User login
mRNA COVID vaccine response found mostly robust in RA, SLE patients
Immunosuppressed patients with autoimmune diseases who received the Moderna mRNA-1273 SARS-CoV-2 two-dose vaccine series had a frequency of adverse events similar to the general population albeit with a somewhat reduced, but still significant, antibody response with no severe vaccine-related disease flares, results of a prospective, nonrandomized open-label comparative trial in Canada demonstrated.
At the same time, patients with RA who were taking rituximab and patients with systemic lupus erythematosus (SLE) who were taking mycophenolate mofetil seemed to have reduced humoral responses after receiving the vaccine, said Ines Colmegna, MD, reporting results of the COVID-19 Vaccine in Immunosuppressed Adults with Autoimmune Disease (COVIAAD) study as a late-breaking poster abstract at the virtual annual meeting of the American College of Rheumatology. Dr. Colmegna is an associate professor of rheumatology in the division of experimental medicine at McGill University, Montreal.
“The frequency of adverse events, specifically the reactogenicity in people with comorbid conditions regardless of their diagnosis, was similar to healthy controls in this study, and their frequency was similar also the initial studies in the general population,” Dr. Colmegna said.
COVIAAD prospectively enrolled 220 fully vaccinated patients, 162 with rheumatic disease (131 with RA, 23 with SLE, and 8 with other diseases) and 58 controls. Adverse events a week and a month after each dose was the primary outcome. The postvaccine presence of the IgG antibody against the SARS-CoV-2 spike protein and the receptor binding domain (IgG-RBD) was the secondary outcome. Dr. Colmegna said that the study will continue evaluating participants after they get a third dose.
The Canadian trial appears to validate the ACR’s COVID-19 vaccine guidance, the fourth version of which was issued in October, said Jeffrey Curtis, MD, MS, MPH, professor of immunology and rheumatology at the University of Alabama at Birmingham and lead of the ACR COVID-19 Vaccine Guidance Task Force. Specifically, the guidance recommends that patients on rituximab or other anti-CD20 B-cell–depleting agents discuss vaccine timing with their rheumatologist.
“A few things changed over time when there was a paucity of evidence for any vaccine, but as time has gone on, mostly we were more correct than we weren’t,” Dr. Curtis said of the task force’s work. “The evidence that now is in this poster with regard to systemic lupus erythematosus and mycophenolate mofetil is [that] you have impaired vaccine response. If you’re on a B-cell drug like rituximab, you really have impaired vaccine response.”
In the study, 100% of controls had immunogenicity in terms of anti-spike and anti-RBD levels after the first and second dose. The rate of immunogenicity after the first and second dose were 67% and 88% in all patients with RA, and 35% and 78% in patients with SLE who were taking mycophenolate mofetil. The subset of patients with RA on rituximab (n = 17) had rates of immunogenicity of 5.9% and 17.6%, respectively.
“Measured antibody response is not the only way in which people develop a response to a vaccine, and there are also similar responses that occur even in people who are on rituximab and have not developed antibodies,” Dr. Colmegna said. “That’s a very important message also that we need to convey to patients: The immune response really extends beyond antibody protection.”
Overall, disease activity in both patients with RA and SLE did not appreciably change from baseline within 7 days and 28 days of each vaccine dose.
The study raises important questions about the timing of the vaccine, particularly in patients on rituximab, Dr. Colmegna said in an interview. “In theory, there is no element to suggest that, if you would schedule the vaccine a month prior to the next dose of rituximab, the effect of the drug would have decreased the number of B cells, and that the possibility of developing antibodies in response to the vaccine might be better if you give rituximab a month later when the amount of the drug and the effect of the drug is maximal,” she said. The average interval between patients receiving rituximab and vaccines was 4.5 months, Dr. Colmegna said in answering a question after her presentation.
Dr. Curtis said that the effect of holding rituximab or the vaccine to boost antibodies “is somewhat yet unknown. We think it will help, but that’s not a guarantee,” he said. “We don’t have direct evidence that just because the drug impairs vaccine response, that holding that drug for a week or 2 is going to take care of the problem.”
The study does arm rheumatologists with more information for discussing COVID vaccines with vaccine-hesitant patients with autoimmune diseases, Dr. Curtis said.
“It gives them evidence that for most of our immunomodulatory drugs the vaccine works pretty well,” he said. “The poster provides evidence that, compared to healthy controls, the vaccine doesn’t work quite as well in some patients, but for most people it actually did work pretty well. That reinforces the message: Go get vaccinated because [you] will mount [an immune] response, even, if that response isn’t quite as brisk as it is in healthy people.”
Dr. Colmegna and Dr. Curtis have no relevant relationships to disclose. The study received funding from Health and Social Services Quebec.
Immunosuppressed patients with autoimmune diseases who received the Moderna mRNA-1273 SARS-CoV-2 two-dose vaccine series had a frequency of adverse events similar to the general population albeit with a somewhat reduced, but still significant, antibody response with no severe vaccine-related disease flares, results of a prospective, nonrandomized open-label comparative trial in Canada demonstrated.
At the same time, patients with RA who were taking rituximab and patients with systemic lupus erythematosus (SLE) who were taking mycophenolate mofetil seemed to have reduced humoral responses after receiving the vaccine, said Ines Colmegna, MD, reporting results of the COVID-19 Vaccine in Immunosuppressed Adults with Autoimmune Disease (COVIAAD) study as a late-breaking poster abstract at the virtual annual meeting of the American College of Rheumatology. Dr. Colmegna is an associate professor of rheumatology in the division of experimental medicine at McGill University, Montreal.
“The frequency of adverse events, specifically the reactogenicity in people with comorbid conditions regardless of their diagnosis, was similar to healthy controls in this study, and their frequency was similar also the initial studies in the general population,” Dr. Colmegna said.
COVIAAD prospectively enrolled 220 fully vaccinated patients, 162 with rheumatic disease (131 with RA, 23 with SLE, and 8 with other diseases) and 58 controls. Adverse events a week and a month after each dose was the primary outcome. The postvaccine presence of the IgG antibody against the SARS-CoV-2 spike protein and the receptor binding domain (IgG-RBD) was the secondary outcome. Dr. Colmegna said that the study will continue evaluating participants after they get a third dose.
The Canadian trial appears to validate the ACR’s COVID-19 vaccine guidance, the fourth version of which was issued in October, said Jeffrey Curtis, MD, MS, MPH, professor of immunology and rheumatology at the University of Alabama at Birmingham and lead of the ACR COVID-19 Vaccine Guidance Task Force. Specifically, the guidance recommends that patients on rituximab or other anti-CD20 B-cell–depleting agents discuss vaccine timing with their rheumatologist.
“A few things changed over time when there was a paucity of evidence for any vaccine, but as time has gone on, mostly we were more correct than we weren’t,” Dr. Curtis said of the task force’s work. “The evidence that now is in this poster with regard to systemic lupus erythematosus and mycophenolate mofetil is [that] you have impaired vaccine response. If you’re on a B-cell drug like rituximab, you really have impaired vaccine response.”
In the study, 100% of controls had immunogenicity in terms of anti-spike and anti-RBD levels after the first and second dose. The rate of immunogenicity after the first and second dose were 67% and 88% in all patients with RA, and 35% and 78% in patients with SLE who were taking mycophenolate mofetil. The subset of patients with RA on rituximab (n = 17) had rates of immunogenicity of 5.9% and 17.6%, respectively.
“Measured antibody response is not the only way in which people develop a response to a vaccine, and there are also similar responses that occur even in people who are on rituximab and have not developed antibodies,” Dr. Colmegna said. “That’s a very important message also that we need to convey to patients: The immune response really extends beyond antibody protection.”
Overall, disease activity in both patients with RA and SLE did not appreciably change from baseline within 7 days and 28 days of each vaccine dose.
The study raises important questions about the timing of the vaccine, particularly in patients on rituximab, Dr. Colmegna said in an interview. “In theory, there is no element to suggest that, if you would schedule the vaccine a month prior to the next dose of rituximab, the effect of the drug would have decreased the number of B cells, and that the possibility of developing antibodies in response to the vaccine might be better if you give rituximab a month later when the amount of the drug and the effect of the drug is maximal,” she said. The average interval between patients receiving rituximab and vaccines was 4.5 months, Dr. Colmegna said in answering a question after her presentation.
Dr. Curtis said that the effect of holding rituximab or the vaccine to boost antibodies “is somewhat yet unknown. We think it will help, but that’s not a guarantee,” he said. “We don’t have direct evidence that just because the drug impairs vaccine response, that holding that drug for a week or 2 is going to take care of the problem.”
The study does arm rheumatologists with more information for discussing COVID vaccines with vaccine-hesitant patients with autoimmune diseases, Dr. Curtis said.
“It gives them evidence that for most of our immunomodulatory drugs the vaccine works pretty well,” he said. “The poster provides evidence that, compared to healthy controls, the vaccine doesn’t work quite as well in some patients, but for most people it actually did work pretty well. That reinforces the message: Go get vaccinated because [you] will mount [an immune] response, even, if that response isn’t quite as brisk as it is in healthy people.”
Dr. Colmegna and Dr. Curtis have no relevant relationships to disclose. The study received funding from Health and Social Services Quebec.
Immunosuppressed patients with autoimmune diseases who received the Moderna mRNA-1273 SARS-CoV-2 two-dose vaccine series had a frequency of adverse events similar to the general population albeit with a somewhat reduced, but still significant, antibody response with no severe vaccine-related disease flares, results of a prospective, nonrandomized open-label comparative trial in Canada demonstrated.
At the same time, patients with RA who were taking rituximab and patients with systemic lupus erythematosus (SLE) who were taking mycophenolate mofetil seemed to have reduced humoral responses after receiving the vaccine, said Ines Colmegna, MD, reporting results of the COVID-19 Vaccine in Immunosuppressed Adults with Autoimmune Disease (COVIAAD) study as a late-breaking poster abstract at the virtual annual meeting of the American College of Rheumatology. Dr. Colmegna is an associate professor of rheumatology in the division of experimental medicine at McGill University, Montreal.
“The frequency of adverse events, specifically the reactogenicity in people with comorbid conditions regardless of their diagnosis, was similar to healthy controls in this study, and their frequency was similar also the initial studies in the general population,” Dr. Colmegna said.
COVIAAD prospectively enrolled 220 fully vaccinated patients, 162 with rheumatic disease (131 with RA, 23 with SLE, and 8 with other diseases) and 58 controls. Adverse events a week and a month after each dose was the primary outcome. The postvaccine presence of the IgG antibody against the SARS-CoV-2 spike protein and the receptor binding domain (IgG-RBD) was the secondary outcome. Dr. Colmegna said that the study will continue evaluating participants after they get a third dose.
The Canadian trial appears to validate the ACR’s COVID-19 vaccine guidance, the fourth version of which was issued in October, said Jeffrey Curtis, MD, MS, MPH, professor of immunology and rheumatology at the University of Alabama at Birmingham and lead of the ACR COVID-19 Vaccine Guidance Task Force. Specifically, the guidance recommends that patients on rituximab or other anti-CD20 B-cell–depleting agents discuss vaccine timing with their rheumatologist.
“A few things changed over time when there was a paucity of evidence for any vaccine, but as time has gone on, mostly we were more correct than we weren’t,” Dr. Curtis said of the task force’s work. “The evidence that now is in this poster with regard to systemic lupus erythematosus and mycophenolate mofetil is [that] you have impaired vaccine response. If you’re on a B-cell drug like rituximab, you really have impaired vaccine response.”
In the study, 100% of controls had immunogenicity in terms of anti-spike and anti-RBD levels after the first and second dose. The rate of immunogenicity after the first and second dose were 67% and 88% in all patients with RA, and 35% and 78% in patients with SLE who were taking mycophenolate mofetil. The subset of patients with RA on rituximab (n = 17) had rates of immunogenicity of 5.9% and 17.6%, respectively.
“Measured antibody response is not the only way in which people develop a response to a vaccine, and there are also similar responses that occur even in people who are on rituximab and have not developed antibodies,” Dr. Colmegna said. “That’s a very important message also that we need to convey to patients: The immune response really extends beyond antibody protection.”
Overall, disease activity in both patients with RA and SLE did not appreciably change from baseline within 7 days and 28 days of each vaccine dose.
The study raises important questions about the timing of the vaccine, particularly in patients on rituximab, Dr. Colmegna said in an interview. “In theory, there is no element to suggest that, if you would schedule the vaccine a month prior to the next dose of rituximab, the effect of the drug would have decreased the number of B cells, and that the possibility of developing antibodies in response to the vaccine might be better if you give rituximab a month later when the amount of the drug and the effect of the drug is maximal,” she said. The average interval between patients receiving rituximab and vaccines was 4.5 months, Dr. Colmegna said in answering a question after her presentation.
Dr. Curtis said that the effect of holding rituximab or the vaccine to boost antibodies “is somewhat yet unknown. We think it will help, but that’s not a guarantee,” he said. “We don’t have direct evidence that just because the drug impairs vaccine response, that holding that drug for a week or 2 is going to take care of the problem.”
The study does arm rheumatologists with more information for discussing COVID vaccines with vaccine-hesitant patients with autoimmune diseases, Dr. Curtis said.
“It gives them evidence that for most of our immunomodulatory drugs the vaccine works pretty well,” he said. “The poster provides evidence that, compared to healthy controls, the vaccine doesn’t work quite as well in some patients, but for most people it actually did work pretty well. That reinforces the message: Go get vaccinated because [you] will mount [an immune] response, even, if that response isn’t quite as brisk as it is in healthy people.”
Dr. Colmegna and Dr. Curtis have no relevant relationships to disclose. The study received funding from Health and Social Services Quebec.
FROM ACR 2021
Infected, vaccinated, or both: How protected am I from COVID-19?
As the United States rounds out its second year of the pandemic, many people are trying to figure out just how vulnerable they may be to COVID-19 infection, and whether it’s finally safe to fully return to all the activities they miss.
On an individual basis, the degree and durability of the immunity a person gets after vaccination versus an infection is not an easy question to answer. But it’s one that science is hotly pursing.
“This virus is teaching us a lot about immunology,” says Gregory Poland, MD, who studies how the body responds to vaccines at the Mayo Clinic in Rochester, Minn. Dr. Poland says this moment in science reminds him of a quote attributed to Ralph Waldo Emerson: “We learn about geology the morning after the earthquake.”
“And that’s the case here. It is and will continue to teach us a lot of immunology,” he says.
It’s vital to understand how a COVID-19 infection reshapes the body’s immune defenses so that researchers can tailor vaccines and therapies to do the same or better.
“Because, of course, it’s much more risky to get infected with the actual virus, than with the vaccine,” says Daniela Weiskopf, PhD, a researcher at the La Jolla Institute for Immunology in California.
What is known so far is that how much protection you get and how long you may have it depends on several factors. Those include your age, whether you’ve had COVID-19 before and how severe your symptoms were, your vaccination status, and how long it has been since you were infected or inoculated. Your underlying health matters, too. Immune protection also depends on the virus and how much it is changing as it evolves to evade all our hard-won defenses.
In a new scientific brief, Here’s what we know so far:
Durability of immunity
The agency’s researchers say if you’ve recovered from a COVID-19 infection or are fully vaccinated, you’re probably in good shape for at least 6 months. That’s why this is the recommended interval for people to consider getting a booster dose.
