User login
Lack of high school education vaccine hesitancy predictor
Lack of a high school education is a predictor of whether a person will be resistant to getting the COVID-19 vaccine, a new study shows.
Researchers from the University of North Carolina looked at vaccination rates in 3,142 counties in the U.S. They compared them to population characteristics based on the CDC Social Vulnerability Index.
They found that more than half of the unvaccinated adults in the U.S. with strong vaccine hesitancy had a high school education or less. Vaccine hesitancy was defined as refusal to be vaccinated even if the COVID-19 vaccine was available.
The other main predictor for vaccine hesitancy was concern about vaccine availability and distribution, the researchers said.
“Our study suggests that low education levels are a major contributor to vaccine hesitancy and ultimately vaccination levels,” the authors wrote. The study was published in the American Journal of Infection Control. “Specifically, low vaccination levels were found in communities with a less educated population and with more concern about vaccine uptake capacity, suggesting that education is an ongoing challenge.”
“Our findings suggest that policy makers and community leaders should tailor vaccine information and efforts to those with limited education and specifically address knowledge concerns that are prevalent and likely more modifiable.”
The study was based on data gathered months ago. It says that as of May 9, 2021, 34.7% of the U.S. population was fully vaccinated and that 8% reported a strong unwillingness to get vaccinated.
At press time, the Centers for Disease Control and Prevention’s COVID Data Tracker showed that 62.5% of the U.S. population was fully vaccinated.
According to the study, other consistent characteristics of people who are vaccine hesitant are that they belong to a racial minority, are 65 or older, live in a household with children 18 or younger, or are unemployed.
When asked why they were vaccine hesitant, people gave these reasons: Lack of trust in COVID-19 vaccines (55%), concerns about side effects (48%), and lack of trust in government (46%).
Lack of access to vaccines, often cited in previous studies about resistance to other vaccines, was not cited as a reason for not getting the COVID-19 vaccine.
“COVID-19 vaccine hesitancy is a public health threat,” the researchers concluded. “Since education levels are not easily modifiable, our results suggest that policymakers would be best served by closing knowledge gaps to overcome negative perceptions of the vaccine through tailored interventions.”
A version of this article first appeared on WebMD.com.
Lack of a high school education is a predictor of whether a person will be resistant to getting the COVID-19 vaccine, a new study shows.
Researchers from the University of North Carolina looked at vaccination rates in 3,142 counties in the U.S. They compared them to population characteristics based on the CDC Social Vulnerability Index.
They found that more than half of the unvaccinated adults in the U.S. with strong vaccine hesitancy had a high school education or less. Vaccine hesitancy was defined as refusal to be vaccinated even if the COVID-19 vaccine was available.
The other main predictor for vaccine hesitancy was concern about vaccine availability and distribution, the researchers said.
“Our study suggests that low education levels are a major contributor to vaccine hesitancy and ultimately vaccination levels,” the authors wrote. The study was published in the American Journal of Infection Control. “Specifically, low vaccination levels were found in communities with a less educated population and with more concern about vaccine uptake capacity, suggesting that education is an ongoing challenge.”
“Our findings suggest that policy makers and community leaders should tailor vaccine information and efforts to those with limited education and specifically address knowledge concerns that are prevalent and likely more modifiable.”
The study was based on data gathered months ago. It says that as of May 9, 2021, 34.7% of the U.S. population was fully vaccinated and that 8% reported a strong unwillingness to get vaccinated.
At press time, the Centers for Disease Control and Prevention’s COVID Data Tracker showed that 62.5% of the U.S. population was fully vaccinated.
According to the study, other consistent characteristics of people who are vaccine hesitant are that they belong to a racial minority, are 65 or older, live in a household with children 18 or younger, or are unemployed.
When asked why they were vaccine hesitant, people gave these reasons: Lack of trust in COVID-19 vaccines (55%), concerns about side effects (48%), and lack of trust in government (46%).
Lack of access to vaccines, often cited in previous studies about resistance to other vaccines, was not cited as a reason for not getting the COVID-19 vaccine.
“COVID-19 vaccine hesitancy is a public health threat,” the researchers concluded. “Since education levels are not easily modifiable, our results suggest that policymakers would be best served by closing knowledge gaps to overcome negative perceptions of the vaccine through tailored interventions.”
A version of this article first appeared on WebMD.com.
Lack of a high school education is a predictor of whether a person will be resistant to getting the COVID-19 vaccine, a new study shows.
Researchers from the University of North Carolina looked at vaccination rates in 3,142 counties in the U.S. They compared them to population characteristics based on the CDC Social Vulnerability Index.
They found that more than half of the unvaccinated adults in the U.S. with strong vaccine hesitancy had a high school education or less. Vaccine hesitancy was defined as refusal to be vaccinated even if the COVID-19 vaccine was available.
The other main predictor for vaccine hesitancy was concern about vaccine availability and distribution, the researchers said.
“Our study suggests that low education levels are a major contributor to vaccine hesitancy and ultimately vaccination levels,” the authors wrote. The study was published in the American Journal of Infection Control. “Specifically, low vaccination levels were found in communities with a less educated population and with more concern about vaccine uptake capacity, suggesting that education is an ongoing challenge.”
“Our findings suggest that policy makers and community leaders should tailor vaccine information and efforts to those with limited education and specifically address knowledge concerns that are prevalent and likely more modifiable.”
The study was based on data gathered months ago. It says that as of May 9, 2021, 34.7% of the U.S. population was fully vaccinated and that 8% reported a strong unwillingness to get vaccinated.
At press time, the Centers for Disease Control and Prevention’s COVID Data Tracker showed that 62.5% of the U.S. population was fully vaccinated.
According to the study, other consistent characteristics of people who are vaccine hesitant are that they belong to a racial minority, are 65 or older, live in a household with children 18 or younger, or are unemployed.
When asked why they were vaccine hesitant, people gave these reasons: Lack of trust in COVID-19 vaccines (55%), concerns about side effects (48%), and lack of trust in government (46%).
Lack of access to vaccines, often cited in previous studies about resistance to other vaccines, was not cited as a reason for not getting the COVID-19 vaccine.
“COVID-19 vaccine hesitancy is a public health threat,” the researchers concluded. “Since education levels are not easily modifiable, our results suggest that policymakers would be best served by closing knowledge gaps to overcome negative perceptions of the vaccine through tailored interventions.”
A version of this article first appeared on WebMD.com.
FROM THE AMERICAN JOURNAL OF INFECTION CONTROL
COVID-19 linked to increased diabetes risk in youth
SARS-CoV-2 infection was associated with an increased risk for diabetes among youth, whereas other acute respiratory infections were not, new data from the U.S. Centers for Disease Control and Prevention indicate.
The results from two large U.S. health claims databases were published in an early release in the CDC’s Morbidity and Mortality Weekly Report by Catherine E. Barrett, PhD, and colleagues of the CDC’s COVID-19 Emergency Response Team and Division of Diabetes Translation.
Clinicians should monitor individuals younger than 18 years in the months following a SARS-CoV-2 infection for new diabetes onset, they advise.
The findings, which are supported by independent studies in adults, “underscore the importance of COVID-19 prevention among all age groups, including vaccination for all eligible children and adolescents, and chronic disease prevention and treatment,” Dr. Barrett and colleagues say.
Diabetes type couldn’t be reliably distinguished from the databases, which is noted as an important study limitation.
“SARS-CoV-2 infection might lead to type 1 or type 2 diabetes through complex and differing mechanisms,” they say.
Emerging evidence began to suggest, in mid-2020, that COVID-19 may trigger the onset of diabetes in healthy people. A new global registry was subsequently established to collect data on patients with COVID-19–related diabetes, called the CoviDiab registry.
Not clear if diabetes after COVID-19 is transient or permanent
From one of the databases used in the new study, known as IQVIA, 80,893 individuals aged younger than 18 years diagnosed with COVID-19 during March 2020 to February 26, 2021, were compared with age- and sex-matched people during that period who did not have COVID-19 and to prepandemic groups with and without a diagnosis of acute respiratory illness during March 1, 2017, to February 26, 2018.
From the second database, HealthVerity, 439,439 youth diagnosed with COVID-19 during March 1, 2020, to June 28, 2021, were compared with age- and sex-matched youth without COVID-19. Here, there was no prepandemic comparison group.
Diabetes diagnoses were coded in 0.08% with COVID-19 vs. 0.03% without COVID-19 in IQVIA and in 0.25% vs. 0.19% in HealthVerity.
Thus, new diabetes diagnoses were 166% and 31% more likely to occur in those with COVID-19 in IQVIA and HealthVerity, respectively. And in IQVIA, those with COVID-19 were 116% more likely to develop diabetes than were those with prepandemic acute respiratory illnesses. Those differences were all significant, whereas non–SARS-CoV-2 respiratory infections were not associated with diabetes, Dr. Barrett and colleagues say.
In both databases, diabetic ketoacidosis (DKA) was more common at diabetes onset among those with, vs. without, COVID-19: 48.5% vs. 13.6% in IQVIA and 40.2% vs. 29.7% in HealthVerity. In IQVIA, 22.0% with prepandemic acute respiratory illness presented with DKA.
Dr. Barrett and colleagues offer several potential explanations for the observed association between COVID-19 and diabetes, including a direct attack on pancreatic beta cells expressing angiotensin-converting enzyme 2 receptors, or via stress hyperglycemia resulting from cytokine storm and alterations in glucose metabolism.
Another possibility is the precipitation to diabetes from prediabetes; the latter is a condition present in one in five U.S. adolescents.
Steroid treatment during hospitalization might have led to transient hyperglycemia, but only 1.5% to 2.2% of diabetes codes were for drug- or chemical-induced diabetes. The majority were for type 1 or 2.
Alternatively, pandemic-associated weight gain might have also contributed to risks for both severe COVID-19 and type 2 diabetes.
“Although this study can provide information on the risk for diabetes following SARS-CoV-2 infection, additional data are needed to understand underlying pathogenic mechanisms, either those caused by SARS-CoV-2 infection itself or resulting from treatments, and whether a COVID-19–associated diabetes diagnosis is transient or leads to a chronic condition,” Dr. Barrett and colleagues conclude.
A version of this article first appeared on Medscape.com.
SARS-CoV-2 infection was associated with an increased risk for diabetes among youth, whereas other acute respiratory infections were not, new data from the U.S. Centers for Disease Control and Prevention indicate.
The results from two large U.S. health claims databases were published in an early release in the CDC’s Morbidity and Mortality Weekly Report by Catherine E. Barrett, PhD, and colleagues of the CDC’s COVID-19 Emergency Response Team and Division of Diabetes Translation.
Clinicians should monitor individuals younger than 18 years in the months following a SARS-CoV-2 infection for new diabetes onset, they advise.
The findings, which are supported by independent studies in adults, “underscore the importance of COVID-19 prevention among all age groups, including vaccination for all eligible children and adolescents, and chronic disease prevention and treatment,” Dr. Barrett and colleagues say.
Diabetes type couldn’t be reliably distinguished from the databases, which is noted as an important study limitation.
“SARS-CoV-2 infection might lead to type 1 or type 2 diabetes through complex and differing mechanisms,” they say.
Emerging evidence began to suggest, in mid-2020, that COVID-19 may trigger the onset of diabetes in healthy people. A new global registry was subsequently established to collect data on patients with COVID-19–related diabetes, called the CoviDiab registry.
Not clear if diabetes after COVID-19 is transient or permanent
From one of the databases used in the new study, known as IQVIA, 80,893 individuals aged younger than 18 years diagnosed with COVID-19 during March 2020 to February 26, 2021, were compared with age- and sex-matched people during that period who did not have COVID-19 and to prepandemic groups with and without a diagnosis of acute respiratory illness during March 1, 2017, to February 26, 2018.
From the second database, HealthVerity, 439,439 youth diagnosed with COVID-19 during March 1, 2020, to June 28, 2021, were compared with age- and sex-matched youth without COVID-19. Here, there was no prepandemic comparison group.
Diabetes diagnoses were coded in 0.08% with COVID-19 vs. 0.03% without COVID-19 in IQVIA and in 0.25% vs. 0.19% in HealthVerity.
Thus, new diabetes diagnoses were 166% and 31% more likely to occur in those with COVID-19 in IQVIA and HealthVerity, respectively. And in IQVIA, those with COVID-19 were 116% more likely to develop diabetes than were those with prepandemic acute respiratory illnesses. Those differences were all significant, whereas non–SARS-CoV-2 respiratory infections were not associated with diabetes, Dr. Barrett and colleagues say.
In both databases, diabetic ketoacidosis (DKA) was more common at diabetes onset among those with, vs. without, COVID-19: 48.5% vs. 13.6% in IQVIA and 40.2% vs. 29.7% in HealthVerity. In IQVIA, 22.0% with prepandemic acute respiratory illness presented with DKA.
Dr. Barrett and colleagues offer several potential explanations for the observed association between COVID-19 and diabetes, including a direct attack on pancreatic beta cells expressing angiotensin-converting enzyme 2 receptors, or via stress hyperglycemia resulting from cytokine storm and alterations in glucose metabolism.
Another possibility is the precipitation to diabetes from prediabetes; the latter is a condition present in one in five U.S. adolescents.
Steroid treatment during hospitalization might have led to transient hyperglycemia, but only 1.5% to 2.2% of diabetes codes were for drug- or chemical-induced diabetes. The majority were for type 1 or 2.
Alternatively, pandemic-associated weight gain might have also contributed to risks for both severe COVID-19 and type 2 diabetes.
“Although this study can provide information on the risk for diabetes following SARS-CoV-2 infection, additional data are needed to understand underlying pathogenic mechanisms, either those caused by SARS-CoV-2 infection itself or resulting from treatments, and whether a COVID-19–associated diabetes diagnosis is transient or leads to a chronic condition,” Dr. Barrett and colleagues conclude.
A version of this article first appeared on Medscape.com.
SARS-CoV-2 infection was associated with an increased risk for diabetes among youth, whereas other acute respiratory infections were not, new data from the U.S. Centers for Disease Control and Prevention indicate.
The results from two large U.S. health claims databases were published in an early release in the CDC’s Morbidity and Mortality Weekly Report by Catherine E. Barrett, PhD, and colleagues of the CDC’s COVID-19 Emergency Response Team and Division of Diabetes Translation.
Clinicians should monitor individuals younger than 18 years in the months following a SARS-CoV-2 infection for new diabetes onset, they advise.
The findings, which are supported by independent studies in adults, “underscore the importance of COVID-19 prevention among all age groups, including vaccination for all eligible children and adolescents, and chronic disease prevention and treatment,” Dr. Barrett and colleagues say.
Diabetes type couldn’t be reliably distinguished from the databases, which is noted as an important study limitation.
“SARS-CoV-2 infection might lead to type 1 or type 2 diabetes through complex and differing mechanisms,” they say.
Emerging evidence began to suggest, in mid-2020, that COVID-19 may trigger the onset of diabetes in healthy people. A new global registry was subsequently established to collect data on patients with COVID-19–related diabetes, called the CoviDiab registry.
Not clear if diabetes after COVID-19 is transient or permanent
From one of the databases used in the new study, known as IQVIA, 80,893 individuals aged younger than 18 years diagnosed with COVID-19 during March 2020 to February 26, 2021, were compared with age- and sex-matched people during that period who did not have COVID-19 and to prepandemic groups with and without a diagnosis of acute respiratory illness during March 1, 2017, to February 26, 2018.
From the second database, HealthVerity, 439,439 youth diagnosed with COVID-19 during March 1, 2020, to June 28, 2021, were compared with age- and sex-matched youth without COVID-19. Here, there was no prepandemic comparison group.
Diabetes diagnoses were coded in 0.08% with COVID-19 vs. 0.03% without COVID-19 in IQVIA and in 0.25% vs. 0.19% in HealthVerity.
Thus, new diabetes diagnoses were 166% and 31% more likely to occur in those with COVID-19 in IQVIA and HealthVerity, respectively. And in IQVIA, those with COVID-19 were 116% more likely to develop diabetes than were those with prepandemic acute respiratory illnesses. Those differences were all significant, whereas non–SARS-CoV-2 respiratory infections were not associated with diabetes, Dr. Barrett and colleagues say.
In both databases, diabetic ketoacidosis (DKA) was more common at diabetes onset among those with, vs. without, COVID-19: 48.5% vs. 13.6% in IQVIA and 40.2% vs. 29.7% in HealthVerity. In IQVIA, 22.0% with prepandemic acute respiratory illness presented with DKA.
