User login
Combination Therapy of Tetracycline and Tacrolimus Resulting in Rapid Resolution of Steroid-Induced Periocular Rosacea
Skin Reaction Following Immunization With Smallpox Vaccine: A Personal Perspective
Smallpox is caused by the variola virus and is capable of causing serious complications. The disease is highly contagious and may present in several forms. The variola major form has a case fatality rate of about 30%.1 The smallpox vaccine was the first immunization used in modern medicine. The first inoculation was given in 1796 by Edward Jenner, MD, who immunized an 8-year-old boy with material taken from cowpox. The inoculation was shown to protect the child from smallpox when he was inoculated 6 weeks later with material that was taken from a smallpox pustule. Since then, mass immunization against smallpox has become a well-established method of fighting the disease. Immunization is based on epidermal inoculation of live vaccinia virus, another poxvirus that provokes immunization against smallpox but does not have the ability to cause the disease. The inoculation method includes putting a droplet of vaccine on the skin and then superficially poking the skin sufficiently to cause minimal blood drops. Disease protection following immunization lasts about 10 years.2
Because humans are the only natural host of the variola virus, the World Health Organization proclaimed smallpox the first disease to be eradicated worldwide. This was accomplished in 1977, when the last naturally occurring case in the world occurred in Somalia. The eradication of smallpox made it possible to stop the use of the vaccine without the threat of natural recurrence of the disease. In many places around the world, immunization was stopped before 1977 because many years had gone by without any documented cases of smallpox. In the United States, the last case was documented in 1949, and routine smallpox immunization was discontinued in 1972. In Israel, it was discontinued in 1980.
Since the events of September 11, 2001, there have been major concerns about the possibility of bioterrorism using the variola virus, which had been kept in laboratories and could be used as an unconventional weapon by countries or militant groups. Because many individuals are not immunized against smallpox, either naturally or artificially, the potential of a major epidemic occurring is significant.3
Because routine immunization against smallpox was discontinued in the United States and Israel, entire populations are vulnerable to the disease. The possibility that Israel will be involved in war became stronger when the United States and its allies targeted Iraq as a country that develops weapons of mass destruction. The use of smallpox as a biological weapon is a major possibility.4
To prepare against smallpox, the Israeli authorities have decided on a 2-step plan. In the first step, emergency personnel are to be vaccinated. These include medical and paramedical personnel, as well as people in other emergency services. The second stage of the plan is to immunize the entire population of Israel if one case of smallpox is diagnosed.
The immunization of healthcare workers in the first stage has 2 purposes. First, medical and other emergency personnel are expected to deal with infected or potentially infected people and thus must be protected against the disease. Second, in case of an epidemic, serious life-threatening disease could be aborted by variola immunoglobulin. All those immunized prior to a smallpox epidemic are the potential donors of human gamma globulin against the disease.
Immunization of medical personnel in Israel started in September 2002. The immunization was voluntary and thus included only a portion of the potential recipients. I was immunized on September 24, 2002, at my workplace. Assuming that many practitioners do not have experience with the vaccine, I decided to keep a diary and take pictures of the changes at the site of the inoculation.
Case Report
On day 1, 3 drops of dried blood (1 mm each) appeared. There was no itching or redness. Days 2 and 3 were the same as day 1, except that the puncture spots became black. A round, red, 4-mm papule appeared on day 4. There was some itching, and the black puncture spots at the area of inoculation remained the same. The papule became a pustule 3 to 4 mm in diameter surrounded by a 2- to 3-mm rim of red papule on day 5, and itching intensified. On day 6, the pustule became 5 to 6 mm, and the red rim around it became 2 to 3 mm. The area was not painful, except when touched. Itching became more obvious, and there was some tenderness of the axillary lymph nodes, with minimal enlargement of the glands. The pustule became 6 to 7 mm, and the red rim became 3 to 4 mm, with an additional 1- to 2-mm pink rim by day 7. Headache was present.
By day 8, the pustule was 10 mm, and the 3 dark 1-mm original puncture spots were still present inside the pustular area. The pustule felt hard when touched. The red rim was 2 to 3 mm, with an additional induration of skin 10 mm in diameter around the rim. The area was somewhat itchy, and headache was present. There was no change on day 9 except that the dark spots within the dry pustule were more obvious, increasing to 2 mm each. The size of the pustule was still the same on day 10, although it was less elevated. Central umbilication was obvious. On day 11, the pustule became dryer, and the black spots inside were 2 to 3 mm. Redness around the pustule was still 2 to 3 mm and was more intense after showering. Some induration of skin remained around the pustule and red area, and it was mildly painful when touched. Itching was lessened, and there was no pain at the axillary lymph nodes (Figure 1). The dry crust began to peel on day 12. The central area was confluent black, and the red rim was 2 to 3 mm, with some itching. On day 13, the crust had no yellow color of dried pus, and the roof of the crust had peeled off completely. The exposed base of the crust was dark black and hard. One to 2 mm of the red rim was still present, with less surrounding induration. There was mild itching but no pain or discharge. Minimal change was seen on day 14, except that the lesion began to shrink slightly.
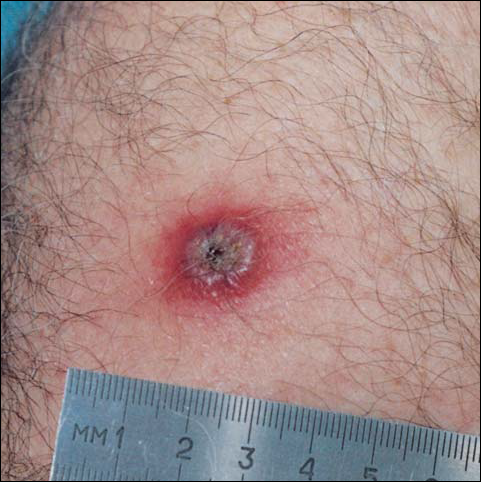
There was no pain on day 15, but the borders of the black crust began to separate from the red rim, and the red rim decreased to 1 to 2 mm. Itching became more intense, and the crust bled slightly at its border when touched. An adhesive bandage was put on the area. When the bandage was removed on day 16, it looked as if there was liquefaction of the crust, which had a yellowish color (Figure 2). The area was covered by gauze to enable air drying. On days 17 and 18, the crust was dry and brown, with no bleeding. It appeared smaller than the day before, the red rim had become pink, and there was mild itching (Figure 3). The skin above the pink rim began to peel on day 19, and there was no change in the scab. On one of the borders, there was a small discharge of serum. The scab fell off after showering (Figure 4). An ulcer 1- to 2-mm deep was obvious at the spot; the rim around the ulcer was red. The ulcer was smaller on day 20, and the rim around the ulcer was pink and still elevated above the surrounding normal skin. There was no discharge. On day 21, the base of the ulcer was dry and brown.
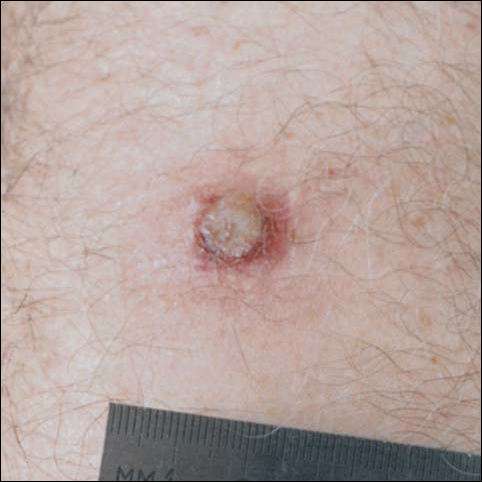


On day 22, the skin around the brown area was dry and pink, and the rim was 1 to 2 mm. Minimal changes were noted from days 23 to 27, and there was no itching. There was still no itching on day 28, and the red rim had disappeared. The central brown area peeled off, leaving a 4-mm pinkish red macule.
From days 29 to 75, the area gradually became pink. The skin looked thin and wrinkled, and there was no umbilication or elevation above the surrounding skin (Figure 5). No scar or induration was present, and symptoms had subsided.
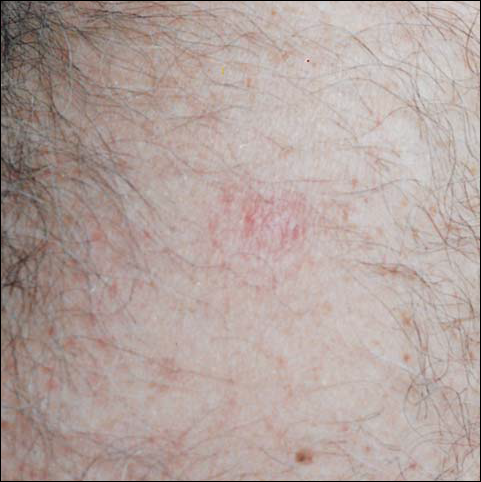
Comment
According to the US Centers for Disease Control and Prevention (CDC), if the smallpox vaccination is successful, a red and itchy bump will develop at the vaccine site in 3 to 4 days. In a week, the bump becomes a large blister, fills with pus, and begins to drain. During week 2, the blister begins to dry up, and a scab forms. The scab falls off during the third week, leaving a small scar.5 The described case follows the general description of the CDC. However, some differences should be mentioned. Itching at the site of vaccination was a major accompanying symptom. Swelling beyond the immediate site of skin reaction was obvious and lasted for more than a week. Axillary lymphadenopathy was noted. Discharge from the pustule was minimal. A scab was formed twice—once as the pus dried up and again after the first scab fell off and the drying of the wet base of the first scab dried up. At the end of the skin reaction, no scar remained.
Could the differences between the case described and the description by the CDC be explained by personal variation in skin reaction? Is the difference dependent on the skin care following the vaccination?
The instructions that were given to the Israeli recipients of the vaccine were to keep the area covered with loose gauze, change the gauze daily, not change regular skin washing habits, not touch the place of vaccination, and not put any topical preparation on the site. I followed the instructions except on day 15, when bleeding began as the scab started to peel off. At that point, a bandage was put over the area, thus limiting the ventilation and drying of the scab.
Complications following smallpox vaccination are relatively rare. Haim et al6 described an overall complication rate of 0.4 per 10,000 young army recruits. The complication rate was higher in primary recipients (those without previous exposure to the disease or vaccine).
Feery7 described an adverse skin reaction rate of 118 per 1 million recipients and a death rate of 1 to 5 per 1 million. The more serious skin reactions were eczema vaccinatum, progressive vaccinia, and neurologic and cardiac complications.
Monitoring the reaction to the vaccine is important in evaluating the intervention. Checking antibody titers postimmunization is possible. The micro–enzyme-linked immunosorbent assay technique was found to be more sensitive than plaque neutralization in finding a 4-fold increase in antibody titers.8 Postimmunization antibody checking is not done routinely, and it is not feasible when mass immunization is done over a short period as a crisis intervention.
As described, one of the reasons for the immunization of health workers in Israel is to build a pool of potential donors for variola immunoglobulin. The CDC description of a successful immunization includes the formation of a scar at the site of vaccination. Barghini9 has described an unusual reaction in an adult after revaccination against smallpox. In his case, there was a delay in the loss of the crust and a lack of cicatrix. In my case, crust appeared twice, and there is no remaining scar.
- Henderson DA, Inglesby TV, Bartlett JG, et al. Smallpox as a biological weapon: medical and public health management. JAMA. 1999;281:2127-2137.
- Katz L, Sagi R, Hurvitz A. Smallpox—past, present and future. Harefuah. 2002;141:43-50.
- Committee on infectious diseases. American Academy of Pediatrics. Smallpox vaccine. Pediatrics. 2002;110:841-845.
- Slater PE, Anis E, Leventhal A. Preparation for an outbreak of smallpox in Israel. Isr Med Assoc J. 2002;4:507-512.
- US Centers for Disease Control and Prevention. Smallpox vaccine overview.
Available at: http://www.bt.cdc.gov/agent/smallpox/vaccination/facts.asp
Accessed October 14, 2002. - Haim M, Gdalevich M, Mimouni D, et al. Adverse reactions to smallpox vaccine: the Israel defense force experience, 1991 to 1996. a comparison with previous surveys. Mil Med. 2000;165:287-289.
