User login
FDA panel backs first pill for COVID-19 by a small margin
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
We physicians must pull together as a knowledge community
The COVID-19 pandemic is a biosocial phenomenon. Patients and doctors alike find themselves assigned to groups designated as responsible and wise, or selfish and irrational, based strictly upon their personal assessments of medical risk. This trend in our culture is represented by threats of disciplinary action issued by medical regulators against physicians who are perceived to be undermining the public health message by spreading “misinformation.”
Our review of the literature reveals many references to “misinformation” but no definition narrow and precise enough to be interpreted consistently in a disciplinary environment. More pressing, this ambiguous word’s use is correlated with negative meaning and innuendo, often discrediting valuable information a priori without actual data points.
The most basic definition available is Merriam Webster’s: “incorrect or misleading information.” This definition includes no point of reference against which competing scientific claims can be measured.
Claudia E. Haupt, PhD, a political scientist and law professor, articulates a useful framework for understanding the relationship between medicine and state regulators. In the Yale Law Journal, Dr. Haupt wrote: “Knowledge communities have specialized expertise and are closest to those affected; they must have the freedom to work things out for themselves. The professions as knowledge communities have a fundamental interest in not having the state (or anyone else, for that matter) corrupt or distort what amounts to the state of the art in their respective fields.”
Injecting the artificial term “misinformation” into the science information ecosystem obfuscates and impedes the very ability of this vital knowledge community to perform its raison d’être. , rather than attending to healing or promoting progress.
Time has certainly shown us that science is anything but settled on all things COVID. If the scientific community accepts disrespect as the response of choice to difference of opinion and practice, we lose the trust in one another as colleagues; we need to keep scientific inquiry and exploration alive. Curiosity, equanimity, and tolerance are key components of the professional attitude as we deftly maneuver against the virus together.
In the face of deadly disease, it is especially imperative that intelligent, thoughtful, highly respected scientists, researchers, and physicians have room to safely share their knowledge and clinical experience. The Association of American Physicians and Surgeons has published a statement on scientific integrity that can be used as a measuring stick for claims about misinformation in medicine. We call on physicians to pull together as a knowledge community. Kindness and respect for patients starts with kindness and respect for one another as colleagues.
Dr. Kohanski is in private practice in Somerset, N.J., and is a diplomate of the American Board of Psychiatry & Neurology. She disclosed no relevant financial relationships. Dr. Emmons is part-time clinical associate professor in the department of psychiatry at the University of Vermont, Burlington, and is a past chair of the Ethics Committee for the Vermont District Branch of the American Psychiatric Association. He is in private practice in Moretown, Vt., and disclosed no relevant financial relationships.
The COVID-19 pandemic is a biosocial phenomenon. Patients and doctors alike find themselves assigned to groups designated as responsible and wise, or selfish and irrational, based strictly upon their personal assessments of medical risk. This trend in our culture is represented by threats of disciplinary action issued by medical regulators against physicians who are perceived to be undermining the public health message by spreading “misinformation.”
Our review of the literature reveals many references to “misinformation” but no definition narrow and precise enough to be interpreted consistently in a disciplinary environment. More pressing, this ambiguous word’s use is correlated with negative meaning and innuendo, often discrediting valuable information a priori without actual data points.
The most basic definition available is Merriam Webster’s: “incorrect or misleading information.” This definition includes no point of reference against which competing scientific claims can be measured.
Claudia E. Haupt, PhD, a political scientist and law professor, articulates a useful framework for understanding the relationship between medicine and state regulators. In the Yale Law Journal, Dr. Haupt wrote: “Knowledge communities have specialized expertise and are closest to those affected; they must have the freedom to work things out for themselves. The professions as knowledge communities have a fundamental interest in not having the state (or anyone else, for that matter) corrupt or distort what amounts to the state of the art in their respective fields.”
Injecting the artificial term “misinformation” into the science information ecosystem obfuscates and impedes the very ability of this vital knowledge community to perform its raison d’être. , rather than attending to healing or promoting progress.
Time has certainly shown us that science is anything but settled on all things COVID. If the scientific community accepts disrespect as the response of choice to difference of opinion and practice, we lose the trust in one another as colleagues; we need to keep scientific inquiry and exploration alive. Curiosity, equanimity, and tolerance are key components of the professional attitude as we deftly maneuver against the virus together.
In the face of deadly disease, it is especially imperative that intelligent, thoughtful, highly respected scientists, researchers, and physicians have room to safely share their knowledge and clinical experience. The Association of American Physicians and Surgeons has published a statement on scientific integrity that can be used as a measuring stick for claims about misinformation in medicine. We call on physicians to pull together as a knowledge community. Kindness and respect for patients starts with kindness and respect for one another as colleagues.
Dr. Kohanski is in private practice in Somerset, N.J., and is a diplomate of the American Board of Psychiatry & Neurology. She disclosed no relevant financial relationships. Dr. Emmons is part-time clinical associate professor in the department of psychiatry at the University of Vermont, Burlington, and is a past chair of the Ethics Committee for the Vermont District Branch of the American Psychiatric Association. He is in private practice in Moretown, Vt., and disclosed no relevant financial relationships.
The COVID-19 pandemic is a biosocial phenomenon. Patients and doctors alike find themselves assigned to groups designated as responsible and wise, or selfish and irrational, based strictly upon their personal assessments of medical risk. This trend in our culture is represented by threats of disciplinary action issued by medical regulators against physicians who are perceived to be undermining the public health message by spreading “misinformation.”
Our review of the literature reveals many references to “misinformation” but no definition narrow and precise enough to be interpreted consistently in a disciplinary environment. More pressing, this ambiguous word’s use is correlated with negative meaning and innuendo, often discrediting valuable information a priori without actual data points.
The most basic definition available is Merriam Webster’s: “incorrect or misleading information.” This definition includes no point of reference against which competing scientific claims can be measured.
Claudia E. Haupt, PhD, a political scientist and law professor, articulates a useful framework for understanding the relationship between medicine and state regulators. In the Yale Law Journal, Dr. Haupt wrote: “Knowledge communities have specialized expertise and are closest to those affected; they must have the freedom to work things out for themselves. The professions as knowledge communities have a fundamental interest in not having the state (or anyone else, for that matter) corrupt or distort what amounts to the state of the art in their respective fields.”
Injecting the artificial term “misinformation” into the science information ecosystem obfuscates and impedes the very ability of this vital knowledge community to perform its raison d’être. , rather than attending to healing or promoting progress.
Time has certainly shown us that science is anything but settled on all things COVID. If the scientific community accepts disrespect as the response of choice to difference of opinion and practice, we lose the trust in one another as colleagues; we need to keep scientific inquiry and exploration alive. Curiosity, equanimity, and tolerance are key components of the professional attitude as we deftly maneuver against the virus together.
In the face of deadly disease, it is especially imperative that intelligent, thoughtful, highly respected scientists, researchers, and physicians have room to safely share their knowledge and clinical experience. The Association of American Physicians and Surgeons has published a statement on scientific integrity that can be used as a measuring stick for claims about misinformation in medicine. We call on physicians to pull together as a knowledge community. Kindness and respect for patients starts with kindness and respect for one another as colleagues.
Dr. Kohanski is in private practice in Somerset, N.J., and is a diplomate of the American Board of Psychiatry & Neurology. She disclosed no relevant financial relationships. Dr. Emmons is part-time clinical associate professor in the department of psychiatry at the University of Vermont, Burlington, and is a past chair of the Ethics Committee for the Vermont District Branch of the American Psychiatric Association. He is in private practice in Moretown, Vt., and disclosed no relevant financial relationships.
Merck’s COVID-19 pill may be less effective than first hoped
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
Pfizer COVID vaccine is 100% effective in adolescents: Study
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Pfizer announced on Nov. 22 that its COVID-19 vaccine provided long-term protection against the virus in a late-stage clinical trial among adolescents ages 12-15.
A two-dose series was 100% effective against COVID-19, which was measured between 7 days and 4 months after the second dose.
“As the global health community works to increase the number of vaccinated people around the world, these additional data provide further confidence in our vaccine safety and effectiveness profile in adolescents,” Albert Bourla, PhD, chairman and CEO of Pfizer, said in a statement.
The clinical trial researchers found no serious safety concerns while following patients for 6 months. The adverse events were consistent with other clinical safety data for the vaccine, the company said.
Pfizer will incorporate the data into its submissions for full regulatory approval of the vaccine for ages 12-15 in the United States and worldwide.
The company will request clearance for a 30-mcg dose of the vaccines for ages 12 and older. The shot received FDA emergency use authorization for ages 12-15 in May and full approval for ages 16 and older in August.
The study included 2,228 clinical trial participants who were monitored between November 2020 and September 2021. There were 30 confirmed symptomatic cases of COVID-19 in the placebo group that didn’t receive the vaccine and 0 COVID-19 cases among the vaccinated group.