Dr. Barrett and colleagues offer several potential explanations for the observed association between COVID-19 and diabetes, including a direct attack on pancreatic beta cells expressing angiotensin-converting enzyme 2 receptors, or via stress hyperglycemia resulting from cytokine storm and alterations in glucose metabolism.
Another possibility is the precipitation to diabetes from prediabetes; the latter is a condition present in one in five U.S. adolescents.
Steroid treatment during hospitalization might have led to transient hyperglycemia, but only 1.5% to 2.2% of diabetes codes were for drug- or chemical-induced diabetes. The majority were for type 1 or 2.
Alternatively, pandemic-associated weight gain might have also contributed to risks for both severe COVID-19 and type 2 diabetes.
“Although this study can provide information on the risk for diabetes following SARS-CoV-2 infection, additional data are needed to understand underlying pathogenic mechanisms, either those caused by SARS-CoV-2 infection itself or resulting from treatments, and whether a COVID-19–associated diabetes diagnosis is transient or leads to a chronic condition,” Dr. Barrett and colleagues conclude.
A version of this article first appeared on Medscape.com.
FROM MMWR
As pandemic regs expire, states get tougher on telehealth: report
Among the most important restrictions that have been reinstated in some states are those barring requirements for insurers to cover telehealth and regulations that prohibit telehealth visits across state lines, unless the physician is licensed in both states.
“Only three states – Arizona, Florida, and Indiana – allow all health care providers to easily practice telehealth across state lines,” says a news release on the think tanks’ report. “Forty-seven others have arbitrary barriers in place that limit patients’ access to specialists and available appointments based purely on residency.”
“Once the [state-based] public health emergency declarations started to end or executive orders were withdrawn, many of the new flexibilities for providers, insurers, and patients were lost overnight,” Vittorio Nastasi, a policy analyst at Reason Foundation and a co-author of the report, says in the news release. “States need to adopt a number of telehealth reforms to provide their residents better access to this safe and effective virtual care.”
On a positive note, the report says, most states have removed the requirement that a patient must first see a provider in person before they can use telehealth services. The exceptions are Tennessee, Alaska, and West Virginia, which require an in-person visit before certain telehealth services can be provided.
In addition, 20 states allow nurse practitioners to conduct telehealth visits without being under the supervision of a physician. Prior to the pandemic, some states allowed only doctors to use telehealth, the report says, but, during the COVID crisis, “the acute shortage of providers in many counties adds to the need for more kinds of providers to be able to use it.”
A number of states place restrictions on the telehealth modalities that can be utilized. Under the definition by the American Telemedicine Association, telehealth includes audio-video visits, remote patient monitoring, and “store and forward” telemedicine, which entails collecting clinical information and sending it to another site for evaluation. The latter method is particularly useful for consultations with specialists, the report notes.
Coverage mandates and payment parity
The report also examines other parameters of telehealth regulations in each state, including whether they have telehealth coverage mandates and whether they require physicians to be paid the same amount for similar types of in-person and telehealth visits.
The report views insurance mandates as beneficial, but not if they require coverage of all virtual services. While telehealth can be a game changer for post-stroke care and for other “treatment-intensive conditions,” the report says, the evidence of better outcomes for other conditions treated through telehealth is far less certain. Therefore, it advises states to “protect flexibility so that new innovative models can emerge.”
Ateev Mehrotra, MD, a professor at Harvard Medical School who studies telehealth, agrees that it offers more value in some clinical situations than in others. “High value is improving quality or outcomes at a reasonable cost,” he told this news organization. “If a telemedicine visit for stroke can save a person’s life and prevent disability, let’s pay for it. A telemedicine visit for a cold may not be necessary. Mom’s chicken soup is fine.”
A little over half of the states still require payment parity, according to the report. While these regulations are intended to promote the use of telehealth, the authors note, they can increase the growth of health care costs. Moreover, they argue, it’s hard to defend equal payments for virtual visits when the overhead required to deliver them – such as office rental, utility, and labor costs – is much lower than that for in-person visits. Also, it makes no sense for health systems to charge facility fees for telehealth visits when these visits can be initiated from anywhere, they say.
Dr. Mehrotra concurs with this view. “If you see someone in your office, your fee includes all the overhead for your office, and it’s a substantial cost,” he says. “For many procedures, it’s more than half of the cost. If you have a telemedicine visit and you’re at home, why would you pay the same amount? The visit may take the same amount of time, but all the money that goes for overhead is not accounted for.”
Telemedicine across state lines
The report’s contention about the difficulty of conducting telehealth encounters across most state lines seems to be at odds with the growth in the Interstate Medical Licensure Compact, which makes it easier for physicians in one compact member state to get licensed in others. Currently, 35 states belong to the compact, Joe Knickrehm, vice president of communications for the Federation of State Medical Boards, told this news organization.
In addition, he says, “12 state boards issue a special purpose license, telemedicine license or certificate, or license to practice medicine across state lines to allow for the practice of telemedicine.”
The catch, Dr. Mehrotra says, is that, despite the streamlining of license applications in compact member states, the fees charged by the state boards are still very high – a point that the report also makes. “If I want to have broad scope of practice, I’d have to pay thousands of dollars to many states. The license fees start to add up. Also, I have to keep track of each state’s CME requirements, which are all different. Keeping up with all of that is an administration burden, and it’s a pain.”
Mr. Knickrehm contends that obtaining multiple licenses via the compact “is generally less expensive for physicians than the cost of requesting transcripts, fingerprints, and other necessary paperwork each time they apply for licensure in a new state. Physicians are seeing the benefits of an expedited process that allows them to begin practicing more quickly [in other states].”
Dr. Mehrotra says he has seen the same retrenchment in state telehealth regulations that the report references. However, he says, “CMS [the Centers for Medicare & Medicaid Services] has signaled that at least through 2022 and maybe into 2023, they’ll continue their extensions of telemedicine [pandemic regulations].” After that, Congress would have to decide whether to make the changes permanent.
“Right now, it’s hard for me to see how a payer is going to pull back on telehealth, unless there’s ample evidence of overuse of telehealth,” he argues. “With the public and providers liking telehealth, it’s hard to say on theoretical grounds that we should stop using it. That’s why Medicare and others have extended it and why Congress will too.”
A version of this article first appeared on Medscape.com.
Among the most important restrictions that have been reinstated in some states are those barring requirements for insurers to cover telehealth and regulations that prohibit telehealth visits across state lines, unless the physician is licensed in both states.
“Only three states – Arizona, Florida, and Indiana – allow all health care providers to easily practice telehealth across state lines,” says a news release on the think tanks’ report. “Forty-seven others have arbitrary barriers in place that limit patients’ access to specialists and available appointments based purely on residency.”
“Once the [state-based] public health emergency declarations started to end or executive orders were withdrawn, many of the new flexibilities for providers, insurers, and patients were lost overnight,” Vittorio Nastasi, a policy analyst at Reason Foundation and a co-author of the report, says in the news release. “States need to adopt a number of telehealth reforms to provide their residents better access to this safe and effective virtual care.”
On a positive note, the report says, most states have removed the requirement that a patient must first see a provider in person before they can use telehealth services. The exceptions are Tennessee, Alaska, and West Virginia, which require an in-person visit before certain telehealth services can be provided.
In addition, 20 states allow nurse practitioners to conduct telehealth visits without being under the supervision of a physician. Prior to the pandemic, some states allowed only doctors to use telehealth, the report says, but, during the COVID crisis, “the acute shortage of providers in many counties adds to the need for more kinds of providers to be able to use it.”
A number of states place restrictions on the telehealth modalities that can be utilized. Under the definition by the American Telemedicine Association, telehealth includes audio-video visits, remote patient monitoring, and “store and forward” telemedicine, which entails collecting clinical information and sending it to another site for evaluation. The latter method is particularly useful for consultations with specialists, the report notes.
Coverage mandates and payment parity
The report also examines other parameters of telehealth regulations in each state, including whether they have telehealth coverage mandates and whether they require physicians to be paid the same amount for similar types of in-person and telehealth visits.
The report views insurance mandates as beneficial, but not if they require coverage of all virtual services. While telehealth can be a game changer for post-stroke care and for other “treatment-intensive conditions,” the report says, the evidence of better outcomes for other conditions treated through telehealth is far less certain. Therefore, it advises states to “protect flexibility so that new innovative models can emerge.”
Ateev Mehrotra, MD, a professor at Harvard Medical School who studies telehealth, agrees that it offers more value in some clinical situations than in others. “High value is improving quality or outcomes at a reasonable cost,” he told this news organization. “If a telemedicine visit for stroke can save a person’s life and prevent disability, let’s pay for it. A telemedicine visit for a cold may not be necessary. Mom’s chicken soup is fine.”
A little over half of the states still require payment parity, according to the report. While these regulations are intended to promote the use of telehealth, the authors note, they can increase the growth of health care costs. Moreover, they argue, it’s hard to defend equal payments for virtual visits when the overhead required to deliver them – such as office rental, utility, and labor costs – is much lower than that for in-person visits. Also, it makes no sense for health systems to charge facility fees for telehealth visits when these visits can be initiated from anywhere, they say.
Dr. Mehrotra concurs with this view. “If you see someone in your office, your fee includes all the overhead for your office, and it’s a substantial cost,” he says. “For many procedures, it’s more than half of the cost. If you have a telemedicine visit and you’re at home, why would you pay the same amount? The visit may take the same amount of time, but all the money that goes for overhead is not accounted for.”
Telemedicine across state lines
The report’s contention about the difficulty of conducting telehealth encounters across most state lines seems to be at odds with the growth in the Interstate Medical Licensure Compact, which makes it easier for physicians in one compact member state to get licensed in others. Currently, 35 states belong to the compact, Joe Knickrehm, vice president of communications for the Federation of State Medical Boards, told this news organization.
In addition, he says, “12 state boards issue a special purpose license, telemedicine license or certificate, or license to practice medicine across state lines to allow for the practice of telemedicine.”
The catch, Dr. Mehrotra says, is that, despite the streamlining of license applications in compact member states, the fees charged by the state boards are still very high – a point that the report also makes. “If I want to have broad scope of practice, I’d have to pay thousands of dollars to many states. The license fees start to add up. Also, I have to keep track of each state’s CME requirements, which are all different. Keeping up with all of that is an administration burden, and it’s a pain.”
Mr. Knickrehm contends that obtaining multiple licenses via the compact “is generally less expensive for physicians than the cost of requesting transcripts, fingerprints, and other necessary paperwork each time they apply for licensure in a new state. Physicians are seeing the benefits of an expedited process that allows them to begin practicing more quickly [in other states].”
Dr. Mehrotra says he has seen the same retrenchment in state telehealth regulations that the report references. However, he says, “CMS [the Centers for Medicare & Medicaid Services] has signaled that at least through 2022 and maybe into 2023, they’ll continue their extensions of telemedicine [pandemic regulations].” After that, Congress would have to decide whether to make the changes permanent.
“Right now, it’s hard for me to see how a payer is going to pull back on telehealth, unless there’s ample evidence of overuse of telehealth,” he argues. “With the public and providers liking telehealth, it’s hard to say on theoretical grounds that we should stop using it. That’s why Medicare and others have extended it and why Congress will too.”
A version of this article first appeared on Medscape.com.
Among the most important restrictions that have been reinstated in some states are those barring requirements for insurers to cover telehealth and regulations that prohibit telehealth visits across state lines, unless the physician is licensed in both states.
“Only three states – Arizona, Florida, and Indiana – allow all health care providers to easily practice telehealth across state lines,” says a news release on the think tanks’ report. “Forty-seven others have arbitrary barriers in place that limit patients’ access to specialists and available appointments based purely on residency.”
“Once the [state-based] public health emergency declarations started to end or executive orders were withdrawn, many of the new flexibilities for providers, insurers, and patients were lost overnight,” Vittorio Nastasi, a policy analyst at Reason Foundation and a co-author of the report, says in the news release. “States need to adopt a number of telehealth reforms to provide their residents better access to this safe and effective virtual care.”
On a positive note, the report says, most states have removed the requirement that a patient must first see a provider in person before they can use telehealth services. The exceptions are Tennessee, Alaska, and West Virginia, which require an in-person visit before certain telehealth services can be provided.
In addition, 20 states allow nurse practitioners to conduct telehealth visits without being under the supervision of a physician. Prior to the pandemic, some states allowed only doctors to use telehealth, the report says, but, during the COVID crisis, “the acute shortage of providers in many counties adds to the need for more kinds of providers to be able to use it.”
A number of states place restrictions on the telehealth modalities that can be utilized. Under the definition by the American Telemedicine Association, telehealth includes audio-video visits, remote patient monitoring, and “store and forward” telemedicine, which entails collecting clinical information and sending it to another site for evaluation. The latter method is particularly useful for consultations with specialists, the report notes.
Coverage mandates and payment parity
The report also examines other parameters of telehealth regulations in each state, including whether they have telehealth coverage mandates and whether they require physicians to be paid the same amount for similar types of in-person and telehealth visits.
The report views insurance mandates as beneficial, but not if they require coverage of all virtual services. While telehealth can be a game changer for post-stroke care and for other “treatment-intensive conditions,” the report says, the evidence of better outcomes for other conditions treated through telehealth is far less certain. Therefore, it advises states to “protect flexibility so that new innovative models can emerge.”
Ateev Mehrotra, MD, a professor at Harvard Medical School who studies telehealth, agrees that it offers more value in some clinical situations than in others. “High value is improving quality or outcomes at a reasonable cost,” he told this news organization. “If a telemedicine visit for stroke can save a person’s life and prevent disability, let’s pay for it. A telemedicine visit for a cold may not be necessary. Mom’s chicken soup is fine.”
A little over half of the states still require payment parity, according to the report. While these regulations are intended to promote the use of telehealth, the authors note, they can increase the growth of health care costs. Moreover, they argue, it’s hard to defend equal payments for virtual visits when the overhead required to deliver them – such as office rental, utility, and labor costs – is much lower than that for in-person visits. Also, it makes no sense for health systems to charge facility fees for telehealth visits when these visits can be initiated from anywhere, they say.
Dr. Mehrotra concurs with this view. “If you see someone in your office, your fee includes all the overhead for your office, and it’s a substantial cost,” he says. “For many procedures, it’s more than half of the cost. If you have a telemedicine visit and you’re at home, why would you pay the same amount? The visit may take the same amount of time, but all the money that goes for overhead is not accounted for.”
Telemedicine across state lines
The report’s contention about the difficulty of conducting telehealth encounters across most state lines seems to be at odds with the growth in the Interstate Medical Licensure Compact, which makes it easier for physicians in one compact member state to get licensed in others. Currently, 35 states belong to the compact, Joe Knickrehm, vice president of communications for the Federation of State Medical Boards, told this news organization.
In addition, he says, “12 state boards issue a special purpose license, telemedicine license or certificate, or license to practice medicine across state lines to allow for the practice of telemedicine.”
The catch, Dr. Mehrotra says, is that, despite the streamlining of license applications in compact member states, the fees charged by the state boards are still very high – a point that the report also makes. “If I want to have broad scope of practice, I’d have to pay thousands of dollars to many states. The license fees start to add up. Also, I have to keep track of each state’s CME requirements, which are all different. Keeping up with all of that is an administration burden, and it’s a pain.”
Mr. Knickrehm contends that obtaining multiple licenses via the compact “is generally less expensive for physicians than the cost of requesting transcripts, fingerprints, and other necessary paperwork each time they apply for licensure in a new state. Physicians are seeing the benefits of an expedited process that allows them to begin practicing more quickly [in other states].”
Dr. Mehrotra says he has seen the same retrenchment in state telehealth regulations that the report references. However, he says, “CMS [the Centers for Medicare & Medicaid Services] has signaled that at least through 2022 and maybe into 2023, they’ll continue their extensions of telemedicine [pandemic regulations].” After that, Congress would have to decide whether to make the changes permanent.
“Right now, it’s hard for me to see how a payer is going to pull back on telehealth, unless there’s ample evidence of overuse of telehealth,” he argues. “With the public and providers liking telehealth, it’s hard to say on theoretical grounds that we should stop using it. That’s why Medicare and others have extended it and why Congress will too.”
A version of this article first appeared on Medscape.com.
