User login
Hair oiling: Practices, benefits, and caveats
including in India and among people of the African diaspora. Hair oiling typically entails combing the hair, followed by applying oil from the roots to the ends about once a week prior to shampooing. Hair oiling daily or every other day, often with a hair braid, is also practiced, using coconut oil or other oils. Oil is found in various hair products and has been popular in the United States, particularly as hot oil treatments in the 1980s.
Oils are used as part of Abhyanga massage, either by an Ayurvedic practitioner or as self-massage, as part of ancient Indian Ayurvedic medicine practice. The type of oil used is determined by the practitioner depending on the individual’s needs; cold-pressed sesame oil or coconut oil is often used as the base oil. Abhyanga is often performed with oil on the entire body as an act of self-care, which includes massage of the hair and scalp. The oil and herbs that are often added to the oil, as well as the scalp massage itself, are explained by practitioners as having therapeutic effects on the hair, including exfoliation of a dry scalp and on overall well-being. Outside of Ayurvedic medicine, hair oiling is also sometimes performed in India as part of routine hair care and can be a bonding experience among parents, grandparents, and children.
Amla oil, which is derived from Indian gooseberry (Phyllanthus emblica L.) and is often used in India on the hair, contains Vitamin C and has been shown to have activity against dermatophytes. In a study conducted in India, amla oil was found to have the most activity against Microsporum canis, M. gypseum, and Trichophyton rubrum, followed by cantharidine and coconut oil, while T. mentagrophytes was most susceptible to coconut oil, followed by amla and cantharidine oil.1 A study conducted in mice in Thailand found that 5-alpha-reductase was reduced with the dried form of the herb Phyllanthus emblica L, as well as with some other traditional Thai herbs used as hair treatments.2
Castor oil has been utilized in many cultures to promote hair growth. Ricinoleic acid, an unsaturated omega-9 fatty acid and hydroxy acid, is the major component of seed oil obtained from mature castor plants. It has anti-inflammatory and antimicrobial effects, and in one study,3 was found to inhibit prostaglandin D2, which has been implicated in the pathogenesis of androgenetic alopecia.4 Jamaican black castor oil is darker in color and has a more alkaline pH compared with traditional castor oil (both contain ricinoleic acid). To produce Jamaican black castor oil, the seed is roasted, ground to a thick paste, boiled in a pot of hot water, then the oil is skimmed off of the top into individual bottles, whereas regular castor oil is cold pressed. The alkalinity of Jamaican black castor oil may play a role in opening up the hair cuticle, which may be beneficial, but application also needs to be followed by a routine that includes closing the cuticle to avoid increasing hair fragility; such a routine could include, for example, leave-in conditioner or rinsing conditioner with colder water.
Castor oil is sometimes used on its own or in combination with other oils, and it is also an ingredient in some hair care products. However, publicly available peer-reviewed research articles demonstrating the effects of castor oil when applied directly to the hair and scalp for hair growth are needed.
Different types of hair oils are used in African American hair care products and, worldwide, by people of the African diaspora as part of regular hair care and hair styling. Common oils include jojoba, coconut, castor, argan, olive, sunflower, and almond oils. Very curly hair often dries out more easily, especially in drier climates; and sebum build-up on the scalp does not occur as quickly, so hair typically does not need to be shampooed frequently. As such, over-shampooing also often dries the hair out faster, especially if hair has been chemically processed. Thus, oils help to coat and protect the hair, and smooth the cuticle. Oils themselves do not moisturize, but can seal moisture into the hair or act as humectants and draw moisture in. Rather than applying on dry hair, oils, when used as daily care as opposed to before shampooing, are often applied on clean hair after shampooing and after a leave-in conditioner has been applied to help seal in moisture for the most benefit. For treatment of scalp conditions, depending on the type of hair care practice performed at home, oils may be preferred over potentially drying solutions and foams if available, for optimum hair care.
Hair oiling is a long-standing practice which, when used correctly, can be beneficial for hair management and health. There are, however, caveats to hair oiling, which include being careful not to excessively brush or comb hair that has a lot of oil in it because the hair is slick, which can cause hair to fall out during combing. Some oils have elevated levels of lauric acid (coconut oil has 50%), which has a high affinity for hair proteins.5 While this can support hair strength, care should be taken not to overuse some oils or other products known as “protein packs,” which should be used as directed. Since hair is protein, excess protein build-up coating the hair can block needed moisture, making the hair more knotted and brittle, potentially resulting in more breakage with brushing or other hair care practices.
Some oil-containing hair products also contain artificial fragrances and/or dyes, which in some individuals, may promote an immunogenic effect, such as contact dermatitis. Certain oils, particularly olive oil, can promote an environment favorable to yeast growth, especially Malassezia species, implicated in seborrheic dermatitis. Practices that involve excessive application of oil to the scalp can also result in build up if not shampooed regularly. Sulfonated castor oil (also known as Turkey Red oil) has been reported to cause contact dermatitis.6
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
including in India and among people of the African diaspora. Hair oiling typically entails combing the hair, followed by applying oil from the roots to the ends about once a week prior to shampooing. Hair oiling daily or every other day, often with a hair braid, is also practiced, using coconut oil or other oils. Oil is found in various hair products and has been popular in the United States, particularly as hot oil treatments in the 1980s.
Oils are used as part of Abhyanga massage, either by an Ayurvedic practitioner or as self-massage, as part of ancient Indian Ayurvedic medicine practice. The type of oil used is determined by the practitioner depending on the individual’s needs; cold-pressed sesame oil or coconut oil is often used as the base oil. Abhyanga is often performed with oil on the entire body as an act of self-care, which includes massage of the hair and scalp. The oil and herbs that are often added to the oil, as well as the scalp massage itself, are explained by practitioners as having therapeutic effects on the hair, including exfoliation of a dry scalp and on overall well-being. Outside of Ayurvedic medicine, hair oiling is also sometimes performed in India as part of routine hair care and can be a bonding experience among parents, grandparents, and children.
Amla oil, which is derived from Indian gooseberry (Phyllanthus emblica L.) and is often used in India on the hair, contains Vitamin C and has been shown to have activity against dermatophytes. In a study conducted in India, amla oil was found to have the most activity against Microsporum canis, M. gypseum, and Trichophyton rubrum, followed by cantharidine and coconut oil, while T. mentagrophytes was most susceptible to coconut oil, followed by amla and cantharidine oil.1 A study conducted in mice in Thailand found that 5-alpha-reductase was reduced with the dried form of the herb Phyllanthus emblica L, as well as with some other traditional Thai herbs used as hair treatments.2
Castor oil has been utilized in many cultures to promote hair growth. Ricinoleic acid, an unsaturated omega-9 fatty acid and hydroxy acid, is the major component of seed oil obtained from mature castor plants. It has anti-inflammatory and antimicrobial effects, and in one study,3 was found to inhibit prostaglandin D2, which has been implicated in the pathogenesis of androgenetic alopecia.4 Jamaican black castor oil is darker in color and has a more alkaline pH compared with traditional castor oil (both contain ricinoleic acid). To produce Jamaican black castor oil, the seed is roasted, ground to a thick paste, boiled in a pot of hot water, then the oil is skimmed off of the top into individual bottles, whereas regular castor oil is cold pressed. The alkalinity of Jamaican black castor oil may play a role in opening up the hair cuticle, which may be beneficial, but application also needs to be followed by a routine that includes closing the cuticle to avoid increasing hair fragility; such a routine could include, for example, leave-in conditioner or rinsing conditioner with colder water.
Castor oil is sometimes used on its own or in combination with other oils, and it is also an ingredient in some hair care products. However, publicly available peer-reviewed research articles demonstrating the effects of castor oil when applied directly to the hair and scalp for hair growth are needed.
Different types of hair oils are used in African American hair care products and, worldwide, by people of the African diaspora as part of regular hair care and hair styling. Common oils include jojoba, coconut, castor, argan, olive, sunflower, and almond oils. Very curly hair often dries out more easily, especially in drier climates; and sebum build-up on the scalp does not occur as quickly, so hair typically does not need to be shampooed frequently. As such, over-shampooing also often dries the hair out faster, especially if hair has been chemically processed. Thus, oils help to coat and protect the hair, and smooth the cuticle. Oils themselves do not moisturize, but can seal moisture into the hair or act as humectants and draw moisture in. Rather than applying on dry hair, oils, when used as daily care as opposed to before shampooing, are often applied on clean hair after shampooing and after a leave-in conditioner has been applied to help seal in moisture for the most benefit. For treatment of scalp conditions, depending on the type of hair care practice performed at home, oils may be preferred over potentially drying solutions and foams if available, for optimum hair care.
Hair oiling is a long-standing practice which, when used correctly, can be beneficial for hair management and health. There are, however, caveats to hair oiling, which include being careful not to excessively brush or comb hair that has a lot of oil in it because the hair is slick, which can cause hair to fall out during combing. Some oils have elevated levels of lauric acid (coconut oil has 50%), which has a high affinity for hair proteins.5 While this can support hair strength, care should be taken not to overuse some oils or other products known as “protein packs,” which should be used as directed. Since hair is protein, excess protein build-up coating the hair can block needed moisture, making the hair more knotted and brittle, potentially resulting in more breakage with brushing or other hair care practices.
Some oil-containing hair products also contain artificial fragrances and/or dyes, which in some individuals, may promote an immunogenic effect, such as contact dermatitis. Certain oils, particularly olive oil, can promote an environment favorable to yeast growth, especially Malassezia species, implicated in seborrheic dermatitis. Practices that involve excessive application of oil to the scalp can also result in build up if not shampooed regularly. Sulfonated castor oil (also known as Turkey Red oil) has been reported to cause contact dermatitis.6
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
including in India and among people of the African diaspora. Hair oiling typically entails combing the hair, followed by applying oil from the roots to the ends about once a week prior to shampooing. Hair oiling daily or every other day, often with a hair braid, is also practiced, using coconut oil or other oils. Oil is found in various hair products and has been popular in the United States, particularly as hot oil treatments in the 1980s.
Oils are used as part of Abhyanga massage, either by an Ayurvedic practitioner or as self-massage, as part of ancient Indian Ayurvedic medicine practice. The type of oil used is determined by the practitioner depending on the individual’s needs; cold-pressed sesame oil or coconut oil is often used as the base oil. Abhyanga is often performed with oil on the entire body as an act of self-care, which includes massage of the hair and scalp. The oil and herbs that are often added to the oil, as well as the scalp massage itself, are explained by practitioners as having therapeutic effects on the hair, including exfoliation of a dry scalp and on overall well-being. Outside of Ayurvedic medicine, hair oiling is also sometimes performed in India as part of routine hair care and can be a bonding experience among parents, grandparents, and children.
Amla oil, which is derived from Indian gooseberry (Phyllanthus emblica L.) and is often used in India on the hair, contains Vitamin C and has been shown to have activity against dermatophytes. In a study conducted in India, amla oil was found to have the most activity against Microsporum canis, M. gypseum, and Trichophyton rubrum, followed by cantharidine and coconut oil, while T. mentagrophytes was most susceptible to coconut oil, followed by amla and cantharidine oil.1 A study conducted in mice in Thailand found that 5-alpha-reductase was reduced with the dried form of the herb Phyllanthus emblica L, as well as with some other traditional Thai herbs used as hair treatments.2
Castor oil has been utilized in many cultures to promote hair growth. Ricinoleic acid, an unsaturated omega-9 fatty acid and hydroxy acid, is the major component of seed oil obtained from mature castor plants. It has anti-inflammatory and antimicrobial effects, and in one study,3 was found to inhibit prostaglandin D2, which has been implicated in the pathogenesis of androgenetic alopecia.4 Jamaican black castor oil is darker in color and has a more alkaline pH compared with traditional castor oil (both contain ricinoleic acid). To produce Jamaican black castor oil, the seed is roasted, ground to a thick paste, boiled in a pot of hot water, then the oil is skimmed off of the top into individual bottles, whereas regular castor oil is cold pressed. The alkalinity of Jamaican black castor oil may play a role in opening up the hair cuticle, which may be beneficial, but application also needs to be followed by a routine that includes closing the cuticle to avoid increasing hair fragility; such a routine could include, for example, leave-in conditioner or rinsing conditioner with colder water.
Castor oil is sometimes used on its own or in combination with other oils, and it is also an ingredient in some hair care products. However, publicly available peer-reviewed research articles demonstrating the effects of castor oil when applied directly to the hair and scalp for hair growth are needed.
Different types of hair oils are used in African American hair care products and, worldwide, by people of the African diaspora as part of regular hair care and hair styling. Common oils include jojoba, coconut, castor, argan, olive, sunflower, and almond oils. Very curly hair often dries out more easily, especially in drier climates; and sebum build-up on the scalp does not occur as quickly, so hair typically does not need to be shampooed frequently. As such, over-shampooing also often dries the hair out faster, especially if hair has been chemically processed. Thus, oils help to coat and protect the hair, and smooth the cuticle. Oils themselves do not moisturize, but can seal moisture into the hair or act as humectants and draw moisture in. Rather than applying on dry hair, oils, when used as daily care as opposed to before shampooing, are often applied on clean hair after shampooing and after a leave-in conditioner has been applied to help seal in moisture for the most benefit. For treatment of scalp conditions, depending on the type of hair care practice performed at home, oils may be preferred over potentially drying solutions and foams if available, for optimum hair care.
Hair oiling is a long-standing practice which, when used correctly, can be beneficial for hair management and health. There are, however, caveats to hair oiling, which include being careful not to excessively brush or comb hair that has a lot of oil in it because the hair is slick, which can cause hair to fall out during combing. Some oils have elevated levels of lauric acid (coconut oil has 50%), which has a high affinity for hair proteins.5 While this can support hair strength, care should be taken not to overuse some oils or other products known as “protein packs,” which should be used as directed. Since hair is protein, excess protein build-up coating the hair can block needed moisture, making the hair more knotted and brittle, potentially resulting in more breakage with brushing or other hair care practices.
Some oil-containing hair products also contain artificial fragrances and/or dyes, which in some individuals, may promote an immunogenic effect, such as contact dermatitis. Certain oils, particularly olive oil, can promote an environment favorable to yeast growth, especially Malassezia species, implicated in seborrheic dermatitis. Practices that involve excessive application of oil to the scalp can also result in build up if not shampooed regularly. Sulfonated castor oil (also known as Turkey Red oil) has been reported to cause contact dermatitis.6
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
More on the history of dermatologists and skin care
Thanks to all the readers of Part 1 (The interesting history of dermatologist-developed skin care), who gave me interesting feedback on my Facebook and LinkedIn accounts. There was so much interest that I will write Part 3 next month. Feel free to DM me your ideas on LinkedIn.
The history of dermatologist-developed skin care continues as more dermatologists become interested in developing a skin care line or retailing skin care in their medical practice. A report in July 2020 showed that physician-dispensed skin care is the largest growing segment of the skin care business with a projected compound annual growth rate of 9.9% from 2020 to 2027. I have not seen national sales numbers since this July report, but we have noticed a large increase in online sales for the doctors using my Skin Type Solutions System. This is most likely because, in a national crisis, the self-care and beauty business segments often see growth. So as you can see, dermatologist-dispensed skin care is becoming a major player in national skin care sales. Let’s get back to the story of how this came to be the case.
Peter Elias, MD. Dr. Elias is professor in the department of dermatology at the University of California, San Francisco. In 1996, Dr. Elias published a landmark paper in the Journal of Investigative Dermatology demonstrating that a 1:1:1 ratio of ceramides, fatty acids, and cholesterol is required to repair a damaged skin barrier. He filed multiple patents for using these lipids in moisturizers as early as 1992. His lipid research has stood the test of time, and this paper is still frequently cited. Dr. Elias has authored over 500 peer reviewed articles on the skin barrier, has edited or coauthored three books on skin barrier science, and developed EpiCeram, a product that utilizes ceramide, the fatty acid linoleic acid, and cholesterol. EpiCeram is the only barrier repair moisturizer approved by the Food and Drug Administration and is available by prescription only.
Kathy Fields, MD, and Katie Rodan, MD. Dr. Fields and Dr. Rodan met at Stanford (Calif.) University. In the 1980s, these entrepreneurial dermatologists realized that patients did not understand the role of preventing acne rather than just treating it. As dermatologists, they knew that a consistent daily routine to prevent acne was much more effective than waiting for an outbreak and spot-treating lesions. They took an already available OTC medication – benzoyl peroxide – and educated consumers through infomercials that they needed to stay ahead of acne instead of waiting for a breakout. Using infomercials to sell skin care, selling skin care kits, and educating patients about the need to prevent acne rather than spot treat it was very unusual at the time. As we all know, reeducating your patients is a huge challenge. Dr. Fields and Dr. Rodan changed consumers thinking in a genius way that continues to resonate today by choosing a brand name to make their point: Proactiv. Their simple 3-step acne kit encouraged patients to be proactive about their acne and encouraged compliance. (Patients love exact skin care steps as demonstrated again by the success of the skin care line from plastic surgeon Suzan Obagi, MD, which became available around 1988).
Dr. Fields and Dr. Rodan first offered Proactiv to Neutrogena, which turned it down. This early disappointment did not deter them and ended up benefiting them because this gave them the idea to do infomercials. Guthy Renker agreed to market and distribute the product, and the first Proactiv infomercial appeared on TV in 1995. It quickly became popular and is still one of the best selling skin care lines of all time. It’s important to note that the “overnight success” of Proactiv took at least a decade of effort.
Dr. Rodan and Dr. Fields started a new skin care line called Rodan and Fields in 2002, which was sold in department stores. This was at a time when department stores were losing market share of the skin care business, and Dr. Rodan and Dr. Fields wisely relaunched in 2007 using a direct sales model similar to Mary Kay and Avon. Their ability to encourage and motivate their team is apparent in the enthusiasm seen in their sales consultants.
Heather Woolery Lloyd, MD. Dr. Woolery Lloyd got her medical degree at the University of Miami where she also completed her dermatology residency. Her interest in skin of color led to her appointment as director of Ethnic Skin Care at the University of Miami, the country’s first cosmetic ethnic skin care department at a major university. She spent years lecturing around the world on skin of color issues and performing clinical trials before she developed the “Specific Beauty” skin care line for melanin rich skin types. Specific Beauty was acquired by Guthy Renker and is available online. It is the most popular dermatologist developed skin care line for skin of color.
In the late 1980s and early 1990s, other dermatologists threw their hats into the ring and came out with skin care lines – some successful and some not. Unfortunately, many skin care lines at that time were based on “pseudoscience” and exaggerated claims, which fueled the fire of those who felt dermatologists should steer clear of these entrepreneurial pursuits. A debate about the ethics of doctors retailing skin care began, and the controversy led to this 2010 statement by the American Medical Association: “In-office sale of health-related products by physicians presents a financial conflict of interest, risks placing undue pressure on the patient, and threatens to erode patient trust and undermine the primary obligation of physicians to serve the interests of their patients before their own.”
Many dermatologists dropped out of the AMA as a result because they felt the organization was no longer representing dermatologist’s interests. After all, we know skin care science better than anyone, and many dermatologists were insulted by the suggestion that we would place our personal financial gain over the best interests of our patients.
This is a perfect example of how the actions of a few unscrupulous dermatologists can affect the entire specialty. I like to focus on the ethical entrepreneurial dermatologists who made great contributions to the skin care industry based on science, efficacy, and patient education and encourage the ethical among us to provide this science-based information to our patients to protect them from pseudoscience-based opportunists. It is obvious that I believe that dermatologists have a responsibility to provide medical advice on skin care to their patients. If not us, who will do it as ethically as we will? But I plead with those of you out there who are promoting foolish stem cell–containing creams and other impossible technologies to remember that you are hurting the credibility of our entire dermatology profession.
In my next column, I will discuss dermatologists who have played a significant role behind the scenes in the development of the skin care industry.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
Castanedo-Tardan MP, Baumann L. Clin Dermatol. Jul-Aug 2009;27(4):355-8.
Gormley DE. Arch Dermatol. 1999 Jul;135(7):765-6.
Miller RC. Arch Dermatol. 1999 Mar;135(3):255-6.
Virtual Mentor. 2010;12(12):925-7.
Thanks to all the readers of Part 1 (The interesting history of dermatologist-developed skin care), who gave me interesting feedback on my Facebook and LinkedIn accounts. There was so much interest that I will write Part 3 next month. Feel free to DM me your ideas on LinkedIn.
The history of dermatologist-developed skin care continues as more dermatologists become interested in developing a skin care line or retailing skin care in their medical practice. A report in July 2020 showed that physician-dispensed skin care is the largest growing segment of the skin care business with a projected compound annual growth rate of 9.9% from 2020 to 2027. I have not seen national sales numbers since this July report, but we have noticed a large increase in online sales for the doctors using my Skin Type Solutions System. This is most likely because, in a national crisis, the self-care and beauty business segments often see growth. So as you can see, dermatologist-dispensed skin care is becoming a major player in national skin care sales. Let’s get back to the story of how this came to be the case.
Peter Elias, MD. Dr. Elias is professor in the department of dermatology at the University of California, San Francisco. In 1996, Dr. Elias published a landmark paper in the Journal of Investigative Dermatology demonstrating that a 1:1:1 ratio of ceramides, fatty acids, and cholesterol is required to repair a damaged skin barrier. He filed multiple patents for using these lipids in moisturizers as early as 1992. His lipid research has stood the test of time, and this paper is still frequently cited. Dr. Elias has authored over 500 peer reviewed articles on the skin barrier, has edited or coauthored three books on skin barrier science, and developed EpiCeram, a product that utilizes ceramide, the fatty acid linoleic acid, and cholesterol. EpiCeram is the only barrier repair moisturizer approved by the Food and Drug Administration and is available by prescription only.
Kathy Fields, MD, and Katie Rodan, MD. Dr. Fields and Dr. Rodan met at Stanford (Calif.) University. In the 1980s, these entrepreneurial dermatologists realized that patients did not understand the role of preventing acne rather than just treating it. As dermatologists, they knew that a consistent daily routine to prevent acne was much more effective than waiting for an outbreak and spot-treating lesions. They took an already available OTC medication – benzoyl peroxide – and educated consumers through infomercials that they needed to stay ahead of acne instead of waiting for a breakout. Using infomercials to sell skin care, selling skin care kits, and educating patients about the need to prevent acne rather than spot treat it was very unusual at the time. As we all know, reeducating your patients is a huge challenge. Dr. Fields and Dr. Rodan changed consumers thinking in a genius way that continues to resonate today by choosing a brand name to make their point: Proactiv. Their simple 3-step acne kit encouraged patients to be proactive about their acne and encouraged compliance. (Patients love exact skin care steps as demonstrated again by the success of the skin care line from plastic surgeon Suzan Obagi, MD, which became available around 1988).
Dr. Fields and Dr. Rodan first offered Proactiv to Neutrogena, which turned it down. This early disappointment did not deter them and ended up benefiting them because this gave them the idea to do infomercials. Guthy Renker agreed to market and distribute the product, and the first Proactiv infomercial appeared on TV in 1995. It quickly became popular and is still one of the best selling skin care lines of all time. It’s important to note that the “overnight success” of Proactiv took at least a decade of effort.
Dr. Rodan and Dr. Fields started a new skin care line called Rodan and Fields in 2002, which was sold in department stores. This was at a time when department stores were losing market share of the skin care business, and Dr. Rodan and Dr. Fields wisely relaunched in 2007 using a direct sales model similar to Mary Kay and Avon. Their ability to encourage and motivate their team is apparent in the enthusiasm seen in their sales consultants.
Heather Woolery Lloyd, MD. Dr. Woolery Lloyd got her medical degree at the University of Miami where she also completed her dermatology residency. Her interest in skin of color led to her appointment as director of Ethnic Skin Care at the University of Miami, the country’s first cosmetic ethnic skin care department at a major university. She spent years lecturing around the world on skin of color issues and performing clinical trials before she developed the “Specific Beauty” skin care line for melanin rich skin types. Specific Beauty was acquired by Guthy Renker and is available online. It is the most popular dermatologist developed skin care line for skin of color.
