User login
Efficacy of Topical Clascoterone for Acne Increased Over Time, Analysis Shows
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
Autoimmune Diseases and Perinatal Depression May Share Two-Way Link
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
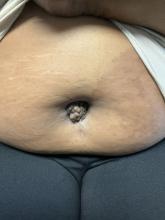
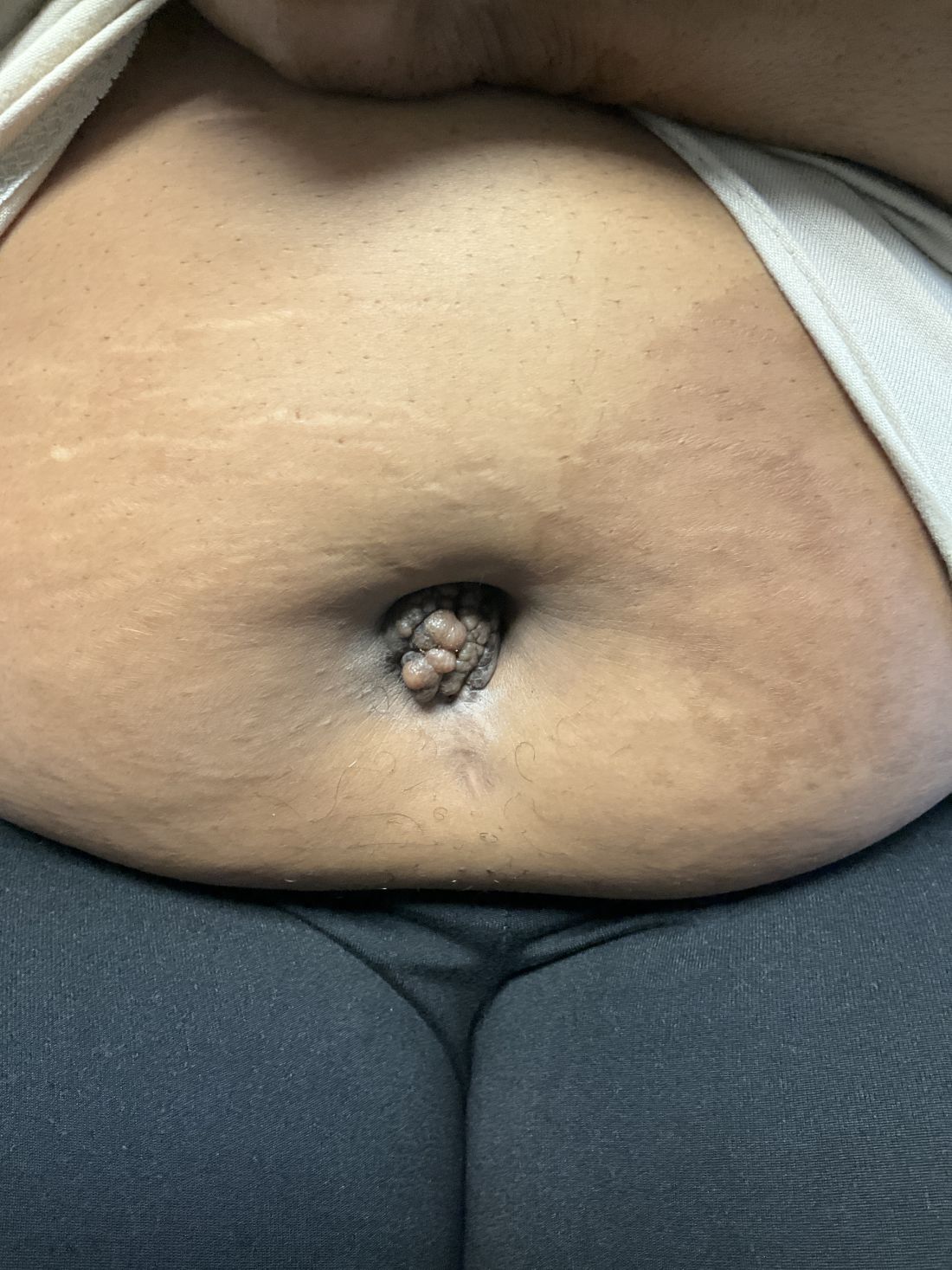
A 27-year-old Haitian woman presented with a painful umbilical mass which had been growing in size for 5 months
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Analysis Finds Risk of Alopecia Areata After COVID-19 Infection
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
FROM JAMA DERMATOLOGY
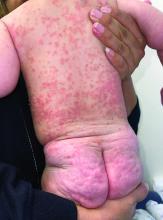
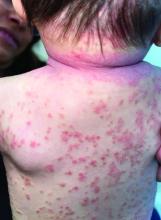
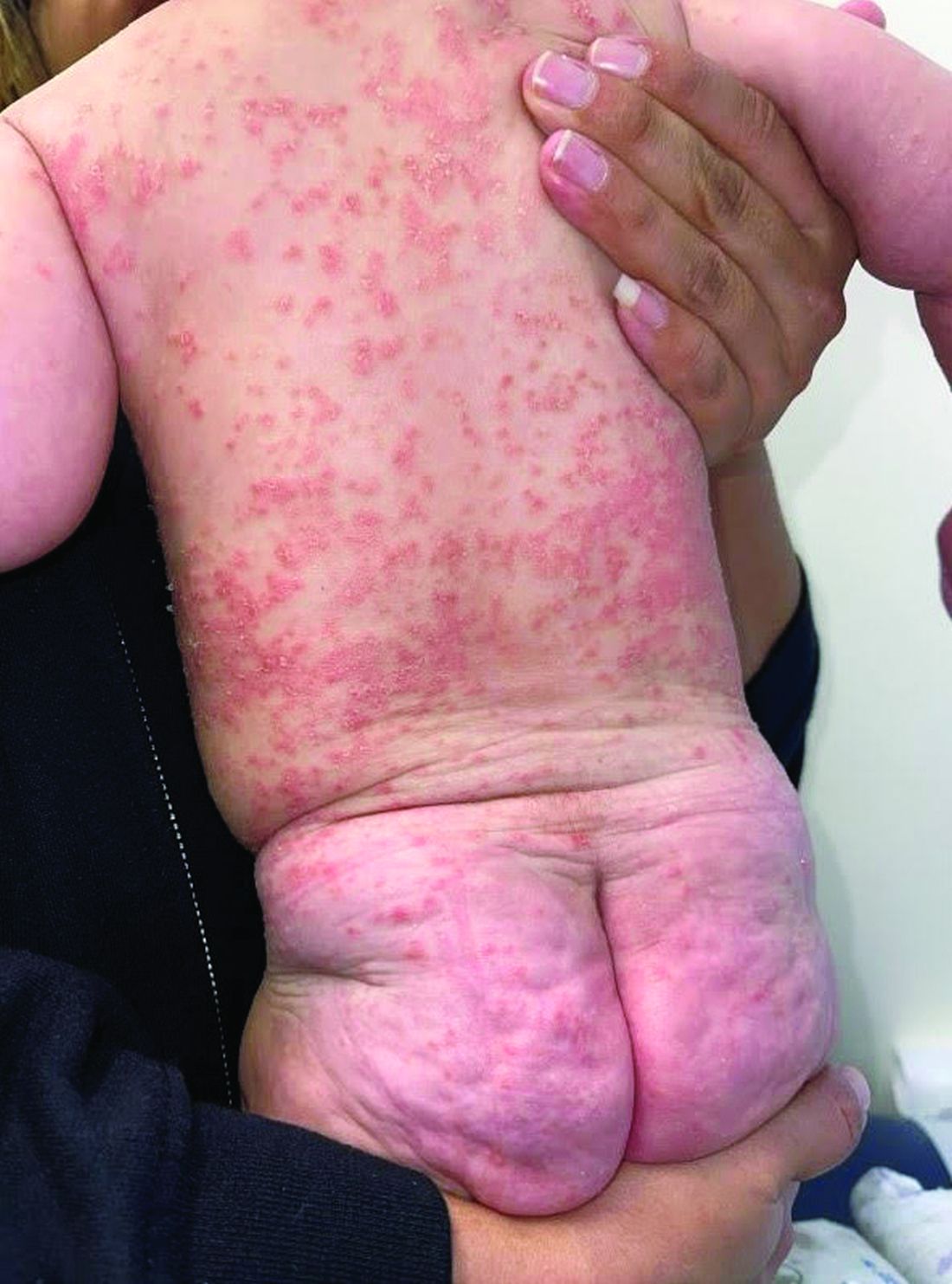
A 4-month-old male was referred for a 3-week history of an itchy generalized rash that started on the neck
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
On physical exam, there was an erythematous patch with overlying areas of macerations on the neck and axilla. The trunk, extremities, and diaper area had multiple psoriasiform erythematous thin plaques with overlying scales.
Impact of Pregnancy on Rosacea Unpredictable, Study Suggests
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
Study Identifies Cardiovascular Comorbidities Associated With Dermatomyositis
TOPLINE:
METHODOLOGY:
- DM is associated with cardiovascular disease (CVD), but US-based data studies on CVD comorbidities in patients with DM are lacking.
- In a cross-sectional analysis of participants in the All of Us research program aged 18 years and older with at least 1 year of electronic health record (EHR) data, researchers identified DM cases and controls with nearest neighbor propensity score matching by age, sex, race/ethnicity, EHR duration, and healthcare visit quantity.
- They used the Pearson’s chi-squared test, Fisher’s exact test, unpaired t-test, or Mann-Whitney U test to compare clinical characteristics and traditional CV comorbidities.
- Multivariable conditional logistic regression was used with backward elimination of comorbidities with P > .1 or evidence of collinearity.
TAKEAWAY:
- Among 235,161 All of Us participants, researchers identified 206 DM cases and 824 matched controls with largely similar demographic characteristics, including smoking status, obesity, and indicators of socioeconomic status.
- Participants with DM were more likely to have a history of atrial fibrillation (10.1% vs 16.0%, respectively), chronic kidney disease (15.2% vs 29.1%), congestive heart failure (9.6% vs 18.0%), coronary artery disease (CAD) (18.2% vs 34.0%), hypertension (52.5% vs 60.7%), myocardial infarction (7.4% vs 15.0), type 2 diabetes (27.3% vs 47.6%), and valvular heart disease (8.7% vs 16.5%) than matched controls.
- In a multivariable analysis that adjusted for potential confounders, three comorbidities remained associated with DM: CAD (odds ratio [OR], 2.0; P < .001), type 2 diabetes (OR, 2.2; P < .001), and chronic kidney disease (OR, 1.7; P = .015).
IN PRACTICE:
“Our findings are important both for prognosis and clinical care, suggesting DM patients should be screened for CVD risk factors to potentially reduce the increased risk for cardiovascular events and CVD-related mortality in DM,” the authors concluded.
SOURCE:
Corresponding author Alisa N. Femia, MD, of the department of dermatology at NYU Grossman School of Medicine, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
How DM treatments might influence CVD development was not addressed. EHRs may have diagnostic inaccuracies and omissions and lack data on clinical features and severity.
DISCLOSURES:
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Femia reported consulting fees from Octagon Therapeutics, Timber Pharmaceuticals, and Guidepoint. Study author Michael S. Garshick, MD, reported consulting fees from AbbVie and Horizon Therapeutics. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- DM is associated with cardiovascular disease (CVD), but US-based data studies on CVD comorbidities in patients with DM are lacking.