- Feery BJ. Adverse reaction after smallpox vaccination. Med J Aust. 1977;2:180-183.
- Lublin-Tennenbaum T, Katzenelson E, El-Ad B, et al. Correlation between cutaneous reaction in vaccines immunized against smallpox and antibody titer determined by plaque neutralization test and ELISA. Viral Immunol. 1990;3:19-25.
- Barghini G. Unusual reaction in an adult after revaccination against smallpox. Ann Sclavo. 1975;17:704-705.
Smallpox is caused by the variola virus and is capable of causing serious complications. The disease is highly contagious and may present in several forms. The variola major form has a case fatality rate of about 30%.1 The smallpox vaccine was the first immunization used in modern medicine. The first inoculation was given in 1796 by Edward Jenner, MD, who immunized an 8-year-old boy with material taken from cowpox. The inoculation was shown to protect the child from smallpox when he was inoculated 6 weeks later with material that was taken from a smallpox pustule. Since then, mass immunization against smallpox has become a well-established method of fighting the disease. Immunization is based on epidermal inoculation of live vaccinia virus, another poxvirus that provokes immunization against smallpox but does not have the ability to cause the disease. The inoculation method includes putting a droplet of vaccine on the skin and then superficially poking the skin sufficiently to cause minimal blood drops. Disease protection following immunization lasts about 10 years.2
Because humans are the only natural host of the variola virus, the World Health Organization proclaimed smallpox the first disease to be eradicated worldwide. This was accomplished in 1977, when the last naturally occurring case in the world occurred in Somalia. The eradication of smallpox made it possible to stop the use of the vaccine without the threat of natural recurrence of the disease. In many places around the world, immunization was stopped before 1977 because many years had gone by without any documented cases of smallpox. In the United States, the last case was documented in 1949, and routine smallpox immunization was discontinued in 1972. In Israel, it was discontinued in 1980.
Since the events of September 11, 2001, there have been major concerns about the possibility of bioterrorism using the variola virus, which had been kept in laboratories and could be used as an unconventional weapon by countries or militant groups. Because many individuals are not immunized against smallpox, either naturally or artificially, the potential of a major epidemic occurring is significant.3
Because routine immunization against smallpox was discontinued in the United States and Israel, entire populations are vulnerable to the disease. The possibility that Israel will be involved in war became stronger when the United States and its allies targeted Iraq as a country that develops weapons of mass destruction. The use of smallpox as a biological weapon is a major possibility.4
To prepare against smallpox, the Israeli authorities have decided on a 2-step plan. In the first step, emergency personnel are to be vaccinated. These include medical and paramedical personnel, as well as people in other emergency services. The second stage of the plan is to immunize the entire population of Israel if one case of smallpox is diagnosed.
The immunization of healthcare workers in the first stage has 2 purposes. First, medical and other emergency personnel are expected to deal with infected or potentially infected people and thus must be protected against the disease. Second, in case of an epidemic, serious life-threatening disease could be aborted by variola immunoglobulin. All those immunized prior to a smallpox epidemic are the potential donors of human gamma globulin against the disease.
Immunization of medical personnel in Israel started in September 2002. The immunization was voluntary and thus included only a portion of the potential recipients. I was immunized on September 24, 2002, at my workplace. Assuming that many practitioners do not have experience with the vaccine, I decided to keep a diary and take pictures of the changes at the site of the inoculation.
Case Report
On day 1, 3 drops of dried blood (1 mm each) appeared. There was no itching or redness. Days 2 and 3 were the same as day 1, except that the puncture spots became black. A round, red, 4-mm papule appeared on day 4. There was some itching, and the black puncture spots at the area of inoculation remained the same. The papule became a pustule 3 to 4 mm in diameter surrounded by a 2- to 3-mm rim of red papule on day 5, and itching intensified. On day 6, the pustule became 5 to 6 mm, and the red rim around it became 2 to 3 mm. The area was not painful, except when touched. Itching became more obvious, and there was some tenderness of the axillary lymph nodes, with minimal enlargement of the glands. The pustule became 6 to 7 mm, and the red rim became 3 to 4 mm, with an additional 1- to 2-mm pink rim by day 7. Headache was present.
By day 8, the pustule was 10 mm, and the 3 dark 1-mm original puncture spots were still present inside the pustular area. The pustule felt hard when touched. The red rim was 2 to 3 mm, with an additional induration of skin 10 mm in diameter around the rim. The area was somewhat itchy, and headache was present. There was no change on day 9 except that the dark spots within the dry pustule were more obvious, increasing to 2 mm each. The size of the pustule was still the same on day 10, although it was less elevated. Central umbilication was obvious. On day 11, the pustule became dryer, and the black spots inside were 2 to 3 mm. Redness around the pustule was still 2 to 3 mm and was more intense after showering. Some induration of skin remained around the pustule and red area, and it was mildly painful when touched. Itching was lessened, and there was no pain at the axillary lymph nodes (Figure 1). The dry crust began to peel on day 12. The central area was confluent black, and the red rim was 2 to 3 mm, with some itching. On day 13, the crust had no yellow color of dried pus, and the roof of the crust had peeled off completely. The exposed base of the crust was dark black and hard. One to 2 mm of the red rim was still present, with less surrounding induration. There was mild itching but no pain or discharge. Minimal change was seen on day 14, except that the lesion began to shrink slightly.

There was no pain on day 15, but the borders of the black crust began to separate from the red rim, and the red rim decreased to 1 to 2 mm. Itching became more intense, and the crust bled slightly at its border when touched. An adhesive bandage was put on the area. When the bandage was removed on day 16, it looked as if there was liquefaction of the crust, which had a yellowish color (Figure 2). The area was covered by gauze to enable air drying. On days 17 and 18, the crust was dry and brown, with no bleeding. It appeared smaller than the day before, the red rim had become pink, and there was mild itching (Figure 3). The skin above the pink rim began to peel on day 19, and there was no change in the scab. On one of the borders, there was a small discharge of serum. The scab fell off after showering (Figure 4). An ulcer 1- to 2-mm deep was obvious at the spot; the rim around the ulcer was red. The ulcer was smaller on day 20, and the rim around the ulcer was pink and still elevated above the surrounding normal skin. There was no discharge. On day 21, the base of the ulcer was dry and brown.



On day 22, the skin around the brown area was dry and pink, and the rim was 1 to 2 mm. Minimal changes were noted from days 23 to 27, and there was no itching. There was still no itching on day 28, and the red rim had disappeared. The central brown area peeled off, leaving a 4-mm pinkish red macule.
From days 29 to 75, the area gradually became pink. The skin looked thin and wrinkled, and there was no umbilication or elevation above the surrounding skin (Figure 5). No scar or induration was present, and symptoms had subsided.

Comment
According to the US Centers for Disease Control and Prevention (CDC), if the smallpox vaccination is successful, a red and itchy bump will develop at the vaccine site in 3 to 4 days. In a week, the bump becomes a large blister, fills with pus, and begins to drain. During week 2, the blister begins to dry up, and a scab forms. The scab falls off during the third week, leaving a small scar.5 The described case follows the general description of the CDC. However, some differences should be mentioned. Itching at the site of vaccination was a major accompanying symptom. Swelling beyond the immediate site of skin reaction was obvious and lasted for more than a week. Axillary lymphadenopathy was noted. Discharge from the pustule was minimal. A scab was formed twice—once as the pus dried up and again after the first scab fell off and the drying of the wet base of the first scab dried up. At the end of the skin reaction, no scar remained.
Could the differences between the case described and the description by the CDC be explained by personal variation in skin reaction? Is the difference dependent on the skin care following the vaccination?
The instructions that were given to the Israeli recipients of the vaccine were to keep the area covered with loose gauze, change the gauze daily, not change regular skin washing habits, not touch the place of vaccination, and not put any topical preparation on the site. I followed the instructions except on day 15, when bleeding began as the scab started to peel off. At that point, a bandage was put over the area, thus limiting the ventilation and drying of the scab.
Complications following smallpox vaccination are relatively rare. Haim et al6 described an overall complication rate of 0.4 per 10,000 young army recruits. The complication rate was higher in primary recipients (those without previous exposure to the disease or vaccine).
Feery7 described an adverse skin reaction rate of 118 per 1 million recipients and a death rate of 1 to 5 per 1 million. The more serious skin reactions were eczema vaccinatum, progressive vaccinia, and neurologic and cardiac complications.
Monitoring the reaction to the vaccine is important in evaluating the intervention. Checking antibody titers postimmunization is possible. The micro–enzyme-linked immunosorbent assay technique was found to be more sensitive than plaque neutralization in finding a 4-fold increase in antibody titers.8 Postimmunization antibody checking is not done routinely, and it is not feasible when mass immunization is done over a short period as a crisis intervention.
As described, one of the reasons for the immunization of health workers in Israel is to build a pool of potential donors for variola immunoglobulin. The CDC description of a successful immunization includes the formation of a scar at the site of vaccination. Barghini9 has described an unusual reaction in an adult after revaccination against smallpox. In his case, there was a delay in the loss of the crust and a lack of cicatrix. In my case, crust appeared twice, and there is no remaining scar.
Smallpox is caused by the variola virus and is capable of causing serious complications. The disease is highly contagious and may present in several forms. The variola major form has a case fatality rate of about 30%.1 The smallpox vaccine was the first immunization used in modern medicine. The first inoculation was given in 1796 by Edward Jenner, MD, who immunized an 8-year-old boy with material taken from cowpox. The inoculation was shown to protect the child from smallpox when he was inoculated 6 weeks later with material that was taken from a smallpox pustule. Since then, mass immunization against smallpox has become a well-established method of fighting the disease. Immunization is based on epidermal inoculation of live vaccinia virus, another poxvirus that provokes immunization against smallpox but does not have the ability to cause the disease. The inoculation method includes putting a droplet of vaccine on the skin and then superficially poking the skin sufficiently to cause minimal blood drops. Disease protection following immunization lasts about 10 years.2
Because humans are the only natural host of the variola virus, the World Health Organization proclaimed smallpox the first disease to be eradicated worldwide. This was accomplished in 1977, when the last naturally occurring case in the world occurred in Somalia. The eradication of smallpox made it possible to stop the use of the vaccine without the threat of natural recurrence of the disease. In many places around the world, immunization was stopped before 1977 because many years had gone by without any documented cases of smallpox. In the United States, the last case was documented in 1949, and routine smallpox immunization was discontinued in 1972. In Israel, it was discontinued in 1980.
Since the events of September 11, 2001, there have been major concerns about the possibility of bioterrorism using the variola virus, which had been kept in laboratories and could be used as an unconventional weapon by countries or militant groups. Because many individuals are not immunized against smallpox, either naturally or artificially, the potential of a major epidemic occurring is significant.3
Because routine immunization against smallpox was discontinued in the United States and Israel, entire populations are vulnerable to the disease. The possibility that Israel will be involved in war became stronger when the United States and its allies targeted Iraq as a country that develops weapons of mass destruction. The use of smallpox as a biological weapon is a major possibility.4
To prepare against smallpox, the Israeli authorities have decided on a 2-step plan. In the first step, emergency personnel are to be vaccinated. These include medical and paramedical personnel, as well as people in other emergency services. The second stage of the plan is to immunize the entire population of Israel if one case of smallpox is diagnosed.
The immunization of healthcare workers in the first stage has 2 purposes. First, medical and other emergency personnel are expected to deal with infected or potentially infected people and thus must be protected against the disease. Second, in case of an epidemic, serious life-threatening disease could be aborted by variola immunoglobulin. All those immunized prior to a smallpox epidemic are the potential donors of human gamma globulin against the disease.
Immunization of medical personnel in Israel started in September 2002. The immunization was voluntary and thus included only a portion of the potential recipients. I was immunized on September 24, 2002, at my workplace. Assuming that many practitioners do not have experience with the vaccine, I decided to keep a diary and take pictures of the changes at the site of the inoculation.
