not listed above, including Mycobacterium tuberculosis, oral anaerobes, atypical mycobacteria, Histoplasma capsulatum, Chlamydophila psittaci, Francisella tularensis, Coxiella burnettii, Pneumocystis jiroveci, Cryptococcus, Aspergillus, Coccidioides, Hantavirus, avian influenza, Burkholderia pseudomallei, severe acute respiratory syndrome virus, Bordetella pertussis, Bacillus anthracis, and Yersinia pestis.
HOW BACTERIA INVADE THE LUNGS
The pathophysiology of CABP involves both host defense and microbial virulence factors.
The airways are most commonly exposed to microbes by microaspiration of upper airway flora, although hematogenous seeding of the lungs in a bacteremic patient or contiguous spread of infection from an adjacent site can also occur.
Mucociliary clearance and the cough reflex are important initial defenses against infection and can be inhibited by neurologic diseases and conditions that impair the mucociliary mechanism. Mucosal immune cells, including macrophages and neutrophils, recognize invading pathogens and generate an antibody response.
Regulation of the host inflammatory response to infection depends on a complex interaction between immune cells, inflammatory cytokines (eg, tumor necrosis factor alpha, interleukin 1-beta, and interleukin 6), and anti-inflammatory cytokines such as interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor type I.22
The interaction and timing of the inflammatory and anti-inflammatory response are essential in manifesting an appropriate host response to infection. An inadequate inflammatory response can lead to sepsis and death, but an excessive, late anti-inflammatory response can lead to a systemic inflammatory response such as ARDS. Polymorphisms within the genes coding for these factors may explain the variation in severity of illness among patients with CABP.23
HOW INFLUENZA DOES ITS DAMAGE
There are three types of influenza virus: A, B, and C. Type A causes most human infections. The influenza A virus envelope comprises a lipid bilayer that contains the projecting glycoproteins hemagglutinin and neuraminidase. Influenza viruses are named on the basis of these proteins and are designated with an H and an N, respectively, each followed by a number referring to the subtype.
Influenza infection begins when the virus makes contact with the epithelium. Hemagglutinin binds to the host cell and allows viral entry, where it begins replication. Neuraminidase prevents viral aggregation and facilitates the release of virus from infected cells.24
Mature virions are released and spread to neighboring host cells; this process is associated with desquamation and inflammation of the airways, causing cough, rhinorrhea, and sore throat. Systemic symptoms are associated with the induction of interferon, which causes fever and myalgia.25
Recovery and immunity to influenza infection occurs through both humoral and cell-mediated immunity, with antibodies directed against the specific hemagglutinin and neuraminidase antigens of the infecting virus. Immunity wanes over time and with antigenic drift of circulating viruses, making the host susceptible to recurrent influenza infection.24
Influenza is often complicated by bacterial superinfection
The influenza virus acts synergistically with certain bacteria to increase infectivity, and this may explain why influenza is often complicated by bacterial superinfection.
Mechanisms leading to bacterial superinfection include increased binding and invasion of bacteria, increased viral replication, and modification of the host inflammatory response. Some S aureus strains produce a protease that directly activates influenza virus hemagglutinin; other bacteria can activate plasminogen to promote influenza replication. The resulting increase in proteases in host tissues promotes activation of influenza through cleavage of hemagglutinin.26
The influenza virus also causes damage to the airway epithelial layer, leading to increased exposure of the binding sites necessary for adherence of S pneumoniae.27
CLINICAL PRESENTATION OF COMMUNITY-ACQUIRED PNEUMONIA
Although CAP is common, agreement on its essential clinical signs and symptoms is surprisingly limited, due in part to heterogeneous patient presentations and in part to interobserver variability. The reader is referred to two excellent reviews on this topic.28,29
The diagnosis of CAP is made on clinical grounds, based on a combination of signs and symptoms. Symptoms of pneumonia can include cough, fever, chills, sputum production, dyspnea, and pleuritic pain. Physical findings can include tachypnea, tachycardia, hypoxemia, and consolidation or rales on auscultation. Laboratory data may show leukocytosis or elevated C-reactive protein, and radiographic studies may show evidence of a new infiltrate.21,30,31
Clinical presentation of influenza
Seasonal influenza as a cause of CAP is difficult to distinguish from bacterial causes. The clinical presentation of seasonal influenza most commonly includes fever or subjective feverishness, cough, myalgia, and weakness.32 In a recent multivariate analysis, five clinical features were shown to be predictive of pandemic H1N1 influenza pneumonia rather than CABP: age younger than 65 years, absence of confusion, white blood cell count less than 12 × 109/L, temperature higher than 38°C (100.4°F), and bilateral opacities on radiography.32,33
Complicated influenza infection can be either primary viral pneumonia or bacterial superinfection.
During the 1918 influenza pandemic, which predated the ability to isolate viruses, two clinical syndromes emerged: an ARDS associated with the rapid onset of cyanosis, delirium, and frothy blood-tinged sputum; and an acute bronchopneumonia characterized by necrosis, hemorrhage, edema, and vasculitis.34,35 The first syndrome has subsequently been shown to be associated with primary viral pneumonia, while the second is caused by bacterial superinfection. Modern reexamination of 1918 data has shown that bacterial superinfection was likely the reason for the distinctly fulminant presentation of that pandemic.36,37
The 2009 H1N1 influenza pandemic caused relatively mild disease in most patients. However, those with severe pneumonia more commonly developed ARDS from primary influenza pneumonia than from bacterial superinfection.17
A third influenza-associated infection is secondary bacterial pneumonia, which follows influenza infection and mimics the presentation of CABP. A typical patient presents with a recent history of influenza-like illness, followed 4 to 14 days later by a recurrence of fever, dyspnea, productive cough, and consolidation on chest radiographs.38 Leukocytosis with an increased number of immature neutrophil forms, prolonged duration of fever, and elevated erythrocyte sedimentation rate are more likely in patients with secondary bacterial pneumonia.39 Isolates from sputum samples commonly include S pneumoniae, S aureus, H influenzae, and other gram-negative rods.40
In recent flu seasons, methicillin-resistant S aureus (MRSA) has emerged as a cause of severe secondary pneumonia. Most of these isolates carry genes for the toxin Panton-Valentine leukocidin; the associated mortality rate is as high as 33%.41,42 Although community-acquired MRSA pneumonia has only been reported in case series, distinct clinical features that have been described include severe pneumonia with high fever, hypotension, shock, respiratory failure, leukopenia, and multilobar and cavitary infiltrates.43
WHEN TO SUSPECT INFLUENZA
The triad of fever, cough, and abrupt onset are the best predictors of influenza, but no single combination of signs and symptoms predict influenza infection with 100% certainty. Therefore, an understanding of local epidemiologic data regarding circulating influenza is essential to maintain a high index of suspicion.44
It is appropriate to suspect influenza in:
- Anyone who is epidemiologically linked to a known outbreak of influenza
- Children, adults, and health care workers who have fever and abrupt onset of respiratory symptoms
- Patients with fever plus exacerbation of underlying pulmonary disease
- Severely ill patients with fever or hypothermia, especially during influenza season.45
DIAGNOSTIC TESTING
Once the diagnosis of pulmonary infection is suggested by clinical features, the initial evaluation should include measurement of vital signs, physical examination, and radiographic imaging of the chest. Additional diagnostic measures to consider include viral testing, blood culture, sputum culture, urinary antigen testing for Legionella and for S pneumoniae, fungal culture, and mycobacterial smear and culture.
Chest radiography (with posterior-anterior and lateral films) is the study that usually demonstrates the presence of a pulmonary infiltrate. If initial chest radiographs do not show an infiltrate, imaging can be repeated after treatment is started if the patient’s clinical presentation still suggests pneumonia. Chest radiographs are of limited value in predicting the pathogen, but they help to determine the extent of pneumonia and to detect parapneumonic effusion.46
A caveat: anterior-posterior, posterior-anterior, and lateral views can miss more than 10% of effusions large enough to warrant thoracentesis, especially when there is lower-lobe consolidation.47
Blood cultures are recommended for patients admitted to the intensive care unit and for those with cavitary infiltrates, leukopenia, alcohol abuse, severe liver disease, asplenia, positive pneumococcal urinary antigen testing, or a pleural effusion.21 However, blood cultures are positive in only 3% to 14% of hospitalized patients with CABP, and the impact of a positive blood culture on management decisions in CABP appears to be quite small.48–50
For the highest yield, blood culture results should be obtained before antibiotics are given. Not only is this good practice, but obtaining blood culture results before starting antibiotics is one of the quality measures evaluated by the Center for Medicare and Medicaid Services.51
Sputum culture is considered optional for outpatients and patients with less-severe pneumonia.21 While it can provide a rapid diagnosis in certain cases, a good-quality sputum sample is obtained in only 39% to 54% of patients with CABP, yields a predominant morphotype in only 45% of cases, and provides a useful microbiologic diagnosis in only 14.4%.52,53 Fungal and mycobacterial cultures are only indicated in certain situations such as cavitary infiltrates or immunosuppression.
Urinary antigen testing for Legionella and S pneumoniae should be done in patients with more severe illness and in those for whom outpatient therapy has failed.21S pneumoniae testing has been shown to allow early diagnosis of pneumococcal pneumonia in 26% more patients than with Gram staining, but it fails to identify 22% of the rapid diagnoses initially identified by Gram staining.54 Thus, a sequential approach is reasonable, with urinary antigen testing for patients at high risk without useful results from sputum Gram staining. Also, recent data suggest that the pneumococcal urinary antigen test may allow optimization of antimicrobial therapy with good clinical outcomes.55
Endotracheal tests. If the patient is intubated, collection of endotracheal aspirates, bronchoscopy, or nonbronchoscopic bronchial lavage (sometimes called “mini-BAL”) should be performed.
Thoracentesis and pleural fluid cultures should be done if a pleural effusion is found. Empyema, large or loculated effusions, and parapneumonic effusions with a pH lower than 7.20, glucose levels less than 3.4 mmol/L (60 mg/dL), or positive results on microbial staining or culture should be drained by chest tube or surgically.56
Testing for influenza should be done if it will change the clinical management, such as the choice of antibiotic or infection control practices. Specimens should be obtained with either a nasopharyngeal swab or aspirate and tested with reverse transcriptase polymerase chain reaction, immunofluorescent staining, or rapid antigen detection, depending on local availability.45
Inflammatory biomarkers such as C-reactive protein and procalcitonin have been receiving interest as ways to predict the etiology and prognosis of CAP and to guide therapy. Several studies have shown that C-reactive protein can help distinguish between CAP and bronchitis, with higher values suggesting more severe pneumonia and pneumonia caused by S pneumoniae or L pneumophila.57 Procalcitonin may help discriminate between severe lower respiratory tract infections of bacterial and 2009 H1N1 origin, although less effectively than C-reactive protein. Low procalcitonin values, particularly when combined with low C-reactive protein levels, suggest that bacterial infection is unlikely.58
RISK STRATIFICATION AND SITE-OF-CARE DECISIONS
Following a presumptive diagnosis of CAP, it is important to decide not only what treatment the patient will receive but whether he or she should be hospitalized. If the patient is to be admitted to the hospital, the clinician must also decide if his or her condition warrants intensive care.
Severity-of-illness scores
A recent meta-analysis compared the performance characteristics of the PSI and CURB-65 scores for predicting mortality in CAP and found no significant differences in overall test performance.61
Another meta-analysis found that the PSI was more sensitive than the CURB-65 and had a low false-negative rate, and so was better at showing which patients do not need to be hospitalized. Conversely, the CURB-65 was more specific and had a higher positive predictive value, and thus was more likely to correctly classify high-risk patients.62
Other scoring systems that aid in deciding about hospital admission and level of care include the CRB-6563 (which can be used instead of the CURB-65 if laboratory values are not available), SMART-COP,64 and SCAP.,65
Guidelines on when to admit to the intensive care unit
Guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) also provide guidance on when intensive care admission is advised,21 and their criteria were recently validated.66 The guidelines advocate direct admission to the intensive care unit for patients requiring vasopressors or mechanical ventilation, and intensive care unit or high-level monitoring for patients with three of the following criteria for severe CAP21:
- Respiratory rate ≥ 30
- Pao2/Fio2 ratio ≤ 250
- Multilobar infiltrates
- Confusion or disorientation
- Uremia (blood urea nitrogen ≥ 20 mg/dL)
- Leukopenia (white blood cell count < 4.0 × 109/L)
- Thrombocytopenia (platelet count < 100 × 109/L)
- Hypothermia (core temperature < 36.0°C [96.8°F])
- Hypotension requiring aggressive fluid resuscitation.
None of these scoring systems or criteria is meant to replace clinical judgment. A recent study has suggested that an oxygen saturation of less than 92% is an appropriate threshold for hospital admission, in view of higher rates of adverse events in outpatients with saturations below this value.67
TREATMENT
Multiple studies have shown that treatment of CAP in accordance with guidelines has led to improved clinical outcomes.21,68–70
How fast must antibiotics be started?
Based on studies that showed a lower mortality rate when antibiotics were started sooner, Medicare and Medicaid adopted a quality measure calling for starting antibiotics within 4 hours in patients being admitted to the hospital.50,71 However, several subsequent studies showed that the diagnosis of pneumonia is often incorrect and that rapid administration of antibiotics could lead to misdiagnosis, overuse of antibiotics, and a higher risk of Clostridium difficile infection.72,73
The current IDSA/ATS guidelines21 recommend that the first antibiotic dose be given while the patient is still in the emergency department, but do not give a specific time within which it should be given. Medicare and Medicaid later updated their quality measure to antibiotic administration within 6 hours.
Which antibiotics should be used?
The selection of antimicrobial agent depends upon the patient’s severity of illness and comorbid conditions.
Although most studies of combination antibiotic therapy have been retrospective and observational, they suggest that a macrolide (ie, one of the “mycins”) added to a beta-lactam antibiotic is beneficial, possibly by covering atypical organisms or via anti-inflammatory action.74–76 The choice of one antibiotic over another appears to be less important, and a recent Cochrane review concluded that there was no significant difference in efficacy among five antibiotic pairs studied.77
Empiric outpatient treatment of a previously healthy patient with CAP and no risk factors for drug-resistant S pneumoniae should include either a macrolide (azithromycin [Zithromax], clarithromycin [Biaxin], or erythromycin) or doxycycline. If the patient has a chronic comorbid condition such as heart, lung, liver, or renal disease, diabetes mellitus, alcoholism, malignancy, asplenia, or immunosuppression or has received antimicrobials within the preceding 3 months, then treatment should include either a respiratory fluoroquinolone (moxifloxacin [Avelox] or levofloxacin [Levaquin]) or a beta-lactam plus a macrolide.21
Overall, published data suggest that the survival rate is about the same with fluoroquinolone monotherapy as with beta-lactam plus macrolide combination therapy, and better than with beta-lactam monotherapy.78
Selection of antibiotics for inpatient treatment of CAP is influenced by severity of illness. Inpatients who do not require intensive care should be treated with either a respiratory fluoroquinolone or combination therapy with a beta-lactam (cefotaxime [Claforan], ceftriaxone [Rocephin], ampicillin, or ertapenem [Invanz]) plus a macrolide or doxycycline.21,76,79
If a specific microbiologic diagnosis is made, then treatment can be narrowed. However in certain cases, such as invasive pneumococcal infection, combination therapy may still be superior.80,81 For patients who need intensive care, treatment should always include a beta-lactam plus either azithromycin or a respiratory fluoroquinolone.21 In certain situations, additional antibiotics may be added as well, such as agents to treat Pseudomonas, community-acquired MRSA, or both.
Switching to oral therapy; short-course therapy
In the interest of avoiding unnecessary antibiotics, numerous studies have addressed the issue of an “early switch” to oral antibiotics and “short-course” therapy for CAP. In general, once clinically stable, patients with CAP, including bacteremic S pneumoniae pneumonia, can be safely switched to oral antibiotics.82
The issue of short-course therapy is more complicated, and the appropriate length of therapy for CAP is not well established. However, 5 days of levofloxacin 750 mg was shown to be as successful as 7 to 10 days of levofloxacin 500 mg.83 In another study, in patients who improved after 3 days of intravenous therapy for CAP, there was no difference in clinical outcome between those who were changed to oral therapy for 5 more days and those who received an oral placebo.84
Most patients who achieve clinical stability in the first week do not need prolonged antibiotic therapy. However, certain conditions, such as S aureus bacteremic pneumonia, complicated pneumonia, and pneumonia due to unusual organisms, may require prolonged treatment.
Other therapies
Additional therapies studied in patients with pneumonia include early mobilization, adjunctive corticosteroids, and statin drugs.
Early mobilization was shown in one study to decrease hospital length of stay without increasing adverse effects.85
Corticosteroids are not supported as a standard of care for patients with severe CAP according to current available studies.86,87 Furthermore, a randomized, controlled trial showed that prednisolone daily for a week did not improve outcomes in hospitalized patients with CAP, and it was associated with increased late failure.88
Statin trials under way. Several observational studies have suggested that statins might be beneficial in managing sepsis through their effects on endothelial cell function, antioxidant effects, anti-inflammatory effects, and immunomodulatory effects.89 However, a recent large prospective multicenter cohort study of hospitalized patients with CAP did not find evidence of a protective effect of statins on clinically meaningful outcomes in CAP or significant differences in circulating biomarkers.90 Several randomized trials of statin therapy in patients with both ventilator-associated pneumonia and CAP are under way.
INFLUENZA TREATMENT: MOST EFFECTIVE WITHIN 48 HOURS
Treatment with antiviral drugs is most effective if started within 48 hours after symptom onset, although some patients with confirmed influenza who are either not improving or who are critically ill may still benefit from treatment started later.
Treatment should be considered in patients with laboratory-confirmed or suspected influenza who are at risk of developing complicated influenza and in otherwise healthy patients who wish to reduce the duration of illness or who have close contact with patients who are at high risk of complications.
Antiviral medications are oseltamivir (Tamiflu), zanamivir (Relenza), and the adamantines amantadine (Symmetrel) and rimantadine (Flumadine).
Due to evolving viral resistance patterns, the choice of antiviral drug depends on the strain. Seasonal H1N1 is best treated with zanamivir or an adamantine, while pandemic 2009 H1N1 and H3N2 are best treated with zanamivir or oseltamivir. When strain typing is not available, empiric therapy should be with either zanamivir monotherapy or a combination of oseltamivir plus rimantadine. Influenza B viruses are resistant to adamantines and should be treated only with either zanamivir or oseltamivir.45
FOLLOW-UP AND PREVENTION
Patients with CAP can generally be expected to improve within 3 to 7 days.91 However, it may be several weeks before they return to baseline.92
Follow-up plans may be guided by the time to clinical stability. For patients who do not achieve clinical stability until more than 72 hours after admission, more aggressive follow-up on discharge is indicated, since they are more likely to experience early readmission and death.93
Pneumococcal vaccination. Because S pneumoniae remains the most common cause of CAP, efforts should be made to vaccinate patients appropriately. The Advisory Committee on Immunization Practices (ACIP) and the US Centers for Disease Control and Prevention recommend that the pneumococcal polysaccharide vaccine (Pneumovax 23; PPSV23) be given to those over age 65. Those who were vaccinated before age 65 should receive another dose at age 65 or later if at least 5 years have passed since their previous dose. Those who receive it at or after age 65 should receive only a single dose. A second dose is recommended 5 years after the first dose for people age 19 to 64 years with functional or anatomic asplenia and for those who are immunocompromised.
Influenza vaccination for all. Of note, the ACIP updated its guidelines on influenza vaccination beginning with the 2010–2011 influenza season. It no longer advocates a risk-stratified approach. Instead, it recommends universal influenza vaccination for everybody more than 6 months old.94
Smoking cessation should be addressed. Smoking cessation is a Medicare and Medicaid quality measure and should be encouraged after an episode of CAP because quitting smoking reduces the risk of pneumococcal disease by approximately 14% each year thereafter.95
General internists need to be able to recognize community-acquired pneumonia (CAP) so that diagnostic and therapeutic interventions can be initiated promptly. It is also important to understand the most likely and possible causes of CAP so that appropriate initial antimicrobial therapy can be chosen. Especially during flu season, influenza can present as CAP and should be included in the differential diagnosis.
When managing a patient with CAP, the internist must decide which level of care, diagnostic tests, antimicrobial agents, and follow-up plans are needed. These topics will be reviewed in this article.
TWO TERMS TO REMEMBER
- CAP refers to pneumonia acquired outside a health care facility. It can be either bacterial or viral.
- CABP (community-acquired bacterial pneumonia) refers only to those cases caused by bacterial pathogens.
NUMBERS AND TRENDS
In the United States, CAP is the number-one cause of death from infection and the sixth leading cause of death overall.1 Each year, it is responsible for about 4.2 million outpatient visits, more than 60,000 deaths, and more than $17 billion in health care expenses.2
Community-acquired bacterial pneumonia: Common, serious
In a population-based US study in 1991, the incidence of CABP requiring hospitalization was 266.8 per 100,000 people.3
Estimates of overall mortality in CABP vary depending on the severity of illness and comorbid conditions. A meta-analysis published in 1996 found the overall mortality rate to be 13.7%, with a range of 5.1% to 36.5% depending on severity.4
In hospitalized patients, mortality rates and length of hospital stay appear to be declining over time. Between 1993 and 2005, the age-adjusted mortality rate decreased from 8.9% to 4.1%, and the average length of stay decreased from 7.5 to 5.7 days, with an overall reduction in hospital cost.5
CABP is more prevalent in older people than in the general population, and it increases with age from 18.2 cases per 1,000 patient-years in patients 60 to 69 years to 52.3 cases per 1,000 patient-years in those older than 85 years.6 Risk factors for pneumonia in the elderly include heart disease, chronic lung disease, immunosuppressive drugs, alcoholism, and increasing age.7 Similar to the trend in the general population, the mortality rate in elderly CABP patients appears to be decreasing over time, possibly thanks to rising rates of pneumococcal and influenza vaccination.8
Among the general population, risk factors for developing CABP also include smoking, occupational dust exposure, history of childhood pneumonia, unemployment, and single marital status.9 The incidence of CABP does not appear to be higher among pregnant women, although it is the most frequent cause of nonobstetric death in this population.10
The use of proton pump inhibitors may be an emerging risk factor for CABP.11 Also, use of nonsteroidal anti-inflammatory drugs among patients with CABP is associated with a blunted inflammatory response as well as a higher risk of pleuropulmonary complications and a delay in presentation.12
Influenza is also common, potentially severe
Influenza is also very common and potentially severe. It can cause a spectrum of disease, from mild upper respiratory tract symptoms to severe viral pneumonia that can be life-threatening and complicated by respiratory failure and the acute respiratory distress syndrome (ARDS).
Influenza infection can also be complicated by subsequent bacterial pneumonia. However, the epidemiology of influenza infection differs from that of CABP in that influenza occurs seasonally.
In the United States, seasonal influenza causes 36,000 deaths and 200,000 hospitalizations annually.13,14 As with CABP, the risk of death from influenza increases with age: it is 16 times greater in people age 85 and older than in those ages 65 to 69.13
During yearly seasonal epidemics, those at the highest risk of hospitalization and death are at the extremes of age. Risk factors for complicated influenza include heart disease, lung disease, diabetes, renal failure, rheumatologic conditions, dementia, and neurologic disease.15,16 During the 2009 H1N1 influenza pandemic, unexpected severity was seen in previously healthy young adults as well as those with obesity, neurodegenerative disease, pregnancy, and asthma.17
PATHOGENS: TYPICAL, ATYPICAL, VIRAL
Identifying the etiologic organism in CAP is confounded by limitations in the available diagnostic tests and also by poor-quality specimens that often are contaminated with bacteria that colonize the upper airways. Given these caveats, the primary pathogens responsible for CAP broadly include typical bacterial pathogens, atypical bacterial pathogens, and viruses.
Typical bacterial pathogens include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis, and, less commonly, a variety of aerobic and anaerobic gram-negative rods including Pseudomonas aeruginosa, Acinetobacter species, and Klebsiella pneumoniae.
Atypical bacterial pathogens include Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella species.18
Viruses implicated in adult CAP include influenza A and B, parainfluenza viruses, respiratory syncytial virus, and adenovirus.19 More recently, human metapneumovirus has been described as a cause of adult CAP.20
Clues to uncommon microbes
Specific historic features or coexisting conditions that may suggest an uncommon microbiologic diagnosis include21:
- Recent travel to the southwestern United States or Southeast Asia
- Ill contacts
- Exposure to birds, bats, rabbits, or farm animals
- Alcoholism
- Chronic obstructive pulmonary disease
- Human immunodeficiency virus infection
- Structural lung disease
- Prolonged cough with whoop or posttussive vomiting
- Aspiration
- Bioterrorism.
In cases in which one or more of these conditions exist, CAP may also be caused by other agents not listed above, including Mycobacterium tuberculosis, oral anaerobes, atypical mycobacteria, Histoplasma capsulatum, Chlamydophila psittaci, Francisella tularensis, Coxiella burnettii, Pneumocystis jiroveci, Cryptococcus, Aspergillus, Coccidioides, Hantavirus, avian influenza, Burkholderia pseudomallei, severe acute respiratory syndrome virus, Bordetella pertussis, Bacillus anthracis, and Yersinia pestis.
HOW BACTERIA INVADE THE LUNGS
The pathophysiology of CABP involves both host defense and microbial virulence factors.
The airways are most commonly exposed to microbes by microaspiration of upper airway flora, although hematogenous seeding of the lungs in a bacteremic patient or contiguous spread of infection from an adjacent site can also occur.
Mucociliary clearance and the cough reflex are important initial defenses against infection and can be inhibited by neurologic diseases and conditions that impair the mucociliary mechanism. Mucosal immune cells, including macrophages and neutrophils, recognize invading pathogens and generate an antibody response.
Regulation of the host inflammatory response to infection depends on a complex interaction between immune cells, inflammatory cytokines (eg, tumor necrosis factor alpha, interleukin 1-beta, and interleukin 6), and anti-inflammatory cytokines such as interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor type I.22
The interaction and timing of the inflammatory and anti-inflammatory response are essential in manifesting an appropriate host response to infection. An inadequate inflammatory response can lead to sepsis and death, but an excessive, late anti-inflammatory response can lead to a systemic inflammatory response such as ARDS. Polymorphisms within the genes coding for these factors may explain the variation in severity of illness among patients with CABP.23
HOW INFLUENZA DOES ITS DAMAGE
There are three types of influenza virus: A, B, and C. Type A causes most human infections. The influenza A virus envelope comprises a lipid bilayer that contains the projecting glycoproteins hemagglutinin and neuraminidase. Influenza viruses are named on the basis of these proteins and are designated with an H and an N, respectively, each followed by a number referring to the subtype.
Influenza infection begins when the virus makes contact with the epithelium. Hemagglutinin binds to the host cell and allows viral entry, where it begins replication. Neuraminidase prevents viral aggregation and facilitates the release of virus from infected cells.24
Mature virions are released and spread to neighboring host cells; this process is associated with desquamation and inflammation of the airways, causing cough, rhinorrhea, and sore throat. Systemic symptoms are associated with the induction of interferon, which causes fever and myalgia.25
Recovery and immunity to influenza infection occurs through both humoral and cell-mediated immunity, with antibodies directed against the specific hemagglutinin and neuraminidase antigens of the infecting virus. Immunity wanes over time and with antigenic drift of circulating viruses, making the host susceptible to recurrent influenza infection.24
Influenza is often complicated by bacterial superinfection
The influenza virus acts synergistically with certain bacteria to increase infectivity, and this may explain why influenza is often complicated by bacterial superinfection.
Mechanisms leading to bacterial superinfection include increased binding and invasion of bacteria, increased viral replication, and modification of the host inflammatory response. Some S aureus strains produce a protease that directly activates influenza virus hemagglutinin; other bacteria can activate plasminogen to promote influenza replication. The resulting increase in proteases in host tissues promotes activation of influenza through cleavage of hemagglutinin.26
The influenza virus also causes damage to the airway epithelial layer, leading to increased exposure of the binding sites necessary for adherence of S pneumoniae.27
CLINICAL PRESENTATION OF COMMUNITY-ACQUIRED PNEUMONIA
Although CAP is common, agreement on its essential clinical signs and symptoms is surprisingly limited, due in part to heterogeneous patient presentations and in part to interobserver variability. The reader is referred to two excellent reviews on this topic.28,29
The diagnosis of CAP is made on clinical grounds, based on a combination of signs and symptoms. Symptoms of pneumonia can include cough, fever, chills, sputum production, dyspnea, and pleuritic pain. Physical findings can include tachypnea, tachycardia, hypoxemia, and consolidation or rales on auscultation. Laboratory data may show leukocytosis or elevated C-reactive protein, and radiographic studies may show evidence of a new infiltrate.21,30,31
Clinical presentation of influenza
Seasonal influenza as a cause of CAP is difficult to distinguish from bacterial causes. The clinical presentation of seasonal influenza most commonly includes fever or subjective feverishness, cough, myalgia, and weakness.32 In a recent multivariate analysis, five clinical features were shown to be predictive of pandemic H1N1 influenza pneumonia rather than CABP: age younger than 65 years, absence of confusion, white blood cell count less than 12 × 109/L, temperature higher than 38°C (100.4°F), and bilateral opacities on radiography.32,33
Complicated influenza infection can be either primary viral pneumonia or bacterial superinfection.
During the 1918 influenza pandemic, which predated the ability to isolate viruses, two clinical syndromes emerged: an ARDS associated with the rapid onset of cyanosis, delirium, and frothy blood-tinged sputum; and an acute bronchopneumonia characterized by necrosis, hemorrhage, edema, and vasculitis.34,35 The first syndrome has subsequently been shown to be associated with primary viral pneumonia, while the second is caused by bacterial superinfection. Modern reexamination of 1918 data has shown that bacterial superinfection was likely the reason for the distinctly fulminant presentation of that pandemic.36,37
The 2009 H1N1 influenza pandemic caused relatively mild disease in most patients. However, those with severe pneumonia more commonly developed ARDS from primary influenza pneumonia than from bacterial superinfection.17
A third influenza-associated infection is secondary bacterial pneumonia, which follows influenza infection and mimics the presentation of CABP. A typical patient presents with a recent history of influenza-like illness, followed 4 to 14 days later by a recurrence of fever, dyspnea, productive cough, and consolidation on chest radiographs.38 Leukocytosis with an increased number of immature neutrophil forms, prolonged duration of fever, and elevated erythrocyte sedimentation rate are more likely in patients with secondary bacterial pneumonia.39 Isolates from sputum samples commonly include S pneumoniae, S aureus, H influenzae, and other gram-negative rods.40
In recent flu seasons, methicillin-resistant S aureus (MRSA) has emerged as a cause of severe secondary pneumonia. Most of these isolates carry genes for the toxin Panton-Valentine leukocidin; the associated mortality rate is as high as 33%.41,42 Although community-acquired MRSA pneumonia has only been reported in case series, distinct clinical features that have been described include severe pneumonia with high fever, hypotension, shock, respiratory failure, leukopenia, and multilobar and cavitary infiltrates.43
WHEN TO SUSPECT INFLUENZA
The triad of fever, cough, and abrupt onset are the best predictors of influenza, but no single combination of signs and symptoms predict influenza infection with 100% certainty. Therefore, an understanding of local epidemiologic data regarding circulating influenza is essential to maintain a high index of suspicion.44
It is appropriate to suspect influenza in:
- Anyone who is epidemiologically linked to a known outbreak of influenza
- Children, adults, and health care workers who have fever and abrupt onset of respiratory symptoms
- Patients with fever plus exacerbation of underlying pulmonary disease
- Severely ill patients with fever or hypothermia, especially during influenza season.45
DIAGNOSTIC TESTING
Once the diagnosis of pulmonary infection is suggested by clinical features, the initial evaluation should include measurement of vital signs, physical examination, and radiographic imaging of the chest. Additional diagnostic measures to consider include viral testing, blood culture, sputum culture, urinary antigen testing for Legionella and for S pneumoniae, fungal culture, and mycobacterial smear and culture.
Chest radiography (with posterior-anterior and lateral films) is the study that usually demonstrates the presence of a pulmonary infiltrate. If initial chest radiographs do not show an infiltrate, imaging can be repeated after treatment is started if the patient’s clinical presentation still suggests pneumonia. Chest radiographs are of limited value in predicting the pathogen, but they help to determine the extent of pneumonia and to detect parapneumonic effusion.46
A caveat: anterior-posterior, posterior-anterior, and lateral views can miss more than 10% of effusions large enough to warrant thoracentesis, especially when there is lower-lobe consolidation.47
Blood cultures are recommended for patients admitted to the intensive care unit and for those with cavitary infiltrates, leukopenia, alcohol abuse, severe liver disease, asplenia, positive pneumococcal urinary antigen testing, or a pleural effusion.21 However, blood cultures are positive in only 3% to 14% of hospitalized patients with CABP, and the impact of a positive blood culture on management decisions in CABP appears to be quite small.48–50
For the highest yield, blood culture results should be obtained before antibiotics are given. Not only is this good practice, but obtaining blood culture results before starting antibiotics is one of the quality measures evaluated by the Center for Medicare and Medicaid Services.51
Sputum culture is considered optional for outpatients and patients with less-severe pneumonia.21 While it can provide a rapid diagnosis in certain cases, a good-quality sputum sample is obtained in only 39% to 54% of patients with CABP, yields a predominant morphotype in only 45% of cases, and provides a useful microbiologic diagnosis in only 14.4%.52,53 Fungal and mycobacterial cultures are only indicated in certain situations such as cavitary infiltrates or immunosuppression.
Urinary antigen testing for Legionella and S pneumoniae should be done in patients with more severe illness and in those for whom outpatient therapy has failed.21S pneumoniae testing has been shown to allow early diagnosis of pneumococcal pneumonia in 26% more patients than with Gram staining, but it fails to identify 22% of the rapid diagnoses initially identified by Gram staining.54 Thus, a sequential approach is reasonable, with urinary antigen testing for patients at high risk without useful results from sputum Gram staining. Also, recent data suggest that the pneumococcal urinary antigen test may allow optimization of antimicrobial therapy with good clinical outcomes.55
Endotracheal tests. If the patient is intubated, collection of endotracheal aspirates, bronchoscopy, or nonbronchoscopic bronchial lavage (sometimes called “mini-BAL”) should be performed.
Thoracentesis and pleural fluid cultures should be done if a pleural effusion is found. Empyema, large or loculated effusions, and parapneumonic effusions with a pH lower than 7.20, glucose levels less than 3.4 mmol/L (60 mg/dL), or positive results on microbial staining or culture should be drained by chest tube or surgically.56
Testing for influenza should be done if it will change the clinical management, such as the choice of antibiotic or infection control practices. Specimens should be obtained with either a nasopharyngeal swab or aspirate and tested with reverse transcriptase polymerase chain reaction, immunofluorescent staining, or rapid antigen detection, depending on local availability.45
Inflammatory biomarkers such as C-reactive protein and procalcitonin have been receiving interest as ways to predict the etiology and prognosis of CAP and to guide therapy. Several studies have shown that C-reactive protein can help distinguish between CAP and bronchitis, with higher values suggesting more severe pneumonia and pneumonia caused by S pneumoniae or L pneumophila.57 Procalcitonin may help discriminate between severe lower respiratory tract infections of bacterial and 2009 H1N1 origin, although less effectively than C-reactive protein. Low procalcitonin values, particularly when combined with low C-reactive protein levels, suggest that bacterial infection is unlikely.58
RISK STRATIFICATION AND SITE-OF-CARE DECISIONS
Following a presumptive diagnosis of CAP, it is important to decide not only what treatment the patient will receive but whether he or she should be hospitalized. If the patient is to be admitted to the hospital, the clinician must also decide if his or her condition warrants intensive care.
Severity-of-illness scores
A recent meta-analysis compared the performance characteristics of the PSI and CURB-65 scores for predicting mortality in CAP and found no significant differences in overall test performance.61
Another meta-analysis found that the PSI was more sensitive than the CURB-65 and had a low false-negative rate, and so was better at showing which patients do not need to be hospitalized. Conversely, the CURB-65 was more specific and had a higher positive predictive value, and thus was more likely to correctly classify high-risk patients.62
Other scoring systems that aid in deciding about hospital admission and level of care include the CRB-6563 (which can be used instead of the CURB-65 if laboratory values are not available), SMART-COP,64 and SCAP.,65
Guidelines on when to admit to the intensive care unit
Guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) also provide guidance on when intensive care admission is advised,21 and their criteria were recently validated.66 The guidelines advocate direct admission to the intensive care unit for patients requiring vasopressors or mechanical ventilation, and intensive care unit or high-level monitoring for patients with three of the following criteria for severe CAP21:
- Respiratory rate ≥ 30
- Pao2/Fio2 ratio ≤ 250
- Multilobar infiltrates
- Confusion or disorientation
- Uremia (blood urea nitrogen ≥ 20 mg/dL)
- Leukopenia (white blood cell count < 4.0 × 109/L)
- Thrombocytopenia (platelet count < 100 × 109/L)
- Hypothermia (core temperature < 36.0°C [96.8°F])
- Hypotension requiring aggressive fluid resuscitation.
None of these scoring systems or criteria is meant to replace clinical judgment. A recent study has suggested that an oxygen saturation of less than 92% is an appropriate threshold for hospital admission, in view of higher rates of adverse events in outpatients with saturations below this value.67
TREATMENT
Multiple studies have shown that treatment of CAP in accordance with guidelines has led to improved clinical outcomes.21,68–70
How fast must antibiotics be started?
Based on studies that showed a lower mortality rate when antibiotics were started sooner, Medicare and Medicaid adopted a quality measure calling for starting antibiotics within 4 hours in patients being admitted to the hospital.50,71 However, several subsequent studies showed that the diagnosis of pneumonia is often incorrect and that rapid administration of antibiotics could lead to misdiagnosis, overuse of antibiotics, and a higher risk of Clostridium difficile infection.72,73
The current IDSA/ATS guidelines21 recommend that the first antibiotic dose be given while the patient is still in the emergency department, but do not give a specific time within which it should be given. Medicare and Medicaid later updated their quality measure to antibiotic administration within 6 hours.
Which antibiotics should be used?
The selection of antimicrobial agent depends upon the patient’s severity of illness and comorbid conditions.
