User login
Acquired Perforating Dermatosis in a Skin Graft
Case Report
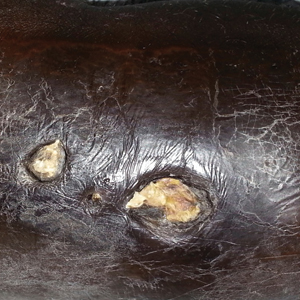
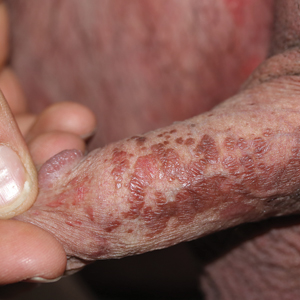
A 57-year-old black woman with a history of dialysis-dependent end-stage renal disease, diabetes mellitus (DM), hypertension, diastolic congestive heart failure, and chronic bronchitis was admitted to Howard University Hospital (Washington, DC) for acute chest pain and shortness of breath. During her hospital stay the dermatology team was consulted for evaluation of two 1.6-cm teardrop-shaped, yellow-white-chalky plaques noted in the center of an atrophic, hyperpigmented, shiny, contracted split-thickness skin graft (STSG) on the right posterior forearm (Figure 1). Twenty years prior, the patient received STSGs on the right and left forearm secondary to caustic burns. Two months before the current admission she noticed 2 adjacent teardrop-shaped white plaques within the center of the STSG on the right forearm. At a 3-month follow-up, she had developed more lesions within both graft sites of the bilateral forearm. There was no notable pruritus associated with the lesions.
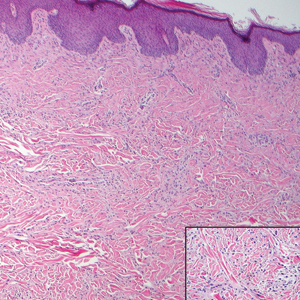
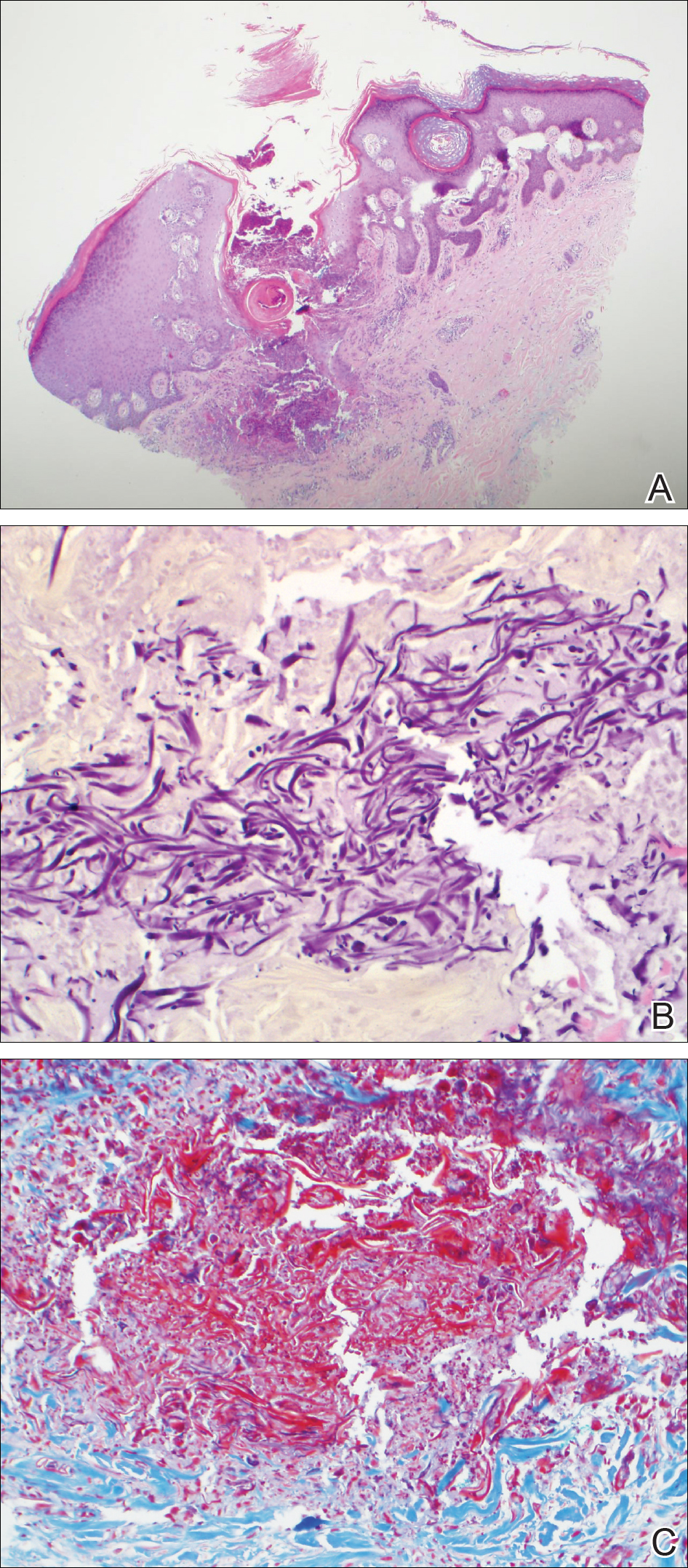
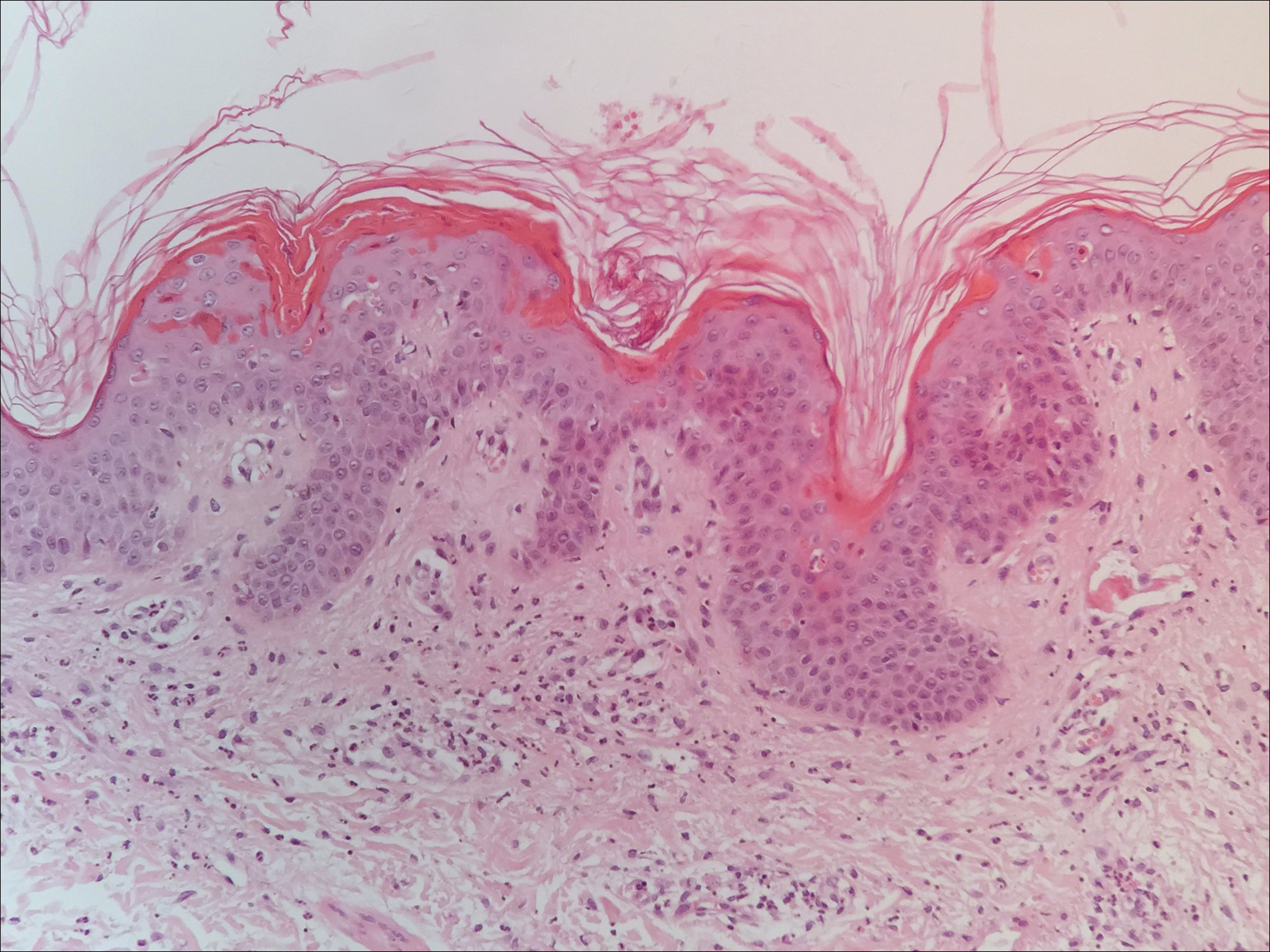
A 4-mm punch biopsy showed an orthokeratotic plug with basophilic inflammatory debris adjacent to acanthotic epidermis, necrotic basophilic debris at the superficial dermis with epidermal canals extending from the base of the lesion superiorly, and transepidermal elimination of elastic fibers (Figure 2A). A Verhoeff-van Gieson stain revealed the necrotic basophilic debris located in the superficial dermis admixed with a cluster of black wavy elastic fibers establishing the identity of the perforating substance (Figure 2B). Masson trichrome stain revealed loss of collagen structure within the aggregate of elastic fibers adjacent to the epidermis and no collagen within epidermal canals (Figure 2C). These histopathologic findings together with the clinical presentation were consistent with a diagnosis of acquired perforating dermatosis (APD).
Comment
Presentation
Acquired perforating dermatosis is a dermatologic condition characterized by multiple pruritic, dome-shaped papules and plaques with central keratotic plugs giving a craterlike appearance.1-4 A green-brown or black crust with an erythematous border typically surrounds the primary lesions.4 Acquired perforating dermatosis favors a distribution over the trunk, gluteal region, and the extensor surfaces of the upper and lower extremities. Palmoplantar, intertriginous, and mucous membrane regions typically are spared.4 Occasionally, APD may present as generalized nodules and papules. Our case consisting of lesions that were localized to STSGs on the forearms supports the typical distribution; however, the presentation of APD occurring within a skin graft is unique.
From an epidemiologic standpoint, APD is more likely to affect men than women (1.5:1 ratio). Additionally, APD’s affected age range is 29 to 96 years (mean, 56.8 years),5 which is consistent with our patient’s age. Acquired perforating dermatosis has no racial predilection, though there is a predominance among black patients with concomitant chronic renal failure, as seen in our patient.3
Pathogenesis
The etiology of APD remains unknown.6 Some believe that the uremic or calcium deposits on the skin of patients with chronic kidney disease may trigger chronic pruritus, leading to epithelial hyperplasia and the development of perforating lesions.1,3 A prominent theory in the literature is that superficial trauma, such as scratching, induces necrosis of tissue, facilitating transepidermal elimination of connective tissue components.7 The Köbner phenomenon, which can easily be induced by scratching the skin, supports this idea.8 Fujimoto et al9 suggested that scratching exposes keratinocytes to advanced glycation end product–modified extracellular matrix proteins, particularly types I and III collagen. This exposure leads to the terminal differentiation of keratinocytes with the advanced glycation end receptor (CD36) followed by the upward movement of keratinocytes with glycated collagen. Others postulate fibronectin, involved in epidermal cell signaling, locomotion, and differentiation, is an antigenic trigger because patients with DM and uremia have increased levels of fibronectin in the serum and at sites of perforating skin lesions.10
Diseases Associated With APD
Acquired perforating dermatosis is an umbrella term for perforating disease found in adults. It is associated with systemic diseases, such as DM and pruritus of renal failure.11 Our patient had both dialysis-dependent end-stage renal disease and DM. Acquired perforating dermatosis is observed in 4.5% to 11% of patients on hemodialysis12,13; however, APD may occur prior to or in the absence of dialysis.3 Other examples of systemic conditions associated with APD include obstructive uropathy, chronic nephritis, anuria, and hypertensive nephrosclerosis. Koebnerization also may trigger lesions to manifest in a linear pattern after localized trauma to the skin.7 Acquired perforating dermatosis is associated with other types of trauma, such as healing herpes zoster, or following exposure to drugs, such as tumor necrosis factor α inhibitors, bevacizumab, telaprevir, sorafenib, sirolimus, and indinavir.14-16 Rarely, there have been associations with a history of insect bites, scabies, lymphoma, and hepatobiliary disease.1-3
Histopathology
Acquired perforating dermatosis is classified as a perforating disease, along with reactive perforating collagenosis, elastosis perforans serpiginosa (EPS), perforating folliculitis, and perforating calcific elastosis. Perforating diseases are histologically characterized by the transepidermal penetration and elimination of altered connective tissue and inflammatory cells.5 Each disease differs based on their clinical and histological characteristics.
Histologic sections of APD show a plug of crusting or hyperkeratosis with variable parakeratosis, acanthosis, and occasional dyskeratotic keratinocytes. In the dermis, aggregates of neutrophils, lymphocytes, macrophages, or multinucleated giant cells may be found.17 The histologic findings vary depending on the stage of evolution of the individual lesion. Early lesions show a concave depression with acanthosis, vacuolation of basal keratinocytes, and dermal inflammation.4 Additionally, transepidermal channels filled with keratin, pyknotic nuclear debris, inflammatory cells, elastin, or collagen can be noted.3 Over time, the elastic fibers, as detected by the Verhoeff-van Gieson stain, dissipate and the collagen acquires a basophilic staining. Adjacent to the channels, the basement membrane remains intact in early lesions but later shows discontinuities and electron-dense fibrinlike material.3 Occasionally, amorphous degenerated material within the perforations is the major histologic finding.11 Usually, the material cannot be clearly identified as collagen or elastin, but sometimes both are present.
In our case, we identified elastin as the perforating substance, which is less common than collagen, the typical perforating substance in APD. Elastin has occasionally been seen to serve as the only perforating substance from APD lesions among patients. Abe et al18 reported that the biopsy of a Japanese patient with keratotic follicular papules and serpiginous-arranged papules demonstrated elimination of atypical elastin fibers from the transepidermal channels. This patient was diagnosed with APD as well as EPS and perforating folliculitis based on the clinical presentation.18 Kim et al19 studied 30 Korean patients with APD. One had serpiginous hyperkeratotic plaques along the upper extremity and trunk that revealed transepidermal channels containing coarse elastic fibers and basophilic debris; however, due to the serpiginous morphology of lesions, both Abe et al18 and Kim et al19 favored a diagnosis of acquired EPS. Saray et al20 conducted a retrospective study of 22 Turkish patients with APD; 1 patient had a painful hyperkeratotic papule on the auricle that on histopathology showed degenerated elastin perforating through the keratotic plug, features similar to our case.
Differential Diagnosis
The differential diagnoses include perforating diseases14,19 as well as other disorders that exhibit the Köbner phenomenon, such as psoriasis, lichen planus, and verruca vulgaris.21,22 Also, it is not uncommon for patients with APD to have coexisting folliculitis or prurigo nodularis.22
Treatment
Management is focused on treating the symptoms. For pruritus, sedating antihistamines and other antipruritic agents are efficacious.23 Topical, intra-lesional, or systemic corticosteroids and topical retinoids have shown variable resolution in APD lesions.24 Some case reports describe topical menthol, salicylic acid, sulfur, benzoyl peroxide, systemic antibiotics (eg, clindamycin, doxycycline), and allopurinol for elevated uric acid levels as effective treatment methods.6 Narrowband UVB phototherapy is beneficial for APD and renal disease.25,26 Renal transplantation has been curative for some patients with APD.27 Given that our patient’s lesions were asymptomatic, no treatment was offered at the time.
Conclusion
Our patient presented with APD localized exclusively to the site of a skin graft, and histologic examination identified elastin as the primary perforating substance. A medical history of DM and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
- Rodney IJ, Taylor CS, Cohen G. Derm Dx: what are these pruritic nodules? The Dermatologist. October 15, 2009. http://www.the-dermatologist.com/content/derm-dx-what-are-these-pruritic-nodules. Accessed September 18, 2018.
- Gagnon, AL, Desai T. Dermatological diseases in patients with chronic kidney disease. J Nephropathol. 2013;2:104-109.
- Kurban MS, Boueiz A, Kibbi AG. Cutaneous manifestations of chronic kidney disease. Clin Dermatol. 2008;26:255-264.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis [published online May 29, 2013]. J Dtsch Dermatol Ges. 2013;11:723-729; 723-730.
- Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592.
- Healy R, Cerio R, Hollingsworth A, et al. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010;35:621-623.
- Cordova KB, Oberg TJ, Malik M, et al. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22:45-55.
- Satchell AC, Crotty K, Lee S. Reactive perforating collagenosis: a condition that may be underdiagnosed. Australas J Dermatol. 2001;42:284-287.
- Fujimoto E, Kobayashi T, Fujimoto N, et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414.
- Bilezikci B, Sechkin D, Demirhan B. Acquired perforating dermatosis in patients with chronic renal failure: a possible role for fibronectin. J Eur Acad Dermatol Venereol. 2003;17:230-232.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Hurwitz RM, Melton ME, Creech FT, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Lübbe J, Sorg O, Malé PJ, et al. Sirolimus-induced inflammatory papules with acquired reactive perforating collagenosis [published online January 9, 2008]. Dermatology. 2008;216:239-242.
- Pernet C, Pageaux GP, Guillot B, et al. Telaprevir-induced acquired perforating dermatosis. JAMA Dermatol. 2014;150:1371-1372.
- Severino-Freire M, Sibaud V, Tournier E, et al. Acquired perforating dermatosis associated with sorafenib therapy [published online September 11, 2014]. J Eur Acad Dermatol Venereol. 2016;30:328-330.
- Zelger B, Hintner H, Auböck J, et al. Acquired perforating dermatosis. transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991;127:695-700.
- Abe R, Murase S, Nomura Y, et al. Acquired perforating dermatosis appearing as elastosis perforans serpiginosa and perforating folliculitis. Clin Exp Dermatol. 2008;33:653-654.
- Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26:162-171.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679-688.
- Carter VH, Constantine VS. Kyrle’s disease. I. clinical findings in five cases and review of literature. Arch Dermatol. 1968;97:624-632.
- Robinson-Bostom L, Digiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975-986.
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Ohe S, Danno K, Sasaki H, et al. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2004;50:892-894.
- Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. 2012;28:50-52.
- Saldanha LF, Gonick HC, Rodriguez HJ, et al. Silicon-related syndrome in dialysis patients. Nephron. 1997;77:48-56.
Case Report
A 57-year-old black woman with a history of dialysis-dependent end-stage renal disease, diabetes mellitus (DM), hypertension, diastolic congestive heart failure, and chronic bronchitis was admitted to Howard University Hospital (Washington, DC) for acute chest pain and shortness of breath. During her hospital stay the dermatology team was consulted for evaluation of two 1.6-cm teardrop-shaped, yellow-white-chalky plaques noted in the center of an atrophic, hyperpigmented, shiny, contracted split-thickness skin graft (STSG) on the right posterior forearm (Figure 1). Twenty years prior, the patient received STSGs on the right and left forearm secondary to caustic burns. Two months before the current admission she noticed 2 adjacent teardrop-shaped white plaques within the center of the STSG on the right forearm. At a 3-month follow-up, she had developed more lesions within both graft sites of the bilateral forearm. There was no notable pruritus associated with the lesions.

A 4-mm punch biopsy showed an orthokeratotic plug with basophilic inflammatory debris adjacent to acanthotic epidermis, necrotic basophilic debris at the superficial dermis with epidermal canals extending from the base of the lesion superiorly, and transepidermal elimination of elastic fibers (Figure 2A). A Verhoeff-van Gieson stain revealed the necrotic basophilic debris located in the superficial dermis admixed with a cluster of black wavy elastic fibers establishing the identity of the perforating substance (Figure 2B). Masson trichrome stain revealed loss of collagen structure within the aggregate of elastic fibers adjacent to the epidermis and no collagen within epidermal canals (Figure 2C). These histopathologic findings together with the clinical presentation were consistent with a diagnosis of acquired perforating dermatosis (APD).

Comment
Presentation
Acquired perforating dermatosis is a dermatologic condition characterized by multiple pruritic, dome-shaped papules and plaques with central keratotic plugs giving a craterlike appearance.1-4 A green-brown or black crust with an erythematous border typically surrounds the primary lesions.4 Acquired perforating dermatosis favors a distribution over the trunk, gluteal region, and the extensor surfaces of the upper and lower extremities. Palmoplantar, intertriginous, and mucous membrane regions typically are spared.4 Occasionally, APD may present as generalized nodules and papules. Our case consisting of lesions that were localized to STSGs on the forearms supports the typical distribution; however, the presentation of APD occurring within a skin graft is unique.
From an epidemiologic standpoint, APD is more likely to affect men than women (1.5:1 ratio). Additionally, APD’s affected age range is 29 to 96 years (mean, 56.8 years),5 which is consistent with our patient’s age. Acquired perforating dermatosis has no racial predilection, though there is a predominance among black patients with concomitant chronic renal failure, as seen in our patient.3
Pathogenesis
The etiology of APD remains unknown.6 Some believe that the uremic or calcium deposits on the skin of patients with chronic kidney disease may trigger chronic pruritus, leading to epithelial hyperplasia and the development of perforating lesions.1,3 A prominent theory in the literature is that superficial trauma, such as scratching, induces necrosis of tissue, facilitating transepidermal elimination of connective tissue components.7 The Köbner phenomenon, which can easily be induced by scratching the skin, supports this idea.8 Fujimoto et al9 suggested that scratching exposes keratinocytes to advanced glycation end product–modified extracellular matrix proteins, particularly types I and III collagen. This exposure leads to the terminal differentiation of keratinocytes with the advanced glycation end receptor (CD36) followed by the upward movement of keratinocytes with glycated collagen. Others postulate fibronectin, involved in epidermal cell signaling, locomotion, and differentiation, is an antigenic trigger because patients with DM and uremia have increased levels of fibronectin in the serum and at sites of perforating skin lesions.10
Diseases Associated With APD
Acquired perforating dermatosis is an umbrella term for perforating disease found in adults. It is associated with systemic diseases, such as DM and pruritus of renal failure.11 Our patient had both dialysis-dependent end-stage renal disease and DM. Acquired perforating dermatosis is observed in 4.5% to 11% of patients on hemodialysis12,13; however, APD may occur prior to or in the absence of dialysis.3 Other examples of systemic conditions associated with APD include obstructive uropathy, chronic nephritis, anuria, and hypertensive nephrosclerosis. Koebnerization also may trigger lesions to manifest in a linear pattern after localized trauma to the skin.7 Acquired perforating dermatosis is associated with other types of trauma, such as healing herpes zoster, or following exposure to drugs, such as tumor necrosis factor α inhibitors, bevacizumab, telaprevir, sorafenib, sirolimus, and indinavir.14-16 Rarely, there have been associations with a history of insect bites, scabies, lymphoma, and hepatobiliary disease.1-3
Histopathology
Acquired perforating dermatosis is classified as a perforating disease, along with reactive perforating collagenosis, elastosis perforans serpiginosa (EPS), perforating folliculitis, and perforating calcific elastosis. Perforating diseases are histologically characterized by the transepidermal penetration and elimination of altered connective tissue and inflammatory cells.5 Each disease differs based on their clinical and histological characteristics.
Histologic sections of APD show a plug of crusting or hyperkeratosis with variable parakeratosis, acanthosis, and occasional dyskeratotic keratinocytes. In the dermis, aggregates of neutrophils, lymphocytes, macrophages, or multinucleated giant cells may be found.17 The histologic findings vary depending on the stage of evolution of the individual lesion. Early lesions show a concave depression with acanthosis, vacuolation of basal keratinocytes, and dermal inflammation.4 Additionally, transepidermal channels filled with keratin, pyknotic nuclear debris, inflammatory cells, elastin, or collagen can be noted.3 Over time, the elastic fibers, as detected by the Verhoeff-van Gieson stain, dissipate and the collagen acquires a basophilic staining. Adjacent to the channels, the basement membrane remains intact in early lesions but later shows discontinuities and electron-dense fibrinlike material.3 Occasionally, amorphous degenerated material within the perforations is the major histologic finding.11 Usually, the material cannot be clearly identified as collagen or elastin, but sometimes both are present.
In our case, we identified elastin as the perforating substance, which is less common than collagen, the typical perforating substance in APD. Elastin has occasionally been seen to serve as the only perforating substance from APD lesions among patients. Abe et al18 reported that the biopsy of a Japanese patient with keratotic follicular papules and serpiginous-arranged papules demonstrated elimination of atypical elastin fibers from the transepidermal channels. This patient was diagnosed with APD as well as EPS and perforating folliculitis based on the clinical presentation.18 Kim et al19 studied 30 Korean patients with APD. One had serpiginous hyperkeratotic plaques along the upper extremity and trunk that revealed transepidermal channels containing coarse elastic fibers and basophilic debris; however, due to the serpiginous morphology of lesions, both Abe et al18 and Kim et al19 favored a diagnosis of acquired EPS. Saray et al20 conducted a retrospective study of 22 Turkish patients with APD; 1 patient had a painful hyperkeratotic papule on the auricle that on histopathology showed degenerated elastin perforating through the keratotic plug, features similar to our case.
Differential Diagnosis
The differential diagnoses include perforating diseases14,19 as well as other disorders that exhibit the Köbner phenomenon, such as psoriasis, lichen planus, and verruca vulgaris.21,22 Also, it is not uncommon for patients with APD to have coexisting folliculitis or prurigo nodularis.22
Treatment
Management is focused on treating the symptoms. For pruritus, sedating antihistamines and other antipruritic agents are efficacious.23 Topical, intra-lesional, or systemic corticosteroids and topical retinoids have shown variable resolution in APD lesions.24 Some case reports describe topical menthol, salicylic acid, sulfur, benzoyl peroxide, systemic antibiotics (eg, clindamycin, doxycycline), and allopurinol for elevated uric acid levels as effective treatment methods.6 Narrowband UVB phototherapy is beneficial for APD and renal disease.25,26 Renal transplantation has been curative for some patients with APD.27 Given that our patient’s lesions were asymptomatic, no treatment was offered at the time.
Conclusion
Our patient presented with APD localized exclusively to the site of a skin graft, and histologic examination identified elastin as the primary perforating substance. A medical history of DM and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
Case Report
A 57-year-old black woman with a history of dialysis-dependent end-stage renal disease, diabetes mellitus (DM), hypertension, diastolic congestive heart failure, and chronic bronchitis was admitted to Howard University Hospital (Washington, DC) for acute chest pain and shortness of breath. During her hospital stay the dermatology team was consulted for evaluation of two 1.6-cm teardrop-shaped, yellow-white-chalky plaques noted in the center of an atrophic, hyperpigmented, shiny, contracted split-thickness skin graft (STSG) on the right posterior forearm (Figure 1). Twenty years prior, the patient received STSGs on the right and left forearm secondary to caustic burns. Two months before the current admission she noticed 2 adjacent teardrop-shaped white plaques within the center of the STSG on the right forearm. At a 3-month follow-up, she had developed more lesions within both graft sites of the bilateral forearm. There was no notable pruritus associated with the lesions.

A 4-mm punch biopsy showed an orthokeratotic plug with basophilic inflammatory debris adjacent to acanthotic epidermis, necrotic basophilic debris at the superficial dermis with epidermal canals extending from the base of the lesion superiorly, and transepidermal elimination of elastic fibers (Figure 2A). A Verhoeff-van Gieson stain revealed the necrotic basophilic debris located in the superficial dermis admixed with a cluster of black wavy elastic fibers establishing the identity of the perforating substance (Figure 2B). Masson trichrome stain revealed loss of collagen structure within the aggregate of elastic fibers adjacent to the epidermis and no collagen within epidermal canals (Figure 2C). These histopathologic findings together with the clinical presentation were consistent with a diagnosis of acquired perforating dermatosis (APD).

