User login
First-in-class device for facial wrinkles, tightening hits the market
DENVER – .
“It’s early yet, but I have treated dozens of patients with this device, and they have been happy with the results,” Mathew M. Avram, MD, JD, said at the annual meeting of the American Society for Dermatologic Surgery. “This is a new technique that offers the ability to remove a significant amount of damaged, lax skin without concern for scarring,” he said.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the first-in-class device is cleared by the Food and Drug Administration for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV. It features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“It doesn’t do anything equivalent to a facelift, but the concept is a facelift by thousands of micro-punch excisions,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “Rather than pulling up the skin and lifting it and cutting the excess skin like we do with a facelift, we are creating thousands of smaller-scale tissue removals with immediate closures to do the same thing. The micro-cores are about the size of a 22-gauge needle and there is no scarring due to the small size of these tissue extractions.”
The device features needle cartridges capable of excising up to 24,000 cores per treatment. According to data from Cytrellis, the manufacturer, the equivalent of about 2 inches of skin can be removed during the procedure, which typically takes fewer than 30 minutes to perform. “There is no heat whatsoever,” Dr. Avram said. “In my experience, it especially helps with jawline definition, the lower medial cheek excess skin, and accordion lines in that area.”
In a pivotal trial of the device, 51 patients with mid to lower face wrinkles (moderately deep or deep wrinkles with well-defined edges) were treated 2-3 times with 7%-8% skin removal and up to a 5-mm needle coring depth). The investigators found that 40% of study participants achieved an improvement of 2 grades on the Lemperle Wrinkle Severity Scale and that the rate of overall satisfaction (slightly, somewhat, and extremely satisfied) was 86%.
In addition, 90% showed improvement of treated sites on the Global Aesthetic Improvement Scale, and 70% were comfortable enough to go out in public or return to work 3 days after treatment. Common side effects that can occur immediately post treatment include redness, swelling, and pinpoint bleeding, which typically clear in a few days.
Dr. Avram, immediate past president of the ASDS, has posted videos to his Instagram feed that show him treating patients with the Ellacor device and he admits that the procedure looks painful. “There are all these tear emojis and people cursing me out,” he said, referring to responses from his Instagram followers.
Proper local anesthesia prior to treatment is key. “I perform nerve blocks and infiltrate the skin,” he said. “You have to cover the whole treatment area. If you don’t, then it’s going to hurt. The average pain score is 1.9 out of 10. The highest pain score I’ve gotten from a patient is a 3 out of 10.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton, and has ownership and/or shareholder interest in Cytrellis.
DENVER – .
“It’s early yet, but I have treated dozens of patients with this device, and they have been happy with the results,” Mathew M. Avram, MD, JD, said at the annual meeting of the American Society for Dermatologic Surgery. “This is a new technique that offers the ability to remove a significant amount of damaged, lax skin without concern for scarring,” he said.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the first-in-class device is cleared by the Food and Drug Administration for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV. It features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“It doesn’t do anything equivalent to a facelift, but the concept is a facelift by thousands of micro-punch excisions,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “Rather than pulling up the skin and lifting it and cutting the excess skin like we do with a facelift, we are creating thousands of smaller-scale tissue removals with immediate closures to do the same thing. The micro-cores are about the size of a 22-gauge needle and there is no scarring due to the small size of these tissue extractions.”
The device features needle cartridges capable of excising up to 24,000 cores per treatment. According to data from Cytrellis, the manufacturer, the equivalent of about 2 inches of skin can be removed during the procedure, which typically takes fewer than 30 minutes to perform. “There is no heat whatsoever,” Dr. Avram said. “In my experience, it especially helps with jawline definition, the lower medial cheek excess skin, and accordion lines in that area.”
In a pivotal trial of the device, 51 patients with mid to lower face wrinkles (moderately deep or deep wrinkles with well-defined edges) were treated 2-3 times with 7%-8% skin removal and up to a 5-mm needle coring depth). The investigators found that 40% of study participants achieved an improvement of 2 grades on the Lemperle Wrinkle Severity Scale and that the rate of overall satisfaction (slightly, somewhat, and extremely satisfied) was 86%.
In addition, 90% showed improvement of treated sites on the Global Aesthetic Improvement Scale, and 70% were comfortable enough to go out in public or return to work 3 days after treatment. Common side effects that can occur immediately post treatment include redness, swelling, and pinpoint bleeding, which typically clear in a few days.
Dr. Avram, immediate past president of the ASDS, has posted videos to his Instagram feed that show him treating patients with the Ellacor device and he admits that the procedure looks painful. “There are all these tear emojis and people cursing me out,” he said, referring to responses from his Instagram followers.
Proper local anesthesia prior to treatment is key. “I perform nerve blocks and infiltrate the skin,” he said. “You have to cover the whole treatment area. If you don’t, then it’s going to hurt. The average pain score is 1.9 out of 10. The highest pain score I’ve gotten from a patient is a 3 out of 10.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton, and has ownership and/or shareholder interest in Cytrellis.
DENVER – .
“It’s early yet, but I have treated dozens of patients with this device, and they have been happy with the results,” Mathew M. Avram, MD, JD, said at the annual meeting of the American Society for Dermatologic Surgery. “This is a new technique that offers the ability to remove a significant amount of damaged, lax skin without concern for scarring,” he said.
A brainchild of dermatologists and plastic surgeons at Massachusetts General Hospital, Boston, the first-in-class device is cleared by the Food and Drug Administration for the treatment of moderate and severe wrinkles in the mid and lower face in adults aged 22 years or older with Fitzpatrick skin types I-IV. It features a proprietary needle design that makes a series of high throughput microexcisions in epidermal and dermal tissue, with minimal downtime and without using thermal energy.
“It doesn’t do anything equivalent to a facelift, but the concept is a facelift by thousands of micro-punch excisions,” said Dr. Avram, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital. “Rather than pulling up the skin and lifting it and cutting the excess skin like we do with a facelift, we are creating thousands of smaller-scale tissue removals with immediate closures to do the same thing. The micro-cores are about the size of a 22-gauge needle and there is no scarring due to the small size of these tissue extractions.”
The device features needle cartridges capable of excising up to 24,000 cores per treatment. According to data from Cytrellis, the manufacturer, the equivalent of about 2 inches of skin can be removed during the procedure, which typically takes fewer than 30 minutes to perform. “There is no heat whatsoever,” Dr. Avram said. “In my experience, it especially helps with jawline definition, the lower medial cheek excess skin, and accordion lines in that area.”
In a pivotal trial of the device, 51 patients with mid to lower face wrinkles (moderately deep or deep wrinkles with well-defined edges) were treated 2-3 times with 7%-8% skin removal and up to a 5-mm needle coring depth). The investigators found that 40% of study participants achieved an improvement of 2 grades on the Lemperle Wrinkle Severity Scale and that the rate of overall satisfaction (slightly, somewhat, and extremely satisfied) was 86%.
In addition, 90% showed improvement of treated sites on the Global Aesthetic Improvement Scale, and 70% were comfortable enough to go out in public or return to work 3 days after treatment. Common side effects that can occur immediately post treatment include redness, swelling, and pinpoint bleeding, which typically clear in a few days.
Dr. Avram, immediate past president of the ASDS, has posted videos to his Instagram feed that show him treating patients with the Ellacor device and he admits that the procedure looks painful. “There are all these tear emojis and people cursing me out,” he said, referring to responses from his Instagram followers.
Proper local anesthesia prior to treatment is key. “I perform nerve blocks and infiltrate the skin,” he said. “You have to cover the whole treatment area. If you don’t, then it’s going to hurt. The average pain score is 1.9 out of 10. The highest pain score I’ve gotten from a patient is a 3 out of 10.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton, and has ownership and/or shareholder interest in Cytrellis.
AT ASDS 2022
Ready or not, hands-free devices are coming
Denver – When Anne Chapas, MD, was asked to help conduct a clinical trial of a wearable, hands-free device for remodeling of the face and submental area, she responded with a healthy dose of skepticism.
“My first thought was, ‘this is crazy. It looks like a Storm Trooper helmet,’ ” Dr. Chapas, founder and medical director of UnionDerm, New York, said at the annual meeting of the American Society for Dermatologic Surgery. “But it’s the first FDA-cleared device that uses bipolar radiofrequency to target the lower third of the face and the submental area of the face. We wanted to see how it works.”
Its bipolar radiofrequency (RF) component reaches 4 mm in depth and travels from central to outer electrodes. The device features real-time temperature monitoring and the ability to delivery energy at lower temps for longer periods of time compared with hands-on approaches. No cooling is required.
“It is able to treat a large surface area simultaneously to achieve maximal tissue contraction,” Dr. Chapas said. “What we’ve learned in decades of RF technology is that it’s not just about heat. It has to be the right amount of heat for the right amount of time. That’s what’s difficult when we’re doing our own individual treatments. How many pulses do we need? How is that heat dissipating? Are we getting the amount of heat we need? Is the patient in pain? We need to take that data from the individual provider and come up with an automated system. That’s what this device is trying to accomplish.”
In a prospective trial, she and her colleagues enrolled 40 patients between the ages of 36 and 75 years with visible signs of facial aging who were seeking skin tightening treatments at one of three centers in the United States. They underwent three biweekly treatments with the Evoke device to the lower face and submental area where a target temperature of 42°-43° C was maintained for 41 minutes, or about 20 minutes for each site.
For the primary safety endpoint, investigators and blinded evaluators used a 4-point Likert scale before treatment, and 1, 3, and 6 months post-treatment. Follow-up visit satisfaction metrics were the patient’s skin appearance evaluation and overall satisfaction, and the investigator improvement rating based on an analysis of volumetric data from 3D imaging software. Chin and cheek discomfort metrics were assessed at all treatments. The subject satisfaction metrics were measured on an 11-point scale where 0 is most comfortable and 10 is most uncomfortable.
In terms of safety, patients tolerated the treatments well and rated their average discomfort from 0.643 to 1.45 on the 11-point Likert scale. “The subject satisfaction rate was about 80%, which is in line with other devices, such as microfocused ultrasound,” said Dr. Chapas, who is also a clinical instructor of dermatology at the Mount Sinai Medical Center, New York.
“The physicians were a little tougher on their assessments. We felt there was about a 65%-70% success rate after the three treatment timepoints.” One possible reason for the disparity between the patient and physician assessments is that patients “may be more accepting of meager results from a hands-free treatment.”
Expect to see more hands-free devices hit the dermatology market in the coming months and years ahead, Dr. Chapas said. Before clinicians incorporate such systems into their practices, she advises them to review existing evidence for the technology, including published data and asking for demonstrations. “If it’s not efficacious, you’ve just wasted everybody’s time,” she said. “Also, is it practical for your office? Do you have the space for it? What staff training is involved? Is it truly automated?”
She added, “If you have a device that’s hands-free but someone must stay in the room with the patient for an hour, does that really help the flow of your practice? And finally, what do your patients want? Do they want to come back multiple times, or do they prefer one-and-done treatments?”
Other questions to consider, she said, include, who benefits from these treatments. Does it fill an unmet need for patients, and for clinicians? Does it help with operator fatigue? How are more consistent treatments achieved? Can the technology be applied to broad body areas?
“The hands-free revolution has been building,” Dr. Chapas commented. “The next generation of lasers and energy devices are going to be coming into our offices, so we should think carefully about how to incorporate them.”
Dr. Chapas disclosed that she is an investigator for InMode (the manufacturer of Evoke), Cutera, and Galderma, and a speaker for Allergan.
Denver – When Anne Chapas, MD, was asked to help conduct a clinical trial of a wearable, hands-free device for remodeling of the face and submental area, she responded with a healthy dose of skepticism.
“My first thought was, ‘this is crazy. It looks like a Storm Trooper helmet,’ ” Dr. Chapas, founder and medical director of UnionDerm, New York, said at the annual meeting of the American Society for Dermatologic Surgery. “But it’s the first FDA-cleared device that uses bipolar radiofrequency to target the lower third of the face and the submental area of the face. We wanted to see how it works.”
Its bipolar radiofrequency (RF) component reaches 4 mm in depth and travels from central to outer electrodes. The device features real-time temperature monitoring and the ability to delivery energy at lower temps for longer periods of time compared with hands-on approaches. No cooling is required.
“It is able to treat a large surface area simultaneously to achieve maximal tissue contraction,” Dr. Chapas said. “What we’ve learned in decades of RF technology is that it’s not just about heat. It has to be the right amount of heat for the right amount of time. That’s what’s difficult when we’re doing our own individual treatments. How many pulses do we need? How is that heat dissipating? Are we getting the amount of heat we need? Is the patient in pain? We need to take that data from the individual provider and come up with an automated system. That’s what this device is trying to accomplish.”
In a prospective trial, she and her colleagues enrolled 40 patients between the ages of 36 and 75 years with visible signs of facial aging who were seeking skin tightening treatments at one of three centers in the United States. They underwent three biweekly treatments with the Evoke device to the lower face and submental area where a target temperature of 42°-43° C was maintained for 41 minutes, or about 20 minutes for each site.
For the primary safety endpoint, investigators and blinded evaluators used a 4-point Likert scale before treatment, and 1, 3, and 6 months post-treatment. Follow-up visit satisfaction metrics were the patient’s skin appearance evaluation and overall satisfaction, and the investigator improvement rating based on an analysis of volumetric data from 3D imaging software. Chin and cheek discomfort metrics were assessed at all treatments. The subject satisfaction metrics were measured on an 11-point scale where 0 is most comfortable and 10 is most uncomfortable.
In terms of safety, patients tolerated the treatments well and rated their average discomfort from 0.643 to 1.45 on the 11-point Likert scale. “The subject satisfaction rate was about 80%, which is in line with other devices, such as microfocused ultrasound,” said Dr. Chapas, who is also a clinical instructor of dermatology at the Mount Sinai Medical Center, New York.
“The physicians were a little tougher on their assessments. We felt there was about a 65%-70% success rate after the three treatment timepoints.” One possible reason for the disparity between the patient and physician assessments is that patients “may be more accepting of meager results from a hands-free treatment.”
Expect to see more hands-free devices hit the dermatology market in the coming months and years ahead, Dr. Chapas said. Before clinicians incorporate such systems into their practices, she advises them to review existing evidence for the technology, including published data and asking for demonstrations. “If it’s not efficacious, you’ve just wasted everybody’s time,” she said. “Also, is it practical for your office? Do you have the space for it? What staff training is involved? Is it truly automated?”
