User login
University taking aim at racial disparities in COVID vaccine trials
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
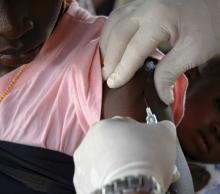
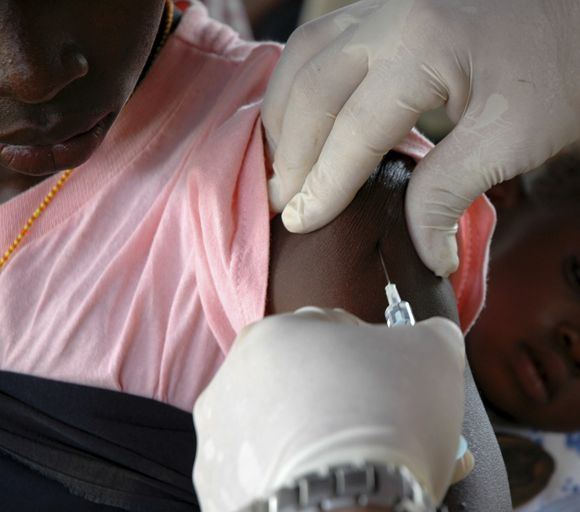
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Although recent months have seen the arrival of several promising vaccines to combat COVID-19, many researchers have been concerned about the shortage of Black and Latinx volunteers in their pivotal trials.
Minority groups have long been underrepresented in clinical research. The pandemic’s inequitable fallout has heightened the need for more inclusive COVID-19 trials. By one estimate, Black Americans are three times more likely to become infected with SARS-Cov-2 and twice as likely to die from it, compared with their White counterparts.
It was therefore welcome news this past November when the Maryland-based biotech company Novavax unveiled their plans to boost participation among specific minority groups during the phase 3 trial of their COVID-19 vaccine candidate NVX-CoV2373. To help them in their efforts, the company tapped Howard University, in Washington, D.C., to be a clinical test site. The goal was to enroll 300 Black and Latinx volunteers through a recruitment registry at the Coronavirus Prevention Network.
“We have seen quite a good number of participants in the registry, and many are African American, who are the ones we are trying to reach in the trial,” explained Siham Mahgoub, MD, medical director of the Center of Infectious Diseases Management and Research and principal investigator for the Novavax trial at Howard University, Washington. “It’s very important for people of color to participate in the trial because we want to make sure these vaccines work in people of color,” Dr. Mahgoub said.
Over the years, Howard University has hosted several important clinical trials and studies, and its participation in the multi-institutional Georgetown–Howard Universities Center for Clinical and Translational Science consortium brings crucial infrastructural value. By bringing this vaccine trial to one of the most esteemed historically Black colleges or universities (HBCUs), researchers hoped to address a sense of hesitancy among possible participants that is prompted in part by the tragic history of medical testing in the Black community.
“The community trusts Howard,” said Dr. Mahgoub. “I think it’s great having Howard and an HBCU host this trial, because these are people who look like them.”
Lisa M. Dunkle, MD, vice president and global medical lead for coronavirus vaccine at Novavax, explained that, in addition to Howard being located close to the company’s headquarters, the university seemed like a great fit for the overall mission.
“As part of our goal to achieve a representative trial population that includes communities who are disproportionately impacted by the pandemic, we sought out some of the HBCUs to include in our trial sites. We hoped that this might encourage people of color to enroll and to increase their comfort level with vaccines in general,” Dr. Dunkle said.
Building more representative clinical trials
For decades, research on some of the most groundbreaking vaccines and treatments have been based on the results of studies conducted with predominately White participants, despite the fact that a much more demographically varied general population would ultimately receive them. This has led to calls to include people of different races and ethnic backgrounds in trials.
Homogeneity in clinical trials is discouraged, but trials are not heavily regulated in this regard. In 1993, Congress passed the Revitalization Act, which requires that trials that are conducted by the National Institutes of Health include women and members of minority groups among their cohorts. However, the number or proportion of such participants is not specified.
Underrepresentation in clinical trials also reflects a general unwillingness by members of ethnic minorities to volunteer because of the deeply unsettling history of such trials in minority communities. Among some Black persons, it is not uncommon for names like Tuskegee, Henrietta Lacks, and J. Marion Simms to be mentioned when giving reasons for not participating.
“There is certainly some dark history in how minorities have been treated by our health care system, and it’s not surprising that there is some fear and distrust,” said Dr. Dunkle. “By recruiting people of color into clinical trials that are governed with strict standards, we can begin to change perceptions and attitudes.”
Vaccine hesitancy is not only rooted in the past. The current state of medical care also has some potential trial participants worried. Misinformation, inequity in health care access, and low health literacy contribute to the current fears of scientific development.
A trial designed to engender trust
Having information about the vaccine come from trusted voices in the community is a key means of overcoming hesitancy. Howard University President Wayne Frederick, MD, reached out to a pastor of a local Black church to have more participants enroll in the trial. One who answered the call to action was Stephanie Williams, an elementary school teacher in Montgomery County, Maryland. When she saw that her pastor was participating in the Novavax trial and when she considered the devastation she had seen from COVID-19, she was on board.
“We had about three sessions where he shared his experiences. He also shared some links to read about it more,” Ms. Williams said. “When I saw that he took it, that gave me a lot of confidence. Since I’m going be going into the classroom, I wanted to be sure that I was well protected.”
Transparency is key to gaining more participation, explained Dr. Maghoub. Webinar-based information sessions have proven particularly important in achieving this.
“We do a lot of explaining in very simple language to make sure everyone understands about the vaccine. The participants have time to ask questions during the webinar, and at any time [during the trial], if a participant feels that it is not right for them, they can stop. They have time to learn about the trial and give consent. People often think they are like guinea pigs in trials, but they are not. They must give consent.”
There are signs that the approach has been successful. Over a period of 4-5 weeks, the Howard site enrolled 150 participants, of whom 30% were Black and 20% were Latinx.
Novavax has been in business for more than 3 decades but hasn’t seen the booming success that their competitors have. The company has noted progress in developing vaccines against Middle East respiratory syndrome and severe acute respiratory syndrome. However, they missed the mark in clinical trials, failing twice in 3 years to develop a respiratory syncytial virus vaccine administered through maternal immunizations.
From being on the verge of closing, Novavax has since made a dramatic turnaround after former President Trump awarded the company $1.6 billion dollars in July 2020 as part of Operation Warp Speed. If trial results are promising, the Novavax vaccine could enter the market in a few months, representing not only a new therapeutic option but perhaps a new model for building inclusivity in clinical trials.
A version of this article first appeared on Medscape.com.
Urine drug screening: A guide to monitoring Tx with controlled substances
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4
A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15
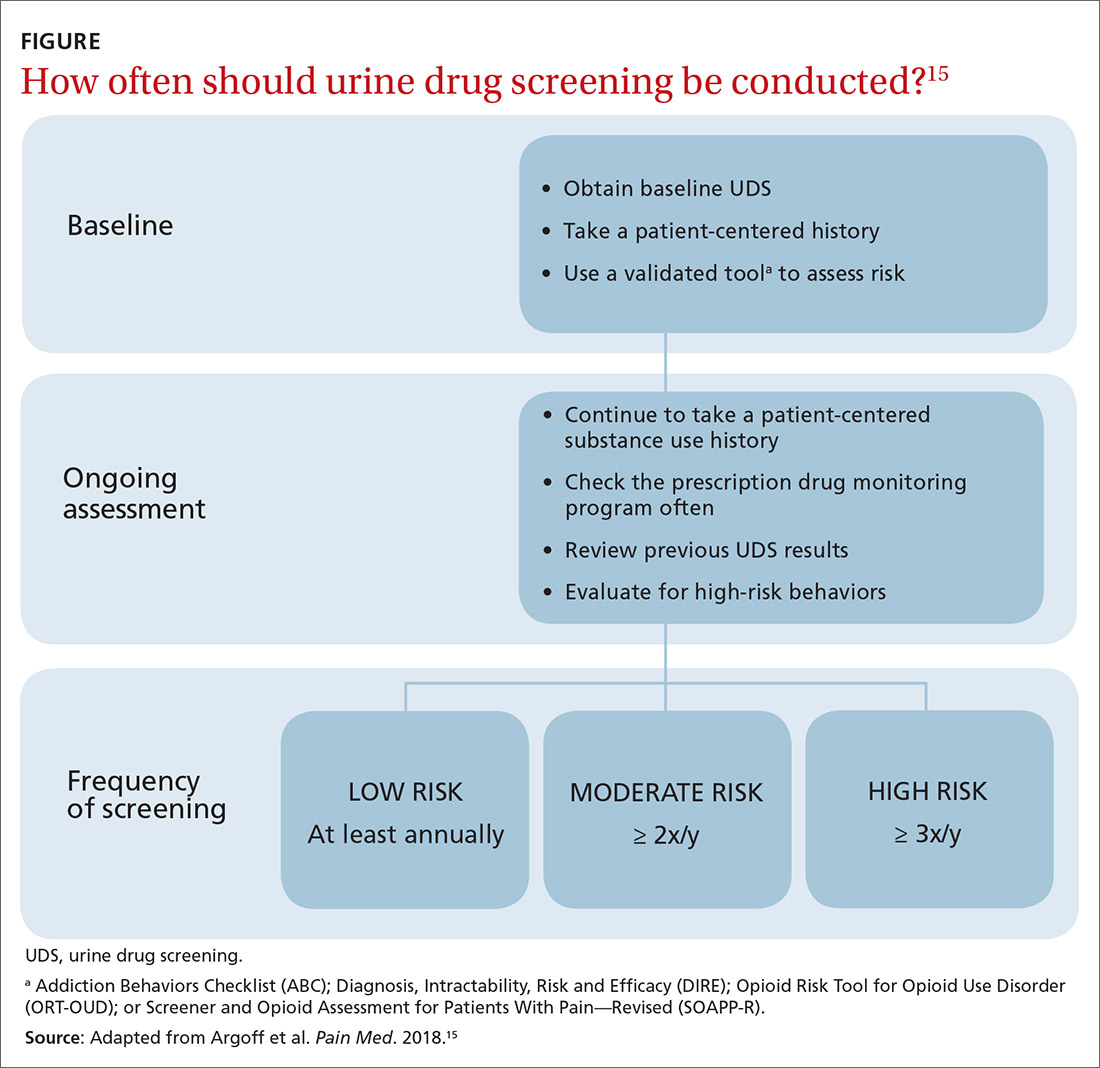
Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19
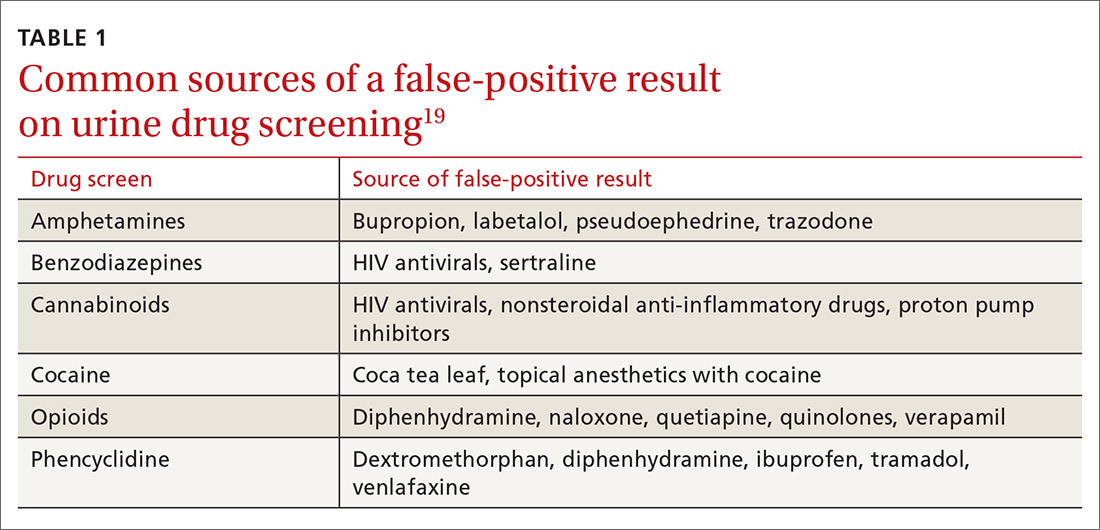
Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20
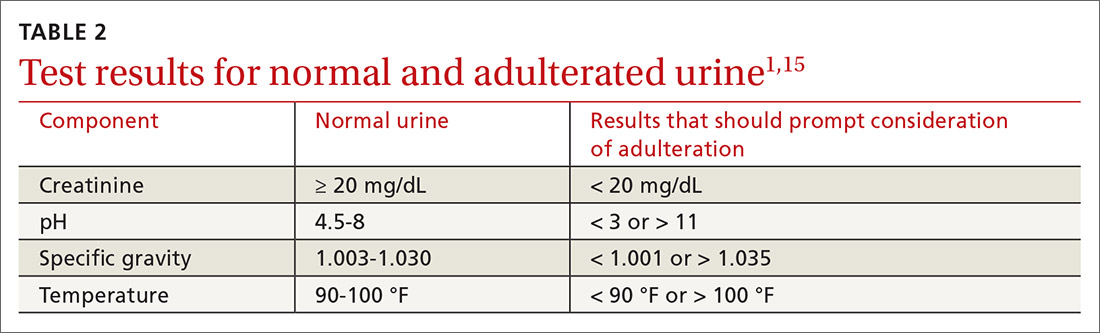
Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).
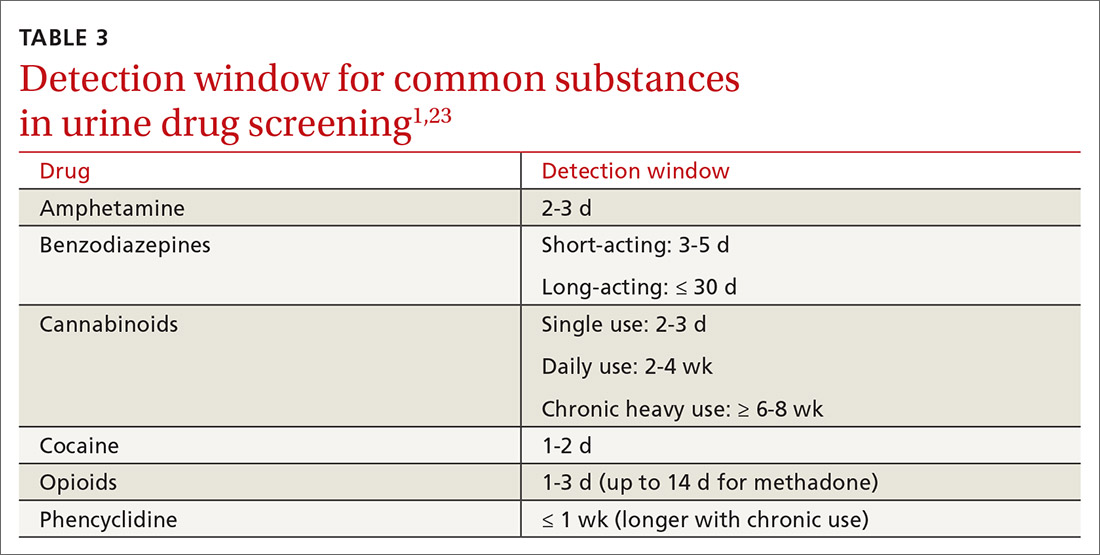
Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4

A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15

Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19

Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20

Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).

Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
An estimated 20 million patients in the United States have a substance use disorder (SUD), with hundreds of millions of prescriptions for controlled substances written annually. Consequently, urine drug screening (UDS) has become widely utilized to evaluate and treat patients with an SUD or on chronic opioid or benzodiazepine therapy.1
Used appropriately, UDS can be a valuable tool; there is ample evidence, however, that it has been misused, by some physicians, to stigmatize patients who use drugs of abuse,2 profile patients racially,2 profit from excessive testing,3 and inappropriately discontinue treatment.4

A patient-centered approach. We have extensive clinical experience in the use and interpretation of urine toxicology, serving as clinical leads in busy family medicine residency practices that care for patients with SUDs, and are often consulted regarding patients on chronic opioid or benzodiazepine therapy. We have encountered countless situations in which the correct interpretation of UDS is critical to providing care.
Over time, and after considerable trial and error, we developed the patient-centered approach to urine toxicology described in this article. We believe that the medical evidence strongly supports our approach to the appropriate use and interpretation of urine toxicology in clinical practice. Our review here is intended as a resource when you consider implementing a UDS protocol or are struggling with the management of unexpected results.
Urine toxicology for therapeutic drug monitoring
Prescribing a controlled substance carries inherent risks, including diversion, nonmedical use, and development of an SUD. Prescribed medications, particularly opioids and benzodiazepines, have been linked to a large increase in overdose deaths over the past decade.5 Several strategies have been investigated to mitigate risk (see “How frequently should a patient be tested?,” later in the article).
Clinical judgment—ie, when a physician orders a drug test upon suspecting that a patient is diverting a prescribed drug or has developed an SUD—has been shown to be highly inaccurate. Implicit racial bias might affect the physician’s judgment, leading to changes in testing and test interpretation. For example, Black patients were found to be 10% more likely to have drug screening ordered while being treated with long-term opioid therapy and 2 to 3 times more likely to have their medication discontinued as a result of a marijuana- or cocaine-positive test.2
Other studies have shown that testing patients for “bad behavior,” so to speak—reporting a prescription lost or stolen, consuming more than the prescribed dosage, visiting the office without an appointment, having multiple drug intolerances and allergies, and making frequent telephone calls to the practice—is ineffective.6 Patients with these behaviors were slightly more likely to unexpectedly test positive, or negative, on their UDS; however, many patients without suspect behavior also were found to have abnormal toxicology results.6 Data do not support therapeutic drug monitoring only of patients selected on the basis of aberrant behavior.6
Continue to: Questions and concerns about urine drug screening
Questions and concerns about urine drug screening
Why not just ask the patient? Studies have evaluated whether patient self-reporting of adherence is a feasible alternative to laboratory drug screening. Regrettably, patients have repeatedly been shown to underreport their use of both prescribed and illicit drugs.7,8
That question leads to another: Why do patients lie to their physician? It is easy to assume malicious intent, but a variety of obstacles might dissuade a patient from being fully truthful with their physician:
- Monetary gain. A small, but real, percentage of medications are diverted by patients for this reason.9
- Addiction, pseudo-addiction due to tolerance, and self-medication for psychological symptoms are clinically treatable syndromes that can lead to underreporting of prescribed and nonprescribed drug and alcohol use.
- Shame. Addiction is a highly stigmatized disease, and patients might simply be ashamed to admit that they need treatment: 13% to 38% of patients receiving chronic opioid therapy in a pain management or primary care setting have a clinically diagnosable SUD.10,11
Is consent needed to test or to share test results? Historically, UDS has been performed on patients without their consent or knowledge.12 Patients give a urine specimen to their physician for a variety of reasons; it seems easy to “add on” UDS. Evidence is clear, however, that confronting a patient about an unexpected test result can make the clinical outcome worse—often resulting in irreparable damage to the patient–physician relationship.12,13 Unless the patient is experiencing a medical emergency, guidelines unanimously recommend obtaining consent prior to testing.1,5,14
Federal law requires written permission from the patient for the physician to disclose information about alcohol or substance use, unless the information is expressly needed to provide care during a medical emergency. Substance use is highly stigmatized, and patients might—legitimately—fear that sharing their history could undermine their care.1,12,14
How frequently should a patient be tested? Experts recommend utilizing a risk-based strategy to determine the frequency of UDS.1,5,15 Validated risk-assessment questionnaires include:
- Opioid Risk Tool for Opioid Use Disorder (ORT-OUD)a
- Screener and Opioid Assessment for Patients With Pain–Revised (SOAPP-R)b
- Diagnosis, Intractability, Risk and Efficacy (DIRE)c
- Addiction Behaviors Checklist (ABC).d
Continue to: Each of these tools...
Each of these tools takes less than 5 minutes to administer and can be used by a primary care physician to objectively quantify the risk of prescribing; there is no evidence for the use of 1 of these screeners over the others.15 It is recommended that you choose a questionnaire that works for you and incorporate the risk assessment into prescribing any high-risk medication.1,5,15
Once you have completed an initial risk assessment, the frequency of UDS can be based on ongoing assessment that incorporates baseline testing, patient self-reporting, toxicology results, behavioral monitoring, and state database monitoring through a prescription drug monitoring program. Annual screening is appropriate in low-risk patients; moderate-risk patients should be screened twice a year, and high-risk patients should be screened at least every 4 months (FIGURE).15

Many state and federal agencies, health systems, employers, and insurers mandate the frequency of testing through guidelines or legislation. These regulations often are inconsistent with the newest medical evidence.15 Consult local guidelines and review the medical evidence and consensus recommendations on UDS.
What are the cost considerations in providing UDS? Insurers have been billed as much as $4000 for definitive chromatography testing (described later).3 This has led to insurance fraud, when drug-testing practices with a financial interest routinely use large and expensive test panels, test too frequently, or unnecessarily send for confirmatory or quantitative analysis of all positive tests.3,14 Often, insurers refuse to pay for unnecessary testing, leaving patients with significant indebtedness.3,14 Take time to review the evidence and consensus recommendations on UDS to avoid waste, potential accusations of fraud, and financial burden on your patients.
Urine toxicology for addiction treatment
UDS protocols in addiction settings are often different from those in which a controlled substance is being prescribed.
Continue to: Routine and random testing
Routine and random testing. Two common practices when treating addiction are to perform UDS on all patients, at every visit, or to test randomly.1 These practices can be problematic, however. Routine testing at every visit can make urine-tampering more likely and is often unnecessary for stable patients. Random testing can reduce the risk of urine-tampering, but it is often difficult for primary care clinics to institute such a protocol. Some clinics have patients provide a urine specimen at every visit and then only send tests to the lab based on randomization.1
Contingency management—a behavioral intervention in which a patient is rewarded, or their performance is reinforced, when they display evidence of positive change—is the most effective strategy used in addiction medicine to determine the frequency of patient visits and UDS.14,16 High-risk patients with self-reported active substance use or UDS results consistent with substance use, or both, are seen more often; as their addiction behavior diminishes, visits and UDS become less frequent. If addiction behavior increases, the patient is seen more often. Keep in mind that addiction behavior decreases over months of treatment, not immediately upon initiation.14,17 For contingency management to be successful, patient-centered interviewing and UDS will need to be employed frequently as the patient works toward meaningful change.14
The technology of urine drug screening
Two general techniques are used for UDS: immunoassay and chromatography. Each plays an important role in clinical practice; physicians must therefore maintain a basic understanding of the mechanism of each technique and their comparable advantages and disadvantages. Such an understanding allows for (1) matching the appropriate technique to the individual clinical scenario and (2) correctly interpreting results.
Immunoassay technology is used for point-of-care and rapid laboratory UDS, using antibodies to detect the drug or drug metabolite of interest. Antibodies utilized in immunoassays are designed to selectively bind a specific antigen—ie, a unique chemical structure within the drug of choice. Once bound, the antigen–antibody complex can be exploited for detection through various methods.
Chromatography–mass spectrometry is considered the gold standard for UDS, yielding confirmatory results. This is a 2-step process: Chromatography separates components within a specimen; mass spectrometry then identifies those components. Most laboratories employ liquid, rather than gas, chromatography. The specificity of the liquid chromatography–mass spectrometry method is such that a false-positive result is, essentially, impossible.18
Continue to: How is the appropriate tests elected for urine drug screening?
How is the appropriate tests elected for urine drug screening?
Variables that influence your choice of the proper test method include the clinical question at hand; cost; the urgency of obtaining results; and the stakes in that decision (ie, will the results be used to simply change the dosage of a medication or, of greater consequence, to determine fitness for employment or inform criminal justice decisions?). Each method of UDS has advantages that can be utilized and disadvantages that must be considered to obtain an accurate and useful result.
Immunoassay provides rapid results, is relatively easy to perform, and is, comparatively, inexpensive.1,14 The speed of results makes this method particularly useful in settings such as the emergency department, where rapid results are crucial. Ease of use makes immunoassay ideal for the office, where non-laboratory staff can be trained to properly administer the test.
A major disadvantage of immunoassay technology, however, is interference resulting in both false-positive and false-negative results, which is discussed in detail in the next section. Immunoassay should be considered a screening test that yields presumptive results.
Liquid chromatography–mass spectrometry is exquisitely specific and provides confirmatory test results—major advantages of the method. However, specificity comes at a price: significantly increased cost and longer wait time for results (typically days, if specimens are sent out to a laboratory). These barriers can make it impractical to employ this method in routine practice.
Interpretation of results: Not so fast
Interpreting UDS results is not as simple as noting a positive or negative result. Physicians must understand the concept of interference, so that results can be appropriately interpreted and confirmed. This is crucial when results influence clinical decisions; inappropriate action, taken on the basis of presumptive results, can have severe consequences for the patient–provider relationship and the treatment plan.1,14
Continue to: Interference falls into 2 categories...
Interference falls into 2 categories: variables inherent in the testing process and patient variables.
Antibody cross-reactivity. A major disadvantage of immunoassay technology is interference that results in false-positive and false-negative results.19,20 The source of this interference is antibody cross-reactivity—the degree to which an antibody binds to structurally similar compounds. Antibody–antigen interactions are incredibly complex; although assay antibodies are engineered to specifically detect a drug class of interest, reactivity with other, structurally similar compounds is unavoidable.
Nevertheless, cross-reactivity is a useful phenomenon that allows broad testing for multiple drugs within a class. For example, most point-of-care tests for benzodiazepines reliably detect diazepam and chlordiazepoxide. Likewise, opiate tests reliably detect natural opiates, such as morphine and codeine. Cross-reactivity is not limitless, however; most benzodiazepine immunoassays have poor reactivity to clonazepam and lorazepam, making it possible that a patient taking clonazepam tests negative for benzodiazepine on an immunoassay.14,20 Similarly, standard opioid tests have only moderate cross-reactivity for semisynthetic opioids, such as hydrocodone and hydromorphone; poor cross-reactivity for oxycodone and oxymorphone; and essentially no cross-reactivity for full synthetics, such as fentanyl and methadone.14
It is the responsibility of the ordering physician to understand cross-reactivity to various drugs within a testing class.
Whereas weak cross-reactivity to drugs within a class can be a source of false-negative results, cross-reactivity to drugs outside the class of interest is a source of false-positive results. An extensive review of drugs that cause false-positive immunoassay screening tests is outside the scope of this article; commonly prescribed medications implicated in false-positive results are listed in TABLE 1.19

Continue to: In general...
In general, amphetamine immunoassays produce frequent false-positive results, whereas cocaine and cannabinoid assays are more specific.1,18 Common over-the-counter medications, including nonsteroidal anti-inflammatory drugs, decongestants, and antacids, can yield false-positive results, highlighting the need to obtain a comprehensive medication list from patients, including over-the-counter and herbal medications, before ordering UDS. Because of the complexity of cross-reactivity, it might not be possible to identify the source of a false-positive result.14
Patient variables. Intentional effort to skew results is another source of interference. The frequency of this effort varies by setting and the potential consequences of results—eg, employment testing or substance use treatment—and a range of attempts have been reported in the literature.21,22 Common practices are dilution, adulteration, and substitution.20,23
- Dilution lowers the concentration of the drug of interest below the detection limit of the assay by directly adding water to the urine specimen, drinking copious amounts of fluid, taking a diuretic, or a combination of these practices.
- Adulteration involves adding a substance to urine that interferes with the testing mechanism: for example, bleach, household cleaners, eye drops, and even commercially available products expressly marketed to interfere with UDS.24
- Substitution involves providing urine or a urine-like substance for testing that did not originate from the patient.
Methods to minimize patient-related interference include observed collection and specimen validity testing for pH, creatinine, and adulterants (TABLE 2).1,15 Efforts to detect patient interference must be balanced against concerns about privacy, personnel resources, and the cost of expanded testing.14,19,20

Additional aspects inherent to the testing process, such as cutoff concentrations and detection windows, can lead to interference. Laboratories must set reporting cutoffs, and specimens with a drug concentration present but below the cutoff value are reported as a negative result. Detection windows are complex and are influenced by inherent properties of the drug, including metabolic pathway and route and frequency of use.1 A given patient might well be using a substance, but if the specimen was obtained outside the detection window, a false-negative result might be reported (TABLE 31,23).

