User login
New York City inpatient detox unit keeps running: Here’s how
Substance use disorder and its daily consequences take no breaks even during a pandemic. The stressors created by COVID-19, including deaths of loved ones and the disruptions to normal life from policies aimed at flattening the curve, seem to have increased substance use.
I practice as a hospitalist with an internal medicine background and specialty in addiction medicine at BronxCare Health System’s inpatient detoxification unit, a 24/7, 20-bed medically-supervised unit in South Bronx in New York City. It is one of the comprehensive services provided by the BronxCare’s life recovery center and addiction services, which also includes an outpatient clinic, opioid treatment program, inpatient rehab, and a half-way house. Inpatient detoxification units like ours are designed to treat serious addictions and chemical dependency and prevent and treat life-threatening withdrawal symptoms and signs or complications. Our patients come from all over the city and its adjoining suburbs, including from emergency room referrals, referral clinics, courts and the justice system, walk-ins, and self-referrals.
At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service. In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.
Individuals with substance use disorder have historically been a vulnerable and underserved population and possess high risk for multiple health problems as well as preexisting conditions. Many have limited life options financially, educationally, and with housing, and encounter barriers to accessing primary health care services, including preventive services. The introduction of the COVID-19 pandemic into these patients’ precarious health situations only made things worse as many of the limited resources for patients with substance use disorder were diverted to battling the pandemic. Numerous inpatient and outpatient addiction services, for example, were temporarily shut down. This has led to an increase in domestic violence, and psychiatric decompensation, including psychosis, suicidal attempts, and worsening of medical comorbidities in these patients.
Our wake-up call came when the first case of COVID-19 was confirmed in New York in early March. Within a short period of time the state became the epicenter for COVID-19. With the projection of millions of cases being positive and the number of new cases doubling every third day at the onset in New York City, we knew we had a battle brewing and needed to radically transform our mode of operation fast.
Our first task was to ensure the safety of our patients and the dedicated health workers attending to them. We streamlined the patient point of entry through one screening site, while also brushing up on our history-taking to intently screen for COVID-19. This included not just focusing on travels from China, but from Europe and other parts of the world.
Yes, we did ask patients about cough, fever, shortness of breath or difficulty breathing, feeling fatigued, severe body ache, and possible contact with someone who is sick or has traveled overseas. But we were also attuned to the increased rate of community spread and the presentation of other symptoms, such as loss of taste and smell, early in the process. Hence we were able to triage patients with suspected cases to the appropriate sections of the hospital for further screening, testing, and evaluation, instead of having those patients admitted to the detox unit.
Early in the process a huddle team was instituted with daily briefing of staff lasting 30 minutes or less. This team consists of physicians, nurses, a physician assistant, a social worker, and a counselor. In addition to discussing treatment plans for the patient, they deliberate on the public health information from the hospital’s COVID-19 command center, New York State Department of Health, the Office of Mental Health, and the Centers for Disease Control and Prevention concerning the latest evidence-based information. These discussions have helped us modify our policies and practices.
We instituted a no visiting rule during a short hospital stay of 5-7 days, and this was initiated weeks in advance of many institutions, including nursing homes with vulnerable populations. Our admitting criteria was reviewed to allow for admission of only those patients who absolutely needed inpatient substance use disorder treatment, including patients with severe withdrawal symptoms and signs, comorbidities, or neuropsychiatric manifestations that made them unsafe for outpatient or home detoxification. Others were triaged to the outpatient services which was amply supported with telemedicine. Rooms and designated areas of the building were earmarked as places for isolation/quarantine if suspected COVID-19 cases were identified pending testing. To assess patients’ risk of COVID-19, we do point-of-care nasopharyngeal swab testing with polymerase chain reaction.
Regarding face masks, patients and staff were fitted with ones early in the process. Additionally, staff were trained on the importance of face mask use and how to ensure you have a tight seal around the mouth and nose and were provided with other appropriate personal protective equipment. Concerning social distancing, we reduced the patient population capacity for the unit down to 50% and offered only single room admissions. Social distancing was encouraged in the unit, including in the television and recreation room and dining room, and during small treatment groups of less than six individuals. Daily temperature checks with noncontact handheld thermometers were enforced for staff and anyone coming into the life recovery center.
Patients are continuously being educated on the presentations of COVID-19 and encouraged to report any symptoms. Any staff feeling sick or having symptoms are encouraged to stay home. Rigorous and continuous cleaning of surfaces, especially of areas subjected to common use, is done frequently by the hospital housekeeping and environmental crew and is the order of the day.
Dr. Fagbemi is a hospitalist at BronxCare Health System, a not-for-profit health and teaching hospital system serving South and Central Bronx in New York. He has no conflicts of interest to disclose.
Substance use disorder and its daily consequences take no breaks even during a pandemic. The stressors created by COVID-19, including deaths of loved ones and the disruptions to normal life from policies aimed at flattening the curve, seem to have increased substance use.
I practice as a hospitalist with an internal medicine background and specialty in addiction medicine at BronxCare Health System’s inpatient detoxification unit, a 24/7, 20-bed medically-supervised unit in South Bronx in New York City. It is one of the comprehensive services provided by the BronxCare’s life recovery center and addiction services, which also includes an outpatient clinic, opioid treatment program, inpatient rehab, and a half-way house. Inpatient detoxification units like ours are designed to treat serious addictions and chemical dependency and prevent and treat life-threatening withdrawal symptoms and signs or complications. Our patients come from all over the city and its adjoining suburbs, including from emergency room referrals, referral clinics, courts and the justice system, walk-ins, and self-referrals.
At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service. In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.
Individuals with substance use disorder have historically been a vulnerable and underserved population and possess high risk for multiple health problems as well as preexisting conditions. Many have limited life options financially, educationally, and with housing, and encounter barriers to accessing primary health care services, including preventive services. The introduction of the COVID-19 pandemic into these patients’ precarious health situations only made things worse as many of the limited resources for patients with substance use disorder were diverted to battling the pandemic. Numerous inpatient and outpatient addiction services, for example, were temporarily shut down. This has led to an increase in domestic violence, and psychiatric decompensation, including psychosis, suicidal attempts, and worsening of medical comorbidities in these patients.
Our wake-up call came when the first case of COVID-19 was confirmed in New York in early March. Within a short period of time the state became the epicenter for COVID-19. With the projection of millions of cases being positive and the number of new cases doubling every third day at the onset in New York City, we knew we had a battle brewing and needed to radically transform our mode of operation fast.
Our first task was to ensure the safety of our patients and the dedicated health workers attending to them. We streamlined the patient point of entry through one screening site, while also brushing up on our history-taking to intently screen for COVID-19. This included not just focusing on travels from China, but from Europe and other parts of the world.
Yes, we did ask patients about cough, fever, shortness of breath or difficulty breathing, feeling fatigued, severe body ache, and possible contact with someone who is sick or has traveled overseas. But we were also attuned to the increased rate of community spread and the presentation of other symptoms, such as loss of taste and smell, early in the process. Hence we were able to triage patients with suspected cases to the appropriate sections of the hospital for further screening, testing, and evaluation, instead of having those patients admitted to the detox unit.
Early in the process a huddle team was instituted with daily briefing of staff lasting 30 minutes or less. This team consists of physicians, nurses, a physician assistant, a social worker, and a counselor. In addition to discussing treatment plans for the patient, they deliberate on the public health information from the hospital’s COVID-19 command center, New York State Department of Health, the Office of Mental Health, and the Centers for Disease Control and Prevention concerning the latest evidence-based information. These discussions have helped us modify our policies and practices.
We instituted a no visiting rule during a short hospital stay of 5-7 days, and this was initiated weeks in advance of many institutions, including nursing homes with vulnerable populations. Our admitting criteria was reviewed to allow for admission of only those patients who absolutely needed inpatient substance use disorder treatment, including patients with severe withdrawal symptoms and signs, comorbidities, or neuropsychiatric manifestations that made them unsafe for outpatient or home detoxification. Others were triaged to the outpatient services which was amply supported with telemedicine. Rooms and designated areas of the building were earmarked as places for isolation/quarantine if suspected COVID-19 cases were identified pending testing. To assess patients’ risk of COVID-19, we do point-of-care nasopharyngeal swab testing with polymerase chain reaction.
Regarding face masks, patients and staff were fitted with ones early in the process. Additionally, staff were trained on the importance of face mask use and how to ensure you have a tight seal around the mouth and nose and were provided with other appropriate personal protective equipment. Concerning social distancing, we reduced the patient population capacity for the unit down to 50% and offered only single room admissions. Social distancing was encouraged in the unit, including in the television and recreation room and dining room, and during small treatment groups of less than six individuals. Daily temperature checks with noncontact handheld thermometers were enforced for staff and anyone coming into the life recovery center.
Patients are continuously being educated on the presentations of COVID-19 and encouraged to report any symptoms. Any staff feeling sick or having symptoms are encouraged to stay home. Rigorous and continuous cleaning of surfaces, especially of areas subjected to common use, is done frequently by the hospital housekeeping and environmental crew and is the order of the day.
Dr. Fagbemi is a hospitalist at BronxCare Health System, a not-for-profit health and teaching hospital system serving South and Central Bronx in New York. He has no conflicts of interest to disclose.
Substance use disorder and its daily consequences take no breaks even during a pandemic. The stressors created by COVID-19, including deaths of loved ones and the disruptions to normal life from policies aimed at flattening the curve, seem to have increased substance use.
I practice as a hospitalist with an internal medicine background and specialty in addiction medicine at BronxCare Health System’s inpatient detoxification unit, a 24/7, 20-bed medically-supervised unit in South Bronx in New York City. It is one of the comprehensive services provided by the BronxCare’s life recovery center and addiction services, which also includes an outpatient clinic, opioid treatment program, inpatient rehab, and a half-way house. Inpatient detoxification units like ours are designed to treat serious addictions and chemical dependency and prevent and treat life-threatening withdrawal symptoms and signs or complications. Our patients come from all over the city and its adjoining suburbs, including from emergency room referrals, referral clinics, courts and the justice system, walk-ins, and self-referrals.
At a time when many inpatient detoxification units within the city were temporarily closed due to fear of inpatient spread of the virus or to provide extra COVID beds in anticipation for the peak surge, we have been able to provide a needed service. In fact, several other inpatient detoxification programs within the city have been able to refer their patients to our facility.
Individuals with substance use disorder have historically been a vulnerable and underserved population and possess high risk for multiple health problems as well as preexisting conditions. Many have limited life options financially, educationally, and with housing, and encounter barriers to accessing primary health care services, including preventive services. The introduction of the COVID-19 pandemic into these patients’ precarious health situations only made things worse as many of the limited resources for patients with substance use disorder were diverted to battling the pandemic. Numerous inpatient and outpatient addiction services, for example, were temporarily shut down. This has led to an increase in domestic violence, and psychiatric decompensation, including psychosis, suicidal attempts, and worsening of medical comorbidities in these patients.
Our wake-up call came when the first case of COVID-19 was confirmed in New York in early March. Within a short period of time the state became the epicenter for COVID-19. With the projection of millions of cases being positive and the number of new cases doubling every third day at the onset in New York City, we knew we had a battle brewing and needed to radically transform our mode of operation fast.
Our first task was to ensure the safety of our patients and the dedicated health workers attending to them. We streamlined the patient point of entry through one screening site, while also brushing up on our history-taking to intently screen for COVID-19. This included not just focusing on travels from China, but from Europe and other parts of the world.
Yes, we did ask patients about cough, fever, shortness of breath or difficulty breathing, feeling fatigued, severe body ache, and possible contact with someone who is sick or has traveled overseas. But we were also attuned to the increased rate of community spread and the presentation of other symptoms, such as loss of taste and smell, early in the process. Hence we were able to triage patients with suspected cases to the appropriate sections of the hospital for further screening, testing, and evaluation, instead of having those patients admitted to the detox unit.
Early in the process a huddle team was instituted with daily briefing of staff lasting 30 minutes or less. This team consists of physicians, nurses, a physician assistant, a social worker, and a counselor. In addition to discussing treatment plans for the patient, they deliberate on the public health information from the hospital’s COVID-19 command center, New York State Department of Health, the Office of Mental Health, and the Centers for Disease Control and Prevention concerning the latest evidence-based information. These discussions have helped us modify our policies and practices.
We instituted a no visiting rule during a short hospital stay of 5-7 days, and this was initiated weeks in advance of many institutions, including nursing homes with vulnerable populations. Our admitting criteria was reviewed to allow for admission of only those patients who absolutely needed inpatient substance use disorder treatment, including patients with severe withdrawal symptoms and signs, comorbidities, or neuropsychiatric manifestations that made them unsafe for outpatient or home detoxification. Others were triaged to the outpatient services which was amply supported with telemedicine. Rooms and designated areas of the building were earmarked as places for isolation/quarantine if suspected COVID-19 cases were identified pending testing. To assess patients’ risk of COVID-19, we do point-of-care nasopharyngeal swab testing with polymerase chain reaction.
Regarding face masks, patients and staff were fitted with ones early in the process. Additionally, staff were trained on the importance of face mask use and how to ensure you have a tight seal around the mouth and nose and were provided with other appropriate personal protective equipment. Concerning social distancing, we reduced the patient population capacity for the unit down to 50% and offered only single room admissions. Social distancing was encouraged in the unit, including in the television and recreation room and dining room, and during small treatment groups of less than six individuals. Daily temperature checks with noncontact handheld thermometers were enforced for staff and anyone coming into the life recovery center.
Patients are continuously being educated on the presentations of COVID-19 and encouraged to report any symptoms. Any staff feeling sick or having symptoms are encouraged to stay home. Rigorous and continuous cleaning of surfaces, especially of areas subjected to common use, is done frequently by the hospital housekeeping and environmental crew and is the order of the day.
Dr. Fagbemi is a hospitalist at BronxCare Health System, a not-for-profit health and teaching hospital system serving South and Central Bronx in New York. He has no conflicts of interest to disclose.
Domestic abuse linked to cardiac disease, mortality in women
Adult female survivors of domestic abuse were at least one-third more likely to develop cardiovascular disease, type 2 diabetes mellitus, and all-cause mortality over a short follow-up period, although they did not face a higher risk of hypertension, a new British study finds.
The study, published in the Journal of the American Heart Association, provides more evidence of a link between domestic abuse and poor health, even in younger women.
“The prevalence of domestic abuse is vast, so any increased risk in cardiometabolic disease may translate into a large burden of potentially preventable illness in society,” said study lead author Joht Singh Chandan, PhD, MBBS, of the University of Birmingham (England) and University of Warwick in Coventry, England, in an interview.
The researchers retrospectively tracked primary care patients in the United Kingdom from 1995-2017. They compared 18,547 adult female survivors of domestic abuse with a group of 72,231 other women who were matched to them at baseline by age, body mass index, smoking status, and a measure known as the Townsend deprivation score.
The average age of women in the groups was 37 years plus or minus 13 in the domestic abuse group and 37 years plus or minus 12 in the unexposed group. In both groups, 45% of women smoked; women in the domestic abuse group were more likely to drink excessively (10%), compared with those in the unexposed group (4%).
Researchers followed the women in the domestic abuse group for an average of 2 years and the unexposed group for 3 years. Those in the domestic abuse group were more likely to fall out of the study because they transferred to other medical practices.
Over the study period, 181 women in the domestic abuse group and 644 women in the unexposed group developed cardiovascular disease outcomes (adjusted incidence rate ratio, 1.31; 95% confidence interval, 1.11-1.55; P = .001). They were also more likely to develop type 2 diabetes (adjusted IRR, 1.51; 95% CI, 1.30-1.76; P less than .001) and all-cause mortality (adjusted IRR, 1.44; 95% CI, 1.24-1.67; P less than.001). But there was no increased risk of hypertension (adjusted IRR, 0.99; 95% CI, 0.88-1.12; P = 0.873).
Why might exposure to domestic abuse boost cardiovascular risk? “Although our study was not able to answer exactly why this relationship exists, Dr. Chandan said. “These can be broadly put into three categories: adoption of poor lifestyle behaviors due to difficult circumstances (physical inactivity, poor diet, disrupted sleep, substance misuse and smoking); associated development of mental ill health; and the alteration of the immune, metabolic, neuroendocrine, and autonomic nervous system due to the impact of stress on the body.”
It’s not clear why the risk of hypertension may be an outlier among cardiovascular outcomes, Dr. Chandan said. However, he pointed to a similar study whose results hinted that survivors of emotional abuse may be more susceptible to a negative impact on hypertension (Ann Epidemiol. 2012 Aug;22[8]:562-7). The new study does not provide information about the type of abuse suffered by subjects.
Adrienne O’Neil, PhD, a family violence practitioner and cardiovascular epidemiologist at Deakin University in Geelong, Australia, said in an interview that the study is “a very useful contribution to the literature.” However, she cautioned that the study might have missed cases of domestic abuse because it relies on reports from primary care practitioners.
As for the findings, she said they’re surprising because of the divergence of major cardiovascular outcomes such as ischemic heart disease and stroke in groups of women with an average age of 37. “These differential health outcomes were observed over a 2-3 period. You probably wouldn’t expect to see a divergence in cardiovascular outcomes for 5-10 years in this age group.”
Dr. O’Neil said that, moving forward, the research can be helpful to understanding the rise of cardiovascular disease in women aged 35-54, especially in the United States. “The way we assess an individual’s risk of having a heart attack in the future is largely guided by evidence based on men. For a long time, this has neglected female-specific risk factors like polycystic ovary syndrome and hypertensive disorders of pregnancy but also conditions and exposures to which young women are especially vulnerable like depression, anxiety and [domestic abuse],” she said.