Even though the protection you get after infection and vaccination is generally strong, it’s not perfect.
Getting COVID-19 after you’ve been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you. And if you do happen to catch COVID, if your immune system has already gotten a heads up about the virus, your infection is much less likely to be one that lands you in the hospital or morgue.
According to CDC data, at the height of the Delta surge in August, fully vaccinated people were six times less likely to get a COVID-19 infection compared with unvaccinated people, and 11 times less likely to die if they did get it.
How strong is immunity after a COVID-19 Infection?
About 90% of people develop some number of protective antibodies after a COVID-19 infection, according to the CDC. But how high those levels climb appears to be all over the map. Studies show peak antibody concentrations can vary as much as 200-fold, or 2,000%.
Where you fall within that very large range will depend on your age and how sick you became from your COVID-19 infection. It also depends on whether you have an underlying health condition or take a medication that blunts immune function.
Our immune system slows down with age. Immunosenescence starts to affect a person’s health around the age of 60. But there’s no bright line for failure. People who exercise and are generally healthy will have better immune function than someone who doesn’t, no matter their age. In general, though, the older you are, the less likely you are to get a robust immune response after an infection or a vaccination. That’s why this group has been prioritized both for first vaccine doses and boosters.
Beyond age, your protection from future infection seems to depend on how ill you were with the first. Several studies have shown that blood levels of antibodies rise faster and reach a higher peak in people with more severe infections.
In general, people with cold-like symptoms who tested positive but recovered at home are better protected than people who didn’t get any symptoms. And people who were hospitalized for their infections are better protected over the long term than people with milder infections. They may have paid a steep price for that protection: Many hospitalized patients continue to have debilitating symptoms that last for months after they go home.
On average, though, protection after infection seems to be comparable to vaccination, at least for a while. Six large studies from different countries have looked into this question, and five of them have used the very sensitive real-time polymerase chain reaction test (RT-PCR) to count people as truly being previously infected. These studies found that for 6 to 9 months after recovery, a person was 80% to 93% less likely to get COVID-19 again.
There are some caveats to mention, though. Early in the pandemic when supplies were scarce, it was hard to get tested unless you were so sick you landed in the hospital. Studies have shown that the concentration of antibodies a person makes after an infection seems to depend on how sick they got in the first place.
People who had milder infections, or who didn’t have any symptoms at all, may not develop as much protection as those who have more severe symptoms. So these studies may reflect the immunity developed by people who were pretty ill during their first infections.
One study of 25,000 health care workers, who were all tested every 2 weeks -- whether they had symptoms or not -- may offer a clearer picture. In this study, health care workers who’d previously tested positive for COVID-19 were 84% less likely to test positive for the virus again. They were 93% less likely to get an infection that made them sick, and 52% less likely to get an infection without symptoms, for at least 6 months after they recovered.
How does protection after infection compare to vaccination?
Two weeks after your final vaccine dose, protection against a COVID-19 infection is high -- around 90% for the Pfizer and Moderna mRNA vaccines and 66% for the one-dose Johnson & Johnson shot. Clinical trials conducted by the manufacturer have shown that a second dose of the Johnson & Johnson vaccine given at least 2 months after vaccination boosts protection against illness in the United States to about 94%, which is why another dose has been recommended for all Johnson & Johnson vaccine recipients 2 months after their first shot.
It’s not yet known how long the COVID-19 vaccines remain protective. There’s some evidence that protection against symptomatic infections wanes a bit over time as antibody levels drop. But protection against severe illness, including hospitalization and death, has remained high so far, even without a booster.
Are antibodies different after infection compared to vaccination?
Yes. And researchers don’t yet understand what these differences mean.
It seems to come down to a question of quality versus quantity. Vaccines seem to produce higher peak antibody levels than natural infections do. But these antibodies are highly specialized, able to recognize only the parts of the virus they were designed to target.
“The mRNA vaccine directs all the immune responses to the single spike protein,” says Alice Cho, PhD, who is studying the differences in vaccine and infection-created immunity at the Rockefeller University in New York. “There’s a lot more to respond to with a virus than there is in a vaccine.”
During an infection, the immune system learns to recognize and grab onto many parts of the virus, not just its spike.
The job of remembering the various pieces and parts of a foreign invader, so that it can be quickly recognized and disarmed should it ever return, falls to memory B cells.
Memory B cells, in turn, make plasma cells that then crank out antibodies that are custom tailored to attach to their targets.
Antibody levels gradually fall over a few months’ time as the plasma cells that make them die off. But memory B cells live for extended periods. One study that was attempting to measure the lifespan of individual memory B cells in mice found that these cells probably live as long as the mouse itself. Memory B cells induced by smallpox vaccination may live at least 60 years -- virtually an entire lifetime.
Dr. Cho’s research team has found that when memory B cells are trained by the vaccine, they become one-hit wonders, cranking out copious amounts of the same kinds of antibodies over and over again.
Memory B cells trained by viral infection, however, are more versatile. They continue to evolve over several months and produce higher quality antibodies that appear to become more potent over time and can even develop activity against future variants.
Still, the researchers stress that it’s not smart to wait to get a COVID-19 infection in hopes of getting these more versatile antibodies.
“While a natural infection may induce maturation of antibodies with broader activity than a vaccine does -- a natural infection can also kill you,” says Michel Nussenzweig, MD, PhD, head of Rockefeller’s Laboratory of Molecular Immunology.
Sure, memory B cells generated by infections may be immunological Swiss Army Knives, but maybe, argues Donna Farber, PhD, an immunologist at Columbia University in New York, we really only need a single blade.
“The thing with the vaccine is that it’s really focused,” she says. “It’s not giving you all these other viral proteins. It’s only giving you the spike.”
“It may be even better than the level of neutralizing spike antibodies you’re going to get from the infection,” she says. “With a viral infection, the immune response really has a lot to do. It’s really being distracted by all these other proteins.”
“Whereas with the vaccine, it’s just saying to the immune response, ‘This is the immunity we need,’” Dr. Farber says. “‘Just generate this immunity.’ So it’s focusing the immune response in a way that’s going to guarantee that you’re going to get that protective response.”
What if you had COVID and later got vaccinated?
This is called hybrid immunity, and it’s the best of both worlds.
“You have the benefit of very deep, but narrow, immunity produced by vaccine, and very broad, but not very deep, immunity produced by infection,” Dr. Poland says. He says you’ve effectively cross-trained your immune system.
In studies of people who recovered from COVID-19 and then went on to get an mRNA vaccine, after one dose, their antibodies were as high as someone who had been fully vaccinated. After two doses, their antibody levels were about double the average levels seen in someone who’d only been vaccinated.
Studies have shown this kind of immunity has real benefits, too. A recent study by researchers at the University of Kentucky and the CDC found that people who’d gotten COVID-19 in 2020, but had not been vaccinated, were about twice as likely to be reinfected in May and June compared with those who recovered and went on to get their vaccines.
What antibody level is protective?
Scientists aren’t exactly sure how high antibody levels need to be for protection, or even which kinds of antibodies or other immune components matter most yet.
But vaccines appear to generate higher antibody levels than infections do. In a recent study published in the journal Science , Dr. Weiskopf and her colleagues at the La Jolla Institute of Immunology detail the findings of a de-escalation study, where they gave people one-quarter of the normal dose of the Moderna mRNA vaccine and then collected blood samples over time to study their immune responses.
Their immune responses were scaled down with the dose.
“We saw that this has the exact same levels as natural infection,” Dr. Weiskopf says. “People who are vaccinated have much higher immune memory than people who are naturally infected,” she says.
Antibody levels are not easy to determine in the real world. Can you take a test to find out how protected you are? The answer is no, because we don’t yet know what antibody level, or even which kind of antibodies, correlate with protection.
Also, there are many different kinds of antibody tests and they all use a slightly different scale, so there’s no broadly agreed upon way to measure them yet. It’s difficult to compare levels test to test.
Weeks or months between doses? Which is best?
Both the Pfizer and Moderna vaccines were tested to be given 3 and 4 weeks apart, respectively. But when the vaccines were first rolling out, shortages prompted some countries to stretch the interval between doses to 4 or more months.
Researchers who have studied the immune responses of people who were inoculated on an extended dosing schedule noticed something interesting: When the interval was stretched, people had better antibody responses. In fact, their antibody responses looked like the sky-high levels people got with hybrid immunity.
Susanna Dunachie, PhD, a global research professor at the University of Oxford in the United Kingdom, wondered why. She’s leading a team of researchers who are doing detailed studies of the immune responses of health care workers after their vaccinations.
“We found that B cells, which are the cells that make antibodies to the viral spike protein after vaccination, carry on increasing in number between 4 and 10 weeks after vaccination,” she says.
Waiting to give the second vaccine 6 to 14 weeks seems to stimulate the immune system when all of its antibody-making factories are finally up and running.
For this reason, giving the second dose at 3 weeks, she says, might be premature.
But there’s a tradeoff involved in waiting. If there are high levels of the virus circulating in a community, you want to get people fully vaccinated as quickly as possible to maximize their protection in the shortest window of time, which is what we decided to do in the United States.
Researchers say it might be a good idea to revisit the dosing interval when it’s less risky to try it.
A version of this article first appeared on WebMD.com.
As the United States rounds out its second year of the pandemic, many people are trying to figure out just how vulnerable they may be to COVID-19 infection, and whether it’s finally safe to fully return to all the activities they miss.
On an individual basis, the degree and durability of the immunity a person gets after vaccination versus an infection is not an easy question to answer. But it’s one that science is hotly pursing.
“This virus is teaching us a lot about immunology,” says Gregory Poland, MD, who studies how the body responds to vaccines at the Mayo Clinic in Rochester, Minn. Dr. Poland says this moment in science reminds him of a quote attributed to Ralph Waldo Emerson: “We learn about geology the morning after the earthquake.”
“And that’s the case here. It is and will continue to teach us a lot of immunology,” he says.
It’s vital to understand how a COVID-19 infection reshapes the body’s immune defenses so that researchers can tailor vaccines and therapies to do the same or better.
“Because, of course, it’s much more risky to get infected with the actual virus, than with the vaccine,” says Daniela Weiskopf, PhD, a researcher at the La Jolla Institute for Immunology in California.
What is known so far is that how much protection you get and how long you may have it depends on several factors. Those include your age, whether you’ve had COVID-19 before and how severe your symptoms were, your vaccination status, and how long it has been since you were infected or inoculated. Your underlying health matters, too. Immune protection also depends on the virus and how much it is changing as it evolves to evade all our hard-won defenses.
In a new scientific brief, Here’s what we know so far:
Durability of immunity
The agency’s researchers say if you’ve recovered from a COVID-19 infection or are fully vaccinated, you’re probably in good shape for at least 6 months. That’s why this is the recommended interval for people to consider getting a booster dose.
Even though the protection you get after infection and vaccination is generally strong, it’s not perfect.
Getting COVID-19 after you’ve been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you. And if you do happen to catch COVID, if your immune system has already gotten a heads up about the virus, your infection is much less likely to be one that lands you in the hospital or morgue.
According to CDC data, at the height of the Delta surge in August, fully vaccinated people were six times less likely to get a COVID-19 infection compared with unvaccinated people, and 11 times less likely to die if they did get it.
How strong is immunity after a COVID-19 Infection?
About 90% of people develop some number of protective antibodies after a COVID-19 infection, according to the CDC. But how high those levels climb appears to be all over the map. Studies show peak antibody concentrations can vary as much as 200-fold, or 2,000%.
Where you fall within that very large range will depend on your age and how sick you became from your COVID-19 infection. It also depends on whether you have an underlying health condition or take a medication that blunts immune function.
Our immune system slows down with age. Immunosenescence starts to affect a person’s health around the age of 60. But there’s no bright line for failure. People who exercise and are generally healthy will have better immune function than someone who doesn’t, no matter their age. In general, though, the older you are, the less likely you are to get a robust immune response after an infection or a vaccination. That’s why this group has been prioritized both for first vaccine doses and boosters.
Beyond age, your protection from future infection seems to depend on how ill you were with the first. Several studies have shown that blood levels of antibodies rise faster and reach a higher peak in people with more severe infections.
In general, people with cold-like symptoms who tested positive but recovered at home are better protected than people who didn’t get any symptoms. And people who were hospitalized for their infections are better protected over the long term than people with milder infections. They may have paid a steep price for that protection: Many hospitalized patients continue to have debilitating symptoms that last for months after they go home.
On average, though, protection after infection seems to be comparable to vaccination, at least for a while. Six large studies from different countries have looked into this question, and five of them have used the very sensitive real-time polymerase chain reaction test (RT-PCR) to count people as truly being previously infected. These studies found that for 6 to 9 months after recovery, a person was 80% to 93% less likely to get COVID-19 again.
There are some caveats to mention, though. Early in the pandemic when supplies were scarce, it was hard to get tested unless you were so sick you landed in the hospital. Studies have shown that the concentration of antibodies a person makes after an infection seems to depend on how sick they got in the first place.
People who had milder infections, or who didn’t have any symptoms at all, may not develop as much protection as those who have more severe symptoms. So these studies may reflect the immunity developed by people who were pretty ill during their first infections.
One study of 25,000 health care workers, who were all tested every 2 weeks -- whether they had symptoms or not -- may offer a clearer picture. In this study, health care workers who’d previously tested positive for COVID-19 were 84% less likely to test positive for the virus again. They were 93% less likely to get an infection that made them sick, and 52% less likely to get an infection without symptoms, for at least 6 months after they recovered.
How does protection after infection compare to vaccination?
Two weeks after your final vaccine dose, protection against a COVID-19 infection is high -- around 90% for the Pfizer and Moderna mRNA vaccines and 66% for the one-dose Johnson & Johnson shot. Clinical trials conducted by the manufacturer have shown that a second dose of the Johnson & Johnson vaccine given at least 2 months after vaccination boosts protection against illness in the United States to about 94%, which is why another dose has been recommended for all Johnson & Johnson vaccine recipients 2 months after their first shot.
It’s not yet known how long the COVID-19 vaccines remain protective. There’s some evidence that protection against symptomatic infections wanes a bit over time as antibody levels drop. But protection against severe illness, including hospitalization and death, has remained high so far, even without a booster.
Are antibodies different after infection compared to vaccination?
Yes. And researchers don’t yet understand what these differences mean.
It seems to come down to a question of quality versus quantity. Vaccines seem to produce higher peak antibody levels than natural infections do. But these antibodies are highly specialized, able to recognize only the parts of the virus they were designed to target.
“The mRNA vaccine directs all the immune responses to the single spike protein,” says Alice Cho, PhD, who is studying the differences in vaccine and infection-created immunity at the Rockefeller University in New York. “There’s a lot more to respond to with a virus than there is in a vaccine.”
During an infection, the immune system learns to recognize and grab onto many parts of the virus, not just its spike.
The job of remembering the various pieces and parts of a foreign invader, so that it can be quickly recognized and disarmed should it ever return, falls to memory B cells.
Memory B cells, in turn, make plasma cells that then crank out antibodies that are custom tailored to attach to their targets.