Managing test results
Appropriate management of UDS results is built on the foundation of understanding the testing mechanism, selecting the correct test, and properly interpreting results. Drug testing is, ultimately, a therapeutic tool used to monitor treatment, provide reinforcement, and explore substance use behavior; results of testing should be employed to achieve those objectives.1,4,14 A negative or expected UDS result can be utilized as positive reinforcement for a patient who is adherent to the treatment plan—much the way objective weight loss in an obese patient can provide encouragement to continue lifestyle changes.
Continue to: Test results should be presented...
Test results should be presented in an objective, nonconfrontational, and compassionate manner, not with stigmatizing language, such as “clean” or “dirty.”1,13,14 Using stigmatizing terms such as “substance abuser” instead of “person with a substance use disorder” has been shown, even among highly trained health care professionals, to have a negative effect on patient care.13
Inevitably, you will encounter an unexpected result, and therefore must develop a rational, systematic, and compassionate management approach. “Unexpected result” is a broad term that includes results that conflict with
- a patient’s self-report
- your understanding of what the patient is taking (using)
- prescribed medications
- a patient’s typical substance use pattern.
When faced with an unexpected test result, first, ensure that the result in question is reliable. If a screening test yields an unanticipated finding—especially if it conflicts with the patient’s self-reporting—make every effort to seek confirmation if you are going to be making a significant clinical decision because of the result.1,14
Second, use your understanding of interference to consider the result in a broader context. If confirmatory results are inconsistent with a patient’s self-report, discuss whether there has been a break in the physician–patient relationship and emphasize that recurrent use or failure to adhere to a treatment plan has clear consequences.1,14 Modify the treatment plan to address the inconsistent finding by escalating care, adjusting medications, and connecting the patient to additional resources.
Third, keep in mind that a positive urine test is not diagnostic of an SUD. Occasional drug use is extremely common17 and should not categorically lead to a change in the treatment plan. Addiction is, fundamentally, a disease of disordered reward, motivation, and behavior that is defined by the consequences of substance use, not substance use per se,25 and an SUD diagnosis is complex, based on clinical history, physical examination, and laboratory testing. Similarly, a negative UDS result does not rule out an SUD.4,10
Continue to: Fourth, patient dismissal...
Fourth, patient dismissal is rarely an appropriate initial response to UDS results. Regrettably, some physicians misinterpret urine toxicology results and inappropriately discharge patients on that basis.
The Centers for Disease Control and Prevention guideline for prescribing opioids has increased utilization of UDS in primary care settings but does not provide the necessary education on proper use of the tool, which has resulted in a rise in misinterpretation and inappropriate discharge.13,26
If recurrent aberrant behavior is detected (by history or urine toxicology), do not abruptly discontinue the patient’s medication(s). Inform the patient of your concern, taper medication, and refer the patient to addiction treatment. Abrupt discontinuation of an opioid or benzodiazepine can lead to significant harm.1,14
CORRESPONDENCE
John Hayes, DO, Department of Family and Community Medicine, Medical College of Wisconsin, 1121 E North Avenue, Milwaukee, WI, 53212; [email protected]
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
1. TAP 32: Clinical drug testing in primary care. Rockville, MD: Substance Abuse and Mental Health Services Administration, US Department of Health & Human Services; 2012. Technical Assistance Publication (TAP) 32; HHS Publication No. (SMA) 12-4668. 2012. Accessed March 19, 2021. https://store.samhsa.gov/sites/default/files/d7/priv/sma12-4668.pdf
2. Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371-376. https://doi.org/10.1016/j.drugalcdep.2018.05.033
3. Segal, David. In pursuit of liquid gold. The New York Times. December 27, 2017. Accessed March 19, 2021. https://nyti.ms/2E2GTOU
4. Ceasar R, Chang J, Zamora K, et al. Primary care providers’ experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37:154-160. https://doi.org/10.1080/08897077.2015.1132293
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49. https://doi.org/10.15585/mmwr.rr6501e1
6. Katz NP, Sherburne S, Beach M, et al. Behavioral monitoring and urine toxicology testing in patients receiving long-term opioid therapy. Anesth Analg. 2003;97:1097-1102. https://doi.org/ 10.1213/01.ane.0000080159.83342.b5
7. Wilcox CE, Bogenschutz MP, Nakazawa M, et al. Concordance between self-report and urine drug screen data in adolescent opioid dependent clinical trial participants. Addict Behav. 2013;38:2568-2574. https://doi.org/10.1016/j.addbeh.2013.05.015
8. Zanis DA, McLellan AT, Randall M. Can you trust patient self-reports of drug use during treatment? Drug Alcohol Depend. 1994;35:127-132. https://doi.org/10.1016/0376-8716(94)90119-8
9. Jones CM, Paulozzi LJ, Mack KA. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: United States, 2008-2011. JAMA Intern Med. 2014;174:802-803. https://doi.org/10.1001/jamainternmed.2013.12809
10. Katz N, Fanciullo GJ. Role of urine toxicology testing in the management of chronic opioid therapy. Clin J Pain. 2002;18(4 suppl):S76-S82. https://doi.org/10.1097/00002508-200207001-00009
11. Vowles KE, McEntee ML, Julnes PS, et al. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. 2015;156:569-576. https://doi.org/10.1097/01.j.pain.0000460357.01998.f1
12. Warner EA, Walker RM, Friedmann PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115:54-58. https://doi.org/10.1016/s0002-9343(03)00236-5
13. Kelly JF, Wakeman SE, Saitz R. Stop talking ‘dirty’: clinicians, language, and quality of care for the leading cause of preventable death in the United States. Am J Med. 2015;128:8-9. https://doi.org/10.1016/j.amjmed.2014.07.043
14. Jarvis M, Williams J, Hurford M, et al. Appropriate use of drug testing in clinical addiction medicine. J Addict Med. 2017;11:163-173. https://doi.org/10.1097/ADM.0000000000000323
15. Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19:97-117. https://doi.org/10.1093/pm/pnx285
16 Ainscough TS, McNeill A, Strang J, et al. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend. 2017;178:318-339. https://doi.org/10.1016/j.drugalcdep.2017.05.028
17. Blum K, Han D, Femino J, et al. Systematic evaluation of “compliance” to prescribed treatment medications and “abstinence” from psychoactive drug abuse in chemical dependence programs: data from the comprehensive analysis of reported drugs. PLoS One. 2014;9:e104275. https://doi.org/10.1371/journal.pone.0104275
18. Miller SC, Fiellin DA, Rosenthal RN, et al. The ASAM Principles of Addiction Medicine. 6th ed. Wolters Kluwer; 2018.
19. Saitman A, Park H-D, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38:387-396. https://doi.org/10.1093/jat/bku075
20. Smith MP, Bluth MH. Common interferences in drug testing. Clin Lab Med. 2016;36:663-671. https://doi.org/10.1016/j.cll.2016.07.006
21. George S, Braithwaite RA. An investigation into the extent of possible dilution of specimens received for urinary drugs of abuse screening. Addiction. 1995;90:967-970. https://doi.org/10.1046/j.1360-0443.1995.9079679.x
22. Beck O, Bohlin M, Bragd F, et al. Adulteration of urine drug testing—an exaggerated cause of concern. [Article in Swedish] Lakartidningen. 2000;97:703-706.
23. Kale N. Urine drug tests: ordering and interpreting results. Am Fam Physician. 2019;99:33-39.
24. Dasgupta A. The effects of adulterants and selected ingested compounds on drugs-of-abuse testing in urine. Am J Clin Pathol. 2007;128:491-503. https://doi.org/10.1309/FQY06F8XKTQPM149
25. Definition of addiction. American Society of Addiction Medicine Web site. Updated October 21, 2019. Accessed February 20, 2021. https://www.asam.org/resources/definition-of-addiction
26. Kroenke K, Alford DP, Argoff C, et al. Challenges with Implementing the Centers for Disease Control and Prevention Opioid Guideline: A Consensus Panel Report. Pain Med. 2019;20:724-735. https://doi.org/10.1093/pm/pny307
PRACTICE RECOMMENDATIONS
› Consider developing a risk-based urine drug testing protocol for all patients who are on chronic opioid therapy. C
› Consider urine drug testing to augment a thorough history when identifying and offering treatment to patients with a substance use disorder. A
› Do not change your management plan based on results of a single screening urine test. Revisit unexpected positive or negative results with a thorough history or confirmatory testing. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Disparities and racism in health care
From Anarcha Westcott to George Floyd to the Atlanta massacre
The Atlanta spa massacre, the commencement of the George Floyd trial, and COVID-19 highlight societal inequalities and health disparities among minority groups. We can only hope that we have arrived at the tipping point to address historical institutional racism and structural violence in this country.
Admittedly, we, as health care professionals, have been at best apathetic and at worst complicit with this tragedy. Dr. James Sims, the father of gynecology, perfected his surgical techniques of vaginal fistula on slaves. Starting in 1845, he performed over thirty surgeries without anesthesia on Anarcha Westcott.1 Moreover, the past century was dotted with similar transgressions such as the Tuskegee Untreated Syphilis Experiment from 1932 to 1972, the use of the cells of Henrietta Lack in 1951, and the disproportionate lack of funding of sickle cell research.2 We must move from complicit/apathetic to being part of the discourse and solution.
The juxtaposition of George Floyd’s cry of “I can’t breathe” and the disproportionate way in which COVID-19 has affected Black communities and people of color highlights how deeply entrenched the problem of systemic racism is in this country. The innumerable reported hate crimes against Asian Americans stemming from xenophobia linked to the COVID-19 pandemic and the stereotyping of Hispanic Americans as criminals during the last U.S. administration demonstrate that all minority racial/ethnic groups are affected. As clinicians who care for the health of our communities and strive to reduce suffering, we have a responsibility to identify discrimination that exists in the health care system – ranging from subtle implicit bias to overt discrimination.3
Unconscious bias and its effect on diversity and inclusion has only recently been recognized and addressed in the realm of health care as applied to clinicians. This is key to structural racism as providers inadvertently use unconscious bias every day to make their medical decisions quick and efficient. As Dayna Bowen Matthews points out in her book, “Just Medicine,” “where health and health care are concerned, even when implicit biases are based on seemingly benign distinctions, or supported by apparently rational or widely held observations, these biases can cause grave individual, group, and societal harm that is commensurate to and even exceeds the harm caused by outright racism.” To deny the prejudices that providers have when making decisions for patients will perpetuate the racism and hinder our ability to overcome health inequity. Americans of racial and ethnic minorities have a higher incidence of chronic diseases and premature death when compared to white Americans.4 These disparities exist even when controlling for individual variations such as availability of health insurance, education, and socioeconomic status.5 Social determinants of health because of racial differences is often talked about as a cause of health care inequity, but given the evidence that providers play a much more active role in this, we need to become more comfortable with the discomfort of using the word “racism” if we intend to bring awareness and create change.
In order to tackle structural racism in health care, organizations must take a multifaceted approach. Evidence-based strategies include: creation of an inclusive workforce, diversification of the workforce to better represent patient populations, and education/training on the effect of implicit bias on equitable health care.6 These aspirations can provide a framework for interventions at all levels of health care organizations.
The JEDI (justice, equity, diversity, and inclusion) committee of the section of hospital medicine at Wake Forest Baptist Health System came into existence in November 2019. The objective for JEDI was to use evidence-based methods to help create an environment that would lead to the creation of a diverse and inclusive hospital medicine group. Prior to establishing our committee, we interviewed providers from traditional minority groups who were part of our practice to bring clarity to the discrimination faced by our providers from colleagues, staff, and patients. The discrimination varied from microaggressions caused by implicit biases to macroaggression from overt discrimination. We initiated our work on this burning platform by following the evidence-based methods mentioned earlier.
Creation of an inclusive workforce. Our working committee included members of varied backgrounds and experiences who were passionate about enhancing equity while focusing on inclusion and wellness. The committee brainstormed ideas for interventions that could make a positive impact for our teammates. Individual providers voted to choose the interventions that would positively impact their inclusion and health. Using a validated survey,7 we were able to measure the degree of inclusion of our work group based on multiple demographics including age, gender, race/ethnicity, training (physician vs. APP), etc. Our intention is to complete the proposed interventions before remeasuring inclusion to understand the effect of our work.
Diversifying the workforce. Although our section of hospital medicine at Wake Forest Baptist Health System consists of providers self-identifying as people of color, we do not adequately mirror the racial composition of the population we serve. To achieve the desired result, we have made changes to our recruiting program. The section of hospital medicine visibly demonstrates our commitment to diversity and displays our values on our website. We intend for this to attract diverse individuals who would intend to be part of our group.
Education and training on impact of implicit bias on equitable health care. Implicit bias training will have to consist of actions that would help our clinicians recognize their own prejudices and find means to mitigate them. We have committed to bystander education that would give practice and words to our providers to speak up in situations where they see discrimination in the workplace that is directed against patients, staff, and colleagues. A series of open and honest conversations about racial and gender discrimination in health care that involves inviting accomplished speakers from around the country has been planned. Continued attention to opportunities to further awareness on this subject is vital.
On Jan. 6, 2021, a day that should have filled citizens with pride and hope with the election of the first Black minister and the first Jewish man to the U.S. Senate in a historically conservative state, as well as the confirmation of the election of a president who pledged to address racial disparities, we instead saw another stark reminder of where we came from and just how far we have to go. White supremacists incited by their perceived threat to a legacy of centuries of suppression transformed into a mob of insurrectionists, blatantly bearing Confederate and Nazi flags, and seemingly easily invaded and desecrated the U.S. Capitol. On March 16, 2021, a white male who was “having a bad day” ended the lives of eight individuals, including six Asian Americans.
These instances have brought forth the reality that many of our interventions have been directed towards subtle prejudices and microaggressions alone. We have skirted around calling out overt discrimination of minority groups and failed to openly acknowledge our own contribution to the problem. This newly found awareness has created an opportunity for more impactful work. The equitable delivery of health care is dependent on creating a patient-provider relationship based on trust; addressing overt discrimination respectfully; and overcoming unconscious bias.
While we have made the commitment to confront structural racism in our workplace and taken important steps to work towards this goal with the initiatives set forth by our JEDI committee, we certainly have a long way to go. George Floyd spent the last 8 minutes and 46 seconds of his life struggling to breathe and asking for his mother. Let’s not waste another second and instead be the change that we seek in health care.
Dr. Nagaraj is medical director, Hospital Medicine, at Lexington (N.C.) Medical Center, assistant professor at Wake Forest School of Medicine, and cochair, JEDI committee for diversity and inclusion, hospital medicine, at Wake Forest Baptist Health, Winston-Salem, NC. Ms. Haller is cochair, JEDI committee for diversity and inclusion, hospital medicine, Wake Forest Baptist Health. Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor at Wake Forest School of Medicine. The authors would like to acknowledge Dr. Julie Freischlag, Dr. Kevin High, and Dr. David McIntosh at Wake Forest Baptist Health System for the support of the JEDI committee and the section on hospital medicine.
References
1. Holland B. The “father of modern gynecology” performed shocking experiments on enslaved women. History. 2017 Aug 29. www.history.com/news/the-father-of-modern-gynecology-performed-shocking-experiments-on-slaves.
2. Buseh AG et al. Community leaders’ perspectives on engaging African Americans in biobanks and other human genetics initiatives. J Community Genet. 2013 Oct;4(4):483-94. doi: 10.1007/s12687-013-0155-z.
3. National Center for Health Statistics. Health, United States, 2015: With special feature on racial and ethnic health disparities. 2016 May. www.cdc.gov/nchs/data/hus/hus15.pdf.
4. Bailey ZD et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017 Apr 8;389(10077):1453-63. doi: 10.1016/S0140-6736(17)30569-X.
5. Arvizo C and Garrison E. Diversity and inclusion: the role of unconscious bias on patient care, health outcomes and the workforce in obstetrics and gynaecology. Curr Opin Obstet Gynecol. 2019 Oct;31(5):356-62. doi: 10.1097/GCO.0000000000000566.
6. Chung BG et al. Work group inclusion: test of a scale and model. Group & Organization Management. 2020;45(1):75-102. doi: 10.1177/1059601119839858.
From Anarcha Westcott to George Floyd to the Atlanta massacre
From Anarcha Westcott to George Floyd to the Atlanta massacre
The Atlanta spa massacre, the commencement of the George Floyd trial, and COVID-19 highlight societal inequalities and health disparities among minority groups. We can only hope that we have arrived at the tipping point to address historical institutional racism and structural violence in this country.
Admittedly, we, as health care professionals, have been at best apathetic and at worst complicit with this tragedy. Dr. James Sims, the father of gynecology, perfected his surgical techniques of vaginal fistula on slaves. Starting in 1845, he performed over thirty surgeries without anesthesia on Anarcha Westcott.1 Moreover, the past century was dotted with similar transgressions such as the Tuskegee Untreated Syphilis Experiment from 1932 to 1972, the use of the cells of Henrietta Lack in 1951, and the disproportionate lack of funding of sickle cell research.2 We must move from complicit/apathetic to being part of the discourse and solution.
The juxtaposition of George Floyd’s cry of “I can’t breathe” and the disproportionate way in which COVID-19 has affected Black communities and people of color highlights how deeply entrenched the problem of systemic racism is in this country. The innumerable reported hate crimes against Asian Americans stemming from xenophobia linked to the COVID-19 pandemic and the stereotyping of Hispanic Americans as criminals during the last U.S. administration demonstrate that all minority racial/ethnic groups are affected. As clinicians who care for the health of our communities and strive to reduce suffering, we have a responsibility to identify discrimination that exists in the health care system – ranging from subtle implicit bias to overt discrimination.3
Unconscious bias and its effect on diversity and inclusion has only recently been recognized and addressed in the realm of health care as applied to clinicians. This is key to structural racism as providers inadvertently use unconscious bias every day to make their medical decisions quick and efficient. As Dayna Bowen Matthews points out in her book, “Just Medicine,” “where health and health care are concerned, even when implicit biases are based on seemingly benign distinctions, or supported by apparently rational or widely held observations, these biases can cause grave individual, group, and societal harm that is commensurate to and even exceeds the harm caused by outright racism.” To deny the prejudices that providers have when making decisions for patients will perpetuate the racism and hinder our ability to overcome health inequity. Americans of racial and ethnic minorities have a higher incidence of chronic diseases and premature death when compared to white Americans.4 These disparities exist even when controlling for individual variations such as availability of health insurance, education, and socioeconomic status.5 Social determinants of health because of racial differences is often talked about as a cause of health care inequity, but given the evidence that providers play a much more active role in this, we need to become more comfortable with the discomfort of using the word “racism” if we intend to bring awareness and create change.
In order to tackle structural racism in health care, organizations must take a multifaceted approach. Evidence-based strategies include: creation of an inclusive workforce, diversification of the workforce to better represent patient populations, and education/training on the effect of implicit bias on equitable health care.6 These aspirations can provide a framework for interventions at all levels of health care organizations.
The JEDI (justice, equity, diversity, and inclusion) committee of the section of hospital medicine at Wake Forest Baptist Health System came into existence in November 2019. The objective for JEDI was to use evidence-based methods to help create an environment that would lead to the creation of a diverse and inclusive hospital medicine group. Prior to establishing our committee, we interviewed providers from traditional minority groups who were part of our practice to bring clarity to the discrimination faced by our providers from colleagues, staff, and patients. The discrimination varied from microaggressions caused by implicit biases to macroaggression from overt discrimination. We initiated our work on this burning platform by following the evidence-based methods mentioned earlier.
Creation of an inclusive workforce. Our working committee included members of varied backgrounds and experiences who were passionate about enhancing equity while focusing on inclusion and wellness. The committee brainstormed ideas for interventions that could make a positive impact for our teammates. Individual providers voted to choose the interventions that would positively impact their inclusion and health. Using a validated survey,7 we were able to measure the degree of inclusion of our work group based on multiple demographics including age, gender, race/ethnicity, training (physician vs. APP), etc. Our intention is to complete the proposed interventions before remeasuring inclusion to understand the effect of our work.
Diversifying the workforce. Although our section of hospital medicine at Wake Forest Baptist Health System consists of providers self-identifying as people of color, we do not adequately mirror the racial composition of the population we serve. To achieve the desired result, we have made changes to our recruiting program. The section of hospital medicine visibly demonstrates our commitment to diversity and displays our values on our website. We intend for this to attract diverse individuals who would intend to be part of our group.
Education and training on impact of implicit bias on equitable health care. Implicit bias training will have to consist of actions that would help our clinicians recognize their own prejudices and find means to mitigate them. We have committed to bystander education that would give practice and words to our providers to speak up in situations where they see discrimination in the workplace that is directed against patients, staff, and colleagues. A series of open and honest conversations about racial and gender discrimination in health care that involves inviting accomplished speakers from around the country has been planned. Continued attention to opportunities to further awareness on this subject is vital.
On Jan. 6, 2021, a day that should have filled citizens with pride and hope with the election of the first Black minister and the first Jewish man to the U.S. Senate in a historically conservative state, as well as the confirmation of the election of a president who pledged to address racial disparities, we instead saw another stark reminder of where we came from and just how far we have to go. White supremacists incited by their perceived threat to a legacy of centuries of suppression transformed into a mob of insurrectionists, blatantly bearing Confederate and Nazi flags, and seemingly easily invaded and desecrated the U.S. Capitol. On March 16, 2021, a white male who was “having a bad day” ended the lives of eight individuals, including six Asian Americans.
These instances have brought forth the reality that many of our interventions have been directed towards subtle prejudices and microaggressions alone. We have skirted around calling out overt discrimination of minority groups and failed to openly acknowledge our own contribution to the problem. This newly found awareness has created an opportunity for more impactful work. The equitable delivery of health care is dependent on creating a patient-provider relationship based on trust; addressing overt discrimination respectfully; and overcoming unconscious bias.
While we have made the commitment to confront structural racism in our workplace and taken important steps to work towards this goal with the initiatives set forth by our JEDI committee, we certainly have a long way to go. George Floyd spent the last 8 minutes and 46 seconds of his life struggling to breathe and asking for his mother. Let’s not waste another second and instead be the change that we seek in health care.
Dr. Nagaraj is medical director, Hospital Medicine, at Lexington (N.C.) Medical Center, assistant professor at Wake Forest School of Medicine, and cochair, JEDI committee for diversity and inclusion, hospital medicine, at Wake Forest Baptist Health, Winston-Salem, NC. Ms. Haller is cochair, JEDI committee for diversity and inclusion, hospital medicine, Wake Forest Baptist Health. Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor at Wake Forest School of Medicine. The authors would like to acknowledge Dr. Julie Freischlag, Dr. Kevin High, and Dr. David McIntosh at Wake Forest Baptist Health System for the support of the JEDI committee and the section on hospital medicine.
References
1. Holland B. The “father of modern gynecology” performed shocking experiments on enslaved women. History. 2017 Aug 29. www.history.com/news/the-father-of-modern-gynecology-performed-shocking-experiments-on-slaves.
2. Buseh AG et al. Community leaders’ perspectives on engaging African Americans in biobanks and other human genetics initiatives. J Community Genet. 2013 Oct;4(4):483-94. doi: 10.1007/s12687-013-0155-z.
3. National Center for Health Statistics. Health, United States, 2015: With special feature on racial and ethnic health disparities. 2016 May. www.cdc.gov/nchs/data/hus/hus15.pdf.
4. Bailey ZD et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017 Apr 8;389(10077):1453-63. doi: 10.1016/S0140-6736(17)30569-X.
5. Arvizo C and Garrison E. Diversity and inclusion: the role of unconscious bias on patient care, health outcomes and the workforce in obstetrics and gynaecology. Curr Opin Obstet Gynecol. 2019 Oct;31(5):356-62. doi: 10.1097/GCO.0000000000000566.
6. Chung BG et al. Work group inclusion: test of a scale and model. Group & Organization Management. 2020;45(1):75-102. doi: 10.1177/1059601119839858.
The Atlanta spa massacre, the commencement of the George Floyd trial, and COVID-19 highlight societal inequalities and health disparities among minority groups. We can only hope that we have arrived at the tipping point to address historical institutional racism and structural violence in this country.
Admittedly, we, as health care professionals, have been at best apathetic and at worst complicit with this tragedy. Dr. James Sims, the father of gynecology, perfected his surgical techniques of vaginal fistula on slaves. Starting in 1845, he performed over thirty surgeries without anesthesia on Anarcha Westcott.1 Moreover, the past century was dotted with similar transgressions such as the Tuskegee Untreated Syphilis Experiment from 1932 to 1972, the use of the cells of Henrietta Lack in 1951, and the disproportionate lack of funding of sickle cell research.2 We must move from complicit/apathetic to being part of the discourse and solution.
The juxtaposition of George Floyd’s cry of “I can’t breathe” and the disproportionate way in which COVID-19 has affected Black communities and people of color highlights how deeply entrenched the problem of systemic racism is in this country. The innumerable reported hate crimes against Asian Americans stemming from xenophobia linked to the COVID-19 pandemic and the stereotyping of Hispanic Americans as criminals during the last U.S. administration demonstrate that all minority racial/ethnic groups are affected. As clinicians who care for the health of our communities and strive to reduce suffering, we have a responsibility to identify discrimination that exists in the health care system – ranging from subtle implicit bias to overt discrimination.3
Unconscious bias and its effect on diversity and inclusion has only recently been recognized and addressed in the realm of health care as applied to clinicians. This is key to structural racism as providers inadvertently use unconscious bias every day to make their medical decisions quick and efficient. As Dayna Bowen Matthews points out in her book, “Just Medicine,” “where health and health care are concerned, even when implicit biases are based on seemingly benign distinctions, or supported by apparently rational or widely held observations, these biases can cause grave individual, group, and societal harm that is commensurate to and even exceeds the harm caused by outright racism.” To deny the prejudices that providers have when making decisions for patients will perpetuate the racism and hinder our ability to overcome health inequity. Americans of racial and ethnic minorities have a higher incidence of chronic diseases and premature death when compared to white Americans.4 These disparities exist even when controlling for individual variations such as availability of health insurance, education, and socioeconomic status.5 Social determinants of health because of racial differences is often talked about as a cause of health care inequity, but given the evidence that providers play a much more active role in this, we need to become more comfortable with the discomfort of using the word “racism” if we intend to bring awareness and create change.
In order to tackle structural racism in health care, organizations must take a multifaceted approach. Evidence-based strategies include: creation of an inclusive workforce, diversification of the workforce to better represent patient populations, and education/training on the effect of implicit bias on equitable health care.6 These aspirations can provide a framework for interventions at all levels of health care organizations.
The JEDI (justice, equity, diversity, and inclusion) committee of the section of hospital medicine at Wake Forest Baptist Health System came into existence in November 2019. The objective for JEDI was to use evidence-based methods to help create an environment that would lead to the creation of a diverse and inclusive hospital medicine group. Prior to establishing our committee, we interviewed providers from traditional minority groups who were part of our practice to bring clarity to the discrimination faced by our providers from colleagues, staff, and patients. The discrimination varied from microaggressions caused by implicit biases to macroaggression from overt discrimination. We initiated our work on this burning platform by following the evidence-based methods mentioned earlier.
Creation of an inclusive workforce. Our working committee included members of varied backgrounds and experiences who were passionate about enhancing equity while focusing on inclusion and wellness. The committee brainstormed ideas for interventions that could make a positive impact for our teammates. Individual providers voted to choose the interventions that would positively impact their inclusion and health. Using a validated survey,7 we were able to measure the degree of inclusion of our work group based on multiple demographics including age, gender, race/ethnicity, training (physician vs. APP), etc. Our intention is to complete the proposed interventions before remeasuring inclusion to understand the effect of our work.
Diversifying the workforce. Although our section of hospital medicine at Wake Forest Baptist Health System consists of providers self-identifying as people of color, we do not adequately mirror the racial composition of the population we serve. To achieve the desired result, we have made changes to our recruiting program. The section of hospital medicine visibly demonstrates our commitment to diversity and displays our values on our website. We intend for this to attract diverse individuals who would intend to be part of our group.
Education and training on impact of implicit bias on equitable health care. Implicit bias training will have to consist of actions that would help our clinicians recognize their own prejudices and find means to mitigate them. We have committed to bystander education that would give practice and words to our providers to speak up in situations where they see discrimination in the workplace that is directed against patients, staff, and colleagues. A series of open and honest conversations about racial and gender discrimination in health care that involves inviting accomplished speakers from around the country has been planned. Continued attention to opportunities to further awareness on this subject is vital.