The efficacy was consistently high across gender, race, ethnicity, and health conditions, the company said.
“This is especially important as we see rates of COVID-19 climbing in this age group in some regions, while vaccine uptake has slowed,” Mr. Bourla said. “We look forward to sharing these data with the FDA and other regulators.”
A version of this article first appeared on WebMD.com.
Fueling an ‘already raging fire’: Fifth COVID surge approaches
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
“A significant rise in cases just before Thanksgiving is not what we want to be seeing,” said Stephen Kissler, PhD, a postdoctoral researcher and data modeler at the Harvard TH Chan School of Public Health in Boston.
Dr. Kissler said he’d rather see increases in daily cases coming 2 weeks after busy travel periods, as that would mean they could come back down as people returned to their routines.
Seeing big increases in cases ahead of the holidays, he said, “is sort of like adding fuel to an already raging fire.”
Last winter, vaccines hadn’t been rolled out as the nation prepared for Thanksgiving. COVID-19 was burning through family gatherings.
But now that two-thirds of Americans over age 5 are fully vaccinated and booster doses are approved for all adults, will a rise in cases translate, once again, into a strain on our still thinly stretched healthcare system?
Experts say the vaccines are keeping people out of the hospital, which will help. And new antiviral pills are coming that seem to be able to cut a COVID-19 infection off at the knees, at least according to early data. A U.S. Food and Drug Administration panel meets next week to discuss the first application for a pill by Merck.
But experts caution that the coming surge will almost certainly tax hospitals again, especially in areas with lower vaccination rates.
And even states where blood testing shows that significant numbers of people have antibodies after a COVID-19 infection aren’t out of the woods, in part because we still don’t know how long the immunity generated by infection may last.
“Erosion of immunity”
“It’s hard to know how much risk is out there,” said Jeffrey Shaman, PhD, professor of environmental health sciences at Columbia University’s Mailman School of Public Health in New York City, who has been modeling the trajectory of the pandemic.
“We’re estimating, unfortunately, and we have for many weeks now, that there is an erosion of immunity,” Dr. Shaman said. “I think it could get bad. How bad? I’m not sure.”
Ali Mokdad, PhD, a professor of health metrics sciences at the University of Washington’s Institute for Health Metrics and Evaluation in Seattle, agrees.
Because there are so few studies on how long immunity from natural infection lasts, Dr. Mokdad and his colleagues are assuming that waning immunity after infection happens at least as quickly as it does after vaccination.
Their model is predicting that the average number of daily cases will peak at around 100,000, with another 100,000 going undetected, and will stay at that level until the end of January, as some states recover from their surges and others pick up steam.
While the number of daily deaths won’t climb to the heights seen during the summer surge, Dr. Mokdad said their model is predicting that daily deaths will climb again to about 1,200 a day.
“We are almost there right now, and it will be with us for a while,” he said. “We are predicting 881,000 deaths by March 1.”
The United States has currently recorded 773,000 COVID-19 deaths, so Dr. Mokdad is predicting about 120,000 more deaths between now and then.
He said his model shows that more than half of those deaths could be prevented if 95% of Americans wore their masks while in close proximity to strangers.
Currently, only about 36% of Americans are consistently wearing masks, according to surveys. While people are moving around more now, mobility is at prepandemic levels in some states.
“The rise that you are seeing right now is high mobility and low mask wearing in the United States,” Dr. Mokdad said.
The solution, he said, is for all adults to get another dose of vaccine — he doesn’t like calling it a booster.
“Because they’re vaccinated and they have two doses they have a false sense of security that they are protected. We needed to come ahead of it immediately and say you need a third dose, and we were late to do so,” Dr. Mokdad said.
A version of this article first appeared on Medscape.com.
30% of docs say they don’t want own kids 5-11 to get COVID vaccine
A Medscape
Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did not want their kids to get the COVID-19 vaccine; 13% were unsure. Among pharmacists, 31% said they would not get them vaccinated and 9% were unsure.
Clinicians were more likely to want vaccinations for their kids 5-11 than were 510 consumers polled by WebMD at the same time. Overall, 49% of the consumers who had kids that age did not want them to get the COVID-19 vaccine.
On November 2, Centers for Disease Control and Prevention (CDC) Director Rochelle P. Walensky, MD, MPH, endorsed the CDC Advisory Committee on Immunization Practices’ recommendation that children 5-11 be vaccinated with the Pfizer-BioNTech pediatric vaccine. That decision expanded vaccine recommendations to about 28 million children in the United States.
The CDC states that, in clinical trials, the Pfizer vaccine had more than 90% efficacy in preventing laboratory-confirmed COVID-19 infection in children 5 to 15 years old, and that the immune response in children ages 5-15 equaled the immune response in people 16 to 25 years old.
The Medscape poll, fielded from November 3 to November 11, included 325 physicians, 793 nurses/APRNs, and 151 pharmacists.
How safe is the vaccine?
Clinicians were asked how confident they were that the vaccine is safe for that age group, and 66% of physicians, 52% of nurses/APRNs, and 66% of pharmacists said they were somewhat or very confident.
Among consumers overall in the WebMD poll, 56% said they were confident or somewhat confident that the vaccine is safe in that age group.
Among adolescents and young adults, rare cases of myocarditis and pericarditis in adolescents and young adults have been reported. According to the CDC, “[I]n one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12-17 years.”
Known and potential benefits of COVID-19 vaccination outweigh the risks, including the possible risk for myocarditis or pericarditis, the CDC states.
Across clinician types, women edged out their male counterparts on confidence in the vaccine’ s safety: 71% vs 65% among physicians, 55% vs 45% among nurses/APRNs, and 68% vs 60% among pharmacists.
Among both physicians and nurses, younger physicians (under 45) tended to have greater confidence in the vaccine’ s safety: 72% vs 64% (physicians), 54% vs 51% (nurses/APRNs), and 71% vs 59% (pharmacists).
The difference in confidence was clear between vaccinated and unvaccinated physicians. All of the unvaccinated physicians who responded to the poll said they had no confidence in the vaccine for kids. Among unvaccinated nurses/APRNs, 2% were somewhat confident in the vaccine for kids under 12.
Knowledge about smaller dosage
The clinicians were asked about whether they were aware, before reading the poll question, that the Pfizer vaccine for children and the proposed Moderna vaccine for children in this age group (5-11) would have a different dosage.
The dose for kids 5-11 is 10 micrograms rather than 30 micrograms for people at least 12 years old. Children 5-11 receive a second dose 21 days or more after their first shot. The formulation comes with an orange cap, and a smaller needle is used.
Knowledge on the lower dose was highest among pharmacists (91% said they knew), followed by physicians (84%) and nurses (79%).
The poll also asked whether the COVID-19 vaccine should be added to the list of childhood immunizations. Responses varied widely and uncertainty was evident.
Notably, female physicians were more likely to say it should be added to the list of immunizations than were their male counterparts: 46% vs 35% (physicians), 26% vs 22% (nurses/APRNs), and 33% vs 30% (pharmacists).
A version of this article first appeared on Medscape.com.
A Medscape
Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did not want their kids to get the COVID-19 vaccine; 13% were unsure. Among pharmacists, 31% said they would not get them vaccinated and 9% were unsure.
Clinicians were more likely to want vaccinations for their kids 5-11 than were 510 consumers polled by WebMD at the same time. Overall, 49% of the consumers who had kids that age did not want them to get the COVID-19 vaccine.
On November 2, Centers for Disease Control and Prevention (CDC) Director Rochelle P. Walensky, MD, MPH, endorsed the CDC Advisory Committee on Immunization Practices’ recommendation that children 5-11 be vaccinated with the Pfizer-BioNTech pediatric vaccine. That decision expanded vaccine recommendations to about 28 million children in the United States.
The CDC states that, in clinical trials, the Pfizer vaccine had more than 90% efficacy in preventing laboratory-confirmed COVID-19 infection in children 5 to 15 years old, and that the immune response in children ages 5-15 equaled the immune response in people 16 to 25 years old.
The Medscape poll, fielded from November 3 to November 11, included 325 physicians, 793 nurses/APRNs, and 151 pharmacists.
How safe is the vaccine?
Clinicians were asked how confident they were that the vaccine is safe for that age group, and 66% of physicians, 52% of nurses/APRNs, and 66% of pharmacists said they were somewhat or very confident.
Among consumers overall in the WebMD poll, 56% said they were confident or somewhat confident that the vaccine is safe in that age group.
Among adolescents and young adults, rare cases of myocarditis and pericarditis in adolescents and young adults have been reported. According to the CDC, “[I]n one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12-17 years.”
Known and potential benefits of COVID-19 vaccination outweigh the risks, including the possible risk for myocarditis or pericarditis, the CDC states.
