User login
We’re dying to tell you about fatigability
Are you tired? Or are you death tired?
When we’re feeling that burnout monster creep in we sometimes say that we’re being worked to death or that we’re dead tired, but what if that feeling could predict when it’s your actual time to go?
In a recent study published in the Journals of Gerontology: Series A, epidemiologists from the University of Pittsburgh were able to associate a level of “physical fatigability” with mortality.
The researchers administered the Pittsburgh Fatigability Scale to almost 3,000 participants aged ≥ 60 years, who ranked from 0 to 5 on how tired they thought they would be after doing activities like light housework or a leisurely 30-minute walk. After accounting for factors such as preexisting conditions and mental health, the researchers found that people who scored 25 or more points were 2.3 times more likely to die in the next 2.7 years, compared with those who scored under 25.
So what does that tell us about the importance of being continuously active? It’s pretty important.
“Previous research indicates that getting more physical activity can reduce a person’s fatigability. Our study is the first to link more severe physical fatigability to an earlier death,” lead author Nancy W. Glynn, PhD, said in a separate statement. The best way to keep physically active, she suggested, is to set manageable goals and a routine.
A nice walk around the neighborhood during golden hour or a little bit of yoga before breakfast could be a great way to keep the body moving, because you know what they say: Use it or lose it.
This work is NFT protected: Do not screenshot
If you’ve been following the nonmedical news, you’ve likely heard the term “NFT” explode in the past few months. Standing for nonfungible token, NFTs are, at least theoretically, a proof of ownership for digital creations that prevents anyone other than the buyer from reselling the artwork. Sounds like a great idea: It protects artists and buyers alike.
Much like its cousin cryptocurrency, however, the NFT world is rife with speculation, scams, misunderstanding, and drawings of bored monkeys. It’s the Wild West out there in the digital art universe: One poor unfortunate accidentally sold a $300k NFT image for $3,000, a group of investors spent $3 million buying an NFT for a rare version of Dune believing it gave them the copyright (it did not), and an Indonesian engineering student’s 5-year series of expressionless selfies is now worth a million dollars.
This is a column detailing weird medical news, however, so with our setup complete (though our understanding of NFTs is very much not), we move to France and meet our hero (?), Emmanuel Masmejean, an orthopedic surgeon who apparently wasn’t making enough money in his lucrative medical career.
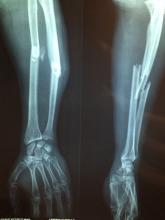
In a move of apocalyptic madness, he threw ethics out the window, delved into his archive, and found an x-ray of a young woman with a bullet lodged in her arm. The woman was a survivor of the Bataclan mass shooting and bombing in 2015, and don’t you worry, our intrepid entrepreneur made sure to identify her as such when he tried selling the x-ray as an NFT on an online art website for $2,776. Yes, this is very much a violation of doctor-patient confidentiality, and no, that’s not a lot of money to risk your medical career on.
Naturally, the woman was horrified and shocked to learn that the image was being sold, her lawyer told the Guardian. When the doctor called her, he merely attempted to justify his action, rather than apologizing or showing any remorse. Dr. Masmejean is now facing legal action and a disciplinary charge for his attempted entry into the NFT world for publishing the image without permission, and the NFT has been removed from the website. Should have stuck with the bored monkeys.
Avatars could be the future
Zoom, FaceTime, and Skype are great when people can’t be together in the same room, state, or country. Not the same as being somewhere in person, but a pretty good replacement during a global pandemic. But what if you had a robot that could be present for you?
Seven-year-old Joshua Martinangeli of Berlin has a severe lung disease and needs to wear a tube in his neck, so he cannot attend school. A robot avatar, donated to Joshua through a private initiative, sits in his seat in the classroom and is able to interact with the students and teacher, according to Reuters. A light on the avatar blinks when Joshua wants to speak and the children can talk with him too. Joshua and his classmates agree that it’s not the same as him really being there to talk and learn, but it’s a great way to keep him included.
“We are the only district in Berlin that has bought four avatars for its schools. The impetus was COVID-19, but I think this will be the future well beyond the pandemic,” Torsten Kuehne, district education councilor, told Reuters.
So where do we get an avatar to go out and run errands? Can we send it to the office instead of Zooming the next meeting? Or maybe our avatar could go to the gym for us. But how do we get the results to show up on our bodies? C’mon science, figure this out.
Futility, thy name is Kiribati
Before we get to the rest of our regularly scheduled hilarity, a brief geography lesson is in order: Kiribati is an island nation – actually 32 atolls and one coral island – in the central Pacific Ocean. Those atolls are spread out across 1.4 million square miles around the intersection of the equator and the International Date Line, so Kiribati is the only country in the world located in all four hemispheres.
Now, back to the news.
Kiribati closed its borders early in the COVID-19 pandemic and recorded only two cases in almost 2 years. Things were going so well that the authorities recently decided to reopen the country to international travelers. Silly authorities.
The first plane was set to arrive on Jan. 14 from Fiji. This being the age of COVID, plans were made and precautions were taken. All 54 passengers quarantined for 2 weeks before the flight and underwent regular testing, the Guardian noted, and “they were only allowed on the flight after returning negative tests.”
You guessed it. Two-thirds of those 54 people tested positive for COVID-19 after landing in Kiribati.
All of the passengers were quarantined, but since then a security guard at the quarantine center has tested positive, as has someone who was not involved in the quarantine. According to NPR, the government said that “there is now an assumption that COVID-19 is now spreading in the community on South Tarawa and Betio.”
Moral of the story? You can’t beat COVID, so never try.
[EDITOR: Is that really the message we want to send to our readers?]
If you can’t beat them, join them.
[EDITOR: Nope. Try again.]
Resistance is futile?
[EDITOR: Sigh. Close enough.]
Are you tired? Or are you death tired?
When we’re feeling that burnout monster creep in we sometimes say that we’re being worked to death or that we’re dead tired, but what if that feeling could predict when it’s your actual time to go?
In a recent study published in the Journals of Gerontology: Series A, epidemiologists from the University of Pittsburgh were able to associate a level of “physical fatigability” with mortality.
The researchers administered the Pittsburgh Fatigability Scale to almost 3,000 participants aged ≥ 60 years, who ranked from 0 to 5 on how tired they thought they would be after doing activities like light housework or a leisurely 30-minute walk. After accounting for factors such as preexisting conditions and mental health, the researchers found that people who scored 25 or more points were 2.3 times more likely to die in the next 2.7 years, compared with those who scored under 25.
So what does that tell us about the importance of being continuously active? It’s pretty important.
“Previous research indicates that getting more physical activity can reduce a person’s fatigability. Our study is the first to link more severe physical fatigability to an earlier death,” lead author Nancy W. Glynn, PhD, said in a separate statement. The best way to keep physically active, she suggested, is to set manageable goals and a routine.
A nice walk around the neighborhood during golden hour or a little bit of yoga before breakfast could be a great way to keep the body moving, because you know what they say: Use it or lose it.
This work is NFT protected: Do not screenshot
If you’ve been following the nonmedical news, you’ve likely heard the term “NFT” explode in the past few months. Standing for nonfungible token, NFTs are, at least theoretically, a proof of ownership for digital creations that prevents anyone other than the buyer from reselling the artwork. Sounds like a great idea: It protects artists and buyers alike.
Much like its cousin cryptocurrency, however, the NFT world is rife with speculation, scams, misunderstanding, and drawings of bored monkeys. It’s the Wild West out there in the digital art universe: One poor unfortunate accidentally sold a $300k NFT image for $3,000, a group of investors spent $3 million buying an NFT for a rare version of Dune believing it gave them the copyright (it did not), and an Indonesian engineering student’s 5-year series of expressionless selfies is now worth a million dollars.
This is a column detailing weird medical news, however, so with our setup complete (though our understanding of NFTs is very much not), we move to France and meet our hero (?), Emmanuel Masmejean, an orthopedic surgeon who apparently wasn’t making enough money in his lucrative medical career.
In a move of apocalyptic madness, he threw ethics out the window, delved into his archive, and found an x-ray of a young woman with a bullet lodged in her arm. The woman was a survivor of the Bataclan mass shooting and bombing in 2015, and don’t you worry, our intrepid entrepreneur made sure to identify her as such when he tried selling the x-ray as an NFT on an online art website for $2,776. Yes, this is very much a violation of doctor-patient confidentiality, and no, that’s not a lot of money to risk your medical career on.
Naturally, the woman was horrified and shocked to learn that the image was being sold, her lawyer told the Guardian. When the doctor called her, he merely attempted to justify his action, rather than apologizing or showing any remorse. Dr. Masmejean is now facing legal action and a disciplinary charge for his attempted entry into the NFT world for publishing the image without permission, and the NFT has been removed from the website. Should have stuck with the bored monkeys.
Avatars could be the future
Zoom, FaceTime, and Skype are great when people can’t be together in the same room, state, or country. Not the same as being somewhere in person, but a pretty good replacement during a global pandemic. But what if you had a robot that could be present for you?
Seven-year-old Joshua Martinangeli of Berlin has a severe lung disease and needs to wear a tube in his neck, so he cannot attend school. A robot avatar, donated to Joshua through a private initiative, sits in his seat in the classroom and is able to interact with the students and teacher, according to Reuters. A light on the avatar blinks when Joshua wants to speak and the children can talk with him too. Joshua and his classmates agree that it’s not the same as him really being there to talk and learn, but it’s a great way to keep him included.
“We are the only district in Berlin that has bought four avatars for its schools. The impetus was COVID-19, but I think this will be the future well beyond the pandemic,” Torsten Kuehne, district education councilor, told Reuters.
So where do we get an avatar to go out and run errands? Can we send it to the office instead of Zooming the next meeting? Or maybe our avatar could go to the gym for us. But how do we get the results to show up on our bodies? C’mon science, figure this out.
Futility, thy name is Kiribati
Before we get to the rest of our regularly scheduled hilarity, a brief geography lesson is in order: Kiribati is an island nation – actually 32 atolls and one coral island – in the central Pacific Ocean. Those atolls are spread out across 1.4 million square miles around the intersection of the equator and the International Date Line, so Kiribati is the only country in the world located in all four hemispheres.
Now, back to the news.
Kiribati closed its borders early in the COVID-19 pandemic and recorded only two cases in almost 2 years. Things were going so well that the authorities recently decided to reopen the country to international travelers. Silly authorities.
The first plane was set to arrive on Jan. 14 from Fiji. This being the age of COVID, plans were made and precautions were taken. All 54 passengers quarantined for 2 weeks before the flight and underwent regular testing, the Guardian noted, and “they were only allowed on the flight after returning negative tests.”
You guessed it. Two-thirds of those 54 people tested positive for COVID-19 after landing in Kiribati.
All of the passengers were quarantined, but since then a security guard at the quarantine center has tested positive, as has someone who was not involved in the quarantine. According to NPR, the government said that “there is now an assumption that COVID-19 is now spreading in the community on South Tarawa and Betio.”
Moral of the story? You can’t beat COVID, so never try.
[EDITOR: Is that really the message we want to send to our readers?]
If you can’t beat them, join them.
[EDITOR: Nope. Try again.]
Resistance is futile?
