User login
When good drugs turn weirdly bad
Many of the adverse effects of the small-molecule drugs such as azathioprine (Imuran) and methotrexate are those expected from chemical toxicity or inhibition of proliferation, eg, aminotransferase elevation, leukopenia, and alopecia. Mycophenolate mofetil (CellCept) uniquely can cause profound anemia, cyclophosphamide (Cytoxan) elicits cystitis, and many of these drugs trigger virus-associated malignancies. In perhaps 8% of patients, azathioprine causes a systemic hypersensitivity reaction with high fevers, variable rash, leukocytosis, and elevated aminotransferase levels shortly after it is started. Yet we are often slow to recognize this syndrome, as we tend to search for an infection and forget that even immunosuppressive drugs can cause systemic allergic-type reactions. A similar syndrome following initiation of phenytoin (Dilantin) would be recognized far more rapidly.
But the biologic agents, which target specific components of the immune system, resulting in focal immunosuppression and a disturbance in the homeostatic balance of the immune system, elicit some of the more challenging and sometimes paradoxical side effects. Interferon alfa, which has antiviral effects, is also used as an immunomodulator to treat Behçet disease and as part of regimens that treat specific malignancies. Perhaps because it up-regulates the expression of major histocompatibility complex class II molecules on antigen-presenting cells, interferon therapy also triggers several organ-specific autoimmune syndromes, including autoimmune thrombocytopenia, hypothyroidism, hemolytic anemia, hepatitis, and psoriasis.
Even more challenging to understand and sometimes to treat are the inflammatory effects of anti-tumor necrosis factor agents. Drugs of this class can evoke a demyelinating syndrome similar to multiple sclerosis. Further, even though they are used to treat psoriasis, they can also provoke a psoriasiform, often palmar and pustular, reaction.
So as we continue to adopt targeted immunologic therapies and revel in their efficacy, we need to remain humbled by what we don’t yet fully understand about the complexity of what the 19th century physiologist Claude Bernard termed the milieu intérieur (homeostasis) and keep in mind that even the most specific of drugs can have untoward biologic effects by weirdly disrupting our finely balanced immune system.
Many of the adverse effects of the small-molecule drugs such as azathioprine (Imuran) and methotrexate are those expected from chemical toxicity or inhibition of proliferation, eg, aminotransferase elevation, leukopenia, and alopecia. Mycophenolate mofetil (CellCept) uniquely can cause profound anemia, cyclophosphamide (Cytoxan) elicits cystitis, and many of these drugs trigger virus-associated malignancies. In perhaps 8% of patients, azathioprine causes a systemic hypersensitivity reaction with high fevers, variable rash, leukocytosis, and elevated aminotransferase levels shortly after it is started. Yet we are often slow to recognize this syndrome, as we tend to search for an infection and forget that even immunosuppressive drugs can cause systemic allergic-type reactions. A similar syndrome following initiation of phenytoin (Dilantin) would be recognized far more rapidly.
But the biologic agents, which target specific components of the immune system, resulting in focal immunosuppression and a disturbance in the homeostatic balance of the immune system, elicit some of the more challenging and sometimes paradoxical side effects. Interferon alfa, which has antiviral effects, is also used as an immunomodulator to treat Behçet disease and as part of regimens that treat specific malignancies. Perhaps because it up-regulates the expression of major histocompatibility complex class II molecules on antigen-presenting cells, interferon therapy also triggers several organ-specific autoimmune syndromes, including autoimmune thrombocytopenia, hypothyroidism, hemolytic anemia, hepatitis, and psoriasis.
Even more challenging to understand and sometimes to treat are the inflammatory effects of anti-tumor necrosis factor agents. Drugs of this class can evoke a demyelinating syndrome similar to multiple sclerosis. Further, even though they are used to treat psoriasis, they can also provoke a psoriasiform, often palmar and pustular, reaction.
So as we continue to adopt targeted immunologic therapies and revel in their efficacy, we need to remain humbled by what we don’t yet fully understand about the complexity of what the 19th century physiologist Claude Bernard termed the milieu intérieur (homeostasis) and keep in mind that even the most specific of drugs can have untoward biologic effects by weirdly disrupting our finely balanced immune system.
Many of the adverse effects of the small-molecule drugs such as azathioprine (Imuran) and methotrexate are those expected from chemical toxicity or inhibition of proliferation, eg, aminotransferase elevation, leukopenia, and alopecia. Mycophenolate mofetil (CellCept) uniquely can cause profound anemia, cyclophosphamide (Cytoxan) elicits cystitis, and many of these drugs trigger virus-associated malignancies. In perhaps 8% of patients, azathioprine causes a systemic hypersensitivity reaction with high fevers, variable rash, leukocytosis, and elevated aminotransferase levels shortly after it is started. Yet we are often slow to recognize this syndrome, as we tend to search for an infection and forget that even immunosuppressive drugs can cause systemic allergic-type reactions. A similar syndrome following initiation of phenytoin (Dilantin) would be recognized far more rapidly.
But the biologic agents, which target specific components of the immune system, resulting in focal immunosuppression and a disturbance in the homeostatic balance of the immune system, elicit some of the more challenging and sometimes paradoxical side effects. Interferon alfa, which has antiviral effects, is also used as an immunomodulator to treat Behçet disease and as part of regimens that treat specific malignancies. Perhaps because it up-regulates the expression of major histocompatibility complex class II molecules on antigen-presenting cells, interferon therapy also triggers several organ-specific autoimmune syndromes, including autoimmune thrombocytopenia, hypothyroidism, hemolytic anemia, hepatitis, and psoriasis.
Even more challenging to understand and sometimes to treat are the inflammatory effects of anti-tumor necrosis factor agents. Drugs of this class can evoke a demyelinating syndrome similar to multiple sclerosis. Further, even though they are used to treat psoriasis, they can also provoke a psoriasiform, often palmar and pustular, reaction.
So as we continue to adopt targeted immunologic therapies and revel in their efficacy, we need to remain humbled by what we don’t yet fully understand about the complexity of what the 19th century physiologist Claude Bernard termed the milieu intérieur (homeostasis) and keep in mind that even the most specific of drugs can have untoward biologic effects by weirdly disrupting our finely balanced immune system.
Rash from hepatitis C treatment
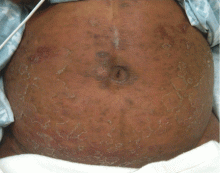
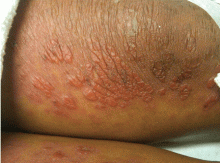
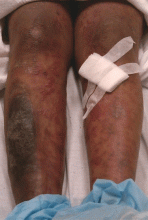
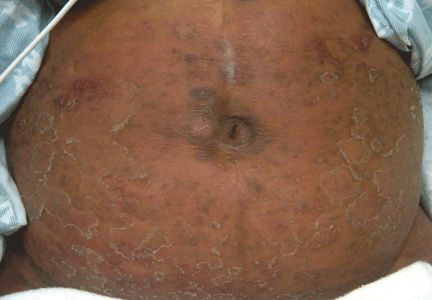
A 54-year-old woman with hepatitis C virus infection presents with generalized rash, pruritus, and fever over the past week. The rash appeared on her left arm after she received her fifth weekly injection of pegylated interferon alfa 2b, in combination with daily oral ribavirin (Copegus, Rebetol). Over the course of 3 days, it spread to her face and the rest of her body.
Q: What is the most likely clinical diagnosis?
- Stevens-Johnson syndrome
- Mixed cryoglobulinemia
- Acute eczematous drug eruption
- Lichen planus
A: Acute eczematous drug eruption is the most likely diagnosis.
The clinical presentation and laboratory findings suggest (the latter by exclusion) that our patient had an allergic drug reaction to the interferon or to the ribavirin therapy, or to both. Although this combination is a standard treatment for chronic hepatitis C, some patients experience adverse reactions that lead to its discontinuation. Local injection-site reactions are the most prevalent, affecting up to 12% of patients, whereas eczematous dermatoses manifest less commonly, occurring in up to 5% of patients.1
While awaiting the results of skin biopsy, a careful evaluation of the clinical features of the physical examination and an appropriate laboratory evaluation can rule out other important conditions in the differential diagnosis.
The absence of mucous membrane involvement steers the diagnosis away from Stevens-Johnson syndrome, a life-threatening hypersensitivity condition often triggered by drugs, malignant tumors, and viral infections, which may also affect internal organs. In this condition, skin biopsy specimens would be distinguished by subepidermal bullae and epidermal cell necrosis—neither of which was seen in our patient.
Mixed cryoglobulinemia should always be considered in hepatitis C patients because of the strong association between this infection and the development of cryoglobulins. The rash usually is purpuric, but it may be pleomorphic.2,3 This vasculitis often manifests with excess cryoglobulins, elevated rheumatoid factor, and low titers of complement in the blood due to consumption by immune complexes. Tissue biopsy would usually show typical vascular changes if performed on fresh lesions.4,5 The normal levels of these components in our patient coupled with the appearance of her skin makes cryoglobulinemia a less likely cause.
Furthermore, hepatitis C infection, whether or not treated with interferon and ribavirin, can cause an onset or recurrence of other dermatologic conditions, notably lichen planus, psoriasis, vitiligo, and systemic lupus erythematosus.1–4
In lichen planus, the rash is often described as flat-topped, pruritic, and violaceous. It may involve the extremities, the genitalia, and the oral cavity.4,5 The difference in quality of the rash compared with the rash in our patient makes lichen planus less likely.
Exclusion of the other conditions in the differential diagnosis, in addition to results from a definitive punch biopsy, solidified the diagnosis in our patient. Skin biopsy of the patient’s lower-extremity lesions revealed spongiotic dermatitis with lymphocytes, neutrophils, and few eosinophils—a finding characteristic of an acute eczematous drug eruption. Improvement of her rash after discontinuation of interferon and ribavirin further supported this conclusion, although it was unclear whether one or both agents were responsible.
OUTCOME
Management of acute eczematous drug eruption entails stopping the offending drug and alleviating the symptoms. Our patient’s non-life-threatening rash improved dramatically with cessation of interferon and ribavirin. She received a single dose of a systemic corticosteroid initially, out of concern for a severe medication-induced reaction (ie, Stevens-Johnson syndrome), but she was otherwise maintained with diphenhydramine (Benadryl) and a multivitamin ointment for the rash throughout her 9-day hospital stay. Her pruritus was well controlled with hydroxyzine (Atarax, Vistaril). At discharge, she was referred back to her hepatologist for further treatment of her hepatitis C, possibly with interferon and ribavirin again.
TAKE-HOME MESSAGE
Adverse reactions to interferon and ribavirin treatment in hepatitis C patients can manifest dermatologically, and the combination therapy should be discontinued to prevent further insult. A broad variety of conditions in the differential diagnosis should be taken into account, but dermatologic conditions that occur or recur specifically in hepatitis C patients should be considered as well.
- Dereure O, Raison-Peyron N, Larrey D, Blanc F, Guilhou JJ. Diffuse inflammatory lesions in patients treated with interferon alfa and ribavirin for hepatitis C: a series of 20 patients. Br J Dermatol 2002; 147:1142–1146.
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol 2002; 55:4–13.
- Faurie P, Broussolle C, Zoulim F, Trepo C, Sève P. Sarcoidosis and hepatitis C: clinical description of 11 cases. Eur J Gastroenterol Hepatol 2010; 22:967–972.
- Shengyuan L, Songpo Y, Wen W, Wenjing T, Haitao Z, Binyou W. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol 2009; 145:1040–1047.
- Aamir S, Ullah Z, Iqbal Z, Khan AA, Yaqub F, Malik K. Cutaneous manifestations of interferon alfa and ribavirin for hepatitis C. J Pak Assoc Dermatol 2008; 18:14–20.
A 54-year-old woman with hepatitis C virus infection presents with generalized rash, pruritus, and fever over the past week. The rash appeared on her left arm after she received her fifth weekly injection of pegylated interferon alfa 2b, in combination with daily oral ribavirin (Copegus, Rebetol). Over the course of 3 days, it spread to her face and the rest of her body.
Q: What is the most likely clinical diagnosis?
- Stevens-Johnson syndrome
- Mixed cryoglobulinemia
- Acute eczematous drug eruption
- Lichen planus
A: Acute eczematous drug eruption is the most likely diagnosis.
The clinical presentation and laboratory findings suggest (the latter by exclusion) that our patient had an allergic drug reaction to the interferon or to the ribavirin therapy, or to both. Although this combination is a standard treatment for chronic hepatitis C, some patients experience adverse reactions that lead to its discontinuation. Local injection-site reactions are the most prevalent, affecting up to 12% of patients, whereas eczematous dermatoses manifest less commonly, occurring in up to 5% of patients.1
While awaiting the results of skin biopsy, a careful evaluation of the clinical features of the physical examination and an appropriate laboratory evaluation can rule out other important conditions in the differential diagnosis.
The absence of mucous membrane involvement steers the diagnosis away from Stevens-Johnson syndrome, a life-threatening hypersensitivity condition often triggered by drugs, malignant tumors, and viral infections, which may also affect internal organs. In this condition, skin biopsy specimens would be distinguished by subepidermal bullae and epidermal cell necrosis—neither of which was seen in our patient.
Mixed cryoglobulinemia should always be considered in hepatitis C patients because of the strong association between this infection and the development of cryoglobulins. The rash usually is purpuric, but it may be pleomorphic.2,3 This vasculitis often manifests with excess cryoglobulins, elevated rheumatoid factor, and low titers of complement in the blood due to consumption by immune complexes. Tissue biopsy would usually show typical vascular changes if performed on fresh lesions.4,5 The normal levels of these components in our patient coupled with the appearance of her skin makes cryoglobulinemia a less likely cause.
Furthermore, hepatitis C infection, whether or not treated with interferon and ribavirin, can cause an onset or recurrence of other dermatologic conditions, notably lichen planus, psoriasis, vitiligo, and systemic lupus erythematosus.1–4
In lichen planus, the rash is often described as flat-topped, pruritic, and violaceous. It may involve the extremities, the genitalia, and the oral cavity.4,5 The difference in quality of the rash compared with the rash in our patient makes lichen planus less likely.
Exclusion of the other conditions in the differential diagnosis, in addition to results from a definitive punch biopsy, solidified the diagnosis in our patient. Skin biopsy of the patient’s lower-extremity lesions revealed spongiotic dermatitis with lymphocytes, neutrophils, and few eosinophils—a finding characteristic of an acute eczematous drug eruption. Improvement of her rash after discontinuation of interferon and ribavirin further supported this conclusion, although it was unclear whether one or both agents were responsible.
OUTCOME
Management of acute eczematous drug eruption entails stopping the offending drug and alleviating the symptoms. Our patient’s non-life-threatening rash improved dramatically with cessation of interferon and ribavirin. She received a single dose of a systemic corticosteroid initially, out of concern for a severe medication-induced reaction (ie, Stevens-Johnson syndrome), but she was otherwise maintained with diphenhydramine (Benadryl) and a multivitamin ointment for the rash throughout her 9-day hospital stay. Her pruritus was well controlled with hydroxyzine (Atarax, Vistaril). At discharge, she was referred back to her hepatologist for further treatment of her hepatitis C, possibly with interferon and ribavirin again.
TAKE-HOME MESSAGE
Adverse reactions to interferon and ribavirin treatment in hepatitis C patients can manifest dermatologically, and the combination therapy should be discontinued to prevent further insult. A broad variety of conditions in the differential diagnosis should be taken into account, but dermatologic conditions that occur or recur specifically in hepatitis C patients should be considered as well.
A 54-year-old woman with hepatitis C virus infection presents with generalized rash, pruritus, and fever over the past week. The rash appeared on her left arm after she received her fifth weekly injection of pegylated interferon alfa 2b, in combination with daily oral ribavirin (Copegus, Rebetol). Over the course of 3 days, it spread to her face and the rest of her body.
Q: What is the most likely clinical diagnosis?
- Stevens-Johnson syndrome
- Mixed cryoglobulinemia
- Acute eczematous drug eruption
- Lichen planus
A: Acute eczematous drug eruption is the most likely diagnosis.
The clinical presentation and laboratory findings suggest (the latter by exclusion) that our patient had an allergic drug reaction to the interferon or to the ribavirin therapy, or to both. Although this combination is a standard treatment for chronic hepatitis C, some patients experience adverse reactions that lead to its discontinuation. Local injection-site reactions are the most prevalent, affecting up to 12% of patients, whereas eczematous dermatoses manifest less commonly, occurring in up to 5% of patients.1
While awaiting the results of skin biopsy, a careful evaluation of the clinical features of the physical examination and an appropriate laboratory evaluation can rule out other important conditions in the differential diagnosis.
The absence of mucous membrane involvement steers the diagnosis away from Stevens-Johnson syndrome, a life-threatening hypersensitivity condition often triggered by drugs, malignant tumors, and viral infections, which may also affect internal organs. In this condition, skin biopsy specimens would be distinguished by subepidermal bullae and epidermal cell necrosis—neither of which was seen in our patient.
Mixed cryoglobulinemia should always be considered in hepatitis C patients because of the strong association between this infection and the development of cryoglobulins. The rash usually is purpuric, but it may be pleomorphic.2,3 This vasculitis often manifests with excess cryoglobulins, elevated rheumatoid factor, and low titers of complement in the blood due to consumption by immune complexes. Tissue biopsy would usually show typical vascular changes if performed on fresh lesions.4,5 The normal levels of these components in our patient coupled with the appearance of her skin makes cryoglobulinemia a less likely cause.
Furthermore, hepatitis C infection, whether or not treated with interferon and ribavirin, can cause an onset or recurrence of other dermatologic conditions, notably lichen planus, psoriasis, vitiligo, and systemic lupus erythematosus.1–4
In lichen planus, the rash is often described as flat-topped, pruritic, and violaceous. It may involve the extremities, the genitalia, and the oral cavity.4,5 The difference in quality of the rash compared with the rash in our patient makes lichen planus less likely.
Exclusion of the other conditions in the differential diagnosis, in addition to results from a definitive punch biopsy, solidified the diagnosis in our patient. Skin biopsy of the patient’s lower-extremity lesions revealed spongiotic dermatitis with lymphocytes, neutrophils, and few eosinophils—a finding characteristic of an acute eczematous drug eruption. Improvement of her rash after discontinuation of interferon and ribavirin further supported this conclusion, although it was unclear whether one or both agents were responsible.
OUTCOME
Management of acute eczematous drug eruption entails stopping the offending drug and alleviating the symptoms. Our patient’s non-life-threatening rash improved dramatically with cessation of interferon and ribavirin. She received a single dose of a systemic corticosteroid initially, out of concern for a severe medication-induced reaction (ie, Stevens-Johnson syndrome), but she was otherwise maintained with diphenhydramine (Benadryl) and a multivitamin ointment for the rash throughout her 9-day hospital stay. Her pruritus was well controlled with hydroxyzine (Atarax, Vistaril). At discharge, she was referred back to her hepatologist for further treatment of her hepatitis C, possibly with interferon and ribavirin again.
TAKE-HOME MESSAGE
Adverse reactions to interferon and ribavirin treatment in hepatitis C patients can manifest dermatologically, and the combination therapy should be discontinued to prevent further insult. A broad variety of conditions in the differential diagnosis should be taken into account, but dermatologic conditions that occur or recur specifically in hepatitis C patients should be considered as well.
- Dereure O, Raison-Peyron N, Larrey D, Blanc F, Guilhou JJ. Diffuse inflammatory lesions in patients treated with interferon alfa and ribavirin for hepatitis C: a series of 20 patients. Br J Dermatol 2002; 147:1142–1146.
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol 2002; 55:4–13.
- Faurie P, Broussolle C, Zoulim F, Trepo C, Sève P. Sarcoidosis and hepatitis C: clinical description of 11 cases. Eur J Gastroenterol Hepatol 2010; 22:967–972.
- Shengyuan L, Songpo Y, Wen W, Wenjing T, Haitao Z, Binyou W. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol 2009; 145:1040–1047.
- Aamir S, Ullah Z, Iqbal Z, Khan AA, Yaqub F, Malik K. Cutaneous manifestations of interferon alfa and ribavirin for hepatitis C. J Pak Assoc Dermatol 2008; 18:14–20.
- Dereure O, Raison-Peyron N, Larrey D, Blanc F, Guilhou JJ. Diffuse inflammatory lesions in patients treated with interferon alfa and ribavirin for hepatitis C: a series of 20 patients. Br J Dermatol 2002; 147:1142–1146.
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol 2002; 55:4–13.
- Faurie P, Broussolle C, Zoulim F, Trepo C, Sève P. Sarcoidosis and hepatitis C: clinical description of 11 cases. Eur J Gastroenterol Hepatol 2010; 22:967–972.
- Shengyuan L, Songpo Y, Wen W, Wenjing T, Haitao Z, Binyou W. Hepatitis C virus and lichen planus: a reciprocal association determined by a meta-analysis. Arch Dermatol 2009; 145:1040–1047.
- Aamir S, Ullah Z, Iqbal Z, Khan AA, Yaqub F, Malik K. Cutaneous manifestations of interferon alfa and ribavirin for hepatitis C. J Pak Assoc Dermatol 2008; 18:14–20.
Statin myopathy: A common dilemma not reflected in clinical trials
When a patient taking a statin complains of muscle aches, is he or she experiencing statin-induced myopathy or some other problem? Should statin therapy be discontinued?
Statins have proven efficacy in preventing heart attacks and death,1 and they are the most widely prescribed drugs worldwide. Nevertheless, they remain underused, with only 50% of those who would benefit from being on a statin receiving one.2,3 In addition, at least 25% of adults who start taking statins stop taking them by 6 months, and up to 60% stop by 2 years.4
Patient and physician fears about myopathy remain a key reason for stopping. Myopathy, a known side effect of statins, is rare in randomized controlled trials, but less so in observational studies and clinical experience. This discrepancy between clinical trials and clinical experience reduces confidence in lipid-lowering therapy and contributes to its underuse.
This review emphasizes clinical aspects of statin myopathy that are important to the practicing physician. We will define myopathy, review its purported mechanisms, and describe a clinical approach to patients with possible toxicity, including risk factors, physical findings, and consideration of alternate diagnoses. Since there is no single test to diagnose statin-induced myopathy, we offer a framework to aid clinicians in stratifying patients based on the likelihood that their symptoms are due to statin toxicity weighed against the likelihood that they will benefit from statin therapy.
DEFINITIONS DIFFER
Little consensus exists on how to define the adverse muscle effects of statins,5 which may contribute to the underdiagnosis of this complication. The magnitude of creatine kinase (CK) elevation required to define rhabdomyolysis has increased from 500 IU/L in 1982,6 to 1,000 IU/L in 1988,7 to 50 times the upper limit of normal in one current definition.8 The American Heart Association, the American College of Cardiology, the National Heart Lung and Blood Institute,9 the National Lipid Association,8 and the US Food and Drug Administration10 all differ in their definitions.
Our definitions
For the purpose of this article, we offer the following definitions:
Myalgia—muscle weakness, soreness, tenderness, stiffness, cramping, or aching, either at rest or with exertion, without any elevation in CK.
Myositis—elevated CK with or without muscle symptoms. The “-itis” suffix is unfortunate since myositis does not correspond to inflammation on biopsy.
Rhabdomyolysis—muscle symptoms with a CK level 10 times the upper limit of normal or higher. Evidence of renal dysfunction is not required for the diagnosis, as preexisting renal disease and hydration status are more closely related to kidney damage than the degree of muscle injury.11
STATIN MYOPATHY IS MORE COMMON IN THE REAL WORLD THAN IN TRIALS
The incidence of statin-induced myopathy is significantly lower in randomized controlled trials of statin efficacy than in observational studies of real-world patients. In randomized clinical trials, myalgia was reported in 1% to 5% of patients in the statin groups and placebo groups alike,9,12 whereas clinical practice would suggest it is more common.
Why is statin-induced myopathy so uncommon in clinical trials?
A reason may be that patients in clinical trials are carefully screened. To minimize toxicity, the clinical trials of statins excluded patients with renal insufficiency, hepatic insufficiency, a history of muscular complaints, and poorly controlled diabetes, as well as patients taking drugs with possible interactions. Large efficacy trials have excluded up to 30% of the participants in active prerandomization phases.13,14
Another reason is that these trials were designed to assess the efficacy of statins and were not sensitive to adverse effects like muscle pain. When they looked at myopathy, they focused on rhabdomyolysis—the most severe form—rather than on myalgia, fatigue, or other minor muscle complaints.15 Additionally, most trials enrolled too few patients and did not have long enough follow-up to reveal infrequent toxicities.
Despite the strict criteria, a significant number of trial patients discontinued statin therapy during the study period. In the Treat to New Targets (TNT) trial, 5% of patients in both the high- and low-dose atorvastatin (Lipitor) groups experienced muscle toxicity, even though 35% of eligible patients had been excluded during the open-label run-in phase.14
Also, physicians may overlook and patients may fail to report symptoms such as fatigue, malaise, or dyspnea that are not commonly accepted as signs of statin toxicity.16
Findings from observational studies
Observational studies in nonselected outpatients show a higher frequency of muscle complaints in the statin groups than in the control groups. These studies suggest the frequency of statin myopathy is 9% to 20%.17–19
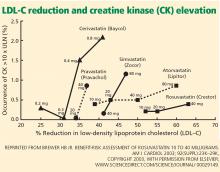
The Prediction of Muscular Risk in Observational Conditions (PRIMO) study20 was one of the largest and best-defined observational studies of muscular symptoms in an unselected population. It included 7,924 French outpatients with hypercholesterolemia, ages 18 to 75 years, on high-dose statins for 3 or more months before the study. Daily statin regimens included atorvastatin 40 to 80 mg, fluvastatin (Lescol) 80 mg, pravastatin (Pravachol) 40 mg, and simvastatin (Zocor) 40 to 80 mg. In this study, 10.5% of patients reported muscle-related symptoms.
Buettner et al,21 in another cross-sectional study, interviewed and examined 3,580 adults over age 40. Of those taking statins, 22% reported having had musculoskeletal pain in at least one anatomic region in the last 30 days, compared with 16.7% of those not taking a statin.
In the United States, where an estimated 33 million adults use statins, musculoskeletal pain can be expected to occur in 7 million people, likely induced by statin therapy in 25% of cases.22
WHAT CAUSES STATIN MYOPATHY?
The causes of statin-induced myopathy are poorly understood.
Historically, statin-induced toxicity was thought to be caused by inhibition of the synthesis of mevalonate, leading to depletion of its metabolites, such as cholesterol, isoprenoids, and ubiquinone (coenzyme Q10). Depletion of intracellular cholesterol may lead to abnormal membrane behaviors; depletion of isoprenoids may affect intracellular signaling; depletion of coenzyme Q10 may in turn reduce mitochondrial respiratory function. Genetic factors may also play a role, contributing to pharmacokinetics and predisposing metabolic muscle disorders.23,24
Statin-induced myopathy seems to be different in randomized efficacy trials than in the clinical setting. In randomized trials, the mechanism appears to involve abnormal pharmacokinetics. The participants are carefully selected to have a low risk of muscle toxicity, but some develop toxicity when statin levels are elevated because of reduced drug breakdown and metabolism. However, in clinical practice, where a much larger group of unselected patients are exposed to statins, toxicity appears to be more related to a metabolic predisposition.25–27
Multiple minor metabolic abnormalities have been described in the muscles of patients with statin-induced muscle toxicity, suggesting that some patients have a predisposition for muscle complaints.23 About 25% of patients with recurrent rhabdomyolysis irrespective of lipid-lowering therapy have an underlying metabolic muscle disorder.28 In these vulnerable patients, minor metabolic defects are exacerbated by any agent that reduces the delivery of fat substrate to muscle, leading to muscle starvation.
This concept explains why patients may develop the same muscle complaint on different lipid-lowering agents.29,30 It also may explain why rhabdomyolysis can occur after apheresis of lipids, when no drugs have been given.31 In vulnerable patients, muscle toxicity may result from any agent that lessens the lipid substrate available to muscle rather than from the reduction of products downstream from mevalonate by statins.
SOME STATINS MAY BE LESS TOXIC
In the PRIMO study, muscle-related symptoms occurred with the various regimens as follows:
- Fluvastatin XL 40 mg—5.1%
- Pravastatin 40 mg—10.9%
- Atorvastatin 40 to 80 mg—14.9%
- Simvastatin 40 to 80 mg—18.2%.
Others have also shown that fluvastatin contributed to the smallest number of reported cases of rhabdomyolysis among statins: 55 (1.6%) of 3,339 cases.34
More recent studies indicate that rosuvastatin (Crestor), the most hydrophilic statin, may be well tolerated in those who do not tolerate other statins,35–37 though no head-to-head trial has been done.
RISK FACTORS FOR STATIN-INDUCED MYOPATHY
APPROACH TO SUSPECTED STATIN-INDUCED MYOPATHY
The recommendations that follow are based on observations from our statin myopathy clinic in more than 650 patients, of whom more than 60 have suffered rhabdomyolysis. This experience is largely anecdotal, since such patients have not been well studied in controlled trials.
Are the symptoms due to the statin?
In our experience, most patients who develop significant weakness and pain on statin therapy have normal CK levels.40
Since there is as yet no test to confirm or reject the diagnosis of statin toxicity, the first objective is to determine the likelihood that the muscle complaint is being caused by statin therapy. Factors that make statin toxicity more likely must be weighed against any features that are atypical or that favor an alternate diagnosis.
The final decision about future statin treatment depends on the balance between the expected benefit of statin therapy for each individual and the likelihood that the symptoms are due to statin therapy. Below, we provide an algorithmic approach based on history, physical examination, and laboratory findings.
Findings from the history that implicate statins
Many muscle symptoms resolve within 2 weeks of starting statin therapy. Therefore, if patients have a normal CK level and can tolerate the symptoms, we ask them to continue therapy and see if their symptoms resolve with continued use.
Symptoms that persist beyond the first 2 weeks of therapy are likely due to the statin. These include symmetric burning or pain in the large muscles during exercise that was not present before lipid-lowering therapy. Any symptom that reproducibly recurs with statin rechallenge and disappears within 2 weeks of discontinuing therapy is more likely to be caused by the statin.
Findings of the PRIMO study are representative of typical statin-induced symptoms that we see in our clinic.20
- Most patients did not identify a trigger, but the 40% who did had engaged in unusual physical exertion or had received a new drug in addition to the statin.
- Heaviness, stiffness, or cramps predominated in 70%, with only a quarter noting weakness and another quarter suffering myalgias during exercise. Pain was diffuse in 60% and more common in the lower extremities than the upper extremities.
- Physically active patients were more likely to suffer muscle symptoms than sedentary patients, echoing the observation by Sinzinger and O’Grady that athletes are especially intolerant of lipid-lowering therapy.41
- Patients who have had muscle complaints with other drug therapies such as bisphosphonates, 42 raloxifene (Evista),43 or diuretics may be having the same provocation of an underlying muscle predisposition by the statin.
- A personal or family history of muscle complaints predisposed patients to statin-induced myopathy.20
Finally, we have repeatedly found that when dyspnea and fatigue are associated with the muscle complaint, they are more likely to be caused by statins.44
Statins can unveil underlying musculoskeletal disorders
Atypical complaints require us to search for an alternate diagnosis before dismissing them as not statin-induced.
Previous episodes of myoglobinuria (dark urine after exertion) would raise the possibility of a metabolic myopathy. Frequent muscle cramps raise the possibility of a metabolic myopathy or motor neuron disease.
Asymmetric pain or pain involving joints and ligaments is less likely to be statin-related but in our experience occasionally occurs.
Some patients with underlying degenerative arthritis or tendinitis repeatedly develop worsening symptoms each time they take statins, perhaps because muscle weakness exacerbates the arthropathy or tendinopathy.45
Although statin-related muscle complaints are almost always symmetric, many patients with underlying peripheral vascular disease have asymmetric pain in the limb with poor vascular supply; the pain is reproducible by statin rechallenge.
In our experience, many patients whose chronic low back pain is due to a lumbar radiculopathy experience exacerbations of that pain whenever they start statins.
Weakness preceding the use of the statins or a family history of neuromuscular disorders may indicate a neurodegenerative disorder and warrants consideration of early neurological consultation.
Although these rules are not absolute, they are helpful in the initial evaluation, which must exclude alternative diagnoses.
Is the patient a vegetarian? A drinker? Taking supplements?
Taking a careful history of diet and supplement use is important to find exposures that may increase the risk of statin-related muscle complaints. Vegetarians may develop carnitine or vitamin B12 deficiencies. Alcohol and vitamin E and other supplements are occasional causes of muscle symptoms falsely attributed to statin therapy. It is also important to remember that red yeast rice contains lovastatin, which can exacerbate myopathy, especially when taken in conjunction with another statin.
Physical examination
The examination of patients with possible statin-induced myopathy begins with a general assessment for signs of hypothyroidism or excess alcohol consumption.
Ankle-brachial indices are used to exclude significant peripheral vascular disease.
The musculoskeletal examination focuses on muscle atrophy, tone, and strength but also excludes tendinopathies, arthropathies, and myofascial pain syndromes, which are often confused with muscle pain.
We conduct quantitative dynamometry, measuring handgrip with a Jamar dynamometer and hip abduction with a Nicholas Manual Muscle Tester. Precise dynamometric measurements are tracked at subsequent visits and are helpful in following recovery from myopathy as well as in tracking strength during subsequent statin rechallenges.
We routinely look for hyperreflexia, fasciculations, extensor-plantar responses, and decreased heel-to-shin movement, which would suggest myelopathy. Reflexes and a sensory examination including vibration and temperature sensation help exclude radiculopathy and peripheral neuropathy.
Laboratory evaluation
In every patient with possibly statin myopathy, the primary care physician should measure:
- The serum CK level (preferably more than 72 hours after exercise)
- The 25-hydroxy vitamin level
- The thyroid-stimulating hormone level.
Further laboratory evaluation depends on the findings and will often be directed by subspecialists. For example, we assess the sedimentation rate, anti-Ro and anti-La antibodies, and the myositis panel in patients with elevated CK whose other findings suggest an autoimmune or inflammatory process. We test serum carnitine levels (free, total, and esterified), fasting serum lactate levels, and serum cortisol in those with findings suggestive of metabolic myopathy. We order electromyography and nerve conduction studies in patients with possible myelopathy, peripheral neuropathy, or inflammatory myopathy.
Ultimately, a muscle biopsy may be necessary to exclude inflammatory or necrotizing myopathies in patients whose CK remains elevated despite withdrawal of statins. It may also be helpful when other findings suggest a metabolic myopathy. When a biopsy is needed, magnetic resonance imaging of the affected limb may identify an affected muscle for biopsy.
MANAGEMENT
Reassess the lipid goal
If the source of a complaint remains unclear after challenge and rechallenge with alternate statins, we generally recommend restarting therapy and trying to achieve the LDL-C goal. If the workup suggests a neurologic or rheumatolic etiology, a referral to a specialist is indicated. However, if the evaluation leads to a diagnosis of statin-induced myopathy, the next task is to reassess the lipid treatment goals.
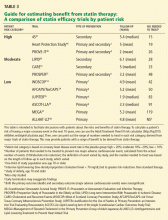
The Adult Treatment Panel (ATP) III guidelines should be used to assess the patient’s risk of having major coronary heart disease in the next 10 years, and to determine appropriate LDL-C goals.46 Some patients with suspected toxicity may have been treated to more aggressive LDL-C levels than recommended by these guidelines. The first step in these patients is to reduce or discontinue the unnecessary statin, even if there is no clear evidence of toxicity.