“This research is important as it gives us clues about who may be at elevated risk to help us guide prevention efforts. Equally, there is some evidence that chest pain presentation may be a useful predictor of domestic abuse victimization so there could be multiple lines of further inquiry.”
Dr. Chandan, the other study authors, and Dr. O’Neil reported no relevant disclosures.
SOURCE: Chandan JS et al. J Am Heart Assoc. 2020. doi: 10.1161/JAHA.119.014580.
Adult female survivors of domestic abuse were at least one-third more likely to develop cardiovascular disease, type 2 diabetes mellitus, and all-cause mortality over a short follow-up period, although they did not face a higher risk of hypertension, a new British study finds.
The study, published in the Journal of the American Heart Association, provides more evidence of a link between domestic abuse and poor health, even in younger women.
“The prevalence of domestic abuse is vast, so any increased risk in cardiometabolic disease may translate into a large burden of potentially preventable illness in society,” said study lead author Joht Singh Chandan, PhD, MBBS, of the University of Birmingham (England) and University of Warwick in Coventry, England, in an interview.
The researchers retrospectively tracked primary care patients in the United Kingdom from 1995-2017. They compared 18,547 adult female survivors of domestic abuse with a group of 72,231 other women who were matched to them at baseline by age, body mass index, smoking status, and a measure known as the Townsend deprivation score.
The average age of women in the groups was 37 years plus or minus 13 in the domestic abuse group and 37 years plus or minus 12 in the unexposed group. In both groups, 45% of women smoked; women in the domestic abuse group were more likely to drink excessively (10%), compared with those in the unexposed group (4%).
Researchers followed the women in the domestic abuse group for an average of 2 years and the unexposed group for 3 years. Those in the domestic abuse group were more likely to fall out of the study because they transferred to other medical practices.
Over the study period, 181 women in the domestic abuse group and 644 women in the unexposed group developed cardiovascular disease outcomes (adjusted incidence rate ratio, 1.31; 95% confidence interval, 1.11-1.55; P = .001). They were also more likely to develop type 2 diabetes (adjusted IRR, 1.51; 95% CI, 1.30-1.76; P less than .001) and all-cause mortality (adjusted IRR, 1.44; 95% CI, 1.24-1.67; P less than.001). But there was no increased risk of hypertension (adjusted IRR, 0.99; 95% CI, 0.88-1.12; P = 0.873).
Why might exposure to domestic abuse boost cardiovascular risk? “Although our study was not able to answer exactly why this relationship exists, Dr. Chandan said. “These can be broadly put into three categories: adoption of poor lifestyle behaviors due to difficult circumstances (physical inactivity, poor diet, disrupted sleep, substance misuse and smoking); associated development of mental ill health; and the alteration of the immune, metabolic, neuroendocrine, and autonomic nervous system due to the impact of stress on the body.”
It’s not clear why the risk of hypertension may be an outlier among cardiovascular outcomes, Dr. Chandan said. However, he pointed to a similar study whose results hinted that survivors of emotional abuse may be more susceptible to a negative impact on hypertension (Ann Epidemiol. 2012 Aug;22[8]:562-7). The new study does not provide information about the type of abuse suffered by subjects.
Adrienne O’Neil, PhD, a family violence practitioner and cardiovascular epidemiologist at Deakin University in Geelong, Australia, said in an interview that the study is “a very useful contribution to the literature.” However, she cautioned that the study might have missed cases of domestic abuse because it relies on reports from primary care practitioners.
As for the findings, she said they’re surprising because of the divergence of major cardiovascular outcomes such as ischemic heart disease and stroke in groups of women with an average age of 37. “These differential health outcomes were observed over a 2-3 period. You probably wouldn’t expect to see a divergence in cardiovascular outcomes for 5-10 years in this age group.”
Dr. O’Neil said that, moving forward, the research can be helpful to understanding the rise of cardiovascular disease in women aged 35-54, especially in the United States. “The way we assess an individual’s risk of having a heart attack in the future is largely guided by evidence based on men. For a long time, this has neglected female-specific risk factors like polycystic ovary syndrome and hypertensive disorders of pregnancy but also conditions and exposures to which young women are especially vulnerable like depression, anxiety and [domestic abuse],” she said.
“This research is important as it gives us clues about who may be at elevated risk to help us guide prevention efforts. Equally, there is some evidence that chest pain presentation may be a useful predictor of domestic abuse victimization so there could be multiple lines of further inquiry.”
Dr. Chandan, the other study authors, and Dr. O’Neil reported no relevant disclosures.
SOURCE: Chandan JS et al. J Am Heart Assoc. 2020. doi: 10.1161/JAHA.119.014580.
Adult female survivors of domestic abuse were at least one-third more likely to develop cardiovascular disease, type 2 diabetes mellitus, and all-cause mortality over a short follow-up period, although they did not face a higher risk of hypertension, a new British study finds.
The study, published in the Journal of the American Heart Association, provides more evidence of a link between domestic abuse and poor health, even in younger women.
“The prevalence of domestic abuse is vast, so any increased risk in cardiometabolic disease may translate into a large burden of potentially preventable illness in society,” said study lead author Joht Singh Chandan, PhD, MBBS, of the University of Birmingham (England) and University of Warwick in Coventry, England, in an interview.
The researchers retrospectively tracked primary care patients in the United Kingdom from 1995-2017. They compared 18,547 adult female survivors of domestic abuse with a group of 72,231 other women who were matched to them at baseline by age, body mass index, smoking status, and a measure known as the Townsend deprivation score.
The average age of women in the groups was 37 years plus or minus 13 in the domestic abuse group and 37 years plus or minus 12 in the unexposed group. In both groups, 45% of women smoked; women in the domestic abuse group were more likely to drink excessively (10%), compared with those in the unexposed group (4%).
Researchers followed the women in the domestic abuse group for an average of 2 years and the unexposed group for 3 years. Those in the domestic abuse group were more likely to fall out of the study because they transferred to other medical practices.
Over the study period, 181 women in the domestic abuse group and 644 women in the unexposed group developed cardiovascular disease outcomes (adjusted incidence rate ratio, 1.31; 95% confidence interval, 1.11-1.55; P = .001). They were also more likely to develop type 2 diabetes (adjusted IRR, 1.51; 95% CI, 1.30-1.76; P less than .001) and all-cause mortality (adjusted IRR, 1.44; 95% CI, 1.24-1.67; P less than.001). But there was no increased risk of hypertension (adjusted IRR, 0.99; 95% CI, 0.88-1.12; P = 0.873).
Why might exposure to domestic abuse boost cardiovascular risk? “Although our study was not able to answer exactly why this relationship exists, Dr. Chandan said. “These can be broadly put into three categories: adoption of poor lifestyle behaviors due to difficult circumstances (physical inactivity, poor diet, disrupted sleep, substance misuse and smoking); associated development of mental ill health; and the alteration of the immune, metabolic, neuroendocrine, and autonomic nervous system due to the impact of stress on the body.”
It’s not clear why the risk of hypertension may be an outlier among cardiovascular outcomes, Dr. Chandan said. However, he pointed to a similar study whose results hinted that survivors of emotional abuse may be more susceptible to a negative impact on hypertension (Ann Epidemiol. 2012 Aug;22[8]:562-7). The new study does not provide information about the type of abuse suffered by subjects.
Adrienne O’Neil, PhD, a family violence practitioner and cardiovascular epidemiologist at Deakin University in Geelong, Australia, said in an interview that the study is “a very useful contribution to the literature.” However, she cautioned that the study might have missed cases of domestic abuse because it relies on reports from primary care practitioners.
As for the findings, she said they’re surprising because of the divergence of major cardiovascular outcomes such as ischemic heart disease and stroke in groups of women with an average age of 37. “These differential health outcomes were observed over a 2-3 period. You probably wouldn’t expect to see a divergence in cardiovascular outcomes for 5-10 years in this age group.”
Dr. O’Neil said that, moving forward, the research can be helpful to understanding the rise of cardiovascular disease in women aged 35-54, especially in the United States. “The way we assess an individual’s risk of having a heart attack in the future is largely guided by evidence based on men. For a long time, this has neglected female-specific risk factors like polycystic ovary syndrome and hypertensive disorders of pregnancy but also conditions and exposures to which young women are especially vulnerable like depression, anxiety and [domestic abuse],” she said.
“This research is important as it gives us clues about who may be at elevated risk to help us guide prevention efforts. Equally, there is some evidence that chest pain presentation may be a useful predictor of domestic abuse victimization so there could be multiple lines of further inquiry.”
Dr. Chandan, the other study authors, and Dr. O’Neil reported no relevant disclosures.
SOURCE: Chandan JS et al. J Am Heart Assoc. 2020. doi: 10.1161/JAHA.119.014580.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Movement-based yoga ‘viable’ for depression in many mental disorders
Movement-based yoga appears to ease depressive symptoms in a wide range of mental health disorders, a new systematic review and meta-analysis suggest.
Results of the research, which included 19 studies and more than 1,000 patients with a variety of mental health diagnoses, showed that those who practiced yoga experienced greater reductions in depressive symptoms versus those undergoing no treatment, usual treatment, or attention-control exercises. In addition, there was a dose-dependent effect such that more weekly yoga sessions were associated with the greatest reduction in depressive symptoms.
“Once we reviewed all the existing science about the mental health benefits of movement-based yoga, we found that movement-based yoga – which is the same thing as postural yoga or asana – helped reduce symptoms of depression,” study investigator Jacinta Brinsley, BClinExPhys, of the University of South Australia, Adelaide, said in an interview.
“We also found those who practiced more frequently had bigger reductions. However, it didn’t matter how long the individual sessions were; what mattered was how many times per week people practiced,” she added.
The researchers noted that the study is the first to focus specifically on movement-based yoga.
“We excluded meditative forms of yoga, which have often been included in previous reviews, yielding mixed findings. The other thing we’ve done a bit differently is pool all the different diagnoses together and then look at depressive symptoms across them,” said Ms. Brinsley.
The study was published online May 18 in the British Journal of Sports Medicine.
Getting clarity
Depressive disorders are currently the world’s leading cause of disability, affecting more than 340 million people.
Most individuals who suffer from depressive disorders also experience a host of physical comorbidities including obesity, type 2 diabetes, metabolic syndrome, and cardiovascular disease.
Perhaps not surprisingly, physical inactivity is also associated with higher levels of depressive symptoms, which may be the reason some international organizations now recommend that physical activity be included as part of routine psychiatric care.
One potential form of exercise is yoga, which has become popular in Western culture, including among psychiatric patients. Although previous systematic reviews and meta-analyses have examined the effects of various yoga interventions on mental health, none has investigated the benefits of yoga across a range of psychiatric diagnoses.
What’s more, the authors of these reviews all urge caution when interpreting their results because of potential heterogeneity of the various yoga interventions, as well as poor methodological reporting.
“As an exercise physiologist, I prescribe evidence-based treatment,” said Ms. Brinsley. “I was interested in seeing if there’s evidence to support movement-based yoga in people who were struggling with mental health or who had a diagnosed mental illness.
“The [previous] findings are quite contradictory and there’s not a clear outcome in terms of intervention results, so we pooled the data and ran the meta-analysis, thinking it would be a great way to add some important evidence to the science,” she added.
To allow for a more comprehensive assessment of yoga’s potential mental health benefits, the investigators included a range of mental health diagnoses.
Dose-dependent effect
Studies were only included in the analysis if they were randomized, controlled trials with a yoga intervention that had a minimum of 50% physical activity during each session in adults with a recognized diagnosed mental disorder. Control conditions were defined as treatment as usual, wait list, or attention controls.
Two investigators independently scanned article titles and abstracts, and a final list of articles for the study was decided by consensus. Study quality was reported using the PEDro checklist; a random-effects meta-analysis was conducted using Comprehensive Meta-Analysis software.
A total of 3,880 records were identified and screened. The investigators assessed full-text versions of 80 articles, 19 of which (1,080 patients) were eligible for inclusion in the review.
Of these, nine studies included patients with a depressive disorder; five trials were in patients with a diagnosis of schizophrenia, three studies included patients with a diagnosis of PTSD, one study included patients diagnosed with alcohol dependence, and one study included patients with a range of psychiatric disorders.
Of the 1,080 patients included in the review, 578 were assigned to yoga and 502 to control conditions. Yoga practice involved a mixture of movement, breathing exercises, and/or mindfulness, but the movement component took up more than half of each session.
The yoga interventions lasted an average of 2.4 months (range, 1.5-2.5 months), with an average of 1.6 sessions per week (range, 1-3 sessions) that lasted an average of 60 minutes (range, 20-90 minutes).
Of the 19 studies (632 patients), 13 reported changes in depressive symptoms and were therefore included in the meta-analysis. The six studies excluded from the quantitative analysis did not report depression symptom scores.
With respect to primary outcomes, individuals who performed yoga showed a greater reduction in depressive symptoms, compared with the three control groups (standardized mean difference, –0.41; 95% CI, –0.65 to –0.17; P < .001).
Specific subgroup analyses showed a moderate effect of yoga on depressive symptoms, compared with wait-list controls (SMD, –0.58; P < .05), treatment as usual (SMD, –0.39; P = .31), and attention controls (SMD, –0.21; P = .22).
Subgroup analyses were also performed with respect to diagnostic category. These data showed a moderate effect of yoga on depressive symptoms in depressive disorders (SMD, –0.40; P < .01), no effect in PTSD (SMD, –0.01; P = .95), a nominal effect in alcohol use disorders (SMD, –0.24; P = .69), and a marked effect in schizophrenia (SMD, –0.90; P < .01).
Movement may be key
Researchers also performed a series of meta-regression analyses, which showed that the number of yoga sessions performed each week had a significant effect on depressive symptoms. Indeed, individuals with higher session frequencies demonstrated a greater improvement in symptoms (beta, –0.44; P < .001).
These findings, said Ms. Brinsley, suggest yoga may be a viable intervention for managing depressive symptoms in patients with a variety of mental disorders.
Based on these findings, along with other conventional forms of exercise.
Equally important was the finding that the number of weekly yoga sessions moderated the effect of depressive symptoms, as it may inform the future design of yoga interventions in patients with mental disorders.
With this in mind, the researchers recommended that such interventions should aim to increase the frequency or weekly sessions rather than the duration of each individual session or the overall duration of the intervention.
However, said Ms. Brinsley, these findings suggest it is the physical aspect of the yoga practice that may be key.
“Yoga comprises several different components, including the movement postures, the breathing component, and the mindfulness or meditative component, but in this meta-analysis we looked specifically at yoga that was at least 50% movement based. So it might have also included mindfulness and breathing, but it had to have the movement,” she said.
Don’t discount meditation
Commenting on the findings, Holger Cramer, MSc, PhD, DSc, who was not involved in the study, noted that the systematic review and meta-analysis builds on a number of previous reviews regarding the benefits of yoga for mental disorders.
“Surprisingly, the largest effect in this analysis was found in schizophrenia, even higher than in patients with depressive disorders,” said Dr. Cramer of the University of Duisburg-Essen (Germany). “This is in strong contradiction to what would otherwise be expected. As the authors point out, only about a quarter of all schizophrenia patients suffer from depression, so there should not be so much room for improvement.”
Dr. Cramer also advised against reducing yoga to simply a physical undertaking. “We have shown in our meta-analysis that those interventions focusing on meditation and/or breathing techniques are the most effective ones,” he added.
As such, he urged that breathing techniques be a part of yoga for treating depression in psychiatric disorders, though care should be taken in patients with PTSD, “since breath control might be perceived as unpleasant.”
For Ms. Brinsley, the findings help solidify yoga’s potential as a genuine treatment option for a variety of mental health patients suffering depressive symptoms.
“It’s about acknowledging that yoga can be a helpful part of treatment and can have a significant effect on mental health,” she noted.
At the same time, practitioners also need to acknowledge that patients suffering from mental health disorders may struggle with motivation when it comes to activities such as yoga.
“Engaging in a new activity can be particularly challenging if you’re struggling with mental health. Nevertheless, it’s important for people to have a choice and do something they enjoy. And yoga can be another tool in their toolbox for managing their mental health,” she said.
The study was funded by the U.K. National Institute for Health Research and Health Education England. Ms. Brinsley and Dr. Cramer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Movement-based yoga appears to ease depressive symptoms in a wide range of mental health disorders, a new systematic review and meta-analysis suggest.
Results of the research, which included 19 studies and more than 1,000 patients with a variety of mental health diagnoses, showed that those who practiced yoga experienced greater reductions in depressive symptoms versus those undergoing no treatment, usual treatment, or attention-control exercises. In addition, there was a dose-dependent effect such that more weekly yoga sessions were associated with the greatest reduction in depressive symptoms.
“Once we reviewed all the existing science about the mental health benefits of movement-based yoga, we found that movement-based yoga – which is the same thing as postural yoga or asana – helped reduce symptoms of depression,” study investigator Jacinta Brinsley, BClinExPhys, of the University of South Australia, Adelaide, said in an interview.
“We also found those who practiced more frequently had bigger reductions. However, it didn’t matter how long the individual sessions were; what mattered was how many times per week people practiced,” she added.
The researchers noted that the study is the first to focus specifically on movement-based yoga.
“We excluded meditative forms of yoga, which have often been included in previous reviews, yielding mixed findings. The other thing we’ve done a bit differently is pool all the different diagnoses together and then look at depressive symptoms across them,” said Ms. Brinsley.
The study was published online May 18 in the British Journal of Sports Medicine.
Getting clarity
Depressive disorders are currently the world’s leading cause of disability, affecting more than 340 million people.