Comment
Presentation
Acquired perforating dermatosis is a dermatologic condition characterized by multiple pruritic, dome-shaped papules and plaques with central keratotic plugs giving a craterlike appearance.1-4 A green-brown or black crust with an erythematous border typically surrounds the primary lesions.4 Acquired perforating dermatosis favors a distribution over the trunk, gluteal region, and the extensor surfaces of the upper and lower extremities. Palmoplantar, intertriginous, and mucous membrane regions typically are spared.4 Occasionally, APD may present as generalized nodules and papules. Our case consisting of lesions that were localized to STSGs on the forearms supports the typical distribution; however, the presentation of APD occurring within a skin graft is unique.
From an epidemiologic standpoint, APD is more likely to affect men than women (1.5:1 ratio). Additionally, APD’s affected age range is 29 to 96 years (mean, 56.8 years),5 which is consistent with our patient’s age. Acquired perforating dermatosis has no racial predilection, though there is a predominance among black patients with concomitant chronic renal failure, as seen in our patient.3
Pathogenesis
The etiology of APD remains unknown.6 Some believe that the uremic or calcium deposits on the skin of patients with chronic kidney disease may trigger chronic pruritus, leading to epithelial hyperplasia and the development of perforating lesions.1,3 A prominent theory in the literature is that superficial trauma, such as scratching, induces necrosis of tissue, facilitating transepidermal elimination of connective tissue components.7 The Köbner phenomenon, which can easily be induced by scratching the skin, supports this idea.8 Fujimoto et al9 suggested that scratching exposes keratinocytes to advanced glycation end product–modified extracellular matrix proteins, particularly types I and III collagen. This exposure leads to the terminal differentiation of keratinocytes with the advanced glycation end receptor (CD36) followed by the upward movement of keratinocytes with glycated collagen. Others postulate fibronectin, involved in epidermal cell signaling, locomotion, and differentiation, is an antigenic trigger because patients with DM and uremia have increased levels of fibronectin in the serum and at sites of perforating skin lesions.10
Diseases Associated With APD
Acquired perforating dermatosis is an umbrella term for perforating disease found in adults. It is associated with systemic diseases, such as DM and pruritus of renal failure.11 Our patient had both dialysis-dependent end-stage renal disease and DM. Acquired perforating dermatosis is observed in 4.5% to 11% of patients on hemodialysis12,13; however, APD may occur prior to or in the absence of dialysis.3 Other examples of systemic conditions associated with APD include obstructive uropathy, chronic nephritis, anuria, and hypertensive nephrosclerosis. Koebnerization also may trigger lesions to manifest in a linear pattern after localized trauma to the skin.7 Acquired perforating dermatosis is associated with other types of trauma, such as healing herpes zoster, or following exposure to drugs, such as tumor necrosis factor α inhibitors, bevacizumab, telaprevir, sorafenib, sirolimus, and indinavir.14-16 Rarely, there have been associations with a history of insect bites, scabies, lymphoma, and hepatobiliary disease.1-3
Histopathology
Acquired perforating dermatosis is classified as a perforating disease, along with reactive perforating collagenosis, elastosis perforans serpiginosa (EPS), perforating folliculitis, and perforating calcific elastosis. Perforating diseases are histologically characterized by the transepidermal penetration and elimination of altered connective tissue and inflammatory cells.5 Each disease differs based on their clinical and histological characteristics.
Histologic sections of APD show a plug of crusting or hyperkeratosis with variable parakeratosis, acanthosis, and occasional dyskeratotic keratinocytes. In the dermis, aggregates of neutrophils, lymphocytes, macrophages, or multinucleated giant cells may be found.17 The histologic findings vary depending on the stage of evolution of the individual lesion. Early lesions show a concave depression with acanthosis, vacuolation of basal keratinocytes, and dermal inflammation.4 Additionally, transepidermal channels filled with keratin, pyknotic nuclear debris, inflammatory cells, elastin, or collagen can be noted.3 Over time, the elastic fibers, as detected by the Verhoeff-van Gieson stain, dissipate and the collagen acquires a basophilic staining. Adjacent to the channels, the basement membrane remains intact in early lesions but later shows discontinuities and electron-dense fibrinlike material.3 Occasionally, amorphous degenerated material within the perforations is the major histologic finding.11 Usually, the material cannot be clearly identified as collagen or elastin, but sometimes both are present.
In our case, we identified elastin as the perforating substance, which is less common than collagen, the typical perforating substance in APD. Elastin has occasionally been seen to serve as the only perforating substance from APD lesions among patients. Abe et al18 reported that the biopsy of a Japanese patient with keratotic follicular papules and serpiginous-arranged papules demonstrated elimination of atypical elastin fibers from the transepidermal channels. This patient was diagnosed with APD as well as EPS and perforating folliculitis based on the clinical presentation.18 Kim et al19 studied 30 Korean patients with APD. One had serpiginous hyperkeratotic plaques along the upper extremity and trunk that revealed transepidermal channels containing coarse elastic fibers and basophilic debris; however, due to the serpiginous morphology of lesions, both Abe et al18 and Kim et al19 favored a diagnosis of acquired EPS. Saray et al20 conducted a retrospective study of 22 Turkish patients with APD; 1 patient had a painful hyperkeratotic papule on the auricle that on histopathology showed degenerated elastin perforating through the keratotic plug, features similar to our case.
Differential Diagnosis
The differential diagnoses include perforating diseases14,19 as well as other disorders that exhibit the Köbner phenomenon, such as psoriasis, lichen planus, and verruca vulgaris.21,22 Also, it is not uncommon for patients with APD to have coexisting folliculitis or prurigo nodularis.22
Treatment
Management is focused on treating the symptoms. For pruritus, sedating antihistamines and other antipruritic agents are efficacious.23 Topical, intra-lesional, or systemic corticosteroids and topical retinoids have shown variable resolution in APD lesions.24 Some case reports describe topical menthol, salicylic acid, sulfur, benzoyl peroxide, systemic antibiotics (eg, clindamycin, doxycycline), and allopurinol for elevated uric acid levels as effective treatment methods.6 Narrowband UVB phototherapy is beneficial for APD and renal disease.25,26 Renal transplantation has been curative for some patients with APD.27 Given that our patient’s lesions were asymptomatic, no treatment was offered at the time.
Conclusion
Our patient presented with APD localized exclusively to the site of a skin graft, and histologic examination identified elastin as the primary perforating substance. A medical history of DM and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
- Rodney IJ, Taylor CS, Cohen G. Derm Dx: what are these pruritic nodules? The Dermatologist. October 15, 2009. http://www.the-dermatologist.com/content/derm-dx-what-are-these-pruritic-nodules. Accessed September 18, 2018.
- Gagnon, AL, Desai T. Dermatological diseases in patients with chronic kidney disease. J Nephropathol. 2013;2:104-109.
- Kurban MS, Boueiz A, Kibbi AG. Cutaneous manifestations of chronic kidney disease. Clin Dermatol. 2008;26:255-264.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis [published online May 29, 2013]. J Dtsch Dermatol Ges. 2013;11:723-729; 723-730.
- Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592.
- Healy R, Cerio R, Hollingsworth A, et al. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010;35:621-623.
- Cordova KB, Oberg TJ, Malik M, et al. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22:45-55.
- Satchell AC, Crotty K, Lee S. Reactive perforating collagenosis: a condition that may be underdiagnosed. Australas J Dermatol. 2001;42:284-287.
- Fujimoto E, Kobayashi T, Fujimoto N, et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414.
- Bilezikci B, Sechkin D, Demirhan B. Acquired perforating dermatosis in patients with chronic renal failure: a possible role for fibronectin. J Eur Acad Dermatol Venereol. 2003;17:230-232.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Hurwitz RM, Melton ME, Creech FT, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Lübbe J, Sorg O, Malé PJ, et al. Sirolimus-induced inflammatory papules with acquired reactive perforating collagenosis [published online January 9, 2008]. Dermatology. 2008;216:239-242.
- Pernet C, Pageaux GP, Guillot B, et al. Telaprevir-induced acquired perforating dermatosis. JAMA Dermatol. 2014;150:1371-1372.
- Severino-Freire M, Sibaud V, Tournier E, et al. Acquired perforating dermatosis associated with sorafenib therapy [published online September 11, 2014]. J Eur Acad Dermatol Venereol. 2016;30:328-330.
- Zelger B, Hintner H, Auböck J, et al. Acquired perforating dermatosis. transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991;127:695-700.
- Abe R, Murase S, Nomura Y, et al. Acquired perforating dermatosis appearing as elastosis perforans serpiginosa and perforating folliculitis. Clin Exp Dermatol. 2008;33:653-654.
- Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26:162-171.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679-688.
- Carter VH, Constantine VS. Kyrle’s disease. I. clinical findings in five cases and review of literature. Arch Dermatol. 1968;97:624-632.
- Robinson-Bostom L, Digiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975-986.
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Ohe S, Danno K, Sasaki H, et al. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2004;50:892-894.
- Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. 2012;28:50-52.
- Saldanha LF, Gonick HC, Rodriguez HJ, et al. Silicon-related syndrome in dialysis patients. Nephron. 1997;77:48-56.
- Rodney IJ, Taylor CS, Cohen G. Derm Dx: what are these pruritic nodules? The Dermatologist. October 15, 2009. http://www.the-dermatologist.com/content/derm-dx-what-are-these-pruritic-nodules. Accessed September 18, 2018.
- Gagnon, AL, Desai T. Dermatological diseases in patients with chronic kidney disease. J Nephropathol. 2013;2:104-109.
- Kurban MS, Boueiz A, Kibbi AG. Cutaneous manifestations of chronic kidney disease. Clin Dermatol. 2008;26:255-264.
- Wagner G, Sachse MM. Acquired reactive perforating dermatosis [published online May 29, 2013]. J Dtsch Dermatol Ges. 2013;11:723-729; 723-730.
- Karpouzis A, Giatromanolaki A, Sivridis E, et al. Acquired reactive perforating collagenosis: current status. J Dermatol. 2010;37:585-592.
- Healy R, Cerio R, Hollingsworth A, et al. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010;35:621-623.
- Cordova KB, Oberg TJ, Malik M, et al. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22:45-55.
- Satchell AC, Crotty K, Lee S. Reactive perforating collagenosis: a condition that may be underdiagnosed. Australas J Dermatol. 2001;42:284-287.
- Fujimoto E, Kobayashi T, Fujimoto N, et al. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: epidermal-dermal interaction in acquired perforating dermatosis. J Invest Dermatol. 2010;130:405-414.
- Bilezikci B, Sechkin D, Demirhan B. Acquired perforating dermatosis in patients with chronic renal failure: a possible role for fibronectin. J Eur Acad Dermatol Venereol. 2003;17:230-232.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Hurwitz RM, Melton ME, Creech FT, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Lübbe J, Sorg O, Malé PJ, et al. Sirolimus-induced inflammatory papules with acquired reactive perforating collagenosis [published online January 9, 2008]. Dermatology. 2008;216:239-242.
- Pernet C, Pageaux GP, Guillot B, et al. Telaprevir-induced acquired perforating dermatosis. JAMA Dermatol. 2014;150:1371-1372.
- Severino-Freire M, Sibaud V, Tournier E, et al. Acquired perforating dermatosis associated with sorafenib therapy [published online September 11, 2014]. J Eur Acad Dermatol Venereol. 2016;30:328-330.
- Zelger B, Hintner H, Auböck J, et al. Acquired perforating dermatosis. transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991;127:695-700.
- Abe R, Murase S, Nomura Y, et al. Acquired perforating dermatosis appearing as elastosis perforans serpiginosa and perforating folliculitis. Clin Exp Dermatol. 2008;33:653-654.
- Kim SW, Kim MS, Lee JH, et al. A clinicopathologic study of thirty cases of acquired perforating dermatosis in Korea. Ann Dermatol. 2014;26:162-171.
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006;20:679-688.
- Carter VH, Constantine VS. Kyrle’s disease. I. clinical findings in five cases and review of literature. Arch Dermatol. 1968;97:624-632.
- Robinson-Bostom L, Digiovanna JJ. Cutaneous manifestations of end-stage renal disease. J Am Acad Dermatol. 2000;43:975-986.
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288.
- Morton CA, Henderson IS, Jones MC, et al. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996;135:671-677.
- Ohe S, Danno K, Sasaki H, et al. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2004;50:892-894.
- Sezer E, Erkek E. Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed. 2012;28:50-52.
- Saldanha LF, Gonick HC, Rodriguez HJ, et al. Silicon-related syndrome in dialysis patients. Nephron. 1997;77:48-56.
Practice Points
- Acquired perforating dermatosis (APD) presents as pruritic crateriform papules and plaques with central keratotic plugs.
- A medical history of diabetes mellitus and chronic kidney disease predisposes patients to APD. This case suggests that skin graft sites may be predisposed to the development of APD.
Multiple Pink Papules on the Chest and Upper Abdomen
The Diagnosis: Cutaneous Metastases
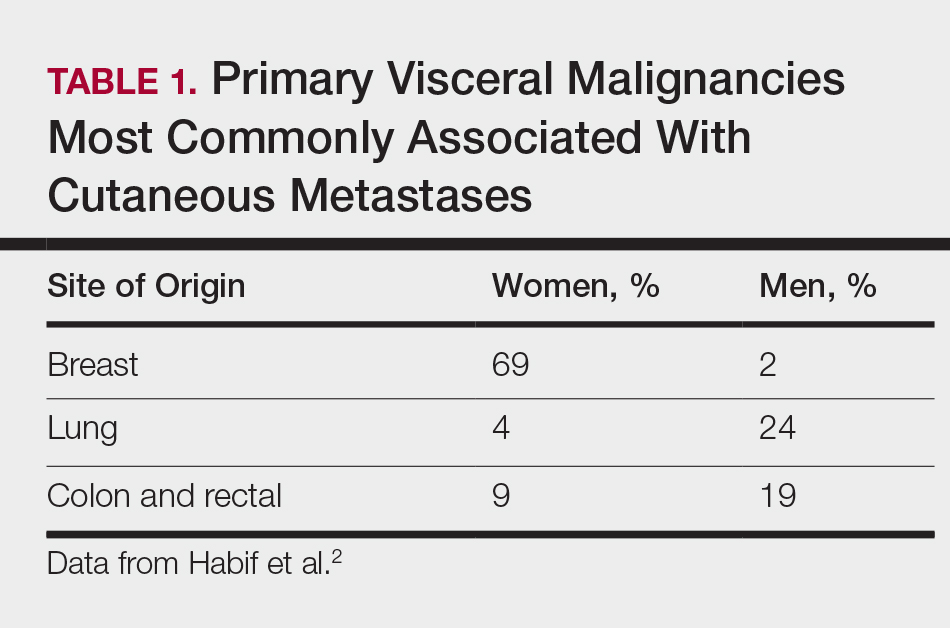
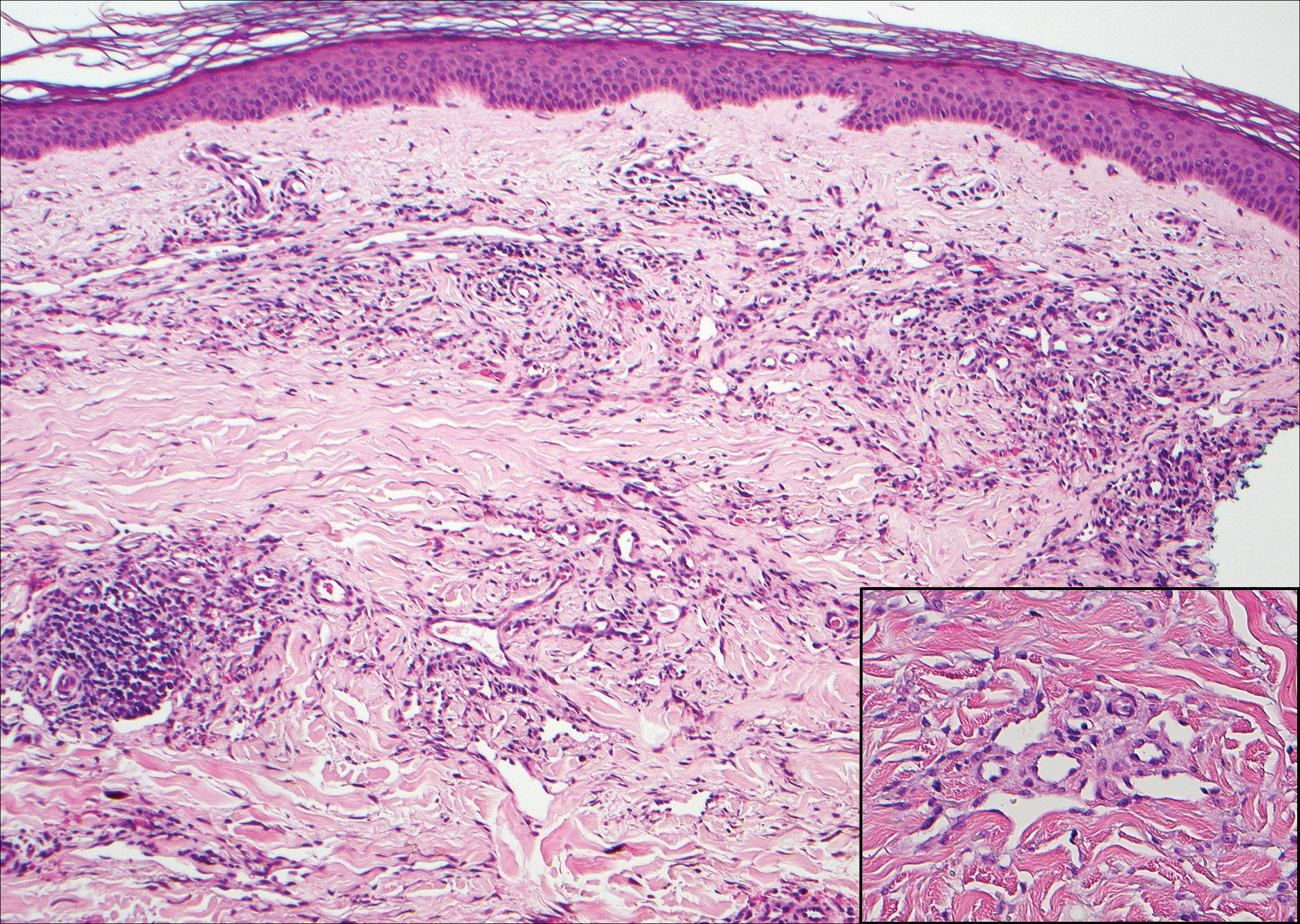
Cutaneous metastases (CMs) can present in an otherwise asymptomatic patient as the only sign of an underlying disease process. In women, the most common cause of CM is breast carcinoma.1-3 Cutaneous metastases are found in approximately 25% of all patients with breast carcinoma,1 and breast carcinomas represent approximately 69% of all CMs found in women (Table 1).2 Cutaneous metastatic breast carcinoma (CMBC) is associated with a poor prognosis with a mean survival of approximately 6 months at the time of diagnosis.1,3 It commonly presents as a collection of flesh-colored, firm, asymptomatic, and rapidly appearing papules and nodules that can resemble cysts or fibrous tumors.1,3,4 They typically are located on the chest wall or abdomen near the site of the underlying malignancy.1-3 The histologic features of CMBC can include hyperchromatic tumor cells infiltrating between the collagen fibers in a characteristic single file manner,3,5 giving the appearance of a busy dermis, a nonspecific term to describe a focally hypercellular dermis at low-power magnification (Table 2).5,6 Cords and clusters of atypical cells with intracytoplasmic vacuoles or well-developed ducts also can be seen (quiz image [inset]). The carcinoma en cuirasse subtype of CMBC is characterized by a fibrotic scarlike plaque on the chest wall.1,3 If a punch biopsy is obtained, the specimen typically appears rectangular rather than tapered because of the sclerotic dermal collagen.6 In contrast, inflammatory carcinoma (carcinoma erysipelatoides) presents as an erythematous plaque resembling cellulitis due to the lymphatics being congested by tumor cells.3 Immunohistochemistry is a valuable tool in diagnosis. Positive staining is seen with cytokeratin 7, gross cystic disease fluid protein-15, mammaglobin, and GATA-3.1,3,6

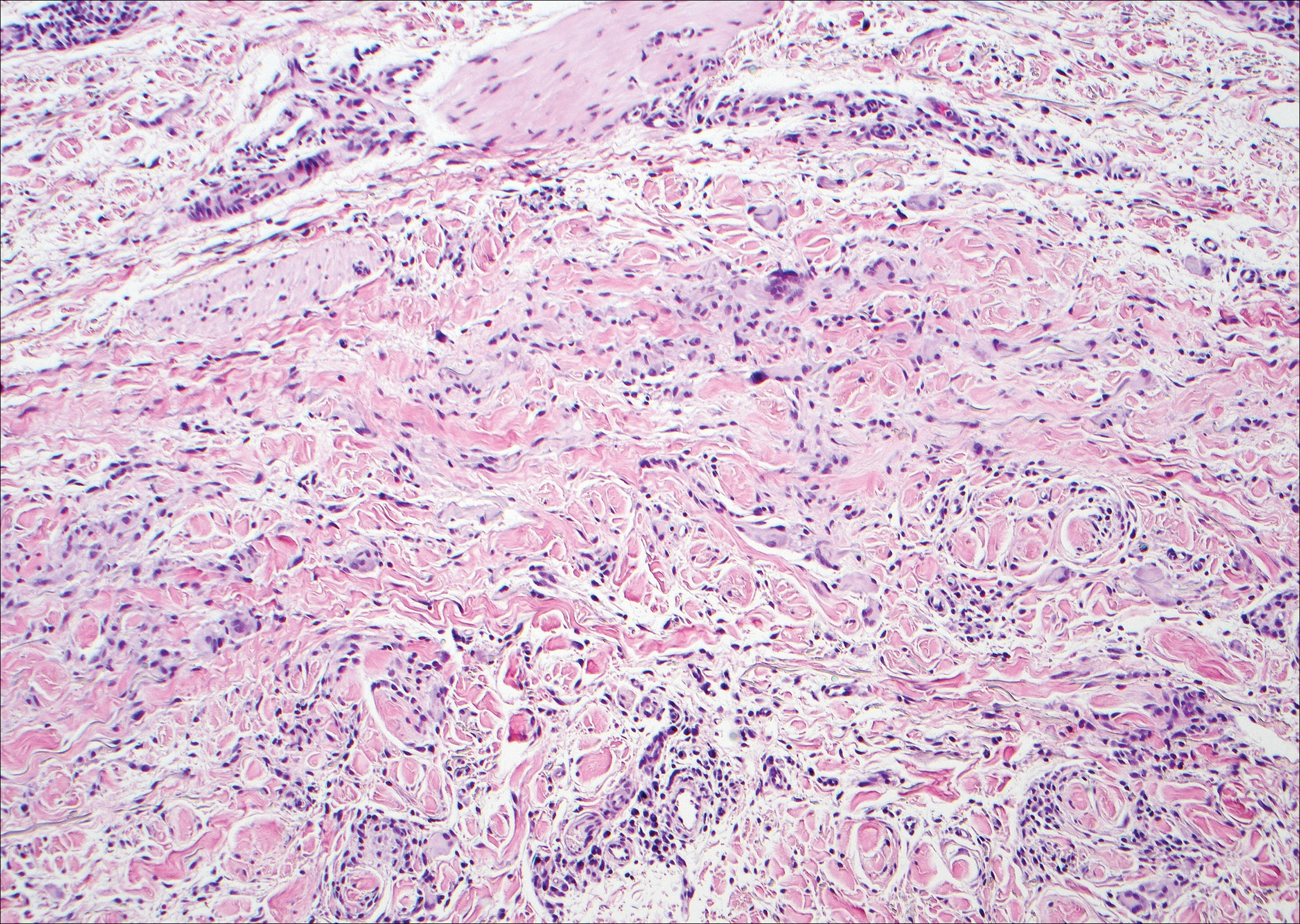
Kaposi sarcoma (KS) is a low-grade endothelial malignancy associated with human herpesvirus 8.3,4 Kaposi sarcoma can be divided into 4 main subtypes: classic KS, African KS, AIDS-related KS, and immunosuppression-associated KS that occurs in patients with diseases such as human immunodeficiency virus. The cutaneous lesions are similar between subtypes and present as dark reddish purple macules that may enlarge or become nodular lesions.3,4 Histologically, 3 distinct stages of progression are described: patch, plaque, and tumor. The plaque stage has the appearance of a busy dermis due to the rapid proliferation of vascular structures within the dermis.3,6 A useful histologic feature known as the promontory sign can be seen as the proliferating tumor causes preexisting structures to project into vascular spaces (Figure 1).6 Immunohistochemistry for the endothelial and lymphatic markers CD31 and D2-40, respectively, are positive and may aid in the diagnosis.3 Staining for the latent nuclear antigen-1 of human herpesvirus 8 is a highly specific marker used to diagnose KS and can further distinguish it from the other busy dermis lesions.3
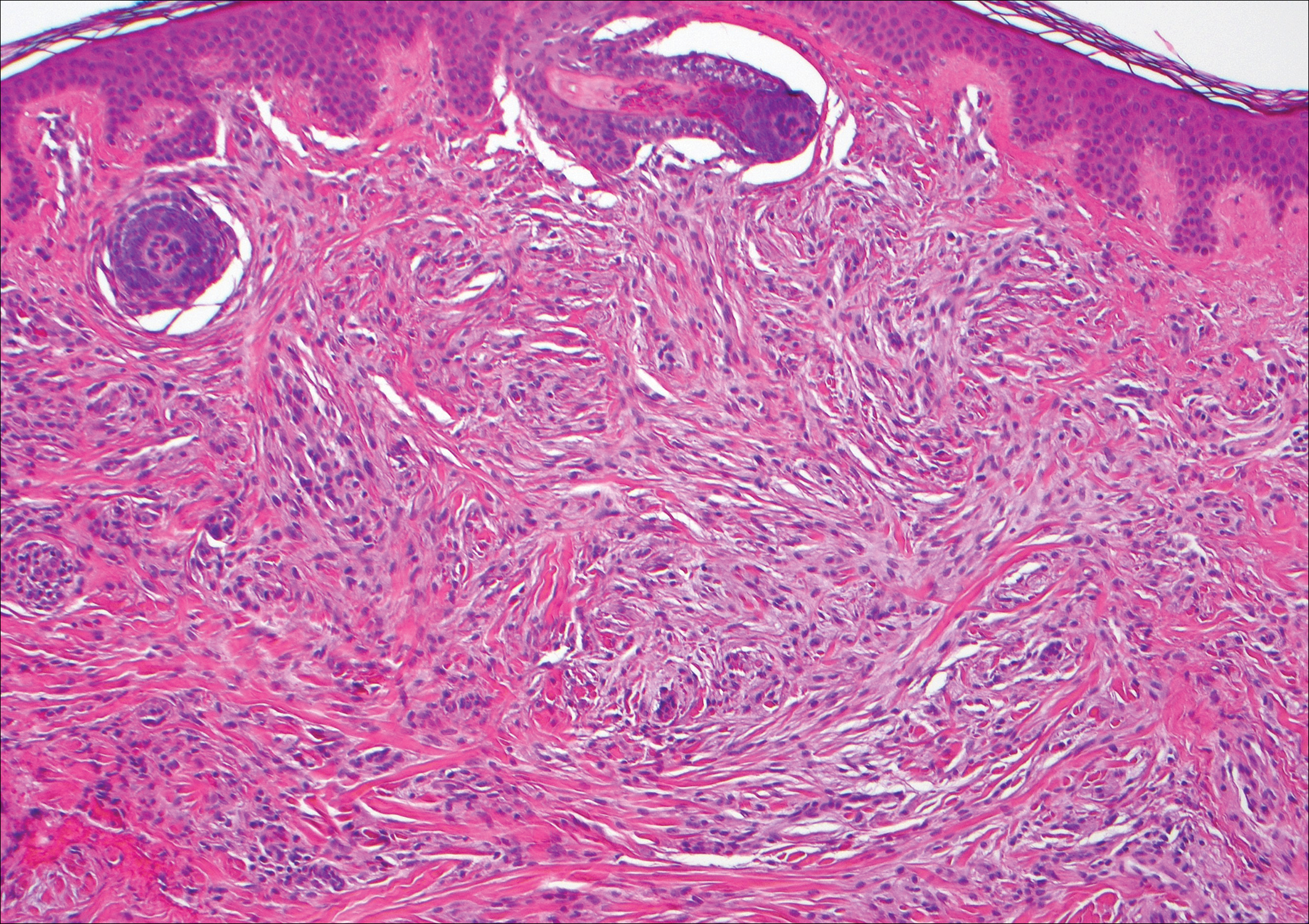
Granuloma annulare (GA) is characterized by rings of small, firm, pink to flesh-colored papules with a variable disease duration.4 Histologically, the interstitial variant of GA is characterized by a scattered inflammatory infiltrate consisting of histiocytes and lymphocytes located between altered collagen fibers in the superficial to mid dermis (Figure 2).3,6 Occasional eosinophils and increased dermal mucin are useful features to distinguish interstitial GA from other entities in the busy dermis differential.7
Scleromyxedema, also known as generalized lichen myxedematosus, is a rare mucinosis.3,8 Although its pathogenesis is unknown, it has been suggested that paraproteins related to the underlying gammopathy act to stimulate fibroblast proliferation and mucin overproduction.8 Clinically, characteristic widespread firm, waxy, dome-shaped papules are present over the head, upper trunk, and extremities.3,8 Histologically, scleromyxedema is characterized by increased dermal fibroblasts, mucin, and fibrosis, leading to the appearance of a busy dermis (Figure 3).3,6
Neurofibromas are common benign peripheral nerve sheath tumors that can occur sporadically or in the setting of neurofibromatosis.3-5 They present as soft, flesh-colored papules or nodules most commonly located on the trunk and limbs.4 Histologically, neurofibromas are nonencapsulated tumors composed of abundant spindle cells with comma-shaped nuclei diffusely arranged in a pale myxoid stroma (Figure 4). Scattered mast cells can be visualized at higher magnification.3,6
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Habif TP, Dinulos JGH, Chapman MS, et al. Skin Disease: Diagnosis and Treatment. 4th ed. Edinburgh, Scotland: Elsevier; 2017.
- Calonje JE, Brenn T, Lazar AJ, et al, eds. McKee's Pathology of the Skin. 4th ed. St. Louis, MO: Elsevier Saunders; 2012.
- Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Philadelphia, PA: Elsevier; 2015.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2016.
- Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Silverman RA, Rabinowitz AD. Eosinophils in the cellular infiltrate of granuloma annulare. J Cutan Pathol. 1985;12:13-17.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
The Diagnosis: Cutaneous Metastases
Cutaneous metastases (CMs) can present in an otherwise asymptomatic patient as the only sign of an underlying disease process. In women, the most common cause of CM is breast carcinoma.1-3 Cutaneous metastases are found in approximately 25% of all patients with breast carcinoma,1 and breast carcinomas represent approximately 69% of all CMs found in women (Table 1).2 Cutaneous metastatic breast carcinoma (CMBC) is associated with a poor prognosis with a mean survival of approximately 6 months at the time of diagnosis.1,3 It commonly presents as a collection of flesh-colored, firm, asymptomatic, and rapidly appearing papules and nodules that can resemble cysts or fibrous tumors.1,3,4 They typically are located on the chest wall or abdomen near the site of the underlying malignancy.1-3 The histologic features of CMBC can include hyperchromatic tumor cells infiltrating between the collagen fibers in a characteristic single file manner,3,5 giving the appearance of a busy dermis, a nonspecific term to describe a focally hypercellular dermis at low-power magnification (Table 2).5,6 Cords and clusters of atypical cells with intracytoplasmic vacuoles or well-developed ducts also can be seen (quiz image [inset]). The carcinoma en cuirasse subtype of CMBC is characterized by a fibrotic scarlike plaque on the chest wall.1,3 If a punch biopsy is obtained, the specimen typically appears rectangular rather than tapered because of the sclerotic dermal collagen.6 In contrast, inflammatory carcinoma (carcinoma erysipelatoides) presents as an erythematous plaque resembling cellulitis due to the lymphatics being congested by tumor cells.3 Immunohistochemistry is a valuable tool in diagnosis. Positive staining is seen with cytokeratin 7, gross cystic disease fluid protein-15, mammaglobin, and GATA-3.1,3,6


Kaposi sarcoma (KS) is a low-grade endothelial malignancy associated with human herpesvirus 8.3,4 Kaposi sarcoma can be divided into 4 main subtypes: classic KS, African KS, AIDS-related KS, and immunosuppression-associated KS that occurs in patients with diseases such as human immunodeficiency virus. The cutaneous lesions are similar between subtypes and present as dark reddish purple macules that may enlarge or become nodular lesions.3,4 Histologically, 3 distinct stages of progression are described: patch, plaque, and tumor. The plaque stage has the appearance of a busy dermis due to the rapid proliferation of vascular structures within the dermis.3,6 A useful histologic feature known as the promontory sign can be seen as the proliferating tumor causes preexisting structures to project into vascular spaces (Figure 1).6 Immunohistochemistry for the endothelial and lymphatic markers CD31 and D2-40, respectively, are positive and may aid in the diagnosis.3 Staining for the latent nuclear antigen-1 of human herpesvirus 8 is a highly specific marker used to diagnose KS and can further distinguish it from the other busy dermis lesions.3

Granuloma annulare (GA) is characterized by rings of small, firm, pink to flesh-colored papules with a variable disease duration.4 Histologically, the interstitial variant of GA is characterized by a scattered inflammatory infiltrate consisting of histiocytes and lymphocytes located between altered collagen fibers in the superficial to mid dermis (Figure 2).3,6 Occasional eosinophils and increased dermal mucin are useful features to distinguish interstitial GA from other entities in the busy dermis differential.7

Scleromyxedema, also known as generalized lichen myxedematosus, is a rare mucinosis.3,8 Although its pathogenesis is unknown, it has been suggested that paraproteins related to the underlying gammopathy act to stimulate fibroblast proliferation and mucin overproduction.8 Clinically, characteristic widespread firm, waxy, dome-shaped papules are present over the head, upper trunk, and extremities.3,8 Histologically, scleromyxedema is characterized by increased dermal fibroblasts, mucin, and fibrosis, leading to the appearance of a busy dermis (Figure 3).3,6

Neurofibromas are common benign peripheral nerve sheath tumors that can occur sporadically or in the setting of neurofibromatosis.3-5 They present as soft, flesh-colored papules or nodules most commonly located on the trunk and limbs.4 Histologically, neurofibromas are nonencapsulated tumors composed of abundant spindle cells with comma-shaped nuclei diffusely arranged in a pale myxoid stroma (Figure 4). Scattered mast cells can be visualized at higher magnification.3,6

The Diagnosis: Cutaneous Metastases
Cutaneous metastases (CMs) can present in an otherwise asymptomatic patient as the only sign of an underlying disease process. In women, the most common cause of CM is breast carcinoma.1-3 Cutaneous metastases are found in approximately 25% of all patients with breast carcinoma,1 and breast carcinomas represent approximately 69% of all CMs found in women (Table 1).2 Cutaneous metastatic breast carcinoma (CMBC) is associated with a poor prognosis with a mean survival of approximately 6 months at the time of diagnosis.1,3 It commonly presents as a collection of flesh-colored, firm, asymptomatic, and rapidly appearing papules and nodules that can resemble cysts or fibrous tumors.1,3,4 They typically are located on the chest wall or abdomen near the site of the underlying malignancy.1-3 The histologic features of CMBC can include hyperchromatic tumor cells infiltrating between the collagen fibers in a characteristic single file manner,3,5 giving the appearance of a busy dermis, a nonspecific term to describe a focally hypercellular dermis at low-power magnification (Table 2).5,6 Cords and clusters of atypical cells with intracytoplasmic vacuoles or well-developed ducts also can be seen (quiz image [inset]). The carcinoma en cuirasse subtype of CMBC is characterized by a fibrotic scarlike plaque on the chest wall.1,3 If a punch biopsy is obtained, the specimen typically appears rectangular rather than tapered because of the sclerotic dermal collagen.6 In contrast, inflammatory carcinoma (carcinoma erysipelatoides) presents as an erythematous plaque resembling cellulitis due to the lymphatics being congested by tumor cells.3 Immunohistochemistry is a valuable tool in diagnosis. Positive staining is seen with cytokeratin 7, gross cystic disease fluid protein-15, mammaglobin, and GATA-3.1,3,6


Kaposi sarcoma (KS) is a low-grade endothelial malignancy associated with human herpesvirus 8.3,4 Kaposi sarcoma can be divided into 4 main subtypes: classic KS, African KS, AIDS-related KS, and immunosuppression-associated KS that occurs in patients with diseases such as human immunodeficiency virus. The cutaneous lesions are similar between subtypes and present as dark reddish purple macules that may enlarge or become nodular lesions.3,4 Histologically, 3 distinct stages of progression are described: patch, plaque, and tumor. The plaque stage has the appearance of a busy dermis due to the rapid proliferation of vascular structures within the dermis.3,6 A useful histologic feature known as the promontory sign can be seen as the proliferating tumor causes preexisting structures to project into vascular spaces (Figure 1).6 Immunohistochemistry for the endothelial and lymphatic markers CD31 and D2-40, respectively, are positive and may aid in the diagnosis.3 Staining for the latent nuclear antigen-1 of human herpesvirus 8 is a highly specific marker used to diagnose KS and can further distinguish it from the other busy dermis lesions.3

Granuloma annulare (GA) is characterized by rings of small, firm, pink to flesh-colored papules with a variable disease duration.4 Histologically, the interstitial variant of GA is characterized by a scattered inflammatory infiltrate consisting of histiocytes and lymphocytes located between altered collagen fibers in the superficial to mid dermis (Figure 2).3,6 Occasional eosinophils and increased dermal mucin are useful features to distinguish interstitial GA from other entities in the busy dermis differential.7

Scleromyxedema, also known as generalized lichen myxedematosus, is a rare mucinosis.3,8 Although its pathogenesis is unknown, it has been suggested that paraproteins related to the underlying gammopathy act to stimulate fibroblast proliferation and mucin overproduction.8 Clinically, characteristic widespread firm, waxy, dome-shaped papules are present over the head, upper trunk, and extremities.3,8 Histologically, scleromyxedema is characterized by increased dermal fibroblasts, mucin, and fibrosis, leading to the appearance of a busy dermis (Figure 3).3,6

Neurofibromas are common benign peripheral nerve sheath tumors that can occur sporadically or in the setting of neurofibromatosis.3-5 They present as soft, flesh-colored papules or nodules most commonly located on the trunk and limbs.4 Histologically, neurofibromas are nonencapsulated tumors composed of abundant spindle cells with comma-shaped nuclei diffusely arranged in a pale myxoid stroma (Figure 4). Scattered mast cells can be visualized at higher magnification.3,6

- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Habif TP, Dinulos JGH, Chapman MS, et al. Skin Disease: Diagnosis and Treatment. 4th ed. Edinburgh, Scotland: Elsevier; 2017.
- Calonje JE, Brenn T, Lazar AJ, et al, eds. McKee's Pathology of the Skin. 4th ed. St. Louis, MO: Elsevier Saunders; 2012.
- Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Philadelphia, PA: Elsevier; 2015.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2016.
- Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Silverman RA, Rabinowitz AD. Eosinophils in the cellular infiltrate of granuloma annulare. J Cutan Pathol. 1985;12:13-17.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Habif TP, Dinulos JGH, Chapman MS, et al. Skin Disease: Diagnosis and Treatment. 4th ed. Edinburgh, Scotland: Elsevier; 2017.
- Calonje JE, Brenn T, Lazar AJ, et al, eds. McKee's Pathology of the Skin. 4th ed. St. Louis, MO: Elsevier Saunders; 2012.
- Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Philadelphia, PA: Elsevier; 2015.
- Patterson JW, Hosler GA. Weedon's Skin Pathology. 4th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2016.
- Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
- Silverman RA, Rabinowitz AD. Eosinophils in the cellular infiltrate of granuloma annulare. J Cutan Pathol. 1985;12:13-17.
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72.
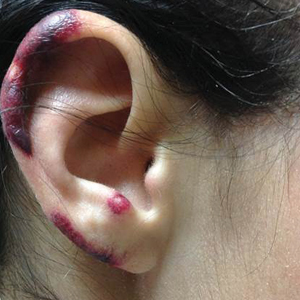
A 56-year-old woman presented with multiple asymptomatic lesions of 2 months' duration. On physical examination firm pink papules were noted dispersed across the upper abdomen, chest, and back. A 5-mm punch biopsy was obtained.
Adult-Onset Still Disease: Persistent Pruritic Papular Rash With Unique Histopathologic Findings
Adult-onset Still disease (AOSD) is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, evanescent skin rash, and lymphadenopathy. 1 The most commonly used criteria for diagnosing AOSD are the Yamaguchi criteria. 2 The major criteria include high fever for more than 1 week, arthralgia for more than 2 weeks, leukocytosis, and an evanescent skin rash. The minor criteria consist of sore throat, lymphadenopathy and/or splenomegaly, liver dysfunction, and negative rheumatoid factor and antinuclear antibodies. Classically, the skin rash is described as an evanescent, salmon-colored erythema involving the extremities. Nevertheless, unusual cutaneous eruptions have been reported in AOSD, including persistent pruritic papules and plaques. 3 Importantly, this atypical rash demonstrates specific histologic findings that are not found on routine histopathology of a typical evanescent rash. We describe 2 patients with this atypical cutaneous eruption along with the unique histopathologic findings of AOSD.
Case Reports
Patient 1
A 23-year-old Chinese woman presented with periodic fevers, persistent rash, and joint pain of 2 years’ duration. Her medical history included splenectomy for hepatosplenomegaly as well as evaluation by hematology for lymphadenopathy; a cervical lymph node biopsy showed lymphoid and follicular hyperplasia.
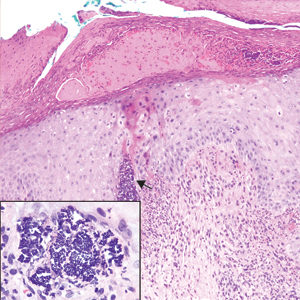
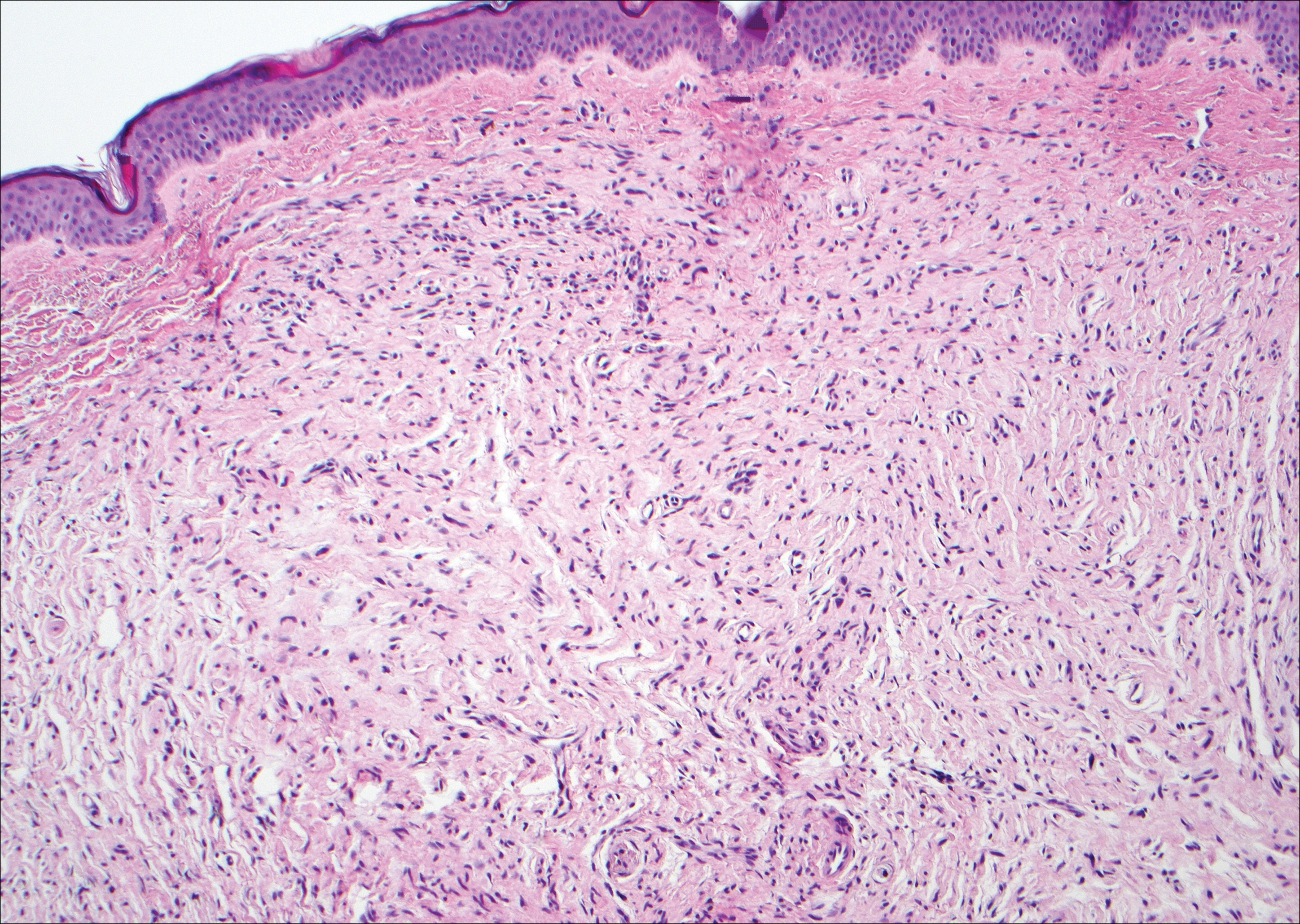
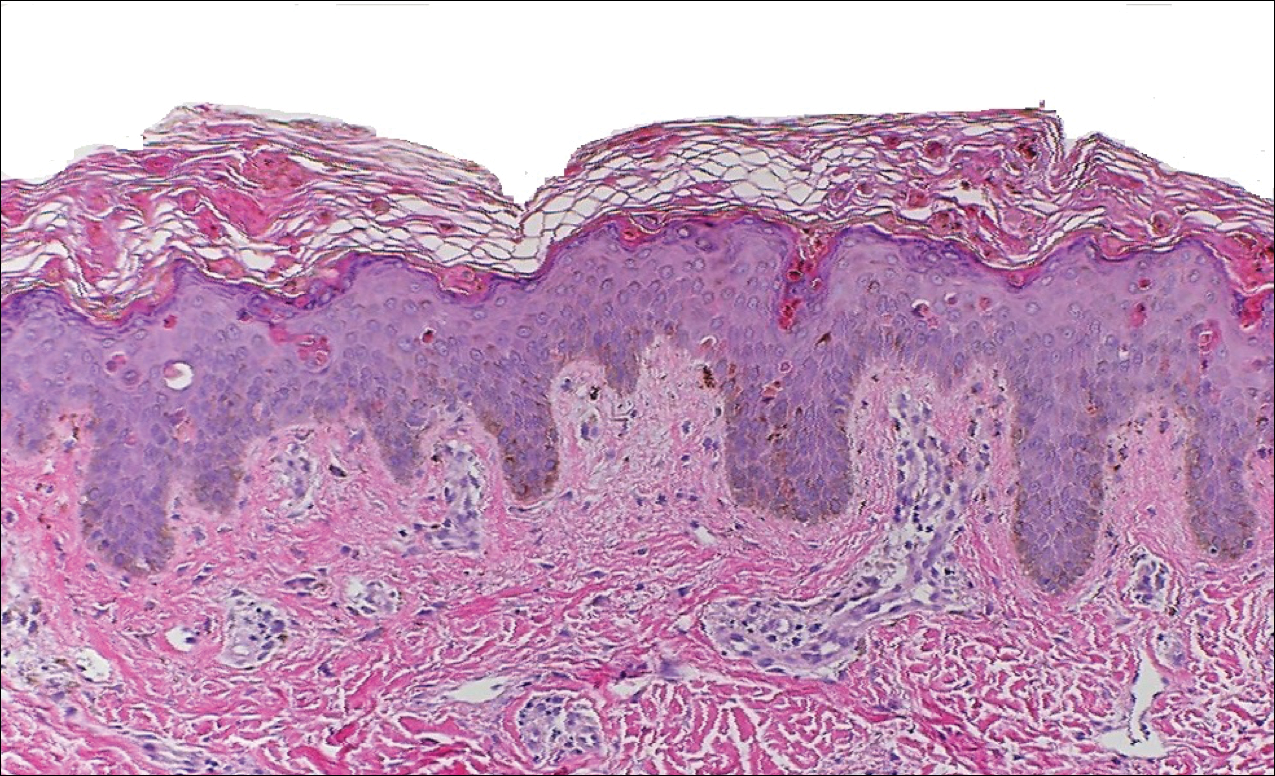
Twenty days later, the patient was referred to the dermatology department for evaluation of the persistent rash. The patient described a history of flushing of the face, severe joint pain in both arms and legs, aching muscles, and persistent sore throat. The patient did not report any history of drug ingestion. Physical examination revealed a fever (temperature, 39.2°C); swollen nontender lymph nodes in the neck, axillae, and groin; and salmon-colored and hyperpigmented patches and thin plaques over the neck, chest, abdomen, and arms (Figure 1). A splenectomy scar also was noted. Peripheral blood was collected for laboratory analyses, which revealed transaminitis and moderate hyperferritinemia (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. The patient was admitted to the hospital, and a skin biopsy was performed. Histology showed superficial dyskeratotic keratinocytes and sparse perivascular infiltration of neutrophils in the upper dermis (Figure 2).
The patient was diagnosed with AOSD based on fulfillment of the Yamaguchi criteria.2 She was treated with methylprednisolone 60 mg daily and was discharged 14 days later. At 16-month follow-up, the patient demonstrated complete resolution of symptoms with a maintenance dose of prednisolone (7.5 mg daily).
Patient 2
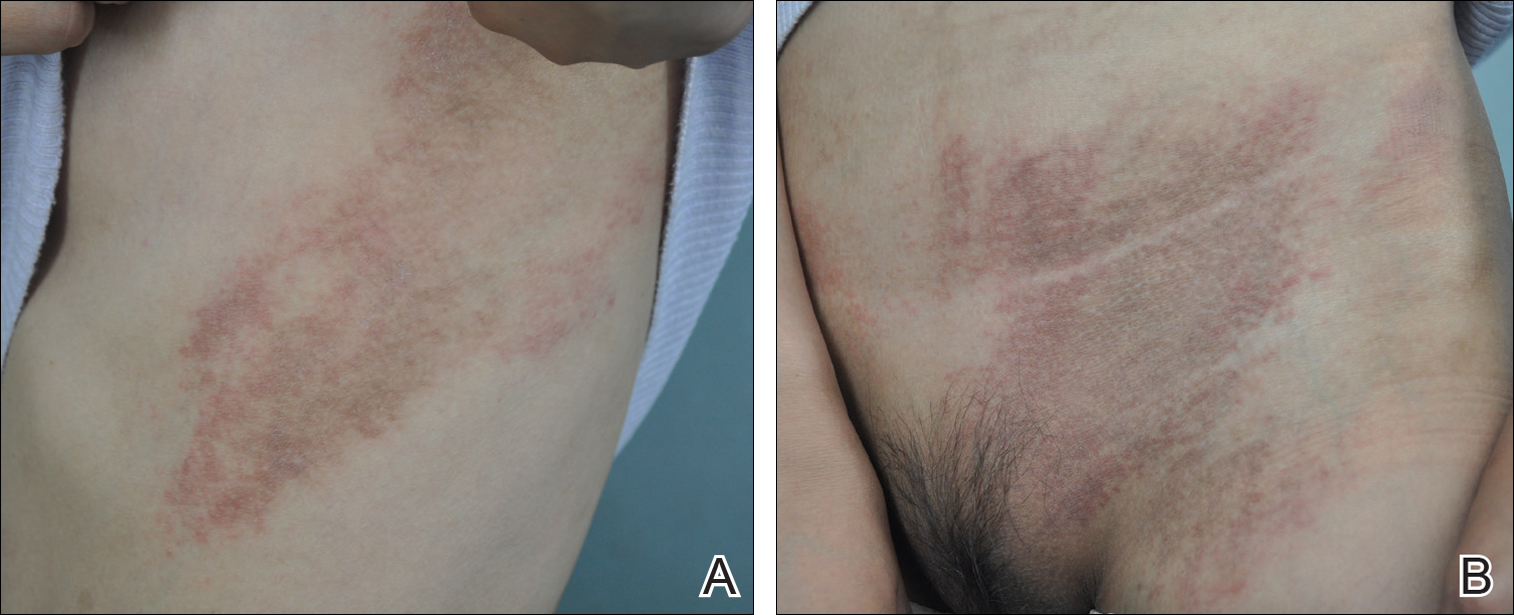
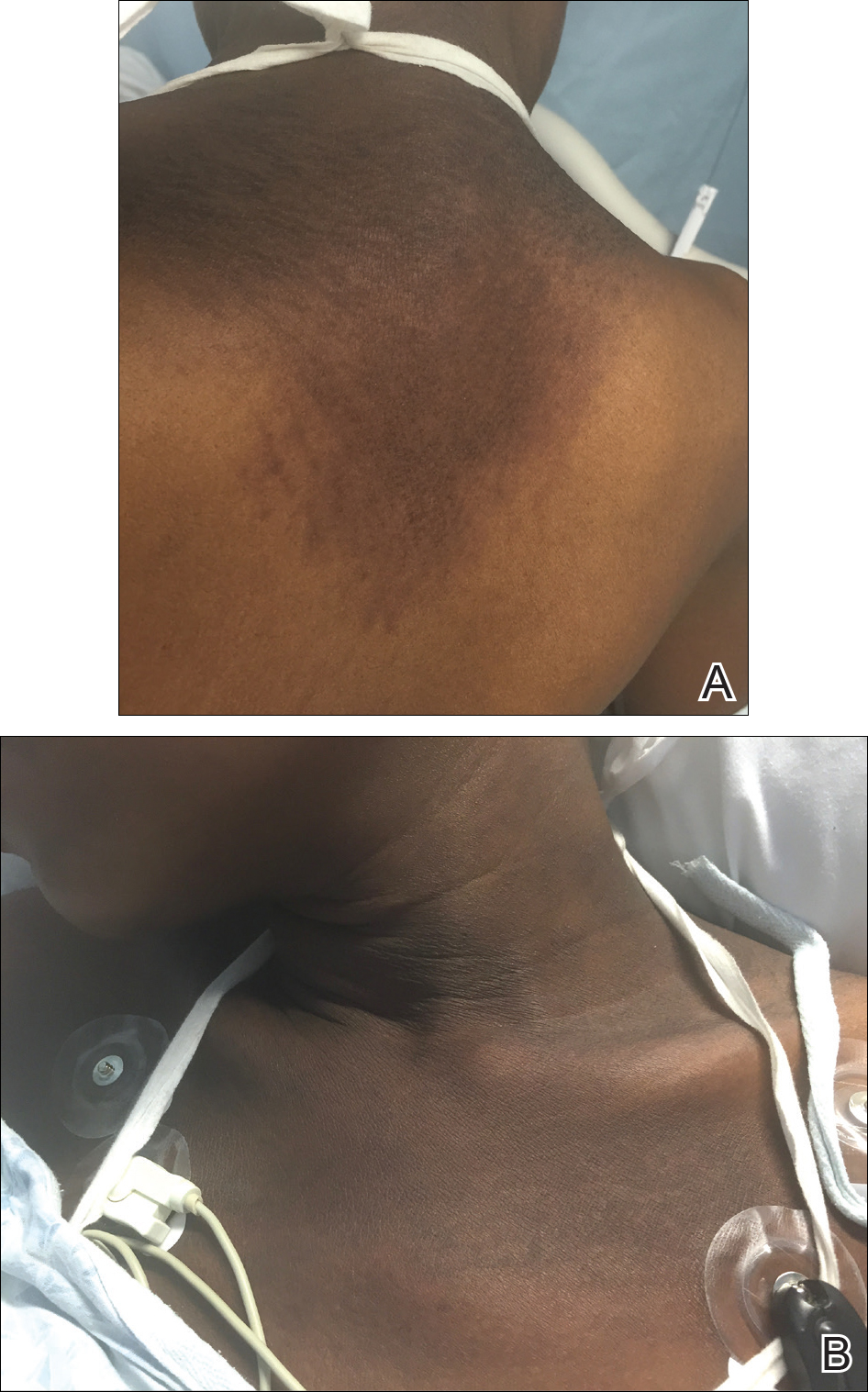
A 23-year-old black woman presented to the emergency department 3 months postpartum with recurrent high fevers, worsening joint pain, and persistent itchy rash of 2 months’ duration. The patient had no history of travel, autoimmune disease, or sick contacts. She occasionally took aspirin for joint pain. Physical examination revealed a fever (temperature, 39.1°C) along with hyperpigmented patches and thin scaly hyperpigmented papules coalescing into a poorly demarcated V-shaped plaque on the upper back and posterior neck, extending to the chest in a shawl-like distribution (Figure 3). Submental lymphadenopathy was present. The spleen was not palpable.
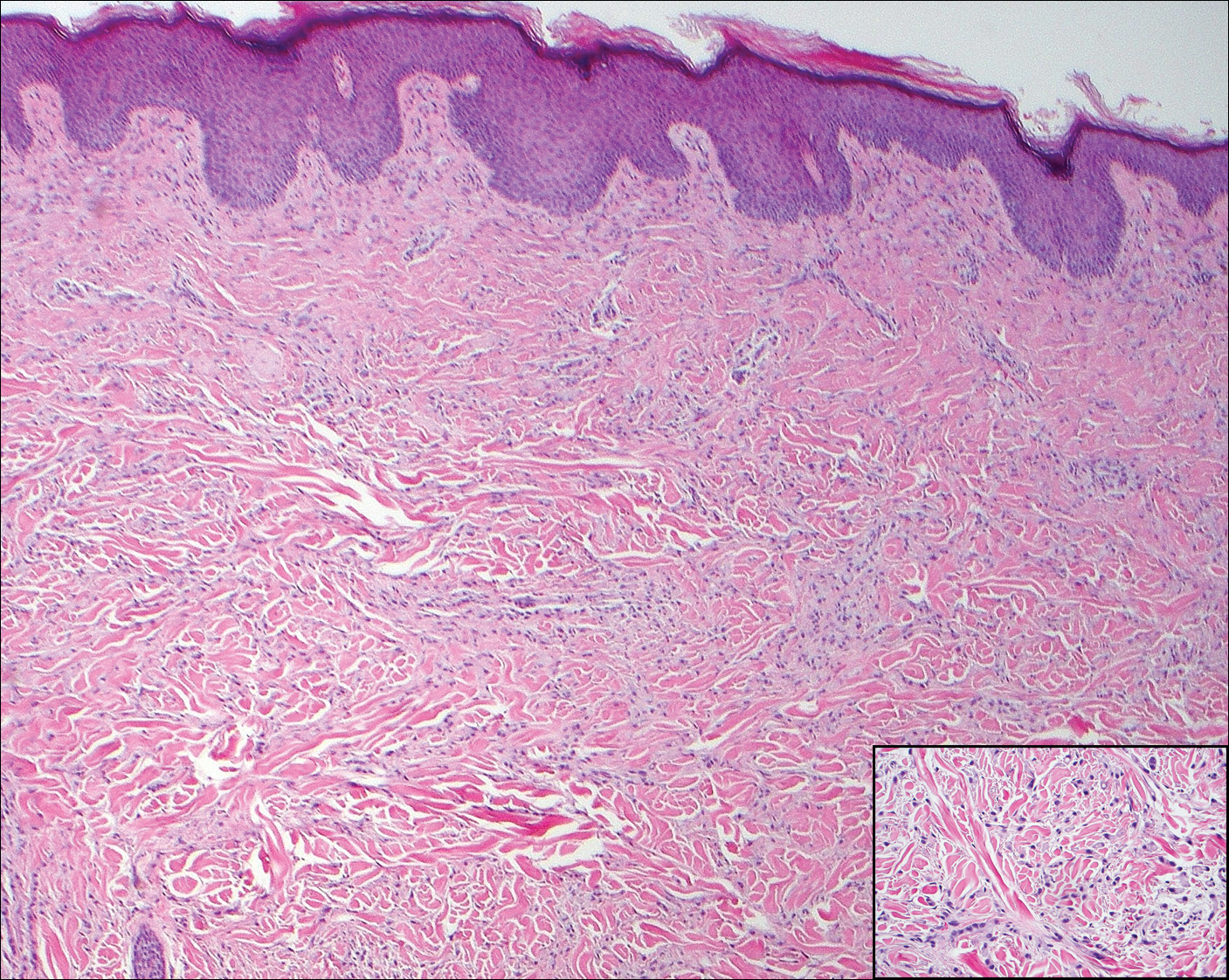
Peripheral blood was collected for laboratory analysis and demonstrated transaminitis and a markedly high ferritin level (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. Skin biopsy was performed and demonstrated many necrotic keratinocytes, singly and in aggregates, distributed from the spinous layer to the stratum corneum. A neutrophilic infiltrate was present in the papillary dermis (Figure 4).
The patient met the Yamaguchi criteria and was subsequently diagnosed with AOSD. She was treated with intravenous methylprednisolone 20 mg every 8 hours and was discharged 1 week later on oral prednisone 60 mg daily to be tapered over a period of months. At 2-week follow-up, the patient continued to experience rash and joint pain; oral methotrexate 10 mg weekly was added to her regimen, as well as vitamin D, calcium, and folic acid supplementation. At the next 2-week follow-up the patient noted improvement in the rash as well as the joint pain, but both still persisted. Prednisone was decreased to 50 mg daily and methotrexate was increased to 15 mg weekly. The patient continued to show improvement over the subsequent 3 months, during which prednisone was tapered to 10 mg daily and methotrexate was increased to 20 mg weekly. The patient showed resolution of symptoms at 3-month follow-up on this regimen, with plans to continue the prednisone taper and maintain methotrexate dosing.
Comment
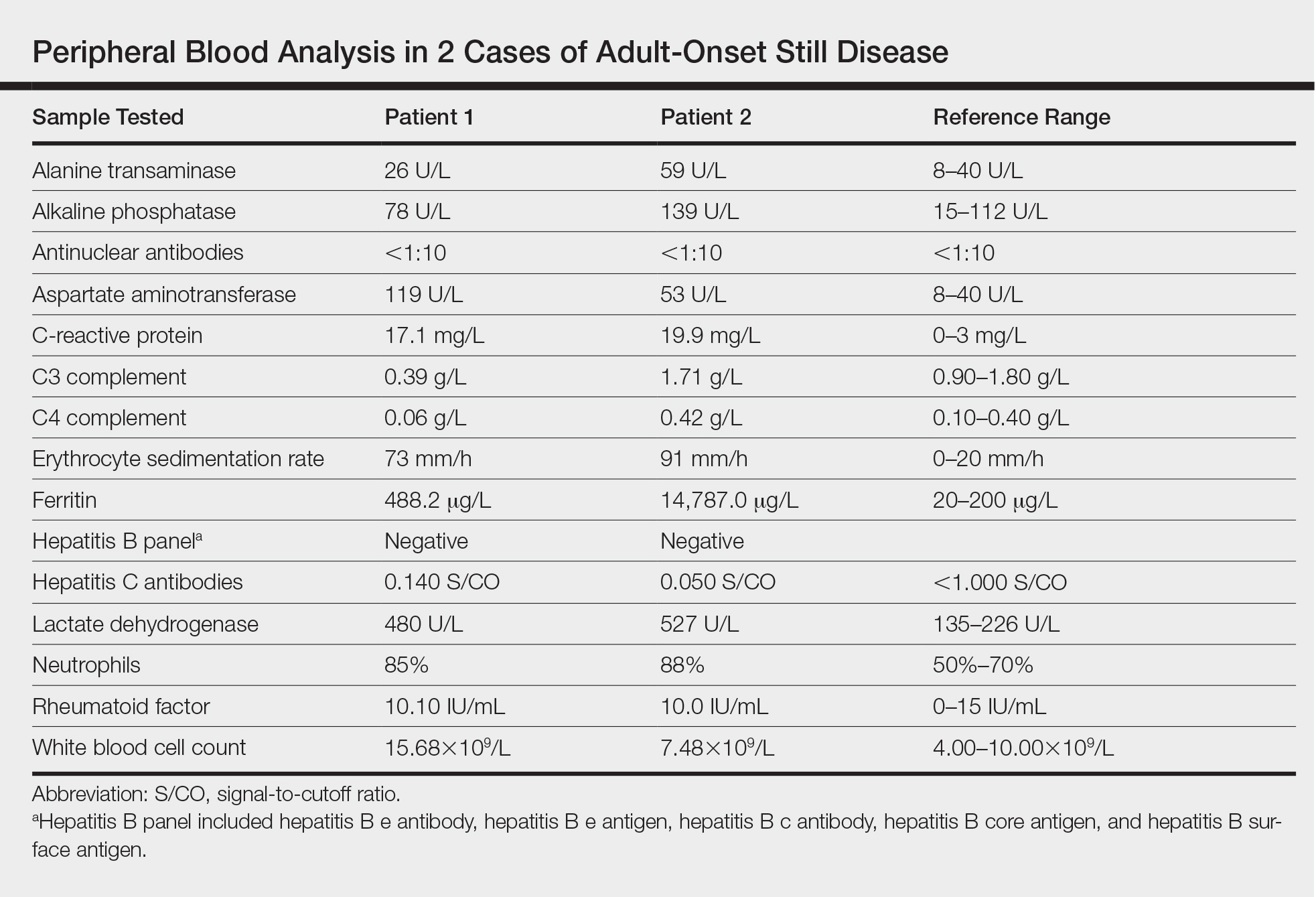
Adult-onset Still disease is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, salmon-pink evanescent erythema, and lymphadenopathy.2 The condition also can cause liver dysfunction, splenomegaly, pericarditis, pleuritis, renal dysfunction, and a reactive hemophagocytic syndrome.1 Furthermore, one review of the literature described an association with delayed-onset malignancy.4 Early diagnosis is important yet challenging, as AOSD is a diagnosis of exclusion. The Yamaguchi criteria are the most widely used method of diagnosis and demonstrate more than 90% sensitivity.In addition to the Yamaguchi criteria, marked hyperferritinemia is characteristic of AOSD and can act as an indicator of disease activity.5 Interestingly, both of our patients had elevated ferritin levels, with patient 2 showing marked elevation (Table). In both patients, all major criteria were fulfilled, except the typical skin rash.
The skin rash in AOSD, classically consisting of an evanescent, salmon-pink erythema predominantly involving the extremities, has been observed in up to 87% of AOSD patients.5 The histology of the typical evanescent rash is nonspecific, characterized by a relatively sparse, perivascular, mixed inflammatory infiltrate. Notably, other skin manifestations may be found in patients with AOSD.1,2,5-16 Persistent pruritic papules and plaques are the most commonly reported nonclassical rash, presenting as erythematous, slightly scaly papules and plaques with a linear configuration typically on the trunk.2 Both of our patients presented with this atypical eruption. Importantly, the histopathology of this unique rash displays distinctive features, which can aid in early diagnosis. Findings include dyskeratotic keratinocytes in the cornified layers as well as in the epidermis, and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis. These findings were evident in both histopathologic studies of our patients (Figures 2 and 4). Although not present in our patients, dermal mucin deposition has been demonstrated in some reports.1,13,15
A 2015 review of the literature yielded 30 cases of AOSD with pruritic persistent papules and plaques.4 The study confirmed a linear, erythematous or brown rash on the back and neck in the majority of cases. Histologic findings were congruent with those reported in our 2 cases: necrotic keratinocytes in the upper epidermis with a neutrophilic infiltrate in the upper dermis without vasculitis. Most patients showed rapid resolution of the rash and symptoms with the use of prednisone, prednisolone, or intravenous pulsed methylprednisolone. Interestingly, a range of presentations were noted, including prurigo pigmentosalike urticarial papules; lichenoid papules; and dermatographismlike, dermatomyositislike, and lichen amyloidosis–like rashes.4 In our report, patient 2 presented with a rash in a dermat-omyositislike shawl distribution. It has been suggested that patients with dermatomyositislike rashes require more potent immunotherapy as compared to patients with other rash morphologies.4 The need for methotrexate in addition to a prednisone taper in the clinical course of patient 2 lends further support to this observation.
Conclusion
A clinically and pathologically distinct form of cutaneous disease—AOSD with persistent pruritic papules and plaques—was observed in our 2 patients. These histopathologic findings facilitated timely diagnosis in both patients. A range of clinical morphologies may exist in AOSD, an awareness of which is paramount. Adult-onset Still disease should be included in the differential diagnosis of a dermatomyositislike presentation in a shawl distribution. Prompt diagnosis is essential to ensure adequate therapy.
- Yamamoto T. Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int. 2012;32:2233-2237.
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-431.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Sun NZ, Brezinski EA, Berliner J, et al. Updates in adult-onset Still disease: atypical cutaneous manifestations and associates with delayed malignancy [published online June 6, 2015]. J Am Acad Dermatol. 2015;73:294-303.
- Schwarz-Eywill M, Heilig B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann Rheum Dis. 1992;51:683-685.
- Ohta A, Yamaguchi M, Tsunematsu T, et al. Adult Still’s disease: a multicenter survey of Japanese patients. J Rheumatol. 1990;17:1058-1063.
- Kaur S, Bambery P, Dhar S. Persistent dermal plaque lesions in adult onset Still’s disease. Dermatology. 1994;188:241-242.
- Lübbe J, Hofer M, Chavaz P, et al. Adult onset Still’s disease with persistent plaques. Br J Dermatol. 1999;141:710-713.
- Suzuki K, Kimura Y, Aoki M, et al. Persistent plaques and linear pigmentation in adult-onset Still’s disease. Dermatology. 2001;202:333-335.
- Fujii K, Konishi K, Kanno Y, et al. Persistent generalized erythema in adult-onset Still’s disease. Int J Dermatol. 2003;42:824-825.
- Thien Huong NT, Pitche P, Minh Hoa T, et al. Persistent pigmented plaques in adult-onset Still’s disease. Ann Dermatol Venereol. 2005;132:693-696.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Wolgamot G, Yoo J, Hurst S, et al. Unique histopathologic findings in a patient with adult-onset Still’s disease. Am J Dermatopathol. 2007;49:194-196.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still’s disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still’s disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Azeck AG, Littlewood SM. Adult-onset Still’s disease with atypical cutaneous features. J Eur Acad Dermatol Venereol. 2005;19:360-363.
Adult-onset Still disease (AOSD) is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, evanescent skin rash, and lymphadenopathy. 1 The most commonly used criteria for diagnosing AOSD are the Yamaguchi criteria. 2 The major criteria include high fever for more than 1 week, arthralgia for more than 2 weeks, leukocytosis, and an evanescent skin rash. The minor criteria consist of sore throat, lymphadenopathy and/or splenomegaly, liver dysfunction, and negative rheumatoid factor and antinuclear antibodies. Classically, the skin rash is described as an evanescent, salmon-colored erythema involving the extremities. Nevertheless, unusual cutaneous eruptions have been reported in AOSD, including persistent pruritic papules and plaques. 3 Importantly, this atypical rash demonstrates specific histologic findings that are not found on routine histopathology of a typical evanescent rash. We describe 2 patients with this atypical cutaneous eruption along with the unique histopathologic findings of AOSD.
Case Reports
Patient 1
A 23-year-old Chinese woman presented with periodic fevers, persistent rash, and joint pain of 2 years’ duration. Her medical history included splenectomy for hepatosplenomegaly as well as evaluation by hematology for lymphadenopathy; a cervical lymph node biopsy showed lymphoid and follicular hyperplasia.
Twenty days later, the patient was referred to the dermatology department for evaluation of the persistent rash. The patient described a history of flushing of the face, severe joint pain in both arms and legs, aching muscles, and persistent sore throat. The patient did not report any history of drug ingestion. Physical examination revealed a fever (temperature, 39.2°C); swollen nontender lymph nodes in the neck, axillae, and groin; and salmon-colored and hyperpigmented patches and thin plaques over the neck, chest, abdomen, and arms (Figure 1). A splenectomy scar also was noted. Peripheral blood was collected for laboratory analyses, which revealed transaminitis and moderate hyperferritinemia (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. The patient was admitted to the hospital, and a skin biopsy was performed. Histology showed superficial dyskeratotic keratinocytes and sparse perivascular infiltration of neutrophils in the upper dermis (Figure 2).



