User login
Endometrial ablation devices: How to make them truly safe
CASE: Leaking fluid causes intraoperative burns
G.S. is a 45-year-old mother of three who is admitted for surgery for persistent menorrhagia. She has experienced at least two menstrual periods every month for several months, each of them associated with heavy bleeding. She has a history of hypothyroidism and hypertension, but no serious disease or surgery, and considers herself to be in good physical and mental health.
G.S. undergoes endometrial hydrothermablation (HTA) under general inhalation anesthesia. After the HTA mechanism is primed, the heating cycle is started, with a good seal and no fluid leaking from the cervix.
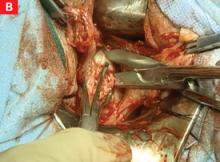
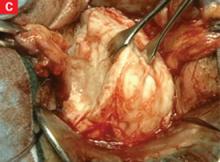
Approximately 8 minutes into the procedure, a 5-mL fluid deficit is noted, and a small amount of hot fluid is observed to be leaking from the cervical os. Examination reveals a thermal injury to the cervix and anterior vaginal wall. The wound is irrigated with cool, sterile saline, and silver sulfadiazine cream is applied. The patient is discharged.
Could this injury have been avoided? Is further treatment warranted?
A minimally invasive operation does not necessarily translate to minimal risk of serious complications. Although few studies of nonhysteroscopic endometrial ablation techniques report any complications,1,2 Baggish and Savells3 found a number of injuries when they searched hospital records and the Food and Drug Administration (FDA) database (TABLE). They identified serious complications associated with the following devices:
- HydroThermablator (Boston Scientific), which utilizes a modified operating hysteroscope to deliver 10 to 12 mL of preheated saline into the uterus under low pressure.4 Complications: 16 adverse events were reported to the FDA, 13 of which involved the retrograde leakage of hot water, causing burns to the cervix, vagina, and vulva. Six additional injuries not reported to the FDA were identified at a single institution.
- Novasure (Cytyc), which employs bipolar electrodes that cover a porous bag.5,6 Complications: 32 injuries, 26 of them uterine perforations.
- Thermachoice (Gynecare), a fluid-distended balloon ablator.7 Complications: 22 injuries included retrograde leakage of hot water after balloon failure and transmural thermal injury, with spread to, and injury of, proximal structures. One death was reported.
- Microsulis (MEA), which uses microwave energy to ablate the endometrium.8-10 Complications: 19 injuries, including 13 thermal injuries to the intestines.
Baggish and Savells3 initiated this study after discovering six adverse events within their own hospital system utilizing a single device (HTA). Because these injuries were not reported to the FDA, the overall number of complications is likely higher than the figures given here.
This article describes the proper use of nonhysteroscopic endometrial ablation devices, the best ways to avert serious injury, and optimal treatment when complication occurs.
TABLE
Complications associated with 4 endometrial ablation devices
| COMPLICATION | HYDRO THERMABLATOR* | THERMACHOICE | NOVASURE | MICROSULIS |
|---|---|---|---|---|
| Uterine perforation | 2 | 3 | 26 | 19 |
| Intestinal injury | 1† | 1† | – | 13† |
| Retrograde leakage burn | 19 | 6 | – | – |
| Infection/sepsis | – | 1† | 2 | 1 |
| Fistula/sinus | – | 1† | 1 | – |
| Transmural uterine burn | – | 1 | – | – |
| Cervical stenosis | – | 8 | 1 | – |
| Cardiac arrest | 1 | – | 1 | – |
| Death | – | 1 | – | – |
| Other major | – | 3 | 1 | 4† |
| Total | 22 | 22 | 32 | 20 |
| * Includes author’s data; 6 retrograde leaks | ||||
| † Collateral injury | ||||
CASE continued: Patient opts for hysterectomy
In the case just described, G.S. was examined 1 week after surgery and found to have an exophytic burn over the entire right half of the cervix, extending into the vagina. She was readmitted for 3 days of intravenous (IV) antibiotic treatment and wound care. Computed tomography imaging showed gas formation within the damaged cervix.
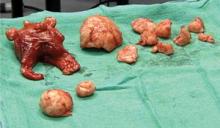
Six weeks after surgery, the patient was still menstruating heavily, but her cervix and vagina had healed. Six months later, she underwent total abdominal hysterectomy for continued menorrhagia.
When is endometrial ablation an option?
Indications for endometrial ablation using a nonhysteroscopic, minimally invasive technique are no different from those for hysteroscopic ablation.11 Abnormal, or dysfunctional, uterine bleeding is the principal reason for this operation. Dysfunctional bleeding is heavy or prolonged menses over 6 months or longer that fail to respond to conservative measures and occur in the absence of tumor, pregnancy, or inflammation (ie, infection).
A woman who meets these criteria should have a desire to retain her uterus if she is to be a candidate for a nonhysteroscopic, minimally invasive technique. She also should understand that ablation can render pregnancy unlikely and even pathologic. Her understanding of this consequence should be documented in the chart! Last, she should be informed that ablation will not necessarily render her sterile, so contraception or sterilization will be required to avoid pregnancy. This should also be clearly documented in the medical record.
Endometrial ablation may also be an alternative to hysterectomy for a mentally retarded woman who is unable to manage menses. Abnormal uterine bleeding in conjunction with bleeding diathesis, significant obesity, or serious medical disorders can also be treated by endometrial ablation.
Avoid endometrial ablation in certain circumstances
These circumstances include the presence of endometrial hyperplasia, endometrial cancer, endocervical neoplasia, cervical stenosis, an undiagnosed adnexal mass, moderate to severe dysmenorrhea, adenomyosis, or a uterine cavity larger than 10 cm.12-15
Valle and Baggish15 reported eight cases in which women developed endometrial carcinoma following ablation, and identified the following major risk factors for postablation cancer:
- endometrial hyperplasia unresponsive to progesterone or progestin therapy
- complex endometrial hyperplasia
- atypical hyperplasia.
These conditions are contraindications to endometrial ablation.
Avoid a rush to ablation
The growing popularity of office-based, minimally invasive, nonhysteroscopic techniques, coupled with an increasing desire for and acceptance of elective cessation of menses, may stretch the indications listed above and cut short the discovery of contraindications. Clearly, thorough endometrial sampling and precise histopathologic interpretation are required before embarking on any type of endometrial ablation, to minimize the risk of complications.
How to prevent injury
Reduce the risk of perforation
Uterine perforation occurs for a variety of reasons:
- position of the uterus is unknown
- uterus has not been gently and carefully sounded
- cervix is insufficiently dilated to permit passage of the probe
- device is too long (large) to be accommodated in an individual patient’s uterus
- uterine cavity is distorted by pathology, such as adhesions, myomas, etc.
Attention to these details before surgery can prevent perforation.
When uterine injury occurs, the bowel is also at risk
The intestines can be injured following perforation or transmural injury of the uterus. Bowel injury has been reported with hysteroscopic ablation and resection as well as with Nd-YAG laser ablation.16-18
Do not activate hot water or electrosurgical energy unless you are 100% certain that the device is within the uterine cavity.
Ideally, manufacturers’ safety studies should guarantee no risk of transtubal spillage of hot liquid.
Hot fluid adds to risk of burns
Devices that permit retrograde leakage of hot fluid, such as the HTA, should be modified to ensure sealing at the level of the external and internal cervical os. The Enable device (Innerdyne), no longer marketed in the United States, had such a sealing mechanism, which minimized retrograde leakage of hot water.
Balloon failure may be an unavoidable injury, but pretesting of the device and careful attention to pressure readings—particularly in a small uterus—may mitigate the risk.
Be alert for electrical leakage
The microwave device operates at the megahertz range of frequency. At this high frequency, the risk of leakage is much greater than with devices that operate in the kilohertz range. Therefore, it is important to pay close attention to grounding sites, such as cardiovascular-monitoring electrodes.
High-power monopolar devices, prolonged application of energy to tissue, and high generator frequency are all associated with leakage and subsequent burns.
- Keep the success rate above 90%
- Minimize complications by proper technique and instrument selection
- Press the market to develop a range of device sizes that will individualize the procedure
- Keep the price of a procedure under $1,000
- Establish and adhere to careful patient selection criteria
Early recognition and treatment are vital to ensure the patient’s safety and reduce the risk of medicolegal liability. I recommend the following steps:
- Stop the procedure immediately if perforation is suspected. If you suspect that hot water has been dispersed within the abdominal cavity, switch to laparotomy and consult a general surgeon to inspect the entire intestine for injury. If perforation occurs during the use of electrosurgical energy, the same action is warranted. If uterine perforation occurs in isolation (ie, there is no thermal energy compounding the problem), admit the patient for careful observation, appropriate blood chemistries and hematologic studies, and radiologic examination.
- When hot liquids are spilled, switch to retrograde flow immediately and generously flush the vulva, vagina, and cervix with cold water. Cleanse the entire area with a soapless detergent, and apply clindamycin cream to the vagina and silver sulfadiazine cream to the vulva. Admit the patient for application of cold compresses, ice packs, and burn therapy, and obtain baseline cultures and hematologic studies and a plastic surgery consult. If third-degree (full thickness) burns are suspected, treat any suspected wound infection aggressively after obtaining cultures. Severe and inordinate pain should be investigated as a possible sign of necrotizing fasciitis. After discharge, follow the patient’s progress at weekly intervals.
- Talk to the patient and her family. It is a good idea to explain the complication in very clear terms. I believe it is reasonable to explain how the complication occurred, without speculation or theatrical explanations. Also be sure to document this conversation, including date and time. It may be useful to have a neutral witness present during the conversation. By and large, the patient and her family are likely to appreciate an honest account of how the complication occurred. Hiding data or attempting to cover up the injury may motivate the patient to seek legal representation.
The long-term success of endometrial ablation devices as a whole depends on several conditions. Foremost, the entire class of devices should demonstrate efficacy on par with hysteroscopic ablation. Currently, efficacy ranges from 80% to 95% (short-term follow-up).11 The goal of minimally invasive procedures should be a sustainable 92% rate of amenorrhea, hypomenorrhea, or light, periodic menses. A long-term failure rate of 25% is unacceptable.22-24 If the devices can, by their simplicity, be adapted to more or less universal office application and attain a 5-year success rate of 90% or higher, they will become the standard of care.
One size does not really fit all
Serious complications from endometrial ablation devices occur with regular frequency and must be eliminated or greatly reduced. Perforation is a significant problem and may be related to the “one-size-fits-all” design of the device. Perhaps a range of sizes needs to be produced and fitted to the individual uterine cavity.
If such complications as perforation and burns to the bowel, cervix, vagina, and vulva can be eliminated or relegated to rarity, then a happy future for these procedures lies beyond the horizon.
Price ceiling should be set at $1,000
If an operation can consistently be performed for less than $1,000 total cost—the cost of in-hospital endometrial ablation—it will gain mass appeal. In hospitals and so-called surgicenters, ablations are expensive and, therefore, less attractive to self- or third-party payers. If fees are based on the volume of cases, then a procedure may be price-efficient.
Outcome depends on patient selection
Poorly screened patients who have underlying hyperplasia may develop postablation carcinoma. Women who have dysmenorrhea before the procedure can be predicted to suffer from it afterward. Older women (ie, 40 years or older) will have better long-term success than younger women. And women with a large uterus or myomas will have a higher failure rate than women with smaller cavities (ie, less than 10 cm in length).
What this means for the individual surgeon
Although minimally invasive techniques are relatively easy to perform and simple to learn, each part of the procedure requires careful application and great attention to detail. Perforation of the uterus and leakage of scalding hot liquid must be avoided. If these complications occur, prompt diagnosis and appropriate treatment are critical. The removal of these procedures from the operating room to the office as well as competitive pricing of instrumentation will make nonhysteroscopic, minimally invasive endometrial ablation more cost-effective.
The modern era of practical endometrial ablation began in 1981, when Goldrath and colleagues19 reported Nd-YAG laser photovaporization of the endometrium via hysteroscopy for treatment of excessive uterine bleeding. Two years later, DeCherney and Polan20 reported hysteroscopic control of abnormal uterine bleeding using the urologic resectoscope.
Over succeeding years, Baggish and Baltoyannis21 and Baggish and Sze22 reported extensive experience with hysteroscopic endometrial ablation in both high- and average-risk patients, including long-term follow-up of 568 cases over 11 years. Garry and colleagues23 reported a large series of 600 cases from the United Kingdom. Not only did these laser techniques prove to be effective, achieving amenorrhea rates ranging from 30% to 60%, but overall control of abnormal bleeding exceeded 90%. In the large series involving approximately 1,200 cases, no uterine perforations were reported.21-23 The major complication: Fluid overload secondary to vascular uptake of distension medium.
In Europe and the United Kingdom, most hysteroscopic treatment of abnormal bleeding involved endometrial resection using the cutting loop of the resectoscope. In the United States, ablation with the ball electrode of the resectoscope largely replaced the Nd-YAG laser because the resectoscopic trigger mechanism required less skill and hand–eye coordination than the hand–finger-controlled movement of the 600- to 1,000-micron laser fiber.24-26
A search for more benign techniques
A 1997 UK survey analyzed 10,686 cases of hysteroscopic endometrial destruction and identified 474 complications.27 Resection alone had a complication rate of 10.9% and an emergency hysterectomy rate of 13 for every 1,000 patients. Laser ablation had a complication rate of 5.5% and an emergency hysterectomy rate of 2 for every 1,000 patients, and the corresponding figures for rollerball ablation were a 4.5% complication rate and 3 emergency hysterectomies for every 1,000 patients. Two deaths occurred (in 10,000 cases) and were associated with loop excision.
Published data indicated that:
- Successful outcomes after endometrial ablation or resection were directly proportional to the skill of the surgeon
- Complications, particularly serious complications, were related to the experience and skill of the surgeon
- Infusion of uterine distension medium, particularly hypo-osmolar solutions, was associated with serious complications when fluid deficits exceeded approximately 500 to 1,000 mL.
As a result, a number of investigators sought to develop new surgical techniques to control abnormal uterine bleeding that would minimize the skill required by the surgeon (requiring only insertion of a cannula into the uterus and a “cookbook” ablation procedure), eliminate the need for distension medium and general anesthesia, and attain efficacy equivalent to earlier techniques.
A quartet of options
Among the devices that resulted were:
- A microwave technique, described by several investigators.8-10 Its chief drawback: High-frequency electrical leakage with the potential to cause thermal burns.
- An intrauterine balloon device distended with sterile water or saline is heated in situ to 85° to 90° Celsius, thereby cooking the endometrium.
- An electrode-bearing device that features an array of monopolar electrodes over the endometrium-facing aspect of a balloon or bipolar electrodes over a porous bag.
- Devices that circulate a small volume of hot saline freely within the uterine cavity. Hydrothermablation delivers 10 to 12 mL of preheated saline into the uterus under low pressure. A similar technique delivers 10 to 12 mL of cool water or saline into the uterus through a sealed cannula, followed by in situ heating and circulation of the fluid at low pressure via a computer-controlled device.
Safety studies were required by the FDA and were performed on all these devices, and the risk of complications appeared to be negligible.1,2 As this article illustrates, that is not the case.
Four devices, four ways of achieving ablation
Since the advent of nonhysteroscopic, minimally invasive endometrial ablation devices, four distinct techniques have gained widespread use
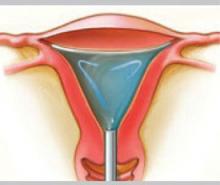
Hydrothermablation
The closed-loop system (HTA) ablates the lining of the endometrium under hysteroscopic visualization by recirculating heated saline within the uterus. The modified hysteroscope allows the operator to view the ablation as it occurs within the uterine cavity.
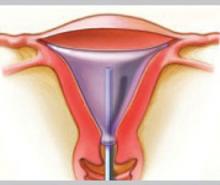
Balloon ablation
Balloon ablation (Thermachoice) features a double-dip balloon construction that conforms to the contours of the uterine cavity. The saline or water in the balloon is heated in situ. This device requires an undistorted uterine cavity, relies on the integrity of the balloon to prevent forward or retrograde spillage of scalding water, and is time-controlled.
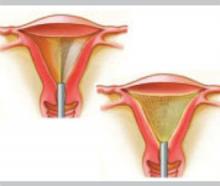
Radiofrequency technology
The three-dimensional gold-plated bipolar mesh electrode (NovaSure) is inserted into the uterine cavity and advanced toward the fundus. Once it is properly positioned (above, left), the system is activated to produce 180 W of bipolar power. A moisture-transport vacuum system draws the endometrium into contact with the mesh to enhance tissue vaporization and evacuate debris.
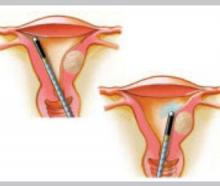
Microwave energy
Microwave energy is emitted from the tip of the device (Microsulis), which is moved back and forth in a sweeping manner, from the fundus to the lower uterine segment. The device directly heats tissue to a depth of 3 mm, with conductive heating of adjacent tissue for an additional 2 to 3 mm. The total 5- to 6-mm depth ensures coagulation and destruction of the basal layer. Microwave energy does not require direct contact with the tissue, as it will “fill the gap” caused by cornual and fibroid distortions.
1. Bustos-Lopez H, Baggish MS, Valle RF, et al. Assessment of the safety of intrauterine instillation of heated saline for endometrial ablation. Fertil Steril. 1998;69:155-160.
2. Baggish MS, Paraiso M, Breznock EM, et al. A computer-controlled, continuously circulating, hot irrigating system for endometrial ablation. Am J Obstet Gynecol. 1995;173:1842-1848.
3. Baggish MS, Savells A. Complications associated with minimally invasive non-hysteroscopic endometrial ablation techniques. J Gynecol Surg. 2007;23:7-12.
4. Goldrath MH. Evaluation of HydroThermablator and rollerball endometrial ablation for menorrhagia: 3 years after treatment. J Am Assoc Gynecol Laparosc. 2003;10:505-511.
5. Abbott J, Hawe J, Hunter D, et al. A double-blind randomized trial comparing the cavaterm and the Novasure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003;80:203-208.
6. Cooper J, Gimpelson R, Laberge P, et al. A randomized, multi-center trial of safety and efficacy of the Novasure system in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418-428.
7. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:429-435.
8. Phipps JH, Lewis BV, Roberts T, et al. Treatment of functional menorrhagia with radiofrequency endometrial ablation. Lancet. 1990;335:374-376.
9. Sharp N, Cronin N, Feldberg I, et al. Microwaves for menorrhagia: a new fast technique for endometrial ablation. Lancet. 1995;346:1003-1004.
10. Thijssen RFA. Radiofrequency-induced endometrial ablation: an update. Br J Obstet Gynaecol. 1997;104:608-613.
11. Baggish MS. Minimally invasive non-hysteroscopic methods for endometrial ablation. In: Baggish MS, Valle RF, Guedj H, eds. Hysteroscopy: Visual Perspectives of Uterine Anatomy, Physiology, and Pathology. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2007:405-415.
12. Dwyer N, Hutton J, Stirrat GM. Randomized controlled trial comparing endometrial resection with abdominal hysterectomy for the surgical treatment of menorrhagia. Br J Obstet Gynaecol. 1993;100:237-243.
13. Raiga J, Mage G, Glowaczower E, et al. Factors affecting risk of failure after endometrial resection. J Gynecol Surg. 1995;11:1-5.
14. Shelly-Jones D, Mooney P, Garry R. Factors influencing the outcome of endometrial laser ablation. J Gynecol Surg. 1994;10:211-215.
15. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;176:569-572.
16. Kanter MH, Kivnick S. Bowel injury from rollerball ablation of the endometrium. Obstet Gynecol. 1992;79:833-835.
17. Perry CP, Daniell JF, Gimpelson RJ. Bowel injury from Nd-YAG endometrial ablation. J Gynecol Surg. 1990;6:1999-2003.
18. Scottish Hysteroscopy Audit Group. A Scottish audit of hysteroscopic surgery for menorrhagia: complications and follow-up. Br J Obstet Gynaecol. 1995;102:239-254.
19. Goldrath MH, Fuller TA, Segal S. Laser photovaporization of the endometrium for the treatment of menorrhagia. Am J Obstet Gynecol. 1981;40:14-19.
20. DeCherney A, Polan ML. Hysteroscopic management of intrauterine lesions and intractable uterine bleeding. Obstet Gynecol. 1983;61:392-396.
21. Baggish MS, Baltoyannis P. New techniques for laser ablation of the endometrium in high-risk patients. Am J Obstet Gynecol. 1988;159:287-292.
22. Baggish MS, Sze EHM. Endometrial ablation: a series of 568 patients treated over an 11-year period. Am J Obstet Gynecol. 1996;174:908-913.
23. Garry R, Shelly-Jones D, Mooney P, et al. Six hundred endometrial laser ablations. Obstet Gynecol. 1995;85:24-29.
24. Magos AL, Bauman R, Lockwood GM, et al. Experience with the first 250 endometrial resections for menorrhagia. Lancet. 1991;337:1074-1078.
25. Wortman M, Daggett A. Hysteroscopic endometrial resection: a new technique for the treatment of menorrhagia. Obstet Gynecol. 1994;83:295-298.
26. Townsend DE, Richart RM, Paskowitz, et al. Rollerball coagulation of the endometrium. Obstet Gynecol. 1990;76:310-313.
27. Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the mistletoe study. Br J Obstet Gynaecol. 1997;104:1351-1359.
CASE: Leaking fluid causes intraoperative burns
G.S. is a 45-year-old mother of three who is admitted for surgery for persistent menorrhagia. She has experienced at least two menstrual periods every month for several months, each of them associated with heavy bleeding. She has a history of hypothyroidism and hypertension, but no serious disease or surgery, and considers herself to be in good physical and mental health.
G.S. undergoes endometrial hydrothermablation (HTA) under general inhalation anesthesia. After the HTA mechanism is primed, the heating cycle is started, with a good seal and no fluid leaking from the cervix.
Approximately 8 minutes into the procedure, a 5-mL fluid deficit is noted, and a small amount of hot fluid is observed to be leaking from the cervical os. Examination reveals a thermal injury to the cervix and anterior vaginal wall. The wound is irrigated with cool, sterile saline, and silver sulfadiazine cream is applied. The patient is discharged.
Could this injury have been avoided? Is further treatment warranted?
A minimally invasive operation does not necessarily translate to minimal risk of serious complications. Although few studies of nonhysteroscopic endometrial ablation techniques report any complications,1,2 Baggish and Savells3 found a number of injuries when they searched hospital records and the Food and Drug Administration (FDA) database (TABLE). They identified serious complications associated with the following devices:
- HydroThermablator (Boston Scientific), which utilizes a modified operating hysteroscope to deliver 10 to 12 mL of preheated saline into the uterus under low pressure.4 Complications: 16 adverse events were reported to the FDA, 13 of which involved the retrograde leakage of hot water, causing burns to the cervix, vagina, and vulva. Six additional injuries not reported to the FDA were identified at a single institution.
- Novasure (Cytyc), which employs bipolar electrodes that cover a porous bag.5,6 Complications: 32 injuries, 26 of them uterine perforations.
- Thermachoice (Gynecare), a fluid-distended balloon ablator.7 Complications: 22 injuries included retrograde leakage of hot water after balloon failure and transmural thermal injury, with spread to, and injury of, proximal structures. One death was reported.
- Microsulis (MEA), which uses microwave energy to ablate the endometrium.8-10 Complications: 19 injuries, including 13 thermal injuries to the intestines.
Baggish and Savells3 initiated this study after discovering six adverse events within their own hospital system utilizing a single device (HTA). Because these injuries were not reported to the FDA, the overall number of complications is likely higher than the figures given here.
This article describes the proper use of nonhysteroscopic endometrial ablation devices, the best ways to avert serious injury, and optimal treatment when complication occurs.
TABLE
Complications associated with 4 endometrial ablation devices
| COMPLICATION | HYDRO THERMABLATOR* | THERMACHOICE | NOVASURE | MICROSULIS |
|---|---|---|---|---|
| Uterine perforation | 2 | 3 | 26 | 19 |
| Intestinal injury | 1† | 1† | – | 13† |
| Retrograde leakage burn | 19 | 6 | – | – |
| Infection/sepsis | – | 1† | 2 | 1 |
| Fistula/sinus | – | 1† | 1 | – |
| Transmural uterine burn | – | 1 | – | – |
| Cervical stenosis | – | 8 | 1 | – |
| Cardiac arrest | 1 | – | 1 | – |
| Death | – | 1 | – | – |
| Other major | – | 3 | 1 | 4† |
| Total | 22 | 22 | 32 | 20 |
| * Includes author’s data; 6 retrograde leaks | ||||
| † Collateral injury | ||||
CASE continued: Patient opts for hysterectomy
In the case just described, G.S. was examined 1 week after surgery and found to have an exophytic burn over the entire right half of the cervix, extending into the vagina. She was readmitted for 3 days of intravenous (IV) antibiotic treatment and wound care. Computed tomography imaging showed gas formation within the damaged cervix.