In the late 1980s and early 1990s, other dermatologists threw their hats into the ring and came out with skin care lines – some successful and some not. Unfortunately, many skin care lines at that time were based on “pseudoscience” and exaggerated claims, which fueled the fire of those who felt dermatologists should steer clear of these entrepreneurial pursuits. A debate about the ethics of doctors retailing skin care began, and the controversy led to this 2010 statement by the American Medical Association: “In-office sale of health-related products by physicians presents a financial conflict of interest, risks placing undue pressure on the patient, and threatens to erode patient trust and undermine the primary obligation of physicians to serve the interests of their patients before their own.”
Many dermatologists dropped out of the AMA as a result because they felt the organization was no longer representing dermatologist’s interests. After all, we know skin care science better than anyone, and many dermatologists were insulted by the suggestion that we would place our personal financial gain over the best interests of our patients.
This is a perfect example of how the actions of a few unscrupulous dermatologists can affect the entire specialty. I like to focus on the ethical entrepreneurial dermatologists who made great contributions to the skin care industry based on science, efficacy, and patient education and encourage the ethical among us to provide this science-based information to our patients to protect them from pseudoscience-based opportunists. It is obvious that I believe that dermatologists have a responsibility to provide medical advice on skin care to their patients. If not us, who will do it as ethically as we will? But I plead with those of you out there who are promoting foolish stem cell–containing creams and other impossible technologies to remember that you are hurting the credibility of our entire dermatology profession.
In my next column, I will discuss dermatologists who have played a significant role behind the scenes in the development of the skin care industry.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
Castanedo-Tardan MP, Baumann L. Clin Dermatol. Jul-Aug 2009;27(4):355-8.
Gormley DE. Arch Dermatol. 1999 Jul;135(7):765-6.
Miller RC. Arch Dermatol. 1999 Mar;135(3):255-6.
Virtual Mentor. 2010;12(12):925-7.
Thanks to all the readers of Part 1 (The interesting history of dermatologist-developed skin care), who gave me interesting feedback on my Facebook and LinkedIn accounts. There was so much interest that I will write Part 3 next month. Feel free to DM me your ideas on LinkedIn.
The history of dermatologist-developed skin care continues as more dermatologists become interested in developing a skin care line or retailing skin care in their medical practice. A report in July 2020 showed that physician-dispensed skin care is the largest growing segment of the skin care business with a projected compound annual growth rate of 9.9% from 2020 to 2027. I have not seen national sales numbers since this July report, but we have noticed a large increase in online sales for the doctors using my Skin Type Solutions System. This is most likely because, in a national crisis, the self-care and beauty business segments often see growth. So as you can see, dermatologist-dispensed skin care is becoming a major player in national skin care sales. Let’s get back to the story of how this came to be the case.
Peter Elias, MD. Dr. Elias is professor in the department of dermatology at the University of California, San Francisco. In 1996, Dr. Elias published a landmark paper in the Journal of Investigative Dermatology demonstrating that a 1:1:1 ratio of ceramides, fatty acids, and cholesterol is required to repair a damaged skin barrier. He filed multiple patents for using these lipids in moisturizers as early as 1992. His lipid research has stood the test of time, and this paper is still frequently cited. Dr. Elias has authored over 500 peer reviewed articles on the skin barrier, has edited or coauthored three books on skin barrier science, and developed EpiCeram, a product that utilizes ceramide, the fatty acid linoleic acid, and cholesterol. EpiCeram is the only barrier repair moisturizer approved by the Food and Drug Administration and is available by prescription only.
Kathy Fields, MD, and Katie Rodan, MD. Dr. Fields and Dr. Rodan met at Stanford (Calif.) University. In the 1980s, these entrepreneurial dermatologists realized that patients did not understand the role of preventing acne rather than just treating it. As dermatologists, they knew that a consistent daily routine to prevent acne was much more effective than waiting for an outbreak and spot-treating lesions. They took an already available OTC medication – benzoyl peroxide – and educated consumers through infomercials that they needed to stay ahead of acne instead of waiting for a breakout. Using infomercials to sell skin care, selling skin care kits, and educating patients about the need to prevent acne rather than spot treat it was very unusual at the time. As we all know, reeducating your patients is a huge challenge. Dr. Fields and Dr. Rodan changed consumers thinking in a genius way that continues to resonate today by choosing a brand name to make their point: Proactiv. Their simple 3-step acne kit encouraged patients to be proactive about their acne and encouraged compliance. (Patients love exact skin care steps as demonstrated again by the success of the skin care line from plastic surgeon Suzan Obagi, MD, which became available around 1988).
Dr. Fields and Dr. Rodan first offered Proactiv to Neutrogena, which turned it down. This early disappointment did not deter them and ended up benefiting them because this gave them the idea to do infomercials. Guthy Renker agreed to market and distribute the product, and the first Proactiv infomercial appeared on TV in 1995. It quickly became popular and is still one of the best selling skin care lines of all time. It’s important to note that the “overnight success” of Proactiv took at least a decade of effort.
Dr. Rodan and Dr. Fields started a new skin care line called Rodan and Fields in 2002, which was sold in department stores. This was at a time when department stores were losing market share of the skin care business, and Dr. Rodan and Dr. Fields wisely relaunched in 2007 using a direct sales model similar to Mary Kay and Avon. Their ability to encourage and motivate their team is apparent in the enthusiasm seen in their sales consultants.
Heather Woolery Lloyd, MD. Dr. Woolery Lloyd got her medical degree at the University of Miami where she also completed her dermatology residency. Her interest in skin of color led to her appointment as director of Ethnic Skin Care at the University of Miami, the country’s first cosmetic ethnic skin care department at a major university. She spent years lecturing around the world on skin of color issues and performing clinical trials before she developed the “Specific Beauty” skin care line for melanin rich skin types. Specific Beauty was acquired by Guthy Renker and is available online. It is the most popular dermatologist developed skin care line for skin of color.
In the late 1980s and early 1990s, other dermatologists threw their hats into the ring and came out with skin care lines – some successful and some not. Unfortunately, many skin care lines at that time were based on “pseudoscience” and exaggerated claims, which fueled the fire of those who felt dermatologists should steer clear of these entrepreneurial pursuits. A debate about the ethics of doctors retailing skin care began, and the controversy led to this 2010 statement by the American Medical Association: “In-office sale of health-related products by physicians presents a financial conflict of interest, risks placing undue pressure on the patient, and threatens to erode patient trust and undermine the primary obligation of physicians to serve the interests of their patients before their own.”
Many dermatologists dropped out of the AMA as a result because they felt the organization was no longer representing dermatologist’s interests. After all, we know skin care science better than anyone, and many dermatologists were insulted by the suggestion that we would place our personal financial gain over the best interests of our patients.
This is a perfect example of how the actions of a few unscrupulous dermatologists can affect the entire specialty. I like to focus on the ethical entrepreneurial dermatologists who made great contributions to the skin care industry based on science, efficacy, and patient education and encourage the ethical among us to provide this science-based information to our patients to protect them from pseudoscience-based opportunists. It is obvious that I believe that dermatologists have a responsibility to provide medical advice on skin care to their patients. If not us, who will do it as ethically as we will? But I plead with those of you out there who are promoting foolish stem cell–containing creams and other impossible technologies to remember that you are hurting the credibility of our entire dermatology profession.
In my next column, I will discuss dermatologists who have played a significant role behind the scenes in the development of the skin care industry.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
Castanedo-Tardan MP, Baumann L. Clin Dermatol. Jul-Aug 2009;27(4):355-8.
Gormley DE. Arch Dermatol. 1999 Jul;135(7):765-6.
Miller RC. Arch Dermatol. 1999 Mar;135(3):255-6.
Virtual Mentor. 2010;12(12):925-7.
Dermatologists play a key role in the transformation of transgender patients
, according to Doris Day, MD.
While clinical management of this patient population has historically been limited to experts in mental health, endocrinology, and select surgeons with experience in sex reassignment surgery, “what dermatologists provide on an aesthetic level through noninvasive or minimally invasive procedures can have a big impact in helping that transformation,” Dr. Day, of the department of dermatology at New York University Langone Health, said during the virtual annual Masters of Aesthetics Symposium. “But, we have to go through a transformation of sorts as well as we care for these patients, because we need to help them in the way that best matches their needs. We need to know about their mental health and the medicines they’re taking as well as their goals for their outcomes. If they’re working with surgeons for sex reassignment, we should have discussions with those clinicians as well.”
Gender-affirming hormone therapy is the primary medical intervention sought by transgender people, she said. This allows the acquisition of secondary sex characteristics more aligned with their gender identity. Feminizing hormone therapy affects the skin by reducing sebaceous gland activity, “which can lead to fewer acne breakouts and smaller pores but also cause drier skin,” Dr. Day said. “We can slow down the growth of body and facial hair and we can perform hair removal treatments. We see decreased male-pattern scalp hair loss, and we see smoother skin as the fat under the skin becomes thicker and the pores become smaller. We can also have increased pigment production, which is always a good thing.”
In a 2016 survey of 327 transgender individuals led by Dr. Day’s mentee, Brian A. Ginsberg, MD, and published in the Journal of the American Academy of Dermatology, most transgender women indicated that their face was most important to have changed, while for men it was the chest. Hair removal was the most common women’s facial procedure, followed by surgery then injectables, mostly performed by plastic surgeons.
Limitations of hormone therapy include the fact that it can take 2 or more years for associated changes to fully develop. “At least here in New York, patients want everything in a New York minute, so that’s always an issue,” she said. “We often recommend that patients wait at least 2 years after beginning hormone therapy before considering drastic feminization surgeries, but there are many options we have for them while they’re waiting for that. Even with hormone therapy, the bone structure of the face is unaffected, so we need to be artistic in creating a more feminized balance in order to help them physically match their gender to their identity.”
Noninvasive aesthetic procedures can compound the effects of hormone therapy, in addition to offering physical transformation beyond hormone therapy. She recalled assisting one of her patients transform from male to female. Over a period of 2 years, Dr. Day added Botox then Juvederm Voluma to the patient’s cheeks and chin, “and she started her transformation to a more feminized gender matching identity,” she said. Next came a hair transplant and the injection of more Voluma and fillers in the lips and cheeks on an as-needed basis.
“During one visit, I felt that we could still do more,” Dr. Day recalled. “She looked at me and said, ‘Actually, I feel so happy. This looks like me as I imagined I would look in my mind.’ I realized that my vision for her wasn’t the same as her vision for herself. She was thrilled with her transformation. I realized that as we see these patients, for all we learn about the science of gender transformation, the emotional aspects of our vision of what we can accomplish for our patients versus their vision of what their happiness level is may not entirely match. We have to be careful to help them celebrate their version of their femininity or masculinity, rather than trying to have our patients match what we think we can accomplish for them with our own sense of what femininity or masculinity is.”
Over time, Dr. Day said, the patient’s acne scars improved with fillers and microneedling treatments, and with the hormone therapy. “As we softened her appearance and as she made changes like the earrings that she wore and the hair style that she chose, she was in line with what her perception of her femininity was,” she said. “Little by little we’ve been watching her grow into her new self. It’s been a beautiful transformation. I was honored to be able to share in that journey with her.”
Dr. Day reported having no relevant financial disclosures.
, according to Doris Day, MD.
While clinical management of this patient population has historically been limited to experts in mental health, endocrinology, and select surgeons with experience in sex reassignment surgery, “what dermatologists provide on an aesthetic level through noninvasive or minimally invasive procedures can have a big impact in helping that transformation,” Dr. Day, of the department of dermatology at New York University Langone Health, said during the virtual annual Masters of Aesthetics Symposium. “But, we have to go through a transformation of sorts as well as we care for these patients, because we need to help them in the way that best matches their needs. We need to know about their mental health and the medicines they’re taking as well as their goals for their outcomes. If they’re working with surgeons for sex reassignment, we should have discussions with those clinicians as well.”
Gender-affirming hormone therapy is the primary medical intervention sought by transgender people, she said. This allows the acquisition of secondary sex characteristics more aligned with their gender identity. Feminizing hormone therapy affects the skin by reducing sebaceous gland activity, “which can lead to fewer acne breakouts and smaller pores but also cause drier skin,” Dr. Day said. “We can slow down the growth of body and facial hair and we can perform hair removal treatments. We see decreased male-pattern scalp hair loss, and we see smoother skin as the fat under the skin becomes thicker and the pores become smaller. We can also have increased pigment production, which is always a good thing.”
In a 2016 survey of 327 transgender individuals led by Dr. Day’s mentee, Brian A. Ginsberg, MD, and published in the Journal of the American Academy of Dermatology, most transgender women indicated that their face was most important to have changed, while for men it was the chest. Hair removal was the most common women’s facial procedure, followed by surgery then injectables, mostly performed by plastic surgeons.
Limitations of hormone therapy include the fact that it can take 2 or more years for associated changes to fully develop. “At least here in New York, patients want everything in a New York minute, so that’s always an issue,” she said. “We often recommend that patients wait at least 2 years after beginning hormone therapy before considering drastic feminization surgeries, but there are many options we have for them while they’re waiting for that. Even with hormone therapy, the bone structure of the face is unaffected, so we need to be artistic in creating a more feminized balance in order to help them physically match their gender to their identity.”
Noninvasive aesthetic procedures can compound the effects of hormone therapy, in addition to offering physical transformation beyond hormone therapy. She recalled assisting one of her patients transform from male to female. Over a period of 2 years, Dr. Day added Botox then Juvederm Voluma to the patient’s cheeks and chin, “and she started her transformation to a more feminized gender matching identity,” she said. Next came a hair transplant and the injection of more Voluma and fillers in the lips and cheeks on an as-needed basis.
“During one visit, I felt that we could still do more,” Dr. Day recalled. “She looked at me and said, ‘Actually, I feel so happy. This looks like me as I imagined I would look in my mind.’ I realized that my vision for her wasn’t the same as her vision for herself. She was thrilled with her transformation. I realized that as we see these patients, for all we learn about the science of gender transformation, the emotional aspects of our vision of what we can accomplish for our patients versus their vision of what their happiness level is may not entirely match. We have to be careful to help them celebrate their version of their femininity or masculinity, rather than trying to have our patients match what we think we can accomplish for them with our own sense of what femininity or masculinity is.”
Over time, Dr. Day said, the patient’s acne scars improved with fillers and microneedling treatments, and with the hormone therapy. “As we softened her appearance and as she made changes like the earrings that she wore and the hair style that she chose, she was in line with what her perception of her femininity was,” she said. “Little by little we’ve been watching her grow into her new self. It’s been a beautiful transformation. I was honored to be able to share in that journey with her.”
Dr. Day reported having no relevant financial disclosures.
, according to Doris Day, MD.
While clinical management of this patient population has historically been limited to experts in mental health, endocrinology, and select surgeons with experience in sex reassignment surgery, “what dermatologists provide on an aesthetic level through noninvasive or minimally invasive procedures can have a big impact in helping that transformation,” Dr. Day, of the department of dermatology at New York University Langone Health, said during the virtual annual Masters of Aesthetics Symposium. “But, we have to go through a transformation of sorts as well as we care for these patients, because we need to help them in the way that best matches their needs. We need to know about their mental health and the medicines they’re taking as well as their goals for their outcomes. If they’re working with surgeons for sex reassignment, we should have discussions with those clinicians as well.”
Gender-affirming hormone therapy is the primary medical intervention sought by transgender people, she said. This allows the acquisition of secondary sex characteristics more aligned with their gender identity. Feminizing hormone therapy affects the skin by reducing sebaceous gland activity, “which can lead to fewer acne breakouts and smaller pores but also cause drier skin,” Dr. Day said. “We can slow down the growth of body and facial hair and we can perform hair removal treatments. We see decreased male-pattern scalp hair loss, and we see smoother skin as the fat under the skin becomes thicker and the pores become smaller. We can also have increased pigment production, which is always a good thing.”
In a 2016 survey of 327 transgender individuals led by Dr. Day’s mentee, Brian A. Ginsberg, MD, and published in the Journal of the American Academy of Dermatology, most transgender women indicated that their face was most important to have changed, while for men it was the chest. Hair removal was the most common women’s facial procedure, followed by surgery then injectables, mostly performed by plastic surgeons.
Limitations of hormone therapy include the fact that it can take 2 or more years for associated changes to fully develop. “At least here in New York, patients want everything in a New York minute, so that’s always an issue,” she said. “We often recommend that patients wait at least 2 years after beginning hormone therapy before considering drastic feminization surgeries, but there are many options we have for them while they’re waiting for that. Even with hormone therapy, the bone structure of the face is unaffected, so we need to be artistic in creating a more feminized balance in order to help them physically match their gender to their identity.”
Noninvasive aesthetic procedures can compound the effects of hormone therapy, in addition to offering physical transformation beyond hormone therapy. She recalled assisting one of her patients transform from male to female. Over a period of 2 years, Dr. Day added Botox then Juvederm Voluma to the patient’s cheeks and chin, “and she started her transformation to a more feminized gender matching identity,” she said. Next came a hair transplant and the injection of more Voluma and fillers in the lips and cheeks on an as-needed basis.
“During one visit, I felt that we could still do more,” Dr. Day recalled. “She looked at me and said, ‘Actually, I feel so happy. This looks like me as I imagined I would look in my mind.’ I realized that my vision for her wasn’t the same as her vision for herself. She was thrilled with her transformation. I realized that as we see these patients, for all we learn about the science of gender transformation, the emotional aspects of our vision of what we can accomplish for our patients versus their vision of what their happiness level is may not entirely match. We have to be careful to help them celebrate their version of their femininity or masculinity, rather than trying to have our patients match what we think we can accomplish for them with our own sense of what femininity or masculinity is.”
Over time, Dr. Day said, the patient’s acne scars improved with fillers and microneedling treatments, and with the hormone therapy. “As we softened her appearance and as she made changes like the earrings that she wore and the hair style that she chose, she was in line with what her perception of her femininity was,” she said. “Little by little we’ve been watching her grow into her new self. It’s been a beautiful transformation. I was honored to be able to share in that journey with her.”
Dr. Day reported having no relevant financial disclosures.
FROM MOA 2020
‘Conservative parameters’ key to maximizing cosmetic laser results in skin of color
with special considerations, according to Andrew F. Alexis, MD, MPH.
“With the devices and approaches we have today, we can achieve safe and favorable outcomes, as long as we keep in mind that there is no one-size-fits all approach,” Dr. Alexis, chair of the department of dermatology at Mount Sinai Morningside and Mount Sinai West, New York, said during the virtual annual Masters of Aesthetics Symposium. “Conservative parameters are key.”
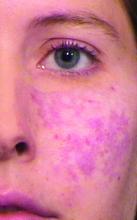
According to 2018 data from the American Society for Aesthetic Plastic Surgery, 30% of all aesthetic procedures in the United States are being performed on self-identified non-White racial ethnic groups. “This is projected to continue to increase given demographic changes as well as changes in our technologies and approaches to aesthetic procedures that allow for safer outcomes across a more diverse range of patients,” said Dr. Alexis, professor of dermatology at Icahn School of Medicine at Mount Sinai, New York. “That being said, even though we have many safe and effective options for all skin types today, we still have to consider that on the whole, there are higher risks of pigmentary and scarring complications when we perform most of our aesthetic procedures in darker skin types. The concept of limiting the degree of injury associated with a procedure remains paramount. Even when we pick the correct device for a give patient’s skin type, if our parameters aren’t optimal, or if our technique isn’t optimal, we can still end up with pigmentary and scarring complications.”
He offered key principles for maximining safety and optimal outcomes:
Know your device. Understand the range of parameters that are safe and effective for the given skin types that you see in your practice. “Don’t just rely on what the manufacturer provides in the manual, because you could have safe parameters as directed by the manual but undertreat some patients because the settings are too conservative,” Dr. Alexis said. “On the other hand, there might be scenarios where following recommended settings for a specific skin type might still wind up with a complication. Doing test spots is key in order to master the device that you are using.”
Know your patient. Don’t assume that you know a patient’s skin phototype or ancestry when that person first presents. “When we do that, we can arrive at erroneous conclusions with respect to phototype and with respect to ancestral background, and with respect to risk of pigmentary and scarring complications,” he said. “Treat your patient as an individual; no cookie-cutter responses, no assumptions.” He makes it a point to ask patients about their ancestry and about how their skin responds to sunlight in terms of tanning ability and to injury and inflammation such as insect bites, acne, and minor abrasions. “What happens to their skin when those things happen?” Dr. Alexis said. “Do they have a tendency to hyperpigment or not? You can easily ask for that or look for evidence of that on their skin. Similarly, asking about a personal or family history of keloids or hypertrophic scars is helpful in determining an overall risk assessment for a patient before you proceed with a given procedure.”
Recognize differences in preferred treatment options and parameters. Often, less is more. For example, he said, with laser hair removal, strive for longer wavelengths, lower fluences, longer pulse durations, and increased epidermal cooling. A study from 2002 in the Journal of the American Academy of Dermatology showed that the maximum tolerated fluence of type VI skin with the 1064 Nd: YAG laser was 50 J/cm2.
According to Dr. Alexis, nonablative fractional resurfacing “set the stage for being able to have safe outcomes for all skin types,” he said. “That being said, the higher the skin phototype, the higher the incidence of postinflammatory hyperpigmentation. How can we reduce this? The most important parameter is the treatment density, even though in a retrospective review from my center, high energies were associated with higher PIH rates too. Using conservative treatment densities lowers the risk of hyperpigmentation.”
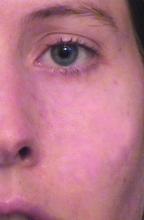
Prophylactic use of hydroquinone prior to resurfacing with fractional lasers is another way to minimize the risk of postinflammatory hyperpigmentation. With this approach, Dr. Alexis asks patients to apply hydroquinone two weeks before treatment and for at least 4 weeks after. “Sun protection is key,” he said. “But when taking all of this into account, using conservative treatment densities in the range of 11%-20% coverage with a 1,550-nm Erbium-doped fractional laser, you can get favorable outcomes across skin types. But sometimes you can wind up with complications even if you do the right things.” He recalled a patient he treated for acne scarring and atrophic scars. After three treatments with the nonablative fractional 1,550-nm Erbium-doped laser set at level 4 (11% coverage), the patient developed hyperpigmentation of the treatment area. Dr. Alexis chose to continue treatment “with a few tweaks to reduce the risk of further hyperpigmentation,” he said. “I reduced the treatment density and the number of passes by half, so that the total energy delivered was halved. I also increased the concentration of hydroquinone from 4% to 6%. With that, the postinflammatory hyperpigmentation resolved.”
Another tool for resurfacing is the microsecond 1,064-nm Nd:YAG laser. “No anesthesia is required, there’s minimal down time, and you can treat all skin types,” Dr. Alexis said. “No pre- or posttreatment prophylaxis with bleaching agents are necessary, but multiple laser treatment sessions are required in order to achieve clinically meaningful results.” His approach to treating types V and VI skin involves a 1,064-nm Nd:YAG laser with a 5-mm spot size, a 0.3-microsecond pulse duration, a fluence of 12-14 J/cm2, a repetition rate of 5-8 Hz, 1,000-2,000 pulses per cosmetic unit, and avoidance of pulse stacking. He generally performs 4-6 treatment sessions 2-6 weeks apart.
An additional option for resurfacing is the 650-microsecond 1,064-nm Nd:YAG laser. The recommend fluence in skin of color is 14-21 J/cm2. A recent review article in the Journal of Drugs in Dermatology described clinical experience using this device for a wide range of conditions in darker skin types, including acne, hyperpigmentation, and melasma.
A more recent approach is using fractional radiofrequency devices, especially those that feature coated pin tips. These tips “protect the epidermis from heat injury and deliver heat to the deeper dermis where we want it, and minimize the risk to the epidermis,” Dr. Alexis said. In a 2018 study in the Journal of Drugs in Dermatology of 35 patients with skin type VI, participants received three sessions of facial treatments, 4 weeks apart using a fractional RF device with 24-pin coated tip. The researchers found that the regimen was safe and effective, and that it resulted in improved wrinkles, acne scars, and overall skin appearance.
Dr. Alexis disclosed that he has served as an adviser to or has received consulting fees from Leo, Novartis, Menlo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Unilever, Celgene, Beiersdorf, Valeant, L’Oreal, BMS, Scientis, Bausch Health, UCB, Foamix, and Cassiopea.
with special considerations, according to Andrew F. Alexis, MD, MPH.
