User login
Screening for adolescent substance use; Changing routines during COVID-19
Screening for adolescent substance use
I want to congratulate Dr. Verma on her article “Opioid use disorder in adolescents: An overview” (Evidence-Based Reviews,
Because evidence suggests there are continued barriers, such as time constraints, in evaluating for adolescent SUD,1,2 I believe the Screen to Brief Intervention (S2BI) and Brief Screener for Tobacco, Alcohol and Drug (BSTAD) should be included.3,4 The S2BI and BSTAD are brief screeners that assess substance use, are validated for adolescent patients, can be completed online, and can assist in identifying DSM-5 criteria for SUD.
The S2BI has demonstrated high sensitivity and specificity for identifying SUD.3 The single screening assessment for “past-year use” is quick and can be administered in a variety of clinical settings. The S2BI begins by asking a patient about his/her frequency of tobacco, alcohol, and/or marijuana use in the past year. If the patient endorses past-year use of any of these substances, the S2BI prompts follow-up questions about the use of prescription medications, illicit drugs, inhalants, and herbal products. A patient’s frequency of use is strongly correlated with the likelihood of having a SUD. Adolescents who report using a substance “once or twice” in the past year are very unlikely to have a SUD. Patients who endorse “monthly” use are more likely to meet the criteria for a mild or moderate SUD, and those reporting “weekly or more” use are more likely to have a severe SUD.
The BSTAD is an electronic, validated, high-sensitivity, high-specificity instrument for identifying SUD.1 It asks a single frequency question about past-year use of tobacco, alcohol, and marijuana, which are the most commonly used substances among adolescents. Patients who report using any of these substances are then asked about additional substance use. Based on the patient’s self-report of past year use, the screen places him/her into 1 of 3 risk categories for SUD: no reported use, lower risk, and higher risk. Each risk level maps to suggested clinical actions that are summarized in the results section.
Kevin M. Simon, MD
Child & Adolescent Psychiatry Fellow
Boston Children’s Hospital
Clinical Fellow in Psychiatry
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Palmer A, Karakus M, Mark T. Barriers faced by physicians in screening for substance use disorders among adolescents. Psychiatr Serv. 2019;70(5):409-412.
2. D’Souza-Li L, Harris SK. The future of screening, brief intervention and referral to treatment in adolescent primary care: research directions and dissemination challenges. Curr Opin Pediatr. 2016;28(4):434-440.
3. Levy S, Weiss R, Sherritt L, et al. An electronic screen for triaging adolescent substance use by risk levels. JAMA Pediatr. 2014;168(9):822-828.
4. Kelly SM, Gryczynski J, Mitchell SG, et al. Validity of brief screening instrument for adolescent tobacco, alcohol, and drug use. Pediatrics. 2014;133(5):819-826.
Continue to: The author responds
The author responds
I thank Dr. Simon for his words of encouragement. I agree that both the S2BI and BSTAD have high sensitivity and specificity and are easy to use for screening for the use of multiple substances. Once substance use is established, both tools recommend administering high-risk assessment with additional scales such as the CRAFFT. During the initial evaluation, many psychiatrists take their patient’s history of substance use in detail, including age of onset, frequency, amount used, severity, and the time of his/her last use, without using a screening instrument. My article focused on instruments that can determine whether there is need for a further detailed evaluation. I agree that the S2BI and BSTAD would assist psychiatrists or physicians in other specialties (eg, pediatrics, family medicine) who might not take a complete substance use history during their initial evaluations.
Shikha Verma, MD
Rogers Behavioral Health
Kenosha, Wisconsin
Assistant Professor
Department of Psychiatry and Behavioral Health
Rosalind Franklin University of Medicine and Science
North Chicago, Illinois
Continue to: Changes as a result of COVID-19
Changes as a result of COVID-19
I thank Dr. Nasrallah for his editorial “During a viral pandemic, anxiety is endemic: The psychiatric aspects of COVID-19” (From the Editor,
I appreciated the editorial because it got me thinking about how the pandemic has changed me and my family:
1. We are engaging more in social media.
2. I feel uncomfortable when I go to the grocery store.
3. I feel better when I don’t access the news about COVID-19.
4. My children need physical socialization with their friends (sports, games, other activities, etc.).
5. My children function better with a schedule, but we find it difficult to keep them on a good schedule. Our teenagers stay up late at night (because all of their friends do), and they sleep in late the next morning.
Here are some positive changes:
1. Creating a weekly family calendar on a dry-erase board, so the family can see what is going on during the week.
2. Creating responsibility for our older children (eg, washing their own clothes, cleaning their bathroom).
3. Eating most meals as a family and organizing meals better, too.
4. Playing games together.
5. Cleaning the house together.
6. Getting outside to walk the dog and appreciate nature more.
7. Exercising.
8. Utilizing positive social media.
9. Getting caught up on life.
Again, I thank Dr. Nasrallah for writing this editorial because it led me to self-reflect on this situation, and helped me feel normal.
Doug Dolenc
Westfield, Indiana
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Screening for adolescent substance use
I want to congratulate Dr. Verma on her article “Opioid use disorder in adolescents: An overview” (Evidence-Based Reviews,
Because evidence suggests there are continued barriers, such as time constraints, in evaluating for adolescent SUD,1,2 I believe the Screen to Brief Intervention (S2BI) and Brief Screener for Tobacco, Alcohol and Drug (BSTAD) should be included.3,4 The S2BI and BSTAD are brief screeners that assess substance use, are validated for adolescent patients, can be completed online, and can assist in identifying DSM-5 criteria for SUD.
The S2BI has demonstrated high sensitivity and specificity for identifying SUD.3 The single screening assessment for “past-year use” is quick and can be administered in a variety of clinical settings. The S2BI begins by asking a patient about his/her frequency of tobacco, alcohol, and/or marijuana use in the past year. If the patient endorses past-year use of any of these substances, the S2BI prompts follow-up questions about the use of prescription medications, illicit drugs, inhalants, and herbal products. A patient’s frequency of use is strongly correlated with the likelihood of having a SUD. Adolescents who report using a substance “once or twice” in the past year are very unlikely to have a SUD. Patients who endorse “monthly” use are more likely to meet the criteria for a mild or moderate SUD, and those reporting “weekly or more” use are more likely to have a severe SUD.
The BSTAD is an electronic, validated, high-sensitivity, high-specificity instrument for identifying SUD.1 It asks a single frequency question about past-year use of tobacco, alcohol, and marijuana, which are the most commonly used substances among adolescents. Patients who report using any of these substances are then asked about additional substance use. Based on the patient’s self-report of past year use, the screen places him/her into 1 of 3 risk categories for SUD: no reported use, lower risk, and higher risk. Each risk level maps to suggested clinical actions that are summarized in the results section.
Kevin M. Simon, MD
Child & Adolescent Psychiatry Fellow
Boston Children’s Hospital
Clinical Fellow in Psychiatry
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Palmer A, Karakus M, Mark T. Barriers faced by physicians in screening for substance use disorders among adolescents. Psychiatr Serv. 2019;70(5):409-412.
2. D’Souza-Li L, Harris SK. The future of screening, brief intervention and referral to treatment in adolescent primary care: research directions and dissemination challenges. Curr Opin Pediatr. 2016;28(4):434-440.
3. Levy S, Weiss R, Sherritt L, et al. An electronic screen for triaging adolescent substance use by risk levels. JAMA Pediatr. 2014;168(9):822-828.
4. Kelly SM, Gryczynski J, Mitchell SG, et al. Validity of brief screening instrument for adolescent tobacco, alcohol, and drug use. Pediatrics. 2014;133(5):819-826.
Continue to: The author responds
The author responds
I thank Dr. Simon for his words of encouragement. I agree that both the S2BI and BSTAD have high sensitivity and specificity and are easy to use for screening for the use of multiple substances. Once substance use is established, both tools recommend administering high-risk assessment with additional scales such as the CRAFFT. During the initial evaluation, many psychiatrists take their patient’s history of substance use in detail, including age of onset, frequency, amount used, severity, and the time of his/her last use, without using a screening instrument. My article focused on instruments that can determine whether there is need for a further detailed evaluation. I agree that the S2BI and BSTAD would assist psychiatrists or physicians in other specialties (eg, pediatrics, family medicine) who might not take a complete substance use history during their initial evaluations.
Shikha Verma, MD
Rogers Behavioral Health
Kenosha, Wisconsin
Assistant Professor
Department of Psychiatry and Behavioral Health
Rosalind Franklin University of Medicine and Science
North Chicago, Illinois
Continue to: Changes as a result of COVID-19
Changes as a result of COVID-19
I thank Dr. Nasrallah for his editorial “During a viral pandemic, anxiety is endemic: The psychiatric aspects of COVID-19” (From the Editor,
I appreciated the editorial because it got me thinking about how the pandemic has changed me and my family:
1. We are engaging more in social media.
2. I feel uncomfortable when I go to the grocery store.
3. I feel better when I don’t access the news about COVID-19.
4. My children need physical socialization with their friends (sports, games, other activities, etc.).
5. My children function better with a schedule, but we find it difficult to keep them on a good schedule. Our teenagers stay up late at night (because all of their friends do), and they sleep in late the next morning.
Here are some positive changes:
1. Creating a weekly family calendar on a dry-erase board, so the family can see what is going on during the week.
2. Creating responsibility for our older children (eg, washing their own clothes, cleaning their bathroom).
3. Eating most meals as a family and organizing meals better, too.
4. Playing games together.
5. Cleaning the house together.
6. Getting outside to walk the dog and appreciate nature more.
7. Exercising.
8. Utilizing positive social media.
9. Getting caught up on life.
Again, I thank Dr. Nasrallah for writing this editorial because it led me to self-reflect on this situation, and helped me feel normal.
Doug Dolenc
Westfield, Indiana
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
Screening for adolescent substance use
I want to congratulate Dr. Verma on her article “Opioid use disorder in adolescents: An overview” (Evidence-Based Reviews,
Because evidence suggests there are continued barriers, such as time constraints, in evaluating for adolescent SUD,1,2 I believe the Screen to Brief Intervention (S2BI) and Brief Screener for Tobacco, Alcohol and Drug (BSTAD) should be included.3,4 The S2BI and BSTAD are brief screeners that assess substance use, are validated for adolescent patients, can be completed online, and can assist in identifying DSM-5 criteria for SUD.
The S2BI has demonstrated high sensitivity and specificity for identifying SUD.3 The single screening assessment for “past-year use” is quick and can be administered in a variety of clinical settings. The S2BI begins by asking a patient about his/her frequency of tobacco, alcohol, and/or marijuana use in the past year. If the patient endorses past-year use of any of these substances, the S2BI prompts follow-up questions about the use of prescription medications, illicit drugs, inhalants, and herbal products. A patient’s frequency of use is strongly correlated with the likelihood of having a SUD. Adolescents who report using a substance “once or twice” in the past year are very unlikely to have a SUD. Patients who endorse “monthly” use are more likely to meet the criteria for a mild or moderate SUD, and those reporting “weekly or more” use are more likely to have a severe SUD.
The BSTAD is an electronic, validated, high-sensitivity, high-specificity instrument for identifying SUD.1 It asks a single frequency question about past-year use of tobacco, alcohol, and marijuana, which are the most commonly used substances among adolescents. Patients who report using any of these substances are then asked about additional substance use. Based on the patient’s self-report of past year use, the screen places him/her into 1 of 3 risk categories for SUD: no reported use, lower risk, and higher risk. Each risk level maps to suggested clinical actions that are summarized in the results section.
Kevin M. Simon, MD
Child & Adolescent Psychiatry Fellow
Boston Children’s Hospital
Clinical Fellow in Psychiatry
Harvard Medical School
Boston, Massachusetts
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
References
1. Palmer A, Karakus M, Mark T. Barriers faced by physicians in screening for substance use disorders among adolescents. Psychiatr Serv. 2019;70(5):409-412.