For the rest of patients with suspected toxicity, the decision to discontinue statin therapy must be weighed against the estimated reduction in risk associated with taking a statin medication.
Prescribe a 6-week ‘statin holiday’ and see if symptoms resolve
In patients whose evaluation suggests statin myopathy, we stop all lipid-lowering therapy for 6 weeks and see if symptoms resolve and if grip and hip strength increase by dynamometry.
We often give these patient supplements of 600 mg daily of a bioavailable source of coenzyme Q10 and fish oil during this statin holiday. The data supporting the use of these supplements are mixed but the risks are minimal.5 In patients whose evaluation suggests a primary disorder in fatty acid oxidation, we add a trial of l-carnitine supplementation if symptoms do not resolve after a 6-week course of the coenzyme Q10 and fish oil.
If symptoms persist or if resolution is unclear at 6 weeks, we extend the holiday for an additional 6 weeks, except in patients with recent unstable coronary disease: for these patients, unless there is evidence of rhabdomyolysis, we believe that the benefits of continued statin therapy exceed the risks.
If the initial evaluation is consistent with statin myopathy and the neuromuscular symptoms (myalgias and weakness) do not respond within a few months of statin withdrawal, neurologic consultation is indicated to evaluate for an underlying neurologic disorder that has become symptomatic during statin therapy but whose existence is independent of the statin therapy. In some cases, the preexisting neurologic disorder may become symptomatic because of the statin therapy and remain symptomatic despite discontinuation of the statin therapy.
Restarting lipid-lowering therapy
Once the myopathy symptoms have abated or are controlled, a rechallenge of statin therapy is in order for those whose risk profile suggests greater benefit from statin therapy.
We consider the complete statin exposure history and any concomitant therapy that may have been competing with cytochrome P450 (CYP) metabolism of statins in designing an alternate lipid-lowering plan.
For patients with known coronary or vascular disease, in whom the survival benefit of statins is greatest, we generally try to find a statin regimen that is tolerable. Long-acting fluvastatin or a statin with less CYP dependence, such as pravastatin, is often successful.59 For patients whose myopathy has recurred with multiple statin rechallenges or whose lipid-lowering goal requires a more potent therapy, rosuvastatin in alternate-day or once- or twice-a-week schedules is efficacious and well tolerated in many patients.36,37,60 Of note, however, although such alternate-day therapies may produce excellent reductions in cholesterol levels, these regimens have not been proven to reduce cardiovascular end points.
Alternative lipid-lowering therapy. Occasionally, a patient cannot tolerate even intermittent rosuvastatin. In these cases, we prescribe resin therapy, which is well tolerated in those with recurrent statin myopathy.61
Although some believe that ezetimibe (Zetia) is an option for these patients, we do not agree, since it often causes similar muscle complaints in the most sensitive statin myopathy patients.30 Furthermore, ezetimibe has not been shown to improve cardiac end points.
Red yeast rice is also not a safe alternative in these patients, in whom muscle complaints and CK elevations frequently develop anew on this unregulated supplement despite its low lovastatin equivalence, 6 mg a day.62 A recent study showed that there is wide variability in the amount of lovastatin in over-the-counter red yeast rice; the median dose was 6 mg, and the maximum dose was 14.5 mg.63
The ultimate lipid-lowering plan for most of these patients will require a compromise between the ideal LDL-C goal and the LDL-C level that is achievable with these alternate attempts at lipid lowering.
While combination therapy may be attractive in patients with combined lipid disorders and no muscle complaints, fibrates are more likely to cause muscle toxicity per dose prescribed than statins, and the addition of fibrates to statin therapy increases the risks of muscle reactions.64,65 The evidence that fibrates reduce cardiovascular end points is much less robust than that for statins, which further reduces enthusiasm for combination therapy in patients with statin muscle toxicity.66,67
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Foley KA, Simpson RJ, Crouse JR, et al. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- O’Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med 2004; 164:1313–1318.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982; 61:141–152.
- Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med 1988; 148:1553–1557.
- McKenney JM, Davidson MH, Jacobson TA, et al. National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–84C.
- Pasternak RC, Smith SC, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002; 40:567–572.
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep 2007; 9:389–396.
- Linares LA, Golomb BA, Jaojoco JA, et al. The modern spectrum of rhabdomyolysis: drug toxicity revealed by creatine kinase screening. Curr Drug Saf 2009; 4:181–187.
- Armitage J. The safety of statins in clinical practice. Lancet 2007; 370:1781–1790.
- Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol 2002; 40:163–171.
- de Sauvage Nolting PR, Buirma RJ, et al. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90:181–184.
- Franc S, Dejager S, Bruckert E, et al. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther 2003; 17:459–465.
- Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation 2006; 114:2788–2797.
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Spatz ES, Canavan ME, Desai MM. From here to JUPITER: identifying new patients for statin therapy using data from the 1999–2004 National Health and Nutrition Examination Survey. Circ Cardiovasc Qual Outcomes 2009; 2:41–48.
- Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve 2006; 34:153–162.
- Vladutiu GD. Genetic predisposition to statin myopathy. Curr Opin Rheumatol 2008; 20:648–655.
- Phillips PS, Phillips CT, Sullivan MJ, et al. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis 2004; 177:183–188.
- Phillips PS, Ciaraldi TP, Kim DL, et al. Myotoxic reactions to lipidlowering therapy are associated with altered oxidation of fatty acids. Endocrine 2009; 35:38–46.
- Phillips PS, Haas RH. Statin myopathy as a metabolic muscle disease. Expert Rev Cardiovasc Ther 2008; 6:971–978.
- Löfberg M, Jänkälä H, Paetau A, et al. Metabolic causes of recurrent rhabdomyolysis. Acta Neurol Scand 1998; 98:268–275.
- Havranek JM, Wolfsen A, Warnke GA, et al. Monotherapy with Ezetimibe Causing Myopathy. Am J Med 2006; 119:285–286.
- Phillips PS. Ezetimibe and statin-associated myopathy. Ann Intern Med 2004; 141:649.
- Durst R, Rund D, Schurr D, et al. One year experience with a low density lipoprotein apheresis system. Isr Med Assoc J 2002; 4:677–680.
- Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol 1997; 80:106–107.
- Brewer HB. Benefit-risk assessment of rosuvastatin 10 to 40 milligrams. Am J Cardiol 2003; 92:23K–29K.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Backes JM, Venero CV, Gibson CA, et al. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother 2008; 42:341–346.
- Backes JM, Moriarty PM, Ruisinger JF, et al. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med 2006; 119:400–409.
- Jacobson TA. Toward “pain-free” statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc 2008; 83:687–700.
- Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002; 137:581–585.
- Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol 2004; 57:525–528.
- Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med 2005; 165:346–347.
- Martino S, Disch D, Dowsett SA, et al. Safety assessment of raloxifene over eight years in a clinical trial setting. Curr Med Res Opin 2005; 21:1441–1452.
- Phillips PS, Kimura BJ, Kelley R, et al. Feasibility of using the internet to perform medical research: observations from a statin-myopathy public information web site. Circulation 2003; 107:e7001–e7039.
- Chazerain P, Hayem G, Hamza S, et al. Four cases of tendinopathy in patients on statin therapy. Joint Bone Spine 2001; 68:430–433.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–732.
- Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998; 97:1453–1460.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333:1301–1307.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368:1155–1163.
- Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHATLLT). JAMA 2002; 288:2998–3007.
- Stein EA, Ballantyne CM, Windler E, et al. Efficacy and tolerability of fluvastatin XL 80 mg alone, ezetimibe alone, and the combination of fluvastatin XL 80 mg with ezetimibe in patients with a history of muscle-related side effects with other statins. Am J Cardiol 2008; 101:490–496.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Phillips PS, Gray NL, McDonald FG, et al. Colesevelam is safe and effective in patients with statin myotoxicity. Arterioscler Thromb Vasc Biol 2005 25:E-97.
- Phillips PS. Balancing randomized trials with anecdote. Ann Intern Med 2009; 150:885–886.
- Gordon RY, Cooperman T, Obermeyer W, et al. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med 2010; 170:1722–1727.
- Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004; 292:2585–2590.
- Gaist D, Rodríguez LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001; 12:565–569.
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–1861.
- ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
When a patient taking a statin complains of muscle aches, is he or she experiencing statin-induced myopathy or some other problem? Should statin therapy be discontinued?
Statins have proven efficacy in preventing heart attacks and death,1 and they are the most widely prescribed drugs worldwide. Nevertheless, they remain underused, with only 50% of those who would benefit from being on a statin receiving one.2,3 In addition, at least 25% of adults who start taking statins stop taking them by 6 months, and up to 60% stop by 2 years.4
Patient and physician fears about myopathy remain a key reason for stopping. Myopathy, a known side effect of statins, is rare in randomized controlled trials, but less so in observational studies and clinical experience. This discrepancy between clinical trials and clinical experience reduces confidence in lipid-lowering therapy and contributes to its underuse.
This review emphasizes clinical aspects of statin myopathy that are important to the practicing physician. We will define myopathy, review its purported mechanisms, and describe a clinical approach to patients with possible toxicity, including risk factors, physical findings, and consideration of alternate diagnoses. Since there is no single test to diagnose statin-induced myopathy, we offer a framework to aid clinicians in stratifying patients based on the likelihood that their symptoms are due to statin toxicity weighed against the likelihood that they will benefit from statin therapy.
DEFINITIONS DIFFER
Little consensus exists on how to define the adverse muscle effects of statins,5 which may contribute to the underdiagnosis of this complication. The magnitude of creatine kinase (CK) elevation required to define rhabdomyolysis has increased from 500 IU/L in 1982,6 to 1,000 IU/L in 1988,7 to 50 times the upper limit of normal in one current definition.8 The American Heart Association, the American College of Cardiology, the National Heart Lung and Blood Institute,9 the National Lipid Association,8 and the US Food and Drug Administration10 all differ in their definitions.
Our definitions
For the purpose of this article, we offer the following definitions:
Myalgia—muscle weakness, soreness, tenderness, stiffness, cramping, or aching, either at rest or with exertion, without any elevation in CK.
Myositis—elevated CK with or without muscle symptoms. The “-itis” suffix is unfortunate since myositis does not correspond to inflammation on biopsy.
Rhabdomyolysis—muscle symptoms with a CK level 10 times the upper limit of normal or higher. Evidence of renal dysfunction is not required for the diagnosis, as preexisting renal disease and hydration status are more closely related to kidney damage than the degree of muscle injury.11
STATIN MYOPATHY IS MORE COMMON IN THE REAL WORLD THAN IN TRIALS
The incidence of statin-induced myopathy is significantly lower in randomized controlled trials of statin efficacy than in observational studies of real-world patients. In randomized clinical trials, myalgia was reported in 1% to 5% of patients in the statin groups and placebo groups alike,9,12 whereas clinical practice would suggest it is more common.
Why is statin-induced myopathy so uncommon in clinical trials?
A reason may be that patients in clinical trials are carefully screened. To minimize toxicity, the clinical trials of statins excluded patients with renal insufficiency, hepatic insufficiency, a history of muscular complaints, and poorly controlled diabetes, as well as patients taking drugs with possible interactions. Large efficacy trials have excluded up to 30% of the participants in active prerandomization phases.13,14
Another reason is that these trials were designed to assess the efficacy of statins and were not sensitive to adverse effects like muscle pain. When they looked at myopathy, they focused on rhabdomyolysis—the most severe form—rather than on myalgia, fatigue, or other minor muscle complaints.15 Additionally, most trials enrolled too few patients and did not have long enough follow-up to reveal infrequent toxicities.
Despite the strict criteria, a significant number of trial patients discontinued statin therapy during the study period. In the Treat to New Targets (TNT) trial, 5% of patients in both the high- and low-dose atorvastatin (Lipitor) groups experienced muscle toxicity, even though 35% of eligible patients had been excluded during the open-label run-in phase.14
Also, physicians may overlook and patients may fail to report symptoms such as fatigue, malaise, or dyspnea that are not commonly accepted as signs of statin toxicity.16
Findings from observational studies
Observational studies in nonselected outpatients show a higher frequency of muscle complaints in the statin groups than in the control groups. These studies suggest the frequency of statin myopathy is 9% to 20%.17–19
The Prediction of Muscular Risk in Observational Conditions (PRIMO) study20 was one of the largest and best-defined observational studies of muscular symptoms in an unselected population. It included 7,924 French outpatients with hypercholesterolemia, ages 18 to 75 years, on high-dose statins for 3 or more months before the study. Daily statin regimens included atorvastatin 40 to 80 mg, fluvastatin (Lescol) 80 mg, pravastatin (Pravachol) 40 mg, and simvastatin (Zocor) 40 to 80 mg. In this study, 10.5% of patients reported muscle-related symptoms.
Buettner et al,21 in another cross-sectional study, interviewed and examined 3,580 adults over age 40. Of those taking statins, 22% reported having had musculoskeletal pain in at least one anatomic region in the last 30 days, compared with 16.7% of those not taking a statin.
In the United States, where an estimated 33 million adults use statins, musculoskeletal pain can be expected to occur in 7 million people, likely induced by statin therapy in 25% of cases.22
WHAT CAUSES STATIN MYOPATHY?
The causes of statin-induced myopathy are poorly understood.
Historically, statin-induced toxicity was thought to be caused by inhibition of the synthesis of mevalonate, leading to depletion of its metabolites, such as cholesterol, isoprenoids, and ubiquinone (coenzyme Q10). Depletion of intracellular cholesterol may lead to abnormal membrane behaviors; depletion of isoprenoids may affect intracellular signaling; depletion of coenzyme Q10 may in turn reduce mitochondrial respiratory function. Genetic factors may also play a role, contributing to pharmacokinetics and predisposing metabolic muscle disorders.23,24
Statin-induced myopathy seems to be different in randomized efficacy trials than in the clinical setting. In randomized trials, the mechanism appears to involve abnormal pharmacokinetics. The participants are carefully selected to have a low risk of muscle toxicity, but some develop toxicity when statin levels are elevated because of reduced drug breakdown and metabolism. However, in clinical practice, where a much larger group of unselected patients are exposed to statins, toxicity appears to be more related to a metabolic predisposition.25–27
Multiple minor metabolic abnormalities have been described in the muscles of patients with statin-induced muscle toxicity, suggesting that some patients have a predisposition for muscle complaints.23 About 25% of patients with recurrent rhabdomyolysis irrespective of lipid-lowering therapy have an underlying metabolic muscle disorder.28 In these vulnerable patients, minor metabolic defects are exacerbated by any agent that reduces the delivery of fat substrate to muscle, leading to muscle starvation.
This concept explains why patients may develop the same muscle complaint on different lipid-lowering agents.29,30 It also may explain why rhabdomyolysis can occur after apheresis of lipids, when no drugs have been given.31 In vulnerable patients, muscle toxicity may result from any agent that lessens the lipid substrate available to muscle rather than from the reduction of products downstream from mevalonate by statins.
SOME STATINS MAY BE LESS TOXIC
In the PRIMO study, muscle-related symptoms occurred with the various regimens as follows:
- Fluvastatin XL 40 mg—5.1%
- Pravastatin 40 mg—10.9%
- Atorvastatin 40 to 80 mg—14.9%
- Simvastatin 40 to 80 mg—18.2%.
Others have also shown that fluvastatin contributed to the smallest number of reported cases of rhabdomyolysis among statins: 55 (1.6%) of 3,339 cases.34
More recent studies indicate that rosuvastatin (Crestor), the most hydrophilic statin, may be well tolerated in those who do not tolerate other statins,35–37 though no head-to-head trial has been done.
RISK FACTORS FOR STATIN-INDUCED MYOPATHY
APPROACH TO SUSPECTED STATIN-INDUCED MYOPATHY
The recommendations that follow are based on observations from our statin myopathy clinic in more than 650 patients, of whom more than 60 have suffered rhabdomyolysis. This experience is largely anecdotal, since such patients have not been well studied in controlled trials.
Are the symptoms due to the statin?
In our experience, most patients who develop significant weakness and pain on statin therapy have normal CK levels.40
Since there is as yet no test to confirm or reject the diagnosis of statin toxicity, the first objective is to determine the likelihood that the muscle complaint is being caused by statin therapy. Factors that make statin toxicity more likely must be weighed against any features that are atypical or that favor an alternate diagnosis.
The final decision about future statin treatment depends on the balance between the expected benefit of statin therapy for each individual and the likelihood that the symptoms are due to statin therapy. Below, we provide an algorithmic approach based on history, physical examination, and laboratory findings.
Findings from the history that implicate statins
Many muscle symptoms resolve within 2 weeks of starting statin therapy. Therefore, if patients have a normal CK level and can tolerate the symptoms, we ask them to continue therapy and see if their symptoms resolve with continued use.
Symptoms that persist beyond the first 2 weeks of therapy are likely due to the statin. These include symmetric burning or pain in the large muscles during exercise that was not present before lipid-lowering therapy. Any symptom that reproducibly recurs with statin rechallenge and disappears within 2 weeks of discontinuing therapy is more likely to be caused by the statin.
Findings of the PRIMO study are representative of typical statin-induced symptoms that we see in our clinic.20
- Most patients did not identify a trigger, but the 40% who did had engaged in unusual physical exertion or had received a new drug in addition to the statin.
- Heaviness, stiffness, or cramps predominated in 70%, with only a quarter noting weakness and another quarter suffering myalgias during exercise. Pain was diffuse in 60% and more common in the lower extremities than the upper extremities.
- Physically active patients were more likely to suffer muscle symptoms than sedentary patients, echoing the observation by Sinzinger and O’Grady that athletes are especially intolerant of lipid-lowering therapy.41
- Patients who have had muscle complaints with other drug therapies such as bisphosphonates, 42 raloxifene (Evista),43 or diuretics may be having the same provocation of an underlying muscle predisposition by the statin.
- A personal or family history of muscle complaints predisposed patients to statin-induced myopathy.20
Finally, we have repeatedly found that when dyspnea and fatigue are associated with the muscle complaint, they are more likely to be caused by statins.44
Statins can unveil underlying musculoskeletal disorders
Atypical complaints require us to search for an alternate diagnosis before dismissing them as not statin-induced.
Previous episodes of myoglobinuria (dark urine after exertion) would raise the possibility of a metabolic myopathy. Frequent muscle cramps raise the possibility of a metabolic myopathy or motor neuron disease.
Asymmetric pain or pain involving joints and ligaments is less likely to be statin-related but in our experience occasionally occurs.
Some patients with underlying degenerative arthritis or tendinitis repeatedly develop worsening symptoms each time they take statins, perhaps because muscle weakness exacerbates the arthropathy or tendinopathy.45
Although statin-related muscle complaints are almost always symmetric, many patients with underlying peripheral vascular disease have asymmetric pain in the limb with poor vascular supply; the pain is reproducible by statin rechallenge.
In our experience, many patients whose chronic low back pain is due to a lumbar radiculopathy experience exacerbations of that pain whenever they start statins.
Weakness preceding the use of the statins or a family history of neuromuscular disorders may indicate a neurodegenerative disorder and warrants consideration of early neurological consultation.
Although these rules are not absolute, they are helpful in the initial evaluation, which must exclude alternative diagnoses.
Is the patient a vegetarian? A drinker? Taking supplements?
Taking a careful history of diet and supplement use is important to find exposures that may increase the risk of statin-related muscle complaints. Vegetarians may develop carnitine or vitamin B12 deficiencies. Alcohol and vitamin E and other supplements are occasional causes of muscle symptoms falsely attributed to statin therapy. It is also important to remember that red yeast rice contains lovastatin, which can exacerbate myopathy, especially when taken in conjunction with another statin.
Physical examination
The examination of patients with possible statin-induced myopathy begins with a general assessment for signs of hypothyroidism or excess alcohol consumption.
Ankle-brachial indices are used to exclude significant peripheral vascular disease.
The musculoskeletal examination focuses on muscle atrophy, tone, and strength but also excludes tendinopathies, arthropathies, and myofascial pain syndromes, which are often confused with muscle pain.
We conduct quantitative dynamometry, measuring handgrip with a Jamar dynamometer and hip abduction with a Nicholas Manual Muscle Tester. Precise dynamometric measurements are tracked at subsequent visits and are helpful in following recovery from myopathy as well as in tracking strength during subsequent statin rechallenges.
We routinely look for hyperreflexia, fasciculations, extensor-plantar responses, and decreased heel-to-shin movement, which would suggest myelopathy. Reflexes and a sensory examination including vibration and temperature sensation help exclude radiculopathy and peripheral neuropathy.
Laboratory evaluation
In every patient with possibly statin myopathy, the primary care physician should measure:
- The serum CK level (preferably more than 72 hours after exercise)
- The 25-hydroxy vitamin level
- The thyroid-stimulating hormone level.
Further laboratory evaluation depends on the findings and will often be directed by subspecialists. For example, we assess the sedimentation rate, anti-Ro and anti-La antibodies, and the myositis panel in patients with elevated CK whose other findings suggest an autoimmune or inflammatory process. We test serum carnitine levels (free, total, and esterified), fasting serum lactate levels, and serum cortisol in those with findings suggestive of metabolic myopathy. We order electromyography and nerve conduction studies in patients with possible myelopathy, peripheral neuropathy, or inflammatory myopathy.
Ultimately, a muscle biopsy may be necessary to exclude inflammatory or necrotizing myopathies in patients whose CK remains elevated despite withdrawal of statins. It may also be helpful when other findings suggest a metabolic myopathy. When a biopsy is needed, magnetic resonance imaging of the affected limb may identify an affected muscle for biopsy.
MANAGEMENT
Reassess the lipid goal
If the source of a complaint remains unclear after challenge and rechallenge with alternate statins, we generally recommend restarting therapy and trying to achieve the LDL-C goal. If the workup suggests a neurologic or rheumatolic etiology, a referral to a specialist is indicated. However, if the evaluation leads to a diagnosis of statin-induced myopathy, the next task is to reassess the lipid treatment goals.
The Adult Treatment Panel (ATP) III guidelines should be used to assess the patient’s risk of having major coronary heart disease in the next 10 years, and to determine appropriate LDL-C goals.46 Some patients with suspected toxicity may have been treated to more aggressive LDL-C levels than recommended by these guidelines. The first step in these patients is to reduce or discontinue the unnecessary statin, even if there is no clear evidence of toxicity.
For the rest of patients with suspected toxicity, the decision to discontinue statin therapy must be weighed against the estimated reduction in risk associated with taking a statin medication.
Prescribe a 6-week ‘statin holiday’ and see if symptoms resolve
In patients whose evaluation suggests statin myopathy, we stop all lipid-lowering therapy for 6 weeks and see if symptoms resolve and if grip and hip strength increase by dynamometry.
We often give these patient supplements of 600 mg daily of a bioavailable source of coenzyme Q10 and fish oil during this statin holiday. The data supporting the use of these supplements are mixed but the risks are minimal.5 In patients whose evaluation suggests a primary disorder in fatty acid oxidation, we add a trial of l-carnitine supplementation if symptoms do not resolve after a 6-week course of the coenzyme Q10 and fish oil.
If symptoms persist or if resolution is unclear at 6 weeks, we extend the holiday for an additional 6 weeks, except in patients with recent unstable coronary disease: for these patients, unless there is evidence of rhabdomyolysis, we believe that the benefits of continued statin therapy exceed the risks.
If the initial evaluation is consistent with statin myopathy and the neuromuscular symptoms (myalgias and weakness) do not respond within a few months of statin withdrawal, neurologic consultation is indicated to evaluate for an underlying neurologic disorder that has become symptomatic during statin therapy but whose existence is independent of the statin therapy. In some cases, the preexisting neurologic disorder may become symptomatic because of the statin therapy and remain symptomatic despite discontinuation of the statin therapy.
Restarting lipid-lowering therapy
Once the myopathy symptoms have abated or are controlled, a rechallenge of statin therapy is in order for those whose risk profile suggests greater benefit from statin therapy.
We consider the complete statin exposure history and any concomitant therapy that may have been competing with cytochrome P450 (CYP) metabolism of statins in designing an alternate lipid-lowering plan.
For patients with known coronary or vascular disease, in whom the survival benefit of statins is greatest, we generally try to find a statin regimen that is tolerable. Long-acting fluvastatin or a statin with less CYP dependence, such as pravastatin, is often successful.59 For patients whose myopathy has recurred with multiple statin rechallenges or whose lipid-lowering goal requires a more potent therapy, rosuvastatin in alternate-day or once- or twice-a-week schedules is efficacious and well tolerated in many patients.36,37,60 Of note, however, although such alternate-day therapies may produce excellent reductions in cholesterol levels, these regimens have not been proven to reduce cardiovascular end points.
Alternative lipid-lowering therapy. Occasionally, a patient cannot tolerate even intermittent rosuvastatin. In these cases, we prescribe resin therapy, which is well tolerated in those with recurrent statin myopathy.61
Although some believe that ezetimibe (Zetia) is an option for these patients, we do not agree, since it often causes similar muscle complaints in the most sensitive statin myopathy patients.30 Furthermore, ezetimibe has not been shown to improve cardiac end points.
Red yeast rice is also not a safe alternative in these patients, in whom muscle complaints and CK elevations frequently develop anew on this unregulated supplement despite its low lovastatin equivalence, 6 mg a day.62 A recent study showed that there is wide variability in the amount of lovastatin in over-the-counter red yeast rice; the median dose was 6 mg, and the maximum dose was 14.5 mg.63
The ultimate lipid-lowering plan for most of these patients will require a compromise between the ideal LDL-C goal and the LDL-C level that is achievable with these alternate attempts at lipid lowering.
While combination therapy may be attractive in patients with combined lipid disorders and no muscle complaints, fibrates are more likely to cause muscle toxicity per dose prescribed than statins, and the addition of fibrates to statin therapy increases the risks of muscle reactions.64,65 The evidence that fibrates reduce cardiovascular end points is much less robust than that for statins, which further reduces enthusiasm for combination therapy in patients with statin muscle toxicity.66,67
When a patient taking a statin complains of muscle aches, is he or she experiencing statin-induced myopathy or some other problem? Should statin therapy be discontinued?
Statins have proven efficacy in preventing heart attacks and death,1 and they are the most widely prescribed drugs worldwide. Nevertheless, they remain underused, with only 50% of those who would benefit from being on a statin receiving one.2,3 In addition, at least 25% of adults who start taking statins stop taking them by 6 months, and up to 60% stop by 2 years.4
Patient and physician fears about myopathy remain a key reason for stopping. Myopathy, a known side effect of statins, is rare in randomized controlled trials, but less so in observational studies and clinical experience. This discrepancy between clinical trials and clinical experience reduces confidence in lipid-lowering therapy and contributes to its underuse.
This review emphasizes clinical aspects of statin myopathy that are important to the practicing physician. We will define myopathy, review its purported mechanisms, and describe a clinical approach to patients with possible toxicity, including risk factors, physical findings, and consideration of alternate diagnoses. Since there is no single test to diagnose statin-induced myopathy, we offer a framework to aid clinicians in stratifying patients based on the likelihood that their symptoms are due to statin toxicity weighed against the likelihood that they will benefit from statin therapy.
DEFINITIONS DIFFER
Little consensus exists on how to define the adverse muscle effects of statins,5 which may contribute to the underdiagnosis of this complication. The magnitude of creatine kinase (CK) elevation required to define rhabdomyolysis has increased from 500 IU/L in 1982,6 to 1,000 IU/L in 1988,7 to 50 times the upper limit of normal in one current definition.8 The American Heart Association, the American College of Cardiology, the National Heart Lung and Blood Institute,9 the National Lipid Association,8 and the US Food and Drug Administration10 all differ in their definitions.
Our definitions
For the purpose of this article, we offer the following definitions:
Myalgia—muscle weakness, soreness, tenderness, stiffness, cramping, or aching, either at rest or with exertion, without any elevation in CK.
Myositis—elevated CK with or without muscle symptoms. The “-itis” suffix is unfortunate since myositis does not correspond to inflammation on biopsy.
Rhabdomyolysis—muscle symptoms with a CK level 10 times the upper limit of normal or higher. Evidence of renal dysfunction is not required for the diagnosis, as preexisting renal disease and hydration status are more closely related to kidney damage than the degree of muscle injury.11
STATIN MYOPATHY IS MORE COMMON IN THE REAL WORLD THAN IN TRIALS
The incidence of statin-induced myopathy is significantly lower in randomized controlled trials of statin efficacy than in observational studies of real-world patients. In randomized clinical trials, myalgia was reported in 1% to 5% of patients in the statin groups and placebo groups alike,9,12 whereas clinical practice would suggest it is more common.
Why is statin-induced myopathy so uncommon in clinical trials?
A reason may be that patients in clinical trials are carefully screened. To minimize toxicity, the clinical trials of statins excluded patients with renal insufficiency, hepatic insufficiency, a history of muscular complaints, and poorly controlled diabetes, as well as patients taking drugs with possible interactions. Large efficacy trials have excluded up to 30% of the participants in active prerandomization phases.13,14
Another reason is that these trials were designed to assess the efficacy of statins and were not sensitive to adverse effects like muscle pain. When they looked at myopathy, they focused on rhabdomyolysis—the most severe form—rather than on myalgia, fatigue, or other minor muscle complaints.15 Additionally, most trials enrolled too few patients and did not have long enough follow-up to reveal infrequent toxicities.
Despite the strict criteria, a significant number of trial patients discontinued statin therapy during the study period. In the Treat to New Targets (TNT) trial, 5% of patients in both the high- and low-dose atorvastatin (Lipitor) groups experienced muscle toxicity, even though 35% of eligible patients had been excluded during the open-label run-in phase.14
Also, physicians may overlook and patients may fail to report symptoms such as fatigue, malaise, or dyspnea that are not commonly accepted as signs of statin toxicity.16
Findings from observational studies
Observational studies in nonselected outpatients show a higher frequency of muscle complaints in the statin groups than in the control groups. These studies suggest the frequency of statin myopathy is 9% to 20%.17–19
The Prediction of Muscular Risk in Observational Conditions (PRIMO) study20 was one of the largest and best-defined observational studies of muscular symptoms in an unselected population. It included 7,924 French outpatients with hypercholesterolemia, ages 18 to 75 years, on high-dose statins for 3 or more months before the study. Daily statin regimens included atorvastatin 40 to 80 mg, fluvastatin (Lescol) 80 mg, pravastatin (Pravachol) 40 mg, and simvastatin (Zocor) 40 to 80 mg. In this study, 10.5% of patients reported muscle-related symptoms.
Buettner et al,21 in another cross-sectional study, interviewed and examined 3,580 adults over age 40. Of those taking statins, 22% reported having had musculoskeletal pain in at least one anatomic region in the last 30 days, compared with 16.7% of those not taking a statin.
In the United States, where an estimated 33 million adults use statins, musculoskeletal pain can be expected to occur in 7 million people, likely induced by statin therapy in 25% of cases.22
WHAT CAUSES STATIN MYOPATHY?
The causes of statin-induced myopathy are poorly understood.
Historically, statin-induced toxicity was thought to be caused by inhibition of the synthesis of mevalonate, leading to depletion of its metabolites, such as cholesterol, isoprenoids, and ubiquinone (coenzyme Q10). Depletion of intracellular cholesterol may lead to abnormal membrane behaviors; depletion of isoprenoids may affect intracellular signaling; depletion of coenzyme Q10 may in turn reduce mitochondrial respiratory function. Genetic factors may also play a role, contributing to pharmacokinetics and predisposing metabolic muscle disorders.23,24
Statin-induced myopathy seems to be different in randomized efficacy trials than in the clinical setting. In randomized trials, the mechanism appears to involve abnormal pharmacokinetics. The participants are carefully selected to have a low risk of muscle toxicity, but some develop toxicity when statin levels are elevated because of reduced drug breakdown and metabolism. However, in clinical practice, where a much larger group of unselected patients are exposed to statins, toxicity appears to be more related to a metabolic predisposition.25–27
Multiple minor metabolic abnormalities have been described in the muscles of patients with statin-induced muscle toxicity, suggesting that some patients have a predisposition for muscle complaints.23 About 25% of patients with recurrent rhabdomyolysis irrespective of lipid-lowering therapy have an underlying metabolic muscle disorder.28 In these vulnerable patients, minor metabolic defects are exacerbated by any agent that reduces the delivery of fat substrate to muscle, leading to muscle starvation.
This concept explains why patients may develop the same muscle complaint on different lipid-lowering agents.29,30 It also may explain why rhabdomyolysis can occur after apheresis of lipids, when no drugs have been given.31 In vulnerable patients, muscle toxicity may result from any agent that lessens the lipid substrate available to muscle rather than from the reduction of products downstream from mevalonate by statins.
SOME STATINS MAY BE LESS TOXIC
In the PRIMO study, muscle-related symptoms occurred with the various regimens as follows:
- Fluvastatin XL 40 mg—5.1%
- Pravastatin 40 mg—10.9%
- Atorvastatin 40 to 80 mg—14.9%
- Simvastatin 40 to 80 mg—18.2%.
Others have also shown that fluvastatin contributed to the smallest number of reported cases of rhabdomyolysis among statins: 55 (1.6%) of 3,339 cases.34
More recent studies indicate that rosuvastatin (Crestor), the most hydrophilic statin, may be well tolerated in those who do not tolerate other statins,35–37 though no head-to-head trial has been done.
RISK FACTORS FOR STATIN-INDUCED MYOPATHY
APPROACH TO SUSPECTED STATIN-INDUCED MYOPATHY
The recommendations that follow are based on observations from our statin myopathy clinic in more than 650 patients, of whom more than 60 have suffered rhabdomyolysis. This experience is largely anecdotal, since such patients have not been well studied in controlled trials.
Are the symptoms due to the statin?
In our experience, most patients who develop significant weakness and pain on statin therapy have normal CK levels.40
Since there is as yet no test to confirm or reject the diagnosis of statin toxicity, the first objective is to determine the likelihood that the muscle complaint is being caused by statin therapy. Factors that make statin toxicity more likely must be weighed against any features that are atypical or that favor an alternate diagnosis.
The final decision about future statin treatment depends on the balance between the expected benefit of statin therapy for each individual and the likelihood that the symptoms are due to statin therapy. Below, we provide an algorithmic approach based on history, physical examination, and laboratory findings.
Findings from the history that implicate statins
Many muscle symptoms resolve within 2 weeks of starting statin therapy. Therefore, if patients have a normal CK level and can tolerate the symptoms, we ask them to continue therapy and see if their symptoms resolve with continued use.
Symptoms that persist beyond the first 2 weeks of therapy are likely due to the statin. These include symmetric burning or pain in the large muscles during exercise that was not present before lipid-lowering therapy. Any symptom that reproducibly recurs with statin rechallenge and disappears within 2 weeks of discontinuing therapy is more likely to be caused by the statin.
Findings of the PRIMO study are representative of typical statin-induced symptoms that we see in our clinic.20
- Most patients did not identify a trigger, but the 40% who did had engaged in unusual physical exertion or had received a new drug in addition to the statin.
- Heaviness, stiffness, or cramps predominated in 70%, with only a quarter noting weakness and another quarter suffering myalgias during exercise. Pain was diffuse in 60% and more common in the lower extremities than the upper extremities.