“With the devices and approaches we have today, we can achieve safe and favorable outcomes, as long as we keep in mind that there is no one-size-fits all approach,” Dr. Alexis, chair of the department of dermatology at Mount Sinai Morningside and Mount Sinai West, New York, said during the virtual annual Masters of Aesthetics Symposium. “Conservative parameters are key.”
According to 2018 data from the American Society for Aesthetic Plastic Surgery, 30% of all aesthetic procedures in the United States are being performed on self-identified non-White racial ethnic groups. “This is projected to continue to increase given demographic changes as well as changes in our technologies and approaches to aesthetic procedures that allow for safer outcomes across a more diverse range of patients,” said Dr. Alexis, professor of dermatology at Icahn School of Medicine at Mount Sinai, New York. “That being said, even though we have many safe and effective options for all skin types today, we still have to consider that on the whole, there are higher risks of pigmentary and scarring complications when we perform most of our aesthetic procedures in darker skin types. The concept of limiting the degree of injury associated with a procedure remains paramount. Even when we pick the correct device for a give patient’s skin type, if our parameters aren’t optimal, or if our technique isn’t optimal, we can still end up with pigmentary and scarring complications.”
He offered key principles for maximining safety and optimal outcomes:
Know your device. Understand the range of parameters that are safe and effective for the given skin types that you see in your practice. “Don’t just rely on what the manufacturer provides in the manual, because you could have safe parameters as directed by the manual but undertreat some patients because the settings are too conservative,” Dr. Alexis said. “On the other hand, there might be scenarios where following recommended settings for a specific skin type might still wind up with a complication. Doing test spots is key in order to master the device that you are using.”
Know your patient. Don’t assume that you know a patient’s skin phototype or ancestry when that person first presents. “When we do that, we can arrive at erroneous conclusions with respect to phototype and with respect to ancestral background, and with respect to risk of pigmentary and scarring complications,” he said. “Treat your patient as an individual; no cookie-cutter responses, no assumptions.” He makes it a point to ask patients about their ancestry and about how their skin responds to sunlight in terms of tanning ability and to injury and inflammation such as insect bites, acne, and minor abrasions. “What happens to their skin when those things happen?” Dr. Alexis said. “Do they have a tendency to hyperpigment or not? You can easily ask for that or look for evidence of that on their skin. Similarly, asking about a personal or family history of keloids or hypertrophic scars is helpful in determining an overall risk assessment for a patient before you proceed with a given procedure.”
Recognize differences in preferred treatment options and parameters. Often, less is more. For example, he said, with laser hair removal, strive for longer wavelengths, lower fluences, longer pulse durations, and increased epidermal cooling. A study from 2002 in the Journal of the American Academy of Dermatology showed that the maximum tolerated fluence of type VI skin with the 1064 Nd: YAG laser was 50 J/cm2.
According to Dr. Alexis, nonablative fractional resurfacing “set the stage for being able to have safe outcomes for all skin types,” he said. “That being said, the higher the skin phototype, the higher the incidence of postinflammatory hyperpigmentation. How can we reduce this? The most important parameter is the treatment density, even though in a retrospective review from my center, high energies were associated with higher PIH rates too. Using conservative treatment densities lowers the risk of hyperpigmentation.”
Prophylactic use of hydroquinone prior to resurfacing with fractional lasers is another way to minimize the risk of postinflammatory hyperpigmentation. With this approach, Dr. Alexis asks patients to apply hydroquinone two weeks before treatment and for at least 4 weeks after. “Sun protection is key,” he said. “But when taking all of this into account, using conservative treatment densities in the range of 11%-20% coverage with a 1,550-nm Erbium-doped fractional laser, you can get favorable outcomes across skin types. But sometimes you can wind up with complications even if you do the right things.” He recalled a patient he treated for acne scarring and atrophic scars. After three treatments with the nonablative fractional 1,550-nm Erbium-doped laser set at level 4 (11% coverage), the patient developed hyperpigmentation of the treatment area. Dr. Alexis chose to continue treatment “with a few tweaks to reduce the risk of further hyperpigmentation,” he said. “I reduced the treatment density and the number of passes by half, so that the total energy delivered was halved. I also increased the concentration of hydroquinone from 4% to 6%. With that, the postinflammatory hyperpigmentation resolved.”
Another tool for resurfacing is the microsecond 1,064-nm Nd:YAG laser. “No anesthesia is required, there’s minimal down time, and you can treat all skin types,” Dr. Alexis said. “No pre- or posttreatment prophylaxis with bleaching agents are necessary, but multiple laser treatment sessions are required in order to achieve clinically meaningful results.” His approach to treating types V and VI skin involves a 1,064-nm Nd:YAG laser with a 5-mm spot size, a 0.3-microsecond pulse duration, a fluence of 12-14 J/cm2, a repetition rate of 5-8 Hz, 1,000-2,000 pulses per cosmetic unit, and avoidance of pulse stacking. He generally performs 4-6 treatment sessions 2-6 weeks apart.
An additional option for resurfacing is the 650-microsecond 1,064-nm Nd:YAG laser. The recommend fluence in skin of color is 14-21 J/cm2. A recent review article in the Journal of Drugs in Dermatology described clinical experience using this device for a wide range of conditions in darker skin types, including acne, hyperpigmentation, and melasma.
A more recent approach is using fractional radiofrequency devices, especially those that feature coated pin tips. These tips “protect the epidermis from heat injury and deliver heat to the deeper dermis where we want it, and minimize the risk to the epidermis,” Dr. Alexis said. In a 2018 study in the Journal of Drugs in Dermatology of 35 patients with skin type VI, participants received three sessions of facial treatments, 4 weeks apart using a fractional RF device with 24-pin coated tip. The researchers found that the regimen was safe and effective, and that it resulted in improved wrinkles, acne scars, and overall skin appearance.
Dr. Alexis disclosed that he has served as an adviser to or has received consulting fees from Leo, Novartis, Menlo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Unilever, Celgene, Beiersdorf, Valeant, L’Oreal, BMS, Scientis, Bausch Health, UCB, Foamix, and Cassiopea.
with special considerations, according to Andrew F. Alexis, MD, MPH.
“With the devices and approaches we have today, we can achieve safe and favorable outcomes, as long as we keep in mind that there is no one-size-fits all approach,” Dr. Alexis, chair of the department of dermatology at Mount Sinai Morningside and Mount Sinai West, New York, said during the virtual annual Masters of Aesthetics Symposium. “Conservative parameters are key.”
According to 2018 data from the American Society for Aesthetic Plastic Surgery, 30% of all aesthetic procedures in the United States are being performed on self-identified non-White racial ethnic groups. “This is projected to continue to increase given demographic changes as well as changes in our technologies and approaches to aesthetic procedures that allow for safer outcomes across a more diverse range of patients,” said Dr. Alexis, professor of dermatology at Icahn School of Medicine at Mount Sinai, New York. “That being said, even though we have many safe and effective options for all skin types today, we still have to consider that on the whole, there are higher risks of pigmentary and scarring complications when we perform most of our aesthetic procedures in darker skin types. The concept of limiting the degree of injury associated with a procedure remains paramount. Even when we pick the correct device for a give patient’s skin type, if our parameters aren’t optimal, or if our technique isn’t optimal, we can still end up with pigmentary and scarring complications.”
He offered key principles for maximining safety and optimal outcomes:
Know your device. Understand the range of parameters that are safe and effective for the given skin types that you see in your practice. “Don’t just rely on what the manufacturer provides in the manual, because you could have safe parameters as directed by the manual but undertreat some patients because the settings are too conservative,” Dr. Alexis said. “On the other hand, there might be scenarios where following recommended settings for a specific skin type might still wind up with a complication. Doing test spots is key in order to master the device that you are using.”
Know your patient. Don’t assume that you know a patient’s skin phototype or ancestry when that person first presents. “When we do that, we can arrive at erroneous conclusions with respect to phototype and with respect to ancestral background, and with respect to risk of pigmentary and scarring complications,” he said. “Treat your patient as an individual; no cookie-cutter responses, no assumptions.” He makes it a point to ask patients about their ancestry and about how their skin responds to sunlight in terms of tanning ability and to injury and inflammation such as insect bites, acne, and minor abrasions. “What happens to their skin when those things happen?” Dr. Alexis said. “Do they have a tendency to hyperpigment or not? You can easily ask for that or look for evidence of that on their skin. Similarly, asking about a personal or family history of keloids or hypertrophic scars is helpful in determining an overall risk assessment for a patient before you proceed with a given procedure.”
Recognize differences in preferred treatment options and parameters. Often, less is more. For example, he said, with laser hair removal, strive for longer wavelengths, lower fluences, longer pulse durations, and increased epidermal cooling. A study from 2002 in the Journal of the American Academy of Dermatology showed that the maximum tolerated fluence of type VI skin with the 1064 Nd: YAG laser was 50 J/cm2.
According to Dr. Alexis, nonablative fractional resurfacing “set the stage for being able to have safe outcomes for all skin types,” he said. “That being said, the higher the skin phototype, the higher the incidence of postinflammatory hyperpigmentation. How can we reduce this? The most important parameter is the treatment density, even though in a retrospective review from my center, high energies were associated with higher PIH rates too. Using conservative treatment densities lowers the risk of hyperpigmentation.”
Prophylactic use of hydroquinone prior to resurfacing with fractional lasers is another way to minimize the risk of postinflammatory hyperpigmentation. With this approach, Dr. Alexis asks patients to apply hydroquinone two weeks before treatment and for at least 4 weeks after. “Sun protection is key,” he said. “But when taking all of this into account, using conservative treatment densities in the range of 11%-20% coverage with a 1,550-nm Erbium-doped fractional laser, you can get favorable outcomes across skin types. But sometimes you can wind up with complications even if you do the right things.” He recalled a patient he treated for acne scarring and atrophic scars. After three treatments with the nonablative fractional 1,550-nm Erbium-doped laser set at level 4 (11% coverage), the patient developed hyperpigmentation of the treatment area. Dr. Alexis chose to continue treatment “with a few tweaks to reduce the risk of further hyperpigmentation,” he said. “I reduced the treatment density and the number of passes by half, so that the total energy delivered was halved. I also increased the concentration of hydroquinone from 4% to 6%. With that, the postinflammatory hyperpigmentation resolved.”
Another tool for resurfacing is the microsecond 1,064-nm Nd:YAG laser. “No anesthesia is required, there’s minimal down time, and you can treat all skin types,” Dr. Alexis said. “No pre- or posttreatment prophylaxis with bleaching agents are necessary, but multiple laser treatment sessions are required in order to achieve clinically meaningful results.” His approach to treating types V and VI skin involves a 1,064-nm Nd:YAG laser with a 5-mm spot size, a 0.3-microsecond pulse duration, a fluence of 12-14 J/cm2, a repetition rate of 5-8 Hz, 1,000-2,000 pulses per cosmetic unit, and avoidance of pulse stacking. He generally performs 4-6 treatment sessions 2-6 weeks apart.
An additional option for resurfacing is the 650-microsecond 1,064-nm Nd:YAG laser. The recommend fluence in skin of color is 14-21 J/cm2. A recent review article in the Journal of Drugs in Dermatology described clinical experience using this device for a wide range of conditions in darker skin types, including acne, hyperpigmentation, and melasma.
A more recent approach is using fractional radiofrequency devices, especially those that feature coated pin tips. These tips “protect the epidermis from heat injury and deliver heat to the deeper dermis where we want it, and minimize the risk to the epidermis,” Dr. Alexis said. In a 2018 study in the Journal of Drugs in Dermatology of 35 patients with skin type VI, participants received three sessions of facial treatments, 4 weeks apart using a fractional RF device with 24-pin coated tip. The researchers found that the regimen was safe and effective, and that it resulted in improved wrinkles, acne scars, and overall skin appearance.
Dr. Alexis disclosed that he has served as an adviser to or has received consulting fees from Leo, Novartis, Menlo, Galderma, Pfizer, Sanofi-Regeneron, Dermavant, Unilever, Celgene, Beiersdorf, Valeant, L’Oreal, BMS, Scientis, Bausch Health, UCB, Foamix, and Cassiopea.
AT MOA 2020
RAP device being investigated as a way to improve appearance of cellulite
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
.
“The procedure is relatively painless, without anesthesia and can easily be delegated with physician oversight,” Mathew M. Avram, MD, JD, said during the virtual annual Masters of Aesthetics Symposium. “Side effects have been minimal and transient to date. There is no down time.”
According to Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples.
“Basically, what you have is a repetition rate and very short rise time that provide microscopic mechanical disruption to the targeted cellular level structures and vacuoles,” Dr. Avram explained. “There’s a high leak pressure and fast repetition rate that exploits the viscoelastic nature of the tissue. You get compressed pulses from electronic filtering and the reflector shape eliminates cavitation, heat, and pain.”
The procedure takes 20-30 minutes to perform and it generates minimal heat and pain, “which is an advantage of the treatment,” he said. “It is completely noninvasive, with no incision whatsoever. No anesthetic is required. There can be physician oversight of delivery, so it is delegable, and there is no recovery time. More study is needed, and we need to stay tuned.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
FROM MOA 2020
No one-size-fits-all approach to tissue-tightening devices
.
“There are many devices on the market, but their efficacy is not consistent,” Catherine M. DiGiorgio, MS, MD, said during the virtual annual Masters of Aesthetics Symposium. “The key to maximizing patient satisfaction is patient selection and setting realistic expectations.”
She avoids recommending the use of tissue-tightening devices for patients who require surgical correction and for those who find the idea of minimal improvement unacceptable. “These are not the treatments for them,” she said. “I also find that when a patient uses her fingers to pull her face back and says, ‘I want to look like this,’ this is not the right patient for these devices. They can get a good amount of improvement, but efficacy is not consistent.”
Still, patients favor noninvasive or minimally invasive procedures for skin tightening now more than ever before. “They are not willing to undergo surgical treatments, and they want something with low downtime,” she said.
Dr. DiGiorgio, who practices at the Boston Center for Facial Rejuvenation, began a review of tissue-tightening devices on the market by discussing the role of ablative fractional lasers such as the carbon dioxide 10,600-nm laser and the Erbium:YAG 2,940-nm laser, which carry risks and downtime. “I don’t view these lasers as a tissue-tightening devices, but they are included because they can provide a little bit of tightening,” she said.
The ideal candidate is someone with skin type I-II and mild skin laxity. “These lasers are really good at improving rhytides,” she noted. “The patient needs to be able to tolerate the discomfort and manage the healing process. Sometimes you can get blepharoplastylike results with some patients. This can be combined with vascular lasers and pigment-targeting lasers to improve the overall texture and tone of the skin. Many combine this with a face-lift or a blepharoplasty. You should wait at least 6-8 weeks after a face-lift before performing this procedure. Some plastic surgeons do combine this with blepharoplasty in the same visit.”
A less invasive option for skin tightening is the delivery of radiofrequency energy, which disrupts hydrogen bonds of the collagen triple helix. This occurs in temperatures greater than 60° C and results in collagen contraction and tightening and neocollagenesis. There are several devices available including transcutaneous monopolar radiofrequency (Thermage, TempSure), subsurface thermistor–controlled monopolar radiofrequency (ThermiTight), and fractional microneedling radiofrequency (Profound RF, Genius RF, Vivace, and Secret RF). The transcutaneous monopolar radiofrequency device delivers energy uniformly via a treatment tip that has contact cooling and coupling fluid. Collagen is denatured at 65° C and fibroblasts are stimulated to form new collagen. The healing process provides additional tightening.
“These treatments are noninvasive; there’s no downtime, and there’s mild discomfort,” Dr. DiGiorgio commented. “Treatments can be done around the eyes, on the face and body. When treating around the eyes with these devices you want to use a corneal plastic eye shield. Contraindications include having a pacemaker, defibrillator, or other electronic implantable device.”
In her opinion, the ideal patient for this device has mild skin laxity or is younger and seeking to maintain a youthful appearance. “It’s great for mild upper eyelid laxity and for temporary improvement of cellulite appearance,” she said. “The patient should not require surgical intervention and the patient should also agree to undergo multiple treatment sessions. Just one treatment session is not going to cut it.”
Another device in this class of technology is subsurface thermistor–controlled monopolar radiofrequency, “which is basically a probe that’s inserted into the skin, most commonly in the submental area,” Dr. DiGiorgio said. An external infrared camera monitors the epidermal temperature, which should not exceed 45°C. This results in a controlled deep dermal and subdermal delivery of thermal energy. “It requires light tumescent anesthesia, and it can be combined with liposuction,” she said. “Common side effects include erythema, edema, and bruising, and sometimes contour irregularities or nodules.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical correction. “You can combine this with liposuction, but you can achieve good results without it,” she said.
The next device in this class of technology that Dr. DiGiorgio discussed is fractional microneedling radiofrequency. Of several such devices on the market, some have adjustable depths up to 4 mm while others have fixed depths. The energy is adjustable, and the tips can be insulated or noninsulated. “Insulated tips make it safer to perform in darker skin types because the proximal portion of the needle is insulated and the epidermis is spared from damage,” she explained. “Some devices are a bit more painful than others. It does require topical anesthesia; some require local injection anesthesia. Patients have erythema for about 24 hours, and treatments are recommended monthly.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical intervention but who seeks to maintain a youthful appearance. “Patients should understand that multiple treatments will be required to achieve optimal results,” she said. “I find that there is less improvement in older patients. This can be combined with thread lifts, vascular lasers, pigment-targeting lasers, and CO2 lasers.”
The next device for skin tightening that she discussed is microfocused ultrasound (Ultherapy), which delivers millisecond domain pulses at three different depths that are determined by the transducer that you use. It can go as deep as 4.5 mm. “Each pulse delivers a focal zone of coagulation to achieve tissue contraction,” Dr. DiGiorgio said. “There’s an ultrasound-imaging device attached to it to ensure proper skin contact and the delivery of energy at an appropriate depth. Patients can have a little bit of pain and erythema and edema, sometime bruising. Usually there is not much downtime with these treatments.”
A newcomer in this class of technology is SoftWave, an intense ultrasound beam array (IUB), which delivers energy precisely to the middermis at a depth of 1.5 mm. “With each pulse, the hand piece has seven transducers that deliver energy in 3-dimensional cylindrical thermal zones,” Dr. DiGiorgio said. “You get greater than 25% tissue coverage in one treatment, and there is no injury to the epidermis or deeper structures. It has unique vectors that are along the lines of facial wrinkles, so you get tightening along those lines.”
The procedure takes about 30 minutes, there is no downtime, and it causes no pain, she said. Pretreatment, patients receive topical anesthesia. “This device has active skin cooling and has an ultrasound gel,” she added. “It does not have an imaging platform like the microfocused ultrasound does, because the depth is fixed. You get significant wrinkle reduction and decrease in submental fullness with improvement in jawline definition, eyebrow position, fine lines, and texture.” In her opinion, the ideal candidate for this device is a patient in the mid-40s to early 50s with mild to moderate elastosis, fullness, texture irregularities, laxity, rhytids, elastosis, and photoaging.
She reported having no financial disclosures.
.
“There are many devices on the market, but their efficacy is not consistent,” Catherine M. DiGiorgio, MS, MD, said during the virtual annual Masters of Aesthetics Symposium. “The key to maximizing patient satisfaction is patient selection and setting realistic expectations.”
She avoids recommending the use of tissue-tightening devices for patients who require surgical correction and for those who find the idea of minimal improvement unacceptable. “These are not the treatments for them,” she said. “I also find that when a patient uses her fingers to pull her face back and says, ‘I want to look like this,’ this is not the right patient for these devices. They can get a good amount of improvement, but efficacy is not consistent.”
Still, patients favor noninvasive or minimally invasive procedures for skin tightening now more than ever before. “They are not willing to undergo surgical treatments, and they want something with low downtime,” she said.
Dr. DiGiorgio, who practices at the Boston Center for Facial Rejuvenation, began a review of tissue-tightening devices on the market by discussing the role of ablative fractional lasers such as the carbon dioxide 10,600-nm laser and the Erbium:YAG 2,940-nm laser, which carry risks and downtime. “I don’t view these lasers as a tissue-tightening devices, but they are included because they can provide a little bit of tightening,” she said.
The ideal candidate is someone with skin type I-II and mild skin laxity. “These lasers are really good at improving rhytides,” she noted. “The patient needs to be able to tolerate the discomfort and manage the healing process. Sometimes you can get blepharoplastylike results with some patients. This can be combined with vascular lasers and pigment-targeting lasers to improve the overall texture and tone of the skin. Many combine this with a face-lift or a blepharoplasty. You should wait at least 6-8 weeks after a face-lift before performing this procedure. Some plastic surgeons do combine this with blepharoplasty in the same visit.”
A less invasive option for skin tightening is the delivery of radiofrequency energy, which disrupts hydrogen bonds of the collagen triple helix. This occurs in temperatures greater than 60° C and results in collagen contraction and tightening and neocollagenesis. There are several devices available including transcutaneous monopolar radiofrequency (Thermage, TempSure), subsurface thermistor–controlled monopolar radiofrequency (ThermiTight), and fractional microneedling radiofrequency (Profound RF, Genius RF, Vivace, and Secret RF). The transcutaneous monopolar radiofrequency device delivers energy uniformly via a treatment tip that has contact cooling and coupling fluid. Collagen is denatured at 65° C and fibroblasts are stimulated to form new collagen. The healing process provides additional tightening.
“These treatments are noninvasive; there’s no downtime, and there’s mild discomfort,” Dr. DiGiorgio commented. “Treatments can be done around the eyes, on the face and body. When treating around the eyes with these devices you want to use a corneal plastic eye shield. Contraindications include having a pacemaker, defibrillator, or other electronic implantable device.”
In her opinion, the ideal patient for this device has mild skin laxity or is younger and seeking to maintain a youthful appearance. “It’s great for mild upper eyelid laxity and for temporary improvement of cellulite appearance,” she said. “The patient should not require surgical intervention and the patient should also agree to undergo multiple treatment sessions. Just one treatment session is not going to cut it.”
Another device in this class of technology is subsurface thermistor–controlled monopolar radiofrequency, “which is basically a probe that’s inserted into the skin, most commonly in the submental area,” Dr. DiGiorgio said. An external infrared camera monitors the epidermal temperature, which should not exceed 45°C. This results in a controlled deep dermal and subdermal delivery of thermal energy. “It requires light tumescent anesthesia, and it can be combined with liposuction,” she said. “Common side effects include erythema, edema, and bruising, and sometimes contour irregularities or nodules.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical correction. “You can combine this with liposuction, but you can achieve good results without it,” she said.
The next device in this class of technology that Dr. DiGiorgio discussed is fractional microneedling radiofrequency. Of several such devices on the market, some have adjustable depths up to 4 mm while others have fixed depths. The energy is adjustable, and the tips can be insulated or noninsulated. “Insulated tips make it safer to perform in darker skin types because the proximal portion of the needle is insulated and the epidermis is spared from damage,” she explained. “Some devices are a bit more painful than others. It does require topical anesthesia; some require local injection anesthesia. Patients have erythema for about 24 hours, and treatments are recommended monthly.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical intervention but who seeks to maintain a youthful appearance. “Patients should understand that multiple treatments will be required to achieve optimal results,” she said. “I find that there is less improvement in older patients. This can be combined with thread lifts, vascular lasers, pigment-targeting lasers, and CO2 lasers.”
The next device for skin tightening that she discussed is microfocused ultrasound (Ultherapy), which delivers millisecond domain pulses at three different depths that are determined by the transducer that you use. It can go as deep as 4.5 mm. “Each pulse delivers a focal zone of coagulation to achieve tissue contraction,” Dr. DiGiorgio said. “There’s an ultrasound-imaging device attached to it to ensure proper skin contact and the delivery of energy at an appropriate depth. Patients can have a little bit of pain and erythema and edema, sometime bruising. Usually there is not much downtime with these treatments.”
A newcomer in this class of technology is SoftWave, an intense ultrasound beam array (IUB), which delivers energy precisely to the middermis at a depth of 1.5 mm. “With each pulse, the hand piece has seven transducers that deliver energy in 3-dimensional cylindrical thermal zones,” Dr. DiGiorgio said. “You get greater than 25% tissue coverage in one treatment, and there is no injury to the epidermis or deeper structures. It has unique vectors that are along the lines of facial wrinkles, so you get tightening along those lines.”