Most individuals who suffer from depressive disorders also experience a host of physical comorbidities including obesity, type 2 diabetes, metabolic syndrome, and cardiovascular disease.
Perhaps not surprisingly, physical inactivity is also associated with higher levels of depressive symptoms, which may be the reason some international organizations now recommend that physical activity be included as part of routine psychiatric care.
One potential form of exercise is yoga, which has become popular in Western culture, including among psychiatric patients. Although previous systematic reviews and meta-analyses have examined the effects of various yoga interventions on mental health, none has investigated the benefits of yoga across a range of psychiatric diagnoses.
What’s more, the authors of these reviews all urge caution when interpreting their results because of potential heterogeneity of the various yoga interventions, as well as poor methodological reporting.
“As an exercise physiologist, I prescribe evidence-based treatment,” said Ms. Brinsley. “I was interested in seeing if there’s evidence to support movement-based yoga in people who were struggling with mental health or who had a diagnosed mental illness.
“The [previous] findings are quite contradictory and there’s not a clear outcome in terms of intervention results, so we pooled the data and ran the meta-analysis, thinking it would be a great way to add some important evidence to the science,” she added.
To allow for a more comprehensive assessment of yoga’s potential mental health benefits, the investigators included a range of mental health diagnoses.
Dose-dependent effect
Studies were only included in the analysis if they were randomized, controlled trials with a yoga intervention that had a minimum of 50% physical activity during each session in adults with a recognized diagnosed mental disorder. Control conditions were defined as treatment as usual, wait list, or attention controls.
Two investigators independently scanned article titles and abstracts, and a final list of articles for the study was decided by consensus. Study quality was reported using the PEDro checklist; a random-effects meta-analysis was conducted using Comprehensive Meta-Analysis software.
A total of 3,880 records were identified and screened. The investigators assessed full-text versions of 80 articles, 19 of which (1,080 patients) were eligible for inclusion in the review.
Of these, nine studies included patients with a depressive disorder; five trials were in patients with a diagnosis of schizophrenia, three studies included patients with a diagnosis of PTSD, one study included patients diagnosed with alcohol dependence, and one study included patients with a range of psychiatric disorders.
Of the 1,080 patients included in the review, 578 were assigned to yoga and 502 to control conditions. Yoga practice involved a mixture of movement, breathing exercises, and/or mindfulness, but the movement component took up more than half of each session.
The yoga interventions lasted an average of 2.4 months (range, 1.5-2.5 months), with an average of 1.6 sessions per week (range, 1-3 sessions) that lasted an average of 60 minutes (range, 20-90 minutes).
Of the 19 studies (632 patients), 13 reported changes in depressive symptoms and were therefore included in the meta-analysis. The six studies excluded from the quantitative analysis did not report depression symptom scores.
With respect to primary outcomes, individuals who performed yoga showed a greater reduction in depressive symptoms, compared with the three control groups (standardized mean difference, –0.41; 95% CI, –0.65 to –0.17; P < .001).
Specific subgroup analyses showed a moderate effect of yoga on depressive symptoms, compared with wait-list controls (SMD, –0.58; P < .05), treatment as usual (SMD, –0.39; P = .31), and attention controls (SMD, –0.21; P = .22).
Subgroup analyses were also performed with respect to diagnostic category. These data showed a moderate effect of yoga on depressive symptoms in depressive disorders (SMD, –0.40; P < .01), no effect in PTSD (SMD, –0.01; P = .95), a nominal effect in alcohol use disorders (SMD, –0.24; P = .69), and a marked effect in schizophrenia (SMD, –0.90; P < .01).
Movement may be key
Researchers also performed a series of meta-regression analyses, which showed that the number of yoga sessions performed each week had a significant effect on depressive symptoms. Indeed, individuals with higher session frequencies demonstrated a greater improvement in symptoms (beta, –0.44; P < .001).
These findings, said Ms. Brinsley, suggest yoga may be a viable intervention for managing depressive symptoms in patients with a variety of mental disorders.
Based on these findings, along with other conventional forms of exercise.
Equally important was the finding that the number of weekly yoga sessions moderated the effect of depressive symptoms, as it may inform the future design of yoga interventions in patients with mental disorders.
With this in mind, the researchers recommended that such interventions should aim to increase the frequency or weekly sessions rather than the duration of each individual session or the overall duration of the intervention.
However, said Ms. Brinsley, these findings suggest it is the physical aspect of the yoga practice that may be key.
“Yoga comprises several different components, including the movement postures, the breathing component, and the mindfulness or meditative component, but in this meta-analysis we looked specifically at yoga that was at least 50% movement based. So it might have also included mindfulness and breathing, but it had to have the movement,” she said.
Don’t discount meditation
Commenting on the findings, Holger Cramer, MSc, PhD, DSc, who was not involved in the study, noted that the systematic review and meta-analysis builds on a number of previous reviews regarding the benefits of yoga for mental disorders.
“Surprisingly, the largest effect in this analysis was found in schizophrenia, even higher than in patients with depressive disorders,” said Dr. Cramer of the University of Duisburg-Essen (Germany). “This is in strong contradiction to what would otherwise be expected. As the authors point out, only about a quarter of all schizophrenia patients suffer from depression, so there should not be so much room for improvement.”
Dr. Cramer also advised against reducing yoga to simply a physical undertaking. “We have shown in our meta-analysis that those interventions focusing on meditation and/or breathing techniques are the most effective ones,” he added.
As such, he urged that breathing techniques be a part of yoga for treating depression in psychiatric disorders, though care should be taken in patients with PTSD, “since breath control might be perceived as unpleasant.”
For Ms. Brinsley, the findings help solidify yoga’s potential as a genuine treatment option for a variety of mental health patients suffering depressive symptoms.
“It’s about acknowledging that yoga can be a helpful part of treatment and can have a significant effect on mental health,” she noted.
At the same time, practitioners also need to acknowledge that patients suffering from mental health disorders may struggle with motivation when it comes to activities such as yoga.
“Engaging in a new activity can be particularly challenging if you’re struggling with mental health. Nevertheless, it’s important for people to have a choice and do something they enjoy. And yoga can be another tool in their toolbox for managing their mental health,” she said.
The study was funded by the U.K. National Institute for Health Research and Health Education England. Ms. Brinsley and Dr. Cramer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Movement-based yoga appears to ease depressive symptoms in a wide range of mental health disorders, a new systematic review and meta-analysis suggest.
Results of the research, which included 19 studies and more than 1,000 patients with a variety of mental health diagnoses, showed that those who practiced yoga experienced greater reductions in depressive symptoms versus those undergoing no treatment, usual treatment, or attention-control exercises. In addition, there was a dose-dependent effect such that more weekly yoga sessions were associated with the greatest reduction in depressive symptoms.
“Once we reviewed all the existing science about the mental health benefits of movement-based yoga, we found that movement-based yoga – which is the same thing as postural yoga or asana – helped reduce symptoms of depression,” study investigator Jacinta Brinsley, BClinExPhys, of the University of South Australia, Adelaide, said in an interview.
“We also found those who practiced more frequently had bigger reductions. However, it didn’t matter how long the individual sessions were; what mattered was how many times per week people practiced,” she added.
The researchers noted that the study is the first to focus specifically on movement-based yoga.
“We excluded meditative forms of yoga, which have often been included in previous reviews, yielding mixed findings. The other thing we’ve done a bit differently is pool all the different diagnoses together and then look at depressive symptoms across them,” said Ms. Brinsley.
The study was published online May 18 in the British Journal of Sports Medicine.
Getting clarity
Depressive disorders are currently the world’s leading cause of disability, affecting more than 340 million people.
Most individuals who suffer from depressive disorders also experience a host of physical comorbidities including obesity, type 2 diabetes, metabolic syndrome, and cardiovascular disease.
Perhaps not surprisingly, physical inactivity is also associated with higher levels of depressive symptoms, which may be the reason some international organizations now recommend that physical activity be included as part of routine psychiatric care.
One potential form of exercise is yoga, which has become popular in Western culture, including among psychiatric patients. Although previous systematic reviews and meta-analyses have examined the effects of various yoga interventions on mental health, none has investigated the benefits of yoga across a range of psychiatric diagnoses.
What’s more, the authors of these reviews all urge caution when interpreting their results because of potential heterogeneity of the various yoga interventions, as well as poor methodological reporting.
“As an exercise physiologist, I prescribe evidence-based treatment,” said Ms. Brinsley. “I was interested in seeing if there’s evidence to support movement-based yoga in people who were struggling with mental health or who had a diagnosed mental illness.
“The [previous] findings are quite contradictory and there’s not a clear outcome in terms of intervention results, so we pooled the data and ran the meta-analysis, thinking it would be a great way to add some important evidence to the science,” she added.
To allow for a more comprehensive assessment of yoga’s potential mental health benefits, the investigators included a range of mental health diagnoses.
Dose-dependent effect
Studies were only included in the analysis if they were randomized, controlled trials with a yoga intervention that had a minimum of 50% physical activity during each session in adults with a recognized diagnosed mental disorder. Control conditions were defined as treatment as usual, wait list, or attention controls.
Two investigators independently scanned article titles and abstracts, and a final list of articles for the study was decided by consensus. Study quality was reported using the PEDro checklist; a random-effects meta-analysis was conducted using Comprehensive Meta-Analysis software.
A total of 3,880 records were identified and screened. The investigators assessed full-text versions of 80 articles, 19 of which (1,080 patients) were eligible for inclusion in the review.
Of these, nine studies included patients with a depressive disorder; five trials were in patients with a diagnosis of schizophrenia, three studies included patients with a diagnosis of PTSD, one study included patients diagnosed with alcohol dependence, and one study included patients with a range of psychiatric disorders.
Of the 1,080 patients included in the review, 578 were assigned to yoga and 502 to control conditions. Yoga practice involved a mixture of movement, breathing exercises, and/or mindfulness, but the movement component took up more than half of each session.
The yoga interventions lasted an average of 2.4 months (range, 1.5-2.5 months), with an average of 1.6 sessions per week (range, 1-3 sessions) that lasted an average of 60 minutes (range, 20-90 minutes).
Of the 19 studies (632 patients), 13 reported changes in depressive symptoms and were therefore included in the meta-analysis. The six studies excluded from the quantitative analysis did not report depression symptom scores.
With respect to primary outcomes, individuals who performed yoga showed a greater reduction in depressive symptoms, compared with the three control groups (standardized mean difference, –0.41; 95% CI, –0.65 to –0.17; P < .001).
Specific subgroup analyses showed a moderate effect of yoga on depressive symptoms, compared with wait-list controls (SMD, –0.58; P < .05), treatment as usual (SMD, –0.39; P = .31), and attention controls (SMD, –0.21; P = .22).
Subgroup analyses were also performed with respect to diagnostic category. These data showed a moderate effect of yoga on depressive symptoms in depressive disorders (SMD, –0.40; P < .01), no effect in PTSD (SMD, –0.01; P = .95), a nominal effect in alcohol use disorders (SMD, –0.24; P = .69), and a marked effect in schizophrenia (SMD, –0.90; P < .01).
Movement may be key
Researchers also performed a series of meta-regression analyses, which showed that the number of yoga sessions performed each week had a significant effect on depressive symptoms. Indeed, individuals with higher session frequencies demonstrated a greater improvement in symptoms (beta, –0.44; P < .001).
These findings, said Ms. Brinsley, suggest yoga may be a viable intervention for managing depressive symptoms in patients with a variety of mental disorders.
Based on these findings, along with other conventional forms of exercise.
Equally important was the finding that the number of weekly yoga sessions moderated the effect of depressive symptoms, as it may inform the future design of yoga interventions in patients with mental disorders.
With this in mind, the researchers recommended that such interventions should aim to increase the frequency or weekly sessions rather than the duration of each individual session or the overall duration of the intervention.
However, said Ms. Brinsley, these findings suggest it is the physical aspect of the yoga practice that may be key.
“Yoga comprises several different components, including the movement postures, the breathing component, and the mindfulness or meditative component, but in this meta-analysis we looked specifically at yoga that was at least 50% movement based. So it might have also included mindfulness and breathing, but it had to have the movement,” she said.
Don’t discount meditation
Commenting on the findings, Holger Cramer, MSc, PhD, DSc, who was not involved in the study, noted that the systematic review and meta-analysis builds on a number of previous reviews regarding the benefits of yoga for mental disorders.
“Surprisingly, the largest effect in this analysis was found in schizophrenia, even higher than in patients with depressive disorders,” said Dr. Cramer of the University of Duisburg-Essen (Germany). “This is in strong contradiction to what would otherwise be expected. As the authors point out, only about a quarter of all schizophrenia patients suffer from depression, so there should not be so much room for improvement.”
Dr. Cramer also advised against reducing yoga to simply a physical undertaking. “We have shown in our meta-analysis that those interventions focusing on meditation and/or breathing techniques are the most effective ones,” he added.
As such, he urged that breathing techniques be a part of yoga for treating depression in psychiatric disorders, though care should be taken in patients with PTSD, “since breath control might be perceived as unpleasant.”
For Ms. Brinsley, the findings help solidify yoga’s potential as a genuine treatment option for a variety of mental health patients suffering depressive symptoms.
“It’s about acknowledging that yoga can be a helpful part of treatment and can have a significant effect on mental health,” she noted.
At the same time, practitioners also need to acknowledge that patients suffering from mental health disorders may struggle with motivation when it comes to activities such as yoga.
“Engaging in a new activity can be particularly challenging if you’re struggling with mental health. Nevertheless, it’s important for people to have a choice and do something they enjoy. And yoga can be another tool in their toolbox for managing their mental health,” she said.
The study was funded by the U.K. National Institute for Health Research and Health Education England. Ms. Brinsley and Dr. Cramer have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Cannabidiol for psychosis: A review of 4 studies
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7
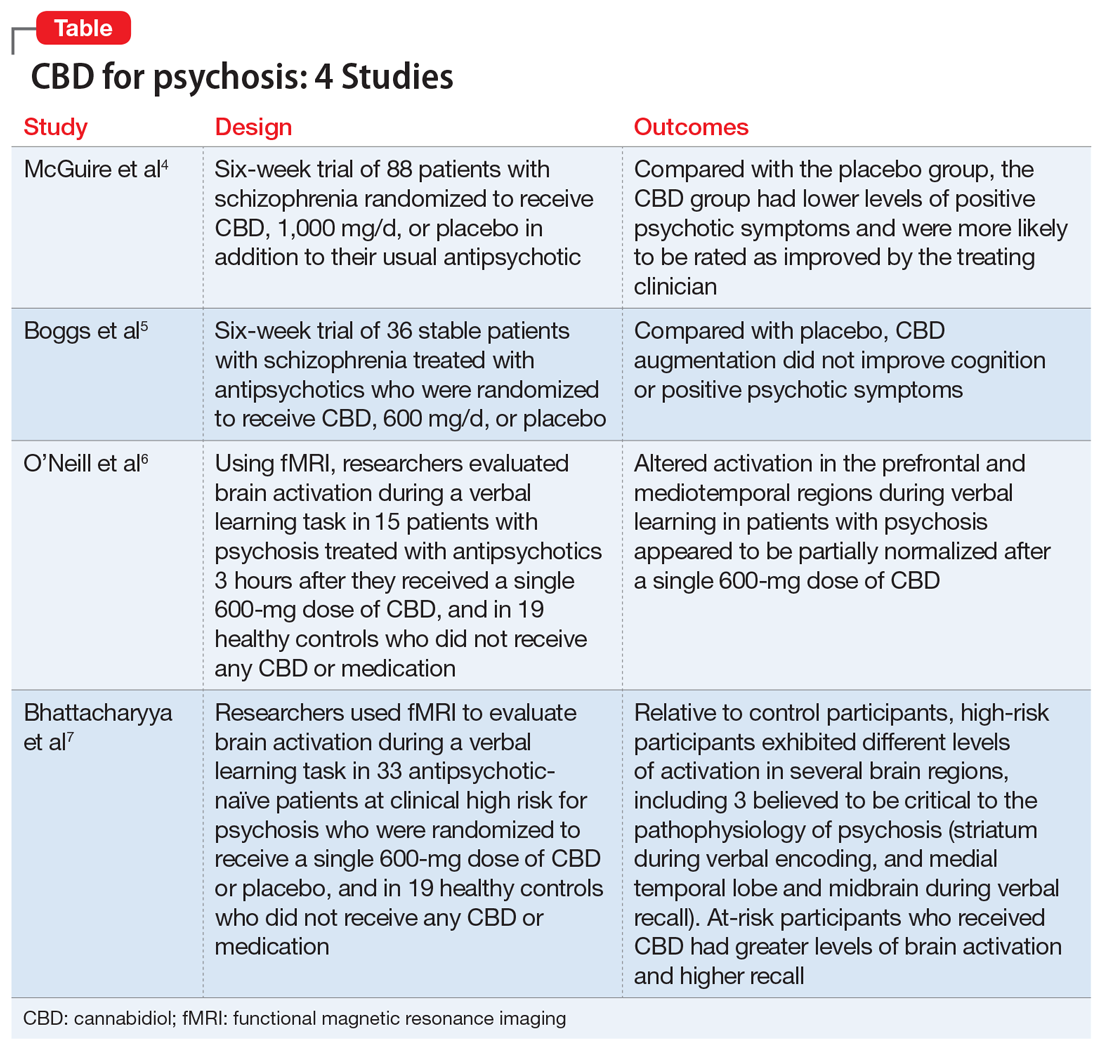
Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7

Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
There has been increasing interest in the medicinal use of cannabidiol (CBD) for a wide variety of health conditions. CBD is one of more than 80 chemicals identified in the Cannabis sativa plant, otherwise known as marijuana or hemp. Delta-9-tetrahydrocannabinol (THC) is the psychoactive ingredient found in marijuana that produces a “high.” CBD, which is one of the most abundant cannabinoids in Cannabis sativa, does not produce any psychotomimetic effects.