Although most studies of combination antibiotic therapy have been retrospective and observational, they suggest that a macrolide (ie, one of the “mycins”) added to a beta-lactam antibiotic is beneficial, possibly by covering atypical organisms or via anti-inflammatory action.74–76 The choice of one antibiotic over another appears to be less important, and a recent Cochrane review concluded that there was no significant difference in efficacy among five antibiotic pairs studied.77
Empiric outpatient treatment of a previously healthy patient with CAP and no risk factors for drug-resistant S pneumoniae should include either a macrolide (azithromycin [Zithromax], clarithromycin [Biaxin], or erythromycin) or doxycycline. If the patient has a chronic comorbid condition such as heart, lung, liver, or renal disease, diabetes mellitus, alcoholism, malignancy, asplenia, or immunosuppression or has received antimicrobials within the preceding 3 months, then treatment should include either a respiratory fluoroquinolone (moxifloxacin [Avelox] or levofloxacin [Levaquin]) or a beta-lactam plus a macrolide.21
Overall, published data suggest that the survival rate is about the same with fluoroquinolone monotherapy as with beta-lactam plus macrolide combination therapy, and better than with beta-lactam monotherapy.78
Selection of antibiotics for inpatient treatment of CAP is influenced by severity of illness. Inpatients who do not require intensive care should be treated with either a respiratory fluoroquinolone or combination therapy with a beta-lactam (cefotaxime [Claforan], ceftriaxone [Rocephin], ampicillin, or ertapenem [Invanz]) plus a macrolide or doxycycline.21,76,79
If a specific microbiologic diagnosis is made, then treatment can be narrowed. However in certain cases, such as invasive pneumococcal infection, combination therapy may still be superior.80,81 For patients who need intensive care, treatment should always include a beta-lactam plus either azithromycin or a respiratory fluoroquinolone.21 In certain situations, additional antibiotics may be added as well, such as agents to treat Pseudomonas, community-acquired MRSA, or both.
Switching to oral therapy; short-course therapy
In the interest of avoiding unnecessary antibiotics, numerous studies have addressed the issue of an “early switch” to oral antibiotics and “short-course” therapy for CAP. In general, once clinically stable, patients with CAP, including bacteremic S pneumoniae pneumonia, can be safely switched to oral antibiotics.82
The issue of short-course therapy is more complicated, and the appropriate length of therapy for CAP is not well established. However, 5 days of levofloxacin 750 mg was shown to be as successful as 7 to 10 days of levofloxacin 500 mg.83 In another study, in patients who improved after 3 days of intravenous therapy for CAP, there was no difference in clinical outcome between those who were changed to oral therapy for 5 more days and those who received an oral placebo.84
Most patients who achieve clinical stability in the first week do not need prolonged antibiotic therapy. However, certain conditions, such as S aureus bacteremic pneumonia, complicated pneumonia, and pneumonia due to unusual organisms, may require prolonged treatment.
Other therapies
Additional therapies studied in patients with pneumonia include early mobilization, adjunctive corticosteroids, and statin drugs.
Early mobilization was shown in one study to decrease hospital length of stay without increasing adverse effects.85
Corticosteroids are not supported as a standard of care for patients with severe CAP according to current available studies.86,87 Furthermore, a randomized, controlled trial showed that prednisolone daily for a week did not improve outcomes in hospitalized patients with CAP, and it was associated with increased late failure.88
Statin trials under way. Several observational studies have suggested that statins might be beneficial in managing sepsis through their effects on endothelial cell function, antioxidant effects, anti-inflammatory effects, and immunomodulatory effects.89 However, a recent large prospective multicenter cohort study of hospitalized patients with CAP did not find evidence of a protective effect of statins on clinically meaningful outcomes in CAP or significant differences in circulating biomarkers.90 Several randomized trials of statin therapy in patients with both ventilator-associated pneumonia and CAP are under way.
INFLUENZA TREATMENT: MOST EFFECTIVE WITHIN 48 HOURS
Treatment with antiviral drugs is most effective if started within 48 hours after symptom onset, although some patients with confirmed influenza who are either not improving or who are critically ill may still benefit from treatment started later.
Treatment should be considered in patients with laboratory-confirmed or suspected influenza who are at risk of developing complicated influenza and in otherwise healthy patients who wish to reduce the duration of illness or who have close contact with patients who are at high risk of complications.
Antiviral medications are oseltamivir (Tamiflu), zanamivir (Relenza), and the adamantines amantadine (Symmetrel) and rimantadine (Flumadine).
Due to evolving viral resistance patterns, the choice of antiviral drug depends on the strain. Seasonal H1N1 is best treated with zanamivir or an adamantine, while pandemic 2009 H1N1 and H3N2 are best treated with zanamivir or oseltamivir. When strain typing is not available, empiric therapy should be with either zanamivir monotherapy or a combination of oseltamivir plus rimantadine. Influenza B viruses are resistant to adamantines and should be treated only with either zanamivir or oseltamivir.45
FOLLOW-UP AND PREVENTION
Patients with CAP can generally be expected to improve within 3 to 7 days.91 However, it may be several weeks before they return to baseline.92
Follow-up plans may be guided by the time to clinical stability. For patients who do not achieve clinical stability until more than 72 hours after admission, more aggressive follow-up on discharge is indicated, since they are more likely to experience early readmission and death.93
Pneumococcal vaccination. Because S pneumoniae remains the most common cause of CAP, efforts should be made to vaccinate patients appropriately. The Advisory Committee on Immunization Practices (ACIP) and the US Centers for Disease Control and Prevention recommend that the pneumococcal polysaccharide vaccine (Pneumovax 23; PPSV23) be given to those over age 65. Those who were vaccinated before age 65 should receive another dose at age 65 or later if at least 5 years have passed since their previous dose. Those who receive it at or after age 65 should receive only a single dose. A second dose is recommended 5 years after the first dose for people age 19 to 64 years with functional or anatomic asplenia and for those who are immunocompromised.
Influenza vaccination for all. Of note, the ACIP updated its guidelines on influenza vaccination beginning with the 2010–2011 influenza season. It no longer advocates a risk-stratified approach. Instead, it recommends universal influenza vaccination for everybody more than 6 months old.94
Smoking cessation should be addressed. Smoking cessation is a Medicare and Medicaid quality measure and should be encouraged after an episode of CAP because quitting smoking reduces the risk of pneumococcal disease by approximately 14% each year thereafter.95
- Mortensen EM, Kapoor WN, Chang CC, Fine MJ. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis 2003; 37:1617–1624.
- File TM, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010; 122:130–141.
- Marston BJ, Plouffe JF, File TM, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med 1997; 157:1709–1718.
- Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. JAMA 1996; 275:134–141.
- Ruhnke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Trends in mortality and medical spending in patients hospitalized for community-acquired pneumonia: 1993–2005. Med Care 2010; 48:1111–1116.
- Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis 2004; 39:1642–1650.
- Koivula I, Sten M, Mäkelä PH. Risk factors for pneumonia in the elderly. Am J Med 1994; 96:313–320.
- Ruhnke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Marked reduction in 30-day mortality among elderly patients with community-acquired pneumonia. Am J Med 2011; 124:171–178.
- Farr BM, Bartlett CL, Wadsworth J, Miller DL. Risk factors for community-acquired pneumonia diagnosed upon hospital admission. British Thoracic Society Pneumonia Study Group. Respir Med 2000; 94:954–963.
- Graves CR. Pneumonia in pregnancy. Clin Obstet Gynecol 2010; 53:329–336.
- Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ 2011; 183:310–319.
- Voiriot G, Dury S, Parrot A, Mayaud C, Fartoukh M. Nonsteroidal antiinflammatory drugs may affect the presentation and course of community-acquired pneumonia. Chest 2011; 139:387–394.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Glezen WP, Decker M, Perrotta DM. Survey of underlying conditions of persons hospitalized with acute respiratory disease during influenza epidemics in Houston, 1978–1981. Am Rev Respir Dis 1987; 136:550–555.
- Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232–239.
- Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 2010; 38(suppl 4):e91–e97.
- Apisarnthanarak A, Mundy LM. Etiology of community-acquired pneumonia. Clin Chest Med 2005; 26:47–55.
- de Roux A, Marcos MA, Garcia E, et al. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest 2004; 125:1343–1351.
- Hamelin ME, Côté S, Laforge J, et al. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis 2005; 41:498–502.
- Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–S72.
- Kolling UK, Hansen F, Braun J, Rink L, Katus HA, Dalhoff K. Leucocyte response and anti-inflammatory cytokines in community acquired pneumonia. Thorax 2001; 56:121–125.
- Wunderink RG, Waterer GW. Community-acquired pneumonia: pathophysiology and host factors with focus on possible new approaches to management of lower respiratory tract infections. Infect Dis Clin North Am 2004; 18:743–759.
- Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine 2002; 20:3068–3087.
- Bender BS, Small PA. Influenza: pathogenesis and host defense. Semin Respir Infect 1992; 7:38–45.
- Scheiblauer H, Reinacher M, Tashiro M, Rott R. Interactions between bacteria and influenza A virus in the development of influenza pneumonia. J Infect Dis 1992; 166:783–791.
- McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19:571–582.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Benbassat J, Baumal R. Narrative review: should teaching of the respiratory physical examination be restricted only to signs with proven reliability and validity? J Gen Intern Med 2010; 25:865–872.
- Kolsuz M, Erginel S, Alatas O, et al. Acute phase reactants and cytokine levels in unilateral community-acquired pneumonia. Respiration 2003; 70:615–622.
- Alves DW, Kennedy MT. Community-acquired pneumonia in casualty: etiology, clinical features, diagnosis, and management (or a look at the “new” in pneumonia since 2002). Curr Opin Pulm Med 2004; 10:166–170.
- Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000; 160:3243–3247.
- Bewick T, Myles P, Greenwood S, et al; Influenza Clinical Information Network. Clinical and laboratory features distinguishing pandemic H1N1 influenza-related pneumonia from interpandemic community-acquired pneumonia in adults. Thorax 2011; 66:247–252.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis 2007; 195:1018–1028.
- Starr I. Influenza in 1918: recollections of the epidemic in Philadelphia. 1976. Ann Intern Med 2006; 145:138–140.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg Infect Dis 2008; 14:1193–1199.
- Treanor J. Influenza virus. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, PA: Elsevier/Churchill Livingstone; 2005:2060–2085.
- Jarstrand C, Tunevall G. The influence of bacterial superinfection on the clinical course of influenza. Studies from the influenza epidemics in Stockholm during the winters 1969–70 and 1971–72. Scand J Infect Dis 1975; 7:243–247.
- Schwarzmann SW, Adler JL, Sullivan RJ, Marine WM. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968–1969. Arch Intern Med 1971; 127:1037–1041.
- Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis 2006; 12:894–899.
- Centers for Disease Control and Prevention (CDC). Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006–January 2007. MMWR Morb Mortal Wkly Rep 2007; 56:325–329.
- Hidron AI, Low CE, Honig EG, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis 2009; 9:384–392.
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005; 293:987–997.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Boersma WG, Daniels JM, Löwenberg A, Boeve WJ, van de Jagt EJ. Reliability of radiographic findings and the relation to etiologic agents in community-acquired pneumonia. Respir Med 2006; 100:926–932.
- Brixey AG, Luo Y, Skouras V, Awdankiewicz A, Light RW. The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology 2011; 16:1000–1004.
- Campbell SG, Marrie TJ, Anstey R, Dickinson G, Ackroyd-Stolarz S. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest 2003; 123:1142–1150.
- Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med 2001; 95:78–82.
- Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med 2004; 164:637–644.
- Information & Quality Healthcare. http://www.IQH.org/attachments/219_CoreMHelpBookletpg4_11_3.pdf. Accessed November 14, 2011.
- Rosón B, Carratalà J, Verdaguer R, Dorca J, Manresa F, Gudiol F. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis 2000; 31:869–874.
- García-Vázquez E, Marcos MA, Mensa J, et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med 2004; 164:1807–1811.
- Rosón B, Fernández-Sabé N, Carratalà J, et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis 2004; 38:222–226.
- Sordé R, Falcó V, Lowak M, et al. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Intern Med 2011; 171:166–172.
- Koegelenberg CFN, Diacon AH, Bolliger CT. Parapneumonic pleural effusion and empyema. Respiration 2008; 75:241–250.
- Almirall J, Bolíbar I, Toran P, et al; Community-Acquired Pneumonia Maresme Study Group. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest 2004; 125:1335–1342.
- Ingram PR, Inglis T, Moxon D, Speers D. Procalcitonin and C-reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med 2010; 36:528–532.
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–250.
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–382.
- Chalmers JD, Singanayagam A, Akram AR, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 2010; 65:878–883.
- Loke YK, Kwok CS, Niruban A, Myint PK. Value of severity scales in predicting mortality from community-acquired pneumonia: systematic review and meta-analysis. Thorax 2010; 65:884–890.
- Capelastegui A, España PP, Quintana JM, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J 2006; 27:151–157.
- Charles PG, Wolfe R, Whitby M, et al; Australian Community-Acquired Pneumonia Study Collaboration. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008; 47:375–384.
- España PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med 2006; 174:1249–1256.
- Chalmers JD, Taylor JK, Mandal P, et al. Validation of the Infectious Diseases Society of America/American Thoracic Society minor criteria for intensive care unit admission in community-acquired pneumonia patients without major criteria or contraindications to intensive care unit care. Clin Infect Dis 2011; 53:503–511.
- Majumdar SR, Eurich DT, Gamble JM, Senthilselvan A, Marrie TJ. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: a population-based cohort study. Clin Infect Dis 2011; 52:325–331.
- Nathwani D, Rubinstein E, Barlow G, Davey P. Do guidelines for community-acquired pneumonia improve the cost-effectiveness of hospital care? Clin Infect Dis 2001; 32:728–741.
- Dean NC, Silver MP, Bateman KA, James B, Hadlock CJ, Hale D. Decreased mortality after implementation of a treatment guideline for community-acquired pneumonia. Am J Med 2001; 110:451–457.
- Capelastegui A, España PP, Quintana JM, et al. Improvement of process-of-care and outcomes after implementing a guideline for the management of community-acquired pneumonia: a controlled before-and-after design study. Clin Infect Dis 2004; 39:955–963.
- Silber SH, Garrett C, Singh R, et al. Early administration of antibiotics does not shorten time to clinical stability in patients with moderate-to-severe community-acquired pneumonia. Chest 2003; 124:1798–1804.
- Welker JA, Huston M, McCue JD. Antibiotic timing and errors in diagnosing pneumonia. Arch Intern Med 2008; 168:351–356.
- Polgreen PM, Chen YY, Cavanaugh JE, et al. An outbreak of severe Clostridium difficile-associated disease possibly related to inappropriate antimicrobial therapy for community-acquired pneumonia. Infect Control Hosp Epidemiol 2007; 28:212–214.
- Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med 2001; 161:1837–1842.
- Lodise TP, Kwa A, Cosler L, Gupta R, Smith RP. Comparison of beta-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother 2007; 51:3977–3982.
- Waterer GW, Rello J, Wunderink RG. Management of community-acquired pneumonia in adults. Am J Respir Crit Care Med 2011; 183:157–164.
- Bjerre LM, Verheij TJ, Kochen MM. Antibiotics for community acquired pneumonia in adult outpatients. Cochrane Database Syst Rev 2009; (4):CD002109.
- Frei CR, Labreche MJ, Attridge RT. Fluoroquinolones in community-acquired pneumonia: guide to selection and appropriate use. Drugs 2011; 71:757–770.
- Weiss K, Tillotson GS. The controversy of combination vs monotherapy in the treatment of hospitalized community-acquired pneumonia. Chest 2005; 128:940–946.
- Martínez JA, Horcajada JP, Almela M, et al. Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis 2003; 36:389–395.
- Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med 2001; 161:1837–1842.
- Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med 2001; 161:848–850.
- Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis 2003; 37:752–760.
- el Moussaoui R, de Borgie CA, van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ 2006; 332:1355.
- Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest 2003; 124:883–889.
- Salluh JI, Póvoa P, Soares M, Castro-Faria-Neto HC, Bozza FA, Bozza PT. The role of corticosteroids in severe community-acquired pneumonia: a systematic review. Crit Care 2008; 12:R76.
- Mikami K, Suzuki M, Kitagawa H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung 2007; 185:249–255.
- Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med 2010; 181:975–982.
- Chopra V, Flanders SA. Does statin use improve pneumonia outcomes? Chest 2009; 136:1381–1388.
- Yende S, Milbrandt EB, Kellum JA, et al. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med 2011; 39:1871–1878.
- Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998; 279:1452–1457.
- Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Feagan BG. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin Infect Dis 2000; 31:1362–1367.
- Aliberti S, Peyrani P, Filardo G, et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community-acquired pneumonia. Chest 2011; 140:482–488.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000; 342:681–689.
- Mortensen EM, Kapoor WN, Chang CC, Fine MJ. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis 2003; 37:1617–1624.
- File TM, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010; 122:130–141.
- Marston BJ, Plouffe JF, File TM, et al. Incidence of community-acquired pneumonia requiring hospitalization. Results of a population-based active surveillance study in Ohio. The Community-Based Pneumonia Incidence Study Group. Arch Intern Med 1997; 157:1709–1718.
- Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. JAMA 1996; 275:134–141.
- Ruhnke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Trends in mortality and medical spending in patients hospitalized for community-acquired pneumonia: 1993–2005. Med Care 2010; 48:1111–1116.
- Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis 2004; 39:1642–1650.
- Koivula I, Sten M, Mäkelä PH. Risk factors for pneumonia in the elderly. Am J Med 1994; 96:313–320.
- Ruhnke GW, Coca-Perraillon M, Kitch BT, Cutler DM. Marked reduction in 30-day mortality among elderly patients with community-acquired pneumonia. Am J Med 2011; 124:171–178.
- Farr BM, Bartlett CL, Wadsworth J, Miller DL. Risk factors for community-acquired pneumonia diagnosed upon hospital admission. British Thoracic Society Pneumonia Study Group. Respir Med 2000; 94:954–963.
- Graves CR. Pneumonia in pregnancy. Clin Obstet Gynecol 2010; 53:329–336.
- Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. CMAJ 2011; 183:310–319.
- Voiriot G, Dury S, Parrot A, Mayaud C, Fartoukh M. Nonsteroidal antiinflammatory drugs may affect the presentation and course of community-acquired pneumonia. Chest 2011; 139:387–394.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Glezen WP, Decker M, Perrotta DM. Survey of underlying conditions of persons hospitalized with acute respiratory disease during influenza epidemics in Houston, 1978–1981. Am Rev Respir Dis 1987; 136:550–555.
- Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232–239.
- Rothberg MB, Haessler SD. Complications of seasonal and pandemic influenza. Crit Care Med 2010; 38(suppl 4):e91–e97.
- Apisarnthanarak A, Mundy LM. Etiology of community-acquired pneumonia. Clin Chest Med 2005; 26:47–55.
- de Roux A, Marcos MA, Garcia E, et al. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest 2004; 125:1343–1351.
- Hamelin ME, Côté S, Laforge J, et al. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis 2005; 41:498–502.
- Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–S72.
- Kolling UK, Hansen F, Braun J, Rink L, Katus HA, Dalhoff K. Leucocyte response and anti-inflammatory cytokines in community acquired pneumonia. Thorax 2001; 56:121–125.
- Wunderink RG, Waterer GW. Community-acquired pneumonia: pathophysiology and host factors with focus on possible new approaches to management of lower respiratory tract infections. Infect Dis Clin North Am 2004; 18:743–759.
- Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine 2002; 20:3068–3087.
- Bender BS, Small PA. Influenza: pathogenesis and host defense. Semin Respir Infect 1992; 7:38–45.
- Scheiblauer H, Reinacher M, Tashiro M, Rott R. Interactions between bacteria and influenza A virus in the development of influenza pneumonia. J Infect Dis 1992; 166:783–791.
- McCullers JA. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 2006; 19:571–582.
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community-acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997; 278:1440–1445.
- Benbassat J, Baumal R. Narrative review: should teaching of the respiratory physical examination be restricted only to signs with proven reliability and validity? J Gen Intern Med 2010; 25:865–872.
- Kolsuz M, Erginel S, Alatas O, et al. Acute phase reactants and cytokine levels in unilateral community-acquired pneumonia. Respiration 2003; 70:615–622.
- Alves DW, Kennedy MT. Community-acquired pneumonia in casualty: etiology, clinical features, diagnosis, and management (or a look at the “new” in pneumonia since 2002). Curr Opin Pulm Med 2004; 10:166–170.
- Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000; 160:3243–3247.
- Bewick T, Myles P, Greenwood S, et al; Influenza Clinical Information Network. Clinical and laboratory features distinguishing pandemic H1N1 influenza-related pneumonia from interpandemic community-acquired pneumonia in adults. Thorax 2011; 66:247–252.
- Morens DM, Fauci AS. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis 2007; 195:1018–1028.
- Starr I. Influenza in 1918: recollections of the epidemic in Philadelphia. 1976. Ann Intern Med 2006; 145:138–140.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918–19 influenza pandemic. Emerg Infect Dis 2008; 14:1193–1199.
- Treanor J. Influenza virus. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, PA: Elsevier/Churchill Livingstone; 2005:2060–2085.
- Jarstrand C, Tunevall G. The influence of bacterial superinfection on the clinical course of influenza. Studies from the influenza epidemics in Stockholm during the winters 1969–70 and 1971–72. Scand J Infect Dis 1975; 7:243–247.
- Schwarzmann SW, Adler JL, Sullivan RJ, Marine WM. Bacterial pneumonia during the Hong Kong influenza epidemic of 1968–1969. Arch Intern Med 1971; 127:1037–1041.
- Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis 2006; 12:894–899.
- Centers for Disease Control and Prevention (CDC). Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006–January 2007. MMWR Morb Mortal Wkly Rep 2007; 56:325–329.
- Hidron AI, Low CE, Honig EG, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. Lancet Infect Dis 2009; 9:384–392.
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005; 293:987–997.
- Harper SA, Bradley JS, Englund JA, et al; Expert Panel of the Infectious Diseases Society of America. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1003–1032.
- Boersma WG, Daniels JM, Löwenberg A, Boeve WJ, van de Jagt EJ. Reliability of radiographic findings and the relation to etiologic agents in community-acquired pneumonia. Respir Med 2006; 100:926–932.
- Brixey AG, Luo Y, Skouras V, Awdankiewicz A, Light RW. The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology 2011; 16:1000–1004.
- Campbell SG, Marrie TJ, Anstey R, Dickinson G, Ackroyd-Stolarz S. The contribution of blood cultures to the clinical management of adult patients admitted to the hospital with community-acquired pneumonia: a prospective observational study. Chest 2003; 123:1142–1150.
- Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med 2001; 95:78–82.
- Houck PM, Bratzler DW, Nsa W, Ma A, Bartlett JG. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch Intern Med 2004; 164:637–644.
- Information & Quality Healthcare. http://www.IQH.org/attachments/219_CoreMHelpBookletpg4_11_3.pdf. Accessed November 14, 2011.
- Rosón B, Carratalà J, Verdaguer R, Dorca J, Manresa F, Gudiol F. Prospective study of the usefulness of sputum Gram stain in the initial approach to community-acquired pneumonia requiring hospitalization. Clin Infect Dis 2000; 31:869–874.
- García-Vázquez E, Marcos MA, Mensa J, et al. Assessment of the usefulness of sputum culture for diagnosis of community-acquired pneumonia using the PORT predictive scoring system. Arch Intern Med 2004; 164:1807–1811.
- Rosón B, Fernández-Sabé N, Carratalà J, et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis 2004; 38:222–226.
- Sordé R, Falcó V, Lowak M, et al. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Intern Med 2011; 171:166–172.
- Koegelenberg CFN, Diacon AH, Bolliger CT. Parapneumonic pleural effusion and empyema. Respiration 2008; 75:241–250.
- Almirall J, Bolíbar I, Toran P, et al; Community-Acquired Pneumonia Maresme Study Group. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest 2004; 125:1335–1342.
- Ingram PR, Inglis T, Moxon D, Speers D. Procalcitonin and C-reactive protein in severe 2009 H1N1 influenza infection. Intensive Care Med 2010; 36:528–532.
- Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–250.
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–382.
- Chalmers JD, Singanayagam A, Akram AR, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax 2010; 65:878–883.
- Loke YK, Kwok CS, Niruban A, Myint PK. Value of severity scales in predicting mortality from community-acquired pneumonia: systematic review and meta-analysis. Thorax 2010; 65:884–890.
- Capelastegui A, España PP, Quintana JM, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J 2006; 27:151–157.
- Charles PG, Wolfe R, Whitby M, et al; Australian Community-Acquired Pneumonia Study Collaboration. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis 2008; 47:375–384.
- España PP, Capelastegui A, Gorordo I, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med 2006; 174:1249–1256.
- Chalmers JD, Taylor JK, Mandal P, et al. Validation of the Infectious Diseases Society of America/American Thoracic Society minor criteria for intensive care unit admission in community-acquired pneumonia patients without major criteria or contraindications to intensive care unit care. Clin Infect Dis 2011; 53:503–511.
- Majumdar SR, Eurich DT, Gamble JM, Senthilselvan A, Marrie TJ. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: a population-based cohort study. Clin Infect Dis 2011; 52:325–331.
- Nathwani D, Rubinstein E, Barlow G, Davey P. Do guidelines for community-acquired pneumonia improve the cost-effectiveness of hospital care? Clin Infect Dis 2001; 32:728–741.
- Dean NC, Silver MP, Bateman KA, James B, Hadlock CJ, Hale D. Decreased mortality after implementation of a treatment guideline for community-acquired pneumonia. Am J Med 2001; 110:451–457.
- Capelastegui A, España PP, Quintana JM, et al. Improvement of process-of-care and outcomes after implementing a guideline for the management of community-acquired pneumonia: a controlled before-and-after design study. Clin Infect Dis 2004; 39:955–963.
- Silber SH, Garrett C, Singh R, et al. Early administration of antibiotics does not shorten time to clinical stability in patients with moderate-to-severe community-acquired pneumonia. Chest 2003; 124:1798–1804.
- Welker JA, Huston M, McCue JD. Antibiotic timing and errors in diagnosing pneumonia. Arch Intern Med 2008; 168:351–356.
- Polgreen PM, Chen YY, Cavanaugh JE, et al. An outbreak of severe Clostridium difficile-associated disease possibly related to inappropriate antimicrobial therapy for community-acquired pneumonia. Infect Control Hosp Epidemiol 2007; 28:212–214.
- Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med 2001; 161:1837–1842.
- Lodise TP, Kwa A, Cosler L, Gupta R, Smith RP. Comparison of beta-lactam and macrolide combination therapy versus fluoroquinolone monotherapy in hospitalized Veterans Affairs patients with community-acquired pneumonia. Antimicrob Agents Chemother 2007; 51:3977–3982.
- Waterer GW, Rello J, Wunderink RG. Management of community-acquired pneumonia in adults. Am J Respir Crit Care Med 2011; 183:157–164.
- Bjerre LM, Verheij TJ, Kochen MM. Antibiotics for community acquired pneumonia in adult outpatients. Cochrane Database Syst Rev 2009; (4):CD002109.
- Frei CR, Labreche MJ, Attridge RT. Fluoroquinolones in community-acquired pneumonia: guide to selection and appropriate use. Drugs 2011; 71:757–770.
- Weiss K, Tillotson GS. The controversy of combination vs monotherapy in the treatment of hospitalized community-acquired pneumonia. Chest 2005; 128:940–946.
- Martínez JA, Horcajada JP, Almela M, et al. Addition of a macrolide to a beta-lactam-based empirical antibiotic regimen is associated with lower in-hospital mortality for patients with bacteremic pneumococcal pneumonia. Clin Infect Dis 2003; 36:389–395.
- Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med 2001; 161:1837–1842.
- Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med 2001; 161:848–850.
- Dunbar LM, Wunderink RG, Habib MP, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis 2003; 37:752–760.
- el Moussaoui R, de Borgie CA, van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ 2006; 332:1355.
- Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest 2003; 124:883–889.
- Salluh JI, Póvoa P, Soares M, Castro-Faria-Neto HC, Bozza FA, Bozza PT. The role of corticosteroids in severe community-acquired pneumonia: a systematic review. Crit Care 2008; 12:R76.
- Mikami K, Suzuki M, Kitagawa H, et al. Efficacy of corticosteroids in the treatment of community-acquired pneumonia requiring hospitalization. Lung 2007; 185:249–255.
- Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med 2010; 181:975–982.
- Chopra V, Flanders SA. Does statin use improve pneumonia outcomes? Chest 2009; 136:1381–1388.
- Yende S, Milbrandt EB, Kellum JA, et al. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med 2011; 39:1871–1878.
- Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998; 279:1452–1457.
- Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Feagan BG. Predictors of symptom resolution in patients with community-acquired pneumonia. Clin Infect Dis 2000; 31:1362–1367.
- Aliberti S, Peyrani P, Filardo G, et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community-acquired pneumonia. Chest 2011; 140:482–488.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med 2000; 342:681–689.
KEY POINTS
- Especially during flu season, clinicians should consider influenza in patients with respiratory symptoms.
- The diagnosis of CAP is based primarily on clinical factors: a combination of signs and symptoms such as cough, fever, chills, sputum production, dyspnea, pleuritic pain, tachypnea, tachycardia, hypoxemia, consolidation or rales on auscultation, and a new infiltrate on chest imaging.
- Empiric outpatient treatment of a previously healthy patient with CABP should include either a macrolide or doxycycline. A fluoroquinolone or beta-lactam plus a macrolide should be used for patients with comorbid conditions.
- Several indices have been validated for use in deciding on inpatient vs outpatient treatment and whether a patient with pneumonia should be admitted to an intensive care unit.
Overcoming barriers to hypertension control in African Americans
High blood pressure takes a devastating toll on African Americans. Better control can go a long way to closing the “mortality gap” between African Americans and white Americans. But which strategies are best to address this complex problem?
In this report, we review the evidence on practice-based approaches to improving blood pressure control, from new styles of patient education to home blood pressure monitoring, focusing on studies in African Americans (Table 1).1–11
BETTER CONTROL IS NEEDED
PATIENT-RELATED BARRIERS
Patient-related barriers24–40 include:
- Poor knowledge about hypertension and its consequences31,32
- Poor adherence to drug therapy (a major factor,24–26 as African Americans have poorer adherence rates than whites,27–29 which may explain some of the racial disparity in blood pressure control30)
- False health beliefs34–37
- Inability to change one’s lifestyle
- Side effects of antihypertensive drugs32
- Unrealistic expectations of treatment (eg, a cure33)
- Demographic factors (eg, socioeconomic status, educational level, age, sex).24,38–40
Perhaps the most salient and easily modifiable of these factors are patients’ reluctance to modify their lifestyle and their misconceptions about the causes, treatment, and prevention of hypertension. Patients whose beliefs are discordant with traditional biomedical concepts of hypertension have poorer blood pressure control than those whose beliefs are concordant.41 This may be more relevant to African Americans, since they are known to have cultural health beliefs that differ from those of Western culture (eg, that hypertension is a curable rather than a chronic illness, and that hypertension is a disease of nerves that often affects the blood and clogs the arteries).42
PHYSICIAN-RELATED BARRIERS
Barriers to effective blood pressure control at the physician level43–48 include:
- Nonadherence to treatment guidelines44
- Failure to intensify the regimen if goals are not met45
- Failure to emphasize therapeutic lifestyle changes.43,46–48
When primary care physicians do not follow evidence-based guidelines, the reason may be that they are not aware of them or that they do not understand them. In a national survey of 1,029 physicians that was designed to explore how well physicians know the indications for specific antihypertensive drugs and how closely their opinions and practice agreed with national guidelines, only 37.3% correctly answered all of the knowledge-related questions.49
Other reasons for nonadherence are that physicians may disagree with the guidelines, may not be able to follow the guidelines, may not believe that following them will achieve the desired effect, or may have no motivation to change their practice.50
Whatever the reason, Hyman et al51 reported that as many as 30% of physicians did not recommend treatment for patients with diastolic blood pressures of 90 to 100 mm Hg, and a higher proportion did not treat patients with systolic blood pressures of 140 to 160 mm Hg.
BARRIERS IN HEALTH CARE SYSTEMS
Although health care systems present barriers to optimal blood pressure control,20,27,31,52 there is evidence that most cases of uncontrolled hypertension occur in patients with good access to care.32,53,54 For example, an NHANES study53 suggested that most patients with uncontrolled hypertension had in fact seen a physician on average at least three times in the previous year. And this may be more pervasive in African Americans: one survey found hypertension was uncontrolled in 75% of hypertensive African American patients despite free access to care, free medications, and regular follow-up visits.41
Thus, the most significant barriers to blood pressure control appear to be patient-related and physician-related.
INTERVENTIONS AIMED AT PATIENTS
The most common approaches to improving blood pressure control at the patient level, regardless of race, are patient education,55–61 home blood pressure monitoring,62–67 and behavioral counseling to address misconceptions about hypertension,68 to improve adherence to drug therapy,69–73 and to encourage lifestyle modifications.74–78
Patient education
Patient education can improve blood pressure control.58,79–82 Its aims are to increase patients’ understanding of the disease83 and to encourage them to be more active in their own care.80,84,85
Patient education has a moderate effect on blood pressure control. The average proportion of patients whose hypertension was under control in community-based trials of various interventions ranged from 60% to 70%, compared with 38% to 46% with usual care.56,80,81
However, these strategies largely did not address misconceptions patients have about hypertension. This issue is especially critical in African Americans, who may have different perceptions of hypertension and different expectations for care41: beliefs that hypertension is “curable,” not chronic, and that medication is needed only for hypertension-related symptoms may translate to poorer rates of medication adherence.
Levine et al1 evaluated the efficacy of home visits by trained community health advisory board workers in a neighborhood in Baltimore, MD, with a high prevalence of hypertension. Participants were randomized to receive either one visit or five visits during the 40-month study period. Both groups had a statistically significant reduction in blood pressure, and in both groups the proportion of patients with adequate blood pressure control increased significantly. The results support the use of a practice- and community-based partnership to improve blood pressure control in African American patients.
Ogedegbe et al2 randomized 190 hypertensive African American patients to receive usual care or quarterly counseling sessions that used motivational interviewing focused on medication adherence. The counseled patients stayed adherent to their medications, whereas adherence declined significantly in those receiving usual care. This effect was associated with a modest, nonsignificant trend toward a net reduction in systolic blood pressure with motivational interviewing.
A novel method of health education is the use of narrative communication—ie, storytelling. It has a good amount of evidence to support it, as culturally appropriate storytelling may allow patients to identify with a story as it relates to their own lives.86–89 Examples of educational storytelling include:
- A woman with hypertension discussing what it means to have high blood pressure, and the benefits of controlling it, such as living long enough to see her grandchildren grow up
- A man discussing the importance of involving family and friends to help control blood pressure, and how dietary modifications can be made to ensure that salt alternatives are used when the family does the cooking.
Storytelling should be done in a culturally appropriate context. For example, storytellers should have the same background as the patient (ie, similar socioeconomic status and ethnic background): patients are more likely to be influenced if they identify with the storyteller and imagine themselves in a similar situation.
Houston et al3 randomized 299 hypertensive African Americans to view either three DVDs that featured patients with hypertension or three “attention-control DVDs” on topics not related to hypertension. The intervention group’s DVDs focused on storytelling and “learning more.” In the storytelling section, patients told personal stories about what it meant to have hypertension and gave advice on how to best interact with health care providers and methods to improve medication adherence. A “learning more” section focused on what high blood pressure is, addressed therapeutic lifestyle changes, and encouraged patients to communicate with their health care providers. The patients who viewed the patient narratives had significantly lower blood pressure at 3 months than those assigned to usual care. Although blood pressure subsequently increased in both groups, the benefits of the intervention still existed at the end of follow-up.
Important to note about two of the above three studies1,3 is that the interventions were done by people other than physicians, thus emphasizing the importance of a team approach to blood pressure control.
Behavioral counseling
The effectiveness of lifestyle modifications such as diet, weight loss, and physical activity in preventing and treating hypertension is well established.74–78 For example:
- In the Dietary Approaches to Stop Hypertension (DASH) trial,76 a healthy diet lowered blood pressure about as much as single drugs do, particularly in African Americans.
- The Trial of Nonpharmacologic Interventions in the Elderly (TONE)74 showed that exercise can lower blood pressure in obese hypertensive patients.
- The PREMIER trial (Lifestyle Interventions for Blood Pressure Control)75 showed that a single brief counseling session could produce substantial decreases in blood pressure in patients with stage 1 hypertension or high-normal blood pressure.
Unfortunately, these results have been hard to translate into primary care practice, especially for African American patients. Several studies have evaluated the impact of lifestyle interventions on blood pressure control in primary care practices with a large population of African American patients.
Bosworth et al,4 in a study of a practice in which almost half the patients were African American, randomized patients to receive usual care, nurse-administered tailored behavioral telephone counseling, home blood pressure monitoring, or home monitoring plus tailored behavioral telephone counseling. The combination of home monitoring and tailored behavioral telephone counseling led to a statistically significant improvement at 24 months compared with baseline.
Home blood pressure monitoring
The effectiveness of self-monitoring in improving blood pressure control is also well documented.62,63,65–67,90–95
Pickering et al62 studied patients with poorly controlled hypertension in a managed-care setting and found a reduction of 7 mm Hg systolic and 5 mm Hg diastolic pressure after 3 to 6 months of home monitoring compared with usual care.
Mengden et al,94 in a similar study, found average blood pressure reductions at 6 months of 19.3/11.9 mm Hg in the home-monitoring group vs 10.6/8.8 mm Hg in the usual-care group.
The effect of home blood pressure monitoring may be greater in African Americans.
Rogers et al93 found it to be more effective at lowering blood pressure than usual care in a group of 121 patients with poorly controlled hypertension followed in primary care practices, and these reductions were twice as large in African American patients than in white patients.93
Bondmass,92 in a study of 33 African American patients with poorly controlled hypertension, reported a 53% control rate within 4 weeks of home monitoring. All patients in the study had uncontrolled blood pressure at baseline (> 140/90 mm Hg).
Artinian et al5 evaluated the effect of nurse-managed telemonitoring on blood pressure control vs enhanced usual care. All participants were African American. The monitored group had a significantly greater reduction in systolic pressure at 12 months compared with those who received enhanced usual care.
