User login
Feds launch COVID-19 worker vaccine mandates
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
Q&A: Meeting the challenge of giving COVID vaccines to younger kids
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
CDC endorses Pfizer’s COVID-19 vaccine for young kids
– meaning the shots are now available for immediate use.
The Nov. 2 decision came mere hours after experts that advise the CDC on vaccinations strongly recommended the vaccine for this age group.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine. As a mom, I encourage parents with questions to talk to their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated,” Dr. Walensky said in a prepared statement.
President Joe Biden applauded Dr. Walensky’s endorsement: “Today, we have reached a turning point in our battle against COVID-19: authorization of a safe, effective vaccine for children age 5 to 11. It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus,” he said in a statement.
The 14 members of the Advisory Committee on Immunization Practices (ACIP) voted unanimously earlier in the day to recommend the vaccine for kids.
“I feel like I have a responsibility to make this vaccine available to children and their parents,” said committee member Beth Bell, MD, MPH, a clinical professor at the University of Washington in Seattle. Bell noted that all evidence the committee had reviewed pointed to a vaccine that was safe and effective for younger children.
“If I had a grandchild, I would certainly get that grandchild vaccinated as soon as possible,” she said.
Their recommendations follow the U.S. Food and Drug Administration’s emergency authorization of Pfizer-BioNTech’s vaccine for this same age group last week.
“I’m voting for this because I think it could have a huge positive impact on [kids’] health and their social and emotional wellbeing,” said Grace Lee, MD, a professor of pediatrics at Stanford University School of Medicine, who chairs the CDC’s ACIP.
She noted that, though masks are available to reduce the risk for kids, they aren’t perfect and transmission still occurs.
“Vaccines are really the only consistent and reliable way to provide that protection,” Lee said.
The vaccine for children is two doses given 3 weeks apart. Each dose is 10 micrograms, which is one-third of the dose used in adults and teens.
To avoid confusion, the smaller dose for kids will come in bottles with orange labels and orange tops. The vaccine for adults is packaged in purple.
The CDC also addressed the question of kids who are close to age 12 when they get their first dose.
In general, pediatricians allow for a 4-day grace period around birthdays to determine which dose is needed. That will be the same with the COVID-19 vaccine.
For kids who are 11 when they start the series, they should get another 10-microgram dose after they turn 12 a few weeks later.
COVID-19 cases in this age group have climbed sharply over the summer and into the fall as schools have fully reopened, sometimes without the benefit of masks.
In the first week of October, roughly 10% of all COVID-19 cases recorded in the United States were among children ages 5 through 11. Since the start of pandemic, about 1.9 million children in this age group have been infected, though that’s almost certainly an undercount. More than 8,300 have been hospitalized, and 94 children have died.
Children of color have been disproportionately impacted. More than two-thirds of hospitalized children have been black or Hispanic.
Weighing benefits and risks
In clinical trials that included more than 4,600 children, the most common adverse events were pain and swelling at the injection site. They could also have side effects like fevers, fatigue, headache, chills, and sometimes swollen lymph nodes.
These kinds of side effects appear to be less common in children ages 5 to 11 than they have been in teens and adults, and they were temporary.
No cases of myocarditis or pericarditis were seen in the studies, but myocarditis is a very rare side effect, and the studies were too small to pick up these cases.
Still, doctors say they’re watching for it. In general, the greatest risk for myocarditis after vaccination has been seen in younger males between the ages of 12 and 30.
Even without COVID-19 or vaccines in the mix, doctors expect to see as many as two cases of myocarditis for every million people over the course of a week. The risk for myocarditis jumps up to about 11 cases for every million doses of mRNA vaccine given to men ages 25 to 30. It’s between 37 and 69 cases per million doses in boys between the ages of 12 and 24.
Still, experts say the possibility of this rare risk shouldn’t deter parents from vaccinating younger children.
Here’s why: The risk for myocarditis is higher after COVID-19 infection than after vaccination. Younger children have a lower risk for myocarditis than teens and young adults, suggesting that this side effect may be less frequent in this age group, although that remains to be seen.
Additionally, the smaller dose authorized for children is expected to minimize the risk for myocarditis even further.
The CDC says parents should call their doctor if a child develops pain in their chest, has trouble breathing, or feels like they have a beating or fluttering heart after vaccination.
What about benefits?
Models looking at the impact of vaccines in this age group predict that, nationally, cases would drop by about 8% if children are vaccinated.
The models also suggested that vaccination of kids this age would slow — but not stop — the emergence of new variants.
For every million doses, the CDC’s modeling predicts that more than 56,000 COVID-19 infections would be prevented in this age group, along with dozens of hospitalizations, and post-COVID conditions like multisystem inflammatory syndrome in children.
CDC experts estimate that just 10 kids would need to be vaccinated over 6 months to prevent a single case of COVID-19.
The CDC pointed out that vaccinating kids may help slow transmission of the virus and would give parents and other caregivers greater confidence in participating in school and extracurricular activities.
CDC experts said they would use a variety of systems, including hospital networks, the open Vaccines and Adverse Events Reporting System (VAERS) database, the cell-phone based V-SAFE app, and insurance claims databases to keep an eye out for any rare adverse events related to the vaccines in children.
This article, a version of which first appeared on Medscape.com, was updated on Nov. 3, 2021.
– meaning the shots are now available for immediate use.
The Nov. 2 decision came mere hours after experts that advise the CDC on vaccinations strongly recommended the vaccine for this age group.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine. As a mom, I encourage parents with questions to talk to their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated,” Dr. Walensky said in a prepared statement.
President Joe Biden applauded Dr. Walensky’s endorsement: “Today, we have reached a turning point in our battle against COVID-19: authorization of a safe, effective vaccine for children age 5 to 11. It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus,” he said in a statement.
The 14 members of the Advisory Committee on Immunization Practices (ACIP) voted unanimously earlier in the day to recommend the vaccine for kids.
“I feel like I have a responsibility to make this vaccine available to children and their parents,” said committee member Beth Bell, MD, MPH, a clinical professor at the University of Washington in Seattle. Bell noted that all evidence the committee had reviewed pointed to a vaccine that was safe and effective for younger children.
“If I had a grandchild, I would certainly get that grandchild vaccinated as soon as possible,” she said.
Their recommendations follow the U.S. Food and Drug Administration’s emergency authorization of Pfizer-BioNTech’s vaccine for this same age group last week.
“I’m voting for this because I think it could have a huge positive impact on [kids’] health and their social and emotional wellbeing,” said Grace Lee, MD, a professor of pediatrics at Stanford University School of Medicine, who chairs the CDC’s ACIP.
She noted that, though masks are available to reduce the risk for kids, they aren’t perfect and transmission still occurs.
“Vaccines are really the only consistent and reliable way to provide that protection,” Lee said.
The vaccine for children is two doses given 3 weeks apart. Each dose is 10 micrograms, which is one-third of the dose used in adults and teens.
To avoid confusion, the smaller dose for kids will come in bottles with orange labels and orange tops. The vaccine for adults is packaged in purple.
The CDC also addressed the question of kids who are close to age 12 when they get their first dose.
In general, pediatricians allow for a 4-day grace period around birthdays to determine which dose is needed. That will be the same with the COVID-19 vaccine.
For kids who are 11 when they start the series, they should get another 10-microgram dose after they turn 12 a few weeks later.
COVID-19 cases in this age group have climbed sharply over the summer and into the fall as schools have fully reopened, sometimes without the benefit of masks.
In the first week of October, roughly 10% of all COVID-19 cases recorded in the United States were among children ages 5 through 11. Since the start of pandemic, about 1.9 million children in this age group have been infected, though that’s almost certainly an undercount. More than 8,300 have been hospitalized, and 94 children have died.
Children of color have been disproportionately impacted. More than two-thirds of hospitalized children have been black or Hispanic.
Weighing benefits and risks
In clinical trials that included more than 4,600 children, the most common adverse events were pain and swelling at the injection site. They could also have side effects like fevers, fatigue, headache, chills, and sometimes swollen lymph nodes.
These kinds of side effects appear to be less common in children ages 5 to 11 than they have been in teens and adults, and they were temporary.
No cases of myocarditis or pericarditis were seen in the studies, but myocarditis is a very rare side effect, and the studies were too small to pick up these cases.
Still, doctors say they’re watching for it. In general, the greatest risk for myocarditis after vaccination has been seen in younger males between the ages of 12 and 30.
Even without COVID-19 or vaccines in the mix, doctors expect to see as many as two cases of myocarditis for every million people over the course of a week. The risk for myocarditis jumps up to about 11 cases for every million doses of mRNA vaccine given to men ages 25 to 30. It’s between 37 and 69 cases per million doses in boys between the ages of 12 and 24.
Still, experts say the possibility of this rare risk shouldn’t deter parents from vaccinating younger children.
Here’s why: The risk for myocarditis is higher after COVID-19 infection than after vaccination. Younger children have a lower risk for myocarditis than teens and young adults, suggesting that this side effect may be less frequent in this age group, although that remains to be seen.
Additionally, the smaller dose authorized for children is expected to minimize the risk for myocarditis even further.
The CDC says parents should call their doctor if a child develops pain in their chest, has trouble breathing, or feels like they have a beating or fluttering heart after vaccination.
What about benefits?
Models looking at the impact of vaccines in this age group predict that, nationally, cases would drop by about 8% if children are vaccinated.
The models also suggested that vaccination of kids this age would slow — but not stop — the emergence of new variants.
For every million doses, the CDC’s modeling predicts that more than 56,000 COVID-19 infections would be prevented in this age group, along with dozens of hospitalizations, and post-COVID conditions like multisystem inflammatory syndrome in children.
CDC experts estimate that just 10 kids would need to be vaccinated over 6 months to prevent a single case of COVID-19.
The CDC pointed out that vaccinating kids may help slow transmission of the virus and would give parents and other caregivers greater confidence in participating in school and extracurricular activities.
CDC experts said they would use a variety of systems, including hospital networks, the open Vaccines and Adverse Events Reporting System (VAERS) database, the cell-phone based V-SAFE app, and insurance claims databases to keep an eye out for any rare adverse events related to the vaccines in children.
This article, a version of which first appeared on Medscape.com, was updated on Nov. 3, 2021.
– meaning the shots are now available for immediate use.
The Nov. 2 decision came mere hours after experts that advise the CDC on vaccinations strongly recommended the vaccine for this age group.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19. We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine. As a mom, I encourage parents with questions to talk to their pediatrician, school nurse, or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated,” Dr. Walensky said in a prepared statement.
President Joe Biden applauded Dr. Walensky’s endorsement: “Today, we have reached a turning point in our battle against COVID-19: authorization of a safe, effective vaccine for children age 5 to 11. It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others. It is a major step forward for our nation in our fight to defeat the virus,” he said in a statement.
The 14 members of the Advisory Committee on Immunization Practices (ACIP) voted unanimously earlier in the day to recommend the vaccine for kids.
“I feel like I have a responsibility to make this vaccine available to children and their parents,” said committee member Beth Bell, MD, MPH, a clinical professor at the University of Washington in Seattle. Bell noted that all evidence the committee had reviewed pointed to a vaccine that was safe and effective for younger children.
“If I had a grandchild, I would certainly get that grandchild vaccinated as soon as possible,” she said.