Antibody levels gradually fall over a few months’ time as the plasma cells that make them die off. But memory B cells live for extended periods. One study that was attempting to measure the lifespan of individual memory B cells in mice found that these cells probably live as long as the mouse itself. Memory B cells induced by smallpox vaccination may live at least 60 years -- virtually an entire lifetime.
Dr. Cho’s research team has found that when memory B cells are trained by the vaccine, they become one-hit wonders, cranking out copious amounts of the same kinds of antibodies over and over again.
Memory B cells trained by viral infection, however, are more versatile. They continue to evolve over several months and produce higher quality antibodies that appear to become more potent over time and can even develop activity against future variants.
Still, the researchers stress that it’s not smart to wait to get a COVID-19 infection in hopes of getting these more versatile antibodies.
“While a natural infection may induce maturation of antibodies with broader activity than a vaccine does -- a natural infection can also kill you,” says Michel Nussenzweig, MD, PhD, head of Rockefeller’s Laboratory of Molecular Immunology.
Sure, memory B cells generated by infections may be immunological Swiss Army Knives, but maybe, argues Donna Farber, PhD, an immunologist at Columbia University in New York, we really only need a single blade.
“The thing with the vaccine is that it’s really focused,” she says. “It’s not giving you all these other viral proteins. It’s only giving you the spike.”
“It may be even better than the level of neutralizing spike antibodies you’re going to get from the infection,” she says. “With a viral infection, the immune response really has a lot to do. It’s really being distracted by all these other proteins.”
“Whereas with the vaccine, it’s just saying to the immune response, ‘This is the immunity we need,’” Dr. Farber says. “‘Just generate this immunity.’ So it’s focusing the immune response in a way that’s going to guarantee that you’re going to get that protective response.”
What if you had COVID and later got vaccinated?
This is called hybrid immunity, and it’s the best of both worlds.
“You have the benefit of very deep, but narrow, immunity produced by vaccine, and very broad, but not very deep, immunity produced by infection,” Dr. Poland says. He says you’ve effectively cross-trained your immune system.
In studies of people who recovered from COVID-19 and then went on to get an mRNA vaccine, after one dose, their antibodies were as high as someone who had been fully vaccinated. After two doses, their antibody levels were about double the average levels seen in someone who’d only been vaccinated.
Studies have shown this kind of immunity has real benefits, too. A recent study by researchers at the University of Kentucky and the CDC found that people who’d gotten COVID-19 in 2020, but had not been vaccinated, were about twice as likely to be reinfected in May and June compared with those who recovered and went on to get their vaccines.
What antibody level is protective?
Scientists aren’t exactly sure how high antibody levels need to be for protection, or even which kinds of antibodies or other immune components matter most yet.
But vaccines appear to generate higher antibody levels than infections do. In a recent study published in the journal Science , Dr. Weiskopf and her colleagues at the La Jolla Institute of Immunology detail the findings of a de-escalation study, where they gave people one-quarter of the normal dose of the Moderna mRNA vaccine and then collected blood samples over time to study their immune responses.
Their immune responses were scaled down with the dose.
“We saw that this has the exact same levels as natural infection,” Dr. Weiskopf says. “People who are vaccinated have much higher immune memory than people who are naturally infected,” she says.
Antibody levels are not easy to determine in the real world. Can you take a test to find out how protected you are? The answer is no, because we don’t yet know what antibody level, or even which kind of antibodies, correlate with protection.
Also, there are many different kinds of antibody tests and they all use a slightly different scale, so there’s no broadly agreed upon way to measure them yet. It’s difficult to compare levels test to test.
Weeks or months between doses? Which is best?
Both the Pfizer and Moderna vaccines were tested to be given 3 and 4 weeks apart, respectively. But when the vaccines were first rolling out, shortages prompted some countries to stretch the interval between doses to 4 or more months.
Researchers who have studied the immune responses of people who were inoculated on an extended dosing schedule noticed something interesting: When the interval was stretched, people had better antibody responses. In fact, their antibody responses looked like the sky-high levels people got with hybrid immunity.
Susanna Dunachie, PhD, a global research professor at the University of Oxford in the United Kingdom, wondered why. She’s leading a team of researchers who are doing detailed studies of the immune responses of health care workers after their vaccinations.
“We found that B cells, which are the cells that make antibodies to the viral spike protein after vaccination, carry on increasing in number between 4 and 10 weeks after vaccination,” she says.
Waiting to give the second vaccine 6 to 14 weeks seems to stimulate the immune system when all of its antibody-making factories are finally up and running.
For this reason, giving the second dose at 3 weeks, she says, might be premature.
But there’s a tradeoff involved in waiting. If there are high levels of the virus circulating in a community, you want to get people fully vaccinated as quickly as possible to maximize their protection in the shortest window of time, which is what we decided to do in the United States.
Researchers say it might be a good idea to revisit the dosing interval when it’s less risky to try it.
A version of this article first appeared on WebMD.com.
As the United States rounds out its second year of the pandemic, many people are trying to figure out just how vulnerable they may be to COVID-19 infection, and whether it’s finally safe to fully return to all the activities they miss.
On an individual basis, the degree and durability of the immunity a person gets after vaccination versus an infection is not an easy question to answer. But it’s one that science is hotly pursing.
“This virus is teaching us a lot about immunology,” says Gregory Poland, MD, who studies how the body responds to vaccines at the Mayo Clinic in Rochester, Minn. Dr. Poland says this moment in science reminds him of a quote attributed to Ralph Waldo Emerson: “We learn about geology the morning after the earthquake.”
“And that’s the case here. It is and will continue to teach us a lot of immunology,” he says.
It’s vital to understand how a COVID-19 infection reshapes the body’s immune defenses so that researchers can tailor vaccines and therapies to do the same or better.
“Because, of course, it’s much more risky to get infected with the actual virus, than with the vaccine,” says Daniela Weiskopf, PhD, a researcher at the La Jolla Institute for Immunology in California.
What is known so far is that how much protection you get and how long you may have it depends on several factors. Those include your age, whether you’ve had COVID-19 before and how severe your symptoms were, your vaccination status, and how long it has been since you were infected or inoculated. Your underlying health matters, too. Immune protection also depends on the virus and how much it is changing as it evolves to evade all our hard-won defenses.
In a new scientific brief, Here’s what we know so far:
Durability of immunity
The agency’s researchers say if you’ve recovered from a COVID-19 infection or are fully vaccinated, you’re probably in good shape for at least 6 months. That’s why this is the recommended interval for people to consider getting a booster dose.
Even though the protection you get after infection and vaccination is generally strong, it’s not perfect.
Getting COVID-19 after you’ve been vaccinated or recovered is still possible. But having some immunity -- whether from infection or vaccination -- really drops the odds of this happening to you. And if you do happen to catch COVID, if your immune system has already gotten a heads up about the virus, your infection is much less likely to be one that lands you in the hospital or morgue.
According to CDC data, at the height of the Delta surge in August, fully vaccinated people were six times less likely to get a COVID-19 infection compared with unvaccinated people, and 11 times less likely to die if they did get it.
How strong is immunity after a COVID-19 Infection?
About 90% of people develop some number of protective antibodies after a COVID-19 infection, according to the CDC. But how high those levels climb appears to be all over the map. Studies show peak antibody concentrations can vary as much as 200-fold, or 2,000%.
Where you fall within that very large range will depend on your age and how sick you became from your COVID-19 infection. It also depends on whether you have an underlying health condition or take a medication that blunts immune function.
Our immune system slows down with age. Immunosenescence starts to affect a person’s health around the age of 60. But there’s no bright line for failure. People who exercise and are generally healthy will have better immune function than someone who doesn’t, no matter their age. In general, though, the older you are, the less likely you are to get a robust immune response after an infection or a vaccination. That’s why this group has been prioritized both for first vaccine doses and boosters.
Beyond age, your protection from future infection seems to depend on how ill you were with the first. Several studies have shown that blood levels of antibodies rise faster and reach a higher peak in people with more severe infections.
In general, people with cold-like symptoms who tested positive but recovered at home are better protected than people who didn’t get any symptoms. And people who were hospitalized for their infections are better protected over the long term than people with milder infections. They may have paid a steep price for that protection: Many hospitalized patients continue to have debilitating symptoms that last for months after they go home.
On average, though, protection after infection seems to be comparable to vaccination, at least for a while. Six large studies from different countries have looked into this question, and five of them have used the very sensitive real-time polymerase chain reaction test (RT-PCR) to count people as truly being previously infected. These studies found that for 6 to 9 months after recovery, a person was 80% to 93% less likely to get COVID-19 again.
There are some caveats to mention, though. Early in the pandemic when supplies were scarce, it was hard to get tested unless you were so sick you landed in the hospital. Studies have shown that the concentration of antibodies a person makes after an infection seems to depend on how sick they got in the first place.
People who had milder infections, or who didn’t have any symptoms at all, may not develop as much protection as those who have more severe symptoms. So these studies may reflect the immunity developed by people who were pretty ill during their first infections.
One study of 25,000 health care workers, who were all tested every 2 weeks -- whether they had symptoms or not -- may offer a clearer picture. In this study, health care workers who’d previously tested positive for COVID-19 were 84% less likely to test positive for the virus again. They were 93% less likely to get an infection that made them sick, and 52% less likely to get an infection without symptoms, for at least 6 months after they recovered.
How does protection after infection compare to vaccination?
Two weeks after your final vaccine dose, protection against a COVID-19 infection is high -- around 90% for the Pfizer and Moderna mRNA vaccines and 66% for the one-dose Johnson & Johnson shot. Clinical trials conducted by the manufacturer have shown that a second dose of the Johnson & Johnson vaccine given at least 2 months after vaccination boosts protection against illness in the United States to about 94%, which is why another dose has been recommended for all Johnson & Johnson vaccine recipients 2 months after their first shot.
It’s not yet known how long the COVID-19 vaccines remain protective. There’s some evidence that protection against symptomatic infections wanes a bit over time as antibody levels drop. But protection against severe illness, including hospitalization and death, has remained high so far, even without a booster.
Are antibodies different after infection compared to vaccination?
Yes. And researchers don’t yet understand what these differences mean.
It seems to come down to a question of quality versus quantity. Vaccines seem to produce higher peak antibody levels than natural infections do. But these antibodies are highly specialized, able to recognize only the parts of the virus they were designed to target.
“The mRNA vaccine directs all the immune responses to the single spike protein,” says Alice Cho, PhD, who is studying the differences in vaccine and infection-created immunity at the Rockefeller University in New York. “There’s a lot more to respond to with a virus than there is in a vaccine.”
During an infection, the immune system learns to recognize and grab onto many parts of the virus, not just its spike.
The job of remembering the various pieces and parts of a foreign invader, so that it can be quickly recognized and disarmed should it ever return, falls to memory B cells.
Memory B cells, in turn, make plasma cells that then crank out antibodies that are custom tailored to attach to their targets.
Antibody levels gradually fall over a few months’ time as the plasma cells that make them die off. But memory B cells live for extended periods. One study that was attempting to measure the lifespan of individual memory B cells in mice found that these cells probably live as long as the mouse itself. Memory B cells induced by smallpox vaccination may live at least 60 years -- virtually an entire lifetime.
Dr. Cho’s research team has found that when memory B cells are trained by the vaccine, they become one-hit wonders, cranking out copious amounts of the same kinds of antibodies over and over again.
Memory B cells trained by viral infection, however, are more versatile. They continue to evolve over several months and produce higher quality antibodies that appear to become more potent over time and can even develop activity against future variants.
Still, the researchers stress that it’s not smart to wait to get a COVID-19 infection in hopes of getting these more versatile antibodies.
“While a natural infection may induce maturation of antibodies with broader activity than a vaccine does -- a natural infection can also kill you,” says Michel Nussenzweig, MD, PhD, head of Rockefeller’s Laboratory of Molecular Immunology.
Sure, memory B cells generated by infections may be immunological Swiss Army Knives, but maybe, argues Donna Farber, PhD, an immunologist at Columbia University in New York, we really only need a single blade.
“The thing with the vaccine is that it’s really focused,” she says. “It’s not giving you all these other viral proteins. It’s only giving you the spike.”
“It may be even better than the level of neutralizing spike antibodies you’re going to get from the infection,” she says. “With a viral infection, the immune response really has a lot to do. It’s really being distracted by all these other proteins.”
“Whereas with the vaccine, it’s just saying to the immune response, ‘This is the immunity we need,’” Dr. Farber says. “‘Just generate this immunity.’ So it’s focusing the immune response in a way that’s going to guarantee that you’re going to get that protective response.”
What if you had COVID and later got vaccinated?
This is called hybrid immunity, and it’s the best of both worlds.
“You have the benefit of very deep, but narrow, immunity produced by vaccine, and very broad, but not very deep, immunity produced by infection,” Dr. Poland says. He says you’ve effectively cross-trained your immune system.
In studies of people who recovered from COVID-19 and then went on to get an mRNA vaccine, after one dose, their antibodies were as high as someone who had been fully vaccinated. After two doses, their antibody levels were about double the average levels seen in someone who’d only been vaccinated.
Studies have shown this kind of immunity has real benefits, too. A recent study by researchers at the University of Kentucky and the CDC found that people who’d gotten COVID-19 in 2020, but had not been vaccinated, were about twice as likely to be reinfected in May and June compared with those who recovered and went on to get their vaccines.
What antibody level is protective?
Scientists aren’t exactly sure how high antibody levels need to be for protection, or even which kinds of antibodies or other immune components matter most yet.
But vaccines appear to generate higher antibody levels than infections do. In a recent study published in the journal Science , Dr. Weiskopf and her colleagues at the La Jolla Institute of Immunology detail the findings of a de-escalation study, where they gave people one-quarter of the normal dose of the Moderna mRNA vaccine and then collected blood samples over time to study their immune responses.
Their immune responses were scaled down with the dose.
“We saw that this has the exact same levels as natural infection,” Dr. Weiskopf says. “People who are vaccinated have much higher immune memory than people who are naturally infected,” she says.
Antibody levels are not easy to determine in the real world. Can you take a test to find out how protected you are? The answer is no, because we don’t yet know what antibody level, or even which kind of antibodies, correlate with protection.
Also, there are many different kinds of antibody tests and they all use a slightly different scale, so there’s no broadly agreed upon way to measure them yet. It’s difficult to compare levels test to test.
Weeks or months between doses? Which is best?
Both the Pfizer and Moderna vaccines were tested to be given 3 and 4 weeks apart, respectively. But when the vaccines were first rolling out, shortages prompted some countries to stretch the interval between doses to 4 or more months.
Researchers who have studied the immune responses of people who were inoculated on an extended dosing schedule noticed something interesting: When the interval was stretched, people had better antibody responses. In fact, their antibody responses looked like the sky-high levels people got with hybrid immunity.
Susanna Dunachie, PhD, a global research professor at the University of Oxford in the United Kingdom, wondered why. She’s leading a team of researchers who are doing detailed studies of the immune responses of health care workers after their vaccinations.
“We found that B cells, which are the cells that make antibodies to the viral spike protein after vaccination, carry on increasing in number between 4 and 10 weeks after vaccination,” she says.
Waiting to give the second vaccine 6 to 14 weeks seems to stimulate the immune system when all of its antibody-making factories are finally up and running.
For this reason, giving the second dose at 3 weeks, she says, might be premature.
