User login
Exophytic Firm Papulonodule on the Labia in a Patient With Nonspecific Gastrointestinal Symptoms
The Diagnosis: Cutaneous Crohn Disease
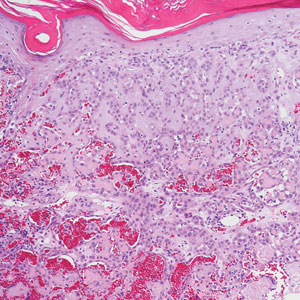
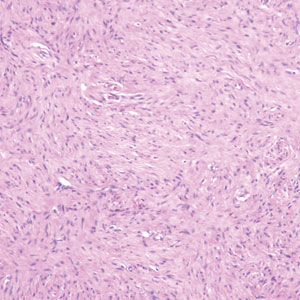
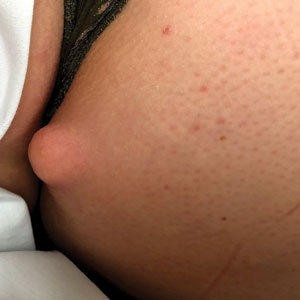
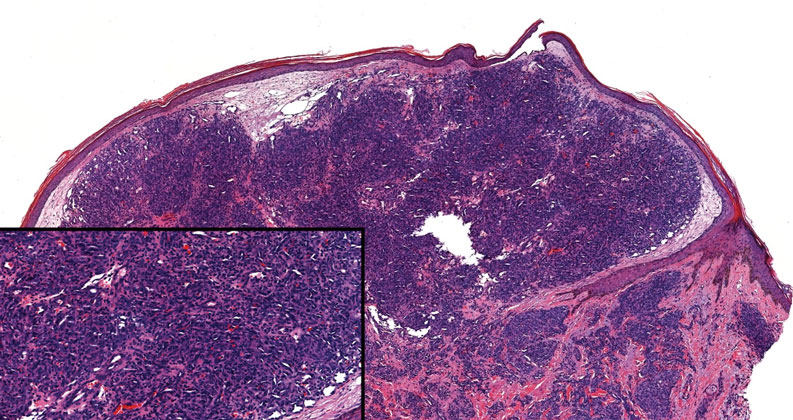
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).
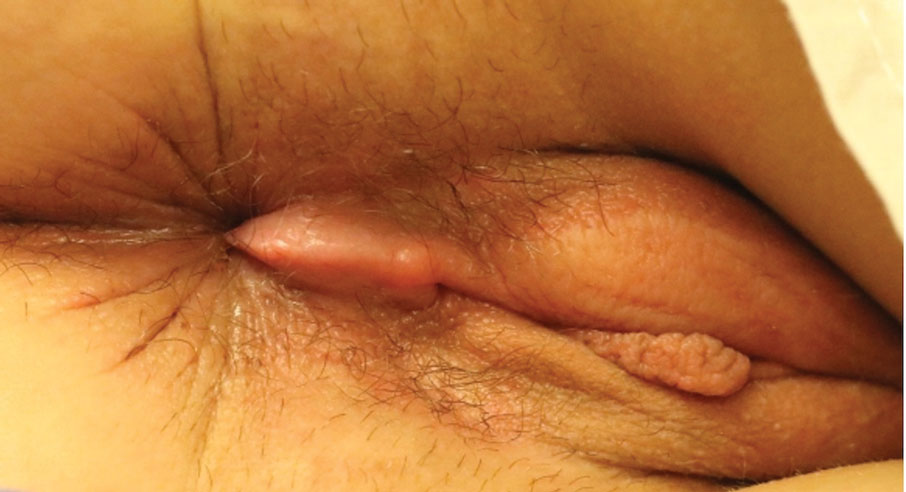
In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.
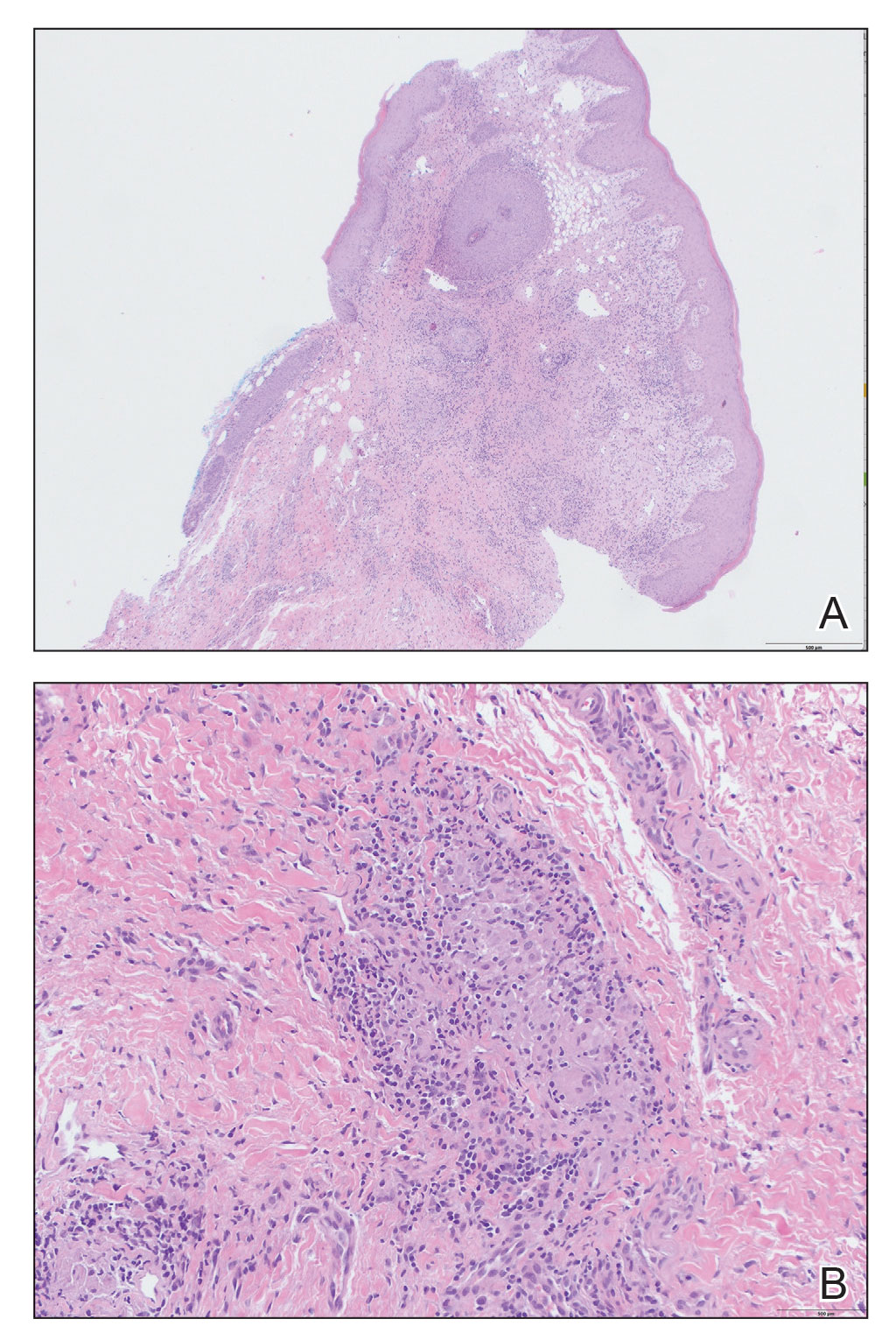
Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
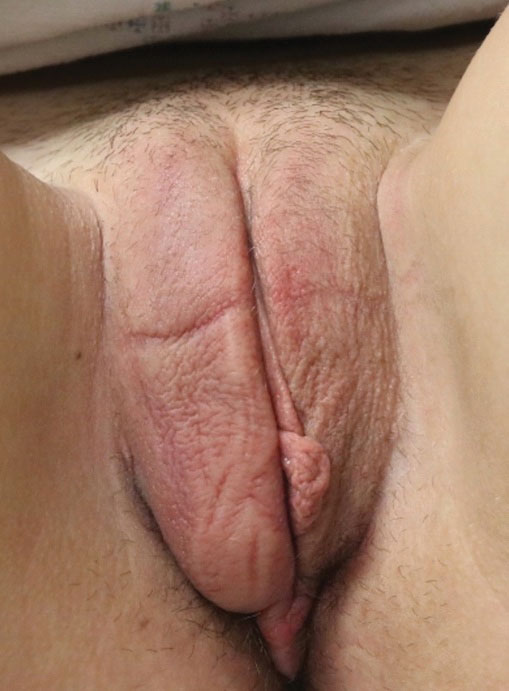
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
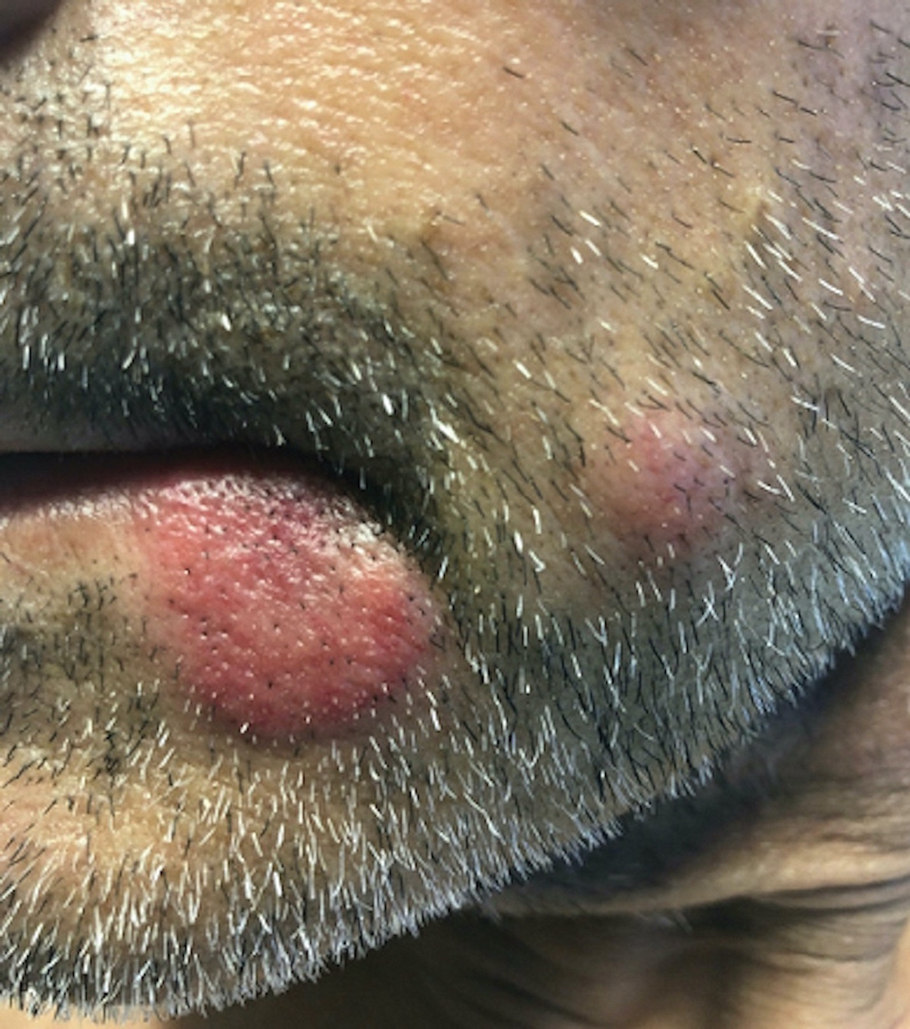
An 18-year-old woman with chronic constipation presented with an enlarging, painful, and edematous “lump” in the perineum of 1 year’s duration. The lesion became firmer and more painful with bowel movements. Physical examination revealed an enlarged right labia majora, as well as a pink to flesh-colored, exophytic, firm papulonodule in the perineum posterior to the right labia. The patient concomitantly was following with gastroenterology due to abdominal pain that worsened with eating, as well as constipation, nausea, weight loss, and rectal bleeding of 5 years’ duration. The patient denied rash, joint arthralgia, or oral ulcers. A biopsy from the labial lesion was performed.
Rapidly Growing Nodule Within a Previously Radiated Area of the Scalp
The Diagnosis: Pseudoangiomatous Squamous Cell Carcinoma
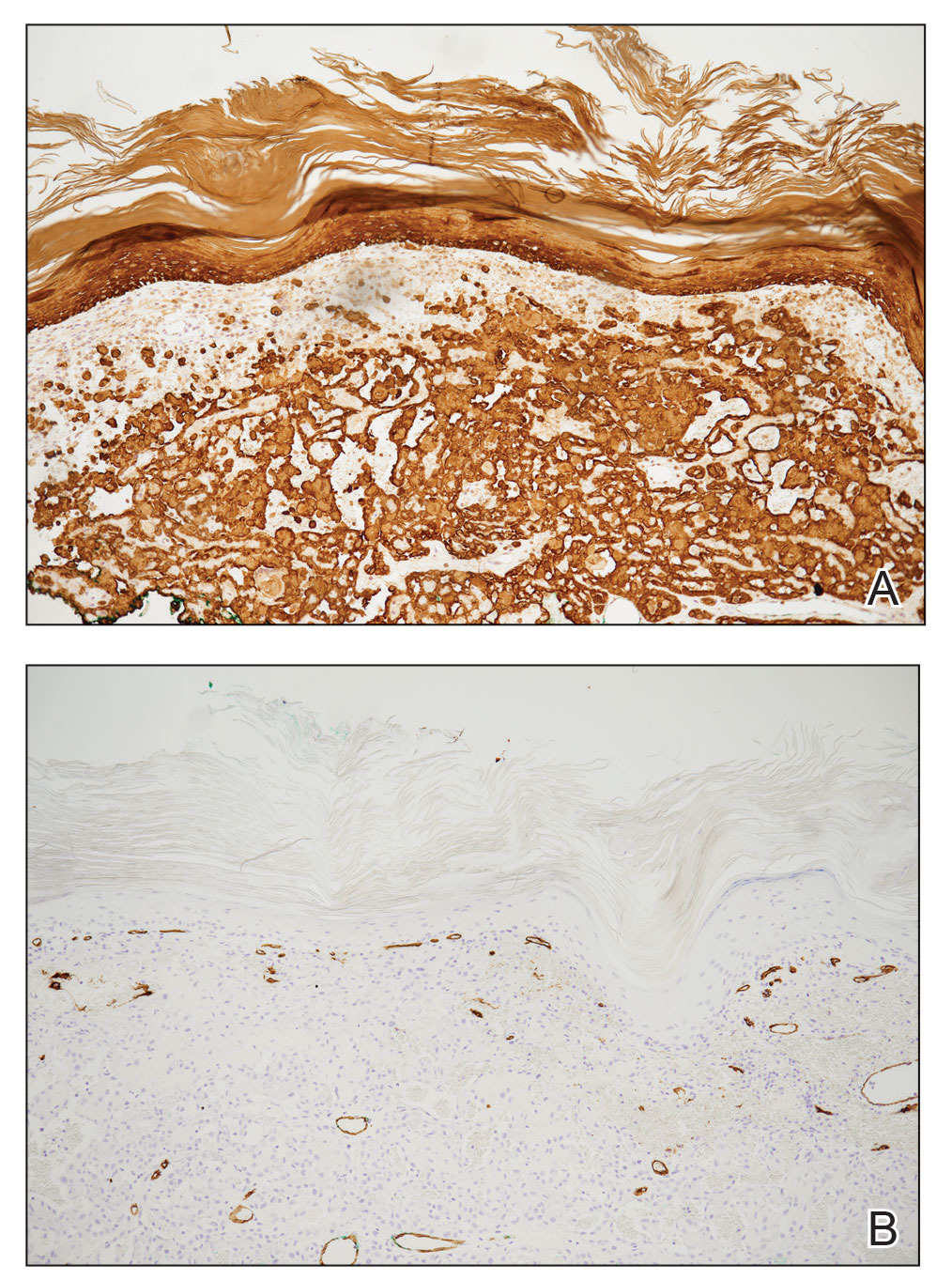
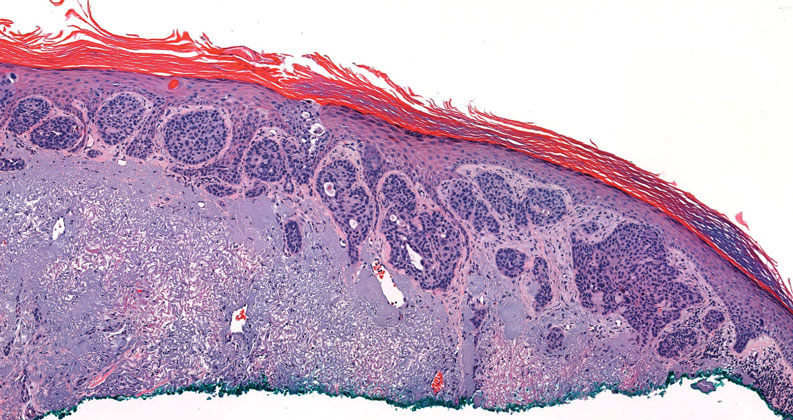
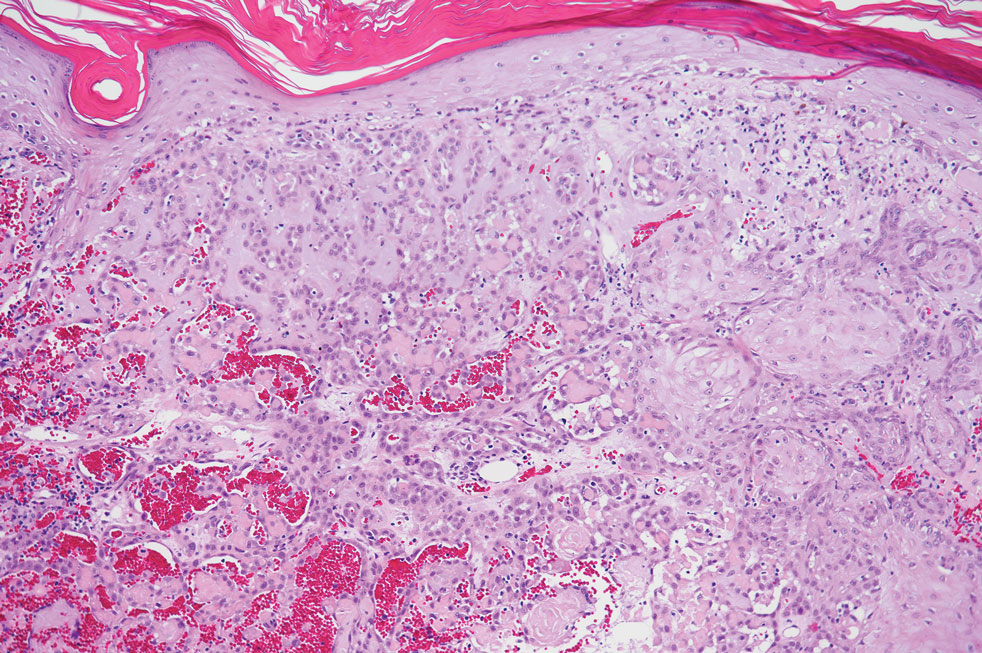
Pseudoangiomatous squamous cell carcinoma (PSCC), a variant of acantholytic squamous cell carcinoma (SCC), is a rare epithelial neoplasm that can mimic angiosarcoma.1 Clinically, PSCC presents as a white-gray ulcer or nodular pink tumor on sun-exposed areas, typically on the head and neck. Due to its increased potential for metastasis, this variant of SCC is considered particularly aggressive. Histologically, PSCC shows nests of acantholytic atypical keratinocytes arranged in anastomosing arrays that form pseudovascular or pseudoglandular structures.2 Acantholytic spaces frequently are filled with erythrocytes. Immunohistochemically, PSCC tumor cells express classic squamous markers such as cytokeratin (CK) 5 and p63 but not vascular markers such as CD31, CD34, and von Willebrand factor.3 In our patient, histopathology of the lesion revealed invasive nests, lobules, and interconnected columns of well-differentiated squamous tumor cells that emanated from the base of the epidermis. The tumor exhibited acantholysis forming ectatic and slitlike spaces, some of which contained erythrocytes. The neoplastic cells, including those lining pseudovascular spaces, positively stained for CK5 (Figure 1A) and nuclear p63 but lacked reactivity to CD31 (Figure 1B) and CD34, corroborating squamous and not vascular differentiation. Current treatment guidelines include Mohs micrographic surgery, excisional surgery, or radiation.4 Our patient’s lesion was completely removed by Mohs micrographic surgery. Three months later, there was no evidence of recurrence.
Angiosarcoma is an aggressive neoplasm associated with a poor prognosis and 5-year survival rate of 30% to 40%. The etiology of angiosarcoma still is unclear, but identified risk factors include prior radiation therapy, lymphedema (Stewart-Treves syndrome), and genetic predisposition.5 In the skin, angiosarcoma often occurs in the head and neck region, accounting for 60% of cutaneous cases.5,6 Early in the disease, most patients present with a bruiselike lesion on the scalp or forehead, often delaying the diagnosis.6 As the cancer progresses, tissue infiltration, edema, and hemorrhage contribute to the formation of violaceous nodules, which eventually prompt for biopsy. Angiosarcoma spans a broad histologic spectrum depending on the cytology of malignant cells (eg, spindle, small round, epithelioid) and their capacity for vasoformation. Welldifferentiated angiosarcoma shows retiform slitlike spaces in between collagen bundles that are lined by hyperchromatic hobnailing endothelial cells (Figure 2).7 Epithelioid angiosarcoma can be mistaken for SCC.8 Immunohistochemically, angiosarcoma stains positively for CD31, CD34, ETS-related gene 1, D2-40, and factor VIII.9 In our patient, the neoplasm was negative for vascular markers CD31 and CD34.
Bacillary angiomatosis (BA), caused by Bartonella henselae, is a rare disease that first was identified in HIV patients with diminished CD4+ T-cell counts. In the skin, BA often manifests as centrally ulcerated, single or clustered, reddish-purple nodules.10 Histologically, it is characterized by highly vascularized, histiocyterich infiltrates with admixed neutrophils and plasma cells (Figure 3). Capillaries often proliferate in a lobular fashion.11 Atypical cytology with areas of necrosis may mimic angiosarcoma.12 The pathognomonic feature of BA is the presence of enlarged histiocytes with pink-purplish cytoplasm corresponding to intracytoplasmic aggregates of bacteria, which can be revealed by Warthin-Starry or Grocott-Gomori methenamine-silver staining. Immunohistochemically, proliferative benign capillaries are highlighted by CD34 and CD31, and histiocytes are decorated by CD68.12 This diagnosis was excluded based on the patient’s history, clinical presentation, and positive staining for CK5 and p63.
Squamoid eccrine ductal carcinoma is an exceedingly rare subtype of eccrine carcinoma that mimics SCC both clinically and histologically.13 It most often occurs on the head and neck of elderly patients. This neoplasm can look similar to SCC and its variants, including PSCC. Histologically, squamoid eccrine ductal carcinoma exhibits a biphasic growth pattern.14 Well-differentiated squamous dysplasia transitions to carcinoma with eccrine duct formation as the tumor percolates deep into the dermis (Figure 4). As a result, superficial skin biopsies often lead to an incorrect diagnosis.15 Unlike SCC, the risk for locoregional and widespread metastasis is elevated. Identifying ducts in the deep aspect of the tumor is critical, thus immunohistochemical staining for carcinoembryonic antigen and epithelial membrane antigen is paramount for the diagnosis.15 Pseudoangiomatous SCC will stain negative for carcinoembryonic antigen, as was the case in our patient.
Pseudoepitheliomatous hyperplasia is a benign histologic reaction that can result from trauma, chronic inflammation (ie, pyoderma gangrenosum), tattoo placement, underlying neoplasia or fungal infection, or a spider bite reaction.14,15 It most commonly is seen as a well-demarcated nodule or plaque associated with scaling or crusting. Papules vary in size from less than 1 cm to several centimeters. Histologically, it is defined by an acanthotic proliferation of the adnexal epithelium and epidermis (Figure 5).16,17 Irregular strands, cords, and nests of squamoid cells can extend into the dermis.18 It can closely mimic SCC, but there are a few key differences. Pseudoepitheliomatous hyperplasia will not display atypical mitotic figures or atypical nuclei and will never invade lymphatics or vascular systems.19 Pseudoepitheliomatous hyperplasia shows identical histology to well-differentiated SCC, and thus clinicopathologic correlation and mindful histologic evaluation are crucial. The presence of an increased influx of neutrophils and histiocytes should prompt for microbial stains or deeper sectioning. A superficial biopsy should be followed by a deep biopsy. In our patient, microorganismal stains were negative.
- Kiyohara T, Miyamoto M, Shijimaya T, et al. Pseudovascular squamous cell carcinoma: a review of the published work and reassessment of prognosis. J Dermatol. 2018;45:1448-1451.
- Nagore E, Sánchez-Motilla JM, Pérez-Vallés A, et al. Pseudovascular squamous cell carcinoma of the skin. Clin Exp Dermatol. 2000;25:206-208.
- Han X, Lin X, Shao X. Pseudovascular adenoid squamous cell carcinoma of the tongue: a case report and literature review. Int J Clin Exp Pathol. 2020;13:1086-1089.
- Singh S, Bisht N, Purkayastha A, et al. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5:165-168.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post‐radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Shilpa K, Leelavathy B, Gorur D, et al. Early-onset epithelioid angiosarcoma: diagnostic enigma, a rare case report. Indian J Dermatopathol Diagn Dermatol. 2019;6:36-38.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe [published online May 4, 2017]. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Hoffman CF, Papadopoulos D, Palmer DM, et al. A case report of bacillary angiomatosis in a patient infected with human immunodeficiency virus. Cutis. 2002;69:175-178.
- Biwer E, Uerlich M, Wimheuer R, et al. Bacillary angiomatosis: an important differential diagnosis in patients with HIV. Am J Dermatopathol. 1994;16:110.
- Medeiros LJ, Miranda RN. Bacillary angiomatosis. In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Elsevier; 2018:58-63.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Mckissack S, Wohltmann W, Dalton S, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;2:19
- Chow P, Goddard L, Greenway H, et al. Squamoid eccrine ductal carcinoma: the Scripps experience. Dermatol Surg. 2021;47:1115-1117.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126.
- Lynch JM. Understanding pseudoepitheliomatous hyperplasia. Pathol Case Rev. 2004;9:36-45.
- Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous aspergillus. Am J Dermatopathol. 2001;23:224-226.
The Diagnosis: Pseudoangiomatous Squamous Cell Carcinoma
Pseudoangiomatous squamous cell carcinoma (PSCC), a variant of acantholytic squamous cell carcinoma (SCC), is a rare epithelial neoplasm that can mimic angiosarcoma.1 Clinically, PSCC presents as a white-gray ulcer or nodular pink tumor on sun-exposed areas, typically on the head and neck. Due to its increased potential for metastasis, this variant of SCC is considered particularly aggressive. Histologically, PSCC shows nests of acantholytic atypical keratinocytes arranged in anastomosing arrays that form pseudovascular or pseudoglandular structures.2 Acantholytic spaces frequently are filled with erythrocytes. Immunohistochemically, PSCC tumor cells express classic squamous markers such as cytokeratin (CK) 5 and p63 but not vascular markers such as CD31, CD34, and von Willebrand factor.3 In our patient, histopathology of the lesion revealed invasive nests, lobules, and interconnected columns of well-differentiated squamous tumor cells that emanated from the base of the epidermis. The tumor exhibited acantholysis forming ectatic and slitlike spaces, some of which contained erythrocytes. The neoplastic cells, including those lining pseudovascular spaces, positively stained for CK5 (Figure 1A) and nuclear p63 but lacked reactivity to CD31 (Figure 1B) and CD34, corroborating squamous and not vascular differentiation. Current treatment guidelines include Mohs micrographic surgery, excisional surgery, or radiation.4 Our patient’s lesion was completely removed by Mohs micrographic surgery. Three months later, there was no evidence of recurrence.