[EDITOR: Sigh. Close enough.]
Are you tired? Or are you death tired?
When we’re feeling that burnout monster creep in we sometimes say that we’re being worked to death or that we’re dead tired, but what if that feeling could predict when it’s your actual time to go?
In a recent study published in the Journals of Gerontology: Series A, epidemiologists from the University of Pittsburgh were able to associate a level of “physical fatigability” with mortality.
The researchers administered the Pittsburgh Fatigability Scale to almost 3,000 participants aged ≥ 60 years, who ranked from 0 to 5 on how tired they thought they would be after doing activities like light housework or a leisurely 30-minute walk. After accounting for factors such as preexisting conditions and mental health, the researchers found that people who scored 25 or more points were 2.3 times more likely to die in the next 2.7 years, compared with those who scored under 25.
So what does that tell us about the importance of being continuously active? It’s pretty important.
“Previous research indicates that getting more physical activity can reduce a person’s fatigability. Our study is the first to link more severe physical fatigability to an earlier death,” lead author Nancy W. Glynn, PhD, said in a separate statement. The best way to keep physically active, she suggested, is to set manageable goals and a routine.
A nice walk around the neighborhood during golden hour or a little bit of yoga before breakfast could be a great way to keep the body moving, because you know what they say: Use it or lose it.
This work is NFT protected: Do not screenshot
If you’ve been following the nonmedical news, you’ve likely heard the term “NFT” explode in the past few months. Standing for nonfungible token, NFTs are, at least theoretically, a proof of ownership for digital creations that prevents anyone other than the buyer from reselling the artwork. Sounds like a great idea: It protects artists and buyers alike.
Much like its cousin cryptocurrency, however, the NFT world is rife with speculation, scams, misunderstanding, and drawings of bored monkeys. It’s the Wild West out there in the digital art universe: One poor unfortunate accidentally sold a $300k NFT image for $3,000, a group of investors spent $3 million buying an NFT for a rare version of Dune believing it gave them the copyright (it did not), and an Indonesian engineering student’s 5-year series of expressionless selfies is now worth a million dollars.
This is a column detailing weird medical news, however, so with our setup complete (though our understanding of NFTs is very much not), we move to France and meet our hero (?), Emmanuel Masmejean, an orthopedic surgeon who apparently wasn’t making enough money in his lucrative medical career.
In a move of apocalyptic madness, he threw ethics out the window, delved into his archive, and found an x-ray of a young woman with a bullet lodged in her arm. The woman was a survivor of the Bataclan mass shooting and bombing in 2015, and don’t you worry, our intrepid entrepreneur made sure to identify her as such when he tried selling the x-ray as an NFT on an online art website for $2,776. Yes, this is very much a violation of doctor-patient confidentiality, and no, that’s not a lot of money to risk your medical career on.
Naturally, the woman was horrified and shocked to learn that the image was being sold, her lawyer told the Guardian. When the doctor called her, he merely attempted to justify his action, rather than apologizing or showing any remorse. Dr. Masmejean is now facing legal action and a disciplinary charge for his attempted entry into the NFT world for publishing the image without permission, and the NFT has been removed from the website. Should have stuck with the bored monkeys.
Avatars could be the future
Zoom, FaceTime, and Skype are great when people can’t be together in the same room, state, or country. Not the same as being somewhere in person, but a pretty good replacement during a global pandemic. But what if you had a robot that could be present for you?
Seven-year-old Joshua Martinangeli of Berlin has a severe lung disease and needs to wear a tube in his neck, so he cannot attend school. A robot avatar, donated to Joshua through a private initiative, sits in his seat in the classroom and is able to interact with the students and teacher, according to Reuters. A light on the avatar blinks when Joshua wants to speak and the children can talk with him too. Joshua and his classmates agree that it’s not the same as him really being there to talk and learn, but it’s a great way to keep him included.
“We are the only district in Berlin that has bought four avatars for its schools. The impetus was COVID-19, but I think this will be the future well beyond the pandemic,” Torsten Kuehne, district education councilor, told Reuters.
So where do we get an avatar to go out and run errands? Can we send it to the office instead of Zooming the next meeting? Or maybe our avatar could go to the gym for us. But how do we get the results to show up on our bodies? C’mon science, figure this out.
Futility, thy name is Kiribati
Before we get to the rest of our regularly scheduled hilarity, a brief geography lesson is in order: Kiribati is an island nation – actually 32 atolls and one coral island – in the central Pacific Ocean. Those atolls are spread out across 1.4 million square miles around the intersection of the equator and the International Date Line, so Kiribati is the only country in the world located in all four hemispheres.
Now, back to the news.
Kiribati closed its borders early in the COVID-19 pandemic and recorded only two cases in almost 2 years. Things were going so well that the authorities recently decided to reopen the country to international travelers. Silly authorities.
The first plane was set to arrive on Jan. 14 from Fiji. This being the age of COVID, plans were made and precautions were taken. All 54 passengers quarantined for 2 weeks before the flight and underwent regular testing, the Guardian noted, and “they were only allowed on the flight after returning negative tests.”
You guessed it. Two-thirds of those 54 people tested positive for COVID-19 after landing in Kiribati.
All of the passengers were quarantined, but since then a security guard at the quarantine center has tested positive, as has someone who was not involved in the quarantine. According to NPR, the government said that “there is now an assumption that COVID-19 is now spreading in the community on South Tarawa and Betio.”
Moral of the story? You can’t beat COVID, so never try.
[EDITOR: Is that really the message we want to send to our readers?]
If you can’t beat them, join them.
[EDITOR: Nope. Try again.]
Resistance is futile?
[EDITOR: Sigh. Close enough.]
35% of employers to proceed with vaccine mandate, poll shows
despite a recent U.S. Supreme Court ruling that blocked the Biden administration’s vaccine-or-test rule for big businesses.
But the poll by Gartner Inc. showed no consensus among employers. About 4% of polled executives said they’re dropping their vaccine mandate, 29% are in a wait-and-see position, and 12% are less likely to impose a mandate now, Bloomberg reported.
Executives were divided on how a vaccine mandate would affect absenteeism and employee morale. Almost 40% of polled employers said they thought a mandate would attract workers, but about 25% said it would do the opposite, Bloomberg said.
“What is more attractive -- to have a mandate or not?” Brian Kropp, PhD, Gartner’s chief of human resources research, said in an interview with Bloomberg. “Most are not exactly sure what to do.”
Big companies have reacted differently since the court’s ruling.
Starbucks announced it was dropping its vaccine-or-test rule for the company’s approximately 228,000 employees. General Electric dropped its mandate after the ruling, but Honeywell International Inc. announced it was staying with its vaccination policy, Bloomberg said.
The Supreme Court ruled Jan. 13 against the Biden administration’s mandate for businesses. The Occupational Safety and Health Administration had proposed that every company with more than 100 employees would be required to ensure workers were either vaccinated or tested weekly for COVID-19.
State governments and business groups immediately appealed, and the court ruled 6-3 against the mandate. The Biden administration officially dropped its rule on Wednesday.
A version of this article first appeared on WebMD.com.
despite a recent U.S. Supreme Court ruling that blocked the Biden administration’s vaccine-or-test rule for big businesses.
But the poll by Gartner Inc. showed no consensus among employers. About 4% of polled executives said they’re dropping their vaccine mandate, 29% are in a wait-and-see position, and 12% are less likely to impose a mandate now, Bloomberg reported.
Executives were divided on how a vaccine mandate would affect absenteeism and employee morale. Almost 40% of polled employers said they thought a mandate would attract workers, but about 25% said it would do the opposite, Bloomberg said.
“What is more attractive -- to have a mandate or not?” Brian Kropp, PhD, Gartner’s chief of human resources research, said in an interview with Bloomberg. “Most are not exactly sure what to do.”
Big companies have reacted differently since the court’s ruling.
Starbucks announced it was dropping its vaccine-or-test rule for the company’s approximately 228,000 employees. General Electric dropped its mandate after the ruling, but Honeywell International Inc. announced it was staying with its vaccination policy, Bloomberg said.
The Supreme Court ruled Jan. 13 against the Biden administration’s mandate for businesses. The Occupational Safety and Health Administration had proposed that every company with more than 100 employees would be required to ensure workers were either vaccinated or tested weekly for COVID-19.
State governments and business groups immediately appealed, and the court ruled 6-3 against the mandate. The Biden administration officially dropped its rule on Wednesday.
A version of this article first appeared on WebMD.com.
despite a recent U.S. Supreme Court ruling that blocked the Biden administration’s vaccine-or-test rule for big businesses.
But the poll by Gartner Inc. showed no consensus among employers. About 4% of polled executives said they’re dropping their vaccine mandate, 29% are in a wait-and-see position, and 12% are less likely to impose a mandate now, Bloomberg reported.
Executives were divided on how a vaccine mandate would affect absenteeism and employee morale. Almost 40% of polled employers said they thought a mandate would attract workers, but about 25% said it would do the opposite, Bloomberg said.
“What is more attractive -- to have a mandate or not?” Brian Kropp, PhD, Gartner’s chief of human resources research, said in an interview with Bloomberg. “Most are not exactly sure what to do.”
Big companies have reacted differently since the court’s ruling.
Starbucks announced it was dropping its vaccine-or-test rule for the company’s approximately 228,000 employees. General Electric dropped its mandate after the ruling, but Honeywell International Inc. announced it was staying with its vaccination policy, Bloomberg said.
The Supreme Court ruled Jan. 13 against the Biden administration’s mandate for businesses. The Occupational Safety and Health Administration had proposed that every company with more than 100 employees would be required to ensure workers were either vaccinated or tested weekly for COVID-19.
State governments and business groups immediately appealed, and the court ruled 6-3 against the mandate. The Biden administration officially dropped its rule on Wednesday.
A version of this article first appeared on WebMD.com.
Omicron survives longer on plastic, skin than other COVID variants
, one possible explanation for why Omicron has spread so rapidly around the world.
In a lab experiment, samples of different variants were applied to pieces of plastic and human skin collected from autopsies, researchers from Kyoto Prefectural University of Medicine wrote in bioRxiv. A variant “survived” until it could no longer be detected on the surface.
“This study showed that the Omicron variant also has the highest environmental stability among VOCs (variants of concern), which suggests that this high stability might also be one of the factors that have allowed the Omicron variant to replace the Delta variant and spread rapidly,” the researchers wrote.
On plastic, the Omicron variant samples survived an average of 193.5 hours, a little more than 8 days. By comparison, the other survival times on plastic were 56 hours for the original COVID strain, 191.3 hours for Alpha, 156.6 hours for Beta, 59.3 hours for Gamma, and 114 hours for Delta.
On skin samples, the Omicron samples survived an average of 21.1 hours. The other variants had these average survival times on skin: 8.6 hours for the original version, 19.6 hours for Alpha, 19.1 hours for Beta, 11 hours for Gamma, and 16.8 hours for Delta.
The study found that the variants had more resistance to ethanol than the original strain of COVID. That said, all COVID samples were inactivated after being exposed to alcohol-based hand sanitizers for 15 seconds.
“Therefore, it is highly recommended that current infection control (hand hygiene) practices use disinfectants ... as proposed by the World Health Organization,” the researchers said.