A COVID-19 Clinical Management Committee to Standardize Care in a 2-Hospital System
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
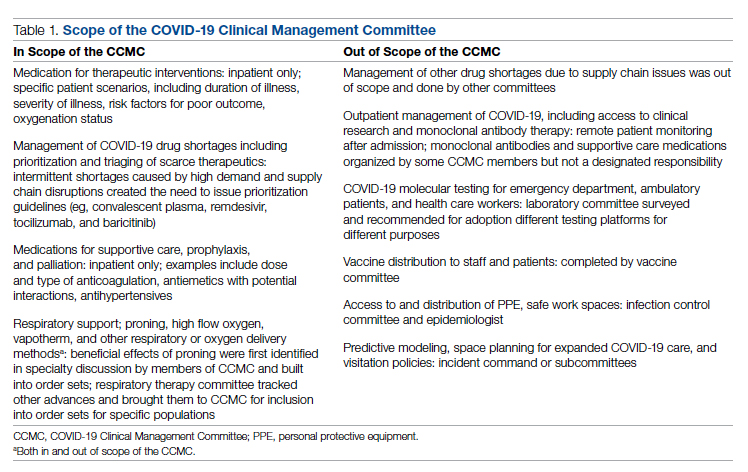
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.
A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
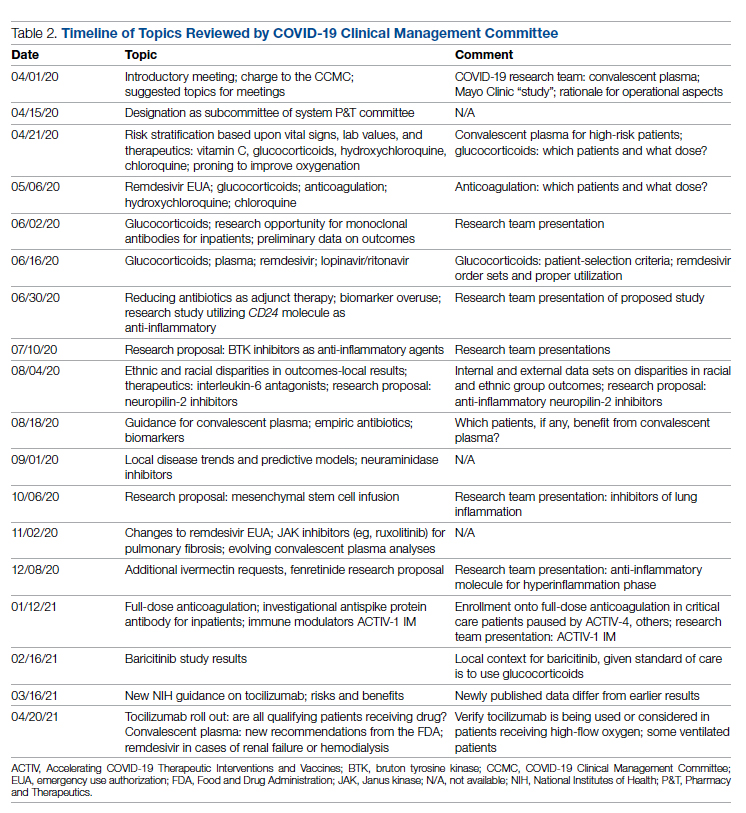
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.
Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
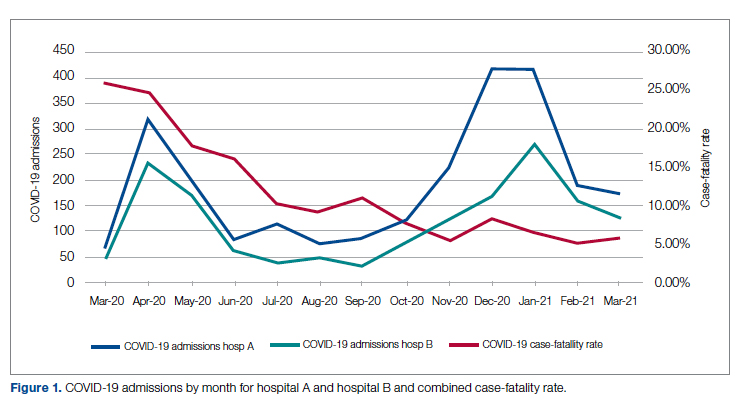
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.
Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
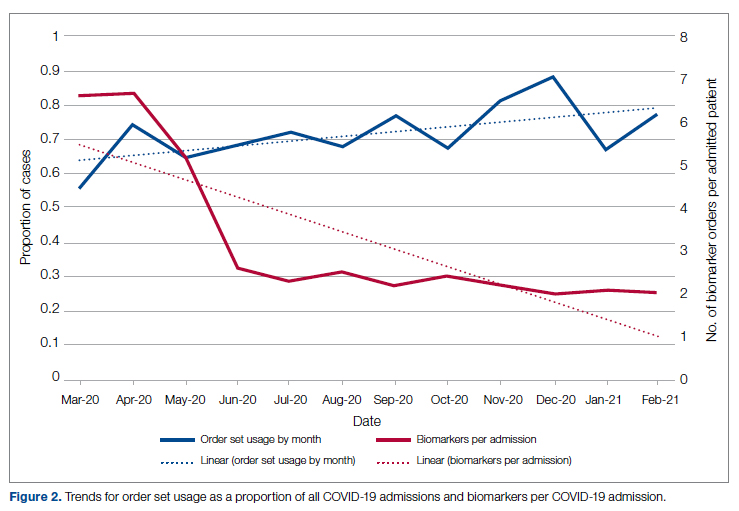
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.
Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)
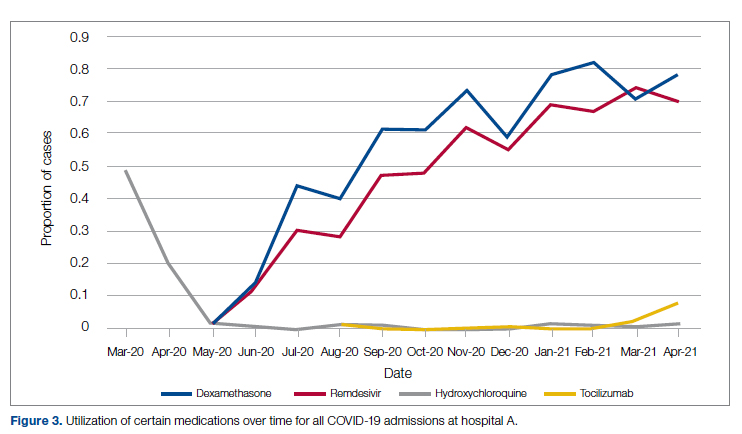
Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
From the Department of Medicine (Drs. Meisenberg, Muganlinskaya, Sharma, Amjadi, Arnold, Barnes, Clance, Khalil, Miller, Mooradian, O’Connell, Patel, Press, Samaras, Shanmugam, Tavadze, and Thompson), Department of Pharmacy (Drs. Jiang, Jarawan, Sheth, and Trinh), Department of Nursing (Dr. Ohnmacht), and Department of Women and Children’s Services (Dr. Raji), Luminis Health, Annapolis, MD, and Lanham, MD.
Objective: The COVID-19 pandemic has been a challenge for hospital medical staffs worldwide due to high volumes of patients acutely ill with novel syndromes and prevailing uncertainty regarding optimum supportive and therapeutic interventions. Additionally, the response to this crisis was driven by a plethora of nontraditional information sources, such as email chains, websites, non–peer-reviewed preprints, and press releases. Care patterns became idiosyncratic and often incorporated unproven interventions driven by these nontraditional information sources. This report evaluates the efforts of a health system to create and empower a multidisciplinary committee to develop, implement, and monitor evidence-based, standardized protocols for patients with COVID-19.
Methods: This report describes the composition of the committee, its scope, and its important interactions with the health system pharmacy and therapeutics committee, research teams, and other work groups planning other aspects of COVID-19 management. It illustrates how the committee was used to demonstrate for trainees the process and value of critically examining evidence, even in a chaotic environment.
Results: Data show successful interventions in reducing excessive ordering of certain laboratory tests, reduction of nonrecommended therapies, and rapid uptake of evidence-based or guidelines-supported interventions.
Conclusions: A multidisciplinary committee dedicated solely to planning, implementing, and monitoring standard approaches that eventually became evidence-based decision-making led to an improved focus on treatment options and outcomes for COVID-19 patients. Data presented illustrate the attainable success that is both adaptable and suitable for similar emergencies in the future.
Keywords: COVID-19; clinical management; pharmacy and therapeutics; treatment; therapy.
The COVID-19 pandemic has spread to nearly all countries, carrying with it high morbidity, mortality, and severe impacts on both well-developed and less-well-developed health systems. Media reports of chaos within overwhelmed hospitals have been prominent.1,2 As of January 5, 2022, SARS-CoV-2 has infected more than 295 million people globally and directly caused the death of more than 5.4 million,3 though this number is likely an undercount even in countries with well-developed mortality tracking.4
Throughout the COVID-19 pandemic, hospital-based medical teams have been confronted with a flood of severely ill patients with novel syndromes. Initially, there were no standards for therapy or supportive care except for treatments borrowed from similar syndromes. In the setting of high volumes, high acuity, and public dismay, it is unsurprising that the usual deliberative methods for weighing evidence and initiating interventions were often pushed aside in favor of the solace of active intervention.5 In this milieu of limited evidence, there was a lamentable, if understandable, tendency to seek guidance from “nontraditional” sources,6 including email chains from colleagues, hospital websites, non–peer-reviewed manuscripts, advanced publication by medical journals,7 and nonscientific media presentations. In many localities, practitioners responded in idiosyncratic ways. For example, findings of high cytokine levels in COVID-19,8 along with reports of in-vitro antiviral activity with drugs like hydroxychloroquine against both SARS9 and SARS-CoV-2,10 drove laboratory test ordering and therapeutic interventions, respectively, carving shortcuts into the traditional clinical trial–dependent standards. Clinical trial results eventually emerged.11COVID-19 created a clinical dilemma for hospital medical staffs in terms of how to organize, standardize, and rapidly adapt to a flood of new information. In this report, we describe how 1 health system responded to these challenges by forming a COVID-19 Clinical Management Committee (CCMC) and empowering this interdisciplinary team to review evidence, create and adjust order sets, educate practitioners, oversee care, and collaborate across teams addressing other aspects of the COVID-19 response.
Program Overview
Health System Description
Luminis Health is a health system with 2 acute care hospitals that was formed in 2019 just before the start of the pandemic. Anne Arundel Medical Center (hospital A) is a 385-bed teaching hospital in Annapolis, MD. It has more than 23 000 discharges annually. Patients with COVID-19 were cared for by either an internal medicine teaching service or nonteaching hospitalist services on cohorted nursing units. Doctor’s Community Medical Center, in Lanham, MD (hospital B), is a 206-bed acute care hospital with more than 10 350 annual discharges. COVID-19 patients were cared for by hospitalist groups, initially in noncohorted units with transition to cohorted nursing units after a few months. The medical staffs are generally distinct, with different leadership structures, though the Luminis Health Department of Medicine has oversight responsibilities at both hospitals. More than 47 physicians attended COVID-19 patients at hospital A (with medical residents) and 30 individual physicians at hospital B, respectively, including intensivists. The nursing and pharmacy staffs are distinct, but there is a shared oversight Pharmacy and Therapeutics (P&T) Committee.
The 2 hospitals had distinct electronic medical records (EMR) until January 2021, when hospital B adopted the same EMR as hospital A (Epic).
Mission and Formation of CCMC
In order to coordinate the therapeutic approach across the health system, it was important for the CCMC to be designated by the health system P&T committee as an official subcommittee so that decisions on restrictions of medications and/or new or revised order sets could be rapidly initiated across the system without waiting for the subsequent P&T meetings. The full committee retained oversight of the CCMC. Some P&T members were also on the CCMC.
The committee reviewed new reports in medical journals and prepublication servers and consulted recommendations of professional societies, such as the National Institutes of Health (NIH) COVID-19 guidelines, Infectious Diseases Society of America, Society of Critical Care Medicine, and US Food and Drug Administration (FDA) Emergency Use Authorizations (EUA), among other sources.
Composition of the CCMC
Physician leaders from both hospitals in the following specialties were solicited for participation: critical care, epidemiology, hospital medicine (internal medicine), emergency medicine, infectious diseases, nephrology, women and children’s services, and medical informatics. Specialists in other areas, such as hematology, were invited for topic-specific discussions. Hospital pharmacists with different specialties and nursing leadership were essential contributors. The committee members were expected to use various communication channels to inform frontline clinicians of new care standards and the existence of new order sets, which were embedded in the EMR.
Clinical Research
An important connection for the CCMC was with theCOVID-19 clinical research team. Three members of the research team were also members of the CCMC. All new study proposals for therapeutics were discussed with the CCMC as they were being considered by the research team. In this way, feedback on the feasibility and acceptance of new study opportunities could be discussed with the CCMC. Occasionally, CCMC decisions impacted clinical research accrual strategies. For example, new data from randomized trials about tocilizumab1,2 demonstrated benefits in some subsets of patients and resulted in a recommendation for use by the NIH guideline committee in these populations.1 The CCMC quickly adopted this recommendation, which required a reprioritization of clinical research enrollment for studies testing other immune-modulating agents. This important dialogue was mediated within the CCMC.
Guideline Distribution, Reinforcement, and Platform for Feedback
New guidelines were disseminated to clinicians via daily brief patient huddles held on COVID units, with participation by nursing and pharmacy, and by weekly meetings with hospitalist leaders and frontline hospital physicians. Order sets and guidelines were maintained on the intranet. Adherence was reinforced by unit-based and central pharmacists. Order sets, including admission order sets, could be created only by designated informatics personnel, thus enforcing standardization. Feedback on the utility of the order sets was obtained during the weekly meetings or huddles, as described above. To ensure a sense of transparency, physicians who had interest in commenting on a particular therapy, or who wished to discuss a particular manuscript, news article, or website, were invited to attend CCMC meetings.
Scope of CCMC
In order to be effective and timely, we limited the scope of our work to the report to the inpatient therapeutic environment, allowing other committees to work on other aspects of the pandemic response. In addition to issuing guidance and creating order sets to direct clinical practice, the CCMC also monitored COVID-19 therapeutic shortages15,16 and advised on prioritization of such treatments as convalescent plasma, remdesivir (prioritization and duration of therapy, 5 vs 10 days), baricitinib, and tocilizumab, depending upon the location of the patient (critical care or not). The CCMC was not involved in the management of non–COVID-19 shortages brought about by supply chain deficiencies.
Table 1 shows some aspects of the health system pandemic-response planning and the committee workforce that undertook that work. Though many items were out of scope for the CCMC, members of the CCMC did participate in the planning work of these other committees and therefore stayed connected to this complementary work.

A Teaching Opportunity About Making Thoughtful Choices
Another important feature of the CCMC was the contributions of residents from both pharmacy and internal medicine. The purpose and operations of the committee were recognized as an opportunity to involve learners in a curriculum based on Kern’s 6-step approach.17 Though the problem identification and general needs assessment were easily defined, the targeted needs assessment, extracted from individual and group interviews with learners and the committee members, pointed at the need to learn how to assess and analyze a rapidly growing body of literature on several relevant clinical aspects of SARS-CoV-2 and COVID-19. To achieve goals and objectives, residents were assigned to present current literature on a particular intervention during a committee meeting, specifically commenting on the merit or deficiencies of the study design, the strength of the data, and applicability to the local context with a recommendation. Prior to the presentations, the residents worked with faculty to identify the best studies or systematic analyses with potential to alter current practices. We thus used the CCMC process as a teaching tool about evidence-based medicine and the dilemma of clinical equipoise. This was imperative, since trainees thrust into the COVID-19 response have often keenly observed a movement away from deliberative decision-making.18 Indeed, including residents in the process of deliberative responses to COVID-19 addresses a recent call to adjust medical education during COVID-19 to “adapt curriculum to current issues in real time.”19
Interventions and Therapies Considered
Table 2 shows the topics reviewed by the CCMC. By the time of the first meeting, nonstandardization of care was already a source of concern for clinicians. Dialogue often continued outside of the formal meetings. Many topics were considered more than once as new guidance developed, changes to EUAs occurred, and new data or new publicity arose.

Methods
The Human Protections Administrator determined that this work constituted “quality improvement, and not research” and was therefore exempt from institutional review board review.
Quantitative Analysis
All admitted patients from March 10, 2020, through April 20, 2021, were considered in the quantitative aspects of this report except as noted. Patients diagnosed with COVID-19 were identified by searching our internal data base using diagnostic codes. Patient admissions with the following diagnostic codes were included (prior to April 1, 2020): J12.89, J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29. After April 1, 2020, the guideline for coding COVID-19 was U07.1.
