User login
Bugs, pundits, evolution, and the New Year
The article on soft-tissue infections in this issue of the Journal by Dr. Sabitha Rajan made me reflect on the relentless march of biology. Pathogens continue to evolve, influenced by human behavior but untouched by self-promoting and partisan dialogue and undaunted by doubting politicians. Several years ago, we could assume that most skin pathogens would readily be controlled by normal body defenses, a few requiring cephalosporin therapy and even fewer needing surgical intervention. But now, environmental pressures, including the zealous use of antibiotics, have altered the microbiology of skin infections. This requires new choices for empiric antibiotic therapy of these infections. With more than just altered susceptibility profiles, these bugs exhibit biologic behaviors distinct from their historic predecessors. The “spider bite” lesion of MRSA and the scarily rapid advance of certain streptococcal infections across tissue planes mandate prompt recognition by astute clinicians—the physical examination still matters.
The brisk evolutionary pace of this new range of infections stokes the urgent need to rapidly develop novel antibiotics, a process caught smack in the middle of our pundits’ political debates. Will the development of drugs for uncommon but serious infections be underwritten by the government, or will companies be required to bear the full expense of developing drugs under the scrutiny of the FDA? Will they then be pressed to price them “affordably” or price them to recoup estimated development costs, only to have payors list them as “third-tier” on the formulary, thus making them unaffordable to many patients? Our ability to medically confront this evolution will be directly affected by the outcome of the current political debate. Will all patients be able to easily access medical care so that early significant infections are recognized for what they are, and will the new antibiotics required for appropriate treatment be affordable? This year is going to be an interesting one.
So, as empiric therapy with cephalexin changes to clindamycin and 2011 rolls into 2012, I and our editorial staff offer our sincere wishes for a healthy, happy, and especially a peaceful New Year.
The article on soft-tissue infections in this issue of the Journal by Dr. Sabitha Rajan made me reflect on the relentless march of biology. Pathogens continue to evolve, influenced by human behavior but untouched by self-promoting and partisan dialogue and undaunted by doubting politicians. Several years ago, we could assume that most skin pathogens would readily be controlled by normal body defenses, a few requiring cephalosporin therapy and even fewer needing surgical intervention. But now, environmental pressures, including the zealous use of antibiotics, have altered the microbiology of skin infections. This requires new choices for empiric antibiotic therapy of these infections. With more than just altered susceptibility profiles, these bugs exhibit biologic behaviors distinct from their historic predecessors. The “spider bite” lesion of MRSA and the scarily rapid advance of certain streptococcal infections across tissue planes mandate prompt recognition by astute clinicians—the physical examination still matters.
The brisk evolutionary pace of this new range of infections stokes the urgent need to rapidly develop novel antibiotics, a process caught smack in the middle of our pundits’ political debates. Will the development of drugs for uncommon but serious infections be underwritten by the government, or will companies be required to bear the full expense of developing drugs under the scrutiny of the FDA? Will they then be pressed to price them “affordably” or price them to recoup estimated development costs, only to have payors list them as “third-tier” on the formulary, thus making them unaffordable to many patients? Our ability to medically confront this evolution will be directly affected by the outcome of the current political debate. Will all patients be able to easily access medical care so that early significant infections are recognized for what they are, and will the new antibiotics required for appropriate treatment be affordable? This year is going to be an interesting one.
So, as empiric therapy with cephalexin changes to clindamycin and 2011 rolls into 2012, I and our editorial staff offer our sincere wishes for a healthy, happy, and especially a peaceful New Year.
The article on soft-tissue infections in this issue of the Journal by Dr. Sabitha Rajan made me reflect on the relentless march of biology. Pathogens continue to evolve, influenced by human behavior but untouched by self-promoting and partisan dialogue and undaunted by doubting politicians. Several years ago, we could assume that most skin pathogens would readily be controlled by normal body defenses, a few requiring cephalosporin therapy and even fewer needing surgical intervention. But now, environmental pressures, including the zealous use of antibiotics, have altered the microbiology of skin infections. This requires new choices for empiric antibiotic therapy of these infections. With more than just altered susceptibility profiles, these bugs exhibit biologic behaviors distinct from their historic predecessors. The “spider bite” lesion of MRSA and the scarily rapid advance of certain streptococcal infections across tissue planes mandate prompt recognition by astute clinicians—the physical examination still matters.
The brisk evolutionary pace of this new range of infections stokes the urgent need to rapidly develop novel antibiotics, a process caught smack in the middle of our pundits’ political debates. Will the development of drugs for uncommon but serious infections be underwritten by the government, or will companies be required to bear the full expense of developing drugs under the scrutiny of the FDA? Will they then be pressed to price them “affordably” or price them to recoup estimated development costs, only to have payors list them as “third-tier” on the formulary, thus making them unaffordable to many patients? Our ability to medically confront this evolution will be directly affected by the outcome of the current political debate. Will all patients be able to easily access medical care so that early significant infections are recognized for what they are, and will the new antibiotics required for appropriate treatment be affordable? This year is going to be an interesting one.
So, as empiric therapy with cephalexin changes to clindamycin and 2011 rolls into 2012, I and our editorial staff offer our sincere wishes for a healthy, happy, and especially a peaceful New Year.
Skin and soft-tissue infections: Classifying and treating a spectrum
Skin and soft-tissue infections (SSTIs) are a common reason for presentation to outpatient practices, emergency rooms, and hospitals.1–5 They account for more than 14 million outpatient visits in the United States each year,1 and visits to the emergency room and admissions to the hospital for them are increasing.2,3 Hospital admissions for SSTIs increased by 29% from 2000 to 2004.3
MORE MRSA NOW, BUT STREPTOCOCCI ARE STILL COMMON
The increase in hospital admissions for SSTIs has been attributed to a rising number of infections with methicillin-resistant Staphylococcus aureus (MRSA).3–5
In addition, strains once seen mostly in the community and other strains that were associated with health care are now being seen more often in both settings. Clinical characteristics do not differ between community-acquired and health-care-associated MRSA, and therefore the distinction between the two is becoming less useful in guiding empiric therapy.6,7
After steadily increasing for several years, the incidence of MRSA has recently stabilized. The US Centers for Disease Control and Prevention maintains a surveillance program and a Web site on MRSA.8
COMPLICATED OR UNCOMPLICATED
The intent of the 1998 guideline was to provide not a clinical framework but rather a guide for industry in designing trials that would include similar groups of infections and therefore be relevant when compared with each other. In 2008, the Anti-Infective Drugs Advisory Committee was convened,11 and subsequently, in August 2010, the FDA released a revision of the guide.12
The revised guidelines specifically exclude many diagnoses, such as bite wounds, bone and joint infections, necrotizing fasciitis, diabetic foot infections, decubitus ulcers, catheter site infections, myonecrosis, and ecthyma gangrenosum. Notably, the word “bacterial” in the title excludes mycobacterial and fungal infections from consideration. The diagnoses that are included include cellulitis, erysipelas, major cutaneous abscess, and burn infections. These are further specified to include 75 cm2 of redness, edema, or induration to standardize the extent of the infection—ie, the infection has to be at least this large or else it is not “complicated.”
The terms “complicated” and “uncomplicated” skin and skin structure infections persist and can be useful adjuncts in describing SSTIs.13–16 However, more specific descriptions of SSTIs based on pathogenesis are more useful to the clinician and are usually the basis for guidelines, such as for preventing surgical site infections or for reducing amputations in diabetic foot infections.
This review will focus on the general categories of SSTI and will not address surgical site infections, pressure ulcers, diabetic foot infections, perirectal wounds, or adjuvant therapies in severe SSTIs, such as negative pressure wound care (vacuum-assisted closure devices) and hyperbaric chambers.
OTHER DISEASES CAN MIMIC SSTIs
SSTIs vary broadly in their location and severity.
Although the classic presentation of erythema, warmth, edema, and tenderness often signals infection, other diseases can mimic SSTIs. Common ones that should be included in the differential diagnosis include gout, thrombophlebitis, deep vein thrombosis, contact dermatitis, carcinoma erysipeloides, drug eruption, and a foreign body reaction.17,18
CLUES FROM THE HISTORY
Wounds. Skin infections are usually precipitated by a break in the skin from a cut, laceration, excoriation, fungal infection, insect or animal bite, or puncture wound.
Impaired response. Patients with diabetes, renal failure, cirrhosis, chronic glucocorticoid use, history of organ transplantation, chronic immunosuppressive therapy, HIV infection, or malnourishment have impaired host responses to infection and are at risk for both more severe infections and recurrent infections. Immunocompromised hosts may also have atypical infections with opportunistic organisms such as Pseudomonas, Proteus, Serratia, Enterobacter, Citrobacter, and anaerobes. Close follow-up of these patients is warranted to ascertain appropriate response to therapy.19
Surgery that includes lymph node dissection or saphenous vein resection for coronary artery bypass can lead to impaired lymphatic drainage and edema, and therefore predisposes patients to SSTIs.
PHYSICAL EXAMINATION
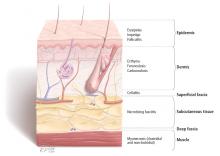
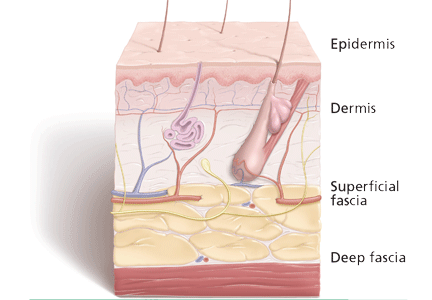
The physical examination should include descriptions of the extent and location of erythema, edema, warmth, and tenderness so that progression or resolution with treatment can be followed in detail.
Crepitus can be felt in gas-forming infections and raises the concern for necrotizing fasciitis and infection with anaerobic organisms such as Clostridium perfringens.
Necrosis can occur in brown recluse spider bites, venous snake bites, or group A streptococcal infections.
Fluctuance indicates fluid and a likely abscess that may need incision and drainage.
Purpura may be present in patients on anticoagulation therapy, but if it is accompanying an SSTI, it also raises the concern for the possibility of sepsis and disseminated intravascular coagulation, especially from streptococcal infections.
Bullae can be seen in impetigo caused by staphylococci or in infection with Vibrio vulnificus or Streptococcus pneumoniae.19
Systemic signs, in addition to fever, can include hypotension and tachycardia, which would prompt closer monitoring and possible hospitalization.
Lymphangitic spread also indicates severe infection.
LABORATORY STUDIES
Simple, localized SSTIs usually do not require laboratory evaluation. Jenkins et al21 recently demonstrated that by using an algorithm for the management of hospitalized patients with cellulitis or cutaneous abscess, they could decrease resource utilization, including laboratory testing, without adversely affecting clinical outcome.
If patients have underlying disease or more extensive infection, then baseline chemistry values, a complete blood cell count, and the C-reactive protein level should be acquired.19 Laboratory findings that suggest more severe disease include low sodium, low bicarbonate (or an anion gap), and high creatinine levels; new anemia; a high or very low white blood cell count; and a high C-reactive protein level. A high C-reactive protein level has been associated with longer hospitalization.22
A score to estimate the risk of necrotizing fasciitis
This tool was developed retrospectively but has been validated prospectively. It has a high sensitivity and a positive predictive value of 92% in patients with a score of six points or more. Its specificity is also high, with a negative predictive value of 96%.20,24
Necrotizing fasciitis has a mortality rate of 23.5%, but this may be reduced to 10% with early detection and prompt surgical intervention.15 Since necrotizing fasciitis is very difficult to diagnose, clinicians must maintain a high level of suspicion and use the LRINEC score to trigger early surgical evaluation. Surgical exploration is the only way to definitively diagnose necrotizing fasciitis.
Blood cultures in some cases
Blood cultures have a low yield and are usually not cost-effective, but they should be obtained in patients who have lymphedema, immune deficiency, fever, pain out of proportion to the findings on examination, tachycardia, or hypotension, as blood cultures are more likely to be positive in more serious infections and can help guide antimicrobial therapy. Blood cultures are also recommended in patients with infections involving specific anatomic sites, such as the mouth and eyes.19
Aspiration, swabs, incision and drainage
Fluid aspirated from abscesses and swabs of debrided ulcerated wounds should be sent for Gram stain and culture. Gram stain and culture have widely varying yields, from less than 5% to 40%, depending on the source and technique.19 Cultures were not routinely obtained before MRSA emerged, but knowing antimicrobial susceptibility is now important to guide antibiotic therapy. Unfortunately, in cellulitis, swabs and aspirates of the leading edge have a low yield of around 10%.25 One prospective study of 25 hospitalized patients did report a higher yield of positive cultures in patients with fever or underlying disease,26 so aspirates may be used in selected cases. In small studies, the yield of punch biopsies was slightly better than that of needle aspirates and was as high as 20% to 30%.27
IMAGING STUDIES
Imaging can be helpful in determining the depth of involvement. Plain radiography can reveal gas or periosteal inflammation and is especially helpful in diabetic foot infections. Ultrasonography can detect abscesses.
Both magnetic resonance imaging (MRI) and computed tomography (CT) are useful to image fascial planes, although MRI is more sensitive. However, in cases of suspected necrotizing fasciitis, imaging should not delay surgical evaluation and debridement or be used as the definitive study. Therefore, the practicality of CT and MRI can be limited.15,16
ANTIMICROBIAL TREATMENT FOR SSTIs IN OUTPATIENTS
For minor skin infections such as impetigo and secondarily infected skin lesions such as eczema, ulcers, or lacerations, mupirocin 2% topical ointment (Bactroban) can be effective.27
For a simple abscess or boil, incision and drainage is the primary treatment, and antibiotics are not needed.
For a complicated abscess or boil. Patients should be given oral or intravenous antibiotic therapy to cover MRSA and, depending on the severity, should be considered for hospitalization if the abscess is associated with severe disease, rapid progression in the presence of associated cellulitis, septic phlebitis, constitutional symptoms, comorbidity (including immunosuppression), or an abscess or boil in an area difficult to drain, such as the face, hands, or genitalia.27
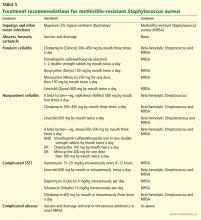
For purulent cellulitis in outpatients, empiric therapy for community-acquired MRSA is recommended, pending culture results. Empiric therapy for streptococcal infection is likely unnecessary. For empiric coverage of community-acquired MRSA in purulent cellulitis, oral antibiotic options include clindamycin (Cleocin), trimethoprim-sulfamethoxazole (Bactrim), doxycycline (Doryx), minocycline (Minocin), and linezolid (Zyvox).
For nonpurulent cellulitis in outpatients, empiric coverage for beta-hemolytic streptococci is warranted. Coverage for community-acquired MRSA should subsequently be added for patients who do not respond to beta-lactam therapy within 48 to 72 hours or who have chills, fever, a new abscess, increasing erythema, or uncontrolled pain.
Options for coverage of both beta-hemolytic streptococci and community-acquired MRSA for outpatient therapy include clindamycin on its own, trimethoprim-sulfamethoxazole or a tetracycline in combination with a beta-lactam, or linezolid on its own.
Increasing rates of resistance to clindamycin, tetracycline, and trimethoprim-sulfamethoxazole in community-acquired MRSA may limit empiric treatment. In areas where resistance is prevalent, culture with antimicrobial susceptibility testing may be required before starting one of these antibiotics.
The use of rifampin (Rifadin) as a single agent is not recommended because resistance is likely to develop. Also, rifampin is not useful as adjunctive therapy, as evidence does not support its efficacy.19,27,29
ANTIMICROBIAL TREATMENT FOR SSTIs IN HOSPITALIZED PATIENTS
For hospitalized patients with a complicated or severe SSTI, empiric therapy for MRSA should be started pending culture results. FDA-approved options are vancomycin, linezolid, daptomycin (Cubicin), tigecycline (Tygacil), and telavancin (Vibativ). Data on clindamycin are very limited in this population. A beta-lactam antibiotic such as cefazolin (Ancef) may be considered in hospitalized patients with nonpurulent cellulitis, and the regimen can be modified to MRSA-active therapy if there is no clinical response. Linezolid, daptomycin, vancomycin, and telavancin have adequate streptococcal coverage in addition to MRSA coverage.
Clindamycin is approved by the FDA for treating serious infections due to S aureus. It has excellent tissue penetration, particularly in bone and abscesses.
Clindamycin resistance in staphylococci can be either constitutive or inducible, and clinicians must be watchful for signs of resistance.
Diarrhea is the most common adverse effect and occurs in up to 20% of patients. Clostridium difficile colitis may occur more frequently with clindamycin than with other oral agents, but it has also has been reported with fluoroquinolones and can be associated with any antibiotic therapy.30
Trimethoprim-sulfamethoxazole is not FDA-approved for treating any staphylococcal infection. However, because 95% to 100% of community-acquired MRSA strains are susceptible to it in vitro, it has become an important option in the outpatient treatment of SSTIs. Caution is advised when using it in elderly patients, particularly those with chronic renal insufficiency, because of an increased risk of hyperkalemia.
Tetracyclines. Doxycycline is FDA-approved for treating SSTIs due to S aureus, although not specifically for MRSA. Minocycline may be an option even when strains are resistant to doxycycline, since it does not induce its own resistance as doxycycline does.
Tigecycline is a glycylcycline (a tetracycline derivative) and is FDA-approved in adults for complicated SSTIs and intra-abdominal infections. It has a large volume of distribution and achieves high concentrations in tissues and low concentrations in serum.
The FDA recently issued a warning to consider alternative agents in patients with serious infections because of higher rates of all-cause mortality noted in phase III and phase IV clinical trials. Due to this warning and the availability of multiple alternatives active against MRSA, tigecycline was not included in the Infectious Diseases Society of America guidelines.31
Linezolid is a synthetic oxazolidinone and is FDA-approved for treating SSTIs and nosocomial pneumonia caused by MRSA. It has 100% oral bioavailability, so parenteral therapy should only be given if there are problems with gastrointestinal absorption or if the patient is unable to take oral medications.
Long-term use of linezolid (> 2 weeks) is limited by hematologic toxicity, especially thrombocytopenia, which occurs more frequently than anemia and neutropenia. Lactic acidosis and peripheral and optic neuropathy are also limiting toxicities. Although myelosuppression is generally reversible, peripheral and optic neuropathy may not be.
Linezolid should not used in patients taking selective serotonin reuptake inhibitors if they cannot stop taking these antidepressant drugs during therapy, as the combination can lead to the serotonin syndrome.
Vancomycin is still the mainstay of parenteral therapy for MRSA infections. However, its efficacy has come into question, with concerns over its slow bactericidal activity and the emergence of resistant strains. The rate of treatment failure is high in those with infection caused by MRSA having minimum inhibitory concentrations of 1 μg/mL or greater. Vancomycin kills staphylococci more slowly than do beta-lactams in vitro and is clearly inferior to beta-lactams for methicillin-sensitive S aureus bacteremia.
Daptomycin is a lipopeptide antibiotic that is FDA-approved for adults with MRSA bacteremia, right-sided infective endocarditis, and complicated SSTI. Elevations in creatinine phosphokinase, which are rarely treatment-limiting, have occurred in patients receiving 6 mg/kg/day but not in those receiving 4 mg/kg/day. Patients should be observed for development of muscle pain or weakness and should have their creatine phosphokinase levels checked weekly, with more frequent monitoring in those with renal insufficiency or who are receiving concomitant statin therapy.
Telavancin is a parenteral lipoglycopeptide that is bactericidal against MRSA. It is FDA-approved for complicated SSTIs in adults. Creatinine levels should be monitored, and the dosage should be adjusted on the basis of creatinine clearance, because nephrotoxicity was more commonly reported among individuals treated with telavancin than among those treated with vancomycin.
Ceftaroline (Teflaro), a fifth-generation cephalosporin, was approved for SSTIs by the FDA in October 2010. It is active against MRSA and gram-negative pathogens.
Cost is a consideration
Cost is a consideration, as it may limit the availability of and access to treatment. In 2008, the expense for 10 days of treatment with generic vancomycin was $183, compared with $1,661 for daptomycin, $1,362 for tigecycline, and $1,560 for linezolid. For outpatient therapy, the contrast was even starker, as generic trimethoprim-sulfamethoxazole cost $9.40 and generic clindamycin cost $95.10.32
INDICATIONS FOR HOSPITALIZATION
Patients who have evidence of tissue necrosis, fever, hypotension, severe pain, altered mental status, an immunocompromised state, or organ failure (respiratory, renal, or hepatic) must be hospitalized.
Although therapy for MRSA is the mainstay of empiric therapy, polymicrobial infections are not uncommon, and gram-negative and anaerobic coverage should be added as appropriate. One study revealed a longer length of stay for hospitalized patients who had inadequate initial empiric coverage.33
Vigilance should be maintained for overlying cellulitis which can mask necrotizing fasciitis, septic joints, or osteomyelitis.
Perianal abscesses and infections, infected decubitus ulcers, and moderate to severe diabetic foot infections are often polymicrobial and warrant coverage for streptococci, MRSA, aerobic gram-negative bacilli, and anaerobes until culture results can guide therapy.
INDICATIONS FOR SURGICAL REFERRAL
Extensive perianal or multiple abscesses may require surgical drainage and debridement.
Surgical site infections should be referred for consideration of opening the incision for drainage.
Necrotizing infections warrant prompt aggressive surgical debridement. Strongly suggestive clinical signs include bullae, crepitus, gas on radiography, hypotension with systolic blood pressure less than 90 mm Hg, or skin necrosis. However, these are late findings, and fewer than 50% of these patients have one of these. Most cases of necrotizing fasciitis originally have an admitting diagnosis of cellulitis and cases of fasciitis are relatively rare, so the diagnosis is easy to miss.15,16 Patients with an LRINEC score of six or more should have prompt surgical evaluation.20,24,34,35
- Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–1591.
- Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008; 51:291–298.
- Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–1518.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007; 357:380–390.
- Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–1771.
- Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis 2011; 52:99–114.
- Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 2007; 44:471–482.
- Centers for Disease Control and Prevention. MRSA Infections. http://www.cdc.gov/mrsa/statistics/MRSA-Surveillance-Summary.html. Accessed December 14, 2011.
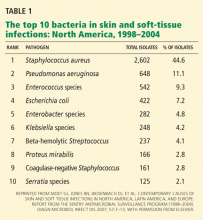
- Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 2007; 57:7–13.
- US Department of Health and Human Services. Guidance for Industry: Uncomplicated and Complicated Skin and Skin Structure Infections—Developing Antimicrobial Drugs for Treatment (draft guidance). July 1998. http://www.fda.gov/ohrms/dockets/98fr/2566dft.pdf. Accessed September 7, 2011.
- US Food and Drug Administration. CDER 2008 Meeting Documents. Anti-Infective Drugs Advisory Committee. http://www.fda.gov/ohrms/dockets/ac/cder08.html#AntiInfective. Accessed September 7, 2011.
- US Department of Health and Human Services. Guidance for Industry: Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment (draft guidance). August 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed December 14, 2011.
- Cornia PB, Davidson HL, Lipsky BA. The evaluation and treatment of complicated skin and skin structure infections. Expert Opin Pharmacother 2008; 9:717–730.
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008; 19:173–184.
- May AK, Stafford RE, Bulger EM, et al; Surgical Infection Society. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt) 2009; 10:467–499.
- Napolitano LM. Severe soft tissue infections. Infect Dis Clin North Am 2009; 23:571–591.
- Papadavid E, Dalamaga M, Stavrianeas N, Papiris SA. Subcutaneous sarcoidosis masquerading as cellulitis. Dermatology 2008; 217:212–214.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32:1535–1541.
- Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med 2011; 171:1072–1079.
- Lazzarini L, Conti E, Tositti G, de Lalla F. Erysipelas and cellulitis: clinical and microbiological spectrum in an Italian tertiary care hospital. J Infect 2005; 51:383–389.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis 2007; 44:705–710.
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ 2005; 330:830–833.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol 1988; 26:401–404.
- Sachs MK. The optimum use of needle aspiration in the bacteriologic diagnosis of cellulitis in adults. Arch Intern Med 1990; 150:1907–1912.
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–e55.
- Hammond SP, Baden LR. Clinical decisions. Management of skin and soft-tissue infection—polling results. N Engl J Med 2008; 359:e20.
- Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med 2008; 168:805–819.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40:1–15.
- US Food and Drug Administration. FDA Drug Safety Communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. September 2010. http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm. Accessed September 7, 2011.
- Moellering RC. A 39-year-old man with a skin infection. JAMA 2008; 299:79–87.
- Zilberberg MD, Shorr AF, Micek ST, et al. Hospitalizations with healthcare-associated complicated skin and skin structure infections: impact of inappropriate empiric therapy on outcomes. J Hosp Med 2010; 5:535–540.
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003; 85:1454–1460.
- Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med 2008; 26:170–175.
Skin and soft-tissue infections (SSTIs) are a common reason for presentation to outpatient practices, emergency rooms, and hospitals.1–5 They account for more than 14 million outpatient visits in the United States each year,1 and visits to the emergency room and admissions to the hospital for them are increasing.2,3 Hospital admissions for SSTIs increased by 29% from 2000 to 2004.3
MORE MRSA NOW, BUT STREPTOCOCCI ARE STILL COMMON
The increase in hospital admissions for SSTIs has been attributed to a rising number of infections with methicillin-resistant Staphylococcus aureus (MRSA).3–5
In addition, strains once seen mostly in the community and other strains that were associated with health care are now being seen more often in both settings. Clinical characteristics do not differ between community-acquired and health-care-associated MRSA, and therefore the distinction between the two is becoming less useful in guiding empiric therapy.6,7
After steadily increasing for several years, the incidence of MRSA has recently stabilized. The US Centers for Disease Control and Prevention maintains a surveillance program and a Web site on MRSA.8
COMPLICATED OR UNCOMPLICATED
The intent of the 1998 guideline was to provide not a clinical framework but rather a guide for industry in designing trials that would include similar groups of infections and therefore be relevant when compared with each other. In 2008, the Anti-Infective Drugs Advisory Committee was convened,11 and subsequently, in August 2010, the FDA released a revision of the guide.12
The revised guidelines specifically exclude many diagnoses, such as bite wounds, bone and joint infections, necrotizing fasciitis, diabetic foot infections, decubitus ulcers, catheter site infections, myonecrosis, and ecthyma gangrenosum. Notably, the word “bacterial” in the title excludes mycobacterial and fungal infections from consideration. The diagnoses that are included include cellulitis, erysipelas, major cutaneous abscess, and burn infections. These are further specified to include 75 cm2 of redness, edema, or induration to standardize the extent of the infection—ie, the infection has to be at least this large or else it is not “complicated.”
The terms “complicated” and “uncomplicated” skin and skin structure infections persist and can be useful adjuncts in describing SSTIs.13–16 However, more specific descriptions of SSTIs based on pathogenesis are more useful to the clinician and are usually the basis for guidelines, such as for preventing surgical site infections or for reducing amputations in diabetic foot infections.
This review will focus on the general categories of SSTI and will not address surgical site infections, pressure ulcers, diabetic foot infections, perirectal wounds, or adjuvant therapies in severe SSTIs, such as negative pressure wound care (vacuum-assisted closure devices) and hyperbaric chambers.
OTHER DISEASES CAN MIMIC SSTIs
SSTIs vary broadly in their location and severity.
Although the classic presentation of erythema, warmth, edema, and tenderness often signals infection, other diseases can mimic SSTIs. Common ones that should be included in the differential diagnosis include gout, thrombophlebitis, deep vein thrombosis, contact dermatitis, carcinoma erysipeloides, drug eruption, and a foreign body reaction.17,18
CLUES FROM THE HISTORY
Wounds. Skin infections are usually precipitated by a break in the skin from a cut, laceration, excoriation, fungal infection, insect or animal bite, or puncture wound.
Impaired response. Patients with diabetes, renal failure, cirrhosis, chronic glucocorticoid use, history of organ transplantation, chronic immunosuppressive therapy, HIV infection, or malnourishment have impaired host responses to infection and are at risk for both more severe infections and recurrent infections. Immunocompromised hosts may also have atypical infections with opportunistic organisms such as Pseudomonas, Proteus, Serratia, Enterobacter, Citrobacter, and anaerobes. Close follow-up of these patients is warranted to ascertain appropriate response to therapy.19
Surgery that includes lymph node dissection or saphenous vein resection for coronary artery bypass can lead to impaired lymphatic drainage and edema, and therefore predisposes patients to SSTIs.
PHYSICAL EXAMINATION
The physical examination should include descriptions of the extent and location of erythema, edema, warmth, and tenderness so that progression or resolution with treatment can be followed in detail.
Crepitus can be felt in gas-forming infections and raises the concern for necrotizing fasciitis and infection with anaerobic organisms such as Clostridium perfringens.
Necrosis can occur in brown recluse spider bites, venous snake bites, or group A streptococcal infections.
Fluctuance indicates fluid and a likely abscess that may need incision and drainage.
Purpura may be present in patients on anticoagulation therapy, but if it is accompanying an SSTI, it also raises the concern for the possibility of sepsis and disseminated intravascular coagulation, especially from streptococcal infections.
Bullae can be seen in impetigo caused by staphylococci or in infection with Vibrio vulnificus or Streptococcus pneumoniae.19
Systemic signs, in addition to fever, can include hypotension and tachycardia, which would prompt closer monitoring and possible hospitalization.
Lymphangitic spread also indicates severe infection.
LABORATORY STUDIES
Simple, localized SSTIs usually do not require laboratory evaluation. Jenkins et al21 recently demonstrated that by using an algorithm for the management of hospitalized patients with cellulitis or cutaneous abscess, they could decrease resource utilization, including laboratory testing, without adversely affecting clinical outcome.
If patients have underlying disease or more extensive infection, then baseline chemistry values, a complete blood cell count, and the C-reactive protein level should be acquired.19 Laboratory findings that suggest more severe disease include low sodium, low bicarbonate (or an anion gap), and high creatinine levels; new anemia; a high or very low white blood cell count; and a high C-reactive protein level. A high C-reactive protein level has been associated with longer hospitalization.22
A score to estimate the risk of necrotizing fasciitis
This tool was developed retrospectively but has been validated prospectively. It has a high sensitivity and a positive predictive value of 92% in patients with a score of six points or more. Its specificity is also high, with a negative predictive value of 96%.20,24
Necrotizing fasciitis has a mortality rate of 23.5%, but this may be reduced to 10% with early detection and prompt surgical intervention.15 Since necrotizing fasciitis is very difficult to diagnose, clinicians must maintain a high level of suspicion and use the LRINEC score to trigger early surgical evaluation. Surgical exploration is the only way to definitively diagnose necrotizing fasciitis.
Blood cultures in some cases
Blood cultures have a low yield and are usually not cost-effective, but they should be obtained in patients who have lymphedema, immune deficiency, fever, pain out of proportion to the findings on examination, tachycardia, or hypotension, as blood cultures are more likely to be positive in more serious infections and can help guide antimicrobial therapy. Blood cultures are also recommended in patients with infections involving specific anatomic sites, such as the mouth and eyes.19
Aspiration, swabs, incision and drainage
Fluid aspirated from abscesses and swabs of debrided ulcerated wounds should be sent for Gram stain and culture. Gram stain and culture have widely varying yields, from less than 5% to 40%, depending on the source and technique.19 Cultures were not routinely obtained before MRSA emerged, but knowing antimicrobial susceptibility is now important to guide antibiotic therapy. Unfortunately, in cellulitis, swabs and aspirates of the leading edge have a low yield of around 10%.25 One prospective study of 25 hospitalized patients did report a higher yield of positive cultures in patients with fever or underlying disease,26 so aspirates may be used in selected cases. In small studies, the yield of punch biopsies was slightly better than that of needle aspirates and was as high as 20% to 30%.27
IMAGING STUDIES
Imaging can be helpful in determining the depth of involvement. Plain radiography can reveal gas or periosteal inflammation and is especially helpful in diabetic foot infections. Ultrasonography can detect abscesses.
Both magnetic resonance imaging (MRI) and computed tomography (CT) are useful to image fascial planes, although MRI is more sensitive. However, in cases of suspected necrotizing fasciitis, imaging should not delay surgical evaluation and debridement or be used as the definitive study. Therefore, the practicality of CT and MRI can be limited.15,16
ANTIMICROBIAL TREATMENT FOR SSTIs IN OUTPATIENTS
For minor skin infections such as impetigo and secondarily infected skin lesions such as eczema, ulcers, or lacerations, mupirocin 2% topical ointment (Bactroban) can be effective.27
For a simple abscess or boil, incision and drainage is the primary treatment, and antibiotics are not needed.
For a complicated abscess or boil. Patients should be given oral or intravenous antibiotic therapy to cover MRSA and, depending on the severity, should be considered for hospitalization if the abscess is associated with severe disease, rapid progression in the presence of associated cellulitis, septic phlebitis, constitutional symptoms, comorbidity (including immunosuppression), or an abscess or boil in an area difficult to drain, such as the face, hands, or genitalia.27
For purulent cellulitis in outpatients, empiric therapy for community-acquired MRSA is recommended, pending culture results. Empiric therapy for streptococcal infection is likely unnecessary. For empiric coverage of community-acquired MRSA in purulent cellulitis, oral antibiotic options include clindamycin (Cleocin), trimethoprim-sulfamethoxazole (Bactrim), doxycycline (Doryx), minocycline (Minocin), and linezolid (Zyvox).
For nonpurulent cellulitis in outpatients, empiric coverage for beta-hemolytic streptococci is warranted. Coverage for community-acquired MRSA should subsequently be added for patients who do not respond to beta-lactam therapy within 48 to 72 hours or who have chills, fever, a new abscess, increasing erythema, or uncontrolled pain.
Options for coverage of both beta-hemolytic streptococci and community-acquired MRSA for outpatient therapy include clindamycin on its own, trimethoprim-sulfamethoxazole or a tetracycline in combination with a beta-lactam, or linezolid on its own.
Increasing rates of resistance to clindamycin, tetracycline, and trimethoprim-sulfamethoxazole in community-acquired MRSA may limit empiric treatment. In areas where resistance is prevalent, culture with antimicrobial susceptibility testing may be required before starting one of these antibiotics.
The use of rifampin (Rifadin) as a single agent is not recommended because resistance is likely to develop. Also, rifampin is not useful as adjunctive therapy, as evidence does not support its efficacy.19,27,29
ANTIMICROBIAL TREATMENT FOR SSTIs IN HOSPITALIZED PATIENTS
For hospitalized patients with a complicated or severe SSTI, empiric therapy for MRSA should be started pending culture results. FDA-approved options are vancomycin, linezolid, daptomycin (Cubicin), tigecycline (Tygacil), and telavancin (Vibativ). Data on clindamycin are very limited in this population. A beta-lactam antibiotic such as cefazolin (Ancef) may be considered in hospitalized patients with nonpurulent cellulitis, and the regimen can be modified to MRSA-active therapy if there is no clinical response. Linezolid, daptomycin, vancomycin, and telavancin have adequate streptococcal coverage in addition to MRSA coverage.
Clindamycin is approved by the FDA for treating serious infections due to S aureus. It has excellent tissue penetration, particularly in bone and abscesses.
Clindamycin resistance in staphylococci can be either constitutive or inducible, and clinicians must be watchful for signs of resistance.
Diarrhea is the most common adverse effect and occurs in up to 20% of patients. Clostridium difficile colitis may occur more frequently with clindamycin than with other oral agents, but it has also has been reported with fluoroquinolones and can be associated with any antibiotic therapy.30
Trimethoprim-sulfamethoxazole is not FDA-approved for treating any staphylococcal infection. However, because 95% to 100% of community-acquired MRSA strains are susceptible to it in vitro, it has become an important option in the outpatient treatment of SSTIs. Caution is advised when using it in elderly patients, particularly those with chronic renal insufficiency, because of an increased risk of hyperkalemia.
Tetracyclines. Doxycycline is FDA-approved for treating SSTIs due to S aureus, although not specifically for MRSA. Minocycline may be an option even when strains are resistant to doxycycline, since it does not induce its own resistance as doxycycline does.
Tigecycline is a glycylcycline (a tetracycline derivative) and is FDA-approved in adults for complicated SSTIs and intra-abdominal infections. It has a large volume of distribution and achieves high concentrations in tissues and low concentrations in serum.
The FDA recently issued a warning to consider alternative agents in patients with serious infections because of higher rates of all-cause mortality noted in phase III and phase IV clinical trials. Due to this warning and the availability of multiple alternatives active against MRSA, tigecycline was not included in the Infectious Diseases Society of America guidelines.31
Linezolid is a synthetic oxazolidinone and is FDA-approved for treating SSTIs and nosocomial pneumonia caused by MRSA. It has 100% oral bioavailability, so parenteral therapy should only be given if there are problems with gastrointestinal absorption or if the patient is unable to take oral medications.
Long-term use of linezolid (> 2 weeks) is limited by hematologic toxicity, especially thrombocytopenia, which occurs more frequently than anemia and neutropenia. Lactic acidosis and peripheral and optic neuropathy are also limiting toxicities. Although myelosuppression is generally reversible, peripheral and optic neuropathy may not be.
Linezolid should not used in patients taking selective serotonin reuptake inhibitors if they cannot stop taking these antidepressant drugs during therapy, as the combination can lead to the serotonin syndrome.
Vancomycin is still the mainstay of parenteral therapy for MRSA infections. However, its efficacy has come into question, with concerns over its slow bactericidal activity and the emergence of resistant strains. The rate of treatment failure is high in those with infection caused by MRSA having minimum inhibitory concentrations of 1 μg/mL or greater. Vancomycin kills staphylococci more slowly than do beta-lactams in vitro and is clearly inferior to beta-lactams for methicillin-sensitive S aureus bacteremia.
Daptomycin is a lipopeptide antibiotic that is FDA-approved for adults with MRSA bacteremia, right-sided infective endocarditis, and complicated SSTI. Elevations in creatinine phosphokinase, which are rarely treatment-limiting, have occurred in patients receiving 6 mg/kg/day but not in those receiving 4 mg/kg/day. Patients should be observed for development of muscle pain or weakness and should have their creatine phosphokinase levels checked weekly, with more frequent monitoring in those with renal insufficiency or who are receiving concomitant statin therapy.
Telavancin is a parenteral lipoglycopeptide that is bactericidal against MRSA. It is FDA-approved for complicated SSTIs in adults. Creatinine levels should be monitored, and the dosage should be adjusted on the basis of creatinine clearance, because nephrotoxicity was more commonly reported among individuals treated with telavancin than among those treated with vancomycin.
Ceftaroline (Teflaro), a fifth-generation cephalosporin, was approved for SSTIs by the FDA in October 2010. It is active against MRSA and gram-negative pathogens.
Cost is a consideration
Cost is a consideration, as it may limit the availability of and access to treatment. In 2008, the expense for 10 days of treatment with generic vancomycin was $183, compared with $1,661 for daptomycin, $1,362 for tigecycline, and $1,560 for linezolid. For outpatient therapy, the contrast was even starker, as generic trimethoprim-sulfamethoxazole cost $9.40 and generic clindamycin cost $95.10.32
INDICATIONS FOR HOSPITALIZATION
Patients who have evidence of tissue necrosis, fever, hypotension, severe pain, altered mental status, an immunocompromised state, or organ failure (respiratory, renal, or hepatic) must be hospitalized.
Although therapy for MRSA is the mainstay of empiric therapy, polymicrobial infections are not uncommon, and gram-negative and anaerobic coverage should be added as appropriate. One study revealed a longer length of stay for hospitalized patients who had inadequate initial empiric coverage.33
Vigilance should be maintained for overlying cellulitis which can mask necrotizing fasciitis, septic joints, or osteomyelitis.
Perianal abscesses and infections, infected decubitus ulcers, and moderate to severe diabetic foot infections are often polymicrobial and warrant coverage for streptococci, MRSA, aerobic gram-negative bacilli, and anaerobes until culture results can guide therapy.
INDICATIONS FOR SURGICAL REFERRAL
Extensive perianal or multiple abscesses may require surgical drainage and debridement.
Surgical site infections should be referred for consideration of opening the incision for drainage.
Necrotizing infections warrant prompt aggressive surgical debridement. Strongly suggestive clinical signs include bullae, crepitus, gas on radiography, hypotension with systolic blood pressure less than 90 mm Hg, or skin necrosis. However, these are late findings, and fewer than 50% of these patients have one of these. Most cases of necrotizing fasciitis originally have an admitting diagnosis of cellulitis and cases of fasciitis are relatively rare, so the diagnosis is easy to miss.15,16 Patients with an LRINEC score of six or more should have prompt surgical evaluation.20,24,34,35
Skin and soft-tissue infections (SSTIs) are a common reason for presentation to outpatient practices, emergency rooms, and hospitals.1–5 They account for more than 14 million outpatient visits in the United States each year,1 and visits to the emergency room and admissions to the hospital for them are increasing.2,3 Hospital admissions for SSTIs increased by 29% from 2000 to 2004.3
MORE MRSA NOW, BUT STREPTOCOCCI ARE STILL COMMON
The increase in hospital admissions for SSTIs has been attributed to a rising number of infections with methicillin-resistant Staphylococcus aureus (MRSA).3–5
In addition, strains once seen mostly in the community and other strains that were associated with health care are now being seen more often in both settings. Clinical characteristics do not differ between community-acquired and health-care-associated MRSA, and therefore the distinction between the two is becoming less useful in guiding empiric therapy.6,7
After steadily increasing for several years, the incidence of MRSA has recently stabilized. The US Centers for Disease Control and Prevention maintains a surveillance program and a Web site on MRSA.8
COMPLICATED OR UNCOMPLICATED
The intent of the 1998 guideline was to provide not a clinical framework but rather a guide for industry in designing trials that would include similar groups of infections and therefore be relevant when compared with each other. In 2008, the Anti-Infective Drugs Advisory Committee was convened,11 and subsequently, in August 2010, the FDA released a revision of the guide.12
The revised guidelines specifically exclude many diagnoses, such as bite wounds, bone and joint infections, necrotizing fasciitis, diabetic foot infections, decubitus ulcers, catheter site infections, myonecrosis, and ecthyma gangrenosum. Notably, the word “bacterial” in the title excludes mycobacterial and fungal infections from consideration. The diagnoses that are included include cellulitis, erysipelas, major cutaneous abscess, and burn infections. These are further specified to include 75 cm2 of redness, edema, or induration to standardize the extent of the infection—ie, the infection has to be at least this large or else it is not “complicated.”
The terms “complicated” and “uncomplicated” skin and skin structure infections persist and can be useful adjuncts in describing SSTIs.13–16 However, more specific descriptions of SSTIs based on pathogenesis are more useful to the clinician and are usually the basis for guidelines, such as for preventing surgical site infections or for reducing amputations in diabetic foot infections.
This review will focus on the general categories of SSTI and will not address surgical site infections, pressure ulcers, diabetic foot infections, perirectal wounds, or adjuvant therapies in severe SSTIs, such as negative pressure wound care (vacuum-assisted closure devices) and hyperbaric chambers.
OTHER DISEASES CAN MIMIC SSTIs
SSTIs vary broadly in their location and severity.
Although the classic presentation of erythema, warmth, edema, and tenderness often signals infection, other diseases can mimic SSTIs. Common ones that should be included in the differential diagnosis include gout, thrombophlebitis, deep vein thrombosis, contact dermatitis, carcinoma erysipeloides, drug eruption, and a foreign body reaction.17,18
CLUES FROM THE HISTORY
Wounds. Skin infections are usually precipitated by a break in the skin from a cut, laceration, excoriation, fungal infection, insect or animal bite, or puncture wound.
Impaired response. Patients with diabetes, renal failure, cirrhosis, chronic glucocorticoid use, history of organ transplantation, chronic immunosuppressive therapy, HIV infection, or malnourishment have impaired host responses to infection and are at risk for both more severe infections and recurrent infections. Immunocompromised hosts may also have atypical infections with opportunistic organisms such as Pseudomonas, Proteus, Serratia, Enterobacter, Citrobacter, and anaerobes. Close follow-up of these patients is warranted to ascertain appropriate response to therapy.19
Surgery that includes lymph node dissection or saphenous vein resection for coronary artery bypass can lead to impaired lymphatic drainage and edema, and therefore predisposes patients to SSTIs.
PHYSICAL EXAMINATION
The physical examination should include descriptions of the extent and location of erythema, edema, warmth, and tenderness so that progression or resolution with treatment can be followed in detail.
Crepitus can be felt in gas-forming infections and raises the concern for necrotizing fasciitis and infection with anaerobic organisms such as Clostridium perfringens.
Necrosis can occur in brown recluse spider bites, venous snake bites, or group A streptococcal infections.
Fluctuance indicates fluid and a likely abscess that may need incision and drainage.
Purpura may be present in patients on anticoagulation therapy, but if it is accompanying an SSTI, it also raises the concern for the possibility of sepsis and disseminated intravascular coagulation, especially from streptococcal infections.
Bullae can be seen in impetigo caused by staphylococci or in infection with Vibrio vulnificus or Streptococcus pneumoniae.19
Systemic signs, in addition to fever, can include hypotension and tachycardia, which would prompt closer monitoring and possible hospitalization.
Lymphangitic spread also indicates severe infection.
LABORATORY STUDIES
Simple, localized SSTIs usually do not require laboratory evaluation. Jenkins et al21 recently demonstrated that by using an algorithm for the management of hospitalized patients with cellulitis or cutaneous abscess, they could decrease resource utilization, including laboratory testing, without adversely affecting clinical outcome.
If patients have underlying disease or more extensive infection, then baseline chemistry values, a complete blood cell count, and the C-reactive protein level should be acquired.19 Laboratory findings that suggest more severe disease include low sodium, low bicarbonate (or an anion gap), and high creatinine levels; new anemia; a high or very low white blood cell count; and a high C-reactive protein level. A high C-reactive protein level has been associated with longer hospitalization.22
A score to estimate the risk of necrotizing fasciitis
This tool was developed retrospectively but has been validated prospectively. It has a high sensitivity and a positive predictive value of 92% in patients with a score of six points or more. Its specificity is also high, with a negative predictive value of 96%.20,24
Necrotizing fasciitis has a mortality rate of 23.5%, but this may be reduced to 10% with early detection and prompt surgical intervention.15 Since necrotizing fasciitis is very difficult to diagnose, clinicians must maintain a high level of suspicion and use the LRINEC score to trigger early surgical evaluation. Surgical exploration is the only way to definitively diagnose necrotizing fasciitis.
Blood cultures in some cases
Blood cultures have a low yield and are usually not cost-effective, but they should be obtained in patients who have lymphedema, immune deficiency, fever, pain out of proportion to the findings on examination, tachycardia, or hypotension, as blood cultures are more likely to be positive in more serious infections and can help guide antimicrobial therapy. Blood cultures are also recommended in patients with infections involving specific anatomic sites, such as the mouth and eyes.19
Aspiration, swabs, incision and drainage
Fluid aspirated from abscesses and swabs of debrided ulcerated wounds should be sent for Gram stain and culture. Gram stain and culture have widely varying yields, from less than 5% to 40%, depending on the source and technique.19 Cultures were not routinely obtained before MRSA emerged, but knowing antimicrobial susceptibility is now important to guide antibiotic therapy. Unfortunately, in cellulitis, swabs and aspirates of the leading edge have a low yield of around 10%.25 One prospective study of 25 hospitalized patients did report a higher yield of positive cultures in patients with fever or underlying disease,26 so aspirates may be used in selected cases. In small studies, the yield of punch biopsies was slightly better than that of needle aspirates and was as high as 20% to 30%.27
IMAGING STUDIES
Imaging can be helpful in determining the depth of involvement. Plain radiography can reveal gas or periosteal inflammation and is especially helpful in diabetic foot infections. Ultrasonography can detect abscesses.
Both magnetic resonance imaging (MRI) and computed tomography (CT) are useful to image fascial planes, although MRI is more sensitive. However, in cases of suspected necrotizing fasciitis, imaging should not delay surgical evaluation and debridement or be used as the definitive study. Therefore, the practicality of CT and MRI can be limited.15,16
ANTIMICROBIAL TREATMENT FOR SSTIs IN OUTPATIENTS
For minor skin infections such as impetigo and secondarily infected skin lesions such as eczema, ulcers, or lacerations, mupirocin 2% topical ointment (Bactroban) can be effective.27
For a simple abscess or boil, incision and drainage is the primary treatment, and antibiotics are not needed.
For a complicated abscess or boil. Patients should be given oral or intravenous antibiotic therapy to cover MRSA and, depending on the severity, should be considered for hospitalization if the abscess is associated with severe disease, rapid progression in the presence of associated cellulitis, septic phlebitis, constitutional symptoms, comorbidity (including immunosuppression), or an abscess or boil in an area difficult to drain, such as the face, hands, or genitalia.27
For purulent cellulitis in outpatients, empiric therapy for community-acquired MRSA is recommended, pending culture results. Empiric therapy for streptococcal infection is likely unnecessary. For empiric coverage of community-acquired MRSA in purulent cellulitis, oral antibiotic options include clindamycin (Cleocin), trimethoprim-sulfamethoxazole (Bactrim), doxycycline (Doryx), minocycline (Minocin), and linezolid (Zyvox).
For nonpurulent cellulitis in outpatients, empiric coverage for beta-hemolytic streptococci is warranted. Coverage for community-acquired MRSA should subsequently be added for patients who do not respond to beta-lactam therapy within 48 to 72 hours or who have chills, fever, a new abscess, increasing erythema, or uncontrolled pain.
Options for coverage of both beta-hemolytic streptococci and community-acquired MRSA for outpatient therapy include clindamycin on its own, trimethoprim-sulfamethoxazole or a tetracycline in combination with a beta-lactam, or linezolid on its own.
Increasing rates of resistance to clindamycin, tetracycline, and trimethoprim-sulfamethoxazole in community-acquired MRSA may limit empiric treatment. In areas where resistance is prevalent, culture with antimicrobial susceptibility testing may be required before starting one of these antibiotics.
The use of rifampin (Rifadin) as a single agent is not recommended because resistance is likely to develop. Also, rifampin is not useful as adjunctive therapy, as evidence does not support its efficacy.19,27,29
ANTIMICROBIAL TREATMENT FOR SSTIs IN HOSPITALIZED PATIENTS
For hospitalized patients with a complicated or severe SSTI, empiric therapy for MRSA should be started pending culture results. FDA-approved options are vancomycin, linezolid, daptomycin (Cubicin), tigecycline (Tygacil), and telavancin (Vibativ). Data on clindamycin are very limited in this population. A beta-lactam antibiotic such as cefazolin (Ancef) may be considered in hospitalized patients with nonpurulent cellulitis, and the regimen can be modified to MRSA-active therapy if there is no clinical response. Linezolid, daptomycin, vancomycin, and telavancin have adequate streptococcal coverage in addition to MRSA coverage.
Clindamycin is approved by the FDA for treating serious infections due to S aureus. It has excellent tissue penetration, particularly in bone and abscesses.
Clindamycin resistance in staphylococci can be either constitutive or inducible, and clinicians must be watchful for signs of resistance.
Diarrhea is the most common adverse effect and occurs in up to 20% of patients. Clostridium difficile colitis may occur more frequently with clindamycin than with other oral agents, but it has also has been reported with fluoroquinolones and can be associated with any antibiotic therapy.30
Trimethoprim-sulfamethoxazole is not FDA-approved for treating any staphylococcal infection. However, because 95% to 100% of community-acquired MRSA strains are susceptible to it in vitro, it has become an important option in the outpatient treatment of SSTIs. Caution is advised when using it in elderly patients, particularly those with chronic renal insufficiency, because of an increased risk of hyperkalemia.
Tetracyclines. Doxycycline is FDA-approved for treating SSTIs due to S aureus, although not specifically for MRSA. Minocycline may be an option even when strains are resistant to doxycycline, since it does not induce its own resistance as doxycycline does.
Tigecycline is a glycylcycline (a tetracycline derivative) and is FDA-approved in adults for complicated SSTIs and intra-abdominal infections. It has a large volume of distribution and achieves high concentrations in tissues and low concentrations in serum.
The FDA recently issued a warning to consider alternative agents in patients with serious infections because of higher rates of all-cause mortality noted in phase III and phase IV clinical trials. Due to this warning and the availability of multiple alternatives active against MRSA, tigecycline was not included in the Infectious Diseases Society of America guidelines.31
Linezolid is a synthetic oxazolidinone and is FDA-approved for treating SSTIs and nosocomial pneumonia caused by MRSA. It has 100% oral bioavailability, so parenteral therapy should only be given if there are problems with gastrointestinal absorption or if the patient is unable to take oral medications.