Six weeks after surgery, the patient was still menstruating heavily, but her cervix and vagina had healed. Six months later, she underwent total abdominal hysterectomy for continued menorrhagia.
When is endometrial ablation an option?
Indications for endometrial ablation using a nonhysteroscopic, minimally invasive technique are no different from those for hysteroscopic ablation.11 Abnormal, or dysfunctional, uterine bleeding is the principal reason for this operation. Dysfunctional bleeding is heavy or prolonged menses over 6 months or longer that fail to respond to conservative measures and occur in the absence of tumor, pregnancy, or inflammation (ie, infection).
A woman who meets these criteria should have a desire to retain her uterus if she is to be a candidate for a nonhysteroscopic, minimally invasive technique. She also should understand that ablation can render pregnancy unlikely and even pathologic. Her understanding of this consequence should be documented in the chart! Last, she should be informed that ablation will not necessarily render her sterile, so contraception or sterilization will be required to avoid pregnancy. This should also be clearly documented in the medical record.
Endometrial ablation may also be an alternative to hysterectomy for a mentally retarded woman who is unable to manage menses. Abnormal uterine bleeding in conjunction with bleeding diathesis, significant obesity, or serious medical disorders can also be treated by endometrial ablation.
Avoid endometrial ablation in certain circumstances
These circumstances include the presence of endometrial hyperplasia, endometrial cancer, endocervical neoplasia, cervical stenosis, an undiagnosed adnexal mass, moderate to severe dysmenorrhea, adenomyosis, or a uterine cavity larger than 10 cm.12-15
Valle and Baggish15 reported eight cases in which women developed endometrial carcinoma following ablation, and identified the following major risk factors for postablation cancer:
- endometrial hyperplasia unresponsive to progesterone or progestin therapy
- complex endometrial hyperplasia
- atypical hyperplasia.
These conditions are contraindications to endometrial ablation.
Avoid a rush to ablation
The growing popularity of office-based, minimally invasive, nonhysteroscopic techniques, coupled with an increasing desire for and acceptance of elective cessation of menses, may stretch the indications listed above and cut short the discovery of contraindications. Clearly, thorough endometrial sampling and precise histopathologic interpretation are required before embarking on any type of endometrial ablation, to minimize the risk of complications.
How to prevent injury
Reduce the risk of perforation
Uterine perforation occurs for a variety of reasons:
- position of the uterus is unknown
- uterus has not been gently and carefully sounded
- cervix is insufficiently dilated to permit passage of the probe
- device is too long (large) to be accommodated in an individual patient’s uterus
- uterine cavity is distorted by pathology, such as adhesions, myomas, etc.
Attention to these details before surgery can prevent perforation.
When uterine injury occurs, the bowel is also at risk
The intestines can be injured following perforation or transmural injury of the uterus. Bowel injury has been reported with hysteroscopic ablation and resection as well as with Nd-YAG laser ablation.16-18
Do not activate hot water or electrosurgical energy unless you are 100% certain that the device is within the uterine cavity.
Ideally, manufacturers’ safety studies should guarantee no risk of transtubal spillage of hot liquid.
Hot fluid adds to risk of burns
Devices that permit retrograde leakage of hot fluid, such as the HTA, should be modified to ensure sealing at the level of the external and internal cervical os. The Enable device (Innerdyne), no longer marketed in the United States, had such a sealing mechanism, which minimized retrograde leakage of hot water.
Balloon failure may be an unavoidable injury, but pretesting of the device and careful attention to pressure readings—particularly in a small uterus—may mitigate the risk.
Be alert for electrical leakage
The microwave device operates at the megahertz range of frequency. At this high frequency, the risk of leakage is much greater than with devices that operate in the kilohertz range. Therefore, it is important to pay close attention to grounding sites, such as cardiovascular-monitoring electrodes.
High-power monopolar devices, prolonged application of energy to tissue, and high generator frequency are all associated with leakage and subsequent burns.
- Keep the success rate above 90%
- Minimize complications by proper technique and instrument selection
- Press the market to develop a range of device sizes that will individualize the procedure
- Keep the price of a procedure under $1,000
- Establish and adhere to careful patient selection criteria
Early recognition and treatment are vital to ensure the patient’s safety and reduce the risk of medicolegal liability. I recommend the following steps:
- Stop the procedure immediately if perforation is suspected. If you suspect that hot water has been dispersed within the abdominal cavity, switch to laparotomy and consult a general surgeon to inspect the entire intestine for injury. If perforation occurs during the use of electrosurgical energy, the same action is warranted. If uterine perforation occurs in isolation (ie, there is no thermal energy compounding the problem), admit the patient for careful observation, appropriate blood chemistries and hematologic studies, and radiologic examination.
- When hot liquids are spilled, switch to retrograde flow immediately and generously flush the vulva, vagina, and cervix with cold water. Cleanse the entire area with a soapless detergent, and apply clindamycin cream to the vagina and silver sulfadiazine cream to the vulva. Admit the patient for application of cold compresses, ice packs, and burn therapy, and obtain baseline cultures and hematologic studies and a plastic surgery consult. If third-degree (full thickness) burns are suspected, treat any suspected wound infection aggressively after obtaining cultures. Severe and inordinate pain should be investigated as a possible sign of necrotizing fasciitis. After discharge, follow the patient’s progress at weekly intervals.
- Talk to the patient and her family. It is a good idea to explain the complication in very clear terms. I believe it is reasonable to explain how the complication occurred, without speculation or theatrical explanations. Also be sure to document this conversation, including date and time. It may be useful to have a neutral witness present during the conversation. By and large, the patient and her family are likely to appreciate an honest account of how the complication occurred. Hiding data or attempting to cover up the injury may motivate the patient to seek legal representation.
The long-term success of endometrial ablation devices as a whole depends on several conditions. Foremost, the entire class of devices should demonstrate efficacy on par with hysteroscopic ablation. Currently, efficacy ranges from 80% to 95% (short-term follow-up).11 The goal of minimally invasive procedures should be a sustainable 92% rate of amenorrhea, hypomenorrhea, or light, periodic menses. A long-term failure rate of 25% is unacceptable.22-24 If the devices can, by their simplicity, be adapted to more or less universal office application and attain a 5-year success rate of 90% or higher, they will become the standard of care.
One size does not really fit all
Serious complications from endometrial ablation devices occur with regular frequency and must be eliminated or greatly reduced. Perforation is a significant problem and may be related to the “one-size-fits-all” design of the device. Perhaps a range of sizes needs to be produced and fitted to the individual uterine cavity.
If such complications as perforation and burns to the bowel, cervix, vagina, and vulva can be eliminated or relegated to rarity, then a happy future for these procedures lies beyond the horizon.
Price ceiling should be set at $1,000
If an operation can consistently be performed for less than $1,000 total cost—the cost of in-hospital endometrial ablation—it will gain mass appeal. In hospitals and so-called surgicenters, ablations are expensive and, therefore, less attractive to self- or third-party payers. If fees are based on the volume of cases, then a procedure may be price-efficient.
Outcome depends on patient selection
Poorly screened patients who have underlying hyperplasia may develop postablation carcinoma. Women who have dysmenorrhea before the procedure can be predicted to suffer from it afterward. Older women (ie, 40 years or older) will have better long-term success than younger women. And women with a large uterus or myomas will have a higher failure rate than women with smaller cavities (ie, less than 10 cm in length).
What this means for the individual surgeon
Although minimally invasive techniques are relatively easy to perform and simple to learn, each part of the procedure requires careful application and great attention to detail. Perforation of the uterus and leakage of scalding hot liquid must be avoided. If these complications occur, prompt diagnosis and appropriate treatment are critical. The removal of these procedures from the operating room to the office as well as competitive pricing of instrumentation will make nonhysteroscopic, minimally invasive endometrial ablation more cost-effective.
The modern era of practical endometrial ablation began in 1981, when Goldrath and colleagues19 reported Nd-YAG laser photovaporization of the endometrium via hysteroscopy for treatment of excessive uterine bleeding. Two years later, DeCherney and Polan20 reported hysteroscopic control of abnormal uterine bleeding using the urologic resectoscope.
Over succeeding years, Baggish and Baltoyannis21 and Baggish and Sze22 reported extensive experience with hysteroscopic endometrial ablation in both high- and average-risk patients, including long-term follow-up of 568 cases over 11 years. Garry and colleagues23 reported a large series of 600 cases from the United Kingdom. Not only did these laser techniques prove to be effective, achieving amenorrhea rates ranging from 30% to 60%, but overall control of abnormal bleeding exceeded 90%. In the large series involving approximately 1,200 cases, no uterine perforations were reported.21-23 The major complication: Fluid overload secondary to vascular uptake of distension medium.
In Europe and the United Kingdom, most hysteroscopic treatment of abnormal bleeding involved endometrial resection using the cutting loop of the resectoscope. In the United States, ablation with the ball electrode of the resectoscope largely replaced the Nd-YAG laser because the resectoscopic trigger mechanism required less skill and hand–eye coordination than the hand–finger-controlled movement of the 600- to 1,000-micron laser fiber.24-26
A search for more benign techniques
A 1997 UK survey analyzed 10,686 cases of hysteroscopic endometrial destruction and identified 474 complications.27 Resection alone had a complication rate of 10.9% and an emergency hysterectomy rate of 13 for every 1,000 patients. Laser ablation had a complication rate of 5.5% and an emergency hysterectomy rate of 2 for every 1,000 patients, and the corresponding figures for rollerball ablation were a 4.5% complication rate and 3 emergency hysterectomies for every 1,000 patients. Two deaths occurred (in 10,000 cases) and were associated with loop excision.
Published data indicated that:
- Successful outcomes after endometrial ablation or resection were directly proportional to the skill of the surgeon
- Complications, particularly serious complications, were related to the experience and skill of the surgeon
- Infusion of uterine distension medium, particularly hypo-osmolar solutions, was associated with serious complications when fluid deficits exceeded approximately 500 to 1,000 mL.
As a result, a number of investigators sought to develop new surgical techniques to control abnormal uterine bleeding that would minimize the skill required by the surgeon (requiring only insertion of a cannula into the uterus and a “cookbook” ablation procedure), eliminate the need for distension medium and general anesthesia, and attain efficacy equivalent to earlier techniques.
A quartet of options
Among the devices that resulted were:
- A microwave technique, described by several investigators.8-10 Its chief drawback: High-frequency electrical leakage with the potential to cause thermal burns.
- An intrauterine balloon device distended with sterile water or saline is heated in situ to 85° to 90° Celsius, thereby cooking the endometrium.
- An electrode-bearing device that features an array of monopolar electrodes over the endometrium-facing aspect of a balloon or bipolar electrodes over a porous bag.
- Devices that circulate a small volume of hot saline freely within the uterine cavity. Hydrothermablation delivers 10 to 12 mL of preheated saline into the uterus under low pressure. A similar technique delivers 10 to 12 mL of cool water or saline into the uterus through a sealed cannula, followed by in situ heating and circulation of the fluid at low pressure via a computer-controlled device.
Safety studies were required by the FDA and were performed on all these devices, and the risk of complications appeared to be negligible.1,2 As this article illustrates, that is not the case.
Four devices, four ways of achieving ablation
Since the advent of nonhysteroscopic, minimally invasive endometrial ablation devices, four distinct techniques have gained widespread use
Hydrothermablation
The closed-loop system (HTA) ablates the lining of the endometrium under hysteroscopic visualization by recirculating heated saline within the uterus. The modified hysteroscope allows the operator to view the ablation as it occurs within the uterine cavity.
Balloon ablation
Balloon ablation (Thermachoice) features a double-dip balloon construction that conforms to the contours of the uterine cavity. The saline or water in the balloon is heated in situ. This device requires an undistorted uterine cavity, relies on the integrity of the balloon to prevent forward or retrograde spillage of scalding water, and is time-controlled.
Radiofrequency technology
The three-dimensional gold-plated bipolar mesh electrode (NovaSure) is inserted into the uterine cavity and advanced toward the fundus. Once it is properly positioned (above, left), the system is activated to produce 180 W of bipolar power. A moisture-transport vacuum system draws the endometrium into contact with the mesh to enhance tissue vaporization and evacuate debris.
Microwave energy
Microwave energy is emitted from the tip of the device (Microsulis), which is moved back and forth in a sweeping manner, from the fundus to the lower uterine segment. The device directly heats tissue to a depth of 3 mm, with conductive heating of adjacent tissue for an additional 2 to 3 mm. The total 5- to 6-mm depth ensures coagulation and destruction of the basal layer. Microwave energy does not require direct contact with the tissue, as it will “fill the gap” caused by cornual and fibroid distortions.
CASE: Leaking fluid causes intraoperative burns
G.S. is a 45-year-old mother of three who is admitted for surgery for persistent menorrhagia. She has experienced at least two menstrual periods every month for several months, each of them associated with heavy bleeding. She has a history of hypothyroidism and hypertension, but no serious disease or surgery, and considers herself to be in good physical and mental health.
G.S. undergoes endometrial hydrothermablation (HTA) under general inhalation anesthesia. After the HTA mechanism is primed, the heating cycle is started, with a good seal and no fluid leaking from the cervix.
Approximately 8 minutes into the procedure, a 5-mL fluid deficit is noted, and a small amount of hot fluid is observed to be leaking from the cervical os. Examination reveals a thermal injury to the cervix and anterior vaginal wall. The wound is irrigated with cool, sterile saline, and silver sulfadiazine cream is applied. The patient is discharged.
Could this injury have been avoided? Is further treatment warranted?
A minimally invasive operation does not necessarily translate to minimal risk of serious complications. Although few studies of nonhysteroscopic endometrial ablation techniques report any complications,1,2 Baggish and Savells3 found a number of injuries when they searched hospital records and the Food and Drug Administration (FDA) database (TABLE). They identified serious complications associated with the following devices:
- HydroThermablator (Boston Scientific), which utilizes a modified operating hysteroscope to deliver 10 to 12 mL of preheated saline into the uterus under low pressure.4 Complications: 16 adverse events were reported to the FDA, 13 of which involved the retrograde leakage of hot water, causing burns to the cervix, vagina, and vulva. Six additional injuries not reported to the FDA were identified at a single institution.
- Novasure (Cytyc), which employs bipolar electrodes that cover a porous bag.5,6 Complications: 32 injuries, 26 of them uterine perforations.
- Thermachoice (Gynecare), a fluid-distended balloon ablator.7 Complications: 22 injuries included retrograde leakage of hot water after balloon failure and transmural thermal injury, with spread to, and injury of, proximal structures. One death was reported.
- Microsulis (MEA), which uses microwave energy to ablate the endometrium.8-10 Complications: 19 injuries, including 13 thermal injuries to the intestines.
Baggish and Savells3 initiated this study after discovering six adverse events within their own hospital system utilizing a single device (HTA). Because these injuries were not reported to the FDA, the overall number of complications is likely higher than the figures given here.
This article describes the proper use of nonhysteroscopic endometrial ablation devices, the best ways to avert serious injury, and optimal treatment when complication occurs.
TABLE
Complications associated with 4 endometrial ablation devices
| COMPLICATION | HYDRO THERMABLATOR* | THERMACHOICE | NOVASURE | MICROSULIS |
|---|---|---|---|---|
| Uterine perforation | 2 | 3 | 26 | 19 |
| Intestinal injury | 1† | 1† | – | 13† |
| Retrograde leakage burn | 19 | 6 | – | – |
| Infection/sepsis | – | 1† | 2 | 1 |
| Fistula/sinus | – | 1† | 1 | – |
| Transmural uterine burn | – | 1 | – | – |
| Cervical stenosis | – | 8 | 1 | – |
| Cardiac arrest | 1 | – | 1 | – |
| Death | – | 1 | – | – |
| Other major | – | 3 | 1 | 4† |
| Total | 22 | 22 | 32 | 20 |
| * Includes author’s data; 6 retrograde leaks | ||||
| † Collateral injury | ||||
CASE continued: Patient opts for hysterectomy
In the case just described, G.S. was examined 1 week after surgery and found to have an exophytic burn over the entire right half of the cervix, extending into the vagina. She was readmitted for 3 days of intravenous (IV) antibiotic treatment and wound care. Computed tomography imaging showed gas formation within the damaged cervix.
Six weeks after surgery, the patient was still menstruating heavily, but her cervix and vagina had healed. Six months later, she underwent total abdominal hysterectomy for continued menorrhagia.
When is endometrial ablation an option?
Indications for endometrial ablation using a nonhysteroscopic, minimally invasive technique are no different from those for hysteroscopic ablation.11 Abnormal, or dysfunctional, uterine bleeding is the principal reason for this operation. Dysfunctional bleeding is heavy or prolonged menses over 6 months or longer that fail to respond to conservative measures and occur in the absence of tumor, pregnancy, or inflammation (ie, infection).
A woman who meets these criteria should have a desire to retain her uterus if she is to be a candidate for a nonhysteroscopic, minimally invasive technique. She also should understand that ablation can render pregnancy unlikely and even pathologic. Her understanding of this consequence should be documented in the chart! Last, she should be informed that ablation will not necessarily render her sterile, so contraception or sterilization will be required to avoid pregnancy. This should also be clearly documented in the medical record.
Endometrial ablation may also be an alternative to hysterectomy for a mentally retarded woman who is unable to manage menses. Abnormal uterine bleeding in conjunction with bleeding diathesis, significant obesity, or serious medical disorders can also be treated by endometrial ablation.
Avoid endometrial ablation in certain circumstances
These circumstances include the presence of endometrial hyperplasia, endometrial cancer, endocervical neoplasia, cervical stenosis, an undiagnosed adnexal mass, moderate to severe dysmenorrhea, adenomyosis, or a uterine cavity larger than 10 cm.12-15
Valle and Baggish15 reported eight cases in which women developed endometrial carcinoma following ablation, and identified the following major risk factors for postablation cancer:
- endometrial hyperplasia unresponsive to progesterone or progestin therapy
- complex endometrial hyperplasia
- atypical hyperplasia.
These conditions are contraindications to endometrial ablation.
Avoid a rush to ablation
The growing popularity of office-based, minimally invasive, nonhysteroscopic techniques, coupled with an increasing desire for and acceptance of elective cessation of menses, may stretch the indications listed above and cut short the discovery of contraindications. Clearly, thorough endometrial sampling and precise histopathologic interpretation are required before embarking on any type of endometrial ablation, to minimize the risk of complications.
How to prevent injury
Reduce the risk of perforation
Uterine perforation occurs for a variety of reasons:
- position of the uterus is unknown
- uterus has not been gently and carefully sounded
- cervix is insufficiently dilated to permit passage of the probe
- device is too long (large) to be accommodated in an individual patient’s uterus
- uterine cavity is distorted by pathology, such as adhesions, myomas, etc.
Attention to these details before surgery can prevent perforation.
When uterine injury occurs, the bowel is also at risk
The intestines can be injured following perforation or transmural injury of the uterus. Bowel injury has been reported with hysteroscopic ablation and resection as well as with Nd-YAG laser ablation.16-18
Do not activate hot water or electrosurgical energy unless you are 100% certain that the device is within the uterine cavity.
Ideally, manufacturers’ safety studies should guarantee no risk of transtubal spillage of hot liquid.
Hot fluid adds to risk of burns
Devices that permit retrograde leakage of hot fluid, such as the HTA, should be modified to ensure sealing at the level of the external and internal cervical os. The Enable device (Innerdyne), no longer marketed in the United States, had such a sealing mechanism, which minimized retrograde leakage of hot water.
Balloon failure may be an unavoidable injury, but pretesting of the device and careful attention to pressure readings—particularly in a small uterus—may mitigate the risk.
Be alert for electrical leakage
The microwave device operates at the megahertz range of frequency. At this high frequency, the risk of leakage is much greater than with devices that operate in the kilohertz range. Therefore, it is important to pay close attention to grounding sites, such as cardiovascular-monitoring electrodes.
High-power monopolar devices, prolonged application of energy to tissue, and high generator frequency are all associated with leakage and subsequent burns.
- Keep the success rate above 90%
- Minimize complications by proper technique and instrument selection
- Press the market to develop a range of device sizes that will individualize the procedure
- Keep the price of a procedure under $1,000
- Establish and adhere to careful patient selection criteria
Early recognition and treatment are vital to ensure the patient’s safety and reduce the risk of medicolegal liability. I recommend the following steps:
- Stop the procedure immediately if perforation is suspected. If you suspect that hot water has been dispersed within the abdominal cavity, switch to laparotomy and consult a general surgeon to inspect the entire intestine for injury. If perforation occurs during the use of electrosurgical energy, the same action is warranted. If uterine perforation occurs in isolation (ie, there is no thermal energy compounding the problem), admit the patient for careful observation, appropriate blood chemistries and hematologic studies, and radiologic examination.
- When hot liquids are spilled, switch to retrograde flow immediately and generously flush the vulva, vagina, and cervix with cold water. Cleanse the entire area with a soapless detergent, and apply clindamycin cream to the vagina and silver sulfadiazine cream to the vulva. Admit the patient for application of cold compresses, ice packs, and burn therapy, and obtain baseline cultures and hematologic studies and a plastic surgery consult. If third-degree (full thickness) burns are suspected, treat any suspected wound infection aggressively after obtaining cultures. Severe and inordinate pain should be investigated as a possible sign of necrotizing fasciitis. After discharge, follow the patient’s progress at weekly intervals.
- Talk to the patient and her family. It is a good idea to explain the complication in very clear terms. I believe it is reasonable to explain how the complication occurred, without speculation or theatrical explanations. Also be sure to document this conversation, including date and time. It may be useful to have a neutral witness present during the conversation. By and large, the patient and her family are likely to appreciate an honest account of how the complication occurred. Hiding data or attempting to cover up the injury may motivate the patient to seek legal representation.
The long-term success of endometrial ablation devices as a whole depends on several conditions. Foremost, the entire class of devices should demonstrate efficacy on par with hysteroscopic ablation. Currently, efficacy ranges from 80% to 95% (short-term follow-up).11 The goal of minimally invasive procedures should be a sustainable 92% rate of amenorrhea, hypomenorrhea, or light, periodic menses. A long-term failure rate of 25% is unacceptable.22-24 If the devices can, by their simplicity, be adapted to more or less universal office application and attain a 5-year success rate of 90% or higher, they will become the standard of care.
One size does not really fit all
Serious complications from endometrial ablation devices occur with regular frequency and must be eliminated or greatly reduced. Perforation is a significant problem and may be related to the “one-size-fits-all” design of the device. Perhaps a range of sizes needs to be produced and fitted to the individual uterine cavity.
If such complications as perforation and burns to the bowel, cervix, vagina, and vulva can be eliminated or relegated to rarity, then a happy future for these procedures lies beyond the horizon.
Price ceiling should be set at $1,000
If an operation can consistently be performed for less than $1,000 total cost—the cost of in-hospital endometrial ablation—it will gain mass appeal. In hospitals and so-called surgicenters, ablations are expensive and, therefore, less attractive to self- or third-party payers. If fees are based on the volume of cases, then a procedure may be price-efficient.
Outcome depends on patient selection
Poorly screened patients who have underlying hyperplasia may develop postablation carcinoma. Women who have dysmenorrhea before the procedure can be predicted to suffer from it afterward. Older women (ie, 40 years or older) will have better long-term success than younger women. And women with a large uterus or myomas will have a higher failure rate than women with smaller cavities (ie, less than 10 cm in length).
What this means for the individual surgeon
Although minimally invasive techniques are relatively easy to perform and simple to learn, each part of the procedure requires careful application and great attention to detail. Perforation of the uterus and leakage of scalding hot liquid must be avoided. If these complications occur, prompt diagnosis and appropriate treatment are critical. The removal of these procedures from the operating room to the office as well as competitive pricing of instrumentation will make nonhysteroscopic, minimally invasive endometrial ablation more cost-effective.