Across clinician types, women edged out their male counterparts on confidence in the vaccine’ s safety: 71% vs 65% among physicians, 55% vs 45% among nurses/APRNs, and 68% vs 60% among pharmacists.
Among both physicians and nurses, younger physicians (under 45) tended to have greater confidence in the vaccine’ s safety: 72% vs 64% (physicians), 54% vs 51% (nurses/APRNs), and 71% vs 59% (pharmacists).
The difference in confidence was clear between vaccinated and unvaccinated physicians. All of the unvaccinated physicians who responded to the poll said they had no confidence in the vaccine for kids. Among unvaccinated nurses/APRNs, 2% were somewhat confident in the vaccine for kids under 12.
Knowledge about smaller dosage
The clinicians were asked about whether they were aware, before reading the poll question, that the Pfizer vaccine for children and the proposed Moderna vaccine for children in this age group (5-11) would have a different dosage.
The dose for kids 5-11 is 10 micrograms rather than 30 micrograms for people at least 12 years old. Children 5-11 receive a second dose 21 days or more after their first shot. The formulation comes with an orange cap, and a smaller needle is used.
Knowledge on the lower dose was highest among pharmacists (91% said they knew), followed by physicians (84%) and nurses (79%).
The poll also asked whether the COVID-19 vaccine should be added to the list of childhood immunizations. Responses varied widely and uncertainty was evident.
Notably, female physicians were more likely to say it should be added to the list of immunizations than were their male counterparts: 46% vs 35% (physicians), 26% vs 22% (nurses/APRNs), and 33% vs 30% (pharmacists).
A version of this article first appeared on Medscape.com.
A Medscape
Among physician respondents who have children in that age group, 30% said they would not want their children to be vaccinated; 9% were unsure. For nurses/advanced practice registered nurses (APRNs), more (45%) said they did not want their kids to get the COVID-19 vaccine; 13% were unsure. Among pharmacists, 31% said they would not get them vaccinated and 9% were unsure.
Clinicians were more likely to want vaccinations for their kids 5-11 than were 510 consumers polled by WebMD at the same time. Overall, 49% of the consumers who had kids that age did not want them to get the COVID-19 vaccine.
On November 2, Centers for Disease Control and Prevention (CDC) Director Rochelle P. Walensky, MD, MPH, endorsed the CDC Advisory Committee on Immunization Practices’ recommendation that children 5-11 be vaccinated with the Pfizer-BioNTech pediatric vaccine. That decision expanded vaccine recommendations to about 28 million children in the United States.
The CDC states that, in clinical trials, the Pfizer vaccine had more than 90% efficacy in preventing laboratory-confirmed COVID-19 infection in children 5 to 15 years old, and that the immune response in children ages 5-15 equaled the immune response in people 16 to 25 years old.
The Medscape poll, fielded from November 3 to November 11, included 325 physicians, 793 nurses/APRNs, and 151 pharmacists.
How safe is the vaccine?
Clinicians were asked how confident they were that the vaccine is safe for that age group, and 66% of physicians, 52% of nurses/APRNs, and 66% of pharmacists said they were somewhat or very confident.
Among consumers overall in the WebMD poll, 56% said they were confident or somewhat confident that the vaccine is safe in that age group.
Among adolescents and young adults, rare cases of myocarditis and pericarditis in adolescents and young adults have been reported. According to the CDC, “[I]n one study, the risk of myocarditis after the second dose of Pfizer-BioNTech in the week following vaccination was around 54 cases per million doses administered to males ages 12-17 years.”
Known and potential benefits of COVID-19 vaccination outweigh the risks, including the possible risk for myocarditis or pericarditis, the CDC states.
Across clinician types, women edged out their male counterparts on confidence in the vaccine’ s safety: 71% vs 65% among physicians, 55% vs 45% among nurses/APRNs, and 68% vs 60% among pharmacists.
Among both physicians and nurses, younger physicians (under 45) tended to have greater confidence in the vaccine’ s safety: 72% vs 64% (physicians), 54% vs 51% (nurses/APRNs), and 71% vs 59% (pharmacists).
The difference in confidence was clear between vaccinated and unvaccinated physicians. All of the unvaccinated physicians who responded to the poll said they had no confidence in the vaccine for kids. Among unvaccinated nurses/APRNs, 2% were somewhat confident in the vaccine for kids under 12.
Knowledge about smaller dosage
The clinicians were asked about whether they were aware, before reading the poll question, that the Pfizer vaccine for children and the proposed Moderna vaccine for children in this age group (5-11) would have a different dosage.
The dose for kids 5-11 is 10 micrograms rather than 30 micrograms for people at least 12 years old. Children 5-11 receive a second dose 21 days or more after their first shot. The formulation comes with an orange cap, and a smaller needle is used.
Knowledge on the lower dose was highest among pharmacists (91% said they knew), followed by physicians (84%) and nurses (79%).
The poll also asked whether the COVID-19 vaccine should be added to the list of childhood immunizations. Responses varied widely and uncertainty was evident.
Notably, female physicians were more likely to say it should be added to the list of immunizations than were their male counterparts: 46% vs 35% (physicians), 26% vs 22% (nurses/APRNs), and 33% vs 30% (pharmacists).
A version of this article first appeared on Medscape.com.
COVID-19 mortality risk factors: An unexpected finding
Schizophrenia and severe mood and anxiety disorders are associated with a significantly lower risk of COVID-19 but are tied to a two- to fourfold increased risk of death from the virus, new research shows.
The study results held after the researchers controlled for other risk factors, and they contradict an earlier study that showed no increased mortality risk associated with mood or anxiety disorders. The findings come as the overall number of deaths in the United States approaches 800,000.
“These patients were less likely to be infected because they were probably less exposed, but once they have the infection, they are more prone to worse outcomes,” lead author Antonio L. Teixeira, MD, PhD, professor of psychiatry with McGovern Medical School at the University of Texas Health Science Center at Houston, said in an interview.
The study was published online Nov. 23 in JAMA Network Open.
Unexpected finding
Researchers analyzed electronic health records for 2.5 million adults with private health insurance who were tested for COVID-19 in 2020.
The overall positivity rate for the entire cohort was 11.91%, and patients with severe psychiatric illness fell below that rate. Positivity rates were 9.86% for people with schizophrenia or mood disorders and 11.17% among those with anxiety disorder.
Despite their lower positivity rate, patients with schizophrenia had the highest odds of death from COVID-19 after adjustment for age, race, body mass index, and comorbidities (aOR, 3.74; 95% confidence interval, 2.66-5.24).
Those results were not very surprising, Dr. Teixeira said, as earlier studies have reported similar findings. However,
Patients with mood disorders were nearly three times as likely to die (aOR, 2.76; 95% CI, 2.00-3.81), and those with anxiety disorders had more than double the mortality risk (aOR, 2.34; 95% CI, 1.68-3.27).
“We were expecting some increase, but there was strong evidence in those populations as well,” he said. “We were especially surprised at the data on patients with anxiety disorders.”
An outstanding question
These findings contradict a study published Jan. 27, 2021, in JAMA Psychiatry, that showed no significant increase in mortality risk among those with mood or anxiety disorders.
Study methodology and timing might explain some of the differences, Katlyn Nemani, MD, a research assistant professor of psychiatry at New York University, who led that earlier study, said in an interview.
Dr. Nemani’s study had a smaller study sample, examined mortality over a 30-day period after a positive COVID-19 test, and was limited to the peak of the pandemic in New York, between March and May 2020. Dr. Teixeira’s team examined a full year of data and assessed mortality for 7 days following a positive test.
“It is possible patients with some psychiatric disorders were less likely to receive or successfully respond to treatment for severe COVD-19 which evolved during the course of the pandemic,” Dr. Nemani said, adding that it’s also possible that differences in mortality in the days following infection became attenuated over time.
While a meta-analysis published in July and reported by this news organization at that time did show higher COVID-19 mortality among patients with mood disorders, the risk was far lower than that reported in this new study. That report, which included 33 studies in 22 countries, also found no increase in risk among those with anxiety disorder.
In October, the Centers for Disease Control and Prevention added mood disorders to the list of medical conditions that increase the risk for more severe COVID-19. Schizophrenia was already on that list.
“The outstanding question is what underlies this increased risk,” Dr. Nemani said. “Future studies focused on immune-mediated mechanisms and other potential explanations will help guide targeted interventions to reduce morbidity and mortality in this vulnerable population.”
Funding for the study was not disclosed. Dr. Teixeira and Dr. Nemani report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Schizophrenia and severe mood and anxiety disorders are associated with a significantly lower risk of COVID-19 but are tied to a two- to fourfold increased risk of death from the virus, new research shows.