The strongest scientific evidence supporting CBD for medicinal purposes is for its effectiveness in treating certain childhood epilepsy syndromes that typically do not respond to antiseizure medications. Currently, the only FDA-approved CBD product is a prescription oil cannabidiol (brand name: Epidiolex) for treating 2 types of epilepsy. Aside from Epidiolex, state laws governing the use of CBD vary. CBD is being studied as a treatment for a wide range of psychiatric conditions, including bipolar disorder, schizophrenia, dystonia, insomnia, and anxiety. Research supporting CBD’s benefits is limited, and the US National Library of Medicine’s MedlinePlus indicates there is “insufficient evidence to rate effectiveness” for these indications.1
Despite having been legalized for medicinal use in many states, CBD is classified as a Schedule I controlled substance by the US Drug Enforcement Agency. Because of this classification, little has been done to regulate and oversee the sale of products containing CBD. In a 2017 study of 84 CBD products sold by 31 companies online, Bonn-Miller et al2 found that nearly 70% percent of products were inaccurately labeled. In this study, blind testing found that only approximately 31% of products contained within 10% of the amount of CBD that was listed on the label. These researchers also found that some products contained components not listed on the label, including THC.2
The relationship between cannabis and psychosis or psychotic symptoms has been investigated for decades. Some recent studies that examined the effects of CBD on psychosis found that individuals who use CBD may experience fewer positive psychotic symptoms compared with placebo. This raises the question of whether CBD may have a role in the treatment of schizophrenia and other psychotic disorders. One of the first studies on this issue was conducted by Leweke et al,3 who compared oral CBD, up to 800 mg/d, with the antipsychotic amisulpride, up to 800 mg/d, in 39 patients with an acute exacerbation of psychotic symptoms. Amisulpride is used outside the United States to treat psychosis, but is FDA-approved only as an antiemetic. Patients were treated for 4 weeks. By Day 28, there was a significant reduction in positive symptoms as measured using the Positive and Negative Syndrome Scale (PANSS), with no significant difference in efficacy between the treatments. Similar findings emerged for negative, total, and general symptoms, with significant reductions by Day 28 in both treatment arms, and no significant between-treatment differences.
These findings were the first robust indication that CBD may have antipsychotic efficacy. However, of greater interest may be CBD’s markedly superior adverse effect profile. Predictably, amisulpride significantly increased extrapyramidal symptoms (EPS), weight gain, and prolactin levels from baseline to Day 28. However, no significant change was found in any of these adverse effects in the CBD group, and the between-treatment difference was significant (all P < .01).
Here we review 4 recent studies that evaluated CBD as a treatment for schizophrenia. These studies are summarized in the Table.4-7

Continue to: McGuire P, et al...
1. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
Antipsychotic medications act through blockade of central dopamine D2 receptors. For most patients, antipsychotics effectively treat positive psychotic symptoms, which are driven by elevated dopamine function. However, these medications have minimal effects on negative symptoms and cognitive impairment, features of schizophrenia that are not driven by elevated dopamine. Compounds exhibiting a mechanism of action unlike that of current antipsychotics may improve the treatment and outcomes of patients with schizophrenia. The mechanism of action of CBD is unclear, but it does not appear to involve the direct antagonism of dopamine receptors. Human and animal research study findings indicate that CBD has antipsychotic properties. McGuire et al4 assessed the safety and effectiveness of CBD as an adjunctive treatment of schizophrenia.
Study design
- In this double-blind parallel-group trial conducted at 15 hospitals in the United Kingdom, Romania, and Poland, 88 patients with schizophrenia received CBD (1,000 mg/d; N = 43) or placebo (N = 45) as adjunct to the antipsychotic medication they had been prescribed. Patients had previously demonstrated at least a partial response to antipsychotic treatment, and were taking stable doses of an antipsychotic for ≥4 weeks.
- Evaluations of symptoms, general functioning, cognitive performance, and EPS were completed at baseline and on Days 8, 22, and 43 (± 3 days). Current substance use was assessed using a semi-structured interview, and reassessed at the end of treatment.
- The key endpoints were the patients’ level of functioning, severity of symptoms, and cognitive performance. Participants were assessed before and after treatment using the PANSS, the Brief Assessment of Cognition in Schizophrenia (BACS), the Global Assessment of Functioning scale (GAF), and the improvement and severity scales of the Clinical Global Impressions Scale (CGI-I and CGI-S, respectively).
- The clinicians’ impression of illness severity and symptom improvement and patient- or caregiver-reported impressions of general functioning and sleep also were noted.
Outcomes
- After 6 weeks, compared with the placebo group, the CBD group had lower levels of positive psychotic symptoms and were more likely to be rated as improved and as not severely unwell by the treating clinician. Patients in the CBD group also showed greater improvements in cognitive performance and in overall functioning, although these were not statistically significant.
- Similar levels of negative psychotic symptoms, overall psychopathology, and general psychopathology were observed in the CBD and placebo groups. The CBD group had a higher proportion of treatment responders (≥20% improvement in PANSS total score) than did the placebo group; however, the total number of responders per group was small (12 and 6 patients, respectively). At baseline, most patients in both groups were classified as moderately, markedly, or severely ill (83.4% in the CBD group vs 79.6% in placebo group). By the end of treatment, this decreased to 54.8% in the CBD group and 63.6% in the placebo group. Clinicians rated 78.6% of patients in the CBD group as “improved” on the CGI-I, compared with 54.6% of patients in the placebo group.
Conclusion
- CBD treatment adjunctive to antipsychotics was associated with significant effects on positive psychotic symptoms and on CGI-I and illness severity. Improvements in cognitive performance and level of overall functioning were also seen, but were not statistically significant.
- Although the effect on positive symptoms was modest, improvement occurred in patients being treated with appropriate dosages of antipsychotics, which suggests CBD provided benefits over and above the effect of antipsychotic treatment. Moreover, the changes in CGI-I and CGI-S scores indicated that the improvement was evident to the treating psychiatrists, and may therefore be clinically meaningful.
Continue to: Boggs DL, et al...
2. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
Schizophrenia is associated with cognitive deficits in learning, recall, attention, working memory, and executive function. The cognitive impairments associated with schizophrenia (CIAS) are independent of phase of illness and often persist after other symptoms have been effectively treated. These impairments are the strongest predictor of functional outcome, even more so than psychotic symptoms.
Antipsychotics have limited efficacy for CIAS, which highlights the need for CIAS treatments that target other nondopaminergic neurotransmitter systems. The endocannabinoid system, which has been implicated in schizophrenia and in cognition, is a potential target. Several cannabinoids impair memory and attention. The main psychoactive component of marijuana, THC, is a cannabinoid receptor type 1 (CB1R) partial agonist. Administration of THC produces significant deficits in verbal learning, attention, and working memory.
Researchers have hypothesized that CB1R blockade or modulation of cannabinoid levels may offer a novel target for treating CIAS. Boggs et al5 compared the cognitive, symptomatic, and adverse effects of CBD vs placebo.
Study design
- In this 6-week, randomized, placebo-controlled study conducted in Connecticut from September 2009 to May 2012, 36 stable patients with schizophrenia who were treated with antipsychotics were randomized to also receive oral CBD, 600 mg/d, or placebo.
- Cognition was assessed using the t score of the MATRICS Consensus Cognitive Battery (MCCB) composite and subscales at baseline and the end of study. An increase in MCCB t score indicates an improvement in cognitive ability. Psychotic symptoms were assessed using the PANSS at baseline, Week 2, Week 4, and Week 6.
Outcomes
- CBD augmentation did not improve MCCB performance or psychotic symptoms. There was no main effect of time or medication on MCCB composite score, but a significant drug × time effect was observed.
- Post-hoc analyses revealed that only patients who received placebo improved over time. The lack of a similar improvement with CBD might be related to the greater incidence of sedation among the CBD group (20%) vs the placebo group (5%). Both the MCCB composite score and reasoning and problem-solving domain scores were higher at baseline and endpoint for patients who received CBD, which suggests that the observed improvement in the placebo group could represent a regression to the mean.
- There was a significant decrease in PANSS scores over time, but there was no significant drug × time interaction.
Conclusion
- CBD augmentation was not associated with an improvement in MCCB score. This is consistent with data from other clinical trials4,8 that suggested that CBD (at a wide range of doses) does not have significant beneficial effects on cognition in patients with schizophrenia.
- Additionally, CBD did not improve psychotic symptoms. These results are in contrast to published case reports9,10 and 2 published clinical trials3,4 that found CBD (800 mg/d) was as efficacious as amisulpride in reducing positive psychotic symptoms, and a small but statistically significant improvement in PANSS positive scores with CBD (1,000 mg/d) compared with placebo. However, these results are similar to those of a separate study11 that evaluated the same 600-mg/d dose of CBD used by Boggs et al.5 At 600 mg/d, CBD produced very small improvements in PANSS total scores (~2.4) that were not statistically significant. A higher CBD dose may be needed to reduce psychotic symptoms in patients with schizophrenia.
Continue to: O’Neill A, et al...
3. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
In addition to their key roles in the psychopathology of psychosis, the mediotemporal and prefrontal cortices are involved in learning and memory, and the striatum plays a role in encoding contextual information associated with memories. Because deficits in verbal learning and memory are one of the most commonly reported impairments in patients with psychosis, O’Neill et al6 used functional MRI (fMRI) to examine brain activity during a verbal learning task in patients with psychosis after taking CBD or placebo.
Study design
- In a double-blind, randomized, placebo-controlled, crossover study, researchers investigated the effects of a single dose of CBD in 15 patients with psychosis who were treated with antipsychotics. Three hours after taking a 600-mg dose of CBD or placebo, these participants were scanned using fMRI while performing a verbal paired associate (VPA) learning task. Nineteen healthy controls underwent fMRI in identical conditions, but without any medication administration.
- The fMRI measured brain activation using the blood oxygen level–dependent (BOLD) hemodynamic responses of the brain. The fMRI signals were studied in the mediotemporal, prefrontal, and striatal regions.
- The VPA task presented word pairs visually, and the accuracy of responses were recorded online. The VPA task was comprised of 3 conditions: encoding, recall, and baseline.
- Results during each phase of the VPA task were compared.
Outcomes
- While completing the VPA task after taking placebo, compared with healthy controls, patients with psychosis demonstrated a different pattern of activity in the prefrontal and mediotemporal brain areas. Specifically, during verbal encoding, the placebo group showed altered activation in prefrontal regions. During verbal recall, the placebo group showed altered activation in prefrontal and mediotemporal regions, as well as increased mediotemporal-striatal functional connectivity.
- After participants received CBD, activation in these brain areas became more like the activation seen in controls. CBD attenuated dysfunction in these regions such that activation was intermediate between the placebo condition and the control group. CBD also attenuated functional connectivity between the hippocampus and striatum, and lead to reduced symptoms in patients with psychosis (as measured by PANSS total score).
Conclusion
- Altered activation in prefrontal and mediotemporal regions during verbal learning in patients with psychosis appeared to be partially normalized after a single 600-mg dose of CBD. Results also showed improvement in PANSS total score with CBD.
- These findings suggest that a single dose of CBD may partially attenuate the dysfunctional prefrontal and mediotemporal activation that is believed to underlie the dopamine dysfunction that leads to psychotic symptoms. These effects, along with a reduction in psychotic symptoms, suggest that normalization of altered prefrontal and mediotemporal function and mediotemporal-striatal connectivity may underlie the antipsychotic effects of CBD in established psychosis.
Continue to: Bhattacharyya S, et al...
4. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
Current preclinical models suggest that psychosis involves a disturbance of activity in the medial temporal lobe (MTL) that drives dopamine dysfunction in the striatum and midbrain. THC, which produces psychotomimetic effects, impacts the function of the striatum (verbal memoryand salience processing) andamygdala (emotional processing), and alters the functional connectivity of these regions. Compared with THC, CBD has broadly opposite neural and behavioral effects, including opposing effects on the activation of these regions. Bhattacharyya et al7 examined the neurocognitive mechanisms that underlie the therapeutic effects of CBD in psychosis and sought to understand whether CBD would attenuate functional abnormalities in the MTL, midbrain, and striatum.
Study design
- A randomized, double-blind, placebo-controlled trial examined 33 antipsychotic-naïve participants at clinical high risk (CHR) for psychosis and 19 healthy controls. The CHR group was randomized to CBD, 600 mg, or placebo.
- Three hours after taking CBD or placebo, CHR participants were studied using fMRI while performing a VPA learning task, which engages verbal learning and recall in the MTL, midbrain and striatum. Control participants did not receive any medication but underwent fMRI while performing the VPA task.
- The VPA task presented word pairs visually, and the accuracy of responses was recorded online. It was comprised of 3 conditions: encoding, recall, and baseline.
Outcomes
- Brain activation was analyzed in 15 participants in the CBD group, 16 in the placebo group, and 19 in the control group. Activation during encoding was observed in the striatum (specifically, the right caudate). Activation during recall was observed in the midbrain and the MTL (specifically, the parahippocampus).
- Brain activation levels in all 3 regions were lowest in the placebo group, intermediate in the CBD group, and greatest in the healthy control group. For all participants, the total recall score was directly correlated with the activation level in the left MTL (parahippocampus) during recall.
Conclusion
- Relative to controls, CHR participants exhibited different levels of activation in several regions, including the 3 areas thought to be critical to the pathophysiology of psychosis: the striatum during verbal encoding, and the MTL and midbrain during verbal recall.
- Compared with those who received placebo, CHR participants who received CBD before completing the VPA task demonstrated greater levels of brain activation and higher recall score.
- These findings suggest that CBD may partially normalize alterations in MTL, striatal, and midbrain function associated with CHR of psychosis. Because these regions are implicated in the pathophysiology of psychosis, the impact of CBD at these sites may contribute to the therapeutic effects of CBD that have been reported by some patients with psychosis.
Continue to: Conflicting data highlights...
Conflicting data highlights the need for longer, larger studies
Research findings on the use of CBD for psychotic symptoms in patients with schizophrenia have been conflicting. Some early research suggests that taking CBD 4 times daily for 4 weeks improves psychotic symptoms and might be as effective as the antipsychotic amisulpride. However, other early research suggests that taking CBD for 14 days is not beneficial. The conflicting results might be related to the CBD dose used and duration of treatment.
Davies and Bhattacharya12 recently reviewed evidence regarding the efficacy of CBD as a potential novel treatment for psychotic disorders.They concluded that CBD represents a promising potential novel treatment for patients with psychosis. It also appears that CBD may improve the disease trajectory of individuals with early psychosis and comorbid cannabis misuse.13 CBD use has also been associated with a decrease in symptoms of psychosis and changes in brain activity during verbal memory tasks in patients at high risk of psychosis.6 However, before CBD can become a viable treatment option for psychosis, the promising findings in these initial clinical studies must be replicated in large-scale trials with appropriate treatment duration.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
1. US National Library of Medicine. MedlinePlus. Cannabidiol (CBD). https://medlineplus.gov/druginfo/natural/1439.html. Accessed May 14, 2020.
2. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708-1709.
3. Leweke FM, Piomelli D, Pahlisch F, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94.
4. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225-231.
5. Boggs DL, Surti T, Gupta A, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923-1932.
6. O’Neill A, Wilson R, Blest-Hopley G, et al. Normalization of mediotemporal and prefrontal activity, and mediotemporal-striatal connectivity, may underlie antipsychotic effects of cannabidiol in psychosis. Psychol Med. 2020;1-11. doi: 10.1017/S0033291719003519.
7. Bhattacharyya S, Wilson R, Appiah-Kusi E, et al. Effect of cannabidiol on medial temporal, midbrain, and striatal dysfunction in people at clinical high risk of psychosis: a randomized clinical trial. JAMA Psychiatry. 2018;75(11):1107-1117.
8. Hallak JE, Machado-de-Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop color word test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Rev Bras Psiquiatr. 2010;32(1):56-61.
9. Zuardi AW, Morais SL, Guimaraes FS, et al. Antipsychotic effect of cannabidiol. J Clin Psychiatry. 1995;56(10):485-486.
10. Zuardi AW, Hallak JE, Dursun SM, et al. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol. 2006;20(5):683-686.
11. Leweke FM, Hellmich M, Pahlisch F, et al. Modulation of the endocannabinoid system as a potential new target in the treatment of schizophrenia. Schizophr Res. 2014; 153(1):S47.
12. Davies C, Bhattacharyya S. Cannabidiol as a potential treatment for psychosis. Ther Adv Psychopharmacol. 2019;9. doi:10.1177/2045125319881916.
13. Hahn B. The potential of cannabidiol treatment for cannabis users with recent-onset psychosis. Schizophr Bull. 2018;44(1):46-53.
Annual U.S. death toll from drugs, alcohol, suicide tops 150,000
Despite decreases in overall opioid overdose deaths in 2018, deaths involving synthetic opioids, cocaine, and other psychostimulants increased sharply in the United States, and alcohol and suicide deaths also rose, new data show.
A report released May 21 by the Trust for America’s Health (TFAH) and the Well Being Trust shows that 151,964 Americans died from alcohol, drugs, and suicide. Experts warn that these “deaths of despair” may well increase in the wake of COVID-19.
A study released earlier in May estimated that an additional 75,000 Americans could die by suicide, drugs, or alcohol abuse because of the pandemic (Petterson S et al. “Projected Deaths of Despair From COVID-19,” Well Being Trust. May 8, 2020. WellBeingTrust.org).