Long-term use of linezolid (> 2 weeks) is limited by hematologic toxicity, especially thrombocytopenia, which occurs more frequently than anemia and neutropenia. Lactic acidosis and peripheral and optic neuropathy are also limiting toxicities. Although myelosuppression is generally reversible, peripheral and optic neuropathy may not be.
Linezolid should not used in patients taking selective serotonin reuptake inhibitors if they cannot stop taking these antidepressant drugs during therapy, as the combination can lead to the serotonin syndrome.
Vancomycin is still the mainstay of parenteral therapy for MRSA infections. However, its efficacy has come into question, with concerns over its slow bactericidal activity and the emergence of resistant strains. The rate of treatment failure is high in those with infection caused by MRSA having minimum inhibitory concentrations of 1 μg/mL or greater. Vancomycin kills staphylococci more slowly than do beta-lactams in vitro and is clearly inferior to beta-lactams for methicillin-sensitive S aureus bacteremia.
Daptomycin is a lipopeptide antibiotic that is FDA-approved for adults with MRSA bacteremia, right-sided infective endocarditis, and complicated SSTI. Elevations in creatinine phosphokinase, which are rarely treatment-limiting, have occurred in patients receiving 6 mg/kg/day but not in those receiving 4 mg/kg/day. Patients should be observed for development of muscle pain or weakness and should have their creatine phosphokinase levels checked weekly, with more frequent monitoring in those with renal insufficiency or who are receiving concomitant statin therapy.
Telavancin is a parenteral lipoglycopeptide that is bactericidal against MRSA. It is FDA-approved for complicated SSTIs in adults. Creatinine levels should be monitored, and the dosage should be adjusted on the basis of creatinine clearance, because nephrotoxicity was more commonly reported among individuals treated with telavancin than among those treated with vancomycin.
Ceftaroline (Teflaro), a fifth-generation cephalosporin, was approved for SSTIs by the FDA in October 2010. It is active against MRSA and gram-negative pathogens.
Cost is a consideration
Cost is a consideration, as it may limit the availability of and access to treatment. In 2008, the expense for 10 days of treatment with generic vancomycin was $183, compared with $1,661 for daptomycin, $1,362 for tigecycline, and $1,560 for linezolid. For outpatient therapy, the contrast was even starker, as generic trimethoprim-sulfamethoxazole cost $9.40 and generic clindamycin cost $95.10.32
INDICATIONS FOR HOSPITALIZATION
Patients who have evidence of tissue necrosis, fever, hypotension, severe pain, altered mental status, an immunocompromised state, or organ failure (respiratory, renal, or hepatic) must be hospitalized.
Although therapy for MRSA is the mainstay of empiric therapy, polymicrobial infections are not uncommon, and gram-negative and anaerobic coverage should be added as appropriate. One study revealed a longer length of stay for hospitalized patients who had inadequate initial empiric coverage.33
Vigilance should be maintained for overlying cellulitis which can mask necrotizing fasciitis, septic joints, or osteomyelitis.
Perianal abscesses and infections, infected decubitus ulcers, and moderate to severe diabetic foot infections are often polymicrobial and warrant coverage for streptococci, MRSA, aerobic gram-negative bacilli, and anaerobes until culture results can guide therapy.
INDICATIONS FOR SURGICAL REFERRAL
Extensive perianal or multiple abscesses may require surgical drainage and debridement.
Surgical site infections should be referred for consideration of opening the incision for drainage.
Necrotizing infections warrant prompt aggressive surgical debridement. Strongly suggestive clinical signs include bullae, crepitus, gas on radiography, hypotension with systolic blood pressure less than 90 mm Hg, or skin necrosis. However, these are late findings, and fewer than 50% of these patients have one of these. Most cases of necrotizing fasciitis originally have an admitting diagnosis of cellulitis and cases of fasciitis are relatively rare, so the diagnosis is easy to miss.15,16 Patients with an LRINEC score of six or more should have prompt surgical evaluation.20,24,34,35
- Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–1591.
- Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008; 51:291–298.
- Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–1518.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007; 357:380–390.
- Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–1771.
- Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis 2011; 52:99–114.
- Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 2007; 44:471–482.
- Centers for Disease Control and Prevention. MRSA Infections. http://www.cdc.gov/mrsa/statistics/MRSA-Surveillance-Summary.html. Accessed December 14, 2011.
- Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 2007; 57:7–13.
- US Department of Health and Human Services. Guidance for Industry: Uncomplicated and Complicated Skin and Skin Structure Infections—Developing Antimicrobial Drugs for Treatment (draft guidance). July 1998. http://www.fda.gov/ohrms/dockets/98fr/2566dft.pdf. Accessed September 7, 2011.
- US Food and Drug Administration. CDER 2008 Meeting Documents. Anti-Infective Drugs Advisory Committee. http://www.fda.gov/ohrms/dockets/ac/cder08.html#AntiInfective. Accessed September 7, 2011.
- US Department of Health and Human Services. Guidance for Industry: Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment (draft guidance). August 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed December 14, 2011.
- Cornia PB, Davidson HL, Lipsky BA. The evaluation and treatment of complicated skin and skin structure infections. Expert Opin Pharmacother 2008; 9:717–730.
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008; 19:173–184.
- May AK, Stafford RE, Bulger EM, et al; Surgical Infection Society. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt) 2009; 10:467–499.
- Napolitano LM. Severe soft tissue infections. Infect Dis Clin North Am 2009; 23:571–591.
- Papadavid E, Dalamaga M, Stavrianeas N, Papiris SA. Subcutaneous sarcoidosis masquerading as cellulitis. Dermatology 2008; 217:212–214.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32:1535–1541.
- Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med 2011; 171:1072–1079.
- Lazzarini L, Conti E, Tositti G, de Lalla F. Erysipelas and cellulitis: clinical and microbiological spectrum in an Italian tertiary care hospital. J Infect 2005; 51:383–389.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis 2007; 44:705–710.
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ 2005; 330:830–833.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol 1988; 26:401–404.
- Sachs MK. The optimum use of needle aspiration in the bacteriologic diagnosis of cellulitis in adults. Arch Intern Med 1990; 150:1907–1912.
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–e55.
- Hammond SP, Baden LR. Clinical decisions. Management of skin and soft-tissue infection—polling results. N Engl J Med 2008; 359:e20.
- Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med 2008; 168:805–819.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40:1–15.
- US Food and Drug Administration. FDA Drug Safety Communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. September 2010. http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm. Accessed September 7, 2011.
- Moellering RC. A 39-year-old man with a skin infection. JAMA 2008; 299:79–87.
- Zilberberg MD, Shorr AF, Micek ST, et al. Hospitalizations with healthcare-associated complicated skin and skin structure infections: impact of inappropriate empiric therapy on outcomes. J Hosp Med 2010; 5:535–540.
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003; 85:1454–1460.
- Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med 2008; 26:170–175.
- Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med 2008; 168:1585–1591.
- Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA. Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2008; 51:291–298.
- Edelsberg J, Taneja C, Zervos M, et al. Trends in US hospital admissions for skin and soft tissue infections. Emerg Infect Dis 2009; 15:1516–1518.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007; 357:380–390.
- Klevens RM, Morrison MA, Nadle J, et al; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763–1771.
- Chua K, Laurent F, Coombs G, Grayson ML, Howden BP. Antimicrobial resistance: not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician’s guide to community MRSA—its evolving antimicrobial resistance and implications for therapy. Clin Infect Dis 2011; 52:99–114.
- Miller LG, Perdreau-Remington F, Bayer AS, et al. Clinical and epidemiologic characteristics cannot distinguish community-associated methicillin-resistant Staphylococcus aureus infection from methicillin-susceptible S. aureus infection: a prospective investigation. Clin Infect Dis 2007; 44:471–482.
- Centers for Disease Control and Prevention. MRSA Infections. http://www.cdc.gov/mrsa/statistics/MRSA-Surveillance-Summary.html. Accessed December 14, 2011.
- Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis 2007; 57:7–13.
- US Department of Health and Human Services. Guidance for Industry: Uncomplicated and Complicated Skin and Skin Structure Infections—Developing Antimicrobial Drugs for Treatment (draft guidance). July 1998. http://www.fda.gov/ohrms/dockets/98fr/2566dft.pdf. Accessed September 7, 2011.
- US Food and Drug Administration. CDER 2008 Meeting Documents. Anti-Infective Drugs Advisory Committee. http://www.fda.gov/ohrms/dockets/ac/cder08.html#AntiInfective. Accessed September 7, 2011.
- US Department of Health and Human Services. Guidance for Industry: Acute Bacterial Skin and Skin Structure Infections: Developing Drugs for Treatment (draft guidance). August 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071185.pdf. Accessed December 14, 2011.
- Cornia PB, Davidson HL, Lipsky BA. The evaluation and treatment of complicated skin and skin structure infections. Expert Opin Pharmacother 2008; 9:717–730.
- Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008; 19:173–184.
- May AK, Stafford RE, Bulger EM, et al; Surgical Infection Society. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt) 2009; 10:467–499.
- Napolitano LM. Severe soft tissue infections. Infect Dis Clin North Am 2009; 23:571–591.
- Papadavid E, Dalamaga M, Stavrianeas N, Papiris SA. Subcutaneous sarcoidosis masquerading as cellulitis. Dermatology 2008; 217:212–214.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004; 32:1535–1541.
- Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med 2011; 171:1072–1079.
- Lazzarini L, Conti E, Tositti G, de Lalla F. Erysipelas and cellulitis: clinical and microbiological spectrum in an Italian tertiary care hospital. J Infect 2005; 51:383–389.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis 2007; 44:705–710.
- Hasham S, Matteucci P, Stanley PR, Hart NB. Necrotising fasciitis. BMJ 2005; 330:830–833.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol 1988; 26:401–404.
- Sachs MK. The optimum use of needle aspiration in the bacteriologic diagnosis of cellulitis in adults. Arch Intern Med 1990; 150:1907–1912.
- Liu C, Bayer A, Cosgrove SE, et al; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–e55.
- Hammond SP, Baden LR. Clinical decisions. Management of skin and soft-tissue infection—polling results. N Engl J Med 2008; 359:e20.
- Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: a systematic review of the literature. Arch Intern Med 2008; 168:805–819.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998; 40:1–15.
- US Food and Drug Administration. FDA Drug Safety Communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. September 2010. http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm. Accessed September 7, 2011.
- Moellering RC. A 39-year-old man with a skin infection. JAMA 2008; 299:79–87.
- Zilberberg MD, Shorr AF, Micek ST, et al. Hospitalizations with healthcare-associated complicated skin and skin structure infections: impact of inappropriate empiric therapy on outcomes. J Hosp Med 2010; 5:535–540.
- Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am 2003; 85:1454–1460.
- Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med 2008; 26:170–175.
KEY POINTS
- Categories and definitions of specific subtypes of infections are evolving and have implications for treatment.
- Methicillin-resistant Staphylococcus aureus (MRSA) and streptococci continue to be the predominant organisms in SSTIs.
- A careful history and examination along with clinical attention are needed to elucidate atypical and severe infections.
- Laboratory data can help characterize the severity of disease and determine the probability of necrotizing fasciitis.
- Although cultures are unfortunately not reliably positive, their yield is higher in severe disease and they should be obtained, given the importance of antimicrobial susceptibility.
- The Infectious Diseases Society of America has recently released guidelines on MRSA, and additional guidelines addressing the spectrum of SSTIs are expected within a year.
Dabigatran
To the Editor: The article “Dabigatran: Will it change clinical practice”1 has a dangerous error. In its Key Points, it says “dabigatran is a potent, reversible direct thrombin inhibitor.” In fact, it is not reversible.2
Shamefully poor editing.
- Wartak SA, Bartholomew JR. Dabigatran: Will it change clinical practice? Cleve Clin J Med 2011; 78:657–664.
- Antithrombotic drugs. Treat Guidel Met Lett 2011; 9:61–66.
To the Editor: The article “Dabigatran: Will it change clinical practice”1 has a dangerous error. In its Key Points, it says “dabigatran is a potent, reversible direct thrombin inhibitor.” In fact, it is not reversible.2
Shamefully poor editing.
To the Editor: The article “Dabigatran: Will it change clinical practice”1 has a dangerous error. In its Key Points, it says “dabigatran is a potent, reversible direct thrombin inhibitor.” In fact, it is not reversible.2
Shamefully poor editing.
- Wartak SA, Bartholomew JR. Dabigatran: Will it change clinical practice? Cleve Clin J Med 2011; 78:657–664.
- Antithrombotic drugs. Treat Guidel Met Lett 2011; 9:61–66.
- Wartak SA, Bartholomew JR. Dabigatran: Will it change clinical practice? Cleve Clin J Med 2011; 78:657–664.
- Antithrombotic drugs. Treat Guidel Met Lett 2011; 9:61–66.
In reply: Dabigatran
In Reply: This is not an error. When we1 and others2 said that dabigatran is a reversible direct thrombin inhibitor, we were referring to its effect at the molecular level, the appropriate description of its mechanism of action. However, we suspect that Dr. Smith means that there is no antidote to give in cases of bleeding or overdose. We share his concern and we discussed this in our article.
Unlike heparin, direct thrombin inhibitors act independently of antithrombin and inhibit thrombin bound to fibrin or fibrin degradation products. There are two types of direct thrombin inhibitors: bivalent (eg, hirudin) and univalent (eg, argatroban, ximelagatran, and dabigatran). The bivalent ones block thrombin at its active site and at an exosite and form an irreversible complex with it. The univalent ones interact with only the active site and reversibly inhibit thrombin, eventually dissociating from it and leaving a small amount of free, enzymatically active thrombin available for hemostatic interactions. Therefore, in contrast to the hirudins, they produce relatively transient thrombin inhibition.2–4
As we pointed out in our article, the lack of an antidote for dabigatran and the lack of experience in treating bleeding complications are major concerns. Fortunately, the drug has a short half-life (12–14 hours) so that the treatment is to withhold the next dose while maintaining adequate diuresis and giving transfusions as indicated. Activated charcoal, given orally to reduce absorption, is under evaluation but must be given within 1 or 2 hours after the dabigatran dose.1 Dabigatran can be removed by dialysis (in part because it is a reversible inhibitor), a measure that may be necessary in life-threatening cases. Recombinant activated factor VII or prothrombin complex concentrates may be additional treatment options.1,4 With time will come experience and, we hope, evidence-based guidelines.
- Wartak SA, Bartholomew JR. Dabigatran: Will it change clinical practice? Cleve Clin J Med 2011; 78:657–664.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med 2005; 353:1028–1040.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
In Reply: This is not an error. When we1 and others2 said that dabigatran is a reversible direct thrombin inhibitor, we were referring to its effect at the molecular level, the appropriate description of its mechanism of action. However, we suspect that Dr. Smith means that there is no antidote to give in cases of bleeding or overdose. We share his concern and we discussed this in our article.
Unlike heparin, direct thrombin inhibitors act independently of antithrombin and inhibit thrombin bound to fibrin or fibrin degradation products. There are two types of direct thrombin inhibitors: bivalent (eg, hirudin) and univalent (eg, argatroban, ximelagatran, and dabigatran). The bivalent ones block thrombin at its active site and at an exosite and form an irreversible complex with it. The univalent ones interact with only the active site and reversibly inhibit thrombin, eventually dissociating from it and leaving a small amount of free, enzymatically active thrombin available for hemostatic interactions. Therefore, in contrast to the hirudins, they produce relatively transient thrombin inhibition.2–4
As we pointed out in our article, the lack of an antidote for dabigatran and the lack of experience in treating bleeding complications are major concerns. Fortunately, the drug has a short half-life (12–14 hours) so that the treatment is to withhold the next dose while maintaining adequate diuresis and giving transfusions as indicated. Activated charcoal, given orally to reduce absorption, is under evaluation but must be given within 1 or 2 hours after the dabigatran dose.1 Dabigatran can be removed by dialysis (in part because it is a reversible inhibitor), a measure that may be necessary in life-threatening cases. Recombinant activated factor VII or prothrombin complex concentrates may be additional treatment options.1,4 With time will come experience and, we hope, evidence-based guidelines.
In Reply: This is not an error. When we1 and others2 said that dabigatran is a reversible direct thrombin inhibitor, we were referring to its effect at the molecular level, the appropriate description of its mechanism of action. However, we suspect that Dr. Smith means that there is no antidote to give in cases of bleeding or overdose. We share his concern and we discussed this in our article.
Unlike heparin, direct thrombin inhibitors act independently of antithrombin and inhibit thrombin bound to fibrin or fibrin degradation products. There are two types of direct thrombin inhibitors: bivalent (eg, hirudin) and univalent (eg, argatroban, ximelagatran, and dabigatran). The bivalent ones block thrombin at its active site and at an exosite and form an irreversible complex with it. The univalent ones interact with only the active site and reversibly inhibit thrombin, eventually dissociating from it and leaving a small amount of free, enzymatically active thrombin available for hemostatic interactions. Therefore, in contrast to the hirudins, they produce relatively transient thrombin inhibition.2–4
As we pointed out in our article, the lack of an antidote for dabigatran and the lack of experience in treating bleeding complications are major concerns. Fortunately, the drug has a short half-life (12–14 hours) so that the treatment is to withhold the next dose while maintaining adequate diuresis and giving transfusions as indicated. Activated charcoal, given orally to reduce absorption, is under evaluation but must be given within 1 or 2 hours after the dabigatran dose.1 Dabigatran can be removed by dialysis (in part because it is a reversible inhibitor), a measure that may be necessary in life-threatening cases. Recombinant activated factor VII or prothrombin complex concentrates may be additional treatment options.1,4 With time will come experience and, we hope, evidence-based guidelines.
- Wartak SA, Bartholomew JR. Dabigatran: Will it change clinical practice? Cleve Clin J Med 2011; 78:657–664.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med 2005; 353:1028–1040.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Wartak SA, Bartholomew JR. Dabigatran: Will it change clinical practice? Cleve Clin J Med 2011; 78:657–664.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med 2005; 353:1028–1040.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
Bioidentical hormone therapy: Clarifying the misconceptions
Recent product endorsements from celebrities on television have brought a new term into the vocabulary of many American women: bioidentical hormone therapy—treatment with hormone products that are identical in molecular structure to those in the human body.
Since 2002, when results of the Women’s Health Initiative1 raised questions about the safety of hormone replacement therapy, women have been inundated by commercials, talk shows, and self-help books that promote bioidentical hormone therapy as a safe and natural way to treat menopausal symptoms—and more.
Although this publicity has helped promote discussion about menopause, it has also perpetuated confusion and misinformation among the lay public and the general medical community concerning menopausal hormone therapy.
Many postmenopausal women suffering from vasomotor symptoms, vaginal dryness, and vaginal atrophy are apprehensive about seeking therapy, owing to concerns resulting from misinterpreted information derived from the Women’s Health Initiative trial.1 (See “What are the known risks of FDA-approved hormone therapy.”2–8) Many others are told to suffer through their symptoms, which may eventually pass. It is not surprising, then, that women turn to unconventional treatments that are claimed to be safer. This unfortunate situation has driven the business of many compounding pharmacies into the multibillion dollar level.
In this paper, we hope to clarify some of the misconceptions surrounding this issue. But first we need to define some terms in what has become a confusing area.

WHAT ARE BIOIDENTICAL HORMONES?
“Bioidentical” means identical in molecular structure to endogenous hormones. However, as we will see, a better distinction should be made between products that are approved and regulated by the US Food and Drug Administration (FDA) and those that are not.
Endogenous reproductive hormones
Women produce various reproductive hormones, including three estrogens—estradiol, estrone, and estriol—as well as progesterone and testosterone.9
17-beta estradiol (E2) is the most bioactive endogenous estrogen. It is primarily produced by the dominant ovarian follicle and the corpus luteum and is synthesized intracellularly through aromatase activity.10,11 The rest of the circulating estradiol is derived from peripheral conversion of estrone to estradiol, and this is the primary source in postmenopausal women not on hormone therapy.11
In postmenopausal women, serum estradiol levels are often below 15 pg/mL. Many physiologic effects of the cellular compartmentalized estradiol contribute to an over-riding force in certain tissues even after menopause.10 With the loss of estradiol, many tissues in postmenopausal women can be affected, particularly resulting in genitourinary atrophy and bone loss.
Estrone (E1), the second dominant human estrogen, is primarily derived from the metabolism of estradiol and from the aromatization of androstenedione in adipose tissue, with a small quantity being secreted directly by the ovary and the adrenal glands.9 In postmenopausal women, mean estrone levels are about 30 pg/mL.11
Estriol (E3), the least active of the endogenous estrogens, is very short-acting.
Progesterone is a 21-carbon steroid secreted by the human ovary.9 It is formed during the transformation of cholesterol to estrogens and androgens and is no longer produced after menopause.9
Testosterone. In premenopausal women, the androgen testosterone is synthesized by the ovary, the adrenal cortex, and the peripheral conversion of circulating androstenedione and dehydroepiandrosterone (DHEA).9 Over a woman’s life span, her androgen levels decline progressively.10 The rate of decline has not been shown to be appreciably affected by the onset of menopause.10
All these hormone therapy products are synthesized
Many nonmedical women’s health books erroneously classify the forms of estrogen used in hormone therapy as either bioidentical or synthetic. In fact, they are all man-made.
Bioidentical hormones are synthesized by chemically extracting diosgenin from plants such as yams and soy.12 Diosgenin is chemically modified to yield the precursor progesterone, which is then used to synthesize bioidentical estrogens and androgens.10
Nonbioidentical estrogen products include conjugated equine estrogens (CEE), which is extracted from the urine of pregnant mares. The two predominant estrogens found in CEE are equilin sulfate (native to horses) and estrone sulfate.10
Other nonbioidentical products include ethinyl estradiol, which is used in most combined oral contraceptives. It is formed after a minor chemical modification of estradiol that makes it one of the most potent estrogens. The ethinyl group at carbon 17 of ring D of the steroid nucleus greatly slows the hepatic and enzymatic degradation of the molecule and, thereby, makes oral ethinyl estradiol 15 to 20 times more active than oral estradiol.
Mestranol is an inactive prodrug that is converted in the body to ethinyl estradiol.
While many women may find the idea of natural bioidentical hormones derived from sweet potatoes or soybeans more acceptable than taking one made from horse’s urine, all the products undergo extensive chemical processing and modification.
Misconception: FDA-regulated products are not bioidentical
WHAT IS CUSTOMIZED COMPOUNDED HORMONAL THERAPY?
There is often confusion between the terms “bioidentical hormones” and “customized compounded therapy,” which are often used interchangeably. Compounded therapy combines ratios of bioidentical hormones into a particular recipe or mixture. Customized compounding can be done by local compounding pharmacies.2
Compounded bioidentical estrogen products
There are several commonly marketed compounded products.
Tri-estrogen (tri-est) is a compounded hormone preparation made up of a mixture of 80% estriol, 10% estrone, and 10% estradiol.12
Bi-estrogen (bi-est) contains estriol and estradiol in a ratio of 8:1 or 9:1.
Although both tri-est and bi-est are largely composed of estriol, given the low potency of estriol, the effects of these products may be solely mediated by their major bioactive component, estradiol.10,12 No large prospective, well-controlled clinical trial has investigated the compounded ratios of these mixtures of estrogens.10
Tri-est and bi-est are frequently promoted as posing less risk of breast or endometrial cancer than FDA-approved agents, although there is no research to back up this claim.12 In fact, estriol may have a stimulatory effect on the breast and endometrium.9
In addition to these “standard” compounded preparations, women can receive more customized compounds.
Valid uses for customized compounded formulations
Some clinical providers use customized compounded formulations when prescribing hormone therapy to women who have allergies to certain ingredients, such as peanut oil (found in the FDA-regulated oral product Prometrium). Customized compounded formulations have also been used when prescribing hormones currently not FDA-approved for women, such as testosterone and DHEA.12 Before oral micronized progesterone was marketed in the United States as Prometrium, it was frequently prescribed as a compounded hormone.
HORMONE THERAPY COMES IN VARIOUS FORMS
Both FDA-regulated hormone therapy and unregulated compounded hormone therapy come in various doses and dosage forms administered by different routes, allowing for individualization for each woman’s specific characteristics.
Estrogens: Oral, transdermal, others
Estrogen therapy can be given orally, transvaginally (as creams, tablets, and rings), transdermally (as patches, gels, and creams), subcutaneously in pellets, intranasally (in Europe), and by injection.11
Most oral contraceptives contain the synthetic estrogen ethinyl estradiol. Ethinyl estradiol is more potent than human estrogens,11 specifically in increasing the production of hepatic proteins (sex-hormone-binding globulin, renin substrate, corticosteroid-binding globulin, and thyroid-binding globulin).11
Bioidentical estradiol, taken orally in tablet form, is first processed through the liver and converted into estrone.12 This stimulates proteins such as C-reactive protein, activated protein C, and clotting factors, which may increase the risk of clotting.12 Estradiol given transdermally by patch or gel or vaginally bypasses the liver and enters the bloodstream as 17-beta estradiol, therefore avoiding stimulation of these proteins.12 Case-control data have shown an associated lower risk of deep venous thromboembolism with transdermal therapy.3
Subcutaneous pellet therapy is a less common, non-FDA-approved method of hormone therapy to relieve postmenopausal symptoms.10 In an outpatient procedure, the pellet is inserted into the subcutaneous fat of the abdomen.10 The crystalline pellet is biodegradable and contains a mixture of testosterone and 17-beta estradiol.10 It is important to remember that endometrial stimulation may be prolonged with this form of therapy and levels may be supraphysiologic.
Progestogens can also be given by different routes
Oral progesterone has poor gastrointestinal absorption and a short half-life.10 Therefore, it is micronized with oil for better absorption. Reported side effects include sedative and anesthetic effects; therefore, it is recommended that oral progesterone be taken at bedtime.9 Medroxyprogesterone acetate may interfere more with estrogen’s positive effects on cholesterol than micronized progesterone does.13
Topical progesterone preparations vary widely in dosage and formulation. Over-the-counter progesterone creams vary in concentration from no active ingredient to 450 mg or more of progesterone per ounce. Application sites for progesterone cream include the inner arm, chest, and inner thigh. No transdermal hormone should be applied to areas of the body that may allow possible contact and transference to others.
Progestogen products
Progestogen products include “natural” progesterone and synthetic progestins. They should be given concurrently with estrogen therapy in women who have an intact uterus to prevent endometrial hyperplasia.9
Bioidentical progesterone is micronized in the laboratory for better absorption in the gut.2
Misconception: Transdermal progesterone protects the endometrium
In general, transdermal progesterone should be avoided, as it does not protect against endometrial cancer.
Many forms of progesterone are available by prescription at compounding pharmacies as lotions, gels, creams, capsules, trochees, and suppositories.9 Transdermal progesterone creams are also available over the counter at health stores. Some of these creams contain only diosgenin, a progesterone precursor derived from wild yams.10 Diosgenin cannot be converted into progesterone within the body and thus does not provide an adequate amount of absorbable progesterone.9 Therefore, progesterone cream that contains only diosgenin is not effective in preventing endometrial hyperplasia and cancer.
To achieve a physiologic response, progesterone levels must be at least in the nanogram range.10 Transdermal progesterone cream has not been shown to reach this level and may not significantly improve vasomotor symptoms.12 Some practitioners prescribe cream that contains more than 400 mg progesterone per ounce. This may achieve physiologic levels of progesterone, but no improvement has been proven for bone mineral density or endometrial protection. In general, no transdermal progesterone cream can be assumed to protect the endometrium against the stimulatory effects of estrogen.
CUSTOM COMPOUNDING AND SALIVA TESTING TO INDIVIDUALIZE THERAPY
Some clinicians who prescribe compounded hormones order saliva tests. They argue the tests help them to establish which hormones are deficient and therefore to customize therapy.12 The basis for this is that saliva is similar to an ultrafiltrate of blood and, theoretically, hormone levels in saliva should represent the bioavailable hormone in serum.10
Unfortunately, this testing is often unreliable due to poor stability of samples in storage and large interassay variability.10 Many factors may alter hormone levels in saliva and make test results unreproducible, including the time of day the sample is collected and dietary habits.10 The FDA states that there is no scientific basis for using salivary testing to adjust hormone levels.2
Levels of drugs with clearance that varies depending on hepatic enzyme activity and plasma binding (capacity-limited metabolism) such as estradiol and testosterone can be monitored with total blood serum concentrations.10 However, many physiologic effects of estrogens are determined intracellularly at the level of tissues.10 Therefore, although levels during therapy with bioidentical estrogens can be monitored more precisely, the FDA states that hormone therapy should be guided by symptom response and findings on physical examination and not by hormone levels alone.2,12 It may be reasonable to order serum levels of estradiol in women being treated with therapeutic doses of bioidentical estrogen but still not achieving symptom relief. If women are being treated with conjugated equine estrogens, serum levels cannot be monitored. Total estrogen can be monitored as a send-out laboratory test.
MISCONCEPTION: HORMONE THERAPY IS A FOUNTAIN OF YOUTH
Customized compounded hormonal therapy is marketed as being able to help with rejuvenation, improve memory, sexual function, and reverse the aging process, essentially promising to be an elixir or fountain of youth.
These claims are not substantiated. However, the actual benefits of hormone therapy in women who have menopausal symptoms include alleviation of moderate to severe vasomotor symptoms and vaginal atrophy that can result in dyspareunia. By alleviating their symptoms, hormone therapy improves women’s quality of life. It also reduces the incidence of postmenopausal osteoporotic fractures.
A research finding that is often overlooked is that postmenopausal women younger than 60 years who started estrogen or estrogenprogestin therapy soon after menopause had a 30% lower rate of death from all causes.2,14 This difference was statistically significant when the estrogen and estrogen-progestin therapy groups were combined. No reduction in the mortality rate was seen if therapy was started after age 60.
MISCONCEPTION: COMPOUNDED THERAPY IS SAFER
Compounded hormone therapy is often marketed as a safer or more effective alternative to government-regulated and approved therapy. Unfortunately, these claims are often false and misleading, and safety information is not consistently provided to patients as is required with FDA-regulated hormone therapy.2
Since these compounds have not been approved by the FDA, there is no guarantee that the ingredients have been tested for purity, potency, and efficacy. There is no batch standardization. These unregulated therapies may use unapproved ingredients, routes of administration, and mixtures with contaminants such as dyes and preservatives.2
Also, custom-compounded prescriptions are considered experimental. Therefore, they are often not covered by insurance, and many women must pay for them out of pocket.11
The North American Menopause Society does not recommend custom-mixed products over well-tested, government-approved commercial products for most women.2 All bioidentical hormone prescriptions should include a patient package insert,11 identical to that required of FDA-approved products.2
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17:242–255.
- Canonico M, Oger E, Plu-Bureau G; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845.
- Risks of postmenopausal hormone replacement (letters). JAMA 2002; 288:2819–2825.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Shumaker SA, Legault C, Rapp SR, et al; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289:2651–2662.
- Chlebowski RT, Anderson GL, Gass M, et al; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010; 304:1684–1692.
- Lobo RA. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 3rd ed. Burlington, MA: Academic Press; 2007.
- Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt) 2007; 16:600–631.
- Menopause Practice: A Clinician’s Guide. 4th ed. Cleveland, OH: The North American Menopause Society; 2010.
- What are bioidentical hormones? Natural. Bioidentical. Compounded. Confusion about these terms is only adding to the confusion over hormone therapy. Harv Womens Health Watch 2006; 13:1–3.
- The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1995; 273:199–208.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
Recent product endorsements from celebrities on television have brought a new term into the vocabulary of many American women: bioidentical hormone therapy—treatment with hormone products that are identical in molecular structure to those in the human body.
Since 2002, when results of the Women’s Health Initiative1 raised questions about the safety of hormone replacement therapy, women have been inundated by commercials, talk shows, and self-help books that promote bioidentical hormone therapy as a safe and natural way to treat menopausal symptoms—and more.
Although this publicity has helped promote discussion about menopause, it has also perpetuated confusion and misinformation among the lay public and the general medical community concerning menopausal hormone therapy.
Many postmenopausal women suffering from vasomotor symptoms, vaginal dryness, and vaginal atrophy are apprehensive about seeking therapy, owing to concerns resulting from misinterpreted information derived from the Women’s Health Initiative trial.1 (See “What are the known risks of FDA-approved hormone therapy.”2–8) Many others are told to suffer through their symptoms, which may eventually pass. It is not surprising, then, that women turn to unconventional treatments that are claimed to be safer. This unfortunate situation has driven the business of many compounding pharmacies into the multibillion dollar level.
In this paper, we hope to clarify some of the misconceptions surrounding this issue. But first we need to define some terms in what has become a confusing area.

WHAT ARE BIOIDENTICAL HORMONES?
“Bioidentical” means identical in molecular structure to endogenous hormones. However, as we will see, a better distinction should be made between products that are approved and regulated by the US Food and Drug Administration (FDA) and those that are not.
Endogenous reproductive hormones
Women produce various reproductive hormones, including three estrogens—estradiol, estrone, and estriol—as well as progesterone and testosterone.9
17-beta estradiol (E2) is the most bioactive endogenous estrogen. It is primarily produced by the dominant ovarian follicle and the corpus luteum and is synthesized intracellularly through aromatase activity.10,11 The rest of the circulating estradiol is derived from peripheral conversion of estrone to estradiol, and this is the primary source in postmenopausal women not on hormone therapy.11
In postmenopausal women, serum estradiol levels are often below 15 pg/mL. Many physiologic effects of the cellular compartmentalized estradiol contribute to an over-riding force in certain tissues even after menopause.10 With the loss of estradiol, many tissues in postmenopausal women can be affected, particularly resulting in genitourinary atrophy and bone loss.
Estrone (E1), the second dominant human estrogen, is primarily derived from the metabolism of estradiol and from the aromatization of androstenedione in adipose tissue, with a small quantity being secreted directly by the ovary and the adrenal glands.9 In postmenopausal women, mean estrone levels are about 30 pg/mL.11
Estriol (E3), the least active of the endogenous estrogens, is very short-acting.
Progesterone is a 21-carbon steroid secreted by the human ovary.9 It is formed during the transformation of cholesterol to estrogens and androgens and is no longer produced after menopause.9
Testosterone. In premenopausal women, the androgen testosterone is synthesized by the ovary, the adrenal cortex, and the peripheral conversion of circulating androstenedione and dehydroepiandrosterone (DHEA).9 Over a woman’s life span, her androgen levels decline progressively.10 The rate of decline has not been shown to be appreciably affected by the onset of menopause.10
All these hormone therapy products are synthesized
Many nonmedical women’s health books erroneously classify the forms of estrogen used in hormone therapy as either bioidentical or synthetic. In fact, they are all man-made.
Bioidentical hormones are synthesized by chemically extracting diosgenin from plants such as yams and soy.12 Diosgenin is chemically modified to yield the precursor progesterone, which is then used to synthesize bioidentical estrogens and androgens.10
Nonbioidentical estrogen products include conjugated equine estrogens (CEE), which is extracted from the urine of pregnant mares. The two predominant estrogens found in CEE are equilin sulfate (native to horses) and estrone sulfate.10
Other nonbioidentical products include ethinyl estradiol, which is used in most combined oral contraceptives. It is formed after a minor chemical modification of estradiol that makes it one of the most potent estrogens. The ethinyl group at carbon 17 of ring D of the steroid nucleus greatly slows the hepatic and enzymatic degradation of the molecule and, thereby, makes oral ethinyl estradiol 15 to 20 times more active than oral estradiol.
Mestranol is an inactive prodrug that is converted in the body to ethinyl estradiol.
While many women may find the idea of natural bioidentical hormones derived from sweet potatoes or soybeans more acceptable than taking one made from horse’s urine, all the products undergo extensive chemical processing and modification.
Misconception: FDA-regulated products are not bioidentical
WHAT IS CUSTOMIZED COMPOUNDED HORMONAL THERAPY?
There is often confusion between the terms “bioidentical hormones” and “customized compounded therapy,” which are often used interchangeably. Compounded therapy combines ratios of bioidentical hormones into a particular recipe or mixture. Customized compounding can be done by local compounding pharmacies.2
Compounded bioidentical estrogen products
There are several commonly marketed compounded products.
Tri-estrogen (tri-est) is a compounded hormone preparation made up of a mixture of 80% estriol, 10% estrone, and 10% estradiol.12
Bi-estrogen (bi-est) contains estriol and estradiol in a ratio of 8:1 or 9:1.
Although both tri-est and bi-est are largely composed of estriol, given the low potency of estriol, the effects of these products may be solely mediated by their major bioactive component, estradiol.10,12 No large prospective, well-controlled clinical trial has investigated the compounded ratios of these mixtures of estrogens.10
Tri-est and bi-est are frequently promoted as posing less risk of breast or endometrial cancer than FDA-approved agents, although there is no research to back up this claim.12 In fact, estriol may have a stimulatory effect on the breast and endometrium.9
In addition to these “standard” compounded preparations, women can receive more customized compounds.
Valid uses for customized compounded formulations
Some clinical providers use customized compounded formulations when prescribing hormone therapy to women who have allergies to certain ingredients, such as peanut oil (found in the FDA-regulated oral product Prometrium). Customized compounded formulations have also been used when prescribing hormones currently not FDA-approved for women, such as testosterone and DHEA.12 Before oral micronized progesterone was marketed in the United States as Prometrium, it was frequently prescribed as a compounded hormone.
HORMONE THERAPY COMES IN VARIOUS FORMS
Both FDA-regulated hormone therapy and unregulated compounded hormone therapy come in various doses and dosage forms administered by different routes, allowing for individualization for each woman’s specific characteristics.
Estrogens: Oral, transdermal, others
Estrogen therapy can be given orally, transvaginally (as creams, tablets, and rings), transdermally (as patches, gels, and creams), subcutaneously in pellets, intranasally (in Europe), and by injection.11
Most oral contraceptives contain the synthetic estrogen ethinyl estradiol. Ethinyl estradiol is more potent than human estrogens,11 specifically in increasing the production of hepatic proteins (sex-hormone-binding globulin, renin substrate, corticosteroid-binding globulin, and thyroid-binding globulin).11
Bioidentical estradiol, taken orally in tablet form, is first processed through the liver and converted into estrone.12 This stimulates proteins such as C-reactive protein, activated protein C, and clotting factors, which may increase the risk of clotting.12 Estradiol given transdermally by patch or gel or vaginally bypasses the liver and enters the bloodstream as 17-beta estradiol, therefore avoiding stimulation of these proteins.12 Case-control data have shown an associated lower risk of deep venous thromboembolism with transdermal therapy.3
Subcutaneous pellet therapy is a less common, non-FDA-approved method of hormone therapy to relieve postmenopausal symptoms.10 In an outpatient procedure, the pellet is inserted into the subcutaneous fat of the abdomen.10 The crystalline pellet is biodegradable and contains a mixture of testosterone and 17-beta estradiol.10 It is important to remember that endometrial stimulation may be prolonged with this form of therapy and levels may be supraphysiologic.
Progestogens can also be given by different routes
Oral progesterone has poor gastrointestinal absorption and a short half-life.10 Therefore, it is micronized with oil for better absorption. Reported side effects include sedative and anesthetic effects; therefore, it is recommended that oral progesterone be taken at bedtime.9 Medroxyprogesterone acetate may interfere more with estrogen’s positive effects on cholesterol than micronized progesterone does.13
Topical progesterone preparations vary widely in dosage and formulation. Over-the-counter progesterone creams vary in concentration from no active ingredient to 450 mg or more of progesterone per ounce. Application sites for progesterone cream include the inner arm, chest, and inner thigh. No transdermal hormone should be applied to areas of the body that may allow possible contact and transference to others.
Progestogen products
Progestogen products include “natural” progesterone and synthetic progestins. They should be given concurrently with estrogen therapy in women who have an intact uterus to prevent endometrial hyperplasia.9
Bioidentical progesterone is micronized in the laboratory for better absorption in the gut.2
Misconception: Transdermal progesterone protects the endometrium
In general, transdermal progesterone should be avoided, as it does not protect against endometrial cancer.
Many forms of progesterone are available by prescription at compounding pharmacies as lotions, gels, creams, capsules, trochees, and suppositories.9 Transdermal progesterone creams are also available over the counter at health stores. Some of these creams contain only diosgenin, a progesterone precursor derived from wild yams.10 Diosgenin cannot be converted into progesterone within the body and thus does not provide an adequate amount of absorbable progesterone.9 Therefore, progesterone cream that contains only diosgenin is not effective in preventing endometrial hyperplasia and cancer.
To achieve a physiologic response, progesterone levels must be at least in the nanogram range.10 Transdermal progesterone cream has not been shown to reach this level and may not significantly improve vasomotor symptoms.12 Some practitioners prescribe cream that contains more than 400 mg progesterone per ounce. This may achieve physiologic levels of progesterone, but no improvement has been proven for bone mineral density or endometrial protection. In general, no transdermal progesterone cream can be assumed to protect the endometrium against the stimulatory effects of estrogen.
CUSTOM COMPOUNDING AND SALIVA TESTING TO INDIVIDUALIZE THERAPY
Some clinicians who prescribe compounded hormones order saliva tests. They argue the tests help them to establish which hormones are deficient and therefore to customize therapy.12 The basis for this is that saliva is similar to an ultrafiltrate of blood and, theoretically, hormone levels in saliva should represent the bioavailable hormone in serum.10
Unfortunately, this testing is often unreliable due to poor stability of samples in storage and large interassay variability.10 Many factors may alter hormone levels in saliva and make test results unreproducible, including the time of day the sample is collected and dietary habits.10 The FDA states that there is no scientific basis for using salivary testing to adjust hormone levels.2
Levels of drugs with clearance that varies depending on hepatic enzyme activity and plasma binding (capacity-limited metabolism) such as estradiol and testosterone can be monitored with total blood serum concentrations.10 However, many physiologic effects of estrogens are determined intracellularly at the level of tissues.10 Therefore, although levels during therapy with bioidentical estrogens can be monitored more precisely, the FDA states that hormone therapy should be guided by symptom response and findings on physical examination and not by hormone levels alone.2,12 It may be reasonable to order serum levels of estradiol in women being treated with therapeutic doses of bioidentical estrogen but still not achieving symptom relief. If women are being treated with conjugated equine estrogens, serum levels cannot be monitored. Total estrogen can be monitored as a send-out laboratory test.
MISCONCEPTION: HORMONE THERAPY IS A FOUNTAIN OF YOUTH
Customized compounded hormonal therapy is marketed as being able to help with rejuvenation, improve memory, sexual function, and reverse the aging process, essentially promising to be an elixir or fountain of youth.
These claims are not substantiated. However, the actual benefits of hormone therapy in women who have menopausal symptoms include alleviation of moderate to severe vasomotor symptoms and vaginal atrophy that can result in dyspareunia. By alleviating their symptoms, hormone therapy improves women’s quality of life. It also reduces the incidence of postmenopausal osteoporotic fractures.
A research finding that is often overlooked is that postmenopausal women younger than 60 years who started estrogen or estrogenprogestin therapy soon after menopause had a 30% lower rate of death from all causes.2,14 This difference was statistically significant when the estrogen and estrogen-progestin therapy groups were combined. No reduction in the mortality rate was seen if therapy was started after age 60.
MISCONCEPTION: COMPOUNDED THERAPY IS SAFER
Compounded hormone therapy is often marketed as a safer or more effective alternative to government-regulated and approved therapy. Unfortunately, these claims are often false and misleading, and safety information is not consistently provided to patients as is required with FDA-regulated hormone therapy.2
Since these compounds have not been approved by the FDA, there is no guarantee that the ingredients have been tested for purity, potency, and efficacy. There is no batch standardization. These unregulated therapies may use unapproved ingredients, routes of administration, and mixtures with contaminants such as dyes and preservatives.2
Also, custom-compounded prescriptions are considered experimental. Therefore, they are often not covered by insurance, and many women must pay for them out of pocket.11
The North American Menopause Society does not recommend custom-mixed products over well-tested, government-approved commercial products for most women.2 All bioidentical hormone prescriptions should include a patient package insert,11 identical to that required of FDA-approved products.2
Recent product endorsements from celebrities on television have brought a new term into the vocabulary of many American women: bioidentical hormone therapy—treatment with hormone products that are identical in molecular structure to those in the human body.
Since 2002, when results of the Women’s Health Initiative1 raised questions about the safety of hormone replacement therapy, women have been inundated by commercials, talk shows, and self-help books that promote bioidentical hormone therapy as a safe and natural way to treat menopausal symptoms—and more.
Although this publicity has helped promote discussion about menopause, it has also perpetuated confusion and misinformation among the lay public and the general medical community concerning menopausal hormone therapy.
Many postmenopausal women suffering from vasomotor symptoms, vaginal dryness, and vaginal atrophy are apprehensive about seeking therapy, owing to concerns resulting from misinterpreted information derived from the Women’s Health Initiative trial.1 (See “What are the known risks of FDA-approved hormone therapy.”2–8) Many others are told to suffer through their symptoms, which may eventually pass. It is not surprising, then, that women turn to unconventional treatments that are claimed to be safer. This unfortunate situation has driven the business of many compounding pharmacies into the multibillion dollar level.
In this paper, we hope to clarify some of the misconceptions surrounding this issue. But first we need to define some terms in what has become a confusing area.

WHAT ARE BIOIDENTICAL HORMONES?
“Bioidentical” means identical in molecular structure to endogenous hormones. However, as we will see, a better distinction should be made between products that are approved and regulated by the US Food and Drug Administration (FDA) and those that are not.
Endogenous reproductive hormones
Women produce various reproductive hormones, including three estrogens—estradiol, estrone, and estriol—as well as progesterone and testosterone.9
17-beta estradiol (E2) is the most bioactive endogenous estrogen. It is primarily produced by the dominant ovarian follicle and the corpus luteum and is synthesized intracellularly through aromatase activity.10,11 The rest of the circulating estradiol is derived from peripheral conversion of estrone to estradiol, and this is the primary source in postmenopausal women not on hormone therapy.11
In postmenopausal women, serum estradiol levels are often below 15 pg/mL. Many physiologic effects of the cellular compartmentalized estradiol contribute to an over-riding force in certain tissues even after menopause.10 With the loss of estradiol, many tissues in postmenopausal women can be affected, particularly resulting in genitourinary atrophy and bone loss.
Estrone (E1), the second dominant human estrogen, is primarily derived from the metabolism of estradiol and from the aromatization of androstenedione in adipose tissue, with a small quantity being secreted directly by the ovary and the adrenal glands.9 In postmenopausal women, mean estrone levels are about 30 pg/mL.11
Estriol (E3), the least active of the endogenous estrogens, is very short-acting.
Progesterone is a 21-carbon steroid secreted by the human ovary.9 It is formed during the transformation of cholesterol to estrogens and androgens and is no longer produced after menopause.9
Testosterone. In premenopausal women, the androgen testosterone is synthesized by the ovary, the adrenal cortex, and the peripheral conversion of circulating androstenedione and dehydroepiandrosterone (DHEA).9 Over a woman’s life span, her androgen levels decline progressively.10 The rate of decline has not been shown to be appreciably affected by the onset of menopause.10
All these hormone therapy products are synthesized
Many nonmedical women’s health books erroneously classify the forms of estrogen used in hormone therapy as either bioidentical or synthetic. In fact, they are all man-made.
Bioidentical hormones are synthesized by chemically extracting diosgenin from plants such as yams and soy.12 Diosgenin is chemically modified to yield the precursor progesterone, which is then used to synthesize bioidentical estrogens and androgens.10
Nonbioidentical estrogen products include conjugated equine estrogens (CEE), which is extracted from the urine of pregnant mares. The two predominant estrogens found in CEE are equilin sulfate (native to horses) and estrone sulfate.10
Other nonbioidentical products include ethinyl estradiol, which is used in most combined oral contraceptives. It is formed after a minor chemical modification of estradiol that makes it one of the most potent estrogens. The ethinyl group at carbon 17 of ring D of the steroid nucleus greatly slows the hepatic and enzymatic degradation of the molecule and, thereby, makes oral ethinyl estradiol 15 to 20 times more active than oral estradiol.
Mestranol is an inactive prodrug that is converted in the body to ethinyl estradiol.
While many women may find the idea of natural bioidentical hormones derived from sweet potatoes or soybeans more acceptable than taking one made from horse’s urine, all the products undergo extensive chemical processing and modification.
Misconception: FDA-regulated products are not bioidentical
WHAT IS CUSTOMIZED COMPOUNDED HORMONAL THERAPY?
There is often confusion between the terms “bioidentical hormones” and “customized compounded therapy,” which are often used interchangeably. Compounded therapy combines ratios of bioidentical hormones into a particular recipe or mixture. Customized compounding can be done by local compounding pharmacies.2
Compounded bioidentical estrogen products
There are several commonly marketed compounded products.
Tri-estrogen (tri-est) is a compounded hormone preparation made up of a mixture of 80% estriol, 10% estrone, and 10% estradiol.12
Bi-estrogen (bi-est) contains estriol and estradiol in a ratio of 8:1 or 9:1.
Although both tri-est and bi-est are largely composed of estriol, given the low potency of estriol, the effects of these products may be solely mediated by their major bioactive component, estradiol.10,12 No large prospective, well-controlled clinical trial has investigated the compounded ratios of these mixtures of estrogens.10
Tri-est and bi-est are frequently promoted as posing less risk of breast or endometrial cancer than FDA-approved agents, although there is no research to back up this claim.12 In fact, estriol may have a stimulatory effect on the breast and endometrium.9
In addition to these “standard” compounded preparations, women can receive more customized compounds.
Valid uses for customized compounded formulations
Some clinical providers use customized compounded formulations when prescribing hormone therapy to women who have allergies to certain ingredients, such as peanut oil (found in the FDA-regulated oral product Prometrium). Customized compounded formulations have also been used when prescribing hormones currently not FDA-approved for women, such as testosterone and DHEA.12 Before oral micronized progesterone was marketed in the United States as Prometrium, it was frequently prescribed as a compounded hormone.
HORMONE THERAPY COMES IN VARIOUS FORMS
Both FDA-regulated hormone therapy and unregulated compounded hormone therapy come in various doses and dosage forms administered by different routes, allowing for individualization for each woman’s specific characteristics.
Estrogens: Oral, transdermal, others
Estrogen therapy can be given orally, transvaginally (as creams, tablets, and rings), transdermally (as patches, gels, and creams), subcutaneously in pellets, intranasally (in Europe), and by injection.11
Most oral contraceptives contain the synthetic estrogen ethinyl estradiol. Ethinyl estradiol is more potent than human estrogens,11 specifically in increasing the production of hepatic proteins (sex-hormone-binding globulin, renin substrate, corticosteroid-binding globulin, and thyroid-binding globulin).11
Bioidentical estradiol, taken orally in tablet form, is first processed through the liver and converted into estrone.12 This stimulates proteins such as C-reactive protein, activated protein C, and clotting factors, which may increase the risk of clotting.12 Estradiol given transdermally by patch or gel or vaginally bypasses the liver and enters the bloodstream as 17-beta estradiol, therefore avoiding stimulation of these proteins.12 Case-control data have shown an associated lower risk of deep venous thromboembolism with transdermal therapy.3
Subcutaneous pellet therapy is a less common, non-FDA-approved method of hormone therapy to relieve postmenopausal symptoms.10 In an outpatient procedure, the pellet is inserted into the subcutaneous fat of the abdomen.10 The crystalline pellet is biodegradable and contains a mixture of testosterone and 17-beta estradiol.10 It is important to remember that endometrial stimulation may be prolonged with this form of therapy and levels may be supraphysiologic.
Progestogens can also be given by different routes
Oral progesterone has poor gastrointestinal absorption and a short half-life.10 Therefore, it is micronized with oil for better absorption. Reported side effects include sedative and anesthetic effects; therefore, it is recommended that oral progesterone be taken at bedtime.9 Medroxyprogesterone acetate may interfere more with estrogen’s positive effects on cholesterol than micronized progesterone does.13
Topical progesterone preparations vary widely in dosage and formulation. Over-the-counter progesterone creams vary in concentration from no active ingredient to 450 mg or more of progesterone per ounce. Application sites for progesterone cream include the inner arm, chest, and inner thigh. No transdermal hormone should be applied to areas of the body that may allow possible contact and transference to others.
Progestogen products
Progestogen products include “natural” progesterone and synthetic progestins. They should be given concurrently with estrogen therapy in women who have an intact uterus to prevent endometrial hyperplasia.9
Bioidentical progesterone is micronized in the laboratory for better absorption in the gut.2
Misconception: Transdermal progesterone protects the endometrium
In general, transdermal progesterone should be avoided, as it does not protect against endometrial cancer.
