User login
Children and COVID: Weekly cases keep rising past 100,000
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.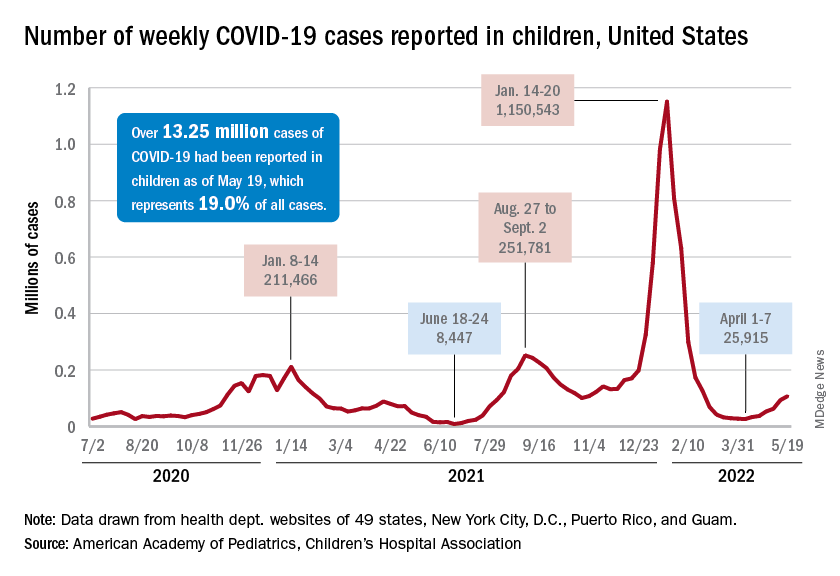
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”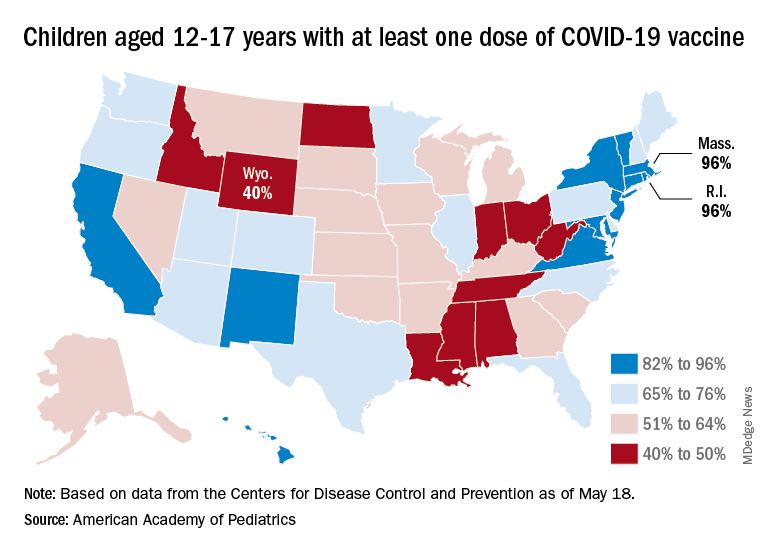
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
, according to the American Academy of Pediatrics and the Children’s Hospital Association.
New cases were up by 14.6% over the previous week to just over 107,000 reported during May 13-16, marking the sixth straight increase since April 1-7, when the count was almost 26,000. Over that period, weekly cases rose 313%, based on data in the latest weekly COVID report from the AAP and CHA.
Rates reported by the Centers for Disease Control and Prevention show the same trend. Weekly cases per 100,000 population, which were down to 34.9 in children aged 0-4 years and 43.1 for those aged 5-11 on March 26, were up to 49.5 and 52.2, respectively, by April 16. The pace picked up right after that, and as of May 14, the rates of new cases were 125.4 per 100,000 in children aged 0-4 years and 143.1 in those aged 5-11, the CDC said.
Hospital admissions continue to rise as well. The rate of new admissions in children aged 0-17 was up to 0.25 per 100,000 population on May 18, nearly double the 0.13 per 100,000 recorded as late as April 13. The latest 7-day average count for new admissions, 163 per day from May 15-21, is down from the previous week’s 175 per day, but the CDC also acknowledges potential reporting delays in the most recent 7-day period.
Both of those weekly averages, however, are far below the peak rate for the pandemic, 914 per day, which occurred Jan. 10-16, 2022, during the Omicron surge. Since the CDC began keeping count at the beginning of August 2020, more than 125,000 children aged 0-17 years have been admitted with confirmed COVID-19, which is about 2.7% of all admissions over that period, the CDC’s data show.
Booster gets the green light
The week brought some positive news on the prevention side, though, as the CDC officially approved a COVID vaccine booster dose for children aged 5-11 years.
Even that good news came with a caveat, however. The vote by the CDC’s Advisory Committee on Immunization Practices was 11:1 in favor, with the negative vote cast by Helen Keipp Talbot, MD, of Vanderbilt University, Nashville, Tenn., who said that “boosters are great once we’ve gotten everyone their first round. That needs to be our priority in this.”
Nationally, in fact, just 35.7% of children aged 5-11 years have received at least one dose of the vaccine and only 29.0% are fully vaccinated. Those figures are nearly doubled among 12- to 17-year-olds: 69.3% have received at least one dose and 59.4% are fully vaccinated, the CDC said on its COVID Data Tracker.
Some states, meanwhile, are well below those national rates. In Wyoming, only 40% of children aged 12-17 have received an initial vaccine dose, and eight other states are below 50%. Among children aged 5-12, there are still five states below 20% in that measure, while the states on the other end of the spectrum – Vermont and Massachusetts – are above 60%, the AAP said in its separate vaccination report.
FDA, AMA prepare for potential COVID-19 shots for children younger than 6
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
Regulators and the nation’s largest physician organization took separate steps in recent days to prepare for expected authorization of use of COVID-19 vaccines in children younger than age 6.
The Food and Drug Administration on May 23 announced its Vaccines and Related Biological Products Advisory Committee will meet June 15 to discuss expanding the use of COVID vaccines from Pfizer and Moderna.
The panel will examine a request from Pfizer and its partner BioNTech for an emergency use authorization (EUA) of its vaccine to cover children ages 6 months through 4 years. The EUA expansion for the Moderna shot would cover children ages 6 months through 5 years, the FDA said.
Many parents and physicians have been urging regulators to clear COVID shots for young children, among whom rates of infection are high.
The American Medical Association in February announced an update of its Current Procedural Terminology (CPT) to prepare for an eventual FDA clearance of the Pfizer-BioNTech shot for children aged 6 months to younger than 5 years. On May 19, the association announced a new CPT update to prepare for FDA clearance for use of the Moderna COVID-19 vaccine for children 6 months through 5 years.
“Extending COVID-19 vaccination protection to approximately 18 million young children will significantly reduce their risk of COVID-19 infection, hospitalization, and death, and give their parents incredible peace of mind,” Gerald Harmon, MD, AMA’s president, said in a statement. “We strongly urge all parents to get their infants and toddlers vaccinated as soon as they are eligible for a COVID-19 vaccine.”
Both the Moderna and the Pfizer-BioNTech COVID vaccines would be given to these young children in low doses.
On May 23, Pfizer announced results from a phase 2/3 trial evaluating a series of three shots of its vaccine in children ages 6 months to younger than 5 years.
Vaccine efficacy, which was a secondary endpoint in this study, was 80.3% in this age group, Pfizer said. The analysis was based on 10 symptomatic cases of COVID-19. The trial’s protocol specifies a formal analysis will be performed when at least 21 cases have accrued from 7 days after the third dose. The company said it would share final data on the effectiveness of the vaccine once the results are available.
Moderna on April 28 issued a statement with details about testing of its vaccine in young children. Vaccine efficacy was estimated at about 51% for children aged 6 months to younger than 2 years and 37% for the children aged 2 years to younger than 6. Paul Burton, MD, Moderna’s chief medical officer, spoke about this rate during a May 1 appearance on CBS’ Face the Nation.
“What it means for parents, for caregivers, is that if they give the Moderna vaccine to these little kids, they would basically cut in half the risk of that child getting symptomatic COVID,” Dr. Burton said in the interview. “Now, the number, 50%, I know is often lower than we are used to seeing with our vaccine, but it’s because this study was conducted during a time of Omicron.”
The FDA’s vaccine advisory committee also will meet on June 14 discuss potential use under an EUA of Moderna’s COVID vaccine for children and teenagers aged 6-17 years. The Pfizer-BioNTech vaccine already is authorized under an EUA for people aged 5 years and older.
The FDA has to date granted both conditional clearances, or EUAs, and regular approvals for COVID vaccines.
EUAs are meant to be temporary, allowing for rapid introduction of medicines in response to public health crises such as the pandemic. The FDA also uses EUAs to provide initial clearances of additional indications for products, as would be the case with the authorizations Moderna and Pfizer-BioNTech are seeking for their COVID vaccines.
Companies that want to continue to sell EUA-cleared products or promote EUA-cleared indications beyond the time of the public health crisis must seek regular approvals.
The FDA cleared the Pfizer-BioNTech and Moderna COVID vaccines under EUAs in December 2020. The agency then granted a regular approval for the Pfizer-BioNTech vaccine for people ages 16 and older in August 2021 based on more robust data. Regular approval for the Moderna vaccine for people ages 18 and older followed in January 2022.
Varied reactions among parents
Attitudes in the United States about pediatric COVID vaccines are far from uniform.
The initial uptake has disappointed physicians and researchers, who have been urging wider use of the COVID vaccination among children and teens for whom the FDA already has granted a clearance. Many parents are hesitating to bring their children for the COVID vaccines, according to the Centers for Disease Control and Prevention. Only 35.4% of children ages 5-11 had received at least one dose of a COVID vaccine, CDC staff said during a meeting.
Yet many other parents are demanding this medicine for their young children, urging the FDA to move quickly to clear COVID shots.
A private Facebook group called “Protect Their Future: A Call to Action for COVID Vaccines in Kids <5” boasts about 6,200 members. Many parents and physicians have used Twitter in recent months to press for a speedy review of COVID vaccines for the youngest children, often using the hashtag #immunizeunder5s. A group called Protect Their Future, which uses @ImmunizeUnder5s as its Twitter handle, had 5,288 followers as of the afternoon of May 23.
A special panel of the House of Representatives, the Select Subcommittee on the Coronavirus Crisis, on May 23 joined those tweeting about the need to soon authorize COVID vaccines for very young children.
“Parents have been waiting many months for vaccines for their young children,” the subcommittee tweeted. “They deserve to hear from @US_FDA why this lengthy process has been in children’s best interests.”
A version of this article first appeared on Medscape.com.
How to manage drug interactions with Paxlovid for COVID-19
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Misinformation about nirmatrelvir/ritonavir (Paxlovid, Pfizer) for treating mild to moderate COVID-19 in patients at high risk for severe disease is feeding misunderstanding among prescribers and patients, two experts from the Infectious Diseases Society of America (IDSA) have said.
They briefed reporters on potential drug interactions and uncommon cases of a “rebound” effect with the drug, which was granted emergency use authorization by the Food and Drug Administration last December for patients at least 12 years old.
The drug combination works “like a pair of scissors chopping up proteins that are made as the virus replicates inside of cells. Inhibiting that enzyme leads to the cessation of replication,” said Jason C. Gallagher, PharmD, of Temple University School of Pharmacy, Philadelphia.
That’s important because other treatments that target the spike protein, such as monoclonal antibodies, can lose their efficacy as the virus changes. He said that while that’s not impossible for Paxlovid, “we have not seen variants emerging that are resistant to it.”
Potential drug interactions
IDSA recently published updated guidance on potential interactions between Paxlovid and the top 100 drugs, and important considerations for prescribing.
“There is a concern that people have not been prescribing it because of fear of these interactions,” Dr. Gallagher said, explaining that, while in some cases those fears may be valid, in many instances the interaction is manageable.