The patient was diagnosed with AOSD based on fulfillment of the Yamaguchi criteria.2 She was treated with methylprednisolone 60 mg daily and was discharged 14 days later. At 16-month follow-up, the patient demonstrated complete resolution of symptoms with a maintenance dose of prednisolone (7.5 mg daily).
Patient 2
A 23-year-old black woman presented to the emergency department 3 months postpartum with recurrent high fevers, worsening joint pain, and persistent itchy rash of 2 months’ duration. The patient had no history of travel, autoimmune disease, or sick contacts. She occasionally took aspirin for joint pain. Physical examination revealed a fever (temperature, 39.1°C) along with hyperpigmented patches and thin scaly hyperpigmented papules coalescing into a poorly demarcated V-shaped plaque on the upper back and posterior neck, extending to the chest in a shawl-like distribution (Figure 3). Submental lymphadenopathy was present. The spleen was not palpable.

Peripheral blood was collected for laboratory analysis and demonstrated transaminitis and a markedly high ferritin level (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. Skin biopsy was performed and demonstrated many necrotic keratinocytes, singly and in aggregates, distributed from the spinous layer to the stratum corneum. A neutrophilic infiltrate was present in the papillary dermis (Figure 4).

The patient met the Yamaguchi criteria and was subsequently diagnosed with AOSD. She was treated with intravenous methylprednisolone 20 mg every 8 hours and was discharged 1 week later on oral prednisone 60 mg daily to be tapered over a period of months. At 2-week follow-up, the patient continued to experience rash and joint pain; oral methotrexate 10 mg weekly was added to her regimen, as well as vitamin D, calcium, and folic acid supplementation. At the next 2-week follow-up the patient noted improvement in the rash as well as the joint pain, but both still persisted. Prednisone was decreased to 50 mg daily and methotrexate was increased to 15 mg weekly. The patient continued to show improvement over the subsequent 3 months, during which prednisone was tapered to 10 mg daily and methotrexate was increased to 20 mg weekly. The patient showed resolution of symptoms at 3-month follow-up on this regimen, with plans to continue the prednisone taper and maintain methotrexate dosing.
Comment
Adult-onset Still disease is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, salmon-pink evanescent erythema, and lymphadenopathy.2 The condition also can cause liver dysfunction, splenomegaly, pericarditis, pleuritis, renal dysfunction, and a reactive hemophagocytic syndrome.1 Furthermore, one review of the literature described an association with delayed-onset malignancy.4 Early diagnosis is important yet challenging, as AOSD is a diagnosis of exclusion. The Yamaguchi criteria are the most widely used method of diagnosis and demonstrate more than 90% sensitivity.In addition to the Yamaguchi criteria, marked hyperferritinemia is characteristic of AOSD and can act as an indicator of disease activity.5 Interestingly, both of our patients had elevated ferritin levels, with patient 2 showing marked elevation (Table). In both patients, all major criteria were fulfilled, except the typical skin rash.
The skin rash in AOSD, classically consisting of an evanescent, salmon-pink erythema predominantly involving the extremities, has been observed in up to 87% of AOSD patients.5 The histology of the typical evanescent rash is nonspecific, characterized by a relatively sparse, perivascular, mixed inflammatory infiltrate. Notably, other skin manifestations may be found in patients with AOSD.1,2,5-16 Persistent pruritic papules and plaques are the most commonly reported nonclassical rash, presenting as erythematous, slightly scaly papules and plaques with a linear configuration typically on the trunk.2 Both of our patients presented with this atypical eruption. Importantly, the histopathology of this unique rash displays distinctive features, which can aid in early diagnosis. Findings include dyskeratotic keratinocytes in the cornified layers as well as in the epidermis, and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis. These findings were evident in both histopathologic studies of our patients (Figures 2 and 4). Although not present in our patients, dermal mucin deposition has been demonstrated in some reports.1,13,15
A 2015 review of the literature yielded 30 cases of AOSD with pruritic persistent papules and plaques.4 The study confirmed a linear, erythematous or brown rash on the back and neck in the majority of cases. Histologic findings were congruent with those reported in our 2 cases: necrotic keratinocytes in the upper epidermis with a neutrophilic infiltrate in the upper dermis without vasculitis. Most patients showed rapid resolution of the rash and symptoms with the use of prednisone, prednisolone, or intravenous pulsed methylprednisolone. Interestingly, a range of presentations were noted, including prurigo pigmentosalike urticarial papules; lichenoid papules; and dermatographismlike, dermatomyositislike, and lichen amyloidosis–like rashes.4 In our report, patient 2 presented with a rash in a dermat-omyositislike shawl distribution. It has been suggested that patients with dermatomyositislike rashes require more potent immunotherapy as compared to patients with other rash morphologies.4 The need for methotrexate in addition to a prednisone taper in the clinical course of patient 2 lends further support to this observation.
Conclusion
A clinically and pathologically distinct form of cutaneous disease—AOSD with persistent pruritic papules and plaques—was observed in our 2 patients. These histopathologic findings facilitated timely diagnosis in both patients. A range of clinical morphologies may exist in AOSD, an awareness of which is paramount. Adult-onset Still disease should be included in the differential diagnosis of a dermatomyositislike presentation in a shawl distribution. Prompt diagnosis is essential to ensure adequate therapy.
Adult-onset Still disease (AOSD) is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, evanescent skin rash, and lymphadenopathy. 1 The most commonly used criteria for diagnosing AOSD are the Yamaguchi criteria. 2 The major criteria include high fever for more than 1 week, arthralgia for more than 2 weeks, leukocytosis, and an evanescent skin rash. The minor criteria consist of sore throat, lymphadenopathy and/or splenomegaly, liver dysfunction, and negative rheumatoid factor and antinuclear antibodies. Classically, the skin rash is described as an evanescent, salmon-colored erythema involving the extremities. Nevertheless, unusual cutaneous eruptions have been reported in AOSD, including persistent pruritic papules and plaques. 3 Importantly, this atypical rash demonstrates specific histologic findings that are not found on routine histopathology of a typical evanescent rash. We describe 2 patients with this atypical cutaneous eruption along with the unique histopathologic findings of AOSD.
Case Reports
Patient 1
A 23-year-old Chinese woman presented with periodic fevers, persistent rash, and joint pain of 2 years’ duration. Her medical history included splenectomy for hepatosplenomegaly as well as evaluation by hematology for lymphadenopathy; a cervical lymph node biopsy showed lymphoid and follicular hyperplasia.
Twenty days later, the patient was referred to the dermatology department for evaluation of the persistent rash. The patient described a history of flushing of the face, severe joint pain in both arms and legs, aching muscles, and persistent sore throat. The patient did not report any history of drug ingestion. Physical examination revealed a fever (temperature, 39.2°C); swollen nontender lymph nodes in the neck, axillae, and groin; and salmon-colored and hyperpigmented patches and thin plaques over the neck, chest, abdomen, and arms (Figure 1). A splenectomy scar also was noted. Peripheral blood was collected for laboratory analyses, which revealed transaminitis and moderate hyperferritinemia (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. The patient was admitted to the hospital, and a skin biopsy was performed. Histology showed superficial dyskeratotic keratinocytes and sparse perivascular infiltration of neutrophils in the upper dermis (Figure 2).



The patient was diagnosed with AOSD based on fulfillment of the Yamaguchi criteria.2 She was treated with methylprednisolone 60 mg daily and was discharged 14 days later. At 16-month follow-up, the patient demonstrated complete resolution of symptoms with a maintenance dose of prednisolone (7.5 mg daily).
Patient 2
A 23-year-old black woman presented to the emergency department 3 months postpartum with recurrent high fevers, worsening joint pain, and persistent itchy rash of 2 months’ duration. The patient had no history of travel, autoimmune disease, or sick contacts. She occasionally took aspirin for joint pain. Physical examination revealed a fever (temperature, 39.1°C) along with hyperpigmented patches and thin scaly hyperpigmented papules coalescing into a poorly demarcated V-shaped plaque on the upper back and posterior neck, extending to the chest in a shawl-like distribution (Figure 3). Submental lymphadenopathy was present. The spleen was not palpable.

Peripheral blood was collected for laboratory analysis and demonstrated transaminitis and a markedly high ferritin level (Table). An autoimmune panel was negative for rheumatoid factor, antinuclear antibodies, and antineutrophil cytoplasmic antibodies. Skin biopsy was performed and demonstrated many necrotic keratinocytes, singly and in aggregates, distributed from the spinous layer to the stratum corneum. A neutrophilic infiltrate was present in the papillary dermis (Figure 4).

The patient met the Yamaguchi criteria and was subsequently diagnosed with AOSD. She was treated with intravenous methylprednisolone 20 mg every 8 hours and was discharged 1 week later on oral prednisone 60 mg daily to be tapered over a period of months. At 2-week follow-up, the patient continued to experience rash and joint pain; oral methotrexate 10 mg weekly was added to her regimen, as well as vitamin D, calcium, and folic acid supplementation. At the next 2-week follow-up the patient noted improvement in the rash as well as the joint pain, but both still persisted. Prednisone was decreased to 50 mg daily and methotrexate was increased to 15 mg weekly. The patient continued to show improvement over the subsequent 3 months, during which prednisone was tapered to 10 mg daily and methotrexate was increased to 20 mg weekly. The patient showed resolution of symptoms at 3-month follow-up on this regimen, with plans to continue the prednisone taper and maintain methotrexate dosing.
Comment
Adult-onset Still disease is a systemic inflammatory condition that clinically manifests as spiking fevers, arthralgia, salmon-pink evanescent erythema, and lymphadenopathy.2 The condition also can cause liver dysfunction, splenomegaly, pericarditis, pleuritis, renal dysfunction, and a reactive hemophagocytic syndrome.1 Furthermore, one review of the literature described an association with delayed-onset malignancy.4 Early diagnosis is important yet challenging, as AOSD is a diagnosis of exclusion. The Yamaguchi criteria are the most widely used method of diagnosis and demonstrate more than 90% sensitivity.In addition to the Yamaguchi criteria, marked hyperferritinemia is characteristic of AOSD and can act as an indicator of disease activity.5 Interestingly, both of our patients had elevated ferritin levels, with patient 2 showing marked elevation (Table). In both patients, all major criteria were fulfilled, except the typical skin rash.
The skin rash in AOSD, classically consisting of an evanescent, salmon-pink erythema predominantly involving the extremities, has been observed in up to 87% of AOSD patients.5 The histology of the typical evanescent rash is nonspecific, characterized by a relatively sparse, perivascular, mixed inflammatory infiltrate. Notably, other skin manifestations may be found in patients with AOSD.1,2,5-16 Persistent pruritic papules and plaques are the most commonly reported nonclassical rash, presenting as erythematous, slightly scaly papules and plaques with a linear configuration typically on the trunk.2 Both of our patients presented with this atypical eruption. Importantly, the histopathology of this unique rash displays distinctive features, which can aid in early diagnosis. Findings include dyskeratotic keratinocytes in the cornified layers as well as in the epidermis, and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis. These findings were evident in both histopathologic studies of our patients (Figures 2 and 4). Although not present in our patients, dermal mucin deposition has been demonstrated in some reports.1,13,15
A 2015 review of the literature yielded 30 cases of AOSD with pruritic persistent papules and plaques.4 The study confirmed a linear, erythematous or brown rash on the back and neck in the majority of cases. Histologic findings were congruent with those reported in our 2 cases: necrotic keratinocytes in the upper epidermis with a neutrophilic infiltrate in the upper dermis without vasculitis. Most patients showed rapid resolution of the rash and symptoms with the use of prednisone, prednisolone, or intravenous pulsed methylprednisolone. Interestingly, a range of presentations were noted, including prurigo pigmentosalike urticarial papules; lichenoid papules; and dermatographismlike, dermatomyositislike, and lichen amyloidosis–like rashes.4 In our report, patient 2 presented with a rash in a dermat-omyositislike shawl distribution. It has been suggested that patients with dermatomyositislike rashes require more potent immunotherapy as compared to patients with other rash morphologies.4 The need for methotrexate in addition to a prednisone taper in the clinical course of patient 2 lends further support to this observation.
Conclusion
A clinically and pathologically distinct form of cutaneous disease—AOSD with persistent pruritic papules and plaques—was observed in our 2 patients. These histopathologic findings facilitated timely diagnosis in both patients. A range of clinical morphologies may exist in AOSD, an awareness of which is paramount. Adult-onset Still disease should be included in the differential diagnosis of a dermatomyositislike presentation in a shawl distribution. Prompt diagnosis is essential to ensure adequate therapy.
- Yamamoto T. Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int. 2012;32:2233-2237.
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-431.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Sun NZ, Brezinski EA, Berliner J, et al. Updates in adult-onset Still disease: atypical cutaneous manifestations and associates with delayed malignancy [published online June 6, 2015]. J Am Acad Dermatol. 2015;73:294-303.
- Schwarz-Eywill M, Heilig B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann Rheum Dis. 1992;51:683-685.
- Ohta A, Yamaguchi M, Tsunematsu T, et al. Adult Still’s disease: a multicenter survey of Japanese patients. J Rheumatol. 1990;17:1058-1063.
- Kaur S, Bambery P, Dhar S. Persistent dermal plaque lesions in adult onset Still’s disease. Dermatology. 1994;188:241-242.
- Lübbe J, Hofer M, Chavaz P, et al. Adult onset Still’s disease with persistent plaques. Br J Dermatol. 1999;141:710-713.
- Suzuki K, Kimura Y, Aoki M, et al. Persistent plaques and linear pigmentation in adult-onset Still’s disease. Dermatology. 2001;202:333-335.
- Fujii K, Konishi K, Kanno Y, et al. Persistent generalized erythema in adult-onset Still’s disease. Int J Dermatol. 2003;42:824-825.
- Thien Huong NT, Pitche P, Minh Hoa T, et al. Persistent pigmented plaques in adult-onset Still’s disease. Ann Dermatol Venereol. 2005;132:693-696.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Wolgamot G, Yoo J, Hurst S, et al. Unique histopathologic findings in a patient with adult-onset Still’s disease. Am J Dermatopathol. 2007;49:194-196.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still’s disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still’s disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Azeck AG, Littlewood SM. Adult-onset Still’s disease with atypical cutaneous features. J Eur Acad Dermatol Venereol. 2005;19:360-363.
- Yamamoto T. Cutaneous manifestations associated with adult-onset Still’s disease: important diagnostic values. Rheumatol Int. 2012;32:2233-2237.
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol. 1992;19:424-431.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Sun NZ, Brezinski EA, Berliner J, et al. Updates in adult-onset Still disease: atypical cutaneous manifestations and associates with delayed malignancy [published online June 6, 2015]. J Am Acad Dermatol. 2015;73:294-303.
- Schwarz-Eywill M, Heilig B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still’s disease activity. Ann Rheum Dis. 1992;51:683-685.
- Ohta A, Yamaguchi M, Tsunematsu T, et al. Adult Still’s disease: a multicenter survey of Japanese patients. J Rheumatol. 1990;17:1058-1063.
- Kaur S, Bambery P, Dhar S. Persistent dermal plaque lesions in adult onset Still’s disease. Dermatology. 1994;188:241-242.
- Lübbe J, Hofer M, Chavaz P, et al. Adult onset Still’s disease with persistent plaques. Br J Dermatol. 1999;141:710-713.
- Suzuki K, Kimura Y, Aoki M, et al. Persistent plaques and linear pigmentation in adult-onset Still’s disease. Dermatology. 2001;202:333-335.
- Fujii K, Konishi K, Kanno Y, et al. Persistent generalized erythema in adult-onset Still’s disease. Int J Dermatol. 2003;42:824-825.
- Thien Huong NT, Pitche P, Minh Hoa T, et al. Persistent pigmented plaques in adult-onset Still’s disease. Ann Dermatol Venereol. 2005;132:693-696.
- Lee JY, Yang CC, Hsu MM. Histopathology of persistent papules and plaques in adult-onset Still’s disease. J Am Acad Dermatol. 2005;52:1003-1008.
- Wolgamot G, Yoo J, Hurst S, et al. Unique histopathologic findings in a patient with adult-onset Still’s disease. Am J Dermatopathol. 2007;49:194-196.
- Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still’s disease. J Cutan Pathol. 2010;37:932-937.
- Yang CC, Lee JY, Liu MF, et al. Adult-onset Still’s disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol. 2006;16:593-594.
- Azeck AG, Littlewood SM. Adult-onset Still’s disease with atypical cutaneous features. J Eur Acad Dermatol Venereol. 2005;19:360-363.
Practice Points
- Serologic testing and skin biopsy are necessary in the timely and appropriate diagnosis of adult-onset Still disease (AOSD).
- In patients with a persistent pruritic papular rash, consider AOSD if there is a supporting history.
- Skin biopsy is diagnostic of AOSD with the unique histopathologic findings of dyskeratotic keratinocytes in the cornified layers as well as in the epidermis and a sparse neutrophilic and/or lymphocytic infiltrate in the papillary dermis without vasculitis.
Reflectance confocal microscopy: The future looks bright
CHICAGO – The future looks bright for to rule out malignancy, Ann M. John, MD, asserted at the annual meeting of the American College of Mohs Surgery.
“With the advent of dermoscopy, dermatologists were able to elucidate both benign and malignant patterns to help further guide their decision to biopsy or not. This increased diagnostic accuracy of suspicious lesions by 30%, while reducing the benign to malignant ratio of biopsies performed from 18:1 to 4:1. However, there are still lesions that are equivocal on dermoscopy, as we all know, and for this, there’s reflectance confocal microscopy,” observed Dr. John, of Robert Wood Johnson Medical School, New Brunswick, N.J.
RCM is a device technology that’s been cleared by the Food and Drug Administration since 2008 for the imaging of clinically suspicious lesions. It employs laser scanning to assess the light-scattering properties of cells in the epidermis and dermis, generating images with resolution comparable to histology.
RCM took a back seat initially while American dermatologists were gradually coming to embrace dermoscopy, which their European colleagues had done years earlier. Now, with the availability of handheld RCM for use in the dermatology clinic, expect RCM to assume a growing role in daily practice.
To illustrate the power of RCM as a diagnostic aid, she presented a single-center retrospective study of 1,189 clinically suspicious skin lesions that were equivocal on dermoscopy and then assessed using RCM with 1 year of subsequent patient follow-up. Overall, 155 lesions were deemed positive for cancer or atypia by RCM, while 1,034 were determined to be benign. Of those 155, 46 lesions were considered false positives because of their benign appearance on histologic inspection of the biopsy sample. Only 2 of the 1,034 lesions identified as negative by RCM proved to be false negatives on the basis of clinical changes within 1 year.
The overall sensitivity and specificity of RCM was 98.2% and 99.8%, respectively, with a positive predictive value of 70.3% and a negative predictive value of 99.8%.
The entire RCM procedure takes a skilled technician 15-20 minutes per lesion. As a practical matter, other investigators have estimated that RCM results in a cost savings of about $308,000 per million health plan members per year by reducing the need for biopsies (Dermatol Clin. 2016 Oct;34[4]:367-75).
In addition to evaluating clinically suspicious lesions, other situations in which RCM offers practical value include its use directly before the first cut during Mohs surgery in order to determine the margins of atypia; ex vivo imaging of Mohs margins, which has been shown to be comparable with frozen sections in accuracy but takes only one-third of the time; and imaging of biopsied lesions in order to determine the diagnosis relatively quickly, Dr. John noted.
She reported having no financial conflicts regarding her study.
CHICAGO – The future looks bright for to rule out malignancy, Ann M. John, MD, asserted at the annual meeting of the American College of Mohs Surgery.
“With the advent of dermoscopy, dermatologists were able to elucidate both benign and malignant patterns to help further guide their decision to biopsy or not. This increased diagnostic accuracy of suspicious lesions by 30%, while reducing the benign to malignant ratio of biopsies performed from 18:1 to 4:1. However, there are still lesions that are equivocal on dermoscopy, as we all know, and for this, there’s reflectance confocal microscopy,” observed Dr. John, of Robert Wood Johnson Medical School, New Brunswick, N.J.
RCM is a device technology that’s been cleared by the Food and Drug Administration since 2008 for the imaging of clinically suspicious lesions. It employs laser scanning to assess the light-scattering properties of cells in the epidermis and dermis, generating images with resolution comparable to histology.
RCM took a back seat initially while American dermatologists were gradually coming to embrace dermoscopy, which their European colleagues had done years earlier. Now, with the availability of handheld RCM for use in the dermatology clinic, expect RCM to assume a growing role in daily practice.
To illustrate the power of RCM as a diagnostic aid, she presented a single-center retrospective study of 1,189 clinically suspicious skin lesions that were equivocal on dermoscopy and then assessed using RCM with 1 year of subsequent patient follow-up. Overall, 155 lesions were deemed positive for cancer or atypia by RCM, while 1,034 were determined to be benign. Of those 155, 46 lesions were considered false positives because of their benign appearance on histologic inspection of the biopsy sample. Only 2 of the 1,034 lesions identified as negative by RCM proved to be false negatives on the basis of clinical changes within 1 year.
The overall sensitivity and specificity of RCM was 98.2% and 99.8%, respectively, with a positive predictive value of 70.3% and a negative predictive value of 99.8%.
The entire RCM procedure takes a skilled technician 15-20 minutes per lesion. As a practical matter, other investigators have estimated that RCM results in a cost savings of about $308,000 per million health plan members per year by reducing the need for biopsies (Dermatol Clin. 2016 Oct;34[4]:367-75).
In addition to evaluating clinically suspicious lesions, other situations in which RCM offers practical value include its use directly before the first cut during Mohs surgery in order to determine the margins of atypia; ex vivo imaging of Mohs margins, which has been shown to be comparable with frozen sections in accuracy but takes only one-third of the time; and imaging of biopsied lesions in order to determine the diagnosis relatively quickly, Dr. John noted.
She reported having no financial conflicts regarding her study.
CHICAGO – The future looks bright for to rule out malignancy, Ann M. John, MD, asserted at the annual meeting of the American College of Mohs Surgery.
“With the advent of dermoscopy, dermatologists were able to elucidate both benign and malignant patterns to help further guide their decision to biopsy or not. This increased diagnostic accuracy of suspicious lesions by 30%, while reducing the benign to malignant ratio of biopsies performed from 18:1 to 4:1. However, there are still lesions that are equivocal on dermoscopy, as we all know, and for this, there’s reflectance confocal microscopy,” observed Dr. John, of Robert Wood Johnson Medical School, New Brunswick, N.J.
RCM is a device technology that’s been cleared by the Food and Drug Administration since 2008 for the imaging of clinically suspicious lesions. It employs laser scanning to assess the light-scattering properties of cells in the epidermis and dermis, generating images with resolution comparable to histology.
RCM took a back seat initially while American dermatologists were gradually coming to embrace dermoscopy, which their European colleagues had done years earlier. Now, with the availability of handheld RCM for use in the dermatology clinic, expect RCM to assume a growing role in daily practice.
To illustrate the power of RCM as a diagnostic aid, she presented a single-center retrospective study of 1,189 clinically suspicious skin lesions that were equivocal on dermoscopy and then assessed using RCM with 1 year of subsequent patient follow-up. Overall, 155 lesions were deemed positive for cancer or atypia by RCM, while 1,034 were determined to be benign. Of those 155, 46 lesions were considered false positives because of their benign appearance on histologic inspection of the biopsy sample. Only 2 of the 1,034 lesions identified as negative by RCM proved to be false negatives on the basis of clinical changes within 1 year.
The overall sensitivity and specificity of RCM was 98.2% and 99.8%, respectively, with a positive predictive value of 70.3% and a negative predictive value of 99.8%.
The entire RCM procedure takes a skilled technician 15-20 minutes per lesion. As a practical matter, other investigators have estimated that RCM results in a cost savings of about $308,000 per million health plan members per year by reducing the need for biopsies (Dermatol Clin. 2016 Oct;34[4]:367-75).
In addition to evaluating clinically suspicious lesions, other situations in which RCM offers practical value include its use directly before the first cut during Mohs surgery in order to determine the margins of atypia; ex vivo imaging of Mohs margins, which has been shown to be comparable with frozen sections in accuracy but takes only one-third of the time; and imaging of biopsied lesions in order to determine the diagnosis relatively quickly, Dr. John noted.
She reported having no financial conflicts regarding her study.
REPORTING FROM THE ACMS ANNUAL MEETING
Key clinical point: The future looks bright for reflectance confocal microscopy in dermatology.
Major finding: The sensitivity and specificity of reflectance confocal microscopy for diagnosis of skin cancer in patients with equivocal dermoscopic findings was 98.2% and 99.8%, respectively.
Study details: This retrospective single center study included 1,189 clinically suspicious skin lesions with equivocal dermoscopy findings, which were then evaluated using reflectance confocal microscopy.
Disclosures: The presenter reported having no financial conflicts regarding her study.
Levamisole-Induced Vasculopathy With Gastric Involvement in a Cocaine User
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.
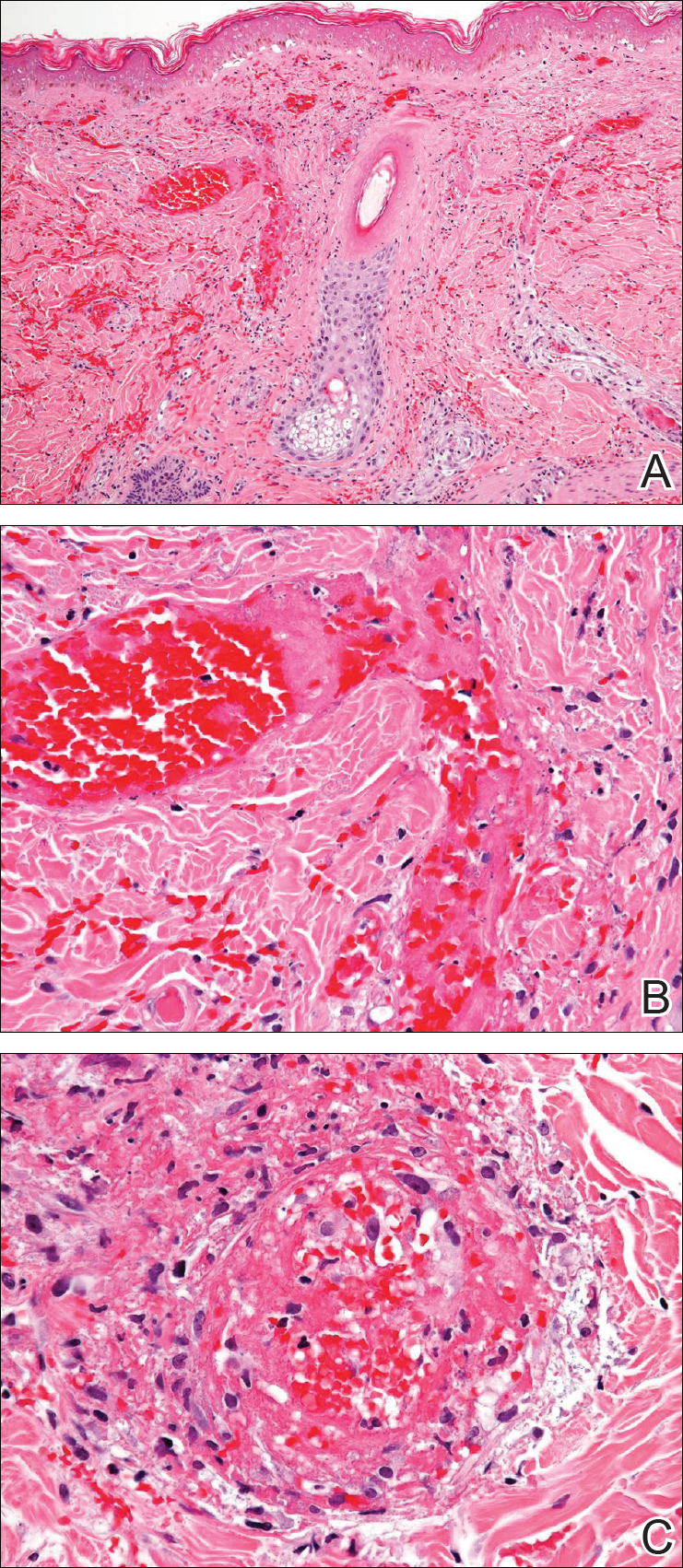
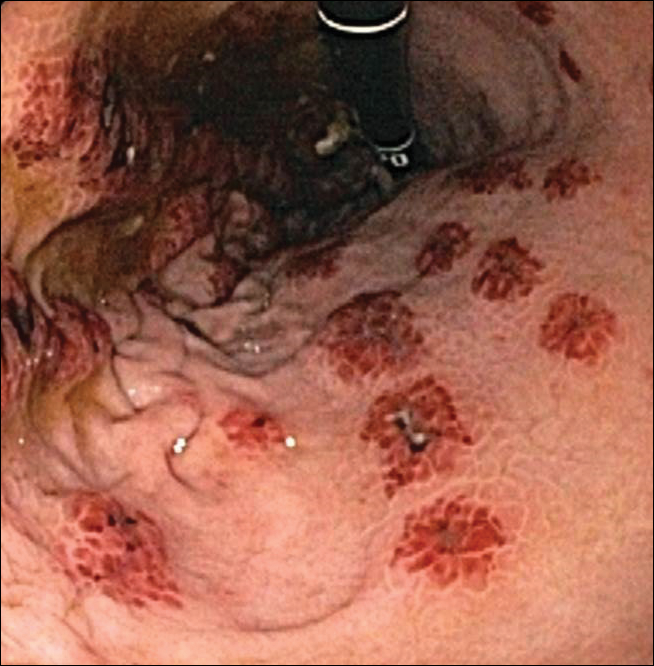
Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.



Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.



Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
Practice Points
- More than half of the cocaine illicitly consumed in the United States is contaminated with levamisole, a veterinary drug that can incite a vasculitic/vasculopathic response in the skin as well as in other organ systems.
- Because dermatologists often are the specialists to make the diagnosis of levamisole-induced vasculopathy, clinicians should be made aware that consumption of levamisole-contaminated cocaine may affect more than the skin alone.
Tetrad Bodies in Skin
The Diagnosis: Bacterial Infection
The tetrad arrangement of organisms seen in this case was classic for Micrococcus and Sarcina species. Both are gram-positive cocci that occur in tetrads, but Micrococcus is aerobic and catalase positive, whereas Sarcina species are anaerobic, catalase negative, acidophilic, and form spores in alkaline pH.1 Although difficult to definitively differentiate on light microscopy, micrococci are smaller in size, ranging from 0.5 to 2.0 μm, and occur in tight clusters, as seen in this case (quiz images), in contrast to Sarcina species, which are relatively larger (1.8-3.0 μm).2 Sarcinae typically are found in soil and air, are considered pathogenic, and are associated with gastric symptoms (Sarcina ventriculi).1 Sarcina species also are reported to colonize the skin of patients with diabetes mellitus, but no pathogenic activity is known in the skin.3 Micrococcus species, with the majority being Micrococcus luteus, are part of the normal flora of the human skin as well as the oral and nasal cavities. Occasional reports of pneumonia, endocarditis, meningitis, arthritis, endophthalmitis, and sepsis have been reported in immunocompromised individuals.4 In the skin, Micrococcus is a commensal organism; however, Micrococcus sedentarius has been associated with pitted keratolysis, and reports of Micrococcus folliculitis in human immunodeficiency virus patients also are described in the literature.5,6 Micrococci are considered opportunistic bacteria and may worsen and prolong a localized cutaneous infection caused by other organisms under favorable conditions.7 Micrococcus luteus is one of the most common bacteria cultured from skin and soft tissue infections caused by fungal organisms.8 Depending on the immune status of an individual, use of broad-spectrum antibiotic and/or elimination of favorable milieu (ie, primary pathogen, breaks in skin) usually treats the infection.
Because of the rarity of infections caused and being part of the normal flora, the clinical implications of subtyping and sensitivity studies via culture or molecular studies may not be important; however, incidental presence of these organisms with unfamiliar morphology may cause confusion for the dermatopathologist. An extremely small size (0.5-2.0 μm) compared to red blood cells (7-8 μm) and white blood cells (10-12 μm) in a tight tetrad arrangement should raise the suspicion for Micrococcus.1 The refractive nature of these organisms from a thick extracellular layer can mimic fungus or plant matter; a negative Grocott-Gomori methenamine-silver stain in this case helped in not only differentiating but also ruling out secondary fungal infection. Finally, a Gram stain with violet staining of these organisms reaffirmed the diagnosis of gram-positive bacterial organisms, most consistent with Micrococcus species (Figure 1). Culture studies were not performed because of contamination of the tissue specimen and resolution of the patient's symptoms.
The presence of foreign material in the skin may be traumatic, occupational, cosmetic, iatrogenic, or self-inflicted, including a wide variety of substances that appear in different morphological forms on hematoxylin and eosin (H&E)-stained sections, depending on their structure and physiochemical properties.9 Although not all foreign bodies may polarize, examining the sample under polarized light is considered an important step to narrow down the differential diagnosis. The tissue reaction is primarily dependent on the nature of the substance and duration, consisting of histiocytes, macrophages, plasma cells, lymphocytes, and fibrosis.9 Activated histiocytes, multinucleated giant cells, and granulomas are classic findings that generally are seen surrounding and engulfing the foreign material (Figure 2). In addition to foreign material, substances such as calcium salts, urate crystals, extruded keratin, ruptured cysts, and hair follicles may act as foreign materials and can incite a tissue response.9 Absence of histiocytic response, granuloma formation, and fibrosis in a lesion of 1 month's duration made the tetrad bodies unlikely to be foreign material.
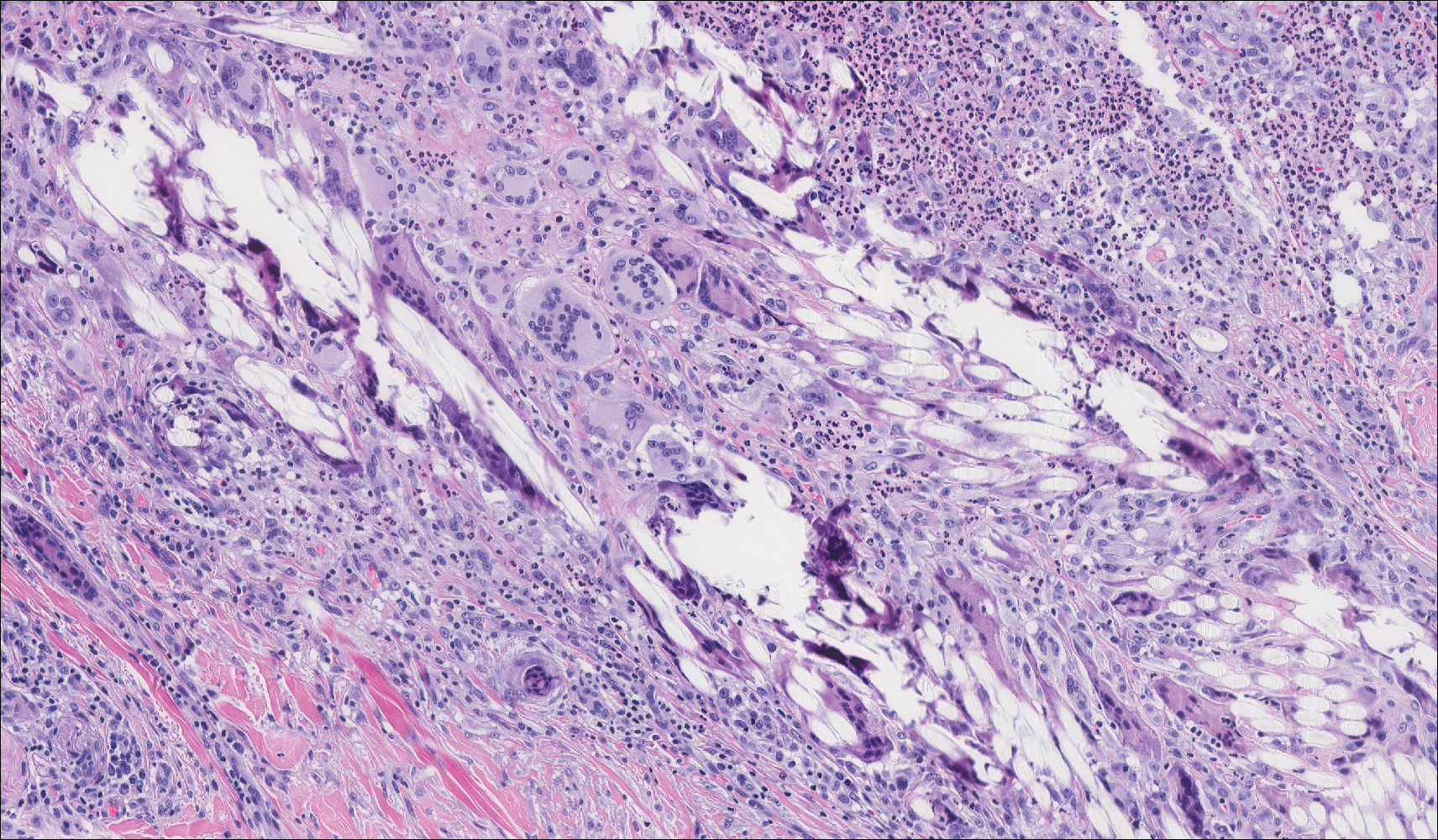
Demodex mites are superficial inhabitants of human skin that are acquired shortly after birth, live in or near pilosebaceous units, and obtain nourishment from skin cells and sebum.10,11 The mites can be recovered on 10% of skin biopsies, most commonly on the face due to high sebum production.10 Adult mites range from 0.1 to 0.4 mm in length and are round to oval in shape. Females lay eggs inside the hair follicle or sebaceous glands.11 They usually are asymptomatic, but their infestation may become pathogenic, especially in immunocompromised individuals.10 The clinical picture may resemble bacterial folliculitis, rosacea, and perioral dermatitis, while histology typically is characterized by spongiosis, lymphohistiocytic inflammation around infested follicles, and mite(s) in follicular infundibula (Figure 3). Sometimes the protrusion of mites and keratin from the follicles is seen as follicular spines on histology and referred to as pityriasis folliculorum.
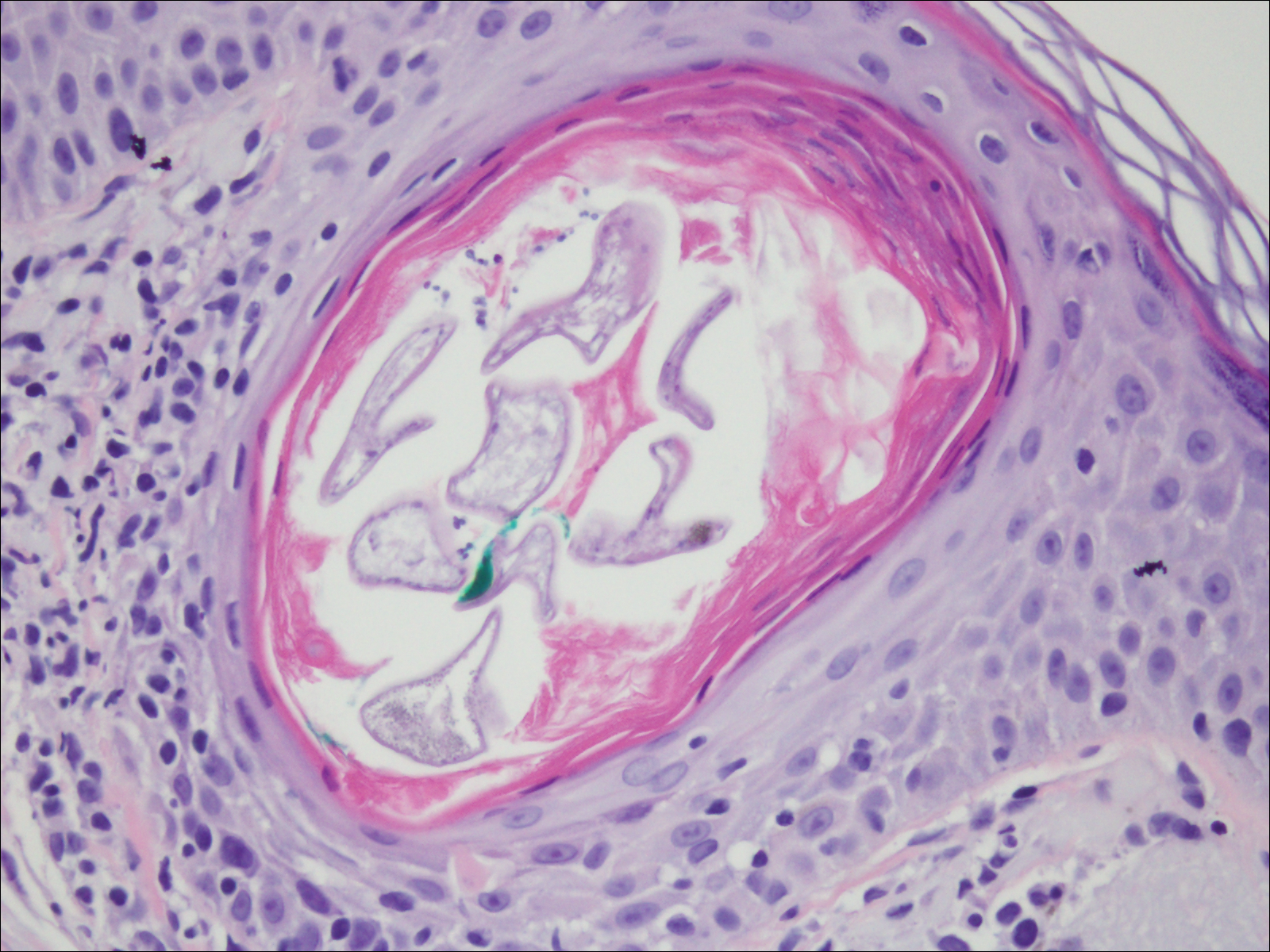
Deposits of urate crystals in skin occur from the elevated serum uric acid levels in gout. The cutaneous deposits are mainly in the dermis and subcutaneous tissue and are extremely painful.12 Urate crystals get dissolved during formalin fixation and leave needlelike clefts in a homogenous, lightly basophilic material on H&E slide (Figure 4). For the same reason, polarized microscopy also is not helpful despite the birefringent nature of urate crystals.12
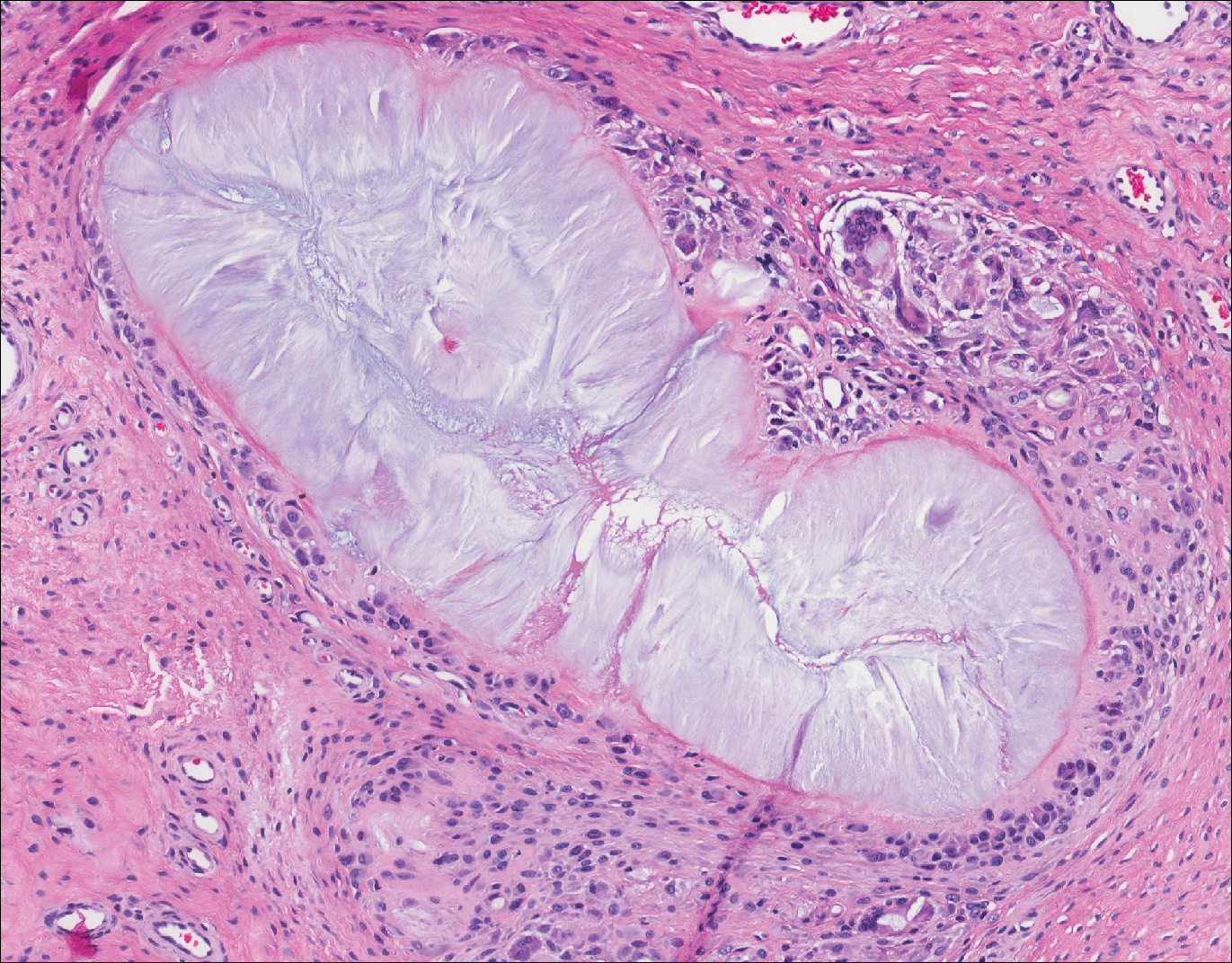
Fungal yeast forms appear round to oval under light microscopy, ranging from 2 to 100 μm in size.13 The common superficial forms involving the epidermis or hair follicles similar to the current case of bacterial infection include Malassezia and dermatophyte infections. Malassezia is part of the normal flora of sebum-rich areas of skin and is associated with superficial infections such as folliculitis, atopic dermatitis, psoriasis, seborrheic dermatitis, and dandruff.14 Malassezia appear as clusters of yeast cells that are pleomorphic and round to oval in shape, ranging from 2 to 6 μm in size. It forms hyphae in its pathogenic form and gives rise to the classic spaghetti and meatball-like appearance that can be highlighted by periodic acid-Schiff (Figure 5) and Grocott-Gomori methenamine-silver special stains. Dermatophytes include 3 genera--Trichophyton, Microsporum, and Epidermophyton--with at least 40 species that causes skin infections in humans.14 Fungal spores and hyphae forms are restricted to the stratum corneum. The hyphae forms may not be apparent on H&E stain, and periodic acid-Schiff staining is helpful in visualizing the fungal elements. The presence of neutrophils in the corneal layer, basket weave hyperkeratosis, and presence of fungal hyphae within the corneal layer fissures (sandwich sign) are clues to the dermatophyte infection.15 Other smaller fungi such as Histoplasma capsulatum (2-4 μm), Candida (3-5 μm), and Pneumocystis (2-5 μm) species can be found in skin in disseminated infections, usually affecting immunocompromised individuals.13 Histoplasma is a basophilic yeast that exhibits narrow-based budding and appears clustered within or outside of macrophages. Candida species generally are dimorphic, and yeasts are found intermingled with filamentous forms. Pneumocystis infection in skin is extremely rare, and the fungi appear as spherical or crescent-shaped bodies in a foamy amorphous material.16

- Al Rasheed MR, Senseng CG. Sarcina ventriculi: review of the literature. Arch Pathol Lab Med. 2016;140:1441-1445.
- Lam-Himlin D, Tsiatis AC, Montgomery E, et al. Sarcina organisms in the gastrointestinal tract: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:1700-1705.
- Somerville DA, Lancaster-Smith M. The aerobic cutaneous microflora of diabetic subjects. Br J Dermatol. 1973;89:395-400.
- Hetem DJ, Rooijakkers S, Ekkelenkamp MB. Staphylococci and Micrococci. In: Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. 4th ed. Vol 2. New York, NY: Elsevier; 2017:1509-1522.
- Nordstrom KM, McGinley KJ, Cappiello L, et al. Pitted keratolysis. the role of Micrococcus sedentarius. Arch Dermatol. 1987;123:1320-1325.
- Smith KJ, Neafie R, Yeager J, et al. Micrococcus folliculitis in HIV-1 disease. Br J Dermatol. 1999;141:558-561.
- van Rensburg JJ, Lin H, Gao X, et al. The human skin microbiome associates with the outcome of and is influenced by bacterial infection. mBio. 2015;6:E01315-15. doi:10.1128/mBio.01315-15.
- Chuku A, Nwankiti OO. Association of bacteria with fungal infection of skin and soft tissue lesions in plateau state, Nigeria. Br Microbiol Res J. 2013;3:470-477.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Rather PA, Hassan I. Human Demodex mite: the versatile mite of dermatological importance. Indian J Dermatol. 2014;59:60-66.
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- White TC, Findley K, Dawson TL Jr, et al. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med. 2014;4. pii:a019802. doi:10.1101/cshperspect.a019802.
- Gottlieb GJ, Ackerman AB. The "sandwich sign" of dermatophytosis. Am J Dermatopathol. 1986;8:347.
- Hennessey NP, Parro EL, Cockerell CJ. Cutaneous Pneumocystis carinii infection in patients with acquired immunodeficiency syndrome. Arch Dermatol. 1991;127:1699-1701.
The Diagnosis: Bacterial Infection
The tetrad arrangement of organisms seen in this case was classic for Micrococcus and Sarcina species. Both are gram-positive cocci that occur in tetrads, but Micrococcus is aerobic and catalase positive, whereas Sarcina species are anaerobic, catalase negative, acidophilic, and form spores in alkaline pH.1 Although difficult to definitively differentiate on light microscopy, micrococci are smaller in size, ranging from 0.5 to 2.0 μm, and occur in tight clusters, as seen in this case (quiz images), in contrast to Sarcina species, which are relatively larger (1.8-3.0 μm).2 Sarcinae typically are found in soil and air, are considered pathogenic, and are associated with gastric symptoms (Sarcina ventriculi).1 Sarcina species also are reported to colonize the skin of patients with diabetes mellitus, but no pathogenic activity is known in the skin.3 Micrococcus species, with the majority being Micrococcus luteus, are part of the normal flora of the human skin as well as the oral and nasal cavities. Occasional reports of pneumonia, endocarditis, meningitis, arthritis, endophthalmitis, and sepsis have been reported in immunocompromised individuals.4 In the skin, Micrococcus is a commensal organism; however, Micrococcus sedentarius has been associated with pitted keratolysis, and reports of Micrococcus folliculitis in human immunodeficiency virus patients also are described in the literature.5,6 Micrococci are considered opportunistic bacteria and may worsen and prolong a localized cutaneous infection caused by other organisms under favorable conditions.7 Micrococcus luteus is one of the most common bacteria cultured from skin and soft tissue infections caused by fungal organisms.8 Depending on the immune status of an individual, use of broad-spectrum antibiotic and/or elimination of favorable milieu (ie, primary pathogen, breaks in skin) usually treats the infection.
Because of the rarity of infections caused and being part of the normal flora, the clinical implications of subtyping and sensitivity studies via culture or molecular studies may not be important; however, incidental presence of these organisms with unfamiliar morphology may cause confusion for the dermatopathologist. An extremely small size (0.5-2.0 μm) compared to red blood cells (7-8 μm) and white blood cells (10-12 μm) in a tight tetrad arrangement should raise the suspicion for Micrococcus.1 The refractive nature of these organisms from a thick extracellular layer can mimic fungus or plant matter; a negative Grocott-Gomori methenamine-silver stain in this case helped in not only differentiating but also ruling out secondary fungal infection. Finally, a Gram stain with violet staining of these organisms reaffirmed the diagnosis of gram-positive bacterial organisms, most consistent with Micrococcus species (Figure 1). Culture studies were not performed because of contamination of the tissue specimen and resolution of the patient's symptoms.

The presence of foreign material in the skin may be traumatic, occupational, cosmetic, iatrogenic, or self-inflicted, including a wide variety of substances that appear in different morphological forms on hematoxylin and eosin (H&E)-stained sections, depending on their structure and physiochemical properties.9 Although not all foreign bodies may polarize, examining the sample under polarized light is considered an important step to narrow down the differential diagnosis. The tissue reaction is primarily dependent on the nature of the substance and duration, consisting of histiocytes, macrophages, plasma cells, lymphocytes, and fibrosis.9 Activated histiocytes, multinucleated giant cells, and granulomas are classic findings that generally are seen surrounding and engulfing the foreign material (Figure 2). In addition to foreign material, substances such as calcium salts, urate crystals, extruded keratin, ruptured cysts, and hair follicles may act as foreign materials and can incite a tissue response.9 Absence of histiocytic response, granuloma formation, and fibrosis in a lesion of 1 month's duration made the tetrad bodies unlikely to be foreign material.

Demodex mites are superficial inhabitants of human skin that are acquired shortly after birth, live in or near pilosebaceous units, and obtain nourishment from skin cells and sebum.10,11 The mites can be recovered on 10% of skin biopsies, most commonly on the face due to high sebum production.10 Adult mites range from 0.1 to 0.4 mm in length and are round to oval in shape. Females lay eggs inside the hair follicle or sebaceous glands.11 They usually are asymptomatic, but their infestation may become pathogenic, especially in immunocompromised individuals.10 The clinical picture may resemble bacterial folliculitis, rosacea, and perioral dermatitis, while histology typically is characterized by spongiosis, lymphohistiocytic inflammation around infested follicles, and mite(s) in follicular infundibula (Figure 3). Sometimes the protrusion of mites and keratin from the follicles is seen as follicular spines on histology and referred to as pityriasis folliculorum.

Deposits of urate crystals in skin occur from the elevated serum uric acid levels in gout. The cutaneous deposits are mainly in the dermis and subcutaneous tissue and are extremely painful.12 Urate crystals get dissolved during formalin fixation and leave needlelike clefts in a homogenous, lightly basophilic material on H&E slide (Figure 4). For the same reason, polarized microscopy also is not helpful despite the birefringent nature of urate crystals.12

Fungal yeast forms appear round to oval under light microscopy, ranging from 2 to 100 μm in size.13 The common superficial forms involving the epidermis or hair follicles similar to the current case of bacterial infection include Malassezia and dermatophyte infections. Malassezia is part of the normal flora of sebum-rich areas of skin and is associated with superficial infections such as folliculitis, atopic dermatitis, psoriasis, seborrheic dermatitis, and dandruff.14 Malassezia appear as clusters of yeast cells that are pleomorphic and round to oval in shape, ranging from 2 to 6 μm in size. It forms hyphae in its pathogenic form and gives rise to the classic spaghetti and meatball-like appearance that can be highlighted by periodic acid-Schiff (Figure 5) and Grocott-Gomori methenamine-silver special stains. Dermatophytes include 3 genera--Trichophyton, Microsporum, and Epidermophyton--with at least 40 species that causes skin infections in humans.14 Fungal spores and hyphae forms are restricted to the stratum corneum. The hyphae forms may not be apparent on H&E stain, and periodic acid-Schiff staining is helpful in visualizing the fungal elements. The presence of neutrophils in the corneal layer, basket weave hyperkeratosis, and presence of fungal hyphae within the corneal layer fissures (sandwich sign) are clues to the dermatophyte infection.15 Other smaller fungi such as Histoplasma capsulatum (2-4 μm), Candida (3-5 μm), and Pneumocystis (2-5 μm) species can be found in skin in disseminated infections, usually affecting immunocompromised individuals.13 Histoplasma is a basophilic yeast that exhibits narrow-based budding and appears clustered within or outside of macrophages. Candida species generally are dimorphic, and yeasts are found intermingled with filamentous forms. Pneumocystis infection in skin is extremely rare, and the fungi appear as spherical or crescent-shaped bodies in a foamy amorphous material.16

The Diagnosis: Bacterial Infection
The tetrad arrangement of organisms seen in this case was classic for Micrococcus and Sarcina species. Both are gram-positive cocci that occur in tetrads, but Micrococcus is aerobic and catalase positive, whereas Sarcina species are anaerobic, catalase negative, acidophilic, and form spores in alkaline pH.1 Although difficult to definitively differentiate on light microscopy, micrococci are smaller in size, ranging from 0.5 to 2.0 μm, and occur in tight clusters, as seen in this case (quiz images), in contrast to Sarcina species, which are relatively larger (1.8-3.0 μm).2 Sarcinae typically are found in soil and air, are considered pathogenic, and are associated with gastric symptoms (Sarcina ventriculi).1 Sarcina species also are reported to colonize the skin of patients with diabetes mellitus, but no pathogenic activity is known in the skin.3 Micrococcus species, with the majority being Micrococcus luteus, are part of the normal flora of the human skin as well as the oral and nasal cavities. Occasional reports of pneumonia, endocarditis, meningitis, arthritis, endophthalmitis, and sepsis have been reported in immunocompromised individuals.4 In the skin, Micrococcus is a commensal organism; however, Micrococcus sedentarius has been associated with pitted keratolysis, and reports of Micrococcus folliculitis in human immunodeficiency virus patients also are described in the literature.5,6 Micrococci are considered opportunistic bacteria and may worsen and prolong a localized cutaneous infection caused by other organisms under favorable conditions.7 Micrococcus luteus is one of the most common bacteria cultured from skin and soft tissue infections caused by fungal organisms.8 Depending on the immune status of an individual, use of broad-spectrum antibiotic and/or elimination of favorable milieu (ie, primary pathogen, breaks in skin) usually treats the infection.
Because of the rarity of infections caused and being part of the normal flora, the clinical implications of subtyping and sensitivity studies via culture or molecular studies may not be important; however, incidental presence of these organisms with unfamiliar morphology may cause confusion for the dermatopathologist. An extremely small size (0.5-2.0 μm) compared to red blood cells (7-8 μm) and white blood cells (10-12 μm) in a tight tetrad arrangement should raise the suspicion for Micrococcus.1 The refractive nature of these organisms from a thick extracellular layer can mimic fungus or plant matter; a negative Grocott-Gomori methenamine-silver stain in this case helped in not only differentiating but also ruling out secondary fungal infection. Finally, a Gram stain with violet staining of these organisms reaffirmed the diagnosis of gram-positive bacterial organisms, most consistent with Micrococcus species (Figure 1). Culture studies were not performed because of contamination of the tissue specimen and resolution of the patient's symptoms.

The presence of foreign material in the skin may be traumatic, occupational, cosmetic, iatrogenic, or self-inflicted, including a wide variety of substances that appear in different morphological forms on hematoxylin and eosin (H&E)-stained sections, depending on their structure and physiochemical properties.9 Although not all foreign bodies may polarize, examining the sample under polarized light is considered an important step to narrow down the differential diagnosis. The tissue reaction is primarily dependent on the nature of the substance and duration, consisting of histiocytes, macrophages, plasma cells, lymphocytes, and fibrosis.9 Activated histiocytes, multinucleated giant cells, and granulomas are classic findings that generally are seen surrounding and engulfing the foreign material (Figure 2). In addition to foreign material, substances such as calcium salts, urate crystals, extruded keratin, ruptured cysts, and hair follicles may act as foreign materials and can incite a tissue response.9 Absence of histiocytic response, granuloma formation, and fibrosis in a lesion of 1 month's duration made the tetrad bodies unlikely to be foreign material.

Demodex mites are superficial inhabitants of human skin that are acquired shortly after birth, live in or near pilosebaceous units, and obtain nourishment from skin cells and sebum.10,11 The mites can be recovered on 10% of skin biopsies, most commonly on the face due to high sebum production.10 Adult mites range from 0.1 to 0.4 mm in length and are round to oval in shape. Females lay eggs inside the hair follicle or sebaceous glands.11 They usually are asymptomatic, but their infestation may become pathogenic, especially in immunocompromised individuals.10 The clinical picture may resemble bacterial folliculitis, rosacea, and perioral dermatitis, while histology typically is characterized by spongiosis, lymphohistiocytic inflammation around infested follicles, and mite(s) in follicular infundibula (Figure 3). Sometimes the protrusion of mites and keratin from the follicles is seen as follicular spines on histology and referred to as pityriasis folliculorum.

Deposits of urate crystals in skin occur from the elevated serum uric acid levels in gout. The cutaneous deposits are mainly in the dermis and subcutaneous tissue and are extremely painful.12 Urate crystals get dissolved during formalin fixation and leave needlelike clefts in a homogenous, lightly basophilic material on H&E slide (Figure 4). For the same reason, polarized microscopy also is not helpful despite the birefringent nature of urate crystals.12

Fungal yeast forms appear round to oval under light microscopy, ranging from 2 to 100 μm in size.13 The common superficial forms involving the epidermis or hair follicles similar to the current case of bacterial infection include Malassezia and dermatophyte infections. Malassezia is part of the normal flora of sebum-rich areas of skin and is associated with superficial infections such as folliculitis, atopic dermatitis, psoriasis, seborrheic dermatitis, and dandruff.14 Malassezia appear as clusters of yeast cells that are pleomorphic and round to oval in shape, ranging from 2 to 6 μm in size. It forms hyphae in its pathogenic form and gives rise to the classic spaghetti and meatball-like appearance that can be highlighted by periodic acid-Schiff (Figure 5) and Grocott-Gomori methenamine-silver special stains. Dermatophytes include 3 genera--Trichophyton, Microsporum, and Epidermophyton--with at least 40 species that causes skin infections in humans.14 Fungal spores and hyphae forms are restricted to the stratum corneum. The hyphae forms may not be apparent on H&E stain, and periodic acid-Schiff staining is helpful in visualizing the fungal elements. The presence of neutrophils in the corneal layer, basket weave hyperkeratosis, and presence of fungal hyphae within the corneal layer fissures (sandwich sign) are clues to the dermatophyte infection.15 Other smaller fungi such as Histoplasma capsulatum (2-4 μm), Candida (3-5 μm), and Pneumocystis (2-5 μm) species can be found in skin in disseminated infections, usually affecting immunocompromised individuals.13 Histoplasma is a basophilic yeast that exhibits narrow-based budding and appears clustered within or outside of macrophages. Candida species generally are dimorphic, and yeasts are found intermingled with filamentous forms. Pneumocystis infection in skin is extremely rare, and the fungi appear as spherical or crescent-shaped bodies in a foamy amorphous material.16