Many forms of progesterone are available by prescription at compounding pharmacies as lotions, gels, creams, capsules, trochees, and suppositories.9 Transdermal progesterone creams are also available over the counter at health stores. Some of these creams contain only diosgenin, a progesterone precursor derived from wild yams.10 Diosgenin cannot be converted into progesterone within the body and thus does not provide an adequate amount of absorbable progesterone.9 Therefore, progesterone cream that contains only diosgenin is not effective in preventing endometrial hyperplasia and cancer.
To achieve a physiologic response, progesterone levels must be at least in the nanogram range.10 Transdermal progesterone cream has not been shown to reach this level and may not significantly improve vasomotor symptoms.12 Some practitioners prescribe cream that contains more than 400 mg progesterone per ounce. This may achieve physiologic levels of progesterone, but no improvement has been proven for bone mineral density or endometrial protection. In general, no transdermal progesterone cream can be assumed to protect the endometrium against the stimulatory effects of estrogen.
CUSTOM COMPOUNDING AND SALIVA TESTING TO INDIVIDUALIZE THERAPY
Some clinicians who prescribe compounded hormones order saliva tests. They argue the tests help them to establish which hormones are deficient and therefore to customize therapy.12 The basis for this is that saliva is similar to an ultrafiltrate of blood and, theoretically, hormone levels in saliva should represent the bioavailable hormone in serum.10
Unfortunately, this testing is often unreliable due to poor stability of samples in storage and large interassay variability.10 Many factors may alter hormone levels in saliva and make test results unreproducible, including the time of day the sample is collected and dietary habits.10 The FDA states that there is no scientific basis for using salivary testing to adjust hormone levels.2
Levels of drugs with clearance that varies depending on hepatic enzyme activity and plasma binding (capacity-limited metabolism) such as estradiol and testosterone can be monitored with total blood serum concentrations.10 However, many physiologic effects of estrogens are determined intracellularly at the level of tissues.10 Therefore, although levels during therapy with bioidentical estrogens can be monitored more precisely, the FDA states that hormone therapy should be guided by symptom response and findings on physical examination and not by hormone levels alone.2,12 It may be reasonable to order serum levels of estradiol in women being treated with therapeutic doses of bioidentical estrogen but still not achieving symptom relief. If women are being treated with conjugated equine estrogens, serum levels cannot be monitored. Total estrogen can be monitored as a send-out laboratory test.
MISCONCEPTION: HORMONE THERAPY IS A FOUNTAIN OF YOUTH
Customized compounded hormonal therapy is marketed as being able to help with rejuvenation, improve memory, sexual function, and reverse the aging process, essentially promising to be an elixir or fountain of youth.
These claims are not substantiated. However, the actual benefits of hormone therapy in women who have menopausal symptoms include alleviation of moderate to severe vasomotor symptoms and vaginal atrophy that can result in dyspareunia. By alleviating their symptoms, hormone therapy improves women’s quality of life. It also reduces the incidence of postmenopausal osteoporotic fractures.
A research finding that is often overlooked is that postmenopausal women younger than 60 years who started estrogen or estrogenprogestin therapy soon after menopause had a 30% lower rate of death from all causes.2,14 This difference was statistically significant when the estrogen and estrogen-progestin therapy groups were combined. No reduction in the mortality rate was seen if therapy was started after age 60.
MISCONCEPTION: COMPOUNDED THERAPY IS SAFER
Compounded hormone therapy is often marketed as a safer or more effective alternative to government-regulated and approved therapy. Unfortunately, these claims are often false and misleading, and safety information is not consistently provided to patients as is required with FDA-regulated hormone therapy.2
Since these compounds have not been approved by the FDA, there is no guarantee that the ingredients have been tested for purity, potency, and efficacy. There is no batch standardization. These unregulated therapies may use unapproved ingredients, routes of administration, and mixtures with contaminants such as dyes and preservatives.2
Also, custom-compounded prescriptions are considered experimental. Therefore, they are often not covered by insurance, and many women must pay for them out of pocket.11
The North American Menopause Society does not recommend custom-mixed products over well-tested, government-approved commercial products for most women.2 All bioidentical hormone prescriptions should include a patient package insert,11 identical to that required of FDA-approved products.2
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17:242–255.
- Canonico M, Oger E, Plu-Bureau G; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845.
- Risks of postmenopausal hormone replacement (letters). JAMA 2002; 288:2819–2825.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Shumaker SA, Legault C, Rapp SR, et al; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289:2651–2662.
- Chlebowski RT, Anderson GL, Gass M, et al; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010; 304:1684–1692.
- Lobo RA. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 3rd ed. Burlington, MA: Academic Press; 2007.
- Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt) 2007; 16:600–631.
- Menopause Practice: A Clinician’s Guide. 4th ed. Cleveland, OH: The North American Menopause Society; 2010.
- What are bioidentical hormones? Natural. Bioidentical. Compounded. Confusion about these terms is only adding to the confusion over hormone therapy. Harv Womens Health Watch 2006; 13:1–3.
- The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1995; 273:199–208.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321–333.
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17:242–255.
- Canonico M, Oger E, Plu-Bureau G; Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007; 115:840–845.
- Risks of postmenopausal hormone replacement (letters). JAMA 2002; 288:2819–2825.
- Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007; 297:1465–1477.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 2000; 133:933–941.
- Shumaker SA, Legault C, Rapp SR, et al; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289:2651–2662.
- Chlebowski RT, Anderson GL, Gass M, et al; WHI Investigators. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA 2010; 304:1684–1692.
- Lobo RA. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 3rd ed. Burlington, MA: Academic Press; 2007.
- Cirigliano M. Bioidentical hormone therapy: a review of the evidence. J Womens Health (Larchmt) 2007; 16:600–631.
- Menopause Practice: A Clinician’s Guide. 4th ed. Cleveland, OH: The North American Menopause Society; 2010.
- What are bioidentical hormones? Natural. Bioidentical. Compounded. Confusion about these terms is only adding to the confusion over hormone therapy. Harv Womens Health Watch 2006; 13:1–3.
- The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1995; 273:199–208.
- Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause 2007; 14:944–957.
KEY POINTS
- Hormone therapy is indicated for relief of menopausal symptoms; claims of reversal of the aging process are unsubstantiated.
- Products that are custom-compounded are not regulated by the US Food and Drug Administration and therefore carry no assurance of purity, safety, or efficacy.
- Transdermal progesterone creams do not achieve high enough serum levels to protect the endometrium.
- Hormone therapy is titrated on the basis of symptom response. Measuring hormone levels in saliva is not called for and is probably not reliable.
The bittersweet of steroid therapy
For many of those long-term effects, such as osteoporosis, cushingoid features, skin fragility, and cataracts, all we can do is hope that they don’t occur, since there is little we can do to screen for or prevent them. We have previously discussed steroid-associated osteoporosis in the Journal,1 and strategies for preventing it have been proposed by specialty societies.2 For other complications such as hypertension, weight gain, and glucose intolerance, we can offer common-sense protective suggestions, monitor for them, and intervene if they occur.
In this issue, Dr. M. Cecilia Lansang and Ms. Leighanne Kramer Hustak3 discuss the management of steroid-induced adrenal suppression and diabetes. They offer practical management suggestions but also point out that the evidence base for our treatment decisions is surprisingly limited.
Nearly all patients chronically receiving high-dose glucocorticoid therapy develop glucose intolerance, but knowing when that is happening is not always easy. In patients destined to develop type 2 diabetes, the laboratory or clinical signs of hyperglycemia appear only when the pancreas can no longer maintain the insulin production necessary to overcome peripheral insulin resistance. Steroid-induced diabetes is characterized by increased gluconeogenesis, insulin resistance, and excessive postprandial surges, so fasting glucose levels are not sensitive for this clinical syndrome.
The degree and duration of the chronic hyperinsulinemia and hyperglycemia dictates the risk of microvascular complications and thus will be linked to duration of steroid therapy (unless the steroid is unmasking preexisting mild diabetes). Although issues surrounding tight control of blood glucose levels in the acute setting remain unresolved, I believe that even short-term significant steroid-induced hyperglycemia should be prevented when reasonably possible, at the least keeping in mind the additive ill effects of hyperglycemia and steroid therapy on the risk of nuisance infections such as oral and vaginal candidiasis and urinary tract infections that, in the setting of high-dose steroid therapy, can rapidly turn nasty.
- Dore RK. How to prevent glucocorticoid-induced osteoporosis. Cleve Clin J Med 2010; 77:529–536.
- American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum 2001; 44:1496–1503.
- Lansang MC, Hustak LK. Glucocorticoid-induced diabetes and adrenal suppression: how to detect and manage them. Cleve Clin J Med 2011; 78:748–756.
For many of those long-term effects, such as osteoporosis, cushingoid features, skin fragility, and cataracts, all we can do is hope that they don’t occur, since there is little we can do to screen for or prevent them. We have previously discussed steroid-associated osteoporosis in the Journal,1 and strategies for preventing it have been proposed by specialty societies.2 For other complications such as hypertension, weight gain, and glucose intolerance, we can offer common-sense protective suggestions, monitor for them, and intervene if they occur.
In this issue, Dr. M. Cecilia Lansang and Ms. Leighanne Kramer Hustak3 discuss the management of steroid-induced adrenal suppression and diabetes. They offer practical management suggestions but also point out that the evidence base for our treatment decisions is surprisingly limited.
Nearly all patients chronically receiving high-dose glucocorticoid therapy develop glucose intolerance, but knowing when that is happening is not always easy. In patients destined to develop type 2 diabetes, the laboratory or clinical signs of hyperglycemia appear only when the pancreas can no longer maintain the insulin production necessary to overcome peripheral insulin resistance. Steroid-induced diabetes is characterized by increased gluconeogenesis, insulin resistance, and excessive postprandial surges, so fasting glucose levels are not sensitive for this clinical syndrome.
The degree and duration of the chronic hyperinsulinemia and hyperglycemia dictates the risk of microvascular complications and thus will be linked to duration of steroid therapy (unless the steroid is unmasking preexisting mild diabetes). Although issues surrounding tight control of blood glucose levels in the acute setting remain unresolved, I believe that even short-term significant steroid-induced hyperglycemia should be prevented when reasonably possible, at the least keeping in mind the additive ill effects of hyperglycemia and steroid therapy on the risk of nuisance infections such as oral and vaginal candidiasis and urinary tract infections that, in the setting of high-dose steroid therapy, can rapidly turn nasty.
For many of those long-term effects, such as osteoporosis, cushingoid features, skin fragility, and cataracts, all we can do is hope that they don’t occur, since there is little we can do to screen for or prevent them. We have previously discussed steroid-associated osteoporosis in the Journal,1 and strategies for preventing it have been proposed by specialty societies.2 For other complications such as hypertension, weight gain, and glucose intolerance, we can offer common-sense protective suggestions, monitor for them, and intervene if they occur.
In this issue, Dr. M. Cecilia Lansang and Ms. Leighanne Kramer Hustak3 discuss the management of steroid-induced adrenal suppression and diabetes. They offer practical management suggestions but also point out that the evidence base for our treatment decisions is surprisingly limited.
Nearly all patients chronically receiving high-dose glucocorticoid therapy develop glucose intolerance, but knowing when that is happening is not always easy. In patients destined to develop type 2 diabetes, the laboratory or clinical signs of hyperglycemia appear only when the pancreas can no longer maintain the insulin production necessary to overcome peripheral insulin resistance. Steroid-induced diabetes is characterized by increased gluconeogenesis, insulin resistance, and excessive postprandial surges, so fasting glucose levels are not sensitive for this clinical syndrome.
The degree and duration of the chronic hyperinsulinemia and hyperglycemia dictates the risk of microvascular complications and thus will be linked to duration of steroid therapy (unless the steroid is unmasking preexisting mild diabetes). Although issues surrounding tight control of blood glucose levels in the acute setting remain unresolved, I believe that even short-term significant steroid-induced hyperglycemia should be prevented when reasonably possible, at the least keeping in mind the additive ill effects of hyperglycemia and steroid therapy on the risk of nuisance infections such as oral and vaginal candidiasis and urinary tract infections that, in the setting of high-dose steroid therapy, can rapidly turn nasty.
- Dore RK. How to prevent glucocorticoid-induced osteoporosis. Cleve Clin J Med 2010; 77:529–536.
- American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum 2001; 44:1496–1503.
- Lansang MC, Hustak LK. Glucocorticoid-induced diabetes and adrenal suppression: how to detect and manage them. Cleve Clin J Med 2011; 78:748–756.
- Dore RK. How to prevent glucocorticoid-induced osteoporosis. Cleve Clin J Med 2010; 77:529–536.
- American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis. Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. Arthritis Rheum 2001; 44:1496–1503.
- Lansang MC, Hustak LK. Glucocorticoid-induced diabetes and adrenal suppression: how to detect and manage them. Cleve Clin J Med 2011; 78:748–756.
Glucocorticoid-induced diabetes and adrenal suppression: How to detect and manage them
Glucocorticoids are commonly prescribed by primary care physicians and specialists alike for multiple medical problems, acute as well as chronic.
However, these useful drugs have adverse effects on multiple endocrine systems, effects that include diabetes (or worsening of hyperglycemia in those with known diabetes), Cushing syndrome, adrenal suppression, osteoporosis (reviewed in the Cleveland Clinic Journal of Medicine in August 2010),1 and dyslipidemia. In addition, suppression of gonadotropins, growth hormone, and, acutely, thyrotropin can ensue.
The focus of this review is on the diabetogenic and adrenal suppressive effects of glucocorticoids and their management. We describe the rationale for choosing specific drugs to counter hyperglycemia, tests for determining adrenal suppression and systemic glucocorticoid absorption, and how and why to taper these drugs.
WIDELY USED DRUGS
Although glucocorticoids (often simply called steroids or corticosteroids, although not all steroids are corticosteroids, and not all corticosteroids are glucocorticoids) are the core treatment for adrenal insufficiency, in most cases they are prescribed for their anti-inflammatory effects. They act through multiple pathways at the cellular and molecular levels, suppressing the cascades that would otherwise result in inflammation and promoting pathways that produce anti-inflammatory proteins.2
In addition to formulations that are intended to have systemic effects, other, “local” formulations are made for specific conditions, such as intra-articular injections for arthritis, epidural injections for lumbar disk pain, eye drops for uveitis, nasal sprays for allergic rhinitis, inhalers for asthma, and topical ointments and creams for eczema. However, as we will discuss, even these preparations can have systemic effects.
GLUCOCORTICOID-INDUCED DIABETES IS COMMON
Glucocorticoids are the most common cause of drug-induced diabetes. Though the exact prevalence is not known, a few observations suggest that glucocorticoid-induced diabetes or hyperglycemia is common:
- In patients with rheumatoid arthritis, mean age 62 years, nearly 9% developed diabetes in the 2 years after starting glucocorticoid treatment, which was a higher rate than expected.3
- In nondiabetic patients with primary renal disease treated with prednisolone 0.75 mg/kg/day, 42% were found to have 2-hour post-lunch plasma glucose concentrations higher than 200 mg/dL but normal fasting glucose levels.4
- In a case-control study, the odds ratio of starting an oral hypoglycemic agent or insulin was 1.77 for patients receiving a hydrocortisone-equivalent dose of 1 to 39 mg/day, 3.02 for 40 to 79 mg/day, 5.82 for 80 to 119 mg/day, and 10.34 for 120 mg/day or more.5 (For a full discussion of glucocorticoid equivalents, see the section below on Cushing syndrome and adrenal suppression.)
- In patients with type 1 diabetes, prednisone 60 mg/day raised the blood glucose levels starting 6 hours after the prednisone dose.6
- Diabetic ketoacidosis and hyperosmolar nonketotic syndrome have been reported as a result of glucocorticoid treatment.7–9
GLUCOCORTICOIDS CAUSE DIABETES MAINLY VIA INSULIN RESISTANCE
The mechanism by which glucocorticoids cause diabetes predominantly involves insulin resistance rather than decreased insulin production. In fact, in a study in healthy volunteers, 10 hydrocortisone infusion resulted in higher insulin production than saline infusion did. (In high doses, however, glucocorticoids have been shown to decrease insulin secretion.11)
Normally, in response to insulin, the liver decreases its output of glucose. Glucocorticoids decrease the liver’s sensitivity to insulin, thereby increasing hepatic glucose output.12 They also inhibit glucose uptake in muscle and fat, reducing insulin sensitivity as much as 60% in healthy volunteers. This seems primarily due to a postreceptor effect, ie, inhibition of glucose transport.13–15
THE PEAK EFFECT OCCURS 4 TO 6 HOURS AFTER DOSING
To understand the optimal time for checking plasma glucose and to apply appropriate treatment, we should consider the pharmacokinetic profile of glucocorticoids.
Studied using the whole-blood lymphocyte proliferation technique, prednisone shows a peak effect at about 4 to 6 hours and a duration of action of 13 to 16 hours.16 This closely resembles what we see in terms of glucose excursion with this drug.17 Two studies of intravenous dexamethasone 10 mg showed that glucose levels rose within 4 hours of injection, but did not pursue this beyond that time frame.18,19
PATIENTS WITHOUT A PREVIOUS DIAGNOSIS OF DIABETES
Be alert for new-onset diabetes
For most diseases treated with glucocorticoids, clinicians can estimate in advance how long the patient will need to take the drug. We can arbitrarily classify the projected exposure as either short-term (3 to 4 weeks or less, such as a 6-day course of methylprednisolone for allergic conditions) or long-term (such as in transplant recipients to prevent rejection or to treat graft-vs-host disease). Hyperglycemia is a potential concern with both short-term and long-term treatment. However, guidelines on checking blood sugar levels, as opposed to relying on symptoms alone, are available only for long-term glucocorticoid treatment.
Patients beginning treatment should be warned of typical diabetes symptoms such as thirst and increased urination and, should these occur, to seek medical attention to have their blood glucose level checked. It is also reasonable to have them return in a week for a random postprandial plasma glucose test in the mid-afternoon.
Why this timing? In most once-daily regimens, glucocorticoids are given in the morning to prevent adrenal suppression (discussed below). In our experience, glucose levels start to rise mid-morning and continue to increase until bedtime. Measuring glucose levels 1 to 2 hours after lunch allows for both the glucocorticoid action and the carbohydrate absorption from lunch to reach their peaks. If hyperglycemia is going to happen, it should be detectable by then. A glucose level of 200 mg/dL or higher should prompt the practitioner to pursue this further.
If glucocorticoid treatment is to continue beyond 3 to 4 weeks, the only population for which there are published guidelines on managing glucocorticoid-related diabetes is transplant recipients. International consensus guidelines, published in 2003, suggest checking the fasting plasma glucose level once a week for the first 4 weeks after transplantation, then at 3 months, at 6 months, and then once a year.20
Though practical, this suggestion does not reflect the fact that glucocorticoids often do not affect fasting plasma glucose, especially if given once daily in the morning at doses of 30 mg or less of prednisone or its equivalent. These guidelines thus may not be applicable to other populations with glucocorticoid-induced diabetes.
The transplant guidelines do mention that an oral glucose tolerance test may be more sensitive, but this is often cumbersome to perform. We believe that checking random postprandial plasma glucose levels is helpful in this regard.
If the patient was at risk of developing diabetes even before receiving a glucocorticoid (for example, if he or she is overweight, has a family history of diabetes, or had a previous hemoglobin A1c of 5.7% or higher), then a fasting plasma glucose level of 126 mg/dL or higher or a hemoglobin A1c of 6.5% or higher might suffice to diagnose diabetes. Results should be confirmed on a separate day in the absence of unequivocal hyperglycemia. Fasting hyperglycemia can also be seen in patients receiving higher once-daily glucocorticoid doses—in our experience, an equivalent of prednisone 40 mg once a day in the morning— or twice-daily dosing.
A hemoglobin A1c checked less than 2 to 3 months after starting glucocorticoid treatment will not be sensitive in picking up glucocorticoid-induced diabetes if the patient did not have underlying diabetes.
Diet and exercise may not be practical
Though diet and exercise are important in managing diabetes, the condition for which the patient is receiving a glucocorticoid may prevent him or her from exercising, at least in the acute phase of the illness.
In addition, though the exact mechanism is not known, glucocorticoids increase hunger, and so decreasing food intake is not easy either. Nonetheless, patients should be familiarized with what carbohydrates are and should be advised to reduce their intake of them.
For suspected type 1 diabetes, start insulin
If type 1 diabetes is suspected, for example, in patients who are lean, younger than 30 years, or who had presented with diabetic ketoacidosis, then insulin should be started. In equivocal cases, insulin therapy can commence while testing is done for C-peptide, glutamic acid decarboxylase antibodies, islet cell antibodies, and insulinoma-associated protein antibodies.
Starting oral antidiabetic drugs
Some patients may have contraindications to specific drugs. For example, metformin (Glucophage) is contraindicated if the serum creatinine level is elevated, an abnormality that renal transplant patients may continue to have.
If the patient has no such contraindications, we have found the following medications suitable in view of their efficacy, low risk of hypoglycemia, or lack of distressing side effects. They will often lower glucose levels enough to achieve capillary blood glucose or fingerstick goals (discussed below). None of them has been specifically approved by the US Food and Drug Administration for glucocorticoid-induced diabetes, but they are approved for type 2 diabetes.
Guidelines from the American Association of Clinical Endocrinologists for type 2 diabetes call for starting monotherapy if the hemoglobin A1c is 6.5% to 7.5%, dual therapy if it is 7.6% to 9%, triple therapy if it is higher than 9% and the patient has no symptoms, and insulin if it is higher than 9% and the patient does have symptoms.22
In terms of estimated average glucose levels, these categories correspond to 140 to 169 mg/dL for monotherapy, 171 to 212 mg/dL for dual therapy, and higher than 212 mg/dL for triple therapy or insulin. Since estimated average levels also include fasting glucose levels (which are lower in glucocorticoid-induced diabetes compared with nonfasting levels), and because we use the American Diabetes Association general hemoglobin A1c goal of less than 7%, we believe that our suggestions below are reasonable.
We divide our recommendations according to initial random (ideally, 1- to 2-hour postprandial) plasma glucose levels.
If the random or 1- to 2-hour post-meal plasma glucose is lower than 220 mg/dL
In this situation the choices are:
- Metformin
- Dipeptidyl peptidase-4 (DPP-4) inhibitors (“gliptins”)
- Meglitinides (“glinides”). The guidelines on new-onset diabetes after transplantation point out that meglitinides may be the safest agents apart from insulin in the renal transplant population, but does acknowledge that efficacies of different oral agents have not been compared in this group.20
- Glucagon-like protein-1 (GLP-1) agonists
- Sulfonylureas. However, the longer-acting forms such as glimepiride (Amaryl) are not suitable if the fasting plasma glucose is not affected.
We have not used thiazolidinediones (“glitazones”) routinely because they can cause weight gain and edema—problems that are already seen with the use of steroids—and have a slower onset of action.
If the random or 1- to 2-hour post-meal plasma glucose is 220 to 300 mg/dL
Often, a combination of drugs or insulin (see below) is needed. However, you can start with one agent and add a second agent within 2 or 3 months (as is recommended for type 2 diabetes).22,23 The following combinations of the agents listed above are supported by published guidelines for type 2 diabetes:
- Metformin plus a sulfonylurea22,23
- Metformin plus a glinide22
- Metformin plus a GLP-1 agonist23
- Metformin plus a DPP-4 inhibitor.22
If the random or 1- to 2-hour post-meal plasma glucose is higher than 300 mg/dL
In our experience, if their plasma glucose levels are this high, patients are experiencing frank symptoms of hyperglycemia.
Insulin addresses those symptoms and avoids the prolonged wait that often results from unsuccessfully starting one agent and then adding another. Of all the available drugs, insulin is the only one that can be used despite multiple underlying illnesses; it does not cause a lot of drug interactions, and the dose can be adjusted upward and downward in increments to fit the patient’s needs, especially when a larger glucocorticoid load is given up front and then is tapered either slowly or rapidly. However, it can cause hypoglycemia and weight gain.
The initial total daily dose of insulin can be based on the patient’s weight. A starting total daily dose of 0.15 to 0.3 U/kg is reasonable— on the lower end if only the postprandial glucose levels are elevated, and on the higher end if both fasting and postprandial glucose levels are affected.
If fasting glucose levels are not elevated, then Neutral Protamine Hagedorn insulin (which is intermediate-acting) or a premixed combination of an intermediate-acting plus a fast- or short-acting insulin can be given once a day before breakfast, or even before lunch if the glucose levels start to rise only after lunch.
If both the fasting and the postprandial glucose levels are elevated, regimens similar to those for type 1 or insulin-requiring type 2 diabetes can be used, except that the ratios of the doses are tilted more toward covering postprandial than fasting hyperglycemia:
- Long-acting insulin plus prandial insulin, in a ratio of 30:70 to 50:50. As glucocorticoids are tapered, the long-acting insulin may have to be discontinued while the prandial doses are continued, since the fasting glucose level decreases first.
- Premixed insulins, with one-half to two-thirds of the dose given before breakfast and the rest before the evening meal, with the possibility of a third injection before lunch. As glucocorticoids are tapered, the evening dose is tapered first.
- Intermediate-acting insulin plus short- or fast-acting insulin in the morning (these two will make up one-half to two-thirds of the total daily dose), short- or fast-acting insulin before the evening meal, and intermediate-acting insulin at bedtime. As glucocorticoids are tapered, the bedtime insulin is tapered first.
Capillary blood glucose (fingerstick) checks
The timing and frequency of fingerstick checks depend on the treatment.
Though postprandial testing is ideal, it is often not practical or convenient. Before lunch, before dinner, and at bedtime are good alternatives since they reflect the pattern of glucose rise throughout the day. For patients on diet and exercise with or without agents other than insulin, testing once or twice a day is reasonable, rotating times before meals (including fasting if this time is affected) and at bedtime.
For patients on insulin, checking two to four times a day initially would help match insulin doses with glucose excursions. For continued care, the American Diabetes Association recommends fingerstick checks three times daily in patients on multiple insulin injections, but it has no specific recommendations for those on once-a-day insulin.21 We have been recommending that our patients on once-daily insulin check at least twice a day.
Goal fingerstick glucose levels that we use are in accordance with the American Diabetes Association guidelines for diabetes in general21:
- Before meals 70 to 130 mg/dL or
- 1 to 2 hours after meals < 180 mg/dL.
During steroid taper, if the glucocorticoid dose is in the lower range (eg, a prednisone-equivalent dose of approximately 7.5 mg per day or less), the fingerstick glucose levels are at the lower end of the target range, and the patient is on a single antidiabetic agent that does not often cause hypoglycemia (eg, metformin), then it is reasonable to ask the patient to not take the antidiabetic medication for 3 to 7 days while continuing to check fingersticks to see if it needs to be resumed. Patients on agents that can cause hypoglycemia need to check more often during the 1 to 3 days after the glucocorticoid dose reduction, as it may take this much time for the glycemic effect to diminish and to adjust the diabetes medication to the appropriate dose.
STARTING GLUCOCORTICOIDS IN PATIENTS WITH KNOWN DIABETES
Fingerstick checks more often
Most patients will already have a glucose meter. They should be instructed to check as discussed above if they do not have a previous diagnosis of diabetes, or to continue as they are doing if they are already checking more often. Patients who have been checking only fasting levels should be instructed to check later in the day, either before or 1 to 2 hours after meals, as discussed above. Patients on oral medications may need additional oral agents or insulin.
Adjust medications if glucose is not at goal
Patients with type 2 diabetes treated with diet and exercise alone can be started on the medications discussed above if their fingerstick readings are not at goal.
If they are already on insulin, we advise them to increase the short- or fast-acting insulins and the morning intermediate-acting insulin by at least 10% to 20% as soon as an elevation in glucose is detected. Long-acting insulin or nighttime intermediate-acting insulin should be increased if fasting glucose levels are affected.
Insulin requirements can double depending on the glucocorticoid dose. In patients with type 1 diabetes who were given prednisone 60 mg orally for 3 days, mean blood glucose levels increased from a baseline of 110 mg/dL at baseline to 149 mg/dL on the days on prednisone.6 The average blood glucose level remained elevated at 141 mg/dL on the day after the last dose of prednisone. The insulin dose increased by 31% to 102% (mean 69%).
CUSHING SYNDROME AND ADRENAL SUPPRESSION
Unlike glucocorticoid-induced diabetes, in which the dilemma is often when to initiate antidiabetic treatment, the question for patients in whom Cushing syndrome or adrenal suppression has developed is when to discontinue glucocorticoids.
Adrenal suppression for the most part goes hand in hand with exogenous Cushing syndrome. If cushingoid features develop, we can infer that the dose of exogenous glucocorticoid exceeds the physiologic needs. This supraphysiologic dosing also leads to suppression of endogenous cortisol production. The suppression occurs at the level of the hypothalamus and pituitary gland, with subsequent atrophy of the part of the adrenal cortex that produces endogenous glucocorticoids.
To understand further the concept of supraphysiologic dosing, the following interconversion of systemic glucocorticoid effects is helpful24,25:
However, there is not much information on interconversion for the local preparations (intra-articular, epidural, inhaled, topical).
Moreover, the definition of supraphysiologic dosing seems to be evolving. Though a total hydrocortisone-equivalent dose of 30 mg/day is still often touted as physiologic replacement, many patients require less. Several studies in the early 1990s, mostly in children and adolescents, showed the mean daily cortisol production rate to be 4.8 to 6.8 mg/m2/day, or closer to 10 to 15 mg/day.26–28 For purposes of this discussion, a physiologic dose will be defined as up to 30 mg hydrocortisone per day or its equivalent.
Adrenal suppression vs insufficiency
Adrenal suppression is often confused with adrenal insufficiency.
Adrenal suppression occurs when cortisol production is decreased because of the presence of exogenous glucocorticoids or other drugs, such as megestrol acetate (Megace), that act on the glucocorticoid receptor. Another situation beyond the scope of this review is excess endogenous cortisol production by an adrenal adenoma or adrenal carcinoma that causes suppression of the contralateral adrenal gland.29
In contrast, adrenal insufficiency is caused by failure of the adrenal gland to produce cortisol as a result of an innate disorder of the adrenal gland (eg, Addison disease) or pituitary gland (eg, pituitary surgery).
Hence, endogenous cortisol production in a patient taking supraphysiologic doses of exogenous glucocorticoids may be suppressed. Recovery of endogenous cortisol production is expected after stopping the exogenous glucocorticoid, though the time to recovery can vary and the patient can be adrenally insufficient if the glucocorticoid is stopped abruptly.
In addition, during times of intercurrent illness, a patient with adrenal suppression may be relatively adrenally insufficient and may need larger doses (“stress doses”) of glucocorticoids, since the adrenal glands may be unable to mount a stress response.29
Local steroids can suppress the adrenal glands
Glucocorticoids are the most common cause of Cushing syndrome. Oral formulations such as dexamethasone, prednisone, and hydrocortisone taken in supraphysiologic doses and for prolonged durations are easily recognized as obvious causes of Cushing syndrome. However, intra-articular, epidural, inhaled, nasal, ocular, and topical steroids—so-called local preparations—have also been linked to Cushing syndrome, and physicians are less likely to recognize them as causes.30–38
In a study in 16 pediatric patients with asthma and 48 controls, inhaled beclomethasone dipropionate (Qvar) 300 to 500 μg daily resulted in adrenal suppression in 100% of patients after 6 to 42 months, as determined by an insulin tolerance test.30
The topical steroid betamethasone (Diprosone) carries a warning that systemic absorption of topical steroids can cause adrenal suppression.39 Intra-articular, intranasal, epidural, and ocular routes are also reported to cause adrenal suppression.32–38
When is adrenal suppression more likely?
Adrenal suppression is more likely in the following situations:
- Longer duration of treatment. Studies have shown that exposure to supraphysiologic steroid doses for 2 weeks or less might already suppress the adrenal glands, but the clinical significance of this is unclear since some recovery already occurs a few days after the glucocorticoids are discontinued.31,40
- Supraphysiologic doses, stronger formulations, longer-acting formulations.41
When is adrenal suppression less likely?
Adrenal suppression is less likely in the following situations:
- Regimens that mimic the diurnal rhythm of cortisol (higher dose in the morning, lower dose in the afternoon)42
- Alternate-day dosing of steroids.43
Steroid withdrawal vs adrenal insufficiency
Another phenomenon that can be confused with adrenal insufficiency or glucocorticoid insufficiency is steroid withdrawal, in which patients experience lethargy, muscle aches, nausea, vomiting, and postural hypotension as glucocorticoids are tapered and their effects wane.42 Increasing the glucocorticoid dose for presumed adrenal insufficiency may delay recovery of the adrenal function and would have to be weighed against the patient’s symptoms.
The following may help distinguish the two: if the patient is on supraphysiologic glucocorticoid doses, then he or she is not glucocorticoid-deficient and is likely suffering from steroid withdrawal. At this point, patients may just need reassurance, symptomatic treatment, or if necessary, a brief (1-week) increase of the previous lowest dose, followed by reevaluation.
With local glucocorticoid preparations that may be systemically absorbed, however, there is no good way of estimating dose equivalence. In these situations, the decision to simply reassure the patient or give symptomatic treatment—as opposed to giving low-dose oral glucocorticoids such as hydrocortisone 5 to 10 mg daily for a week followed by reevaluation— depends on the severity of symptoms and whether the patient has quick access to medical attention should he or she develop an intercurrent illness.
Identifying patients at risk of adrenal suppression
Patients presenting with weight gain or symptoms suggesting Cushing syndrome should be asked about steroid intake and should be prompted to recall possible nonoral routes. In addition, patients presenting with muscle aches and fatigue—symptoms of steroid withdrawal— may have received unrecognized local glucocorticoids that were systemically absorbed, now with diminishing effects.
The ACTH stimulation test for adrenal recovery
Testing can be done to see if the adrenal glands have recovered and glucocorticoid therapy can be discontinued (see Tapering from glucocorticoids, below).
The test most often used is the corticotropin (ACTH) stimulation test. Since the suppression is at the level of the hypothalamus and the pituitary gland, the ACTH stimulation test is an indirect method of assessing hypothalamic and pituitary function in the context of glucocorticoid-induced adrenal suppression. It has good correlation with the insulin tolerance test, the gold-standard test for an intact hypothalamic-pituitary-adrenal axis.
The synthetic ACTH cosyntropin (Cortrosyn) 250 μg is injected intravenously or intramuscularly, and a cortisol level is drawn at baseline and 30 and 60 minutes later. Other doses such as 1 μg or 10 μg have been reported but are not yet widely accepted. A cortisol level of greater than 18 to 20 μg/dL at any time point shows that the adrenals have regained function and the steroids may be discontinued.42 If adrenal suppression persists, weaning from steroids should continue.
In reality, it may not be possible or practical to do an ACTH stimulation test, as not all physicians’ offices have a supply of cosyntropin or the manpower to perform the test correctly. In these cases, weaning can progress with monitoring of symptoms.
Testing for synthetic glucocorticoids in the urine and serum can demonstrate systemic absorption and may be helpful in patients who do not recall receiving steroids.33
Tapering from glucocorticoids
Several tapering schedules have been suggested (although not necessarily validated). Whether and how to taper depend on how long the glucocorticoid has been taken.
If taken for less than 1 week, glucocorticoids can be stopped without tapering, regardless of the dose.
If taken for 1 to 3 weeks, the decision to taper depends on the clinician’s assessment of the patient’s general health or constitution and the illness for which the glucocorticoid was prescribed. For example, if the underlying disease is less likely to flare with a gradual dose reduction, then tapering would be suitable.44
If taken for more than 3 weeks, the practice has been a more rapid taper at the beginning until a physiologic dose is reached. How quickly to reduce the dose depends on whether the underlying illness is expected to flare up, or if the patient might experience steroid withdrawal symptoms.
One schedule is to lower the glucocorticoid dose by an amount equivalent to prednisolone 2.5 mg every 3 to 4 days when above the physiologic dose, then to taper more slowly by 1 mg every 2 to 4 weeks.44 Once the physiologic dose is reached, one can switch to the equivalent dose of hydrocortisone and decrease the dose by 2.5 mg a week until a daily dose of 10 mg a day is reached and maintained for 2 to 3 months, and then perform a test of adrenal function (see above).44 Passing the test implies that the adrenal glands have recovered and the glucocorticoid can be stopped.
Another option is to switch to alternate-day therapy once a physiologic dose is reached and to test 8:00 am cortisol levels, continuing the glucocorticoid and retesting in 4 to 6 weeks if the value is less than 3 μg/dL; stopping the glucocorticoid if the value is higher than 20 μg/dL; and performing an ACTH stimulation test for values in between.45
A review of other tapering regimens for chronic diseases, mostly pulmonary, did not find enough evidence to recommend one particular schedule over another.46 The tapering schedule may have to be adjusted to prevent disease flare and symptoms of steroid withdrawal.
Locally administered steroids. Since the equivalence of systemically absorbed local glucocorticoids is not known, these patients are likely to present when they have symptoms of steroid withdrawal. In this situation, testing adrenal function will help.
- Dore RK. How to prevent glucocorticoid-induced osteoporosis. Cleve Clin J Med 2010; 77:529–536.
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 2005; 353:1711–1723.
- Panthakalam S, Bhatnagar D, Klimiuk P. The prevalence and management of hyperglycaemia in patients with rheumatoid arthritis on corticosteroid therapy. Scott Med J 2004; 49:139–141.
- Uzu T, Harada T, Sakaguchi M, et al. Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract 2007; 105:c54–c57.
- Gurwitz JH, Bohn RL, Glynn RJ, Monane M, Mogun H, Avorn J. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med 1994; 154:97–101.
- Bevier WC, Zisser HC, Jovanovic L, et al. Use of continuous glucose monitoring to estimate insulin requirements in patients with type 1 diabetes mellitus during a short course of prednisone. J Diabetes Sci Technol 2008; 2:578–583.
- Cagdas DN, Paç FA, Cakal E. Glucocorticoid-induced diabetic ketoacidosis in acute rheumatic fever. J Cardiovasc Pharmacol Ther 2008; 13:298–300.
- Bedalov A, Balasubramanyam A. Glucocorticoid-induced ketoacidosis in gestational diabetes: sequela of the acute treatment of preterm labor. A case report. Diabetes Care 1997; 20:922–924.
- Yang JY, Cui XL, He XJ. Non-ketotic hyperosmolar coma complicating steroid treatment in childhood nephrosis. Pediatr Nephrol 1995; 9:621–622.
- Nielsen MF, Caumo A, Chandramouli V, et al. Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercortisolemia in healthy subjects. Am J Physiol Endocrinol Metab 2004; 286:E102–E110.
- Matsumoto K, Yamasaki H, Akazawa S, et al. High-dose but not low-dose dexamethasone impairs glucose tolerance by inducing compensatory failure of pancreatic beta-cells in normal men. J Clin Endocrinol Metab 1996; 81:2621–2626.
- Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab 1982; 54:131–138.
- Meyuhas O, Reshef L, Gunn JM, Hanson RW, Ballard FJ. Regulation of phosphoenolpyruvate carboxykinase (GTP) in adipose tissue in vivo by glucocorticoids and insulin. Biochem J 1976; 158:1–7.
- Tappy L, Randin D, Vollenweider P, et al. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J Clin Endocrinol Metab 1994; 79:1063–1069.
- Pagano G, Cavallo-Perin P, Cassader M, et al. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest 1983; 72:1814–1820.
- Magee MH, Blum RA, Lates CD, Jusko WJ. Pharmacokinetic/pharmaco-dynamic model for prednisolone inhibition of whole blood lymphocyte proliferation. Br J Clin Pharmacol 2002; 53:474–484.
- Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab 2011; 96:1789–1796.
- Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth 2006; 97:164–170.
- Pasternak JJ, McGregor DG, Lanier WL. Effect of single-dose dexamethasone on blood glucose concentration in patients undergoing craniotomy. J Neurosurg Anesthesiol 2004; 16:122–125.
- Davidson J, Wilkinson A, Dantal J, et al; International Expert Panel. New-onset diabetes after transplantation: 2003 international consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 2003; 75(suppl 10):SS3–SS24.
- American Diabetes Association. Standards of medical care in diabetes— 2011. Diabetes Care 2011; 34(suppl 1):S11–S61.
- Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009; 15:540–559.
- Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Axelrod L. Corticosteroid therapy. In:Becker KL, editor. Principles and Practice of Endocrinology and Metabolism. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2000:752–763.
- Ferri FF, editor. Practical Guide to the Care of the Medical Patient. 8th ed. Philadelphia, PA: Mosby/Elsevier; 2011.
- Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab 1993; 76:1505–1510.
- Linder BL, Esteban NV, Yergey AL, Winterer JC, Loriaux DL, Cassorla F. Cortisol production rate in childhood and adolescence. J Pediatr 1990; 117:892–896.
- Esteban NV, Loughlin T, Yergey AL, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 1991; 72:39–45.
- Lansang MC, Quinn SL. Adrenal suppression. BMJ BestPractice 2010. http://bestpractice.bmj.com/best-practice/monograph/863/diagnosis/stepby-step.html. Accessed August 19, 2011.
- Zöllner EW. Hypothalamic-pituitary-adrenal axis suppression in asthmatic children on inhaled corticosteroids (part 2)—the risk as determined by gold standard adrenal function tests: a systematic review. Pediatr Allergy Immunol 2007; 18:469–474.
- Schuetz P, Christ-Crain M, Schild U, et al. Effect of a 14-day course of systemic corticosteroids on the hypothalamic-pituitary-adrenal-axis in patients with acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med 2008; 8:1.
- Kay J, Findling JW, Raff H. Epidural triamcinolone suppresses the pituitary-adrenal axis in human subjects. Anesth Analg 1994; 79:501–505.
- Lansang MC, Farmer T, Kennedy L. Diagnosing the unrecognized systemic absorption of intra-articular and epidural steroid injections. Endocr Pract 2009; 15:225–228.
- Duclos M, Guinot M, Colsy M, et al. High risk of adrenal insufficiency after a single articular steroid injection in athletes. Med Sci Sports Exerc 2007; 39:1036–1043.
- Bong JL, Connell JM, Lever R. Intranasal betamethasone induced acne and adrenal suppression. Br J Dermatol 2000; 142:579–580.
- Atabek ME, Pirgon O, Unal E. Pituitary-adrenal axis suppression due to topical steroid administration in an infant. Pediatr Int 2007; 49:242–244.
- Ozerdem U, Levi L, Cheng L, Song MK, Scher C, Freeman WR. Systemic toxicity of topical and periocular corticosteroid therapy in an 11-year-old male with posterior uveitis. Am J Ophthalmol 2000; 130:240–241.
- Chiang MY, Sarkar M, Koppens JM, Milles J, Shah P. Exogenous Cushing’s syndrome and topical ocular steroids. Eye (Lond) 2006; 20:725–727.
- Diprolene prescribing information. Schering Corp 2005. www.theodora.com/drugs/diprolene_gel_005_schering.html. Accessed September 27, 2011.
- Villabona CV, Koh C, Panergo J, Reddy A, Fogelfeld L. Adrenocorticotropic hormone stimulation test during high-dose glucocorticoid therapy. Endocr Pract 2009; 15:122–127.
- Ortega E, Rodriguez C, Strand LJ, Segre E. Effects of cloprednol and other corticosteroids on hypothalamic-pituitary-adrenal axis function. J Int Med Res 1976; 4:326–337.
- Axelrod L. Glucocorticoid therapy. Medicine (Baltimore) 1976; 55:39–65.
- Schürmeyer TH, Tsokos GC, Avgerinos PC, et al. Pituitary-adrenal responsiveness to corticotropin-releasing hormone in patients receiving chronic, alternate day glucocorticoid therapy. J Clin Endocrinol Metab 1985; 61:22–27.
- Stewart PM. The adrenal cortex. In:Kronenberg HM, editor. Williams Textbook of Endocrinology. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2008.
- Hopkins RL, Leinung MC. Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol Metab Clin North Am 2005; 34:371–384.
- Richter B, Neises G, Clar C. Glucocorticoid withdrawal schemes in chronic medical disorders. A systematic review. Endocrinol Metab Clin North Am 2002; 31:751–778.
Glucocorticoids are commonly prescribed by primary care physicians and specialists alike for multiple medical problems, acute as well as chronic.
However, these useful drugs have adverse effects on multiple endocrine systems, effects that include diabetes (or worsening of hyperglycemia in those with known diabetes), Cushing syndrome, adrenal suppression, osteoporosis (reviewed in the Cleveland Clinic Journal of Medicine in August 2010),1 and dyslipidemia. In addition, suppression of gonadotropins, growth hormone, and, acutely, thyrotropin can ensue.
The focus of this review is on the diabetogenic and adrenal suppressive effects of glucocorticoids and their management. We describe the rationale for choosing specific drugs to counter hyperglycemia, tests for determining adrenal suppression and systemic glucocorticoid absorption, and how and why to taper these drugs.
WIDELY USED DRUGS
Although glucocorticoids (often simply called steroids or corticosteroids, although not all steroids are corticosteroids, and not all corticosteroids are glucocorticoids) are the core treatment for adrenal insufficiency, in most cases they are prescribed for their anti-inflammatory effects. They act through multiple pathways at the cellular and molecular levels, suppressing the cascades that would otherwise result in inflammation and promoting pathways that produce anti-inflammatory proteins.2
In addition to formulations that are intended to have systemic effects, other, “local” formulations are made for specific conditions, such as intra-articular injections for arthritis, epidural injections for lumbar disk pain, eye drops for uveitis, nasal sprays for allergic rhinitis, inhalers for asthma, and topical ointments and creams for eczema. However, as we will discuss, even these preparations can have systemic effects.
GLUCOCORTICOID-INDUCED DIABETES IS COMMON
Glucocorticoids are the most common cause of drug-induced diabetes. Though the exact prevalence is not known, a few observations suggest that glucocorticoid-induced diabetes or hyperglycemia is common:
- In patients with rheumatoid arthritis, mean age 62 years, nearly 9% developed diabetes in the 2 years after starting glucocorticoid treatment, which was a higher rate than expected.3
- In nondiabetic patients with primary renal disease treated with prednisolone 0.75 mg/kg/day, 42% were found to have 2-hour post-lunch plasma glucose concentrations higher than 200 mg/dL but normal fasting glucose levels.4
- In a case-control study, the odds ratio of starting an oral hypoglycemic agent or insulin was 1.77 for patients receiving a hydrocortisone-equivalent dose of 1 to 39 mg/day, 3.02 for 40 to 79 mg/day, 5.82 for 80 to 119 mg/day, and 10.34 for 120 mg/day or more.5 (For a full discussion of glucocorticoid equivalents, see the section below on Cushing syndrome and adrenal suppression.)
- In patients with type 1 diabetes, prednisone 60 mg/day raised the blood glucose levels starting 6 hours after the prednisone dose.6
- Diabetic ketoacidosis and hyperosmolar nonketotic syndrome have been reported as a result of glucocorticoid treatment.7–9
GLUCOCORTICOIDS CAUSE DIABETES MAINLY VIA INSULIN RESISTANCE
The mechanism by which glucocorticoids cause diabetes predominantly involves insulin resistance rather than decreased insulin production. In fact, in a study in healthy volunteers, 10 hydrocortisone infusion resulted in higher insulin production than saline infusion did. (In high doses, however, glucocorticoids have been shown to decrease insulin secretion.11)
Normally, in response to insulin, the liver decreases its output of glucose. Glucocorticoids decrease the liver’s sensitivity to insulin, thereby increasing hepatic glucose output.12 They also inhibit glucose uptake in muscle and fat, reducing insulin sensitivity as much as 60% in healthy volunteers. This seems primarily due to a postreceptor effect, ie, inhibition of glucose transport.13–15
THE PEAK EFFECT OCCURS 4 TO 6 HOURS AFTER DOSING
To understand the optimal time for checking plasma glucose and to apply appropriate treatment, we should consider the pharmacokinetic profile of glucocorticoids.
Studied using the whole-blood lymphocyte proliferation technique, prednisone shows a peak effect at about 4 to 6 hours and a duration of action of 13 to 16 hours.16 This closely resembles what we see in terms of glucose excursion with this drug.17 Two studies of intravenous dexamethasone 10 mg showed that glucose levels rose within 4 hours of injection, but did not pursue this beyond that time frame.18,19
PATIENTS WITHOUT A PREVIOUS DIAGNOSIS OF DIABETES
Be alert for new-onset diabetes
For most diseases treated with glucocorticoids, clinicians can estimate in advance how long the patient will need to take the drug. We can arbitrarily classify the projected exposure as either short-term (3 to 4 weeks or less, such as a 6-day course of methylprednisolone for allergic conditions) or long-term (such as in transplant recipients to prevent rejection or to treat graft-vs-host disease). Hyperglycemia is a potential concern with both short-term and long-term treatment. However, guidelines on checking blood sugar levels, as opposed to relying on symptoms alone, are available only for long-term glucocorticoid treatment.
Patients beginning treatment should be warned of typical diabetes symptoms such as thirst and increased urination and, should these occur, to seek medical attention to have their blood glucose level checked. It is also reasonable to have them return in a week for a random postprandial plasma glucose test in the mid-afternoon.
Why this timing? In most once-daily regimens, glucocorticoids are given in the morning to prevent adrenal suppression (discussed below). In our experience, glucose levels start to rise mid-morning and continue to increase until bedtime. Measuring glucose levels 1 to 2 hours after lunch allows for both the glucocorticoid action and the carbohydrate absorption from lunch to reach their peaks. If hyperglycemia is going to happen, it should be detectable by then. A glucose level of 200 mg/dL or higher should prompt the practitioner to pursue this further.
If glucocorticoid treatment is to continue beyond 3 to 4 weeks, the only population for which there are published guidelines on managing glucocorticoid-related diabetes is transplant recipients. International consensus guidelines, published in 2003, suggest checking the fasting plasma glucose level once a week for the first 4 weeks after transplantation, then at 3 months, at 6 months, and then once a year.20
Though practical, this suggestion does not reflect the fact that glucocorticoids often do not affect fasting plasma glucose, especially if given once daily in the morning at doses of 30 mg or less of prednisone or its equivalent. These guidelines thus may not be applicable to other populations with glucocorticoid-induced diabetes.
The transplant guidelines do mention that an oral glucose tolerance test may be more sensitive, but this is often cumbersome to perform. We believe that checking random postprandial plasma glucose levels is helpful in this regard.
If the patient was at risk of developing diabetes even before receiving a glucocorticoid (for example, if he or she is overweight, has a family history of diabetes, or had a previous hemoglobin A1c of 5.7% or higher), then a fasting plasma glucose level of 126 mg/dL or higher or a hemoglobin A1c of 6.5% or higher might suffice to diagnose diabetes. Results should be confirmed on a separate day in the absence of unequivocal hyperglycemia. Fasting hyperglycemia can also be seen in patients receiving higher once-daily glucocorticoid doses—in our experience, an equivalent of prednisone 40 mg once a day in the morning— or twice-daily dosing.
A hemoglobin A1c checked less than 2 to 3 months after starting glucocorticoid treatment will not be sensitive in picking up glucocorticoid-induced diabetes if the patient did not have underlying diabetes.
Diet and exercise may not be practical
Though diet and exercise are important in managing diabetes, the condition for which the patient is receiving a glucocorticoid may prevent him or her from exercising, at least in the acute phase of the illness.
In addition, though the exact mechanism is not known, glucocorticoids increase hunger, and so decreasing food intake is not easy either. Nonetheless, patients should be familiarized with what carbohydrates are and should be advised to reduce their intake of them.
For suspected type 1 diabetes, start insulin
If type 1 diabetes is suspected, for example, in patients who are lean, younger than 30 years, or who had presented with diabetic ketoacidosis, then insulin should be started. In equivocal cases, insulin therapy can commence while testing is done for C-peptide, glutamic acid decarboxylase antibodies, islet cell antibodies, and insulinoma-associated protein antibodies.
Starting oral antidiabetic drugs
Some patients may have contraindications to specific drugs. For example, metformin (Glucophage) is contraindicated if the serum creatinine level is elevated, an abnormality that renal transplant patients may continue to have.
If the patient has no such contraindications, we have found the following medications suitable in view of their efficacy, low risk of hypoglycemia, or lack of distressing side effects. They will often lower glucose levels enough to achieve capillary blood glucose or fingerstick goals (discussed below). None of them has been specifically approved by the US Food and Drug Administration for glucocorticoid-induced diabetes, but they are approved for type 2 diabetes.