Angiosarcoma is an aggressive neoplasm associated with a poor prognosis and 5-year survival rate of 30% to 40%. The etiology of angiosarcoma still is unclear, but identified risk factors include prior radiation therapy, lymphedema (Stewart-Treves syndrome), and genetic predisposition.5 In the skin, angiosarcoma often occurs in the head and neck region, accounting for 60% of cutaneous cases.5,6 Early in the disease, most patients present with a bruiselike lesion on the scalp or forehead, often delaying the diagnosis.6 As the cancer progresses, tissue infiltration, edema, and hemorrhage contribute to the formation of violaceous nodules, which eventually prompt for biopsy. Angiosarcoma spans a broad histologic spectrum depending on the cytology of malignant cells (eg, spindle, small round, epithelioid) and their capacity for vasoformation. Welldifferentiated angiosarcoma shows retiform slitlike spaces in between collagen bundles that are lined by hyperchromatic hobnailing endothelial cells (Figure 2).7 Epithelioid angiosarcoma can be mistaken for SCC.8 Immunohistochemically, angiosarcoma stains positively for CD31, CD34, ETS-related gene 1, D2-40, and factor VIII.9 In our patient, the neoplasm was negative for vascular markers CD31 and CD34.

Bacillary angiomatosis (BA), caused by Bartonella henselae, is a rare disease that first was identified in HIV patients with diminished CD4+ T-cell counts. In the skin, BA often manifests as centrally ulcerated, single or clustered, reddish-purple nodules.10 Histologically, it is characterized by highly vascularized, histiocyterich infiltrates with admixed neutrophils and plasma cells (Figure 3). Capillaries often proliferate in a lobular fashion.11 Atypical cytology with areas of necrosis may mimic angiosarcoma.12 The pathognomonic feature of BA is the presence of enlarged histiocytes with pink-purplish cytoplasm corresponding to intracytoplasmic aggregates of bacteria, which can be revealed by Warthin-Starry or Grocott-Gomori methenamine-silver staining. Immunohistochemically, proliferative benign capillaries are highlighted by CD34 and CD31, and histiocytes are decorated by CD68.12 This diagnosis was excluded based on the patient’s history, clinical presentation, and positive staining for CK5 and p63.

Squamoid eccrine ductal carcinoma is an exceedingly rare subtype of eccrine carcinoma that mimics SCC both clinically and histologically.13 It most often occurs on the head and neck of elderly patients. This neoplasm can look similar to SCC and its variants, including PSCC. Histologically, squamoid eccrine ductal carcinoma exhibits a biphasic growth pattern.14 Well-differentiated squamous dysplasia transitions to carcinoma with eccrine duct formation as the tumor percolates deep into the dermis (Figure 4). As a result, superficial skin biopsies often lead to an incorrect diagnosis.15 Unlike SCC, the risk for locoregional and widespread metastasis is elevated. Identifying ducts in the deep aspect of the tumor is critical, thus immunohistochemical staining for carcinoembryonic antigen and epithelial membrane antigen is paramount for the diagnosis.15 Pseudoangiomatous SCC will stain negative for carcinoembryonic antigen, as was the case in our patient.

Pseudoepitheliomatous hyperplasia is a benign histologic reaction that can result from trauma, chronic inflammation (ie, pyoderma gangrenosum), tattoo placement, underlying neoplasia or fungal infection, or a spider bite reaction.14,15 It most commonly is seen as a well-demarcated nodule or plaque associated with scaling or crusting. Papules vary in size from less than 1 cm to several centimeters. Histologically, it is defined by an acanthotic proliferation of the adnexal epithelium and epidermis (Figure 5).16,17 Irregular strands, cords, and nests of squamoid cells can extend into the dermis.18 It can closely mimic SCC, but there are a few key differences. Pseudoepitheliomatous hyperplasia will not display atypical mitotic figures or atypical nuclei and will never invade lymphatics or vascular systems.19 Pseudoepitheliomatous hyperplasia shows identical histology to well-differentiated SCC, and thus clinicopathologic correlation and mindful histologic evaluation are crucial. The presence of an increased influx of neutrophils and histiocytes should prompt for microbial stains or deeper sectioning. A superficial biopsy should be followed by a deep biopsy. In our patient, microorganismal stains were negative.

The Diagnosis: Pseudoangiomatous Squamous Cell Carcinoma
Pseudoangiomatous squamous cell carcinoma (PSCC), a variant of acantholytic squamous cell carcinoma (SCC), is a rare epithelial neoplasm that can mimic angiosarcoma.1 Clinically, PSCC presents as a white-gray ulcer or nodular pink tumor on sun-exposed areas, typically on the head and neck. Due to its increased potential for metastasis, this variant of SCC is considered particularly aggressive. Histologically, PSCC shows nests of acantholytic atypical keratinocytes arranged in anastomosing arrays that form pseudovascular or pseudoglandular structures.2 Acantholytic spaces frequently are filled with erythrocytes. Immunohistochemically, PSCC tumor cells express classic squamous markers such as cytokeratin (CK) 5 and p63 but not vascular markers such as CD31, CD34, and von Willebrand factor.3 In our patient, histopathology of the lesion revealed invasive nests, lobules, and interconnected columns of well-differentiated squamous tumor cells that emanated from the base of the epidermis. The tumor exhibited acantholysis forming ectatic and slitlike spaces, some of which contained erythrocytes. The neoplastic cells, including those lining pseudovascular spaces, positively stained for CK5 (Figure 1A) and nuclear p63 but lacked reactivity to CD31 (Figure 1B) and CD34, corroborating squamous and not vascular differentiation. Current treatment guidelines include Mohs micrographic surgery, excisional surgery, or radiation.4 Our patient’s lesion was completely removed by Mohs micrographic surgery. Three months later, there was no evidence of recurrence.

Angiosarcoma is an aggressive neoplasm associated with a poor prognosis and 5-year survival rate of 30% to 40%. The etiology of angiosarcoma still is unclear, but identified risk factors include prior radiation therapy, lymphedema (Stewart-Treves syndrome), and genetic predisposition.5 In the skin, angiosarcoma often occurs in the head and neck region, accounting for 60% of cutaneous cases.5,6 Early in the disease, most patients present with a bruiselike lesion on the scalp or forehead, often delaying the diagnosis.6 As the cancer progresses, tissue infiltration, edema, and hemorrhage contribute to the formation of violaceous nodules, which eventually prompt for biopsy. Angiosarcoma spans a broad histologic spectrum depending on the cytology of malignant cells (eg, spindle, small round, epithelioid) and their capacity for vasoformation. Welldifferentiated angiosarcoma shows retiform slitlike spaces in between collagen bundles that are lined by hyperchromatic hobnailing endothelial cells (Figure 2).7 Epithelioid angiosarcoma can be mistaken for SCC.8 Immunohistochemically, angiosarcoma stains positively for CD31, CD34, ETS-related gene 1, D2-40, and factor VIII.9 In our patient, the neoplasm was negative for vascular markers CD31 and CD34.

Bacillary angiomatosis (BA), caused by Bartonella henselae, is a rare disease that first was identified in HIV patients with diminished CD4+ T-cell counts. In the skin, BA often manifests as centrally ulcerated, single or clustered, reddish-purple nodules.10 Histologically, it is characterized by highly vascularized, histiocyterich infiltrates with admixed neutrophils and plasma cells (Figure 3). Capillaries often proliferate in a lobular fashion.11 Atypical cytology with areas of necrosis may mimic angiosarcoma.12 The pathognomonic feature of BA is the presence of enlarged histiocytes with pink-purplish cytoplasm corresponding to intracytoplasmic aggregates of bacteria, which can be revealed by Warthin-Starry or Grocott-Gomori methenamine-silver staining. Immunohistochemically, proliferative benign capillaries are highlighted by CD34 and CD31, and histiocytes are decorated by CD68.12 This diagnosis was excluded based on the patient’s history, clinical presentation, and positive staining for CK5 and p63.

Squamoid eccrine ductal carcinoma is an exceedingly rare subtype of eccrine carcinoma that mimics SCC both clinically and histologically.13 It most often occurs on the head and neck of elderly patients. This neoplasm can look similar to SCC and its variants, including PSCC. Histologically, squamoid eccrine ductal carcinoma exhibits a biphasic growth pattern.14 Well-differentiated squamous dysplasia transitions to carcinoma with eccrine duct formation as the tumor percolates deep into the dermis (Figure 4). As a result, superficial skin biopsies often lead to an incorrect diagnosis.15 Unlike SCC, the risk for locoregional and widespread metastasis is elevated. Identifying ducts in the deep aspect of the tumor is critical, thus immunohistochemical staining for carcinoembryonic antigen and epithelial membrane antigen is paramount for the diagnosis.15 Pseudoangiomatous SCC will stain negative for carcinoembryonic antigen, as was the case in our patient.

Pseudoepitheliomatous hyperplasia is a benign histologic reaction that can result from trauma, chronic inflammation (ie, pyoderma gangrenosum), tattoo placement, underlying neoplasia or fungal infection, or a spider bite reaction.14,15 It most commonly is seen as a well-demarcated nodule or plaque associated with scaling or crusting. Papules vary in size from less than 1 cm to several centimeters. Histologically, it is defined by an acanthotic proliferation of the adnexal epithelium and epidermis (Figure 5).16,17 Irregular strands, cords, and nests of squamoid cells can extend into the dermis.18 It can closely mimic SCC, but there are a few key differences. Pseudoepitheliomatous hyperplasia will not display atypical mitotic figures or atypical nuclei and will never invade lymphatics or vascular systems.19 Pseudoepitheliomatous hyperplasia shows identical histology to well-differentiated SCC, and thus clinicopathologic correlation and mindful histologic evaluation are crucial. The presence of an increased influx of neutrophils and histiocytes should prompt for microbial stains or deeper sectioning. A superficial biopsy should be followed by a deep biopsy. In our patient, microorganismal stains were negative.