- Physically active patients were more likely to suffer muscle symptoms than sedentary patients, echoing the observation by Sinzinger and O’Grady that athletes are especially intolerant of lipid-lowering therapy.41
- Patients who have had muscle complaints with other drug therapies such as bisphosphonates, 42 raloxifene (Evista),43 or diuretics may be having the same provocation of an underlying muscle predisposition by the statin.
- A personal or family history of muscle complaints predisposed patients to statin-induced myopathy.20
Finally, we have repeatedly found that when dyspnea and fatigue are associated with the muscle complaint, they are more likely to be caused by statins.44
Statins can unveil underlying musculoskeletal disorders
Atypical complaints require us to search for an alternate diagnosis before dismissing them as not statin-induced.
Previous episodes of myoglobinuria (dark urine after exertion) would raise the possibility of a metabolic myopathy. Frequent muscle cramps raise the possibility of a metabolic myopathy or motor neuron disease.
Asymmetric pain or pain involving joints and ligaments is less likely to be statin-related but in our experience occasionally occurs.
Some patients with underlying degenerative arthritis or tendinitis repeatedly develop worsening symptoms each time they take statins, perhaps because muscle weakness exacerbates the arthropathy or tendinopathy.45
Although statin-related muscle complaints are almost always symmetric, many patients with underlying peripheral vascular disease have asymmetric pain in the limb with poor vascular supply; the pain is reproducible by statin rechallenge.
In our experience, many patients whose chronic low back pain is due to a lumbar radiculopathy experience exacerbations of that pain whenever they start statins.
Weakness preceding the use of the statins or a family history of neuromuscular disorders may indicate a neurodegenerative disorder and warrants consideration of early neurological consultation.
Although these rules are not absolute, they are helpful in the initial evaluation, which must exclude alternative diagnoses.
Is the patient a vegetarian? A drinker? Taking supplements?
Taking a careful history of diet and supplement use is important to find exposures that may increase the risk of statin-related muscle complaints. Vegetarians may develop carnitine or vitamin B12 deficiencies. Alcohol and vitamin E and other supplements are occasional causes of muscle symptoms falsely attributed to statin therapy. It is also important to remember that red yeast rice contains lovastatin, which can exacerbate myopathy, especially when taken in conjunction with another statin.
Physical examination
The examination of patients with possible statin-induced myopathy begins with a general assessment for signs of hypothyroidism or excess alcohol consumption.
Ankle-brachial indices are used to exclude significant peripheral vascular disease.
The musculoskeletal examination focuses on muscle atrophy, tone, and strength but also excludes tendinopathies, arthropathies, and myofascial pain syndromes, which are often confused with muscle pain.
We conduct quantitative dynamometry, measuring handgrip with a Jamar dynamometer and hip abduction with a Nicholas Manual Muscle Tester. Precise dynamometric measurements are tracked at subsequent visits and are helpful in following recovery from myopathy as well as in tracking strength during subsequent statin rechallenges.
We routinely look for hyperreflexia, fasciculations, extensor-plantar responses, and decreased heel-to-shin movement, which would suggest myelopathy. Reflexes and a sensory examination including vibration and temperature sensation help exclude radiculopathy and peripheral neuropathy.
Laboratory evaluation
In every patient with possibly statin myopathy, the primary care physician should measure:
- The serum CK level (preferably more than 72 hours after exercise)
- The 25-hydroxy vitamin level
- The thyroid-stimulating hormone level.
Further laboratory evaluation depends on the findings and will often be directed by subspecialists. For example, we assess the sedimentation rate, anti-Ro and anti-La antibodies, and the myositis panel in patients with elevated CK whose other findings suggest an autoimmune or inflammatory process. We test serum carnitine levels (free, total, and esterified), fasting serum lactate levels, and serum cortisol in those with findings suggestive of metabolic myopathy. We order electromyography and nerve conduction studies in patients with possible myelopathy, peripheral neuropathy, or inflammatory myopathy.
Ultimately, a muscle biopsy may be necessary to exclude inflammatory or necrotizing myopathies in patients whose CK remains elevated despite withdrawal of statins. It may also be helpful when other findings suggest a metabolic myopathy. When a biopsy is needed, magnetic resonance imaging of the affected limb may identify an affected muscle for biopsy.
MANAGEMENT
Reassess the lipid goal
If the source of a complaint remains unclear after challenge and rechallenge with alternate statins, we generally recommend restarting therapy and trying to achieve the LDL-C goal. If the workup suggests a neurologic or rheumatolic etiology, a referral to a specialist is indicated. However, if the evaluation leads to a diagnosis of statin-induced myopathy, the next task is to reassess the lipid treatment goals.
The Adult Treatment Panel (ATP) III guidelines should be used to assess the patient’s risk of having major coronary heart disease in the next 10 years, and to determine appropriate LDL-C goals.46 Some patients with suspected toxicity may have been treated to more aggressive LDL-C levels than recommended by these guidelines. The first step in these patients is to reduce or discontinue the unnecessary statin, even if there is no clear evidence of toxicity.
For the rest of patients with suspected toxicity, the decision to discontinue statin therapy must be weighed against the estimated reduction in risk associated with taking a statin medication.
Prescribe a 6-week ‘statin holiday’ and see if symptoms resolve
In patients whose evaluation suggests statin myopathy, we stop all lipid-lowering therapy for 6 weeks and see if symptoms resolve and if grip and hip strength increase by dynamometry.
We often give these patient supplements of 600 mg daily of a bioavailable source of coenzyme Q10 and fish oil during this statin holiday. The data supporting the use of these supplements are mixed but the risks are minimal.5 In patients whose evaluation suggests a primary disorder in fatty acid oxidation, we add a trial of l-carnitine supplementation if symptoms do not resolve after a 6-week course of the coenzyme Q10 and fish oil.
If symptoms persist or if resolution is unclear at 6 weeks, we extend the holiday for an additional 6 weeks, except in patients with recent unstable coronary disease: for these patients, unless there is evidence of rhabdomyolysis, we believe that the benefits of continued statin therapy exceed the risks.
If the initial evaluation is consistent with statin myopathy and the neuromuscular symptoms (myalgias and weakness) do not respond within a few months of statin withdrawal, neurologic consultation is indicated to evaluate for an underlying neurologic disorder that has become symptomatic during statin therapy but whose existence is independent of the statin therapy. In some cases, the preexisting neurologic disorder may become symptomatic because of the statin therapy and remain symptomatic despite discontinuation of the statin therapy.
Restarting lipid-lowering therapy
Once the myopathy symptoms have abated or are controlled, a rechallenge of statin therapy is in order for those whose risk profile suggests greater benefit from statin therapy.
We consider the complete statin exposure history and any concomitant therapy that may have been competing with cytochrome P450 (CYP) metabolism of statins in designing an alternate lipid-lowering plan.
For patients with known coronary or vascular disease, in whom the survival benefit of statins is greatest, we generally try to find a statin regimen that is tolerable. Long-acting fluvastatin or a statin with less CYP dependence, such as pravastatin, is often successful.59 For patients whose myopathy has recurred with multiple statin rechallenges or whose lipid-lowering goal requires a more potent therapy, rosuvastatin in alternate-day or once- or twice-a-week schedules is efficacious and well tolerated in many patients.36,37,60 Of note, however, although such alternate-day therapies may produce excellent reductions in cholesterol levels, these regimens have not been proven to reduce cardiovascular end points.
Alternative lipid-lowering therapy. Occasionally, a patient cannot tolerate even intermittent rosuvastatin. In these cases, we prescribe resin therapy, which is well tolerated in those with recurrent statin myopathy.61
Although some believe that ezetimibe (Zetia) is an option for these patients, we do not agree, since it often causes similar muscle complaints in the most sensitive statin myopathy patients.30 Furthermore, ezetimibe has not been shown to improve cardiac end points.
Red yeast rice is also not a safe alternative in these patients, in whom muscle complaints and CK elevations frequently develop anew on this unregulated supplement despite its low lovastatin equivalence, 6 mg a day.62 A recent study showed that there is wide variability in the amount of lovastatin in over-the-counter red yeast rice; the median dose was 6 mg, and the maximum dose was 14.5 mg.63
The ultimate lipid-lowering plan for most of these patients will require a compromise between the ideal LDL-C goal and the LDL-C level that is achievable with these alternate attempts at lipid lowering.
While combination therapy may be attractive in patients with combined lipid disorders and no muscle complaints, fibrates are more likely to cause muscle toxicity per dose prescribed than statins, and the addition of fibrates to statin therapy increases the risks of muscle reactions.64,65 The evidence that fibrates reduce cardiovascular end points is much less robust than that for statins, which further reduces enthusiasm for combination therapy in patients with statin muscle toxicity.66,67
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Foley KA, Simpson RJ, Crouse JR, et al. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- O’Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med 2004; 164:1313–1318.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982; 61:141–152.
- Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med 1988; 148:1553–1557.
- McKenney JM, Davidson MH, Jacobson TA, et al. National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–84C.
- Pasternak RC, Smith SC, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002; 40:567–572.
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep 2007; 9:389–396.
- Linares LA, Golomb BA, Jaojoco JA, et al. The modern spectrum of rhabdomyolysis: drug toxicity revealed by creatine kinase screening. Curr Drug Saf 2009; 4:181–187.
- Armitage J. The safety of statins in clinical practice. Lancet 2007; 370:1781–1790.
- Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol 2002; 40:163–171.
- de Sauvage Nolting PR, Buirma RJ, et al. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90:181–184.
- Franc S, Dejager S, Bruckert E, et al. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther 2003; 17:459–465.
- Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation 2006; 114:2788–2797.
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Spatz ES, Canavan ME, Desai MM. From here to JUPITER: identifying new patients for statin therapy using data from the 1999–2004 National Health and Nutrition Examination Survey. Circ Cardiovasc Qual Outcomes 2009; 2:41–48.
- Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve 2006; 34:153–162.
- Vladutiu GD. Genetic predisposition to statin myopathy. Curr Opin Rheumatol 2008; 20:648–655.
- Phillips PS, Phillips CT, Sullivan MJ, et al. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis 2004; 177:183–188.
- Phillips PS, Ciaraldi TP, Kim DL, et al. Myotoxic reactions to lipidlowering therapy are associated with altered oxidation of fatty acids. Endocrine 2009; 35:38–46.
- Phillips PS, Haas RH. Statin myopathy as a metabolic muscle disease. Expert Rev Cardiovasc Ther 2008; 6:971–978.
- Löfberg M, Jänkälä H, Paetau A, et al. Metabolic causes of recurrent rhabdomyolysis. Acta Neurol Scand 1998; 98:268–275.
- Havranek JM, Wolfsen A, Warnke GA, et al. Monotherapy with Ezetimibe Causing Myopathy. Am J Med 2006; 119:285–286.
- Phillips PS. Ezetimibe and statin-associated myopathy. Ann Intern Med 2004; 141:649.
- Durst R, Rund D, Schurr D, et al. One year experience with a low density lipoprotein apheresis system. Isr Med Assoc J 2002; 4:677–680.
- Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol 1997; 80:106–107.
- Brewer HB. Benefit-risk assessment of rosuvastatin 10 to 40 milligrams. Am J Cardiol 2003; 92:23K–29K.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Backes JM, Venero CV, Gibson CA, et al. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother 2008; 42:341–346.
- Backes JM, Moriarty PM, Ruisinger JF, et al. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med 2006; 119:400–409.
- Jacobson TA. Toward “pain-free” statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc 2008; 83:687–700.
- Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002; 137:581–585.
- Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol 2004; 57:525–528.
- Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med 2005; 165:346–347.
- Martino S, Disch D, Dowsett SA, et al. Safety assessment of raloxifene over eight years in a clinical trial setting. Curr Med Res Opin 2005; 21:1441–1452.
- Phillips PS, Kimura BJ, Kelley R, et al. Feasibility of using the internet to perform medical research: observations from a statin-myopathy public information web site. Circulation 2003; 107:e7001–e7039.
- Chazerain P, Hayem G, Hamza S, et al. Four cases of tendinopathy in patients on statin therapy. Joint Bone Spine 2001; 68:430–433.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–732.
- Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998; 97:1453–1460.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333:1301–1307.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368:1155–1163.
- Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHATLLT). JAMA 2002; 288:2998–3007.
- Stein EA, Ballantyne CM, Windler E, et al. Efficacy and tolerability of fluvastatin XL 80 mg alone, ezetimibe alone, and the combination of fluvastatin XL 80 mg with ezetimibe in patients with a history of muscle-related side effects with other statins. Am J Cardiol 2008; 101:490–496.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Phillips PS, Gray NL, McDonald FG, et al. Colesevelam is safe and effective in patients with statin myotoxicity. Arterioscler Thromb Vasc Biol 2005 25:E-97.
- Phillips PS. Balancing randomized trials with anecdote. Ann Intern Med 2009; 150:885–886.
- Gordon RY, Cooperman T, Obermeyer W, et al. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med 2010; 170:1722–1727.
- Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004; 292:2585–2590.
- Gaist D, Rodríguez LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001; 12:565–569.
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–1861.
- ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- Foley KA, Simpson RJ, Crouse JR, et al. Effectiveness of statin titration on low-density lipoprotein cholesterol goal attainment in patients at high risk of atherogenic events. Am J Cardiol 2003; 92:79–81.
- O’Meara JG, Kardia SL, Armon JJ, et al. Ethnic and sex differences in the prevalence, treatment, and control of dyslipidemia among hypertensive adults in the GENOA study. Arch Intern Med 2004; 164:1313–1318.
- Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002; 288:462–467.
- Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med 2009; 150:858–868.
- Gabow PA, Kaehny WD, Kelleher SP. The spectrum of rhabdomyolysis. Medicine (Baltimore) 1982; 61:141–152.
- Ward MM. Factors predictive of acute renal failure in rhabdomyolysis. Arch Intern Med 1988; 148:1553–1557.
- McKenney JM, Davidson MH, Jacobson TA, et al. National Lipid Association Statin Safety Assessment Task Force. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol 2006; 97:89C–84C.
- Pasternak RC, Smith SC, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002; 40:567–572.
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep 2007; 9:389–396.
- Linares LA, Golomb BA, Jaojoco JA, et al. The modern spectrum of rhabdomyolysis: drug toxicity revealed by creatine kinase screening. Curr Drug Saf 2009; 4:181–187.
- Armitage J. The safety of statins in clinical practice. Lancet 2007; 370:1781–1790.
- Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003; 361:2005–2016.
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–1435.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Sinzinger H, Wolfram R, Peskar BA. Muscular side effects of statins. J Cardiovasc Pharmacol 2002; 40:163–171.
- de Sauvage Nolting PR, Buirma RJ, et al. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90:181–184.
- Franc S, Dejager S, Bruckert E, et al. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther 2003; 17:459–465.
- Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation 2006; 114:2788–2797.
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005; 19:403–414.
- Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med 2008; 23:1182–1186.
- Spatz ES, Canavan ME, Desai MM. From here to JUPITER: identifying new patients for statin therapy using data from the 1999–2004 National Health and Nutrition Examination Survey. Circ Cardiovasc Qual Outcomes 2009; 2:41–48.
- Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve 2006; 34:153–162.
- Vladutiu GD. Genetic predisposition to statin myopathy. Curr Opin Rheumatol 2008; 20:648–655.
- Phillips PS, Phillips CT, Sullivan MJ, et al. Statin myotoxicity is associated with changes in the cardiopulmonary function. Atherosclerosis 2004; 177:183–188.
- Phillips PS, Ciaraldi TP, Kim DL, et al. Myotoxic reactions to lipidlowering therapy are associated with altered oxidation of fatty acids. Endocrine 2009; 35:38–46.
- Phillips PS, Haas RH. Statin myopathy as a metabolic muscle disease. Expert Rev Cardiovasc Ther 2008; 6:971–978.
- Löfberg M, Jänkälä H, Paetau A, et al. Metabolic causes of recurrent rhabdomyolysis. Acta Neurol Scand 1998; 98:268–275.
- Havranek JM, Wolfsen A, Warnke GA, et al. Monotherapy with Ezetimibe Causing Myopathy. Am J Med 2006; 119:285–286.
- Phillips PS. Ezetimibe and statin-associated myopathy. Ann Intern Med 2004; 141:649.
- Durst R, Rund D, Schurr D, et al. One year experience with a low density lipoprotein apheresis system. Isr Med Assoc J 2002; 4:677–680.
- Roberts WC. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am J Cardiol 1997; 80:106–107.
- Brewer HB. Benefit-risk assessment of rosuvastatin 10 to 40 milligrams. Am J Cardiol 2003; 92:23K–29K.
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA 2003; 289:1681–1690.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Backes JM, Venero CV, Gibson CA, et al. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother 2008; 42:341–346.
- Backes JM, Moriarty PM, Ruisinger JF, et al. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol 2007; 100:554–555.
- Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med 2006; 119:400–409.
- Jacobson TA. Toward “pain-free” statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc 2008; 83:687–700.
- Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med 2002; 137:581–585.
- Sinzinger H, O’Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol 2004; 57:525–528.
- Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med 2005; 165:346–347.
- Martino S, Disch D, Dowsett SA, et al. Safety assessment of raloxifene over eight years in a clinical trial setting. Curr Med Res Opin 2005; 21:1441–1452.
- Phillips PS, Kimura BJ, Kelley R, et al. Feasibility of using the internet to perform medical research: observations from a statin-myopathy public information web site. Circulation 2003; 107:e7001–e7039.
- Chazerain P, Hayem G, Hamza S, et al. Four cases of tendinopathy in patients on statin therapy. Joint Bone Spine 2001; 68:430–433.
- Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol 2004; 44:720–732.
- Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation 1998; 97:1453–1460.
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350:1495–1504.
- The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998; 339:1349–1357.
- Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996; 335:1001–1009.
- Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–1630.
- Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995; 333:1301–1307.
- Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622.
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003; 361:1149–1158.
- Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006; 368:1155–1163.
- Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHATLLT). JAMA 2002; 288:2998–3007.
- Stein EA, Ballantyne CM, Windler E, et al. Efficacy and tolerability of fluvastatin XL 80 mg alone, ezetimibe alone, and the combination of fluvastatin XL 80 mg with ezetimibe in patients with a history of muscle-related side effects with other statins. Am J Cardiol 2008; 101:490–496.
- Glueck CJ, Aregawi D, Agloria M, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther 2006; 28:933–942.
- Phillips PS, Gray NL, McDonald FG, et al. Colesevelam is safe and effective in patients with statin myotoxicity. Arterioscler Thromb Vasc Biol 2005 25:E-97.
- Phillips PS. Balancing randomized trials with anecdote. Ann Intern Med 2009; 150:885–886.
- Gordon RY, Cooperman T, Obermeyer W, et al. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med 2010; 170:1722–1727.
- Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004; 292:2585–2590.
- Gaist D, Rodríguez LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001; 12:565–569.
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005; 366:1849–1861.
- ACCORD Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
KEY POINTS
- There is little consensus on the definition of statin-induced myopathy, and it is underdiagnosed. The incidence of statin-induced muscle toxicity in randomized controlled trials is lower than in clinical practice.
- Abnormal pharmacokinetic activity contributes to toxicity, but some patients may be predisposed by underlying metabolic muscle disorders.
- A focused history and neuromusculoskeletal examination are important in the evaluation of muscle complaints that may be induced by statins.
- In patients with possible statin-induced myopathy, assessing the risks and benefits of statin therapy is essential.
- For patients who cannot tolerate statin therapy, alternatives include a “statin holiday” followed by a rechallenge with a different statin, intermittent rosuvastatin (Crestor), or resin therapy. Sometimes the best alternative is a compromise between the goal level for low-density-lipoprotein cholesterol and the level achievable with alternative therapy.
Insulin treatment for type 2 diabetes: When to start, which to use
Many patients with type 2 diabetes eventually need insulin, as their ability to produce their own insulin from pancreatic beta cells declines progressively.1 The questions remain as to when insulin therapy should be started, and which regimen is the most appropriate.
Guidelines from professional societies differ on these points,2,3 as do individual clinicians. Moreover, antidiabetic treatment is an evolving topic. Many new drugs—oral agents as well as injectable analogues of glucagon-like peptide-1 (GLP1) and insulin formulations—have become available in the last 15 years.
In this paper, I advocate an individualized approach and review the indications for insulin treatment, the available preparations, the pros and cons of each regimen, and how the properties of each type of insulin influence attempts to intensify the regimen.
Coexisting physiologic and medical conditions such as pregnancy and chronic renal failure and drugs such as glucocorticoids may alter insulin requirements. I will not cover these special situations, as they deserve separate, detailed discussions.
WHEN SHOULD INSULIN BE STARTED? TWO VIEWS
Early on, patients can be adequately managed with lifestyle modifications and oral hypoglycemic agents or injections of a GLP1 analogue, either alone or in combination with oral medication. Later, some patients reach a point at which insulin therapy becomes the main treatment, similar to patients with type 1 diabetes.
The American Diabetes Association (ADA), in a consensus statement,2 has called for using insulin early in the disease if lifestyle management and monotherapy with metformin (Glucophage) fail to control glucose or if lifestyle management is not adequate and metformin is contraindicated. The ADA’s goal hemoglobin A1c level is less than 7% for most patients.
The American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE), in another consensus statement, use an algorithm stratified by hemoglobin A1c level, in which insulin is mostly reserved for when combination therapy fails.3 Their goal hemoglobin A1c level is 6.5% or less for most patients.
Comment. Both consensus statements make exceptions for patients presenting with very high blood glucose and hemoglobin A1c levels and those who have contraindications to drugs other than insulin. These patients should start insulin immediately, along with lifestyle management.2,3
Both consensus statements give priority to safety. The AACE/ACE statement gives more weight to the risk of hypoglycemia with insulin treatment, whereas the ADA gives more weight to the risk of edema and congestive heart failure with thiazolidinedione drugs (although both insulin and thiazolidinediones cause weight gain) and to adequate validation of treatments in clinical trials.
Ongoing clinical trials may add insight to this issue. For example, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) study is investigating the effects of the long-acting insulin glargine (Lantus) in early diabetes with regard to glycemic control, safety, and cardiovascular outcomes.4 This study is expected to end this year (2011). The safety of alternative treatment options is also under investigation and scrutiny. In the interim, individualized treatment should be considered, as we will see below.

MY VIEW: AN INDIVIDUALIZED APPROACH
The decision to start insulin therapy should be made individually, based on several factors:
- Whether the patient is willing to try it
- The degree of hyperglycemia
- How relevant the potential side effects of insulin are to the patient compared with those of other hypoglycemic agents
- Whether oral hypoglycemic agents with or without GLP1 analogues are expected to provide the desired benefit
- The patient’s work schedule and lifestyle factors
- Cost
- The availability of nurses, diabetes educators, and others to implement and follow the insulin treatment.
Will patients accept insulin?
Factors that affect whether patients comply with a treatment include the number of pills or injections they must take per day, how often they must check their blood glucose, adverse effects, lifestyle limitations caused by the treatment (especially insulin), and cost. Most patients feel better when their glucose levels are under good control, which is a major motivation for initiating and adhering to insulin. The anticipated reduction of diabetic complications further enhances compliance.
Education promotes compliance. Patients need to know that type 2 diabetes tends to progress and that in time their treatment will have to be intensified, with higher doses of their current drugs and new drugs added or substituted, possibly including insulin. This information is best given early, ie, when the diagnosis is made, even if hyperglycemia is mild at that time.
With newer insulin preparations and delivery devices available, more patients are finding insulin treatment acceptable.
The glycemic goal should be individualized
The key issue is glycemic control. If glycemic control is worsening or if the hemoglobin A1c level remains above the goal, then the treatment strategy should be readdressed.
In general, one should try to achieve the best possible glycemic control with the few est adverse effects. Adequate dietary management with a regular meal schedule and predictable carbohydrate intake for each meal helps to avoid or at least minimize the two most important adverse effects of insulin, ie, weight gain and hypoglycemia.
For most patients, I believe a goal hemoglobin A1c level of less than 7% is reasonable.2 For others, a less stringent goal might be more appropriate, such as 7.5%. Several factors affect this decision, including whether the patient is willing to follow a complex insulin regimen (such as a basal-bolus regimen), his or her work schedule, other lifestyle factors, the duration of diabetes, the type or types of insulin used, coexisting medical conditions, the frequency of hypoglycemia, unawareness of hypoglycemia, age, prognosis, life expectancy, and cost.5
If hyperglycemia is severe (Table 1),2 the goal might not be clear when insulin therapy is started. It should become obvious with ongoing follow-up.
Previously untreated patients presenting with severe hyperglycemia are a heterogeneous group. Many of them have had diabetes for a relatively short time and were recently diagnosed. These patients are likely to safely achieve near-normal glycemic control. Some of them might be adequately treated with oral hypoglycemic agents; if insulin is used, transitioning from insulin to oral hypoglycemic agents may be feasible.2
Some untreated patients may have had diabetes for several years and have advanced disease and therefore might be more difficult to treat. Only 21 (57%) of 37 previously untreated patients intensively treated with insulin reached the goal fasting glucose level of less than 126 mg/dL in one study.6 The only way to evaluate the feasibility of achieving near-normal glycemia safely is by following the patient’s progress over time.
The patient’s glycemic goal should be reevaluated periodically and may need to be adjusted over time, based on changes in any of the factors discussed above.
Risk of hypoglycemia
The goal should be looser in difficult-to-treat patients, ie, those with frequent hypoglycemia and decreased awareness of hypoglycemia.
Patients with advanced diabetes whose glucose levels continue to fluctuate widely after lifestyle management and the insulin regimen have been addressed should also have a looser goal. These fluctuations of glucose levels are surrogate markers for the degree of insulin deficiency. Attempting to achieve near-normal glycemic levels in this situation would be associated with a higher risk of hypoglycemia.
A higher risk of hypoglycemia and its complications (eg, falling and accidents, especially among operators of heavy machinery, construction workers, and drivers) is another reason for adopting a relaxed goal of glycemic control.
ADDING BASAL INSULIN TO ORAL HYPOGLYCEMIC THERAPY
When glycemic control worsens or is not adequate despite the use of oral hypoglycemic agents, often the next step is to add basal insulin therapy, ie, once-daily doses of a long-acting insulin.
NPH, detemir, or glargine?
Most often, glargine or detemir (Levemir) insulin is used. Detemir can also be given twice daily if needed. If cost is a concern, neutral protamine Hagedorn (NPH, Humulin N, Novolin N) insulin once daily at bedtime or twice daily is a reasonable alternative.
Costs of basal insulins are $22 to $50 per 1,000-unit vial for NPH, $70 to $90 per 1,000-unit vial for detemir and glargine, and $170 to $200 for a box of five detemir or glargine pens (containing 1,500 units total). Complicating this issue, vials should not be used for more than 1 month, and thus, the cost of vials vs pens depends on dosage.
Detemir vs NPH. In a trial in patients with inadequately controlled type 2 diabetes who had never taken insulin before and who were taking one or more oral hypoglycemic drugs, the addition of detemir insulin once daily or NPH at bedtime resulted in similar improvements in hemoglobin A1c (a decrease of about 1.5%).10
Detemir had several advantages over NPH. First, the incidence of nocturnal hypoglycemia was 50% lower with detemir at bedtime than with NPH at bedtime, and 87% lower with detemir in the morning than with bedtime NPH.10 In another trial,11 the risk of hypoglycemia at any time of day was 47% lower with insulin detemir than with NPH, and the risk of nocturnal hypoglycemia was 55% lower.
The risk of nocturnal hypoglycemia is lower if detemir is taken in the morning than at bedtime, although the total frequency of hypoglycemic episodes is the same.10 Therefore, another decision after starting basal insulin, based on the patient’s glucose trends and frequency of hypoglycemic events, would be whether insulin should be taken in the morning or at bedtime.
The second advantage of detemir is that it causes less weight gain: 0.7 kg at 20 weeks with detemir at bedtime vs 1.6 kg with NPH at bedtime.10
Further, detemir insulin was associated with less within-subject variability in the fasting glucose level than with NPH when these insulins were used in a basal-bolus regimen.12
Hermansen et al11 found that if the dosage of basal insulin was aggressively increased, 70% of patients achieved a hemoglobin A1c target of less than 7% with either NPH or detemir insulin, with fewer hypoglycemic episodes in patients treated with detemir.
Therefore, adding basal insulin to oral therapy is adequate for many patients who are new to insulin. Many patients would need more, such as the addition of insulin before meals.
Glargine vs NPH. Compared with adding NPH, adding glargine to a regimen of oral hypoglycemic agents controls blood glucose levels better and with less fluctuation in glucose levels, a lower risk of hypoglycemia, and less weight gain.13–15 These results were the same when using glargine with either metformin13 or glimeperide (Amaryl).14
Glargine is usually given once daily at bedtime. One study suggested that giving it in the morning is more effective.14
Detemir vs glargine. Studies that compared detemir and glargine revealed more similarities than differences in their clinical benefits.16,17 Both preparations effectively lower glucose levels and improve quality of life.18
Titrating the insulin regimen is a key in achieving adequate glycemic control. This includes teaching patients how to adjust their insulin, for example by increasing the dosage of glargine or detemir by 2 units every 4 to 7 days until adequate glycemic control is achieved, unless hypoglycemia becomes a barrier.
BASAL VS PRANDIAL INSULIN
Once-daily insulin injection is relatively convenient, but it comes with a limitation: it does not adequately control postprandial hyperglycemia. A solution is insulin before meals, ie, prandial insulin.
Kazda et al19 compared three regimens in patients not taking oral hypoglycemic agents: rapid-acting insulin lispro (Humalog) before each meal, a mix of 50% lispro and 50% protamine lispro (Humalog Mix 50/50) (the protamine delays its release) before each meal, and glargine at bedtime. The absolute change in hemoglobin A1c was −0.3% in the glargine group, −1.1% in the lispro group, and −1.2% in the lispro mix group. The glargine group had better control of fasting glucose.
Similar advantages of better glycemic control and fewer nocturnal hypoglycemic episodes were seen in trials of a mixture of 25% lispro and 75% protamine lispro before meals compared with glargine insulin in patients on simultaneous treatment with oral hypoglycemic agents.20,21 Buse et al21 reported that more patients achieved a hemoglobin A1c level below 7% with this lispro mix (47%) than with glargine (40%). The absolute difference in mean hemoglobin A1c between the two groups was minimal, although it reached statistical significance. As expected, weight gain was less in the glargine group.21
Kann et al22 reported that glycemic control was also better with a mixture of 30% aspart and 70% protamine aspart (NovoLog Mix 70/30) twice a day along with metformin than with glargine insulin once a day along with oral glimepiride, a sulfonylurea. Further, in this study, weight gain was noted in the glargine-glimepiride group only.22 Therefore, the advantage of less weight gain has not been always reproducible in glargine studies.
Comment. These studies point to the contribution of postprandial glucose to hemoglobin A1c.23–25 In patients with satisfactory glycemic control, the postprandial glucose level seems to be the major contributor to hemoglobin A1c. When glycemic control worsens, the contribution of fasting glucose to hemoglobin A1c increases.23
Premixed insulins (lispro mix and aspart mix) provide basal coverage and control postprandial hyperglycemia. Therefore, prandial premixed insulin therapy is expected to be superior to basal insulin therapy. Premixed insulin could be considered as a simplified basal-bolus regimen (see below).
The superiority of prandial (rapid-acting) insulin alone over basal insulin therapy, as seen in the study by Kazda et al,19 has not been reproducible in other studies. For example, in one study, once-daily glargine resulted in a similar improvement in hemoglobin A1c, a lower rate of hypoglycemic episodes, and greater patient satisfaction with treatment compared with lispro insulin before meals.26 This issue remains debatable because all the trials have been open-label and thus are subject to limitations.
The main lesson is that either glargine or lispro monotherapy is a reasonable option and results in better glycemic control in patients for whom two oral hypoglycemic agents have failed. Further, both fasting and postprandial hyperglycemia are important to address. In patients with severe hyperglycemia, a combination of prandial and basal insulin may be indicated. One would expect neither basal nor prandial (bolus) insulin to be adequate in this situation.
In conclusion, adding basal insulin to oral hypoglycemic agents is a reasonable option in the advancement of diabetes therapy and has become a common way to introduce insulin. It is simple and less labor-intensive for patients and medical groups than a basal-bolus regimen. Patients usually find it acceptable. The future availability of an easy-to-deliver, safe, and effective prandial insulin may change the current treatment paradigm; several newer prandial insulins are under investigation.
In advanced diabetes, both prandial and fasting glucose levels are crucial to address. Some patients may need to be started on both basal and prandial insulin simultaneously, depending on their degree of hyperglycemia, the duration of diabetes, coexisting medical conditions, and the goal of glycemic control.
BASAL-BOLUS INSULIN REGIMENS
In the advanced stages of type 2 diabetes, as insulin deficiency worsens, patients need to start giving themselves injections of a rapid-acting insulin—regular, lispro, aspart, or glulisine (Apidra) before meals, in addition to once- or twice-daily basal insulin injections. Such a “basal-bolus” regimen could also be used for newly diagnosed patients presenting with severe hyperglycemia. In addition, some patients on basal insulin plus oral hypoglycemic drugs may develop contraindications to their oral drugs. Adding bolus insulin becomes the main option for these patients too.
For others, a basal-bolus regimen might be chosen purely because of cost. For example, a regimen of NPH and regular insulin (multiple daily injections or premixed) would be significantly less expensive than multiple oral hypoglycemic agents.
Currently, only a few classes of oral hypoglycemic drugs are available in generic formulations. For example, generic glimeperide and metformin cost as little as $4 to $12 per month, while the costs of brand-name oral hypoglycemic agents are in the range of $170 to $200 per month. In contrast, premixed NPH plus regular insulin such as Novolin 70/30 and Humulin 70/30 cost between $22 and $70 per vial.
A basal-bolus regimen should provide 50% of the total daily insulin in the form of basal insulin. A regimen of 50% basal and 50% bolus seemed to provide better glycemic control than a regimen of 35% basal and 65% bolus in several studies.27,28
In patients already taking a single daily dose of basal insulin along with oral hypoglycemic agents, the dosage of basal insulin is usually raised gradually until adequate glycemic control is achieved. A main question is when to add prandial insulin. There is no clear cutoff for a basal insulin dosage at which prandial insulin should be added.
In the Treat-to-Target Trial,29 almost 60% of patients achieved a hemoglobin A1c level of 7% or less with the addition of either glargine or NPH insulin (basal insulin only) to oral hypoglycemic agents during 24 weeks of follow-up. As expected, glargine caused less nocturnal hypoglycemia. Fewer than half the patients who achieved a hemoglobin A1c level less than 7% had no documented nocturnal hypoglycemia (33% of glargine-treated patients and 27% of NPH-treated patients).
Type 2 diabetes is progressive1; over time, patients treated with once-daily basal insulin often require multiple daily injections.
Adding prandial to basal insulin clearly results in better glycemic control and less glucose variability.19,20,22,30–33 Two major factors in deciding to start prandial insulin are the degree of hyperglycemia and the patient’s acceptance of multiple daily injections. The higher the blood glucose levels, the sooner prandial insulin should be added, especially if hyperglycemia is influencing the prognosis of a coexisting condition or a diabetic complication (eg, an infected foot ulcer).
Adding prandial insulin should be also considered if the dosage of basal insulin has progressively been increased and the hemoglobin A1c level is not improving, especially if a patient has both inadequate glycemic control and frequent hypoglycemia, or if the morning glucose level is within the desired range (indicating there is no room for a further increase in the basal insulin dose) in association with inadequate control of hemoglobin A1c.