But there’s a tradeoff involved in waiting. If there are high levels of the virus circulating in a community, you want to get people fully vaccinated as quickly as possible to maximize their protection in the shortest window of time, which is what we decided to do in the United States.
Researchers say it might be a good idea to revisit the dosing interval when it’s less risky to try it.
A version of this article first appeared on WebMD.com.
Pfizer seeks EUA expansion for COVID-19 booster
Pfizer and its European partner BioNTech on Nov. 9 asked the U.S. government to expand emergency use authorization (EUA) to allow everybody over 18 to receive their COVID-19 booster shots.
If the request is approved, He announced the goal last August but backed off after some scientists said younger people may not need boosters, especially with large parts of the world unvaccinated.
Pfizer is submitting a study of booster effects on 10,000 people to make its case, according to The Associated Press.
This would be Pfizer’s second attempt. In September, a Food and Drug Administration advisory panel turned down Pfizer’s idea of booster shots for everybody over 18.
However, the committee recommended Pfizer booster shots for people 65 and over, essential workers, and people with underlying health conditions.
The FDA and the Centers for Disease Control and Prevention authorized the Pfizer booster for those other groups and later authorization was granted for the same groups with Moderna and Johnson & Johnson boosters. People who got the two-shot Pfizer or Moderna vaccines should get a booster 6 months after the second dose and people who got the one-dose J&J vaccine should get a booster 2 months later.
The pro-booster argument has strengthened because new data have come in from Israel that confirm boosters provide protection as vaccine effectiveness wanes over time, The Washington Post reported. Also, health officials are worried about a post-holiday surge and because COVID-19 case counts and deaths are not dropping in every part of the country, though they are declining overall, according to the The Post report.
The regulatory path for a booster-for-all application is unclear. The Post, citing two unnamed officials, said the FDA probably won’t send the Pfizer application to the FDA advisory committee this time because the committee has already had extensive discussions about boosters. If the FDA gives the green light, CDC Director Rochelle Walensky, MD, would have to make updated recommendations on boosters, The Post article noted.
A version of this article first appeared on WebMD.com.
Pfizer and its European partner BioNTech on Nov. 9 asked the U.S. government to expand emergency use authorization (EUA) to allow everybody over 18 to receive their COVID-19 booster shots.
If the request is approved, He announced the goal last August but backed off after some scientists said younger people may not need boosters, especially with large parts of the world unvaccinated.
Pfizer is submitting a study of booster effects on 10,000 people to make its case, according to The Associated Press.
This would be Pfizer’s second attempt. In September, a Food and Drug Administration advisory panel turned down Pfizer’s idea of booster shots for everybody over 18.
However, the committee recommended Pfizer booster shots for people 65 and over, essential workers, and people with underlying health conditions.
The FDA and the Centers for Disease Control and Prevention authorized the Pfizer booster for those other groups and later authorization was granted for the same groups with Moderna and Johnson & Johnson boosters. People who got the two-shot Pfizer or Moderna vaccines should get a booster 6 months after the second dose and people who got the one-dose J&J vaccine should get a booster 2 months later.
The pro-booster argument has strengthened because new data have come in from Israel that confirm boosters provide protection as vaccine effectiveness wanes over time, The Washington Post reported. Also, health officials are worried about a post-holiday surge and because COVID-19 case counts and deaths are not dropping in every part of the country, though they are declining overall, according to the The Post report.
The regulatory path for a booster-for-all application is unclear. The Post, citing two unnamed officials, said the FDA probably won’t send the Pfizer application to the FDA advisory committee this time because the committee has already had extensive discussions about boosters. If the FDA gives the green light, CDC Director Rochelle Walensky, MD, would have to make updated recommendations on boosters, The Post article noted.
A version of this article first appeared on WebMD.com.
Pfizer and its European partner BioNTech on Nov. 9 asked the U.S. government to expand emergency use authorization (EUA) to allow everybody over 18 to receive their COVID-19 booster shots.
If the request is approved, He announced the goal last August but backed off after some scientists said younger people may not need boosters, especially with large parts of the world unvaccinated.
Pfizer is submitting a study of booster effects on 10,000 people to make its case, according to The Associated Press.
This would be Pfizer’s second attempt. In September, a Food and Drug Administration advisory panel turned down Pfizer’s idea of booster shots for everybody over 18.
However, the committee recommended Pfizer booster shots for people 65 and over, essential workers, and people with underlying health conditions.
The FDA and the Centers for Disease Control and Prevention authorized the Pfizer booster for those other groups and later authorization was granted for the same groups with Moderna and Johnson & Johnson boosters. People who got the two-shot Pfizer or Moderna vaccines should get a booster 6 months after the second dose and people who got the one-dose J&J vaccine should get a booster 2 months later.
The pro-booster argument has strengthened because new data have come in from Israel that confirm boosters provide protection as vaccine effectiveness wanes over time, The Washington Post reported. Also, health officials are worried about a post-holiday surge and because COVID-19 case counts and deaths are not dropping in every part of the country, though they are declining overall, according to the The Post report.
The regulatory path for a booster-for-all application is unclear. The Post, citing two unnamed officials, said the FDA probably won’t send the Pfizer application to the FDA advisory committee this time because the committee has already had extensive discussions about boosters. If the FDA gives the green light, CDC Director Rochelle Walensky, MD, would have to make updated recommendations on boosters, The Post article noted.
A version of this article first appeared on WebMD.com.
A house divided cannot stand
The United States of America are not united. Politics have polarized the competing monologues and the policy making around vaccines, masks, children returning to school, what children are taught in school, and whether the federal government (or the National Football League) can or should create universal mandates enforcing one extreme of any of those policy disputes. Public health and health care have become so entangled in polarized politics that the role of science has often been pushed aside.
Polarization is not a novel event in the history of governments. The partition of India in 1947 divided most of its Hindu and Muslim inhabitants into separate countries, but that hasn’t stopped the recent resurgence of Hindu nationalism in India. The Thirty Years’ War in Europe sought to decide whether Catholics or Protestants would dominate Western Christianity. Those two sides decided in 1648 that coexistence was wiser than continuing into the abyss of mutual annihilation. Current conflicts between Israelis and Palestinians, between Shia and Sunni Arab states, between China and the Uyghurs, and within Sudan and Ethiopia together demonstrate that polarization to the point of genocide can occur regardless of religion, race, and nationality.
Abraham Lincoln, a lawyer in Illinois with a habit of losing elections, was nominated in 1858 to be the Republican nominee in the U.S. Senate race. His speech accepting the nomination spoke a truth that resonated across the nation and across time. It is known as the House Divided speech. He said: “A house divided against itself cannot stand. I believe this government cannot endure, permanently half slave and half free. I do not expect the Union to be dissolved – I do not expect the house to fall – but I do expect it will cease to be divided. It will become all one thing or all the other.”
The Republican Lincoln, supported by antislavery groups, lost that election to the Democrat Stephen A. Douglas, whose party espoused popular sovereignty and local decision-making about slavery. Lincoln’s acceptance speech propelled him 2 years later to be nominated for and elected President of the United States. Lincoln’s first inaugural address as the President of the United States on March 4, 1861, focused on the issue of division and secession. This time, Lincoln placed much more emphasis on preserving the Union. He specifically renounced any federal efforts to use force to abolish slavery in the states that permitted it. He declared: “I have no purpose, directly or indirectly, to interfere with the institution of slavery in the States where it exists. I believe I have no lawful right to do so, and I have no inclination to do so.”
President Lincoln’s approach might not meet muster in today’s cancel culture. He was facing a precariously divided nation not unlike the current day, so his speech contains insights and wisdom important for today. Lincoln saw government as “a majority held in restraint by constitutional checks and limitations.” I am loath to further quote out of context or paraphrase his masterful words. Go read the original, in its balanced entirety.
I have written previous columns about the importance of taking time to reflect on one’s life and one’s career. Reflection is both a wellness check and a moral compass check. Some call it mindfulness. I lean toward calling it thankfulness and gratitude. Hence, November is a convenient time for pediatricians if flu and respiratory syncytial virus seasons haven’t started.
The Gettysburg Address extols the virtue of dedication. Lincoln’s second inaugural address promotes mercy and forgiveness. His Farewell Address to Springfield in 1861 in a single paragraph captures grief, faith, and hope. Those speeches are my perennial favorites. But this year it is the two aforementioned addresses that must be mined for wisdom.
I advocate vaccine and mask mandates, but I am not enamored with the idea of President Biden using the unchecked power of the executive branch to promulgate a single federal regulation that overreaches into every moderate-size business nationwide. The 1861 inaugural address concurs. Lincoln’s prophecy that division will be solved when one side ultimately wins is not the model I seek. It hasn’t worked for gun control. It hasn’t worked for abortion as we approach the 50th anniversary of Roe v. Wade. The present 50+1 vote majority in the U.S. Senate does not have a mandate to overhaul society, especially when those majorities are transient. One should have the courage to seek change, but beware of creating large divisions with small majorities.
Facebook profits when you meditate in the echo chambers of large, outraged groups. Avoid that. Hebrew tradition has some reflection occurring in groups of two or three, rather than solo. Truth is revealed in community. Voltaire said: “Cherish those who seek the truth but beware of those who find it.” As a scientist, my experience is that humility, skepticism, and a dedication to finding truth have served me well for a lifetime.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
The United States of America are not united. Politics have polarized the competing monologues and the policy making around vaccines, masks, children returning to school, what children are taught in school, and whether the federal government (or the National Football League) can or should create universal mandates enforcing one extreme of any of those policy disputes. Public health and health care have become so entangled in polarized politics that the role of science has often been pushed aside.
Polarization is not a novel event in the history of governments. The partition of India in 1947 divided most of its Hindu and Muslim inhabitants into separate countries, but that hasn’t stopped the recent resurgence of Hindu nationalism in India. The Thirty Years’ War in Europe sought to decide whether Catholics or Protestants would dominate Western Christianity. Those two sides decided in 1648 that coexistence was wiser than continuing into the abyss of mutual annihilation. Current conflicts between Israelis and Palestinians, between Shia and Sunni Arab states, between China and the Uyghurs, and within Sudan and Ethiopia together demonstrate that polarization to the point of genocide can occur regardless of religion, race, and nationality.
Abraham Lincoln, a lawyer in Illinois with a habit of losing elections, was nominated in 1858 to be the Republican nominee in the U.S. Senate race. His speech accepting the nomination spoke a truth that resonated across the nation and across time. It is known as the House Divided speech. He said: “A house divided against itself cannot stand. I believe this government cannot endure, permanently half slave and half free. I do not expect the Union to be dissolved – I do not expect the house to fall – but I do expect it will cease to be divided. It will become all one thing or all the other.”
The Republican Lincoln, supported by antislavery groups, lost that election to the Democrat Stephen A. Douglas, whose party espoused popular sovereignty and local decision-making about slavery. Lincoln’s acceptance speech propelled him 2 years later to be nominated for and elected President of the United States. Lincoln’s first inaugural address as the President of the United States on March 4, 1861, focused on the issue of division and secession. This time, Lincoln placed much more emphasis on preserving the Union. He specifically renounced any federal efforts to use force to abolish slavery in the states that permitted it. He declared: “I have no purpose, directly or indirectly, to interfere with the institution of slavery in the States where it exists. I believe I have no lawful right to do so, and I have no inclination to do so.”
President Lincoln’s approach might not meet muster in today’s cancel culture. He was facing a precariously divided nation not unlike the current day, so his speech contains insights and wisdom important for today. Lincoln saw government as “a majority held in restraint by constitutional checks and limitations.” I am loath to further quote out of context or paraphrase his masterful words. Go read the original, in its balanced entirety.
I have written previous columns about the importance of taking time to reflect on one’s life and one’s career. Reflection is both a wellness check and a moral compass check. Some call it mindfulness. I lean toward calling it thankfulness and gratitude. Hence, November is a convenient time for pediatricians if flu and respiratory syncytial virus seasons haven’t started.
The Gettysburg Address extols the virtue of dedication. Lincoln’s second inaugural address promotes mercy and forgiveness. His Farewell Address to Springfield in 1861 in a single paragraph captures grief, faith, and hope. Those speeches are my perennial favorites. But this year it is the two aforementioned addresses that must be mined for wisdom.
I advocate vaccine and mask mandates, but I am not enamored with the idea of President Biden using the unchecked power of the executive branch to promulgate a single federal regulation that overreaches into every moderate-size business nationwide. The 1861 inaugural address concurs. Lincoln’s prophecy that division will be solved when one side ultimately wins is not the model I seek. It hasn’t worked for gun control. It hasn’t worked for abortion as we approach the 50th anniversary of Roe v. Wade. The present 50+1 vote majority in the U.S. Senate does not have a mandate to overhaul society, especially when those majorities are transient. One should have the courage to seek change, but beware of creating large divisions with small majorities.
Facebook profits when you meditate in the echo chambers of large, outraged groups. Avoid that. Hebrew tradition has some reflection occurring in groups of two or three, rather than solo. Truth is revealed in community. Voltaire said: “Cherish those who seek the truth but beware of those who find it.” As a scientist, my experience is that humility, skepticism, and a dedication to finding truth have served me well for a lifetime.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
The United States of America are not united. Politics have polarized the competing monologues and the policy making around vaccines, masks, children returning to school, what children are taught in school, and whether the federal government (or the National Football League) can or should create universal mandates enforcing one extreme of any of those policy disputes. Public health and health care have become so entangled in polarized politics that the role of science has often been pushed aside.
Polarization is not a novel event in the history of governments. The partition of India in 1947 divided most of its Hindu and Muslim inhabitants into separate countries, but that hasn’t stopped the recent resurgence of Hindu nationalism in India. The Thirty Years’ War in Europe sought to decide whether Catholics or Protestants would dominate Western Christianity. Those two sides decided in 1648 that coexistence was wiser than continuing into the abyss of mutual annihilation. Current conflicts between Israelis and Palestinians, between Shia and Sunni Arab states, between China and the Uyghurs, and within Sudan and Ethiopia together demonstrate that polarization to the point of genocide can occur regardless of religion, race, and nationality.
Abraham Lincoln, a lawyer in Illinois with a habit of losing elections, was nominated in 1858 to be the Republican nominee in the U.S. Senate race. His speech accepting the nomination spoke a truth that resonated across the nation and across time. It is known as the House Divided speech. He said: “A house divided against itself cannot stand. I believe this government cannot endure, permanently half slave and half free. I do not expect the Union to be dissolved – I do not expect the house to fall – but I do expect it will cease to be divided. It will become all one thing or all the other.”
The Republican Lincoln, supported by antislavery groups, lost that election to the Democrat Stephen A. Douglas, whose party espoused popular sovereignty and local decision-making about slavery. Lincoln’s acceptance speech propelled him 2 years later to be nominated for and elected President of the United States. Lincoln’s first inaugural address as the President of the United States on March 4, 1861, focused on the issue of division and secession. This time, Lincoln placed much more emphasis on preserving the Union. He specifically renounced any federal efforts to use force to abolish slavery in the states that permitted it. He declared: “I have no purpose, directly or indirectly, to interfere with the institution of slavery in the States where it exists. I believe I have no lawful right to do so, and I have no inclination to do so.”