The study results held after the researchers controlled for other risk factors, and they contradict an earlier study that showed no increased mortality risk associated with mood or anxiety disorders. The findings come as the overall number of deaths in the United States approaches 800,000.
“These patients were less likely to be infected because they were probably less exposed, but once they have the infection, they are more prone to worse outcomes,” lead author Antonio L. Teixeira, MD, PhD, professor of psychiatry with McGovern Medical School at the University of Texas Health Science Center at Houston, said in an interview.
The study was published online Nov. 23 in JAMA Network Open.
Unexpected finding
Researchers analyzed electronic health records for 2.5 million adults with private health insurance who were tested for COVID-19 in 2020.
The overall positivity rate for the entire cohort was 11.91%, and patients with severe psychiatric illness fell below that rate. Positivity rates were 9.86% for people with schizophrenia or mood disorders and 11.17% among those with anxiety disorder.
Despite their lower positivity rate, patients with schizophrenia had the highest odds of death from COVID-19 after adjustment for age, race, body mass index, and comorbidities (aOR, 3.74; 95% confidence interval, 2.66-5.24).
Those results were not very surprising, Dr. Teixeira said, as earlier studies have reported similar findings. However,
Patients with mood disorders were nearly three times as likely to die (aOR, 2.76; 95% CI, 2.00-3.81), and those with anxiety disorders had more than double the mortality risk (aOR, 2.34; 95% CI, 1.68-3.27).
“We were expecting some increase, but there was strong evidence in those populations as well,” he said. “We were especially surprised at the data on patients with anxiety disorders.”
An outstanding question
These findings contradict a study published Jan. 27, 2021, in JAMA Psychiatry, that showed no significant increase in mortality risk among those with mood or anxiety disorders.
Study methodology and timing might explain some of the differences, Katlyn Nemani, MD, a research assistant professor of psychiatry at New York University, who led that earlier study, said in an interview.
Dr. Nemani’s study had a smaller study sample, examined mortality over a 30-day period after a positive COVID-19 test, and was limited to the peak of the pandemic in New York, between March and May 2020. Dr. Teixeira’s team examined a full year of data and assessed mortality for 7 days following a positive test.
“It is possible patients with some psychiatric disorders were less likely to receive or successfully respond to treatment for severe COVD-19 which evolved during the course of the pandemic,” Dr. Nemani said, adding that it’s also possible that differences in mortality in the days following infection became attenuated over time.
While a meta-analysis published in July and reported by this news organization at that time did show higher COVID-19 mortality among patients with mood disorders, the risk was far lower than that reported in this new study. That report, which included 33 studies in 22 countries, also found no increase in risk among those with anxiety disorder.
In October, the Centers for Disease Control and Prevention added mood disorders to the list of medical conditions that increase the risk for more severe COVID-19. Schizophrenia was already on that list.
“The outstanding question is what underlies this increased risk,” Dr. Nemani said. “Future studies focused on immune-mediated mechanisms and other potential explanations will help guide targeted interventions to reduce morbidity and mortality in this vulnerable population.”
Funding for the study was not disclosed. Dr. Teixeira and Dr. Nemani report no conflicts of interest.
A version of this article first appeared on Medscape.com.
Schizophrenia and severe mood and anxiety disorders are associated with a significantly lower risk of COVID-19 but are tied to a two- to fourfold increased risk of death from the virus, new research shows.
The study results held after the researchers controlled for other risk factors, and they contradict an earlier study that showed no increased mortality risk associated with mood or anxiety disorders. The findings come as the overall number of deaths in the United States approaches 800,000.
“These patients were less likely to be infected because they were probably less exposed, but once they have the infection, they are more prone to worse outcomes,” lead author Antonio L. Teixeira, MD, PhD, professor of psychiatry with McGovern Medical School at the University of Texas Health Science Center at Houston, said in an interview.
The study was published online Nov. 23 in JAMA Network Open.
Unexpected finding
Researchers analyzed electronic health records for 2.5 million adults with private health insurance who were tested for COVID-19 in 2020.
The overall positivity rate for the entire cohort was 11.91%, and patients with severe psychiatric illness fell below that rate. Positivity rates were 9.86% for people with schizophrenia or mood disorders and 11.17% among those with anxiety disorder.
Despite their lower positivity rate, patients with schizophrenia had the highest odds of death from COVID-19 after adjustment for age, race, body mass index, and comorbidities (aOR, 3.74; 95% confidence interval, 2.66-5.24).
Those results were not very surprising, Dr. Teixeira said, as earlier studies have reported similar findings. However,
Patients with mood disorders were nearly three times as likely to die (aOR, 2.76; 95% CI, 2.00-3.81), and those with anxiety disorders had more than double the mortality risk (aOR, 2.34; 95% CI, 1.68-3.27).
“We were expecting some increase, but there was strong evidence in those populations as well,” he said. “We were especially surprised at the data on patients with anxiety disorders.”
An outstanding question
These findings contradict a study published Jan. 27, 2021, in JAMA Psychiatry, that showed no significant increase in mortality risk among those with mood or anxiety disorders.
Study methodology and timing might explain some of the differences, Katlyn Nemani, MD, a research assistant professor of psychiatry at New York University, who led that earlier study, said in an interview.
Dr. Nemani’s study had a smaller study sample, examined mortality over a 30-day period after a positive COVID-19 test, and was limited to the peak of the pandemic in New York, between March and May 2020. Dr. Teixeira’s team examined a full year of data and assessed mortality for 7 days following a positive test.
“It is possible patients with some psychiatric disorders were less likely to receive or successfully respond to treatment for severe COVD-19 which evolved during the course of the pandemic,” Dr. Nemani said, adding that it’s also possible that differences in mortality in the days following infection became attenuated over time.
While a meta-analysis published in July and reported by this news organization at that time did show higher COVID-19 mortality among patients with mood disorders, the risk was far lower than that reported in this new study. That report, which included 33 studies in 22 countries, also found no increase in risk among those with anxiety disorder.
In October, the Centers for Disease Control and Prevention added mood disorders to the list of medical conditions that increase the risk for more severe COVID-19. Schizophrenia was already on that list.
“The outstanding question is what underlies this increased risk,” Dr. Nemani said. “Future studies focused on immune-mediated mechanisms and other potential explanations will help guide targeted interventions to reduce morbidity and mortality in this vulnerable population.”
Funding for the study was not disclosed. Dr. Teixeira and Dr. Nemani report no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Headache is a common post–COVID-19 complaint
The Centers for Disease Control and Prevention has identified it as a sentinel symptom of COVID-19 disease. “A lot of the recommendations surrounding post-COVID headache is that if you identify a patient who has headaches associated with fever, and myalgia, and other systemic symptoms, the specificity of a COVID-19 diagnosis goes up. So [COVID-19] is a really important feature to look out for in patients with headache,” Deena Kuruvilla, MD, said during a presentation on post–COVID-19 headache at the 2021 Scottsdale Headache Symposium.
Estimates of the prevalence of headache in COVID-19 range widely, from 6.5% to 71%, but Dr. Kuruvilla has plenty of personal experience with it. “During my stint on the inpatient neurology service during the peak of COVID, I saw patients with headache being one of the most frequent complaints, [along with] dizziness, stroke, and seizure among many other neurological manifestations,” said Dr. Kuruvilla, director of the Westport (Conn.) Headache Institute.
One meta-analysis showed that 47% of patients with COVID-19 complain of headache within 30 days of diagnosis, and this drops to around 10% at 60-90 days, and around 8% at 180 days.
A survey of 3,458 patients, published in the Journal of Headache Pain, found that migraine is the most common type of post–COVID-19 headache phenotype, and patients reporting anosmia-ageusia were more likely to have post–COVID-19 headache (odds ratio [OR], 5.39; 95% confidence interval, 1.66-17.45).
A case-control study of post–COVID-19 headache patients with and without a history of migraine found that those with a history of migraine were more likely to have post–COVID-19 symptoms (OR, 1.70; P < .001) and fatigue (OR, 2.89; P = .008). “Interestingly, they found no difference in headache as post-COVID symptoms in people who had a history of migraine compared with people without a history of migraine,” said Dr. Kuruvilla.
Headache and COVID-19: What is the connection?
Several mechanisms have been proposed for direct invasion of the central nervous system, either via infection through the angiotensin-converting enzyme 2 (ACE-2) receptor, which is expressed in brain regions including the motor cortex, the posterior cingulate cortex, and the olfactory bulb, among other locations. Another potential mechanism is direct entry through the olfactory nerve and the associated olfactory epithelium. There are various potential mechanisms for spread among the peripheral nervous system, and the blood-brain barrier can be compromised by infection of vascular endothelial cells. According to the literature, neuronal damage seems to occur directly from viral damage rather than from the immune response, said Dr. Kuruvilla.