She added, “If you have a device that’s hands-free but someone must stay in the room with the patient for an hour, does that really help the flow of your practice? And finally, what do your patients want? Do they want to come back multiple times, or do they prefer one-and-done treatments?”
Other questions to consider, she said, include, who benefits from these treatments. Does it fill an unmet need for patients, and for clinicians? Does it help with operator fatigue? How are more consistent treatments achieved? Can the technology be applied to broad body areas?
“The hands-free revolution has been building,” Dr. Chapas commented. “The next generation of lasers and energy devices are going to be coming into our offices, so we should think carefully about how to incorporate them.”
Dr. Chapas disclosed that she is an investigator for InMode (the manufacturer of Evoke), Cutera, and Galderma, and a speaker for Allergan.
Denver – When Anne Chapas, MD, was asked to help conduct a clinical trial of a wearable, hands-free device for remodeling of the face and submental area, she responded with a healthy dose of skepticism.
“My first thought was, ‘this is crazy. It looks like a Storm Trooper helmet,’ ” Dr. Chapas, founder and medical director of UnionDerm, New York, said at the annual meeting of the American Society for Dermatologic Surgery. “But it’s the first FDA-cleared device that uses bipolar radiofrequency to target the lower third of the face and the submental area of the face. We wanted to see how it works.”
Its bipolar radiofrequency (RF) component reaches 4 mm in depth and travels from central to outer electrodes. The device features real-time temperature monitoring and the ability to delivery energy at lower temps for longer periods of time compared with hands-on approaches. No cooling is required.
“It is able to treat a large surface area simultaneously to achieve maximal tissue contraction,” Dr. Chapas said. “What we’ve learned in decades of RF technology is that it’s not just about heat. It has to be the right amount of heat for the right amount of time. That’s what’s difficult when we’re doing our own individual treatments. How many pulses do we need? How is that heat dissipating? Are we getting the amount of heat we need? Is the patient in pain? We need to take that data from the individual provider and come up with an automated system. That’s what this device is trying to accomplish.”
In a prospective trial, she and her colleagues enrolled 40 patients between the ages of 36 and 75 years with visible signs of facial aging who were seeking skin tightening treatments at one of three centers in the United States. They underwent three biweekly treatments with the Evoke device to the lower face and submental area where a target temperature of 42°-43° C was maintained for 41 minutes, or about 20 minutes for each site.
For the primary safety endpoint, investigators and blinded evaluators used a 4-point Likert scale before treatment, and 1, 3, and 6 months post-treatment. Follow-up visit satisfaction metrics were the patient’s skin appearance evaluation and overall satisfaction, and the investigator improvement rating based on an analysis of volumetric data from 3D imaging software. Chin and cheek discomfort metrics were assessed at all treatments. The subject satisfaction metrics were measured on an 11-point scale where 0 is most comfortable and 10 is most uncomfortable.
In terms of safety, patients tolerated the treatments well and rated their average discomfort from 0.643 to 1.45 on the 11-point Likert scale. “The subject satisfaction rate was about 80%, which is in line with other devices, such as microfocused ultrasound,” said Dr. Chapas, who is also a clinical instructor of dermatology at the Mount Sinai Medical Center, New York.
“The physicians were a little tougher on their assessments. We felt there was about a 65%-70% success rate after the three treatment timepoints.” One possible reason for the disparity between the patient and physician assessments is that patients “may be more accepting of meager results from a hands-free treatment.”
Expect to see more hands-free devices hit the dermatology market in the coming months and years ahead, Dr. Chapas said. Before clinicians incorporate such systems into their practices, she advises them to review existing evidence for the technology, including published data and asking for demonstrations. “If it’s not efficacious, you’ve just wasted everybody’s time,” she said. “Also, is it practical for your office? Do you have the space for it? What staff training is involved? Is it truly automated?”
She added, “If you have a device that’s hands-free but someone must stay in the room with the patient for an hour, does that really help the flow of your practice? And finally, what do your patients want? Do they want to come back multiple times, or do they prefer one-and-done treatments?”
Other questions to consider, she said, include, who benefits from these treatments. Does it fill an unmet need for patients, and for clinicians? Does it help with operator fatigue? How are more consistent treatments achieved? Can the technology be applied to broad body areas?
“The hands-free revolution has been building,” Dr. Chapas commented. “The next generation of lasers and energy devices are going to be coming into our offices, so we should think carefully about how to incorporate them.”
Dr. Chapas disclosed that she is an investigator for InMode (the manufacturer of Evoke), Cutera, and Galderma, and a speaker for Allergan.
AT ASDS 2022
Vision loss may be a risk with PRP facial injections
A systematic review was recently conducted by Wu and colleagues examining the risk of blindness associated with platelet-rich plasma (PRP) injection. In dermatology, PRP is used more commonly now than 5 years ago to promote hair growth with injections on the scalp, as an adjunct to microneedling procedures, and sometimes – in a similar way to facial fillers – to improve volume loss, and skin tone and texture (particularly to the tear trough region).
Total unilateral blindness occurred in all cases. In one of the seven reported cases, the patient experienced recovery of vision after 3 months, but with some residual deficits noted on the ophthalmologist examination. In this case, the patient was evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
In addition, four cases were reported from Venezuela, one from the United States, one from the United Kingdom, and one from Malaysia. Similar to reports of blindness with facial fillers, the most common injection site reported with this adverse effect was the glabella (five cases);
Other reports involved injections of the forehead (two), followed by the nasolabial fold (one), lateral canthus (one), and temporomandibular joint (one). Two of the seven patients received injections at more than one site, resulting in the total number of injections reported (10) being higher than the number of patients.
The risk of blindness is inherent with deep injection into a vessel that anastomoses with the blood supply to the eye. No mention was made as to whether PRP or platelet-rich fibrin was used. Other details are lacking from the original articles as to injection technique and whether or not cannula injection was used. No treatment was attempted in four of seven cases.
As plasma is native to the arteries and dissolves in the blood stream naturally, the mechanism as to why retinal artery occlusion or blindness would occur is not completely clear. One theory is that it is volume related and results from the speed of injection, causing a large rapid bolus that temporarily occludes or compresses an involved vessel.
Another theory is that damage to the vessel results from the injection itself or injection technique, leading to a clotting cascade and clot of the involved vessel with subsequent retrograde flow or blockade of the retinal artery. But if this were the case, we would expect to hear about more cases of clots leading to vascular occlusion or skin necrosis, which does not typically occur or we do not hear about.
Details about proper collection materials and technique or mixing with some other materials are also unknown in these cases, thus leaving the possibility that a more occlusive material may have been injected, as opposed to the fluid-like composition of the typical PRP preparation.With regards to risk with scalp PRP injection, the frontal scalp does receive blood supply from the supratrochlear artery that anastomoses with the angular artery of the face – both of which anastomose with the retinal artery (where occlusion would occur via back flow). The scalp tributaries are small and far enough away from the retina at that point that risk of back flow the to retinal artery should be minimal. Additionally, no reports of vascular occlusion from PRP scalp injection leading to skin necrosis have ever been reported. Of note, this is also not a risk that has been reported with the use of PRP with microneedling procedures, where PRP is placed on top of the skin before, during and after microneedling.
Anything that occludes the blood supply to the eye, whether it be fat, filler, or PRP, has an inherent risk of blindness. As there is no reversal agent or designated treatment for PRP occlusion, care must be taken to minimize risk, including awareness of anatomy and avoidance of injection into high risk areas, and cannula use where appropriate. Gentle, slow, low-volume administration, and when possible, use of a retrograde injection technique, may also be helpful.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A systematic review was recently conducted by Wu and colleagues examining the risk of blindness associated with platelet-rich plasma (PRP) injection. In dermatology, PRP is used more commonly now than 5 years ago to promote hair growth with injections on the scalp, as an adjunct to microneedling procedures, and sometimes – in a similar way to facial fillers – to improve volume loss, and skin tone and texture (particularly to the tear trough region).
Total unilateral blindness occurred in all cases. In one of the seven reported cases, the patient experienced recovery of vision after 3 months, but with some residual deficits noted on the ophthalmologist examination. In this case, the patient was evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
In addition, four cases were reported from Venezuela, one from the United States, one from the United Kingdom, and one from Malaysia. Similar to reports of blindness with facial fillers, the most common injection site reported with this adverse effect was the glabella (five cases);
Other reports involved injections of the forehead (two), followed by the nasolabial fold (one), lateral canthus (one), and temporomandibular joint (one). Two of the seven patients received injections at more than one site, resulting in the total number of injections reported (10) being higher than the number of patients.
The risk of blindness is inherent with deep injection into a vessel that anastomoses with the blood supply to the eye. No mention was made as to whether PRP or platelet-rich fibrin was used. Other details are lacking from the original articles as to injection technique and whether or not cannula injection was used. No treatment was attempted in four of seven cases.
As plasma is native to the arteries and dissolves in the blood stream naturally, the mechanism as to why retinal artery occlusion or blindness would occur is not completely clear. One theory is that it is volume related and results from the speed of injection, causing a large rapid bolus that temporarily occludes or compresses an involved vessel.
Another theory is that damage to the vessel results from the injection itself or injection technique, leading to a clotting cascade and clot of the involved vessel with subsequent retrograde flow or blockade of the retinal artery. But if this were the case, we would expect to hear about more cases of clots leading to vascular occlusion or skin necrosis, which does not typically occur or we do not hear about.
Details about proper collection materials and technique or mixing with some other materials are also unknown in these cases, thus leaving the possibility that a more occlusive material may have been injected, as opposed to the fluid-like composition of the typical PRP preparation.With regards to risk with scalp PRP injection, the frontal scalp does receive blood supply from the supratrochlear artery that anastomoses with the angular artery of the face – both of which anastomose with the retinal artery (where occlusion would occur via back flow). The scalp tributaries are small and far enough away from the retina at that point that risk of back flow the to retinal artery should be minimal. Additionally, no reports of vascular occlusion from PRP scalp injection leading to skin necrosis have ever been reported. Of note, this is also not a risk that has been reported with the use of PRP with microneedling procedures, where PRP is placed on top of the skin before, during and after microneedling.
Anything that occludes the blood supply to the eye, whether it be fat, filler, or PRP, has an inherent risk of blindness. As there is no reversal agent or designated treatment for PRP occlusion, care must be taken to minimize risk, including awareness of anatomy and avoidance of injection into high risk areas, and cannula use where appropriate. Gentle, slow, low-volume administration, and when possible, use of a retrograde injection technique, may also be helpful.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
A systematic review was recently conducted by Wu and colleagues examining the risk of blindness associated with platelet-rich plasma (PRP) injection. In dermatology, PRP is used more commonly now than 5 years ago to promote hair growth with injections on the scalp, as an adjunct to microneedling procedures, and sometimes – in a similar way to facial fillers – to improve volume loss, and skin tone and texture (particularly to the tear trough region).
Total unilateral blindness occurred in all cases. In one of the seven reported cases, the patient experienced recovery of vision after 3 months, but with some residual deficits noted on the ophthalmologist examination. In this case, the patient was evaluated and treated by an ophthalmologist within 3 hours of symptom onset.
In addition, four cases were reported from Venezuela, one from the United States, one from the United Kingdom, and one from Malaysia. Similar to reports of blindness with facial fillers, the most common injection site reported with this adverse effect was the glabella (five cases);
Other reports involved injections of the forehead (two), followed by the nasolabial fold (one), lateral canthus (one), and temporomandibular joint (one). Two of the seven patients received injections at more than one site, resulting in the total number of injections reported (10) being higher than the number of patients.
The risk of blindness is inherent with deep injection into a vessel that anastomoses with the blood supply to the eye. No mention was made as to whether PRP or platelet-rich fibrin was used. Other details are lacking from the original articles as to injection technique and whether or not cannula injection was used. No treatment was attempted in four of seven cases.
As plasma is native to the arteries and dissolves in the blood stream naturally, the mechanism as to why retinal artery occlusion or blindness would occur is not completely clear. One theory is that it is volume related and results from the speed of injection, causing a large rapid bolus that temporarily occludes or compresses an involved vessel.
Another theory is that damage to the vessel results from the injection itself or injection technique, leading to a clotting cascade and clot of the involved vessel with subsequent retrograde flow or blockade of the retinal artery. But if this were the case, we would expect to hear about more cases of clots leading to vascular occlusion or skin necrosis, which does not typically occur or we do not hear about.
Details about proper collection materials and technique or mixing with some other materials are also unknown in these cases, thus leaving the possibility that a more occlusive material may have been injected, as opposed to the fluid-like composition of the typical PRP preparation.With regards to risk with scalp PRP injection, the frontal scalp does receive blood supply from the supratrochlear artery that anastomoses with the angular artery of the face – both of which anastomose with the retinal artery (where occlusion would occur via back flow). The scalp tributaries are small and far enough away from the retina at that point that risk of back flow the to retinal artery should be minimal. Additionally, no reports of vascular occlusion from PRP scalp injection leading to skin necrosis have ever been reported. Of note, this is also not a risk that has been reported with the use of PRP with microneedling procedures, where PRP is placed on top of the skin before, during and after microneedling.
Anything that occludes the blood supply to the eye, whether it be fat, filler, or PRP, has an inherent risk of blindness. As there is no reversal agent or designated treatment for PRP occlusion, care must be taken to minimize risk, including awareness of anatomy and avoidance of injection into high risk areas, and cannula use where appropriate. Gentle, slow, low-volume administration, and when possible, use of a retrograde injection technique, may also be helpful.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Ossification and Migration of a Nodule Following Calcium Hydroxylapatite Injection
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.