Their recommendations follow the U.S. Food and Drug Administration’s emergency authorization of Pfizer-BioNTech’s vaccine for this same age group last week.
“I’m voting for this because I think it could have a huge positive impact on [kids’] health and their social and emotional wellbeing,” said Grace Lee, MD, a professor of pediatrics at Stanford University School of Medicine, who chairs the CDC’s ACIP.
She noted that, though masks are available to reduce the risk for kids, they aren’t perfect and transmission still occurs.
“Vaccines are really the only consistent and reliable way to provide that protection,” Lee said.
The vaccine for children is two doses given 3 weeks apart. Each dose is 10 micrograms, which is one-third of the dose used in adults and teens.
To avoid confusion, the smaller dose for kids will come in bottles with orange labels and orange tops. The vaccine for adults is packaged in purple.
The CDC also addressed the question of kids who are close to age 12 when they get their first dose.
In general, pediatricians allow for a 4-day grace period around birthdays to determine which dose is needed. That will be the same with the COVID-19 vaccine.
For kids who are 11 when they start the series, they should get another 10-microgram dose after they turn 12 a few weeks later.
COVID-19 cases in this age group have climbed sharply over the summer and into the fall as schools have fully reopened, sometimes without the benefit of masks.
In the first week of October, roughly 10% of all COVID-19 cases recorded in the United States were among children ages 5 through 11. Since the start of pandemic, about 1.9 million children in this age group have been infected, though that’s almost certainly an undercount. More than 8,300 have been hospitalized, and 94 children have died.
Children of color have been disproportionately impacted. More than two-thirds of hospitalized children have been black or Hispanic.
Weighing benefits and risks
In clinical trials that included more than 4,600 children, the most common adverse events were pain and swelling at the injection site. They could also have side effects like fevers, fatigue, headache, chills, and sometimes swollen lymph nodes.
These kinds of side effects appear to be less common in children ages 5 to 11 than they have been in teens and adults, and they were temporary.
No cases of myocarditis or pericarditis were seen in the studies, but myocarditis is a very rare side effect, and the studies were too small to pick up these cases.
Still, doctors say they’re watching for it. In general, the greatest risk for myocarditis after vaccination has been seen in younger males between the ages of 12 and 30.
Even without COVID-19 or vaccines in the mix, doctors expect to see as many as two cases of myocarditis for every million people over the course of a week. The risk for myocarditis jumps up to about 11 cases for every million doses of mRNA vaccine given to men ages 25 to 30. It’s between 37 and 69 cases per million doses in boys between the ages of 12 and 24.
Still, experts say the possibility of this rare risk shouldn’t deter parents from vaccinating younger children.
Here’s why: The risk for myocarditis is higher after COVID-19 infection than after vaccination. Younger children have a lower risk for myocarditis than teens and young adults, suggesting that this side effect may be less frequent in this age group, although that remains to be seen.
Additionally, the smaller dose authorized for children is expected to minimize the risk for myocarditis even further.
The CDC says parents should call their doctor if a child develops pain in their chest, has trouble breathing, or feels like they have a beating or fluttering heart after vaccination.
What about benefits?
Models looking at the impact of vaccines in this age group predict that, nationally, cases would drop by about 8% if children are vaccinated.
The models also suggested that vaccination of kids this age would slow — but not stop — the emergence of new variants.
For every million doses, the CDC’s modeling predicts that more than 56,000 COVID-19 infections would be prevented in this age group, along with dozens of hospitalizations, and post-COVID conditions like multisystem inflammatory syndrome in children.
CDC experts estimate that just 10 kids would need to be vaccinated over 6 months to prevent a single case of COVID-19.
The CDC pointed out that vaccinating kids may help slow transmission of the virus and would give parents and other caregivers greater confidence in participating in school and extracurricular activities.
CDC experts said they would use a variety of systems, including hospital networks, the open Vaccines and Adverse Events Reporting System (VAERS) database, the cell-phone based V-SAFE app, and insurance claims databases to keep an eye out for any rare adverse events related to the vaccines in children.
This article, a version of which first appeared on Medscape.com, was updated on Nov. 3, 2021.
Rural hospitalists confront COVID-19
Unique demands of patient care in small hospitals
In 2018, Atashi Mandal, MD, a hospitalist residing in Orange County, Calif., was recruited along with several other doctors to fill hospitalist positions in rural Bishop, Calif. She has since driven 600 miles round trip every month for a week of hospital medicine shifts at Northern Inyo Hospital.
Dr. Mandal said she has really enjoyed her time at the small rural hospital and found it professionally fulfilling to participate so fully in the health of its local community. She was building personal bonds and calling the experience the pinnacle of her career when the COVID-19 pandemic swept across America and the world, even reaching up into Bishop, population 3,760, in the isolated Owens Valley.
The 25-bed hospital has seen at least 100 COVID patients in the past year and some months. Responsibility for taking care of these patients has been both humbling and gratifying, Dr. Mandal said. The facility’s hospitalists made a commitment to keep working through the pandemic. “We were able to come together (around COVID) as a team and our teamwork really made a difference,” she said.
“One of the advantages in a smaller hospital is you can have greater cohesiveness and your communication can be tighter. That played a big role in how we were able to accomplish so much with fewer resources as a rural hospital.” But staffing shortages, recruitment, and retention remain a perennial challenge for rural hospitals. “And COVID only exacerbated the problems,” she said. “I’ve had my challenges trying to make proper treatment plans without access to specialists.”
It was also difficult to witness so many patients severely ill or dying from COVID, Dr. Mandal said, especially since patients were not allowed family visitors – even though that was for a good reason, to minimize the virus’s spread.
HM in rural communities
Hospital medicine continues to extend into rural communities and small rural hospitals. In 2018, 35.7% of all rural counties in America had hospitals staffed with hospitalists, and 63.3% of rural hospitals had hospitalist programs (compared with 79.2% of urban hospitals). These numbers come from Medicare resources files from the Department of Health & Human Services, analyzed by Peiyin Hung, PhD, assistant professor of health services management and policy at the University of South Carolina, Columbia.1 Hospitalist penetration rates rose steadily from 2011 to 2017, with a slight dip in 2018, Dr. Hung said in an interview.
A total of 138 rural hospitals have closed since 2010, according to the Cecil G. Sheps Center for Health Services Research in Chapel Hill, N.C. Nineteen rural hospitals closed in 2020 alone, although many of those were caused by factors predating the pandemic. Only one has closed so far in 2021. But financial pressures, including low patient volumes and loss of revenue from canceled routine services like elective surgeries during the pandemic, have added to hospitals’ difficulties. Pandemic relief funding may have helped some hospitals stay open, but that support eventually will go away.
Experts emphasize the diversity of rural America and its health care systems. Rural economies are volatile and more diverse than is often appreciated. The hospital may be a cornerstone of the local economy; when one closes, it can devastate the community. Workforce is one of the chief components of a hospital’s ability to meet its strategic vision, and hospitalists are a big part in that. But while hospitalists are valued and appreciated, if the hospital is suffering severe financial problems, that will impact its doctors’ jobs and livelihoods.
“Bandwidth” varies widely for rural hospitalists and their hospitalist groups, said Ken Simone, DO, SFHM, executive chair of SHM’s Rural Special Interest Group and founder and principal of KGS Consultants, a Hospital Medicine and Primary Care Practice Management Consulting company. They may face scarce resources, scarce clinical staffing, lack of support staff to help operations run smoothly, lack of access to specialists locally, and lack of technology. While practicing in a rural setting presents various challenges, it can be rewarding for those clinicians who embrace its autonomy and broad scope of services, Dr. Simone said.
SHM’s Rural SIG focuses on the unique needs of rural hospitalists, providing them with an opportunity to share their concerns, challenges and solutions through roundtable discussions every other month and a special interest forum held in conjunction with the SHM Converge annual conference, Dr. Simone said. (The next SHM Converge will be April 7-10, 2022, in Nashville, Tenn.) The Rural SIG also collaborates with other hospital medicine SIGs and committees and is working on a white paper, “Key Principles and Characteristics of an Effective Rural Hospital Medicine Group.” It is also looking to develop a rural mentorship exchange program.
COVID reaches rural America
Early COVID caseloads tended to be in urban areas, but subsequent surges of infections have spread to many rural areas. Some rural settings became epicenters for the pandemic in November and December 2020. More recent troubling rises in COVID cases, particularly in areas with lower vaccination rates – suggest that the challenges of the pandemic are still not behind us.
“By no means is the crisis done in rural America,” said Alan Morgan, CEO of the National Rural Health Association, in a Virtual Rural Health Journalism workshop on rural health care sponsored by the Association of Health Care Journalists.2
Mr. Morgan’s colleague, Brock Slabach, NRHA’s chief operations officer, said in an interview that, while 453 of the 1,800 hospitals in rural areas fit NRHA’s criteria as being vulnerable to closure, the rest are not, and are fulfilling their missions for their communities. Hospitalists are becoming more common in these hospitals, he said, and rural hospitalists can be an important asset in attracting primary care physicians – who might not appreciate being perpetually on call for their hospitalized patients – to rural communities.
In many cases, traveling doctors like Dr. Mandal or telemedicine backup, particularly for after-hours coverage or ICU beds, are important pieces of the puzzle for smaller hospitals. There are different ways to use the spectrum of telemedicine services to interact with a hospital’s daytime and night routines. In some isolated locations, nurse practitioners or physician assistants provide on-the-ground coverage with virtual backup. Rural hospitals often affiliate with telemedicine networks within health systems – or else contract with independent specialized providers of telemedicine consultation.
Mr. Slabach said another alternative for staffing hospitals with smaller ED and inpatient volumes is to have one doctor on duty who can cover both departments simultaneously. Meanwhile, the new federal Rural Emergency Hospital Program proposes to allow rural hospitals to become essentially freestanding EDs – starting Jan. 1, 2023 – that can manage patients for a maximum of 24 hours.3
Community connections and proactive staffing
Lisa Kaufmann, MD, works as a hospitalist for a two-hospital system in North Carolina, Appalachian Regional Health Care. She practices at Watauga Medical Center, with 100 licensed beds in Boone, and at Cannon Memorial Hospital, a critical access hospital in unincorporated Linville. “We are proud of what we have been able to accomplish during the pandemic,” she said.
A former critical care unit at Watauga had been shut down, but its wiring remained intact. “We turned it into a COVID unit in three days. Then we opened another COVID unit with 18 beds, but that still wasn’t enough. We converted half of our med/surg capacity into a COVID unit. At one point almost half of all of our acute beds were for COVID patients. We made plans for what we would do if it got worse, since we had almost run out of beds,” she said. Demand peaked at the end of January 2021.
“The biggest barrier for us was if someone needed to be transferred, for example, if they needed ECMO [extracorporeal membrane oxygenation], and we couldn’t find another hospital to provide that technology.” In ARHC’s mountainous region – known as the “High Country” – weather can also make it difficult to transport patients. “Sometimes the ambulance can’t make it off the mountain, and half of the time the medical helicopter can’t fly. So we have to be prepared to keep people who we might think ought to be transferred,” she said.
Like many rural communities, the High Country is tightly knit, and its hospitals are really connected to their communities, Dr. Kaufmann said. The health system already had a lot of community connections beyond acute care, and that meant the pandemic wasn’t experienced as severely as it was in some other rural communities. “But without hospitalists in our hospitals, it would have been much more difficult.”