On Jan. 6, 2021, a day that should have filled citizens with pride and hope with the election of the first Black minister and the first Jewish man to the U.S. Senate in a historically conservative state, as well as the confirmation of the election of a president who pledged to address racial disparities, we instead saw another stark reminder of where we came from and just how far we have to go. White supremacists incited by their perceived threat to a legacy of centuries of suppression transformed into a mob of insurrectionists, blatantly bearing Confederate and Nazi flags, and seemingly easily invaded and desecrated the U.S. Capitol. On March 16, 2021, a white male who was “having a bad day” ended the lives of eight individuals, including six Asian Americans.
These instances have brought forth the reality that many of our interventions have been directed towards subtle prejudices and microaggressions alone. We have skirted around calling out overt discrimination of minority groups and failed to openly acknowledge our own contribution to the problem. This newly found awareness has created an opportunity for more impactful work. The equitable delivery of health care is dependent on creating a patient-provider relationship based on trust; addressing overt discrimination respectfully; and overcoming unconscious bias.
While we have made the commitment to confront structural racism in our workplace and taken important steps to work towards this goal with the initiatives set forth by our JEDI committee, we certainly have a long way to go. George Floyd spent the last 8 minutes and 46 seconds of his life struggling to breathe and asking for his mother. Let’s not waste another second and instead be the change that we seek in health care.
Dr. Nagaraj is medical director, Hospital Medicine, at Lexington (N.C.) Medical Center, assistant professor at Wake Forest School of Medicine, and cochair, JEDI committee for diversity and inclusion, hospital medicine, at Wake Forest Baptist Health, Winston-Salem, NC. Ms. Haller is cochair, JEDI committee for diversity and inclusion, hospital medicine, Wake Forest Baptist Health. Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor at Wake Forest School of Medicine. The authors would like to acknowledge Dr. Julie Freischlag, Dr. Kevin High, and Dr. David McIntosh at Wake Forest Baptist Health System for the support of the JEDI committee and the section on hospital medicine.
References
1. Holland B. The “father of modern gynecology” performed shocking experiments on enslaved women. History. 2017 Aug 29. www.history.com/news/the-father-of-modern-gynecology-performed-shocking-experiments-on-slaves.
2. Buseh AG et al. Community leaders’ perspectives on engaging African Americans in biobanks and other human genetics initiatives. J Community Genet. 2013 Oct;4(4):483-94. doi: 10.1007/s12687-013-0155-z.
3. National Center for Health Statistics. Health, United States, 2015: With special feature on racial and ethnic health disparities. 2016 May. www.cdc.gov/nchs/data/hus/hus15.pdf.
4. Bailey ZD et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017 Apr 8;389(10077):1453-63. doi: 10.1016/S0140-6736(17)30569-X.
5. Arvizo C and Garrison E. Diversity and inclusion: the role of unconscious bias on patient care, health outcomes and the workforce in obstetrics and gynaecology. Curr Opin Obstet Gynecol. 2019 Oct;31(5):356-62. doi: 10.1097/GCO.0000000000000566.
6. Chung BG et al. Work group inclusion: test of a scale and model. Group & Organization Management. 2020;45(1):75-102. doi: 10.1177/1059601119839858.
Cardiovascular risks elevated in transgender youth
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiovascular and metabolic risk factors are increased among transgender youths, compared with youths who are not transgender. Elevations in lipid levels and body mass index (BMI) also occur in adult transgender patients, new research shows.
“This is the first study of its size in the United States of which we are aware that looks at the odds of youth with a diagnosis of gender dysphoria having medical diagnoses that relate to overall metabolic and cardiovascular health,” first author Anna Valentine, MD, of Children’s Hospital Colorado, Aurora, said in a press statement.
Although previous studies have shown that among transgender adults, BMI is higher and there is an increased risk for cardiovascular events, such as stroke or heart attack, compared with nontransgender people, research on adolescent transgender patients has been lacking.
With a recent survey showing that nearly 2% of adolescents identify as transgender, interest in health outcomes among younger patients is high.
To investigate, Dr. Valentine, and colleagues evaluated data from the PEDSnet pediatric database on 4,177 youths who had received a diagnosis of gender dysphoria. The participants had been enrolled at six sites from 2009 to 2019. The researchers compared these patients in a ratio of 1:4 with 16,664 control persons who had not been diagnosed with gender dysphoria. They reported their findings as a poster at the annual meeting of the Endocrine Society.
For the propensity-score analysis, participants were matched according to year of birth, age at last visit, site, race, ethnicity, insurance status, and duration in the database.
In both the transgender and control groups, about 66% were female at birth, 73% were White, and 9% Hispanic. For both groups, the average age was 16.2 years at the last visit. The average duration in the database was 7 years.
Study didn’t distinguish between those receiving and those not receiving gender-affirming hormones
In the retrospective study, among those who identified as transgender, the rates of diagnoses of dyslipidemia (odds ratio, 1.6; P < .0001) and metabolic syndrome (OR, 1.9; P = .0086) were significantly higher, compared with those without gender dysphoria.
Among the transgender male patients (born female) but not transgender female patients (born male), rates of diagnoses of overweight/obesity (OR, 1.7; P < .0001) and polycystic ovary syndrome were higher (OR, 1.9, P = .0006), compared with controls.
Gender-affirming hormone therapy, such as with testosterone or estradiol, is among the suspected culprits for the cardiovascular effects. However, importantly, this study did not differentiate between patients who had received estradiol or testosterone for gender affirmation and those who had not, Dr. Valentine said.
“We don’t know [whether gender-affirming hormone therapy is a cause], as we have not looked at this yet,” she said in an interview. “We are looking at that in our next analyses and will be including that in our future publication.
“We’ll also be looking at the relationship between having overweight/obesity and the other diagnoses that influence cardiovascular health (high blood pressure, liver dysfunction, and abnormal cholesterol), as that could certainly be playing a role as well,” she said.
For many transgender patients, gender-affirming hormone therapy is lifelong. One question that needs to be evaluated concerns whether the dose of such therapy has a role on cardiovascular effects and if so, whether adjustments could be made without compromising the therapeutic effect, Dr. Valentine noted.
“This is an important question, and future research is needed to evaluate whether doses [of gender-affirming hormones] are related to cardiometabolic outcomes,” she said.
Potential confounders in the study include the fact that rates of overweight and obesity are higher among youths with gender dysphoria. This can in itself can increase the risk for other disorders, Dr. Valentine noted.
Furthermore, rates of mental health comorbidities are higher among youths with gender dysphoria. One consequence of this may be that they engage in less physical activity, she said.
Hormone therapy, health care disparities, or both could explain risk
In commenting on the study, Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery, the Mount Sinai Health System, New York, said that although similar cardiovascular effects are known to occur in transgender adults as well, they may or may not be hormone related. Other factors can increase the risk.
“With transgender adults, any differences in lipids or cardiac risk factors relative to cisgender people might be attributable either to hormone therapy or to health care disparities,” he said in an interview.
“The data are mixed. It may be that most differences relate to lack of access to care and to mistreatment by society,” he said. “Even studies that focus on hormones see a worsened situation for trans women versus trans men.”
Other recent research that shows potential cardiovascular effects among adult transgender men includes a study of more than 1,000 transgender men (born female) who received testosterone. That study, which was also presented at the ENDO meeting and was reported by this news organization, found an increased risk for high hematocrit levels, which could lead to a thrombotic event.
However, a study published in Pediatrics, which was also reported by this news organization, that included 611 transgender youths who had taken gender-affirming hormone therapy for more than a year found no increased risk for thrombosis, even in the presence of thrombosis risk factors, including obesity, tobacco use, and family history of thrombosis. However, the senior author of that study pointed out that the duration of follow-up in that study was relatively short, which may have been why they did not find an increased risk for thrombosis.
Dr. Safer noted that transgender youths and adults alike face a host of cultural factors that could play a role in increased cardiovascular risks.
“For adults, the major candidate explanations for worse BMI and cardiac risk factors are societal mistreatment, and for trans women specifically, progestins. For youth, the major candidate explanations are societal mistreatment and lack of access to athletics,” he said.
The authors and Dr. Safer disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Black inpatients at higher risk of poor safety outcomes
One expert says these findings should be a call to action for hospitals and physicians.
The Urban Institute, which is funded in part by the Robert Wood Johnson Foundation, looked at differences in Black and White patient safety measures among adults receiving inpatient care in 26 states.
Care quality was measured by the rate of preventable adverse hospital patient safety events per 1,000 at-risk discharges using data from the Agency for Healthcare Research and Quality (AHRQ).
Researchers compared experience by race on 11 patient safety indicators – four related to general patient safety, and seven linked to risk of adverse events with surgical procedures.
Surgical risk differences significant
The gaps were widest surrounding surgical care. Black patients were 7.9 percentage points more likely to be in a hospital considered low quality across all surgical safety measures. They were 4.9 percentage points more likely to be admitted to a hospital considered low quality across all general safety indicators.
“If you’re a Black patient getting surgery – relative to a White patient – in my study, you were 25% less likely to be in a hospital that prevented hemorrhage during surgery; you were 26% less likely to be in a hospital that prevented postoperative respiratory failure; and you were more than 30% less likely to be in a hospital that is effective in preventing postoperative sepsis,” Anuj Gangopadhyaya, PhD, senior research associate at the Urban Institute, said in an interview.
According to the report, Black patients were also 31.9% less likely than were White patients to be admitted into hospitals considered high quality in preventing pressure ulcers and 22.8% less likely to be in a hospital good at preventing iatrogenic pneumothorax.
Dr. Gangopadhyaya said this may be the first study to compare the numbers after the inception of the Affordable Care Act. These data were collected in 2017, 3 years after the core elements of the ACA kicked in.
He said that although the ACA has done much to narrow the racial gap in terms of insurance coverage, it has not been effective in reducing the heightened safety risk to Black patients in the hospital.
‘Shocking, though not surprising’
Uché Blackstock, MD, founder and CEO of Advancing Health Equity in New York City, called the findings “shocking, though not surprising.”
Though these data were collected before COVID-19, the pandemic has exposed profound racial inequities, she noted.
She cited the example of Susan Moore, MD, a Black physician in Carmel, Ind., who died from COVID-19 at age 52 in December after experiencing what she said was systemic racism in her care.
“We saw in the death of Dr. Susan Moore that even having a formal education and being a physician is not protective for Black patients. These findings only reaffirm what we already know – that Black patients receive worse and lower-quality care than White patients,” Dr. Blackstock said in an interview.
“These findings are not a result of Black patients’ individual choices as is often suggested, but rather the results of a health care system that has devalued the lives of Black patients and inherently provides poorer quality of care to them.”
Dr. Blackstock said this report represents a call to action.
Health care institutions must, she said, “look inward at the intentional and critical antiracism work that must be done on provider, organizational, and systems levels by allocating the necessary resources, continuing to track disaggregated health metrics, and committing to structural change within health care systems.”
Resources instead of penalties?
Dr. Gangopadhyaya said the second phase of the research will compare safety outcomes between Black and White patients in the same hospital. Those results will shed more light on what’s driving the differences in risk on safety measures.
He acknowledged that, particularly in an emergency, there is little choice involved with which hospital a patient enters. Patients typically go to a hospital in their neighborhood. And it’s well established that ZIP codes can determine health care outcomes.
But he suspects the differences cannot be explained simply by socioeconomic factors.
He pointed out that previous research has found disparities among Black and White patients in the same neighborhoods.
In one part of this study, researchers narrowed the comparison to Black and White adults with Medicare coverage, with similar provider networks and reimbursement structure, to test whether insurance was playing a significant role.
“Even among that group, you still see the persistent differences in the safety risks driven by the hospitals patients are admitted to,” Dr. Gangopadhyaya said.
He suggests two policy approaches to address the gaps: Either find ways for high-quality hospitals to reach more people of color, or find out what’s keeping the low-quality hospitals from implementing the practices that are effective in high-quality hospitals.
Currently, the ACA has penalties in place when hospitals score low for specific safety risks, he noted, saying that approach doesn’t appear to be working.
“Perhaps instead of penalizing hospitals, we might want to consider providing resources to hospitals that help them better adopt the successful protocols in their high-quality counterparts,” he said.
Dr. Gangopadhyaya has disclosed no relevant financial relationships. Dr. Blackstock has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This article was updated 4/2/21.
One expert says these findings should be a call to action for hospitals and physicians.
The Urban Institute, which is funded in part by the Robert Wood Johnson Foundation, looked at differences in Black and White patient safety measures among adults receiving inpatient care in 26 states.
Care quality was measured by the rate of preventable adverse hospital patient safety events per 1,000 at-risk discharges using data from the Agency for Healthcare Research and Quality (AHRQ).
Researchers compared experience by race on 11 patient safety indicators – four related to general patient safety, and seven linked to risk of adverse events with surgical procedures.
Surgical risk differences significant
The gaps were widest surrounding surgical care. Black patients were 7.9 percentage points more likely to be in a hospital considered low quality across all surgical safety measures. They were 4.9 percentage points more likely to be admitted to a hospital considered low quality across all general safety indicators.
“If you’re a Black patient getting surgery – relative to a White patient – in my study, you were 25% less likely to be in a hospital that prevented hemorrhage during surgery; you were 26% less likely to be in a hospital that prevented postoperative respiratory failure; and you were more than 30% less likely to be in a hospital that is effective in preventing postoperative sepsis,” Anuj Gangopadhyaya, PhD, senior research associate at the Urban Institute, said in an interview.
According to the report, Black patients were also 31.9% less likely than were White patients to be admitted into hospitals considered high quality in preventing pressure ulcers and 22.8% less likely to be in a hospital good at preventing iatrogenic pneumothorax.
Dr. Gangopadhyaya said this may be the first study to compare the numbers after the inception of the Affordable Care Act. These data were collected in 2017, 3 years after the core elements of the ACA kicked in.
He said that although the ACA has done much to narrow the racial gap in terms of insurance coverage, it has not been effective in reducing the heightened safety risk to Black patients in the hospital.
‘Shocking, though not surprising’
Uché Blackstock, MD, founder and CEO of Advancing Health Equity in New York City, called the findings “shocking, though not surprising.”
Though these data were collected before COVID-19, the pandemic has exposed profound racial inequities, she noted.
She cited the example of Susan Moore, MD, a Black physician in Carmel, Ind., who died from COVID-19 at age 52 in December after experiencing what she said was systemic racism in her care.
“We saw in the death of Dr. Susan Moore that even having a formal education and being a physician is not protective for Black patients. These findings only reaffirm what we already know – that Black patients receive worse and lower-quality care than White patients,” Dr. Blackstock said in an interview.
“These findings are not a result of Black patients’ individual choices as is often suggested, but rather the results of a health care system that has devalued the lives of Black patients and inherently provides poorer quality of care to them.”
Dr. Blackstock said this report represents a call to action.
Health care institutions must, she said, “look inward at the intentional and critical antiracism work that must be done on provider, organizational, and systems levels by allocating the necessary resources, continuing to track disaggregated health metrics, and committing to structural change within health care systems.”
Resources instead of penalties?
Dr. Gangopadhyaya said the second phase of the research will compare safety outcomes between Black and White patients in the same hospital. Those results will shed more light on what’s driving the differences in risk on safety measures.
He acknowledged that, particularly in an emergency, there is little choice involved with which hospital a patient enters. Patients typically go to a hospital in their neighborhood. And it’s well established that ZIP codes can determine health care outcomes.
But he suspects the differences cannot be explained simply by socioeconomic factors.
He pointed out that previous research has found disparities among Black and White patients in the same neighborhoods.
In one part of this study, researchers narrowed the comparison to Black and White adults with Medicare coverage, with similar provider networks and reimbursement structure, to test whether insurance was playing a significant role.
“Even among that group, you still see the persistent differences in the safety risks driven by the hospitals patients are admitted to,” Dr. Gangopadhyaya said.
He suggests two policy approaches to address the gaps: Either find ways for high-quality hospitals to reach more people of color, or find out what’s keeping the low-quality hospitals from implementing the practices that are effective in high-quality hospitals.
Currently, the ACA has penalties in place when hospitals score low for specific safety risks, he noted, saying that approach doesn’t appear to be working.
“Perhaps instead of penalizing hospitals, we might want to consider providing resources to hospitals that help them better adopt the successful protocols in their high-quality counterparts,” he said.
Dr. Gangopadhyaya has disclosed no relevant financial relationships. Dr. Blackstock has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This article was updated 4/2/21.
One expert says these findings should be a call to action for hospitals and physicians.
The Urban Institute, which is funded in part by the Robert Wood Johnson Foundation, looked at differences in Black and White patient safety measures among adults receiving inpatient care in 26 states.
Care quality was measured by the rate of preventable adverse hospital patient safety events per 1,000 at-risk discharges using data from the Agency for Healthcare Research and Quality (AHRQ).
Researchers compared experience by race on 11 patient safety indicators – four related to general patient safety, and seven linked to risk of adverse events with surgical procedures.
Surgical risk differences significant
The gaps were widest surrounding surgical care. Black patients were 7.9 percentage points more likely to be in a hospital considered low quality across all surgical safety measures. They were 4.9 percentage points more likely to be admitted to a hospital considered low quality across all general safety indicators.
“If you’re a Black patient getting surgery – relative to a White patient – in my study, you were 25% less likely to be in a hospital that prevented hemorrhage during surgery; you were 26% less likely to be in a hospital that prevented postoperative respiratory failure; and you were more than 30% less likely to be in a hospital that is effective in preventing postoperative sepsis,” Anuj Gangopadhyaya, PhD, senior research associate at the Urban Institute, said in an interview.
According to the report, Black patients were also 31.9% less likely than were White patients to be admitted into hospitals considered high quality in preventing pressure ulcers and 22.8% less likely to be in a hospital good at preventing iatrogenic pneumothorax.
Dr. Gangopadhyaya said this may be the first study to compare the numbers after the inception of the Affordable Care Act. These data were collected in 2017, 3 years after the core elements of the ACA kicked in.
He said that although the ACA has done much to narrow the racial gap in terms of insurance coverage, it has not been effective in reducing the heightened safety risk to Black patients in the hospital.
‘Shocking, though not surprising’
Uché Blackstock, MD, founder and CEO of Advancing Health Equity in New York City, called the findings “shocking, though not surprising.”
Though these data were collected before COVID-19, the pandemic has exposed profound racial inequities, she noted.
She cited the example of Susan Moore, MD, a Black physician in Carmel, Ind., who died from COVID-19 at age 52 in December after experiencing what she said was systemic racism in her care.
“We saw in the death of Dr. Susan Moore that even having a formal education and being a physician is not protective for Black patients. These findings only reaffirm what we already know – that Black patients receive worse and lower-quality care than White patients,” Dr. Blackstock said in an interview.
“These findings are not a result of Black patients’ individual choices as is often suggested, but rather the results of a health care system that has devalued the lives of Black patients and inherently provides poorer quality of care to them.”
Dr. Blackstock said this report represents a call to action.
Health care institutions must, she said, “look inward at the intentional and critical antiracism work that must be done on provider, organizational, and systems levels by allocating the necessary resources, continuing to track disaggregated health metrics, and committing to structural change within health care systems.”
Resources instead of penalties?
Dr. Gangopadhyaya said the second phase of the research will compare safety outcomes between Black and White patients in the same hospital. Those results will shed more light on what’s driving the differences in risk on safety measures.
He acknowledged that, particularly in an emergency, there is little choice involved with which hospital a patient enters. Patients typically go to a hospital in their neighborhood. And it’s well established that ZIP codes can determine health care outcomes.
But he suspects the differences cannot be explained simply by socioeconomic factors.
He pointed out that previous research has found disparities among Black and White patients in the same neighborhoods.
In one part of this study, researchers narrowed the comparison to Black and White adults with Medicare coverage, with similar provider networks and reimbursement structure, to test whether insurance was playing a significant role.
“Even among that group, you still see the persistent differences in the safety risks driven by the hospitals patients are admitted to,” Dr. Gangopadhyaya said.
He suggests two policy approaches to address the gaps: Either find ways for high-quality hospitals to reach more people of color, or find out what’s keeping the low-quality hospitals from implementing the practices that are effective in high-quality hospitals.
Currently, the ACA has penalties in place when hospitals score low for specific safety risks, he noted, saying that approach doesn’t appear to be working.
“Perhaps instead of penalizing hospitals, we might want to consider providing resources to hospitals that help them better adopt the successful protocols in their high-quality counterparts,” he said.
Dr. Gangopadhyaya has disclosed no relevant financial relationships. Dr. Blackstock has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This article was updated 4/2/21.
Many unknowns on fertility preservation in transgender patients
Unknowns around the long-term effects of gender-affirming hormonal treatment on fertility in transgender individuals, especially adolescents, and what this means for fertility preservation, should be red flags for clinicians, according to one expert addressing the issue at the recent virtual ENDO 2021 meeting.
“One of the main concerns regarding fertility preservation in this population is that the decision to seek gender-affirming therapy is often made early in the reproductive lifespan, and for many patients this is well before the consideration of … child-bearing,” remarked Marie Menke, MD, an ob/gyn from University of Michigan, Ann Arbor, presenting in a session dedicated to state-of-the-art approaches to gamete preservation.
“These patients need to consider simultaneously their desire for gender-affirming therapy and their desire for child-bearing,” she added, explaining that gender-affirming therapy typically requires suppression of the hormonal axis that supports reproduction.
“This level of shared decision-making requires time and multidisciplinary involvement in the face of … limited data, and even with the best of counseling it can be quite overwhelming,” Dr. Menke stressed.
Specifically, the effects of gender-affirming therapy on both fertility and fertility preservation options in transgender individuals in comparison to the general population are areas that require much more research, she emphasized.
On the topic of adolescents specifically, she said they are “a special population,” as many seeking medical therapy for gender dysphoria have never considered long-term fertility goals or desires. Reports of such discussions during pediatric gender care vary greatly depending on the age of the patient and their geographic location.
And where such conversations have happened, “often there is no recollection by patients of such discussion prior to referral to endocrinology,” she emphasized.
Session co-moderator Irene Su, MD, a reproductive endocrinologist at the University of California, San Diego, said shared decisions with patients have to be made every day, even though data are limited.
“Little is known about both the adverse medical impact of gender-affirming hormonal therapy on fertility potential, as well as the psychosocial impact of interrupting/reversing gender-affirming hormonal therapy in the future to attempt fertility,” she told this news organization.
However, “because there are reasons to be concerned about an adverse impact on fertility, transgender individuals need access to fertility risk and preservation counseling,” she stressed.
Dr. Su has a special interest in improving reproductive health in young cancer survivors, and this involves similar discussions around fertility preservation – a medical subspecialty known as “oncofertility.”
There is a greater pool of knowledge in this field compared with fertility preservation and family planning in transgender patients, Dr. Su noted.
“While we need similar data in transgender individuals, what we’ve learned from the cancer survivor population is that they and their families want to know about known and unknown fertility risks and options, even if they ultimately do not choose to undertake fertility preservation procedures,” she explains.
Desire for future kids, but <10% currently preserve fertility
Dr. Menke said the estimated prevalence of individuals who identify as transgender is around 0.7% of the U.S. population, and she observed that, “by and large, fertility management involves tissue cryopreservation.”
She presented survey data showing that between 33%-54% of transgender and nonbinary individuals report a desire to have biological children currently, or in the future, and 94.6% are also strongly in support of transgender people having access to fertility preservation procedures.
Likewise, an online cross-sectional survey of over 1,100 people in the general population found that 76.2% agree that transgender individuals should be offered fertility preservation, and 60% support fertility preservation in minors.
Multiple professional societies support counseling in regard to options for fertility preservation and recommend that it should be offered to transgender individuals.
The American Society for Reproductive Medicine (ASRM), the American College of Obstetricians and Gynecologists (ACOG), the World Professional Association for Transgender Health (WPATH), and the Endocrine Society all advocate that individuals seeking gender-affirming medical treatment should receive multidisciplinary counseling regarding fertility preservation prior to puberty suppression in adolescents, and prior to cross-sex hormone treatment in both adolescents and adults.
But despite all of these recommendations and the survey findings, fertility preservation rates in transgender patients are low, “at less than 10%,” reported Dr. Menke.
Fertility preservation counseling and management ideally needs to begin prior to initiation of hormone therapy, stressed Dr. Menke.
Given the limited data on the long-term effects of gender-affirming therapy on fertility and its preservation, such counseling often leads to a myriad of questions, she further explained.
“Patients ask ‘What are the chances of having biological children if I don’t pursue fertility preservation?’, and ‘How likely am I to have a biological child if I do pursue fertility preservation?’, as well as issues around access to care, with patients asking, ‘Will I be able to pursue this option [of fertility preservation]?’”
“The chance of having a biological child if fertility preservation is pursued is similar to those [patients with cancer] who receive ‘oncofertility’ care, which has a good prognosis,” she explained.
However, issues around access to care, and the cost of it, can be barriers.
What does a transgender male, born female, need to do?
For transgender males, options for fertility preservation include the recommended option of cryopreservation of the eggs (oocytes), although freezing of embryos and/or ovarian tissue are also possible.
The latter would be required in a prepubertal individual if they wanted to start puberty blockers and then go straight onto cross-sex hormones, Dr. Menke noted, although she said it’s not definitively known if prepubertal ovarian tissue is capable of being stimulated in the future to produce viable mature oocytes.
In someone who has gone through puberty, the ideal time to freeze eggs is before beginning gender-affirming hormone therapy, Dr. Menke explained. This is because it is not known whether testosterone has any adverse impact on oocyte development.
“We just don’t have definitive data that long-term testosterone isn’t gonadotoxic,” she said in response to a question about this after her talk.
Assessment of the reproductive consequences of gender-affirming therapy in transgender males can also be complicated by coexisting conditions, Dr. Menke explained.
For example, up to 58% of transgender males have polycystic ovary syndrome (PCOS) prior to transitioning, she noted. PCOS itself, and/or the gender-affirming therapy, may cause histologic changes of the ovarian tissue – for example, hyperplasia of ovarian stroma – and it’s not yet known to what extent this may impact future fertility, if present, she noted.
For oocyte preservation in female-to-male transgender individuals, stimulation with gonadotropins for 2-3 weeks is needed, and the procedure is invasive, requiring repeated vaginal ultrasounds. During this period, estradiol levels are supraphysiologic, and there is potential for breast development and vaginal bleeding post-retrieval, which individuals will need to be counseled about, Dr. Menke noted.
The cost of this also needs to be factored into the equation. Depending on insurance coverage, costs may be covered – and where there is no precedent, individuals can try referring their insurance companies to the ‘oncofertility consortium access-to-care model’, Dr. Menke advised.
If there is no coverage, the average cost for one egg-freezing cycle ranges from $10,000-$17,000 in the U.S., and often two to three cycles are needed to generate sufficient oocytes to be sure of a pregnancy. In addition, there are storage costs. Plus, there will be the cost of any future intervention to achieve a pregnancy, she stressed.
How long frozen oocytes remain viable is also still a matter of scientific debate, although “as the technology changes from slow-freeze to vitrification,” this time period should lengthen, Dr. Menke said.
In transgender males who have not preserved oocytes or embryos prior to transitioning, it’s necessary to stop testosterone to have the best chance of harvesting viable gametes, Dr. Menke said. Furthermore, individuals undertaking this procedure need to take into account all of the above-mentioned side effects of egg harvesting.
Although there have been reports of successful pregnancies with eggs retrieved from transgender males who have temporarily stopped testosterone, fertilization and embryo development following discontinuation of testosterone still require “additional investigation,” she observed.
Furthermore, “there are case reports of oocyte stimulation and retrieval of mature oocytes while patients continue testosterone therapy, and this may be an option in the future,” she noted, again stressing that it’s not known if excess testosterone is gonadotoxic.
Other options for fertility preservation in the transgender male include embryo cryopreservation, but this still involves hormonal stimulation and invasive procedures and would require the use of a sperm donor in a person who doesn’t currently have a partner (or who has one, but not necessarily one with whom they want to create a child).
For transgender males there is also the possibility of using a surrogate mother for the pregnancy, she noted.
What about transgender women, assigned male at birth?
For those assigned male at birth who wish to take puberty blockers, fertility preservation would require cryopreservation of testicular tissue, although Dr. Menke stressed that this is still considered “experimental.”
In the postpubertal period, the simplest option is to cryopreserve semen, with this ideally being performed prior to the individual commencing gender-affirming hormone therapy, Dr. Menke said.