What is the best basal insulin for a basal-bolus regimen?
Glargine and detemir were shown to be equally effective as the basal component of a basal-bolus regimen.34,35 Findings were similar to those of studies comparing NPH, detemir, and glargine added, by themselves, to oral hypoglycemic agents. When possible, either glargine or detemir is favored because of less hypoglycemia and less weight gain than with NPH. Weight gain is the least with detemir.
Adding prandial insulin to a basal regimen
In general, whether to add prandial insulin can be decided on the basis of the patient’s record of blood glucose monitoring. Insulin could be added before breakfast if the pre-lunch glucose level is elevated, or before lunch if the dinnertime blood glucose level is elevated, or before dinner if the bedtime blood glucose level is elevated—or a combination of these. Prandial insulin can be started at a low dose (4–6 units) and increased gradually.
In the case of poor glycemic control on a high dosage of basal insulin, a reasonable first step would be to change the regimen to a basal-bolus regimen (about 50% basal and 50% bolus) with no change or a small decrease in the total daily dosage of insulin to avoid hypoglycemia. For example, in a patient on 80 units of glargine or detemir insulin who has inadequate control, the regimen could be changed to 35 units of either glargine or detemir and 10 to 12 units of lispro, aspart, or glulisine before each meal as the bolus component.
Further adjustments of the insulin dosage can be made according to the results of glucose monitoring before each meal and at bedtime. In all case scenarios, the insulin regimen should be re-evaluated routinely during the advancement of therapy from single daily injection of basal insulin to multiple daily injections. Redistribution of total insulin dosage to 50% basal and 50% bolus (divided into three doses before meals) should be considered for patients who continue to have fluctuations of glucose levels, inadequate control, or frequent hypoglycemia. This ratio seems to provide better control for most patients.27,28
Starting with a basal-bolus regimen
For patients new to insulin who are starting a basal-bolus regimen, a dosage based on total body weight could be considered. The requirements vary significantly based on dietary management, level of physical activity, stress (especially illnesses), use of oral hypoglycemic agents, and degree of hyperglycemia.
A lower dosage of insulin (0.2 units per kg) should be considered for people with mild stress, with milder hyperglycemia, or on treatment with oral hypoglycemic agents. Elderly patients and patients with renal or liver failure are at higher risk of hypoglycemia and should also receive a lower dosage of insulin, at least to start with.
Others could be started on a dosage of 0.3 to 0.5 units/kg. Fifty percent of the calculated dosage could be given as basal insulin and 50% given as bolus (divided into three doses, before meals). Subsequently, the dosage would need to be titrated on the basis of the record of glucose monitoring.
Choosing a prandial insulin
Rapid-acting insulin analogues (lispro, aspart, and glulisine) control postprandial glucose levels better than regular insulin and cause less hypoglycemia. Their pharmacokinetics enable them to be taken within a few minutes of the start of a meal, or even after the meal if the patient forgets to take an injection before the meal.
For example, in one study,36 taking aspart immediately before the meal provided better glycemic control than taking regular insulin 30 minutes before meals. In a basal-bolus regimen, the use of aspart along with detemir resulted in glycemic control similar to that provided by twice-daily NPH and regular insulin, with less hypoglycemia.37
The dosage of prandial insulin can be adjusted according to the amount of carbohydrates in each meal (the insulin-to-carbohydrate ratio), as in patients with type 1 diabetes. This approach was associated with less weight gain.38
IS THERE STILL A ROLE FOR PREMIXED INSULIN PREPARATIONS?
Basal-bolus insulin regimens have gained popularity because the prandial doses can easily be adjusted according to carbohydrate intake, glucose level (on a sliding scale), variations in meal time, missed meals (eg, when having a procedure), and exercise. For example, the dose of prandial insulin can be reduced if the patient expects to exercise within 2 or 3 hours after the meal.
Some patients may not accept giving themselves four or five injections per day with a basal-bolus regimen. They may accept a simpler regimen, ie, giving themselves three injections of a premixed insulin per day, one before each meal.
Compared with a basal-bolus regimen, the possibility of achieving adequate glycemic control using lispro mix (50% lispro, 50% lispro protamine suspension) before meals seemed to depend on the goal of glycemic control. Its use in one study showed similar ability to achieve hemoglobin A1c less than 7.5% compared with a basal-bolus regimen of glargine and lispro. For a goal hemoglobin A1c level of less than 7%, the use of glargine and lispro was superior. The rate of hypoglycemia was similar with both strategies.39 These findings imply that the goal hemoglobin A1c should be more relaxed (< 7.5%) when using lispro mix (50% lispro) three times daily before meals.
Biphasic insulin aspart (a mix of aspart and protamine aspart) given three times daily provided similar improvement in glycemic control with no difference in the frequency of hypoglycemia compared with a basal-bolus regimen of NPH and aspart.40 Further, the use of biphasic insulin aspart seemed to provide better glycemic control with less weight gain compared with premixed human insulin (70% NPH, 30% regular insulin).41
Therefore, simpler premixed insulin regimens remain reasonable options for selected patients who do not accept a more complex insulin regimen (basal-bolus) or cannot adhere to it for any reason, especially if premixed insulin is given before meals three times daily. In fact, recent studies have focused on comparing premixed insulin three times daily with basal-bolus regimens (detemir or glargine as basal insulin along with pre-meal insulin analogue).
Glycemic control is harder to achieve with premixed insulin twice daily, mainly because of a higher frequency of hypoglycemia.42 In Europe, the use of premixed insulin three times daily is a popular option, whereas in the United States, a twice-daily schedule has been more common.
COST VS CONTROL
Newer insulin analogues make insulin treatment safer and more accepted by patients. The availability of several options for insulin regimens allows individualization of the treatment according to the patient’s acceptance, the safety profile, and the cost.
Patient selection and insulin titration are key issues in ensuring the achievement of adequate control with the fewest side effects. Lifestyle management (diet and physical activity) enhances the efficacy of insulin therapy and reduces the chances of side effects, namely fluctuation of glucose levels, hypoglycemic episodes, and weight gain.
Human insulins (NPH and regular) remain the least expensive, especially when using premixed NPH-regular insulin 70/30. Their use should be considered when the cost of medication is a major concern for the patient. A more relaxed goal of glycemic control may be considered in order to avoid hypoglycemia when using those insulin preparations, such as a hemoglobin A1c level less than 7.5% or even in the range of 7.5% to 8.5%, depending on the expected seasonal variation of hemoglobin A1c (which is higher in winter43), individual factors, and whether the premixed insulin is used twice or three times daily.
RE-EVALUATE THE REGIMEN ROUTINELY
The insulin regimen should be re-evaluated routinely. It might need to be changed in response to the dynamic multifactorial process of progression of diabetes, change in stress level, presence or resolution of intercurrent illnesses, risk of hypoglycemia, concerns about weight gain, and cost.
Finally, adjustment of the regimen should be considered in response to improvement of glycemic control related to improvement of dietary management, exercising, weight loss, and medical therapies.
- UK Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes 1995; 44:1249–1258.
- Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009; 15:540–558.
- ClinicalTrials.gov. The ORIGIN Trial (Outcome Reduction With Initial Glargine Intervention). http://clinicaltrials.gov/ct2/show/NCT00069784. Accessed 2/11/11.
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010; 33(suppl 1):S11–S61.
- Retnakaran R, Qi Y, Opsteen C, Vivero E, Zinman B. Initial short-term intensive insulin therapy as a strategy for evaluating the preservation of beta-cell function with oral antidiabetic medications: a pilot study with sitagliptin. Diabetes Obes Metab 2010; 12:909–915.
- Zoungas S, Patel A, Chalmers J, et al; ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363:1410–1418.
- Akram K, Pedersen-Bjergaard U, Borch-Johnsen K, Thorsteinsson B. Frequency and risk factors of severe hypoglycemia in insulin-treated type 2 diabetes: a literature survey. J Diabetes Complications 2006; 20:402–408.
- Cryer PE. Chapter 19. Hypoglycemia. In: Jameson JL, editor. Harrison’s Endocrinology. McGraw Hill, 2006:355–363.
- Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006; 28:1569–1581. Erratum in: Clin Ther 2006; 28:1967.
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006; 29:1269–1274. Erratum in: Diabetes Care 2007; 30:1035.
- Haak T, Tiengo A, Draeger E, Suntum M, Waldhäusl W. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab 2005; 7:56–64.
- Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetalogia 2006; 49:442–451.
- Fritsche A, Schweitzer MA, Häring HU; 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med 2003; 138:952–959.
- Rosenstock J, Schwartz SL, Clark CM, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001; 24:631–636.
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008; 51:408–416.
- King AB. Once-daily insulin detemir is comparable to once-daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: a double-blind, randomized, crossover study. Diabetes Obes Metab 2009; 11:69–71.
- Swinnen SG, Snoek FJ, Dain MP, DeVries JH, Hoekstra JB, Holleman F. Rationale, design, and baseline data of the insulin glargine (Lantus) versus insulin detemir (Levemir) Treat-To-Target (L2T3) study: a multinational, randomized noninferiority trial of basal insulin initiation in type 2 diabetes. Diabetes Technol Ther 2009; 11:739–743.
- Kazda C, Hülstrunk H, Helsberg K, Langer F, Forst T, Hanefeld M. Prandial insulin substitution with insulin lispro or insulin lispro mid mixture vs. basal therapy with insulin glargine: a randomized controlled trial in patients with type 2 diabetes beginning insulin therapy. J Diabetes Complications 2006; 20:145–152.
- Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with type 2 diabetes. Diabet Med 2005; 22:374–381.
- Buse JB, Wolffenbuttel BH, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care 2009; 32:1007–1013.
- Kann PH, Wascher T, Zackova V, et al. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes 2006; 114:527–532.
- Monnier L, Colette C, Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract 2006; 12(suppl 1):42–46.
- Woerle HJ, Pimenta WP, Meyer C, et al. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin A1c values. Arch Intern Med 2004; 164:1627–1632.
- Schrot RJ. Targeting plasma glucose: preprandial versus postprandial. Clinical Diabetes 2004; 22:169–172.
- Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008; 371:1073–1084.
- Tamaki M, Shimizu T, Kanazawa A, Fujitani Y, Watada H, Kawamori R, Hirose T. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract 2008; 81:e1–e3.
- Yokoyama H, Tada J, Kamikawa F, Kanno S, Yokota Y, Kuramitsu M. Efficacy of conversion from bedtime NPH insulin to morning insulin glargine in type 2 diabetic patients on basal-prandial insulin therapy. Diabetes Res Clin Pract 2006; 73:35–40.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The Treat-To-Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Davies M, Sinnassamy P, Storms F, Gomis R; ATLANTUS Study Group. Insulin glargine-based therapy improves glycemic control in patients with type 2 diabetes sub-optimally controlled on premixed insulin therapies. Diabetes Res Clin Pract 2008; 79:368–375.
- Jacober SJ, Scism-Bacon JL, Zagar AJ. A comparison of intensive mixture therapy with basal insulin therapy in insulin-naïve patients with type 2 diabetes receiving oral antidiabetes agents. Diabetes Obes Metab 2006; 8:448–455.
- Hirsch IB, Yuan H, Campaigne BN, Tan MH. Impact of prandial plus basal vs basal insulin on glycemic variability in type 2 diabetic patients. Endocr Pract 2009; 15:343–348.
- Robbins DC, Beisswenger PJ, Ceriello A, et al. Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison. Clin Ther 2007; 29:2349–2364.
- Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther 2008; 30:1976–1987.
- Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev 2009; 25:542–548.
- Perriello G, Pampanelli S, Porcellati F, et al. Insulin aspart improves meal time glycaemic control in patients with type 2 diabetes: a randomized, stratified, double-blind and cross-over trial. Diabet Med 2005; 22:606–611.
- Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:564–569.
- Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008; 31:1305–1310.
- Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 2008; 31:20–25.
- Ligthelm RJ, Mouritzen U, Lynggaard H, et al. Biphasic insulin aspart given thrice daily is as efficacious as a basal-bolus insulin regimen with four daily injections: a randomised open-label parallel group four months comparison in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2006; 114:511–519.
- Velojic-Golubovic M, Mikic D, Pesic M, Dimic D, Radenkovic S, Antic S. Biphasic insulin aspart 30: better glycemic control than with premixed human insulin 30 in obese patients with type 2 diabetes. J Endocrinol Invest 2009; 32:23–27.
- Holman RR, Farmer AJ, Davies MJ, et al; 4-T Study Group. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl Med 2009; 361:1736–1747.
- Tseng CL, Brimacombe M, Xie M, et al. Seasonal patterns in monthly hemoglobin A1c values. Am J Epidemiol 2005; 161:565–574.
Many patients with type 2 diabetes eventually need insulin, as their ability to produce their own insulin from pancreatic beta cells declines progressively.1 The questions remain as to when insulin therapy should be started, and which regimen is the most appropriate.
Guidelines from professional societies differ on these points,2,3 as do individual clinicians. Moreover, antidiabetic treatment is an evolving topic. Many new drugs—oral agents as well as injectable analogues of glucagon-like peptide-1 (GLP1) and insulin formulations—have become available in the last 15 years.
In this paper, I advocate an individualized approach and review the indications for insulin treatment, the available preparations, the pros and cons of each regimen, and how the properties of each type of insulin influence attempts to intensify the regimen.
Coexisting physiologic and medical conditions such as pregnancy and chronic renal failure and drugs such as glucocorticoids may alter insulin requirements. I will not cover these special situations, as they deserve separate, detailed discussions.
WHEN SHOULD INSULIN BE STARTED? TWO VIEWS
Early on, patients can be adequately managed with lifestyle modifications and oral hypoglycemic agents or injections of a GLP1 analogue, either alone or in combination with oral medication. Later, some patients reach a point at which insulin therapy becomes the main treatment, similar to patients with type 1 diabetes.
The American Diabetes Association (ADA), in a consensus statement,2 has called for using insulin early in the disease if lifestyle management and monotherapy with metformin (Glucophage) fail to control glucose or if lifestyle management is not adequate and metformin is contraindicated. The ADA’s goal hemoglobin A1c level is less than 7% for most patients.
The American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE), in another consensus statement, use an algorithm stratified by hemoglobin A1c level, in which insulin is mostly reserved for when combination therapy fails.3 Their goal hemoglobin A1c level is 6.5% or less for most patients.
Comment. Both consensus statements make exceptions for patients presenting with very high blood glucose and hemoglobin A1c levels and those who have contraindications to drugs other than insulin. These patients should start insulin immediately, along with lifestyle management.2,3
Both consensus statements give priority to safety. The AACE/ACE statement gives more weight to the risk of hypoglycemia with insulin treatment, whereas the ADA gives more weight to the risk of edema and congestive heart failure with thiazolidinedione drugs (although both insulin and thiazolidinediones cause weight gain) and to adequate validation of treatments in clinical trials.
Ongoing clinical trials may add insight to this issue. For example, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) study is investigating the effects of the long-acting insulin glargine (Lantus) in early diabetes with regard to glycemic control, safety, and cardiovascular outcomes.4 This study is expected to end this year (2011). The safety of alternative treatment options is also under investigation and scrutiny. In the interim, individualized treatment should be considered, as we will see below.

MY VIEW: AN INDIVIDUALIZED APPROACH
The decision to start insulin therapy should be made individually, based on several factors:
- Whether the patient is willing to try it
- The degree of hyperglycemia
- How relevant the potential side effects of insulin are to the patient compared with those of other hypoglycemic agents
- Whether oral hypoglycemic agents with or without GLP1 analogues are expected to provide the desired benefit
- The patient’s work schedule and lifestyle factors
- Cost
- The availability of nurses, diabetes educators, and others to implement and follow the insulin treatment.
Will patients accept insulin?
Factors that affect whether patients comply with a treatment include the number of pills or injections they must take per day, how often they must check their blood glucose, adverse effects, lifestyle limitations caused by the treatment (especially insulin), and cost. Most patients feel better when their glucose levels are under good control, which is a major motivation for initiating and adhering to insulin. The anticipated reduction of diabetic complications further enhances compliance.
Education promotes compliance. Patients need to know that type 2 diabetes tends to progress and that in time their treatment will have to be intensified, with higher doses of their current drugs and new drugs added or substituted, possibly including insulin. This information is best given early, ie, when the diagnosis is made, even if hyperglycemia is mild at that time.
With newer insulin preparations and delivery devices available, more patients are finding insulin treatment acceptable.
The glycemic goal should be individualized
The key issue is glycemic control. If glycemic control is worsening or if the hemoglobin A1c level remains above the goal, then the treatment strategy should be readdressed.
In general, one should try to achieve the best possible glycemic control with the few est adverse effects. Adequate dietary management with a regular meal schedule and predictable carbohydrate intake for each meal helps to avoid or at least minimize the two most important adverse effects of insulin, ie, weight gain and hypoglycemia.
For most patients, I believe a goal hemoglobin A1c level of less than 7% is reasonable.2 For others, a less stringent goal might be more appropriate, such as 7.5%. Several factors affect this decision, including whether the patient is willing to follow a complex insulin regimen (such as a basal-bolus regimen), his or her work schedule, other lifestyle factors, the duration of diabetes, the type or types of insulin used, coexisting medical conditions, the frequency of hypoglycemia, unawareness of hypoglycemia, age, prognosis, life expectancy, and cost.5
If hyperglycemia is severe (Table 1),2 the goal might not be clear when insulin therapy is started. It should become obvious with ongoing follow-up.
Previously untreated patients presenting with severe hyperglycemia are a heterogeneous group. Many of them have had diabetes for a relatively short time and were recently diagnosed. These patients are likely to safely achieve near-normal glycemic control. Some of them might be adequately treated with oral hypoglycemic agents; if insulin is used, transitioning from insulin to oral hypoglycemic agents may be feasible.2
Some untreated patients may have had diabetes for several years and have advanced disease and therefore might be more difficult to treat. Only 21 (57%) of 37 previously untreated patients intensively treated with insulin reached the goal fasting glucose level of less than 126 mg/dL in one study.6 The only way to evaluate the feasibility of achieving near-normal glycemia safely is by following the patient’s progress over time.
The patient’s glycemic goal should be reevaluated periodically and may need to be adjusted over time, based on changes in any of the factors discussed above.
Risk of hypoglycemia
The goal should be looser in difficult-to-treat patients, ie, those with frequent hypoglycemia and decreased awareness of hypoglycemia.
Patients with advanced diabetes whose glucose levels continue to fluctuate widely after lifestyle management and the insulin regimen have been addressed should also have a looser goal. These fluctuations of glucose levels are surrogate markers for the degree of insulin deficiency. Attempting to achieve near-normal glycemic levels in this situation would be associated with a higher risk of hypoglycemia.
A higher risk of hypoglycemia and its complications (eg, falling and accidents, especially among operators of heavy machinery, construction workers, and drivers) is another reason for adopting a relaxed goal of glycemic control.
ADDING BASAL INSULIN TO ORAL HYPOGLYCEMIC THERAPY
When glycemic control worsens or is not adequate despite the use of oral hypoglycemic agents, often the next step is to add basal insulin therapy, ie, once-daily doses of a long-acting insulin.
NPH, detemir, or glargine?
Most often, glargine or detemir (Levemir) insulin is used. Detemir can also be given twice daily if needed. If cost is a concern, neutral protamine Hagedorn (NPH, Humulin N, Novolin N) insulin once daily at bedtime or twice daily is a reasonable alternative.
Costs of basal insulins are $22 to $50 per 1,000-unit vial for NPH, $70 to $90 per 1,000-unit vial for detemir and glargine, and $170 to $200 for a box of five detemir or glargine pens (containing 1,500 units total). Complicating this issue, vials should not be used for more than 1 month, and thus, the cost of vials vs pens depends on dosage.
Detemir vs NPH. In a trial in patients with inadequately controlled type 2 diabetes who had never taken insulin before and who were taking one or more oral hypoglycemic drugs, the addition of detemir insulin once daily or NPH at bedtime resulted in similar improvements in hemoglobin A1c (a decrease of about 1.5%).10
Detemir had several advantages over NPH. First, the incidence of nocturnal hypoglycemia was 50% lower with detemir at bedtime than with NPH at bedtime, and 87% lower with detemir in the morning than with bedtime NPH.10 In another trial,11 the risk of hypoglycemia at any time of day was 47% lower with insulin detemir than with NPH, and the risk of nocturnal hypoglycemia was 55% lower.
The risk of nocturnal hypoglycemia is lower if detemir is taken in the morning than at bedtime, although the total frequency of hypoglycemic episodes is the same.10 Therefore, another decision after starting basal insulin, based on the patient’s glucose trends and frequency of hypoglycemic events, would be whether insulin should be taken in the morning or at bedtime.
The second advantage of detemir is that it causes less weight gain: 0.7 kg at 20 weeks with detemir at bedtime vs 1.6 kg with NPH at bedtime.10
Further, detemir insulin was associated with less within-subject variability in the fasting glucose level than with NPH when these insulins were used in a basal-bolus regimen.12
Hermansen et al11 found that if the dosage of basal insulin was aggressively increased, 70% of patients achieved a hemoglobin A1c target of less than 7% with either NPH or detemir insulin, with fewer hypoglycemic episodes in patients treated with detemir.
Therefore, adding basal insulin to oral therapy is adequate for many patients who are new to insulin. Many patients would need more, such as the addition of insulin before meals.
Glargine vs NPH. Compared with adding NPH, adding glargine to a regimen of oral hypoglycemic agents controls blood glucose levels better and with less fluctuation in glucose levels, a lower risk of hypoglycemia, and less weight gain.13–15 These results were the same when using glargine with either metformin13 or glimeperide (Amaryl).14
Glargine is usually given once daily at bedtime. One study suggested that giving it in the morning is more effective.14
Detemir vs glargine. Studies that compared detemir and glargine revealed more similarities than differences in their clinical benefits.16,17 Both preparations effectively lower glucose levels and improve quality of life.18
Titrating the insulin regimen is a key in achieving adequate glycemic control. This includes teaching patients how to adjust their insulin, for example by increasing the dosage of glargine or detemir by 2 units every 4 to 7 days until adequate glycemic control is achieved, unless hypoglycemia becomes a barrier.
BASAL VS PRANDIAL INSULIN
Once-daily insulin injection is relatively convenient, but it comes with a limitation: it does not adequately control postprandial hyperglycemia. A solution is insulin before meals, ie, prandial insulin.
Kazda et al19 compared three regimens in patients not taking oral hypoglycemic agents: rapid-acting insulin lispro (Humalog) before each meal, a mix of 50% lispro and 50% protamine lispro (Humalog Mix 50/50) (the protamine delays its release) before each meal, and glargine at bedtime. The absolute change in hemoglobin A1c was −0.3% in the glargine group, −1.1% in the lispro group, and −1.2% in the lispro mix group. The glargine group had better control of fasting glucose.
Similar advantages of better glycemic control and fewer nocturnal hypoglycemic episodes were seen in trials of a mixture of 25% lispro and 75% protamine lispro before meals compared with glargine insulin in patients on simultaneous treatment with oral hypoglycemic agents.20,21 Buse et al21 reported that more patients achieved a hemoglobin A1c level below 7% with this lispro mix (47%) than with glargine (40%). The absolute difference in mean hemoglobin A1c between the two groups was minimal, although it reached statistical significance. As expected, weight gain was less in the glargine group.21
Kann et al22 reported that glycemic control was also better with a mixture of 30% aspart and 70% protamine aspart (NovoLog Mix 70/30) twice a day along with metformin than with glargine insulin once a day along with oral glimepiride, a sulfonylurea. Further, in this study, weight gain was noted in the glargine-glimepiride group only.22 Therefore, the advantage of less weight gain has not been always reproducible in glargine studies.
Comment. These studies point to the contribution of postprandial glucose to hemoglobin A1c.23–25 In patients with satisfactory glycemic control, the postprandial glucose level seems to be the major contributor to hemoglobin A1c. When glycemic control worsens, the contribution of fasting glucose to hemoglobin A1c increases.23
Premixed insulins (lispro mix and aspart mix) provide basal coverage and control postprandial hyperglycemia. Therefore, prandial premixed insulin therapy is expected to be superior to basal insulin therapy. Premixed insulin could be considered as a simplified basal-bolus regimen (see below).
The superiority of prandial (rapid-acting) insulin alone over basal insulin therapy, as seen in the study by Kazda et al,19 has not been reproducible in other studies. For example, in one study, once-daily glargine resulted in a similar improvement in hemoglobin A1c, a lower rate of hypoglycemic episodes, and greater patient satisfaction with treatment compared with lispro insulin before meals.26 This issue remains debatable because all the trials have been open-label and thus are subject to limitations.
The main lesson is that either glargine or lispro monotherapy is a reasonable option and results in better glycemic control in patients for whom two oral hypoglycemic agents have failed. Further, both fasting and postprandial hyperglycemia are important to address. In patients with severe hyperglycemia, a combination of prandial and basal insulin may be indicated. One would expect neither basal nor prandial (bolus) insulin to be adequate in this situation.
In conclusion, adding basal insulin to oral hypoglycemic agents is a reasonable option in the advancement of diabetes therapy and has become a common way to introduce insulin. It is simple and less labor-intensive for patients and medical groups than a basal-bolus regimen. Patients usually find it acceptable. The future availability of an easy-to-deliver, safe, and effective prandial insulin may change the current treatment paradigm; several newer prandial insulins are under investigation.
In advanced diabetes, both prandial and fasting glucose levels are crucial to address. Some patients may need to be started on both basal and prandial insulin simultaneously, depending on their degree of hyperglycemia, the duration of diabetes, coexisting medical conditions, and the goal of glycemic control.
BASAL-BOLUS INSULIN REGIMENS
In the advanced stages of type 2 diabetes, as insulin deficiency worsens, patients need to start giving themselves injections of a rapid-acting insulin—regular, lispro, aspart, or glulisine (Apidra) before meals, in addition to once- or twice-daily basal insulin injections. Such a “basal-bolus” regimen could also be used for newly diagnosed patients presenting with severe hyperglycemia. In addition, some patients on basal insulin plus oral hypoglycemic drugs may develop contraindications to their oral drugs. Adding bolus insulin becomes the main option for these patients too.
For others, a basal-bolus regimen might be chosen purely because of cost. For example, a regimen of NPH and regular insulin (multiple daily injections or premixed) would be significantly less expensive than multiple oral hypoglycemic agents.
Currently, only a few classes of oral hypoglycemic drugs are available in generic formulations. For example, generic glimeperide and metformin cost as little as $4 to $12 per month, while the costs of brand-name oral hypoglycemic agents are in the range of $170 to $200 per month. In contrast, premixed NPH plus regular insulin such as Novolin 70/30 and Humulin 70/30 cost between $22 and $70 per vial.
A basal-bolus regimen should provide 50% of the total daily insulin in the form of basal insulin. A regimen of 50% basal and 50% bolus seemed to provide better glycemic control than a regimen of 35% basal and 65% bolus in several studies.27,28
In patients already taking a single daily dose of basal insulin along with oral hypoglycemic agents, the dosage of basal insulin is usually raised gradually until adequate glycemic control is achieved. A main question is when to add prandial insulin. There is no clear cutoff for a basal insulin dosage at which prandial insulin should be added.
In the Treat-to-Target Trial,29 almost 60% of patients achieved a hemoglobin A1c level of 7% or less with the addition of either glargine or NPH insulin (basal insulin only) to oral hypoglycemic agents during 24 weeks of follow-up. As expected, glargine caused less nocturnal hypoglycemia. Fewer than half the patients who achieved a hemoglobin A1c level less than 7% had no documented nocturnal hypoglycemia (33% of glargine-treated patients and 27% of NPH-treated patients).
Type 2 diabetes is progressive1; over time, patients treated with once-daily basal insulin often require multiple daily injections.
Adding prandial to basal insulin clearly results in better glycemic control and less glucose variability.19,20,22,30–33 Two major factors in deciding to start prandial insulin are the degree of hyperglycemia and the patient’s acceptance of multiple daily injections. The higher the blood glucose levels, the sooner prandial insulin should be added, especially if hyperglycemia is influencing the prognosis of a coexisting condition or a diabetic complication (eg, an infected foot ulcer).
Adding prandial insulin should be also considered if the dosage of basal insulin has progressively been increased and the hemoglobin A1c level is not improving, especially if a patient has both inadequate glycemic control and frequent hypoglycemia, or if the morning glucose level is within the desired range (indicating there is no room for a further increase in the basal insulin dose) in association with inadequate control of hemoglobin A1c.
What is the best basal insulin for a basal-bolus regimen?
Glargine and detemir were shown to be equally effective as the basal component of a basal-bolus regimen.34,35 Findings were similar to those of studies comparing NPH, detemir, and glargine added, by themselves, to oral hypoglycemic agents. When possible, either glargine or detemir is favored because of less hypoglycemia and less weight gain than with NPH. Weight gain is the least with detemir.
Adding prandial insulin to a basal regimen
In general, whether to add prandial insulin can be decided on the basis of the patient’s record of blood glucose monitoring. Insulin could be added before breakfast if the pre-lunch glucose level is elevated, or before lunch if the dinnertime blood glucose level is elevated, or before dinner if the bedtime blood glucose level is elevated—or a combination of these. Prandial insulin can be started at a low dose (4–6 units) and increased gradually.
In the case of poor glycemic control on a high dosage of basal insulin, a reasonable first step would be to change the regimen to a basal-bolus regimen (about 50% basal and 50% bolus) with no change or a small decrease in the total daily dosage of insulin to avoid hypoglycemia. For example, in a patient on 80 units of glargine or detemir insulin who has inadequate control, the regimen could be changed to 35 units of either glargine or detemir and 10 to 12 units of lispro, aspart, or glulisine before each meal as the bolus component.
Further adjustments of the insulin dosage can be made according to the results of glucose monitoring before each meal and at bedtime. In all case scenarios, the insulin regimen should be re-evaluated routinely during the advancement of therapy from single daily injection of basal insulin to multiple daily injections. Redistribution of total insulin dosage to 50% basal and 50% bolus (divided into three doses before meals) should be considered for patients who continue to have fluctuations of glucose levels, inadequate control, or frequent hypoglycemia. This ratio seems to provide better control for most patients.27,28
Starting with a basal-bolus regimen
For patients new to insulin who are starting a basal-bolus regimen, a dosage based on total body weight could be considered. The requirements vary significantly based on dietary management, level of physical activity, stress (especially illnesses), use of oral hypoglycemic agents, and degree of hyperglycemia.
A lower dosage of insulin (0.2 units per kg) should be considered for people with mild stress, with milder hyperglycemia, or on treatment with oral hypoglycemic agents. Elderly patients and patients with renal or liver failure are at higher risk of hypoglycemia and should also receive a lower dosage of insulin, at least to start with.
Others could be started on a dosage of 0.3 to 0.5 units/kg. Fifty percent of the calculated dosage could be given as basal insulin and 50% given as bolus (divided into three doses, before meals). Subsequently, the dosage would need to be titrated on the basis of the record of glucose monitoring.
Choosing a prandial insulin
Rapid-acting insulin analogues (lispro, aspart, and glulisine) control postprandial glucose levels better than regular insulin and cause less hypoglycemia. Their pharmacokinetics enable them to be taken within a few minutes of the start of a meal, or even after the meal if the patient forgets to take an injection before the meal.
For example, in one study,36 taking aspart immediately before the meal provided better glycemic control than taking regular insulin 30 minutes before meals. In a basal-bolus regimen, the use of aspart along with detemir resulted in glycemic control similar to that provided by twice-daily NPH and regular insulin, with less hypoglycemia.37
The dosage of prandial insulin can be adjusted according to the amount of carbohydrates in each meal (the insulin-to-carbohydrate ratio), as in patients with type 1 diabetes. This approach was associated with less weight gain.38
IS THERE STILL A ROLE FOR PREMIXED INSULIN PREPARATIONS?
Basal-bolus insulin regimens have gained popularity because the prandial doses can easily be adjusted according to carbohydrate intake, glucose level (on a sliding scale), variations in meal time, missed meals (eg, when having a procedure), and exercise. For example, the dose of prandial insulin can be reduced if the patient expects to exercise within 2 or 3 hours after the meal.
Some patients may not accept giving themselves four or five injections per day with a basal-bolus regimen. They may accept a simpler regimen, ie, giving themselves three injections of a premixed insulin per day, one before each meal.
Compared with a basal-bolus regimen, the possibility of achieving adequate glycemic control using lispro mix (50% lispro, 50% lispro protamine suspension) before meals seemed to depend on the goal of glycemic control. Its use in one study showed similar ability to achieve hemoglobin A1c less than 7.5% compared with a basal-bolus regimen of glargine and lispro. For a goal hemoglobin A1c level of less than 7%, the use of glargine and lispro was superior. The rate of hypoglycemia was similar with both strategies.39 These findings imply that the goal hemoglobin A1c should be more relaxed (< 7.5%) when using lispro mix (50% lispro) three times daily before meals.
Biphasic insulin aspart (a mix of aspart and protamine aspart) given three times daily provided similar improvement in glycemic control with no difference in the frequency of hypoglycemia compared with a basal-bolus regimen of NPH and aspart.40 Further, the use of biphasic insulin aspart seemed to provide better glycemic control with less weight gain compared with premixed human insulin (70% NPH, 30% regular insulin).41
Therefore, simpler premixed insulin regimens remain reasonable options for selected patients who do not accept a more complex insulin regimen (basal-bolus) or cannot adhere to it for any reason, especially if premixed insulin is given before meals three times daily. In fact, recent studies have focused on comparing premixed insulin three times daily with basal-bolus regimens (detemir or glargine as basal insulin along with pre-meal insulin analogue).
Glycemic control is harder to achieve with premixed insulin twice daily, mainly because of a higher frequency of hypoglycemia.42 In Europe, the use of premixed insulin three times daily is a popular option, whereas in the United States, a twice-daily schedule has been more common.
COST VS CONTROL
Newer insulin analogues make insulin treatment safer and more accepted by patients. The availability of several options for insulin regimens allows individualization of the treatment according to the patient’s acceptance, the safety profile, and the cost.
Patient selection and insulin titration are key issues in ensuring the achievement of adequate control with the fewest side effects. Lifestyle management (diet and physical activity) enhances the efficacy of insulin therapy and reduces the chances of side effects, namely fluctuation of glucose levels, hypoglycemic episodes, and weight gain.
Human insulins (NPH and regular) remain the least expensive, especially when using premixed NPH-regular insulin 70/30. Their use should be considered when the cost of medication is a major concern for the patient. A more relaxed goal of glycemic control may be considered in order to avoid hypoglycemia when using those insulin preparations, such as a hemoglobin A1c level less than 7.5% or even in the range of 7.5% to 8.5%, depending on the expected seasonal variation of hemoglobin A1c (which is higher in winter43), individual factors, and whether the premixed insulin is used twice or three times daily.
RE-EVALUATE THE REGIMEN ROUTINELY
The insulin regimen should be re-evaluated routinely. It might need to be changed in response to the dynamic multifactorial process of progression of diabetes, change in stress level, presence or resolution of intercurrent illnesses, risk of hypoglycemia, concerns about weight gain, and cost.
Finally, adjustment of the regimen should be considered in response to improvement of glycemic control related to improvement of dietary management, exercising, weight loss, and medical therapies.
Many patients with type 2 diabetes eventually need insulin, as their ability to produce their own insulin from pancreatic beta cells declines progressively.1 The questions remain as to when insulin therapy should be started, and which regimen is the most appropriate.