Descriptive statistics were used to measure utilization rates of certain medications and laboratory tests of interest over time. These data were adjusted for number of unique admissions. In a few cases, not all data elements were available from both hospitals due to differences in the EMR.
Case fatality rate was calculated based upon whether the patient died or was admitted to inpatient hospice as a result of COVID-19. Four patients transferred out of hospital A and 18 transferred out of hospital B were censored from case-fatality-rate determination.
Figure 1 shows the number of admissions for each acute care hospital in the health system and the combined COVID-19 case-fatality rate over time.

Results
A total of 5955 consecutive COVID-19 patients admitted from March 10, 2020, through April 30, 2021, were analyzed. Patients with International Statistical Classification of Diseases, Tenth Revision codes J12.89. J20.8, J40, J22, J98.8, J80, each with the additional code of B97.29 (or the code UO7.1 after April 1, 2020), were included in the analysis. The median age of admitted patients was 65 years (range 19-91 years). Using the NIH classification system for severity,20 the distribution of severity during the first 24 hours after the time of hospital admission was as follows: asymptomatic/presymptomatic, 0.5%; mild illness, 5.3%; moderate illness, 37.1%; severe illness, 55.5%; and critical illness, 1.1%.
The impact of the CCMC can be estimated by looking at care patterns over time. Since the work of the CCMC was aimed at influencing and standardizing physician ordering and therapy choices through order set creation and other forms of oversight, we measured the use of the CCMC-approved order sets at both hospitals and the use of certain laboratory tests and therapies that the CCMC sought either to limit or increase. These counts were adjusted for number of unique COVID-19 admissions. But the limits of the case collection tool meant it also collected cases that were not eligible for some of the interventions. For example, COVID-19 admissions without hypoxemia would not have been eligible for remdesivir or glucocorticoids. When admitted, some patients were already on steroids for other medical indications and did not receive the prescribed dexamethasone dose that we measured in pharmacy databases. Similarly, a few patients were hospitalized for indications unrelated to COVID-19, such as surgery or childbirth, and were found to be SARS-CoV-2-positive on routine screening.
Figure 2 shows the utilization of CCMC-approved standard COVID-19 admission order sets as a proportion of all COVID-19 admissions over time. The trend reveals a modest increase in usage (R2 = 0.34), but these data do not reflect the progressive build of content into order sets over time. One of the goals of the order sets was to standardize and reduce the ordering of certain biomarkers: C-reactive protein, serum ferritin, and D-dimer, which were ordered frequently in many early patients. Orders for these 3 laboratory tests are combined and expressed as an average number of labs per COVID-19 admission in Figure 2. A downward trend, with an R2 value of 0.65, is suggestive of impact from the order sets, though other explanations are possible.

Medication guidance was also a goal of the CCMC, simultaneously discouraging poorly supported interventions and driving uptake of the recommended evidence-based interventions in appropriate patients. Figure 3 shows the utilization pattern for some drugs of interest over the course of the pandemic, specifically the proportion of patients receiving at least 1 dose of medication among all COVID-19 admissions by month. (Data for hospital B was excluded from this analysis because it did not include all admitted patients.)

Hydroxychloroquine, which enjoyed a wave of popularity early on during the pandemic, was a target of successful order stewardship through the CCMC. Use of hydroxychloroquine as a COVID-19 therapeutic option after the first 2 months of the pandemic stopped, and subsequent use at low levels likely represented continuation therapy for outpatients who took hydroxychloroquine for rheumatologic indications.
Dexamethasone, as used in the RECOVERY trial,21 had a swift uptake among physicians after it was incorporated into order sets and its use encouraged. Similarly, uptake was immediate for remdesivir when, in May 2020, preliminary reports showed at least some benefits, confirmed by later analysis,22 and it received an FDA EUA.
Our data also show successful stewardship of the interleukin-6 antagonist toclilizumab, which was discouraged early on by the CCMC due to lack of data or negative results. But in March 2021, with new studies releasing data12,13 and new recommendations14 for its use in some subsets of patients with COVID-19, this drug was encouraged in appropriate subsets. A new order set with qualifying indications was prepared by the CCMC and new educational efforts made to encourage its use in appropriate patients.
Ivermectin was nonformulary at the start of the pandemic. This drug enjoyed much publicity from media sources and was promoted by certain physicians and on websites,23 based on in-vitro activity against coronaviruses. Eventually, the World Health Organization24 and the FDA25 found it necessary to issue advisory statements to the public against its use outside of clinical trials. The CCMC had requests from physicians to incorporate ivermectin but declined to add it to the formulary and recommended not approving nonformulary requests due to lack of data. As a result, ivermectin was not used at either hospital.
Discussion
COVID-19 represents many challenges to health systems all over the world. For Luminis Health, the high volume of acutely ill patients with novel syndromes was a particular challenge for the hospital-based care teams. A flood of information from preprints, press releases, preliminary reports, and many other nontraditional sources made deliberative management decisions difficult for individual physicians. Much commentary has appeared around the phenomenon but with less practical advice about how to make day-to-day care decisions at a time of scientific uncertainty and intense pressure to intervene.26,27 The CCMC was designed to overcome the information management dilemma. The need to coordinate, standardize, and oversee care was necessary given the large number of physicians who cared for COVID-19 patients on inpatient services.
It should be noted that creating order sets and issuing guidance is necessary, but not sufficient, to achieve our goals of being updated and consistent. This is especially true with large cadres of health care workers attending COVID-19 patients. Guidelines and recommendations were reinforced by unit-based oversight and stewardship from pharmacy and other leaders who constituted the CCMC.
The reduction in COVID-19 mortality over time experienced in this health care system was not unique and cannot necessarily be attributed to standardization of care. Similar improvements in mortality have been reported at many US hospitals in aggregate.28 Many other factors, including changes in patient characteristics, may be responsible for reduction in mortality over time.
Throughout this report we have relied upon an implicit assumption that standardization of medical therapeutics is desirable and leads to better outcomes as compared with allowing unlimited empiricism by individual physicians, either consultants or hospitalists. Our program represents a single health system with 2 acute care hospitals located 25 miles apart and which thus were similarly impacted by the different phases of the pandemic. Generalizability to health systems either smaller or larger, or in different geographical areas, has not been established. Data limitations have already been discussed.
We did not measure user satisfaction with the program either from physicians or nurses. However, the high rate of compliance suggests general agreement with the content and process.
We cannot definitively ascribe reduction in utilization of some nonrecommended treatments and increased utilization of the recommended therapies to the work of the CCMC. Individual physicians may have made these adjustments on their own or under the influence of other sources.
Finally, it should be noted that the mission to rapidly respond to data from well-conducted trials might be thwarted by too rigid a process or a committee’s lack of a sense of urgency. Organizing a committee and then empowering it to act is no guarantee of success; commitment to the mission is.
Conclusion
COVID-19 represented a challenge to medical staffs everywhere, inundating them with high volumes of acutely ill patients presenting with unfamiliar syndromes. Initial responses were characterized by idiosyncratic management approaches based on nontraditional sources of opinion and influences.
This report describes how a complex medical system brought order and standardization through a deliberative, but urgent, multidisciplinary committee with responsibility for planning, implementing, and monitoring standard approaches that eventually became evidence based. The composition of the committee and its scope of influence, limited to inpatient management, were important elements of success, allowing for better focus on the many treatment decisions. The important connection between the management committee and the system P&T committee, the clinical research effort, and teaching programs in both medicine and pharmacy are offered as exemplars of coordination. The data presented show success in achieving standardized, guideline-directed care. The approach is adoptable and suitable for similar emergencies in the future.
Acknowledgments: The authors thank Gary Scabis, Kip Waite, John Moxley, Angela Clubb, and David Woodley for their assistance in gathering data. We express appreciation and admiration for all our colleagues at the bedside.
Corresponding author: Barry R. Meisenberg, MD, Department of Medicine, Luminis Health, 2001 Medical Pkwy, Annapolis, MD 21401; [email protected].
Financial disclosures: None.
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
1. Gettleman J, Raj S, Kumar H. India’s health system cracks under the strain as coronavirus cases surge. The New York Times. April 22, 2021. https://www.nytimes.com/2021/04/21/world/asia/india-coronavirus-oxygen.html
2. Rappleye H, Lehren AW, Strickler L, Fitzpatrick S. ‘This system is doomed’: doctors, nurses sound off in NBC News coronavirus survey. NBC News. March 20, 2020. https://www.nbcnews.com/news/us-news/system-doomed-doctors-nurses-sound-nbc-news-coronavirus-survey-n1164841
3. Johns Hopkins Coronavirus Resource Center. Accessed January 5, 2022. https://coronavirus.jhu.edu/map.html
4. Fineberg HV. The toll of COVID-19. JAMA. 2020;324(15):1502-1503. doi:10.1001/jama.2020.20019
5. Meisenberg BR. Medical staffs response to COVID-19 ‘data’: have we misplaced our skeptic’s eye? Am J Med. 2021;134(2):151-152. doi:10.1016/j.amjmed.2020.09.013
6. McMahon JH, Lydeamore MH, Stewardson AJ. Bringing evidence from press release to the clinic in the era of COVID-19. J Antimicrob Chemother. 2021;76(3):547-549. doi:10.1093/jac/dkaa506
7. Rubin EJ, Baden LR, Morrissey S, Campion EW. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382(9):866. doi:10.1056/NEJMe2001329
8. Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi:10.1016/j.jcv.2020.104370
9. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi:10.1186/1743-422X-2-69
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269-271. doi:10.1038/s41422-020-0282-0
11. RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030-2040. doi:10.1056/NEJMoa2022926
12. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial [preprint]. February 11, 2021. doi:10.1101/2021.02.11.21249258 https://www.medrxiv.org/content/10.1101/2021.02.11.21249258v1
13. REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
14. National Institutes of Health. COVID-19 treatment guidelines: interleukin-6 inhibitors. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/interleukin-6-inhibitors/
15. Deana C, Vetrugno L, Tonizzo A, et al. Drug supply during COVID-19 pandemic: remember not to run with your tank empty. Hosp Pharm. 2021;56(5):405-407. doi:10.1177/0018578720931749
16. Choe J, Crane M, Greene J, et al. The Pandemic and the Supply Chain: Addressing Gaps in Pharmaceutical Production and Distribution. Johns Hopkins University, November 2020. https://www.jhsph.edu/research/affiliated-programs/johns-hopkins-drug-access-and-affordability-initiative/publications/Pandemic_Supply_Chain.pdf
17. Kern DE. Overview: a six-step approach to curriculum development. In: Kern DE, Thornton PA, Hughes MT, eds. Curriculum Development for Medical Education: A Six-Step Approach. 3rd ed. Johns Hopkins University Press; 2016.
18. Rice TW, Janz DR. In defense of evidence-based medicine for the treatment of COVID-19 acute respiratory distress syndrome. Ann Am Thorac Soc. 2020;17(7):787-789. doi:10.1513/AnnalsATS.202004-325IP
19. Lucey CR, Johnston SC. The transformational effects of COVID-19 on medical education. JAMA. 2020;324(11):1033-1034. doi:10.1001/jama.2020.14136
20. National Institutes of Health. COVID-19 treatment guidelines: clinical spectrum of SARS-CoV-2 infection. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
21. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693-704. doi:10.1056/NEJMoa2021436
22. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19—final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764
23. Jiminez D. Ivermectin and Covid-19: how a cheap antiparasitic became political. April 19, 2021. https://www.pharmaceutical-technology.com/features/ivermectin-covid-19-antiparasitic-political/
24. World Health Organization. WHO advises that ivermectin only be used to treat COVID-19 within clinical trials. March 31, 2021. https://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials
25. U.S. Food and Drug Administration. Why you should not use ivermectin to treat or prevent COVID-19. March 5, 2021. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
26. Seymour CW, McCreary EK, Stegenga J. Sensible medicine-balancing intervention and inaction during the COVID-19 pandemic. JAMA. 2020;324(18):1827-1828. doi:10.1001/jama.2020.20271
27. Flanagin A, Fontanarosa PB, Bauchner H. Preprints involving medical research—do the benefits outweigh the challenges? JAMA. 2020;324(18):1840-1843. doi:10.1001/jama.2020.20674
28. Asch DA, Shells NE, Islam N, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471-478. doi:10.1001/jamainternmed.2020.8193
Mayo Clinic fires 700 employees for refusing COVID vaccine
The medical center, which is Minnesota’s largest employer, has major campuses in Arizona, Florida, and Minnesota and operates hospitals in Iowa and Wisconsin.
Employees had until Jan. 3 to get vaccinated or receive approval for an exemption. On Jan. 4, the hospital fired those who didn’t meet the requirement, according to Action News Jax, a CBS affiliate in Florida.
The 700 employees make up about 1% of Mayo Clinic’s 73,000-person workforce. So far, none of the employees at the campus in Jacksonville, Fla., have been affected, the news outlet reported.
“Florida staff who are not in compliance with our vaccination program remain employed pending the outcome of litigation related to the Centers for Medicare & Medicaid Services requirements,” a Mayo Clinic spokesperson told Action News Jax.
The federal government and Florida remain at odds over vaccine mandates, and several lawsuits are winding through the court system. Florida Gov. Ron DeSantis signed legislation in November that bans private Florida employers from requiring all employees to get vaccinated and calls for various exemption options, according to The Florida Times-Union. The state law clashes with a federal rule that requires vaccinations for all health care workers at hospitals that receive Medicare and Medicaid funding.
The Mayo Clinic mandate required employees to receive at least one COVID-19 vaccine dose and not be “overdue” for a second dose, according to the statement. Only medical and religious exemptions were allowed, and most medical and religious exemptions were approved.
“While Mayo Clinic is saddened to lose valuable employees, we need to take all steps necessary to keep our patients, workforce, visitors, and communities safe,” Mayo Clinic wrote in its statement. “If individuals released from employment choose to get vaccinated at a later date, the opportunity exists for them to apply and return to Mayo Clinic for future job openings.”
With the latest surge in COVID-19 cases from the Omicron variant, the Mayo Clinic also encouraged unvaccinated people to get a shot and those who are eligible for a booster to get one “as soon as possible.”
“Based on science and data, it’s clear that vaccination keeps people out of the hospital and saves lives,” according to the statement. “That’s true for everyone in our communities – and it’s especially true for the many patients with serious or complex diseases who seek care at Mayo Clinic each day.”
A version of this article first appeared on WebMD.com.
The medical center, which is Minnesota’s largest employer, has major campuses in Arizona, Florida, and Minnesota and operates hospitals in Iowa and Wisconsin.
Employees had until Jan. 3 to get vaccinated or receive approval for an exemption. On Jan. 4, the hospital fired those who didn’t meet the requirement, according to Action News Jax, a CBS affiliate in Florida.
The 700 employees make up about 1% of Mayo Clinic’s 73,000-person workforce. So far, none of the employees at the campus in Jacksonville, Fla., have been affected, the news outlet reported.
“Florida staff who are not in compliance with our vaccination program remain employed pending the outcome of litigation related to the Centers for Medicare & Medicaid Services requirements,” a Mayo Clinic spokesperson told Action News Jax.
The federal government and Florida remain at odds over vaccine mandates, and several lawsuits are winding through the court system. Florida Gov. Ron DeSantis signed legislation in November that bans private Florida employers from requiring all employees to get vaccinated and calls for various exemption options, according to The Florida Times-Union. The state law clashes with a federal rule that requires vaccinations for all health care workers at hospitals that receive Medicare and Medicaid funding.
The Mayo Clinic mandate required employees to receive at least one COVID-19 vaccine dose and not be “overdue” for a second dose, according to the statement. Only medical and religious exemptions were allowed, and most medical and religious exemptions were approved.
“While Mayo Clinic is saddened to lose valuable employees, we need to take all steps necessary to keep our patients, workforce, visitors, and communities safe,” Mayo Clinic wrote in its statement. “If individuals released from employment choose to get vaccinated at a later date, the opportunity exists for them to apply and return to Mayo Clinic for future job openings.”
With the latest surge in COVID-19 cases from the Omicron variant, the Mayo Clinic also encouraged unvaccinated people to get a shot and those who are eligible for a booster to get one “as soon as possible.”