The study has not been peer-reviewed.
A version of this article first appeared on WebMD.com.
, one possible explanation for why Omicron has spread so rapidly around the world.
In a lab experiment, samples of different variants were applied to pieces of plastic and human skin collected from autopsies, researchers from Kyoto Prefectural University of Medicine wrote in bioRxiv. A variant “survived” until it could no longer be detected on the surface.
“This study showed that the Omicron variant also has the highest environmental stability among VOCs (variants of concern), which suggests that this high stability might also be one of the factors that have allowed the Omicron variant to replace the Delta variant and spread rapidly,” the researchers wrote.
On plastic, the Omicron variant samples survived an average of 193.5 hours, a little more than 8 days. By comparison, the other survival times on plastic were 56 hours for the original COVID strain, 191.3 hours for Alpha, 156.6 hours for Beta, 59.3 hours for Gamma, and 114 hours for Delta.
On skin samples, the Omicron samples survived an average of 21.1 hours. The other variants had these average survival times on skin: 8.6 hours for the original version, 19.6 hours for Alpha, 19.1 hours for Beta, 11 hours for Gamma, and 16.8 hours for Delta.
The study found that the variants had more resistance to ethanol than the original strain of COVID. That said, all COVID samples were inactivated after being exposed to alcohol-based hand sanitizers for 15 seconds.
“Therefore, it is highly recommended that current infection control (hand hygiene) practices use disinfectants ... as proposed by the World Health Organization,” the researchers said.
The study has not been peer-reviewed.
A version of this article first appeared on WebMD.com.
, one possible explanation for why Omicron has spread so rapidly around the world.
In a lab experiment, samples of different variants were applied to pieces of plastic and human skin collected from autopsies, researchers from Kyoto Prefectural University of Medicine wrote in bioRxiv. A variant “survived” until it could no longer be detected on the surface.
“This study showed that the Omicron variant also has the highest environmental stability among VOCs (variants of concern), which suggests that this high stability might also be one of the factors that have allowed the Omicron variant to replace the Delta variant and spread rapidly,” the researchers wrote.
On plastic, the Omicron variant samples survived an average of 193.5 hours, a little more than 8 days. By comparison, the other survival times on plastic were 56 hours for the original COVID strain, 191.3 hours for Alpha, 156.6 hours for Beta, 59.3 hours for Gamma, and 114 hours for Delta.
On skin samples, the Omicron samples survived an average of 21.1 hours. The other variants had these average survival times on skin: 8.6 hours for the original version, 19.6 hours for Alpha, 19.1 hours for Beta, 11 hours for Gamma, and 16.8 hours for Delta.
The study found that the variants had more resistance to ethanol than the original strain of COVID. That said, all COVID samples were inactivated after being exposed to alcohol-based hand sanitizers for 15 seconds.
“Therefore, it is highly recommended that current infection control (hand hygiene) practices use disinfectants ... as proposed by the World Health Organization,” the researchers said.
The study has not been peer-reviewed.
A version of this article first appeared on WebMD.com.
Presence of autoantibodies most predictive of long COVID in study
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
Other significant early predictors of prolonged COVID symptoms – which the researchers called postacute sequelae – were having type 2 diabetes, SARS-CoV-2 RNAemia, and Epstein-Barr virus (EBV) viremia, Yapeng Su, PhD, of the Institute for Systems Biology (ISB) in Seattle, and colleagues wrote in Cell.
Having EBV viremia suggested that latent EBV has been reactivated, the authors noted.
“The most important postacute sequelae [that is conditions that are consequences of a disease] of COVID is the presence of autoantibodies,” James R. Heath, PhD, president of ISB and a bioengineering professor at the University of Washington, Seattle, said in an interview. “It’s about two times more important than the others.”
Dr. Heath and coauthors said early detection of this and other variables could prompt earlier aggressive treatment in patients susceptible to long COVID and ward off lingering symptoms.
“These predictive measures of long COVID can also help to better inform patients of their possible disease course,” study coauthor Daniel G. Chen, an undergraduate researcher at ISB, said in an interview. “We were also able to partially resolve the immunological underpinnings of some postacute sequelae of COVID in a way that suggested potential therapies, and the timing of those therapies.”
For example, he continued, the use of antivirals very early in the infectious course may mitigate the later development of long COVID. “This will, of course, have to be explored in an appropriately designed clinical trial.
“We also identified biomarkers of certain types of long COVID, such as neurological sequelae. Those biomarkers can help define the condition, which is a first step towards developing treatments.”
Study findings
With COVID patients monitored for 2 or 3 months, the study findings of the international “multiomic profiling” analysis include:
- Subclinical patient autoantibodies that reduce anti–SARS-CoV-2 antibodies suggest there is immune dysregulation during COVID-19 infection.
- Reactivation of latent other viruses during initial infection may be contributing to long COVID.
- Gastrointestinal postacute sequelae of COVID presents with a unique postacute expansion of cytotoxic T cells.
- SARS-CoV-2–specific and cytomegalovirus-specific CD8+ T cells displayed unique dynamics during recovery from infection.
According to the authors, as many as 69% of COVID-19 patients suffer from long COVID – a range of new, recurrent, or ongoing problems 4 or more weeks following initial SARS-CoV-2 infection. These may include memory loss, gastrointestinal distress, fatigue, anosmia, and shortness of breath.
Long COVID has been associated with acute disease severity, and is suspected to be related to autoimmune factors and unresolved viral fragments, according to the paper.
Research methods
The international study did a deep and detailed dive into multiple molecular markers of long COVID. It enrolled 209 COVID-19 patients with varying degrees of disease severity and matched them to 457 healthy controls. The researchers’ goal was to identify discrete and quantifiable long COVID factors and guide possible preemptive treatment.
Patients were assessed at three time points: at initial diagnosis, during the acute disease phase about a week later, and again 2 to 3 months post onset of symptoms after recovery from the acute phase of COVID. At the third assessment, some patients had lingering symptoms such as fatigue (52% ), cough (25%), and loss of taste or sense of smell (18%).
Blood draws were analyzed for autoantibodies and SARS-CoV-2–specific antibodies, global plasma proteomic and metabolomic profiles, and single-cell multiomic characterizations of peripheral blood mononuclear cells.
Each blood draw was paired with nasal-swab and plasma measurements of SARS-CoV-2 viral load and the data sets were integrated with electronic health records and self-reported patient symptoms to guide the interpretation of the molecular signatures of long COVID.
Author conclusions
The authors found an association between T2 hyperinflammation and long COVID–anticipating autoantibodies. This association further implies that hyperinflammation-controlling therapies in the acute stage of COVID may influence whether a patient experiences long COVID. “However, the detailed timing and context of these therapies matter, and, thus, future well-controlled studies will be needed to test these and other therapeutic implications,” Dr. Su and colleagues wrote.
Moreover, the negative correlations between anti–SARS-CoV-2 IgG and certain autoantibodies may suggest that patients with elevated autoantibody levels are more susceptible to breakthrough infections, the authors said.
“Many patients with high autoantibodies simultaneously have low protective antibodies that neutralize SARS-CoV-2, and that’s going to make them more susceptible to breakthrough infections,” Mr. Chen explained.*
“Detectability of most [long COVID-19 factors] at COVID diagnosis emphasizes the importance of early disease measurements for understanding emergent chronic conditions and suggests [long COVID] treatment strategies,” they wrote.
According to Mr. Chen, there are clear similarities in underlying immunobiology between patients with COVID autoantibodies and patients with systemic lupus erythematosus.
“These findings are also helping us frame our thinking around other chronic autoimmune conditions, such as postacute Lyme syndrome, for example,” said Dr. Heath.
The bottom line, said Mr. Chen, is that measuring early long COVID indicators may result in preventive treatments. “An example is the cortisol deficiency we see in certain long COVID patients. There are known treatments such as cortisol replacement therapy that should be explored for this group.”
Outside expert’s take on findings
Commenting on the study, Sherry Hsiang-Yi Chou, MD, who was not involved in the research, called the study a very important first step in understanding the path of this complex phenomenon and perhaps other conditions with long-term side effects.
“The researchers have done huge amount of innovative scientific work. They’ve shown the DNA signature of how our bodies respond to this disease,” said Dr. Chou, who is chief of the division of neurocritical care at Northwestern Medicine in Chicago.
“This type of research will help us scientifically understand and differentiate the various syndromes within long COVID. It will help identify who’s at risk for different aspects of this syndrome and lead to following them for longer periods in clinical trials,” she added.
The authors acknowledged that lengthier studies in larger cohorts were needed to see which patients will develop long-term chronic postacute sequelae of COVID.
This research was supported by the Wilke Family Foundation, the Parker Institute for Cancer Immunotherapy, Merck, and the Biomedical Advanced Research and Development Authority. Other support came from the National Institutes of Health, the Bill and Melinda Gates Foundation, Saint John’s Cancer Center, Fred Hutchinson Cancer Research Center, and the European Union’s Horizon 2020 research and innovation program. Dr. Heath is a cofounder of Pact Pharma. He and several coauthors disclosed various ties to multiple private-sector companies. Mr. Chen and Dr. Chou had no competing interests.
*Correction, 1/28: An earlier version of this story misidentified Daniel G. Chen, an undergraduate researcher at ISB.
FROM CELL
Children and COVID: United States passes 10 million total cases
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.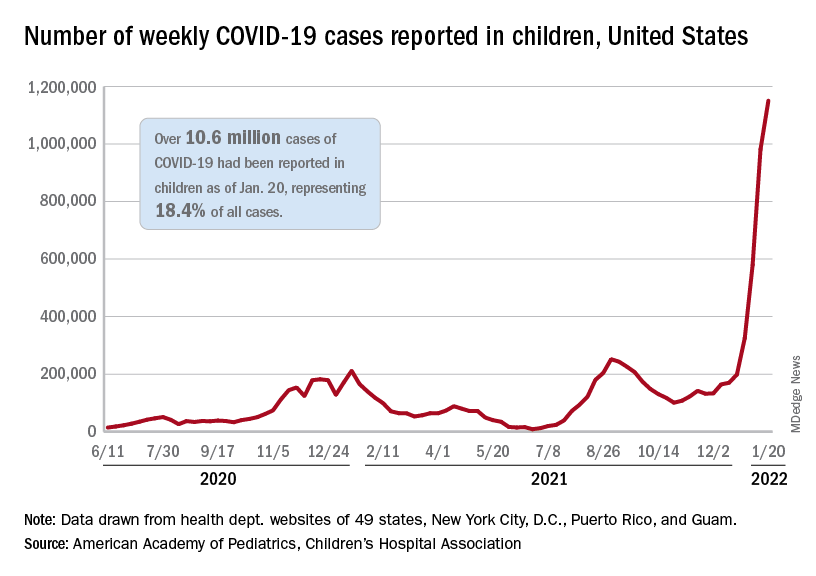
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.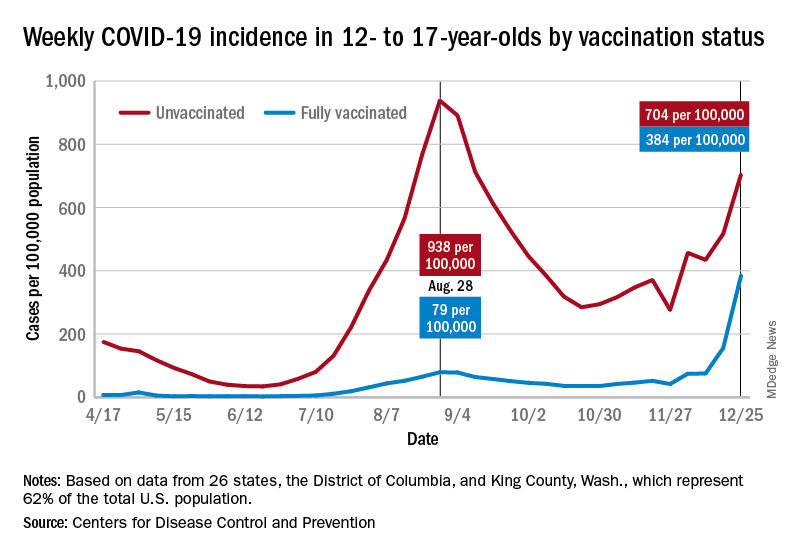
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
Weekly COVID-19 cases in children topped 1 million for the first time as the cumulative count surpassed 10 million since the start of the pandemic, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
the AAP and CHA said in their weekly COVID report. Those 10.6 million child cases represent 18.4% of all cases, and the latest 1.15 million represented 25.5% of all cases for the week.