One example is in two popular statins for heart disease, lovastatin and simvastatin.
“That’s an interaction that can be managed by holding [those drugs] for the 5 days that someone receives Paxlovid,” he said.
Misinformation also is circulating about distribution status of Paxlovid, Dr. Gallagher said.
“We’re in a very different state from that standpoint than we were a month or 2 months ago,” he said, adding that it is widely available in not all but a large number of pharmacies throughout the United States.
He emphasized the importance of drug reconciliation, as many patients will go to a different pharmacy for Paxlovid than they might for their usual prescriptions, so without a full accounting of prescriptions and supplements potential interactions may be missed.
Important interactions to watch
Melanie Thompson, MD, cochair of the HIVMA/IDSA HIV Primary Care Guidance Panel, highlighted some classes of drugs to watch, among them the antiarrhythmics, most of which are contraindicated with Paxlovid.
There are also important interactions with a number of cancer drugs, and consults with oncologists will be critical, she said.
“Likewise, people who have had transplants are likely to be on drugs that have significant ritonavir interactions,” Dr. Thompson said.
People on ergot drugs for migraine cannot take Paxlovid, she said, and “people who take colchicine for gout have to be very careful.”
She said it’s better not to use colchicine while taking Paxlovid, as it is contraindicated, “but it can be managed in certain circumstances with substantial dose reduction.”
A number of mental health drugs can be managed with Paxlovid, Dr. Thompson said. For the antipsychotic drug quetiapine, (Seroquel), a “substantial decrease in dose is required.”
Viagra for ED can be managed
Use of Viagra depends on why it’s being used, Dr. Thompson said. If it’s used for pulmonary hypertension, it is used at a very high dose and that is contraindicated. But if used for erectile dysfunction, the dose needs to be managed when people are on Paxlovid.
She said prescribers must know the kidney function of patients.
“There is a dose reduction that is required if people have impaired kidney function but below a certain level of function, which is 30 mL/min, it’s not recommended to give Paxlovid.”
Dr. Thompson highlighted two other websites for thorough, printable information on drug-drug interactions with Paxlovid: the University of Liverpool’s drug interaction checker and a printable handout from the University of Waterloo in Ontario, Canada.
“We need a 24/7 clinician hotline for Paxlovid to really make it accessible,” she said.
No data yet on ‘rebound’ effect
As to a few recent reports of a “rebound” effect, of people developing COVID-19 symptoms after completing a course of Paxlovid, there are not enough data yet to determine a clear pattern or cause.
“All we have are anecdotal data,” Dr. Thompson said. Current questions for study include whether the 5-day course is not long enough, she said, and whether people more at risk should be given a second course of Paxlovid if they do rebound.
Dr. Gallagher said it’s important to remember that the therapy goal of the drug is to prevent hospitalizations and deaths, and while any rebound is problematic, “it’s possible the use of the medication has already saved a life.”
Dr. Gallagher and Dr. Thompson report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anxiety in America: COVID ‘takes a backseat’ to global events
NEW ORLEANS – With 2 years of COVID-19 in the rearview mirror, anxiety among U.S. adults has turned instead toward global events, results from the annual Healthy Minds Poll from the American Psychiatric Association show.
“It’s not surprising that recent events, such as the war in Ukraine, racially motivated mass shootings, or the impacts of climate change, are weighing heavily on Americans’ minds,” APA president Vivian Pender, MD, said in a news release.
“COVID-19 in a way has taken a back seat, but the pandemic and its mental health effects are very much still with us. It’s important that we are cognizant of that and continue to work to ensure people who need psychiatric care, whether the causes are tied to the pandemic or to other issues, can access it,” Dr. Pender added.
Results from the 2022’s poll were released May 22 during the annual meeting of the APA.
Record low COVID anxiety
The poll was conducted by Morning Consult between April 23-24 and included 2,210 adult participants.
Results showed that This was down from 65% in 2021 and from 75% in 2020.
Instead, nearly three-quarters (73%) of adults are somewhat or extremely anxious about current events happening around the world, 64% are anxious about keeping themselves or their families safe, and 60% worry about their health in general.
Overall, about one-third (32%) reported being more anxious now than in 2021, 46% reported no change in their anxiety level, and 18% were less anxious.
About one-quarter (26%) have spoken with a mental health care professional in the past few years, which is down from 34% in 2021. In addition, Hispanic (36%) and Black (35%) adults were more likely to have reached out for help than White (25%) adults.
Despite the U.S. Surgeon General’s recent advisory on the mental health crisis among children, the poll results also showed that Americans are less concerned about their children’s mental health than in 2021. A total of 41% of parents expressed concern about this topic, which was down from 53% in 2021.
Still, 40% of parents said their children had received help from a mental health professional since the pandemic hit. Of that group, 36% sought help before the pandemic, whereas half said the pandemic had caused mental health issues for their children.
“While the overall level of concern has dropped, still 4 in 10 parents are worried about how their children are doing, and a third are having issues with access to care,” Saul Levin, MD, CEO and medical director of the APA, said in the release.
“This is unacceptable and as a nation, we need to invest in the kind of systems that will ensure any parent who’s worried about their child has access to lifesaving treatment,” Dr. Levin added.
Workplace mental health
In addition, the poll showed employees often have a tough time getting mental health support from employers, or are hesitant to ask for help.
“What’s troubling about the results of this poll is that, even as the pandemic has continued and its mental health effects wear on, fewer employees are reporting that they have access to mental health services,” Dr. Pender said.
“Workplaces need to ensure that they are paying attention to what their employees need, particularly now, and moving away from mental health benefits isn’t the right move,” she added.
About half (48%) of those polled said they can discuss mental health openly and honestly with their supervisor, down from 56% in 2021 and 62% in 2020.
Only about half (52%) said they feel comfortable using mental health services with their current employer, compared with 64% in 2021 and 67% in 2020.
In addition, fewer workers felt their employer is offering sufficient mental health resources and benefits. In 2022, 53% of workers thought resources and benefits were adequate, which was down from 65% in 2021 and 68% in 2020.
“It’s quite concerning to see that fewer people feel comfortable discussing mental health with a supervisor, at a time when people experiencing symptoms of anxiety, depression, and other conditions are on the rise and impact nearly every aspect of work, including productivity, performance, retention, and overall health care costs,” said Darcy Gruttadaro, JD, director of the APA Foundation’s Center for Workplace Mental Health.
“As rates of these conditions rise, we should see more employees knowing about available workplace mental health resources, not less,” Ms. Gruttadaro said.
Strong bipartisan support
Perhaps unexpectedly, the poll shows strong support among Democrats, Republicans, and Independents for three APA-backed approaches to improve timely access to mental health care and treatment.
Specifically, about three-quarters of those polled supported making it easier to see a mental health professional via telehealth, allowing patients to receive mental health care through a primary care provider, and funding mental health care professionals to work in rural or urban communities that are traditionally underserved.
“We’re in a moment when mental health is a big part of the national conversation, and clearly political party doesn’t matter as much on this issue,” Dr. Pender noted.
“It’s a rare thing in Washington these days to see such a resounding endorsement, but there is strong support for these practical workable solutions that mean more access to mental health care,” she said.
“What you see in this poll is agreement: It’s hard to access mental [health care] but we do have great solutions that could work across party lines,” Dr. Levin added.
“Many policy makers, in the administration and in Congress, are already putting these ideas into action, and they should feel encouraged that the public wants to see Congress act on them,” he said.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – With 2 years of COVID-19 in the rearview mirror, anxiety among U.S. adults has turned instead toward global events, results from the annual Healthy Minds Poll from the American Psychiatric Association show.
“It’s not surprising that recent events, such as the war in Ukraine, racially motivated mass shootings, or the impacts of climate change, are weighing heavily on Americans’ minds,” APA president Vivian Pender, MD, said in a news release.
“COVID-19 in a way has taken a back seat, but the pandemic and its mental health effects are very much still with us. It’s important that we are cognizant of that and continue to work to ensure people who need psychiatric care, whether the causes are tied to the pandemic or to other issues, can access it,” Dr. Pender added.
Results from the 2022’s poll were released May 22 during the annual meeting of the APA.
Record low COVID anxiety
The poll was conducted by Morning Consult between April 23-24 and included 2,210 adult participants.
Results showed that This was down from 65% in 2021 and from 75% in 2020.
Instead, nearly three-quarters (73%) of adults are somewhat or extremely anxious about current events happening around the world, 64% are anxious about keeping themselves or their families safe, and 60% worry about their health in general.
Overall, about one-third (32%) reported being more anxious now than in 2021, 46% reported no change in their anxiety level, and 18% were less anxious.
About one-quarter (26%) have spoken with a mental health care professional in the past few years, which is down from 34% in 2021. In addition, Hispanic (36%) and Black (35%) adults were more likely to have reached out for help than White (25%) adults.
Despite the U.S. Surgeon General’s recent advisory on the mental health crisis among children, the poll results also showed that Americans are less concerned about their children’s mental health than in 2021. A total of 41% of parents expressed concern about this topic, which was down from 53% in 2021.
Still, 40% of parents said their children had received help from a mental health professional since the pandemic hit. Of that group, 36% sought help before the pandemic, whereas half said the pandemic had caused mental health issues for their children.
“While the overall level of concern has dropped, still 4 in 10 parents are worried about how their children are doing, and a third are having issues with access to care,” Saul Levin, MD, CEO and medical director of the APA, said in the release.
“This is unacceptable and as a nation, we need to invest in the kind of systems that will ensure any parent who’s worried about their child has access to lifesaving treatment,” Dr. Levin added.
Workplace mental health
In addition, the poll showed employees often have a tough time getting mental health support from employers, or are hesitant to ask for help.
“What’s troubling about the results of this poll is that, even as the pandemic has continued and its mental health effects wear on, fewer employees are reporting that they have access to mental health services,” Dr. Pender said.
“Workplaces need to ensure that they are paying attention to what their employees need, particularly now, and moving away from mental health benefits isn’t the right move,” she added.
About half (48%) of those polled said they can discuss mental health openly and honestly with their supervisor, down from 56% in 2021 and 62% in 2020.
Only about half (52%) said they feel comfortable using mental health services with their current employer, compared with 64% in 2021 and 67% in 2020.
In addition, fewer workers felt their employer is offering sufficient mental health resources and benefits. In 2022, 53% of workers thought resources and benefits were adequate, which was down from 65% in 2021 and 68% in 2020.
“It’s quite concerning to see that fewer people feel comfortable discussing mental health with a supervisor, at a time when people experiencing symptoms of anxiety, depression, and other conditions are on the rise and impact nearly every aspect of work, including productivity, performance, retention, and overall health care costs,” said Darcy Gruttadaro, JD, director of the APA Foundation’s Center for Workplace Mental Health.
“As rates of these conditions rise, we should see more employees knowing about available workplace mental health resources, not less,” Ms. Gruttadaro said.
Strong bipartisan support
Perhaps unexpectedly, the poll shows strong support among Democrats, Republicans, and Independents for three APA-backed approaches to improve timely access to mental health care and treatment.
Specifically, about three-quarters of those polled supported making it easier to see a mental health professional via telehealth, allowing patients to receive mental health care through a primary care provider, and funding mental health care professionals to work in rural or urban communities that are traditionally underserved.
“We’re in a moment when mental health is a big part of the national conversation, and clearly political party doesn’t matter as much on this issue,” Dr. Pender noted.
“It’s a rare thing in Washington these days to see such a resounding endorsement, but there is strong support for these practical workable solutions that mean more access to mental health care,” she said.
“What you see in this poll is agreement: It’s hard to access mental [health care] but we do have great solutions that could work across party lines,” Dr. Levin added.
“Many policy makers, in the administration and in Congress, are already putting these ideas into action, and they should feel encouraged that the public wants to see Congress act on them,” he said.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – With 2 years of COVID-19 in the rearview mirror, anxiety among U.S. adults has turned instead toward global events, results from the annual Healthy Minds Poll from the American Psychiatric Association show.