Guidelines from the American Association of Clinical Endocrinologists for type 2 diabetes call for starting monotherapy if the hemoglobin A1c is 6.5% to 7.5%, dual therapy if it is 7.6% to 9%, triple therapy if it is higher than 9% and the patient has no symptoms, and insulin if it is higher than 9% and the patient does have symptoms.22
In terms of estimated average glucose levels, these categories correspond to 140 to 169 mg/dL for monotherapy, 171 to 212 mg/dL for dual therapy, and higher than 212 mg/dL for triple therapy or insulin. Since estimated average levels also include fasting glucose levels (which are lower in glucocorticoid-induced diabetes compared with nonfasting levels), and because we use the American Diabetes Association general hemoglobin A1c goal of less than 7%, we believe that our suggestions below are reasonable.
We divide our recommendations according to initial random (ideally, 1- to 2-hour postprandial) plasma glucose levels.
If the random or 1- to 2-hour post-meal plasma glucose is lower than 220 mg/dL
In this situation the choices are:
- Metformin
- Dipeptidyl peptidase-4 (DPP-4) inhibitors (“gliptins”)
- Meglitinides (“glinides”). The guidelines on new-onset diabetes after transplantation point out that meglitinides may be the safest agents apart from insulin in the renal transplant population, but does acknowledge that efficacies of different oral agents have not been compared in this group.20
- Glucagon-like protein-1 (GLP-1) agonists
- Sulfonylureas. However, the longer-acting forms such as glimepiride (Amaryl) are not suitable if the fasting plasma glucose is not affected.
We have not used thiazolidinediones (“glitazones”) routinely because they can cause weight gain and edema—problems that are already seen with the use of steroids—and have a slower onset of action.
If the random or 1- to 2-hour post-meal plasma glucose is 220 to 300 mg/dL
Often, a combination of drugs or insulin (see below) is needed. However, you can start with one agent and add a second agent within 2 or 3 months (as is recommended for type 2 diabetes).22,23 The following combinations of the agents listed above are supported by published guidelines for type 2 diabetes:
- Metformin plus a sulfonylurea22,23
- Metformin plus a glinide22
- Metformin plus a GLP-1 agonist23
- Metformin plus a DPP-4 inhibitor.22
If the random or 1- to 2-hour post-meal plasma glucose is higher than 300 mg/dL
In our experience, if their plasma glucose levels are this high, patients are experiencing frank symptoms of hyperglycemia.
Insulin addresses those symptoms and avoids the prolonged wait that often results from unsuccessfully starting one agent and then adding another. Of all the available drugs, insulin is the only one that can be used despite multiple underlying illnesses; it does not cause a lot of drug interactions, and the dose can be adjusted upward and downward in increments to fit the patient’s needs, especially when a larger glucocorticoid load is given up front and then is tapered either slowly or rapidly. However, it can cause hypoglycemia and weight gain.
The initial total daily dose of insulin can be based on the patient’s weight. A starting total daily dose of 0.15 to 0.3 U/kg is reasonable— on the lower end if only the postprandial glucose levels are elevated, and on the higher end if both fasting and postprandial glucose levels are affected.
If fasting glucose levels are not elevated, then Neutral Protamine Hagedorn insulin (which is intermediate-acting) or a premixed combination of an intermediate-acting plus a fast- or short-acting insulin can be given once a day before breakfast, or even before lunch if the glucose levels start to rise only after lunch.
If both the fasting and the postprandial glucose levels are elevated, regimens similar to those for type 1 or insulin-requiring type 2 diabetes can be used, except that the ratios of the doses are tilted more toward covering postprandial than fasting hyperglycemia:
- Long-acting insulin plus prandial insulin, in a ratio of 30:70 to 50:50. As glucocorticoids are tapered, the long-acting insulin may have to be discontinued while the prandial doses are continued, since the fasting glucose level decreases first.
- Premixed insulins, with one-half to two-thirds of the dose given before breakfast and the rest before the evening meal, with the possibility of a third injection before lunch. As glucocorticoids are tapered, the evening dose is tapered first.
- Intermediate-acting insulin plus short- or fast-acting insulin in the morning (these two will make up one-half to two-thirds of the total daily dose), short- or fast-acting insulin before the evening meal, and intermediate-acting insulin at bedtime. As glucocorticoids are tapered, the bedtime insulin is tapered first.
Capillary blood glucose (fingerstick) checks
The timing and frequency of fingerstick checks depend on the treatment.
Though postprandial testing is ideal, it is often not practical or convenient. Before lunch, before dinner, and at bedtime are good alternatives since they reflect the pattern of glucose rise throughout the day. For patients on diet and exercise with or without agents other than insulin, testing once or twice a day is reasonable, rotating times before meals (including fasting if this time is affected) and at bedtime.
For patients on insulin, checking two to four times a day initially would help match insulin doses with glucose excursions. For continued care, the American Diabetes Association recommends fingerstick checks three times daily in patients on multiple insulin injections, but it has no specific recommendations for those on once-a-day insulin.21 We have been recommending that our patients on once-daily insulin check at least twice a day.
Goal fingerstick glucose levels that we use are in accordance with the American Diabetes Association guidelines for diabetes in general21:
- Before meals 70 to 130 mg/dL or
- 1 to 2 hours after meals < 180 mg/dL.
During steroid taper, if the glucocorticoid dose is in the lower range (eg, a prednisone-equivalent dose of approximately 7.5 mg per day or less), the fingerstick glucose levels are at the lower end of the target range, and the patient is on a single antidiabetic agent that does not often cause hypoglycemia (eg, metformin), then it is reasonable to ask the patient to not take the antidiabetic medication for 3 to 7 days while continuing to check fingersticks to see if it needs to be resumed. Patients on agents that can cause hypoglycemia need to check more often during the 1 to 3 days after the glucocorticoid dose reduction, as it may take this much time for the glycemic effect to diminish and to adjust the diabetes medication to the appropriate dose.
STARTING GLUCOCORTICOIDS IN PATIENTS WITH KNOWN DIABETES
Fingerstick checks more often
Most patients will already have a glucose meter. They should be instructed to check as discussed above if they do not have a previous diagnosis of diabetes, or to continue as they are doing if they are already checking more often. Patients who have been checking only fasting levels should be instructed to check later in the day, either before or 1 to 2 hours after meals, as discussed above. Patients on oral medications may need additional oral agents or insulin.
Adjust medications if glucose is not at goal
Patients with type 2 diabetes treated with diet and exercise alone can be started on the medications discussed above if their fingerstick readings are not at goal.
If they are already on insulin, we advise them to increase the short- or fast-acting insulins and the morning intermediate-acting insulin by at least 10% to 20% as soon as an elevation in glucose is detected. Long-acting insulin or nighttime intermediate-acting insulin should be increased if fasting glucose levels are affected.
Insulin requirements can double depending on the glucocorticoid dose. In patients with type 1 diabetes who were given prednisone 60 mg orally for 3 days, mean blood glucose levels increased from a baseline of 110 mg/dL at baseline to 149 mg/dL on the days on prednisone.6 The average blood glucose level remained elevated at 141 mg/dL on the day after the last dose of prednisone. The insulin dose increased by 31% to 102% (mean 69%).
CUSHING SYNDROME AND ADRENAL SUPPRESSION
Unlike glucocorticoid-induced diabetes, in which the dilemma is often when to initiate antidiabetic treatment, the question for patients in whom Cushing syndrome or adrenal suppression has developed is when to discontinue glucocorticoids.
Adrenal suppression for the most part goes hand in hand with exogenous Cushing syndrome. If cushingoid features develop, we can infer that the dose of exogenous glucocorticoid exceeds the physiologic needs. This supraphysiologic dosing also leads to suppression of endogenous cortisol production. The suppression occurs at the level of the hypothalamus and pituitary gland, with subsequent atrophy of the part of the adrenal cortex that produces endogenous glucocorticoids.
To understand further the concept of supraphysiologic dosing, the following interconversion of systemic glucocorticoid effects is helpful24,25:
However, there is not much information on interconversion for the local preparations (intra-articular, epidural, inhaled, topical).
Moreover, the definition of supraphysiologic dosing seems to be evolving. Though a total hydrocortisone-equivalent dose of 30 mg/day is still often touted as physiologic replacement, many patients require less. Several studies in the early 1990s, mostly in children and adolescents, showed the mean daily cortisol production rate to be 4.8 to 6.8 mg/m2/day, or closer to 10 to 15 mg/day.26–28 For purposes of this discussion, a physiologic dose will be defined as up to 30 mg hydrocortisone per day or its equivalent.
Adrenal suppression vs insufficiency
Adrenal suppression is often confused with adrenal insufficiency.
Adrenal suppression occurs when cortisol production is decreased because of the presence of exogenous glucocorticoids or other drugs, such as megestrol acetate (Megace), that act on the glucocorticoid receptor. Another situation beyond the scope of this review is excess endogenous cortisol production by an adrenal adenoma or adrenal carcinoma that causes suppression of the contralateral adrenal gland.29
In contrast, adrenal insufficiency is caused by failure of the adrenal gland to produce cortisol as a result of an innate disorder of the adrenal gland (eg, Addison disease) or pituitary gland (eg, pituitary surgery).
Hence, endogenous cortisol production in a patient taking supraphysiologic doses of exogenous glucocorticoids may be suppressed. Recovery of endogenous cortisol production is expected after stopping the exogenous glucocorticoid, though the time to recovery can vary and the patient can be adrenally insufficient if the glucocorticoid is stopped abruptly.
In addition, during times of intercurrent illness, a patient with adrenal suppression may be relatively adrenally insufficient and may need larger doses (“stress doses”) of glucocorticoids, since the adrenal glands may be unable to mount a stress response.29
Local steroids can suppress the adrenal glands
Glucocorticoids are the most common cause of Cushing syndrome. Oral formulations such as dexamethasone, prednisone, and hydrocortisone taken in supraphysiologic doses and for prolonged durations are easily recognized as obvious causes of Cushing syndrome. However, intra-articular, epidural, inhaled, nasal, ocular, and topical steroids—so-called local preparations—have also been linked to Cushing syndrome, and physicians are less likely to recognize them as causes.30–38
In a study in 16 pediatric patients with asthma and 48 controls, inhaled beclomethasone dipropionate (Qvar) 300 to 500 μg daily resulted in adrenal suppression in 100% of patients after 6 to 42 months, as determined by an insulin tolerance test.30
The topical steroid betamethasone (Diprosone) carries a warning that systemic absorption of topical steroids can cause adrenal suppression.39 Intra-articular, intranasal, epidural, and ocular routes are also reported to cause adrenal suppression.32–38
When is adrenal suppression more likely?
Adrenal suppression is more likely in the following situations:
- Longer duration of treatment. Studies have shown that exposure to supraphysiologic steroid doses for 2 weeks or less might already suppress the adrenal glands, but the clinical significance of this is unclear since some recovery already occurs a few days after the glucocorticoids are discontinued.31,40
- Supraphysiologic doses, stronger formulations, longer-acting formulations.41
When is adrenal suppression less likely?
Adrenal suppression is less likely in the following situations:
- Regimens that mimic the diurnal rhythm of cortisol (higher dose in the morning, lower dose in the afternoon)42
- Alternate-day dosing of steroids.43
Steroid withdrawal vs adrenal insufficiency
Another phenomenon that can be confused with adrenal insufficiency or glucocorticoid insufficiency is steroid withdrawal, in which patients experience lethargy, muscle aches, nausea, vomiting, and postural hypotension as glucocorticoids are tapered and their effects wane.42 Increasing the glucocorticoid dose for presumed adrenal insufficiency may delay recovery of the adrenal function and would have to be weighed against the patient’s symptoms.
The following may help distinguish the two: if the patient is on supraphysiologic glucocorticoid doses, then he or she is not glucocorticoid-deficient and is likely suffering from steroid withdrawal. At this point, patients may just need reassurance, symptomatic treatment, or if necessary, a brief (1-week) increase of the previous lowest dose, followed by reevaluation.
With local glucocorticoid preparations that may be systemically absorbed, however, there is no good way of estimating dose equivalence. In these situations, the decision to simply reassure the patient or give symptomatic treatment—as opposed to giving low-dose oral glucocorticoids such as hydrocortisone 5 to 10 mg daily for a week followed by reevaluation— depends on the severity of symptoms and whether the patient has quick access to medical attention should he or she develop an intercurrent illness.
Identifying patients at risk of adrenal suppression
Patients presenting with weight gain or symptoms suggesting Cushing syndrome should be asked about steroid intake and should be prompted to recall possible nonoral routes. In addition, patients presenting with muscle aches and fatigue—symptoms of steroid withdrawal— may have received unrecognized local glucocorticoids that were systemically absorbed, now with diminishing effects.
The ACTH stimulation test for adrenal recovery
Testing can be done to see if the adrenal glands have recovered and glucocorticoid therapy can be discontinued (see Tapering from glucocorticoids, below).
The test most often used is the corticotropin (ACTH) stimulation test. Since the suppression is at the level of the hypothalamus and the pituitary gland, the ACTH stimulation test is an indirect method of assessing hypothalamic and pituitary function in the context of glucocorticoid-induced adrenal suppression. It has good correlation with the insulin tolerance test, the gold-standard test for an intact hypothalamic-pituitary-adrenal axis.
The synthetic ACTH cosyntropin (Cortrosyn) 250 μg is injected intravenously or intramuscularly, and a cortisol level is drawn at baseline and 30 and 60 minutes later. Other doses such as 1 μg or 10 μg have been reported but are not yet widely accepted. A cortisol level of greater than 18 to 20 μg/dL at any time point shows that the adrenals have regained function and the steroids may be discontinued.42 If adrenal suppression persists, weaning from steroids should continue.
In reality, it may not be possible or practical to do an ACTH stimulation test, as not all physicians’ offices have a supply of cosyntropin or the manpower to perform the test correctly. In these cases, weaning can progress with monitoring of symptoms.
Testing for synthetic glucocorticoids in the urine and serum can demonstrate systemic absorption and may be helpful in patients who do not recall receiving steroids.33
Tapering from glucocorticoids
Several tapering schedules have been suggested (although not necessarily validated). Whether and how to taper depend on how long the glucocorticoid has been taken.
If taken for less than 1 week, glucocorticoids can be stopped without tapering, regardless of the dose.
If taken for 1 to 3 weeks, the decision to taper depends on the clinician’s assessment of the patient’s general health or constitution and the illness for which the glucocorticoid was prescribed. For example, if the underlying disease is less likely to flare with a gradual dose reduction, then tapering would be suitable.44
If taken for more than 3 weeks, the practice has been a more rapid taper at the beginning until a physiologic dose is reached. How quickly to reduce the dose depends on whether the underlying illness is expected to flare up, or if the patient might experience steroid withdrawal symptoms.
One schedule is to lower the glucocorticoid dose by an amount equivalent to prednisolone 2.5 mg every 3 to 4 days when above the physiologic dose, then to taper more slowly by 1 mg every 2 to 4 weeks.44 Once the physiologic dose is reached, one can switch to the equivalent dose of hydrocortisone and decrease the dose by 2.5 mg a week until a daily dose of 10 mg a day is reached and maintained for 2 to 3 months, and then perform a test of adrenal function (see above).44 Passing the test implies that the adrenal glands have recovered and the glucocorticoid can be stopped.
Another option is to switch to alternate-day therapy once a physiologic dose is reached and to test 8:00 am cortisol levels, continuing the glucocorticoid and retesting in 4 to 6 weeks if the value is less than 3 μg/dL; stopping the glucocorticoid if the value is higher than 20 μg/dL; and performing an ACTH stimulation test for values in between.45
A review of other tapering regimens for chronic diseases, mostly pulmonary, did not find enough evidence to recommend one particular schedule over another.46 The tapering schedule may have to be adjusted to prevent disease flare and symptoms of steroid withdrawal.
Locally administered steroids. Since the equivalence of systemically absorbed local glucocorticoids is not known, these patients are likely to present when they have symptoms of steroid withdrawal. In this situation, testing adrenal function will help.
Glucocorticoids are commonly prescribed by primary care physicians and specialists alike for multiple medical problems, acute as well as chronic.
However, these useful drugs have adverse effects on multiple endocrine systems, effects that include diabetes (or worsening of hyperglycemia in those with known diabetes), Cushing syndrome, adrenal suppression, osteoporosis (reviewed in the Cleveland Clinic Journal of Medicine in August 2010),1 and dyslipidemia. In addition, suppression of gonadotropins, growth hormone, and, acutely, thyrotropin can ensue.
The focus of this review is on the diabetogenic and adrenal suppressive effects of glucocorticoids and their management. We describe the rationale for choosing specific drugs to counter hyperglycemia, tests for determining adrenal suppression and systemic glucocorticoid absorption, and how and why to taper these drugs.
WIDELY USED DRUGS
Although glucocorticoids (often simply called steroids or corticosteroids, although not all steroids are corticosteroids, and not all corticosteroids are glucocorticoids) are the core treatment for adrenal insufficiency, in most cases they are prescribed for their anti-inflammatory effects. They act through multiple pathways at the cellular and molecular levels, suppressing the cascades that would otherwise result in inflammation and promoting pathways that produce anti-inflammatory proteins.2
In addition to formulations that are intended to have systemic effects, other, “local” formulations are made for specific conditions, such as intra-articular injections for arthritis, epidural injections for lumbar disk pain, eye drops for uveitis, nasal sprays for allergic rhinitis, inhalers for asthma, and topical ointments and creams for eczema. However, as we will discuss, even these preparations can have systemic effects.
GLUCOCORTICOID-INDUCED DIABETES IS COMMON
Glucocorticoids are the most common cause of drug-induced diabetes. Though the exact prevalence is not known, a few observations suggest that glucocorticoid-induced diabetes or hyperglycemia is common:
- In patients with rheumatoid arthritis, mean age 62 years, nearly 9% developed diabetes in the 2 years after starting glucocorticoid treatment, which was a higher rate than expected.3
- In nondiabetic patients with primary renal disease treated with prednisolone 0.75 mg/kg/day, 42% were found to have 2-hour post-lunch plasma glucose concentrations higher than 200 mg/dL but normal fasting glucose levels.4
- In a case-control study, the odds ratio of starting an oral hypoglycemic agent or insulin was 1.77 for patients receiving a hydrocortisone-equivalent dose of 1 to 39 mg/day, 3.02 for 40 to 79 mg/day, 5.82 for 80 to 119 mg/day, and 10.34 for 120 mg/day or more.5 (For a full discussion of glucocorticoid equivalents, see the section below on Cushing syndrome and adrenal suppression.)
- In patients with type 1 diabetes, prednisone 60 mg/day raised the blood glucose levels starting 6 hours after the prednisone dose.6
- Diabetic ketoacidosis and hyperosmolar nonketotic syndrome have been reported as a result of glucocorticoid treatment.7–9
GLUCOCORTICOIDS CAUSE DIABETES MAINLY VIA INSULIN RESISTANCE
The mechanism by which glucocorticoids cause diabetes predominantly involves insulin resistance rather than decreased insulin production. In fact, in a study in healthy volunteers, 10 hydrocortisone infusion resulted in higher insulin production than saline infusion did. (In high doses, however, glucocorticoids have been shown to decrease insulin secretion.11)
Normally, in response to insulin, the liver decreases its output of glucose. Glucocorticoids decrease the liver’s sensitivity to insulin, thereby increasing hepatic glucose output.12 They also inhibit glucose uptake in muscle and fat, reducing insulin sensitivity as much as 60% in healthy volunteers. This seems primarily due to a postreceptor effect, ie, inhibition of glucose transport.13–15
THE PEAK EFFECT OCCURS 4 TO 6 HOURS AFTER DOSING
To understand the optimal time for checking plasma glucose and to apply appropriate treatment, we should consider the pharmacokinetic profile of glucocorticoids.
Studied using the whole-blood lymphocyte proliferation technique, prednisone shows a peak effect at about 4 to 6 hours and a duration of action of 13 to 16 hours.16 This closely resembles what we see in terms of glucose excursion with this drug.17 Two studies of intravenous dexamethasone 10 mg showed that glucose levels rose within 4 hours of injection, but did not pursue this beyond that time frame.18,19
PATIENTS WITHOUT A PREVIOUS DIAGNOSIS OF DIABETES
Be alert for new-onset diabetes
For most diseases treated with glucocorticoids, clinicians can estimate in advance how long the patient will need to take the drug. We can arbitrarily classify the projected exposure as either short-term (3 to 4 weeks or less, such as a 6-day course of methylprednisolone for allergic conditions) or long-term (such as in transplant recipients to prevent rejection or to treat graft-vs-host disease). Hyperglycemia is a potential concern with both short-term and long-term treatment. However, guidelines on checking blood sugar levels, as opposed to relying on symptoms alone, are available only for long-term glucocorticoid treatment.
Patients beginning treatment should be warned of typical diabetes symptoms such as thirst and increased urination and, should these occur, to seek medical attention to have their blood glucose level checked. It is also reasonable to have them return in a week for a random postprandial plasma glucose test in the mid-afternoon.
Why this timing? In most once-daily regimens, glucocorticoids are given in the morning to prevent adrenal suppression (discussed below). In our experience, glucose levels start to rise mid-morning and continue to increase until bedtime. Measuring glucose levels 1 to 2 hours after lunch allows for both the glucocorticoid action and the carbohydrate absorption from lunch to reach their peaks. If hyperglycemia is going to happen, it should be detectable by then. A glucose level of 200 mg/dL or higher should prompt the practitioner to pursue this further.
If glucocorticoid treatment is to continue beyond 3 to 4 weeks, the only population for which there are published guidelines on managing glucocorticoid-related diabetes is transplant recipients. International consensus guidelines, published in 2003, suggest checking the fasting plasma glucose level once a week for the first 4 weeks after transplantation, then at 3 months, at 6 months, and then once a year.20
Though practical, this suggestion does not reflect the fact that glucocorticoids often do not affect fasting plasma glucose, especially if given once daily in the morning at doses of 30 mg or less of prednisone or its equivalent. These guidelines thus may not be applicable to other populations with glucocorticoid-induced diabetes.
The transplant guidelines do mention that an oral glucose tolerance test may be more sensitive, but this is often cumbersome to perform. We believe that checking random postprandial plasma glucose levels is helpful in this regard.
If the patient was at risk of developing diabetes even before receiving a glucocorticoid (for example, if he or she is overweight, has a family history of diabetes, or had a previous hemoglobin A1c of 5.7% or higher), then a fasting plasma glucose level of 126 mg/dL or higher or a hemoglobin A1c of 6.5% or higher might suffice to diagnose diabetes. Results should be confirmed on a separate day in the absence of unequivocal hyperglycemia. Fasting hyperglycemia can also be seen in patients receiving higher once-daily glucocorticoid doses—in our experience, an equivalent of prednisone 40 mg once a day in the morning— or twice-daily dosing.
A hemoglobin A1c checked less than 2 to 3 months after starting glucocorticoid treatment will not be sensitive in picking up glucocorticoid-induced diabetes if the patient did not have underlying diabetes.
Diet and exercise may not be practical
Though diet and exercise are important in managing diabetes, the condition for which the patient is receiving a glucocorticoid may prevent him or her from exercising, at least in the acute phase of the illness.
In addition, though the exact mechanism is not known, glucocorticoids increase hunger, and so decreasing food intake is not easy either. Nonetheless, patients should be familiarized with what carbohydrates are and should be advised to reduce their intake of them.
For suspected type 1 diabetes, start insulin
If type 1 diabetes is suspected, for example, in patients who are lean, younger than 30 years, or who had presented with diabetic ketoacidosis, then insulin should be started. In equivocal cases, insulin therapy can commence while testing is done for C-peptide, glutamic acid decarboxylase antibodies, islet cell antibodies, and insulinoma-associated protein antibodies.
Starting oral antidiabetic drugs
Some patients may have contraindications to specific drugs. For example, metformin (Glucophage) is contraindicated if the serum creatinine level is elevated, an abnormality that renal transplant patients may continue to have.
If the patient has no such contraindications, we have found the following medications suitable in view of their efficacy, low risk of hypoglycemia, or lack of distressing side effects. They will often lower glucose levels enough to achieve capillary blood glucose or fingerstick goals (discussed below). None of them has been specifically approved by the US Food and Drug Administration for glucocorticoid-induced diabetes, but they are approved for type 2 diabetes.
Guidelines from the American Association of Clinical Endocrinologists for type 2 diabetes call for starting monotherapy if the hemoglobin A1c is 6.5% to 7.5%, dual therapy if it is 7.6% to 9%, triple therapy if it is higher than 9% and the patient has no symptoms, and insulin if it is higher than 9% and the patient does have symptoms.22
In terms of estimated average glucose levels, these categories correspond to 140 to 169 mg/dL for monotherapy, 171 to 212 mg/dL for dual therapy, and higher than 212 mg/dL for triple therapy or insulin. Since estimated average levels also include fasting glucose levels (which are lower in glucocorticoid-induced diabetes compared with nonfasting levels), and because we use the American Diabetes Association general hemoglobin A1c goal of less than 7%, we believe that our suggestions below are reasonable.
We divide our recommendations according to initial random (ideally, 1- to 2-hour postprandial) plasma glucose levels.
If the random or 1- to 2-hour post-meal plasma glucose is lower than 220 mg/dL
In this situation the choices are:
- Metformin
- Dipeptidyl peptidase-4 (DPP-4) inhibitors (“gliptins”)
- Meglitinides (“glinides”). The guidelines on new-onset diabetes after transplantation point out that meglitinides may be the safest agents apart from insulin in the renal transplant population, but does acknowledge that efficacies of different oral agents have not been compared in this group.20
- Glucagon-like protein-1 (GLP-1) agonists
- Sulfonylureas. However, the longer-acting forms such as glimepiride (Amaryl) are not suitable if the fasting plasma glucose is not affected.
We have not used thiazolidinediones (“glitazones”) routinely because they can cause weight gain and edema—problems that are already seen with the use of steroids—and have a slower onset of action.
If the random or 1- to 2-hour post-meal plasma glucose is 220 to 300 mg/dL
Often, a combination of drugs or insulin (see below) is needed. However, you can start with one agent and add a second agent within 2 or 3 months (as is recommended for type 2 diabetes).22,23 The following combinations of the agents listed above are supported by published guidelines for type 2 diabetes:
- Metformin plus a sulfonylurea22,23
- Metformin plus a glinide22
- Metformin plus a GLP-1 agonist23
- Metformin plus a DPP-4 inhibitor.22
If the random or 1- to 2-hour post-meal plasma glucose is higher than 300 mg/dL
In our experience, if their plasma glucose levels are this high, patients are experiencing frank symptoms of hyperglycemia.
Insulin addresses those symptoms and avoids the prolonged wait that often results from unsuccessfully starting one agent and then adding another. Of all the available drugs, insulin is the only one that can be used despite multiple underlying illnesses; it does not cause a lot of drug interactions, and the dose can be adjusted upward and downward in increments to fit the patient’s needs, especially when a larger glucocorticoid load is given up front and then is tapered either slowly or rapidly. However, it can cause hypoglycemia and weight gain.
The initial total daily dose of insulin can be based on the patient’s weight. A starting total daily dose of 0.15 to 0.3 U/kg is reasonable— on the lower end if only the postprandial glucose levels are elevated, and on the higher end if both fasting and postprandial glucose levels are affected.
If fasting glucose levels are not elevated, then Neutral Protamine Hagedorn insulin (which is intermediate-acting) or a premixed combination of an intermediate-acting plus a fast- or short-acting insulin can be given once a day before breakfast, or even before lunch if the glucose levels start to rise only after lunch.
If both the fasting and the postprandial glucose levels are elevated, regimens similar to those for type 1 or insulin-requiring type 2 diabetes can be used, except that the ratios of the doses are tilted more toward covering postprandial than fasting hyperglycemia:
- Long-acting insulin plus prandial insulin, in a ratio of 30:70 to 50:50. As glucocorticoids are tapered, the long-acting insulin may have to be discontinued while the prandial doses are continued, since the fasting glucose level decreases first.
- Premixed insulins, with one-half to two-thirds of the dose given before breakfast and the rest before the evening meal, with the possibility of a third injection before lunch. As glucocorticoids are tapered, the evening dose is tapered first.
- Intermediate-acting insulin plus short- or fast-acting insulin in the morning (these two will make up one-half to two-thirds of the total daily dose), short- or fast-acting insulin before the evening meal, and intermediate-acting insulin at bedtime. As glucocorticoids are tapered, the bedtime insulin is tapered first.
Capillary blood glucose (fingerstick) checks
The timing and frequency of fingerstick checks depend on the treatment.
Though postprandial testing is ideal, it is often not practical or convenient. Before lunch, before dinner, and at bedtime are good alternatives since they reflect the pattern of glucose rise throughout the day. For patients on diet and exercise with or without agents other than insulin, testing once or twice a day is reasonable, rotating times before meals (including fasting if this time is affected) and at bedtime.
For patients on insulin, checking two to four times a day initially would help match insulin doses with glucose excursions. For continued care, the American Diabetes Association recommends fingerstick checks three times daily in patients on multiple insulin injections, but it has no specific recommendations for those on once-a-day insulin.21 We have been recommending that our patients on once-daily insulin check at least twice a day.
Goal fingerstick glucose levels that we use are in accordance with the American Diabetes Association guidelines for diabetes in general21:
- Before meals 70 to 130 mg/dL or
- 1 to 2 hours after meals < 180 mg/dL.
During steroid taper, if the glucocorticoid dose is in the lower range (eg, a prednisone-equivalent dose of approximately 7.5 mg per day or less), the fingerstick glucose levels are at the lower end of the target range, and the patient is on a single antidiabetic agent that does not often cause hypoglycemia (eg, metformin), then it is reasonable to ask the patient to not take the antidiabetic medication for 3 to 7 days while continuing to check fingersticks to see if it needs to be resumed. Patients on agents that can cause hypoglycemia need to check more often during the 1 to 3 days after the glucocorticoid dose reduction, as it may take this much time for the glycemic effect to diminish and to adjust the diabetes medication to the appropriate dose.
STARTING GLUCOCORTICOIDS IN PATIENTS WITH KNOWN DIABETES
Fingerstick checks more often
Most patients will already have a glucose meter. They should be instructed to check as discussed above if they do not have a previous diagnosis of diabetes, or to continue as they are doing if they are already checking more often. Patients who have been checking only fasting levels should be instructed to check later in the day, either before or 1 to 2 hours after meals, as discussed above. Patients on oral medications may need additional oral agents or insulin.
Adjust medications if glucose is not at goal
Patients with type 2 diabetes treated with diet and exercise alone can be started on the medications discussed above if their fingerstick readings are not at goal.
If they are already on insulin, we advise them to increase the short- or fast-acting insulins and the morning intermediate-acting insulin by at least 10% to 20% as soon as an elevation in glucose is detected. Long-acting insulin or nighttime intermediate-acting insulin should be increased if fasting glucose levels are affected.
Insulin requirements can double depending on the glucocorticoid dose. In patients with type 1 diabetes who were given prednisone 60 mg orally for 3 days, mean blood glucose levels increased from a baseline of 110 mg/dL at baseline to 149 mg/dL on the days on prednisone.6 The average blood glucose level remained elevated at 141 mg/dL on the day after the last dose of prednisone. The insulin dose increased by 31% to 102% (mean 69%).
CUSHING SYNDROME AND ADRENAL SUPPRESSION
Unlike glucocorticoid-induced diabetes, in which the dilemma is often when to initiate antidiabetic treatment, the question for patients in whom Cushing syndrome or adrenal suppression has developed is when to discontinue glucocorticoids.
Adrenal suppression for the most part goes hand in hand with exogenous Cushing syndrome. If cushingoid features develop, we can infer that the dose of exogenous glucocorticoid exceeds the physiologic needs. This supraphysiologic dosing also leads to suppression of endogenous cortisol production. The suppression occurs at the level of the hypothalamus and pituitary gland, with subsequent atrophy of the part of the adrenal cortex that produces endogenous glucocorticoids.
To understand further the concept of supraphysiologic dosing, the following interconversion of systemic glucocorticoid effects is helpful24,25:
However, there is not much information on interconversion for the local preparations (intra-articular, epidural, inhaled, topical).
Moreover, the definition of supraphysiologic dosing seems to be evolving. Though a total hydrocortisone-equivalent dose of 30 mg/day is still often touted as physiologic replacement, many patients require less. Several studies in the early 1990s, mostly in children and adolescents, showed the mean daily cortisol production rate to be 4.8 to 6.8 mg/m2/day, or closer to 10 to 15 mg/day.26–28 For purposes of this discussion, a physiologic dose will be defined as up to 30 mg hydrocortisone per day or its equivalent.
Adrenal suppression vs insufficiency
Adrenal suppression is often confused with adrenal insufficiency.
Adrenal suppression occurs when cortisol production is decreased because of the presence of exogenous glucocorticoids or other drugs, such as megestrol acetate (Megace), that act on the glucocorticoid receptor. Another situation beyond the scope of this review is excess endogenous cortisol production by an adrenal adenoma or adrenal carcinoma that causes suppression of the contralateral adrenal gland.29
In contrast, adrenal insufficiency is caused by failure of the adrenal gland to produce cortisol as a result of an innate disorder of the adrenal gland (eg, Addison disease) or pituitary gland (eg, pituitary surgery).
Hence, endogenous cortisol production in a patient taking supraphysiologic doses of exogenous glucocorticoids may be suppressed. Recovery of endogenous cortisol production is expected after stopping the exogenous glucocorticoid, though the time to recovery can vary and the patient can be adrenally insufficient if the glucocorticoid is stopped abruptly.
In addition, during times of intercurrent illness, a patient with adrenal suppression may be relatively adrenally insufficient and may need larger doses (“stress doses”) of glucocorticoids, since the adrenal glands may be unable to mount a stress response.29
Local steroids can suppress the adrenal glands
Glucocorticoids are the most common cause of Cushing syndrome. Oral formulations such as dexamethasone, prednisone, and hydrocortisone taken in supraphysiologic doses and for prolonged durations are easily recognized as obvious causes of Cushing syndrome. However, intra-articular, epidural, inhaled, nasal, ocular, and topical steroids—so-called local preparations—have also been linked to Cushing syndrome, and physicians are less likely to recognize them as causes.30–38
In a study in 16 pediatric patients with asthma and 48 controls, inhaled beclomethasone dipropionate (Qvar) 300 to 500 μg daily resulted in adrenal suppression in 100% of patients after 6 to 42 months, as determined by an insulin tolerance test.30
The topical steroid betamethasone (Diprosone) carries a warning that systemic absorption of topical steroids can cause adrenal suppression.39 Intra-articular, intranasal, epidural, and ocular routes are also reported to cause adrenal suppression.32–38
When is adrenal suppression more likely?
Adrenal suppression is more likely in the following situations:
- Longer duration of treatment. Studies have shown that exposure to supraphysiologic steroid doses for 2 weeks or less might already suppress the adrenal glands, but the clinical significance of this is unclear since some recovery already occurs a few days after the glucocorticoids are discontinued.31,40
- Supraphysiologic doses, stronger formulations, longer-acting formulations.41
When is adrenal suppression less likely?
Adrenal suppression is less likely in the following situations:
- Regimens that mimic the diurnal rhythm of cortisol (higher dose in the morning, lower dose in the afternoon)42
- Alternate-day dosing of steroids.43
Steroid withdrawal vs adrenal insufficiency
Another phenomenon that can be confused with adrenal insufficiency or glucocorticoid insufficiency is steroid withdrawal, in which patients experience lethargy, muscle aches, nausea, vomiting, and postural hypotension as glucocorticoids are tapered and their effects wane.42 Increasing the glucocorticoid dose for presumed adrenal insufficiency may delay recovery of the adrenal function and would have to be weighed against the patient’s symptoms.
The following may help distinguish the two: if the patient is on supraphysiologic glucocorticoid doses, then he or she is not glucocorticoid-deficient and is likely suffering from steroid withdrawal. At this point, patients may just need reassurance, symptomatic treatment, or if necessary, a brief (1-week) increase of the previous lowest dose, followed by reevaluation.
With local glucocorticoid preparations that may be systemically absorbed, however, there is no good way of estimating dose equivalence. In these situations, the decision to simply reassure the patient or give symptomatic treatment—as opposed to giving low-dose oral glucocorticoids such as hydrocortisone 5 to 10 mg daily for a week followed by reevaluation— depends on the severity of symptoms and whether the patient has quick access to medical attention should he or she develop an intercurrent illness.
Identifying patients at risk of adrenal suppression
Patients presenting with weight gain or symptoms suggesting Cushing syndrome should be asked about steroid intake and should be prompted to recall possible nonoral routes. In addition, patients presenting with muscle aches and fatigue—symptoms of steroid withdrawal— may have received unrecognized local glucocorticoids that were systemically absorbed, now with diminishing effects.
The ACTH stimulation test for adrenal recovery
Testing can be done to see if the adrenal glands have recovered and glucocorticoid therapy can be discontinued (see Tapering from glucocorticoids, below).
The test most often used is the corticotropin (ACTH) stimulation test. Since the suppression is at the level of the hypothalamus and the pituitary gland, the ACTH stimulation test is an indirect method of assessing hypothalamic and pituitary function in the context of glucocorticoid-induced adrenal suppression. It has good correlation with the insulin tolerance test, the gold-standard test for an intact hypothalamic-pituitary-adrenal axis.
The synthetic ACTH cosyntropin (Cortrosyn) 250 μg is injected intravenously or intramuscularly, and a cortisol level is drawn at baseline and 30 and 60 minutes later. Other doses such as 1 μg or 10 μg have been reported but are not yet widely accepted. A cortisol level of greater than 18 to 20 μg/dL at any time point shows that the adrenals have regained function and the steroids may be discontinued.42 If adrenal suppression persists, weaning from steroids should continue.
In reality, it may not be possible or practical to do an ACTH stimulation test, as not all physicians’ offices have a supply of cosyntropin or the manpower to perform the test correctly. In these cases, weaning can progress with monitoring of symptoms.
Testing for synthetic glucocorticoids in the urine and serum can demonstrate systemic absorption and may be helpful in patients who do not recall receiving steroids.33
Tapering from glucocorticoids
Several tapering schedules have been suggested (although not necessarily validated). Whether and how to taper depend on how long the glucocorticoid has been taken.
If taken for less than 1 week, glucocorticoids can be stopped without tapering, regardless of the dose.
If taken for 1 to 3 weeks, the decision to taper depends on the clinician’s assessment of the patient’s general health or constitution and the illness for which the glucocorticoid was prescribed. For example, if the underlying disease is less likely to flare with a gradual dose reduction, then tapering would be suitable.44
If taken for more than 3 weeks, the practice has been a more rapid taper at the beginning until a physiologic dose is reached. How quickly to reduce the dose depends on whether the underlying illness is expected to flare up, or if the patient might experience steroid withdrawal symptoms.
One schedule is to lower the glucocorticoid dose by an amount equivalent to prednisolone 2.5 mg every 3 to 4 days when above the physiologic dose, then to taper more slowly by 1 mg every 2 to 4 weeks.44 Once the physiologic dose is reached, one can switch to the equivalent dose of hydrocortisone and decrease the dose by 2.5 mg a week until a daily dose of 10 mg a day is reached and maintained for 2 to 3 months, and then perform a test of adrenal function (see above).44 Passing the test implies that the adrenal glands have recovered and the glucocorticoid can be stopped.
Another option is to switch to alternate-day therapy once a physiologic dose is reached and to test 8:00 am cortisol levels, continuing the glucocorticoid and retesting in 4 to 6 weeks if the value is less than 3 μg/dL; stopping the glucocorticoid if the value is higher than 20 μg/dL; and performing an ACTH stimulation test for values in between.45
A review of other tapering regimens for chronic diseases, mostly pulmonary, did not find enough evidence to recommend one particular schedule over another.46 The tapering schedule may have to be adjusted to prevent disease flare and symptoms of steroid withdrawal.
Locally administered steroids. Since the equivalence of systemically absorbed local glucocorticoids is not known, these patients are likely to present when they have symptoms of steroid withdrawal. In this situation, testing adrenal function will help.
- Dore RK. How to prevent glucocorticoid-induced osteoporosis. Cleve Clin J Med 2010; 77:529–536.
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 2005; 353:1711–1723.
- Panthakalam S, Bhatnagar D, Klimiuk P. The prevalence and management of hyperglycaemia in patients with rheumatoid arthritis on corticosteroid therapy. Scott Med J 2004; 49:139–141.
- Uzu T, Harada T, Sakaguchi M, et al. Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract 2007; 105:c54–c57.
- Gurwitz JH, Bohn RL, Glynn RJ, Monane M, Mogun H, Avorn J. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med 1994; 154:97–101.
- Bevier WC, Zisser HC, Jovanovic L, et al. Use of continuous glucose monitoring to estimate insulin requirements in patients with type 1 diabetes mellitus during a short course of prednisone. J Diabetes Sci Technol 2008; 2:578–583.
- Cagdas DN, Paç FA, Cakal E. Glucocorticoid-induced diabetic ketoacidosis in acute rheumatic fever. J Cardiovasc Pharmacol Ther 2008; 13:298–300.
- Bedalov A, Balasubramanyam A. Glucocorticoid-induced ketoacidosis in gestational diabetes: sequela of the acute treatment of preterm labor. A case report. Diabetes Care 1997; 20:922–924.
- Yang JY, Cui XL, He XJ. Non-ketotic hyperosmolar coma complicating steroid treatment in childhood nephrosis. Pediatr Nephrol 1995; 9:621–622.
- Nielsen MF, Caumo A, Chandramouli V, et al. Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercortisolemia in healthy subjects. Am J Physiol Endocrinol Metab 2004; 286:E102–E110.
- Matsumoto K, Yamasaki H, Akazawa S, et al. High-dose but not low-dose dexamethasone impairs glucose tolerance by inducing compensatory failure of pancreatic beta-cells in normal men. J Clin Endocrinol Metab 1996; 81:2621–2626.
- Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab 1982; 54:131–138.
- Meyuhas O, Reshef L, Gunn JM, Hanson RW, Ballard FJ. Regulation of phosphoenolpyruvate carboxykinase (GTP) in adipose tissue in vivo by glucocorticoids and insulin. Biochem J 1976; 158:1–7.
- Tappy L, Randin D, Vollenweider P, et al. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J Clin Endocrinol Metab 1994; 79:1063–1069.
- Pagano G, Cavallo-Perin P, Cassader M, et al. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest 1983; 72:1814–1820.
- Magee MH, Blum RA, Lates CD, Jusko WJ. Pharmacokinetic/pharmaco-dynamic model for prednisolone inhibition of whole blood lymphocyte proliferation. Br J Clin Pharmacol 2002; 53:474–484.
- Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab 2011; 96:1789–1796.
- Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth 2006; 97:164–170.
- Pasternak JJ, McGregor DG, Lanier WL. Effect of single-dose dexamethasone on blood glucose concentration in patients undergoing craniotomy. J Neurosurg Anesthesiol 2004; 16:122–125.
- Davidson J, Wilkinson A, Dantal J, et al; International Expert Panel. New-onset diabetes after transplantation: 2003 international consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 2003; 75(suppl 10):SS3–SS24.
- American Diabetes Association. Standards of medical care in diabetes— 2011. Diabetes Care 2011; 34(suppl 1):S11–S61.
- Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009; 15:540–559.
- Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Axelrod L. Corticosteroid therapy. In:Becker KL, editor. Principles and Practice of Endocrinology and Metabolism. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2000:752–763.
- Ferri FF, editor. Practical Guide to the Care of the Medical Patient. 8th ed. Philadelphia, PA: Mosby/Elsevier; 2011.
- Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab 1993; 76:1505–1510.
- Linder BL, Esteban NV, Yergey AL, Winterer JC, Loriaux DL, Cassorla F. Cortisol production rate in childhood and adolescence. J Pediatr 1990; 117:892–896.
- Esteban NV, Loughlin T, Yergey AL, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 1991; 72:39–45.
- Lansang MC, Quinn SL. Adrenal suppression. BMJ BestPractice 2010. http://bestpractice.bmj.com/best-practice/monograph/863/diagnosis/stepby-step.html. Accessed August 19, 2011.
- Zöllner EW. Hypothalamic-pituitary-adrenal axis suppression in asthmatic children on inhaled corticosteroids (part 2)—the risk as determined by gold standard adrenal function tests: a systematic review. Pediatr Allergy Immunol 2007; 18:469–474.
- Schuetz P, Christ-Crain M, Schild U, et al. Effect of a 14-day course of systemic corticosteroids on the hypothalamic-pituitary-adrenal-axis in patients with acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med 2008; 8:1.
- Kay J, Findling JW, Raff H. Epidural triamcinolone suppresses the pituitary-adrenal axis in human subjects. Anesth Analg 1994; 79:501–505.
- Lansang MC, Farmer T, Kennedy L. Diagnosing the unrecognized systemic absorption of intra-articular and epidural steroid injections. Endocr Pract 2009; 15:225–228.
- Duclos M, Guinot M, Colsy M, et al. High risk of adrenal insufficiency after a single articular steroid injection in athletes. Med Sci Sports Exerc 2007; 39:1036–1043.
- Bong JL, Connell JM, Lever R. Intranasal betamethasone induced acne and adrenal suppression. Br J Dermatol 2000; 142:579–580.
- Atabek ME, Pirgon O, Unal E. Pituitary-adrenal axis suppression due to topical steroid administration in an infant. Pediatr Int 2007; 49:242–244.
- Ozerdem U, Levi L, Cheng L, Song MK, Scher C, Freeman WR. Systemic toxicity of topical and periocular corticosteroid therapy in an 11-year-old male with posterior uveitis. Am J Ophthalmol 2000; 130:240–241.
- Chiang MY, Sarkar M, Koppens JM, Milles J, Shah P. Exogenous Cushing’s syndrome and topical ocular steroids. Eye (Lond) 2006; 20:725–727.
- Diprolene prescribing information. Schering Corp 2005. www.theodora.com/drugs/diprolene_gel_005_schering.html. Accessed September 27, 2011.
- Villabona CV, Koh C, Panergo J, Reddy A, Fogelfeld L. Adrenocorticotropic hormone stimulation test during high-dose glucocorticoid therapy. Endocr Pract 2009; 15:122–127.
- Ortega E, Rodriguez C, Strand LJ, Segre E. Effects of cloprednol and other corticosteroids on hypothalamic-pituitary-adrenal axis function. J Int Med Res 1976; 4:326–337.
- Axelrod L. Glucocorticoid therapy. Medicine (Baltimore) 1976; 55:39–65.
- Schürmeyer TH, Tsokos GC, Avgerinos PC, et al. Pituitary-adrenal responsiveness to corticotropin-releasing hormone in patients receiving chronic, alternate day glucocorticoid therapy. J Clin Endocrinol Metab 1985; 61:22–27.
- Stewart PM. The adrenal cortex. In:Kronenberg HM, editor. Williams Textbook of Endocrinology. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2008.
- Hopkins RL, Leinung MC. Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol Metab Clin North Am 2005; 34:371–384.
- Richter B, Neises G, Clar C. Glucocorticoid withdrawal schemes in chronic medical disorders. A systematic review. Endocrinol Metab Clin North Am 2002; 31:751–778.
- Dore RK. How to prevent glucocorticoid-induced osteoporosis. Cleve Clin J Med 2010; 77:529–536.
- Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 2005; 353:1711–1723.
- Panthakalam S, Bhatnagar D, Klimiuk P. The prevalence and management of hyperglycaemia in patients with rheumatoid arthritis on corticosteroid therapy. Scott Med J 2004; 49:139–141.
- Uzu T, Harada T, Sakaguchi M, et al. Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract 2007; 105:c54–c57.
- Gurwitz JH, Bohn RL, Glynn RJ, Monane M, Mogun H, Avorn J. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med 1994; 154:97–101.
- Bevier WC, Zisser HC, Jovanovic L, et al. Use of continuous glucose monitoring to estimate insulin requirements in patients with type 1 diabetes mellitus during a short course of prednisone. J Diabetes Sci Technol 2008; 2:578–583.
- Cagdas DN, Paç FA, Cakal E. Glucocorticoid-induced diabetic ketoacidosis in acute rheumatic fever. J Cardiovasc Pharmacol Ther 2008; 13:298–300.
- Bedalov A, Balasubramanyam A. Glucocorticoid-induced ketoacidosis in gestational diabetes: sequela of the acute treatment of preterm labor. A case report. Diabetes Care 1997; 20:922–924.
- Yang JY, Cui XL, He XJ. Non-ketotic hyperosmolar coma complicating steroid treatment in childhood nephrosis. Pediatr Nephrol 1995; 9:621–622.
- Nielsen MF, Caumo A, Chandramouli V, et al. Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercortisolemia in healthy subjects. Am J Physiol Endocrinol Metab 2004; 286:E102–E110.
- Matsumoto K, Yamasaki H, Akazawa S, et al. High-dose but not low-dose dexamethasone impairs glucose tolerance by inducing compensatory failure of pancreatic beta-cells in normal men. J Clin Endocrinol Metab 1996; 81:2621–2626.
- Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab 1982; 54:131–138.
- Meyuhas O, Reshef L, Gunn JM, Hanson RW, Ballard FJ. Regulation of phosphoenolpyruvate carboxykinase (GTP) in adipose tissue in vivo by glucocorticoids and insulin. Biochem J 1976; 158:1–7.
- Tappy L, Randin D, Vollenweider P, et al. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J Clin Endocrinol Metab 1994; 79:1063–1069.
- Pagano G, Cavallo-Perin P, Cassader M, et al. An in vivo and in vitro study of the mechanism of prednisone-induced insulin resistance in healthy subjects. J Clin Invest 1983; 72:1814–1820.
- Magee MH, Blum RA, Lates CD, Jusko WJ. Pharmacokinetic/pharmaco-dynamic model for prednisolone inhibition of whole blood lymphocyte proliferation. Br J Clin Pharmacol 2002; 53:474–484.
- Burt MG, Roberts GW, Aguilar-Loza NR, Frith P, Stranks SN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab 2011; 96:1789–1796.
- Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth 2006; 97:164–170.
- Pasternak JJ, McGregor DG, Lanier WL. Effect of single-dose dexamethasone on blood glucose concentration in patients undergoing craniotomy. J Neurosurg Anesthesiol 2004; 16:122–125.
- Davidson J, Wilkinson A, Dantal J, et al; International Expert Panel. New-onset diabetes after transplantation: 2003 international consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 2003; 75(suppl 10):SS3–SS24.
- American Diabetes Association. Standards of medical care in diabetes— 2011. Diabetes Care 2011; 34(suppl 1):S11–S61.
- Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009; 15:540–559.
- Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Axelrod L. Corticosteroid therapy. In:Becker KL, editor. Principles and Practice of Endocrinology and Metabolism. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2000:752–763.
- Ferri FF, editor. Practical Guide to the Care of the Medical Patient. 8th ed. Philadelphia, PA: Mosby/Elsevier; 2011.
- Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab 1993; 76:1505–1510.
- Linder BL, Esteban NV, Yergey AL, Winterer JC, Loriaux DL, Cassorla F. Cortisol production rate in childhood and adolescence. J Pediatr 1990; 117:892–896.
- Esteban NV, Loughlin T, Yergey AL, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 1991; 72:39–45.
- Lansang MC, Quinn SL. Adrenal suppression. BMJ BestPractice 2010. http://bestpractice.bmj.com/best-practice/monograph/863/diagnosis/stepby-step.html. Accessed August 19, 2011.
- Zöllner EW. Hypothalamic-pituitary-adrenal axis suppression in asthmatic children on inhaled corticosteroids (part 2)—the risk as determined by gold standard adrenal function tests: a systematic review. Pediatr Allergy Immunol 2007; 18:469–474.
- Schuetz P, Christ-Crain M, Schild U, et al. Effect of a 14-day course of systemic corticosteroids on the hypothalamic-pituitary-adrenal-axis in patients with acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med 2008; 8:1.
- Kay J, Findling JW, Raff H. Epidural triamcinolone suppresses the pituitary-adrenal axis in human subjects. Anesth Analg 1994; 79:501–505.
- Lansang MC, Farmer T, Kennedy L. Diagnosing the unrecognized systemic absorption of intra-articular and epidural steroid injections. Endocr Pract 2009; 15:225–228.
- Duclos M, Guinot M, Colsy M, et al. High risk of adrenal insufficiency after a single articular steroid injection in athletes. Med Sci Sports Exerc 2007; 39:1036–1043.