- Kiyohara T, Miyamoto M, Shijimaya T, et al. Pseudovascular squamous cell carcinoma: a review of the published work and reassessment of prognosis. J Dermatol. 2018;45:1448-1451.
- Nagore E, Sánchez-Motilla JM, Pérez-Vallés A, et al. Pseudovascular squamous cell carcinoma of the skin. Clin Exp Dermatol. 2000;25:206-208.
- Han X, Lin X, Shao X. Pseudovascular adenoid squamous cell carcinoma of the tongue: a case report and literature review. Int J Clin Exp Pathol. 2020;13:1086-1089.
- Singh S, Bisht N, Purkayastha A, et al. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5:165-168.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post‐radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Shilpa K, Leelavathy B, Gorur D, et al. Early-onset epithelioid angiosarcoma: diagnostic enigma, a rare case report. Indian J Dermatopathol Diagn Dermatol. 2019;6:36-38.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe [published online May 4, 2017]. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Hoffman CF, Papadopoulos D, Palmer DM, et al. A case report of bacillary angiomatosis in a patient infected with human immunodeficiency virus. Cutis. 2002;69:175-178.
- Biwer E, Uerlich M, Wimheuer R, et al. Bacillary angiomatosis: an important differential diagnosis in patients with HIV. Am J Dermatopathol. 1994;16:110.
- Medeiros LJ, Miranda RN. Bacillary angiomatosis. In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Elsevier; 2018:58-63.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Mckissack S, Wohltmann W, Dalton S, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;2:19
- Chow P, Goddard L, Greenway H, et al. Squamoid eccrine ductal carcinoma: the Scripps experience. Dermatol Surg. 2021;47:1115-1117.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126.
- Lynch JM. Understanding pseudoepitheliomatous hyperplasia. Pathol Case Rev. 2004;9:36-45.
- Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous aspergillus. Am J Dermatopathol. 2001;23:224-226.
- Kiyohara T, Miyamoto M, Shijimaya T, et al. Pseudovascular squamous cell carcinoma: a review of the published work and reassessment of prognosis. J Dermatol. 2018;45:1448-1451.
- Nagore E, Sánchez-Motilla JM, Pérez-Vallés A, et al. Pseudovascular squamous cell carcinoma of the skin. Clin Exp Dermatol. 2000;25:206-208.
- Han X, Lin X, Shao X. Pseudovascular adenoid squamous cell carcinoma of the tongue: a case report and literature review. Int J Clin Exp Pathol. 2020;13:1086-1089.
- Singh S, Bisht N, Purkayastha A, et al. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5:165-168.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post‐radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Shilpa K, Leelavathy B, Gorur D, et al. Early-onset epithelioid angiosarcoma: diagnostic enigma, a rare case report. Indian J Dermatopathol Diagn Dermatol. 2019;6:36-38.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe [published online May 4, 2017]. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Hoffman CF, Papadopoulos D, Palmer DM, et al. A case report of bacillary angiomatosis in a patient infected with human immunodeficiency virus. Cutis. 2002;69:175-178.
- Biwer E, Uerlich M, Wimheuer R, et al. Bacillary angiomatosis: an important differential diagnosis in patients with HIV. Am J Dermatopathol. 1994;16:110.
- Medeiros LJ, Miranda RN. Bacillary angiomatosis. In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Elsevier; 2018:58-63.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Mckissack S, Wohltmann W, Dalton S, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;2:19
- Chow P, Goddard L, Greenway H, et al. Squamoid eccrine ductal carcinoma: the Scripps experience. Dermatol Surg. 2021;47:1115-1117.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126.
- Lynch JM. Understanding pseudoepitheliomatous hyperplasia. Pathol Case Rev. 2004;9:36-45.
- Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous aspergillus. Am J Dermatopathol. 2001;23:224-226.
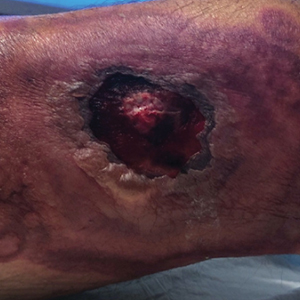
An 84-year-old man with a history of nonmelanoma skin cancer presented to our clinic with a 1.6×1.5-cm exophytic lesion on the left posterior parietal scalp. The lesion nearly doubled in size over the last 4 months. The patient received radiation therapy in this area for the treatment of basal cell carcinoma 7 years prior to presentation. A shave biopsy was performed.
Atypical Keratotic Nodule on the Knuckle
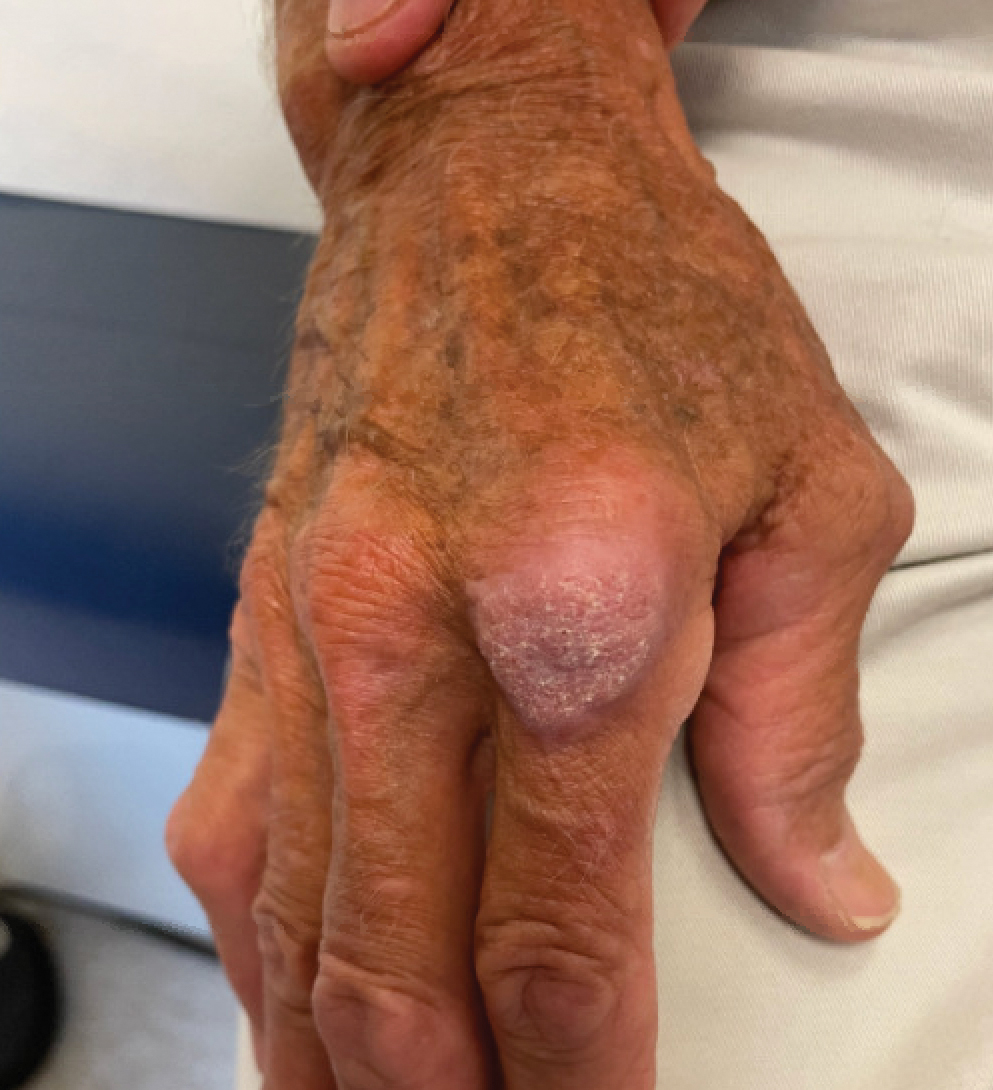
The Diagnosis: Atypical Mycobacterial Infection
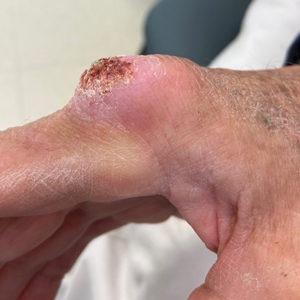
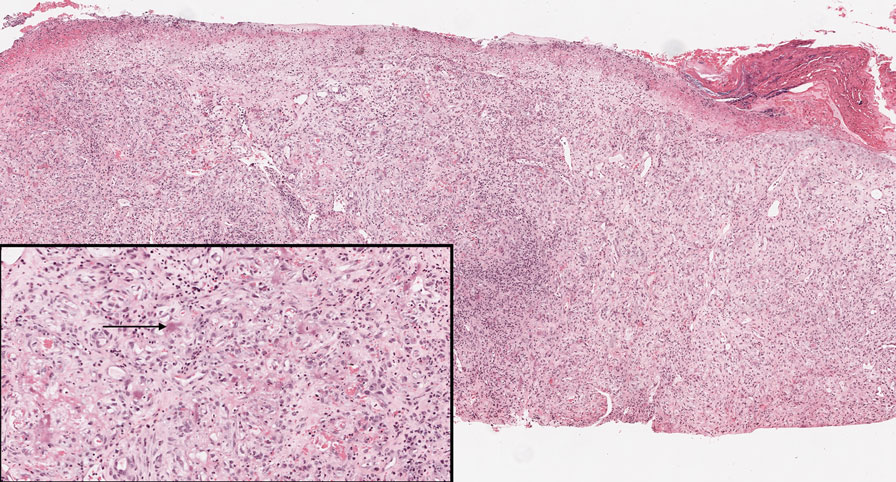
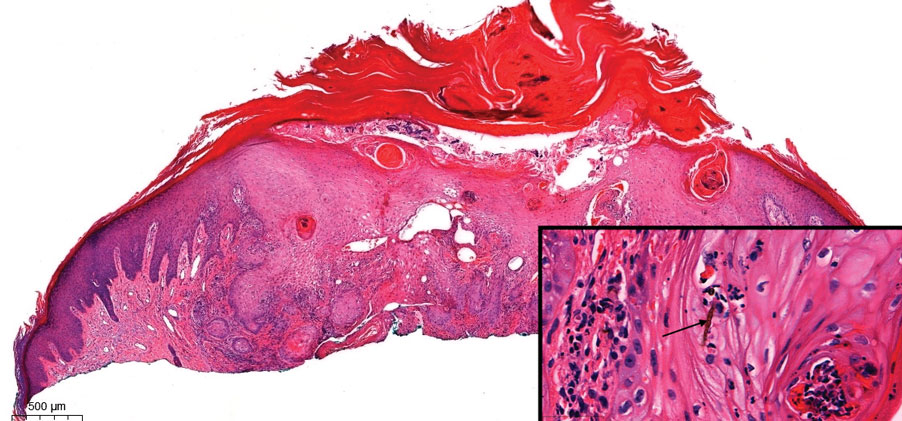
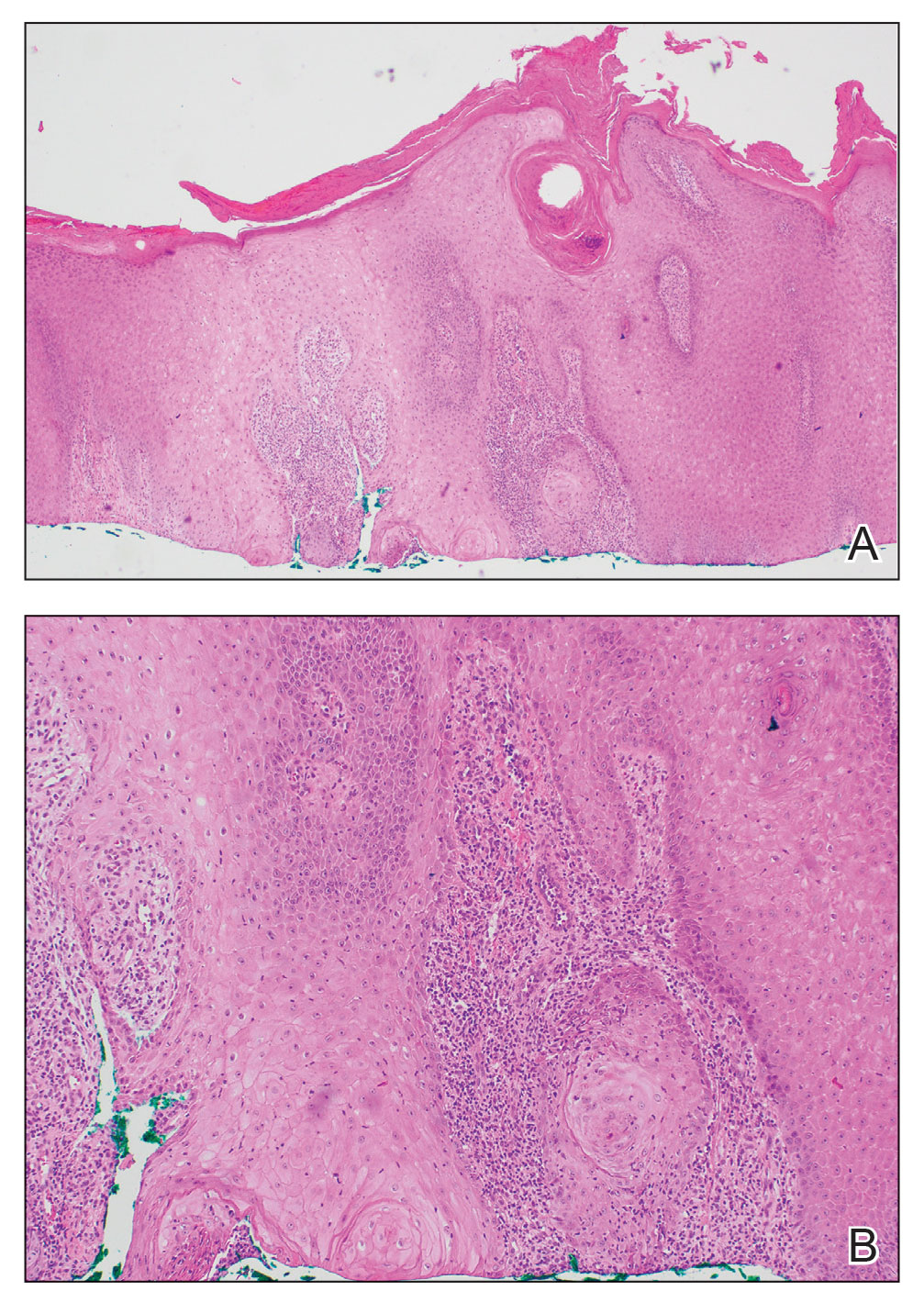
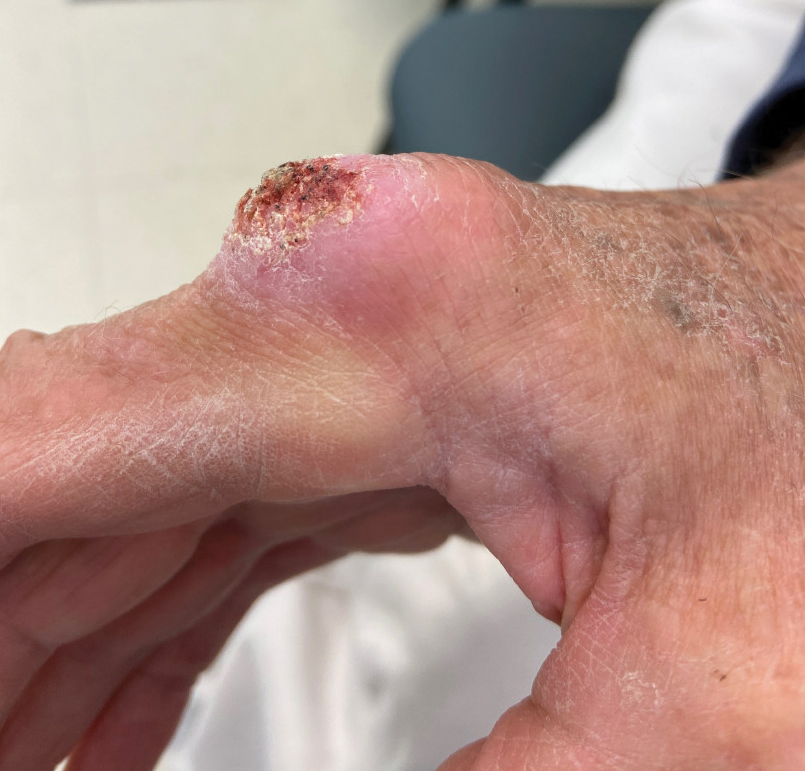
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.
The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2
Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
The Diagnosis: Atypical Mycobacterial Infection
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.

The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2

Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
The Diagnosis: Atypical Mycobacterial Infection
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.