“It’s not surprising that recent events, such as the war in Ukraine, racially motivated mass shootings, or the impacts of climate change, are weighing heavily on Americans’ minds,” APA president Vivian Pender, MD, said in a news release.
“COVID-19 in a way has taken a back seat, but the pandemic and its mental health effects are very much still with us. It’s important that we are cognizant of that and continue to work to ensure people who need psychiatric care, whether the causes are tied to the pandemic or to other issues, can access it,” Dr. Pender added.
Results from the 2022’s poll were released May 22 during the annual meeting of the APA.
Record low COVID anxiety
The poll was conducted by Morning Consult between April 23-24 and included 2,210 adult participants.
Results showed that This was down from 65% in 2021 and from 75% in 2020.
Instead, nearly three-quarters (73%) of adults are somewhat or extremely anxious about current events happening around the world, 64% are anxious about keeping themselves or their families safe, and 60% worry about their health in general.
Overall, about one-third (32%) reported being more anxious now than in 2021, 46% reported no change in their anxiety level, and 18% were less anxious.
About one-quarter (26%) have spoken with a mental health care professional in the past few years, which is down from 34% in 2021. In addition, Hispanic (36%) and Black (35%) adults were more likely to have reached out for help than White (25%) adults.
Despite the U.S. Surgeon General’s recent advisory on the mental health crisis among children, the poll results also showed that Americans are less concerned about their children’s mental health than in 2021. A total of 41% of parents expressed concern about this topic, which was down from 53% in 2021.
Still, 40% of parents said their children had received help from a mental health professional since the pandemic hit. Of that group, 36% sought help before the pandemic, whereas half said the pandemic had caused mental health issues for their children.
“While the overall level of concern has dropped, still 4 in 10 parents are worried about how their children are doing, and a third are having issues with access to care,” Saul Levin, MD, CEO and medical director of the APA, said in the release.
“This is unacceptable and as a nation, we need to invest in the kind of systems that will ensure any parent who’s worried about their child has access to lifesaving treatment,” Dr. Levin added.
Workplace mental health
In addition, the poll showed employees often have a tough time getting mental health support from employers, or are hesitant to ask for help.
“What’s troubling about the results of this poll is that, even as the pandemic has continued and its mental health effects wear on, fewer employees are reporting that they have access to mental health services,” Dr. Pender said.
“Workplaces need to ensure that they are paying attention to what their employees need, particularly now, and moving away from mental health benefits isn’t the right move,” she added.
About half (48%) of those polled said they can discuss mental health openly and honestly with their supervisor, down from 56% in 2021 and 62% in 2020.
Only about half (52%) said they feel comfortable using mental health services with their current employer, compared with 64% in 2021 and 67% in 2020.
In addition, fewer workers felt their employer is offering sufficient mental health resources and benefits. In 2022, 53% of workers thought resources and benefits were adequate, which was down from 65% in 2021 and 68% in 2020.
“It’s quite concerning to see that fewer people feel comfortable discussing mental health with a supervisor, at a time when people experiencing symptoms of anxiety, depression, and other conditions are on the rise and impact nearly every aspect of work, including productivity, performance, retention, and overall health care costs,” said Darcy Gruttadaro, JD, director of the APA Foundation’s Center for Workplace Mental Health.
“As rates of these conditions rise, we should see more employees knowing about available workplace mental health resources, not less,” Ms. Gruttadaro said.
Strong bipartisan support
Perhaps unexpectedly, the poll shows strong support among Democrats, Republicans, and Independents for three APA-backed approaches to improve timely access to mental health care and treatment.
Specifically, about three-quarters of those polled supported making it easier to see a mental health professional via telehealth, allowing patients to receive mental health care through a primary care provider, and funding mental health care professionals to work in rural or urban communities that are traditionally underserved.
“We’re in a moment when mental health is a big part of the national conversation, and clearly political party doesn’t matter as much on this issue,” Dr. Pender noted.
“It’s a rare thing in Washington these days to see such a resounding endorsement, but there is strong support for these practical workable solutions that mean more access to mental health care,” she said.
“What you see in this poll is agreement: It’s hard to access mental [health care] but we do have great solutions that could work across party lines,” Dr. Levin added.
“Many policy makers, in the administration and in Congress, are already putting these ideas into action, and they should feel encouraged that the public wants to see Congress act on them,” he said.
A version of this article first appeared on Medscape.com.
FROM APA 2022
Does COVID-19 raise the risk for diabetes?
This transcript has been edited for clarity.
Does having had a COVID-19 infection increase your risk for the development of diabetes subsequently? Some data say yes and other data say no. No matter what, it’s obviously important to screen people for diabetes routinely, pandemic or not. Remember, screening should start at age 35.
For over a decade, we have known that SARS-type viruses bind to beta cells. This could cause either direct damage to the beta cell or in some way trigger beta cell autoimmunity. We also know that COVID-19 infection increases the levels of inflammatory mediators, which could cause damage to beta cells and potentially to insulin receptors. There is a potential that having had a COVID-19 infection could increase rates of developing type 1 and/or type 2 diabetes.
However, there are other possible causes for people to develop diabetes after having a COVID-19 infection. A COVID-19 infection could cause one to seek medical care, unmasking latent type 1 and/or type 2 diabetes by causing infection-related insulin resistance and worsening preexisting mild hypoglycemia. In addition, people could have sought more medical care in the years since the pandemic has been ebbing, which may make it look like cases have increased.
For example, during the worst of the pandemic, I had multiple referrals for “COVID-19–caused new-onset diabetes” only to find that the patient had an A1c level above 10% and a history of mildly elevated blood glucose levels. This suggests to me that COVID-19 did not cause the diabetes per se but rather worsened an underlying glucose abnormality.
Since the pandemic has improved, I have also seen people diagnosed with type 2 diabetes that I think is associated with pandemic-related weight gain and inactivity.
The bigger issue is what is happening to people after COVID-19 infection who lack risk factors. What about those who we didn’t think were at high risk to get diabetes to begin with and didn’t have prediabetes?
An article by Xie and Al-Aly in The Lancet Diabetes & Endocrinology showed an increase in rates of diabetes in a large VA cohort among those who had a COVID-19 infection compared with both a contemporaneous control who did not have COVID-19 and a historical control. The researchers looked at the patient data 1 year after they’d had COVID-19, so it wasn’t the immediate post–COVID-19 phase but several months later.
They found that the risk for incident type 2 diabetes development was increased by 40% after adjusting for many risk factors. This included individuals who didn’t have traditional risk factors before they developed type 2 diabetes.
What does this mean clinically? First, pandemic or not, people need screening for diabetes and encouragement to have a healthy lifestyle. There may be an increased risk for the diagnosis of type 2 diabetes after COVID-19 infection due to a variety of different mechanisms.
As for people with type 1 diabetes, we also don’t know if having a COVID-19 infection increases their risk. We do know that there was an increase in the severity of diabetic ketoacidosis presentation during the pandemic, so we need to be sure that we reinforce sick-day rules with our patients with type 1 diabetes and that all individuals with type 1 diabetes have the ability to test their ketone levels at home.
In people with new-onset diabetes, whether type 1 or type 2, caused by COVID-19 or not, we need to treat appropriately based on their clinical situation.
Data from registries started during the pandemic will provide more definitive answers and help us find out if there is a relationship between having had COVID-19 infection and developing diabetes.
Perhaps that can help us better understand the mechanisms behind the development of diabetes overall.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Does having had a COVID-19 infection increase your risk for the development of diabetes subsequently? Some data say yes and other data say no. No matter what, it’s obviously important to screen people for diabetes routinely, pandemic or not. Remember, screening should start at age 35.
For over a decade, we have known that SARS-type viruses bind to beta cells. This could cause either direct damage to the beta cell or in some way trigger beta cell autoimmunity. We also know that COVID-19 infection increases the levels of inflammatory mediators, which could cause damage to beta cells and potentially to insulin receptors. There is a potential that having had a COVID-19 infection could increase rates of developing type 1 and/or type 2 diabetes.
However, there are other possible causes for people to develop diabetes after having a COVID-19 infection. A COVID-19 infection could cause one to seek medical care, unmasking latent type 1 and/or type 2 diabetes by causing infection-related insulin resistance and worsening preexisting mild hypoglycemia. In addition, people could have sought more medical care in the years since the pandemic has been ebbing, which may make it look like cases have increased.
For example, during the worst of the pandemic, I had multiple referrals for “COVID-19–caused new-onset diabetes” only to find that the patient had an A1c level above 10% and a history of mildly elevated blood glucose levels. This suggests to me that COVID-19 did not cause the diabetes per se but rather worsened an underlying glucose abnormality.
Since the pandemic has improved, I have also seen people diagnosed with type 2 diabetes that I think is associated with pandemic-related weight gain and inactivity.
The bigger issue is what is happening to people after COVID-19 infection who lack risk factors. What about those who we didn’t think were at high risk to get diabetes to begin with and didn’t have prediabetes?
An article by Xie and Al-Aly in The Lancet Diabetes & Endocrinology showed an increase in rates of diabetes in a large VA cohort among those who had a COVID-19 infection compared with both a contemporaneous control who did not have COVID-19 and a historical control. The researchers looked at the patient data 1 year after they’d had COVID-19, so it wasn’t the immediate post–COVID-19 phase but several months later.
They found that the risk for incident type 2 diabetes development was increased by 40% after adjusting for many risk factors. This included individuals who didn’t have traditional risk factors before they developed type 2 diabetes.
What does this mean clinically? First, pandemic or not, people need screening for diabetes and encouragement to have a healthy lifestyle. There may be an increased risk for the diagnosis of type 2 diabetes after COVID-19 infection due to a variety of different mechanisms.
As for people with type 1 diabetes, we also don’t know if having a COVID-19 infection increases their risk. We do know that there was an increase in the severity of diabetic ketoacidosis presentation during the pandemic, so we need to be sure that we reinforce sick-day rules with our patients with type 1 diabetes and that all individuals with type 1 diabetes have the ability to test their ketone levels at home.
In people with new-onset diabetes, whether type 1 or type 2, caused by COVID-19 or not, we need to treat appropriately based on their clinical situation.
Data from registries started during the pandemic will provide more definitive answers and help us find out if there is a relationship between having had COVID-19 infection and developing diabetes.
Perhaps that can help us better understand the mechanisms behind the development of diabetes overall.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Does having had a COVID-19 infection increase your risk for the development of diabetes subsequently? Some data say yes and other data say no. No matter what, it’s obviously important to screen people for diabetes routinely, pandemic or not. Remember, screening should start at age 35.
For over a decade, we have known that SARS-type viruses bind to beta cells. This could cause either direct damage to the beta cell or in some way trigger beta cell autoimmunity. We also know that COVID-19 infection increases the levels of inflammatory mediators, which could cause damage to beta cells and potentially to insulin receptors. There is a potential that having had a COVID-19 infection could increase rates of developing type 1 and/or type 2 diabetes.
However, there are other possible causes for people to develop diabetes after having a COVID-19 infection. A COVID-19 infection could cause one to seek medical care, unmasking latent type 1 and/or type 2 diabetes by causing infection-related insulin resistance and worsening preexisting mild hypoglycemia. In addition, people could have sought more medical care in the years since the pandemic has been ebbing, which may make it look like cases have increased.
For example, during the worst of the pandemic, I had multiple referrals for “COVID-19–caused new-onset diabetes” only to find that the patient had an A1c level above 10% and a history of mildly elevated blood glucose levels. This suggests to me that COVID-19 did not cause the diabetes per se but rather worsened an underlying glucose abnormality.
Since the pandemic has improved, I have also seen people diagnosed with type 2 diabetes that I think is associated with pandemic-related weight gain and inactivity.
The bigger issue is what is happening to people after COVID-19 infection who lack risk factors. What about those who we didn’t think were at high risk to get diabetes to begin with and didn’t have prediabetes?