Proactive supply fulfillment meant that her hospitals never ran out of personal protective equipment. “Staffing was a challenge, but we were proactive in getting traveling doctors to come here. We also utilized extra doctors from the local community,” she said. Another key was well-established disaster planning, with regular drills, and a robust incident command structure, which just needed to be activated in the crisis. “Small hospitals need to be prepared for disaster,” Dr. Kaufmann said.
For Dale Wiersma, MD, a hospitalist with Spectrum Health, a 14-hospital system in western Michigan, telemedicine services are coordinated across 8 rural regional hospitals. “We don’t tend to use it for direct hospitalist work during daytime hours, unless a facility is swamped, in which case we can cross-cover. We do more telemedicine at night. But during daytime hours we have access to stroke neurology, cardiology, psychiatry, critical care and infectious disease specialists who are able to offer virtual consults,” Dr. Wiersma said. A virtual critical care team of doctor and nurse is often the only intensivist service covering Spectrum’s rural hospitals.
“In our system, the pandemic accelerated the adoption of telemedicine,” Dr. Wiersma said. “We had been working on the tele-ICU program, trying to get it rolled out. When the pandemic hit, we launched it in just 6 weeks.”
There have been several COVID surges in Michigan, he said. “We were stretched pretty close to our limit several times, but never to the breaking point. For our physicians, it was the protracted nature of the pandemic that was fatiguing for everyone involved. Our system worked hard to staff up as well as it could, to make sure our people didn’t go over the edge.” It was also hard for hospitals that typically might see one or two deaths in a month to suddenly have five in a week.
Another Spectrum hospitalist, Christopher Skinner, MD, works at two rural Michigan hospitals 15 minutes apart in Big Rapids and Reed City. “I prefer working in rural areas. I’ve never had an ambition to be a top dog. I like the style of practice where you don’t have all of the medical subspecialties on site. It frees you up to use all your skills,” Dr. Skinner said.
But that approach was put to the test by the pandemic, since it was harder to transfer those patients who normally would not have stayed at these rural hospitals. “We had to make do,” he said, although virtual backup and second opinions from Spectrum’s virtual critical care team helped.
“It was a great collaboration, which helped us to handle critical care cases that we hadn’t had to manage pre-COVID. We’ve gotten used to it, with the backup, so I expect we’ll still be taking care of these kind of sick ventilator patients even after the pandemic ends,” Dr. Skinner said. “We’ve gotten pretty good at it.”
Sukhbir Pannu, MD, a hospitalist in Denver and CEO and founder of Rural Physicians Group, said the pandemic was highly impactful, operationally and logistically, for his firm, which contracts with 54 hospitals to provide their hospitalist staffing. “There was no preparation. Everything had to be done on the fly. Initially, it was felt that rural areas weren’t at as great a risk for COVID, but that proved not to be true. Many experienced a sudden increase in very sick patients. We set up a task force to manage daily census in all of our contracted facilities.”
How did Rural Physicians Group manage through the crisis? “The short answer is telemedicine,” he said. “We had physicians on the ground in these hospitals. But we needed intensivists at the other end of the line to support them.” A lot of conversations about telemedicine were already going on in the company, but the pandemic provided the impetus to launch its network, which has grown to include rheumatologists, pulmonologists, cardiologists, infection medicine, neurology, and psychiatry, all reachable through a central command structure.
Telemedicine is not a cure-all, Dr. Pannu said. It doesn’t work in a vacuum. It requires both a provider on the ground and specialists available remotely. “But it can be a massive multiplier.”
Critical medicine
Other hospitals, including small and rural ones, have reported taking on the challenge of covering critical care with nonintensivist physicians because the pandemic demanded it. David Aymond, MD, a hospitalist at 60-bed Byrd Regional Hospital in Leesville, La., population 6,612, has advocated for years for expanded training and credentialing opportunities in intensive care medicine beyond the traditional path of becoming a board-certified intensivist. Some rural hospitalists were already experienced in providing critical care for ICU patients even before the pandemic hit.
“What COVID did was to highlight the problem that there aren’t enough intensivists in this country, particular for smaller hospitals,” Dr. Aymond said. Some hospitalists who stepped into crisis roles in ICUs during COVID surges showed that they could take care of COVID patients very well.
Dr. Aymond, who is a fellowship-trained hospitalist with primary training in family medicine, has used his ICU experience in both fellowship and practice to make a thorough study of critical care medicine, which he put to good use when the seven-bed ICU at Byrd Memorial filled with COVID patients. “Early on, we were managing multiple ventilators throughout the hospital,” he said. “But we were having good outcomes. Our COVID patients were surviving.” That led to Dr. Aymond being interviewed by local news media, which led to other patients across the state asking to be transferred to “the COVID specialist who practices at Byrd.”
Dr. Aymond would like to see opportunities for abbreviated 1-year critical care fellowships for hospitalists who have amassed enough ICU experience in practice or in residency, and to make room for family medicine physicians in such programs. He is also working through SHM with the Society of Critical Care Medicine to generate educational ICU content. SHM now has a critical care lecture series at: www.hospitalmedicine.org/clinical-topics/critical-care/.
Dr. Mandal, who also works as a pediatric hospitalist, said that experience gave her more familiarity with using noninvasive methods for delivering respiratory therapies like high-flow oxygen. “When I saw a COVID patient who had hypoxia but was still able to talk, I didn’t hesitate to deliver oxygen through noninvasive means.” Eventually hospital practice generally for COVID caught up with this approach.
But she ran into personal difficulties because N95 face masks didn’t fit her face. Instead, she had to wear a portable respirator, which made it hard to hear what her patients were saying. “I formulated a lot of workarounds, such as interviewing the patient over the phone before going into the room for the physical exam.”
Throughout the pandemic, she never wavered in her commitment to rural hospital medicine and its opportunities for working in a small and wonderful community, where she could practice at the top of her license, with a degree of autonomy not granted in other settings. For doctors who want that kind of practice, she said, “the rewards will be paid back in spades. That’s been my experience.”
For more information on SHM’s Rural SIG and its supports for rural hospitalists, contact its executive chair, Kenneth Simone, DO, at [email protected].
References
1. Personal communication from Peiyin Hung, June 2021.
2. Association of Health Care Journalists. Rural Health Journalism Workshop 2021. June 21, 2021. https://healthjournalism.org/calendar-details.php?id=2369.
3. Congress Establishes New Medicare Provider Category and Reimbursement for Rural Emergency Hospitals. National Law Review. Jan. 5, 2021. https://www.natlawreview.com/article/congress-establishes-new-medicare-provider-category-and-reimbursement-rural.
Unique demands of patient care in small hospitals
Unique demands of patient care in small hospitals
In 2018, Atashi Mandal, MD, a hospitalist residing in Orange County, Calif., was recruited along with several other doctors to fill hospitalist positions in rural Bishop, Calif. She has since driven 600 miles round trip every month for a week of hospital medicine shifts at Northern Inyo Hospital.
Dr. Mandal said she has really enjoyed her time at the small rural hospital and found it professionally fulfilling to participate so fully in the health of its local community. She was building personal bonds and calling the experience the pinnacle of her career when the COVID-19 pandemic swept across America and the world, even reaching up into Bishop, population 3,760, in the isolated Owens Valley.
The 25-bed hospital has seen at least 100 COVID patients in the past year and some months. Responsibility for taking care of these patients has been both humbling and gratifying, Dr. Mandal said. The facility’s hospitalists made a commitment to keep working through the pandemic. “We were able to come together (around COVID) as a team and our teamwork really made a difference,” she said.
“One of the advantages in a smaller hospital is you can have greater cohesiveness and your communication can be tighter. That played a big role in how we were able to accomplish so much with fewer resources as a rural hospital.” But staffing shortages, recruitment, and retention remain a perennial challenge for rural hospitals. “And COVID only exacerbated the problems,” she said. “I’ve had my challenges trying to make proper treatment plans without access to specialists.”
It was also difficult to witness so many patients severely ill or dying from COVID, Dr. Mandal said, especially since patients were not allowed family visitors – even though that was for a good reason, to minimize the virus’s spread.
HM in rural communities
Hospital medicine continues to extend into rural communities and small rural hospitals. In 2018, 35.7% of all rural counties in America had hospitals staffed with hospitalists, and 63.3% of rural hospitals had hospitalist programs (compared with 79.2% of urban hospitals). These numbers come from Medicare resources files from the Department of Health & Human Services, analyzed by Peiyin Hung, PhD, assistant professor of health services management and policy at the University of South Carolina, Columbia.1 Hospitalist penetration rates rose steadily from 2011 to 2017, with a slight dip in 2018, Dr. Hung said in an interview.
A total of 138 rural hospitals have closed since 2010, according to the Cecil G. Sheps Center for Health Services Research in Chapel Hill, N.C. Nineteen rural hospitals closed in 2020 alone, although many of those were caused by factors predating the pandemic. Only one has closed so far in 2021. But financial pressures, including low patient volumes and loss of revenue from canceled routine services like elective surgeries during the pandemic, have added to hospitals’ difficulties. Pandemic relief funding may have helped some hospitals stay open, but that support eventually will go away.
Experts emphasize the diversity of rural America and its health care systems. Rural economies are volatile and more diverse than is often appreciated. The hospital may be a cornerstone of the local economy; when one closes, it can devastate the community. Workforce is one of the chief components of a hospital’s ability to meet its strategic vision, and hospitalists are a big part in that. But while hospitalists are valued and appreciated, if the hospital is suffering severe financial problems, that will impact its doctors’ jobs and livelihoods.
“Bandwidth” varies widely for rural hospitalists and their hospitalist groups, said Ken Simone, DO, SFHM, executive chair of SHM’s Rural Special Interest Group and founder and principal of KGS Consultants, a Hospital Medicine and Primary Care Practice Management Consulting company. They may face scarce resources, scarce clinical staffing, lack of support staff to help operations run smoothly, lack of access to specialists locally, and lack of technology. While practicing in a rural setting presents various challenges, it can be rewarding for those clinicians who embrace its autonomy and broad scope of services, Dr. Simone said.
SHM’s Rural SIG focuses on the unique needs of rural hospitalists, providing them with an opportunity to share their concerns, challenges and solutions through roundtable discussions every other month and a special interest forum held in conjunction with the SHM Converge annual conference, Dr. Simone said. (The next SHM Converge will be April 7-10, 2022, in Nashville, Tenn.) The Rural SIG also collaborates with other hospital medicine SIGs and committees and is working on a white paper, “Key Principles and Characteristics of an Effective Rural Hospital Medicine Group.” It is also looking to develop a rural mentorship exchange program.
COVID reaches rural America
Early COVID caseloads tended to be in urban areas, but subsequent surges of infections have spread to many rural areas. Some rural settings became epicenters for the pandemic in November and December 2020. More recent troubling rises in COVID cases, particularly in areas with lower vaccination rates – suggest that the challenges of the pandemic are still not behind us.