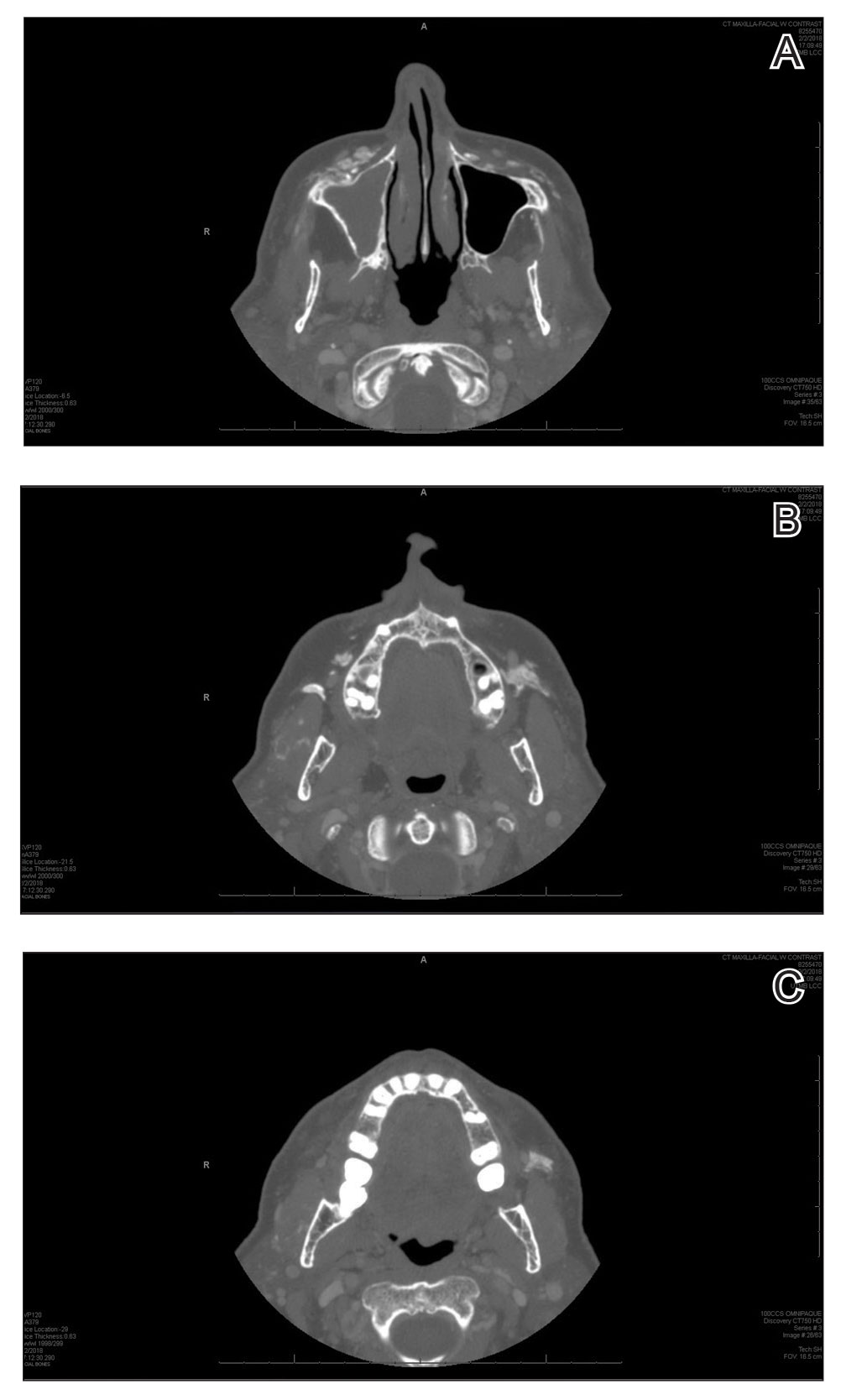
We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.

We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
To the Editor:
Calcium hydroxylapatite is an injectable filler approved by the US Food and Drug Administration for moderate to severe rhytides of the face and the treatment of facial lipodystrophy in patients with HIV.1 This long-lasting filler generally is well tolerated with minimal side effects; however, there have been reports of nodules or granulomatous formation following injection.2 We present a case of a migrating nodule following injection of a calcium hydroxylapatite filler that appeared ossified on radiographic imaging. We highlight this rarely reported phenomenon to increase awareness of this complication.
A 72-year-old woman presented to our clinic with a mass on the left cheek. The patient had a history of treatment with facial fillers but no notable medical conditions. She initially received hyaluronic acid injectable gel dermal filler twice—3 years apart—before switching to calcium hydroxylapatite injections twice—4 months apart—from an outside provider. One month after the second treatment, she noticed a mass on the left cheek and promptly returned to the provider who performed the calcium hydroxylapatite injections. The provider, who had originally injected in the infraorbital area, stated it was unlikely that the filler would have migrated to the mid cheek and referred the patient to a general dentist who suspected salivary gland pathology. The patient was referred to an oral and maxillofacial surgeon who suspected the mass was related to the parotid gland. Maxillofacial computed tomography (CT) revealed heterotopic ossification vs myositis ossificans, possibly related to the recent injection. The patient was eventually referred to the Division of Plastic Surgery, Department of Surgery, at the University of Texas Medical Branch (Galveston, Texas) for further evaluation. Physical examination revealed a 2×1-cm firm, mobile, nontender mass in the left cheek in the area of the buccinator muscles. The mass did not express any fluid and was most easily palpable from the oral cavity. Radiography findings showed that the calcium hydroxylapatite filler had migrated to this location and formed a nodule (Figure). Because calcium hydroxylapatite fillers generally last 12 to 18 months, we opted to observe the lesion for spontaneous resolution. Four months later, the patient presented to our clinic for follow-up and the mass had reduced in size and appeared to be spontaneously resolving.

We present a unique case of a migrating nodule that occurred after injection with calcium hydroxylapatite, which led to concern for neoplastic tumor formation. This complication is rare, and it is important for practitioners who inject calcium hydroxylapatite as well as those who these patients may be referred to for evaluation to be aware that migrating nodules can occur. This awareness can help reduce unnecessary referrals, medical procedures, and anxiety.
Calcium hydroxylapatite filler is composed of 30% calcium hydroxylapatite microspheres suspended in a 70% sodium carboxymethylcellulose gel. The water-soluble gel rapidly becomes absorbed upon injection; however, the microspheres form a scaffold for the production of newly synthesized collagen. The filling effect generally lasts 12 to 18 months.1
Calcium hydroxylapatite, similar to most fillers, generally is well tolerated with a low complication rate of 3%.1 Although nodule formation with calcium hydroxylapatite is rare, it is the most common adverse event and encompasses 96% of complications. The remaining 4% of complications include persistent inflammation, swelling, erythema, and technical mistakes leading to overcorrection.1 Migrating nodules are rare; however, Beer3 reported a similar case.
Treatment of calcium hydroxylapatite nodules depends on differentiating a cause based on the time of onset. Early nodules that occur within 1 to 2 weeks of the injection usually represent incorrect positioning of the filler and can be treated by massaging the nodule. Other more invasive techniques involve aspiration or injection of sterile water. Late-onset nodules have shown response to corticosteroid injections. For inflammatory nodules of infectious origin, antibiotics can be useful. Surgical excision of the nodule rarely is required, as most nodules will resolve spontaneously, even without intervention.1,2
Radiologic findings of calcium hydroxylapatite appear as high-attenuation linear streaks or masses on CT (280–700 HU) and as low to intermediate signal intensity on T1- or T2-weighted sequences on magnetic resonance imaging. Oftentimes, calcium hydroxylapatite has a similar radiographic appearance to bone and can persist for 2 years or more on radiographic imaging, longer than they are clinically visible.4 The nodule formation from injection with calcium hydroxylapatite can mimic pathologic conditions such as miliary osteomas, myositis ossificans, heterotrophic/dystrophic calcifications, and foreign bodies on CT. Our patient’s CT findings of high attenuation linear streaks and nodules of similar signal intensity to bone were consistent with those previously described in the radiographic literature.
Calcium hydroxylapatite fillers have a good safety profile, but it is important to recognize that nodule formation is a common adverse event and that migration of nodules can occur. Practitioners should recognize this possibility in patients presenting with new masses after filler injection before advocating for potentially invasive and costly procedures and diagnostic modalities.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
- Kadouch JA. Calcium hydroxylapatite: a review on safety and complications. J Cosmet Dermatol. 2017;16:152-161.
- Moulinets I, Arnaud E, Bui P, et al. Foreign body reaction to Radiesse: 2 cases. Am J Dermatopathol. 2013;35:e37-40.
- Beer KR. Radiesse nodule of the lips from a distant injection site: report of a case and consideration of etiology and management. J Drugs Dermatol. 2007;6:846-847.
- Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013;34:1488-1495.
Practice Points
- Calcium hydroxylapatite filler can migrate and form nodules in distant locations from the original injection site.
- Practitioners of calcium hydroxylapatite fillers should be aware of the potential for nodule migration to avoid costly, time-consuming, and invasive referrals and procedures.
Poor evidence for vaginal laser therapy
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
Despite a lack of evidence and high cost, laser therapy continues to attract many women seeking “vaginal rejuvenation” to help reverse the physical symptoms of menopause.
Recent reviews of the medical literature continue to show that laser treatment appears to be less effective than estrogen at improving vaginal dryness and pain during sex, according to Cheryl B. Iglesia, MD, a professor of ob.gyn. and urology at Georgetown University, Washington.
“Laser for GSM [genitourinary syndrome of menopause] is showing some promise, but patients need to be offered [Food and Drug Administration]–approved treatments prior to considering laser, and users need to know how to do speculum and pelvic exams and understand vulvovaginal anatomy and pathology,” Dr. Iglesia, who directs the section of female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, said in an interview, adding that patients should avoid “vaginal rejuvenation” treatments offered at med-spas.
Dr. Iglesia reviewed how these lasers work and then discussed the controversy over their marketing and the evidence for their use at the annual meeting of the North American Menopause Society.
By 3 years after menopause, more than half of women experience atrophy in their vagina resulting from a lack of estrogen. Marked by a thinning of the epithelium, reduced blood supply, and loss of glycogen, vulvovaginal atrophy is to blame for GSM.
Vaginal laser therapy has been a popular option for women for the last decade, despite a lack of evidence supporting its use or approval from regulators.
The FDA has issued broad clearance for laser therapy for incision, ablation, vaporization, and coagulation of body soft tissues, such as dysplasia, vulvar or anal neoplasia, endometriosis, condylomas, and other disorders. However, the agency has not approved the use of laser therapy for vulvovaginal atrophy, GSM, vaginal dryness, or dyspareunia.
Evidence regarding vaginal laser therapy
According to Dr. Iglesia, the evidence for vaginal laser therapy is mixed and of generally low quality. A systematic review published in the Journal of Sexual Medicine (2022 Jan 29. doi: 10.1016/j.jsxm.2021.12.010) presented mostly low-quality evidence from 25 studies and found promising data for genitourinary symptoms but not enough to justify its use for genitourinary symptoms just yet. Dr. Iglesia discussed her own small, multisite study of 62 participants, which compared vaginal laser with vaginal estrogen and found no differences between the two for multiple outcomes. (The study would have been larger if not for interruption from an FDA warning for an Investigational Device Exemption.)
A JAMA study from Australia found no difference between laser therapy and sham laser therapy, but the most recent systematic review, from JAMA Network Open, found no significant difference between vaginal laser and vaginal estrogen for vaginal and sexual function symptoms. This review, however, covered only the six existing randomized controlled trials, including Dr. Iglesia’s, which were small and had a follow-up period of only 3-6 months.
“There have only been a few randomized controlled trials comparing laser to vaginal estrogen therapy, and most of those did not include a placebo or sham arm,” Monica Christmas, MD, director of the Center for Women’s Integrated Health at the University of Chicago Medicine, said in an interview. “This is extremely important, as most of the trials that did include a sham arm did not find that laser was better than the sham.” Dr. Christmas was not a part of the presentation but attended it at NAMS.
The bottom line, she said, is that “current evidence is not sufficient to make conclusions on long-term safety or sustainability, nor is there compelling evidence to make claims on equivalence to vaginal estrogen therapy.” Currently, committee opinions from a half-dozen medical societies, including NAMS, oppose using vaginal laser therapy until rigorous, robust trials on long-term safety and efficacy have been conducted. The International Continence Society and International Society for the Study of Vulvovaginal Disease issued a joint statement in 2018 that emphasized that histologic changes from lasers do not necessarily equate with changes in function. The statement noted the lack of evidence for laser treatment of incontinence and prolapse and stated that it should not be used for vulvodynia or lichen sclerosus.
A 2020 statement from NAMS found “insufficient placebo-controlled trials of energy-based therapies, including laser, to draw conclusions of efficacy or safety or to make treatment recommendations.” A slightly more optimistic statement from the American Urogynecologic Society concluded that energy-based devices have shown short-term efficacy for menopause-related vaginal atrophy and dyspareunia, including effects lasting up to 1 year from fractionated laser for treat dyspareunia, but also noted that studies up to that time were small and measure various outcomes.
Recommendations on vaginal laser therapy
Given this landscape of uneven and poor-quality evidence, Dr. Iglesia provided several “common sense” recommendations for energy-based therapies, starting with the need for any practitioner to have working knowledge of vulvovaginal anatomy. Contraindications for laser therapy include any malignancy – especially gynecologic – undiagnosed bleeding, active herpes or other infections, radiation, and vaginal mesh, particularly transvaginal mesh. The provider also must discuss the limited data on long-term function and treatment alternatives, including FDA-approved therapies like topical estrogen, dehydroepiandrosterone sulfate (DHEA-S), ospemifene, and moisturizers, Dr. Iglesia said.
Adverse events associated with laser therapy, such as scarring or burning, are rare but do occur, and cost remains an issue, Dr. Iglesia said.
“Vaginal estrogen therapy is well established as a safe and effective treatment option based on high quality evidence,” Dr. Christmas said. “This is not the case for laser therapy. Rare, but serious harms are reported with vaginal laser, including burns, scarring, dyspareunia, pain, and potential irreversible damage.”
Dr. Iglesia also cautioned that clinicians should take extra care with vulnerable populations, particularly cancer patients and others with contraindications for estrogen treatment.
For those in whom vaginal estrogen is contraindicated, Dr. Christmas recommended vaginal moisturizers, lubricants, dilators, and physical therapy for the pelvic floor.
“In patients who fail those nonhormonal approaches, short courses of vaginal estrogen therapy or DHEA-S suppository may be employed with approval from their oncologist,” Dr. Christmas said.
Dr. Iglesia finally reviewed the major research questions that remain with laser therapy:
- What are outcomes for laser versus sham studies?
- What are long-term outcomes (beyond 6 months)
- What pretreatment is necessary?
- Could laser be used as a drug delivery mechanism for estrogen, and could this provide a synergistic effect?