Regionally, the South had the most cases with over 380,000 for the week of Jan. 14-20, while the West was next with close to 350,000, followed by the Midwest and then the East. Among the states, the largest percent increases – on the order of 30% – came in New England (Massachusetts, Rhode Island, and Vermont), as well as Virginia and California, the AAP and CHA said.
Examining all those cases by vaccination status shows an obvious difference between the Omicron and Delta variants: The fully vaccinated have been hit much harder than before. For the week ending Dec. 25, 2021, the incidence of COVID-19 in children aged 12-17 years was 704 per 100,000 among those were unvaccinated and 384 per 100,000 in those who were fully vaccinated. During the Delta surge in the summer of 2021, the peak rates were 938 (unvaccinated) and 79 (vaccinated), the Centers for Disease Control and Prevention said.
Hospitalizations are also at record levels, but two separate CDC databases seem to show a decline in child admissions over the last available week or so of data, which follows the trend among all ages. The peak among children aged 0-17 years came on Jan. 15, when the rate of new admissions reached 1.25 per 100,000, based on reporting to the CDC from 5,265 hospitals nationwide.
The second database, the COVID-19–Associated Hospitalization Surveillance Network (COVID-NET), indicates that children aged 0-4 years had the highest admission rate, 14.5 per 100,000, for the week ending Jan. 8, compared with 5.5 per 100,000 for 12- to 17-year-olds and 2.3 per 100,000 for those aged 5-11 years. COVID-NET covers almost 100 counties in 10 states, along with 4 entire states, and represents about 10% of the U.S. population.
Vaccinations rose briefly in late December and into January to meet the Omicron surge, but the numbers for the latest week show a return to their earlier levels. In children aged 5-11 years, new vaccinations went from 381,000 for the week of Dec. 20-26 to 524,000 for Jan. 3-9, but fell to just 260,000 during Jan. 17-23. The response was a little later for those aged 12-17, with the big week coming Jan. 10-16, but there was still a 38% drop for Jan. 17-23, according to the CDC’s COVID Data Tracker.
Currently, 29.3% of all 5- to 11-year-olds have received at least one dose of the COVID vaccine, and an even 20.0% are fully vaccinated. For children aged 12-17, the corresponding figures are 65.8% and 55.1%, the CDC said.
Statewide vaccination rates vary from Vermont’s high of 61% for those aged 5-11 to 12% for Alabama, Louisiana, and Mississippi, while Hawaii has the highest rate for 12- to 17-year-olds at 92% and Wyoming has the lowest at 39%, the AAP reported.
COVID brain fog is a ‘true neurologic condition’
early research suggests. Investigators found abnormalities in cerebrospinal fluid (CSF) and other risk factors, including diabetes and hypertension, present in individuals with mild COVID-19 experiencing persistent cognitive problems, often referred to as “brain fog.”
“We’re seeing changes to the [CSF] in the brain of most people who report cognitive changes,” said Joanna Hellmuth, MD, assistant professor of neurology, Memory and Aging Center, University of California, San Francisco. “We’re just in the beginning stages, but I hope this study will provide some legitimacy to this being a true neurologic condition.”
The study was published online Jan. 18, 2022, in Annals of Clinical and Translational Neurology.
No guidance
There is currently no guidance on how to identify patients with COVID-related cognitive changes, said Dr. Hellmuth. “The term ‘brain fog’ is not based in science or medicine, but that’s the most common term we use to describe this.”
The analysis included adults with confirmed SARS-CoV-2 infection not requiring hospitalization who were enrolled in the Long-term Impact of Infection with Novel Coronavirus study.
Participants underwent a structured interview that covered COVID-19 illness, past medical history, preexisting cognitive risk factors, medications, and cognitive symptoms following onset of COVID-19. They also completed an in-person battery of cognitive tests.
The analysis included 22 participants with at least one new cognitive symptom who had cognitive post-acute sequelae of SARS-CoV-2 infection (PASC). Ten cognitive controls reported no new cognitive symptoms after acute infection.
Participants were a median age of 41 years, had a median of 16 years of education, and were assessed a median of 10.1 months from their first COVID-19 symptom. There were no group differences in terms of age, gender, years of education, or distribution of race/ethnicity (all P > .05).
Among those with cognitive PASC, 43% reported cognitive symptoms starting 1 or more months after the first COVID symptom. About 29% reported cognitive changes started 2 or more months after their first COVID symptom.
“The immune system could be altered in some way after the infection, and perhaps that’s what’s contributing to these delayed onset cognitive changes,” said Dr. Hellmuth.
Compared with controls, participants with cognitive PASC had more preexisting cognitive risk factors (a median of 2.5 vs. 0; P = .03). These included hypertension and diabetes, which increase the risk of stroke, mild cognitive impairment, vascular dementia, traumatic brain injury, (TBI), learning disabilities, anxiety, depression, stimulant use, and ADHD, which may make the brain more vulnerable to executive functioning problems.
Dr. Hellmuth noted that the study wasn’t powered to determine whether any individual risk factor was associated with risk of cognitive changes.
As there are no published neuropsychological testing criteria for cognitive PASC, the researchers applied the equivalent criteria for HIV-associated neurocognitive disorder (HAND), a similar, virally associated cognitive disorder. Only 59% of those with cognitive PASC met equivalent HAND criteria for objective cognitive impairment versus 70% of cognitive controls. This, the investigators noted, highlights “the challenges and incongruities of using subjective, versus objective cognitive assessments for diagnosis.”
Is self-report enough?
While there is currently “nothing objective doctors can hang their hats on to say ‘you do’ or ‘you don’t’ have cognitive changes related to COVID,” using the HAND criteria is “not particularly helpful,” said Dr. Hellmuth. “Comparing an individual to a population-based norm in this case is really nuanced, and we shouldn’t rely on this solely to determine whether they do, or don’t, have cognitive changes.”
Perhaps self-reports in this case are “enough” said Dr. Hellmuth. “People know their brains better than anyone else, better than any doctor will.”
A total of 13 in the cognitive PASC group and 4 in the control group consented to a lumbar puncture. Cognitive PASC participants were older than controls (median of 47 vs. 28 years; P = .03) with no other between-group differences.
Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC, which Dr. Hellmuth said is typically a marker of inflammation.
Researchers also noted abnormal oligoclonal banding, a collection of antibodies, in the blood or brain fluid. These were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls (P = .03).
“When we find this pattern in both blood and brain, it suggests a systemic inflammatory disorder,” although “we have no idea what these antibodies are targeting,” said Dr. Hellmuth.
The study represents “the very beginning stages” of PASC becoming a medical diagnosis “where doctors know what to call it, how to treat it, and how to do blood and cerebrospinal fluid tests to diagnose it,” said Dr. Hellmuth.
She hopes PASC will receive medical legitimacy just as TBI has. In years past, a player was hit on the head or had their “bell rung,” simply returned to the field. “Now that we understand the science, we call it a mild TBI or concussion, and we have a very different medical approach to it.”
A limitation of the study was the small sample size, which may hinder the results’ validity. In addition, the study demographics may not reflect the broader population of those impacted by PASC.
‘A first substantial step’
Commenting on the research, William Schaffner, MD, professor, division of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said the new results represent “a first substantial step on the road to trying to find out what’s going on” with COVID patients dealing with cognitive issues.
Dr. Schaffner noted that elevated protein levels, identified in some study subjects, “is usually a consequence of previous inflammation” and is “a very interesting” finding. “In people who are otherwise normal, if you do a lumbar puncture, you don’t find elevated proteins.”
However, he noted the “diversity of results” from CSF examinations. “A single pattern does not leap out.”
What the researchers are observing “is not just a phenomenon of the mind or just something psychological,” said Dr. Schaffner. “Something physical is going on here.”
The study was funded by grants from the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. Dr. Hellmuth received grant support from the National Institutes of Health/NIMH supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner has disclosed not relevant financial relationships.
A version of this article first appeared on Medscape.com.
early research suggests. Investigators found abnormalities in cerebrospinal fluid (CSF) and other risk factors, including diabetes and hypertension, present in individuals with mild COVID-19 experiencing persistent cognitive problems, often referred to as “brain fog.”
“We’re seeing changes to the [CSF] in the brain of most people who report cognitive changes,” said Joanna Hellmuth, MD, assistant professor of neurology, Memory and Aging Center, University of California, San Francisco. “We’re just in the beginning stages, but I hope this study will provide some legitimacy to this being a true neurologic condition.”
The study was published online Jan. 18, 2022, in Annals of Clinical and Translational Neurology.
No guidance
There is currently no guidance on how to identify patients with COVID-related cognitive changes, said Dr. Hellmuth. “The term ‘brain fog’ is not based in science or medicine, but that’s the most common term we use to describe this.”
The analysis included adults with confirmed SARS-CoV-2 infection not requiring hospitalization who were enrolled in the Long-term Impact of Infection with Novel Coronavirus study.
Participants underwent a structured interview that covered COVID-19 illness, past medical history, preexisting cognitive risk factors, medications, and cognitive symptoms following onset of COVID-19. They also completed an in-person battery of cognitive tests.
The analysis included 22 participants with at least one new cognitive symptom who had cognitive post-acute sequelae of SARS-CoV-2 infection (PASC). Ten cognitive controls reported no new cognitive symptoms after acute infection.
Participants were a median age of 41 years, had a median of 16 years of education, and were assessed a median of 10.1 months from their first COVID-19 symptom. There were no group differences in terms of age, gender, years of education, or distribution of race/ethnicity (all P > .05).
Among those with cognitive PASC, 43% reported cognitive symptoms starting 1 or more months after the first COVID symptom. About 29% reported cognitive changes started 2 or more months after their first COVID symptom.