“By no means is the crisis done in rural America,” said Alan Morgan, CEO of the National Rural Health Association, in a Virtual Rural Health Journalism workshop on rural health care sponsored by the Association of Health Care Journalists.2
Mr. Morgan’s colleague, Brock Slabach, NRHA’s chief operations officer, said in an interview that, while 453 of the 1,800 hospitals in rural areas fit NRHA’s criteria as being vulnerable to closure, the rest are not, and are fulfilling their missions for their communities. Hospitalists are becoming more common in these hospitals, he said, and rural hospitalists can be an important asset in attracting primary care physicians – who might not appreciate being perpetually on call for their hospitalized patients – to rural communities.
In many cases, traveling doctors like Dr. Mandal or telemedicine backup, particularly for after-hours coverage or ICU beds, are important pieces of the puzzle for smaller hospitals. There are different ways to use the spectrum of telemedicine services to interact with a hospital’s daytime and night routines. In some isolated locations, nurse practitioners or physician assistants provide on-the-ground coverage with virtual backup. Rural hospitals often affiliate with telemedicine networks within health systems – or else contract with independent specialized providers of telemedicine consultation.
Mr. Slabach said another alternative for staffing hospitals with smaller ED and inpatient volumes is to have one doctor on duty who can cover both departments simultaneously. Meanwhile, the new federal Rural Emergency Hospital Program proposes to allow rural hospitals to become essentially freestanding EDs – starting Jan. 1, 2023 – that can manage patients for a maximum of 24 hours.3
Community connections and proactive staffing
Lisa Kaufmann, MD, works as a hospitalist for a two-hospital system in North Carolina, Appalachian Regional Health Care. She practices at Watauga Medical Center, with 100 licensed beds in Boone, and at Cannon Memorial Hospital, a critical access hospital in unincorporated Linville. “We are proud of what we have been able to accomplish during the pandemic,” she said.
A former critical care unit at Watauga had been shut down, but its wiring remained intact. “We turned it into a COVID unit in three days. Then we opened another COVID unit with 18 beds, but that still wasn’t enough. We converted half of our med/surg capacity into a COVID unit. At one point almost half of all of our acute beds were for COVID patients. We made plans for what we would do if it got worse, since we had almost run out of beds,” she said. Demand peaked at the end of January 2021.
“The biggest barrier for us was if someone needed to be transferred, for example, if they needed ECMO [extracorporeal membrane oxygenation], and we couldn’t find another hospital to provide that technology.” In ARHC’s mountainous region – known as the “High Country” – weather can also make it difficult to transport patients. “Sometimes the ambulance can’t make it off the mountain, and half of the time the medical helicopter can’t fly. So we have to be prepared to keep people who we might think ought to be transferred,” she said.
Like many rural communities, the High Country is tightly knit, and its hospitals are really connected to their communities, Dr. Kaufmann said. The health system already had a lot of community connections beyond acute care, and that meant the pandemic wasn’t experienced as severely as it was in some other rural communities. “But without hospitalists in our hospitals, it would have been much more difficult.”
Proactive supply fulfillment meant that her hospitals never ran out of personal protective equipment. “Staffing was a challenge, but we were proactive in getting traveling doctors to come here. We also utilized extra doctors from the local community,” she said. Another key was well-established disaster planning, with regular drills, and a robust incident command structure, which just needed to be activated in the crisis. “Small hospitals need to be prepared for disaster,” Dr. Kaufmann said.
For Dale Wiersma, MD, a hospitalist with Spectrum Health, a 14-hospital system in western Michigan, telemedicine services are coordinated across 8 rural regional hospitals. “We don’t tend to use it for direct hospitalist work during daytime hours, unless a facility is swamped, in which case we can cross-cover. We do more telemedicine at night. But during daytime hours we have access to stroke neurology, cardiology, psychiatry, critical care and infectious disease specialists who are able to offer virtual consults,” Dr. Wiersma said. A virtual critical care team of doctor and nurse is often the only intensivist service covering Spectrum’s rural hospitals.
“In our system, the pandemic accelerated the adoption of telemedicine,” Dr. Wiersma said. “We had been working on the tele-ICU program, trying to get it rolled out. When the pandemic hit, we launched it in just 6 weeks.”
There have been several COVID surges in Michigan, he said. “We were stretched pretty close to our limit several times, but never to the breaking point. For our physicians, it was the protracted nature of the pandemic that was fatiguing for everyone involved. Our system worked hard to staff up as well as it could, to make sure our people didn’t go over the edge.” It was also hard for hospitals that typically might see one or two deaths in a month to suddenly have five in a week.
Another Spectrum hospitalist, Christopher Skinner, MD, works at two rural Michigan hospitals 15 minutes apart in Big Rapids and Reed City. “I prefer working in rural areas. I’ve never had an ambition to be a top dog. I like the style of practice where you don’t have all of the medical subspecialties on site. It frees you up to use all your skills,” Dr. Skinner said.
But that approach was put to the test by the pandemic, since it was harder to transfer those patients who normally would not have stayed at these rural hospitals. “We had to make do,” he said, although virtual backup and second opinions from Spectrum’s virtual critical care team helped.
“It was a great collaboration, which helped us to handle critical care cases that we hadn’t had to manage pre-COVID. We’ve gotten used to it, with the backup, so I expect we’ll still be taking care of these kind of sick ventilator patients even after the pandemic ends,” Dr. Skinner said. “We’ve gotten pretty good at it.”
Sukhbir Pannu, MD, a hospitalist in Denver and CEO and founder of Rural Physicians Group, said the pandemic was highly impactful, operationally and logistically, for his firm, which contracts with 54 hospitals to provide their hospitalist staffing. “There was no preparation. Everything had to be done on the fly. Initially, it was felt that rural areas weren’t at as great a risk for COVID, but that proved not to be true. Many experienced a sudden increase in very sick patients. We set up a task force to manage daily census in all of our contracted facilities.”
How did Rural Physicians Group manage through the crisis? “The short answer is telemedicine,” he said. “We had physicians on the ground in these hospitals. But we needed intensivists at the other end of the line to support them.” A lot of conversations about telemedicine were already going on in the company, but the pandemic provided the impetus to launch its network, which has grown to include rheumatologists, pulmonologists, cardiologists, infection medicine, neurology, and psychiatry, all reachable through a central command structure.
Telemedicine is not a cure-all, Dr. Pannu said. It doesn’t work in a vacuum. It requires both a provider on the ground and specialists available remotely. “But it can be a massive multiplier.”
Critical medicine
Other hospitals, including small and rural ones, have reported taking on the challenge of covering critical care with nonintensivist physicians because the pandemic demanded it. David Aymond, MD, a hospitalist at 60-bed Byrd Regional Hospital in Leesville, La., population 6,612, has advocated for years for expanded training and credentialing opportunities in intensive care medicine beyond the traditional path of becoming a board-certified intensivist. Some rural hospitalists were already experienced in providing critical care for ICU patients even before the pandemic hit.
“What COVID did was to highlight the problem that there aren’t enough intensivists in this country, particular for smaller hospitals,” Dr. Aymond said. Some hospitalists who stepped into crisis roles in ICUs during COVID surges showed that they could take care of COVID patients very well.
Dr. Aymond, who is a fellowship-trained hospitalist with primary training in family medicine, has used his ICU experience in both fellowship and practice to make a thorough study of critical care medicine, which he put to good use when the seven-bed ICU at Byrd Memorial filled with COVID patients. “Early on, we were managing multiple ventilators throughout the hospital,” he said. “But we were having good outcomes. Our COVID patients were surviving.” That led to Dr. Aymond being interviewed by local news media, which led to other patients across the state asking to be transferred to “the COVID specialist who practices at Byrd.”
Dr. Aymond would like to see opportunities for abbreviated 1-year critical care fellowships for hospitalists who have amassed enough ICU experience in practice or in residency, and to make room for family medicine physicians in such programs. He is also working through SHM with the Society of Critical Care Medicine to generate educational ICU content. SHM now has a critical care lecture series at: www.hospitalmedicine.org/clinical-topics/critical-care/.
Dr. Mandal, who also works as a pediatric hospitalist, said that experience gave her more familiarity with using noninvasive methods for delivering respiratory therapies like high-flow oxygen. “When I saw a COVID patient who had hypoxia but was still able to talk, I didn’t hesitate to deliver oxygen through noninvasive means.” Eventually hospital practice generally for COVID caught up with this approach.
But she ran into personal difficulties because N95 face masks didn’t fit her face. Instead, she had to wear a portable respirator, which made it hard to hear what her patients were saying. “I formulated a lot of workarounds, such as interviewing the patient over the phone before going into the room for the physical exam.”
Throughout the pandemic, she never wavered in her commitment to rural hospital medicine and its opportunities for working in a small and wonderful community, where she could practice at the top of her license, with a degree of autonomy not granted in other settings. For doctors who want that kind of practice, she said, “the rewards will be paid back in spades. That’s been my experience.”
For more information on SHM’s Rural SIG and its supports for rural hospitalists, contact its executive chair, Kenneth Simone, DO, at [email protected].
References
1. Personal communication from Peiyin Hung, June 2021.
2. Association of Health Care Journalists. Rural Health Journalism Workshop 2021. June 21, 2021. https://healthjournalism.org/calendar-details.php?id=2369.
3. Congress Establishes New Medicare Provider Category and Reimbursement for Rural Emergency Hospitals. National Law Review. Jan. 5, 2021. https://www.natlawreview.com/article/congress-establishes-new-medicare-provider-category-and-reimbursement-rural.
In 2018, Atashi Mandal, MD, a hospitalist residing in Orange County, Calif., was recruited along with several other doctors to fill hospitalist positions in rural Bishop, Calif. She has since driven 600 miles round trip every month for a week of hospital medicine shifts at Northern Inyo Hospital.
Dr. Mandal said she has really enjoyed her time at the small rural hospital and found it professionally fulfilling to participate so fully in the health of its local community. She was building personal bonds and calling the experience the pinnacle of her career when the COVID-19 pandemic swept across America and the world, even reaching up into Bishop, population 3,760, in the isolated Owens Valley.
The 25-bed hospital has seen at least 100 COVID patients in the past year and some months. Responsibility for taking care of these patients has been both humbling and gratifying, Dr. Mandal said. The facility’s hospitalists made a commitment to keep working through the pandemic. “We were able to come together (around COVID) as a team and our teamwork really made a difference,” she said.
“One of the advantages in a smaller hospital is you can have greater cohesiveness and your communication can be tighter. That played a big role in how we were able to accomplish so much with fewer resources as a rural hospital.” But staffing shortages, recruitment, and retention remain a perennial challenge for rural hospitals. “And COVID only exacerbated the problems,” she said. “I’ve had my challenges trying to make proper treatment plans without access to specialists.”
It was also difficult to witness so many patients severely ill or dying from COVID, Dr. Mandal said, especially since patients were not allowed family visitors – even though that was for a good reason, to minimize the virus’s spread.
HM in rural communities
Hospital medicine continues to extend into rural communities and small rural hospitals. In 2018, 35.7% of all rural counties in America had hospitals staffed with hospitalists, and 63.3% of rural hospitals had hospitalist programs (compared with 79.2% of urban hospitals). These numbers come from Medicare resources files from the Department of Health & Human Services, analyzed by Peiyin Hung, PhD, assistant professor of health services management and policy at the University of South Carolina, Columbia.1 Hospitalist penetration rates rose steadily from 2011 to 2017, with a slight dip in 2018, Dr. Hung said in an interview.