Case Report
On day 1, 3 drops of dried blood (1 mm each) appeared. There was no itching or redness. Days 2 and 3 were the same as day 1, except that the puncture spots became black. A round, red, 4-mm papule appeared on day 4. There was some itching, and the black puncture spots at the area of inoculation remained the same. The papule became a pustule 3 to 4 mm in diameter surrounded by a 2- to 3-mm rim of red papule on day 5, and itching intensified. On day 6, the pustule became 5 to 6 mm, and the red rim around it became 2 to 3 mm. The area was not painful, except when touched. Itching became more obvious, and there was some tenderness of the axillary lymph nodes, with minimal enlargement of the glands. The pustule became 6 to 7 mm, and the red rim became 3 to 4 mm, with an additional 1- to 2-mm pink rim by day 7. Headache was present.
By day 8, the pustule was 10 mm, and the 3 dark 1-mm original puncture spots were still present inside the pustular area. The pustule felt hard when touched. The red rim was 2 to 3 mm, with an additional induration of skin 10 mm in diameter around the rim. The area was somewhat itchy, and headache was present. There was no change on day 9 except that the dark spots within the dry pustule were more obvious, increasing to 2 mm each. The size of the pustule was still the same on day 10, although it was less elevated. Central umbilication was obvious. On day 11, the pustule became dryer, and the black spots inside were 2 to 3 mm. Redness around the pustule was still 2 to 3 mm and was more intense after showering. Some induration of skin remained around the pustule and red area, and it was mildly painful when touched. Itching was lessened, and there was no pain at the axillary lymph nodes (Figure 1). The dry crust began to peel on day 12. The central area was confluent black, and the red rim was 2 to 3 mm, with some itching. On day 13, the crust had no yellow color of dried pus, and the roof of the crust had peeled off completely. The exposed base of the crust was dark black and hard. One to 2 mm of the red rim was still present, with less surrounding induration. There was mild itching but no pain or discharge. Minimal change was seen on day 14, except that the lesion began to shrink slightly.

There was no pain on day 15, but the borders of the black crust began to separate from the red rim, and the red rim decreased to 1 to 2 mm. Itching became more intense, and the crust bled slightly at its border when touched. An adhesive bandage was put on the area. When the bandage was removed on day 16, it looked as if there was liquefaction of the crust, which had a yellowish color (Figure 2). The area was covered by gauze to enable air drying. On days 17 and 18, the crust was dry and brown, with no bleeding. It appeared smaller than the day before, the red rim had become pink, and there was mild itching (Figure 3). The skin above the pink rim began to peel on day 19, and there was no change in the scab. On one of the borders, there was a small discharge of serum. The scab fell off after showering (Figure 4). An ulcer 1- to 2-mm deep was obvious at the spot; the rim around the ulcer was red. The ulcer was smaller on day 20, and the rim around the ulcer was pink and still elevated above the surrounding normal skin. There was no discharge. On day 21, the base of the ulcer was dry and brown.



On day 22, the skin around the brown area was dry and pink, and the rim was 1 to 2 mm. Minimal changes were noted from days 23 to 27, and there was no itching. There was still no itching on day 28, and the red rim had disappeared. The central brown area peeled off, leaving a 4-mm pinkish red macule.
From days 29 to 75, the area gradually became pink. The skin looked thin and wrinkled, and there was no umbilication or elevation above the surrounding skin (Figure 5). No scar or induration was present, and symptoms had subsided.

Comment
According to the US Centers for Disease Control and Prevention (CDC), if the smallpox vaccination is successful, a red and itchy bump will develop at the vaccine site in 3 to 4 days. In a week, the bump becomes a large blister, fills with pus, and begins to drain. During week 2, the blister begins to dry up, and a scab forms. The scab falls off during the third week, leaving a small scar.5 The described case follows the general description of the CDC. However, some differences should be mentioned. Itching at the site of vaccination was a major accompanying symptom. Swelling beyond the immediate site of skin reaction was obvious and lasted for more than a week. Axillary lymphadenopathy was noted. Discharge from the pustule was minimal. A scab was formed twice—once as the pus dried up and again after the first scab fell off and the drying of the wet base of the first scab dried up. At the end of the skin reaction, no scar remained.
Could the differences between the case described and the description by the CDC be explained by personal variation in skin reaction? Is the difference dependent on the skin care following the vaccination?
The instructions that were given to the Israeli recipients of the vaccine were to keep the area covered with loose gauze, change the gauze daily, not change regular skin washing habits, not touch the place of vaccination, and not put any topical preparation on the site. I followed the instructions except on day 15, when bleeding began as the scab started to peel off. At that point, a bandage was put over the area, thus limiting the ventilation and drying of the scab.
Complications following smallpox vaccination are relatively rare. Haim et al6 described an overall complication rate of 0.4 per 10,000 young army recruits. The complication rate was higher in primary recipients (those without previous exposure to the disease or vaccine).
Feery7 described an adverse skin reaction rate of 118 per 1 million recipients and a death rate of 1 to 5 per 1 million. The more serious skin reactions were eczema vaccinatum, progressive vaccinia, and neurologic and cardiac complications.
Monitoring the reaction to the vaccine is important in evaluating the intervention. Checking antibody titers postimmunization is possible. The micro–enzyme-linked immunosorbent assay technique was found to be more sensitive than plaque neutralization in finding a 4-fold increase in antibody titers.8 Postimmunization antibody checking is not done routinely, and it is not feasible when mass immunization is done over a short period as a crisis intervention.
As described, one of the reasons for the immunization of health workers in Israel is to build a pool of potential donors for variola immunoglobulin. The CDC description of a successful immunization includes the formation of a scar at the site of vaccination. Barghini9 has described an unusual reaction in an adult after revaccination against smallpox. In his case, there was a delay in the loss of the crust and a lack of cicatrix. In my case, crust appeared twice, and there is no remaining scar.
- Henderson DA, Inglesby TV, Bartlett JG, et al. Smallpox as a biological weapon: medical and public health management. JAMA. 1999;281:2127-2137.
- Katz L, Sagi R, Hurvitz A. Smallpox—past, present and future. Harefuah. 2002;141:43-50.
- Committee on infectious diseases. American Academy of Pediatrics. Smallpox vaccine. Pediatrics. 2002;110:841-845.
- Slater PE, Anis E, Leventhal A. Preparation for an outbreak of smallpox in Israel. Isr Med Assoc J. 2002;4:507-512.
- US Centers for Disease Control and Prevention. Smallpox vaccine overview.
Available at: http://www.bt.cdc.gov/agent/smallpox/vaccination/facts.asp
Accessed October 14, 2002. - Haim M, Gdalevich M, Mimouni D, et al. Adverse reactions to smallpox vaccine: the Israel defense force experience, 1991 to 1996. a comparison with previous surveys. Mil Med. 2000;165:287-289.
- Feery BJ. Adverse reaction after smallpox vaccination. Med J Aust. 1977;2:180-183.
- Lublin-Tennenbaum T, Katzenelson E, El-Ad B, et al. Correlation between cutaneous reaction in vaccines immunized against smallpox and antibody titer determined by plaque neutralization test and ELISA. Viral Immunol. 1990;3:19-25.
- Barghini G. Unusual reaction in an adult after revaccination against smallpox. Ann Sclavo. 1975;17:704-705.
- Henderson DA, Inglesby TV, Bartlett JG, et al. Smallpox as a biological weapon: medical and public health management. JAMA. 1999;281:2127-2137.
- Katz L, Sagi R, Hurvitz A. Smallpox—past, present and future. Harefuah. 2002;141:43-50.
- Committee on infectious diseases. American Academy of Pediatrics. Smallpox vaccine. Pediatrics. 2002;110:841-845.
- Slater PE, Anis E, Leventhal A. Preparation for an outbreak of smallpox in Israel. Isr Med Assoc J. 2002;4:507-512.
- US Centers for Disease Control and Prevention. Smallpox vaccine overview.
Available at: http://www.bt.cdc.gov/agent/smallpox/vaccination/facts.asp
Accessed October 14, 2002. - Haim M, Gdalevich M, Mimouni D, et al. Adverse reactions to smallpox vaccine: the Israel defense force experience, 1991 to 1996. a comparison with previous surveys. Mil Med. 2000;165:287-289.
- Feery BJ. Adverse reaction after smallpox vaccination. Med J Aust. 1977;2:180-183.
- Lublin-Tennenbaum T, Katzenelson E, El-Ad B, et al. Correlation between cutaneous reaction in vaccines immunized against smallpox and antibody titer determined by plaque neutralization test and ELISA. Viral Immunol. 1990;3:19-25.
- Barghini G. Unusual reaction in an adult after revaccination against smallpox. Ann Sclavo. 1975;17:704-705.
What's Eating You? Megalopyge opercularis
Pet Hamsters as a Source of Rat Mite Dermatitis
Rat mite dermatitis is characterized by pruritic papules in a patient exposed to the tropical rat mite Ornithonyssus bacoti. We report a case of a woman with rat mite dermatitis who developed this eruption after exposure to her pet hamster. Mites were collected from the hamster and identified as O bacoti. Reported sources of rat mites, as well as avian mites and other mites that bite humans, are reviewed.
Rat mite dermatitis is a pruritic eruption in humans caused by bites from the tropical rat mite Ornithonyssus bacoti. Other biting mite species that have been reported to cause a similar dermatitis in humans include Dermanyssus gallinae (red mite or poultry mite), Ornithonyssus sylviarum (northern fowl mite), and Ornithonyssus bursa (tropical fowl mite).1-6 The eruptions caused by these mites are clinically indistinguishable. Initial case reports of mite dermatitis identified the sources of these mites to be rat-infested homes or bird nests around the home.1-6 Rat mite dermatitis also was reported in a patient who had contact with mite-infested laboratory mice.7 More recently, avian mite dermatitis was reported in patients who had mite-infested pet gerbils.8 This report describes a patient with rat mite dermatitis acquired from a pet hamster. Based on the variety of mites and sources of infestations, mite dermatitis may be more common than generally thought. back to top
CASE REPORT
A 48-year-old healthy woman presented with a complaint of pruritic papules on the wrists (Figure 1) and waist for several weeks. History revealed she maintained a small menagerie of animals including horses, dogs, cats, and hamsters. She was informed that her skin lesions were most likely the result of insect bites and she should evaluate her animals and their environment for evidence of infestation. She returned 2 days later and reported that her hamster had died the previous day. When she went to bury it, she noticed numerous red specks in its fur. She placed the hamster in a plastic bag in the freezer until she could bring it in for examination. Examination of the hamster (Figure 2) revealed numerous red mites (Figure 3). The patient's symptoms resolved over the following few weeks. The mites were identified as the tropical rat mite O bacoti. No necropsy was performed on the hamster, and a specific cause of death was never determined. This mite ingests blood and can cause debility, anemia, decreased reproduction, and death in small animals, suggesting that it may have contributed to the hamster's death.
Comment
Mites are arthropods in the class Arachnida, which includes ticks, spiders, and scorpions. The arachnids are characterized by 4 pairs of legs and 2 body regions, a cephalothorax and an abdomen. Mites and ticks are further classified in the subclass Acari. Mites of medical importance can be grouped by their pathology in humans.9 House dust mites (Dermatophagoides and Euroglyphus ssp) cause respiratory allergies, whereas human follicle mites (Demodex spp) infest hair follicles and associated sebaceous glands. Neither group of mites causes cutaneous lesions in the form of bites or burrows. The scabies mite (Sarcoptes scabiei) is a primary human parasitic mite in which the adult mite burrows and feeds on skin cells. Chiggers (family Trombiculidae) and common animal mites bite humans but do not reside on humans as a primary host. Many mite species are opportunistic, often feeding on various hosts they encounter.10 The common animal mites that bite humans include several avian mites, the rodent mites, and fur mites of rabbits (Cheyletiella parasitivorax), dogs (Cheyletiella yasguri), and cats (Cheyletiella blakei). Mites infesting grain, hay, and straw occasionally cause dermatitis in humans.