- What is the optimal number and interval for laser treatments?
Dr. Iglesia had no industry disclosures but received honoraria for consulting at UpToDate. Dr. Christmas is a consultant for Materna. The presentation did not rely on any external funding.
FROM NAMS 2022
Vaccinium myrtillus (bilberry seed oil) extract
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
A member of the Ericaceae family, bilberry (Vaccinium myrtillus) is native to northern Europe and North America, and its fruit is known to contain myriad polyphenols that display potent antioxidant and anti-inflammatory activity.1,2 Also known as European blueberry or whortleberry, this perennial deciduous shrub is also one of the richest sources of the polyphenolic pigments anthocyanins.3-5 Indeed, anthocyanins impart the blue/black color to bilberries and other berries and are thought to be the primary bioactive constituents of berries associated with numerous health benefits.3,6 They are also known to confer anti-allergic, anticancer, and wound healing activity.4 Overall, bilberry has also been reported to exert anti-inflammatory, lipid-lowering, and antimicrobial activity.3 In this column, the focus will be on the chemical constituents and properties of V. myrtillus that indicate potential or applicability for skin care.
Active ingredients of bilberry
Bilberry seed oil contains unsaturated fatty acids such as linoleic acid and alpha-linolenic acid, which exhibit anti-inflammatory activity and contribute to the suppression of tyrosinase. For instance, Ando et al. showed, in 1998, that linoleic and alpha-linolenic acids lighten UV-induced skin hyperpigmentation. Their in vitro experiments using cultured murine melanoma cells and in vivo study of the topical application of either acid to the UV-induced hyperpigmented dorsal skin of guinea pigs revealed pigment-lightening effects that they partly ascribed to inhibited melanin synthesis by active melanocytes and accelerated desquamation of epidermal melanin pigment.7
A 2009 comparative study of the anthocyanin composition as well as antimicrobial and antioxidant activities delivered by bilberry and blueberry fruits and their skins by Burdulis et al. revealed robust functions in both fruits. Cyanidin was found to be an active anthocyanidin in bilberry. Cultivars of both fruits demonstrated antimicrobial and antioxidant activity, with bilberry fruit skin demonstrating potent antiradical activity.8
The anthocyanins of V. myrtillus are reputed to impart protection against cardiovascular disorders, age-induced oxidative stress, inflammatory responses, and various degenerative conditions, as well ameliorate neuronal and cognitive brain functions and ocular health.6
In 2012, Bornsek et al. demonstrated that bilberry (and blueberry) anthocyanins function as potent intracellular antioxidants, which may account for their noted health benefits despite relatively low bioavailability.9
Six years later, a chemical composition study of wild bilberry found in Montenegro, Brasanac-Vukanovic et al. determined that chlorogenic acid was the most prevalent phenolic constituent, followed by protocatechuic acid, with resveratrol, isoquercetin, quercetin, and hyperoside also found to be abundant. In vitro assays indicated significant antioxidant activity exhibited by these compounds.10
Activity against allergic contact dermatitis
Yamaura et al. used a mouse model, in 2011, to determine that the anthocyanins from a bilberry extract attenuated various symptoms of chronic allergic contact dermatitis, particularly alleviating pruritus.8 A year later, Yamaura et al. used a BALB/c mouse model of allergic contact dermatitis to compare the antipruritic effect of anthocyanin-rich quality-controlled bilberry extract and anthocyanidin-rich degraded extract. The investigators found that anthocyanins, but not anthocyanidins, derived from bilberry exert an antipruritic effect, likely through their inhibitory action on mast cell degranulation. They concluded that anthocyanin-rich bilberry extract could act as an effective oral supplement to treat pruritic symptoms of skin disorders such as chronic allergic contact dermatitis and atopic dermatitis.11
Antioxidant and anti-inflammatory activity
Bilberries, consumed since ancient times, are reputed to function as potent antioxidants because of a wide array of phenolic constituents, and this fruit is gaining interest for use in pharmaceuticals.12
In 2008, Svobodová et al. assessed possible UVA preventive properties of V. myrtillus fruit extract in a human keratinocyte cell line (HaCaT), finding that pre- or posttreatment mitigated UVA-induced harm. They also observed a significant decrease in UVA-caused reactive oxygen species (ROS) formation and the prevention or attenuation of UVA-stimulated peroxidation of membrane lipids. Intracellular glutathione was also protected. The investigators attributed the array of cytoprotective effects conferred by V. myrtillus extract primarily to its constituent anthocyanins.2 A year later, they found that the phenolic fraction of V. myrtillus fruits inhibited UVB-induced damage to HaCaT keratinocytes in vitro.13
In 2014, Calò and Marabini used HaCaT keratinocytes to ascertain whether a water-soluble V. myrtillus extract could mitigate UVA- and UVB-induced damage. They found that the extract diminished UVB-induced cytotoxicity and genotoxicity at lower doses, decreasing lipid peroxidation but exerting no effect on reactive oxygen species generated by UVB. The extract attenuated genotoxicity induced by UVA as well as ROS and apoptosis. Overall, the investigators concluded that V. myrtillus extract demonstrated antioxidant activity, particularly against UVA exposure.14
Four years later, Bucci et al. developed nanoberries, an ultradeformable liposome carrying V. myrtillus ethanolic extract, and determined that the preparation could penetrate the stratum corneum safely and suggested potential for yielding protection against photodamage.15
Skin preparations
In 2021, Tadic et al. developed an oil-in-water (O/W) cream containing wild bilberry leaf extracts and seed oil. The leaves contained copious phenolic acids (particularly chlorogenic acid), flavonoids (especially isoquercetin), and resveratrol. The seed oil was rife with alpha-linolenic, linoleic, and oleic acids. The investigators conducted an in vivo study over 30 days in 25 healthy volunteers (20 women, 5 men; mean age 23.36 ± 0.64 years). They found that the O/W cream successfully increased stratum corneum hydration, enhanced skin barrier function, and maintained skin pH after topical application. The cream was also well tolerated. In vitro assays also indicated that the bilberry isolates displayed notable antioxidant capacity (stronger in the case of the leaves). Tadic et al. suggested that skin disorders characterized by oxidative stress and/or xerosis may be appropriate targets for topically applied bilberry cream.1
Early in 2022, Ruscinc et al. reported on their efforts to incorporate V. myrtillus extract into a multifunctional sunscreen. In vitro and in vivo tests revealed that while sun protection factor was lowered in the presence of the extract, the samples were safe and photostable. The researchers concluded that further study is necessary to elucidate the effect of V. myrtillus extract on photoprotection.16
V. myrtillus has been consumed by human beings for many generations. Skin care formulations based on this ingredient have not been associated with adverse events. Notably, the Environmental Working Group has rated V. myrtillus (bilberry seed) oil as very safe.17
Summary
While research, particularly in the form of randomized controlled trials, is called for, because the fatty acids it contains have been shown to suppress tyrosinase. Currently, this botanical agent seems to be most suited for sensitive, aging skin and for skin with an uneven tone, particularly postinflammatory pigmentation and melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, an SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Tadic VM et al. Antioxidants (Basel). 2021 Mar 16;10(3):465.
2. Svobodová A et al. Biofactors. 2008;33(4):249-66.
3. Chu WK et al. Bilberry (Vaccinium myrtillus L.), in Benzie IFF, Wachtel-Galor S, eds., “Herbal Medicine: Biomolecular and Clinical Aspects,” 2nd ed. (Boca Raton, Fla.: CRC Press/Taylor & Francis, 2011, Chapter 4).
4. Yamaura K et al. Pharmacognosy Res. 2011 Jul;3(3):173-7.
5. Stefanescu BE et al. Molecules. 2019 May 29;24(11):2046.
6. Smeriglio A et al. Mini Rev Med Chem. 2014;14(7):567-84.
7. Ando H et al. Arch Dermatol Res. 1998 Jul;290(7):375-81.
8. Burdulis D et al. Acta Pol Pharm. 2009 Jul-Aug;66(4):399-408.
9. Bornsek SM et al. Food Chem. 2012 Oct 15;134(4):1878-84.
10. Brasanac-Vukanovic S et al. Molecules. 2018 Jul 26;23(8):1864.
11. Yamaura K et al. J Food Sci. 2012 Dec;77(12):H262-7.
12. Pires TCSP et al. Curr Pharm Des. 2020;26(16):1917-28.
13. Svobodová A et al. J Dermatol Sci. 2009 Dec;56(3):196-204.
14. Calò R, Marabini L. J Photochem Photobiol B. 2014 Mar 5;132:27-35.
15. Bucci P et al. J Cosmet Dermatol. 2018 Oct;17(5):889-99.
16. Ruscinc N et al. J Cosmet Dermatol. 2022 Jan 13.
17. Environmental Working Group’s Skin Deep website. Vaccinium Myrtillus Bilberry Seed Oil. Accessed October 18, 2022.
Novel stepwise method found to benefit patients with severe rhinophyma
DENVER –
Rhinophyma occurs primarily in the sixth and seventh decades of life and is marked by facial hypertrophy that leads to tumor-like growth, inflammation, fibrosis, and loss of the cosmetic nasal subunits. “When it becomes severe it leads to a degree of embarrassment as well,” one of the study authors, Patricia Richey, MD, said during an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery. “We found that our method has been efficacious, but most often, and more importantly, leads to an improvement in the patient’s quality of life.”
To date, clinicians have used fully ablative lasers to treat varying degrees of rhinophyma but at a cost of prolonged healing time and higher rates of scarring and pigment or textural changes. However, not all dermatologists use full-field ablative lasers in their practices.
“Fractionated ablative lasers have been used in the past for mild to moderate rhinophyma, but they cannot ablate to 100% density, which would be necessary to debulk the marked hypertrophy present in our patients,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “That’s why we added a surgical component.”
She and colleague Mathew M. Avram, MD, JD, developed a three-step method for treating severe rhinophyma that they performed on three elderly patients. Step 1 is the surgical debulk. Following infiltration of local anesthesia, a razor blade or 15-blade is used to excise the most prominent lobules of hypertrophied sebaceous tissue down to the fibrofatty layer of the nose as a partial thickness excision that does not reach the level of the perichondrium or cartilage. “Hemostasis is achieved with electrocoagulation and application of petrolatum ointment, followed by a pressure dressing,” Dr. Richey said. “The location of the debulk varies by patient.”
Step 2 involves fractionated ablative laser treatment 4 weeks later with either the CO2 or erbium:YAG (Er:YAG) 2,940-nm laser. According to Dr. Richey, the typical setting for the fractionated CO2 is a fluence of 70mJ/cm2 and a high density, performing six out of four passes with 60 seconds between each pass, “though these settings may vary based on the patient presentation,” she said.
The treatment level ranges from 5 (14% density) to 10 (70% density, for the most severe cases). Meanwhile, a representative setting for the ablative fractionated Er:YAG 2,940-nm laser is 250 mcm, no coagulation, 5.5% density, and one pass. “If a second surgical debulk is performed on the same day as ablative laser treatment, the sites of shave removal are typically avoided with the laser,” she said. If a certain portion of the nose has recently healed following surgical debulk 4 weeks prior, they may perform only two passes in this region.
In an interview, Dr. Avram, who directs the MGH Dermatology Laser and Cosmetic Center, characterized the staged method as providing “transformative change to severe, cosmetically disfiguring rhinophyma. The ablative fractional laser provides more fine-tuned contouring.”
The three patients studied had an average of three to four monthly treatments. “There is typically a great deal of improvement by the second treatment,” Dr. Richey said. Add-on treatments may include low voltage electrodessication at 1.8 watts for patients with well-demarcated papules of sebaceous hyperplasia, and a vascular laser such as the pulsed dye laser if telangiectasias are present.
One limitation of the stepwise method, she said, is that the surgical debulk typically results in a scar, “but it’s rarely noticeable if carefully performed, likely due to fractionated ablative use during the scar remodeling period. It’s important to set expectations with your patient at the initial consult. We always discuss treatment goals and that while we aim achieve the most desirable outcome possible, we’re never going to get them back to having a completely normal nose. They’re always going to have some mild or moderate rhinophymatous changes present.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on these results, characterized the stepwise method as promising. “Though more treatments are required, the easier recovery, safe outcomes in the case presented and excellent cosmetic result made it an interesting alternative when fully ablative resurfacing is daunting, either for patients or physicians involved,” he said in an interview.
The researchers reported having no relevant disclosures. Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory board for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
DENVER –
Rhinophyma occurs primarily in the sixth and seventh decades of life and is marked by facial hypertrophy that leads to tumor-like growth, inflammation, fibrosis, and loss of the cosmetic nasal subunits. “When it becomes severe it leads to a degree of embarrassment as well,” one of the study authors, Patricia Richey, MD, said during an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery. “We found that our method has been efficacious, but most often, and more importantly, leads to an improvement in the patient’s quality of life.”
To date, clinicians have used fully ablative lasers to treat varying degrees of rhinophyma but at a cost of prolonged healing time and higher rates of scarring and pigment or textural changes. However, not all dermatologists use full-field ablative lasers in their practices.
“Fractionated ablative lasers have been used in the past for mild to moderate rhinophyma, but they cannot ablate to 100% density, which would be necessary to debulk the marked hypertrophy present in our patients,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “That’s why we added a surgical component.”
She and colleague Mathew M. Avram, MD, JD, developed a three-step method for treating severe rhinophyma that they performed on three elderly patients. Step 1 is the surgical debulk. Following infiltration of local anesthesia, a razor blade or 15-blade is used to excise the most prominent lobules of hypertrophied sebaceous tissue down to the fibrofatty layer of the nose as a partial thickness excision that does not reach the level of the perichondrium or cartilage. “Hemostasis is achieved with electrocoagulation and application of petrolatum ointment, followed by a pressure dressing,” Dr. Richey said. “The location of the debulk varies by patient.”
Step 2 involves fractionated ablative laser treatment 4 weeks later with either the CO2 or erbium:YAG (Er:YAG) 2,940-nm laser. According to Dr. Richey, the typical setting for the fractionated CO2 is a fluence of 70mJ/cm2 and a high density, performing six out of four passes with 60 seconds between each pass, “though these settings may vary based on the patient presentation,” she said.