President Lincoln’s approach might not meet muster in today’s cancel culture. He was facing a precariously divided nation not unlike the current day, so his speech contains insights and wisdom important for today. Lincoln saw government as “a majority held in restraint by constitutional checks and limitations.” I am loath to further quote out of context or paraphrase his masterful words. Go read the original, in its balanced entirety.
I have written previous columns about the importance of taking time to reflect on one’s life and one’s career. Reflection is both a wellness check and a moral compass check. Some call it mindfulness. I lean toward calling it thankfulness and gratitude. Hence, November is a convenient time for pediatricians if flu and respiratory syncytial virus seasons haven’t started.
The Gettysburg Address extols the virtue of dedication. Lincoln’s second inaugural address promotes mercy and forgiveness. His Farewell Address to Springfield in 1861 in a single paragraph captures grief, faith, and hope. Those speeches are my perennial favorites. But this year it is the two aforementioned addresses that must be mined for wisdom.
I advocate vaccine and mask mandates, but I am not enamored with the idea of President Biden using the unchecked power of the executive branch to promulgate a single federal regulation that overreaches into every moderate-size business nationwide. The 1861 inaugural address concurs. Lincoln’s prophecy that division will be solved when one side ultimately wins is not the model I seek. It hasn’t worked for gun control. It hasn’t worked for abortion as we approach the 50th anniversary of Roe v. Wade. The present 50+1 vote majority in the U.S. Senate does not have a mandate to overhaul society, especially when those majorities are transient. One should have the courage to seek change, but beware of creating large divisions with small majorities.
Facebook profits when you meditate in the echo chambers of large, outraged groups. Avoid that. Hebrew tradition has some reflection occurring in groups of two or three, rather than solo. Truth is revealed in community. Voltaire said: “Cherish those who seek the truth but beware of those who find it.” As a scientist, my experience is that humility, skepticism, and a dedication to finding truth have served me well for a lifetime.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Unvaccinated people 20 times more likely to die from COVID: Texas study
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
During the month of September, , according to a new study from the Texas Department of State Health Services.
The data also showed that unvaccinated people were 13 times more likely to test positive for COVID-19 than people who were fully vaccinated.
“This analysis quantifies what we’ve known for months,” Jennifer Shuford, MD, the state’s chief epidemiologist, told The Dallas Morning News.
“The COVID-19 vaccines are doing an excellent job of protecting people from getting sick and from dying from COVID-19,” she said. “Vaccination remains the best way to keep yourself and the people close to you safe from this deadly disease.”
As part of the study, researchers analyzed electronic lab reports, death certificates, and state immunization records, with a particular focus on September when the contagious Delta variant surged across Texas. The research marks the state’s first statistical analysis of COVID-19 vaccinations in Texas and the effects, the newspaper reported.
The protective effect of vaccination was most noticeable among younger groups. During September, the risk of COVID-19 death was 23 times higher in unvaccinated people in their 30s and 55 times higher for unvaccinated people in their 40s.
In addition, there were fewer than 10 COVID-19 deaths in September among fully vaccinated people between ages 18-29, as compared with 339 deaths among unvaccinated people in the same age group.
Then, looking at a longer time period -- from Jan. 15 to Oct. 1 -- the researchers found that unvaccinated people were 45 times more likely to contract COVID-19 than fully vaccinated people. The protective effect of vaccination against infection was strong across all adult age groups but greatest among ages 12-17.
“All authorized COVID-19 vaccines in the United States are highly effective at protecting people from getting sick or severely ill with COVID-19, including those infected with Delta and other known variants,” the study authors wrote. “Real world data from Texas clearly shows these benefits.”
About 15.6 million people in Texas have been fully vaccinated against COVID-19 in a state of about 29 million residents, according to state data. About 66% of the population has received at least one dose, while 58% is fully vaccinated.
A version of this article first appeared on WebMD.com.
Practicing telepsychiatry: Include backup plans, ground rules
For psychiatrists embarking on a telemedicine consultation, it might be helpful to review a checklist of steps that will reduce the risk of problems when things go wrong, according to an overview of the dangers at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sponsored by Medscape Live.
Ideally, telepsychiatry will function much like an inpatient office visit, but the dynamics differ – as do the things that can go wrong, according to Sanjay Gupta, MD, chief medical officer, BryLin Behavioral Health System, Buffalo, N.Y. “Issues can arise suddenly. You need contingency planning.”
At the outset, psychiatrists should establish the location of the patient. This is necessary at every telemedicine encounter. With a remote device, a patient could be essentially anywhere on Earth. Patients might not even remember to mention that they are vacationing in Australia.
The location of the patient is important in the event of an unexpected crisis. This is not only relevant to an unstable patient at risk of dangerous behavior, such as actively attempting suicide, but to patients who have a seizure or some other emergency that inhibits communication. , and this requires confirming that the patient is where he or she was expected to be.
In addition, there should be a plan for technological failure. As everyone knows, these failures, such as dysfunction of a device, a poor connection, or an Internet outage, can happen at any time. Both the clinician and the patient can derive reassurance from at least one if not two or more plans to reconnect in the event of these failures.
The visit should also begin with questions that will establish the patient has a sense of adequate privacy. This is one of the most common obstacles to an effective telemedicine consultation. Dr. Gupta pointed out that phone or computer cameras do not typically permit the clinician to exclude the presence of another individual sitting even a few feet away from the patient. With spouses and children nearby, there might be a tenuous sense of privacy even if they are unlikely to overhear the telemedicine visit.
One strategy that can be used to assess the patient’s level of comfort is to ask for a description of the patient’s surroundings and any other people at the location. Dr. Gupta also said it is appropriate to establish ground rules about recording of the session, which has its own potential to inhibit the interaction.
Warning that some form of consent to a telemedicine visit is mandatory in most states, Dr. Gupta also cautioned that a formal identification check is appropriate for a first-time visit. The risk of an individual offering a false identification is likely to be low, but it can be eliminated entirely by a protocol that verifies consent and identify before the clinical work begins.
Because of the importance of engaging patients quickly, Dr. Gupta called the first few minutes of a telemedicine visit “crucial.” By initiating the visit with a warm and respectful tone, by relaying a competent and professional appearance, and by establishing an atmosphere that encourages communication, the initial minutes of the call can set a tone that facilitates an effective visit.
Simple and established telehealth etiquette strategies should be employed, according to Dr. Gupta. He suggested paying attention to such issues as lighting, background, and camera position. Descriptions of what constitutes adequate lighting and background are easily obtained on free how-to websites, but the goal is to provide patients with a nondistracting and clear view of the clinician.
During a telemedicine visit, the clinician’s focus should remain on the patient, according to Dr. Gupta. He advised against taking notes or documenting the visit on an electronic health record during the course of the visit. Rather, he advised positioning the camera in a way that the patient feels eye contact is being made.
“It can be helpful to periodically summarize what the patient has said to demonstrate that you are fully engaged,” Dr. Gupta suggested.
Telemedicine is very effective for many but not all patients. Some, such as those with active psychosis, are not suited to this approach, but others are simply uncomfortable with this form of communication. Dr. Gupta suggested that clinicians should be mindful of the advantages and the limitations of telepsychiatry.
Ultimately, Dr. Gupta believes that the substantial expansion of telepsychiatry that took place during the COVID-19 pandemic is likely to persist when the pandemic ends, even if many of the changes that permitted its expansion, such as a relaxation of HIPPA requirements, are withdrawn. However, parity reimbursement for visits offered by telemedicine relative to those that are face-to-face, which greatly facilitated the growth of telepsychiatry, is not guaranteed, so this remains an unanswered question.
“The question is what will happen to the billing codes when we see COVID-19 in the rearview mirror, and the answer is that no one knows,” he said.
Uncertainty about future use
Other experts in this field agreed. James (Jay) H. Shore, MD, MPH, director of telemedicine, Helen and Arthur E. Johnson Depression Center, University of Colorado at Denver, Aurora, has long been an advocate for the value of telepsychiatry for reaching patients with limited psychosocial services. The attention drawn to this practice by the COVID-19 pandemic has been welcome, but he does not know how it will affect the future.
“There is too much uncertainty in the system to make a good prediction of where this may end up,” he said.
It is not just reimbursement that is at risk, according to Peter Yellowlees, MBBS, MD, chief wellness officer at the University of California, Davis. Also a longtime advocate of telepsychiatry, particularly to reach the underserved, Dr. Yellowlees pointed out that the ability to prescribe controlled substances through telemedicine and the ability to consult with patients across state lines might also be in jeopardy if and when rules for telemedicine are revisited after the pandemic.
“Many organizations are lobbying to make the pandemic changes permanent because they greatly support telemedicine delivery,” Dr. Yellowlees said, but agreed about the uncertainty regarding what policy makers will do.
Jayasudha Gude, MD, who is completing her residency in psychiatry at Zucker Hillside Hospital, Northwell Health, New York, recently led a literature review evaluating the needs and viability of telepsychiatry during and after the COVID-19 era (Cureus. 2021 Aug;13:e16974). Based on the benefits she identified in her review, she said, “I would definitely want to advocate for the continued use of telepsychiatry after the pandemic is over.” She hopes that psychiatrists who now have experience in this area will join her.
“I am hopeful that a lot of mental health providers will also be advocating since they have experience, and many will want to continue its use,” she said. Medscape Live and this news organization are owned by the same parent company. Dr. Gupta, Dr. Shore, Dr. Yellowlees, and Dr. Gude reported no potential conflicts of interest.
For psychiatrists embarking on a telemedicine consultation, it might be helpful to review a checklist of steps that will reduce the risk of problems when things go wrong, according to an overview of the dangers at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sponsored by Medscape Live.
Ideally, telepsychiatry will function much like an inpatient office visit, but the dynamics differ – as do the things that can go wrong, according to Sanjay Gupta, MD, chief medical officer, BryLin Behavioral Health System, Buffalo, N.Y. “Issues can arise suddenly. You need contingency planning.”
At the outset, psychiatrists should establish the location of the patient. This is necessary at every telemedicine encounter. With a remote device, a patient could be essentially anywhere on Earth. Patients might not even remember to mention that they are vacationing in Australia.
The location of the patient is important in the event of an unexpected crisis. This is not only relevant to an unstable patient at risk of dangerous behavior, such as actively attempting suicide, but to patients who have a seizure or some other emergency that inhibits communication. , and this requires confirming that the patient is where he or she was expected to be.
In addition, there should be a plan for technological failure. As everyone knows, these failures, such as dysfunction of a device, a poor connection, or an Internet outage, can happen at any time. Both the clinician and the patient can derive reassurance from at least one if not two or more plans to reconnect in the event of these failures.
The visit should also begin with questions that will establish the patient has a sense of adequate privacy. This is one of the most common obstacles to an effective telemedicine consultation. Dr. Gupta pointed out that phone or computer cameras do not typically permit the clinician to exclude the presence of another individual sitting even a few feet away from the patient. With spouses and children nearby, there might be a tenuous sense of privacy even if they are unlikely to overhear the telemedicine visit.
One strategy that can be used to assess the patient’s level of comfort is to ask for a description of the patient’s surroundings and any other people at the location. Dr. Gupta also said it is appropriate to establish ground rules about recording of the session, which has its own potential to inhibit the interaction.
Warning that some form of consent to a telemedicine visit is mandatory in most states, Dr. Gupta also cautioned that a formal identification check is appropriate for a first-time visit. The risk of an individual offering a false identification is likely to be low, but it can be eliminated entirely by a protocol that verifies consent and identify before the clinical work begins.
Because of the importance of engaging patients quickly, Dr. Gupta called the first few minutes of a telemedicine visit “crucial.” By initiating the visit with a warm and respectful tone, by relaying a competent and professional appearance, and by establishing an atmosphere that encourages communication, the initial minutes of the call can set a tone that facilitates an effective visit.
Simple and established telehealth etiquette strategies should be employed, according to Dr. Gupta. He suggested paying attention to such issues as lighting, background, and camera position. Descriptions of what constitutes adequate lighting and background are easily obtained on free how-to websites, but the goal is to provide patients with a nondistracting and clear view of the clinician.
During a telemedicine visit, the clinician’s focus should remain on the patient, according to Dr. Gupta. He advised against taking notes or documenting the visit on an electronic health record during the course of the visit. Rather, he advised positioning the camera in a way that the patient feels eye contact is being made.
“It can be helpful to periodically summarize what the patient has said to demonstrate that you are fully engaged,” Dr. Gupta suggested.
Telemedicine is very effective for many but not all patients. Some, such as those with active psychosis, are not suited to this approach, but others are simply uncomfortable with this form of communication. Dr. Gupta suggested that clinicians should be mindful of the advantages and the limitations of telepsychiatry.
Ultimately, Dr. Gupta believes that the substantial expansion of telepsychiatry that took place during the COVID-19 pandemic is likely to persist when the pandemic ends, even if many of the changes that permitted its expansion, such as a relaxation of HIPPA requirements, are withdrawn. However, parity reimbursement for visits offered by telemedicine relative to those that are face-to-face, which greatly facilitated the growth of telepsychiatry, is not guaranteed, so this remains an unanswered question.
“The question is what will happen to the billing codes when we see COVID-19 in the rearview mirror, and the answer is that no one knows,” he said.
Uncertainty about future use
Other experts in this field agreed. James (Jay) H. Shore, MD, MPH, director of telemedicine, Helen and Arthur E. Johnson Depression Center, University of Colorado at Denver, Aurora, has long been an advocate for the value of telepsychiatry for reaching patients with limited psychosocial services. The attention drawn to this practice by the COVID-19 pandemic has been welcome, but he does not know how it will affect the future.
“There is too much uncertainty in the system to make a good prediction of where this may end up,” he said.
It is not just reimbursement that is at risk, according to Peter Yellowlees, MBBS, MD, chief wellness officer at the University of California, Davis. Also a longtime advocate of telepsychiatry, particularly to reach the underserved, Dr. Yellowlees pointed out that the ability to prescribe controlled substances through telemedicine and the ability to consult with patients across state lines might also be in jeopardy if and when rules for telemedicine are revisited after the pandemic.
“Many organizations are lobbying to make the pandemic changes permanent because they greatly support telemedicine delivery,” Dr. Yellowlees said, but agreed about the uncertainty regarding what policy makers will do.
Jayasudha Gude, MD, who is completing her residency in psychiatry at Zucker Hillside Hospital, Northwell Health, New York, recently led a literature review evaluating the needs and viability of telepsychiatry during and after the COVID-19 era (Cureus. 2021 Aug;13:e16974). Based on the benefits she identified in her review, she said, “I would definitely want to advocate for the continued use of telepsychiatry after the pandemic is over.” She hopes that psychiatrists who now have experience in this area will join her.
“I am hopeful that a lot of mental health providers will also be advocating since they have experience, and many will want to continue its use,” she said. Medscape Live and this news organization are owned by the same parent company. Dr. Gupta, Dr. Shore, Dr. Yellowlees, and Dr. Gude reported no potential conflicts of interest.
For psychiatrists embarking on a telemedicine consultation, it might be helpful to review a checklist of steps that will reduce the risk of problems when things go wrong, according to an overview of the dangers at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists, sponsored by Medscape Live.