An article by Xie and Al-Aly in The Lancet Diabetes & Endocrinology showed an increase in rates of diabetes in a large VA cohort among those who had a COVID-19 infection compared with both a contemporaneous control who did not have COVID-19 and a historical control. The researchers looked at the patient data 1 year after they’d had COVID-19, so it wasn’t the immediate post–COVID-19 phase but several months later.
They found that the risk for incident type 2 diabetes development was increased by 40% after adjusting for many risk factors. This included individuals who didn’t have traditional risk factors before they developed type 2 diabetes.
What does this mean clinically? First, pandemic or not, people need screening for diabetes and encouragement to have a healthy lifestyle. There may be an increased risk for the diagnosis of type 2 diabetes after COVID-19 infection due to a variety of different mechanisms.
As for people with type 1 diabetes, we also don’t know if having a COVID-19 infection increases their risk. We do know that there was an increase in the severity of diabetic ketoacidosis presentation during the pandemic, so we need to be sure that we reinforce sick-day rules with our patients with type 1 diabetes and that all individuals with type 1 diabetes have the ability to test their ketone levels at home.
In people with new-onset diabetes, whether type 1 or type 2, caused by COVID-19 or not, we need to treat appropriately based on their clinical situation.
Data from registries started during the pandemic will provide more definitive answers and help us find out if there is a relationship between having had COVID-19 infection and developing diabetes.
Perhaps that can help us better understand the mechanisms behind the development of diabetes overall.
Dr. Peters is professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She disclosed ties with Abbott Diabetes Care, AstraZeneca, Becton Dickinson, Boehringer Ingelheim Pharmaceuticals, Dexcom, Eli Lilly, Lexicon Pharmaceuticals, Livongo, MannKind Corporation, Medscape, Merck, Novo Nordisk, Omada Health, OptumHealth, Sanofi, and Zafgen. A version of this article first appeared on Medscape.com.
COVID-19 burnout? Turn off your mind, relax, and float downstream
SAN FRANCISCO – Along with first responders, health care workers in pulmonary and critical care have borne the brunt of the COVID-19 pandemic, and it’s not surprising that a large proportion have suffered from burnout, a syndrome characterized by chronic workplace stress, emotional exhaustion, cynicism about the job, and a reduced sense of personal accomplishment.
“Prior to the pandemic, 50% of providers reported burnout, and that, of course, has been exacerbated, with recent surveys showing up to 80% of health care workers reporting burnout,” said Sangeeta Joshi, MD, of the division of pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C.
In a randomized clinical trial, Dr. Joshi and colleagues showed that transcendental meditation (TM) can significantly improve burnout symptoms of emotional exhaustion, anxiety, and insomnia compared with other interventions, albeit without significant improvement in acute psychological distress.
Dr. Joshi reported the results of the trial at the American Thoracic Society’s international conference.
Mind-body intervention
TM, popularized in the 1960s by the Beatles and their guru, Maharishi Mahesh Yogi, is a nonpharmacologic mind-body intervention that has been shown to reduce sympathetic arousal and to promote a state of relaxation, Dr. Joshi said.
Although the mechanism of action is not fully understood, proposed explanations for its efficacy include increased alpha coherence, as seen on electroencephalography, and increases in blood flow to the prefrontal cortex, as visualized on functional MRI.
TM has been shown to be effective for reducing symptoms of posttraumatic stress disorder in veterans and for reducing stress and burnout symptoms in teachers, Dr. Joshi noted.
Randomized trial
To see whether TM could make a difference for health care providers, Dr. Joshi and colleagues screened candidates for burnout with the single-item Columbia–Suicide Severity Rating Scale and digital autonomic reactivity, a measure of the depth of physiologic stimulus.
Their study included 80 eligible participants, who were randomly assigned to receive either TM or treatment as usual.
The participants who received the intervention were assigned to attend four TM instruction sessions over 4 consecutive days, followed by four virtual follow-up sessions over the 3-month period. The investigators hypothesized that these participants would have significant improvements in symptoms of burnout over baseline compared with those assigned to standard treatments. Participants who underwent the intervention were encouraged to perform TM at home for 20 minutes twice each day.
Participants were evaluated at baseline and at 3-month follow-up with the Brief Symptom Inventory–18 (BSI), the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire–9 (PHQ-9), the Generalized Anxiety Disorder–7, the Insomnia Severity Index (ISI), and the Connor Davidson Resilience Scale (CD-RISC)–25.
At baseline, 70% of all participants reported a history of visiting a psychiatrist or other mental health worker, and 91% reported onset of a mental health condition. Only 30% reported that they had had a mental health condition that resolved with treatment.
At 3 months, there were significant improvements over baseline in the TM group compared with the treatment-as-usual group for the MBI emotional exhaustion item (P = .005), insomnia (P = .029), and anxiety (P = .010). There was trend toward significance on the PHQ-9 (P = .057), but no significant difference in the Global Severity Index (the total score of BSI items).
There were improvements in both study arms in both the MBI professional accomplishment item and in the CD-RISC scale, but the between-group differences were not significant.
The results show that “TM is a feasible, efficacious intervention in health care workers, especially during a pandemic,” Dr. Joshi said.
Future studies of TM in this setting should expand the number of participants and recruitment sites so as to have the necessary power to detect statistically significant changes in the numerical scales, she said.
Integrating TM into employee wellness
“These results are really encouraging,” said Seppo Rinne, MD, PhD, assistant professor of medicine at Boston University, who comoderated the oral abstract session in which the data were presented but was not involved in the study.
Commenting on the fact that TM is not more widely offered as part of a package of services for treating employees with symptoms of burnout, he noted that “in the burnout literature, we have a tendency to dichotomize these individual vs. organizational interventions, and the reality is that they are probably more integrated, and it’s not really helpful for us to think about these as totally separate.
“We need organizational interventions that support individual wellness,” he said.
The trial was sponsored by Duke University. Dr. Joshi and Dr. Rinne reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Along with first responders, health care workers in pulmonary and critical care have borne the brunt of the COVID-19 pandemic, and it’s not surprising that a large proportion have suffered from burnout, a syndrome characterized by chronic workplace stress, emotional exhaustion, cynicism about the job, and a reduced sense of personal accomplishment.
“Prior to the pandemic, 50% of providers reported burnout, and that, of course, has been exacerbated, with recent surveys showing up to 80% of health care workers reporting burnout,” said Sangeeta Joshi, MD, of the division of pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C.
In a randomized clinical trial, Dr. Joshi and colleagues showed that transcendental meditation (TM) can significantly improve burnout symptoms of emotional exhaustion, anxiety, and insomnia compared with other interventions, albeit without significant improvement in acute psychological distress.
Dr. Joshi reported the results of the trial at the American Thoracic Society’s international conference.
Mind-body intervention
TM, popularized in the 1960s by the Beatles and their guru, Maharishi Mahesh Yogi, is a nonpharmacologic mind-body intervention that has been shown to reduce sympathetic arousal and to promote a state of relaxation, Dr. Joshi said.
Although the mechanism of action is not fully understood, proposed explanations for its efficacy include increased alpha coherence, as seen on electroencephalography, and increases in blood flow to the prefrontal cortex, as visualized on functional MRI.
TM has been shown to be effective for reducing symptoms of posttraumatic stress disorder in veterans and for reducing stress and burnout symptoms in teachers, Dr. Joshi noted.
Randomized trial
To see whether TM could make a difference for health care providers, Dr. Joshi and colleagues screened candidates for burnout with the single-item Columbia–Suicide Severity Rating Scale and digital autonomic reactivity, a measure of the depth of physiologic stimulus.
Their study included 80 eligible participants, who were randomly assigned to receive either TM or treatment as usual.
The participants who received the intervention were assigned to attend four TM instruction sessions over 4 consecutive days, followed by four virtual follow-up sessions over the 3-month period. The investigators hypothesized that these participants would have significant improvements in symptoms of burnout over baseline compared with those assigned to standard treatments. Participants who underwent the intervention were encouraged to perform TM at home for 20 minutes twice each day.
Participants were evaluated at baseline and at 3-month follow-up with the Brief Symptom Inventory–18 (BSI), the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire–9 (PHQ-9), the Generalized Anxiety Disorder–7, the Insomnia Severity Index (ISI), and the Connor Davidson Resilience Scale (CD-RISC)–25.
At baseline, 70% of all participants reported a history of visiting a psychiatrist or other mental health worker, and 91% reported onset of a mental health condition. Only 30% reported that they had had a mental health condition that resolved with treatment.
At 3 months, there were significant improvements over baseline in the TM group compared with the treatment-as-usual group for the MBI emotional exhaustion item (P = .005), insomnia (P = .029), and anxiety (P = .010). There was trend toward significance on the PHQ-9 (P = .057), but no significant difference in the Global Severity Index (the total score of BSI items).
There were improvements in both study arms in both the MBI professional accomplishment item and in the CD-RISC scale, but the between-group differences were not significant.
The results show that “TM is a feasible, efficacious intervention in health care workers, especially during a pandemic,” Dr. Joshi said.
Future studies of TM in this setting should expand the number of participants and recruitment sites so as to have the necessary power to detect statistically significant changes in the numerical scales, she said.
Integrating TM into employee wellness
“These results are really encouraging,” said Seppo Rinne, MD, PhD, assistant professor of medicine at Boston University, who comoderated the oral abstract session in which the data were presented but was not involved in the study.
Commenting on the fact that TM is not more widely offered as part of a package of services for treating employees with symptoms of burnout, he noted that “in the burnout literature, we have a tendency to dichotomize these individual vs. organizational interventions, and the reality is that they are probably more integrated, and it’s not really helpful for us to think about these as totally separate.
“We need organizational interventions that support individual wellness,” he said.
The trial was sponsored by Duke University. Dr. Joshi and Dr. Rinne reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Along with first responders, health care workers in pulmonary and critical care have borne the brunt of the COVID-19 pandemic, and it’s not surprising that a large proportion have suffered from burnout, a syndrome characterized by chronic workplace stress, emotional exhaustion, cynicism about the job, and a reduced sense of personal accomplishment.
“Prior to the pandemic, 50% of providers reported burnout, and that, of course, has been exacerbated, with recent surveys showing up to 80% of health care workers reporting burnout,” said Sangeeta Joshi, MD, of the division of pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C.
In a randomized clinical trial, Dr. Joshi and colleagues showed that transcendental meditation (TM) can significantly improve burnout symptoms of emotional exhaustion, anxiety, and insomnia compared with other interventions, albeit without significant improvement in acute psychological distress.
Dr. Joshi reported the results of the trial at the American Thoracic Society’s international conference.
Mind-body intervention
TM, popularized in the 1960s by the Beatles and their guru, Maharishi Mahesh Yogi, is a nonpharmacologic mind-body intervention that has been shown to reduce sympathetic arousal and to promote a state of relaxation, Dr. Joshi said.
Although the mechanism of action is not fully understood, proposed explanations for its efficacy include increased alpha coherence, as seen on electroencephalography, and increases in blood flow to the prefrontal cortex, as visualized on functional MRI.
TM has been shown to be effective for reducing symptoms of posttraumatic stress disorder in veterans and for reducing stress and burnout symptoms in teachers, Dr. Joshi noted.
Randomized trial
To see whether TM could make a difference for health care providers, Dr. Joshi and colleagues screened candidates for burnout with the single-item Columbia–Suicide Severity Rating Scale and digital autonomic reactivity, a measure of the depth of physiologic stimulus.
Their study included 80 eligible participants, who were randomly assigned to receive either TM or treatment as usual.
The participants who received the intervention were assigned to attend four TM instruction sessions over 4 consecutive days, followed by four virtual follow-up sessions over the 3-month period. The investigators hypothesized that these participants would have significant improvements in symptoms of burnout over baseline compared with those assigned to standard treatments. Participants who underwent the intervention were encouraged to perform TM at home for 20 minutes twice each day.