The modern era of practical endometrial ablation began in 1981, when Goldrath and colleagues19 reported Nd-YAG laser photovaporization of the endometrium via hysteroscopy for treatment of excessive uterine bleeding. Two years later, DeCherney and Polan20 reported hysteroscopic control of abnormal uterine bleeding using the urologic resectoscope.
Over succeeding years, Baggish and Baltoyannis21 and Baggish and Sze22 reported extensive experience with hysteroscopic endometrial ablation in both high- and average-risk patients, including long-term follow-up of 568 cases over 11 years. Garry and colleagues23 reported a large series of 600 cases from the United Kingdom. Not only did these laser techniques prove to be effective, achieving amenorrhea rates ranging from 30% to 60%, but overall control of abnormal bleeding exceeded 90%. In the large series involving approximately 1,200 cases, no uterine perforations were reported.21-23 The major complication: Fluid overload secondary to vascular uptake of distension medium.
In Europe and the United Kingdom, most hysteroscopic treatment of abnormal bleeding involved endometrial resection using the cutting loop of the resectoscope. In the United States, ablation with the ball electrode of the resectoscope largely replaced the Nd-YAG laser because the resectoscopic trigger mechanism required less skill and hand–eye coordination than the hand–finger-controlled movement of the 600- to 1,000-micron laser fiber.24-26
A search for more benign techniques
A 1997 UK survey analyzed 10,686 cases of hysteroscopic endometrial destruction and identified 474 complications.27 Resection alone had a complication rate of 10.9% and an emergency hysterectomy rate of 13 for every 1,000 patients. Laser ablation had a complication rate of 5.5% and an emergency hysterectomy rate of 2 for every 1,000 patients, and the corresponding figures for rollerball ablation were a 4.5% complication rate and 3 emergency hysterectomies for every 1,000 patients. Two deaths occurred (in 10,000 cases) and were associated with loop excision.
Published data indicated that:
- Successful outcomes after endometrial ablation or resection were directly proportional to the skill of the surgeon
- Complications, particularly serious complications, were related to the experience and skill of the surgeon
- Infusion of uterine distension medium, particularly hypo-osmolar solutions, was associated with serious complications when fluid deficits exceeded approximately 500 to 1,000 mL.
As a result, a number of investigators sought to develop new surgical techniques to control abnormal uterine bleeding that would minimize the skill required by the surgeon (requiring only insertion of a cannula into the uterus and a “cookbook” ablation procedure), eliminate the need for distension medium and general anesthesia, and attain efficacy equivalent to earlier techniques.
A quartet of options
Among the devices that resulted were:
- A microwave technique, described by several investigators.8-10 Its chief drawback: High-frequency electrical leakage with the potential to cause thermal burns.
- An intrauterine balloon device distended with sterile water or saline is heated in situ to 85° to 90° Celsius, thereby cooking the endometrium.
- An electrode-bearing device that features an array of monopolar electrodes over the endometrium-facing aspect of a balloon or bipolar electrodes over a porous bag.
- Devices that circulate a small volume of hot saline freely within the uterine cavity. Hydrothermablation delivers 10 to 12 mL of preheated saline into the uterus under low pressure. A similar technique delivers 10 to 12 mL of cool water or saline into the uterus through a sealed cannula, followed by in situ heating and circulation of the fluid at low pressure via a computer-controlled device.
Safety studies were required by the FDA and were performed on all these devices, and the risk of complications appeared to be negligible.1,2 As this article illustrates, that is not the case.
Four devices, four ways of achieving ablation
Since the advent of nonhysteroscopic, minimally invasive endometrial ablation devices, four distinct techniques have gained widespread use
Hydrothermablation
The closed-loop system (HTA) ablates the lining of the endometrium under hysteroscopic visualization by recirculating heated saline within the uterus. The modified hysteroscope allows the operator to view the ablation as it occurs within the uterine cavity.
Balloon ablation
Balloon ablation (Thermachoice) features a double-dip balloon construction that conforms to the contours of the uterine cavity. The saline or water in the balloon is heated in situ. This device requires an undistorted uterine cavity, relies on the integrity of the balloon to prevent forward or retrograde spillage of scalding water, and is time-controlled.
Radiofrequency technology
The three-dimensional gold-plated bipolar mesh electrode (NovaSure) is inserted into the uterine cavity and advanced toward the fundus. Once it is properly positioned (above, left), the system is activated to produce 180 W of bipolar power. A moisture-transport vacuum system draws the endometrium into contact with the mesh to enhance tissue vaporization and evacuate debris.
Microwave energy
Microwave energy is emitted from the tip of the device (Microsulis), which is moved back and forth in a sweeping manner, from the fundus to the lower uterine segment. The device directly heats tissue to a depth of 3 mm, with conductive heating of adjacent tissue for an additional 2 to 3 mm. The total 5- to 6-mm depth ensures coagulation and destruction of the basal layer. Microwave energy does not require direct contact with the tissue, as it will “fill the gap” caused by cornual and fibroid distortions.
1. Bustos-Lopez H, Baggish MS, Valle RF, et al. Assessment of the safety of intrauterine instillation of heated saline for endometrial ablation. Fertil Steril. 1998;69:155-160.
2. Baggish MS, Paraiso M, Breznock EM, et al. A computer-controlled, continuously circulating, hot irrigating system for endometrial ablation. Am J Obstet Gynecol. 1995;173:1842-1848.
3. Baggish MS, Savells A. Complications associated with minimally invasive non-hysteroscopic endometrial ablation techniques. J Gynecol Surg. 2007;23:7-12.
4. Goldrath MH. Evaluation of HydroThermablator and rollerball endometrial ablation for menorrhagia: 3 years after treatment. J Am Assoc Gynecol Laparosc. 2003;10:505-511.
5. Abbott J, Hawe J, Hunter D, et al. A double-blind randomized trial comparing the cavaterm and the Novasure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003;80:203-208.
6. Cooper J, Gimpelson R, Laberge P, et al. A randomized, multi-center trial of safety and efficacy of the Novasure system in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418-428.
7. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:429-435.
8. Phipps JH, Lewis BV, Roberts T, et al. Treatment of functional menorrhagia with radiofrequency endometrial ablation. Lancet. 1990;335:374-376.
9. Sharp N, Cronin N, Feldberg I, et al. Microwaves for menorrhagia: a new fast technique for endometrial ablation. Lancet. 1995;346:1003-1004.
10. Thijssen RFA. Radiofrequency-induced endometrial ablation: an update. Br J Obstet Gynaecol. 1997;104:608-613.
11. Baggish MS. Minimally invasive non-hysteroscopic methods for endometrial ablation. In: Baggish MS, Valle RF, Guedj H, eds. Hysteroscopy: Visual Perspectives of Uterine Anatomy, Physiology, and Pathology. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2007:405-415.
12. Dwyer N, Hutton J, Stirrat GM. Randomized controlled trial comparing endometrial resection with abdominal hysterectomy for the surgical treatment of menorrhagia. Br J Obstet Gynaecol. 1993;100:237-243.
13. Raiga J, Mage G, Glowaczower E, et al. Factors affecting risk of failure after endometrial resection. J Gynecol Surg. 1995;11:1-5.
14. Shelly-Jones D, Mooney P, Garry R. Factors influencing the outcome of endometrial laser ablation. J Gynecol Surg. 1994;10:211-215.
15. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;176:569-572.
16. Kanter MH, Kivnick S. Bowel injury from rollerball ablation of the endometrium. Obstet Gynecol. 1992;79:833-835.
17. Perry CP, Daniell JF, Gimpelson RJ. Bowel injury from Nd-YAG endometrial ablation. J Gynecol Surg. 1990;6:1999-2003.
18. Scottish Hysteroscopy Audit Group. A Scottish audit of hysteroscopic surgery for menorrhagia: complications and follow-up. Br J Obstet Gynaecol. 1995;102:239-254.
19. Goldrath MH, Fuller TA, Segal S. Laser photovaporization of the endometrium for the treatment of menorrhagia. Am J Obstet Gynecol. 1981;40:14-19.
20. DeCherney A, Polan ML. Hysteroscopic management of intrauterine lesions and intractable uterine bleeding. Obstet Gynecol. 1983;61:392-396.
21. Baggish MS, Baltoyannis P. New techniques for laser ablation of the endometrium in high-risk patients. Am J Obstet Gynecol. 1988;159:287-292.
22. Baggish MS, Sze EHM. Endometrial ablation: a series of 568 patients treated over an 11-year period. Am J Obstet Gynecol. 1996;174:908-913.
23. Garry R, Shelly-Jones D, Mooney P, et al. Six hundred endometrial laser ablations. Obstet Gynecol. 1995;85:24-29.
24. Magos AL, Bauman R, Lockwood GM, et al. Experience with the first 250 endometrial resections for menorrhagia. Lancet. 1991;337:1074-1078.
25. Wortman M, Daggett A. Hysteroscopic endometrial resection: a new technique for the treatment of menorrhagia. Obstet Gynecol. 1994;83:295-298.
26. Townsend DE, Richart RM, Paskowitz, et al. Rollerball coagulation of the endometrium. Obstet Gynecol. 1990;76:310-313.
27. Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the mistletoe study. Br J Obstet Gynaecol. 1997;104:1351-1359.
1. Bustos-Lopez H, Baggish MS, Valle RF, et al. Assessment of the safety of intrauterine instillation of heated saline for endometrial ablation. Fertil Steril. 1998;69:155-160.
2. Baggish MS, Paraiso M, Breznock EM, et al. A computer-controlled, continuously circulating, hot irrigating system for endometrial ablation. Am J Obstet Gynecol. 1995;173:1842-1848.
3. Baggish MS, Savells A. Complications associated with minimally invasive non-hysteroscopic endometrial ablation techniques. J Gynecol Surg. 2007;23:7-12.
4. Goldrath MH. Evaluation of HydroThermablator and rollerball endometrial ablation for menorrhagia: 3 years after treatment. J Am Assoc Gynecol Laparosc. 2003;10:505-511.
5. Abbott J, Hawe J, Hunter D, et al. A double-blind randomized trial comparing the cavaterm and the Novasure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003;80:203-208.
6. Cooper J, Gimpelson R, Laberge P, et al. A randomized, multi-center trial of safety and efficacy of the Novasure system in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418-428.
7. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:429-435.
8. Phipps JH, Lewis BV, Roberts T, et al. Treatment of functional menorrhagia with radiofrequency endometrial ablation. Lancet. 1990;335:374-376.
9. Sharp N, Cronin N, Feldberg I, et al. Microwaves for menorrhagia: a new fast technique for endometrial ablation. Lancet. 1995;346:1003-1004.
10. Thijssen RFA. Radiofrequency-induced endometrial ablation: an update. Br J Obstet Gynaecol. 1997;104:608-613.
11. Baggish MS. Minimally invasive non-hysteroscopic methods for endometrial ablation. In: Baggish MS, Valle RF, Guedj H, eds. Hysteroscopy: Visual Perspectives of Uterine Anatomy, Physiology, and Pathology. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2007:405-415.
12. Dwyer N, Hutton J, Stirrat GM. Randomized controlled trial comparing endometrial resection with abdominal hysterectomy for the surgical treatment of menorrhagia. Br J Obstet Gynaecol. 1993;100:237-243.
13. Raiga J, Mage G, Glowaczower E, et al. Factors affecting risk of failure after endometrial resection. J Gynecol Surg. 1995;11:1-5.
14. Shelly-Jones D, Mooney P, Garry R. Factors influencing the outcome of endometrial laser ablation. J Gynecol Surg. 1994;10:211-215.
15. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;176:569-572.
16. Kanter MH, Kivnick S. Bowel injury from rollerball ablation of the endometrium. Obstet Gynecol. 1992;79:833-835.
17. Perry CP, Daniell JF, Gimpelson RJ. Bowel injury from Nd-YAG endometrial ablation. J Gynecol Surg. 1990;6:1999-2003.
18. Scottish Hysteroscopy Audit Group. A Scottish audit of hysteroscopic surgery for menorrhagia: complications and follow-up. Br J Obstet Gynaecol. 1995;102:239-254.
19. Goldrath MH, Fuller TA, Segal S. Laser photovaporization of the endometrium for the treatment of menorrhagia. Am J Obstet Gynecol. 1981;40:14-19.
20. DeCherney A, Polan ML. Hysteroscopic management of intrauterine lesions and intractable uterine bleeding. Obstet Gynecol. 1983;61:392-396.
21. Baggish MS, Baltoyannis P. New techniques for laser ablation of the endometrium in high-risk patients. Am J Obstet Gynecol. 1988;159:287-292.
22. Baggish MS, Sze EHM. Endometrial ablation: a series of 568 patients treated over an 11-year period. Am J Obstet Gynecol. 1996;174:908-913.
23. Garry R, Shelly-Jones D, Mooney P, et al. Six hundred endometrial laser ablations. Obstet Gynecol. 1995;85:24-29.
24. Magos AL, Bauman R, Lockwood GM, et al. Experience with the first 250 endometrial resections for menorrhagia. Lancet. 1991;337:1074-1078.
25. Wortman M, Daggett A. Hysteroscopic endometrial resection: a new technique for the treatment of menorrhagia. Obstet Gynecol. 1994;83:295-298.
26. Townsend DE, Richart RM, Paskowitz, et al. Rollerball coagulation of the endometrium. Obstet Gynecol. 1990;76:310-313.
27. Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the mistletoe study. Br J Obstet Gynaecol. 1997;104:1351-1359.
How to manage the cuff at vaginal hysterectomy
CASE 1 What procedures should accompany hysterectomy?
A.E., 44, mother of one, complains of heavy irregular bleeding with no sensation of a vaginal bulge. She has tried oral contraceptives, but they did not improve her bleeding pattern. She also has undergone dilatation and curettage and hysteroscopy (benign findings), also with no improvement.
Examination reveals a 9- to 10-week-size fibroid uterus, which is confirmed by ultrasonography. Pelvic support appears to be excellent.
After a discussion of the options, the patient elects to undergo vaginal hysterectomy. Are other procedures warranted?
Ask a gynecologic surgeon to name the most significant challenges he or she faces, and the answer is likely to include preventing pelvic organ prolapse after surgical intervention. Approximately one third of operations for pelvic organ prolapse involve patients whose prolapse has recurred after previous surgery.1 Although we have advanced our understanding of the anatomy of pelvic support and the pathophysiology of support defects, the various surgical strategies remain largely untested and unproven.
Even women with good pelvic support who are undergoing hysterectomy—like the patient described above—are vulnerable. One particular area of concern: the risk of enterocele or vaginal apical prolapse, or both, after hysterectomy. In this article, I describe a technique to reduce the risk of these defects after vaginal hysterectomy: high uterosacral suspension, or modified McCall culdoplasty.
Enterocele and apical prolapse do not always coexist
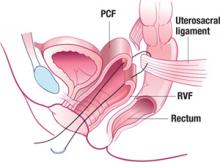
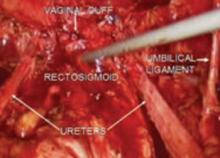
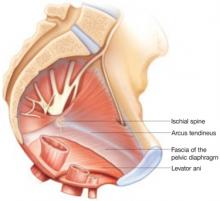
Enterocele and apical prolapse are distinct entities. The latter represents a deficiency in the level I supporting structures described by DeLancey2—primarily the uterosacral and cardinal ligaments (FIGURE 1). Enterocele, or peritoneocele, is a herniation of the cul-de-sac peritoneum, with or without intestinal contents. In women who have undergone hysterectomy, enterocele is usually caused by a lack of continuity of level II fibers, namely, the failure to approximate the pubocervical and rectovaginal connective tissues at the time of hysterectomy.3 Careful attention to the vaginal cuff and cul-de-sac at the time of hysterectomy is therefore imperative.
FIGURE 1 Three levels of support
The endopelvic fascia of a posthysterectomy patient divided into DeLancey’s biomechanical levels: level I—proximal suspension, level II—lateral attachment, and level III—distal fusion.
The McCall culdoplasty: 50 years “young”
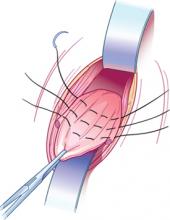
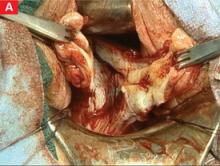
In 1957, Milton McCall, MD, described a technique to manage the cul-de-sac at the time of vaginal hysterectomy.4 The McCall technique of posterior culdoplasty differs from other approaches by omitting dissection and excision of the hernia sac, or excess cul-de-sac peritoneum. The original McCall culdoplasty begins with the placement of several rows (average of 3) of nonabsorbable suture (“internal” McCall sutures), starting at the left uterosacral ligament about 2 cm above its cut edge, and proceeding across the redundant cul-de-sac to terminate in the right uterosacral ligament. Each subsequent row is placed superior to the first, by applying traction to the previously placed sutures.
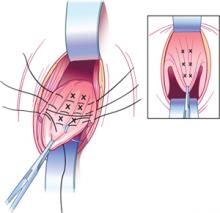
Prior to the tying of these sutures, 3 “external” absorbable sutures are placed. These sutures incorporate posterior vaginal epithelium, each uterosacral ligament, and the contralateral vaginal epithelium in a mirror image of the first pass through the vagina. Again, several rows are placed, each more superior to the last, to move the newly created vaginal apex to the highest point on the uterosacral ligaments once all the sutures are tied.
Tying the internal sutures not only creates a firm, shelf-like midline structure, but obliterates the redundant cul-de-sac. The external sutures move the vaginal apex to the uterosacral bridge and are tied at the conclusion of the procedure (FIGURES 2 and 3).
FIGURE 2 Internal McCall sutures
Traction on the most dependent portion of the cul-de-sac and posterior vaginal epithelium allows placement of 3 rows of sutures across the cul-de-sac from one uterosacral ligament to the other.
FIGURE 3 External McCall sutures
Three additional rows of absorbable sutures incorporate vaginal epithelium and uterosacral ligaments to move the vaginal cuff superiorly.
Modifications enhance durability and support
When the surgical indication is significant apical vaginal prolapse, the efficacy of the McCall procedure as both treatment and prevention is uncertain, because we lack adequate studies in this population. However, assuming that identifiable defects or breaks in the uterosacral ligaments lead to apical prolapse,3 use of the portion of the uterosacral ligament nearest the vagina appears unlikely to create a durable repair.
Thus, the concept of a “high” uterosacral attachment came to be proposed to provide a strong midline site of support for the vaginal apex.5,6 Further modifications include attachment of the uterosacral ligaments to pubocervical and rectovaginal connective tissues to create continuity of these level II fibers and prevent subsequent enterocele.7
With a high uterosacral attachment, the uterosacral ligaments need not be brought together in the midline.
Technique for modified approach
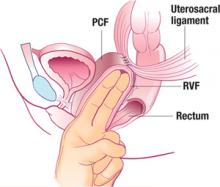
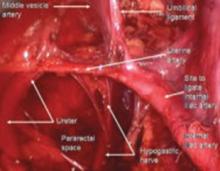
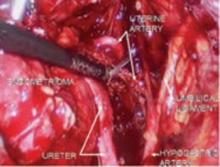
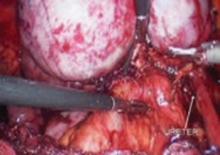
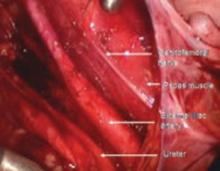
To locate each ligament, place traction on the vaginal apex toward the contralateral side. Palpate the pelvic structures posterior and medial to the ischial spines, at the 4 and 8 o’clock positions, to identify the strong tissue emanating from the sacrum.
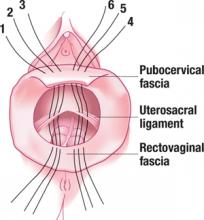
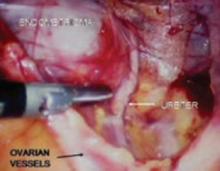
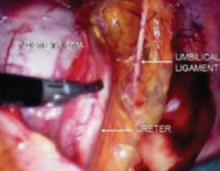
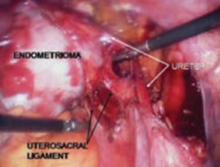
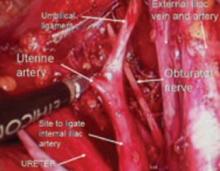
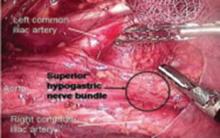
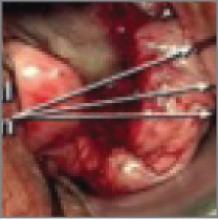
Place several nonabsorbable sutures through the medial aspect of each uterosacral ligament, working from lateral to medial to minimize the risk of ureteral trauma. Then place 1 strand of each suture through the pubocervical and rectovaginal connective tissues. Tie the sutures to move the vaginal apex to the proximal segment of the uterosacral ligament (near the sacrum) and establish continuity of the pubocervical and rectovaginal connective tissues (FIGURES 4-6).
FIGURE 4 Suture placement is bilateral
Three sutures are placed in the uterosacral ligament pedicles on each side, with 1 arm of each suture placed in the transverse portion of the pubocervical and rectovaginal fascia.
FIGURE 5 Suspensory suture
Sagittal view of a suspensory suture in left uterosacral ligament with 1 arm through pubocervical fascia (PCF) and 1 arm through rectovaginal fascia (RVF).
FIGURE 6 The suspended vaginal vault
Sagittal view of pubocervical fascia (PCF) and rectovaginal fascia (RVF) suspended from uterosacral ligaments.
Main concern is ureteral injury
The ureter lies near the anterior margin of the uterosacral ligament, with a mean distance of 4.1±0.6 cm at the level of the sacrum and 2.3±0.9 cm at the level of the ischial spine.8 In 1 series of high uterosacral ligament suspension with site-specific endopelvic fascia defect repair, ureteral complications occurred in 11% of patients.5 Other series have reported rates of ureteral trauma in the range of 0.7%9 to 2.4%.6
Evaluate ureteral patency
After performing this procedure, the surgeon should ensure ureteral patency. For this reason, I believe that only surgeons skilled in cystoscopy and able to treat ureteral injury (or with ready access to those capable of treating this complication) should undertake high uterosacral suspension.
How the McCall procedure compares with other approaches
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180:859–865
Although many techniques and modifications have been described for management of the cul-de-sac and vaginal cuff, few comparative data exist. In McCall’s original series,4 43 patients undergoing vaginal hysterectomy with posterior culdoplasty were followed for a minimum of 3 months and a maximum of 3 years. Mean and median lengths of follow-up were not provided, nor were the indications for hysterectomy or the method of assessing the patient for recurrent defects. McCall reported that no patients developed an enterocele after the surgery.
The first and only randomized study
Cruikshank and Kovac performed the only prospective, randomized comparison of procedures used at the time of hysterectomy to prevent enterocele. In their study, 100 patients undergoing vaginal hysterectomy for various indications (excluding prolapse of the posterior superior segment of the vagina) were randomized to 1 of 3 surgical methods to prevent enterocele:
- Moschcowitz-type closure (n=33), in which the peritoneum was closed using a purse-string technique, incorporating the distal ends of the uterosacral and cardinal ligaments and thereby drawing these structures to the midline
- modified McCall culdoplasty (n=33), in which a higher purse-string closure of the peritoneum was performed superior to the “yellow fat line,” incorporating the uterosacral-cardinal ligament complex (thus drawing these structures to the midline) and including the vagina (similar to the external McCall sutures) to move the apex superiorly
- simple closure of the peritoneum with a purse-string suture (n=34), with none of the uterosacral-cardinal ligaments incorporated into the repair.
Three years of follow-up
Of the 100 patients, 98 were followed with serial examinations for 3 years, and the outcomes at all vaginal segments were documented, with particular attention to the posterior superior segment, using staging from the Pelvic Organ Prolapse Quantification (POP-Q) system.10
McCall procedure was most effective
Overall, 11 patients (11.2%) were found to have stage II prolapse of the posterior superior vagina, none of them in the McCall group. An additional 14 patients (14.3%) had stage I prolapse, with only 2 (2%) of these in the McCall group.
The McCall repair was significantly more effective than the other 2 types of repair, with a 6.1% risk of subsequent prolapse, versus 30.3% in women who had a Moschcowitz-type closure and 39.4% in those who underwent simple closure of the peritoneum. No patients in any group had prolapse greater than stage II at follow-up.