The usual hosts of the tropical rat mite O bacoti are the brown rat (Rattus norvegicus) and the black rat (Rattus rattus). This mite is yellow to dark red, when blood-fed, and ranges in size from 0.75 to 1.4 mm. It will feed on humans when its rodent hosts are killed or abandon their nests.11-16 O bacoti also infests mice and hamsters in research laboratories.7
The common avian mites D gallinae, O sylviarum, and O bursa occur on both domestic and wild bird species, including chickens, ducks, pigeons, sparrows, canaries, starlings, robins, tiger finches, and doves.1 D gallinae has been identified on commensal and laboratory rodents, and in one case it was found on a farm dog.17-19 The species are similar in size and appearance, but differ in their life cycle. The adult mite of these species ranges in color from brown to red and in size from 1 to 3 mm. D gallinae lives most of its life cycle off the hosts in nests, crevices, and cracks in buildings. It feeds on the host nocturnally for 1 to 2 hours at a time, and may live up to 8 months without a host. O sylviarum and O bursa spend their entire life cycle on the host. The mites will leave the host and bite humans in close proximity, especially in heavily infested quarters. The Ornithonyssus species live only 2 to 3 weeks without a host.9
Mite bites typically produce urticarial, pruritic papules on the skin. These papules result from an inflammatory cutaneous reaction to mite saliva as it takes a blood meal. Clinically, the bites are nonspecific, but pruritus is the most consistent feature. The lesions may be vesicular, urticarial, eczematous, or any combination of these. Secondary lesions such as persistent nodules, postinflammatory hyperpigmentation, excoriations, and secondary infection may be present. The bites tend to occur in asymmetric groups, most commonly on the abdomen and extremities. Often a patient presents with a combination of these clinical features, but denies a history of any "bites."
On pathologic examination, the lesions are nonspecific mild arthropod reactions with superficial and mid-dermal perivascular infiltrate. Eosinophils may be present. The epidermis may be mildly spongiotic.
The diagnosis of mite dermatitis should be considered in unexplained pruritic dermatitis. The rodent and avian mites are rarely found on the human host because the mites leave after feeding. History of exposure can include bird handling, bird nests or roosts near the home, rat infestations, pets, and occupational exposure to laboratory rodents. The mite may be discovered in abandoned bird or rodent nests, on pets, or in pet bedding. Speciation of the mite usually requires assistance of an entomologist or acarologist. To facilitate microscopic identification of mites, specimens can be temporarily slide-mounted in a drop of mineral oil under a coverglass. However, if they are to be sent to an acarologist or other specialist for identification, it is best to place them in 70% alcohol, rather than mineral oil. It is very difficult to remove mineral oil from mite specimens before clearing and slide-mounting them in other appropriate mounting media. This may be required to discern fine structural details needed for making species determinations.
Cheyletiella mite dermatitis also may present as a nonspecific pruritic dermatitis. These mites are parasitic on dogs, cats, and rabbits and may be discovered on the pet as "walking dandruff."20
Bites from chiggers, or red bugs, may be distinguished from other mites by the location of bites at sites of clothing constriction, such as the waistline, sock line, and beneath undergarments. These bites typically appear as papules with hemorrhagic puncta.
The scabies mite burrows in the skin and thus can be distinguished from other mites that cause dermatitis. In addition, scabies may be distinguished clinically by lesions in the interdigital web spaces and on the genitals. The mite, its eggs, and its feces can be visualized by a routine scabies preparation during the patient visit.
Other arthropod bites, including those from fleas, human body lice, and pubic lice, should be included in the differential diagnosis of mite dermatitis. In addition, systemic pruritus with excoriations, drug hypersensitivity reaction, and neurodermatitis should be considered.
Treatment focuses on reducing or eliminating problem mites in infested areas, often requiring involvement of veterinarians and pest control agencies. In the case of D gallinae, which does not live on the host, acaricides must penetrate into crevices and cracks in buildings.21 Both the host and the area of infestation must be treated to exterminate O sylviarum and O bursa. Elimination of rats and removal of their nests are important for controlling O bacoti.22 Patients may be treated with antihistamines and topical corticosteroids for symptomatic relief. The dermatitis is self-limited when the exposure is eliminated.
Two important mite-borne diseases of humans are tsutsugamushi disease (scrub typhus) and rickettsialpox, caused by the rickettsial organisms Orienta tsutsugamushi and Rickettsia akari, respectively. In the case of tsutsugamushi disease, chiggers are the vectors, whereas in rickettsialpox, the vector is the house-mouse mite (Liponyssoides sanguineus). These are the only 2 groups of mites that play a significant role in transmission of human pathogens.23
Mite dermatitis should be considered in any unexplained dermatitis. When considering a diagnosis of mite dermatitis, it is important to determine if there is a history of exposure to mice, hamsters, other rodents, or birds. Although the mites are rarely found on the patient, they may be discovered around the home or on pets. Demonstrating the presence of mites is important in diagnosing cases of mite-induced dermatitis. In many cases, reliable identification of the mite species is important in not only confirming the diagnosis but also identifying the sources of mite infestations so that they can be eliminated. The diagnosis should not be overlooked simply because the patient denies having rats or birds in the home.
- Schulze KE, Cohen PR. Dove-associated gamasoidosis: a case of avian mite dermatitis. J Am Acad Derm. 1994;30:278-280.
- Hidano A, Asanuma K. Acariasis caused by bird mites. Arch Dermatol. 1976;112:882-883.
- Gupta AK, Billings JK, Ellis CN. Chronic pruritus: an uncommon cause. avian mite dermatitis caused by Ornithonyssus sylviarum (Northern fowl mite). Arch Dermatol. 1988;124:1105-1106.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Aiba S, Suetake T, Tagami H. Multiple infestations with avian mites within a family. Int J Dermatol. 1994;33:566-567.
- Lodha KR. The occurrence of tropical fowl mite, Ornithonyssus (Bdellonyssus, Liponyssus) bursa on man in Rajasthan (India). Vet Rec. 1969;84:363-365.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol. 1982;118:676-678.
- Lucky AW, Sayers C, Argus JD, et al. Avian mite bites acquired from a new source—pet gerbils: report of 2 cases and review of the literature. Arch Dermatol. 2001;137:167-170.
- Goddard J. Physician's Guide to Arthropods of Medical Importance. Boca Raton, Fla: CRC Press; 2000.
- Strickland GT. Hunter's Tropical Medicine and Emerging Infectious Diseases. Philadelphia, Pa: WB Saunders Co; 1991.
- Chung SL, Hwang SJ, Kwon SB, et al. Outbreak of rat mite dermatitis in medical students. Int J Dermatol. 1998;37:591-594.
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis. 1998;27:1465-1469.
- Theis J, Lavoipierre MM, LaPerriere R, et al. Tropical rat mite dermatitis. report of six cases and review of other mite infestations. Arch Dermatol. 1981;117:341-343.
- Charlesworth EN, Clegern RW. Tropical rat mite dermatitis. Arch Dermatol. 1977;133:937-939.
- Fishman HC. Rat mite dermatitis. Cutis. 1988;42:414-416.
- Hetherington GW, Holder WR, Smith EB. Rat mite dermatitis. JAMA. 1971;215:1499-1500.
- Bakr ME, Morsy TA, Nassef NE, et al. Mites infesting commensal rodents in Shebin El Kom, Menoufia G, Egypt. J Egypt Soc Parasitol. 1995;25:853-859.
- Durden LA, Turell MJ. Inefficient mechanical transmission of Langat (tick-borne encephalitis virus complex) virus by blood-feeding mites (Acari) to laboratory mice. J Med Entomol. 1993;30:639-641.
- Ramsay GW, Mason PC, Hunter AC. Letter: chicken mite (Dermanyssus gallinae) infesting a dog. N Z Vet J. 1975;23:155-156.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:130-133.
- Chauve C. Th
Rat mite dermatitis is characterized by pruritic papules in a patient exposed to the tropical rat mite Ornithonyssus bacoti. We report a case of a woman with rat mite dermatitis who developed this eruption after exposure to her pet hamster. Mites were collected from the hamster and identified as O bacoti. Reported sources of rat mites, as well as avian mites and other mites that bite humans, are reviewed.
Rat mite dermatitis is a pruritic eruption in humans caused by bites from the tropical rat mite Ornithonyssus bacoti. Other biting mite species that have been reported to cause a similar dermatitis in humans include Dermanyssus gallinae (red mite or poultry mite), Ornithonyssus sylviarum (northern fowl mite), and Ornithonyssus bursa (tropical fowl mite).1-6 The eruptions caused by these mites are clinically indistinguishable. Initial case reports of mite dermatitis identified the sources of these mites to be rat-infested homes or bird nests around the home.1-6 Rat mite dermatitis also was reported in a patient who had contact with mite-infested laboratory mice.7 More recently, avian mite dermatitis was reported in patients who had mite-infested pet gerbils.8 This report describes a patient with rat mite dermatitis acquired from a pet hamster. Based on the variety of mites and sources of infestations, mite dermatitis may be more common than generally thought. back to top
CASE REPORT
A 48-year-old healthy woman presented with a complaint of pruritic papules on the wrists (Figure 1) and waist for several weeks. History revealed she maintained a small menagerie of animals including horses, dogs, cats, and hamsters. She was informed that her skin lesions were most likely the result of insect bites and she should evaluate her animals and their environment for evidence of infestation. She returned 2 days later and reported that her hamster had died the previous day. When she went to bury it, she noticed numerous red specks in its fur. She placed the hamster in a plastic bag in the freezer until she could bring it in for examination. Examination of the hamster (Figure 2) revealed numerous red mites (Figure 3). The patient's symptoms resolved over the following few weeks. The mites were identified as the tropical rat mite O bacoti. No necropsy was performed on the hamster, and a specific cause of death was never determined. This mite ingests blood and can cause debility, anemia, decreased reproduction, and death in small animals, suggesting that it may have contributed to the hamster's death.
Comment
Mites are arthropods in the class Arachnida, which includes ticks, spiders, and scorpions. The arachnids are characterized by 4 pairs of legs and 2 body regions, a cephalothorax and an abdomen. Mites and ticks are further classified in the subclass Acari. Mites of medical importance can be grouped by their pathology in humans.9 House dust mites (Dermatophagoides and Euroglyphus ssp) cause respiratory allergies, whereas human follicle mites (Demodex spp) infest hair follicles and associated sebaceous glands. Neither group of mites causes cutaneous lesions in the form of bites or burrows. The scabies mite (Sarcoptes scabiei) is a primary human parasitic mite in which the adult mite burrows and feeds on skin cells. Chiggers (family Trombiculidae) and common animal mites bite humans but do not reside on humans as a primary host. Many mite species are opportunistic, often feeding on various hosts they encounter.10 The common animal mites that bite humans include several avian mites, the rodent mites, and fur mites of rabbits (Cheyletiella parasitivorax), dogs (Cheyletiella yasguri), and cats (Cheyletiella blakei). Mites infesting grain, hay, and straw occasionally cause dermatitis in humans.
The usual hosts of the tropical rat mite O bacoti are the brown rat (Rattus norvegicus) and the black rat (Rattus rattus). This mite is yellow to dark red, when blood-fed, and ranges in size from 0.75 to 1.4 mm. It will feed on humans when its rodent hosts are killed or abandon their nests.11-16 O bacoti also infests mice and hamsters in research laboratories.7
The common avian mites D gallinae, O sylviarum, and O bursa occur on both domestic and wild bird species, including chickens, ducks, pigeons, sparrows, canaries, starlings, robins, tiger finches, and doves.1 D gallinae has been identified on commensal and laboratory rodents, and in one case it was found on a farm dog.17-19 The species are similar in size and appearance, but differ in their life cycle. The adult mite of these species ranges in color from brown to red and in size from 1 to 3 mm. D gallinae lives most of its life cycle off the hosts in nests, crevices, and cracks in buildings. It feeds on the host nocturnally for 1 to 2 hours at a time, and may live up to 8 months without a host. O sylviarum and O bursa spend their entire life cycle on the host. The mites will leave the host and bite humans in close proximity, especially in heavily infested quarters. The Ornithonyssus species live only 2 to 3 weeks without a host.9
Mite bites typically produce urticarial, pruritic papules on the skin. These papules result from an inflammatory cutaneous reaction to mite saliva as it takes a blood meal. Clinically, the bites are nonspecific, but pruritus is the most consistent feature. The lesions may be vesicular, urticarial, eczematous, or any combination of these. Secondary lesions such as persistent nodules, postinflammatory hyperpigmentation, excoriations, and secondary infection may be present. The bites tend to occur in asymmetric groups, most commonly on the abdomen and extremities. Often a patient presents with a combination of these clinical features, but denies a history of any "bites."