Participants were evaluated at baseline and at 3-month follow-up with the Brief Symptom Inventory–18 (BSI), the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire–9 (PHQ-9), the Generalized Anxiety Disorder–7, the Insomnia Severity Index (ISI), and the Connor Davidson Resilience Scale (CD-RISC)–25.
At baseline, 70% of all participants reported a history of visiting a psychiatrist or other mental health worker, and 91% reported onset of a mental health condition. Only 30% reported that they had had a mental health condition that resolved with treatment.
At 3 months, there were significant improvements over baseline in the TM group compared with the treatment-as-usual group for the MBI emotional exhaustion item (P = .005), insomnia (P = .029), and anxiety (P = .010). There was trend toward significance on the PHQ-9 (P = .057), but no significant difference in the Global Severity Index (the total score of BSI items).
There were improvements in both study arms in both the MBI professional accomplishment item and in the CD-RISC scale, but the between-group differences were not significant.
The results show that “TM is a feasible, efficacious intervention in health care workers, especially during a pandemic,” Dr. Joshi said.
Future studies of TM in this setting should expand the number of participants and recruitment sites so as to have the necessary power to detect statistically significant changes in the numerical scales, she said.
Integrating TM into employee wellness
“These results are really encouraging,” said Seppo Rinne, MD, PhD, assistant professor of medicine at Boston University, who comoderated the oral abstract session in which the data were presented but was not involved in the study.
Commenting on the fact that TM is not more widely offered as part of a package of services for treating employees with symptoms of burnout, he noted that “in the burnout literature, we have a tendency to dichotomize these individual vs. organizational interventions, and the reality is that they are probably more integrated, and it’s not really helpful for us to think about these as totally separate.
“We need organizational interventions that support individual wellness,” he said.
The trial was sponsored by Duke University. Dr. Joshi and Dr. Rinne reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ATS 2022
CDC signs off on COVID boosters in children ages 5-11
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Centers for Disease Control and Prevention Director Rochelle Walensky, MD, signed off May 19 on an advisory panel’s recommendation that children ages 5 to 11 years should receive a Pfizer-BioNTech COVID-19 vaccine booster dose at least 5 months after completion of the primary series.
The CDC’s Advisory Committee on Immunization Practices (ACIP) voted 11:1, with one abstention, on a question about whether it recommended these additional shots in this age group.
The U.S. Food and Drug Administration on May 17 amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine to cover a single booster dose for administration to individuals 5 through 11 years of age.
At the request of CDC staff, ACIP members considered whether there should be softer wording for this recommendation, stating that children in this age group “may” receive a booster. This kind of phrasing would better reflect uncertainty about the course of COVID in the months ahead and allow flexibility for a stronger recommendation in the fall.
ACIP panelists and members of key groups argued strongly for a “should” recommendation, despite the uncertainties.
They also called for stronger efforts to make sure eligible children received their initial COVID-19 shots. Data gathered between November and April show only 14.4% of children ages 5 to 11 in rural areas have received at least one dose of COVID-19 vaccination, with top rates of 39.8% in large urban communities and 36% in larger suburban regions, CDC staff said.
CDC staff also said nearly 40% of parents in rural areas reported that their children’s pediatricians did not recommend COVID-19 vaccinations, compared with only 8% of parents in urban communities. These figures concerned ACIP members and liaisons from medical associations who take part in the panel’s deliberations but not in its votes.
“People will hear the word ‘m-a-y’ as ‘m-e-h’,” said Patricia Stinchfield, RN, MS, who served as the liaison for National Association of Pediatric Nurse Practitioners to ACIP. “I think we need to add urgency” to efforts to increase use of COVID vaccinations, she said.
Voting no on Thursday was Helen Keipp Talbot, MD, of Vanderbilt University. She explained after the vote that she is in favor of having young children vaccinated, but she’s concerned about the low rates of initial uptake of the COVID-19 shots.
“Boosters are great once we’ve gotten everyone their first round,” she said. “That needs to be our priority in this.”
Sandra Fryhofer, MD, the American Medical Association’s liaison to ACIP, stressed the add-on benefits from more widespread vaccination of children against COVID. Dr. Fryhofer said she serves adults in her practice as an internal medicine physician, with many of her patients being at high risk for complications from COVID.
Too many people are assuming the spread of infections in the community has lessened the risk of the virus, Dr. Fryhofer said.
“Not everyone’s had COVID yet, and my patients will be likely to get COVID if their grandchildren get it. We’re going through pandemic fatigue in this country,” she said. “Unfortunately, masks are now more off than on. Winter’s coming. They’re more variants” of the virus likely to emerge.
The data emerging so far suggests COVID vaccines will become a three-dose medicine, as is already accepted for other shots like hepatitis B vaccine, Dr. Fryhofer said.
Data gathered to date show the vaccine decreases risk of hospitalization for COVID and for complications such as multisystem inflammatory syndrome in children (MIS-C), she said.
“The bottom line is children in this age group are getting COVID,” Dr. Fryhofer said of the 5- to 11-year-olds. “Some do fine. Some are getting real sick. Some are hospitalized, some have died.”
At the meeting, CDC staff cited data from a paper published in the New England Journal of Medicine in March showing that vaccination had reduced the risk of hospitalization for COVID-19 among children 5 to 11 years of age by two-thirds during the Omicron period; most children with critical COVID-19 were unvaccinated.
COVID-19 led to 66 deaths among children ages 5 to 11 in the October 2020 to October 2021 timeframe, said ACIP member Matthew F. Daley, MD, of Kaiser Permanente Colorado during a presentation to his fellow panel members.
Parents may underestimate children’s risk from COVID and thus hold off on vaccinations, stressed AMA President Gerald E. Harmon, MD, in a statement issued after the meeting.
“It is concerning that only 1 in 3 children between the ages of 5 and 11 in the United States have received two doses of the vaccine, in part because parents believe them to be at lower risk for severe disease than adults,” Dr. Harmon said. “But the Omicron variant brought about change that should alter that calculus.”
Responding to early data
As Dr. Fryhofer put it, the medical community has been learning in “real time” about how COVID vaccines work and how to use them.
The EUA granted on May 17 for booster shots for children ages 5 to 11 was based on an analysis of immune response data in a subset of children from an ongoing randomized placebo-controlled trial, the FDA said.
Antibody responses were evaluated in 67 study participants who received a booster dose 7 to 9 months after completing a two-dose primary series of the Pfizer-BioNTech COVID-19 Vaccine. The EUA for the booster shot was intended to respond to emerging data that suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine, the FDA said.
CDC seeks help tracking vaccine complications
At the ACIP meeting, a top CDC vaccine-safety official, Tom Shimabukuro, MD, MPH, MBA, asked physicians to make sure their patients know about the agency’s V-Safe program for gathering reports from the public about their experiences with COVID vaccines. This is intended to help the CDC monitor for side effects of these medications.
“We need your help,” he said during a presentation about adverse events reported to date in children ages 5 to 11 who took the Pfizer vaccine.
About 18.1 million doses of Pfizer-BioNTech vaccine have been administered to children ages 5 to 11 years in the United States so far. Most of the reports of adverse events following vaccination were not serious, he said. But there were 20 reports of myocarditis verified to meet CDC case definition among children ages 5 to 11 years.
One case involved a death with histopathologic evidence of myocarditis on autopsy. The CDC continues to assist with case review, he said.
A version of this article first appeared on Medscape.com.
Pancreatic involvement in COVID-19: What do we know?
MADRID – It involves the relationship between COVID-19 and new diagnoses of diabetes and blood glucose disorders, among others, in the post–COVID-19 period. These topics were addressed at the XXXIII National Congress of the Spanish Diabetes Society. They were also the central theme of the inaugural conference, Pancreatic Involvement During COVID-19: From Preclinical Studies to Clinical Relevance, which was led by Alexander Kleger, MD, PhD, head of the department of pancreatology at the Ulm (Germany) University Clinic for Internal Medicine.
The chair of the scientific committee of the congress, Franz Martín, MD, launched the conference by noting that the work of Dr. Kleger and his team has made it possible to ascertain that SARS-CoV-2 can infect pancreatic beta cells that produce insulin. This observation may help in understanding why patients with COVID-19 sometimes experience symptoms related to greater difficulty regulating blood glucose.
“In addition, the German expert and his group have described the abnormalities that occur in beta cells when they are infected by SARS-CoV-2, something especially important, given that knowledge of these abnormalities may be of great importance to understanding the possible appearance of more cases of diabetes in the future,” Dr. Martín added.
“Our data identify the human pancreas as a target of SARS-CoV-2 infection and suggest that pancreatic beta cell involvement could contribute to the metabolic dysregulation seen in COVID-19 patients,” Dr. Kleger pointed out.
In his speech, Dr. Kleger reviewed the evidence on the effects of SARS-CoV-2 that has been garnered since the start of the pandemic, and he presented his research group’s findings on the impact at the pancreatic level.
“Since March 2020, it has been seen that COVID-19 affected the pancreas, and studies published in August of that same year clearly spoke of both a worsening of diabetes and an increase in new cases of this disease diagnosed after SARS-CoV-2 infection. Also, the data showed how hospitalized patients with no previous history of diabetes experienced rapid increases in glucose levels 5 days after admission,” Dr. Kleger said.
Angiotensin-converting enzyme 2
As an example of the pace at which evidence on the pancreatic impact of this virus has been evolving, Dr. Kleger referred to early studies that found no angiotensin-converting enzyme 2 receptor on cells of the endocrine and exocrine pancreas. “To our surprise, in our work, we did observe the obvious presence of angiotensin-converting enzyme 2 specifically expressed in human pancreatic beta cells, something confirmed by other investigations. Another surprising aspect was verifying that the viral infection lasts longer in the pancreas than in the lungs,” said the expert.
These findings caused the researchers to realize that SARS-CoV-2 may be directly or indirectly associated with diabetes. “It is currently the subject of debate whether it may be a direct effect, infecting or directly reaching the pancreatic beta cells, or whether this involvement is a result of the effect of the infection at systemic level, in the context of the cytokine storm and the proinflammatory environment derived from it. Our current challenge is to confirm whether this virus can really replicate in pancreatic beta cells and to assess the possible existence of reinfections, among other aspects,” said Dr. Kleger.
Along with these “developing areas of knowledge,” there are several certainties regarding the link between diabetes and COVID-19. Dr. Kleger summarized the most relevant one. “Preexisting diabetes is known to be a highly prevalent comorbidity seen in 11%-22% of patients and increases the risk of severe disease and mortality.
“SARS-CoV-2 infection has also been shown to affect the exocrine pancreas, manifesting as pancreatitis in 5% of critically ill patients with COVID-19, as well as enlargement of the pancreas and abnormal levels of amylase or lipase in 7.5%-17% of patients.
“Furthermore, it is obvious that SARS-CoV-2 infection produces glycometabolic dysfunction in these patients, with increased hyperglycemia in people with type 2 diabetes and ketoacidosis in 2%-6.4% of patients with and without diabetes.”
After recovery
The most recent research reveals the persistence of this dysregulation long after recovery from COVID-19. “We’ve seen that in a significant proportion of patients, hyperglycemia is maintained for some time; in the specific case of hospitalized patients [without the need for assisted ventilation or other intensive care requirements], for up to more than 2 months after overcoming the illness.
“In the same way, there are studies that have shown that insulin resistance and hyperstimulation of pancreatic beta cells remain at pathological levels in the post–COVID-19 phase. And in line with increased insulin resistance, signs of hyperinflammation have also been detected in these patients.”
Dr. Kleger noted that another research area is the increased incidence of newly diagnosed diabetes after recovery from SARS-CoV-2 infection, “something that seems to be correlated with how severely the disease has been experienced and also depending on whether hospitalization or intensive care was needed. Likewise, retrospective studies have shown that the risk of developing type 2 diabetes is higher in COVID-19 patients, compared with those with other respiratory infections. Regarding the incidence of type 1 diabetes, there is evidence, particularly in the case of children, of a clear correlation between the pandemic waves and the increase in cases.