PHYSICIAN-LEVEL INTERVENTIONS
Most interventions to improve how physicians manage patients with hypertension are designed to improve adherence to treatment guidelines. In most cases, these interventions are based on continuous quality improvement and disease management concepts such as physician education and academic detailing, reminders, feedback on performance measures, and risk-assessment tools.96,97
Physician education
Interest is increasing in physician educational interventions for blood pressure control.24,98
Inui et al,99 in an early study in a primary care practice, found that patients of physicians who received tutorials on hypertension management were more compliant with their drug regimens and had better blood pressure control than patients of physicians in the control group.
Jennett et al,100 in a similar randomized clinical trial, found that physicians who participated in an education activity were more adherent to treatment guidelines at 6 and 12 months compared with those who did not participate.
Maue et al101 showed that rates of blood pressure control improved from 41% to 52% after a 6-month educational intervention for physicians in a managed-care setting.
Tu et al102 reviewed 12 studies in which seven different physician educational interventions were used either alone or in combination and concluded that physician education improves compliance with guidelines for managing hypertension.
Unfortunately, these studies did not report outcomes separately for African American and white patients.
Hicks et al6 found that disease management approaches that target physicians whose patients with hypertension are mostly African American did not yield clinically relevant improvement in these patients, and that minority patients were significantly less likely to have their blood pressure controlled at the end of the study compared with their non-Hispanic white counterparts.
Feedback to providers
Several studies have shown that, given reminders and feedback systems, physicians will change their practice.103–106
Mashru and Lant104 combined chart audits and physician education in primary care practices and found they improved physician performance measures such as accuracy of diagnosis, number of patients who received cardiovascular risk assessment, and number of patients whose treatment was based on clinical laboratory assessments.
Feedback takes many forms but consists mostly of computerized information107 or peer-to-peer academic detailing with opinion leaders.108–110
Dickinson et al,106 for instance, showed that computer-generated listings of patients’ blood pressures combined with a physician education program on clinical management of hypertension led to increased knowledge and better follow-up on their patients.
Again, however, these studies did not distinguish between African American and white patients, which makes it difficult to judge whether or not these approaches work differently for physicians with a large proportion of African American patients.
Computerized decision-support systems
Computerized decision-support systems have proliferated in primary care practices.111
McAlister et al103 found that general practitioners randomized to manage hypertension with the assistance of a computer obtained better outcomes than with usual care.
Montgomery and Fahey,107 in a systematic review, found improved blood pressure control in two of the three trials that compared computer-generated feedback reports and reminders to usual care. Specifically, 51% of patients whose physicians received reminders either had controlled blood pressure or were at least receiving treatment vs 33% in the control group at 12 months. This difference was even higher at 24 months.
Montgomery et al7 later randomized primary care practices to use a computer-based decision-support system and a cardiovascular risk chart, the risk chart alone, or to continue as usual. Results indicated no reduction in cardiovascular risk in the computer-system or the chart-only group, whereas patients in the chart-only group had a significant reduction in systolic pressure and were prescribed more cardiovascular drugs. This study indicates that use of a computerized decision-support system is not superior to chart review and audit feedback alone.
Evidence that computerized decision systems improve blood pressure control in African Americans is scant. However, when one looks at the evidence from studies of African Americans, the outcomes do not seem to differ between African American and white patients.
Hicks et al6 examined the effectiveness of computerized decision support in improving hypertension care in a racially diverse population. Physicians were randomized to receive computerized decision support or to provide usual care without computerized support. Both groups improved significantly in prescribing appropriate drugs but not in overall blood pressure control. Furthermore, the study showed no reduction in racial disparities of care and blood pressure control.
A potential explanation for the lack of improvement in blood pressure was that the intervention dealt with making sure the appropriate drugs were prescribed rather than making sure physicians also appropriately intensified antihypertensive management when necessary.
INTERVENTIONS TARGETING PATIENTS AND PHYSICIANS
Several studies have targeted both patient and physician-level barriers to blood pressure control in practice-based settings.
Roumie et al8 randomized physicians to one of three intervention groups:
- “Provider education” consisting of an email message with a Web-based link to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7)
- Provider education plus a computer alert with information about their patient’s blood pressure
- Provider education, a computer alert, and patient education (ie, patients received a letter encouraging adherence to drug therapy, changing their lifestyle, and talking with their doctor about their blood pressure).
Patients whose providers were randomized to the third group had better blood pressure control. The report did not differentiate African American vs white patients. The data, however, did show the effectiveness of adding patient education to provider education to improve blood pressure control.
Bosworth et al,112 in a study in which 40% of patients were African American, randomized patients to usual care or to bimonthly nurse-delivered behavioral telephone counseling. They also randomized providers either to receive computer-generated decision support designed to improve adherence to guidelines or to receive no support.
There were no significant differences in rates of blood pressure control in the intervention groups compared with a control group. Although differences in blood pressure control between groups were not significant, patients randomized to behavioral intervention had significantly better blood pressure control at the 24-month follow-up than at baseline.
Svetkey et al9 evaluated the effects of physician intervention, patient intervention, and physician intervention plus patient intervention compared with control on systolic blood pressure at 6 months. They found that an intensive behavioral lifestyle intervention led to a significant reduction in systolic pressure at 6 months. By itself, the physician intervention did not have a meaningful effect, but patients in the combined physician-and-patient-intervention group experienced the greatest reduction (9.7 ± 12.7 mm Hg).
It takes a team
Physicians should not be the only focus in helping patients achieve blood pressure control. Although physician and patient factors need to be addressed to improve blood pressure control in African Americans, emphasis should also be placed on interdisciplinary, team-based care utilizing health care providers such as nurses, physician assistants, and pharmacists. Team-based care has been shown to have the greatest impact of all the strategies for improving blood pressure control.113 There is a good amount of evidence involving interventions with a focus on health care providers other than physicians, although the data lack a sufficient focus on African Americans.
Carter et al,10 in a randomized controlled trial in which 26.3% of the patients were African American, found that an intervention consisting of clinical pharmacists giving physicians drug therapy recommendations based on national guidelines resulted in a significantly lower blood pressure compared with a control group: the mean reduction was 20.7/9.7 mm Hg in the intervention group vs 6.8/4.5 mm Hg in the control group.
Carter et al114 performed a meta-analysis of 37 studies and found that two strategies led to a significant reduction in blood pressure: a pharmacist-led intervention with treatment recommendations to physicians resulted in a systolic pressure reduction of 9.30 mm Hg; and nurse-led interventions resulted in a systolic pressure reduction of 4.80 mm Hg. Again, many of the studies cited in this meta-analysis lacked a focus on African Americans.
Hunt et al11 conducted a randomized controlled trial in which pharmacists actively participated in the management of blood pressure. They were involved with every aspect of care, including reviewing medications and adverse drug reactions, assessing lifestyle behaviors and barriers to adherence, making dosing adjustments, and adding medications. Patients randomized to the intervention group achieved significantly lower systolic and diastolic pressures (137/75 vs 143/78 mm Hg in the control group). However, information about race was not included.
The above studies are just a few out of a large body of evidence demonstrating the value of team-based care to improve blood pressure control. It has yet to be determined whether these models can improve blood pressure control specifically in African Americans, since so many of these trials lacked a focus on this group. Promising is an ongoing randomized prospective trial by Carter et al115 evaluating a model of collaboration between physicians and pharmacists, with a focus on patients in underrepresented minorities.
SO WHAT WORKS?
Although there is a growing body of literature on interventions to try to reduce disparities in hypertension and blood pressure control between African Americans and whites, only a few randomized controlled trials have focused on African Americans, and several have not reported their results.116 So the question remains: How should we interpret the available data, which are aggregated across racial groups, and put it into practice when caring for hypertensive African American patients?
Patient education. In trying to overcome patient-related barriers, emphasis should be on patient education, in particular addressing misconceptions about hypertension and promoting adherence to antihypertensive therapy. This is evident from the narrative storytelling intervention by Houston et al.3 Although this is the first study of its kind, this strategy may be something to consider if future studies replicate these findings. Culturally appropriate storytelling may allow patients to identify with the stories as they relate to their own personal lives. It can be an effective way to address patient education and change behaviors.
Self-monitoring with a home blood pressure monitor has also proven effective in the management of hypertension in African Americans. Indeed, the few studies that reported findings in African Americans showed impressive reductions in blood pressure. The benefits of home monitoring are well documented, and the effect on physician-related barriers such as clinical inertia are also quite impressive.117 However, most of these studies did not assess the long-term impact or cost-effectiveness of home monitoring on blood pressure control.
Behavioral counseling. Although we have good evidence of the effectiveness of behavioral counseling, whether this is sustained long-term has been less studied in African Americans. Thus, while interventions that targeted African Americans have reported impressive reductions in blood pressure, the effect tends to be greatest during the first few months of implementation, with the benefits disappearing over time.
Physician-related interventions. With regard to physician-level interventions, research has focused on physician education, utilizing alerts and computerized clinical decision-support systems. Evidence is scant on whether the use of computerized systems results in improves hypertension care in African Americans. However, a closer look at the data from studies that report outcomes in African American and white patients shows that the results do not seem to differ between these groups. Still, there is insufficient information about the impact on hypertensive African Americans.6
Strategies that address both patient- and physician-related barriers can improve overall blood pressure control; however, there is a lack of data comparing outcomes in hypertensive African Americans with those of whites, making it difficult to know if this would be an effective strategy in African American patients alone.
More studies needed that focus on African Americans
Developing interventions to improve blood pressure control in African Americans should be an ongoing priority for research if we intend to address racial disparities in cardiovascular disease. Although it is reassuring that there is a growing body of evidence and research with this focus,118–121 more research is needed to determine effective strategies that address barriers related to physician practice and to the health care system overall as they relate to blood pressure control in African Americans. More importantly, these strategies should also emphasize a team-based approach that includes nurses, pharmacists, and physician assistants. Developing targeted interventions for hypertensive African Americans will help reduce disparities in the rates of cardiovascular illness and death in this patient population.
- Levine DM, Bone LR, Hill MN, et al. The effectiveness of a community/academic health center partnership in decreasing the level of blood pressure in an urban African-American population. Ethn Dis 2003; 13:354–361.
- Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens 2008; 21:1137–1143.
- Houston TK, Allison JJ, Sussman M, et al. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med 2011; 154:77–84.
- Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009; 151:687–695.
- Artinian NT, Flack JM, Nordstrom CK, et al. Effects of nurse-managed telemonitoring on blood pressure at 12-month follow-up among urban African Americans. Nurs Res 2007; 56:312–322.
- Hicks LS, Sequist TD, Ayanian JZ, et al. Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med 2008; 23:429–441.
- Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ 2000; 320:686–690.
- Roumie CL, Elasy TA, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med 2006; 145:165–175.
- Svetkey LP, Pollak KI, Yancy WS, et al. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension 2009; 54:1226–1233.
- Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med 2009; 169:1996–2002.
- Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med 2008; 23:1966–1972.
- US Department of Health and Human Services: Office of Disease Prevention and Health Promotion—Healthy People 2010. Nasnewsletter 2000; 15:3.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- US Centers for Disease Control and Prevention. Age-specific excess deaths associated with stroke among racial/ethnic minority populations–United States, 1997. JAMA 2000; 283:2382–2383.
- Giles WH, Kittner SJ, Hebel JR, Losonczy KG, Sherwin RW. Determinants of black-white differences in the risk of cerebral infarction. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med 1995; 155:1319–1324.
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 1997; 277:1293–1298.
- Pavlik VN, Hyman DJ, Vallbona C, Toronjo C, Louis K. Hypertension awareness and control in an inner-city African-American sample. J Hum Hypertens 1997; 11:277–283.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association. Circulation Feb 1; 123( 4):e18–e209.
- Chobanian AV, Bakris GL, Black HR, et al., Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bone LR, Hill MN, Stallings R, et al. Community health survey in an urban African-American neighborhood: distribution and correlates of elevated blood pressure. Ethn Dis 2000; 10:87–95.
- Weber MA. Strategies for improving blood pressure control. Am J Hypertens 1998; 11:897–899.
- Hill MN, Sutton BS. Barriers to hypertension care and control. Curr Hypertens Rep 2000; 2:445–450.
- Alderman MH. Barriers to blood pressure control. Am J Hypertens 1999; 12:1268–1269.
- Chobanian AV. Control of hypertension—an important national priority. N Engl J Med 2001; 345:534–535.
- Miller NH, Hill M, Kottke T, Ockene IS. The multilevel compliance challenge: recommendations for a call to action. A statement for healthcare professionals. Circulation 1997; 95:1085–1090.
- Sackett DL, Snow JC. The magnitude of compliance and noncompliance. In:Haynes RB, Taylor DW, Sackett DL, eds. Compliance in Health Care. Baltimore, MD: John Hopkins University Press; 1979:11–22.
- Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Correlates of nonadherence to hypertension treatment in an inner-city minority population. Am J Public Health 1992; 82:1607–1612.
- Kirscht JP, Rosenstock IM. Patient adherence to antihypertensive medical regimens. J Community Health. 1977; 3:115–124.
- Hershey JC, Morton BG, Davis JB, Reichgott MJ. Patient compliance with antihypertensive medication. Am J Public Health 1980; 70:1081–1089.
- Bosworth HB, Powers B, Grubber JM, et al. Racial differences in blood pressure control: potential explanatory factors. J Gen Intern Med 2008; 23:692–698.
- Douglas JG, Ferdinand KC, Bakris GL, Sowers JR. Barriers to blood pressure control in African Americans. Overcoming obstacles is challenging, but target goals can be attained. Postgrad Med 2002; 112:51–52,55,59–62passim.
- Knight EL, Bohn RL, Wang PS, Glynn RJ, Mogun H, Avorn J. Predictors of uncontrolled hypertension in ambulatory patients. Hypertension 2001; 38:809–814.
- Ogedegbe G, Mancuso CA, Allegrante JP. Expectations of blood pressure management in hypertensive African-American patients: a qualitative study. J Natl Med Assoc 2004; 96:442–449.
- Blumhagen D. Hypertension: a folk illness with a medical name. Cult Med Psychiatry 1980; 4:197–224.
- Meyer D, Leventhal H, Gutmann M. Common-sense models of illness: the example of hypertension. Health Psychol 1985; 4:115–135.
- Nelson EC, Stason WB, Neutra RR, Solomon HS, McArdle PJ. Impact of patient perceptions on compliance with treatment for hypertension. Med Care 1978; 16:893–906.
- Heurtin-Roberts S. ‘High-pertension’—the uses of a chronic folk illness for personal adaptation. Soc Sci Med 1993; 37:285–294.
- Lang T. Social and economic factors as obstacles to blood pressure control. Am J Hypertens 1998; 11:900–902.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Stamler R, Shipley M, Elliott P, Dyer A, Sans S, Stamler J. Higher blood pressure in adults with less education. Some explanations from INTERSALT. Hypertension 1992; 19:237–241.
- Heurtin-Roberts S, Reisin E. The relation of culturally influenced lay models of hypertension to compliance with treatment. Am J Hypertens 1992; 5:787–792.
- Snow LF. Folk medical beliefs and their implications for care of patients. A review bases on studies among black Americans. Ann Intern Med 1974; 81:82–96.
- Hicks LS, Shaykevich S, Bates DW, Ayanian JZ. Determinants of racial/ethnic differences in blood pressure management among hypertensive patients. BMC Cardiovasc Disord 2005; 5:16.
- Mehta SS, Wilcox CS, Schulman KA. Treatment of hypertension in patients with comorbidities: results from the study of hypertensive prescribing practices (SHyPP). Am J Hypertens 1999; 12:333–340.
- Ballard DJ, Strogatz DS, Wagner EH, et al. Hypertension control in a rural southern community: medical care process and dropping out. Am J Prev Med 1988; 4:133–139.
- Hajjar I, Miller K, Hirth V. Age-related bias in the management of hypertension: a national survey of physicians’ opinions on hypertension in elderly adults. J Gerontol A Biol Sci Med Sci 2002; 57:M487–M491.
- McAlister FA, Laupacis A, Teo KK, Hamilton PG, Montague TJ. A survey of clinician attitudes and management practices in hypertension. J Hum Hypertens 1997; 11:413–419.
- Trilling JS, Froom J. The urgent need to improve hypertension care. Arch Fam Med 2000; 9:794–801.
- Huse DM, Roht LH, Alpert JS, Hartz SC. Physicians’ knowledge, attitudes, and practice of pharmacologic treatment of hypertension. Ann Pharmacother 2001; 35:1173–1179.
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282:1458–1465.
- Hyman DJ, Pavlik VN, Vallbona C. Physician role in lack of awareness and control of hypertension. J Clin Hypertens (Greenwich) 2000; 2:324–330.
- Morley Kotchen J, Walker WE, Kotchen TA. Rationale for a community approach to hypertension control among inner city minority populations. Heart Dis Stroke 1994; 3:61–62.
- Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med 2001; 345:479–486.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Pierce JP, Watson DS, Knights S, Gliddon T, Williams S, Watson R. A controlled trial of health education in the physician’s office. Prev Med 1984; 13:185–194.
- Morisky DE, DeMuth NM, Field-Fass M, Green LW, Levine DM. Evaluation of family health education to build social support for long-term control of high blood pressure. Health Educ Q 1985; 12:35–50.
- Lorgelly P, Siatis I, Brooks A, et al. Is ambulatory blood pressure monitoring cost-effective in the routine surveillance of treated hypertensive patients in primary care? Br J Gen Pract 2003; 53:794–796.
- Green LW, Levine DM, Wolle J, Deeds S. Development of randomized patient education experiments with urban poor hypertensives. Patient Couns Health Educ 1979; 1:106–111.
- Gruesser M, Hartmann P, Schlottmann N, Lohmann FW, Sawicki PT, Joergens V. Structured patient education for out-patients with hypertension in general practice: a model project in Germany. J Hum Hypertens 1997; 11:501–506.
- Mühlhauser I, Sawicki PT, Didjurgeit U, Jörgens V, Trampisch HJ, Berger M. Evaluation of a structured treatment and teaching programme on hypertension in general practice. Clin Exp Hypertens 1993; 15:125–142.
- Roca B, Nadal E, Rovira RE, Valls S, Lapuebla C, Lloría N. Usefulness of a hypertension education program. South Med J 2003; 96:1133–1137.
- Pickering TG, Gerin W, Holland JK. Home blood pressure teletransmission for better diagnosis and treatment. Curr Hypertens Rep 1999; 1:489–494.
- Yarows SA, Julius S, Pickering TG. Home blood pressure monitoring. Arch Intern Med 2000; 160:1251–1257.
- Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet 1976; 1:1265–1268.
- Johnson AL, Taylor DW, Sackett DL, Dunnett CW, Shimizu AG. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J 1978; 119:1034–1039.
- Carnahan JE, Nugent CA. The effects of self-monitoring by patients on the control of hypertension. Am J Med Sci 1975; 269:69–73.
- Stahl SM, Kelley CR, Neill PJ, Grim CE, Mamlin J. Effects of home blood pressure measurement on long-term BP control. Am J Public Health 1984; 74:704–709.
- Boulware LE, Daumit GL, Frick KD, Minkovitz CS, Lawrence RS, Powe NR. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med 2001; 21:221–232.
- Haynes RB, Mattson ME, Engebretson TO. Patient compliance to prescribed antihypertensive medication regimens: a report to the National Heart, Lung, and Blood institute. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, 1980. NIH publication 81-2102.
- Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med 1997; 19:239–263.
- Dunbar-Jacob J, Dwyer K, Dunning EJ. Compliance with antihypertensive regimen: a review of the research in the 1980s. Ann Behav Med 1991; 13:31–39.
- Haynes RB, Montague P, Oliver T, McKibbon KA, Brouwers MC, Kanani R. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev 2000; ( 2):CD000011.
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care 1998; 36:1138–1161.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Appel LJ, Champagne CM, Harsha DW, et al; Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003; 289:2083–2093.
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336:1117–1124.
- Moore TJ, Conlin PR, Ard J, Svetkey LP. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension 2001; 38:155–158.
- Stevens VJ, Obarzanek E, Cook NR, et al; Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134:1–11.
- Sawicki PT, Mühlhauser I, Didjurgeit U, Berger M. Improvement of hypertension care by a structured treatment and teaching programme. J Hum Hypertens 1993; 7:571–573.
- Morisky DE, Bowler MH, Finlay JS. An educational and behavioral approach toward increasing patient activation in hypertension management. J Community Health 1982; 7:171–182.
- Levine DM, Green LW, Deeds SG, Chwalow J, Russell RP, Finlay J. Health education for hypertensive patients. JAMA 1979; 241:1700–1703.
- Iso H, Shimamoto T, Yokota K, Sankai T, Jacobs DR, Komachi Y. Community-based education classes for hypertension control. A 1.5-year randomized controlled trial. Hypertension 1996; 27:968–974.
- Cuspidi C, Sampieri L, Macca G, et al. Improvement of patients’ knowledge by a single educational meeting on hypertension. J Hum Hypertens 2001; 15:57–61.
- Nessman DG, Carnahan JE, Nugent CA. Increasing compliance. Patient-operated hypertension groups. Arch Intern Med 1980; 140:1427–1430.
- Casasanta L, Patel S. Outcomes of an educational component of a disease management program for hypertension. Manag Care Interface 1999; 12:70–73.
- McAdams DP. The Stories We Live By: Personal Myths and the Making of the Self. New York NY: The Guilford Press; 1993.
- Bruner J. Acts of Meaning. Cambridge, MA: Harvard Univ Pr; 1990.
- Slater MD, Rouner D. Entertainment—education and elaboration likelihood: Understanding the processing of narrative persuasion. Commun Theory 2002; 12:173–191.
- Dal CS, Zanna MP, Fong GT. Narrative persuasion and overcoming resistance. In:Knowles ES, Linn J, eds. Resistance and Persuasion. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004:175–191.
- Artinian NT, Washington OG, Templin TN. Effects of home telemonitoring and community-based monitoring on blood pressure control in urban African Americans: a pilot study. Heart Lung 2001; 30:191–199.
- Bailey B, Carney SL, Gillies AA, Smith AJ. Antihypertensive drug treatment: a comparison of usual care with self blood pressure measurement. J Hum Hypertens 1999; 13:147–150.
- Bondmass M. The effect of home monitoring and telemanagement on blood pressure control among African Americans. Telemed J 2000; 6:15–23.
- Rogers MA, Small D, Buchan DA, et al. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med 2001; 134:1024–1032.
- Mengden T, Uen S, Baulmann J, Vetter H. Significance of blood pressure self-measurement as compared with office blood pressure measurement and ambulatory 24-hour blood pressure measurement in pharmacological studies. Blood Press Monit 2003; 8:169–172.
- Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens 1996; 9:285–292.
- Oxman AD, Thomson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995; 153:1423–1431.
- Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract 1998; 48:991–997.
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995; 274:700–705.
- Inui TS, Yourtee EL, Williamson JW. Improved outcomes in hypertension after physician tutorials. A controlled trial. Ann Intern Med 1976; 84:646–651.
- Jennett PA, Wilson TW, Hayton RC, Mainprize GW, Laxdal OE. Desirable behaviours in the office management of hypertension addressed through continuing medical education. Can J Public Health 1989; 80:359–362.
- Maue SK, Rivo ML, Weiss B, Farrelly EW, Brower-Stenger S. Effect of a primary care physician-focused, population-based approach to blood pressure control. Fam Med 2002; 34:508–513.
- Tu K, Davis D. Can we alter physician behavior by educational methods? Lessons learned from studies of the management and follow-up of hypertension. J Contin Educ Health Prof 2002; 22:11–22.
- McAlister NH, Covvey HD, Tong C, Lee A, Wigle ED. Randomised controlled trial of computer assisted management of hypertension in primary care. Br Med J (Clin Res Ed) 1986; 293:670–674.
- Mashru M, Lant A. Interpractice audit of diagnosis and management of hypertension in primary care: educational intervention and review of medical records. BMJ 1997; 314:942–946.
- Degoulet P, Menard J, Berger C, Plouin PF, Devries C, Hirel JC. Hypertension management: the computer as a participant. Am J Med 1980; 68:559–567.
- Dickinson JC, Warshaw GA, Gehlbach SH, Bobula JA, Muhlbaier LH, Parkerson GR. Improving hypertension control: impact of computer feedback and physician education. Med Care 1981; 19:843–854.
- Montgomery AA, Fahey T. A systematic review of the use of computers in the management of hypertension. J Epidemiol Community Health 1998; 52:520–525.
- Coleman MT, Lott JA, Sharma S. Use of continuous quality improvement to identify barriers in the management of hypertension. Am J Med Qual 2000; 15:72–77.
- Goldberg HI, Wagner EH, Fihn SD, et al. A randomized controlled trial of CQI teams and academic detailing: can they alter compliance with guidelines? Jt Comm J Qual Improv 1998; 24:130–142.
- Horowitz CR, Goldberg HI, Martin DP, et al. Conducting a randomized controlled trial of CQI and academic detailing to implement clinical guidelines. Jt Comm J Qual Improv 1996; 22:734–750.
- Johnson B, McNair D, Kailasam K, et al. Discern—an integrated prospective decision support system. Proc Annu Symp Comput Appl Med Care 1994; 969.
- Bosworth HB, Olsen MK, Dudley T, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. Am Heart J 2009; 157:450–456.
- Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care 2006; 44:646–657.
- Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med 2009; 169:1748–1755.
- Carter BL, Clarke W, Ardery G, et al; Collaboration Among Pharmacists Physicians To Improve Outcomes Now (CAPTION) Trial Investigators. A cluster-randomized effectiveness trial of a physician-pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:418–423.
- Einhorn PT. National heart, lung, and blood institute-initiated program “interventions to improve hypertension control rates in African Americans”: background and implementation. Circ Cardiovasc Qual Outcomes 2009; 2:236–240.
- Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011; 57:29–38.
- Bosworth HB, Olsen MK, Neary A, et al. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns 2008; 70:338–347.
- Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans’ study to improve the control of hypertension (V-STITCH): design and methodology. Contemp Clin Trials 2005; 26:155–168.
- Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to Control Hypertension (CAATCH) trial: a multi-level intervention to improve blood pressure control in hypertensive blacks. Circ Cardiovasc Qual Outcomes 2009; 2:249–256.
- Bosworth HB, Almirall D, Weiner BJ, et al. The implementation of a translational study involving a primary care based behavioral program to improve blood pressure control: The HTN-IMPROVE study protocol (01295). Implement Sci 2010; 5:54.
High blood pressure takes a devastating toll on African Americans. Better control can go a long way to closing the “mortality gap” between African Americans and white Americans. But which strategies are best to address this complex problem?
In this report, we review the evidence on practice-based approaches to improving blood pressure control, from new styles of patient education to home blood pressure monitoring, focusing on studies in African Americans (Table 1).1–11
BETTER CONTROL IS NEEDED
PATIENT-RELATED BARRIERS
Patient-related barriers24–40 include:
- Poor knowledge about hypertension and its consequences31,32
- Poor adherence to drug therapy (a major factor,24–26 as African Americans have poorer adherence rates than whites,27–29 which may explain some of the racial disparity in blood pressure control30)
- False health beliefs34–37
- Inability to change one’s lifestyle
- Side effects of antihypertensive drugs32
- Unrealistic expectations of treatment (eg, a cure33)
- Demographic factors (eg, socioeconomic status, educational level, age, sex).24,38–40
Perhaps the most salient and easily modifiable of these factors are patients’ reluctance to modify their lifestyle and their misconceptions about the causes, treatment, and prevention of hypertension. Patients whose beliefs are discordant with traditional biomedical concepts of hypertension have poorer blood pressure control than those whose beliefs are concordant.41 This may be more relevant to African Americans, since they are known to have cultural health beliefs that differ from those of Western culture (eg, that hypertension is a curable rather than a chronic illness, and that hypertension is a disease of nerves that often affects the blood and clogs the arteries).42
PHYSICIAN-RELATED BARRIERS
Barriers to effective blood pressure control at the physician level43–48 include:
- Nonadherence to treatment guidelines44
- Failure to intensify the regimen if goals are not met45
- Failure to emphasize therapeutic lifestyle changes.43,46–48
When primary care physicians do not follow evidence-based guidelines, the reason may be that they are not aware of them or that they do not understand them. In a national survey of 1,029 physicians that was designed to explore how well physicians know the indications for specific antihypertensive drugs and how closely their opinions and practice agreed with national guidelines, only 37.3% correctly answered all of the knowledge-related questions.49
Other reasons for nonadherence are that physicians may disagree with the guidelines, may not be able to follow the guidelines, may not believe that following them will achieve the desired effect, or may have no motivation to change their practice.50
Whatever the reason, Hyman et al51 reported that as many as 30% of physicians did not recommend treatment for patients with diastolic blood pressures of 90 to 100 mm Hg, and a higher proportion did not treat patients with systolic blood pressures of 140 to 160 mm Hg.
BARRIERS IN HEALTH CARE SYSTEMS
Although health care systems present barriers to optimal blood pressure control,20,27,31,52 there is evidence that most cases of uncontrolled hypertension occur in patients with good access to care.32,53,54 For example, an NHANES study53 suggested that most patients with uncontrolled hypertension had in fact seen a physician on average at least three times in the previous year. And this may be more pervasive in African Americans: one survey found hypertension was uncontrolled in 75% of hypertensive African American patients despite free access to care, free medications, and regular follow-up visits.41
Thus, the most significant barriers to blood pressure control appear to be patient-related and physician-related.
INTERVENTIONS AIMED AT PATIENTS
The most common approaches to improving blood pressure control at the patient level, regardless of race, are patient education,55–61 home blood pressure monitoring,62–67 and behavioral counseling to address misconceptions about hypertension,68 to improve adherence to drug therapy,69–73 and to encourage lifestyle modifications.74–78
Patient education
Patient education can improve blood pressure control.58,79–82 Its aims are to increase patients’ understanding of the disease83 and to encourage them to be more active in their own care.80,84,85
Patient education has a moderate effect on blood pressure control. The average proportion of patients whose hypertension was under control in community-based trials of various interventions ranged from 60% to 70%, compared with 38% to 46% with usual care.56,80,81
However, these strategies largely did not address misconceptions patients have about hypertension. This issue is especially critical in African Americans, who may have different perceptions of hypertension and different expectations for care41: beliefs that hypertension is “curable,” not chronic, and that medication is needed only for hypertension-related symptoms may translate to poorer rates of medication adherence.
Levine et al1 evaluated the efficacy of home visits by trained community health advisory board workers in a neighborhood in Baltimore, MD, with a high prevalence of hypertension. Participants were randomized to receive either one visit or five visits during the 40-month study period. Both groups had a statistically significant reduction in blood pressure, and in both groups the proportion of patients with adequate blood pressure control increased significantly. The results support the use of a practice- and community-based partnership to improve blood pressure control in African American patients.
Ogedegbe et al2 randomized 190 hypertensive African American patients to receive usual care or quarterly counseling sessions that used motivational interviewing focused on medication adherence. The counseled patients stayed adherent to their medications, whereas adherence declined significantly in those receiving usual care. This effect was associated with a modest, nonsignificant trend toward a net reduction in systolic blood pressure with motivational interviewing.
A novel method of health education is the use of narrative communication—ie, storytelling. It has a good amount of evidence to support it, as culturally appropriate storytelling may allow patients to identify with a story as it relates to their own lives.86–89 Examples of educational storytelling include:
- A woman with hypertension discussing what it means to have high blood pressure, and the benefits of controlling it, such as living long enough to see her grandchildren grow up
- A man discussing the importance of involving family and friends to help control blood pressure, and how dietary modifications can be made to ensure that salt alternatives are used when the family does the cooking.
Storytelling should be done in a culturally appropriate context. For example, storytellers should have the same background as the patient (ie, similar socioeconomic status and ethnic background): patients are more likely to be influenced if they identify with the storyteller and imagine themselves in a similar situation.
Houston et al3 randomized 299 hypertensive African Americans to view either three DVDs that featured patients with hypertension or three “attention-control DVDs” on topics not related to hypertension. The intervention group’s DVDs focused on storytelling and “learning more.” In the storytelling section, patients told personal stories about what it meant to have hypertension and gave advice on how to best interact with health care providers and methods to improve medication adherence. A “learning more” section focused on what high blood pressure is, addressed therapeutic lifestyle changes, and encouraged patients to communicate with their health care providers. The patients who viewed the patient narratives had significantly lower blood pressure at 3 months than those assigned to usual care. Although blood pressure subsequently increased in both groups, the benefits of the intervention still existed at the end of follow-up.
Important to note about two of the above three studies1,3 is that the interventions were done by people other than physicians, thus emphasizing the importance of a team approach to blood pressure control.
Behavioral counseling
The effectiveness of lifestyle modifications such as diet, weight loss, and physical activity in preventing and treating hypertension is well established.74–78 For example:
- In the Dietary Approaches to Stop Hypertension (DASH) trial,76 a healthy diet lowered blood pressure about as much as single drugs do, particularly in African Americans.
- The Trial of Nonpharmacologic Interventions in the Elderly (TONE)74 showed that exercise can lower blood pressure in obese hypertensive patients.
- The PREMIER trial (Lifestyle Interventions for Blood Pressure Control)75 showed that a single brief counseling session could produce substantial decreases in blood pressure in patients with stage 1 hypertension or high-normal blood pressure.
Unfortunately, these results have been hard to translate into primary care practice, especially for African American patients. Several studies have evaluated the impact of lifestyle interventions on blood pressure control in primary care practices with a large population of African American patients.
Bosworth et al,4 in a study of a practice in which almost half the patients were African American, randomized patients to receive usual care, nurse-administered tailored behavioral telephone counseling, home blood pressure monitoring, or home monitoring plus tailored behavioral telephone counseling. The combination of home monitoring and tailored behavioral telephone counseling led to a statistically significant improvement at 24 months compared with baseline.
Home blood pressure monitoring
The effectiveness of self-monitoring in improving blood pressure control is also well documented.62,63,65–67,90–95
Pickering et al62 studied patients with poorly controlled hypertension in a managed-care setting and found a reduction of 7 mm Hg systolic and 5 mm Hg diastolic pressure after 3 to 6 months of home monitoring compared with usual care.
Mengden et al,94 in a similar study, found average blood pressure reductions at 6 months of 19.3/11.9 mm Hg in the home-monitoring group vs 10.6/8.8 mm Hg in the usual-care group.
The effect of home blood pressure monitoring may be greater in African Americans.
Rogers et al93 found it to be more effective at lowering blood pressure than usual care in a group of 121 patients with poorly controlled hypertension followed in primary care practices, and these reductions were twice as large in African American patients than in white patients.93
Bondmass,92 in a study of 33 African American patients with poorly controlled hypertension, reported a 53% control rate within 4 weeks of home monitoring. All patients in the study had uncontrolled blood pressure at baseline (> 140/90 mm Hg).
Artinian et al5 evaluated the effect of nurse-managed telemonitoring on blood pressure control vs enhanced usual care. All participants were African American. The monitored group had a significantly greater reduction in systolic pressure at 12 months compared with those who received enhanced usual care.
PHYSICIAN-LEVEL INTERVENTIONS
Most interventions to improve how physicians manage patients with hypertension are designed to improve adherence to treatment guidelines. In most cases, these interventions are based on continuous quality improvement and disease management concepts such as physician education and academic detailing, reminders, feedback on performance measures, and risk-assessment tools.96,97
Physician education
Interest is increasing in physician educational interventions for blood pressure control.24,98
Inui et al,99 in an early study in a primary care practice, found that patients of physicians who received tutorials on hypertension management were more compliant with their drug regimens and had better blood pressure control than patients of physicians in the control group.
Jennett et al,100 in a similar randomized clinical trial, found that physicians who participated in an education activity were more adherent to treatment guidelines at 6 and 12 months compared with those who did not participate.
Maue et al101 showed that rates of blood pressure control improved from 41% to 52% after a 6-month educational intervention for physicians in a managed-care setting.
Tu et al102 reviewed 12 studies in which seven different physician educational interventions were used either alone or in combination and concluded that physician education improves compliance with guidelines for managing hypertension.
Unfortunately, these studies did not report outcomes separately for African American and white patients.
Hicks et al6 found that disease management approaches that target physicians whose patients with hypertension are mostly African American did not yield clinically relevant improvement in these patients, and that minority patients were significantly less likely to have their blood pressure controlled at the end of the study compared with their non-Hispanic white counterparts.
Feedback to providers
Several studies have shown that, given reminders and feedback systems, physicians will change their practice.103–106
Mashru and Lant104 combined chart audits and physician education in primary care practices and found they improved physician performance measures such as accuracy of diagnosis, number of patients who received cardiovascular risk assessment, and number of patients whose treatment was based on clinical laboratory assessments.
Feedback takes many forms but consists mostly of computerized information107 or peer-to-peer academic detailing with opinion leaders.108–110
Dickinson et al,106 for instance, showed that computer-generated listings of patients’ blood pressures combined with a physician education program on clinical management of hypertension led to increased knowledge and better follow-up on their patients.
Again, however, these studies did not distinguish between African American and white patients, which makes it difficult to judge whether or not these approaches work differently for physicians with a large proportion of African American patients.
Computerized decision-support systems
Computerized decision-support systems have proliferated in primary care practices.111
McAlister et al103 found that general practitioners randomized to manage hypertension with the assistance of a computer obtained better outcomes than with usual care.
Montgomery and Fahey,107 in a systematic review, found improved blood pressure control in two of the three trials that compared computer-generated feedback reports and reminders to usual care. Specifically, 51% of patients whose physicians received reminders either had controlled blood pressure or were at least receiving treatment vs 33% in the control group at 12 months. This difference was even higher at 24 months.
Montgomery et al7 later randomized primary care practices to use a computer-based decision-support system and a cardiovascular risk chart, the risk chart alone, or to continue as usual. Results indicated no reduction in cardiovascular risk in the computer-system or the chart-only group, whereas patients in the chart-only group had a significant reduction in systolic pressure and were prescribed more cardiovascular drugs. This study indicates that use of a computerized decision-support system is not superior to chart review and audit feedback alone.
Evidence that computerized decision systems improve blood pressure control in African Americans is scant. However, when one looks at the evidence from studies of African Americans, the outcomes do not seem to differ between African American and white patients.
Hicks et al6 examined the effectiveness of computerized decision support in improving hypertension care in a racially diverse population. Physicians were randomized to receive computerized decision support or to provide usual care without computerized support. Both groups improved significantly in prescribing appropriate drugs but not in overall blood pressure control. Furthermore, the study showed no reduction in racial disparities of care and blood pressure control.