“The immune system could be altered in some way after the infection, and perhaps that’s what’s contributing to these delayed onset cognitive changes,” said Dr. Hellmuth.
Compared with controls, participants with cognitive PASC had more preexisting cognitive risk factors (a median of 2.5 vs. 0; P = .03). These included hypertension and diabetes, which increase the risk of stroke, mild cognitive impairment, vascular dementia, traumatic brain injury, (TBI), learning disabilities, anxiety, depression, stimulant use, and ADHD, which may make the brain more vulnerable to executive functioning problems.
Dr. Hellmuth noted that the study wasn’t powered to determine whether any individual risk factor was associated with risk of cognitive changes.
As there are no published neuropsychological testing criteria for cognitive PASC, the researchers applied the equivalent criteria for HIV-associated neurocognitive disorder (HAND), a similar, virally associated cognitive disorder. Only 59% of those with cognitive PASC met equivalent HAND criteria for objective cognitive impairment versus 70% of cognitive controls. This, the investigators noted, highlights “the challenges and incongruities of using subjective, versus objective cognitive assessments for diagnosis.”
Is self-report enough?
While there is currently “nothing objective doctors can hang their hats on to say ‘you do’ or ‘you don’t’ have cognitive changes related to COVID,” using the HAND criteria is “not particularly helpful,” said Dr. Hellmuth. “Comparing an individual to a population-based norm in this case is really nuanced, and we shouldn’t rely on this solely to determine whether they do, or don’t, have cognitive changes.”
Perhaps self-reports in this case are “enough” said Dr. Hellmuth. “People know their brains better than anyone else, better than any doctor will.”
A total of 13 in the cognitive PASC group and 4 in the control group consented to a lumbar puncture. Cognitive PASC participants were older than controls (median of 47 vs. 28 years; P = .03) with no other between-group differences.
Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC, which Dr. Hellmuth said is typically a marker of inflammation.
Researchers also noted abnormal oligoclonal banding, a collection of antibodies, in the blood or brain fluid. These were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls (P = .03).
“When we find this pattern in both blood and brain, it suggests a systemic inflammatory disorder,” although “we have no idea what these antibodies are targeting,” said Dr. Hellmuth.
The study represents “the very beginning stages” of PASC becoming a medical diagnosis “where doctors know what to call it, how to treat it, and how to do blood and cerebrospinal fluid tests to diagnose it,” said Dr. Hellmuth.
She hopes PASC will receive medical legitimacy just as TBI has. In years past, a player was hit on the head or had their “bell rung,” simply returned to the field. “Now that we understand the science, we call it a mild TBI or concussion, and we have a very different medical approach to it.”
A limitation of the study was the small sample size, which may hinder the results’ validity. In addition, the study demographics may not reflect the broader population of those impacted by PASC.
‘A first substantial step’
Commenting on the research, William Schaffner, MD, professor, division of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said the new results represent “a first substantial step on the road to trying to find out what’s going on” with COVID patients dealing with cognitive issues.
Dr. Schaffner noted that elevated protein levels, identified in some study subjects, “is usually a consequence of previous inflammation” and is “a very interesting” finding. “In people who are otherwise normal, if you do a lumbar puncture, you don’t find elevated proteins.”
However, he noted the “diversity of results” from CSF examinations. “A single pattern does not leap out.”
What the researchers are observing “is not just a phenomenon of the mind or just something psychological,” said Dr. Schaffner. “Something physical is going on here.”
The study was funded by grants from the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. Dr. Hellmuth received grant support from the National Institutes of Health/NIMH supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner has disclosed not relevant financial relationships.
A version of this article first appeared on Medscape.com.
early research suggests. Investigators found abnormalities in cerebrospinal fluid (CSF) and other risk factors, including diabetes and hypertension, present in individuals with mild COVID-19 experiencing persistent cognitive problems, often referred to as “brain fog.”
“We’re seeing changes to the [CSF] in the brain of most people who report cognitive changes,” said Joanna Hellmuth, MD, assistant professor of neurology, Memory and Aging Center, University of California, San Francisco. “We’re just in the beginning stages, but I hope this study will provide some legitimacy to this being a true neurologic condition.”
The study was published online Jan. 18, 2022, in Annals of Clinical and Translational Neurology.
No guidance
There is currently no guidance on how to identify patients with COVID-related cognitive changes, said Dr. Hellmuth. “The term ‘brain fog’ is not based in science or medicine, but that’s the most common term we use to describe this.”
The analysis included adults with confirmed SARS-CoV-2 infection not requiring hospitalization who were enrolled in the Long-term Impact of Infection with Novel Coronavirus study.
Participants underwent a structured interview that covered COVID-19 illness, past medical history, preexisting cognitive risk factors, medications, and cognitive symptoms following onset of COVID-19. They also completed an in-person battery of cognitive tests.
The analysis included 22 participants with at least one new cognitive symptom who had cognitive post-acute sequelae of SARS-CoV-2 infection (PASC). Ten cognitive controls reported no new cognitive symptoms after acute infection.
Participants were a median age of 41 years, had a median of 16 years of education, and were assessed a median of 10.1 months from their first COVID-19 symptom. There were no group differences in terms of age, gender, years of education, or distribution of race/ethnicity (all P > .05).
Among those with cognitive PASC, 43% reported cognitive symptoms starting 1 or more months after the first COVID symptom. About 29% reported cognitive changes started 2 or more months after their first COVID symptom.
“The immune system could be altered in some way after the infection, and perhaps that’s what’s contributing to these delayed onset cognitive changes,” said Dr. Hellmuth.
Compared with controls, participants with cognitive PASC had more preexisting cognitive risk factors (a median of 2.5 vs. 0; P = .03). These included hypertension and diabetes, which increase the risk of stroke, mild cognitive impairment, vascular dementia, traumatic brain injury, (TBI), learning disabilities, anxiety, depression, stimulant use, and ADHD, which may make the brain more vulnerable to executive functioning problems.
Dr. Hellmuth noted that the study wasn’t powered to determine whether any individual risk factor was associated with risk of cognitive changes.
As there are no published neuropsychological testing criteria for cognitive PASC, the researchers applied the equivalent criteria for HIV-associated neurocognitive disorder (HAND), a similar, virally associated cognitive disorder. Only 59% of those with cognitive PASC met equivalent HAND criteria for objective cognitive impairment versus 70% of cognitive controls. This, the investigators noted, highlights “the challenges and incongruities of using subjective, versus objective cognitive assessments for diagnosis.”
Is self-report enough?
While there is currently “nothing objective doctors can hang their hats on to say ‘you do’ or ‘you don’t’ have cognitive changes related to COVID,” using the HAND criteria is “not particularly helpful,” said Dr. Hellmuth. “Comparing an individual to a population-based norm in this case is really nuanced, and we shouldn’t rely on this solely to determine whether they do, or don’t, have cognitive changes.”
Perhaps self-reports in this case are “enough” said Dr. Hellmuth. “People know their brains better than anyone else, better than any doctor will.”
A total of 13 in the cognitive PASC group and 4 in the control group consented to a lumbar puncture. Cognitive PASC participants were older than controls (median of 47 vs. 28 years; P = .03) with no other between-group differences.
Overall, 77% of participants with cognitive PASC had a CSF abnormality, compared with 0% of cognitive controls (P = .01). CSF abnormalities included elevated protein levels with no other explainable cause in 2 of the 13 subjects with PASC, which Dr. Hellmuth said is typically a marker of inflammation.
Researchers also noted abnormal oligoclonal banding, a collection of antibodies, in the blood or brain fluid. These were identified in 69% of participants with cognitive PASC, compared with 0% of cognitive controls (P = .03).
“When we find this pattern in both blood and brain, it suggests a systemic inflammatory disorder,” although “we have no idea what these antibodies are targeting,” said Dr. Hellmuth.
The study represents “the very beginning stages” of PASC becoming a medical diagnosis “where doctors know what to call it, how to treat it, and how to do blood and cerebrospinal fluid tests to diagnose it,” said Dr. Hellmuth.
She hopes PASC will receive medical legitimacy just as TBI has. In years past, a player was hit on the head or had their “bell rung,” simply returned to the field. “Now that we understand the science, we call it a mild TBI or concussion, and we have a very different medical approach to it.”
A limitation of the study was the small sample size, which may hinder the results’ validity. In addition, the study demographics may not reflect the broader population of those impacted by PASC.
‘A first substantial step’
Commenting on the research, William Schaffner, MD, professor, division of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said the new results represent “a first substantial step on the road to trying to find out what’s going on” with COVID patients dealing with cognitive issues.
Dr. Schaffner noted that elevated protein levels, identified in some study subjects, “is usually a consequence of previous inflammation” and is “a very interesting” finding. “In people who are otherwise normal, if you do a lumbar puncture, you don’t find elevated proteins.”
However, he noted the “diversity of results” from CSF examinations. “A single pattern does not leap out.”
What the researchers are observing “is not just a phenomenon of the mind or just something psychological,” said Dr. Schaffner. “Something physical is going on here.”
The study was funded by grants from the National Institute of Mental Health and the National Institute of Neurological Disorders and Stroke. Dr. Hellmuth received grant support from the National Institutes of Health/NIMH supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner has disclosed not relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF CLINICAL AND TRANSLATIONAL NEUROLOGY
Watch, but don’t worry yet, about new Omicron subvariant
In the meantime, it’s worth watching BA.2, the World Health Organization said. The subvariant has been identified across at least 40 countries, including three cases reported in Houston and several in Washington state.
BA.2 accounts for only a small minority of reported cases so far, including 5% in India, 4% of those in the United Kingdom, and 2% each of cases in Sweden and Singapore.
The one exception is Denmark, a country with robust genetic sequencing abilities, where estimates range from 50% to 81% of cases.
The news throws a little more uncertainty into an already uncertain situation, including how close the world might be to a less life-altering infectious disease.
For example, the world is at an ideal point for a new variant to emerge, WHO Director General Tedros Adhanom Ghebreyesus, PhD, said during a Jan. 24 meeting of the WHO executive board. He also said it’s too early to call an “end game” to the pandemic.
Similarly, Anthony S. Fauci, MD, said on Jan. 19 that it remained “an open question” whether the Omicron variant could hasten endemic COVID-19, a situation where the virus still circulates but is much less disruptive to everyday life.
No Pi for you
This could be the first time a coronavirus subvariant rises to the level of a household name, or – if previous variants of the moment have shown us – it could recede from the spotlight.
For example, a lot of focus on the potential of the Mu variant to wreak havoc fizzled out a few weeks after the WHO listed it as a variant of interest on Aug. 30.
Subvariants can feature mutations and other small differences but are not distinct enough from an existing strain to be called a variant on their own and be named after the next letter in the Greek alphabet. That’s why BA.2 is not called the “Pi variant.”
Predicting what’s next for the coronavirus has puzzled many experts throughout the pandemic. That is why many public health officials wait for the WHO to officially designate a strain as a variant of interest or variant of concern before taking action.
At the moment with BA.2, it seems close monitoring is warranted.
Because it’s too early to call, expert predictions about BA.2 vary widely, from worry to cautious optimism.
For example, early data indicates that BA.2 could be more worrisome than original Omicron, Eric Feigl-Ding, ScD, an epidemiologist and health economist, said on Twitter.