The procedure takes about 30 minutes, there is no downtime, and it causes no pain, she said. Pretreatment, patients receive topical anesthesia. “This device has active skin cooling and has an ultrasound gel,” she added. “It does not have an imaging platform like the microfocused ultrasound does, because the depth is fixed. You get significant wrinkle reduction and decrease in submental fullness with improvement in jawline definition, eyebrow position, fine lines, and texture.” In her opinion, the ideal candidate for this device is a patient in the mid-40s to early 50s with mild to moderate elastosis, fullness, texture irregularities, laxity, rhytids, elastosis, and photoaging.
She reported having no financial disclosures.
.
“There are many devices on the market, but their efficacy is not consistent,” Catherine M. DiGiorgio, MS, MD, said during the virtual annual Masters of Aesthetics Symposium. “The key to maximizing patient satisfaction is patient selection and setting realistic expectations.”
She avoids recommending the use of tissue-tightening devices for patients who require surgical correction and for those who find the idea of minimal improvement unacceptable. “These are not the treatments for them,” she said. “I also find that when a patient uses her fingers to pull her face back and says, ‘I want to look like this,’ this is not the right patient for these devices. They can get a good amount of improvement, but efficacy is not consistent.”
Still, patients favor noninvasive or minimally invasive procedures for skin tightening now more than ever before. “They are not willing to undergo surgical treatments, and they want something with low downtime,” she said.
Dr. DiGiorgio, who practices at the Boston Center for Facial Rejuvenation, began a review of tissue-tightening devices on the market by discussing the role of ablative fractional lasers such as the carbon dioxide 10,600-nm laser and the Erbium:YAG 2,940-nm laser, which carry risks and downtime. “I don’t view these lasers as a tissue-tightening devices, but they are included because they can provide a little bit of tightening,” she said.
The ideal candidate is someone with skin type I-II and mild skin laxity. “These lasers are really good at improving rhytides,” she noted. “The patient needs to be able to tolerate the discomfort and manage the healing process. Sometimes you can get blepharoplastylike results with some patients. This can be combined with vascular lasers and pigment-targeting lasers to improve the overall texture and tone of the skin. Many combine this with a face-lift or a blepharoplasty. You should wait at least 6-8 weeks after a face-lift before performing this procedure. Some plastic surgeons do combine this with blepharoplasty in the same visit.”
A less invasive option for skin tightening is the delivery of radiofrequency energy, which disrupts hydrogen bonds of the collagen triple helix. This occurs in temperatures greater than 60° C and results in collagen contraction and tightening and neocollagenesis. There are several devices available including transcutaneous monopolar radiofrequency (Thermage, TempSure), subsurface thermistor–controlled monopolar radiofrequency (ThermiTight), and fractional microneedling radiofrequency (Profound RF, Genius RF, Vivace, and Secret RF). The transcutaneous monopolar radiofrequency device delivers energy uniformly via a treatment tip that has contact cooling and coupling fluid. Collagen is denatured at 65° C and fibroblasts are stimulated to form new collagen. The healing process provides additional tightening.
“These treatments are noninvasive; there’s no downtime, and there’s mild discomfort,” Dr. DiGiorgio commented. “Treatments can be done around the eyes, on the face and body. When treating around the eyes with these devices you want to use a corneal plastic eye shield. Contraindications include having a pacemaker, defibrillator, or other electronic implantable device.”
In her opinion, the ideal patient for this device has mild skin laxity or is younger and seeking to maintain a youthful appearance. “It’s great for mild upper eyelid laxity and for temporary improvement of cellulite appearance,” she said. “The patient should not require surgical intervention and the patient should also agree to undergo multiple treatment sessions. Just one treatment session is not going to cut it.”
Another device in this class of technology is subsurface thermistor–controlled monopolar radiofrequency, “which is basically a probe that’s inserted into the skin, most commonly in the submental area,” Dr. DiGiorgio said. An external infrared camera monitors the epidermal temperature, which should not exceed 45°C. This results in a controlled deep dermal and subdermal delivery of thermal energy. “It requires light tumescent anesthesia, and it can be combined with liposuction,” she said. “Common side effects include erythema, edema, and bruising, and sometimes contour irregularities or nodules.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical correction. “You can combine this with liposuction, but you can achieve good results without it,” she said.
The next device in this class of technology that Dr. DiGiorgio discussed is fractional microneedling radiofrequency. Of several such devices on the market, some have adjustable depths up to 4 mm while others have fixed depths. The energy is adjustable, and the tips can be insulated or noninsulated. “Insulated tips make it safer to perform in darker skin types because the proximal portion of the needle is insulated and the epidermis is spared from damage,” she explained. “Some devices are a bit more painful than others. It does require topical anesthesia; some require local injection anesthesia. Patients have erythema for about 24 hours, and treatments are recommended monthly.” In her opinion, the ideal candidate for this device is someone with mild to moderate skin laxity who does not require surgical intervention but who seeks to maintain a youthful appearance. “Patients should understand that multiple treatments will be required to achieve optimal results,” she said. “I find that there is less improvement in older patients. This can be combined with thread lifts, vascular lasers, pigment-targeting lasers, and CO2 lasers.”
The next device for skin tightening that she discussed is microfocused ultrasound (Ultherapy), which delivers millisecond domain pulses at three different depths that are determined by the transducer that you use. It can go as deep as 4.5 mm. “Each pulse delivers a focal zone of coagulation to achieve tissue contraction,” Dr. DiGiorgio said. “There’s an ultrasound-imaging device attached to it to ensure proper skin contact and the delivery of energy at an appropriate depth. Patients can have a little bit of pain and erythema and edema, sometime bruising. Usually there is not much downtime with these treatments.”
A newcomer in this class of technology is SoftWave, an intense ultrasound beam array (IUB), which delivers energy precisely to the middermis at a depth of 1.5 mm. “With each pulse, the hand piece has seven transducers that deliver energy in 3-dimensional cylindrical thermal zones,” Dr. DiGiorgio said. “You get greater than 25% tissue coverage in one treatment, and there is no injury to the epidermis or deeper structures. It has unique vectors that are along the lines of facial wrinkles, so you get tightening along those lines.”
The procedure takes about 30 minutes, there is no downtime, and it causes no pain, she said. Pretreatment, patients receive topical anesthesia. “This device has active skin cooling and has an ultrasound gel,” she added. “It does not have an imaging platform like the microfocused ultrasound does, because the depth is fixed. You get significant wrinkle reduction and decrease in submental fullness with improvement in jawline definition, eyebrow position, fine lines, and texture.” In her opinion, the ideal candidate for this device is a patient in the mid-40s to early 50s with mild to moderate elastosis, fullness, texture irregularities, laxity, rhytids, elastosis, and photoaging.
She reported having no financial disclosures.
REPORTING FROM MOA 2020
Innovator banks on ‘truly smart’ robotic lasers in dermatology
Dr. Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, conceived and developed many of the nonscarring laser treatments now widely used in dermatology. These include selective photothermolysis for birthmarks, microvascular and pigmented lesions, and tattoo and permanent hair removal. He also contributed to laser lithotripsy, laser angioplasty, photodynamic therapy, and optical diagnostics. The highest-resolution imaging device approved for human use, an infrared confocal microscope, came from his laboratory. Dr. Anderson has also contributed to basic knowledge of human photobiology, drug photosensitization mechanisms, tissue optics and laser-tissue interactions. In this Q&A with Doug Brunk, he reflects on his achievements and on the future of lasers in dermatology.
In published interviews you have described yourself as more of a problem solver than an inventor. How did your upbringing foster your affinity for problem solving?
I grew up in Central Illinois during the 1950s and early 1960s, an area known for corn, soybeans, and hogs. At an early age I learned to be interested in other things because it’s possible to die of boredom there. By the time I was 12 years old, I was an amateur radio operator, and I was building rockets to see how high they would go.
Problem solving comes naturally to me. I enjoy very much finding a problem that is worth solving, which means getting passionate about it and brainstorming. Half the time you don’t come up with a potential route to solve the problem. I attended the Massachusetts Institute of Technology at the age of 17, which was a real eye-opener. I had never been east of the Wabash River prior to that. I studied physics for a while, then decided to flip into biology. That combination has served me well. My special sauce is to have some intuitive and academic rigorous feeling for physical processes. But we physicians have a front row seat to nature’s human drama. There is no lack of problems to solve. I can sit around and obsess about things theoretically, but at the end of the day I want to work on things that ultimately benefit people.
What inspired you most early in your career as a physician scientist?
After I made a commitment to medicine, Dr. John A. Parrish, and Dr. Thomas B. Fitzpatrick were key mentors to me. I was 30 years old when I started medical school, but they took me under their wing even before that. I took a part-time, temporary job with them, which turned into a permanent job. That turned into a love for the work they did. Instead of going into a graduate program in a laboratory and studying bacteria and genetics, the whole idea of working with people and on people was awesome. Dr. Parrish really mentored me. I won a lifetime achievement award from the American Academy of Dermatology a few years ago. I found myself on stage and it rolled out of my mouth that John Parrish believed in me before I believed in myself. It’s really true. He somehow recognized that I had some talents. I was very young and a combination of naive and humble, I guess.
What was the initial genesis for your idea of selective photothermolysis?
I was interested in going to medical school and working with Dr. Fitzpatrick and Dr. Parrish on things related to light. They were mostly interested in PUVA and UVB; it was the heyday of modern phototherapy. I attended a lecture at the Beth Israel Hospital in Boston given by a plastic surgeon, Dr. Joel Mark Noe. He was talking about using lasers to treat port-wine stains in children. The gist of the talk was that argon lasers were being used, and that the results were sometimes decent, but not great. Often children would have burn scars after the treatment. Dr. Noe was talking about how you had to choose the color of the wavelength of the laser to be absorbed by hemoglobin, but he wasn’t talking about what happens to the heat once it’s created. My background in physics led me to recognize that he wasn’t capturing the full picture. Selective disruption of a target in the skin by light is half of the story. The confinement of heat in the target is the other half of the story. Literally on a bus on the way home from that lecture to my apartment in Cambridge, I hatched the idea for selective photothermolysis and wrote down some equations. I also wrote down the ideal wavelength region, how much energy was needed, and what the pulse duration would have to be like to damage target vessels that small. I showed John Parrish what I had written. He took me seriously and said, “Let’s see if we can find a light source that can accomplish this.” We traveled around the country looking at various lasers, but we wound up building the first pulsed dye laser for treating port-wine stains. To me, the surprise was that we didn’t kill the skin. If you treat an area of skin with a laser and hurt all the blood vessels, you think, “Wait a minute. Are we going to kill the skin because it has no blood supply?” The questions of the day were so basic, and we just got lucky. It took 6-8 years before we ramped up the clinical studies showing efficacy and safety of this technology.
I presume that you experimented on your own skin while developing some of the nonscarring laser treatments now widely used in dermatology. What “war story” stands out to you most from that part of your work?
I’m right handed, so I’d grab a laser with my right hand and treat my left arm, so that arm sports a bit of history. In 1994, while working with Dr. Melanie Grossman on the development of laser hair removal, I used a ruby laser to self-treat a patch of hair on my left arm. I still have the world’s oldest laser-induced bald spot on that arm. It’s been 26 years now. I still look at it and count the hairs, because one of the big questions is, is laser hair removal permanent? In all these years I have grown two hairs.
What technology that you conceived of or developed has most surprised you, in term of its ultimate clinical impact?
I would say confocal microscopy. In the mid-1990s I worked with a physicist named Robert H. Webb, who invented an imaging system for the retina. We got together, noodled about it, and decided we would modify his ophthalmoscope system to see if we could get images from inside the skin. It worked pretty well. It was truly surprising from many points of view. First, it wasn’t clear at all that we’d get any images this way. Now, reflectance confocal microscopy is a standard tool in both clinical and research dermatology. But there were odd discoveries early on. For example, the darker your skin, the brighter it appeared in the microscope. You might think that melanin absorbs light and that you would get poor images in dark skin. It was the exact opposite; melanin acts as a natural contrast agent.
We worked with a small company to make the first confocal microscope. Initially, it had no clinical applications but what was fascinating to me was the incredible value of being able to see inside human skin harmlessly, and just see what’s going on. It became a potent research tool, and recently CPT codes were established for its use in evaluating skin cancer margins. I wouldn’t be surprised if 30 years from now, taking a skin biopsy is seemingly barbaric. A forerunner of all these new imaging tools for the skin was the confocal microscope developed in my lab in 1994.
During a 2011 TED talk, you said that nevus of Ota is your favorite thing to treat, because the outcome is usually perfect skin. Are there other technologies or devices you played a role in developing that make you proud at this stage in your career?
The reason I love treating nevus of Ota is that you have a lifelong facial disfigurement, and the only treatment for it is a laser we came up with, and it always works. How perfect could it be? The flip side of the same coin is, there are lesions of the skin that just don’t respond. One of the things we don’t know enough about is the connection between the biologic aspects of repair of various lesions and the treatments that we come up with. The most recent example of selective photothermolysis is a new laser we’re building right now for acne that is based on sebaceous gland injury. You’ll see this coming out in the next year or two. My heart goes out to people with nodular cystic acne. For young men it’s highly associated with suicide. So, I’m excited about optimizing and learning what happens when we target sebaceous glands.
One of the other big stories in laser dermatology is the fractional laser. I developed this with Dr. Dieter Manstein when he was a postdoc in my lab. One of the most pleasing things from this technology is how well you can rehabilitate scars, particularly burn scars in children. Over the last few years, I have trained plastic surgeons at the Shriners Hospital for Children in Boston on how to use fractional lasers to improve the lives of these kids. Another technology I developed with Dr. Manstein is cryolipolysis, which is removing fat from the body by cooling it. There are no lasers involved with this technology. I like to say that I’ve spent most of my career studying light and heat, and now we’ve come up with something that’s cold in the dark. We are now working on derivatives of cryolipolysis, to determine if what we’ve learned about targeting fat that might be applicable elsewhere.
Who inspires you most in your work today?
In addition to Dr. John Parrish and Dr. Thomas Fitzpatrick, the late Dr. Albert M. Kligman also influenced me. He never accepted dogma and he loved to ask questions, like, “What if?” as opposed to just accumulating a fund of knowledge. Understanding things is not just based on how much you know. It’s based on critical thinking and the ability to question. I also admire Albert Einstein, his ability to sit down with nothing more than pencil and paper and change our view of the universe. I love physics because it’s the science of everything. I also love poetry. My favorite poet is Stanley Kunitz. He had amazing insight and was named United States Poet Laureate in 1974 and in 2000. I have plenty of antiheroes as well, mostly politicians.
I understand that you play the banjo. How long have you been playing, and what do you enjoy about it?
You cannot sit down and play the banjo and have your mind on much else. It’s a wonderful moving meditation. Before my medical career, I was a schoolteacher in Vermont. There was a guy on the staff there who played banjo. He came from a small town in Georgia. I just picked it up and started plunking. It’s a happy instrument. It’s awfully hard to make the banjo sound melancholy.
What novel use of lasers and light in dermatology are you most excited about in the next 5 years?
The marriage of therapeutic devices with diagnostic and imaging devices has not happened yet. They are not even in the honeymoon moment. But I think that having truly smart robotic systems in our hands for treating patients will become a reality. These days, dermatologists have to buy a certain type of laser to treat a certain type of lesion. For example, the Q-switched alexandrite laser you buy for treating Nevus of Ota won’t do anything for a port-wine stain; it’s the wrong pulse duration. This means that clinicians who practice a lot of laser dermatology end up with a dozen lasers in their practice. In the future, I think it will be possible to have a software laser, so when you want to acquire another target, you load an App as opposed to buying a new laser. This means that you would have software programmable targeting, and you would not have the requirement of having selective absorption. So, I’m excited by the idea of guided fractional lasers. None of them exist now. We have to start from scratch.
Dr. Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, conceived and developed many of the nonscarring laser treatments now widely used in dermatology. These include selective photothermolysis for birthmarks, microvascular and pigmented lesions, and tattoo and permanent hair removal. He also contributed to laser lithotripsy, laser angioplasty, photodynamic therapy, and optical diagnostics. The highest-resolution imaging device approved for human use, an infrared confocal microscope, came from his laboratory. Dr. Anderson has also contributed to basic knowledge of human photobiology, drug photosensitization mechanisms, tissue optics and laser-tissue interactions. In this Q&A with Doug Brunk, he reflects on his achievements and on the future of lasers in dermatology.
In published interviews you have described yourself as more of a problem solver than an inventor. How did your upbringing foster your affinity for problem solving?
I grew up in Central Illinois during the 1950s and early 1960s, an area known for corn, soybeans, and hogs. At an early age I learned to be interested in other things because it’s possible to die of boredom there. By the time I was 12 years old, I was an amateur radio operator, and I was building rockets to see how high they would go.
Problem solving comes naturally to me. I enjoy very much finding a problem that is worth solving, which means getting passionate about it and brainstorming. Half the time you don’t come up with a potential route to solve the problem. I attended the Massachusetts Institute of Technology at the age of 17, which was a real eye-opener. I had never been east of the Wabash River prior to that. I studied physics for a while, then decided to flip into biology. That combination has served me well. My special sauce is to have some intuitive and academic rigorous feeling for physical processes. But we physicians have a front row seat to nature’s human drama. There is no lack of problems to solve. I can sit around and obsess about things theoretically, but at the end of the day I want to work on things that ultimately benefit people.
What inspired you most early in your career as a physician scientist?
After I made a commitment to medicine, Dr. John A. Parrish, and Dr. Thomas B. Fitzpatrick were key mentors to me. I was 30 years old when I started medical school, but they took me under their wing even before that. I took a part-time, temporary job with them, which turned into a permanent job. That turned into a love for the work they did. Instead of going into a graduate program in a laboratory and studying bacteria and genetics, the whole idea of working with people and on people was awesome. Dr. Parrish really mentored me. I won a lifetime achievement award from the American Academy of Dermatology a few years ago. I found myself on stage and it rolled out of my mouth that John Parrish believed in me before I believed in myself. It’s really true. He somehow recognized that I had some talents. I was very young and a combination of naive and humble, I guess.
What was the initial genesis for your idea of selective photothermolysis?
I was interested in going to medical school and working with Dr. Fitzpatrick and Dr. Parrish on things related to light. They were mostly interested in PUVA and UVB; it was the heyday of modern phototherapy. I attended a lecture at the Beth Israel Hospital in Boston given by a plastic surgeon, Dr. Joel Mark Noe. He was talking about using lasers to treat port-wine stains in children. The gist of the talk was that argon lasers were being used, and that the results were sometimes decent, but not great. Often children would have burn scars after the treatment. Dr. Noe was talking about how you had to choose the color of the wavelength of the laser to be absorbed by hemoglobin, but he wasn’t talking about what happens to the heat once it’s created. My background in physics led me to recognize that he wasn’t capturing the full picture. Selective disruption of a target in the skin by light is half of the story. The confinement of heat in the target is the other half of the story. Literally on a bus on the way home from that lecture to my apartment in Cambridge, I hatched the idea for selective photothermolysis and wrote down some equations. I also wrote down the ideal wavelength region, how much energy was needed, and what the pulse duration would have to be like to damage target vessels that small. I showed John Parrish what I had written. He took me seriously and said, “Let’s see if we can find a light source that can accomplish this.” We traveled around the country looking at various lasers, but we wound up building the first pulsed dye laser for treating port-wine stains. To me, the surprise was that we didn’t kill the skin. If you treat an area of skin with a laser and hurt all the blood vessels, you think, “Wait a minute. Are we going to kill the skin because it has no blood supply?” The questions of the day were so basic, and we just got lucky. It took 6-8 years before we ramped up the clinical studies showing efficacy and safety of this technology.
I presume that you experimented on your own skin while developing some of the nonscarring laser treatments now widely used in dermatology. What “war story” stands out to you most from that part of your work?
I’m right handed, so I’d grab a laser with my right hand and treat my left arm, so that arm sports a bit of history. In 1994, while working with Dr. Melanie Grossman on the development of laser hair removal, I used a ruby laser to self-treat a patch of hair on my left arm. I still have the world’s oldest laser-induced bald spot on that arm. It’s been 26 years now. I still look at it and count the hairs, because one of the big questions is, is laser hair removal permanent? In all these years I have grown two hairs.
What technology that you conceived of or developed has most surprised you, in term of its ultimate clinical impact?
I would say confocal microscopy. In the mid-1990s I worked with a physicist named Robert H. Webb, who invented an imaging system for the retina. We got together, noodled about it, and decided we would modify his ophthalmoscope system to see if we could get images from inside the skin. It worked pretty well. It was truly surprising from many points of view. First, it wasn’t clear at all that we’d get any images this way. Now, reflectance confocal microscopy is a standard tool in both clinical and research dermatology. But there were odd discoveries early on. For example, the darker your skin, the brighter it appeared in the microscope. You might think that melanin absorbs light and that you would get poor images in dark skin. It was the exact opposite; melanin acts as a natural contrast agent.
We worked with a small company to make the first confocal microscope. Initially, it had no clinical applications but what was fascinating to me was the incredible value of being able to see inside human skin harmlessly, and just see what’s going on. It became a potent research tool, and recently CPT codes were established for its use in evaluating skin cancer margins. I wouldn’t be surprised if 30 years from now, taking a skin biopsy is seemingly barbaric. A forerunner of all these new imaging tools for the skin was the confocal microscope developed in my lab in 1994.
During a 2011 TED talk, you said that nevus of Ota is your favorite thing to treat, because the outcome is usually perfect skin. Are there other technologies or devices you played a role in developing that make you proud at this stage in your career?
The reason I love treating nevus of Ota is that you have a lifelong facial disfigurement, and the only treatment for it is a laser we came up with, and it always works. How perfect could it be? The flip side of the same coin is, there are lesions of the skin that just don’t respond. One of the things we don’t know enough about is the connection between the biologic aspects of repair of various lesions and the treatments that we come up with. The most recent example of selective photothermolysis is a new laser we’re building right now for acne that is based on sebaceous gland injury. You’ll see this coming out in the next year or two. My heart goes out to people with nodular cystic acne. For young men it’s highly associated with suicide. So, I’m excited about optimizing and learning what happens when we target sebaceous glands.
One of the other big stories in laser dermatology is the fractional laser. I developed this with Dr. Dieter Manstein when he was a postdoc in my lab. One of the most pleasing things from this technology is how well you can rehabilitate scars, particularly burn scars in children. Over the last few years, I have trained plastic surgeons at the Shriners Hospital for Children in Boston on how to use fractional lasers to improve the lives of these kids. Another technology I developed with Dr. Manstein is cryolipolysis, which is removing fat from the body by cooling it. There are no lasers involved with this technology. I like to say that I’ve spent most of my career studying light and heat, and now we’ve come up with something that’s cold in the dark. We are now working on derivatives of cryolipolysis, to determine if what we’ve learned about targeting fat that might be applicable elsewhere.
Who inspires you most in your work today?
In addition to Dr. John Parrish and Dr. Thomas Fitzpatrick, the late Dr. Albert M. Kligman also influenced me. He never accepted dogma and he loved to ask questions, like, “What if?” as opposed to just accumulating a fund of knowledge. Understanding things is not just based on how much you know. It’s based on critical thinking and the ability to question. I also admire Albert Einstein, his ability to sit down with nothing more than pencil and paper and change our view of the universe. I love physics because it’s the science of everything. I also love poetry. My favorite poet is Stanley Kunitz. He had amazing insight and was named United States Poet Laureate in 1974 and in 2000. I have plenty of antiheroes as well, mostly politicians.
I understand that you play the banjo. How long have you been playing, and what do you enjoy about it?
You cannot sit down and play the banjo and have your mind on much else. It’s a wonderful moving meditation. Before my medical career, I was a schoolteacher in Vermont. There was a guy on the staff there who played banjo. He came from a small town in Georgia. I just picked it up and started plunking. It’s a happy instrument. It’s awfully hard to make the banjo sound melancholy.
What novel use of lasers and light in dermatology are you most excited about in the next 5 years?