Guidelines from professional societies differ on these points,2,3 as do individual clinicians. Moreover, antidiabetic treatment is an evolving topic. Many new drugs—oral agents as well as injectable analogues of glucagon-like peptide-1 (GLP1) and insulin formulations—have become available in the last 15 years.
In this paper, I advocate an individualized approach and review the indications for insulin treatment, the available preparations, the pros and cons of each regimen, and how the properties of each type of insulin influence attempts to intensify the regimen.
Coexisting physiologic and medical conditions such as pregnancy and chronic renal failure and drugs such as glucocorticoids may alter insulin requirements. I will not cover these special situations, as they deserve separate, detailed discussions.
WHEN SHOULD INSULIN BE STARTED? TWO VIEWS
Early on, patients can be adequately managed with lifestyle modifications and oral hypoglycemic agents or injections of a GLP1 analogue, either alone or in combination with oral medication. Later, some patients reach a point at which insulin therapy becomes the main treatment, similar to patients with type 1 diabetes.
The American Diabetes Association (ADA), in a consensus statement,2 has called for using insulin early in the disease if lifestyle management and monotherapy with metformin (Glucophage) fail to control glucose or if lifestyle management is not adequate and metformin is contraindicated. The ADA’s goal hemoglobin A1c level is less than 7% for most patients.
The American Association of Clinical Endocrinologists (AACE) and the American College of Endocrinology (ACE), in another consensus statement, use an algorithm stratified by hemoglobin A1c level, in which insulin is mostly reserved for when combination therapy fails.3 Their goal hemoglobin A1c level is 6.5% or less for most patients.
Comment. Both consensus statements make exceptions for patients presenting with very high blood glucose and hemoglobin A1c levels and those who have contraindications to drugs other than insulin. These patients should start insulin immediately, along with lifestyle management.2,3
Both consensus statements give priority to safety. The AACE/ACE statement gives more weight to the risk of hypoglycemia with insulin treatment, whereas the ADA gives more weight to the risk of edema and congestive heart failure with thiazolidinedione drugs (although both insulin and thiazolidinediones cause weight gain) and to adequate validation of treatments in clinical trials.
Ongoing clinical trials may add insight to this issue. For example, the Outcome Reduction With Initial Glargine Intervention (ORIGIN) study is investigating the effects of the long-acting insulin glargine (Lantus) in early diabetes with regard to glycemic control, safety, and cardiovascular outcomes.4 This study is expected to end this year (2011). The safety of alternative treatment options is also under investigation and scrutiny. In the interim, individualized treatment should be considered, as we will see below.

MY VIEW: AN INDIVIDUALIZED APPROACH
The decision to start insulin therapy should be made individually, based on several factors:
- Whether the patient is willing to try it
- The degree of hyperglycemia
- How relevant the potential side effects of insulin are to the patient compared with those of other hypoglycemic agents
- Whether oral hypoglycemic agents with or without GLP1 analogues are expected to provide the desired benefit
- The patient’s work schedule and lifestyle factors
- Cost
- The availability of nurses, diabetes educators, and others to implement and follow the insulin treatment.
Will patients accept insulin?
Factors that affect whether patients comply with a treatment include the number of pills or injections they must take per day, how often they must check their blood glucose, adverse effects, lifestyle limitations caused by the treatment (especially insulin), and cost. Most patients feel better when their glucose levels are under good control, which is a major motivation for initiating and adhering to insulin. The anticipated reduction of diabetic complications further enhances compliance.
Education promotes compliance. Patients need to know that type 2 diabetes tends to progress and that in time their treatment will have to be intensified, with higher doses of their current drugs and new drugs added or substituted, possibly including insulin. This information is best given early, ie, when the diagnosis is made, even if hyperglycemia is mild at that time.
With newer insulin preparations and delivery devices available, more patients are finding insulin treatment acceptable.
The glycemic goal should be individualized
The key issue is glycemic control. If glycemic control is worsening or if the hemoglobin A1c level remains above the goal, then the treatment strategy should be readdressed.
In general, one should try to achieve the best possible glycemic control with the few est adverse effects. Adequate dietary management with a regular meal schedule and predictable carbohydrate intake for each meal helps to avoid or at least minimize the two most important adverse effects of insulin, ie, weight gain and hypoglycemia.
For most patients, I believe a goal hemoglobin A1c level of less than 7% is reasonable.2 For others, a less stringent goal might be more appropriate, such as 7.5%. Several factors affect this decision, including whether the patient is willing to follow a complex insulin regimen (such as a basal-bolus regimen), his or her work schedule, other lifestyle factors, the duration of diabetes, the type or types of insulin used, coexisting medical conditions, the frequency of hypoglycemia, unawareness of hypoglycemia, age, prognosis, life expectancy, and cost.5
If hyperglycemia is severe (Table 1),2 the goal might not be clear when insulin therapy is started. It should become obvious with ongoing follow-up.
Previously untreated patients presenting with severe hyperglycemia are a heterogeneous group. Many of them have had diabetes for a relatively short time and were recently diagnosed. These patients are likely to safely achieve near-normal glycemic control. Some of them might be adequately treated with oral hypoglycemic agents; if insulin is used, transitioning from insulin to oral hypoglycemic agents may be feasible.2
Some untreated patients may have had diabetes for several years and have advanced disease and therefore might be more difficult to treat. Only 21 (57%) of 37 previously untreated patients intensively treated with insulin reached the goal fasting glucose level of less than 126 mg/dL in one study.6 The only way to evaluate the feasibility of achieving near-normal glycemia safely is by following the patient’s progress over time.
The patient’s glycemic goal should be reevaluated periodically and may need to be adjusted over time, based on changes in any of the factors discussed above.
Risk of hypoglycemia
The goal should be looser in difficult-to-treat patients, ie, those with frequent hypoglycemia and decreased awareness of hypoglycemia.
Patients with advanced diabetes whose glucose levels continue to fluctuate widely after lifestyle management and the insulin regimen have been addressed should also have a looser goal. These fluctuations of glucose levels are surrogate markers for the degree of insulin deficiency. Attempting to achieve near-normal glycemic levels in this situation would be associated with a higher risk of hypoglycemia.
A higher risk of hypoglycemia and its complications (eg, falling and accidents, especially among operators of heavy machinery, construction workers, and drivers) is another reason for adopting a relaxed goal of glycemic control.
ADDING BASAL INSULIN TO ORAL HYPOGLYCEMIC THERAPY
When glycemic control worsens or is not adequate despite the use of oral hypoglycemic agents, often the next step is to add basal insulin therapy, ie, once-daily doses of a long-acting insulin.
NPH, detemir, or glargine?
Most often, glargine or detemir (Levemir) insulin is used. Detemir can also be given twice daily if needed. If cost is a concern, neutral protamine Hagedorn (NPH, Humulin N, Novolin N) insulin once daily at bedtime or twice daily is a reasonable alternative.
Costs of basal insulins are $22 to $50 per 1,000-unit vial for NPH, $70 to $90 per 1,000-unit vial for detemir and glargine, and $170 to $200 for a box of five detemir or glargine pens (containing 1,500 units total). Complicating this issue, vials should not be used for more than 1 month, and thus, the cost of vials vs pens depends on dosage.
Detemir vs NPH. In a trial in patients with inadequately controlled type 2 diabetes who had never taken insulin before and who were taking one or more oral hypoglycemic drugs, the addition of detemir insulin once daily or NPH at bedtime resulted in similar improvements in hemoglobin A1c (a decrease of about 1.5%).10
Detemir had several advantages over NPH. First, the incidence of nocturnal hypoglycemia was 50% lower with detemir at bedtime than with NPH at bedtime, and 87% lower with detemir in the morning than with bedtime NPH.10 In another trial,11 the risk of hypoglycemia at any time of day was 47% lower with insulin detemir than with NPH, and the risk of nocturnal hypoglycemia was 55% lower.
The risk of nocturnal hypoglycemia is lower if detemir is taken in the morning than at bedtime, although the total frequency of hypoglycemic episodes is the same.10 Therefore, another decision after starting basal insulin, based on the patient’s glucose trends and frequency of hypoglycemic events, would be whether insulin should be taken in the morning or at bedtime.
The second advantage of detemir is that it causes less weight gain: 0.7 kg at 20 weeks with detemir at bedtime vs 1.6 kg with NPH at bedtime.10
Further, detemir insulin was associated with less within-subject variability in the fasting glucose level than with NPH when these insulins were used in a basal-bolus regimen.12
Hermansen et al11 found that if the dosage of basal insulin was aggressively increased, 70% of patients achieved a hemoglobin A1c target of less than 7% with either NPH or detemir insulin, with fewer hypoglycemic episodes in patients treated with detemir.
Therefore, adding basal insulin to oral therapy is adequate for many patients who are new to insulin. Many patients would need more, such as the addition of insulin before meals.
Glargine vs NPH. Compared with adding NPH, adding glargine to a regimen of oral hypoglycemic agents controls blood glucose levels better and with less fluctuation in glucose levels, a lower risk of hypoglycemia, and less weight gain.13–15 These results were the same when using glargine with either metformin13 or glimeperide (Amaryl).14
Glargine is usually given once daily at bedtime. One study suggested that giving it in the morning is more effective.14
Detemir vs glargine. Studies that compared detemir and glargine revealed more similarities than differences in their clinical benefits.16,17 Both preparations effectively lower glucose levels and improve quality of life.18
Titrating the insulin regimen is a key in achieving adequate glycemic control. This includes teaching patients how to adjust their insulin, for example by increasing the dosage of glargine or detemir by 2 units every 4 to 7 days until adequate glycemic control is achieved, unless hypoglycemia becomes a barrier.
BASAL VS PRANDIAL INSULIN
Once-daily insulin injection is relatively convenient, but it comes with a limitation: it does not adequately control postprandial hyperglycemia. A solution is insulin before meals, ie, prandial insulin.
Kazda et al19 compared three regimens in patients not taking oral hypoglycemic agents: rapid-acting insulin lispro (Humalog) before each meal, a mix of 50% lispro and 50% protamine lispro (Humalog Mix 50/50) (the protamine delays its release) before each meal, and glargine at bedtime. The absolute change in hemoglobin A1c was −0.3% in the glargine group, −1.1% in the lispro group, and −1.2% in the lispro mix group. The glargine group had better control of fasting glucose.
Similar advantages of better glycemic control and fewer nocturnal hypoglycemic episodes were seen in trials of a mixture of 25% lispro and 75% protamine lispro before meals compared with glargine insulin in patients on simultaneous treatment with oral hypoglycemic agents.20,21 Buse et al21 reported that more patients achieved a hemoglobin A1c level below 7% with this lispro mix (47%) than with glargine (40%). The absolute difference in mean hemoglobin A1c between the two groups was minimal, although it reached statistical significance. As expected, weight gain was less in the glargine group.21
Kann et al22 reported that glycemic control was also better with a mixture of 30% aspart and 70% protamine aspart (NovoLog Mix 70/30) twice a day along with metformin than with glargine insulin once a day along with oral glimepiride, a sulfonylurea. Further, in this study, weight gain was noted in the glargine-glimepiride group only.22 Therefore, the advantage of less weight gain has not been always reproducible in glargine studies.
Comment. These studies point to the contribution of postprandial glucose to hemoglobin A1c.23–25 In patients with satisfactory glycemic control, the postprandial glucose level seems to be the major contributor to hemoglobin A1c. When glycemic control worsens, the contribution of fasting glucose to hemoglobin A1c increases.23
Premixed insulins (lispro mix and aspart mix) provide basal coverage and control postprandial hyperglycemia. Therefore, prandial premixed insulin therapy is expected to be superior to basal insulin therapy. Premixed insulin could be considered as a simplified basal-bolus regimen (see below).
The superiority of prandial (rapid-acting) insulin alone over basal insulin therapy, as seen in the study by Kazda et al,19 has not been reproducible in other studies. For example, in one study, once-daily glargine resulted in a similar improvement in hemoglobin A1c, a lower rate of hypoglycemic episodes, and greater patient satisfaction with treatment compared with lispro insulin before meals.26 This issue remains debatable because all the trials have been open-label and thus are subject to limitations.
The main lesson is that either glargine or lispro monotherapy is a reasonable option and results in better glycemic control in patients for whom two oral hypoglycemic agents have failed. Further, both fasting and postprandial hyperglycemia are important to address. In patients with severe hyperglycemia, a combination of prandial and basal insulin may be indicated. One would expect neither basal nor prandial (bolus) insulin to be adequate in this situation.
In conclusion, adding basal insulin to oral hypoglycemic agents is a reasonable option in the advancement of diabetes therapy and has become a common way to introduce insulin. It is simple and less labor-intensive for patients and medical groups than a basal-bolus regimen. Patients usually find it acceptable. The future availability of an easy-to-deliver, safe, and effective prandial insulin may change the current treatment paradigm; several newer prandial insulins are under investigation.
In advanced diabetes, both prandial and fasting glucose levels are crucial to address. Some patients may need to be started on both basal and prandial insulin simultaneously, depending on their degree of hyperglycemia, the duration of diabetes, coexisting medical conditions, and the goal of glycemic control.
BASAL-BOLUS INSULIN REGIMENS
In the advanced stages of type 2 diabetes, as insulin deficiency worsens, patients need to start giving themselves injections of a rapid-acting insulin—regular, lispro, aspart, or glulisine (Apidra) before meals, in addition to once- or twice-daily basal insulin injections. Such a “basal-bolus” regimen could also be used for newly diagnosed patients presenting with severe hyperglycemia. In addition, some patients on basal insulin plus oral hypoglycemic drugs may develop contraindications to their oral drugs. Adding bolus insulin becomes the main option for these patients too.
For others, a basal-bolus regimen might be chosen purely because of cost. For example, a regimen of NPH and regular insulin (multiple daily injections or premixed) would be significantly less expensive than multiple oral hypoglycemic agents.
Currently, only a few classes of oral hypoglycemic drugs are available in generic formulations. For example, generic glimeperide and metformin cost as little as $4 to $12 per month, while the costs of brand-name oral hypoglycemic agents are in the range of $170 to $200 per month. In contrast, premixed NPH plus regular insulin such as Novolin 70/30 and Humulin 70/30 cost between $22 and $70 per vial.
A basal-bolus regimen should provide 50% of the total daily insulin in the form of basal insulin. A regimen of 50% basal and 50% bolus seemed to provide better glycemic control than a regimen of 35% basal and 65% bolus in several studies.27,28
In patients already taking a single daily dose of basal insulin along with oral hypoglycemic agents, the dosage of basal insulin is usually raised gradually until adequate glycemic control is achieved. A main question is when to add prandial insulin. There is no clear cutoff for a basal insulin dosage at which prandial insulin should be added.
In the Treat-to-Target Trial,29 almost 60% of patients achieved a hemoglobin A1c level of 7% or less with the addition of either glargine or NPH insulin (basal insulin only) to oral hypoglycemic agents during 24 weeks of follow-up. As expected, glargine caused less nocturnal hypoglycemia. Fewer than half the patients who achieved a hemoglobin A1c level less than 7% had no documented nocturnal hypoglycemia (33% of glargine-treated patients and 27% of NPH-treated patients).
Type 2 diabetes is progressive1; over time, patients treated with once-daily basal insulin often require multiple daily injections.
Adding prandial to basal insulin clearly results in better glycemic control and less glucose variability.19,20,22,30–33 Two major factors in deciding to start prandial insulin are the degree of hyperglycemia and the patient’s acceptance of multiple daily injections. The higher the blood glucose levels, the sooner prandial insulin should be added, especially if hyperglycemia is influencing the prognosis of a coexisting condition or a diabetic complication (eg, an infected foot ulcer).
Adding prandial insulin should be also considered if the dosage of basal insulin has progressively been increased and the hemoglobin A1c level is not improving, especially if a patient has both inadequate glycemic control and frequent hypoglycemia, or if the morning glucose level is within the desired range (indicating there is no room for a further increase in the basal insulin dose) in association with inadequate control of hemoglobin A1c.
What is the best basal insulin for a basal-bolus regimen?
Glargine and detemir were shown to be equally effective as the basal component of a basal-bolus regimen.34,35 Findings were similar to those of studies comparing NPH, detemir, and glargine added, by themselves, to oral hypoglycemic agents. When possible, either glargine or detemir is favored because of less hypoglycemia and less weight gain than with NPH. Weight gain is the least with detemir.
Adding prandial insulin to a basal regimen
In general, whether to add prandial insulin can be decided on the basis of the patient’s record of blood glucose monitoring. Insulin could be added before breakfast if the pre-lunch glucose level is elevated, or before lunch if the dinnertime blood glucose level is elevated, or before dinner if the bedtime blood glucose level is elevated—or a combination of these. Prandial insulin can be started at a low dose (4–6 units) and increased gradually.
In the case of poor glycemic control on a high dosage of basal insulin, a reasonable first step would be to change the regimen to a basal-bolus regimen (about 50% basal and 50% bolus) with no change or a small decrease in the total daily dosage of insulin to avoid hypoglycemia. For example, in a patient on 80 units of glargine or detemir insulin who has inadequate control, the regimen could be changed to 35 units of either glargine or detemir and 10 to 12 units of lispro, aspart, or glulisine before each meal as the bolus component.
Further adjustments of the insulin dosage can be made according to the results of glucose monitoring before each meal and at bedtime. In all case scenarios, the insulin regimen should be re-evaluated routinely during the advancement of therapy from single daily injection of basal insulin to multiple daily injections. Redistribution of total insulin dosage to 50% basal and 50% bolus (divided into three doses before meals) should be considered for patients who continue to have fluctuations of glucose levels, inadequate control, or frequent hypoglycemia. This ratio seems to provide better control for most patients.27,28
Starting with a basal-bolus regimen
For patients new to insulin who are starting a basal-bolus regimen, a dosage based on total body weight could be considered. The requirements vary significantly based on dietary management, level of physical activity, stress (especially illnesses), use of oral hypoglycemic agents, and degree of hyperglycemia.
A lower dosage of insulin (0.2 units per kg) should be considered for people with mild stress, with milder hyperglycemia, or on treatment with oral hypoglycemic agents. Elderly patients and patients with renal or liver failure are at higher risk of hypoglycemia and should also receive a lower dosage of insulin, at least to start with.
Others could be started on a dosage of 0.3 to 0.5 units/kg. Fifty percent of the calculated dosage could be given as basal insulin and 50% given as bolus (divided into three doses, before meals). Subsequently, the dosage would need to be titrated on the basis of the record of glucose monitoring.
Choosing a prandial insulin
Rapid-acting insulin analogues (lispro, aspart, and glulisine) control postprandial glucose levels better than regular insulin and cause less hypoglycemia. Their pharmacokinetics enable them to be taken within a few minutes of the start of a meal, or even after the meal if the patient forgets to take an injection before the meal.
For example, in one study,36 taking aspart immediately before the meal provided better glycemic control than taking regular insulin 30 minutes before meals. In a basal-bolus regimen, the use of aspart along with detemir resulted in glycemic control similar to that provided by twice-daily NPH and regular insulin, with less hypoglycemia.37
The dosage of prandial insulin can be adjusted according to the amount of carbohydrates in each meal (the insulin-to-carbohydrate ratio), as in patients with type 1 diabetes. This approach was associated with less weight gain.38
IS THERE STILL A ROLE FOR PREMIXED INSULIN PREPARATIONS?
Basal-bolus insulin regimens have gained popularity because the prandial doses can easily be adjusted according to carbohydrate intake, glucose level (on a sliding scale), variations in meal time, missed meals (eg, when having a procedure), and exercise. For example, the dose of prandial insulin can be reduced if the patient expects to exercise within 2 or 3 hours after the meal.
Some patients may not accept giving themselves four or five injections per day with a basal-bolus regimen. They may accept a simpler regimen, ie, giving themselves three injections of a premixed insulin per day, one before each meal.
Compared with a basal-bolus regimen, the possibility of achieving adequate glycemic control using lispro mix (50% lispro, 50% lispro protamine suspension) before meals seemed to depend on the goal of glycemic control. Its use in one study showed similar ability to achieve hemoglobin A1c less than 7.5% compared with a basal-bolus regimen of glargine and lispro. For a goal hemoglobin A1c level of less than 7%, the use of glargine and lispro was superior. The rate of hypoglycemia was similar with both strategies.39 These findings imply that the goal hemoglobin A1c should be more relaxed (< 7.5%) when using lispro mix (50% lispro) three times daily before meals.
Biphasic insulin aspart (a mix of aspart and protamine aspart) given three times daily provided similar improvement in glycemic control with no difference in the frequency of hypoglycemia compared with a basal-bolus regimen of NPH and aspart.40 Further, the use of biphasic insulin aspart seemed to provide better glycemic control with less weight gain compared with premixed human insulin (70% NPH, 30% regular insulin).41
Therefore, simpler premixed insulin regimens remain reasonable options for selected patients who do not accept a more complex insulin regimen (basal-bolus) or cannot adhere to it for any reason, especially if premixed insulin is given before meals three times daily. In fact, recent studies have focused on comparing premixed insulin three times daily with basal-bolus regimens (detemir or glargine as basal insulin along with pre-meal insulin analogue).
Glycemic control is harder to achieve with premixed insulin twice daily, mainly because of a higher frequency of hypoglycemia.42 In Europe, the use of premixed insulin three times daily is a popular option, whereas in the United States, a twice-daily schedule has been more common.
COST VS CONTROL
Newer insulin analogues make insulin treatment safer and more accepted by patients. The availability of several options for insulin regimens allows individualization of the treatment according to the patient’s acceptance, the safety profile, and the cost.
Patient selection and insulin titration are key issues in ensuring the achievement of adequate control with the fewest side effects. Lifestyle management (diet and physical activity) enhances the efficacy of insulin therapy and reduces the chances of side effects, namely fluctuation of glucose levels, hypoglycemic episodes, and weight gain.
Human insulins (NPH and regular) remain the least expensive, especially when using premixed NPH-regular insulin 70/30. Their use should be considered when the cost of medication is a major concern for the patient. A more relaxed goal of glycemic control may be considered in order to avoid hypoglycemia when using those insulin preparations, such as a hemoglobin A1c level less than 7.5% or even in the range of 7.5% to 8.5%, depending on the expected seasonal variation of hemoglobin A1c (which is higher in winter43), individual factors, and whether the premixed insulin is used twice or three times daily.
RE-EVALUATE THE REGIMEN ROUTINELY
The insulin regimen should be re-evaluated routinely. It might need to be changed in response to the dynamic multifactorial process of progression of diabetes, change in stress level, presence or resolution of intercurrent illnesses, risk of hypoglycemia, concerns about weight gain, and cost.
Finally, adjustment of the regimen should be considered in response to improvement of glycemic control related to improvement of dietary management, exercising, weight loss, and medical therapies.
- UK Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes 1995; 44:1249–1258.
- Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009; 15:540–558.
- ClinicalTrials.gov. The ORIGIN Trial (Outcome Reduction With Initial Glargine Intervention). http://clinicaltrials.gov/ct2/show/NCT00069784. Accessed 2/11/11.
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010; 33(suppl 1):S11–S61.
- Retnakaran R, Qi Y, Opsteen C, Vivero E, Zinman B. Initial short-term intensive insulin therapy as a strategy for evaluating the preservation of beta-cell function with oral antidiabetic medications: a pilot study with sitagliptin. Diabetes Obes Metab 2010; 12:909–915.
- Zoungas S, Patel A, Chalmers J, et al; ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363:1410–1418.
- Akram K, Pedersen-Bjergaard U, Borch-Johnsen K, Thorsteinsson B. Frequency and risk factors of severe hypoglycemia in insulin-treated type 2 diabetes: a literature survey. J Diabetes Complications 2006; 20:402–408.
- Cryer PE. Chapter 19. Hypoglycemia. In: Jameson JL, editor. Harrison’s Endocrinology. McGraw Hill, 2006:355–363.
- Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006; 28:1569–1581. Erratum in: Clin Ther 2006; 28:1967.
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006; 29:1269–1274. Erratum in: Diabetes Care 2007; 30:1035.
- Haak T, Tiengo A, Draeger E, Suntum M, Waldhäusl W. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab 2005; 7:56–64.
- Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetalogia 2006; 49:442–451.
- Fritsche A, Schweitzer MA, Häring HU; 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med 2003; 138:952–959.
- Rosenstock J, Schwartz SL, Clark CM, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001; 24:631–636.
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008; 51:408–416.
- King AB. Once-daily insulin detemir is comparable to once-daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: a double-blind, randomized, crossover study. Diabetes Obes Metab 2009; 11:69–71.
- Swinnen SG, Snoek FJ, Dain MP, DeVries JH, Hoekstra JB, Holleman F. Rationale, design, and baseline data of the insulin glargine (Lantus) versus insulin detemir (Levemir) Treat-To-Target (L2T3) study: a multinational, randomized noninferiority trial of basal insulin initiation in type 2 diabetes. Diabetes Technol Ther 2009; 11:739–743.
- Kazda C, Hülstrunk H, Helsberg K, Langer F, Forst T, Hanefeld M. Prandial insulin substitution with insulin lispro or insulin lispro mid mixture vs. basal therapy with insulin glargine: a randomized controlled trial in patients with type 2 diabetes beginning insulin therapy. J Diabetes Complications 2006; 20:145–152.
- Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with type 2 diabetes. Diabet Med 2005; 22:374–381.
- Buse JB, Wolffenbuttel BH, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care 2009; 32:1007–1013.
- Kann PH, Wascher T, Zackova V, et al. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes 2006; 114:527–532.
- Monnier L, Colette C, Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract 2006; 12(suppl 1):42–46.
- Woerle HJ, Pimenta WP, Meyer C, et al. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin A1c values. Arch Intern Med 2004; 164:1627–1632.
- Schrot RJ. Targeting plasma glucose: preprandial versus postprandial. Clinical Diabetes 2004; 22:169–172.
- Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008; 371:1073–1084.
- Tamaki M, Shimizu T, Kanazawa A, Fujitani Y, Watada H, Kawamori R, Hirose T. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract 2008; 81:e1–e3.
- Yokoyama H, Tada J, Kamikawa F, Kanno S, Yokota Y, Kuramitsu M. Efficacy of conversion from bedtime NPH insulin to morning insulin glargine in type 2 diabetic patients on basal-prandial insulin therapy. Diabetes Res Clin Pract 2006; 73:35–40.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The Treat-To-Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Davies M, Sinnassamy P, Storms F, Gomis R; ATLANTUS Study Group. Insulin glargine-based therapy improves glycemic control in patients with type 2 diabetes sub-optimally controlled on premixed insulin therapies. Diabetes Res Clin Pract 2008; 79:368–375.
- Jacober SJ, Scism-Bacon JL, Zagar AJ. A comparison of intensive mixture therapy with basal insulin therapy in insulin-naïve patients with type 2 diabetes receiving oral antidiabetes agents. Diabetes Obes Metab 2006; 8:448–455.
- Hirsch IB, Yuan H, Campaigne BN, Tan MH. Impact of prandial plus basal vs basal insulin on glycemic variability in type 2 diabetic patients. Endocr Pract 2009; 15:343–348.
- Robbins DC, Beisswenger PJ, Ceriello A, et al. Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison. Clin Ther 2007; 29:2349–2364.
- Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther 2008; 30:1976–1987.
- Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev 2009; 25:542–548.
- Perriello G, Pampanelli S, Porcellati F, et al. Insulin aspart improves meal time glycaemic control in patients with type 2 diabetes: a randomized, stratified, double-blind and cross-over trial. Diabet Med 2005; 22:606–611.
- Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:564–569.
- Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008; 31:1305–1310.
- Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 2008; 31:20–25.
- Ligthelm RJ, Mouritzen U, Lynggaard H, et al. Biphasic insulin aspart given thrice daily is as efficacious as a basal-bolus insulin regimen with four daily injections: a randomised open-label parallel group four months comparison in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2006; 114:511–519.
- Velojic-Golubovic M, Mikic D, Pesic M, Dimic D, Radenkovic S, Antic S. Biphasic insulin aspart 30: better glycemic control than with premixed human insulin 30 in obese patients with type 2 diabetes. J Endocrinol Invest 2009; 32:23–27.
- Holman RR, Farmer AJ, Davies MJ, et al; 4-T Study Group. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl Med 2009; 361:1736–1747.
- Tseng CL, Brimacombe M, Xie M, et al. Seasonal patterns in monthly hemoglobin A1c values. Am J Epidemiol 2005; 161:565–574.
- UK Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group. Diabetes 1995; 44:1249–1258.
- Nathan DM, Buse JB, Davidson MB, et al; American Diabetes Association. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32:193–203.
- Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract 2009; 15:540–558.
- ClinicalTrials.gov. The ORIGIN Trial (Outcome Reduction With Initial Glargine Intervention). http://clinicaltrials.gov/ct2/show/NCT00069784. Accessed 2/11/11.
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010; 33(suppl 1):S11–S61.
- Retnakaran R, Qi Y, Opsteen C, Vivero E, Zinman B. Initial short-term intensive insulin therapy as a strategy for evaluating the preservation of beta-cell function with oral antidiabetic medications: a pilot study with sitagliptin. Diabetes Obes Metab 2010; 12:909–915.
- Zoungas S, Patel A, Chalmers J, et al; ADVANCE Collaborative Group. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363:1410–1418.
- Akram K, Pedersen-Bjergaard U, Borch-Johnsen K, Thorsteinsson B. Frequency and risk factors of severe hypoglycemia in insulin-treated type 2 diabetes: a literature survey. J Diabetes Complications 2006; 20:402–408.
- Cryer PE. Chapter 19. Hypoglycemia. In: Jameson JL, editor. Harrison’s Endocrinology. McGraw Hill, 2006:355–363.
- Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006; 28:1569–1581. Erratum in: Clin Ther 2006; 28:1967.
- Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006; 29:1269–1274. Erratum in: Diabetes Care 2007; 30:1035.
- Haak T, Tiengo A, Draeger E, Suntum M, Waldhäusl W. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab 2005; 7:56–64.
- Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetalogia 2006; 49:442–451.
- Fritsche A, Schweitzer MA, Häring HU; 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med 2003; 138:952–959.
- Rosenstock J, Schwartz SL, Clark CM, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001; 24:631–636.
- Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008; 51:408–416.
- King AB. Once-daily insulin detemir is comparable to once-daily insulin glargine in providing glycaemic control over 24 h in patients with type 2 diabetes: a double-blind, randomized, crossover study. Diabetes Obes Metab 2009; 11:69–71.
- Swinnen SG, Snoek FJ, Dain MP, DeVries JH, Hoekstra JB, Holleman F. Rationale, design, and baseline data of the insulin glargine (Lantus) versus insulin detemir (Levemir) Treat-To-Target (L2T3) study: a multinational, randomized noninferiority trial of basal insulin initiation in type 2 diabetes. Diabetes Technol Ther 2009; 11:739–743.
- Kazda C, Hülstrunk H, Helsberg K, Langer F, Forst T, Hanefeld M. Prandial insulin substitution with insulin lispro or insulin lispro mid mixture vs. basal therapy with insulin glargine: a randomized controlled trial in patients with type 2 diabetes beginning insulin therapy. J Diabetes Complications 2006; 20:145–152.
- Malone JK, Bai S, Campaigne BN, Reviriego J, Augendre-Ferrante B. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycaemic control in patients with type 2 diabetes. Diabet Med 2005; 22:374–381.
- Buse JB, Wolffenbuttel BH, Herman WH, et al. DURAbility of basal versus lispro mix 75/25 insulin efficacy (DURABLE) trial 24-week results: safety and efficacy of insulin lispro mix 75/25 versus insulin glargine added to oral antihyperglycemic drugs in patients with type 2 diabetes. Diabetes Care 2009; 32:1007–1013.
- Kann PH, Wascher T, Zackova V, et al. Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes 2006; 114:527–532.
- Monnier L, Colette C, Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract 2006; 12(suppl 1):42–46.
- Woerle HJ, Pimenta WP, Meyer C, et al. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and hemoglobin A1c values. Arch Intern Med 2004; 164:1627–1632.
- Schrot RJ. Targeting plasma glucose: preprandial versus postprandial. Clinical Diabetes 2004; 22:169–172.
- Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008; 371:1073–1084.
- Tamaki M, Shimizu T, Kanazawa A, Fujitani Y, Watada H, Kawamori R, Hirose T. Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract 2008; 81:e1–e3.
- Yokoyama H, Tada J, Kamikawa F, Kanno S, Yokota Y, Kuramitsu M. Efficacy of conversion from bedtime NPH insulin to morning insulin glargine in type 2 diabetic patients on basal-prandial insulin therapy. Diabetes Res Clin Pract 2006; 73:35–40.
- Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The Treat-To-Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003; 26:3080–3086.
- Davies M, Sinnassamy P, Storms F, Gomis R; ATLANTUS Study Group. Insulin glargine-based therapy improves glycemic control in patients with type 2 diabetes sub-optimally controlled on premixed insulin therapies. Diabetes Res Clin Pract 2008; 79:368–375.
- Jacober SJ, Scism-Bacon JL, Zagar AJ. A comparison of intensive mixture therapy with basal insulin therapy in insulin-naïve patients with type 2 diabetes receiving oral antidiabetes agents. Diabetes Obes Metab 2006; 8:448–455.
- Hirsch IB, Yuan H, Campaigne BN, Tan MH. Impact of prandial plus basal vs basal insulin on glycemic variability in type 2 diabetic patients. Endocr Pract 2009; 15:343–348.
- Robbins DC, Beisswenger PJ, Ceriello A, et al. Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison. Clin Ther 2007; 29:2349–2364.
- Hollander P, Cooper J, Bregnhøj J, Pedersen CB. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther 2008; 30:1976–1987.
- Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev 2009; 25:542–548.
- Perriello G, Pampanelli S, Porcellati F, et al. Insulin aspart improves meal time glycaemic control in patients with type 2 diabetes: a randomized, stratified, double-blind and cross-over trial. Diabet Med 2005; 22:606–611.
- Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009; 94:564–569.
- Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008; 31:1305–1310.
- Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S. Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents: prandial premixed (insulin lispro protamine suspension/lispro) versus basal/bolus (glargine/lispro) therapy. Diabetes Care 2008; 31:20–25.
- Ligthelm RJ, Mouritzen U, Lynggaard H, et al. Biphasic insulin aspart given thrice daily is as efficacious as a basal-bolus insulin regimen with four daily injections: a randomised open-label parallel group four months comparison in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2006; 114:511–519.
- Velojic-Golubovic M, Mikic D, Pesic M, Dimic D, Radenkovic S, Antic S. Biphasic insulin aspart 30: better glycemic control than with premixed human insulin 30 in obese patients with type 2 diabetes. J Endocrinol Invest 2009; 32:23–27.
- Holman RR, Farmer AJ, Davies MJ, et al; 4-T Study Group. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl Med 2009; 361:1736–1747.
- Tseng CL, Brimacombe M, Xie M, et al. Seasonal patterns in monthly hemoglobin A1c values. Am J Epidemiol 2005; 161:565–574.
KEY POINTS
- Whether to start insulin therapy and which regimen to use depend on a number of factors, including the patient’s acceptance and willingness to adhere to the therapy.
- A common way to start is to add a once-daily dose of a long-acting insulin at bedtime (basal insulin) to the patient’s antidiabetic regimen.