Ideally, telepsychiatry will function much like an inpatient office visit, but the dynamics differ – as do the things that can go wrong, according to Sanjay Gupta, MD, chief medical officer, BryLin Behavioral Health System, Buffalo, N.Y. “Issues can arise suddenly. You need contingency planning.”
At the outset, psychiatrists should establish the location of the patient. This is necessary at every telemedicine encounter. With a remote device, a patient could be essentially anywhere on Earth. Patients might not even remember to mention that they are vacationing in Australia.
The location of the patient is important in the event of an unexpected crisis. This is not only relevant to an unstable patient at risk of dangerous behavior, such as actively attempting suicide, but to patients who have a seizure or some other emergency that inhibits communication. , and this requires confirming that the patient is where he or she was expected to be.
In addition, there should be a plan for technological failure. As everyone knows, these failures, such as dysfunction of a device, a poor connection, or an Internet outage, can happen at any time. Both the clinician and the patient can derive reassurance from at least one if not two or more plans to reconnect in the event of these failures.
The visit should also begin with questions that will establish the patient has a sense of adequate privacy. This is one of the most common obstacles to an effective telemedicine consultation. Dr. Gupta pointed out that phone or computer cameras do not typically permit the clinician to exclude the presence of another individual sitting even a few feet away from the patient. With spouses and children nearby, there might be a tenuous sense of privacy even if they are unlikely to overhear the telemedicine visit.
One strategy that can be used to assess the patient’s level of comfort is to ask for a description of the patient’s surroundings and any other people at the location. Dr. Gupta also said it is appropriate to establish ground rules about recording of the session, which has its own potential to inhibit the interaction.
Warning that some form of consent to a telemedicine visit is mandatory in most states, Dr. Gupta also cautioned that a formal identification check is appropriate for a first-time visit. The risk of an individual offering a false identification is likely to be low, but it can be eliminated entirely by a protocol that verifies consent and identify before the clinical work begins.
Because of the importance of engaging patients quickly, Dr. Gupta called the first few minutes of a telemedicine visit “crucial.” By initiating the visit with a warm and respectful tone, by relaying a competent and professional appearance, and by establishing an atmosphere that encourages communication, the initial minutes of the call can set a tone that facilitates an effective visit.
Simple and established telehealth etiquette strategies should be employed, according to Dr. Gupta. He suggested paying attention to such issues as lighting, background, and camera position. Descriptions of what constitutes adequate lighting and background are easily obtained on free how-to websites, but the goal is to provide patients with a nondistracting and clear view of the clinician.
During a telemedicine visit, the clinician’s focus should remain on the patient, according to Dr. Gupta. He advised against taking notes or documenting the visit on an electronic health record during the course of the visit. Rather, he advised positioning the camera in a way that the patient feels eye contact is being made.
“It can be helpful to periodically summarize what the patient has said to demonstrate that you are fully engaged,” Dr. Gupta suggested.
Telemedicine is very effective for many but not all patients. Some, such as those with active psychosis, are not suited to this approach, but others are simply uncomfortable with this form of communication. Dr. Gupta suggested that clinicians should be mindful of the advantages and the limitations of telepsychiatry.
Ultimately, Dr. Gupta believes that the substantial expansion of telepsychiatry that took place during the COVID-19 pandemic is likely to persist when the pandemic ends, even if many of the changes that permitted its expansion, such as a relaxation of HIPPA requirements, are withdrawn. However, parity reimbursement for visits offered by telemedicine relative to those that are face-to-face, which greatly facilitated the growth of telepsychiatry, is not guaranteed, so this remains an unanswered question.
“The question is what will happen to the billing codes when we see COVID-19 in the rearview mirror, and the answer is that no one knows,” he said.
Uncertainty about future use
Other experts in this field agreed. James (Jay) H. Shore, MD, MPH, director of telemedicine, Helen and Arthur E. Johnson Depression Center, University of Colorado at Denver, Aurora, has long been an advocate for the value of telepsychiatry for reaching patients with limited psychosocial services. The attention drawn to this practice by the COVID-19 pandemic has been welcome, but he does not know how it will affect the future.
“There is too much uncertainty in the system to make a good prediction of where this may end up,” he said.
It is not just reimbursement that is at risk, according to Peter Yellowlees, MBBS, MD, chief wellness officer at the University of California, Davis. Also a longtime advocate of telepsychiatry, particularly to reach the underserved, Dr. Yellowlees pointed out that the ability to prescribe controlled substances through telemedicine and the ability to consult with patients across state lines might also be in jeopardy if and when rules for telemedicine are revisited after the pandemic.
“Many organizations are lobbying to make the pandemic changes permanent because they greatly support telemedicine delivery,” Dr. Yellowlees said, but agreed about the uncertainty regarding what policy makers will do.
Jayasudha Gude, MD, who is completing her residency in psychiatry at Zucker Hillside Hospital, Northwell Health, New York, recently led a literature review evaluating the needs and viability of telepsychiatry during and after the COVID-19 era (Cureus. 2021 Aug;13:e16974). Based on the benefits she identified in her review, she said, “I would definitely want to advocate for the continued use of telepsychiatry after the pandemic is over.” She hopes that psychiatrists who now have experience in this area will join her.
“I am hopeful that a lot of mental health providers will also be advocating since they have experience, and many will want to continue its use,” she said. Medscape Live and this news organization are owned by the same parent company. Dr. Gupta, Dr. Shore, Dr. Yellowlees, and Dr. Gude reported no potential conflicts of interest.
FROM PSYCHOPHARMACOLOGY UPDATE
Cities, states offer to pay kids to get vaccinated
As millions of children between ages 5-11 became eligible to receive a COVID-19 vaccine recently, .
In New York City, for instance, children can claim $100 if they receive their first dose of the Pfizer vaccine at a city-operated vaccine site. As an alternate choice, they can receive tickets to city attractions such as the Statue of Liberty or the Brooklyn Cyclones baseball team.
“We really want kids to take advantage, families take advantage of that,” Mayor Bill de Blasio said Nov. 4.
“Everyone could use a little more money around the holidays,” he said. “But, most importantly, we want our kids and our families to be safe.”
In Chicago, health officials are offering $100 gift cards to children who get vaccinated at public health events or clinics. The Chicago school district is also closing on Nov. 12 for Vaccination Awareness Day so students can get shots.
“It is rare that we make a late change to the school calendar, but we see this as an important investment in the future of this school year and the health and well-being of our students, staff, and families,” Pedro Martinez, the CEO for Chicago Public Schools, said in a message to parents.
The Centers for Disease Control and Prevention cleared the COVID-19 shot for children as young as age 5 on Nov. 2, making most Americans eligible for the vaccine. Ages 5-11 receive one-third of the dose given to adults and teens.
Other states and cities are offering incentives as well:
- San Antonio: Parents and guardians who take their children to get vaccinated at a Metro Health clinic can receive a $100 gift card for H-E-B grocery stores.
- Louisiana: As part of the “Shot for $100” program, anyone who receives their first shot is eligible for $100. Children between ages 5-11 can receive the cash incentive but require parental consent to get the vaccine.
- Minnesota: As part of the “Kids Deserve a Shot!” program, ages 12-17 can receive a $200 gift card and the opportunity to enter a raffle for a $100,000 college scholarship.
A version of this article first appeared on WebMD.com.
As millions of children between ages 5-11 became eligible to receive a COVID-19 vaccine recently, .
In New York City, for instance, children can claim $100 if they receive their first dose of the Pfizer vaccine at a city-operated vaccine site. As an alternate choice, they can receive tickets to city attractions such as the Statue of Liberty or the Brooklyn Cyclones baseball team.
“We really want kids to take advantage, families take advantage of that,” Mayor Bill de Blasio said Nov. 4.
“Everyone could use a little more money around the holidays,” he said. “But, most importantly, we want our kids and our families to be safe.”
In Chicago, health officials are offering $100 gift cards to children who get vaccinated at public health events or clinics. The Chicago school district is also closing on Nov. 12 for Vaccination Awareness Day so students can get shots.
“It is rare that we make a late change to the school calendar, but we see this as an important investment in the future of this school year and the health and well-being of our students, staff, and families,” Pedro Martinez, the CEO for Chicago Public Schools, said in a message to parents.
The Centers for Disease Control and Prevention cleared the COVID-19 shot for children as young as age 5 on Nov. 2, making most Americans eligible for the vaccine. Ages 5-11 receive one-third of the dose given to adults and teens.
Other states and cities are offering incentives as well:
- San Antonio: Parents and guardians who take their children to get vaccinated at a Metro Health clinic can receive a $100 gift card for H-E-B grocery stores.
- Louisiana: As part of the “Shot for $100” program, anyone who receives their first shot is eligible for $100. Children between ages 5-11 can receive the cash incentive but require parental consent to get the vaccine.
- Minnesota: As part of the “Kids Deserve a Shot!” program, ages 12-17 can receive a $200 gift card and the opportunity to enter a raffle for a $100,000 college scholarship.
A version of this article first appeared on WebMD.com.
As millions of children between ages 5-11 became eligible to receive a COVID-19 vaccine recently, .
In New York City, for instance, children can claim $100 if they receive their first dose of the Pfizer vaccine at a city-operated vaccine site. As an alternate choice, they can receive tickets to city attractions such as the Statue of Liberty or the Brooklyn Cyclones baseball team.
“We really want kids to take advantage, families take advantage of that,” Mayor Bill de Blasio said Nov. 4.
“Everyone could use a little more money around the holidays,” he said. “But, most importantly, we want our kids and our families to be safe.”
In Chicago, health officials are offering $100 gift cards to children who get vaccinated at public health events or clinics. The Chicago school district is also closing on Nov. 12 for Vaccination Awareness Day so students can get shots.
“It is rare that we make a late change to the school calendar, but we see this as an important investment in the future of this school year and the health and well-being of our students, staff, and families,” Pedro Martinez, the CEO for Chicago Public Schools, said in a message to parents.
The Centers for Disease Control and Prevention cleared the COVID-19 shot for children as young as age 5 on Nov. 2, making most Americans eligible for the vaccine. Ages 5-11 receive one-third of the dose given to adults and teens.
Other states and cities are offering incentives as well:
- San Antonio: Parents and guardians who take their children to get vaccinated at a Metro Health clinic can receive a $100 gift card for H-E-B grocery stores.
- Louisiana: As part of the “Shot for $100” program, anyone who receives their first shot is eligible for $100. Children between ages 5-11 can receive the cash incentive but require parental consent to get the vaccine.
- Minnesota: As part of the “Kids Deserve a Shot!” program, ages 12-17 can receive a $200 gift card and the opportunity to enter a raffle for a $100,000 college scholarship.
A version of this article first appeared on WebMD.com.
Children and COVID: New cases up again after dropping for 8 weeks
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.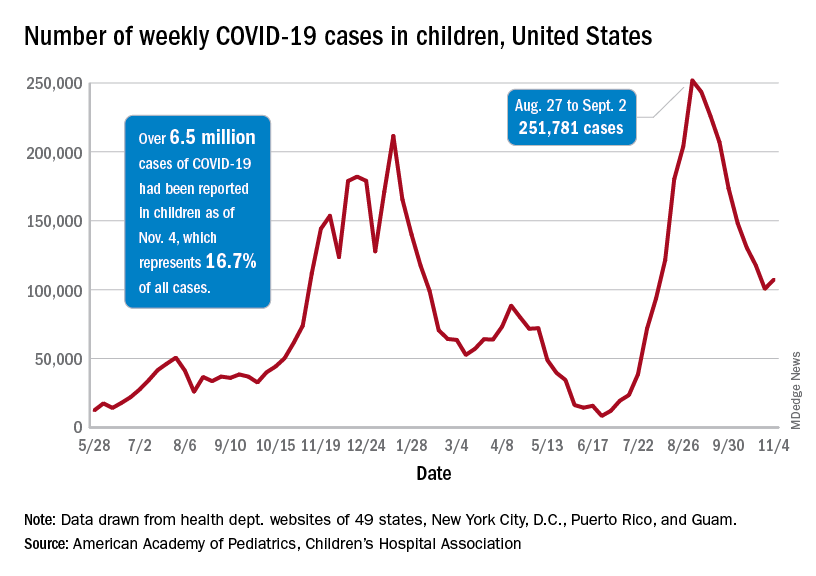
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
Early trials underway to test mushrooms as COVID treatment
The U.S. Food and Drug Administration (FDA) approved the MACH-19 trials (the acronym for Mushrooms and Chinese Herbs for COVID-19) after researchers applied for approval in April.
The first two phase 1 randomized, double-blind, placebo-controlled trials have begun at UCLA and the University of California San Diego to treat COVID-19 patients quarantining at home with mild to moderate symptoms. A third trial is investigating the use of medicinal mushrooms as an adjuvant to COVID-19 vaccines.
The researchers have also launched a fourth trial testing the mushrooms against placebo as an adjunct to a COVID booster shot. It looks at the effect in people who have comorbidities that would reduce their vaccine response. An article in JAMA described the trials.
The two mushroom varieties being tested — turkey tail and agarikon — are available as over-the-counter supplements, according to the report. They are a separate class from hallucinogenic or “magic” mushrooms being tested for other uses in medicine.
“They are not even as psychoactive as a cup of tea,” Gordon Saxe, MD, PhD, MPH, principal investigator for the MACH-19 trials, told this news organization.
For each of the MACH-19 treatment trials, researchers plan to recruit 66 people who are quarantined at home with mild to moderate COVID-19 symptoms. Participants will be randomly assigned either to receive the mushroom combination, the Chinese herbs, or a placebo for 2 weeks, according to the JAMA paper.
D. Craig Hopp, PhD, deputy director of the division of extramural research at the National Center for Complementary and Integrative Health (NCCIH), told JAMA in an interview that he was “mildly concerned” about using mushrooms to treat people with active SARS-CoV-2 infection.
“We know that a cytokine storm poses the greatest risk of COVID mortality, not the virus itself,” Dr. Hopp said. “The danger is that an immune-stimulating agent like mushrooms might supercharge an individual’s immune response, leading to a cytokine storm.”
Stephen Wilson, PhD, an immunologist who consulted on the trials when he was chief operating officer of the La Jolla Institute for Immunology, says in the JAMA article that a cytokine storm is unlikely for these patients because the mushroom components “don’t mimic inflammatory cytokines.” Dr. Wilson is now chief innovations officer at Statera Biopharma.
“We think the mushrooms increase the number of immunologic opportunities to better see and respond to a specific threat. In the doses used, the mushrooms perturb the immune system in a good way but fall far short of driving hyper or sustained inflammation,” Dr. Wilson said.
Dr. Saxe said the FDA process was extensive and rigorous and FDA investigators also asked about potential cytokine storms before approving the trials. Cytokine storm is not an issue with a healthy response, Dr. Saxe pointed out. It’s a response that’s not balanced or modulated.
“Mushrooms are immunomodulatory,” he said. “In some ways they very specifically enhance immunity. In other ways they calm down overimmunity.” Dr. Saxe noted that they did a sentinel study for the storm potential “and we didn’t see any evidence for it.”
“Not a crazy concept”
Dr. Saxe pointed out that one of the mushrooms in the combo they use — agarikon — was used to treat pulmonary infections 2,300 years ago.