If this is not done prior to beginning hormonal treatment, estrogen will need to be discontinued for fertility preservation, she noted.
Return of sperm function following cessation of estrogen may be limited – “expect at least 3 months before return of reproductive function,” Dr. Menke said. And even this may not be sufficient to restore normal spermatogenesis, she cautioned. “Absent or reduced spermatogenesis or morphological changes to Sertoli cells [have been reported in transgender women].”
Also, “there are needs for multiple attempts at ejaculation and storage requirements” for this approach. Cost for freezing sperm in the U.S., if not covered by insurance, is around $400, she noted, with storage costs ranging from $100 to up to $800 a year.
“Case reports using cryopreserved sperm [in transgender individuals] are promising overall … with clinical pregnancy rates following [in vitro fertilization] (IVF) with cryopreserved sperm … equivalent to patients without evidence of male factor fertility,” Dr. Menke reported.
However, she emphasized the fact that IVF, or intracytoplasmic sperm injection (ICSI), will still be necessary for conception, with potential additional costs.
Some individuals may also need to undergo surgical removal of sperm postpuberty; this is typically performed where there is evidence of male factor infertility, for example.
Embryo cryopreservation requires a partner or use of donor oocytes and, again, will have cost implications.
In conclusion, Dr. Menke reiterated that the use of fertility preservation techniques among transgender people is low, and it is more frequently accessed by transgender females. Among the identified barriers to fertility preservation are cost, lack of information, invasiveness of procedures, and desire not to delay medical transition.
Dr. Menke has disclosed no relevant financial relationships. Dr. Su has received a speaker honorarium from Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Unknowns around the long-term effects of gender-affirming hormonal treatment on fertility in transgender individuals, especially adolescents, and what this means for fertility preservation, should be red flags for clinicians, according to one expert addressing the issue at the recent virtual ENDO 2021 meeting.
“One of the main concerns regarding fertility preservation in this population is that the decision to seek gender-affirming therapy is often made early in the reproductive lifespan, and for many patients this is well before the consideration of … child-bearing,” remarked Marie Menke, MD, an ob/gyn from University of Michigan, Ann Arbor, presenting in a session dedicated to state-of-the-art approaches to gamete preservation.
“These patients need to consider simultaneously their desire for gender-affirming therapy and their desire for child-bearing,” she added, explaining that gender-affirming therapy typically requires suppression of the hormonal axis that supports reproduction.
“This level of shared decision-making requires time and multidisciplinary involvement in the face of … limited data, and even with the best of counseling it can be quite overwhelming,” Dr. Menke stressed.
Specifically, the effects of gender-affirming therapy on both fertility and fertility preservation options in transgender individuals in comparison to the general population are areas that require much more research, she emphasized.
On the topic of adolescents specifically, she said they are “a special population,” as many seeking medical therapy for gender dysphoria have never considered long-term fertility goals or desires. Reports of such discussions during pediatric gender care vary greatly depending on the age of the patient and their geographic location.
And where such conversations have happened, “often there is no recollection by patients of such discussion prior to referral to endocrinology,” she emphasized.
Session co-moderator Irene Su, MD, a reproductive endocrinologist at the University of California, San Diego, said shared decisions with patients have to be made every day, even though data are limited.
“Little is known about both the adverse medical impact of gender-affirming hormonal therapy on fertility potential, as well as the psychosocial impact of interrupting/reversing gender-affirming hormonal therapy in the future to attempt fertility,” she told this news organization.
However, “because there are reasons to be concerned about an adverse impact on fertility, transgender individuals need access to fertility risk and preservation counseling,” she stressed.
Dr. Su has a special interest in improving reproductive health in young cancer survivors, and this involves similar discussions around fertility preservation – a medical subspecialty known as “oncofertility.”
There is a greater pool of knowledge in this field compared with fertility preservation and family planning in transgender patients, Dr. Su noted.
“While we need similar data in transgender individuals, what we’ve learned from the cancer survivor population is that they and their families want to know about known and unknown fertility risks and options, even if they ultimately do not choose to undertake fertility preservation procedures,” she explains.
Desire for future kids, but <10% currently preserve fertility
Dr. Menke said the estimated prevalence of individuals who identify as transgender is around 0.7% of the U.S. population, and she observed that, “by and large, fertility management involves tissue cryopreservation.”
She presented survey data showing that between 33%-54% of transgender and nonbinary individuals report a desire to have biological children currently, or in the future, and 94.6% are also strongly in support of transgender people having access to fertility preservation procedures.
Likewise, an online cross-sectional survey of over 1,100 people in the general population found that 76.2% agree that transgender individuals should be offered fertility preservation, and 60% support fertility preservation in minors.
Multiple professional societies support counseling in regard to options for fertility preservation and recommend that it should be offered to transgender individuals.
The American Society for Reproductive Medicine (ASRM), the American College of Obstetricians and Gynecologists (ACOG), the World Professional Association for Transgender Health (WPATH), and the Endocrine Society all advocate that individuals seeking gender-affirming medical treatment should receive multidisciplinary counseling regarding fertility preservation prior to puberty suppression in adolescents, and prior to cross-sex hormone treatment in both adolescents and adults.
But despite all of these recommendations and the survey findings, fertility preservation rates in transgender patients are low, “at less than 10%,” reported Dr. Menke.
Fertility preservation counseling and management ideally needs to begin prior to initiation of hormone therapy, stressed Dr. Menke.
Given the limited data on the long-term effects of gender-affirming therapy on fertility and its preservation, such counseling often leads to a myriad of questions, she further explained.
“Patients ask ‘What are the chances of having biological children if I don’t pursue fertility preservation?’, and ‘How likely am I to have a biological child if I do pursue fertility preservation?’, as well as issues around access to care, with patients asking, ‘Will I be able to pursue this option [of fertility preservation]?’”
“The chance of having a biological child if fertility preservation is pursued is similar to those [patients with cancer] who receive ‘oncofertility’ care, which has a good prognosis,” she explained.
However, issues around access to care, and the cost of it, can be barriers.
What does a transgender male, born female, need to do?
For transgender males, options for fertility preservation include the recommended option of cryopreservation of the eggs (oocytes), although freezing of embryos and/or ovarian tissue are also possible.
The latter would be required in a prepubertal individual if they wanted to start puberty blockers and then go straight onto cross-sex hormones, Dr. Menke noted, although she said it’s not definitively known if prepubertal ovarian tissue is capable of being stimulated in the future to produce viable mature oocytes.
In someone who has gone through puberty, the ideal time to freeze eggs is before beginning gender-affirming hormone therapy, Dr. Menke explained. This is because it is not known whether testosterone has any adverse impact on oocyte development.
“We just don’t have definitive data that long-term testosterone isn’t gonadotoxic,” she said in response to a question about this after her talk.
Assessment of the reproductive consequences of gender-affirming therapy in transgender males can also be complicated by coexisting conditions, Dr. Menke explained.
For example, up to 58% of transgender males have polycystic ovary syndrome (PCOS) prior to transitioning, she noted. PCOS itself, and/or the gender-affirming therapy, may cause histologic changes of the ovarian tissue – for example, hyperplasia of ovarian stroma – and it’s not yet known to what extent this may impact future fertility, if present, she noted.
For oocyte preservation in female-to-male transgender individuals, stimulation with gonadotropins for 2-3 weeks is needed, and the procedure is invasive, requiring repeated vaginal ultrasounds. During this period, estradiol levels are supraphysiologic, and there is potential for breast development and vaginal bleeding post-retrieval, which individuals will need to be counseled about, Dr. Menke noted.
The cost of this also needs to be factored into the equation. Depending on insurance coverage, costs may be covered – and where there is no precedent, individuals can try referring their insurance companies to the ‘oncofertility consortium access-to-care model’, Dr. Menke advised.
If there is no coverage, the average cost for one egg-freezing cycle ranges from $10,000-$17,000 in the U.S., and often two to three cycles are needed to generate sufficient oocytes to be sure of a pregnancy. In addition, there are storage costs. Plus, there will be the cost of any future intervention to achieve a pregnancy, she stressed.
How long frozen oocytes remain viable is also still a matter of scientific debate, although “as the technology changes from slow-freeze to vitrification,” this time period should lengthen, Dr. Menke said.
In transgender males who have not preserved oocytes or embryos prior to transitioning, it’s necessary to stop testosterone to have the best chance of harvesting viable gametes, Dr. Menke said. Furthermore, individuals undertaking this procedure need to take into account all of the above-mentioned side effects of egg harvesting.
Although there have been reports of successful pregnancies with eggs retrieved from transgender males who have temporarily stopped testosterone, fertilization and embryo development following discontinuation of testosterone still require “additional investigation,” she observed.
Furthermore, “there are case reports of oocyte stimulation and retrieval of mature oocytes while patients continue testosterone therapy, and this may be an option in the future,” she noted, again stressing that it’s not known if excess testosterone is gonadotoxic.
Other options for fertility preservation in the transgender male include embryo cryopreservation, but this still involves hormonal stimulation and invasive procedures and would require the use of a sperm donor in a person who doesn’t currently have a partner (or who has one, but not necessarily one with whom they want to create a child).
For transgender males there is also the possibility of using a surrogate mother for the pregnancy, she noted.
What about transgender women, assigned male at birth?
For those assigned male at birth who wish to take puberty blockers, fertility preservation would require cryopreservation of testicular tissue, although Dr. Menke stressed that this is still considered “experimental.”
In the postpubertal period, the simplest option is to cryopreserve semen, with this ideally being performed prior to the individual commencing gender-affirming hormone therapy, Dr. Menke said.
If this is not done prior to beginning hormonal treatment, estrogen will need to be discontinued for fertility preservation, she noted.
Return of sperm function following cessation of estrogen may be limited – “expect at least 3 months before return of reproductive function,” Dr. Menke said. And even this may not be sufficient to restore normal spermatogenesis, she cautioned. “Absent or reduced spermatogenesis or morphological changes to Sertoli cells [have been reported in transgender women].”
Also, “there are needs for multiple attempts at ejaculation and storage requirements” for this approach. Cost for freezing sperm in the U.S., if not covered by insurance, is around $400, she noted, with storage costs ranging from $100 to up to $800 a year.
“Case reports using cryopreserved sperm [in transgender individuals] are promising overall … with clinical pregnancy rates following [in vitro fertilization] (IVF) with cryopreserved sperm … equivalent to patients without evidence of male factor fertility,” Dr. Menke reported.
However, she emphasized the fact that IVF, or intracytoplasmic sperm injection (ICSI), will still be necessary for conception, with potential additional costs.
Some individuals may also need to undergo surgical removal of sperm postpuberty; this is typically performed where there is evidence of male factor infertility, for example.
Embryo cryopreservation requires a partner or use of donor oocytes and, again, will have cost implications.
In conclusion, Dr. Menke reiterated that the use of fertility preservation techniques among transgender people is low, and it is more frequently accessed by transgender females. Among the identified barriers to fertility preservation are cost, lack of information, invasiveness of procedures, and desire not to delay medical transition.
Dr. Menke has disclosed no relevant financial relationships. Dr. Su has received a speaker honorarium from Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Unknowns around the long-term effects of gender-affirming hormonal treatment on fertility in transgender individuals, especially adolescents, and what this means for fertility preservation, should be red flags for clinicians, according to one expert addressing the issue at the recent virtual ENDO 2021 meeting.
“One of the main concerns regarding fertility preservation in this population is that the decision to seek gender-affirming therapy is often made early in the reproductive lifespan, and for many patients this is well before the consideration of … child-bearing,” remarked Marie Menke, MD, an ob/gyn from University of Michigan, Ann Arbor, presenting in a session dedicated to state-of-the-art approaches to gamete preservation.
“These patients need to consider simultaneously their desire for gender-affirming therapy and their desire for child-bearing,” she added, explaining that gender-affirming therapy typically requires suppression of the hormonal axis that supports reproduction.
“This level of shared decision-making requires time and multidisciplinary involvement in the face of … limited data, and even with the best of counseling it can be quite overwhelming,” Dr. Menke stressed.
Specifically, the effects of gender-affirming therapy on both fertility and fertility preservation options in transgender individuals in comparison to the general population are areas that require much more research, she emphasized.
On the topic of adolescents specifically, she said they are “a special population,” as many seeking medical therapy for gender dysphoria have never considered long-term fertility goals or desires. Reports of such discussions during pediatric gender care vary greatly depending on the age of the patient and their geographic location.
And where such conversations have happened, “often there is no recollection by patients of such discussion prior to referral to endocrinology,” she emphasized.
Session co-moderator Irene Su, MD, a reproductive endocrinologist at the University of California, San Diego, said shared decisions with patients have to be made every day, even though data are limited.
“Little is known about both the adverse medical impact of gender-affirming hormonal therapy on fertility potential, as well as the psychosocial impact of interrupting/reversing gender-affirming hormonal therapy in the future to attempt fertility,” she told this news organization.
However, “because there are reasons to be concerned about an adverse impact on fertility, transgender individuals need access to fertility risk and preservation counseling,” she stressed.
Dr. Su has a special interest in improving reproductive health in young cancer survivors, and this involves similar discussions around fertility preservation – a medical subspecialty known as “oncofertility.”
There is a greater pool of knowledge in this field compared with fertility preservation and family planning in transgender patients, Dr. Su noted.
“While we need similar data in transgender individuals, what we’ve learned from the cancer survivor population is that they and their families want to know about known and unknown fertility risks and options, even if they ultimately do not choose to undertake fertility preservation procedures,” she explains.
Desire for future kids, but <10% currently preserve fertility
Dr. Menke said the estimated prevalence of individuals who identify as transgender is around 0.7% of the U.S. population, and she observed that, “by and large, fertility management involves tissue cryopreservation.”
She presented survey data showing that between 33%-54% of transgender and nonbinary individuals report a desire to have biological children currently, or in the future, and 94.6% are also strongly in support of transgender people having access to fertility preservation procedures.
Likewise, an online cross-sectional survey of over 1,100 people in the general population found that 76.2% agree that transgender individuals should be offered fertility preservation, and 60% support fertility preservation in minors.
Multiple professional societies support counseling in regard to options for fertility preservation and recommend that it should be offered to transgender individuals.
The American Society for Reproductive Medicine (ASRM), the American College of Obstetricians and Gynecologists (ACOG), the World Professional Association for Transgender Health (WPATH), and the Endocrine Society all advocate that individuals seeking gender-affirming medical treatment should receive multidisciplinary counseling regarding fertility preservation prior to puberty suppression in adolescents, and prior to cross-sex hormone treatment in both adolescents and adults.
But despite all of these recommendations and the survey findings, fertility preservation rates in transgender patients are low, “at less than 10%,” reported Dr. Menke.
Fertility preservation counseling and management ideally needs to begin prior to initiation of hormone therapy, stressed Dr. Menke.
Given the limited data on the long-term effects of gender-affirming therapy on fertility and its preservation, such counseling often leads to a myriad of questions, she further explained.
“Patients ask ‘What are the chances of having biological children if I don’t pursue fertility preservation?’, and ‘How likely am I to have a biological child if I do pursue fertility preservation?’, as well as issues around access to care, with patients asking, ‘Will I be able to pursue this option [of fertility preservation]?’”
“The chance of having a biological child if fertility preservation is pursued is similar to those [patients with cancer] who receive ‘oncofertility’ care, which has a good prognosis,” she explained.
However, issues around access to care, and the cost of it, can be barriers.
What does a transgender male, born female, need to do?
For transgender males, options for fertility preservation include the recommended option of cryopreservation of the eggs (oocytes), although freezing of embryos and/or ovarian tissue are also possible.
The latter would be required in a prepubertal individual if they wanted to start puberty blockers and then go straight onto cross-sex hormones, Dr. Menke noted, although she said it’s not definitively known if prepubertal ovarian tissue is capable of being stimulated in the future to produce viable mature oocytes.
In someone who has gone through puberty, the ideal time to freeze eggs is before beginning gender-affirming hormone therapy, Dr. Menke explained. This is because it is not known whether testosterone has any adverse impact on oocyte development.
“We just don’t have definitive data that long-term testosterone isn’t gonadotoxic,” she said in response to a question about this after her talk.
Assessment of the reproductive consequences of gender-affirming therapy in transgender males can also be complicated by coexisting conditions, Dr. Menke explained.
For example, up to 58% of transgender males have polycystic ovary syndrome (PCOS) prior to transitioning, she noted. PCOS itself, and/or the gender-affirming therapy, may cause histologic changes of the ovarian tissue – for example, hyperplasia of ovarian stroma – and it’s not yet known to what extent this may impact future fertility, if present, she noted.
For oocyte preservation in female-to-male transgender individuals, stimulation with gonadotropins for 2-3 weeks is needed, and the procedure is invasive, requiring repeated vaginal ultrasounds. During this period, estradiol levels are supraphysiologic, and there is potential for breast development and vaginal bleeding post-retrieval, which individuals will need to be counseled about, Dr. Menke noted.
The cost of this also needs to be factored into the equation. Depending on insurance coverage, costs may be covered – and where there is no precedent, individuals can try referring their insurance companies to the ‘oncofertility consortium access-to-care model’, Dr. Menke advised.
If there is no coverage, the average cost for one egg-freezing cycle ranges from $10,000-$17,000 in the U.S., and often two to three cycles are needed to generate sufficient oocytes to be sure of a pregnancy. In addition, there are storage costs. Plus, there will be the cost of any future intervention to achieve a pregnancy, she stressed.
How long frozen oocytes remain viable is also still a matter of scientific debate, although “as the technology changes from slow-freeze to vitrification,” this time period should lengthen, Dr. Menke said.
In transgender males who have not preserved oocytes or embryos prior to transitioning, it’s necessary to stop testosterone to have the best chance of harvesting viable gametes, Dr. Menke said. Furthermore, individuals undertaking this procedure need to take into account all of the above-mentioned side effects of egg harvesting.
Although there have been reports of successful pregnancies with eggs retrieved from transgender males who have temporarily stopped testosterone, fertilization and embryo development following discontinuation of testosterone still require “additional investigation,” she observed.
Furthermore, “there are case reports of oocyte stimulation and retrieval of mature oocytes while patients continue testosterone therapy, and this may be an option in the future,” she noted, again stressing that it’s not known if excess testosterone is gonadotoxic.
Other options for fertility preservation in the transgender male include embryo cryopreservation, but this still involves hormonal stimulation and invasive procedures and would require the use of a sperm donor in a person who doesn’t currently have a partner (or who has one, but not necessarily one with whom they want to create a child).
For transgender males there is also the possibility of using a surrogate mother for the pregnancy, she noted.
What about transgender women, assigned male at birth?
For those assigned male at birth who wish to take puberty blockers, fertility preservation would require cryopreservation of testicular tissue, although Dr. Menke stressed that this is still considered “experimental.”
In the postpubertal period, the simplest option is to cryopreserve semen, with this ideally being performed prior to the individual commencing gender-affirming hormone therapy, Dr. Menke said.
If this is not done prior to beginning hormonal treatment, estrogen will need to be discontinued for fertility preservation, she noted.
Return of sperm function following cessation of estrogen may be limited – “expect at least 3 months before return of reproductive function,” Dr. Menke said. And even this may not be sufficient to restore normal spermatogenesis, she cautioned. “Absent or reduced spermatogenesis or morphological changes to Sertoli cells [have been reported in transgender women].”
Also, “there are needs for multiple attempts at ejaculation and storage requirements” for this approach. Cost for freezing sperm in the U.S., if not covered by insurance, is around $400, she noted, with storage costs ranging from $100 to up to $800 a year.
“Case reports using cryopreserved sperm [in transgender individuals] are promising overall … with clinical pregnancy rates following [in vitro fertilization] (IVF) with cryopreserved sperm … equivalent to patients without evidence of male factor fertility,” Dr. Menke reported.
However, she emphasized the fact that IVF, or intracytoplasmic sperm injection (ICSI), will still be necessary for conception, with potential additional costs.
Some individuals may also need to undergo surgical removal of sperm postpuberty; this is typically performed where there is evidence of male factor infertility, for example.
Embryo cryopreservation requires a partner or use of donor oocytes and, again, will have cost implications.
In conclusion, Dr. Menke reiterated that the use of fertility preservation techniques among transgender people is low, and it is more frequently accessed by transgender females. Among the identified barriers to fertility preservation are cost, lack of information, invasiveness of procedures, and desire not to delay medical transition.
Dr. Menke has disclosed no relevant financial relationships. Dr. Su has received a speaker honorarium from Ferring Pharmaceuticals.
A version of this article first appeared on Medscape.com.
“Thank You for Not Letting Me Crash and Burn”: The Imperative of Quality Physician Onboarding to Foster Job Satisfaction, Strengthen Workplace Culture, and Advance the Quadruple Aim
From The Ohio State University College of Medicine Department of Family and Community Medicine, Columbus, OH (Candy Magaña, Jná Báez, Christine Junk, Drs. Ahmad, Conroy, and Olayiwola); The Ohio State University College of Medicine Center for Primary Care Innovation and Transformation (Candy Magaña, Jná Báez, and Dr. Olayiwola); and The Ohio State University Wexner Medical Center (Christine Harsh, Erica Esposito).
Much has been discussed about the growing crisis of professional dissatisfaction among physicians, with increasing efforts being made to incorporate physician wellness into health system strategies that move from the Triple to the Quadruple Aim.1 For many years, our health care system has been focused on improving the health of populations, optimizing the patient experience, and reducing the cost of care (Triple Aim). The inclusion of the fourth aim, improving the experience of the teams that deliver care, has become paramount in achieving the other aims.
An area often overlooked in this focus on wellness, however, is the importance of the earliest days of employment to shape and predict long-term career contentment. This is a missed opportunity, as data suggest that organizations with standardized onboarding programs boast a 62% increased productivity rate and a 50% greater retention rate among new hires.2,3 Moreover, a study by the International Institute for Management Development found that businesses lose an estimated $37 billion annually because employees do not fully understand their jobs.4 The report ties losses to “actions taken by employees who have misunderstood or misinterpreted company policies, business processes, job function, or a combination of the three.” Additionally, onboarding programs that focus strictly on technical or functional orientation tasks miss important opportunities for culture integration during the onboarding process.5 It is therefore imperative to look to effective models of employee onboarding to develop systems that position physicians and practices for success.
Challenges With Traditional Physician Onboarding
In recent years, the Department of Family and Community Medicine at The Ohio State University College of Medicine has experienced rapid organizational change. Like many primary care systems nationwide responding to disruption in health care and changing demands on the clinical workforce, the department has hired new leadership, revised strategic priorities, and witnessed an influx of faculty and staff. It has also planned an expansion of ambulatory services that will more than double the clinical workforce over the next 3 years. While an exciting time, there has been a growing need to align strategy, culture, and human capital during these changes.
As we entered this phase of transformation, we recognized that our highly individualized, ad hoc orientation system presented shortcomings. During the act of revamping our physician recruitment process, stakeholder workgroup members specifically noted that improvement efforts were needed regarding new physician orientation, as no consistent structures were previously in place. New physician orientation had been a major gap for years, resulting in dissatisfaction in the first few months of physician practice, early physician turnover, and staff frustration. For physicians, we continued to learn about their frustration and unanswered questions regarding expectations, norms, structures, and processes.
Many new hires were left with a kind of “trial by fire” entry into their roles. On the first day of clinic, a new physician would most likely need to simultaneously see patients, learn the nuances of the electronic health record (EHR), figure out where the break room was located, and quickly learn population health issues for the patients they were serving. Opportunities to meet key clinic site leadership would be at random, and new physicians might not have the opportunity to meet leadership or staff until months into their tenure; this did not allow for a sense of belonging or understanding of the many resources available to them. We learned that the quality of these ad hoc orientations also varied based on the experience and priorities of each practice’s clinic and administrative leaders, who themselves felt ill-equipped to provide a consistent, robust, and confidence-building experience. In addition, practice site management was rarely given advance time to prepare for the arrival of new physicians, which resulted in physicians perceiving practices to be unwelcoming and disorganized. Their first days were often spent with patients in clinic with no structured orientation and without understanding workflows or having systems practice knowledge.
Institutionally, the interview process satisfied some transfer of knowledge, but we were unclear of what was being consistently shared and understood in the multiple ambulatory locations where our physicians enter practice. More importantly, we knew we were missing a critical opportunity to use orientation to imbue other values of diversity and inclusion, health equity, and operational excellence into the workforce. Based on anecdotal insights from employees and our own review of successful onboarding approaches from other industries, we also knew a more structured welcoming process would predict greater long-term career satisfaction for physicians and create a foundation for providing optimal care for patients when clinical encounters began.
Reengineering Physician Onboarding
In 2019, our department developed a multipronged approach to physician onboarding, which is already paying dividends in easing acculturation and fostering team cohesion. The department tapped its Center for Primary Care Innovation and Transformation (PCIT) to direct this effort, based on its expertise in practice transformation, clinical transformation and adaptations, and workflow efficiency through process and quality improvement. The PCIT team provides support to the department and the entire health system focused on technology and innovation, health equity, and health care efficiency.6 They applied many of the tools used in the Clinical Transformation in Technology approach to lead this initiative.7
The PCIT team began identifying key stakeholders (department, clinical and ambulatory leadership, clinicians and clinical staff, community partners, human resources, and resident physicians), and then engaging those individuals in dialogue surrounding orientation needs. During scheduled in-person and virtual work sessions, stakeholders were asked to provide input on pain points for new physicians and clinic leadership and were then empowered to create an onboarding program. Applying health care quality improvement techniques, we leveraged workflow mapping, current and future state planning, and goal setting, led by the skilled process improvement and clinical transformation specialists. We coordinated a multidisciplinary process improvement team that included clinic administrators, medical directors, human resources, administrative staff, ambulatory and resident leadership, clinical leadership, and recruitment liaisons. This diverse group of leadership and staff was brought together to address these critical identified gaps and weaknesses in new physician onboarding.
Through a series of learning sessions, the workgroup provided input that was used to form an itemized physician onboarding schedule, which was then leveraged to develop Plan-Do-Study-Act (PDSA) cycles, collecting feedback in real time. Some issues that seem small can cause major distress for new physicians. For example, in our inaugural orientation implementation, a physician provided feedback that they wanted to obtain information on setting up their work email on their personal devices and was having considerable trouble figuring out how to do so. This particular topic was not initially included in the first iteration of the Department’s orientation program. We rapidly sought out different ways to embed that into the onboarding experience. The first PDSA involved integrating the university information technology team (IT) into the process but was not successful because it required extra work for the new physician and reliance on the IT schedule. The next attempt was to have IT train a department staff member, but again, this still required that the physician find time to connect with that staff member. Finally, we decided to obtain a useful tip sheet that clearly outlined the process and could be included in orientation materials. This gave the new physicians control over how and when they would work on this issue. Based on these learnings, this was incorporated as a standing agenda item and resource for incoming physicians.
Essential Elements of Effective Onboarding
The new physician onboarding program consists of 5 key elements: (1) 2-week acclimation period; (2) peer learning and connection; (3) training before beginning patient care; (4) standardization, transparency, and accountability in all processes; (5) ongoing feedback for continued program improvement with individual support (Figure).