The treatment level ranges from 5 (14% density) to 10 (70% density, for the most severe cases). Meanwhile, a representative setting for the ablative fractionated Er:YAG 2,940-nm laser is 250 mcm, no coagulation, 5.5% density, and one pass. “If a second surgical debulk is performed on the same day as ablative laser treatment, the sites of shave removal are typically avoided with the laser,” she said. If a certain portion of the nose has recently healed following surgical debulk 4 weeks prior, they may perform only two passes in this region.
In an interview, Dr. Avram, who directs the MGH Dermatology Laser and Cosmetic Center, characterized the staged method as providing “transformative change to severe, cosmetically disfiguring rhinophyma. The ablative fractional laser provides more fine-tuned contouring.”
The three patients studied had an average of three to four monthly treatments. “There is typically a great deal of improvement by the second treatment,” Dr. Richey said. Add-on treatments may include low voltage electrodessication at 1.8 watts for patients with well-demarcated papules of sebaceous hyperplasia, and a vascular laser such as the pulsed dye laser if telangiectasias are present.
One limitation of the stepwise method, she said, is that the surgical debulk typically results in a scar, “but it’s rarely noticeable if carefully performed, likely due to fractionated ablative use during the scar remodeling period. It’s important to set expectations with your patient at the initial consult. We always discuss treatment goals and that while we aim achieve the most desirable outcome possible, we’re never going to get them back to having a completely normal nose. They’re always going to have some mild or moderate rhinophymatous changes present.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on these results, characterized the stepwise method as promising. “Though more treatments are required, the easier recovery, safe outcomes in the case presented and excellent cosmetic result made it an interesting alternative when fully ablative resurfacing is daunting, either for patients or physicians involved,” he said in an interview.
The researchers reported having no relevant disclosures. Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory board for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
DENVER –
Rhinophyma occurs primarily in the sixth and seventh decades of life and is marked by facial hypertrophy that leads to tumor-like growth, inflammation, fibrosis, and loss of the cosmetic nasal subunits. “When it becomes severe it leads to a degree of embarrassment as well,” one of the study authors, Patricia Richey, MD, said during an oral abstract session at the annual meeting of the American Society for Dermatologic Surgery. “We found that our method has been efficacious, but most often, and more importantly, leads to an improvement in the patient’s quality of life.”
To date, clinicians have used fully ablative lasers to treat varying degrees of rhinophyma but at a cost of prolonged healing time and higher rates of scarring and pigment or textural changes. However, not all dermatologists use full-field ablative lasers in their practices.
“Fractionated ablative lasers have been used in the past for mild to moderate rhinophyma, but they cannot ablate to 100% density, which would be necessary to debulk the marked hypertrophy present in our patients,” said Dr. Richey, who practices Mohs surgery and cosmetic dermatology in Washington, D.C., and conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston. “That’s why we added a surgical component.”
She and colleague Mathew M. Avram, MD, JD, developed a three-step method for treating severe rhinophyma that they performed on three elderly patients. Step 1 is the surgical debulk. Following infiltration of local anesthesia, a razor blade or 15-blade is used to excise the most prominent lobules of hypertrophied sebaceous tissue down to the fibrofatty layer of the nose as a partial thickness excision that does not reach the level of the perichondrium or cartilage. “Hemostasis is achieved with electrocoagulation and application of petrolatum ointment, followed by a pressure dressing,” Dr. Richey said. “The location of the debulk varies by patient.”
Step 2 involves fractionated ablative laser treatment 4 weeks later with either the CO2 or erbium:YAG (Er:YAG) 2,940-nm laser. According to Dr. Richey, the typical setting for the fractionated CO2 is a fluence of 70mJ/cm2 and a high density, performing six out of four passes with 60 seconds between each pass, “though these settings may vary based on the patient presentation,” she said.
The treatment level ranges from 5 (14% density) to 10 (70% density, for the most severe cases). Meanwhile, a representative setting for the ablative fractionated Er:YAG 2,940-nm laser is 250 mcm, no coagulation, 5.5% density, and one pass. “If a second surgical debulk is performed on the same day as ablative laser treatment, the sites of shave removal are typically avoided with the laser,” she said. If a certain portion of the nose has recently healed following surgical debulk 4 weeks prior, they may perform only two passes in this region.
In an interview, Dr. Avram, who directs the MGH Dermatology Laser and Cosmetic Center, characterized the staged method as providing “transformative change to severe, cosmetically disfiguring rhinophyma. The ablative fractional laser provides more fine-tuned contouring.”
The three patients studied had an average of three to four monthly treatments. “There is typically a great deal of improvement by the second treatment,” Dr. Richey said. Add-on treatments may include low voltage electrodessication at 1.8 watts for patients with well-demarcated papules of sebaceous hyperplasia, and a vascular laser such as the pulsed dye laser if telangiectasias are present.
One limitation of the stepwise method, she said, is that the surgical debulk typically results in a scar, “but it’s rarely noticeable if carefully performed, likely due to fractionated ablative use during the scar remodeling period. It’s important to set expectations with your patient at the initial consult. We always discuss treatment goals and that while we aim achieve the most desirable outcome possible, we’re never going to get them back to having a completely normal nose. They’re always going to have some mild or moderate rhinophymatous changes present.”
Vincent Richer, MD, a Vancouver-based medical and cosmetic dermatologist who was asked to comment on these results, characterized the stepwise method as promising. “Though more treatments are required, the easier recovery, safe outcomes in the case presented and excellent cosmetic result made it an interesting alternative when fully ablative resurfacing is daunting, either for patients or physicians involved,” he said in an interview.
The researchers reported having no relevant disclosures. Dr. Richer disclosed that he performs clinical trials for AbbVie/Allergan, Galderma, Leo Pharma, Pfizer, and is a member of advisory board for Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, and Sanofi. He is also a consultant to AbbVie/Allergan, Bausch, Celgene, Eli Lilly, Galderma, Janssen, Johnson & Johnson, Leo Pharma, L’Oréal, Merz, and Sanofi.
AT ASDS 2022
Dermatologists embrace low-dose oral minoxidil as hair loss adjunctive therapy
It’s not a new drug – it’s been available in topical form for hair loss since 1988 and was approved as an antihypertensive in 1979 – but .
The number of scholarly publications examining its use for hair loss has grown dramatically in the last 2 years: There were 2 in 2019, and that jumped to 17 in 2020 and 20 in 2021, with another 16 published so far this year, according to a PubMed search. An August article in The New York Times touting it as a potential cheap magic bullet is likely to drum up even more interest, said dermatologists.
The low-dose formulation is especially exciting for women, as there have been few great oral options for them, clinicians said.
Female hair loss “is devastating,” said Lily Talakoub, MD, adding that topical minoxidil (Rogaine), topical serums, and supplements “really do not provide the considerable growth that women really want to see.” Oral minoxidil is not approved by the U.S. Food and Drug Administration for hair loss, but “it has been shown in studies to cause the hairs to grow,” and has become a “lifeline” for women, said Dr. Talakoub, a dermatologist who is in private practice in McLean, Va.
“For many years we haven’t had anything new to tell patients medically,” said Lynne J. Goldberg, MD, professor of dermatology and pathology at Boston University School of Medicine. “Now, all of the sudden there’s a cheap, widely available efficacious medicine. That’s huge for female-pattern hair loss,” said Dr. Goldberg, who is also the director of the Boston Medical Center’s Hair Clinic.
“I’ve been using oral minoxidil for about 4 years with great success,” said dermatologist Eva Simmons-O’Brien, MD, who is in private practice in Towson, Md. She has used it primarily in women, mainly because she sees more women than men for hair loss.
Dr. Simmons-O’Brien said the excitement about low-dose oral minoxidil follows an increasing recognition in the medical and scientific community that hair loss is more than just a cosmetic issue.
Mechanism not fully understood
When minoxidil was first brought to market as an antihypertensive, clinicians noted hair growth in “balding patients,” which led to the development of the topical form. Even though it has been used for hair growth for decades, its mechanism of action is not fully understood. It is known that minoxidil is a vasodilator; it may also increase DNA synthesis and enhance cell proliferation, according to a review published in 2019.
“The positive effect of minoxidil on hair growth is mainly due to its metabolite, minoxidil sulfate, and the enzyme responsible for this conversion is sulfotransferase, which is located in hair follicles and varies in production among individuals,” write the authors, all affiliated with Mahidol University in Bangkok, Thailand.
Writing in the American Academy of Dermatology’s Dermatology World Insights and Inquiries, Warren R. Heymann, MD, observed that “even after decades of use,” how minoxidil improves alopecia is still not completely understood. He noted that a 2020 review found that minoxidil’s vasodilatory effects “are propagated by upregulation of vascular endothelial growth factor (VEGF), increasing cutaneous blood flow with resultant increase in oxygen and growth factor delivery to the hair follicle.” The medication prolongs the anagen phase and shortens the telogen phase, added Dr. Heymann, head of dermatology at Rowan University, Camden, N.J.
As an antihypertensive, minoxidil is given at 5-40 mg daily. Those doses have produced serious side effects such as sodium and fluid retention, ischemic heart disease, pericardial effusion, and pulmonary hypertension, according to the Thai researchers.
Those side effects have appeared to be rare with low-dose oral minoxidil. However, in JAAD Case Reports, South African researchers reported a case in which low-dose oral minoxidil may have led to cardiac side effects. A healthy 40-year-old woman, who after 3 weeks of treatment with 5% topical minoxidil, tacrolimus ointment 0.1%, clobetasol propionate ointment, 100 mg of doxycycline twice daily, and 0.25 mg of oral minoxidil daily, was hospitalized with full-body edema. An ultrasound showed fluid collections in the pericardium, pleural space, and abdomen. She also had a pleural effusion. The patient was given 40 mg of intravenous furosemide daily for 4 days, and the edema resolved.
“Having excluded other causes of pericardial effusion and anasarca in the previously healthy, young woman, we concluded that LDOM [low-dose oral minoxidil] was responsible for her clinical presentation,” write the authors.
A review of 17 studies published on-line in 2020 in the Journal of the American Academy of Dermatology found low-dose minoxidil to be safe and effective. Androgenetic alopecia was the most commonly studied, with doses of 0.25-1.25 mg proving to be effective and safe. It was also safe and effective for female-pattern hair loss, traction alopecia, chronic telogen effluvium, lichen planopilaris, alopecia areata, and permanent chemotherapy-induced alopecia.
The most common adverse effect was hypertrichosis. Other adverse events included postural hypotension and dizziness, lower-limb edema, and mild blood pressure changes.
In another multicenter, 1,404-patient safety study published in 2021 in JAAD, the authors found that hypertrichosis was the most frequent adverse event, reported by 15% of patients. Systemic adverse events included lightheadedness (1.7% of patients), fluid retention (1.3%), tachycardia (0.9%), headache (0.4%), periorbital edema (0.3%), and insomnia (0.2%). Only 29 patients (1.2%) withdrew because of these side effects.
“It definitely helps, and it’s relatively safe,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University in Washington. “But I wouldn’t want to call it a game-changer,” he said, adding that it works best when used in combination with other therapies. He often uses it with a 5-alpha reductase inhibitor – finasteride (Propecia) or dutasteride (Avodart) – “rather than as a monotherapy,” said Dr. Friedman.
From Australia to around the globe
The first publication on low-dose oral minoxidil for hair loss was in December 2017. The pilot study in female-pattern hair loss was published in the International Journal of Dermatology by Rodney Sinclair, MBBS, MD, a Melbourne, Australia–based dermatologist.
Amy McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she first heard Dr. Sinclair present his findings at an alopecia research meeting in Japan shortly before his initial publication.
“After that, I think all of us said, ‘Huh, this is interesting, and let’s try it, because we’re always looking for something more to help our patients,’” Dr. McMichael said, adding that she’s been prescribing low-dose minoxidil to her patients for 5 years.
She and colleagues at Wake Forest, along with Jerry Cooley, MD, a dermatologist in private practice in Charlotte, published a retrospective case series in March, looking at 105 adult patients – 80 women (ages 24-80) and 25 men (ages 19-63) – who were treated for androgenetic alopecia and/or telogen effluvium with oral minoxidil (dose range of 0.625–2.5 mg) once daily for a year, matched to 105 case controls.
Efficacy was based on the clinician’s assessment of clinical response and clinical photographic evaluation using a 3-point scale (worsening, stabilization, and improvement). Half of those treated demonstrated clinical improvement and 43% demonstrated stabilization. There was a significant difference (P < .001) in clinical response between those who received minoxidil and the controls.
Ideal patients?
Given its ease of use and low cost – $4-$12 for a 30-day supply of 2.5 mg tablets, according to GoodRX – low-dose minoxidil is a good fit for many patients, said dermatologists.
The best candidate is “a woman who’s perimenopausal or menopausal who’s got what we would say is moderate to severe loss of hair that’s kind of just starting,” said Dr. Simmons-O’Brien. The medication is not likely to grow hair where there is scarring already, however, she said.
“I tend to use it in people who either don’t want to do the topical minoxidil or have used it and have a lot of potential side effects from it,” like itching and irritation, said Dr. McMichael. She said oral minoxidil can also be helpful as an adjunct in patients with alopecia areata and that it can be used after anti-inflammatory treatments in central centrifugal cicatricial alopecia.
Dr. Goldberg said low-dose minoxidil would not be her first choice for female-pattern hair loss but that it’s “a great alternative” for people who can’t tolerate the topical form. Most of the women she has prescribed it to “have been pretty happy,” she added.
“I would be a little cautious in patients on a number of other medications,” Dr. Goldberg said, noting minoxidil’s potential systemic side effects.
Clinicians said they generally consult with a patient’s internist when they are starting them on oral minoxidil. “I always want to touch base with the primary care physician first,” said Dr. Friedman.
“If they’re on oral antihypertensive medications already, then I would ask them to talk to either their primary care physician or their cardiologist to make sure it’s okay to give this low dose,” said Dr. McMichael.
At the low doses, minoxidil rarely has any blood pressure–lowering effects, dermatologists said.
Women are usually started on 1.25 mg, while men can start at a higher, 2.5-mg dose, said clinicians.
Dr. Goldberg and Dr. Simmons-O’Brien said that recent additional warnings for finasteride about sexual side effects and the potential for suicide have changed the way they approach its use in young men, and that it has highlighted the potential for oral minoxidil as an alternative.
Oral minoxidil is rarely used as a monotherapy. “It takes a village” to address hair loss, said Dr. Simmons-O’Brien, noting that she likes to evaluate nutrition, vitamin D levels, and whether a patient is anemic or has thyroid disease when determining a course of action.
