User login
Granuloma Faciale in Woman With Levamisole-Induced Vasculitis
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
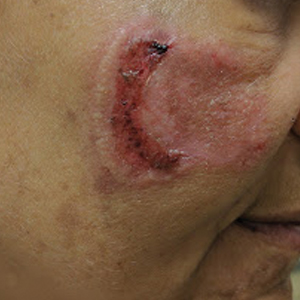
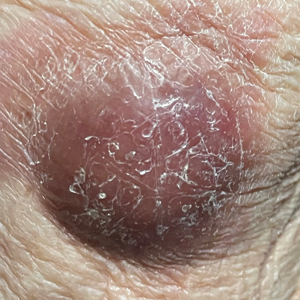
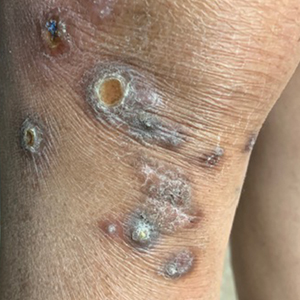
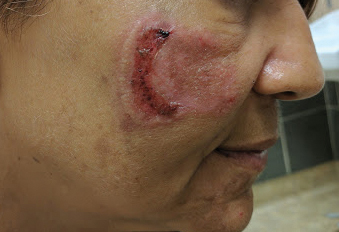
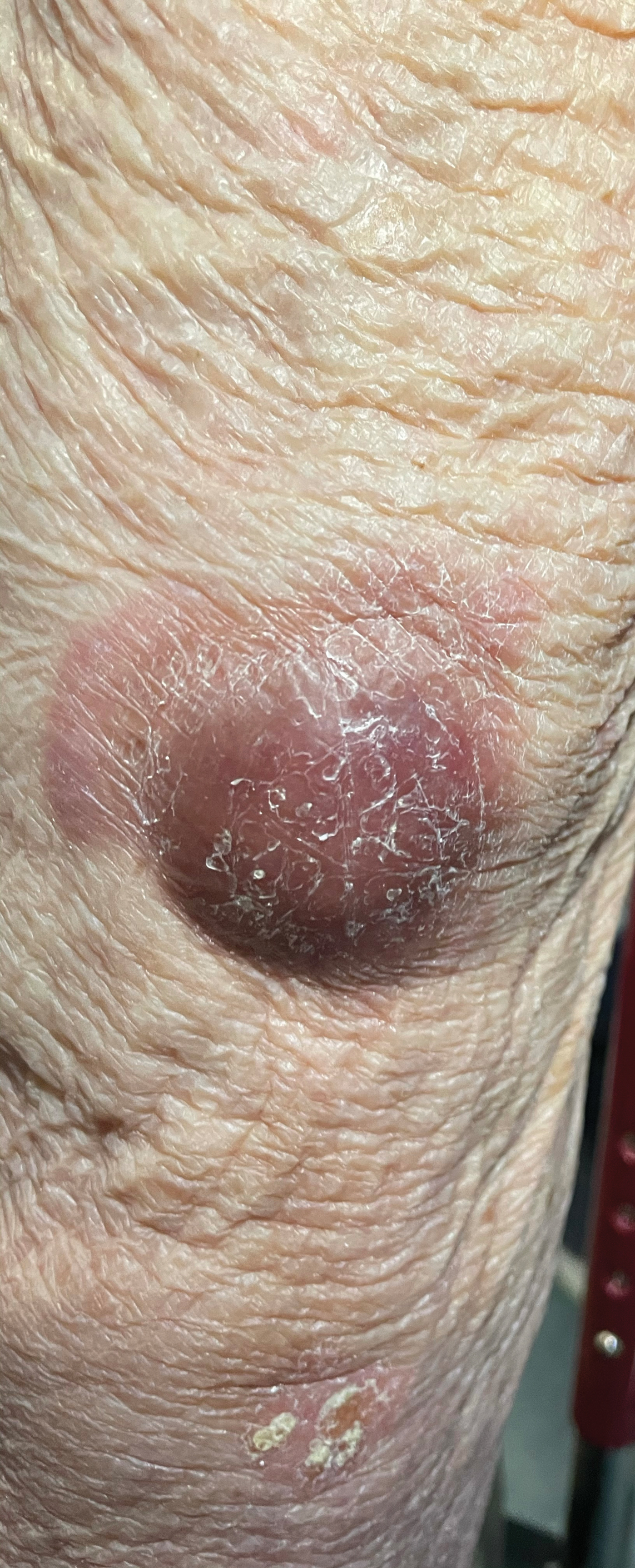
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.
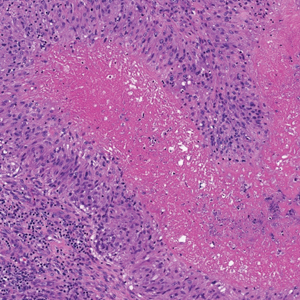
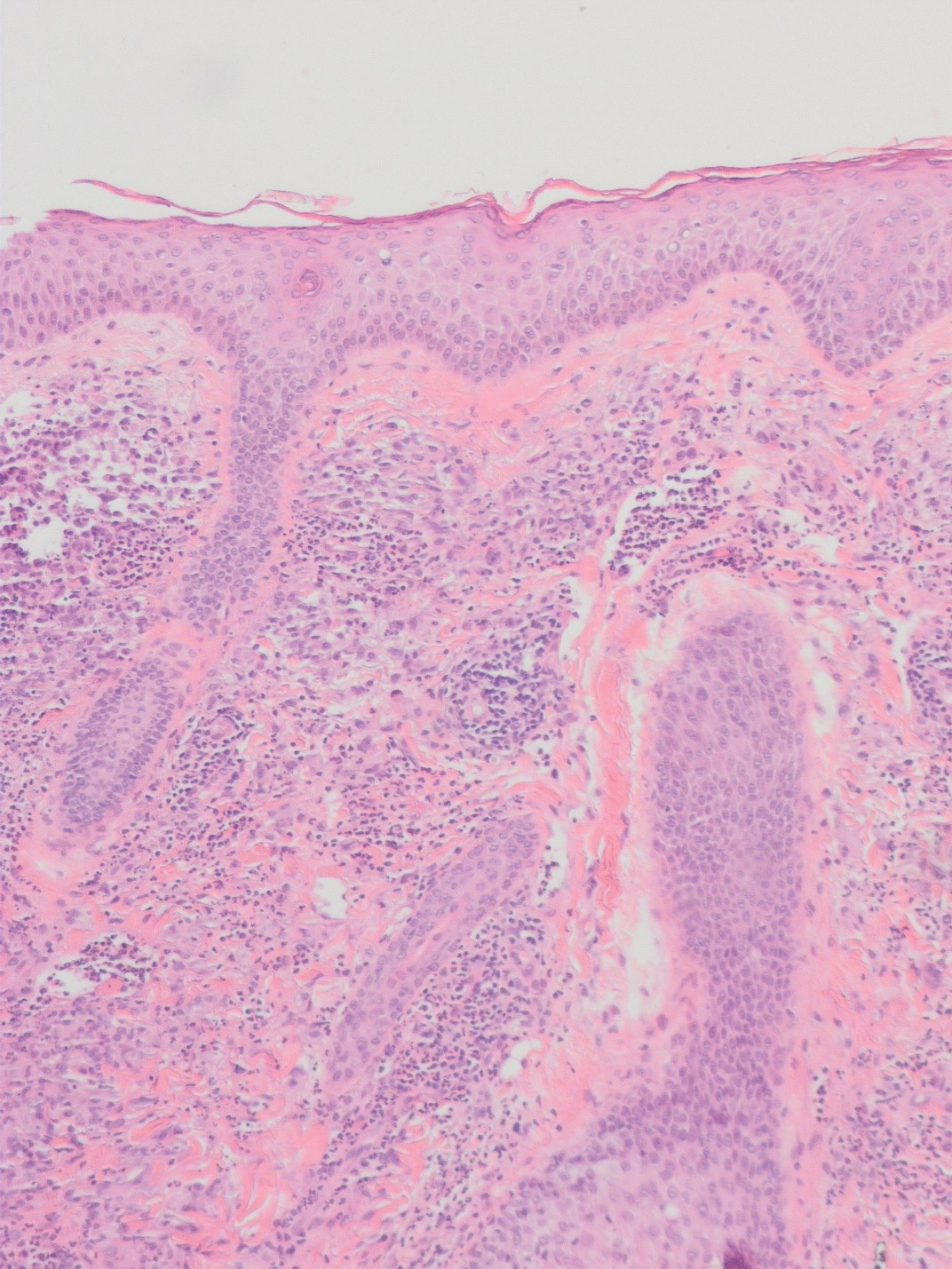
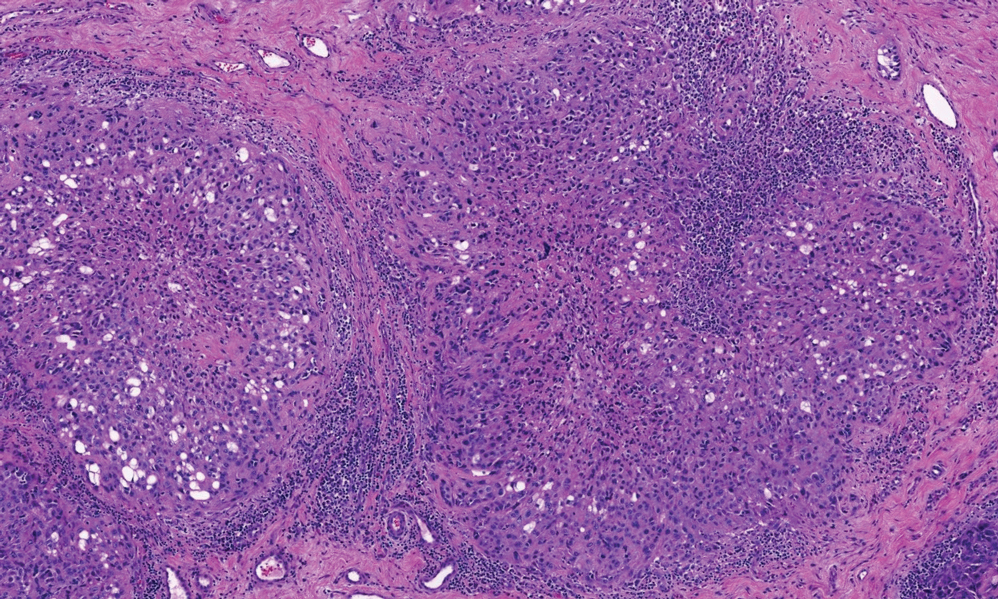
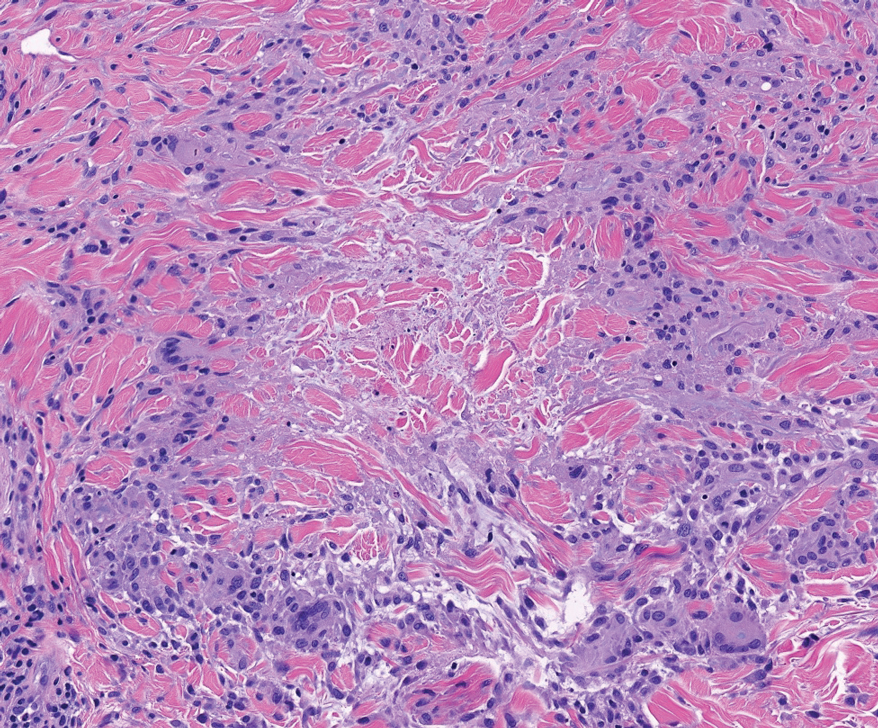
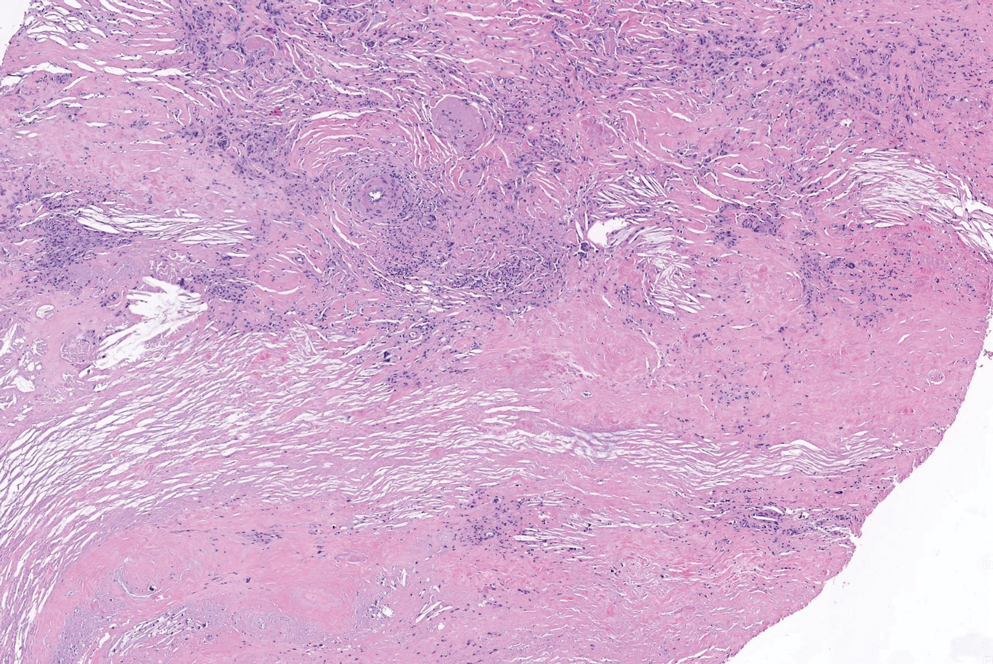
There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.
Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.

There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.

Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.

There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.

Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
Practice Points
- Granuloma faciale is a benign dermal process presenting with a red-brown plaque on the face of adults that typically is not ulcerated unless physically manipulated.
- Skin biopsy often is required for correct diagnosis.
- Granuloma faciale does not resolve spontaneously and tends to be chronic.
Violaceous Nodules on the Lower Leg
The Diagnosis: Cutaneous B-cell Lymphoma
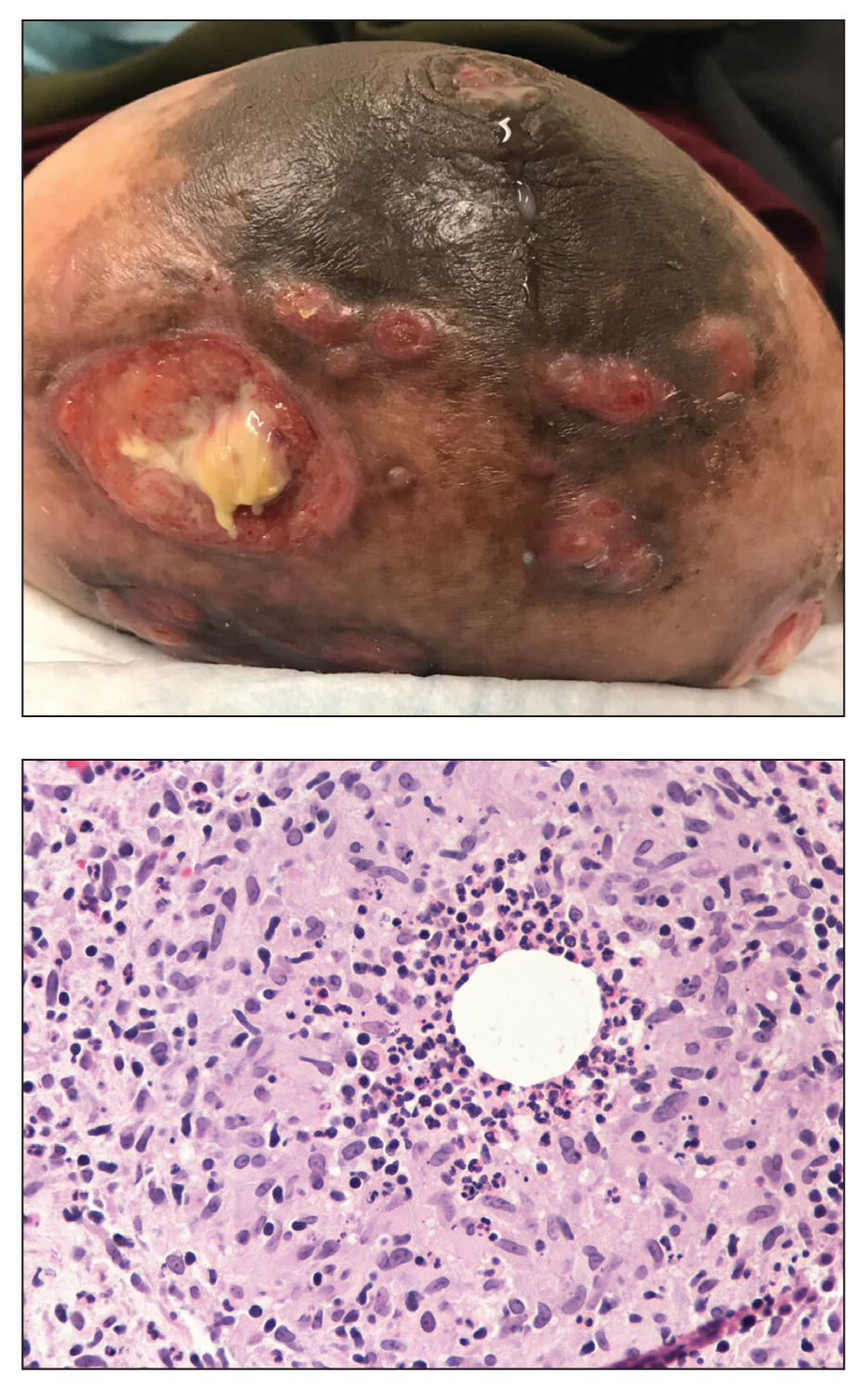
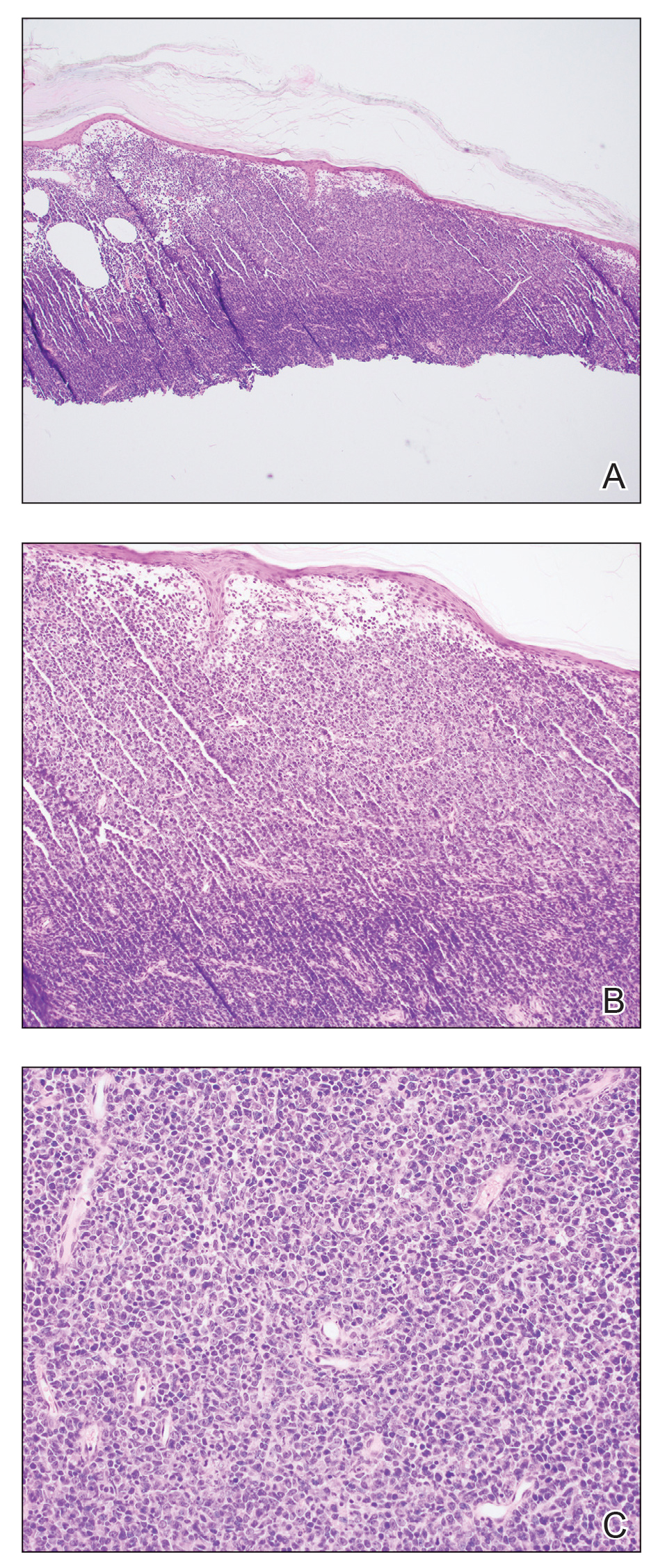
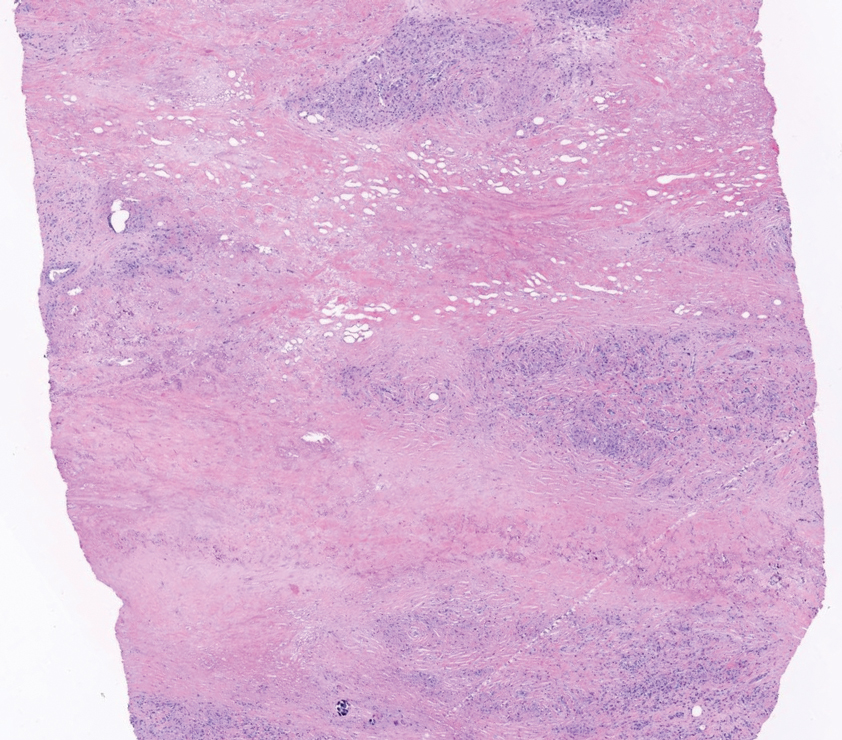
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.
Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
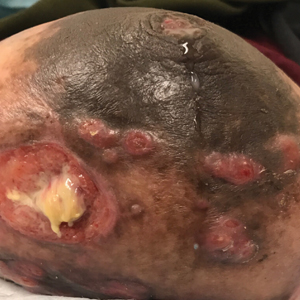
A 79-year-old man presented to the dermatology clinic with 4 enlarging, asymptomatic, violaceous, desquamating nodules on the left pretibial region and calf of 3 months’ duration. He denied any constitutional symptoms such as night sweats or weight loss. His medical history included a malignant melanoma on the left ear that was excised 5 years prior. He also had a history of peripheral edema, hypertension, and rheumatoid arthritis, as well as a 50-pack-year history of smoking. Physical examination revealed 2 large nodules measuring 3.0×3.0 cm each and 2 smaller nodules measuring 1.0×1.0 cm each. There was no appreciable lymphadenopathy.
PLA testing brings nuance to the diagnosis of early-stage melanoma
BOSTON – Although
One such test, the Pigmented Lesional Assay (PLA) uses adhesive patches applied to lesions of concern at the bedside to extract RNA from the stratum corneum to help determine the risk for melanoma.
At the annual meeting of the American Academy of Dermatology, Caroline C. Kim, MD, director of melanoma and pigmented lesion clinics at Newton Wellesley Dermatology, Wellesley Hills, Mass., and Tufts Medical Center, Boston, spoke about the PLA, which uses genetic expression profiling to measure the expression level of specific genes that are associated with melanoma: PRAME (preferentially expressed antigen in melanoma) and LINC00518 (LINC). There are four possible results of the test: Aberrant expression of both LINC and PRAME (high risk); aberrant expression of a single gene (moderate risk); aberrant expression of neither gene (low risk); or inconclusive.
Validation data have shown a sensitivity of 91% and a specificity of 69% for the PLA, with a 99% negative predictive value; so a lesion that tested negative by PLA has a less than 1% chance of being melanoma. In addition, a study published in 2020 found that the addition of TERT (telomerase reverse transcriptase) mutation analyses increased the sensitivity of the PLA to 97%.
While the high negative predictive value is helpful to consider in clinical scenarios to rule-out melanoma for borderline lesions, one must consider the positive predictive value as well and how this may impact clinical care, Dr. Kim said. In a study examining outcomes of 381 lesions, 51 were PLA positive (single or double) and were biopsied, of which 19 (37%) revealed a melanoma diagnosis. In a large U.S. registry study of 3,418 lesions, 324 lesions that were PLA double positive were biopsied, with 18.7% revealing a melanoma diagnosis.
“No test is perfect, and this applies to PLA, even if you get a double-positive or double-negative test result,” Dr. Kim said. “You want to make sure that patients are aware of false positives and negatives. However, PLA could be an additional piece of data to inform your decision to proceed with biopsy on select borderline suspicious pigmented lesions. More studies are needed to better understand the approach to single- and double-positive PLA results.”
The PLA kit contains adhesive patches and supplies and a FedEx envelope for return to DermTech, the test’s manufacturer, for processing. The patches can be applied to lesions at least 4 mm in diameter; multiple kits are recommended for those greater than 16 mm in diameter. The test is not validated for lesions located on mucous membranes, palms, soles, nails, or on ulcerated or bleeding lesions, nor for those that have been previously biopsied. It is also not validated for use in pediatric patients or in those with skin types IV or higher. Results are returned in 2-3 days. If insurance does not cover the test, the cost to the patient is approximately $50 per lesion or a maximum of $150, according to Dr. Kim.
Use in clinical practice
In Dr. Kim’s clinical experience, the PLA can be considered for suspicious pigmented lesions on cosmetically sensitive areas and for suspicious lesions in areas difficult to biopsy or excise. For example, she discussed the case of a 72-year-old woman with a family history of melanoma, who presented to her clinic with a longstanding pigmented lesion on her right upper and lower eyelids that had previously been treated with laser. She had undergone multiple prior biopsies over 12 years, which caused mild to moderate atypical melanocytic proliferation. The PLA result was double negative for PRAME and LINC in her upper and lower eyelid, “which provided reassurance to the patient,” Dr. Kim said. The patient continues to be followed closely for any clinical changes.
Another patient, a 67-year-old woman, was referred to Dr. Kim from out of state for a teledermatology visit early in the COVID-19 pandemic. The patient had a lesion on her right calf that was hard, raised, and pink, did not resemble other lesions on her body, and had been present for a few weeks. “Her husband had recently passed away from brain cancer and she was very concerned about melanoma,” Dr. Kim recalled. “She lived alone, and the adult son was with her during the teledermatology call to assist. The patient asked about the PLA test, and given her difficulty going to a medical office at the time, we agreed to help her with this test.” The patient and her son arranged another teledermatology visit with Dr. Kim after receiving the kit in the mail from DermTech, and Dr. Kim coached them on how to properly administer the test. The results came back as PRAME negative and LINC positive. A biopsy with a local provider was recommended and the pathology results showed an inflamed seborrheic keratosis.
“This case exemplifies a false-positive result. We should be sure to make patients aware of this possibility,” Dr. Kim said.
Incorporating PLA into clinical practice requires certain workflow considerations, with paperwork to fill out in addition to performing the adhesive test, collection of insurance information, mailing the kit via FedEx, retrieving the results, and following up with the patient, said Dr. Kim. “For select borderline pigmented lesions, I discuss the rationale of the test with patients, the possibility of false-positive and false-negative results and the need to return for a biopsy when there is positive result. Clinical follow-up is recommended for negative results. There is also the possibility of charge to the patient if the test is not covered by their insurance.”
Skin biopsy still the gold standard
Despite the availability of the PLA as an assessment tool, Dr. Kim emphasized that skin biopsy remains the gold standard for diagnosing melanoma. “Future prospective randomized clinical trials are needed to examine the role of genetic expression profiling in staging and managing patients,” she said.
In 2019, she and her colleagues surveyed 42 pigmented lesion experts in the United States about why they ordered one of three molecular tests on the market or not and how results affected patient treatment. The proportion of clinicians who ordered the tests ranged from 21% to 29%. The top 2 reasons respondents chose for not ordering the PLA test specifically were: “Feel that further validation studies are necessary” (20%) and “do not feel it would be useful in my practice” (18%).
Results of a larger follow-up survey on usage patterns of PLA of both pigmented lesion experts and general clinicians on this topic are expected to be published shortly.
Dr. Kim reported having no disclosures related to her presentation.
BOSTON – Although
One such test, the Pigmented Lesional Assay (PLA) uses adhesive patches applied to lesions of concern at the bedside to extract RNA from the stratum corneum to help determine the risk for melanoma.
At the annual meeting of the American Academy of Dermatology, Caroline C. Kim, MD, director of melanoma and pigmented lesion clinics at Newton Wellesley Dermatology, Wellesley Hills, Mass., and Tufts Medical Center, Boston, spoke about the PLA, which uses genetic expression profiling to measure the expression level of specific genes that are associated with melanoma: PRAME (preferentially expressed antigen in melanoma) and LINC00518 (LINC). There are four possible results of the test: Aberrant expression of both LINC and PRAME (high risk); aberrant expression of a single gene (moderate risk); aberrant expression of neither gene (low risk); or inconclusive.
Validation data have shown a sensitivity of 91% and a specificity of 69% for the PLA, with a 99% negative predictive value; so a lesion that tested negative by PLA has a less than 1% chance of being melanoma. In addition, a study published in 2020 found that the addition of TERT (telomerase reverse transcriptase) mutation analyses increased the sensitivity of the PLA to 97%.
While the high negative predictive value is helpful to consider in clinical scenarios to rule-out melanoma for borderline lesions, one must consider the positive predictive value as well and how this may impact clinical care, Dr. Kim said. In a study examining outcomes of 381 lesions, 51 were PLA positive (single or double) and were biopsied, of which 19 (37%) revealed a melanoma diagnosis. In a large U.S. registry study of 3,418 lesions, 324 lesions that were PLA double positive were biopsied, with 18.7% revealing a melanoma diagnosis.
“No test is perfect, and this applies to PLA, even if you get a double-positive or double-negative test result,” Dr. Kim said. “You want to make sure that patients are aware of false positives and negatives. However, PLA could be an additional piece of data to inform your decision to proceed with biopsy on select borderline suspicious pigmented lesions. More studies are needed to better understand the approach to single- and double-positive PLA results.”
The PLA kit contains adhesive patches and supplies and a FedEx envelope for return to DermTech, the test’s manufacturer, for processing. The patches can be applied to lesions at least 4 mm in diameter; multiple kits are recommended for those greater than 16 mm in diameter. The test is not validated for lesions located on mucous membranes, palms, soles, nails, or on ulcerated or bleeding lesions, nor for those that have been previously biopsied. It is also not validated for use in pediatric patients or in those with skin types IV or higher. Results are returned in 2-3 days. If insurance does not cover the test, the cost to the patient is approximately $50 per lesion or a maximum of $150, according to Dr. Kim.