Limitations of the study
Although Cruikshank and Kovac designed their study to analyze appropriate prophylaxis against enterocele in patients without prolapse, several patients did have some form of prolapse—although it was unrelated to the posterior superior vagina. Therefore, several patients underwent concomitant reconstructive procedures that included: anterior colporrhaphy (13), posterior colporrhaphy (4), sacrospinous ligament fixation (3), bilateral paravaginal repair (10), and anti-incontinence procedures (10).
It is not clear which groups these patients fell into and whether the distribution was similar across all groups.
CASE 2 Is McCall procedure appropriate?
B.D., 57, complains of increasing pelvic pressure and a noticeable vaginal bulge. Her 2 children were delivered vaginally, the largest weighing 8 lb. B.D. reports that she remains sexually active.
Physical examination reveals the cervix to be at the level of the introitus, but it descends 2 cm beyond the introitus when the patient performs the valsalva maneuver. Although there is also some descent of the anterior and posterior vaginal walls (1 cm superior to the hymen with strain; Pelvic Organ Prolapse Quantification [POP-Q] value=-1), the predominant component of prolapse is an elongated cervix. The posterior vaginal fornix (POP-Q point D), representing apical support, descends to 7 cm superior to the hymen with strain, with a total vaginal length of 9 cm.
At surgery, the uterosacral ligaments do not appear to be attenuated. After vaginal hysterectomy, the apex of the vaginal vault is superior to the level of the ischial spines.
How do you proceed?
Given the relatively good support at the apex, this patient is a good candidate for a McCall-type culdoplasty. Whether or not this procedure will be truly prophylactic (because there is already some descent of the apex, albeit mild) is perhaps only a matter of semantics.
The author reports no financial relationships relevant to this article.
1. Olson AL, Smith VJ, Bergstrom JO, Coiling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
3. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;1:214-221.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
6. Karram M, Goldwasser S, Kleeman S, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1342.
7. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
8. Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
9. Aronson MP, Aronson PK, Howard AE, et al. Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol. 2005;192:1530-1536.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
CASE 1 What procedures should accompany hysterectomy?
A.E., 44, mother of one, complains of heavy irregular bleeding with no sensation of a vaginal bulge. She has tried oral contraceptives, but they did not improve her bleeding pattern. She also has undergone dilatation and curettage and hysteroscopy (benign findings), also with no improvement.
Examination reveals a 9- to 10-week-size fibroid uterus, which is confirmed by ultrasonography. Pelvic support appears to be excellent.
After a discussion of the options, the patient elects to undergo vaginal hysterectomy. Are other procedures warranted?
Ask a gynecologic surgeon to name the most significant challenges he or she faces, and the answer is likely to include preventing pelvic organ prolapse after surgical intervention. Approximately one third of operations for pelvic organ prolapse involve patients whose prolapse has recurred after previous surgery.1 Although we have advanced our understanding of the anatomy of pelvic support and the pathophysiology of support defects, the various surgical strategies remain largely untested and unproven.
Even women with good pelvic support who are undergoing hysterectomy—like the patient described above—are vulnerable. One particular area of concern: the risk of enterocele or vaginal apical prolapse, or both, after hysterectomy. In this article, I describe a technique to reduce the risk of these defects after vaginal hysterectomy: high uterosacral suspension, or modified McCall culdoplasty.
Enterocele and apical prolapse do not always coexist
Enterocele and apical prolapse are distinct entities. The latter represents a deficiency in the level I supporting structures described by DeLancey2—primarily the uterosacral and cardinal ligaments (FIGURE 1). Enterocele, or peritoneocele, is a herniation of the cul-de-sac peritoneum, with or without intestinal contents. In women who have undergone hysterectomy, enterocele is usually caused by a lack of continuity of level II fibers, namely, the failure to approximate the pubocervical and rectovaginal connective tissues at the time of hysterectomy.3 Careful attention to the vaginal cuff and cul-de-sac at the time of hysterectomy is therefore imperative.
FIGURE 1 Three levels of support
The endopelvic fascia of a posthysterectomy patient divided into DeLancey’s biomechanical levels: level I—proximal suspension, level II—lateral attachment, and level III—distal fusion.
The McCall culdoplasty: 50 years “young”
In 1957, Milton McCall, MD, described a technique to manage the cul-de-sac at the time of vaginal hysterectomy.4 The McCall technique of posterior culdoplasty differs from other approaches by omitting dissection and excision of the hernia sac, or excess cul-de-sac peritoneum. The original McCall culdoplasty begins with the placement of several rows (average of 3) of nonabsorbable suture (“internal” McCall sutures), starting at the left uterosacral ligament about 2 cm above its cut edge, and proceeding across the redundant cul-de-sac to terminate in the right uterosacral ligament. Each subsequent row is placed superior to the first, by applying traction to the previously placed sutures.
Prior to the tying of these sutures, 3 “external” absorbable sutures are placed. These sutures incorporate posterior vaginal epithelium, each uterosacral ligament, and the contralateral vaginal epithelium in a mirror image of the first pass through the vagina. Again, several rows are placed, each more superior to the last, to move the newly created vaginal apex to the highest point on the uterosacral ligaments once all the sutures are tied.
Tying the internal sutures not only creates a firm, shelf-like midline structure, but obliterates the redundant cul-de-sac. The external sutures move the vaginal apex to the uterosacral bridge and are tied at the conclusion of the procedure (FIGURES 2 and 3).
FIGURE 2 Internal McCall sutures
Traction on the most dependent portion of the cul-de-sac and posterior vaginal epithelium allows placement of 3 rows of sutures across the cul-de-sac from one uterosacral ligament to the other.
FIGURE 3 External McCall sutures
Three additional rows of absorbable sutures incorporate vaginal epithelium and uterosacral ligaments to move the vaginal cuff superiorly.
Modifications enhance durability and support
When the surgical indication is significant apical vaginal prolapse, the efficacy of the McCall procedure as both treatment and prevention is uncertain, because we lack adequate studies in this population. However, assuming that identifiable defects or breaks in the uterosacral ligaments lead to apical prolapse,3 use of the portion of the uterosacral ligament nearest the vagina appears unlikely to create a durable repair.
Thus, the concept of a “high” uterosacral attachment came to be proposed to provide a strong midline site of support for the vaginal apex.5,6 Further modifications include attachment of the uterosacral ligaments to pubocervical and rectovaginal connective tissues to create continuity of these level II fibers and prevent subsequent enterocele.7
With a high uterosacral attachment, the uterosacral ligaments need not be brought together in the midline.
Technique for modified approach
To locate each ligament, place traction on the vaginal apex toward the contralateral side. Palpate the pelvic structures posterior and medial to the ischial spines, at the 4 and 8 o’clock positions, to identify the strong tissue emanating from the sacrum.
Place several nonabsorbable sutures through the medial aspect of each uterosacral ligament, working from lateral to medial to minimize the risk of ureteral trauma. Then place 1 strand of each suture through the pubocervical and rectovaginal connective tissues. Tie the sutures to move the vaginal apex to the proximal segment of the uterosacral ligament (near the sacrum) and establish continuity of the pubocervical and rectovaginal connective tissues (FIGURES 4-6).
FIGURE 4 Suture placement is bilateral
Three sutures are placed in the uterosacral ligament pedicles on each side, with 1 arm of each suture placed in the transverse portion of the pubocervical and rectovaginal fascia.
FIGURE 5 Suspensory suture
Sagittal view of a suspensory suture in left uterosacral ligament with 1 arm through pubocervical fascia (PCF) and 1 arm through rectovaginal fascia (RVF).
FIGURE 6 The suspended vaginal vault
Sagittal view of pubocervical fascia (PCF) and rectovaginal fascia (RVF) suspended from uterosacral ligaments.
Main concern is ureteral injury
The ureter lies near the anterior margin of the uterosacral ligament, with a mean distance of 4.1±0.6 cm at the level of the sacrum and 2.3±0.9 cm at the level of the ischial spine.8 In 1 series of high uterosacral ligament suspension with site-specific endopelvic fascia defect repair, ureteral complications occurred in 11% of patients.5 Other series have reported rates of ureteral trauma in the range of 0.7%9 to 2.4%.6
Evaluate ureteral patency
After performing this procedure, the surgeon should ensure ureteral patency. For this reason, I believe that only surgeons skilled in cystoscopy and able to treat ureteral injury (or with ready access to those capable of treating this complication) should undertake high uterosacral suspension.
How the McCall procedure compares with other approaches
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180:859–865
Although many techniques and modifications have been described for management of the cul-de-sac and vaginal cuff, few comparative data exist. In McCall’s original series,4 43 patients undergoing vaginal hysterectomy with posterior culdoplasty were followed for a minimum of 3 months and a maximum of 3 years. Mean and median lengths of follow-up were not provided, nor were the indications for hysterectomy or the method of assessing the patient for recurrent defects. McCall reported that no patients developed an enterocele after the surgery.
The first and only randomized study
Cruikshank and Kovac performed the only prospective, randomized comparison of procedures used at the time of hysterectomy to prevent enterocele. In their study, 100 patients undergoing vaginal hysterectomy for various indications (excluding prolapse of the posterior superior segment of the vagina) were randomized to 1 of 3 surgical methods to prevent enterocele:
- Moschcowitz-type closure (n=33), in which the peritoneum was closed using a purse-string technique, incorporating the distal ends of the uterosacral and cardinal ligaments and thereby drawing these structures to the midline
- modified McCall culdoplasty (n=33), in which a higher purse-string closure of the peritoneum was performed superior to the “yellow fat line,” incorporating the uterosacral-cardinal ligament complex (thus drawing these structures to the midline) and including the vagina (similar to the external McCall sutures) to move the apex superiorly
- simple closure of the peritoneum with a purse-string suture (n=34), with none of the uterosacral-cardinal ligaments incorporated into the repair.
Three years of follow-up
Of the 100 patients, 98 were followed with serial examinations for 3 years, and the outcomes at all vaginal segments were documented, with particular attention to the posterior superior segment, using staging from the Pelvic Organ Prolapse Quantification (POP-Q) system.10
McCall procedure was most effective
Overall, 11 patients (11.2%) were found to have stage II prolapse of the posterior superior vagina, none of them in the McCall group. An additional 14 patients (14.3%) had stage I prolapse, with only 2 (2%) of these in the McCall group.
The McCall repair was significantly more effective than the other 2 types of repair, with a 6.1% risk of subsequent prolapse, versus 30.3% in women who had a Moschcowitz-type closure and 39.4% in those who underwent simple closure of the peritoneum. No patients in any group had prolapse greater than stage II at follow-up.
Limitations of the study
Although Cruikshank and Kovac designed their study to analyze appropriate prophylaxis against enterocele in patients without prolapse, several patients did have some form of prolapse—although it was unrelated to the posterior superior vagina. Therefore, several patients underwent concomitant reconstructive procedures that included: anterior colporrhaphy (13), posterior colporrhaphy (4), sacrospinous ligament fixation (3), bilateral paravaginal repair (10), and anti-incontinence procedures (10).
It is not clear which groups these patients fell into and whether the distribution was similar across all groups.
CASE 2 Is McCall procedure appropriate?
B.D., 57, complains of increasing pelvic pressure and a noticeable vaginal bulge. Her 2 children were delivered vaginally, the largest weighing 8 lb. B.D. reports that she remains sexually active.
Physical examination reveals the cervix to be at the level of the introitus, but it descends 2 cm beyond the introitus when the patient performs the valsalva maneuver. Although there is also some descent of the anterior and posterior vaginal walls (1 cm superior to the hymen with strain; Pelvic Organ Prolapse Quantification [POP-Q] value=-1), the predominant component of prolapse is an elongated cervix. The posterior vaginal fornix (POP-Q point D), representing apical support, descends to 7 cm superior to the hymen with strain, with a total vaginal length of 9 cm.
At surgery, the uterosacral ligaments do not appear to be attenuated. After vaginal hysterectomy, the apex of the vaginal vault is superior to the level of the ischial spines.
How do you proceed?
Given the relatively good support at the apex, this patient is a good candidate for a McCall-type culdoplasty. Whether or not this procedure will be truly prophylactic (because there is already some descent of the apex, albeit mild) is perhaps only a matter of semantics.
The author reports no financial relationships relevant to this article.
CASE 1 What procedures should accompany hysterectomy?
A.E., 44, mother of one, complains of heavy irregular bleeding with no sensation of a vaginal bulge. She has tried oral contraceptives, but they did not improve her bleeding pattern. She also has undergone dilatation and curettage and hysteroscopy (benign findings), also with no improvement.
Examination reveals a 9- to 10-week-size fibroid uterus, which is confirmed by ultrasonography. Pelvic support appears to be excellent.
After a discussion of the options, the patient elects to undergo vaginal hysterectomy. Are other procedures warranted?
Ask a gynecologic surgeon to name the most significant challenges he or she faces, and the answer is likely to include preventing pelvic organ prolapse after surgical intervention. Approximately one third of operations for pelvic organ prolapse involve patients whose prolapse has recurred after previous surgery.1 Although we have advanced our understanding of the anatomy of pelvic support and the pathophysiology of support defects, the various surgical strategies remain largely untested and unproven.
Even women with good pelvic support who are undergoing hysterectomy—like the patient described above—are vulnerable. One particular area of concern: the risk of enterocele or vaginal apical prolapse, or both, after hysterectomy. In this article, I describe a technique to reduce the risk of these defects after vaginal hysterectomy: high uterosacral suspension, or modified McCall culdoplasty.
Enterocele and apical prolapse do not always coexist
Enterocele and apical prolapse are distinct entities. The latter represents a deficiency in the level I supporting structures described by DeLancey2—primarily the uterosacral and cardinal ligaments (FIGURE 1). Enterocele, or peritoneocele, is a herniation of the cul-de-sac peritoneum, with or without intestinal contents. In women who have undergone hysterectomy, enterocele is usually caused by a lack of continuity of level II fibers, namely, the failure to approximate the pubocervical and rectovaginal connective tissues at the time of hysterectomy.3 Careful attention to the vaginal cuff and cul-de-sac at the time of hysterectomy is therefore imperative.
FIGURE 1 Three levels of support
The endopelvic fascia of a posthysterectomy patient divided into DeLancey’s biomechanical levels: level I—proximal suspension, level II—lateral attachment, and level III—distal fusion.
The McCall culdoplasty: 50 years “young”
In 1957, Milton McCall, MD, described a technique to manage the cul-de-sac at the time of vaginal hysterectomy.4 The McCall technique of posterior culdoplasty differs from other approaches by omitting dissection and excision of the hernia sac, or excess cul-de-sac peritoneum. The original McCall culdoplasty begins with the placement of several rows (average of 3) of nonabsorbable suture (“internal” McCall sutures), starting at the left uterosacral ligament about 2 cm above its cut edge, and proceeding across the redundant cul-de-sac to terminate in the right uterosacral ligament. Each subsequent row is placed superior to the first, by applying traction to the previously placed sutures.
Prior to the tying of these sutures, 3 “external” absorbable sutures are placed. These sutures incorporate posterior vaginal epithelium, each uterosacral ligament, and the contralateral vaginal epithelium in a mirror image of the first pass through the vagina. Again, several rows are placed, each more superior to the last, to move the newly created vaginal apex to the highest point on the uterosacral ligaments once all the sutures are tied.
Tying the internal sutures not only creates a firm, shelf-like midline structure, but obliterates the redundant cul-de-sac. The external sutures move the vaginal apex to the uterosacral bridge and are tied at the conclusion of the procedure (FIGURES 2 and 3).
FIGURE 2 Internal McCall sutures
Traction on the most dependent portion of the cul-de-sac and posterior vaginal epithelium allows placement of 3 rows of sutures across the cul-de-sac from one uterosacral ligament to the other.
FIGURE 3 External McCall sutures
Three additional rows of absorbable sutures incorporate vaginal epithelium and uterosacral ligaments to move the vaginal cuff superiorly.
Modifications enhance durability and support
When the surgical indication is significant apical vaginal prolapse, the efficacy of the McCall procedure as both treatment and prevention is uncertain, because we lack adequate studies in this population. However, assuming that identifiable defects or breaks in the uterosacral ligaments lead to apical prolapse,3 use of the portion of the uterosacral ligament nearest the vagina appears unlikely to create a durable repair.
Thus, the concept of a “high” uterosacral attachment came to be proposed to provide a strong midline site of support for the vaginal apex.5,6 Further modifications include attachment of the uterosacral ligaments to pubocervical and rectovaginal connective tissues to create continuity of these level II fibers and prevent subsequent enterocele.7
With a high uterosacral attachment, the uterosacral ligaments need not be brought together in the midline.
Technique for modified approach
To locate each ligament, place traction on the vaginal apex toward the contralateral side. Palpate the pelvic structures posterior and medial to the ischial spines, at the 4 and 8 o’clock positions, to identify the strong tissue emanating from the sacrum.
Place several nonabsorbable sutures through the medial aspect of each uterosacral ligament, working from lateral to medial to minimize the risk of ureteral trauma. Then place 1 strand of each suture through the pubocervical and rectovaginal connective tissues. Tie the sutures to move the vaginal apex to the proximal segment of the uterosacral ligament (near the sacrum) and establish continuity of the pubocervical and rectovaginal connective tissues (FIGURES 4-6).
FIGURE 4 Suture placement is bilateral
Three sutures are placed in the uterosacral ligament pedicles on each side, with 1 arm of each suture placed in the transverse portion of the pubocervical and rectovaginal fascia.
FIGURE 5 Suspensory suture
Sagittal view of a suspensory suture in left uterosacral ligament with 1 arm through pubocervical fascia (PCF) and 1 arm through rectovaginal fascia (RVF).
FIGURE 6 The suspended vaginal vault
Sagittal view of pubocervical fascia (PCF) and rectovaginal fascia (RVF) suspended from uterosacral ligaments.
Main concern is ureteral injury
The ureter lies near the anterior margin of the uterosacral ligament, with a mean distance of 4.1±0.6 cm at the level of the sacrum and 2.3±0.9 cm at the level of the ischial spine.8 In 1 series of high uterosacral ligament suspension with site-specific endopelvic fascia defect repair, ureteral complications occurred in 11% of patients.5 Other series have reported rates of ureteral trauma in the range of 0.7%9 to 2.4%.6
Evaluate ureteral patency
After performing this procedure, the surgeon should ensure ureteral patency. For this reason, I believe that only surgeons skilled in cystoscopy and able to treat ureteral injury (or with ready access to those capable of treating this complication) should undertake high uterosacral suspension.
How the McCall procedure compares with other approaches
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180:859–865
Although many techniques and modifications have been described for management of the cul-de-sac and vaginal cuff, few comparative data exist. In McCall’s original series,4 43 patients undergoing vaginal hysterectomy with posterior culdoplasty were followed for a minimum of 3 months and a maximum of 3 years. Mean and median lengths of follow-up were not provided, nor were the indications for hysterectomy or the method of assessing the patient for recurrent defects. McCall reported that no patients developed an enterocele after the surgery.
The first and only randomized study
Cruikshank and Kovac performed the only prospective, randomized comparison of procedures used at the time of hysterectomy to prevent enterocele. In their study, 100 patients undergoing vaginal hysterectomy for various indications (excluding prolapse of the posterior superior segment of the vagina) were randomized to 1 of 3 surgical methods to prevent enterocele:
- Moschcowitz-type closure (n=33), in which the peritoneum was closed using a purse-string technique, incorporating the distal ends of the uterosacral and cardinal ligaments and thereby drawing these structures to the midline
- modified McCall culdoplasty (n=33), in which a higher purse-string closure of the peritoneum was performed superior to the “yellow fat line,” incorporating the uterosacral-cardinal ligament complex (thus drawing these structures to the midline) and including the vagina (similar to the external McCall sutures) to move the apex superiorly
- simple closure of the peritoneum with a purse-string suture (n=34), with none of the uterosacral-cardinal ligaments incorporated into the repair.
Three years of follow-up
Of the 100 patients, 98 were followed with serial examinations for 3 years, and the outcomes at all vaginal segments were documented, with particular attention to the posterior superior segment, using staging from the Pelvic Organ Prolapse Quantification (POP-Q) system.10
McCall procedure was most effective
Overall, 11 patients (11.2%) were found to have stage II prolapse of the posterior superior vagina, none of them in the McCall group. An additional 14 patients (14.3%) had stage I prolapse, with only 2 (2%) of these in the McCall group.
The McCall repair was significantly more effective than the other 2 types of repair, with a 6.1% risk of subsequent prolapse, versus 30.3% in women who had a Moschcowitz-type closure and 39.4% in those who underwent simple closure of the peritoneum. No patients in any group had prolapse greater than stage II at follow-up.
Limitations of the study
Although Cruikshank and Kovac designed their study to analyze appropriate prophylaxis against enterocele in patients without prolapse, several patients did have some form of prolapse—although it was unrelated to the posterior superior vagina. Therefore, several patients underwent concomitant reconstructive procedures that included: anterior colporrhaphy (13), posterior colporrhaphy (4), sacrospinous ligament fixation (3), bilateral paravaginal repair (10), and anti-incontinence procedures (10).
It is not clear which groups these patients fell into and whether the distribution was similar across all groups.
CASE 2 Is McCall procedure appropriate?
B.D., 57, complains of increasing pelvic pressure and a noticeable vaginal bulge. Her 2 children were delivered vaginally, the largest weighing 8 lb. B.D. reports that she remains sexually active.
Physical examination reveals the cervix to be at the level of the introitus, but it descends 2 cm beyond the introitus when the patient performs the valsalva maneuver. Although there is also some descent of the anterior and posterior vaginal walls (1 cm superior to the hymen with strain; Pelvic Organ Prolapse Quantification [POP-Q] value=-1), the predominant component of prolapse is an elongated cervix. The posterior vaginal fornix (POP-Q point D), representing apical support, descends to 7 cm superior to the hymen with strain, with a total vaginal length of 9 cm.
At surgery, the uterosacral ligaments do not appear to be attenuated. After vaginal hysterectomy, the apex of the vaginal vault is superior to the level of the ischial spines.
How do you proceed?
Given the relatively good support at the apex, this patient is a good candidate for a McCall-type culdoplasty. Whether or not this procedure will be truly prophylactic (because there is already some descent of the apex, albeit mild) is perhaps only a matter of semantics.
The author reports no financial relationships relevant to this article.
1. Olson AL, Smith VJ, Bergstrom JO, Coiling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
3. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;1:214-221.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
6. Karram M, Goldwasser S, Kleeman S, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1342.
7. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
8. Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
9. Aronson MP, Aronson PK, Howard AE, et al. Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol. 2005;192:1530-1536.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
1. Olson AL, Smith VJ, Bergstrom JO, Coiling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
3. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;1:214-221.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
6. Karram M, Goldwasser S, Kleeman S, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1342.
7. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
8. Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
9. Aronson MP, Aronson PK, Howard AE, et al. Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol. 2005;192:1530-1536.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
IN THIS ARTICLE
Secrets to successful vaginal hysterectomy
M.K. is a 43-year-old gravida 2 para 2 who is undergoing a vaginal hysterectomy for menorrhagia. A preoperative pelvic exam and ultrasound suggested a 12-week-size uterus with several small leiomyomata. Her gynecologist estimates the uterine weight at 240 g and notes that the uterus is mobile. M.K. asks that her ovaries be removed at the time of hysterectomy because of a family history of ovarian cancer.
During the initial dissection, the surgeon is unable to enter the anterior cul-de-sac due to distortion created by an anterior fibroid. The surgeon has entered the posterior cul-de-sac, but the uterus is too large to manipulate a finger around anteriorly to identify the peritoneal fold. Although he feels confident that the bladder has been adequately mobilized from the cervix, the surgeon is strongly considering abandoning the vaginal approach and completing the hysterectomy abdominally.
How should he proceed?
Entry into the peritoneal cavity through the anterior or posterior cul-de-sac can sometimes be challenging, as this case illustrates. However, there is no need for the surgeon to abandon the vaginal approach just yet. In my experience, the anterior peritoneal fold can be high or distorted by fibroids in some women. The key to successful surgery is a pause in activity to consider the case at hand and determine whether additional progress can be made safely without changing the approach.
Avoid blind entry at all costs
No less an authority than Heaney1 advised against blind attempts to enter the anterior cul-de-sac. Such attempts are often frustrating, can involve bleeding, and raise the risk of injury to the bladder. However, once the surgeon is confident that the bladder is free and retracted out of the way, he or she can proceed without intraperitoneal entry. This is especially true if the posterior cul-de-sac has been entered safely.