A total of 138 rural hospitals have closed since 2010, according to the Cecil G. Sheps Center for Health Services Research in Chapel Hill, N.C. Nineteen rural hospitals closed in 2020 alone, although many of those were caused by factors predating the pandemic. Only one has closed so far in 2021. But financial pressures, including low patient volumes and loss of revenue from canceled routine services like elective surgeries during the pandemic, have added to hospitals’ difficulties. Pandemic relief funding may have helped some hospitals stay open, but that support eventually will go away.
Experts emphasize the diversity of rural America and its health care systems. Rural economies are volatile and more diverse than is often appreciated. The hospital may be a cornerstone of the local economy; when one closes, it can devastate the community. Workforce is one of the chief components of a hospital’s ability to meet its strategic vision, and hospitalists are a big part in that. But while hospitalists are valued and appreciated, if the hospital is suffering severe financial problems, that will impact its doctors’ jobs and livelihoods.
“Bandwidth” varies widely for rural hospitalists and their hospitalist groups, said Ken Simone, DO, SFHM, executive chair of SHM’s Rural Special Interest Group and founder and principal of KGS Consultants, a Hospital Medicine and Primary Care Practice Management Consulting company. They may face scarce resources, scarce clinical staffing, lack of support staff to help operations run smoothly, lack of access to specialists locally, and lack of technology. While practicing in a rural setting presents various challenges, it can be rewarding for those clinicians who embrace its autonomy and broad scope of services, Dr. Simone said.
SHM’s Rural SIG focuses on the unique needs of rural hospitalists, providing them with an opportunity to share their concerns, challenges and solutions through roundtable discussions every other month and a special interest forum held in conjunction with the SHM Converge annual conference, Dr. Simone said. (The next SHM Converge will be April 7-10, 2022, in Nashville, Tenn.) The Rural SIG also collaborates with other hospital medicine SIGs and committees and is working on a white paper, “Key Principles and Characteristics of an Effective Rural Hospital Medicine Group.” It is also looking to develop a rural mentorship exchange program.
COVID reaches rural America
Early COVID caseloads tended to be in urban areas, but subsequent surges of infections have spread to many rural areas. Some rural settings became epicenters for the pandemic in November and December 2020. More recent troubling rises in COVID cases, particularly in areas with lower vaccination rates – suggest that the challenges of the pandemic are still not behind us.
“By no means is the crisis done in rural America,” said Alan Morgan, CEO of the National Rural Health Association, in a Virtual Rural Health Journalism workshop on rural health care sponsored by the Association of Health Care Journalists.2
Mr. Morgan’s colleague, Brock Slabach, NRHA’s chief operations officer, said in an interview that, while 453 of the 1,800 hospitals in rural areas fit NRHA’s criteria as being vulnerable to closure, the rest are not, and are fulfilling their missions for their communities. Hospitalists are becoming more common in these hospitals, he said, and rural hospitalists can be an important asset in attracting primary care physicians – who might not appreciate being perpetually on call for their hospitalized patients – to rural communities.
In many cases, traveling doctors like Dr. Mandal or telemedicine backup, particularly for after-hours coverage or ICU beds, are important pieces of the puzzle for smaller hospitals. There are different ways to use the spectrum of telemedicine services to interact with a hospital’s daytime and night routines. In some isolated locations, nurse practitioners or physician assistants provide on-the-ground coverage with virtual backup. Rural hospitals often affiliate with telemedicine networks within health systems – or else contract with independent specialized providers of telemedicine consultation.
Mr. Slabach said another alternative for staffing hospitals with smaller ED and inpatient volumes is to have one doctor on duty who can cover both departments simultaneously. Meanwhile, the new federal Rural Emergency Hospital Program proposes to allow rural hospitals to become essentially freestanding EDs – starting Jan. 1, 2023 – that can manage patients for a maximum of 24 hours.3
Community connections and proactive staffing
Lisa Kaufmann, MD, works as a hospitalist for a two-hospital system in North Carolina, Appalachian Regional Health Care. She practices at Watauga Medical Center, with 100 licensed beds in Boone, and at Cannon Memorial Hospital, a critical access hospital in unincorporated Linville. “We are proud of what we have been able to accomplish during the pandemic,” she said.
A former critical care unit at Watauga had been shut down, but its wiring remained intact. “We turned it into a COVID unit in three days. Then we opened another COVID unit with 18 beds, but that still wasn’t enough. We converted half of our med/surg capacity into a COVID unit. At one point almost half of all of our acute beds were for COVID patients. We made plans for what we would do if it got worse, since we had almost run out of beds,” she said. Demand peaked at the end of January 2021.
“The biggest barrier for us was if someone needed to be transferred, for example, if they needed ECMO [extracorporeal membrane oxygenation], and we couldn’t find another hospital to provide that technology.” In ARHC’s mountainous region – known as the “High Country” – weather can also make it difficult to transport patients. “Sometimes the ambulance can’t make it off the mountain, and half of the time the medical helicopter can’t fly. So we have to be prepared to keep people who we might think ought to be transferred,” she said.
Like many rural communities, the High Country is tightly knit, and its hospitals are really connected to their communities, Dr. Kaufmann said. The health system already had a lot of community connections beyond acute care, and that meant the pandemic wasn’t experienced as severely as it was in some other rural communities. “But without hospitalists in our hospitals, it would have been much more difficult.”
Proactive supply fulfillment meant that her hospitals never ran out of personal protective equipment. “Staffing was a challenge, but we were proactive in getting traveling doctors to come here. We also utilized extra doctors from the local community,” she said. Another key was well-established disaster planning, with regular drills, and a robust incident command structure, which just needed to be activated in the crisis. “Small hospitals need to be prepared for disaster,” Dr. Kaufmann said.
For Dale Wiersma, MD, a hospitalist with Spectrum Health, a 14-hospital system in western Michigan, telemedicine services are coordinated across 8 rural regional hospitals. “We don’t tend to use it for direct hospitalist work during daytime hours, unless a facility is swamped, in which case we can cross-cover. We do more telemedicine at night. But during daytime hours we have access to stroke neurology, cardiology, psychiatry, critical care and infectious disease specialists who are able to offer virtual consults,” Dr. Wiersma said. A virtual critical care team of doctor and nurse is often the only intensivist service covering Spectrum’s rural hospitals.
“In our system, the pandemic accelerated the adoption of telemedicine,” Dr. Wiersma said. “We had been working on the tele-ICU program, trying to get it rolled out. When the pandemic hit, we launched it in just 6 weeks.”
There have been several COVID surges in Michigan, he said. “We were stretched pretty close to our limit several times, but never to the breaking point. For our physicians, it was the protracted nature of the pandemic that was fatiguing for everyone involved. Our system worked hard to staff up as well as it could, to make sure our people didn’t go over the edge.” It was also hard for hospitals that typically might see one or two deaths in a month to suddenly have five in a week.
Another Spectrum hospitalist, Christopher Skinner, MD, works at two rural Michigan hospitals 15 minutes apart in Big Rapids and Reed City. “I prefer working in rural areas. I’ve never had an ambition to be a top dog. I like the style of practice where you don’t have all of the medical subspecialties on site. It frees you up to use all your skills,” Dr. Skinner said.
But that approach was put to the test by the pandemic, since it was harder to transfer those patients who normally would not have stayed at these rural hospitals. “We had to make do,” he said, although virtual backup and second opinions from Spectrum’s virtual critical care team helped.
“It was a great collaboration, which helped us to handle critical care cases that we hadn’t had to manage pre-COVID. We’ve gotten used to it, with the backup, so I expect we’ll still be taking care of these kind of sick ventilator patients even after the pandemic ends,” Dr. Skinner said. “We’ve gotten pretty good at it.”
Sukhbir Pannu, MD, a hospitalist in Denver and CEO and founder of Rural Physicians Group, said the pandemic was highly impactful, operationally and logistically, for his firm, which contracts with 54 hospitals to provide their hospitalist staffing. “There was no preparation. Everything had to be done on the fly. Initially, it was felt that rural areas weren’t at as great a risk for COVID, but that proved not to be true. Many experienced a sudden increase in very sick patients. We set up a task force to manage daily census in all of our contracted facilities.”
How did Rural Physicians Group manage through the crisis? “The short answer is telemedicine,” he said. “We had physicians on the ground in these hospitals. But we needed intensivists at the other end of the line to support them.” A lot of conversations about telemedicine were already going on in the company, but the pandemic provided the impetus to launch its network, which has grown to include rheumatologists, pulmonologists, cardiologists, infection medicine, neurology, and psychiatry, all reachable through a central command structure.
Telemedicine is not a cure-all, Dr. Pannu said. It doesn’t work in a vacuum. It requires both a provider on the ground and specialists available remotely. “But it can be a massive multiplier.”
Critical medicine
Other hospitals, including small and rural ones, have reported taking on the challenge of covering critical care with nonintensivist physicians because the pandemic demanded it. David Aymond, MD, a hospitalist at 60-bed Byrd Regional Hospital in Leesville, La., population 6,612, has advocated for years for expanded training and credentialing opportunities in intensive care medicine beyond the traditional path of becoming a board-certified intensivist. Some rural hospitalists were already experienced in providing critical care for ICU patients even before the pandemic hit.
“What COVID did was to highlight the problem that there aren’t enough intensivists in this country, particular for smaller hospitals,” Dr. Aymond said. Some hospitalists who stepped into crisis roles in ICUs during COVID surges showed that they could take care of COVID patients very well.
Dr. Aymond, who is a fellowship-trained hospitalist with primary training in family medicine, has used his ICU experience in both fellowship and practice to make a thorough study of critical care medicine, which he put to good use when the seven-bed ICU at Byrd Memorial filled with COVID patients. “Early on, we were managing multiple ventilators throughout the hospital,” he said. “But we were having good outcomes. Our COVID patients were surviving.” That led to Dr. Aymond being interviewed by local news media, which led to other patients across the state asking to be transferred to “the COVID specialist who practices at Byrd.”
Dr. Aymond would like to see opportunities for abbreviated 1-year critical care fellowships for hospitalists who have amassed enough ICU experience in practice or in residency, and to make room for family medicine physicians in such programs. He is also working through SHM with the Society of Critical Care Medicine to generate educational ICU content. SHM now has a critical care lecture series at: www.hospitalmedicine.org/clinical-topics/critical-care/.
Dr. Mandal, who also works as a pediatric hospitalist, said that experience gave her more familiarity with using noninvasive methods for delivering respiratory therapies like high-flow oxygen. “When I saw a COVID patient who had hypoxia but was still able to talk, I didn’t hesitate to deliver oxygen through noninvasive means.” Eventually hospital practice generally for COVID caught up with this approach.
But she ran into personal difficulties because N95 face masks didn’t fit her face. Instead, she had to wear a portable respirator, which made it hard to hear what her patients were saying. “I formulated a lot of workarounds, such as interviewing the patient over the phone before going into the room for the physical exam.”
Throughout the pandemic, she never wavered in her commitment to rural hospital medicine and its opportunities for working in a small and wonderful community, where she could practice at the top of her license, with a degree of autonomy not granted in other settings. For doctors who want that kind of practice, she said, “the rewards will be paid back in spades. That’s been my experience.”
For more information on SHM’s Rural SIG and its supports for rural hospitalists, contact its executive chair, Kenneth Simone, DO, at [email protected].