A potential explanation for the lack of improvement in blood pressure was that the intervention dealt with making sure the appropriate drugs were prescribed rather than making sure physicians also appropriately intensified antihypertensive management when necessary.
INTERVENTIONS TARGETING PATIENTS AND PHYSICIANS
Several studies have targeted both patient and physician-level barriers to blood pressure control in practice-based settings.
Roumie et al8 randomized physicians to one of three intervention groups:
- “Provider education” consisting of an email message with a Web-based link to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7)
- Provider education plus a computer alert with information about their patient’s blood pressure
- Provider education, a computer alert, and patient education (ie, patients received a letter encouraging adherence to drug therapy, changing their lifestyle, and talking with their doctor about their blood pressure).
Patients whose providers were randomized to the third group had better blood pressure control. The report did not differentiate African American vs white patients. The data, however, did show the effectiveness of adding patient education to provider education to improve blood pressure control.
Bosworth et al,112 in a study in which 40% of patients were African American, randomized patients to usual care or to bimonthly nurse-delivered behavioral telephone counseling. They also randomized providers either to receive computer-generated decision support designed to improve adherence to guidelines or to receive no support.
There were no significant differences in rates of blood pressure control in the intervention groups compared with a control group. Although differences in blood pressure control between groups were not significant, patients randomized to behavioral intervention had significantly better blood pressure control at the 24-month follow-up than at baseline.
Svetkey et al9 evaluated the effects of physician intervention, patient intervention, and physician intervention plus patient intervention compared with control on systolic blood pressure at 6 months. They found that an intensive behavioral lifestyle intervention led to a significant reduction in systolic pressure at 6 months. By itself, the physician intervention did not have a meaningful effect, but patients in the combined physician-and-patient-intervention group experienced the greatest reduction (9.7 ± 12.7 mm Hg).
It takes a team
Physicians should not be the only focus in helping patients achieve blood pressure control. Although physician and patient factors need to be addressed to improve blood pressure control in African Americans, emphasis should also be placed on interdisciplinary, team-based care utilizing health care providers such as nurses, physician assistants, and pharmacists. Team-based care has been shown to have the greatest impact of all the strategies for improving blood pressure control.113 There is a good amount of evidence involving interventions with a focus on health care providers other than physicians, although the data lack a sufficient focus on African Americans.
Carter et al,10 in a randomized controlled trial in which 26.3% of the patients were African American, found that an intervention consisting of clinical pharmacists giving physicians drug therapy recommendations based on national guidelines resulted in a significantly lower blood pressure compared with a control group: the mean reduction was 20.7/9.7 mm Hg in the intervention group vs 6.8/4.5 mm Hg in the control group.
Carter et al114 performed a meta-analysis of 37 studies and found that two strategies led to a significant reduction in blood pressure: a pharmacist-led intervention with treatment recommendations to physicians resulted in a systolic pressure reduction of 9.30 mm Hg; and nurse-led interventions resulted in a systolic pressure reduction of 4.80 mm Hg. Again, many of the studies cited in this meta-analysis lacked a focus on African Americans.
Hunt et al11 conducted a randomized controlled trial in which pharmacists actively participated in the management of blood pressure. They were involved with every aspect of care, including reviewing medications and adverse drug reactions, assessing lifestyle behaviors and barriers to adherence, making dosing adjustments, and adding medications. Patients randomized to the intervention group achieved significantly lower systolic and diastolic pressures (137/75 vs 143/78 mm Hg in the control group). However, information about race was not included.
The above studies are just a few out of a large body of evidence demonstrating the value of team-based care to improve blood pressure control. It has yet to be determined whether these models can improve blood pressure control specifically in African Americans, since so many of these trials lacked a focus on this group. Promising is an ongoing randomized prospective trial by Carter et al115 evaluating a model of collaboration between physicians and pharmacists, with a focus on patients in underrepresented minorities.
SO WHAT WORKS?
Although there is a growing body of literature on interventions to try to reduce disparities in hypertension and blood pressure control between African Americans and whites, only a few randomized controlled trials have focused on African Americans, and several have not reported their results.116 So the question remains: How should we interpret the available data, which are aggregated across racial groups, and put it into practice when caring for hypertensive African American patients?
Patient education. In trying to overcome patient-related barriers, emphasis should be on patient education, in particular addressing misconceptions about hypertension and promoting adherence to antihypertensive therapy. This is evident from the narrative storytelling intervention by Houston et al.3 Although this is the first study of its kind, this strategy may be something to consider if future studies replicate these findings. Culturally appropriate storytelling may allow patients to identify with the stories as they relate to their own personal lives. It can be an effective way to address patient education and change behaviors.
Self-monitoring with a home blood pressure monitor has also proven effective in the management of hypertension in African Americans. Indeed, the few studies that reported findings in African Americans showed impressive reductions in blood pressure. The benefits of home monitoring are well documented, and the effect on physician-related barriers such as clinical inertia are also quite impressive.117 However, most of these studies did not assess the long-term impact or cost-effectiveness of home monitoring on blood pressure control.
Behavioral counseling. Although we have good evidence of the effectiveness of behavioral counseling, whether this is sustained long-term has been less studied in African Americans. Thus, while interventions that targeted African Americans have reported impressive reductions in blood pressure, the effect tends to be greatest during the first few months of implementation, with the benefits disappearing over time.
Physician-related interventions. With regard to physician-level interventions, research has focused on physician education, utilizing alerts and computerized clinical decision-support systems. Evidence is scant on whether the use of computerized systems results in improves hypertension care in African Americans. However, a closer look at the data from studies that report outcomes in African American and white patients shows that the results do not seem to differ between these groups. Still, there is insufficient information about the impact on hypertensive African Americans.6
Strategies that address both patient- and physician-related barriers can improve overall blood pressure control; however, there is a lack of data comparing outcomes in hypertensive African Americans with those of whites, making it difficult to know if this would be an effective strategy in African American patients alone.
More studies needed that focus on African Americans
Developing interventions to improve blood pressure control in African Americans should be an ongoing priority for research if we intend to address racial disparities in cardiovascular disease. Although it is reassuring that there is a growing body of evidence and research with this focus,118–121 more research is needed to determine effective strategies that address barriers related to physician practice and to the health care system overall as they relate to blood pressure control in African Americans. More importantly, these strategies should also emphasize a team-based approach that includes nurses, pharmacists, and physician assistants. Developing targeted interventions for hypertensive African Americans will help reduce disparities in the rates of cardiovascular illness and death in this patient population.
High blood pressure takes a devastating toll on African Americans. Better control can go a long way to closing the “mortality gap” between African Americans and white Americans. But which strategies are best to address this complex problem?
In this report, we review the evidence on practice-based approaches to improving blood pressure control, from new styles of patient education to home blood pressure monitoring, focusing on studies in African Americans (Table 1).1–11
BETTER CONTROL IS NEEDED
PATIENT-RELATED BARRIERS
Patient-related barriers24–40 include:
- Poor knowledge about hypertension and its consequences31,32
- Poor adherence to drug therapy (a major factor,24–26 as African Americans have poorer adherence rates than whites,27–29 which may explain some of the racial disparity in blood pressure control30)
- False health beliefs34–37
- Inability to change one’s lifestyle
- Side effects of antihypertensive drugs32
- Unrealistic expectations of treatment (eg, a cure33)
- Demographic factors (eg, socioeconomic status, educational level, age, sex).24,38–40
Perhaps the most salient and easily modifiable of these factors are patients’ reluctance to modify their lifestyle and their misconceptions about the causes, treatment, and prevention of hypertension. Patients whose beliefs are discordant with traditional biomedical concepts of hypertension have poorer blood pressure control than those whose beliefs are concordant.41 This may be more relevant to African Americans, since they are known to have cultural health beliefs that differ from those of Western culture (eg, that hypertension is a curable rather than a chronic illness, and that hypertension is a disease of nerves that often affects the blood and clogs the arteries).42
PHYSICIAN-RELATED BARRIERS
Barriers to effective blood pressure control at the physician level43–48 include:
- Nonadherence to treatment guidelines44
- Failure to intensify the regimen if goals are not met45
- Failure to emphasize therapeutic lifestyle changes.43,46–48
When primary care physicians do not follow evidence-based guidelines, the reason may be that they are not aware of them or that they do not understand them. In a national survey of 1,029 physicians that was designed to explore how well physicians know the indications for specific antihypertensive drugs and how closely their opinions and practice agreed with national guidelines, only 37.3% correctly answered all of the knowledge-related questions.49
Other reasons for nonadherence are that physicians may disagree with the guidelines, may not be able to follow the guidelines, may not believe that following them will achieve the desired effect, or may have no motivation to change their practice.50
Whatever the reason, Hyman et al51 reported that as many as 30% of physicians did not recommend treatment for patients with diastolic blood pressures of 90 to 100 mm Hg, and a higher proportion did not treat patients with systolic blood pressures of 140 to 160 mm Hg.
BARRIERS IN HEALTH CARE SYSTEMS
Although health care systems present barriers to optimal blood pressure control,20,27,31,52 there is evidence that most cases of uncontrolled hypertension occur in patients with good access to care.32,53,54 For example, an NHANES study53 suggested that most patients with uncontrolled hypertension had in fact seen a physician on average at least three times in the previous year. And this may be more pervasive in African Americans: one survey found hypertension was uncontrolled in 75% of hypertensive African American patients despite free access to care, free medications, and regular follow-up visits.41
Thus, the most significant barriers to blood pressure control appear to be patient-related and physician-related.
INTERVENTIONS AIMED AT PATIENTS
The most common approaches to improving blood pressure control at the patient level, regardless of race, are patient education,55–61 home blood pressure monitoring,62–67 and behavioral counseling to address misconceptions about hypertension,68 to improve adherence to drug therapy,69–73 and to encourage lifestyle modifications.74–78
Patient education
Patient education can improve blood pressure control.58,79–82 Its aims are to increase patients’ understanding of the disease83 and to encourage them to be more active in their own care.80,84,85
Patient education has a moderate effect on blood pressure control. The average proportion of patients whose hypertension was under control in community-based trials of various interventions ranged from 60% to 70%, compared with 38% to 46% with usual care.56,80,81
However, these strategies largely did not address misconceptions patients have about hypertension. This issue is especially critical in African Americans, who may have different perceptions of hypertension and different expectations for care41: beliefs that hypertension is “curable,” not chronic, and that medication is needed only for hypertension-related symptoms may translate to poorer rates of medication adherence.
Levine et al1 evaluated the efficacy of home visits by trained community health advisory board workers in a neighborhood in Baltimore, MD, with a high prevalence of hypertension. Participants were randomized to receive either one visit or five visits during the 40-month study period. Both groups had a statistically significant reduction in blood pressure, and in both groups the proportion of patients with adequate blood pressure control increased significantly. The results support the use of a practice- and community-based partnership to improve blood pressure control in African American patients.
Ogedegbe et al2 randomized 190 hypertensive African American patients to receive usual care or quarterly counseling sessions that used motivational interviewing focused on medication adherence. The counseled patients stayed adherent to their medications, whereas adherence declined significantly in those receiving usual care. This effect was associated with a modest, nonsignificant trend toward a net reduction in systolic blood pressure with motivational interviewing.
A novel method of health education is the use of narrative communication—ie, storytelling. It has a good amount of evidence to support it, as culturally appropriate storytelling may allow patients to identify with a story as it relates to their own lives.86–89 Examples of educational storytelling include:
- A woman with hypertension discussing what it means to have high blood pressure, and the benefits of controlling it, such as living long enough to see her grandchildren grow up
- A man discussing the importance of involving family and friends to help control blood pressure, and how dietary modifications can be made to ensure that salt alternatives are used when the family does the cooking.
Storytelling should be done in a culturally appropriate context. For example, storytellers should have the same background as the patient (ie, similar socioeconomic status and ethnic background): patients are more likely to be influenced if they identify with the storyteller and imagine themselves in a similar situation.
Houston et al3 randomized 299 hypertensive African Americans to view either three DVDs that featured patients with hypertension or three “attention-control DVDs” on topics not related to hypertension. The intervention group’s DVDs focused on storytelling and “learning more.” In the storytelling section, patients told personal stories about what it meant to have hypertension and gave advice on how to best interact with health care providers and methods to improve medication adherence. A “learning more” section focused on what high blood pressure is, addressed therapeutic lifestyle changes, and encouraged patients to communicate with their health care providers. The patients who viewed the patient narratives had significantly lower blood pressure at 3 months than those assigned to usual care. Although blood pressure subsequently increased in both groups, the benefits of the intervention still existed at the end of follow-up.
Important to note about two of the above three studies1,3 is that the interventions were done by people other than physicians, thus emphasizing the importance of a team approach to blood pressure control.
Behavioral counseling
The effectiveness of lifestyle modifications such as diet, weight loss, and physical activity in preventing and treating hypertension is well established.74–78 For example:
- In the Dietary Approaches to Stop Hypertension (DASH) trial,76 a healthy diet lowered blood pressure about as much as single drugs do, particularly in African Americans.
- The Trial of Nonpharmacologic Interventions in the Elderly (TONE)74 showed that exercise can lower blood pressure in obese hypertensive patients.
- The PREMIER trial (Lifestyle Interventions for Blood Pressure Control)75 showed that a single brief counseling session could produce substantial decreases in blood pressure in patients with stage 1 hypertension or high-normal blood pressure.
Unfortunately, these results have been hard to translate into primary care practice, especially for African American patients. Several studies have evaluated the impact of lifestyle interventions on blood pressure control in primary care practices with a large population of African American patients.
Bosworth et al,4 in a study of a practice in which almost half the patients were African American, randomized patients to receive usual care, nurse-administered tailored behavioral telephone counseling, home blood pressure monitoring, or home monitoring plus tailored behavioral telephone counseling. The combination of home monitoring and tailored behavioral telephone counseling led to a statistically significant improvement at 24 months compared with baseline.
Home blood pressure monitoring
The effectiveness of self-monitoring in improving blood pressure control is also well documented.62,63,65–67,90–95
Pickering et al62 studied patients with poorly controlled hypertension in a managed-care setting and found a reduction of 7 mm Hg systolic and 5 mm Hg diastolic pressure after 3 to 6 months of home monitoring compared with usual care.
Mengden et al,94 in a similar study, found average blood pressure reductions at 6 months of 19.3/11.9 mm Hg in the home-monitoring group vs 10.6/8.8 mm Hg in the usual-care group.
The effect of home blood pressure monitoring may be greater in African Americans.
Rogers et al93 found it to be more effective at lowering blood pressure than usual care in a group of 121 patients with poorly controlled hypertension followed in primary care practices, and these reductions were twice as large in African American patients than in white patients.93
Bondmass,92 in a study of 33 African American patients with poorly controlled hypertension, reported a 53% control rate within 4 weeks of home monitoring. All patients in the study had uncontrolled blood pressure at baseline (> 140/90 mm Hg).
Artinian et al5 evaluated the effect of nurse-managed telemonitoring on blood pressure control vs enhanced usual care. All participants were African American. The monitored group had a significantly greater reduction in systolic pressure at 12 months compared with those who received enhanced usual care.
PHYSICIAN-LEVEL INTERVENTIONS
Most interventions to improve how physicians manage patients with hypertension are designed to improve adherence to treatment guidelines. In most cases, these interventions are based on continuous quality improvement and disease management concepts such as physician education and academic detailing, reminders, feedback on performance measures, and risk-assessment tools.96,97
Physician education
Interest is increasing in physician educational interventions for blood pressure control.24,98
Inui et al,99 in an early study in a primary care practice, found that patients of physicians who received tutorials on hypertension management were more compliant with their drug regimens and had better blood pressure control than patients of physicians in the control group.
Jennett et al,100 in a similar randomized clinical trial, found that physicians who participated in an education activity were more adherent to treatment guidelines at 6 and 12 months compared with those who did not participate.
Maue et al101 showed that rates of blood pressure control improved from 41% to 52% after a 6-month educational intervention for physicians in a managed-care setting.
Tu et al102 reviewed 12 studies in which seven different physician educational interventions were used either alone or in combination and concluded that physician education improves compliance with guidelines for managing hypertension.
Unfortunately, these studies did not report outcomes separately for African American and white patients.
Hicks et al6 found that disease management approaches that target physicians whose patients with hypertension are mostly African American did not yield clinically relevant improvement in these patients, and that minority patients were significantly less likely to have their blood pressure controlled at the end of the study compared with their non-Hispanic white counterparts.
Feedback to providers
Several studies have shown that, given reminders and feedback systems, physicians will change their practice.103–106
Mashru and Lant104 combined chart audits and physician education in primary care practices and found they improved physician performance measures such as accuracy of diagnosis, number of patients who received cardiovascular risk assessment, and number of patients whose treatment was based on clinical laboratory assessments.
Feedback takes many forms but consists mostly of computerized information107 or peer-to-peer academic detailing with opinion leaders.108–110
Dickinson et al,106 for instance, showed that computer-generated listings of patients’ blood pressures combined with a physician education program on clinical management of hypertension led to increased knowledge and better follow-up on their patients.
Again, however, these studies did not distinguish between African American and white patients, which makes it difficult to judge whether or not these approaches work differently for physicians with a large proportion of African American patients.
Computerized decision-support systems
Computerized decision-support systems have proliferated in primary care practices.111
McAlister et al103 found that general practitioners randomized to manage hypertension with the assistance of a computer obtained better outcomes than with usual care.
Montgomery and Fahey,107 in a systematic review, found improved blood pressure control in two of the three trials that compared computer-generated feedback reports and reminders to usual care. Specifically, 51% of patients whose physicians received reminders either had controlled blood pressure or were at least receiving treatment vs 33% in the control group at 12 months. This difference was even higher at 24 months.
Montgomery et al7 later randomized primary care practices to use a computer-based decision-support system and a cardiovascular risk chart, the risk chart alone, or to continue as usual. Results indicated no reduction in cardiovascular risk in the computer-system or the chart-only group, whereas patients in the chart-only group had a significant reduction in systolic pressure and were prescribed more cardiovascular drugs. This study indicates that use of a computerized decision-support system is not superior to chart review and audit feedback alone.
Evidence that computerized decision systems improve blood pressure control in African Americans is scant. However, when one looks at the evidence from studies of African Americans, the outcomes do not seem to differ between African American and white patients.
Hicks et al6 examined the effectiveness of computerized decision support in improving hypertension care in a racially diverse population. Physicians were randomized to receive computerized decision support or to provide usual care without computerized support. Both groups improved significantly in prescribing appropriate drugs but not in overall blood pressure control. Furthermore, the study showed no reduction in racial disparities of care and blood pressure control.
A potential explanation for the lack of improvement in blood pressure was that the intervention dealt with making sure the appropriate drugs were prescribed rather than making sure physicians also appropriately intensified antihypertensive management when necessary.
INTERVENTIONS TARGETING PATIENTS AND PHYSICIANS
Several studies have targeted both patient and physician-level barriers to blood pressure control in practice-based settings.
Roumie et al8 randomized physicians to one of three intervention groups:
- “Provider education” consisting of an email message with a Web-based link to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7)
- Provider education plus a computer alert with information about their patient’s blood pressure
- Provider education, a computer alert, and patient education (ie, patients received a letter encouraging adherence to drug therapy, changing their lifestyle, and talking with their doctor about their blood pressure).
Patients whose providers were randomized to the third group had better blood pressure control. The report did not differentiate African American vs white patients. The data, however, did show the effectiveness of adding patient education to provider education to improve blood pressure control.
Bosworth et al,112 in a study in which 40% of patients were African American, randomized patients to usual care or to bimonthly nurse-delivered behavioral telephone counseling. They also randomized providers either to receive computer-generated decision support designed to improve adherence to guidelines or to receive no support.
There were no significant differences in rates of blood pressure control in the intervention groups compared with a control group. Although differences in blood pressure control between groups were not significant, patients randomized to behavioral intervention had significantly better blood pressure control at the 24-month follow-up than at baseline.
Svetkey et al9 evaluated the effects of physician intervention, patient intervention, and physician intervention plus patient intervention compared with control on systolic blood pressure at 6 months. They found that an intensive behavioral lifestyle intervention led to a significant reduction in systolic pressure at 6 months. By itself, the physician intervention did not have a meaningful effect, but patients in the combined physician-and-patient-intervention group experienced the greatest reduction (9.7 ± 12.7 mm Hg).
It takes a team
Physicians should not be the only focus in helping patients achieve blood pressure control. Although physician and patient factors need to be addressed to improve blood pressure control in African Americans, emphasis should also be placed on interdisciplinary, team-based care utilizing health care providers such as nurses, physician assistants, and pharmacists. Team-based care has been shown to have the greatest impact of all the strategies for improving blood pressure control.113 There is a good amount of evidence involving interventions with a focus on health care providers other than physicians, although the data lack a sufficient focus on African Americans.
Carter et al,10 in a randomized controlled trial in which 26.3% of the patients were African American, found that an intervention consisting of clinical pharmacists giving physicians drug therapy recommendations based on national guidelines resulted in a significantly lower blood pressure compared with a control group: the mean reduction was 20.7/9.7 mm Hg in the intervention group vs 6.8/4.5 mm Hg in the control group.
Carter et al114 performed a meta-analysis of 37 studies and found that two strategies led to a significant reduction in blood pressure: a pharmacist-led intervention with treatment recommendations to physicians resulted in a systolic pressure reduction of 9.30 mm Hg; and nurse-led interventions resulted in a systolic pressure reduction of 4.80 mm Hg. Again, many of the studies cited in this meta-analysis lacked a focus on African Americans.
Hunt et al11 conducted a randomized controlled trial in which pharmacists actively participated in the management of blood pressure. They were involved with every aspect of care, including reviewing medications and adverse drug reactions, assessing lifestyle behaviors and barriers to adherence, making dosing adjustments, and adding medications. Patients randomized to the intervention group achieved significantly lower systolic and diastolic pressures (137/75 vs 143/78 mm Hg in the control group). However, information about race was not included.
The above studies are just a few out of a large body of evidence demonstrating the value of team-based care to improve blood pressure control. It has yet to be determined whether these models can improve blood pressure control specifically in African Americans, since so many of these trials lacked a focus on this group. Promising is an ongoing randomized prospective trial by Carter et al115 evaluating a model of collaboration between physicians and pharmacists, with a focus on patients in underrepresented minorities.
SO WHAT WORKS?
Although there is a growing body of literature on interventions to try to reduce disparities in hypertension and blood pressure control between African Americans and whites, only a few randomized controlled trials have focused on African Americans, and several have not reported their results.116 So the question remains: How should we interpret the available data, which are aggregated across racial groups, and put it into practice when caring for hypertensive African American patients?
Patient education. In trying to overcome patient-related barriers, emphasis should be on patient education, in particular addressing misconceptions about hypertension and promoting adherence to antihypertensive therapy. This is evident from the narrative storytelling intervention by Houston et al.3 Although this is the first study of its kind, this strategy may be something to consider if future studies replicate these findings. Culturally appropriate storytelling may allow patients to identify with the stories as they relate to their own personal lives. It can be an effective way to address patient education and change behaviors.
Self-monitoring with a home blood pressure monitor has also proven effective in the management of hypertension in African Americans. Indeed, the few studies that reported findings in African Americans showed impressive reductions in blood pressure. The benefits of home monitoring are well documented, and the effect on physician-related barriers such as clinical inertia are also quite impressive.117 However, most of these studies did not assess the long-term impact or cost-effectiveness of home monitoring on blood pressure control.
Behavioral counseling. Although we have good evidence of the effectiveness of behavioral counseling, whether this is sustained long-term has been less studied in African Americans. Thus, while interventions that targeted African Americans have reported impressive reductions in blood pressure, the effect tends to be greatest during the first few months of implementation, with the benefits disappearing over time.
Physician-related interventions. With regard to physician-level interventions, research has focused on physician education, utilizing alerts and computerized clinical decision-support systems. Evidence is scant on whether the use of computerized systems results in improves hypertension care in African Americans. However, a closer look at the data from studies that report outcomes in African American and white patients shows that the results do not seem to differ between these groups. Still, there is insufficient information about the impact on hypertensive African Americans.6
Strategies that address both patient- and physician-related barriers can improve overall blood pressure control; however, there is a lack of data comparing outcomes in hypertensive African Americans with those of whites, making it difficult to know if this would be an effective strategy in African American patients alone.
More studies needed that focus on African Americans
Developing interventions to improve blood pressure control in African Americans should be an ongoing priority for research if we intend to address racial disparities in cardiovascular disease. Although it is reassuring that there is a growing body of evidence and research with this focus,118–121 more research is needed to determine effective strategies that address barriers related to physician practice and to the health care system overall as they relate to blood pressure control in African Americans. More importantly, these strategies should also emphasize a team-based approach that includes nurses, pharmacists, and physician assistants. Developing targeted interventions for hypertensive African Americans will help reduce disparities in the rates of cardiovascular illness and death in this patient population.
- Levine DM, Bone LR, Hill MN, et al. The effectiveness of a community/academic health center partnership in decreasing the level of blood pressure in an urban African-American population. Ethn Dis 2003; 13:354–361.
- Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens 2008; 21:1137–1143.
- Houston TK, Allison JJ, Sussman M, et al. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med 2011; 154:77–84.
- Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009; 151:687–695.
- Artinian NT, Flack JM, Nordstrom CK, et al. Effects of nurse-managed telemonitoring on blood pressure at 12-month follow-up among urban African Americans. Nurs Res 2007; 56:312–322.
- Hicks LS, Sequist TD, Ayanian JZ, et al. Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med 2008; 23:429–441.
- Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ 2000; 320:686–690.
- Roumie CL, Elasy TA, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med 2006; 145:165–175.
- Svetkey LP, Pollak KI, Yancy WS, et al. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension 2009; 54:1226–1233.
- Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med 2009; 169:1996–2002.
- Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med 2008; 23:1966–1972.
- US Department of Health and Human Services: Office of Disease Prevention and Health Promotion—Healthy People 2010. Nasnewsletter 2000; 15:3.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- US Centers for Disease Control and Prevention. Age-specific excess deaths associated with stroke among racial/ethnic minority populations–United States, 1997. JAMA 2000; 283:2382–2383.
- Giles WH, Kittner SJ, Hebel JR, Losonczy KG, Sherwin RW. Determinants of black-white differences in the risk of cerebral infarction. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med 1995; 155:1319–1324.
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 1997; 277:1293–1298.
- Pavlik VN, Hyman DJ, Vallbona C, Toronjo C, Louis K. Hypertension awareness and control in an inner-city African-American sample. J Hum Hypertens 1997; 11:277–283.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association. Circulation Feb 1; 123( 4):e18–e209.
- Chobanian AV, Bakris GL, Black HR, et al., Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bone LR, Hill MN, Stallings R, et al. Community health survey in an urban African-American neighborhood: distribution and correlates of elevated blood pressure. Ethn Dis 2000; 10:87–95.
- Weber MA. Strategies for improving blood pressure control. Am J Hypertens 1998; 11:897–899.
- Hill MN, Sutton BS. Barriers to hypertension care and control. Curr Hypertens Rep 2000; 2:445–450.
- Alderman MH. Barriers to blood pressure control. Am J Hypertens 1999; 12:1268–1269.
- Chobanian AV. Control of hypertension—an important national priority. N Engl J Med 2001; 345:534–535.
- Miller NH, Hill M, Kottke T, Ockene IS. The multilevel compliance challenge: recommendations for a call to action. A statement for healthcare professionals. Circulation 1997; 95:1085–1090.
- Sackett DL, Snow JC. The magnitude of compliance and noncompliance. In:Haynes RB, Taylor DW, Sackett DL, eds. Compliance in Health Care. Baltimore, MD: John Hopkins University Press; 1979:11–22.
- Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Correlates of nonadherence to hypertension treatment in an inner-city minority population. Am J Public Health 1992; 82:1607–1612.
- Kirscht JP, Rosenstock IM. Patient adherence to antihypertensive medical regimens. J Community Health. 1977; 3:115–124.
- Hershey JC, Morton BG, Davis JB, Reichgott MJ. Patient compliance with antihypertensive medication. Am J Public Health 1980; 70:1081–1089.
- Bosworth HB, Powers B, Grubber JM, et al. Racial differences in blood pressure control: potential explanatory factors. J Gen Intern Med 2008; 23:692–698.
- Douglas JG, Ferdinand KC, Bakris GL, Sowers JR. Barriers to blood pressure control in African Americans. Overcoming obstacles is challenging, but target goals can be attained. Postgrad Med 2002; 112:51–52,55,59–62passim.
- Knight EL, Bohn RL, Wang PS, Glynn RJ, Mogun H, Avorn J. Predictors of uncontrolled hypertension in ambulatory patients. Hypertension 2001; 38:809–814.
- Ogedegbe G, Mancuso CA, Allegrante JP. Expectations of blood pressure management in hypertensive African-American patients: a qualitative study. J Natl Med Assoc 2004; 96:442–449.
- Blumhagen D. Hypertension: a folk illness with a medical name. Cult Med Psychiatry 1980; 4:197–224.
- Meyer D, Leventhal H, Gutmann M. Common-sense models of illness: the example of hypertension. Health Psychol 1985; 4:115–135.
- Nelson EC, Stason WB, Neutra RR, Solomon HS, McArdle PJ. Impact of patient perceptions on compliance with treatment for hypertension. Med Care 1978; 16:893–906.
- Heurtin-Roberts S. ‘High-pertension’—the uses of a chronic folk illness for personal adaptation. Soc Sci Med 1993; 37:285–294.
- Lang T. Social and economic factors as obstacles to blood pressure control. Am J Hypertens 1998; 11:900–902.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Stamler R, Shipley M, Elliott P, Dyer A, Sans S, Stamler J. Higher blood pressure in adults with less education. Some explanations from INTERSALT. Hypertension 1992; 19:237–241.
- Heurtin-Roberts S, Reisin E. The relation of culturally influenced lay models of hypertension to compliance with treatment. Am J Hypertens 1992; 5:787–792.
- Snow LF. Folk medical beliefs and their implications for care of patients. A review bases on studies among black Americans. Ann Intern Med 1974; 81:82–96.
- Hicks LS, Shaykevich S, Bates DW, Ayanian JZ. Determinants of racial/ethnic differences in blood pressure management among hypertensive patients. BMC Cardiovasc Disord 2005; 5:16.
- Mehta SS, Wilcox CS, Schulman KA. Treatment of hypertension in patients with comorbidities: results from the study of hypertensive prescribing practices (SHyPP). Am J Hypertens 1999; 12:333–340.
- Ballard DJ, Strogatz DS, Wagner EH, et al. Hypertension control in a rural southern community: medical care process and dropping out. Am J Prev Med 1988; 4:133–139.
- Hajjar I, Miller K, Hirth V. Age-related bias in the management of hypertension: a national survey of physicians’ opinions on hypertension in elderly adults. J Gerontol A Biol Sci Med Sci 2002; 57:M487–M491.
- McAlister FA, Laupacis A, Teo KK, Hamilton PG, Montague TJ. A survey of clinician attitudes and management practices in hypertension. J Hum Hypertens 1997; 11:413–419.
- Trilling JS, Froom J. The urgent need to improve hypertension care. Arch Fam Med 2000; 9:794–801.
- Huse DM, Roht LH, Alpert JS, Hartz SC. Physicians’ knowledge, attitudes, and practice of pharmacologic treatment of hypertension. Ann Pharmacother 2001; 35:1173–1179.
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282:1458–1465.
- Hyman DJ, Pavlik VN, Vallbona C. Physician role in lack of awareness and control of hypertension. J Clin Hypertens (Greenwich) 2000; 2:324–330.
- Morley Kotchen J, Walker WE, Kotchen TA. Rationale for a community approach to hypertension control among inner city minority populations. Heart Dis Stroke 1994; 3:61–62.
- Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med 2001; 345:479–486.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Pierce JP, Watson DS, Knights S, Gliddon T, Williams S, Watson R. A controlled trial of health education in the physician’s office. Prev Med 1984; 13:185–194.
- Morisky DE, DeMuth NM, Field-Fass M, Green LW, Levine DM. Evaluation of family health education to build social support for long-term control of high blood pressure. Health Educ Q 1985; 12:35–50.
- Lorgelly P, Siatis I, Brooks A, et al. Is ambulatory blood pressure monitoring cost-effective in the routine surveillance of treated hypertensive patients in primary care? Br J Gen Pract 2003; 53:794–796.
- Green LW, Levine DM, Wolle J, Deeds S. Development of randomized patient education experiments with urban poor hypertensives. Patient Couns Health Educ 1979; 1:106–111.
- Gruesser M, Hartmann P, Schlottmann N, Lohmann FW, Sawicki PT, Joergens V. Structured patient education for out-patients with hypertension in general practice: a model project in Germany. J Hum Hypertens 1997; 11:501–506.
- Mühlhauser I, Sawicki PT, Didjurgeit U, Jörgens V, Trampisch HJ, Berger M. Evaluation of a structured treatment and teaching programme on hypertension in general practice. Clin Exp Hypertens 1993; 15:125–142.
- Roca B, Nadal E, Rovira RE, Valls S, Lapuebla C, Lloría N. Usefulness of a hypertension education program. South Med J 2003; 96:1133–1137.
- Pickering TG, Gerin W, Holland JK. Home blood pressure teletransmission for better diagnosis and treatment. Curr Hypertens Rep 1999; 1:489–494.
- Yarows SA, Julius S, Pickering TG. Home blood pressure monitoring. Arch Intern Med 2000; 160:1251–1257.
- Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet 1976; 1:1265–1268.
- Johnson AL, Taylor DW, Sackett DL, Dunnett CW, Shimizu AG. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J 1978; 119:1034–1039.
- Carnahan JE, Nugent CA. The effects of self-monitoring by patients on the control of hypertension. Am J Med Sci 1975; 269:69–73.
- Stahl SM, Kelley CR, Neill PJ, Grim CE, Mamlin J. Effects of home blood pressure measurement on long-term BP control. Am J Public Health 1984; 74:704–709.
- Boulware LE, Daumit GL, Frick KD, Minkovitz CS, Lawrence RS, Powe NR. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med 2001; 21:221–232.
- Haynes RB, Mattson ME, Engebretson TO. Patient compliance to prescribed antihypertensive medication regimens: a report to the National Heart, Lung, and Blood institute. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, 1980. NIH publication 81-2102.
- Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med 1997; 19:239–263.
- Dunbar-Jacob J, Dwyer K, Dunning EJ. Compliance with antihypertensive regimen: a review of the research in the 1980s. Ann Behav Med 1991; 13:31–39.
- Haynes RB, Montague P, Oliver T, McKibbon KA, Brouwers MC, Kanani R. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev 2000; ( 2):CD000011.
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care 1998; 36:1138–1161.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Appel LJ, Champagne CM, Harsha DW, et al; Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003; 289:2083–2093.
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336:1117–1124.
- Moore TJ, Conlin PR, Ard J, Svetkey LP. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension 2001; 38:155–158.
- Stevens VJ, Obarzanek E, Cook NR, et al; Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134:1–11.
- Sawicki PT, Mühlhauser I, Didjurgeit U, Berger M. Improvement of hypertension care by a structured treatment and teaching programme. J Hum Hypertens 1993; 7:571–573.
- Morisky DE, Bowler MH, Finlay JS. An educational and behavioral approach toward increasing patient activation in hypertension management. J Community Health 1982; 7:171–182.
- Levine DM, Green LW, Deeds SG, Chwalow J, Russell RP, Finlay J. Health education for hypertensive patients. JAMA 1979; 241:1700–1703.
- Iso H, Shimamoto T, Yokota K, Sankai T, Jacobs DR, Komachi Y. Community-based education classes for hypertension control. A 1.5-year randomized controlled trial. Hypertension 1996; 27:968–974.
- Cuspidi C, Sampieri L, Macca G, et al. Improvement of patients’ knowledge by a single educational meeting on hypertension. J Hum Hypertens 2001; 15:57–61.
- Nessman DG, Carnahan JE, Nugent CA. Increasing compliance. Patient-operated hypertension groups. Arch Intern Med 1980; 140:1427–1430.
- Casasanta L, Patel S. Outcomes of an educational component of a disease management program for hypertension. Manag Care Interface 1999; 12:70–73.
- McAdams DP. The Stories We Live By: Personal Myths and the Making of the Self. New York NY: The Guilford Press; 1993.
- Bruner J. Acts of Meaning. Cambridge, MA: Harvard Univ Pr; 1990.
- Slater MD, Rouner D. Entertainment—education and elaboration likelihood: Understanding the processing of narrative persuasion. Commun Theory 2002; 12:173–191.
- Dal CS, Zanna MP, Fong GT. Narrative persuasion and overcoming resistance. In:Knowles ES, Linn J, eds. Resistance and Persuasion. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004:175–191.
- Artinian NT, Washington OG, Templin TN. Effects of home telemonitoring and community-based monitoring on blood pressure control in urban African Americans: a pilot study. Heart Lung 2001; 30:191–199.
- Bailey B, Carney SL, Gillies AA, Smith AJ. Antihypertensive drug treatment: a comparison of usual care with self blood pressure measurement. J Hum Hypertens 1999; 13:147–150.
- Bondmass M. The effect of home monitoring and telemanagement on blood pressure control among African Americans. Telemed J 2000; 6:15–23.
- Rogers MA, Small D, Buchan DA, et al. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med 2001; 134:1024–1032.
- Mengden T, Uen S, Baulmann J, Vetter H. Significance of blood pressure self-measurement as compared with office blood pressure measurement and ambulatory 24-hour blood pressure measurement in pharmacological studies. Blood Press Monit 2003; 8:169–172.
- Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens 1996; 9:285–292.
- Oxman AD, Thomson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995; 153:1423–1431.
- Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract 1998; 48:991–997.
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995; 274:700–705.
- Inui TS, Yourtee EL, Williamson JW. Improved outcomes in hypertension after physician tutorials. A controlled trial. Ann Intern Med 1976; 84:646–651.
- Jennett PA, Wilson TW, Hayton RC, Mainprize GW, Laxdal OE. Desirable behaviours in the office management of hypertension addressed through continuing medical education. Can J Public Health 1989; 80:359–362.
- Maue SK, Rivo ML, Weiss B, Farrelly EW, Brower-Stenger S. Effect of a primary care physician-focused, population-based approach to blood pressure control. Fam Med 2002; 34:508–513.