Dermatologists said they use oral minoxidil in combination with spironolactone, topical minoxidil, finasteride, or dutasteride. If patients are already on antihypertensives or at risk for excessive blood pressure–lowering effects of a combination that includes spironolactone, the dermatologists said again they will consult with a patient’s primary care physician first.
For women, the main limiting factor with oral minoxidil may be unwanted hair growth, usually on the face. Most of the clinicians interviewed for this story said they did not use spironolactone to counteract that hypertrichosis.
Dr. McMichael said she cautions African American women or women of African descent – who tend to have more body hair at baseline – that they should be aware of the potential for excess hair growth associated with low-dose minoxidil. She and other dermatologists interviewed for this story said they urge patients who are bothered by the excess hair to shave or wax or use other nonpharmacologic approaches.
The excess hair growth is less bothersome for men, they said.
Not a magic wand
Despite the increased profile and interest, oral minoxidil is not a cure-all, clinicians said.
“It’s important for patients to realize that hair loss can be complicated and there is no one magic wand,” said Dr. Simmons-O’Brien. Clinicians typically “are using several things to help encourage these follicular units to not miniaturize and disappear and create scars,” she said.
Dr. Friedman said he finds that patients have a hard time hearing that to continue to maintain growth, they have to take a medication for the rest of their life. “If you stop, you will have to start again,” he said.
Oral minoxidil, when used in combination with other therapies, will improve hair growth, said Dr. Goldberg. But it will not take someone back a decade, she said. “I try to temper expectations – promise a little and achieve more,” Dr. Goldberg said.
The study was independently supported. Dr. Smith and Dr. Jones report no relevant financial relationships. Dr. Simmons-O’Brien reports that she has received speaking fees from Isdin. Dr. McMichael disclosed relationships with Eli Lilly, Pfizer, Nutrafol, Revian, and UCB Pharma. Dr. Friedman, Dr. Goldberg, and Dr. Talakoub reported no disclosures.
A version of this article first appeared on Medscape.com.
It’s not a new drug – it’s been available in topical form for hair loss since 1988 and was approved as an antihypertensive in 1979 – but .
The number of scholarly publications examining its use for hair loss has grown dramatically in the last 2 years: There were 2 in 2019, and that jumped to 17 in 2020 and 20 in 2021, with another 16 published so far this year, according to a PubMed search. An August article in The New York Times touting it as a potential cheap magic bullet is likely to drum up even more interest, said dermatologists.
The low-dose formulation is especially exciting for women, as there have been few great oral options for them, clinicians said.
Female hair loss “is devastating,” said Lily Talakoub, MD, adding that topical minoxidil (Rogaine), topical serums, and supplements “really do not provide the considerable growth that women really want to see.” Oral minoxidil is not approved by the U.S. Food and Drug Administration for hair loss, but “it has been shown in studies to cause the hairs to grow,” and has become a “lifeline” for women, said Dr. Talakoub, a dermatologist who is in private practice in McLean, Va.
“For many years we haven’t had anything new to tell patients medically,” said Lynne J. Goldberg, MD, professor of dermatology and pathology at Boston University School of Medicine. “Now, all of the sudden there’s a cheap, widely available efficacious medicine. That’s huge for female-pattern hair loss,” said Dr. Goldberg, who is also the director of the Boston Medical Center’s Hair Clinic.
“I’ve been using oral minoxidil for about 4 years with great success,” said dermatologist Eva Simmons-O’Brien, MD, who is in private practice in Towson, Md. She has used it primarily in women, mainly because she sees more women than men for hair loss.
Dr. Simmons-O’Brien said the excitement about low-dose oral minoxidil follows an increasing recognition in the medical and scientific community that hair loss is more than just a cosmetic issue.
Mechanism not fully understood
When minoxidil was first brought to market as an antihypertensive, clinicians noted hair growth in “balding patients,” which led to the development of the topical form. Even though it has been used for hair growth for decades, its mechanism of action is not fully understood. It is known that minoxidil is a vasodilator; it may also increase DNA synthesis and enhance cell proliferation, according to a review published in 2019.
“The positive effect of minoxidil on hair growth is mainly due to its metabolite, minoxidil sulfate, and the enzyme responsible for this conversion is sulfotransferase, which is located in hair follicles and varies in production among individuals,” write the authors, all affiliated with Mahidol University in Bangkok, Thailand.
Writing in the American Academy of Dermatology’s Dermatology World Insights and Inquiries, Warren R. Heymann, MD, observed that “even after decades of use,” how minoxidil improves alopecia is still not completely understood. He noted that a 2020 review found that minoxidil’s vasodilatory effects “are propagated by upregulation of vascular endothelial growth factor (VEGF), increasing cutaneous blood flow with resultant increase in oxygen and growth factor delivery to the hair follicle.” The medication prolongs the anagen phase and shortens the telogen phase, added Dr. Heymann, head of dermatology at Rowan University, Camden, N.J.
As an antihypertensive, minoxidil is given at 5-40 mg daily. Those doses have produced serious side effects such as sodium and fluid retention, ischemic heart disease, pericardial effusion, and pulmonary hypertension, according to the Thai researchers.
Those side effects have appeared to be rare with low-dose oral minoxidil. However, in JAAD Case Reports, South African researchers reported a case in which low-dose oral minoxidil may have led to cardiac side effects. A healthy 40-year-old woman, who after 3 weeks of treatment with 5% topical minoxidil, tacrolimus ointment 0.1%, clobetasol propionate ointment, 100 mg of doxycycline twice daily, and 0.25 mg of oral minoxidil daily, was hospitalized with full-body edema. An ultrasound showed fluid collections in the pericardium, pleural space, and abdomen. She also had a pleural effusion. The patient was given 40 mg of intravenous furosemide daily for 4 days, and the edema resolved.
“Having excluded other causes of pericardial effusion and anasarca in the previously healthy, young woman, we concluded that LDOM [low-dose oral minoxidil] was responsible for her clinical presentation,” write the authors.
A review of 17 studies published on-line in 2020 in the Journal of the American Academy of Dermatology found low-dose minoxidil to be safe and effective. Androgenetic alopecia was the most commonly studied, with doses of 0.25-1.25 mg proving to be effective and safe. It was also safe and effective for female-pattern hair loss, traction alopecia, chronic telogen effluvium, lichen planopilaris, alopecia areata, and permanent chemotherapy-induced alopecia.
The most common adverse effect was hypertrichosis. Other adverse events included postural hypotension and dizziness, lower-limb edema, and mild blood pressure changes.
In another multicenter, 1,404-patient safety study published in 2021 in JAAD, the authors found that hypertrichosis was the most frequent adverse event, reported by 15% of patients. Systemic adverse events included lightheadedness (1.7% of patients), fluid retention (1.3%), tachycardia (0.9%), headache (0.4%), periorbital edema (0.3%), and insomnia (0.2%). Only 29 patients (1.2%) withdrew because of these side effects.
“It definitely helps, and it’s relatively safe,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University in Washington. “But I wouldn’t want to call it a game-changer,” he said, adding that it works best when used in combination with other therapies. He often uses it with a 5-alpha reductase inhibitor – finasteride (Propecia) or dutasteride (Avodart) – “rather than as a monotherapy,” said Dr. Friedman.
From Australia to around the globe
The first publication on low-dose oral minoxidil for hair loss was in December 2017. The pilot study in female-pattern hair loss was published in the International Journal of Dermatology by Rodney Sinclair, MBBS, MD, a Melbourne, Australia–based dermatologist.
Amy McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she first heard Dr. Sinclair present his findings at an alopecia research meeting in Japan shortly before his initial publication.
“After that, I think all of us said, ‘Huh, this is interesting, and let’s try it, because we’re always looking for something more to help our patients,’” Dr. McMichael said, adding that she’s been prescribing low-dose minoxidil to her patients for 5 years.
She and colleagues at Wake Forest, along with Jerry Cooley, MD, a dermatologist in private practice in Charlotte, published a retrospective case series in March, looking at 105 adult patients – 80 women (ages 24-80) and 25 men (ages 19-63) – who were treated for androgenetic alopecia and/or telogen effluvium with oral minoxidil (dose range of 0.625–2.5 mg) once daily for a year, matched to 105 case controls.
Efficacy was based on the clinician’s assessment of clinical response and clinical photographic evaluation using a 3-point scale (worsening, stabilization, and improvement). Half of those treated demonstrated clinical improvement and 43% demonstrated stabilization. There was a significant difference (P < .001) in clinical response between those who received minoxidil and the controls.
Ideal patients?
Given its ease of use and low cost – $4-$12 for a 30-day supply of 2.5 mg tablets, according to GoodRX – low-dose minoxidil is a good fit for many patients, said dermatologists.
The best candidate is “a woman who’s perimenopausal or menopausal who’s got what we would say is moderate to severe loss of hair that’s kind of just starting,” said Dr. Simmons-O’Brien. The medication is not likely to grow hair where there is scarring already, however, she said.
“I tend to use it in people who either don’t want to do the topical minoxidil or have used it and have a lot of potential side effects from it,” like itching and irritation, said Dr. McMichael. She said oral minoxidil can also be helpful as an adjunct in patients with alopecia areata and that it can be used after anti-inflammatory treatments in central centrifugal cicatricial alopecia.
Dr. Goldberg said low-dose minoxidil would not be her first choice for female-pattern hair loss but that it’s “a great alternative” for people who can’t tolerate the topical form. Most of the women she has prescribed it to “have been pretty happy,” she added.
“I would be a little cautious in patients on a number of other medications,” Dr. Goldberg said, noting minoxidil’s potential systemic side effects.
Clinicians said they generally consult with a patient’s internist when they are starting them on oral minoxidil. “I always want to touch base with the primary care physician first,” said Dr. Friedman.
“If they’re on oral antihypertensive medications already, then I would ask them to talk to either their primary care physician or their cardiologist to make sure it’s okay to give this low dose,” said Dr. McMichael.
At the low doses, minoxidil rarely has any blood pressure–lowering effects, dermatologists said.
Women are usually started on 1.25 mg, while men can start at a higher, 2.5-mg dose, said clinicians.
Dr. Goldberg and Dr. Simmons-O’Brien said that recent additional warnings for finasteride about sexual side effects and the potential for suicide have changed the way they approach its use in young men, and that it has highlighted the potential for oral minoxidil as an alternative.
Oral minoxidil is rarely used as a monotherapy. “It takes a village” to address hair loss, said Dr. Simmons-O’Brien, noting that she likes to evaluate nutrition, vitamin D levels, and whether a patient is anemic or has thyroid disease when determining a course of action.
Dermatologists said they use oral minoxidil in combination with spironolactone, topical minoxidil, finasteride, or dutasteride. If patients are already on antihypertensives or at risk for excessive blood pressure–lowering effects of a combination that includes spironolactone, the dermatologists said again they will consult with a patient’s primary care physician first.
For women, the main limiting factor with oral minoxidil may be unwanted hair growth, usually on the face. Most of the clinicians interviewed for this story said they did not use spironolactone to counteract that hypertrichosis.
Dr. McMichael said she cautions African American women or women of African descent – who tend to have more body hair at baseline – that they should be aware of the potential for excess hair growth associated with low-dose minoxidil. She and other dermatologists interviewed for this story said they urge patients who are bothered by the excess hair to shave or wax or use other nonpharmacologic approaches.
The excess hair growth is less bothersome for men, they said.
Not a magic wand
Despite the increased profile and interest, oral minoxidil is not a cure-all, clinicians said.
“It’s important for patients to realize that hair loss can be complicated and there is no one magic wand,” said Dr. Simmons-O’Brien. Clinicians typically “are using several things to help encourage these follicular units to not miniaturize and disappear and create scars,” she said.
Dr. Friedman said he finds that patients have a hard time hearing that to continue to maintain growth, they have to take a medication for the rest of their life. “If you stop, you will have to start again,” he said.
Oral minoxidil, when used in combination with other therapies, will improve hair growth, said Dr. Goldberg. But it will not take someone back a decade, she said. “I try to temper expectations – promise a little and achieve more,” Dr. Goldberg said.
The study was independently supported. Dr. Smith and Dr. Jones report no relevant financial relationships. Dr. Simmons-O’Brien reports that she has received speaking fees from Isdin. Dr. McMichael disclosed relationships with Eli Lilly, Pfizer, Nutrafol, Revian, and UCB Pharma. Dr. Friedman, Dr. Goldberg, and Dr. Talakoub reported no disclosures.
A version of this article first appeared on Medscape.com.
It’s not a new drug – it’s been available in topical form for hair loss since 1988 and was approved as an antihypertensive in 1979 – but .
The number of scholarly publications examining its use for hair loss has grown dramatically in the last 2 years: There were 2 in 2019, and that jumped to 17 in 2020 and 20 in 2021, with another 16 published so far this year, according to a PubMed search. An August article in The New York Times touting it as a potential cheap magic bullet is likely to drum up even more interest, said dermatologists.
The low-dose formulation is especially exciting for women, as there have been few great oral options for them, clinicians said.
Female hair loss “is devastating,” said Lily Talakoub, MD, adding that topical minoxidil (Rogaine), topical serums, and supplements “really do not provide the considerable growth that women really want to see.” Oral minoxidil is not approved by the U.S. Food and Drug Administration for hair loss, but “it has been shown in studies to cause the hairs to grow,” and has become a “lifeline” for women, said Dr. Talakoub, a dermatologist who is in private practice in McLean, Va.
“For many years we haven’t had anything new to tell patients medically,” said Lynne J. Goldberg, MD, professor of dermatology and pathology at Boston University School of Medicine. “Now, all of the sudden there’s a cheap, widely available efficacious medicine. That’s huge for female-pattern hair loss,” said Dr. Goldberg, who is also the director of the Boston Medical Center’s Hair Clinic.
“I’ve been using oral minoxidil for about 4 years with great success,” said dermatologist Eva Simmons-O’Brien, MD, who is in private practice in Towson, Md. She has used it primarily in women, mainly because she sees more women than men for hair loss.
Dr. Simmons-O’Brien said the excitement about low-dose oral minoxidil follows an increasing recognition in the medical and scientific community that hair loss is more than just a cosmetic issue.