Use in clinical practice
In Dr. Kim’s clinical experience, the PLA can be considered for suspicious pigmented lesions on cosmetically sensitive areas and for suspicious lesions in areas difficult to biopsy or excise. For example, she discussed the case of a 72-year-old woman with a family history of melanoma, who presented to her clinic with a longstanding pigmented lesion on her right upper and lower eyelids that had previously been treated with laser. She had undergone multiple prior biopsies over 12 years, which caused mild to moderate atypical melanocytic proliferation. The PLA result was double negative for PRAME and LINC in her upper and lower eyelid, “which provided reassurance to the patient,” Dr. Kim said. The patient continues to be followed closely for any clinical changes.
Another patient, a 67-year-old woman, was referred to Dr. Kim from out of state for a teledermatology visit early in the COVID-19 pandemic. The patient had a lesion on her right calf that was hard, raised, and pink, did not resemble other lesions on her body, and had been present for a few weeks. “Her husband had recently passed away from brain cancer and she was very concerned about melanoma,” Dr. Kim recalled. “She lived alone, and the adult son was with her during the teledermatology call to assist. The patient asked about the PLA test, and given her difficulty going to a medical office at the time, we agreed to help her with this test.” The patient and her son arranged another teledermatology visit with Dr. Kim after receiving the kit in the mail from DermTech, and Dr. Kim coached them on how to properly administer the test. The results came back as PRAME negative and LINC positive. A biopsy with a local provider was recommended and the pathology results showed an inflamed seborrheic keratosis.
“This case exemplifies a false-positive result. We should be sure to make patients aware of this possibility,” Dr. Kim said.
Incorporating PLA into clinical practice requires certain workflow considerations, with paperwork to fill out in addition to performing the adhesive test, collection of insurance information, mailing the kit via FedEx, retrieving the results, and following up with the patient, said Dr. Kim. “For select borderline pigmented lesions, I discuss the rationale of the test with patients, the possibility of false-positive and false-negative results and the need to return for a biopsy when there is positive result. Clinical follow-up is recommended for negative results. There is also the possibility of charge to the patient if the test is not covered by their insurance.”
Skin biopsy still the gold standard
Despite the availability of the PLA as an assessment tool, Dr. Kim emphasized that skin biopsy remains the gold standard for diagnosing melanoma. “Future prospective randomized clinical trials are needed to examine the role of genetic expression profiling in staging and managing patients,” she said.
In 2019, she and her colleagues surveyed 42 pigmented lesion experts in the United States about why they ordered one of three molecular tests on the market or not and how results affected patient treatment. The proportion of clinicians who ordered the tests ranged from 21% to 29%. The top 2 reasons respondents chose for not ordering the PLA test specifically were: “Feel that further validation studies are necessary” (20%) and “do not feel it would be useful in my practice” (18%).
Results of a larger follow-up survey on usage patterns of PLA of both pigmented lesion experts and general clinicians on this topic are expected to be published shortly.
Dr. Kim reported having no disclosures related to her presentation.
BOSTON – Although
One such test, the Pigmented Lesional Assay (PLA) uses adhesive patches applied to lesions of concern at the bedside to extract RNA from the stratum corneum to help determine the risk for melanoma.
At the annual meeting of the American Academy of Dermatology, Caroline C. Kim, MD, director of melanoma and pigmented lesion clinics at Newton Wellesley Dermatology, Wellesley Hills, Mass., and Tufts Medical Center, Boston, spoke about the PLA, which uses genetic expression profiling to measure the expression level of specific genes that are associated with melanoma: PRAME (preferentially expressed antigen in melanoma) and LINC00518 (LINC). There are four possible results of the test: Aberrant expression of both LINC and PRAME (high risk); aberrant expression of a single gene (moderate risk); aberrant expression of neither gene (low risk); or inconclusive.
Validation data have shown a sensitivity of 91% and a specificity of 69% for the PLA, with a 99% negative predictive value; so a lesion that tested negative by PLA has a less than 1% chance of being melanoma. In addition, a study published in 2020 found that the addition of TERT (telomerase reverse transcriptase) mutation analyses increased the sensitivity of the PLA to 97%.
While the high negative predictive value is helpful to consider in clinical scenarios to rule-out melanoma for borderline lesions, one must consider the positive predictive value as well and how this may impact clinical care, Dr. Kim said. In a study examining outcomes of 381 lesions, 51 were PLA positive (single or double) and were biopsied, of which 19 (37%) revealed a melanoma diagnosis. In a large U.S. registry study of 3,418 lesions, 324 lesions that were PLA double positive were biopsied, with 18.7% revealing a melanoma diagnosis.
“No test is perfect, and this applies to PLA, even if you get a double-positive or double-negative test result,” Dr. Kim said. “You want to make sure that patients are aware of false positives and negatives. However, PLA could be an additional piece of data to inform your decision to proceed with biopsy on select borderline suspicious pigmented lesions. More studies are needed to better understand the approach to single- and double-positive PLA results.”
The PLA kit contains adhesive patches and supplies and a FedEx envelope for return to DermTech, the test’s manufacturer, for processing. The patches can be applied to lesions at least 4 mm in diameter; multiple kits are recommended for those greater than 16 mm in diameter. The test is not validated for lesions located on mucous membranes, palms, soles, nails, or on ulcerated or bleeding lesions, nor for those that have been previously biopsied. It is also not validated for use in pediatric patients or in those with skin types IV or higher. Results are returned in 2-3 days. If insurance does not cover the test, the cost to the patient is approximately $50 per lesion or a maximum of $150, according to Dr. Kim.
Use in clinical practice
In Dr. Kim’s clinical experience, the PLA can be considered for suspicious pigmented lesions on cosmetically sensitive areas and for suspicious lesions in areas difficult to biopsy or excise. For example, she discussed the case of a 72-year-old woman with a family history of melanoma, who presented to her clinic with a longstanding pigmented lesion on her right upper and lower eyelids that had previously been treated with laser. She had undergone multiple prior biopsies over 12 years, which caused mild to moderate atypical melanocytic proliferation. The PLA result was double negative for PRAME and LINC in her upper and lower eyelid, “which provided reassurance to the patient,” Dr. Kim said. The patient continues to be followed closely for any clinical changes.
Another patient, a 67-year-old woman, was referred to Dr. Kim from out of state for a teledermatology visit early in the COVID-19 pandemic. The patient had a lesion on her right calf that was hard, raised, and pink, did not resemble other lesions on her body, and had been present for a few weeks. “Her husband had recently passed away from brain cancer and she was very concerned about melanoma,” Dr. Kim recalled. “She lived alone, and the adult son was with her during the teledermatology call to assist. The patient asked about the PLA test, and given her difficulty going to a medical office at the time, we agreed to help her with this test.” The patient and her son arranged another teledermatology visit with Dr. Kim after receiving the kit in the mail from DermTech, and Dr. Kim coached them on how to properly administer the test. The results came back as PRAME negative and LINC positive. A biopsy with a local provider was recommended and the pathology results showed an inflamed seborrheic keratosis.
“This case exemplifies a false-positive result. We should be sure to make patients aware of this possibility,” Dr. Kim said.
Incorporating PLA into clinical practice requires certain workflow considerations, with paperwork to fill out in addition to performing the adhesive test, collection of insurance information, mailing the kit via FedEx, retrieving the results, and following up with the patient, said Dr. Kim. “For select borderline pigmented lesions, I discuss the rationale of the test with patients, the possibility of false-positive and false-negative results and the need to return for a biopsy when there is positive result. Clinical follow-up is recommended for negative results. There is also the possibility of charge to the patient if the test is not covered by their insurance.”
Skin biopsy still the gold standard
Despite the availability of the PLA as an assessment tool, Dr. Kim emphasized that skin biopsy remains the gold standard for diagnosing melanoma. “Future prospective randomized clinical trials are needed to examine the role of genetic expression profiling in staging and managing patients,” she said.
In 2019, she and her colleagues surveyed 42 pigmented lesion experts in the United States about why they ordered one of three molecular tests on the market or not and how results affected patient treatment. The proportion of clinicians who ordered the tests ranged from 21% to 29%. The top 2 reasons respondents chose for not ordering the PLA test specifically were: “Feel that further validation studies are necessary” (20%) and “do not feel it would be useful in my practice” (18%).
Results of a larger follow-up survey on usage patterns of PLA of both pigmented lesion experts and general clinicians on this topic are expected to be published shortly.
Dr. Kim reported having no disclosures related to her presentation.
AT AAD 22
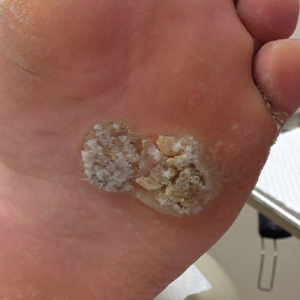
Ulcerating Nodule on the Foot
The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.
Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11
Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13
Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.

Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11

Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13

Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).

The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.

Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11

Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13

Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).

- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
A 59-year-old woman with a history of joint pain presented with a foot nodule that developed over the course of 2 years. Physical examination revealed a firm, mobile, mildly tender, 3-cm, deep red nodule on the dorsal aspect of the left foot (top [inset]) with an overlying central epidermal defect and thick keratinaceous debris. The remainder of the physical examination was unremarkable. Empiric treatments with oral antibiotics and intralesional corticosteroids were unsuccessful. Incisional biopsy was performed for histologic review, and tissue culture studies were negative.
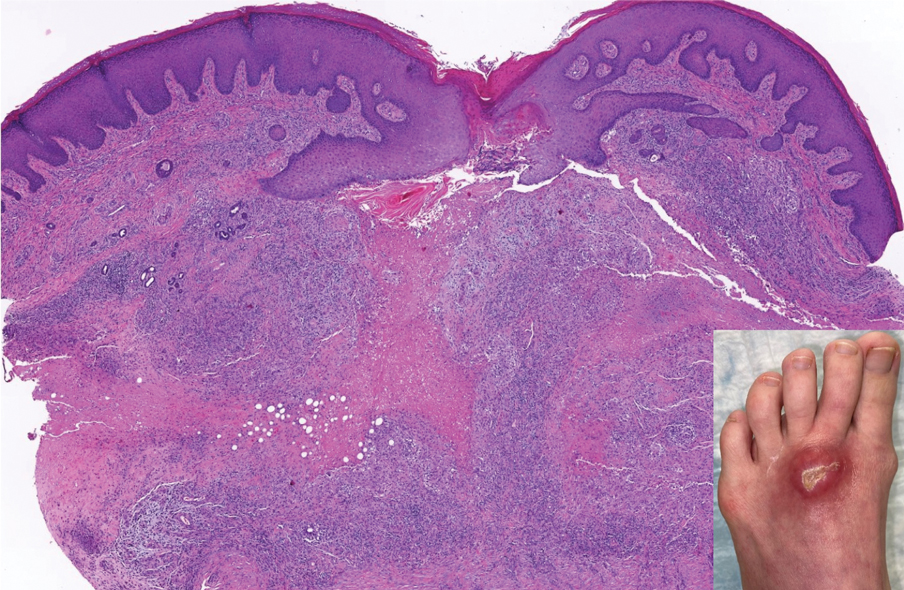
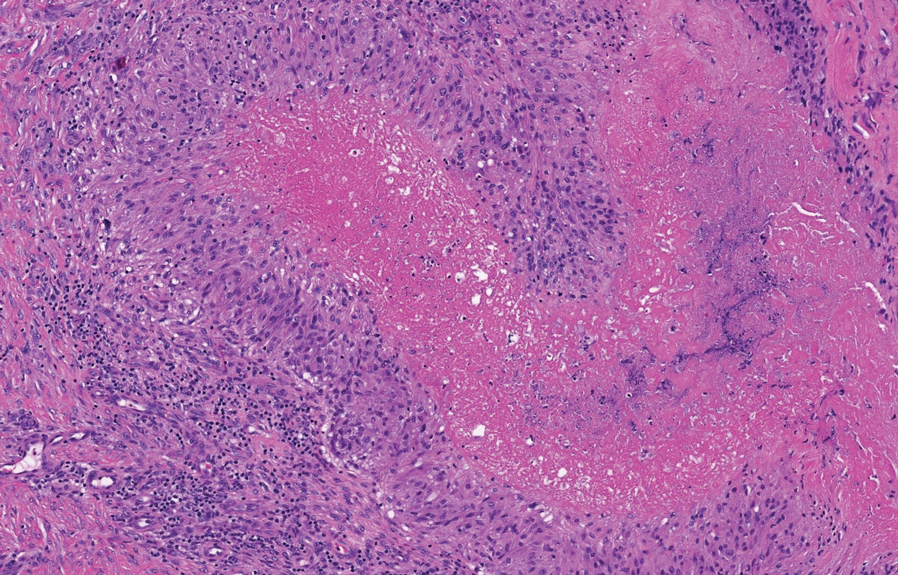
Excoriated Papules and Plaques on the Arms and Legs
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) may be either acquired or inherited. It is 1 of 4 classical forms of transepithelial elimination, which also includes elastosis perforans serpiginosa (EPS) as well as perforating folliculitis and Kyrle disease. These 4 forms of transepithelial elimination share characteristics of the elimination of altered dermal components through the epidermis.1 The acquired subtype of RPC frequently occurs in patients with diabetes mellitus and end-stage renal disease,2 both present in our patient.
Clinical presentation typically shows pruritic hyperkeratotic papules with a central crater filled with crust that frequently are distributed on the extensor surfaces of the extremities, often in a linear pattern.3 The perforating papules and nodules occasionally may involve the trunk and face.4 Histopathologic examination is characterized by the elimination of altered collagen through the epidermis. Established lesions may show a cup-shaped depression of the epidermis filled with a keratin plug. The underlying dermis will show vertically oriented basophilic collagen fibers with focal extrusion through the epidermis, and elastic fibers will be absent.5 The exact pathophysiology of this disease is unknown, but it may represent a cutaneous response to superficial trauma caused by intense scratching.6
Standard treatment protocols are not well established for this condition, but some evidence shows that a combination of treatments can help ameliorate symptoms, even if they are not curative.7 Treatments without strong evidence have included a wide range of topical, systemic, and other therapies. Case series and anecdotal reports have used retinoids, corticosteroids, menthol, antibiotics, allopurinol antihistamines, cryotherapy, and lasers.8 One case was treated with a combination of narrowband UVB phototherapy and doxycycline with resolution in approximately 6 weeks.9 Other cases have been cured using triple therapy with antihistamines, topical or injected steroids, and emollients or oral antibiotics.7 Evidence shows that there may be benefit to combining multiple different treatment types that target pruritus, inflammation, and collagen damage.7,9 This disease usually cannot be cured, but it may be improved by the available treatments.
The differential diagnosis includes delusional parasitosis, EPS, perforating folliculitis, and prurigo nodularis. Delusional parasitosis also can be characterized by excoriated plaques and a sensation of parasites infesting the skin, as our patient described.10 However, it can be differentiated from RPC by the fact that it is a diagnosis of exclusion, which would not have the histopathologic findings of the elimination of collagen from the epidermis, as was demonstrated in our patient.11 Elastosis perforans serpiginosa is in the same family of perforating diseases as RPC; however, EPS typically appears in children or young adults and often is associated with other genetic disorders. Physical examination in a patient with EPS would reveal keratotic papules in a serpiginous pattern, whereas our patient had discrete lesions without any serpiginous pattern. The histopathologic appearance of EPS would reveal plugs of elastic fibers rather than collagen fibers, as was demonstrated in our patient.8 Perforating folliculitis, while also demonstrating transepithelial elimination similar to RPC, would appear as erythematous follicular papules with small central keratotic plugs and histopathologic findings of a widely dilated follicle with a mass of keratotic debris.12 Prurigo nodularis would appear as dome-shaped papulonodules with varying degrees of scale, crust, and erosion, with a histopathologic appearance of hyperplasia and thick hyperkeratosis.11
Overall, the histopathology is paramount in differentiating RPC from the alternative diagnoses, with the extrusion of collagen from the epidermis not being seen in these other conditions. The coupling of the medical history (type 2 diabetes mellitus and end-stage renal disease) with the clinical presentation and skin biopsy findings confirmed the diagnosis of RPC.
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305.
- Matsui A, Nakano H, Aizu T, et al. Treatment of acquired reactive perforating collagenosis with 308‐nm excimer laser. Clin Exp Dermatol. 2016;41:820-821.
- Dey AK. Reactive perforating collagenosis: an important differential diagnosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2018;29:422-425.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 9th ed. McGraw-Hill Education LLC; 2012.
- Plaza JA, Prieto VG. Inflammatory Skin Disorders. Demos Medical Publishing LLC; 2012.
- Kreuter A, Gambichler T. Acquired reactive perforating collagenosis. CMAJ. 2010;182:E184.
- Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:E20391.
- Rapini RP. Perforating diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1690-1696.
- Gao L, Gu L, Chen Z, et al. Doxycycline combined with NB-UVB phototherapy for acquired reactive perforating collagenosis. Ther Clin Risk Manag. 2020;16:917-921.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Psychocutaneous disorders. Dermatology Essentials. Elsevier; 2014:50-55. 11. Bolognia JL, Schaffer JV, Duncan KO, et al. Pruritus and dysesthesia. Dermatology Essentials. Elsevier; 2014:39-49. 12. Rubio FA, Herranz P, Robayna G, et al. Perforating folliculitis: report of a case in an HIV-infected man. J Am Acad Dermatol. 1999;40:300-302.
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) may be either acquired or inherited. It is 1 of 4 classical forms of transepithelial elimination, which also includes elastosis perforans serpiginosa (EPS) as well as perforating folliculitis and Kyrle disease. These 4 forms of transepithelial elimination share characteristics of the elimination of altered dermal components through the epidermis.1 The acquired subtype of RPC frequently occurs in patients with diabetes mellitus and end-stage renal disease,2 both present in our patient.
Clinical presentation typically shows pruritic hyperkeratotic papules with a central crater filled with crust that frequently are distributed on the extensor surfaces of the extremities, often in a linear pattern.3 The perforating papules and nodules occasionally may involve the trunk and face.4 Histopathologic examination is characterized by the elimination of altered collagen through the epidermis. Established lesions may show a cup-shaped depression of the epidermis filled with a keratin plug. The underlying dermis will show vertically oriented basophilic collagen fibers with focal extrusion through the epidermis, and elastic fibers will be absent.5 The exact pathophysiology of this disease is unknown, but it may represent a cutaneous response to superficial trauma caused by intense scratching.6
Standard treatment protocols are not well established for this condition, but some evidence shows that a combination of treatments can help ameliorate symptoms, even if they are not curative.7 Treatments without strong evidence have included a wide range of topical, systemic, and other therapies. Case series and anecdotal reports have used retinoids, corticosteroids, menthol, antibiotics, allopurinol antihistamines, cryotherapy, and lasers.8 One case was treated with a combination of narrowband UVB phototherapy and doxycycline with resolution in approximately 6 weeks.9 Other cases have been cured using triple therapy with antihistamines, topical or injected steroids, and emollients or oral antibiotics.7 Evidence shows that there may be benefit to combining multiple different treatment types that target pruritus, inflammation, and collagen damage.7,9 This disease usually cannot be cured, but it may be improved by the available treatments.
The differential diagnosis includes delusional parasitosis, EPS, perforating folliculitis, and prurigo nodularis. Delusional parasitosis also can be characterized by excoriated plaques and a sensation of parasites infesting the skin, as our patient described.10 However, it can be differentiated from RPC by the fact that it is a diagnosis of exclusion, which would not have the histopathologic findings of the elimination of collagen from the epidermis, as was demonstrated in our patient.11 Elastosis perforans serpiginosa is in the same family of perforating diseases as RPC; however, EPS typically appears in children or young adults and often is associated with other genetic disorders. Physical examination in a patient with EPS would reveal keratotic papules in a serpiginous pattern, whereas our patient had discrete lesions without any serpiginous pattern. The histopathologic appearance of EPS would reveal plugs of elastic fibers rather than collagen fibers, as was demonstrated in our patient.8 Perforating folliculitis, while also demonstrating transepithelial elimination similar to RPC, would appear as erythematous follicular papules with small central keratotic plugs and histopathologic findings of a widely dilated follicle with a mass of keratotic debris.12 Prurigo nodularis would appear as dome-shaped papulonodules with varying degrees of scale, crust, and erosion, with a histopathologic appearance of hyperplasia and thick hyperkeratosis.11