The virus may also gain entry to the CNS indirectly, as a result of hypoxia and metabolic disturbances, as well as dehydration and systematic inflammation. The cytokine storm associated with COVID-19 infection can activate C-reactive protein and calcitonin gene-related peptide (CGRP), which plays a key role in migraine pathology. The CGRP receptor antagonist vazegepant is being studied in a phase 2 clinical trial for the treatment of COVID-19–related lung inflammation.
Testing and treatment
“If I see patients with new headache, worsening headache from their baseline, or headache with systemic symptoms, I often consider screening them for COVID. If that screening is positive, I proceed with PCR testing. I also consider an MRI of the brain with and without gadolinium just to rule out any secondary causes for headache,” said Dr. Kuruvilla, noting that she has diagnosed patients with venous sinus thrombosis, ischemic stroke, and meningitis following COVID-19.
The existing literature suggests that lumbar puncture in patients with SARS-CoV-2 typically returns normal results, but Dr. Kuruvilla proceeds with it anyway with viral, bacterial, fungal, and autoimmune studies to rule out potential secondary causes for headache.
There are few studies on how to treat post–COVID-19 headache, and the general recommendation is that headache phenotype should drive treatment decisions.
In a case series, three patients with persistent headache following mild COVID-19 infection were treated with onabotulinumtoxinA and amitriptyline. They had daily headaches, along with post–COVID-19 symptoms including fatigue and insomnia. After treatment, each patient converted to episodic headaches.
One retrospective study of 37 patients found that a 5-day course of indomethacin 50 mg twice per day and pantoprazole 40 mg once per day was associated with a 50% or greater improvement in headache on the third day in 36 of the 37 patients. Five patients were free of pain by day 5.
A common problem
Neurologists have been involved in the treatment of COVID-19 since the beginning, and post–COVID-19 headache has added another layer. “It’s been a remarkably common clinical problem. And the fact that it’s actually reached the level of headache specialist actually shows that in some cases, it’s really quite a significant problem, in both its severity and persistence. So I think it’s a very, very significant issue,” said Andrew Charles, MD, professor of neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program.
Dr. Kuruvilla also discussed the question of whether neurological damage is due to direct damage from the virus, or indirect damage from an immune response. This was debated during the Q&A session following Dr. Kuruvilla’s talk, and it was pointed out that headache is a frequent side effect of the Pfizer and Moderna vaccines.
“It’s a huge open question about how much is direct invasion or damage or not even damage, but just change in function with the viral infection, as opposed to inflammation. The fact that very often the response to the vaccine is similar to what you see with COVID suggests that at least some component of it is inflammation. I wouldn’t commit to one mechanism or the other, but I’d say that it’s possible that it’s really both,” said Dr. Charles.
Dr. Kuruvilla has consulted for Cefaly, Neurolief, Theranica, Now What Media, and KX advisors. She has been on the speakers bureau for Abbvie/Allergan, Amgen/Novartis, and Lilly. She has been on advisory boards for Abbvie/Allergan, Lilly, Theranica, and Amgen/Novartis. Dr. Charles has no relevant financial disclosures.
The Centers for Disease Control and Prevention has identified it as a sentinel symptom of COVID-19 disease. “A lot of the recommendations surrounding post-COVID headache is that if you identify a patient who has headaches associated with fever, and myalgia, and other systemic symptoms, the specificity of a COVID-19 diagnosis goes up. So [COVID-19] is a really important feature to look out for in patients with headache,” Deena Kuruvilla, MD, said during a presentation on post–COVID-19 headache at the 2021 Scottsdale Headache Symposium.
Estimates of the prevalence of headache in COVID-19 range widely, from 6.5% to 71%, but Dr. Kuruvilla has plenty of personal experience with it. “During my stint on the inpatient neurology service during the peak of COVID, I saw patients with headache being one of the most frequent complaints, [along with] dizziness, stroke, and seizure among many other neurological manifestations,” said Dr. Kuruvilla, director of the Westport (Conn.) Headache Institute.
One meta-analysis showed that 47% of patients with COVID-19 complain of headache within 30 days of diagnosis, and this drops to around 10% at 60-90 days, and around 8% at 180 days.
A survey of 3,458 patients, published in the Journal of Headache Pain, found that migraine is the most common type of post–COVID-19 headache phenotype, and patients reporting anosmia-ageusia were more likely to have post–COVID-19 headache (odds ratio [OR], 5.39; 95% confidence interval, 1.66-17.45).
A case-control study of post–COVID-19 headache patients with and without a history of migraine found that those with a history of migraine were more likely to have post–COVID-19 symptoms (OR, 1.70; P < .001) and fatigue (OR, 2.89; P = .008). “Interestingly, they found no difference in headache as post-COVID symptoms in people who had a history of migraine compared with people without a history of migraine,” said Dr. Kuruvilla.
Headache and COVID-19: What is the connection?
Several mechanisms have been proposed for direct invasion of the central nervous system, either via infection through the angiotensin-converting enzyme 2 (ACE-2) receptor, which is expressed in brain regions including the motor cortex, the posterior cingulate cortex, and the olfactory bulb, among other locations. Another potential mechanism is direct entry through the olfactory nerve and the associated olfactory epithelium. There are various potential mechanisms for spread among the peripheral nervous system, and the blood-brain barrier can be compromised by infection of vascular endothelial cells. According to the literature, neuronal damage seems to occur directly from viral damage rather than from the immune response, said Dr. Kuruvilla.
The virus may also gain entry to the CNS indirectly, as a result of hypoxia and metabolic disturbances, as well as dehydration and systematic inflammation. The cytokine storm associated with COVID-19 infection can activate C-reactive protein and calcitonin gene-related peptide (CGRP), which plays a key role in migraine pathology. The CGRP receptor antagonist vazegepant is being studied in a phase 2 clinical trial for the treatment of COVID-19–related lung inflammation.
Testing and treatment
“If I see patients with new headache, worsening headache from their baseline, or headache with systemic symptoms, I often consider screening them for COVID. If that screening is positive, I proceed with PCR testing. I also consider an MRI of the brain with and without gadolinium just to rule out any secondary causes for headache,” said Dr. Kuruvilla, noting that she has diagnosed patients with venous sinus thrombosis, ischemic stroke, and meningitis following COVID-19.
The existing literature suggests that lumbar puncture in patients with SARS-CoV-2 typically returns normal results, but Dr. Kuruvilla proceeds with it anyway with viral, bacterial, fungal, and autoimmune studies to rule out potential secondary causes for headache.
There are few studies on how to treat post–COVID-19 headache, and the general recommendation is that headache phenotype should drive treatment decisions.
In a case series, three patients with persistent headache following mild COVID-19 infection were treated with onabotulinumtoxinA and amitriptyline. They had daily headaches, along with post–COVID-19 symptoms including fatigue and insomnia. After treatment, each patient converted to episodic headaches.
One retrospective study of 37 patients found that a 5-day course of indomethacin 50 mg twice per day and pantoprazole 40 mg once per day was associated with a 50% or greater improvement in headache on the third day in 36 of the 37 patients. Five patients were free of pain by day 5.
A common problem
Neurologists have been involved in the treatment of COVID-19 since the beginning, and post–COVID-19 headache has added another layer. “It’s been a remarkably common clinical problem. And the fact that it’s actually reached the level of headache specialist actually shows that in some cases, it’s really quite a significant problem, in both its severity and persistence. So I think it’s a very, very significant issue,” said Andrew Charles, MD, professor of neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program.
Dr. Kuruvilla also discussed the question of whether neurological damage is due to direct damage from the virus, or indirect damage from an immune response. This was debated during the Q&A session following Dr. Kuruvilla’s talk, and it was pointed out that headache is a frequent side effect of the Pfizer and Moderna vaccines.
“It’s a huge open question about how much is direct invasion or damage or not even damage, but just change in function with the viral infection, as opposed to inflammation. The fact that very often the response to the vaccine is similar to what you see with COVID suggests that at least some component of it is inflammation. I wouldn’t commit to one mechanism or the other, but I’d say that it’s possible that it’s really both,” said Dr. Charles.
Dr. Kuruvilla has consulted for Cefaly, Neurolief, Theranica, Now What Media, and KX advisors. She has been on the speakers bureau for Abbvie/Allergan, Amgen/Novartis, and Lilly. She has been on advisory boards for Abbvie/Allergan, Lilly, Theranica, and Amgen/Novartis. Dr. Charles has no relevant financial disclosures.
The Centers for Disease Control and Prevention has identified it as a sentinel symptom of COVID-19 disease. “A lot of the recommendations surrounding post-COVID headache is that if you identify a patient who has headaches associated with fever, and myalgia, and other systemic symptoms, the specificity of a COVID-19 diagnosis goes up. So [COVID-19] is a really important feature to look out for in patients with headache,” Deena Kuruvilla, MD, said during a presentation on post–COVID-19 headache at the 2021 Scottsdale Headache Symposium.