- Bong JL, Connell JM, Lever R. Intranasal betamethasone induced acne and adrenal suppression. Br J Dermatol 2000; 142:579–580.
- Atabek ME, Pirgon O, Unal E. Pituitary-adrenal axis suppression due to topical steroid administration in an infant. Pediatr Int 2007; 49:242–244.
- Ozerdem U, Levi L, Cheng L, Song MK, Scher C, Freeman WR. Systemic toxicity of topical and periocular corticosteroid therapy in an 11-year-old male with posterior uveitis. Am J Ophthalmol 2000; 130:240–241.
- Chiang MY, Sarkar M, Koppens JM, Milles J, Shah P. Exogenous Cushing’s syndrome and topical ocular steroids. Eye (Lond) 2006; 20:725–727.
- Diprolene prescribing information. Schering Corp 2005. www.theodora.com/drugs/diprolene_gel_005_schering.html. Accessed September 27, 2011.
- Villabona CV, Koh C, Panergo J, Reddy A, Fogelfeld L. Adrenocorticotropic hormone stimulation test during high-dose glucocorticoid therapy. Endocr Pract 2009; 15:122–127.
- Ortega E, Rodriguez C, Strand LJ, Segre E. Effects of cloprednol and other corticosteroids on hypothalamic-pituitary-adrenal axis function. J Int Med Res 1976; 4:326–337.
- Axelrod L. Glucocorticoid therapy. Medicine (Baltimore) 1976; 55:39–65.
- Schürmeyer TH, Tsokos GC, Avgerinos PC, et al. Pituitary-adrenal responsiveness to corticotropin-releasing hormone in patients receiving chronic, alternate day glucocorticoid therapy. J Clin Endocrinol Metab 1985; 61:22–27.
- Stewart PM. The adrenal cortex. In:Kronenberg HM, editor. Williams Textbook of Endocrinology. 11th ed. Philadelphia, PA: Saunders/Elsevier; 2008.
- Hopkins RL, Leinung MC. Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol Metab Clin North Am 2005; 34:371–384.
- Richter B, Neises G, Clar C. Glucocorticoid withdrawal schemes in chronic medical disorders. A systematic review. Endocrinol Metab Clin North Am 2002; 31:751–778.
KEY POINTS
- Nonfasting plasma glucose levels are more sensitive than fasting levels for detecting glucocorticoid-induced diabetes, and antidiabetic agents that have greater effects on random postprandial plasma glucose levels are more suitable than those that mostly affect fasting levels.
- Even those glucocorticoid formulations that are not intended to have systemic effects (eg, eye drops, inhaled corticosteroids, creams, intra-articular injections) can cause adrenal suppression and, therefore, if they are discontinued, steroid withdrawal and adrenal insufficiency.
- Needed are studies comparing antidiabetic regimens for glucocorticoid-induced hyperglycemia and studies comparing glucocorticoid tapering schedules for adrenal suppression to determine the best way to manage these adverse effects.
Dabigatran: Will it change clinical practice?
Dabigatran etexilate (Pradaxa) is a new oral anticoagulant that has distinct advantages over warfarin (Coumadin) in terms of its ease of administration, efficacy, and safety.
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY trial),1 in patients with nonvalvular atrial fibrillation, dabigatran 110 mg twice a day was found to be as good as warfarin in preventing systemic embolization and stroke (the primary outcome of the study), and at 150 mg twice a day it was superior.1 It has also shown efficacy in treating acute deep vein thrombosis and pulmonary embolism and in preventing these complications in orthopedic surgical patients.2–4
Dabigatran has been approved in 75 countries. It carries the trade name Pradaxa in Europe and the United States and Pradax in Canada. In October 2010, the US Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee endorsed two twice-daily doses (75 mg and 150 mg) of dabigatran for the prevention of systemic embolization and stroke in patients with nonvalvular atrial fibrillation.
However, dabigatran is relatively expensive, and its current high cost might be a barrier to its wider use.
MANY PATIENTS NEED ANTICOAGULATION
Anticoagulation plays a vital role in the primary and secondary prevention of stroke in patients with atrial fibrillation and of pulmonary embolism in patients with venous thromboembolism. It is also used during cardiothoracic and vascular surgery, endovascular procedures, and dialysis and in patients with mechanical heart valves and hypercoagulable conditions.
Atrial fibrillation affects 3.03 million people in the United States (2005 figures), and this number is predicted to be as high as 7.56 million by 2050.5 More than 10% of people over the age of 80 years have it, and the lifetime risk of developing it is approximately 25%.6,7 Its most serious complication is ischemic stroke (the risk of which increases with age) and systemic embolization.5,8
Until the recent introduction of dabigatran, the only oral anticoagulant available in the United States for treating patients with atrial fibrillation was warfarin. Although warfarin has a number of disadvantages (see below), it is actually very effective for preventing ischemic stroke, reducing the incidence by as much as 65%.9,10
Venous thromboembolism is the third most common cardiovascular disorder after myocardial infarction and stroke.11 Although its exact incidence is unknown, nearly 1 million cases of it (incident or recurrent, fatal and nonfatal events) occur in the United States each year.12 Many patients with venous thromboembolism need oral anticoagulation long-term, and currently warfarin remains the only option for them as well.
NEEDED: A BETTER ANTICOAGULANT
Warfarin has been the most commonly prescribed oral anticoagulant in the United States for more than 60 years. As of 2004, more than 30 million outpatient prescriptions for it were filled annually in this country alone.13 However, warfarin has several important limitations.
Warfarin has a narrow therapeutic index. Patients taking it require monitoring of their international normalized ratio (INR) and frequent dose adjustments, and this is time-consuming and inconvenient. The target INR for patients with venous thromboembolism and atrial fibrillation is 2.0 to 3.0, whereas patients with a mechanical heart valve need a higher INR (2.5 to 3.5). If the INR is below these ranges, warfarin is less effective, with a risk of new thrombosis. On the other hand, if the INR is too high, there is a risk of bleeding.14 In fact, the most important side effect of warfarin is the risk of major and minor bleeding.13 However, even in well-designed clinical trials in which patients are closely managed, only 55% to 60% of patients regularly achieve their therapeutic target INR.1,2,14,15
Warfarin also interacts with many drugs and with some foods. Compliance is difficult. It has a slow onset of action. Genetic variations require dose adjustments. When switching from a parenteral anticoagulant, overlapping is required. Skin necrosis is a possible side effect. And warfarin is teratogenic.
Despite these limitations, the American College of Chest Physicians endorses warfarin to prevent or treat venous thromboembolism, and to prevent stroke in patients with atrial fibrillation.16
DABIGATRAN, A THROMBIN INHIBITOR
A prodrug, dabigatran is rapidly absorbed and converted to its active form. Its plasma concentration reaches a peak 1.5 to 3 hours after an oral dose, and it has an elimination half-life of 12 to 14 hours. About 80% of its excretion is by the kidneys and the remaining 20% is through bile.
Dabigatran is not metabolized by cytochrome P450 isoenzymes, and therefore it has few major interactions with other drugs. An exception is rifampin, a P-glycoprotein inducer that blocks dabigatran’s absorption in the gut, so this combination should be avoided. Another is quinidine, a strong P-glycoprotein inhibitor that is contraindicated for use with dabigatran. Also, amiodarone (Cordarone), another P-glycoprotein inhibitor, increases blood levels of dabigatran, and therefore a lower dose of dabigatran is recommended if these drugs are given together.18–20
DOES DABIGATRAN NEED MONITORING? CAN IT EVEN BE MONITORED?
Dabigatran has a predictable pharmacodynamic effect, and current data indicate it does not need regular monitoring.18–20 However, one may need to be able to measure the drug’s activity in certain situations, such as suspected overdose, bleeding, need for emergency surgery, impaired renal function, pregnancy, and obesity, and in children.20
Dabigatran has little effect on the prothrombin time or the INR, even at therapeutic concentrations.19 Further, its effect on the activated partial thromboplastin time (aPTT) is neither linear nor dose-dependent, and the aPTT reaches a plateau and becomes less sensitive at very high concentrations. Therefore, the aPTT does not appear to be an appropriate test to monitor dabigatran’s therapeutic anticoagulant effect, although it does provide a qualitative indication of anticoagulant activity.18,19
The thrombin time is a very sensitive method for determining if dabigatran is present, but the test lacks standardization; the ecarin clotting time provides better evidence of the dose but is not readily available at most institutions.18,19,21
EVALUATED IN CLINICAL TRIALS
DABIGATRAN IS EXPENSIVE BUT MAY BE COST-EFFECTIVE
The estimated price of dabigatran 150 mg twice a day in the United States is about $6.75 to $8.00 per day.26,27
Warfarin, in contrast, costs as little as $50 per year.28 However, this low price does not include the cost of monitoring the INR (office visits and laboratory testing), and these combined expenses are much higher than the price of the warfarin itself.29 In addition, warfarin requires time-consuming management when bridging to a parenteral anticoagulant (for reversal of its anticoagulant action) before routine health maintenance procedures such as dental work and colonoscopy and interventional procedures and surgery. Any bleeding complication will also add to its cost and will be associated with a decrease in the patient’s perceived health and quality of life, but this is true for both drugs.30
In today’s health care environment, controlling costs is a universal priority, but it may be unfair to compare the cost of dabigatran with that of warfarin alone. The expense and morbidity associated with stroke and intracranial bleeding are high, and if patients on dabigatran have fewer strokes (as seen in the RE-LY trial with dabigatran 150 mg twice a day) and no added expense of monitoring, then dabigatran may be cost-effective.
Freeman et al31 analyzed the cost-effectiveness of dabigatran, using an estimated cost of $13.70 per day and data from the RE-LY trial. They concluded that dabigatran may be a cost-effective alternative to warfarin in preventing ischemic stroke in patients considered at higher risk for ischemic stroke or intracranial hemorrhage, ie, those with a CHADS2 score of 1 or higher or equivalent. (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack.)
As more new-generation oral anticoagulants become available (see below), the price of dabigatran will undoubtedly decrease. Until then, warfarin will remain a cost-effective and cost-saving drug that cannot yet be considered obsolete.
WHO SHOULD RECEIVE DABIGATRAN?
The ideal patient for dabigatran treatment is not yet defined. The decision to convert a patient’s treatment from warfarin to dabigatran will likely depend on several factors, including the patient’s response to warfarin and the physician’s comfort with this new drug.
Many patients do extremely well with warfarin, requiring infrequent monitoring to maintain a therapeutic INR and having no bleeding complications. For them, it would be more practical to continue warfarin. Another reason for staying with warfarin would be if twice-a-day dosing would pose a problem.
Dabigatran would be a reasonable choice for a patient whose INR is erratic, who requires more frequent monitoring, for whom cost is not an issue, and for whom there is concern about dietary or drug interactions.
Another consideration is whether the patient has access to a health care facility for warfarin monitoring: this is difficult for those who cannot drive, who depend on others for transportation, and who live in rural areas.
Additionally, dabigatran may be a cost-effective alternative to warfarin for a patient with a high CHADS2 score who is considered at a higher risk for stroke.31
In all cases, the option should be considered only after an open discussion with the patient about the risks and benefits of this new drug.
WHO SHOULD NOT RECEIVE IT?
Dabigatran is a twice-daily drug with a short half-life. No patient with a history of poor compliance will be a good candidate for dabigatran. Since there are no practical laboratory tests for monitoring compliance, one will have to reinforce at every visit the importance of taking this medication according to instructions.
Patients with underlying kidney disease will need close monitoring of their creatinine clearance, with dose adjustment if renal function deteriorates.
Additionally, one should use caution when prescribing dabigatran to obese patients, pregnant women, or children until more is known about its use in these populations.
ADVANTAGES AND DISADVANTAGES OF DABIGATRAN
A reason may be that patients with atrial fibrillation and poor INR control have higher rates of death, stroke, myocardial infarction, and major bleeding.14 In most clinical trials, only 55% to 60% of patients achieve a therapeutic INR on warfarin, leaving them at risk of thrombosis or, conversely, bleeding.1,2,15,32 Dabigatran has predictable pharmacokinetics, and its twice-daily dosing allows for less variability in its anticoagulant effect, making it more consistently therapeutic with less potential for bleeding or thrombosis.1
The Canadian Cardiovascular Society included dabigatran in its 2010 guidelines on atrial fibrillation, recommending it or warfarin.33 The American College of Cardiology, the American Heart Association, and the Heart Rhythm Society now give dabigatran a class I B recommendation (benefit greater than risk, but limited populations studied) in secondary stroke prevention.34
On the other hand, major concerns are the lack of an antidote for dabigatran and a lack of experience in treating bleeding complications. Since dabigatran is not monitored, physicians may be uncertain if we are overdosing or undertreating. As we gain experience, we will learn how to treat bleeding complications. Until then, it will be important to anticipate this problem and to develop an algorithm based on the best available evidence in managing this complication.
Although the overall rates of bleeding in the RE-LY trial were lower with dabigatran than with warfarin, there were more gastrointestinal bleeding events with the 150-mg dose of dabigatran, which was not readily explained.
Further, the rate of dyspepsia was almost twice as high with dabigatran than with warfarin, regardless of the dose of dabigatran. There were also more dropouts in the 2nd year of follow-up in the dabigatran groups, with gastrointestinal intolerance being one of the major reasons. Therefore, dyspepsia may cause intolerance and noncompliance.1
Dabigatran must be taken twice a day and has a relatively short half-life. For a noncompliant patient, missing one or two doses will cause a reversal of its anticoagulation effect, leaving the patient susceptible to thrombosis. In comparison, warfarin has a longer half-life and is taken once a day, so missing a dose is less likely to result in a similar reversal of its anticoagulant effect.
SPECIAL CONDITIONS
Switching from other anticoagulants to dabigatran
When making the transition from a subcutaneously administered anticoagulant, ie, a low-molecular-weight heparin or the anti-Xa inhibitor fondaparinux (Arixtra), dabigatran should be started 0 to 2 hours before the next subcutaneous dose of the parenteral anticoagulant was to be given.21,35
When switching from unfractionated heparin given by continuous intravenous infusion, the first dose of dabigatran should be given at the time the infusion is stopped.
When switching from warfarin, dabigatran should be started once the patient’s INR is less than 2.0.
Switching from dabigatran to a parenteral anticoagulant
When switching from dabigatran back to a parenteral anticoagulant, allow 12 to 24 hours after the last dabigatran dose before starting the parenteral agent.21,35
Elective surgery or invasive procedures
The manufacturer recommends stopping dabigatran 1 to 2 days before elective surgery for patients who have normal renal function and a low risk of bleeding, or 3 to 5 days before surgery for patients who have a creatinine clearance of 50 mL/min or less. Before major surgery or placement of a spinal or epidural catheter, the manufacturer recommends that dabigatran be held even longer.35
If emergency surgery is needed
If emergency surgery is needed, the clinician must use his or her judgment as to the risks of bleeding vs those of postponing the surgery.21,35
Overdose or bleeding
No antidote for dabigatran is currently available. It has a short half-life (12–14 hours), and the treatment for overdose or bleeding is to discontinue it immediately, maintain adequate diuresis, and transfuse fresh-frozen plasma or red blood cells as indicated.
The role of activated charcoal given orally to reduce absorption is under evaluation, but the charcoal must be given within 1 to 2 hours after the overdose is taken.21
Dabigatran does not bind very much to plasma proteins and hence is dialyzable—an approach that may be necessary in cases of persistent or life-threatening bleeding.
Recombinant activated factor VII or prothrombin complex concentrates may be additional options in cases of severe bleeding.18,21
TOPICS OF FUTURE RESEARCH
A limitation of the dabigatran trials was that they did not enroll patients who had renal or liver impairment, cancer, or other comorbidities; pregnant women; or children. Other topics of future research include its use in patients weighing less than 48 kg or more than 110 kg, its efficacy in patients with thrombophilia, in patients with mechanical heart valves, and in long-term follow-up and the use of thrombolytics in patients with acute stroke who are on dabigatran.
WILL DABIGATRAN CHANGE CLINICAL PRACTICE?
Despite some of the challenges listed above, we believe that dabigatran is likely to change medical practice in patients requiring anticoagulation.
Dabigatran’s biggest use will most likely be in patients with atrial fibrillation, mainly because this is the largest group of people receiving anticoagulation. In addition, the incidence of atrial fibrillation rises with age, the US population is living longer, and patients generally require life-long anticoagulation once this condition develops.
Dabigatran may be approved for additional indications in the near future. It has already shown efficacy in primary and secondary prevention of venous thromboembolism. Other important areas to be studied include its use in patients with mechanical heart valves and thrombophilia.
Whether dabigatran will be a worthy substitute for the parenteral anticoagulants (heparin, low-molecular-weight heparins, or factor Xa inhibitors) is not yet known, but it will have an enormous impact on anticoagulation management if proved efficacious.
If dabigatran becomes a major substitute for warfarin, it will affect the anticoagulation clinics, with their well-trained staff, that are currently monitoring millions of patients in the United States. These clinics would no longer be needed, and laboratory and technical costs could be saved. A downside is that patients on dabigatran will not be as closely supervised and reminded to take their medication as patients on warfarin are now at these clinics. Instead, they will likely be supervised by their own physician (or assistants), who will need to become familiar with this anticoagulant. This may affect compliance with dabigatran.
OTHER NEW ORAL ANTICOAGULANTS ARE ON THE WAY
Other oral anticoagulants, including rivaroxaban (Xarelto) and apixaban (Eliquis), have been under study and show promise in preventing both thrombotic stroke and venous thromboembolism. They will likely compete with dabigatran once they are approved.
Rivaroxaban, an oral direct factor Xa inhibitor, is being investigated for stroke prevention in patients with atrial fibrillation. It has also been shown to be not inferior to (and to be less expensive than) enoxaparin in treating and preventing venous thromboembolism in patients undergoing hip or knee arthroplasty.32,36,37 Rivaroxaban has recently been approved by the FDA for this indication.
Apixaban, another direct factor Xa inhibitor, is also being studied for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. To date, there are no head-to-head trials comparing dabigatran with either of these new oral anticoagulants.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- RE-MOBILIZE Writing Committee; Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24:1–9.
- Wolowacz SE, Roskell NS, Plumb JM, Caprini JA, Eriksson BI. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009; 101:77–85.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med 1995; 98:476–484.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991; 22:983–988.
- Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003; 290:2685–2692.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Goldhaber SZ. Pulmonary embolism thrombolysis: a clarion call for international collaboration. J Am Coll Cardiol 1992; 19:246–247.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007; 167:1414–1419.
- White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007; 167:239–245.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008; 133(6 suppl):381S–453S.
- Mungall D. BIBR-1048 Boehringer Ingelheim. Curr Opin Investig Drugs 2002; 3:905–907.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Eisert WG, Hauel N, Stangier J, Wienen W, Clemens A, van Ryn J. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol 2010; 30:1885–1889.
- Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against. J Thromb Haemost 2010; 8:627–630.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Eriksson BI, Dahl OE, Buller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost 2005; 3:103–111.
- Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO study). Am J Cardiol 2007; 100:1419–1426.
- Burger L. Bayer rival Boehringer prices blood pill at $6.75. Reuters, October 26, 2010. Available at http://www.reuters.com. Accessed September 12, 2011.
- Drugstore.com. Pradaxa. http://www.drugstore.com/pradaxa/bottle-60-150mg-capsules/qxn00597013554. Accessed September 10, 2011.
- Wal-Mart Stores, Inc. Retail Prescription Program Drug List. http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed September 10, 2011.
- Teachey DT. Dabigatran versus warfarin for venous thromboembolism (letter). N Engl J Med 2010; 362:1050; author reply1050–1051.
- Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991; 151:1944–1949.
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011; 154:1–11.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M; CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic embolization in atrial fibrillation and flutter. Can J Cardiol 2011; 27:74–90.
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:1144–1150.
- Boehringer Ingelheim. Pradaxa prescribing information. http://www.pradaxa.com. Accessed September 8, 2011.
- Huisman MV, Quinlan DJ, Dahl OE, Schulman S. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes 2010; 3:652–660.
- McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics 2009; 27:829–846.
Dabigatran etexilate (Pradaxa) is a new oral anticoagulant that has distinct advantages over warfarin (Coumadin) in terms of its ease of administration, efficacy, and safety.
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY trial),1 in patients with nonvalvular atrial fibrillation, dabigatran 110 mg twice a day was found to be as good as warfarin in preventing systemic embolization and stroke (the primary outcome of the study), and at 150 mg twice a day it was superior.1 It has also shown efficacy in treating acute deep vein thrombosis and pulmonary embolism and in preventing these complications in orthopedic surgical patients.2–4
Dabigatran has been approved in 75 countries. It carries the trade name Pradaxa in Europe and the United States and Pradax in Canada. In October 2010, the US Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee endorsed two twice-daily doses (75 mg and 150 mg) of dabigatran for the prevention of systemic embolization and stroke in patients with nonvalvular atrial fibrillation.
However, dabigatran is relatively expensive, and its current high cost might be a barrier to its wider use.
MANY PATIENTS NEED ANTICOAGULATION
Anticoagulation plays a vital role in the primary and secondary prevention of stroke in patients with atrial fibrillation and of pulmonary embolism in patients with venous thromboembolism. It is also used during cardiothoracic and vascular surgery, endovascular procedures, and dialysis and in patients with mechanical heart valves and hypercoagulable conditions.
Atrial fibrillation affects 3.03 million people in the United States (2005 figures), and this number is predicted to be as high as 7.56 million by 2050.5 More than 10% of people over the age of 80 years have it, and the lifetime risk of developing it is approximately 25%.6,7 Its most serious complication is ischemic stroke (the risk of which increases with age) and systemic embolization.5,8
Until the recent introduction of dabigatran, the only oral anticoagulant available in the United States for treating patients with atrial fibrillation was warfarin. Although warfarin has a number of disadvantages (see below), it is actually very effective for preventing ischemic stroke, reducing the incidence by as much as 65%.9,10
Venous thromboembolism is the third most common cardiovascular disorder after myocardial infarction and stroke.11 Although its exact incidence is unknown, nearly 1 million cases of it (incident or recurrent, fatal and nonfatal events) occur in the United States each year.12 Many patients with venous thromboembolism need oral anticoagulation long-term, and currently warfarin remains the only option for them as well.
NEEDED: A BETTER ANTICOAGULANT
Warfarin has been the most commonly prescribed oral anticoagulant in the United States for more than 60 years. As of 2004, more than 30 million outpatient prescriptions for it were filled annually in this country alone.13 However, warfarin has several important limitations.
Warfarin has a narrow therapeutic index. Patients taking it require monitoring of their international normalized ratio (INR) and frequent dose adjustments, and this is time-consuming and inconvenient. The target INR for patients with venous thromboembolism and atrial fibrillation is 2.0 to 3.0, whereas patients with a mechanical heart valve need a higher INR (2.5 to 3.5). If the INR is below these ranges, warfarin is less effective, with a risk of new thrombosis. On the other hand, if the INR is too high, there is a risk of bleeding.14 In fact, the most important side effect of warfarin is the risk of major and minor bleeding.13 However, even in well-designed clinical trials in which patients are closely managed, only 55% to 60% of patients regularly achieve their therapeutic target INR.1,2,14,15
Warfarin also interacts with many drugs and with some foods. Compliance is difficult. It has a slow onset of action. Genetic variations require dose adjustments. When switching from a parenteral anticoagulant, overlapping is required. Skin necrosis is a possible side effect. And warfarin is teratogenic.
Despite these limitations, the American College of Chest Physicians endorses warfarin to prevent or treat venous thromboembolism, and to prevent stroke in patients with atrial fibrillation.16
DABIGATRAN, A THROMBIN INHIBITOR
A prodrug, dabigatran is rapidly absorbed and converted to its active form. Its plasma concentration reaches a peak 1.5 to 3 hours after an oral dose, and it has an elimination half-life of 12 to 14 hours. About 80% of its excretion is by the kidneys and the remaining 20% is through bile.
Dabigatran is not metabolized by cytochrome P450 isoenzymes, and therefore it has few major interactions with other drugs. An exception is rifampin, a P-glycoprotein inducer that blocks dabigatran’s absorption in the gut, so this combination should be avoided. Another is quinidine, a strong P-glycoprotein inhibitor that is contraindicated for use with dabigatran. Also, amiodarone (Cordarone), another P-glycoprotein inhibitor, increases blood levels of dabigatran, and therefore a lower dose of dabigatran is recommended if these drugs are given together.18–20
DOES DABIGATRAN NEED MONITORING? CAN IT EVEN BE MONITORED?
Dabigatran has a predictable pharmacodynamic effect, and current data indicate it does not need regular monitoring.18–20 However, one may need to be able to measure the drug’s activity in certain situations, such as suspected overdose, bleeding, need for emergency surgery, impaired renal function, pregnancy, and obesity, and in children.20
Dabigatran has little effect on the prothrombin time or the INR, even at therapeutic concentrations.19 Further, its effect on the activated partial thromboplastin time (aPTT) is neither linear nor dose-dependent, and the aPTT reaches a plateau and becomes less sensitive at very high concentrations. Therefore, the aPTT does not appear to be an appropriate test to monitor dabigatran’s therapeutic anticoagulant effect, although it does provide a qualitative indication of anticoagulant activity.18,19
The thrombin time is a very sensitive method for determining if dabigatran is present, but the test lacks standardization; the ecarin clotting time provides better evidence of the dose but is not readily available at most institutions.18,19,21
EVALUATED IN CLINICAL TRIALS
DABIGATRAN IS EXPENSIVE BUT MAY BE COST-EFFECTIVE
The estimated price of dabigatran 150 mg twice a day in the United States is about $6.75 to $8.00 per day.26,27
Warfarin, in contrast, costs as little as $50 per year.28 However, this low price does not include the cost of monitoring the INR (office visits and laboratory testing), and these combined expenses are much higher than the price of the warfarin itself.29 In addition, warfarin requires time-consuming management when bridging to a parenteral anticoagulant (for reversal of its anticoagulant action) before routine health maintenance procedures such as dental work and colonoscopy and interventional procedures and surgery. Any bleeding complication will also add to its cost and will be associated with a decrease in the patient’s perceived health and quality of life, but this is true for both drugs.30
In today’s health care environment, controlling costs is a universal priority, but it may be unfair to compare the cost of dabigatran with that of warfarin alone. The expense and morbidity associated with stroke and intracranial bleeding are high, and if patients on dabigatran have fewer strokes (as seen in the RE-LY trial with dabigatran 150 mg twice a day) and no added expense of monitoring, then dabigatran may be cost-effective.
Freeman et al31 analyzed the cost-effectiveness of dabigatran, using an estimated cost of $13.70 per day and data from the RE-LY trial. They concluded that dabigatran may be a cost-effective alternative to warfarin in preventing ischemic stroke in patients considered at higher risk for ischemic stroke or intracranial hemorrhage, ie, those with a CHADS2 score of 1 or higher or equivalent. (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack.)
As more new-generation oral anticoagulants become available (see below), the price of dabigatran will undoubtedly decrease. Until then, warfarin will remain a cost-effective and cost-saving drug that cannot yet be considered obsolete.
WHO SHOULD RECEIVE DABIGATRAN?
The ideal patient for dabigatran treatment is not yet defined. The decision to convert a patient’s treatment from warfarin to dabigatran will likely depend on several factors, including the patient’s response to warfarin and the physician’s comfort with this new drug.
Many patients do extremely well with warfarin, requiring infrequent monitoring to maintain a therapeutic INR and having no bleeding complications. For them, it would be more practical to continue warfarin. Another reason for staying with warfarin would be if twice-a-day dosing would pose a problem.
Dabigatran would be a reasonable choice for a patient whose INR is erratic, who requires more frequent monitoring, for whom cost is not an issue, and for whom there is concern about dietary or drug interactions.
Another consideration is whether the patient has access to a health care facility for warfarin monitoring: this is difficult for those who cannot drive, who depend on others for transportation, and who live in rural areas.
Additionally, dabigatran may be a cost-effective alternative to warfarin for a patient with a high CHADS2 score who is considered at a higher risk for stroke.31
In all cases, the option should be considered only after an open discussion with the patient about the risks and benefits of this new drug.
WHO SHOULD NOT RECEIVE IT?
Dabigatran is a twice-daily drug with a short half-life. No patient with a history of poor compliance will be a good candidate for dabigatran. Since there are no practical laboratory tests for monitoring compliance, one will have to reinforce at every visit the importance of taking this medication according to instructions.
Patients with underlying kidney disease will need close monitoring of their creatinine clearance, with dose adjustment if renal function deteriorates.
Additionally, one should use caution when prescribing dabigatran to obese patients, pregnant women, or children until more is known about its use in these populations.
ADVANTAGES AND DISADVANTAGES OF DABIGATRAN
A reason may be that patients with atrial fibrillation and poor INR control have higher rates of death, stroke, myocardial infarction, and major bleeding.14 In most clinical trials, only 55% to 60% of patients achieve a therapeutic INR on warfarin, leaving them at risk of thrombosis or, conversely, bleeding.1,2,15,32 Dabigatran has predictable pharmacokinetics, and its twice-daily dosing allows for less variability in its anticoagulant effect, making it more consistently therapeutic with less potential for bleeding or thrombosis.1
The Canadian Cardiovascular Society included dabigatran in its 2010 guidelines on atrial fibrillation, recommending it or warfarin.33 The American College of Cardiology, the American Heart Association, and the Heart Rhythm Society now give dabigatran a class I B recommendation (benefit greater than risk, but limited populations studied) in secondary stroke prevention.34
On the other hand, major concerns are the lack of an antidote for dabigatran and a lack of experience in treating bleeding complications. Since dabigatran is not monitored, physicians may be uncertain if we are overdosing or undertreating. As we gain experience, we will learn how to treat bleeding complications. Until then, it will be important to anticipate this problem and to develop an algorithm based on the best available evidence in managing this complication.
Although the overall rates of bleeding in the RE-LY trial were lower with dabigatran than with warfarin, there were more gastrointestinal bleeding events with the 150-mg dose of dabigatran, which was not readily explained.
Further, the rate of dyspepsia was almost twice as high with dabigatran than with warfarin, regardless of the dose of dabigatran. There were also more dropouts in the 2nd year of follow-up in the dabigatran groups, with gastrointestinal intolerance being one of the major reasons. Therefore, dyspepsia may cause intolerance and noncompliance.1
Dabigatran must be taken twice a day and has a relatively short half-life. For a noncompliant patient, missing one or two doses will cause a reversal of its anticoagulation effect, leaving the patient susceptible to thrombosis. In comparison, warfarin has a longer half-life and is taken once a day, so missing a dose is less likely to result in a similar reversal of its anticoagulant effect.
SPECIAL CONDITIONS
Switching from other anticoagulants to dabigatran
When making the transition from a subcutaneously administered anticoagulant, ie, a low-molecular-weight heparin or the anti-Xa inhibitor fondaparinux (Arixtra), dabigatran should be started 0 to 2 hours before the next subcutaneous dose of the parenteral anticoagulant was to be given.21,35
When switching from unfractionated heparin given by continuous intravenous infusion, the first dose of dabigatran should be given at the time the infusion is stopped.
When switching from warfarin, dabigatran should be started once the patient’s INR is less than 2.0.
Switching from dabigatran to a parenteral anticoagulant
When switching from dabigatran back to a parenteral anticoagulant, allow 12 to 24 hours after the last dabigatran dose before starting the parenteral agent.21,35
Elective surgery or invasive procedures
The manufacturer recommends stopping dabigatran 1 to 2 days before elective surgery for patients who have normal renal function and a low risk of bleeding, or 3 to 5 days before surgery for patients who have a creatinine clearance of 50 mL/min or less. Before major surgery or placement of a spinal or epidural catheter, the manufacturer recommends that dabigatran be held even longer.35
If emergency surgery is needed
If emergency surgery is needed, the clinician must use his or her judgment as to the risks of bleeding vs those of postponing the surgery.21,35
Overdose or bleeding
No antidote for dabigatran is currently available. It has a short half-life (12–14 hours), and the treatment for overdose or bleeding is to discontinue it immediately, maintain adequate diuresis, and transfuse fresh-frozen plasma or red blood cells as indicated.
The role of activated charcoal given orally to reduce absorption is under evaluation, but the charcoal must be given within 1 to 2 hours after the overdose is taken.21
Dabigatran does not bind very much to plasma proteins and hence is dialyzable—an approach that may be necessary in cases of persistent or life-threatening bleeding.
Recombinant activated factor VII or prothrombin complex concentrates may be additional options in cases of severe bleeding.18,21
TOPICS OF FUTURE RESEARCH
A limitation of the dabigatran trials was that they did not enroll patients who had renal or liver impairment, cancer, or other comorbidities; pregnant women; or children. Other topics of future research include its use in patients weighing less than 48 kg or more than 110 kg, its efficacy in patients with thrombophilia, in patients with mechanical heart valves, and in long-term follow-up and the use of thrombolytics in patients with acute stroke who are on dabigatran.
WILL DABIGATRAN CHANGE CLINICAL PRACTICE?
Despite some of the challenges listed above, we believe that dabigatran is likely to change medical practice in patients requiring anticoagulation.
Dabigatran’s biggest use will most likely be in patients with atrial fibrillation, mainly because this is the largest group of people receiving anticoagulation. In addition, the incidence of atrial fibrillation rises with age, the US population is living longer, and patients generally require life-long anticoagulation once this condition develops.
Dabigatran may be approved for additional indications in the near future. It has already shown efficacy in primary and secondary prevention of venous thromboembolism. Other important areas to be studied include its use in patients with mechanical heart valves and thrombophilia.
Whether dabigatran will be a worthy substitute for the parenteral anticoagulants (heparin, low-molecular-weight heparins, or factor Xa inhibitors) is not yet known, but it will have an enormous impact on anticoagulation management if proved efficacious.
If dabigatran becomes a major substitute for warfarin, it will affect the anticoagulation clinics, with their well-trained staff, that are currently monitoring millions of patients in the United States. These clinics would no longer be needed, and laboratory and technical costs could be saved. A downside is that patients on dabigatran will not be as closely supervised and reminded to take their medication as patients on warfarin are now at these clinics. Instead, they will likely be supervised by their own physician (or assistants), who will need to become familiar with this anticoagulant. This may affect compliance with dabigatran.
OTHER NEW ORAL ANTICOAGULANTS ARE ON THE WAY
Other oral anticoagulants, including rivaroxaban (Xarelto) and apixaban (Eliquis), have been under study and show promise in preventing both thrombotic stroke and venous thromboembolism. They will likely compete with dabigatran once they are approved.
Rivaroxaban, an oral direct factor Xa inhibitor, is being investigated for stroke prevention in patients with atrial fibrillation. It has also been shown to be not inferior to (and to be less expensive than) enoxaparin in treating and preventing venous thromboembolism in patients undergoing hip or knee arthroplasty.32,36,37 Rivaroxaban has recently been approved by the FDA for this indication.
Apixaban, another direct factor Xa inhibitor, is also being studied for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. To date, there are no head-to-head trials comparing dabigatran with either of these new oral anticoagulants.
Dabigatran etexilate (Pradaxa) is a new oral anticoagulant that has distinct advantages over warfarin (Coumadin) in terms of its ease of administration, efficacy, and safety.
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY trial),1 in patients with nonvalvular atrial fibrillation, dabigatran 110 mg twice a day was found to be as good as warfarin in preventing systemic embolization and stroke (the primary outcome of the study), and at 150 mg twice a day it was superior.1 It has also shown efficacy in treating acute deep vein thrombosis and pulmonary embolism and in preventing these complications in orthopedic surgical patients.2–4
Dabigatran has been approved in 75 countries. It carries the trade name Pradaxa in Europe and the United States and Pradax in Canada. In October 2010, the US Food and Drug Administration (FDA) Cardiovascular and Renal Drugs Advisory Committee endorsed two twice-daily doses (75 mg and 150 mg) of dabigatran for the prevention of systemic embolization and stroke in patients with nonvalvular atrial fibrillation.
However, dabigatran is relatively expensive, and its current high cost might be a barrier to its wider use.
MANY PATIENTS NEED ANTICOAGULATION
Anticoagulation plays a vital role in the primary and secondary prevention of stroke in patients with atrial fibrillation and of pulmonary embolism in patients with venous thromboembolism. It is also used during cardiothoracic and vascular surgery, endovascular procedures, and dialysis and in patients with mechanical heart valves and hypercoagulable conditions.
Atrial fibrillation affects 3.03 million people in the United States (2005 figures), and this number is predicted to be as high as 7.56 million by 2050.5 More than 10% of people over the age of 80 years have it, and the lifetime risk of developing it is approximately 25%.6,7 Its most serious complication is ischemic stroke (the risk of which increases with age) and systemic embolization.5,8
Until the recent introduction of dabigatran, the only oral anticoagulant available in the United States for treating patients with atrial fibrillation was warfarin. Although warfarin has a number of disadvantages (see below), it is actually very effective for preventing ischemic stroke, reducing the incidence by as much as 65%.9,10
Venous thromboembolism is the third most common cardiovascular disorder after myocardial infarction and stroke.11 Although its exact incidence is unknown, nearly 1 million cases of it (incident or recurrent, fatal and nonfatal events) occur in the United States each year.12 Many patients with venous thromboembolism need oral anticoagulation long-term, and currently warfarin remains the only option for them as well.
NEEDED: A BETTER ANTICOAGULANT
Warfarin has been the most commonly prescribed oral anticoagulant in the United States for more than 60 years. As of 2004, more than 30 million outpatient prescriptions for it were filled annually in this country alone.13 However, warfarin has several important limitations.
Warfarin has a narrow therapeutic index. Patients taking it require monitoring of their international normalized ratio (INR) and frequent dose adjustments, and this is time-consuming and inconvenient. The target INR for patients with venous thromboembolism and atrial fibrillation is 2.0 to 3.0, whereas patients with a mechanical heart valve need a higher INR (2.5 to 3.5). If the INR is below these ranges, warfarin is less effective, with a risk of new thrombosis. On the other hand, if the INR is too high, there is a risk of bleeding.14 In fact, the most important side effect of warfarin is the risk of major and minor bleeding.13 However, even in well-designed clinical trials in which patients are closely managed, only 55% to 60% of patients regularly achieve their therapeutic target INR.1,2,14,15
Warfarin also interacts with many drugs and with some foods. Compliance is difficult. It has a slow onset of action. Genetic variations require dose adjustments. When switching from a parenteral anticoagulant, overlapping is required. Skin necrosis is a possible side effect. And warfarin is teratogenic.
Despite these limitations, the American College of Chest Physicians endorses warfarin to prevent or treat venous thromboembolism, and to prevent stroke in patients with atrial fibrillation.16
DABIGATRAN, A THROMBIN INHIBITOR
A prodrug, dabigatran is rapidly absorbed and converted to its active form. Its plasma concentration reaches a peak 1.5 to 3 hours after an oral dose, and it has an elimination half-life of 12 to 14 hours. About 80% of its excretion is by the kidneys and the remaining 20% is through bile.
Dabigatran is not metabolized by cytochrome P450 isoenzymes, and therefore it has few major interactions with other drugs. An exception is rifampin, a P-glycoprotein inducer that blocks dabigatran’s absorption in the gut, so this combination should be avoided. Another is quinidine, a strong P-glycoprotein inhibitor that is contraindicated for use with dabigatran. Also, amiodarone (Cordarone), another P-glycoprotein inhibitor, increases blood levels of dabigatran, and therefore a lower dose of dabigatran is recommended if these drugs are given together.18–20
DOES DABIGATRAN NEED MONITORING? CAN IT EVEN BE MONITORED?
Dabigatran has a predictable pharmacodynamic effect, and current data indicate it does not need regular monitoring.18–20 However, one may need to be able to measure the drug’s activity in certain situations, such as suspected overdose, bleeding, need for emergency surgery, impaired renal function, pregnancy, and obesity, and in children.20
Dabigatran has little effect on the prothrombin time or the INR, even at therapeutic concentrations.19 Further, its effect on the activated partial thromboplastin time (aPTT) is neither linear nor dose-dependent, and the aPTT reaches a plateau and becomes less sensitive at very high concentrations. Therefore, the aPTT does not appear to be an appropriate test to monitor dabigatran’s therapeutic anticoagulant effect, although it does provide a qualitative indication of anticoagulant activity.18,19
The thrombin time is a very sensitive method for determining if dabigatran is present, but the test lacks standardization; the ecarin clotting time provides better evidence of the dose but is not readily available at most institutions.18,19,21
EVALUATED IN CLINICAL TRIALS
DABIGATRAN IS EXPENSIVE BUT MAY BE COST-EFFECTIVE
The estimated price of dabigatran 150 mg twice a day in the United States is about $6.75 to $8.00 per day.26,27
Warfarin, in contrast, costs as little as $50 per year.28 However, this low price does not include the cost of monitoring the INR (office visits and laboratory testing), and these combined expenses are much higher than the price of the warfarin itself.29 In addition, warfarin requires time-consuming management when bridging to a parenteral anticoagulant (for reversal of its anticoagulant action) before routine health maintenance procedures such as dental work and colonoscopy and interventional procedures and surgery. Any bleeding complication will also add to its cost and will be associated with a decrease in the patient’s perceived health and quality of life, but this is true for both drugs.30
In today’s health care environment, controlling costs is a universal priority, but it may be unfair to compare the cost of dabigatran with that of warfarin alone. The expense and morbidity associated with stroke and intracranial bleeding are high, and if patients on dabigatran have fewer strokes (as seen in the RE-LY trial with dabigatran 150 mg twice a day) and no added expense of monitoring, then dabigatran may be cost-effective.
Freeman et al31 analyzed the cost-effectiveness of dabigatran, using an estimated cost of $13.70 per day and data from the RE-LY trial. They concluded that dabigatran may be a cost-effective alternative to warfarin in preventing ischemic stroke in patients considered at higher risk for ischemic stroke or intracranial hemorrhage, ie, those with a CHADS2 score of 1 or higher or equivalent. (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age 75 or older, and diabetes mellitus; 2 points for prior stroke or transient ischemic attack.)
As more new-generation oral anticoagulants become available (see below), the price of dabigatran will undoubtedly decrease. Until then, warfarin will remain a cost-effective and cost-saving drug that cannot yet be considered obsolete.
WHO SHOULD RECEIVE DABIGATRAN?
The ideal patient for dabigatran treatment is not yet defined. The decision to convert a patient’s treatment from warfarin to dabigatran will likely depend on several factors, including the patient’s response to warfarin and the physician’s comfort with this new drug.
Many patients do extremely well with warfarin, requiring infrequent monitoring to maintain a therapeutic INR and having no bleeding complications. For them, it would be more practical to continue warfarin. Another reason for staying with warfarin would be if twice-a-day dosing would pose a problem.
Dabigatran would be a reasonable choice for a patient whose INR is erratic, who requires more frequent monitoring, for whom cost is not an issue, and for whom there is concern about dietary or drug interactions.
Another consideration is whether the patient has access to a health care facility for warfarin monitoring: this is difficult for those who cannot drive, who depend on others for transportation, and who live in rural areas.
Additionally, dabigatran may be a cost-effective alternative to warfarin for a patient with a high CHADS2 score who is considered at a higher risk for stroke.31
In all cases, the option should be considered only after an open discussion with the patient about the risks and benefits of this new drug.
WHO SHOULD NOT RECEIVE IT?
Dabigatran is a twice-daily drug with a short half-life. No patient with a history of poor compliance will be a good candidate for dabigatran. Since there are no practical laboratory tests for monitoring compliance, one will have to reinforce at every visit the importance of taking this medication according to instructions.
Patients with underlying kidney disease will need close monitoring of their creatinine clearance, with dose adjustment if renal function deteriorates.
Additionally, one should use caution when prescribing dabigatran to obese patients, pregnant women, or children until more is known about its use in these populations.
ADVANTAGES AND DISADVANTAGES OF DABIGATRAN
A reason may be that patients with atrial fibrillation and poor INR control have higher rates of death, stroke, myocardial infarction, and major bleeding.14 In most clinical trials, only 55% to 60% of patients achieve a therapeutic INR on warfarin, leaving them at risk of thrombosis or, conversely, bleeding.1,2,15,32 Dabigatran has predictable pharmacokinetics, and its twice-daily dosing allows for less variability in its anticoagulant effect, making it more consistently therapeutic with less potential for bleeding or thrombosis.1
The Canadian Cardiovascular Society included dabigatran in its 2010 guidelines on atrial fibrillation, recommending it or warfarin.33 The American College of Cardiology, the American Heart Association, and the Heart Rhythm Society now give dabigatran a class I B recommendation (benefit greater than risk, but limited populations studied) in secondary stroke prevention.34
On the other hand, major concerns are the lack of an antidote for dabigatran and a lack of experience in treating bleeding complications. Since dabigatran is not monitored, physicians may be uncertain if we are overdosing or undertreating. As we gain experience, we will learn how to treat bleeding complications. Until then, it will be important to anticipate this problem and to develop an algorithm based on the best available evidence in managing this complication.
Although the overall rates of bleeding in the RE-LY trial were lower with dabigatran than with warfarin, there were more gastrointestinal bleeding events with the 150-mg dose of dabigatran, which was not readily explained.
Further, the rate of dyspepsia was almost twice as high with dabigatran than with warfarin, regardless of the dose of dabigatran. There were also more dropouts in the 2nd year of follow-up in the dabigatran groups, with gastrointestinal intolerance being one of the major reasons. Therefore, dyspepsia may cause intolerance and noncompliance.1
Dabigatran must be taken twice a day and has a relatively short half-life. For a noncompliant patient, missing one or two doses will cause a reversal of its anticoagulation effect, leaving the patient susceptible to thrombosis. In comparison, warfarin has a longer half-life and is taken once a day, so missing a dose is less likely to result in a similar reversal of its anticoagulant effect.
SPECIAL CONDITIONS
Switching from other anticoagulants to dabigatran
When making the transition from a subcutaneously administered anticoagulant, ie, a low-molecular-weight heparin or the anti-Xa inhibitor fondaparinux (Arixtra), dabigatran should be started 0 to 2 hours before the next subcutaneous dose of the parenteral anticoagulant was to be given.21,35
When switching from unfractionated heparin given by continuous intravenous infusion, the first dose of dabigatran should be given at the time the infusion is stopped.
When switching from warfarin, dabigatran should be started once the patient’s INR is less than 2.0.
Switching from dabigatran to a parenteral anticoagulant
When switching from dabigatran back to a parenteral anticoagulant, allow 12 to 24 hours after the last dabigatran dose before starting the parenteral agent.21,35
Elective surgery or invasive procedures
The manufacturer recommends stopping dabigatran 1 to 2 days before elective surgery for patients who have normal renal function and a low risk of bleeding, or 3 to 5 days before surgery for patients who have a creatinine clearance of 50 mL/min or less. Before major surgery or placement of a spinal or epidural catheter, the manufacturer recommends that dabigatran be held even longer.35
If emergency surgery is needed
If emergency surgery is needed, the clinician must use his or her judgment as to the risks of bleeding vs those of postponing the surgery.21,35
Overdose or bleeding
No antidote for dabigatran is currently available. It has a short half-life (12–14 hours), and the treatment for overdose or bleeding is to discontinue it immediately, maintain adequate diuresis, and transfuse fresh-frozen plasma or red blood cells as indicated.
The role of activated charcoal given orally to reduce absorption is under evaluation, but the charcoal must be given within 1 to 2 hours after the overdose is taken.21
Dabigatran does not bind very much to plasma proteins and hence is dialyzable—an approach that may be necessary in cases of persistent or life-threatening bleeding.
Recombinant activated factor VII or prothrombin complex concentrates may be additional options in cases of severe bleeding.18,21
TOPICS OF FUTURE RESEARCH
A limitation of the dabigatran trials was that they did not enroll patients who had renal or liver impairment, cancer, or other comorbidities; pregnant women; or children. Other topics of future research include its use in patients weighing less than 48 kg or more than 110 kg, its efficacy in patients with thrombophilia, in patients with mechanical heart valves, and in long-term follow-up and the use of thrombolytics in patients with acute stroke who are on dabigatran.
WILL DABIGATRAN CHANGE CLINICAL PRACTICE?
Despite some of the challenges listed above, we believe that dabigatran is likely to change medical practice in patients requiring anticoagulation.
Dabigatran’s biggest use will most likely be in patients with atrial fibrillation, mainly because this is the largest group of people receiving anticoagulation. In addition, the incidence of atrial fibrillation rises with age, the US population is living longer, and patients generally require life-long anticoagulation once this condition develops.
Dabigatran may be approved for additional indications in the near future. It has already shown efficacy in primary and secondary prevention of venous thromboembolism. Other important areas to be studied include its use in patients with mechanical heart valves and thrombophilia.
Whether dabigatran will be a worthy substitute for the parenteral anticoagulants (heparin, low-molecular-weight heparins, or factor Xa inhibitors) is not yet known, but it will have an enormous impact on anticoagulation management if proved efficacious.
If dabigatran becomes a major substitute for warfarin, it will affect the anticoagulation clinics, with their well-trained staff, that are currently monitoring millions of patients in the United States. These clinics would no longer be needed, and laboratory and technical costs could be saved. A downside is that patients on dabigatran will not be as closely supervised and reminded to take their medication as patients on warfarin are now at these clinics. Instead, they will likely be supervised by their own physician (or assistants), who will need to become familiar with this anticoagulant. This may affect compliance with dabigatran.
OTHER NEW ORAL ANTICOAGULANTS ARE ON THE WAY
Other oral anticoagulants, including rivaroxaban (Xarelto) and apixaban (Eliquis), have been under study and show promise in preventing both thrombotic stroke and venous thromboembolism. They will likely compete with dabigatran once they are approved.
Rivaroxaban, an oral direct factor Xa inhibitor, is being investigated for stroke prevention in patients with atrial fibrillation. It has also been shown to be not inferior to (and to be less expensive than) enoxaparin in treating and preventing venous thromboembolism in patients undergoing hip or knee arthroplasty.32,36,37 Rivaroxaban has recently been approved by the FDA for this indication.
Apixaban, another direct factor Xa inhibitor, is also being studied for the prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. To date, there are no head-to-head trials comparing dabigatran with either of these new oral anticoagulants.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- RE-MOBILIZE Writing Committee; Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24:1–9.
- Wolowacz SE, Roskell NS, Plumb JM, Caprini JA, Eriksson BI. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009; 101:77–85.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med 1995; 98:476–484.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991; 22:983–988.
- Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003; 290:2685–2692.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Goldhaber SZ. Pulmonary embolism thrombolysis: a clarion call for international collaboration. J Am Coll Cardiol 1992; 19:246–247.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007; 167:1414–1419.
- White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007; 167:239–245.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008; 133(6 suppl):381S–453S.
- Mungall D. BIBR-1048 Boehringer Ingelheim. Curr Opin Investig Drugs 2002; 3:905–907.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Eisert WG, Hauel N, Stangier J, Wienen W, Clemens A, van Ryn J. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol 2010; 30:1885–1889.
- Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against. J Thromb Haemost 2010; 8:627–630.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Eriksson BI, Dahl OE, Buller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost 2005; 3:103–111.
- Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO study). Am J Cardiol 2007; 100:1419–1426.
- Burger L. Bayer rival Boehringer prices blood pill at $6.75. Reuters, October 26, 2010. Available at http://www.reuters.com. Accessed September 12, 2011.
- Drugstore.com. Pradaxa. http://www.drugstore.com/pradaxa/bottle-60-150mg-capsules/qxn00597013554. Accessed September 10, 2011.
- Wal-Mart Stores, Inc. Retail Prescription Program Drug List. http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed September 10, 2011.
- Teachey DT. Dabigatran versus warfarin for venous thromboembolism (letter). N Engl J Med 2010; 362:1050; author reply1050–1051.
- Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991; 151:1944–1949.
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011; 154:1–11.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M; CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic embolization in atrial fibrillation and flutter. Can J Cardiol 2011; 27:74–90.
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:1144–1150.
- Boehringer Ingelheim. Pradaxa prescribing information. http://www.pradaxa.com. Accessed September 8, 2011.
- Huisman MV, Quinlan DJ, Dahl OE, Schulman S. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes 2010; 3:652–660.
- McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics 2009; 27:829–846.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- RE-MOBILIZE Writing Committee; Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24:1–9.
- Wolowacz SE, Roskell NS, Plumb JM, Caprini JA, Eriksson BI. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009; 101:77–85.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med 1995; 98:476–484.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004; 110:1042–1046.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991; 22:983–988.
- Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 2003; 290:2685–2692.
- Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med 2009; 151:297–305.
- Goldhaber SZ. Pulmonary embolism thrombolysis: a clarion call for international collaboration. J Am Coll Cardiol 1992; 19:246–247.
- Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol 2008; 28:370–372.
- Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007; 167:1414–1419.
- White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med 2007; 167:239–245.
- ACTIVE Writing Group of the ACTIVE Investigators; Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006; 367:1903–1912.
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008; 133(6 suppl):381S–453S.
- Mungall D. BIBR-1048 Boehringer Ingelheim. Curr Opin Investig Drugs 2002; 3:905–907.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Eisert WG, Hauel N, Stangier J, Wienen W, Clemens A, van Ryn J. Dabigatran: an oral novel potent reversible nonpeptide inhibitor of thrombin. Arterioscler Thromb Vasc Biol 2010; 30:1885–1889.
- Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against. J Thromb Haemost 2010; 8:627–630.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Eriksson BI, Dahl OE, Buller HR, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost 2005; 3:103–111.
- Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO study). Am J Cardiol 2007; 100:1419–1426.
- Burger L. Bayer rival Boehringer prices blood pill at $6.75. Reuters, October 26, 2010. Available at http://www.reuters.com. Accessed September 12, 2011.
- Drugstore.com. Pradaxa. http://www.drugstore.com/pradaxa/bottle-60-150mg-capsules/qxn00597013554. Accessed September 10, 2011.
- Wal-Mart Stores, Inc. Retail Prescription Program Drug List. http://i.walmartimages.com/i/if/hmp/fusion/customer_list.pdf. Accessed September 10, 2011.
- Teachey DT. Dabigatran versus warfarin for venous thromboembolism (letter). N Engl J Med 2010; 362:1050; author reply1050–1051.
- Lancaster TR, Singer DE, Sheehan MA, et al. The impact of long-term warfarin therapy on quality of life. Evidence from a randomized trial. Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. Arch Intern Med 1991; 151:1944–1949.
- Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011; 154:1–11.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- Cairns JA, Connolly S, McMurtry S, Stephenson M, Talajic M; CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic embolization in atrial fibrillation and flutter. Can J Cardiol 2011; 27:74–90.
- Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011; 123:1144–1150.
- Boehringer Ingelheim. Pradaxa prescribing information. http://www.pradaxa.com. Accessed September 8, 2011.
- Huisman MV, Quinlan DJ, Dahl OE, Schulman S. Enoxaparin versus dabigatran or rivaroxaban for thromboprophylaxis after hip or knee arthroplasty: results of separate pooled analyses of phase III multicenter randomized trials. Circ Cardiovasc Qual Outcomes 2010; 3:652–660.
- McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics 2009; 27:829–846.
KEY POINTS
- Dabigatran is a potent, reversible, direct thrombin inhibitor. Available only in oral form, it has a rapid onset of action, a predictable anticoagulant response, and few major interactions.
- Dabigatran does not require dose adjustments (except for renal insufficiency) or monitoring of its effect during treatment.
- In trials in patients with nonvalvular atrial fibrillation, two different doses of dabigatran were compared with warfarin. Less bleeding occurred with the lower dose than with warfarin, while the higher dose was more effective than warfarin in preventing stroke and systemic embolization.
- The American College of Cardiology, the American Heart Association, and the Heart Rhythm Society have given dabigatran a class I B recommendation for secondary stroke prevention in patients with nonvalvular atrial fibrillation.
What is the optimal duration of bisphosphonate therapy?
Almost all the data about the safety and efficacy of bisphosphonate drugs for treating osteoporosis are from patients who took them for less than 5 years.
Reports of adverse effects with prolonged use have caused concern about the long-term safety of this class of drugs. This is particularly important because these drugs are retained in the skeleton longer than 10 years, because there are physiologic reasons why excessive bisphosphonate-induced inhibition of bone turnover could be damaging, and because many healthy postmenopausal women have been prescribed bisphosphonates in the hope of preventing fractures that are not expected to occur for 20 to 30 years.
Because information from trials is scant, opinions differ over whether bisphosphonates should be continued indefinitely. In this article, I summarize the physiologic mechanisms of these drugs, review the scant existing data about their effects beyond 5 years, and describe my approach to bisphosphonate therapy (while waiting for better evidence).
MORE THAN 4 MILLION WOMEN TAKE BISPHOSPHONATES
The first medical use of a bisphosphonate was in 1967, when a girl with myositis ossificans was given etidronate (Didronel) because it inhibited mineralization. Two years later, it was given to patients with Paget disease of bone because it was found to inhibit bone resorption.1 Etidronate could not be given for longer than 6 months, however, because patients developed osteomalacia.
Adding a nitrogen to the molecule dramatically increased its potency and led to the second generation of bisphosphonates. Alendronate (Fosamax), the first amino-bisphosphonate, became available in 1995, It was followed by risedronate (Actonel), ibandronate (Boniva), and zoledronic acid (Reclast). These drugs are potent inhibitors of bone resorption; however, in clinical doses they do not inhibit mineralization and therefore do not cause osteomalacia.
Randomized clinical trials involving more than 30,000 patients have provided grade A evidence that these drugs reduce the incidence of fragility fractures in patients with osteoporosis.2 Furthermore, observational studies have confirmed that they prevent fractures and have a good safety profile in clinical practice.
Therefore, the use of these drugs has become common. In 2008, an estimated 4 million women in the United States were taking them.3
BISPHOSPHONATES STRENGTHEN BONE BY INHIBITING RESORPTION
On a molecular level, bisphosphonates inhibit farnesyl pyrophosphate synthase, an enzyme necessary for formation of the cytoskeleton in osteoclasts. Thus, they strongly inhibit bone resorption. They do not appear to directly inhibit osteoblasts, the cells that form new bone, but they substantially decrease bone formation indirectly.4
To understand how inhibition of bone resorption affects bone physiology, it is necessary to appreciate the nature of bone remodeling. Bone is not like the skin, which is continually forming a new layer and sloughing off the old. Instead, bone is renewed in small units. It takes about 5 years to remodel cancellous bone and 13 years to remodel cortical bone5; at any one time, about 8% of the surface is being remodeled.
The first step occurs at a spot on the surface, where the osteoclasts resorb some bone to form a pit that looks like a pothole. Then a team of osteoblasts is formed and fills the pit with new bone over the next 3 to 6 months. When first formed, the new bone is mainly collagen and, like the tip of the nose, is not very stiff, but with mineral deposition the bone becomes stronger, like the bridge of the nose. The new bone gradually accumulates mineral and becomes harder and denser over the next 3 years.
When a bisphosphonate is given, the osteoclasts abruptly stop resorbing the bone, but osteoblasts continue to fill the pits that were there when the bisphosphonate was started. For the next several months, while the previous pits are being filled, the bone volume increases slightly. Thereafter, rates of both bone resorption and bone formation are very low.
A misconception: Bisphosphonates build bone
While semantically it is true that the bone formation rate in patients taking bisphosphonates is within the normal premenopausal range, this often-repeated statement is essentially misleading.
With continued bisphosphonate use, the bone gradually becomes more dense. There is no further new bone, but the existing bone matrix is packed more tightly with mineral crystals.9 The old bone is not resorbed. The bone density, measured radiographically, increases most rapidly during the first 6 months (while resorption pits are filling in) and more gradually over the next 3 years (while bone is becoming more mineralized).
Another common misunderstanding is that the bone density increases because the drugs are “building bone.” After 3 years, the bone density in the femur reaches a plateau.10 I have seen patients who were very worried because their bone density was no longer increasing, and their physicians did not realize that this is the expected pattern. The spinal bone density continues to increase modestly, but some of this may be from disk space narrowing, harder bone edges, and soft-tissue calcifications. Spinal bone density frequently increases even in those on placebo.
Bisphosphonates suppress markers of bone turnover
These changes in bone remodeling with bisphosphonates are reflected by changes in markers of bone formation and resorption. The levels of markers of bone resorption—N-telopeptide cross-linked type I collagen (NTx) and C-telopeptide cross-linked type I collagen (CTx)—decrease rapidly and remain low. The markers of bone formation—propeptide of type I collagen, bone alkaline phosphatase, and osteocalcin—decrease gradually over 3 to 6 months and then remain low. As measured directly at the bone, bone formation appears to be more suppressed than as measured by biochemical markers in the serum.
In a risedronate trial,11 the fracture rate decreased as the biochemical markers of bone turnover decreased, except when the markers were very low, in which case the fracture rate increased.
Without remodeling, cracks can accumulate
The bisphosphonates do not significantly increase bone volume, but they prevent microscopic architectural deterioration of the bone, as shown on microscopic computed tomographic imaging.12 This prevents fractures for at least 5 years.
But bisphosphonates may have long-term negative effects. One purpose of bone remodeling is to refresh the bone and to repair the microscopic damage that accumulates within any structure. Without remodeling, cracks can accumulate. Because the development and repair of microcracks is complex, it is difficult to predict what will happen with long-term bisphosphonate use. Studies of biopsies from women taking bisphosphonates long-term are inconsistent: one study found accumulation of microcracks,13 but another did not.8
STUDIES OF LONG-TERM USE: FOCUS ON FRACTURES
For this review, I consider long-term bisphosphonate use to be greater than 5 years, and I will focus on fractures. Bone density is only a surrogate end point. Unfortunately, this fact is often not emphasized in the training of young physicians.
The best illustration of this point was in a randomized clinical trial of fluoride,14 in which the bone density of the treated group increased by 8% per year for 4 years, for a total increase of 32%. This is more than we ever see with current therapies. But the patients had more fractures with fluoride than with placebo. This is because the quality of bone produced after fluoride treatment is poor, and although the bone is denser, it is weaker.
Observational studies of fracture incidence in patients who continued taking bisphosphonates compared with those who stopped provide some weak evidence about long-term effectiveness.
Curtis et al15 found, in 9,063 women who were prescribed bisphosphonates, that those who stopped taking them during the first 2 years had higher rates of hip fracture than compliant patients. Those who took bisphosphonates for 3 years and then stopped had a rate of hip fracture during the next year similar to that of those who continued taking the drugs.
Meijer et al16 used a database in the Netherlands to examine the fracture rates in 14,750 women who started taking a bisphosphonate for osteoporosis between 1996 and 2004. More than half of the women stopped taking the drug during the first year, and they served as the control group. Those who took bisphosphonates for 3 to 4 years had significantly fewer fractures than those who stopped during the first year (odds ratio 0.54). However, those who took them for 5 to 6 years had slightly more fractures than those who took them for less than a year.
Mellström et al17 performed a 2-year uncontrolled extension of a 5-year trial of risedronate that had blinded controls.18 Initially, 407 women were in the risedronate group; 68 completed 7 years.
The vertebral fracture rate in the placebo group was 7.6% per year during years 0 through 3. In the risedronate group, the rate was 4.7% per year during years 0 through 3 and 3.8% per year during years 6 and 7. Nonvertebral fractures occurred in 10.9% of risedronate-treated patients during the first 3 years and in 6% during the last 2 years. Markers of bone turnover remained reduced throughout the 7 years. Bone mineral density of the spine and hip did not change from years 5 to 7. The study did not include those who took risedronate for 5 years and then discontinued it.
Bone et al19 performed a similar, 10-year uncontrolled extension of a 3-year controlled trial of alendronate.20 There were 398 patients randomly assigned to alendronate, and 164 remained in the study for 8 to 10 years.
During years 8 through 10, bone mineral density of the spine increased by about 2%; no change was seen in the hip or total body. The nonvertebral fracture rate was similar in years 0 through 3 and years 6 through 10. Vertebral fractures occurred in approximately 3% of women in the first 3 years and in 9% in the last 5 years.
The FLEX trial: Continuing alendronate vs stopping
Only one study compared continuing a bisphosphonate vs stopping it. The Fracture Intervention Trial Long-Term Extension (FLEX)10 was an extension of the Fracture Intervention Trial (FIT)21,22 of alendronate. I am reviewing this study in detail because it is the only one that randomized patients and was double-blinded.
In the original trial,21,22 3,236 women were in the alendronate group. After a mean of 5 years on alendronate, 1,099 of them were randomized into the alendronate or placebo group.10 Those with T scores lower than −3.5 or who had lost bone density during the first 5 years were excluded.
The bone mineral density of the hip in the placebo group decreased by 3.4%, whereas in the alendronate group it decreased by 1.0%. At the spine, the placebo group gained less than the alendronate group.
Despite these differences in bone density, no significant difference was noted in the rates of all clinical fractures, nonvertebral fractures, vertebral fractures as measured on radiographs taken for the study (“morphometric” fractures, 11.3% vs 9.8%), or in the number of severe vertebral fractures (those with more than a two-grade change on radiography) between those who took alendronate for 10 years and those who took it for 5 years followed by placebo for 5 years.
However, fewer “clinical spine fractures” were observed in the group continuing alendronate (2.4% vs 5.3%). A clinical spine fracture was one diagnosed by the patient’s personal physician.
In FIT, these clinical fractures were painful in 90% of patients, and although the community radiographs were reviewed by a central radiologist, only 73% of the fractures were confirmed by subsequent measurements on the per protocol radiographs done at the study centers. About one-fourth of the morphometric fractures were also clinical fractures.23 Therefore, I think morphometric fractures provide the best evidence about the effects of treatment—ie, that treatment beyond 5 years is not beneficial. Other physicians, however, disagree, emphasizing the 55% reduction in clinical fractures.24
Markers of bone turnover gradually increased after discontinuation but remained lower than baseline even after 5 years without alendronate.10 There were no significant differences in fracture rates between the placebo and alendronate groups in those with baseline bone mineral density T scores less than −2.5.10 Also, after age adjustment, the fracture incidence was similar in the FIT and the FLEX studies.
Several years later, the authors published a post hoc subgroup analysis of these data.25 The patients were divided into six subgroups based on bone density and the presence of vertebral fractures at baseline. This is weak evidence, but I include it because reviews in the literature have emphasized only the positive findings, or have misquoted the data: Schwartz et al stated that in those with T scores of −2.5 or below, the risk of nonvertebral fracture was reduced by 50%25; and Shane26 concluded in an editorial that the use of alendronate for 10 years, rather than for 5 years, was associated with significantly fewer new vertebral fractures and nonvertebral fractures in patients with a bone mineral density T score of −2.5 or below.26
ATYPICAL FEMUR FRACTURES
By March 2011, there were 55 papers describing a total of 283 cases, and about 85 individual cases (listed online in Ott SM. Osteoporosis and Bone Physiology. http://courses.washington.edu/bonephys/opsubtroch.html. Accessed 7/30/2011).
The mean age of the patients was 65, bisphosphonate use was longer than 5 years in 77% of cases, and bilateral fractures were seen in 48%.
The fractures occur with minor trauma, such as tripping, stepping off an elevator, or being jolted by a subway stop, and a disproportionate number of cases involve no trauma. They are often preceded by leg pain, typically in the mid-thigh.
These fractures are characterized by radiographic findings of a transverse fracture, with thickened cortices near the site of the fracture. Often, there is a peak on the cortex that may precede the fracture. These fractures initiate on the lateral side, and it is striking that they occur in the same horizontal plane on the contralateral side.
Radiographs and bone scans show stress fractures on the lateral side of the femur that resemble Looser zones (ie, dark lines seen radiographically). These radiographic features are not typical in osteoporosis but are reminiscent of the stress fractures seen with hypophosphatasia, an inherited disease characterized by severely decreased bone formation.31
Bone biopsy specimens show very low bone formation rates, but this is not a necessary feature. At the fracture site itself there is bone activity. For example, pathologists from St. Louis reviewed all iliac crest bone biopsies from patients seen between 2004 and 2007 who had an unusual cortical fracture while taking a bisphosphonate. An absence of double tetracycline labels was seen in 11 of the 16 patients.32
The first reports were anecdotal cases, then some centers reported systematic surveys of their patients. In a key report, Neviaser et al33 reviewed all low-trauma subtrochanteric fractures in their large hospital and found 20 cases with the atypical radiographic appearance; 19 of the patients in these cases had been taking a bisphosphonate. A similar survey in Australia found 41 cases with atypical radiographic features (out of 79 subtrochanteric low-trauma fractures), and all of the patients had been taking a bisphosphonate.34
By now, more than 230 cases have been reported. The estimated incidence is 1 in 1,000, based on a review of operative cases and radiographs.35
However, just because the drugs are associated with the fractures does not mean they caused the fractures, because the patients who took bisphosphonates were more likely to get a fracture in the first place. This confounding by indication makes it difficult to prove beyond a doubt that bisphosphonates cause atypical fractures.
Further, some studies have found no association between bisphosphonates and subtrochanteric fractures.36,37 These database analyses have relied on the coding of the International Classification of Diseases, Ninth Revision (ICD-9), and not on the examination of radiographs. We reviewed the ability of ICD-9 codes to identify subtrochanteric fractures and found that the predictive ability was only 36%.38 Even for fractures in the correct location, the codes cannot tell which cases have the typical spiral or comminuted fractures seen in osteoporosis and which have the unusual features of the bisphosphonate-associated fractures. Subtrochanteric and shaft fractures are about 10 times less common than hip fractures, and the atypical ones are about 10 times less common than typical ones, so studies based on ICD-9 codes cannot exonerate bisphosphonates.
A report of nearly 15,000 patients from randomized clinical trials did not find a significant incidence of subtrochanteric fractures, but the radiographs were not examined and only 500 of the patients had taken the medication for longer than 5 years.39
A population-based, nested case-control study using a database from Ontario, Canada, found an increased risk of diaphyseal femoral fractures in patients who had taken bisphosphonates longer than 5 years. The study included only women who had started bisphosphonates when they were older than 68, so many of the atypical fractures would have been missed. The investigators did not review the radiographs, so they combined both osteoporotic and atypical diaphyseal fractures in their analysis.40
At the 2010 meeting of the American Society for Bone and Mineral Research, preliminary data were presented from a systematic review of radiographs of patients with fractures of the femur from a health care plan with data about the use of medications. The incidence of atypical fractures increased progressively with the duration of bisphosphonate use, and was significantly higher after 5 years compared with less than 3 years.28
OTHER POSSIBLE ADVERSE EFFECTS
There have been conflicting reports about esophageal cancer with bisphosphonate use.41,42
Another possible adverse effect, osteonecrosis of the jaw, may have occurred in 1.4% of patients with cancer who were treated for 3 years with high intravenous doses of bisphosphonates (about 10 to 12 times the doses recommended for osteoporosis).43 This adverse effect is rare in patients with osteoporosis, occurring in less than 1 in 10,000 exposed patients.44
BISPHOSPHONATES SHOULD BE USED WHEN THEY ARE INDICATED
The focus of this paper is on the duration of use, but concern about long-term use should not discourage physicians or patients from using these drugs when there is a high risk of an osteoporotic fracture within the next 10 years, particularly in elderly patients who have experienced a vertebral compression fracture or a hip fracture. Patients with a vertebral fracture have a one-in-five chance of fracturing another vertebra, which is a far higher risk than any of the known long-term side effects from treatment, and bisphosphonates are effective at reducing the risk.
Low bone density alone can be used as an indication for bisphosphonates if the hip T score is lower than −2.5. A cost-effectiveness study concluded that alendronate was beneficial in these cases.45 In the FIT patients without a vertebral fracture at baseline, the overall fracture rate was significantly decreased by 36% with alendronate in those with a hip T score lower than −2.5, but there was no difference between placebo and alendronate in those with T scores between −2 and −2.5, and a 14% (nonsignificant) higher fracture rate when the T score was better than −2.0.22
A new method of calculating the risk of an osteoporotic fracture is the FRAX prediction tool (http://www.shef.ac.uk/FRAX), and one group has suggested that treatment is indicated when the 10-year risk of a hip fracture is greater than 3%.46 Another group, from the United Kingdom, suggests using a sliding scale depending on the fracture risk and the age.47
It is not always clear what to do when the hip fracture risk is greater than 3% for the next decade but the T score is better than −2.5. These patients have other factors that contribute to fracture risk. Their therapy must be individualized, and if they are at risk of fracture because of low weight, smoking, or alcohol use, it makes more sense to focus the approach on those treatable factors.
Women who have osteopenia and have not had a fragility fracture are often treated with bisphosphonates with the intent of preventing osteoporosis in the distant future. This approach is based on hope, not evidence, and several editorial reviews have concluded that these women do not need drug therapy.48–50
MY RECOMMENDATION: STOP AFTER 5 YEARS
Bisphosphonates reduce the incidence of devastating osteoporotic fractures in patients with osteoporosis, but that does not mean they should be used indefinitely.
After 5 years, the overall fracture risk is the same in patients who keep taking bisphosphonates as in patients who discontinue them. Therefore, I think these drugs are no longer necessary after 5 years. The post hoc subgroup analysis that showed benefit in only one of six groups of the FLEX study does not provide compelling evidence to continue taking bisphosphonates.
While awaiting better studies, we use the approach shown in the algorithm in Figure 4.
Follow the patient with bone resorption markers
In patients who have shown some improvement in bone density during 5 years of bisphosphonate treatment and who have not had any fractures, I measure a marker of bone resorption at the end of 5 years.
The use of a biochemical marker to assess patients treated with anti-turnover drugs has not been studied in a formal trial, so we have no grade A evidence for recommending it. However, there have been many papers describing the effects of bisphosphonates on these markers, and it makes physiologic sense to use them in situations where decisions must be made when there is not enough evidence.
In FIT (a trial of alendronate), we reported that the change in bone turnover markers was significantly related to the reduction in fracture risk, and the effect was at least as strong as that observed with a 1-year change in bone density. Those with a 30% decrease in bone alkaline phosphatase had a significant reduction in fracture risk.51
Furthermore, in those patients who were compliant with bisphosphonate treatment, the reduction in fractures with alendronate treatment was significantly better in those who initially had a high bone turnover.52
Similarly, with risedronate, the change in NTx accounted for half of the effect on fracture reduction during the clinical trial, and there was little further improvement in fracture benefit below a decrease of 35% to 40%.10
The baseline NTx level in these clinical trials was about 70 nmol bone collagen equivalents per millimole of creatinine (nmol BCE/mmol Cr) in the risedronate study and 60 in the alendronate study, and in both the fracture reduction was seen at a level of about 40. The FLEX study measured NTx after 5 years, and the average was 19 nmol BCE/mmol Cr. This increased to 22 after 3 years without alendronate.53 At 5 years, the turnover markers had gradually increased but were still 7% to 24% lower than baseline.10
These markers have a diurnal rhythm and daily variation, but despite these limitations they do help identify low bone resorption.
In our hospital, NTx is the most economical marker, and my patients prefer a urine sample to a blood test. Therefore, we measure the NTx and consider values lower than 40 nmol BCE/mmol Cr to be satisfactory.
If the NTx is as low as expected, I discontinue the bisphosphonate. The patient remains on 1,200 mg/day of calcium and 1,000 U/day vitamin D supplementation and is encouraged to exercise.
Bone density tends to be stable for 1 or 2 years after stopping a bisphosphonate, and the biochemical markers of bone resorption remain reduced for several years. We remeasure the urine NTx level annually, and if it increases to more than 40 nmol BCE/mmol Cr an antiresorptive medication is given: either the bisphosphonate is restarted or raloxifene (Evista), calcitonin (Miacalcin), or denosumab (Prolia) is used.
Bone density is less helpful, but reassuring
Bone density is less helpful because it decreases even though the markers of bone resorption remain low. Although one could argue that bone density is not helpful in monitoring patients on therapy, I think it is reassuring to know the patient is not excessively losing bone.
Checking at 2-year intervals is reasonable. If the bone density shows a consistent decrease greater than 6% (which is greater than the difference we can see from patients walking around the room), then we would re-evaluate the patient and consider adding another medication.
If the bone density decreases but the biomarkers are low, then clinical judgment must be used. The bone density result may be erroneous due to different positioning or different regions of interest.
If turnover markers are not reduced
If a patient has been prescribed a bisphosphonate for 5 years but the NTx level is not reduced, I reevaluate the patient. Some are not taking the medication or are not taking it properly. The absorption of oral bisphosphonates is quite low in terms of bioavailability, and this decreases to nearly zero if the medication is taken with food. Some patients may have another disease, such as hyperparathyroidism, malignancy, hyperthyroidism, weight loss, malabsorption, celiac sprue, or vitamin D deficiency.
If repeated biochemical tests show high bone resorption and if the bone density response is suboptimal without a secondary cause, I often switch to an intravenous form of bisphosphonate because some patients do not seem to absorb the oral doses.
If a patient has had a fracture
If a patient has had a fracture despite several years of bisphosphonate therapy, I first check for any other medical problems. The bone markers are, unfortunately, not very helpful because they increase after a fracture and stay elevated for at least 4 months.54 If there are no contraindications, treatment with teriparatide (Forteo) is a reasonable choice. There is evidence from human biopsy studies that teriparatide can reduce the number of microcracks that were related to bisphosphonate treatment,13 and can increase the bone formation rate even when there has been prior bisphosphonate treatment.55–57 Although the anabolic response is blunted, it is still there.58
If the patient remains at high risk
A frail patient with a high risk of fracture presents a challenge, especially one who needs treatment with glucocorticoids or who still has a hip T score below −3. Many physicians are uneasy about discontinuing all osteoporosis-specific drugs, even after 5 years of successful bisphosphonate treatment. In these patients anabolic medications make the most sense. Currently, teriparatide is the only one available, but others are being developed. Bone becomes resistant to the anabolic effects of teriparatide after about 18 months, so this drug cannot be used indefinitely. What we really need are longer-lasting anabolic medicines!
If the patient has thigh pain
Finally, in patients with thigh pain, radiography of the femur should be done to check for a stress fracture. Magnetic resonance imaging or computed tomography may be needed to diagnose a hairline fracture.
If there are already radiographic changes that precede the atypical fractures, then bisphosphonates should be discontinued. In a follow-up observational study of 16 patients who already had one fracture, all four whose contralateral side showed a fracture line (the “dreaded black line”) eventually completed the fracture.59
Another study found that five of six incomplete fractures went on to a complete fracture if not surgically stabilized with rods.60 This is an indication for prophylactic rodding of the femur.
Teriparatide use and rodding of a femur with thickening but not a fracture line must be decided on an individual basis and should be considered more strongly in those with pain in the thigh.
- Francis MD, Valent DJ. Historical perspectives on the clinical development of bisphosphonates in the treatment of bone diseases. J Musculoskelet Neuronal Interact 2007; 7:2–8.
- Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med 2009; 122(suppl 2):S14–S21.
- Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res 2011; 26:3–11.
- Russell RG, Xia Z, Dunford JE, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci 2007; 1117:209–257.
- Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone 2002; 30:807–809.
- Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res 1997; 12:498–508.
- Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest 1997; 100:1475–1480.
- Chapurlat RD, Arlot M, Burt-Pichat B, et al. Microcrack frequency and bone remodeling in postmenopausal osteoporotic women on long-term bisphosphonates: a bone biopsy study. J Bone Miner Res 2007; 22:1502–1509.
- Boivin G, Meunier PJ. Effects of bisphosphonates on matrix mineralization. J Musculoskelet Neuronal Interact 2002; 2:538–543.
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- Eastell R, Hannon RA, Garnero P, Campbell MJ, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate: review of statistical analysis. J Bone Miner Res 2007; 22:1656–1660.
- Borah B, Dufresne TE, Chmielewski PA, Johnson TD, Chines A, Manhart MD. Risedronate preserves bone architecture in postmenopausal women with osteoporosis as measured by three-dimensional microcomputed tomography. Bone 2004; 34:736–746.
- Stepan JJ, Dobnig H, Burr DB, et al. Histomorphometric changes by teriparatide in alendronate pre-treated women with osteoporosis (abstract). Presented at the Annual Meeting of the American Society of Bone and Mineral Research, Montreal 2008: #1019.
- Riggs BL, Hodgson SF, O’Fallon WM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990; 322:802–809.
- Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int 2008; 19:1613–1620.
- Meijer WM, Penning-van Beest FJ, Olson M, Herings RM. Relationship between duration of compliant bisphosphonate use and the risk of osteoporotic fractures. Curr Med Res Opin 2008; 24:3217–3222.
- Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004; 75:462–468.
- Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11:83–91.
- Bone HG, Hosking D, Devogelaer JP, et al; Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004; 350:1189–1199.
- Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995; 333:1437–1443.
- Black DM, Cummings SR, Karpf DB, et al; Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996; 348:1535–1541.
- Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998; 280:2077–2082.
- Fink HA, Milavetz DL, Palermo L, et al. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res 2005; 20:1216–1222.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 2010; 95:1555–1565.
- Schwartz AV, Bauer DC, Cummings SR, et al; FLEX Research Group. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res 2010; 25:976–982.
- Shane E. Evolving data about subtrochanteric fractures and bisphosphonates (editorial). N Engl J Med 2010; 362:1825–1827.
- Sellmeyer DE. Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA 2010; 304:1480–1484.
- Shane E, Burr D, Ebeling PR, et al; American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010; 25:2267–2294.
- Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone 2010; 47:169–180.
- Rizzoli R, Akesson K, Bouxsein M, et al. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int 2011; 22:373–390.
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24:1132–1134.
- Armamento-Villareal R, Napoli N, Panwar V, Novack D. Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med 2006; 355:2048–2050.
- Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 2008; 22:346–350.
- Isaacs JD, Shidiak L, Harris IA, Szomor ZL. Femoral insufficiency fractures associated with prolonged bisphosphonate therapy. Clin Orthop Relat Res 2010; 468:3384–3392.
- Schilcher J, Aspenberg P. Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate. Acta Orthop 2009; 80:413–415.
- Abrahamsen B, Eiken P, Eastell R. Cumulative alendronate dose and the long-term absolute risk of subtrochanteric and diaphyseal femur fractures: a register-based national cohort analysis. J Clin Endocrinol Metab 2010; 95:5258–5265.
- Kim SY, Schneeweiss S, Katz JN, Levin R, Solomon DH. Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort. J Bone Miner Res 2010. [Epub ahead of print]
- Spangler L, Ott SM, Scholes D. Utility of automated data in identifying femoral shaft and subtrochanteric (diaphyseal) fractures. Osteoporos Int. 2010. [Epub ahead of print]
- Black DM, Kelly MP, Genant HK, et al; Fracture Intervention Trial Steering Committee; HORIZON Pivotal Fracture Trial Steering Committee. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 2010; 362:1761–1771.
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305:783–789.
- Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ 2010; 341:c4444.
- Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA 2010; 304:657–663.
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010; 28:5132–5139.
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22:1479–1491.
- Schousboe JT, Ensrud KE, Nyman JA, Kane RL, Melton LJ. Cost-effectiveness of vertebral fracture assessment to detect prevalent vertebral deformity and select postmenopausal women with a femoral neck T-score > −2.5 for alendronate therapy: a modeling study. J Clin Densitom 2006; 9:133–143.
- Dawson-Hughes B; National Osteoporosis Foundation Guide Committee. A revised clinician’s guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab 2008; 93:2463–2465.
- Compston J, Cooper A, Cooper C, et al; the National Osteoporosis Guideline Group (NOGG). Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas 2009; 62:105–108.
- Cummings SR. A 55-year-old woman with osteopenia. JAMA 2006; 296:2601–2610.
- Khosla S, Melton LJ. Clinical practice. Osteopenia. N Engl J Med 2007; 356:2293–2300.
- McClung MR. Osteopenia: to treat or not to treat? Ann Intern Med 2005; 142:796–797.
- Bauer DC, Black DM, Garnero P, et al; Fracture Intervention Trial Study Group. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 2004; 19:1250–1258.
- Bauer DC, Garnero P, Hochberg MC, et al; for the Fracture Intervention Research Group. Pretreatment levels of bone turnover and the anti-fracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 2006; 21:292–299.
- Ensrud KE, Barrett-Connor EL, Schwartz A, et al; Fracture Intervention Trial Long-Term Extension Research Group. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res 2004; 19:1259–1269.
- Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res 2007; 22:1155–1164.
- Cosman F, Nieves JW, Zion M, Barbuto N, Lindsay R. Retreatment with teriparatide one year after the first teriparatide course in patients on continued long-term alendronate. J Bone Miner Res 2009; 24:1110–1115.
- Jobke B, Pfeifer M, Minne HW. Teriparatide following bisphosphonates: initial and long-term effects on microarchitecture and bone remodeling at the human iliac crest. Connect Tissue Res 2009; 50:46–54.
- Miller PD, Delmas PD, Lindsay R, et al; Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide Investigators. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 2008; 93:3785–3793.
- Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004; 19:745–751.
- Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma 2010; 24:75–81.
- Banffy MB, Vrahas MS, Ready JE, Abraham JA. Nonoperative versus prophylactic treatment of bisphosphonate-associated femoral stress fractures. Clin Orthop Relat Res 2011; 469:2028–2034.
Almost all the data about the safety and efficacy of bisphosphonate drugs for treating osteoporosis are from patients who took them for less than 5 years.
Reports of adverse effects with prolonged use have caused concern about the long-term safety of this class of drugs. This is particularly important because these drugs are retained in the skeleton longer than 10 years, because there are physiologic reasons why excessive bisphosphonate-induced inhibition of bone turnover could be damaging, and because many healthy postmenopausal women have been prescribed bisphosphonates in the hope of preventing fractures that are not expected to occur for 20 to 30 years.
Because information from trials is scant, opinions differ over whether bisphosphonates should be continued indefinitely. In this article, I summarize the physiologic mechanisms of these drugs, review the scant existing data about their effects beyond 5 years, and describe my approach to bisphosphonate therapy (while waiting for better evidence).
MORE THAN 4 MILLION WOMEN TAKE BISPHOSPHONATES
The first medical use of a bisphosphonate was in 1967, when a girl with myositis ossificans was given etidronate (Didronel) because it inhibited mineralization. Two years later, it was given to patients with Paget disease of bone because it was found to inhibit bone resorption.1 Etidronate could not be given for longer than 6 months, however, because patients developed osteomalacia.
Adding a nitrogen to the molecule dramatically increased its potency and led to the second generation of bisphosphonates. Alendronate (Fosamax), the first amino-bisphosphonate, became available in 1995, It was followed by risedronate (Actonel), ibandronate (Boniva), and zoledronic acid (Reclast). These drugs are potent inhibitors of bone resorption; however, in clinical doses they do not inhibit mineralization and therefore do not cause osteomalacia.
Randomized clinical trials involving more than 30,000 patients have provided grade A evidence that these drugs reduce the incidence of fragility fractures in patients with osteoporosis.2 Furthermore, observational studies have confirmed that they prevent fractures and have a good safety profile in clinical practice.
Therefore, the use of these drugs has become common. In 2008, an estimated 4 million women in the United States were taking them.3
BISPHOSPHONATES STRENGTHEN BONE BY INHIBITING RESORPTION
On a molecular level, bisphosphonates inhibit farnesyl pyrophosphate synthase, an enzyme necessary for formation of the cytoskeleton in osteoclasts. Thus, they strongly inhibit bone resorption. They do not appear to directly inhibit osteoblasts, the cells that form new bone, but they substantially decrease bone formation indirectly.4
To understand how inhibition of bone resorption affects bone physiology, it is necessary to appreciate the nature of bone remodeling. Bone is not like the skin, which is continually forming a new layer and sloughing off the old. Instead, bone is renewed in small units. It takes about 5 years to remodel cancellous bone and 13 years to remodel cortical bone5; at any one time, about 8% of the surface is being remodeled.
The first step occurs at a spot on the surface, where the osteoclasts resorb some bone to form a pit that looks like a pothole. Then a team of osteoblasts is formed and fills the pit with new bone over the next 3 to 6 months. When first formed, the new bone is mainly collagen and, like the tip of the nose, is not very stiff, but with mineral deposition the bone becomes stronger, like the bridge of the nose. The new bone gradually accumulates mineral and becomes harder and denser over the next 3 years.
When a bisphosphonate is given, the osteoclasts abruptly stop resorbing the bone, but osteoblasts continue to fill the pits that were there when the bisphosphonate was started. For the next several months, while the previous pits are being filled, the bone volume increases slightly. Thereafter, rates of both bone resorption and bone formation are very low.
A misconception: Bisphosphonates build bone
While semantically it is true that the bone formation rate in patients taking bisphosphonates is within the normal premenopausal range, this often-repeated statement is essentially misleading.
With continued bisphosphonate use, the bone gradually becomes more dense. There is no further new bone, but the existing bone matrix is packed more tightly with mineral crystals.9 The old bone is not resorbed. The bone density, measured radiographically, increases most rapidly during the first 6 months (while resorption pits are filling in) and more gradually over the next 3 years (while bone is becoming more mineralized).
Another common misunderstanding is that the bone density increases because the drugs are “building bone.” After 3 years, the bone density in the femur reaches a plateau.10 I have seen patients who were very worried because their bone density was no longer increasing, and their physicians did not realize that this is the expected pattern. The spinal bone density continues to increase modestly, but some of this may be from disk space narrowing, harder bone edges, and soft-tissue calcifications. Spinal bone density frequently increases even in those on placebo.
Bisphosphonates suppress markers of bone turnover
These changes in bone remodeling with bisphosphonates are reflected by changes in markers of bone formation and resorption. The levels of markers of bone resorption—N-telopeptide cross-linked type I collagen (NTx) and C-telopeptide cross-linked type I collagen (CTx)—decrease rapidly and remain low. The markers of bone formation—propeptide of type I collagen, bone alkaline phosphatase, and osteocalcin—decrease gradually over 3 to 6 months and then remain low. As measured directly at the bone, bone formation appears to be more suppressed than as measured by biochemical markers in the serum.
In a risedronate trial,11 the fracture rate decreased as the biochemical markers of bone turnover decreased, except when the markers were very low, in which case the fracture rate increased.
Without remodeling, cracks can accumulate
The bisphosphonates do not significantly increase bone volume, but they prevent microscopic architectural deterioration of the bone, as shown on microscopic computed tomographic imaging.12 This prevents fractures for at least 5 years.
But bisphosphonates may have long-term negative effects. One purpose of bone remodeling is to refresh the bone and to repair the microscopic damage that accumulates within any structure. Without remodeling, cracks can accumulate. Because the development and repair of microcracks is complex, it is difficult to predict what will happen with long-term bisphosphonate use. Studies of biopsies from women taking bisphosphonates long-term are inconsistent: one study found accumulation of microcracks,13 but another did not.8
STUDIES OF LONG-TERM USE: FOCUS ON FRACTURES
For this review, I consider long-term bisphosphonate use to be greater than 5 years, and I will focus on fractures. Bone density is only a surrogate end point. Unfortunately, this fact is often not emphasized in the training of young physicians.
The best illustration of this point was in a randomized clinical trial of fluoride,14 in which the bone density of the treated group increased by 8% per year for 4 years, for a total increase of 32%. This is more than we ever see with current therapies. But the patients had more fractures with fluoride than with placebo. This is because the quality of bone produced after fluoride treatment is poor, and although the bone is denser, it is weaker.
Observational studies of fracture incidence in patients who continued taking bisphosphonates compared with those who stopped provide some weak evidence about long-term effectiveness.
Curtis et al15 found, in 9,063 women who were prescribed bisphosphonates, that those who stopped taking them during the first 2 years had higher rates of hip fracture than compliant patients. Those who took bisphosphonates for 3 years and then stopped had a rate of hip fracture during the next year similar to that of those who continued taking the drugs.
Meijer et al16 used a database in the Netherlands to examine the fracture rates in 14,750 women who started taking a bisphosphonate for osteoporosis between 1996 and 2004. More than half of the women stopped taking the drug during the first year, and they served as the control group. Those who took bisphosphonates for 3 to 4 years had significantly fewer fractures than those who stopped during the first year (odds ratio 0.54). However, those who took them for 5 to 6 years had slightly more fractures than those who took them for less than a year.
Mellström et al17 performed a 2-year uncontrolled extension of a 5-year trial of risedronate that had blinded controls.18 Initially, 407 women were in the risedronate group; 68 completed 7 years.
The vertebral fracture rate in the placebo group was 7.6% per year during years 0 through 3. In the risedronate group, the rate was 4.7% per year during years 0 through 3 and 3.8% per year during years 6 and 7. Nonvertebral fractures occurred in 10.9% of risedronate-treated patients during the first 3 years and in 6% during the last 2 years. Markers of bone turnover remained reduced throughout the 7 years. Bone mineral density of the spine and hip did not change from years 5 to 7. The study did not include those who took risedronate for 5 years and then discontinued it.
Bone et al19 performed a similar, 10-year uncontrolled extension of a 3-year controlled trial of alendronate.20 There were 398 patients randomly assigned to alendronate, and 164 remained in the study for 8 to 10 years.
During years 8 through 10, bone mineral density of the spine increased by about 2%; no change was seen in the hip or total body. The nonvertebral fracture rate was similar in years 0 through 3 and years 6 through 10. Vertebral fractures occurred in approximately 3% of women in the first 3 years and in 9% in the last 5 years.
The FLEX trial: Continuing alendronate vs stopping
Only one study compared continuing a bisphosphonate vs stopping it. The Fracture Intervention Trial Long-Term Extension (FLEX)10 was an extension of the Fracture Intervention Trial (FIT)21,22 of alendronate. I am reviewing this study in detail because it is the only one that randomized patients and was double-blinded.
In the original trial,21,22 3,236 women were in the alendronate group. After a mean of 5 years on alendronate, 1,099 of them were randomized into the alendronate or placebo group.10 Those with T scores lower than −3.5 or who had lost bone density during the first 5 years were excluded.
The bone mineral density of the hip in the placebo group decreased by 3.4%, whereas in the alendronate group it decreased by 1.0%. At the spine, the placebo group gained less than the alendronate group.
Despite these differences in bone density, no significant difference was noted in the rates of all clinical fractures, nonvertebral fractures, vertebral fractures as measured on radiographs taken for the study (“morphometric” fractures, 11.3% vs 9.8%), or in the number of severe vertebral fractures (those with more than a two-grade change on radiography) between those who took alendronate for 10 years and those who took it for 5 years followed by placebo for 5 years.
However, fewer “clinical spine fractures” were observed in the group continuing alendronate (2.4% vs 5.3%). A clinical spine fracture was one diagnosed by the patient’s personal physician.
In FIT, these clinical fractures were painful in 90% of patients, and although the community radiographs were reviewed by a central radiologist, only 73% of the fractures were confirmed by subsequent measurements on the per protocol radiographs done at the study centers. About one-fourth of the morphometric fractures were also clinical fractures.23 Therefore, I think morphometric fractures provide the best evidence about the effects of treatment—ie, that treatment beyond 5 years is not beneficial. Other physicians, however, disagree, emphasizing the 55% reduction in clinical fractures.24
Markers of bone turnover gradually increased after discontinuation but remained lower than baseline even after 5 years without alendronate.10 There were no significant differences in fracture rates between the placebo and alendronate groups in those with baseline bone mineral density T scores less than −2.5.10 Also, after age adjustment, the fracture incidence was similar in the FIT and the FLEX studies.
Several years later, the authors published a post hoc subgroup analysis of these data.25 The patients were divided into six subgroups based on bone density and the presence of vertebral fractures at baseline. This is weak evidence, but I include it because reviews in the literature have emphasized only the positive findings, or have misquoted the data: Schwartz et al stated that in those with T scores of −2.5 or below, the risk of nonvertebral fracture was reduced by 50%25; and Shane26 concluded in an editorial that the use of alendronate for 10 years, rather than for 5 years, was associated with significantly fewer new vertebral fractures and nonvertebral fractures in patients with a bone mineral density T score of −2.5 or below.26
ATYPICAL FEMUR FRACTURES
By March 2011, there were 55 papers describing a total of 283 cases, and about 85 individual cases (listed online in Ott SM. Osteoporosis and Bone Physiology. http://courses.washington.edu/bonephys/opsubtroch.html. Accessed 7/30/2011).
The mean age of the patients was 65, bisphosphonate use was longer than 5 years in 77% of cases, and bilateral fractures were seen in 48%.
The fractures occur with minor trauma, such as tripping, stepping off an elevator, or being jolted by a subway stop, and a disproportionate number of cases involve no trauma. They are often preceded by leg pain, typically in the mid-thigh.
These fractures are characterized by radiographic findings of a transverse fracture, with thickened cortices near the site of the fracture. Often, there is a peak on the cortex that may precede the fracture. These fractures initiate on the lateral side, and it is striking that they occur in the same horizontal plane on the contralateral side.
Radiographs and bone scans show stress fractures on the lateral side of the femur that resemble Looser zones (ie, dark lines seen radiographically). These radiographic features are not typical in osteoporosis but are reminiscent of the stress fractures seen with hypophosphatasia, an inherited disease characterized by severely decreased bone formation.31
Bone biopsy specimens show very low bone formation rates, but this is not a necessary feature. At the fracture site itself there is bone activity. For example, pathologists from St. Louis reviewed all iliac crest bone biopsies from patients seen between 2004 and 2007 who had an unusual cortical fracture while taking a bisphosphonate. An absence of double tetracycline labels was seen in 11 of the 16 patients.32
The first reports were anecdotal cases, then some centers reported systematic surveys of their patients. In a key report, Neviaser et al33 reviewed all low-trauma subtrochanteric fractures in their large hospital and found 20 cases with the atypical radiographic appearance; 19 of the patients in these cases had been taking a bisphosphonate. A similar survey in Australia found 41 cases with atypical radiographic features (out of 79 subtrochanteric low-trauma fractures), and all of the patients had been taking a bisphosphonate.34
By now, more than 230 cases have been reported. The estimated incidence is 1 in 1,000, based on a review of operative cases and radiographs.35
However, just because the drugs are associated with the fractures does not mean they caused the fractures, because the patients who took bisphosphonates were more likely to get a fracture in the first place. This confounding by indication makes it difficult to prove beyond a doubt that bisphosphonates cause atypical fractures.
Further, some studies have found no association between bisphosphonates and subtrochanteric fractures.36,37 These database analyses have relied on the coding of the International Classification of Diseases, Ninth Revision (ICD-9), and not on the examination of radiographs. We reviewed the ability of ICD-9 codes to identify subtrochanteric fractures and found that the predictive ability was only 36%.38 Even for fractures in the correct location, the codes cannot tell which cases have the typical spiral or comminuted fractures seen in osteoporosis and which have the unusual features of the bisphosphonate-associated fractures. Subtrochanteric and shaft fractures are about 10 times less common than hip fractures, and the atypical ones are about 10 times less common than typical ones, so studies based on ICD-9 codes cannot exonerate bisphosphonates.
A report of nearly 15,000 patients from randomized clinical trials did not find a significant incidence of subtrochanteric fractures, but the radiographs were not examined and only 500 of the patients had taken the medication for longer than 5 years.39
A population-based, nested case-control study using a database from Ontario, Canada, found an increased risk of diaphyseal femoral fractures in patients who had taken bisphosphonates longer than 5 years. The study included only women who had started bisphosphonates when they were older than 68, so many of the atypical fractures would have been missed. The investigators did not review the radiographs, so they combined both osteoporotic and atypical diaphyseal fractures in their analysis.40
At the 2010 meeting of the American Society for Bone and Mineral Research, preliminary data were presented from a systematic review of radiographs of patients with fractures of the femur from a health care plan with data about the use of medications. The incidence of atypical fractures increased progressively with the duration of bisphosphonate use, and was significantly higher after 5 years compared with less than 3 years.28
OTHER POSSIBLE ADVERSE EFFECTS
There have been conflicting reports about esophageal cancer with bisphosphonate use.41,42
Another possible adverse effect, osteonecrosis of the jaw, may have occurred in 1.4% of patients with cancer who were treated for 3 years with high intravenous doses of bisphosphonates (about 10 to 12 times the doses recommended for osteoporosis).43 This adverse effect is rare in patients with osteoporosis, occurring in less than 1 in 10,000 exposed patients.44
BISPHOSPHONATES SHOULD BE USED WHEN THEY ARE INDICATED
The focus of this paper is on the duration of use, but concern about long-term use should not discourage physicians or patients from using these drugs when there is a high risk of an osteoporotic fracture within the next 10 years, particularly in elderly patients who have experienced a vertebral compression fracture or a hip fracture. Patients with a vertebral fracture have a one-in-five chance of fracturing another vertebra, which is a far higher risk than any of the known long-term side effects from treatment, and bisphosphonates are effective at reducing the risk.
Low bone density alone can be used as an indication for bisphosphonates if the hip T score is lower than −2.5. A cost-effectiveness study concluded that alendronate was beneficial in these cases.45 In the FIT patients without a vertebral fracture at baseline, the overall fracture rate was significantly decreased by 36% with alendronate in those with a hip T score lower than −2.5, but there was no difference between placebo and alendronate in those with T scores between −2 and −2.5, and a 14% (nonsignificant) higher fracture rate when the T score was better than −2.0.22
A new method of calculating the risk of an osteoporotic fracture is the FRAX prediction tool (http://www.shef.ac.uk/FRAX), and one group has suggested that treatment is indicated when the 10-year risk of a hip fracture is greater than 3%.46 Another group, from the United Kingdom, suggests using a sliding scale depending on the fracture risk and the age.47
It is not always clear what to do when the hip fracture risk is greater than 3% for the next decade but the T score is better than −2.5. These patients have other factors that contribute to fracture risk. Their therapy must be individualized, and if they are at risk of fracture because of low weight, smoking, or alcohol use, it makes more sense to focus the approach on those treatable factors.
Women who have osteopenia and have not had a fragility fracture are often treated with bisphosphonates with the intent of preventing osteoporosis in the distant future. This approach is based on hope, not evidence, and several editorial reviews have concluded that these women do not need drug therapy.48–50
MY RECOMMENDATION: STOP AFTER 5 YEARS
Bisphosphonates reduce the incidence of devastating osteoporotic fractures in patients with osteoporosis, but that does not mean they should be used indefinitely.
After 5 years, the overall fracture risk is the same in patients who keep taking bisphosphonates as in patients who discontinue them. Therefore, I think these drugs are no longer necessary after 5 years. The post hoc subgroup analysis that showed benefit in only one of six groups of the FLEX study does not provide compelling evidence to continue taking bisphosphonates.
While awaiting better studies, we use the approach shown in the algorithm in Figure 4.
Follow the patient with bone resorption markers
In patients who have shown some improvement in bone density during 5 years of bisphosphonate treatment and who have not had any fractures, I measure a marker of bone resorption at the end of 5 years.
The use of a biochemical marker to assess patients treated with anti-turnover drugs has not been studied in a formal trial, so we have no grade A evidence for recommending it. However, there have been many papers describing the effects of bisphosphonates on these markers, and it makes physiologic sense to use them in situations where decisions must be made when there is not enough evidence.
In FIT (a trial of alendronate), we reported that the change in bone turnover markers was significantly related to the reduction in fracture risk, and the effect was at least as strong as that observed with a 1-year change in bone density. Those with a 30% decrease in bone alkaline phosphatase had a significant reduction in fracture risk.51
Furthermore, in those patients who were compliant with bisphosphonate treatment, the reduction in fractures with alendronate treatment was significantly better in those who initially had a high bone turnover.52
Similarly, with risedronate, the change in NTx accounted for half of the effect on fracture reduction during the clinical trial, and there was little further improvement in fracture benefit below a decrease of 35% to 40%.10
The baseline NTx level in these clinical trials was about 70 nmol bone collagen equivalents per millimole of creatinine (nmol BCE/mmol Cr) in the risedronate study and 60 in the alendronate study, and in both the fracture reduction was seen at a level of about 40. The FLEX study measured NTx after 5 years, and the average was 19 nmol BCE/mmol Cr. This increased to 22 after 3 years without alendronate.53 At 5 years, the turnover markers had gradually increased but were still 7% to 24% lower than baseline.10
These markers have a diurnal rhythm and daily variation, but despite these limitations they do help identify low bone resorption.
In our hospital, NTx is the most economical marker, and my patients prefer a urine sample to a blood test. Therefore, we measure the NTx and consider values lower than 40 nmol BCE/mmol Cr to be satisfactory.
If the NTx is as low as expected, I discontinue the bisphosphonate. The patient remains on 1,200 mg/day of calcium and 1,000 U/day vitamin D supplementation and is encouraged to exercise.
Bone density tends to be stable for 1 or 2 years after stopping a bisphosphonate, and the biochemical markers of bone resorption remain reduced for several years. We remeasure the urine NTx level annually, and if it increases to more than 40 nmol BCE/mmol Cr an antiresorptive medication is given: either the bisphosphonate is restarted or raloxifene (Evista), calcitonin (Miacalcin), or denosumab (Prolia) is used.