2. D’Souza-Li L, Harris SK. The future of screening, brief intervention and referral to treatment in adolescent primary care: research directions and dissemination challenges. Curr Opin Pediatr. 2016;28(4):434-440.
3. Levy S, Weiss R, Sherritt L, et al. An electronic screen for triaging adolescent substance use by risk levels. JAMA Pediatr. 2014;168(9):822-828.
4. Kelly SM, Gryczynski J, Mitchell SG, et al. Validity of brief screening instrument for adolescent tobacco, alcohol, and drug use. Pediatrics. 2014;133(5):819-826.
Continue to: The author responds
The author responds
I thank Dr. Simon for his words of encouragement. I agree that both the S2BI and BSTAD have high sensitivity and specificity and are easy to use for screening for the use of multiple substances. Once substance use is established, both tools recommend administering high-risk assessment with additional scales such as the CRAFFT. During the initial evaluation, many psychiatrists take their patient’s history of substance use in detail, including age of onset, frequency, amount used, severity, and the time of his/her last use, without using a screening instrument. My article focused on instruments that can determine whether there is need for a further detailed evaluation. I agree that the S2BI and BSTAD would assist psychiatrists or physicians in other specialties (eg, pediatrics, family medicine) who might not take a complete substance use history during their initial evaluations.
Shikha Verma, MD
Rogers Behavioral Health
Kenosha, Wisconsin
Assistant Professor
Department of Psychiatry and Behavioral Health
Rosalind Franklin University of Medicine and Science
North Chicago, Illinois
Continue to: Changes as a result of COVID-19
Changes as a result of COVID-19
I thank Dr. Nasrallah for his editorial “During a viral pandemic, anxiety is endemic: The psychiatric aspects of COVID-19” (From the Editor,
I appreciated the editorial because it got me thinking about how the pandemic has changed me and my family:
1. We are engaging more in social media.
2. I feel uncomfortable when I go to the grocery store.
3. I feel better when I don’t access the news about COVID-19.
4. My children need physical socialization with their friends (sports, games, other activities, etc.).
5. My children function better with a schedule, but we find it difficult to keep them on a good schedule. Our teenagers stay up late at night (because all of their friends do), and they sleep in late the next morning.
Here are some positive changes:
1. Creating a weekly family calendar on a dry-erase board, so the family can see what is going on during the week.
2. Creating responsibility for our older children (eg, washing their own clothes, cleaning their bathroom).
3. Eating most meals as a family and organizing meals better, too.
4. Playing games together.
5. Cleaning the house together.
6. Getting outside to walk the dog and appreciate nature more.
7. Exercising.
8. Utilizing positive social media.
9. Getting caught up on life.
Again, I thank Dr. Nasrallah for writing this editorial because it led me to self-reflect on this situation, and helped me feel normal.
Doug Dolenc
Westfield, Indiana
Disclosure: The author reports no financial relationships with any companies whose products are mentioned in this article, or with manufacturers of competing products.
COVID-19: An opportunity, challenge for addiction treatment, NIDA boss says
The COVID-19 pandemic is posing significant challenges while also providing unique opportunities for patients with substance use disorders (SUD), a leading expert says.
Nora Volkow, MD, director of the National Institute on Drug Abuse, said that the pandemic has accelerated the use of telemedicine, making it easier for patients with SUD to access treatment. It has also led to the proliferation of more mental health hotlines, which is critical since the vast majority of these patients have comorbid mental illness.
In addition, COVID-19 has resulted in increased availability of “alternative” peer support mechanisms via cellphones or computers to aid individuals’ sobriety.
Dr. Volkow spoke at the virtual American Psychiatric Association Spring Highlights Meeting 2020, which is replacing the organization’s canceled annual meeting.
While methadone clinics have had to close during the pandemic, making it challenging for those on medically assisted treatment to receive methadone or buprenorphine, some of the rules and regulations have been relaxed in order to make these medications accessible without the need for in-person attendance at a clinic. In addition, the Substance Abuse and Mental Health Services Administration has relaxed some of its own regulations regarding telehealth and opioid treatment programs.
Social isolation, stigma intensified
A pandemic increases anxiety in the general population, but for patients with SUD who may be also be struggling with homelessness and comorbid mental illness, the situation can further exacerbate social stigma and isolation – leading to relapse, more overdoses, and overdose deaths, Dr. Volkow said. Social interaction is “extraordinarily important” for patients and “one of the most powerful tools we have” to build resilience.
Right now, said Dr. Volkow, “we are in the dark as to how COVID infections have affected the number of overdose deaths.”
However, she noted that
“So even through this devastation, we can actually extract something that may help others in future,” she said.
Dr. Volkow noted that during the pandemic it is critical to reinforce the importance of engaging in – and remaining in – treatment to SUD patients. It’s also crucial to make patients aware of social support systems and behavioral interventions to help them cope with stress and to mitigate relapse risk.
COVID-19 and relapse
Elie G. Aoun, MD, assistant professor of psychiatry at New York University and vice chair of the APA’s Council on Addiction Psychiatry, said in an interview that Dr. Volkow’s presentation provided “exactly the kind of accessible information” clinicians need.
Dr. Aoun said he sees the impact of the COVID-19 crisis in his practice every day. Patients with SUD “are getting the short end of the stick.”
Social distancing measures prompted by the pandemic can be “very triggering” for SUD patients, he said. One of his patients told him the current isolation, loneliness, movement restrictions, and boredom remind her of the way she felt when she used drugs.
Dr. Aoun said four of his patients have relapsed since the pandemic began. Two of them had just started treatment after years of using drugs, so this was a “major setback” for them.
He and his colleagues were “not really prepared” to provide care via video link, which he believes is not as effective as in-person sessions.
In addition to disrupting patient care, said Dr. Aoun, the pandemic is forcing the medical community to face social determinants of health, such as poverty and homelessness, as they relate to addiction disorders and whether or not someone receives care.
This article originally appeared on Medscape.com.
The COVID-19 pandemic is posing significant challenges while also providing unique opportunities for patients with substance use disorders (SUD), a leading expert says.
Nora Volkow, MD, director of the National Institute on Drug Abuse, said that the pandemic has accelerated the use of telemedicine, making it easier for patients with SUD to access treatment. It has also led to the proliferation of more mental health hotlines, which is critical since the vast majority of these patients have comorbid mental illness.
In addition, COVID-19 has resulted in increased availability of “alternative” peer support mechanisms via cellphones or computers to aid individuals’ sobriety.
Dr. Volkow spoke at the virtual American Psychiatric Association Spring Highlights Meeting 2020, which is replacing the organization’s canceled annual meeting.
While methadone clinics have had to close during the pandemic, making it challenging for those on medically assisted treatment to receive methadone or buprenorphine, some of the rules and regulations have been relaxed in order to make these medications accessible without the need for in-person attendance at a clinic. In addition, the Substance Abuse and Mental Health Services Administration has relaxed some of its own regulations regarding telehealth and opioid treatment programs.
Social isolation, stigma intensified
A pandemic increases anxiety in the general population, but for patients with SUD who may be also be struggling with homelessness and comorbid mental illness, the situation can further exacerbate social stigma and isolation – leading to relapse, more overdoses, and overdose deaths, Dr. Volkow said. Social interaction is “extraordinarily important” for patients and “one of the most powerful tools we have” to build resilience.
Right now, said Dr. Volkow, “we are in the dark as to how COVID infections have affected the number of overdose deaths.”
However, she noted that
“So even through this devastation, we can actually extract something that may help others in future,” she said.
Dr. Volkow noted that during the pandemic it is critical to reinforce the importance of engaging in – and remaining in – treatment to SUD patients. It’s also crucial to make patients aware of social support systems and behavioral interventions to help them cope with stress and to mitigate relapse risk.
COVID-19 and relapse
Elie G. Aoun, MD, assistant professor of psychiatry at New York University and vice chair of the APA’s Council on Addiction Psychiatry, said in an interview that Dr. Volkow’s presentation provided “exactly the kind of accessible information” clinicians need.
Dr. Aoun said he sees the impact of the COVID-19 crisis in his practice every day. Patients with SUD “are getting the short end of the stick.”
Social distancing measures prompted by the pandemic can be “very triggering” for SUD patients, he said. One of his patients told him the current isolation, loneliness, movement restrictions, and boredom remind her of the way she felt when she used drugs.
Dr. Aoun said four of his patients have relapsed since the pandemic began. Two of them had just started treatment after years of using drugs, so this was a “major setback” for them.
He and his colleagues were “not really prepared” to provide care via video link, which he believes is not as effective as in-person sessions.
In addition to disrupting patient care, said Dr. Aoun, the pandemic is forcing the medical community to face social determinants of health, such as poverty and homelessness, as they relate to addiction disorders and whether or not someone receives care.
This article originally appeared on Medscape.com.
The COVID-19 pandemic is posing significant challenges while also providing unique opportunities for patients with substance use disorders (SUD), a leading expert says.
Nora Volkow, MD, director of the National Institute on Drug Abuse, said that the pandemic has accelerated the use of telemedicine, making it easier for patients with SUD to access treatment. It has also led to the proliferation of more mental health hotlines, which is critical since the vast majority of these patients have comorbid mental illness.
In addition, COVID-19 has resulted in increased availability of “alternative” peer support mechanisms via cellphones or computers to aid individuals’ sobriety.
Dr. Volkow spoke at the virtual American Psychiatric Association Spring Highlights Meeting 2020, which is replacing the organization’s canceled annual meeting.
While methadone clinics have had to close during the pandemic, making it challenging for those on medically assisted treatment to receive methadone or buprenorphine, some of the rules and regulations have been relaxed in order to make these medications accessible without the need for in-person attendance at a clinic. In addition, the Substance Abuse and Mental Health Services Administration has relaxed some of its own regulations regarding telehealth and opioid treatment programs.
Social isolation, stigma intensified
A pandemic increases anxiety in the general population, but for patients with SUD who may be also be struggling with homelessness and comorbid mental illness, the situation can further exacerbate social stigma and isolation – leading to relapse, more overdoses, and overdose deaths, Dr. Volkow said. Social interaction is “extraordinarily important” for patients and “one of the most powerful tools we have” to build resilience.
Right now, said Dr. Volkow, “we are in the dark as to how COVID infections have affected the number of overdose deaths.”
However, she noted that
“So even through this devastation, we can actually extract something that may help others in future,” she said.
Dr. Volkow noted that during the pandemic it is critical to reinforce the importance of engaging in – and remaining in – treatment to SUD patients. It’s also crucial to make patients aware of social support systems and behavioral interventions to help them cope with stress and to mitigate relapse risk.
COVID-19 and relapse
Elie G. Aoun, MD, assistant professor of psychiatry at New York University and vice chair of the APA’s Council on Addiction Psychiatry, said in an interview that Dr. Volkow’s presentation provided “exactly the kind of accessible information” clinicians need.
Dr. Aoun said he sees the impact of the COVID-19 crisis in his practice every day. Patients with SUD “are getting the short end of the stick.”
Social distancing measures prompted by the pandemic can be “very triggering” for SUD patients, he said. One of his patients told him the current isolation, loneliness, movement restrictions, and boredom remind her of the way she felt when she used drugs.
Dr. Aoun said four of his patients have relapsed since the pandemic began. Two of them had just started treatment after years of using drugs, so this was a “major setback” for them.
He and his colleagues were “not really prepared” to provide care via video link, which he believes is not as effective as in-person sessions.
In addition to disrupting patient care, said Dr. Aoun, the pandemic is forcing the medical community to face social determinants of health, such as poverty and homelessness, as they relate to addiction disorders and whether or not someone receives care.
This article originally appeared on Medscape.com.