The “climb up” technique
In some cases, the surgeon may safely proceed extraperitoneally even if neither cul-de-sac has been opened. Krige2 coined the term “climb up” to describe the extraperitoneal approach to the inaccessible posterior cul-de-sac. He performed extensive extraperitoneal dissection that, if necessary, included both uterosacral and cardinal ligaments as well as uterine vessels. A surgeon may carry a total extraperitoneal dissection completely to the uterine fundus as long as the bladder and rectum are free.3
In M.K.’s case, the surgeon should proceed to take the uterosacral and cardinal ligaments posteriorly without swinging the clamps around to the anterior aspect of the cervix, if possible. Once these ligaments are taken, the uterus often descends enough that the anterior peritoneal fold becomes accessible. Once it is identified, the anterior cul-de-sac can be entered safely.
If safe entry still is not possible, the surgeon can take the uterine vessels if he or she is confident that the bladder is out of harm’s way. If the fold still cannot be identified after this bite, proceed with broad-ligament clamps, which usually lead to eventual opening of the peritoneal fold.
CASE 1 Some progress, then surgery stalls
The surgeon proceeds to operate extraperitoneally, as described above, and successfully enters the anterior cul-de-sac after the uterine vessels are ligated. However, after several additional bites of broad ligament on each side, progress stalls because of uterine size. The surgeon seems to be stuck and is growing increasingly frustrated.
What is the best way around this impasse?
Morcellation can involve a range of techniques
Whenever a large uterus prevents further progress, and the uterine vessels have been ligated, uterine morcellation can be performed. Morcellation techniques originated when vaginal hysterectomy was the archetypal gynecologic operation,4-7 and include uterine bisection,8-11 Lash intramyometrial coring,6,8,9 myomectomy,10,11 and wedge debulking.9 Although every surgeon has a favorite, some or all of these procedures may be necessary in the same patient.12-15 In all cases it is mandatory that the uterine vessels be ligated before any morcellation procedure is initiated.
In my experience, a uterus in the range of 240 g usually lends itself very nicely to Lash intramyometrial coring. This technique is a nearly bloodless procedure that does not violate the endometrial cavity when it is performed properly. In addition, any intramyometrial fibroids can be easily removed.
If coring does not decompress the uterus enough for safe delivery, the core can be cut off and the remaining uterus can be further morcellated by removing wedges of myometrium or by bivalving the uterus. Since there is usually more room in the posterior vagina than in the anterior vagina, as much of the wedge morcellation as possible should be done posteriorly.
CASE 1 Ovaries appear out of reach
After Lash intramyometrial coring, the surgeon successfully removes the uterus. He then turns his attention to the bilateral adnexectomy. Unfortunately, the ovaries are higher than anticipated, and he once again considers switching to the abdominal route to remove them.
Is a change in route the best option?
Focus on the round ligaments
The key to safe removal of the adnexa, especially in difficult cases, is the separate transection and ligation of the round ligaments. Many authors have reported high success rates for vaginal oophorectomy using this technique, especially in premenopausal women.16-19
Separate transection of the round ligament allows the surgeon to accomplish 2 very important tasks:
In many hysterectomy cases when oophorectomy is planned, this maneuver can be carried out prior to removal of the uterus. Once the round ligaments have been reached, the surgeon can deliver the uterine fundus anteriorly, allowing the round ligaments to be clamped and cut. It is not uncommon to be able to remove the uterus with both adnexa still attached.
With a large uterus, it may be necessary to clamp and transect the round ligament after the uterus is out. This does not preclude identification and transection of the round ligament to carry out the maneuvers described above.
Consider your tools
In very difficult cases, specialized clamps or sutures may be necessary. I find long, sturdy, right-angle clamps to be most useful. In addition, endoloop-type sutures often facilitate ligation of the vascular pedicle. The use of newer specialized bipolar electrosurgical instruments may be helpful, although I have no personal experience using them in vaginal surgery.
CASE 1 At closure, concerns about injury
After successful removal of both adnexa using the round-ligament technique, the surgeon is satisfied that he has achieved hemostasis and proceeds with his usual closure. However, he has nagging concerns about the possibility of undetected complications, because this case turned out to be more of a challenge than he had expected. He wonders if there is anything else he can do to ensure that everything is OK.
What would you do?
Besides ensuring satisfactory hemostasis, confirming the integrity of the urinary tract is the most important goal to achieve before leaving the operating room. Unrecognized injuries to the bladder or ureters are unacceptable and will lead to significant morbidity for the patient. I would certainly recommend that the surgeon in M.K.’s case perform cystoscopy after giving the patient intravenous indigo carmine to assure both ureteral patency and integrity of the bladder. I perform cystoscopy after all vaginal hysterectomies.
CASE 2 History of cesarean delivery
C.S. is a 38-year-old gravida 3 para 3 who presents with menometrorrhagia and dysmenorrhea unresponsive to medical therapy. Her first pregnancy resulted in vaginal delivery of a full-term infant without complications. Her second child was delivered via low-segment transverse cesarean section due to a persistent breech presentation at term. Her last child was delivered vaginally, also at term. Two years later C.S. underwent a laparoscopic tubal ligation without complications. That was 4 years ago. She began seeing her current gynecologist 2 years ago, when she moved to a new community.
Pelvic examination reveals a 6-week–size uterus and normal adnexa. Her uterus is mobile, and there does not appear to be any ventral fixation of the uterus to the abdominal wall from the cesarean section. Endometrial biopsy reveals proliferative endometrium only. Saline ultrasound demonstrates a 2-cm submucosal leiomyoma.
C.S. refuses hysteroscopic resection of the myoma and prefers hysterectomy as definitive therapy. She is the business manager for her family’s construction business, and she would like to be able to return to work as soon as possible after her surgery. She requests vaginal hysterectomy with conservation of her ovaries.
What is the best way to proceed at this point?
Many gynecologic surgeons regard previous pelvic surgery, including cesarean delivery, as a relative contraindication to vaginal hysterectomy. Although the major concern seems to be a potential for bladder injury during the bladder dissection, other problems such as ventral fixation of the uterus to the previous abdominal incision also are possible.
Vaginal hysterectomy requires a mobile uterus
All patients who will be undergoing vaginal hysterectomy must have demonstrated mobility of the uterus upon pelvic examination. This is particularly important in the case of prior pelvic surgery. In this case, the gynecologist also should make every attempt to obtain her surgical records—especially those from her laparoscopic tubal ligation—to exclude major adhesive disease in the pelvis.
Laparoscopic adhesiolysis may facilitate vaginal hysterectomy
If there is any concern that the uterus is fixed to the abdominal wall, abdominal hysterectomy should be considered. Even more preferable is laparoscopic adhesiolysis, which can make it possible to proceed with vaginal hysterectomy. I have used this approach in women with as many as 5 previous cesarean deliveries and severe ventral fixation of the uterus.20 After adhesiolysis, the remainder of the hysterectomy can usually be performed solely through the vaginal route.
CASE 2 Medical records suggest the vaginal route is feasible
The gynecologist obtains C.S.’s previous medical records, which confirm that the cesarean delivery was uncomplicated. They also indicate that, at the time of the sterilization procedure, there was no evidence of ventral fixation of the uterus or other major adhesive disease.
The physician decides to proceed with vaginal hysterectomy, but remains very concerned about the possibility of bladder injury. How can she avoid inadvertent cystotomy?
Difficulty identifying and safely dissecting the bladder—because of distortion of the vesicouterine space from the previous cesarean delivery—is a legitimate concern. However, injury to a scarred and densely adherent bladder is a risk even with abdominal dissection.
The vaginal approach to the distal vesicouterine space has a clear advantage: The vesicouterine space closest to the initial vaginal dissection is unaffected by the previous operation on the lower uterine segment. In contrast, with the abdominal approach, dissection begins in the area of scar, and only after penetrating the scar does one find the unaffected space. With the vaginal approach, dissection begins in the correct surgical plane, which aids in identification of the location of the bladder and cesarean scar.
Sharp dissection is a must to protect the bladder
Once the correct surgical plane is encountered, sharp dissection is necessary to prevent tears of the adherent bladder, which can occur with blunt dissection.
Although sharp dissection is the key to success under these circumstances, other maneuvers may be helpful in some cases.
Nichols21 suggested performing dissection of the bladder after it has been filled with a dilute indigo carmine solution to stain the bladder tissues and help prevent bladder injury.
Hoffman and Jaeger22 describe the placement of a bent uterine sound in the posterior cul-de-sac. The sound is then brought around to the anterior cul-de-sac as an aid to dissection of the bladder and the vesicouterine peritoneal fold.
Sheth and Malpani23 describe developing a lateral “window” through the broad ligament to the bladder dissection when there are dense midline adhesions.
Although these are all valuable suggestions, I have found that they are rarely needed with careful sharp dissection. Remember, it is essential to avoid the temptation of blunt dissection when performing vaginal hysterectomy in women with a prior cesarean delivery.
CASE 2 Procedure is a success
The vaginal hysterectomy is carried out without incident, and cystoscopy following the hysterectomy is negative for any bladder injury; both ureteral orifices promptly efflux indigo carmine.
The surgeon encountered little difficulty during the bladder dissection, which was performed sharply. In fact, she was surprised at how well she could actually identify the hysterotomy scar and bladder. The patient goes home after 24 hours and is back at work in 2 weeks.
As noted in both cases presented here, the gynecologic surgeon must be certain that the urinary tract is intact and uninjured prior to leaving the operating room. This includes careful inspection of the bladder grossly for any sign of injury, as well as cystoscopy.
Most bladder injuries that occur with hysterectomy—either vaginal or abdominal—are usually well above the trigone and can be carefully repaired by the gynecologic surgeon. Complex injuries to the bladder involving the trigone or ureters usually require urologic intraoperative consultation.
The author reports no financial relationships relevant to this article.
REFERNCES
1. Heaney NS. Vaginal hysterectomy-its indications and technique. Am J Surg. 1940;48:284-288.
2. Krige CF. Vaginal hysterectomy and genital prolapse repair. Johannesburg, South Africa: Witwatersrand University Press; 1965: 57-70.
3. Unger JB. The extraperitoneal approach to vaginal hysterectomy. J Pelvic Surg. 1997;3:240-245.
4. Garceau E. Vaginal hysterectomy as done in France. Am J Obstet Dis Women Child. 1895;31:305-346.
5. Heaney NS. A report of 565 vaginal hysterectomies performed for benign disease. Am J Obstet Gynecol. 1934;28:751-755.
6. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
7. Allen E, Peterson LF. Versatility of vaginal hysterectomy technic. Obstet Gynecol. 1954;3:240-247.
8. Nichols DH, Randall CL. Vaginal Surgery. 4th ed. Baltimore: Williams and Wilkins;1996.
9. Stovall TG. Vaginal, abdominal, and laparoscopic-assisted hysterectomy. In: Mann WJ, Stovall TG, eds. Gynecologic Surgery. New York: Churchill Livingstone;1996: 403-404.
10. Lee RA. Atlas of Gynecologic Surgery. Philadelphia: WB Saunders; 1992.
11. Reiffenstuhl G, Platzer W, Knapstein PG, Imig JR. Vaginal operations–surgical anatomy and technique. 2nd ed. Baltimore: Williams and Wilkins; 1996.
12. Unger JB. Vaginal hysterectomy for the woman with a moderately enlarged uterus weighing 200 to 700 grams. Am J Obstet Gynecol. 1999;180:1337-1344.
13. Magos A, Bournas N, Sinha R, Richardson RE, O’Connor H. Vaginal hysterectomy for the large uterus. Br J Obstet Gynaecol. 1996;103:246-251.
14. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico Hospital’s experience. Obstet Gynecol. 1996;88:560-563.
15. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, nonmorbid procedure. Obstet Gynecol. 1995;86:60-64.
16. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
17. Ballard LA, Walters MD. Transvaginal mobilization and removal of the ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
18. Davies A, O’Connor H, Magos AL. A prospective study to evaluate oophorectomy at the time of vaginal hysterectomy. Br J Obstet Gynaecol. 1996;103:915-920.
19. Unger JB. Planned prophylactic oophorectomy at vaginal hysterectomy: clamp technique with separate division of the round and infundibulopelvic ligaments. J Pelvic Surg. 1999;5:151-155.
20. Unger JB, Meeks GR. Vaginal hysterectomy in women with history of previous cesarean delivery. Am J Obstet Gynecol. 1998;179:1473-1478.
21. Nichols DH. Vaginal versus abdominal hysterectomy. In: Stovall TG, ed. Current topics in obstetrics and gynecology: hysterectomy. New York: Elsevier; 1993:27-33.
22. Hoffman MS, Jaeger M. A new method for gaining entry into the scarred anterior cul-de-sac during transvaginal hysterectomy. Am J Obstet Gynecol. 1990;162:1269-1270.
23. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynecol Obstet. 1995;50:165-169.
M.K. is a 43-year-old gravida 2 para 2 who is undergoing a vaginal hysterectomy for menorrhagia. A preoperative pelvic exam and ultrasound suggested a 12-week-size uterus with several small leiomyomata. Her gynecologist estimates the uterine weight at 240 g and notes that the uterus is mobile. M.K. asks that her ovaries be removed at the time of hysterectomy because of a family history of ovarian cancer.
During the initial dissection, the surgeon is unable to enter the anterior cul-de-sac due to distortion created by an anterior fibroid. The surgeon has entered the posterior cul-de-sac, but the uterus is too large to manipulate a finger around anteriorly to identify the peritoneal fold. Although he feels confident that the bladder has been adequately mobilized from the cervix, the surgeon is strongly considering abandoning the vaginal approach and completing the hysterectomy abdominally.
How should he proceed?
Entry into the peritoneal cavity through the anterior or posterior cul-de-sac can sometimes be challenging, as this case illustrates. However, there is no need for the surgeon to abandon the vaginal approach just yet. In my experience, the anterior peritoneal fold can be high or distorted by fibroids in some women. The key to successful surgery is a pause in activity to consider the case at hand and determine whether additional progress can be made safely without changing the approach.
Avoid blind entry at all costs
No less an authority than Heaney1 advised against blind attempts to enter the anterior cul-de-sac. Such attempts are often frustrating, can involve bleeding, and raise the risk of injury to the bladder. However, once the surgeon is confident that the bladder is free and retracted out of the way, he or she can proceed without intraperitoneal entry. This is especially true if the posterior cul-de-sac has been entered safely.
The “climb up” technique
In some cases, the surgeon may safely proceed extraperitoneally even if neither cul-de-sac has been opened. Krige2 coined the term “climb up” to describe the extraperitoneal approach to the inaccessible posterior cul-de-sac. He performed extensive extraperitoneal dissection that, if necessary, included both uterosacral and cardinal ligaments as well as uterine vessels. A surgeon may carry a total extraperitoneal dissection completely to the uterine fundus as long as the bladder and rectum are free.3
In M.K.’s case, the surgeon should proceed to take the uterosacral and cardinal ligaments posteriorly without swinging the clamps around to the anterior aspect of the cervix, if possible. Once these ligaments are taken, the uterus often descends enough that the anterior peritoneal fold becomes accessible. Once it is identified, the anterior cul-de-sac can be entered safely.
If safe entry still is not possible, the surgeon can take the uterine vessels if he or she is confident that the bladder is out of harm’s way. If the fold still cannot be identified after this bite, proceed with broad-ligament clamps, which usually lead to eventual opening of the peritoneal fold.
CASE 1 Some progress, then surgery stalls
The surgeon proceeds to operate extraperitoneally, as described above, and successfully enters the anterior cul-de-sac after the uterine vessels are ligated. However, after several additional bites of broad ligament on each side, progress stalls because of uterine size. The surgeon seems to be stuck and is growing increasingly frustrated.
What is the best way around this impasse?
Morcellation can involve a range of techniques
Whenever a large uterus prevents further progress, and the uterine vessels have been ligated, uterine morcellation can be performed. Morcellation techniques originated when vaginal hysterectomy was the archetypal gynecologic operation,4-7 and include uterine bisection,8-11 Lash intramyometrial coring,6,8,9 myomectomy,10,11 and wedge debulking.9 Although every surgeon has a favorite, some or all of these procedures may be necessary in the same patient.12-15 In all cases it is mandatory that the uterine vessels be ligated before any morcellation procedure is initiated.
In my experience, a uterus in the range of 240 g usually lends itself very nicely to Lash intramyometrial coring. This technique is a nearly bloodless procedure that does not violate the endometrial cavity when it is performed properly. In addition, any intramyometrial fibroids can be easily removed.
If coring does not decompress the uterus enough for safe delivery, the core can be cut off and the remaining uterus can be further morcellated by removing wedges of myometrium or by bivalving the uterus. Since there is usually more room in the posterior vagina than in the anterior vagina, as much of the wedge morcellation as possible should be done posteriorly.
CASE 1 Ovaries appear out of reach
After Lash intramyometrial coring, the surgeon successfully removes the uterus. He then turns his attention to the bilateral adnexectomy. Unfortunately, the ovaries are higher than anticipated, and he once again considers switching to the abdominal route to remove them.
Is a change in route the best option?
Focus on the round ligaments
The key to safe removal of the adnexa, especially in difficult cases, is the separate transection and ligation of the round ligaments. Many authors have reported high success rates for vaginal oophorectomy using this technique, especially in premenopausal women.16-19
Separate transection of the round ligament allows the surgeon to accomplish 2 very important tasks:
In many hysterectomy cases when oophorectomy is planned, this maneuver can be carried out prior to removal of the uterus. Once the round ligaments have been reached, the surgeon can deliver the uterine fundus anteriorly, allowing the round ligaments to be clamped and cut. It is not uncommon to be able to remove the uterus with both adnexa still attached.
With a large uterus, it may be necessary to clamp and transect the round ligament after the uterus is out. This does not preclude identification and transection of the round ligament to carry out the maneuvers described above.
Consider your tools
In very difficult cases, specialized clamps or sutures may be necessary. I find long, sturdy, right-angle clamps to be most useful. In addition, endoloop-type sutures often facilitate ligation of the vascular pedicle. The use of newer specialized bipolar electrosurgical instruments may be helpful, although I have no personal experience using them in vaginal surgery.
CASE 1 At closure, concerns about injury
After successful removal of both adnexa using the round-ligament technique, the surgeon is satisfied that he has achieved hemostasis and proceeds with his usual closure. However, he has nagging concerns about the possibility of undetected complications, because this case turned out to be more of a challenge than he had expected. He wonders if there is anything else he can do to ensure that everything is OK.
What would you do?
Besides ensuring satisfactory hemostasis, confirming the integrity of the urinary tract is the most important goal to achieve before leaving the operating room. Unrecognized injuries to the bladder or ureters are unacceptable and will lead to significant morbidity for the patient. I would certainly recommend that the surgeon in M.K.’s case perform cystoscopy after giving the patient intravenous indigo carmine to assure both ureteral patency and integrity of the bladder. I perform cystoscopy after all vaginal hysterectomies.
CASE 2 History of cesarean delivery
C.S. is a 38-year-old gravida 3 para 3 who presents with menometrorrhagia and dysmenorrhea unresponsive to medical therapy. Her first pregnancy resulted in vaginal delivery of a full-term infant without complications. Her second child was delivered via low-segment transverse cesarean section due to a persistent breech presentation at term. Her last child was delivered vaginally, also at term. Two years later C.S. underwent a laparoscopic tubal ligation without complications. That was 4 years ago. She began seeing her current gynecologist 2 years ago, when she moved to a new community.
Pelvic examination reveals a 6-week–size uterus and normal adnexa. Her uterus is mobile, and there does not appear to be any ventral fixation of the uterus to the abdominal wall from the cesarean section. Endometrial biopsy reveals proliferative endometrium only. Saline ultrasound demonstrates a 2-cm submucosal leiomyoma.
C.S. refuses hysteroscopic resection of the myoma and prefers hysterectomy as definitive therapy. She is the business manager for her family’s construction business, and she would like to be able to return to work as soon as possible after her surgery. She requests vaginal hysterectomy with conservation of her ovaries.
What is the best way to proceed at this point?
Many gynecologic surgeons regard previous pelvic surgery, including cesarean delivery, as a relative contraindication to vaginal hysterectomy. Although the major concern seems to be a potential for bladder injury during the bladder dissection, other problems such as ventral fixation of the uterus to the previous abdominal incision also are possible.
Vaginal hysterectomy requires a mobile uterus
All patients who will be undergoing vaginal hysterectomy must have demonstrated mobility of the uterus upon pelvic examination. This is particularly important in the case of prior pelvic surgery. In this case, the gynecologist also should make every attempt to obtain her surgical records—especially those from her laparoscopic tubal ligation—to exclude major adhesive disease in the pelvis.
Laparoscopic adhesiolysis may facilitate vaginal hysterectomy
If there is any concern that the uterus is fixed to the abdominal wall, abdominal hysterectomy should be considered. Even more preferable is laparoscopic adhesiolysis, which can make it possible to proceed with vaginal hysterectomy. I have used this approach in women with as many as 5 previous cesarean deliveries and severe ventral fixation of the uterus.20 After adhesiolysis, the remainder of the hysterectomy can usually be performed solely through the vaginal route.
CASE 2 Medical records suggest the vaginal route is feasible
The gynecologist obtains C.S.’s previous medical records, which confirm that the cesarean delivery was uncomplicated. They also indicate that, at the time of the sterilization procedure, there was no evidence of ventral fixation of the uterus or other major adhesive disease.
The physician decides to proceed with vaginal hysterectomy, but remains very concerned about the possibility of bladder injury. How can she avoid inadvertent cystotomy?
Difficulty identifying and safely dissecting the bladder—because of distortion of the vesicouterine space from the previous cesarean delivery—is a legitimate concern. However, injury to a scarred and densely adherent bladder is a risk even with abdominal dissection.
The vaginal approach to the distal vesicouterine space has a clear advantage: The vesicouterine space closest to the initial vaginal dissection is unaffected by the previous operation on the lower uterine segment. In contrast, with the abdominal approach, dissection begins in the area of scar, and only after penetrating the scar does one find the unaffected space. With the vaginal approach, dissection begins in the correct surgical plane, which aids in identification of the location of the bladder and cesarean scar.
Sharp dissection is a must to protect the bladder
Once the correct surgical plane is encountered, sharp dissection is necessary to prevent tears of the adherent bladder, which can occur with blunt dissection.
Although sharp dissection is the key to success under these circumstances, other maneuvers may be helpful in some cases.
Nichols21 suggested performing dissection of the bladder after it has been filled with a dilute indigo carmine solution to stain the bladder tissues and help prevent bladder injury.
Hoffman and Jaeger22 describe the placement of a bent uterine sound in the posterior cul-de-sac. The sound is then brought around to the anterior cul-de-sac as an aid to dissection of the bladder and the vesicouterine peritoneal fold.
Sheth and Malpani23 describe developing a lateral “window” through the broad ligament to the bladder dissection when there are dense midline adhesions.
Although these are all valuable suggestions, I have found that they are rarely needed with careful sharp dissection. Remember, it is essential to avoid the temptation of blunt dissection when performing vaginal hysterectomy in women with a prior cesarean delivery.
CASE 2 Procedure is a success
The vaginal hysterectomy is carried out without incident, and cystoscopy following the hysterectomy is negative for any bladder injury; both ureteral orifices promptly efflux indigo carmine.
The surgeon encountered little difficulty during the bladder dissection, which was performed sharply. In fact, she was surprised at how well she could actually identify the hysterotomy scar and bladder. The patient goes home after 24 hours and is back at work in 2 weeks.
As noted in both cases presented here, the gynecologic surgeon must be certain that the urinary tract is intact and uninjured prior to leaving the operating room. This includes careful inspection of the bladder grossly for any sign of injury, as well as cystoscopy.
Most bladder injuries that occur with hysterectomy—either vaginal or abdominal—are usually well above the trigone and can be carefully repaired by the gynecologic surgeon. Complex injuries to the bladder involving the trigone or ureters usually require urologic intraoperative consultation.
The author reports no financial relationships relevant to this article.
M.K. is a 43-year-old gravida 2 para 2 who is undergoing a vaginal hysterectomy for menorrhagia. A preoperative pelvic exam and ultrasound suggested a 12-week-size uterus with several small leiomyomata. Her gynecologist estimates the uterine weight at 240 g and notes that the uterus is mobile. M.K. asks that her ovaries be removed at the time of hysterectomy because of a family history of ovarian cancer.