The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2

Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
A 75-year-old man presented with a lesion on the knuckle of 5 months’ duration. He reported that the lesion initially grew very quickly before shrinking down to its current size. He denied any bleeding or pain but thought he may have had a splinter in the area around the time the lesion appeared. He reported spending a lot of time outdoors and noted several recent insect and tick bites. He also owned a boat and frequently went fishing. He previously had been treated for actinic keratoses but had no history of skin cancer and no family history of melanoma. Physical examination revealed a 2-cm erythematous nodule with central hyperkeratosis overlying the metacarpophalangeal joint of the right index finger. A shave biopsy was performed.
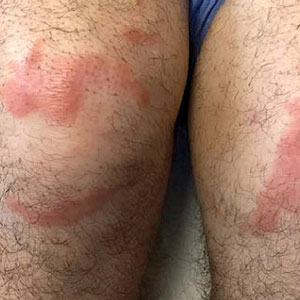
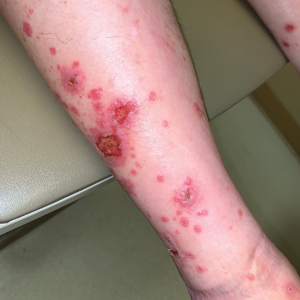
Violaceous-Purpuric Targetoid Macules and Patches With Bullae and Ulceration
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
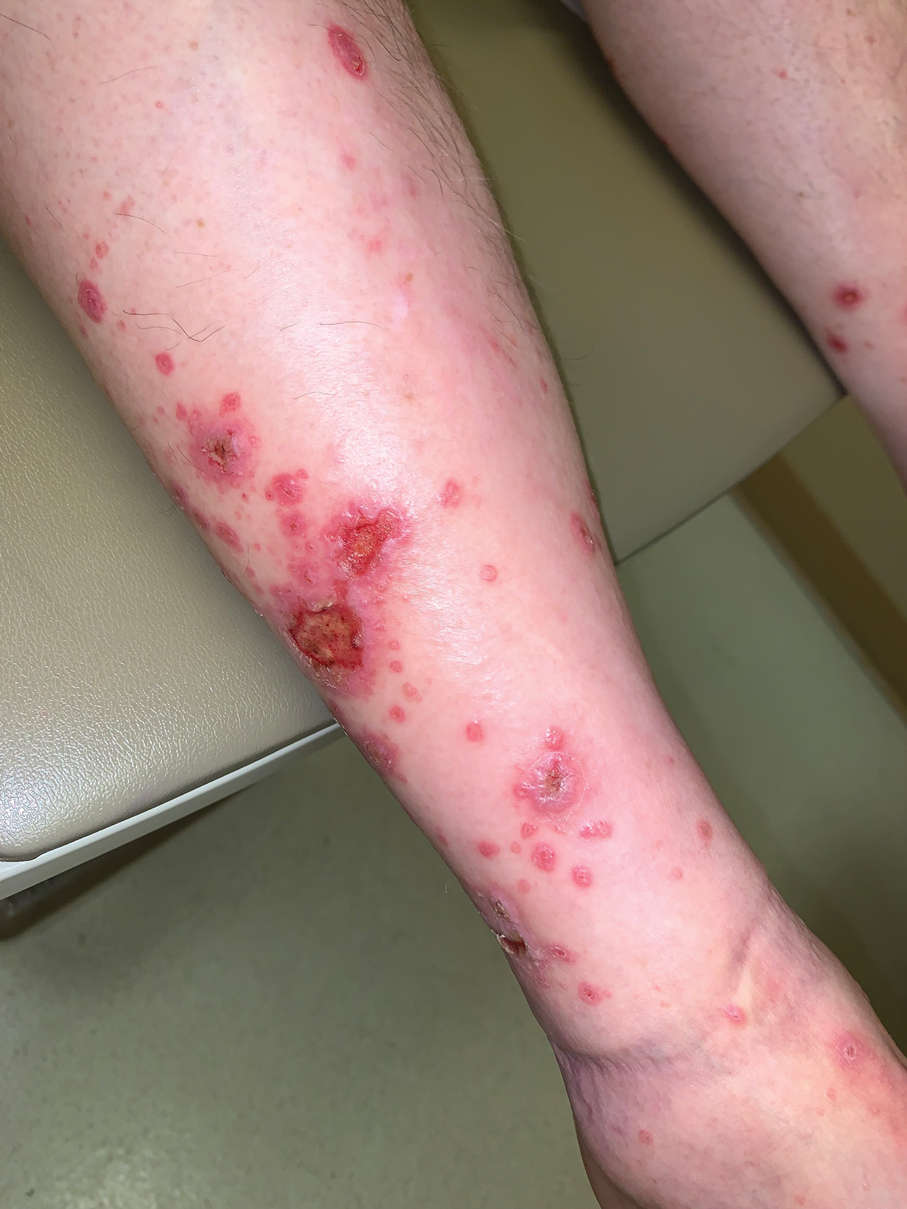
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.
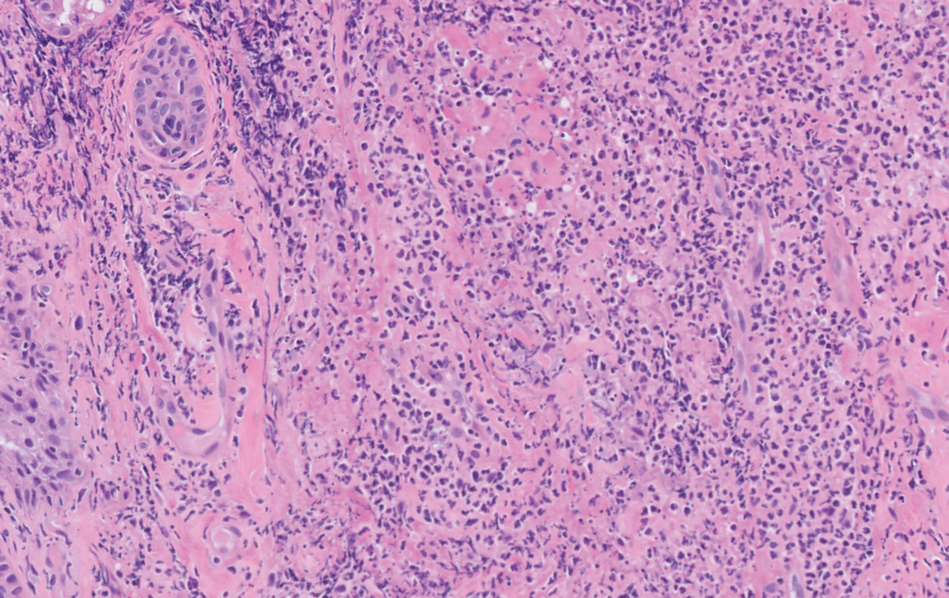
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1
The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
A 64-year-old man with long-standing myelofibrosis presented with neutropenic fevers as well as progressive painful lesions of 3 days’ duration on the legs. A bone marrow biopsy during this hospitalization demonstrated a recent progression of the patient’s myelofibrosis to acute myeloid leukemia. Physical examination revealed round to oval, violaceous, targetoid plaques. Within a week, new erythematous and nodular lesions appeared on the right arm and left vermilion border. The lesions on the legs enlarged, formed bullae, and ulcerated.
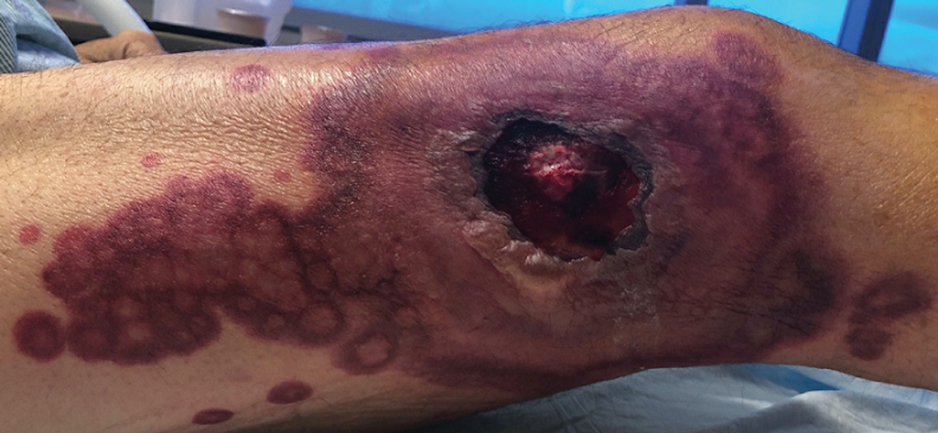
Mobile Enlarging Scalp Nodule
The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6
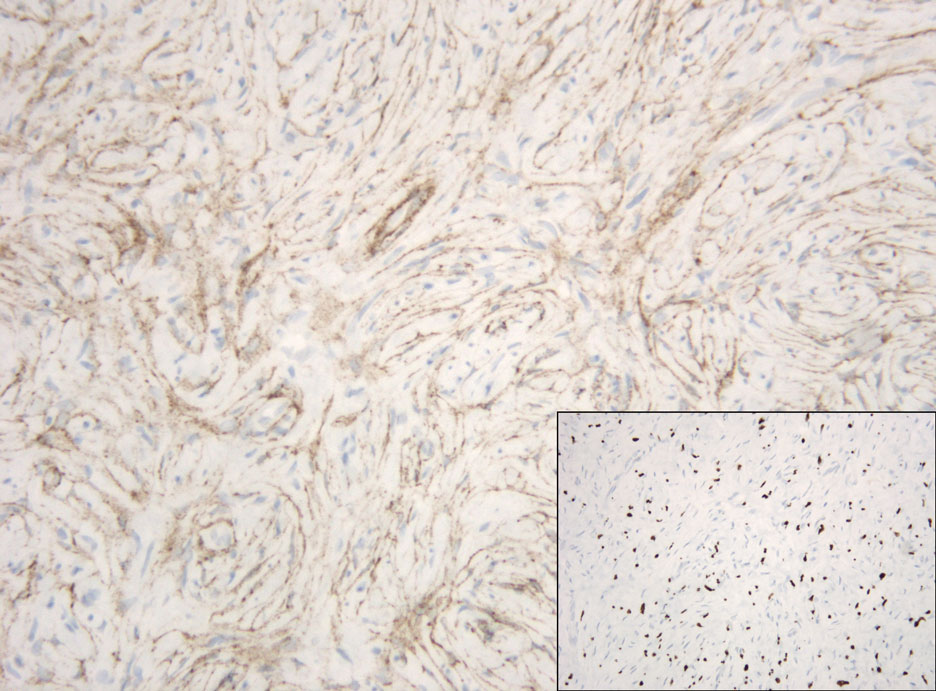
Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9
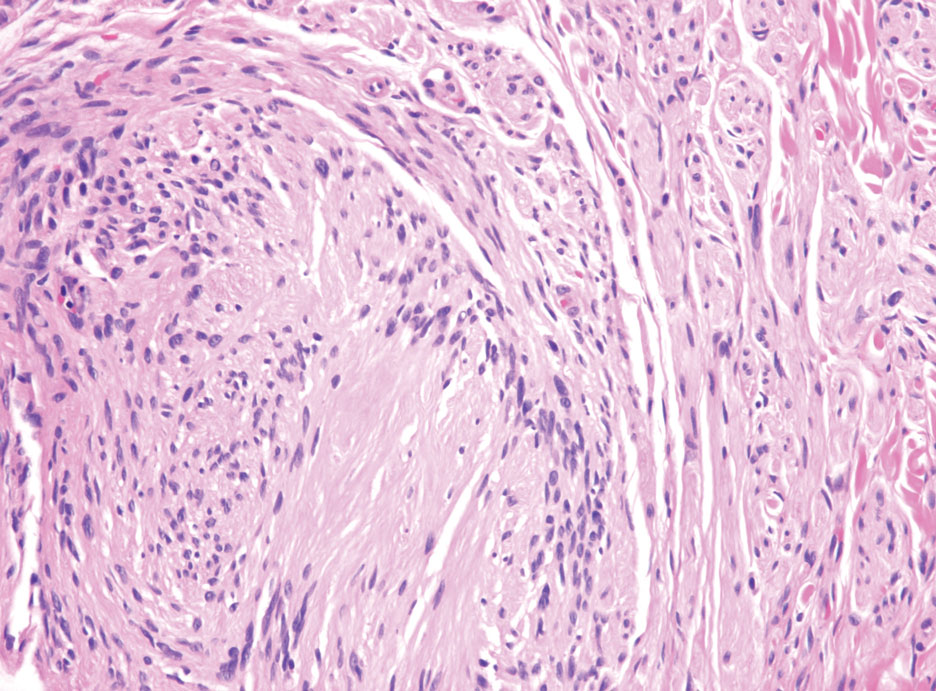
Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7
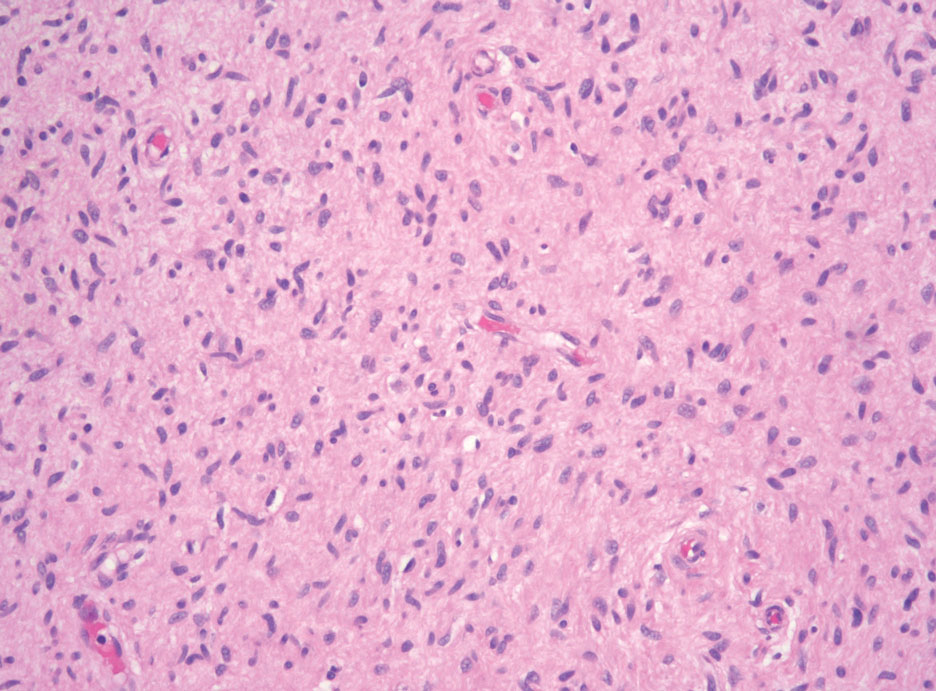
Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15
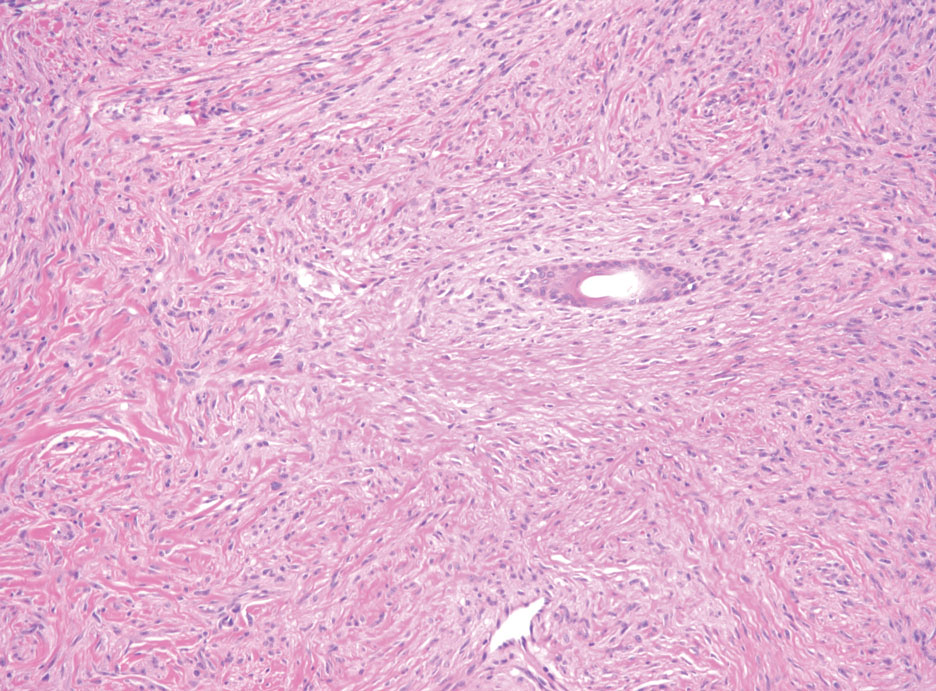
Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20
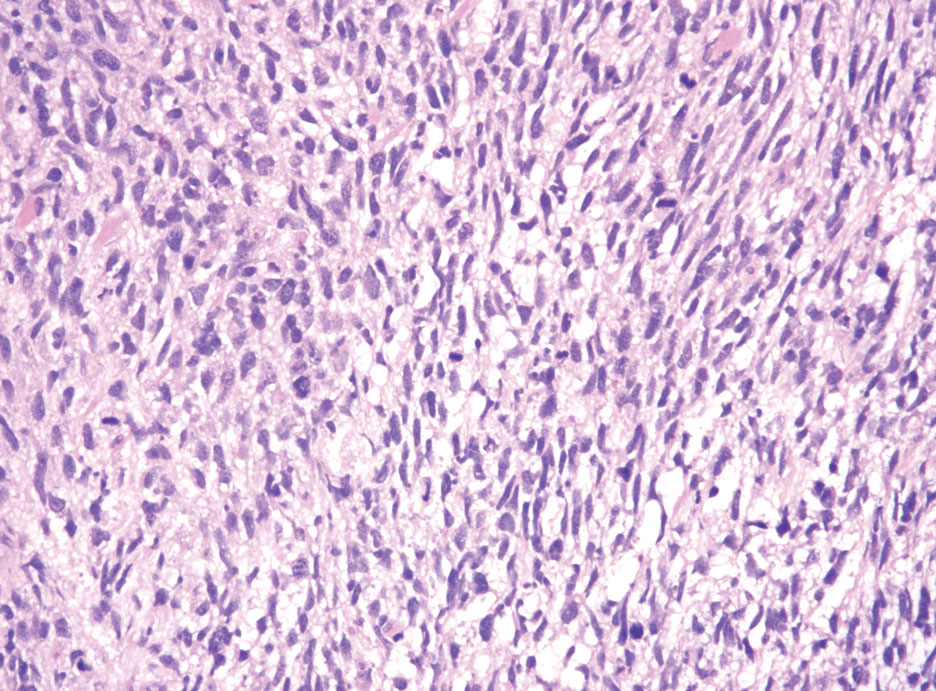
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
A 50-year-old man presented with a 2.5-cm, subcutaneous, freely mobile nodule on the occipital scalp that first appeared 35 years prior but recently had started enlarging. Histologically the lesion was well circumscribed. Immunohistochemical staining was positive for SRY-box transcription factor 10 in some of the spindle cells, and staining for epithelial membrane antigen was positive in a separate population of intermixed spindle cells.