“These data are a clarion call to action,” TFAH President and CEO John Auerbach said in a news release.
“We know what works to address deaths of despair but progress has been uneven and death rates continue to climb, with communities of color experiencing higher rates of increases in drug-induced and alcohol deaths,” he said.
“And there’s another immediate concern: The COVID-19 crisis has increased the health burdens and economic pressures on many communities of color,” said Mr. Auerbach.
According to the report, the 2018 national rate for alcohol, drug, and suicide deaths combined was only slightly lower than that reported in 2017 (46.4 vs 46.6 per 100,000).
Among the key findings in the report:
- 37,329 Americans died from alcohol-induced causes in 2018; the rate was up 4% over 2017.
- Alcohol-induced deaths were highest among American Indians (30.0 per 100,000) and adults aged 55 to 74 (27.6 per 100,000). For all population groups, rates of alcohol-related deaths were higher in 2018 than in 2017 except for people aged 17 years and younger, for whom the rate held steady.
- Despite a 4% decline in all drug-induced deaths and a 2% drop in all opioid-related deaths, 2018 saw sharp increases in deaths involving synthetic opioids (up 10%), cocaine (up 5%), and other psychostimulants, such as methamphetamine, ecstasy, amphetamine, and prescription stimulants (up 22%).
- Suicide claimed the lives of 48,344 Americans in 2018. The suicide rate in 2018 was 2% higher than in 2017 and 25% higher than in 2008.
- Suicide rates increased across all demographics except for adults aged 18-54 years, among whom the rate remained stable. Suicide death rates were highest in males (23.4 per 100,000), rural residents (19.7 per 100,000), whites (16.8 per 100,000), and American Indian/Alaska Natives (14.1 per 100,000).
- Between 2017 and 2018, 27 states had higher rates (above 0.04%) of alcohol, drug, and suicide deaths; 23 states and the District of Columbia had lower rates of deaths from those causes.
- States with the highest alcohol, drug, and suicide death rates in 2018 were West Virginia (84.9 per 100,000), New Mexico (82.8 per 100,000), New Hampshire (68.2 per 100,000), and Alaska (67.8 per 100,000).
- States with the lowest rates in 2018 were Texas (31.7 per 100,000), Mississippi (31.7 per 100,000), and Hawaii (34.6 per 100,000).
“Quite simply, too many Americans are dying from preventable causes. The profound racial health disparities seen in these data show that many ethnic minority groups are being left behind in our response efforts,” Benjamin F. Miller, PsyD, Well Being Trust chief strategy officer, said in the release.
“The nation needs a comprehensive framework for excellence in mental health and well-being, one that intentionally provides solutions for American Indians, blacks, Asians and Latinos. said Dr. Miller.
Policy recommendations outlined in the report include investing in prevention; reducing risk factors and promoting resilience in children, families, and communities; engaging all sectors of society to address mental health and substance use disorders; limiting access to lethal means of suicide; and promoting safe storage of medications and firearms.
A version of this article originally appeared on Medscape.com.
Despite decreases in overall opioid overdose deaths in 2018, deaths involving synthetic opioids, cocaine, and other psychostimulants increased sharply in the United States, and alcohol and suicide deaths also rose, new data show.
A report released May 21 by the Trust for America’s Health (TFAH) and the Well Being Trust shows that 151,964 Americans died from alcohol, drugs, and suicide. Experts warn that these “deaths of despair” may well increase in the wake of COVID-19.
A study released earlier in May estimated that an additional 75,000 Americans could die by suicide, drugs, or alcohol abuse because of the pandemic (Petterson S et al. “Projected Deaths of Despair From COVID-19,” Well Being Trust. May 8, 2020. WellBeingTrust.org).
“These data are a clarion call to action,” TFAH President and CEO John Auerbach said in a news release.
“We know what works to address deaths of despair but progress has been uneven and death rates continue to climb, with communities of color experiencing higher rates of increases in drug-induced and alcohol deaths,” he said.
“And there’s another immediate concern: The COVID-19 crisis has increased the health burdens and economic pressures on many communities of color,” said Mr. Auerbach.
According to the report, the 2018 national rate for alcohol, drug, and suicide deaths combined was only slightly lower than that reported in 2017 (46.4 vs 46.6 per 100,000).
Among the key findings in the report:
- 37,329 Americans died from alcohol-induced causes in 2018; the rate was up 4% over 2017.
- Alcohol-induced deaths were highest among American Indians (30.0 per 100,000) and adults aged 55 to 74 (27.6 per 100,000). For all population groups, rates of alcohol-related deaths were higher in 2018 than in 2017 except for people aged 17 years and younger, for whom the rate held steady.
- Despite a 4% decline in all drug-induced deaths and a 2% drop in all opioid-related deaths, 2018 saw sharp increases in deaths involving synthetic opioids (up 10%), cocaine (up 5%), and other psychostimulants, such as methamphetamine, ecstasy, amphetamine, and prescription stimulants (up 22%).
- Suicide claimed the lives of 48,344 Americans in 2018. The suicide rate in 2018 was 2% higher than in 2017 and 25% higher than in 2008.
- Suicide rates increased across all demographics except for adults aged 18-54 years, among whom the rate remained stable. Suicide death rates were highest in males (23.4 per 100,000), rural residents (19.7 per 100,000), whites (16.8 per 100,000), and American Indian/Alaska Natives (14.1 per 100,000).
- Between 2017 and 2018, 27 states had higher rates (above 0.04%) of alcohol, drug, and suicide deaths; 23 states and the District of Columbia had lower rates of deaths from those causes.
- States with the highest alcohol, drug, and suicide death rates in 2018 were West Virginia (84.9 per 100,000), New Mexico (82.8 per 100,000), New Hampshire (68.2 per 100,000), and Alaska (67.8 per 100,000).
- States with the lowest rates in 2018 were Texas (31.7 per 100,000), Mississippi (31.7 per 100,000), and Hawaii (34.6 per 100,000).
“Quite simply, too many Americans are dying from preventable causes. The profound racial health disparities seen in these data show that many ethnic minority groups are being left behind in our response efforts,” Benjamin F. Miller, PsyD, Well Being Trust chief strategy officer, said in the release.
“The nation needs a comprehensive framework for excellence in mental health and well-being, one that intentionally provides solutions for American Indians, blacks, Asians and Latinos. said Dr. Miller.
Policy recommendations outlined in the report include investing in prevention; reducing risk factors and promoting resilience in children, families, and communities; engaging all sectors of society to address mental health and substance use disorders; limiting access to lethal means of suicide; and promoting safe storage of medications and firearms.
A version of this article originally appeared on Medscape.com.
Despite decreases in overall opioid overdose deaths in 2018, deaths involving synthetic opioids, cocaine, and other psychostimulants increased sharply in the United States, and alcohol and suicide deaths also rose, new data show.
A report released May 21 by the Trust for America’s Health (TFAH) and the Well Being Trust shows that 151,964 Americans died from alcohol, drugs, and suicide. Experts warn that these “deaths of despair” may well increase in the wake of COVID-19.
A study released earlier in May estimated that an additional 75,000 Americans could die by suicide, drugs, or alcohol abuse because of the pandemic (Petterson S et al. “Projected Deaths of Despair From COVID-19,” Well Being Trust. May 8, 2020. WellBeingTrust.org).
“These data are a clarion call to action,” TFAH President and CEO John Auerbach said in a news release.
“We know what works to address deaths of despair but progress has been uneven and death rates continue to climb, with communities of color experiencing higher rates of increases in drug-induced and alcohol deaths,” he said.
“And there’s another immediate concern: The COVID-19 crisis has increased the health burdens and economic pressures on many communities of color,” said Mr. Auerbach.
According to the report, the 2018 national rate for alcohol, drug, and suicide deaths combined was only slightly lower than that reported in 2017 (46.4 vs 46.6 per 100,000).
Among the key findings in the report:
- 37,329 Americans died from alcohol-induced causes in 2018; the rate was up 4% over 2017.
- Alcohol-induced deaths were highest among American Indians (30.0 per 100,000) and adults aged 55 to 74 (27.6 per 100,000). For all population groups, rates of alcohol-related deaths were higher in 2018 than in 2017 except for people aged 17 years and younger, for whom the rate held steady.
- Despite a 4% decline in all drug-induced deaths and a 2% drop in all opioid-related deaths, 2018 saw sharp increases in deaths involving synthetic opioids (up 10%), cocaine (up 5%), and other psychostimulants, such as methamphetamine, ecstasy, amphetamine, and prescription stimulants (up 22%).
- Suicide claimed the lives of 48,344 Americans in 2018. The suicide rate in 2018 was 2% higher than in 2017 and 25% higher than in 2008.
- Suicide rates increased across all demographics except for adults aged 18-54 years, among whom the rate remained stable. Suicide death rates were highest in males (23.4 per 100,000), rural residents (19.7 per 100,000), whites (16.8 per 100,000), and American Indian/Alaska Natives (14.1 per 100,000).
- Between 2017 and 2018, 27 states had higher rates (above 0.04%) of alcohol, drug, and suicide deaths; 23 states and the District of Columbia had lower rates of deaths from those causes.
- States with the highest alcohol, drug, and suicide death rates in 2018 were West Virginia (84.9 per 100,000), New Mexico (82.8 per 100,000), New Hampshire (68.2 per 100,000), and Alaska (67.8 per 100,000).
- States with the lowest rates in 2018 were Texas (31.7 per 100,000), Mississippi (31.7 per 100,000), and Hawaii (34.6 per 100,000).
“Quite simply, too many Americans are dying from preventable causes. The profound racial health disparities seen in these data show that many ethnic minority groups are being left behind in our response efforts,” Benjamin F. Miller, PsyD, Well Being Trust chief strategy officer, said in the release.
“The nation needs a comprehensive framework for excellence in mental health and well-being, one that intentionally provides solutions for American Indians, blacks, Asians and Latinos. said Dr. Miller.
Policy recommendations outlined in the report include investing in prevention; reducing risk factors and promoting resilience in children, families, and communities; engaging all sectors of society to address mental health and substance use disorders; limiting access to lethal means of suicide; and promoting safe storage of medications and firearms.
A version of this article originally appeared on Medscape.com.
Novel program for preventing addiction-related suicide
A single 3-hour-long group psychosocial intervention designed specifically for patients in intensive outpatient programs for addiction treatment to prevent future suicide resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up in a large multicenter randomized effectiveness study, reported Richard K. Ries, MD.
There is an enormous unmet need for evidence-based strategies for preventing addiction-related suicide, since people with substance use disorders have a 10-fold increased risk of suicide. Based upon these new study findings, the Preventing Addiction Related Suicide (PARS) program can now be considered the first such evidence-based intervention for this extremely high-risk population, Dr. Ries said at the virtual annual meeting of the American Association of Suicidology.
“We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Dr. Ries, professor of psychiatry at the University of Washington, Seattle, and director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center.
The workbook-based PARS program developed by Dr. Ries and colleagues adapted empirically supported suicide prevention best practices from other settings for use in group-based intensive outpatient addiction treatment, which is the most common form of treatment for chemical dependency in the United States. Dr. Ries recognized the need for a program such as PARS because addiction counselors often feel out of their depth regarding suicide-related issues.
he explained. “We designed the PARS intervention to be integrated right into the counselors’ workflow. They get trained right in their setting in a one-shot deal that takes 2-3 hours. The training was highly rated by counselors as acceptable and effective in their day-to-day work, not ivory tower-type stuff.”
Once the counselors were trained in PARS, they then trained their alcohol- and drug-addicted patients. Elements of the PARS program include information on suicide risk and protective factors, myths and facts about suicide, action steps to take when warning signs of suicide are observed, and local crisis resources.
The effectiveness study was a randomized, stepped-wedge cluster design intervention that included 905 patients in 15 busy community group–based intensive outpatient addiction treatment programs in Western Washington. Patients were randomized to counselor-delivered PARS or treatment as usual, with follow-up assessments at 2 weeks and 1, 3, and 6 months.
There was no attempt to enrich the study population for suicidality by prescreening potential enrollees, since participation in a drug and alcohol treatment program already places an individual in a high-risk group. For example, 74% of study participants indicated they had thought of suicide at least once in the past year, compared with a background rate of 4% in the U.S. general population. And 29% of study participants reported a lifetime history of one or more suicide attempts, versus roughly 5% in the general population.
Dr. Ries’ coinvestigator, Katherine Anne Comtois, PhD, MPH, presented the study results. The three key outcomes were improvement on structured measures of suicide knowledge, attitudes, and help-seeking behavior. The PARS recipients showed statistically significant improvement compared with baseline on two of the three: they displayed greater knowledge about suicide and its close relationship with addiction, and less stigmatization and other maladaptive attitudes toward suicide. Scores on all three measures remained unchanged over time in the control group.
“Overall, we had small to medium effect sizes, comparable to what you might see in studies measuring antidepressant effect sizes. I think these were meaningful improvements in knowledge and attitudes,” said Dr. Comtois of the University of Washington.
The PARS group showed a small increase in the third endpoint – willingness to seek professional help for themselves, friends, or family if depressed or suicidal, but this didn’t achieve statistical significance. However, Dr. Comtois noted that the study outcomes were assessed per protocol using an intent-to-treat analysis. This likely underestimated the true effectiveness of the PARS intervention, given that 40% of patients randomized to PARS didn’t actually attend the intervention session.
“People with drug and alcohol problems have complicated lives,” she said by way of explanation for the high no-show rate.
The investigators are now performing a per-protocol analysis of the data, restricted to those subjects who actually attended their session. A long-term look at suicide events and outcomes in the study population is planned.
Dr. Ries and Dr. Comtois reported having no financial conflicts regarding the study, which was funded by a multiyear grant from the National Institute on Drug Abuse.
A single 3-hour-long group psychosocial intervention designed specifically for patients in intensive outpatient programs for addiction treatment to prevent future suicide resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up in a large multicenter randomized effectiveness study, reported Richard K. Ries, MD.
There is an enormous unmet need for evidence-based strategies for preventing addiction-related suicide, since people with substance use disorders have a 10-fold increased risk of suicide. Based upon these new study findings, the Preventing Addiction Related Suicide (PARS) program can now be considered the first such evidence-based intervention for this extremely high-risk population, Dr. Ries said at the virtual annual meeting of the American Association of Suicidology.
“We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Dr. Ries, professor of psychiatry at the University of Washington, Seattle, and director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center.
The workbook-based PARS program developed by Dr. Ries and colleagues adapted empirically supported suicide prevention best practices from other settings for use in group-based intensive outpatient addiction treatment, which is the most common form of treatment for chemical dependency in the United States. Dr. Ries recognized the need for a program such as PARS because addiction counselors often feel out of their depth regarding suicide-related issues.
he explained. “We designed the PARS intervention to be integrated right into the counselors’ workflow. They get trained right in their setting in a one-shot deal that takes 2-3 hours. The training was highly rated by counselors as acceptable and effective in their day-to-day work, not ivory tower-type stuff.”
Once the counselors were trained in PARS, they then trained their alcohol- and drug-addicted patients. Elements of the PARS program include information on suicide risk and protective factors, myths and facts about suicide, action steps to take when warning signs of suicide are observed, and local crisis resources.
The effectiveness study was a randomized, stepped-wedge cluster design intervention that included 905 patients in 15 busy community group–based intensive outpatient addiction treatment programs in Western Washington. Patients were randomized to counselor-delivered PARS or treatment as usual, with follow-up assessments at 2 weeks and 1, 3, and 6 months.
There was no attempt to enrich the study population for suicidality by prescreening potential enrollees, since participation in a drug and alcohol treatment program already places an individual in a high-risk group. For example, 74% of study participants indicated they had thought of suicide at least once in the past year, compared with a background rate of 4% in the U.S. general population. And 29% of study participants reported a lifetime history of one or more suicide attempts, versus roughly 5% in the general population.
Dr. Ries’ coinvestigator, Katherine Anne Comtois, PhD, MPH, presented the study results. The three key outcomes were improvement on structured measures of suicide knowledge, attitudes, and help-seeking behavior. The PARS recipients showed statistically significant improvement compared with baseline on two of the three: they displayed greater knowledge about suicide and its close relationship with addiction, and less stigmatization and other maladaptive attitudes toward suicide. Scores on all three measures remained unchanged over time in the control group.
“Overall, we had small to medium effect sizes, comparable to what you might see in studies measuring antidepressant effect sizes. I think these were meaningful improvements in knowledge and attitudes,” said Dr. Comtois of the University of Washington.
The PARS group showed a small increase in the third endpoint – willingness to seek professional help for themselves, friends, or family if depressed or suicidal, but this didn’t achieve statistical significance. However, Dr. Comtois noted that the study outcomes were assessed per protocol using an intent-to-treat analysis. This likely underestimated the true effectiveness of the PARS intervention, given that 40% of patients randomized to PARS didn’t actually attend the intervention session.
“People with drug and alcohol problems have complicated lives,” she said by way of explanation for the high no-show rate.
The investigators are now performing a per-protocol analysis of the data, restricted to those subjects who actually attended their session. A long-term look at suicide events and outcomes in the study population is planned.
Dr. Ries and Dr. Comtois reported having no financial conflicts regarding the study, which was funded by a multiyear grant from the National Institute on Drug Abuse.
A single 3-hour-long group psychosocial intervention designed specifically for patients in intensive outpatient programs for addiction treatment to prevent future suicide resulted in significantly improved knowledge and attitudes regarding suicide that persisted at 6 months of follow-up in a large multicenter randomized effectiveness study, reported Richard K. Ries, MD.