Information from Denmark seems to show BA.2 either has “much faster transmission or it evades immunity even more,” he said.
The same day, Jan. 23, Dr. Feigl-Ding tweeted that other data shows the subvariant can spread twice as fast as Omicron, which was already much more contagious than previous versions of the virus.
At the same time, other experts appear less concerned. Robert Garry, PhD, a virologist at Tulane University, New Orleans, told the Washington Post that there is no reason to think BA.2 will be any worse than the original Omicron strain.
So which expert predictions will come closer to BA.2’s potential? For now, it’s just a watch-and-see situation.
For updated information, the website outbreak.info tracks BA.2’s average daily and cumulative prevalence in the United States and in other locations.
Also, if and when WHO experts decide to elevate BA.2 to a variant of interest or a variant of concern, it will be noted on its coronavirus variant tracking website.
A version of this article first appeared on WebMD.com.
In the meantime, it’s worth watching BA.2, the World Health Organization said. The subvariant has been identified across at least 40 countries, including three cases reported in Houston and several in Washington state.
BA.2 accounts for only a small minority of reported cases so far, including 5% in India, 4% of those in the United Kingdom, and 2% each of cases in Sweden and Singapore.
The one exception is Denmark, a country with robust genetic sequencing abilities, where estimates range from 50% to 81% of cases.
The news throws a little more uncertainty into an already uncertain situation, including how close the world might be to a less life-altering infectious disease.
For example, the world is at an ideal point for a new variant to emerge, WHO Director General Tedros Adhanom Ghebreyesus, PhD, said during a Jan. 24 meeting of the WHO executive board. He also said it’s too early to call an “end game” to the pandemic.
Similarly, Anthony S. Fauci, MD, said on Jan. 19 that it remained “an open question” whether the Omicron variant could hasten endemic COVID-19, a situation where the virus still circulates but is much less disruptive to everyday life.
No Pi for you
This could be the first time a coronavirus subvariant rises to the level of a household name, or – if previous variants of the moment have shown us – it could recede from the spotlight.
For example, a lot of focus on the potential of the Mu variant to wreak havoc fizzled out a few weeks after the WHO listed it as a variant of interest on Aug. 30.
Subvariants can feature mutations and other small differences but are not distinct enough from an existing strain to be called a variant on their own and be named after the next letter in the Greek alphabet. That’s why BA.2 is not called the “Pi variant.”
Predicting what’s next for the coronavirus has puzzled many experts throughout the pandemic. That is why many public health officials wait for the WHO to officially designate a strain as a variant of interest or variant of concern before taking action.
At the moment with BA.2, it seems close monitoring is warranted.
Because it’s too early to call, expert predictions about BA.2 vary widely, from worry to cautious optimism.
For example, early data indicates that BA.2 could be more worrisome than original Omicron, Eric Feigl-Ding, ScD, an epidemiologist and health economist, said on Twitter.
Information from Denmark seems to show BA.2 either has “much faster transmission or it evades immunity even more,” he said.
The same day, Jan. 23, Dr. Feigl-Ding tweeted that other data shows the subvariant can spread twice as fast as Omicron, which was already much more contagious than previous versions of the virus.
At the same time, other experts appear less concerned. Robert Garry, PhD, a virologist at Tulane University, New Orleans, told the Washington Post that there is no reason to think BA.2 will be any worse than the original Omicron strain.
So which expert predictions will come closer to BA.2’s potential? For now, it’s just a watch-and-see situation.
For updated information, the website outbreak.info tracks BA.2’s average daily and cumulative prevalence in the United States and in other locations.
Also, if and when WHO experts decide to elevate BA.2 to a variant of interest or a variant of concern, it will be noted on its coronavirus variant tracking website.
A version of this article first appeared on WebMD.com.
In the meantime, it’s worth watching BA.2, the World Health Organization said. The subvariant has been identified across at least 40 countries, including three cases reported in Houston and several in Washington state.
BA.2 accounts for only a small minority of reported cases so far, including 5% in India, 4% of those in the United Kingdom, and 2% each of cases in Sweden and Singapore.
The one exception is Denmark, a country with robust genetic sequencing abilities, where estimates range from 50% to 81% of cases.
The news throws a little more uncertainty into an already uncertain situation, including how close the world might be to a less life-altering infectious disease.
For example, the world is at an ideal point for a new variant to emerge, WHO Director General Tedros Adhanom Ghebreyesus, PhD, said during a Jan. 24 meeting of the WHO executive board. He also said it’s too early to call an “end game” to the pandemic.
Similarly, Anthony S. Fauci, MD, said on Jan. 19 that it remained “an open question” whether the Omicron variant could hasten endemic COVID-19, a situation where the virus still circulates but is much less disruptive to everyday life.
No Pi for you
This could be the first time a coronavirus subvariant rises to the level of a household name, or – if previous variants of the moment have shown us – it could recede from the spotlight.
For example, a lot of focus on the potential of the Mu variant to wreak havoc fizzled out a few weeks after the WHO listed it as a variant of interest on Aug. 30.
Subvariants can feature mutations and other small differences but are not distinct enough from an existing strain to be called a variant on their own and be named after the next letter in the Greek alphabet. That’s why BA.2 is not called the “Pi variant.”
Predicting what’s next for the coronavirus has puzzled many experts throughout the pandemic. That is why many public health officials wait for the WHO to officially designate a strain as a variant of interest or variant of concern before taking action.
At the moment with BA.2, it seems close monitoring is warranted.
Because it’s too early to call, expert predictions about BA.2 vary widely, from worry to cautious optimism.
For example, early data indicates that BA.2 could be more worrisome than original Omicron, Eric Feigl-Ding, ScD, an epidemiologist and health economist, said on Twitter.
Information from Denmark seems to show BA.2 either has “much faster transmission or it evades immunity even more,” he said.
The same day, Jan. 23, Dr. Feigl-Ding tweeted that other data shows the subvariant can spread twice as fast as Omicron, which was already much more contagious than previous versions of the virus.
At the same time, other experts appear less concerned. Robert Garry, PhD, a virologist at Tulane University, New Orleans, told the Washington Post that there is no reason to think BA.2 will be any worse than the original Omicron strain.
So which expert predictions will come closer to BA.2’s potential? For now, it’s just a watch-and-see situation.
For updated information, the website outbreak.info tracks BA.2’s average daily and cumulative prevalence in the United States and in other locations.
Also, if and when WHO experts decide to elevate BA.2 to a variant of interest or a variant of concern, it will be noted on its coronavirus variant tracking website.
A version of this article first appeared on WebMD.com.
Ways to make sure 2022 doesn’t stink for docs
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Depending on the data you’re looking at, 40%-60% of physicians are burned out.
Research studies and the eye test reveal the painfully obvious: Colleagues are tired, winded, spent, and at times way past burned out. People aren’t asking me if they’re burned out. They know they’re burned out; heck, they can even recite the Maslach burnout inventory, forward and backward, in a mask, or while completing a COVID quarantine. A fair share of people know the key steps to prevent burnout and promote recovery.
What I’m starting to see more of is, “Why should I even bother to recover from this? Why pick myself up again just to get another occupational stress injury (burnout, demoralization, moral injury, etc.)?” In other words, it’s not just simply about negating burnout; it’s about supporting and facilitating the motivation to work.
We’ve been through so much with COVID that it might be challenging to remember when you saw a truly engaged work environment. No doubt, we have outstanding professionals across medicine who answer the bell every day. However, if you’ve been looking closely, many teams/units have lost a bit of the zip and pep. The synergy and trust aren’t as smooth, and at noon, everyone counts the hours to the end of the shift.
You may be thinking, Well, of course, they are; we’re still amid a pandemic, and people have been through hell. Your observation would be correct, except I’ve personally seen some teams weather the pandemic storm and still remain engaged (some even more involved).
The No. 1 consult result for the GW Resiliency and Well-Being Center, where I work, has been on lectures for burnout. The R&WC has given so many of these lectures that my dreams take the form of a PowerPoint presentation. Overall the talks have gone very well. We’ve added skills sections on practices of whole-person care. We’ve blitzed the daylights out of restorative sleep, yet I know we are still searching for the correct narrative.
Motivated staff, faculty, and students will genuinely take in the information and follow the recommendations; however, they still struggle to find that drive and zest for work. Yes, moving from burnout to neutral is reasonable but likely won’t move the needle of your professional or personal life. We need to have the emotional energy and the clear desire to utilize that energy for a meaningful purpose.
Talking about burnout in specific ways is straightforward and, in my opinion, much easier than talking about engagement. Part of the challenge when trying to discuss engagement is that people can feel invalidated or that you’re telling them to be stoic. Or worse yet, that the problem of burnout primarily lies with them. It’s essential to recognize the role of an organizational factor in burnout (approximately 80%, depending on the study); still, even if you address burnout, people may not be miserable, but it doesn’t mean they will stay at their current job (please cue intro music for the Great Resignation).
Engagement models have existed for some time and certainly have gained much more attention in health care settings over the past 2 decades. Engagement can be described as having three components: dedication, vigor, and absorption. When a person is filling all three of these components over time, presto – you get the much-sought-after state of the supremely engaged professional.
These models definitely give us excellent starting points to approach engagement from a pre-COVID era. In COVID and beyond, I’m not sure how these models will stand up in a hybrid work environment, where autonomy and flexibility could be more valued than ever. Personally, COVID revealed some things I was missing in my work pre-COVID:
- Time to think and process. This was one of the great things about being a consultation-liaison psychiatrist; it was literally feast or famine.
- Doing what I’m talented at and really enjoy.
- Time is short, and I want to be more present in the life of my family.
The list above isn’t exhaustive, but I’ve found them to be my own personal recipe for being engaged. Over the next series of articles, I’m going to focus on engagement and factors related to key resilience. These articles will be informed by a front-line view from my colleagues, and hopefully start to separate the myth from reality on the subject of health professional engagement and resilience.
Everyone be safe and well!
A version of this article first appeared on Medscape.com.
Does COVID-19 induce type 1 diabetes in kids? Jury still out
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
Two new studies from different parts of the world have identified an increase in the incidence of type 1 diabetes in children since the COVID-19 pandemic began, but the reasons still aren’t clear.
The findings from the two studies, in Germany and the United States, align closely, endocrinologist Jane J. Kim, MD, professor of pediatrics and principal investigator of the U.S. study, told this news organization. “I think that the general conclusion based on their data and our data is that there appears to be an increased rate of new type 1 diabetes diagnoses in children since the onset of the pandemic.”
Dr. Kim noted that because her group’s data pertain to just a single center, she is “heartened to see that the [German team’s] general conclusions are the same as ours.” Moreover, she pointed out that other studies examining this question came from Europe early in the pandemic, whereas “now both they [the German group] and we have had the opportunity to look at what’s happening over a longer period of time.”
But the reason for the association remains unclear. Some answers may be forthcoming from a database designed in mid-2020 specifically to examine the relationship between COVID-19 and new-onset diabetes. Called CoviDiab, the registry aims “to establish the extent and characteristics of new-onset, COVID-19–related diabetes and to investigate its pathogenesis, management, and outcomes,” according to the website.