“Therefore, and in view of this data, we could say that, with regard to the involvement of SARS-CoV-2 in pancreatic beta cells, something is up, but we are not yet able to fully understand what it is. What can be confirmed based on the numerous studies carried out in this regard is that COVID-19 produces a metabolic dysregulation [hyperglycemia, insulin resistance, diabetic ketoacidosis] which in turn favors the development of diabetes in patients with no history of this disease,” said Dr. Kleger.
“Likewise, everything points to the existence of a definitively feasible infection in pancreatic beta cells associated with SARS-CoV-2, but there are still unknown aspects of the physiology that explain this effect that remain the subject of debate and deserve future studies,” he concluded.
Consequences of the pandemic
The experts agreed that, although COVID-19 is no longer at the center of specialist care, it is still a subject of investigation. On the conference’s opening day, an update was made on the approach to diabetes.
Care activity is gradually recovering as the time that professionals devote to COVID-19 care is reduced, “but it will take time to catch up with the care activities not carried out during the pandemic, and, unfortunately, in the coming years, we will see the repercussion of the lack or reduction of care during these years,” stressed the SED chair, Antonio Pérez Pérez, MD, director of endocrinology and nutrition of Hospital de la Santa Creu i Sant Pau, Barcelona.
Dr. Pérez stressed that the pandemic has revealed health system deficiencies in diabetes care. He added that the impact of COVID-19 on diabetes (resulting from the effects of the infection itself or from the inadequacy of prevention, diagnosis, and treatment measures) fostered a deterioration of metabolic control and a delay in the diagnosis of the disease and its complications.
“All this contributes to the fact that we currently continue to see patients with complications, especially in the case of type 2 diabetes, with more serious decompensations and diagnoses in more advanced stages of the disease. This impact has been more significant in older people from disadvantaged areas and with less capacity for self-monitoring and self-adjustment of treatment,” he added.
Describing lessons learned through the experiences accumulated in diabetes care during the pandemic, Dr. Pérez highlighted the push for virtual consultations, accessibility to drugs prescribed in electronic prescriptions, and the use of educational resources online and of telemedicine tools. “The need to invest in the health sector has also been assumed, endowing it with robustness in well-trained health personnel, to promote health education, boost efficient health organization, and invest in innovation aimed at facilitating care.”
Dr. Kleger and Dr. Pérez disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MADRID – It involves the relationship between COVID-19 and new diagnoses of diabetes and blood glucose disorders, among others, in the post–COVID-19 period. These topics were addressed at the XXXIII National Congress of the Spanish Diabetes Society. They were also the central theme of the inaugural conference, Pancreatic Involvement During COVID-19: From Preclinical Studies to Clinical Relevance, which was led by Alexander Kleger, MD, PhD, head of the department of pancreatology at the Ulm (Germany) University Clinic for Internal Medicine.
The chair of the scientific committee of the congress, Franz Martín, MD, launched the conference by noting that the work of Dr. Kleger and his team has made it possible to ascertain that SARS-CoV-2 can infect pancreatic beta cells that produce insulin. This observation may help in understanding why patients with COVID-19 sometimes experience symptoms related to greater difficulty regulating blood glucose.
“In addition, the German expert and his group have described the abnormalities that occur in beta cells when they are infected by SARS-CoV-2, something especially important, given that knowledge of these abnormalities may be of great importance to understanding the possible appearance of more cases of diabetes in the future,” Dr. Martín added.
“Our data identify the human pancreas as a target of SARS-CoV-2 infection and suggest that pancreatic beta cell involvement could contribute to the metabolic dysregulation seen in COVID-19 patients,” Dr. Kleger pointed out.
In his speech, Dr. Kleger reviewed the evidence on the effects of SARS-CoV-2 that has been garnered since the start of the pandemic, and he presented his research group’s findings on the impact at the pancreatic level.
“Since March 2020, it has been seen that COVID-19 affected the pancreas, and studies published in August of that same year clearly spoke of both a worsening of diabetes and an increase in new cases of this disease diagnosed after SARS-CoV-2 infection. Also, the data showed how hospitalized patients with no previous history of diabetes experienced rapid increases in glucose levels 5 days after admission,” Dr. Kleger said.
Angiotensin-converting enzyme 2
As an example of the pace at which evidence on the pancreatic impact of this virus has been evolving, Dr. Kleger referred to early studies that found no angiotensin-converting enzyme 2 receptor on cells of the endocrine and exocrine pancreas. “To our surprise, in our work, we did observe the obvious presence of angiotensin-converting enzyme 2 specifically expressed in human pancreatic beta cells, something confirmed by other investigations. Another surprising aspect was verifying that the viral infection lasts longer in the pancreas than in the lungs,” said the expert.
These findings caused the researchers to realize that SARS-CoV-2 may be directly or indirectly associated with diabetes. “It is currently the subject of debate whether it may be a direct effect, infecting or directly reaching the pancreatic beta cells, or whether this involvement is a result of the effect of the infection at systemic level, in the context of the cytokine storm and the proinflammatory environment derived from it. Our current challenge is to confirm whether this virus can really replicate in pancreatic beta cells and to assess the possible existence of reinfections, among other aspects,” said Dr. Kleger.
Along with these “developing areas of knowledge,” there are several certainties regarding the link between diabetes and COVID-19. Dr. Kleger summarized the most relevant one. “Preexisting diabetes is known to be a highly prevalent comorbidity seen in 11%-22% of patients and increases the risk of severe disease and mortality.
“SARS-CoV-2 infection has also been shown to affect the exocrine pancreas, manifesting as pancreatitis in 5% of critically ill patients with COVID-19, as well as enlargement of the pancreas and abnormal levels of amylase or lipase in 7.5%-17% of patients.
“Furthermore, it is obvious that SARS-CoV-2 infection produces glycometabolic dysfunction in these patients, with increased hyperglycemia in people with type 2 diabetes and ketoacidosis in 2%-6.4% of patients with and without diabetes.”
After recovery
The most recent research reveals the persistence of this dysregulation long after recovery from COVID-19. “We’ve seen that in a significant proportion of patients, hyperglycemia is maintained for some time; in the specific case of hospitalized patients [without the need for assisted ventilation or other intensive care requirements], for up to more than 2 months after overcoming the illness.
“In the same way, there are studies that have shown that insulin resistance and hyperstimulation of pancreatic beta cells remain at pathological levels in the post–COVID-19 phase. And in line with increased insulin resistance, signs of hyperinflammation have also been detected in these patients.”
Dr. Kleger noted that another research area is the increased incidence of newly diagnosed diabetes after recovery from SARS-CoV-2 infection, “something that seems to be correlated with how severely the disease has been experienced and also depending on whether hospitalization or intensive care was needed. Likewise, retrospective studies have shown that the risk of developing type 2 diabetes is higher in COVID-19 patients, compared with those with other respiratory infections. Regarding the incidence of type 1 diabetes, there is evidence, particularly in the case of children, of a clear correlation between the pandemic waves and the increase in cases.
“Therefore, and in view of this data, we could say that, with regard to the involvement of SARS-CoV-2 in pancreatic beta cells, something is up, but we are not yet able to fully understand what it is. What can be confirmed based on the numerous studies carried out in this regard is that COVID-19 produces a metabolic dysregulation [hyperglycemia, insulin resistance, diabetic ketoacidosis] which in turn favors the development of diabetes in patients with no history of this disease,” said Dr. Kleger.
“Likewise, everything points to the existence of a definitively feasible infection in pancreatic beta cells associated with SARS-CoV-2, but there are still unknown aspects of the physiology that explain this effect that remain the subject of debate and deserve future studies,” he concluded.
Consequences of the pandemic
The experts agreed that, although COVID-19 is no longer at the center of specialist care, it is still a subject of investigation. On the conference’s opening day, an update was made on the approach to diabetes.
Care activity is gradually recovering as the time that professionals devote to COVID-19 care is reduced, “but it will take time to catch up with the care activities not carried out during the pandemic, and, unfortunately, in the coming years, we will see the repercussion of the lack or reduction of care during these years,” stressed the SED chair, Antonio Pérez Pérez, MD, director of endocrinology and nutrition of Hospital de la Santa Creu i Sant Pau, Barcelona.
Dr. Pérez stressed that the pandemic has revealed health system deficiencies in diabetes care. He added that the impact of COVID-19 on diabetes (resulting from the effects of the infection itself or from the inadequacy of prevention, diagnosis, and treatment measures) fostered a deterioration of metabolic control and a delay in the diagnosis of the disease and its complications.
“All this contributes to the fact that we currently continue to see patients with complications, especially in the case of type 2 diabetes, with more serious decompensations and diagnoses in more advanced stages of the disease. This impact has been more significant in older people from disadvantaged areas and with less capacity for self-monitoring and self-adjustment of treatment,” he added.
Describing lessons learned through the experiences accumulated in diabetes care during the pandemic, Dr. Pérez highlighted the push for virtual consultations, accessibility to drugs prescribed in electronic prescriptions, and the use of educational resources online and of telemedicine tools. “The need to invest in the health sector has also been assumed, endowing it with robustness in well-trained health personnel, to promote health education, boost efficient health organization, and invest in innovation aimed at facilitating care.”
Dr. Kleger and Dr. Pérez disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
MADRID – It involves the relationship between COVID-19 and new diagnoses of diabetes and blood glucose disorders, among others, in the post–COVID-19 period. These topics were addressed at the XXXIII National Congress of the Spanish Diabetes Society. They were also the central theme of the inaugural conference, Pancreatic Involvement During COVID-19: From Preclinical Studies to Clinical Relevance, which was led by Alexander Kleger, MD, PhD, head of the department of pancreatology at the Ulm (Germany) University Clinic for Internal Medicine.
The chair of the scientific committee of the congress, Franz Martín, MD, launched the conference by noting that the work of Dr. Kleger and his team has made it possible to ascertain that SARS-CoV-2 can infect pancreatic beta cells that produce insulin. This observation may help in understanding why patients with COVID-19 sometimes experience symptoms related to greater difficulty regulating blood glucose.
“In addition, the German expert and his group have described the abnormalities that occur in beta cells when they are infected by SARS-CoV-2, something especially important, given that knowledge of these abnormalities may be of great importance to understanding the possible appearance of more cases of diabetes in the future,” Dr. Martín added.
“Our data identify the human pancreas as a target of SARS-CoV-2 infection and suggest that pancreatic beta cell involvement could contribute to the metabolic dysregulation seen in COVID-19 patients,” Dr. Kleger pointed out.
In his speech, Dr. Kleger reviewed the evidence on the effects of SARS-CoV-2 that has been garnered since the start of the pandemic, and he presented his research group’s findings on the impact at the pancreatic level.
“Since March 2020, it has been seen that COVID-19 affected the pancreas, and studies published in August of that same year clearly spoke of both a worsening of diabetes and an increase in new cases of this disease diagnosed after SARS-CoV-2 infection. Also, the data showed how hospitalized patients with no previous history of diabetes experienced rapid increases in glucose levels 5 days after admission,” Dr. Kleger said.
Angiotensin-converting enzyme 2
As an example of the pace at which evidence on the pancreatic impact of this virus has been evolving, Dr. Kleger referred to early studies that found no angiotensin-converting enzyme 2 receptor on cells of the endocrine and exocrine pancreas. “To our surprise, in our work, we did observe the obvious presence of angiotensin-converting enzyme 2 specifically expressed in human pancreatic beta cells, something confirmed by other investigations. Another surprising aspect was verifying that the viral infection lasts longer in the pancreas than in the lungs,” said the expert.