Estimates of the prevalence of headache in COVID-19 range widely, from 6.5% to 71%, but Dr. Kuruvilla has plenty of personal experience with it. “During my stint on the inpatient neurology service during the peak of COVID, I saw patients with headache being one of the most frequent complaints, [along with] dizziness, stroke, and seizure among many other neurological manifestations,” said Dr. Kuruvilla, director of the Westport (Conn.) Headache Institute.
One meta-analysis showed that 47% of patients with COVID-19 complain of headache within 30 days of diagnosis, and this drops to around 10% at 60-90 days, and around 8% at 180 days.
A survey of 3,458 patients, published in the Journal of Headache Pain, found that migraine is the most common type of post–COVID-19 headache phenotype, and patients reporting anosmia-ageusia were more likely to have post–COVID-19 headache (odds ratio [OR], 5.39; 95% confidence interval, 1.66-17.45).
A case-control study of post–COVID-19 headache patients with and without a history of migraine found that those with a history of migraine were more likely to have post–COVID-19 symptoms (OR, 1.70; P < .001) and fatigue (OR, 2.89; P = .008). “Interestingly, they found no difference in headache as post-COVID symptoms in people who had a history of migraine compared with people without a history of migraine,” said Dr. Kuruvilla.
Headache and COVID-19: What is the connection?
Several mechanisms have been proposed for direct invasion of the central nervous system, either via infection through the angiotensin-converting enzyme 2 (ACE-2) receptor, which is expressed in brain regions including the motor cortex, the posterior cingulate cortex, and the olfactory bulb, among other locations. Another potential mechanism is direct entry through the olfactory nerve and the associated olfactory epithelium. There are various potential mechanisms for spread among the peripheral nervous system, and the blood-brain barrier can be compromised by infection of vascular endothelial cells. According to the literature, neuronal damage seems to occur directly from viral damage rather than from the immune response, said Dr. Kuruvilla.
The virus may also gain entry to the CNS indirectly, as a result of hypoxia and metabolic disturbances, as well as dehydration and systematic inflammation. The cytokine storm associated with COVID-19 infection can activate C-reactive protein and calcitonin gene-related peptide (CGRP), which plays a key role in migraine pathology. The CGRP receptor antagonist vazegepant is being studied in a phase 2 clinical trial for the treatment of COVID-19–related lung inflammation.
Testing and treatment
“If I see patients with new headache, worsening headache from their baseline, or headache with systemic symptoms, I often consider screening them for COVID. If that screening is positive, I proceed with PCR testing. I also consider an MRI of the brain with and without gadolinium just to rule out any secondary causes for headache,” said Dr. Kuruvilla, noting that she has diagnosed patients with venous sinus thrombosis, ischemic stroke, and meningitis following COVID-19.
The existing literature suggests that lumbar puncture in patients with SARS-CoV-2 typically returns normal results, but Dr. Kuruvilla proceeds with it anyway with viral, bacterial, fungal, and autoimmune studies to rule out potential secondary causes for headache.
There are few studies on how to treat post–COVID-19 headache, and the general recommendation is that headache phenotype should drive treatment decisions.
In a case series, three patients with persistent headache following mild COVID-19 infection were treated with onabotulinumtoxinA and amitriptyline. They had daily headaches, along with post–COVID-19 symptoms including fatigue and insomnia. After treatment, each patient converted to episodic headaches.
One retrospective study of 37 patients found that a 5-day course of indomethacin 50 mg twice per day and pantoprazole 40 mg once per day was associated with a 50% or greater improvement in headache on the third day in 36 of the 37 patients. Five patients were free of pain by day 5.
A common problem
Neurologists have been involved in the treatment of COVID-19 since the beginning, and post–COVID-19 headache has added another layer. “It’s been a remarkably common clinical problem. And the fact that it’s actually reached the level of headache specialist actually shows that in some cases, it’s really quite a significant problem, in both its severity and persistence. So I think it’s a very, very significant issue,” said Andrew Charles, MD, professor of neurology at the University of California, Los Angeles, and director of the UCLA Goldberg Migraine Program.
Dr. Kuruvilla also discussed the question of whether neurological damage is due to direct damage from the virus, or indirect damage from an immune response. This was debated during the Q&A session following Dr. Kuruvilla’s talk, and it was pointed out that headache is a frequent side effect of the Pfizer and Moderna vaccines.
“It’s a huge open question about how much is direct invasion or damage or not even damage, but just change in function with the viral infection, as opposed to inflammation. The fact that very often the response to the vaccine is similar to what you see with COVID suggests that at least some component of it is inflammation. I wouldn’t commit to one mechanism or the other, but I’d say that it’s possible that it’s really both,” said Dr. Charles.
Dr. Kuruvilla has consulted for Cefaly, Neurolief, Theranica, Now What Media, and KX advisors. She has been on the speakers bureau for Abbvie/Allergan, Amgen/Novartis, and Lilly. She has been on advisory boards for Abbvie/Allergan, Lilly, Theranica, and Amgen/Novartis. Dr. Charles has no relevant financial disclosures.
FROM 2021 SCOTTSDALE HEADACHE SYMPOSIUM
The Use of Nasogastric Tube Bridle Kits in COVID-19 Intensive Care Unit Patients
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).
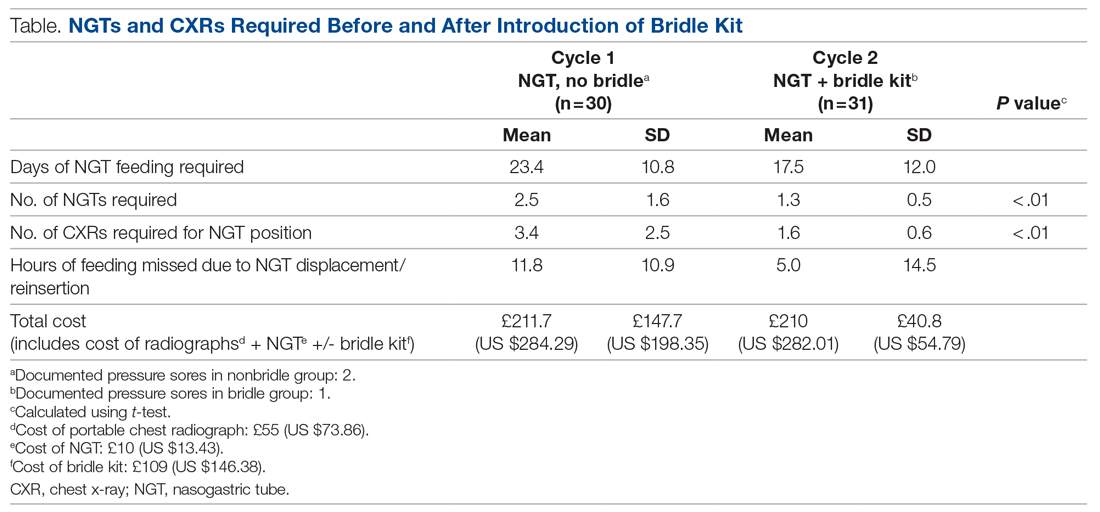
The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; [email protected].
Financial disclosures: None.
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).

The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; [email protected].
Financial disclosures: None.
From Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham, United Kingdom.
Objective: To ascertain the extent of nasogastric tube (NGT) dislodgment in COVID-19 intensive care unit (ICU) patients after the introduction of NGT bridle kits as a standard of practice, to see whether this would reduce the number of NGT insertions, patient irradiation, missed feeds, and overall cost.
Background: Nasogastric feeding is the mainstay of enteral feeding for ICU patients. The usual standard of practice is to secure the tube using adhesive tape. Studies show this method has a 40% to 48% dislodgment rate. The COVID-19 ICU patient population may be at even greater risk due to the need for proning, long duration of invasive ventilation, and emergence delirium.
Design: This was a 2-cycle quality improvement project. The first cycle was done retrospectively, looking at the contemporaneous standard of practice where bridle kits were not used. This gave an objective measure of the extent of NGT displacement, associated costs, and missed feeds. The second cycle was carried out prospectively, with the use of NGT bridle kits as the new standard of practice.
Setting: A large United Kingdom teaching hospital with a 100-bed, single-floor ICU.
Participants: Patients admitted to the ICU with COVID-19 who subsequently required sedation and invasive ventilation.
Measurements: Measurements included days of feeding required, hours of feeding missed due to NGT dislodgment, total number of nasogastric tubes required per ICU stay, and number of chest radiographs for NGT position confirmation. NGT-related pressure sores were also recorded.