- Tu K, Davis D. Can we alter physician behavior by educational methods? Lessons learned from studies of the management and follow-up of hypertension. J Contin Educ Health Prof 2002; 22:11–22.
- McAlister NH, Covvey HD, Tong C, Lee A, Wigle ED. Randomised controlled trial of computer assisted management of hypertension in primary care. Br Med J (Clin Res Ed) 1986; 293:670–674.
- Mashru M, Lant A. Interpractice audit of diagnosis and management of hypertension in primary care: educational intervention and review of medical records. BMJ 1997; 314:942–946.
- Degoulet P, Menard J, Berger C, Plouin PF, Devries C, Hirel JC. Hypertension management: the computer as a participant. Am J Med 1980; 68:559–567.
- Dickinson JC, Warshaw GA, Gehlbach SH, Bobula JA, Muhlbaier LH, Parkerson GR. Improving hypertension control: impact of computer feedback and physician education. Med Care 1981; 19:843–854.
- Montgomery AA, Fahey T. A systematic review of the use of computers in the management of hypertension. J Epidemiol Community Health 1998; 52:520–525.
- Coleman MT, Lott JA, Sharma S. Use of continuous quality improvement to identify barriers in the management of hypertension. Am J Med Qual 2000; 15:72–77.
- Goldberg HI, Wagner EH, Fihn SD, et al. A randomized controlled trial of CQI teams and academic detailing: can they alter compliance with guidelines? Jt Comm J Qual Improv 1998; 24:130–142.
- Horowitz CR, Goldberg HI, Martin DP, et al. Conducting a randomized controlled trial of CQI and academic detailing to implement clinical guidelines. Jt Comm J Qual Improv 1996; 22:734–750.
- Johnson B, McNair D, Kailasam K, et al. Discern—an integrated prospective decision support system. Proc Annu Symp Comput Appl Med Care 1994; 969.
- Bosworth HB, Olsen MK, Dudley T, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. Am Heart J 2009; 157:450–456.
- Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care 2006; 44:646–657.
- Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med 2009; 169:1748–1755.
- Carter BL, Clarke W, Ardery G, et al; Collaboration Among Pharmacists Physicians To Improve Outcomes Now (CAPTION) Trial Investigators. A cluster-randomized effectiveness trial of a physician-pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:418–423.
- Einhorn PT. National heart, lung, and blood institute-initiated program “interventions to improve hypertension control rates in African Americans”: background and implementation. Circ Cardiovasc Qual Outcomes 2009; 2:236–240.
- Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011; 57:29–38.
- Bosworth HB, Olsen MK, Neary A, et al. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns 2008; 70:338–347.
- Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans’ study to improve the control of hypertension (V-STITCH): design and methodology. Contemp Clin Trials 2005; 26:155–168.
- Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to Control Hypertension (CAATCH) trial: a multi-level intervention to improve blood pressure control in hypertensive blacks. Circ Cardiovasc Qual Outcomes 2009; 2:249–256.
- Bosworth HB, Almirall D, Weiner BJ, et al. The implementation of a translational study involving a primary care based behavioral program to improve blood pressure control: The HTN-IMPROVE study protocol (01295). Implement Sci 2010; 5:54.
- Levine DM, Bone LR, Hill MN, et al. The effectiveness of a community/academic health center partnership in decreasing the level of blood pressure in an urban African-American population. Ethn Dis 2003; 13:354–361.
- Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens 2008; 21:1137–1143.
- Houston TK, Allison JJ, Sussman M, et al. Culturally appropriate storytelling to improve blood pressure: a randomized trial. Ann Intern Med 2011; 154:77–84.
- Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009; 151:687–695.
- Artinian NT, Flack JM, Nordstrom CK, et al. Effects of nurse-managed telemonitoring on blood pressure at 12-month follow-up among urban African Americans. Nurs Res 2007; 56:312–322.
- Hicks LS, Sequist TD, Ayanian JZ, et al. Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med 2008; 23:429–441.
- Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ 2000; 320:686–690.
- Roumie CL, Elasy TA, Greevy R, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med 2006; 145:165–175.
- Svetkey LP, Pollak KI, Yancy WS, et al. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension 2009; 54:1226–1233.
- Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med 2009; 169:1996–2002.
- Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med 2008; 23:1966–1972.
- US Department of Health and Human Services: Office of Disease Prevention and Health Promotion—Healthy People 2010. Nasnewsletter 2000; 15:3.
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010; 303:2043–2050.
- US Centers for Disease Control and Prevention. Age-specific excess deaths associated with stroke among racial/ethnic minority populations–United States, 1997. JAMA 2000; 283:2382–2383.
- Giles WH, Kittner SJ, Hebel JR, Losonczy KG, Sherwin RW. Determinants of black-white differences in the risk of cerebral infarction. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med 1995; 155:1319–1324.
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 1997; 277:1293–1298.
- Pavlik VN, Hyman DJ, Vallbona C, Toronjo C, Louis K. Hypertension awareness and control in an inner-city African-American sample. J Hum Hypertens 1997; 11:277–283.
- Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics—2011 Update: A Report From the American Heart Association. Circulation Feb 1; 123( 4):e18–e209.
- Chobanian AV, Bakris GL, Black HR, et al., Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bone LR, Hill MN, Stallings R, et al. Community health survey in an urban African-American neighborhood: distribution and correlates of elevated blood pressure. Ethn Dis 2000; 10:87–95.
- Weber MA. Strategies for improving blood pressure control. Am J Hypertens 1998; 11:897–899.
- Hill MN, Sutton BS. Barriers to hypertension care and control. Curr Hypertens Rep 2000; 2:445–450.
- Alderman MH. Barriers to blood pressure control. Am J Hypertens 1999; 12:1268–1269.
- Chobanian AV. Control of hypertension—an important national priority. N Engl J Med 2001; 345:534–535.
- Miller NH, Hill M, Kottke T, Ockene IS. The multilevel compliance challenge: recommendations for a call to action. A statement for healthcare professionals. Circulation 1997; 95:1085–1090.
- Sackett DL, Snow JC. The magnitude of compliance and noncompliance. In:Haynes RB, Taylor DW, Sackett DL, eds. Compliance in Health Care. Baltimore, MD: John Hopkins University Press; 1979:11–22.
- Shea S, Misra D, Ehrlich MH, Field L, Francis CK. Correlates of nonadherence to hypertension treatment in an inner-city minority population. Am J Public Health 1992; 82:1607–1612.
- Kirscht JP, Rosenstock IM. Patient adherence to antihypertensive medical regimens. J Community Health. 1977; 3:115–124.
- Hershey JC, Morton BG, Davis JB, Reichgott MJ. Patient compliance with antihypertensive medication. Am J Public Health 1980; 70:1081–1089.
- Bosworth HB, Powers B, Grubber JM, et al. Racial differences in blood pressure control: potential explanatory factors. J Gen Intern Med 2008; 23:692–698.
- Douglas JG, Ferdinand KC, Bakris GL, Sowers JR. Barriers to blood pressure control in African Americans. Overcoming obstacles is challenging, but target goals can be attained. Postgrad Med 2002; 112:51–52,55,59–62passim.
- Knight EL, Bohn RL, Wang PS, Glynn RJ, Mogun H, Avorn J. Predictors of uncontrolled hypertension in ambulatory patients. Hypertension 2001; 38:809–814.
- Ogedegbe G, Mancuso CA, Allegrante JP. Expectations of blood pressure management in hypertensive African-American patients: a qualitative study. J Natl Med Assoc 2004; 96:442–449.
- Blumhagen D. Hypertension: a folk illness with a medical name. Cult Med Psychiatry 1980; 4:197–224.
- Meyer D, Leventhal H, Gutmann M. Common-sense models of illness: the example of hypertension. Health Psychol 1985; 4:115–135.
- Nelson EC, Stason WB, Neutra RR, Solomon HS, McArdle PJ. Impact of patient perceptions on compliance with treatment for hypertension. Med Care 1978; 16:893–906.
- Heurtin-Roberts S. ‘High-pertension’—the uses of a chronic folk illness for personal adaptation. Soc Sci Med 1993; 37:285–294.
- Lang T. Social and economic factors as obstacles to blood pressure control. Am J Hypertens 1998; 11:900–902.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Stamler R, Shipley M, Elliott P, Dyer A, Sans S, Stamler J. Higher blood pressure in adults with less education. Some explanations from INTERSALT. Hypertension 1992; 19:237–241.
- Heurtin-Roberts S, Reisin E. The relation of culturally influenced lay models of hypertension to compliance with treatment. Am J Hypertens 1992; 5:787–792.
- Snow LF. Folk medical beliefs and their implications for care of patients. A review bases on studies among black Americans. Ann Intern Med 1974; 81:82–96.
- Hicks LS, Shaykevich S, Bates DW, Ayanian JZ. Determinants of racial/ethnic differences in blood pressure management among hypertensive patients. BMC Cardiovasc Disord 2005; 5:16.
- Mehta SS, Wilcox CS, Schulman KA. Treatment of hypertension in patients with comorbidities: results from the study of hypertensive prescribing practices (SHyPP). Am J Hypertens 1999; 12:333–340.
- Ballard DJ, Strogatz DS, Wagner EH, et al. Hypertension control in a rural southern community: medical care process and dropping out. Am J Prev Med 1988; 4:133–139.
- Hajjar I, Miller K, Hirth V. Age-related bias in the management of hypertension: a national survey of physicians’ opinions on hypertension in elderly adults. J Gerontol A Biol Sci Med Sci 2002; 57:M487–M491.
- McAlister FA, Laupacis A, Teo KK, Hamilton PG, Montague TJ. A survey of clinician attitudes and management practices in hypertension. J Hum Hypertens 1997; 11:413–419.
- Trilling JS, Froom J. The urgent need to improve hypertension care. Arch Fam Med 2000; 9:794–801.
- Huse DM, Roht LH, Alpert JS, Hartz SC. Physicians’ knowledge, attitudes, and practice of pharmacologic treatment of hypertension. Ann Pharmacother 2001; 35:1173–1179.
- Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282:1458–1465.
- Hyman DJ, Pavlik VN, Vallbona C. Physician role in lack of awareness and control of hypertension. J Clin Hypertens (Greenwich) 2000; 2:324–330.
- Morley Kotchen J, Walker WE, Kotchen TA. Rationale for a community approach to hypertension control among inner city minority populations. Heart Dis Stroke 1994; 3:61–62.
- Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med 2001; 345:479–486.
- Lang T. Factors that appear as obstacles to the control of high blood pressure. Ethn Dis 2000; 10:125–130.
- Pierce JP, Watson DS, Knights S, Gliddon T, Williams S, Watson R. A controlled trial of health education in the physician’s office. Prev Med 1984; 13:185–194.
- Morisky DE, DeMuth NM, Field-Fass M, Green LW, Levine DM. Evaluation of family health education to build social support for long-term control of high blood pressure. Health Educ Q 1985; 12:35–50.
- Lorgelly P, Siatis I, Brooks A, et al. Is ambulatory blood pressure monitoring cost-effective in the routine surveillance of treated hypertensive patients in primary care? Br J Gen Pract 2003; 53:794–796.
- Green LW, Levine DM, Wolle J, Deeds S. Development of randomized patient education experiments with urban poor hypertensives. Patient Couns Health Educ 1979; 1:106–111.
- Gruesser M, Hartmann P, Schlottmann N, Lohmann FW, Sawicki PT, Joergens V. Structured patient education for out-patients with hypertension in general practice: a model project in Germany. J Hum Hypertens 1997; 11:501–506.
- Mühlhauser I, Sawicki PT, Didjurgeit U, Jörgens V, Trampisch HJ, Berger M. Evaluation of a structured treatment and teaching programme on hypertension in general practice. Clin Exp Hypertens 1993; 15:125–142.
- Roca B, Nadal E, Rovira RE, Valls S, Lapuebla C, Lloría N. Usefulness of a hypertension education program. South Med J 2003; 96:1133–1137.
- Pickering TG, Gerin W, Holland JK. Home blood pressure teletransmission for better diagnosis and treatment. Curr Hypertens Rep 1999; 1:489–494.
- Yarows SA, Julius S, Pickering TG. Home blood pressure monitoring. Arch Intern Med 2000; 160:1251–1257.
- Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet 1976; 1:1265–1268.
- Johnson AL, Taylor DW, Sackett DL, Dunnett CW, Shimizu AG. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J 1978; 119:1034–1039.
- Carnahan JE, Nugent CA. The effects of self-monitoring by patients on the control of hypertension. Am J Med Sci 1975; 269:69–73.
- Stahl SM, Kelley CR, Neill PJ, Grim CE, Mamlin J. Effects of home blood pressure measurement on long-term BP control. Am J Public Health 1984; 74:704–709.
- Boulware LE, Daumit GL, Frick KD, Minkovitz CS, Lawrence RS, Powe NR. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med 2001; 21:221–232.
- Haynes RB, Mattson ME, Engebretson TO. Patient compliance to prescribed antihypertensive medication regimens: a report to the National Heart, Lung, and Blood institute. Bethesda, MD: US Department of Health and Human Services, Public Health Service, National Institutes of Health, 1980. NIH publication 81-2102.
- Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med 1997; 19:239–263.
- Dunbar-Jacob J, Dwyer K, Dunning EJ. Compliance with antihypertensive regimen: a review of the research in the 1980s. Ann Behav Med 1991; 13:31–39.
- Haynes RB, Montague P, Oliver T, McKibbon KA, Brouwers MC, Kanani R. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev 2000; ( 2):CD000011.
- Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care 1998; 36:1138–1161.
- Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693.
- Appel LJ, Champagne CM, Harsha DW, et al; Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA 2003; 289:2083–2093.
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336:1117–1124.
- Moore TJ, Conlin PR, Ard J, Svetkey LP. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension 2001; 38:155–158.
- Stevens VJ, Obarzanek E, Cook NR, et al; Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134:1–11.
- Sawicki PT, Mühlhauser I, Didjurgeit U, Berger M. Improvement of hypertension care by a structured treatment and teaching programme. J Hum Hypertens 1993; 7:571–573.
- Morisky DE, Bowler MH, Finlay JS. An educational and behavioral approach toward increasing patient activation in hypertension management. J Community Health 1982; 7:171–182.
- Levine DM, Green LW, Deeds SG, Chwalow J, Russell RP, Finlay J. Health education for hypertensive patients. JAMA 1979; 241:1700–1703.
- Iso H, Shimamoto T, Yokota K, Sankai T, Jacobs DR, Komachi Y. Community-based education classes for hypertension control. A 1.5-year randomized controlled trial. Hypertension 1996; 27:968–974.
- Cuspidi C, Sampieri L, Macca G, et al. Improvement of patients’ knowledge by a single educational meeting on hypertension. J Hum Hypertens 2001; 15:57–61.
- Nessman DG, Carnahan JE, Nugent CA. Increasing compliance. Patient-operated hypertension groups. Arch Intern Med 1980; 140:1427–1430.
- Casasanta L, Patel S. Outcomes of an educational component of a disease management program for hypertension. Manag Care Interface 1999; 12:70–73.
- McAdams DP. The Stories We Live By: Personal Myths and the Making of the Self. New York NY: The Guilford Press; 1993.
- Bruner J. Acts of Meaning. Cambridge, MA: Harvard Univ Pr; 1990.
- Slater MD, Rouner D. Entertainment—education and elaboration likelihood: Understanding the processing of narrative persuasion. Commun Theory 2002; 12:173–191.
- Dal CS, Zanna MP, Fong GT. Narrative persuasion and overcoming resistance. In:Knowles ES, Linn J, eds. Resistance and Persuasion. Mahwah, NJ: Lawrence Erlbaum Assoc; 2004:175–191.
- Artinian NT, Washington OG, Templin TN. Effects of home telemonitoring and community-based monitoring on blood pressure control in urban African Americans: a pilot study. Heart Lung 2001; 30:191–199.
- Bailey B, Carney SL, Gillies AA, Smith AJ. Antihypertensive drug treatment: a comparison of usual care with self blood pressure measurement. J Hum Hypertens 1999; 13:147–150.
- Bondmass M. The effect of home monitoring and telemanagement on blood pressure control among African Americans. Telemed J 2000; 6:15–23.
- Rogers MA, Small D, Buchan DA, et al. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med 2001; 134:1024–1032.
- Mengden T, Uen S, Baulmann J, Vetter H. Significance of blood pressure self-measurement as compared with office blood pressure measurement and ambulatory 24-hour blood pressure measurement in pharmacological studies. Blood Press Monit 2003; 8:169–172.
- Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens 1996; 9:285–292.
- Oxman AD, Thomson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995; 153:1423–1431.
- Wensing M, van der Weijden T, Grol R. Implementing guidelines and innovations in general practice: which interventions are effective? Br J Gen Pract 1998; 48:991–997.
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995; 274:700–705.
- Inui TS, Yourtee EL, Williamson JW. Improved outcomes in hypertension after physician tutorials. A controlled trial. Ann Intern Med 1976; 84:646–651.
- Jennett PA, Wilson TW, Hayton RC, Mainprize GW, Laxdal OE. Desirable behaviours in the office management of hypertension addressed through continuing medical education. Can J Public Health 1989; 80:359–362.
- Maue SK, Rivo ML, Weiss B, Farrelly EW, Brower-Stenger S. Effect of a primary care physician-focused, population-based approach to blood pressure control. Fam Med 2002; 34:508–513.
- Tu K, Davis D. Can we alter physician behavior by educational methods? Lessons learned from studies of the management and follow-up of hypertension. J Contin Educ Health Prof 2002; 22:11–22.
- McAlister NH, Covvey HD, Tong C, Lee A, Wigle ED. Randomised controlled trial of computer assisted management of hypertension in primary care. Br Med J (Clin Res Ed) 1986; 293:670–674.
- Mashru M, Lant A. Interpractice audit of diagnosis and management of hypertension in primary care: educational intervention and review of medical records. BMJ 1997; 314:942–946.
- Degoulet P, Menard J, Berger C, Plouin PF, Devries C, Hirel JC. Hypertension management: the computer as a participant. Am J Med 1980; 68:559–567.
- Dickinson JC, Warshaw GA, Gehlbach SH, Bobula JA, Muhlbaier LH, Parkerson GR. Improving hypertension control: impact of computer feedback and physician education. Med Care 1981; 19:843–854.
- Montgomery AA, Fahey T. A systematic review of the use of computers in the management of hypertension. J Epidemiol Community Health 1998; 52:520–525.
- Coleman MT, Lott JA, Sharma S. Use of continuous quality improvement to identify barriers in the management of hypertension. Am J Med Qual 2000; 15:72–77.
- Goldberg HI, Wagner EH, Fihn SD, et al. A randomized controlled trial of CQI teams and academic detailing: can they alter compliance with guidelines? Jt Comm J Qual Improv 1998; 24:130–142.
- Horowitz CR, Goldberg HI, Martin DP, et al. Conducting a randomized controlled trial of CQI and academic detailing to implement clinical guidelines. Jt Comm J Qual Improv 1996; 22:734–750.
- Johnson B, McNair D, Kailasam K, et al. Discern—an integrated prospective decision support system. Proc Annu Symp Comput Appl Med Care 1994; 969.
- Bosworth HB, Olsen MK, Dudley T, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. Am Heart J 2009; 157:450–456.
- Walsh JM, McDonald KM, Shojania KG, et al. Quality improvement strategies for hypertension management: a systematic review. Med Care 2006; 44:646–657.
- Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med 2009; 169:1748–1755.
- Carter BL, Clarke W, Ardery G, et al; Collaboration Among Pharmacists Physicians To Improve Outcomes Now (CAPTION) Trial Investigators. A cluster-randomized effectiveness trial of a physician-pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:418–423.
- Einhorn PT. National heart, lung, and blood institute-initiated program “interventions to improve hypertension control rates in African Americans”: background and implementation. Circ Cardiovasc Qual Outcomes 2009; 2:236–240.
- Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension 2011; 57:29–38.
- Bosworth HB, Olsen MK, Neary A, et al. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns 2008; 70:338–347.
- Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans’ study to improve the control of hypertension (V-STITCH): design and methodology. Contemp Clin Trials 2005; 26:155–168.
- Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to Control Hypertension (CAATCH) trial: a multi-level intervention to improve blood pressure control in hypertensive blacks. Circ Cardiovasc Qual Outcomes 2009; 2:249–256.
- Bosworth HB, Almirall D, Weiner BJ, et al. The implementation of a translational study involving a primary care based behavioral program to improve blood pressure control: The HTN-IMPROVE study protocol (01295). Implement Sci 2010; 5:54.
KEY POINTS
- Rates of cardiovascular disease and related death are disparately high in African Americans.
- Ways to improve how physicians manage blood pressure in this patient population may include chart audit with feedback, a computerized clinical decision-support system, and keeping up-to-date with treatment guidelines. However, more data are needed to determine the effectiveness of these interventions.
- A novel method of health education is the use of narrative communication—ie, storytelling. Culturally appropriate storytelling may allow patients to identify with a story as it relates to their own lives.
- A team-based approach to blood pressure control that involves nurses, pharmacists, and physician assistants should be emphasized, even though studies that have shown positive results did not focus specifically on African Americans.
Is niacin ineffective? Or did AIM-HIGH miss its target?
The recent publication of the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes)1 has thrown the use of niacin as a lipid-modifying therapy into question. The trial was stopped early because an interim analysis found that the patients who took extended-release niacin had no clinical benefit. In addition, it found a trend toward more ischemic strokes, though this finding was later found not to be statistically significant.
Complicating the interpretation, while both the treatment group and the control group in the study received statin therapy, the researchers attempted to keep low-density lipoprotein cholesterol (LDL-C) levels equal, meaning that patients in the control group received more intensive statin therapy than those in the treatment group. And the placebo that the control patients received was actually a low dose of niacin, to induce flushing and thus to blind study participants and their physicians to which drug they were taking.
In the article that follows, I will explore the background, design, findings, and implications of this key trial and try to untangle the many questions about how to interpret it.
LOWERING LDL-C REDUCES RISK, BUT DOES NOT ELIMINATE IT
Large randomized controlled trials have consistently shown that lowering the level of LDL-C reduces cardiovascular event rates by 25% to 45% both in people who are known to have coronary artery disease and in those who are not.2–4 As a result, guidelines for preventing cardiovascular disease have increasingly emphasized maintaining low LDL-C levels. This has led to a proliferation in the use of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) in patients at high cardiovascular risk.
However, these agents only reduce the risk—they do not eliminate it. Needed are additional therapies to complement existing LDL-C-lowering approaches to lower the cardiovascular risk even further.
Raising HDL-C: The next frontier
One such strategy for further lowering cardiovascular risk that has received considerable interest is to promote the biological activity of the “good” cholesterol.
Studies have consistently shown that the higher the plasma level of high-density lipoprotein cholesterol (HDL-C), the lower the risk of cardiovascular events, suggesting that raising HDL-C may be beneficial.5 Studies in animals with atherosclerosis show that raising HDL-C via genetic modification of the animal or direct infusion of the molecule has a favorable impact on both the size and the structure of experimental plaque.6,7
Accordingly, much activity has focused on developing new therapies that raise HDL-C more effectively than current ones.
Why niacin should protect the heart
For more than 50 years, niacin has been used to manage dyslipidemia.
In addition to raising HDL-C levels more effectively than any other agent available today, niacin also lowers the levels of LDL-C, triglycerides, and lipoprotein (a).8 Before statins were available, the Coronary Drug Project found that niacin reduced the rate of nonfatal myocardial infarction and the 15-year mortality rate.9 In addition, niacin has been shown to slow the progression of carotid intimal-medial thickness and coronary atherosclerosis, and even to reverse these processes in some trials.10–12
However, a number of issues remain about using niacin to prevent cardiovascular events. Nearly all patients who take it experience flushing, which limits its tolerability and, thus, our ability to titrate doses to levels needed for adequate lipid changes. While a number of modifications of niacin administration have been developed (eg, extended-release formulations and products that inhibit flushing), no large study has tested the clinical efficacy of these strategies. Furthermore, until AIM-HIGH, no large-scale trial had directly evaluated the impact of niacin therapy on a background of statin therapy.
AIM-HIGH STUDY DESIGN
The intent of the AIM-HIGH trial was to determine whether extended-release niacin (Niaspan) would reduce the risk of cardiovascular events when added to therapy with a statin—in this case, simvastatin (Zocor) supplemented with ezetimibe (Zetia).1
The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) and by Abbott Laboratories, which also supplied the extended-release niacin and the ezetimibe. Merck donated the simvastatin.
Patient characteristics
The patients were all at least 45 years of age with established, stable coronary heart disease, cerebrovascular or carotid arterial disease, or peripheral arterial disease. They also had to have low levels of HDL-C (< 40 mg/dL in men, < 50 mg/dL in women), elevated triglycerides (150–400 mg/dL), and LDL-C levels lower than 180 mg/dL if they were not taking a statin at entry.
The mean age of the patients was 64 years, 85% were men, and 92% were white. They had a high prevalence of cardiovascular risk factors: 34% had diabetes, 71% had hypertension, and 81% had metabolic syndrome. Nearly all (94%) of the patients were taking a statin at entry; 76% had been taking one for more than 1 year, and 40% had been taking one for more than 5 years.1
Simvastatin, ezetimibe, and either niacin or placebo
All lipid-modifying agents except statins and ezetimibe were stopped for least 4 weeks after enrollment.
All patients then entered a 4- to 8-week open-label period, during which they took simvastatin 40 mg daily and extended-release niacin starting at 500 mg and increased weekly up to 2,000 mg daily. Patients who could tolerate at least 1,500 mg daily were randomly assigned to treatment with either niacin 1,500 to 2,000 mg or matching placebo. Both groups continued to receive simvastatin. The placebo contained a small dose of immediate-release niacin (50 mg) in each tablet to induce flushing and to maintain blinding of treatment.
Given that niacin also lowers LDL-C, an algorithm was used to try to keep LDL-C levels roughly the same in both treatment groups. This involved adjusting the simvastatin dose and permitting the use of ezetimibe 10 mg to keep the LDL-C level between 40 and 80 mg/dL. Accordingly, participating physicians were told their patients’ LDL-C levels but were blinded to their HDL-C and triglyceride levels throughout the study.
Every 6 months, patients had a follow-up visit in the clinic, and midway through each 6-month interval they received a phone call from the investigators.1
AIM-HIGH end points
The primary end point was the composite of the first event of death due to coronary heart disease, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven revascularization of the coronary or cerebral arteries.
Secondary end points were:
- Death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or hospitalization for acute coronary syndrome
- Death from coronary heart disease, nonfatal myocardial infarction, or ischemic stroke
- Death from cardiovascular causes.
Tertiary end points included:
- Death from any cause
- Individual components of the primary end point
- Prespecified subgroups according to sex, history or no history of diabetes, and presence or absence of the metabolic syndrome.1
All clinical events were adjudicated by a central committee.
STUDY HALTED EARLY
The study was planned to run for a mean of 4.6 years, during which 800 primary end point events were expected. With these numbers, the investigators calculated that the study had 85% power to detect a 25% reduction in the primary end point, at a one-sided alpha level of 0.025.
The plan called for an interim analysis when 50% of the anticipated events had occurred, with prespecified stopping boundaries based on either efficacy or futility. The boundary for lack of efficacy required an observed hazard ratio of at least 1.02 with a probability of less than .001.
In the interim analysis, after a median follow-up of only 3 years, the data and safety monitoring board recommended stopping the study early because the boundary for futility had been crossed and, unexpectedly, the rate of ischemic stroke was higher in the niacin-treated patients than in those receiving placebo.
MAJOR FINDINGS OF AIM-HIGH
Of 4,273 patients who began open-label treatment with niacin, 3,414 were randomized to treatment with niacin or placebo.1
HDL-C levels went up in both groups
At 2 years:
- HDL-C levels had increased by 25.0% (to 42 mg/dL) in the niacin group and by 9.8% (to 38 mg/dL) in the placebo group
- Triglycerides had decreased by 28.6% with niacin and by 8.1% with placebo
- LDL-C had decreased by 12.0% with niacin and by 5.5% with placebo.
Patients in the placebo group were more likely to have subsequently received the maximum dose of simvastatin, ie, 80 mg/day (24.7% vs 17.5%), and to have received ezetimibe (21.5% vs 9.5%). More patients in the niacin group required either dose reduction of the study drug (6.3% vs 3.4%) or drug discontinuation (25.4% vs 20.1%).1
No difference in the primary end point
There was no difference between the two treatment groups in the rate of the primary end point, which occurred in 282 (16.4%) of the 1,718 patients in the niacin group and 272 (16.2%) of the 1,696 patients in the placebo group (P = .79; hazard ratio 1.02, 95% confidence interval 0.87–1.21).1
However, more patients in the niacin group than in the placebo group who reached the primary end point did so by having a first ischemic stroke: 27 patients (1.6%) vs 15 patients (0.9%). Eight of these patients, all in the niacin group, had their stroke between 2 months and 4 years after they had stopped taking the study drug.
Further analysis that included all ischemic strokes revealed the same trend: 29 vs 18 patients (P = .11).1
No benefit was observed for niacin-treated patients in terms of any of the secondary or tertiary end points.
Subgroup analysis revealed no evidence of statistical heterogeneity: ie, niacin seemed to lack efficacy in all the prespecified subgroups studied (age 65 and older vs younger, men vs women, and those with or without diabetes, metabolic syndrome, prior myocardial infarction, or statin use at entry).
In general, niacin was well tolerated in the active-treatment group, with a low incidence of liver and muscle abnormalities.
PUTTING AIM-HIGH IN CONTEXT
How should practicing clinicians interpret these outcomes?
Ever since the NHLBI reported (in an urgent press release) that it was stopping the study early due to futility and a potential excess of strokes,13 there has been considerable debate as to which factors contributed to these outcomes. In the wake of the publication of more detailed information about the trial,1 this debate is likely to continue.
The AIM-HIGH results can be interpreted in several ways:
- Perhaps niacin is no good as a preventive agent
- Perhaps raising HDL-C is flawed as a preventive strategy
- Perhaps AIM-HIGH had methodologic flaws, such as looking at the wrong patient cohort or using a treatment protocol that set itself up for failure
- Perhaps statins are so good that, once you prescribe one, anything else you give provides no additional benefit.
Which of these is correct?
Is niacin no good?
In its most simple form, AIM-HIGH has always been seen as a clinical trial of niacin. While the early trials of immediate-release niacin were encouraging in terms of its effects on lipids, atherosclerotic plaque, and cardiovascular outcomes, using it in clinical practice has always been challenging, largely because many patients cannot tolerate it in doses high enough to be effective. A number of developments have improved niacin’s tolerability, but its clinical impact in the statin era has not been evaluated.
Niacin’s lack of efficacy in this trial will ultimately be viewed as a failure of the drug itself, but is this the case?
AIM-HIGH was not simply a direct comparison of niacin vs placebo on top of standard medical practice. The investigators recognized that niacin has additional effects—in particular, lowering levels of atherogenic lipids—and they attempted to control for these effects by titrating the other LDL-C-lowering therapies during the study. As a result, the trial was actually a comparison between niacin plus low-dose simvastatin on the one hand, and placebo plus high-dose simvastatin (and, more often, also ezetimibe) on the other.
Furthermore, the placebo-treated patients received small doses of immediate-release niacin to induce flushing and maintain blinding. It is therefore hard to conclude that this clinical trial was a direct evaluation of the impact of niacin.
In contrast, the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study is currently evaluating extended-release niacin in combination with laropiprant, a prostaglandin receptor antagonist, vs placebo in more than 24,000 statin-treated patients.14 Without any in-trial titration of lipids, this study provides a more direct comparison of the effects of niacin in the statin era.
Niacin continues to attract interest, largely because it can raise HDL-C by 20% to 30% when given at doses of 1,500 mg or more. Also, consistent observations from population studies of an inverse relationship between HDL-C levels and cardiovascular risk5 have stimulated interest in developing novel agents that substantially raise HDL-C.
Is raising HDL-C a flawed strategy?
The failure of HDL-C-raising therapies in clinical trials15,16 has fueled concern that HDL may not be the magic elixir that many have sought. Given that niacin is the most effective HDL-C-raising agent currently available, its lack of efficacy in AIM-HIGH could be perceived as another nail in the coffin of the hypothesis that raising the HDL-C level with pharmacologic agents is beneficial.
AIM-HIGH was designed to examine the effects of raising HDL-C. To this end, it was performed exclusively in patients with low HDL-C levels, and the investigators tried to isolate the potential effects of raising HDL-C by equalizing the LDL-C levels in the treatment groups.
However, the HDL-C changes observed in AIM-HIGH are likely to have undermined the study objective. While niacin predictably increased HDL-C levels by 25%, an unexpected increase in HDL-C of 9.8% in the placebo-treated patients resulted in a difference in achieved HDL-C levels of only 4 mg/dL between the groups. This was far less than anticipated, and it likely had a major impact on an already underpowered study.
AIM-HIGH was designed to have 85% power to demonstrate a 25% reduction in clinical events, which was an optimistic estimate. On the basis of population studies, a difference of 4 mg/dL in HDL-C would be anticipated to result in no more than a 10% lower rate of clinical events, far beyond AIM-HIGH’s limit of detection.
The reasons for the increase in HDL-C in the placebo group are unknown, but they likely reflect the use of higher doses of simvastatin, some regression to the mean, and, possibly, the small doses of immediate-release niacin that the placebo contained. (Contrary to the belief of the investigators, there have been some reports of lipid changes with such doses,17 which may have contributed to the observed HDL-C-raising.)
Given that the HDL-C difference between the groups was relatively small and that niacin has additional effects beyond raising HDL-C and lowering LDL-C, it is unlikely that the futility of AIM-HIGH reflects a major indictment of HDL-C-raising. For the time being, the jury is still out on this question.
Was AIM-HIGH methodologically flawed?
A number of methodologic issues may have affected AIM-HIGH’s ability to adequately address its objectives.
The wrong cohort? In planning a study such as AIM-HIGH, the need for a relatively small sample size and the need to detect the greatest relative risk reduction with niacin would require enrollment of patients at the highest risk of cardiovascular events despite the use of statins. These needs were satisfied by only including patients who had atherosclerotic cardiovascular disease and low HDL-C levels. The inclusion of patients with low levels of HDL-C was also expected to promote greater increases in this lipid, and potentially event reduction, with niacin.
But no benefit was observed. It remains to be determined whether the inclusion of a high proportion of patients with the metabolic syndrome adversely affected the ability to detect a benefit with niacin. While post hoc analyses of studies of carotid intimal-medial thickness demonstrated no relationship between raising HDL-C with niacin and slowing of disease progression in patients with the metabolic syndrome,18 it remains to be determined whether this would translate to any effect on cardiovascular event rates.
Inadequate statistical power? An underpowered study would leave very little room for error, a pertinent point given the variability in therapeutic response in both actively treated and placebo-treated patients typically encountered in clinical trials. Giving low doses of immediate-release niacin and titrating the simvastatin dose to control LDL-C, resulting in imbalances in lipid-modifying therapies, represent additional flaws in the study design.
Stopped too soon? The early cessation of the study was somewhat questionable. The study crossed the prespecified boundary for lack of efficacy at the time of the interim analysis, and initial review by the data and safety monitoring board suggested an excess rate of ischemic stroke with niacin. The inclusion of this latter finding in the press release prompted considerable speculation regarding potential mechanisms and also concern among patients currently taking niacin. The subsequent finding that this signal was not statistically significant serves as an important warning for those conducting clinical trials not to prematurely overstate preliminary observations.
The implications for agents used in clinical practice are considerable: negative findings should not be overemphasized without robust evidence.
Do statins make everything else irrelevant?
The final factor to consider is the relative modifiability of residual clinical risk in statin-treated patients.
While residual risk is often cited as the reason to develop new antiatherosclerotic therapies, it is unknown how many of these ongoing events can be prevented. Several nonmodifiable factors such as age and concomitant disease are likely to contribute to these clinical events, which may limit our ability to further reduce event rates in patients who have already achieved low LDL-C levels with statin therapy. This may underscore the observation that no major clinical trial has demonstrated clinical benefit of an antiatherosclerotic agent on top of background medical care that included statins.
The finding that atherosclerosis continues to progress in many patients even though they take statins in high doses or achieve low LDL-C levels suggests that there is still room for improvement.
WHAT FUTURE FOR NIACIN?
So what does the future hold for niacin? The ongoing HPS2-THRIVE study provides another opportunity to evaluate the potential clinical efficacy of niacin in statin-treated patients. For now, we must wait for the results of this study.
In the meantime, it would seem reasonable to continue treatment with niacin in patients who need it for its multiple lipid-modifying effects. Whether clinicians will be less likely to initiate niacin therapy until there is clear evidence of clinical benefit remains uncertain. As for HDL-C, it remains to be determined whether any therapy targeting either quantitative or qualitative changes will be beneficial.
Over the last 3 decades, clinical trials have provided important insights into the prevention of cardiovascular events and have had a profound impact on clinical practice. Such studies simply evaluate whether one strategy is better or worse than the existing standard of care. They do not provide mechanistic insights, and when attempts have been made to address mechanisms in the study design, the trial, as in the case of AIM-HIGH, leaves more questions than answers.
Future trials will provide more clarity as to the optimal way to treat patients, but they must be based on a robust design that permits the study question to be adequately addressed.
- The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977; 62:707–714.
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 1991; 353:265–267.
- Nicholls SJ, Cutri B, Worthley SG, et al. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 2005; 25:2416–2421.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao X-Q, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- US Department of Health and Human Services. NIH stops clinical trial on combination cholesterol treatment. http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2792. Accessed November 30, 2011.
- Brown BG, Zhao XQ. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am J Cardiol 2008; 101:58B–62B.
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Luria MH, Sapoznikov D. Raising HDL cholesterol with low-dose nicotinic acid and bezafibrate: preliminary experience. Postgrad Med J 1993; 69:296–299.
- Taylor AJ, Zhu D, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Relationship between glycemic status and progression of carotid intima-media thickness during treatment with combined statin and extended-release niacin in ARBITER 2. Vasc Health Risk Manag 2007; 3:159–164.
The recent publication of the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes)1 has thrown the use of niacin as a lipid-modifying therapy into question. The trial was stopped early because an interim analysis found that the patients who took extended-release niacin had no clinical benefit. In addition, it found a trend toward more ischemic strokes, though this finding was later found not to be statistically significant.