- Basal regimens do not control postprandial hyperglycemia very well. Another option is to take a long-acting (basal) insulin along with a rapid-acting (prandial or bolus) insulin before meals. Multiple formulations of premixed insulins are available and are convenient to use among new users.
- Basal-bolus regimens, which involve injections of rapid-acting insulin before meals and long-acting insulin at bedtime, are gaining popularity. Their cost and the number of injections may affect patient acceptance of this treatment.
Recognizing the unusual: The diagnostic epiphany
This group had a patient with unexplained abdominal pain who ultimately underwent laparotomy, which did not reveal the diagnosis. I can reconstruct the thought processes that led to the decision for surgery, but far more intriguing is what provoked the “aha” moment when the true diagnosis—ACE inhibitor-associated angioedema—finally occurred to someone.
This is a rare complication of a common therapy, perhaps read about but not reasonable to expect all physicians to recall. If that is true, why can’t we incorporate technology into our care system to intelligently supplement the individual physician’s memory? What would have been the result if a “smart” electronic record had flagged the combination of ACE inhibitor therapy and recurrent abdominal pain and provided a citation on visceral angioedema?
We have all experienced a diagnostic epiphany, the sudden recognition of an arcane or unexpected diagnosis—as on the TV show House, but without the sneer or commercials. Some epiphanies result from suddenly seeing theretofore disconnected dots as a recognizable pattern. Some result from sudden recall of “I saw something like this once.” The superb diagnosticians seem to have these experiences more than the rest of us. Their powers of clinical reasoning are not always transparent. Some are based on the gestalt born of perception and experience, others are the result of incredibly compulsive structured analysis. Both require experience, contextual knowledge, and accurate historical information. These components will need to be incorporated into any diagnostic assistive software. But is this possible?
Those who have read my previous commentaries know that I value highly the clinical skills of history-taking and examination. I believe that these fundamental processes should be used to direct laboratory and imaging studies. I also optimistically expect that electronic medical records will evolve to become far more useful than most currently are, ultimately acting as true auxiliary brains, able to remind us of facts that we can’t recall (eg, that visceral angioedema is associated with ACE inhibitors). But there will never be a substitute for the artful and compulsive interview that establishes whether our patient is actually taking his or her medication, and whether there is a relationship between when a medication is ingested and when symptoms appear. The quality of the data entered into our electronic medical record (or other auxiliary brains), to then be associated with various informational databases, will always depend on the skill of the listening and examining clinician.
This group had a patient with unexplained abdominal pain who ultimately underwent laparotomy, which did not reveal the diagnosis. I can reconstruct the thought processes that led to the decision for surgery, but far more intriguing is what provoked the “aha” moment when the true diagnosis—ACE inhibitor-associated angioedema—finally occurred to someone.
This is a rare complication of a common therapy, perhaps read about but not reasonable to expect all physicians to recall. If that is true, why can’t we incorporate technology into our care system to intelligently supplement the individual physician’s memory? What would have been the result if a “smart” electronic record had flagged the combination of ACE inhibitor therapy and recurrent abdominal pain and provided a citation on visceral angioedema?
We have all experienced a diagnostic epiphany, the sudden recognition of an arcane or unexpected diagnosis—as on the TV show House, but without the sneer or commercials. Some epiphanies result from suddenly seeing theretofore disconnected dots as a recognizable pattern. Some result from sudden recall of “I saw something like this once.” The superb diagnosticians seem to have these experiences more than the rest of us. Their powers of clinical reasoning are not always transparent. Some are based on the gestalt born of perception and experience, others are the result of incredibly compulsive structured analysis. Both require experience, contextual knowledge, and accurate historical information. These components will need to be incorporated into any diagnostic assistive software. But is this possible?
Those who have read my previous commentaries know that I value highly the clinical skills of history-taking and examination. I believe that these fundamental processes should be used to direct laboratory and imaging studies. I also optimistically expect that electronic medical records will evolve to become far more useful than most currently are, ultimately acting as true auxiliary brains, able to remind us of facts that we can’t recall (eg, that visceral angioedema is associated with ACE inhibitors). But there will never be a substitute for the artful and compulsive interview that establishes whether our patient is actually taking his or her medication, and whether there is a relationship between when a medication is ingested and when symptoms appear. The quality of the data entered into our electronic medical record (or other auxiliary brains), to then be associated with various informational databases, will always depend on the skill of the listening and examining clinician.
This group had a patient with unexplained abdominal pain who ultimately underwent laparotomy, which did not reveal the diagnosis. I can reconstruct the thought processes that led to the decision for surgery, but far more intriguing is what provoked the “aha” moment when the true diagnosis—ACE inhibitor-associated angioedema—finally occurred to someone.
This is a rare complication of a common therapy, perhaps read about but not reasonable to expect all physicians to recall. If that is true, why can’t we incorporate technology into our care system to intelligently supplement the individual physician’s memory? What would have been the result if a “smart” electronic record had flagged the combination of ACE inhibitor therapy and recurrent abdominal pain and provided a citation on visceral angioedema?
We have all experienced a diagnostic epiphany, the sudden recognition of an arcane or unexpected diagnosis—as on the TV show House, but without the sneer or commercials. Some epiphanies result from suddenly seeing theretofore disconnected dots as a recognizable pattern. Some result from sudden recall of “I saw something like this once.” The superb diagnosticians seem to have these experiences more than the rest of us. Their powers of clinical reasoning are not always transparent. Some are based on the gestalt born of perception and experience, others are the result of incredibly compulsive structured analysis. Both require experience, contextual knowledge, and accurate historical information. These components will need to be incorporated into any diagnostic assistive software. But is this possible?
Those who have read my previous commentaries know that I value highly the clinical skills of history-taking and examination. I believe that these fundamental processes should be used to direct laboratory and imaging studies. I also optimistically expect that electronic medical records will evolve to become far more useful than most currently are, ultimately acting as true auxiliary brains, able to remind us of facts that we can’t recall (eg, that visceral angioedema is associated with ACE inhibitors). But there will never be a substitute for the artful and compulsive interview that establishes whether our patient is actually taking his or her medication, and whether there is a relationship between when a medication is ingested and when symptoms appear. The quality of the data entered into our electronic medical record (or other auxiliary brains), to then be associated with various informational databases, will always depend on the skill of the listening and examining clinician.
Visceral angioedema due to angiotensin-converting enzyme inhibitor therapy
A 57-year-old black woman presented to the emergency department with severe, dull abdominal pain associated with nonbilious vomiting and nausea. She had diabetes mellitus and hypertension, for which she had been taking metformin (Glucophage) 500 mg twice a day and lisinopril (available as Prinivil and Zestril) 20 mg daily for the last 4 years.
Multiple admissions in the past 4 years
The patient started taking lisinopril 10 mg daily in 2005, and she presented to her medical provider 2 weeks later with abdominal discomfort. Colonoscopy was performed, which revealed a benign polyp. She continued taking her medications, including lisinopril.
She continued to occasionally have abdominal pain of variable severity, but it was tolerable until 6 months later, when she presented to the emergency department with severe recurrent abdominal pain.
In view of the clinical picture, her physicians decided to treat her for small bowel obstruction, and an exploratory laparotomy was performed. The surgeons noted that she had moderate ascites, adhesions on the omentum, and a thickened high loop of the small bowel that was unequivocally viable and hyperemic, with thickening of the mesentery. Ascitic fluid was evacuated, adhesions were lysed, and the abdomen was closed. She was discharged with the same medications, including lisinopril; the dose was subsequently increased for better control of her hypertension.
The woman was admitted three more times within the same year for the same symptoms and underwent multiple workups for pancreatitis, gastritis, small-bowel obstruction, and other common gastrointestinal diseases.
Present admission
On review of systems, she denied any dry cough, weight loss or gain, food allergies, new medications, or hematochezia.
On physical examination, she had hypoactive bowel sounds and diffuse tenderness with guarding around the epigastric area.
Laboratory tests did not reveal any abnormalities; in particular, her C1 esterase concentration was normal. Stool studies were negative for infectious diseases.
Plain radiography of the abdomen showed a nonobstructive bowel-gas pattern.
She was diagnosed with gastrointestinal angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Her lisinopril was discontinued, and the symptoms resolved completely in 24 hours. On follow-up 8 weeks and 16 months later, her symptoms had not returned.
A RARE COMPLICATION OF ACE-INHIBITOR THERAPY
Angioedema occurs in 0.1% to 0.7% of patients taking ACE inhibitors, and it can affect about 1 of 2,500 patients during the first week of exposure.1–3 It usually manifests as swelling of the face, tongue, and lips, and in rare cases, the gastrointestinal wall. Thus, visceral angioedema is a rare complication of ACE-inhibitor therapy.
Because angioedema is less obvious when it involves abdominal organs, it presents a diagnostic challenge. It is placed lower in the differential diagnosis, as other, more common, and occasionally more high-risk medical conditions are generally considered first. Most of the time, the diagnosis is missed. Some physicians may not be aware of this problem, since only a few case reports have been published. Nevertheless, this potential complication needs to be considered when any patient receiving ACE inhibitors for treatment of hypertension, myocardial infarction, heart failure, or diabetic nephropathy presents with diffuse abdominal pain, diarrhea, or edema of the upper airways.4–8
If a high level of suspicion is applied along with good clinical judgment, then hospitalizations, unnecessary procedures, patient discomfort, and unnecessary health care costs can be prevented.
A MEDLINE SEARCH
To investigate the characteristics associated with this unusual presentation, including the time of symptom onset, the types of symptoms, and the diagnostic studies performed on the patients with visceral angioedema, we performed a MEDLINE search to identify case reports and case series published in English from 1980 to 2010 on the topic of abdominal or visceral angioedema. The search terms used were “visceral,” “intestinal angioedema,” “ACE-inhibitor side effects,” and the names of various ACE inhibitors.
Pertinent articles were identified, and clinical characteristics were collected, including demographics, onset of symptoms, the drug’s name, and others. In our summary below, data are presented as the mean and standard deviation for continuous variables and percentages for categorical variables.
SUMMARY OF REPORTED CASES
Our search revealed 27 reported cases of visceral angioedema associated with ACE inhibitors (a table summarizing our findings is available).9–34 The drug most often involved was lisinopril (11 cases), followed by enalapril (Vasotec) (8 cases).
Twenty-three (82%) of the cases were in women. The mean age of the patients was 49.5 ± 12.2 years (range 29–77 years); the mean age was 46.7 ± 11.7 years in women and 57 ± 13 years in men. Unfortunately, the race and ethnicity of the patients was documented in only some cases.
In 15 (54%) of the cases, the patient presented to a physician or emergency department within 72 hours (41.1 ± 17.4) of starting therapy, and in 8 cases the patient presented between 2 weeks and 18 months.
In 10 cases (including the case we are reporting here), the patients were kept on ACE inhibitors from 2 to 9 years after the initial presentation, as the diagnosis was missed.9,12,14,18,20,31,32 In 2 cases, the dose of the ACE inhibitor had been increased after the patient presented with the abdominal pain.
All of the patients were hospitalized for further diagnostic workup.
As for the presenting symptoms, all the patients had abdominal pain, 24 (86%) had emesis, 14 (50%) had diarrhea, and 20 (71%) had ascites. Laboratory results were mostly nonspecific. Twelve (44%) of the patients had leukocytosis. The C1 esterase inhibitor concentration was measured in 18 patients, and the results were normal in all of them.
Twenty-four (86%) of the patients underwent abdominal and pelvic CT or ultrasonography as part of the initial diagnostic evaluation, and intestinal wall-thickening was found in 21 (87.5%) of them.
Either surgery or gastrointestinal biopsy was performed in 16 (57%) of the patients; the surgical procedures included 2 cholecystectomies and 1 bone marrow biopsy. Only 1 case was diagnosed on the basis of clinical suspicion and abdominal radiographs alone.
The combination of intestinal and stomach angioedema was found in only 2 cases.
Two patients were kept on an ACE inhibitor in spite of symptoms and intestinal wall edema that showed a migratory pattern on imaging after chronic exposure.
The thickening involved the jejunum in 14 patients (50%), the ileum in 8 (29%), the duodenum in 5 (18%), the stomach in 2, and the sigmoid colon in 1.
In 12 cases (43%), visceral angioedema and its symptoms resolved within 48 hours of stopping the ACE inhibitor.
A DIAGNOSIS TO KEEP IN MIND
As we have seen, the diagnosis of visceral angioedema needs to be kept in mind when a patient—especially a middle-aged woman—taking an ACE inhibitor presents with abdominal pain, vomiting, diarrhea, leukocytosis, ascites, and wall-thickening of the small bowel on imaging studies.9,35,36
The diagnosis is hard to establish, and in the interim the patient may undergo invasive and unnecessary procedures, which can be avoided by a heightened awareness of this complication. In all of the reported cases, the patients required hospitalization because of the severity of symptoms and attempts to exclude other possible diseases.36
POSSIBLY DUE TO BRADYKININ
Several theories have been proposed to explain how visceral angioedema is induced by ACE inhibitors. The possible mechanisms that have been described include the following:
- The accumulation of bradykinin and substance P secondary to the effect of the ACE inhibitor, which may lead to the inflammatory response, therefore increasing permeability of the vascular compartment
- Deficiency of complement and the enzymes carboxypeptidase N and alpha-1 antitrypsin
- An antibody-antigen reaction37
- Hormones such as estrogen and progesterone (suggested by the greater number of women represented38)
- Contrast media used for imaging39
- Genetic predisposition
- Inflammation due to acute-phase proteins
- C1-inhibitor deficiency or dysfunction (however, the levels of C1/C4 and the C1-esterase inhibitor functional activity usually are normal2,10,40).
Many other theories are being explored.11,12,38,41–53
The most plausible mechanism is an increase in the levels of bradykinin and its metabolites.45 The absence of ACE can lead to breakdown of bradykinin to des-Arg bradykinin via the minor pathway, which can lead to more pronounced vasodilation and vascular permeability.54,55 During an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level.56
Moreover, C-reactive protein levels were higher (mean 4.42 mg/dL ± 0.15 mg/dL) in patients with ACE-inhibitor-induced angioedema than in those with other causes of angioedema (P < .0001).52 The patients taking ACE inhibitors without any previous angioedema had normal C-reactive protein levels (0.39 mg/dL ± 0.1 mg/dL).52
INCIDENCE RATES
In our review of the literature, all of the patients were taking an ACE inhibitor, and some were taking both an ACE inhibitor and an angiotensin-receptor blocker (ARB).
Initially, the incidence rate of angioedema was thought to be 0.1% to 0.2%, but recently the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) trial had more than 12,000 patients on enalapril and reported the incidence of angioedema to be 0.68%,57 with a higher risk in women than in men (0.84% vs 0.54%)58 and a relative risk of 3.03 for blacks compared with whites.59
Even though ARBs seem to be safer, angioedema can recur in up to one-third of patients who switch from an ACE inhibitor to an ARB.60–63
Moreover, one study in the United States found that the frequency of hospital admission of patients with angioedema increased from 8,839 per year in 1998 to 11,925 in 2005, and the cost was estimated to be close to $123 million in 2005.64
Interestingly, when angioedema involved the face, it developed within the first week in 60% of cases,65 whereas when visceral angioedema developed, it did so within the first week in 59% of cases. Therefore, the timing of the onset is similar regardless of the body area involved.
Smokers who developed ACE-inhibitor-induced cough had a higher risk of ACE-inhibitor-induced angioedema in a retrospective cohort study by Morimoto,66 but no relationship to the area of involvement was made.
ON IMAGING, A THICKENED BOWEL WALL
Computed tomography can reveal bowel edema and ascites more reliably than plain radiography or barium studies. Edema thickens the bowel wall, with increased contrast enhancement that makes mesenteric vessels show up on the study. In some instances edema is so significant that edematous submucosa can be differentiated from the serosa due to impressive thickening of the mucosal wall.15,16 Oral contrast can be seen in the middle of the lumen, giving it a target-sign appearance. Edema of the small bowel and ascites can lead to fluid sequestration in the abdomen, resulting in a presentation with shock.67
Magnetic resonance imaging can be even more useful in identifying gastrointestinal angioedema, but it would not be cost-effective, and based on our study, CT and ultrasonography of the abdomen were diagnostic in most cases.
AVOIDING UNNECESSARY TESTING
Hemodynamic instability and abdominal pain usually trigger a surgical consult and a more extensive workup, but with a good clinical approach, unnecessary testing and invasive diagnostic procedures can be avoided under the right circumstances.
Numerous surgical procedures have been reported in patients presenting with visceral angioedema secondary to ACE inhibitors.67 Although a thorough history and physical examination can give us a clue in the diagnosis of drug-induced gastrointestinal angioedema, CT is extremely helpful, as it shows dilated loops, thickened mucosal folds, perihepatic fluid, ascites, mesenteric edema, and a “doughnut” or “stacked coin” appearance.17,68
So far, there have been only two reports of angioedema of the stomach (the case reported by Shahzad et al10 and the current report). Angioedema can affect any visceral organ, but we usually see involvement of the jejunum followed by the ileum and duodenum.40
FINDINGS ON ENDOSCOPY
Usually, endoscopic examination of the upper and lower gastrointestinal tract does not reveal any specific pathology, but endoscopy and biopsy can rule out other causes of abdominal pain, such as Crohn disease, ulcerative colitis, infection, malignancy, granuloma, and vasculitis. Also, hereditary or acquired C1-esterase deficiency and other autoimmune disorders should be considered in the workup.18,69 In the reported cases, endoscopy revealed petechial bleeding with generalized edema.19
Biopsy often demonstrates an expanded edematous submucosal layer with inflammatory cell infiltration and protrusion of the proper muscular layer into the submucosal layer.15 A proper muscular layer and an edematous submucosal layer can produce edema so severe as to obstruct the intestine.15
Ultrasonography or CT provides essential information as to location, structure, and size, and it rules out other diagnoses. Therefore, consideration should be given to noninvasive imaging studies and laboratory testing (C1-esterase inhibitor, complement, antinuclear antibody, complete metabolic panel, complete blood cell count) before resorting to endoscopy or exploratory laparotomy.20,70 In three case reports,29,30,32 abdominal ultrasonography did not show any thickening of the small-bowel wall. Several cases have been diagnosed with the help of endoscopy.
Symptoms usually resolve when the ACE inhibitor is stopped
There is no standard treatment for ACE-inhibitor-induced visceral angioedema. In most patients, stopping the drug, giving nothing by mouth, and giving intravenous fluids to prevent dehydration are sufficient. Symptoms usually resolve within 48 hours.
In several case reports, fresh-frozen plasma was used to increase the levels of kininase II, which can degrade high levels of bradykinin.51,71,72 However, no randomized controlled trial of fresh-frozen plasma for ACE-inhibitor-induced angioedema has been published.
Drugs for hereditary angioedema—eg, recombinant C1-INH, the kallikrein inhibitor ecallantide (Kalbitor), and the BKR-2-antagonist icatibant (Firazyr)73—have not been prospectively studied in gastrointestinal angioedema associated with ACE inhibitors. Icatibant has been shown to be effective in the treatment of hereditary angioedema and could be promising in treating angioedema secondary to ACE inhibitors.8 Rosenberg et al21 described a patient who was on prednisone when she developed intestinal angioedema, thus calling into question the efficacy of steroids in the treatment of visceral angioedema.
RAISING AWARENESS
More than 40 million patients are currently taking ACE inhibitors or ARBs.9 Therefore, we suggest that patients with a known history of angioedema in response to these drugs should wear an identification bracelet to increase awareness and to prevent recurrence of angioedema.
- Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA 1997; 278:232–233.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117:234–242.
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angiooedema. Lancet 2000; 356:608–609.
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–2018.
- Jessup M. The less familiar face of heart failure. J Am Coll Cardiol 2003; 41:224–226.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357:789–796.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 2008; 51:1465–1467.
- Oudit G, Girgrah N, Allard J. ACE inhibitor-induced angioedema of the intestine: Case report, incidence, pathophysiology, diagnosis and management. Can J Gastroenterol 2001; 15:827–832.
- Shahzad G, Korsten MA, Blatt C, Motwani P. Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. Mt Sinai J Med 2006; 73:1123–1125.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol 2000; 31:254–257.
- Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust 1996; 165:319–321.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Smoger SH, Sayed MA. Simultaneous mucosal and small bowel angioedema due to captopril. South Med J 1998; 91:1060–1063.
- Tojo A, Onozato ML, Fujita T. Repeated subileus due to angioedema during renin-angiotensin system blockade. Am J Med Sci 2006; 332:36–38.
- De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol 2001; 176:649–652.
- Marmery H, Mirvis SE. Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clin Radiol 2006; 61:979–982.
- Orr KK, Myers JR. Intermittent visceral edema induced by long-term enalapril administration. Ann Pharmacother 2004; 38:825–827.
- Spahn TW, Grosse-Thie W, Mueller MK. Endoscopic visualization of angiotensin-converting enzyme inhibitor-induced small bowel angioedema as a cause of relapsing abdominal pain using double-balloon enteroscopy. Dig Dis Sci 2008; 53:1257–1260.
- Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc 2000; 75:1201–1204.
- Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75:730–732.
- Salloum H, Locher C, Chenard A, et al. [Small bowel angioedema due to perindopril]. Gastroenterol Clin Biol 2005; 29:1180–1181.
- Arakawa M, Murata Y, Rikimaru Y, Sasaki Y. Drug-induced isolated visceral angioneurotic edema. Intern Med 2005; 44:975–978.
- Abdelmalek MF, Douglas DD. Lisinopril-induced isolated visceral angioedema: review of ACE-inhibitor-induced small bowel angioedema. Dig Dis Sci 1997; 42:847–850.
- Gregory KW, Davis RC. Images in clinical medicine. Angioedema of the intestine. N Engl J Med 1996; 334:1641.
- Farraye FA, Peppercorn MA, Steer ML, Joffe N, Rees M. Acute small-bowel mucosal edema following enalapril use. JAMA 1988; 259:3131.
- Jacobs RL, Hoberman LJ, Goldstein HM. Angioedema of the small bowel caused by an angiotensin-converting enzyme inhibitor. Am J Gastroenterol 1994; 89:127–128.
- Herman L, Jocums SB, Coleman MD. A 29-year-old woman with crampy abdominal pain. Tenn Med 1999; 92:272–273.
- Guy C, Cathébras P, Rousset H. Suspected angioedema of abdominal viscera. Ann Intern Med 1994; 121:900.
- Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen]. Arch Mal Coeur Vaiss 1994; 87:1371–1374.
- Jardine DL, Anderson JC, McClintock AD. Delayed diagnosis of recurrent visceral angio-oedema secondary to ACE inhibitor therapy. Aust N Z J Med 1999; 29:377–378.
- Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med 1993; 32:424–426.
- Khan MU, Baig MA, Javed RA, et al. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol 2007; 118:e68–e69.
- Adhikari SP, Schneider JI. An unusual cause of abdominal pain and hypotension: angioedema of the bowel. J Emerg Med 2009; 36:23–25.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med 2005; 118:1428–1329.
- Bi CK, Soltani K, Sloan JB, Weber RR, Elliott WJ, Murphy MB. Tissue-specific autoantibodies induced by captopril. Clin Res 1987; 35:922A.
- Bork K, Dewald G. Hereditary angioedema type III, angioedema associated with angiotensin II receptor antagonists, and female sex. Am J Med 2004; 116:644–645.
- Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med 1973; 119:832–840.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM. Angioedema presenting as chronic gastrointestinal symptoms. Am J Gastroenterol 1993; 88:436–439.
- Coleman JW, Yeung JH, Roberts DH, Breckenridge AM, Park BK. Drug-specific antibodies in patients receiving captopril. Br J Clin Pharmacol 1986; 22:161–165.
- Kallenberg CG. Autoantibodies during captopril treatment. Arthritis Rheum 1985; 28:597–598.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. BMJ 1988; 297:826–829.
- Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002; 39:460–464.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angiooedema on ACE inhibitors. Lancet 2002; 359:2088–2089.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 2000; 106:546–550.
- Yeung JH, Coleman JW, Park BK. Drug-protein conjugates—IX. Immunogenicity of captopril-protein conjugates. Biochem Pharmacol 1985; 34:4005–4012.
- Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol 1999; 82:473–476.
- Pichler WJ, Lehner R, Späth PJ. Recurrent angioedema associated with hypogonadism or anti-androgen therapy. Ann Allergy 1989; 63:301–305.
- Bass G, Honan D. Octaplas is not equivalent to fresh frozen plasma in the treatment of acute angioedema. Eur J Anaesthesiol 2007; 24:1062–1063.
- Bas M, Hoffmann TK, Bier H, Kojda G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br J Clin Pharmacol 2005; 59:233–238.
- Herman AG. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am J Cardiol 1992; 70:102C–108C.
- Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immunol 2001; 69:6796–6803.
- Cunnion KM, Wagner E, Frank MM. Complement and kinins. In:Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology. 10th ed. New York, NY: Lange Medical Books; 2001:186–188.
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin-converting enzyme inhibition in human subjects. Clin Sci (Lond) 1994; 87:567–574.
- Bristol-Myers Squibb Pharmaceutical Research Institute. FDA Advisory Committee Briefing Book for OMAPATRILAT Tablets NDA 21-188. www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf. Accessed 2/4/2011.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Mahoney EJ, Devaiah AK. Angioedema and angiotensin-converting enzyme inhibitors: are demographics a risk? Otolaryngol Head Neck Surg 2008; 139:105–108.
- Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 2000; 34:526–528.
- Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg 2004; 130:1416–1419.
- MacLean JA, Hannaway PJ. Angioedema and AT1 receptor blockers: proceed with caution. Arch Intern Med 2003; 163:1488–1489,
- Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy 2002; 22:1173–1175.
- Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol 2008; 101:185–192.
- Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA 1988; 260:967–970.
- Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10:499–509.
- Cohen N, Sharon A, Golik A, Zaidenstein R, Modai D. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol 1993; 16:237–239.
- Ciaccia D, Brazer SR, Baker ME. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR Am J Roentgenol 1993; 161:1215–1216.
- Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn’s disease. Med J Aust 1999; 171:418–420.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 2002; 109:370–371.
- Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 2004; 92:573–575.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62:842–856.
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999; 44:21–25.
A 57-year-old black woman presented to the emergency department with severe, dull abdominal pain associated with nonbilious vomiting and nausea. She had diabetes mellitus and hypertension, for which she had been taking metformin (Glucophage) 500 mg twice a day and lisinopril (available as Prinivil and Zestril) 20 mg daily for the last 4 years.
Multiple admissions in the past 4 years
The patient started taking lisinopril 10 mg daily in 2005, and she presented to her medical provider 2 weeks later with abdominal discomfort. Colonoscopy was performed, which revealed a benign polyp. She continued taking her medications, including lisinopril.
She continued to occasionally have abdominal pain of variable severity, but it was tolerable until 6 months later, when she presented to the emergency department with severe recurrent abdominal pain.
In view of the clinical picture, her physicians decided to treat her for small bowel obstruction, and an exploratory laparotomy was performed. The surgeons noted that she had moderate ascites, adhesions on the omentum, and a thickened high loop of the small bowel that was unequivocally viable and hyperemic, with thickening of the mesentery. Ascitic fluid was evacuated, adhesions were lysed, and the abdomen was closed. She was discharged with the same medications, including lisinopril; the dose was subsequently increased for better control of her hypertension.
The woman was admitted three more times within the same year for the same symptoms and underwent multiple workups for pancreatitis, gastritis, small-bowel obstruction, and other common gastrointestinal diseases.
Present admission
On review of systems, she denied any dry cough, weight loss or gain, food allergies, new medications, or hematochezia.
On physical examination, she had hypoactive bowel sounds and diffuse tenderness with guarding around the epigastric area.
Laboratory tests did not reveal any abnormalities; in particular, her C1 esterase concentration was normal. Stool studies were negative for infectious diseases.
Plain radiography of the abdomen showed a nonobstructive bowel-gas pattern.
She was diagnosed with gastrointestinal angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Her lisinopril was discontinued, and the symptoms resolved completely in 24 hours. On follow-up 8 weeks and 16 months later, her symptoms had not returned.
A RARE COMPLICATION OF ACE-INHIBITOR THERAPY
Angioedema occurs in 0.1% to 0.7% of patients taking ACE inhibitors, and it can affect about 1 of 2,500 patients during the first week of exposure.1–3 It usually manifests as swelling of the face, tongue, and lips, and in rare cases, the gastrointestinal wall. Thus, visceral angioedema is a rare complication of ACE-inhibitor therapy.
Because angioedema is less obvious when it involves abdominal organs, it presents a diagnostic challenge. It is placed lower in the differential diagnosis, as other, more common, and occasionally more high-risk medical conditions are generally considered first. Most of the time, the diagnosis is missed. Some physicians may not be aware of this problem, since only a few case reports have been published. Nevertheless, this potential complication needs to be considered when any patient receiving ACE inhibitors for treatment of hypertension, myocardial infarction, heart failure, or diabetic nephropathy presents with diffuse abdominal pain, diarrhea, or edema of the upper airways.4–8
If a high level of suspicion is applied along with good clinical judgment, then hospitalizations, unnecessary procedures, patient discomfort, and unnecessary health care costs can be prevented.
A MEDLINE SEARCH
To investigate the characteristics associated with this unusual presentation, including the time of symptom onset, the types of symptoms, and the diagnostic studies performed on the patients with visceral angioedema, we performed a MEDLINE search to identify case reports and case series published in English from 1980 to 2010 on the topic of abdominal or visceral angioedema. The search terms used were “visceral,” “intestinal angioedema,” “ACE-inhibitor side effects,” and the names of various ACE inhibitors.
Pertinent articles were identified, and clinical characteristics were collected, including demographics, onset of symptoms, the drug’s name, and others. In our summary below, data are presented as the mean and standard deviation for continuous variables and percentages for categorical variables.
SUMMARY OF REPORTED CASES
Our search revealed 27 reported cases of visceral angioedema associated with ACE inhibitors (a table summarizing our findings is available).9–34 The drug most often involved was lisinopril (11 cases), followed by enalapril (Vasotec) (8 cases).
Twenty-three (82%) of the cases were in women. The mean age of the patients was 49.5 ± 12.2 years (range 29–77 years); the mean age was 46.7 ± 11.7 years in women and 57 ± 13 years in men. Unfortunately, the race and ethnicity of the patients was documented in only some cases.
In 15 (54%) of the cases, the patient presented to a physician or emergency department within 72 hours (41.1 ± 17.4) of starting therapy, and in 8 cases the patient presented between 2 weeks and 18 months.
In 10 cases (including the case we are reporting here), the patients were kept on ACE inhibitors from 2 to 9 years after the initial presentation, as the diagnosis was missed.9,12,14,18,20,31,32 In 2 cases, the dose of the ACE inhibitor had been increased after the patient presented with the abdominal pain.
All of the patients were hospitalized for further diagnostic workup.
As for the presenting symptoms, all the patients had abdominal pain, 24 (86%) had emesis, 14 (50%) had diarrhea, and 20 (71%) had ascites. Laboratory results were mostly nonspecific. Twelve (44%) of the patients had leukocytosis. The C1 esterase inhibitor concentration was measured in 18 patients, and the results were normal in all of them.
Twenty-four (86%) of the patients underwent abdominal and pelvic CT or ultrasonography as part of the initial diagnostic evaluation, and intestinal wall-thickening was found in 21 (87.5%) of them.
Either surgery or gastrointestinal biopsy was performed in 16 (57%) of the patients; the surgical procedures included 2 cholecystectomies and 1 bone marrow biopsy. Only 1 case was diagnosed on the basis of clinical suspicion and abdominal radiographs alone.
The combination of intestinal and stomach angioedema was found in only 2 cases.
Two patients were kept on an ACE inhibitor in spite of symptoms and intestinal wall edema that showed a migratory pattern on imaging after chronic exposure.
The thickening involved the jejunum in 14 patients (50%), the ileum in 8 (29%), the duodenum in 5 (18%), the stomach in 2, and the sigmoid colon in 1.
In 12 cases (43%), visceral angioedema and its symptoms resolved within 48 hours of stopping the ACE inhibitor.
A DIAGNOSIS TO KEEP IN MIND
As we have seen, the diagnosis of visceral angioedema needs to be kept in mind when a patient—especially a middle-aged woman—taking an ACE inhibitor presents with abdominal pain, vomiting, diarrhea, leukocytosis, ascites, and wall-thickening of the small bowel on imaging studies.9,35,36
The diagnosis is hard to establish, and in the interim the patient may undergo invasive and unnecessary procedures, which can be avoided by a heightened awareness of this complication. In all of the reported cases, the patients required hospitalization because of the severity of symptoms and attempts to exclude other possible diseases.36
POSSIBLY DUE TO BRADYKININ
Several theories have been proposed to explain how visceral angioedema is induced by ACE inhibitors. The possible mechanisms that have been described include the following:
- The accumulation of bradykinin and substance P secondary to the effect of the ACE inhibitor, which may lead to the inflammatory response, therefore increasing permeability of the vascular compartment
- Deficiency of complement and the enzymes carboxypeptidase N and alpha-1 antitrypsin
- An antibody-antigen reaction37
- Hormones such as estrogen and progesterone (suggested by the greater number of women represented38)
- Contrast media used for imaging39
- Genetic predisposition
- Inflammation due to acute-phase proteins
- C1-inhibitor deficiency or dysfunction (however, the levels of C1/C4 and the C1-esterase inhibitor functional activity usually are normal2,10,40).
Many other theories are being explored.11,12,38,41–53
The most plausible mechanism is an increase in the levels of bradykinin and its metabolites.45 The absence of ACE can lead to breakdown of bradykinin to des-Arg bradykinin via the minor pathway, which can lead to more pronounced vasodilation and vascular permeability.54,55 During an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level.56
Moreover, C-reactive protein levels were higher (mean 4.42 mg/dL ± 0.15 mg/dL) in patients with ACE-inhibitor-induced angioedema than in those with other causes of angioedema (P < .0001).52 The patients taking ACE inhibitors without any previous angioedema had normal C-reactive protein levels (0.39 mg/dL ± 0.1 mg/dL).52
INCIDENCE RATES
In our review of the literature, all of the patients were taking an ACE inhibitor, and some were taking both an ACE inhibitor and an angiotensin-receptor blocker (ARB).
Initially, the incidence rate of angioedema was thought to be 0.1% to 0.2%, but recently the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) trial had more than 12,000 patients on enalapril and reported the incidence of angioedema to be 0.68%,57 with a higher risk in women than in men (0.84% vs 0.54%)58 and a relative risk of 3.03 for blacks compared with whites.59
Even though ARBs seem to be safer, angioedema can recur in up to one-third of patients who switch from an ACE inhibitor to an ARB.60–63
Moreover, one study in the United States found that the frequency of hospital admission of patients with angioedema increased from 8,839 per year in 1998 to 11,925 in 2005, and the cost was estimated to be close to $123 million in 2005.64
Interestingly, when angioedema involved the face, it developed within the first week in 60% of cases,65 whereas when visceral angioedema developed, it did so within the first week in 59% of cases. Therefore, the timing of the onset is similar regardless of the body area involved.
Smokers who developed ACE-inhibitor-induced cough had a higher risk of ACE-inhibitor-induced angioedema in a retrospective cohort study by Morimoto,66 but no relationship to the area of involvement was made.