Researchers investigate impact of smoking on COVID-19 risk
but quitting smoking is likely to lower the risk of developing more severe or fatal cases of the infection, according to research from several recent papers.
Interest in how tobacco use affects COVID-19 infection rates stems from research showing that men at the epicenter of the outbreak in China having a higher early mortality rate. Early reports from China showed a case fatality rate of 4.7% for men, compared with 2.8% for women, according to the World Health Organization. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2, is suspected to enter a cell using the ACE2 receptor. Since smoking up-regulates this receptor, one popular theory is that smoking can increase the risk of COVID-19 or exacerbate symptoms of an existing infection (Eur Respir J. 2020 Apr 8. doi: 10.1183/13993003.00688-2020). In China, about half of men are active smokers, compared with 2.7% of women (Transl Lung Cancer Res. 2019;8[Suppl 1]:S21-30), so this association would explain the severe cases and increased mortality in this group. In response to potential risk for public health, the World Health Organization, Centers for Disease Control and Prevention, the Attorney General of Massachusetts, and other organizations have warned that smoking may increase one’s risk of transmitting and developing COVID-19 or may worsen the infection.
“While it is easy to jump to the conclusion that more ACE2 means more susceptibility to severe infection, there is no evidence to support this,” Brandon Michael Henry, MD, of the cardiac intensive care unit and the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in an interview. “Moreover, some would argue (including myself) that increased ACE2 may in fact be protective, as ACE2 decreases the levels of angiotensin-2 which likely plays a significant role in the pathophysiology of ARDS.”
Some researchers have examined the limited evidence of smoking on COVID-19 risk and come to preliminary conclusions. In a letter to the editor recently published in the European Journal of Internal Medicine, Dr. Henry and Giuseppe Lippi, MD, of the section of clinical biochemistry in the department of neuroscience, biomedicine, and movement at the University of Verona (Italy), performed a meta-analysis of papers examining smoking and COVID-19 up to March 9, 2020 and identified five articles with 1,399 COVID-19 cases (Eur J Intern Med. 2020 Mar 16. doi: 10.1016/j.ejim.2020.03.014).
“Given the fact that COVID-19 is a primarily respiratory illness, smoking was one of first risk factors we examined,” Dr. Henry said.
They noted that a study by Liu et al. in the Chinese Medical Journal was the only paper that showed a significant association between smoking status and COVID-19 case severity (Chin Med J [Engl]. 2020 Feb 28. doi: 10.1097/CM9.0000000000000775), while the four other studies showed no significant association. The pooled data of all five studies showed an association that was not statistically significant (odds ratio, 1.69; 95% confidence interval, 0.41-6.92; P = .254). When Dr. Lippi and Dr. Henry performed the analysis again after removing a paper by Guan et al. (N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032) comprising 89.5% of patients in the pooled analysis, there was no significant association (OR, 4.35; 95% CI, 0.86-21.86; P = .129).
Constantine I. Vardavas, MD, FCCP, of the department of oral health policy and epidemiology at Harvard School of Dental Medicine, Boston, and Katerina Nikitara, of the University of Crete in Heraklion, Greece, also published a systematic review in Tobacco Induced Diseases of five studies evaluating smoking and COVID-19 (Tob Induc Dis. 2020. doi: 10.18332/tid/119324). Of the studies chosen for the review, four were shared with the paper by Dr. Lippi and Dr. Henry. They found “a higher percentage of smokers” made up severe COVID-19 cases, but acknowledged the majority of these were from the largest study by Guan et al. Overall, they calculated smokers carried a risk ratio of 1.4 (95% CI, 0.98-2.00) for developing severe COVID-19 symptoms, and were over twice as likely to be admitted to an ICU, require a mechanical ventilator, or die from COVID-19, compared with patients who did not smoke (RR, 2.4; 95% CI, 1.43-4.04).
“Although further research is warranted as the weight of the evidence increases, with the limited available data, and although the above results are unadjusted for other factors that may impact disease progression, smoking is most likely associated with the negative progression and adverse outcomes of COVID-19,” Dr. Vardavas and Ms. Nikitara concluded.
However, the association between smoking and severe disease was not significant, and it is not immediately clear how the analysis was performed based on the details in the editorial. “Both of our reports were limited by a lack of data adjusted for age, sex, and comorbidities which may influence any analysis on smoking,” Dr. Henry said.
Some researchers have proposed collecting information on smoking status and conducting further research on whether vaping devices like e-cigarettes also impact COVID-19 cases. An editorial by Samuel Brake and colleagues published in the Journal of Clinical Medicine proposed the ACE2-receptor binding site as an area of interest for COVID-19 and as a potential therapeutic target (J Clin Med. 2020 Mar 20. doi: 10.3390/jcm9030841).
Ultimately, whether smoking itself is associated with COVID-19 is still an open question. Nonetheless, encouraging patients to quit smoking should be a priority because long-term sequelae of smoking have been linked to worsened or fatal COVID-19 cases, said Dr. Henry.
“There is a lack of definitive data on smoking to date. Nonetheless, we do know that many illnesses associated with smoking, such as [chronic obstructive pulmonary disease, hypertension, and heart disease are all strong risk factors for severe and fatal COVID-19,” he said. “Thus, absolutely we should encourage the public to quit smoking, especially for older individuals and those with comorbidities.”
The papers by Lippi et al., Vardavas et al., and Brake et al. had no funding source, and the authors reported no relevant conflicts of interest.
but quitting smoking is likely to lower the risk of developing more severe or fatal cases of the infection, according to research from several recent papers.
Interest in how tobacco use affects COVID-19 infection rates stems from research showing that men at the epicenter of the outbreak in China having a higher early mortality rate. Early reports from China showed a case fatality rate of 4.7% for men, compared with 2.8% for women, according to the World Health Organization. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2, is suspected to enter a cell using the ACE2 receptor. Since smoking up-regulates this receptor, one popular theory is that smoking can increase the risk of COVID-19 or exacerbate symptoms of an existing infection (Eur Respir J. 2020 Apr 8. doi: 10.1183/13993003.00688-2020). In China, about half of men are active smokers, compared with 2.7% of women (Transl Lung Cancer Res. 2019;8[Suppl 1]:S21-30), so this association would explain the severe cases and increased mortality in this group. In response to potential risk for public health, the World Health Organization, Centers for Disease Control and Prevention, the Attorney General of Massachusetts, and other organizations have warned that smoking may increase one’s risk of transmitting and developing COVID-19 or may worsen the infection.
“While it is easy to jump to the conclusion that more ACE2 means more susceptibility to severe infection, there is no evidence to support this,” Brandon Michael Henry, MD, of the cardiac intensive care unit and the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in an interview. “Moreover, some would argue (including myself) that increased ACE2 may in fact be protective, as ACE2 decreases the levels of angiotensin-2 which likely plays a significant role in the pathophysiology of ARDS.”
Some researchers have examined the limited evidence of smoking on COVID-19 risk and come to preliminary conclusions. In a letter to the editor recently published in the European Journal of Internal Medicine, Dr. Henry and Giuseppe Lippi, MD, of the section of clinical biochemistry in the department of neuroscience, biomedicine, and movement at the University of Verona (Italy), performed a meta-analysis of papers examining smoking and COVID-19 up to March 9, 2020 and identified five articles with 1,399 COVID-19 cases (Eur J Intern Med. 2020 Mar 16. doi: 10.1016/j.ejim.2020.03.014).
“Given the fact that COVID-19 is a primarily respiratory illness, smoking was one of first risk factors we examined,” Dr. Henry said.
They noted that a study by Liu et al. in the Chinese Medical Journal was the only paper that showed a significant association between smoking status and COVID-19 case severity (Chin Med J [Engl]. 2020 Feb 28. doi: 10.1097/CM9.0000000000000775), while the four other studies showed no significant association. The pooled data of all five studies showed an association that was not statistically significant (odds ratio, 1.69; 95% confidence interval, 0.41-6.92; P = .254). When Dr. Lippi and Dr. Henry performed the analysis again after removing a paper by Guan et al. (N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032) comprising 89.5% of patients in the pooled analysis, there was no significant association (OR, 4.35; 95% CI, 0.86-21.86; P = .129).
Constantine I. Vardavas, MD, FCCP, of the department of oral health policy and epidemiology at Harvard School of Dental Medicine, Boston, and Katerina Nikitara, of the University of Crete in Heraklion, Greece, also published a systematic review in Tobacco Induced Diseases of five studies evaluating smoking and COVID-19 (Tob Induc Dis. 2020. doi: 10.18332/tid/119324). Of the studies chosen for the review, four were shared with the paper by Dr. Lippi and Dr. Henry. They found “a higher percentage of smokers” made up severe COVID-19 cases, but acknowledged the majority of these were from the largest study by Guan et al. Overall, they calculated smokers carried a risk ratio of 1.4 (95% CI, 0.98-2.00) for developing severe COVID-19 symptoms, and were over twice as likely to be admitted to an ICU, require a mechanical ventilator, or die from COVID-19, compared with patients who did not smoke (RR, 2.4; 95% CI, 1.43-4.04).
“Although further research is warranted as the weight of the evidence increases, with the limited available data, and although the above results are unadjusted for other factors that may impact disease progression, smoking is most likely associated with the negative progression and adverse outcomes of COVID-19,” Dr. Vardavas and Ms. Nikitara concluded.
However, the association between smoking and severe disease was not significant, and it is not immediately clear how the analysis was performed based on the details in the editorial. “Both of our reports were limited by a lack of data adjusted for age, sex, and comorbidities which may influence any analysis on smoking,” Dr. Henry said.
Some researchers have proposed collecting information on smoking status and conducting further research on whether vaping devices like e-cigarettes also impact COVID-19 cases. An editorial by Samuel Brake and colleagues published in the Journal of Clinical Medicine proposed the ACE2-receptor binding site as an area of interest for COVID-19 and as a potential therapeutic target (J Clin Med. 2020 Mar 20. doi: 10.3390/jcm9030841).
Ultimately, whether smoking itself is associated with COVID-19 is still an open question. Nonetheless, encouraging patients to quit smoking should be a priority because long-term sequelae of smoking have been linked to worsened or fatal COVID-19 cases, said Dr. Henry.
“There is a lack of definitive data on smoking to date. Nonetheless, we do know that many illnesses associated with smoking, such as [chronic obstructive pulmonary disease, hypertension, and heart disease are all strong risk factors for severe and fatal COVID-19,” he said. “Thus, absolutely we should encourage the public to quit smoking, especially for older individuals and those with comorbidities.”
The papers by Lippi et al., Vardavas et al., and Brake et al. had no funding source, and the authors reported no relevant conflicts of interest.
but quitting smoking is likely to lower the risk of developing more severe or fatal cases of the infection, according to research from several recent papers.
Interest in how tobacco use affects COVID-19 infection rates stems from research showing that men at the epicenter of the outbreak in China having a higher early mortality rate. Early reports from China showed a case fatality rate of 4.7% for men, compared with 2.8% for women, according to the World Health Organization. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2, is suspected to enter a cell using the ACE2 receptor. Since smoking up-regulates this receptor, one popular theory is that smoking can increase the risk of COVID-19 or exacerbate symptoms of an existing infection (Eur Respir J. 2020 Apr 8. doi: 10.1183/13993003.00688-2020). In China, about half of men are active smokers, compared with 2.7% of women (Transl Lung Cancer Res. 2019;8[Suppl 1]:S21-30), so this association would explain the severe cases and increased mortality in this group. In response to potential risk for public health, the World Health Organization, Centers for Disease Control and Prevention, the Attorney General of Massachusetts, and other organizations have warned that smoking may increase one’s risk of transmitting and developing COVID-19 or may worsen the infection.