- Al Rasheed MR, Senseng CG. Sarcina ventriculi: review of the literature. Arch Pathol Lab Med. 2016;140:1441-1445.
- Lam-Himlin D, Tsiatis AC, Montgomery E, et al. Sarcina organisms in the gastrointestinal tract: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:1700-1705.
- Somerville DA, Lancaster-Smith M. The aerobic cutaneous microflora of diabetic subjects. Br J Dermatol. 1973;89:395-400.
- Hetem DJ, Rooijakkers S, Ekkelenkamp MB. Staphylococci and Micrococci. In: Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. 4th ed. Vol 2. New York, NY: Elsevier; 2017:1509-1522.
- Nordstrom KM, McGinley KJ, Cappiello L, et al. Pitted keratolysis. the role of Micrococcus sedentarius. Arch Dermatol. 1987;123:1320-1325.
- Smith KJ, Neafie R, Yeager J, et al. Micrococcus folliculitis in HIV-1 disease. Br J Dermatol. 1999;141:558-561.
- van Rensburg JJ, Lin H, Gao X, et al. The human skin microbiome associates with the outcome of and is influenced by bacterial infection. mBio. 2015;6:E01315-15. doi:10.1128/mBio.01315-15.
- Chuku A, Nwankiti OO. Association of bacteria with fungal infection of skin and soft tissue lesions in plateau state, Nigeria. Br Microbiol Res J. 2013;3:470-477.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Rather PA, Hassan I. Human Demodex mite: the versatile mite of dermatological importance. Indian J Dermatol. 2014;59:60-66.
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- White TC, Findley K, Dawson TL Jr, et al. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med. 2014;4. pii:a019802. doi:10.1101/cshperspect.a019802.
- Gottlieb GJ, Ackerman AB. The "sandwich sign" of dermatophytosis. Am J Dermatopathol. 1986;8:347.
- Hennessey NP, Parro EL, Cockerell CJ. Cutaneous Pneumocystis carinii infection in patients with acquired immunodeficiency syndrome. Arch Dermatol. 1991;127:1699-1701.
- Al Rasheed MR, Senseng CG. Sarcina ventriculi: review of the literature. Arch Pathol Lab Med. 2016;140:1441-1445.
- Lam-Himlin D, Tsiatis AC, Montgomery E, et al. Sarcina organisms in the gastrointestinal tract: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:1700-1705.
- Somerville DA, Lancaster-Smith M. The aerobic cutaneous microflora of diabetic subjects. Br J Dermatol. 1973;89:395-400.
- Hetem DJ, Rooijakkers S, Ekkelenkamp MB. Staphylococci and Micrococci. In: Cohen J, Powderly WG, Opal SM, eds. Infectious Diseases. 4th ed. Vol 2. New York, NY: Elsevier; 2017:1509-1522.
- Nordstrom KM, McGinley KJ, Cappiello L, et al. Pitted keratolysis. the role of Micrococcus sedentarius. Arch Dermatol. 1987;123:1320-1325.
- Smith KJ, Neafie R, Yeager J, et al. Micrococcus folliculitis in HIV-1 disease. Br J Dermatol. 1999;141:558-561.
- van Rensburg JJ, Lin H, Gao X, et al. The human skin microbiome associates with the outcome of and is influenced by bacterial infection. mBio. 2015;6:E01315-15. doi:10.1128/mBio.01315-15.
- Chuku A, Nwankiti OO. Association of bacteria with fungal infection of skin and soft tissue lesions in plateau state, Nigeria. Br Microbiol Res J. 2013;3:470-477.
- Molina-Ruiz AM, Requena L. Foreign body granulomas. Dermatol Clin. 2015;33:497-523.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Rather PA, Hassan I. Human Demodex mite: the versatile mite of dermatological importance. Indian J Dermatol. 2014;59:60-66.
- Gaviria JL, Ortega VG, Gaona J, et al. Unusual dermatological manifestations of gout: review of literature and a case report. Plast Reconstr Surg Glob Open. 2015;3:E445.
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280.
- White TC, Findley K, Dawson TL Jr, et al. Fungi on the skin: dermatophytes and Malassezia. Cold Spring Harb Perspect Med. 2014;4. pii:a019802. doi:10.1101/cshperspect.a019802.
- Gottlieb GJ, Ackerman AB. The "sandwich sign" of dermatophytosis. Am J Dermatopathol. 1986;8:347.
- Hennessey NP, Parro EL, Cockerell CJ. Cutaneous Pneumocystis carinii infection in patients with acquired immunodeficiency syndrome. Arch Dermatol. 1991;127:1699-1701.
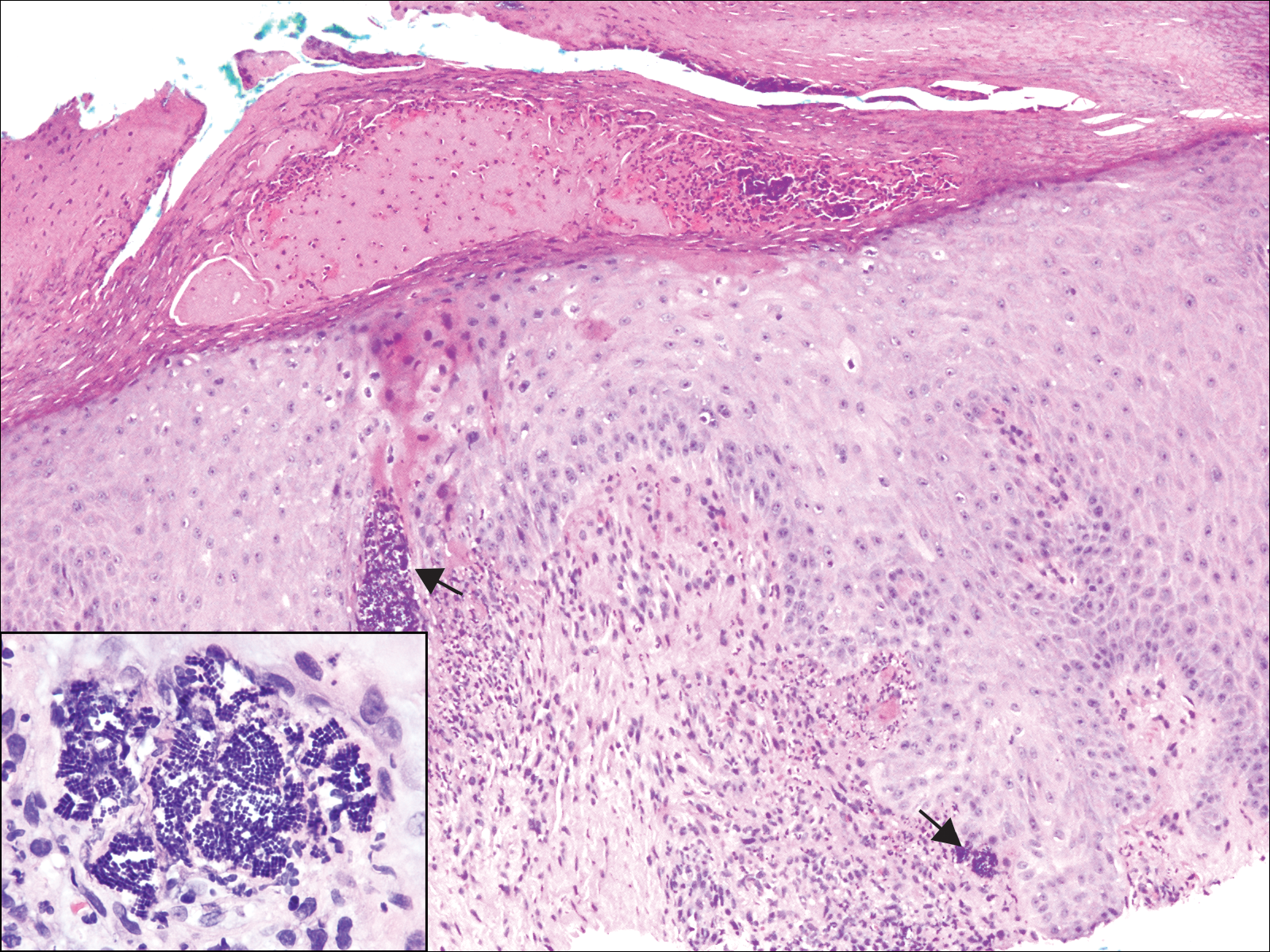
A 72-year-old woman with a medical history notable for multiple sclerosis and intravenous drug abuse presented to the dermatology clinic with a 0.6×0.5-cm, pruritic, wartlike, inflamed, keratotic papule on the palmar aspect of the right finger of more than 1 month's duration. A shave biopsy was performed that showed excoriation with serum crust, parakeratosis, and neutrophilic infiltrate in the papillary dermis. Within the serum crust and at the dermoepidermal junction, clusters of refractive basophilic bodies (arrows) in tetrad arrangement also were noted (inset). The papule resolved after the biopsy without any additional treatment.
Bedside Microscopy for the Beginner
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.
Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2
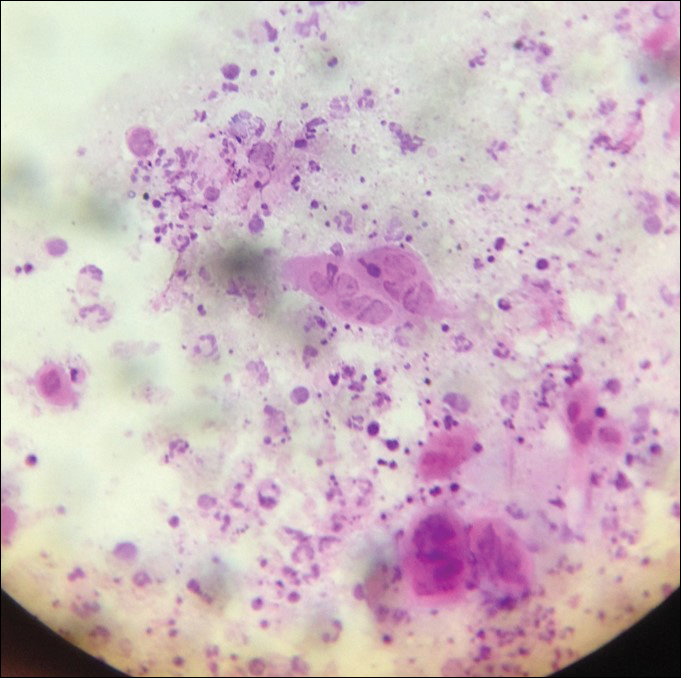
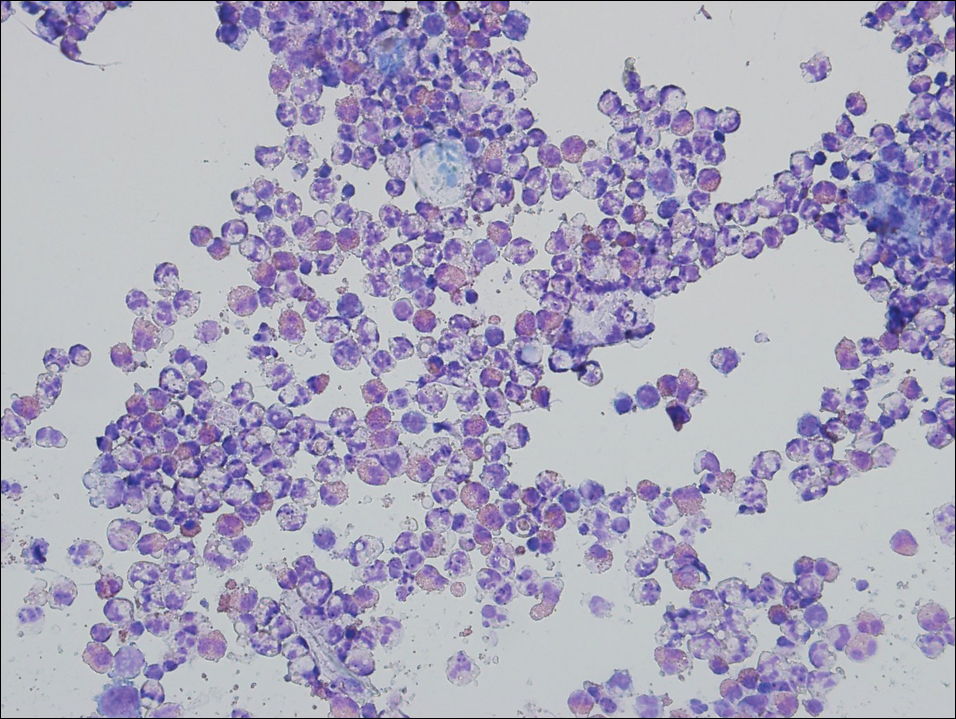
Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12
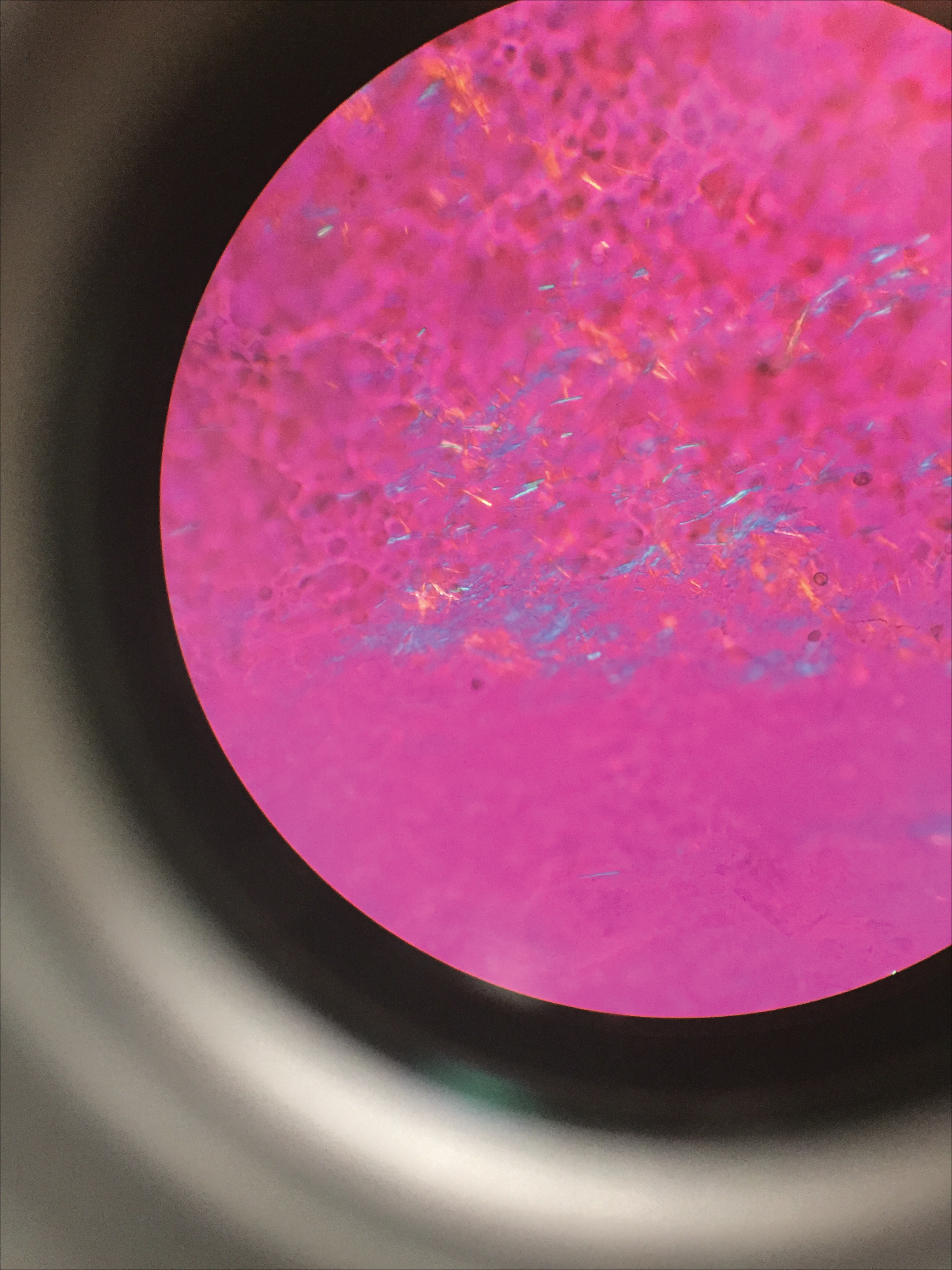
Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.
Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.


Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2


Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12

Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.

Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
Dermatologists are uniquely equipped amongst clinicians to make bedside diagnoses because of the focus on histopathology and microscopy inherent in our training. This skill is highly valuable in both an inpatient and outpatient setting because it may lead to a rapid diagnosis or be a useful adjunct in the initial clinical decision-making process. Although expert microscopists may be able to garner relevant information from scraping almost any type of lesion, bedside microscopy primarily is used by dermatologists in the United States for consideration of infectious etiologies of a variety of cutaneous manifestations.1,2
Basic Principles
Lesions that should be considered for bedside microscopic analysis in outpatient settings are scaly lesions, vesiculobullous lesions, inflammatory papules, and pustules1; microscopic evaluation also can be useful for myriad trichoscopic considerations.3,4 In some instances, direct visualization of the pathogen is possible (eg, cutaneous fungal infections, demodicidosis, scabetic infections), and in other circumstances reactive changes of keratinocytes or the presence of specific cell types can aid in diagnosis (eg, ballooning degeneration and multinucleation of keratinocytes in herpetic lesions, an abundance of eosinophils in erythema toxicum neonatorum). Different types of media are used to best prepare tissue based on the suspected etiology of the condition.
One major stumbling block for residents when beginning to perform bedside testing is the lack of dimensional understanding of the structures they are searching for; for example, medical students and residents often may mistake fibers for dermatophytes, which typically are much larger than fungal hyphae. Familiarizing oneself with the basic dimensions of different cell types or pathogens in relation to each other (Table) will help further refine the beginner’s ability to effectively search for and identify pathogenic features. This concept is further schematized in Figure 1 to help visualize scale differences.


Examination of the Specimen
Slide preparation depends on the primary lesion in consideration and will be discussed in greater detail in the following sections. Once the slide is prepared, place it on the microscope stage and adjust the condenser and light source for optimal visualization. Scan the specimen in a gridlike fashion on low power (usually ×10) and then inspect suspicious findings on higher power (×40 or higher).
Dermatomycoses
Fungal infections of the skin can present as annular papulosquamous lesions, follicular pustules or papules, bullous lesions, hypopigmented patches, and mucosal exudate or erosions, among other manifestations.5 Potassium hydroxide (KOH) is the classic medium used in preparation of lesions being assessed for evidence of fungus because it leads to lysis of keratinocytes for better visualization of fungal hyphae and spores. Other media that contain KOH and additional substrates such as dimethyl sulfoxide or chlorazol black E can be used to better highlight fungal elements.6
Dermatophytosis
Dermatophytes lead to superficial infection of the epidermis and epidermal appendages and present in a variety of ways, including site-specific infections manifesting typically as erythematous, annular or arcuate scaling (eg, tinea faciei, tinea corporis, tinea cruris, tinea manus, tinea pedis), alopecia with broken hair shafts, black dots, boggy nodules and/or scaling of the scalp (eg, tinea capitis, favus, kerion), and dystrophic nails (eg, onychomycosis).5,7 For examination of lesional skin scrapings, one can either use clear cellophane tape against the skin to remove scale, which is especially useful in the case of pediatric patients, and then press the tape against a slide prepared with several drops of a KOH-based medium to directly visualize without a coverslip, or scrape the lesion with a No. 15 blade and place the scales onto the glass slide, with further preparation as described below.8 For assessment of alopecia or dystrophic nails, scrape lesional skin with a No. 15 blade to obtain affected hair follicles and proximal subungual debris, respectively.6,9
Once the cellular debris has been obtained and placed on the slide, a coverslip can be overlaid and KOH applied laterally to be taken up across the slide by capillary action. Allow the slide to sit for at least 5 minutes before analyzing to better visualize fungal elements. Both tinea and onychomycosis will show branching septate hyphae extending across keratinocytes; a common false-positive is identifying overlapping keratinocyte edges, which are a similar size, but they can be distinguished from fungi because they do not cross multiple keratinocytes.1,8 Tinea capitis may demonstrate similar findings or may reveal hair shafts with spores contained within or surrounding it, corresponding to endothrix or ectothrix infection, respectively.5
Pityriasis Versicolor and Malassezia Folliculitis
Pityriasis versicolor presents with hypopigmented to pink, finely scaling ovoid papules, usually on the upper back, shoulders, and neck, and is caused by Malassezia furfur and other Malassezia species.5 Malassezia folliculitis also is caused by this fungus and presents with monomorphic follicular papules and pustules. Scrapings from the scaly papules will demonstrate keratinocytes with the classic “spaghetti and meatballs” fungal elements, whereas Malassezia folliculitis demonstrates only spores.5,7
Candidiasis
One possible outpatient presentation of candidiasis is oral thrush, which can exhibit white mucosal exudate or erythematous patches. A tongue blade can be used to scrape the tongue or cheek wall, with subsequent preparatory steps with application of KOH as described for dermatophytes. Cutaneous candidiasis most often develops in intertriginous regions and will exhibit erosive painful lesions with satellite pustules. In both cases, analysis of the specimen will show shorter fatter hyphal elements than seen in dermatophytosis, with pseudohyphae, blunted ends, and potentially yeast forms.5
Vesiculobullous Lesions
The Tzanck smear has been used since the 1940s to differentiate between etiologies of blistering disorders and is now most commonly used for the quick identification of herpetic lesions.1 The test is performed by scraping the base of a deroofed vesicle, pustule, or bulla, and smearing the cellular materials onto a glass slide. The most commonly utilized media for staining in the outpatient setting at my institution (University of Texas Dell Medical School, Austin) is Giemsa, which is composed of azure II–eosin, glycerin, and methanol. It stains nuclei a reddish blue to pink and the cytoplasm blue.10 After being applied to the slide, the cells are allowed to air-dry for 5 to 10 minutes, and Giemsa stain is subsequently applied and allowed to incubate for 15 minutes, then rinsed carefully with water and directly examined.
Other stains that can be used to perform the Tzanck smear include commercial preparations that may be more accessible in the inpatient settings such as the Wright-Giemsa, Quik-Dip, and Diff-Quick.1,10
Examination of a Tzanck smear from a herpetic lesion will yield acantholytic, enlarged keratinocytes up to twice their usual size (referred to as ballooning degeneration), and multinucleation. In addition, molding of the nuclei to each other within the multinucleated cells and margination of the nuclear chromatin may be appreciated (Figure 2). Intranuclear inclusion bodies, also known as Cowdry type A bodies, can be seen that are nearly the size of red blood cells but are rare to find, with only 10% of specimens exhibiting this finding in a prospective review of 299 patients with herpetic vesiculobullous lesions.11 Evaluation of the contents of blisters caused by bullous pemphigoid and erythema toxicum neonatorum may yield high densities of eosinophils with normal keratinocyte morphology (Figure 3). Other blistering eruptions such as pemphigus vulgaris and bullous drug eruptions also have characteristic findings.1,2


Gout Preparation
Gout is a systemic disease caused by uric acid accumulation that can present with joint pain and white to red nodules on digits, joints, and ears (known as tophi). Material may be expressed from tophi and examined immediately by polarized light microscopy to confirm the diagnosis.5 Specimens will demonstrate needle-shaped, negatively birefringent monosodium urate crystals on polarized light microscopy (Figure 4). An ordinary light microscope can be converted for such use with the lenses of inexpensive polarized sunglasses, placing one lens between the light source and specimen and the other lens between the examiner’s eye and the specimen.12

Parasitic Infections
Two common parasitic infections identified in outpatient dermatology clinics are scabies mites and Demodex mites. Human scabies is extremely pruritic and caused by infestation with Sarcoptes scabiei var hominis; the typical presentation in an adult is erythematous and crusted papules, linear burrows, and vesiculopustules, especially of the interdigital spaces, wrists, axillae, umbilicus, and genital region.1,13 Demodicidosis presents with papules and pustules on the face, usually in a patient with background rosacea and diffuse erythema.1,5,14
If either of these conditions are suspected, mineral oil should be used to prepare the slide because it will maintain viability of the organisms, which are visualized better in motion. Adult scabies mites are roughly 10 times larger than keratinocytes, measuring approximately 250 to 450 µm in length with 8 legs.13 Eggs also may be visualized within the cellular debris and typically are 100 to 150 µm in size and ovoid in shape. Of note, polariscopic examination may be a useful adjunct for evaluation of scabies because scabetic spines and scybala (or fecal material) are polarizable.15
Two types of Demodex mites typically are found in the skin: Demodex folliculorum, which are similarly sized to scabies mites with a more oblong body and occur most commonly in mature hair follicles (eg, eyelashes), and Demodex brevis, which are about half the size (150–200 µm) and live in the sebaceous glands of vellus hairs (Figure 5).14 Both of these mites have 8 legs, similar to the scabies mite.

Hair Preparations
Hair preparations for bulbar examination (eg, trichogram) may prove useful in the evaluation of many types of alopecia, and elaboration on this topic is beyond the scope of this article. Microscopic evaluation of the hair shaft may be an underutilized technique in the outpatient setting and is capable of yielding a variety of diagnoses, including monilethrix, pili torti, and pili trianguli et canaliculi, among others.3 One particularly useful scenario for hair shaft examination (usually of the eyebrow) is in the setting of a patient with severe atopic dermatitis or a baby with ichthyosiform erythroderma, as discovery of trichorrhexis invaginata is pathognomonic for the diagnosis of Netherton syndrome.16 Lastly, evaluation of the hair shaft in patients with patchy and diffuse hair loss whose clinical impression is reminiscent of alopecia areata, or those with concerns of inability to grow hair beyond a short length, may lead to diagnosis of loose anagen syndrome, especially if more than 70% of hair fibers examined exhibit the classic findings of a ruffled proximal cuticle and lack of root sheath.4
Final Thoughts
Bedside microscopy is a rapid and cost-sensitive way to confirm diagnoses that are clinically suspected and remains a valuable tool to acquire during residency training.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
- Wanat KA, Dominguez AR, Carter Z, et al. Bedside diagnostics in dermatology: viral, bacterial, and fungal infections. J Am Acad Dermatol. 2017;77:197-218.
- Micheletti RG, Dominguez AR, Wanat KA. Bedside diagnostics in dermatology: parasitic and noninfectious diseases. J Am Acad Dermatol. 2017;77:221-230.
- Whiting DA, Dy LC. Office diagnosis of hair shaft defects. Semin Cutan Med Surg. 2006;25:24-34.
- Tosti A. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521-522.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Philadelphia PA: Elsevier; 2017.
- Lilly KK, Koshnick RL, Grill JP, et al. Cost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated-measure, single-blinded, cross-sectional evaluation of 7 diagnostic tests. J Am Acad Dermatol. 2006;55:620-626.
- Elder DE, ed. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009.
- Raghukumar S, Ravikumar BC. Potassium hydroxide mount with cellophane adhesive: a method for direct diagnosis of dermatophyte skin infections [published online May 29, 2018]. Clin Exp Dermatol. doi:10.1111/ced.13573.
- Bhat YJ, Zeerak S, Kanth F, et al. Clinicoepidemiological and mycological study of tinea capitis in the pediatric population of Kashmir Valley: a study from a tertiary care centre. Indian Dermatol Online J. 2017;8:100-103.
- Gupta LK, Singhi MK. Tzanck smear: a useful diagnostic tool. Indian J Dermatol Venereol Leprol. 2005;71:295-299.
- Durdu M, Baba M, Seçkin D. The value of Tzanck smear test in diagnosis of erosive, vesicular, bullous, and pustular skin lesions. J Am Acad Dermatol. 2008;59:958-964.
- Fagan TJ, Lidsky MD. Compensated polarized light microscopy using cellophane adhesive tape. Arthritis Rheum. 1974;17:256-262.
- Walton SF, Currie BJ. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev. 2007;20:268-279.
- Desch C, Nutting WB. Demodex folliculorum (Simon) and D. brevis akbulatova of man: redescription and reevaluation. J Parasitol. 1972;58:169-177.
- Foo CW, Florell SR, Bowen AR. Polarizable elements in scabies infestation: a clue to diagnosis. J Cutan Pathol. 2013;40:6-10.
- Akkurt ZM, Tuncel T, Ayhan E, et al. Rapid and easy diagnosis of Netherton syndrome with dermoscopy. J Cutan Med Surg. 2014;18:280-282.
Brown Papules on the Penis
The Diagnosis: Bowenoid Papulosis
A 4-mm punch biopsy was performed from the active border of brown plaques on the dorsal penis. Histopathology revealed parakeratotic hyperkeratosis, acanthosis, loss of maturation in epithelium, and full-size atypia (Figure 1). Ki-67 index was 90% positive in the epidermis (Figure 2). Staining for p16 and human papillomavirus (HPV) screening was positive for HPV type 16 (Figure 3). Serologic tests for other sexually transmitted infections were negative. A diagnosis of penile bowenoid papulosis (BP) with grade 3 penile intraepithelial neoplasia was made, and treatment with topical 5-fluorouracil (5-FU) was initiated. Almost total regression was appreciated at 1-month follow-up (Figure 4), and he also was recurrence free at 1-year follow-up.

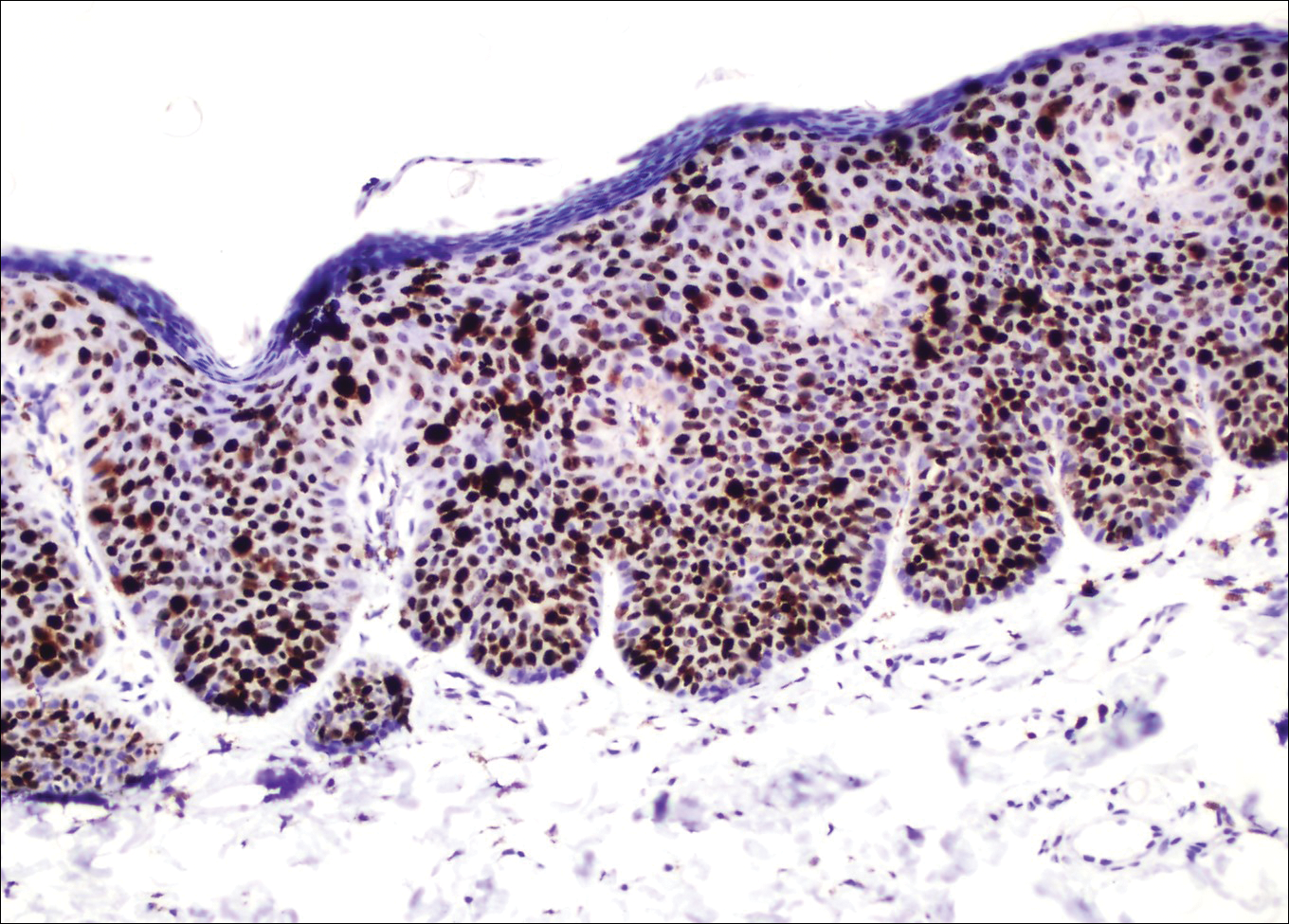
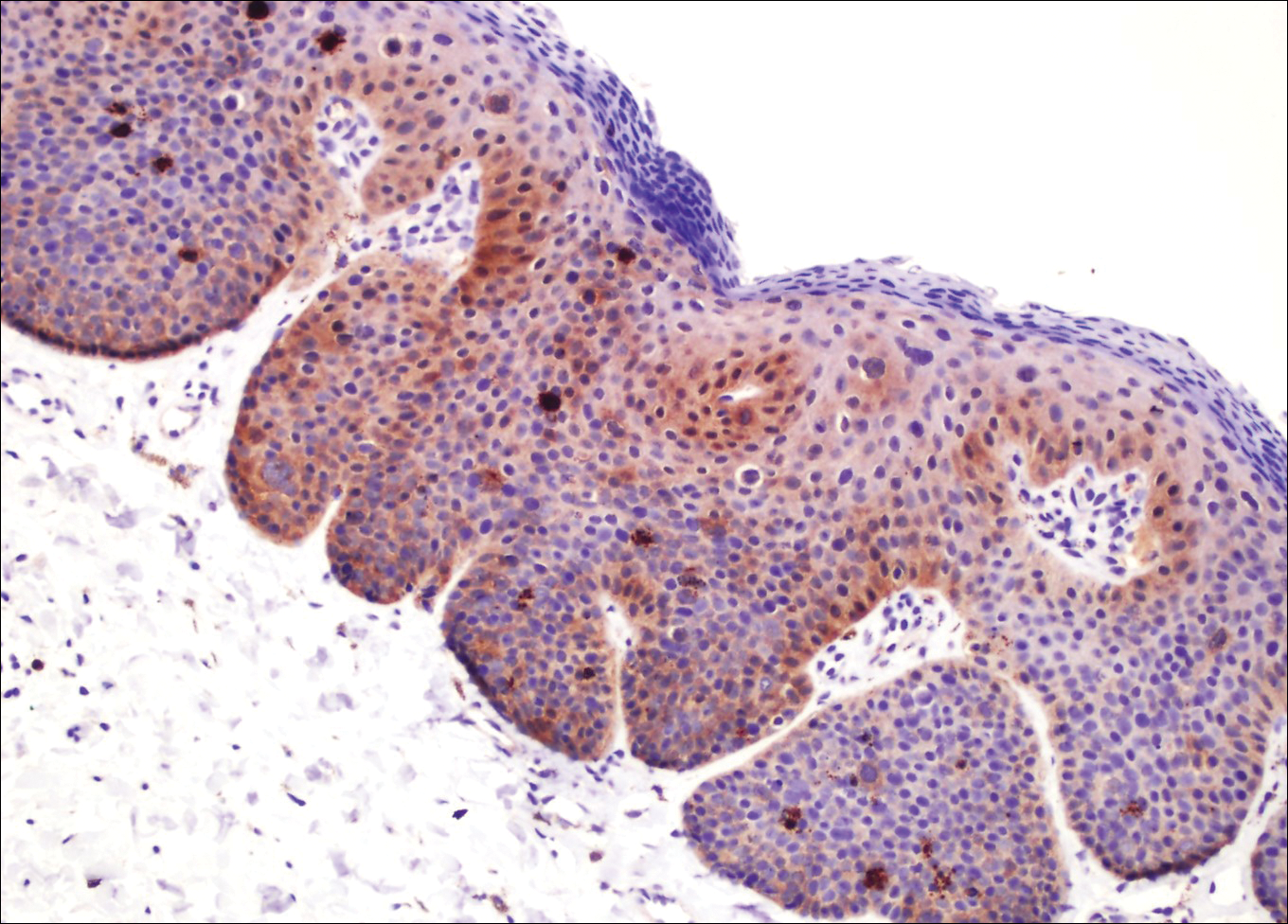
Penile intraepithelial neoplasia (PIN), or penile squamous cell carcinoma in situ, is a rare disease with high morbidity and mortality rates. Clinically, PIN is comprised of a clinical spectrum including 3 different entities: erythroplasia of Queyrat, Bowen disease, and BP.1 Histologically, PIN also is classified into 3 subtypes according to histological depth of epidermal atypia.1
Bowenoid papulosis usually is characterized by multiple red-brown or flesh-colored papules that most commonly appear on the shaft or glans of the penis. Bowenoid papulosis frequently is associated with high-risk types of HPV, such as HPV type 16, and is sometimes difficult to differentiate clinically from pigmented condyloma acuminatum. The clinical lesions of BP usually are less papillomatous, smoother topped, more polymorphic, and more coalescent compared to common genital viral condyloma acuminatum.2 Bowenoid papulosis usually is seen in young (<30 years of age) sexually active men, unlike the patches or plaques of erythroplasia of Queyrat or Bowen disease, which are seen in older men aged 45 to 75 years. Bowenoid papulosis also has a lower malignancy potential than erythroplasia of Queyrat and Bowen disease.2
Penile melanosis, penile lentigo, and seborrheic keratosis comprise the differential diagnosis of dark spots on the penis and also should be kept in mind. Penile melanosis is the most common cause of dark spots on the penis. When the dark spots have irregular borders and change in color, they may be misdiagnosed as malignant lesions such as melanoma.3 In most cases, biopsy is indicated. Histologically, penile melanosis is characterized by hyperpigmentation of the basal cell layer with no melanocytic hyperplasia. Treatment is unnecessary in most cases.
Penile lentigo presents as small flat pigmented spots on the penile skin with clearly defined margins surrounded by normal-appearing skin. Histologically, it is characterized by hyperplasia of melanocytes above the basement membrane of the epidermis.3
Penile pigmented seborrheic keratosis is a rare clinical entity that can be easily misinterpreted as condyloma acuminatum. Histologically, it is characterized by basal cell hyperplasia with cystic formation in the thickened epidermis. Excisional biopsy may be the only way to rule out malignant disease.
Treatment options for PIN include cryotherapy, CO2 or Nd:YAG lasers, photodynamic therapy, topical 5-FU or imiquimod therapy, and surgical excision such as Mohs micrographic surgery.4-9 Although these therapeutic modalities usually are effective, recurrence is common.6 The patients' discomfort and poor cosmetic and functional outcomes from the surgical removal of lesions also present a challenge in treatment planning.
In our patient, we quickly achieved a good result with topical 5-FU, though the disease was in local advanced stage. It is important for clinicians to consider 5-FU as an effective treatment option for PIN before planning surgery.
- Deen K, Burdon-Jones D. Imiquimod in the treatment of penile intraepithelial neoplasia: an update. Australas J Dermatol. 2017;58:86-92.
- Porter WM, Francis N, Hawkins D, et al. Penile intraepithelial neoplasia: clinical spectrum and treatment of 35 cases. Br J Dermatol. 2002;147:1159-1165.
- Fahmy M. Dermatological disease of the penis. In: Fahmy M. Congenital Anomalies of the Penis. Cham, Switzerland: Springer; 2017:257-264.
- Shimizu A, Kato M, Ishikawa O. Bowenoid papulosis successfully treated with imiquimod 5% cream. J Dermatol. 2014;41:545-546.
- Lucky M, Murthy KV, Rogers B, et al. The treatment of penile carcinoma in situ (CIS) within a UK supra-regional network [published online December 15, 2014]. BJU Int. 2015;115:595-598.
- Alnajjar HM, Lam W, Bolgeri M, et al. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. Eur Urol. 2012;62:923-928.
- Wang XL, Wang HW, Guo MX, et al. Combination of immunotherapy and photodynamic therapy in the treatment of bowenoid papulosis. Photodiagnosis Photodyn Ther. 2007;4:88-93.
- Zreik A, Rewhorn M, Vint R, et al. Carbon dioxide laser treatment of penile intraepithelial neoplasia [published online December 7, 2016]. Surgeon. 2017;15:321-324.
- Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936-944.
The Diagnosis: Bowenoid Papulosis
A 4-mm punch biopsy was performed from the active border of brown plaques on the dorsal penis. Histopathology revealed parakeratotic hyperkeratosis, acanthosis, loss of maturation in epithelium, and full-size atypia (Figure 1). Ki-67 index was 90% positive in the epidermis (Figure 2). Staining for p16 and human papillomavirus (HPV) screening was positive for HPV type 16 (Figure 3). Serologic tests for other sexually transmitted infections were negative. A diagnosis of penile bowenoid papulosis (BP) with grade 3 penile intraepithelial neoplasia was made, and treatment with topical 5-fluorouracil (5-FU) was initiated. Almost total regression was appreciated at 1-month follow-up (Figure 4), and he also was recurrence free at 1-year follow-up.