“Hippocrates, the father of western medicine, used mushrooms,” he said. “Penicillin comes from fungi. It’s not a crazy concept. Most people who oppose this or are skeptics — to some extent, it’s a lack of information.”
Dr. Saxe explained that there are receptors on human cells that bind specific mushroom polysaccharides.
“There’s a hand-in-glove fit there,” Dr. Saxe said, and that’s one way mushrooms can modulate immune cell behavior, which could have an effect against SARS-CoV-2.
Daniel Kuritzkes, MD, chief of the division of infectious diseases at Brigham and Women’s Hospital in Boston, who was not part of the study, told this news organization that he wasn’t surprised the FDA approved moving forward with the trials.
“As long as you can demonstrate that there is a rationale for doing the trial and that you have some safety data or a plan to collect safety data, they are fairly liberal about doing early-phase studies. It would be a much different issue, I think, if they were proposing to do a study for actual licensing or approval of a drug,” Dr. Kuritzkes said.
As yet unanswered, he noted, is which component of the mushrooms or herbs is having the effect. It will be a challenge, he said, to know from one batch of the compound to the next that you have the same amount of material and that it’s going to have the same potency among lots.
Another challenge is how the mushrooms and herbs might interact with other therapies, Dr. Kuritzkes said.
He gave the example of St. John’s Wort, which has been problematic in HIV treatment.
“If someone is on certain HIV medicines and they also are taking St. John’s Wort, they basically are causing the liver to eat up the HIV drug and they don’t get adequate levels of the drug,” he said.
Though there are many challenges ahead, Dr. Kuritzkes acknowledged, but added that “this is a great starting point.”
He, too, pointed out that many traditional medicines were discovered from plants.
“The most famous of these is quinine, which came from cinchona bark that was used to treat malaria.” Dr. Kuritzkes said. Digitalis, often used to treat heart failure, comes from the fox glove plant, he added.
He said it’s important to remember that “people shouldn’t be seeking experimental therapies in place of proven therapies, they should be thinking of them in addition to proven therapies.»
A co-author reports an investment in the dietary supplement company Mycomedica Life Sciences, for which he also serves as an unpaid scientific adviser. Another co-author is a medical consultant for Evergreen Herbs and Medical Supplies. Dr. Hopp, Dr. Saxe, and Dr. Wilson have disclosed no relevant financial relationships. Dr. Kuritzkes consults for Merck, Gilead, and GlaxoSmithKline.
The U.S. Food and Drug Administration (FDA) approved the MACH-19 trials (the acronym for Mushrooms and Chinese Herbs for COVID-19) after researchers applied for approval in April.
The first two phase 1 randomized, double-blind, placebo-controlled trials have begun at UCLA and the University of California San Diego to treat COVID-19 patients quarantining at home with mild to moderate symptoms. A third trial is investigating the use of medicinal mushrooms as an adjuvant to COVID-19 vaccines.
The researchers have also launched a fourth trial testing the mushrooms against placebo as an adjunct to a COVID booster shot. It looks at the effect in people who have comorbidities that would reduce their vaccine response. An article in JAMA described the trials.
The two mushroom varieties being tested — turkey tail and agarikon — are available as over-the-counter supplements, according to the report. They are a separate class from hallucinogenic or “magic” mushrooms being tested for other uses in medicine.
“They are not even as psychoactive as a cup of tea,” Gordon Saxe, MD, PhD, MPH, principal investigator for the MACH-19 trials, told this news organization.
For each of the MACH-19 treatment trials, researchers plan to recruit 66 people who are quarantined at home with mild to moderate COVID-19 symptoms. Participants will be randomly assigned either to receive the mushroom combination, the Chinese herbs, or a placebo for 2 weeks, according to the JAMA paper.
D. Craig Hopp, PhD, deputy director of the division of extramural research at the National Center for Complementary and Integrative Health (NCCIH), told JAMA in an interview that he was “mildly concerned” about using mushrooms to treat people with active SARS-CoV-2 infection.
“We know that a cytokine storm poses the greatest risk of COVID mortality, not the virus itself,” Dr. Hopp said. “The danger is that an immune-stimulating agent like mushrooms might supercharge an individual’s immune response, leading to a cytokine storm.”
Stephen Wilson, PhD, an immunologist who consulted on the trials when he was chief operating officer of the La Jolla Institute for Immunology, says in the JAMA article that a cytokine storm is unlikely for these patients because the mushroom components “don’t mimic inflammatory cytokines.” Dr. Wilson is now chief innovations officer at Statera Biopharma.
“We think the mushrooms increase the number of immunologic opportunities to better see and respond to a specific threat. In the doses used, the mushrooms perturb the immune system in a good way but fall far short of driving hyper or sustained inflammation,” Dr. Wilson said.
Dr. Saxe said the FDA process was extensive and rigorous and FDA investigators also asked about potential cytokine storms before approving the trials. Cytokine storm is not an issue with a healthy response, Dr. Saxe pointed out. It’s a response that’s not balanced or modulated.
“Mushrooms are immunomodulatory,” he said. “In some ways they very specifically enhance immunity. In other ways they calm down overimmunity.” Dr. Saxe noted that they did a sentinel study for the storm potential “and we didn’t see any evidence for it.”
“Not a crazy concept”
Dr. Saxe pointed out that one of the mushrooms in the combo they use — agarikon — was used to treat pulmonary infections 2,300 years ago.
“Hippocrates, the father of western medicine, used mushrooms,” he said. “Penicillin comes from fungi. It’s not a crazy concept. Most people who oppose this or are skeptics — to some extent, it’s a lack of information.”
Dr. Saxe explained that there are receptors on human cells that bind specific mushroom polysaccharides.
“There’s a hand-in-glove fit there,” Dr. Saxe said, and that’s one way mushrooms can modulate immune cell behavior, which could have an effect against SARS-CoV-2.
Daniel Kuritzkes, MD, chief of the division of infectious diseases at Brigham and Women’s Hospital in Boston, who was not part of the study, told this news organization that he wasn’t surprised the FDA approved moving forward with the trials.
“As long as you can demonstrate that there is a rationale for doing the trial and that you have some safety data or a plan to collect safety data, they are fairly liberal about doing early-phase studies. It would be a much different issue, I think, if they were proposing to do a study for actual licensing or approval of a drug,” Dr. Kuritzkes said.
As yet unanswered, he noted, is which component of the mushrooms or herbs is having the effect. It will be a challenge, he said, to know from one batch of the compound to the next that you have the same amount of material and that it’s going to have the same potency among lots.
Another challenge is how the mushrooms and herbs might interact with other therapies, Dr. Kuritzkes said.
He gave the example of St. John’s Wort, which has been problematic in HIV treatment.
“If someone is on certain HIV medicines and they also are taking St. John’s Wort, they basically are causing the liver to eat up the HIV drug and they don’t get adequate levels of the drug,” he said.
Though there are many challenges ahead, Dr. Kuritzkes acknowledged, but added that “this is a great starting point.”
He, too, pointed out that many traditional medicines were discovered from plants.
“The most famous of these is quinine, which came from cinchona bark that was used to treat malaria.” Dr. Kuritzkes said. Digitalis, often used to treat heart failure, comes from the fox glove plant, he added.
He said it’s important to remember that “people shouldn’t be seeking experimental therapies in place of proven therapies, they should be thinking of them in addition to proven therapies.»
A co-author reports an investment in the dietary supplement company Mycomedica Life Sciences, for which he also serves as an unpaid scientific adviser. Another co-author is a medical consultant for Evergreen Herbs and Medical Supplies. Dr. Hopp, Dr. Saxe, and Dr. Wilson have disclosed no relevant financial relationships. Dr. Kuritzkes consults for Merck, Gilead, and GlaxoSmithKline.
The U.S. Food and Drug Administration (FDA) approved the MACH-19 trials (the acronym for Mushrooms and Chinese Herbs for COVID-19) after researchers applied for approval in April.
The first two phase 1 randomized, double-blind, placebo-controlled trials have begun at UCLA and the University of California San Diego to treat COVID-19 patients quarantining at home with mild to moderate symptoms. A third trial is investigating the use of medicinal mushrooms as an adjuvant to COVID-19 vaccines.
The researchers have also launched a fourth trial testing the mushrooms against placebo as an adjunct to a COVID booster shot. It looks at the effect in people who have comorbidities that would reduce their vaccine response. An article in JAMA described the trials.
The two mushroom varieties being tested — turkey tail and agarikon — are available as over-the-counter supplements, according to the report. They are a separate class from hallucinogenic or “magic” mushrooms being tested for other uses in medicine.
“They are not even as psychoactive as a cup of tea,” Gordon Saxe, MD, PhD, MPH, principal investigator for the MACH-19 trials, told this news organization.
For each of the MACH-19 treatment trials, researchers plan to recruit 66 people who are quarantined at home with mild to moderate COVID-19 symptoms. Participants will be randomly assigned either to receive the mushroom combination, the Chinese herbs, or a placebo for 2 weeks, according to the JAMA paper.
D. Craig Hopp, PhD, deputy director of the division of extramural research at the National Center for Complementary and Integrative Health (NCCIH), told JAMA in an interview that he was “mildly concerned” about using mushrooms to treat people with active SARS-CoV-2 infection.
“We know that a cytokine storm poses the greatest risk of COVID mortality, not the virus itself,” Dr. Hopp said. “The danger is that an immune-stimulating agent like mushrooms might supercharge an individual’s immune response, leading to a cytokine storm.”
Stephen Wilson, PhD, an immunologist who consulted on the trials when he was chief operating officer of the La Jolla Institute for Immunology, says in the JAMA article that a cytokine storm is unlikely for these patients because the mushroom components “don’t mimic inflammatory cytokines.” Dr. Wilson is now chief innovations officer at Statera Biopharma.
“We think the mushrooms increase the number of immunologic opportunities to better see and respond to a specific threat. In the doses used, the mushrooms perturb the immune system in a good way but fall far short of driving hyper or sustained inflammation,” Dr. Wilson said.
Dr. Saxe said the FDA process was extensive and rigorous and FDA investigators also asked about potential cytokine storms before approving the trials. Cytokine storm is not an issue with a healthy response, Dr. Saxe pointed out. It’s a response that’s not balanced or modulated.
“Mushrooms are immunomodulatory,” he said. “In some ways they very specifically enhance immunity. In other ways they calm down overimmunity.” Dr. Saxe noted that they did a sentinel study for the storm potential “and we didn’t see any evidence for it.”
“Not a crazy concept”
Dr. Saxe pointed out that one of the mushrooms in the combo they use — agarikon — was used to treat pulmonary infections 2,300 years ago.
“Hippocrates, the father of western medicine, used mushrooms,” he said. “Penicillin comes from fungi. It’s not a crazy concept. Most people who oppose this or are skeptics — to some extent, it’s a lack of information.”
Dr. Saxe explained that there are receptors on human cells that bind specific mushroom polysaccharides.
“There’s a hand-in-glove fit there,” Dr. Saxe said, and that’s one way mushrooms can modulate immune cell behavior, which could have an effect against SARS-CoV-2.
Daniel Kuritzkes, MD, chief of the division of infectious diseases at Brigham and Women’s Hospital in Boston, who was not part of the study, told this news organization that he wasn’t surprised the FDA approved moving forward with the trials.
“As long as you can demonstrate that there is a rationale for doing the trial and that you have some safety data or a plan to collect safety data, they are fairly liberal about doing early-phase studies. It would be a much different issue, I think, if they were proposing to do a study for actual licensing or approval of a drug,” Dr. Kuritzkes said.
As yet unanswered, he noted, is which component of the mushrooms or herbs is having the effect. It will be a challenge, he said, to know from one batch of the compound to the next that you have the same amount of material and that it’s going to have the same potency among lots.
Another challenge is how the mushrooms and herbs might interact with other therapies, Dr. Kuritzkes said.
He gave the example of St. John’s Wort, which has been problematic in HIV treatment.
“If someone is on certain HIV medicines and they also are taking St. John’s Wort, they basically are causing the liver to eat up the HIV drug and they don’t get adequate levels of the drug,” he said.
Though there are many challenges ahead, Dr. Kuritzkes acknowledged, but added that “this is a great starting point.”
He, too, pointed out that many traditional medicines were discovered from plants.
“The most famous of these is quinine, which came from cinchona bark that was used to treat malaria.” Dr. Kuritzkes said. Digitalis, often used to treat heart failure, comes from the fox glove plant, he added.
He said it’s important to remember that “people shouldn’t be seeking experimental therapies in place of proven therapies, they should be thinking of them in addition to proven therapies.»
A co-author reports an investment in the dietary supplement company Mycomedica Life Sciences, for which he also serves as an unpaid scientific adviser. Another co-author is a medical consultant for Evergreen Herbs and Medical Supplies. Dr. Hopp, Dr. Saxe, and Dr. Wilson have disclosed no relevant financial relationships. Dr. Kuritzkes consults for Merck, Gilead, and GlaxoSmithKline.
FROM JAMA
As constituents clamor for ivermectin, Republican politicians embrace the cause
When state senators in South Carolina held two hearings in September about COVID-19 treatments, they got an earful on the benefits of ivermectin — which many of the lawmakers echoed, sharing experiences of their own loved ones.
The demands for access to the drug were loud and insistent, despite federal regulators’ recent warning against using the drug to treat COVID.
Ivermectin is a generic drug that has been used for decades to treat river blindness, scabies, and even head lice. Veterinarians also use it, in different formulations and dosages, to treat animals for parasites like worms.
At one of the South Carolina hearings, Pressley Stutts III reminded the panel that his father, a prominent GOP leader in the state, had died of COVID a month earlier. He believed ivermectin could have helped him. But doctors at the hospital wouldn’t discuss it.
“I went every bit as far as I could without getting myself thrown in jail trying to save my father’s life,” he told the panel, as lawmakers offered condolences.
“What is going on here?” he asked, with the passion in his voice growing. “My dad’s dead!”
After the pandemic began, scientists launched clinical trials to see if ivermectin could help as a treatment for COVID. Some are still ongoing. But providers in mainstream medicine have rejected it as a COVID treatment, citing the poor quality of the studies to date, and two notorious “preprint” studies that were circulated before they were peer-reviewed, and later taken off the internet because of inaccurate and flawed data.
On Aug. 26, the Centers for Disease Control and Prevention advised clinicians not to use ivermectin, citing insufficient evidence of benefit and pointing out that unauthorized use had led to accidental poisonings. Vaccination, the CDC reiterated, is still the best way to avoid serious illness and death from the coronavirus.
But many Americans remain convinced ivermectin could be beneficial, and some politicians appear to be listening to them.
“If we have medications out here that are working — or seem to be working — I think it’s absolutely horrible that we’re not trying them,” said Republican state Sen. Tom Corbin in South Carolina. He questioned doctors who had come to the Statehouse to counter efforts to move ivermectin into mainstream use.
The doctors challenged the implied insult that they weren’t following best practices: “Any implication that any of us would do anything to withhold effective treatments from our patients is really insulting to our profession,” said Dr. Annie Andrews, a professor at the Medical University of South Carolina who has cared for COVID patients throughout the pandemic.
Instead of listening to the medical consensus, some politicians in states like South Carolina seem to be taking cues from doctors on the fringe. During one September hearing, state senators patched in a call from Dr. Pierre Kory.