Complicating the interpretation, while both the treatment group and the control group in the study received statin therapy, the researchers attempted to keep low-density lipoprotein cholesterol (LDL-C) levels equal, meaning that patients in the control group received more intensive statin therapy than those in the treatment group. And the placebo that the control patients received was actually a low dose of niacin, to induce flushing and thus to blind study participants and their physicians to which drug they were taking.
In the article that follows, I will explore the background, design, findings, and implications of this key trial and try to untangle the many questions about how to interpret it.
LOWERING LDL-C REDUCES RISK, BUT DOES NOT ELIMINATE IT
Large randomized controlled trials have consistently shown that lowering the level of LDL-C reduces cardiovascular event rates by 25% to 45% both in people who are known to have coronary artery disease and in those who are not.2–4 As a result, guidelines for preventing cardiovascular disease have increasingly emphasized maintaining low LDL-C levels. This has led to a proliferation in the use of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) in patients at high cardiovascular risk.
However, these agents only reduce the risk—they do not eliminate it. Needed are additional therapies to complement existing LDL-C-lowering approaches to lower the cardiovascular risk even further.
Raising HDL-C: The next frontier
One such strategy for further lowering cardiovascular risk that has received considerable interest is to promote the biological activity of the “good” cholesterol.
Studies have consistently shown that the higher the plasma level of high-density lipoprotein cholesterol (HDL-C), the lower the risk of cardiovascular events, suggesting that raising HDL-C may be beneficial.5 Studies in animals with atherosclerosis show that raising HDL-C via genetic modification of the animal or direct infusion of the molecule has a favorable impact on both the size and the structure of experimental plaque.6,7
Accordingly, much activity has focused on developing new therapies that raise HDL-C more effectively than current ones.
Why niacin should protect the heart
For more than 50 years, niacin has been used to manage dyslipidemia.
In addition to raising HDL-C levels more effectively than any other agent available today, niacin also lowers the levels of LDL-C, triglycerides, and lipoprotein (a).8 Before statins were available, the Coronary Drug Project found that niacin reduced the rate of nonfatal myocardial infarction and the 15-year mortality rate.9 In addition, niacin has been shown to slow the progression of carotid intimal-medial thickness and coronary atherosclerosis, and even to reverse these processes in some trials.10–12
However, a number of issues remain about using niacin to prevent cardiovascular events. Nearly all patients who take it experience flushing, which limits its tolerability and, thus, our ability to titrate doses to levels needed for adequate lipid changes. While a number of modifications of niacin administration have been developed (eg, extended-release formulations and products that inhibit flushing), no large study has tested the clinical efficacy of these strategies. Furthermore, until AIM-HIGH, no large-scale trial had directly evaluated the impact of niacin therapy on a background of statin therapy.
AIM-HIGH STUDY DESIGN
The intent of the AIM-HIGH trial was to determine whether extended-release niacin (Niaspan) would reduce the risk of cardiovascular events when added to therapy with a statin—in this case, simvastatin (Zocor) supplemented with ezetimibe (Zetia).1
The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) and by Abbott Laboratories, which also supplied the extended-release niacin and the ezetimibe. Merck donated the simvastatin.
Patient characteristics
The patients were all at least 45 years of age with established, stable coronary heart disease, cerebrovascular or carotid arterial disease, or peripheral arterial disease. They also had to have low levels of HDL-C (< 40 mg/dL in men, < 50 mg/dL in women), elevated triglycerides (150–400 mg/dL), and LDL-C levels lower than 180 mg/dL if they were not taking a statin at entry.
The mean age of the patients was 64 years, 85% were men, and 92% were white. They had a high prevalence of cardiovascular risk factors: 34% had diabetes, 71% had hypertension, and 81% had metabolic syndrome. Nearly all (94%) of the patients were taking a statin at entry; 76% had been taking one for more than 1 year, and 40% had been taking one for more than 5 years.1
Simvastatin, ezetimibe, and either niacin or placebo
All lipid-modifying agents except statins and ezetimibe were stopped for least 4 weeks after enrollment.
All patients then entered a 4- to 8-week open-label period, during which they took simvastatin 40 mg daily and extended-release niacin starting at 500 mg and increased weekly up to 2,000 mg daily. Patients who could tolerate at least 1,500 mg daily were randomly assigned to treatment with either niacin 1,500 to 2,000 mg or matching placebo. Both groups continued to receive simvastatin. The placebo contained a small dose of immediate-release niacin (50 mg) in each tablet to induce flushing and to maintain blinding of treatment.
Given that niacin also lowers LDL-C, an algorithm was used to try to keep LDL-C levels roughly the same in both treatment groups. This involved adjusting the simvastatin dose and permitting the use of ezetimibe 10 mg to keep the LDL-C level between 40 and 80 mg/dL. Accordingly, participating physicians were told their patients’ LDL-C levels but were blinded to their HDL-C and triglyceride levels throughout the study.
Every 6 months, patients had a follow-up visit in the clinic, and midway through each 6-month interval they received a phone call from the investigators.1
AIM-HIGH end points
The primary end point was the composite of the first event of death due to coronary heart disease, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven revascularization of the coronary or cerebral arteries.
Secondary end points were:
- Death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or hospitalization for acute coronary syndrome
- Death from coronary heart disease, nonfatal myocardial infarction, or ischemic stroke
- Death from cardiovascular causes.
Tertiary end points included:
- Death from any cause
- Individual components of the primary end point
- Prespecified subgroups according to sex, history or no history of diabetes, and presence or absence of the metabolic syndrome.1
All clinical events were adjudicated by a central committee.
STUDY HALTED EARLY
The study was planned to run for a mean of 4.6 years, during which 800 primary end point events were expected. With these numbers, the investigators calculated that the study had 85% power to detect a 25% reduction in the primary end point, at a one-sided alpha level of 0.025.
The plan called for an interim analysis when 50% of the anticipated events had occurred, with prespecified stopping boundaries based on either efficacy or futility. The boundary for lack of efficacy required an observed hazard ratio of at least 1.02 with a probability of less than .001.
In the interim analysis, after a median follow-up of only 3 years, the data and safety monitoring board recommended stopping the study early because the boundary for futility had been crossed and, unexpectedly, the rate of ischemic stroke was higher in the niacin-treated patients than in those receiving placebo.
MAJOR FINDINGS OF AIM-HIGH
Of 4,273 patients who began open-label treatment with niacin, 3,414 were randomized to treatment with niacin or placebo.1
HDL-C levels went up in both groups
At 2 years:
- HDL-C levels had increased by 25.0% (to 42 mg/dL) in the niacin group and by 9.8% (to 38 mg/dL) in the placebo group
- Triglycerides had decreased by 28.6% with niacin and by 8.1% with placebo
- LDL-C had decreased by 12.0% with niacin and by 5.5% with placebo.
Patients in the placebo group were more likely to have subsequently received the maximum dose of simvastatin, ie, 80 mg/day (24.7% vs 17.5%), and to have received ezetimibe (21.5% vs 9.5%). More patients in the niacin group required either dose reduction of the study drug (6.3% vs 3.4%) or drug discontinuation (25.4% vs 20.1%).1
No difference in the primary end point
There was no difference between the two treatment groups in the rate of the primary end point, which occurred in 282 (16.4%) of the 1,718 patients in the niacin group and 272 (16.2%) of the 1,696 patients in the placebo group (P = .79; hazard ratio 1.02, 95% confidence interval 0.87–1.21).1
However, more patients in the niacin group than in the placebo group who reached the primary end point did so by having a first ischemic stroke: 27 patients (1.6%) vs 15 patients (0.9%). Eight of these patients, all in the niacin group, had their stroke between 2 months and 4 years after they had stopped taking the study drug.
Further analysis that included all ischemic strokes revealed the same trend: 29 vs 18 patients (P = .11).1
No benefit was observed for niacin-treated patients in terms of any of the secondary or tertiary end points.
Subgroup analysis revealed no evidence of statistical heterogeneity: ie, niacin seemed to lack efficacy in all the prespecified subgroups studied (age 65 and older vs younger, men vs women, and those with or without diabetes, metabolic syndrome, prior myocardial infarction, or statin use at entry).
In general, niacin was well tolerated in the active-treatment group, with a low incidence of liver and muscle abnormalities.
PUTTING AIM-HIGH IN CONTEXT
How should practicing clinicians interpret these outcomes?
Ever since the NHLBI reported (in an urgent press release) that it was stopping the study early due to futility and a potential excess of strokes,13 there has been considerable debate as to which factors contributed to these outcomes. In the wake of the publication of more detailed information about the trial,1 this debate is likely to continue.
The AIM-HIGH results can be interpreted in several ways:
- Perhaps niacin is no good as a preventive agent
- Perhaps raising HDL-C is flawed as a preventive strategy
- Perhaps AIM-HIGH had methodologic flaws, such as looking at the wrong patient cohort or using a treatment protocol that set itself up for failure
- Perhaps statins are so good that, once you prescribe one, anything else you give provides no additional benefit.
Which of these is correct?
Is niacin no good?
In its most simple form, AIM-HIGH has always been seen as a clinical trial of niacin. While the early trials of immediate-release niacin were encouraging in terms of its effects on lipids, atherosclerotic plaque, and cardiovascular outcomes, using it in clinical practice has always been challenging, largely because many patients cannot tolerate it in doses high enough to be effective. A number of developments have improved niacin’s tolerability, but its clinical impact in the statin era has not been evaluated.
Niacin’s lack of efficacy in this trial will ultimately be viewed as a failure of the drug itself, but is this the case?
AIM-HIGH was not simply a direct comparison of niacin vs placebo on top of standard medical practice. The investigators recognized that niacin has additional effects—in particular, lowering levels of atherogenic lipids—and they attempted to control for these effects by titrating the other LDL-C-lowering therapies during the study. As a result, the trial was actually a comparison between niacin plus low-dose simvastatin on the one hand, and placebo plus high-dose simvastatin (and, more often, also ezetimibe) on the other.
Furthermore, the placebo-treated patients received small doses of immediate-release niacin to induce flushing and maintain blinding. It is therefore hard to conclude that this clinical trial was a direct evaluation of the impact of niacin.
In contrast, the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study is currently evaluating extended-release niacin in combination with laropiprant, a prostaglandin receptor antagonist, vs placebo in more than 24,000 statin-treated patients.14 Without any in-trial titration of lipids, this study provides a more direct comparison of the effects of niacin in the statin era.
Niacin continues to attract interest, largely because it can raise HDL-C by 20% to 30% when given at doses of 1,500 mg or more. Also, consistent observations from population studies of an inverse relationship between HDL-C levels and cardiovascular risk5 have stimulated interest in developing novel agents that substantially raise HDL-C.
Is raising HDL-C a flawed strategy?
The failure of HDL-C-raising therapies in clinical trials15,16 has fueled concern that HDL may not be the magic elixir that many have sought. Given that niacin is the most effective HDL-C-raising agent currently available, its lack of efficacy in AIM-HIGH could be perceived as another nail in the coffin of the hypothesis that raising the HDL-C level with pharmacologic agents is beneficial.
AIM-HIGH was designed to examine the effects of raising HDL-C. To this end, it was performed exclusively in patients with low HDL-C levels, and the investigators tried to isolate the potential effects of raising HDL-C by equalizing the LDL-C levels in the treatment groups.
However, the HDL-C changes observed in AIM-HIGH are likely to have undermined the study objective. While niacin predictably increased HDL-C levels by 25%, an unexpected increase in HDL-C of 9.8% in the placebo-treated patients resulted in a difference in achieved HDL-C levels of only 4 mg/dL between the groups. This was far less than anticipated, and it likely had a major impact on an already underpowered study.
AIM-HIGH was designed to have 85% power to demonstrate a 25% reduction in clinical events, which was an optimistic estimate. On the basis of population studies, a difference of 4 mg/dL in HDL-C would be anticipated to result in no more than a 10% lower rate of clinical events, far beyond AIM-HIGH’s limit of detection.
The reasons for the increase in HDL-C in the placebo group are unknown, but they likely reflect the use of higher doses of simvastatin, some regression to the mean, and, possibly, the small doses of immediate-release niacin that the placebo contained. (Contrary to the belief of the investigators, there have been some reports of lipid changes with such doses,17 which may have contributed to the observed HDL-C-raising.)
Given that the HDL-C difference between the groups was relatively small and that niacin has additional effects beyond raising HDL-C and lowering LDL-C, it is unlikely that the futility of AIM-HIGH reflects a major indictment of HDL-C-raising. For the time being, the jury is still out on this question.
Was AIM-HIGH methodologically flawed?
A number of methodologic issues may have affected AIM-HIGH’s ability to adequately address its objectives.
The wrong cohort? In planning a study such as AIM-HIGH, the need for a relatively small sample size and the need to detect the greatest relative risk reduction with niacin would require enrollment of patients at the highest risk of cardiovascular events despite the use of statins. These needs were satisfied by only including patients who had atherosclerotic cardiovascular disease and low HDL-C levels. The inclusion of patients with low levels of HDL-C was also expected to promote greater increases in this lipid, and potentially event reduction, with niacin.
But no benefit was observed. It remains to be determined whether the inclusion of a high proportion of patients with the metabolic syndrome adversely affected the ability to detect a benefit with niacin. While post hoc analyses of studies of carotid intimal-medial thickness demonstrated no relationship between raising HDL-C with niacin and slowing of disease progression in patients with the metabolic syndrome,18 it remains to be determined whether this would translate to any effect on cardiovascular event rates.
Inadequate statistical power? An underpowered study would leave very little room for error, a pertinent point given the variability in therapeutic response in both actively treated and placebo-treated patients typically encountered in clinical trials. Giving low doses of immediate-release niacin and titrating the simvastatin dose to control LDL-C, resulting in imbalances in lipid-modifying therapies, represent additional flaws in the study design.
Stopped too soon? The early cessation of the study was somewhat questionable. The study crossed the prespecified boundary for lack of efficacy at the time of the interim analysis, and initial review by the data and safety monitoring board suggested an excess rate of ischemic stroke with niacin. The inclusion of this latter finding in the press release prompted considerable speculation regarding potential mechanisms and also concern among patients currently taking niacin. The subsequent finding that this signal was not statistically significant serves as an important warning for those conducting clinical trials not to prematurely overstate preliminary observations.
The implications for agents used in clinical practice are considerable: negative findings should not be overemphasized without robust evidence.
Do statins make everything else irrelevant?
The final factor to consider is the relative modifiability of residual clinical risk in statin-treated patients.
While residual risk is often cited as the reason to develop new antiatherosclerotic therapies, it is unknown how many of these ongoing events can be prevented. Several nonmodifiable factors such as age and concomitant disease are likely to contribute to these clinical events, which may limit our ability to further reduce event rates in patients who have already achieved low LDL-C levels with statin therapy. This may underscore the observation that no major clinical trial has demonstrated clinical benefit of an antiatherosclerotic agent on top of background medical care that included statins.
The finding that atherosclerosis continues to progress in many patients even though they take statins in high doses or achieve low LDL-C levels suggests that there is still room for improvement.
WHAT FUTURE FOR NIACIN?
So what does the future hold for niacin? The ongoing HPS2-THRIVE study provides another opportunity to evaluate the potential clinical efficacy of niacin in statin-treated patients. For now, we must wait for the results of this study.
In the meantime, it would seem reasonable to continue treatment with niacin in patients who need it for its multiple lipid-modifying effects. Whether clinicians will be less likely to initiate niacin therapy until there is clear evidence of clinical benefit remains uncertain. As for HDL-C, it remains to be determined whether any therapy targeting either quantitative or qualitative changes will be beneficial.
Over the last 3 decades, clinical trials have provided important insights into the prevention of cardiovascular events and have had a profound impact on clinical practice. Such studies simply evaluate whether one strategy is better or worse than the existing standard of care. They do not provide mechanistic insights, and when attempts have been made to address mechanisms in the study design, the trial, as in the case of AIM-HIGH, leaves more questions than answers.
Future trials will provide more clarity as to the optimal way to treat patients, but they must be based on a robust design that permits the study question to be adequately addressed.
The recent publication of the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes)1 has thrown the use of niacin as a lipid-modifying therapy into question. The trial was stopped early because an interim analysis found that the patients who took extended-release niacin had no clinical benefit. In addition, it found a trend toward more ischemic strokes, though this finding was later found not to be statistically significant.
Complicating the interpretation, while both the treatment group and the control group in the study received statin therapy, the researchers attempted to keep low-density lipoprotein cholesterol (LDL-C) levels equal, meaning that patients in the control group received more intensive statin therapy than those in the treatment group. And the placebo that the control patients received was actually a low dose of niacin, to induce flushing and thus to blind study participants and their physicians to which drug they were taking.
In the article that follows, I will explore the background, design, findings, and implications of this key trial and try to untangle the many questions about how to interpret it.
LOWERING LDL-C REDUCES RISK, BUT DOES NOT ELIMINATE IT
Large randomized controlled trials have consistently shown that lowering the level of LDL-C reduces cardiovascular event rates by 25% to 45% both in people who are known to have coronary artery disease and in those who are not.2–4 As a result, guidelines for preventing cardiovascular disease have increasingly emphasized maintaining low LDL-C levels. This has led to a proliferation in the use of inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (statins) in patients at high cardiovascular risk.
However, these agents only reduce the risk—they do not eliminate it. Needed are additional therapies to complement existing LDL-C-lowering approaches to lower the cardiovascular risk even further.
Raising HDL-C: The next frontier
One such strategy for further lowering cardiovascular risk that has received considerable interest is to promote the biological activity of the “good” cholesterol.
Studies have consistently shown that the higher the plasma level of high-density lipoprotein cholesterol (HDL-C), the lower the risk of cardiovascular events, suggesting that raising HDL-C may be beneficial.5 Studies in animals with atherosclerosis show that raising HDL-C via genetic modification of the animal or direct infusion of the molecule has a favorable impact on both the size and the structure of experimental plaque.6,7
Accordingly, much activity has focused on developing new therapies that raise HDL-C more effectively than current ones.
Why niacin should protect the heart
For more than 50 years, niacin has been used to manage dyslipidemia.
In addition to raising HDL-C levels more effectively than any other agent available today, niacin also lowers the levels of LDL-C, triglycerides, and lipoprotein (a).8 Before statins were available, the Coronary Drug Project found that niacin reduced the rate of nonfatal myocardial infarction and the 15-year mortality rate.9 In addition, niacin has been shown to slow the progression of carotid intimal-medial thickness and coronary atherosclerosis, and even to reverse these processes in some trials.10–12
However, a number of issues remain about using niacin to prevent cardiovascular events. Nearly all patients who take it experience flushing, which limits its tolerability and, thus, our ability to titrate doses to levels needed for adequate lipid changes. While a number of modifications of niacin administration have been developed (eg, extended-release formulations and products that inhibit flushing), no large study has tested the clinical efficacy of these strategies. Furthermore, until AIM-HIGH, no large-scale trial had directly evaluated the impact of niacin therapy on a background of statin therapy.
AIM-HIGH STUDY DESIGN
The intent of the AIM-HIGH trial was to determine whether extended-release niacin (Niaspan) would reduce the risk of cardiovascular events when added to therapy with a statin—in this case, simvastatin (Zocor) supplemented with ezetimibe (Zetia).1
The trial was funded by the National Heart, Lung, and Blood Institute (NHLBI) and by Abbott Laboratories, which also supplied the extended-release niacin and the ezetimibe. Merck donated the simvastatin.
Patient characteristics
The patients were all at least 45 years of age with established, stable coronary heart disease, cerebrovascular or carotid arterial disease, or peripheral arterial disease. They also had to have low levels of HDL-C (< 40 mg/dL in men, < 50 mg/dL in women), elevated triglycerides (150–400 mg/dL), and LDL-C levels lower than 180 mg/dL if they were not taking a statin at entry.
The mean age of the patients was 64 years, 85% were men, and 92% were white. They had a high prevalence of cardiovascular risk factors: 34% had diabetes, 71% had hypertension, and 81% had metabolic syndrome. Nearly all (94%) of the patients were taking a statin at entry; 76% had been taking one for more than 1 year, and 40% had been taking one for more than 5 years.1
Simvastatin, ezetimibe, and either niacin or placebo
All lipid-modifying agents except statins and ezetimibe were stopped for least 4 weeks after enrollment.
All patients then entered a 4- to 8-week open-label period, during which they took simvastatin 40 mg daily and extended-release niacin starting at 500 mg and increased weekly up to 2,000 mg daily. Patients who could tolerate at least 1,500 mg daily were randomly assigned to treatment with either niacin 1,500 to 2,000 mg or matching placebo. Both groups continued to receive simvastatin. The placebo contained a small dose of immediate-release niacin (50 mg) in each tablet to induce flushing and to maintain blinding of treatment.
Given that niacin also lowers LDL-C, an algorithm was used to try to keep LDL-C levels roughly the same in both treatment groups. This involved adjusting the simvastatin dose and permitting the use of ezetimibe 10 mg to keep the LDL-C level between 40 and 80 mg/dL. Accordingly, participating physicians were told their patients’ LDL-C levels but were blinded to their HDL-C and triglyceride levels throughout the study.
Every 6 months, patients had a follow-up visit in the clinic, and midway through each 6-month interval they received a phone call from the investigators.1
AIM-HIGH end points
The primary end point was the composite of the first event of death due to coronary heart disease, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven revascularization of the coronary or cerebral arteries.
Secondary end points were:
- Death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, or hospitalization for acute coronary syndrome
- Death from coronary heart disease, nonfatal myocardial infarction, or ischemic stroke
- Death from cardiovascular causes.
Tertiary end points included:
- Death from any cause
- Individual components of the primary end point
- Prespecified subgroups according to sex, history or no history of diabetes, and presence or absence of the metabolic syndrome.1
All clinical events were adjudicated by a central committee.
STUDY HALTED EARLY
The study was planned to run for a mean of 4.6 years, during which 800 primary end point events were expected. With these numbers, the investigators calculated that the study had 85% power to detect a 25% reduction in the primary end point, at a one-sided alpha level of 0.025.
The plan called for an interim analysis when 50% of the anticipated events had occurred, with prespecified stopping boundaries based on either efficacy or futility. The boundary for lack of efficacy required an observed hazard ratio of at least 1.02 with a probability of less than .001.
In the interim analysis, after a median follow-up of only 3 years, the data and safety monitoring board recommended stopping the study early because the boundary for futility had been crossed and, unexpectedly, the rate of ischemic stroke was higher in the niacin-treated patients than in those receiving placebo.
MAJOR FINDINGS OF AIM-HIGH
Of 4,273 patients who began open-label treatment with niacin, 3,414 were randomized to treatment with niacin or placebo.1
HDL-C levels went up in both groups
At 2 years:
- HDL-C levels had increased by 25.0% (to 42 mg/dL) in the niacin group and by 9.8% (to 38 mg/dL) in the placebo group
- Triglycerides had decreased by 28.6% with niacin and by 8.1% with placebo
- LDL-C had decreased by 12.0% with niacin and by 5.5% with placebo.
Patients in the placebo group were more likely to have subsequently received the maximum dose of simvastatin, ie, 80 mg/day (24.7% vs 17.5%), and to have received ezetimibe (21.5% vs 9.5%). More patients in the niacin group required either dose reduction of the study drug (6.3% vs 3.4%) or drug discontinuation (25.4% vs 20.1%).1
No difference in the primary end point
There was no difference between the two treatment groups in the rate of the primary end point, which occurred in 282 (16.4%) of the 1,718 patients in the niacin group and 272 (16.2%) of the 1,696 patients in the placebo group (P = .79; hazard ratio 1.02, 95% confidence interval 0.87–1.21).1
However, more patients in the niacin group than in the placebo group who reached the primary end point did so by having a first ischemic stroke: 27 patients (1.6%) vs 15 patients (0.9%). Eight of these patients, all in the niacin group, had their stroke between 2 months and 4 years after they had stopped taking the study drug.
Further analysis that included all ischemic strokes revealed the same trend: 29 vs 18 patients (P = .11).1
No benefit was observed for niacin-treated patients in terms of any of the secondary or tertiary end points.
Subgroup analysis revealed no evidence of statistical heterogeneity: ie, niacin seemed to lack efficacy in all the prespecified subgroups studied (age 65 and older vs younger, men vs women, and those with or without diabetes, metabolic syndrome, prior myocardial infarction, or statin use at entry).
In general, niacin was well tolerated in the active-treatment group, with a low incidence of liver and muscle abnormalities.
PUTTING AIM-HIGH IN CONTEXT
How should practicing clinicians interpret these outcomes?
Ever since the NHLBI reported (in an urgent press release) that it was stopping the study early due to futility and a potential excess of strokes,13 there has been considerable debate as to which factors contributed to these outcomes. In the wake of the publication of more detailed information about the trial,1 this debate is likely to continue.
The AIM-HIGH results can be interpreted in several ways:
- Perhaps niacin is no good as a preventive agent
- Perhaps raising HDL-C is flawed as a preventive strategy
- Perhaps AIM-HIGH had methodologic flaws, such as looking at the wrong patient cohort or using a treatment protocol that set itself up for failure
- Perhaps statins are so good that, once you prescribe one, anything else you give provides no additional benefit.
Which of these is correct?
Is niacin no good?
In its most simple form, AIM-HIGH has always been seen as a clinical trial of niacin. While the early trials of immediate-release niacin were encouraging in terms of its effects on lipids, atherosclerotic plaque, and cardiovascular outcomes, using it in clinical practice has always been challenging, largely because many patients cannot tolerate it in doses high enough to be effective. A number of developments have improved niacin’s tolerability, but its clinical impact in the statin era has not been evaluated.
Niacin’s lack of efficacy in this trial will ultimately be viewed as a failure of the drug itself, but is this the case?
AIM-HIGH was not simply a direct comparison of niacin vs placebo on top of standard medical practice. The investigators recognized that niacin has additional effects—in particular, lowering levels of atherogenic lipids—and they attempted to control for these effects by titrating the other LDL-C-lowering therapies during the study. As a result, the trial was actually a comparison between niacin plus low-dose simvastatin on the one hand, and placebo plus high-dose simvastatin (and, more often, also ezetimibe) on the other.
Furthermore, the placebo-treated patients received small doses of immediate-release niacin to induce flushing and maintain blinding. It is therefore hard to conclude that this clinical trial was a direct evaluation of the impact of niacin.
In contrast, the Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study is currently evaluating extended-release niacin in combination with laropiprant, a prostaglandin receptor antagonist, vs placebo in more than 24,000 statin-treated patients.14 Without any in-trial titration of lipids, this study provides a more direct comparison of the effects of niacin in the statin era.
Niacin continues to attract interest, largely because it can raise HDL-C by 20% to 30% when given at doses of 1,500 mg or more. Also, consistent observations from population studies of an inverse relationship between HDL-C levels and cardiovascular risk5 have stimulated interest in developing novel agents that substantially raise HDL-C.
Is raising HDL-C a flawed strategy?
The failure of HDL-C-raising therapies in clinical trials15,16 has fueled concern that HDL may not be the magic elixir that many have sought. Given that niacin is the most effective HDL-C-raising agent currently available, its lack of efficacy in AIM-HIGH could be perceived as another nail in the coffin of the hypothesis that raising the HDL-C level with pharmacologic agents is beneficial.
AIM-HIGH was designed to examine the effects of raising HDL-C. To this end, it was performed exclusively in patients with low HDL-C levels, and the investigators tried to isolate the potential effects of raising HDL-C by equalizing the LDL-C levels in the treatment groups.
However, the HDL-C changes observed in AIM-HIGH are likely to have undermined the study objective. While niacin predictably increased HDL-C levels by 25%, an unexpected increase in HDL-C of 9.8% in the placebo-treated patients resulted in a difference in achieved HDL-C levels of only 4 mg/dL between the groups. This was far less than anticipated, and it likely had a major impact on an already underpowered study.
AIM-HIGH was designed to have 85% power to demonstrate a 25% reduction in clinical events, which was an optimistic estimate. On the basis of population studies, a difference of 4 mg/dL in HDL-C would be anticipated to result in no more than a 10% lower rate of clinical events, far beyond AIM-HIGH’s limit of detection.
The reasons for the increase in HDL-C in the placebo group are unknown, but they likely reflect the use of higher doses of simvastatin, some regression to the mean, and, possibly, the small doses of immediate-release niacin that the placebo contained. (Contrary to the belief of the investigators, there have been some reports of lipid changes with such doses,17 which may have contributed to the observed HDL-C-raising.)
Given that the HDL-C difference between the groups was relatively small and that niacin has additional effects beyond raising HDL-C and lowering LDL-C, it is unlikely that the futility of AIM-HIGH reflects a major indictment of HDL-C-raising. For the time being, the jury is still out on this question.
Was AIM-HIGH methodologically flawed?
A number of methodologic issues may have affected AIM-HIGH’s ability to adequately address its objectives.
The wrong cohort? In planning a study such as AIM-HIGH, the need for a relatively small sample size and the need to detect the greatest relative risk reduction with niacin would require enrollment of patients at the highest risk of cardiovascular events despite the use of statins. These needs were satisfied by only including patients who had atherosclerotic cardiovascular disease and low HDL-C levels. The inclusion of patients with low levels of HDL-C was also expected to promote greater increases in this lipid, and potentially event reduction, with niacin.
But no benefit was observed. It remains to be determined whether the inclusion of a high proportion of patients with the metabolic syndrome adversely affected the ability to detect a benefit with niacin. While post hoc analyses of studies of carotid intimal-medial thickness demonstrated no relationship between raising HDL-C with niacin and slowing of disease progression in patients with the metabolic syndrome,18 it remains to be determined whether this would translate to any effect on cardiovascular event rates.
Inadequate statistical power? An underpowered study would leave very little room for error, a pertinent point given the variability in therapeutic response in both actively treated and placebo-treated patients typically encountered in clinical trials. Giving low doses of immediate-release niacin and titrating the simvastatin dose to control LDL-C, resulting in imbalances in lipid-modifying therapies, represent additional flaws in the study design.
Stopped too soon? The early cessation of the study was somewhat questionable. The study crossed the prespecified boundary for lack of efficacy at the time of the interim analysis, and initial review by the data and safety monitoring board suggested an excess rate of ischemic stroke with niacin. The inclusion of this latter finding in the press release prompted considerable speculation regarding potential mechanisms and also concern among patients currently taking niacin. The subsequent finding that this signal was not statistically significant serves as an important warning for those conducting clinical trials not to prematurely overstate preliminary observations.
The implications for agents used in clinical practice are considerable: negative findings should not be overemphasized without robust evidence.
Do statins make everything else irrelevant?
The final factor to consider is the relative modifiability of residual clinical risk in statin-treated patients.
While residual risk is often cited as the reason to develop new antiatherosclerotic therapies, it is unknown how many of these ongoing events can be prevented. Several nonmodifiable factors such as age and concomitant disease are likely to contribute to these clinical events, which may limit our ability to further reduce event rates in patients who have already achieved low LDL-C levels with statin therapy. This may underscore the observation that no major clinical trial has demonstrated clinical benefit of an antiatherosclerotic agent on top of background medical care that included statins.
The finding that atherosclerosis continues to progress in many patients even though they take statins in high doses or achieve low LDL-C levels suggests that there is still room for improvement.
WHAT FUTURE FOR NIACIN?
So what does the future hold for niacin? The ongoing HPS2-THRIVE study provides another opportunity to evaluate the potential clinical efficacy of niacin in statin-treated patients. For now, we must wait for the results of this study.
In the meantime, it would seem reasonable to continue treatment with niacin in patients who need it for its multiple lipid-modifying effects. Whether clinicians will be less likely to initiate niacin therapy until there is clear evidence of clinical benefit remains uncertain. As for HDL-C, it remains to be determined whether any therapy targeting either quantitative or qualitative changes will be beneficial.
Over the last 3 decades, clinical trials have provided important insights into the prevention of cardiovascular events and have had a profound impact on clinical practice. Such studies simply evaluate whether one strategy is better or worse than the existing standard of care. They do not provide mechanistic insights, and when attempts have been made to address mechanisms in the study design, the trial, as in the case of AIM-HIGH, leaves more questions than answers.
Future trials will provide more clarity as to the optimal way to treat patients, but they must be based on a robust design that permits the study question to be adequately addressed.
- The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977; 62:707–714.
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 1991; 353:265–267.
- Nicholls SJ, Cutri B, Worthley SG, et al. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 2005; 25:2416–2421.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao X-Q, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- US Department of Health and Human Services. NIH stops clinical trial on combination cholesterol treatment. http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2792. Accessed November 30, 2011.
- Brown BG, Zhao XQ. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am J Cardiol 2008; 101:58B–62B.
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Luria MH, Sapoznikov D. Raising HDL cholesterol with low-dose nicotinic acid and bezafibrate: preliminary experience. Postgrad Med J 1993; 69:296–299.
- Taylor AJ, Zhu D, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Relationship between glycemic status and progression of carotid intima-media thickness during treatment with combined statin and extended-release niacin in ARBITER 2. Vasc Health Risk Manag 2007; 3:159–164.
- The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med 1977; 62:707–714.
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 1991; 353:265–267.
- Nicholls SJ, Cutri B, Worthley SG, et al. Impact of short-term administration of high-density lipoproteins and atorvastatin on atherosclerosis in rabbits. Arterioscler Thromb Vasc Biol 2005; 25:2416–2421.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao X-Q, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- US Department of Health and Human Services. NIH stops clinical trial on combination cholesterol treatment. http://public.nhlbi.nih.gov/newsroom/home/GetPressRelease.aspx?id=2792. Accessed November 30, 2011.
- Brown BG, Zhao XQ. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am J Cardiol 2008; 101:58B–62B.
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Luria MH, Sapoznikov D. Raising HDL cholesterol with low-dose nicotinic acid and bezafibrate: preliminary experience. Postgrad Med J 1993; 69:296–299.
- Taylor AJ, Zhu D, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Relationship between glycemic status and progression of carotid intima-media thickness during treatment with combined statin and extended-release niacin in ARBITER 2. Vasc Health Risk Manag 2007; 3:159–164.
KEY POINTS
- The study was stopped early because of the concerns raised by the interim analysis.
- The AIM-HIGH results can be interpreted in several ways: perhaps niacin is no good as a preventive agent; perhaps raising levels of high-density lipoprotein cholesterol (HDL-C) is flawed as a preventive strategy; perhaps AIM-HIGH had methodologic flaws; or perhaps statins are so good that, once you prescribe one, anything else you do will not make much of a difference.
- It seems reasonable to continue niacin treatment in patients who need its multiple lipid-modifying effects. It is uncertain if clinicians will be less likely to prescribe niacin therapy until we have clear evidence of clinical benefit. As for HDL-C, it remains to be determined whether any therapy targeting quantitative or qualitative changes will be beneficial.
Updates in the medical management of Parkinson disease
More than a dozen drugs have been approved by the US Food and Drug Administration (FDA) for treating Parkinson disease, and more are expected in the near future. Many are currently in clinical trials, with the goals of finding ways to better control the disease with fewer adverse effects and, ultimately, to provide neuroprotection.
This article will review the features of Parkinson disease, the treatment options, and the complications in moderate to advanced disease.
PARKINSON DISEASE IS MULTIFACTORIAL
Although the cure for Parkinson disease is still elusive, much has been learned over the nearly 200 years since it was first described by James Parkinson in 1817. It is now understood to be a progressive neurodegenerative disease of multifactorial etiology: although a small proportion of patients have a direct inherited mutation that causes it, multiple genetic predisposition factors and environmental factors are more commonly involved.
The central pathology is dopaminergic loss in the basal ganglia, but other neurotransmitters are also involved and the disease extends to other areas of the brain.
CARDINAL MOTOR SYMPTOMS
In general, Parkinson disease is easy to identify. The classic patient has1:
- Tremor at rest, which can be subtle—such as only involving a thumb or a few fingers—and is absent in 20% of patients at presentation.
- Rigidity, which is felt by the examiner rather than seen by an observer.
- Bradykinesia (slow movements), which is characteristic of all Parkinson patients.
- Gait and balance problems, which usually arise after a few years, although occasionally patients present with them. Patients typically walk with small steps with occasional freezing, as if their foot were stuck. Balance problems are the most difficult to treat among the motor problems.
Asymmetry of motor problems is apparent in 75% of patients at presentation, although problems become bilateral later in the course of the disease.
NONMOTOR FEATURES CAN BE MORE DISABLING
Pain is common, but years ago it was not recognized as a specific feature of Parkinson disease. The pain from other conditions may also worsen.
Fatigue is very common and, if present, is usually one of the most disabling features.
Neuropsychiatric disturbances are among the most difficult problems, and they become increasingly common as motor symptoms are better controlled with treatment and patients live longer.
INCREASINGLY PREVALENT AS THE POPULATION AGES
Parkinson disease can present from the teenage years up to age 90, but it is most often diagnosed in patients from 60 to 70 years old (mean onset, 62.5 years). A different nomenclature is used depending on the age of onset:
- 10 to 20 years: juvenile-onset
- 21 to 40 years: young-onset.
Parkinson disease is now an epidemic, with an estimated 1 million people having it in the United States, representing 0.3% of the population and 1% of those older than 60 years.2 More people can be expected to develop it as our population ages in the next decades. It is estimated that in 2040 more people will die from Parkinson disease, Alzheimer disease, and amyotrophic lateral sclerosis (all of which are neurodegenerative diseases) than from kidney cancer, malignant melanoma, colon cancer, and lung cancer combined.
DIAGNOSIS IS STILL MAINLY CLINICAL
The diagnosis of Parkinson disease remains clinical. In addition to the motor features, the best test is a clear response to dopaminergic treatment with levodopa. If all these features are present, the diagnosis of Parkinson disease is usually correct.3
Imaging useful in select patients
The FDA recently approved a radiopharmaceutical contrast agent, DaTscan, to use with single-photon emission computed tomography (SPECT) to help diagnose Parkinson disease. DaTscan is a dopamine transporter ligand that tags presynaptic dopaminergic neurons in the basal ganglia; a patient with Parkinson disease has less signal.
The test can be used to distinguish parkinsonian syndromes from disorders that can mimic them, such as essential tremor or a psychogenic disorder. However, it cannot differentiate various Parkinson-plus syndromes (see below) such as multiple system atrophy or progressive nuclear palsy. It also cannot be used to detect drug-induced or vascular parkinsonism.