There is an enormous unmet need for evidence-based strategies for preventing addiction-related suicide, since people with substance use disorders have a 10-fold increased risk of suicide. Based upon these new study findings, the Preventing Addiction Related Suicide (PARS) program can now be considered the first such evidence-based intervention for this extremely high-risk population, Dr. Ries said at the virtual annual meeting of the American Association of Suicidology.
“We’ve shown that suicide prevention in intensive outpatient program addiction groups is feasible, easy to train, and highly rated by counselors, and I’d say it’s very adaptable, easy to go national in almost any addiction treatment program, right out of the box,” said Dr. Ries, professor of psychiatry at the University of Washington, Seattle, and director of outpatient psychiatry as well as the psychiatry addiction division at Harborview Medical Center.
The workbook-based PARS program developed by Dr. Ries and colleagues adapted empirically supported suicide prevention best practices from other settings for use in group-based intensive outpatient addiction treatment, which is the most common form of treatment for chemical dependency in the United States. Dr. Ries recognized the need for a program such as PARS because addiction counselors often feel out of their depth regarding suicide-related issues.
he explained. “We designed the PARS intervention to be integrated right into the counselors’ workflow. They get trained right in their setting in a one-shot deal that takes 2-3 hours. The training was highly rated by counselors as acceptable and effective in their day-to-day work, not ivory tower-type stuff.”
Once the counselors were trained in PARS, they then trained their alcohol- and drug-addicted patients. Elements of the PARS program include information on suicide risk and protective factors, myths and facts about suicide, action steps to take when warning signs of suicide are observed, and local crisis resources.
The effectiveness study was a randomized, stepped-wedge cluster design intervention that included 905 patients in 15 busy community group–based intensive outpatient addiction treatment programs in Western Washington. Patients were randomized to counselor-delivered PARS or treatment as usual, with follow-up assessments at 2 weeks and 1, 3, and 6 months.
There was no attempt to enrich the study population for suicidality by prescreening potential enrollees, since participation in a drug and alcohol treatment program already places an individual in a high-risk group. For example, 74% of study participants indicated they had thought of suicide at least once in the past year, compared with a background rate of 4% in the U.S. general population. And 29% of study participants reported a lifetime history of one or more suicide attempts, versus roughly 5% in the general population.
Dr. Ries’ coinvestigator, Katherine Anne Comtois, PhD, MPH, presented the study results. The three key outcomes were improvement on structured measures of suicide knowledge, attitudes, and help-seeking behavior. The PARS recipients showed statistically significant improvement compared with baseline on two of the three: they displayed greater knowledge about suicide and its close relationship with addiction, and less stigmatization and other maladaptive attitudes toward suicide. Scores on all three measures remained unchanged over time in the control group.
“Overall, we had small to medium effect sizes, comparable to what you might see in studies measuring antidepressant effect sizes. I think these were meaningful improvements in knowledge and attitudes,” said Dr. Comtois of the University of Washington.
The PARS group showed a small increase in the third endpoint – willingness to seek professional help for themselves, friends, or family if depressed or suicidal, but this didn’t achieve statistical significance. However, Dr. Comtois noted that the study outcomes were assessed per protocol using an intent-to-treat analysis. This likely underestimated the true effectiveness of the PARS intervention, given that 40% of patients randomized to PARS didn’t actually attend the intervention session.
“People with drug and alcohol problems have complicated lives,” she said by way of explanation for the high no-show rate.
The investigators are now performing a per-protocol analysis of the data, restricted to those subjects who actually attended their session. A long-term look at suicide events and outcomes in the study population is planned.
Dr. Ries and Dr. Comtois reported having no financial conflicts regarding the study, which was funded by a multiyear grant from the National Institute on Drug Abuse.
FROM AAS20
New ‘atlas’ maps links between mental disorders, physical illnesses
Mental illnesses are associated with a significantly increased risk of subsequent physical diseases, new research shows.
An international team of researchers has created an “atlas” that maps the relationship between specific mental disorders and the risk of subsequent physical illnesses.
The researchers found that, following the diagnosis of a mental disorder, psychiatric patients are significantly more likely than the general population to develop potentially life-threatening conditions, including heart disease and stroke.
These findings, the investigators noted, highlight the need for better medical care in this vulnerable population. They have created a website with detailed information about the risks of specific physical ailments and the link to particular mental disorders.
“We found that women with anxiety disorders have a 50% increased risk of developing a heart condition or stroke – over 15 years, one in three women with anxiety disorders will develop these medical disorders,” lead investigator John McGrath, MD, PhD, University of Queensland’s Brain Institute, Brisbane, Australia, and Aarhus (Denmark) University, said in a statement.
“We also looked at men with substance use disorders such as alcohol-related disorders and found they have a 400% increased risk of gut or liver disorders, while over 15 years, one in five of them will develop gut or liver conditions,” he added.
The study was published in the New England Journal of Medicine.
New ‘atlas’
It’s well known that patients with mental disorders have decreased quality of life, increased health care utilization, and a shorter life expectancy than individuals in the general population – about 10 years for men and 7 years for women.
However, the investigators noted, previous research examining the relationship between mental disorders and medical conditions only focused on “particular pairs or a small set of mental disorders and medical conditions.”
“We needed a comprehensive study to map the links between different types of mental disorders versus different types of general medical conditions. Our study has provided this atlas,” Dr. McGrath said in an interview.
The clinical utility of such a map could provide comprehensive data on relative and absolute risks of various medical conditions after a diagnosis of a mental disorder. This information, the researchers noted, would “help clinicians and health care planners identify the primary prevention needs of their patients.”
The study included 5.9 million people born in Denmark between 1900 and 2015 and followed them from 2000 to 2016, a total of 83.9 million person-years. The researchers followed patients for up to 17 years (2000-2016) for medical diagnoses and up to 48 years (1969-2016) for diagnoses of mental disorders.
The study’s large sample size allowed investigators to assess 10 broad types of mental disorders and 9 broad categories of medical conditions that encompassed 31 specific conditions.
Categories of medical conditions included circulatory, endocrine, pulmonary, gastrointestinal, urogenital, musculoskeletal, hematologic, neurologic, and cancer. Mental disorder categories included organic disorders such as Alzheimer’s, substance abuse disorders, schizophrenia, mood disorders, neurotic disorders, eating disorders, personality disorders, developmental disorders, behavioral/emotional disorders, and intellectual disabilities.
The researchers estimated associations between 90 pairs of mental disorders and broad-category medical conditions, as well as 310 pairs of mental disorders and specific medical conditions.
‘Curious’ finding
Individuals with mental disorders showed a higher risk of medical conditions in 76 out of 90 specific mental disorder–medical condition pairs.
After adjusting for sex, age, calendar time, and previous coexisting mental disorders, the median hazard ratio for a subsequent medical condition was 1.37 in patients with a mental disorder.
The lowest HR was 0.82 for organic mental disorders and the broad category of cancer (95% confidence interval, 0.80-0.84), and the highest was 3.62 for eating disorders and urogenital conditions (95% CI, 3.11-4.22). On the other hand, schizophrenia was associated with a reduced risk of developing musculoskeletal conditions (HR, 0.87; 95% CI, 0.84-0.91).
Dr. McGrath described this finding as “curious” and speculated it “may be related to underlying genetic risk factors.”
compared with the matched reference group without a mood disorder (40.9% vs. 32.6%, respectively).
The risk of developing subsequent medical conditions after a mental disorder diagnosis did not remain steady over time. For instance, although mood disorders were associated with an increased risk of developing circulatory problems (HR, 1.32; 95% CI, 1.31-1.34), the highest risk occurred during the first 6 months following diagnosis and gradually decreased over the next 15 years (HR, 2.39; 95% CI, 2.29-2.48 and HR, 1.18; 95% CI, 1.17-1.20, respectively).
“Many people with mental disorders have unhealthy lifestyle, including low exercise, poor diet, smoking, and alcohol, which may account for the increased risk of physical illness, and also they may not seek and/or may not get quick treatment for their health conditions,” said Dr. McGrath.
Additionally, “perhaps some genetic and early life exposures, such as trauma, may increase the risk of both medical conditions and mental disorders,” he added. “We need better treatments for mental disorders, so that they do not slip into unemployment or poverty.”
A strong case
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto and head of the mood disorders psychopharmacology unit, University Health Network, said that the research “really makes a strong case for the fact that persons who have mental disorders are at higher risk of chronic diseases, and it’s the chronic diseases that decrease their lifespan.”
Dr. McIntyre, who is also director of the Depression and Bipolar Support Alliance, said that the “takeaway message is that mental disorders are not just brain disorders but are multisystem disorders.”
For this reason, “the most appropriate way to provide care would be to provide a holistic approach to treat and prevent the chronic diseases that lead to increase in mortality,” recommended Dr. McIntyre, who was not involved with the current study.
The study was supported by grants from the Danish National Research Foundation, the National Health and Medical Research Council, the Novo Nordisk Foundation , the European Union’s Horizon 2020 Research and Innovation Program, the Aarhus University Research Foundation, the Lundbeck Foundation, the National Institutes of Health, the European Commission, Helsefonden, the Danish Council for Independent Research, the Independent Research Fund Denmark, the National Health and Medical Research Council of Australia, and the National Institute on Drug Abuse.
Dr. McGrath has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reports receiving grants from Stanley Medical Research Institute; the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation; and receiving speaking/consultation fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva.
A version of this article originally appeared on Medscape.com.
Mental illnesses are associated with a significantly increased risk of subsequent physical diseases, new research shows.
An international team of researchers has created an “atlas” that maps the relationship between specific mental disorders and the risk of subsequent physical illnesses.
The researchers found that, following the diagnosis of a mental disorder, psychiatric patients are significantly more likely than the general population to develop potentially life-threatening conditions, including heart disease and stroke.
These findings, the investigators noted, highlight the need for better medical care in this vulnerable population. They have created a website with detailed information about the risks of specific physical ailments and the link to particular mental disorders.
“We found that women with anxiety disorders have a 50% increased risk of developing a heart condition or stroke – over 15 years, one in three women with anxiety disorders will develop these medical disorders,” lead investigator John McGrath, MD, PhD, University of Queensland’s Brain Institute, Brisbane, Australia, and Aarhus (Denmark) University, said in a statement.
“We also looked at men with substance use disorders such as alcohol-related disorders and found they have a 400% increased risk of gut or liver disorders, while over 15 years, one in five of them will develop gut or liver conditions,” he added.
The study was published in the New England Journal of Medicine.
New ‘atlas’
It’s well known that patients with mental disorders have decreased quality of life, increased health care utilization, and a shorter life expectancy than individuals in the general population – about 10 years for men and 7 years for women.
However, the investigators noted, previous research examining the relationship between mental disorders and medical conditions only focused on “particular pairs or a small set of mental disorders and medical conditions.”
“We needed a comprehensive study to map the links between different types of mental disorders versus different types of general medical conditions. Our study has provided this atlas,” Dr. McGrath said in an interview.
The clinical utility of such a map could provide comprehensive data on relative and absolute risks of various medical conditions after a diagnosis of a mental disorder. This information, the researchers noted, would “help clinicians and health care planners identify the primary prevention needs of their patients.”
The study included 5.9 million people born in Denmark between 1900 and 2015 and followed them from 2000 to 2016, a total of 83.9 million person-years. The researchers followed patients for up to 17 years (2000-2016) for medical diagnoses and up to 48 years (1969-2016) for diagnoses of mental disorders.
The study’s large sample size allowed investigators to assess 10 broad types of mental disorders and 9 broad categories of medical conditions that encompassed 31 specific conditions.
Categories of medical conditions included circulatory, endocrine, pulmonary, gastrointestinal, urogenital, musculoskeletal, hematologic, neurologic, and cancer. Mental disorder categories included organic disorders such as Alzheimer’s, substance abuse disorders, schizophrenia, mood disorders, neurotic disorders, eating disorders, personality disorders, developmental disorders, behavioral/emotional disorders, and intellectual disabilities.
The researchers estimated associations between 90 pairs of mental disorders and broad-category medical conditions, as well as 310 pairs of mental disorders and specific medical conditions.
‘Curious’ finding
Individuals with mental disorders showed a higher risk of medical conditions in 76 out of 90 specific mental disorder–medical condition pairs.
After adjusting for sex, age, calendar time, and previous coexisting mental disorders, the median hazard ratio for a subsequent medical condition was 1.37 in patients with a mental disorder.
The lowest HR was 0.82 for organic mental disorders and the broad category of cancer (95% confidence interval, 0.80-0.84), and the highest was 3.62 for eating disorders and urogenital conditions (95% CI, 3.11-4.22). On the other hand, schizophrenia was associated with a reduced risk of developing musculoskeletal conditions (HR, 0.87; 95% CI, 0.84-0.91).
Dr. McGrath described this finding as “curious” and speculated it “may be related to underlying genetic risk factors.”
compared with the matched reference group without a mood disorder (40.9% vs. 32.6%, respectively).
The risk of developing subsequent medical conditions after a mental disorder diagnosis did not remain steady over time. For instance, although mood disorders were associated with an increased risk of developing circulatory problems (HR, 1.32; 95% CI, 1.31-1.34), the highest risk occurred during the first 6 months following diagnosis and gradually decreased over the next 15 years (HR, 2.39; 95% CI, 2.29-2.48 and HR, 1.18; 95% CI, 1.17-1.20, respectively).
“Many people with mental disorders have unhealthy lifestyle, including low exercise, poor diet, smoking, and alcohol, which may account for the increased risk of physical illness, and also they may not seek and/or may not get quick treatment for their health conditions,” said Dr. McGrath.
Additionally, “perhaps some genetic and early life exposures, such as trauma, may increase the risk of both medical conditions and mental disorders,” he added. “We need better treatments for mental disorders, so that they do not slip into unemployment or poverty.”
A strong case
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto and head of the mood disorders psychopharmacology unit, University Health Network, said that the research “really makes a strong case for the fact that persons who have mental disorders are at higher risk of chronic diseases, and it’s the chronic diseases that decrease their lifespan.”
Dr. McIntyre, who is also director of the Depression and Bipolar Support Alliance, said that the “takeaway message is that mental disorders are not just brain disorders but are multisystem disorders.”
For this reason, “the most appropriate way to provide care would be to provide a holistic approach to treat and prevent the chronic diseases that lead to increase in mortality,” recommended Dr. McIntyre, who was not involved with the current study.
The study was supported by grants from the Danish National Research Foundation, the National Health and Medical Research Council, the Novo Nordisk Foundation , the European Union’s Horizon 2020 Research and Innovation Program, the Aarhus University Research Foundation, the Lundbeck Foundation, the National Institutes of Health, the European Commission, Helsefonden, the Danish Council for Independent Research, the Independent Research Fund Denmark, the National Health and Medical Research Council of Australia, and the National Institute on Drug Abuse.
Dr. McGrath has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reports receiving grants from Stanley Medical Research Institute; the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation; and receiving speaking/consultation fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva.
A version of this article originally appeared on Medscape.com.
Mental illnesses are associated with a significantly increased risk of subsequent physical diseases, new research shows.
An international team of researchers has created an “atlas” that maps the relationship between specific mental disorders and the risk of subsequent physical illnesses.
The researchers found that, following the diagnosis of a mental disorder, psychiatric patients are significantly more likely than the general population to develop potentially life-threatening conditions, including heart disease and stroke.
These findings, the investigators noted, highlight the need for better medical care in this vulnerable population. They have created a website with detailed information about the risks of specific physical ailments and the link to particular mental disorders.
“We found that women with anxiety disorders have a 50% increased risk of developing a heart condition or stroke – over 15 years, one in three women with anxiety disorders will develop these medical disorders,” lead investigator John McGrath, MD, PhD, University of Queensland’s Brain Institute, Brisbane, Australia, and Aarhus (Denmark) University, said in a statement.
“We also looked at men with substance use disorders such as alcohol-related disorders and found they have a 400% increased risk of gut or liver disorders, while over 15 years, one in five of them will develop gut or liver conditions,” he added.
The study was published in the New England Journal of Medicine.
New ‘atlas’
It’s well known that patients with mental disorders have decreased quality of life, increased health care utilization, and a shorter life expectancy than individuals in the general population – about 10 years for men and 7 years for women.
However, the investigators noted, previous research examining the relationship between mental disorders and medical conditions only focused on “particular pairs or a small set of mental disorders and medical conditions.”
“We needed a comprehensive study to map the links between different types of mental disorders versus different types of general medical conditions. Our study has provided this atlas,” Dr. McGrath said in an interview.
The clinical utility of such a map could provide comprehensive data on relative and absolute risks of various medical conditions after a diagnosis of a mental disorder. This information, the researchers noted, would “help clinicians and health care planners identify the primary prevention needs of their patients.”
The study included 5.9 million people born in Denmark between 1900 and 2015 and followed them from 2000 to 2016, a total of 83.9 million person-years. The researchers followed patients for up to 17 years (2000-2016) for medical diagnoses and up to 48 years (1969-2016) for diagnoses of mental disorders.
The study’s large sample size allowed investigators to assess 10 broad types of mental disorders and 9 broad categories of medical conditions that encompassed 31 specific conditions.
Categories of medical conditions included circulatory, endocrine, pulmonary, gastrointestinal, urogenital, musculoskeletal, hematologic, neurologic, and cancer. Mental disorder categories included organic disorders such as Alzheimer’s, substance abuse disorders, schizophrenia, mood disorders, neurotic disorders, eating disorders, personality disorders, developmental disorders, behavioral/emotional disorders, and intellectual disabilities.
The researchers estimated associations between 90 pairs of mental disorders and broad-category medical conditions, as well as 310 pairs of mental disorders and specific medical conditions.