“Based on science and data, it’s clear that vaccination keeps people out of the hospital and saves lives,” according to the statement. “That’s true for everyone in our communities – and it’s especially true for the many patients with serious or complex diseases who seek care at Mayo Clinic each day.”
A version of this article first appeared on WebMD.com.
The medical center, which is Minnesota’s largest employer, has major campuses in Arizona, Florida, and Minnesota and operates hospitals in Iowa and Wisconsin.
Employees had until Jan. 3 to get vaccinated or receive approval for an exemption. On Jan. 4, the hospital fired those who didn’t meet the requirement, according to Action News Jax, a CBS affiliate in Florida.
The 700 employees make up about 1% of Mayo Clinic’s 73,000-person workforce. So far, none of the employees at the campus in Jacksonville, Fla., have been affected, the news outlet reported.
“Florida staff who are not in compliance with our vaccination program remain employed pending the outcome of litigation related to the Centers for Medicare & Medicaid Services requirements,” a Mayo Clinic spokesperson told Action News Jax.
The federal government and Florida remain at odds over vaccine mandates, and several lawsuits are winding through the court system. Florida Gov. Ron DeSantis signed legislation in November that bans private Florida employers from requiring all employees to get vaccinated and calls for various exemption options, according to The Florida Times-Union. The state law clashes with a federal rule that requires vaccinations for all health care workers at hospitals that receive Medicare and Medicaid funding.
The Mayo Clinic mandate required employees to receive at least one COVID-19 vaccine dose and not be “overdue” for a second dose, according to the statement. Only medical and religious exemptions were allowed, and most medical and religious exemptions were approved.
“While Mayo Clinic is saddened to lose valuable employees, we need to take all steps necessary to keep our patients, workforce, visitors, and communities safe,” Mayo Clinic wrote in its statement. “If individuals released from employment choose to get vaccinated at a later date, the opportunity exists for them to apply and return to Mayo Clinic for future job openings.”
With the latest surge in COVID-19 cases from the Omicron variant, the Mayo Clinic also encouraged unvaccinated people to get a shot and those who are eligible for a booster to get one “as soon as possible.”
“Based on science and data, it’s clear that vaccination keeps people out of the hospital and saves lives,” according to the statement. “That’s true for everyone in our communities – and it’s especially true for the many patients with serious or complex diseases who seek care at Mayo Clinic each day.”
A version of this article first appeared on WebMD.com.
A Simple Message
I do not usually have difficulty writing editorials. However, this month was different. I kept coming up with grand ideas that flopped. First, I thought I would write a column entitled, “For What Should We Hope For?” When I started exploring the concept of hope, I quickly learned that there was extensive literature from multiple disciplines and even several centers and research projects dedicated to studying it.1 It seemed unlikely that I would have anything worthwhile to add to that literature. Then I thought I would discuss new year’s resolutions for federal practitioners. There was not much written about that topic, yet it seemed to be overly self-indulgent and superficial to discuss eating less and exercising more amid a pandemic and a climate change crisis. Finally, I wanted to opine on the futility of telling people to be resilient when we are all exhausted and demoralized, and yet that seemed too ponderous and paradoxical for our beleaguered state. With the third strike, I finally realized I was trying too hard. And perhaps that was exactly what I needed to say, at least to myself, and maybe some readers would benefit from reading that simple message as well.
I was surprised—though I probably should not have been given the explosion of media—to find that Americans were surveyed about what months they hate most. A 2021 poll of more than 15,000 adults found that January was the most disliked month.2 It’s not hard to figure out why. Characterized by a postholiday let down, these months in the middle of winter marked by either too much precipitation or if you live in the West not enough; short days and gray nights that are dark and cold. It is a long time to wait before spring with few holidays to break up the quotidian routine of work and school. January is a hard enough month in a good or even ordinary year. And 2022 is shaping up to be neither. We are entering the third year of a prolonged pandemic. Every time we have hope we are coming to the end of this long ordeal or at least things are moving toward normality, a new variant emerges, and we are back to living in fear and uncertainty.
COVID-19 is only the most relentless and deadly of our current disasters: There are rumors of wars, tornadoes, droughts, floods, shootings in schools and churches, political turmoil, and police violence. American society and the very planet seem to be in a perilous situation more than ever. No wonder then, that in the last month, several people have asked me, “Do you think this is the end of the world?” I suppose they think I am so old that I have become wise. And though I should cite a brilliant philosopher or renowned theologian: I am going to revert to my youth as a rock musician and quote R.E.M.: “It is the end of the world as we know it.” And “most of us do not feel fine!”
The world of 2022 is far more constricted and confined than it was before we heard the word COVID-19. We have less freedom of movement and fewer opportunities for companionship and gathering, for advancement and enjoyment. To thrive, and even to survive, in this cramped existence of limited possibilities, we need different values and attitudes than those that made us happy and successful in the open, hurried world before 2019. No generation since World War II has confronted such shortages of automobiles, paper goods, food, and even medicines as we have.
That is the first of the important simple messages I want to convey. Find something to be grateful for: your loved ones, your companion animals, your friends. Cherish the rainy or sunny day depending on how your climate has changed. Treasure the most basic and enduring pleasures, homemade cookies, favorite music, talking to a good friend even virtually, reading an actual book on a Sunday afternoon. These are things even the pandemic cannot take away from us unless we let our own inability to accept the conditions of our time ruin even what the meager, harsh Master of History has spared us.
The second of these simple messages is even more essential to finding any peace or joy in our current tense and somber existence: to show compassion for others and kindness to yourself. The most consistent report I have heard from people all over the country is that their fellow citizens are angry and selfish. We all understand, and even in some measure empathize with this the frustration and impatience with all the extraordinary pressure of having to function under these challenging conditions. Though we can take it out on the stranger at the grocery store or the family of the patient who has different views of masks and vaccines; it likely will not make the line shorter, the family any less demanding or seemingly unreasonable and probably will waste the little energy we have left to get home with the groceries or take care of the patient.
You never know what burden the person annoying you is carrying; it may perhaps be heavier than yours. And how we react to each other makes the weight of world weariness we all bear either easier or harder to shoulder. It sounds trite and trivial to say, yet tell people you care, and value, and love them. Although no less than Pope Francis in a Christmas present to marriages under strain from the stress of the pandemic that the 3 key words to remember are please, sorry, and thank you.3 I am applying that sage advice liberally to all relationships and interactions in the daily grind of work and home. The cost is little, the reward priceless.
It is good and right to have high hopes. We all need to take care of ourselves, whether we make resolutions to do so or not. Though more than anything else what we need is to be kind to ourselves. It is presumptuous of me to tell you what wellness means for your individual struggle, as it is inhuman of me to deign to tell you to be resilient when many of you face intolerable working conditions.4 As Jackson Browne sang in “Rock Me on the Water”, “Everyone must have some thought that’s going to pull them through somehow. Find your own thought, the reason you keep getting up and going to care for patients who increasingly respond with the rage of denial and resentment. Amid what morally distressed public health professionals have called so many unnecessary deaths,choose what gives you reason to keep serving that other side of this life full of healing.5 And if like so many of my fellow health care professionals, you are so spent and bent, that you feel that you can no longer practice without becoming someone you do not want to be, then let go with grace, get the help you deserve and perhaps one day when rested and mended, find another way to give.6
I rarely self-disclose but I want to end this column with a personal story that exemplifies more than all these words living this simple message. My spouse is a health care practitioner at a Veterans Affairs medical center. Like all of you on the front lines they work far too long hours in difficult conditions, with challenging patients and not enough staff to care for them. My partner had not an hour to get any gifts for me or our furry children. On Christmas Eve, before a long shift, they went to a packed Walgreens to buy our huskies each a toy and me a pair of fuzzy slippers. We sat by the tree and opened the hastily wrapped packages, and nothing could have been more memorable or meaningful. All of us at Federal Practitioner wish you, our readers, find in 2022 many such moments to sustain you.
1. The Center for the Advanced Study and Practice of Hope. T Denny Sanford School of Social and Family Dynamics, Arizona State University. Accessed January 3, 2022. https://thesanfordschool.asu.edu/research/centers-initiatives/hope-center
2. Ballard J. What is America’s favorite (and least favorite) month?” Published March 1, 2021. Accessed January 3, 2022. https://today.yougov.com/topics/lifestyle/articles-reports/2021/03/01/favorite-least-favorite-month-poll
3. Winlfield N. Pope’s 3 key words for a marriage: ‘please, thanks sorry.’ Associated Press. December 26, 2021. Accessed January 3, 2022. https://apnews.com/article/pope-francis-lifestyle-religion-relationships-couples-23c81169982e50c35d1c1fc7bfef8cbc
4. Dineen K. Why resilience isn’t always the answer to coping with challenging times. Published September 29, 2020. Accessed January 3, 2022. https://theconversation.com/why-resilience-isnt-always-the-answer-to-coping-with-challenging-times-145796
5. Caldwell T. ‘Everyone of those deaths is unnecessary,’ expert says of rising COVID-19 U.S. death toll as tens of millions remain unvaccinated. Published October 3, 2021. Accessed December 29, 2021. https://www.cnn.com/2021/10/03/health/us-coronavirus-sunday/index.html 6. Yong E. Why healthcare professionals are quitting in droves. The Atlantic. November 16, 2021. Accessed December 29, 2021. https://www.theatlantic.com/health/archive/2021/11/the-mass-exodus-of-americas-health-care-workers/620713/
I do not usually have difficulty writing editorials. However, this month was different. I kept coming up with grand ideas that flopped. First, I thought I would write a column entitled, “For What Should We Hope For?” When I started exploring the concept of hope, I quickly learned that there was extensive literature from multiple disciplines and even several centers and research projects dedicated to studying it.1 It seemed unlikely that I would have anything worthwhile to add to that literature. Then I thought I would discuss new year’s resolutions for federal practitioners. There was not much written about that topic, yet it seemed to be overly self-indulgent and superficial to discuss eating less and exercising more amid a pandemic and a climate change crisis. Finally, I wanted to opine on the futility of telling people to be resilient when we are all exhausted and demoralized, and yet that seemed too ponderous and paradoxical for our beleaguered state. With the third strike, I finally realized I was trying too hard. And perhaps that was exactly what I needed to say, at least to myself, and maybe some readers would benefit from reading that simple message as well.
I was surprised—though I probably should not have been given the explosion of media—to find that Americans were surveyed about what months they hate most. A 2021 poll of more than 15,000 adults found that January was the most disliked month.2 It’s not hard to figure out why. Characterized by a postholiday let down, these months in the middle of winter marked by either too much precipitation or if you live in the West not enough; short days and gray nights that are dark and cold. It is a long time to wait before spring with few holidays to break up the quotidian routine of work and school. January is a hard enough month in a good or even ordinary year. And 2022 is shaping up to be neither. We are entering the third year of a prolonged pandemic. Every time we have hope we are coming to the end of this long ordeal or at least things are moving toward normality, a new variant emerges, and we are back to living in fear and uncertainty.
COVID-19 is only the most relentless and deadly of our current disasters: There are rumors of wars, tornadoes, droughts, floods, shootings in schools and churches, political turmoil, and police violence. American society and the very planet seem to be in a perilous situation more than ever. No wonder then, that in the last month, several people have asked me, “Do you think this is the end of the world?” I suppose they think I am so old that I have become wise. And though I should cite a brilliant philosopher or renowned theologian: I am going to revert to my youth as a rock musician and quote R.E.M.: “It is the end of the world as we know it.” And “most of us do not feel fine!”
The world of 2022 is far more constricted and confined than it was before we heard the word COVID-19. We have less freedom of movement and fewer opportunities for companionship and gathering, for advancement and enjoyment. To thrive, and even to survive, in this cramped existence of limited possibilities, we need different values and attitudes than those that made us happy and successful in the open, hurried world before 2019. No generation since World War II has confronted such shortages of automobiles, paper goods, food, and even medicines as we have.
That is the first of the important simple messages I want to convey. Find something to be grateful for: your loved ones, your companion animals, your friends. Cherish the rainy or sunny day depending on how your climate has changed. Treasure the most basic and enduring pleasures, homemade cookies, favorite music, talking to a good friend even virtually, reading an actual book on a Sunday afternoon. These are things even the pandemic cannot take away from us unless we let our own inability to accept the conditions of our time ruin even what the meager, harsh Master of History has spared us.
The second of these simple messages is even more essential to finding any peace or joy in our current tense and somber existence: to show compassion for others and kindness to yourself. The most consistent report I have heard from people all over the country is that their fellow citizens are angry and selfish. We all understand, and even in some measure empathize with this the frustration and impatience with all the extraordinary pressure of having to function under these challenging conditions. Though we can take it out on the stranger at the grocery store or the family of the patient who has different views of masks and vaccines; it likely will not make the line shorter, the family any less demanding or seemingly unreasonable and probably will waste the little energy we have left to get home with the groceries or take care of the patient.
You never know what burden the person annoying you is carrying; it may perhaps be heavier than yours. And how we react to each other makes the weight of world weariness we all bear either easier or harder to shoulder. It sounds trite and trivial to say, yet tell people you care, and value, and love them. Although no less than Pope Francis in a Christmas present to marriages under strain from the stress of the pandemic that the 3 key words to remember are please, sorry, and thank you.3 I am applying that sage advice liberally to all relationships and interactions in the daily grind of work and home. The cost is little, the reward priceless.
It is good and right to have high hopes. We all need to take care of ourselves, whether we make resolutions to do so or not. Though more than anything else what we need is to be kind to ourselves. It is presumptuous of me to tell you what wellness means for your individual struggle, as it is inhuman of me to deign to tell you to be resilient when many of you face intolerable working conditions.4 As Jackson Browne sang in “Rock Me on the Water”, “Everyone must have some thought that’s going to pull them through somehow. Find your own thought, the reason you keep getting up and going to care for patients who increasingly respond with the rage of denial and resentment. Amid what morally distressed public health professionals have called so many unnecessary deaths,choose what gives you reason to keep serving that other side of this life full of healing.5 And if like so many of my fellow health care professionals, you are so spent and bent, that you feel that you can no longer practice without becoming someone you do not want to be, then let go with grace, get the help you deserve and perhaps one day when rested and mended, find another way to give.6
I rarely self-disclose but I want to end this column with a personal story that exemplifies more than all these words living this simple message. My spouse is a health care practitioner at a Veterans Affairs medical center. Like all of you on the front lines they work far too long hours in difficult conditions, with challenging patients and not enough staff to care for them. My partner had not an hour to get any gifts for me or our furry children. On Christmas Eve, before a long shift, they went to a packed Walgreens to buy our huskies each a toy and me a pair of fuzzy slippers. We sat by the tree and opened the hastily wrapped packages, and nothing could have been more memorable or meaningful. All of us at Federal Practitioner wish you, our readers, find in 2022 many such moments to sustain you.
I do not usually have difficulty writing editorials. However, this month was different. I kept coming up with grand ideas that flopped. First, I thought I would write a column entitled, “For What Should We Hope For?” When I started exploring the concept of hope, I quickly learned that there was extensive literature from multiple disciplines and even several centers and research projects dedicated to studying it.1 It seemed unlikely that I would have anything worthwhile to add to that literature. Then I thought I would discuss new year’s resolutions for federal practitioners. There was not much written about that topic, yet it seemed to be overly self-indulgent and superficial to discuss eating less and exercising more amid a pandemic and a climate change crisis. Finally, I wanted to opine on the futility of telling people to be resilient when we are all exhausted and demoralized, and yet that seemed too ponderous and paradoxical for our beleaguered state. With the third strike, I finally realized I was trying too hard. And perhaps that was exactly what I needed to say, at least to myself, and maybe some readers would benefit from reading that simple message as well.
I was surprised—though I probably should not have been given the explosion of media—to find that Americans were surveyed about what months they hate most. A 2021 poll of more than 15,000 adults found that January was the most disliked month.2 It’s not hard to figure out why. Characterized by a postholiday let down, these months in the middle of winter marked by either too much precipitation or if you live in the West not enough; short days and gray nights that are dark and cold. It is a long time to wait before spring with few holidays to break up the quotidian routine of work and school. January is a hard enough month in a good or even ordinary year. And 2022 is shaping up to be neither. We are entering the third year of a prolonged pandemic. Every time we have hope we are coming to the end of this long ordeal or at least things are moving toward normality, a new variant emerges, and we are back to living in fear and uncertainty.