Overall, the histopathology is paramount in differentiating RPC from the alternative diagnoses, with the extrusion of collagen from the epidermis not being seen in these other conditions. The coupling of the medical history (type 2 diabetes mellitus and end-stage renal disease) with the clinical presentation and skin biopsy findings confirmed the diagnosis of RPC.
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) may be either acquired or inherited. It is 1 of 4 classical forms of transepithelial elimination, which also includes elastosis perforans serpiginosa (EPS) as well as perforating folliculitis and Kyrle disease. These 4 forms of transepithelial elimination share characteristics of the elimination of altered dermal components through the epidermis.1 The acquired subtype of RPC frequently occurs in patients with diabetes mellitus and end-stage renal disease,2 both present in our patient.
Clinical presentation typically shows pruritic hyperkeratotic papules with a central crater filled with crust that frequently are distributed on the extensor surfaces of the extremities, often in a linear pattern.3 The perforating papules and nodules occasionally may involve the trunk and face.4 Histopathologic examination is characterized by the elimination of altered collagen through the epidermis. Established lesions may show a cup-shaped depression of the epidermis filled with a keratin plug. The underlying dermis will show vertically oriented basophilic collagen fibers with focal extrusion through the epidermis, and elastic fibers will be absent.5 The exact pathophysiology of this disease is unknown, but it may represent a cutaneous response to superficial trauma caused by intense scratching.6
Standard treatment protocols are not well established for this condition, but some evidence shows that a combination of treatments can help ameliorate symptoms, even if they are not curative.7 Treatments without strong evidence have included a wide range of topical, systemic, and other therapies. Case series and anecdotal reports have used retinoids, corticosteroids, menthol, antibiotics, allopurinol antihistamines, cryotherapy, and lasers.8 One case was treated with a combination of narrowband UVB phototherapy and doxycycline with resolution in approximately 6 weeks.9 Other cases have been cured using triple therapy with antihistamines, topical or injected steroids, and emollients or oral antibiotics.7 Evidence shows that there may be benefit to combining multiple different treatment types that target pruritus, inflammation, and collagen damage.7,9 This disease usually cannot be cured, but it may be improved by the available treatments.
The differential diagnosis includes delusional parasitosis, EPS, perforating folliculitis, and prurigo nodularis. Delusional parasitosis also can be characterized by excoriated plaques and a sensation of parasites infesting the skin, as our patient described.10 However, it can be differentiated from RPC by the fact that it is a diagnosis of exclusion, which would not have the histopathologic findings of the elimination of collagen from the epidermis, as was demonstrated in our patient.11 Elastosis perforans serpiginosa is in the same family of perforating diseases as RPC; however, EPS typically appears in children or young adults and often is associated with other genetic disorders. Physical examination in a patient with EPS would reveal keratotic papules in a serpiginous pattern, whereas our patient had discrete lesions without any serpiginous pattern. The histopathologic appearance of EPS would reveal plugs of elastic fibers rather than collagen fibers, as was demonstrated in our patient.8 Perforating folliculitis, while also demonstrating transepithelial elimination similar to RPC, would appear as erythematous follicular papules with small central keratotic plugs and histopathologic findings of a widely dilated follicle with a mass of keratotic debris.12 Prurigo nodularis would appear as dome-shaped papulonodules with varying degrees of scale, crust, and erosion, with a histopathologic appearance of hyperplasia and thick hyperkeratosis.11
Overall, the histopathology is paramount in differentiating RPC from the alternative diagnoses, with the extrusion of collagen from the epidermis not being seen in these other conditions. The coupling of the medical history (type 2 diabetes mellitus and end-stage renal disease) with the clinical presentation and skin biopsy findings confirmed the diagnosis of RPC.
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305.
- Matsui A, Nakano H, Aizu T, et al. Treatment of acquired reactive perforating collagenosis with 308‐nm excimer laser. Clin Exp Dermatol. 2016;41:820-821.
- Dey AK. Reactive perforating collagenosis: an important differential diagnosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2018;29:422-425.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 9th ed. McGraw-Hill Education LLC; 2012.
- Plaza JA, Prieto VG. Inflammatory Skin Disorders. Demos Medical Publishing LLC; 2012.
- Kreuter A, Gambichler T. Acquired reactive perforating collagenosis. CMAJ. 2010;182:E184.
- Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:E20391.
- Rapini RP. Perforating diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1690-1696.
- Gao L, Gu L, Chen Z, et al. Doxycycline combined with NB-UVB phototherapy for acquired reactive perforating collagenosis. Ther Clin Risk Manag. 2020;16:917-921.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Psychocutaneous disorders. Dermatology Essentials. Elsevier; 2014:50-55. 11. Bolognia JL, Schaffer JV, Duncan KO, et al. Pruritus and dysesthesia. Dermatology Essentials. Elsevier; 2014:39-49. 12. Rubio FA, Herranz P, Robayna G, et al. Perforating folliculitis: report of a case in an HIV-infected man. J Am Acad Dermatol. 1999;40:300-302.
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305.
- Matsui A, Nakano H, Aizu T, et al. Treatment of acquired reactive perforating collagenosis with 308‐nm excimer laser. Clin Exp Dermatol. 2016;41:820-821.
- Dey AK. Reactive perforating collagenosis: an important differential diagnosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2018;29:422-425.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 9th ed. McGraw-Hill Education LLC; 2012.
- Plaza JA, Prieto VG. Inflammatory Skin Disorders. Demos Medical Publishing LLC; 2012.
- Kreuter A, Gambichler T. Acquired reactive perforating collagenosis. CMAJ. 2010;182:E184.
- Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:E20391.
- Rapini RP. Perforating diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1690-1696.
- Gao L, Gu L, Chen Z, et al. Doxycycline combined with NB-UVB phototherapy for acquired reactive perforating collagenosis. Ther Clin Risk Manag. 2020;16:917-921.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Psychocutaneous disorders. Dermatology Essentials. Elsevier; 2014:50-55. 11. Bolognia JL, Schaffer JV, Duncan KO, et al. Pruritus and dysesthesia. Dermatology Essentials. Elsevier; 2014:39-49. 12. Rubio FA, Herranz P, Robayna G, et al. Perforating folliculitis: report of a case in an HIV-infected man. J Am Acad Dermatol. 1999;40:300-302.
A 73-year-old woman presented for evaluation of a rash on the arms and legs of 3 months’ duration. The rash had developed abruptly, and she believed it was caused by bugs in the skin; her husband noted that she constantly picked at her arms and legs. She had a medical history of hypertension, type 2 diabetes mellitus, and endstage renal disease on dialysis. Physical examination revealed multiple pigmented papules and plaques, some with keratotic scale, on the lower legs (left) and arms, with greater involvement on the left arm (right). The lesions were of various sizes and shapes, some with a central keratotic core, and several lesions demonstrated erosion, excoriation, or ulceration. Histopathologic examination revealed slight attenuation of the epidermis with loss of normal rete peg architecture, alternating areas of hypergranulosis and hypogranulosis, central ulceration with inflammatory cells, and a basophilic hue to the ulcer base with sweeping up of the collagen fibers.
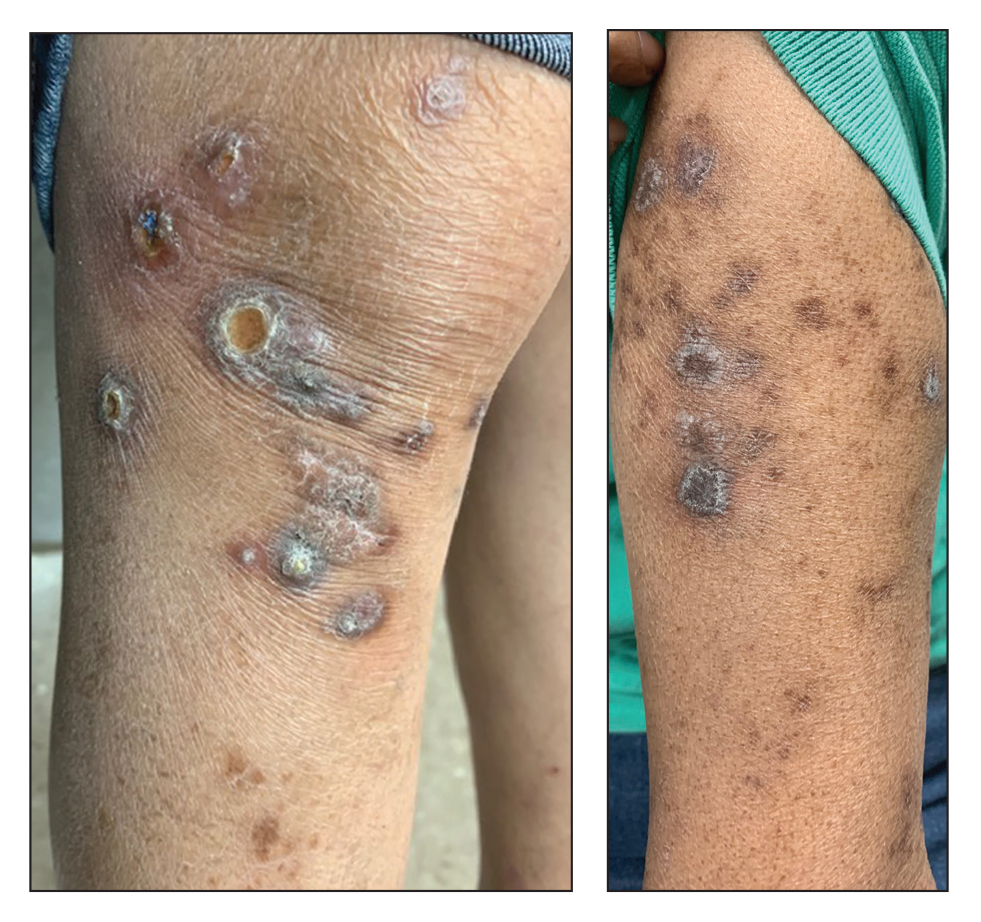
Verrucous Carcinoma of the Foot: A Retrospective Study of 19 Cases and Analysis of Prognostic Factors Influencing Recurrence
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.
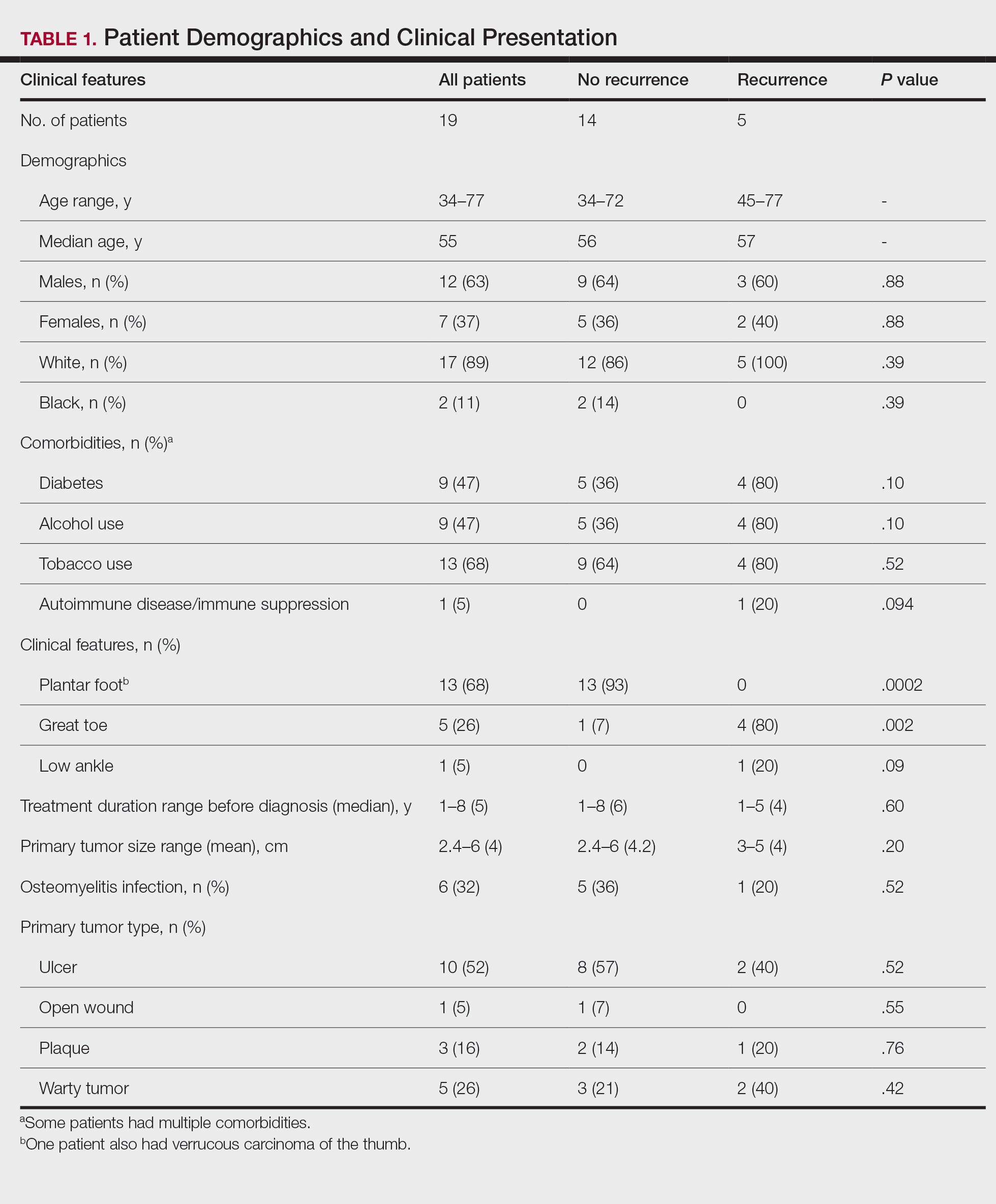
Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).
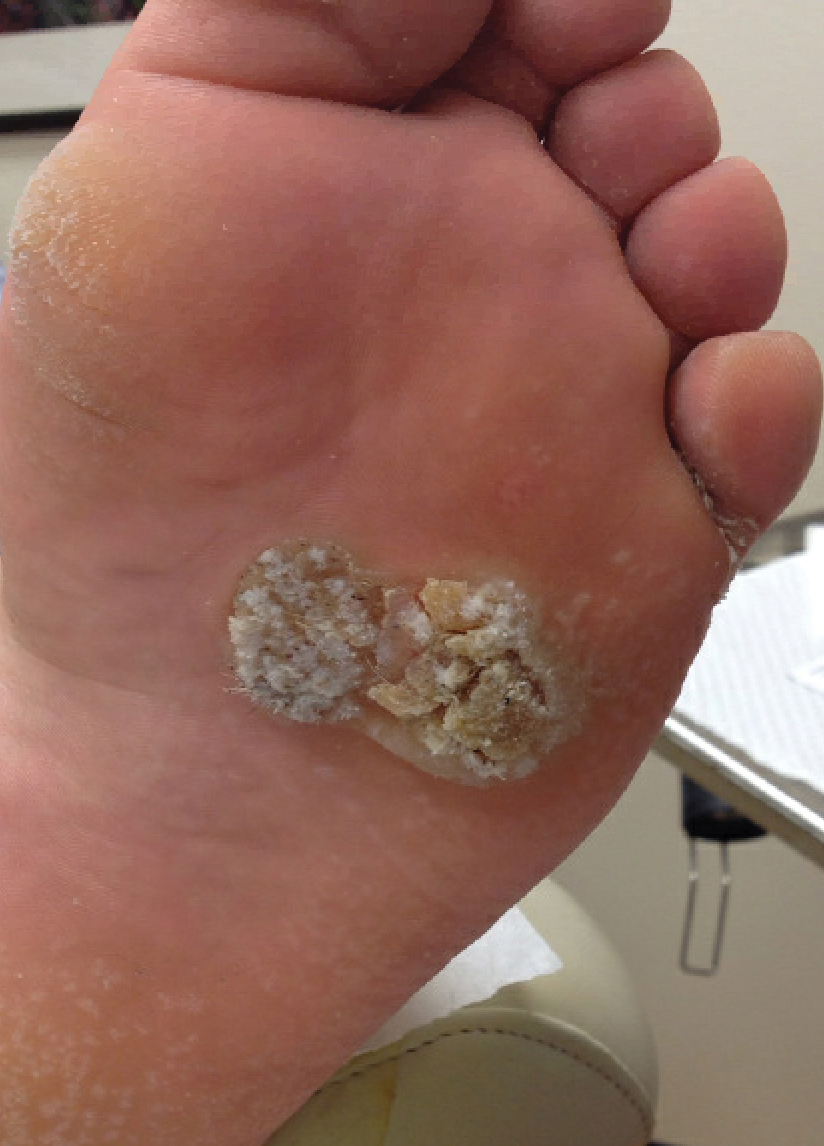
Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.
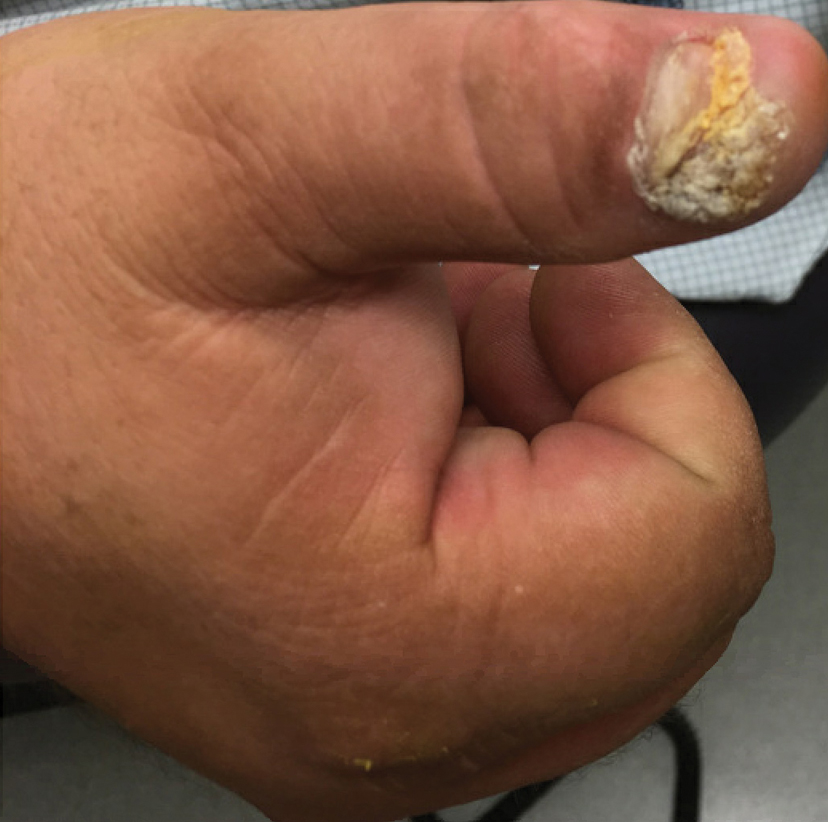
Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation
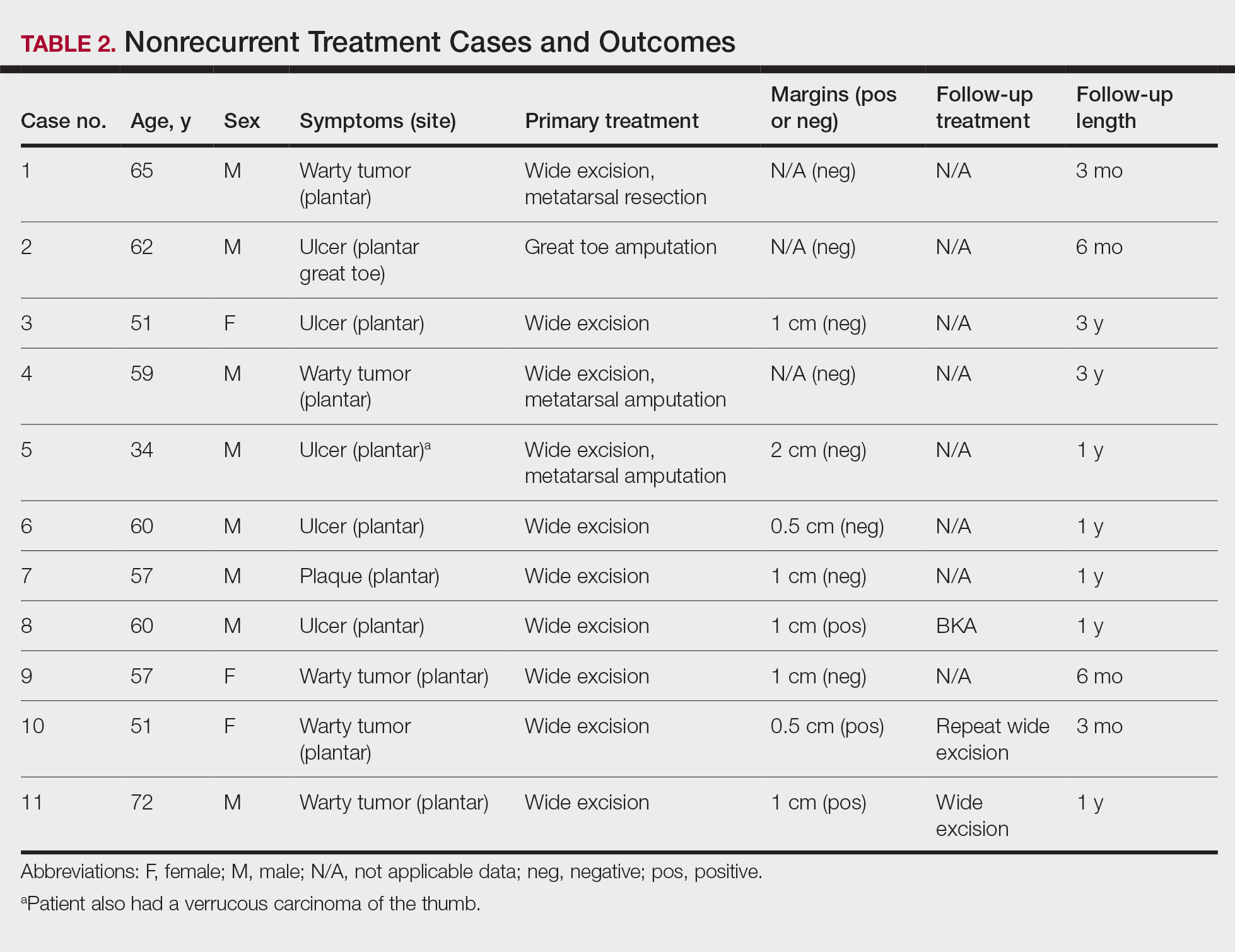
Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).
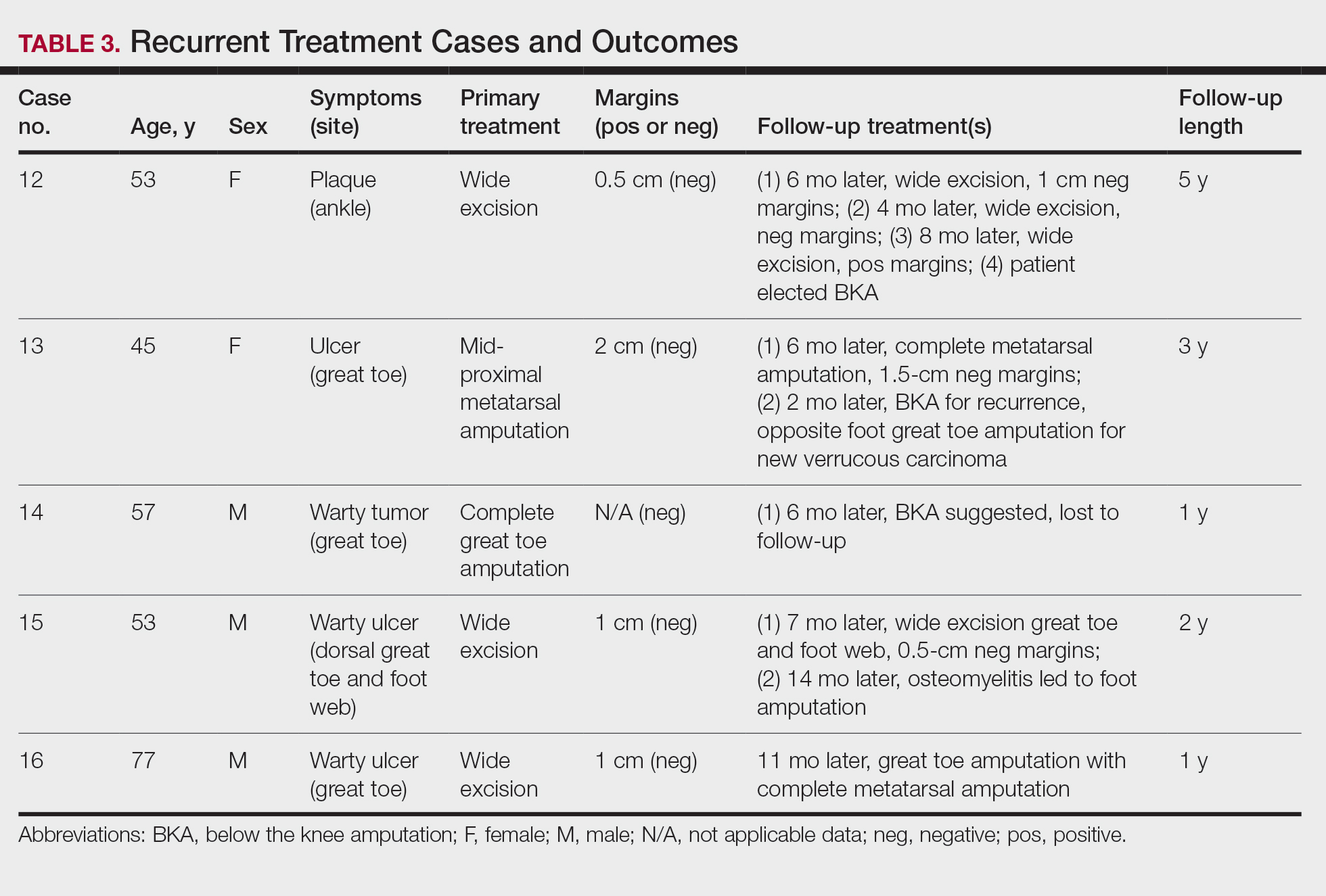
The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.
Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.
Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.

Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).

Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.

Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation

Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).

The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.

Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.

Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.

Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).

Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.

Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation

Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).

The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.

Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.

Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Practice Points
- Clinicians should have a high suspicion for verrucous carcinoma in the setting of a chronic ulceration or warty lesion that is resistant to traditional treatment. Early biopsy with tissue collection of the raised ulcer borders and the deep dermis layer of warty lesions is imperative for diagnosis.
- Verrucous carcinoma originating on the nonglabrous surface of the foot may have a higher rate of recurrence often occurring within months of previous treatment. Patients presenting with nonhealing surgical sites in this area should be treated with a high level of suspicion for recurrence.
Chronic Vulvar Plaque in a Patient With Severe Hidradenitis Suppurativa
The Diagnosis: Acquired Lymphangioma Circumscriptum
A skin biopsy of the plaque on the right labium majus showed a proliferation of well-formed, dilated lymphatic vessels lined by benign-appearing endothelial cells in the papillary dermis (Figure). These findings were consistent with a diagnosis of acquired lymphangioma circumscriptum (ALC) in the setting of severe hidradenitis suppurativa (HS).
Acquired lymphangioma circumscriptum (also known as acquired lymphangiectasia or secondary lymphangioma1) is a rare skin finding resulting from chronic lymphatic obstruction that leads to dilated lymphatic vessels within the dermis.2,3 There also is a distinct congenital form of lymphangioma circumscriptum caused by lymphatic malformations present at birth.2,4 Acquired lymphangioma circumscriptum of the vulva is a rare phenomenon.3 Identified causes include radiation or surgery for carcinoma, solid gynecologic tumors, lymphadenectomy, Crohn disease, and tuberculosis and other infections, all of which can disrupt normal lymphatics to cause ALC.2-4 Hidradenitis suppurativa is not a widely recognized cause of ALC; however, this phenomenon is reported in the literature. A long-standing history of severe HS complicated by lymphedema seems to precede the development of ALC in the reported cases, as in our patient.5-7
Acquired lymphangioma circumscriptum of the vulva can appear in women of all ages as frog spawn or cobblestone papules or vesicles, sometimes with a hyperkeratotic or verrucous appearance.2,4 Associated symptoms include serous drainage, edema, pruritus, and discomfort. The lesions may become eroded, which can predispose patients to secondary infections.1,2 Acquired lymphangioma circumscriptum of the vulva can be difficult to diagnose, as the time interval between the initial cause and the appearance of skin findings can be years, leading to the misdiagnosis of ALC as other similar-appearing genital skin conditions such as squamous cell carcinoma or condyloma.4,8 When misidentified as an infection, diagnosis can lead to substantial distress, abstinence from sexual activity, and unnecessary and painful treatments.
Skin biopsy is helpful in distinguishing ALC from other differential diagnoses such as condylomata acuminata, squamous cell carcinoma, and condyloma lata. Histopathology in ALC is notable for dilated lymphatic vessels filled with hypocellular fluid and lined with endothelial cells in the superficial dermis; the epidermis can appear hyperplastic, hyperkeratotic, or eroded.3-5,9 These lymphatic vessels stain positively for CD31 and D2-40, markers for endothelial cells and lymphatic endothelium, respectively, and negative for CD34, a marker for vascular endothelium.3,4,9 Features suggestive of condylomata acuminata such as rounded parakeratosis, hypergranulosis, and vacuolated keratinocytes9 are not present. The giant condyloma of Buschke-Löwenstein, a clinical variant of verrucous squamous cell carcinoma, also can present as a warty ulcerated papule or plaque in the genital region, but the characteristic rounded eosinophilic keratinocytes pushing down into the dermis9 are not seen in ALC. Secondary syphilis is associated with condyloma lata, which are verrucous or fleshy-appearing papules often coalescing into plaques located in the anogenital region. Pathologic features of secondary syphilis include vacuolar interface dermatitis and acanthosis with long slender rete ridges.9 Squamous cell carcinoma, which can arise from inflammation associated with long-standing HS, must be ruled out, as it is associated with a high risk of mortality in patients with HS.10
It is noteworthy to recognize the various, often confusing nomenclature used to describe cutaneous lymphatic conditions. The terms acquired lymphangioma circumscriptum, secondary lymphangioma, and lymphangiectasia are used interchangeably to describe dilated lymphatic vessels in the skin.1 The term atypical vascular lesion refers to lymphectasias of the skin of the breast due to prior radiation therapy most often used in the treatment of breast carcinoma; clinically, these present as red-brown or flesh-colored papules or telangiectatic plaques on the breast.11,12 Lymphedema also may occur alongside atypical vascular lesions, as prior radiation or surgical lymph node dissection can predispose patients to impaired lymphatic drainage.13 The lymphatic histopathologic subtype of atypical vascular lesions may appear similar to ALC; however, the vascular subtype will demonstrate collections of capillary-sized vessels and extravasated erythrocytes.11,12 Unlike ALC, the benign nature of atypical vascular lesions has been questioned, as they may be associated with a small risk for progression to angiosarcoma.11-13 It also is important to distinguish ALC from lymphangiomatosis, a generalized lymphatic anomaly that is characterized by extensive lymphatic malformations involving numerous internal organs, including the lungs and gastrointestinal tract. This condition is associated with notable morbidity and mortality.13
Although the suffix of the term lymphangioma suggests a neoplastic process, ALC is not a neoplasm and can be managed expectantly in many cases.2,3,8 However, due to cosmetic appearance, pain, discomfort, and recurrent bacterial superinfections, many patients pursue treatment. Treatment options for ALC include sclerotherapy, electrocautery, radiofrequency or carbon dioxide laser ablation, and excision, though recurrence can arise.3-5,7,8 Our patient elected to manage her asymptomatic ALC expectantly.
- Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487.
- Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
- Sims SM, McLean FW, Davis JD, et al. Vulvar lymphangioma circumscriptum: a report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010; 14:234-237.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;6:1223-1224.
- Piernick DM 2nd, Mahmood SH, Daveluy S. Acquired lymphangioma circumscriptum of the genitals in an individual with chronic hidradenitis suppurativa. JAAD Case Rep. 2018;1:64-66.
- Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;1:118-120.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2019.
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
The Diagnosis: Acquired Lymphangioma Circumscriptum
A skin biopsy of the plaque on the right labium majus showed a proliferation of well-formed, dilated lymphatic vessels lined by benign-appearing endothelial cells in the papillary dermis (Figure). These findings were consistent with a diagnosis of acquired lymphangioma circumscriptum (ALC) in the setting of severe hidradenitis suppurativa (HS).

Acquired lymphangioma circumscriptum (also known as acquired lymphangiectasia or secondary lymphangioma1) is a rare skin finding resulting from chronic lymphatic obstruction that leads to dilated lymphatic vessels within the dermis.2,3 There also is a distinct congenital form of lymphangioma circumscriptum caused by lymphatic malformations present at birth.2,4 Acquired lymphangioma circumscriptum of the vulva is a rare phenomenon.3 Identified causes include radiation or surgery for carcinoma, solid gynecologic tumors, lymphadenectomy, Crohn disease, and tuberculosis and other infections, all of which can disrupt normal lymphatics to cause ALC.2-4 Hidradenitis suppurativa is not a widely recognized cause of ALC; however, this phenomenon is reported in the literature. A long-standing history of severe HS complicated by lymphedema seems to precede the development of ALC in the reported cases, as in our patient.5-7
Acquired lymphangioma circumscriptum of the vulva can appear in women of all ages as frog spawn or cobblestone papules or vesicles, sometimes with a hyperkeratotic or verrucous appearance.2,4 Associated symptoms include serous drainage, edema, pruritus, and discomfort. The lesions may become eroded, which can predispose patients to secondary infections.1,2 Acquired lymphangioma circumscriptum of the vulva can be difficult to diagnose, as the time interval between the initial cause and the appearance of skin findings can be years, leading to the misdiagnosis of ALC as other similar-appearing genital skin conditions such as squamous cell carcinoma or condyloma.4,8 When misidentified as an infection, diagnosis can lead to substantial distress, abstinence from sexual activity, and unnecessary and painful treatments.
Skin biopsy is helpful in distinguishing ALC from other differential diagnoses such as condylomata acuminata, squamous cell carcinoma, and condyloma lata. Histopathology in ALC is notable for dilated lymphatic vessels filled with hypocellular fluid and lined with endothelial cells in the superficial dermis; the epidermis can appear hyperplastic, hyperkeratotic, or eroded.3-5,9 These lymphatic vessels stain positively for CD31 and D2-40, markers for endothelial cells and lymphatic endothelium, respectively, and negative for CD34, a marker for vascular endothelium.3,4,9 Features suggestive of condylomata acuminata such as rounded parakeratosis, hypergranulosis, and vacuolated keratinocytes9 are not present. The giant condyloma of Buschke-Löwenstein, a clinical variant of verrucous squamous cell carcinoma, also can present as a warty ulcerated papule or plaque in the genital region, but the characteristic rounded eosinophilic keratinocytes pushing down into the dermis9 are not seen in ALC. Secondary syphilis is associated with condyloma lata, which are verrucous or fleshy-appearing papules often coalescing into plaques located in the anogenital region. Pathologic features of secondary syphilis include vacuolar interface dermatitis and acanthosis with long slender rete ridges.9 Squamous cell carcinoma, which can arise from inflammation associated with long-standing HS, must be ruled out, as it is associated with a high risk of mortality in patients with HS.10
It is noteworthy to recognize the various, often confusing nomenclature used to describe cutaneous lymphatic conditions. The terms acquired lymphangioma circumscriptum, secondary lymphangioma, and lymphangiectasia are used interchangeably to describe dilated lymphatic vessels in the skin.1 The term atypical vascular lesion refers to lymphectasias of the skin of the breast due to prior radiation therapy most often used in the treatment of breast carcinoma; clinically, these present as red-brown or flesh-colored papules or telangiectatic plaques on the breast.11,12 Lymphedema also may occur alongside atypical vascular lesions, as prior radiation or surgical lymph node dissection can predispose patients to impaired lymphatic drainage.13 The lymphatic histopathologic subtype of atypical vascular lesions may appear similar to ALC; however, the vascular subtype will demonstrate collections of capillary-sized vessels and extravasated erythrocytes.11,12 Unlike ALC, the benign nature of atypical vascular lesions has been questioned, as they may be associated with a small risk for progression to angiosarcoma.11-13 It also is important to distinguish ALC from lymphangiomatosis, a generalized lymphatic anomaly that is characterized by extensive lymphatic malformations involving numerous internal organs, including the lungs and gastrointestinal tract. This condition is associated with notable morbidity and mortality.13
Although the suffix of the term lymphangioma suggests a neoplastic process, ALC is not a neoplasm and can be managed expectantly in many cases.2,3,8 However, due to cosmetic appearance, pain, discomfort, and recurrent bacterial superinfections, many patients pursue treatment. Treatment options for ALC include sclerotherapy, electrocautery, radiofrequency or carbon dioxide laser ablation, and excision, though recurrence can arise.3-5,7,8 Our patient elected to manage her asymptomatic ALC expectantly.
The Diagnosis: Acquired Lymphangioma Circumscriptum
A skin biopsy of the plaque on the right labium majus showed a proliferation of well-formed, dilated lymphatic vessels lined by benign-appearing endothelial cells in the papillary dermis (Figure). These findings were consistent with a diagnosis of acquired lymphangioma circumscriptum (ALC) in the setting of severe hidradenitis suppurativa (HS).