Penile intraepithelial neoplasia (PIN), or penile squamous cell carcinoma in situ, is a rare disease with high morbidity and mortality rates. Clinically, PIN is comprised of a clinical spectrum including 3 different entities: erythroplasia of Queyrat, Bowen disease, and BP.1 Histologically, PIN also is classified into 3 subtypes according to histological depth of epidermal atypia.1
Bowenoid papulosis usually is characterized by multiple red-brown or flesh-colored papules that most commonly appear on the shaft or glans of the penis. Bowenoid papulosis frequently is associated with high-risk types of HPV, such as HPV type 16, and is sometimes difficult to differentiate clinically from pigmented condyloma acuminatum. The clinical lesions of BP usually are less papillomatous, smoother topped, more polymorphic, and more coalescent compared to common genital viral condyloma acuminatum.2 Bowenoid papulosis usually is seen in young (<30 years of age) sexually active men, unlike the patches or plaques of erythroplasia of Queyrat or Bowen disease, which are seen in older men aged 45 to 75 years. Bowenoid papulosis also has a lower malignancy potential than erythroplasia of Queyrat and Bowen disease.2
Penile melanosis, penile lentigo, and seborrheic keratosis comprise the differential diagnosis of dark spots on the penis and also should be kept in mind. Penile melanosis is the most common cause of dark spots on the penis. When the dark spots have irregular borders and change in color, they may be misdiagnosed as malignant lesions such as melanoma.3 In most cases, biopsy is indicated. Histologically, penile melanosis is characterized by hyperpigmentation of the basal cell layer with no melanocytic hyperplasia. Treatment is unnecessary in most cases.
Penile lentigo presents as small flat pigmented spots on the penile skin with clearly defined margins surrounded by normal-appearing skin. Histologically, it is characterized by hyperplasia of melanocytes above the basement membrane of the epidermis.3
Penile pigmented seborrheic keratosis is a rare clinical entity that can be easily misinterpreted as condyloma acuminatum. Histologically, it is characterized by basal cell hyperplasia with cystic formation in the thickened epidermis. Excisional biopsy may be the only way to rule out malignant disease.
Treatment options for PIN include cryotherapy, CO2 or Nd:YAG lasers, photodynamic therapy, topical 5-FU or imiquimod therapy, and surgical excision such as Mohs micrographic surgery.4-9 Although these therapeutic modalities usually are effective, recurrence is common.6 The patients' discomfort and poor cosmetic and functional outcomes from the surgical removal of lesions also present a challenge in treatment planning.
In our patient, we quickly achieved a good result with topical 5-FU, though the disease was in local advanced stage. It is important for clinicians to consider 5-FU as an effective treatment option for PIN before planning surgery.
The Diagnosis: Bowenoid Papulosis
A 4-mm punch biopsy was performed from the active border of brown plaques on the dorsal penis. Histopathology revealed parakeratotic hyperkeratosis, acanthosis, loss of maturation in epithelium, and full-size atypia (Figure 1). Ki-67 index was 90% positive in the epidermis (Figure 2). Staining for p16 and human papillomavirus (HPV) screening was positive for HPV type 16 (Figure 3). Serologic tests for other sexually transmitted infections were negative. A diagnosis of penile bowenoid papulosis (BP) with grade 3 penile intraepithelial neoplasia was made, and treatment with topical 5-fluorouracil (5-FU) was initiated. Almost total regression was appreciated at 1-month follow-up (Figure 4), and he also was recurrence free at 1-year follow-up.




Penile intraepithelial neoplasia (PIN), or penile squamous cell carcinoma in situ, is a rare disease with high morbidity and mortality rates. Clinically, PIN is comprised of a clinical spectrum including 3 different entities: erythroplasia of Queyrat, Bowen disease, and BP.1 Histologically, PIN also is classified into 3 subtypes according to histological depth of epidermal atypia.1
Bowenoid papulosis usually is characterized by multiple red-brown or flesh-colored papules that most commonly appear on the shaft or glans of the penis. Bowenoid papulosis frequently is associated with high-risk types of HPV, such as HPV type 16, and is sometimes difficult to differentiate clinically from pigmented condyloma acuminatum. The clinical lesions of BP usually are less papillomatous, smoother topped, more polymorphic, and more coalescent compared to common genital viral condyloma acuminatum.2 Bowenoid papulosis usually is seen in young (<30 years of age) sexually active men, unlike the patches or plaques of erythroplasia of Queyrat or Bowen disease, which are seen in older men aged 45 to 75 years. Bowenoid papulosis also has a lower malignancy potential than erythroplasia of Queyrat and Bowen disease.2
Penile melanosis, penile lentigo, and seborrheic keratosis comprise the differential diagnosis of dark spots on the penis and also should be kept in mind. Penile melanosis is the most common cause of dark spots on the penis. When the dark spots have irregular borders and change in color, they may be misdiagnosed as malignant lesions such as melanoma.3 In most cases, biopsy is indicated. Histologically, penile melanosis is characterized by hyperpigmentation of the basal cell layer with no melanocytic hyperplasia. Treatment is unnecessary in most cases.
Penile lentigo presents as small flat pigmented spots on the penile skin with clearly defined margins surrounded by normal-appearing skin. Histologically, it is characterized by hyperplasia of melanocytes above the basement membrane of the epidermis.3
Penile pigmented seborrheic keratosis is a rare clinical entity that can be easily misinterpreted as condyloma acuminatum. Histologically, it is characterized by basal cell hyperplasia with cystic formation in the thickened epidermis. Excisional biopsy may be the only way to rule out malignant disease.
Treatment options for PIN include cryotherapy, CO2 or Nd:YAG lasers, photodynamic therapy, topical 5-FU or imiquimod therapy, and surgical excision such as Mohs micrographic surgery.4-9 Although these therapeutic modalities usually are effective, recurrence is common.6 The patients' discomfort and poor cosmetic and functional outcomes from the surgical removal of lesions also present a challenge in treatment planning.
In our patient, we quickly achieved a good result with topical 5-FU, though the disease was in local advanced stage. It is important for clinicians to consider 5-FU as an effective treatment option for PIN before planning surgery.
- Deen K, Burdon-Jones D. Imiquimod in the treatment of penile intraepithelial neoplasia: an update. Australas J Dermatol. 2017;58:86-92.
- Porter WM, Francis N, Hawkins D, et al. Penile intraepithelial neoplasia: clinical spectrum and treatment of 35 cases. Br J Dermatol. 2002;147:1159-1165.
- Fahmy M. Dermatological disease of the penis. In: Fahmy M. Congenital Anomalies of the Penis. Cham, Switzerland: Springer; 2017:257-264.
- Shimizu A, Kato M, Ishikawa O. Bowenoid papulosis successfully treated with imiquimod 5% cream. J Dermatol. 2014;41:545-546.
- Lucky M, Murthy KV, Rogers B, et al. The treatment of penile carcinoma in situ (CIS) within a UK supra-regional network [published online December 15, 2014]. BJU Int. 2015;115:595-598.
- Alnajjar HM, Lam W, Bolgeri M, et al. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. Eur Urol. 2012;62:923-928.
- Wang XL, Wang HW, Guo MX, et al. Combination of immunotherapy and photodynamic therapy in the treatment of bowenoid papulosis. Photodiagnosis Photodyn Ther. 2007;4:88-93.
- Zreik A, Rewhorn M, Vint R, et al. Carbon dioxide laser treatment of penile intraepithelial neoplasia [published online December 7, 2016]. Surgeon. 2017;15:321-324.
- Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936-944.
- Deen K, Burdon-Jones D. Imiquimod in the treatment of penile intraepithelial neoplasia: an update. Australas J Dermatol. 2017;58:86-92.
- Porter WM, Francis N, Hawkins D, et al. Penile intraepithelial neoplasia: clinical spectrum and treatment of 35 cases. Br J Dermatol. 2002;147:1159-1165.
- Fahmy M. Dermatological disease of the penis. In: Fahmy M. Congenital Anomalies of the Penis. Cham, Switzerland: Springer; 2017:257-264.
- Shimizu A, Kato M, Ishikawa O. Bowenoid papulosis successfully treated with imiquimod 5% cream. J Dermatol. 2014;41:545-546.
- Lucky M, Murthy KV, Rogers B, et al. The treatment of penile carcinoma in situ (CIS) within a UK supra-regional network [published online December 15, 2014]. BJU Int. 2015;115:595-598.
- Alnajjar HM, Lam W, Bolgeri M, et al. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. Eur Urol. 2012;62:923-928.
- Wang XL, Wang HW, Guo MX, et al. Combination of immunotherapy and photodynamic therapy in the treatment of bowenoid papulosis. Photodiagnosis Photodyn Ther. 2007;4:88-93.
- Zreik A, Rewhorn M, Vint R, et al. Carbon dioxide laser treatment of penile intraepithelial neoplasia [published online December 7, 2016]. Surgeon. 2017;15:321-324.
- Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936-944.
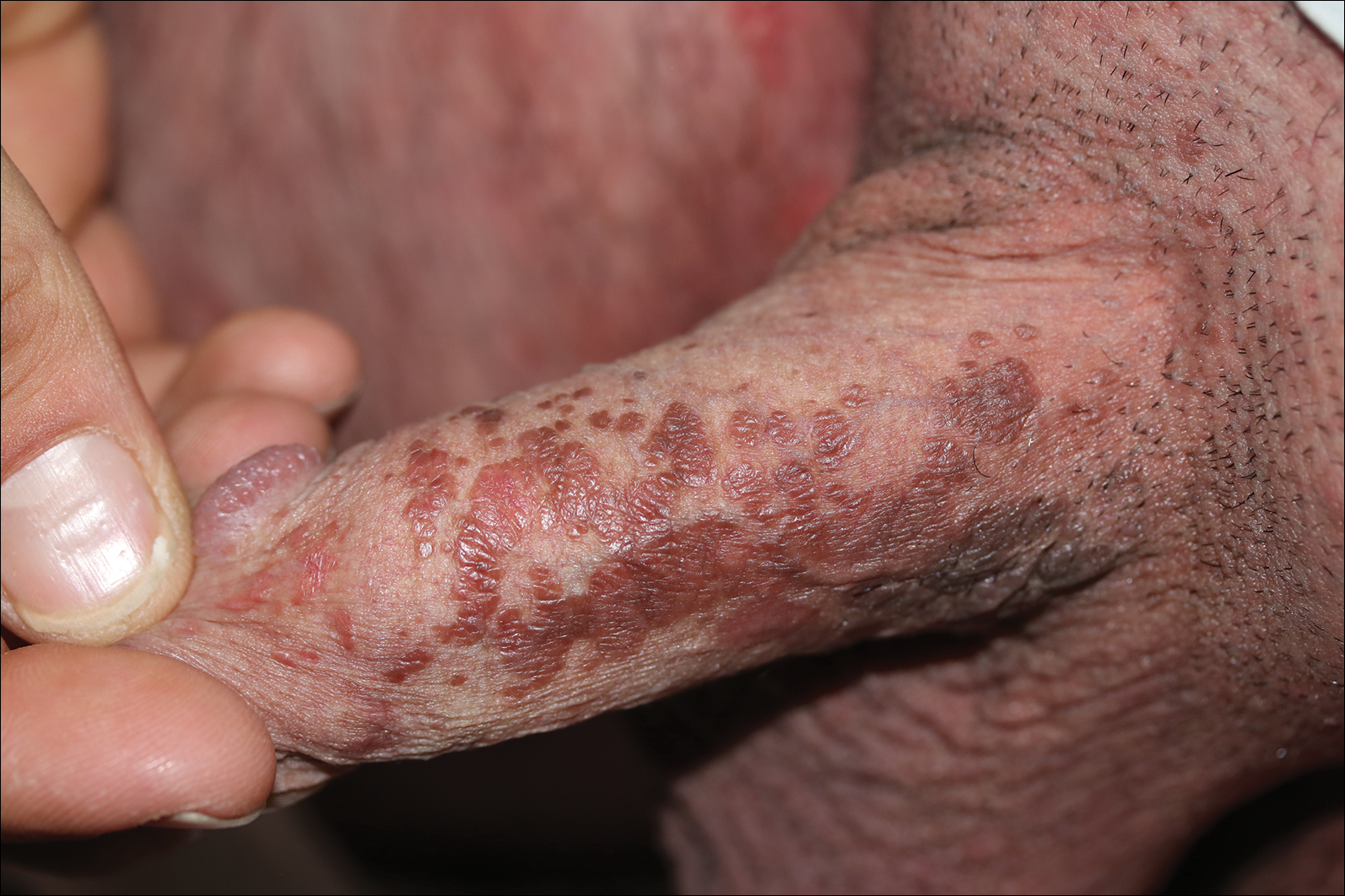
A 32-year-old man presented to the outpatient clinic with reddish brown lesions on the penis of 5 months' duration. Dermatologic examination revealed multiple mildly infiltrated, bright reddish brown papules and plaques on the dorsal penis.
Agminated Heterogeneous Papules on the Neck
The Diagnosis: Eruptive Blue Nevus
All biopsies demonstrated similar histologic features, including an intradermal proliferation of heavily pigmented, spindle-shaped dendritic melanocytes (Figure). The dermal pigment was most pronounced in the grossly darker papules, and there was not a substantial amount of background pigmentation at the stratum basale. Cytologic atypia, foci of necrosis, and mitotic activity were absent from all sections. There was no definitive junctional component identified, no multinucleated giant cells, and there was no overlying epidermal aberration. With some background pigmentation seen histologically, nevus spilus was considered, but because this acute eruption occurred in a young adult without appreciable gross background hyperpigmentation, the clinical context led to a diagnosis of eruptive blue nevus. After communicating the findings to the patient, he declined further treatment.
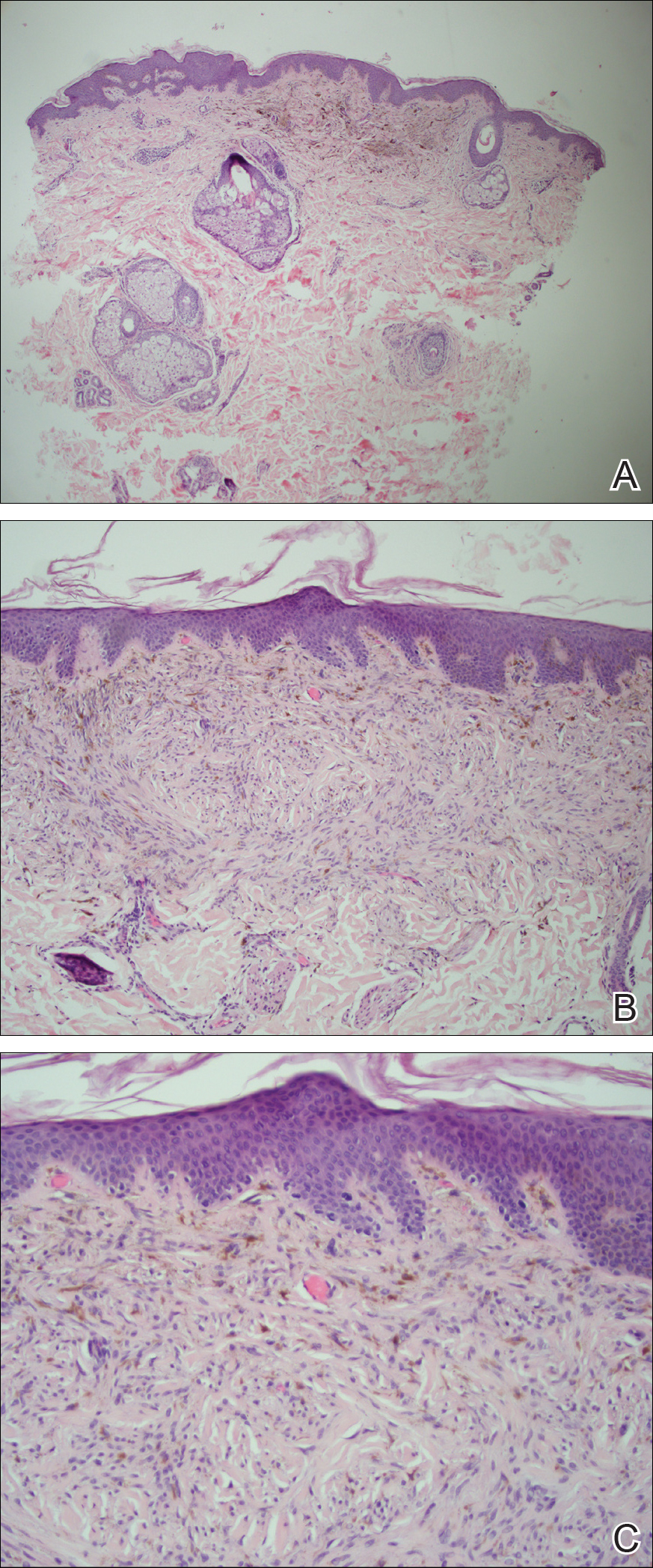
Eruptive blue nevus is an exceptionally rare subtype of blue nevus with few cases reported since the 1940s.1-9 Generally, each case report found a triggering event that could possibly have precipitated the acute proliferation and evolution of nevi. Triggering events can include bullous processes such as erythema multiforme2 and Stevens-Johnson syndrome,3 severe sunburn,4 trauma,5 immunosuppression,6 and a variety of endocrinopathies. No such history could be identified in our patient, except the biopsy.
Common blue nevi are benign, usually congenital, well-circumscribed, solitary, blue-gray macules or papules. Half of blue nevi cases are found on the dorsal aspects of the hands and feet but can present anywhere (eg, face, scalp, wrists, sacrum, buttocks). The blue-gray color appreciated clinically is attributed to the Tyndall effect, which occurs when long-wavelength light--red, orange, and yellow--is absorbed by the melanin deep in the dermis, while short-wavelength visible light--blue, violet, and indigo--is reflected with backscattering. On polarized dermoscopy, a homogeneous blue-gray hue is appreciated, but lighter segments may be present when collagen deposition is robust. Histopathologic findings confirm spindle-shaped dendritic melanocytes in the dermis without epidermal involvement. It generally is accepted that the etiology of these benign nevi is a failed migration of neural crest cells to the epidermis.10,11 Although the common blue nevus may be simple to diagnose, several subtypes have been described in the literature, including combined blue nevus, desmoplastic blue nevus, hypomelanotic/amelanotic blue nevus, and epithelioid blue nevus of Carney complex, and excluding a malignant process is of monumental importance.7,12
Biopsy is recommended for common blue nevi in the evaluation of newly acquired lesions, expansion of previously stable nevi, or for nevi larger than 10 mm in diameter. The nature of eruptive blue nevi warrants a biopsy to exclude melanoma or another malignant process. While the Becker nevus may manifest in adolescent males, it is clinically distinct from an eruptive blue nevus due to the size, relative homogeneity, and presence of hair within the lesion. Cutaneous amyloidosis may appear clinically similar to an eruptive blue nevus, but globular or amorphous material was not present in the papillary dermis of biopsied lesions in our patient. Since there was no cellular atypia or mitotic activity, melanoma and other malignancies were ruled out. Lastly, NAME syndrome by definition must include atrial myxomas, myxoid neurofibromas, and ephelides in addition to the nevi; however, our patient had only nevi and few ephelides. Once the diagnosis is established and benign nature confirmed, treatment is not necessarily required. If the patient elects to remove the lesion for aesthetic reasons, an excision into the subcutaneous fat is required to ensure complete removal of deep dermal melanocytes. Prior excisions of eruptive blue nevi have had no recurrence after more than 10 months.8,9
- Krause M, Bonnekoh B, Weisshaar E, et al. Coincidence of multiple, disseminated, tardive-eruptive blue nevi with cutis marmorata teleangiectatica congenita. Dermatology. 2000;200:134-138.
- Soltani K, Bernstein J, Lorincz A. Eruptive nevocytic nevi after erythema multiforme. J Am Acad Dermatol. 1979;1:503-505.
- Shoji T, Cockerell C, Koff A, et al. Eruptive melanocytic nevi after Stevens-Johnson syndrome. J Am Acad Dermatol. 1997;37:337-339.
- Hendricks W. Eruptive blue nevi. J Am Acad Dermatol. 1981;4:50-53.
- Kesty K, Zargari O. Eruptive blue nevi. Indian J Dermatol Venereol Leprol. 2015;81:198-201.
- Chen T, Kurwa H, Trotter M, et al. Agminated blue nevi in a patient with dermatomyositis. J Am Acad Dermatol. 2013;68:52-53.
- Walsh M. Correspondence: eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;141:581-582.
- Nardini P, De Giorgi V, Massi D, et al. Eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;140:178-180.
- de Giorgi V, Massi D, Brunasso G, et al. Eruptive multiple blue nevi of the penis: a clinical dermoscopic pathologic case study. J Cutan Pathol. 2004;31:185-188.
- Zimmermann AH, Becker SA. Precursors of epidermal melanocytes in the negro fetus. In: Gordon M, ed. Pigment Cell Biology. New York, NY: Academic Press Inc; 1959:159-170.
- Leopold JG, Richards DB. The interrelationship of blue and common naevi. J Pathol Bacteriol. 1968;95:37-46.
- Zembowicz A, Phadke P. Blue nevi and variants: an update. Arch Pathol Lab Med. 2011;135:327-336.
The Diagnosis: Eruptive Blue Nevus
All biopsies demonstrated similar histologic features, including an intradermal proliferation of heavily pigmented, spindle-shaped dendritic melanocytes (Figure). The dermal pigment was most pronounced in the grossly darker papules, and there was not a substantial amount of background pigmentation at the stratum basale. Cytologic atypia, foci of necrosis, and mitotic activity were absent from all sections. There was no definitive junctional component identified, no multinucleated giant cells, and there was no overlying epidermal aberration. With some background pigmentation seen histologically, nevus spilus was considered, but because this acute eruption occurred in a young adult without appreciable gross background hyperpigmentation, the clinical context led to a diagnosis of eruptive blue nevus. After communicating the findings to the patient, he declined further treatment.

Eruptive blue nevus is an exceptionally rare subtype of blue nevus with few cases reported since the 1940s.1-9 Generally, each case report found a triggering event that could possibly have precipitated the acute proliferation and evolution of nevi. Triggering events can include bullous processes such as erythema multiforme2 and Stevens-Johnson syndrome,3 severe sunburn,4 trauma,5 immunosuppression,6 and a variety of endocrinopathies. No such history could be identified in our patient, except the biopsy.
Common blue nevi are benign, usually congenital, well-circumscribed, solitary, blue-gray macules or papules. Half of blue nevi cases are found on the dorsal aspects of the hands and feet but can present anywhere (eg, face, scalp, wrists, sacrum, buttocks). The blue-gray color appreciated clinically is attributed to the Tyndall effect, which occurs when long-wavelength light--red, orange, and yellow--is absorbed by the melanin deep in the dermis, while short-wavelength visible light--blue, violet, and indigo--is reflected with backscattering. On polarized dermoscopy, a homogeneous blue-gray hue is appreciated, but lighter segments may be present when collagen deposition is robust. Histopathologic findings confirm spindle-shaped dendritic melanocytes in the dermis without epidermal involvement. It generally is accepted that the etiology of these benign nevi is a failed migration of neural crest cells to the epidermis.10,11 Although the common blue nevus may be simple to diagnose, several subtypes have been described in the literature, including combined blue nevus, desmoplastic blue nevus, hypomelanotic/amelanotic blue nevus, and epithelioid blue nevus of Carney complex, and excluding a malignant process is of monumental importance.7,12
Biopsy is recommended for common blue nevi in the evaluation of newly acquired lesions, expansion of previously stable nevi, or for nevi larger than 10 mm in diameter. The nature of eruptive blue nevi warrants a biopsy to exclude melanoma or another malignant process. While the Becker nevus may manifest in adolescent males, it is clinically distinct from an eruptive blue nevus due to the size, relative homogeneity, and presence of hair within the lesion. Cutaneous amyloidosis may appear clinically similar to an eruptive blue nevus, but globular or amorphous material was not present in the papillary dermis of biopsied lesions in our patient. Since there was no cellular atypia or mitotic activity, melanoma and other malignancies were ruled out. Lastly, NAME syndrome by definition must include atrial myxomas, myxoid neurofibromas, and ephelides in addition to the nevi; however, our patient had only nevi and few ephelides. Once the diagnosis is established and benign nature confirmed, treatment is not necessarily required. If the patient elects to remove the lesion for aesthetic reasons, an excision into the subcutaneous fat is required to ensure complete removal of deep dermal melanocytes. Prior excisions of eruptive blue nevi have had no recurrence after more than 10 months.8,9
The Diagnosis: Eruptive Blue Nevus
All biopsies demonstrated similar histologic features, including an intradermal proliferation of heavily pigmented, spindle-shaped dendritic melanocytes (Figure). The dermal pigment was most pronounced in the grossly darker papules, and there was not a substantial amount of background pigmentation at the stratum basale. Cytologic atypia, foci of necrosis, and mitotic activity were absent from all sections. There was no definitive junctional component identified, no multinucleated giant cells, and there was no overlying epidermal aberration. With some background pigmentation seen histologically, nevus spilus was considered, but because this acute eruption occurred in a young adult without appreciable gross background hyperpigmentation, the clinical context led to a diagnosis of eruptive blue nevus. After communicating the findings to the patient, he declined further treatment.