- In a cross-sectional analysis of participants in the All of Us research program aged 18 years and older with at least 1 year of electronic health record (EHR) data, researchers identified DM cases and controls with nearest neighbor propensity score matching by age, sex, race/ethnicity, EHR duration, and healthcare visit quantity.
- They used the Pearson’s chi-squared test, Fisher’s exact test, unpaired t-test, or Mann-Whitney U test to compare clinical characteristics and traditional CV comorbidities.
- Multivariable conditional logistic regression was used with backward elimination of comorbidities with P > .1 or evidence of collinearity.
TAKEAWAY:
- Among 235,161 All of Us participants, researchers identified 206 DM cases and 824 matched controls with largely similar demographic characteristics, including smoking status, obesity, and indicators of socioeconomic status.
- Participants with DM were more likely to have a history of atrial fibrillation (10.1% vs 16.0%, respectively), chronic kidney disease (15.2% vs 29.1%), congestive heart failure (9.6% vs 18.0%), coronary artery disease (CAD) (18.2% vs 34.0%), hypertension (52.5% vs 60.7%), myocardial infarction (7.4% vs 15.0), type 2 diabetes (27.3% vs 47.6%), and valvular heart disease (8.7% vs 16.5%) than matched controls.
- In a multivariable analysis that adjusted for potential confounders, three comorbidities remained associated with DM: CAD (odds ratio [OR], 2.0; P < .001), type 2 diabetes (OR, 2.2; P < .001), and chronic kidney disease (OR, 1.7; P = .015).
IN PRACTICE:
“Our findings are important both for prognosis and clinical care, suggesting DM patients should be screened for CVD risk factors to potentially reduce the increased risk for cardiovascular events and CVD-related mortality in DM,” the authors concluded.
SOURCE:
Corresponding author Alisa N. Femia, MD, of the department of dermatology at NYU Grossman School of Medicine, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
How DM treatments might influence CVD development was not addressed. EHRs may have diagnostic inaccuracies and omissions and lack data on clinical features and severity.
DISCLOSURES:
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Femia reported consulting fees from Octagon Therapeutics, Timber Pharmaceuticals, and Guidepoint. Study author Michael S. Garshick, MD, reported consulting fees from AbbVie and Horizon Therapeutics. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- DM is associated with cardiovascular disease (CVD), but US-based data studies on CVD comorbidities in patients with DM are lacking.
- In a cross-sectional analysis of participants in the All of Us research program aged 18 years and older with at least 1 year of electronic health record (EHR) data, researchers identified DM cases and controls with nearest neighbor propensity score matching by age, sex, race/ethnicity, EHR duration, and healthcare visit quantity.
- They used the Pearson’s chi-squared test, Fisher’s exact test, unpaired t-test, or Mann-Whitney U test to compare clinical characteristics and traditional CV comorbidities.
- Multivariable conditional logistic regression was used with backward elimination of comorbidities with P > .1 or evidence of collinearity.
TAKEAWAY:
- Among 235,161 All of Us participants, researchers identified 206 DM cases and 824 matched controls with largely similar demographic characteristics, including smoking status, obesity, and indicators of socioeconomic status.
- Participants with DM were more likely to have a history of atrial fibrillation (10.1% vs 16.0%, respectively), chronic kidney disease (15.2% vs 29.1%), congestive heart failure (9.6% vs 18.0%), coronary artery disease (CAD) (18.2% vs 34.0%), hypertension (52.5% vs 60.7%), myocardial infarction (7.4% vs 15.0), type 2 diabetes (27.3% vs 47.6%), and valvular heart disease (8.7% vs 16.5%) than matched controls.
- In a multivariable analysis that adjusted for potential confounders, three comorbidities remained associated with DM: CAD (odds ratio [OR], 2.0; P < .001), type 2 diabetes (OR, 2.2; P < .001), and chronic kidney disease (OR, 1.7; P = .015).