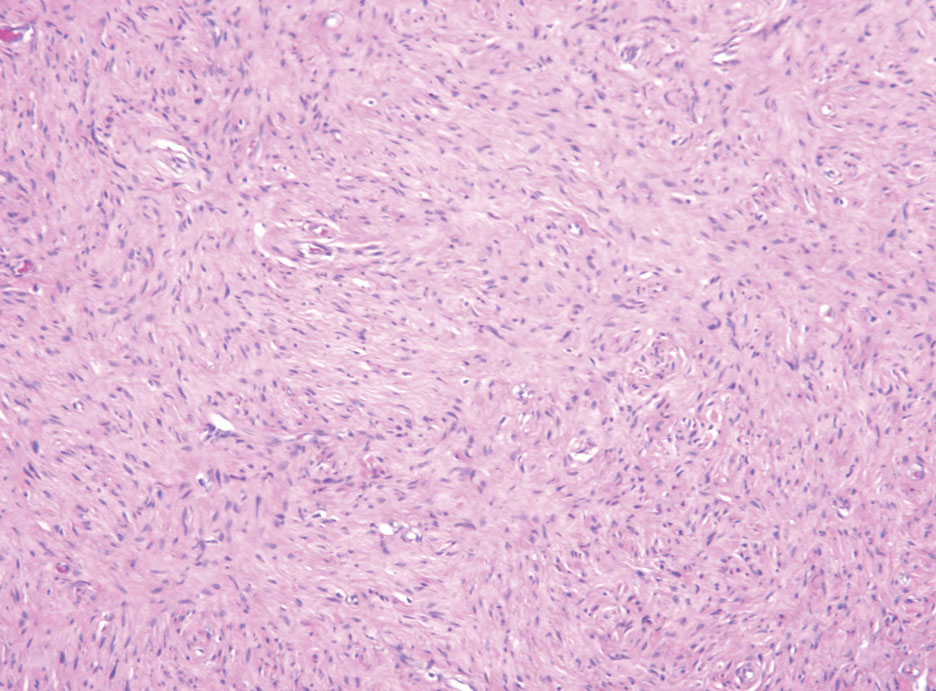
Advocacy Update: Ringing in 2023
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.
Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2
Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5
The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.
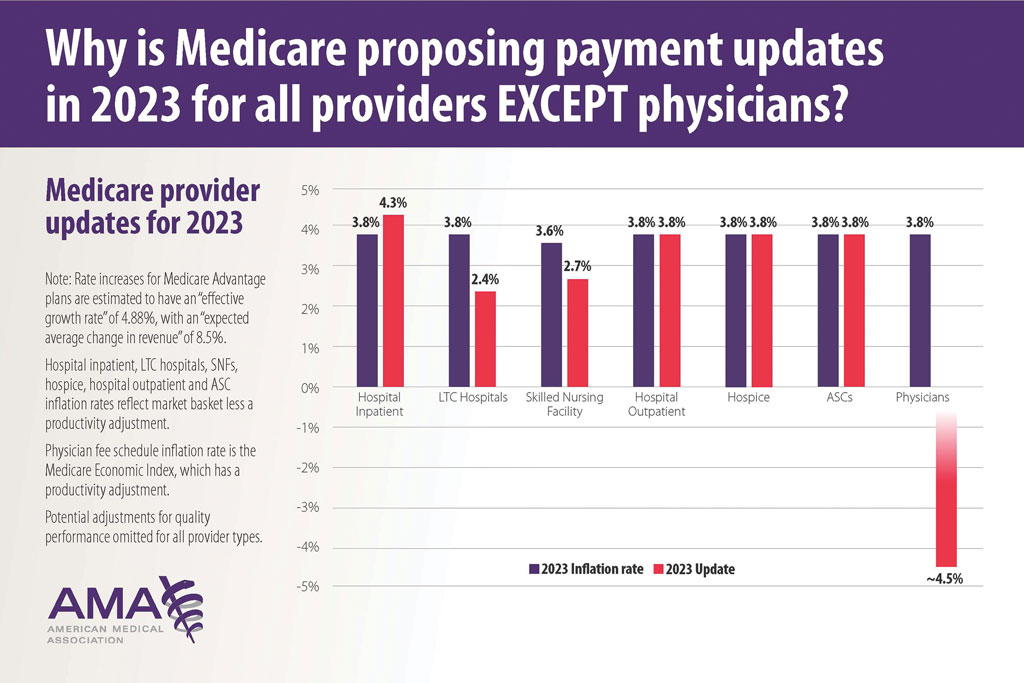
In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
Practice Points
- New digital pathology codes proposed by the American Medical Association can be used starting January 1, 2023.
- A proposed 2023 fee schedule negatively impacting dermatology practices was published by the Centers for Medicare & Medicaid Services in July 2022.
- Advocacy involvement provides a collaborative collective voice for our specialty to help our patients improve their care.
Multiple Annular Erythematous Plaques
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.
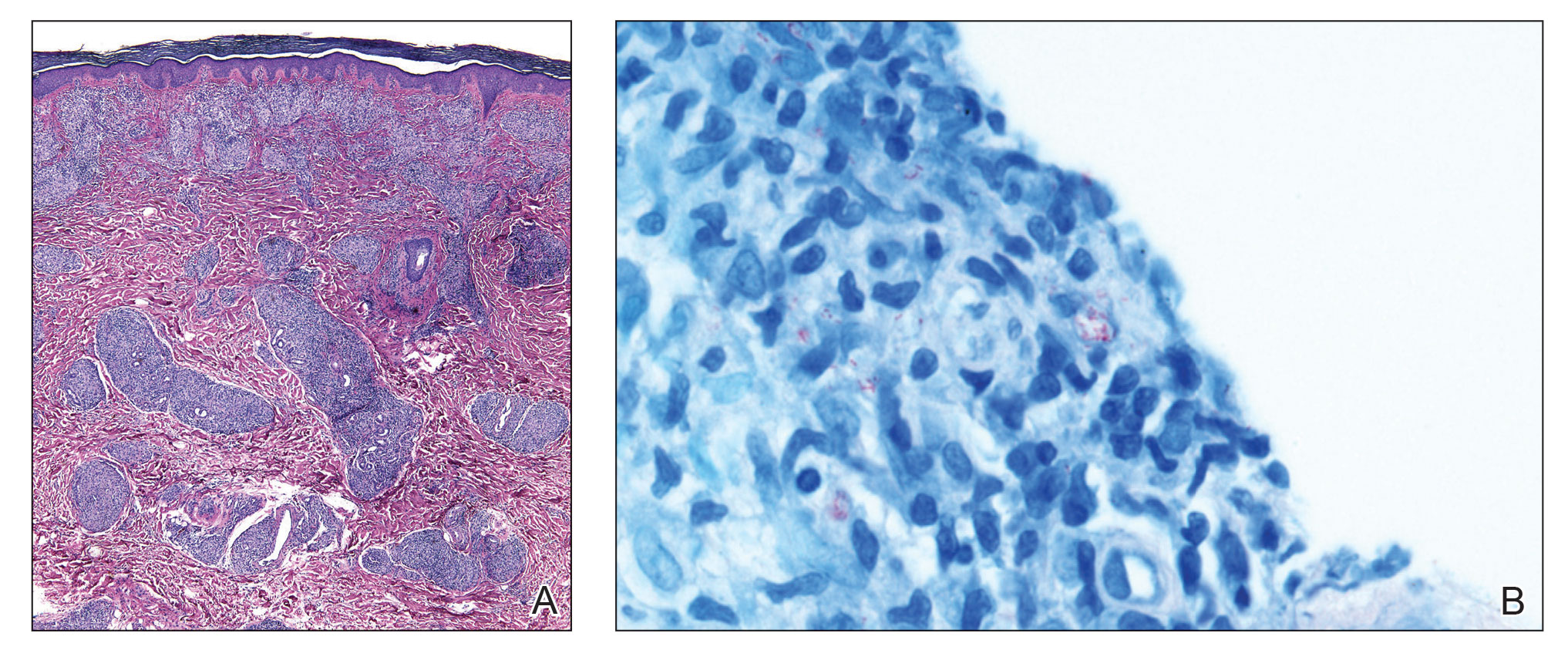
Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.
Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
The Diagnosis: Mid-Borderline Multibacillary Leprosy
The biopsies showed a granulomatous dermatitis involving the dermis and subcutaneous adipose tissue (Figure, A). Fite staining also revealed numerous acid-fast bacilli (AFB) throughout the dermis (Figure, B); however, polymerase chain reaction (PCR) for Mycobacterium tuberculosis was negative, and concomitant AFB tissue culture showed no growth after 8 weeks of incubation from the left wrist biopsy (Table). Interestingly, a left inguinal lymph node biopsy performed 6 months prior to presentation that helped to establish the diagnosis of follicular lymphoma also revealed nonnecrotizing granulomas and the presence of rare AFB; this formalin-fixed specimen subsequently tested negative for M tuberculosis and nontuberculous mycobacteria (NTM) by broad-range PCR. Due to a high index of suspicion, another unpreserved skin biopsy of the right knee was sent for NTM testing with PCR. Primers to 16S ribosomal RNA and the beta subunit of RNA polymerase, rpoB, gene detected Mycobacterium leprae DNA, leading to the diagnosis of mid-borderline (or borderline-borderline) multibacillary leprosy. Our patient subsequently reported subtle hypoesthesia of the plaques on the knees. He recalled eating undercooked armadillo meat in the southern United States more than 30 years prior to admission. In addition, he had a history of being incarcerated in the northeastern United States. This case was reported to the National Hansen’s Disease Program, and our patient was started on a 2-year course of daily clarithromycin, daily minocycline, and once-monthly moxifloxacin. His family also was evaluated and did not have any skin lesions concerning for leprosy.