During the initial dissection, the surgeon is unable to enter the anterior cul-de-sac due to distortion created by an anterior fibroid. The surgeon has entered the posterior cul-de-sac, but the uterus is too large to manipulate a finger around anteriorly to identify the peritoneal fold. Although he feels confident that the bladder has been adequately mobilized from the cervix, the surgeon is strongly considering abandoning the vaginal approach and completing the hysterectomy abdominally.
How should he proceed?
Entry into the peritoneal cavity through the anterior or posterior cul-de-sac can sometimes be challenging, as this case illustrates. However, there is no need for the surgeon to abandon the vaginal approach just yet. In my experience, the anterior peritoneal fold can be high or distorted by fibroids in some women. The key to successful surgery is a pause in activity to consider the case at hand and determine whether additional progress can be made safely without changing the approach.
Avoid blind entry at all costs
No less an authority than Heaney1 advised against blind attempts to enter the anterior cul-de-sac. Such attempts are often frustrating, can involve bleeding, and raise the risk of injury to the bladder. However, once the surgeon is confident that the bladder is free and retracted out of the way, he or she can proceed without intraperitoneal entry. This is especially true if the posterior cul-de-sac has been entered safely.
The “climb up” technique
In some cases, the surgeon may safely proceed extraperitoneally even if neither cul-de-sac has been opened. Krige2 coined the term “climb up” to describe the extraperitoneal approach to the inaccessible posterior cul-de-sac. He performed extensive extraperitoneal dissection that, if necessary, included both uterosacral and cardinal ligaments as well as uterine vessels. A surgeon may carry a total extraperitoneal dissection completely to the uterine fundus as long as the bladder and rectum are free.3
In M.K.’s case, the surgeon should proceed to take the uterosacral and cardinal ligaments posteriorly without swinging the clamps around to the anterior aspect of the cervix, if possible. Once these ligaments are taken, the uterus often descends enough that the anterior peritoneal fold becomes accessible. Once it is identified, the anterior cul-de-sac can be entered safely.
If safe entry still is not possible, the surgeon can take the uterine vessels if he or she is confident that the bladder is out of harm’s way. If the fold still cannot be identified after this bite, proceed with broad-ligament clamps, which usually lead to eventual opening of the peritoneal fold.
CASE 1 Some progress, then surgery stalls
The surgeon proceeds to operate extraperitoneally, as described above, and successfully enters the anterior cul-de-sac after the uterine vessels are ligated. However, after several additional bites of broad ligament on each side, progress stalls because of uterine size. The surgeon seems to be stuck and is growing increasingly frustrated.
What is the best way around this impasse?
Morcellation can involve a range of techniques
Whenever a large uterus prevents further progress, and the uterine vessels have been ligated, uterine morcellation can be performed. Morcellation techniques originated when vaginal hysterectomy was the archetypal gynecologic operation,4-7 and include uterine bisection,8-11 Lash intramyometrial coring,6,8,9 myomectomy,10,11 and wedge debulking.9 Although every surgeon has a favorite, some or all of these procedures may be necessary in the same patient.12-15 In all cases it is mandatory that the uterine vessels be ligated before any morcellation procedure is initiated.
In my experience, a uterus in the range of 240 g usually lends itself very nicely to Lash intramyometrial coring. This technique is a nearly bloodless procedure that does not violate the endometrial cavity when it is performed properly. In addition, any intramyometrial fibroids can be easily removed.
If coring does not decompress the uterus enough for safe delivery, the core can be cut off and the remaining uterus can be further morcellated by removing wedges of myometrium or by bivalving the uterus. Since there is usually more room in the posterior vagina than in the anterior vagina, as much of the wedge morcellation as possible should be done posteriorly.
CASE 1 Ovaries appear out of reach
After Lash intramyometrial coring, the surgeon successfully removes the uterus. He then turns his attention to the bilateral adnexectomy. Unfortunately, the ovaries are higher than anticipated, and he once again considers switching to the abdominal route to remove them.
Is a change in route the best option?
Focus on the round ligaments
The key to safe removal of the adnexa, especially in difficult cases, is the separate transection and ligation of the round ligaments. Many authors have reported high success rates for vaginal oophorectomy using this technique, especially in premenopausal women.16-19
Separate transection of the round ligament allows the surgeon to accomplish 2 very important tasks:
In many hysterectomy cases when oophorectomy is planned, this maneuver can be carried out prior to removal of the uterus. Once the round ligaments have been reached, the surgeon can deliver the uterine fundus anteriorly, allowing the round ligaments to be clamped and cut. It is not uncommon to be able to remove the uterus with both adnexa still attached.
With a large uterus, it may be necessary to clamp and transect the round ligament after the uterus is out. This does not preclude identification and transection of the round ligament to carry out the maneuvers described above.
Consider your tools
In very difficult cases, specialized clamps or sutures may be necessary. I find long, sturdy, right-angle clamps to be most useful. In addition, endoloop-type sutures often facilitate ligation of the vascular pedicle. The use of newer specialized bipolar electrosurgical instruments may be helpful, although I have no personal experience using them in vaginal surgery.
CASE 1 At closure, concerns about injury
After successful removal of both adnexa using the round-ligament technique, the surgeon is satisfied that he has achieved hemostasis and proceeds with his usual closure. However, he has nagging concerns about the possibility of undetected complications, because this case turned out to be more of a challenge than he had expected. He wonders if there is anything else he can do to ensure that everything is OK.
What would you do?
Besides ensuring satisfactory hemostasis, confirming the integrity of the urinary tract is the most important goal to achieve before leaving the operating room. Unrecognized injuries to the bladder or ureters are unacceptable and will lead to significant morbidity for the patient. I would certainly recommend that the surgeon in M.K.’s case perform cystoscopy after giving the patient intravenous indigo carmine to assure both ureteral patency and integrity of the bladder. I perform cystoscopy after all vaginal hysterectomies.
CASE 2 History of cesarean delivery
C.S. is a 38-year-old gravida 3 para 3 who presents with menometrorrhagia and dysmenorrhea unresponsive to medical therapy. Her first pregnancy resulted in vaginal delivery of a full-term infant without complications. Her second child was delivered via low-segment transverse cesarean section due to a persistent breech presentation at term. Her last child was delivered vaginally, also at term. Two years later C.S. underwent a laparoscopic tubal ligation without complications. That was 4 years ago. She began seeing her current gynecologist 2 years ago, when she moved to a new community.
Pelvic examination reveals a 6-week–size uterus and normal adnexa. Her uterus is mobile, and there does not appear to be any ventral fixation of the uterus to the abdominal wall from the cesarean section. Endometrial biopsy reveals proliferative endometrium only. Saline ultrasound demonstrates a 2-cm submucosal leiomyoma.
C.S. refuses hysteroscopic resection of the myoma and prefers hysterectomy as definitive therapy. She is the business manager for her family’s construction business, and she would like to be able to return to work as soon as possible after her surgery. She requests vaginal hysterectomy with conservation of her ovaries.
What is the best way to proceed at this point?
Many gynecologic surgeons regard previous pelvic surgery, including cesarean delivery, as a relative contraindication to vaginal hysterectomy. Although the major concern seems to be a potential for bladder injury during the bladder dissection, other problems such as ventral fixation of the uterus to the previous abdominal incision also are possible.
Vaginal hysterectomy requires a mobile uterus
All patients who will be undergoing vaginal hysterectomy must have demonstrated mobility of the uterus upon pelvic examination. This is particularly important in the case of prior pelvic surgery. In this case, the gynecologist also should make every attempt to obtain her surgical records—especially those from her laparoscopic tubal ligation—to exclude major adhesive disease in the pelvis.
Laparoscopic adhesiolysis may facilitate vaginal hysterectomy
If there is any concern that the uterus is fixed to the abdominal wall, abdominal hysterectomy should be considered. Even more preferable is laparoscopic adhesiolysis, which can make it possible to proceed with vaginal hysterectomy. I have used this approach in women with as many as 5 previous cesarean deliveries and severe ventral fixation of the uterus.20 After adhesiolysis, the remainder of the hysterectomy can usually be performed solely through the vaginal route.
CASE 2 Medical records suggest the vaginal route is feasible
The gynecologist obtains C.S.’s previous medical records, which confirm that the cesarean delivery was uncomplicated. They also indicate that, at the time of the sterilization procedure, there was no evidence of ventral fixation of the uterus or other major adhesive disease.
The physician decides to proceed with vaginal hysterectomy, but remains very concerned about the possibility of bladder injury. How can she avoid inadvertent cystotomy?
Difficulty identifying and safely dissecting the bladder—because of distortion of the vesicouterine space from the previous cesarean delivery—is a legitimate concern. However, injury to a scarred and densely adherent bladder is a risk even with abdominal dissection.
The vaginal approach to the distal vesicouterine space has a clear advantage: The vesicouterine space closest to the initial vaginal dissection is unaffected by the previous operation on the lower uterine segment. In contrast, with the abdominal approach, dissection begins in the area of scar, and only after penetrating the scar does one find the unaffected space. With the vaginal approach, dissection begins in the correct surgical plane, which aids in identification of the location of the bladder and cesarean scar.
Sharp dissection is a must to protect the bladder
Once the correct surgical plane is encountered, sharp dissection is necessary to prevent tears of the adherent bladder, which can occur with blunt dissection.
Although sharp dissection is the key to success under these circumstances, other maneuvers may be helpful in some cases.
Nichols21 suggested performing dissection of the bladder after it has been filled with a dilute indigo carmine solution to stain the bladder tissues and help prevent bladder injury.
Hoffman and Jaeger22 describe the placement of a bent uterine sound in the posterior cul-de-sac. The sound is then brought around to the anterior cul-de-sac as an aid to dissection of the bladder and the vesicouterine peritoneal fold.
Sheth and Malpani23 describe developing a lateral “window” through the broad ligament to the bladder dissection when there are dense midline adhesions.
Although these are all valuable suggestions, I have found that they are rarely needed with careful sharp dissection. Remember, it is essential to avoid the temptation of blunt dissection when performing vaginal hysterectomy in women with a prior cesarean delivery.
CASE 2 Procedure is a success
The vaginal hysterectomy is carried out without incident, and cystoscopy following the hysterectomy is negative for any bladder injury; both ureteral orifices promptly efflux indigo carmine.
The surgeon encountered little difficulty during the bladder dissection, which was performed sharply. In fact, she was surprised at how well she could actually identify the hysterotomy scar and bladder. The patient goes home after 24 hours and is back at work in 2 weeks.
As noted in both cases presented here, the gynecologic surgeon must be certain that the urinary tract is intact and uninjured prior to leaving the operating room. This includes careful inspection of the bladder grossly for any sign of injury, as well as cystoscopy.
Most bladder injuries that occur with hysterectomy—either vaginal or abdominal—are usually well above the trigone and can be carefully repaired by the gynecologic surgeon. Complex injuries to the bladder involving the trigone or ureters usually require urologic intraoperative consultation.
The author reports no financial relationships relevant to this article.
REFERNCES
1. Heaney NS. Vaginal hysterectomy-its indications and technique. Am J Surg. 1940;48:284-288.
2. Krige CF. Vaginal hysterectomy and genital prolapse repair. Johannesburg, South Africa: Witwatersrand University Press; 1965: 57-70.
3. Unger JB. The extraperitoneal approach to vaginal hysterectomy. J Pelvic Surg. 1997;3:240-245.
4. Garceau E. Vaginal hysterectomy as done in France. Am J Obstet Dis Women Child. 1895;31:305-346.
5. Heaney NS. A report of 565 vaginal hysterectomies performed for benign disease. Am J Obstet Gynecol. 1934;28:751-755.
6. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
7. Allen E, Peterson LF. Versatility of vaginal hysterectomy technic. Obstet Gynecol. 1954;3:240-247.
8. Nichols DH, Randall CL. Vaginal Surgery. 4th ed. Baltimore: Williams and Wilkins;1996.
9. Stovall TG. Vaginal, abdominal, and laparoscopic-assisted hysterectomy. In: Mann WJ, Stovall TG, eds. Gynecologic Surgery. New York: Churchill Livingstone;1996: 403-404.
10. Lee RA. Atlas of Gynecologic Surgery. Philadelphia: WB Saunders; 1992.
11. Reiffenstuhl G, Platzer W, Knapstein PG, Imig JR. Vaginal operations–surgical anatomy and technique. 2nd ed. Baltimore: Williams and Wilkins; 1996.
12. Unger JB. Vaginal hysterectomy for the woman with a moderately enlarged uterus weighing 200 to 700 grams. Am J Obstet Gynecol. 1999;180:1337-1344.
13. Magos A, Bournas N, Sinha R, Richardson RE, O’Connor H. Vaginal hysterectomy for the large uterus. Br J Obstet Gynaecol. 1996;103:246-251.
14. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico Hospital’s experience. Obstet Gynecol. 1996;88:560-563.
15. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, nonmorbid procedure. Obstet Gynecol. 1995;86:60-64.
16. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
17. Ballard LA, Walters MD. Transvaginal mobilization and removal of the ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
18. Davies A, O’Connor H, Magos AL. A prospective study to evaluate oophorectomy at the time of vaginal hysterectomy. Br J Obstet Gynaecol. 1996;103:915-920.
19. Unger JB. Planned prophylactic oophorectomy at vaginal hysterectomy: clamp technique with separate division of the round and infundibulopelvic ligaments. J Pelvic Surg. 1999;5:151-155.
20. Unger JB, Meeks GR. Vaginal hysterectomy in women with history of previous cesarean delivery. Am J Obstet Gynecol. 1998;179:1473-1478.
21. Nichols DH. Vaginal versus abdominal hysterectomy. In: Stovall TG, ed. Current topics in obstetrics and gynecology: hysterectomy. New York: Elsevier; 1993:27-33.
22. Hoffman MS, Jaeger M. A new method for gaining entry into the scarred anterior cul-de-sac during transvaginal hysterectomy. Am J Obstet Gynecol. 1990;162:1269-1270.
23. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynecol Obstet. 1995;50:165-169.
REFERNCES
1. Heaney NS. Vaginal hysterectomy-its indications and technique. Am J Surg. 1940;48:284-288.
2. Krige CF. Vaginal hysterectomy and genital prolapse repair. Johannesburg, South Africa: Witwatersrand University Press; 1965: 57-70.
3. Unger JB. The extraperitoneal approach to vaginal hysterectomy. J Pelvic Surg. 1997;3:240-245.
4. Garceau E. Vaginal hysterectomy as done in France. Am J Obstet Dis Women Child. 1895;31:305-346.
5. Heaney NS. A report of 565 vaginal hysterectomies performed for benign disease. Am J Obstet Gynecol. 1934;28:751-755.
6. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
7. Allen E, Peterson LF. Versatility of vaginal hysterectomy technic. Obstet Gynecol. 1954;3:240-247.
8. Nichols DH, Randall CL. Vaginal Surgery. 4th ed. Baltimore: Williams and Wilkins;1996.
9. Stovall TG. Vaginal, abdominal, and laparoscopic-assisted hysterectomy. In: Mann WJ, Stovall TG, eds. Gynecologic Surgery. New York: Churchill Livingstone;1996: 403-404.
10. Lee RA. Atlas of Gynecologic Surgery. Philadelphia: WB Saunders; 1992.
11. Reiffenstuhl G, Platzer W, Knapstein PG, Imig JR. Vaginal operations–surgical anatomy and technique. 2nd ed. Baltimore: Williams and Wilkins; 1996.
12. Unger JB. Vaginal hysterectomy for the woman with a moderately enlarged uterus weighing 200 to 700 grams. Am J Obstet Gynecol. 1999;180:1337-1344.
13. Magos A, Bournas N, Sinha R, Richardson RE, O’Connor H. Vaginal hysterectomy for the large uterus. Br J Obstet Gynaecol. 1996;103:246-251.
14. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico Hospital’s experience. Obstet Gynecol. 1996;88:560-563.
15. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, nonmorbid procedure. Obstet Gynecol. 1995;86:60-64.
16. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
17. Ballard LA, Walters MD. Transvaginal mobilization and removal of the ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
18. Davies A, O’Connor H, Magos AL. A prospective study to evaluate oophorectomy at the time of vaginal hysterectomy. Br J Obstet Gynaecol. 1996;103:915-920.
19. Unger JB. Planned prophylactic oophorectomy at vaginal hysterectomy: clamp technique with separate division of the round and infundibulopelvic ligaments. J Pelvic Surg. 1999;5:151-155.
20. Unger JB, Meeks GR. Vaginal hysterectomy in women with history of previous cesarean delivery. Am J Obstet Gynecol. 1998;179:1473-1478.
21. Nichols DH. Vaginal versus abdominal hysterectomy. In: Stovall TG, ed. Current topics in obstetrics and gynecology: hysterectomy. New York: Elsevier; 1993:27-33.
22. Hoffman MS, Jaeger M. A new method for gaining entry into the scarred anterior cul-de-sac during transvaginal hysterectomy. Am J Obstet Gynecol. 1990;162:1269-1270.
23. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynecol Obstet. 1995;50:165-169.
IN THIS ARTICLE
Vaginal hysterectomy: 6 challenges, an arsenal of solutions
Newer codes for vaginal hysterectomy capture the work of removing larger uteri without laparoscopy
True or false: When it comes to hysterectomy, surgeons tend to use the route that is safest, least invasive, and most economical.
Sadly, the statement is false. Although vaginal hysterectomy tops all 3 categories, it is the least utilized of surgical routes. The number of vaginal hysterectomies may have increased slightly over the past decade, likely due to the incorporation of laparoscopically assisted vaginal hysterectomy into the mainstream and increased practice with the vaginal component, but fewer than 30% of hysterectomies are performed vaginally.
This article addresses 6 common challenges at vaginal hysterectomy and offers strategies to overcome them.
Laparoscopic strategies ease vaginal hysterectomy, too
Laparoscopic hysterectomy became widely accepted when surgical instruments were developed to overcome the technical challenges inherent in operating with limited access. By incorporating some of the techniques we routinely use for laparoscopic surgery, we can overcome many of the challenges faced during difficult vaginal surgery.
VAGINAL HYSTERECTOMY CHALLENGE 1: Obesity
Unfortunately, our population is increasingly rotund. This is not only a significant risk factor for the patient’s health in general, but it poses some unique challenges for surgeons. I must say that, as tough as it may be to complete a hysterectomy vaginally in a morbidly obese woman, I would much rather approach her pelvic organs through the cul-de-sac, which contains no fat cells, than through the abdominal wall—either laparoscopically or abdominally! The trick is gaining access to the posterior cul-de-sac.
How to enter the cul-de-sac
It seems to be a perverse rule of nature, but a tight upper vaginal ring seems almost universal in obese women. Added to the redundant sidewalls and the large buttocks, this tightness makes entry into the anterior or posterior cul-de-sac problematic. Several tricks make peritoneal access possible:
Position the patient to increase access, with the buttocks well over the edge of the operating table. This brings the operative field a bit closer to the surgeon, and permits the use of long-handled retractors posteriorly.
Use candy-cane stirrups to allow assistants better access to the operative field. Adequate assistance is essential in attempting vaginal surgery in the morbidly obese.
Avoid the Trendelenburg position. Although it might seem that this position would facilitate visualization and placement of a posterior weighted speculum, all it does is allow the patient to slide up on the table, making placement of alternate retractors difficult.
Use the right tools. If the posterior weighted speculum will not stay in place or does not afford access to the cul-de-sac due to an upper vaginal ring, use a narrow Deaver retractor posteriorly (without sidewall or anterior retraction). Use a Jacob’s tenaculum on the posterior lip of the cervix and have your assistant pull straight up on the tenaculum while using the Deaver retractor to see the area between the uterosacral ligaments.
Use the uterosacral ligaments as a guide. Another perversity in morbidly obese women: Despite multiparity, they seem to have little or no apical prolapse but lots of vaginal wall redundancy. The cervix is often elongated, but the uterosacral ligaments are sky high.
I palpate these ligaments, injecting them with a combination of vasopressin diluted 1:5 with bupivacaine and epinephrine (for enhanced hemostasis and preemptive analgesia), then use a pencil electrosurgical electrode to rapidly open the vaginal epithelium between the ligaments.
I then use a long, toothed tissue forceps to tent the peritoneum at 90 degrees to the plane of the posterior cul-de-sac and use Mayo scissors to enter the peritoneal cavity. Usually there is a spurt of fluid to mark appropriate entry into the peritoneum.
I then use the blades of my scissors to stretch the peritoneum between the ligaments and place a moistened 4×4 sponge into the incision.
At the onset of the procedure, inject indigo carmine dye intravenously so that any injury to the bladder will be immediately recognized. I have the circulating nurse empty the bladder while she is prepping the patient, but do not leave an indwelling catheter in place during the operation. I find it cumbersome to work around the catheter.
Problematic entries
When entry into the posterior cul-de-sac is difficult, I stop dissection, place a 4×4 sponge into the incision to reduce bleeding from the vagina, and proceed to attempt anterior entry.
I place the Deaver retractor into the anterior space and move the tenaculum to the anterior lip of the cervix. This gives maximal space for downward traction on the cervix while anterior entry is attempted.
Once again, I inject the tissue with the bupivacaine solution before incising the vaginal epithelium at the level of the internal os. I use sharp dissection only when creating a plane between the lower uterine segment and the bladder.
Ensuring room to move and good visualization
If neither the anterior nor the posterior cul-de-sac can be accessed, it may be time to rethink the vaginal approach—but there is no harm in taking the uterosacral and cardinal ligaments extraperitoneally in an effort to gain some mobility. Hugging the uterus and leaving the anterior retractor in place to lift the bladder superiorly are essential steps to protect the ureters. The cervix can then be split in the midline (12 to 6 o’clock) to easily identify the peritoneal reflections.
Other tips
Sidewall retractors are rarely needed. They significantly impair placement of clamps and sutures by creating a long, narrow, parallel passageway. An alternative trick is to use the suction tip to retract the vaginal sidewall as the surgeon is working.
A disposable, fiberoptic, lighted suction irrigator is another option. The light can be directed precisely where it is needed, and the irrigation helps keep the field clean and tidy, simplifying identification of anatomy.
Skip the sutures whenever possible. Because it is difficult to place sutures with precision in a tight, poorly illuminated space, I use a vessel sealer for all pedicles above the uterosacral ligaments. Some of these instruments were designed specifically for vaginal hysterectomy in the same shape and size as Heaney clamps. They are remarkably efficient and permit the completion of a vaginal procedure when suture placement is difficult.
Use a Heaney needleholder, with the suture loaded precisely in the center of the needle curve, along with the lighted suction irrigator to retract redundant tissue away from the track of the needle, to facilitate suturing high in the pelvis.
VAGINAL HYSTERECTOMY CHALLENGE 2: Nulliparity
Many of us are reluctant to attempt vaginal hysterectomy in a woman who has never had children. Although this situation can be challenging at times, in my experience, the access issues tend to be more difficult in obese, multiparous women.
The same tricks and techniques addressed above will permit the vaginal approach in almost all nulliparous women.
VAGINAL HYSTERECTOMY CHALLENGE 3: Previous cesarean section
A prior cesarean delivery is sometimes considered an indication for laparoscopic hysterectomy. There are 2 concerns here: The patient may have a small pelvis, and there may be significant scar tissue and difficulty gaining access to the anterior cul-de-sac, as a result of repeated dissection between the lower uterine segment and the bladder. Several tricks may be useful:
Examine the patient under anesthesia to ensure that the fundus is not stuck to the anterior abdominal wall. This can occur if the peritoneum was not closed during the last cesarean section.
Empty the bladder before beginning the hysterectomy, and inject indigo carmine dye intravenously with the induction of anesthesia.
Use careful sharp dissection between the bladder and lower uterine segment using fine Metzenbaum scissors, with the tips pointed toward the uterus. Dissect only as far as you can easily see.
Secure the uterosacral, cardinal, and broad ligaments, if necessary, before pursuing entry into the anterior cul-de-sac. It is not essential to gain anterior access before taking these pedicles. The additional mobility and descensus enable safe sharp dissection.
If pedicles have been secured up to the fundus, and the anterior cul-de-sac remains difficult to assess, flip the fundus through the posterior cul-de-sac and reach your finger or an instrument around the top of the fundus to identify the peritoneum anteriorly, then incise it under direct vision.
VAGINAL HYSTERECTOMY CHALLENGE 4: Previous abdominal/pelvic surgery
This history is another often-cited rationale for avoiding the vaginal approach. In reality, the adhesions created from prior surgery tend to arise between the anterior abdominal wall and the omentum or small bowel. This situation makes laparoscopic or open abdominal entry riskier than vaginal peritoneal access through the cul-de-sac.