These findings caused the researchers to realize that SARS-CoV-2 may be directly or indirectly associated with diabetes. “It is currently the subject of debate whether it may be a direct effect, infecting or directly reaching the pancreatic beta cells, or whether this involvement is a result of the effect of the infection at systemic level, in the context of the cytokine storm and the proinflammatory environment derived from it. Our current challenge is to confirm whether this virus can really replicate in pancreatic beta cells and to assess the possible existence of reinfections, among other aspects,” said Dr. Kleger.
Along with these “developing areas of knowledge,” there are several certainties regarding the link between diabetes and COVID-19. Dr. Kleger summarized the most relevant one. “Preexisting diabetes is known to be a highly prevalent comorbidity seen in 11%-22% of patients and increases the risk of severe disease and mortality.
“SARS-CoV-2 infection has also been shown to affect the exocrine pancreas, manifesting as pancreatitis in 5% of critically ill patients with COVID-19, as well as enlargement of the pancreas and abnormal levels of amylase or lipase in 7.5%-17% of patients.
“Furthermore, it is obvious that SARS-CoV-2 infection produces glycometabolic dysfunction in these patients, with increased hyperglycemia in people with type 2 diabetes and ketoacidosis in 2%-6.4% of patients with and without diabetes.”
After recovery
The most recent research reveals the persistence of this dysregulation long after recovery from COVID-19. “We’ve seen that in a significant proportion of patients, hyperglycemia is maintained for some time; in the specific case of hospitalized patients [without the need for assisted ventilation or other intensive care requirements], for up to more than 2 months after overcoming the illness.
“In the same way, there are studies that have shown that insulin resistance and hyperstimulation of pancreatic beta cells remain at pathological levels in the post–COVID-19 phase. And in line with increased insulin resistance, signs of hyperinflammation have also been detected in these patients.”
Dr. Kleger noted that another research area is the increased incidence of newly diagnosed diabetes after recovery from SARS-CoV-2 infection, “something that seems to be correlated with how severely the disease has been experienced and also depending on whether hospitalization or intensive care was needed. Likewise, retrospective studies have shown that the risk of developing type 2 diabetes is higher in COVID-19 patients, compared with those with other respiratory infections. Regarding the incidence of type 1 diabetes, there is evidence, particularly in the case of children, of a clear correlation between the pandemic waves and the increase in cases.
“Therefore, and in view of this data, we could say that, with regard to the involvement of SARS-CoV-2 in pancreatic beta cells, something is up, but we are not yet able to fully understand what it is. What can be confirmed based on the numerous studies carried out in this regard is that COVID-19 produces a metabolic dysregulation [hyperglycemia, insulin resistance, diabetic ketoacidosis] which in turn favors the development of diabetes in patients with no history of this disease,” said Dr. Kleger.
“Likewise, everything points to the existence of a definitively feasible infection in pancreatic beta cells associated with SARS-CoV-2, but there are still unknown aspects of the physiology that explain this effect that remain the subject of debate and deserve future studies,” he concluded.
Consequences of the pandemic
The experts agreed that, although COVID-19 is no longer at the center of specialist care, it is still a subject of investigation. On the conference’s opening day, an update was made on the approach to diabetes.
Care activity is gradually recovering as the time that professionals devote to COVID-19 care is reduced, “but it will take time to catch up with the care activities not carried out during the pandemic, and, unfortunately, in the coming years, we will see the repercussion of the lack or reduction of care during these years,” stressed the SED chair, Antonio Pérez Pérez, MD, director of endocrinology and nutrition of Hospital de la Santa Creu i Sant Pau, Barcelona.
Dr. Pérez stressed that the pandemic has revealed health system deficiencies in diabetes care. He added that the impact of COVID-19 on diabetes (resulting from the effects of the infection itself or from the inadequacy of prevention, diagnosis, and treatment measures) fostered a deterioration of metabolic control and a delay in the diagnosis of the disease and its complications.
“All this contributes to the fact that we currently continue to see patients with complications, especially in the case of type 2 diabetes, with more serious decompensations and diagnoses in more advanced stages of the disease. This impact has been more significant in older people from disadvantaged areas and with less capacity for self-monitoring and self-adjustment of treatment,” he added.
Describing lessons learned through the experiences accumulated in diabetes care during the pandemic, Dr. Pérez highlighted the push for virtual consultations, accessibility to drugs prescribed in electronic prescriptions, and the use of educational resources online and of telemedicine tools. “The need to invest in the health sector has also been assumed, endowing it with robustness in well-trained health personnel, to promote health education, boost efficient health organization, and invest in innovation aimed at facilitating care.”
Dr. Kleger and Dr. Pérez disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com. This article was translated from the Medscape Spanish edition.
Finding ‘bright lights’: Why family physician continues to love practicing mid-career
A few years ago I tracked down my medical school interviewer to thank him for giving me the opportunity to do what I felt I was called to do. I was surprised that, after 15 years, he actually remembered me and remembered details like walking to the courtyard to meet my father who’d driven me to the interview.
Sharing my gratitude and letting him know I was happy felt important to me.
Choosing to practice family medicine has a lot to do with why I am happy in my career today.
One of my frustrations with health care had been its emphasis on treatment of sickness, rather than a broader one that incorporated prevention of sickness. During my third year of medical school, I was following a family and sports medicine faculty member who was focusing on aspects of lifestyle medicine to help a patient remain active and age gracefully. Seeing opportunities to practice preventative medicine in family medicine made me realize the specialty was the perfect fit for me.
Food as medicine
While participating in rotations I also realized you can find a subspecialty within family medicine.
During my fourth year of medical school, I followed an attending who was seeing a patient for hypertension, prediabetes and hypercholesterolemia. The attending told the patient to eat “healthier,” gave her a handout, and scheduled a follow up appointment for 6 months later.
My thoughts were: “That’s it? That’s how we counsel patients to improve their dietary habits?”
As the patient was leaving the exam room, I asked her what type of oil she cooked with, and I proceeded to share culinary tips from my mother – who was a self-taught and early adopter of the food-as-medicine movement.
Once I started my residency, I knew I’d want to incorporate lifestyle and dietary approaches at many of my patient visits.
I scheduled patients every month to monitor their weight, follow up on chronic conditions, but more importantly, to engage them in their health and empower them to make small lifestyle changes each month and report their efforts. I felt like I was their health coach and cheerleader.
My career in family medicine
Entering the job market allowed me to form my philosophy of treating patients with a mind, body, and spirit approach. I chose to practice value-based care, which aligns with my lifestyle and preventative medicine approach .
I currently practice in a small family medicine–only clinic that is part of a larger multispecialty system. Primary care specialties in my organization are valued, respected and central to a patient’s well being and care. We are encouraged to spend time with patients, assess barriers to care and work collaboratively with our healthcare team, so that preventative medicine approaches take the lead in a patient’s health. This supportive culture and environment is one where my passion for food as medicine has thrived.
One day I forgot to pack a lunch and instead brought a grocery bag of items to make a salad. When I realized I made too much, I sent an email to my staff to get some “free salad in my office.” This serendipitous moment started an informal office “salad club” each week. Continued support from my staff and leadership, allowed me to consider further extending this teaching to my patients and my colleagues.
Three years ago, I helped adopt a sustainable plant-forward menu for our physician meetings, complete with a recipe from the menu for physicians to replicate at home or give to their patients.
I also pursued adoption of shared medical appointments for our medical group. These appointments apply the “see one, do one, teach one” model in medicine, but with culinary medicine as the focus.
Knowing that my patients are all connected to their families through food, I sought this as an opportunity to dive in further with wellness opportunities at their next meal. After almost 2 years of working on this project, I was able to host my first shared medical appointment with a group of patients on March 12, 2020. The next day schools closed, lockdowns occurred, and the world changed.
Opportunities highlighted by the pandemic
We always knew health care was broken but adding the increasingly longer hours and COVID vaccine–hesitant patients that the pandemic brought made everything look dark at times. What has helped me stay hopeful and energetic for system changes is feeling gratitude and seeking bright lights.
My experiences seeing patients in telehealth visits are examples of some of the bright lights I found in the pandemic. During these visits, patients showed me something from their pantry, and we’d go over nutritional labels together.
Additionally, my patients became engaged with their own conditions and wanted to improve them because of news articles highlighting risk factors for COVID-19, such as obesity. I had an active audience when it came to talking about food-as-medicine approaches to improving risk factors and immunity. And since everyone was listening, I didn’t stop at food. I also talked about physical health, stress resiliency, planetary diets, sleep, connections, and lastly vaccines!
Once the vaccines were distributed, I naturally gravitated to having those conversations with patients and colleagues and on social media. Plus, the pandemic gave us moments of simple times to slow down, take more rests, be less overscheduled, consider work-life priorities, and, lastly, to be okay with not being totally okay.
In practicing primary care, we have a unique role in seeing medicine from a whole body, whole person, whole family perspective. There is an opportunity to highlight what is broken in medicine and aim to make it whole.
I’m currently looking at shared medical appointments as a new standard way to provide care to all patients, because it improves access, provides better quality visits and aligns my values, mission, and purpose.
In the midst of the pandemic, I helped advocate for a sustainable plant-forward menu that was launched throughout four different hospitals in the Sharp HealthCare system, in California, in 2020. Knowing that patients were served a menu I played a role in, gave me solace.
As part of the hospital food and nutrition team, I am grateful for the opportunity I have to work on a broader mission to address social determinants of health and seek opportunities to help the system work for our patients.
Public health communication has been lacking in the pandemic, but another bright light is that we were still the trusted messengers to our patients and our communities. I’m continually honored and humbled to be trusted with a whole family’s health.
Dr. Neison practices family medicine and culinary medicine at Sharp Rees-Stealy Medical Group in San Diego, and is cochair of climate and planetary health for SRS Medical Group. You can follow her on Instagram, LinkedIn, and Facebook @Flavors4WellnessMD.
A few years ago I tracked down my medical school interviewer to thank him for giving me the opportunity to do what I felt I was called to do. I was surprised that, after 15 years, he actually remembered me and remembered details like walking to the courtyard to meet my father who’d driven me to the interview.
Sharing my gratitude and letting him know I was happy felt important to me.
Choosing to practice family medicine has a lot to do with why I am happy in my career today.
One of my frustrations with health care had been its emphasis on treatment of sickness, rather than a broader one that incorporated prevention of sickness. During my third year of medical school, I was following a family and sports medicine faculty member who was focusing on aspects of lifestyle medicine to help a patient remain active and age gracefully. Seeing opportunities to practice preventative medicine in family medicine made me realize the specialty was the perfect fit for me.
Food as medicine
While participating in rotations I also realized you can find a subspecialty within family medicine.
During my fourth year of medical school, I followed an attending who was seeing a patient for hypertension, prediabetes and hypercholesterolemia. The attending told the patient to eat “healthier,” gave her a handout, and scheduled a follow up appointment for 6 months later.
My thoughts were: “That’s it? That’s how we counsel patients to improve their dietary habits?”
As the patient was leaving the exam room, I asked her what type of oil she cooked with, and I proceeded to share culinary tips from my mother – who was a self-taught and early adopter of the food-as-medicine movement.
Once I started my residency, I knew I’d want to incorporate lifestyle and dietary approaches at many of my patient visits.
I scheduled patients every month to monitor their weight, follow up on chronic conditions, but more importantly, to engage them in their health and empower them to make small lifestyle changes each month and report their efforts. I felt like I was their health coach and cheerleader.
My career in family medicine
Entering the job market allowed me to form my philosophy of treating patients with a mind, body, and spirit approach. I chose to practice value-based care, which aligns with my lifestyle and preventative medicine approach .
I currently practice in a small family medicine–only clinic that is part of a larger multispecialty system. Primary care specialties in my organization are valued, respected and central to a patient’s well being and care. We are encouraged to spend time with patients, assess barriers to care and work collaboratively with our healthcare team, so that preventative medicine approaches take the lead in a patient’s health. This supportive culture and environment is one where my passion for food as medicine has thrived.