The marriage of therapeutic devices with diagnostic and imaging devices has not happened yet. They are not even in the honeymoon moment. But I think that having truly smart robotic systems in our hands for treating patients will become a reality. These days, dermatologists have to buy a certain type of laser to treat a certain type of lesion. For example, the Q-switched alexandrite laser you buy for treating Nevus of Ota won’t do anything for a port-wine stain; it’s the wrong pulse duration. This means that clinicians who practice a lot of laser dermatology end up with a dozen lasers in their practice. In the future, I think it will be possible to have a software laser, so when you want to acquire another target, you load an App as opposed to buying a new laser. This means that you would have software programmable targeting, and you would not have the requirement of having selective absorption. So, I’m excited by the idea of guided fractional lasers. None of them exist now. We have to start from scratch.
Dr. Anderson, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, conceived and developed many of the nonscarring laser treatments now widely used in dermatology. These include selective photothermolysis for birthmarks, microvascular and pigmented lesions, and tattoo and permanent hair removal. He also contributed to laser lithotripsy, laser angioplasty, photodynamic therapy, and optical diagnostics. The highest-resolution imaging device approved for human use, an infrared confocal microscope, came from his laboratory. Dr. Anderson has also contributed to basic knowledge of human photobiology, drug photosensitization mechanisms, tissue optics and laser-tissue interactions. In this Q&A with Doug Brunk, he reflects on his achievements and on the future of lasers in dermatology.
In published interviews you have described yourself as more of a problem solver than an inventor. How did your upbringing foster your affinity for problem solving?
I grew up in Central Illinois during the 1950s and early 1960s, an area known for corn, soybeans, and hogs. At an early age I learned to be interested in other things because it’s possible to die of boredom there. By the time I was 12 years old, I was an amateur radio operator, and I was building rockets to see how high they would go.
Problem solving comes naturally to me. I enjoy very much finding a problem that is worth solving, which means getting passionate about it and brainstorming. Half the time you don’t come up with a potential route to solve the problem. I attended the Massachusetts Institute of Technology at the age of 17, which was a real eye-opener. I had never been east of the Wabash River prior to that. I studied physics for a while, then decided to flip into biology. That combination has served me well. My special sauce is to have some intuitive and academic rigorous feeling for physical processes. But we physicians have a front row seat to nature’s human drama. There is no lack of problems to solve. I can sit around and obsess about things theoretically, but at the end of the day I want to work on things that ultimately benefit people.
What inspired you most early in your career as a physician scientist?
After I made a commitment to medicine, Dr. John A. Parrish, and Dr. Thomas B. Fitzpatrick were key mentors to me. I was 30 years old when I started medical school, but they took me under their wing even before that. I took a part-time, temporary job with them, which turned into a permanent job. That turned into a love for the work they did. Instead of going into a graduate program in a laboratory and studying bacteria and genetics, the whole idea of working with people and on people was awesome. Dr. Parrish really mentored me. I won a lifetime achievement award from the American Academy of Dermatology a few years ago. I found myself on stage and it rolled out of my mouth that John Parrish believed in me before I believed in myself. It’s really true. He somehow recognized that I had some talents. I was very young and a combination of naive and humble, I guess.
What was the initial genesis for your idea of selective photothermolysis?
I was interested in going to medical school and working with Dr. Fitzpatrick and Dr. Parrish on things related to light. They were mostly interested in PUVA and UVB; it was the heyday of modern phototherapy. I attended a lecture at the Beth Israel Hospital in Boston given by a plastic surgeon, Dr. Joel Mark Noe. He was talking about using lasers to treat port-wine stains in children. The gist of the talk was that argon lasers were being used, and that the results were sometimes decent, but not great. Often children would have burn scars after the treatment. Dr. Noe was talking about how you had to choose the color of the wavelength of the laser to be absorbed by hemoglobin, but he wasn’t talking about what happens to the heat once it’s created. My background in physics led me to recognize that he wasn’t capturing the full picture. Selective disruption of a target in the skin by light is half of the story. The confinement of heat in the target is the other half of the story. Literally on a bus on the way home from that lecture to my apartment in Cambridge, I hatched the idea for selective photothermolysis and wrote down some equations. I also wrote down the ideal wavelength region, how much energy was needed, and what the pulse duration would have to be like to damage target vessels that small. I showed John Parrish what I had written. He took me seriously and said, “Let’s see if we can find a light source that can accomplish this.” We traveled around the country looking at various lasers, but we wound up building the first pulsed dye laser for treating port-wine stains. To me, the surprise was that we didn’t kill the skin. If you treat an area of skin with a laser and hurt all the blood vessels, you think, “Wait a minute. Are we going to kill the skin because it has no blood supply?” The questions of the day were so basic, and we just got lucky. It took 6-8 years before we ramped up the clinical studies showing efficacy and safety of this technology.
I presume that you experimented on your own skin while developing some of the nonscarring laser treatments now widely used in dermatology. What “war story” stands out to you most from that part of your work?
I’m right handed, so I’d grab a laser with my right hand and treat my left arm, so that arm sports a bit of history. In 1994, while working with Dr. Melanie Grossman on the development of laser hair removal, I used a ruby laser to self-treat a patch of hair on my left arm. I still have the world’s oldest laser-induced bald spot on that arm. It’s been 26 years now. I still look at it and count the hairs, because one of the big questions is, is laser hair removal permanent? In all these years I have grown two hairs.
What technology that you conceived of or developed has most surprised you, in term of its ultimate clinical impact?
I would say confocal microscopy. In the mid-1990s I worked with a physicist named Robert H. Webb, who invented an imaging system for the retina. We got together, noodled about it, and decided we would modify his ophthalmoscope system to see if we could get images from inside the skin. It worked pretty well. It was truly surprising from many points of view. First, it wasn’t clear at all that we’d get any images this way. Now, reflectance confocal microscopy is a standard tool in both clinical and research dermatology. But there were odd discoveries early on. For example, the darker your skin, the brighter it appeared in the microscope. You might think that melanin absorbs light and that you would get poor images in dark skin. It was the exact opposite; melanin acts as a natural contrast agent.
We worked with a small company to make the first confocal microscope. Initially, it had no clinical applications but what was fascinating to me was the incredible value of being able to see inside human skin harmlessly, and just see what’s going on. It became a potent research tool, and recently CPT codes were established for its use in evaluating skin cancer margins. I wouldn’t be surprised if 30 years from now, taking a skin biopsy is seemingly barbaric. A forerunner of all these new imaging tools for the skin was the confocal microscope developed in my lab in 1994.
During a 2011 TED talk, you said that nevus of Ota is your favorite thing to treat, because the outcome is usually perfect skin. Are there other technologies or devices you played a role in developing that make you proud at this stage in your career?
The reason I love treating nevus of Ota is that you have a lifelong facial disfigurement, and the only treatment for it is a laser we came up with, and it always works. How perfect could it be? The flip side of the same coin is, there are lesions of the skin that just don’t respond. One of the things we don’t know enough about is the connection between the biologic aspects of repair of various lesions and the treatments that we come up with. The most recent example of selective photothermolysis is a new laser we’re building right now for acne that is based on sebaceous gland injury. You’ll see this coming out in the next year or two. My heart goes out to people with nodular cystic acne. For young men it’s highly associated with suicide. So, I’m excited about optimizing and learning what happens when we target sebaceous glands.
One of the other big stories in laser dermatology is the fractional laser. I developed this with Dr. Dieter Manstein when he was a postdoc in my lab. One of the most pleasing things from this technology is how well you can rehabilitate scars, particularly burn scars in children. Over the last few years, I have trained plastic surgeons at the Shriners Hospital for Children in Boston on how to use fractional lasers to improve the lives of these kids. Another technology I developed with Dr. Manstein is cryolipolysis, which is removing fat from the body by cooling it. There are no lasers involved with this technology. I like to say that I’ve spent most of my career studying light and heat, and now we’ve come up with something that’s cold in the dark. We are now working on derivatives of cryolipolysis, to determine if what we’ve learned about targeting fat that might be applicable elsewhere.
Who inspires you most in your work today?
In addition to Dr. John Parrish and Dr. Thomas Fitzpatrick, the late Dr. Albert M. Kligman also influenced me. He never accepted dogma and he loved to ask questions, like, “What if?” as opposed to just accumulating a fund of knowledge. Understanding things is not just based on how much you know. It’s based on critical thinking and the ability to question. I also admire Albert Einstein, his ability to sit down with nothing more than pencil and paper and change our view of the universe. I love physics because it’s the science of everything. I also love poetry. My favorite poet is Stanley Kunitz. He had amazing insight and was named United States Poet Laureate in 1974 and in 2000. I have plenty of antiheroes as well, mostly politicians.
I understand that you play the banjo. How long have you been playing, and what do you enjoy about it?
You cannot sit down and play the banjo and have your mind on much else. It’s a wonderful moving meditation. Before my medical career, I was a schoolteacher in Vermont. There was a guy on the staff there who played banjo. He came from a small town in Georgia. I just picked it up and started plunking. It’s a happy instrument. It’s awfully hard to make the banjo sound melancholy.
What novel use of lasers and light in dermatology are you most excited about in the next 5 years?
The marriage of therapeutic devices with diagnostic and imaging devices has not happened yet. They are not even in the honeymoon moment. But I think that having truly smart robotic systems in our hands for treating patients will become a reality. These days, dermatologists have to buy a certain type of laser to treat a certain type of lesion. For example, the Q-switched alexandrite laser you buy for treating Nevus of Ota won’t do anything for a port-wine stain; it’s the wrong pulse duration. This means that clinicians who practice a lot of laser dermatology end up with a dozen lasers in their practice. In the future, I think it will be possible to have a software laser, so when you want to acquire another target, you load an App as opposed to buying a new laser. This means that you would have software programmable targeting, and you would not have the requirement of having selective absorption. So, I’m excited by the idea of guided fractional lasers. None of them exist now. We have to start from scratch.
Visionary reflects on the importance of teamwork in advancing technology
When John A. Parrish, MD, worked with R. Rox Anderson, MD, and a team of clinicians and scientists in the early 1980s to develop the first pulsed dye laser for dermatologic use, it became clear that the Food and Drug Administration required convincing that their prototype would be safe.
“Laser medicine was new, and lasers had some specific frightening risks like blindness and bleeding from laser suturing,” recalled Dr. Parrish, founder of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The main issue was eye risk. Because the operator and the patient were at risk for eye injury, the FDA was reluctant to press on with laser treatments of skin.”
To make the FDA more comfortable with their efforts, Dr. Parrish and his colleagues drew from the published work of ophthalmologists, who were ahead of dermatologists in the clinical use of lasers. “A lot of the animal experiments and the human understanding of laser-tissue interactions came from ophthalmologists,” he said. “We worked with a fellow named David H. Sliney, PhD. He was very interested in laser safety of the eye, so we worked closely with him to measure the boundary conditions that could be used without injuring the eye.”
To Dr. Parrish, forging that partnership illustrated a key principle in developing novel diagnostics and therapeutics that use lasers and light: You need a multidisciplinary team. “You need a pathologist, clinicians, physicists, technologists, and engineers, because all of the barriers to figure out how to deliver a new treatment safely often don’t rest in one person’s mind, so early on we had to be very collaborative and find experts who would help us solve problems,” he said. “That’s how the Wellman Labs got started. All of the new treatments were explored by multidisciplinary teams so that we didn’t have to hope that the expertise to get past all the barriers was in one person’s mind. That was often not the case.”
Dr. Parrish credits his mentor, the late Thomas B. Fitzpatrick, MD, PhD, who in 1975 devised the Fitzpatrick scale of skin phototypes, with inspiring his career path. Dr. Fitzpatrick, who is widely considered the father of modern academic dermatology, was professor and chief of dermatology at Harvard Medical School when Dr. Parrish began his dermatology training there. “He was a great clinician who loved patient care and he was a very curious investigator,” said Dr. Parrish, who cofounded the Consortia for Improving Medicine with Innovation and Technology (CIMIT). “He not only trained me, but I became his collaborator during my early faculty time. What I learned most from him was the joy of work, curiosity, and serious commitment to patient care. It was almost contagious.”
Of all the devices he’s played a role in developing in the past 50 years, Dr. Parrish said that he remains most surprised by the impact of pulsed lasers in dermatology. “It took us a while to understand the capabilities of pulsed lasers in that they could confine injury to small spots and treat multiple areas at once,” he said. “A lot of that did not come because we were so wise to think about that, but we did a lot of work in the early days with a free-electron laser, a pulsed laser which had a tunable wavelength and a tunable pulse duration. That gave us the capability of looking at very specific injuries and the host responses that heal without scarring.”
Dr. Parrish’s interest in dermatology was piqued in 1968, when he was assigned to Oak Knoll Naval Hospital in Oakland, Calif., after a year of serving in the U.S. Marine Corps as a battlefield doctor in Vietnam. (He wrote about his wartime experience in two books, most recently “Autopsy of War: A Personal History” [New York: Thomas Dunne Books, 2012].) Prior to serving in Vietnam he had completed early internal medicine training, but once at Oak Knoll he discovered that he had a propensity for diagnosing and treating disorders of the skin. “When I came back to resume my residency, I asked if I could train in dermatology,” he said. “It was by happenstance. I felt like I could understand skin disease and that I could make a difference. In internal medicine you often change blood pressure medicines around. I felt like I was a better diagnostician than in internal medicine and that I could most often make a difference. I liked seeing all ages of patients, and most of them got better, so it was more fun.”
When John A. Parrish, MD, worked with R. Rox Anderson, MD, and a team of clinicians and scientists in the early 1980s to develop the first pulsed dye laser for dermatologic use, it became clear that the Food and Drug Administration required convincing that their prototype would be safe.
“Laser medicine was new, and lasers had some specific frightening risks like blindness and bleeding from laser suturing,” recalled Dr. Parrish, founder of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The main issue was eye risk. Because the operator and the patient were at risk for eye injury, the FDA was reluctant to press on with laser treatments of skin.”
To make the FDA more comfortable with their efforts, Dr. Parrish and his colleagues drew from the published work of ophthalmologists, who were ahead of dermatologists in the clinical use of lasers. “A lot of the animal experiments and the human understanding of laser-tissue interactions came from ophthalmologists,” he said. “We worked with a fellow named David H. Sliney, PhD. He was very interested in laser safety of the eye, so we worked closely with him to measure the boundary conditions that could be used without injuring the eye.”
To Dr. Parrish, forging that partnership illustrated a key principle in developing novel diagnostics and therapeutics that use lasers and light: You need a multidisciplinary team. “You need a pathologist, clinicians, physicists, technologists, and engineers, because all of the barriers to figure out how to deliver a new treatment safely often don’t rest in one person’s mind, so early on we had to be very collaborative and find experts who would help us solve problems,” he said. “That’s how the Wellman Labs got started. All of the new treatments were explored by multidisciplinary teams so that we didn’t have to hope that the expertise to get past all the barriers was in one person’s mind. That was often not the case.”
Dr. Parrish credits his mentor, the late Thomas B. Fitzpatrick, MD, PhD, who in 1975 devised the Fitzpatrick scale of skin phototypes, with inspiring his career path. Dr. Fitzpatrick, who is widely considered the father of modern academic dermatology, was professor and chief of dermatology at Harvard Medical School when Dr. Parrish began his dermatology training there. “He was a great clinician who loved patient care and he was a very curious investigator,” said Dr. Parrish, who cofounded the Consortia for Improving Medicine with Innovation and Technology (CIMIT). “He not only trained me, but I became his collaborator during my early faculty time. What I learned most from him was the joy of work, curiosity, and serious commitment to patient care. It was almost contagious.”
Of all the devices he’s played a role in developing in the past 50 years, Dr. Parrish said that he remains most surprised by the impact of pulsed lasers in dermatology. “It took us a while to understand the capabilities of pulsed lasers in that they could confine injury to small spots and treat multiple areas at once,” he said. “A lot of that did not come because we were so wise to think about that, but we did a lot of work in the early days with a free-electron laser, a pulsed laser which had a tunable wavelength and a tunable pulse duration. That gave us the capability of looking at very specific injuries and the host responses that heal without scarring.”
Dr. Parrish’s interest in dermatology was piqued in 1968, when he was assigned to Oak Knoll Naval Hospital in Oakland, Calif., after a year of serving in the U.S. Marine Corps as a battlefield doctor in Vietnam. (He wrote about his wartime experience in two books, most recently “Autopsy of War: A Personal History” [New York: Thomas Dunne Books, 2012].) Prior to serving in Vietnam he had completed early internal medicine training, but once at Oak Knoll he discovered that he had a propensity for diagnosing and treating disorders of the skin. “When I came back to resume my residency, I asked if I could train in dermatology,” he said. “It was by happenstance. I felt like I could understand skin disease and that I could make a difference. In internal medicine you often change blood pressure medicines around. I felt like I was a better diagnostician than in internal medicine and that I could most often make a difference. I liked seeing all ages of patients, and most of them got better, so it was more fun.”
When John A. Parrish, MD, worked with R. Rox Anderson, MD, and a team of clinicians and scientists in the early 1980s to develop the first pulsed dye laser for dermatologic use, it became clear that the Food and Drug Administration required convincing that their prototype would be safe.
“Laser medicine was new, and lasers had some specific frightening risks like blindness and bleeding from laser suturing,” recalled Dr. Parrish, founder of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “The main issue was eye risk. Because the operator and the patient were at risk for eye injury, the FDA was reluctant to press on with laser treatments of skin.”
To make the FDA more comfortable with their efforts, Dr. Parrish and his colleagues drew from the published work of ophthalmologists, who were ahead of dermatologists in the clinical use of lasers. “A lot of the animal experiments and the human understanding of laser-tissue interactions came from ophthalmologists,” he said. “We worked with a fellow named David H. Sliney, PhD. He was very interested in laser safety of the eye, so we worked closely with him to measure the boundary conditions that could be used without injuring the eye.”
To Dr. Parrish, forging that partnership illustrated a key principle in developing novel diagnostics and therapeutics that use lasers and light: You need a multidisciplinary team. “You need a pathologist, clinicians, physicists, technologists, and engineers, because all of the barriers to figure out how to deliver a new treatment safely often don’t rest in one person’s mind, so early on we had to be very collaborative and find experts who would help us solve problems,” he said. “That’s how the Wellman Labs got started. All of the new treatments were explored by multidisciplinary teams so that we didn’t have to hope that the expertise to get past all the barriers was in one person’s mind. That was often not the case.”
Dr. Parrish credits his mentor, the late Thomas B. Fitzpatrick, MD, PhD, who in 1975 devised the Fitzpatrick scale of skin phototypes, with inspiring his career path. Dr. Fitzpatrick, who is widely considered the father of modern academic dermatology, was professor and chief of dermatology at Harvard Medical School when Dr. Parrish began his dermatology training there. “He was a great clinician who loved patient care and he was a very curious investigator,” said Dr. Parrish, who cofounded the Consortia for Improving Medicine with Innovation and Technology (CIMIT). “He not only trained me, but I became his collaborator during my early faculty time. What I learned most from him was the joy of work, curiosity, and serious commitment to patient care. It was almost contagious.”
Of all the devices he’s played a role in developing in the past 50 years, Dr. Parrish said that he remains most surprised by the impact of pulsed lasers in dermatology. “It took us a while to understand the capabilities of pulsed lasers in that they could confine injury to small spots and treat multiple areas at once,” he said. “A lot of that did not come because we were so wise to think about that, but we did a lot of work in the early days with a free-electron laser, a pulsed laser which had a tunable wavelength and a tunable pulse duration. That gave us the capability of looking at very specific injuries and the host responses that heal without scarring.”
Dr. Parrish’s interest in dermatology was piqued in 1968, when he was assigned to Oak Knoll Naval Hospital in Oakland, Calif., after a year of serving in the U.S. Marine Corps as a battlefield doctor in Vietnam. (He wrote about his wartime experience in two books, most recently “Autopsy of War: A Personal History” [New York: Thomas Dunne Books, 2012].) Prior to serving in Vietnam he had completed early internal medicine training, but once at Oak Knoll he discovered that he had a propensity for diagnosing and treating disorders of the skin. “When I came back to resume my residency, I asked if I could train in dermatology,” he said. “It was by happenstance. I felt like I could understand skin disease and that I could make a difference. In internal medicine you often change blood pressure medicines around. I felt like I was a better diagnostician than in internal medicine and that I could most often make a difference. I liked seeing all ages of patients, and most of them got better, so it was more fun.”
Experts reflect on the past 50 years of lasers in dermatology
During her dermatology residency at Yale University in the late 1980s, Tina S. Alster, MD, met a 44-year-old woman who changed the trajectory of her professional career.
During her clinic visit, the woman explained that she always wore heavy facial makeup to hide her port-wine stain birthmark – a vascular malformation that she kept secret from her husband and teenage son. “She was very good about covering it,” recalled Dr. Alster, who is the founding director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington. “She removed a small amount of makeup for me so I could take a look at it. I had just finished reading an article about using a laser for birthmarks; it had just been published. I told her, ‘There’s something new; we don’t have it at Yale, but I read about treatment that could hone in on birthmarks.’ I promised her I would find out more details.”
A few days later, Dr. Alster pored through stacks of medical journals at Yale’s library and relocated the article she’d seen by first author Oon Tian Tan, MD, PhD, of the department of dermatology at Boston University Medical Center. It described use of the flashpump-pulsed tunable dye laser to treat port-wine stains in 35 children (N Engl J Med. 1989;320:416-21). After giving the article a more thorough read, Dr. Alster became so intrigued by the technology it described that she moved to Boston the following year for a dermatology fellowship with Dr. Tan and joined the ranks of early clinicians who used lasers for treating port-wine stains and other dermatologic conditions.
“That was at a time when there were only a handful of pulsed dye lasers in the world, and the first time I used it was when I went to Boston,” she said. “It was life-changing. You think, ‘Isn’t this great for children with port-wine stains.’ Your heart breaks for them, but I also felt compassion for adults who had suffered a lifetime of stares, including the woman who propelled me to look into this. She ended up coming to Boston during my fellowship and had her birthmark removed, so I changed her life, but she changed mine as well.”
The real credit for that series of events, Dr. Alster continued, belongs to John A. Parrish, MD, and R. Rox Anderson, MD, who in 1983 published the concept of selective photothermolysis, a seminal work that shifted the paradigm for how lasers and other light sources are designed for skin diseases and conditions (Science. 1983 Apr 29;220(4596):524-7). The first pulsed dye laser was built on this concept, an approach that minimized or eliminated the unwanted tissue damage and significant scarring that impeded therapeutic use of laser energy for port-wine stains and other lesions prior to that time. “Lasers that were built subsequent to that seminal paper focused our attention on building lasers that were specific for treatment of certain skin conditions,” Dr. Alster said. “Selective photothermolysis catapulted not only our understanding of how lasers interact with the skin, but allowed us to identify things in the skin that we could potentially target with this new laser technology, and to build laser systems that were specific to those purposes.”
In the late 1970s, Dr. Parrish, who played a key role in making psoralens plus ultraviolet A safe and effective for patients with severe psoriasis, turned his attention to studying lasers in his lab at Harvard Medical School. He hired R. Rox Anderson, a recent graduate of the Massachusetts Institute of Technology, as a technician. “Rox then got interested in medicine and went to medical school at Harvard, got interested in dermatology, and then worked in my lab a little bit more,” said Dr. Parrish, who founded the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
“Rox was interested in port-wine stains because of his rotation through pediatrics and was theorizing about how lasers could improve port-wine stains and hemangiomas. I think he first thought of that through the physics of what would be needed. He was thinking, ‘What are these hemangiomas under the microscope? What does the target look like, and what do you need to do to promote healing without scarring? You would have to be able to heat for this duration and this time and at this wavelength.’ He matched the physics of lasers with the pathophysiology of port-wine stains, and together we figured out how to deliver the right energy at the right wavelength at the right time. In fact, at the time, there was no ideal laser. We had to convince a laser manufacturer to build a tunable dye laser, which is what we ended up using around the specifications that we wanted for this treatment.”
Prior to the theory of selective photothermolysis, lasers were a blunt instrument. “They would target the skin but you wouldn’t just selectively target something; you’d get a result you didn’t want,” said Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital.