COVID-19 is only the most relentless and deadly of our current disasters: There are rumors of wars, tornadoes, droughts, floods, shootings in schools and churches, political turmoil, and police violence. American society and the very planet seem to be in a perilous situation more than ever. No wonder then, that in the last month, several people have asked me, “Do you think this is the end of the world?” I suppose they think I am so old that I have become wise. And though I should cite a brilliant philosopher or renowned theologian: I am going to revert to my youth as a rock musician and quote R.E.M.: “It is the end of the world as we know it.” And “most of us do not feel fine!”
The world of 2022 is far more constricted and confined than it was before we heard the word COVID-19. We have less freedom of movement and fewer opportunities for companionship and gathering, for advancement and enjoyment. To thrive, and even to survive, in this cramped existence of limited possibilities, we need different values and attitudes than those that made us happy and successful in the open, hurried world before 2019. No generation since World War II has confronted such shortages of automobiles, paper goods, food, and even medicines as we have.
That is the first of the important simple messages I want to convey. Find something to be grateful for: your loved ones, your companion animals, your friends. Cherish the rainy or sunny day depending on how your climate has changed. Treasure the most basic and enduring pleasures, homemade cookies, favorite music, talking to a good friend even virtually, reading an actual book on a Sunday afternoon. These are things even the pandemic cannot take away from us unless we let our own inability to accept the conditions of our time ruin even what the meager, harsh Master of History has spared us.
The second of these simple messages is even more essential to finding any peace or joy in our current tense and somber existence: to show compassion for others and kindness to yourself. The most consistent report I have heard from people all over the country is that their fellow citizens are angry and selfish. We all understand, and even in some measure empathize with this the frustration and impatience with all the extraordinary pressure of having to function under these challenging conditions. Though we can take it out on the stranger at the grocery store or the family of the patient who has different views of masks and vaccines; it likely will not make the line shorter, the family any less demanding or seemingly unreasonable and probably will waste the little energy we have left to get home with the groceries or take care of the patient.
You never know what burden the person annoying you is carrying; it may perhaps be heavier than yours. And how we react to each other makes the weight of world weariness we all bear either easier or harder to shoulder. It sounds trite and trivial to say, yet tell people you care, and value, and love them. Although no less than Pope Francis in a Christmas present to marriages under strain from the stress of the pandemic that the 3 key words to remember are please, sorry, and thank you.3 I am applying that sage advice liberally to all relationships and interactions in the daily grind of work and home. The cost is little, the reward priceless.
It is good and right to have high hopes. We all need to take care of ourselves, whether we make resolutions to do so or not. Though more than anything else what we need is to be kind to ourselves. It is presumptuous of me to tell you what wellness means for your individual struggle, as it is inhuman of me to deign to tell you to be resilient when many of you face intolerable working conditions.4 As Jackson Browne sang in “Rock Me on the Water”, “Everyone must have some thought that’s going to pull them through somehow. Find your own thought, the reason you keep getting up and going to care for patients who increasingly respond with the rage of denial and resentment. Amid what morally distressed public health professionals have called so many unnecessary deaths,choose what gives you reason to keep serving that other side of this life full of healing.5 And if like so many of my fellow health care professionals, you are so spent and bent, that you feel that you can no longer practice without becoming someone you do not want to be, then let go with grace, get the help you deserve and perhaps one day when rested and mended, find another way to give.6
I rarely self-disclose but I want to end this column with a personal story that exemplifies more than all these words living this simple message. My spouse is a health care practitioner at a Veterans Affairs medical center. Like all of you on the front lines they work far too long hours in difficult conditions, with challenging patients and not enough staff to care for them. My partner had not an hour to get any gifts for me or our furry children. On Christmas Eve, before a long shift, they went to a packed Walgreens to buy our huskies each a toy and me a pair of fuzzy slippers. We sat by the tree and opened the hastily wrapped packages, and nothing could have been more memorable or meaningful. All of us at Federal Practitioner wish you, our readers, find in 2022 many such moments to sustain you.
1. The Center for the Advanced Study and Practice of Hope. T Denny Sanford School of Social and Family Dynamics, Arizona State University. Accessed January 3, 2022. https://thesanfordschool.asu.edu/research/centers-initiatives/hope-center
2. Ballard J. What is America’s favorite (and least favorite) month?” Published March 1, 2021. Accessed January 3, 2022. https://today.yougov.com/topics/lifestyle/articles-reports/2021/03/01/favorite-least-favorite-month-poll
3. Winlfield N. Pope’s 3 key words for a marriage: ‘please, thanks sorry.’ Associated Press. December 26, 2021. Accessed January 3, 2022. https://apnews.com/article/pope-francis-lifestyle-religion-relationships-couples-23c81169982e50c35d1c1fc7bfef8cbc
4. Dineen K. Why resilience isn’t always the answer to coping with challenging times. Published September 29, 2020. Accessed January 3, 2022. https://theconversation.com/why-resilience-isnt-always-the-answer-to-coping-with-challenging-times-145796
5. Caldwell T. ‘Everyone of those deaths is unnecessary,’ expert says of rising COVID-19 U.S. death toll as tens of millions remain unvaccinated. Published October 3, 2021. Accessed December 29, 2021. https://www.cnn.com/2021/10/03/health/us-coronavirus-sunday/index.html 6. Yong E. Why healthcare professionals are quitting in droves. The Atlantic. November 16, 2021. Accessed December 29, 2021. https://www.theatlantic.com/health/archive/2021/11/the-mass-exodus-of-americas-health-care-workers/620713/
1. The Center for the Advanced Study and Practice of Hope. T Denny Sanford School of Social and Family Dynamics, Arizona State University. Accessed January 3, 2022. https://thesanfordschool.asu.edu/research/centers-initiatives/hope-center
2. Ballard J. What is America’s favorite (and least favorite) month?” Published March 1, 2021. Accessed January 3, 2022. https://today.yougov.com/topics/lifestyle/articles-reports/2021/03/01/favorite-least-favorite-month-poll
3. Winlfield N. Pope’s 3 key words for a marriage: ‘please, thanks sorry.’ Associated Press. December 26, 2021. Accessed January 3, 2022. https://apnews.com/article/pope-francis-lifestyle-religion-relationships-couples-23c81169982e50c35d1c1fc7bfef8cbc
4. Dineen K. Why resilience isn’t always the answer to coping with challenging times. Published September 29, 2020. Accessed January 3, 2022. https://theconversation.com/why-resilience-isnt-always-the-answer-to-coping-with-challenging-times-145796
5. Caldwell T. ‘Everyone of those deaths is unnecessary,’ expert says of rising COVID-19 U.S. death toll as tens of millions remain unvaccinated. Published October 3, 2021. Accessed December 29, 2021. https://www.cnn.com/2021/10/03/health/us-coronavirus-sunday/index.html 6. Yong E. Why healthcare professionals are quitting in droves. The Atlantic. November 16, 2021. Accessed December 29, 2021. https://www.theatlantic.com/health/archive/2021/11/the-mass-exodus-of-americas-health-care-workers/620713/
COVID-19 vaccination has little impact on menstrual cycle
Women may rest a bit easier thanks to results from a study showing that vaccination against the SARS-CoV-2 virus has almost no impact on a woman’s menstrual cycle. The issue is significant, as regular menstruation is a sign of health and fertility, and fears of disturbances might increase vaccination hesitancy as COVID-19 cases continue to surge.
Alison Edelman, MD, MPH, a professor of obstetrics and gynecology at Oregon Health & Science University, Portland, led a group studying prospective data on almost 24,000 menstrual cycles reported by almost 4,000 U.S. women.
The investigators found that COVID-19 vaccination was associated with a less than 1-day change in cycle length for the menstrual cycles after the first and second inoculations, compared with prevaccine cycles. Vaccination had no effect on the actual number of days menstrual bleeding lasted.
The study looked at the menstrual patterns of women aged 18-45 years with normal cycle lengths of 24-38 days for the three consecutive cycles before the first vaccine dose and for three consecutive postvaccine cycles. The final sample included 2,403 vaccinated and 1,556 unvaccinated individuals.
In vaccinated women, the study initially found a slight average increase in cycle length after dose one of 71% of a day and 91% of a day after dose two. Following adjustments, those increases dropped to 64% of a day after the first dose and 79% of a day after the second dose.
In unvaccinated women, the study looked at six cycles over a similar time period and found no significant changes from baseline.
“Coronavirus disease 2019 vaccination is associated with a small change in cycle length but not menses length,” Dr. Edelman’s group concluded in Obstetrics and Gynecology.
In the rare instance that a woman received two vaccine doses within the same menstrual cycle, the change in length could increase to 2 days. These variations appear to resolve quickly, possibly as soon as the next cycle after vaccination and do not indicate any cause for long-term physical or reproductive health concern, according to the authors.
Reports by women on social media, however, have suggested that postvaccine menstrual disruptions are more common with, for example, heavier and breakthrough bleeding. But it appears such changes are temporary and resolve quickly.
“These findings are reassuring and validating,” Dr. Edelman said in an interview. On a population level, the changes indicate no cause for concern for long-term physical or reproductive health and no reason to avoid vaccination. “On a personal level, people want this information so they know what to expect when they get vaccinated, and not worry about a pregnancy scare or be disappointed if they were trying for pregnancy.”
According to the International Federation of Gynecologists and Obstetricians, variations in cycle length of fewer than 8 days are considered normal, said Christine Metz, PhD, a research biologist and a professor of molecular medicine at the Feinstein Institutes for Medical Research in Manhasset, N.Y. “Thus, the extra 17 hours added to the menstrual cycle length in the vaccination group in this study is well within the ‘normal’ range.”
In a group of about 1,600 menstruating women being studied at Dr. Metz’s center, some have anecdotally reported transient cycle changes post vaccination for COVID-19, including delays in menstruation onset and changes in bleeding patterns.
Exactly how vaccination might alter menstrual cycle length is not known and has not been studied with vaccination against other infections such as influenza and meningococcal disease.
“Many factors are known to affect menstrual cycle length including changes in diet, sleep, and exercise, as well as sickness, travel, and stress,” Dr. Metz said. The COVID-19 vaccines have affected people in different ways, with side effects ranging from injection-site pain to nausea, aches, fever, and fatigue. “Vaccination side effects, particularly if severe, could lead to changes in diet, exercise, and sleep, and feelings of sickness and/or stress.”
These stressors can alter hormone production and stability, as well as the body’s response to hormones such as estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and other hormones associated with female reproduction. “Because these hormones regulate the menstrual cycle, variations in these hormones can either shorten or lengthen the cycle,” Dr. Metz explained.
More research needs to be done at the global level, according to the authors. “Questions remain about other possible changes in menstrual cycles, such as menstrual symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding.”
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health’s Office of Research on Women’s Health. Dr. Edelman reported support from the American College of Obstetrics and Gynecology, the World Health Organization, Gynuity, and the Karolinska Institute as well as royalties from UpToDate. Other study authors reported similar relationships with not-for-profit and private-sector companies. Three coauthors are employees of Natural Cycles, a fertility tracking device that was used in the study. Dr. Metz disclosed no conflicts of interest with regard to her comments.
Women may rest a bit easier thanks to results from a study showing that vaccination against the SARS-CoV-2 virus has almost no impact on a woman’s menstrual cycle. The issue is significant, as regular menstruation is a sign of health and fertility, and fears of disturbances might increase vaccination hesitancy as COVID-19 cases continue to surge.
Alison Edelman, MD, MPH, a professor of obstetrics and gynecology at Oregon Health & Science University, Portland, led a group studying prospective data on almost 24,000 menstrual cycles reported by almost 4,000 U.S. women.
The investigators found that COVID-19 vaccination was associated with a less than 1-day change in cycle length for the menstrual cycles after the first and second inoculations, compared with prevaccine cycles. Vaccination had no effect on the actual number of days menstrual bleeding lasted.
The study looked at the menstrual patterns of women aged 18-45 years with normal cycle lengths of 24-38 days for the three consecutive cycles before the first vaccine dose and for three consecutive postvaccine cycles. The final sample included 2,403 vaccinated and 1,556 unvaccinated individuals.
In vaccinated women, the study initially found a slight average increase in cycle length after dose one of 71% of a day and 91% of a day after dose two. Following adjustments, those increases dropped to 64% of a day after the first dose and 79% of a day after the second dose.
In unvaccinated women, the study looked at six cycles over a similar time period and found no significant changes from baseline.
“Coronavirus disease 2019 vaccination is associated with a small change in cycle length but not menses length,” Dr. Edelman’s group concluded in Obstetrics and Gynecology.
In the rare instance that a woman received two vaccine doses within the same menstrual cycle, the change in length could increase to 2 days. These variations appear to resolve quickly, possibly as soon as the next cycle after vaccination and do not indicate any cause for long-term physical or reproductive health concern, according to the authors.
Reports by women on social media, however, have suggested that postvaccine menstrual disruptions are more common with, for example, heavier and breakthrough bleeding. But it appears such changes are temporary and resolve quickly.
“These findings are reassuring and validating,” Dr. Edelman said in an interview. On a population level, the changes indicate no cause for concern for long-term physical or reproductive health and no reason to avoid vaccination. “On a personal level, people want this information so they know what to expect when they get vaccinated, and not worry about a pregnancy scare or be disappointed if they were trying for pregnancy.”
According to the International Federation of Gynecologists and Obstetricians, variations in cycle length of fewer than 8 days are considered normal, said Christine Metz, PhD, a research biologist and a professor of molecular medicine at the Feinstein Institutes for Medical Research in Manhasset, N.Y. “Thus, the extra 17 hours added to the menstrual cycle length in the vaccination group in this study is well within the ‘normal’ range.”
In a group of about 1,600 menstruating women being studied at Dr. Metz’s center, some have anecdotally reported transient cycle changes post vaccination for COVID-19, including delays in menstruation onset and changes in bleeding patterns.
Exactly how vaccination might alter menstrual cycle length is not known and has not been studied with vaccination against other infections such as influenza and meningococcal disease.
“Many factors are known to affect menstrual cycle length including changes in diet, sleep, and exercise, as well as sickness, travel, and stress,” Dr. Metz said. The COVID-19 vaccines have affected people in different ways, with side effects ranging from injection-site pain to nausea, aches, fever, and fatigue. “Vaccination side effects, particularly if severe, could lead to changes in diet, exercise, and sleep, and feelings of sickness and/or stress.”
These stressors can alter hormone production and stability, as well as the body’s response to hormones such as estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and other hormones associated with female reproduction. “Because these hormones regulate the menstrual cycle, variations in these hormones can either shorten or lengthen the cycle,” Dr. Metz explained.
More research needs to be done at the global level, according to the authors. “Questions remain about other possible changes in menstrual cycles, such as menstrual symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding.”
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health’s Office of Research on Women’s Health. Dr. Edelman reported support from the American College of Obstetrics and Gynecology, the World Health Organization, Gynuity, and the Karolinska Institute as well as royalties from UpToDate. Other study authors reported similar relationships with not-for-profit and private-sector companies. Three coauthors are employees of Natural Cycles, a fertility tracking device that was used in the study. Dr. Metz disclosed no conflicts of interest with regard to her comments.
Women may rest a bit easier thanks to results from a study showing that vaccination against the SARS-CoV-2 virus has almost no impact on a woman’s menstrual cycle. The issue is significant, as regular menstruation is a sign of health and fertility, and fears of disturbances might increase vaccination hesitancy as COVID-19 cases continue to surge.
Alison Edelman, MD, MPH, a professor of obstetrics and gynecology at Oregon Health & Science University, Portland, led a group studying prospective data on almost 24,000 menstrual cycles reported by almost 4,000 U.S. women.
The investigators found that COVID-19 vaccination was associated with a less than 1-day change in cycle length for the menstrual cycles after the first and second inoculations, compared with prevaccine cycles. Vaccination had no effect on the actual number of days menstrual bleeding lasted.
The study looked at the menstrual patterns of women aged 18-45 years with normal cycle lengths of 24-38 days for the three consecutive cycles before the first vaccine dose and for three consecutive postvaccine cycles. The final sample included 2,403 vaccinated and 1,556 unvaccinated individuals.
In vaccinated women, the study initially found a slight average increase in cycle length after dose one of 71% of a day and 91% of a day after dose two. Following adjustments, those increases dropped to 64% of a day after the first dose and 79% of a day after the second dose.
In unvaccinated women, the study looked at six cycles over a similar time period and found no significant changes from baseline.
“Coronavirus disease 2019 vaccination is associated with a small change in cycle length but not menses length,” Dr. Edelman’s group concluded in Obstetrics and Gynecology.