IN PRACTICE:
“Our findings are important both for prognosis and clinical care, suggesting DM patients should be screened for CVD risk factors to potentially reduce the increased risk for cardiovascular events and CVD-related mortality in DM,” the authors concluded.
SOURCE:
Corresponding author Alisa N. Femia, MD, of the department of dermatology at NYU Grossman School of Medicine, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
How DM treatments might influence CVD development was not addressed. EHRs may have diagnostic inaccuracies and omissions and lack data on clinical features and severity.
DISCLOSURES:
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Femia reported consulting fees from Octagon Therapeutics, Timber Pharmaceuticals, and Guidepoint. Study author Michael S. Garshick, MD, reported consulting fees from AbbVie and Horizon Therapeutics. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Myo-inositol is one of the components of an integrative approach to acne
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
FROM IDS 2023
FDA Gives Nod to Berdazimer Gel for Molluscum Contagiosum
On January 5, the Food and Drug Administration (FDA) approved berdazimer gel 10.3% for the treatment of molluscum contagiosum (MC) in adults and children aged 1 year or older.
Approval of berdazimer, a topical nitric oxide–releasing agent, was based largely on a 12-week pivotal phase 3 trial known as B-SIMPLE4, in which 891 patients with a mean age of 6.6 years (range, 0.9-47.5 years) were randomly assigned to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily. At 12 weeks, 32.4% of patients in the berdazimer group achieved complete clearance of MC lesions compared with 19.7% of those in the vehicle group (P < .001).
Only 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events in both groups were application-site pain and erythema, and most of these were mild or moderate.
According to a press release announcing the approval from Ligand Pharmaceuticals, which acquired berdazimer topical gel from Novan in September 2023, the development makes berdazimer topical gel 10.3% the first and only topical prescription medication that can be applied by patients, parents, or caregivers at home; outside of a physician›s office; or outside of other medical settings to treat MC. Nitric oxide has been shown to have antiviral effects, although the mechanism of action of berdazimer for treating molluscum “is unknown,” the company said in the release.
The drug will be marketed under the name Zelsuvmi and is expected to be available in the second half of 2024.
On July 21, 2023, topical cantharidin became the first approved treatment of MC for adults and pediatric patients aged 2 years or older, with the FDA approval of a drug-device combination (Ycanth) that contains a formulation of cantharidin solution 0.7% and is administered by healthcare professionals.
A version of this article appeared on Medscape.com.
On January 5, the Food and Drug Administration (FDA) approved berdazimer gel 10.3% for the treatment of molluscum contagiosum (MC) in adults and children aged 1 year or older.
Approval of berdazimer, a topical nitric oxide–releasing agent, was based largely on a 12-week pivotal phase 3 trial known as B-SIMPLE4, in which 891 patients with a mean age of 6.6 years (range, 0.9-47.5 years) were randomly assigned to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily. At 12 weeks, 32.4% of patients in the berdazimer group achieved complete clearance of MC lesions compared with 19.7% of those in the vehicle group (P < .001).
Only 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events in both groups were application-site pain and erythema, and most of these were mild or moderate.
According to a press release announcing the approval from Ligand Pharmaceuticals, which acquired berdazimer topical gel from Novan in September 2023, the development makes berdazimer topical gel 10.3% the first and only topical prescription medication that can be applied by patients, parents, or caregivers at home; outside of a physician›s office; or outside of other medical settings to treat MC. Nitric oxide has been shown to have antiviral effects, although the mechanism of action of berdazimer for treating molluscum “is unknown,” the company said in the release.
The drug will be marketed under the name Zelsuvmi and is expected to be available in the second half of 2024.
On July 21, 2023, topical cantharidin became the first approved treatment of MC for adults and pediatric patients aged 2 years or older, with the FDA approval of a drug-device combination (Ycanth) that contains a formulation of cantharidin solution 0.7% and is administered by healthcare professionals.
A version of this article appeared on Medscape.com.