ON IMAGING, A THICKENED BOWEL WALL
Computed tomography can reveal bowel edema and ascites more reliably than plain radiography or barium studies. Edema thickens the bowel wall, with increased contrast enhancement that makes mesenteric vessels show up on the study. In some instances edema is so significant that edematous submucosa can be differentiated from the serosa due to impressive thickening of the mucosal wall.15,16 Oral contrast can be seen in the middle of the lumen, giving it a target-sign appearance. Edema of the small bowel and ascites can lead to fluid sequestration in the abdomen, resulting in a presentation with shock.67
Magnetic resonance imaging can be even more useful in identifying gastrointestinal angioedema, but it would not be cost-effective, and based on our study, CT and ultrasonography of the abdomen were diagnostic in most cases.
AVOIDING UNNECESSARY TESTING
Hemodynamic instability and abdominal pain usually trigger a surgical consult and a more extensive workup, but with a good clinical approach, unnecessary testing and invasive diagnostic procedures can be avoided under the right circumstances.
Numerous surgical procedures have been reported in patients presenting with visceral angioedema secondary to ACE inhibitors.67 Although a thorough history and physical examination can give us a clue in the diagnosis of drug-induced gastrointestinal angioedema, CT is extremely helpful, as it shows dilated loops, thickened mucosal folds, perihepatic fluid, ascites, mesenteric edema, and a “doughnut” or “stacked coin” appearance.17,68
So far, there have been only two reports of angioedema of the stomach (the case reported by Shahzad et al10 and the current report). Angioedema can affect any visceral organ, but we usually see involvement of the jejunum followed by the ileum and duodenum.40
FINDINGS ON ENDOSCOPY
Usually, endoscopic examination of the upper and lower gastrointestinal tract does not reveal any specific pathology, but endoscopy and biopsy can rule out other causes of abdominal pain, such as Crohn disease, ulcerative colitis, infection, malignancy, granuloma, and vasculitis. Also, hereditary or acquired C1-esterase deficiency and other autoimmune disorders should be considered in the workup.18,69 In the reported cases, endoscopy revealed petechial bleeding with generalized edema.19
Biopsy often demonstrates an expanded edematous submucosal layer with inflammatory cell infiltration and protrusion of the proper muscular layer into the submucosal layer.15 A proper muscular layer and an edematous submucosal layer can produce edema so severe as to obstruct the intestine.15
Ultrasonography or CT provides essential information as to location, structure, and size, and it rules out other diagnoses. Therefore, consideration should be given to noninvasive imaging studies and laboratory testing (C1-esterase inhibitor, complement, antinuclear antibody, complete metabolic panel, complete blood cell count) before resorting to endoscopy or exploratory laparotomy.20,70 In three case reports,29,30,32 abdominal ultrasonography did not show any thickening of the small-bowel wall. Several cases have been diagnosed with the help of endoscopy.
Symptoms usually resolve when the ACE inhibitor is stopped
There is no standard treatment for ACE-inhibitor-induced visceral angioedema. In most patients, stopping the drug, giving nothing by mouth, and giving intravenous fluids to prevent dehydration are sufficient. Symptoms usually resolve within 48 hours.
In several case reports, fresh-frozen plasma was used to increase the levels of kininase II, which can degrade high levels of bradykinin.51,71,72 However, no randomized controlled trial of fresh-frozen plasma for ACE-inhibitor-induced angioedema has been published.
Drugs for hereditary angioedema—eg, recombinant C1-INH, the kallikrein inhibitor ecallantide (Kalbitor), and the BKR-2-antagonist icatibant (Firazyr)73—have not been prospectively studied in gastrointestinal angioedema associated with ACE inhibitors. Icatibant has been shown to be effective in the treatment of hereditary angioedema and could be promising in treating angioedema secondary to ACE inhibitors.8 Rosenberg et al21 described a patient who was on prednisone when she developed intestinal angioedema, thus calling into question the efficacy of steroids in the treatment of visceral angioedema.
RAISING AWARENESS
More than 40 million patients are currently taking ACE inhibitors or ARBs.9 Therefore, we suggest that patients with a known history of angioedema in response to these drugs should wear an identification bracelet to increase awareness and to prevent recurrence of angioedema.
A 57-year-old black woman presented to the emergency department with severe, dull abdominal pain associated with nonbilious vomiting and nausea. She had diabetes mellitus and hypertension, for which she had been taking metformin (Glucophage) 500 mg twice a day and lisinopril (available as Prinivil and Zestril) 20 mg daily for the last 4 years.
Multiple admissions in the past 4 years
The patient started taking lisinopril 10 mg daily in 2005, and she presented to her medical provider 2 weeks later with abdominal discomfort. Colonoscopy was performed, which revealed a benign polyp. She continued taking her medications, including lisinopril.
She continued to occasionally have abdominal pain of variable severity, but it was tolerable until 6 months later, when she presented to the emergency department with severe recurrent abdominal pain.
In view of the clinical picture, her physicians decided to treat her for small bowel obstruction, and an exploratory laparotomy was performed. The surgeons noted that she had moderate ascites, adhesions on the omentum, and a thickened high loop of the small bowel that was unequivocally viable and hyperemic, with thickening of the mesentery. Ascitic fluid was evacuated, adhesions were lysed, and the abdomen was closed. She was discharged with the same medications, including lisinopril; the dose was subsequently increased for better control of her hypertension.
The woman was admitted three more times within the same year for the same symptoms and underwent multiple workups for pancreatitis, gastritis, small-bowel obstruction, and other common gastrointestinal diseases.
Present admission
On review of systems, she denied any dry cough, weight loss or gain, food allergies, new medications, or hematochezia.
On physical examination, she had hypoactive bowel sounds and diffuse tenderness with guarding around the epigastric area.
Laboratory tests did not reveal any abnormalities; in particular, her C1 esterase concentration was normal. Stool studies were negative for infectious diseases.
Plain radiography of the abdomen showed a nonobstructive bowel-gas pattern.
She was diagnosed with gastrointestinal angioedema secondary to angiotensin-converting enzyme (ACE) inhibitor therapy. Her lisinopril was discontinued, and the symptoms resolved completely in 24 hours. On follow-up 8 weeks and 16 months later, her symptoms had not returned.
A RARE COMPLICATION OF ACE-INHIBITOR THERAPY
Angioedema occurs in 0.1% to 0.7% of patients taking ACE inhibitors, and it can affect about 1 of 2,500 patients during the first week of exposure.1–3 It usually manifests as swelling of the face, tongue, and lips, and in rare cases, the gastrointestinal wall. Thus, visceral angioedema is a rare complication of ACE-inhibitor therapy.
Because angioedema is less obvious when it involves abdominal organs, it presents a diagnostic challenge. It is placed lower in the differential diagnosis, as other, more common, and occasionally more high-risk medical conditions are generally considered first. Most of the time, the diagnosis is missed. Some physicians may not be aware of this problem, since only a few case reports have been published. Nevertheless, this potential complication needs to be considered when any patient receiving ACE inhibitors for treatment of hypertension, myocardial infarction, heart failure, or diabetic nephropathy presents with diffuse abdominal pain, diarrhea, or edema of the upper airways.4–8
If a high level of suspicion is applied along with good clinical judgment, then hospitalizations, unnecessary procedures, patient discomfort, and unnecessary health care costs can be prevented.
A MEDLINE SEARCH
To investigate the characteristics associated with this unusual presentation, including the time of symptom onset, the types of symptoms, and the diagnostic studies performed on the patients with visceral angioedema, we performed a MEDLINE search to identify case reports and case series published in English from 1980 to 2010 on the topic of abdominal or visceral angioedema. The search terms used were “visceral,” “intestinal angioedema,” “ACE-inhibitor side effects,” and the names of various ACE inhibitors.
Pertinent articles were identified, and clinical characteristics were collected, including demographics, onset of symptoms, the drug’s name, and others. In our summary below, data are presented as the mean and standard deviation for continuous variables and percentages for categorical variables.
SUMMARY OF REPORTED CASES
Our search revealed 27 reported cases of visceral angioedema associated with ACE inhibitors (a table summarizing our findings is available).9–34 The drug most often involved was lisinopril (11 cases), followed by enalapril (Vasotec) (8 cases).
Twenty-three (82%) of the cases were in women. The mean age of the patients was 49.5 ± 12.2 years (range 29–77 years); the mean age was 46.7 ± 11.7 years in women and 57 ± 13 years in men. Unfortunately, the race and ethnicity of the patients was documented in only some cases.
In 15 (54%) of the cases, the patient presented to a physician or emergency department within 72 hours (41.1 ± 17.4) of starting therapy, and in 8 cases the patient presented between 2 weeks and 18 months.
In 10 cases (including the case we are reporting here), the patients were kept on ACE inhibitors from 2 to 9 years after the initial presentation, as the diagnosis was missed.9,12,14,18,20,31,32 In 2 cases, the dose of the ACE inhibitor had been increased after the patient presented with the abdominal pain.
All of the patients were hospitalized for further diagnostic workup.
As for the presenting symptoms, all the patients had abdominal pain, 24 (86%) had emesis, 14 (50%) had diarrhea, and 20 (71%) had ascites. Laboratory results were mostly nonspecific. Twelve (44%) of the patients had leukocytosis. The C1 esterase inhibitor concentration was measured in 18 patients, and the results were normal in all of them.
Twenty-four (86%) of the patients underwent abdominal and pelvic CT or ultrasonography as part of the initial diagnostic evaluation, and intestinal wall-thickening was found in 21 (87.5%) of them.
Either surgery or gastrointestinal biopsy was performed in 16 (57%) of the patients; the surgical procedures included 2 cholecystectomies and 1 bone marrow biopsy. Only 1 case was diagnosed on the basis of clinical suspicion and abdominal radiographs alone.
The combination of intestinal and stomach angioedema was found in only 2 cases.
Two patients were kept on an ACE inhibitor in spite of symptoms and intestinal wall edema that showed a migratory pattern on imaging after chronic exposure.
The thickening involved the jejunum in 14 patients (50%), the ileum in 8 (29%), the duodenum in 5 (18%), the stomach in 2, and the sigmoid colon in 1.
In 12 cases (43%), visceral angioedema and its symptoms resolved within 48 hours of stopping the ACE inhibitor.
A DIAGNOSIS TO KEEP IN MIND
As we have seen, the diagnosis of visceral angioedema needs to be kept in mind when a patient—especially a middle-aged woman—taking an ACE inhibitor presents with abdominal pain, vomiting, diarrhea, leukocytosis, ascites, and wall-thickening of the small bowel on imaging studies.9,35,36
The diagnosis is hard to establish, and in the interim the patient may undergo invasive and unnecessary procedures, which can be avoided by a heightened awareness of this complication. In all of the reported cases, the patients required hospitalization because of the severity of symptoms and attempts to exclude other possible diseases.36
POSSIBLY DUE TO BRADYKININ
Several theories have been proposed to explain how visceral angioedema is induced by ACE inhibitors. The possible mechanisms that have been described include the following:
- The accumulation of bradykinin and substance P secondary to the effect of the ACE inhibitor, which may lead to the inflammatory response, therefore increasing permeability of the vascular compartment
- Deficiency of complement and the enzymes carboxypeptidase N and alpha-1 antitrypsin
- An antibody-antigen reaction37
- Hormones such as estrogen and progesterone (suggested by the greater number of women represented38)
- Contrast media used for imaging39
- Genetic predisposition
- Inflammation due to acute-phase proteins
- C1-inhibitor deficiency or dysfunction (however, the levels of C1/C4 and the C1-esterase inhibitor functional activity usually are normal2,10,40).
Many other theories are being explored.11,12,38,41–53
The most plausible mechanism is an increase in the levels of bradykinin and its metabolites.45 The absence of ACE can lead to breakdown of bradykinin to des-Arg bradykinin via the minor pathway, which can lead to more pronounced vasodilation and vascular permeability.54,55 During an acute attack of angioedema secondary to ACE inhibition, the bradykinin concentration can increase to more than 10 times the normal level.56
Moreover, C-reactive protein levels were higher (mean 4.42 mg/dL ± 0.15 mg/dL) in patients with ACE-inhibitor-induced angioedema than in those with other causes of angioedema (P < .0001).52 The patients taking ACE inhibitors without any previous angioedema had normal C-reactive protein levels (0.39 mg/dL ± 0.1 mg/dL).52
INCIDENCE RATES
In our review of the literature, all of the patients were taking an ACE inhibitor, and some were taking both an ACE inhibitor and an angiotensin-receptor blocker (ARB).
Initially, the incidence rate of angioedema was thought to be 0.1% to 0.2%, but recently the Omapatrilat Cardiovascular Treatment Assessment vs Enalapril (OCTAVE) trial had more than 12,000 patients on enalapril and reported the incidence of angioedema to be 0.68%,57 with a higher risk in women than in men (0.84% vs 0.54%)58 and a relative risk of 3.03 for blacks compared with whites.59
Even though ARBs seem to be safer, angioedema can recur in up to one-third of patients who switch from an ACE inhibitor to an ARB.60–63
Moreover, one study in the United States found that the frequency of hospital admission of patients with angioedema increased from 8,839 per year in 1998 to 11,925 in 2005, and the cost was estimated to be close to $123 million in 2005.64
Interestingly, when angioedema involved the face, it developed within the first week in 60% of cases,65 whereas when visceral angioedema developed, it did so within the first week in 59% of cases. Therefore, the timing of the onset is similar regardless of the body area involved.
Smokers who developed ACE-inhibitor-induced cough had a higher risk of ACE-inhibitor-induced angioedema in a retrospective cohort study by Morimoto,66 but no relationship to the area of involvement was made.
ON IMAGING, A THICKENED BOWEL WALL
Computed tomography can reveal bowel edema and ascites more reliably than plain radiography or barium studies. Edema thickens the bowel wall, with increased contrast enhancement that makes mesenteric vessels show up on the study. In some instances edema is so significant that edematous submucosa can be differentiated from the serosa due to impressive thickening of the mucosal wall.15,16 Oral contrast can be seen in the middle of the lumen, giving it a target-sign appearance. Edema of the small bowel and ascites can lead to fluid sequestration in the abdomen, resulting in a presentation with shock.67
Magnetic resonance imaging can be even more useful in identifying gastrointestinal angioedema, but it would not be cost-effective, and based on our study, CT and ultrasonography of the abdomen were diagnostic in most cases.
AVOIDING UNNECESSARY TESTING
Hemodynamic instability and abdominal pain usually trigger a surgical consult and a more extensive workup, but with a good clinical approach, unnecessary testing and invasive diagnostic procedures can be avoided under the right circumstances.
Numerous surgical procedures have been reported in patients presenting with visceral angioedema secondary to ACE inhibitors.67 Although a thorough history and physical examination can give us a clue in the diagnosis of drug-induced gastrointestinal angioedema, CT is extremely helpful, as it shows dilated loops, thickened mucosal folds, perihepatic fluid, ascites, mesenteric edema, and a “doughnut” or “stacked coin” appearance.17,68
So far, there have been only two reports of angioedema of the stomach (the case reported by Shahzad et al10 and the current report). Angioedema can affect any visceral organ, but we usually see involvement of the jejunum followed by the ileum and duodenum.40
FINDINGS ON ENDOSCOPY
Usually, endoscopic examination of the upper and lower gastrointestinal tract does not reveal any specific pathology, but endoscopy and biopsy can rule out other causes of abdominal pain, such as Crohn disease, ulcerative colitis, infection, malignancy, granuloma, and vasculitis. Also, hereditary or acquired C1-esterase deficiency and other autoimmune disorders should be considered in the workup.18,69 In the reported cases, endoscopy revealed petechial bleeding with generalized edema.19
Biopsy often demonstrates an expanded edematous submucosal layer with inflammatory cell infiltration and protrusion of the proper muscular layer into the submucosal layer.15 A proper muscular layer and an edematous submucosal layer can produce edema so severe as to obstruct the intestine.15
Ultrasonography or CT provides essential information as to location, structure, and size, and it rules out other diagnoses. Therefore, consideration should be given to noninvasive imaging studies and laboratory testing (C1-esterase inhibitor, complement, antinuclear antibody, complete metabolic panel, complete blood cell count) before resorting to endoscopy or exploratory laparotomy.20,70 In three case reports,29,30,32 abdominal ultrasonography did not show any thickening of the small-bowel wall. Several cases have been diagnosed with the help of endoscopy.
Symptoms usually resolve when the ACE inhibitor is stopped
There is no standard treatment for ACE-inhibitor-induced visceral angioedema. In most patients, stopping the drug, giving nothing by mouth, and giving intravenous fluids to prevent dehydration are sufficient. Symptoms usually resolve within 48 hours.
In several case reports, fresh-frozen plasma was used to increase the levels of kininase II, which can degrade high levels of bradykinin.51,71,72 However, no randomized controlled trial of fresh-frozen plasma for ACE-inhibitor-induced angioedema has been published.
Drugs for hereditary angioedema—eg, recombinant C1-INH, the kallikrein inhibitor ecallantide (Kalbitor), and the BKR-2-antagonist icatibant (Firazyr)73—have not been prospectively studied in gastrointestinal angioedema associated with ACE inhibitors. Icatibant has been shown to be effective in the treatment of hereditary angioedema and could be promising in treating angioedema secondary to ACE inhibitors.8 Rosenberg et al21 described a patient who was on prednisone when she developed intestinal angioedema, thus calling into question the efficacy of steroids in the treatment of visceral angioedema.
RAISING AWARENESS
More than 40 million patients are currently taking ACE inhibitors or ARBs.9 Therefore, we suggest that patients with a known history of angioedema in response to these drugs should wear an identification bracelet to increase awareness and to prevent recurrence of angioedema.
- Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA 1997; 278:232–233.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117:234–242.
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angiooedema. Lancet 2000; 356:608–609.
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–2018.
- Jessup M. The less familiar face of heart failure. J Am Coll Cardiol 2003; 41:224–226.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357:789–796.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 2008; 51:1465–1467.
- Oudit G, Girgrah N, Allard J. ACE inhibitor-induced angioedema of the intestine: Case report, incidence, pathophysiology, diagnosis and management. Can J Gastroenterol 2001; 15:827–832.
- Shahzad G, Korsten MA, Blatt C, Motwani P. Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. Mt Sinai J Med 2006; 73:1123–1125.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol 2000; 31:254–257.
- Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust 1996; 165:319–321.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Smoger SH, Sayed MA. Simultaneous mucosal and small bowel angioedema due to captopril. South Med J 1998; 91:1060–1063.
- Tojo A, Onozato ML, Fujita T. Repeated subileus due to angioedema during renin-angiotensin system blockade. Am J Med Sci 2006; 332:36–38.
- De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol 2001; 176:649–652.
- Marmery H, Mirvis SE. Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clin Radiol 2006; 61:979–982.
- Orr KK, Myers JR. Intermittent visceral edema induced by long-term enalapril administration. Ann Pharmacother 2004; 38:825–827.
- Spahn TW, Grosse-Thie W, Mueller MK. Endoscopic visualization of angiotensin-converting enzyme inhibitor-induced small bowel angioedema as a cause of relapsing abdominal pain using double-balloon enteroscopy. Dig Dis Sci 2008; 53:1257–1260.
- Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc 2000; 75:1201–1204.
- Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75:730–732.
- Salloum H, Locher C, Chenard A, et al. [Small bowel angioedema due to perindopril]. Gastroenterol Clin Biol 2005; 29:1180–1181.
- Arakawa M, Murata Y, Rikimaru Y, Sasaki Y. Drug-induced isolated visceral angioneurotic edema. Intern Med 2005; 44:975–978.
- Abdelmalek MF, Douglas DD. Lisinopril-induced isolated visceral angioedema: review of ACE-inhibitor-induced small bowel angioedema. Dig Dis Sci 1997; 42:847–850.
- Gregory KW, Davis RC. Images in clinical medicine. Angioedema of the intestine. N Engl J Med 1996; 334:1641.
- Farraye FA, Peppercorn MA, Steer ML, Joffe N, Rees M. Acute small-bowel mucosal edema following enalapril use. JAMA 1988; 259:3131.
- Jacobs RL, Hoberman LJ, Goldstein HM. Angioedema of the small bowel caused by an angiotensin-converting enzyme inhibitor. Am J Gastroenterol 1994; 89:127–128.
- Herman L, Jocums SB, Coleman MD. A 29-year-old woman with crampy abdominal pain. Tenn Med 1999; 92:272–273.
- Guy C, Cathébras P, Rousset H. Suspected angioedema of abdominal viscera. Ann Intern Med 1994; 121:900.
- Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen]. Arch Mal Coeur Vaiss 1994; 87:1371–1374.
- Jardine DL, Anderson JC, McClintock AD. Delayed diagnosis of recurrent visceral angio-oedema secondary to ACE inhibitor therapy. Aust N Z J Med 1999; 29:377–378.
- Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med 1993; 32:424–426.
- Khan MU, Baig MA, Javed RA, et al. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol 2007; 118:e68–e69.
- Adhikari SP, Schneider JI. An unusual cause of abdominal pain and hypotension: angioedema of the bowel. J Emerg Med 2009; 36:23–25.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med 2005; 118:1428–1329.
- Bi CK, Soltani K, Sloan JB, Weber RR, Elliott WJ, Murphy MB. Tissue-specific autoantibodies induced by captopril. Clin Res 1987; 35:922A.
- Bork K, Dewald G. Hereditary angioedema type III, angioedema associated with angiotensin II receptor antagonists, and female sex. Am J Med 2004; 116:644–645.
- Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med 1973; 119:832–840.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM. Angioedema presenting as chronic gastrointestinal symptoms. Am J Gastroenterol 1993; 88:436–439.
- Coleman JW, Yeung JH, Roberts DH, Breckenridge AM, Park BK. Drug-specific antibodies in patients receiving captopril. Br J Clin Pharmacol 1986; 22:161–165.
- Kallenberg CG. Autoantibodies during captopril treatment. Arthritis Rheum 1985; 28:597–598.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. BMJ 1988; 297:826–829.
- Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002; 39:460–464.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angiooedema on ACE inhibitors. Lancet 2002; 359:2088–2089.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 2000; 106:546–550.
- Yeung JH, Coleman JW, Park BK. Drug-protein conjugates—IX. Immunogenicity of captopril-protein conjugates. Biochem Pharmacol 1985; 34:4005–4012.
- Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol 1999; 82:473–476.
- Pichler WJ, Lehner R, Späth PJ. Recurrent angioedema associated with hypogonadism or anti-androgen therapy. Ann Allergy 1989; 63:301–305.
- Bass G, Honan D. Octaplas is not equivalent to fresh frozen plasma in the treatment of acute angioedema. Eur J Anaesthesiol 2007; 24:1062–1063.
- Bas M, Hoffmann TK, Bier H, Kojda G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br J Clin Pharmacol 2005; 59:233–238.
- Herman AG. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am J Cardiol 1992; 70:102C–108C.
- Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immunol 2001; 69:6796–6803.
- Cunnion KM, Wagner E, Frank MM. Complement and kinins. In:Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology. 10th ed. New York, NY: Lange Medical Books; 2001:186–188.
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin-converting enzyme inhibition in human subjects. Clin Sci (Lond) 1994; 87:567–574.
- Bristol-Myers Squibb Pharmaceutical Research Institute. FDA Advisory Committee Briefing Book for OMAPATRILAT Tablets NDA 21-188. www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf. Accessed 2/4/2011.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Mahoney EJ, Devaiah AK. Angioedema and angiotensin-converting enzyme inhibitors: are demographics a risk? Otolaryngol Head Neck Surg 2008; 139:105–108.
- Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 2000; 34:526–528.
- Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg 2004; 130:1416–1419.
- MacLean JA, Hannaway PJ. Angioedema and AT1 receptor blockers: proceed with caution. Arch Intern Med 2003; 163:1488–1489,
- Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy 2002; 22:1173–1175.
- Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol 2008; 101:185–192.
- Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA 1988; 260:967–970.
- Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10:499–509.
- Cohen N, Sharon A, Golik A, Zaidenstein R, Modai D. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol 1993; 16:237–239.
- Ciaccia D, Brazer SR, Baker ME. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR Am J Roentgenol 1993; 161:1215–1216.
- Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn’s disease. Med J Aust 1999; 171:418–420.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 2002; 109:370–371.
- Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 2004; 92:573–575.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62:842–856.
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999; 44:21–25.
- Brown NJ, Snowden M, Griffin MR. Recurrent angiotensin-converting enzyme inhibitor–associated angioedema. JAMA 1997; 278:232–233.
- Israili ZH, Hall WD. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy. A review of the literature and pathophysiology. Ann Intern Med 1992; 117:234–242.
- Messerli FH, Nussberger J. Vasopeptidase inhibition and angiooedema. Lancet 2000; 356:608–609.
- Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–2018.
- Jessup M. The less familiar face of heart failure. J Am Coll Cardiol 2003; 41:224–226.
- Chobanian AV. Clinical practice. Isolated systolic hypertension in the elderly. N Engl J Med 2007; 357:789–796.
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005; 366:2026–2033.
- Weber MA, Messerli FH. Angiotensin-converting enzyme inhibitors and angioedema: estimating the risk. Hypertension 2008; 51:1465–1467.
- Oudit G, Girgrah N, Allard J. ACE inhibitor-induced angioedema of the intestine: Case report, incidence, pathophysiology, diagnosis and management. Can J Gastroenterol 2001; 15:827–832.
- Shahzad G, Korsten MA, Blatt C, Motwani P. Angiotensin-converting enzyme (ACE) inhibitor-associated angioedema of the stomach and small intestine: a case report. Mt Sinai J Med 2006; 73:1123–1125.
- Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol 2000; 31:254–257.
- Mullins RJ, Shanahan TM, Dobson RT. Visceral angioedema related to treatment with an ACE inhibitor. Med J Aust 1996; 165:319–321.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Smoger SH, Sayed MA. Simultaneous mucosal and small bowel angioedema due to captopril. South Med J 1998; 91:1060–1063.
- Tojo A, Onozato ML, Fujita T. Repeated subileus due to angioedema during renin-angiotensin system blockade. Am J Med Sci 2006; 332:36–38.
- De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol 2001; 176:649–652.
- Marmery H, Mirvis SE. Angiotensin-converting enzyme inhibitor-induced visceral angioedema. Clin Radiol 2006; 61:979–982.
- Orr KK, Myers JR. Intermittent visceral edema induced by long-term enalapril administration. Ann Pharmacother 2004; 38:825–827.
- Spahn TW, Grosse-Thie W, Mueller MK. Endoscopic visualization of angiotensin-converting enzyme inhibitor-induced small bowel angioedema as a cause of relapsing abdominal pain using double-balloon enteroscopy. Dig Dis Sci 2008; 53:1257–1260.
- Byrne TJ, Douglas DD, Landis ME, Heppell JP. Isolated visceral angioedema: an underdiagnosed complication of ACE inhibitors? Mayo Clin Proc 2000; 75:1201–1204.
- Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation 2003; 75:730–732.
- Salloum H, Locher C, Chenard A, et al. [Small bowel angioedema due to perindopril]. Gastroenterol Clin Biol 2005; 29:1180–1181.
- Arakawa M, Murata Y, Rikimaru Y, Sasaki Y. Drug-induced isolated visceral angioneurotic edema. Intern Med 2005; 44:975–978.
- Abdelmalek MF, Douglas DD. Lisinopril-induced isolated visceral angioedema: review of ACE-inhibitor-induced small bowel angioedema. Dig Dis Sci 1997; 42:847–850.
- Gregory KW, Davis RC. Images in clinical medicine. Angioedema of the intestine. N Engl J Med 1996; 334:1641.
- Farraye FA, Peppercorn MA, Steer ML, Joffe N, Rees M. Acute small-bowel mucosal edema following enalapril use. JAMA 1988; 259:3131.
- Jacobs RL, Hoberman LJ, Goldstein HM. Angioedema of the small bowel caused by an angiotensin-converting enzyme inhibitor. Am J Gastroenterol 1994; 89:127–128.
- Herman L, Jocums SB, Coleman MD. A 29-year-old woman with crampy abdominal pain. Tenn Med 1999; 92:272–273.
- Guy C, Cathébras P, Rousset H. Suspected angioedema of abdominal viscera. Ann Intern Med 1994; 121:900.
- Dupasquier E. [A rare clinical form of angioneurotic edema caused by enalapril: acute abdomen]. Arch Mal Coeur Vaiss 1994; 87:1371–1374.
- Jardine DL, Anderson JC, McClintock AD. Delayed diagnosis of recurrent visceral angio-oedema secondary to ACE inhibitor therapy. Aust N Z J Med 1999; 29:377–378.
- Matsumura M, Haruki K, Kajinami K, Takada T. Angioedema likely related to angiotensin converting enzyme inhibitors. Intern Med 1993; 32:424–426.
- Khan MU, Baig MA, Javed RA, et al. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol 2007; 118:e68–e69.
- Adhikari SP, Schneider JI. An unusual cause of abdominal pain and hypotension: angioedema of the bowel. J Emerg Med 2009; 36:23–25.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Johnsen SP, Jacobsen J, Monster TB, Friis S, McLaughlin JK, Sørensen HT. Risk of first-time hospitalization for angioedema among users of ACE inhibitors and angiotensin receptor antagonists. Am J Med 2005; 118:1428–1329.
- Bi CK, Soltani K, Sloan JB, Weber RR, Elliott WJ, Murphy MB. Tissue-specific autoantibodies induced by captopril. Clin Res 1987; 35:922A.
- Bork K, Dewald G. Hereditary angioedema type III, angioedema associated with angiotensin II receptor antagonists, and female sex. Am J Med 2004; 116:644–645.
- Witten DM, Hirsch FD, Hartman GW. Acute reactions to urographic contrast medium: incidence, clinical characteristics and relationship to history of hypersensitivity states. Am J Roentgenol Radium Ther Nucl Med 1973; 119:832–840.
- Eck SL, Morse JH, Janssen DA, Emerson SG, Markovitz DM. Angioedema presenting as chronic gastrointestinal symptoms. Am J Gastroenterol 1993; 88:436–439.
- Coleman JW, Yeung JH, Roberts DH, Breckenridge AM, Park BK. Drug-specific antibodies in patients receiving captopril. Br J Clin Pharmacol 1986; 22:161–165.
- Kallenberg CG. Autoantibodies during captopril treatment. Arthritis Rheum 1985; 28:597–598.
- Inman WH, Rawson NS, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I: Results of prescription-event monitoring. BMJ 1988; 297:826–829.
- Lefebvre J, Murphey LJ, Hartert TV, Jiao Shan R, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension 2002; 39:460–464.
- Molinaro G, Cugno M, Perez M, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther 2002; 303:232–237.
- Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angiooedema on ACE inhibitors. Lancet 2002; 359:2088–2089.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol 2000; 106:546–550.
- Yeung JH, Coleman JW, Park BK. Drug-protein conjugates—IX. Immunogenicity of captopril-protein conjugates. Biochem Pharmacol 1985; 34:4005–4012.
- Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol 1999; 82:473–476.
- Pichler WJ, Lehner R, Späth PJ. Recurrent angioedema associated with hypogonadism or anti-androgen therapy. Ann Allergy 1989; 63:301–305.
- Bass G, Honan D. Octaplas is not equivalent to fresh frozen plasma in the treatment of acute angioedema. Eur J Anaesthesiol 2007; 24:1062–1063.
- Bas M, Hoffmann TK, Bier H, Kojda G. Increased C-reactive protein in ACE-inhibitor-induced angioedema. Br J Clin Pharmacol 2005; 59:233–238.
- Herman AG. Differences in structure of angiotensin-converting enzyme inhibitors might predict differences in action. Am J Cardiol 1992; 70:102C–108C.
- Cunnion KM, Lee JC, Frank MM. Capsule production and growth phase influence binding of complement to Staphylococcus aureus. Infect Immunol 2001; 69:6796–6803.
- Cunnion KM, Wagner E, Frank MM. Complement and kinins. In:Parlow TG, Stites DP, Imboden JB, editors. Medical Immunology. 10th ed. New York, NY: Lange Medical Books; 2001:186–188.
- Pellacani A, Brunner HR, Nussberger J. Plasma kinins increase after angiotensin-converting enzyme inhibition in human subjects. Clin Sci (Lond) 1994; 87:567–574.
- Bristol-Myers Squibb Pharmaceutical Research Institute. FDA Advisory Committee Briefing Book for OMAPATRILAT Tablets NDA 21-188. www.fda.gov/ohrms/dockets/ac/02/briefing/3877B2_01_BristolMeyersSquibb.pdf. Accessed 2/4/2011.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Mahoney EJ, Devaiah AK. Angioedema and angiotensin-converting enzyme inhibitors: are demographics a risk? Otolaryngol Head Neck Surg 2008; 139:105–108.
- Warner KK, Visconti JA, Tschampel MM. Angiotensin II receptor blockers in patients with ACE inhibitor-induced angioedema. Ann Pharmacother 2000; 34:526–528.
- Kyrmizakis DE, Papadakis CE, Liolios AD, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Arch Otolaryngol Head Neck Surg 2004; 130:1416–1419.
- MacLean JA, Hannaway PJ. Angioedema and AT1 receptor blockers: proceed with caution. Arch Intern Med 2003; 163:1488–1489,
- Abdi R, Dong VM, Lee CJ, Ntoso KA. Angiotensin II receptor blocker-associated angioedema: on the heels of ACE inhibitor angioedema. Pharmacotherapy 2002; 22:1173–1175.
- Lin RY, Shah SN. Increasing hospitalizations due to angioedema in the United States. Ann Allergy Asthma Immunol 2008; 101:185–192.
- Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA 1988; 260:967–970.
- Morimoto T, Gandhi TK, Fiskio JM, et al. An evaluation of risk factors for adverse drug events associated with angiotensin-converting enzyme inhibitors. J Eval Clin Pract 2004; 10:499–509.
- Cohen N, Sharon A, Golik A, Zaidenstein R, Modai D. Hereditary angioneurotic edema with severe hypovolemic shock. J Clin Gastroenterol 1993; 16:237–239.
- Ciaccia D, Brazer SR, Baker ME. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. AJR Am J Roentgenol 1993; 161:1215–1216.
- Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn’s disease. Med J Aust 1999; 171:418–420.
- Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci 2002; 324:106–108.
- Karim MY, Masood A. Fresh-frozen plasma as a treatment for life-threatening ACE-inhibitor angioedema. J Allergy Clin Immunol 2002; 109:370–371.
- Warrier MR, Copilevitz CA, Dykewicz MS, Slavin RG. Fresh frozen plasma in the treatment of resistant angiotensin-converting enzyme inhibitor angioedema. Ann Allergy Asthma Immunol 2004; 92:573–575.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy 2007; 62:842–856.
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffré D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999; 44:21–25.
KEY POINTS
- Visceral angioedema due to ACE-inhibitor therapy can easily be diagnosed by clinical suspicion and abdominal computed tomography (CT).
- Many physicians are not aware of this condition and so may subject patients to unnecessary invasive procedures, including surgery and endoscopy.
- If a middle-aged woman taking an ACE inhibitor presents with abdominal pain and emesis, the differential diagnosis should include visceral angioedema, and CT should be strongly considered.
In reply: Coadministration of clopidogrel and proton pump inhibitors
In Reply: I thank Dr. Keller for his interest in my review on the side effects and drug interactions of proton pump inhibitors (PPIs).1 In particular, the concern about the potentially increased risk of a cardiovascular event in patients taking a PPI while on clopidogrel is a matter of active research. Since the prevention of death, myocardial infarction, or stroke is the desired outcome in patients receiving antiplatelet therapy, any reduction in the antiplatelet effect of clopidogrel could put patients at increased risk. Because of the enormous number of patients on both PPIs and clopidogrel, investigators are studying the effect of PPIs on clopidogrel to determine the true significance in day-to-day practice. We should expect that the data will continue to evolve in the coming years as more research is done on this important interaction.