On pathologic examination, the lesions are nonspecific mild arthropod reactions with superficial and mid-dermal perivascular infiltrate. Eosinophils may be present. The epidermis may be mildly spongiotic.
The diagnosis of mite dermatitis should be considered in unexplained pruritic dermatitis. The rodent and avian mites are rarely found on the human host because the mites leave after feeding. History of exposure can include bird handling, bird nests or roosts near the home, rat infestations, pets, and occupational exposure to laboratory rodents. The mite may be discovered in abandoned bird or rodent nests, on pets, or in pet bedding. Speciation of the mite usually requires assistance of an entomologist or acarologist. To facilitate microscopic identification of mites, specimens can be temporarily slide-mounted in a drop of mineral oil under a coverglass. However, if they are to be sent to an acarologist or other specialist for identification, it is best to place them in 70% alcohol, rather than mineral oil. It is very difficult to remove mineral oil from mite specimens before clearing and slide-mounting them in other appropriate mounting media. This may be required to discern fine structural details needed for making species determinations.
Cheyletiella mite dermatitis also may present as a nonspecific pruritic dermatitis. These mites are parasitic on dogs, cats, and rabbits and may be discovered on the pet as "walking dandruff."20
Bites from chiggers, or red bugs, may be distinguished from other mites by the location of bites at sites of clothing constriction, such as the waistline, sock line, and beneath undergarments. These bites typically appear as papules with hemorrhagic puncta.
The scabies mite burrows in the skin and thus can be distinguished from other mites that cause dermatitis. In addition, scabies may be distinguished clinically by lesions in the interdigital web spaces and on the genitals. The mite, its eggs, and its feces can be visualized by a routine scabies preparation during the patient visit.
Other arthropod bites, including those from fleas, human body lice, and pubic lice, should be included in the differential diagnosis of mite dermatitis. In addition, systemic pruritus with excoriations, drug hypersensitivity reaction, and neurodermatitis should be considered.
Treatment focuses on reducing or eliminating problem mites in infested areas, often requiring involvement of veterinarians and pest control agencies. In the case of D gallinae, which does not live on the host, acaricides must penetrate into crevices and cracks in buildings.21 Both the host and the area of infestation must be treated to exterminate O sylviarum and O bursa. Elimination of rats and removal of their nests are important for controlling O bacoti.22 Patients may be treated with antihistamines and topical corticosteroids for symptomatic relief. The dermatitis is self-limited when the exposure is eliminated.
Two important mite-borne diseases of humans are tsutsugamushi disease (scrub typhus) and rickettsialpox, caused by the rickettsial organisms Orienta tsutsugamushi and Rickettsia akari, respectively. In the case of tsutsugamushi disease, chiggers are the vectors, whereas in rickettsialpox, the vector is the house-mouse mite (Liponyssoides sanguineus). These are the only 2 groups of mites that play a significant role in transmission of human pathogens.23
Mite dermatitis should be considered in any unexplained dermatitis. When considering a diagnosis of mite dermatitis, it is important to determine if there is a history of exposure to mice, hamsters, other rodents, or birds. Although the mites are rarely found on the patient, they may be discovered around the home or on pets. Demonstrating the presence of mites is important in diagnosing cases of mite-induced dermatitis. In many cases, reliable identification of the mite species is important in not only confirming the diagnosis but also identifying the sources of mite infestations so that they can be eliminated. The diagnosis should not be overlooked simply because the patient denies having rats or birds in the home.
Rat mite dermatitis is characterized by pruritic papules in a patient exposed to the tropical rat mite Ornithonyssus bacoti. We report a case of a woman with rat mite dermatitis who developed this eruption after exposure to her pet hamster. Mites were collected from the hamster and identified as O bacoti. Reported sources of rat mites, as well as avian mites and other mites that bite humans, are reviewed.
Rat mite dermatitis is a pruritic eruption in humans caused by bites from the tropical rat mite Ornithonyssus bacoti. Other biting mite species that have been reported to cause a similar dermatitis in humans include Dermanyssus gallinae (red mite or poultry mite), Ornithonyssus sylviarum (northern fowl mite), and Ornithonyssus bursa (tropical fowl mite).1-6 The eruptions caused by these mites are clinically indistinguishable. Initial case reports of mite dermatitis identified the sources of these mites to be rat-infested homes or bird nests around the home.1-6 Rat mite dermatitis also was reported in a patient who had contact with mite-infested laboratory mice.7 More recently, avian mite dermatitis was reported in patients who had mite-infested pet gerbils.8 This report describes a patient with rat mite dermatitis acquired from a pet hamster. Based on the variety of mites and sources of infestations, mite dermatitis may be more common than generally thought. back to top
CASE REPORT
A 48-year-old healthy woman presented with a complaint of pruritic papules on the wrists (Figure 1) and waist for several weeks. History revealed she maintained a small menagerie of animals including horses, dogs, cats, and hamsters. She was informed that her skin lesions were most likely the result of insect bites and she should evaluate her animals and their environment for evidence of infestation. She returned 2 days later and reported that her hamster had died the previous day. When she went to bury it, she noticed numerous red specks in its fur. She placed the hamster in a plastic bag in the freezer until she could bring it in for examination. Examination of the hamster (Figure 2) revealed numerous red mites (Figure 3). The patient's symptoms resolved over the following few weeks. The mites were identified as the tropical rat mite O bacoti. No necropsy was performed on the hamster, and a specific cause of death was never determined. This mite ingests blood and can cause debility, anemia, decreased reproduction, and death in small animals, suggesting that it may have contributed to the hamster's death.
Comment
Mites are arthropods in the class Arachnida, which includes ticks, spiders, and scorpions. The arachnids are characterized by 4 pairs of legs and 2 body regions, a cephalothorax and an abdomen. Mites and ticks are further classified in the subclass Acari. Mites of medical importance can be grouped by their pathology in humans.9 House dust mites (Dermatophagoides and Euroglyphus ssp) cause respiratory allergies, whereas human follicle mites (Demodex spp) infest hair follicles and associated sebaceous glands. Neither group of mites causes cutaneous lesions in the form of bites or burrows. The scabies mite (Sarcoptes scabiei) is a primary human parasitic mite in which the adult mite burrows and feeds on skin cells. Chiggers (family Trombiculidae) and common animal mites bite humans but do not reside on humans as a primary host. Many mite species are opportunistic, often feeding on various hosts they encounter.10 The common animal mites that bite humans include several avian mites, the rodent mites, and fur mites of rabbits (Cheyletiella parasitivorax), dogs (Cheyletiella yasguri), and cats (Cheyletiella blakei). Mites infesting grain, hay, and straw occasionally cause dermatitis in humans.
The usual hosts of the tropical rat mite O bacoti are the brown rat (Rattus norvegicus) and the black rat (Rattus rattus). This mite is yellow to dark red, when blood-fed, and ranges in size from 0.75 to 1.4 mm. It will feed on humans when its rodent hosts are killed or abandon their nests.11-16 O bacoti also infests mice and hamsters in research laboratories.7
The common avian mites D gallinae, O sylviarum, and O bursa occur on both domestic and wild bird species, including chickens, ducks, pigeons, sparrows, canaries, starlings, robins, tiger finches, and doves.1 D gallinae has been identified on commensal and laboratory rodents, and in one case it was found on a farm dog.17-19 The species are similar in size and appearance, but differ in their life cycle. The adult mite of these species ranges in color from brown to red and in size from 1 to 3 mm. D gallinae lives most of its life cycle off the hosts in nests, crevices, and cracks in buildings. It feeds on the host nocturnally for 1 to 2 hours at a time, and may live up to 8 months without a host. O sylviarum and O bursa spend their entire life cycle on the host. The mites will leave the host and bite humans in close proximity, especially in heavily infested quarters. The Ornithonyssus species live only 2 to 3 weeks without a host.9
Mite bites typically produce urticarial, pruritic papules on the skin. These papules result from an inflammatory cutaneous reaction to mite saliva as it takes a blood meal. Clinically, the bites are nonspecific, but pruritus is the most consistent feature. The lesions may be vesicular, urticarial, eczematous, or any combination of these. Secondary lesions such as persistent nodules, postinflammatory hyperpigmentation, excoriations, and secondary infection may be present. The bites tend to occur in asymmetric groups, most commonly on the abdomen and extremities. Often a patient presents with a combination of these clinical features, but denies a history of any "bites."
On pathologic examination, the lesions are nonspecific mild arthropod reactions with superficial and mid-dermal perivascular infiltrate. Eosinophils may be present. The epidermis may be mildly spongiotic.
The diagnosis of mite dermatitis should be considered in unexplained pruritic dermatitis. The rodent and avian mites are rarely found on the human host because the mites leave after feeding. History of exposure can include bird handling, bird nests or roosts near the home, rat infestations, pets, and occupational exposure to laboratory rodents. The mite may be discovered in abandoned bird or rodent nests, on pets, or in pet bedding. Speciation of the mite usually requires assistance of an entomologist or acarologist. To facilitate microscopic identification of mites, specimens can be temporarily slide-mounted in a drop of mineral oil under a coverglass. However, if they are to be sent to an acarologist or other specialist for identification, it is best to place them in 70% alcohol, rather than mineral oil. It is very difficult to remove mineral oil from mite specimens before clearing and slide-mounting them in other appropriate mounting media. This may be required to discern fine structural details needed for making species determinations.
Cheyletiella mite dermatitis also may present as a nonspecific pruritic dermatitis. These mites are parasitic on dogs, cats, and rabbits and may be discovered on the pet as "walking dandruff."20
Bites from chiggers, or red bugs, may be distinguished from other mites by the location of bites at sites of clothing constriction, such as the waistline, sock line, and beneath undergarments. These bites typically appear as papules with hemorrhagic puncta.
The scabies mite burrows in the skin and thus can be distinguished from other mites that cause dermatitis. In addition, scabies may be distinguished clinically by lesions in the interdigital web spaces and on the genitals. The mite, its eggs, and its feces can be visualized by a routine scabies preparation during the patient visit.
Other arthropod bites, including those from fleas, human body lice, and pubic lice, should be included in the differential diagnosis of mite dermatitis. In addition, systemic pruritus with excoriations, drug hypersensitivity reaction, and neurodermatitis should be considered.
Treatment focuses on reducing or eliminating problem mites in infested areas, often requiring involvement of veterinarians and pest control agencies. In the case of D gallinae, which does not live on the host, acaricides must penetrate into crevices and cracks in buildings.21 Both the host and the area of infestation must be treated to exterminate O sylviarum and O bursa. Elimination of rats and removal of their nests are important for controlling O bacoti.22 Patients may be treated with antihistamines and topical corticosteroids for symptomatic relief. The dermatitis is self-limited when the exposure is eliminated.
Two important mite-borne diseases of humans are tsutsugamushi disease (scrub typhus) and rickettsialpox, caused by the rickettsial organisms Orienta tsutsugamushi and Rickettsia akari, respectively. In the case of tsutsugamushi disease, chiggers are the vectors, whereas in rickettsialpox, the vector is the house-mouse mite (Liponyssoides sanguineus). These are the only 2 groups of mites that play a significant role in transmission of human pathogens.23
Mite dermatitis should be considered in any unexplained dermatitis. When considering a diagnosis of mite dermatitis, it is important to determine if there is a history of exposure to mice, hamsters, other rodents, or birds. Although the mites are rarely found on the patient, they may be discovered around the home or on pets. Demonstrating the presence of mites is important in diagnosing cases of mite-induced dermatitis. In many cases, reliable identification of the mite species is important in not only confirming the diagnosis but also identifying the sources of mite infestations so that they can be eliminated. The diagnosis should not be overlooked simply because the patient denies having rats or birds in the home.