‘Curious’ finding
Individuals with mental disorders showed a higher risk of medical conditions in 76 out of 90 specific mental disorder–medical condition pairs.
After adjusting for sex, age, calendar time, and previous coexisting mental disorders, the median hazard ratio for a subsequent medical condition was 1.37 in patients with a mental disorder.
The lowest HR was 0.82 for organic mental disorders and the broad category of cancer (95% confidence interval, 0.80-0.84), and the highest was 3.62 for eating disorders and urogenital conditions (95% CI, 3.11-4.22). On the other hand, schizophrenia was associated with a reduced risk of developing musculoskeletal conditions (HR, 0.87; 95% CI, 0.84-0.91).
Dr. McGrath described this finding as “curious” and speculated it “may be related to underlying genetic risk factors.”
compared with the matched reference group without a mood disorder (40.9% vs. 32.6%, respectively).
The risk of developing subsequent medical conditions after a mental disorder diagnosis did not remain steady over time. For instance, although mood disorders were associated with an increased risk of developing circulatory problems (HR, 1.32; 95% CI, 1.31-1.34), the highest risk occurred during the first 6 months following diagnosis and gradually decreased over the next 15 years (HR, 2.39; 95% CI, 2.29-2.48 and HR, 1.18; 95% CI, 1.17-1.20, respectively).
“Many people with mental disorders have unhealthy lifestyle, including low exercise, poor diet, smoking, and alcohol, which may account for the increased risk of physical illness, and also they may not seek and/or may not get quick treatment for their health conditions,” said Dr. McGrath.
Additionally, “perhaps some genetic and early life exposures, such as trauma, may increase the risk of both medical conditions and mental disorders,” he added. “We need better treatments for mental disorders, so that they do not slip into unemployment or poverty.”
A strong case
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto and head of the mood disorders psychopharmacology unit, University Health Network, said that the research “really makes a strong case for the fact that persons who have mental disorders are at higher risk of chronic diseases, and it’s the chronic diseases that decrease their lifespan.”
Dr. McIntyre, who is also director of the Depression and Bipolar Support Alliance, said that the “takeaway message is that mental disorders are not just brain disorders but are multisystem disorders.”
For this reason, “the most appropriate way to provide care would be to provide a holistic approach to treat and prevent the chronic diseases that lead to increase in mortality,” recommended Dr. McIntyre, who was not involved with the current study.
The study was supported by grants from the Danish National Research Foundation, the National Health and Medical Research Council, the Novo Nordisk Foundation , the European Union’s Horizon 2020 Research and Innovation Program, the Aarhus University Research Foundation, the Lundbeck Foundation, the National Institutes of Health, the European Commission, Helsefonden, the Danish Council for Independent Research, the Independent Research Fund Denmark, the National Health and Medical Research Council of Australia, and the National Institute on Drug Abuse.
Dr. McGrath has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reports receiving grants from Stanley Medical Research Institute; the Canadian Institutes of Health Research/Global Alliance for Chronic Diseases/Chinese National Natural Research Foundation; and receiving speaking/consultation fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, and Minerva.
A version of this article originally appeared on Medscape.com.
E-cigarette users topped 8 million in 2018
according to a report from the National Center for Health Statistics.
Those 8.1 million individuals who were using e-cigarettes either every day or some days represented 3.2% of the total adult population, based on data from the 2018 National Health Interview Survey. An even larger proportion, 14.9%, said that they had at least tried an e-cigarette, Maria A. Villarroel, PhD, and associates at the NCHS said in a recent data brief.
Most cigarette smokers, both current and former, were even more likely to use e-cigarettes, they noted.
Former cigarette smokers who had quit within the last year were the most likely to use e-cigarettes – 57.3% had ever used one and 25.2% were current users – while current cigarette users (49.4% ever use and 9.7% current use) and former smokers who had quit 1-5 years before (48.6% ever use, 17.3% current) also were above-average e-cigarette consumers, they reported.
Use was significantly lower, however, among former cigarette smokers who had quit 5 or more years earlier (9.0% and 1.7%, respectively) and those who had never smoked (6.5% and 1.1%), the NCHS investigators said.
The survey data also showed much variation among the sociodemographic subgroups:
- E-cigarette ever/current use was significantly higher in men (17.9% and 4.2%) than women (12.3% and 2.3%).
- Whites were significantly more likely to use e-cigarettes (16.9% and 3.7%), compared with Hispanic (11.5% and 2.5%), black (10.0% and 1.6%), and Asian (10.2% and 2.2%) adults.
- There was significant trend of decreasing use from age 18-24 years (25.8% and 7.6%) to 65 years and older (4.7% and 0.8%).
SOURCE: Villarroel MA et al. NCHS Data Brief No. 365, April 2020.
according to a report from the National Center for Health Statistics.

Those 8.1 million individuals who were using e-cigarettes either every day or some days represented 3.2% of the total adult population, based on data from the 2018 National Health Interview Survey. An even larger proportion, 14.9%, said that they had at least tried an e-cigarette, Maria A. Villarroel, PhD, and associates at the NCHS said in a recent data brief.
Most cigarette smokers, both current and former, were even more likely to use e-cigarettes, they noted.
Former cigarette smokers who had quit within the last year were the most likely to use e-cigarettes – 57.3% had ever used one and 25.2% were current users – while current cigarette users (49.4% ever use and 9.7% current use) and former smokers who had quit 1-5 years before (48.6% ever use, 17.3% current) also were above-average e-cigarette consumers, they reported.
Use was significantly lower, however, among former cigarette smokers who had quit 5 or more years earlier (9.0% and 1.7%, respectively) and those who had never smoked (6.5% and 1.1%), the NCHS investigators said.
The survey data also showed much variation among the sociodemographic subgroups:
- E-cigarette ever/current use was significantly higher in men (17.9% and 4.2%) than women (12.3% and 2.3%).
- Whites were significantly more likely to use e-cigarettes (16.9% and 3.7%), compared with Hispanic (11.5% and 2.5%), black (10.0% and 1.6%), and Asian (10.2% and 2.2%) adults.
- There was significant trend of decreasing use from age 18-24 years (25.8% and 7.6%) to 65 years and older (4.7% and 0.8%).
SOURCE: Villarroel MA et al. NCHS Data Brief No. 365, April 2020.
according to a report from the National Center for Health Statistics.

Those 8.1 million individuals who were using e-cigarettes either every day or some days represented 3.2% of the total adult population, based on data from the 2018 National Health Interview Survey. An even larger proportion, 14.9%, said that they had at least tried an e-cigarette, Maria A. Villarroel, PhD, and associates at the NCHS said in a recent data brief.
Most cigarette smokers, both current and former, were even more likely to use e-cigarettes, they noted.
Former cigarette smokers who had quit within the last year were the most likely to use e-cigarettes – 57.3% had ever used one and 25.2% were current users – while current cigarette users (49.4% ever use and 9.7% current use) and former smokers who had quit 1-5 years before (48.6% ever use, 17.3% current) also were above-average e-cigarette consumers, they reported.
Use was significantly lower, however, among former cigarette smokers who had quit 5 or more years earlier (9.0% and 1.7%, respectively) and those who had never smoked (6.5% and 1.1%), the NCHS investigators said.
The survey data also showed much variation among the sociodemographic subgroups:
- E-cigarette ever/current use was significantly higher in men (17.9% and 4.2%) than women (12.3% and 2.3%).
- Whites were significantly more likely to use e-cigarettes (16.9% and 3.7%), compared with Hispanic (11.5% and 2.5%), black (10.0% and 1.6%), and Asian (10.2% and 2.2%) adults.
- There was significant trend of decreasing use from age 18-24 years (25.8% and 7.6%) to 65 years and older (4.7% and 0.8%).
SOURCE: Villarroel MA et al. NCHS Data Brief No. 365, April 2020.
NSDUH data might underestimate substance use by pregnant women
New study suggests rate of alcohol use might be almost 19%
The use of alcohol, tobacco products, and drugs by pregnant women is a substantial problem that may be more prevalent than previously thought, according to researcher Kimberly Yonkers, MD.
Higher levels of substance use during pregnancy means more negative impacts on maternal and fetal, neonatal, and child health. However, one bit of good news is that pregnant women still are less likely than nonpregnant women to engage in such behavior, said Dr. Yonkers, director of psychological medicine and the Center for Wellbeing of Women and Mothers at Yale University, New Haven, Conn.
Dr. Yonkers said in a featured presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Tobacco predominates among substances of concern used by pregnant women, with 11.6% reporting past-month use in 2018, according to the National Survey on Drug Use and Health, Dr. Yonkers said. Alcohol was next, with 9.9% of pregnant women reporting use in the past month, followed by drugs at 5.4%, of which marijuana was the most common.
Those numbers may jump much higher when focusing on substance use that’s biologically verified, she added, referring to a recent three-center cross-sectional study she and her colleagues published in Addiction (2019 Jun 19. doi: 10.1111/add.14651). In that study, alcohol use was as high as 18.9% among pregnant women who either had positive urine or self-reported use. Similarly, rates of nicotine or nicotine byproduct detected were 27% at one center in the study, and tetrahydrocannabinol reached 29.4% at that same center.
“These numbers are impressive,” Dr. Yonkers told attendees at the meeting. “So what we may be seeing in terms of the National Survey on Drug Use and Health, as valuable as it is, is in all likelihood it underestimates the use of substances in pregnancy.”
Substance use goes down in pregnancy as some women become more mindful of perinatal health, though unfortunately, that abstinence is offset by a dramatic rise in substance use in the 6-12 months’ post partum, research suggests.
Interestingly, big differences are found in both abstinence and relapse rates, with some data sets showing that, while alcohol is stopped fairly early, cigarettes are stopped much later, if at all.
On the postpartum side of the equation, relapse rates look similar for cigarettes, alcohol, and marijuana, but for some reason, cocaine relapse rates are much lower “That’s kind of nice, and we’d like to be able to understand what it is about this whole process that enabled that relative period of wellness,” Dr. Yonkers said.
Opioid use disorder is rising among pregnant women, just like it is in the general population, and 50% – or possibly even as high as 80% – of babies born to these women will experience neonatal opioid withdrawal, Dr. Yonkers said.
Maternal mortality in the United States increased by 34% from 2008 to 2016; while that’s a sobering statistic, Dr. Yonkers said, opioid-related maternal mortality doubled over that same time period.
“We really have to be mindful that we’re not just talking about taking care of kids and offspring, but we have to take care of moms – it’s really critical,” she said.
With the increasing legalization of cannabis, it’s expected that a lot more cannabis-exposed pregnancies will be seen in clinical practice, and some studies are starting to show an increase in prevalence in the preconception, prenatal, and postpartum period.
While some people feel that cannabis is benign, more data are needed, according to Dr. Yonkers, who said that cannabis and its metabolites cross the blood/placenta and blood/milk barriers, and that cannabinoid receptors are “very important” to fertility, implantation, and fetal development. One study recently published linked cannabis use in pregnancy to significant increases in preterm birth rates (JAMA. 2019 Jun 18;322[2]:145-52).
“We don’t really have a context for this, so we don’t really know what’s going to have an impact, and what’s not,” Dr. Yonkers said.
While both standard and novel treatments could help in the quest to achieve and maintain well-being in this unique patient population, Dr. Yonkers said that health equity and universal approaches to care might be needed to more comprehensively address the problem, which means taking a close look at how much money women have, the resources available to them, and where they live.
In many communities, eliminating inequalities in care will be critical to successfully addressing substance use issues in pregnant women, agreed Maureen Sayres Van Niel, MD, a specialist in women’s psychiatry in Boston and president of the Women’s Caucus of the APA.
“What we see is such a disparity in the delivery of care to women who are poor and living in communities where the socioeconomic and financial problems are very severe,” Dr. Van Niel said in an interview. “Unless we address these disparities, women will not be getting the kind of health care that they really need to have in the perinatal period.”
Dr. Yonkers reported a disclosure related to UpToDate.
New study suggests rate of alcohol use might be almost 19%
New study suggests rate of alcohol use might be almost 19%
The use of alcohol, tobacco products, and drugs by pregnant women is a substantial problem that may be more prevalent than previously thought, according to researcher Kimberly Yonkers, MD.
Higher levels of substance use during pregnancy means more negative impacts on maternal and fetal, neonatal, and child health. However, one bit of good news is that pregnant women still are less likely than nonpregnant women to engage in such behavior, said Dr. Yonkers, director of psychological medicine and the Center for Wellbeing of Women and Mothers at Yale University, New Haven, Conn.
Dr. Yonkers said in a featured presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Tobacco predominates among substances of concern used by pregnant women, with 11.6% reporting past-month use in 2018, according to the National Survey on Drug Use and Health, Dr. Yonkers said. Alcohol was next, with 9.9% of pregnant women reporting use in the past month, followed by drugs at 5.4%, of which marijuana was the most common.
Those numbers may jump much higher when focusing on substance use that’s biologically verified, she added, referring to a recent three-center cross-sectional study she and her colleagues published in Addiction (2019 Jun 19. doi: 10.1111/add.14651). In that study, alcohol use was as high as 18.9% among pregnant women who either had positive urine or self-reported use. Similarly, rates of nicotine or nicotine byproduct detected were 27% at one center in the study, and tetrahydrocannabinol reached 29.4% at that same center.
“These numbers are impressive,” Dr. Yonkers told attendees at the meeting. “So what we may be seeing in terms of the National Survey on Drug Use and Health, as valuable as it is, is in all likelihood it underestimates the use of substances in pregnancy.”
Substance use goes down in pregnancy as some women become more mindful of perinatal health, though unfortunately, that abstinence is offset by a dramatic rise in substance use in the 6-12 months’ post partum, research suggests.
Interestingly, big differences are found in both abstinence and relapse rates, with some data sets showing that, while alcohol is stopped fairly early, cigarettes are stopped much later, if at all.
On the postpartum side of the equation, relapse rates look similar for cigarettes, alcohol, and marijuana, but for some reason, cocaine relapse rates are much lower “That’s kind of nice, and we’d like to be able to understand what it is about this whole process that enabled that relative period of wellness,” Dr. Yonkers said.
Opioid use disorder is rising among pregnant women, just like it is in the general population, and 50% – or possibly even as high as 80% – of babies born to these women will experience neonatal opioid withdrawal, Dr. Yonkers said.
Maternal mortality in the United States increased by 34% from 2008 to 2016; while that’s a sobering statistic, Dr. Yonkers said, opioid-related maternal mortality doubled over that same time period.
“We really have to be mindful that we’re not just talking about taking care of kids and offspring, but we have to take care of moms – it’s really critical,” she said.
With the increasing legalization of cannabis, it’s expected that a lot more cannabis-exposed pregnancies will be seen in clinical practice, and some studies are starting to show an increase in prevalence in the preconception, prenatal, and postpartum period.
While some people feel that cannabis is benign, more data are needed, according to Dr. Yonkers, who said that cannabis and its metabolites cross the blood/placenta and blood/milk barriers, and that cannabinoid receptors are “very important” to fertility, implantation, and fetal development. One study recently published linked cannabis use in pregnancy to significant increases in preterm birth rates (JAMA. 2019 Jun 18;322[2]:145-52).
“We don’t really have a context for this, so we don’t really know what’s going to have an impact, and what’s not,” Dr. Yonkers said.
While both standard and novel treatments could help in the quest to achieve and maintain well-being in this unique patient population, Dr. Yonkers said that health equity and universal approaches to care might be needed to more comprehensively address the problem, which means taking a close look at how much money women have, the resources available to them, and where they live.
In many communities, eliminating inequalities in care will be critical to successfully addressing substance use issues in pregnant women, agreed Maureen Sayres Van Niel, MD, a specialist in women’s psychiatry in Boston and president of the Women’s Caucus of the APA.
“What we see is such a disparity in the delivery of care to women who are poor and living in communities where the socioeconomic and financial problems are very severe,” Dr. Van Niel said in an interview. “Unless we address these disparities, women will not be getting the kind of health care that they really need to have in the perinatal period.”
Dr. Yonkers reported a disclosure related to UpToDate.
The use of alcohol, tobacco products, and drugs by pregnant women is a substantial problem that may be more prevalent than previously thought, according to researcher Kimberly Yonkers, MD.
Higher levels of substance use during pregnancy means more negative impacts on maternal and fetal, neonatal, and child health. However, one bit of good news is that pregnant women still are less likely than nonpregnant women to engage in such behavior, said Dr. Yonkers, director of psychological medicine and the Center for Wellbeing of Women and Mothers at Yale University, New Haven, Conn.
Dr. Yonkers said in a featured presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
Tobacco predominates among substances of concern used by pregnant women, with 11.6% reporting past-month use in 2018, according to the National Survey on Drug Use and Health, Dr. Yonkers said. Alcohol was next, with 9.9% of pregnant women reporting use in the past month, followed by drugs at 5.4%, of which marijuana was the most common.
Those numbers may jump much higher when focusing on substance use that’s biologically verified, she added, referring to a recent three-center cross-sectional study she and her colleagues published in Addiction (2019 Jun 19. doi: 10.1111/add.14651). In that study, alcohol use was as high as 18.9% among pregnant women who either had positive urine or self-reported use. Similarly, rates of nicotine or nicotine byproduct detected were 27% at one center in the study, and tetrahydrocannabinol reached 29.4% at that same center.
“These numbers are impressive,” Dr. Yonkers told attendees at the meeting. “So what we may be seeing in terms of the National Survey on Drug Use and Health, as valuable as it is, is in all likelihood it underestimates the use of substances in pregnancy.”
Substance use goes down in pregnancy as some women become more mindful of perinatal health, though unfortunately, that abstinence is offset by a dramatic rise in substance use in the 6-12 months’ post partum, research suggests.