Two possible exceptions: a patient who has had surgery for deeply infiltrating endometriosis in the cul-de-sac or a woman who has undergone myomectomies with posterior incisions. These patients may have dense scarring in the cul-de-sac, which would preclude a vaginal approach. Whatever the surgical route, appropriate bowel preparation is necessary to permit simple closure of any intestinal injury at the time of hysterectomy. Why not begin vaginally if the exam under anesthesia demonstrates an accessible and reasonably free cul-de-sac?
VAGINAL HYSTERECTOMY CHALLENGE 5: Large myomatous uterus
A uterus of any size can be removed vaginally as long as there is mobility with access to the uterine arteries. The one exception: a patient with a small, normal uterus and a massive pedunculated myoma arising from the top of the fundus. If the fibroid cannot be pulled into the true pelvis for morcellation, it cannot be removed transvaginally. Fortunately, this situation is quite rare.
Keep the uterine serosa intact to maintain orientation.
Split the uterus from 12 to 6 o’clock (bivalving). Protect the bladder with a Deaver retractor anteriorly, and protect the posterior vaginal epithelium with the weighted speculum posteriorly.
Remove chunks of tissue from the inside of the specimen. Orient your scalpel blade so that you are always cutting toward the center of the specimen. That way, if the blade slips, you will not accidentally injure tissue on the pelvic sidewall.
Use Lahey thyroid clamps to place the tissue you plan to remove under tension. Finding the capsule of each myoma and gently separating it from the surrounding myometrium facilitates delivery of larger fibroids into the endometrial cavity. Some myomas require morcellation themselves for removal.
Replace the scalpel blade periodically to keep it sharp. Calcified fibroids can dull the blade rapidly.
Work systematically to remove as much central tissue as possible. Try to keep a clean, sharp margin of tissue around the edges for easy grasping. Torn, irregular tissue is very difficult to grab and may cause significant frustration.
If access becomes limited, try clamping additional pedicles on each side of the specimen. A tiny amount of additional descensus can make a huge difference.
Do not administer GnRH agonists prior to surgery. The uterus may shrink, but the myomas tend to become quite soft and difficult to remove. If the patient is seriously anemic, give norethindrone acetate, 5 to 20 mg daily, to stop bleeding and allow the patient’s red blood cell volume to improve before elective surgery.
Use a vessel-sealing instrument to control the pedicles. This strategy produces optimal hemostasis to permit a dry field during morcellation. Moreover, the seals do not get disrupted when the large uterus is pulled past them. Placing suture around pedicles when there is a large, bulky uterus in the pelvis is challenging at best, and it is frustrating to see significant bleeding after removal of the specimen. This problem does not seem to occur with the sealing devices.
Know when to quit! We should not promise any patient a minimally invasive operation. If there is uncontrolled bleeding or no progress after 5 to 10 minutes, convert to a laparoscopic or abdominal approach.
I schedule cases I know will be challenging as “possible” laparoscopic or open hysterectomy. This alerts the OR staff to have additional equipment ready and nearby should we need it. It is not a surgical failure or complication to convert a minimally invasive hysterectomy to a more invasive technique when appropriate. Better to have tried and failed than never to have tried at all!
VAGINAL HYSTERECTOMY CHALLENGE 6: Avoiding complications
The most common complications of vaginal hysterectomy are bleeding, infection, and injury to the bladder. Ureteral injury is less common at vaginal hysterectomy than with the abdominal or laparoscopic approaches. Thus, I do not think routine cystoscopy is essential after uncomplicated vaginal hysterectomy, although I recommend intravenous administration of indigo carmine dye at the beginning of the procedure to enable rapid recognition of even a small bladder laceration. Sharp, careful dissection of the bladder off the lower uterine segment and the avoidance of finger dissection (especially with a gauze sponge) keep these injuries to a minimum.
Minimize bleeding by using newer vessel-sealing technologies rather than suture for most of the pedicles. I attach the uterosacral–cardinal ligament pedicles to the vaginal cuff at closure with suture. I suture the first pedicle once I have entered the posterior cul-de-sac and hold that suture to stay oriented.
Pay attention to patient positioning. Careful positioning will help you avoid neurological injuries. Avoid hyperflexion at the hips, which stretches the femoral nerve. Large nerves have comparatively little blood supply, so stretching them for prolonged periods can cause hypoxic injury. Although such injuries are almost always rapidly reversible, they are disconcerting for both the patient and her surgeon.
When operating on a very thin woman with a bony sacrum, I like to place egg-crate foam beneath the buttocks to provide some cushioning. I am also very careful with these women to keep their legs in a neutral position, and I watch my surgical assistants to be sure they are not leaning on the patient during the procedure.
Prophylactic antibiotics are a must to avoid postoperative vaginal cuff infections and pelvic abscess. Smokers and women with preexisting bacterial vaginosis are at highest risk for infection. I ask women to discontinue smoking at least 2 weeks prior to surgery and inform all smokers that their risk of infection is heightened. I treat anaerobic overgrowth in the vagina prior to surgery to help prevent infections in women with bacterial vaginosis.
The timing of prophylactic antibiotics is important. Intravenous first-generation cephalosporins must be administered within 60 minutes of the initial incision, but it is important to give them early enough for them to adequately disseminate to tissue before the colpotomy incision.
DVT prophylaxis is especially important for women with large uteri. Routine use of sequential compression stockings is both cost-effective and equivalent to the prophylactic use of subcutaneous heparin, so I use them for all patients undergoing vaginal hysterectomy. Early ambulation (usually within 2 hours of surgery) is also helpful in avoiding thromboses.
90% of hysterectomies can be performed vaginally
Using the techniques described in this article, I have been able to perform over 90% of the hysterectomies in my practice vaginally. More than 50% of my patients are either morbidly obese, nulliparous, or have had previous abdominal surgery of some type.
The instruments I find most useful are the lighted suction irrigator and the vessel-sealing Heaney-type clamp.
Establishing a routine and approaching technically challenging cases with a systematic and standardized set of techniques make the vaginal route possible for the vast majority of patients with benign disease.
Dr. Levy has served as a consultant to ValleyLab.
SUGGESTED READING
1. Abostini A, Vejux N, Colette E, et al. Risk of bladder injury during vaginal hysterectomy in women with a previous cesarean section. J Reprod Med. 2005;50:940-942.
2. Clave H, Barr H, Niccolai P. Painless vaginal hysterectomy with thermal hemostasis (results of a series of 152 cases). Gynecol Surg. 2005;2:101-105.
3. Isik-Akbay EF, Harmanli OH, Panganamamula UR, et al. Hysterectomy in obese women: a comparison of abdominal and vaginal routes. Obstet Gynecol. 2004;104:710-714.
4. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
5. O’Neal MG, Beste T, Shackelford DP. Utility of preemptive local analgesia in vaginal hysterectomy. Am J Obstet Gynecol. 2003;189:1539-1542.
6. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005;(1):CD003677.-
Newer codes for vaginal hysterectomy capture the work of removing larger uteri without laparoscopy
True or false: When it comes to hysterectomy, surgeons tend to use the route that is safest, least invasive, and most economical.
Sadly, the statement is false. Although vaginal hysterectomy tops all 3 categories, it is the least utilized of surgical routes. The number of vaginal hysterectomies may have increased slightly over the past decade, likely due to the incorporation of laparoscopically assisted vaginal hysterectomy into the mainstream and increased practice with the vaginal component, but fewer than 30% of hysterectomies are performed vaginally.
This article addresses 6 common challenges at vaginal hysterectomy and offers strategies to overcome them.
Laparoscopic strategies ease vaginal hysterectomy, too
Laparoscopic hysterectomy became widely accepted when surgical instruments were developed to overcome the technical challenges inherent in operating with limited access. By incorporating some of the techniques we routinely use for laparoscopic surgery, we can overcome many of the challenges faced during difficult vaginal surgery.
VAGINAL HYSTERECTOMY CHALLENGE 1: Obesity
Unfortunately, our population is increasingly rotund. This is not only a significant risk factor for the patient’s health in general, but it poses some unique challenges for surgeons. I must say that, as tough as it may be to complete a hysterectomy vaginally in a morbidly obese woman, I would much rather approach her pelvic organs through the cul-de-sac, which contains no fat cells, than through the abdominal wall—either laparoscopically or abdominally! The trick is gaining access to the posterior cul-de-sac.
How to enter the cul-de-sac
It seems to be a perverse rule of nature, but a tight upper vaginal ring seems almost universal in obese women. Added to the redundant sidewalls and the large buttocks, this tightness makes entry into the anterior or posterior cul-de-sac problematic. Several tricks make peritoneal access possible:
Position the patient to increase access, with the buttocks well over the edge of the operating table. This brings the operative field a bit closer to the surgeon, and permits the use of long-handled retractors posteriorly.
Use candy-cane stirrups to allow assistants better access to the operative field. Adequate assistance is essential in attempting vaginal surgery in the morbidly obese.
Avoid the Trendelenburg position. Although it might seem that this position would facilitate visualization and placement of a posterior weighted speculum, all it does is allow the patient to slide up on the table, making placement of alternate retractors difficult.
Use the right tools. If the posterior weighted speculum will not stay in place or does not afford access to the cul-de-sac due to an upper vaginal ring, use a narrow Deaver retractor posteriorly (without sidewall or anterior retraction). Use a Jacob’s tenaculum on the posterior lip of the cervix and have your assistant pull straight up on the tenaculum while using the Deaver retractor to see the area between the uterosacral ligaments.
Use the uterosacral ligaments as a guide. Another perversity in morbidly obese women: Despite multiparity, they seem to have little or no apical prolapse but lots of vaginal wall redundancy. The cervix is often elongated, but the uterosacral ligaments are sky high.
I palpate these ligaments, injecting them with a combination of vasopressin diluted 1:5 with bupivacaine and epinephrine (for enhanced hemostasis and preemptive analgesia), then use a pencil electrosurgical electrode to rapidly open the vaginal epithelium between the ligaments.
I then use a long, toothed tissue forceps to tent the peritoneum at 90 degrees to the plane of the posterior cul-de-sac and use Mayo scissors to enter the peritoneal cavity. Usually there is a spurt of fluid to mark appropriate entry into the peritoneum.
I then use the blades of my scissors to stretch the peritoneum between the ligaments and place a moistened 4×4 sponge into the incision.
At the onset of the procedure, inject indigo carmine dye intravenously so that any injury to the bladder will be immediately recognized. I have the circulating nurse empty the bladder while she is prepping the patient, but do not leave an indwelling catheter in place during the operation. I find it cumbersome to work around the catheter.
Problematic entries
When entry into the posterior cul-de-sac is difficult, I stop dissection, place a 4×4 sponge into the incision to reduce bleeding from the vagina, and proceed to attempt anterior entry.
I place the Deaver retractor into the anterior space and move the tenaculum to the anterior lip of the cervix. This gives maximal space for downward traction on the cervix while anterior entry is attempted.
Once again, I inject the tissue with the bupivacaine solution before incising the vaginal epithelium at the level of the internal os. I use sharp dissection only when creating a plane between the lower uterine segment and the bladder.
Ensuring room to move and good visualization
If neither the anterior nor the posterior cul-de-sac can be accessed, it may be time to rethink the vaginal approach—but there is no harm in taking the uterosacral and cardinal ligaments extraperitoneally in an effort to gain some mobility. Hugging the uterus and leaving the anterior retractor in place to lift the bladder superiorly are essential steps to protect the ureters. The cervix can then be split in the midline (12 to 6 o’clock) to easily identify the peritoneal reflections.
Other tips
Sidewall retractors are rarely needed. They significantly impair placement of clamps and sutures by creating a long, narrow, parallel passageway. An alternative trick is to use the suction tip to retract the vaginal sidewall as the surgeon is working.
A disposable, fiberoptic, lighted suction irrigator is another option. The light can be directed precisely where it is needed, and the irrigation helps keep the field clean and tidy, simplifying identification of anatomy.
Skip the sutures whenever possible. Because it is difficult to place sutures with precision in a tight, poorly illuminated space, I use a vessel sealer for all pedicles above the uterosacral ligaments. Some of these instruments were designed specifically for vaginal hysterectomy in the same shape and size as Heaney clamps. They are remarkably efficient and permit the completion of a vaginal procedure when suture placement is difficult.
Use a Heaney needleholder, with the suture loaded precisely in the center of the needle curve, along with the lighted suction irrigator to retract redundant tissue away from the track of the needle, to facilitate suturing high in the pelvis.
VAGINAL HYSTERECTOMY CHALLENGE 2: Nulliparity
Many of us are reluctant to attempt vaginal hysterectomy in a woman who has never had children. Although this situation can be challenging at times, in my experience, the access issues tend to be more difficult in obese, multiparous women.
The same tricks and techniques addressed above will permit the vaginal approach in almost all nulliparous women.
VAGINAL HYSTERECTOMY CHALLENGE 3: Previous cesarean section
A prior cesarean delivery is sometimes considered an indication for laparoscopic hysterectomy. There are 2 concerns here: The patient may have a small pelvis, and there may be significant scar tissue and difficulty gaining access to the anterior cul-de-sac, as a result of repeated dissection between the lower uterine segment and the bladder. Several tricks may be useful:
Examine the patient under anesthesia to ensure that the fundus is not stuck to the anterior abdominal wall. This can occur if the peritoneum was not closed during the last cesarean section.
Empty the bladder before beginning the hysterectomy, and inject indigo carmine dye intravenously with the induction of anesthesia.
Use careful sharp dissection between the bladder and lower uterine segment using fine Metzenbaum scissors, with the tips pointed toward the uterus. Dissect only as far as you can easily see.
Secure the uterosacral, cardinal, and broad ligaments, if necessary, before pursuing entry into the anterior cul-de-sac. It is not essential to gain anterior access before taking these pedicles. The additional mobility and descensus enable safe sharp dissection.
If pedicles have been secured up to the fundus, and the anterior cul-de-sac remains difficult to assess, flip the fundus through the posterior cul-de-sac and reach your finger or an instrument around the top of the fundus to identify the peritoneum anteriorly, then incise it under direct vision.
VAGINAL HYSTERECTOMY CHALLENGE 4: Previous abdominal/pelvic surgery
This history is another often-cited rationale for avoiding the vaginal approach. In reality, the adhesions created from prior surgery tend to arise between the anterior abdominal wall and the omentum or small bowel. This situation makes laparoscopic or open abdominal entry riskier than vaginal peritoneal access through the cul-de-sac.
Two possible exceptions: a patient who has had surgery for deeply infiltrating endometriosis in the cul-de-sac or a woman who has undergone myomectomies with posterior incisions. These patients may have dense scarring in the cul-de-sac, which would preclude a vaginal approach. Whatever the surgical route, appropriate bowel preparation is necessary to permit simple closure of any intestinal injury at the time of hysterectomy. Why not begin vaginally if the exam under anesthesia demonstrates an accessible and reasonably free cul-de-sac?
VAGINAL HYSTERECTOMY CHALLENGE 5: Large myomatous uterus
A uterus of any size can be removed vaginally as long as there is mobility with access to the uterine arteries. The one exception: a patient with a small, normal uterus and a massive pedunculated myoma arising from the top of the fundus. If the fibroid cannot be pulled into the true pelvis for morcellation, it cannot be removed transvaginally. Fortunately, this situation is quite rare.
Keep the uterine serosa intact to maintain orientation.
Split the uterus from 12 to 6 o’clock (bivalving). Protect the bladder with a Deaver retractor anteriorly, and protect the posterior vaginal epithelium with the weighted speculum posteriorly.
Remove chunks of tissue from the inside of the specimen. Orient your scalpel blade so that you are always cutting toward the center of the specimen. That way, if the blade slips, you will not accidentally injure tissue on the pelvic sidewall.
Use Lahey thyroid clamps to place the tissue you plan to remove under tension. Finding the capsule of each myoma and gently separating it from the surrounding myometrium facilitates delivery of larger fibroids into the endometrial cavity. Some myomas require morcellation themselves for removal.
Replace the scalpel blade periodically to keep it sharp. Calcified fibroids can dull the blade rapidly.
Work systematically to remove as much central tissue as possible. Try to keep a clean, sharp margin of tissue around the edges for easy grasping. Torn, irregular tissue is very difficult to grab and may cause significant frustration.
If access becomes limited, try clamping additional pedicles on each side of the specimen. A tiny amount of additional descensus can make a huge difference.
Do not administer GnRH agonists prior to surgery. The uterus may shrink, but the myomas tend to become quite soft and difficult to remove. If the patient is seriously anemic, give norethindrone acetate, 5 to 20 mg daily, to stop bleeding and allow the patient’s red blood cell volume to improve before elective surgery.
Use a vessel-sealing instrument to control the pedicles. This strategy produces optimal hemostasis to permit a dry field during morcellation. Moreover, the seals do not get disrupted when the large uterus is pulled past them. Placing suture around pedicles when there is a large, bulky uterus in the pelvis is challenging at best, and it is frustrating to see significant bleeding after removal of the specimen. This problem does not seem to occur with the sealing devices.
Know when to quit! We should not promise any patient a minimally invasive operation. If there is uncontrolled bleeding or no progress after 5 to 10 minutes, convert to a laparoscopic or abdominal approach.
I schedule cases I know will be challenging as “possible” laparoscopic or open hysterectomy. This alerts the OR staff to have additional equipment ready and nearby should we need it. It is not a surgical failure or complication to convert a minimally invasive hysterectomy to a more invasive technique when appropriate. Better to have tried and failed than never to have tried at all!
VAGINAL HYSTERECTOMY CHALLENGE 6: Avoiding complications
The most common complications of vaginal hysterectomy are bleeding, infection, and injury to the bladder. Ureteral injury is less common at vaginal hysterectomy than with the abdominal or laparoscopic approaches. Thus, I do not think routine cystoscopy is essential after uncomplicated vaginal hysterectomy, although I recommend intravenous administration of indigo carmine dye at the beginning of the procedure to enable rapid recognition of even a small bladder laceration. Sharp, careful dissection of the bladder off the lower uterine segment and the avoidance of finger dissection (especially with a gauze sponge) keep these injuries to a minimum.
Minimize bleeding by using newer vessel-sealing technologies rather than suture for most of the pedicles. I attach the uterosacral–cardinal ligament pedicles to the vaginal cuff at closure with suture. I suture the first pedicle once I have entered the posterior cul-de-sac and hold that suture to stay oriented.
Pay attention to patient positioning. Careful positioning will help you avoid neurological injuries. Avoid hyperflexion at the hips, which stretches the femoral nerve. Large nerves have comparatively little blood supply, so stretching them for prolonged periods can cause hypoxic injury. Although such injuries are almost always rapidly reversible, they are disconcerting for both the patient and her surgeon.
When operating on a very thin woman with a bony sacrum, I like to place egg-crate foam beneath the buttocks to provide some cushioning. I am also very careful with these women to keep their legs in a neutral position, and I watch my surgical assistants to be sure they are not leaning on the patient during the procedure.
Prophylactic antibiotics are a must to avoid postoperative vaginal cuff infections and pelvic abscess. Smokers and women with preexisting bacterial vaginosis are at highest risk for infection. I ask women to discontinue smoking at least 2 weeks prior to surgery and inform all smokers that their risk of infection is heightened. I treat anaerobic overgrowth in the vagina prior to surgery to help prevent infections in women with bacterial vaginosis.
The timing of prophylactic antibiotics is important. Intravenous first-generation cephalosporins must be administered within 60 minutes of the initial incision, but it is important to give them early enough for them to adequately disseminate to tissue before the colpotomy incision.
DVT prophylaxis is especially important for women with large uteri. Routine use of sequential compression stockings is both cost-effective and equivalent to the prophylactic use of subcutaneous heparin, so I use them for all patients undergoing vaginal hysterectomy. Early ambulation (usually within 2 hours of surgery) is also helpful in avoiding thromboses.
90% of hysterectomies can be performed vaginally
Using the techniques described in this article, I have been able to perform over 90% of the hysterectomies in my practice vaginally. More than 50% of my patients are either morbidly obese, nulliparous, or have had previous abdominal surgery of some type.
The instruments I find most useful are the lighted suction irrigator and the vessel-sealing Heaney-type clamp.
Establishing a routine and approaching technically challenging cases with a systematic and standardized set of techniques make the vaginal route possible for the vast majority of patients with benign disease.
Dr. Levy has served as a consultant to ValleyLab.
Newer codes for vaginal hysterectomy capture the work of removing larger uteri without laparoscopy
True or false: When it comes to hysterectomy, surgeons tend to use the route that is safest, least invasive, and most economical.
Sadly, the statement is false. Although vaginal hysterectomy tops all 3 categories, it is the least utilized of surgical routes. The number of vaginal hysterectomies may have increased slightly over the past decade, likely due to the incorporation of laparoscopically assisted vaginal hysterectomy into the mainstream and increased practice with the vaginal component, but fewer than 30% of hysterectomies are performed vaginally.
This article addresses 6 common challenges at vaginal hysterectomy and offers strategies to overcome them.
Laparoscopic strategies ease vaginal hysterectomy, too
Laparoscopic hysterectomy became widely accepted when surgical instruments were developed to overcome the technical challenges inherent in operating with limited access. By incorporating some of the techniques we routinely use for laparoscopic surgery, we can overcome many of the challenges faced during difficult vaginal surgery.
VAGINAL HYSTERECTOMY CHALLENGE 1: Obesity
Unfortunately, our population is increasingly rotund. This is not only a significant risk factor for the patient’s health in general, but it poses some unique challenges for surgeons. I must say that, as tough as it may be to complete a hysterectomy vaginally in a morbidly obese woman, I would much rather approach her pelvic organs through the cul-de-sac, which contains no fat cells, than through the abdominal wall—either laparoscopically or abdominally! The trick is gaining access to the posterior cul-de-sac.
How to enter the cul-de-sac
It seems to be a perverse rule of nature, but a tight upper vaginal ring seems almost universal in obese women. Added to the redundant sidewalls and the large buttocks, this tightness makes entry into the anterior or posterior cul-de-sac problematic. Several tricks make peritoneal access possible:
Position the patient to increase access, with the buttocks well over the edge of the operating table. This brings the operative field a bit closer to the surgeon, and permits the use of long-handled retractors posteriorly.
Use candy-cane stirrups to allow assistants better access to the operative field. Adequate assistance is essential in attempting vaginal surgery in the morbidly obese.
Avoid the Trendelenburg position. Although it might seem that this position would facilitate visualization and placement of a posterior weighted speculum, all it does is allow the patient to slide up on the table, making placement of alternate retractors difficult.
Use the right tools. If the posterior weighted speculum will not stay in place or does not afford access to the cul-de-sac due to an upper vaginal ring, use a narrow Deaver retractor posteriorly (without sidewall or anterior retraction). Use a Jacob’s tenaculum on the posterior lip of the cervix and have your assistant pull straight up on the tenaculum while using the Deaver retractor to see the area between the uterosacral ligaments.
Use the uterosacral ligaments as a guide. Another perversity in morbidly obese women: Despite multiparity, they seem to have little or no apical prolapse but lots of vaginal wall redundancy. The cervix is often elongated, but the uterosacral ligaments are sky high.
I palpate these ligaments, injecting them with a combination of vasopressin diluted 1:5 with bupivacaine and epinephrine (for enhanced hemostasis and preemptive analgesia), then use a pencil electrosurgical electrode to rapidly open the vaginal epithelium between the ligaments.
I then use a long, toothed tissue forceps to tent the peritoneum at 90 degrees to the plane of the posterior cul-de-sac and use Mayo scissors to enter the peritoneal cavity. Usually there is a spurt of fluid to mark appropriate entry into the peritoneum.
I then use the blades of my scissors to stretch the peritoneum between the ligaments and place a moistened 4×4 sponge into the incision.
At the onset of the procedure, inject indigo carmine dye intravenously so that any injury to the bladder will be immediately recognized. I have the circulating nurse empty the bladder while she is prepping the patient, but do not leave an indwelling catheter in place during the operation. I find it cumbersome to work around the catheter.
Problematic entries
When entry into the posterior cul-de-sac is difficult, I stop dissection, place a 4×4 sponge into the incision to reduce bleeding from the vagina, and proceed to attempt anterior entry.
I place the Deaver retractor into the anterior space and move the tenaculum to the anterior lip of the cervix. This gives maximal space for downward traction on the cervix while anterior entry is attempted.