Check for Wilson disease or brain tumors in young or atypical cases
For most patients, no imaging or blood tests are needed to make the diagnosis. However, in patients younger than 50, Wilson disease, a rare inherited disorder characterized by excess copper accumulation, must be considered. Testing for Wilson disease includes serum ceruloplasmin, 24-hour urinary copper excretion, and an ophthalmologic slit-lamp examination for Kaiser-Fleischer rings.
For patients who do not quite fit the picture of Parkinson disease, such as those who have spasticity with little tremor, or who have a minimal response to levodopa, magnetic resonance imaging should be done to see if a structural lesion is present.
Consider secondary parkinsonism
Although idiopathic Parkinson disease is by far the most common form of parkinsonism in the United States and in most developing countries, secondary causes must also be considered in a patient presenting with symptoms of parkinsonism. They include:
- Dopamine-receptor blocking agents: metoclopramide (Reglan), prochlorperazine (Compazine), haloperidol (Haldol), thioridazine (Mellaril), risperidone (Risperdal), olanzapine (Zyprexa)
- Strokes in the basal ganglia
- Normal pressure hydrocephalus.
Parkinson-plus syndromes
Parkinson-plus syndromes have other features in addition to the classic features of idiopathic Parkinson disease. They occur commonly and can be difficult to distinguish from Parkinson disease and from each other.
Parkinson-plus syndromes include:
- Progressive supranuclear palsy
- Multiple system atrophy
- Corticobasal degeneration
- Lewy body dementia.
Clinical features that suggest a diagnosis other than Parkinson disease include poor response to adequate dosages of levodopa, early onset of postural instability, axial more than appendicular rigidity, early dementia, and inability to look up or down without needing to move the head (supranuclear palsy).4
MANAGING PARKINSON DISEASE
Nonpharmacologic therapy is very important. Because patients tend to live longer because of better treatment, education is particularly important. The benefits of exercise go beyond general conditioning and cardiovascular health. People who exercise vigorously at least three times a week for 30 to 45 minutes are less likely to develop Parkinson disease and, if they develop it, they tend to have slower progression.
Prevention with neuroprotective drugs is not yet an option but hopefully will be in the near future.
Drug treatment generally starts when the patient is functionally impaired. If so, either levodopa or a dopamine agonist is started, depending on the patient’s age and the severity of symptoms. With increasing severity, other drugs can be added, and when those fail to control symptoms, surgery should be considered.
Deep brain stimulation surgery can make a tremendous difference in a patient’s quality of life. Other than levodopa, it is probably the best therapy available; however, it is very expensive and is not without risks.
Levodopa: The most effective drug, until it wears off
All current drugs for Parkinson disease activate dopamine neurotransmission in the brain. The most effective—and the cheapest—is still carbidopa/levodopa (Sinemet, Parcopa, Atamet). Levodopa converts to dopamine both peripherally and after it crosses the blood-brain barrier. Carbidopa prevents the peripheral conversion of levodopa to dopamine, reducing the peripheral adverse effects of levodopa, such as nausea and vomiting. The combination drug is usually given three times a day, with different doses available (10 mg carbidopa/100 mg levodopa, 25/100, 50/200, and 25/250) and as immediate-release and controlled-release formulations as well as an orally dissolving form (Parcopa) for patients with difficulty swallowing.
The major problem with levodopa is that after 4 to 6 years of treatment, about 40% of patients develop motor fluctuations and dyskinesias.5 If treatment is started too soon or at too high a dose, these problems tend to develop even earlier, especially among younger patients.
Motor fluctuations can take many forms: slow wearing-off, abrupt loss of effectiveness, and random on-and-off effectiveness (“yo-yoing”).
Dyskinesias typically involve constant chorea (dance-like) movements and occur at peak dose. Although chorea is easily treated by lowering the dosage, patients generally prefer having these movements rather than the Parkinson symptoms that recur from underdosing.
Dopamine agonists may be best for younger patients in early stages
The next most effective class of drugs are the dopamine agonists: pramipexole (Mirapex), ropinirole (Requip), and bromocriptine (Parlodel). A fourth drug, pergolide, is no longer available because of associated valvular heart complications. Each can be used as monotherapy in mild, early Parkinson disease or as an additional drug for moderate to severe disease. They are longer-acting than levodopa and can be taken once daily. Although they are less likely than levodopa to cause wearing-off or dyskinesias, they are associated with more nonmotor side effects: nausea and vomiting, hallucinations, confusion, somnolence or sleep attacks, low blood pressure, edema, and impulse control disorders.
Multiple clinical trials have been conducted to test the efficacy of dopamine agonists vs levodopa for treating Parkinson disease.6–9 Almost always, levodopa is more effective but involves more wearing-off and dyskinesias. For this reason, for patients with milder parkinsonism who may not need the strongest drug available, trying one of the dopamine agonists first may be worthwhile.
In addition, patients younger than age 60 are more prone to develop motor fluctuations and dyskinesias, so a dopamine agonist should be tried first in patients in that age group. For patients over age 65 for whom cost may be of concern, levodopa is the preferred starting drug.
Anticholinergic drugs for tremor
Before 1969, only anticholinergic drugs were available to treat Parkinson disease. Examples include trihexyphenidyl (Artane, Trihexane) and benztropine (Cogentin). These drugs are effective for treating tremor and drooling but are much less useful against rigidity, bradykinesia, and balance problems. Side effects include confusion, dry mouth, constipation, blurred vision, urinary retention, and cognitive impairment.
Anticholinergics should only be considered for young patients in whom tremor is a large problem and who have not responded well to the traditional Parkinson drugs. Because tremor is mostly a cosmetic problem, anticholinergics can also be useful for treating actors, musicians, and other patients with a public role.
Monoamine oxidase B inhibitors are well tolerated but less effective
In the brain, dopamine is broken down by monoamine oxidase B (MAO-B); therefore, inhibiting this enzyme increases dopamine’s availability. The MAO-B inhibitors selegiline (Eldepryl, Zelapar) and rasagiline (Azilect) are effective for monotherapy for Parkinson disease but are not as effective as levodopa. Most physicians feel MAO-B inhibitors are also less effective than dopamine agonists, although double-blind, randomized clinical trials have not proven this.6,10,11
MAO-B inhibitors have a long half-life, allowing once-daily dosing, and they are very well tolerated, with a side-effect profile similar to that of placebo. As with all MAO inhibitors, caution is needed regarding drug and food interactions.
EFFECTIVE NEUROPROTECTIVE AGENTS REMAIN ELUSIVE
Although numerous drugs are now available to treat the symptoms of Parkinson disease, the ability to slow the progression of the disease remains elusive. The only factor consistently shown by epidemiologic evidence to be protective is cigarette smoking, but we don’t recommend it.
A number of agents have been tested for neuroprotective efficacy:
Coenzyme Q10 has been tested at low and high dosages but was not found to be effective.
Pramipexole, a dopamine agonist, has also been studied without success.
Creatine is currently being studied and shows promise, possibly because of its effects on complex-I, part of the electron transport chain in mitochondria, which may be disrupted in Parkinson disease.
Inosine, which elevates uric acid, is also promising. The link between high uric acid and Parkinson disease was serendipitously discovered: when evaluating numerous blood panels taken from patients with Parkinson disease who were in clinical trials (using what turned out to be ineffective agents), it was noted that patients with the slowest progression of disease tended to have the highest uric acid levels. This has led to trials evaluating the effect of elevating uric acid to a pre-gout threshold.
Calcium channel blockers may be protective, according to epidemiologic evidence. Experiments involving injecting isradipine (DynaCirc) in rat models of Parkinson disease have indicated that the drug is promising.
Rasagiline: Protective effects still unknown
A large study of the neuroprotective effects of the MAO-B inhibitor rasagiline has just been completed, but the results are uncertain.12 A unique “delayed-start” clinical trial design was used to try to evaluate whether this agent that is known to reduce symptoms may also be neuroprotective. More than 1,000 people with untreated Parkinson disease from 14 countries were randomly assigned to receive rasagiline (the early-start group) or placebo (the delayed-start group) for 36 weeks. Afterward, both groups were given rasagiline for another 36 weeks. Rasagiline was given in a daily dose of either 1 mg or 2 mg.
The investigators anticipated that if the benefits of rasagiline were purely symptomatic, the early- and delayed-start groups would have equivalent disease severity at the end of the study. If rasagiline were protective, the early-start group would be better off at the end of the study. Unfortunately, the results were ambiguous: the early- and delayed-start groups were equivalent at the end of the study if they received the 2-mg daily dose, apparently indicating no protective effect. But at the 1-mg daily dose, the delayed-start group developed more severe disease at 36 weeks and did not catch up to the early-start group after treatment with rasagiline, apparently indicating a protective benefit. As a result, no definitive conclusion can be drawn.
EXTENDING TREATMENT EFFECTS IN ADVANCED PARKINSON DISEASE
For most patients, the first 5 years after being diagnosed with Parkinson disease is the “honeymoon phase,” when almost any treatment is effective. During this time, patients tend to have enough surviving dopaminergic neurons to store levodopa, despite its very short half-life of only 60 minutes.
As the disease progresses, fewer dopaminergic neurons survive, the therapeutic window narrows, and dosing becomes a balancing act: too much dopamine causes dyskinesias, hallucinations, delusions, and impulsive behavior, and too little dopamine causes worsening of Parkinson symptoms, freezing, and wearing-off, with ensuing falls and fractures. At this stage, some patients are prescribed levodopa every 1.5 or 2 hours.
Drugs are now available that extend the half-life of levodopa by slowing the breakdown of dopamine.
Catechol-O-methyltransferase (COMT) inhibitors—including tolcapone (Tasmar) and entacapone (Comtan) (also available as combined cardidopa, entacapone, and levodopa [Stalevo])—reduce off periods by about 1 hour per day.13 Given that the price is about $2,500 per year, the cost and benefits to the patient must be considered.14–17
Rasagiline, an MAO-B inhibitor, can also be added to levodopa to extend the “on” time for about 1 hour a day and to reduce freezing of gait. Clinical trials have shown it to be well tolerated, although common side effects include worsening dyskinesias and nausea.18,19
Apomorphine (Apokyn) is a dopamine agonist given by subcutaneous injection, allowing it to avoid first-pass metabolism by the liver. The benefits start just 10 minutes after injection, but only last for about 1 hour. It is a good option for rescue therapy for patients who cannot swallow or who have severe, unpredictable, or painful off-periods. It is also useful for situations in which it is especially inconvenient to have an off-period, such as being away from home.
Many agents have been tested for improving the off-period, but most work for about 1 to 2 hours, which is not nearly as effective as deep brain stimulation.
Managing dyskinesias
Dyskinesias can be managed by giving lower doses of levodopa more often. If wearing-off is a problem, a dopamine agonist or MAO-B inhibitor can be added. For patients at this stage, a specialist should be consulted.
Amantadine (Symmetrel), an N-methyl-d-aspartate (NMDA) receptor antagonist and dopamine-releasing agent used to treat influenza, is also effective against dyskinesias. Adverse effects include anxiety, insomnia, nightmares, anticholinergic effects, and livedo reticularis.20,21
Deep brain stimulation is the best treatment for dyskinesias in a patient for whom the procedure is appropriate and who has medical insurance that covers it.
NONMOTOR FEATURES OF PARKINSON DISEASE
Dementia: One of the most limiting nonmotor features
Often the most limiting nonmotor feature of Parkinson disease is dementia, which develops at about four to six times the rate for age-matched controls. At a given time, about 40% of patients with Parkinson disease have dementia, and the risk is 80% over 15 years of the disease.
If dementia is present, many of the drugs effective against Parkinson disease cannot be used because of exacerbating side effects. Treatment is mainly restricted to levodopa.
The only FDA-approved drug to treat dementia in Parkinson disease is the same drug for Alzheimer disease, rivastigmine (Exelon). Its effects are only modest, and its cholinergic side effects may transiently worsen parkinsonian features.22
Psychosis: Also very common
About half of patients with Parkinson disease have an episode of hallucinations or delusions in their lifetime, and about 20% are actively psychotic at any time. Delusions typically have the theme of spousal infidelity. Psychosis is associated with a higher rate of death compared with patients with Parkinson disease who do not develop it. Rebound psychosis may occur on withdrawal of antipsychotic medication.23–27
Patients who develop psychosis should have a physical examination and laboratory evaluation to determine if an infection or electrolyte imbalance is the cause. Medications should be discontinued in the following order: anticholinergic drug, amantadine, MAO-B inhibitor, dopamine agonist, and COMT inhibitor. Levodopa and carbidopa should be reduced to the minimum tolerable yet effective dosages.
For a patient who still has psychosis despite a minimum Parkinson drug regimen, an atypical antipsychotic drug should be used. Although clozapine (Clozaril, FazaClo) is very effective without worsening parkinsonism, it requires weekly monitoring with a complete blood count because of the small (< 1%) risk of agranulocytosis. For that reason, the first-line drug is quetiapine (Seroquel). Most double-blind studies have not found it to be effective, yet it is the drug most often used. No other antipsychotic drugs are safe to treat Parkinson psychosis.
Many patients with Parkinson disease who are hospitalized become agitated and confused soon after they are admitted to the hospital. The best treatment is quetiapine if an oral drug can be prescribed. A benzodiazepine—eg, clonazepam (Klonopin), lorazepam (Ativan), diazepam (Valium)—at a low dose may also be effective. Haloperidol, risperidone, and olanzapine should not be given, as they block dopamine receptors and worsen rigidity.
Mood disturbances
Depression occurs in about half of patients with Parkinson disease and is a significant cause of functional impairment. About 25% of patients have anxiety, and 20% are apathetic.
Depression appears to be secondary to underlying neuroanatomic degeneration rather than a reaction to disability.28 Fortunately, most antidepressants are effective in patients with Parkinson disease.29,30 Bupropion (Wellbutrin) is a dopamine reuptake inhibitor and so increases the availability of dopamine, and it should also have antiparkinsonian effects, but unfortunately it does not. Conversely, selective serotonin reuptake inhibitors (SSRIs) theoretically can worsen or cause parkinsonism, but evidence shows that they are safe to use in patients with Parkinson disease. Some evidence indicates that tricyclic antidepressants may be superior to SSRIs for treating depression in patients with Parkinson disease, so they might be the better choice in patients who can tolerate them.
Compulsive behaviors such as punding (prolonged performance of repetitive, mechanical tasks, such as disassembling and reassembling household objects) may occur from levodopa.
In addition, impulse control disorders involving pathologic gambling, hypersexuality, compulsive shopping, or binge eating occur in about 8% of patients with Parkinson disease taking dopamine agonists. These behaviors are more likely to arise in young, single patients, who are also more likely to have a family history of impulsive control disorder.31
THE FUTURE OF DRUG THERAPY
Clinical trials are now testing new therapies that work the traditional way through dopaminergic mechanisms, as well as those that work in novel ways.
A large international trial is studying patients with newly diagnosed Parkinson disease to try to discover a biomarker. Parkinson disease is unlike many other diseases in that physicians can only use clinical features to measure improvement, which is very crude. Identifying a biomarker will make evaluating and monitoring treatment a more exact science, and will lead to faster development of effective treatments.
- Adler CH, Ahlskog JE. Parkinson’s Disease and Movement Disorders: Diagnosis and Treatment Guidelines for The Practicing Physician. Totowa, NJ: Humana Press; 2000.
- Nutt JG, Wooten GF. Clinical practice. Diagnosis and initial management of Parkinson’s disease. N Engl J Med 2005; 353:1021–1027.
- Litvan I, Bhatia KP, Burn DJ, et al; Movement Disorders Society Scientific Issues Committee. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 2003; 18:467–486.
- Wenning GK, Ben-Shlomo Y, Hughes A, Daniel SE, Lees A, Quinn NP. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000; 68:434–440.
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001; 16:448–458.
- Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group. JAMA 2000; 284:1931–1938.
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 2000; 342:1484–1491.
- Oertel WH, Wolters E, Sampaio C, et al. Pergolide versus levodopa monotherapy in early Parkinson’s disease patients: The PELMOPET study. Mov Disord 2006; 21:343–353.
- Lees AJ, Katzenschlager R, Head J, Ben-Shlomo Y. Ten-year follow-up of three different initial treatments in de-novo PD: a randomized trial. Neurology 2001; 57:1687–1694.
- Fowler JS, Volkow ND, Logan J, et al. Slow recovery of human brain MAO B after L-deprenyl (selegeline) withdrawal. Synapse 1994; 18:86–93.
- Elmer LW, Bertoni JM. The increasing role of monoamine oxidase type B inhibitors in Parkinson’s disease therapy. Expert Opin Pharmacother 2008; 9:2759–2772.
- Olanow CW, Rascol O, Hauser R, et al; ADAGIO Study Investigators. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 2009; 361:1268–1278. Erratum in: N Engl J Med 2011; 364:1882.
- Stocchi F, Barbato L, Nordera G, Bolner A, Caraceni T. Entacapone improves the pharmacokinetic and therapeutic response of controlled release levodopa/carbidopa in Parkinson’s patients. J Neural Transm 2004; 111:173–180.
- Brooks DJ, Sagar HUK-Irish Entacapone Study Group. Entacapone is beneficial in both fluctuating and non-fluctuating patients with Parkinson’s disease: a randomised, placebo controlled, double blind six month study. J Neurol Neurosurg Psychiatry 2003; 74:1071–1079.
- Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M; Celomen Study Group. Efficacy and safety of entacapone in Parkinson’s disease patients with soboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurol Scand 2002; 105:245–255.
- Rinne UK, Larsen JP, Siden A, Worm-Petersen J. Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Nomecomt Study Group. Neurology 1998; 51:1309–1314.
- Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Parkinson Study Group. Ann Neurol 1997; 42:747–755.
- Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol 2005; 62:241–248.
- Rascol O, Brooks DJ, Melamed E, et al; LARGO study group. Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet 2005; 365:947–954.
- Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol 1999; 56:1383–1386.
- Snow BJ, Macdonald L, Mcauley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 2000; 23:82–85.
- Almaraz AC, Driver-Dunckley ED, Woodruff BK, et al. Efficacy of rivastigmine for cognitive symptoms in Parkinson disease with dementia. Neurologist 2009; 15:234–237.
- Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 2000; 123:733–745.
- Fernandez HH, Donnelly EM, Friedman JH. Long-term outcome of clozapine use for psychosis in parkinsonian patients. Mov Disord 2004; 19:831–833.
- Goetz CG, Wuu J, Curgian LM, Leurgans S. Hallucinations and sleep disorders in PD: six-year prospective longitudinal study. Neurology 2005; 64:81–86.
- Tollefson GD, Dellva MA, Mattler CA, Kane JM, Wirshing DA, Kinon BJ. Controlled, double-blind investigation of the clozapine discontinuation symptoms with conversion to either olanzapine or placebo. The Collaborative Crossover Study Group. J Clin Psychopharmacol 1999; 19:435–443.
- Fernandez HH, Trieschmann ME, Okun MS. Rebound psychosis: effect of discontinuation of antipsychotics in Parkinson’s disease. Mov Disord 2005; 20:104–105.
- McDonald WM, Richard IH, DeLong MR. Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol Psychiatry 2003; 54:363–375.
- Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2008; 23:850–857.
- Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009; 72:886–892.
- Voon V, Sohr M, Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case-control study. Ann Neurol 2011; 69:986–996. .
More than a dozen drugs have been approved by the US Food and Drug Administration (FDA) for treating Parkinson disease, and more are expected in the near future. Many are currently in clinical trials, with the goals of finding ways to better control the disease with fewer adverse effects and, ultimately, to provide neuroprotection.
This article will review the features of Parkinson disease, the treatment options, and the complications in moderate to advanced disease.
PARKINSON DISEASE IS MULTIFACTORIAL
Although the cure for Parkinson disease is still elusive, much has been learned over the nearly 200 years since it was first described by James Parkinson in 1817. It is now understood to be a progressive neurodegenerative disease of multifactorial etiology: although a small proportion of patients have a direct inherited mutation that causes it, multiple genetic predisposition factors and environmental factors are more commonly involved.
The central pathology is dopaminergic loss in the basal ganglia, but other neurotransmitters are also involved and the disease extends to other areas of the brain.
CARDINAL MOTOR SYMPTOMS
In general, Parkinson disease is easy to identify. The classic patient has1:
- Tremor at rest, which can be subtle—such as only involving a thumb or a few fingers—and is absent in 20% of patients at presentation.
- Rigidity, which is felt by the examiner rather than seen by an observer.
- Bradykinesia (slow movements), which is characteristic of all Parkinson patients.
- Gait and balance problems, which usually arise after a few years, although occasionally patients present with them. Patients typically walk with small steps with occasional freezing, as if their foot were stuck. Balance problems are the most difficult to treat among the motor problems.
Asymmetry of motor problems is apparent in 75% of patients at presentation, although problems become bilateral later in the course of the disease.
NONMOTOR FEATURES CAN BE MORE DISABLING
Pain is common, but years ago it was not recognized as a specific feature of Parkinson disease. The pain from other conditions may also worsen.
Fatigue is very common and, if present, is usually one of the most disabling features.
Neuropsychiatric disturbances are among the most difficult problems, and they become increasingly common as motor symptoms are better controlled with treatment and patients live longer.
INCREASINGLY PREVALENT AS THE POPULATION AGES
Parkinson disease can present from the teenage years up to age 90, but it is most often diagnosed in patients from 60 to 70 years old (mean onset, 62.5 years). A different nomenclature is used depending on the age of onset:
- 10 to 20 years: juvenile-onset
- 21 to 40 years: young-onset.
Parkinson disease is now an epidemic, with an estimated 1 million people having it in the United States, representing 0.3% of the population and 1% of those older than 60 years.2 More people can be expected to develop it as our population ages in the next decades. It is estimated that in 2040 more people will die from Parkinson disease, Alzheimer disease, and amyotrophic lateral sclerosis (all of which are neurodegenerative diseases) than from kidney cancer, malignant melanoma, colon cancer, and lung cancer combined.
DIAGNOSIS IS STILL MAINLY CLINICAL
The diagnosis of Parkinson disease remains clinical. In addition to the motor features, the best test is a clear response to dopaminergic treatment with levodopa. If all these features are present, the diagnosis of Parkinson disease is usually correct.3
Imaging useful in select patients
The FDA recently approved a radiopharmaceutical contrast agent, DaTscan, to use with single-photon emission computed tomography (SPECT) to help diagnose Parkinson disease. DaTscan is a dopamine transporter ligand that tags presynaptic dopaminergic neurons in the basal ganglia; a patient with Parkinson disease has less signal.
The test can be used to distinguish parkinsonian syndromes from disorders that can mimic them, such as essential tremor or a psychogenic disorder. However, it cannot differentiate various Parkinson-plus syndromes (see below) such as multiple system atrophy or progressive nuclear palsy. It also cannot be used to detect drug-induced or vascular parkinsonism.
Check for Wilson disease or brain tumors in young or atypical cases
For most patients, no imaging or blood tests are needed to make the diagnosis. However, in patients younger than 50, Wilson disease, a rare inherited disorder characterized by excess copper accumulation, must be considered. Testing for Wilson disease includes serum ceruloplasmin, 24-hour urinary copper excretion, and an ophthalmologic slit-lamp examination for Kaiser-Fleischer rings.
For patients who do not quite fit the picture of Parkinson disease, such as those who have spasticity with little tremor, or who have a minimal response to levodopa, magnetic resonance imaging should be done to see if a structural lesion is present.
Consider secondary parkinsonism
Although idiopathic Parkinson disease is by far the most common form of parkinsonism in the United States and in most developing countries, secondary causes must also be considered in a patient presenting with symptoms of parkinsonism. They include:
- Dopamine-receptor blocking agents: metoclopramide (Reglan), prochlorperazine (Compazine), haloperidol (Haldol), thioridazine (Mellaril), risperidone (Risperdal), olanzapine (Zyprexa)
- Strokes in the basal ganglia
- Normal pressure hydrocephalus.
Parkinson-plus syndromes
Parkinson-plus syndromes have other features in addition to the classic features of idiopathic Parkinson disease. They occur commonly and can be difficult to distinguish from Parkinson disease and from each other.
Parkinson-plus syndromes include:
- Progressive supranuclear palsy
- Multiple system atrophy
- Corticobasal degeneration
- Lewy body dementia.
Clinical features that suggest a diagnosis other than Parkinson disease include poor response to adequate dosages of levodopa, early onset of postural instability, axial more than appendicular rigidity, early dementia, and inability to look up or down without needing to move the head (supranuclear palsy).4
MANAGING PARKINSON DISEASE
Nonpharmacologic therapy is very important. Because patients tend to live longer because of better treatment, education is particularly important. The benefits of exercise go beyond general conditioning and cardiovascular health. People who exercise vigorously at least three times a week for 30 to 45 minutes are less likely to develop Parkinson disease and, if they develop it, they tend to have slower progression.
Prevention with neuroprotective drugs is not yet an option but hopefully will be in the near future.
Drug treatment generally starts when the patient is functionally impaired. If so, either levodopa or a dopamine agonist is started, depending on the patient’s age and the severity of symptoms. With increasing severity, other drugs can be added, and when those fail to control symptoms, surgery should be considered.
Deep brain stimulation surgery can make a tremendous difference in a patient’s quality of life. Other than levodopa, it is probably the best therapy available; however, it is very expensive and is not without risks.
Levodopa: The most effective drug, until it wears off
All current drugs for Parkinson disease activate dopamine neurotransmission in the brain. The most effective—and the cheapest—is still carbidopa/levodopa (Sinemet, Parcopa, Atamet). Levodopa converts to dopamine both peripherally and after it crosses the blood-brain barrier. Carbidopa prevents the peripheral conversion of levodopa to dopamine, reducing the peripheral adverse effects of levodopa, such as nausea and vomiting. The combination drug is usually given three times a day, with different doses available (10 mg carbidopa/100 mg levodopa, 25/100, 50/200, and 25/250) and as immediate-release and controlled-release formulations as well as an orally dissolving form (Parcopa) for patients with difficulty swallowing.
The major problem with levodopa is that after 4 to 6 years of treatment, about 40% of patients develop motor fluctuations and dyskinesias.5 If treatment is started too soon or at too high a dose, these problems tend to develop even earlier, especially among younger patients.
Motor fluctuations can take many forms: slow wearing-off, abrupt loss of effectiveness, and random on-and-off effectiveness (“yo-yoing”).
Dyskinesias typically involve constant chorea (dance-like) movements and occur at peak dose. Although chorea is easily treated by lowering the dosage, patients generally prefer having these movements rather than the Parkinson symptoms that recur from underdosing.
Dopamine agonists may be best for younger patients in early stages
The next most effective class of drugs are the dopamine agonists: pramipexole (Mirapex), ropinirole (Requip), and bromocriptine (Parlodel). A fourth drug, pergolide, is no longer available because of associated valvular heart complications. Each can be used as monotherapy in mild, early Parkinson disease or as an additional drug for moderate to severe disease. They are longer-acting than levodopa and can be taken once daily. Although they are less likely than levodopa to cause wearing-off or dyskinesias, they are associated with more nonmotor side effects: nausea and vomiting, hallucinations, confusion, somnolence or sleep attacks, low blood pressure, edema, and impulse control disorders.
Multiple clinical trials have been conducted to test the efficacy of dopamine agonists vs levodopa for treating Parkinson disease.6–9 Almost always, levodopa is more effective but involves more wearing-off and dyskinesias. For this reason, for patients with milder parkinsonism who may not need the strongest drug available, trying one of the dopamine agonists first may be worthwhile.
In addition, patients younger than age 60 are more prone to develop motor fluctuations and dyskinesias, so a dopamine agonist should be tried first in patients in that age group. For patients over age 65 for whom cost may be of concern, levodopa is the preferred starting drug.
Anticholinergic drugs for tremor
Before 1969, only anticholinergic drugs were available to treat Parkinson disease. Examples include trihexyphenidyl (Artane, Trihexane) and benztropine (Cogentin). These drugs are effective for treating tremor and drooling but are much less useful against rigidity, bradykinesia, and balance problems. Side effects include confusion, dry mouth, constipation, blurred vision, urinary retention, and cognitive impairment.
Anticholinergics should only be considered for young patients in whom tremor is a large problem and who have not responded well to the traditional Parkinson drugs. Because tremor is mostly a cosmetic problem, anticholinergics can also be useful for treating actors, musicians, and other patients with a public role.
Monoamine oxidase B inhibitors are well tolerated but less effective
In the brain, dopamine is broken down by monoamine oxidase B (MAO-B); therefore, inhibiting this enzyme increases dopamine’s availability. The MAO-B inhibitors selegiline (Eldepryl, Zelapar) and rasagiline (Azilect) are effective for monotherapy for Parkinson disease but are not as effective as levodopa. Most physicians feel MAO-B inhibitors are also less effective than dopamine agonists, although double-blind, randomized clinical trials have not proven this.6,10,11
MAO-B inhibitors have a long half-life, allowing once-daily dosing, and they are very well tolerated, with a side-effect profile similar to that of placebo. As with all MAO inhibitors, caution is needed regarding drug and food interactions.
EFFECTIVE NEUROPROTECTIVE AGENTS REMAIN ELUSIVE
Although numerous drugs are now available to treat the symptoms of Parkinson disease, the ability to slow the progression of the disease remains elusive. The only factor consistently shown by epidemiologic evidence to be protective is cigarette smoking, but we don’t recommend it.
A number of agents have been tested for neuroprotective efficacy:
Coenzyme Q10 has been tested at low and high dosages but was not found to be effective.
Pramipexole, a dopamine agonist, has also been studied without success.
Creatine is currently being studied and shows promise, possibly because of its effects on complex-I, part of the electron transport chain in mitochondria, which may be disrupted in Parkinson disease.
Inosine, which elevates uric acid, is also promising. The link between high uric acid and Parkinson disease was serendipitously discovered: when evaluating numerous blood panels taken from patients with Parkinson disease who were in clinical trials (using what turned out to be ineffective agents), it was noted that patients with the slowest progression of disease tended to have the highest uric acid levels. This has led to trials evaluating the effect of elevating uric acid to a pre-gout threshold.
Calcium channel blockers may be protective, according to epidemiologic evidence. Experiments involving injecting isradipine (DynaCirc) in rat models of Parkinson disease have indicated that the drug is promising.
Rasagiline: Protective effects still unknown
A large study of the neuroprotective effects of the MAO-B inhibitor rasagiline has just been completed, but the results are uncertain.12 A unique “delayed-start” clinical trial design was used to try to evaluate whether this agent that is known to reduce symptoms may also be neuroprotective. More than 1,000 people with untreated Parkinson disease from 14 countries were randomly assigned to receive rasagiline (the early-start group) or placebo (the delayed-start group) for 36 weeks. Afterward, both groups were given rasagiline for another 36 weeks. Rasagiline was given in a daily dose of either 1 mg or 2 mg.
The investigators anticipated that if the benefits of rasagiline were purely symptomatic, the early- and delayed-start groups would have equivalent disease severity at the end of the study. If rasagiline were protective, the early-start group would be better off at the end of the study. Unfortunately, the results were ambiguous: the early- and delayed-start groups were equivalent at the end of the study if they received the 2-mg daily dose, apparently indicating no protective effect. But at the 1-mg daily dose, the delayed-start group developed more severe disease at 36 weeks and did not catch up to the early-start group after treatment with rasagiline, apparently indicating a protective benefit. As a result, no definitive conclusion can be drawn.
EXTENDING TREATMENT EFFECTS IN ADVANCED PARKINSON DISEASE
For most patients, the first 5 years after being diagnosed with Parkinson disease is the “honeymoon phase,” when almost any treatment is effective. During this time, patients tend to have enough surviving dopaminergic neurons to store levodopa, despite its very short half-life of only 60 minutes.
As the disease progresses, fewer dopaminergic neurons survive, the therapeutic window narrows, and dosing becomes a balancing act: too much dopamine causes dyskinesias, hallucinations, delusions, and impulsive behavior, and too little dopamine causes worsening of Parkinson symptoms, freezing, and wearing-off, with ensuing falls and fractures. At this stage, some patients are prescribed levodopa every 1.5 or 2 hours.
Drugs are now available that extend the half-life of levodopa by slowing the breakdown of dopamine.
Catechol-O-methyltransferase (COMT) inhibitors—including tolcapone (Tasmar) and entacapone (Comtan) (also available as combined cardidopa, entacapone, and levodopa [Stalevo])—reduce off periods by about 1 hour per day.13 Given that the price is about $2,500 per year, the cost and benefits to the patient must be considered.14–17
Rasagiline, an MAO-B inhibitor, can also be added to levodopa to extend the “on” time for about 1 hour a day and to reduce freezing of gait. Clinical trials have shown it to be well tolerated, although common side effects include worsening dyskinesias and nausea.18,19
Apomorphine (Apokyn) is a dopamine agonist given by subcutaneous injection, allowing it to avoid first-pass metabolism by the liver. The benefits start just 10 minutes after injection, but only last for about 1 hour. It is a good option for rescue therapy for patients who cannot swallow or who have severe, unpredictable, or painful off-periods. It is also useful for situations in which it is especially inconvenient to have an off-period, such as being away from home.
Many agents have been tested for improving the off-period, but most work for about 1 to 2 hours, which is not nearly as effective as deep brain stimulation.
Managing dyskinesias
Dyskinesias can be managed by giving lower doses of levodopa more often. If wearing-off is a problem, a dopamine agonist or MAO-B inhibitor can be added. For patients at this stage, a specialist should be consulted.
Amantadine (Symmetrel), an N-methyl-d-aspartate (NMDA) receptor antagonist and dopamine-releasing agent used to treat influenza, is also effective against dyskinesias. Adverse effects include anxiety, insomnia, nightmares, anticholinergic effects, and livedo reticularis.20,21
Deep brain stimulation is the best treatment for dyskinesias in a patient for whom the procedure is appropriate and who has medical insurance that covers it.
NONMOTOR FEATURES OF PARKINSON DISEASE
Dementia: One of the most limiting nonmotor features
Often the most limiting nonmotor feature of Parkinson disease is dementia, which develops at about four to six times the rate for age-matched controls. At a given time, about 40% of patients with Parkinson disease have dementia, and the risk is 80% over 15 years of the disease.
If dementia is present, many of the drugs effective against Parkinson disease cannot be used because of exacerbating side effects. Treatment is mainly restricted to levodopa.
The only FDA-approved drug to treat dementia in Parkinson disease is the same drug for Alzheimer disease, rivastigmine (Exelon). Its effects are only modest, and its cholinergic side effects may transiently worsen parkinsonian features.22
Psychosis: Also very common
About half of patients with Parkinson disease have an episode of hallucinations or delusions in their lifetime, and about 20% are actively psychotic at any time. Delusions typically have the theme of spousal infidelity. Psychosis is associated with a higher rate of death compared with patients with Parkinson disease who do not develop it. Rebound psychosis may occur on withdrawal of antipsychotic medication.23–27
Patients who develop psychosis should have a physical examination and laboratory evaluation to determine if an infection or electrolyte imbalance is the cause. Medications should be discontinued in the following order: anticholinergic drug, amantadine, MAO-B inhibitor, dopamine agonist, and COMT inhibitor. Levodopa and carbidopa should be reduced to the minimum tolerable yet effective dosages.
For a patient who still has psychosis despite a minimum Parkinson drug regimen, an atypical antipsychotic drug should be used. Although clozapine (Clozaril, FazaClo) is very effective without worsening parkinsonism, it requires weekly monitoring with a complete blood count because of the small (< 1%) risk of agranulocytosis. For that reason, the first-line drug is quetiapine (Seroquel). Most double-blind studies have not found it to be effective, yet it is the drug most often used. No other antipsychotic drugs are safe to treat Parkinson psychosis.
Many patients with Parkinson disease who are hospitalized become agitated and confused soon after they are admitted to the hospital. The best treatment is quetiapine if an oral drug can be prescribed. A benzodiazepine—eg, clonazepam (Klonopin), lorazepam (Ativan), diazepam (Valium)—at a low dose may also be effective. Haloperidol, risperidone, and olanzapine should not be given, as they block dopamine receptors and worsen rigidity.
Mood disturbances
Depression occurs in about half of patients with Parkinson disease and is a significant cause of functional impairment. About 25% of patients have anxiety, and 20% are apathetic.
Depression appears to be secondary to underlying neuroanatomic degeneration rather than a reaction to disability.28 Fortunately, most antidepressants are effective in patients with Parkinson disease.29,30 Bupropion (Wellbutrin) is a dopamine reuptake inhibitor and so increases the availability of dopamine, and it should also have antiparkinsonian effects, but unfortunately it does not. Conversely, selective serotonin reuptake inhibitors (SSRIs) theoretically can worsen or cause parkinsonism, but evidence shows that they are safe to use in patients with Parkinson disease. Some evidence indicates that tricyclic antidepressants may be superior to SSRIs for treating depression in patients with Parkinson disease, so they might be the better choice in patients who can tolerate them.
Compulsive behaviors such as punding (prolonged performance of repetitive, mechanical tasks, such as disassembling and reassembling household objects) may occur from levodopa.
In addition, impulse control disorders involving pathologic gambling, hypersexuality, compulsive shopping, or binge eating occur in about 8% of patients with Parkinson disease taking dopamine agonists. These behaviors are more likely to arise in young, single patients, who are also more likely to have a family history of impulsive control disorder.31
THE FUTURE OF DRUG THERAPY
Clinical trials are now testing new therapies that work the traditional way through dopaminergic mechanisms, as well as those that work in novel ways.
A large international trial is studying patients with newly diagnosed Parkinson disease to try to discover a biomarker. Parkinson disease is unlike many other diseases in that physicians can only use clinical features to measure improvement, which is very crude. Identifying a biomarker will make evaluating and monitoring treatment a more exact science, and will lead to faster development of effective treatments.
More than a dozen drugs have been approved by the US Food and Drug Administration (FDA) for treating Parkinson disease, and more are expected in the near future. Many are currently in clinical trials, with the goals of finding ways to better control the disease with fewer adverse effects and, ultimately, to provide neuroprotection.
This article will review the features of Parkinson disease, the treatment options, and the complications in moderate to advanced disease.
PARKINSON DISEASE IS MULTIFACTORIAL
Although the cure for Parkinson disease is still elusive, much has been learned over the nearly 200 years since it was first described by James Parkinson in 1817. It is now understood to be a progressive neurodegenerative disease of multifactorial etiology: although a small proportion of patients have a direct inherited mutation that causes it, multiple genetic predisposition factors and environmental factors are more commonly involved.