Once pulsed dye lasers that incorporated principles of selective photothermolysis hit the marketplace, clinicians could treat and improve port-wine stains without scarring the skin. They could even improve scarring from port-wine stains that had been previously treated with the argon laser, the subject of early published work by Dr. Alster (Lasers Surg Med. 1993;13[3]:368-73). “When we treated port-wine stains with the pulsed dye laser on top of the argon laser scars, we observed that the scars were looking better,” Dr. Alster said. “From that observation, we were able to demonstrate improvement of a wide range of scars: traumatic and burn scars, surgical scars, acne scars, and scars caused by other lasers. But it all started with the pulsed dye laser for treating port-wine stains that had scars in them.”
, which enabled the user to deliver even shorter pulse widths in the nanosecond domain. “That changed tattoo treatment,” said Dr. Avram, who is also a past president of the American Society for Laser Medicine and Surgery. “Prior to that, for tattoos and brown spots you would use ablative lasers like CO2 or dermabrasion. They would cause scarring and not really get rid of the tattoo ink or the brown spots. With the Q-switched nanosecond lasers and the picosecond lasers, which came about 15 years later, you had the ability to remove spots with a week of down time, and [they worked] for things like Nevus of Ota, where someone has a disfiguring blue-brown discoloration of their cheek. There’s no surgical treatment for that whatsoever. It’s not like you can take it out.”
Another key advancement was the introduction of “scanning” technology in the early 1990s for CO2 and erbium YAG lasers, which enabled precise computerized control of laser beams. Dr. Avram characterized the CO2 laser as “the gold standard for facial rejuvenation, for sun-damaged skin. The downside of CO2 lasers is that they really need to be in skilled hands. There can be serious side effects such as scarring if it’s not done appropriately or there is not appropriate follow-up. The CO2 lasers have been used in fractional modes for scars. I think it’s the best treatment for scars.”
Dr. Anderson and Melanie Grossman, MD, who practices in New York City, developed the ruby laser for hair removal in the 1990s, and today that procedure ranks as the most common laser treatment in medicine, according to Dr. Avram. He described it as “safe and effective in skilled hands,” requiring about six treatments. Indications are for hypertrichosis, hirsutism (sometimes in the setting of polycystic ovary syndrome), pseudofolliculitis barbae, pilonidal cysts, and gender reassignment surgery.
Another game-changing technology developed by Dr. Anderson came in the early 2000s with the Food and Drug Administration clearance of the Fraxel laser, which is based on the concept of fractional photothermolysis. With this technology, “instead of treating skin to a certain depth, you treat a fraction of it, anywhere from 5% to 40% of the skin,” Dr. Avram explained. “You go in deeper, but you leave surrounding viable tissue that is not affected by the laser. That serves as viable tissue to promote healing. The laser goes in deeper but it’s fractional, so there are skip zones in between the lasers that are going into the skin. You can do this with the CO2 and erbium YAG lasers.” Since hitting the marketplace, the FDA has cleared the use of Fraxel for a number of indications, from periorbital wrinkles and acne scars to surgical scars and melasma.
Dr. Parrish predicted that the next frontier for the advancement of lasers in dermatology will involve the treatment of photodamaged skin. “I’m not sure which technology is going to win,” he said.
Dr. Avram anticipates that dermatologic lasers of the future are going to be more effective, safer, and result in less downtime for patients. “I think we are going to be able to treat skin of color more safely and more effectively, and I think we’re going to become much more successful,” he said. “At some point, the standard of care of treatment for skin cancer will involve lasers and light sources. With all the advances that have happened in the last 50 years, sometimes you wonder, are we at a time to pause, or is most of the story behind us? I think that the advances in innovation that are occurring are going to accelerate greatly as we pass the 50th anniversary. In due credit, laser therapy has completely revolutionized the field of dermatology and has completely revolutionized the way we practice medicine. That will only accelerate in the future.”
Dr. Alster emphasized a “safety first” approach to her hopes for the future. “My wish is that we educate people to know that, while lasers have become ubiquitous and we’ve made them safe, they’re still only safe in the right hands,” she said. “There’s not a day that goes by when I don’t have somebody referred to me who’s been mishandled. There’s no reason for that. With proper training, the risk of bad side effects or complications is markedly reduced.”
During her dermatology residency at Yale University in the late 1980s, Tina S. Alster, MD, met a 44-year-old woman who changed the trajectory of her professional career.
During her clinic visit, the woman explained that she always wore heavy facial makeup to hide her port-wine stain birthmark – a vascular malformation that she kept secret from her husband and teenage son. “She was very good about covering it,” recalled Dr. Alster, who is the founding director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington. “She removed a small amount of makeup for me so I could take a look at it. I had just finished reading an article about using a laser for birthmarks; it had just been published. I told her, ‘There’s something new; we don’t have it at Yale, but I read about treatment that could hone in on birthmarks.’ I promised her I would find out more details.”
A few days later, Dr. Alster pored through stacks of medical journals at Yale’s library and relocated the article she’d seen by first author Oon Tian Tan, MD, PhD, of the department of dermatology at Boston University Medical Center. It described use of the flashpump-pulsed tunable dye laser to treat port-wine stains in 35 children (N Engl J Med. 1989;320:416-21). After giving the article a more thorough read, Dr. Alster became so intrigued by the technology it described that she moved to Boston the following year for a dermatology fellowship with Dr. Tan and joined the ranks of early clinicians who used lasers for treating port-wine stains and other dermatologic conditions.
“That was at a time when there were only a handful of pulsed dye lasers in the world, and the first time I used it was when I went to Boston,” she said. “It was life-changing. You think, ‘Isn’t this great for children with port-wine stains.’ Your heart breaks for them, but I also felt compassion for adults who had suffered a lifetime of stares, including the woman who propelled me to look into this. She ended up coming to Boston during my fellowship and had her birthmark removed, so I changed her life, but she changed mine as well.”
The real credit for that series of events, Dr. Alster continued, belongs to John A. Parrish, MD, and R. Rox Anderson, MD, who in 1983 published the concept of selective photothermolysis, a seminal work that shifted the paradigm for how lasers and other light sources are designed for skin diseases and conditions (Science. 1983 Apr 29;220(4596):524-7). The first pulsed dye laser was built on this concept, an approach that minimized or eliminated the unwanted tissue damage and significant scarring that impeded therapeutic use of laser energy for port-wine stains and other lesions prior to that time. “Lasers that were built subsequent to that seminal paper focused our attention on building lasers that were specific for treatment of certain skin conditions,” Dr. Alster said. “Selective photothermolysis catapulted not only our understanding of how lasers interact with the skin, but allowed us to identify things in the skin that we could potentially target with this new laser technology, and to build laser systems that were specific to those purposes.”
In the late 1970s, Dr. Parrish, who played a key role in making psoralens plus ultraviolet A safe and effective for patients with severe psoriasis, turned his attention to studying lasers in his lab at Harvard Medical School. He hired R. Rox Anderson, a recent graduate of the Massachusetts Institute of Technology, as a technician. “Rox then got interested in medicine and went to medical school at Harvard, got interested in dermatology, and then worked in my lab a little bit more,” said Dr. Parrish, who founded the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
“Rox was interested in port-wine stains because of his rotation through pediatrics and was theorizing about how lasers could improve port-wine stains and hemangiomas. I think he first thought of that through the physics of what would be needed. He was thinking, ‘What are these hemangiomas under the microscope? What does the target look like, and what do you need to do to promote healing without scarring? You would have to be able to heat for this duration and this time and at this wavelength.’ He matched the physics of lasers with the pathophysiology of port-wine stains, and together we figured out how to deliver the right energy at the right wavelength at the right time. In fact, at the time, there was no ideal laser. We had to convince a laser manufacturer to build a tunable dye laser, which is what we ended up using around the specifications that we wanted for this treatment.”
Prior to the theory of selective photothermolysis, lasers were a blunt instrument. “They would target the skin but you wouldn’t just selectively target something; you’d get a result you didn’t want,” said Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital.
Once pulsed dye lasers that incorporated principles of selective photothermolysis hit the marketplace, clinicians could treat and improve port-wine stains without scarring the skin. They could even improve scarring from port-wine stains that had been previously treated with the argon laser, the subject of early published work by Dr. Alster (Lasers Surg Med. 1993;13[3]:368-73). “When we treated port-wine stains with the pulsed dye laser on top of the argon laser scars, we observed that the scars were looking better,” Dr. Alster said. “From that observation, we were able to demonstrate improvement of a wide range of scars: traumatic and burn scars, surgical scars, acne scars, and scars caused by other lasers. But it all started with the pulsed dye laser for treating port-wine stains that had scars in them.”
, which enabled the user to deliver even shorter pulse widths in the nanosecond domain. “That changed tattoo treatment,” said Dr. Avram, who is also a past president of the American Society for Laser Medicine and Surgery. “Prior to that, for tattoos and brown spots you would use ablative lasers like CO2 or dermabrasion. They would cause scarring and not really get rid of the tattoo ink or the brown spots. With the Q-switched nanosecond lasers and the picosecond lasers, which came about 15 years later, you had the ability to remove spots with a week of down time, and [they worked] for things like Nevus of Ota, where someone has a disfiguring blue-brown discoloration of their cheek. There’s no surgical treatment for that whatsoever. It’s not like you can take it out.”
Another key advancement was the introduction of “scanning” technology in the early 1990s for CO2 and erbium YAG lasers, which enabled precise computerized control of laser beams. Dr. Avram characterized the CO2 laser as “the gold standard for facial rejuvenation, for sun-damaged skin. The downside of CO2 lasers is that they really need to be in skilled hands. There can be serious side effects such as scarring if it’s not done appropriately or there is not appropriate follow-up. The CO2 lasers have been used in fractional modes for scars. I think it’s the best treatment for scars.”
Dr. Anderson and Melanie Grossman, MD, who practices in New York City, developed the ruby laser for hair removal in the 1990s, and today that procedure ranks as the most common laser treatment in medicine, according to Dr. Avram. He described it as “safe and effective in skilled hands,” requiring about six treatments. Indications are for hypertrichosis, hirsutism (sometimes in the setting of polycystic ovary syndrome), pseudofolliculitis barbae, pilonidal cysts, and gender reassignment surgery.
Another game-changing technology developed by Dr. Anderson came in the early 2000s with the Food and Drug Administration clearance of the Fraxel laser, which is based on the concept of fractional photothermolysis. With this technology, “instead of treating skin to a certain depth, you treat a fraction of it, anywhere from 5% to 40% of the skin,” Dr. Avram explained. “You go in deeper, but you leave surrounding viable tissue that is not affected by the laser. That serves as viable tissue to promote healing. The laser goes in deeper but it’s fractional, so there are skip zones in between the lasers that are going into the skin. You can do this with the CO2 and erbium YAG lasers.” Since hitting the marketplace, the FDA has cleared the use of Fraxel for a number of indications, from periorbital wrinkles and acne scars to surgical scars and melasma.
Dr. Parrish predicted that the next frontier for the advancement of lasers in dermatology will involve the treatment of photodamaged skin. “I’m not sure which technology is going to win,” he said.
Dr. Avram anticipates that dermatologic lasers of the future are going to be more effective, safer, and result in less downtime for patients. “I think we are going to be able to treat skin of color more safely and more effectively, and I think we’re going to become much more successful,” he said. “At some point, the standard of care of treatment for skin cancer will involve lasers and light sources. With all the advances that have happened in the last 50 years, sometimes you wonder, are we at a time to pause, or is most of the story behind us? I think that the advances in innovation that are occurring are going to accelerate greatly as we pass the 50th anniversary. In due credit, laser therapy has completely revolutionized the field of dermatology and has completely revolutionized the way we practice medicine. That will only accelerate in the future.”
Dr. Alster emphasized a “safety first” approach to her hopes for the future. “My wish is that we educate people to know that, while lasers have become ubiquitous and we’ve made them safe, they’re still only safe in the right hands,” she said. “There’s not a day that goes by when I don’t have somebody referred to me who’s been mishandled. There’s no reason for that. With proper training, the risk of bad side effects or complications is markedly reduced.”
During her dermatology residency at Yale University in the late 1980s, Tina S. Alster, MD, met a 44-year-old woman who changed the trajectory of her professional career.
During her clinic visit, the woman explained that she always wore heavy facial makeup to hide her port-wine stain birthmark – a vascular malformation that she kept secret from her husband and teenage son. “She was very good about covering it,” recalled Dr. Alster, who is the founding director of the Washington Institute of Dermatologic Laser Surgery and clinical professor of dermatology at Georgetown University, Washington. “She removed a small amount of makeup for me so I could take a look at it. I had just finished reading an article about using a laser for birthmarks; it had just been published. I told her, ‘There’s something new; we don’t have it at Yale, but I read about treatment that could hone in on birthmarks.’ I promised her I would find out more details.”
A few days later, Dr. Alster pored through stacks of medical journals at Yale’s library and relocated the article she’d seen by first author Oon Tian Tan, MD, PhD, of the department of dermatology at Boston University Medical Center. It described use of the flashpump-pulsed tunable dye laser to treat port-wine stains in 35 children (N Engl J Med. 1989;320:416-21). After giving the article a more thorough read, Dr. Alster became so intrigued by the technology it described that she moved to Boston the following year for a dermatology fellowship with Dr. Tan and joined the ranks of early clinicians who used lasers for treating port-wine stains and other dermatologic conditions.
“That was at a time when there were only a handful of pulsed dye lasers in the world, and the first time I used it was when I went to Boston,” she said. “It was life-changing. You think, ‘Isn’t this great for children with port-wine stains.’ Your heart breaks for them, but I also felt compassion for adults who had suffered a lifetime of stares, including the woman who propelled me to look into this. She ended up coming to Boston during my fellowship and had her birthmark removed, so I changed her life, but she changed mine as well.”
The real credit for that series of events, Dr. Alster continued, belongs to John A. Parrish, MD, and R. Rox Anderson, MD, who in 1983 published the concept of selective photothermolysis, a seminal work that shifted the paradigm for how lasers and other light sources are designed for skin diseases and conditions (Science. 1983 Apr 29;220(4596):524-7). The first pulsed dye laser was built on this concept, an approach that minimized or eliminated the unwanted tissue damage and significant scarring that impeded therapeutic use of laser energy for port-wine stains and other lesions prior to that time. “Lasers that were built subsequent to that seminal paper focused our attention on building lasers that were specific for treatment of certain skin conditions,” Dr. Alster said. “Selective photothermolysis catapulted not only our understanding of how lasers interact with the skin, but allowed us to identify things in the skin that we could potentially target with this new laser technology, and to build laser systems that were specific to those purposes.”
In the late 1970s, Dr. Parrish, who played a key role in making psoralens plus ultraviolet A safe and effective for patients with severe psoriasis, turned his attention to studying lasers in his lab at Harvard Medical School. He hired R. Rox Anderson, a recent graduate of the Massachusetts Institute of Technology, as a technician. “Rox then got interested in medicine and went to medical school at Harvard, got interested in dermatology, and then worked in my lab a little bit more,” said Dr. Parrish, who founded the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston.
“Rox was interested in port-wine stains because of his rotation through pediatrics and was theorizing about how lasers could improve port-wine stains and hemangiomas. I think he first thought of that through the physics of what would be needed. He was thinking, ‘What are these hemangiomas under the microscope? What does the target look like, and what do you need to do to promote healing without scarring? You would have to be able to heat for this duration and this time and at this wavelength.’ He matched the physics of lasers with the pathophysiology of port-wine stains, and together we figured out how to deliver the right energy at the right wavelength at the right time. In fact, at the time, there was no ideal laser. We had to convince a laser manufacturer to build a tunable dye laser, which is what we ended up using around the specifications that we wanted for this treatment.”
Prior to the theory of selective photothermolysis, lasers were a blunt instrument. “They would target the skin but you wouldn’t just selectively target something; you’d get a result you didn’t want,” said Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital.
Once pulsed dye lasers that incorporated principles of selective photothermolysis hit the marketplace, clinicians could treat and improve port-wine stains without scarring the skin. They could even improve scarring from port-wine stains that had been previously treated with the argon laser, the subject of early published work by Dr. Alster (Lasers Surg Med. 1993;13[3]:368-73). “When we treated port-wine stains with the pulsed dye laser on top of the argon laser scars, we observed that the scars were looking better,” Dr. Alster said. “From that observation, we were able to demonstrate improvement of a wide range of scars: traumatic and burn scars, surgical scars, acne scars, and scars caused by other lasers. But it all started with the pulsed dye laser for treating port-wine stains that had scars in them.”
, which enabled the user to deliver even shorter pulse widths in the nanosecond domain. “That changed tattoo treatment,” said Dr. Avram, who is also a past president of the American Society for Laser Medicine and Surgery. “Prior to that, for tattoos and brown spots you would use ablative lasers like CO2 or dermabrasion. They would cause scarring and not really get rid of the tattoo ink or the brown spots. With the Q-switched nanosecond lasers and the picosecond lasers, which came about 15 years later, you had the ability to remove spots with a week of down time, and [they worked] for things like Nevus of Ota, where someone has a disfiguring blue-brown discoloration of their cheek. There’s no surgical treatment for that whatsoever. It’s not like you can take it out.”
Another key advancement was the introduction of “scanning” technology in the early 1990s for CO2 and erbium YAG lasers, which enabled precise computerized control of laser beams. Dr. Avram characterized the CO2 laser as “the gold standard for facial rejuvenation, for sun-damaged skin. The downside of CO2 lasers is that they really need to be in skilled hands. There can be serious side effects such as scarring if it’s not done appropriately or there is not appropriate follow-up. The CO2 lasers have been used in fractional modes for scars. I think it’s the best treatment for scars.”
Dr. Anderson and Melanie Grossman, MD, who practices in New York City, developed the ruby laser for hair removal in the 1990s, and today that procedure ranks as the most common laser treatment in medicine, according to Dr. Avram. He described it as “safe and effective in skilled hands,” requiring about six treatments. Indications are for hypertrichosis, hirsutism (sometimes in the setting of polycystic ovary syndrome), pseudofolliculitis barbae, pilonidal cysts, and gender reassignment surgery.
Another game-changing technology developed by Dr. Anderson came in the early 2000s with the Food and Drug Administration clearance of the Fraxel laser, which is based on the concept of fractional photothermolysis. With this technology, “instead of treating skin to a certain depth, you treat a fraction of it, anywhere from 5% to 40% of the skin,” Dr. Avram explained. “You go in deeper, but you leave surrounding viable tissue that is not affected by the laser. That serves as viable tissue to promote healing. The laser goes in deeper but it’s fractional, so there are skip zones in between the lasers that are going into the skin. You can do this with the CO2 and erbium YAG lasers.” Since hitting the marketplace, the FDA has cleared the use of Fraxel for a number of indications, from periorbital wrinkles and acne scars to surgical scars and melasma.
Dr. Parrish predicted that the next frontier for the advancement of lasers in dermatology will involve the treatment of photodamaged skin. “I’m not sure which technology is going to win,” he said.
Dr. Avram anticipates that dermatologic lasers of the future are going to be more effective, safer, and result in less downtime for patients. “I think we are going to be able to treat skin of color more safely and more effectively, and I think we’re going to become much more successful,” he said. “At some point, the standard of care of treatment for skin cancer will involve lasers and light sources. With all the advances that have happened in the last 50 years, sometimes you wonder, are we at a time to pause, or is most of the story behind us? I think that the advances in innovation that are occurring are going to accelerate greatly as we pass the 50th anniversary. In due credit, laser therapy has completely revolutionized the field of dermatology and has completely revolutionized the way we practice medicine. That will only accelerate in the future.”
Dr. Alster emphasized a “safety first” approach to her hopes for the future. “My wish is that we educate people to know that, while lasers have become ubiquitous and we’ve made them safe, they’re still only safe in the right hands,” she said. “There’s not a day that goes by when I don’t have somebody referred to me who’s been mishandled. There’s no reason for that. With proper training, the risk of bad side effects or complications is markedly reduced.”
Skin Tightening
Minimally and noninvasive skin tightening has become one of the most requested cosmetic procedures. Skin laxity often is apparent in areas of the face, neck, jawline, hands, abdomen, and thighs, with features of fine lines, wrinkles, and cellulite. Intrinsic and extrinsic factors contribute to the development of skin laxity. Intrinsic aspects include chronological age, stress, and genetics, whereas extrinsic influences include exposure to solar radiation, environmental toxins, and smoking.1,2 These factors affect the production and maintenance of both collagen and elastic proteins, which are the main components that help the skin stay firm and smooth. With a goal of improving skin laxity, multiple skin tightening modalities have been developed.
Traditionally, skin laxity was treated by invasive surgical skin procedures (eg, rhytidectomy), which carry a high financial cost, require an operating room and general anesthesia, have a prolonged recovery time with notable postoperative care, and have possible risk of unwanted scars.3,4 The risks associated with invasive procedures have spurned a growing demand for minimally invasive and noninvasive methods, which have fostered the development of several skin laxity reversal modalities over the last decade. Although the achieved results of these technologies are less dramatic and require more treatments, they do not possess the associated risks and adverse effects seen in invasive surgical procedures. As such, demand for these techniques has been growing among cosmetic patients.
There are multiple technologies that currently are employed to achieve noninvasive skin tightening. Laser therapy, radiofrequency (RF), ultrasound, and intense pulsed light (IPL) are methods that focus targeted energy to elevate temperatures in the deeper layers of the skin. Elevated thermal energy causes denaturing of collagen with preservation of heat-stable intermolecular cross-links. Skin tightening is achieved through physical shortening of the collagen fibers with preservation of the heat-stable intermolecular hydrogen bonds, which leads to an increase in the rubber elastic properties of the collagen polymer and stimulation of new collagen formation.5,6 The temperature at which this process occurs has been frequently reported as approximately 65°C.7,8 Alternative noninvasive therapies that do not focus on elevated thermal energy for skin tightening include chemical peels and skin care products.
Given the multitude of treatment methods that have been developed to counteract skin laxity, this article seeks to provide an overview of some technologies, devices, and commonly used therapies to help dermatologists choose the appropriate modalities for their cosmetic patients.
Laser Therapy
Since its approval in the 1980s, laser therapy has become an alternative to invasive surgical skin tightening.9 Laser therapy utilized for treatment can be subcategorized into 2 types: ablative and nonablative.
Traditional ablative skin tightening utilized CO2 or erbium:YAG lasers. These lasers caused skin tightening by first ablating the epidermis cleanly off the dermis, with a partially coagulated area in the dermis, which triggered a wound-healing cascade followed by neocollagenesis and remodeling.10,11 Although this treatment displays notable retightening of the skin, traditional ablative lasers are not routinely used, likely because of lengthy recovery periods, risk for scar development, flares of acne and herpes simplex virus, hyperpigmentation, and delayed-onset hypopigmentation.9,12,13
Fractional ablative laser treatments soon emerged as an effective alternative to traditional ablative lasers. Various studies have noted better recovery times and side-effect profiles.14-18 This improvement is believed to be due to the method of wound healing in fractional ablative laser treatments. Ablative fractional photothermolysis works by generating deeply narrow focal ablations that involve the dermis and epidermis while leaving the surrounding skin unscathed, which allows for rapid re-epithelization, filling in of the dermal pockets, and stimulation of dermal remodeling.10,11,18,19 Studies have demonstrated a range of improvement in skin laxity from 56% to 65.3% at 6 months posttreatment.20,21 Although the incidence of reported side effects is better than with the traditional ablative laser, fractional ablative lasers have documented reports of similar types of side effects as traditional lasers due in part to ablation of the skin.22,23
Nonablative lasers were developed as alternatives to ablative laser treatments. This class of lasers produces a milder effect compared with its ablative counterpart. Studies show a quantitative improvement range of 8.9% to 11% in skin laxity 3 months posttreatment.24,25 Nonablative lasers induce controlled tissue injury in the dermis, which leads to stimulation of dermal remodeling and collagen production.11 Although the effects of nonablative lasers are milder compared with their ablative counterparts, they possess the superior benefit of minimal adverse events. Most studies reported transient erythema posttreatment, but no long-term adverse effects have been noted,26-31 in part due to preservation of the epidermal layer.