“While it is easy to jump to the conclusion that more ACE2 means more susceptibility to severe infection, there is no evidence to support this,” Brandon Michael Henry, MD, of the cardiac intensive care unit and the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in an interview. “Moreover, some would argue (including myself) that increased ACE2 may in fact be protective, as ACE2 decreases the levels of angiotensin-2 which likely plays a significant role in the pathophysiology of ARDS.”
Some researchers have examined the limited evidence of smoking on COVID-19 risk and come to preliminary conclusions. In a letter to the editor recently published in the European Journal of Internal Medicine, Dr. Henry and Giuseppe Lippi, MD, of the section of clinical biochemistry in the department of neuroscience, biomedicine, and movement at the University of Verona (Italy), performed a meta-analysis of papers examining smoking and COVID-19 up to March 9, 2020 and identified five articles with 1,399 COVID-19 cases (Eur J Intern Med. 2020 Mar 16. doi: 10.1016/j.ejim.2020.03.014).
“Given the fact that COVID-19 is a primarily respiratory illness, smoking was one of first risk factors we examined,” Dr. Henry said.
They noted that a study by Liu et al. in the Chinese Medical Journal was the only paper that showed a significant association between smoking status and COVID-19 case severity (Chin Med J [Engl]. 2020 Feb 28. doi: 10.1097/CM9.0000000000000775), while the four other studies showed no significant association. The pooled data of all five studies showed an association that was not statistically significant (odds ratio, 1.69; 95% confidence interval, 0.41-6.92; P = .254). When Dr. Lippi and Dr. Henry performed the analysis again after removing a paper by Guan et al. (N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMoa2002032) comprising 89.5% of patients in the pooled analysis, there was no significant association (OR, 4.35; 95% CI, 0.86-21.86; P = .129).
Constantine I. Vardavas, MD, FCCP, of the department of oral health policy and epidemiology at Harvard School of Dental Medicine, Boston, and Katerina Nikitara, of the University of Crete in Heraklion, Greece, also published a systematic review in Tobacco Induced Diseases of five studies evaluating smoking and COVID-19 (Tob Induc Dis. 2020. doi: 10.18332/tid/119324). Of the studies chosen for the review, four were shared with the paper by Dr. Lippi and Dr. Henry. They found “a higher percentage of smokers” made up severe COVID-19 cases, but acknowledged the majority of these were from the largest study by Guan et al. Overall, they calculated smokers carried a risk ratio of 1.4 (95% CI, 0.98-2.00) for developing severe COVID-19 symptoms, and were over twice as likely to be admitted to an ICU, require a mechanical ventilator, or die from COVID-19, compared with patients who did not smoke (RR, 2.4; 95% CI, 1.43-4.04).
“Although further research is warranted as the weight of the evidence increases, with the limited available data, and although the above results are unadjusted for other factors that may impact disease progression, smoking is most likely associated with the negative progression and adverse outcomes of COVID-19,” Dr. Vardavas and Ms. Nikitara concluded.
However, the association between smoking and severe disease was not significant, and it is not immediately clear how the analysis was performed based on the details in the editorial. “Both of our reports were limited by a lack of data adjusted for age, sex, and comorbidities which may influence any analysis on smoking,” Dr. Henry said.
Some researchers have proposed collecting information on smoking status and conducting further research on whether vaping devices like e-cigarettes also impact COVID-19 cases. An editorial by Samuel Brake and colleagues published in the Journal of Clinical Medicine proposed the ACE2-receptor binding site as an area of interest for COVID-19 and as a potential therapeutic target (J Clin Med. 2020 Mar 20. doi: 10.3390/jcm9030841).
Ultimately, whether smoking itself is associated with COVID-19 is still an open question. Nonetheless, encouraging patients to quit smoking should be a priority because long-term sequelae of smoking have been linked to worsened or fatal COVID-19 cases, said Dr. Henry.
“There is a lack of definitive data on smoking to date. Nonetheless, we do know that many illnesses associated with smoking, such as [chronic obstructive pulmonary disease, hypertension, and heart disease are all strong risk factors for severe and fatal COVID-19,” he said. “Thus, absolutely we should encourage the public to quit smoking, especially for older individuals and those with comorbidities.”
The papers by Lippi et al., Vardavas et al., and Brake et al. had no funding source, and the authors reported no relevant conflicts of interest.
Incidence of Chronic Opioid Use in Previously Opioid-Naïve Patients Receiving Opioids for Analgesia in the Intensive Care Unit
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
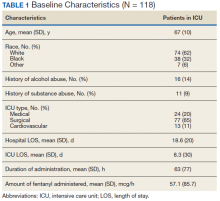
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
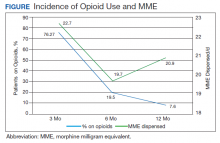
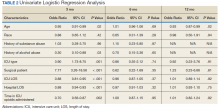
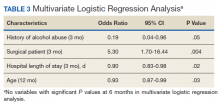
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
Chronic pain is a worldwide cause of impairment. According to data from the 2016 National Health Interview Survey, an estimated 50 million American adults suffer from chronic pain, with 19.6 million adults suffering from high-impact chronic pain.1 This phenomenon is particularly prevalent in the older population. More than 25% of adults aged 65 to 74 years reported that they were often in pain in the past 3 months compared with just 10% of those adults between the ages of 18 and 44 years.2
The economic burdens of chronic pain disorders are well known. In 2010, Gaskin and Richard found that chronic pain has far-reaching consequences for the US economy, ranging from direct health care costs to lost productivity. This study estimated additional health care costs at about $300 billion yearly and lost productivity at $300 billion, bringing total annual costs to about $600 billion. This expense is more than heart disease alone ($309 billion), and cancer and diabetes mellitus ($243 billion and $188 billion respectively) combined.3
Opioid medications are powerful and effective pain-reducing agents that are indicated for short-term acute pain or long-term in the management of chronic, severe cancer-related pain.4 Although efficacious, use of these medications carries with it the inherent risks of abuse, misuse, addiction, and overdose.5 Since 1999, opioid-related overdose deaths have been on the rise. The CDC estimated that > 15,000 deaths were attributable specifically to prescription opioids in 2015.6 The estimates had risen to > 17,000 deaths in 2017, with the number increasing since that time.7 Cumulatively, the CDC estimates that > 200,000 deaths in the US between 1999 and 2017 are attributed to prescription opioid overdose, clearly marking this trend as a growing nationwide epidemic.8
In 2016, Florence and colleagues estimated costs associated with opioid overdose to be just shy of $80 billion in 2013 dollars.9 In October 2017, the US Department of Health and Human Services declared the opioid epidemic a public health emergency and committed $900 million to combating the crisis.10
An abundance of data exist analyzing outpatient prescribing and its impacts on opioid dependence, particularly postoperatively. A study by Brummett and colleagues indicated that the incidence of new persistent opioid use in patients who underwent surgery was 5.9% to 6.5% and did not differ between major and minor surgical procedures. This study concluded that new opioid use could be considered one of the most common complications after elective surgery.11 Similarly, in 2017 Makary and colleagues found that surgeons tend to overprescribe pain medications after procedures; some prescribing as many as 50 to 60 tablets to control pain after simple procedures.12 This is in stark contrast to pain guideline recommendations of no more than 10 tablets for most standard operative procedures.13
Sun and colleagues conducted a retrospective analysis of health care claims data in more than 18 million opioid-naïve patients who did and did not undergo surgery. Seven of the 11 surgical procedures were associated with an increased risk of chronic opioid use. The highest incidence of chronic opioid use in the first postoperative year was for total hip arthroplasty (1.4%, OR 5.10; 95% CI, 1.29-1.53). The study found that the risk factors most associated with chronic opioid use after surgery were male sex, aged > 50 years, and preoperative history of drug abuse, alcohol abuse, or depression, along with benzodiazepine use or antidepressant use.14 In a 2018 cohort study that evaluated predictors associated with transitioning to incident chronic opioid therapy, 4 factors were identified. These included opioid duration of action (adjusted odds ratio [AOR], 12.28; 95% CI, 8.1-06-18.72), the parent opioid compound (eg, tramadol vs codeine; AOR, 7.26; 95% CI, 5.20-10.13), the presence of conditions that are very likely to cause chronic pain (AOR, 5.47; 95% CI, 3.89-7.68), and drug use disorders (AOR, 4.02; 95% CI, 2.53-6.40).15
While there has been research into outpatient risk factors and medical practices that may contribute to chronic opioid use, a relative paucity of data exists on the contribution of hospitalization and inpatient opioid use on patient outcomes. A 2014 Canadian study assessed the impact of opioid use in the intensive care unit (ICU) on opioid use after discharge.16 This study included more than 2,500 patients who were admitted to a Canadian ICU between 2005 and 2008, and then followed after discharge for 48 months to quantify chronic opioid use. Nonopioid users increased from 87.8% in the early post-ICU period to 95.6% at 48 months after discharge. Preadmission chronic opioid use and prolonged hospital length of stay (LOS) were found to be associated with an increased risk of chronic opioid use after discharge.16 To date, there are no published studies that analyze the incidence of opioid-naïve veterans who convert to chronic opioid use after receiving opioids during an acute hospitalization.
In this retrospective analysis, we analyze the incidence of chronic opioid use after administration of opioids in the ICU as well as a variety of risk factors that may influence conversion to chronic opioid use.
Methods
This analysis was a single center, retrospective chart review conducted for patients admitted between July 1, 2017 and December 31, 2017 at the US Department of Veterans Affairs (VA) Michael E. DeBakey VA Medical Center (MEDVAMC) in Houston, Texas. MEDVAMC is a 538-bed academic\teaching hospital serving about 130,000 veterans in Southeast Texas. The hospital has 3 ICUs (medical, cardiovascular, and surgical) and 38 total ICU beds. The study was approved by the Baylor College of Medicine Institutional Review Board and MEDVAMC Research and Development Review Board. Informed consent was not required.
Inclusion criteria consisted of patients aged ≥ 18 years admitted to the ICU in the above-specified time frame, who were administered a continuous infusion of an opioid for at least 12 hours. Patients were excluded if they were not opioid naïve prior to admission, defined as receiving > 30 days of opioids in the prior 12 months. Patients who died during hospital admission, never received an opioid despite having an active order, were hospital-to-hospital transfers, or were still admitted at the time of data collection were excluded from the analysis.
All pertinent data were collected using the VA Computerized Patient Record System (CPRS) and the Critical Care Manager (Picis Clinical Solutions) ICU monitoring application. Critical Care Manager was used to track the time frame, duration, and amounts of opioid infusions administered in the ICU. Patient demographic and preadmission data, including date of birth, age, race, history of substance use/alcohol use disorder (defined as a previous diagnosis) and previous opioid prescriptions within the past year were recorded. For the inpatient admission, the ICU LOS, hospital LOS, primary admission diagnosis, type of opioid medication administered, and total duration and dose of opioid administered were collected. After discharge, opioid medication fill data at 3, 6, and 12 months were collected. This information included names of any outpatient opioids filled, dosage unit, quantity, day supply, and number of refills.
The primary outcome for this study was to determine the incidence of chronic opioid use at 3, 6, and 12 months after discharge, defined as the percentage of patients receiving outpatient opioid prescriptions at each time point. Analyses were conducted to observe the effect of age, race, history of substance use or history of alcohol use (International Classification of Diseases documented diagnosis, 9th edition), ICU type (medical, surgical, or cardiovascular), surgical/nonsurgical admission, ICU LOS, hospital LOS, total time, and amount of opioids administered during admission on risk of conversion to chronic opioid use.
Descriptive statistics were calculated to analyze the incidence of chronic opioid use. Univariate logistic regression analysis, including ORs, 95% CIs, and P values, was conducted to determine the effects of the risk factors noted earlier on chronic opioid use at each time point. A multivariate logistic regression model was performed to assess the effect of multiple independent variables on opioid use at 3, 6, and 12 months. Statistical analysis was performed using StataCorp Stata SE.