- Schulze KE, Cohen PR. Dove-associated gamasoidosis: a case of avian mite dermatitis. J Am Acad Derm. 1994;30:278-280.
- Hidano A, Asanuma K. Acariasis caused by bird mites. Arch Dermatol. 1976;112:882-883.
- Gupta AK, Billings JK, Ellis CN. Chronic pruritus: an uncommon cause. avian mite dermatitis caused by Ornithonyssus sylviarum (Northern fowl mite). Arch Dermatol. 1988;124:1105-1106.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Aiba S, Suetake T, Tagami H. Multiple infestations with avian mites within a family. Int J Dermatol. 1994;33:566-567.
- Lodha KR. The occurrence of tropical fowl mite, Ornithonyssus (Bdellonyssus, Liponyssus) bursa on man in Rajasthan (India). Vet Rec. 1969;84:363-365.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol. 1982;118:676-678.
- Lucky AW, Sayers C, Argus JD, et al. Avian mite bites acquired from a new source—pet gerbils: report of 2 cases and review of the literature. Arch Dermatol. 2001;137:167-170.
- Goddard J. Physician's Guide to Arthropods of Medical Importance. Boca Raton, Fla: CRC Press; 2000.
- Strickland GT. Hunter's Tropical Medicine and Emerging Infectious Diseases. Philadelphia, Pa: WB Saunders Co; 1991.
- Chung SL, Hwang SJ, Kwon SB, et al. Outbreak of rat mite dermatitis in medical students. Int J Dermatol. 1998;37:591-594.
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis. 1998;27:1465-1469.
- Theis J, Lavoipierre MM, LaPerriere R, et al. Tropical rat mite dermatitis. report of six cases and review of other mite infestations. Arch Dermatol. 1981;117:341-343.
- Charlesworth EN, Clegern RW. Tropical rat mite dermatitis. Arch Dermatol. 1977;133:937-939.
- Fishman HC. Rat mite dermatitis. Cutis. 1988;42:414-416.
- Hetherington GW, Holder WR, Smith EB. Rat mite dermatitis. JAMA. 1971;215:1499-1500.
- Bakr ME, Morsy TA, Nassef NE, et al. Mites infesting commensal rodents in Shebin El Kom, Menoufia G, Egypt. J Egypt Soc Parasitol. 1995;25:853-859.
- Durden LA, Turell MJ. Inefficient mechanical transmission of Langat (tick-borne encephalitis virus complex) virus by blood-feeding mites (Acari) to laboratory mice. J Med Entomol. 1993;30:639-641.
- Ramsay GW, Mason PC, Hunter AC. Letter: chicken mite (Dermanyssus gallinae) infesting a dog. N Z Vet J. 1975;23:155-156.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:130-133.
- Chauve C. Th
- Schulze KE, Cohen PR. Dove-associated gamasoidosis: a case of avian mite dermatitis. J Am Acad Derm. 1994;30:278-280.
- Hidano A, Asanuma K. Acariasis caused by bird mites. Arch Dermatol. 1976;112:882-883.
- Gupta AK, Billings JK, Ellis CN. Chronic pruritus: an uncommon cause. avian mite dermatitis caused by Ornithonyssus sylviarum (Northern fowl mite). Arch Dermatol. 1988;124:1105-1106.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Aiba S, Suetake T, Tagami H. Multiple infestations with avian mites within a family. Int J Dermatol. 1994;33:566-567.
- Lodha KR. The occurrence of tropical fowl mite, Ornithonyssus (Bdellonyssus, Liponyssus) bursa on man in Rajasthan (India). Vet Rec. 1969;84:363-365.
- Fox JG. Outbreak of tropical rat mite dermatitis in laboratory personnel. Arch Dermatol. 1982;118:676-678.
- Lucky AW, Sayers C, Argus JD, et al. Avian mite bites acquired from a new source—pet gerbils: report of 2 cases and review of the literature. Arch Dermatol. 2001;137:167-170.
- Goddard J. Physician's Guide to Arthropods of Medical Importance. Boca Raton, Fla: CRC Press; 2000.
- Strickland GT. Hunter's Tropical Medicine and Emerging Infectious Diseases. Philadelphia, Pa: WB Saunders Co; 1991.
- Chung SL, Hwang SJ, Kwon SB, et al. Outbreak of rat mite dermatitis in medical students. Int J Dermatol. 1998;37:591-594.
- Engel PM, Welzel J, Maass M, et al. Tropical rat mite dermatitis: case report and review. Clin Infect Dis. 1998;27:1465-1469.
- Theis J, Lavoipierre MM, LaPerriere R, et al. Tropical rat mite dermatitis. report of six cases and review of other mite infestations. Arch Dermatol. 1981;117:341-343.
- Charlesworth EN, Clegern RW. Tropical rat mite dermatitis. Arch Dermatol. 1977;133:937-939.
- Fishman HC. Rat mite dermatitis. Cutis. 1988;42:414-416.
- Hetherington GW, Holder WR, Smith EB. Rat mite dermatitis. JAMA. 1971;215:1499-1500.
- Bakr ME, Morsy TA, Nassef NE, et al. Mites infesting commensal rodents in Shebin El Kom, Menoufia G, Egypt. J Egypt Soc Parasitol. 1995;25:853-859.
- Durden LA, Turell MJ. Inefficient mechanical transmission of Langat (tick-borne encephalitis virus complex) virus by blood-feeding mites (Acari) to laboratory mice. J Med Entomol. 1993;30:639-641.
- Ramsay GW, Mason PC, Hunter AC. Letter: chicken mite (Dermanyssus gallinae) infesting a dog. N Z Vet J. 1975;23:155-156.
- Rivers JK, Martin J, Pukay B. Walking dandruff and Cheyletiella dermatitis. J Am Acad Dermatol. 1986;15:130-133.
- Chauve C. Th
Botanical Briefs: Tulips—Tulipa Species L.
Betel Quid–Induced Oral Lichen Planus: A Case Report
Epidemiology of Skin Diseases in People of Color
Trachyonychia: A Case Report and Review of Manifestations, Associations, and Treatments
Trachyonychia (“rough nails”) is best considered a reaction or morphologic pattern with a variety of clinical presentations and etiologies. It may involve only 1 or as many as 20 nails (20-nail dystrophy). It can be a manifestation of lichen planus, psoriasis, alopecia areata, immunoglobulin A deficiency, atopic dermatitis, and ichthyosis vulgaris. Nail matrix biopsy results and physical examination findings help in establishing the cause of this condition, though often trachyonychia is an isolated finding. When trachyonychia occurs in childhood as a manifestation of lichen planus, it tends to resolve with time. We review a case of trachyonychia, its association, its diagnostic evaluation, and treatment options.
Trachyonychia means "rough nails." This condition may involve only 1 or as many as 20 nails. It is best considered a reaction or morphologic pattern with a variety of clinical presentations and etiologies. Clinical presentations are rough nails with a sandpapered appearance and numerous small superficial pits that make the nails shiny1; onychorrhexis, onychoschizia, distal chipping, and yellow onychauxis of the great toenail; and closely arranged longitudinal ridges, distal notching, and layered splitting.2,3 Nail matrix biopsy results combined with clinical findings have linked trachyonychia with lichen planus generally,4 lichen planus in children,5 psoriasis,6 alopecia areata,7 IgA deficiency,8 atopic dermatitis,9 and ichthyosis vulgaris.10 The term 20-nail dystrophy of childhood11 refers to a trachyonychia variant likely caused by lichen planus. Some who consider the term a misnomer—in part because not all nails are necessarily involved—think that perhaps it should be abandoned.12 back to top
Case Report A 10-year-old girl presented with a 1-year history of worsening nail dystrophy. The patient had no history of psoriasis, atopic dermatitis, alopecia, or other skin disease, and family history was unremarkable. Except for dystrophy and hyperkeratosis identified on nails of both hands and both feet (Figure), physical examination findings were normal. Results of a fungal nail culture were negative, and the nail matrix biopsy specimen showed a bandlike lymphocytic infiltrate in the superficial dermis, with vacuolar alteration of the basal level. The diagnosis was trachyonychia secondary to lichen planus. Daily use of flurandrenolone tape and monthly intralesional injections of triamcinolone 2.5 mg/mL did not improve this patient's condition. After 4 months of injections in the distal nail folds, she was lost to follow-up.
Comment Often, the onset of trachyonychia is insidious. The condition usually develops on all nails simultaneously. Trachyonychia also can occur on individual nails over many months. Peak age of onset is 3 to 12 years. Trachyonychia occurs, however, in multigenerational families,13 in all age groups, in twins in the United States14 and Europe,15 in both sexes, and in all ethnic groups. This condition has been associated with ichthyosis vulgaris combined with alopecia universalis,16 ungual lichen planus and alopecia areata,17 koilonychia,18 primary biliary cirrhosis,19 and vitiligo.20 In chronic graft versus host (GVH) disease, trachyonychia can be an isolated finding21 or part of a constellation of cutaneous symptoms.22 It may be associated with dystrophy, atrophy, and, often, ulceration of the lunula.23 In the proper setting, the nail findings and clinical presentation of chronic GVH disease can resemble those of dyskeratosis congentia.24 A mother and her 7-year-old daughter with chronic GVH disease had balanced translocation 46, XX, t(6q13;10p13).25 A 15-year-old white boy with chronic GVH disease had recurrent episodes of immune thrombocytopenic purpura, autoimmune hemolytic anemia, and mild depression of immunoglobulin levels.26
Nail matrix biopsy results and physical examination findings help in establishing the cause of trachyonychia, though this condition often is an isolated finding.27 In the case of lichen planus,28 some patients also have flat polished purple papules on the body and white lacy or reticulated plaques in the mouth.29 Nail biopsy specimens can show hyperkeratosis, hypergranulosis, and acanthosis in the ventral portion of the proximal nail fold and in the nail matrix; a bandlike lymphocytic infiltrate in the superficial dermis; and vacuolar alterations in the basal layer. Nail abnormalities can develop in 1% to 10% of patients with lichen planus.30 In the case of psoriasis, psoriasiform plaques sometimes develop on other body areas, and nail biopsy specimens can show psoriasis evidence such as psoriasiform hyperplasia and neutrophils. In the case of atopic dermatitis, spongiosis31 (intercellular edema of the epidermis) also can occur in nail matrix biopsy specimens.32 In the case of alopecia areata, lymphocytes can be present in the nail matrix, patches of nonscarring alopecia can develop on the scalp, and nail pits can develop in a gridlike pattern (giving a pounded brass appearance) on the nail plates. Evaluation of trachyonychia should include a check for fungus—a fungal culture or periodic acid–Schiff staining of a nail clipping. Some authors have suggested that longitudinal nail biopsy may be a useful diagnostic tool in certain cases of acquired nail dystrophy.33
Hazelrigg et al11 stated that trachyonychia is self-limited and self-resolving in children. Specifically, trachyonychia tends to resolve with time when it occurs in childhood as a manifestation of lichen planus. Rarely, there is nail destruction in 20-nail dystrophy. If destruction occurs, the diagnosis is lichen planus—a form not restricted to the proximal nail fold but extended to the matrix. If the matrix is involved in lichen planus, a pterygium can develop—a manifestation rarely seen in 20-nail dystrophy.
Treatments for trachyonychia include intralesional injections of triamcinolone 2.5 to 3 mg/mL into the proximal nail folds.2,34 Injections are painful and thus difficult in children. Medications for systemic treatment include prednisolone,35 antimalarials,36 and etretinate.37 Seven-month therapy with topical psoralen and UVA light is reported effective.38 In treating psoriatic nail disease, topical 5-fluorouracil39 and cyclosporine40 are useful. Clear nail hardeners can be applied to nails to improve their appearance.
In a study of 15 children, intramuscularly injected triamcinolone acetonide 0.5 to 1 mg/kg per month was prescribed for children with typical nail lichen planus.41 Therapy duration was increased from 3 to 6 months, until the proximal half of the nail was normal. No treatment was prescribed for patients with 20-nail dystrophy or idiopathic atrophy of the nails. Treatment with systemic corticosteroids was effective in curing typical nail lichen planus. For 2 children, the disease recurred during follow-up. Recurrences were always responsive to therapy. Two children with 20-nail dystrophy improved without any therapy. Nail lesions caused by idiopathic atrophy of the nails remained unchanged during follow-up.