On January 5, the Food and Drug Administration (FDA) approved berdazimer gel 10.3% for the treatment of molluscum contagiosum (MC) in adults and children aged 1 year or older.
Approval of berdazimer, a topical nitric oxide–releasing agent, was based largely on a 12-week pivotal phase 3 trial known as B-SIMPLE4, in which 891 patients with a mean age of 6.6 years (range, 0.9-47.5 years) were randomly assigned to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily. At 12 weeks, 32.4% of patients in the berdazimer group achieved complete clearance of MC lesions compared with 19.7% of those in the vehicle group (P < .001).
Only 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events in both groups were application-site pain and erythema, and most of these were mild or moderate.
According to a press release announcing the approval from Ligand Pharmaceuticals, which acquired berdazimer topical gel from Novan in September 2023, the development makes berdazimer topical gel 10.3% the first and only topical prescription medication that can be applied by patients, parents, or caregivers at home; outside of a physician›s office; or outside of other medical settings to treat MC. Nitric oxide has been shown to have antiviral effects, although the mechanism of action of berdazimer for treating molluscum “is unknown,” the company said in the release.
The drug will be marketed under the name Zelsuvmi and is expected to be available in the second half of 2024.
On July 21, 2023, topical cantharidin became the first approved treatment of MC for adults and pediatric patients aged 2 years or older, with the FDA approval of a drug-device combination (Ycanth) that contains a formulation of cantharidin solution 0.7% and is administered by healthcare professionals.
A version of this article appeared on Medscape.com.
AAAAI/ACAAI Joint Task Force Issues Updated ‘Practice-Changing’ Guidelines to Manage AD
and new methodological standards for making recommendations. The new guidelines update 2012 recommendations.
The JTFPP AD guidelines represent “an evolution” in trustworthy allergy guidelines and provide systematic reviews of the evidence with multidisciplinary panelist engagement, adherence to a rigorous guideline development process, the involvement of the patient and caregiver voice from start to finish, clear translation of evidence to clinically actionable and contextual recommendations, and novel approaches to facilitate knowledge translation, task force cochair Derek K. Chu, MD, PhD, said in an interview. Dr. Chu, director of the Evidence in Allergy research group at McMaster University, Hamilton, Ontario, Canada, cochaired the task force with Lynda Schneider, MD, section chief of the allergy and asthma program at Boston Children’s Hospital.
The new guidelines were published online on December 17, 2023, in Annals of Allergy, Asthma, & Immunology. They include 25 recommendations and address optimal use of topical treatments, such as topical corticosteroids, topical calcineurin inhibitors, topical JAK inhibitors, topical crisaborole, and topical antimicrobials; dilute bleach baths; dietary elimination; allergen immunotherapy by subcutaneous (SCIT) and sublingual (SLIT) routes; and systemic treatments with dupilumab and tralokinumab, cyclosporine, azathioprine, methotrexate, mycophenolate, oral JAK inhibitors, systemic corticosteroids; and phototherapy.
“There’s something in here for all clinicians — from primary care to AD experts— and patients may benefit as well, so the key individual recommendations will vary,” Dr. Chu told this news organization.
“Throughout the guideline, we emphasize shared decision-making, key factors to consider for each recommendation, and the specific evidence behind each recommendation,” he said. “There is a major focus on addressing equity, diversity, inclusiveness; and addressing health disparities, and key gaps to address in future research.”
Among the changes to the 2012 JTFPP guidelines, the 2023 update suggests using dilute bleach baths for patients with AD with moderate to severe disease as an additive therapy and suggests using allergen immunotherapy (AIT) for moderate to severe AD.
In other changes, the 2023 update suggests against using elimination diets for AD; recommends against very low dose baricitinib (1 mg); suggests against azathioprine, methotrexate, and mycophenolate mofetil; and suggests against adding topical JAK inhibitors, such as ruxolitinib, for patients with mild to moderate AD refractory to moisturization alone.