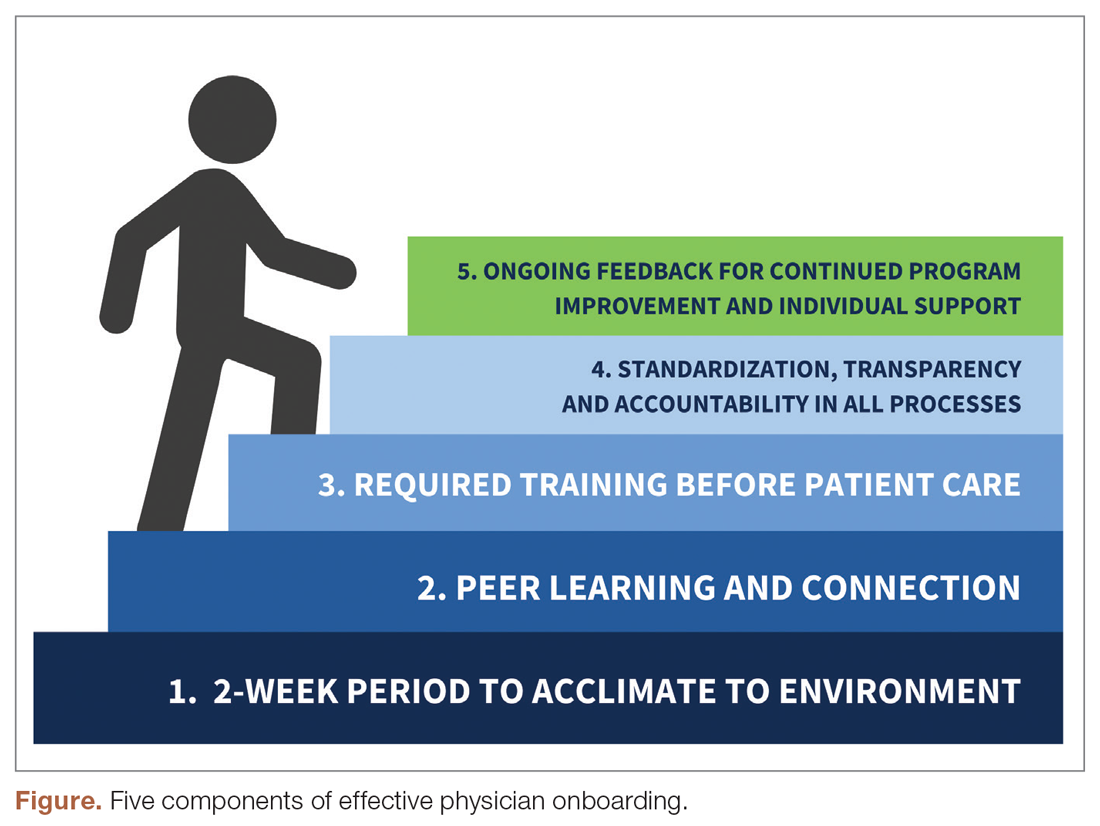
The program begins with a 2-week period of intentional investment in individual success, during which time no patients are scheduled. In week 1, we work with new hires to set expectations for performance, understand departmental norms, and introduce culture. Physicians meet formally and informally with department and institutional leadership, as well as attend team meetings and trainings that include a range of administrative and compliance requirements, such as quality standards and expectations, compliance, billing and coding specific to family medicine, EHR management, and institutionally mandated orientations. We are also adding implicit bias and antiracism training during this period, which are essential to creating a culture of unity and belonging.
During week 2, we focus on clinic-level orientation, assigning new hires an orientation buddy and a department sponsor, such as a physician lead or medical director. Physicians spend time with leadership at their clinic as they nurture relationships important for mentorship, sponsorship, and peer support. They also meet care team members, including front desk associates, medical assistants, behavioral health clinicians, nutritionists, social workers, pharmacists, and other key colleagues and care team members. This introduces the physician to the clinical environment and physical space as well as acclimates the physician to workflows and feedback loops for regular interaction.
When physicians ultimately begin patient care, they begin with an expected productivity rate of 50%, followed by an expected productivity rate of 75%, and then an expected productivity rate of 100%. This steady increase occurs over 3 to 4 weeks depending on the physician’s comfort level. They are also provided monthly reports on work relative value unit performance so that they can track and adapt practice patterns as necessary.More details on the program can be found in Appendix 1.
Takeaways From the Implementation of the New Program
Give time for new physicians to focus on acclimating to the role and environment.
The initial 2-week period of transition—without direct patient care—ensures that physicians feel comfortable in their new ecosystem. This also supports personal transitions, as many new hires are managing relocation and acclimating themselves and their families to new settings. Even residents from our training program who returned as attending physicians found this flexibility and slow reentry essential. This also gives the clinic time to orient to an additional provider, nurture them into the team culture, and develop relationships with the care team.
Cultivate spaces for shared learning, problem-solving, and peer connection.
Orientation is delivered primarily through group learning sessions with cohorts of new physicians, thus developing spaces for networking, fostering psychological safety, encouraging personal and professional rapport, emphasizing interactive learning, and reinforcing scheduling blocks at the departmental level. New hires also participate in peer shadowing to develop clinical competencies and are assigned a workplace buddy to foster a sense of belonging and create opportunities for additional knowledge sharing and cross-training.
Strengthen physician knowledge base, confidence, and comfort in the workplace before beginning direct patient care.
Without fluency in the workflows, culture, and operations of a practice, the urgency to have physicians begin clinical care can result in frustration for the physician, patients, and clinical and administrative staff. Therefore, we complete essential training prior to seeing any patients. This includes clinical workflows, referral processes, use of alternate modalities of care (eg, telehealth, eConsults), billing protocols, population health training, patient resources, office resources, and other essential daily processes and tools. This creates efficiency in administrative management, increased productivity, and better understanding of resources available for patients’ medical, social, and behavioral needs when patient care begins.
Embrace standardization, transparency, and accountability in as many processes as possible.
Standardized knowledge-sharing and checklists are mandated at every step of the orientation process, requiring sign off from the physician lead, practice manager, and new physicians upon completion. This offers all parties the opportunity to play a role in the delivery of and accountability for skills transfer and empowers new hires to press pause if they feel unsure about any domain in the training. It is also essential in guaranteeing that all physicians—regardless of which ambulatory location they practice in—receive consistent information and expectations. A sample checklist can be found in Appendix 2.
Commit to collecting and acting on feedback for continued program improvement and individual support.
As physicians complete the program, it is necessary to create structures to measure and enhance its impact, as well as evaluate how physicians are faring following the program. Each physician completes surveys at the end of the orientation program, attends a 90-day post-program check-in with the department chair, and receives follow-up trainings on advanced topics as they become more deeply embedded in the organization.
Lessons Learned
Feedback from surveys and 90-day check-ins with leadership and physicians reflect a high degree of clarity on job roles and duties, a sense of team camaraderie, easier system navigation, and a strong sense of support. We do recognize that sustaining change takes time and our study is limited by data demonstrating the impact of these efforts. We look forward to sharing more robust data from surveys and qualitative interviews with physicians, clinical leadership, and staff in the future. Our team will conduct interviews at 90-day and 180-day checkpoints with new physicians who have gone through this program, followed by a check-in after 1 year. Additionally, new physicians as well as key stakeholders, such as physician leads, practice managers, and members of the recruitment team, have started to participate in short surveys. These are designed to better understand their experiences, what worked well, what can be improved, and the overall satisfaction of the physician and other members of the extended care team.
What follows are some comments made by the initial group of physicians that went through this program and participated in follow-up interviews:
“I really feel like part of a bigger team.”
“I knew exactly what do to when I walked into the exam room on clinic Day 1.”
“It was great to make deep connections during the early process of joining.”
“Having a buddy to direct questions and ideas to is amazing and empowering.”
“Even though the orientation was long, I felt that I learned so much that I would not have otherwise.”
“Thank you for not letting me crash and burn!”
“Great culture! I love understanding our values of health equity, diversity, and inclusion.”
In the months since our endeavor began, we have learned just how essential it is to fully and effectively integrate new hires into the organization for their own satisfaction and success—and ours. Indeed, we cannot expect to achieve the Quadruple Aim without investing in the kind of transparent and intentional orientation process that defines expectations, aligns cultural values, mitigates costly and stressful operational misunderstandings, and communicates to physicians that, not only do they belong, but their sense of belonging is our priority. While we have yet to understand the impact of this program on the fourth aim of the Quadruple Aim, we are hopeful that the benefits will be far-reaching.
It is our ultimate hope that programs like this: (1) give physicians the confidence needed to create impactful patient-centered experiences; (2) enable physicians to become more cost-effective and efficient in care delivery; (3) allow physicians to understand the populations they are serving and access tools available to mitigate health disparities and other barriers; and (4) improve the collective experience of every member of the care team, practice leadership, and clinician-patient partnership.
Corresponding author: J. Nwando Olayiwola, MD, MPH, FAAFP, The Ohio State University College of Medicine, Department of Family and Community Medicine, 2231 N High St, Ste 250, Columbus, OH 43210; [email protected].
Financial disclosures: None.
Keywords: physician onboarding; Quadruple Aim; leadership; clinician satisfaction; care team satisfaction.
1. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6): 573-576.
2. Maurer R. Onboarding key to retaining, engaging talent. Society for Human Resource Management. April 16, 2015. Accessed January 8, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/onboarding-key-retaining-engaging-talent.aspx
3. Boston AG. New hire onboarding standardization and automation powers productivity gains. GlobeNewswire. March 8, 2011. Accessed January 8, 2021. http://www.globenewswire.com/news-release/2011/03/08/994239/0/en/New-Hire-Onboarding-Standardization-and-Automation-Powers-Productivity-Gains.html
4. $37 billion – US and UK business count the cost of employee misunderstanding. HR.com – Maximizing Human Potential. June 18, 2008. Accessed March 10, 2021. https://www.hr.com/en/communities/staffing_and_recruitment/37-billion---us-and-uk-businesses-count-the-cost-o_fhnduq4d.html
5. Employers risk driving new hires away with poor onboarding. Society for Human Resource Management. February 23, 2018. Accessed March 10, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/employers-new-hires-poor-onboarding.aspx
6. Center for Primary Care Innovation and Transformation. The Ohio State University College of Medicine. Accessed January 8, 2021. https://wexnermedical.osu.edu/departments/family-medicine/pcit
7. Olayiwola, J.N. and Magaña, C. Clinical transformation in technology: a fresh change management approach for primary care. Harvard Health Policy Review. February 2, 2019. Accessed March 10, 2021. http://www.hhpronline.org/articles/2019/2/2/clinical-transformation-in-technology-a-fresh-change-management-approach-for-primary-care
From The Ohio State University College of Medicine Department of Family and Community Medicine, Columbus, OH (Candy Magaña, Jná Báez, Christine Junk, Drs. Ahmad, Conroy, and Olayiwola); The Ohio State University College of Medicine Center for Primary Care Innovation and Transformation (Candy Magaña, Jná Báez, and Dr. Olayiwola); and The Ohio State University Wexner Medical Center (Christine Harsh, Erica Esposito).
Much has been discussed about the growing crisis of professional dissatisfaction among physicians, with increasing efforts being made to incorporate physician wellness into health system strategies that move from the Triple to the Quadruple Aim.1 For many years, our health care system has been focused on improving the health of populations, optimizing the patient experience, and reducing the cost of care (Triple Aim). The inclusion of the fourth aim, improving the experience of the teams that deliver care, has become paramount in achieving the other aims.
An area often overlooked in this focus on wellness, however, is the importance of the earliest days of employment to shape and predict long-term career contentment. This is a missed opportunity, as data suggest that organizations with standardized onboarding programs boast a 62% increased productivity rate and a 50% greater retention rate among new hires.2,3 Moreover, a study by the International Institute for Management Development found that businesses lose an estimated $37 billion annually because employees do not fully understand their jobs.4 The report ties losses to “actions taken by employees who have misunderstood or misinterpreted company policies, business processes, job function, or a combination of the three.” Additionally, onboarding programs that focus strictly on technical or functional orientation tasks miss important opportunities for culture integration during the onboarding process.5 It is therefore imperative to look to effective models of employee onboarding to develop systems that position physicians and practices for success.
Challenges With Traditional Physician Onboarding
In recent years, the Department of Family and Community Medicine at The Ohio State University College of Medicine has experienced rapid organizational change. Like many primary care systems nationwide responding to disruption in health care and changing demands on the clinical workforce, the department has hired new leadership, revised strategic priorities, and witnessed an influx of faculty and staff. It has also planned an expansion of ambulatory services that will more than double the clinical workforce over the next 3 years. While an exciting time, there has been a growing need to align strategy, culture, and human capital during these changes.
As we entered this phase of transformation, we recognized that our highly individualized, ad hoc orientation system presented shortcomings. During the act of revamping our physician recruitment process, stakeholder workgroup members specifically noted that improvement efforts were needed regarding new physician orientation, as no consistent structures were previously in place. New physician orientation had been a major gap for years, resulting in dissatisfaction in the first few months of physician practice, early physician turnover, and staff frustration. For physicians, we continued to learn about their frustration and unanswered questions regarding expectations, norms, structures, and processes.
Many new hires were left with a kind of “trial by fire” entry into their roles. On the first day of clinic, a new physician would most likely need to simultaneously see patients, learn the nuances of the electronic health record (EHR), figure out where the break room was located, and quickly learn population health issues for the patients they were serving. Opportunities to meet key clinic site leadership would be at random, and new physicians might not have the opportunity to meet leadership or staff until months into their tenure; this did not allow for a sense of belonging or understanding of the many resources available to them. We learned that the quality of these ad hoc orientations also varied based on the experience and priorities of each practice’s clinic and administrative leaders, who themselves felt ill-equipped to provide a consistent, robust, and confidence-building experience. In addition, practice site management was rarely given advance time to prepare for the arrival of new physicians, which resulted in physicians perceiving practices to be unwelcoming and disorganized. Their first days were often spent with patients in clinic with no structured orientation and without understanding workflows or having systems practice knowledge.
Institutionally, the interview process satisfied some transfer of knowledge, but we were unclear of what was being consistently shared and understood in the multiple ambulatory locations where our physicians enter practice. More importantly, we knew we were missing a critical opportunity to use orientation to imbue other values of diversity and inclusion, health equity, and operational excellence into the workforce. Based on anecdotal insights from employees and our own review of successful onboarding approaches from other industries, we also knew a more structured welcoming process would predict greater long-term career satisfaction for physicians and create a foundation for providing optimal care for patients when clinical encounters began.
Reengineering Physician Onboarding
In 2019, our department developed a multipronged approach to physician onboarding, which is already paying dividends in easing acculturation and fostering team cohesion. The department tapped its Center for Primary Care Innovation and Transformation (PCIT) to direct this effort, based on its expertise in practice transformation, clinical transformation and adaptations, and workflow efficiency through process and quality improvement. The PCIT team provides support to the department and the entire health system focused on technology and innovation, health equity, and health care efficiency.6 They applied many of the tools used in the Clinical Transformation in Technology approach to lead this initiative.7
The PCIT team began identifying key stakeholders (department, clinical and ambulatory leadership, clinicians and clinical staff, community partners, human resources, and resident physicians), and then engaging those individuals in dialogue surrounding orientation needs. During scheduled in-person and virtual work sessions, stakeholders were asked to provide input on pain points for new physicians and clinic leadership and were then empowered to create an onboarding program. Applying health care quality improvement techniques, we leveraged workflow mapping, current and future state planning, and goal setting, led by the skilled process improvement and clinical transformation specialists. We coordinated a multidisciplinary process improvement team that included clinic administrators, medical directors, human resources, administrative staff, ambulatory and resident leadership, clinical leadership, and recruitment liaisons. This diverse group of leadership and staff was brought together to address these critical identified gaps and weaknesses in new physician onboarding.
Through a series of learning sessions, the workgroup provided input that was used to form an itemized physician onboarding schedule, which was then leveraged to develop Plan-Do-Study-Act (PDSA) cycles, collecting feedback in real time. Some issues that seem small can cause major distress for new physicians. For example, in our inaugural orientation implementation, a physician provided feedback that they wanted to obtain information on setting up their work email on their personal devices and was having considerable trouble figuring out how to do so. This particular topic was not initially included in the first iteration of the Department’s orientation program. We rapidly sought out different ways to embed that into the onboarding experience. The first PDSA involved integrating the university information technology team (IT) into the process but was not successful because it required extra work for the new physician and reliance on the IT schedule. The next attempt was to have IT train a department staff member, but again, this still required that the physician find time to connect with that staff member. Finally, we decided to obtain a useful tip sheet that clearly outlined the process and could be included in orientation materials. This gave the new physicians control over how and when they would work on this issue. Based on these learnings, this was incorporated as a standing agenda item and resource for incoming physicians.
Essential Elements of Effective Onboarding
The new physician onboarding program consists of 5 key elements: (1) 2-week acclimation period; (2) peer learning and connection; (3) training before beginning patient care; (4) standardization, transparency, and accountability in all processes; (5) ongoing feedback for continued program improvement with individual support (Figure).

The program begins with a 2-week period of intentional investment in individual success, during which time no patients are scheduled. In week 1, we work with new hires to set expectations for performance, understand departmental norms, and introduce culture. Physicians meet formally and informally with department and institutional leadership, as well as attend team meetings and trainings that include a range of administrative and compliance requirements, such as quality standards and expectations, compliance, billing and coding specific to family medicine, EHR management, and institutionally mandated orientations. We are also adding implicit bias and antiracism training during this period, which are essential to creating a culture of unity and belonging.
During week 2, we focus on clinic-level orientation, assigning new hires an orientation buddy and a department sponsor, such as a physician lead or medical director. Physicians spend time with leadership at their clinic as they nurture relationships important for mentorship, sponsorship, and peer support. They also meet care team members, including front desk associates, medical assistants, behavioral health clinicians, nutritionists, social workers, pharmacists, and other key colleagues and care team members. This introduces the physician to the clinical environment and physical space as well as acclimates the physician to workflows and feedback loops for regular interaction.
When physicians ultimately begin patient care, they begin with an expected productivity rate of 50%, followed by an expected productivity rate of 75%, and then an expected productivity rate of 100%. This steady increase occurs over 3 to 4 weeks depending on the physician’s comfort level. They are also provided monthly reports on work relative value unit performance so that they can track and adapt practice patterns as necessary.More details on the program can be found in Appendix 1.
Takeaways From the Implementation of the New Program
Give time for new physicians to focus on acclimating to the role and environment.
The initial 2-week period of transition—without direct patient care—ensures that physicians feel comfortable in their new ecosystem. This also supports personal transitions, as many new hires are managing relocation and acclimating themselves and their families to new settings. Even residents from our training program who returned as attending physicians found this flexibility and slow reentry essential. This also gives the clinic time to orient to an additional provider, nurture them into the team culture, and develop relationships with the care team.
Cultivate spaces for shared learning, problem-solving, and peer connection.
Orientation is delivered primarily through group learning sessions with cohorts of new physicians, thus developing spaces for networking, fostering psychological safety, encouraging personal and professional rapport, emphasizing interactive learning, and reinforcing scheduling blocks at the departmental level. New hires also participate in peer shadowing to develop clinical competencies and are assigned a workplace buddy to foster a sense of belonging and create opportunities for additional knowledge sharing and cross-training.
Strengthen physician knowledge base, confidence, and comfort in the workplace before beginning direct patient care.
Without fluency in the workflows, culture, and operations of a practice, the urgency to have physicians begin clinical care can result in frustration for the physician, patients, and clinical and administrative staff. Therefore, we complete essential training prior to seeing any patients. This includes clinical workflows, referral processes, use of alternate modalities of care (eg, telehealth, eConsults), billing protocols, population health training, patient resources, office resources, and other essential daily processes and tools. This creates efficiency in administrative management, increased productivity, and better understanding of resources available for patients’ medical, social, and behavioral needs when patient care begins.
Embrace standardization, transparency, and accountability in as many processes as possible.
Standardized knowledge-sharing and checklists are mandated at every step of the orientation process, requiring sign off from the physician lead, practice manager, and new physicians upon completion. This offers all parties the opportunity to play a role in the delivery of and accountability for skills transfer and empowers new hires to press pause if they feel unsure about any domain in the training. It is also essential in guaranteeing that all physicians—regardless of which ambulatory location they practice in—receive consistent information and expectations. A sample checklist can be found in Appendix 2.
Commit to collecting and acting on feedback for continued program improvement and individual support.
As physicians complete the program, it is necessary to create structures to measure and enhance its impact, as well as evaluate how physicians are faring following the program. Each physician completes surveys at the end of the orientation program, attends a 90-day post-program check-in with the department chair, and receives follow-up trainings on advanced topics as they become more deeply embedded in the organization.
Lessons Learned
Feedback from surveys and 90-day check-ins with leadership and physicians reflect a high degree of clarity on job roles and duties, a sense of team camaraderie, easier system navigation, and a strong sense of support. We do recognize that sustaining change takes time and our study is limited by data demonstrating the impact of these efforts. We look forward to sharing more robust data from surveys and qualitative interviews with physicians, clinical leadership, and staff in the future. Our team will conduct interviews at 90-day and 180-day checkpoints with new physicians who have gone through this program, followed by a check-in after 1 year. Additionally, new physicians as well as key stakeholders, such as physician leads, practice managers, and members of the recruitment team, have started to participate in short surveys. These are designed to better understand their experiences, what worked well, what can be improved, and the overall satisfaction of the physician and other members of the extended care team.
What follows are some comments made by the initial group of physicians that went through this program and participated in follow-up interviews:
“I really feel like part of a bigger team.”
“I knew exactly what do to when I walked into the exam room on clinic Day 1.”
“It was great to make deep connections during the early process of joining.”
“Having a buddy to direct questions and ideas to is amazing and empowering.”
“Even though the orientation was long, I felt that I learned so much that I would not have otherwise.”
“Thank you for not letting me crash and burn!”
“Great culture! I love understanding our values of health equity, diversity, and inclusion.”
In the months since our endeavor began, we have learned just how essential it is to fully and effectively integrate new hires into the organization for their own satisfaction and success—and ours. Indeed, we cannot expect to achieve the Quadruple Aim without investing in the kind of transparent and intentional orientation process that defines expectations, aligns cultural values, mitigates costly and stressful operational misunderstandings, and communicates to physicians that, not only do they belong, but their sense of belonging is our priority. While we have yet to understand the impact of this program on the fourth aim of the Quadruple Aim, we are hopeful that the benefits will be far-reaching.
It is our ultimate hope that programs like this: (1) give physicians the confidence needed to create impactful patient-centered experiences; (2) enable physicians to become more cost-effective and efficient in care delivery; (3) allow physicians to understand the populations they are serving and access tools available to mitigate health disparities and other barriers; and (4) improve the collective experience of every member of the care team, practice leadership, and clinician-patient partnership.
Corresponding author: J. Nwando Olayiwola, MD, MPH, FAAFP, The Ohio State University College of Medicine, Department of Family and Community Medicine, 2231 N High St, Ste 250, Columbus, OH 43210; [email protected].
Financial disclosures: None.
Keywords: physician onboarding; Quadruple Aim; leadership; clinician satisfaction; care team satisfaction.
From The Ohio State University College of Medicine Department of Family and Community Medicine, Columbus, OH (Candy Magaña, Jná Báez, Christine Junk, Drs. Ahmad, Conroy, and Olayiwola); The Ohio State University College of Medicine Center for Primary Care Innovation and Transformation (Candy Magaña, Jná Báez, and Dr. Olayiwola); and The Ohio State University Wexner Medical Center (Christine Harsh, Erica Esposito).
Much has been discussed about the growing crisis of professional dissatisfaction among physicians, with increasing efforts being made to incorporate physician wellness into health system strategies that move from the Triple to the Quadruple Aim.1 For many years, our health care system has been focused on improving the health of populations, optimizing the patient experience, and reducing the cost of care (Triple Aim). The inclusion of the fourth aim, improving the experience of the teams that deliver care, has become paramount in achieving the other aims.
An area often overlooked in this focus on wellness, however, is the importance of the earliest days of employment to shape and predict long-term career contentment. This is a missed opportunity, as data suggest that organizations with standardized onboarding programs boast a 62% increased productivity rate and a 50% greater retention rate among new hires.2,3 Moreover, a study by the International Institute for Management Development found that businesses lose an estimated $37 billion annually because employees do not fully understand their jobs.4 The report ties losses to “actions taken by employees who have misunderstood or misinterpreted company policies, business processes, job function, or a combination of the three.” Additionally, onboarding programs that focus strictly on technical or functional orientation tasks miss important opportunities for culture integration during the onboarding process.5 It is therefore imperative to look to effective models of employee onboarding to develop systems that position physicians and practices for success.
Challenges With Traditional Physician Onboarding
In recent years, the Department of Family and Community Medicine at The Ohio State University College of Medicine has experienced rapid organizational change. Like many primary care systems nationwide responding to disruption in health care and changing demands on the clinical workforce, the department has hired new leadership, revised strategic priorities, and witnessed an influx of faculty and staff. It has also planned an expansion of ambulatory services that will more than double the clinical workforce over the next 3 years. While an exciting time, there has been a growing need to align strategy, culture, and human capital during these changes.
As we entered this phase of transformation, we recognized that our highly individualized, ad hoc orientation system presented shortcomings. During the act of revamping our physician recruitment process, stakeholder workgroup members specifically noted that improvement efforts were needed regarding new physician orientation, as no consistent structures were previously in place. New physician orientation had been a major gap for years, resulting in dissatisfaction in the first few months of physician practice, early physician turnover, and staff frustration. For physicians, we continued to learn about their frustration and unanswered questions regarding expectations, norms, structures, and processes.
Many new hires were left with a kind of “trial by fire” entry into their roles. On the first day of clinic, a new physician would most likely need to simultaneously see patients, learn the nuances of the electronic health record (EHR), figure out where the break room was located, and quickly learn population health issues for the patients they were serving. Opportunities to meet key clinic site leadership would be at random, and new physicians might not have the opportunity to meet leadership or staff until months into their tenure; this did not allow for a sense of belonging or understanding of the many resources available to them. We learned that the quality of these ad hoc orientations also varied based on the experience and priorities of each practice’s clinic and administrative leaders, who themselves felt ill-equipped to provide a consistent, robust, and confidence-building experience. In addition, practice site management was rarely given advance time to prepare for the arrival of new physicians, which resulted in physicians perceiving practices to be unwelcoming and disorganized. Their first days were often spent with patients in clinic with no structured orientation and without understanding workflows or having systems practice knowledge.
Institutionally, the interview process satisfied some transfer of knowledge, but we were unclear of what was being consistently shared and understood in the multiple ambulatory locations where our physicians enter practice. More importantly, we knew we were missing a critical opportunity to use orientation to imbue other values of diversity and inclusion, health equity, and operational excellence into the workforce. Based on anecdotal insights from employees and our own review of successful onboarding approaches from other industries, we also knew a more structured welcoming process would predict greater long-term career satisfaction for physicians and create a foundation for providing optimal care for patients when clinical encounters began.
Reengineering Physician Onboarding
In 2019, our department developed a multipronged approach to physician onboarding, which is already paying dividends in easing acculturation and fostering team cohesion. The department tapped its Center for Primary Care Innovation and Transformation (PCIT) to direct this effort, based on its expertise in practice transformation, clinical transformation and adaptations, and workflow efficiency through process and quality improvement. The PCIT team provides support to the department and the entire health system focused on technology and innovation, health equity, and health care efficiency.6 They applied many of the tools used in the Clinical Transformation in Technology approach to lead this initiative.7
The PCIT team began identifying key stakeholders (department, clinical and ambulatory leadership, clinicians and clinical staff, community partners, human resources, and resident physicians), and then engaging those individuals in dialogue surrounding orientation needs. During scheduled in-person and virtual work sessions, stakeholders were asked to provide input on pain points for new physicians and clinic leadership and were then empowered to create an onboarding program. Applying health care quality improvement techniques, we leveraged workflow mapping, current and future state planning, and goal setting, led by the skilled process improvement and clinical transformation specialists. We coordinated a multidisciplinary process improvement team that included clinic administrators, medical directors, human resources, administrative staff, ambulatory and resident leadership, clinical leadership, and recruitment liaisons. This diverse group of leadership and staff was brought together to address these critical identified gaps and weaknesses in new physician onboarding.
Through a series of learning sessions, the workgroup provided input that was used to form an itemized physician onboarding schedule, which was then leveraged to develop Plan-Do-Study-Act (PDSA) cycles, collecting feedback in real time. Some issues that seem small can cause major distress for new physicians. For example, in our inaugural orientation implementation, a physician provided feedback that they wanted to obtain information on setting up their work email on their personal devices and was having considerable trouble figuring out how to do so. This particular topic was not initially included in the first iteration of the Department’s orientation program. We rapidly sought out different ways to embed that into the onboarding experience. The first PDSA involved integrating the university information technology team (IT) into the process but was not successful because it required extra work for the new physician and reliance on the IT schedule. The next attempt was to have IT train a department staff member, but again, this still required that the physician find time to connect with that staff member. Finally, we decided to obtain a useful tip sheet that clearly outlined the process and could be included in orientation materials. This gave the new physicians control over how and when they would work on this issue. Based on these learnings, this was incorporated as a standing agenda item and resource for incoming physicians.
Essential Elements of Effective Onboarding
The new physician onboarding program consists of 5 key elements: (1) 2-week acclimation period; (2) peer learning and connection; (3) training before beginning patient care; (4) standardization, transparency, and accountability in all processes; (5) ongoing feedback for continued program improvement with individual support (Figure).