Results
During the study period, 330 patients were admitted to the ICU. After applying inclusion/exclusion criteria, 118 patients were included in the final analysis. The most frequent reasons for exclusion from the study were patient death (n = 77), a past history of opioid use (n = 56), and not having received an opioid infusion for at least 12 hours (n = 68). The average age of the patients included was 67 years (Table 1). A total of 14% and 9% of patients, respectively, had a diagnosis of alcohol use disorder or substance use disorder recorded in CPRS. After admission, the most common location for these patients was the surgical ICU (65%). All patients were male. The average hospital LOS was 18.6 days , and the ICU LOS was 8.3 days. The average duration of administration for the opioid (fentanyl) infusion was 63 hours, and the average amount of fentanyl administered to each patient was 57.1 mcg/h.
The incidence of opioid-naïve patients receiving opioids after discharge was 76.3% (n = 90) at 3 months, 19.5% (n = 23) at 6 months and 7.6% (n = 9) at 12 months (Figure). The daily morphine milligram equivalent (MME) of patients prescribed opioids at 3, 6, and 12 months was similar (3 months, 22.7; 6 months, 19.7; 12 months, 20.9). In the univariate regression analysis, several variables were found to be associated with converting to chronic opioid use. Prior history of alcohol use disorder (OR, 0.3; 95% CI, 0.10-0.88; P = .03), ICU type (OR, 3.9; 95% CI, 1.73-8.75; P = .001) and ICU LOS (OR, 0.88; 95% CI, 0.81-0.95; P = .01) had a statistically significant association on opioid use at 3 months. (Table 2). No variables evaluated had a statistically significant effect on chronic opioid use at 6 months, and only age (OR 0.93; 95% CI. 0.87-0.99; P = .02) was statistically significant at 12 months. In the multivariate logistic regression analysis, history of alcohol abuse, admission for surgery, and hospital LOS were significant at 3 months (Table 3).
Discussion
In this single-center analysis conducted at a VA academic hospital of opioid-naïve patients who were administered opioids in the ICU, the incidence of patients subsequently receiving outpatient opioid prescriptions at 12 months after discharge was 7.6%. There also was a decrease in the amount of opioids received by patients (daily MME) after discharge at 3, 6, and 12 months. This trend did not demonstrate the propensity for inpatient opioid use to convert opioid-naïve patients to chronic opioid users.
The most common outpatient opioids prescribed were hydrocodone/acetaminophen, morphine, and tramadol. Logistic regression showed few factors that correlated significantly with opioid use in the long-term (12 month) period. This finding is a deviation from the findings of Yaffe and colleagues who found hospital LOS to be one of the only predictors of long-term opioid use in their population (defined as use at 48 months).16 One important difference between our study and the Yaffe and colleagues study was that they evaluated all patients who were admitted to the ICU, regardless of the exposure to opioids during their inpatient stay. Consequently, this difference may have resulted in the differences in findings.
Strengths and Limitations
Location was a strength of our study, as this analysis was conducted at an integrated health care system that provides comprehensive inpatient and outpatient care. The VA uses a closed electronic health record, which allowed patients to be followed both in the inpatient and outpatient settings to determine which medications were prescribed at each time. In other health care systems this information would have been difficult to follow as patients often fill outpatient prescriptions at community pharmacies not affiliated with the treating hospital. However, any patient not using a VA prescriber for subsequent opioid prescriptions or patients who received opioids through other sources would not have had their continued opioid use captured. These data may be available in the states prescription monitoring program, but it was not available to query for research at this time.
This study also excluded chronic opioid users, which could have been another confounding factor to account for when analyzing the results. However, the primary objective of the study was to determine the impact of opioids prescribed in the ICU on converting previous opioid-naïve patients to chronic users. Finally, a multivariate logistic regression was incorporated to assess for factors that may predispose certain patients to convert to chronic opioid users. This analysis served to extend the applicability of our study by not only analyzing whether receiving opioids in the ICU contributed to chronic opioid use in the long-term, but also which populations may be at greatest risk. This information can be used in the future to target harm-reduction efforts toward high-risk hospitalized patients.
One limitation of this study was that it was conducted as a retrospective, single-center chart review in Houston, Texas. Because this was not a randomized controlled trial, it is difficult to imply any causation between exposure to opioids in the ICU and chronic use. In addition, because this study was conducted at a single site, the results may not be able to be generalized to other populations. VA populations tend to be elderly and predominantly male, as was reflected by the study population. These factors, along with regional variability in patient characteristics, may limit the generalizability of this study to older male patients located in Southeast Texas or other similar populations. Other limitations of this study also included the small sample size, limited period of follow-up obtained, and that other comorbidity information (pain scores during stay, use of nonopioid pain medications, past history of anxiety or depression, or other acute illnesses or surgeries that may have required opioid therapy during admission) was not collected.
This study was only able to review 118 patients for a follow-up duration of 1 year. In the Yaffe and colleagues study, more than 2,500 patients were followed over 4 years, which provided a more long-term overview of the clinical course of these patients and may be another reason for discrepant findings. However, this study did not actually assess the impact on administration of opioids on the development of chronic opioid use.16 Finally, the biggest limitation to this study may be the potential for confounding discharge prescriptions. After discharge, patients’ prescriptions were evaluated from discharge to 3 months, in between 3 and 6 months, and between 6 and 12 months for the presence of an opioid prescription. Due to this methodology, any opioid prescription a patient was discharged with was counted in the 3-month time point. Since many patients included in the study were admitted to the surgical ICU (65%), it was logical that they were discharged with opioids after their procedure. While including the immediate postdischarge prescription data was useful for evaluating the decrease in opioid use and incidence over time, it did cause the 3-month time point to appear overly inflated, potentially signaling that at 3 months after discharge many of these patients were still requiring opioid use.
The Society of Critical Care Medicine still recommends opioids as first-line therapy for non-neuropathic pain in the ICU setting.17 Additionally, postoperative pain can be difficult to manage in the surgical population and is often treated with opioids, though treatment with multimodal pain regimens is becoming more common.18 It is difficult to imagine that a finding that implicates opioid use in the hospital with conversion to chronic opioid use would prompt a cessation in the use of opioid in these settings, especially in the context of analgosedation related to mechanically ventilated patients. However, it would be plausible to use this knowledge to advocate for opioid-sparing therapies and consideration for weaning individuals at high risk for misuse after discharge from opioid-containing sedation or analgesia regimens in a timelier manner.
Though our findings did not show a clinically relevant increase in chronic opioid users, clinicians can still use this information to encourage targeted education and closer monitoring for those patients deemed as high risk at discharge to prevent unnecessary prolonged opioid use. By having more frequent follow-up in pain clinics, switching patients to nonopioid therapies after discharge, and ensuring high-risk patients are discharged with naloxone rescue kits, it would be possible to drastically reduce the number of potential overdoses for patients who previously required opioid therapy in the ICU.
Conclusion
After discharge, 7.6% of previously opioid-naïve patients who were treated with opioids in the ICU were still receiving prescriptions for opioids at 12 months. These findings did not suggest a clinically significant increase in the incidence of chronic opioid use after inpatient administration of opioids. However, these results prompt the need for larger, prospective, multicenter studies to evaluate the effect on hospitalization on converting to chronic opioid use and a deeper evaluation of other potential risk factors that may be present.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006.
2. Centers for Disease Control and Prevention. QuickStats: percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6217a10.htm. Published May 3, 2103. Accessed February 7, 2020.
3. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715-724.
4. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-68.
5. DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM. Pharmacotherapy: A Pathophysiologic Approach, 9e. McGraw Hill Professional; 2014.
6. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-1452.
7. Ahmad FB, Rossen LM, Spencer M, Warner M, Sutton P. Provisional drug overdose death counts. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Reviewed February 12, 2020. Accessed February 18, 2020.
8. National Institute on Drug Abuse. Overdose death rates. https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Revised January 2019. Accessed February 10, 2020.
9. Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care. 2016;54(10):901-906.
10. HHS Acting Secretary declares public health emergency to address national opioid crisis [news release]. https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Published October 26, 2017. Accessed February 7, 2020.
11. Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
12. Makary MA, Overton HN, Wang P. Overprescribing is major contributor to opioid crisis. BMJ. 2017;359:j4792.
13. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
14. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286-93.
15. Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among working-age adults in the United States. Am Health Drug Benefits. 2018;11(1):12-21.
16. Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;327(7):429-435.
17. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873.
18. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
Elderly Americans carry heavier opioid burden
according to the Agency for Healthcare Quality and Research.
Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
New ASAM guideline released amid COVID-19 concerns
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
Home-based buprenorphine induction deemed safe for OUD
Home-based buprenorphine induction deemed safe for OUD
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
The American Society of Addiction Medicine has released an updated practice guideline for patients with opioid use disorder.
The guideline, called a focused update, advances ASAM’s 2015 National Practice Guidelines for the Treament of Opioid Use Disorder. “During the ongoing COVID-19 pandemic and the associated need for social distancing, it is especially important that clinicians and health care providers across the country take steps to ensure that individuals with OUD can continue to receive evidence-based care,” said Paul H. Earley, MD, president of ASAM, in a press release announcing the new guideline.
The guideline specifies that home-based buprenorphine induction is safe and effective for treatment of opioid use disorder and that no individual entering the criminal justice system should be subjected to opioid withdrawal.
“The research is clear, providing methadone or buprenorphine, even without psychosocial treatment, reduces the patient’s risk of death,” said Kyle Kampman, MD, chair of the group’s Guideline Writing Committee, in the release. “Ultimately, keeping patients with the disease of addiction alive and engaged to become ready for recovery is absolutely critical in the context of the deadly overdose epidemic that has struck communities across our country.”
The society released this focused update to reflect new medications and formulations, published evidence, and clinical guidance related to treatment of OUD. This update includes the addition of 13 new recommendations and major revisions to 35 existing recommendations. One concern the society has is how to help patients being treated for OUD who are limited in their ability to leave their homes. Because of these same concerns, the Substance Abuse and Mental Health Services Administration relaxed regulations on March 16 regarding patient eligibility for take-home medications, such as buprenorphine and methadone, which dovetails with the society’s guidance regarding home-based induction.
, continuing on to pharmacologic treatment even if the patient declines recommended psychosocial treatment, keeping naloxone kits available in correctional facilities, and more. Additional information about this update can be found on ASAM’s website.
COVID-19 prompts ‘lifesaving’ policy change for opioid addiction
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
In the face of the US COVID-19 pandemic, the US Substance Abuse and Mental Health Services Administration (SAMHSA) has announced policy changes to allow some patients in opioid treatment programs (OTP) to take home their medication.
According to the agency, states may request “blanket exceptions” for all stable patients in an OTP to receive a 28-day supply of take-home doses of medications such as methadone and buprenorphine, which are used to treat opioid use disorder (OUD).
States may request up to 14 days of take-home medication for patients who are less stable but who can, in the judgment of OTP clinicians, safely handle this level of take-home medication.
“SAMHSA recognizes the evolving issues surrounding COVID-19 and the emerging needs OTPs continue to face,” the agency writes in its updated guidance.
“SAMHSA affirms its commitment to supporting OTPs in any way possible during this time. As such, we are expanding our previous guidance to provide increased flexibility,” the agency said.
A ‘Lifesaving’ Decision
Commenting on the SAMHSA policy change, Richard Saitz, MD, professor and chair of the department of community health sciences, Boston University School of Public Health, said, the policy “is not only a good idea, it is critical and lifesaving.”
“This approach had to be done now. With the reduction in face-to-face visits, patients with opioid use disorder need a way to access treatment. If they cannot get opioid agonists, they would withdraw and return to illicit opioid use and high overdose risk and it would be cruel,” said Saitz.