Results: When compared to the bridled group, the unbridled group required a higher number of NGTs (2.5 vs 1.3; P < .001) and chest radiographs (3.4 vs 1.6; P < .001), had more hours of feeding missed (11.8 vs 5.0), and accumulated a slightly higher total cost (cost of NGT, chest radiographs +/- bridle kit: £211.67 vs £210, [US $284.25 vs US $282.01]).
Conclusions: The use of NGT bridle kits reduces the number of NGT insertions patients require and subsequently reduces the number of chest radiographs for each patient. These patients also miss fewer feeds, with no appreciable increase in cost.
Keywords: nasogastric, bridle, enteral, COVID-19, intensive care, quality improvement, safety.
The COVID-19 pandemic has led to a large influx of patients to critical care units in the United Kingdom (UK) and across the world. Figures from the Intensive Care National Audit & Research Centre in May 2020 show that the median length of stay for COVID-19 survivors requiring invasive ventilatory support while on the intensive care unit (ICU) was 15 days.1 For these days at the very least, patients are completely reliant on enteral feeding in order to meet their nutritional requirements.The standard method of enteral feeding when a patient is sedated and ventilated is via a nasogastric tube (NGT). Incorrect placement of an NGT can have devastating consequences, including pneumothorax, fistula formation, ulceration, sepsis, and death. Between September 2011 and March 2016, the National Patient Safety Agency in the UK recorded 95 incidents of feeding into the respiratory tract as a result of incorrect NGT placement.2 With the onset of the pandemic, the prevalence of NGT misplacement increased, with the NHS Improvement team reporting 7 cases of misplaced NGTs within just 3 months (April 1, 2020, through June 30, 2020).3 With over 3 million nasogastric or orogastric tubes inserted each year in the UK, the risk of adverse events is very real.
NGT dislodgment is common, with 1 study putting this figure at 40%.4 Recurrent dislodgment of NGTs disrupts nutrition and may lead to the patient missing a feed in a time where nutrition is vital during acute illness. Research has showed that NGT bridling reduces the rate of dislodgment significantly (from 40% to 14%).5 Moreover, a 2018 systematic review looking specifically at NGT dislodgment found 10 out of 11 studies showed a significant reduction in dislodgment following use of a bridle kit.6 Bridling an NGT has been shown to significantly reduce the need for percutaneous endoscopic gastrostomy insertion.7 NGT bridle kits have already been used successfully in ICU burn patients, where sloughed skin makes securement particularly difficult with traditional methods.8 With each repeated insertion comes the risk of incorrect placement. COVID-19 ICU patients had specific risk factors for their NGTs becoming dislodged: duration of NGT feeding (in the ICU and on the ward), requirement for proning and de-proning, and post-emergence confusion related to long duration of sedation. Repeated NGT insertion comes with potential risks to the patient and staff, as well as a financial cost. Patient-specific risks include potential for incorrect placement, missed feedings, irradiation (from the patient’s own chest radiograph and from others), and discomfort from manual handling and repeat reinsertions. Staff risk factors include radiation scatter from portable radiographs (especially when dealing with more than 1 patient per bed space), manual handling, and increased pressure on radiographers. Finally, financial costs are related to the NGTs themselves as well as the portable chest radiograph, which our Superintendent Radiographer estimates to be £55 (US $73.86).
The objective of this study was to ascertain the extent of NGT dislodgment in COVID-19 ICU patients after the introduction of NGT bridle kits as a standard of practice and to determine whether this would reduce the number of NGT insertions, patient irradiation, missed feedings, and overall costs. With the introduction of bridle kits, incidence of pressure sores related to the bridle kit were also recorded.
Methods
Data were collected over 2 cycles, the first retrospectively and the second prospectively, once NGT bridle kits were introduced as an intervention.
Cycle 1. Analyzing the current standard of practice: regular NGT insertion with no use of bridle kit
Cycle 1 was done retrospectively, looking at 30 patient notes of COVID-19 patients admitted to the critical care unit (CCU) between March 11, 2020, and April 20, 2020, at Queen Elizabeth Hospital Birmingham, Birmingham, UK. All patients admitted to the ICU with COVID-19 requiring invasive ventilation were eligible for inclusion in the study. A total of 32 patients were admitted during this time; however, 2 patients were excluded due to NGTs being inserted prior to ICU admission.
Individual patient notes were searched for:
- days of feeding required during their inpatient stay (this included NGT feeding on the ward post-ICU discharge).
- hours of feeding missed while waiting for NGT reinsertion or chest radiograph due to dislodged or displaced NGTs (during the entire period of enteral feeding, ICU, and ward).
- number of NGT insertions.
- number of chest radiographs purely for NGT position.
Each patient’s first day of feeding and NGT insertion were noted. Following that, the patient electronic note system, the Prescribing Information and Communication System, was used to look for any further chest radiograph requests, which were primarily for NGT position. Using the date and time, the “critical care observations” tab was used to look at fluids and to calculate how long NGT feeding was stopped while NGT position-check x-rays were being awaited. The notes were also checked at this date and time to work out whether a new NGT was inserted or whether an existing tube had been dislodged (if not evident from the x-ray request). Data collection was stopped once either of the following occurred:
- patient no longer required NGT feeding.
- patient was transferred to another hospital.
- death.
The cost of the NGT was averaged between the cost of size 8 and 12, which worked out to be £10 (US $13.43). As mentioned earlier, each radiograph cost was determined by the Superintendent Radiographer (£55).
Cycle 2. Implementing a change: introduction of NGT bridle kit (Applied Medical Technology Bridle) as standard of practice
The case notes of 54 patients admitted to the COVID-19 CCU at the Queen Elizabeth Hospital Birmingham, Birmingham, UK, were retrospectively reviewed between February 8, 2021, and April 17, 2021. The inclusion criteria consisted of: admitted to the CCU due to COVID-19, required NGT feeding, and was bridled on admission. Case notes were retrospectively reviewed for:
- Length of CCU stay
- Days of feeding required during the hospital stay
- Hours of feeding missed while waiting for a chest radiograph due to displaced NGTs
- Number of NGT insertions
- Number of chest radiographs to confirm NGT position
- Bridling of NGTs
- Documented pressure sores related to the bridle or NGT, or referrals for wound management advice (Tissue Viability Team) as a consequence of the NGT bridle
Results
Of the 54 patients admitted, 31 had their NGTs bridled. Data were collected as in the first cycle, with individual notes analyzed on the online system (Table). Additionally, notes were reviewed for documentation of pressure sores related to NGT bridling, and the “requests” tab as well as the “noting” function were used to identify referrals for “Wound Management Advice” (Tissue Viability Review).

The average length of stay for this ICU cohort was 17.6 days. This reiterates the reliance on NGT feeding of patients admitted to the CCU. The results from this project can be summarized as follows: The use of NGT bridle kits leads to a significant reduction in the total number of NGTs a patient requires during intensive care. As a result, there is a significant reduction in the number of chest radiographs required to confirm NGT position. Feedings missed can also be reduced by using a bridle kit. These advantages all come with no additional cost.
On average, bridled patients required 1.3 NGTs, compared to 2.5 before bridles were introduced. The fewer NGTs inserted, the less chance of an NGT-associated injury occurring.
The number of chest radiographs required to confirm NGT position after resiting also fell, from 3.4 to 1.6. This has numerous advantages. There is a financial savings of £99 (US $133.04) per patient from the reduced number of chest x-rays. Although this does not offset the price of the bridle kit itself, there are other less easily quantifiable costs that are reduced. For instance, patients are highly catabolic during severe infection, and their predominant energy source comes from their feedings. Missed feedings are associated with longer length of stay in the ICU and in the hospital in general.9 Bridle kits have the potential to reduce the number of missed feedings by ensuring the NGT remains in the correct position.
Discussion
Many of the results are aligned with what is already known in the literature. A meta-analysis from 2014 concluded that dislodgment is reduced with the use of a bridle kit.6 This change is what underpins many of the advantages seen, as an NGT that stays in place means additional radiographs are not required and feeding is not delayed.
COVID-19 critical care patients are very fragile and are dependent on ventilators for the majority of their stay. They are often on very high levels of ventilator support and moving the patient can lead to desaturation or difficulties in ventilation. Therefore, reduction in any manual handling occurring as a result of the need for portable chest radiographs minimizes the chances of further negative events. Furthermore, nursing staff, along with the radiographers, are often the ones who must move these patients in order for the x-ray film to be placed behind the patient. This task is not easy, especially with limited personnel, and has the potential to cause injuries to both patients and staff members.
The knock-on effect of reduced NGTs and x-rays is also a reduction of work for the portable radiography team, in what is a very time- and resource-consuming process of coming onto the COVID-19 CCU. Not only does the machine itself need to be wiped down thoroughly after use, but also the individual must use personal protective equipment (PPE) each time. There is a cost associated with PPE itself, as well as the time it takes to don and doff appropriately.