Bone density is less helpful, but reassuring
Bone density is less helpful because it decreases even though the markers of bone resorption remain low. Although one could argue that bone density is not helpful in monitoring patients on therapy, I think it is reassuring to know the patient is not excessively losing bone.
Checking at 2-year intervals is reasonable. If the bone density shows a consistent decrease greater than 6% (which is greater than the difference we can see from patients walking around the room), then we would re-evaluate the patient and consider adding another medication.
If the bone density decreases but the biomarkers are low, then clinical judgment must be used. The bone density result may be erroneous due to different positioning or different regions of interest.
If turnover markers are not reduced
If a patient has been prescribed a bisphosphonate for 5 years but the NTx level is not reduced, I reevaluate the patient. Some are not taking the medication or are not taking it properly. The absorption of oral bisphosphonates is quite low in terms of bioavailability, and this decreases to nearly zero if the medication is taken with food. Some patients may have another disease, such as hyperparathyroidism, malignancy, hyperthyroidism, weight loss, malabsorption, celiac sprue, or vitamin D deficiency.
If repeated biochemical tests show high bone resorption and if the bone density response is suboptimal without a secondary cause, I often switch to an intravenous form of bisphosphonate because some patients do not seem to absorb the oral doses.
If a patient has had a fracture
If a patient has had a fracture despite several years of bisphosphonate therapy, I first check for any other medical problems. The bone markers are, unfortunately, not very helpful because they increase after a fracture and stay elevated for at least 4 months.54 If there are no contraindications, treatment with teriparatide (Forteo) is a reasonable choice. There is evidence from human biopsy studies that teriparatide can reduce the number of microcracks that were related to bisphosphonate treatment,13 and can increase the bone formation rate even when there has been prior bisphosphonate treatment.55–57 Although the anabolic response is blunted, it is still there.58
If the patient remains at high risk
A frail patient with a high risk of fracture presents a challenge, especially one who needs treatment with glucocorticoids or who still has a hip T score below −3. Many physicians are uneasy about discontinuing all osteoporosis-specific drugs, even after 5 years of successful bisphosphonate treatment. In these patients anabolic medications make the most sense. Currently, teriparatide is the only one available, but others are being developed. Bone becomes resistant to the anabolic effects of teriparatide after about 18 months, so this drug cannot be used indefinitely. What we really need are longer-lasting anabolic medicines!
If the patient has thigh pain
Finally, in patients with thigh pain, radiography of the femur should be done to check for a stress fracture. Magnetic resonance imaging or computed tomography may be needed to diagnose a hairline fracture.
If there are already radiographic changes that precede the atypical fractures, then bisphosphonates should be discontinued. In a follow-up observational study of 16 patients who already had one fracture, all four whose contralateral side showed a fracture line (the “dreaded black line”) eventually completed the fracture.59
Another study found that five of six incomplete fractures went on to a complete fracture if not surgically stabilized with rods.60 This is an indication for prophylactic rodding of the femur.
Teriparatide use and rodding of a femur with thickening but not a fracture line must be decided on an individual basis and should be considered more strongly in those with pain in the thigh.
Almost all the data about the safety and efficacy of bisphosphonate drugs for treating osteoporosis are from patients who took them for less than 5 years.
Reports of adverse effects with prolonged use have caused concern about the long-term safety of this class of drugs. This is particularly important because these drugs are retained in the skeleton longer than 10 years, because there are physiologic reasons why excessive bisphosphonate-induced inhibition of bone turnover could be damaging, and because many healthy postmenopausal women have been prescribed bisphosphonates in the hope of preventing fractures that are not expected to occur for 20 to 30 years.
Because information from trials is scant, opinions differ over whether bisphosphonates should be continued indefinitely. In this article, I summarize the physiologic mechanisms of these drugs, review the scant existing data about their effects beyond 5 years, and describe my approach to bisphosphonate therapy (while waiting for better evidence).
MORE THAN 4 MILLION WOMEN TAKE BISPHOSPHONATES
The first medical use of a bisphosphonate was in 1967, when a girl with myositis ossificans was given etidronate (Didronel) because it inhibited mineralization. Two years later, it was given to patients with Paget disease of bone because it was found to inhibit bone resorption.1 Etidronate could not be given for longer than 6 months, however, because patients developed osteomalacia.
Adding a nitrogen to the molecule dramatically increased its potency and led to the second generation of bisphosphonates. Alendronate (Fosamax), the first amino-bisphosphonate, became available in 1995, It was followed by risedronate (Actonel), ibandronate (Boniva), and zoledronic acid (Reclast). These drugs are potent inhibitors of bone resorption; however, in clinical doses they do not inhibit mineralization and therefore do not cause osteomalacia.
Randomized clinical trials involving more than 30,000 patients have provided grade A evidence that these drugs reduce the incidence of fragility fractures in patients with osteoporosis.2 Furthermore, observational studies have confirmed that they prevent fractures and have a good safety profile in clinical practice.
Therefore, the use of these drugs has become common. In 2008, an estimated 4 million women in the United States were taking them.3
BISPHOSPHONATES STRENGTHEN BONE BY INHIBITING RESORPTION
On a molecular level, bisphosphonates inhibit farnesyl pyrophosphate synthase, an enzyme necessary for formation of the cytoskeleton in osteoclasts. Thus, they strongly inhibit bone resorption. They do not appear to directly inhibit osteoblasts, the cells that form new bone, but they substantially decrease bone formation indirectly.4
To understand how inhibition of bone resorption affects bone physiology, it is necessary to appreciate the nature of bone remodeling. Bone is not like the skin, which is continually forming a new layer and sloughing off the old. Instead, bone is renewed in small units. It takes about 5 years to remodel cancellous bone and 13 years to remodel cortical bone5; at any one time, about 8% of the surface is being remodeled.
The first step occurs at a spot on the surface, where the osteoclasts resorb some bone to form a pit that looks like a pothole. Then a team of osteoblasts is formed and fills the pit with new bone over the next 3 to 6 months. When first formed, the new bone is mainly collagen and, like the tip of the nose, is not very stiff, but with mineral deposition the bone becomes stronger, like the bridge of the nose. The new bone gradually accumulates mineral and becomes harder and denser over the next 3 years.
When a bisphosphonate is given, the osteoclasts abruptly stop resorbing the bone, but osteoblasts continue to fill the pits that were there when the bisphosphonate was started. For the next several months, while the previous pits are being filled, the bone volume increases slightly. Thereafter, rates of both bone resorption and bone formation are very low.
A misconception: Bisphosphonates build bone
While semantically it is true that the bone formation rate in patients taking bisphosphonates is within the normal premenopausal range, this often-repeated statement is essentially misleading.
With continued bisphosphonate use, the bone gradually becomes more dense. There is no further new bone, but the existing bone matrix is packed more tightly with mineral crystals.9 The old bone is not resorbed. The bone density, measured radiographically, increases most rapidly during the first 6 months (while resorption pits are filling in) and more gradually over the next 3 years (while bone is becoming more mineralized).
Another common misunderstanding is that the bone density increases because the drugs are “building bone.” After 3 years, the bone density in the femur reaches a plateau.10 I have seen patients who were very worried because their bone density was no longer increasing, and their physicians did not realize that this is the expected pattern. The spinal bone density continues to increase modestly, but some of this may be from disk space narrowing, harder bone edges, and soft-tissue calcifications. Spinal bone density frequently increases even in those on placebo.
Bisphosphonates suppress markers of bone turnover
These changes in bone remodeling with bisphosphonates are reflected by changes in markers of bone formation and resorption. The levels of markers of bone resorption—N-telopeptide cross-linked type I collagen (NTx) and C-telopeptide cross-linked type I collagen (CTx)—decrease rapidly and remain low. The markers of bone formation—propeptide of type I collagen, bone alkaline phosphatase, and osteocalcin—decrease gradually over 3 to 6 months and then remain low. As measured directly at the bone, bone formation appears to be more suppressed than as measured by biochemical markers in the serum.
In a risedronate trial,11 the fracture rate decreased as the biochemical markers of bone turnover decreased, except when the markers were very low, in which case the fracture rate increased.
Without remodeling, cracks can accumulate
The bisphosphonates do not significantly increase bone volume, but they prevent microscopic architectural deterioration of the bone, as shown on microscopic computed tomographic imaging.12 This prevents fractures for at least 5 years.
But bisphosphonates may have long-term negative effects. One purpose of bone remodeling is to refresh the bone and to repair the microscopic damage that accumulates within any structure. Without remodeling, cracks can accumulate. Because the development and repair of microcracks is complex, it is difficult to predict what will happen with long-term bisphosphonate use. Studies of biopsies from women taking bisphosphonates long-term are inconsistent: one study found accumulation of microcracks,13 but another did not.8
STUDIES OF LONG-TERM USE: FOCUS ON FRACTURES
For this review, I consider long-term bisphosphonate use to be greater than 5 years, and I will focus on fractures. Bone density is only a surrogate end point. Unfortunately, this fact is often not emphasized in the training of young physicians.
The best illustration of this point was in a randomized clinical trial of fluoride,14 in which the bone density of the treated group increased by 8% per year for 4 years, for a total increase of 32%. This is more than we ever see with current therapies. But the patients had more fractures with fluoride than with placebo. This is because the quality of bone produced after fluoride treatment is poor, and although the bone is denser, it is weaker.
Observational studies of fracture incidence in patients who continued taking bisphosphonates compared with those who stopped provide some weak evidence about long-term effectiveness.
Curtis et al15 found, in 9,063 women who were prescribed bisphosphonates, that those who stopped taking them during the first 2 years had higher rates of hip fracture than compliant patients. Those who took bisphosphonates for 3 years and then stopped had a rate of hip fracture during the next year similar to that of those who continued taking the drugs.
Meijer et al16 used a database in the Netherlands to examine the fracture rates in 14,750 women who started taking a bisphosphonate for osteoporosis between 1996 and 2004. More than half of the women stopped taking the drug during the first year, and they served as the control group. Those who took bisphosphonates for 3 to 4 years had significantly fewer fractures than those who stopped during the first year (odds ratio 0.54). However, those who took them for 5 to 6 years had slightly more fractures than those who took them for less than a year.
Mellström et al17 performed a 2-year uncontrolled extension of a 5-year trial of risedronate that had blinded controls.18 Initially, 407 women were in the risedronate group; 68 completed 7 years.
The vertebral fracture rate in the placebo group was 7.6% per year during years 0 through 3. In the risedronate group, the rate was 4.7% per year during years 0 through 3 and 3.8% per year during years 6 and 7. Nonvertebral fractures occurred in 10.9% of risedronate-treated patients during the first 3 years and in 6% during the last 2 years. Markers of bone turnover remained reduced throughout the 7 years. Bone mineral density of the spine and hip did not change from years 5 to 7. The study did not include those who took risedronate for 5 years and then discontinued it.
Bone et al19 performed a similar, 10-year uncontrolled extension of a 3-year controlled trial of alendronate.20 There were 398 patients randomly assigned to alendronate, and 164 remained in the study for 8 to 10 years.
During years 8 through 10, bone mineral density of the spine increased by about 2%; no change was seen in the hip or total body. The nonvertebral fracture rate was similar in years 0 through 3 and years 6 through 10. Vertebral fractures occurred in approximately 3% of women in the first 3 years and in 9% in the last 5 years.
The FLEX trial: Continuing alendronate vs stopping
Only one study compared continuing a bisphosphonate vs stopping it. The Fracture Intervention Trial Long-Term Extension (FLEX)10 was an extension of the Fracture Intervention Trial (FIT)21,22 of alendronate. I am reviewing this study in detail because it is the only one that randomized patients and was double-blinded.
In the original trial,21,22 3,236 women were in the alendronate group. After a mean of 5 years on alendronate, 1,099 of them were randomized into the alendronate or placebo group.10 Those with T scores lower than −3.5 or who had lost bone density during the first 5 years were excluded.
The bone mineral density of the hip in the placebo group decreased by 3.4%, whereas in the alendronate group it decreased by 1.0%. At the spine, the placebo group gained less than the alendronate group.
Despite these differences in bone density, no significant difference was noted in the rates of all clinical fractures, nonvertebral fractures, vertebral fractures as measured on radiographs taken for the study (“morphometric” fractures, 11.3% vs 9.8%), or in the number of severe vertebral fractures (those with more than a two-grade change on radiography) between those who took alendronate for 10 years and those who took it for 5 years followed by placebo for 5 years.
However, fewer “clinical spine fractures” were observed in the group continuing alendronate (2.4% vs 5.3%). A clinical spine fracture was one diagnosed by the patient’s personal physician.
In FIT, these clinical fractures were painful in 90% of patients, and although the community radiographs were reviewed by a central radiologist, only 73% of the fractures were confirmed by subsequent measurements on the per protocol radiographs done at the study centers. About one-fourth of the morphometric fractures were also clinical fractures.23 Therefore, I think morphometric fractures provide the best evidence about the effects of treatment—ie, that treatment beyond 5 years is not beneficial. Other physicians, however, disagree, emphasizing the 55% reduction in clinical fractures.24
Markers of bone turnover gradually increased after discontinuation but remained lower than baseline even after 5 years without alendronate.10 There were no significant differences in fracture rates between the placebo and alendronate groups in those with baseline bone mineral density T scores less than −2.5.10 Also, after age adjustment, the fracture incidence was similar in the FIT and the FLEX studies.
Several years later, the authors published a post hoc subgroup analysis of these data.25 The patients were divided into six subgroups based on bone density and the presence of vertebral fractures at baseline. This is weak evidence, but I include it because reviews in the literature have emphasized only the positive findings, or have misquoted the data: Schwartz et al stated that in those with T scores of −2.5 or below, the risk of nonvertebral fracture was reduced by 50%25; and Shane26 concluded in an editorial that the use of alendronate for 10 years, rather than for 5 years, was associated with significantly fewer new vertebral fractures and nonvertebral fractures in patients with a bone mineral density T score of −2.5 or below.26
ATYPICAL FEMUR FRACTURES
By March 2011, there were 55 papers describing a total of 283 cases, and about 85 individual cases (listed online in Ott SM. Osteoporosis and Bone Physiology. http://courses.washington.edu/bonephys/opsubtroch.html. Accessed 7/30/2011).
The mean age of the patients was 65, bisphosphonate use was longer than 5 years in 77% of cases, and bilateral fractures were seen in 48%.
The fractures occur with minor trauma, such as tripping, stepping off an elevator, or being jolted by a subway stop, and a disproportionate number of cases involve no trauma. They are often preceded by leg pain, typically in the mid-thigh.
These fractures are characterized by radiographic findings of a transverse fracture, with thickened cortices near the site of the fracture. Often, there is a peak on the cortex that may precede the fracture. These fractures initiate on the lateral side, and it is striking that they occur in the same horizontal plane on the contralateral side.
Radiographs and bone scans show stress fractures on the lateral side of the femur that resemble Looser zones (ie, dark lines seen radiographically). These radiographic features are not typical in osteoporosis but are reminiscent of the stress fractures seen with hypophosphatasia, an inherited disease characterized by severely decreased bone formation.31
Bone biopsy specimens show very low bone formation rates, but this is not a necessary feature. At the fracture site itself there is bone activity. For example, pathologists from St. Louis reviewed all iliac crest bone biopsies from patients seen between 2004 and 2007 who had an unusual cortical fracture while taking a bisphosphonate. An absence of double tetracycline labels was seen in 11 of the 16 patients.32
The first reports were anecdotal cases, then some centers reported systematic surveys of their patients. In a key report, Neviaser et al33 reviewed all low-trauma subtrochanteric fractures in their large hospital and found 20 cases with the atypical radiographic appearance; 19 of the patients in these cases had been taking a bisphosphonate. A similar survey in Australia found 41 cases with atypical radiographic features (out of 79 subtrochanteric low-trauma fractures), and all of the patients had been taking a bisphosphonate.34
By now, more than 230 cases have been reported. The estimated incidence is 1 in 1,000, based on a review of operative cases and radiographs.35
However, just because the drugs are associated with the fractures does not mean they caused the fractures, because the patients who took bisphosphonates were more likely to get a fracture in the first place. This confounding by indication makes it difficult to prove beyond a doubt that bisphosphonates cause atypical fractures.
Further, some studies have found no association between bisphosphonates and subtrochanteric fractures.36,37 These database analyses have relied on the coding of the International Classification of Diseases, Ninth Revision (ICD-9), and not on the examination of radiographs. We reviewed the ability of ICD-9 codes to identify subtrochanteric fractures and found that the predictive ability was only 36%.38 Even for fractures in the correct location, the codes cannot tell which cases have the typical spiral or comminuted fractures seen in osteoporosis and which have the unusual features of the bisphosphonate-associated fractures. Subtrochanteric and shaft fractures are about 10 times less common than hip fractures, and the atypical ones are about 10 times less common than typical ones, so studies based on ICD-9 codes cannot exonerate bisphosphonates.
A report of nearly 15,000 patients from randomized clinical trials did not find a significant incidence of subtrochanteric fractures, but the radiographs were not examined and only 500 of the patients had taken the medication for longer than 5 years.39
A population-based, nested case-control study using a database from Ontario, Canada, found an increased risk of diaphyseal femoral fractures in patients who had taken bisphosphonates longer than 5 years. The study included only women who had started bisphosphonates when they were older than 68, so many of the atypical fractures would have been missed. The investigators did not review the radiographs, so they combined both osteoporotic and atypical diaphyseal fractures in their analysis.40
At the 2010 meeting of the American Society for Bone and Mineral Research, preliminary data were presented from a systematic review of radiographs of patients with fractures of the femur from a health care plan with data about the use of medications. The incidence of atypical fractures increased progressively with the duration of bisphosphonate use, and was significantly higher after 5 years compared with less than 3 years.28
OTHER POSSIBLE ADVERSE EFFECTS
There have been conflicting reports about esophageal cancer with bisphosphonate use.41,42
Another possible adverse effect, osteonecrosis of the jaw, may have occurred in 1.4% of patients with cancer who were treated for 3 years with high intravenous doses of bisphosphonates (about 10 to 12 times the doses recommended for osteoporosis).43 This adverse effect is rare in patients with osteoporosis, occurring in less than 1 in 10,000 exposed patients.44
BISPHOSPHONATES SHOULD BE USED WHEN THEY ARE INDICATED
The focus of this paper is on the duration of use, but concern about long-term use should not discourage physicians or patients from using these drugs when there is a high risk of an osteoporotic fracture within the next 10 years, particularly in elderly patients who have experienced a vertebral compression fracture or a hip fracture. Patients with a vertebral fracture have a one-in-five chance of fracturing another vertebra, which is a far higher risk than any of the known long-term side effects from treatment, and bisphosphonates are effective at reducing the risk.
Low bone density alone can be used as an indication for bisphosphonates if the hip T score is lower than −2.5. A cost-effectiveness study concluded that alendronate was beneficial in these cases.45 In the FIT patients without a vertebral fracture at baseline, the overall fracture rate was significantly decreased by 36% with alendronate in those with a hip T score lower than −2.5, but there was no difference between placebo and alendronate in those with T scores between −2 and −2.5, and a 14% (nonsignificant) higher fracture rate when the T score was better than −2.0.22
A new method of calculating the risk of an osteoporotic fracture is the FRAX prediction tool (http://www.shef.ac.uk/FRAX), and one group has suggested that treatment is indicated when the 10-year risk of a hip fracture is greater than 3%.46 Another group, from the United Kingdom, suggests using a sliding scale depending on the fracture risk and the age.47
It is not always clear what to do when the hip fracture risk is greater than 3% for the next decade but the T score is better than −2.5. These patients have other factors that contribute to fracture risk. Their therapy must be individualized, and if they are at risk of fracture because of low weight, smoking, or alcohol use, it makes more sense to focus the approach on those treatable factors.
Women who have osteopenia and have not had a fragility fracture are often treated with bisphosphonates with the intent of preventing osteoporosis in the distant future. This approach is based on hope, not evidence, and several editorial reviews have concluded that these women do not need drug therapy.48–50
MY RECOMMENDATION: STOP AFTER 5 YEARS
Bisphosphonates reduce the incidence of devastating osteoporotic fractures in patients with osteoporosis, but that does not mean they should be used indefinitely.
After 5 years, the overall fracture risk is the same in patients who keep taking bisphosphonates as in patients who discontinue them. Therefore, I think these drugs are no longer necessary after 5 years. The post hoc subgroup analysis that showed benefit in only one of six groups of the FLEX study does not provide compelling evidence to continue taking bisphosphonates.
While awaiting better studies, we use the approach shown in the algorithm in Figure 4.
Follow the patient with bone resorption markers
In patients who have shown some improvement in bone density during 5 years of bisphosphonate treatment and who have not had any fractures, I measure a marker of bone resorption at the end of 5 years.
The use of a biochemical marker to assess patients treated with anti-turnover drugs has not been studied in a formal trial, so we have no grade A evidence for recommending it. However, there have been many papers describing the effects of bisphosphonates on these markers, and it makes physiologic sense to use them in situations where decisions must be made when there is not enough evidence.
In FIT (a trial of alendronate), we reported that the change in bone turnover markers was significantly related to the reduction in fracture risk, and the effect was at least as strong as that observed with a 1-year change in bone density. Those with a 30% decrease in bone alkaline phosphatase had a significant reduction in fracture risk.51
Furthermore, in those patients who were compliant with bisphosphonate treatment, the reduction in fractures with alendronate treatment was significantly better in those who initially had a high bone turnover.52
Similarly, with risedronate, the change in NTx accounted for half of the effect on fracture reduction during the clinical trial, and there was little further improvement in fracture benefit below a decrease of 35% to 40%.10
The baseline NTx level in these clinical trials was about 70 nmol bone collagen equivalents per millimole of creatinine (nmol BCE/mmol Cr) in the risedronate study and 60 in the alendronate study, and in both the fracture reduction was seen at a level of about 40. The FLEX study measured NTx after 5 years, and the average was 19 nmol BCE/mmol Cr. This increased to 22 after 3 years without alendronate.53 At 5 years, the turnover markers had gradually increased but were still 7% to 24% lower than baseline.10
These markers have a diurnal rhythm and daily variation, but despite these limitations they do help identify low bone resorption.
In our hospital, NTx is the most economical marker, and my patients prefer a urine sample to a blood test. Therefore, we measure the NTx and consider values lower than 40 nmol BCE/mmol Cr to be satisfactory.
If the NTx is as low as expected, I discontinue the bisphosphonate. The patient remains on 1,200 mg/day of calcium and 1,000 U/day vitamin D supplementation and is encouraged to exercise.
Bone density tends to be stable for 1 or 2 years after stopping a bisphosphonate, and the biochemical markers of bone resorption remain reduced for several years. We remeasure the urine NTx level annually, and if it increases to more than 40 nmol BCE/mmol Cr an antiresorptive medication is given: either the bisphosphonate is restarted or raloxifene (Evista), calcitonin (Miacalcin), or denosumab (Prolia) is used.
Bone density is less helpful, but reassuring
Bone density is less helpful because it decreases even though the markers of bone resorption remain low. Although one could argue that bone density is not helpful in monitoring patients on therapy, I think it is reassuring to know the patient is not excessively losing bone.
Checking at 2-year intervals is reasonable. If the bone density shows a consistent decrease greater than 6% (which is greater than the difference we can see from patients walking around the room), then we would re-evaluate the patient and consider adding another medication.
If the bone density decreases but the biomarkers are low, then clinical judgment must be used. The bone density result may be erroneous due to different positioning or different regions of interest.
If turnover markers are not reduced
If a patient has been prescribed a bisphosphonate for 5 years but the NTx level is not reduced, I reevaluate the patient. Some are not taking the medication or are not taking it properly. The absorption of oral bisphosphonates is quite low in terms of bioavailability, and this decreases to nearly zero if the medication is taken with food. Some patients may have another disease, such as hyperparathyroidism, malignancy, hyperthyroidism, weight loss, malabsorption, celiac sprue, or vitamin D deficiency.
If repeated biochemical tests show high bone resorption and if the bone density response is suboptimal without a secondary cause, I often switch to an intravenous form of bisphosphonate because some patients do not seem to absorb the oral doses.
If a patient has had a fracture
If a patient has had a fracture despite several years of bisphosphonate therapy, I first check for any other medical problems. The bone markers are, unfortunately, not very helpful because they increase after a fracture and stay elevated for at least 4 months.54 If there are no contraindications, treatment with teriparatide (Forteo) is a reasonable choice. There is evidence from human biopsy studies that teriparatide can reduce the number of microcracks that were related to bisphosphonate treatment,13 and can increase the bone formation rate even when there has been prior bisphosphonate treatment.55–57 Although the anabolic response is blunted, it is still there.58
If the patient remains at high risk
A frail patient with a high risk of fracture presents a challenge, especially one who needs treatment with glucocorticoids or who still has a hip T score below −3. Many physicians are uneasy about discontinuing all osteoporosis-specific drugs, even after 5 years of successful bisphosphonate treatment. In these patients anabolic medications make the most sense. Currently, teriparatide is the only one available, but others are being developed. Bone becomes resistant to the anabolic effects of teriparatide after about 18 months, so this drug cannot be used indefinitely. What we really need are longer-lasting anabolic medicines!
If the patient has thigh pain
Finally, in patients with thigh pain, radiography of the femur should be done to check for a stress fracture. Magnetic resonance imaging or computed tomography may be needed to diagnose a hairline fracture.
If there are already radiographic changes that precede the atypical fractures, then bisphosphonates should be discontinued. In a follow-up observational study of 16 patients who already had one fracture, all four whose contralateral side showed a fracture line (the “dreaded black line”) eventually completed the fracture.59
Another study found that five of six incomplete fractures went on to a complete fracture if not surgically stabilized with rods.60 This is an indication for prophylactic rodding of the femur.
Teriparatide use and rodding of a femur with thickening but not a fracture line must be decided on an individual basis and should be considered more strongly in those with pain in the thigh.
- Francis MD, Valent DJ. Historical perspectives on the clinical development of bisphosphonates in the treatment of bone diseases. J Musculoskelet Neuronal Interact 2007; 7:2–8.
- Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med 2009; 122(suppl 2):S14–S21.
- Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res 2011; 26:3–11.
- Russell RG, Xia Z, Dunford JE, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci 2007; 1117:209–257.
- Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone 2002; 30:807–809.
- Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res 1997; 12:498–508.
- Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest 1997; 100:1475–1480.
- Chapurlat RD, Arlot M, Burt-Pichat B, et al. Microcrack frequency and bone remodeling in postmenopausal osteoporotic women on long-term bisphosphonates: a bone biopsy study. J Bone Miner Res 2007; 22:1502–1509.
- Boivin G, Meunier PJ. Effects of bisphosphonates on matrix mineralization. J Musculoskelet Neuronal Interact 2002; 2:538–543.
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- Eastell R, Hannon RA, Garnero P, Campbell MJ, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate: review of statistical analysis. J Bone Miner Res 2007; 22:1656–1660.
- Borah B, Dufresne TE, Chmielewski PA, Johnson TD, Chines A, Manhart MD. Risedronate preserves bone architecture in postmenopausal women with osteoporosis as measured by three-dimensional microcomputed tomography. Bone 2004; 34:736–746.
- Stepan JJ, Dobnig H, Burr DB, et al. Histomorphometric changes by teriparatide in alendronate pre-treated women with osteoporosis (abstract). Presented at the Annual Meeting of the American Society of Bone and Mineral Research, Montreal 2008: #1019.
- Riggs BL, Hodgson SF, O’Fallon WM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990; 322:802–809.
- Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int 2008; 19:1613–1620.
- Meijer WM, Penning-van Beest FJ, Olson M, Herings RM. Relationship between duration of compliant bisphosphonate use and the risk of osteoporotic fractures. Curr Med Res Opin 2008; 24:3217–3222.
- Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004; 75:462–468.
- Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11:83–91.
- Bone HG, Hosking D, Devogelaer JP, et al; Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004; 350:1189–1199.
- Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995; 333:1437–1443.
- Black DM, Cummings SR, Karpf DB, et al; Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996; 348:1535–1541.
- Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998; 280:2077–2082.
- Fink HA, Milavetz DL, Palermo L, et al. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res 2005; 20:1216–1222.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 2010; 95:1555–1565.
- Schwartz AV, Bauer DC, Cummings SR, et al; FLEX Research Group. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res 2010; 25:976–982.
- Shane E. Evolving data about subtrochanteric fractures and bisphosphonates (editorial). N Engl J Med 2010; 362:1825–1827.
- Sellmeyer DE. Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA 2010; 304:1480–1484.
- Shane E, Burr D, Ebeling PR, et al; American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010; 25:2267–2294.
- Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone 2010; 47:169–180.
- Rizzoli R, Akesson K, Bouxsein M, et al. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int 2011; 22:373–390.
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24:1132–1134.
- Armamento-Villareal R, Napoli N, Panwar V, Novack D. Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med 2006; 355:2048–2050.
- Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 2008; 22:346–350.
- Isaacs JD, Shidiak L, Harris IA, Szomor ZL. Femoral insufficiency fractures associated with prolonged bisphosphonate therapy. Clin Orthop Relat Res 2010; 468:3384–3392.
- Schilcher J, Aspenberg P. Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate. Acta Orthop 2009; 80:413–415.
- Abrahamsen B, Eiken P, Eastell R. Cumulative alendronate dose and the long-term absolute risk of subtrochanteric and diaphyseal femur fractures: a register-based national cohort analysis. J Clin Endocrinol Metab 2010; 95:5258–5265.
- Kim SY, Schneeweiss S, Katz JN, Levin R, Solomon DH. Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort. J Bone Miner Res 2010. [Epub ahead of print]
- Spangler L, Ott SM, Scholes D. Utility of automated data in identifying femoral shaft and subtrochanteric (diaphyseal) fractures. Osteoporos Int. 2010. [Epub ahead of print]
- Black DM, Kelly MP, Genant HK, et al; Fracture Intervention Trial Steering Committee; HORIZON Pivotal Fracture Trial Steering Committee. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 2010; 362:1761–1771.
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305:783–789.
- Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ 2010; 341:c4444.
- Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA 2010; 304:657–663.
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010; 28:5132–5139.
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22:1479–1491.
- Schousboe JT, Ensrud KE, Nyman JA, Kane RL, Melton LJ. Cost-effectiveness of vertebral fracture assessment to detect prevalent vertebral deformity and select postmenopausal women with a femoral neck T-score > −2.5 for alendronate therapy: a modeling study. J Clin Densitom 2006; 9:133–143.
- Dawson-Hughes B; National Osteoporosis Foundation Guide Committee. A revised clinician’s guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab 2008; 93:2463–2465.
- Compston J, Cooper A, Cooper C, et al; the National Osteoporosis Guideline Group (NOGG). Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas 2009; 62:105–108.
- Cummings SR. A 55-year-old woman with osteopenia. JAMA 2006; 296:2601–2610.
- Khosla S, Melton LJ. Clinical practice. Osteopenia. N Engl J Med 2007; 356:2293–2300.
- McClung MR. Osteopenia: to treat or not to treat? Ann Intern Med 2005; 142:796–797.
- Bauer DC, Black DM, Garnero P, et al; Fracture Intervention Trial Study Group. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 2004; 19:1250–1258.
- Bauer DC, Garnero P, Hochberg MC, et al; for the Fracture Intervention Research Group. Pretreatment levels of bone turnover and the anti-fracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 2006; 21:292–299.
- Ensrud KE, Barrett-Connor EL, Schwartz A, et al; Fracture Intervention Trial Long-Term Extension Research Group. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res 2004; 19:1259–1269.
- Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res 2007; 22:1155–1164.
- Cosman F, Nieves JW, Zion M, Barbuto N, Lindsay R. Retreatment with teriparatide one year after the first teriparatide course in patients on continued long-term alendronate. J Bone Miner Res 2009; 24:1110–1115.
- Jobke B, Pfeifer M, Minne HW. Teriparatide following bisphosphonates: initial and long-term effects on microarchitecture and bone remodeling at the human iliac crest. Connect Tissue Res 2009; 50:46–54.
- Miller PD, Delmas PD, Lindsay R, et al; Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide Investigators. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 2008; 93:3785–3793.
- Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004; 19:745–751.
- Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma 2010; 24:75–81.
- Banffy MB, Vrahas MS, Ready JE, Abraham JA. Nonoperative versus prophylactic treatment of bisphosphonate-associated femoral stress fractures. Clin Orthop Relat Res 2011; 469:2028–2034.
- Francis MD, Valent DJ. Historical perspectives on the clinical development of bisphosphonates in the treatment of bone diseases. J Musculoskelet Neuronal Interact 2007; 7:2–8.
- Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med 2009; 122(suppl 2):S14–S21.
- Siris ES, Pasquale MK, Wang Y, Watts NB. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res 2011; 26:3–11.
- Russell RG, Xia Z, Dunford JE, et al. Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci 2007; 1117:209–257.
- Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone 2002; 30:807–809.
- Han ZH, Palnitkar S, Rao DS, Nelson D, Parfitt AM. Effects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res 1997; 12:498–508.
- Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest 1997; 100:1475–1480.
- Chapurlat RD, Arlot M, Burt-Pichat B, et al. Microcrack frequency and bone remodeling in postmenopausal osteoporotic women on long-term bisphosphonates: a bone biopsy study. J Bone Miner Res 2007; 22:1502–1509.
- Boivin G, Meunier PJ. Effects of bisphosphonates on matrix mineralization. J Musculoskelet Neuronal Interact 2002; 2:538–543.
- Black DM, Schwartz AV, Ensrud KE, et al; FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 2006; 296:2927–2938.
- Eastell R, Hannon RA, Garnero P, Campbell MJ, Delmas PD. Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate: review of statistical analysis. J Bone Miner Res 2007; 22:1656–1660.
- Borah B, Dufresne TE, Chmielewski PA, Johnson TD, Chines A, Manhart MD. Risedronate preserves bone architecture in postmenopausal women with osteoporosis as measured by three-dimensional microcomputed tomography. Bone 2004; 34:736–746.
- Stepan JJ, Dobnig H, Burr DB, et al. Histomorphometric changes by teriparatide in alendronate pre-treated women with osteoporosis (abstract). Presented at the Annual Meeting of the American Society of Bone and Mineral Research, Montreal 2008: #1019.
- Riggs BL, Hodgson SF, O’Fallon WM, et al. Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 1990; 322:802–809.
- Curtis JR, Westfall AO, Cheng H, Delzell E, Saag KG. Risk of hip fracture after bisphosphonate discontinuation: implications for a drug holiday. Osteoporos Int 2008; 19:1613–1620.
- Meijer WM, Penning-van Beest FJ, Olson M, Herings RM. Relationship between duration of compliant bisphosphonate use and the risk of osteoporotic fractures. Curr Med Res Opin 2008; 24:3217–3222.
- Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA. Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 2004; 75:462–468.
- Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 2000; 11:83–91.
- Bone HG, Hosking D, Devogelaer JP, et al; Alendronate Phase III Osteoporosis Treatment Study Group. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 2004; 350:1189–1199.
- Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995; 333:1437–1443.
- Black DM, Cummings SR, Karpf DB, et al; Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996; 348:1535–1541.
- Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998; 280:2077–2082.
- Fink HA, Milavetz DL, Palermo L, et al. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res 2005; 20:1216–1222.
- Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab 2010; 95:1555–1565.
- Schwartz AV, Bauer DC, Cummings SR, et al; FLEX Research Group. Efficacy of continued alendronate for fractures in women with and without prevalent vertebral fracture: the FLEX trial. J Bone Miner Res 2010; 25:976–982.
- Shane E. Evolving data about subtrochanteric fractures and bisphosphonates (editorial). N Engl J Med 2010; 362:1825–1827.
- Sellmeyer DE. Atypical fractures as a potential complication of long-term bisphosphonate therapy. JAMA 2010; 304:1480–1484.
- Shane E, Burr D, Ebeling PR, et al; American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2010; 25:2267–2294.
- Giusti A, Hamdy NA, Papapoulos SE. Atypical fractures of the femur and bisphosphonate therapy: a systematic review of case/case series studies. Bone 2010; 47:169–180.
- Rizzoli R, Akesson K, Bouxsein M, et al. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int 2011; 22:373–390.
- Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 2009; 24:1132–1134.
- Armamento-Villareal R, Napoli N, Panwar V, Novack D. Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med 2006; 355:2048–2050.
- Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 2008; 22:346–350.
- Isaacs JD, Shidiak L, Harris IA, Szomor ZL. Femoral insufficiency fractures associated with prolonged bisphosphonate therapy. Clin Orthop Relat Res 2010; 468:3384–3392.
- Schilcher J, Aspenberg P. Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate. Acta Orthop 2009; 80:413–415.
- Abrahamsen B, Eiken P, Eastell R. Cumulative alendronate dose and the long-term absolute risk of subtrochanteric and diaphyseal femur fractures: a register-based national cohort analysis. J Clin Endocrinol Metab 2010; 95:5258–5265.
- Kim SY, Schneeweiss S, Katz JN, Levin R, Solomon DH. Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort. J Bone Miner Res 2010. [Epub ahead of print]
- Spangler L, Ott SM, Scholes D. Utility of automated data in identifying femoral shaft and subtrochanteric (diaphyseal) fractures. Osteoporos Int. 2010. [Epub ahead of print]
- Black DM, Kelly MP, Genant HK, et al; Fracture Intervention Trial Steering Committee; HORIZON Pivotal Fracture Trial Steering Committee. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 2010; 362:1761–1771.
- Park-Wyllie LY, Mamdani MM, Juurlink DN, et al. Bisphosphonate use and the risk of subtrochanteric or femoral shaft fractures in older women. JAMA 2011; 305:783–789.
- Green J, Czanner G, Reeves G, Watson J, Wise L, Beral V. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ 2010; 341:c4444.
- Cardwell CR, Abnet CC, Cantwell MM, Murray LJ. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA 2010; 304:657–663.
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010; 28:5132–5139.
- Khosla S, Burr D, Cauley J, et al; American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2007; 22:1479–1491.
- Schousboe JT, Ensrud KE, Nyman JA, Kane RL, Melton LJ. Cost-effectiveness of vertebral fracture assessment to detect prevalent vertebral deformity and select postmenopausal women with a femoral neck T-score > −2.5 for alendronate therapy: a modeling study. J Clin Densitom 2006; 9:133–143.
- Dawson-Hughes B; National Osteoporosis Foundation Guide Committee. A revised clinician’s guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab 2008; 93:2463–2465.
- Compston J, Cooper A, Cooper C, et al; the National Osteoporosis Guideline Group (NOGG). Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. Maturitas 2009; 62:105–108.
- Cummings SR. A 55-year-old woman with osteopenia. JAMA 2006; 296:2601–2610.
- Khosla S, Melton LJ. Clinical practice. Osteopenia. N Engl J Med 2007; 356:2293–2300.
- McClung MR. Osteopenia: to treat or not to treat? Ann Intern Med 2005; 142:796–797.
- Bauer DC, Black DM, Garnero P, et al; Fracture Intervention Trial Study Group. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 2004; 19:1250–1258.
- Bauer DC, Garnero P, Hochberg MC, et al; for the Fracture Intervention Research Group. Pretreatment levels of bone turnover and the anti-fracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 2006; 21:292–299.
- Ensrud KE, Barrett-Connor EL, Schwartz A, et al; Fracture Intervention Trial Long-Term Extension Research Group. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res 2004; 19:1259–1269.
- Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res 2007; 22:1155–1164.
- Cosman F, Nieves JW, Zion M, Barbuto N, Lindsay R. Retreatment with teriparatide one year after the first teriparatide course in patients on continued long-term alendronate. J Bone Miner Res 2009; 24:1110–1115.
- Jobke B, Pfeifer M, Minne HW. Teriparatide following bisphosphonates: initial and long-term effects on microarchitecture and bone remodeling at the human iliac crest. Connect Tissue Res 2009; 50:46–54.
- Miller PD, Delmas PD, Lindsay R, et al; Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide Investigators. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 2008; 93:3785–3793.
- Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 2004; 19:745–751.
- Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma 2010; 24:75–81.
- Banffy MB, Vrahas MS, Ready JE, Abraham JA. Nonoperative versus prophylactic treatment of bisphosphonate-associated femoral stress fractures. Clin Orthop Relat Res 2011; 469:2028–2034.
KEY POINTS
- Bisphosphonates reduce the risk of osteoporotic fractures, including devastating hip and spine fractures.
- As with any drugs, bisphosphonates should not be used indiscriminately. They are indicated for patients at high risk of fracture, especially those with vertebral fractures or a hip bone density T score lower than −2.5.
- There is little evidence to guide physicians about the duration of bisphosphonate therapy beyond 5 years. One study with marginal power did not show any difference in fracture rates between those who continued taking alendronate and those who discontinued after 5 years (JAMA 2006; 296:2927–2938).
- Evidence is accumulating that the risk of atypical fracture of the femur increases after 5 years of bisphosphonate use.
- Anabolic drugs are needed; the only one currently available is teriparatide (Forteo), which can be used when fractures occur despite (or perhaps because of) bisphosphonate use.
Vancomycin: A 50-something-year-old antibiotic we still don’t understand
In the past half-century, vancomycin has gone from near-orphan status to being one of the most often used antibiotics in our formulary. The driving force for its use is clear: the evolution of Staphylococcus aureus. At first, vancomycin was used to treat infections caused by penicillin-resistant strains. However, the discovery of methicillin curbed its use for more than 2 decades.1
Then, as methicillin-resistant S aureus (MRSA) began to spread in the 1980s, the use of vancomycin began to increase, and with the rise in community-associated MRSA infections in the 1990s, it became even more widely prescribed. The recent Infectious Diseases Society of America (IDSA) guidelines for treatment of infections due to MRSA are replete with references to the use of vancomycin.2
Another factor driving the use of vancomycin is the increased prevalence of device-associated infections, many of which are caused by coagulase-negative staphylococci and other organisms that colonize the skin.3 Many of these bacteria are susceptible only to vancomycin; they may be associated with infections of vascular catheters, cardiac valves, pacemakers, implantable cardioverter-defibrillators, orthopedic implants, neurosurgical devices, and other devices.
To use vancomycin appropriately, we need to recognize the changing minimum inhibitory concentrations (MICs), to select proper doses and dosing intervals, and to know how to monitor its use. Despite more than 50 years of experience with vancomycin, we sometimes find ourselves with more questions than answers about its optimal use.
WHAT IS VANCOMYCIN?
Vancomycin is a glycopeptide antibiotic isolated from a strain of Streptomyces orientalis discovered in a soil sample from Borneo in the mid-1950s.1 It exerts its action by binding to a d-alanyl-d-alanine cell wall precursor necessary for peptidoglycan cross-linking and, therefore, for inhibiting bacterial cell wall synthesis.
Vancomycin is bactericidal against most gram-positive species, including streptococci and staphylococci, with the exception of Enterococcus species, for which it is bacteriostatic. Though it is bactericidal, it appears to kill bacteria more slowly than beta-lactam antibiotics, and therefore it may take longer to clear bacteremia.4
WHAT IS THE BEST WAY TO DOSE VANCOMYCIN?
Vancomycin is widely distributed to most tissues, with an approximate volume of distribution of 0.4 to 1 L/kg; 50% to 55% is protein-bound. Because of this large volume of distribution, vancomycin’s dosing is based on actual body weight.
Vancomycin is not metabolized and is primarily excreted unchanged in the urine via glomerular filtration. It therefore requires dosage adjustments for renal insufficiency.
Vancomycin’s molecular weight is 1,485.73 Da, making it less susceptible to removal by dialysis than smaller molecules. Dosing of vancomycin in patients on hemodialysis depends on many factors specific to the dialysis center, including but not limited to the type of filter used, the duration of filtration, and whether high-flux filtration is used.
Is continuous intravenous infusion better than standard dosing?
Giving vancomycin by continuous infusion has been suggested as a way to optimize its serum concentration and improve its clinical effectiveness.
Wysocki et al5 conducted a multicenter, prospective, randomized study comparing continuous and intermittent intravenous infusions of vancomycin (the latter every 12 hours) to treat severe hospital-acquired MRSA infections, including bloodstream infections and pneumonia. Although blood concentrations above 10 μg/mL were reached more than 30 hours faster with continuous infusions than with intermittent ones, the microbiologic and clinical outcomes were similar with either method.
James et al6 compared the pharmacodynamics of conventional dosing of vancomycin (ie, 1 g every 12 hours) and continuous infusion in 10 patients with suspected or documented gram-positive infections in a prospective, randomized, crossover study. While no adverse effects were observed, the authors also found no statistically significant difference between the treatment groups in the pharmacodynamic variables investigated, including the area under the curve (AUC) divided by the MIC (the AUC-MIC ratio).
In view of the currently available data, the guidelines for monitoring vancomycin therapy note that there does not appear to be any difference in patient outcomes with continuous infusion vs intermittent dosing.7
Should a loading dose be given?
Another proposed strategy for optimizing vancomycin’s effectiveness is to give a higher initial dose, ie, a loading dose.
Wang et al8 performed a single-center study in 28 patients who received a 25 mg/kg loading dose at a rate of 500 mg/hour. This loading dose was safe, but the authors did not evaluate its efficacy.
Mohammedi et al9 compared loading doses of 500 mg and 15 mg/kg in critically ill patients receiving vancomycin by continuous infusion. The weight-based loading dose produced higher post-dose levels and a significantly higher rate of clinical cure, but there was no significant difference in the rate of survival to discharge from the intensive care unit.
While the use of a loading dose appears to be safe and likely leads to more rapid attainment of therapeutic blood levels, we lack data on whether it improves clinical outcomes, and further study is needed to determine its role.
WHAT IS THE BEST WAY TO MONITOR VANCOMYCIN THERAPY?
Whether and how to use the serum vancomycin concentration to adjust the dosing has been a matter of debate for many years. Convincing evidence that vancomycin levels predict clinical outcomes or that measuring them prevents toxicity is lacking.7
A consensus statement from the American Society of Health-System Pharmacists, the IDSA, and the Society of Infectious Diseases Pharmacists7 contains recommendations for monitoring vancomycin therapy, based on a critical evaluation of the available scientific evidence. Their recommendations:
- Vancomycin serum concentrations should be checked to optimize therapy and used as a surrogate marker of effectiveness.
- Trough, rather than peak, levels should be monitored.
- Trough levels should be checked just before the fourth dose, when steady-state levels are likely to have been achieved. More frequent monitoring may be considered in patients with fluctuating renal function.
- Trough levels should be higher than 10 mg/L to prevent the development of resistance.
- To improve antibiotic penetration and optimize the likelihood of achieving pharmacokinetic and pharmacodynamic targets, trough levels of 15 to 20 mg/L are recommended for pathogens with a vancomycin MIC of 1 mg/L or higher and for complicated infections such as endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia.
- For prolonged courses, it is appropriate to check vancomycin levels weekly in hemodynamically stable patients and more often in those who are not hemodynamically stable.
IS VANCOMYCIN NEPHROTOXIC?
In the 1950s, vancomycin formulations were sometimes called “Mississippi mud” because of the many impurities they contained.1 These impurities were associated with significant nephrotoxicity. Better purification methods used in the manufacture of current formulations mitigate this problem, resulting in a lower incidence of nephrotoxicity.
Over the last several years, organizations such as the American Thoracic Society and the IDSA have recommended targeting higher vancomycin trough concentrations.10 The consequent widespread use of higher doses has renewed interest in vancomycin’s potential nephrotoxicity.
Lodise et al,11 in a cohort study, examined the incidence of nephrotoxicity with higher daily doses of vancomycin (≥ 4 g/day), lower daily doses (< 4 g/day), and linezolid (Zyvox). They defined nephrotoxicity as an increase in serum creatinine of 0.5 mg/dL or a decrease in calculated creatinine clearance of 50% from baseline on 2 consecutive days.
The incidence of nephrotoxicity was significantly higher in the high-dose vancomycin group (34.6%) than in the low-dose vancomycin group (10.9%) and in the linezolid group (6.7%) (P = .001). Additional factors associated with nephrotoxicity in this study included baseline creatinine clearance less than 86.6 mL/minute, weight greater than 101.4 kg (223.5 lb), and being in an intensive care unit.
Hidayat et al12 investigated outcomes in patients with high vs low vancomycin trough levels (≥ 15 mg/L vs < 15 mg/L) in a prospective cohort study. Sixty-three patients achieved an average vancomycin trough of 15 to 20 mg/L, and of these, 11 developed nephrotoxicity, compared with no patients in the low-trough group (P = .01). Of the 11 who developed nephrotoxicity, 10 were concomitantly taking other potentially nephrotoxic agents.
Comment. The data on vancomycin and nephrotoxicity are mostly from studies that had limitations such as small numbers of patients, retrospective design, and variable definitions of nephrotoxicity. Many of the patients in these studies had additional factors contributing to nephrotoxicity, including hemodynamic instability and concomitant exposure to other nephrotoxins. Additionally, the sequence of events (nephrotoxicity leading to elevated vancomycin levels vs elevated vancomycin levels causing nephrotoxicity) is still debatable.
The incidence of nephrotoxicity associated with vancomycin therapy is difficult to determine. However, based on current information, the incidence of nephrotoxicity appears to be low when vancomycin is used as monotherapy.
IS S AUREUS BECOMING RESISTANT TO VANCOMYCIN?
An issue of increasing importance in health care settings is the emergence of vancomycin-intermediate S aureus (VISA) and vancomycin-resistant S aureus (VRSA). Eleven cases of VRSA were identified in the United States from 2002 to 2005.13 All cases of VRSA in the United States have involved the incorporation of enterococcal vanA cassette into the S aureus genome.14 While true VRSA isolates remain rare, VISA isolates are becoming more common.
Heteroresistant VISA: An emerging subpopulation of MRSA
Another population of S aureus that has emerged is heteroresistant vancomycin-intermediate S aureus (hVISA). It is defined as the presence of subpopulations of VISA within a population of MRSA at a rate of one organism per 105 to 106 organisms. With traditional testing methods, the vancomycin MIC for the entire population of the strain is within the susceptible range.15 These hVISA populations are thought to be precursors to the development of VISA.16
The resistance to vancomycin in hVISA and VISA populations is due to increased cell wall thickness, altered penicillin-binding protein profiles, and decreased cell wall autolysis.
While the true prevalence of hVISA is difficult to predict because of challenges in microbiological detection and probably varies between geographic regions and individual institutions, different studies have reported hVISA rates between 2% and 13% of all MRSA isolates.15–17
Reduced vancomycin susceptibility can develop regardless of methicillin susceptibility.18
While hVISA is not common, its presence is thought to be a predictor of failing vancomycin therapy.15
Factors associated with hVISA bacteremia include high-bacterial-load infections, treatment failure (including persistent bacteremia for more than 7 days), and initially low serum vancomycin levels.15
‘MIC creep’: Is it real?
Also worrisome, the average vancomycin MIC for S aureus has been shifting upward, based on reports from several institutions, although it is still within the susceptible range.19,20 However, this “MIC creep” likely reflects, at least in part, differences in MIC testing and varying methods used to analyze the data.19,20
Holmes and Jorgensen,21 in a single-institution study of MRSA isolates recovered from bacteremic patients from 1999 to 2006, determined that no MIC creep existed when they tested vancomycin MICs using the broth microdilution method. The authors found the MIC90 (ie, the MIC in at least 90% of the isolates) remained less than 1 mg/L during each year of the study.
Sader et al,22 in a multicenter study, evaluated 1,800 MRSA bloodstream isolates from nine hospitals across the United States from 2002 to 2006. Vancomycin MICs were again measured by broth microdilution methods. The mode MIC remained stable at 0.625 mg/L during the study period, and the authors did not detect a trend of rising MICs.
The inconsistency between reports of MIC creep at single institutions and the absence of this phenomenon in large, multicenter studies seems to imply that vancomycin MIC creep is not occurring on a grand scale.
Vancomycin tolerance
Another troubling matter with S aureus and vancomycin is the issue of tolerance. Vancomycin tolerance, defined in terms of increased minimum bactericidal concentration, represents a loss of bactericidal activity. Tolerance to vancomycin can occur even if the MIC remains in the susceptible range.23
Safdar and Rolston,24 in an observational study from a cancer center, reported that of eight cases of bacteremia that was resistant to vancomycin therapy, three were caused by S aureus.
Sakoulas et al25 found that higher levels of vancomycin bactericidal activity were associated with higher rates of clinical success; however, they found no effect on the mortality rate.
The issue of vancomycin tolerance remains controversial, and because testing for it is impractical in clinical microbiology laboratories, its implications outside the research arena are difficult to ascertain at present.
IS VANCOMYCIN STILL THE BEST DRUG FOR S AUREUS?