The program begins with a 2-week period of intentional investment in individual success, during which time no patients are scheduled. In week 1, we work with new hires to set expectations for performance, understand departmental norms, and introduce culture. Physicians meet formally and informally with department and institutional leadership, as well as attend team meetings and trainings that include a range of administrative and compliance requirements, such as quality standards and expectations, compliance, billing and coding specific to family medicine, EHR management, and institutionally mandated orientations. We are also adding implicit bias and antiracism training during this period, which are essential to creating a culture of unity and belonging.
During week 2, we focus on clinic-level orientation, assigning new hires an orientation buddy and a department sponsor, such as a physician lead or medical director. Physicians spend time with leadership at their clinic as they nurture relationships important for mentorship, sponsorship, and peer support. They also meet care team members, including front desk associates, medical assistants, behavioral health clinicians, nutritionists, social workers, pharmacists, and other key colleagues and care team members. This introduces the physician to the clinical environment and physical space as well as acclimates the physician to workflows and feedback loops for regular interaction.
When physicians ultimately begin patient care, they begin with an expected productivity rate of 50%, followed by an expected productivity rate of 75%, and then an expected productivity rate of 100%. This steady increase occurs over 3 to 4 weeks depending on the physician’s comfort level. They are also provided monthly reports on work relative value unit performance so that they can track and adapt practice patterns as necessary.More details on the program can be found in Appendix 1.
Takeaways From the Implementation of the New Program
Give time for new physicians to focus on acclimating to the role and environment.
The initial 2-week period of transition—without direct patient care—ensures that physicians feel comfortable in their new ecosystem. This also supports personal transitions, as many new hires are managing relocation and acclimating themselves and their families to new settings. Even residents from our training program who returned as attending physicians found this flexibility and slow reentry essential. This also gives the clinic time to orient to an additional provider, nurture them into the team culture, and develop relationships with the care team.
Cultivate spaces for shared learning, problem-solving, and peer connection.
Orientation is delivered primarily through group learning sessions with cohorts of new physicians, thus developing spaces for networking, fostering psychological safety, encouraging personal and professional rapport, emphasizing interactive learning, and reinforcing scheduling blocks at the departmental level. New hires also participate in peer shadowing to develop clinical competencies and are assigned a workplace buddy to foster a sense of belonging and create opportunities for additional knowledge sharing and cross-training.
Strengthen physician knowledge base, confidence, and comfort in the workplace before beginning direct patient care.
Without fluency in the workflows, culture, and operations of a practice, the urgency to have physicians begin clinical care can result in frustration for the physician, patients, and clinical and administrative staff. Therefore, we complete essential training prior to seeing any patients. This includes clinical workflows, referral processes, use of alternate modalities of care (eg, telehealth, eConsults), billing protocols, population health training, patient resources, office resources, and other essential daily processes and tools. This creates efficiency in administrative management, increased productivity, and better understanding of resources available for patients’ medical, social, and behavioral needs when patient care begins.
Embrace standardization, transparency, and accountability in as many processes as possible.
Standardized knowledge-sharing and checklists are mandated at every step of the orientation process, requiring sign off from the physician lead, practice manager, and new physicians upon completion. This offers all parties the opportunity to play a role in the delivery of and accountability for skills transfer and empowers new hires to press pause if they feel unsure about any domain in the training. It is also essential in guaranteeing that all physicians—regardless of which ambulatory location they practice in—receive consistent information and expectations. A sample checklist can be found in Appendix 2.
Commit to collecting and acting on feedback for continued program improvement and individual support.
As physicians complete the program, it is necessary to create structures to measure and enhance its impact, as well as evaluate how physicians are faring following the program. Each physician completes surveys at the end of the orientation program, attends a 90-day post-program check-in with the department chair, and receives follow-up trainings on advanced topics as they become more deeply embedded in the organization.
Lessons Learned
Feedback from surveys and 90-day check-ins with leadership and physicians reflect a high degree of clarity on job roles and duties, a sense of team camaraderie, easier system navigation, and a strong sense of support. We do recognize that sustaining change takes time and our study is limited by data demonstrating the impact of these efforts. We look forward to sharing more robust data from surveys and qualitative interviews with physicians, clinical leadership, and staff in the future. Our team will conduct interviews at 90-day and 180-day checkpoints with new physicians who have gone through this program, followed by a check-in after 1 year. Additionally, new physicians as well as key stakeholders, such as physician leads, practice managers, and members of the recruitment team, have started to participate in short surveys. These are designed to better understand their experiences, what worked well, what can be improved, and the overall satisfaction of the physician and other members of the extended care team.
What follows are some comments made by the initial group of physicians that went through this program and participated in follow-up interviews:
“I really feel like part of a bigger team.”
“I knew exactly what do to when I walked into the exam room on clinic Day 1.”
“It was great to make deep connections during the early process of joining.”
“Having a buddy to direct questions and ideas to is amazing and empowering.”
“Even though the orientation was long, I felt that I learned so much that I would not have otherwise.”
“Thank you for not letting me crash and burn!”
“Great culture! I love understanding our values of health equity, diversity, and inclusion.”
In the months since our endeavor began, we have learned just how essential it is to fully and effectively integrate new hires into the organization for their own satisfaction and success—and ours. Indeed, we cannot expect to achieve the Quadruple Aim without investing in the kind of transparent and intentional orientation process that defines expectations, aligns cultural values, mitigates costly and stressful operational misunderstandings, and communicates to physicians that, not only do they belong, but their sense of belonging is our priority. While we have yet to understand the impact of this program on the fourth aim of the Quadruple Aim, we are hopeful that the benefits will be far-reaching.
It is our ultimate hope that programs like this: (1) give physicians the confidence needed to create impactful patient-centered experiences; (2) enable physicians to become more cost-effective and efficient in care delivery; (3) allow physicians to understand the populations they are serving and access tools available to mitigate health disparities and other barriers; and (4) improve the collective experience of every member of the care team, practice leadership, and clinician-patient partnership.
Corresponding author: J. Nwando Olayiwola, MD, MPH, FAAFP, The Ohio State University College of Medicine, Department of Family and Community Medicine, 2231 N High St, Ste 250, Columbus, OH 43210; [email protected].
Financial disclosures: None.
Keywords: physician onboarding; Quadruple Aim; leadership; clinician satisfaction; care team satisfaction.
1. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6): 573-576.
2. Maurer R. Onboarding key to retaining, engaging talent. Society for Human Resource Management. April 16, 2015. Accessed January 8, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/onboarding-key-retaining-engaging-talent.aspx
3. Boston AG. New hire onboarding standardization and automation powers productivity gains. GlobeNewswire. March 8, 2011. Accessed January 8, 2021. http://www.globenewswire.com/news-release/2011/03/08/994239/0/en/New-Hire-Onboarding-Standardization-and-Automation-Powers-Productivity-Gains.html
4. $37 billion – US and UK business count the cost of employee misunderstanding. HR.com – Maximizing Human Potential. June 18, 2008. Accessed March 10, 2021. https://www.hr.com/en/communities/staffing_and_recruitment/37-billion---us-and-uk-businesses-count-the-cost-o_fhnduq4d.html
5. Employers risk driving new hires away with poor onboarding. Society for Human Resource Management. February 23, 2018. Accessed March 10, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/employers-new-hires-poor-onboarding.aspx
6. Center for Primary Care Innovation and Transformation. The Ohio State University College of Medicine. Accessed January 8, 2021. https://wexnermedical.osu.edu/departments/family-medicine/pcit
7. Olayiwola, J.N. and Magaña, C. Clinical transformation in technology: a fresh change management approach for primary care. Harvard Health Policy Review. February 2, 2019. Accessed March 10, 2021. http://www.hhpronline.org/articles/2019/2/2/clinical-transformation-in-technology-a-fresh-change-management-approach-for-primary-care
1. Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6): 573-576.
2. Maurer R. Onboarding key to retaining, engaging talent. Society for Human Resource Management. April 16, 2015. Accessed January 8, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/onboarding-key-retaining-engaging-talent.aspx
3. Boston AG. New hire onboarding standardization and automation powers productivity gains. GlobeNewswire. March 8, 2011. Accessed January 8, 2021. http://www.globenewswire.com/news-release/2011/03/08/994239/0/en/New-Hire-Onboarding-Standardization-and-Automation-Powers-Productivity-Gains.html
4. $37 billion – US and UK business count the cost of employee misunderstanding. HR.com – Maximizing Human Potential. June 18, 2008. Accessed March 10, 2021. https://www.hr.com/en/communities/staffing_and_recruitment/37-billion---us-and-uk-businesses-count-the-cost-o_fhnduq4d.html
5. Employers risk driving new hires away with poor onboarding. Society for Human Resource Management. February 23, 2018. Accessed March 10, 2021. https://www.shrm.org/resourcesandtools/hr-topics/talent-acquisition/pages/employers-new-hires-poor-onboarding.aspx
6. Center for Primary Care Innovation and Transformation. The Ohio State University College of Medicine. Accessed January 8, 2021. https://wexnermedical.osu.edu/departments/family-medicine/pcit
7. Olayiwola, J.N. and Magaña, C. Clinical transformation in technology: a fresh change management approach for primary care. Harvard Health Policy Review. February 2, 2019. Accessed March 10, 2021. http://www.hhpronline.org/articles/2019/2/2/clinical-transformation-in-technology-a-fresh-change-management-approach-for-primary-care
An Analysis of the Involvement and Attitudes of Resident Physicians in Reporting Errors in Patient Care
From Adelante Healthcare, Mesa, AZ (Dr. Chin), University Hospitals of Cleveland, Cleveland, OH (Drs. Delozier, Bascug, Levine, Bejanishvili, and Wynbrandt and Janet C. Peachey, Rachel M. Cerminara, and Sharon M. Darkovich), and Houston Methodist Hospitals, Houston, TX (Dr. Bhakta).
Abstract
Background: Resident physicians play an active role in the reporting of errors that occur in patient care. Previous studies indicate that residents significantly underreport errors in patient care.
Methods: Fifty-four of 80 eligible residents enrolled at University Hospitals–Regional Hospitals (UH-RH) during the 2018-2019 academic year completed a survey assessing their knowledge and experience in completing Patient Advocacy and Shared Stories (PASS) reports, which serve as incident reports in the UH health system in reporting errors in patient care. A series of interventions aimed at educating residents about the PASS report system were then conducted. The 54 residents who completed the first survey received it again 4 months later.
Results: Residents demonstrated greater understanding of when filing PASS reports was appropriate after the intervention, as significantly more residents reported having been involved in a situation where they should have filed a PASS report but did not (P = 0.036).
Conclusion: In this study, residents often did not report errors in patient care because they simply did not know the process for doing so. In addition, many residents often felt that the reporting of patient errors could be used as a form of retaliation.
Keywords: resident physicians; quality improvement; high-value care; medical errors; patient safety.
Resident physicians play a critical role in patient care. Residents undergo extensive supervised training in order to one day be able to practice medicine in an unsupervised setting, with the goal of providing the highest quality of care possible. One study reported that primary care provided by residents in a training program is of similar or higher quality than that provided by attending physicians.1
Besides providing high-quality care, it is important that residents play an active role in the reporting of errors that occur regarding patient care as well as in identifying events that may compromise patient safety and quality.2 In fact, increased reporting of patient errors has been shown to decrease liability-related costs for hospitals.3 Unfortunately, physicians, and residents in particular, have historically been poor reporters of errors in patient care.4 This is especially true when comparing physicians to other health professionals, such as nurses, in error reporting.5
Several studies have examined the involvement of residents in reporting errors in patient care. One recent study showed that a graduate medical education financial incentive program significantly increased the number of patient safety events reported by residents and fellows.6 This study, along with several others, supports the concept of using incentives to help improve the reporting of errors in patient care for physicians in training.7-10 Another study used Quality Improvement Knowledge Assessment Tool (QIKAT) scores to assess quality improvement (QI) knowledge. The study demonstrated that self-assessment scores of QI skills using QIKAT scores improved following a targeted intervention.11 Because further information on the involvement and attitudes of residents in reporting errors in patient care is needed, University Hospitals of Cleveland (UH) designed and implemented a QI study during the 2018-2019 academic year. This prospective study used anonymous surveys to objectively examine the involvement and attitudes of residents in reporting errors in patient care.
Methods
The UH health system uses Patient Advocacy and Shared Stories (PASS) reports as incident reports to not only disclose errors in patient care but also to identify any events that may compromise patient safety and quality. Based on preliminary review, nurses, ancillary staff, and administrators file the majority of PASS reports.
The study group consisted of residents at University Hospitals–Regional Hospitals (UH-RH), which is comprised of 2 hospitals: University Hospitals–Richmond Medical Center (UH-RMC) and University Hospitals –Bedford Medical Center (UH-BMC). UH-RMC and UH-BMC are 2 medium-sized university-affiliated community hospitals located in the Cleveland metropolitan area in Northeast Ohio. Both serve as clinical training sites for Case Western Reserve University School of Medicine and Lake Erie College of Osteopathic Medicine, the latter of which helped fund this study. The study was submitted to the Institutional Review Board (IRB) of University Hospitals of Cleveland and granted “not human subjects research” status as a QI study.
Surveys
UH-RH offers residency programs in dermatology, emergency medicine, family medicine, internal medicine, orthopedic surgery, and physical medicine and rehabilitation, along with a 1-year transitional/preliminary year. A total of 80 residents enrolled at UH-RH during the 2018-2019 academic year. All 80 residents at UH-RH received an email in December 2018 asking them to complete an anonymous survey regarding the PASS report system. The survey was administered using the REDCap software system and consisted of 15 multiple-choice questions. As an incentive for completing the survey, residents were offered a $10 Amazon gift card. The gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey. At the end of the week, 54 of 80 residents completed the first survey.
Following the first survey, efforts were undertaken by the study authors, in conjunction with the quality improvement department at UH-RH, to educate residents about the PASS report system. These interventions included giving a lecture on the PASS report system during resident didactic sessions, sending an email to all residents about the PASS report system, and providing residents an opportunity to complete an optional online training course regarding the PASS report system. As an incentive for completing the online training course, residents were offered a $10 Amazon gift card. As before, the gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine.
A second survey was administered in April 2019, 4 months after the first survey. To determine whether the intervention made an impact on the involvement and attitudes of residents in the reporting errors in patient care, only residents who completed the first survey were sent the second survey. The second survey consisted of the same questions as the first survey and was also administered using the REDCap software system. As an incentive for completing the survey, residents were offered another $10 Amazon gift card, again were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey.
Analysis
Chi-square analyses were utilized to examine differences between preintervention and postintervention responses across categories. All analyses were conducted using R statistical software, version 3.6.1 (R Foundation for Statistical Computing).
Results
A total of 54 of 80 eligible residents responded to the first survey (Table). Twenty-nine of 54 eligible residents responded to the second survey. Postintervention, significantly more residents indicated being involved in a situation where they should have filed a PASS report but did not (58.6% vs 53.7%; P = 0.036). Improvement was seen in PASS knowledge postintervention, where fewer residents reported not knowing how to file a PASS report (31.5% vs 55.2%; P = 0.059). No other improvements were significant, nor were there significant differences in responses between any other categories pre- and postintervention.
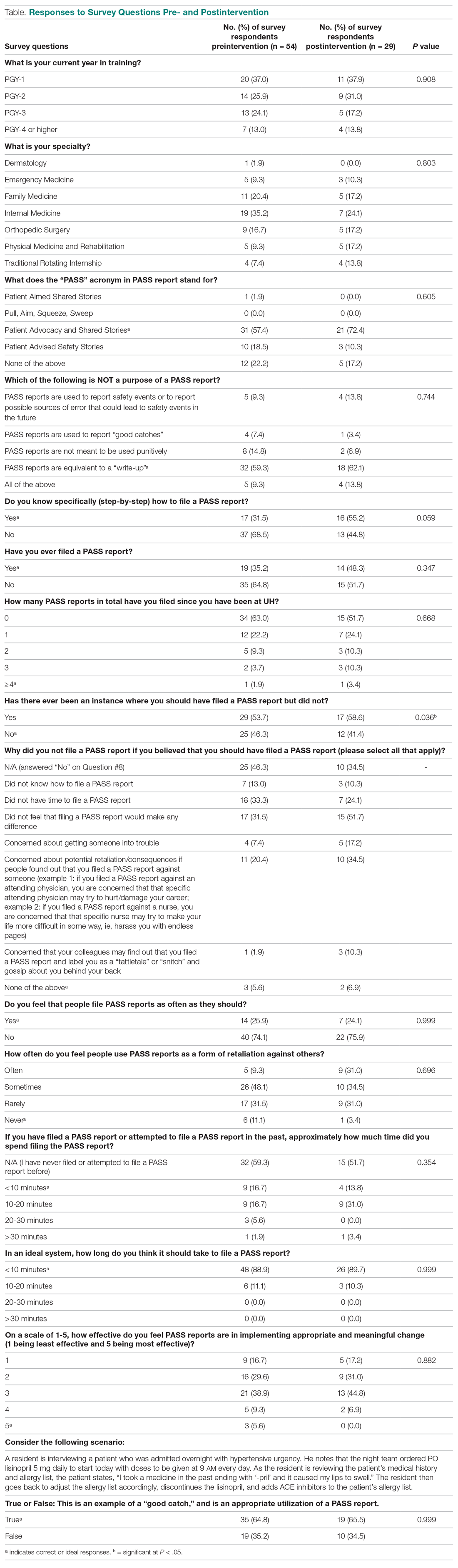
Discussion
Errors in patient care are a common occurrence in the hospital setting. Reporting errors when they happen is important for hospitals to gain data and better care for patients, but studies show that patient errors are usually underreported. This is concerning, as data on errors and other aspects of patient care are needed to inform quality improvement programs.
This study measured residents’ attitudes and knowledge regarding the filing of a PASS report. It also aimed to increase both the frequency of and knowledge about filing a PASS report with interventions. The results from each survey indicated a statistically significant increase in knowledge of when to file a PASS report. In the first survey, 53.7% of residents responded they they were involved in an instance where they should have filed a PASS report but did not. In the second survey, 58.5% of residents reported being involved in an instance where they should have filed a PASS report but did not. This difference was statistically significant (P = 0.036), sugesting that the intervention was successful at increasing residents’ knowledge regarding PASS reports and the appropriate times to file a PASS report.
The survey results also showed a trend toward increasing aggregate knowledge level of how to file PASS reports on the first survey and second surveys (from 31.5% vs 55.2%. This demonstrates an increase in knowledge of how to file a PASS report among residents at our hospital after the intervention. It should be noted that the intervention that was performed in this study was simple, easy to perform, and can be completed at any hospital system that uses a similar system for reporting patient errors.
Another important trend indicating the effectiveness of the intervention was a 15% increase in knowledge of what the PASS report acronym stands for, along with a 13.1% aggregate increase in the number of residents who filed a PASS report. This indicated that residents may have wanted to file a PASS report previously but simply did not know how to until the intervention. In addition, there was also a decrease in the aggregate percentages of residents who had never filed a PASS report and an increase in how many PASS reports were filed.
While PASS reports are a great way for hospitals to gain data and insight into problems at their sites, there was also a negative view of PASS reports. For example, a large percentage of residents indicated that filing a PASS report would not make any difference and that PASS reports are often used as a form of retaliation, either against themselves as the submitter or the person(s) mentioned in the PASS report. More specifically, more than 50% of residents felt that PASS reports were sometimes or often used as a form of retaliation against others. While many residents correctly identified in the survey that PASS reports are not equivalent to a “write-up,” it is concerning that they still feel there is a strong potential for retaliation when filing a PASS report. This finding is unfortunate but matches the results of a multicenter study that found that 44.6% of residents felt uncomfortable reporting patient errors, possibly secondary to fear of retaliation, along with issues with the reporting system.12
It is interesting to note that a minority of residents indicated that they feel that PASS reports are filed as often as they should be (25.9% on first survey and 24.1% on second survey). This is concerning, as the data gathered through PASS reports is used to improve patient care. However, the percentage reported in our study, although low, is higher than that reported in a similar study involving patients with Medicare insurance, which showed that only 14% of patient safety events were reported.13 These results demonstrate that further interventions are necessary in order to ensure that a PASS report is filed each time a patient safety event occurs.
Another finding of note is that the majority of residents also feel that the process of filing a PASS report is too time consuming. The majority of residents who have completed a PASS report stated that it took them between 10 and 20 minutes to complete a PASS report, but those same individuals also feel that it should take < 10 minutes to complete a PASS report. This is an important issue for hospital systems to address. Reducing the time it takes to file a PASS report may facilitate an increase in the amount of PASS reports filed.
We administered our surveys using email outreach to residents asking them to complete an anonymous online survey regarding the PASS report system using the REDCap software system. Researchers have various ways of administering surveys, ranging from paper surveys, emails, and even mobile apps. One study showed that online surveys tend to have higher response rates compared to non-online surveys, such as paper surveys and telephone surveys, which is likely due to the ease of use of online surveys.14 At the same time, unsolicited email surveys have been shown to have a negative influence on response rates. Mobile apps are a new way of administering surveys. However, research has not found any significant difference in the time required to complete the survey using mobile apps compared to other forms of administering surveys. In addition, surveys using mobile apps did not have increased response rates compared to other forms of administering surveys.15
To increase the response rate of our surveys, we offered gift cards to the study population for completing the survey. Studies have shown that surveys that offer incentives tend to have higher response rates than surveys that do not.16 Also, in addition to serving as a method for gathering data from our study population, we used our surveys as an intervention to increase awareness of PASS reporting, as reported in other studies. For example, another study used the HABITS questionnaire to not only gather information about children’s diet, but also to promote behavioral change towards healthy eating habits.17
This study had several limitations. First, the study was conducted using an anonymous online survey, which means we could not clarify questions that residents found confusing or needed further explanation. For example, 17 residents indicated in the first survey that they knew how to PASS report, but 19 residents indicated in the same survey that they have filed a PASS report in the past.
A second limitation of the study was that fewer residents completed the second survey (29 of 54 eligible residents) compared to the first survey (54 of 80 eligible residents). This may have impacted the results of the analysis, as certain findings were not statistically significant, despite trends in the data.
A third limitation of the study is that not all of the residents that completed the first and second surveys completed the entire intervention. For example, some residents did not attend the didactic lecture discussing PASS reports, and as such may not have received the appropriate training prior to completing the second survey.
The findings from this study can be used by the residency programs at UH-RH and by residency programs across the country to improve the involvement and attitudes of residents in reporting errors in patient care. Hospital staff need to be encouraged and educated on how to better report patient errors and the importance of reporting these errors. It would benefit hospital systems to provide continued and targeted training to familiarize physicians with the process of reporting patient errors, and take steps to reduce the time it takes to report patient errors. By increasing the reporting of errors, hospitals will be able to improve patient care through initiatives aimed at preventing errors.
Conclusion
Residents play an important role in providing high-quality care for patients. Part of providing high-quality care is the reporting of errors in patient care when they occur. Physicians, and in particular, residents, have historically underreported errors in patient care. Part of this underreporting results from residents not knowing or understanding the process of filing a report and feeling that the reports could be used as a form of retaliation. For hospital systems to continue to improve patient care, it is important for residents to not only know how to report errors in patient care but to feel comfortable doing so.
Corresponding author: Andrew J. Chin, DO, MS, MPH, Department of Internal Medicine, Adelante Healthcare, 1705 W Main St, Mesa, AZ 85201; [email protected].
Financial disclosures: None.
Funding: This study was funded by a research grant provided by Lake Eric College of Osteopathic Medicine to Andrew J. Chin and Anish Bhakta.
1. Zallman L, Ma J, Xiao L, Lasser KE. Quality of US primary care delivered by resident and staff physicians. J Gen Intern Med. 2010;25(11):1193-1197.
2. Bagain JP. The future of graduate medical education: a systems-based approach to ensure patient safety. Acad Med. 2015;90(9):1199-1202.
3. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical disclosure program. Ann Intern Med. 2010;153(4):213-221.
4. Kaldjian LC, Jones EW, Wu BJ, et al. Reporting medical errors to improve patient safety: a survey of physicians in teaching hospitals. Arch Intern Med. 2008;168(1):40-46.
5. Rowin EJ, Lucier D, Pauker SG, et al. Does error and adverse event reporting by physicians and nurses differ? Jt Comm J Qual Patient Saf. 2008;34(9):537-545.
6. Turner DA, Bae J, Cheely G, et al. Improving resident and fellow engagement in patient safety through a graduate medical education incentive program. J Grad Med Educ. 2018;10(6):671-675.
7. Macht R, Balen A, McAneny D, Hess D. A multifaceted intervention to increase surgery resident engagement in reporting adverse events. J Surg Educ. 2015;72(6):e117-e122.
8. Scott DR, Weimer M, English C, et al. A novel approach to increase residents’ involvement in reporting adverse events. Acad Med. 2011;86(6):742-746.
9. Stewart DA, Junn J, Adams MA, et al. House staff participation in patient safety reporting: identification of predominant barriers and implementation of a pilot program. South Med J. 2016;109(7):395-400.
10. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468.
11. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252.
12. Wijesekera TP, Sanders L, Windish DM. Education and reporting of diagnostic errors among physicians in internal medicine training programs. JAMA Intern Med. 2018;178(11):1548-1549.
13. Levinson DR. Hospital incident reporting systems do not capture most patient harm. Washington, D.C.: U.S. Department of Health and Human Services Office of the Inspector General. January 2012. Report No. OEI-06-09-00091.
14. Evans JR, Mathur A. The value of online surveys. Internet Research. 2005;15(2):192-219.
15. Marcano Belisario JS, Jamsek J, Huckvale K, et al. Comparison of self‐administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database of Syst Rev. 2015;7:MR000042.
16. Manfreda KL, Batagelj Z, Vehovar V. Design of web survey questionnaires: three basic experiments. J Comput Mediat Commun. 2002;7(3):JCMC731.
17. Wright ND, Groisman‐Perelstein AE, Wylie‐Rosett J, et al. A lifestyle assessment and intervention tool for pediatric weight management: the HABITS questionnaire. J Hum Nutr Diet. 2011;24(1):96-100.
From Adelante Healthcare, Mesa, AZ (Dr. Chin), University Hospitals of Cleveland, Cleveland, OH (Drs. Delozier, Bascug, Levine, Bejanishvili, and Wynbrandt and Janet C. Peachey, Rachel M. Cerminara, and Sharon M. Darkovich), and Houston Methodist Hospitals, Houston, TX (Dr. Bhakta).
Abstract
Background: Resident physicians play an active role in the reporting of errors that occur in patient care. Previous studies indicate that residents significantly underreport errors in patient care.
Methods: Fifty-four of 80 eligible residents enrolled at University Hospitals–Regional Hospitals (UH-RH) during the 2018-2019 academic year completed a survey assessing their knowledge and experience in completing Patient Advocacy and Shared Stories (PASS) reports, which serve as incident reports in the UH health system in reporting errors in patient care. A series of interventions aimed at educating residents about the PASS report system were then conducted. The 54 residents who completed the first survey received it again 4 months later.
Results: Residents demonstrated greater understanding of when filing PASS reports was appropriate after the intervention, as significantly more residents reported having been involved in a situation where they should have filed a PASS report but did not (P = 0.036).
Conclusion: In this study, residents often did not report errors in patient care because they simply did not know the process for doing so. In addition, many residents often felt that the reporting of patient errors could be used as a form of retaliation.
Keywords: resident physicians; quality improvement; high-value care; medical errors; patient safety.
Resident physicians play a critical role in patient care. Residents undergo extensive supervised training in order to one day be able to practice medicine in an unsupervised setting, with the goal of providing the highest quality of care possible. One study reported that primary care provided by residents in a training program is of similar or higher quality than that provided by attending physicians.1
Besides providing high-quality care, it is important that residents play an active role in the reporting of errors that occur regarding patient care as well as in identifying events that may compromise patient safety and quality.2 In fact, increased reporting of patient errors has been shown to decrease liability-related costs for hospitals.3 Unfortunately, physicians, and residents in particular, have historically been poor reporters of errors in patient care.4 This is especially true when comparing physicians to other health professionals, such as nurses, in error reporting.5
Several studies have examined the involvement of residents in reporting errors in patient care. One recent study showed that a graduate medical education financial incentive program significantly increased the number of patient safety events reported by residents and fellows.6 This study, along with several others, supports the concept of using incentives to help improve the reporting of errors in patient care for physicians in training.7-10 Another study used Quality Improvement Knowledge Assessment Tool (QIKAT) scores to assess quality improvement (QI) knowledge. The study demonstrated that self-assessment scores of QI skills using QIKAT scores improved following a targeted intervention.11 Because further information on the involvement and attitudes of residents in reporting errors in patient care is needed, University Hospitals of Cleveland (UH) designed and implemented a QI study during the 2018-2019 academic year. This prospective study used anonymous surveys to objectively examine the involvement and attitudes of residents in reporting errors in patient care.