Acquired lymphangioma circumscriptum (also known as acquired lymphangiectasia or secondary lymphangioma1) is a rare skin finding resulting from chronic lymphatic obstruction that leads to dilated lymphatic vessels within the dermis.2,3 There also is a distinct congenital form of lymphangioma circumscriptum caused by lymphatic malformations present at birth.2,4 Acquired lymphangioma circumscriptum of the vulva is a rare phenomenon.3 Identified causes include radiation or surgery for carcinoma, solid gynecologic tumors, lymphadenectomy, Crohn disease, and tuberculosis and other infections, all of which can disrupt normal lymphatics to cause ALC.2-4 Hidradenitis suppurativa is not a widely recognized cause of ALC; however, this phenomenon is reported in the literature. A long-standing history of severe HS complicated by lymphedema seems to precede the development of ALC in the reported cases, as in our patient.5-7
Acquired lymphangioma circumscriptum of the vulva can appear in women of all ages as frog spawn or cobblestone papules or vesicles, sometimes with a hyperkeratotic or verrucous appearance.2,4 Associated symptoms include serous drainage, edema, pruritus, and discomfort. The lesions may become eroded, which can predispose patients to secondary infections.1,2 Acquired lymphangioma circumscriptum of the vulva can be difficult to diagnose, as the time interval between the initial cause and the appearance of skin findings can be years, leading to the misdiagnosis of ALC as other similar-appearing genital skin conditions such as squamous cell carcinoma or condyloma.4,8 When misidentified as an infection, diagnosis can lead to substantial distress, abstinence from sexual activity, and unnecessary and painful treatments.
Skin biopsy is helpful in distinguishing ALC from other differential diagnoses such as condylomata acuminata, squamous cell carcinoma, and condyloma lata. Histopathology in ALC is notable for dilated lymphatic vessels filled with hypocellular fluid and lined with endothelial cells in the superficial dermis; the epidermis can appear hyperplastic, hyperkeratotic, or eroded.3-5,9 These lymphatic vessels stain positively for CD31 and D2-40, markers for endothelial cells and lymphatic endothelium, respectively, and negative for CD34, a marker for vascular endothelium.3,4,9 Features suggestive of condylomata acuminata such as rounded parakeratosis, hypergranulosis, and vacuolated keratinocytes9 are not present. The giant condyloma of Buschke-Löwenstein, a clinical variant of verrucous squamous cell carcinoma, also can present as a warty ulcerated papule or plaque in the genital region, but the characteristic rounded eosinophilic keratinocytes pushing down into the dermis9 are not seen in ALC. Secondary syphilis is associated with condyloma lata, which are verrucous or fleshy-appearing papules often coalescing into plaques located in the anogenital region. Pathologic features of secondary syphilis include vacuolar interface dermatitis and acanthosis with long slender rete ridges.9 Squamous cell carcinoma, which can arise from inflammation associated with long-standing HS, must be ruled out, as it is associated with a high risk of mortality in patients with HS.10
It is noteworthy to recognize the various, often confusing nomenclature used to describe cutaneous lymphatic conditions. The terms acquired lymphangioma circumscriptum, secondary lymphangioma, and lymphangiectasia are used interchangeably to describe dilated lymphatic vessels in the skin.1 The term atypical vascular lesion refers to lymphectasias of the skin of the breast due to prior radiation therapy most often used in the treatment of breast carcinoma; clinically, these present as red-brown or flesh-colored papules or telangiectatic plaques on the breast.11,12 Lymphedema also may occur alongside atypical vascular lesions, as prior radiation or surgical lymph node dissection can predispose patients to impaired lymphatic drainage.13 The lymphatic histopathologic subtype of atypical vascular lesions may appear similar to ALC; however, the vascular subtype will demonstrate collections of capillary-sized vessels and extravasated erythrocytes.11,12 Unlike ALC, the benign nature of atypical vascular lesions has been questioned, as they may be associated with a small risk for progression to angiosarcoma.11-13 It also is important to distinguish ALC from lymphangiomatosis, a generalized lymphatic anomaly that is characterized by extensive lymphatic malformations involving numerous internal organs, including the lungs and gastrointestinal tract. This condition is associated with notable morbidity and mortality.13
Although the suffix of the term lymphangioma suggests a neoplastic process, ALC is not a neoplasm and can be managed expectantly in many cases.2,3,8 However, due to cosmetic appearance, pain, discomfort, and recurrent bacterial superinfections, many patients pursue treatment. Treatment options for ALC include sclerotherapy, electrocautery, radiofrequency or carbon dioxide laser ablation, and excision, though recurrence can arise.3-5,7,8 Our patient elected to manage her asymptomatic ALC expectantly.
- Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487.
- Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
- Sims SM, McLean FW, Davis JD, et al. Vulvar lymphangioma circumscriptum: a report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010; 14:234-237.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;6:1223-1224.
- Piernick DM 2nd, Mahmood SH, Daveluy S. Acquired lymphangioma circumscriptum of the genitals in an individual with chronic hidradenitis suppurativa. JAAD Case Rep. 2018;1:64-66.
- Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;1:118-120.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2019.
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
- Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487.
- Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
- Sims SM, McLean FW, Davis JD, et al. Vulvar lymphangioma circumscriptum: a report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010; 14:234-237.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;6:1223-1224.
- Piernick DM 2nd, Mahmood SH, Daveluy S. Acquired lymphangioma circumscriptum of the genitals in an individual with chronic hidradenitis suppurativa. JAAD Case Rep. 2018;1:64-66.
- Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;1:118-120.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2019.
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
A 38-year-old woman with long-standing severe hidradenitis suppurativa presented to our dermatology clinic with an asymptomatic, slowly enlarging growth on the right labium majus of 2 years’ duration. She also had severe persistent drainage from nodules and sinus tracts involving the abdominal pannus, inguinal folds, vulva, perineum, buttocks, and upper thighs. After treatment failure with oral antibiotics and adalimumab, her regimen included infliximab-dyyb, chronic systemic steroids, spironolactone, topical clindamycin, and benzoyl peroxide, with plans for eventual surgical intervention. Physical examination revealed the patient had numerous pink papules coalescing into a plaque on the right labium majus. She also had innumerable papulonodules, sinus tracts, and indurated scars in the inguinal folds, genitalia, and perineal region from severe hidradenitis suppurativa.
A Fixed Drug Eruption to Medroxyprogesterone Acetate Injectable Suspension
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).
An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3
Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).

An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3

Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
To the Editor:
A fixed drug eruption (FDE) is a well-documented form of cutaneous hypersensitivity that typically manifests as a sharply demarcated, dusky, round to oval, edematous, red-violaceous macule or patch on the skin and mucous membranes. The lesion often resolves with residual postinflammatory hyperpigmentation, most commonly as a reaction to ingested drugs or drug components.1 Lesions generally occur at the same anatomic site with repeated exposure to the offending drug. Typically, a single site is affected, but additional sites with more generalized involvement have been reported to occur with subsequent exposure to the offending medication. The diagnosis usually is clinical, but histopathologic findings can help confirm the diagnosis in unusual presentations. We present a novel case of a patient with an FDE from medroxyprogesterone acetate, a contraceptive injection that contains the hormone progestin.
A 35-year-old woman presented to the dermatology clinic for evaluation of a lesion on the left lower buttock of 1 year’s duration. She reported periodic swelling and associated pruritus of the lesion. She denied any growth in size, and no other similar lesions were present. The patient reported a medication history of medroxyprogesterone acetate for birth control, but she denied any other prescription or over-the-counter medication, oral supplements, or recreational drug use. Upon further inquiry, she reported that the recurrence of symptoms appeared to coincide with each administration of medroxyprogesterone acetate, which occurred approximately every 3 months. The eruption cleared between injections and recurred in the same location following subsequent injections. The lesion appeared approximately 2 weeks after the first injection (approximately 1 year prior to presentation to dermatology) and within 2 to 3 days after each subsequent injection. Physical examination revealed a 2×2-cm, circular, slightly violaceous patch on the left buttock (Figure 1). A biopsy was recommended to aid in diagnosis, and the patient was offered a topical steroid for symptomatic relief. A punch biopsy revealed subtle interface dermatitis with superficial perivascular lymphoid infiltrate and marked pigmentary incontinence consistent with an FDE (Figure 2).

An FDE was first reported in 1889 by Bourns,2 and over time more implicated agents and varying clinical presentations have been linked to the disease. The FDE can be accompanied by symptoms of pruritus or paresthesia. Most cases are devoid of systemic symptoms. An FDE can be located anywhere on the body, but it most frequently manifests on the lips, face, hands, feet, and genitalia. Although the eruption often heals with residual postinflammatory hyperpigmentation, a nonpigmenting FDE due to pseudoephedrine has been reported.3