Mechanism not fully understood
When minoxidil was first brought to market as an antihypertensive, clinicians noted hair growth in “balding patients,” which led to the development of the topical form. Even though it has been used for hair growth for decades, its mechanism of action is not fully understood. It is known that minoxidil is a vasodilator; it may also increase DNA synthesis and enhance cell proliferation, according to a review published in 2019.
“The positive effect of minoxidil on hair growth is mainly due to its metabolite, minoxidil sulfate, and the enzyme responsible for this conversion is sulfotransferase, which is located in hair follicles and varies in production among individuals,” write the authors, all affiliated with Mahidol University in Bangkok, Thailand.
Writing in the American Academy of Dermatology’s Dermatology World Insights and Inquiries, Warren R. Heymann, MD, observed that “even after decades of use,” how minoxidil improves alopecia is still not completely understood. He noted that a 2020 review found that minoxidil’s vasodilatory effects “are propagated by upregulation of vascular endothelial growth factor (VEGF), increasing cutaneous blood flow with resultant increase in oxygen and growth factor delivery to the hair follicle.” The medication prolongs the anagen phase and shortens the telogen phase, added Dr. Heymann, head of dermatology at Rowan University, Camden, N.J.
As an antihypertensive, minoxidil is given at 5-40 mg daily. Those doses have produced serious side effects such as sodium and fluid retention, ischemic heart disease, pericardial effusion, and pulmonary hypertension, according to the Thai researchers.
Those side effects have appeared to be rare with low-dose oral minoxidil. However, in JAAD Case Reports, South African researchers reported a case in which low-dose oral minoxidil may have led to cardiac side effects. A healthy 40-year-old woman, who after 3 weeks of treatment with 5% topical minoxidil, tacrolimus ointment 0.1%, clobetasol propionate ointment, 100 mg of doxycycline twice daily, and 0.25 mg of oral minoxidil daily, was hospitalized with full-body edema. An ultrasound showed fluid collections in the pericardium, pleural space, and abdomen. She also had a pleural effusion. The patient was given 40 mg of intravenous furosemide daily for 4 days, and the edema resolved.
“Having excluded other causes of pericardial effusion and anasarca in the previously healthy, young woman, we concluded that LDOM [low-dose oral minoxidil] was responsible for her clinical presentation,” write the authors.
A review of 17 studies published on-line in 2020 in the Journal of the American Academy of Dermatology found low-dose minoxidil to be safe and effective. Androgenetic alopecia was the most commonly studied, with doses of 0.25-1.25 mg proving to be effective and safe. It was also safe and effective for female-pattern hair loss, traction alopecia, chronic telogen effluvium, lichen planopilaris, alopecia areata, and permanent chemotherapy-induced alopecia.
The most common adverse effect was hypertrichosis. Other adverse events included postural hypotension and dizziness, lower-limb edema, and mild blood pressure changes.
In another multicenter, 1,404-patient safety study published in 2021 in JAAD, the authors found that hypertrichosis was the most frequent adverse event, reported by 15% of patients. Systemic adverse events included lightheadedness (1.7% of patients), fluid retention (1.3%), tachycardia (0.9%), headache (0.4%), periorbital edema (0.3%), and insomnia (0.2%). Only 29 patients (1.2%) withdrew because of these side effects.
“It definitely helps, and it’s relatively safe,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University in Washington. “But I wouldn’t want to call it a game-changer,” he said, adding that it works best when used in combination with other therapies. He often uses it with a 5-alpha reductase inhibitor – finasteride (Propecia) or dutasteride (Avodart) – “rather than as a monotherapy,” said Dr. Friedman.
From Australia to around the globe
The first publication on low-dose oral minoxidil for hair loss was in December 2017. The pilot study in female-pattern hair loss was published in the International Journal of Dermatology by Rodney Sinclair, MBBS, MD, a Melbourne, Australia–based dermatologist.
Amy McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., said she first heard Dr. Sinclair present his findings at an alopecia research meeting in Japan shortly before his initial publication.
“After that, I think all of us said, ‘Huh, this is interesting, and let’s try it, because we’re always looking for something more to help our patients,’” Dr. McMichael said, adding that she’s been prescribing low-dose minoxidil to her patients for 5 years.
She and colleagues at Wake Forest, along with Jerry Cooley, MD, a dermatologist in private practice in Charlotte, published a retrospective case series in March, looking at 105 adult patients – 80 women (ages 24-80) and 25 men (ages 19-63) – who were treated for androgenetic alopecia and/or telogen effluvium with oral minoxidil (dose range of 0.625–2.5 mg) once daily for a year, matched to 105 case controls.
Efficacy was based on the clinician’s assessment of clinical response and clinical photographic evaluation using a 3-point scale (worsening, stabilization, and improvement). Half of those treated demonstrated clinical improvement and 43% demonstrated stabilization. There was a significant difference (P < .001) in clinical response between those who received minoxidil and the controls.
Ideal patients?
Given its ease of use and low cost – $4-$12 for a 30-day supply of 2.5 mg tablets, according to GoodRX – low-dose minoxidil is a good fit for many patients, said dermatologists.
The best candidate is “a woman who’s perimenopausal or menopausal who’s got what we would say is moderate to severe loss of hair that’s kind of just starting,” said Dr. Simmons-O’Brien. The medication is not likely to grow hair where there is scarring already, however, she said.
“I tend to use it in people who either don’t want to do the topical minoxidil or have used it and have a lot of potential side effects from it,” like itching and irritation, said Dr. McMichael. She said oral minoxidil can also be helpful as an adjunct in patients with alopecia areata and that it can be used after anti-inflammatory treatments in central centrifugal cicatricial alopecia.
Dr. Goldberg said low-dose minoxidil would not be her first choice for female-pattern hair loss but that it’s “a great alternative” for people who can’t tolerate the topical form. Most of the women she has prescribed it to “have been pretty happy,” she added.
“I would be a little cautious in patients on a number of other medications,” Dr. Goldberg said, noting minoxidil’s potential systemic side effects.
Clinicians said they generally consult with a patient’s internist when they are starting them on oral minoxidil. “I always want to touch base with the primary care physician first,” said Dr. Friedman.
“If they’re on oral antihypertensive medications already, then I would ask them to talk to either their primary care physician or their cardiologist to make sure it’s okay to give this low dose,” said Dr. McMichael.
At the low doses, minoxidil rarely has any blood pressure–lowering effects, dermatologists said.
Women are usually started on 1.25 mg, while men can start at a higher, 2.5-mg dose, said clinicians.
Dr. Goldberg and Dr. Simmons-O’Brien said that recent additional warnings for finasteride about sexual side effects and the potential for suicide have changed the way they approach its use in young men, and that it has highlighted the potential for oral minoxidil as an alternative.
Oral minoxidil is rarely used as a monotherapy. “It takes a village” to address hair loss, said Dr. Simmons-O’Brien, noting that she likes to evaluate nutrition, vitamin D levels, and whether a patient is anemic or has thyroid disease when determining a course of action.
Dermatologists said they use oral minoxidil in combination with spironolactone, topical minoxidil, finasteride, or dutasteride. If patients are already on antihypertensives or at risk for excessive blood pressure–lowering effects of a combination that includes spironolactone, the dermatologists said again they will consult with a patient’s primary care physician first.
For women, the main limiting factor with oral minoxidil may be unwanted hair growth, usually on the face. Most of the clinicians interviewed for this story said they did not use spironolactone to counteract that hypertrichosis.
Dr. McMichael said she cautions African American women or women of African descent – who tend to have more body hair at baseline – that they should be aware of the potential for excess hair growth associated with low-dose minoxidil. She and other dermatologists interviewed for this story said they urge patients who are bothered by the excess hair to shave or wax or use other nonpharmacologic approaches.
The excess hair growth is less bothersome for men, they said.
Not a magic wand
Despite the increased profile and interest, oral minoxidil is not a cure-all, clinicians said.
“It’s important for patients to realize that hair loss can be complicated and there is no one magic wand,” said Dr. Simmons-O’Brien. Clinicians typically “are using several things to help encourage these follicular units to not miniaturize and disappear and create scars,” she said.
Dr. Friedman said he finds that patients have a hard time hearing that to continue to maintain growth, they have to take a medication for the rest of their life. “If you stop, you will have to start again,” he said.
Oral minoxidil, when used in combination with other therapies, will improve hair growth, said Dr. Goldberg. But it will not take someone back a decade, she said. “I try to temper expectations – promise a little and achieve more,” Dr. Goldberg said.
The study was independently supported. Dr. Smith and Dr. Jones report no relevant financial relationships. Dr. Simmons-O’Brien reports that she has received speaking fees from Isdin. Dr. McMichael disclosed relationships with Eli Lilly, Pfizer, Nutrafol, Revian, and UCB Pharma. Dr. Friedman, Dr. Goldberg, and Dr. Talakoub reported no disclosures.
A version of this article first appeared on Medscape.com.
Noninvasive combination procedure effective for upper arm fat reduction, muscle toning
DENVER – , according to results from a study that analyzed results with MRI and other measures at two dermatology practices.
Simultaneous use of HIFEM and RF has been shown to be safe and effective “for fat reduction and muscle toning in various body parts,” lead study author Carolyn Jacob, MD, founder and director of Chicago Cosmetic Surgery and Dermatology, wrote in an abstract presented at the annual meeting of the American Society for Dermatologic Surgery. This study investigated the effect of the HIFEM and RF procedure on muscle toning and adipose tissue in the upper arms.
In what Dr. Jacob described as the first study of its kind because magnetic resonance imaging (MRI) was used to evaluate results, she and her coauthors enrolled 34 patients aged 23-72 years at two centers who had a BMI in the range of 18.5-33.9 kg/m2. The patients underwent four 30-minute bilateral procedures over the upper arms spaced 1 week apart with the Emsculpt NEO (BTL Aesthetics), which simultaneously delivers HIFEM and RF therapy.
NEO small sized applicators were used, which at the time of the study were under investigation but have since been cleared for use with the device. According to the manufacturer’s website, Emsculpt NEO is indicated for noninvasive lipolysis of the abdomen and thighs and reduction in the circumference of the abdomen and thighs in patients with skin types I-VI; and for noninvasive lipolysis of the upper arms “limited to skin types II and III and BMI 30 or under.”
The investigators measured changes in fat and triceps muscle tissue via MRI at baseline, 1-month, and 3-month follow-up visits. They also obtained digital photographs, administered patient questionnaires regarding comfort and satisfaction, and monitored safety of the treatments.
Of the 28 patients who completed their 1-month follow-up visit, analysis of MRI images showed a 22.3% average decrease in fat tissue from baseline MRIs (a decrease of 4.0 ± 1.2 mm; P < .01) and a 21.5% average increase in muscle mass (an increase of 8.2 ± 2.3 mm; P < .001). For the 25 patients who completed their 3-month follow-up visit, analysis of MRI images showed a 25.5% average decrease in fat tissue (a decrease of 4.9 ± 1.5 mm; P < .01) and a 23.9% average increase in muscle mass (an increase of 8.9 ± 2.0 mm; P < .001).
The analysis of questionnaires revealed high patient satisfaction with the results (87.1%), high comfort during the treatment (91.2%), and a low Visual Analogue Scale (VAS) score (1.6 ± 2.0) used to evaluate pain.
“This study shows that HIFEM and RF consistently increases muscle and decreases fat,” Dr. Jacob said in an interview. “It’s the only study on the triceps showing MRI evidence of fat loss with a nonsurgical body shaping device.”
She characterized the learning curve for the Emsculpt NEO as “small, as the previous Emsculpt small applicators have a similar fit.”
Pooja Sodha, MD, director of the center for laser and cosmetic dermatology at George Washington University, Washington, who was asked to comment on the study, said that the combination of radiofrequency energy and high-intensity focused electromagnetic technology triggers heat-induced damage of adipose tissue and muscle strengthening, respectively, to improve overall appearance and tone.
“Simultaneous delivery is the key here, and the real technological superhero, allowing us to take advantage of the synergistic effects of the muscle contractions and the tissue heating,” Dr. Sodha told this news organization. “Earlier this year, we saw published data on success with abdominal contouring with similar fat reduction and muscle enhancement as reported in this study, and these results persisted at 6 months,” with some declines noted at that time, she said.
“It is very encouraging and exciting to have similar effectiveness and safety for the arms, with such high satisfaction and comfort,” she added.
Dr. Jacob disclosed that she has conducted research studies for BTL Aesthetics since 2017 and is a member of the company’s advisory board. Dr. Sodha reported having no financial disclosures.
DENVER – , according to results from a study that analyzed results with MRI and other measures at two dermatology practices.
Simultaneous use of HIFEM and RF has been shown to be safe and effective “for fat reduction and muscle toning in various body parts,” lead study author Carolyn Jacob, MD, founder and director of Chicago Cosmetic Surgery and Dermatology, wrote in an abstract presented at the annual meeting of the American Society for Dermatologic Surgery. This study investigated the effect of the HIFEM and RF procedure on muscle toning and adipose tissue in the upper arms.
In what Dr. Jacob described as the first study of its kind because magnetic resonance imaging (MRI) was used to evaluate results, she and her coauthors enrolled 34 patients aged 23-72 years at two centers who had a BMI in the range of 18.5-33.9 kg/m2. The patients underwent four 30-minute bilateral procedures over the upper arms spaced 1 week apart with the Emsculpt NEO (BTL Aesthetics), which simultaneously delivers HIFEM and RF therapy.
NEO small sized applicators were used, which at the time of the study were under investigation but have since been cleared for use with the device. According to the manufacturer’s website, Emsculpt NEO is indicated for noninvasive lipolysis of the abdomen and thighs and reduction in the circumference of the abdomen and thighs in patients with skin types I-VI; and for noninvasive lipolysis of the upper arms “limited to skin types II and III and BMI 30 or under.”
The investigators measured changes in fat and triceps muscle tissue via MRI at baseline, 1-month, and 3-month follow-up visits. They also obtained digital photographs, administered patient questionnaires regarding comfort and satisfaction, and monitored safety of the treatments.
Of the 28 patients who completed their 1-month follow-up visit, analysis of MRI images showed a 22.3% average decrease in fat tissue from baseline MRIs (a decrease of 4.0 ± 1.2 mm; P < .01) and a 21.5% average increase in muscle mass (an increase of 8.2 ± 2.3 mm; P < .001). For the 25 patients who completed their 3-month follow-up visit, analysis of MRI images showed a 25.5% average decrease in fat tissue (a decrease of 4.9 ± 1.5 mm; P < .01) and a 23.9% average increase in muscle mass (an increase of 8.9 ± 2.0 mm; P < .001).