In the rare instance that a woman received two vaccine doses within the same menstrual cycle, the change in length could increase to 2 days. These variations appear to resolve quickly, possibly as soon as the next cycle after vaccination and do not indicate any cause for long-term physical or reproductive health concern, according to the authors.
Reports by women on social media, however, have suggested that postvaccine menstrual disruptions are more common with, for example, heavier and breakthrough bleeding. But it appears such changes are temporary and resolve quickly.
“These findings are reassuring and validating,” Dr. Edelman said in an interview. On a population level, the changes indicate no cause for concern for long-term physical or reproductive health and no reason to avoid vaccination. “On a personal level, people want this information so they know what to expect when they get vaccinated, and not worry about a pregnancy scare or be disappointed if they were trying for pregnancy.”
According to the International Federation of Gynecologists and Obstetricians, variations in cycle length of fewer than 8 days are considered normal, said Christine Metz, PhD, a research biologist and a professor of molecular medicine at the Feinstein Institutes for Medical Research in Manhasset, N.Y. “Thus, the extra 17 hours added to the menstrual cycle length in the vaccination group in this study is well within the ‘normal’ range.”
In a group of about 1,600 menstruating women being studied at Dr. Metz’s center, some have anecdotally reported transient cycle changes post vaccination for COVID-19, including delays in menstruation onset and changes in bleeding patterns.
Exactly how vaccination might alter menstrual cycle length is not known and has not been studied with vaccination against other infections such as influenza and meningococcal disease.
“Many factors are known to affect menstrual cycle length including changes in diet, sleep, and exercise, as well as sickness, travel, and stress,” Dr. Metz said. The COVID-19 vaccines have affected people in different ways, with side effects ranging from injection-site pain to nausea, aches, fever, and fatigue. “Vaccination side effects, particularly if severe, could lead to changes in diet, exercise, and sleep, and feelings of sickness and/or stress.”
These stressors can alter hormone production and stability, as well as the body’s response to hormones such as estrogen, progesterone, follicle-stimulating hormone, luteinizing hormone, and other hormones associated with female reproduction. “Because these hormones regulate the menstrual cycle, variations in these hormones can either shorten or lengthen the cycle,” Dr. Metz explained.
More research needs to be done at the global level, according to the authors. “Questions remain about other possible changes in menstrual cycles, such as menstrual symptoms, unscheduled bleeding, and changes in the quality and quantity of menstrual bleeding.”
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health’s Office of Research on Women’s Health. Dr. Edelman reported support from the American College of Obstetrics and Gynecology, the World Health Organization, Gynuity, and the Karolinska Institute as well as royalties from UpToDate. Other study authors reported similar relationships with not-for-profit and private-sector companies. Three coauthors are employees of Natural Cycles, a fertility tracking device that was used in the study. Dr. Metz disclosed no conflicts of interest with regard to her comments.
FROM OBSTETRICS & GYNECOLOGY
Teledermatology During the COVID-19 Pandemic: Lessons Learned and Future Directions
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
Although teledermatology utilization in the United States traditionally has lagged behind other countries,1,2 the COVID-19 pandemic upended this trend by creating the need for a massive teledermatology experiment. Recently reported survey results from a large representative sample of US dermatologists (5000 participants) on perceptions of teledermatology during COVID-19 indicated that only 14.1% of participants used teledermatology prior to the COVID-19 pandemic vs 54.1% of dermatologists in Europe.2,3 Since the pandemic started, 97% of US dermatologists reported teledermatology use,3 demonstrating a huge shift in utilization. This trend is notable, as teledermatology has been shown to increase access to dermatology in underserved areas, reduce patient travel times, improve patient triage, and even reduce carbon footprints.1,4 Thus, to sustain the momentum, insights from the recent teledermatology experience during the pandemic should inform future development.
Notably, the COVID-19 pandemic led to a rapid shift in focus from store-and-forward teledermatology to live video–based models.1,2 Logistically, live video visits are challenging, require more time and resources, and often are diagnostically limited, with concerns regarding technology, connectivity, reimbursement, and appropriate use.3 Prior to COVID-19, formal Health Insurance Portability and Accountability Act–compliant teledermatology platforms often were costly to establish and maintain, largely relegating use to academic centers and Veterans Affairs hospitals. Thus, many fewer private practice dermatologists had used teledermatology compared to academic dermatologists in the United States (11.4% vs 27.6%).3 Government regulations—a key barrier to the adoption of teledermatology in private practice before COVID-19—were greatly relaxed during the pandemic. The Centers for Medicare and Medicaid Services removed restrictions on where patients could be seen, improved reimbursement for video visits, and allowed the use of platforms that are not Health Insurance Portability and Accountability Act compliant. Many states also relaxed medical licensing rules.
Overall, the general outlook on telehealth seems positive. Reimbursement has been found to be a primary factor in dermatologists’ willingness to use teledermatology.3 Thus, sustainable use of teledermatology likely will depend on continued reimbursement parity for live video as well as store-and-forward consultations, which have several advantages but currently are de-incentivized by low reimbursement. The survey also found that 70% of respondents felt that teledermatology use will continue after COVID-19, while 58% intended to continue use—nearly 5-fold more than before the pandemic.3 We suspect the discrepancy between participants’ predictions regarding future use of teledermatology and their personal intent to use it highlights perceived barriers and limitations of the long-term success of teledermatology. Aside from reimbursement, connectivity and functionality were common concerns, emphasizing the need for innovative technological solutions.3 Moving forward, we anticipate that dermatologists will need to establish consistent workflows to establish consistent triage for the most appropriate visit—in-person visits vs teledermatology, which may include augmented, intelligence-enhanced solutions. Similar to prior clinician perspectives about which types of visits are conducive to teledermatology,2 most survey participants believed virtual visits were effective for acne, routine follow-ups, medication monitoring, and some inflammatory conditions.3
Importantly, we must be mindful of patients who may be left behind by the digital divide, such as those with lack of access to a smartphone or the internet, language barriers, or limited telehealth experience.5 Systems should be designed to provide these patients with technologic and health literacy aid or alternate modalities to access care. For example, structured methods could be introduced to provide training and instructions on how to access phone applications, computer-based programs, and more. Likewise, for those with hearing or vision deficits, it will be important to improve sound amplification and accessibility for headphones or hearing aid connectivity, as well as appropriate font size, button size, and application navigation. In remote areas, existing clinics may be used to help field specialty consultation teleconferences. Certainly, applications and platforms devised for teledermatology must be designed to serve diverse patient groups, with special consideration for the elderly, those who speak languages other than English, and those with disabilities that may make telehealth use more challenging.
Large-scale regulatory changes and reimbursement parity can have a substantial impact on future teledermatology use. Advocacy efforts continue to push for fair valuation of telemedicine, coverage of store-and-forward teledermatology codes, and coverage for all models of care. It is imperative for the dermatology community to continue discussions on implementation and methodology to best leverage this technology for the most patient benefit.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Tensen E, van der Heijden JP, Jaspers MWM, et al. Two decades of teledermatology: current status and integration in national healthcare systems. Curr Dermatol Rep. 2016;5:96-104.
- Moscarella E, Pasquali P, Cinotti E, et al. A survey on teledermatology use and doctors’ perception in times of COVID-19 [published online August 17, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E772-E773.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Bonsall A. Unleashing carbon emissions savings with regular teledermatology clinics. Clin Exp Dermatol. 2021;46:574-575.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
First ‘flurona’ cases reported in the U.S.
The first known case was detected in Israel, but until the first week of January no cases had been reported in the United States.
In Los Angeles, a teenaged boy tested positive for both illnesses at a COVID testing site in Brentwood, the Los Angeles Times reported. The child’s mother tested positive for COVID the next day.
“This is the first one that we’re aware of,” Steve Farzam, chief operating officer of 911 COVID Testing, told the LA Times. “In and of itself, it’s not overly concerning; however, it is concerning and can be problematic for someone who has pre-existing medical conditions, anyone who is immunocompromised.”
The teen and his family of five had just returned from vacation in Cabo San Lucas, Mexico. All said they tested negative before the trip, but they tested again when they got home because one of the children had a runny nose, Mr. Farzam said.
The boy, who had not been vaccinated for COVID or the flu, doesn’t have serious symptoms and is recovering at home.
In Houston, a 17-year-old boy, his siblings, and his father felt sick a few days before Christmas and went in for testing, TV station KTRK reported. The teen tested positive for both the flu and COVID.
“I ended up getting tested the day before Christmas for strep throat, flu and COVID,” the teenager, Alec Zierlein, told KTRK. “I didn’t think I had any of the three. It felt like a mild cold.”
Health officials reported Jan. 5 that a flurona case was detected in Hays, Kan., TV station WIBW reported. The patient was being treated in the ICU. No other details were provided. In Israel, flurona was first found in an unvaccinated pregnant woman at Rabin Medical Center in Petach Tikva, according to the Times of Israel. She tested positive for both viruses when she arrived at the medical center, and doctors double-checked to confirm her diagnosis. The woman had mild symptoms and was released in good condition, the news outlet reported.
Public health officials in Israel said they are concerned that an increase in both viruses at the same time could lead to many hospitalizations.
A version of this article first appeared on WebMD.com.
The first known case was detected in Israel, but until the first week of January no cases had been reported in the United States.
In Los Angeles, a teenaged boy tested positive for both illnesses at a COVID testing site in Brentwood, the Los Angeles Times reported. The child’s mother tested positive for COVID the next day.
“This is the first one that we’re aware of,” Steve Farzam, chief operating officer of 911 COVID Testing, told the LA Times. “In and of itself, it’s not overly concerning; however, it is concerning and can be problematic for someone who has pre-existing medical conditions, anyone who is immunocompromised.”
The teen and his family of five had just returned from vacation in Cabo San Lucas, Mexico. All said they tested negative before the trip, but they tested again when they got home because one of the children had a runny nose, Mr. Farzam said.
The boy, who had not been vaccinated for COVID or the flu, doesn’t have serious symptoms and is recovering at home.
In Houston, a 17-year-old boy, his siblings, and his father felt sick a few days before Christmas and went in for testing, TV station KTRK reported. The teen tested positive for both the flu and COVID.
“I ended up getting tested the day before Christmas for strep throat, flu and COVID,” the teenager, Alec Zierlein, told KTRK. “I didn’t think I had any of the three. It felt like a mild cold.”
Health officials reported Jan. 5 that a flurona case was detected in Hays, Kan., TV station WIBW reported. The patient was being treated in the ICU. No other details were provided. In Israel, flurona was first found in an unvaccinated pregnant woman at Rabin Medical Center in Petach Tikva, according to the Times of Israel. She tested positive for both viruses when she arrived at the medical center, and doctors double-checked to confirm her diagnosis. The woman had mild symptoms and was released in good condition, the news outlet reported.
Public health officials in Israel said they are concerned that an increase in both viruses at the same time could lead to many hospitalizations.
A version of this article first appeared on WebMD.com.
The first known case was detected in Israel, but until the first week of January no cases had been reported in the United States.
In Los Angeles, a teenaged boy tested positive for both illnesses at a COVID testing site in Brentwood, the Los Angeles Times reported. The child’s mother tested positive for COVID the next day.
“This is the first one that we’re aware of,” Steve Farzam, chief operating officer of 911 COVID Testing, told the LA Times. “In and of itself, it’s not overly concerning; however, it is concerning and can be problematic for someone who has pre-existing medical conditions, anyone who is immunocompromised.”
The teen and his family of five had just returned from vacation in Cabo San Lucas, Mexico. All said they tested negative before the trip, but they tested again when they got home because one of the children had a runny nose, Mr. Farzam said.
The boy, who had not been vaccinated for COVID or the flu, doesn’t have serious symptoms and is recovering at home.
In Houston, a 17-year-old boy, his siblings, and his father felt sick a few days before Christmas and went in for testing, TV station KTRK reported. The teen tested positive for both the flu and COVID.
“I ended up getting tested the day before Christmas for strep throat, flu and COVID,” the teenager, Alec Zierlein, told KTRK. “I didn’t think I had any of the three. It felt like a mild cold.”
Health officials reported Jan. 5 that a flurona case was detected in Hays, Kan., TV station WIBW reported. The patient was being treated in the ICU. No other details were provided. In Israel, flurona was first found in an unvaccinated pregnant woman at Rabin Medical Center in Petach Tikva, according to the Times of Israel. She tested positive for both viruses when she arrived at the medical center, and doctors double-checked to confirm her diagnosis. The woman had mild symptoms and was released in good condition, the news outlet reported.
Public health officials in Israel said they are concerned that an increase in both viruses at the same time could lead to many hospitalizations.
A version of this article first appeared on WebMD.com.
At-home geriatric assessment offers cost-effective alternative to hospital
The comprehensive geriatric assessment (CGA) is an established strategy for guiding care of older adults in a hospital setting, but its use in other settings has not been well studied, Surya Singh, PhD, of the University of Oxford (England), and colleagues wrote in their paper published in Age and Ageing. Hospital at home is active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care, for a limited time period.
Interest in providing health care in the home as an alternative to hospitalization is on the rise as a way to improve patient outcomes and reduce costs, but actual cost-effectiveness data on HAH interventions are limited, the authors said. “Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements.”
In this study, the researchers conducted a cost-effectiveness analysis alongside a randomized trial of an admission avoidance CGA hospital at home (CGAHAH) service as an alternative to hospital admission. They identified individuals aged 65 years and older who were living in the community but being considered for an unplanned hospital admission in the United Kingdom. A total of 700 individuals were randomized to CGAHAH and 355 to hospital care using a 2:1 ratio. Patients were assessed at baseline in the community or in an acute care setting before being transferred to CGAHAH service. These services included access to social workers, home care, district nursing, community rehabilitation, community mental health services and acute hospital services, such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists, and community pharmacists. There were at least daily virtual ward rounds
Comparison between HAH and in-hospital groups
Patients in the CGAHAH group had a mean of 7.17 days of care, and those in hospital had a mean of 4.92 hospital days. At 6 months’ follow-up, the mean number of care days was 9.47 in the CGAHAH group and 10.58 in the hospital group, which was a nonsignificant difference.
“For complete cases, we found that allocation to CGAHAH resulted in 3 fewer days in hospital, a difference that was reduced to 1 day at 6 months follow-up,” the researchers wrote.
Overall, after adjusting for baseline variables, the health and social care costs after 6 months were less for CGAHAH than admission to hospital. The average cost differences between the two were approximately $3,000 or 2,265 pounds. The cost difference remained and increased to a mean difference of 2,840 pounds in favor of HAH after adding informal care/societal costs.
In addition, patients randomized to CGAHAH were less likely to have been admitted to long-term residential care at 6 months follow-up, compared with the hospital group; the mean days in residential care at 6 months were 3.43 and 6.14, respectively.
Both groups showed an approximate 15% decrease in measures of quality of life from baseline to 6 months, and no differences were noted in quality-adjusted survival between the groups.
Pandemic ‘has accelerated interest’ in HAH
“Health systems around the world are exploring alternatives to hospital admission, such as hospital at home, to act as a buffer to the increasing demand for hospital care,” corresponding author Sasha Shepperd, MSc, DPhil, said in an interview. “This is partly due to a growing older population with increased health needs, but also an emphasis on providing health care that limits a decline in capacity for the older population. Inevitably, the COVID-19 pandemic has accelerated interest in hospital at home to create additional acute health care capacity.”
The take home-message supports the home service option. “If you can access a hospital-at-home service, consider this as an option for older people who would otherwise be admitted to hospital and are eligible for hospital at home care. However, is important that the provision of hospital at home is adequately resourced, and that families and caregivers are supported,” she said.
“Barriers include delivering a different type of service that requires easy access to hospital services, including admission if required; a trained workforce to provide multidisciplinary care in a patient’s home; and ensuring a good fit with existing health and social care services,” Dr. Shepperd said.
Future research areas include the demands placed on caregivers from hospital-at-home services, and how the provision of hospital at home impacts hospital and community services, she added.
Findings support use of HAH
The data from the current study support the use of a hospital at home concept, especially in the geriatric age population, for acute health conditions that could be managed at home rather than acutely in a hospital-based environment,” Noel Deep, MD, emphasized in an interview.
Dr. Deep, who is a general internist in group practice in Antigo, Wisc., said he was not surprised by the study findings.
“I am a big proponent of the hospital at home approach to taking care of patients who can be safely and appropriately managed in the familiarity and comfort of their own home environment with help from physicians, nurses, and other home health care services,” he said. “It is a valuable option for appropriately screened and selected patients to be provided this approach to management of their acute health care situations.”