Methods
The UH health system uses Patient Advocacy and Shared Stories (PASS) reports as incident reports to not only disclose errors in patient care but also to identify any events that may compromise patient safety and quality. Based on preliminary review, nurses, ancillary staff, and administrators file the majority of PASS reports.
The study group consisted of residents at University Hospitals–Regional Hospitals (UH-RH), which is comprised of 2 hospitals: University Hospitals–Richmond Medical Center (UH-RMC) and University Hospitals –Bedford Medical Center (UH-BMC). UH-RMC and UH-BMC are 2 medium-sized university-affiliated community hospitals located in the Cleveland metropolitan area in Northeast Ohio. Both serve as clinical training sites for Case Western Reserve University School of Medicine and Lake Erie College of Osteopathic Medicine, the latter of which helped fund this study. The study was submitted to the Institutional Review Board (IRB) of University Hospitals of Cleveland and granted “not human subjects research” status as a QI study.
Surveys
UH-RH offers residency programs in dermatology, emergency medicine, family medicine, internal medicine, orthopedic surgery, and physical medicine and rehabilitation, along with a 1-year transitional/preliminary year. A total of 80 residents enrolled at UH-RH during the 2018-2019 academic year. All 80 residents at UH-RH received an email in December 2018 asking them to complete an anonymous survey regarding the PASS report system. The survey was administered using the REDCap software system and consisted of 15 multiple-choice questions. As an incentive for completing the survey, residents were offered a $10 Amazon gift card. The gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey. At the end of the week, 54 of 80 residents completed the first survey.
Following the first survey, efforts were undertaken by the study authors, in conjunction with the quality improvement department at UH-RH, to educate residents about the PASS report system. These interventions included giving a lecture on the PASS report system during resident didactic sessions, sending an email to all residents about the PASS report system, and providing residents an opportunity to complete an optional online training course regarding the PASS report system. As an incentive for completing the online training course, residents were offered a $10 Amazon gift card. As before, the gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine.
A second survey was administered in April 2019, 4 months after the first survey. To determine whether the intervention made an impact on the involvement and attitudes of residents in the reporting errors in patient care, only residents who completed the first survey were sent the second survey. The second survey consisted of the same questions as the first survey and was also administered using the REDCap software system. As an incentive for completing the survey, residents were offered another $10 Amazon gift card, again were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey.
Analysis
Chi-square analyses were utilized to examine differences between preintervention and postintervention responses across categories. All analyses were conducted using R statistical software, version 3.6.1 (R Foundation for Statistical Computing).
Results
A total of 54 of 80 eligible residents responded to the first survey (Table). Twenty-nine of 54 eligible residents responded to the second survey. Postintervention, significantly more residents indicated being involved in a situation where they should have filed a PASS report but did not (58.6% vs 53.7%; P = 0.036). Improvement was seen in PASS knowledge postintervention, where fewer residents reported not knowing how to file a PASS report (31.5% vs 55.2%; P = 0.059). No other improvements were significant, nor were there significant differences in responses between any other categories pre- and postintervention.

Discussion
Errors in patient care are a common occurrence in the hospital setting. Reporting errors when they happen is important for hospitals to gain data and better care for patients, but studies show that patient errors are usually underreported. This is concerning, as data on errors and other aspects of patient care are needed to inform quality improvement programs.
This study measured residents’ attitudes and knowledge regarding the filing of a PASS report. It also aimed to increase both the frequency of and knowledge about filing a PASS report with interventions. The results from each survey indicated a statistically significant increase in knowledge of when to file a PASS report. In the first survey, 53.7% of residents responded they they were involved in an instance where they should have filed a PASS report but did not. In the second survey, 58.5% of residents reported being involved in an instance where they should have filed a PASS report but did not. This difference was statistically significant (P = 0.036), sugesting that the intervention was successful at increasing residents’ knowledge regarding PASS reports and the appropriate times to file a PASS report.
The survey results also showed a trend toward increasing aggregate knowledge level of how to file PASS reports on the first survey and second surveys (from 31.5% vs 55.2%. This demonstrates an increase in knowledge of how to file a PASS report among residents at our hospital after the intervention. It should be noted that the intervention that was performed in this study was simple, easy to perform, and can be completed at any hospital system that uses a similar system for reporting patient errors.
Another important trend indicating the effectiveness of the intervention was a 15% increase in knowledge of what the PASS report acronym stands for, along with a 13.1% aggregate increase in the number of residents who filed a PASS report. This indicated that residents may have wanted to file a PASS report previously but simply did not know how to until the intervention. In addition, there was also a decrease in the aggregate percentages of residents who had never filed a PASS report and an increase in how many PASS reports were filed.
While PASS reports are a great way for hospitals to gain data and insight into problems at their sites, there was also a negative view of PASS reports. For example, a large percentage of residents indicated that filing a PASS report would not make any difference and that PASS reports are often used as a form of retaliation, either against themselves as the submitter or the person(s) mentioned in the PASS report. More specifically, more than 50% of residents felt that PASS reports were sometimes or often used as a form of retaliation against others. While many residents correctly identified in the survey that PASS reports are not equivalent to a “write-up,” it is concerning that they still feel there is a strong potential for retaliation when filing a PASS report. This finding is unfortunate but matches the results of a multicenter study that found that 44.6% of residents felt uncomfortable reporting patient errors, possibly secondary to fear of retaliation, along with issues with the reporting system.12
It is interesting to note that a minority of residents indicated that they feel that PASS reports are filed as often as they should be (25.9% on first survey and 24.1% on second survey). This is concerning, as the data gathered through PASS reports is used to improve patient care. However, the percentage reported in our study, although low, is higher than that reported in a similar study involving patients with Medicare insurance, which showed that only 14% of patient safety events were reported.13 These results demonstrate that further interventions are necessary in order to ensure that a PASS report is filed each time a patient safety event occurs.
Another finding of note is that the majority of residents also feel that the process of filing a PASS report is too time consuming. The majority of residents who have completed a PASS report stated that it took them between 10 and 20 minutes to complete a PASS report, but those same individuals also feel that it should take < 10 minutes to complete a PASS report. This is an important issue for hospital systems to address. Reducing the time it takes to file a PASS report may facilitate an increase in the amount of PASS reports filed.
We administered our surveys using email outreach to residents asking them to complete an anonymous online survey regarding the PASS report system using the REDCap software system. Researchers have various ways of administering surveys, ranging from paper surveys, emails, and even mobile apps. One study showed that online surveys tend to have higher response rates compared to non-online surveys, such as paper surveys and telephone surveys, which is likely due to the ease of use of online surveys.14 At the same time, unsolicited email surveys have been shown to have a negative influence on response rates. Mobile apps are a new way of administering surveys. However, research has not found any significant difference in the time required to complete the survey using mobile apps compared to other forms of administering surveys. In addition, surveys using mobile apps did not have increased response rates compared to other forms of administering surveys.15
To increase the response rate of our surveys, we offered gift cards to the study population for completing the survey. Studies have shown that surveys that offer incentives tend to have higher response rates than surveys that do not.16 Also, in addition to serving as a method for gathering data from our study population, we used our surveys as an intervention to increase awareness of PASS reporting, as reported in other studies. For example, another study used the HABITS questionnaire to not only gather information about children’s diet, but also to promote behavioral change towards healthy eating habits.17
This study had several limitations. First, the study was conducted using an anonymous online survey, which means we could not clarify questions that residents found confusing or needed further explanation. For example, 17 residents indicated in the first survey that they knew how to PASS report, but 19 residents indicated in the same survey that they have filed a PASS report in the past.
A second limitation of the study was that fewer residents completed the second survey (29 of 54 eligible residents) compared to the first survey (54 of 80 eligible residents). This may have impacted the results of the analysis, as certain findings were not statistically significant, despite trends in the data.
A third limitation of the study is that not all of the residents that completed the first and second surveys completed the entire intervention. For example, some residents did not attend the didactic lecture discussing PASS reports, and as such may not have received the appropriate training prior to completing the second survey.
The findings from this study can be used by the residency programs at UH-RH and by residency programs across the country to improve the involvement and attitudes of residents in reporting errors in patient care. Hospital staff need to be encouraged and educated on how to better report patient errors and the importance of reporting these errors. It would benefit hospital systems to provide continued and targeted training to familiarize physicians with the process of reporting patient errors, and take steps to reduce the time it takes to report patient errors. By increasing the reporting of errors, hospitals will be able to improve patient care through initiatives aimed at preventing errors.
Conclusion
Residents play an important role in providing high-quality care for patients. Part of providing high-quality care is the reporting of errors in patient care when they occur. Physicians, and in particular, residents, have historically underreported errors in patient care. Part of this underreporting results from residents not knowing or understanding the process of filing a report and feeling that the reports could be used as a form of retaliation. For hospital systems to continue to improve patient care, it is important for residents to not only know how to report errors in patient care but to feel comfortable doing so.
Corresponding author: Andrew J. Chin, DO, MS, MPH, Department of Internal Medicine, Adelante Healthcare, 1705 W Main St, Mesa, AZ 85201; [email protected].
Financial disclosures: None.
Funding: This study was funded by a research grant provided by Lake Eric College of Osteopathic Medicine to Andrew J. Chin and Anish Bhakta.
From Adelante Healthcare, Mesa, AZ (Dr. Chin), University Hospitals of Cleveland, Cleveland, OH (Drs. Delozier, Bascug, Levine, Bejanishvili, and Wynbrandt and Janet C. Peachey, Rachel M. Cerminara, and Sharon M. Darkovich), and Houston Methodist Hospitals, Houston, TX (Dr. Bhakta).
Abstract
Background: Resident physicians play an active role in the reporting of errors that occur in patient care. Previous studies indicate that residents significantly underreport errors in patient care.
Methods: Fifty-four of 80 eligible residents enrolled at University Hospitals–Regional Hospitals (UH-RH) during the 2018-2019 academic year completed a survey assessing their knowledge and experience in completing Patient Advocacy and Shared Stories (PASS) reports, which serve as incident reports in the UH health system in reporting errors in patient care. A series of interventions aimed at educating residents about the PASS report system were then conducted. The 54 residents who completed the first survey received it again 4 months later.
Results: Residents demonstrated greater understanding of when filing PASS reports was appropriate after the intervention, as significantly more residents reported having been involved in a situation where they should have filed a PASS report but did not (P = 0.036).
Conclusion: In this study, residents often did not report errors in patient care because they simply did not know the process for doing so. In addition, many residents often felt that the reporting of patient errors could be used as a form of retaliation.
Keywords: resident physicians; quality improvement; high-value care; medical errors; patient safety.
Resident physicians play a critical role in patient care. Residents undergo extensive supervised training in order to one day be able to practice medicine in an unsupervised setting, with the goal of providing the highest quality of care possible. One study reported that primary care provided by residents in a training program is of similar or higher quality than that provided by attending physicians.1
Besides providing high-quality care, it is important that residents play an active role in the reporting of errors that occur regarding patient care as well as in identifying events that may compromise patient safety and quality.2 In fact, increased reporting of patient errors has been shown to decrease liability-related costs for hospitals.3 Unfortunately, physicians, and residents in particular, have historically been poor reporters of errors in patient care.4 This is especially true when comparing physicians to other health professionals, such as nurses, in error reporting.5
Several studies have examined the involvement of residents in reporting errors in patient care. One recent study showed that a graduate medical education financial incentive program significantly increased the number of patient safety events reported by residents and fellows.6 This study, along with several others, supports the concept of using incentives to help improve the reporting of errors in patient care for physicians in training.7-10 Another study used Quality Improvement Knowledge Assessment Tool (QIKAT) scores to assess quality improvement (QI) knowledge. The study demonstrated that self-assessment scores of QI skills using QIKAT scores improved following a targeted intervention.11 Because further information on the involvement and attitudes of residents in reporting errors in patient care is needed, University Hospitals of Cleveland (UH) designed and implemented a QI study during the 2018-2019 academic year. This prospective study used anonymous surveys to objectively examine the involvement and attitudes of residents in reporting errors in patient care.
Methods
The UH health system uses Patient Advocacy and Shared Stories (PASS) reports as incident reports to not only disclose errors in patient care but also to identify any events that may compromise patient safety and quality. Based on preliminary review, nurses, ancillary staff, and administrators file the majority of PASS reports.
The study group consisted of residents at University Hospitals–Regional Hospitals (UH-RH), which is comprised of 2 hospitals: University Hospitals–Richmond Medical Center (UH-RMC) and University Hospitals –Bedford Medical Center (UH-BMC). UH-RMC and UH-BMC are 2 medium-sized university-affiliated community hospitals located in the Cleveland metropolitan area in Northeast Ohio. Both serve as clinical training sites for Case Western Reserve University School of Medicine and Lake Erie College of Osteopathic Medicine, the latter of which helped fund this study. The study was submitted to the Institutional Review Board (IRB) of University Hospitals of Cleveland and granted “not human subjects research” status as a QI study.
Surveys
UH-RH offers residency programs in dermatology, emergency medicine, family medicine, internal medicine, orthopedic surgery, and physical medicine and rehabilitation, along with a 1-year transitional/preliminary year. A total of 80 residents enrolled at UH-RH during the 2018-2019 academic year. All 80 residents at UH-RH received an email in December 2018 asking them to complete an anonymous survey regarding the PASS report system. The survey was administered using the REDCap software system and consisted of 15 multiple-choice questions. As an incentive for completing the survey, residents were offered a $10 Amazon gift card. The gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey. At the end of the week, 54 of 80 residents completed the first survey.
Following the first survey, efforts were undertaken by the study authors, in conjunction with the quality improvement department at UH-RH, to educate residents about the PASS report system. These interventions included giving a lecture on the PASS report system during resident didactic sessions, sending an email to all residents about the PASS report system, and providing residents an opportunity to complete an optional online training course regarding the PASS report system. As an incentive for completing the online training course, residents were offered a $10 Amazon gift card. As before, the gift cards were funded through a research grant from Lake Erie College of Osteopathic Medicine.
A second survey was administered in April 2019, 4 months after the first survey. To determine whether the intervention made an impact on the involvement and attitudes of residents in the reporting errors in patient care, only residents who completed the first survey were sent the second survey. The second survey consisted of the same questions as the first survey and was also administered using the REDCap software system. As an incentive for completing the survey, residents were offered another $10 Amazon gift card, again were funded through a research grant from Lake Erie College of Osteopathic Medicine. Residents were given 1 week to complete the survey.
Analysis
Chi-square analyses were utilized to examine differences between preintervention and postintervention responses across categories. All analyses were conducted using R statistical software, version 3.6.1 (R Foundation for Statistical Computing).
Results
A total of 54 of 80 eligible residents responded to the first survey (Table). Twenty-nine of 54 eligible residents responded to the second survey. Postintervention, significantly more residents indicated being involved in a situation where they should have filed a PASS report but did not (58.6% vs 53.7%; P = 0.036). Improvement was seen in PASS knowledge postintervention, where fewer residents reported not knowing how to file a PASS report (31.5% vs 55.2%; P = 0.059). No other improvements were significant, nor were there significant differences in responses between any other categories pre- and postintervention.

Discussion
Errors in patient care are a common occurrence in the hospital setting. Reporting errors when they happen is important for hospitals to gain data and better care for patients, but studies show that patient errors are usually underreported. This is concerning, as data on errors and other aspects of patient care are needed to inform quality improvement programs.
This study measured residents’ attitudes and knowledge regarding the filing of a PASS report. It also aimed to increase both the frequency of and knowledge about filing a PASS report with interventions. The results from each survey indicated a statistically significant increase in knowledge of when to file a PASS report. In the first survey, 53.7% of residents responded they they were involved in an instance where they should have filed a PASS report but did not. In the second survey, 58.5% of residents reported being involved in an instance where they should have filed a PASS report but did not. This difference was statistically significant (P = 0.036), sugesting that the intervention was successful at increasing residents’ knowledge regarding PASS reports and the appropriate times to file a PASS report.
The survey results also showed a trend toward increasing aggregate knowledge level of how to file PASS reports on the first survey and second surveys (from 31.5% vs 55.2%. This demonstrates an increase in knowledge of how to file a PASS report among residents at our hospital after the intervention. It should be noted that the intervention that was performed in this study was simple, easy to perform, and can be completed at any hospital system that uses a similar system for reporting patient errors.
Another important trend indicating the effectiveness of the intervention was a 15% increase in knowledge of what the PASS report acronym stands for, along with a 13.1% aggregate increase in the number of residents who filed a PASS report. This indicated that residents may have wanted to file a PASS report previously but simply did not know how to until the intervention. In addition, there was also a decrease in the aggregate percentages of residents who had never filed a PASS report and an increase in how many PASS reports were filed.
While PASS reports are a great way for hospitals to gain data and insight into problems at their sites, there was also a negative view of PASS reports. For example, a large percentage of residents indicated that filing a PASS report would not make any difference and that PASS reports are often used as a form of retaliation, either against themselves as the submitter or the person(s) mentioned in the PASS report. More specifically, more than 50% of residents felt that PASS reports were sometimes or often used as a form of retaliation against others. While many residents correctly identified in the survey that PASS reports are not equivalent to a “write-up,” it is concerning that they still feel there is a strong potential for retaliation when filing a PASS report. This finding is unfortunate but matches the results of a multicenter study that found that 44.6% of residents felt uncomfortable reporting patient errors, possibly secondary to fear of retaliation, along with issues with the reporting system.12
It is interesting to note that a minority of residents indicated that they feel that PASS reports are filed as often as they should be (25.9% on first survey and 24.1% on second survey). This is concerning, as the data gathered through PASS reports is used to improve patient care. However, the percentage reported in our study, although low, is higher than that reported in a similar study involving patients with Medicare insurance, which showed that only 14% of patient safety events were reported.13 These results demonstrate that further interventions are necessary in order to ensure that a PASS report is filed each time a patient safety event occurs.
Another finding of note is that the majority of residents also feel that the process of filing a PASS report is too time consuming. The majority of residents who have completed a PASS report stated that it took them between 10 and 20 minutes to complete a PASS report, but those same individuals also feel that it should take < 10 minutes to complete a PASS report. This is an important issue for hospital systems to address. Reducing the time it takes to file a PASS report may facilitate an increase in the amount of PASS reports filed.
We administered our surveys using email outreach to residents asking them to complete an anonymous online survey regarding the PASS report system using the REDCap software system. Researchers have various ways of administering surveys, ranging from paper surveys, emails, and even mobile apps. One study showed that online surveys tend to have higher response rates compared to non-online surveys, such as paper surveys and telephone surveys, which is likely due to the ease of use of online surveys.14 At the same time, unsolicited email surveys have been shown to have a negative influence on response rates. Mobile apps are a new way of administering surveys. However, research has not found any significant difference in the time required to complete the survey using mobile apps compared to other forms of administering surveys. In addition, surveys using mobile apps did not have increased response rates compared to other forms of administering surveys.15
To increase the response rate of our surveys, we offered gift cards to the study population for completing the survey. Studies have shown that surveys that offer incentives tend to have higher response rates than surveys that do not.16 Also, in addition to serving as a method for gathering data from our study population, we used our surveys as an intervention to increase awareness of PASS reporting, as reported in other studies. For example, another study used the HABITS questionnaire to not only gather information about children’s diet, but also to promote behavioral change towards healthy eating habits.17
This study had several limitations. First, the study was conducted using an anonymous online survey, which means we could not clarify questions that residents found confusing or needed further explanation. For example, 17 residents indicated in the first survey that they knew how to PASS report, but 19 residents indicated in the same survey that they have filed a PASS report in the past.
A second limitation of the study was that fewer residents completed the second survey (29 of 54 eligible residents) compared to the first survey (54 of 80 eligible residents). This may have impacted the results of the analysis, as certain findings were not statistically significant, despite trends in the data.
A third limitation of the study is that not all of the residents that completed the first and second surveys completed the entire intervention. For example, some residents did not attend the didactic lecture discussing PASS reports, and as such may not have received the appropriate training prior to completing the second survey.
The findings from this study can be used by the residency programs at UH-RH and by residency programs across the country to improve the involvement and attitudes of residents in reporting errors in patient care. Hospital staff need to be encouraged and educated on how to better report patient errors and the importance of reporting these errors. It would benefit hospital systems to provide continued and targeted training to familiarize physicians with the process of reporting patient errors, and take steps to reduce the time it takes to report patient errors. By increasing the reporting of errors, hospitals will be able to improve patient care through initiatives aimed at preventing errors.
Conclusion
Residents play an important role in providing high-quality care for patients. Part of providing high-quality care is the reporting of errors in patient care when they occur. Physicians, and in particular, residents, have historically underreported errors in patient care. Part of this underreporting results from residents not knowing or understanding the process of filing a report and feeling that the reports could be used as a form of retaliation. For hospital systems to continue to improve patient care, it is important for residents to not only know how to report errors in patient care but to feel comfortable doing so.
Corresponding author: Andrew J. Chin, DO, MS, MPH, Department of Internal Medicine, Adelante Healthcare, 1705 W Main St, Mesa, AZ 85201; [email protected].
Financial disclosures: None.
Funding: This study was funded by a research grant provided by Lake Eric College of Osteopathic Medicine to Andrew J. Chin and Anish Bhakta.
1. Zallman L, Ma J, Xiao L, Lasser KE. Quality of US primary care delivered by resident and staff physicians. J Gen Intern Med. 2010;25(11):1193-1197.
2. Bagain JP. The future of graduate medical education: a systems-based approach to ensure patient safety. Acad Med. 2015;90(9):1199-1202.
3. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical disclosure program. Ann Intern Med. 2010;153(4):213-221.
4. Kaldjian LC, Jones EW, Wu BJ, et al. Reporting medical errors to improve patient safety: a survey of physicians in teaching hospitals. Arch Intern Med. 2008;168(1):40-46.
5. Rowin EJ, Lucier D, Pauker SG, et al. Does error and adverse event reporting by physicians and nurses differ? Jt Comm J Qual Patient Saf. 2008;34(9):537-545.
6. Turner DA, Bae J, Cheely G, et al. Improving resident and fellow engagement in patient safety through a graduate medical education incentive program. J Grad Med Educ. 2018;10(6):671-675.
7. Macht R, Balen A, McAneny D, Hess D. A multifaceted intervention to increase surgery resident engagement in reporting adverse events. J Surg Educ. 2015;72(6):e117-e122.
8. Scott DR, Weimer M, English C, et al. A novel approach to increase residents’ involvement in reporting adverse events. Acad Med. 2011;86(6):742-746.
9. Stewart DA, Junn J, Adams MA, et al. House staff participation in patient safety reporting: identification of predominant barriers and implementation of a pilot program. South Med J. 2016;109(7):395-400.
10. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468.
11. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252.
12. Wijesekera TP, Sanders L, Windish DM. Education and reporting of diagnostic errors among physicians in internal medicine training programs. JAMA Intern Med. 2018;178(11):1548-1549.
13. Levinson DR. Hospital incident reporting systems do not capture most patient harm. Washington, D.C.: U.S. Department of Health and Human Services Office of the Inspector General. January 2012. Report No. OEI-06-09-00091.
14. Evans JR, Mathur A. The value of online surveys. Internet Research. 2005;15(2):192-219.
15. Marcano Belisario JS, Jamsek J, Huckvale K, et al. Comparison of self‐administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database of Syst Rev. 2015;7:MR000042.
16. Manfreda KL, Batagelj Z, Vehovar V. Design of web survey questionnaires: three basic experiments. J Comput Mediat Commun. 2002;7(3):JCMC731.
17. Wright ND, Groisman‐Perelstein AE, Wylie‐Rosett J, et al. A lifestyle assessment and intervention tool for pediatric weight management: the HABITS questionnaire. J Hum Nutr Diet. 2011;24(1):96-100.
1. Zallman L, Ma J, Xiao L, Lasser KE. Quality of US primary care delivered by resident and staff physicians. J Gen Intern Med. 2010;25(11):1193-1197.
2. Bagain JP. The future of graduate medical education: a systems-based approach to ensure patient safety. Acad Med. 2015;90(9):1199-1202.
3. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical disclosure program. Ann Intern Med. 2010;153(4):213-221.
4. Kaldjian LC, Jones EW, Wu BJ, et al. Reporting medical errors to improve patient safety: a survey of physicians in teaching hospitals. Arch Intern Med. 2008;168(1):40-46.
5. Rowin EJ, Lucier D, Pauker SG, et al. Does error and adverse event reporting by physicians and nurses differ? Jt Comm J Qual Patient Saf. 2008;34(9):537-545.
6. Turner DA, Bae J, Cheely G, et al. Improving resident and fellow engagement in patient safety through a graduate medical education incentive program. J Grad Med Educ. 2018;10(6):671-675.
7. Macht R, Balen A, McAneny D, Hess D. A multifaceted intervention to increase surgery resident engagement in reporting adverse events. J Surg Educ. 2015;72(6):e117-e122.
8. Scott DR, Weimer M, English C, et al. A novel approach to increase residents’ involvement in reporting adverse events. Acad Med. 2011;86(6):742-746.
9. Stewart DA, Junn J, Adams MA, et al. House staff participation in patient safety reporting: identification of predominant barriers and implementation of a pilot program. South Med J. 2016;109(7):395-400.
10. Vidyarthi AR, Green AL, Rosenbluth G, Baron RB. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89(3):460-468.
11. Fok MC, Wong RY. Impact of a competency based curriculum on quality improvement among internal medicine residents. BMC Med Educ. 2014;14:252.
12. Wijesekera TP, Sanders L, Windish DM. Education and reporting of diagnostic errors among physicians in internal medicine training programs. JAMA Intern Med. 2018;178(11):1548-1549.
13. Levinson DR. Hospital incident reporting systems do not capture most patient harm. Washington, D.C.: U.S. Department of Health and Human Services Office of the Inspector General. January 2012. Report No. OEI-06-09-00091.
14. Evans JR, Mathur A. The value of online surveys. Internet Research. 2005;15(2):192-219.
15. Marcano Belisario JS, Jamsek J, Huckvale K, et al. Comparison of self‐administered survey questionnaire responses collected using mobile apps versus other methods. Cochrane Database of Syst Rev. 2015;7:MR000042.
16. Manfreda KL, Batagelj Z, Vehovar V. Design of web survey questionnaires: three basic experiments. J Comput Mediat Commun. 2002;7(3):JCMC731.
17. Wright ND, Groisman‐Perelstein AE, Wylie‐Rosett J, et al. A lifestyle assessment and intervention tool for pediatric weight management: the HABITS questionnaire. J Hum Nutr Diet. 2011;24(1):96-100.
Top JAMA editor on leave amid podcast investigation
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
Paving the way for diversity in clinical trials
“I’m the first person in my circle of family and friends to participate in a clinical trial.”
Five years ago, Rhonda Long was diagnosed with cholangiocarcinoma, a rare bile duct cancer that’s seen in only about 8,000 Americans each year.
At the time, Mrs. Long, who is Black, said her doctor in Dayton, Ohio, told her she was not a candidate for surgery and suggested palliative care. After seeking a second opinion at Duke University Medical Center, Durham, N.C., where her sister worked, the 51-year-old wife and mother of two had surgery, radiation, and chemotherapy there in North Carolina. When the chemo stopped working after 3 months, her oncologist at Duke referred her to a colleague at Massachusetts General Hospital in Boston, where she was accepted into a clinical trial.
“In 2019, I traveled to Boston from Dayton, Ohio, every 3 weeks for labs and scans, to make sure that the drug wasn’t doing more harm than good, making sure that the drug as developed was maintaining, shrinking, or even eliminating the disease. Physically and financially, it takes a toll on you and loved ones.”
Her medical insurance did not cover the direct expenses from the clinical trial, and she was spending $1,000-$1,500 each trip. Sometimes they drove the 15 hours to Boston, and sometimes they flew on the cheapest flight they could find.
It’s not an unfamiliar story: people traveling, often long distances, to take part in clinical trials they hope will save their lives.
The Lazarex Cancer Foundation of Danville, Calif., helped Mrs. Long do just that.
Marya Shegog, PhD, health equity and diversity coordinator at Lazarex, said that a patient travels an average of 500 miles to participate in a trial.