The analysis of questionnaires revealed high patient satisfaction with the results (87.1%), high comfort during the treatment (91.2%), and a low Visual Analogue Scale (VAS) score (1.6 ± 2.0) used to evaluate pain.
“This study shows that HIFEM and RF consistently increases muscle and decreases fat,” Dr. Jacob said in an interview. “It’s the only study on the triceps showing MRI evidence of fat loss with a nonsurgical body shaping device.”
She characterized the learning curve for the Emsculpt NEO as “small, as the previous Emsculpt small applicators have a similar fit.”
Pooja Sodha, MD, director of the center for laser and cosmetic dermatology at George Washington University, Washington, who was asked to comment on the study, said that the combination of radiofrequency energy and high-intensity focused electromagnetic technology triggers heat-induced damage of adipose tissue and muscle strengthening, respectively, to improve overall appearance and tone.
“Simultaneous delivery is the key here, and the real technological superhero, allowing us to take advantage of the synergistic effects of the muscle contractions and the tissue heating,” Dr. Sodha told this news organization. “Earlier this year, we saw published data on success with abdominal contouring with similar fat reduction and muscle enhancement as reported in this study, and these results persisted at 6 months,” with some declines noted at that time, she said.
“It is very encouraging and exciting to have similar effectiveness and safety for the arms, with such high satisfaction and comfort,” she added.
Dr. Jacob disclosed that she has conducted research studies for BTL Aesthetics since 2017 and is a member of the company’s advisory board. Dr. Sodha reported having no financial disclosures.
DENVER – , according to results from a study that analyzed results with MRI and other measures at two dermatology practices.
Simultaneous use of HIFEM and RF has been shown to be safe and effective “for fat reduction and muscle toning in various body parts,” lead study author Carolyn Jacob, MD, founder and director of Chicago Cosmetic Surgery and Dermatology, wrote in an abstract presented at the annual meeting of the American Society for Dermatologic Surgery. This study investigated the effect of the HIFEM and RF procedure on muscle toning and adipose tissue in the upper arms.
In what Dr. Jacob described as the first study of its kind because magnetic resonance imaging (MRI) was used to evaluate results, she and her coauthors enrolled 34 patients aged 23-72 years at two centers who had a BMI in the range of 18.5-33.9 kg/m2. The patients underwent four 30-minute bilateral procedures over the upper arms spaced 1 week apart with the Emsculpt NEO (BTL Aesthetics), which simultaneously delivers HIFEM and RF therapy.
NEO small sized applicators were used, which at the time of the study were under investigation but have since been cleared for use with the device. According to the manufacturer’s website, Emsculpt NEO is indicated for noninvasive lipolysis of the abdomen and thighs and reduction in the circumference of the abdomen and thighs in patients with skin types I-VI; and for noninvasive lipolysis of the upper arms “limited to skin types II and III and BMI 30 or under.”
The investigators measured changes in fat and triceps muscle tissue via MRI at baseline, 1-month, and 3-month follow-up visits. They also obtained digital photographs, administered patient questionnaires regarding comfort and satisfaction, and monitored safety of the treatments.
Of the 28 patients who completed their 1-month follow-up visit, analysis of MRI images showed a 22.3% average decrease in fat tissue from baseline MRIs (a decrease of 4.0 ± 1.2 mm; P < .01) and a 21.5% average increase in muscle mass (an increase of 8.2 ± 2.3 mm; P < .001). For the 25 patients who completed their 3-month follow-up visit, analysis of MRI images showed a 25.5% average decrease in fat tissue (a decrease of 4.9 ± 1.5 mm; P < .01) and a 23.9% average increase in muscle mass (an increase of 8.9 ± 2.0 mm; P < .001).
The analysis of questionnaires revealed high patient satisfaction with the results (87.1%), high comfort during the treatment (91.2%), and a low Visual Analogue Scale (VAS) score (1.6 ± 2.0) used to evaluate pain.
“This study shows that HIFEM and RF consistently increases muscle and decreases fat,” Dr. Jacob said in an interview. “It’s the only study on the triceps showing MRI evidence of fat loss with a nonsurgical body shaping device.”
She characterized the learning curve for the Emsculpt NEO as “small, as the previous Emsculpt small applicators have a similar fit.”
Pooja Sodha, MD, director of the center for laser and cosmetic dermatology at George Washington University, Washington, who was asked to comment on the study, said that the combination of radiofrequency energy and high-intensity focused electromagnetic technology triggers heat-induced damage of adipose tissue and muscle strengthening, respectively, to improve overall appearance and tone.
“Simultaneous delivery is the key here, and the real technological superhero, allowing us to take advantage of the synergistic effects of the muscle contractions and the tissue heating,” Dr. Sodha told this news organization. “Earlier this year, we saw published data on success with abdominal contouring with similar fat reduction and muscle enhancement as reported in this study, and these results persisted at 6 months,” with some declines noted at that time, she said.
“It is very encouraging and exciting to have similar effectiveness and safety for the arms, with such high satisfaction and comfort,” she added.
Dr. Jacob disclosed that she has conducted research studies for BTL Aesthetics since 2017 and is a member of the company’s advisory board. Dr. Sodha reported having no financial disclosures.
AT ASDS 2022
Liquid injectable silicone safe for acne scarring in dark-skinned patients, study finds
DENVER – Highly , results from a recent study showed.
“Acne is pervasive, and acne scarring disproportionately affects darker skin types,” lead study author Nicole Salame, MD, told this news organization in advance of the annual meeting of the American Society for Dermatologic Surgery, where she presented the results of the study. “Treatment of acne scarring in darker skin is also particularly challenging since resurfacing can be problematic. Numerous treatment options exist but vary in effectiveness, sustainability, and side-effect profile, especially for patients with darker skin.”
Highly purified liquid injectable silicone (also known as LIS) is approved by the Food and Drug Administration for treating intraocular tamponade of retinal detachment, and has been used off label for skin augmentation. A 2005 study of LIS for five patients with acne scarring, with up to 30 years of follow-up, showed efficacy and preservation of product without complications for depressed, broad-based acne scars .
“Use of LIS as a permanent treatment for acne scarring in darker skin types has yet to be evaluated,” said Dr. Salame, a 4th-year dermatology resident at Emory University, Atlanta. “Our study is the first to retrospectively evaluate the safety and efficacy of highly purified LIS for the treatment of acne scars in all skin types.”
Dr. Salame and coauthor Harold J. Brody, MD, evaluated the charts of 96 patients with a mean age of 51 years who received highly purified LIS for the treatment of acne scars at Dr. Brody’s Atlanta-based private dermatology practice between July 2010 and March 2021. Of the 96 patients, 31 had darker skin types (20 were Fitzpatrick skin type IV and 11 were Fitzpatrick skin type V). Dr. Brody performed all treatments: a total of 206 in the 96 patients.
The average time of follow-up was 6.31 years; 19 patients had a follow-up of 1-3 years, 25 had a follow-up of 3-5 years, and 52 had a follow-up of greater than 5 years. The researchers did not observe any complications along the course of the patients’ treatments, and no patients reported complications or dissatisfaction with treatment.
“Among the most impressive findings of our study was the permanence of effectiveness of LIS for acne scarring in patients who had treatment over a decade before,” Dr. Salame said. “Our longest follow up was 12 years. These patients continued to show improvement in their acne scarring years after treatment with LIS, even as they lost collagen and volume in their face with advancing age.”
In addition, she said, none of the patients experienced complications of granulomatous reactions, migration, or extrusion of product, which were previously documented with the use of macrodroplet injectable silicone techniques. “This is likely due to the consistent use of the microdroplet injection technique in our study – less than 0.01 cc per injection at minimum 6- to 8-week intervals or more,” Dr. Salame said.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the findings “show safety and durability of highly purified microdroplet liquid silicone to treat acne scars. The numbers of patients reviewed are small and selective (one highly skilled dermatologist), but with the right material (highly purified liquid silicone) and in a qualified and experienced physician’s hand, this treatment seems like a great option.”
Dr. Salame acknowledged certain limitations of the study, including its single-center, retrospective design. “Future prospective studies with larger patient populations of all skin types recruited from multiple centers may be needed,” she said.
The researchers reported having no relevant conflicts of interest or funding sources to disclose. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
DENVER – Highly , results from a recent study showed.
“Acne is pervasive, and acne scarring disproportionately affects darker skin types,” lead study author Nicole Salame, MD, told this news organization in advance of the annual meeting of the American Society for Dermatologic Surgery, where she presented the results of the study. “Treatment of acne scarring in darker skin is also particularly challenging since resurfacing can be problematic. Numerous treatment options exist but vary in effectiveness, sustainability, and side-effect profile, especially for patients with darker skin.”
Highly purified liquid injectable silicone (also known as LIS) is approved by the Food and Drug Administration for treating intraocular tamponade of retinal detachment, and has been used off label for skin augmentation. A 2005 study of LIS for five patients with acne scarring, with up to 30 years of follow-up, showed efficacy and preservation of product without complications for depressed, broad-based acne scars .
“Use of LIS as a permanent treatment for acne scarring in darker skin types has yet to be evaluated,” said Dr. Salame, a 4th-year dermatology resident at Emory University, Atlanta. “Our study is the first to retrospectively evaluate the safety and efficacy of highly purified LIS for the treatment of acne scars in all skin types.”
Dr. Salame and coauthor Harold J. Brody, MD, evaluated the charts of 96 patients with a mean age of 51 years who received highly purified LIS for the treatment of acne scars at Dr. Brody’s Atlanta-based private dermatology practice between July 2010 and March 2021. Of the 96 patients, 31 had darker skin types (20 were Fitzpatrick skin type IV and 11 were Fitzpatrick skin type V). Dr. Brody performed all treatments: a total of 206 in the 96 patients.
The average time of follow-up was 6.31 years; 19 patients had a follow-up of 1-3 years, 25 had a follow-up of 3-5 years, and 52 had a follow-up of greater than 5 years. The researchers did not observe any complications along the course of the patients’ treatments, and no patients reported complications or dissatisfaction with treatment.
“Among the most impressive findings of our study was the permanence of effectiveness of LIS for acne scarring in patients who had treatment over a decade before,” Dr. Salame said. “Our longest follow up was 12 years. These patients continued to show improvement in their acne scarring years after treatment with LIS, even as they lost collagen and volume in their face with advancing age.”
In addition, she said, none of the patients experienced complications of granulomatous reactions, migration, or extrusion of product, which were previously documented with the use of macrodroplet injectable silicone techniques. “This is likely due to the consistent use of the microdroplet injection technique in our study – less than 0.01 cc per injection at minimum 6- to 8-week intervals or more,” Dr. Salame said.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the findings “show safety and durability of highly purified microdroplet liquid silicone to treat acne scars. The numbers of patients reviewed are small and selective (one highly skilled dermatologist), but with the right material (highly purified liquid silicone) and in a qualified and experienced physician’s hand, this treatment seems like a great option.”
Dr. Salame acknowledged certain limitations of the study, including its single-center, retrospective design. “Future prospective studies with larger patient populations of all skin types recruited from multiple centers may be needed,” she said.
The researchers reported having no relevant conflicts of interest or funding sources to disclose. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
DENVER – Highly , results from a recent study showed.
“Acne is pervasive, and acne scarring disproportionately affects darker skin types,” lead study author Nicole Salame, MD, told this news organization in advance of the annual meeting of the American Society for Dermatologic Surgery, where she presented the results of the study. “Treatment of acne scarring in darker skin is also particularly challenging since resurfacing can be problematic. Numerous treatment options exist but vary in effectiveness, sustainability, and side-effect profile, especially for patients with darker skin.”
Highly purified liquid injectable silicone (also known as LIS) is approved by the Food and Drug Administration for treating intraocular tamponade of retinal detachment, and has been used off label for skin augmentation. A 2005 study of LIS for five patients with acne scarring, with up to 30 years of follow-up, showed efficacy and preservation of product without complications for depressed, broad-based acne scars .
“Use of LIS as a permanent treatment for acne scarring in darker skin types has yet to be evaluated,” said Dr. Salame, a 4th-year dermatology resident at Emory University, Atlanta. “Our study is the first to retrospectively evaluate the safety and efficacy of highly purified LIS for the treatment of acne scars in all skin types.”
Dr. Salame and coauthor Harold J. Brody, MD, evaluated the charts of 96 patients with a mean age of 51 years who received highly purified LIS for the treatment of acne scars at Dr. Brody’s Atlanta-based private dermatology practice between July 2010 and March 2021. Of the 96 patients, 31 had darker skin types (20 were Fitzpatrick skin type IV and 11 were Fitzpatrick skin type V). Dr. Brody performed all treatments: a total of 206 in the 96 patients.
The average time of follow-up was 6.31 years; 19 patients had a follow-up of 1-3 years, 25 had a follow-up of 3-5 years, and 52 had a follow-up of greater than 5 years. The researchers did not observe any complications along the course of the patients’ treatments, and no patients reported complications or dissatisfaction with treatment.
“Among the most impressive findings of our study was the permanence of effectiveness of LIS for acne scarring in patients who had treatment over a decade before,” Dr. Salame said. “Our longest follow up was 12 years. These patients continued to show improvement in their acne scarring years after treatment with LIS, even as they lost collagen and volume in their face with advancing age.”
In addition, she said, none of the patients experienced complications of granulomatous reactions, migration, or extrusion of product, which were previously documented with the use of macrodroplet injectable silicone techniques. “This is likely due to the consistent use of the microdroplet injection technique in our study – less than 0.01 cc per injection at minimum 6- to 8-week intervals or more,” Dr. Salame said.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the findings “show safety and durability of highly purified microdroplet liquid silicone to treat acne scars. The numbers of patients reviewed are small and selective (one highly skilled dermatologist), but with the right material (highly purified liquid silicone) and in a qualified and experienced physician’s hand, this treatment seems like a great option.”
Dr. Salame acknowledged certain limitations of the study, including its single-center, retrospective design. “Future prospective studies with larger patient populations of all skin types recruited from multiple centers may be needed,” she said.
The researchers reported having no relevant conflicts of interest or funding sources to disclose. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
AT ASDS 2022


