Eruptive blue nevus is an exceptionally rare subtype of blue nevus with few cases reported since the 1940s.1-9 Generally, each case report found a triggering event that could possibly have precipitated the acute proliferation and evolution of nevi. Triggering events can include bullous processes such as erythema multiforme2 and Stevens-Johnson syndrome,3 severe sunburn,4 trauma,5 immunosuppression,6 and a variety of endocrinopathies. No such history could be identified in our patient, except the biopsy.
Common blue nevi are benign, usually congenital, well-circumscribed, solitary, blue-gray macules or papules. Half of blue nevi cases are found on the dorsal aspects of the hands and feet but can present anywhere (eg, face, scalp, wrists, sacrum, buttocks). The blue-gray color appreciated clinically is attributed to the Tyndall effect, which occurs when long-wavelength light--red, orange, and yellow--is absorbed by the melanin deep in the dermis, while short-wavelength visible light--blue, violet, and indigo--is reflected with backscattering. On polarized dermoscopy, a homogeneous blue-gray hue is appreciated, but lighter segments may be present when collagen deposition is robust. Histopathologic findings confirm spindle-shaped dendritic melanocytes in the dermis without epidermal involvement. It generally is accepted that the etiology of these benign nevi is a failed migration of neural crest cells to the epidermis.10,11 Although the common blue nevus may be simple to diagnose, several subtypes have been described in the literature, including combined blue nevus, desmoplastic blue nevus, hypomelanotic/amelanotic blue nevus, and epithelioid blue nevus of Carney complex, and excluding a malignant process is of monumental importance.7,12
Biopsy is recommended for common blue nevi in the evaluation of newly acquired lesions, expansion of previously stable nevi, or for nevi larger than 10 mm in diameter. The nature of eruptive blue nevi warrants a biopsy to exclude melanoma or another malignant process. While the Becker nevus may manifest in adolescent males, it is clinically distinct from an eruptive blue nevus due to the size, relative homogeneity, and presence of hair within the lesion. Cutaneous amyloidosis may appear clinically similar to an eruptive blue nevus, but globular or amorphous material was not present in the papillary dermis of biopsied lesions in our patient. Since there was no cellular atypia or mitotic activity, melanoma and other malignancies were ruled out. Lastly, NAME syndrome by definition must include atrial myxomas, myxoid neurofibromas, and ephelides in addition to the nevi; however, our patient had only nevi and few ephelides. Once the diagnosis is established and benign nature confirmed, treatment is not necessarily required. If the patient elects to remove the lesion for aesthetic reasons, an excision into the subcutaneous fat is required to ensure complete removal of deep dermal melanocytes. Prior excisions of eruptive blue nevi have had no recurrence after more than 10 months.8,9
- Krause M, Bonnekoh B, Weisshaar E, et al. Coincidence of multiple, disseminated, tardive-eruptive blue nevi with cutis marmorata teleangiectatica congenita. Dermatology. 2000;200:134-138.
- Soltani K, Bernstein J, Lorincz A. Eruptive nevocytic nevi after erythema multiforme. J Am Acad Dermatol. 1979;1:503-505.
- Shoji T, Cockerell C, Koff A, et al. Eruptive melanocytic nevi after Stevens-Johnson syndrome. J Am Acad Dermatol. 1997;37:337-339.
- Hendricks W. Eruptive blue nevi. J Am Acad Dermatol. 1981;4:50-53.
- Kesty K, Zargari O. Eruptive blue nevi. Indian J Dermatol Venereol Leprol. 2015;81:198-201.
- Chen T, Kurwa H, Trotter M, et al. Agminated blue nevi in a patient with dermatomyositis. J Am Acad Dermatol. 2013;68:52-53.
- Walsh M. Correspondence: eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;141:581-582.
- Nardini P, De Giorgi V, Massi D, et al. Eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;140:178-180.
- de Giorgi V, Massi D, Brunasso G, et al. Eruptive multiple blue nevi of the penis: a clinical dermoscopic pathologic case study. J Cutan Pathol. 2004;31:185-188.
- Zimmermann AH, Becker SA. Precursors of epidermal melanocytes in the negro fetus. In: Gordon M, ed. Pigment Cell Biology. New York, NY: Academic Press Inc; 1959:159-170.
- Leopold JG, Richards DB. The interrelationship of blue and common naevi. J Pathol Bacteriol. 1968;95:37-46.
- Zembowicz A, Phadke P. Blue nevi and variants: an update. Arch Pathol Lab Med. 2011;135:327-336.
- Krause M, Bonnekoh B, Weisshaar E, et al. Coincidence of multiple, disseminated, tardive-eruptive blue nevi with cutis marmorata teleangiectatica congenita. Dermatology. 2000;200:134-138.
- Soltani K, Bernstein J, Lorincz A. Eruptive nevocytic nevi after erythema multiforme. J Am Acad Dermatol. 1979;1:503-505.
- Shoji T, Cockerell C, Koff A, et al. Eruptive melanocytic nevi after Stevens-Johnson syndrome. J Am Acad Dermatol. 1997;37:337-339.
- Hendricks W. Eruptive blue nevi. J Am Acad Dermatol. 1981;4:50-53.
- Kesty K, Zargari O. Eruptive blue nevi. Indian J Dermatol Venereol Leprol. 2015;81:198-201.
- Chen T, Kurwa H, Trotter M, et al. Agminated blue nevi in a patient with dermatomyositis. J Am Acad Dermatol. 2013;68:52-53.
- Walsh M. Correspondence: eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;141:581-582.
- Nardini P, De Giorgi V, Massi D, et al. Eruptive disseminated blue naevi of the scalp. Br J Dermatol. 1999;140:178-180.
- de Giorgi V, Massi D, Brunasso G, et al. Eruptive multiple blue nevi of the penis: a clinical dermoscopic pathologic case study. J Cutan Pathol. 2004;31:185-188.
- Zimmermann AH, Becker SA. Precursors of epidermal melanocytes in the negro fetus. In: Gordon M, ed. Pigment Cell Biology. New York, NY: Academic Press Inc; 1959:159-170.
- Leopold JG, Richards DB. The interrelationship of blue and common naevi. J Pathol Bacteriol. 1968;95:37-46.
- Zembowicz A, Phadke P. Blue nevi and variants: an update. Arch Pathol Lab Med. 2011;135:327-336.
A 19-year-old man presented to the dermatology clinic for evaluation of several new dark papules on the neck of 1 year's duration. He denied any personal or family history of skin cancer, cardiac abnormalities, or endocrine dysfunction. He also denied any recent changes in health or use of medication. A biopsy was performed at the site 2 years prior for a single blue nevus, but the patient denied history of other trauma or cutaneous eruptions localized to the area. Physical examination revealed numerous dark brown, blue, white, and flesh-colored papules and macules agminated into a well-circumscribed plaque on the left posterolateral neck without background hyperpigmentation. The total area of the plaque was roughly 3×4 cm. There was no associated edema or erythema. Cardiac murmur, thyromegaly, exophthalmos, neurologic deficits, regional lymphadenopathy, and similar skin findings on other areas of the body were not appreciated. Three scouting punch biopsies were taken of the various morphologies present.
Metastatic Vulvovaginal Crohn Disease in the Setting of Well-Controlled Intestinal Disease
The cutaneous manifestations of Crohn disease (CD) are varied, including pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). First described by Parks et al,1 MCD is defined as the occurrence of granulomatous lesions at a skin site distant from the gastrointestinal tract.1-20 Metastatic CD presents a diagnostic challenge because it is a rare component in the spectrum of inflammatory bowel disease complications, and many physicians are unaware of its existence. It may precede, coincide with, or develop after the diagnosis of intestinal disease.2-5 Vulvoperineal involvement is particularly problematic because a multitude of other, more likely disease processes are considered first. Typically it is initially diagnosed as a presumed infection prompting reflexive treatment with antivirals, antifungals, and antibiotics. Patients may experience symptoms for years prior to correct diagnosis and institution of proper therapy. A variety of clinical presentations have been described, including nonspecific pain and swelling, erythematous papules and plaques, and nonhealing ulcers. Skin biopsy characteristically confirms the diagnosis and reveals dermal noncaseating granulomas. Multiple oral and parenteral therapies are available, with surgical intervention reserved for resistant cases. We present a case of vulvovaginal MCD in the setting of well-controlled intestinal disease. We also provide a review of the literature regarding genital CD and emphasize the need to keep MCD in the differential of vulvoperineal pathology.
Case Report
A 29-year-old woman was referred to the dermatology clinic with vulvar pain, swelling, and pruritus of 14 months’ duration. Her medical history was remarkable for CD following a colectomy with colostomy. Prior therapies included methotrexate with infliximab for 5 years followed by a 2-year regimen with adalimumab, which induced remission of the intestinal disease.
The patient previously had taken a variety of topical and oral antimicrobials based on treatment from a primary care physician because fungal, bacterial, and viral infections initially were suspected; however, the vulvar disease persisted, and drug-induced immunosuppression was considered to be an underlying factor. Thus, adalimumab was discontinued. Despite elimination of the biologic, the vulvar disease progressed, which prompted referral to the dermatology clinic.
Physical examination revealed diffuse vulvar edema with overlying erythema and scale (Figure 1A). Upon closer inspection, scattered violaceous papules atop a backdrop of lichenification were evident, imparting a cobblestone appearance (Figure 1B). Additionally, a fissure was present on the gluteal cleft. Biopsy from the left labia majora demonstrated well-formed granulomas within a fibrotic reticular dermis (Figures 2A and 2B). The granulomas consisted of both mononucleated and multinucleated histiocytes, rimmed peripherally by lymphocytes and plasma cells (Figure 2C). Periodic acid–Schiff–diastase and acid-fast bacilli stains as well as polarizing microscopy were negative.
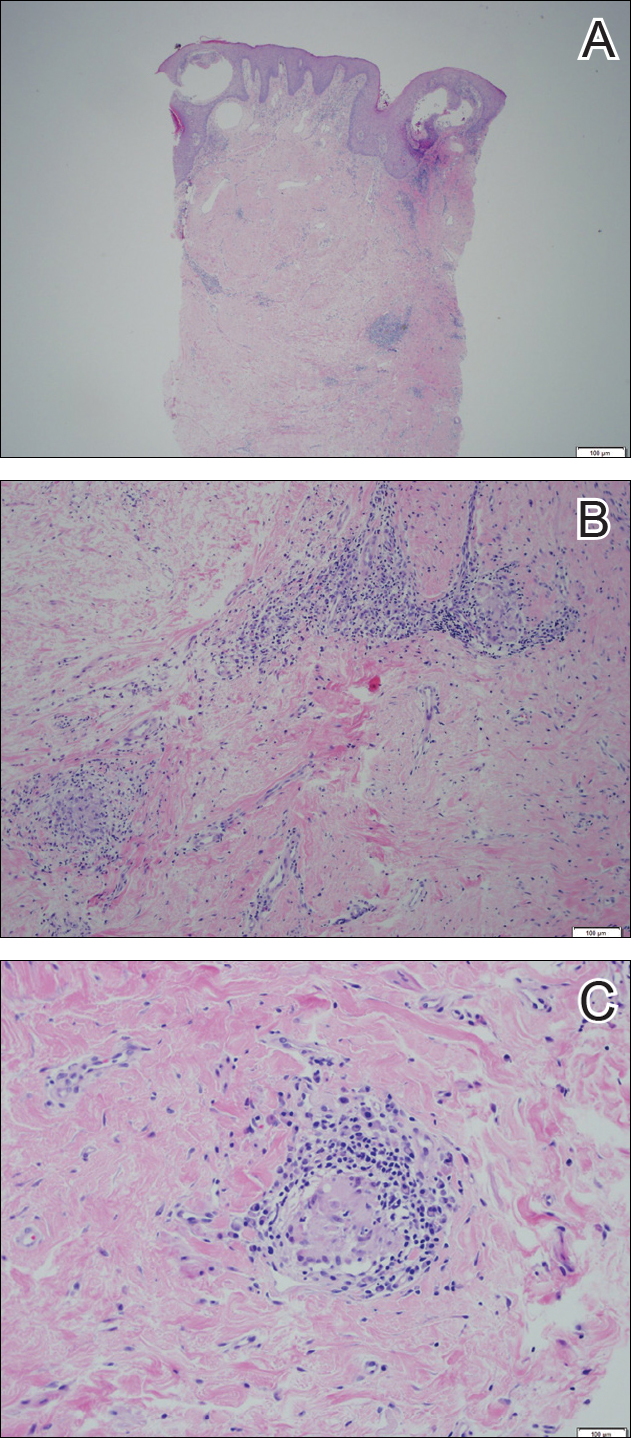
Given the patient’s history, a diagnosis of vulvoperineal MCD was rendered. The patient was started on oral metronidazole 250 mg 3 times daily with topical fluocinonide and tacrolimus. She responded well to this treatment regimen and was referred back to the gastroenterologist for management of the intestinal disease.
Comment
Crohn disease is an idiopathic chronic inflammatory condition that primarily affects the gastrointestinal tract, anywhere from the mouth to the anus. It is characterized by transmural inflammation and fissures that can extend beyond the muscularis propria.4,6 Extraintestinal manifestations are common.3
Cutaneous CD often presents as perianal, perifistular, or peristomal inflammation or ulceration.7 Other skin manifestations include pyoderma gangrenosum, erythema nodosum, erythema multiforme, epidermolysis bullosa acquisita, and palmar erythema.7 Metastatic CD involves skin noncontiguous with the gastrointestinal tract1-20 and may involve any portion of the cutis. Although rare, MCD is the typical etiology underlying vulvar CD.8
Approximately 20% of MCD patients have cutaneous lesions without a history of gastrointestinal disease. More than half of cases in adults and approximately two-thirds in children involve the genitalia. Although more common in adults, vulvar involvement has been reported in children as young as 6 years of age.2 Diagnosis is especially challenging when bowel symptoms are absent; those patients should be evaluated and followed for subsequent intestinal involvement.6
Clinically, symptoms may include general discomfort, pain, pruritus, and dyspareunia. Psychosocial and sexual dysfunction are prevalent and also should be addressed.9 Depending on the stage of the disease, physical examination may reveal erythema, edema, papules, pustules, nodules, condylomatous lesions, abscesses, fissures, fistulas, ulceration, acrochordons, and scarring.2-6,10,11
A host of infections (ie, mycobacterial, actinomycosis, deep fungal, sexually transmitted, schistosomiasis), inflammatory conditions (ie, sarcoid, hidradenitis suppurativa), foreign body reactions, Melkersson-Rosenthal syndrome, and sexual abuse should be included in the differential diagnosis.2,6,10-12 Once infection, sarcoid, and foreign body reaction have been ruled out, noncaseating granulomas in skin are highly suggestive of CD.7
Histopathologic findings of MCD reveal myriad morphological reaction patterns,5,13 including high-grade dysplasia and carcinoma of the vulva; therefore, it may be imprudent to withhold diagnosis based on the absence of the historically pathognomonic noncaseating granulomas.5
The etiopathogenesis of MCD remains an enigma. Dermatopathologic examinations consistently reveal a vascular injury syndrome,13 implicating a possible circulatory system contribution via deposition of immune complexes or antigens in skin.7 Bacterial infection has been implicated in the intestinal manifestations of CD; however, failure to detect microbial ribosomal RNA in MCD biopsies refutes theories of hematogenous spread of microbes.13 Another plausible explanation is that antibodies are formed to conserved microbial epitopes following loss of tolerance to gut flora, which results in an excessive immunologic response at distinct sites in susceptible individuals.13 A T-lymphocyte–mediated type IV hypersensitivity reaction also has been proposed via cross-reactivity of lymphocytes, with skin antigens precipitating extraintestinal granuloma formation and vascular injury.3 Clearly, further investigation is needed.
Magnetic resonanance imaging can identify the extent and anatomy of intestinal and pelvic disease and can assist in the diagnosis of vulvar CD.10,11,14 For these reasons, some experts propose that imaging should be instituted prior to therapy,12,15,16 especially when direct extension is suspected.17
Treatment is challenging and often involves collaboration among several specialties.12 Many treatment options exist because therapeutic responses vary and genital MCD is frequently recalcitrant to therapy.4 Medical therapy includes antibiotics such as metronidazole, corticosteroids (ie, topical, intralesional, systemic), and immune modulators (eg, azathioprine, 6-mercaptopurine, cyclosporine, methotrexate, mycophenolate mofetil, tumor necrosis factor α inhibitors).2,3,6,10,16,18 Thalidomide has been used for refractory cases.19 These treatments can be used alone or in combination. Patients should be monitored for side effects and informed that many treatment regimens may be required before a sustained response is achieved.4,16,18 Surgery is reserved for the most resistant cases. Extensive radical excision of the involved area is the best approach, as limited local excision often is followed by recurrence.20
Conclusion
Our case highlights that vulvar CD can develop in the setting of well-controlled intestinal disease. Vulvoperineal CD should be considered in the differential diagnosis of chronic vulvar pain, swelling, and pruritus, especially in cases resistant to standard therapies and regardless of whether or not gastrointestinal tract symptoms are present. Physicians must be cognizant that vulvar signs and symptoms may precede, coincide with, or follow the diagnosis of intestinal CD. Increased awareness of this entity may facilitate its early recognition and prompt more timely treatment among women with vulvar disease caused by MCD.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn’s disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulvar metastatic Crohn’s disease. Dig Dis Sci. 2009;54:1565-1571.
- Foo WC, Papalas JA, Robboy SJ, et al. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol. 2001;33:588-593.
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21:211-214.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol. 1981;5:689-695.
- Andreani SM, Ratnasingham K, Dang HH, et al. Crohn’s disease of the vulva. Int J Surg. 2010;8:2-5.
- Feller E, Ribaudo S, Jackson N. Gynecologic aspects of Crohn’s disease. Am Fam Physician. 2001;64:1725-1728.
- Corbett SL, Walsh CM, Spitzer RF, et al. Vulvar inflammation as the only clinical manifestation of Crohn disease in an 8-year-old girl [published online May 10, 2010]. Pediatrics. 2010;125:E1518-E1522.
- Tonolini M, Villa C, Campari A, et al. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41.
- Bhaduri S, Jenkinson S, Lewis F. Vulval Crohn’s disease—a multi-specialty approach. Int J STD AIDS. 2005;16:512-514.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn’s disease, its spectrum, and its pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn’s disease. Hum Pathol. 2003;34:1185-1192.
- Pai D, Dillman JR, Mahani MG, et al. MRI of vulvar Crohn disease. Pediatr Radiol. 2011;41:537-541.
- Madnani NA, Desai D, Gandhi N, et al. Isolated Crohn’s disease of the vulva. Indian J Dermatol Venereol Leprol. 2011;77:342-344.
- Makhija S, Trotter M, Wagner E, et al. Refractory Crohn’s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835-837.
- Fahmy N, Kalidindi M, Khan R. Direct colo-labial Crohn’s abscess mimicking bartholinitis. Am J Obstret Gynecol. 2010;30:741-742.
- Preston PW, Hudson N, Lewis FM. Treatment of vulval Crohn’s disease with infliximab. Clin Exp Derm. 2006;31:378-380.
- Kolivras A, De Maubeuge J, André J, et al. Thalidomide in refractory vulvar ulcerations associated with Crohn’s disease. Dermatology. 2003;206:381-383.
- Kao MS, Paulson JD, Askin FB. Crohn’s disease of the vulva. Obstet Gynecol. 1975;46:329-333.
The cutaneous manifestations of Crohn disease (CD) are varied, including pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). First described by Parks et al,1 MCD is defined as the occurrence of granulomatous lesions at a skin site distant from the gastrointestinal tract.1-20 Metastatic CD presents a diagnostic challenge because it is a rare component in the spectrum of inflammatory bowel disease complications, and many physicians are unaware of its existence. It may precede, coincide with, or develop after the diagnosis of intestinal disease.2-5 Vulvoperineal involvement is particularly problematic because a multitude of other, more likely disease processes are considered first. Typically it is initially diagnosed as a presumed infection prompting reflexive treatment with antivirals, antifungals, and antibiotics. Patients may experience symptoms for years prior to correct diagnosis and institution of proper therapy. A variety of clinical presentations have been described, including nonspecific pain and swelling, erythematous papules and plaques, and nonhealing ulcers. Skin biopsy characteristically confirms the diagnosis and reveals dermal noncaseating granulomas. Multiple oral and parenteral therapies are available, with surgical intervention reserved for resistant cases. We present a case of vulvovaginal MCD in the setting of well-controlled intestinal disease. We also provide a review of the literature regarding genital CD and emphasize the need to keep MCD in the differential of vulvoperineal pathology.
Case Report
A 29-year-old woman was referred to the dermatology clinic with vulvar pain, swelling, and pruritus of 14 months’ duration. Her medical history was remarkable for CD following a colectomy with colostomy. Prior therapies included methotrexate with infliximab for 5 years followed by a 2-year regimen with adalimumab, which induced remission of the intestinal disease.
The patient previously had taken a variety of topical and oral antimicrobials based on treatment from a primary care physician because fungal, bacterial, and viral infections initially were suspected; however, the vulvar disease persisted, and drug-induced immunosuppression was considered to be an underlying factor. Thus, adalimumab was discontinued. Despite elimination of the biologic, the vulvar disease progressed, which prompted referral to the dermatology clinic.
Physical examination revealed diffuse vulvar edema with overlying erythema and scale (Figure 1A). Upon closer inspection, scattered violaceous papules atop a backdrop of lichenification were evident, imparting a cobblestone appearance (Figure 1B). Additionally, a fissure was present on the gluteal cleft. Biopsy from the left labia majora demonstrated well-formed granulomas within a fibrotic reticular dermis (Figures 2A and 2B). The granulomas consisted of both mononucleated and multinucleated histiocytes, rimmed peripherally by lymphocytes and plasma cells (Figure 2C). Periodic acid–Schiff–diastase and acid-fast bacilli stains as well as polarizing microscopy were negative.


Given the patient’s history, a diagnosis of vulvoperineal MCD was rendered. The patient was started on oral metronidazole 250 mg 3 times daily with topical fluocinonide and tacrolimus. She responded well to this treatment regimen and was referred back to the gastroenterologist for management of the intestinal disease.
Comment
Crohn disease is an idiopathic chronic inflammatory condition that primarily affects the gastrointestinal tract, anywhere from the mouth to the anus. It is characterized by transmural inflammation and fissures that can extend beyond the muscularis propria.4,6 Extraintestinal manifestations are common.3
Cutaneous CD often presents as perianal, perifistular, or peristomal inflammation or ulceration.7 Other skin manifestations include pyoderma gangrenosum, erythema nodosum, erythema multiforme, epidermolysis bullosa acquisita, and palmar erythema.7 Metastatic CD involves skin noncontiguous with the gastrointestinal tract1-20 and may involve any portion of the cutis. Although rare, MCD is the typical etiology underlying vulvar CD.8
Approximately 20% of MCD patients have cutaneous lesions without a history of gastrointestinal disease. More than half of cases in adults and approximately two-thirds in children involve the genitalia. Although more common in adults, vulvar involvement has been reported in children as young as 6 years of age.2 Diagnosis is especially challenging when bowel symptoms are absent; those patients should be evaluated and followed for subsequent intestinal involvement.6
Clinically, symptoms may include general discomfort, pain, pruritus, and dyspareunia. Psychosocial and sexual dysfunction are prevalent and also should be addressed.9 Depending on the stage of the disease, physical examination may reveal erythema, edema, papules, pustules, nodules, condylomatous lesions, abscesses, fissures, fistulas, ulceration, acrochordons, and scarring.2-6,10,11
A host of infections (ie, mycobacterial, actinomycosis, deep fungal, sexually transmitted, schistosomiasis), inflammatory conditions (ie, sarcoid, hidradenitis suppurativa), foreign body reactions, Melkersson-Rosenthal syndrome, and sexual abuse should be included in the differential diagnosis.2,6,10-12 Once infection, sarcoid, and foreign body reaction have been ruled out, noncaseating granulomas in skin are highly suggestive of CD.7
Histopathologic findings of MCD reveal myriad morphological reaction patterns,5,13 including high-grade dysplasia and carcinoma of the vulva; therefore, it may be imprudent to withhold diagnosis based on the absence of the historically pathognomonic noncaseating granulomas.5
The etiopathogenesis of MCD remains an enigma. Dermatopathologic examinations consistently reveal a vascular injury syndrome,13 implicating a possible circulatory system contribution via deposition of immune complexes or antigens in skin.7 Bacterial infection has been implicated in the intestinal manifestations of CD; however, failure to detect microbial ribosomal RNA in MCD biopsies refutes theories of hematogenous spread of microbes.13 Another plausible explanation is that antibodies are formed to conserved microbial epitopes following loss of tolerance to gut flora, which results in an excessive immunologic response at distinct sites in susceptible individuals.13 A T-lymphocyte–mediated type IV hypersensitivity reaction also has been proposed via cross-reactivity of lymphocytes, with skin antigens precipitating extraintestinal granuloma formation and vascular injury.3 Clearly, further investigation is needed.
Magnetic resonanance imaging can identify the extent and anatomy of intestinal and pelvic disease and can assist in the diagnosis of vulvar CD.10,11,14 For these reasons, some experts propose that imaging should be instituted prior to therapy,12,15,16 especially when direct extension is suspected.17
Treatment is challenging and often involves collaboration among several specialties.12 Many treatment options exist because therapeutic responses vary and genital MCD is frequently recalcitrant to therapy.4 Medical therapy includes antibiotics such as metronidazole, corticosteroids (ie, topical, intralesional, systemic), and immune modulators (eg, azathioprine, 6-mercaptopurine, cyclosporine, methotrexate, mycophenolate mofetil, tumor necrosis factor α inhibitors).2,3,6,10,16,18 Thalidomide has been used for refractory cases.19 These treatments can be used alone or in combination. Patients should be monitored for side effects and informed that many treatment regimens may be required before a sustained response is achieved.4,16,18 Surgery is reserved for the most resistant cases. Extensive radical excision of the involved area is the best approach, as limited local excision often is followed by recurrence.20
Conclusion
Our case highlights that vulvar CD can develop in the setting of well-controlled intestinal disease. Vulvoperineal CD should be considered in the differential diagnosis of chronic vulvar pain, swelling, and pruritus, especially in cases resistant to standard therapies and regardless of whether or not gastrointestinal tract symptoms are present. Physicians must be cognizant that vulvar signs and symptoms may precede, coincide with, or follow the diagnosis of intestinal CD. Increased awareness of this entity may facilitate its early recognition and prompt more timely treatment among women with vulvar disease caused by MCD.
The cutaneous manifestations of Crohn disease (CD) are varied, including pyoderma gangrenosum, erythema nodosum, and metastatic CD (MCD). First described by Parks et al,1 MCD is defined as the occurrence of granulomatous lesions at a skin site distant from the gastrointestinal tract.1-20 Metastatic CD presents a diagnostic challenge because it is a rare component in the spectrum of inflammatory bowel disease complications, and many physicians are unaware of its existence. It may precede, coincide with, or develop after the diagnosis of intestinal disease.2-5 Vulvoperineal involvement is particularly problematic because a multitude of other, more likely disease processes are considered first. Typically it is initially diagnosed as a presumed infection prompting reflexive treatment with antivirals, antifungals, and antibiotics. Patients may experience symptoms for years prior to correct diagnosis and institution of proper therapy. A variety of clinical presentations have been described, including nonspecific pain and swelling, erythematous papules and plaques, and nonhealing ulcers. Skin biopsy characteristically confirms the diagnosis and reveals dermal noncaseating granulomas. Multiple oral and parenteral therapies are available, with surgical intervention reserved for resistant cases. We present a case of vulvovaginal MCD in the setting of well-controlled intestinal disease. We also provide a review of the literature regarding genital CD and emphasize the need to keep MCD in the differential of vulvoperineal pathology.
Case Report
A 29-year-old woman was referred to the dermatology clinic with vulvar pain, swelling, and pruritus of 14 months’ duration. Her medical history was remarkable for CD following a colectomy with colostomy. Prior therapies included methotrexate with infliximab for 5 years followed by a 2-year regimen with adalimumab, which induced remission of the intestinal disease.
The patient previously had taken a variety of topical and oral antimicrobials based on treatment from a primary care physician because fungal, bacterial, and viral infections initially were suspected; however, the vulvar disease persisted, and drug-induced immunosuppression was considered to be an underlying factor. Thus, adalimumab was discontinued. Despite elimination of the biologic, the vulvar disease progressed, which prompted referral to the dermatology clinic.
Physical examination revealed diffuse vulvar edema with overlying erythema and scale (Figure 1A). Upon closer inspection, scattered violaceous papules atop a backdrop of lichenification were evident, imparting a cobblestone appearance (Figure 1B). Additionally, a fissure was present on the gluteal cleft. Biopsy from the left labia majora demonstrated well-formed granulomas within a fibrotic reticular dermis (Figures 2A and 2B). The granulomas consisted of both mononucleated and multinucleated histiocytes, rimmed peripherally by lymphocytes and plasma cells (Figure 2C). Periodic acid–Schiff–diastase and acid-fast bacilli stains as well as polarizing microscopy were negative.


Given the patient’s history, a diagnosis of vulvoperineal MCD was rendered. The patient was started on oral metronidazole 250 mg 3 times daily with topical fluocinonide and tacrolimus. She responded well to this treatment regimen and was referred back to the gastroenterologist for management of the intestinal disease.
Comment
Crohn disease is an idiopathic chronic inflammatory condition that primarily affects the gastrointestinal tract, anywhere from the mouth to the anus. It is characterized by transmural inflammation and fissures that can extend beyond the muscularis propria.4,6 Extraintestinal manifestations are common.3
Cutaneous CD often presents as perianal, perifistular, or peristomal inflammation or ulceration.7 Other skin manifestations include pyoderma gangrenosum, erythema nodosum, erythema multiforme, epidermolysis bullosa acquisita, and palmar erythema.7 Metastatic CD involves skin noncontiguous with the gastrointestinal tract1-20 and may involve any portion of the cutis. Although rare, MCD is the typical etiology underlying vulvar CD.8
Approximately 20% of MCD patients have cutaneous lesions without a history of gastrointestinal disease. More than half of cases in adults and approximately two-thirds in children involve the genitalia. Although more common in adults, vulvar involvement has been reported in children as young as 6 years of age.2 Diagnosis is especially challenging when bowel symptoms are absent; those patients should be evaluated and followed for subsequent intestinal involvement.6
Clinically, symptoms may include general discomfort, pain, pruritus, and dyspareunia. Psychosocial and sexual dysfunction are prevalent and also should be addressed.9 Depending on the stage of the disease, physical examination may reveal erythema, edema, papules, pustules, nodules, condylomatous lesions, abscesses, fissures, fistulas, ulceration, acrochordons, and scarring.2-6,10,11
A host of infections (ie, mycobacterial, actinomycosis, deep fungal, sexually transmitted, schistosomiasis), inflammatory conditions (ie, sarcoid, hidradenitis suppurativa), foreign body reactions, Melkersson-Rosenthal syndrome, and sexual abuse should be included in the differential diagnosis.2,6,10-12 Once infection, sarcoid, and foreign body reaction have been ruled out, noncaseating granulomas in skin are highly suggestive of CD.7
Histopathologic findings of MCD reveal myriad morphological reaction patterns,5,13 including high-grade dysplasia and carcinoma of the vulva; therefore, it may be imprudent to withhold diagnosis based on the absence of the historically pathognomonic noncaseating granulomas.5
The etiopathogenesis of MCD remains an enigma. Dermatopathologic examinations consistently reveal a vascular injury syndrome,13 implicating a possible circulatory system contribution via deposition of immune complexes or antigens in skin.7 Bacterial infection has been implicated in the intestinal manifestations of CD; however, failure to detect microbial ribosomal RNA in MCD biopsies refutes theories of hematogenous spread of microbes.13 Another plausible explanation is that antibodies are formed to conserved microbial epitopes following loss of tolerance to gut flora, which results in an excessive immunologic response at distinct sites in susceptible individuals.13 A T-lymphocyte–mediated type IV hypersensitivity reaction also has been proposed via cross-reactivity of lymphocytes, with skin antigens precipitating extraintestinal granuloma formation and vascular injury.3 Clearly, further investigation is needed.
Magnetic resonanance imaging can identify the extent and anatomy of intestinal and pelvic disease and can assist in the diagnosis of vulvar CD.10,11,14 For these reasons, some experts propose that imaging should be instituted prior to therapy,12,15,16 especially when direct extension is suspected.17
Treatment is challenging and often involves collaboration among several specialties.12 Many treatment options exist because therapeutic responses vary and genital MCD is frequently recalcitrant to therapy.4 Medical therapy includes antibiotics such as metronidazole, corticosteroids (ie, topical, intralesional, systemic), and immune modulators (eg, azathioprine, 6-mercaptopurine, cyclosporine, methotrexate, mycophenolate mofetil, tumor necrosis factor α inhibitors).2,3,6,10,16,18 Thalidomide has been used for refractory cases.19 These treatments can be used alone or in combination. Patients should be monitored for side effects and informed that many treatment regimens may be required before a sustained response is achieved.4,16,18 Surgery is reserved for the most resistant cases. Extensive radical excision of the involved area is the best approach, as limited local excision often is followed by recurrence.20
Conclusion
Our case highlights that vulvar CD can develop in the setting of well-controlled intestinal disease. Vulvoperineal CD should be considered in the differential diagnosis of chronic vulvar pain, swelling, and pruritus, especially in cases resistant to standard therapies and regardless of whether or not gastrointestinal tract symptoms are present. Physicians must be cognizant that vulvar signs and symptoms may precede, coincide with, or follow the diagnosis of intestinal CD. Increased awareness of this entity may facilitate its early recognition and prompt more timely treatment among women with vulvar disease caused by MCD.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn’s disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulvar metastatic Crohn’s disease. Dig Dis Sci. 2009;54:1565-1571.
- Foo WC, Papalas JA, Robboy SJ, et al. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol. 2001;33:588-593.
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21:211-214.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol. 1981;5:689-695.
- Andreani SM, Ratnasingham K, Dang HH, et al. Crohn’s disease of the vulva. Int J Surg. 2010;8:2-5.
- Feller E, Ribaudo S, Jackson N. Gynecologic aspects of Crohn’s disease. Am Fam Physician. 2001;64:1725-1728.
- Corbett SL, Walsh CM, Spitzer RF, et al. Vulvar inflammation as the only clinical manifestation of Crohn disease in an 8-year-old girl [published online May 10, 2010]. Pediatrics. 2010;125:E1518-E1522.
- Tonolini M, Villa C, Campari A, et al. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41.
- Bhaduri S, Jenkinson S, Lewis F. Vulval Crohn’s disease—a multi-specialty approach. Int J STD AIDS. 2005;16:512-514.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn’s disease, its spectrum, and its pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn’s disease. Hum Pathol. 2003;34:1185-1192.
- Pai D, Dillman JR, Mahani MG, et al. MRI of vulvar Crohn disease. Pediatr Radiol. 2011;41:537-541.
- Madnani NA, Desai D, Gandhi N, et al. Isolated Crohn’s disease of the vulva. Indian J Dermatol Venereol Leprol. 2011;77:342-344.
- Makhija S, Trotter M, Wagner E, et al. Refractory Crohn’s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835-837.
- Fahmy N, Kalidindi M, Khan R. Direct colo-labial Crohn’s abscess mimicking bartholinitis. Am J Obstret Gynecol. 2010;30:741-742.
- Preston PW, Hudson N, Lewis FM. Treatment of vulval Crohn’s disease with infliximab. Clin Exp Derm. 2006;31:378-380.
- Kolivras A, De Maubeuge J, André J, et al. Thalidomide in refractory vulvar ulcerations associated with Crohn’s disease. Dermatology. 2003;206:381-383.
- Kao MS, Paulson JD, Askin FB. Crohn’s disease of the vulva. Obstet Gynecol. 1975;46:329-333.
- Parks AG, Morson BC, Pegum JS. Crohn’s disease with cutaneous involvement. Proc R Soc Med. 1965;58:241-242.
- Ploysangam T, Heubi JE, Eisen D, et al. Cutaneous Crohn’s disease in children. J Am Acad Dermatol. 1997;36:697-704.
- Palamaras I, El-Jabbour J, Pietropaolo N, et al. Metastatic Crohn’s disease: a review. J Eur Acad Dermatol Venereol. 2008;22:1033-1043.
- Leu S, Sun PK, Collyer J, et al. Clinical spectrum of vulvar metastatic Crohn’s disease. Dig Dis Sci. 2009;54:1565-1571.
- Foo WC, Papalas JA, Robboy SJ, et al. Vulvar manifestations of Crohn’s disease. Am J Dermatopathol. 2001;33:588-593.
- Urbanek M, Neill SM, McKee PH. Vulval Crohn’s disease: difficulties in diagnosis. Clin Exp Dermatol. 1996;21:211-214.
- Burgdorf W. Cutaneous manifestations of Crohn’s disease. J Am Acad Dermatol. 1981;5:689-695.
- Andreani SM, Ratnasingham K, Dang HH, et al. Crohn’s disease of the vulva. Int J Surg. 2010;8:2-5.
- Feller E, Ribaudo S, Jackson N. Gynecologic aspects of Crohn’s disease. Am Fam Physician. 2001;64:1725-1728.
- Corbett SL, Walsh CM, Spitzer RF, et al. Vulvar inflammation as the only clinical manifestation of Crohn disease in an 8-year-old girl [published online May 10, 2010]. Pediatrics. 2010;125:E1518-E1522.
- Tonolini M, Villa C, Campari A, et al. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41.
- Bhaduri S, Jenkinson S, Lewis F. Vulval Crohn’s disease—a multi-specialty approach. Int J STD AIDS. 2005;16:512-514.
- Crowson AN, Nuovo GJ, Mihm MC Jr, et al. Cutaneous manifestations of Crohn’s disease, its spectrum, and its pathogenesis: intracellular consensus bacterial 16S rRNA is associated with the gastrointestinal but not the cutaneous manifestations of Crohn’s disease. Hum Pathol. 2003;34:1185-1192.
- Pai D, Dillman JR, Mahani MG, et al. MRI of vulvar Crohn disease. Pediatr Radiol. 2011;41:537-541.
- Madnani NA, Desai D, Gandhi N, et al. Isolated Crohn’s disease of the vulva. Indian J Dermatol Venereol Leprol. 2011;77:342-344.
- Makhija S, Trotter M, Wagner E, et al. Refractory Crohn’s disease of the vulva treated with infliximab: a case report. Can J Gastroenterol. 2007;21:835-837.
- Fahmy N, Kalidindi M, Khan R. Direct colo-labial Crohn’s abscess mimicking bartholinitis. Am J Obstret Gynecol. 2010;30:741-742.
- Preston PW, Hudson N, Lewis FM. Treatment of vulval Crohn’s disease with infliximab. Clin Exp Derm. 2006;31:378-380.
- Kolivras A, De Maubeuge J, André J, et al. Thalidomide in refractory vulvar ulcerations associated with Crohn’s disease. Dermatology. 2003;206:381-383.
- Kao MS, Paulson JD, Askin FB. Crohn’s disease of the vulva. Obstet Gynecol. 1975;46:329-333.