References
1. Personal communication from Peiyin Hung, June 2021.
2. Association of Health Care Journalists. Rural Health Journalism Workshop 2021. June 21, 2021. https://healthjournalism.org/calendar-details.php?id=2369.
3. Congress Establishes New Medicare Provider Category and Reimbursement for Rural Emergency Hospitals. National Law Review. Jan. 5, 2021. https://www.natlawreview.com/article/congress-establishes-new-medicare-provider-category-and-reimbursement-rural.
Children and COVID: A look at the pace of vaccination
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?
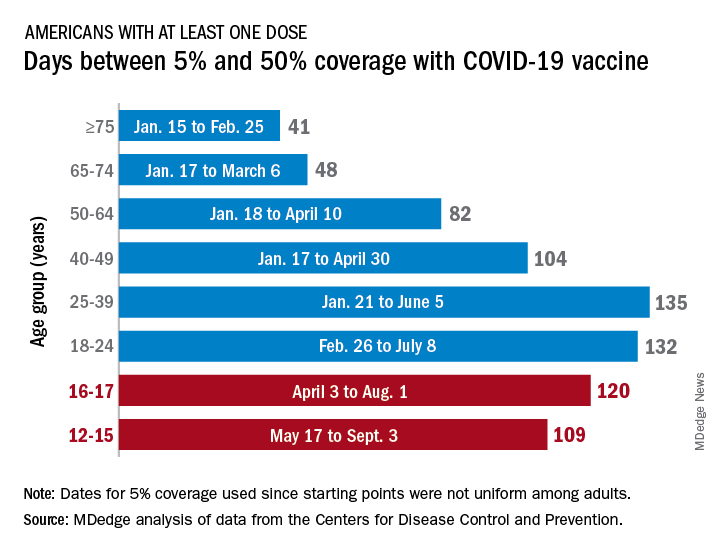
Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?

Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?

Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
COVID-19 vaccines provide 5 times the protection of natural immunity, CDC study says
, according to a new study published recently in the CDC’s Morbidity and Mortality Weekly Report.
The research team concluded that vaccination can provide a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least six months.
“We now have additional evidence that reaffirms the importance of COVID-19 vaccines, even if you have had prior infection,” Rochelle Walensky, MD, director of the CDC, said in a statement.
“This study adds more to the body of knowledge demonstrating the protection of vaccines against severe disease from COVID-19,” she said. “The best way to stop COVID-19, including the emergence of variants, is with widespread COVID-19 vaccination and with disease prevention actions such as mask wearing, washing hands often, physical distancing and staying home when sick.”
Researchers looked at data from the VISION Network, which included more than 201,000 hospitalizations for COVID-like illness at 187 hospitals across nine states between Jan. 1 to Sept. 2. Among those, more than 94,000 had rapid testing for the coronavirus, and 7,300 had a lab-confirmed test for COVID-19.
The research team found that unvaccinated people with a prior infection within 3 to 6 months were about 5-1/2 times more likely to have laboratory-confirmed COVID-19 than those who were fully vaccinated within 3 to 6 months with the Pfizer or Moderna shots. They found similar results when looking at the months that the Delta variant was the dominant strain of the coronavirus.
Protection from the Moderna vaccine “appeared to be higher” than for the Pfizer vaccine, the study authors wrote. The boost in protection also “trended higher” among older adults, as compared to those under age 65.
Importantly, the research team noted, these estimates may change over time as immunity wanes. Future studies should consider infection-induced and vaccine-induced immunity as time passes during the pandemic, they wrote.
Additional research is also needed for the Johnson & Johnson vaccine, they wrote. Those who have received the Johnson & Johnson vaccine are currently recommended to receive a booster shot at least two months after the first shot.
Overall, “all eligible persons should be vaccinated against COVID-19 as soon as possible, including unvaccinated persons previously infected,” the research team concluded.
A version of this article first appeared on WebMD.com.
, according to a new study published recently in the CDC’s Morbidity and Mortality Weekly Report.
The research team concluded that vaccination can provide a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least six months.
“We now have additional evidence that reaffirms the importance of COVID-19 vaccines, even if you have had prior infection,” Rochelle Walensky, MD, director of the CDC, said in a statement.
“This study adds more to the body of knowledge demonstrating the protection of vaccines against severe disease from COVID-19,” she said. “The best way to stop COVID-19, including the emergence of variants, is with widespread COVID-19 vaccination and with disease prevention actions such as mask wearing, washing hands often, physical distancing and staying home when sick.”
Researchers looked at data from the VISION Network, which included more than 201,000 hospitalizations for COVID-like illness at 187 hospitals across nine states between Jan. 1 to Sept. 2. Among those, more than 94,000 had rapid testing for the coronavirus, and 7,300 had a lab-confirmed test for COVID-19.
The research team found that unvaccinated people with a prior infection within 3 to 6 months were about 5-1/2 times more likely to have laboratory-confirmed COVID-19 than those who were fully vaccinated within 3 to 6 months with the Pfizer or Moderna shots. They found similar results when looking at the months that the Delta variant was the dominant strain of the coronavirus.
Protection from the Moderna vaccine “appeared to be higher” than for the Pfizer vaccine, the study authors wrote. The boost in protection also “trended higher” among older adults, as compared to those under age 65.
Importantly, the research team noted, these estimates may change over time as immunity wanes. Future studies should consider infection-induced and vaccine-induced immunity as time passes during the pandemic, they wrote.
Additional research is also needed for the Johnson & Johnson vaccine, they wrote. Those who have received the Johnson & Johnson vaccine are currently recommended to receive a booster shot at least two months after the first shot.
Overall, “all eligible persons should be vaccinated against COVID-19 as soon as possible, including unvaccinated persons previously infected,” the research team concluded.
A version of this article first appeared on WebMD.com.
, according to a new study published recently in the CDC’s Morbidity and Mortality Weekly Report.
The research team concluded that vaccination can provide a higher, stronger, and more consistent level of immunity against COVID-19 hospitalization than infection alone for at least six months.
“We now have additional evidence that reaffirms the importance of COVID-19 vaccines, even if you have had prior infection,” Rochelle Walensky, MD, director of the CDC, said in a statement.
“This study adds more to the body of knowledge demonstrating the protection of vaccines against severe disease from COVID-19,” she said. “The best way to stop COVID-19, including the emergence of variants, is with widespread COVID-19 vaccination and with disease prevention actions such as mask wearing, washing hands often, physical distancing and staying home when sick.”
Researchers looked at data from the VISION Network, which included more than 201,000 hospitalizations for COVID-like illness at 187 hospitals across nine states between Jan. 1 to Sept. 2. Among those, more than 94,000 had rapid testing for the coronavirus, and 7,300 had a lab-confirmed test for COVID-19.
The research team found that unvaccinated people with a prior infection within 3 to 6 months were about 5-1/2 times more likely to have laboratory-confirmed COVID-19 than those who were fully vaccinated within 3 to 6 months with the Pfizer or Moderna shots. They found similar results when looking at the months that the Delta variant was the dominant strain of the coronavirus.
Protection from the Moderna vaccine “appeared to be higher” than for the Pfizer vaccine, the study authors wrote. The boost in protection also “trended higher” among older adults, as compared to those under age 65.
Importantly, the research team noted, these estimates may change over time as immunity wanes. Future studies should consider infection-induced and vaccine-induced immunity as time passes during the pandemic, they wrote.
Additional research is also needed for the Johnson & Johnson vaccine, they wrote. Those who have received the Johnson & Johnson vaccine are currently recommended to receive a booster shot at least two months after the first shot.
Overall, “all eligible persons should be vaccinated against COVID-19 as soon as possible, including unvaccinated persons previously infected,” the research team concluded.
A version of this article first appeared on WebMD.com.
Video of nurse escorted from hospital for refusing vaccine goes viral
It had been viewed more than 6 million times at press time.
The 5-minute video of the nurse leaving the hospital, both of which are unidentified in the clip, was a peaceful protest by the frontline worker against COVID-19 vaccine mandates, which many employers globally are enforcing.
In her video, which was originally posted October 30 on Rumble, the nurse explained, “I am being escorted out of Kaiser Permanente hospital for my religious beliefs because I don’t want to get the jab. And I asked all day for someone to explain to me why my sincerely held religious beliefs are not good enough for Kaiser. And no one was able to do that for me,” she continued.
“So now they’re escorting me out because I wanted an answer. And I’m not leaving without an answer. I have some nurses here who are standing with me in solidarity, and I appreciate that.”
The nurse, seen walking through the halls of the hospital surrounded by masked personnel, including a security guard, further stated that she had been put on unpaid administrative leave. Kaiser Permanente had not responded at press time to media requests for comment.
“I just want all of you to count the costs,” she said. “I want you to watch this and think, what really matters to me? Because I am willing to lose my safety and security, my house, everything, for my freedom. And I want you to think about that.”
While waiting for an elevator, she also posed questions to a few random people about their views on the subject. “Let me ask you, do you believe in religious freedom?” Offscreen, those responding indicated affirmatively. “Well, Kaiser doesn’t. Because they are not accepting my religious exemption based on my sincerely held religious beliefs. So that’s a problem.”
Also on the video she stated that she has worked since the beginning of the pandemic, “when we didn’t know what was going on,” and that she had shown up the day of her expulsion “happy to work.” She also touted Kaiser for paying well. She even quoted the company’s signs in a parking garage that encourages employees to climb the stairs for exercise as she went with the security guard who was escorting her up seven flights to the top of the parking garage.
“It’s a sad day. I don’t know what kind of a pandemic it is if they’re firing nurses who are willing to work. I don’t know,” she concluded. “It doesn’t make sense to me. So you have got to ask yourself that question: what kind of world are we living in when we have a pandemic where my kids have to wear masks at school and they have to get a vaccine for something that they are not at risk of dying from at all.”
A version of this article first appeared on Medscape.com.
It had been viewed more than 6 million times at press time.
The 5-minute video of the nurse leaving the hospital, both of which are unidentified in the clip, was a peaceful protest by the frontline worker against COVID-19 vaccine mandates, which many employers globally are enforcing.
In her video, which was originally posted October 30 on Rumble, the nurse explained, “I am being escorted out of Kaiser Permanente hospital for my religious beliefs because I don’t want to get the jab. And I asked all day for someone to explain to me why my sincerely held religious beliefs are not good enough for Kaiser. And no one was able to do that for me,” she continued.
“So now they’re escorting me out because I wanted an answer. And I’m not leaving without an answer. I have some nurses here who are standing with me in solidarity, and I appreciate that.”
The nurse, seen walking through the halls of the hospital surrounded by masked personnel, including a security guard, further stated that she had been put on unpaid administrative leave. Kaiser Permanente had not responded at press time to media requests for comment.
“I just want all of you to count the costs,” she said. “I want you to watch this and think, what really matters to me? Because I am willing to lose my safety and security, my house, everything, for my freedom. And I want you to think about that.”
While waiting for an elevator, she also posed questions to a few random people about their views on the subject. “Let me ask you, do you believe in religious freedom?” Offscreen, those responding indicated affirmatively. “Well, Kaiser doesn’t. Because they are not accepting my religious exemption based on my sincerely held religious beliefs. So that’s a problem.”