Trachyonychia and 20-nail dystrophy continue to present difficulties in classification, diagnosis, and treatment. With the advent of new immunomodulators, it is hoped that more effective treatments will be developed. Prompt diagnosis of these conditions aids in patient education and therapy. back to top
- Tosti A, Bardazzi F, Paraccini BM. Idiopathic trachyonychia (twenty-nail dystrophy): a pathological study of 23 patients. Br J Dermatol. 1994;131:866-872.
- Samman PD. Trachyonychia (rough nails). Br J Dermatol. 1979;101:701-705.
- Kechijian P. Twenty-nail dystrophy of childhood: a reappraisal. Cutis. 1985;35:38-41.
- Scher RK, Fischbein R, Ackerman AB. Twenty-nail dystrophy: a variant of lichen planus. Arch Dermatol. 1978;114:612-613.
- Silverman RA, Rhodes AR. Twenty-nail dystrophy of childhood: a sign of localized lichen planus. Pediatr Dermatol. 1984;1:207-210.
- Schissel DJ, Elston DM. Topical 5-fluorouracil treatment for psoriatic trachyonychia. Cutis. 1998:62:27-28.
- Horn RT Jr, Odom RB. Twenty-nail dystrophy of alopecia areata. Arch Dermatol. 1980;116;573-574.
- Leong AB, Gange RW, O'Connor RD. Twenty-nail dystrophy (trachyonychia) associated with selective IgA deficiency. J Pediatr. 1982;100:418-420.
- Braun-Falco O, Dorn M, Neubert U, et al. Trachyonychia: 20-nail dystrophy. Hautarzt. 1981;32:17-22.
- James WD, Odom RB, Horn RT. Twenty-nail dystrophy and ichthyosis vulgaris. Arch Dermatol. 1981;117:316.
- Hazelrigg DE, Duncan WC, Jarratt M. Twenty-nail dystrophy of childhood. Arch Dermatol. 1977;113:73-75.
- Baran R, Dawber R. Twenty-nail dystrophy of childhood: a misnamed syndrome. Cutis. 1987;39:481-482.
- Arias AM, Yung CW, Rendler S, et al. Familial severe twenty-nail dystrophy in identical twins. Pediatr Dermatol. 1988;5:117-119.
- Commens CA. Twenty nail dystrophy in identical twins. Pediatr Dermatol. 1988;5:117-119.
- Crosby DL, Swanson SL, Fleischer AB. Twenty-nail dystrophy of childhood with koilonychia. Clin Pediatr (Phila). 1991;30:117-119.
- Karakayali G, Lenk N, Gungor E, et al. Twenty-nail dystrophy in monozygotic twins. J Eur Acad Dermatol Venereol. 1995;33:903-905.
- Taniguchi S, Kutsuna H, Tani Y, et al. Twenty-nail dystrophy (trachyonychia) caused by lichen planus in a patient with alopecia universalis and ichthyosis vulgaris. J Am Acad Dermatol. 1995;33(5 pt 2):903-905.
- Kanwar AJ, Ghosh S, Thami GP, et al. Twenty-nail dystrophy due to lichen planus in a patient with alopecia areata. Clin Exp Dermatol. 1993;18:293-294.
- Jeanmougin M, Civatte J. Sandy nails and twenty-nail dystrophy of childhood: apropos of 2 cases. Dermatologica. 1984;168:242-246.
- Sowden JM, Cartwright PH, Green JR, et al. Isolated lichen planus of the nails associated with primary biliary cirrhosis. Br J Dermatol. 1989;121:659-662.
- Khandpur S, Reddy BS. An association of twenty-nail dystrophy with vitiligo. J Dermatol. 2001;28:38-42.
- Palencia SI, Rodriguez-Peralto JL, Castano E, et al. Lichenoid nail changes as sole external manifestation of graft vs. host dise
Trachyonychia (“rough nails”) is best considered a reaction or morphologic pattern with a variety of clinical presentations and etiologies. It may involve only 1 or as many as 20 nails (20-nail dystrophy). It can be a manifestation of lichen planus, psoriasis, alopecia areata, immunoglobulin A deficiency, atopic dermatitis, and ichthyosis vulgaris. Nail matrix biopsy results and physical examination findings help in establishing the cause of this condition, though often trachyonychia is an isolated finding. When trachyonychia occurs in childhood as a manifestation of lichen planus, it tends to resolve with time. We review a case of trachyonychia, its association, its diagnostic evaluation, and treatment options.
Trachyonychia means "rough nails." This condition may involve only 1 or as many as 20 nails. It is best considered a reaction or morphologic pattern with a variety of clinical presentations and etiologies. Clinical presentations are rough nails with a sandpapered appearance and numerous small superficial pits that make the nails shiny1; onychorrhexis, onychoschizia, distal chipping, and yellow onychauxis of the great toenail; and closely arranged longitudinal ridges, distal notching, and layered splitting.2,3 Nail matrix biopsy results combined with clinical findings have linked trachyonychia with lichen planus generally,4 lichen planus in children,5 psoriasis,6 alopecia areata,7 IgA deficiency,8 atopic dermatitis,9 and ichthyosis vulgaris.10 The term 20-nail dystrophy of childhood11 refers to a trachyonychia variant likely caused by lichen planus. Some who consider the term a misnomer—in part because not all nails are necessarily involved—think that perhaps it should be abandoned.12 back to top
Case Report A 10-year-old girl presented with a 1-year history of worsening nail dystrophy. The patient had no history of psoriasis, atopic dermatitis, alopecia, or other skin disease, and family history was unremarkable. Except for dystrophy and hyperkeratosis identified on nails of both hands and both feet (Figure), physical examination findings were normal. Results of a fungal nail culture were negative, and the nail matrix biopsy specimen showed a bandlike lymphocytic infiltrate in the superficial dermis, with vacuolar alteration of the basal level. The diagnosis was trachyonychia secondary to lichen planus. Daily use of flurandrenolone tape and monthly intralesional injections of triamcinolone 2.5 mg/mL did not improve this patient's condition. After 4 months of injections in the distal nail folds, she was lost to follow-up.
Comment Often, the onset of trachyonychia is insidious. The condition usually develops on all nails simultaneously. Trachyonychia also can occur on individual nails over many months. Peak age of onset is 3 to 12 years. Trachyonychia occurs, however, in multigenerational families,13 in all age groups, in twins in the United States14 and Europe,15 in both sexes, and in all ethnic groups. This condition has been associated with ichthyosis vulgaris combined with alopecia universalis,16 ungual lichen planus and alopecia areata,17 koilonychia,18 primary biliary cirrhosis,19 and vitiligo.20 In chronic graft versus host (GVH) disease, trachyonychia can be an isolated finding21 or part of a constellation of cutaneous symptoms.22 It may be associated with dystrophy, atrophy, and, often, ulceration of the lunula.23 In the proper setting, the nail findings and clinical presentation of chronic GVH disease can resemble those of dyskeratosis congentia.24 A mother and her 7-year-old daughter with chronic GVH disease had balanced translocation 46, XX, t(6q13;10p13).25 A 15-year-old white boy with chronic GVH disease had recurrent episodes of immune thrombocytopenic purpura, autoimmune hemolytic anemia, and mild depression of immunoglobulin levels.26
Nail matrix biopsy results and physical examination findings help in establishing the cause of trachyonychia, though this condition often is an isolated finding.27 In the case of lichen planus,28 some patients also have flat polished purple papules on the body and white lacy or reticulated plaques in the mouth.29 Nail biopsy specimens can show hyperkeratosis, hypergranulosis, and acanthosis in the ventral portion of the proximal nail fold and in the nail matrix; a bandlike lymphocytic infiltrate in the superficial dermis; and vacuolar alterations in the basal layer. Nail abnormalities can develop in 1% to 10% of patients with lichen planus.30 In the case of psoriasis, psoriasiform plaques sometimes develop on other body areas, and nail biopsy specimens can show psoriasis evidence such as psoriasiform hyperplasia and neutrophils. In the case of atopic dermatitis, spongiosis31 (intercellular edema of the epidermis) also can occur in nail matrix biopsy specimens.32 In the case of alopecia areata, lymphocytes can be present in the nail matrix, patches of nonscarring alopecia can develop on the scalp, and nail pits can develop in a gridlike pattern (giving a pounded brass appearance) on the nail plates. Evaluation of trachyonychia should include a check for fungus—a fungal culture or periodic acid–Schiff staining of a nail clipping. Some authors have suggested that longitudinal nail biopsy may be a useful diagnostic tool in certain cases of acquired nail dystrophy.33
Hazelrigg et al11 stated that trachyonychia is self-limited and self-resolving in children. Specifically, trachyonychia tends to resolve with time when it occurs in childhood as a manifestation of lichen planus. Rarely, there is nail destruction in 20-nail dystrophy. If destruction occurs, the diagnosis is lichen planus—a form not restricted to the proximal nail fold but extended to the matrix. If the matrix is involved in lichen planus, a pterygium can develop—a manifestation rarely seen in 20-nail dystrophy.
Treatments for trachyonychia include intralesional injections of triamcinolone 2.5 to 3 mg/mL into the proximal nail folds.2,34 Injections are painful and thus difficult in children. Medications for systemic treatment include prednisolone,35 antimalarials,36 and etretinate.37 Seven-month therapy with topical psoralen and UVA light is reported effective.38 In treating psoriatic nail disease, topical 5-fluorouracil39 and cyclosporine40 are useful. Clear nail hardeners can be applied to nails to improve their appearance.
In a study of 15 children, intramuscularly injected triamcinolone acetonide 0.5 to 1 mg/kg per month was prescribed for children with typical nail lichen planus.41 Therapy duration was increased from 3 to 6 months, until the proximal half of the nail was normal. No treatment was prescribed for patients with 20-nail dystrophy or idiopathic atrophy of the nails. Treatment with systemic corticosteroids was effective in curing typical nail lichen planus. For 2 children, the disease recurred during follow-up. Recurrences were always responsive to therapy. Two children with 20-nail dystrophy improved without any therapy. Nail lesions caused by idiopathic atrophy of the nails remained unchanged during follow-up.
Trachyonychia and 20-nail dystrophy continue to present difficulties in classification, diagnosis, and treatment. With the advent of new immunomodulators, it is hoped that more effective treatments will be developed. Prompt diagnosis of these conditions aids in patient education and therapy. back to top
Trachyonychia (“rough nails”) is best considered a reaction or morphologic pattern with a variety of clinical presentations and etiologies. It may involve only 1 or as many as 20 nails (20-nail dystrophy). It can be a manifestation of lichen planus, psoriasis, alopecia areata, immunoglobulin A deficiency, atopic dermatitis, and ichthyosis vulgaris. Nail matrix biopsy results and physical examination findings help in establishing the cause of this condition, though often trachyonychia is an isolated finding. When trachyonychia occurs in childhood as a manifestation of lichen planus, it tends to resolve with time. We review a case of trachyonychia, its association, its diagnostic evaluation, and treatment options.
Trachyonychia means "rough nails." This condition may involve only 1 or as many as 20 nails. It is best considered a reaction or morphologic pattern with a variety of clinical presentations and etiologies. Clinical presentations are rough nails with a sandpapered appearance and numerous small superficial pits that make the nails shiny1; onychorrhexis, onychoschizia, distal chipping, and yellow onychauxis of the great toenail; and closely arranged longitudinal ridges, distal notching, and layered splitting.2,3 Nail matrix biopsy results combined with clinical findings have linked trachyonychia with lichen planus generally,4 lichen planus in children,5 psoriasis,6 alopecia areata,7 IgA deficiency,8 atopic dermatitis,9 and ichthyosis vulgaris.10 The term 20-nail dystrophy of childhood11 refers to a trachyonychia variant likely caused by lichen planus. Some who consider the term a misnomer—in part because not all nails are necessarily involved—think that perhaps it should be abandoned.12 back to top
Case Report A 10-year-old girl presented with a 1-year history of worsening nail dystrophy. The patient had no history of psoriasis, atopic dermatitis, alopecia, or other skin disease, and family history was unremarkable. Except for dystrophy and hyperkeratosis identified on nails of both hands and both feet (Figure), physical examination findings were normal. Results of a fungal nail culture were negative, and the nail matrix biopsy specimen showed a bandlike lymphocytic infiltrate in the superficial dermis, with vacuolar alteration of the basal level. The diagnosis was trachyonychia secondary to lichen planus. Daily use of flurandrenolone tape and monthly intralesional injections of triamcinolone 2.5 mg/mL did not improve this patient's condition. After 4 months of injections in the distal nail folds, she was lost to follow-up.