Common culprits include antibiotics (eg, sulfonamides, trimethoprim, fluoroquinolones, tetracyclines), nonsteroidal anti-inflammatory medications (eg, naproxen sodium, ibuprofen, celecoxib), barbiturates, antimalarials, and anticonvulsants. Rare cases of FDE induced by foods and food additives also have been reported.4 Oral fluconazole, levocetirizine dihydrochloride, loperamide, and multivitamin-mineral preparations are other rare inducers of FDE.5-8 In 2004, Ritter and Meffert9 described an FDE to the green dye used in inactive oral contraceptive pills. A similar case was reported by Rea et al10 that described an FDE from the inactive sugar pills in ethinyl estradiol and levonorgestrel, which is another combined oral contraceptive.
The time between ingestion of the offending agent and the manifestation of the disease usually is 1 to 2 weeks; however, upon subsequent exposure, the disease has been reported to manifest within hours.1 CD8+ memory T cells have been shown to be major players in the development of FDE and can be found along the dermoepidermal junction as part of a delayed type IV hypersensitivity reaction.11 Histopathology reveals superficial and deep interstitial and perivascular infiltrates consisting of lymphocytes with admixed eosinophils and possibly neutrophils in the dermis. In the epidermis, necrotic keratinocytes can be present. In rare cases, FDE may have atypical features, such as in generalized bullous FDE and nonpigmenting FDE, the latter of which more commonly is associated with pseudoephedrine.1
The differential diagnosis for FDE includes erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis, autoimmune progesterone dermatitis, and large plaque parapsoriasis. The number and morphology of lesions in erythema multiforme help differentiate it from FDE, as erythema multiforme presents with multiple targetoid lesions. The lesions of generalized bullous FDE can be similar to those of Stevens-Johnson syndrome/toxic epidermal necrolysis, and the pigmented patches of FDE can resemble large plaque parapsoriasis.
It is important to consider any medication ingested in the 1- to 2-week period before FDE onset, including over-the-counter medications, health food supplements, and prescription medications. Discontinuation of the implicated medication or any medication potentially cross-reacting with another medication is the most important step in management. Wound care may be needed for any bullous or eroded lesions. Lesions typically resolve within a few days to weeks of stopping the offending agent. Importantly, patients should be counseled on the secondary pigment alterations that may be persistent for several months. Other treatment for FDEs is aimed at symptomatic relief and may include topical corticosteroids and oral antihistamines.1
Medroxyprogesterone acetate is a highly effective contraceptive drug with low rates of failure.12 It is a weak androgenic progestin that is administered as a single 150-mg intramuscular injection every 3 months and inhibits gonadotropins. Common side effects include local injection-site reactions, unscheduled bleeding, amenorrhea, weight gain, headache, and mood changes. However, FDE has not been reported as an adverse effect to medroxyprogesterone acetate, both in official US Food and Drug Administration information and in the current literature.12
Autoimmune progesterone dermatitis (also known as progestin hypersensitivity) is a well-characterized cyclic hypersensitivity reaction to the hormone progesterone that occurs during the luteal phase of the menstrual cycle. It is known to have a variable clinical presentation including urticaria, erythema multiforme, eczema, and angioedema.13 Autoimmune progesterone dermatitis also has been reported to present as an FDE.14-16 The onset of the cutaneous manifestation often starts a few days before the onset of menses, with spontaneous resolution occurring after the onset of menstruation. The mechanism by which endogenous progesterone or other secretory products become antigenic is unknown. It has been suggested that there is an alteration in the properties of the hormone that would predispose it to be antigenic as it would not be considered self. In 2001, Warin17 proposed the following diagnostic criteria for autoimmune progesterone dermatitis: (1) skin lesions associated with menstrual cycle (premenstrual flare); (2) a positive response to the progesterone intradermal or intramuscular test; and (3) symptomatic improvement after inhibiting progesterone secretion by suppressing ovulation.17 The treatment includes antiallergy medications, progesterone desensitization, omalizumab injection, and leuprolide acetate injection.
Our case represents FDE from medroxyprogesterone acetate. Although we did not formally investigate the antigenicity of the exogenous progesterone, we postulate that the pathophysiology likely is similar to an FDE associated with endogenous progesterone. This reasoning is supported by the time course of the patient’s lesion as well as the worsening of symptoms in the days following the administration of the medication. Additionally, the patient had no history of skin lesions prior to the initiation of medroxyprogesterone acetate or similar lesions associated with her menstrual cycles.
A careful and detailed review of medication history is necessary to evaluate FDEs. Our case emphasizes that not only endogenous but also exogenous forms of progesterone may cause hypersensitivity, leading to an FDE. With more than 2 million prescriptions of medroxyprogesterone acetate written every year, dermatologists should be aware of the rare but potential risk for an FDE in patients using this medication.18
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
- Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Mosby; 2008.
- Bourns DCG. Unusual effects of antipyrine. Br Med J. 1889;2:818-820.
- Shelley WB, Shelley ED. Nonpigmenting fixed drug eruption as a distinctive reaction pattern: examples caused by sensitivity to pseudoephedrine hydrochloride and tetrahydrozoline. J Am Acad Dermatol. 1987;17:403-407.
- Sohn KH, Kim BK, Kim JY, et al. Fixed food eruption caused by Actinidia arguta (hardy kiwi): a case report and literature review. Allergy Asthma Immunol Res. 2017;9:182-184.
- Nakai N, Katoh N. Fixed drug eruption caused by fluconazole: a case report and mini-review of the literature. Allergol Int. 2013;6:139-141.
- An I, Demir V, Ibiloglu I, et al. Fixed drug eruption induced by levocetirizine. Indian Dermatol Online J. 2017;8:276-278.
- Matarredona J, Borrás Blasco J, Navarro-Ruiz A, et al. Fixed drug eruption associated to loperamide [in Spanish]. Med Clin (Barc). 2005;124:198-199.
- Gohel D. Fixed drug eruption due to multi-vitamin multi-mineral preparation. J Assoc Physicians India. 2000;48:268.
- Ritter SE, Meffert J. A refractory fixed drug reaction to a dye used in an oral contraceptive. Cutis. 2004;74:243-244.
- Rea S, McMeniman E, Darch K, et al. A fixed drug eruption to the sugar pills of a combined oral contraceptive. Poster presented at: The Australasian College of Dermatologists 51st Annual Scientific Meeting; May 22, 2018; Queensland, Australia.
- Shiohara T, Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur J Dermatol. 2007;17:201-208.
- Depo-Provera CI. Prescribing information. Pfizer; 2020. Accessed March 10, 2022. https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=522
- George R, Badawy SZ. Autoimmune progesterone dermatitis: a case report. Case Rep Obstet Gynecol. 2012;2012:757854.
- Mokhtari R, Sepaskhah M, Aslani FS, et al. Autoimmune progesterone dermatitis presenting as fixed drug eruption: a case report. Dermatol Online J. 2017;23:13030/qt685685p4.
- Asai J, Katoh N, Nakano M, et al. Case of autoimmune progesterone dermatitis presenting as fixed drug eruption. J Dermatol. 2009;36:643-645.
- Bhardwaj N, Jindal R, Chauhan P. Autoimmune progesterone dermatitis presenting as fixed drug eruption. BMJ Case Rep. 2019;12:E231873.
- Warin AP. Case 2. diagnosis: erythema multiforme as a presentation of autoimmune progesterone dermatitis. Clin Exp Dermatol. 2001;26:107-108.
- Medroxyprogesterone Drug Usage Statistics, United States, 2013-2019. ClinCalc website. Updated September 15, 2021. Accessed March 17, 2022. https://clincalc.com/DrugStats/Drugs/Medroxyprogesterone
Practice Points
- Exogenous progesterone from the administration of the contraceptive injectable medroxyprogesterone acetate has the potential to cause a cutaneous hypersensitivity reaction in the form of a fixed drug eruption (FDE).
- Dermatologists should perform a careful and detailed review of medication history to evaluate drug eruptions.
Q&A With JAAD Editor Dirk M. Elston, MD
who has authored more than 600 peer-reviewed publications and 92 textbook chapters.
After earning his undergraduate degree from Pennsylvania State University and his medical degree from Jefferson Medical College in Philadelphia, Dr. Elston completed an internship and a dermatology residency at Walter Reed Army Medical Center in Washington, as well as a dermatopathology fellowship at the Cleveland Clinic. He currently is professor and chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina in Charleston.
Dr. Elston is one of five authors of “Andrews’ Diseases of the Skin),” coauthor with Tammie Ferringer, MD, of the “Dermatopathology” textbook, and editor in chief of the Requisites in Dermatology series of textbooks. In 2018, he succeeded Bruce H. Thiers, MD, as editor of the Journal of the American Academy of Dermatology and in 2021, received the AAD’s Gold Medal Award, which is the academy’s highest honor.
In an interview, Dr. Elston reflected on his mentors, shared how he manages his many responsibilities as a clinician, teacher, and editor, and talked about the promising future of dermatology.
Who inspired you most to pursue a career in medicine? My grandmother, Annie Elston, was a physician and dedicated her life to helping others. She was a front-line medic during World War I, helped to run a neonatal syphilis ward after the war, and practiced pediatrics in New York City until her death. She was a great role model.
Did you enter medical school knowing that you wanted to become a dermatologist? If not, what was the turning point for you? I didn’t really know much about dermatology when I entered medical school. I fell in love with the specialty during a rotation.
What was the most memorable experience from your dermatology residency at Walter Reed Army Medical Center? There were so many interesting patients, including many tropical diseases.
Why did you choose to pursue a fellowship in dermatopathology? What was it about this subspeciality that piqued your interest? Great teachers, including Tim Berger, MD, George Lupton, MD, and Dean Pearson, MD. They inspired me to seek a dermpath fellowship and I was lucky enough to train with Wilma Bergfeld, MD.
In your opinion, what’s been the most important advance in dermatopathology to date?
Immunohistochemistry changed the specialty. Now molecular diagnostics is a second wave of major advancement.
How do you stay passionate about both dermatology and dermatopathology? The patients, residents, and fellows keep it interesting. It’s a two-way street. I learn as much as I teach.
You’ve had a remarkable run at the Journal of the American Academy of Dermatology, starting as deputy editor in 2008 before becoming editor in 2018. What’s been most rewarding about this role for you? It is a labor of love and such a privilege to see everyone’s best work.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint? Fear of the unknown is always a challenge with a new epidemic and worse with a pandemic. The patients still needed to be seen but it was a challenge with some buildings closed and some personnel afraid to come to work.
Is there anything you would tell your younger self in terms of career advice? Enjoy every step of the journey.
Considering your various work responsibilities as a clinician, teacher, and editor, what’s your strategy for achieving a work-life balance? A good friend of mine is fond of saying that balance is an illusion. There is only resilience. I believe the truth lies somewhere in between. Make time for family, and decide what has to get done today and what can wait until tomorrow.
What development in dermatology are you most excited about in the next 5 years? We are in a golden age of therapeutic innovations that are life changing and lifesaving for our patients. I never would have believed I would see complete cures of patients with widely metastatic melanoma. From psoriasis to eczema to malignancy, our therapeutic armamentarium is dramatically better each year. It makes the practice of medicine exciting.
who has authored more than 600 peer-reviewed publications and 92 textbook chapters.
After earning his undergraduate degree from Pennsylvania State University and his medical degree from Jefferson Medical College in Philadelphia, Dr. Elston completed an internship and a dermatology residency at Walter Reed Army Medical Center in Washington, as well as a dermatopathology fellowship at the Cleveland Clinic. He currently is professor and chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina in Charleston.
Dr. Elston is one of five authors of “Andrews’ Diseases of the Skin),” coauthor with Tammie Ferringer, MD, of the “Dermatopathology” textbook, and editor in chief of the Requisites in Dermatology series of textbooks. In 2018, he succeeded Bruce H. Thiers, MD, as editor of the Journal of the American Academy of Dermatology and in 2021, received the AAD’s Gold Medal Award, which is the academy’s highest honor.
In an interview, Dr. Elston reflected on his mentors, shared how he manages his many responsibilities as a clinician, teacher, and editor, and talked about the promising future of dermatology.
Who inspired you most to pursue a career in medicine? My grandmother, Annie Elston, was a physician and dedicated her life to helping others. She was a front-line medic during World War I, helped to run a neonatal syphilis ward after the war, and practiced pediatrics in New York City until her death. She was a great role model.
Did you enter medical school knowing that you wanted to become a dermatologist? If not, what was the turning point for you? I didn’t really know much about dermatology when I entered medical school. I fell in love with the specialty during a rotation.
What was the most memorable experience from your dermatology residency at Walter Reed Army Medical Center? There were so many interesting patients, including many tropical diseases.
Why did you choose to pursue a fellowship in dermatopathology? What was it about this subspeciality that piqued your interest? Great teachers, including Tim Berger, MD, George Lupton, MD, and Dean Pearson, MD. They inspired me to seek a dermpath fellowship and I was lucky enough to train with Wilma Bergfeld, MD.
In your opinion, what’s been the most important advance in dermatopathology to date?
Immunohistochemistry changed the specialty. Now molecular diagnostics is a second wave of major advancement.
How do you stay passionate about both dermatology and dermatopathology? The patients, residents, and fellows keep it interesting. It’s a two-way street. I learn as much as I teach.
You’ve had a remarkable run at the Journal of the American Academy of Dermatology, starting as deputy editor in 2008 before becoming editor in 2018. What’s been most rewarding about this role for you? It is a labor of love and such a privilege to see everyone’s best work.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint? Fear of the unknown is always a challenge with a new epidemic and worse with a pandemic. The patients still needed to be seen but it was a challenge with some buildings closed and some personnel afraid to come to work.
Is there anything you would tell your younger self in terms of career advice? Enjoy every step of the journey.
Considering your various work responsibilities as a clinician, teacher, and editor, what’s your strategy for achieving a work-life balance? A good friend of mine is fond of saying that balance is an illusion. There is only resilience. I believe the truth lies somewhere in between. Make time for family, and decide what has to get done today and what can wait until tomorrow.
What development in dermatology are you most excited about in the next 5 years? We are in a golden age of therapeutic innovations that are life changing and lifesaving for our patients. I never would have believed I would see complete cures of patients with widely metastatic melanoma. From psoriasis to eczema to malignancy, our therapeutic armamentarium is dramatically better each year. It makes the practice of medicine exciting.
who has authored more than 600 peer-reviewed publications and 92 textbook chapters.
After earning his undergraduate degree from Pennsylvania State University and his medical degree from Jefferson Medical College in Philadelphia, Dr. Elston completed an internship and a dermatology residency at Walter Reed Army Medical Center in Washington, as well as a dermatopathology fellowship at the Cleveland Clinic. He currently is professor and chair of the department of dermatology and dermatologic surgery at the Medical University of South Carolina in Charleston.
Dr. Elston is one of five authors of “Andrews’ Diseases of the Skin),” coauthor with Tammie Ferringer, MD, of the “Dermatopathology” textbook, and editor in chief of the Requisites in Dermatology series of textbooks. In 2018, he succeeded Bruce H. Thiers, MD, as editor of the Journal of the American Academy of Dermatology and in 2021, received the AAD’s Gold Medal Award, which is the academy’s highest honor.
In an interview, Dr. Elston reflected on his mentors, shared how he manages his many responsibilities as a clinician, teacher, and editor, and talked about the promising future of dermatology.
Who inspired you most to pursue a career in medicine? My grandmother, Annie Elston, was a physician and dedicated her life to helping others. She was a front-line medic during World War I, helped to run a neonatal syphilis ward after the war, and practiced pediatrics in New York City until her death. She was a great role model.
Did you enter medical school knowing that you wanted to become a dermatologist? If not, what was the turning point for you? I didn’t really know much about dermatology when I entered medical school. I fell in love with the specialty during a rotation.
What was the most memorable experience from your dermatology residency at Walter Reed Army Medical Center? There were so many interesting patients, including many tropical diseases.
Why did you choose to pursue a fellowship in dermatopathology? What was it about this subspeciality that piqued your interest? Great teachers, including Tim Berger, MD, George Lupton, MD, and Dean Pearson, MD. They inspired me to seek a dermpath fellowship and I was lucky enough to train with Wilma Bergfeld, MD.
In your opinion, what’s been the most important advance in dermatopathology to date?
Immunohistochemistry changed the specialty. Now molecular diagnostics is a second wave of major advancement.
How do you stay passionate about both dermatology and dermatopathology? The patients, residents, and fellows keep it interesting. It’s a two-way street. I learn as much as I teach.
You’ve had a remarkable run at the Journal of the American Academy of Dermatology, starting as deputy editor in 2008 before becoming editor in 2018. What’s been most rewarding about this role for you? It is a labor of love and such a privilege to see everyone’s best work.
During the peak of the COVID-19 pandemic, what were your most significant challenges from both a clinical and a personal standpoint? Fear of the unknown is always a challenge with a new epidemic and worse with a pandemic. The patients still needed to be seen but it was a challenge with some buildings closed and some personnel afraid to come to work.
Is there anything you would tell your younger self in terms of career advice? Enjoy every step of the journey.
Considering your various work responsibilities as a clinician, teacher, and editor, what’s your strategy for achieving a work-life balance? A good friend of mine is fond of saying that balance is an illusion. There is only resilience. I believe the truth lies somewhere in between. Make time for family, and decide what has to get done today and what can wait until tomorrow.
What development in dermatology are you most excited about in the next 5 years? We are in a golden age of therapeutic innovations that are life changing and lifesaving for our patients. I never would have believed I would see complete cures of patients with widely metastatic melanoma. From psoriasis to eczema to malignancy, our therapeutic armamentarium is dramatically better each year. It makes the practice of medicine exciting.
Painful Ulcerating Lesions on the Breast
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
The Diagnosis: Cystic Neutrophilic Granulomatous Mastitis
The histopathologic findings in our patient were characteristic of cystic neutrophilic granulomatous mastitis (CNGM), a rare granulomatous mastitis associated with Corynebacterium and suppurative lipogranulomas. Although not seen in our patient, the lipid vacuoles may contain gram-positive bacilli.1 The surrounding mixed inflammatory infiltrate contains Langerhans giant cells, lymphocytes, and neutrophils. Cystic neutrophilic granulomatous mastitis is seen in parous women of reproductive age. Physical examination demonstrates a palpable painful mass on the breast. Wound cultures frequently are negative, likely due to difficulty culturing Corynebacterium and prophylactic antibiotic treatment. Given the association with Corynebacterium species, early diagnosis of CNGM is essential in offering patients the most appropriate treatment. Prolonged antibiotic therapy specifically directed to corynebacteria is required, sometimes even beyond resolution of clinical symptoms. The diagnosis of CNGM often is missed or delayed due to its rarity and many potential mimickers. Clinically, CNGM may be virtually impossible to discern from invasive carcinoma.1
Our patient was treated with vancomycin and cefepime with incision and drainage as an inpatient. Upon discharge, she was started on prednisone 1 mg/kg daily tapered by 10 mg every 5 days over 1 month and doxycycline 100 mg twice daily. She was then transitioned to topical hydrocortisone and bacitracin; she reported decreased swelling and pain. No new lesions formed after the initiation of therapy; however, most lesions remained open. Cystic neutrophilic granulomatous mastitis remains a challenging entity to treat, with a variable response rate reported in the literature for antibiotics such as doxycycline and systemic and topical steroids as well as immunosuppressants including methotrexate.2,3
Cystic neutrophilic granulomatous mastitis can be distinguished from hidradenitis suppurativa clinically because ulcerating lesions can involve the superior portions of the breast in CNGM, whereas hidradenitis suppurativa typically is restricted to the lower intertriginous parts of the breast. Other mimics of CNGM can be distinguished with biopsy. Histology of pyoderma gangrenosum lacks prominent granuloma formation. Although sarcoidosis and mycobacterial infection show prominent granulomas, neither show the characteristic lipogranulomas seen in CNGM. Additionally, the granulomas of sarcoidosis are much larger and deeper than CNGM. Mycobacterial granulomas also typically reveal bacilli with acid-fast bacilli staining or via wound culture.
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
- Wu JM, Turashvili G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. 2020;73:445-453. doi:10.1136/jclinpath-2019-206180
- Steuer AB, Stern MJ, Cobos G, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156:460-464. doi:10.1001/jamadermatol.2019.4516
- Dobinson HC, Anderson TP, Chambers ST, et al. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species [published online July 1, 2015]. J Clin Microbiol. 2015;53:2895-2899. doi:10.1128/JCM.00760-15
A 36-year-old puerperal woman presented with painful, unilateral, ulcerating breast lesions (top) of 3 months’ duration that developed during pregnancy and drained pus with blood. No improvement was seen with antibiotics or incision and drainage. Biopsy of a lesion showed stellate granulomas with cystic spaces and suppurative lipogranulomas where central lipid vacuoles were rimmed by neutrophils and an outer cuff of epithelioid histiocytes (bottom). Acid-fast bacilli, Grocott-Gomori methenamine-silver, Gram, and Steiner staining did not reveal any microorganisms. Additionally, wound cultures were negative.