The first new study, a multicenter German diabetes registry study, was published online Jan. 17 in Diabetes Care by Clemens Kamrath, MD, of Justus Liebig University, Giessen, Germany, and colleagues.
The other, from Rady Children’s Hospital of San Diego, was published online Jan. 24 in JAMA Pediatrics by Bethany L. Gottesman, MD, and colleagues, all with the University of California, San Diego.
Mechanisms likely to differ for type 1 versus type 2 diabetes
Neither the German nor the U.S. investigators were able to directly correlate current or prior SARS-CoV-2 infection in children with the subsequent development of type 1 diabetes.
Earlier this month, a study from the U.S. Centers for Disease Control and Prevention did examine that issue, but it also included youth with type 2 diabetes and did not separate out the two groups.
Dr. Kim said her institution has also seen an increase in type 2 diabetes among youth since the COVID-19 pandemic began but did not include that in their current article.
“When we started looking at our data, diabetes and COVID-19 in adults had been relatively well established. To see an increase in type 2 [diabetes] was not so surprising to our group. But we had the sense we were seeing more patients with type 1, and when we looked at our hospital that was very much the case. I think that was a surprise to people,” said Dr. Kim.
Although a direct effect of SARS-CoV-2 on pancreatic beta cells has been proposed, in both the German and San Diego datasets the diagnosis of type 1 diabetes was confirmed with autoantibodies that are typically present years prior to the onset of clinical symptoms.
The German group suggests possible other explanations for the link, including the lack of immune system exposure to other common pediatric infections during pandemic-necessitated social distancing – the so-called hygiene hypothesis – as well as the possible role of psychological stress, which several studies have linked to type 1 diabetes.
But as of now, Dr. Kim said, “Nobody really knows.”
Is the effect direct or indirect?
Using data from the multicenter German Diabetes Prospective Follow-up Registry, Dr. Kamrath and colleagues compared the incidence of type 1 diabetes in children and adolescents from Jan. 1, 2020 through June 30, 2021 with the incidence in 2011-2019.
During the pandemic period, a total of 5,162 youth were newly diagnosed with type 1 diabetes at 236 German centers. That incidence, 24.4 per 100,000 patient-years, was significantly higher than the 21.2 per 100,000 patient-years expected based on the prior decade, with an incidence rate ratio of 1.15 (P < .001). The increase was similar in both males and females.
There was a difference by age, however, as the phenomenon appeared to be limited to the preadolescent age groups. The incidence rate ratios (IRRs) for ages below 6 years and 6-11 years were 1.23 and 1.18 (both P < .001), respectively, compared to a nonsignificant IRR of 1.06 (P = .13) in those aged 12-17 years.
Compared with the expected monthly incidence, the observed incidence was significantly higher in June 2020 (IRR, 1.43; P = .003), July 2020 (IRR, 1.48; P < 0.001), March 2021 (IRR, 1.29; P = .028), and June 2021 (IRR, 1.39; P = .01).
Among the 3,851 patients for whom data on type 1 diabetes-associated autoantibodies were available, the adjusted rates of autoantibody negativity did not differ from 2018-2019 during the entire pandemic period or during the year 2020 or the first half of 2021.
“Therefore, the increase in the incidence of type 1 diabetes in children appears to be due to immune-mediated type 1 diabetes. However, because autoimmunity and progressive beta-cell destruction typically begin long before the clinical diagnosis of type 1 diabetes, we were surprised to see the incidence of type 1 diabetes followed the peak incidence of COVID-19 and also the pandemic containment measures by only approximately 3 months,” Dr. Kamrath and colleagues write.
Taken together, they say, the data suggest that “the impact on type 1 diabetes incidence is not due to infection with SARS-CoV-2 but rather a consequence of environmental changes resulting from the pandemic itself or pandemic containment measures.”
Similar findings at a U.S. children’s hospital
In the cross-sectional study in San Diego, Dr. Gottesman and colleagues looked at the electronic medical records (EMRs) at Rady Children’s Hospital for patients aged younger than 19 years with at least one positive type 1 diabetes antibody titer.
During March 19, 2020 to March 18, 2021, a total of 187 children were admitted for new-onset type 1 diabetes, compared with just 119 the previous year, a 57% increase.
From July 2020 through February 2021, the number of new type 1 diabetes diagnoses significantly exceeded the number expected based on a quarterly moving average of each of the preceding 5 years.
Only four of the 187 patients (2.1%) diagnosed during the pandemic period had a COVID-19 infection at the time of presentation. Antibody testing to assess prior infection wasn’t feasible, and now that children are receiving the vaccine – and therefore most will have antibodies – “we’ve lost our window of opportunity to look at that question,” Dr. Kim noted.
As has been previously shown, there was an increase in the percentage of patients presenting with diabetic ketoacidosis during the pandemic compared with the prior 5 years (49.7% vs. 40.7% requiring insulin infusion). However, there was no difference in mean age at presentation, body mass index, A1c, or percentage requiring admission to intensive care.
Because these data only go through March 2021, Dr. Kim noted, “We need to see what’s happening with these different variants. We’ll have a chance to look in a month or two to see the effects of Omicron on the rates of diabetes in the hospital.”
Will CoviDiab answer the question?
Data from CoviDiab will include diabetes type in adults and children, registry coprincipal investigator Francesco Rubino, MD, of King’s College London, told this news organization.
“We aimed at having as many as possible cases of new-onset diabetes for which we can have also a minimum set of clinical data including type of diabetes and A1c. By looking at this information we can infer whether a role of COVID-19 in triggering diabetes is clinically plausible – or not – and what type of diabetes is most frequently associated with COVID-19 as this also speaks about mechanisms of action.”
Dr. Rubino said that the CoviDiab team is approaching the data with the assumption that, at least in adults diagnosed with type 2 diabetes, the explanation might be that the person already had undiagnosed diabetes or that the hyperglycemia may be stress-induced and temporary.
“We’re looking at this question with a skeptical eye ... Is it just an association, or does the virus have a role in inducing diabetes from scratch, or can the virus advance pathophysiology in a way that it ends up in full-blown diabetes in predisposed individuals?”
While no single study will prove that SARS-CoV-2 causes diabetes, “combining observations from various studies and approaches we may get a higher degree of certainty,” Dr. Rubino said, noting that the CoviDiab team plans to publish data from the first 800 cases “soon.”
Dr. Kim has reported no relevant financial relationships. Dr. Rubino has reported receiving grants from Ethicon and Medtronic, personal fees from GI Dynamic, Keyron, Novo Nordisk, Ethicon, and Medtronic.
A version of this article first appeared on Medscape.com.
Gut bacteria linked with long COVID
While links have been found between the gut’s microbiome and COVID-19, as well as other diseases, this is the first published research to show a link specifically to COVID’s long-term effects, the investigators, based at the Chinese University of Hong Kong, wrote in Gut.
“To our knowledge, this is the first study to show that altered gut microbiome composition is strongly associated with persistent symptoms in patients with COVID-19 up to 6 months after clearance of SARS-CoV-2 virus,” said Siew Ng, MBBS, PhD, associate director at the university’s Center for Gut Microbiota Research.
At three hospitals, the researchers enrolled 106 patients with COVID-19 from February to August 2020 with stool samples at admission and at 1 month and 6 months after discharge, and compared them with people who did not have COVID, recruited in 2019. The severity of COVID in the enrolled patients was mostly mild to moderate.
At 3 months, 86 of the patients with COVID had post–acute COVID-19 syndrome (PACS) – defined as at least one persistent, otherwise unexplained symptom 4 weeks after clearance of the virus. And 81 patients had PACS at 6 months, most commonly fatigue, poor memory, hair loss, anxiety, and trouble sleeping.
Using stool samples for their analysis, the researchers found that, broadly, the diversity of the types of bacteria, and the abundance of these bacteria, were significantly lower at 6 months for those with PACS, compared with those without PACS and with controls (P < .05 and P < .0001, respectively). Among those with PACS, 28 bacteria species were diminished and 14 were enriched, both at baseline and follow-up. Those patients who had COVID but not PACS showed just 25 alterations of bacteria species at the time of hospital admission, and they all normalized by 6 months.
Having respiratory symptoms at 6 months was linked with higher levels of opportunistic pathogens such as Streptococcus anginosus and S. vestibularis. Neuropsychiatric symptoms and fatigue were associated with nosocomial pathogens that are linked to opportunistic infections, such as Clostridium innocuum and Actinomyces naeslundii (P < .05).
Bacteria known for producing butyrate, a beneficial fatty acid, were significantly depleted in those patients with hair loss. And certain of these bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, had the largest inverse correlations with PACS at 6 months (P < .05), the researchers found.
“Particular gut microbial profiles may indicate heightened susceptibility,” Dr. Ng said.
Although the findings were drawn from patients with earlier strains of the COVID-19 virus, the findings still apply to new variants, including Omicron, since these pose the same problem of persistent disruption of the immune system, Dr. Ng said.
Her group is conducting trials to look at how modulating the microbiome might prevent long COVID and boost antibodies after vaccination in high-risk people, she said.
“Gut microbiota influences the health of the host,” Dr. Ng said. “It provides crucial benefits in the form of immune system development, prevention of infections, nutrient acquisition, and brain and nervous system functionality. Considering the millions of people infected during the ongoing pandemic, our findings are a strong impetus for consideration of microbiota modulation to facilitate timely recovery and reduce the burden of post–acute COVID-19 syndrome.”
John Haran, MD, PhD, associate professor of microbiology and physiological systems and emergency medicine at the University of Massachusetts, Worcester, said the research adds to the evidence base on the gut microbiome’s links to COVID, but there was likely be no clinical impact yet. Still, he said the findings linking specific species to specific symptoms was particularly interesting.
“Very early on during hospitalization, [the researchers] saw these differences and correlated out with people who have longer symptoms, and especially different groups of people that have longer symptoms, too,” said Dr. Haran, who has done research on the topic. “It’s very different if you have different symptoms, for example, you keep coughing for months versus you have brain fog and fatigue, or other debilitating symptoms.”
Dr. Haran noted that the findings didn’t identify bacteria types especially linked to COVID, but rather species that have already been found to be associated with a “bad” microbiome. He also pointed out that the patients enrolled in the study were not vaccinated, because vaccines weren’t available at the time. Still, further study to see whether modulation of gut bacteria can be a therapy seems worthwhile.
“Microbiome modulation is pretty safe, and that’s really the next big step that needs to be taken in this,” he said.
For now, the findings don’t give the clinician much new ammunition for treatment.
“We’re not there yet,” he added. “It’s not as if clinicians are going to tell their COVID patients: ‘Go out and buy some kale.’ ”
Eugene Chang, MD, professor of medicine at the University of Chicago, who has studied the gut microbiome and gastrointestinal disease, said it’s “too preliminary” to say whether the findings could lead to a clinical impact. The measures used merely identify the microbes present, but not what they are doing.
“These measures are unlikely to perform well enough to be useful for risk assessment or predicting clinical outcomes,” he said. “That being said, advances in technology are being made where next generations of metrics could be developed and useful as stratifiers and predictors of risk.”
Seeing shifting patterns associated with certain symptoms, he said, is “notable because it suggests that the disturbances of the gut microbiota in PACS are significant.”