Comment Often, the onset of trachyonychia is insidious. The condition usually develops on all nails simultaneously. Trachyonychia also can occur on individual nails over many months. Peak age of onset is 3 to 12 years. Trachyonychia occurs, however, in multigenerational families,13 in all age groups, in twins in the United States14 and Europe,15 in both sexes, and in all ethnic groups. This condition has been associated with ichthyosis vulgaris combined with alopecia universalis,16 ungual lichen planus and alopecia areata,17 koilonychia,18 primary biliary cirrhosis,19 and vitiligo.20 In chronic graft versus host (GVH) disease, trachyonychia can be an isolated finding21 or part of a constellation of cutaneous symptoms.22 It may be associated with dystrophy, atrophy, and, often, ulceration of the lunula.23 In the proper setting, the nail findings and clinical presentation of chronic GVH disease can resemble those of dyskeratosis congentia.24 A mother and her 7-year-old daughter with chronic GVH disease had balanced translocation 46, XX, t(6q13;10p13).25 A 15-year-old white boy with chronic GVH disease had recurrent episodes of immune thrombocytopenic purpura, autoimmune hemolytic anemia, and mild depression of immunoglobulin levels.26
Nail matrix biopsy results and physical examination findings help in establishing the cause of trachyonychia, though this condition often is an isolated finding.27 In the case of lichen planus,28 some patients also have flat polished purple papules on the body and white lacy or reticulated plaques in the mouth.29 Nail biopsy specimens can show hyperkeratosis, hypergranulosis, and acanthosis in the ventral portion of the proximal nail fold and in the nail matrix; a bandlike lymphocytic infiltrate in the superficial dermis; and vacuolar alterations in the basal layer. Nail abnormalities can develop in 1% to 10% of patients with lichen planus.30 In the case of psoriasis, psoriasiform plaques sometimes develop on other body areas, and nail biopsy specimens can show psoriasis evidence such as psoriasiform hyperplasia and neutrophils. In the case of atopic dermatitis, spongiosis31 (intercellular edema of the epidermis) also can occur in nail matrix biopsy specimens.32 In the case of alopecia areata, lymphocytes can be present in the nail matrix, patches of nonscarring alopecia can develop on the scalp, and nail pits can develop in a gridlike pattern (giving a pounded brass appearance) on the nail plates. Evaluation of trachyonychia should include a check for fungus—a fungal culture or periodic acid–Schiff staining of a nail clipping. Some authors have suggested that longitudinal nail biopsy may be a useful diagnostic tool in certain cases of acquired nail dystrophy.33
Hazelrigg et al11 stated that trachyonychia is self-limited and self-resolving in children. Specifically, trachyonychia tends to resolve with time when it occurs in childhood as a manifestation of lichen planus. Rarely, there is nail destruction in 20-nail dystrophy. If destruction occurs, the diagnosis is lichen planus—a form not restricted to the proximal nail fold but extended to the matrix. If the matrix is involved in lichen planus, a pterygium can develop—a manifestation rarely seen in 20-nail dystrophy.
Treatments for trachyonychia include intralesional injections of triamcinolone 2.5 to 3 mg/mL into the proximal nail folds.2,34 Injections are painful and thus difficult in children. Medications for systemic treatment include prednisolone,35 antimalarials,36 and etretinate.37 Seven-month therapy with topical psoralen and UVA light is reported effective.38 In treating psoriatic nail disease, topical 5-fluorouracil39 and cyclosporine40 are useful. Clear nail hardeners can be applied to nails to improve their appearance.
In a study of 15 children, intramuscularly injected triamcinolone acetonide 0.5 to 1 mg/kg per month was prescribed for children with typical nail lichen planus.41 Therapy duration was increased from 3 to 6 months, until the proximal half of the nail was normal. No treatment was prescribed for patients with 20-nail dystrophy or idiopathic atrophy of the nails. Treatment with systemic corticosteroids was effective in curing typical nail lichen planus. For 2 children, the disease recurred during follow-up. Recurrences were always responsive to therapy. Two children with 20-nail dystrophy improved without any therapy. Nail lesions caused by idiopathic atrophy of the nails remained unchanged during follow-up.
Trachyonychia and 20-nail dystrophy continue to present difficulties in classification, diagnosis, and treatment. With the advent of new immunomodulators, it is hoped that more effective treatments will be developed. Prompt diagnosis of these conditions aids in patient education and therapy. back to top
- Tosti A, Bardazzi F, Paraccini BM. Idiopathic trachyonychia (twenty-nail dystrophy): a pathological study of 23 patients. Br J Dermatol. 1994;131:866-872.
- Samman PD. Trachyonychia (rough nails). Br J Dermatol. 1979;101:701-705.
- Kechijian P. Twenty-nail dystrophy of childhood: a reappraisal. Cutis. 1985;35:38-41.
- Scher RK, Fischbein R, Ackerman AB. Twenty-nail dystrophy: a variant of lichen planus. Arch Dermatol. 1978;114:612-613.
- Silverman RA, Rhodes AR. Twenty-nail dystrophy of childhood: a sign of localized lichen planus. Pediatr Dermatol. 1984;1:207-210.
- Schissel DJ, Elston DM. Topical 5-fluorouracil treatment for psoriatic trachyonychia. Cutis. 1998:62:27-28.
- Horn RT Jr, Odom RB. Twenty-nail dystrophy of alopecia areata. Arch Dermatol. 1980;116;573-574.
- Leong AB, Gange RW, O'Connor RD. Twenty-nail dystrophy (trachyonychia) associated with selective IgA deficiency. J Pediatr. 1982;100:418-420.
- Braun-Falco O, Dorn M, Neubert U, et al. Trachyonychia: 20-nail dystrophy. Hautarzt. 1981;32:17-22.
- James WD, Odom RB, Horn RT. Twenty-nail dystrophy and ichthyosis vulgaris. Arch Dermatol. 1981;117:316.
- Hazelrigg DE, Duncan WC, Jarratt M. Twenty-nail dystrophy of childhood. Arch Dermatol. 1977;113:73-75.
- Baran R, Dawber R. Twenty-nail dystrophy of childhood: a misnamed syndrome. Cutis. 1987;39:481-482.
- Arias AM, Yung CW, Rendler S, et al. Familial severe twenty-nail dystrophy in identical twins. Pediatr Dermatol. 1988;5:117-119.
- Commens CA. Twenty nail dystrophy in identical twins. Pediatr Dermatol. 1988;5:117-119.
- Crosby DL, Swanson SL, Fleischer AB. Twenty-nail dystrophy of childhood with koilonychia. Clin Pediatr (Phila). 1991;30:117-119.
- Karakayali G, Lenk N, Gungor E, et al. Twenty-nail dystrophy in monozygotic twins. J Eur Acad Dermatol Venereol. 1995;33:903-905.
- Taniguchi S, Kutsuna H, Tani Y, et al. Twenty-nail dystrophy (trachyonychia) caused by lichen planus in a patient with alopecia universalis and ichthyosis vulgaris. J Am Acad Dermatol. 1995;33(5 pt 2):903-905.
- Kanwar AJ, Ghosh S, Thami GP, et al. Twenty-nail dystrophy due to lichen planus in a patient with alopecia areata. Clin Exp Dermatol. 1993;18:293-294.
- Jeanmougin M, Civatte J. Sandy nails and twenty-nail dystrophy of childhood: apropos of 2 cases. Dermatologica. 1984;168:242-246.
- Sowden JM, Cartwright PH, Green JR, et al. Isolated lichen planus of the nails associated with primary biliary cirrhosis. Br J Dermatol. 1989;121:659-662.
- Khandpur S, Reddy BS. An association of twenty-nail dystrophy with vitiligo. J Dermatol. 2001;28:38-42.
- Palencia SI, Rodriguez-Peralto JL, Castano E, et al. Lichenoid nail changes as sole external manifestation of graft vs. host dise
- Tosti A, Bardazzi F, Paraccini BM. Idiopathic trachyonychia (twenty-nail dystrophy): a pathological study of 23 patients. Br J Dermatol. 1994;131:866-872.
- Samman PD. Trachyonychia (rough nails). Br J Dermatol. 1979;101:701-705.
- Kechijian P. Twenty-nail dystrophy of childhood: a reappraisal. Cutis. 1985;35:38-41.
- Scher RK, Fischbein R, Ackerman AB. Twenty-nail dystrophy: a variant of lichen planus. Arch Dermatol. 1978;114:612-613.
- Silverman RA, Rhodes AR. Twenty-nail dystrophy of childhood: a sign of localized lichen planus. Pediatr Dermatol. 1984;1:207-210.
- Schissel DJ, Elston DM. Topical 5-fluorouracil treatment for psoriatic trachyonychia. Cutis. 1998:62:27-28.
- Horn RT Jr, Odom RB. Twenty-nail dystrophy of alopecia areata. Arch Dermatol. 1980;116;573-574.
- Leong AB, Gange RW, O'Connor RD. Twenty-nail dystrophy (trachyonychia) associated with selective IgA deficiency. J Pediatr. 1982;100:418-420.
- Braun-Falco O, Dorn M, Neubert U, et al. Trachyonychia: 20-nail dystrophy. Hautarzt. 1981;32:17-22.
- James WD, Odom RB, Horn RT. Twenty-nail dystrophy and ichthyosis vulgaris. Arch Dermatol. 1981;117:316.
- Hazelrigg DE, Duncan WC, Jarratt M. Twenty-nail dystrophy of childhood. Arch Dermatol. 1977;113:73-75.
- Baran R, Dawber R. Twenty-nail dystrophy of childhood: a misnamed syndrome. Cutis. 1987;39:481-482.
- Arias AM, Yung CW, Rendler S, et al. Familial severe twenty-nail dystrophy in identical twins. Pediatr Dermatol. 1988;5:117-119.
- Commens CA. Twenty nail dystrophy in identical twins. Pediatr Dermatol. 1988;5:117-119.
- Crosby DL, Swanson SL, Fleischer AB. Twenty-nail dystrophy of childhood with koilonychia. Clin Pediatr (Phila). 1991;30:117-119.
- Karakayali G, Lenk N, Gungor E, et al. Twenty-nail dystrophy in monozygotic twins. J Eur Acad Dermatol Venereol. 1995;33:903-905.
- Taniguchi S, Kutsuna H, Tani Y, et al. Twenty-nail dystrophy (trachyonychia) caused by lichen planus in a patient with alopecia universalis and ichthyosis vulgaris. J Am Acad Dermatol. 1995;33(5 pt 2):903-905.
- Kanwar AJ, Ghosh S, Thami GP, et al. Twenty-nail dystrophy due to lichen planus in a patient with alopecia areata. Clin Exp Dermatol. 1993;18:293-294.
- Jeanmougin M, Civatte J. Sandy nails and twenty-nail dystrophy of childhood: apropos of 2 cases. Dermatologica. 1984;168:242-246.
- Sowden JM, Cartwright PH, Green JR, et al. Isolated lichen planus of the nails associated with primary biliary cirrhosis. Br J Dermatol. 1989;121:659-662.
- Khandpur S, Reddy BS. An association of twenty-nail dystrophy with vitiligo. J Dermatol. 2001;28:38-42.
- Palencia SI, Rodriguez-Peralto JL, Castano E, et al. Lichenoid nail changes as sole external manifestation of graft vs. host dise