One day I forgot to pack a lunch and instead brought a grocery bag of items to make a salad. When I realized I made too much, I sent an email to my staff to get some “free salad in my office.” This serendipitous moment started an informal office “salad club” each week. Continued support from my staff and leadership, allowed me to consider further extending this teaching to my patients and my colleagues.
Three years ago, I helped adopt a sustainable plant-forward menu for our physician meetings, complete with a recipe from the menu for physicians to replicate at home or give to their patients.
I also pursued adoption of shared medical appointments for our medical group. These appointments apply the “see one, do one, teach one” model in medicine, but with culinary medicine as the focus.
Knowing that my patients are all connected to their families through food, I sought this as an opportunity to dive in further with wellness opportunities at their next meal. After almost 2 years of working on this project, I was able to host my first shared medical appointment with a group of patients on March 12, 2020. The next day schools closed, lockdowns occurred, and the world changed.
Opportunities highlighted by the pandemic
We always knew health care was broken but adding the increasingly longer hours and COVID vaccine–hesitant patients that the pandemic brought made everything look dark at times. What has helped me stay hopeful and energetic for system changes is feeling gratitude and seeking bright lights.
My experiences seeing patients in telehealth visits are examples of some of the bright lights I found in the pandemic. During these visits, patients showed me something from their pantry, and we’d go over nutritional labels together.
Additionally, my patients became engaged with their own conditions and wanted to improve them because of news articles highlighting risk factors for COVID-19, such as obesity. I had an active audience when it came to talking about food-as-medicine approaches to improving risk factors and immunity. And since everyone was listening, I didn’t stop at food. I also talked about physical health, stress resiliency, planetary diets, sleep, connections, and lastly vaccines!
Once the vaccines were distributed, I naturally gravitated to having those conversations with patients and colleagues and on social media. Plus, the pandemic gave us moments of simple times to slow down, take more rests, be less overscheduled, consider work-life priorities, and, lastly, to be okay with not being totally okay.
In practicing primary care, we have a unique role in seeing medicine from a whole body, whole person, whole family perspective. There is an opportunity to highlight what is broken in medicine and aim to make it whole.
I’m currently looking at shared medical appointments as a new standard way to provide care to all patients, because it improves access, provides better quality visits and aligns my values, mission, and purpose.
In the midst of the pandemic, I helped advocate for a sustainable plant-forward menu that was launched throughout four different hospitals in the Sharp HealthCare system, in California, in 2020. Knowing that patients were served a menu I played a role in, gave me solace.
As part of the hospital food and nutrition team, I am grateful for the opportunity I have to work on a broader mission to address social determinants of health and seek opportunities to help the system work for our patients.
Public health communication has been lacking in the pandemic, but another bright light is that we were still the trusted messengers to our patients and our communities. I’m continually honored and humbled to be trusted with a whole family’s health.
Dr. Neison practices family medicine and culinary medicine at Sharp Rees-Stealy Medical Group in San Diego, and is cochair of climate and planetary health for SRS Medical Group. You can follow her on Instagram, LinkedIn, and Facebook @Flavors4WellnessMD.
A few years ago I tracked down my medical school interviewer to thank him for giving me the opportunity to do what I felt I was called to do. I was surprised that, after 15 years, he actually remembered me and remembered details like walking to the courtyard to meet my father who’d driven me to the interview.
Sharing my gratitude and letting him know I was happy felt important to me.
Choosing to practice family medicine has a lot to do with why I am happy in my career today.
One of my frustrations with health care had been its emphasis on treatment of sickness, rather than a broader one that incorporated prevention of sickness. During my third year of medical school, I was following a family and sports medicine faculty member who was focusing on aspects of lifestyle medicine to help a patient remain active and age gracefully. Seeing opportunities to practice preventative medicine in family medicine made me realize the specialty was the perfect fit for me.
Food as medicine
While participating in rotations I also realized you can find a subspecialty within family medicine.
During my fourth year of medical school, I followed an attending who was seeing a patient for hypertension, prediabetes and hypercholesterolemia. The attending told the patient to eat “healthier,” gave her a handout, and scheduled a follow up appointment for 6 months later.
My thoughts were: “That’s it? That’s how we counsel patients to improve their dietary habits?”
As the patient was leaving the exam room, I asked her what type of oil she cooked with, and I proceeded to share culinary tips from my mother – who was a self-taught and early adopter of the food-as-medicine movement.
Once I started my residency, I knew I’d want to incorporate lifestyle and dietary approaches at many of my patient visits.
I scheduled patients every month to monitor their weight, follow up on chronic conditions, but more importantly, to engage them in their health and empower them to make small lifestyle changes each month and report their efforts. I felt like I was their health coach and cheerleader.
My career in family medicine
Entering the job market allowed me to form my philosophy of treating patients with a mind, body, and spirit approach. I chose to practice value-based care, which aligns with my lifestyle and preventative medicine approach .
I currently practice in a small family medicine–only clinic that is part of a larger multispecialty system. Primary care specialties in my organization are valued, respected and central to a patient’s well being and care. We are encouraged to spend time with patients, assess barriers to care and work collaboratively with our healthcare team, so that preventative medicine approaches take the lead in a patient’s health. This supportive culture and environment is one where my passion for food as medicine has thrived.
One day I forgot to pack a lunch and instead brought a grocery bag of items to make a salad. When I realized I made too much, I sent an email to my staff to get some “free salad in my office.” This serendipitous moment started an informal office “salad club” each week. Continued support from my staff and leadership, allowed me to consider further extending this teaching to my patients and my colleagues.
Three years ago, I helped adopt a sustainable plant-forward menu for our physician meetings, complete with a recipe from the menu for physicians to replicate at home or give to their patients.
I also pursued adoption of shared medical appointments for our medical group. These appointments apply the “see one, do one, teach one” model in medicine, but with culinary medicine as the focus.
Knowing that my patients are all connected to their families through food, I sought this as an opportunity to dive in further with wellness opportunities at their next meal. After almost 2 years of working on this project, I was able to host my first shared medical appointment with a group of patients on March 12, 2020. The next day schools closed, lockdowns occurred, and the world changed.
Opportunities highlighted by the pandemic
We always knew health care was broken but adding the increasingly longer hours and COVID vaccine–hesitant patients that the pandemic brought made everything look dark at times. What has helped me stay hopeful and energetic for system changes is feeling gratitude and seeking bright lights.
My experiences seeing patients in telehealth visits are examples of some of the bright lights I found in the pandemic. During these visits, patients showed me something from their pantry, and we’d go over nutritional labels together.
Additionally, my patients became engaged with their own conditions and wanted to improve them because of news articles highlighting risk factors for COVID-19, such as obesity. I had an active audience when it came to talking about food-as-medicine approaches to improving risk factors and immunity. And since everyone was listening, I didn’t stop at food. I also talked about physical health, stress resiliency, planetary diets, sleep, connections, and lastly vaccines!
Once the vaccines were distributed, I naturally gravitated to having those conversations with patients and colleagues and on social media. Plus, the pandemic gave us moments of simple times to slow down, take more rests, be less overscheduled, consider work-life priorities, and, lastly, to be okay with not being totally okay.
In practicing primary care, we have a unique role in seeing medicine from a whole body, whole person, whole family perspective. There is an opportunity to highlight what is broken in medicine and aim to make it whole.
I’m currently looking at shared medical appointments as a new standard way to provide care to all patients, because it improves access, provides better quality visits and aligns my values, mission, and purpose.
In the midst of the pandemic, I helped advocate for a sustainable plant-forward menu that was launched throughout four different hospitals in the Sharp HealthCare system, in California, in 2020. Knowing that patients were served a menu I played a role in, gave me solace.
As part of the hospital food and nutrition team, I am grateful for the opportunity I have to work on a broader mission to address social determinants of health and seek opportunities to help the system work for our patients.
Public health communication has been lacking in the pandemic, but another bright light is that we were still the trusted messengers to our patients and our communities. I’m continually honored and humbled to be trusted with a whole family’s health.
Dr. Neison practices family medicine and culinary medicine at Sharp Rees-Stealy Medical Group in San Diego, and is cochair of climate and planetary health for SRS Medical Group. You can follow her on Instagram, LinkedIn, and Facebook @Flavors4WellnessMD.
Omicron breakthrough cases boost protection, studies say
two preprint studies show.
The University of Washington, Seattle, working with Vir Biotechnology of San Francisco, looked at blood samples of vaccinated people who had breakthrough cases of Delta or Omicron and compared the samples with three other groups: people who caught COVID and were later vaccinated, vaccinated people who were never infected, and people who were infected and never vaccinated.
The vaccinated people who had a breakthrough case of Omicron produced antibodies that helped protect against coronavirus variants, whereas unvaccinated people who caught Omicron didn’t produce as many antibodies, the study showed.
BioNTech, the German biotechnology company, found that people who’d been double and triple vaccinated and then became infected with Omicron had a better B-cell response than people who’d gotten a booster shot but had not been infected.
The University of Washington research team also came up with similar findings about B cells.
The findings don’t mean people should deliberately try to become infected with COVID, said Alexandra Walls, PhD, one of the University of Washington scientists, according to Business Standard.
But the study does indicate “that we are at the point where we may want to consider having a different vaccine to boost people,” said David Veesler, PhD, of the University of Washington team.
“We should think about breakthrough infections as essentially equivalent to another dose of vaccine,” John Wherry, PhD, a professor and director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, told Business Standard. Dr. Wherry was not involved in the studies but reviewed the BioNTech study.
A version of this article first appeared on WebMD.com.
two preprint studies show.
The University of Washington, Seattle, working with Vir Biotechnology of San Francisco, looked at blood samples of vaccinated people who had breakthrough cases of Delta or Omicron and compared the samples with three other groups: people who caught COVID and were later vaccinated, vaccinated people who were never infected, and people who were infected and never vaccinated.
The vaccinated people who had a breakthrough case of Omicron produced antibodies that helped protect against coronavirus variants, whereas unvaccinated people who caught Omicron didn’t produce as many antibodies, the study showed.
BioNTech, the German biotechnology company, found that people who’d been double and triple vaccinated and then became infected with Omicron had a better B-cell response than people who’d gotten a booster shot but had not been infected.
The University of Washington research team also came up with similar findings about B cells.
The findings don’t mean people should deliberately try to become infected with COVID, said Alexandra Walls, PhD, one of the University of Washington scientists, according to Business Standard.
But the study does indicate “that we are at the point where we may want to consider having a different vaccine to boost people,” said David Veesler, PhD, of the University of Washington team.
“We should think about breakthrough infections as essentially equivalent to another dose of vaccine,” John Wherry, PhD, a professor and director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, told Business Standard. Dr. Wherry was not involved in the studies but reviewed the BioNTech study.
A version of this article first appeared on WebMD.com.
two preprint studies show.
The University of Washington, Seattle, working with Vir Biotechnology of San Francisco, looked at blood samples of vaccinated people who had breakthrough cases of Delta or Omicron and compared the samples with three other groups: people who caught COVID and were later vaccinated, vaccinated people who were never infected, and people who were infected and never vaccinated.
The vaccinated people who had a breakthrough case of Omicron produced antibodies that helped protect against coronavirus variants, whereas unvaccinated people who caught Omicron didn’t produce as many antibodies, the study showed.
BioNTech, the German biotechnology company, found that people who’d been double and triple vaccinated and then became infected with Omicron had a better B-cell response than people who’d gotten a booster shot but had not been infected.
The University of Washington research team also came up with similar findings about B cells.
The findings don’t mean people should deliberately try to become infected with COVID, said Alexandra Walls, PhD, one of the University of Washington scientists, according to Business Standard.
But the study does indicate “that we are at the point where we may want to consider having a different vaccine to boost people,” said David Veesler, PhD, of the University of Washington team.
“We should think about breakthrough infections as essentially equivalent to another dose of vaccine,” John Wherry, PhD, a professor and director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, told Business Standard. Dr. Wherry was not involved in the studies but reviewed the BioNTech study.
A version of this article first appeared on WebMD.com.