Radiofrequency
Radiofrequency technology was the first method marketed for noninvasive skin tightening. Radiofrequency devices work by generating heat through tissue resistance to an applied alternating electrical current, which leads to collagen contraction and remodeling along with neocollgenesis.32 The major electrode configurations used in these technologies are monopolar, bipolar, and multipolar, which differ by the electric field they produce. Reported side effects include erythema that arose 1 week following completion of treatment and resolved by 6-month follow-up, as well as hypertrophic scarring, transient postinflammatory hyperpigmentation, and pain.33,34
Monopolar systems were the first among these devices to be developed for use in skin tightening and remain the most extensively studied technology for treatment of skin laxity. Developed in 2001, the Thermage device (Solta Medical, Valent Pharmaceuticals) remains the most extensively studied technology for the treatment of skin laxity.35 In a trial performed by Fitzpatrick et al,36 treatment of skin laxity of the periorbital area with ThermaCool TC (Thermage, Inc) demonstrated an 83.2% improvement in at least 1 point treated and an overall 28.9% improvement of the entire treatment area at 6-month follow-up. Additionally, a survey study of 5700 patients who received monopolar RF skin tightening treatments demonstrated that 26% of patients experienced immediate tightening following treatment, and 54% observed tightening 6 months posttreatment.37
Bipolar and multipolar devices were developed following the success of monopolar devices in the treatment for skin laxity. In a study evaluating multipolar RF for the face and neck, all 11 patients were determined to have improvement of their skin laxity following weekly treatments for 8 weeks.38
Ultrasound
The use of ultrasound for skin tightening was first approved in 2009.39 The primary mechanism of skin tightening is through thermally induced contraction of collagen with subsequent collagen neogenesis achieved through absorption of the vibrational acoustic energy into target tissue.40 There are 2 types of ultrasound methods: microfocused and high-intensity focused. Microfocused ultrasound focuses on delivering lower-energy pulses to the deep reticular dermal and subdermal layers that lead to disruption of the underlying architecture of the skin, promoting increases in distensibility, elasticity, and viscoelasticity.41 To date, microfocused ultrasound is approved for treating skin laxity of the eyebrow and submental area and wrinkles of the décolleté. Currently, there are 2 devices approved by the US Food and Drug Administration for the treatment of skin laxity with ultrasound. These devices are the Ulthera System (Merz Pharmaceuticals) and the Sofwave system (Sofwave Medical Ltd).42 Oni et al43 evaluated 93 patients following treatment using Ulthera for skin laxity in the lower face. There was a noticeable improvement of 63.6% at 90 days following treatment. Brobst et al44 showed improvement in laxity at 6 months and 1.5 years following last treatment. The most commonly reported posttreatment side effects include transient purpura, transient edema, and transient postinflammatory pigmentation.42,45 Serious complications are rare and include development of palpable subcutaneous nodules and motor nerve paresis.42,46
High-intensity focused ultrasound has been more recently introduced as a modality for skin tightening and rejuvenation. This method focuses on applying heat to areas through acoustic energy to areas of the deep dermis, subdermal connective tissue, and fibromuscular layer in targeted microcoagulation zones without effect to the epidermis.47 The targeted thermal effects and microcoagulation are believed to cause skin tightening through collagen contraction and remodeling. Future studies are needed to determine the overall benefits in skin laxity to achieve approval by the US Food and Drug Administration for use as a treatment option.
IPL Therapy
Intense pulsed light therapy is different from lasers in that it utilizes a wider variety of wavelengths ranging from approximately 500 to 1200 nm.48 The process of skin tightening is achieved through selective photothermolysis in which thermal damage is focused solely on pigmented targets at the cellular or tissue levels in the epidermis and dermis.49 Intense pulsed light penetrates the tissues and is selectively absorbed by melanin and hemoglobin, thereby producing photothermal effects. The photothermal effects lead to reversible thermal damage to surrounding collagen and induction contraction of collagen fibers and fiber remodeling.50 Clinical studies on the effectiveness on skin tightening have shown incongruent results. Multiple studies have noted improvement in skin elasticity as well as increased deposits of collagen in treated areas. Other studies have shown no improvement of rhytides or wrinkle reduction. The side effects noted were transient pain, swelling, and erythema, along with rare instances of blisters and crusting.48,51-54 Due to the inhomogeneous results, the use of IPL is largely reserved for treatment of acne, hyperpigmentation, hypertrichosis, and superficial vascular malformations.
Chemical Peels
Chemical peels are used in the treatment of skin laxity through a process similar to ablative lasers. Unlike other methods described in this article, this type of treatment is only reserved for the facial areas. The peel must penetrate to the lower papillary dermis or deeper to allow for adequate collagen synthesis.55 As such, medium to deep peeling agents should be used.56 Peels cause coagulation of membrane proteins and necrosis of the epidermis and dermis, thereby stimulating collagen synthesis and keratinocyte regeneration. Additionally, there is an increase in the deposition of glycosaminoglycans, which play a major role in providing hydration for the skin because of their water-binding capacity.56 Deep peels have the added effect of restoring dermal architecture to its native state. Medium-depth peels work up to the layer of the epidermis and dermis.57 Trichloroacetic acid (TCA) 35% is the main ingredient used in these types of peels. Some examples include Monet combination (Jessner solution with 35% TCA), Brody combination (solid CO2 plus 35% TCA), and Coleman combination (70% glycolic acid and 35% TCA). Deep peels penetrate to the levels of the reticular dermis.58 The formulation of these peels contain croton oil and phenols in various concentrations.57,58 A study by Brody59 noted clinical improvement of skin laxity–attributed histologic depth achieved by medium-depth peels. The results of the study demonstrated that the depth of wounding from 3 consecutive applications of TCA led to greater epidermal hyperplasia and a more dense formation of dermal elastic fiber formation on histologic examination. Side effects noted in the study included transient erythema, edema, and erosions that resolved without scar formation at 30-day follow-up.59 Another study performed by Oresajo et al60 demonstrated that patients treated with either a chemical peel of 41% capryloyl salicylic acid or 30% glycolic acid led to notable reduction of fine lines/wrinkles vs baseline. Side effects noted included pruritus, erythema, increased skin sensitivity, epidermolysis, allergic and irritant contact dermatitis, and postinflammatory hyperpigmentation.60
Skin Care
Skin care products have been developed over the years and marketed to aid in the treatment of skin laxity. Some studied methods include photoprotection products, antioxidant-based products, and vitamin A products. Photoprotection plays a crucial role in the prevention of skin laxity. Unprotected sun exposure can induce damage to previously treated skin, leading to minimized or cancelled rejuvenation measures.61
Oxidation is a major contributor in the development of skin laxity. The skin naturally possesses endogenous antioxidant defense mechanisms that protect its cells from free radical damage. However, these mechanisms are reduced as skin ages and are further diminished with photodamage. Ascorbic acid is a collagen stimulator that is known to have antioxidant properties. In the appropriate formulations, topical vitamin C directly supplements the skin’s antioxidant reservoir.61
The use of vitamin A, a retinoic acid, for treatment of skin laxity is based on its ability to improve the production of procollagen and elastic fiber components, resulting in the restoration of dermal matrix proteins.61-65 Vitamin A in the skin plays a key role in the regulation and control of proliferation and differentiation of all major cell types found in the epidermis and dermis.61 Studies have shown that the long-term use of topical vitamin A improves fine and coarse wrinkling.65
Final Thoughts
Various technologies have been developed to provide clinically significant skin laxity reversal. Laser, RF, ultrasound, IPL, and topical therapies provide numerous options at our disposal. Although many devices are available, it is important to consider the desired outcome, cost, and adverse events when discussing therapeutic options for treating skin laxity (eTable). Patients should be advised that multiple treatment sessions over the course of months will likely be necessary. With the development of numerous technologies, we now have many options to offer our patients who desire minimally or noninvasive skin tightening.
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323-331.
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Berlin, Germany: Springer, Heidelberg; 2006:9-21.
- Ramanadham SR, Costa CR, Narasimhan K, et al. Refining the anesthesia management of the face-lift patient: lessons learned from 1089 consecutive face lifts. Plast Reconstr Surg. 2015;135:723-730.
- Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1-13.
- le Lous M, Flandin F, Herbage D, et al. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295-300.
- Ross EV, Yashar SS, Naseef GS, et al. A pilot study of in vivo immediate tissue contraction with CO2 skin laser resurfacing in a live farm pig. Dermatol Surg. 1999;25:851-856.
- Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
- Hsu TS, Kaminer MS. The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg. 2003;22:115-123.
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619-632; discussion 633-634.
- Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49-60.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Bernstein LJ, Kauvar AN, Grossman MC, et al. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. an evaluation of 500 patients. Dermatol Surg. 1998;24:315-320.
- Ortiz AE, Tremaine AM, Zachary CB. Long‐term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168-170.
- Rahman Z, MacFalls H, Jiang K, et al. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med. 2009;41:78-86.
- Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301-305; discussion 305-307.
- Fisher GH, Geronemus RG. Short‐term side effects of fractional photothermolysis. Dermatol Surg. 2005;31:1245-1249.
- Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: traditional versus fractional. Dermatol Surg. 2014;40(suppl 12):S147-S151.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169-176.
- Tierney EP, Hanke CW, Petersen J. Ablative fractionated CO2 laser treatment of photoaging: a clinical and histologic study. Dermatol Surg. 2012;38:1777-1789.
- Tierney EP, Hanke CW, Watkins L. Treatment of lower eyelid rhytids and laxity with ablative fractionated carbon-dioxide laser resurfacing: case series and review of the literature. J Am Acad Dermatol. 2011;64:730-740.
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: four cases. Lasers Surg Med. 2009;41:179-184.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299-306.
- Miller L, Mishra V, Alsaad S, et al. Clinical evaluation of a non-ablative 1940 nm fractional laser. J Drugs Dermatol. 2014;13:1324-1329.
- Alexiades-Armenakas M. Nonablative skin tightening with a variable depth heating 1310-nm wavelength laser in combination with surface cooling. J Drugs Dermatol. 2007;6:1096-1103.
- Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35:813-815.
- Fournier N, Lagarde JM, Turlier V, et al. A 35-month profilometric and clinical evaluation of non-ablative remodeling using a 1540-nm Er:glass laser. J Cosmet Laser Ther. 2004;6:126-130.
- Hædersdal M, Moreau KER, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189-195.
- Lupton JR, Williams CM, Alster TS. Nonablative laser skin resurfacing using a 1540 nm erbium glass laser: a clinical and histologic analysis. Dermatol Surg. 2002;28:833-835.
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585‐nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg. 2003;29:997-999, discussion 999-1000.
- Pollock H, Pollock TA. NLite laser: nonablative wrinkle reduction.Aesthet Surg J. 2001;21:371-372.
- Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638-642.
- Weiss RA, Weiss MA, Munavelli G, et al. Monopolar radiofrequency facial tightening: a retrospective analysis of efficacy and safety in over 600 treatments. J Drugs Dermatol. 2006;5:707-712.
- Sadick NS, Makino Y. Selective electro‐thermolysis in aesthetic medicine: a review. Lasers Surg Med. 2004;34:91-97.
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol. 2007;25:487-491.
- Fitzpatrick R, Geronemus R, Goldberg D, et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232-242.
- Dover JS, Zelickson B, 14-Physician Multispecialty Consensus Panel. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low‐energy multiple‐pass technique leading to a clinical end point algorithm. Dermatol Surg. 2007;33:900-907.
- de Oliveira TC, Rocha SF, Ramos DG, et al. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for face and neck rejuvenation [published online March 8, 2017]. Dermatol Res Pract. doi:10.1155/2017/4146391.
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Van Leenders GJ, Beerlage HP, Ruijter ET, et al. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269-275.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 201332:18-25.
- Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099-1110.
- Brobst RW, Ferguson M, Perkins SW. Noninvasive treatment of the neck. Facial Plast Surg North Am. 2014;22:191-202.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754-759.
- Missel L. Prevention of potential adverse events associated with use of Ulthera device. Tech Bull. 2011;32:18-25.
- Bove T, Zawada T, Serup J, et al. High‐frequency (20‐MHz) high‐intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217-228.
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78-87.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Faucz LL, Will SE, Rodrigues CJ, et al. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg Med. 2018;50:644-650.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med. 2000;26:196-200.
- Li Y-H, Wu Y, Chen JZ, et al. Application of a new intense pulsed light device in the treatment of photoaging skin in Asian patients. Dermatol Surg. 2008;34:1459-1464.
- Shin J-W, Lee D-H, Choi S-Y, et al. Objective and non‐invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25:516-522.
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28:1115-1119.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313-324.
- Brody HJ. Do chemical peels tighten the skin? Dermatol Surg. 2014;40(suppl):S129-S133.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Brody HJ. Variations and comparisons in medium‐depth chemical peeling. J Dermatol Surg Oncol. 1989;15:953-963.
- Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7:259-262.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- Griffiths C. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613-618.
- Darlenski R, Surber C, Fluhr J. Topical retinoids in the management of photodamaged skin: from theory to evidence‐based practical approach. Br J Dermatol. 2010;163:1157-1165.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6:245-253.
- Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17:265-276.
- American Society of Plastic Surgeons. Average surgeon/physician fees. https://www.plasticsurgery.org/documents/News/Statistics/2019/cosmetic-procedures-average-cost-2019.pdf. Accessed August 24, 2020.
Minimally and noninvasive skin tightening has become one of the most requested cosmetic procedures. Skin laxity often is apparent in areas of the face, neck, jawline, hands, abdomen, and thighs, with features of fine lines, wrinkles, and cellulite. Intrinsic and extrinsic factors contribute to the development of skin laxity. Intrinsic aspects include chronological age, stress, and genetics, whereas extrinsic influences include exposure to solar radiation, environmental toxins, and smoking.1,2 These factors affect the production and maintenance of both collagen and elastic proteins, which are the main components that help the skin stay firm and smooth. With a goal of improving skin laxity, multiple skin tightening modalities have been developed.
Traditionally, skin laxity was treated by invasive surgical skin procedures (eg, rhytidectomy), which carry a high financial cost, require an operating room and general anesthesia, have a prolonged recovery time with notable postoperative care, and have possible risk of unwanted scars.3,4 The risks associated with invasive procedures have spurned a growing demand for minimally invasive and noninvasive methods, which have fostered the development of several skin laxity reversal modalities over the last decade. Although the achieved results of these technologies are less dramatic and require more treatments, they do not possess the associated risks and adverse effects seen in invasive surgical procedures. As such, demand for these techniques has been growing among cosmetic patients.
There are multiple technologies that currently are employed to achieve noninvasive skin tightening. Laser therapy, radiofrequency (RF), ultrasound, and intense pulsed light (IPL) are methods that focus targeted energy to elevate temperatures in the deeper layers of the skin. Elevated thermal energy causes denaturing of collagen with preservation of heat-stable intermolecular cross-links. Skin tightening is achieved through physical shortening of the collagen fibers with preservation of the heat-stable intermolecular hydrogen bonds, which leads to an increase in the rubber elastic properties of the collagen polymer and stimulation of new collagen formation.5,6 The temperature at which this process occurs has been frequently reported as approximately 65°C.7,8 Alternative noninvasive therapies that do not focus on elevated thermal energy for skin tightening include chemical peels and skin care products.
Given the multitude of treatment methods that have been developed to counteract skin laxity, this article seeks to provide an overview of some technologies, devices, and commonly used therapies to help dermatologists choose the appropriate modalities for their cosmetic patients.
Laser Therapy
Since its approval in the 1980s, laser therapy has become an alternative to invasive surgical skin tightening.9 Laser therapy utilized for treatment can be subcategorized into 2 types: ablative and nonablative.
Traditional ablative skin tightening utilized CO2 or erbium:YAG lasers. These lasers caused skin tightening by first ablating the epidermis cleanly off the dermis, with a partially coagulated area in the dermis, which triggered a wound-healing cascade followed by neocollagenesis and remodeling.10,11 Although this treatment displays notable retightening of the skin, traditional ablative lasers are not routinely used, likely because of lengthy recovery periods, risk for scar development, flares of acne and herpes simplex virus, hyperpigmentation, and delayed-onset hypopigmentation.9,12,13
Fractional ablative laser treatments soon emerged as an effective alternative to traditional ablative lasers. Various studies have noted better recovery times and side-effect profiles.14-18 This improvement is believed to be due to the method of wound healing in fractional ablative laser treatments. Ablative fractional photothermolysis works by generating deeply narrow focal ablations that involve the dermis and epidermis while leaving the surrounding skin unscathed, which allows for rapid re-epithelization, filling in of the dermal pockets, and stimulation of dermal remodeling.10,11,18,19 Studies have demonstrated a range of improvement in skin laxity from 56% to 65.3% at 6 months posttreatment.20,21 Although the incidence of reported side effects is better than with the traditional ablative laser, fractional ablative lasers have documented reports of similar types of side effects as traditional lasers due in part to ablation of the skin.22,23
Nonablative lasers were developed as alternatives to ablative laser treatments. This class of lasers produces a milder effect compared with its ablative counterpart. Studies show a quantitative improvement range of 8.9% to 11% in skin laxity 3 months posttreatment.24,25 Nonablative lasers induce controlled tissue injury in the dermis, which leads to stimulation of dermal remodeling and collagen production.11 Although the effects of nonablative lasers are milder compared with their ablative counterparts, they possess the superior benefit of minimal adverse events. Most studies reported transient erythema posttreatment, but no long-term adverse effects have been noted,26-31 in part due to preservation of the epidermal layer.
Radiofrequency
Radiofrequency technology was the first method marketed for noninvasive skin tightening. Radiofrequency devices work by generating heat through tissue resistance to an applied alternating electrical current, which leads to collagen contraction and remodeling along with neocollgenesis.32 The major electrode configurations used in these technologies are monopolar, bipolar, and multipolar, which differ by the electric field they produce. Reported side effects include erythema that arose 1 week following completion of treatment and resolved by 6-month follow-up, as well as hypertrophic scarring, transient postinflammatory hyperpigmentation, and pain.33,34
Monopolar systems were the first among these devices to be developed for use in skin tightening and remain the most extensively studied technology for treatment of skin laxity. Developed in 2001, the Thermage device (Solta Medical, Valent Pharmaceuticals) remains the most extensively studied technology for the treatment of skin laxity.35 In a trial performed by Fitzpatrick et al,36 treatment of skin laxity of the periorbital area with ThermaCool TC (Thermage, Inc) demonstrated an 83.2% improvement in at least 1 point treated and an overall 28.9% improvement of the entire treatment area at 6-month follow-up. Additionally, a survey study of 5700 patients who received monopolar RF skin tightening treatments demonstrated that 26% of patients experienced immediate tightening following treatment, and 54% observed tightening 6 months posttreatment.37
Bipolar and multipolar devices were developed following the success of monopolar devices in the treatment for skin laxity. In a study evaluating multipolar RF for the face and neck, all 11 patients were determined to have improvement of their skin laxity following weekly treatments for 8 weeks.38
Ultrasound
The use of ultrasound for skin tightening was first approved in 2009.39 The primary mechanism of skin tightening is through thermally induced contraction of collagen with subsequent collagen neogenesis achieved through absorption of the vibrational acoustic energy into target tissue.40 There are 2 types of ultrasound methods: microfocused and high-intensity focused. Microfocused ultrasound focuses on delivering lower-energy pulses to the deep reticular dermal and subdermal layers that lead to disruption of the underlying architecture of the skin, promoting increases in distensibility, elasticity, and viscoelasticity.41 To date, microfocused ultrasound is approved for treating skin laxity of the eyebrow and submental area and wrinkles of the décolleté. Currently, there are 2 devices approved by the US Food and Drug Administration for the treatment of skin laxity with ultrasound. These devices are the Ulthera System (Merz Pharmaceuticals) and the Sofwave system (Sofwave Medical Ltd).42 Oni et al43 evaluated 93 patients following treatment using Ulthera for skin laxity in the lower face. There was a noticeable improvement of 63.6% at 90 days following treatment. Brobst et al44 showed improvement in laxity at 6 months and 1.5 years following last treatment. The most commonly reported posttreatment side effects include transient purpura, transient edema, and transient postinflammatory pigmentation.42,45 Serious complications are rare and include development of palpable subcutaneous nodules and motor nerve paresis.42,46
High-intensity focused ultrasound has been more recently introduced as a modality for skin tightening and rejuvenation. This method focuses on applying heat to areas through acoustic energy to areas of the deep dermis, subdermal connective tissue, and fibromuscular layer in targeted microcoagulation zones without effect to the epidermis.47 The targeted thermal effects and microcoagulation are believed to cause skin tightening through collagen contraction and remodeling. Future studies are needed to determine the overall benefits in skin laxity to achieve approval by the US Food and Drug Administration for use as a treatment option.
IPL Therapy
Intense pulsed light therapy is different from lasers in that it utilizes a wider variety of wavelengths ranging from approximately 500 to 1200 nm.48 The process of skin tightening is achieved through selective photothermolysis in which thermal damage is focused solely on pigmented targets at the cellular or tissue levels in the epidermis and dermis.49 Intense pulsed light penetrates the tissues and is selectively absorbed by melanin and hemoglobin, thereby producing photothermal effects. The photothermal effects lead to reversible thermal damage to surrounding collagen and induction contraction of collagen fibers and fiber remodeling.50 Clinical studies on the effectiveness on skin tightening have shown incongruent results. Multiple studies have noted improvement in skin elasticity as well as increased deposits of collagen in treated areas. Other studies have shown no improvement of rhytides or wrinkle reduction. The side effects noted were transient pain, swelling, and erythema, along with rare instances of blisters and crusting.48,51-54 Due to the inhomogeneous results, the use of IPL is largely reserved for treatment of acne, hyperpigmentation, hypertrichosis, and superficial vascular malformations.
Chemical Peels
Chemical peels are used in the treatment of skin laxity through a process similar to ablative lasers. Unlike other methods described in this article, this type of treatment is only reserved for the facial areas. The peel must penetrate to the lower papillary dermis or deeper to allow for adequate collagen synthesis.55 As such, medium to deep peeling agents should be used.56 Peels cause coagulation of membrane proteins and necrosis of the epidermis and dermis, thereby stimulating collagen synthesis and keratinocyte regeneration. Additionally, there is an increase in the deposition of glycosaminoglycans, which play a major role in providing hydration for the skin because of their water-binding capacity.56 Deep peels have the added effect of restoring dermal architecture to its native state. Medium-depth peels work up to the layer of the epidermis and dermis.57 Trichloroacetic acid (TCA) 35% is the main ingredient used in these types of peels. Some examples include Monet combination (Jessner solution with 35% TCA), Brody combination (solid CO2 plus 35% TCA), and Coleman combination (70% glycolic acid and 35% TCA). Deep peels penetrate to the levels of the reticular dermis.58 The formulation of these peels contain croton oil and phenols in various concentrations.57,58 A study by Brody59 noted clinical improvement of skin laxity–attributed histologic depth achieved by medium-depth peels. The results of the study demonstrated that the depth of wounding from 3 consecutive applications of TCA led to greater epidermal hyperplasia and a more dense formation of dermal elastic fiber formation on histologic examination. Side effects noted in the study included transient erythema, edema, and erosions that resolved without scar formation at 30-day follow-up.59 Another study performed by Oresajo et al60 demonstrated that patients treated with either a chemical peel of 41% capryloyl salicylic acid or 30% glycolic acid led to notable reduction of fine lines/wrinkles vs baseline. Side effects noted included pruritus, erythema, increased skin sensitivity, epidermolysis, allergic and irritant contact dermatitis, and postinflammatory hyperpigmentation.60
Skin Care
Skin care products have been developed over the years and marketed to aid in the treatment of skin laxity. Some studied methods include photoprotection products, antioxidant-based products, and vitamin A products. Photoprotection plays a crucial role in the prevention of skin laxity. Unprotected sun exposure can induce damage to previously treated skin, leading to minimized or cancelled rejuvenation measures.61
Oxidation is a major contributor in the development of skin laxity. The skin naturally possesses endogenous antioxidant defense mechanisms that protect its cells from free radical damage. However, these mechanisms are reduced as skin ages and are further diminished with photodamage. Ascorbic acid is a collagen stimulator that is known to have antioxidant properties. In the appropriate formulations, topical vitamin C directly supplements the skin’s antioxidant reservoir.61
The use of vitamin A, a retinoic acid, for treatment of skin laxity is based on its ability to improve the production of procollagen and elastic fiber components, resulting in the restoration of dermal matrix proteins.61-65 Vitamin A in the skin plays a key role in the regulation and control of proliferation and differentiation of all major cell types found in the epidermis and dermis.61 Studies have shown that the long-term use of topical vitamin A improves fine and coarse wrinkling.65
Final Thoughts
Various technologies have been developed to provide clinically significant skin laxity reversal. Laser, RF, ultrasound, IPL, and topical therapies provide numerous options at our disposal. Although many devices are available, it is important to consider the desired outcome, cost, and adverse events when discussing therapeutic options for treating skin laxity (eTable). Patients should be advised that multiple treatment sessions over the course of months will likely be necessary. With the development of numerous technologies, we now have many options to offer our patients who desire minimally or noninvasive skin tightening.