But he said it’s important to know whether these changes are a cause of PACS in some way or just an effect of it.
“If causative or contributory – this has to be proven – then ‘microbiota modulation’ would make sense and could be a priority for development,” he said. “If merely an effect, these metrics and better ones to come could be useful as predictors or measures of the patient’s general state of health.”
As seen in his group’s work and other work, he said, “the gut microbiota is highly sensitive to changes in their ecosystem, which is influenced by the health state of the patient.”
Dr. Ng, Dr. Haran, and Dr. Chang reported no relevant disclosures.
This article was updated Jan. 27, 2022.
While links have been found between the gut’s microbiome and COVID-19, as well as other diseases, this is the first published research to show a link specifically to COVID’s long-term effects, the investigators, based at the Chinese University of Hong Kong, wrote in Gut.
“To our knowledge, this is the first study to show that altered gut microbiome composition is strongly associated with persistent symptoms in patients with COVID-19 up to 6 months after clearance of SARS-CoV-2 virus,” said Siew Ng, MBBS, PhD, associate director at the university’s Center for Gut Microbiota Research.
At three hospitals, the researchers enrolled 106 patients with COVID-19 from February to August 2020 with stool samples at admission and at 1 month and 6 months after discharge, and compared them with people who did not have COVID, recruited in 2019. The severity of COVID in the enrolled patients was mostly mild to moderate.
At 3 months, 86 of the patients with COVID had post–acute COVID-19 syndrome (PACS) – defined as at least one persistent, otherwise unexplained symptom 4 weeks after clearance of the virus. And 81 patients had PACS at 6 months, most commonly fatigue, poor memory, hair loss, anxiety, and trouble sleeping.
Using stool samples for their analysis, the researchers found that, broadly, the diversity of the types of bacteria, and the abundance of these bacteria, were significantly lower at 6 months for those with PACS, compared with those without PACS and with controls (P < .05 and P < .0001, respectively). Among those with PACS, 28 bacteria species were diminished and 14 were enriched, both at baseline and follow-up. Those patients who had COVID but not PACS showed just 25 alterations of bacteria species at the time of hospital admission, and they all normalized by 6 months.
Having respiratory symptoms at 6 months was linked with higher levels of opportunistic pathogens such as Streptococcus anginosus and S. vestibularis. Neuropsychiatric symptoms and fatigue were associated with nosocomial pathogens that are linked to opportunistic infections, such as Clostridium innocuum and Actinomyces naeslundii (P < .05).
Bacteria known for producing butyrate, a beneficial fatty acid, were significantly depleted in those patients with hair loss. And certain of these bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, had the largest inverse correlations with PACS at 6 months (P < .05), the researchers found.
“Particular gut microbial profiles may indicate heightened susceptibility,” Dr. Ng said.
Although the findings were drawn from patients with earlier strains of the COVID-19 virus, the findings still apply to new variants, including Omicron, since these pose the same problem of persistent disruption of the immune system, Dr. Ng said.
Her group is conducting trials to look at how modulating the microbiome might prevent long COVID and boost antibodies after vaccination in high-risk people, she said.
“Gut microbiota influences the health of the host,” Dr. Ng said. “It provides crucial benefits in the form of immune system development, prevention of infections, nutrient acquisition, and brain and nervous system functionality. Considering the millions of people infected during the ongoing pandemic, our findings are a strong impetus for consideration of microbiota modulation to facilitate timely recovery and reduce the burden of post–acute COVID-19 syndrome.”
John Haran, MD, PhD, associate professor of microbiology and physiological systems and emergency medicine at the University of Massachusetts, Worcester, said the research adds to the evidence base on the gut microbiome’s links to COVID, but there was likely be no clinical impact yet. Still, he said the findings linking specific species to specific symptoms was particularly interesting.
“Very early on during hospitalization, [the researchers] saw these differences and correlated out with people who have longer symptoms, and especially different groups of people that have longer symptoms, too,” said Dr. Haran, who has done research on the topic. “It’s very different if you have different symptoms, for example, you keep coughing for months versus you have brain fog and fatigue, or other debilitating symptoms.”
Dr. Haran noted that the findings didn’t identify bacteria types especially linked to COVID, but rather species that have already been found to be associated with a “bad” microbiome. He also pointed out that the patients enrolled in the study were not vaccinated, because vaccines weren’t available at the time. Still, further study to see whether modulation of gut bacteria can be a therapy seems worthwhile.
“Microbiome modulation is pretty safe, and that’s really the next big step that needs to be taken in this,” he said.
For now, the findings don’t give the clinician much new ammunition for treatment.
“We’re not there yet,” he added. “It’s not as if clinicians are going to tell their COVID patients: ‘Go out and buy some kale.’ ”
Eugene Chang, MD, professor of medicine at the University of Chicago, who has studied the gut microbiome and gastrointestinal disease, said it’s “too preliminary” to say whether the findings could lead to a clinical impact. The measures used merely identify the microbes present, but not what they are doing.
“These measures are unlikely to perform well enough to be useful for risk assessment or predicting clinical outcomes,” he said. “That being said, advances in technology are being made where next generations of metrics could be developed and useful as stratifiers and predictors of risk.”
Seeing shifting patterns associated with certain symptoms, he said, is “notable because it suggests that the disturbances of the gut microbiota in PACS are significant.”
But he said it’s important to know whether these changes are a cause of PACS in some way or just an effect of it.
“If causative or contributory – this has to be proven – then ‘microbiota modulation’ would make sense and could be a priority for development,” he said. “If merely an effect, these metrics and better ones to come could be useful as predictors or measures of the patient’s general state of health.”
As seen in his group’s work and other work, he said, “the gut microbiota is highly sensitive to changes in their ecosystem, which is influenced by the health state of the patient.”
Dr. Ng, Dr. Haran, and Dr. Chang reported no relevant disclosures.
This article was updated Jan. 27, 2022.
While links have been found between the gut’s microbiome and COVID-19, as well as other diseases, this is the first published research to show a link specifically to COVID’s long-term effects, the investigators, based at the Chinese University of Hong Kong, wrote in Gut.
“To our knowledge, this is the first study to show that altered gut microbiome composition is strongly associated with persistent symptoms in patients with COVID-19 up to 6 months after clearance of SARS-CoV-2 virus,” said Siew Ng, MBBS, PhD, associate director at the university’s Center for Gut Microbiota Research.
At three hospitals, the researchers enrolled 106 patients with COVID-19 from February to August 2020 with stool samples at admission and at 1 month and 6 months after discharge, and compared them with people who did not have COVID, recruited in 2019. The severity of COVID in the enrolled patients was mostly mild to moderate.
At 3 months, 86 of the patients with COVID had post–acute COVID-19 syndrome (PACS) – defined as at least one persistent, otherwise unexplained symptom 4 weeks after clearance of the virus. And 81 patients had PACS at 6 months, most commonly fatigue, poor memory, hair loss, anxiety, and trouble sleeping.
Using stool samples for their analysis, the researchers found that, broadly, the diversity of the types of bacteria, and the abundance of these bacteria, were significantly lower at 6 months for those with PACS, compared with those without PACS and with controls (P < .05 and P < .0001, respectively). Among those with PACS, 28 bacteria species were diminished and 14 were enriched, both at baseline and follow-up. Those patients who had COVID but not PACS showed just 25 alterations of bacteria species at the time of hospital admission, and they all normalized by 6 months.
Having respiratory symptoms at 6 months was linked with higher levels of opportunistic pathogens such as Streptococcus anginosus and S. vestibularis. Neuropsychiatric symptoms and fatigue were associated with nosocomial pathogens that are linked to opportunistic infections, such as Clostridium innocuum and Actinomyces naeslundii (P < .05).
Bacteria known for producing butyrate, a beneficial fatty acid, were significantly depleted in those patients with hair loss. And certain of these bacteria, including Bifidobacterium pseudocatenulatum and Faecalibacterium prausnitzii, had the largest inverse correlations with PACS at 6 months (P < .05), the researchers found.
“Particular gut microbial profiles may indicate heightened susceptibility,” Dr. Ng said.
Although the findings were drawn from patients with earlier strains of the COVID-19 virus, the findings still apply to new variants, including Omicron, since these pose the same problem of persistent disruption of the immune system, Dr. Ng said.
Her group is conducting trials to look at how modulating the microbiome might prevent long COVID and boost antibodies after vaccination in high-risk people, she said.
“Gut microbiota influences the health of the host,” Dr. Ng said. “It provides crucial benefits in the form of immune system development, prevention of infections, nutrient acquisition, and brain and nervous system functionality. Considering the millions of people infected during the ongoing pandemic, our findings are a strong impetus for consideration of microbiota modulation to facilitate timely recovery and reduce the burden of post–acute COVID-19 syndrome.”
John Haran, MD, PhD, associate professor of microbiology and physiological systems and emergency medicine at the University of Massachusetts, Worcester, said the research adds to the evidence base on the gut microbiome’s links to COVID, but there was likely be no clinical impact yet. Still, he said the findings linking specific species to specific symptoms was particularly interesting.
“Very early on during hospitalization, [the researchers] saw these differences and correlated out with people who have longer symptoms, and especially different groups of people that have longer symptoms, too,” said Dr. Haran, who has done research on the topic. “It’s very different if you have different symptoms, for example, you keep coughing for months versus you have brain fog and fatigue, or other debilitating symptoms.”
Dr. Haran noted that the findings didn’t identify bacteria types especially linked to COVID, but rather species that have already been found to be associated with a “bad” microbiome. He also pointed out that the patients enrolled in the study were not vaccinated, because vaccines weren’t available at the time. Still, further study to see whether modulation of gut bacteria can be a therapy seems worthwhile.
“Microbiome modulation is pretty safe, and that’s really the next big step that needs to be taken in this,” he said.
For now, the findings don’t give the clinician much new ammunition for treatment.
“We’re not there yet,” he added. “It’s not as if clinicians are going to tell their COVID patients: ‘Go out and buy some kale.’ ”
Eugene Chang, MD, professor of medicine at the University of Chicago, who has studied the gut microbiome and gastrointestinal disease, said it’s “too preliminary” to say whether the findings could lead to a clinical impact. The measures used merely identify the microbes present, but not what they are doing.
“These measures are unlikely to perform well enough to be useful for risk assessment or predicting clinical outcomes,” he said. “That being said, advances in technology are being made where next generations of metrics could be developed and useful as stratifiers and predictors of risk.”
Seeing shifting patterns associated with certain symptoms, he said, is “notable because it suggests that the disturbances of the gut microbiota in PACS are significant.”
But he said it’s important to know whether these changes are a cause of PACS in some way or just an effect of it.
“If causative or contributory – this has to be proven – then ‘microbiota modulation’ would make sense and could be a priority for development,” he said. “If merely an effect, these metrics and better ones to come could be useful as predictors or measures of the patient’s general state of health.”
As seen in his group’s work and other work, he said, “the gut microbiota is highly sensitive to changes in their ecosystem, which is influenced by the health state of the patient.”
Dr. Ng, Dr. Haran, and Dr. Chang reported no relevant disclosures.
This article was updated Jan. 27, 2022.
FROM GUT