The 38-page guidelines include an infographic that summarizes comparative effects of systemic treatments on patient-important outcomes for AD that are important to patients, and includes other key summary tables that can be used at the point of care.
In addition to addressing evidence underlying each recommendation, the guideline’s eAppendix contains 1- to 2-page handouts that address practical issues for each treatment and can be used to facilitate shared decision making.
Dr. Chu said that the updated guidelines “provide important changes to almost all aspects of AD care — my own and my colleagues’ — and I strongly recommend all clinicians treating AD to read the full guidelines and use them in clinical practice. We’re grateful to all our contributors, especially our patient and caregiver partners, for helping make these guidelines. We will continue to periodically update the guidelines as part of maintaining them as living guidelines.”
The guidelines incorporate the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence.
The work was funded by the AAAAI/ACAAI JTFPP. Dr. Chu disclosed that he has received a faculty development award from the AAAAI Foundation and research grants to McMaster from the Canadian Institutes of Health Research, the Ontario Ministry of Health, and the Ontario Medical Association.
and new methodological standards for making recommendations. The new guidelines update 2012 recommendations.
The JTFPP AD guidelines represent “an evolution” in trustworthy allergy guidelines and provide systematic reviews of the evidence with multidisciplinary panelist engagement, adherence to a rigorous guideline development process, the involvement of the patient and caregiver voice from start to finish, clear translation of evidence to clinically actionable and contextual recommendations, and novel approaches to facilitate knowledge translation, task force cochair Derek K. Chu, MD, PhD, said in an interview. Dr. Chu, director of the Evidence in Allergy research group at McMaster University, Hamilton, Ontario, Canada, cochaired the task force with Lynda Schneider, MD, section chief of the allergy and asthma program at Boston Children’s Hospital.
The new guidelines were published online on December 17, 2023, in Annals of Allergy, Asthma, & Immunology. They include 25 recommendations and address optimal use of topical treatments, such as topical corticosteroids, topical calcineurin inhibitors, topical JAK inhibitors, topical crisaborole, and topical antimicrobials; dilute bleach baths; dietary elimination; allergen immunotherapy by subcutaneous (SCIT) and sublingual (SLIT) routes; and systemic treatments with dupilumab and tralokinumab, cyclosporine, azathioprine, methotrexate, mycophenolate, oral JAK inhibitors, systemic corticosteroids; and phototherapy.
“There’s something in here for all clinicians — from primary care to AD experts— and patients may benefit as well, so the key individual recommendations will vary,” Dr. Chu told this news organization.
“Throughout the guideline, we emphasize shared decision-making, key factors to consider for each recommendation, and the specific evidence behind each recommendation,” he said. “There is a major focus on addressing equity, diversity, inclusiveness; and addressing health disparities, and key gaps to address in future research.”
Among the changes to the 2012 JTFPP guidelines, the 2023 update suggests using dilute bleach baths for patients with AD with moderate to severe disease as an additive therapy and suggests using allergen immunotherapy (AIT) for moderate to severe AD.
In other changes, the 2023 update suggests against using elimination diets for AD; recommends against very low dose baricitinib (1 mg); suggests against azathioprine, methotrexate, and mycophenolate mofetil; and suggests against adding topical JAK inhibitors, such as ruxolitinib, for patients with mild to moderate AD refractory to moisturization alone.
The 38-page guidelines include an infographic that summarizes comparative effects of systemic treatments on patient-important outcomes for AD that are important to patients, and includes other key summary tables that can be used at the point of care.
In addition to addressing evidence underlying each recommendation, the guideline’s eAppendix contains 1- to 2-page handouts that address practical issues for each treatment and can be used to facilitate shared decision making.
Dr. Chu said that the updated guidelines “provide important changes to almost all aspects of AD care — my own and my colleagues’ — and I strongly recommend all clinicians treating AD to read the full guidelines and use them in clinical practice. We’re grateful to all our contributors, especially our patient and caregiver partners, for helping make these guidelines. We will continue to periodically update the guidelines as part of maintaining them as living guidelines.”