A reduction in chest radiographs reduces the irradiation of the patient and the potential irradiation of staff members. With bridling of the NGT, the radiation exposure is more than halved for the patient. Because the COVID ICU is often very busy, with patients in some cases being doubled up in a bed space, the scatter radiation is high. This can be reduced if fewer chest radiographs are required.
An additional benefit of a reduction in the mean number of NGT insertions per patient is also illustrated by anecdotal evidence. Over the studied period, we identified 2 traumatic pneumothoraces related to NGT insertion on the COVID-19 CCU, highlighting the potential risks of NGT insertion and the need to reduce its frequency, if possible.
One concern noted was that bridles could cause increased incidence of pressure sores. In the patients represented in this study, only 1 suffered a pressure sore (grade 2) directly related to the bridle. A subpopulation of patients not bridled was also noted. This was significantly smaller than the main group; however, we had noted 2 incidences of pressure sores from their standard NGT and securement devices. Some studies have alluded to the potential for increased skin complications with bridle kits; however, studies looking specifically at kits using umbilical tape (as in this study) show no significant increase in skin damage.10 This leaves us confident that there is no increased risk of pressure sores related to the bridling of patients when umbilical tape is used with the bridle kit.
NGT bridles require training to insert safely. With the introduction of bridling, our hospital’s nursing staff underwent training in order to be proficient with the bridle kits. This comes with a time commitment, and, like other equipment usage, it takes time to build confidence. However, in this study, there were no concerns raised from nursing staff regarding difficulty of insertion or the time taken to do so.
Our study adds an objective measure of the benefits provided by bridle kits. Not only was there a reduction in the number of NGT insertions required, but we were also able to show a significant reduction in the number of chest radiographs required as well in the amount of time feeding is missed. While apprehension regarding bridle kits may be focused on cost, this study has shown that the savings more than make up for the initial cost of the kit itself.
Although the patient demographics, systemic effects, and treatment of COVID-19 are similar between different ICUs, a single-center study does have limitations. One of these is the potential for an intervention in a single-center study to lead to a larger effect than that of multicenter studies.11 But as seen in previous studies, the dislodgment of NGTs is not just an issue in this ICU.12 COVID-19–specific risk factors for NGT dislodgment also apply to all patients requiring invasive ventilation and proning.
Identification of whether a new NGT was inserted, or whether the existing NGT was replaced following dislodging of an NGT, relied on accurate documentation by the relevant staff. The case notes did not always make this explicitly clear. Unlike other procedures commonly performed, documentation of NGT insertion is not formally done under the procedures heading, and, on occasion is not done at all. We recognize that manually searching notes only yields NGT insertions that have been formally documented. There is a potential for the number recorded to be lower than the actual number of NGTs inserted. However, when x-ray requests are cross-referenced with the notes, there is a significant degree of confidence that the vast majority of insertions are picked up.
One patient identified in the study required a Ryle’s tube as part of their critical care treatment. While similar in nature to an NGT, these are unable to fit into a bridle and are at increased risk of dislodging during the patient’s critical care stay. The intended benefit of the bridle kit does not therefore extend to patients with Ryle’s tubes.
Conclusion
The COVID-19 critical care population requires significant time on invasive ventilation and remains dependent on NGT feeding during this process. The risk of NGT dislodgment can be mitigated by using a bridle kit, as the number of NGT insertions a patient requires is significantly reduced. Not only does this reduce the risk of inadvertent misplacement but also has a cost savings, as well as increasing safety for staff and patients. From this study, the risk of pressure injuries is not significant. The benefit of NGT bridling may be extended to other non-COVID long-stay ICU patients.
Future research looking at the efficacy of bridle kits in larger patient groups will help confirm the benefits seen in this study and will also provide better information with regard to any long-term complications associated with bridles.
Corresponding author: Rajveer Atkar, MBBS, Queen Elizabeth Hospital Birmingham, Mindelsohn Way, Birmingham B15 2GW, United Kingdom; [email protected].
Financial disclosures: None.
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
1. Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 15 May 2020. https://www.icnarc.org/DataServices/Attachments/Download/cbcb6217-f698-ea11-9125-00505601089b
2. NHS. Nasogastric tube misplacement: continuing risk of death and severe harm. July 22, 2016. https://www.england.nhs.uk/2016/07/nasogastric-tube-misplacement-continuing-risk-of-death-severe-harm/
3. NHS. Provisional publication of never events reported as occurring between 1 April and 30 June 2020. https://www.england.nhs.uk/wp-content/uploads/2020/08/Provisional_publication_-_NE_1_April_-_30_June_2020.pdf
4. Meer JA. Inadvertent dislodgement of nasoenteral feeding tubes: incidence and prevention. JPEN J Parenter Enteral Nutr. 1987;11(2):187- 189. doi:10.1177/0148607187011002187
5. Bechtold ML, Nguyen DL, Palmer L, et al. Nasal bridles for securing nasoenteric tubes: a meta-analysis. Nutr Clin Pract. 2014;29(5):667-671. doi:10.1177/0884533614536737
6. Lynch A, Tang CS, Jeganathan LS, Rockey JG. A systematic review of the effectiveness and complications of using nasal bridles to secure nasoenteral feeding tubes. Aust J Otolaryngol. 2018;1:8. doi:10.21037/ajo.2018.01.01
7. Johnston R, O’Dell L, Patrick M, Cole OT, Cunliffe N. Outcome of patients fed via a nasogastric tube retained with a bridle loop: Do bridle loops reduce the requirement for percutaneous endoscopic gastrostomy insertion and 30-day mortality? Proc Nutr Soc. 2008;67:E116. doi:10.1017/S0029665108007489
8. Li AY, Rustad KC, Long C, et al. Reduced incidence of feeding tube dislodgement and missed feeds in burn patients with nasal bridle securement. Burns. 2018;44(5):1203-1209. doi:10.1016/j.burns.2017.05.025
9. Peev MP, Yeh DD, Quraishi SA, et al. Causes and consequences of interrupted enteral nutrition: a prospective observational study in critically ill surgical patients. JPEN J Parenter Enteral Nutr. 2015;39(1):21-27. doi:10.1177/0148607114526887
10. Seder CW, Janczyk R. The routine bridling of nasjejunal tubes is a safe and effective method of reducing dislodgement in the intensive care unit. Nutr Clin Pract. 2008;23(6):651-654. doi:10.1177/0148607114526887
11. Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155:39-51. doi:10.7326/0003-4819-155-1-201107050-00006
12. Morton B, Hall R, Ridgway T, Al-Rawi O. Nasogastric tube dislodgement: a problem on our ICU. Crit Care. 2013;17(suppl 2):P242. doi:10.1186/cc12180
Children and COVID: New cases increase for third straight week
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.
There were almost 142,000 new cases reported during the week of Nov. 12-18, marking an increase of 16% over the previous week and the 15th straight week with a weekly total over 100,000, the American Academy of Pediatrics and the Children’s Hospital Association said.
Regional data show that the Midwest has experienced the largest share of this latest surge, followed by the Northeast. Cases increased in the South during the week of Nov. 12-18 after holding steady over the previous 2 weeks, while new cases in the West dropped in the last week. At the state level, Maine, New Hampshire, and Vermont again reported the largest percent increases, with Michigan, Minnesota, and New Mexico also above average, the AAP and CHA said in their weekly COVID report.
Data from the Centers for Disease Control and Prevention show similar trends for both emergency department visits and hospital admissions, as both have risen in November after declines that began in late August and early September.
The cumulative number of pediatric cases is 6.77 million since the pandemic began, based on the AAP/CHA accounting of state cases, although Alabama, Nebraska, and Texas stopped reporting over the summer, suggesting the actual number is higher. The CDC puts the total number of COVID cases in children at 5.96 million, but there are age discrepancies between the CDC and the AAP/CHA’s state-based data.
The vaccine gap is closing
Vaccinations among the recently eligible 5- to 11-year-olds have steadily increased following a somewhat slow start. The initial pace was behind that of the 12- to 15-years-olds through the first postapproval week but has since closed the gap, based on data from the CDC’s COVID Data Tracker.
The tally of children who received at least one dose of the COVID vaccine among the 5- to 11-year-olds was behind the older group by almost 1.2 million on day 7 after the CDC’s Nov. 2 approval, but by day 18 the deficit was down to about 650,000, the CDC reported.
Altogether, just over 3 million children aged 5-11 have received at least one dose, which is 10.7% of that age group’s total population. Among children aged 12-17, the proportions are 60.7% with at least one dose and 51.1% at full vaccination. Children aged 5-11, who make up 8.7% of the total U.S. population, represented 42.8% of all vaccinations initiated over the 2 weeks ending Nov. 21, compared with 4.2% for those aged 12-17, the CDC said.