Primary care physicians should explore using HAH when faced with the decision of admitting an elderly individual to the hospital for management of an acute worsening of a chronic medical condition or a reversible acute illness, said Dr. Deep, who serves on the editorial advisory board of Internal Medicine News.
The current study reinforces previous studies and data showing the benefits of managing acute health problems of elderly individuals in their home environment. These benefits include “an opportunity to free up the emergency rooms and hospitals for providing care to those individuals who truly would be best served by being admitted to the hospital,” Dr. Deep explained. Home care for the elderly “would also lead to decreased utilization of the personal protective equipment and limit exposure of the vulnerable elderly individuals to the coronavirus. Primary care physicians should always explore this possibility of providing care to the patients in their homes if it is a viable option.
“While our practice environment [in the United States] is slightly different than that referenced in this article, many, if not almost all, of our primary care physicians provide care to the geriatric age population and provide assessment and management which would be comparable to this comprehensive geriatric assessment that is discussed in the article,” and many primary care physicians have seen similar results in outcomes that the study shows, said Dr. Deep. The available research and expert opinions are quite similar and agree upon the positive outcomes in terms of providing the CGAHAH approach.
Study is important but raises questions
The study is important because patient-centered, effective care should be the goal of any health system, William Golden, MD, of the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
Dr. Golden also noted that the study raised a number of questions. How each patient entered the treatment protocol was not clear. “Similarly, it is not clear whether admission criteria and resource costs in England cross to the United States experience.”
“Having close follow up of patients at home as opposed to an ‘observation status’ could be a nice innovation, but more details are needed to consider implementation in a specific community setting,” he emphasized.
As for the clinical value of the study for primary care, “primary care professionals should welcome well-staffed alternatives to inpatient care for select patient presentations,” said Dr. Golden, who is also a member of the editorial advisory board of Internal Medicine News.
The current study does not identify the conditions that were treated at home and the logistics of delivering such services, which limits comparison with what experts have seen in practice in terms of outcomes using the CGAHAH, he said. “Interested practitioners would benefit from literature detailing the staffing and decision support tools that form the core framework of this innovation.”
Limitations and strengths of study, according to authors
The study findings were limited by several factors including the calculation of CGAHAH based on service budgets, rather than from collecting information on the actual resources used; potential errors in patients’ estimation of their informal care; and lack of data on a differential impact of CGAHAH for underserved communities, the researchers noted.
However, the results were strengthened by the large study population and randomized design, and support the value of CGAHAH, which addresses the need for management of multiple long-term conditions and the potential decline in functional and cognitive ability in older adults, they said. Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multidimensional assessment, is one option that might reduce reliance on hospitalization and residential care and at a lower cost.
The study was supported by the National Institute for Health Research, and several coauthors received individual grants from the NIHR, with no other financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose.
The comprehensive geriatric assessment (CGA) is an established strategy for guiding care of older adults in a hospital setting, but its use in other settings has not been well studied, Surya Singh, PhD, of the University of Oxford (England), and colleagues wrote in their paper published in Age and Ageing. Hospital at home is active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care, for a limited time period.
Interest in providing health care in the home as an alternative to hospitalization is on the rise as a way to improve patient outcomes and reduce costs, but actual cost-effectiveness data on HAH interventions are limited, the authors said. “Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements.”
In this study, the researchers conducted a cost-effectiveness analysis alongside a randomized trial of an admission avoidance CGA hospital at home (CGAHAH) service as an alternative to hospital admission. They identified individuals aged 65 years and older who were living in the community but being considered for an unplanned hospital admission in the United Kingdom. A total of 700 individuals were randomized to CGAHAH and 355 to hospital care using a 2:1 ratio. Patients were assessed at baseline in the community or in an acute care setting before being transferred to CGAHAH service. These services included access to social workers, home care, district nursing, community rehabilitation, community mental health services and acute hospital services, such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists, and community pharmacists. There were at least daily virtual ward rounds
Comparison between HAH and in-hospital groups
Patients in the CGAHAH group had a mean of 7.17 days of care, and those in hospital had a mean of 4.92 hospital days. At 6 months’ follow-up, the mean number of care days was 9.47 in the CGAHAH group and 10.58 in the hospital group, which was a nonsignificant difference.
“For complete cases, we found that allocation to CGAHAH resulted in 3 fewer days in hospital, a difference that was reduced to 1 day at 6 months follow-up,” the researchers wrote.
Overall, after adjusting for baseline variables, the health and social care costs after 6 months were less for CGAHAH than admission to hospital. The average cost differences between the two were approximately $3,000 or 2,265 pounds. The cost difference remained and increased to a mean difference of 2,840 pounds in favor of HAH after adding informal care/societal costs.
In addition, patients randomized to CGAHAH were less likely to have been admitted to long-term residential care at 6 months follow-up, compared with the hospital group; the mean days in residential care at 6 months were 3.43 and 6.14, respectively.
Both groups showed an approximate 15% decrease in measures of quality of life from baseline to 6 months, and no differences were noted in quality-adjusted survival between the groups.
Pandemic ‘has accelerated interest’ in HAH
“Health systems around the world are exploring alternatives to hospital admission, such as hospital at home, to act as a buffer to the increasing demand for hospital care,” corresponding author Sasha Shepperd, MSc, DPhil, said in an interview. “This is partly due to a growing older population with increased health needs, but also an emphasis on providing health care that limits a decline in capacity for the older population. Inevitably, the COVID-19 pandemic has accelerated interest in hospital at home to create additional acute health care capacity.”
The take home-message supports the home service option. “If you can access a hospital-at-home service, consider this as an option for older people who would otherwise be admitted to hospital and are eligible for hospital at home care. However, is important that the provision of hospital at home is adequately resourced, and that families and caregivers are supported,” she said.
“Barriers include delivering a different type of service that requires easy access to hospital services, including admission if required; a trained workforce to provide multidisciplinary care in a patient’s home; and ensuring a good fit with existing health and social care services,” Dr. Shepperd said.
Future research areas include the demands placed on caregivers from hospital-at-home services, and how the provision of hospital at home impacts hospital and community services, she added.
Findings support use of HAH
The data from the current study support the use of a hospital at home concept, especially in the geriatric age population, for acute health conditions that could be managed at home rather than acutely in a hospital-based environment,” Noel Deep, MD, emphasized in an interview.
Dr. Deep, who is a general internist in group practice in Antigo, Wisc., said he was not surprised by the study findings.
“I am a big proponent of the hospital at home approach to taking care of patients who can be safely and appropriately managed in the familiarity and comfort of their own home environment with help from physicians, nurses, and other home health care services,” he said. “It is a valuable option for appropriately screened and selected patients to be provided this approach to management of their acute health care situations.”
Primary care physicians should explore using HAH when faced with the decision of admitting an elderly individual to the hospital for management of an acute worsening of a chronic medical condition or a reversible acute illness, said Dr. Deep, who serves on the editorial advisory board of Internal Medicine News.
The current study reinforces previous studies and data showing the benefits of managing acute health problems of elderly individuals in their home environment. These benefits include “an opportunity to free up the emergency rooms and hospitals for providing care to those individuals who truly would be best served by being admitted to the hospital,” Dr. Deep explained. Home care for the elderly “would also lead to decreased utilization of the personal protective equipment and limit exposure of the vulnerable elderly individuals to the coronavirus. Primary care physicians should always explore this possibility of providing care to the patients in their homes if it is a viable option.
“While our practice environment [in the United States] is slightly different than that referenced in this article, many, if not almost all, of our primary care physicians provide care to the geriatric age population and provide assessment and management which would be comparable to this comprehensive geriatric assessment that is discussed in the article,” and many primary care physicians have seen similar results in outcomes that the study shows, said Dr. Deep. The available research and expert opinions are quite similar and agree upon the positive outcomes in terms of providing the CGAHAH approach.
Study is important but raises questions
The study is important because patient-centered, effective care should be the goal of any health system, William Golden, MD, of the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
Dr. Golden also noted that the study raised a number of questions. How each patient entered the treatment protocol was not clear. “Similarly, it is not clear whether admission criteria and resource costs in England cross to the United States experience.”
“Having close follow up of patients at home as opposed to an ‘observation status’ could be a nice innovation, but more details are needed to consider implementation in a specific community setting,” he emphasized.
As for the clinical value of the study for primary care, “primary care professionals should welcome well-staffed alternatives to inpatient care for select patient presentations,” said Dr. Golden, who is also a member of the editorial advisory board of Internal Medicine News.
The current study does not identify the conditions that were treated at home and the logistics of delivering such services, which limits comparison with what experts have seen in practice in terms of outcomes using the CGAHAH, he said. “Interested practitioners would benefit from literature detailing the staffing and decision support tools that form the core framework of this innovation.”
Limitations and strengths of study, according to authors
The study findings were limited by several factors including the calculation of CGAHAH based on service budgets, rather than from collecting information on the actual resources used; potential errors in patients’ estimation of their informal care; and lack of data on a differential impact of CGAHAH for underserved communities, the researchers noted.
However, the results were strengthened by the large study population and randomized design, and support the value of CGAHAH, which addresses the need for management of multiple long-term conditions and the potential decline in functional and cognitive ability in older adults, they said. Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multidimensional assessment, is one option that might reduce reliance on hospitalization and residential care and at a lower cost.
The study was supported by the National Institute for Health Research, and several coauthors received individual grants from the NIHR, with no other financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose.
The comprehensive geriatric assessment (CGA) is an established strategy for guiding care of older adults in a hospital setting, but its use in other settings has not been well studied, Surya Singh, PhD, of the University of Oxford (England), and colleagues wrote in their paper published in Age and Ageing. Hospital at home is active treatment by health care professionals in the patient’s home for a condition that otherwise would require acute hospital inpatient care, for a limited time period.
Interest in providing health care in the home as an alternative to hospitalization is on the rise as a way to improve patient outcomes and reduce costs, but actual cost-effectiveness data on HAH interventions are limited, the authors said. “Wide scale implementation of such services has also been constrained by the practical difficulties of designing and delivering services that cut across primary and secondary care, might involve social care and require different workforce and funding arrangements.”
In this study, the researchers conducted a cost-effectiveness analysis alongside a randomized trial of an admission avoidance CGA hospital at home (CGAHAH) service as an alternative to hospital admission. They identified individuals aged 65 years and older who were living in the community but being considered for an unplanned hospital admission in the United Kingdom. A total of 700 individuals were randomized to CGAHAH and 355 to hospital care using a 2:1 ratio. Patients were assessed at baseline in the community or in an acute care setting before being transferred to CGAHAH service. These services included access to social workers, home care, district nursing, community rehabilitation, community mental health services and acute hospital services, such as diagnostic tests and transfer to hospital. The core workforce usually included consultant geriatricians, junior doctors, nurse practitioners, health care assistants or support workers, physiotherapists, occupational therapists, and community pharmacists. There were at least daily virtual ward rounds
Comparison between HAH and in-hospital groups
Patients in the CGAHAH group had a mean of 7.17 days of care, and those in hospital had a mean of 4.92 hospital days. At 6 months’ follow-up, the mean number of care days was 9.47 in the CGAHAH group and 10.58 in the hospital group, which was a nonsignificant difference.
“For complete cases, we found that allocation to CGAHAH resulted in 3 fewer days in hospital, a difference that was reduced to 1 day at 6 months follow-up,” the researchers wrote.
Overall, after adjusting for baseline variables, the health and social care costs after 6 months were less for CGAHAH than admission to hospital. The average cost differences between the two were approximately $3,000 or 2,265 pounds. The cost difference remained and increased to a mean difference of 2,840 pounds in favor of HAH after adding informal care/societal costs.
In addition, patients randomized to CGAHAH were less likely to have been admitted to long-term residential care at 6 months follow-up, compared with the hospital group; the mean days in residential care at 6 months were 3.43 and 6.14, respectively.
Both groups showed an approximate 15% decrease in measures of quality of life from baseline to 6 months, and no differences were noted in quality-adjusted survival between the groups.
Pandemic ‘has accelerated interest’ in HAH
“Health systems around the world are exploring alternatives to hospital admission, such as hospital at home, to act as a buffer to the increasing demand for hospital care,” corresponding author Sasha Shepperd, MSc, DPhil, said in an interview. “This is partly due to a growing older population with increased health needs, but also an emphasis on providing health care that limits a decline in capacity for the older population. Inevitably, the COVID-19 pandemic has accelerated interest in hospital at home to create additional acute health care capacity.”
The take home-message supports the home service option. “If you can access a hospital-at-home service, consider this as an option for older people who would otherwise be admitted to hospital and are eligible for hospital at home care. However, is important that the provision of hospital at home is adequately resourced, and that families and caregivers are supported,” she said.
“Barriers include delivering a different type of service that requires easy access to hospital services, including admission if required; a trained workforce to provide multidisciplinary care in a patient’s home; and ensuring a good fit with existing health and social care services,” Dr. Shepperd said.
Future research areas include the demands placed on caregivers from hospital-at-home services, and how the provision of hospital at home impacts hospital and community services, she added.
Findings support use of HAH
The data from the current study support the use of a hospital at home concept, especially in the geriatric age population, for acute health conditions that could be managed at home rather than acutely in a hospital-based environment,” Noel Deep, MD, emphasized in an interview.
Dr. Deep, who is a general internist in group practice in Antigo, Wisc., said he was not surprised by the study findings.
“I am a big proponent of the hospital at home approach to taking care of patients who can be safely and appropriately managed in the familiarity and comfort of their own home environment with help from physicians, nurses, and other home health care services,” he said. “It is a valuable option for appropriately screened and selected patients to be provided this approach to management of their acute health care situations.”
Primary care physicians should explore using HAH when faced with the decision of admitting an elderly individual to the hospital for management of an acute worsening of a chronic medical condition or a reversible acute illness, said Dr. Deep, who serves on the editorial advisory board of Internal Medicine News.
The current study reinforces previous studies and data showing the benefits of managing acute health problems of elderly individuals in their home environment. These benefits include “an opportunity to free up the emergency rooms and hospitals for providing care to those individuals who truly would be best served by being admitted to the hospital,” Dr. Deep explained. Home care for the elderly “would also lead to decreased utilization of the personal protective equipment and limit exposure of the vulnerable elderly individuals to the coronavirus. Primary care physicians should always explore this possibility of providing care to the patients in their homes if it is a viable option.
“While our practice environment [in the United States] is slightly different than that referenced in this article, many, if not almost all, of our primary care physicians provide care to the geriatric age population and provide assessment and management which would be comparable to this comprehensive geriatric assessment that is discussed in the article,” and many primary care physicians have seen similar results in outcomes that the study shows, said Dr. Deep. The available research and expert opinions are quite similar and agree upon the positive outcomes in terms of providing the CGAHAH approach.
Study is important but raises questions
The study is important because patient-centered, effective care should be the goal of any health system, William Golden, MD, of the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
Dr. Golden also noted that the study raised a number of questions. How each patient entered the treatment protocol was not clear. “Similarly, it is not clear whether admission criteria and resource costs in England cross to the United States experience.”
“Having close follow up of patients at home as opposed to an ‘observation status’ could be a nice innovation, but more details are needed to consider implementation in a specific community setting,” he emphasized.
As for the clinical value of the study for primary care, “primary care professionals should welcome well-staffed alternatives to inpatient care for select patient presentations,” said Dr. Golden, who is also a member of the editorial advisory board of Internal Medicine News.
The current study does not identify the conditions that were treated at home and the logistics of delivering such services, which limits comparison with what experts have seen in practice in terms of outcomes using the CGAHAH, he said. “Interested practitioners would benefit from literature detailing the staffing and decision support tools that form the core framework of this innovation.”
Limitations and strengths of study, according to authors
The study findings were limited by several factors including the calculation of CGAHAH based on service budgets, rather than from collecting information on the actual resources used; potential errors in patients’ estimation of their informal care; and lack of data on a differential impact of CGAHAH for underserved communities, the researchers noted.
However, the results were strengthened by the large study population and randomized design, and support the value of CGAHAH, which addresses the need for management of multiple long-term conditions and the potential decline in functional and cognitive ability in older adults, they said. Providing CGAHAH as an alternative to admission to hospital for older people, with a focus on multidimensional assessment, is one option that might reduce reliance on hospitalization and residential care and at a lower cost.
The study was supported by the National Institute for Health Research, and several coauthors received individual grants from the NIHR, with no other financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose.
FROM AGE AND AGEING