MIC break points have been lowered
In 2006, the Clinical Laboratories and Standards Institute lowered its break points for vancomycin MIC categories for S aureus:
- Susceptible: ≤ 2 mg/L (formerly ≤ 4 mg/L)
- Intermediate: 4–8 mg/L (formerly 8–16 mg/L)
- Resistant: ≥ 16 mg/L (formerly ≥ 32 mg/L).
The rationales for these changes were that the lower break points would better detect hVISA, and that cases have been reported of clinical treatment failure of S aureus infections in which the MICs for vancomycin were 4 mg/L.26
Since 2006, the question has been raised whether to lower the break points even further. A reason for this proposal comes from an enhanced understanding of the pharmacokinetics and pharmacodynamics of vancomycin.
The variable most closely associated with clinical response to vancomycin is the AUC-MIC ratio. An AUC-MIC ratio of 400 or higher may be associated with better outcomes in patients with serious S aureus infection. A study of 108 patients with S aureus infection of the lower respiratory tract indicated that organism eradication was more likely if the AUC-MIC ratio was 400 or greater compared with values less than 400, and this was statistically significant.27 However, in cases of S aureus infection with a vancomycin MIC of 2 mg/L or higher, this ratio may not be achievable.
A prospective study of 414 MRSA bacteremia episodes found a vancomycin MIC of 2 mg/L to be a predictor of death.28 The authors concluded that vancomycin may not be the optimal treatment for MRSA with a vancomycin MIC of 2 mg/L.28 Additional studies have also suggested a possible decrease in response to vancomycin in MRSA isolates with elevated MICs within the susceptible range.25,29
Recent guidelines from the IDSA recommend using the clinical response, regardless of the MIC, to guide antimicrobial selection for isolates with MICs in the susceptible range.2
Combination therapy with vancomycin
As vancomycin use has increased, therapeutic failures with vancomycin have become apparent. Combination therapy has been suggested as an option to increase the efficacy of vancomycin when treating complicated infections.
Rifampin plus vancomycin is controversial.30 The combination is theoretically beneficial, especially in infections associated with prosthetic devices. However, clinical studies have failed to convincingly support its use, and some have suggested that it might prolong bacteremia. In addition, it has numerous drug interactions to consider and adverse effects.31
Gentamicin plus vancomycin. The evidence supporting the use of this combination is weak at best. It appears that clinicians may have extrapolated from the success reported by Korzeniowski and Sande,32 who found that methicillin-susceptible S aureus bacteremia was cleared faster if gentamicin was added to nafcillin. A more recent study33 that compared daptomycin (Cubicin) monotherapy with combined vancomycin and gentamicin to treat MRSA bacteremia and endocarditis showed a better overall success rate with daptomycin (44% vs 32.6%), but the difference was not statistically significant.
Gentamicin has some toxicity. Even short-term use (for the first 4 days of therapy) at low doses for bacteremia and endocarditis due to staphylococci has been associated with a higher rate of renal adverse events, including a significant decrease in creatinine clearance.34
Clindamycin or linezolid plus vancomycin is used to decrease toxin production by S aureus.30
While combination therapy with vancomycin is recommended in specific clinical situations, and the combinations are synergistic in vitro, information is lacking about clinical outcomes to support their use.
Don’t use vancomycin when another drug would be better
Vancomycin continues to be the drug of choice in many circumstances, but in some instances its role is under scrutiny and another drug might be better.
Beta-lactams. In patients with infection due to methicillin-susceptible S aureus, failure rates are higher with vancomycin than with beta-lactam therapy, specifically nafcillin.35–37 Beta-lactam antibiotics are thus the drugs of choice for treating infection with beta-lactam-susceptible strains of S aureus.
Linezolid. In theory, linezolid’s ability to decrease production of the S aureus Panton-Valentine leukocidin (PVL) toxin may be an advantage over vancomycin for treating necrotizing pneumonias. For the treatment of MRSA pneumonia, however, controversy exists as to whether linezolid is superior to vancomycin. An analysis of two prospective, randomized, double-blind studies of patients with MRSA pneumonia suggested that initial therapy with linezolid was associated with better survival and clinical cure rates,38 but a subsequent meta-analysis did not substantiate this finding.39 An additional comparative study has been completed, and analysis of the results is in progress.
Daptomycin, approved for skin and soft-tissue infections and bacteremias, including those with right-sided endocarditis, is a lipopeptide antibiotic with a spectrum of action similar to that of vancomycin.40 Daptomycin is also active against many strains of vancomycin-resistant enterococci. As noted above, in the MRSA subgroup of the pivotal comparative study of treatment for S aureus bacteremia and endocarditis, the success rate for daptomycin-treated patients (44.4%) was better than that for patients treated with vancomycin plus gentamicin (32.6%), but the difference was not statistically significant.33,41
The creatine phosphokinase concentration should be monitored weekly in patients on daptomycin.42 Daptomycin is inactivated by lung surfactant and should not be used to treat pneumonia.
Other treatment options approved by the US Food and Drug Administration (FDA) for MRSA infections include tigecycline (Tygacil), quinupristin-dalfopristin (Synercid), telavancin (Vibativ), and ceftaroline (Teflaro).
Tigecycline is a glycylcycline with bacteriostatic activity against S aureus and wide distribution to the tissues.43
Quinupristin-dalfopristin, a streptogramin antibiotic, has activity against S aureus. Its use may be associated with severe myalgias, sometimes leading patients to stop taking it.
Telavancin, recently approved by the FDA, is a lipoglycopeptide antibiotic.44 It is currently approved to treat complicated skin and skin structure infections and was found to be not inferior to vancomycin. An important side effect of this agent is nephrotoxicity. A negative pregnancy test is required before using this agent in women of childbearing potential.
Ceftaroline, a fifth-generation cephalosporin active against MRSA, has been approved by the FDA for the treatment of skin and skin structure infections and community-acquired pneumonia.45
- Murray BE, Nannini EC. Glycopeptides (vancomycin and teicoplanin), streptogramins (quinupristin-dalfopristin), and lipopeptides (daptomycin). In:Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010:449–468.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:285–292.
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 2001; 45:2460–2467.
- James JK, Palmer SM, Levine DP, Rybak MJ. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 1996; 40:696–700.
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98.
- Wang JT, Fang CT, Chen YC, Chang SC. Necessity of a loading dose when using vancomycin in critically ill patients (letter). J Antimicrob Chemother 2001; 47:246.
- Mohammedi I, Descloux E, Argaud L, Le Scanff J, Robert D. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int J Antimicrob Agents 2006; 27:259–262.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52:1330–1336.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–2144.
- Centers for Disease Control and Prevention. CDC reminds clinical laboratories and healthcare infection preventionists of their role in the search and containment of vancomycin-resistant Staphylococcus aureus (VRSA), May 2010. http://emergency.cdc.gov/coca/reminders/2010/2010may06.asp. Accessed June 7, 2011.
- Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 2008; 46:668–674.
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–451.
- Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–3045.
- Sader HS, Jones RN, Rossi KL, Rybak MJ. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine USA hospitals. J Antimicrob Chemother 2009; 64:1024–1028.
- Pillai SK, Wennersten C, Venkataraman L, Eliopoulos GM, Moellering RC, Karchmer AW. Development of reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 2009; 49:1169–1174.
- Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 2006; 44:3883–3886.
- Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in nonvancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother 2007; 60:788–794.
- Holmes RL, Jorgensen JH. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob Agents Chemother 2008; 52:757–760.
- Sader HS, Fey PD, Limaye AP, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother 2009; 53:4127–4132.
- May J, Shannon K, King A, French G. Glycopeptide tolerance in Staphylococcus aureus. J Antimicrob Chemother 1998; 42:189–197.
- Safdar A, Rolston KV. Vancomycin tolerance, a potential mechanism for refractory gram-positive bacteremia observational study in patients with cancer. Cancer 2006; 106:1815–1820.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Tenover FC, Moellering RC. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007; 44:1208–1215.
- Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43:925–942.
- Soriano A, Marco F, Martínez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:193–200.
- Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008; 52:3315–3320.
- Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2009; 49:1072–1079.
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–680.
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med 1982; 97:496–503.
- Rehm SJ, Boucher H, Levine D, et al. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: subset analysis of patients infected with methicillin-resistant isolates. J Antimicrob Chemother 2008; 62:1413–1421.
- Cosgrove SE, Vigliani GA, Fowler VG, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009; 48:713–721.
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–1231.
- Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 1997; 17:990–997.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003; 124:1789–1797.
- Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 2010; 38:1802–1808.
- Kosmidis C, Levine DP. Daptomycin: pharmacology and clinical use. Expert Opin Pharmacother 2010; 11:615–625.
- Fowler VG, Boucher HW, Corey GR, et al; S aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–665.
- Daptomycin package insert. Lexington, MA. Cubist Pharmaceuticals, Inc. November 2010. www.cubicin.com/pdf/PrescribingInformation.pdf. Accessed June 7, 2011.
- Peterson LR. A review of tigecycline—the first glycylcycline. Int J Antimicrob Agents 2008; 32(suppl 4):S215–S222.
- Saravolatz LD, Stein GE, Johnson LB. Telavancin: a novel lipoglycopeptide. Clin Infect Dis 2009; 49:1908–1914.
- Ceftaroline package insert. St. Louis, MO. Forest Pharmaceuticals. October 2010.
In the past half-century, vancomycin has gone from near-orphan status to being one of the most often used antibiotics in our formulary. The driving force for its use is clear: the evolution of Staphylococcus aureus. At first, vancomycin was used to treat infections caused by penicillin-resistant strains. However, the discovery of methicillin curbed its use for more than 2 decades.1
Then, as methicillin-resistant S aureus (MRSA) began to spread in the 1980s, the use of vancomycin began to increase, and with the rise in community-associated MRSA infections in the 1990s, it became even more widely prescribed. The recent Infectious Diseases Society of America (IDSA) guidelines for treatment of infections due to MRSA are replete with references to the use of vancomycin.2
Another factor driving the use of vancomycin is the increased prevalence of device-associated infections, many of which are caused by coagulase-negative staphylococci and other organisms that colonize the skin.3 Many of these bacteria are susceptible only to vancomycin; they may be associated with infections of vascular catheters, cardiac valves, pacemakers, implantable cardioverter-defibrillators, orthopedic implants, neurosurgical devices, and other devices.
To use vancomycin appropriately, we need to recognize the changing minimum inhibitory concentrations (MICs), to select proper doses and dosing intervals, and to know how to monitor its use. Despite more than 50 years of experience with vancomycin, we sometimes find ourselves with more questions than answers about its optimal use.
WHAT IS VANCOMYCIN?
Vancomycin is a glycopeptide antibiotic isolated from a strain of Streptomyces orientalis discovered in a soil sample from Borneo in the mid-1950s.1 It exerts its action by binding to a d-alanyl-d-alanine cell wall precursor necessary for peptidoglycan cross-linking and, therefore, for inhibiting bacterial cell wall synthesis.
Vancomycin is bactericidal against most gram-positive species, including streptococci and staphylococci, with the exception of Enterococcus species, for which it is bacteriostatic. Though it is bactericidal, it appears to kill bacteria more slowly than beta-lactam antibiotics, and therefore it may take longer to clear bacteremia.4
WHAT IS THE BEST WAY TO DOSE VANCOMYCIN?
Vancomycin is widely distributed to most tissues, with an approximate volume of distribution of 0.4 to 1 L/kg; 50% to 55% is protein-bound. Because of this large volume of distribution, vancomycin’s dosing is based on actual body weight.
Vancomycin is not metabolized and is primarily excreted unchanged in the urine via glomerular filtration. It therefore requires dosage adjustments for renal insufficiency.
Vancomycin’s molecular weight is 1,485.73 Da, making it less susceptible to removal by dialysis than smaller molecules. Dosing of vancomycin in patients on hemodialysis depends on many factors specific to the dialysis center, including but not limited to the type of filter used, the duration of filtration, and whether high-flux filtration is used.
Is continuous intravenous infusion better than standard dosing?
Giving vancomycin by continuous infusion has been suggested as a way to optimize its serum concentration and improve its clinical effectiveness.
Wysocki et al5 conducted a multicenter, prospective, randomized study comparing continuous and intermittent intravenous infusions of vancomycin (the latter every 12 hours) to treat severe hospital-acquired MRSA infections, including bloodstream infections and pneumonia. Although blood concentrations above 10 μg/mL were reached more than 30 hours faster with continuous infusions than with intermittent ones, the microbiologic and clinical outcomes were similar with either method.
James et al6 compared the pharmacodynamics of conventional dosing of vancomycin (ie, 1 g every 12 hours) and continuous infusion in 10 patients with suspected or documented gram-positive infections in a prospective, randomized, crossover study. While no adverse effects were observed, the authors also found no statistically significant difference between the treatment groups in the pharmacodynamic variables investigated, including the area under the curve (AUC) divided by the MIC (the AUC-MIC ratio).
In view of the currently available data, the guidelines for monitoring vancomycin therapy note that there does not appear to be any difference in patient outcomes with continuous infusion vs intermittent dosing.7
Should a loading dose be given?
Another proposed strategy for optimizing vancomycin’s effectiveness is to give a higher initial dose, ie, a loading dose.
Wang et al8 performed a single-center study in 28 patients who received a 25 mg/kg loading dose at a rate of 500 mg/hour. This loading dose was safe, but the authors did not evaluate its efficacy.
Mohammedi et al9 compared loading doses of 500 mg and 15 mg/kg in critically ill patients receiving vancomycin by continuous infusion. The weight-based loading dose produced higher post-dose levels and a significantly higher rate of clinical cure, but there was no significant difference in the rate of survival to discharge from the intensive care unit.
While the use of a loading dose appears to be safe and likely leads to more rapid attainment of therapeutic blood levels, we lack data on whether it improves clinical outcomes, and further study is needed to determine its role.
WHAT IS THE BEST WAY TO MONITOR VANCOMYCIN THERAPY?
Whether and how to use the serum vancomycin concentration to adjust the dosing has been a matter of debate for many years. Convincing evidence that vancomycin levels predict clinical outcomes or that measuring them prevents toxicity is lacking.7
A consensus statement from the American Society of Health-System Pharmacists, the IDSA, and the Society of Infectious Diseases Pharmacists7 contains recommendations for monitoring vancomycin therapy, based on a critical evaluation of the available scientific evidence. Their recommendations:
- Vancomycin serum concentrations should be checked to optimize therapy and used as a surrogate marker of effectiveness.
- Trough, rather than peak, levels should be monitored.
- Trough levels should be checked just before the fourth dose, when steady-state levels are likely to have been achieved. More frequent monitoring may be considered in patients with fluctuating renal function.
- Trough levels should be higher than 10 mg/L to prevent the development of resistance.
- To improve antibiotic penetration and optimize the likelihood of achieving pharmacokinetic and pharmacodynamic targets, trough levels of 15 to 20 mg/L are recommended for pathogens with a vancomycin MIC of 1 mg/L or higher and for complicated infections such as endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia.
- For prolonged courses, it is appropriate to check vancomycin levels weekly in hemodynamically stable patients and more often in those who are not hemodynamically stable.
IS VANCOMYCIN NEPHROTOXIC?
In the 1950s, vancomycin formulations were sometimes called “Mississippi mud” because of the many impurities they contained.1 These impurities were associated with significant nephrotoxicity. Better purification methods used in the manufacture of current formulations mitigate this problem, resulting in a lower incidence of nephrotoxicity.
Over the last several years, organizations such as the American Thoracic Society and the IDSA have recommended targeting higher vancomycin trough concentrations.10 The consequent widespread use of higher doses has renewed interest in vancomycin’s potential nephrotoxicity.
Lodise et al,11 in a cohort study, examined the incidence of nephrotoxicity with higher daily doses of vancomycin (≥ 4 g/day), lower daily doses (< 4 g/day), and linezolid (Zyvox). They defined nephrotoxicity as an increase in serum creatinine of 0.5 mg/dL or a decrease in calculated creatinine clearance of 50% from baseline on 2 consecutive days.
The incidence of nephrotoxicity was significantly higher in the high-dose vancomycin group (34.6%) than in the low-dose vancomycin group (10.9%) and in the linezolid group (6.7%) (P = .001). Additional factors associated with nephrotoxicity in this study included baseline creatinine clearance less than 86.6 mL/minute, weight greater than 101.4 kg (223.5 lb), and being in an intensive care unit.
Hidayat et al12 investigated outcomes in patients with high vs low vancomycin trough levels (≥ 15 mg/L vs < 15 mg/L) in a prospective cohort study. Sixty-three patients achieved an average vancomycin trough of 15 to 20 mg/L, and of these, 11 developed nephrotoxicity, compared with no patients in the low-trough group (P = .01). Of the 11 who developed nephrotoxicity, 10 were concomitantly taking other potentially nephrotoxic agents.
Comment. The data on vancomycin and nephrotoxicity are mostly from studies that had limitations such as small numbers of patients, retrospective design, and variable definitions of nephrotoxicity. Many of the patients in these studies had additional factors contributing to nephrotoxicity, including hemodynamic instability and concomitant exposure to other nephrotoxins. Additionally, the sequence of events (nephrotoxicity leading to elevated vancomycin levels vs elevated vancomycin levels causing nephrotoxicity) is still debatable.
The incidence of nephrotoxicity associated with vancomycin therapy is difficult to determine. However, based on current information, the incidence of nephrotoxicity appears to be low when vancomycin is used as monotherapy.
IS S AUREUS BECOMING RESISTANT TO VANCOMYCIN?
An issue of increasing importance in health care settings is the emergence of vancomycin-intermediate S aureus (VISA) and vancomycin-resistant S aureus (VRSA). Eleven cases of VRSA were identified in the United States from 2002 to 2005.13 All cases of VRSA in the United States have involved the incorporation of enterococcal vanA cassette into the S aureus genome.14 While true VRSA isolates remain rare, VISA isolates are becoming more common.
Heteroresistant VISA: An emerging subpopulation of MRSA
Another population of S aureus that has emerged is heteroresistant vancomycin-intermediate S aureus (hVISA). It is defined as the presence of subpopulations of VISA within a population of MRSA at a rate of one organism per 105 to 106 organisms. With traditional testing methods, the vancomycin MIC for the entire population of the strain is within the susceptible range.15 These hVISA populations are thought to be precursors to the development of VISA.16
The resistance to vancomycin in hVISA and VISA populations is due to increased cell wall thickness, altered penicillin-binding protein profiles, and decreased cell wall autolysis.
While the true prevalence of hVISA is difficult to predict because of challenges in microbiological detection and probably varies between geographic regions and individual institutions, different studies have reported hVISA rates between 2% and 13% of all MRSA isolates.15–17
Reduced vancomycin susceptibility can develop regardless of methicillin susceptibility.18
While hVISA is not common, its presence is thought to be a predictor of failing vancomycin therapy.15
Factors associated with hVISA bacteremia include high-bacterial-load infections, treatment failure (including persistent bacteremia for more than 7 days), and initially low serum vancomycin levels.15
‘MIC creep’: Is it real?
Also worrisome, the average vancomycin MIC for S aureus has been shifting upward, based on reports from several institutions, although it is still within the susceptible range.19,20 However, this “MIC creep” likely reflects, at least in part, differences in MIC testing and varying methods used to analyze the data.19,20
Holmes and Jorgensen,21 in a single-institution study of MRSA isolates recovered from bacteremic patients from 1999 to 2006, determined that no MIC creep existed when they tested vancomycin MICs using the broth microdilution method. The authors found the MIC90 (ie, the MIC in at least 90% of the isolates) remained less than 1 mg/L during each year of the study.
Sader et al,22 in a multicenter study, evaluated 1,800 MRSA bloodstream isolates from nine hospitals across the United States from 2002 to 2006. Vancomycin MICs were again measured by broth microdilution methods. The mode MIC remained stable at 0.625 mg/L during the study period, and the authors did not detect a trend of rising MICs.
The inconsistency between reports of MIC creep at single institutions and the absence of this phenomenon in large, multicenter studies seems to imply that vancomycin MIC creep is not occurring on a grand scale.
Vancomycin tolerance
Another troubling matter with S aureus and vancomycin is the issue of tolerance. Vancomycin tolerance, defined in terms of increased minimum bactericidal concentration, represents a loss of bactericidal activity. Tolerance to vancomycin can occur even if the MIC remains in the susceptible range.23
Safdar and Rolston,24 in an observational study from a cancer center, reported that of eight cases of bacteremia that was resistant to vancomycin therapy, three were caused by S aureus.
Sakoulas et al25 found that higher levels of vancomycin bactericidal activity were associated with higher rates of clinical success; however, they found no effect on the mortality rate.
The issue of vancomycin tolerance remains controversial, and because testing for it is impractical in clinical microbiology laboratories, its implications outside the research arena are difficult to ascertain at present.
IS VANCOMYCIN STILL THE BEST DRUG FOR S AUREUS?
MIC break points have been lowered
In 2006, the Clinical Laboratories and Standards Institute lowered its break points for vancomycin MIC categories for S aureus:
- Susceptible: ≤ 2 mg/L (formerly ≤ 4 mg/L)
- Intermediate: 4–8 mg/L (formerly 8–16 mg/L)
- Resistant: ≥ 16 mg/L (formerly ≥ 32 mg/L).
The rationales for these changes were that the lower break points would better detect hVISA, and that cases have been reported of clinical treatment failure of S aureus infections in which the MICs for vancomycin were 4 mg/L.26
Since 2006, the question has been raised whether to lower the break points even further. A reason for this proposal comes from an enhanced understanding of the pharmacokinetics and pharmacodynamics of vancomycin.
The variable most closely associated with clinical response to vancomycin is the AUC-MIC ratio. An AUC-MIC ratio of 400 or higher may be associated with better outcomes in patients with serious S aureus infection. A study of 108 patients with S aureus infection of the lower respiratory tract indicated that organism eradication was more likely if the AUC-MIC ratio was 400 or greater compared with values less than 400, and this was statistically significant.27 However, in cases of S aureus infection with a vancomycin MIC of 2 mg/L or higher, this ratio may not be achievable.
A prospective study of 414 MRSA bacteremia episodes found a vancomycin MIC of 2 mg/L to be a predictor of death.28 The authors concluded that vancomycin may not be the optimal treatment for MRSA with a vancomycin MIC of 2 mg/L.28 Additional studies have also suggested a possible decrease in response to vancomycin in MRSA isolates with elevated MICs within the susceptible range.25,29
Recent guidelines from the IDSA recommend using the clinical response, regardless of the MIC, to guide antimicrobial selection for isolates with MICs in the susceptible range.2
Combination therapy with vancomycin
As vancomycin use has increased, therapeutic failures with vancomycin have become apparent. Combination therapy has been suggested as an option to increase the efficacy of vancomycin when treating complicated infections.
Rifampin plus vancomycin is controversial.30 The combination is theoretically beneficial, especially in infections associated with prosthetic devices. However, clinical studies have failed to convincingly support its use, and some have suggested that it might prolong bacteremia. In addition, it has numerous drug interactions to consider and adverse effects.31
Gentamicin plus vancomycin. The evidence supporting the use of this combination is weak at best. It appears that clinicians may have extrapolated from the success reported by Korzeniowski and Sande,32 who found that methicillin-susceptible S aureus bacteremia was cleared faster if gentamicin was added to nafcillin. A more recent study33 that compared daptomycin (Cubicin) monotherapy with combined vancomycin and gentamicin to treat MRSA bacteremia and endocarditis showed a better overall success rate with daptomycin (44% vs 32.6%), but the difference was not statistically significant.
Gentamicin has some toxicity. Even short-term use (for the first 4 days of therapy) at low doses for bacteremia and endocarditis due to staphylococci has been associated with a higher rate of renal adverse events, including a significant decrease in creatinine clearance.34
Clindamycin or linezolid plus vancomycin is used to decrease toxin production by S aureus.30
While combination therapy with vancomycin is recommended in specific clinical situations, and the combinations are synergistic in vitro, information is lacking about clinical outcomes to support their use.
Don’t use vancomycin when another drug would be better
Vancomycin continues to be the drug of choice in many circumstances, but in some instances its role is under scrutiny and another drug might be better.
Beta-lactams. In patients with infection due to methicillin-susceptible S aureus, failure rates are higher with vancomycin than with beta-lactam therapy, specifically nafcillin.35–37 Beta-lactam antibiotics are thus the drugs of choice for treating infection with beta-lactam-susceptible strains of S aureus.
Linezolid. In theory, linezolid’s ability to decrease production of the S aureus Panton-Valentine leukocidin (PVL) toxin may be an advantage over vancomycin for treating necrotizing pneumonias. For the treatment of MRSA pneumonia, however, controversy exists as to whether linezolid is superior to vancomycin. An analysis of two prospective, randomized, double-blind studies of patients with MRSA pneumonia suggested that initial therapy with linezolid was associated with better survival and clinical cure rates,38 but a subsequent meta-analysis did not substantiate this finding.39 An additional comparative study has been completed, and analysis of the results is in progress.
Daptomycin, approved for skin and soft-tissue infections and bacteremias, including those with right-sided endocarditis, is a lipopeptide antibiotic with a spectrum of action similar to that of vancomycin.40 Daptomycin is also active against many strains of vancomycin-resistant enterococci. As noted above, in the MRSA subgroup of the pivotal comparative study of treatment for S aureus bacteremia and endocarditis, the success rate for daptomycin-treated patients (44.4%) was better than that for patients treated with vancomycin plus gentamicin (32.6%), but the difference was not statistically significant.33,41
The creatine phosphokinase concentration should be monitored weekly in patients on daptomycin.42 Daptomycin is inactivated by lung surfactant and should not be used to treat pneumonia.
Other treatment options approved by the US Food and Drug Administration (FDA) for MRSA infections include tigecycline (Tygacil), quinupristin-dalfopristin (Synercid), telavancin (Vibativ), and ceftaroline (Teflaro).
Tigecycline is a glycylcycline with bacteriostatic activity against S aureus and wide distribution to the tissues.43
Quinupristin-dalfopristin, a streptogramin antibiotic, has activity against S aureus. Its use may be associated with severe myalgias, sometimes leading patients to stop taking it.
Telavancin, recently approved by the FDA, is a lipoglycopeptide antibiotic.44 It is currently approved to treat complicated skin and skin structure infections and was found to be not inferior to vancomycin. An important side effect of this agent is nephrotoxicity. A negative pregnancy test is required before using this agent in women of childbearing potential.
Ceftaroline, a fifth-generation cephalosporin active against MRSA, has been approved by the FDA for the treatment of skin and skin structure infections and community-acquired pneumonia.45
In the past half-century, vancomycin has gone from near-orphan status to being one of the most often used antibiotics in our formulary. The driving force for its use is clear: the evolution of Staphylococcus aureus. At first, vancomycin was used to treat infections caused by penicillin-resistant strains. However, the discovery of methicillin curbed its use for more than 2 decades.1
Then, as methicillin-resistant S aureus (MRSA) began to spread in the 1980s, the use of vancomycin began to increase, and with the rise in community-associated MRSA infections in the 1990s, it became even more widely prescribed. The recent Infectious Diseases Society of America (IDSA) guidelines for treatment of infections due to MRSA are replete with references to the use of vancomycin.2
Another factor driving the use of vancomycin is the increased prevalence of device-associated infections, many of which are caused by coagulase-negative staphylococci and other organisms that colonize the skin.3 Many of these bacteria are susceptible only to vancomycin; they may be associated with infections of vascular catheters, cardiac valves, pacemakers, implantable cardioverter-defibrillators, orthopedic implants, neurosurgical devices, and other devices.
To use vancomycin appropriately, we need to recognize the changing minimum inhibitory concentrations (MICs), to select proper doses and dosing intervals, and to know how to monitor its use. Despite more than 50 years of experience with vancomycin, we sometimes find ourselves with more questions than answers about its optimal use.
WHAT IS VANCOMYCIN?
Vancomycin is a glycopeptide antibiotic isolated from a strain of Streptomyces orientalis discovered in a soil sample from Borneo in the mid-1950s.1 It exerts its action by binding to a d-alanyl-d-alanine cell wall precursor necessary for peptidoglycan cross-linking and, therefore, for inhibiting bacterial cell wall synthesis.
Vancomycin is bactericidal against most gram-positive species, including streptococci and staphylococci, with the exception of Enterococcus species, for which it is bacteriostatic. Though it is bactericidal, it appears to kill bacteria more slowly than beta-lactam antibiotics, and therefore it may take longer to clear bacteremia.4
WHAT IS THE BEST WAY TO DOSE VANCOMYCIN?
Vancomycin is widely distributed to most tissues, with an approximate volume of distribution of 0.4 to 1 L/kg; 50% to 55% is protein-bound. Because of this large volume of distribution, vancomycin’s dosing is based on actual body weight.
Vancomycin is not metabolized and is primarily excreted unchanged in the urine via glomerular filtration. It therefore requires dosage adjustments for renal insufficiency.
Vancomycin’s molecular weight is 1,485.73 Da, making it less susceptible to removal by dialysis than smaller molecules. Dosing of vancomycin in patients on hemodialysis depends on many factors specific to the dialysis center, including but not limited to the type of filter used, the duration of filtration, and whether high-flux filtration is used.
Is continuous intravenous infusion better than standard dosing?
Giving vancomycin by continuous infusion has been suggested as a way to optimize its serum concentration and improve its clinical effectiveness.
Wysocki et al5 conducted a multicenter, prospective, randomized study comparing continuous and intermittent intravenous infusions of vancomycin (the latter every 12 hours) to treat severe hospital-acquired MRSA infections, including bloodstream infections and pneumonia. Although blood concentrations above 10 μg/mL were reached more than 30 hours faster with continuous infusions than with intermittent ones, the microbiologic and clinical outcomes were similar with either method.
James et al6 compared the pharmacodynamics of conventional dosing of vancomycin (ie, 1 g every 12 hours) and continuous infusion in 10 patients with suspected or documented gram-positive infections in a prospective, randomized, crossover study. While no adverse effects were observed, the authors also found no statistically significant difference between the treatment groups in the pharmacodynamic variables investigated, including the area under the curve (AUC) divided by the MIC (the AUC-MIC ratio).
In view of the currently available data, the guidelines for monitoring vancomycin therapy note that there does not appear to be any difference in patient outcomes with continuous infusion vs intermittent dosing.7
Should a loading dose be given?
Another proposed strategy for optimizing vancomycin’s effectiveness is to give a higher initial dose, ie, a loading dose.
Wang et al8 performed a single-center study in 28 patients who received a 25 mg/kg loading dose at a rate of 500 mg/hour. This loading dose was safe, but the authors did not evaluate its efficacy.
Mohammedi et al9 compared loading doses of 500 mg and 15 mg/kg in critically ill patients receiving vancomycin by continuous infusion. The weight-based loading dose produced higher post-dose levels and a significantly higher rate of clinical cure, but there was no significant difference in the rate of survival to discharge from the intensive care unit.
While the use of a loading dose appears to be safe and likely leads to more rapid attainment of therapeutic blood levels, we lack data on whether it improves clinical outcomes, and further study is needed to determine its role.
WHAT IS THE BEST WAY TO MONITOR VANCOMYCIN THERAPY?
Whether and how to use the serum vancomycin concentration to adjust the dosing has been a matter of debate for many years. Convincing evidence that vancomycin levels predict clinical outcomes or that measuring them prevents toxicity is lacking.7
A consensus statement from the American Society of Health-System Pharmacists, the IDSA, and the Society of Infectious Diseases Pharmacists7 contains recommendations for monitoring vancomycin therapy, based on a critical evaluation of the available scientific evidence. Their recommendations:
- Vancomycin serum concentrations should be checked to optimize therapy and used as a surrogate marker of effectiveness.
- Trough, rather than peak, levels should be monitored.
- Trough levels should be checked just before the fourth dose, when steady-state levels are likely to have been achieved. More frequent monitoring may be considered in patients with fluctuating renal function.
- Trough levels should be higher than 10 mg/L to prevent the development of resistance.
- To improve antibiotic penetration and optimize the likelihood of achieving pharmacokinetic and pharmacodynamic targets, trough levels of 15 to 20 mg/L are recommended for pathogens with a vancomycin MIC of 1 mg/L or higher and for complicated infections such as endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia.
- For prolonged courses, it is appropriate to check vancomycin levels weekly in hemodynamically stable patients and more often in those who are not hemodynamically stable.
IS VANCOMYCIN NEPHROTOXIC?
In the 1950s, vancomycin formulations were sometimes called “Mississippi mud” because of the many impurities they contained.1 These impurities were associated with significant nephrotoxicity. Better purification methods used in the manufacture of current formulations mitigate this problem, resulting in a lower incidence of nephrotoxicity.
Over the last several years, organizations such as the American Thoracic Society and the IDSA have recommended targeting higher vancomycin trough concentrations.10 The consequent widespread use of higher doses has renewed interest in vancomycin’s potential nephrotoxicity.
Lodise et al,11 in a cohort study, examined the incidence of nephrotoxicity with higher daily doses of vancomycin (≥ 4 g/day), lower daily doses (< 4 g/day), and linezolid (Zyvox). They defined nephrotoxicity as an increase in serum creatinine of 0.5 mg/dL or a decrease in calculated creatinine clearance of 50% from baseline on 2 consecutive days.
The incidence of nephrotoxicity was significantly higher in the high-dose vancomycin group (34.6%) than in the low-dose vancomycin group (10.9%) and in the linezolid group (6.7%) (P = .001). Additional factors associated with nephrotoxicity in this study included baseline creatinine clearance less than 86.6 mL/minute, weight greater than 101.4 kg (223.5 lb), and being in an intensive care unit.
Hidayat et al12 investigated outcomes in patients with high vs low vancomycin trough levels (≥ 15 mg/L vs < 15 mg/L) in a prospective cohort study. Sixty-three patients achieved an average vancomycin trough of 15 to 20 mg/L, and of these, 11 developed nephrotoxicity, compared with no patients in the low-trough group (P = .01). Of the 11 who developed nephrotoxicity, 10 were concomitantly taking other potentially nephrotoxic agents.
Comment. The data on vancomycin and nephrotoxicity are mostly from studies that had limitations such as small numbers of patients, retrospective design, and variable definitions of nephrotoxicity. Many of the patients in these studies had additional factors contributing to nephrotoxicity, including hemodynamic instability and concomitant exposure to other nephrotoxins. Additionally, the sequence of events (nephrotoxicity leading to elevated vancomycin levels vs elevated vancomycin levels causing nephrotoxicity) is still debatable.
The incidence of nephrotoxicity associated with vancomycin therapy is difficult to determine. However, based on current information, the incidence of nephrotoxicity appears to be low when vancomycin is used as monotherapy.
IS S AUREUS BECOMING RESISTANT TO VANCOMYCIN?
An issue of increasing importance in health care settings is the emergence of vancomycin-intermediate S aureus (VISA) and vancomycin-resistant S aureus (VRSA). Eleven cases of VRSA were identified in the United States from 2002 to 2005.13 All cases of VRSA in the United States have involved the incorporation of enterococcal vanA cassette into the S aureus genome.14 While true VRSA isolates remain rare, VISA isolates are becoming more common.
Heteroresistant VISA: An emerging subpopulation of MRSA
Another population of S aureus that has emerged is heteroresistant vancomycin-intermediate S aureus (hVISA). It is defined as the presence of subpopulations of VISA within a population of MRSA at a rate of one organism per 105 to 106 organisms. With traditional testing methods, the vancomycin MIC for the entire population of the strain is within the susceptible range.15 These hVISA populations are thought to be precursors to the development of VISA.16
The resistance to vancomycin in hVISA and VISA populations is due to increased cell wall thickness, altered penicillin-binding protein profiles, and decreased cell wall autolysis.
While the true prevalence of hVISA is difficult to predict because of challenges in microbiological detection and probably varies between geographic regions and individual institutions, different studies have reported hVISA rates between 2% and 13% of all MRSA isolates.15–17
Reduced vancomycin susceptibility can develop regardless of methicillin susceptibility.18
While hVISA is not common, its presence is thought to be a predictor of failing vancomycin therapy.15
Factors associated with hVISA bacteremia include high-bacterial-load infections, treatment failure (including persistent bacteremia for more than 7 days), and initially low serum vancomycin levels.15
‘MIC creep’: Is it real?
Also worrisome, the average vancomycin MIC for S aureus has been shifting upward, based on reports from several institutions, although it is still within the susceptible range.19,20 However, this “MIC creep” likely reflects, at least in part, differences in MIC testing and varying methods used to analyze the data.19,20
Holmes and Jorgensen,21 in a single-institution study of MRSA isolates recovered from bacteremic patients from 1999 to 2006, determined that no MIC creep existed when they tested vancomycin MICs using the broth microdilution method. The authors found the MIC90 (ie, the MIC in at least 90% of the isolates) remained less than 1 mg/L during each year of the study.
Sader et al,22 in a multicenter study, evaluated 1,800 MRSA bloodstream isolates from nine hospitals across the United States from 2002 to 2006. Vancomycin MICs were again measured by broth microdilution methods. The mode MIC remained stable at 0.625 mg/L during the study period, and the authors did not detect a trend of rising MICs.
The inconsistency between reports of MIC creep at single institutions and the absence of this phenomenon in large, multicenter studies seems to imply that vancomycin MIC creep is not occurring on a grand scale.
Vancomycin tolerance
Another troubling matter with S aureus and vancomycin is the issue of tolerance. Vancomycin tolerance, defined in terms of increased minimum bactericidal concentration, represents a loss of bactericidal activity. Tolerance to vancomycin can occur even if the MIC remains in the susceptible range.23
Safdar and Rolston,24 in an observational study from a cancer center, reported that of eight cases of bacteremia that was resistant to vancomycin therapy, three were caused by S aureus.
Sakoulas et al25 found that higher levels of vancomycin bactericidal activity were associated with higher rates of clinical success; however, they found no effect on the mortality rate.
The issue of vancomycin tolerance remains controversial, and because testing for it is impractical in clinical microbiology laboratories, its implications outside the research arena are difficult to ascertain at present.
IS VANCOMYCIN STILL THE BEST DRUG FOR S AUREUS?
MIC break points have been lowered
In 2006, the Clinical Laboratories and Standards Institute lowered its break points for vancomycin MIC categories for S aureus:
- Susceptible: ≤ 2 mg/L (formerly ≤ 4 mg/L)
- Intermediate: 4–8 mg/L (formerly 8–16 mg/L)
- Resistant: ≥ 16 mg/L (formerly ≥ 32 mg/L).
The rationales for these changes were that the lower break points would better detect hVISA, and that cases have been reported of clinical treatment failure of S aureus infections in which the MICs for vancomycin were 4 mg/L.26
Since 2006, the question has been raised whether to lower the break points even further. A reason for this proposal comes from an enhanced understanding of the pharmacokinetics and pharmacodynamics of vancomycin.
The variable most closely associated with clinical response to vancomycin is the AUC-MIC ratio. An AUC-MIC ratio of 400 or higher may be associated with better outcomes in patients with serious S aureus infection. A study of 108 patients with S aureus infection of the lower respiratory tract indicated that organism eradication was more likely if the AUC-MIC ratio was 400 or greater compared with values less than 400, and this was statistically significant.27 However, in cases of S aureus infection with a vancomycin MIC of 2 mg/L or higher, this ratio may not be achievable.
A prospective study of 414 MRSA bacteremia episodes found a vancomycin MIC of 2 mg/L to be a predictor of death.28 The authors concluded that vancomycin may not be the optimal treatment for MRSA with a vancomycin MIC of 2 mg/L.28 Additional studies have also suggested a possible decrease in response to vancomycin in MRSA isolates with elevated MICs within the susceptible range.25,29
Recent guidelines from the IDSA recommend using the clinical response, regardless of the MIC, to guide antimicrobial selection for isolates with MICs in the susceptible range.2
Combination therapy with vancomycin
As vancomycin use has increased, therapeutic failures with vancomycin have become apparent. Combination therapy has been suggested as an option to increase the efficacy of vancomycin when treating complicated infections.
Rifampin plus vancomycin is controversial.30 The combination is theoretically beneficial, especially in infections associated with prosthetic devices. However, clinical studies have failed to convincingly support its use, and some have suggested that it might prolong bacteremia. In addition, it has numerous drug interactions to consider and adverse effects.31
Gentamicin plus vancomycin. The evidence supporting the use of this combination is weak at best. It appears that clinicians may have extrapolated from the success reported by Korzeniowski and Sande,32 who found that methicillin-susceptible S aureus bacteremia was cleared faster if gentamicin was added to nafcillin. A more recent study33 that compared daptomycin (Cubicin) monotherapy with combined vancomycin and gentamicin to treat MRSA bacteremia and endocarditis showed a better overall success rate with daptomycin (44% vs 32.6%), but the difference was not statistically significant.
Gentamicin has some toxicity. Even short-term use (for the first 4 days of therapy) at low doses for bacteremia and endocarditis due to staphylococci has been associated with a higher rate of renal adverse events, including a significant decrease in creatinine clearance.34
Clindamycin or linezolid plus vancomycin is used to decrease toxin production by S aureus.30
While combination therapy with vancomycin is recommended in specific clinical situations, and the combinations are synergistic in vitro, information is lacking about clinical outcomes to support their use.
Don’t use vancomycin when another drug would be better
Vancomycin continues to be the drug of choice in many circumstances, but in some instances its role is under scrutiny and another drug might be better.
Beta-lactams. In patients with infection due to methicillin-susceptible S aureus, failure rates are higher with vancomycin than with beta-lactam therapy, specifically nafcillin.35–37 Beta-lactam antibiotics are thus the drugs of choice for treating infection with beta-lactam-susceptible strains of S aureus.
Linezolid. In theory, linezolid’s ability to decrease production of the S aureus Panton-Valentine leukocidin (PVL) toxin may be an advantage over vancomycin for treating necrotizing pneumonias. For the treatment of MRSA pneumonia, however, controversy exists as to whether linezolid is superior to vancomycin. An analysis of two prospective, randomized, double-blind studies of patients with MRSA pneumonia suggested that initial therapy with linezolid was associated with better survival and clinical cure rates,38 but a subsequent meta-analysis did not substantiate this finding.39 An additional comparative study has been completed, and analysis of the results is in progress.
Daptomycin, approved for skin and soft-tissue infections and bacteremias, including those with right-sided endocarditis, is a lipopeptide antibiotic with a spectrum of action similar to that of vancomycin.40 Daptomycin is also active against many strains of vancomycin-resistant enterococci. As noted above, in the MRSA subgroup of the pivotal comparative study of treatment for S aureus bacteremia and endocarditis, the success rate for daptomycin-treated patients (44.4%) was better than that for patients treated with vancomycin plus gentamicin (32.6%), but the difference was not statistically significant.33,41
The creatine phosphokinase concentration should be monitored weekly in patients on daptomycin.42 Daptomycin is inactivated by lung surfactant and should not be used to treat pneumonia.
Other treatment options approved by the US Food and Drug Administration (FDA) for MRSA infections include tigecycline (Tygacil), quinupristin-dalfopristin (Synercid), telavancin (Vibativ), and ceftaroline (Teflaro).
Tigecycline is a glycylcycline with bacteriostatic activity against S aureus and wide distribution to the tissues.43
Quinupristin-dalfopristin, a streptogramin antibiotic, has activity against S aureus. Its use may be associated with severe myalgias, sometimes leading patients to stop taking it.
Telavancin, recently approved by the FDA, is a lipoglycopeptide antibiotic.44 It is currently approved to treat complicated skin and skin structure infections and was found to be not inferior to vancomycin. An important side effect of this agent is nephrotoxicity. A negative pregnancy test is required before using this agent in women of childbearing potential.
Ceftaroline, a fifth-generation cephalosporin active against MRSA, has been approved by the FDA for the treatment of skin and skin structure infections and community-acquired pneumonia.45
- Murray BE, Nannini EC. Glycopeptides (vancomycin and teicoplanin), streptogramins (quinupristin-dalfopristin), and lipopeptides (daptomycin). In:Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010:449–468.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:285–292.
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 2001; 45:2460–2467.
- James JK, Palmer SM, Levine DP, Rybak MJ. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 1996; 40:696–700.
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98.
- Wang JT, Fang CT, Chen YC, Chang SC. Necessity of a loading dose when using vancomycin in critically ill patients (letter). J Antimicrob Chemother 2001; 47:246.
- Mohammedi I, Descloux E, Argaud L, Le Scanff J, Robert D. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int J Antimicrob Agents 2006; 27:259–262.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52:1330–1336.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–2144.
- Centers for Disease Control and Prevention. CDC reminds clinical laboratories and healthcare infection preventionists of their role in the search and containment of vancomycin-resistant Staphylococcus aureus (VRSA), May 2010. http://emergency.cdc.gov/coca/reminders/2010/2010may06.asp. Accessed June 7, 2011.
- Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 2008; 46:668–674.
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–451.
- Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–3045.
- Sader HS, Jones RN, Rossi KL, Rybak MJ. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine USA hospitals. J Antimicrob Chemother 2009; 64:1024–1028.
- Pillai SK, Wennersten C, Venkataraman L, Eliopoulos GM, Moellering RC, Karchmer AW. Development of reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 2009; 49:1169–1174.
- Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 2006; 44:3883–3886.
- Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in nonvancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother 2007; 60:788–794.
- Holmes RL, Jorgensen JH. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob Agents Chemother 2008; 52:757–760.
- Sader HS, Fey PD, Limaye AP, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother 2009; 53:4127–4132.
- May J, Shannon K, King A, French G. Glycopeptide tolerance in Staphylococcus aureus. J Antimicrob Chemother 1998; 42:189–197.
- Safdar A, Rolston KV. Vancomycin tolerance, a potential mechanism for refractory gram-positive bacteremia observational study in patients with cancer. Cancer 2006; 106:1815–1820.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Tenover FC, Moellering RC. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007; 44:1208–1215.
- Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43:925–942.
- Soriano A, Marco F, Martínez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:193–200.
- Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008; 52:3315–3320.
- Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2009; 49:1072–1079.
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–680.
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med 1982; 97:496–503.
- Rehm SJ, Boucher H, Levine D, et al. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: subset analysis of patients infected with methicillin-resistant isolates. J Antimicrob Chemother 2008; 62:1413–1421.
- Cosgrove SE, Vigliani GA, Fowler VG, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009; 48:713–721.
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–1231.
- Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 1997; 17:990–997.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003; 124:1789–1797.
- Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 2010; 38:1802–1808.
- Kosmidis C, Levine DP. Daptomycin: pharmacology and clinical use. Expert Opin Pharmacother 2010; 11:615–625.
- Fowler VG, Boucher HW, Corey GR, et al; S aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–665.
- Daptomycin package insert. Lexington, MA. Cubist Pharmaceuticals, Inc. November 2010. www.cubicin.com/pdf/PrescribingInformation.pdf. Accessed June 7, 2011.
- Peterson LR. A review of tigecycline—the first glycylcycline. Int J Antimicrob Agents 2008; 32(suppl 4):S215–S222.
- Saravolatz LD, Stein GE, Johnson LB. Telavancin: a novel lipoglycopeptide. Clin Infect Dis 2009; 49:1908–1914.
- Ceftaroline package insert. St. Louis, MO. Forest Pharmaceuticals. October 2010.
- Murray BE, Nannini EC. Glycopeptides (vancomycin and teicoplanin), streptogramins (quinupristin-dalfopristin), and lipopeptides (daptomycin). In:Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010:449–468.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:285–292.
- Baddour LM, Epstein AE, Erickson CC, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 2001; 45:2460–2467.
- James JK, Palmer SM, Levine DP, Rybak MJ. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented gram-positive infections. Antimicrob Agents Chemother 1996; 40:696–700.
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66:82–98.
- Wang JT, Fang CT, Chen YC, Chang SC. Necessity of a loading dose when using vancomycin in critically ill patients (letter). J Antimicrob Chemother 2001; 47:246.
- Mohammedi I, Descloux E, Argaud L, Le Scanff J, Robert D. Loading dose of vancomycin in critically ill patients: 15 mg/kg is a better choice than 500 mg. Int J Antimicrob Agents 2006; 27:259–262.
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416.
- Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 2008; 52:1330–1336.
- Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–2144.
- Centers for Disease Control and Prevention. CDC reminds clinical laboratories and healthcare infection preventionists of their role in the search and containment of vancomycin-resistant Staphylococcus aureus (VRSA), May 2010. http://emergency.cdc.gov/coca/reminders/2010/2010may06.asp. Accessed June 7, 2011.
- Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 2008; 46:668–674.
- Charles PG, Ward PB, Johnson PD, Howden BP, Grayson ML. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin Infect Dis 2004; 38:448–451.
- Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrob Agents Chemother 2003; 47:3040–3045.
- Sader HS, Jones RN, Rossi KL, Rybak MJ. Occurrence of vancomycin-tolerant and heterogeneous vancomycin-intermediate strains (hVISA) among Staphylococcus aureus causing bloodstream infections in nine USA hospitals. J Antimicrob Chemother 2009; 64:1024–1028.
- Pillai SK, Wennersten C, Venkataraman L, Eliopoulos GM, Moellering RC, Karchmer AW. Development of reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus. Clin Infect Dis 2009; 49:1169–1174.
- Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol 2006; 44:3883–3886.
- Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in nonvancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. J Antimicrob Chemother 2007; 60:788–794.
- Holmes RL, Jorgensen JH. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob Agents Chemother 2008; 52:757–760.
- Sader HS, Fey PD, Limaye AP, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother 2009; 53:4127–4132.
- May J, Shannon K, King A, French G. Glycopeptide tolerance in Staphylococcus aureus. J Antimicrob Chemother 1998; 42:189–197.
- Safdar A, Rolston KV. Vancomycin tolerance, a potential mechanism for refractory gram-positive bacteremia observational study in patients with cancer. Cancer 2006; 106:1815–1820.
- Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol 2004; 42:2398–2402.
- Tenover FC, Moellering RC. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007; 44:1208–1215.
- Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43:925–942.
- Soriano A, Marco F, Martínez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:193–200.
- Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother 2008; 52:3315–3320.
- Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis 2009; 49:1072–1079.
- Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–680.
- Korzeniowski O, Sande MA. Combination antimicrobial therapy for Staphylococcus aureus endocarditis in patients addicted to parenteral drugs and in nonaddicts: a prospective study. Ann Intern Med 1982; 97:496–503.
- Rehm SJ, Boucher H, Levine D, et al. Daptomycin versus vancomycin plus gentamicin for treatment of bacteraemia and endocarditis due to Staphylococcus aureus: subset analysis of patients infected with methicillin-resistant isolates. J Antimicrob Chemother 2008; 62:1413–1421.
- Cosgrove SE, Vigliani GA, Fowler VG, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009; 48:713–721.
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990; 34:1227–1231.
- Gentry CA, Rodvold KA, Novak RM, Hershow RC, Naderer OJ. Retrospective evaluation of therapies for Staphylococcus aureus endocarditis. Pharmacotherapy 1997; 17:990–997.
- Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 2003; 82:333–339.
- Wunderink RG, Rello J, Cammarata SK, Croos-Dabrera RV, Kollef MH. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 2003; 124:1789–1797.
- Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 2010; 38:1802–1808.
- Kosmidis C, Levine DP. Daptomycin: pharmacology and clinical use. Expert Opin Pharmacother 2010; 11:615–625.
- Fowler VG, Boucher HW, Corey GR, et al; S aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–665.
- Daptomycin package insert. Lexington, MA. Cubist Pharmaceuticals, Inc. November 2010. www.cubicin.com/pdf/PrescribingInformation.pdf. Accessed June 7, 2011.
- Peterson LR. A review of tigecycline—the first glycylcycline. Int J Antimicrob Agents 2008; 32(suppl 4):S215–S222.
- Saravolatz LD, Stein GE, Johnson LB. Telavancin: a novel lipoglycopeptide. Clin Infect Dis 2009; 49:1908–1914.
- Ceftaroline package insert. St. Louis, MO. Forest Pharmaceuticals. October 2010.
KEY POINTS
- Giving vancomycin by continuous infusion appears to offer no advantage over giving it every 12 hours.
- Therapeutic blood levels can be reached more quickly if a loading dose is given, but whether this offers a clinical advantage is unclear.
- The trough vancomycin serum concentration should be greater than 10 mg/L to prevent the development of resistance, and trough levels of 15 to 20 mg/L are recommended if the minimum inhibitory concentration (MIC) is 1 mg/L or higher.
- Whether S aureus is becoming resistant to vancomycin is not clear.
- The variable most closely associated with clinical response to vancomycin is the area under the curve (AUC) divided by the MIC (the AUC-MIC ratio), which should be greater than 400.