“It is possible that there will be some diversion and some risk of overdose or misuse, but even for less stable patients the benefit likely far outweighs the risk,” he told Medscape Medical News.
Saitz believes policy changes like this should have been made before a crisis.
“Honestly, this is perhaps a silver lining of the crisis” and could lead to permanent change in how OUD is treated in the US, he said.
“Just like we are learning what can be done without a medical in-person visit, we will learn that it is perfectly fine to treat patients with addiction more like we treat patients with other chronic diseases who take medication that has risks and benefits,” Saitz said.
in cases when a patient is quarantined because of coronavirus.
Typically, only licensed practitioners can dispense or administer OUD medications to patients, but during the COVID-19 crisis, treatment program staff members, law enforcement officers, and national guard personnel will be allowed to deliver OUD medications to an approved “lockbox” at the patient’s doorstep. The change applies only while the coronavirus public health emergency lasts.
“This is also an excellent idea,” Saitz said.
ASAM Also Responds
In addition, the American Society of Addiction Medicine (ASAM) released a focused update to its National Practice Guideline for the Treatment of Opioid Use Disorder (NPG).
The update is “especially critical in the context of the ongoing COVID-19 emergency, which threatens to curtail patient access to evidence-based treatment,” the organization said in a news release. The new document updates the 2015 NPG. It includes 13 new recommendations and major revisions to 35 existing recommendations.
One new recommendation states that comprehensive assessment of a patient is critical for treatment planning, but completing all assessments should not delay or preclude initiating pharmacotherapy for OUD. Another new recommendation states that there is no recommended time limit for pharmacotherapy.
ASAM continues to recommend that patients’ psychosocial needs be assessed and psychosocial treatment offered. However, if patients can’t access psychosocial treatment because they are in isolation or have other risk factors that preclude external interactions, clinicians should not delay initiation of medication for the treatment of addiction.
Expanding the use of telemedicine might also be appropriate for many patients, ASAM announced.
They note that the NPG is the first to address in a single document all medications currently approved by the US Food and Drug Administration to treat OUD and opioid withdrawal, including all available buprenorphine formulations.
“All of the updated recommendations are designed to both improve the quality and consistency of care and reduce barriers to access to care for Americans living with OUD. The updated recommendations aim to support initiation of buprenorphine treatment in the emergency department and other urgent care settings,” the society said in the release.
“In addition, [the recommendations] provide greater flexibility on dosing during the initiation of buprenorphine treatment and for initiation of buprenorphine at home (which is also an important change in the midst of the COVID-19 crisis).”
The full document is available online.
This article first appeared on Medscape.com.
Should physicians with OUDs return to practice after treatment?
New review points to importance of sustained recovery
A new article in the Journal of the Neurological Sciences provides an impressive review of research on the complex impairments produced by a wide range of drugs of abuse with a close look at physicians and other health care professionals.1
This review breaks new ground in outlining fitness for duty as an important outcome of the state physician health programs (PHPs). In addition, the review and case report by Alexandria G. Polles, MD, and colleagues are a response to the growing call for the state PHP system of care management to explicitly endorse the use of medication-assisted treatment, specifically the use of buprenorphine and methadone, in the treatment of physicians diagnosed with opioid use disorder (OUD). , because of the elevated rate of substance use disorders among physicians and the safety-sensitive nature of the practice of medicine.
Medication-assisted treatment (MAT)2 for opioid use disorders now dominates the field of treatment in terms of prescribing and also funding to address the opioid overdose crisis. MAT generally includes naltrexone and injectable naltrexone, though those antagonist medications have been used successfully for many decades by PHPs.3 However, to understand the controversy over the use of MAT in the care management of physicians first requires an understanding of state PHPs and how those programs oversee the care of physicians diagnosed with substance use disorders (SUDs), including OUDs.
A national blueprint study of PHPs showed that care begins with a formal diagnostic evaluation.4 Only when a diagnosis of an SUD is established is a physician referred to the attention of a state PHP, and a monitoring contract is signed. PHPs typically do not offer any direct treatment; instead, they manage the care of physician participants in programs in which the PHPs have confidence. Formal addiction treatment most often is 30 days of residential treatment, but many physicians receive intensive outpatient treatment.
After completing an episode of formal treatment, physicians are closely monitored, usually for 5 years, through random drug and alcohol tests, and work site monitors. They are required to engage in intensive recovery support, typically 12-step fellowships but also other alternative recovery support programs. Comorbid conditions, including mental health disorders, are also treated. Managing PHPs have no sanctions for noncompliance; however, importantly, they do offer a safe haven from state medical licensing boards for physicians who are compliant with their recommendations and who remain abstinent from any use of alcohol, marijuana, illicit drugs, or other nonmedical drug use.
The national blueprint study included 16 state PHPs and reviewed single episodes of PHP care for 908 physicians. Complete abstinence from any use of alcohol, marijuana, or other drugs was required of all physicians for monitoring periods of at least 5 years. During the extended period, 78% of the physicians did not have a single positive or missed test. Two-thirds of physicians who had one positive or missed test did not have a second. About a dozen publications have resulted from this national study, including an analysis of the roughly one-third of the physicians who were diagnosed with OUD.5
A sample of 702 PHP participants was grouped based on primary drug at intake: alcohol only, any opioid with or without alcohol, and nonopioid drugs. No significant differences were found among these groups in the percentage who completed PHP contracts, failed to complete their contract, or extended their contract and continued to be monitored. Only one physician received methadone to treat chronic pain. None received opioid agonists to treat their opioid use disorder. Opioid antagonist medication (naltrexone) was used for 40 physicians, or 5.7% of the total sample: 2 physicians (1%) from the alcohol-only group; 35 physicians (10.3%) from the any opioid group, and 3 physicians (1.9%) from nonopioid group.
The second fact that needs to be understood is that medical practice in relationship to SUDs is treated by state licensing boards as a safety-sensitive job, analogous to commercial airline pilots who have the Human Intervention Motivation Study (HIMS),6 which is their own care management program analogous to that of PHPs. A similar program exists for attorneys known as Commission on Lawyer Assistance Programs (CoLAP).7 Fitness for duty and prevention of harm are major concerns in occupations such as those of physicians, commercial truck drivers, and people working in the nuclear power industry, all of whom have similar safety protections requiring no drug use.
A third fact that deserves special attention is that the unique system of care management for physicians began in the early 1970s. It grew out of employee assistance programs, led then and often now by physicians who are themselves in recovery from SUDs. Many of the successful addiction treatment tools used today come from extensive research of their use in PHPs. Contingency management, 12 steps, caduceus recovery, cognitive-behavioral therapy, and treatment outcomes defined in years are examples in which PHP research helped change treatment and long-term management of SUDs in non-PHP populations.
Dr. Polles and colleagues provide an impressive and comprehensive summary of the issues involved in the new interest in providing the physicians with OUD under PHP care management the option of using buprenorphine or methadone. Such a model within an abstinence-based framework is now being pioneered by a variety of programs, from COAT8 at West Virginia University, Morgantown, to the Hazelden Betty Ford Foundation.9 In those programs, patients with OUD are offered the option of using buprenorphine, methadone, or naltrexone as well as the option of using none of those medications in an extended abstinence-based intensive treatment. The authors impressively and fairly summarize the evidence on whether there are cognitive or behavioral deficits associated with the therapeutic use of either buprenorphine or methadone, which might make them unacceptable for physicians. The strongest evidence that these medicines are not necessary in the treatment of OUDs in PHPs is the outstanding outcomes PHPs produce without use of these two medications. If skeptical of the use of medications for OUD treatment in PHP care management, Dr. Polles and colleagues are open to experiments to test the effects of this option just as Florida PHP programs pioneered contracts that included mandatory naltrexone.10 West Virginia University, the Hazelden Betty Ford Foundation, and other programs should be tested to evaluate just how safe, effective, and attractive such an option would be to physicians.
Many, if not most, SUD treatment programs that use MAT are not associated with the intensive psychological treatment or extended participation in recovery support, such as the 12-step fellowships. MAT is viewed as a harm reduction strategy rather than conceptualized as an abstinence-oriented treatment. For example, there is seldom a “sobriety date” among individuals in MAT, i.e., the last day the individual used any substance of abuse, including alcohol and marijuana. These are, however, central features of PHP care, and they are features of the Hazelden Betty Ford Foundation’s definition of recovery11 and use of MAT.
Dr. Polles and colleagues call attention to the unique care management of the PHP for all SUDs, not just for OUDs, because the PHPs set the standard for returning physicians to work who have the fitness and cognitive skills to first do no harm. They emphasize the importance of making sustained recovery the expected outcome of SUD treatment. There is a robust literature on the ways in which this distinctive system of care management shows the path forward for addiction treatment generally to regularly achieve 5-year recovery.12 The current controversy over the potential use of buprenorphine and buprenorphine plus naloxone in PHPs is a useful entry into this far larger issue of the potential for PHPs to show the path forward for the addiction treatment field.
Dr. DuPont, the first director of the National Institute on Drug Abuse (NIDA), is president of the Institute for Behavior and Health Inc., a nonprofit drug-policy research organization in Rockville, Md. He has no disclosures. Dr. Gold is professor of psychiatry (adjunct) at Washington University in St. Louis. He is also the 17th Distinguished Alumni Professor at the University of Florida Gainesville. He has no disclosures.
References
1. Polles AG et al. J Neurol Sci. 2020 Jan 30;411:116714.
2. Oesterle TS et al. Mayo Clin Proc. 2019 Oct;94(10):2072-86.
3. Srivastava AB and Gold MS. Cerebrum. 2018 Sep-Oct; cer-13-8.
4. DuPont RL et al. J Subst Abuse Treat. 2009 Mar 1;36(2):159-71.
5. Merlo LJ et al. J Subst Abuse Treat. 2016 May 1;64:47-54.
6. Human Intervention Motivation Study (HIMS): An Occupational Substance Abuse Treatment Program.
7. Commission on Lawyer Assistance Programs (CoLAP).
8. Lander LR et al. J Neurol Sci. 2020;411:116712-8.
9. Klein AA et al. J Subst Abuse Treat. 2019;104:51-63.
10. Merlo LJ et al. J Addict Med. 2012;5(4):279-83.
11. Betty Ford Consensus Panel. J Subst Abuse Treat. 2007 Oct;33(3):221-8.
12. Carr GD et al. “Physician health programs: The U.S. model.” In KJ Brower and MB Riba, (eds.) Physician Mental Health and Well-Being (pp. 265-94). Cham, Switzerland: Springer International Publishing, 2017.
New review points to importance of sustained recovery
New review points to importance of sustained recovery
A new article in the Journal of the Neurological Sciences provides an impressive review of research on the complex impairments produced by a wide range of drugs of abuse with a close look at physicians and other health care professionals.1
This review breaks new ground in outlining fitness for duty as an important outcome of the state physician health programs (PHPs). In addition, the review and case report by Alexandria G. Polles, MD, and colleagues are a response to the growing call for the state PHP system of care management to explicitly endorse the use of medication-assisted treatment, specifically the use of buprenorphine and methadone, in the treatment of physicians diagnosed with opioid use disorder (OUD). , because of the elevated rate of substance use disorders among physicians and the safety-sensitive nature of the practice of medicine.
Medication-assisted treatment (MAT)2 for opioid use disorders now dominates the field of treatment in terms of prescribing and also funding to address the opioid overdose crisis. MAT generally includes naltrexone and injectable naltrexone, though those antagonist medications have been used successfully for many decades by PHPs.3 However, to understand the controversy over the use of MAT in the care management of physicians first requires an understanding of state PHPs and how those programs oversee the care of physicians diagnosed with substance use disorders (SUDs), including OUDs.