Minimally and noninvasive skin tightening has become one of the most requested cosmetic procedures. Skin laxity often is apparent in areas of the face, neck, jawline, hands, abdomen, and thighs, with features of fine lines, wrinkles, and cellulite. Intrinsic and extrinsic factors contribute to the development of skin laxity. Intrinsic aspects include chronological age, stress, and genetics, whereas extrinsic influences include exposure to solar radiation, environmental toxins, and smoking.1,2 These factors affect the production and maintenance of both collagen and elastic proteins, which are the main components that help the skin stay firm and smooth. With a goal of improving skin laxity, multiple skin tightening modalities have been developed.
Traditionally, skin laxity was treated by invasive surgical skin procedures (eg, rhytidectomy), which carry a high financial cost, require an operating room and general anesthesia, have a prolonged recovery time with notable postoperative care, and have possible risk of unwanted scars.3,4 The risks associated with invasive procedures have spurned a growing demand for minimally invasive and noninvasive methods, which have fostered the development of several skin laxity reversal modalities over the last decade. Although the achieved results of these technologies are less dramatic and require more treatments, they do not possess the associated risks and adverse effects seen in invasive surgical procedures. As such, demand for these techniques has been growing among cosmetic patients.
There are multiple technologies that currently are employed to achieve noninvasive skin tightening. Laser therapy, radiofrequency (RF), ultrasound, and intense pulsed light (IPL) are methods that focus targeted energy to elevate temperatures in the deeper layers of the skin. Elevated thermal energy causes denaturing of collagen with preservation of heat-stable intermolecular cross-links. Skin tightening is achieved through physical shortening of the collagen fibers with preservation of the heat-stable intermolecular hydrogen bonds, which leads to an increase in the rubber elastic properties of the collagen polymer and stimulation of new collagen formation.5,6 The temperature at which this process occurs has been frequently reported as approximately 65°C.7,8 Alternative noninvasive therapies that do not focus on elevated thermal energy for skin tightening include chemical peels and skin care products.
Given the multitude of treatment methods that have been developed to counteract skin laxity, this article seeks to provide an overview of some technologies, devices, and commonly used therapies to help dermatologists choose the appropriate modalities for their cosmetic patients.
Laser Therapy
Since its approval in the 1980s, laser therapy has become an alternative to invasive surgical skin tightening.9 Laser therapy utilized for treatment can be subcategorized into 2 types: ablative and nonablative.
Traditional ablative skin tightening utilized CO2 or erbium:YAG lasers. These lasers caused skin tightening by first ablating the epidermis cleanly off the dermis, with a partially coagulated area in the dermis, which triggered a wound-healing cascade followed by neocollagenesis and remodeling.10,11 Although this treatment displays notable retightening of the skin, traditional ablative lasers are not routinely used, likely because of lengthy recovery periods, risk for scar development, flares of acne and herpes simplex virus, hyperpigmentation, and delayed-onset hypopigmentation.9,12,13
Fractional ablative laser treatments soon emerged as an effective alternative to traditional ablative lasers. Various studies have noted better recovery times and side-effect profiles.14-18 This improvement is believed to be due to the method of wound healing in fractional ablative laser treatments. Ablative fractional photothermolysis works by generating deeply narrow focal ablations that involve the dermis and epidermis while leaving the surrounding skin unscathed, which allows for rapid re-epithelization, filling in of the dermal pockets, and stimulation of dermal remodeling.10,11,18,19 Studies have demonstrated a range of improvement in skin laxity from 56% to 65.3% at 6 months posttreatment.20,21 Although the incidence of reported side effects is better than with the traditional ablative laser, fractional ablative lasers have documented reports of similar types of side effects as traditional lasers due in part to ablation of the skin.22,23
Nonablative lasers were developed as alternatives to ablative laser treatments. This class of lasers produces a milder effect compared with its ablative counterpart. Studies show a quantitative improvement range of 8.9% to 11% in skin laxity 3 months posttreatment.24,25 Nonablative lasers induce controlled tissue injury in the dermis, which leads to stimulation of dermal remodeling and collagen production.11 Although the effects of nonablative lasers are milder compared with their ablative counterparts, they possess the superior benefit of minimal adverse events. Most studies reported transient erythema posttreatment, but no long-term adverse effects have been noted,26-31 in part due to preservation of the epidermal layer.
Radiofrequency
Radiofrequency technology was the first method marketed for noninvasive skin tightening. Radiofrequency devices work by generating heat through tissue resistance to an applied alternating electrical current, which leads to collagen contraction and remodeling along with neocollgenesis.32 The major electrode configurations used in these technologies are monopolar, bipolar, and multipolar, which differ by the electric field they produce. Reported side effects include erythema that arose 1 week following completion of treatment and resolved by 6-month follow-up, as well as hypertrophic scarring, transient postinflammatory hyperpigmentation, and pain.33,34
Monopolar systems were the first among these devices to be developed for use in skin tightening and remain the most extensively studied technology for treatment of skin laxity. Developed in 2001, the Thermage device (Solta Medical, Valent Pharmaceuticals) remains the most extensively studied technology for the treatment of skin laxity.35 In a trial performed by Fitzpatrick et al,36 treatment of skin laxity of the periorbital area with ThermaCool TC (Thermage, Inc) demonstrated an 83.2% improvement in at least 1 point treated and an overall 28.9% improvement of the entire treatment area at 6-month follow-up. Additionally, a survey study of 5700 patients who received monopolar RF skin tightening treatments demonstrated that 26% of patients experienced immediate tightening following treatment, and 54% observed tightening 6 months posttreatment.37
Bipolar and multipolar devices were developed following the success of monopolar devices in the treatment for skin laxity. In a study evaluating multipolar RF for the face and neck, all 11 patients were determined to have improvement of their skin laxity following weekly treatments for 8 weeks.38
Ultrasound
The use of ultrasound for skin tightening was first approved in 2009.39 The primary mechanism of skin tightening is through thermally induced contraction of collagen with subsequent collagen neogenesis achieved through absorption of the vibrational acoustic energy into target tissue.40 There are 2 types of ultrasound methods: microfocused and high-intensity focused. Microfocused ultrasound focuses on delivering lower-energy pulses to the deep reticular dermal and subdermal layers that lead to disruption of the underlying architecture of the skin, promoting increases in distensibility, elasticity, and viscoelasticity.41 To date, microfocused ultrasound is approved for treating skin laxity of the eyebrow and submental area and wrinkles of the décolleté. Currently, there are 2 devices approved by the US Food and Drug Administration for the treatment of skin laxity with ultrasound. These devices are the Ulthera System (Merz Pharmaceuticals) and the Sofwave system (Sofwave Medical Ltd).42 Oni et al43 evaluated 93 patients following treatment using Ulthera for skin laxity in the lower face. There was a noticeable improvement of 63.6% at 90 days following treatment. Brobst et al44 showed improvement in laxity at 6 months and 1.5 years following last treatment. The most commonly reported posttreatment side effects include transient purpura, transient edema, and transient postinflammatory pigmentation.42,45 Serious complications are rare and include development of palpable subcutaneous nodules and motor nerve paresis.42,46
High-intensity focused ultrasound has been more recently introduced as a modality for skin tightening and rejuvenation. This method focuses on applying heat to areas through acoustic energy to areas of the deep dermis, subdermal connective tissue, and fibromuscular layer in targeted microcoagulation zones without effect to the epidermis.47 The targeted thermal effects and microcoagulation are believed to cause skin tightening through collagen contraction and remodeling. Future studies are needed to determine the overall benefits in skin laxity to achieve approval by the US Food and Drug Administration for use as a treatment option.
IPL Therapy
Intense pulsed light therapy is different from lasers in that it utilizes a wider variety of wavelengths ranging from approximately 500 to 1200 nm.48 The process of skin tightening is achieved through selective photothermolysis in which thermal damage is focused solely on pigmented targets at the cellular or tissue levels in the epidermis and dermis.49 Intense pulsed light penetrates the tissues and is selectively absorbed by melanin and hemoglobin, thereby producing photothermal effects. The photothermal effects lead to reversible thermal damage to surrounding collagen and induction contraction of collagen fibers and fiber remodeling.50 Clinical studies on the effectiveness on skin tightening have shown incongruent results. Multiple studies have noted improvement in skin elasticity as well as increased deposits of collagen in treated areas. Other studies have shown no improvement of rhytides or wrinkle reduction. The side effects noted were transient pain, swelling, and erythema, along with rare instances of blisters and crusting.48,51-54 Due to the inhomogeneous results, the use of IPL is largely reserved for treatment of acne, hyperpigmentation, hypertrichosis, and superficial vascular malformations.
Chemical Peels
Chemical peels are used in the treatment of skin laxity through a process similar to ablative lasers. Unlike other methods described in this article, this type of treatment is only reserved for the facial areas. The peel must penetrate to the lower papillary dermis or deeper to allow for adequate collagen synthesis.55 As such, medium to deep peeling agents should be used.56 Peels cause coagulation of membrane proteins and necrosis of the epidermis and dermis, thereby stimulating collagen synthesis and keratinocyte regeneration. Additionally, there is an increase in the deposition of glycosaminoglycans, which play a major role in providing hydration for the skin because of their water-binding capacity.56 Deep peels have the added effect of restoring dermal architecture to its native state. Medium-depth peels work up to the layer of the epidermis and dermis.57 Trichloroacetic acid (TCA) 35% is the main ingredient used in these types of peels. Some examples include Monet combination (Jessner solution with 35% TCA), Brody combination (solid CO2 plus 35% TCA), and Coleman combination (70% glycolic acid and 35% TCA). Deep peels penetrate to the levels of the reticular dermis.58 The formulation of these peels contain croton oil and phenols in various concentrations.57,58 A study by Brody59 noted clinical improvement of skin laxity–attributed histologic depth achieved by medium-depth peels. The results of the study demonstrated that the depth of wounding from 3 consecutive applications of TCA led to greater epidermal hyperplasia and a more dense formation of dermal elastic fiber formation on histologic examination. Side effects noted in the study included transient erythema, edema, and erosions that resolved without scar formation at 30-day follow-up.59 Another study performed by Oresajo et al60 demonstrated that patients treated with either a chemical peel of 41% capryloyl salicylic acid or 30% glycolic acid led to notable reduction of fine lines/wrinkles vs baseline. Side effects noted included pruritus, erythema, increased skin sensitivity, epidermolysis, allergic and irritant contact dermatitis, and postinflammatory hyperpigmentation.60
Skin Care
Skin care products have been developed over the years and marketed to aid in the treatment of skin laxity. Some studied methods include photoprotection products, antioxidant-based products, and vitamin A products. Photoprotection plays a crucial role in the prevention of skin laxity. Unprotected sun exposure can induce damage to previously treated skin, leading to minimized or cancelled rejuvenation measures.61
Oxidation is a major contributor in the development of skin laxity. The skin naturally possesses endogenous antioxidant defense mechanisms that protect its cells from free radical damage. However, these mechanisms are reduced as skin ages and are further diminished with photodamage. Ascorbic acid is a collagen stimulator that is known to have antioxidant properties. In the appropriate formulations, topical vitamin C directly supplements the skin’s antioxidant reservoir.61
The use of vitamin A, a retinoic acid, for treatment of skin laxity is based on its ability to improve the production of procollagen and elastic fiber components, resulting in the restoration of dermal matrix proteins.61-65 Vitamin A in the skin plays a key role in the regulation and control of proliferation and differentiation of all major cell types found in the epidermis and dermis.61 Studies have shown that the long-term use of topical vitamin A improves fine and coarse wrinkling.65
Final Thoughts
Various technologies have been developed to provide clinically significant skin laxity reversal. Laser, RF, ultrasound, IPL, and topical therapies provide numerous options at our disposal. Although many devices are available, it is important to consider the desired outcome, cost, and adverse events when discussing therapeutic options for treating skin laxity (eTable). Patients should be advised that multiple treatment sessions over the course of months will likely be necessary. With the development of numerous technologies, we now have many options to offer our patients who desire minimally or noninvasive skin tightening.
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323-331.
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Berlin, Germany: Springer, Heidelberg; 2006:9-21.
- Ramanadham SR, Costa CR, Narasimhan K, et al. Refining the anesthesia management of the face-lift patient: lessons learned from 1089 consecutive face lifts. Plast Reconstr Surg. 2015;135:723-730.
- Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1-13.
- le Lous M, Flandin F, Herbage D, et al. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295-300.
- Ross EV, Yashar SS, Naseef GS, et al. A pilot study of in vivo immediate tissue contraction with CO2 skin laser resurfacing in a live farm pig. Dermatol Surg. 1999;25:851-856.
- Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
- Hsu TS, Kaminer MS. The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg. 2003;22:115-123.
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619-632; discussion 633-634.
- Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49-60.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Bernstein LJ, Kauvar AN, Grossman MC, et al. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. an evaluation of 500 patients. Dermatol Surg. 1998;24:315-320.
- Ortiz AE, Tremaine AM, Zachary CB. Long‐term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168-170.
- Rahman Z, MacFalls H, Jiang K, et al. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med. 2009;41:78-86.
- Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301-305; discussion 305-307.
- Fisher GH, Geronemus RG. Short‐term side effects of fractional photothermolysis. Dermatol Surg. 2005;31:1245-1249.
- Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: traditional versus fractional. Dermatol Surg. 2014;40(suppl 12):S147-S151.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169-176.
- Tierney EP, Hanke CW, Petersen J. Ablative fractionated CO2 laser treatment of photoaging: a clinical and histologic study. Dermatol Surg. 2012;38:1777-1789.
- Tierney EP, Hanke CW, Watkins L. Treatment of lower eyelid rhytids and laxity with ablative fractionated carbon-dioxide laser resurfacing: case series and review of the literature. J Am Acad Dermatol. 2011;64:730-740.
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: four cases. Lasers Surg Med. 2009;41:179-184.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299-306.
- Miller L, Mishra V, Alsaad S, et al. Clinical evaluation of a non-ablative 1940 nm fractional laser. J Drugs Dermatol. 2014;13:1324-1329.
- Alexiades-Armenakas M. Nonablative skin tightening with a variable depth heating 1310-nm wavelength laser in combination with surface cooling. J Drugs Dermatol. 2007;6:1096-1103.
- Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35:813-815.
- Fournier N, Lagarde JM, Turlier V, et al. A 35-month profilometric and clinical evaluation of non-ablative remodeling using a 1540-nm Er:glass laser. J Cosmet Laser Ther. 2004;6:126-130.
- Hædersdal M, Moreau KER, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189-195.
- Lupton JR, Williams CM, Alster TS. Nonablative laser skin resurfacing using a 1540 nm erbium glass laser: a clinical and histologic analysis. Dermatol Surg. 2002;28:833-835.
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585‐nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg. 2003;29:997-999, discussion 999-1000.
- Pollock H, Pollock TA. NLite laser: nonablative wrinkle reduction.Aesthet Surg J. 2001;21:371-372.
- Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638-642.
- Weiss RA, Weiss MA, Munavelli G, et al. Monopolar radiofrequency facial tightening: a retrospective analysis of efficacy and safety in over 600 treatments. J Drugs Dermatol. 2006;5:707-712.
- Sadick NS, Makino Y. Selective electro‐thermolysis in aesthetic medicine: a review. Lasers Surg Med. 2004;34:91-97.
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol. 2007;25:487-491.
- Fitzpatrick R, Geronemus R, Goldberg D, et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232-242.
- Dover JS, Zelickson B, 14-Physician Multispecialty Consensus Panel. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low‐energy multiple‐pass technique leading to a clinical end point algorithm. Dermatol Surg. 2007;33:900-907.
- de Oliveira TC, Rocha SF, Ramos DG, et al. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for face and neck rejuvenation [published online March 8, 2017]. Dermatol Res Pract. doi:10.1155/2017/4146391.
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Van Leenders GJ, Beerlage HP, Ruijter ET, et al. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269-275.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 201332:18-25.
- Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099-1110.
- Brobst RW, Ferguson M, Perkins SW. Noninvasive treatment of the neck. Facial Plast Surg North Am. 2014;22:191-202.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754-759.
- Missel L. Prevention of potential adverse events associated with use of Ulthera device. Tech Bull. 2011;32:18-25.
- Bove T, Zawada T, Serup J, et al. High‐frequency (20‐MHz) high‐intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217-228.
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78-87.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Faucz LL, Will SE, Rodrigues CJ, et al. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg Med. 2018;50:644-650.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med. 2000;26:196-200.
- Li Y-H, Wu Y, Chen JZ, et al. Application of a new intense pulsed light device in the treatment of photoaging skin in Asian patients. Dermatol Surg. 2008;34:1459-1464.
- Shin J-W, Lee D-H, Choi S-Y, et al. Objective and non‐invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25:516-522.
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28:1115-1119.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313-324.
- Brody HJ. Do chemical peels tighten the skin? Dermatol Surg. 2014;40(suppl):S129-S133.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Brody HJ. Variations and comparisons in medium‐depth chemical peeling. J Dermatol Surg Oncol. 1989;15:953-963.
- Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7:259-262.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- Griffiths C. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613-618.
- Darlenski R, Surber C, Fluhr J. Topical retinoids in the management of photodamaged skin: from theory to evidence‐based practical approach. Br J Dermatol. 2010;163:1157-1165.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6:245-253.
- Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17:265-276.
- American Society of Plastic Surgeons. Average surgeon/physician fees. https://www.plasticsurgery.org/documents/News/Statistics/2019/cosmetic-procedures-average-cost-2019.pdf. Accessed August 24, 2020.
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323-331.
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Berlin, Germany: Springer, Heidelberg; 2006:9-21.
- Ramanadham SR, Costa CR, Narasimhan K, et al. Refining the anesthesia management of the face-lift patient: lessons learned from 1089 consecutive face lifts. Plast Reconstr Surg. 2015;135:723-730.
- Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1-13.
- le Lous M, Flandin F, Herbage D, et al. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295-300.
- Ross EV, Yashar SS, Naseef GS, et al. A pilot study of in vivo immediate tissue contraction with CO2 skin laser resurfacing in a live farm pig. Dermatol Surg. 1999;25:851-856.
- Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
- Hsu TS, Kaminer MS. The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg. 2003;22:115-123.
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619-632; discussion 633-634.
- Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49-60.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Bernstein LJ, Kauvar AN, Grossman MC, et al. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. an evaluation of 500 patients. Dermatol Surg. 1998;24:315-320.
- Ortiz AE, Tremaine AM, Zachary CB. Long‐term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168-170.
- Rahman Z, MacFalls H, Jiang K, et al. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med. 2009;41:78-86.
- Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301-305; discussion 305-307.
- Fisher GH, Geronemus RG. Short‐term side effects of fractional photothermolysis. Dermatol Surg. 2005;31:1245-1249.
- Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: traditional versus fractional. Dermatol Surg. 2014;40(suppl 12):S147-S151.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169-176.
- Tierney EP, Hanke CW, Petersen J. Ablative fractionated CO2 laser treatment of photoaging: a clinical and histologic study. Dermatol Surg. 2012;38:1777-1789.
- Tierney EP, Hanke CW, Watkins L. Treatment of lower eyelid rhytids and laxity with ablative fractionated carbon-dioxide laser resurfacing: case series and review of the literature. J Am Acad Dermatol. 2011;64:730-740.
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: four cases. Lasers Surg Med. 2009;41:179-184.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299-306.
- Miller L, Mishra V, Alsaad S, et al. Clinical evaluation of a non-ablative 1940 nm fractional laser. J Drugs Dermatol. 2014;13:1324-1329.
- Alexiades-Armenakas M. Nonablative skin tightening with a variable depth heating 1310-nm wavelength laser in combination with surface cooling. J Drugs Dermatol. 2007;6:1096-1103.
- Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35:813-815.
- Fournier N, Lagarde JM, Turlier V, et al. A 35-month profilometric and clinical evaluation of non-ablative remodeling using a 1540-nm Er:glass laser. J Cosmet Laser Ther. 2004;6:126-130.
- Hædersdal M, Moreau KER, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189-195.
- Lupton JR, Williams CM, Alster TS. Nonablative laser skin resurfacing using a 1540 nm erbium glass laser: a clinical and histologic analysis. Dermatol Surg. 2002;28:833-835.
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585‐nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg. 2003;29:997-999, discussion 999-1000.
- Pollock H, Pollock TA. NLite laser: nonablative wrinkle reduction.Aesthet Surg J. 2001;21:371-372.
- Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638-642.
- Weiss RA, Weiss MA, Munavelli G, et al. Monopolar radiofrequency facial tightening: a retrospective analysis of efficacy and safety in over 600 treatments. J Drugs Dermatol. 2006;5:707-712.
- Sadick NS, Makino Y. Selective electro‐thermolysis in aesthetic medicine: a review. Lasers Surg Med. 2004;34:91-97.
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol. 2007;25:487-491.
- Fitzpatrick R, Geronemus R, Goldberg D, et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232-242.
- Dover JS, Zelickson B, 14-Physician Multispecialty Consensus Panel. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low‐energy multiple‐pass technique leading to a clinical end point algorithm. Dermatol Surg. 2007;33:900-907.
- de Oliveira TC, Rocha SF, Ramos DG, et al. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for face and neck rejuvenation [published online March 8, 2017]. Dermatol Res Pract. doi:10.1155/2017/4146391.
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Van Leenders GJ, Beerlage HP, Ruijter ET, et al. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269-275.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 201332:18-25.
- Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099-1110.
- Brobst RW, Ferguson M, Perkins SW. Noninvasive treatment of the neck. Facial Plast Surg North Am. 2014;22:191-202.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754-759.
- Missel L. Prevention of potential adverse events associated with use of Ulthera device. Tech Bull. 2011;32:18-25.
- Bove T, Zawada T, Serup J, et al. High‐frequency (20‐MHz) high‐intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217-228.
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78-87.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Faucz LL, Will SE, Rodrigues CJ, et al. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg Med. 2018;50:644-650.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med. 2000;26:196-200.
- Li Y-H, Wu Y, Chen JZ, et al. Application of a new intense pulsed light device in the treatment of photoaging skin in Asian patients. Dermatol Surg. 2008;34:1459-1464.
- Shin J-W, Lee D-H, Choi S-Y, et al. Objective and non‐invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25:516-522.
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28:1115-1119.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313-324.
- Brody HJ. Do chemical peels tighten the skin? Dermatol Surg. 2014;40(suppl):S129-S133.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Brody HJ. Variations and comparisons in medium‐depth chemical peeling. J Dermatol Surg Oncol. 1989;15:953-963.
- Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7:259-262.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- Griffiths C. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613-618.
- Darlenski R, Surber C, Fluhr J. Topical retinoids in the management of photodamaged skin: from theory to evidence‐based practical approach. Br J Dermatol. 2010;163:1157-1165.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6:245-253.
- Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17:265-276.
- American Society of Plastic Surgeons. Average surgeon/physician fees. https://www.plasticsurgery.org/documents/News/Statistics/2019/cosmetic-procedures-average-cost-2019.pdf. Accessed August 24, 2020.
Practice Points
- There are a multitude of noninvasive modalities available to treat skin laxity.
- Understanding the mechanisms of each modality is crucial to selecting the appropriate treatment for your patients.
- Treatments should be tailored to the individual patient based on desired outcome, possible adverse events, patient preferences, and cost.