The FDA Web site that Dr. Keller brings up2 was posted a few months after the submission of my manuscript. But even with the FDA’s cautionary words, it is important to realize that the risk that purportedly exists with the interaction of omeprazole and clopidogrel and the suggestion for the alternative use of pantoprazole are both based on pharmacokinetic, pharmacodynamic, and epidemiologic studies, not on clinical outcome data.
As much as we would like to rely on such studies, pharmacokinetic and pharmacodynamic studies do not address clinical outcomes, and observational studies cannot account for every confounder, because patients in these studies are not randomly assigned to the intervention, which is the rationale behind the necessity for a prospective trial. The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) study,3 a prospective randomized controlled trial with 3,761 analyzed patients, found no differences in adjudicated cardiovascular outcomes between groups who received a clopidogrel plus omeprazole vs clopidogrel alone.3 Although the COGENT study ended prematurely because of bankruptcy of the funding source, these outcomes represent the only randomized prospective data that can be found to date on PubMed. With such large numbers of patients in each group (1,876 and 1,885, respectively) and no differences in outcomes, it stands to reason that only a study with massive sample sizes would be able to detect a statistically significant difference. Differences between clopidogrel-treated patients taking and not taking omeprazole are likely be found in a well-designed prospective trial; however, it would be virtually impossible to find differences among PPIs.
To make matters even less convincing that therapy should be altered, the Working Group on High On-treatment Platelet Reactivity stated in their recent consensus paper that there are “limited data to support that alteration of therapy based on platelet function measurements actually improves outcomes.” 4 Additionally, a recent multisociety Expert Consensus Document discussing the concomitant use of PPIs and thienopyridine drugs to reduce gastrointestinal complications further supports this argument.5 Therefore, it is difficult to justify a marked increase in cost of the PPI selected (pantoprazole costs nearly seven times more per dose than omeprazole, according to one Web site6) for a benefit that is supported only by theoretical and observational data, not by outcome data.
As Dr. Keller also mentions, Aggrenox can be used for secondary stroke prophylaxis, but a discussion about a therapeutic exchange between clopidogrel and other antiplatelet agents was beyond the scope of my review. A recently published joint guideline of the American Heart Association and the American Stroke Association guideline should be consulted for further information.7
Other gastroprotective therapies are available. However, misoprostol (as mentioned) is associated with significant gastrointestinal side effects and must be taken four times a day. H2-receptor antagonists are not considered to be as effective as PPIs.8,9
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–33.
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines:a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010; 105:2533–2549.
- HealthWarehouse. www.healthwarehouse.com. Accessed March 23, 2011.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents.Circulation 2008; 118:1894–1909.
- Lanza FL, Chan FK, Quigley EM, et al. Guidelines forprevention of NSAID-related ulcer complications. Am JGastroenterol 2009; 104:728–738.
In Reply: I thank Dr. Keller for his interest in my review on the side effects and drug interactions of proton pump inhibitors (PPIs).1 In particular, the concern about the potentially increased risk of a cardiovascular event in patients taking a PPI while on clopidogrel is a matter of active research. Since the prevention of death, myocardial infarction, or stroke is the desired outcome in patients receiving antiplatelet therapy, any reduction in the antiplatelet effect of clopidogrel could put patients at increased risk. Because of the enormous number of patients on both PPIs and clopidogrel, investigators are studying the effect of PPIs on clopidogrel to determine the true significance in day-to-day practice. We should expect that the data will continue to evolve in the coming years as more research is done on this important interaction.
The FDA Web site that Dr. Keller brings up2 was posted a few months after the submission of my manuscript. But even with the FDA’s cautionary words, it is important to realize that the risk that purportedly exists with the interaction of omeprazole and clopidogrel and the suggestion for the alternative use of pantoprazole are both based on pharmacokinetic, pharmacodynamic, and epidemiologic studies, not on clinical outcome data.
As much as we would like to rely on such studies, pharmacokinetic and pharmacodynamic studies do not address clinical outcomes, and observational studies cannot account for every confounder, because patients in these studies are not randomly assigned to the intervention, which is the rationale behind the necessity for a prospective trial. The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) study,3 a prospective randomized controlled trial with 3,761 analyzed patients, found no differences in adjudicated cardiovascular outcomes between groups who received a clopidogrel plus omeprazole vs clopidogrel alone.3 Although the COGENT study ended prematurely because of bankruptcy of the funding source, these outcomes represent the only randomized prospective data that can be found to date on PubMed. With such large numbers of patients in each group (1,876 and 1,885, respectively) and no differences in outcomes, it stands to reason that only a study with massive sample sizes would be able to detect a statistically significant difference. Differences between clopidogrel-treated patients taking and not taking omeprazole are likely be found in a well-designed prospective trial; however, it would be virtually impossible to find differences among PPIs.
To make matters even less convincing that therapy should be altered, the Working Group on High On-treatment Platelet Reactivity stated in their recent consensus paper that there are “limited data to support that alteration of therapy based on platelet function measurements actually improves outcomes.” 4 Additionally, a recent multisociety Expert Consensus Document discussing the concomitant use of PPIs and thienopyridine drugs to reduce gastrointestinal complications further supports this argument.5 Therefore, it is difficult to justify a marked increase in cost of the PPI selected (pantoprazole costs nearly seven times more per dose than omeprazole, according to one Web site6) for a benefit that is supported only by theoretical and observational data, not by outcome data.
As Dr. Keller also mentions, Aggrenox can be used for secondary stroke prophylaxis, but a discussion about a therapeutic exchange between clopidogrel and other antiplatelet agents was beyond the scope of my review. A recently published joint guideline of the American Heart Association and the American Stroke Association guideline should be consulted for further information.7
Other gastroprotective therapies are available. However, misoprostol (as mentioned) is associated with significant gastrointestinal side effects and must be taken four times a day. H2-receptor antagonists are not considered to be as effective as PPIs.8,9
In Reply: I thank Dr. Keller for his interest in my review on the side effects and drug interactions of proton pump inhibitors (PPIs).1 In particular, the concern about the potentially increased risk of a cardiovascular event in patients taking a PPI while on clopidogrel is a matter of active research. Since the prevention of death, myocardial infarction, or stroke is the desired outcome in patients receiving antiplatelet therapy, any reduction in the antiplatelet effect of clopidogrel could put patients at increased risk. Because of the enormous number of patients on both PPIs and clopidogrel, investigators are studying the effect of PPIs on clopidogrel to determine the true significance in day-to-day practice. We should expect that the data will continue to evolve in the coming years as more research is done on this important interaction.
The FDA Web site that Dr. Keller brings up2 was posted a few months after the submission of my manuscript. But even with the FDA’s cautionary words, it is important to realize that the risk that purportedly exists with the interaction of omeprazole and clopidogrel and the suggestion for the alternative use of pantoprazole are both based on pharmacokinetic, pharmacodynamic, and epidemiologic studies, not on clinical outcome data.
As much as we would like to rely on such studies, pharmacokinetic and pharmacodynamic studies do not address clinical outcomes, and observational studies cannot account for every confounder, because patients in these studies are not randomly assigned to the intervention, which is the rationale behind the necessity for a prospective trial. The Clopidogrel and the Optimization of Gastrointestinal Events (COGENT) study,3 a prospective randomized controlled trial with 3,761 analyzed patients, found no differences in adjudicated cardiovascular outcomes between groups who received a clopidogrel plus omeprazole vs clopidogrel alone.3 Although the COGENT study ended prematurely because of bankruptcy of the funding source, these outcomes represent the only randomized prospective data that can be found to date on PubMed. With such large numbers of patients in each group (1,876 and 1,885, respectively) and no differences in outcomes, it stands to reason that only a study with massive sample sizes would be able to detect a statistically significant difference. Differences between clopidogrel-treated patients taking and not taking omeprazole are likely be found in a well-designed prospective trial; however, it would be virtually impossible to find differences among PPIs.
To make matters even less convincing that therapy should be altered, the Working Group on High On-treatment Platelet Reactivity stated in their recent consensus paper that there are “limited data to support that alteration of therapy based on platelet function measurements actually improves outcomes.” 4 Additionally, a recent multisociety Expert Consensus Document discussing the concomitant use of PPIs and thienopyridine drugs to reduce gastrointestinal complications further supports this argument.5 Therefore, it is difficult to justify a marked increase in cost of the PPI selected (pantoprazole costs nearly seven times more per dose than omeprazole, according to one Web site6) for a benefit that is supported only by theoretical and observational data, not by outcome data.
As Dr. Keller also mentions, Aggrenox can be used for secondary stroke prophylaxis, but a discussion about a therapeutic exchange between clopidogrel and other antiplatelet agents was beyond the scope of my review. A recently published joint guideline of the American Heart Association and the American Stroke Association guideline should be consulted for further information.7
Other gastroprotective therapies are available. However, misoprostol (as mentioned) is associated with significant gastrointestinal side effects and must be taken four times a day. H2-receptor antagonists are not considered to be as effective as PPIs.8,9
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–33.
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines:a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010; 105:2533–2549.
- HealthWarehouse. www.healthwarehouse.com. Accessed March 23, 2011.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents.Circulation 2008; 118:1894–1909.
- Lanza FL, Chan FK, Quigley EM, et al. Guidelines forprevention of NSAID-related ulcer complications. Am JGastroenterol 2009; 104:728–738.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 2010; 363:1909–1917.
- Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56:919–33.
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines:a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2010; 105:2533–2549.
- HealthWarehouse. www.healthwarehouse.com. Accessed March 23, 2011.
- Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42:227–276.
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation task force on clinical expert consensus documents.Circulation 2008; 118:1894–1909.
- Lanza FL, Chan FK, Quigley EM, et al. Guidelines forprevention of NSAID-related ulcer complications. Am JGastroenterol 2009; 104:728–738.
Coadministration of clopidogrel and proton pump inhibitors
To the Editor: Thank you for the excellent review on proton pump inhibitors (PPIs) in the January 2011 issue.1 I would like to make the following comments about Dr. Madanick’s suggested algorithm (see Figure 2 in the article) for deciding whether to use a PPI in patients requiring clopidogrel:
A posting dated October 27, 2010, on the Web site of the US Food and Drug Administration (FDA) states the following: “With regard to the proton pump inhibitor (PPI) drug class, this recommendation [against the concomitant use of Plavix (clopidogrel) and omeprazole (Prilosec)] applies only to omeprazole and not to all PPIs. Not all PPIs have the same inhibitory effect on the enzyme [CYP2C19] that is crucial for conversion of Plavix into its active form. Pantoprazole (Protonix) may be an alternative PPI for consideration. It is a weak inhibitor of CYP2C19 and has less effect on the pharmacological activity of Plavix than omeprazole.”2 Thus, when it is deemed necessary to coadminister clopidogrel with a PPI, pantoprazole appears to be the preferred PPI.
If the patient is taking clopidogrel for stroke prophylaxis, one can consider switching to Aggrenox (aspirin plus extended-release dipyridamole), which has no warnings regarding coadministration with PPIs.
Patients taking aspirin plus clopidogrel may benefit by the addition of misoprostol (Cytotec), which is indicated for reducing the risk of aspirin-induced gastric ulcers in patients at high risk of complications from gastric ulcer.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
To the Editor: Thank you for the excellent review on proton pump inhibitors (PPIs) in the January 2011 issue.1 I would like to make the following comments about Dr. Madanick’s suggested algorithm (see Figure 2 in the article) for deciding whether to use a PPI in patients requiring clopidogrel:
A posting dated October 27, 2010, on the Web site of the US Food and Drug Administration (FDA) states the following: “With regard to the proton pump inhibitor (PPI) drug class, this recommendation [against the concomitant use of Plavix (clopidogrel) and omeprazole (Prilosec)] applies only to omeprazole and not to all PPIs. Not all PPIs have the same inhibitory effect on the enzyme [CYP2C19] that is crucial for conversion of Plavix into its active form. Pantoprazole (Protonix) may be an alternative PPI for consideration. It is a weak inhibitor of CYP2C19 and has less effect on the pharmacological activity of Plavix than omeprazole.”2 Thus, when it is deemed necessary to coadminister clopidogrel with a PPI, pantoprazole appears to be the preferred PPI.
If the patient is taking clopidogrel for stroke prophylaxis, one can consider switching to Aggrenox (aspirin plus extended-release dipyridamole), which has no warnings regarding coadministration with PPIs.
Patients taking aspirin plus clopidogrel may benefit by the addition of misoprostol (Cytotec), which is indicated for reducing the risk of aspirin-induced gastric ulcers in patients at high risk of complications from gastric ulcer.
To the Editor: Thank you for the excellent review on proton pump inhibitors (PPIs) in the January 2011 issue.1 I would like to make the following comments about Dr. Madanick’s suggested algorithm (see Figure 2 in the article) for deciding whether to use a PPI in patients requiring clopidogrel:
A posting dated October 27, 2010, on the Web site of the US Food and Drug Administration (FDA) states the following: “With regard to the proton pump inhibitor (PPI) drug class, this recommendation [against the concomitant use of Plavix (clopidogrel) and omeprazole (Prilosec)] applies only to omeprazole and not to all PPIs. Not all PPIs have the same inhibitory effect on the enzyme [CYP2C19] that is crucial for conversion of Plavix into its active form. Pantoprazole (Protonix) may be an alternative PPI for consideration. It is a weak inhibitor of CYP2C19 and has less effect on the pharmacological activity of Plavix than omeprazole.”2 Thus, when it is deemed necessary to coadminister clopidogrel with a PPI, pantoprazole appears to be the preferred PPI.
If the patient is taking clopidogrel for stroke prophylaxis, one can consider switching to Aggrenox (aspirin plus extended-release dipyridamole), which has no warnings regarding coadministration with PPIs.
Patients taking aspirin plus clopidogrel may benefit by the addition of misoprostol (Cytotec), which is indicated for reducing the risk of aspirin-induced gastric ulcers in patients at high risk of complications from gastric ulcer.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
- Madanick RD. Proton pump inhibitor side effects and drug interactions: much ado about nothing? Cleve Clin J Med 2011; 78:39–49.
- US Food and Drug Administration. FDA reminder to avoid concomitant use of Plavix (clopidogrel) and omeprazole. www.fda.gov/Drugs/DrugSafety/ucm231161.htm. Accessed March 23, 2011.
Gene-based, rational drug-dosing: An evolving, complex opportunity
For some drugs there is a key step in metabolism, often in a rate-limiting pathway, with an enzyme that has known and detectable polymorphisms that differ dramatically in their ability to affect the drug’s degradation. In theory, by determining the patient’s specific genotype ahead of time, the initial dose of the drug can be determined more rationally. In this issue of the Journal, Kitzmiller et al describe several drugs for which this may be true.
However, for this approach to be practical and cost-effective, several conditions should be met. The drug must be one that needs to be dosed to its therapeutic level rapidly: if there is time to titrate slowly, then there is little need for the extra expense associated with genotyping in order to titrate it more rapidly. Also, it should be proven that dosing based on advance knowledge of the genotype of the target actually results in safer or more efficacious dosing.
For carbamazepine (Tegretol, Equetro) and allopurinol (Zyloprim), specific human leukocyte antigen haplotypes are associated with a strikingly increased frequency of serious hypersensitivity reactions. In some patients, these should be checked before giving the drug.
But the concept of pharmacogenomics is broad, and it may yet explain many vagaries of drug-responsiveness in individual patients. Polymorphisms in renal anion transporters may dictate the level of anionic drugs. Drug-receptor polymorphisms may determine the affinity of a drug for its target and, hence, its efficacy. Cell-membrane transporters, which may have functionally different stable alleles or polymorphisms, may regulate intracellular drug levels by pumping the drug into or out of cells with different efficiencies.
As the entire human genome is dissected and analyzed, and as more and more genes (with their polymorphisms) are linked to specific functions readily detectable in specific patients, we will have more opportunities to match the right drug and dose to the right patient. We are not there yet, but that day is coming.
For some drugs there is a key step in metabolism, often in a rate-limiting pathway, with an enzyme that has known and detectable polymorphisms that differ dramatically in their ability to affect the drug’s degradation. In theory, by determining the patient’s specific genotype ahead of time, the initial dose of the drug can be determined more rationally. In this issue of the Journal, Kitzmiller et al describe several drugs for which this may be true.
However, for this approach to be practical and cost-effective, several conditions should be met. The drug must be one that needs to be dosed to its therapeutic level rapidly: if there is time to titrate slowly, then there is little need for the extra expense associated with genotyping in order to titrate it more rapidly. Also, it should be proven that dosing based on advance knowledge of the genotype of the target actually results in safer or more efficacious dosing.
For carbamazepine (Tegretol, Equetro) and allopurinol (Zyloprim), specific human leukocyte antigen haplotypes are associated with a strikingly increased frequency of serious hypersensitivity reactions. In some patients, these should be checked before giving the drug.
But the concept of pharmacogenomics is broad, and it may yet explain many vagaries of drug-responsiveness in individual patients. Polymorphisms in renal anion transporters may dictate the level of anionic drugs. Drug-receptor polymorphisms may determine the affinity of a drug for its target and, hence, its efficacy. Cell-membrane transporters, which may have functionally different stable alleles or polymorphisms, may regulate intracellular drug levels by pumping the drug into or out of cells with different efficiencies.
As the entire human genome is dissected and analyzed, and as more and more genes (with their polymorphisms) are linked to specific functions readily detectable in specific patients, we will have more opportunities to match the right drug and dose to the right patient. We are not there yet, but that day is coming.
For some drugs there is a key step in metabolism, often in a rate-limiting pathway, with an enzyme that has known and detectable polymorphisms that differ dramatically in their ability to affect the drug’s degradation. In theory, by determining the patient’s specific genotype ahead of time, the initial dose of the drug can be determined more rationally. In this issue of the Journal, Kitzmiller et al describe several drugs for which this may be true.
However, for this approach to be practical and cost-effective, several conditions should be met. The drug must be one that needs to be dosed to its therapeutic level rapidly: if there is time to titrate slowly, then there is little need for the extra expense associated with genotyping in order to titrate it more rapidly. Also, it should be proven that dosing based on advance knowledge of the genotype of the target actually results in safer or more efficacious dosing.
For carbamazepine (Tegretol, Equetro) and allopurinol (Zyloprim), specific human leukocyte antigen haplotypes are associated with a strikingly increased frequency of serious hypersensitivity reactions. In some patients, these should be checked before giving the drug.
But the concept of pharmacogenomics is broad, and it may yet explain many vagaries of drug-responsiveness in individual patients. Polymorphisms in renal anion transporters may dictate the level of anionic drugs. Drug-receptor polymorphisms may determine the affinity of a drug for its target and, hence, its efficacy. Cell-membrane transporters, which may have functionally different stable alleles or polymorphisms, may regulate intracellular drug levels by pumping the drug into or out of cells with different efficiencies.
As the entire human genome is dissected and analyzed, and as more and more genes (with their polymorphisms) are linked to specific functions readily detectable in specific patients, we will have more opportunities to match the right drug and dose to the right patient. We are not there yet, but that day is coming.
Pharmacogenomics for the primary care provider: Why should we care?
Since the human genome was sequenced in 2000, the American public has continued to hold hope that our growing understanding of genetics will revolutionize the practice of medicine.
One way genetics promises to improve the quality and value of health care is in personalized medicine, by helping us tailor treatment to a person’s individual genetic makeup. One such approach is called pharmacogenomics.
Pharmacogenomics uses knowledge of a person’s genetics to understand how a particular drug will work, or not work, in his or her body. For instance, some people might carry genes that make them more sensitive than average to a drug, and therefore they would require a lower dose. Others might have genes that make them resistant to the drug, meaning the drug is ineffective unless they receive a higher dose.
Adverse drug reactions are a leading cause of death in hospitalized patients in the United States and are responsible for billions of dollars in health care costs.1,2 Our current practice of prescribing for adult patients is largely trial-and-error, with the same dose given to all patients, in many cases with little regard even to sex, height, or weight.
Pharmacogenomics promises to change this way of prescribing to a customized approach that uses genetic information to predict an individual’s response to medications. It is one piece of an overall initiative to personalize patient care based on the patient’s individual characteristics and preferences.
OVERCOMING BARRIERS TO USING PHARMACOGENOMICS IN PRACTICE
If personalized medicine has promised to improve the quality and value of health care for our patients, why have we been so slow to adopt this information in clinical practice?
The usual barriers to clinical adoption certainly exist. We need further studies to determine whether genetic-based prescribing is truly valid, and for which patient populations. We need to determine whether this approach is cost-effective and better than the current standard of care. We need to work on payment options.
However, one of the largest barriers for busy primary care physicians is the lack of time to keep up with new information. Many practicing physicians were taught little about formal genetics in medical school. The body of scientific literature on pharmacogenomics is fragmented, and it crosses disease states and specialties, making it difficult to unite. Given the breadth of diseases treated and drugs prescribed by primary care physicians, it is unrealistic for most to keep track of the vast body of literature of pharmacogenomic testing and to decipher how to apply this to clinical practice.
In this issue of the Journal, Kitzmiller et al3 provide one solution to this problem, giving an overview of pharmacogenomic applications that might be pertinent to practicing physicians. However, as we try to make pharmacogenomics accessible to busy physicians, we need other solutions to integrate pharmacogenomic information efficiently into the clinical work flow. One approach might be to build pharmacogenomics into the electronic medical record. We can also store the integrated information in research databases and provide clinical recommendations on Internet sites such as www.pharmgkb.org, and we can develop applications to run on cell phones and iPads.
QUESTIONS REMAIN
Kitzmiller et al discuss an important step in this process, highlighting several key questions:
Should we seek genetics-based information to personalize drug selection? Based on the information presented in the literature and in the Kitzmiller paper, there may be circumstances when it is appropriate to consider doing so. While the evidence is not yet compelling to order these tests on a regular basis in clinical practice, this information might be helpful in some situations, such as for patients who have had adverse effects from minimal doses of antidepressants.
For now, clinicians should not abandon their current practice of personalizing patient care on the basis of personal, cultural, and economic preferences. Rather, they should consider pharmacogenomic information an additional piece of information when selecting drug therapy. We should also encourage health care systems and interested providers to be early adopters and to study how their outcomes compare with the standard of care.
Once we have this information, what is our obligation to use it? An increasing number of patients already have genetic information in their health record, either ordered by or provided to their physicians. However, there is little in the scientific literature to guide us in this arena.
Yet most of us would agree that if we have information (genetic or otherwise) that can help to select a drug type or dose or reduce adverse events or costs, we should consider this information in our decision-making. Several circumstances are documented in this paper and in the literature in which prior knowledge about drug metabolism can help in selecting a dose of medication. One example would be the 50% recommended reduction in tricyclic antidepressant dose if the patient is a CYP2D6 poor metabolizer.4
MOVING FORWARD AS A TEAM
In summary, Kitzmiller et al bring to light the promise and the uncertainties that currently exist in the field of pharmacogenomics. While it is unclear if we should incorporate pharmacogenomic tests into standard medical practice at this time, it is clear that this information is becoming more readily available, whether or not we have requested it. Some would argue that, once we have the information, we have an obligation to use it, just as we use other information in our clinical decision-making. This means we need to develop tools and resources to help practitioners evaluate pharmacogenomic data and incorporate it into clinical care in an efficient manner.
The authors also highlight the need for more education about drug metabolism in general, and they cite several instances in which knowledge of drug interactions and metabolism can clearly influence decision-making. An example is paroxetine (Paxil) inhibition of tamoxifen (Nolvadex).5
Lastly, regardless of our personal feelings about the clinical usefulness of genetic testing in large populations, we need to work together to determine clinical utility and validity and to develop efficient ways to put into practice findings that could affect patient care. As we move forward, we need to work as a team, utilizing our clinical partners—pharmacists, pharmacologists, metabolism and health information technology experts, and medical geneticists. Working as a team, pooling our resources and tools, we move closer to providing world-class personalized health care.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.
- Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events among older adults in the ambulatory setting. Med Care 2005; 43:1171–1176.
- Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice. Why drugs work in some patients but not others. Cleve Clin J Med 2011; 78:243–257.
- Thuerauf N, Lunkenheimer J. The impact of the CYP2D6-polymorphism on dose recommendations for current antidepressants. Eur Arch Psychiatry Clin Neurosci 2006; 256:287–293.
- Schwarz EB, McNamara M, Miller RG, Walsh JM. Update in women’s health for the general internist. J Gen Intern Med201; 26:207–213.
Since the human genome was sequenced in 2000, the American public has continued to hold hope that our growing understanding of genetics will revolutionize the practice of medicine.
One way genetics promises to improve the quality and value of health care is in personalized medicine, by helping us tailor treatment to a person’s individual genetic makeup. One such approach is called pharmacogenomics.
Pharmacogenomics uses knowledge of a person’s genetics to understand how a particular drug will work, or not work, in his or her body. For instance, some people might carry genes that make them more sensitive than average to a drug, and therefore they would require a lower dose. Others might have genes that make them resistant to the drug, meaning the drug is ineffective unless they receive a higher dose.
Adverse drug reactions are a leading cause of death in hospitalized patients in the United States and are responsible for billions of dollars in health care costs.1,2 Our current practice of prescribing for adult patients is largely trial-and-error, with the same dose given to all patients, in many cases with little regard even to sex, height, or weight.
Pharmacogenomics promises to change this way of prescribing to a customized approach that uses genetic information to predict an individual’s response to medications. It is one piece of an overall initiative to personalize patient care based on the patient’s individual characteristics and preferences.
OVERCOMING BARRIERS TO USING PHARMACOGENOMICS IN PRACTICE
If personalized medicine has promised to improve the quality and value of health care for our patients, why have we been so slow to adopt this information in clinical practice?
The usual barriers to clinical adoption certainly exist. We need further studies to determine whether genetic-based prescribing is truly valid, and for which patient populations. We need to determine whether this approach is cost-effective and better than the current standard of care. We need to work on payment options.
However, one of the largest barriers for busy primary care physicians is the lack of time to keep up with new information. Many practicing physicians were taught little about formal genetics in medical school. The body of scientific literature on pharmacogenomics is fragmented, and it crosses disease states and specialties, making it difficult to unite. Given the breadth of diseases treated and drugs prescribed by primary care physicians, it is unrealistic for most to keep track of the vast body of literature of pharmacogenomic testing and to decipher how to apply this to clinical practice.
In this issue of the Journal, Kitzmiller et al3 provide one solution to this problem, giving an overview of pharmacogenomic applications that might be pertinent to practicing physicians. However, as we try to make pharmacogenomics accessible to busy physicians, we need other solutions to integrate pharmacogenomic information efficiently into the clinical work flow. One approach might be to build pharmacogenomics into the electronic medical record. We can also store the integrated information in research databases and provide clinical recommendations on Internet sites such as www.pharmgkb.org, and we can develop applications to run on cell phones and iPads.
QUESTIONS REMAIN
Kitzmiller et al discuss an important step in this process, highlighting several key questions:
Should we seek genetics-based information to personalize drug selection? Based on the information presented in the literature and in the Kitzmiller paper, there may be circumstances when it is appropriate to consider doing so. While the evidence is not yet compelling to order these tests on a regular basis in clinical practice, this information might be helpful in some situations, such as for patients who have had adverse effects from minimal doses of antidepressants.
For now, clinicians should not abandon their current practice of personalizing patient care on the basis of personal, cultural, and economic preferences. Rather, they should consider pharmacogenomic information an additional piece of information when selecting drug therapy. We should also encourage health care systems and interested providers to be early adopters and to study how their outcomes compare with the standard of care.
Once we have this information, what is our obligation to use it? An increasing number of patients already have genetic information in their health record, either ordered by or provided to their physicians. However, there is little in the scientific literature to guide us in this arena.
Yet most of us would agree that if we have information (genetic or otherwise) that can help to select a drug type or dose or reduce adverse events or costs, we should consider this information in our decision-making. Several circumstances are documented in this paper and in the literature in which prior knowledge about drug metabolism can help in selecting a dose of medication. One example would be the 50% recommended reduction in tricyclic antidepressant dose if the patient is a CYP2D6 poor metabolizer.4
MOVING FORWARD AS A TEAM
In summary, Kitzmiller et al bring to light the promise and the uncertainties that currently exist in the field of pharmacogenomics. While it is unclear if we should incorporate pharmacogenomic tests into standard medical practice at this time, it is clear that this information is becoming more readily available, whether or not we have requested it. Some would argue that, once we have the information, we have an obligation to use it, just as we use other information in our clinical decision-making. This means we need to develop tools and resources to help practitioners evaluate pharmacogenomic data and incorporate it into clinical care in an efficient manner.
The authors also highlight the need for more education about drug metabolism in general, and they cite several instances in which knowledge of drug interactions and metabolism can clearly influence decision-making. An example is paroxetine (Paxil) inhibition of tamoxifen (Nolvadex).5
Lastly, regardless of our personal feelings about the clinical usefulness of genetic testing in large populations, we need to work together to determine clinical utility and validity and to develop efficient ways to put into practice findings that could affect patient care. As we move forward, we need to work as a team, utilizing our clinical partners—pharmacists, pharmacologists, metabolism and health information technology experts, and medical geneticists. Working as a team, pooling our resources and tools, we move closer to providing world-class personalized health care.
Since the human genome was sequenced in 2000, the American public has continued to hold hope that our growing understanding of genetics will revolutionize the practice of medicine.
One way genetics promises to improve the quality and value of health care is in personalized medicine, by helping us tailor treatment to a person’s individual genetic makeup. One such approach is called pharmacogenomics.
Pharmacogenomics uses knowledge of a person’s genetics to understand how a particular drug will work, or not work, in his or her body. For instance, some people might carry genes that make them more sensitive than average to a drug, and therefore they would require a lower dose. Others might have genes that make them resistant to the drug, meaning the drug is ineffective unless they receive a higher dose.
Adverse drug reactions are a leading cause of death in hospitalized patients in the United States and are responsible for billions of dollars in health care costs.1,2 Our current practice of prescribing for adult patients is largely trial-and-error, with the same dose given to all patients, in many cases with little regard even to sex, height, or weight.
Pharmacogenomics promises to change this way of prescribing to a customized approach that uses genetic information to predict an individual’s response to medications. It is one piece of an overall initiative to personalize patient care based on the patient’s individual characteristics and preferences.
OVERCOMING BARRIERS TO USING PHARMACOGENOMICS IN PRACTICE
If personalized medicine has promised to improve the quality and value of health care for our patients, why have we been so slow to adopt this information in clinical practice?
The usual barriers to clinical adoption certainly exist. We need further studies to determine whether genetic-based prescribing is truly valid, and for which patient populations. We need to determine whether this approach is cost-effective and better than the current standard of care. We need to work on payment options.
However, one of the largest barriers for busy primary care physicians is the lack of time to keep up with new information. Many practicing physicians were taught little about formal genetics in medical school. The body of scientific literature on pharmacogenomics is fragmented, and it crosses disease states and specialties, making it difficult to unite. Given the breadth of diseases treated and drugs prescribed by primary care physicians, it is unrealistic for most to keep track of the vast body of literature of pharmacogenomic testing and to decipher how to apply this to clinical practice.
In this issue of the Journal, Kitzmiller et al3 provide one solution to this problem, giving an overview of pharmacogenomic applications that might be pertinent to practicing physicians. However, as we try to make pharmacogenomics accessible to busy physicians, we need other solutions to integrate pharmacogenomic information efficiently into the clinical work flow. One approach might be to build pharmacogenomics into the electronic medical record. We can also store the integrated information in research databases and provide clinical recommendations on Internet sites such as www.pharmgkb.org, and we can develop applications to run on cell phones and iPads.
QUESTIONS REMAIN
Kitzmiller et al discuss an important step in this process, highlighting several key questions:
Should we seek genetics-based information to personalize drug selection? Based on the information presented in the literature and in the Kitzmiller paper, there may be circumstances when it is appropriate to consider doing so. While the evidence is not yet compelling to order these tests on a regular basis in clinical practice, this information might be helpful in some situations, such as for patients who have had adverse effects from minimal doses of antidepressants.
For now, clinicians should not abandon their current practice of personalizing patient care on the basis of personal, cultural, and economic preferences. Rather, they should consider pharmacogenomic information an additional piece of information when selecting drug therapy. We should also encourage health care systems and interested providers to be early adopters and to study how their outcomes compare with the standard of care.
Once we have this information, what is our obligation to use it? An increasing number of patients already have genetic information in their health record, either ordered by or provided to their physicians. However, there is little in the scientific literature to guide us in this arena.
Yet most of us would agree that if we have information (genetic or otherwise) that can help to select a drug type or dose or reduce adverse events or costs, we should consider this information in our decision-making. Several circumstances are documented in this paper and in the literature in which prior knowledge about drug metabolism can help in selecting a dose of medication. One example would be the 50% recommended reduction in tricyclic antidepressant dose if the patient is a CYP2D6 poor metabolizer.4
MOVING FORWARD AS A TEAM
In summary, Kitzmiller et al bring to light the promise and the uncertainties that currently exist in the field of pharmacogenomics. While it is unclear if we should incorporate pharmacogenomic tests into standard medical practice at this time, it is clear that this information is becoming more readily available, whether or not we have requested it. Some would argue that, once we have the information, we have an obligation to use it, just as we use other information in our clinical decision-making. This means we need to develop tools and resources to help practitioners evaluate pharmacogenomic data and incorporate it into clinical care in an efficient manner.
The authors also highlight the need for more education about drug metabolism in general, and they cite several instances in which knowledge of drug interactions and metabolism can clearly influence decision-making. An example is paroxetine (Paxil) inhibition of tamoxifen (Nolvadex).5
Lastly, regardless of our personal feelings about the clinical usefulness of genetic testing in large populations, we need to work together to determine clinical utility and validity and to develop efficient ways to put into practice findings that could affect patient care. As we move forward, we need to work as a team, utilizing our clinical partners—pharmacists, pharmacologists, metabolism and health information technology experts, and medical geneticists. Working as a team, pooling our resources and tools, we move closer to providing world-class personalized health care.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.
- Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events among older adults in the ambulatory setting. Med Care 2005; 43:1171–1176.
- Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice. Why drugs work in some patients but not others. Cleve Clin J Med 2011; 78:243–257.
- Thuerauf N, Lunkenheimer J. The impact of the CYP2D6-polymorphism on dose recommendations for current antidepressants. Eur Arch Psychiatry Clin Neurosci 2006; 256:287–293.
- Schwarz EB, McNamara M, Miller RG, Walsh JM. Update in women’s health for the general internist. J Gen Intern Med201; 26:207–213.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279:1200–1205.
- Field TS, Gilman BH, Subramanian S, Fuller JC, Bates DW, Gurwitz JH. The costs associated with adverse drug events among older adults in the ambulatory setting. Med Care 2005; 43:1171–1176.
- Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice. Why drugs work in some patients but not others. Cleve Clin J Med 2011; 78:243–257.
- Thuerauf N, Lunkenheimer J. The impact of the CYP2D6-polymorphism on dose recommendations for current antidepressants. Eur Arch Psychiatry Clin Neurosci 2006; 256:287–293.
- Schwarz EB, McNamara M, Miller RG, Walsh JM. Update in women’s health for the general internist. J Gen Intern Med201; 26:207–213.