Interestingly, big differences are found in both abstinence and relapse rates, with some data sets showing that, while alcohol is stopped fairly early, cigarettes are stopped much later, if at all.
On the postpartum side of the equation, relapse rates look similar for cigarettes, alcohol, and marijuana, but for some reason, cocaine relapse rates are much lower “That’s kind of nice, and we’d like to be able to understand what it is about this whole process that enabled that relative period of wellness,” Dr. Yonkers said.
Opioid use disorder is rising among pregnant women, just like it is in the general population, and 50% – or possibly even as high as 80% – of babies born to these women will experience neonatal opioid withdrawal, Dr. Yonkers said.
Maternal mortality in the United States increased by 34% from 2008 to 2016; while that’s a sobering statistic, Dr. Yonkers said, opioid-related maternal mortality doubled over that same time period.
“We really have to be mindful that we’re not just talking about taking care of kids and offspring, but we have to take care of moms – it’s really critical,” she said.
With the increasing legalization of cannabis, it’s expected that a lot more cannabis-exposed pregnancies will be seen in clinical practice, and some studies are starting to show an increase in prevalence in the preconception, prenatal, and postpartum period.
While some people feel that cannabis is benign, more data are needed, according to Dr. Yonkers, who said that cannabis and its metabolites cross the blood/placenta and blood/milk barriers, and that cannabinoid receptors are “very important” to fertility, implantation, and fetal development. One study recently published linked cannabis use in pregnancy to significant increases in preterm birth rates (JAMA. 2019 Jun 18;322[2]:145-52).
“We don’t really have a context for this, so we don’t really know what’s going to have an impact, and what’s not,” Dr. Yonkers said.
While both standard and novel treatments could help in the quest to achieve and maintain well-being in this unique patient population, Dr. Yonkers said that health equity and universal approaches to care might be needed to more comprehensively address the problem, which means taking a close look at how much money women have, the resources available to them, and where they live.
In many communities, eliminating inequalities in care will be critical to successfully addressing substance use issues in pregnant women, agreed Maureen Sayres Van Niel, MD, a specialist in women’s psychiatry in Boston and president of the Women’s Caucus of the APA.
“What we see is such a disparity in the delivery of care to women who are poor and living in communities where the socioeconomic and financial problems are very severe,” Dr. Van Niel said in an interview. “Unless we address these disparities, women will not be getting the kind of health care that they really need to have in the perinatal period.”
Dr. Yonkers reported a disclosure related to UpToDate.
EXPERT ANALYSIS FROM APA 2020
Reframing AUD as treatable may reduce stigma
As alcohol-related death and disease rates rise, framing alcohol use disorder as a treatable disease with neurobiologic underpinnings might help reduce the stigma that many patients endure, according to George F. Koob, PhD, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
“Alcohol misuse and alcohol use disorder (AUD) have not gone away during the opioid crisis, and [they have] not gone away during the current (COVID-19) pandemic,” Dr. Koob said in a presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
There are at least 14 million individuals in the United States with AUD now, compared with 2 million with opioid use disorder, Dr. Koob said.
– just like hypertension or diabetes are treatable chronic conditions.
However, framing AUD as a treatable chronic condition is just one of many issues that need to be addressed, he said, adding that rates of screening and referral for AUD need to be increased among patients with other mental health conditions.
Psychiatrists can play a key role in reducing that screening and treatment gap, though concerningly, data suggest fewer than half of psychiatric patients with substance use disorders (SUDs) are being diagnosed or treated, said Andrew J. Saxon, MD, director of the Center of Excellence in Substance Abuse Treatment and Education (CESATE) VA Puget Sound Health Care System in Seattle.
Only about 9% of psychiatrist office visits from 2012 to 2015 involved a substance use disorder diagnosis or prescribed medication, whereas at least 20% of adults with mental health conditions also have an SUD, according to authors of a recent study in Psychiatric Services (2018 Jan 16. doi: 10.1176/appi.ps.201700457).
Better efforts are needed to improve training or somehow better incentivize psychiatrists to screen for alcohol use disorder and make sure patients get treatment for addiction, Dr. Saxon said in an interview.
“What we have is a lack of subspecialists in addiction psychiatry,” said Dr. Saxon, former director of the addiction psychiatry residency program at the University of Washington. “That becomes self-perpetuating, because we don’t have the knowledge experts to train the residents, and therefore, the residency programs don’t provide a rich enough experience.”
Changes in alcohol-related deaths
A new report (Alcoholism Clin Exper Res. 2020 Jan;44[1]:178-87) highlights the gravity of the AUD problem, showing that alcohol-related deaths have doubled over the past few decades, Dr. Koob said in his presentation.
Among individuals 16 years of age or older, the number of alcohol-related deaths in the United States rose from 35,914 in 1999 to 72,558 (or about 2.6% of all U.S. deaths) in 2017, according to that report, which was based on U.S. mortality data from the National Center for Health Statistics. The largest increase was seen in non-Hispanic white females, according to the investigators.
Alcohol is playing a more prominent role in “deaths of despair,” said Dr. Koob, noting that it contributes to about one-quarter of suicides and up to 20% of drug overdoses. “Probably even more salient is that half of liver disease in the United States is now caused by alcohol,” he added.
Misuse of alcohol is correlated with poor mental health, an observation that Dr. Koob said was particularly relevant to the current COVID-19 pandemic, he said, since alcohol is commonly used to cope with stress and symptoms of mental health conditions.
“In the end, it makes the prognosis worse,” he said.
Addressing AUD stigma
A better understanding of the neurobiology of addiction may reduce the stigma associated with AUD, helping reframe the issue as a “health condition, rather than as a moral failing,” Dr. Koob said.
Stigma remains a major barrier to AUD treatment, he added, explaining that factors contributing to stigma include shame patients may feel for what they perceive as a personal failure, and lack of knowledge about treatment options.
Separating AUD treatment from primary care exacerbates that problem, perpetuating the sense that AUD is somehow a “different” kind of issue, he said.
Health care clinicians in primary care can help alleviate the stigma by engaging in screening and offering referral to treatment, he said, adding that the NIAAA offers a navigator website designed to help individuals negotiate the process of choosing a treatment approach for AUD.
Language matters, according to Dr. Koob, who suggested using nonstigmatizing “person-first” terminology to refer to affected individuals not as alcoholics, but as “persons with AUD.”
Challenges ahead for AUD
There’s still a lot of work to be done to understand differences in alcohol pathology between men and women, especially as gaps narrow between the sexes for AUD incidence, early-onset drinking, frequency and intensity of drinking, and self-reported consequences, Dr. Koob said.
Age differences are also important to study. On one hand, older individuals appear to be more sensitive to the effects of alcohol, he said, because of metabolism changes, neurocognitive decline, and “inflamm-aging,” or the chronic and low level inflammatory state associated with aging.
Adolescents are also an increased-risk population of research interest, since brain wiring connections are “particularly sensitive” to alcohol in the teen years, potentially setting up changes in vulnerability to AUD that last into adulthood.
Other challenges include the unmet need for better and more individualized AUD treatments, the issue of alcohol tolerance, which Dr. Koob said has been “ignored for many years” by researchers, the contribution of pain to AUD, and the way that dysregulated sleep contributes to AUD, and vice versa.
Research likewise remains “challenging” regarding conditions that are frequently found in conjunction with AUD, such as major depressive episodes, anxiety disorders, and posttraumatic stress disorder: “These are all areas that we’re intensely interested in as comorbidities with AUD,” Dr. Koob said.
Dr. Koob reported no disclosures.
SOURCE: Koob GF. APA 2020, Abstract.
As alcohol-related death and disease rates rise, framing alcohol use disorder as a treatable disease with neurobiologic underpinnings might help reduce the stigma that many patients endure, according to George F. Koob, PhD, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
“Alcohol misuse and alcohol use disorder (AUD) have not gone away during the opioid crisis, and [they have] not gone away during the current (COVID-19) pandemic,” Dr. Koob said in a presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
There are at least 14 million individuals in the United States with AUD now, compared with 2 million with opioid use disorder, Dr. Koob said.
– just like hypertension or diabetes are treatable chronic conditions.
However, framing AUD as a treatable chronic condition is just one of many issues that need to be addressed, he said, adding that rates of screening and referral for AUD need to be increased among patients with other mental health conditions.
Psychiatrists can play a key role in reducing that screening and treatment gap, though concerningly, data suggest fewer than half of psychiatric patients with substance use disorders (SUDs) are being diagnosed or treated, said Andrew J. Saxon, MD, director of the Center of Excellence in Substance Abuse Treatment and Education (CESATE) VA Puget Sound Health Care System in Seattle.
Only about 9% of psychiatrist office visits from 2012 to 2015 involved a substance use disorder diagnosis or prescribed medication, whereas at least 20% of adults with mental health conditions also have an SUD, according to authors of a recent study in Psychiatric Services (2018 Jan 16. doi: 10.1176/appi.ps.201700457).
Better efforts are needed to improve training or somehow better incentivize psychiatrists to screen for alcohol use disorder and make sure patients get treatment for addiction, Dr. Saxon said in an interview.
“What we have is a lack of subspecialists in addiction psychiatry,” said Dr. Saxon, former director of the addiction psychiatry residency program at the University of Washington. “That becomes self-perpetuating, because we don’t have the knowledge experts to train the residents, and therefore, the residency programs don’t provide a rich enough experience.”
Changes in alcohol-related deaths
A new report (Alcoholism Clin Exper Res. 2020 Jan;44[1]:178-87) highlights the gravity of the AUD problem, showing that alcohol-related deaths have doubled over the past few decades, Dr. Koob said in his presentation.
Among individuals 16 years of age or older, the number of alcohol-related deaths in the United States rose from 35,914 in 1999 to 72,558 (or about 2.6% of all U.S. deaths) in 2017, according to that report, which was based on U.S. mortality data from the National Center for Health Statistics. The largest increase was seen in non-Hispanic white females, according to the investigators.
Alcohol is playing a more prominent role in “deaths of despair,” said Dr. Koob, noting that it contributes to about one-quarter of suicides and up to 20% of drug overdoses. “Probably even more salient is that half of liver disease in the United States is now caused by alcohol,” he added.
Misuse of alcohol is correlated with poor mental health, an observation that Dr. Koob said was particularly relevant to the current COVID-19 pandemic, he said, since alcohol is commonly used to cope with stress and symptoms of mental health conditions.
“In the end, it makes the prognosis worse,” he said.
Addressing AUD stigma
A better understanding of the neurobiology of addiction may reduce the stigma associated with AUD, helping reframe the issue as a “health condition, rather than as a moral failing,” Dr. Koob said.
Stigma remains a major barrier to AUD treatment, he added, explaining that factors contributing to stigma include shame patients may feel for what they perceive as a personal failure, and lack of knowledge about treatment options.
Separating AUD treatment from primary care exacerbates that problem, perpetuating the sense that AUD is somehow a “different” kind of issue, he said.
Health care clinicians in primary care can help alleviate the stigma by engaging in screening and offering referral to treatment, he said, adding that the NIAAA offers a navigator website designed to help individuals negotiate the process of choosing a treatment approach for AUD.
Language matters, according to Dr. Koob, who suggested using nonstigmatizing “person-first” terminology to refer to affected individuals not as alcoholics, but as “persons with AUD.”
Challenges ahead for AUD
There’s still a lot of work to be done to understand differences in alcohol pathology between men and women, especially as gaps narrow between the sexes for AUD incidence, early-onset drinking, frequency and intensity of drinking, and self-reported consequences, Dr. Koob said.
Age differences are also important to study. On one hand, older individuals appear to be more sensitive to the effects of alcohol, he said, because of metabolism changes, neurocognitive decline, and “inflamm-aging,” or the chronic and low level inflammatory state associated with aging.
Adolescents are also an increased-risk population of research interest, since brain wiring connections are “particularly sensitive” to alcohol in the teen years, potentially setting up changes in vulnerability to AUD that last into adulthood.
Other challenges include the unmet need for better and more individualized AUD treatments, the issue of alcohol tolerance, which Dr. Koob said has been “ignored for many years” by researchers, the contribution of pain to AUD, and the way that dysregulated sleep contributes to AUD, and vice versa.
Research likewise remains “challenging” regarding conditions that are frequently found in conjunction with AUD, such as major depressive episodes, anxiety disorders, and posttraumatic stress disorder: “These are all areas that we’re intensely interested in as comorbidities with AUD,” Dr. Koob said.
Dr. Koob reported no disclosures.
SOURCE: Koob GF. APA 2020, Abstract.
As alcohol-related death and disease rates rise, framing alcohol use disorder as a treatable disease with neurobiologic underpinnings might help reduce the stigma that many patients endure, according to George F. Koob, PhD, director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
“Alcohol misuse and alcohol use disorder (AUD) have not gone away during the opioid crisis, and [they have] not gone away during the current (COVID-19) pandemic,” Dr. Koob said in a presentation at the annual meeting of the American Psychiatric Association, which was held as a virtual live event.
There are at least 14 million individuals in the United States with AUD now, compared with 2 million with opioid use disorder, Dr. Koob said.
– just like hypertension or diabetes are treatable chronic conditions.
However, framing AUD as a treatable chronic condition is just one of many issues that need to be addressed, he said, adding that rates of screening and referral for AUD need to be increased among patients with other mental health conditions.
Psychiatrists can play a key role in reducing that screening and treatment gap, though concerningly, data suggest fewer than half of psychiatric patients with substance use disorders (SUDs) are being diagnosed or treated, said Andrew J. Saxon, MD, director of the Center of Excellence in Substance Abuse Treatment and Education (CESATE) VA Puget Sound Health Care System in Seattle.
Only about 9% of psychiatrist office visits from 2012 to 2015 involved a substance use disorder diagnosis or prescribed medication, whereas at least 20% of adults with mental health conditions also have an SUD, according to authors of a recent study in Psychiatric Services (2018 Jan 16. doi: 10.1176/appi.ps.201700457).
Better efforts are needed to improve training or somehow better incentivize psychiatrists to screen for alcohol use disorder and make sure patients get treatment for addiction, Dr. Saxon said in an interview.
“What we have is a lack of subspecialists in addiction psychiatry,” said Dr. Saxon, former director of the addiction psychiatry residency program at the University of Washington. “That becomes self-perpetuating, because we don’t have the knowledge experts to train the residents, and therefore, the residency programs don’t provide a rich enough experience.”
Changes in alcohol-related deaths
A new report (Alcoholism Clin Exper Res. 2020 Jan;44[1]:178-87) highlights the gravity of the AUD problem, showing that alcohol-related deaths have doubled over the past few decades, Dr. Koob said in his presentation.
Among individuals 16 years of age or older, the number of alcohol-related deaths in the United States rose from 35,914 in 1999 to 72,558 (or about 2.6% of all U.S. deaths) in 2017, according to that report, which was based on U.S. mortality data from the National Center for Health Statistics. The largest increase was seen in non-Hispanic white females, according to the investigators.
Alcohol is playing a more prominent role in “deaths of despair,” said Dr. Koob, noting that it contributes to about one-quarter of suicides and up to 20% of drug overdoses. “Probably even more salient is that half of liver disease in the United States is now caused by alcohol,” he added.
Misuse of alcohol is correlated with poor mental health, an observation that Dr. Koob said was particularly relevant to the current COVID-19 pandemic, he said, since alcohol is commonly used to cope with stress and symptoms of mental health conditions.
“In the end, it makes the prognosis worse,” he said.
Addressing AUD stigma
A better understanding of the neurobiology of addiction may reduce the stigma associated with AUD, helping reframe the issue as a “health condition, rather than as a moral failing,” Dr. Koob said.
Stigma remains a major barrier to AUD treatment, he added, explaining that factors contributing to stigma include shame patients may feel for what they perceive as a personal failure, and lack of knowledge about treatment options.
Separating AUD treatment from primary care exacerbates that problem, perpetuating the sense that AUD is somehow a “different” kind of issue, he said.
Health care clinicians in primary care can help alleviate the stigma by engaging in screening and offering referral to treatment, he said, adding that the NIAAA offers a navigator website designed to help individuals negotiate the process of choosing a treatment approach for AUD.
Language matters, according to Dr. Koob, who suggested using nonstigmatizing “person-first” terminology to refer to affected individuals not as alcoholics, but as “persons with AUD.”
Challenges ahead for AUD
There’s still a lot of work to be done to understand differences in alcohol pathology between men and women, especially as gaps narrow between the sexes for AUD incidence, early-onset drinking, frequency and intensity of drinking, and self-reported consequences, Dr. Koob said.
Age differences are also important to study. On one hand, older individuals appear to be more sensitive to the effects of alcohol, he said, because of metabolism changes, neurocognitive decline, and “inflamm-aging,” or the chronic and low level inflammatory state associated with aging.
Adolescents are also an increased-risk population of research interest, since brain wiring connections are “particularly sensitive” to alcohol in the teen years, potentially setting up changes in vulnerability to AUD that last into adulthood.
Other challenges include the unmet need for better and more individualized AUD treatments, the issue of alcohol tolerance, which Dr. Koob said has been “ignored for many years” by researchers, the contribution of pain to AUD, and the way that dysregulated sleep contributes to AUD, and vice versa.
Research likewise remains “challenging” regarding conditions that are frequently found in conjunction with AUD, such as major depressive episodes, anxiety disorders, and posttraumatic stress disorder: “These are all areas that we’re intensely interested in as comorbidities with AUD,” Dr. Koob said.
Dr. Koob reported no disclosures.
SOURCE: Koob GF. APA 2020, Abstract.
FROM APA 2020