Leprosy is a major global health concern, transmitted via breaks in the skin, respiratory secretions, and contact with armadillos. It continues to be endemic in India, Brazil, and Indonesia.1 In the United States where leprosy is nonendemic, 159 new cases were detected in 2020; the most notable risk factors in the United States are armadillo exposure and travel history.2,3 Mycobacterium leprae are intracellular bacilli that preferentially infect macrophages and Schwann cells, resulting in erythematous or hypopigmented skin lesions that often are anesthetic. Mycobacterium leprae has the longest doubling time of all bacteria with unknown in vitro growth requirements and a typical in vivo incubation period of 2 to 10 years.4 Therefore, in vitro cultures will yield no growth, as seen in our case. In our patient, Fite stain showed acid-fast organisms in multiple tissue specimens, but AFB cultures demonstrated no growth after 8 weeks of incubation. Although clinicopathologic correlation is most important, PCR analysis can help to assist in the diagnosis of leprosy. Unpreserved tissue should be used when possible, as the fixation process may adversely affect the analytic sensitivity of subsequent PCR-based assays.5 In our case, NTM were not detected by PCR in the inguinal lymph node specimen despite demonstrating rare AFB staining. This result likely was multifactorial, including the effect of formalin fixation and paraffin embedding as well as concomitant low biomass.