Also on the video she stated that she has worked since the beginning of the pandemic, “when we didn’t know what was going on,” and that she had shown up the day of her expulsion “happy to work.” She also touted Kaiser for paying well. She even quoted the company’s signs in a parking garage that encourages employees to climb the stairs for exercise as she went with the security guard who was escorting her up seven flights to the top of the parking garage.
“It’s a sad day. I don’t know what kind of a pandemic it is if they’re firing nurses who are willing to work. I don’t know,” she concluded. “It doesn’t make sense to me. So you have got to ask yourself that question: what kind of world are we living in when we have a pandemic where my kids have to wear masks at school and they have to get a vaccine for something that they are not at risk of dying from at all.”
A version of this article first appeared on Medscape.com.
It had been viewed more than 6 million times at press time.
The 5-minute video of the nurse leaving the hospital, both of which are unidentified in the clip, was a peaceful protest by the frontline worker against COVID-19 vaccine mandates, which many employers globally are enforcing.
In her video, which was originally posted October 30 on Rumble, the nurse explained, “I am being escorted out of Kaiser Permanente hospital for my religious beliefs because I don’t want to get the jab. And I asked all day for someone to explain to me why my sincerely held religious beliefs are not good enough for Kaiser. And no one was able to do that for me,” she continued.
“So now they’re escorting me out because I wanted an answer. And I’m not leaving without an answer. I have some nurses here who are standing with me in solidarity, and I appreciate that.”
The nurse, seen walking through the halls of the hospital surrounded by masked personnel, including a security guard, further stated that she had been put on unpaid administrative leave. Kaiser Permanente had not responded at press time to media requests for comment.
“I just want all of you to count the costs,” she said. “I want you to watch this and think, what really matters to me? Because I am willing to lose my safety and security, my house, everything, for my freedom. And I want you to think about that.”
While waiting for an elevator, she also posed questions to a few random people about their views on the subject. “Let me ask you, do you believe in religious freedom?” Offscreen, those responding indicated affirmatively. “Well, Kaiser doesn’t. Because they are not accepting my religious exemption based on my sincerely held religious beliefs. So that’s a problem.”
Also on the video she stated that she has worked since the beginning of the pandemic, “when we didn’t know what was going on,” and that she had shown up the day of her expulsion “happy to work.” She also touted Kaiser for paying well. She even quoted the company’s signs in a parking garage that encourages employees to climb the stairs for exercise as she went with the security guard who was escorting her up seven flights to the top of the parking garage.
“It’s a sad day. I don’t know what kind of a pandemic it is if they’re firing nurses who are willing to work. I don’t know,” she concluded. “It doesn’t make sense to me. So you have got to ask yourself that question: what kind of world are we living in when we have a pandemic where my kids have to wear masks at school and they have to get a vaccine for something that they are not at risk of dying from at all.”
A version of this article first appeared on Medscape.com.
With a Captive Audience, a Hospitalist Tries to Reach the Unvaccinated
Cheryl K. Lee, MD, an Assistant Professor Of Medicine at Northwestern Feinberg School of Medicine, practices internal medicine and pediatrics at Northwestern Memorial and the Ann & Robert H. Lurie Children's Hospital, both in Chicago, IL. She also serves on the Northwestern Medicine Covid Quality Committee and as core clinical faculty in the Internal Medicine Residency.
Dr. Lee reported no disclosures.
You have been treating COVID-19 patients since before the US Food and Drug Administration (FDA) granted emergency authorization to 3 pharma vaccine producers. But now you have patients, on oxygen or under observation, who have foregone vaccination. What do you think about that?
This question raises a good point that is often missed: how the unvaccinated are often portrayed. The reasons these patients remain unvaccinated are not necessarily uniform.
What we know based on attitude surveys done by the Kaiser Family Foundation1 is that people are vaccine hesitant for varied reasons. And this finding isn’t unique. The pediatric literature shows that those who are opposed to childhood vaccination do not share the same motivations.2 Yes, some are strident about their beliefs against vaccination, usually in concert with popularized myths. Many unvaccinated people are hesitant based on misconceptions, do not have access to a clinician who can answer their questions, can’t afford to lose a day of work due to the vaccine’s expected side effects, or understandably mistrust the healthcare community based on personal or historical context.
What do the unvaccinated have in common? Education levels, income levels?
We know from surveys3 that generally, more men than women are hesitant. Those who are uninsured or underinsured4 and those of lower socioeconomic status are more hesitant than their counterparts. It's changing a bit, but those who are in minority communities, Black and Latinx communities, are more likely to be unvaccinated compared to other groups. Even in Chicago, where we have a relatively good vaccination rate (59%),5 Black and Latinx communities are under vaccinated as compared to those who are White or Asian. The reasons for this are complex and include historical disinvestment in communities and decreased access to medical care. Some wonderful agencies are pairing up with community leaders in target neighborhoods to address this equity gap.
What do you say to these patients, if anything, about their status?
It’s not what you might expect. At first, I listen. I find that most are well-intentioned people trying to make the right decision for themselves and their family. It is, therefore, helpful to hear what their motivations and fears are first, before delving into facts. Furthermore, although facts are wonderful and necessary, what is more persuasive is a personal anecdote. I will tell folks my personal story about deciding to be vaccinated. I talk about how I found accurate information about the vaccine and what a relief it was afterwards to know that I would be safe, especially as a mom. I even talk about feeling tired and achy after the second shot, which means that the vaccine is working. I joke that it is the only time I’ve felt so relieved to feel sick. Last, I often say that it’s okay to feel scared or apprehensive, and that they deserve to get the best information. What’s important is that these conversations feel genuine.
Can you share an anecdote or two?
A few months ago, I took care of an unvaccinated gentleman who was in the hospital for a chronic medical condition. Before this hospitalization, his personal physicians had tried to convince him to get the vaccine over a period of several months.
It would have been easy to assume that he would remain unvaccinated and that I should put my energy into convincing someone else. However, I found him surprisingly open to discussion, and we were able to have many conversations about what he'd heard from nonmedical sources. We bonded over the sheer volume of available information and how difficult it is to know what is true. We then walked through what was truth vs fiction, and I tailored the discussion to how the vaccine could specifically improve his quality of life and his family's. He confided that what made his decision more difficult was the fact that he hadn’t met anyone who had gotten the vaccine among his friends and family. He ultimately did decide to get vaccinated, along with a family member. We made the appointment for the week after he was discharged. What a feeling it was to get a text message from his clinic physician saying that he got his first shot and that it went great!
I wasn’t the only physician who had spoken to this patient about getting vaccinated; others had done the same before he came to the hospital. It is a good reminder that each conversation can act like a gentle nudge in the right direction.
In terms of the data on the unvaccinated–reasons they stay away, what their backgrounds are and so forth–how close do those data play out in real life?
It is not advisable to assume why someone would be unvaccinated based on first impressions. I find the reasons are highly specific to that individual, ranging from false impressions about fertility to concerns about missing work. In my experience, several patients simply wanted to get more facts from a healthcare worker directly before signing up. Pregnancy is particularly important to talk about, considering how devastating the Delta variant has been to this group of women. One gentleman that I spoke to was worried about affecting his wife’s pregnancy with the vaccine. We know now that vaccines are safe and prevent pregnant patients from getting seriously ill and dying, but that knowledge isn’t widely known to the public. So many kind and well-meaning people have foregone vaccination because they're concerned about doing anything to upset the pregnancy.
How long, generally, does it take for unvaccinated patients to discuss the reasons for their choice?
It takes time, and that's a real barrier for many healthcare professionals, especially in a clinic setting where the luxury of extra time is nonexistent. How much time differs for everyone, and usually a change of heart takes more than one conversation.
Truly, the first conversation is just to listen, to understand their hesitation, and to develop trust. For anyone to really hear what I have to say, they must trust that what I'm saying is solely motivated by caring about what happens to them and their family.
One gentleman said something pointed during our first conversation: Thank you for listening. When I tell people I am not vaccinated I can feel them judging me, that they've already decided what to think of me.
I always tell people that they have good questions because they do. I respect the fact that they're feeling open enough to share what they're hearing or what they're afraid of. It's a privilege for me to be involved in that conversation.
What advice would you give other hospitalists in terms of treating and counseling patients who are unvaccinated?
Every hospitalization, whether it’s COVID-related or not, is an opportunity to speak with those who are still unvaccinated. Every encounter can be used to further the conversation about vaccines, by increasing their trust in the healthcare community, answering their questions, and providing facts in place of confusion. Using those opportunities is the best way to get us out of this pandemic.
That said, it's been a long two years, so it's okay if physicians don't have the emotional bandwidth or the time to have these discussions. Maybe save that conversation for another day. But for some providers, perhaps knowing that those who are unvaccinated can change and that anxiety could be preventing some from getting their shot will motivate them to start these conversations with their patients.
References
1. Does the public want to get a Covid-19 vaccine? When? Kaiser Family Foundation. Sept. 13-22, 2021. Accessed October 26, 2021. https://www.kff.org/coronavirus-covid-19/dashboard/kff-covid-19-vaccine-monitor-dashboard/#concernsorbarriers
2. Report of the SAGE Working Group on Vaccine Hesitancy. World Health Organization. November 12, 2014. Accessed October 25, 2021. https://www.who.int/immunization/sage/meetings/2014/october/SAGE_working_group_revised_report_vaccine_hesitancy.pdf?ua=1
3. Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225-228. Erratum in: Nat Med. 2021;27:354.
Cheryl K. Lee, MD, an Assistant Professor Of Medicine at Northwestern Feinberg School of Medicine, practices internal medicine and pediatrics at Northwestern Memorial and the Ann & Robert H. Lurie Children's Hospital, both in Chicago, IL. She also serves on the Northwestern Medicine Covid Quality Committee and as core clinical faculty in the Internal Medicine Residency.
Dr. Lee reported no disclosures.
You have been treating COVID-19 patients since before the US Food and Drug Administration (FDA) granted emergency authorization to 3 pharma vaccine producers. But now you have patients, on oxygen or under observation, who have foregone vaccination. What do you think about that?
This question raises a good point that is often missed: how the unvaccinated are often portrayed. The reasons these patients remain unvaccinated are not necessarily uniform.
What we know based on attitude surveys done by the Kaiser Family Foundation1 is that people are vaccine hesitant for varied reasons. And this finding isn’t unique. The pediatric literature shows that those who are opposed to childhood vaccination do not share the same motivations.2 Yes, some are strident about their beliefs against vaccination, usually in concert with popularized myths. Many unvaccinated people are hesitant based on misconceptions, do not have access to a clinician who can answer their questions, can’t afford to lose a day of work due to the vaccine’s expected side effects, or understandably mistrust the healthcare community based on personal or historical context.
What do the unvaccinated have in common? Education levels, income levels?
We know from surveys3 that generally, more men than women are hesitant. Those who are uninsured or underinsured4 and those of lower socioeconomic status are more hesitant than their counterparts. It's changing a bit, but those who are in minority communities, Black and Latinx communities, are more likely to be unvaccinated compared to other groups. Even in Chicago, where we have a relatively good vaccination rate (59%),5 Black and Latinx communities are under vaccinated as compared to those who are White or Asian. The reasons for this are complex and include historical disinvestment in communities and decreased access to medical care. Some wonderful agencies are pairing up with community leaders in target neighborhoods to address this equity gap.
What do you say to these patients, if anything, about their status?