The central pathology is dopaminergic loss in the basal ganglia, but other neurotransmitters are also involved and the disease extends to other areas of the brain.
CARDINAL MOTOR SYMPTOMS
In general, Parkinson disease is easy to identify. The classic patient has1:
- Tremor at rest, which can be subtle—such as only involving a thumb or a few fingers—and is absent in 20% of patients at presentation.
- Rigidity, which is felt by the examiner rather than seen by an observer.
- Bradykinesia (slow movements), which is characteristic of all Parkinson patients.
- Gait and balance problems, which usually arise after a few years, although occasionally patients present with them. Patients typically walk with small steps with occasional freezing, as if their foot were stuck. Balance problems are the most difficult to treat among the motor problems.
Asymmetry of motor problems is apparent in 75% of patients at presentation, although problems become bilateral later in the course of the disease.
NONMOTOR FEATURES CAN BE MORE DISABLING
Pain is common, but years ago it was not recognized as a specific feature of Parkinson disease. The pain from other conditions may also worsen.
Fatigue is very common and, if present, is usually one of the most disabling features.
Neuropsychiatric disturbances are among the most difficult problems, and they become increasingly common as motor symptoms are better controlled with treatment and patients live longer.
INCREASINGLY PREVALENT AS THE POPULATION AGES
Parkinson disease can present from the teenage years up to age 90, but it is most often diagnosed in patients from 60 to 70 years old (mean onset, 62.5 years). A different nomenclature is used depending on the age of onset:
- 10 to 20 years: juvenile-onset
- 21 to 40 years: young-onset.
Parkinson disease is now an epidemic, with an estimated 1 million people having it in the United States, representing 0.3% of the population and 1% of those older than 60 years.2 More people can be expected to develop it as our population ages in the next decades. It is estimated that in 2040 more people will die from Parkinson disease, Alzheimer disease, and amyotrophic lateral sclerosis (all of which are neurodegenerative diseases) than from kidney cancer, malignant melanoma, colon cancer, and lung cancer combined.
DIAGNOSIS IS STILL MAINLY CLINICAL
The diagnosis of Parkinson disease remains clinical. In addition to the motor features, the best test is a clear response to dopaminergic treatment with levodopa. If all these features are present, the diagnosis of Parkinson disease is usually correct.3
Imaging useful in select patients
The FDA recently approved a radiopharmaceutical contrast agent, DaTscan, to use with single-photon emission computed tomography (SPECT) to help diagnose Parkinson disease. DaTscan is a dopamine transporter ligand that tags presynaptic dopaminergic neurons in the basal ganglia; a patient with Parkinson disease has less signal.
The test can be used to distinguish parkinsonian syndromes from disorders that can mimic them, such as essential tremor or a psychogenic disorder. However, it cannot differentiate various Parkinson-plus syndromes (see below) such as multiple system atrophy or progressive nuclear palsy. It also cannot be used to detect drug-induced or vascular parkinsonism.
Check for Wilson disease or brain tumors in young or atypical cases
For most patients, no imaging or blood tests are needed to make the diagnosis. However, in patients younger than 50, Wilson disease, a rare inherited disorder characterized by excess copper accumulation, must be considered. Testing for Wilson disease includes serum ceruloplasmin, 24-hour urinary copper excretion, and an ophthalmologic slit-lamp examination for Kaiser-Fleischer rings.
For patients who do not quite fit the picture of Parkinson disease, such as those who have spasticity with little tremor, or who have a minimal response to levodopa, magnetic resonance imaging should be done to see if a structural lesion is present.
Consider secondary parkinsonism
Although idiopathic Parkinson disease is by far the most common form of parkinsonism in the United States and in most developing countries, secondary causes must also be considered in a patient presenting with symptoms of parkinsonism. They include:
- Dopamine-receptor blocking agents: metoclopramide (Reglan), prochlorperazine (Compazine), haloperidol (Haldol), thioridazine (Mellaril), risperidone (Risperdal), olanzapine (Zyprexa)
- Strokes in the basal ganglia
- Normal pressure hydrocephalus.
Parkinson-plus syndromes
Parkinson-plus syndromes have other features in addition to the classic features of idiopathic Parkinson disease. They occur commonly and can be difficult to distinguish from Parkinson disease and from each other.
Parkinson-plus syndromes include:
- Progressive supranuclear palsy
- Multiple system atrophy
- Corticobasal degeneration
- Lewy body dementia.
Clinical features that suggest a diagnosis other than Parkinson disease include poor response to adequate dosages of levodopa, early onset of postural instability, axial more than appendicular rigidity, early dementia, and inability to look up or down without needing to move the head (supranuclear palsy).4
MANAGING PARKINSON DISEASE
Nonpharmacologic therapy is very important. Because patients tend to live longer because of better treatment, education is particularly important. The benefits of exercise go beyond general conditioning and cardiovascular health. People who exercise vigorously at least three times a week for 30 to 45 minutes are less likely to develop Parkinson disease and, if they develop it, they tend to have slower progression.
Prevention with neuroprotective drugs is not yet an option but hopefully will be in the near future.
Drug treatment generally starts when the patient is functionally impaired. If so, either levodopa or a dopamine agonist is started, depending on the patient’s age and the severity of symptoms. With increasing severity, other drugs can be added, and when those fail to control symptoms, surgery should be considered.
Deep brain stimulation surgery can make a tremendous difference in a patient’s quality of life. Other than levodopa, it is probably the best therapy available; however, it is very expensive and is not without risks.
Levodopa: The most effective drug, until it wears off
All current drugs for Parkinson disease activate dopamine neurotransmission in the brain. The most effective—and the cheapest—is still carbidopa/levodopa (Sinemet, Parcopa, Atamet). Levodopa converts to dopamine both peripherally and after it crosses the blood-brain barrier. Carbidopa prevents the peripheral conversion of levodopa to dopamine, reducing the peripheral adverse effects of levodopa, such as nausea and vomiting. The combination drug is usually given three times a day, with different doses available (10 mg carbidopa/100 mg levodopa, 25/100, 50/200, and 25/250) and as immediate-release and controlled-release formulations as well as an orally dissolving form (Parcopa) for patients with difficulty swallowing.
The major problem with levodopa is that after 4 to 6 years of treatment, about 40% of patients develop motor fluctuations and dyskinesias.5 If treatment is started too soon or at too high a dose, these problems tend to develop even earlier, especially among younger patients.
Motor fluctuations can take many forms: slow wearing-off, abrupt loss of effectiveness, and random on-and-off effectiveness (“yo-yoing”).
Dyskinesias typically involve constant chorea (dance-like) movements and occur at peak dose. Although chorea is easily treated by lowering the dosage, patients generally prefer having these movements rather than the Parkinson symptoms that recur from underdosing.
Dopamine agonists may be best for younger patients in early stages
The next most effective class of drugs are the dopamine agonists: pramipexole (Mirapex), ropinirole (Requip), and bromocriptine (Parlodel). A fourth drug, pergolide, is no longer available because of associated valvular heart complications. Each can be used as monotherapy in mild, early Parkinson disease or as an additional drug for moderate to severe disease. They are longer-acting than levodopa and can be taken once daily. Although they are less likely than levodopa to cause wearing-off or dyskinesias, they are associated with more nonmotor side effects: nausea and vomiting, hallucinations, confusion, somnolence or sleep attacks, low blood pressure, edema, and impulse control disorders.
Multiple clinical trials have been conducted to test the efficacy of dopamine agonists vs levodopa for treating Parkinson disease.6–9 Almost always, levodopa is more effective but involves more wearing-off and dyskinesias. For this reason, for patients with milder parkinsonism who may not need the strongest drug available, trying one of the dopamine agonists first may be worthwhile.
In addition, patients younger than age 60 are more prone to develop motor fluctuations and dyskinesias, so a dopamine agonist should be tried first in patients in that age group. For patients over age 65 for whom cost may be of concern, levodopa is the preferred starting drug.
Anticholinergic drugs for tremor
Before 1969, only anticholinergic drugs were available to treat Parkinson disease. Examples include trihexyphenidyl (Artane, Trihexane) and benztropine (Cogentin). These drugs are effective for treating tremor and drooling but are much less useful against rigidity, bradykinesia, and balance problems. Side effects include confusion, dry mouth, constipation, blurred vision, urinary retention, and cognitive impairment.
Anticholinergics should only be considered for young patients in whom tremor is a large problem and who have not responded well to the traditional Parkinson drugs. Because tremor is mostly a cosmetic problem, anticholinergics can also be useful for treating actors, musicians, and other patients with a public role.
Monoamine oxidase B inhibitors are well tolerated but less effective
In the brain, dopamine is broken down by monoamine oxidase B (MAO-B); therefore, inhibiting this enzyme increases dopamine’s availability. The MAO-B inhibitors selegiline (Eldepryl, Zelapar) and rasagiline (Azilect) are effective for monotherapy for Parkinson disease but are not as effective as levodopa. Most physicians feel MAO-B inhibitors are also less effective than dopamine agonists, although double-blind, randomized clinical trials have not proven this.6,10,11
MAO-B inhibitors have a long half-life, allowing once-daily dosing, and they are very well tolerated, with a side-effect profile similar to that of placebo. As with all MAO inhibitors, caution is needed regarding drug and food interactions.
EFFECTIVE NEUROPROTECTIVE AGENTS REMAIN ELUSIVE
Although numerous drugs are now available to treat the symptoms of Parkinson disease, the ability to slow the progression of the disease remains elusive. The only factor consistently shown by epidemiologic evidence to be protective is cigarette smoking, but we don’t recommend it.
A number of agents have been tested for neuroprotective efficacy:
Coenzyme Q10 has been tested at low and high dosages but was not found to be effective.
Pramipexole, a dopamine agonist, has also been studied without success.
Creatine is currently being studied and shows promise, possibly because of its effects on complex-I, part of the electron transport chain in mitochondria, which may be disrupted in Parkinson disease.
Inosine, which elevates uric acid, is also promising. The link between high uric acid and Parkinson disease was serendipitously discovered: when evaluating numerous blood panels taken from patients with Parkinson disease who were in clinical trials (using what turned out to be ineffective agents), it was noted that patients with the slowest progression of disease tended to have the highest uric acid levels. This has led to trials evaluating the effect of elevating uric acid to a pre-gout threshold.
Calcium channel blockers may be protective, according to epidemiologic evidence. Experiments involving injecting isradipine (DynaCirc) in rat models of Parkinson disease have indicated that the drug is promising.
Rasagiline: Protective effects still unknown
A large study of the neuroprotective effects of the MAO-B inhibitor rasagiline has just been completed, but the results are uncertain.12 A unique “delayed-start” clinical trial design was used to try to evaluate whether this agent that is known to reduce symptoms may also be neuroprotective. More than 1,000 people with untreated Parkinson disease from 14 countries were randomly assigned to receive rasagiline (the early-start group) or placebo (the delayed-start group) for 36 weeks. Afterward, both groups were given rasagiline for another 36 weeks. Rasagiline was given in a daily dose of either 1 mg or 2 mg.
The investigators anticipated that if the benefits of rasagiline were purely symptomatic, the early- and delayed-start groups would have equivalent disease severity at the end of the study. If rasagiline were protective, the early-start group would be better off at the end of the study. Unfortunately, the results were ambiguous: the early- and delayed-start groups were equivalent at the end of the study if they received the 2-mg daily dose, apparently indicating no protective effect. But at the 1-mg daily dose, the delayed-start group developed more severe disease at 36 weeks and did not catch up to the early-start group after treatment with rasagiline, apparently indicating a protective benefit. As a result, no definitive conclusion can be drawn.
EXTENDING TREATMENT EFFECTS IN ADVANCED PARKINSON DISEASE
For most patients, the first 5 years after being diagnosed with Parkinson disease is the “honeymoon phase,” when almost any treatment is effective. During this time, patients tend to have enough surviving dopaminergic neurons to store levodopa, despite its very short half-life of only 60 minutes.
As the disease progresses, fewer dopaminergic neurons survive, the therapeutic window narrows, and dosing becomes a balancing act: too much dopamine causes dyskinesias, hallucinations, delusions, and impulsive behavior, and too little dopamine causes worsening of Parkinson symptoms, freezing, and wearing-off, with ensuing falls and fractures. At this stage, some patients are prescribed levodopa every 1.5 or 2 hours.
Drugs are now available that extend the half-life of levodopa by slowing the breakdown of dopamine.
Catechol-O-methyltransferase (COMT) inhibitors—including tolcapone (Tasmar) and entacapone (Comtan) (also available as combined cardidopa, entacapone, and levodopa [Stalevo])—reduce off periods by about 1 hour per day.13 Given that the price is about $2,500 per year, the cost and benefits to the patient must be considered.14–17
Rasagiline, an MAO-B inhibitor, can also be added to levodopa to extend the “on” time for about 1 hour a day and to reduce freezing of gait. Clinical trials have shown it to be well tolerated, although common side effects include worsening dyskinesias and nausea.18,19
Apomorphine (Apokyn) is a dopamine agonist given by subcutaneous injection, allowing it to avoid first-pass metabolism by the liver. The benefits start just 10 minutes after injection, but only last for about 1 hour. It is a good option for rescue therapy for patients who cannot swallow or who have severe, unpredictable, or painful off-periods. It is also useful for situations in which it is especially inconvenient to have an off-period, such as being away from home.
Many agents have been tested for improving the off-period, but most work for about 1 to 2 hours, which is not nearly as effective as deep brain stimulation.
Managing dyskinesias
Dyskinesias can be managed by giving lower doses of levodopa more often. If wearing-off is a problem, a dopamine agonist or MAO-B inhibitor can be added. For patients at this stage, a specialist should be consulted.
Amantadine (Symmetrel), an N-methyl-d-aspartate (NMDA) receptor antagonist and dopamine-releasing agent used to treat influenza, is also effective against dyskinesias. Adverse effects include anxiety, insomnia, nightmares, anticholinergic effects, and livedo reticularis.20,21
Deep brain stimulation is the best treatment for dyskinesias in a patient for whom the procedure is appropriate and who has medical insurance that covers it.
NONMOTOR FEATURES OF PARKINSON DISEASE
Dementia: One of the most limiting nonmotor features
Often the most limiting nonmotor feature of Parkinson disease is dementia, which develops at about four to six times the rate for age-matched controls. At a given time, about 40% of patients with Parkinson disease have dementia, and the risk is 80% over 15 years of the disease.
If dementia is present, many of the drugs effective against Parkinson disease cannot be used because of exacerbating side effects. Treatment is mainly restricted to levodopa.
The only FDA-approved drug to treat dementia in Parkinson disease is the same drug for Alzheimer disease, rivastigmine (Exelon). Its effects are only modest, and its cholinergic side effects may transiently worsen parkinsonian features.22
Psychosis: Also very common
About half of patients with Parkinson disease have an episode of hallucinations or delusions in their lifetime, and about 20% are actively psychotic at any time. Delusions typically have the theme of spousal infidelity. Psychosis is associated with a higher rate of death compared with patients with Parkinson disease who do not develop it. Rebound psychosis may occur on withdrawal of antipsychotic medication.23–27
Patients who develop psychosis should have a physical examination and laboratory evaluation to determine if an infection or electrolyte imbalance is the cause. Medications should be discontinued in the following order: anticholinergic drug, amantadine, MAO-B inhibitor, dopamine agonist, and COMT inhibitor. Levodopa and carbidopa should be reduced to the minimum tolerable yet effective dosages.
For a patient who still has psychosis despite a minimum Parkinson drug regimen, an atypical antipsychotic drug should be used. Although clozapine (Clozaril, FazaClo) is very effective without worsening parkinsonism, it requires weekly monitoring with a complete blood count because of the small (< 1%) risk of agranulocytosis. For that reason, the first-line drug is quetiapine (Seroquel). Most double-blind studies have not found it to be effective, yet it is the drug most often used. No other antipsychotic drugs are safe to treat Parkinson psychosis.
Many patients with Parkinson disease who are hospitalized become agitated and confused soon after they are admitted to the hospital. The best treatment is quetiapine if an oral drug can be prescribed. A benzodiazepine—eg, clonazepam (Klonopin), lorazepam (Ativan), diazepam (Valium)—at a low dose may also be effective. Haloperidol, risperidone, and olanzapine should not be given, as they block dopamine receptors and worsen rigidity.
Mood disturbances
Depression occurs in about half of patients with Parkinson disease and is a significant cause of functional impairment. About 25% of patients have anxiety, and 20% are apathetic.
Depression appears to be secondary to underlying neuroanatomic degeneration rather than a reaction to disability.28 Fortunately, most antidepressants are effective in patients with Parkinson disease.29,30 Bupropion (Wellbutrin) is a dopamine reuptake inhibitor and so increases the availability of dopamine, and it should also have antiparkinsonian effects, but unfortunately it does not. Conversely, selective serotonin reuptake inhibitors (SSRIs) theoretically can worsen or cause parkinsonism, but evidence shows that they are safe to use in patients with Parkinson disease. Some evidence indicates that tricyclic antidepressants may be superior to SSRIs for treating depression in patients with Parkinson disease, so they might be the better choice in patients who can tolerate them.
Compulsive behaviors such as punding (prolonged performance of repetitive, mechanical tasks, such as disassembling and reassembling household objects) may occur from levodopa.
In addition, impulse control disorders involving pathologic gambling, hypersexuality, compulsive shopping, or binge eating occur in about 8% of patients with Parkinson disease taking dopamine agonists. These behaviors are more likely to arise in young, single patients, who are also more likely to have a family history of impulsive control disorder.31
THE FUTURE OF DRUG THERAPY
Clinical trials are now testing new therapies that work the traditional way through dopaminergic mechanisms, as well as those that work in novel ways.
A large international trial is studying patients with newly diagnosed Parkinson disease to try to discover a biomarker. Parkinson disease is unlike many other diseases in that physicians can only use clinical features to measure improvement, which is very crude. Identifying a biomarker will make evaluating and monitoring treatment a more exact science, and will lead to faster development of effective treatments.
- Adler CH, Ahlskog JE. Parkinson’s Disease and Movement Disorders: Diagnosis and Treatment Guidelines for The Practicing Physician. Totowa, NJ: Humana Press; 2000.
- Nutt JG, Wooten GF. Clinical practice. Diagnosis and initial management of Parkinson’s disease. N Engl J Med 2005; 353:1021–1027.
- Litvan I, Bhatia KP, Burn DJ, et al; Movement Disorders Society Scientific Issues Committee. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 2003; 18:467–486.
- Wenning GK, Ben-Shlomo Y, Hughes A, Daniel SE, Lees A, Quinn NP. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000; 68:434–440.
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001; 16:448–458.
- Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group. JAMA 2000; 284:1931–1938.
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 2000; 342:1484–1491.
- Oertel WH, Wolters E, Sampaio C, et al. Pergolide versus levodopa monotherapy in early Parkinson’s disease patients: The PELMOPET study. Mov Disord 2006; 21:343–353.
- Lees AJ, Katzenschlager R, Head J, Ben-Shlomo Y. Ten-year follow-up of three different initial treatments in de-novo PD: a randomized trial. Neurology 2001; 57:1687–1694.
- Fowler JS, Volkow ND, Logan J, et al. Slow recovery of human brain MAO B after L-deprenyl (selegeline) withdrawal. Synapse 1994; 18:86–93.
- Elmer LW, Bertoni JM. The increasing role of monoamine oxidase type B inhibitors in Parkinson’s disease therapy. Expert Opin Pharmacother 2008; 9:2759–2772.
- Olanow CW, Rascol O, Hauser R, et al; ADAGIO Study Investigators. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 2009; 361:1268–1278. Erratum in: N Engl J Med 2011; 364:1882.
- Stocchi F, Barbato L, Nordera G, Bolner A, Caraceni T. Entacapone improves the pharmacokinetic and therapeutic response of controlled release levodopa/carbidopa in Parkinson’s patients. J Neural Transm 2004; 111:173–180.
- Brooks DJ, Sagar HUK-Irish Entacapone Study Group. Entacapone is beneficial in both fluctuating and non-fluctuating patients with Parkinson’s disease: a randomised, placebo controlled, double blind six month study. J Neurol Neurosurg Psychiatry 2003; 74:1071–1079.
- Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M; Celomen Study Group. Efficacy and safety of entacapone in Parkinson’s disease patients with soboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurol Scand 2002; 105:245–255.
- Rinne UK, Larsen JP, Siden A, Worm-Petersen J. Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Nomecomt Study Group. Neurology 1998; 51:1309–1314.
- Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Parkinson Study Group. Ann Neurol 1997; 42:747–755.
- Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol 2005; 62:241–248.
- Rascol O, Brooks DJ, Melamed E, et al; LARGO study group. Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet 2005; 365:947–954.
- Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol 1999; 56:1383–1386.
- Snow BJ, Macdonald L, Mcauley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 2000; 23:82–85.
- Almaraz AC, Driver-Dunckley ED, Woodruff BK, et al. Efficacy of rivastigmine for cognitive symptoms in Parkinson disease with dementia. Neurologist 2009; 15:234–237.
- Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 2000; 123:733–745.
- Fernandez HH, Donnelly EM, Friedman JH. Long-term outcome of clozapine use for psychosis in parkinsonian patients. Mov Disord 2004; 19:831–833.
- Goetz CG, Wuu J, Curgian LM, Leurgans S. Hallucinations and sleep disorders in PD: six-year prospective longitudinal study. Neurology 2005; 64:81–86.
- Tollefson GD, Dellva MA, Mattler CA, Kane JM, Wirshing DA, Kinon BJ. Controlled, double-blind investigation of the clozapine discontinuation symptoms with conversion to either olanzapine or placebo. The Collaborative Crossover Study Group. J Clin Psychopharmacol 1999; 19:435–443.
- Fernandez HH, Trieschmann ME, Okun MS. Rebound psychosis: effect of discontinuation of antipsychotics in Parkinson’s disease. Mov Disord 2005; 20:104–105.
- McDonald WM, Richard IH, DeLong MR. Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol Psychiatry 2003; 54:363–375.
- Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2008; 23:850–857.
- Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009; 72:886–892.
- Voon V, Sohr M, Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case-control study. Ann Neurol 2011; 69:986–996. .
- Adler CH, Ahlskog JE. Parkinson’s Disease and Movement Disorders: Diagnosis and Treatment Guidelines for The Practicing Physician. Totowa, NJ: Humana Press; 2000.
- Nutt JG, Wooten GF. Clinical practice. Diagnosis and initial management of Parkinson’s disease. N Engl J Med 2005; 353:1021–1027.
- Litvan I, Bhatia KP, Burn DJ, et al; Movement Disorders Society Scientific Issues Committee. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord 2003; 18:467–486.
- Wenning GK, Ben-Shlomo Y, Hughes A, Daniel SE, Lees A, Quinn NP. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000; 68:434–440.
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001; 16:448–458.
- Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. Parkinson Study Group. JAMA 2000; 284:1931–1938.
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE. A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 2000; 342:1484–1491.
- Oertel WH, Wolters E, Sampaio C, et al. Pergolide versus levodopa monotherapy in early Parkinson’s disease patients: The PELMOPET study. Mov Disord 2006; 21:343–353.
- Lees AJ, Katzenschlager R, Head J, Ben-Shlomo Y. Ten-year follow-up of three different initial treatments in de-novo PD: a randomized trial. Neurology 2001; 57:1687–1694.
- Fowler JS, Volkow ND, Logan J, et al. Slow recovery of human brain MAO B after L-deprenyl (selegeline) withdrawal. Synapse 1994; 18:86–93.
- Elmer LW, Bertoni JM. The increasing role of monoamine oxidase type B inhibitors in Parkinson’s disease therapy. Expert Opin Pharmacother 2008; 9:2759–2772.
- Olanow CW, Rascol O, Hauser R, et al; ADAGIO Study Investigators. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 2009; 361:1268–1278. Erratum in: N Engl J Med 2011; 364:1882.
- Stocchi F, Barbato L, Nordera G, Bolner A, Caraceni T. Entacapone improves the pharmacokinetic and therapeutic response of controlled release levodopa/carbidopa in Parkinson’s patients. J Neural Transm 2004; 111:173–180.
- Brooks DJ, Sagar HUK-Irish Entacapone Study Group. Entacapone is beneficial in both fluctuating and non-fluctuating patients with Parkinson’s disease: a randomised, placebo controlled, double blind six month study. J Neurol Neurosurg Psychiatry 2003; 74:1071–1079.
- Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M; Celomen Study Group. Efficacy and safety of entacapone in Parkinson’s disease patients with soboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurol Scand 2002; 105:245–255.
- Rinne UK, Larsen JP, Siden A, Worm-Petersen J. Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Nomecomt Study Group. Neurology 1998; 51:1309–1314.
- Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Parkinson Study Group. Ann Neurol 1997; 42:747–755.
- Parkinson Study Group. A randomized placebo-controlled trial of rasagiline in levodopa-treated patients with Parkinson disease and motor fluctuations: the PRESTO study. Arch Neurol 2005; 62:241–248.
- Rascol O, Brooks DJ, Melamed E, et al; LARGO study group. Rasagiline as an adjunct to levodopa in patients with Parkinson’s disease and motor fluctuations (LARGO, Lasting effect in Adjunct therapy with Rasagiline Given Once daily, study): a randomised, double-blind, parallel-group trial. Lancet 2005; 365:947–954.
- Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol 1999; 56:1383–1386.
- Snow BJ, Macdonald L, Mcauley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 2000; 23:82–85.
- Almaraz AC, Driver-Dunckley ED, Woodruff BK, et al. Efficacy of rivastigmine for cognitive symptoms in Parkinson disease with dementia. Neurologist 2009; 15:234–237.
- Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 2000; 123:733–745.
- Fernandez HH, Donnelly EM, Friedman JH. Long-term outcome of clozapine use for psychosis in parkinsonian patients. Mov Disord 2004; 19:831–833.
- Goetz CG, Wuu J, Curgian LM, Leurgans S. Hallucinations and sleep disorders in PD: six-year prospective longitudinal study. Neurology 2005; 64:81–86.
- Tollefson GD, Dellva MA, Mattler CA, Kane JM, Wirshing DA, Kinon BJ. Controlled, double-blind investigation of the clozapine discontinuation symptoms with conversion to either olanzapine or placebo. The Collaborative Crossover Study Group. J Clin Psychopharmacol 1999; 19:435–443.
- Fernandez HH, Trieschmann ME, Okun MS. Rebound psychosis: effect of discontinuation of antipsychotics in Parkinson’s disease. Mov Disord 2005; 20:104–105.
- McDonald WM, Richard IH, DeLong MR. Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol Psychiatry 2003; 54:363–375.
- Devos D, Dujardin K, Poirot I, et al. Comparison of desipramine and citalopram treatments for depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2008; 23:850–857.
- Menza M, Dobkin RD, Marin H, et al. A controlled trial of antidepressants in patients with Parkinson disease and depression. Neurology 2009; 72:886–892.
- Voon V, Sohr M, Lang AE, et al. Impulse control disorders in Parkinson disease: a multicenter case-control study. Ann Neurol 2011; 69:986–996. .
KEY POINTS
- Parkinson disease can usually be diagnosed on the basis of clinical features: slow movement, resting tremor, rigidity, and asymmetrical presentation, as well as alleviation of symptoms with dopaminergic therapy.
- Early disease can be treated with levodopa, dopamine agonists, anticholinergics, and monoamine oxidase-B inhibitors.
- Advanced Parkinson disease may require a catechol-O-methyltransferase (COMT) inhibitor, apomorphine, and amantadine (Symmetrel). Side effects include motor fluctuations, dyskinesias, and cognitive problems.
Cytomegalovirus colitis
A 21-year-old woman with Crohn disease presented to the hospital after 5 days of diffuse abdominal pain, nausea, vomiting, and watery diarrhea despite taking azathioprine (Imuran) 100 mg daily as maintenance therapy. She had been hospitalized 2 weeks previously at another hospital for a Crohn disease flare, which was treated with intravenous methylprednisolone (Solu-Medrol).
On admission to our hospital, her temperature was 38.4°C (101.1°F), heart rate 78 per minute, respiratory rate 18 per minute, blood pressure 110/50 mm Hg, and oxygen saturation 98% while breathing room air. She had diffuse abdominal tenderness without rebound tenderness.
Because Clostridium difficile has a high prevalence in our hospital, treament for C difficile diarrhea was started empirically directly upon hospital admission; it was stopped 48 hours later when stool cultures came back negative for C difficile.
COLITIS AND CYTOMEGALOVIRUS INFECTION
CMV colitis is common in patients with inflammatory bowel disease (ie, Crohn disease or ulcerative colitis) who are on long-term immunosuppressive therapy. Heightened suspicion for it is needed when treating patients with inflammatory bowel disease, as they tend to present with atypical symptoms and signs.
It is also important to keep a wide differential diagnosis in mind, as acute fever and diarrhea in patients with inflammatory bowel disease are not always related to the underlying disease. In these patients, a variety of diagnostic tests may be necessary to exclude an opportunistic infection and an unrelated intercurrent illness.
Human CMV is a member of the family of herpes viruses, which persist for life after a primary infection. In exacerbations of inflammatory bowel disease, it is not clear whether CMV is a nonpathogenic bystander or a true pathogen.1 Most CMV infections in patients with inflammatory bowel disease are due to reactivation of the virus, as levels of inflammatory cytokines such as tumor necrosis factor are increased in the intestinal mucosa in active inflammatory bowel disease, and these cytokines are known to trigger reactivation.2
In patients with chronic inflammatory bowel disease, CMV colitis usually presents with abdominal pain, diarrhea, intestinal bleeding, and fever. The gold standard for diagnosis is immunohistochemical testing of colon biopsy samples using monoclonal antibodies against CMV. Owl’s eye inclusion bodies on histopathologic sections are highly specific for CMV infection. Other diagnostic studies include endoscopy and serologic testing.
The gastrointestinal tract is thought to contain latent CMV after a primary infection, and long-term treatment with immunomodulatory drugs such as azathioprine and corticosteroids can cause local reactivation of the latent virus.3
CMV infection in patients with inflammatory bowel disease is associated with poor outcomes, such as the need for colectomy.4 The prevalence of CMV infection in patients with inflammatory bowel disease has been reported as 5% to 36%, and higher in patients with disease refractory to steroid therapy.1,5
When a patient with inflammatory bowel disease is diagnosed with CMV infection, the immunomodulatory drugs should be stopped and the corticosteroids should be tapered to the lowest possible dose. Treatment of the infection is intravenous ganciclovir at 5 mg per kilogram of body weight twice daily for 14 days, followed by oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks.
After receiving intravenous ganciclovir for 14 days, our patient received oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks. Her azathioprine was stopped while she was taking the antivirals, and it was resumed the day after she completed the course of valacyclovir.
The response to treatment is monitored with a cytomegalovirus pp 65 antigenemia assay. Immunomodulatory therapy can be reintroduced slowly if needed.
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–2865.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest 1997; 100:3154–3163.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993; 119:924–935.
- Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol 2001; 96:773–775.
- Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance, and outcome. J Med Microbiol 2004; 53:1155–1160.
A 21-year-old woman with Crohn disease presented to the hospital after 5 days of diffuse abdominal pain, nausea, vomiting, and watery diarrhea despite taking azathioprine (Imuran) 100 mg daily as maintenance therapy. She had been hospitalized 2 weeks previously at another hospital for a Crohn disease flare, which was treated with intravenous methylprednisolone (Solu-Medrol).
On admission to our hospital, her temperature was 38.4°C (101.1°F), heart rate 78 per minute, respiratory rate 18 per minute, blood pressure 110/50 mm Hg, and oxygen saturation 98% while breathing room air. She had diffuse abdominal tenderness without rebound tenderness.
Because Clostridium difficile has a high prevalence in our hospital, treament for C difficile diarrhea was started empirically directly upon hospital admission; it was stopped 48 hours later when stool cultures came back negative for C difficile.
COLITIS AND CYTOMEGALOVIRUS INFECTION
CMV colitis is common in patients with inflammatory bowel disease (ie, Crohn disease or ulcerative colitis) who are on long-term immunosuppressive therapy. Heightened suspicion for it is needed when treating patients with inflammatory bowel disease, as they tend to present with atypical symptoms and signs.
It is also important to keep a wide differential diagnosis in mind, as acute fever and diarrhea in patients with inflammatory bowel disease are not always related to the underlying disease. In these patients, a variety of diagnostic tests may be necessary to exclude an opportunistic infection and an unrelated intercurrent illness.
Human CMV is a member of the family of herpes viruses, which persist for life after a primary infection. In exacerbations of inflammatory bowel disease, it is not clear whether CMV is a nonpathogenic bystander or a true pathogen.1 Most CMV infections in patients with inflammatory bowel disease are due to reactivation of the virus, as levels of inflammatory cytokines such as tumor necrosis factor are increased in the intestinal mucosa in active inflammatory bowel disease, and these cytokines are known to trigger reactivation.2
In patients with chronic inflammatory bowel disease, CMV colitis usually presents with abdominal pain, diarrhea, intestinal bleeding, and fever. The gold standard for diagnosis is immunohistochemical testing of colon biopsy samples using monoclonal antibodies against CMV. Owl’s eye inclusion bodies on histopathologic sections are highly specific for CMV infection. Other diagnostic studies include endoscopy and serologic testing.
The gastrointestinal tract is thought to contain latent CMV after a primary infection, and long-term treatment with immunomodulatory drugs such as azathioprine and corticosteroids can cause local reactivation of the latent virus.3
CMV infection in patients with inflammatory bowel disease is associated with poor outcomes, such as the need for colectomy.4 The prevalence of CMV infection in patients with inflammatory bowel disease has been reported as 5% to 36%, and higher in patients with disease refractory to steroid therapy.1,5
When a patient with inflammatory bowel disease is diagnosed with CMV infection, the immunomodulatory drugs should be stopped and the corticosteroids should be tapered to the lowest possible dose. Treatment of the infection is intravenous ganciclovir at 5 mg per kilogram of body weight twice daily for 14 days, followed by oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks.
After receiving intravenous ganciclovir for 14 days, our patient received oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks. Her azathioprine was stopped while she was taking the antivirals, and it was resumed the day after she completed the course of valacyclovir.
The response to treatment is monitored with a cytomegalovirus pp 65 antigenemia assay. Immunomodulatory therapy can be reintroduced slowly if needed.
A 21-year-old woman with Crohn disease presented to the hospital after 5 days of diffuse abdominal pain, nausea, vomiting, and watery diarrhea despite taking azathioprine (Imuran) 100 mg daily as maintenance therapy. She had been hospitalized 2 weeks previously at another hospital for a Crohn disease flare, which was treated with intravenous methylprednisolone (Solu-Medrol).
On admission to our hospital, her temperature was 38.4°C (101.1°F), heart rate 78 per minute, respiratory rate 18 per minute, blood pressure 110/50 mm Hg, and oxygen saturation 98% while breathing room air. She had diffuse abdominal tenderness without rebound tenderness.
Because Clostridium difficile has a high prevalence in our hospital, treament for C difficile diarrhea was started empirically directly upon hospital admission; it was stopped 48 hours later when stool cultures came back negative for C difficile.
COLITIS AND CYTOMEGALOVIRUS INFECTION
CMV colitis is common in patients with inflammatory bowel disease (ie, Crohn disease or ulcerative colitis) who are on long-term immunosuppressive therapy. Heightened suspicion for it is needed when treating patients with inflammatory bowel disease, as they tend to present with atypical symptoms and signs.
It is also important to keep a wide differential diagnosis in mind, as acute fever and diarrhea in patients with inflammatory bowel disease are not always related to the underlying disease. In these patients, a variety of diagnostic tests may be necessary to exclude an opportunistic infection and an unrelated intercurrent illness.
Human CMV is a member of the family of herpes viruses, which persist for life after a primary infection. In exacerbations of inflammatory bowel disease, it is not clear whether CMV is a nonpathogenic bystander or a true pathogen.1 Most CMV infections in patients with inflammatory bowel disease are due to reactivation of the virus, as levels of inflammatory cytokines such as tumor necrosis factor are increased in the intestinal mucosa in active inflammatory bowel disease, and these cytokines are known to trigger reactivation.2
In patients with chronic inflammatory bowel disease, CMV colitis usually presents with abdominal pain, diarrhea, intestinal bleeding, and fever. The gold standard for diagnosis is immunohistochemical testing of colon biopsy samples using monoclonal antibodies against CMV. Owl’s eye inclusion bodies on histopathologic sections are highly specific for CMV infection. Other diagnostic studies include endoscopy and serologic testing.
The gastrointestinal tract is thought to contain latent CMV after a primary infection, and long-term treatment with immunomodulatory drugs such as azathioprine and corticosteroids can cause local reactivation of the latent virus.3
CMV infection in patients with inflammatory bowel disease is associated with poor outcomes, such as the need for colectomy.4 The prevalence of CMV infection in patients with inflammatory bowel disease has been reported as 5% to 36%, and higher in patients with disease refractory to steroid therapy.1,5
When a patient with inflammatory bowel disease is diagnosed with CMV infection, the immunomodulatory drugs should be stopped and the corticosteroids should be tapered to the lowest possible dose. Treatment of the infection is intravenous ganciclovir at 5 mg per kilogram of body weight twice daily for 14 days, followed by oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks.
After receiving intravenous ganciclovir for 14 days, our patient received oral valacyclovir (Valtrex) 450 mg twice daily for 4 weeks. Her azathioprine was stopped while she was taking the antivirals, and it was resumed the day after she completed the course of valacyclovir.
The response to treatment is monitored with a cytomegalovirus pp 65 antigenemia assay. Immunomodulatory therapy can be reintroduced slowly if needed.
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–2865.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest 1997; 100:3154–3163.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993; 119:924–935.
- Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol 2001; 96:773–775.
- Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance, and outcome. J Med Microbiol 2004; 53:1155–1160.
- Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol 2006; 101:2857–2865.
- Söderberg-Nauclér C, Fish KN, Nelson JA. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Invest 1997; 100:3154–3163.
- Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993; 119:924–935.
- Cottone M, Pietrosi G, Martorana G, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn’s colitis. Am J Gastroenterol 2001; 96:773–775.
- Kishore J, Ghoshal U, Ghoshal UC, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance, and outcome. J Med Microbiol 2004; 53:1155–1160.