Last year, Dr. Kory started a nonprofit called the Front Line COVID-19 Critical Care Alliance, which promotes ivermectin. He said he’s not making money by prescribing the drug, though the nonprofit does solicit donations and has not yet filed required financial documents with the IRS.
Dr. Kory acknowledged his medical opinions have landed him on “an island.”
He first testified about ivermectin to a U.S. Senate committee in December. That video went viral. Although it was taken down by YouTube, his Senate testimony prompted patients across the country to ask for ivermectin when they fell ill.
By late August, outpatient prescriptions had jumped 24-fold. Calls to poison control hotlines had tripled, mostly related to people taking ivermectin formulations meant for livestock.
Dr. Kory said he has effectively lost two jobs over his views on ivermectin. At his current hospital in Wisconsin, where he runs the intensive care unit two weeks a month, managers called him to a meeting in September, where he was informed he could no longer prescribe ivermectin. He’d been giving it to “every patient with COVID,” he said.
“After the pharma-geddon that was unleashed, yeah, they shut it down,” he told the South Carolina lawmakers. “And I will tell you that many hospitals across the country had already shut it down months ago.”
Framing the ivermectin fight as a battle against faceless federal agencies and big pharmaceutical corporations appealed to Americans already suspicious of the science behind the pandemic and the approved COVID vaccines.
Dr. Kory suggests success stories with COVID treatments in other parts of the world have been suppressed to instead promote the vaccines.
In an interview with NPR, Dr. Kory said he regrets the flashpoint he helped ignite.
“I feel really bad for the patients, and I feel really bad for the doctors,” he told NPR. “Both of them — both the patients and doctors — are trapped.”
Patients are still demanding the treatment, but doctors sympathetic to their wishes are being told by their health systems not to try it.
Now conservatives in elected office are sensing political payoff if they step in to help patients get the drug. State legislatures, including those in Tennessee and Alaska, are debating various ways to increase access to ivermectin — with proposals such as shielding doctors from repercussions for prescribing it, or forcing pharmacists to fill questionable prescriptions.
The Montana State News Bureau reported that the state’s Republican attorney general dispatched a state trooper to a hospital in Helena where a politically connected patient was dying of COVID. Her family was asking for ivermectin.
In a statement, St. Peter’s Hospital said doctors and nurses were “harassed and threatened by three public officials.”
“These officials have no medical training or experience, yet they were insisting our providers give treatments for COVID-19 that are not authorized, clinically approved, or within the guidelines established by the FDA and the CDC,” the statement added.
On Oct. 14, the Republican attorney general in Nebraska addressed the controversy, issuing a nearly 50-page legal opinion arguing that doctors who consider the “off-label” use of ivermectin and hydroxychloroquine for COVID are acting within the parameters of their state medical licenses, as long as the physician obtains appropriate informed consent from a patient.
Some patients have filed lawsuits to obtain ivermectin, with mixed success. A patient in Illinois was denied. But other hospitals, including one in Ohio, have been forced to administer the drug against the objections of their physicians.
Even as they gain powerful political supporters, some ivermectin fans say they’re now avoiding the health care system — because they’ve lost faith in it.
Lesa Berry, of Richmond, Va., had a friend who died earlier this year of COVID. The doctors refused to use ivermectin, despite requests from Ms. Berry and the patient’s daughter.
They know better now, she said.
“My first attempt would have been to keep her out of the hospital,” Ms. Berry said. “Because right now when you go to the hospital, they only give you what’s on the CDC protocol.”
Ms. Berry and her husband have purchased their own supply of ivermectin, which they keep at home.
This story is from a partnership that includes NPR, Nashville Public Radio and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When state senators in South Carolina held two hearings in September about COVID-19 treatments, they got an earful on the benefits of ivermectin — which many of the lawmakers echoed, sharing experiences of their own loved ones.
The demands for access to the drug were loud and insistent, despite federal regulators’ recent warning against using the drug to treat COVID.
Ivermectin is a generic drug that has been used for decades to treat river blindness, scabies, and even head lice. Veterinarians also use it, in different formulations and dosages, to treat animals for parasites like worms.
At one of the South Carolina hearings, Pressley Stutts III reminded the panel that his father, a prominent GOP leader in the state, had died of COVID a month earlier. He believed ivermectin could have helped him. But doctors at the hospital wouldn’t discuss it.
“I went every bit as far as I could without getting myself thrown in jail trying to save my father’s life,” he told the panel, as lawmakers offered condolences.
“What is going on here?” he asked, with the passion in his voice growing. “My dad’s dead!”
After the pandemic began, scientists launched clinical trials to see if ivermectin could help as a treatment for COVID. Some are still ongoing. But providers in mainstream medicine have rejected it as a COVID treatment, citing the poor quality of the studies to date, and two notorious “preprint” studies that were circulated before they were peer-reviewed, and later taken off the internet because of inaccurate and flawed data.
On Aug. 26, the Centers for Disease Control and Prevention advised clinicians not to use ivermectin, citing insufficient evidence of benefit and pointing out that unauthorized use had led to accidental poisonings. Vaccination, the CDC reiterated, is still the best way to avoid serious illness and death from the coronavirus.
But many Americans remain convinced ivermectin could be beneficial, and some politicians appear to be listening to them.
“If we have medications out here that are working — or seem to be working — I think it’s absolutely horrible that we’re not trying them,” said Republican state Sen. Tom Corbin in South Carolina. He questioned doctors who had come to the Statehouse to counter efforts to move ivermectin into mainstream use.
The doctors challenged the implied insult that they weren’t following best practices: “Any implication that any of us would do anything to withhold effective treatments from our patients is really insulting to our profession,” said Dr. Annie Andrews, a professor at the Medical University of South Carolina who has cared for COVID patients throughout the pandemic.
Instead of listening to the medical consensus, some politicians in states like South Carolina seem to be taking cues from doctors on the fringe. During one September hearing, state senators patched in a call from Dr. Pierre Kory.
Last year, Dr. Kory started a nonprofit called the Front Line COVID-19 Critical Care Alliance, which promotes ivermectin. He said he’s not making money by prescribing the drug, though the nonprofit does solicit donations and has not yet filed required financial documents with the IRS.
Dr. Kory acknowledged his medical opinions have landed him on “an island.”
He first testified about ivermectin to a U.S. Senate committee in December. That video went viral. Although it was taken down by YouTube, his Senate testimony prompted patients across the country to ask for ivermectin when they fell ill.
By late August, outpatient prescriptions had jumped 24-fold. Calls to poison control hotlines had tripled, mostly related to people taking ivermectin formulations meant for livestock.
Dr. Kory said he has effectively lost two jobs over his views on ivermectin. At his current hospital in Wisconsin, where he runs the intensive care unit two weeks a month, managers called him to a meeting in September, where he was informed he could no longer prescribe ivermectin. He’d been giving it to “every patient with COVID,” he said.
“After the pharma-geddon that was unleashed, yeah, they shut it down,” he told the South Carolina lawmakers. “And I will tell you that many hospitals across the country had already shut it down months ago.”
Framing the ivermectin fight as a battle against faceless federal agencies and big pharmaceutical corporations appealed to Americans already suspicious of the science behind the pandemic and the approved COVID vaccines.
Dr. Kory suggests success stories with COVID treatments in other parts of the world have been suppressed to instead promote the vaccines.
In an interview with NPR, Dr. Kory said he regrets the flashpoint he helped ignite.
“I feel really bad for the patients, and I feel really bad for the doctors,” he told NPR. “Both of them — both the patients and doctors — are trapped.”
Patients are still demanding the treatment, but doctors sympathetic to their wishes are being told by their health systems not to try it.
Now conservatives in elected office are sensing political payoff if they step in to help patients get the drug. State legislatures, including those in Tennessee and Alaska, are debating various ways to increase access to ivermectin — with proposals such as shielding doctors from repercussions for prescribing it, or forcing pharmacists to fill questionable prescriptions.
The Montana State News Bureau reported that the state’s Republican attorney general dispatched a state trooper to a hospital in Helena where a politically connected patient was dying of COVID. Her family was asking for ivermectin.
In a statement, St. Peter’s Hospital said doctors and nurses were “harassed and threatened by three public officials.”
“These officials have no medical training or experience, yet they were insisting our providers give treatments for COVID-19 that are not authorized, clinically approved, or within the guidelines established by the FDA and the CDC,” the statement added.
On Oct. 14, the Republican attorney general in Nebraska addressed the controversy, issuing a nearly 50-page legal opinion arguing that doctors who consider the “off-label” use of ivermectin and hydroxychloroquine for COVID are acting within the parameters of their state medical licenses, as long as the physician obtains appropriate informed consent from a patient.
Some patients have filed lawsuits to obtain ivermectin, with mixed success. A patient in Illinois was denied. But other hospitals, including one in Ohio, have been forced to administer the drug against the objections of their physicians.
Even as they gain powerful political supporters, some ivermectin fans say they’re now avoiding the health care system — because they’ve lost faith in it.
Lesa Berry, of Richmond, Va., had a friend who died earlier this year of COVID. The doctors refused to use ivermectin, despite requests from Ms. Berry and the patient’s daughter.
They know better now, she said.
“My first attempt would have been to keep her out of the hospital,” Ms. Berry said. “Because right now when you go to the hospital, they only give you what’s on the CDC protocol.”
Ms. Berry and her husband have purchased their own supply of ivermectin, which they keep at home.
This story is from a partnership that includes NPR, Nashville Public Radio and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
When state senators in South Carolina held two hearings in September about COVID-19 treatments, they got an earful on the benefits of ivermectin — which many of the lawmakers echoed, sharing experiences of their own loved ones.
The demands for access to the drug were loud and insistent, despite federal regulators’ recent warning against using the drug to treat COVID.
Ivermectin is a generic drug that has been used for decades to treat river blindness, scabies, and even head lice. Veterinarians also use it, in different formulations and dosages, to treat animals for parasites like worms.
At one of the South Carolina hearings, Pressley Stutts III reminded the panel that his father, a prominent GOP leader in the state, had died of COVID a month earlier. He believed ivermectin could have helped him. But doctors at the hospital wouldn’t discuss it.
“I went every bit as far as I could without getting myself thrown in jail trying to save my father’s life,” he told the panel, as lawmakers offered condolences.
“What is going on here?” he asked, with the passion in his voice growing. “My dad’s dead!”
After the pandemic began, scientists launched clinical trials to see if ivermectin could help as a treatment for COVID. Some are still ongoing. But providers in mainstream medicine have rejected it as a COVID treatment, citing the poor quality of the studies to date, and two notorious “preprint” studies that were circulated before they were peer-reviewed, and later taken off the internet because of inaccurate and flawed data.
On Aug. 26, the Centers for Disease Control and Prevention advised clinicians not to use ivermectin, citing insufficient evidence of benefit and pointing out that unauthorized use had led to accidental poisonings. Vaccination, the CDC reiterated, is still the best way to avoid serious illness and death from the coronavirus.
But many Americans remain convinced ivermectin could be beneficial, and some politicians appear to be listening to them.
“If we have medications out here that are working — or seem to be working — I think it’s absolutely horrible that we’re not trying them,” said Republican state Sen. Tom Corbin in South Carolina. He questioned doctors who had come to the Statehouse to counter efforts to move ivermectin into mainstream use.
The doctors challenged the implied insult that they weren’t following best practices: “Any implication that any of us would do anything to withhold effective treatments from our patients is really insulting to our profession,” said Dr. Annie Andrews, a professor at the Medical University of South Carolina who has cared for COVID patients throughout the pandemic.
Instead of listening to the medical consensus, some politicians in states like South Carolina seem to be taking cues from doctors on the fringe. During one September hearing, state senators patched in a call from Dr. Pierre Kory.
Last year, Dr. Kory started a nonprofit called the Front Line COVID-19 Critical Care Alliance, which promotes ivermectin. He said he’s not making money by prescribing the drug, though the nonprofit does solicit donations and has not yet filed required financial documents with the IRS.
Dr. Kory acknowledged his medical opinions have landed him on “an island.”
He first testified about ivermectin to a U.S. Senate committee in December. That video went viral. Although it was taken down by YouTube, his Senate testimony prompted patients across the country to ask for ivermectin when they fell ill.
By late August, outpatient prescriptions had jumped 24-fold. Calls to poison control hotlines had tripled, mostly related to people taking ivermectin formulations meant for livestock.
Dr. Kory said he has effectively lost two jobs over his views on ivermectin. At his current hospital in Wisconsin, where he runs the intensive care unit two weeks a month, managers called him to a meeting in September, where he was informed he could no longer prescribe ivermectin. He’d been giving it to “every patient with COVID,” he said.
“After the pharma-geddon that was unleashed, yeah, they shut it down,” he told the South Carolina lawmakers. “And I will tell you that many hospitals across the country had already shut it down months ago.”
Framing the ivermectin fight as a battle against faceless federal agencies and big pharmaceutical corporations appealed to Americans already suspicious of the science behind the pandemic and the approved COVID vaccines.
Dr. Kory suggests success stories with COVID treatments in other parts of the world have been suppressed to instead promote the vaccines.
In an interview with NPR, Dr. Kory said he regrets the flashpoint he helped ignite.
“I feel really bad for the patients, and I feel really bad for the doctors,” he told NPR. “Both of them — both the patients and doctors — are trapped.”
Patients are still demanding the treatment, but doctors sympathetic to their wishes are being told by their health systems not to try it.
Now conservatives in elected office are sensing political payoff if they step in to help patients get the drug. State legislatures, including those in Tennessee and Alaska, are debating various ways to increase access to ivermectin — with proposals such as shielding doctors from repercussions for prescribing it, or forcing pharmacists to fill questionable prescriptions.
The Montana State News Bureau reported that the state’s Republican attorney general dispatched a state trooper to a hospital in Helena where a politically connected patient was dying of COVID. Her family was asking for ivermectin.
In a statement, St. Peter’s Hospital said doctors and nurses were “harassed and threatened by three public officials.”
“These officials have no medical training or experience, yet they were insisting our providers give treatments for COVID-19 that are not authorized, clinically approved, or within the guidelines established by the FDA and the CDC,” the statement added.
On Oct. 14, the Republican attorney general in Nebraska addressed the controversy, issuing a nearly 50-page legal opinion arguing that doctors who consider the “off-label” use of ivermectin and hydroxychloroquine for COVID are acting within the parameters of their state medical licenses, as long as the physician obtains appropriate informed consent from a patient.
Some patients have filed lawsuits to obtain ivermectin, with mixed success. A patient in Illinois was denied. But other hospitals, including one in Ohio, have been forced to administer the drug against the objections of their physicians.
Even as they gain powerful political supporters, some ivermectin fans say they’re now avoiding the health care system — because they’ve lost faith in it.
Lesa Berry, of Richmond, Va., had a friend who died earlier this year of COVID. The doctors refused to use ivermectin, despite requests from Ms. Berry and the patient’s daughter.
They know better now, she said.
“My first attempt would have been to keep her out of the hospital,” Ms. Berry said. “Because right now when you go to the hospital, they only give you what’s on the CDC protocol.”
Ms. Berry and her husband have purchased their own supply of ivermectin, which they keep at home.
This story is from a partnership that includes NPR, Nashville Public Radio and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.