Leprosy is known as a great imitator, and clinical manifestations (both neurologic and cutaneous) depend on host immune response to the mycobacteria. Although tuberculoid leprosy (associated with T helper type 1 immune response) is distinguished by few asymmetric, well-demarcated, and often hypopigmented plaques, lepromatous leprosy (associated with T helper type 2 response) is characterized by numerous symmetric and poorly defined lesions. Borderline leprosy, as seen in our patient, is the most common type of leprosy and shows features of both tuberculoid and lepromatous leprosy.4 It also may be particularly difficult to diagnose.6,7 Borderline-borderline leprosy involves lesions that mostly are of the lepromatous type and symmetric but also may include raised plaques, as in tuberculoid leprosy.4 Plaques in an annular configuration with central clearing, as seen in our patient, are considered suggestive.8 Histopathology of borderline-borderline leprosy lesions shows subepidermal clear zones, and granulomas are more diffuse than in tuberculoid leprosy.4
Given the noncaseating granulomatous dermatitis seen on histopathology and the relatively higher incidence of sarcoidosis in our region of practice, our initial differential included sarcoidosis and other granulomatous disorders such as granuloma annulare. Interestingly, sarcoidosis has been misdiagnosed as leprosy on multiple occasions in countries where leprosy is endemic.9,10 Localized cutaneous leishmaniasis typically presents with infiltrated plaques and nodules that may ulcerate; diffuse and disseminated as well as mucocutaneous presentations may occur depending on the species and severity of infection. Parasitized macrophages containing amastigotes may be seen in the dermis highlighted by CD1a immunostaining. Mycosis fungoides presents as papulosquamous patches or plaques, often favoring sunprotected sites; the hypopigmented variant may mimic the central clearing seen in leprosy.
The diagnosis of leprosy can be challenging due to varying clinical presentation; indolent growth of the causative organism; and indeterminate nature of stains, including the Fite stain. Although leprosy is an uncommon diagnosis, this case underscores the need to keep it in the differential of granulomatous dermatoses in the appropriate clinical setting, particularly in patients with risk factors for exposure.8
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
- Blok DJ, De Vlas SJ, Richardus JH. Global elimination of leprosy by 2020: are we on track? Parasit Vectors. 2015;8:548. doi:10.1186/s13071-015-1143-4
- National Hansen’s disease (leprosy) program caring and curing since 1894. Health Resources and Services Administration website. Published April 13, 2017. Accessed November 17, 2022. https://www.hrsa.gov/hansens-disease/index.html
- Aslam S, Peraza J, Mekaiel A, et al. Major risk factors for leprosy in a non-endemic area of the United States: a case series. IDCases. 2019;17:E00557. doi:10.1016/j.idcr.2019.e00557
- Kundakci N, Erdem C. Leprosy: a great imitator. Clin Dermatol. 2019;37:200-212. doi:10.1016/j.clindermatol.2019.01.002
- Marchetti G, Gori A, Catozzi L, et al. Evaluation of PCR in detection of Mycobacterium tuberculosis from formalin-fixed, paraffin-embedded tissues: comparison of four amplification assays. J Clin Microbiol. 1998;36:1512-1517.
- Pawar M, Zawar V. Mid-borderline leprosy masquerading as an overlap syndrome. Rheumatology (Oxford). 2018;57:1686-1688. doi:10.1093 /rheumatology/key125
- Day W, Prodanovic E. Borderline lepromatous leprosy masking as tinea versicolor. Int J Dermatol. 2019;58:E125-E126. doi:10.1111/ijd.14439
- Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects: part 1. An Bras Dermatol. 2014;89:205-218. doi:10.1590/abd1806-4841.20142450
- Kaushik A, Vinay K, Narang T, et al. Ichthyosiform sarcoidosis: a mimic of leprosy? Clin Exp Dermatol. 2019;44:677-680. doi:10.1111/ced.13863
- Chowdhary KN, Rao R, Priya P, et al. Cutaneous sarcoidosis misdiagnosed as leprosy. report of two cases and review of literature. Indian J Lepr. 2016;88:177-183.
A 59-year-old man was admitted to the medical ward with multiple annular erythematous plaques and polyarthralgia of several months’ duration. His medical history included low-grade stage IIA follicular lymphoma diagnosed 6 months prior to presentation, substance abuse with opiates and cocaine, coronary artery disease, ascending aortic aneurysm, and chronic lower back pain. Physical examination revealed multiple red to red-brown papules and plaques, some in an annular configuration, that were distributed on the cheeks, left wrist, knees, dorsal feet, and soles. Bilateral inguinal lymphadenopathy also was noted. Serological testing for HIV, hepatitis B and C viruses, Treponema pallidum, Borrelia burgdorferi, and tuberculosis assay were negative. Arthrocentesis of the left wrist 1 week prior to admission noted 5333 nucleated cells/μL (reference range, <3000 cells/μL) and no crystals; culture of the fluid was sterile. Skin biopsies of plaques on the left wrist, left dorsal foot, and right knee were obtained for histopathologic analysis.
Yellow Nodule on the Scalp
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4
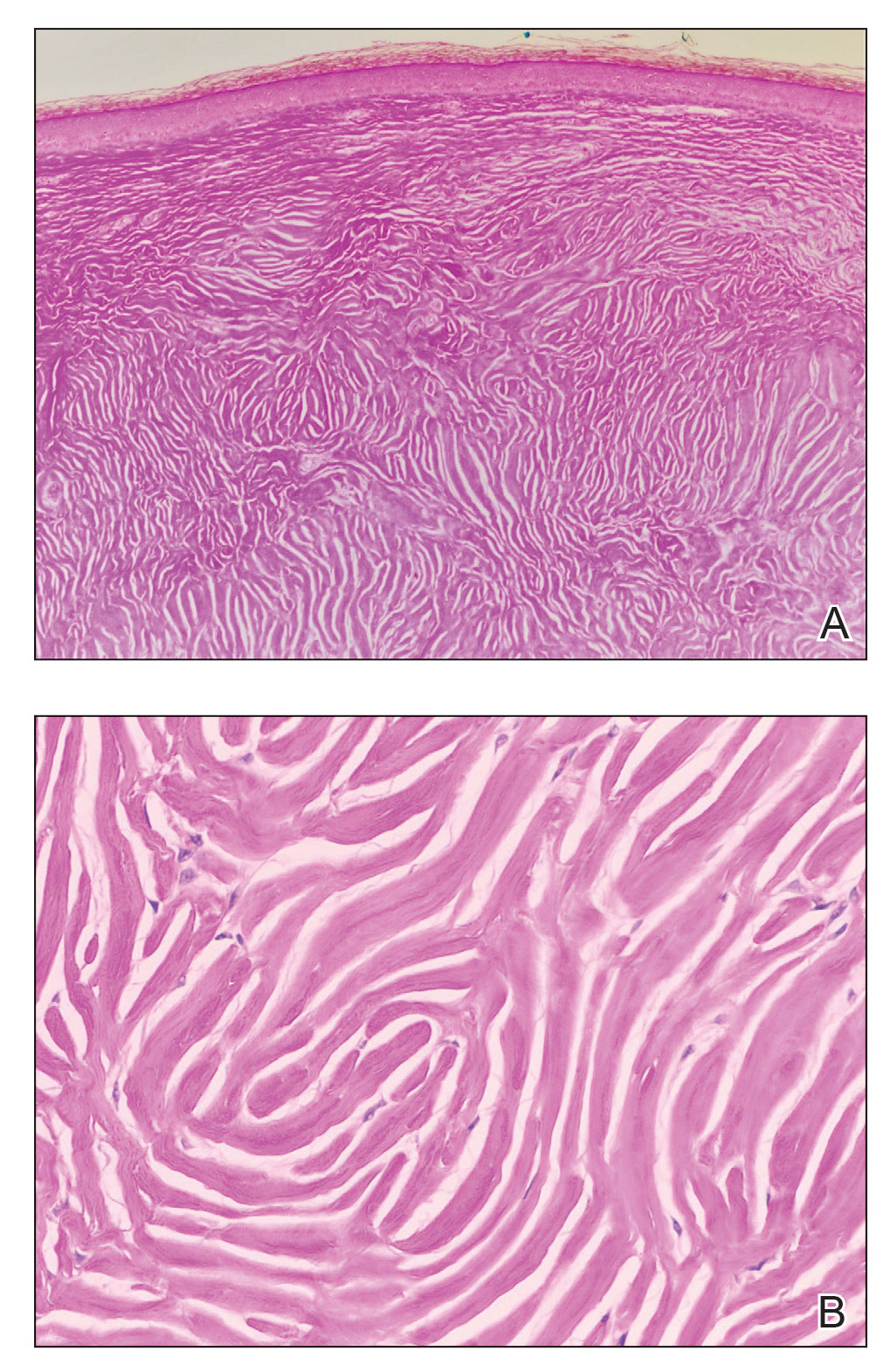
The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
The Diagnosis: Solitary Sclerotic Fibroma
Based on the clinical and histologic findings, the patient was diagnosed with solitary sclerotic fibroma (SF). Sclerotic fibroma is a rare benign tumor that first was described in 1972 by Weary et al1 in the oral mucosa of a patient with Cowden syndrome, a genodermatosis associated with multiple benign and malignant tumors. Rapini and Golitz2 reported solitary SF in 11 otherwise-healthy individuals with no signs of multiple hamartoma syndrome. Solitary SF is a sporadic benign condition, whereas multiple lesions are suggestive of Cowden syndrome. Solitary SF most commonly appears as an asymptomatic white-yellow papule or nodule on the head or neck, though larger tumors have been reported on the trunk and extremities.3 Histologic features of solitary SF include a well-circumscribed dermal nodule composed of eosinophilic dense collagen bundles arranged in a plywoodlike pattern (Figure). Immunohistochemistry is positive for CD34 and vimentin but negative for S-100, epithelial membrane antigen, and neuron-specific enolase.4

The differential diagnosis of solitary SF of the head and neck includes sebaceous adenoma, pilar cyst, nodular basal cell carcinoma, and giant molluscum contagiosum. Sebaceous adenomas usually are solitary yellow nodules less than 1 cm in diameter and located on the head and neck. They are the most common sebaceous neoplasm associated with Muir-Torre syndrome, an autosomal-dominant disorder characterized by sebaceous adenoma or carcinoma and colorectal cancer. Histopathology demonstrates well-circumscribed, round aggregations of mature lipid-filled sebocytes with a rim of basaloid germinative cells at the periphery. Pilar cysts typically are flesh-colored subcutaneous nodules on the scalp that are freely mobile over underlying tissue. Histopathology shows stratified squamous epithelium lining and trichilemmal keratinization. Nodular basal cell carcinoma has a pearly translucent appearance and arborizing telangiectases. Histopathology demonstrates nests of basaloid cells with palisading of the cells at the periphery. Giant solitary molluscum contagiosum is a dome-shaped, flesh-colored nodule with central umbilication. Histopathology reveals hyperplastic squamous epithelium with characteristic eosinophilic inclusion bodies above the basal layer.
Solitary SF can be difficult to diagnose based solely on the clinical presentation; thus biopsy with histologic evaluation is recommended. If SF is confirmed, the clinician should inquire about a family history of Cowden syndrome and then perform a total-body skin examination to check for multiple SF and other clinical hamartomas of Cowden syndrome such as trichilemmomas, acral keratosis, and oral papillomas.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
- Weary PE, Gorlin RJ, Gentry Jr WC, et al. Multiple hamartoma syndrome (Cowden’s disease). Arch Dermatol. 1972;106:682-690.
- Rapini RP, Golitz LE. Sclerotic fibromas of the skin. J Am Acad Dermatol. 1989;20(2 pt 1):266-271.
- Tosa M, Ansai S, Kuwahara H, et al. Two cases of sclerotic fibroma of the skin that mimicked keloids clinically. J Nippon Med Sch. 2018;85:283-286.
- High WA, Stewart D, Essary LR, et al. Sclerotic fibroma-like changes in various neoplastic and inflammatory skin lesions: is sclerotic fibroma a distinct entity? J Cutan Pathol. 2004;31:373-378.
A 45-year-old woman was referred to dermatology by a primary care physician for evaluation of a raised skin lesion on the scalp. She was otherwise healthy. The lesion had been present for many years but recently grew in size. The patient reported that the lesion was subject to recurrent physical trauma and she wanted it removed. Physical examination revealed a 6×6-mm, domeshaped, yellow nodule on the left inferior parietal scalp. There were no similar lesions located elsewhere on the body. A shave removal was performed and sent for histopathologic evaluation.