A national blueprint study of PHPs showed that care begins with a formal diagnostic evaluation.4 Only when a diagnosis of an SUD is established is a physician referred to the attention of a state PHP, and a monitoring contract is signed. PHPs typically do not offer any direct treatment; instead, they manage the care of physician participants in programs in which the PHPs have confidence. Formal addiction treatment most often is 30 days of residential treatment, but many physicians receive intensive outpatient treatment.
After completing an episode of formal treatment, physicians are closely monitored, usually for 5 years, through random drug and alcohol tests, and work site monitors. They are required to engage in intensive recovery support, typically 12-step fellowships but also other alternative recovery support programs. Comorbid conditions, including mental health disorders, are also treated. Managing PHPs have no sanctions for noncompliance; however, importantly, they do offer a safe haven from state medical licensing boards for physicians who are compliant with their recommendations and who remain abstinent from any use of alcohol, marijuana, illicit drugs, or other nonmedical drug use.
The national blueprint study included 16 state PHPs and reviewed single episodes of PHP care for 908 physicians. Complete abstinence from any use of alcohol, marijuana, or other drugs was required of all physicians for monitoring periods of at least 5 years. During the extended period, 78% of the physicians did not have a single positive or missed test. Two-thirds of physicians who had one positive or missed test did not have a second. About a dozen publications have resulted from this national study, including an analysis of the roughly one-third of the physicians who were diagnosed with OUD.5
A sample of 702 PHP participants was grouped based on primary drug at intake: alcohol only, any opioid with or without alcohol, and nonopioid drugs. No significant differences were found among these groups in the percentage who completed PHP contracts, failed to complete their contract, or extended their contract and continued to be monitored. Only one physician received methadone to treat chronic pain. None received opioid agonists to treat their opioid use disorder. Opioid antagonist medication (naltrexone) was used for 40 physicians, or 5.7% of the total sample: 2 physicians (1%) from the alcohol-only group; 35 physicians (10.3%) from the any opioid group, and 3 physicians (1.9%) from nonopioid group.
The second fact that needs to be understood is that medical practice in relationship to SUDs is treated by state licensing boards as a safety-sensitive job, analogous to commercial airline pilots who have the Human Intervention Motivation Study (HIMS),6 which is their own care management program analogous to that of PHPs. A similar program exists for attorneys known as Commission on Lawyer Assistance Programs (CoLAP).7 Fitness for duty and prevention of harm are major concerns in occupations such as those of physicians, commercial truck drivers, and people working in the nuclear power industry, all of whom have similar safety protections requiring no drug use.
A third fact that deserves special attention is that the unique system of care management for physicians began in the early 1970s. It grew out of employee assistance programs, led then and often now by physicians who are themselves in recovery from SUDs. Many of the successful addiction treatment tools used today come from extensive research of their use in PHPs. Contingency management, 12 steps, caduceus recovery, cognitive-behavioral therapy, and treatment outcomes defined in years are examples in which PHP research helped change treatment and long-term management of SUDs in non-PHP populations.
Dr. Polles and colleagues provide an impressive and comprehensive summary of the issues involved in the new interest in providing the physicians with OUD under PHP care management the option of using buprenorphine or methadone. Such a model within an abstinence-based framework is now being pioneered by a variety of programs, from COAT8 at West Virginia University, Morgantown, to the Hazelden Betty Ford Foundation.9 In those programs, patients with OUD are offered the option of using buprenorphine, methadone, or naltrexone as well as the option of using none of those medications in an extended abstinence-based intensive treatment. The authors impressively and fairly summarize the evidence on whether there are cognitive or behavioral deficits associated with the therapeutic use of either buprenorphine or methadone, which might make them unacceptable for physicians. The strongest evidence that these medicines are not necessary in the treatment of OUDs in PHPs is the outstanding outcomes PHPs produce without use of these two medications. If skeptical of the use of medications for OUD treatment in PHP care management, Dr. Polles and colleagues are open to experiments to test the effects of this option just as Florida PHP programs pioneered contracts that included mandatory naltrexone.10 West Virginia University, the Hazelden Betty Ford Foundation, and other programs should be tested to evaluate just how safe, effective, and attractive such an option would be to physicians.
Many, if not most, SUD treatment programs that use MAT are not associated with the intensive psychological treatment or extended participation in recovery support, such as the 12-step fellowships. MAT is viewed as a harm reduction strategy rather than conceptualized as an abstinence-oriented treatment. For example, there is seldom a “sobriety date” among individuals in MAT, i.e., the last day the individual used any substance of abuse, including alcohol and marijuana. These are, however, central features of PHP care, and they are features of the Hazelden Betty Ford Foundation’s definition of recovery11 and use of MAT.
Dr. Polles and colleagues call attention to the unique care management of the PHP for all SUDs, not just for OUDs, because the PHPs set the standard for returning physicians to work who have the fitness and cognitive skills to first do no harm. They emphasize the importance of making sustained recovery the expected outcome of SUD treatment. There is a robust literature on the ways in which this distinctive system of care management shows the path forward for addiction treatment generally to regularly achieve 5-year recovery.12 The current controversy over the potential use of buprenorphine and buprenorphine plus naloxone in PHPs is a useful entry into this far larger issue of the potential for PHPs to show the path forward for the addiction treatment field.
Dr. DuPont, the first director of the National Institute on Drug Abuse (NIDA), is president of the Institute for Behavior and Health Inc., a nonprofit drug-policy research organization in Rockville, Md. He has no disclosures. Dr. Gold is professor of psychiatry (adjunct) at Washington University in St. Louis. He is also the 17th Distinguished Alumni Professor at the University of Florida Gainesville. He has no disclosures.
References
1. Polles AG et al. J Neurol Sci. 2020 Jan 30;411:116714.
2. Oesterle TS et al. Mayo Clin Proc. 2019 Oct;94(10):2072-86.
3. Srivastava AB and Gold MS. Cerebrum. 2018 Sep-Oct; cer-13-8.
4. DuPont RL et al. J Subst Abuse Treat. 2009 Mar 1;36(2):159-71.
5. Merlo LJ et al. J Subst Abuse Treat. 2016 May 1;64:47-54.
6. Human Intervention Motivation Study (HIMS): An Occupational Substance Abuse Treatment Program.
7. Commission on Lawyer Assistance Programs (CoLAP).
8. Lander LR et al. J Neurol Sci. 2020;411:116712-8.
9. Klein AA et al. J Subst Abuse Treat. 2019;104:51-63.
10. Merlo LJ et al. J Addict Med. 2012;5(4):279-83.
11. Betty Ford Consensus Panel. J Subst Abuse Treat. 2007 Oct;33(3):221-8.
12. Carr GD et al. “Physician health programs: The U.S. model.” In KJ Brower and MB Riba, (eds.) Physician Mental Health and Well-Being (pp. 265-94). Cham, Switzerland: Springer International Publishing, 2017.
A new article in the Journal of the Neurological Sciences provides an impressive review of research on the complex impairments produced by a wide range of drugs of abuse with a close look at physicians and other health care professionals.1
This review breaks new ground in outlining fitness for duty as an important outcome of the state physician health programs (PHPs). In addition, the review and case report by Alexandria G. Polles, MD, and colleagues are a response to the growing call for the state PHP system of care management to explicitly endorse the use of medication-assisted treatment, specifically the use of buprenorphine and methadone, in the treatment of physicians diagnosed with opioid use disorder (OUD). , because of the elevated rate of substance use disorders among physicians and the safety-sensitive nature of the practice of medicine.
Medication-assisted treatment (MAT)2 for opioid use disorders now dominates the field of treatment in terms of prescribing and also funding to address the opioid overdose crisis. MAT generally includes naltrexone and injectable naltrexone, though those antagonist medications have been used successfully for many decades by PHPs.3 However, to understand the controversy over the use of MAT in the care management of physicians first requires an understanding of state PHPs and how those programs oversee the care of physicians diagnosed with substance use disorders (SUDs), including OUDs.
A national blueprint study of PHPs showed that care begins with a formal diagnostic evaluation.4 Only when a diagnosis of an SUD is established is a physician referred to the attention of a state PHP, and a monitoring contract is signed. PHPs typically do not offer any direct treatment; instead, they manage the care of physician participants in programs in which the PHPs have confidence. Formal addiction treatment most often is 30 days of residential treatment, but many physicians receive intensive outpatient treatment.
After completing an episode of formal treatment, physicians are closely monitored, usually for 5 years, through random drug and alcohol tests, and work site monitors. They are required to engage in intensive recovery support, typically 12-step fellowships but also other alternative recovery support programs. Comorbid conditions, including mental health disorders, are also treated. Managing PHPs have no sanctions for noncompliance; however, importantly, they do offer a safe haven from state medical licensing boards for physicians who are compliant with their recommendations and who remain abstinent from any use of alcohol, marijuana, illicit drugs, or other nonmedical drug use.
The national blueprint study included 16 state PHPs and reviewed single episodes of PHP care for 908 physicians. Complete abstinence from any use of alcohol, marijuana, or other drugs was required of all physicians for monitoring periods of at least 5 years. During the extended period, 78% of the physicians did not have a single positive or missed test. Two-thirds of physicians who had one positive or missed test did not have a second. About a dozen publications have resulted from this national study, including an analysis of the roughly one-third of the physicians who were diagnosed with OUD.5
A sample of 702 PHP participants was grouped based on primary drug at intake: alcohol only, any opioid with or without alcohol, and nonopioid drugs. No significant differences were found among these groups in the percentage who completed PHP contracts, failed to complete their contract, or extended their contract and continued to be monitored. Only one physician received methadone to treat chronic pain. None received opioid agonists to treat their opioid use disorder. Opioid antagonist medication (naltrexone) was used for 40 physicians, or 5.7% of the total sample: 2 physicians (1%) from the alcohol-only group; 35 physicians (10.3%) from the any opioid group, and 3 physicians (1.9%) from nonopioid group.
The second fact that needs to be understood is that medical practice in relationship to SUDs is treated by state licensing boards as a safety-sensitive job, analogous to commercial airline pilots who have the Human Intervention Motivation Study (HIMS),6 which is their own care management program analogous to that of PHPs. A similar program exists for attorneys known as Commission on Lawyer Assistance Programs (CoLAP).7 Fitness for duty and prevention of harm are major concerns in occupations such as those of physicians, commercial truck drivers, and people working in the nuclear power industry, all of whom have similar safety protections requiring no drug use.
A third fact that deserves special attention is that the unique system of care management for physicians began in the early 1970s. It grew out of employee assistance programs, led then and often now by physicians who are themselves in recovery from SUDs. Many of the successful addiction treatment tools used today come from extensive research of their use in PHPs. Contingency management, 12 steps, caduceus recovery, cognitive-behavioral therapy, and treatment outcomes defined in years are examples in which PHP research helped change treatment and long-term management of SUDs in non-PHP populations.
Dr. Polles and colleagues provide an impressive and comprehensive summary of the issues involved in the new interest in providing the physicians with OUD under PHP care management the option of using buprenorphine or methadone. Such a model within an abstinence-based framework is now being pioneered by a variety of programs, from COAT8 at West Virginia University, Morgantown, to the Hazelden Betty Ford Foundation.9 In those programs, patients with OUD are offered the option of using buprenorphine, methadone, or naltrexone as well as the option of using none of those medications in an extended abstinence-based intensive treatment. The authors impressively and fairly summarize the evidence on whether there are cognitive or behavioral deficits associated with the therapeutic use of either buprenorphine or methadone, which might make them unacceptable for physicians. The strongest evidence that these medicines are not necessary in the treatment of OUDs in PHPs is the outstanding outcomes PHPs produce without use of these two medications. If skeptical of the use of medications for OUD treatment in PHP care management, Dr. Polles and colleagues are open to experiments to test the effects of this option just as Florida PHP programs pioneered contracts that included mandatory naltrexone.10 West Virginia University, the Hazelden Betty Ford Foundation, and other programs should be tested to evaluate just how safe, effective, and attractive such an option would be to physicians.