It’s not what you might expect. At first, I listen. I find that most are well-intentioned people trying to make the right decision for themselves and their family. It is, therefore, helpful to hear what their motivations and fears are first, before delving into facts. Furthermore, although facts are wonderful and necessary, what is more persuasive is a personal anecdote. I will tell folks my personal story about deciding to be vaccinated. I talk about how I found accurate information about the vaccine and what a relief it was afterwards to know that I would be safe, especially as a mom. I even talk about feeling tired and achy after the second shot, which means that the vaccine is working. I joke that it is the only time I’ve felt so relieved to feel sick. Last, I often say that it’s okay to feel scared or apprehensive, and that they deserve to get the best information. What’s important is that these conversations feel genuine.
Can you share an anecdote or two?
A few months ago, I took care of an unvaccinated gentleman who was in the hospital for a chronic medical condition. Before this hospitalization, his personal physicians had tried to convince him to get the vaccine over a period of several months.
It would have been easy to assume that he would remain unvaccinated and that I should put my energy into convincing someone else. However, I found him surprisingly open to discussion, and we were able to have many conversations about what he'd heard from nonmedical sources. We bonded over the sheer volume of available information and how difficult it is to know what is true. We then walked through what was truth vs fiction, and I tailored the discussion to how the vaccine could specifically improve his quality of life and his family's. He confided that what made his decision more difficult was the fact that he hadn’t met anyone who had gotten the vaccine among his friends and family. He ultimately did decide to get vaccinated, along with a family member. We made the appointment for the week after he was discharged. What a feeling it was to get a text message from his clinic physician saying that he got his first shot and that it went great!
I wasn’t the only physician who had spoken to this patient about getting vaccinated; others had done the same before he came to the hospital. It is a good reminder that each conversation can act like a gentle nudge in the right direction.
In terms of the data on the unvaccinated–reasons they stay away, what their backgrounds are and so forth–how close do those data play out in real life?
It is not advisable to assume why someone would be unvaccinated based on first impressions. I find the reasons are highly specific to that individual, ranging from false impressions about fertility to concerns about missing work. In my experience, several patients simply wanted to get more facts from a healthcare worker directly before signing up. Pregnancy is particularly important to talk about, considering how devastating the Delta variant has been to this group of women. One gentleman that I spoke to was worried about affecting his wife’s pregnancy with the vaccine. We know now that vaccines are safe and prevent pregnant patients from getting seriously ill and dying, but that knowledge isn’t widely known to the public. So many kind and well-meaning people have foregone vaccination because they're concerned about doing anything to upset the pregnancy.
How long, generally, does it take for unvaccinated patients to discuss the reasons for their choice?
It takes time, and that's a real barrier for many healthcare professionals, especially in a clinic setting where the luxury of extra time is nonexistent. How much time differs for everyone, and usually a change of heart takes more than one conversation.
Truly, the first conversation is just to listen, to understand their hesitation, and to develop trust. For anyone to really hear what I have to say, they must trust that what I'm saying is solely motivated by caring about what happens to them and their family.
One gentleman said something pointed during our first conversation: Thank you for listening. When I tell people I am not vaccinated I can feel them judging me, that they've already decided what to think of me.
I always tell people that they have good questions because they do. I respect the fact that they're feeling open enough to share what they're hearing or what they're afraid of. It's a privilege for me to be involved in that conversation.
What advice would you give other hospitalists in terms of treating and counseling patients who are unvaccinated?
Every hospitalization, whether it’s COVID-related or not, is an opportunity to speak with those who are still unvaccinated. Every encounter can be used to further the conversation about vaccines, by increasing their trust in the healthcare community, answering their questions, and providing facts in place of confusion. Using those opportunities is the best way to get us out of this pandemic.
That said, it's been a long two years, so it's okay if physicians don't have the emotional bandwidth or the time to have these discussions. Maybe save that conversation for another day. But for some providers, perhaps knowing that those who are unvaccinated can change and that anxiety could be preventing some from getting their shot will motivate them to start these conversations with their patients.
Cheryl K. Lee, MD, an Assistant Professor Of Medicine at Northwestern Feinberg School of Medicine, practices internal medicine and pediatrics at Northwestern Memorial and the Ann & Robert H. Lurie Children's Hospital, both in Chicago, IL. She also serves on the Northwestern Medicine Covid Quality Committee and as core clinical faculty in the Internal Medicine Residency.
Dr. Lee reported no disclosures.
You have been treating COVID-19 patients since before the US Food and Drug Administration (FDA) granted emergency authorization to 3 pharma vaccine producers. But now you have patients, on oxygen or under observation, who have foregone vaccination. What do you think about that?
This question raises a good point that is often missed: how the unvaccinated are often portrayed. The reasons these patients remain unvaccinated are not necessarily uniform.
What we know based on attitude surveys done by the Kaiser Family Foundation1 is that people are vaccine hesitant for varied reasons. And this finding isn’t unique. The pediatric literature shows that those who are opposed to childhood vaccination do not share the same motivations.2 Yes, some are strident about their beliefs against vaccination, usually in concert with popularized myths. Many unvaccinated people are hesitant based on misconceptions, do not have access to a clinician who can answer their questions, can’t afford to lose a day of work due to the vaccine’s expected side effects, or understandably mistrust the healthcare community based on personal or historical context.
What do the unvaccinated have in common? Education levels, income levels?
We know from surveys3 that generally, more men than women are hesitant. Those who are uninsured or underinsured4 and those of lower socioeconomic status are more hesitant than their counterparts. It's changing a bit, but those who are in minority communities, Black and Latinx communities, are more likely to be unvaccinated compared to other groups. Even in Chicago, where we have a relatively good vaccination rate (59%),5 Black and Latinx communities are under vaccinated as compared to those who are White or Asian. The reasons for this are complex and include historical disinvestment in communities and decreased access to medical care. Some wonderful agencies are pairing up with community leaders in target neighborhoods to address this equity gap.
What do you say to these patients, if anything, about their status?
It’s not what you might expect. At first, I listen. I find that most are well-intentioned people trying to make the right decision for themselves and their family. It is, therefore, helpful to hear what their motivations and fears are first, before delving into facts. Furthermore, although facts are wonderful and necessary, what is more persuasive is a personal anecdote. I will tell folks my personal story about deciding to be vaccinated. I talk about how I found accurate information about the vaccine and what a relief it was afterwards to know that I would be safe, especially as a mom. I even talk about feeling tired and achy after the second shot, which means that the vaccine is working. I joke that it is the only time I’ve felt so relieved to feel sick. Last, I often say that it’s okay to feel scared or apprehensive, and that they deserve to get the best information. What’s important is that these conversations feel genuine.
Can you share an anecdote or two?
A few months ago, I took care of an unvaccinated gentleman who was in the hospital for a chronic medical condition. Before this hospitalization, his personal physicians had tried to convince him to get the vaccine over a period of several months.
It would have been easy to assume that he would remain unvaccinated and that I should put my energy into convincing someone else. However, I found him surprisingly open to discussion, and we were able to have many conversations about what he'd heard from nonmedical sources. We bonded over the sheer volume of available information and how difficult it is to know what is true. We then walked through what was truth vs fiction, and I tailored the discussion to how the vaccine could specifically improve his quality of life and his family's. He confided that what made his decision more difficult was the fact that he hadn’t met anyone who had gotten the vaccine among his friends and family. He ultimately did decide to get vaccinated, along with a family member. We made the appointment for the week after he was discharged. What a feeling it was to get a text message from his clinic physician saying that he got his first shot and that it went great!
I wasn’t the only physician who had spoken to this patient about getting vaccinated; others had done the same before he came to the hospital. It is a good reminder that each conversation can act like a gentle nudge in the right direction.
In terms of the data on the unvaccinated–reasons they stay away, what their backgrounds are and so forth–how close do those data play out in real life?
It is not advisable to assume why someone would be unvaccinated based on first impressions. I find the reasons are highly specific to that individual, ranging from false impressions about fertility to concerns about missing work. In my experience, several patients simply wanted to get more facts from a healthcare worker directly before signing up. Pregnancy is particularly important to talk about, considering how devastating the Delta variant has been to this group of women. One gentleman that I spoke to was worried about affecting his wife’s pregnancy with the vaccine. We know now that vaccines are safe and prevent pregnant patients from getting seriously ill and dying, but that knowledge isn’t widely known to the public. So many kind and well-meaning people have foregone vaccination because they're concerned about doing anything to upset the pregnancy.
How long, generally, does it take for unvaccinated patients to discuss the reasons for their choice?
It takes time, and that's a real barrier for many healthcare professionals, especially in a clinic setting where the luxury of extra time is nonexistent. How much time differs for everyone, and usually a change of heart takes more than one conversation.
Truly, the first conversation is just to listen, to understand their hesitation, and to develop trust. For anyone to really hear what I have to say, they must trust that what I'm saying is solely motivated by caring about what happens to them and their family.
One gentleman said something pointed during our first conversation: Thank you for listening. When I tell people I am not vaccinated I can feel them judging me, that they've already decided what to think of me.
I always tell people that they have good questions because they do. I respect the fact that they're feeling open enough to share what they're hearing or what they're afraid of. It's a privilege for me to be involved in that conversation.
What advice would you give other hospitalists in terms of treating and counseling patients who are unvaccinated?
Every hospitalization, whether it’s COVID-related or not, is an opportunity to speak with those who are still unvaccinated. Every encounter can be used to further the conversation about vaccines, by increasing their trust in the healthcare community, answering their questions, and providing facts in place of confusion. Using those opportunities is the best way to get us out of this pandemic.
That said, it's been a long two years, so it's okay if physicians don't have the emotional bandwidth or the time to have these discussions. Maybe save that conversation for another day. But for some providers, perhaps knowing that those who are unvaccinated can change and that anxiety could be preventing some from getting their shot will motivate them to start these conversations with their patients.
References
1. Does the public want to get a Covid-19 vaccine? When? Kaiser Family Foundation. Sept. 13-22, 2021. Accessed October 26, 2021. https://www.kff.org/coronavirus-covid-19/dashboard/kff-covid-19-vaccine-monitor-dashboard/#concernsorbarriers
2. Report of the SAGE Working Group on Vaccine Hesitancy. World Health Organization. November 12, 2014. Accessed October 25, 2021. https://www.who.int/immunization/sage/meetings/2014/october/SAGE_working_group_revised_report_vaccine_hesitancy.pdf?ua=1
3. Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225-228. Erratum in: Nat Med. 2021;27:354.
References
1. Does the public want to get a Covid-19 vaccine? When? Kaiser Family Foundation. Sept. 13-22, 2021. Accessed October 26, 2021. https://www.kff.org/coronavirus-covid-19/dashboard/kff-covid-19-vaccine-monitor-dashboard/#concernsorbarriers
2. Report of the SAGE Working Group on Vaccine Hesitancy. World Health Organization. November 12, 2014. Accessed October 25, 2021. https://www.who.int/immunization/sage/meetings/2014/october/SAGE_working_group_revised_report_vaccine_hesitancy.pdf?ua=1
3. Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225-228. Erratum in: Nat Med. 2021;27:354.
FDA authorizes Pfizer’s COVID-19 vaccine for kids
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
Antidepressant may cut COVID-19–related hospitalization, mortality: TOGETHER
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.