A Trauma-Induced Fatty Mass: The Facts About Posttraumatic Pseudolipomas
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.
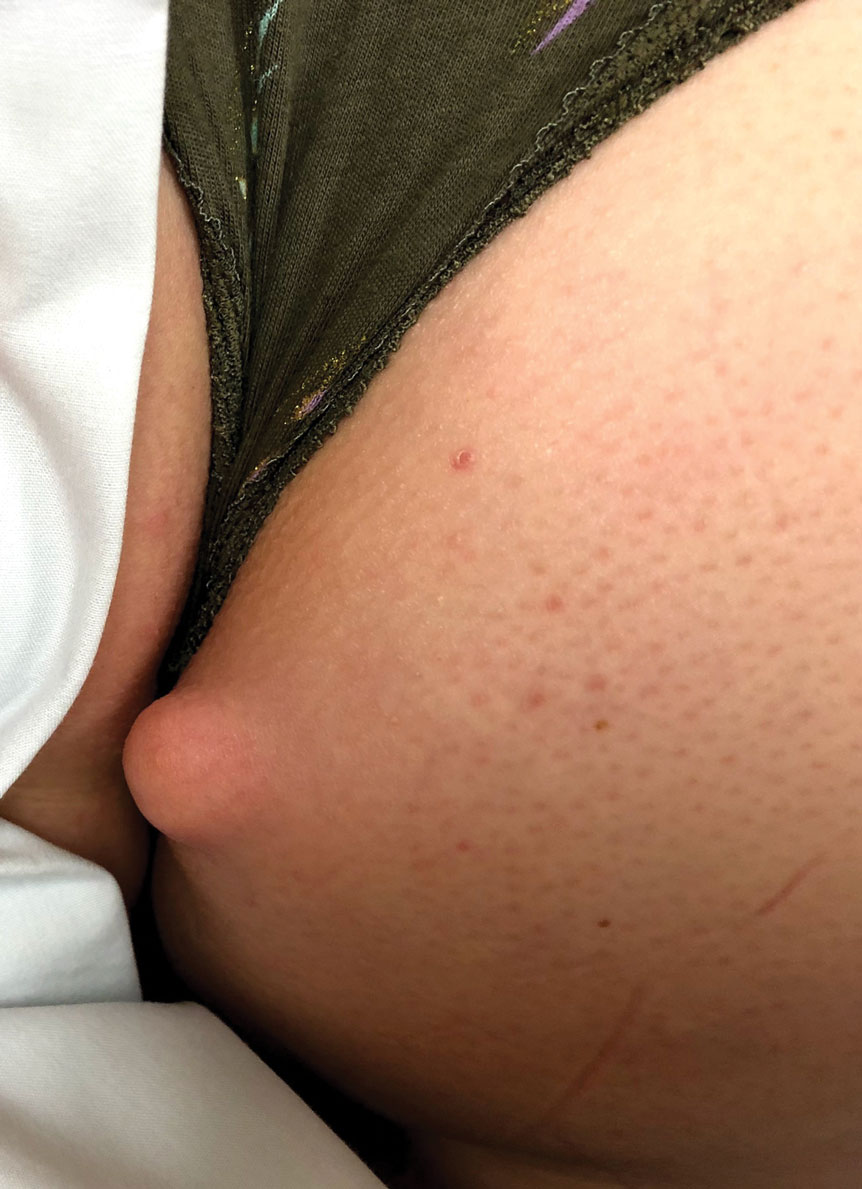
In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5
Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
Practice Points
- Physicians should include pseudolipoma in the differential diagnosis when evaluating masses that develop in patients at sites of blunt or prolonged trauma.
- A pseudolipoma is an unencapsulated, round, or fusiform fatty mass that differs from a traditional lipoma by the absence of a capsule.
- Further research may elucidate the pathogenesis of these adiposities.
Diffuse Papular Eruption With Erosions and Ulcerations
The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.
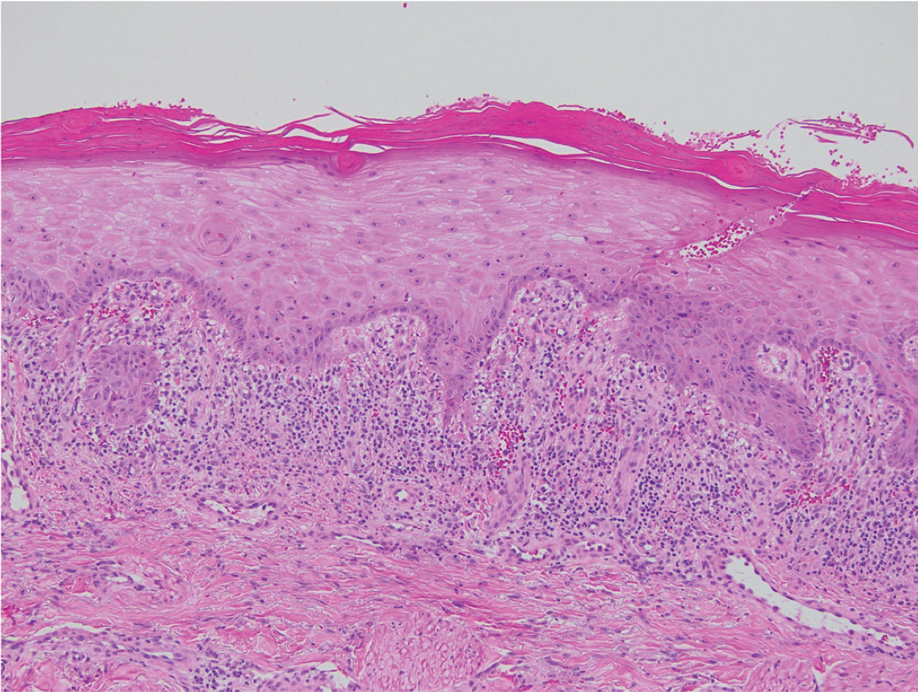
Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.
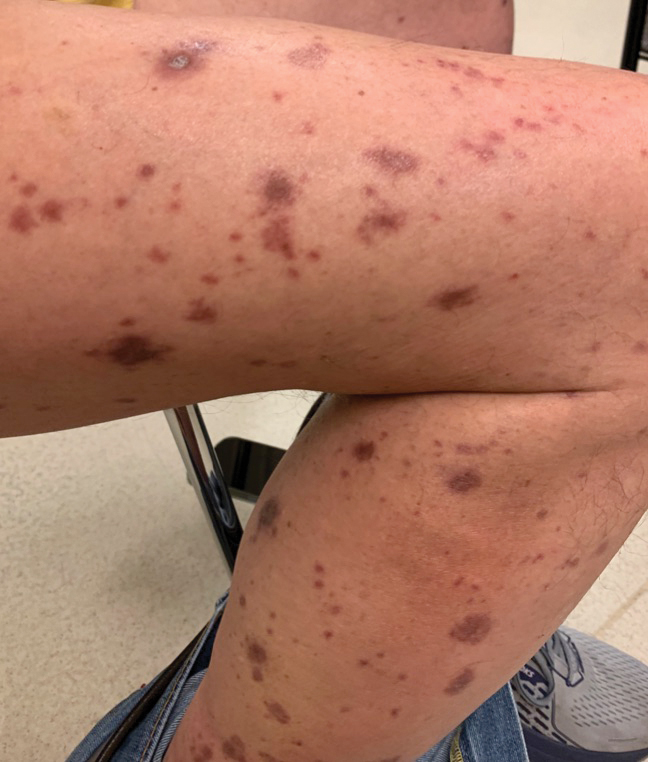
- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

The Diagnosis: Immunotherapy-Related Lichenoid Drug Eruption
Direct immunofluorescence was negative, and histopathology revealed a lichenoid interface dermatitis, minimal parakeratosis, and saw-toothed rete ridges (Figure 1). He was diagnosed with an immunotherapyrelated lichenoid drug eruption based on the morphology of the skin lesions and clinicopathologic correlation. Bullous pemphigoid and lichen planus pemphigoides were ruled out given the negative direct immunofluorescence findings. Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) was not consistent with the clinical presentation, especially given the lack of mucosal findings. The histology also was not consistent, as the biopsy specimen lacked apoptotic and necrotic keratinocytes to the degree seen in SJS/TEN and also had a greater degree of inflammatory infiltrate. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome was ruled out given the lack of systemic findings, including facial swelling and lymphadenopathy and the clinical appearance of the rash. No morbilliform features were present, which is the most common presentation of DRESS syndrome.

Checkpoint inhibitor (CPI) therapy has become the cornerstone in management of certain advanced malignancies.1 Checkpoint inhibitors block cytotoxic T lymphocyte–associated protein 4, programmed cell death-1, and/or programmed cell death ligand-1, allowing activated T cells to infiltrate the tumor microenvironment and destroy malignant cells. Checkpoint inhibitors are approved for the treatment of melanoma, cutaneous squamous cell carcinoma, and Merkel cell carcinoma and are being investigated in various other cutaneous and soft tissue malignancies.1-3
Although CPIs have shown substantial efficacy in the management of advanced malignancies, immune-related adverse events (AEs) are common due to nonspecific immune activation.2 Immune-related cutaneous AEs are the most common immune-related AEs, occurring in 30% to 50% of patients who undergo treatment.2-5 Common immune-related cutaneous AEs include maculopapular, psoriasiform, and lichenoid dermatitis, as well as pruritus without dermatitis.2,3,6 Other reactions include but are not limited to bullous pemphigoid, vitiligolike depigmentation, and alopecia.2,3 Immune-related cutaneous AEs usually are self-limited; however, severe life-threatening reactions such as the spectrum of SJS/TEN and DRESS syndrome also can occur.2-4 Immune-related cutaneous AEs are graded based on the Common Terminology Criteria for Adverse Events: grade 1 reactions are asymptomatic and cover less than 10% of the patient’s body surface area (BSA), grade 2 reactions have mild symptoms and cover 10% to 30% of the patient’s BSA, grade 3 reactions have moderate to severe symptoms and cover greater than 30% of the patient’s BSA, and grade 4 reactions are life-threatening.2,3 With prompt recognition and adequate treatment, mild to moderate immune-related cutaneous AEs—grades 1 and 2—largely are reversible, and less than 5% require discontinuation of therapy.2,3,6 It has been suggested that immune-related cutaneous AEs may be a positive prognostic factor in the treatment of underlying malignancy, indicating adequate immune activation targeting the malignant cells.6
Although our patient had some typical violaceous, flat-topped papules and plaques with Wickham striae, he also had atypical findings for a lichenoid reaction. Given the endorsement of blisters, it is possible that some of these lesions initially were bullous and subsequently ruptured, leaving behind erosions. However, in other areas, there also were eroded papules and ulcerations without a reported history of excoriation, scratching, picking, or prior bullae, including difficult-to-reach areas such as the back. It is favored that these lesions represented a robust lichenoid dermatitis leading to erosive and ulcerated lesions, similar to the formation of bullous lichen planus. Lichenoid eruptions secondary to immunotherapy are well-known phenomena, but a PubMed search of articles indexed for MEDLINE using the terms ulcer, lichenoid, and immunotherapy revealed only 2 cases of ulcerative lichenoid eruptions: a localized digital erosive lichenoid dermatitis and a widespread ulcerative lichenoid drug eruption without true erosions.7,8 However, widespread erosive and ulcerated lichenoid reactions are rare.
Lichenoid eruptions most strongly are associated with anti–programmed cell death-1/ programmed cell death ligand-1 therapy, occurring in 20% of patients undergoing treatment.3 Lichenoid eruptions present as discrete, pruritic, erythematous, violaceous papules and plaques on the chest and back and rarely may involve the limbs, palmoplantar surfaces, and oral mucosa.2,3,6 Histopathologic features include a dense bandlike lymphocytic infiltrate in the dermis with scattered apoptotic keratinocytes in the basal layer of the epidermis.2,4,6 Grades 1 to 2 lesions can be managed with high-potency topical corticosteroids without CPI dose interruption, with more extensive grade 2 lesions requiring systemic corticosteroids.2,6,9 Lichenoid eruptions grade 3 or higher also require systemic corticosteroid therapy CPI therapy cessation until the eruption has receded to grade 0 to 1.2 Alternative treatment options for high-grade toxicity include phototherapy and acitretin.2,4,9
Our patient was treated with cessation of immunotherapy and initiation of a systemic corticosteroid taper, acitretin, and narrowband UVB therapy. After 6 weeks of treatment, the pain and pruritus improved and the rash had resolved in some areas while it had taken on a more classic lichenoid appearance with violaceous scaly papules and plaques (Figure 2) in areas of prior ulcers and erosions. He no longer had any bullae, erosions, or ulcers.

- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
- Barrios DM, Do MH, Phillips GS, et al. Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol. 2020;83:1239-1253. doi:10.1016/j.jaad.2020.03.131
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93:123-132.
- Si X, He C, Zhang L, et al. Management of immune checkpoint inhibitor-related dermatologic adverse events. Thorac Cancer. 2020;11:488-492. doi:10.1111/1759-7714.13275
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2020;6:519-527. doi:10.1001 /jamaoncol.2019.5570
- Sibaud V, Meyer N, Lamant L, et al. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. Curr Opin Oncol. 2016;28:254-263. doi:10.1097/CCO.0000000000000290
- Martínez-Doménech Á, García-Legaz Martínez M, Magdaleno-Tapial J, et al. Digital ulcerative lichenoid dermatitis in a patient receiving anti-PD-1 therapy. Dermatol Online J. 2019;25:13030/qt8sm0j7t7.
- Davis MJ, Wilken R, Fung MA, et al. Debilitating erosive lichenoid interface dermatitis from checkpoint inhibitor therapy. Dermatol Online J. 2018;24:13030/qt3vq6b04v.
- Apalla Z, Papageorgiou C, Lallas A, et al. Cutaneous adverse events of immune checkpoint inhibitors: a literature review [published online January 29, 2021]. Dermatol Pract Concept. 2021;11:E2021155. doi:10.5826/dpc.1101a155
A 70-year-old man presented with a painful, pruritic, diffuse eruption on the trunk, legs, and arms of 2 months’ duration. He had a history of stage IV pleomorphic cell sarcoma of the retroperitoneum and was started on pembrolizumab therapy 6 weeks prior to the eruption. Physical examination revealed violaceous papules and plaques with shiny reticulated scaling as well as multiple scattered eroded papules and shallow ulcerations. The oral mucosa and genitals were spared. The patient endorsed blisters followed by open sores that were both itchy and painful. He denied self-infliction. Both the patient and his wife denied scratching. Two biopsies for direct immunofluorescence and histopathology were performed.