Many, if not most, SUD treatment programs that use MAT are not associated with the intensive psychological treatment or extended participation in recovery support, such as the 12-step fellowships. MAT is viewed as a harm reduction strategy rather than conceptualized as an abstinence-oriented treatment. For example, there is seldom a “sobriety date” among individuals in MAT, i.e., the last day the individual used any substance of abuse, including alcohol and marijuana. These are, however, central features of PHP care, and they are features of the Hazelden Betty Ford Foundation’s definition of recovery11 and use of MAT.
Dr. Polles and colleagues call attention to the unique care management of the PHP for all SUDs, not just for OUDs, because the PHPs set the standard for returning physicians to work who have the fitness and cognitive skills to first do no harm. They emphasize the importance of making sustained recovery the expected outcome of SUD treatment. There is a robust literature on the ways in which this distinctive system of care management shows the path forward for addiction treatment generally to regularly achieve 5-year recovery.12 The current controversy over the potential use of buprenorphine and buprenorphine plus naloxone in PHPs is a useful entry into this far larger issue of the potential for PHPs to show the path forward for the addiction treatment field.
Dr. DuPont, the first director of the National Institute on Drug Abuse (NIDA), is president of the Institute for Behavior and Health Inc., a nonprofit drug-policy research organization in Rockville, Md. He has no disclosures. Dr. Gold is professor of psychiatry (adjunct) at Washington University in St. Louis. He is also the 17th Distinguished Alumni Professor at the University of Florida Gainesville. He has no disclosures.
References
1. Polles AG et al. J Neurol Sci. 2020 Jan 30;411:116714.
2. Oesterle TS et al. Mayo Clin Proc. 2019 Oct;94(10):2072-86.
3. Srivastava AB and Gold MS. Cerebrum. 2018 Sep-Oct; cer-13-8.
4. DuPont RL et al. J Subst Abuse Treat. 2009 Mar 1;36(2):159-71.
5. Merlo LJ et al. J Subst Abuse Treat. 2016 May 1;64:47-54.
6. Human Intervention Motivation Study (HIMS): An Occupational Substance Abuse Treatment Program.
7. Commission on Lawyer Assistance Programs (CoLAP).
8. Lander LR et al. J Neurol Sci. 2020;411:116712-8.
9. Klein AA et al. J Subst Abuse Treat. 2019;104:51-63.
10. Merlo LJ et al. J Addict Med. 2012;5(4):279-83.
11. Betty Ford Consensus Panel. J Subst Abuse Treat. 2007 Oct;33(3):221-8.
12. Carr GD et al. “Physician health programs: The U.S. model.” In KJ Brower and MB Riba, (eds.) Physician Mental Health and Well-Being (pp. 265-94). Cham, Switzerland: Springer International Publishing, 2017.
What psychiatrists can do to prepare for the coming pandemic
Coronavirus fever is gripping the world. What I hope to do here is open a discussion of what psychiatrists and other clinicians can do to mitigate the psychological consequences of COVID-19. I am focusing on the right now.
The psychological consequences are fear of the disease, effects of possible quarantine, and the potential effects of the economic slowdown on the world economy.
Fear of the disease is gripping the nation. With invisible diseases, that is not irrational. If you do not know whether you are exposed and/or spreading it to coworkers, children, or aged parents, then the fear of contagion is logical. So I would not “poo-poo” the “worried well.” – especially if you have parents in nursing homes.
The quarantine issue is harder. I have long thought that quarantine would be harder to implement in the United States than in nations like China. But self or home quarantine is currently the de facto solution for those who have been exposed. What are some remedies?
For everybody, having an adequate supply of basic supplies at home is essential. As in preparing for a snowstorm or hurricane, adequate food, water, and yes, toilet paper, is important to relieve anxiety.
Psychiatrists can encourage patients to have an adequate supply of their medications. That may mean that we prescribe more pills. If the patient has suicidal tendencies, we can ask other family members to safeguard those medications.
A salient question is how likely people who are addicted to alcohol or opiates are to stay in place if they are withdrawing. In previous presentations, delivered some 20 years ago, I have (facetiously) suggested horse-drawn wagons of beer to avoid people breaking quarantine in search of the substances they are physically dependent on.
For people in methadone clinics who require daily visits that kind of approach may be harder. I do not have a solution, other than to plan for the eventuality of large-scale withdrawal and the behavioral consequences, which, unfortunately, often involve crime. Telemedicine may be a solution, but we are not yet equipped for it.
The longer-term psychological impacts of a major economic slowdown are not yet known. Based on past epidemics and other disasters, they might include unemployment and the related consequences of domestic violence and suicide.
COVID-19 is spreading fast. As clinicians, we must take steps to protect ourselves and our patients. Because this is a new virus, we have a lot to learn about it. We must be agile, because our actions will need to change over time.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington.
Coronavirus fever is gripping the world. What I hope to do here is open a discussion of what psychiatrists and other clinicians can do to mitigate the psychological consequences of COVID-19. I am focusing on the right now.
The psychological consequences are fear of the disease, effects of possible quarantine, and the potential effects of the economic slowdown on the world economy.
Fear of the disease is gripping the nation. With invisible diseases, that is not irrational. If you do not know whether you are exposed and/or spreading it to coworkers, children, or aged parents, then the fear of contagion is logical. So I would not “poo-poo” the “worried well.” – especially if you have parents in nursing homes.
The quarantine issue is harder. I have long thought that quarantine would be harder to implement in the United States than in nations like China. But self or home quarantine is currently the de facto solution for those who have been exposed. What are some remedies?
For everybody, having an adequate supply of basic supplies at home is essential. As in preparing for a snowstorm or hurricane, adequate food, water, and yes, toilet paper, is important to relieve anxiety.
Psychiatrists can encourage patients to have an adequate supply of their medications. That may mean that we prescribe more pills. If the patient has suicidal tendencies, we can ask other family members to safeguard those medications.
A salient question is how likely people who are addicted to alcohol or opiates are to stay in place if they are withdrawing. In previous presentations, delivered some 20 years ago, I have (facetiously) suggested horse-drawn wagons of beer to avoid people breaking quarantine in search of the substances they are physically dependent on.
For people in methadone clinics who require daily visits that kind of approach may be harder. I do not have a solution, other than to plan for the eventuality of large-scale withdrawal and the behavioral consequences, which, unfortunately, often involve crime. Telemedicine may be a solution, but we are not yet equipped for it.
The longer-term psychological impacts of a major economic slowdown are not yet known. Based on past epidemics and other disasters, they might include unemployment and the related consequences of domestic violence and suicide.
COVID-19 is spreading fast. As clinicians, we must take steps to protect ourselves and our patients. Because this is a new virus, we have a lot to learn about it. We must be agile, because our actions will need to change over time.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington.
Coronavirus fever is gripping the world. What I hope to do here is open a discussion of what psychiatrists and other clinicians can do to mitigate the psychological consequences of COVID-19. I am focusing on the right now.
The psychological consequences are fear of the disease, effects of possible quarantine, and the potential effects of the economic slowdown on the world economy.
Fear of the disease is gripping the nation. With invisible diseases, that is not irrational. If you do not know whether you are exposed and/or spreading it to coworkers, children, or aged parents, then the fear of contagion is logical. So I would not “poo-poo” the “worried well.” – especially if you have parents in nursing homes.
The quarantine issue is harder. I have long thought that quarantine would be harder to implement in the United States than in nations like China. But self or home quarantine is currently the de facto solution for those who have been exposed. What are some remedies?
For everybody, having an adequate supply of basic supplies at home is essential. As in preparing for a snowstorm or hurricane, adequate food, water, and yes, toilet paper, is important to relieve anxiety.
Psychiatrists can encourage patients to have an adequate supply of their medications. That may mean that we prescribe more pills. If the patient has suicidal tendencies, we can ask other family members to safeguard those medications.
A salient question is how likely people who are addicted to alcohol or opiates are to stay in place if they are withdrawing. In previous presentations, delivered some 20 years ago, I have (facetiously) suggested horse-drawn wagons of beer to avoid people breaking quarantine in search of the substances they are physically dependent on.
For people in methadone clinics who require daily visits that kind of approach may be harder. I do not have a solution, other than to plan for the eventuality of large-scale withdrawal and the behavioral consequences, which, unfortunately, often involve crime. Telemedicine may be a solution, but we are not yet equipped for it.
The longer-term psychological impacts of a major economic slowdown are not yet known. Based on past epidemics and other disasters, they might include unemployment and the related consequences of domestic violence and suicide.
COVID-19 is spreading fast. As clinicians, we must take steps to protect ourselves and our patients. Because this is a new virus, we have a lot to learn about it. We must be agile, because our actions will need to change over time.
Dr. Ritchie is chair of psychiatry at Medstar Washington Hospital Center and professor of psychiatry at Georgetown University, Washington.
A rarely discussed aspect of the opioid crisis
Your article, “A patient-centered approach to tapering opioids” (J Fam Pract. 2019;68:548-556) by Davis et al is the most thoughtful article I have seen on opioids. The patient-centered ap-proach takes this article to a place that is rarely discussed in the opioid crisis.
If we could really understand and treat chronic psychic and physical pain better, we might begin to have a real impact on this crisis. I completely agree that evidence-based intensive trauma treatment is generally unavailable in the United States. I have been working with women in a residential chemical dependency treatment program for the past 15 years and more than 90% of them were sexually abused. Trauma can lead to all forms of addiction, and trauma induced hyperalgesia is not the same as nociceptive pain.
We have so many unaddressed mental health issues in our country and your article emphasized the importance of understanding people and their mental health issues rather than taking a formulaic approach and replacing one opioid with another. It is clear to me that we will not win this battle with medication-assisted treatment alone.
Richard Usatine, MD
San Antonio, TX
Associate Editor, The Journal of Family Practice
Your article, “A patient-centered approach to tapering opioids” (J Fam Pract. 2019;68:548-556) by Davis et al is the most thoughtful article I have seen on opioids. The patient-centered ap-proach takes this article to a place that is rarely discussed in the opioid crisis.
If we could really understand and treat chronic psychic and physical pain better, we might begin to have a real impact on this crisis. I completely agree that evidence-based intensive trauma treatment is generally unavailable in the United States. I have been working with women in a residential chemical dependency treatment program for the past 15 years and more than 90% of them were sexually abused. Trauma can lead to all forms of addiction, and trauma induced hyperalgesia is not the same as nociceptive pain.
We have so many unaddressed mental health issues in our country and your article emphasized the importance of understanding people and their mental health issues rather than taking a formulaic approach and replacing one opioid with another. It is clear to me that we will not win this battle with medication-assisted treatment alone.
Richard Usatine, MD
San Antonio, TX
Associate Editor, The Journal of Family Practice
Your article, “A patient-centered approach to tapering opioids” (J Fam Pract. 2019;68:548-556) by Davis et al is the most thoughtful article I have seen on opioids. The patient-centered ap-proach takes this article to a place that is rarely discussed in the opioid crisis.
If we could really understand and treat chronic psychic and physical pain better, we might begin to have a real impact on this crisis. I completely agree that evidence-based intensive trauma treatment is generally unavailable in the United States. I have been working with women in a residential chemical dependency treatment program for the past 15 years and more than 90% of them were sexually abused. Trauma can lead to all forms of addiction, and trauma induced hyperalgesia is not the same as nociceptive pain.
We have so many unaddressed mental health issues in our country and your article emphasized the importance of understanding people and their mental health issues rather than taking a formulaic approach and replacing one opioid with another. It is clear to me that we will not win this battle with medication-assisted treatment alone.
Richard Usatine, MD
San Antonio, TX
Associate Editor, The Journal of Family Practice