The guidelines incorporate the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence.
The work was funded by the AAAAI/ACAAI JTFPP. Dr. Chu disclosed that he has received a faculty development award from the AAAAI Foundation and research grants to McMaster from the Canadian Institutes of Health Research, the Ontario Ministry of Health, and the Ontario Medical Association.
and new methodological standards for making recommendations. The new guidelines update 2012 recommendations.
The JTFPP AD guidelines represent “an evolution” in trustworthy allergy guidelines and provide systematic reviews of the evidence with multidisciplinary panelist engagement, adherence to a rigorous guideline development process, the involvement of the patient and caregiver voice from start to finish, clear translation of evidence to clinically actionable and contextual recommendations, and novel approaches to facilitate knowledge translation, task force cochair Derek K. Chu, MD, PhD, said in an interview. Dr. Chu, director of the Evidence in Allergy research group at McMaster University, Hamilton, Ontario, Canada, cochaired the task force with Lynda Schneider, MD, section chief of the allergy and asthma program at Boston Children’s Hospital.
The new guidelines were published online on December 17, 2023, in Annals of Allergy, Asthma, & Immunology. They include 25 recommendations and address optimal use of topical treatments, such as topical corticosteroids, topical calcineurin inhibitors, topical JAK inhibitors, topical crisaborole, and topical antimicrobials; dilute bleach baths; dietary elimination; allergen immunotherapy by subcutaneous (SCIT) and sublingual (SLIT) routes; and systemic treatments with dupilumab and tralokinumab, cyclosporine, azathioprine, methotrexate, mycophenolate, oral JAK inhibitors, systemic corticosteroids; and phototherapy.
“There’s something in here for all clinicians — from primary care to AD experts— and patients may benefit as well, so the key individual recommendations will vary,” Dr. Chu told this news organization.
“Throughout the guideline, we emphasize shared decision-making, key factors to consider for each recommendation, and the specific evidence behind each recommendation,” he said. “There is a major focus on addressing equity, diversity, inclusiveness; and addressing health disparities, and key gaps to address in future research.”
Among the changes to the 2012 JTFPP guidelines, the 2023 update suggests using dilute bleach baths for patients with AD with moderate to severe disease as an additive therapy and suggests using allergen immunotherapy (AIT) for moderate to severe AD.
In other changes, the 2023 update suggests against using elimination diets for AD; recommends against very low dose baricitinib (1 mg); suggests against azathioprine, methotrexate, and mycophenolate mofetil; and suggests against adding topical JAK inhibitors, such as ruxolitinib, for patients with mild to moderate AD refractory to moisturization alone.
The 38-page guidelines include an infographic that summarizes comparative effects of systemic treatments on patient-important outcomes for AD that are important to patients, and includes other key summary tables that can be used at the point of care.
In addition to addressing evidence underlying each recommendation, the guideline’s eAppendix contains 1- to 2-page handouts that address practical issues for each treatment and can be used to facilitate shared decision making.
Dr. Chu said that the updated guidelines “provide important changes to almost all aspects of AD care — my own and my colleagues’ — and I strongly recommend all clinicians treating AD to read the full guidelines and use them in clinical practice. We’re grateful to all our contributors, especially our patient and caregiver partners, for helping make these guidelines. We will continue to periodically update the guidelines as part of maintaining them as living guidelines.”
The guidelines incorporate the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence.
The work was funded by the AAAAI/ACAAI JTFPP. Dr. Chu disclosed that he has received a faculty development award from the AAAAI Foundation and research grants to McMaster from the Canadian Institutes of Health Research, the Ontario Ministry of Health, and the Ontario Medical Association.
FROM ANNALS OF ALLERGY, ASTHMA, AND IMMUNOLOGY