The financial hurdles often prevent patients from taking part in clinical trials, Dr. Shegog said. “When you are sick, and you have a disease that may be terminal, you start thinking about setting your things in order.”
Many patients have to make a decision.
“Do I bankrupt my family on trying and hoping that this drug works and helps me live longer, or do I start setting things in order so that when I’m gone, they’re okay or at least better than if I wouldn’t have spent all the money traveling back and forth.”
Dr. Shegog, a 17-year cancer survivor, says when she was battling cervical cancer, a clinical trial was never offered or explored.
Lazarex has been helping cancer patients who have run out of options for 15 years. It identifies clinical trial opportunities and reimburses patients for all travel costs. Last year, Lazarex reimbursed more than 1,000 cancer patients. And it has supported more than 6,000 people since opening its doors.
“Lazarex exists to help remove the barriers of people not being able to participate in trials,” Dr. Shegog said. “It’s systemic that the medical system does not treat patients the same and oftentimes does not offer or make aware the opportunities for African Americans to participate.”
But now, thanks in part to COVID-19, new possibilities are taking shape. The pandemic has changed the landscape for trials, forcing many of them to go virtual, which allows patients to schedule telehealth visits and get some services like bloodwork and CT or MRI scans closer to home. Mrs. Long’s trial eventually went virtual.
“It was absolutely fantastic,” she said. “Having the trial locally, it saves us money, it saves wear and tear on my body. Being in the car, being in an airport or in a plane and in a hotel, all of that wears on you physically.”
The move to virtual studies may have lasting effects on research and treatment.
“The current pandemic has forced us to reexamine all of the traditional burdens we place on patients as it relates to receiving cancer treatment,” said Hala Borno, MD, an assistant professor of medicine at the University of California, San Francisco. “Whether they’re coming to our health care facility to see a clinician, for diagnostics such as blood draws and scans, or to receive therapy, this pandemic has challenged us to explore other possibilities that minimize the risk of exposure to SARS-CoV-2. What I find striking is that it has helped us operationalize use of telemedicine and the delivery of care closer to home.”
This is especially encouraging news for minority patients whose participation in trials has for years lagged well behind that of Whites.
But travel is not the only reason. Racial disparities in clinical trials have long been an issue that’s just another part of the implicit bias in health care.
Compared with White people, Black people are largely at higher risk for heart disease, cancer, stroke, diabetes, asthma, and even mental health problems.
And it’s not just African Americans. Asians, Hispanics, Native Americans, and Alaska Natives are all underrepresented in trials at a time when there is growing evidence that drugs may have different effects on different populations.
Dr. Borno is an oncologist who specializes in prostate cancer, a disease that she says shows a “significant disparity,” where Black men are two times more likely to die from advanced prostate cancer, compared with white men. Yet Black men make up just 3% of advanced therapeutic trials.
“A lack of diversity and inclusion in clinical trials is unacceptable,” she said. “If we continue to underrecruit racial/ethnic minorities and older adults to therapeutic clinical trials, we will not be powered to make valid conclusions regarding safety and efficacy in those patient populations. As a result, we can do harm.”
Dr. Borno said that telehealth and telemedicine are not cure-alls, and digital health solutions don’t work for all patients. Approaches, she says, must be tailored to the individual, or disparities could worsen.
In 2020, the Food and Drug Administration approved 53 new drugs. Overall, 32,000 patients took part in these trials. On average, 75% were White, 8% were Black, 6% were Asian, and 11% were Hispanic.
Here’s one stark example of the issue. In 2015, the FDA approved ixazomib (Ninlaro), a promising new drug for multiple myeloma, a blood cancer that affects Black people at disproportionately higher rates than White people. In the United States, one in five people diagnosed with multiple myeloma are Black people. They are more than twice as likely to get the disease as White people. Yet during the clinical trial of 722 participants, only 13 patients, or 1.8%, were Black.
The American Cancer Society estimates that more than 600,000 Americans will die from cancer this year. Historically, Black Americans have the highest death rate and the shortest survival of any racial or ethnic group, stemming largely, it concluded, from centuries of structural racism.
According to Jamie Freedman, MD, head of U.S. medical affairs at Genentech, a global pharmaceutical company, the lack of diversity is often tied to where studies are run.
“Companies tend to choose major academic medical centers where there is a high volume of clinical trial work. When you go to the same tried and true hospitals repeatedly, the pool of patients becomes very homogeneous and tends to be primarily white,” he said. “It’s critical to bring more trials into the community setting by including new sites that can reach underrepresented groups, and Genentech is making significant progress in that area.”
Dr. Freedman believes that, while access is a big hurdle, it doesn’t end there.
“Many patients have a lack of trust in the health care system,” he said. “There are also issues around underserved communities being able to afford quality care, so it’s important to keep time and financial burdens in mind when designing trials to help mitigate barriers such as travel, parking, time off work, and child care.”
Genentech started its diversity and inclusion effort several years ago. Dr. Freeman said that, until more trials become diverse, Black Americans will continue to pay the price. “I think they’re losing their lives in part due to lack of access to these trials. And that is why Genentech and all of us in the health care industry need to change how we design and enroll these studies. We have a long way to go, but I think the steps we’re taking are leading us in the right direction.”
Jennifer Jones-McMeans, PhD, director of global clinical affairs at Abbott Pharmaceuticals, is a clinical research scientist who has designed and led many clinical trials.
She said that Abbott is actively working on solutions.
“We have designed our trials to reduce the barriers to participation and expand access,” she said. “This can be as simple as providing transportation services or home visits for those who are housebound. We’re taking it a step further and providing home health services where someone comes to the home and provides follow-up visits there.”
They also provide interpretation services to address any language barriers.
“We are reaching out to a new set of talented investigators who work closely with underrepresented communities. They are very much wedded and supportive of the communities they treat. By working with doctors within these communities, it expands access to new therapies.”
Spokesperson Keanna Ghazvini said that Pfizer Pharmaceuticals is also committed to increasing minority participation in trials.
“We know that if historically underserved populations are left out of clinical trials, they risk not benefiting from medical breakthroughs down the line,” she said.
The National Institutes of Health’s National Library of Medicine maintains the clinicaltrials.gov database.
There, you can find information on nearly 372,000 publicly and privately supported clinical trials happening in all 50 states and 219 countries. Many are funded by the NIH, but not all of these studies have been evaluated by the U.S. government.
Andrea Denicoff, a nurse consultant at the National Cancer Institute and head of clinical trials operations for the NCI’s National Clinical Trials Network, has been involved in clinical research at the NIH for 35 years.
“It’s really important that our publicly funded trials represent the people of the country,” she said. “There are some cancers that we’re doing a good job in enrolling minorities, and other cancers we need to do a much better job in having a diverse representation in our trials.”
Ms. Denicoff believed opening trials in places where people live is key, but having a diverse clinical trials team is as important.
“We need to reinforce that cancer centers across the country have open doors, and anyone with cancer feels comfortable getting care at that center, and that also includes discussing the option to participate in clinical trials when one might be available. We know from research that when people are invited and asked about trial participation and educated about them, they’ll be much more interested in joining them.”
Ms. Denicoff said that, during the pandemic, the NCI quickly came up with guidance to allow trial sites to send patients their oral study drugs and set up virtual visits. She believes it may help increase future access.
‘Lola Fashoyin-Aje, MD, associate director for science and policy to address disparities in the Oncology Center of Excellence at the FDA, says the agency firmly believes clinical trials should represent the patients who will ultimately get the drug if it’s approved.
But the FDA’s power to require diversity in trials is limited.
“It is important to point out that there are legal constraints which limit’s FDA’s authority to require specific proportional representation in clinical trials by demographic factors,” Dr. Fashoyin-Aje said.
Still, some researchers feel the FDA should play a bigger role. The question is: Should diversity be mandated?
Rhonda Long is now back in Boston to start a new trial, with a new drug that targets her specific mutation. She will be there for 2 months. Once again, Lazarex will help cover some of the cost.
She wants people of color to understand that they are missing out on the promise of new cancer drugs and extended life.
“I feel like there’s not enough emphasis on clinical trials, I don’t believe there’s enough emphasis on second opinions, I don’t think there’s enough emphasis that medicine happens outside our borders, outside of our communities. Clinical trials that don’t have a broad range of participants, how do we know how effective they are if Black and brown people, Asian or Latin American people aren’t represented in the trial?”
And with more trials adopting virtual elements, she said it’s time for minorities to get on board.
Dr. Freedman believed the groundwork is being laid for that to happen. “I don’t think we’ll ever return back to the way we used to do things, where everything has to be done at the clinical trial site. I just don’t think we’re ever going back.”
A version of this article first appeared on WebMD.com.
“I’m the first person in my circle of family and friends to participate in a clinical trial.”
Five years ago, Rhonda Long was diagnosed with cholangiocarcinoma, a rare bile duct cancer that’s seen in only about 8,000 Americans each year.
At the time, Mrs. Long, who is Black, said her doctor in Dayton, Ohio, told her she was not a candidate for surgery and suggested palliative care. After seeking a second opinion at Duke University Medical Center, Durham, N.C., where her sister worked, the 51-year-old wife and mother of two had surgery, radiation, and chemotherapy there in North Carolina. When the chemo stopped working after 3 months, her oncologist at Duke referred her to a colleague at Massachusetts General Hospital in Boston, where she was accepted into a clinical trial.
“In 2019, I traveled to Boston from Dayton, Ohio, every 3 weeks for labs and scans, to make sure that the drug wasn’t doing more harm than good, making sure that the drug as developed was maintaining, shrinking, or even eliminating the disease. Physically and financially, it takes a toll on you and loved ones.”
Her medical insurance did not cover the direct expenses from the clinical trial, and she was spending $1,000-$1,500 each trip. Sometimes they drove the 15 hours to Boston, and sometimes they flew on the cheapest flight they could find.
It’s not an unfamiliar story: people traveling, often long distances, to take part in clinical trials they hope will save their lives.
The Lazarex Cancer Foundation of Danville, Calif., helped Mrs. Long do just that.
Marya Shegog, PhD, health equity and diversity coordinator at Lazarex, said that a patient travels an average of 500 miles to participate in a trial.
The financial hurdles often prevent patients from taking part in clinical trials, Dr. Shegog said. “When you are sick, and you have a disease that may be terminal, you start thinking about setting your things in order.”
Many patients have to make a decision.
“Do I bankrupt my family on trying and hoping that this drug works and helps me live longer, or do I start setting things in order so that when I’m gone, they’re okay or at least better than if I wouldn’t have spent all the money traveling back and forth.”
Dr. Shegog, a 17-year cancer survivor, says when she was battling cervical cancer, a clinical trial was never offered or explored.
Lazarex has been helping cancer patients who have run out of options for 15 years. It identifies clinical trial opportunities and reimburses patients for all travel costs. Last year, Lazarex reimbursed more than 1,000 cancer patients. And it has supported more than 6,000 people since opening its doors.
“Lazarex exists to help remove the barriers of people not being able to participate in trials,” Dr. Shegog said. “It’s systemic that the medical system does not treat patients the same and oftentimes does not offer or make aware the opportunities for African Americans to participate.”
But now, thanks in part to COVID-19, new possibilities are taking shape. The pandemic has changed the landscape for trials, forcing many of them to go virtual, which allows patients to schedule telehealth visits and get some services like bloodwork and CT or MRI scans closer to home. Mrs. Long’s trial eventually went virtual.
“It was absolutely fantastic,” she said. “Having the trial locally, it saves us money, it saves wear and tear on my body. Being in the car, being in an airport or in a plane and in a hotel, all of that wears on you physically.”
The move to virtual studies may have lasting effects on research and treatment.
“The current pandemic has forced us to reexamine all of the traditional burdens we place on patients as it relates to receiving cancer treatment,” said Hala Borno, MD, an assistant professor of medicine at the University of California, San Francisco. “Whether they’re coming to our health care facility to see a clinician, for diagnostics such as blood draws and scans, or to receive therapy, this pandemic has challenged us to explore other possibilities that minimize the risk of exposure to SARS-CoV-2. What I find striking is that it has helped us operationalize use of telemedicine and the delivery of care closer to home.”
This is especially encouraging news for minority patients whose participation in trials has for years lagged well behind that of Whites.
But travel is not the only reason. Racial disparities in clinical trials have long been an issue that’s just another part of the implicit bias in health care.
Compared with White people, Black people are largely at higher risk for heart disease, cancer, stroke, diabetes, asthma, and even mental health problems.
And it’s not just African Americans. Asians, Hispanics, Native Americans, and Alaska Natives are all underrepresented in trials at a time when there is growing evidence that drugs may have different effects on different populations.
Dr. Borno is an oncologist who specializes in prostate cancer, a disease that she says shows a “significant disparity,” where Black men are two times more likely to die from advanced prostate cancer, compared with white men. Yet Black men make up just 3% of advanced therapeutic trials.
“A lack of diversity and inclusion in clinical trials is unacceptable,” she said. “If we continue to underrecruit racial/ethnic minorities and older adults to therapeutic clinical trials, we will not be powered to make valid conclusions regarding safety and efficacy in those patient populations. As a result, we can do harm.”
Dr. Borno said that telehealth and telemedicine are not cure-alls, and digital health solutions don’t work for all patients. Approaches, she says, must be tailored to the individual, or disparities could worsen.
In 2020, the Food and Drug Administration approved 53 new drugs. Overall, 32,000 patients took part in these trials. On average, 75% were White, 8% were Black, 6% were Asian, and 11% were Hispanic.
Here’s one stark example of the issue. In 2015, the FDA approved ixazomib (Ninlaro), a promising new drug for multiple myeloma, a blood cancer that affects Black people at disproportionately higher rates than White people. In the United States, one in five people diagnosed with multiple myeloma are Black people. They are more than twice as likely to get the disease as White people. Yet during the clinical trial of 722 participants, only 13 patients, or 1.8%, were Black.
The American Cancer Society estimates that more than 600,000 Americans will die from cancer this year. Historically, Black Americans have the highest death rate and the shortest survival of any racial or ethnic group, stemming largely, it concluded, from centuries of structural racism.
According to Jamie Freedman, MD, head of U.S. medical affairs at Genentech, a global pharmaceutical company, the lack of diversity is often tied to where studies are run.
“Companies tend to choose major academic medical centers where there is a high volume of clinical trial work. When you go to the same tried and true hospitals repeatedly, the pool of patients becomes very homogeneous and tends to be primarily white,” he said. “It’s critical to bring more trials into the community setting by including new sites that can reach underrepresented groups, and Genentech is making significant progress in that area.”
Dr. Freedman believes that, while access is a big hurdle, it doesn’t end there.
“Many patients have a lack of trust in the health care system,” he said. “There are also issues around underserved communities being able to afford quality care, so it’s important to keep time and financial burdens in mind when designing trials to help mitigate barriers such as travel, parking, time off work, and child care.”
Genentech started its diversity and inclusion effort several years ago. Dr. Freeman said that, until more trials become diverse, Black Americans will continue to pay the price. “I think they’re losing their lives in part due to lack of access to these trials. And that is why Genentech and all of us in the health care industry need to change how we design and enroll these studies. We have a long way to go, but I think the steps we’re taking are leading us in the right direction.”
Jennifer Jones-McMeans, PhD, director of global clinical affairs at Abbott Pharmaceuticals, is a clinical research scientist who has designed and led many clinical trials.
She said that Abbott is actively working on solutions.
“We have designed our trials to reduce the barriers to participation and expand access,” she said. “This can be as simple as providing transportation services or home visits for those who are housebound. We’re taking it a step further and providing home health services where someone comes to the home and provides follow-up visits there.”
They also provide interpretation services to address any language barriers.
“We are reaching out to a new set of talented investigators who work closely with underrepresented communities. They are very much wedded and supportive of the communities they treat. By working with doctors within these communities, it expands access to new therapies.”
Spokesperson Keanna Ghazvini said that Pfizer Pharmaceuticals is also committed to increasing minority participation in trials.
“We know that if historically underserved populations are left out of clinical trials, they risk not benefiting from medical breakthroughs down the line,” she said.
The National Institutes of Health’s National Library of Medicine maintains the clinicaltrials.gov database.
There, you can find information on nearly 372,000 publicly and privately supported clinical trials happening in all 50 states and 219 countries. Many are funded by the NIH, but not all of these studies have been evaluated by the U.S. government.
Andrea Denicoff, a nurse consultant at the National Cancer Institute and head of clinical trials operations for the NCI’s National Clinical Trials Network, has been involved in clinical research at the NIH for 35 years.
“It’s really important that our publicly funded trials represent the people of the country,” she said. “There are some cancers that we’re doing a good job in enrolling minorities, and other cancers we need to do a much better job in having a diverse representation in our trials.”
Ms. Denicoff believed opening trials in places where people live is key, but having a diverse clinical trials team is as important.
“We need to reinforce that cancer centers across the country have open doors, and anyone with cancer feels comfortable getting care at that center, and that also includes discussing the option to participate in clinical trials when one might be available. We know from research that when people are invited and asked about trial participation and educated about them, they’ll be much more interested in joining them.”
Ms. Denicoff said that, during the pandemic, the NCI quickly came up with guidance to allow trial sites to send patients their oral study drugs and set up virtual visits. She believes it may help increase future access.
‘Lola Fashoyin-Aje, MD, associate director for science and policy to address disparities in the Oncology Center of Excellence at the FDA, says the agency firmly believes clinical trials should represent the patients who will ultimately get the drug if it’s approved.
But the FDA’s power to require diversity in trials is limited.
“It is important to point out that there are legal constraints which limit’s FDA’s authority to require specific proportional representation in clinical trials by demographic factors,” Dr. Fashoyin-Aje said.
Still, some researchers feel the FDA should play a bigger role. The question is: Should diversity be mandated?
Rhonda Long is now back in Boston to start a new trial, with a new drug that targets her specific mutation. She will be there for 2 months. Once again, Lazarex will help cover some of the cost.
She wants people of color to understand that they are missing out on the promise of new cancer drugs and extended life.
“I feel like there’s not enough emphasis on clinical trials, I don’t believe there’s enough emphasis on second opinions, I don’t think there’s enough emphasis that medicine happens outside our borders, outside of our communities. Clinical trials that don’t have a broad range of participants, how do we know how effective they are if Black and brown people, Asian or Latin American people aren’t represented in the trial?”
And with more trials adopting virtual elements, she said it’s time for minorities to get on board.
Dr. Freedman believed the groundwork is being laid for that to happen. “I don’t think we’ll ever return back to the way we used to do things, where everything has to be done at the clinical trial site. I just don’t think we’re ever going back.”
A version of this article first appeared on WebMD.com.
“I’m the first person in my circle of family and friends to participate in a clinical trial.”
Five years ago, Rhonda Long was diagnosed with cholangiocarcinoma, a rare bile duct cancer that’s seen in only about 8,000 Americans each year.
At the time, Mrs. Long, who is Black, said her doctor in Dayton, Ohio, told her she was not a candidate for surgery and suggested palliative care. After seeking a second opinion at Duke University Medical Center, Durham, N.C., where her sister worked, the 51-year-old wife and mother of two had surgery, radiation, and chemotherapy there in North Carolina. When the chemo stopped working after 3 months, her oncologist at Duke referred her to a colleague at Massachusetts General Hospital in Boston, where she was accepted into a clinical trial.
“In 2019, I traveled to Boston from Dayton, Ohio, every 3 weeks for labs and scans, to make sure that the drug wasn’t doing more harm than good, making sure that the drug as developed was maintaining, shrinking, or even eliminating the disease. Physically and financially, it takes a toll on you and loved ones.”
Her medical insurance did not cover the direct expenses from the clinical trial, and she was spending $1,000-$1,500 each trip. Sometimes they drove the 15 hours to Boston, and sometimes they flew on the cheapest flight they could find.
It’s not an unfamiliar story: people traveling, often long distances, to take part in clinical trials they hope will save their lives.
The Lazarex Cancer Foundation of Danville, Calif., helped Mrs. Long do just that.
Marya Shegog, PhD, health equity and diversity coordinator at Lazarex, said that a patient travels an average of 500 miles to participate in a trial.
The financial hurdles often prevent patients from taking part in clinical trials, Dr. Shegog said. “When you are sick, and you have a disease that may be terminal, you start thinking about setting your things in order.”
Many patients have to make a decision.
“Do I bankrupt my family on trying and hoping that this drug works and helps me live longer, or do I start setting things in order so that when I’m gone, they’re okay or at least better than if I wouldn’t have spent all the money traveling back and forth.”
Dr. Shegog, a 17-year cancer survivor, says when she was battling cervical cancer, a clinical trial was never offered or explored.
Lazarex has been helping cancer patients who have run out of options for 15 years. It identifies clinical trial opportunities and reimburses patients for all travel costs. Last year, Lazarex reimbursed more than 1,000 cancer patients. And it has supported more than 6,000 people since opening its doors.
“Lazarex exists to help remove the barriers of people not being able to participate in trials,” Dr. Shegog said. “It’s systemic that the medical system does not treat patients the same and oftentimes does not offer or make aware the opportunities for African Americans to participate.”
But now, thanks in part to COVID-19, new possibilities are taking shape. The pandemic has changed the landscape for trials, forcing many of them to go virtual, which allows patients to schedule telehealth visits and get some services like bloodwork and CT or MRI scans closer to home. Mrs. Long’s trial eventually went virtual.
“It was absolutely fantastic,” she said. “Having the trial locally, it saves us money, it saves wear and tear on my body. Being in the car, being in an airport or in a plane and in a hotel, all of that wears on you physically.”
The move to virtual studies may have lasting effects on research and treatment.
“The current pandemic has forced us to reexamine all of the traditional burdens we place on patients as it relates to receiving cancer treatment,” said Hala Borno, MD, an assistant professor of medicine at the University of California, San Francisco. “Whether they’re coming to our health care facility to see a clinician, for diagnostics such as blood draws and scans, or to receive therapy, this pandemic has challenged us to explore other possibilities that minimize the risk of exposure to SARS-CoV-2. What I find striking is that it has helped us operationalize use of telemedicine and the delivery of care closer to home.”
This is especially encouraging news for minority patients whose participation in trials has for years lagged well behind that of Whites.
But travel is not the only reason. Racial disparities in clinical trials have long been an issue that’s just another part of the implicit bias in health care.
Compared with White people, Black people are largely at higher risk for heart disease, cancer, stroke, diabetes, asthma, and even mental health problems.
And it’s not just African Americans. Asians, Hispanics, Native Americans, and Alaska Natives are all underrepresented in trials at a time when there is growing evidence that drugs may have different effects on different populations.
Dr. Borno is an oncologist who specializes in prostate cancer, a disease that she says shows a “significant disparity,” where Black men are two times more likely to die from advanced prostate cancer, compared with white men. Yet Black men make up just 3% of advanced therapeutic trials.
“A lack of diversity and inclusion in clinical trials is unacceptable,” she said. “If we continue to underrecruit racial/ethnic minorities and older adults to therapeutic clinical trials, we will not be powered to make valid conclusions regarding safety and efficacy in those patient populations. As a result, we can do harm.”
Dr. Borno said that telehealth and telemedicine are not cure-alls, and digital health solutions don’t work for all patients. Approaches, she says, must be tailored to the individual, or disparities could worsen.
In 2020, the Food and Drug Administration approved 53 new drugs. Overall, 32,000 patients took part in these trials. On average, 75% were White, 8% were Black, 6% were Asian, and 11% were Hispanic.
Here’s one stark example of the issue. In 2015, the FDA approved ixazomib (Ninlaro), a promising new drug for multiple myeloma, a blood cancer that affects Black people at disproportionately higher rates than White people. In the United States, one in five people diagnosed with multiple myeloma are Black people. They are more than twice as likely to get the disease as White people. Yet during the clinical trial of 722 participants, only 13 patients, or 1.8%, were Black.
The American Cancer Society estimates that more than 600,000 Americans will die from cancer this year. Historically, Black Americans have the highest death rate and the shortest survival of any racial or ethnic group, stemming largely, it concluded, from centuries of structural racism.
According to Jamie Freedman, MD, head of U.S. medical affairs at Genentech, a global pharmaceutical company, the lack of diversity is often tied to where studies are run.
“Companies tend to choose major academic medical centers where there is a high volume of clinical trial work. When you go to the same tried and true hospitals repeatedly, the pool of patients becomes very homogeneous and tends to be primarily white,” he said. “It’s critical to bring more trials into the community setting by including new sites that can reach underrepresented groups, and Genentech is making significant progress in that area.”
Dr. Freedman believes that, while access is a big hurdle, it doesn’t end there.
“Many patients have a lack of trust in the health care system,” he said. “There are also issues around underserved communities being able to afford quality care, so it’s important to keep time and financial burdens in mind when designing trials to help mitigate barriers such as travel, parking, time off work, and child care.”
Genentech started its diversity and inclusion effort several years ago. Dr. Freeman said that, until more trials become diverse, Black Americans will continue to pay the price. “I think they’re losing their lives in part due to lack of access to these trials. And that is why Genentech and all of us in the health care industry need to change how we design and enroll these studies. We have a long way to go, but I think the steps we’re taking are leading us in the right direction.”
Jennifer Jones-McMeans, PhD, director of global clinical affairs at Abbott Pharmaceuticals, is a clinical research scientist who has designed and led many clinical trials.
She said that Abbott is actively working on solutions.
“We have designed our trials to reduce the barriers to participation and expand access,” she said. “This can be as simple as providing transportation services or home visits for those who are housebound. We’re taking it a step further and providing home health services where someone comes to the home and provides follow-up visits there.”
They also provide interpretation services to address any language barriers.
“We are reaching out to a new set of talented investigators who work closely with underrepresented communities. They are very much wedded and supportive of the communities they treat. By working with doctors within these communities, it expands access to new therapies.”
Spokesperson Keanna Ghazvini said that Pfizer Pharmaceuticals is also committed to increasing minority participation in trials.
“We know that if historically underserved populations are left out of clinical trials, they risk not benefiting from medical breakthroughs down the line,” she said.
The National Institutes of Health’s National Library of Medicine maintains the clinicaltrials.gov database.
There, you can find information on nearly 372,000 publicly and privately supported clinical trials happening in all 50 states and 219 countries. Many are funded by the NIH, but not all of these studies have been evaluated by the U.S. government.
Andrea Denicoff, a nurse consultant at the National Cancer Institute and head of clinical trials operations for the NCI’s National Clinical Trials Network, has been involved in clinical research at the NIH for 35 years.
“It’s really important that our publicly funded trials represent the people of the country,” she said. “There are some cancers that we’re doing a good job in enrolling minorities, and other cancers we need to do a much better job in having a diverse representation in our trials.”
Ms. Denicoff believed opening trials in places where people live is key, but having a diverse clinical trials team is as important.
“We need to reinforce that cancer centers across the country have open doors, and anyone with cancer feels comfortable getting care at that center, and that also includes discussing the option to participate in clinical trials when one might be available. We know from research that when people are invited and asked about trial participation and educated about them, they’ll be much more interested in joining them.”
Ms. Denicoff said that, during the pandemic, the NCI quickly came up with guidance to allow trial sites to send patients their oral study drugs and set up virtual visits. She believes it may help increase future access.
‘Lola Fashoyin-Aje, MD, associate director for science and policy to address disparities in the Oncology Center of Excellence at the FDA, says the agency firmly believes clinical trials should represent the patients who will ultimately get the drug if it’s approved.
But the FDA’s power to require diversity in trials is limited.
“It is important to point out that there are legal constraints which limit’s FDA’s authority to require specific proportional representation in clinical trials by demographic factors,” Dr. Fashoyin-Aje said.
Still, some researchers feel the FDA should play a bigger role. The question is: Should diversity be mandated?
Rhonda Long is now back in Boston to start a new trial, with a new drug that targets her specific mutation. She will be there for 2 months. Once again, Lazarex will help cover some of the cost.
She wants people of color to understand that they are missing out on the promise of new cancer drugs and extended life.
“I feel like there’s not enough emphasis on clinical trials, I don’t believe there’s enough emphasis on second opinions, I don’t think there’s enough emphasis that medicine happens outside our borders, outside of our communities. Clinical trials that don’t have a broad range of participants, how do we know how effective they are if Black and brown people, Asian or Latin American people aren’t represented in the trial?”
And with more trials adopting virtual elements, she said it’s time for minorities to get on board.
Dr. Freedman believed the groundwork is being laid for that to happen. “I don’t think we’ll ever return back to the way we used to do things, where everything has to be done at the clinical trial site. I just don’t think we’re ever going back.”
A version of this article first appeared on WebMD.com.