Once again, I inject the tissue with the bupivacaine solution before incising the vaginal epithelium at the level of the internal os. I use sharp dissection only when creating a plane between the lower uterine segment and the bladder.
Ensuring room to move and good visualization
If neither the anterior nor the posterior cul-de-sac can be accessed, it may be time to rethink the vaginal approach—but there is no harm in taking the uterosacral and cardinal ligaments extraperitoneally in an effort to gain some mobility. Hugging the uterus and leaving the anterior retractor in place to lift the bladder superiorly are essential steps to protect the ureters. The cervix can then be split in the midline (12 to 6 o’clock) to easily identify the peritoneal reflections.
Other tips
Sidewall retractors are rarely needed. They significantly impair placement of clamps and sutures by creating a long, narrow, parallel passageway. An alternative trick is to use the suction tip to retract the vaginal sidewall as the surgeon is working.
A disposable, fiberoptic, lighted suction irrigator is another option. The light can be directed precisely where it is needed, and the irrigation helps keep the field clean and tidy, simplifying identification of anatomy.
Skip the sutures whenever possible. Because it is difficult to place sutures with precision in a tight, poorly illuminated space, I use a vessel sealer for all pedicles above the uterosacral ligaments. Some of these instruments were designed specifically for vaginal hysterectomy in the same shape and size as Heaney clamps. They are remarkably efficient and permit the completion of a vaginal procedure when suture placement is difficult.
Use a Heaney needleholder, with the suture loaded precisely in the center of the needle curve, along with the lighted suction irrigator to retract redundant tissue away from the track of the needle, to facilitate suturing high in the pelvis.
VAGINAL HYSTERECTOMY CHALLENGE 2: Nulliparity
Many of us are reluctant to attempt vaginal hysterectomy in a woman who has never had children. Although this situation can be challenging at times, in my experience, the access issues tend to be more difficult in obese, multiparous women.
The same tricks and techniques addressed above will permit the vaginal approach in almost all nulliparous women.
VAGINAL HYSTERECTOMY CHALLENGE 3: Previous cesarean section
A prior cesarean delivery is sometimes considered an indication for laparoscopic hysterectomy. There are 2 concerns here: The patient may have a small pelvis, and there may be significant scar tissue and difficulty gaining access to the anterior cul-de-sac, as a result of repeated dissection between the lower uterine segment and the bladder. Several tricks may be useful:
Examine the patient under anesthesia to ensure that the fundus is not stuck to the anterior abdominal wall. This can occur if the peritoneum was not closed during the last cesarean section.
Empty the bladder before beginning the hysterectomy, and inject indigo carmine dye intravenously with the induction of anesthesia.
Use careful sharp dissection between the bladder and lower uterine segment using fine Metzenbaum scissors, with the tips pointed toward the uterus. Dissect only as far as you can easily see.
Secure the uterosacral, cardinal, and broad ligaments, if necessary, before pursuing entry into the anterior cul-de-sac. It is not essential to gain anterior access before taking these pedicles. The additional mobility and descensus enable safe sharp dissection.
If pedicles have been secured up to the fundus, and the anterior cul-de-sac remains difficult to assess, flip the fundus through the posterior cul-de-sac and reach your finger or an instrument around the top of the fundus to identify the peritoneum anteriorly, then incise it under direct vision.
VAGINAL HYSTERECTOMY CHALLENGE 4: Previous abdominal/pelvic surgery
This history is another often-cited rationale for avoiding the vaginal approach. In reality, the adhesions created from prior surgery tend to arise between the anterior abdominal wall and the omentum or small bowel. This situation makes laparoscopic or open abdominal entry riskier than vaginal peritoneal access through the cul-de-sac.
Two possible exceptions: a patient who has had surgery for deeply infiltrating endometriosis in the cul-de-sac or a woman who has undergone myomectomies with posterior incisions. These patients may have dense scarring in the cul-de-sac, which would preclude a vaginal approach. Whatever the surgical route, appropriate bowel preparation is necessary to permit simple closure of any intestinal injury at the time of hysterectomy. Why not begin vaginally if the exam under anesthesia demonstrates an accessible and reasonably free cul-de-sac?
VAGINAL HYSTERECTOMY CHALLENGE 5: Large myomatous uterus
A uterus of any size can be removed vaginally as long as there is mobility with access to the uterine arteries. The one exception: a patient with a small, normal uterus and a massive pedunculated myoma arising from the top of the fundus. If the fibroid cannot be pulled into the true pelvis for morcellation, it cannot be removed transvaginally. Fortunately, this situation is quite rare.
Keep the uterine serosa intact to maintain orientation.
Split the uterus from 12 to 6 o’clock (bivalving). Protect the bladder with a Deaver retractor anteriorly, and protect the posterior vaginal epithelium with the weighted speculum posteriorly.
Remove chunks of tissue from the inside of the specimen. Orient your scalpel blade so that you are always cutting toward the center of the specimen. That way, if the blade slips, you will not accidentally injure tissue on the pelvic sidewall.
Use Lahey thyroid clamps to place the tissue you plan to remove under tension. Finding the capsule of each myoma and gently separating it from the surrounding myometrium facilitates delivery of larger fibroids into the endometrial cavity. Some myomas require morcellation themselves for removal.
Replace the scalpel blade periodically to keep it sharp. Calcified fibroids can dull the blade rapidly.
Work systematically to remove as much central tissue as possible. Try to keep a clean, sharp margin of tissue around the edges for easy grasping. Torn, irregular tissue is very difficult to grab and may cause significant frustration.
If access becomes limited, try clamping additional pedicles on each side of the specimen. A tiny amount of additional descensus can make a huge difference.
Do not administer GnRH agonists prior to surgery. The uterus may shrink, but the myomas tend to become quite soft and difficult to remove. If the patient is seriously anemic, give norethindrone acetate, 5 to 20 mg daily, to stop bleeding and allow the patient’s red blood cell volume to improve before elective surgery.
Use a vessel-sealing instrument to control the pedicles. This strategy produces optimal hemostasis to permit a dry field during morcellation. Moreover, the seals do not get disrupted when the large uterus is pulled past them. Placing suture around pedicles when there is a large, bulky uterus in the pelvis is challenging at best, and it is frustrating to see significant bleeding after removal of the specimen. This problem does not seem to occur with the sealing devices.
Know when to quit! We should not promise any patient a minimally invasive operation. If there is uncontrolled bleeding or no progress after 5 to 10 minutes, convert to a laparoscopic or abdominal approach.
I schedule cases I know will be challenging as “possible” laparoscopic or open hysterectomy. This alerts the OR staff to have additional equipment ready and nearby should we need it. It is not a surgical failure or complication to convert a minimally invasive hysterectomy to a more invasive technique when appropriate. Better to have tried and failed than never to have tried at all!
VAGINAL HYSTERECTOMY CHALLENGE 6: Avoiding complications
The most common complications of vaginal hysterectomy are bleeding, infection, and injury to the bladder. Ureteral injury is less common at vaginal hysterectomy than with the abdominal or laparoscopic approaches. Thus, I do not think routine cystoscopy is essential after uncomplicated vaginal hysterectomy, although I recommend intravenous administration of indigo carmine dye at the beginning of the procedure to enable rapid recognition of even a small bladder laceration. Sharp, careful dissection of the bladder off the lower uterine segment and the avoidance of finger dissection (especially with a gauze sponge) keep these injuries to a minimum.
Minimize bleeding by using newer vessel-sealing technologies rather than suture for most of the pedicles. I attach the uterosacral–cardinal ligament pedicles to the vaginal cuff at closure with suture. I suture the first pedicle once I have entered the posterior cul-de-sac and hold that suture to stay oriented.
Pay attention to patient positioning. Careful positioning will help you avoid neurological injuries. Avoid hyperflexion at the hips, which stretches the femoral nerve. Large nerves have comparatively little blood supply, so stretching them for prolonged periods can cause hypoxic injury. Although such injuries are almost always rapidly reversible, they are disconcerting for both the patient and her surgeon.
When operating on a very thin woman with a bony sacrum, I like to place egg-crate foam beneath the buttocks to provide some cushioning. I am also very careful with these women to keep their legs in a neutral position, and I watch my surgical assistants to be sure they are not leaning on the patient during the procedure.
Prophylactic antibiotics are a must to avoid postoperative vaginal cuff infections and pelvic abscess. Smokers and women with preexisting bacterial vaginosis are at highest risk for infection. I ask women to discontinue smoking at least 2 weeks prior to surgery and inform all smokers that their risk of infection is heightened. I treat anaerobic overgrowth in the vagina prior to surgery to help prevent infections in women with bacterial vaginosis.
The timing of prophylactic antibiotics is important. Intravenous first-generation cephalosporins must be administered within 60 minutes of the initial incision, but it is important to give them early enough for them to adequately disseminate to tissue before the colpotomy incision.
DVT prophylaxis is especially important for women with large uteri. Routine use of sequential compression stockings is both cost-effective and equivalent to the prophylactic use of subcutaneous heparin, so I use them for all patients undergoing vaginal hysterectomy. Early ambulation (usually within 2 hours of surgery) is also helpful in avoiding thromboses.
90% of hysterectomies can be performed vaginally
Using the techniques described in this article, I have been able to perform over 90% of the hysterectomies in my practice vaginally. More than 50% of my patients are either morbidly obese, nulliparous, or have had previous abdominal surgery of some type.
The instruments I find most useful are the lighted suction irrigator and the vessel-sealing Heaney-type clamp.
Establishing a routine and approaching technically challenging cases with a systematic and standardized set of techniques make the vaginal route possible for the vast majority of patients with benign disease.
Dr. Levy has served as a consultant to ValleyLab.
SUGGESTED READING
1. Abostini A, Vejux N, Colette E, et al. Risk of bladder injury during vaginal hysterectomy in women with a previous cesarean section. J Reprod Med. 2005;50:940-942.
2. Clave H, Barr H, Niccolai P. Painless vaginal hysterectomy with thermal hemostasis (results of a series of 152 cases). Gynecol Surg. 2005;2:101-105.
3. Isik-Akbay EF, Harmanli OH, Panganamamula UR, et al. Hysterectomy in obese women: a comparison of abdominal and vaginal routes. Obstet Gynecol. 2004;104:710-714.
4. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
5. O’Neal MG, Beste T, Shackelford DP. Utility of preemptive local analgesia in vaginal hysterectomy. Am J Obstet Gynecol. 2003;189:1539-1542.
6. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005;(1):CD003677.-
SUGGESTED READING
1. Abostini A, Vejux N, Colette E, et al. Risk of bladder injury during vaginal hysterectomy in women with a previous cesarean section. J Reprod Med. 2005;50:940-942.
2. Clave H, Barr H, Niccolai P. Painless vaginal hysterectomy with thermal hemostasis (results of a series of 152 cases). Gynecol Surg. 2005;2:101-105.
3. Isik-Akbay EF, Harmanli OH, Panganamamula UR, et al. Hysterectomy in obese women: a comparison of abdominal and vaginal routes. Obstet Gynecol. 2004;104:710-714.
4. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
5. O’Neal MG, Beste T, Shackelford DP. Utility of preemptive local analgesia in vaginal hysterectomy. Am J Obstet Gynecol. 2003;189:1539-1542.
6. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005;(1):CD003677.-
The Retroperitoneal Space: Keeping vital structures out of harm’s way
The accomplished gynecologic surgeon must know the anatomy of the retroperitoneal space in order to avoid damage to normal structures, as well as remove pathology. Many disease processes involve the pelvic peritoneum, uterosacral ligaments, rectosigmoid or ovarian pedicles, and require the surgeon to enter the retroperitoneal space to identify the ureters and blood vessels and keep them out of harm’s way. The challenges are complex:
- Badly distorted anatomy and the anterior and posterior cul-de-sac necessitate mobilization of the rectosigmoid and bladder.
- Intraligamentous fibroids require knowledge of the blood supply in the retroperitoneal space. Malignant disorders mandate that the lymph nodes be dissected to determine extent of disease and as part of treatment.
Is training adequate?
Every training program should teach the surgical anatomy of the retroperitoneal space, since every surgeon needs to be comfortable exposing the anatomy, both to prevent injury and to accomplish the needed surgery. Videotapes and cadaver courses can prepare the resident for the operating room.
The “landmark” umbilical ligament
The umbilical ligament was the umbilical artery in fetal life and courses along the edge of the bladder to the anterior abdominal wall up to the umbilicus. It is a useful guide into the perivesicle space. Lateral to it are the iliac vessels, and medial is the bladder. It is also a good marker for finding the right spot to open the round ligament.
FIGURE 1 Opening the round ligament over the umbilical ligament
The round ligament is the key to exposing the retroperitoneal space. It should be open at the pelvic sidewall, just medial to the external iliac vessels. The umbilical ligament can be used as a landmark.
FIGURE 2 Opening lateral to the ovarian vessels
The divided round ligament is retracted ventrally and medially to place the ovarian vessels under traction. The peritoneum lateral to the ovarian vessels is divided up to the pelvic brim.
FIGURE 3 Right pelvic sidewall anatomy
Medial traction of the peritoneum around the ovarian vessels at the pelvic brim will expose the ureter coming over the iliac vessels at their bifurcation into external and internal branches.
FIGURE 4 Relationship of ureter to the umbilical ligament
The ureter comes off medial with the fold of peritoneum. Once the space is developed, the operator’s finger or the laparoscopic probe can be introduced along the medial side of the internal iliac artery and ventral to the curve of the sacrum. This will open the pararectal space. The ureter and the anterior branch of the internal iliac artery are nearly parallel as they course through the pelvis.
FIGURE 5 Hypogastric nerve
The anterior branch of the internal iliac artery gives off the uterine artery and the middle and superior vesicle arteries before continuing on as the umbilical ligament.
Endometriosis may imperil the ureter
Endometriomas and peritoneal implants are among the most common reasons for accessing the retroperitoneal space. Frequently the peritoneum between the ovarian vessels and the uterosacral ligaments (ovarian fossa) is thickened and retracted within the endometriosis implants. This alters the pelvic anatomy and puts the ureter at risk for injury.
Definitive surgical treatment for endometriosis includes removal of this diseased peritoneum. The ureter is best identified using the technique described, and then dissected off the peritoneum down to the uterine artery.
FIGURE 6 Finding the ureter lateral to an endometrioma
FIGURE 7 Relationship of ureter to the umbilical ligament
FIGURE 8 Dissecting the ureter out of the uterosacral ligament
The ureter may be placed on a Penrose drain to better isolate it and keep it under direct visualization. The cul-de-sac of Douglas is another site of endometrial implants. The fundus of the uterus is often adhesed to the rectosigmoid reflection and even the sigmoid colon. Nodules of endometriosis may infiltrate the uterosacral ligaments and extend into the rectovaginal space. To manage these implants, isolate the ureter to the point where it passes under the uterine artery.
FIGURE 9 Divide uterine artery lateral to endometrioma
Here, the uterine artery is taken lateral to the mass, enabling the surgeon to remove all of the uterosacral implants. The bladder flap will be developed and the bladder advanced caudad. By dissecting medial to the ureter as it courses under the uterine artery, it will be retracted laterally and provide the space necessary to resect the uterosacral implants.
FIGURE 10 Rectosigmoid reflection with endometrioma nodule
FIGURE 11 Postoperative appearance of endometriosis resection
As dissection proceeds medially, use the perirectal fatty plane to remove implants between the uterosacral area and rectum. In the midline, remove implants below the rectosigmoid peritoneal reflexion, taking care not to injure the rectum.
Implants above the peritoneal reflexion are often attached to the sigmoid tinea coli and cannot be removed without taking a portion of the bowel. Thus, patients with extensive endometriosis should have a bowel prep with two 10-ounce bottles of magnesium citrate the day before surgery.
If bowel resection is necessary, consult a general surgeon or gynecologic oncologist. A history of painful defecation or a finding of nodules in the cul-de-sac with rectal dimpling warrants preoperative consultation with a specialist skilled at bowel resection.
Ureteral injuries occur in 0.5% to 2.5% of women undergoing gynecologic surgery.1 The most common predisposing condition is previous pelvic surgery.2,3 The usual sites of injury are at the pelvic rim close to the ovarian vessels, at the level of the uterine artery, and lateral to the vaginal cuff. Neither preoperative excretory urograms nor placement of ureteral catheters preoperatively have been found to be effective prevention measures.2,4-6 Using the steps described above, the ureter can be identified and mobilized.
Blood supply to the ureter comes from the plexus of vessels that form a network along the length of the ureter. This plexus is fed by arteries from the renal pelvis, common iliac, internal iliac, uterine artery, and the base of the bladder. Complete mobilization of the ureter away from its peritoneal attachments and these lateral blood supply sources can be accomplished as long as the vascular plexus is not disrupted by cautery, crushing, or tearing. A clean transection can be re-anastomosed or reimplanted with the expectation of normal healing. Innervation of the ureter is from the inferior mesenteric plexus superiorly and the inferior hypogastric plexus in the pelvis. Ureteral peristalsis will continue, even if the ureter is completely divided or ligated.
Protecting pelvic blood vessels
The majority of gynecologic operations involve the ovarian vessels and the uterine vessels. The operator rarely needs to explore the lateral pelvis to identify the rest of the vessels. When faced with a large endometrioma or cancer, knowledge of the anatomy of the lateral blood vessels is vital.
Since the pathology is usually deeper in the pelvis, it is wise to identify the anatomy of the pelvis starting at the pelvic brim, where the common iliac bifurcates into the external and internal iliacs. There is a safe dissection plane medial to the internal iliac artery all the way to the uterine artery. This exposes the pararectal space, which can be opened without risk of major bleeding (FIGURE 5).
The obliterated umbilical vessel is the other friendly marker just distal to the uterine artery. It can be placed on traction and the uterine artery isolated. The inferior and superior vesical arteries are generally not dissected, as they are adjacent to the bladder and the surgery takes place medial to them in the prevesical fascial space.
Hypogastric artery ligations are rarely performed today, as interventional radiology is the standard of care for patients with postoperative and postpartum bleeding. When it becomes necessary, the hypogastric artery can be isolated and tied using the right-angle clamp to pass the tie. The superior gluteal artery branches so close to the bifurcation of the common iliac artery that it is not visualized. The inferior gluteal artery is the largest distal branch, which might be visualized during the ligation. It is not necessary to identify it.
FIGURE 12 Right genitofemoral nerve
The genitofemoral nerve runs along the medial aspect of the body of the psoas muscle. It is sometimes injured by the self-retaining retractors placed at the time of laparotomy. This leads to some numbness and burning of the skin of the anterior thigh.
FIGURE 13 Right pelvic sidewall anatomy
The obturator nerve is in the obturator space and typically far lateral to the usual dissection. Metastatic cancer to the obturator lymph nodes may entrap it, or it may be injured during a node dissection, causing loss of internal rotation of the anterior thigh.
The sciatic nerve is seen only during exenterative surgery. Pressure on the lateral pelvis by advanced pelvic tumors can lead to sciatic pain and motor weakness—even loss of motor function to the lower leg, which commonly leads to foot drop.
FIGURE 14 Superior hypogastric nerve bundle
The hypogastric plexus of nerves is sometimes damaged during surgery for endometriosis or for malignancy. The superior hypergastric plexus can be identified between the 2 common iliac arteries at the sacral promontory. The left common iliac vein runs underneath it.
FIGURE 15 Hypogastric nerve descending into the right pelvis
The right and left hypogastric nerves leave the hypogastric plexus and descend into the pelvis parallel to the ureter and 2 cm medial. It passes dorsal to the ureter as it goes through the cardinal ligament (FIGURE 5).
This plexus then supplies autonomic innervation of the bladder, rectum, uterus, and ureter. Complete disruption of the hypogastric nerve will lead to a hypertonic, noncontractile bladder and the necessity for self-catheterization to eliminate urine. Preservation of this nerve during radical hysterectomy or endometriosis resection is a high priority.
Laparoscopic uterosacral nerve ablation procedures divide the uterosacral ligament medial and caudad to the ureter and do not disrupt the main hypogastric nerve. Only the medial branches to the uterus are affected. Successful uterosacral nerve ablation has been reported in approximately 44% of women who have dysmenorrhea without visible endometriosis and approximately 62% of women who have visible endometriosis.7,8 The efficacy of this procedure is controversial, however. Removal of the superior hypogastric plexus (presacral neurectomy) has not proved to be more effective in controlling pelvic pain than conservative surgery that only destroys endometrial implants. Presacral neurectomy is no longer advised.9
1. American College of Obstetricians and Gynecologists. Educational Bulletin #238: Lower Urinary Tract Operative Injuries. Washington, DC: ACOG; 1985:1.
2. Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
3. Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155:878-881.
4. Symmonds RE. Ureteral injuries associated with gynecologic surgery: prevention and management. Clin Obstet Gynecol. 19676;19:623-644.
5. Higgins CC. Ureteral injuries during surgery: a review of 87 cases. JAMA 1967;199:82-88.
6. Fry DE, Milholen L, Harbrecht PJ. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118:454-457.
7. Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med. 1987;32:37-41.
8. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696.-
9. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
Dr. Hatch receives research/grant support from Merck; is a consultant to Ethicon, Merck, and Quest Laboratory; and is a speaker for Cytyc, Digene, Ethicon, and Merck.
The accomplished gynecologic surgeon must know the anatomy of the retroperitoneal space in order to avoid damage to normal structures, as well as remove pathology. Many disease processes involve the pelvic peritoneum, uterosacral ligaments, rectosigmoid or ovarian pedicles, and require the surgeon to enter the retroperitoneal space to identify the ureters and blood vessels and keep them out of harm’s way. The challenges are complex:
- Badly distorted anatomy and the anterior and posterior cul-de-sac necessitate mobilization of the rectosigmoid and bladder.
- Intraligamentous fibroids require knowledge of the blood supply in the retroperitoneal space. Malignant disorders mandate that the lymph nodes be dissected to determine extent of disease and as part of treatment.
Is training adequate?
Every training program should teach the surgical anatomy of the retroperitoneal space, since every surgeon needs to be comfortable exposing the anatomy, both to prevent injury and to accomplish the needed surgery. Videotapes and cadaver courses can prepare the resident for the operating room.
The “landmark” umbilical ligament
The umbilical ligament was the umbilical artery in fetal life and courses along the edge of the bladder to the anterior abdominal wall up to the umbilicus. It is a useful guide into the perivesicle space. Lateral to it are the iliac vessels, and medial is the bladder. It is also a good marker for finding the right spot to open the round ligament.
FIGURE 1 Opening the round ligament over the umbilical ligament
The round ligament is the key to exposing the retroperitoneal space. It should be open at the pelvic sidewall, just medial to the external iliac vessels. The umbilical ligament can be used as a landmark.
FIGURE 2 Opening lateral to the ovarian vessels
The divided round ligament is retracted ventrally and medially to place the ovarian vessels under traction. The peritoneum lateral to the ovarian vessels is divided up to the pelvic brim.
FIGURE 3 Right pelvic sidewall anatomy
Medial traction of the peritoneum around the ovarian vessels at the pelvic brim will expose the ureter coming over the iliac vessels at their bifurcation into external and internal branches.
FIGURE 4 Relationship of ureter to the umbilical ligament
The ureter comes off medial with the fold of peritoneum. Once the space is developed, the operator’s finger or the laparoscopic probe can be introduced along the medial side of the internal iliac artery and ventral to the curve of the sacrum. This will open the pararectal space. The ureter and the anterior branch of the internal iliac artery are nearly parallel as they course through the pelvis.
FIGURE 5 Hypogastric nerve
The anterior branch of the internal iliac artery gives off the uterine artery and the middle and superior vesicle arteries before continuing on as the umbilical ligament.
Endometriosis may imperil the ureter
Endometriomas and peritoneal implants are among the most common reasons for accessing the retroperitoneal space. Frequently the peritoneum between the ovarian vessels and the uterosacral ligaments (ovarian fossa) is thickened and retracted within the endometriosis implants. This alters the pelvic anatomy and puts the ureter at risk for injury.
Definitive surgical treatment for endometriosis includes removal of this diseased peritoneum. The ureter is best identified using the technique described, and then dissected off the peritoneum down to the uterine artery.
FIGURE 6 Finding the ureter lateral to an endometrioma
FIGURE 7 Relationship of ureter to the umbilical ligament
FIGURE 8 Dissecting the ureter out of the uterosacral ligament
The ureter may be placed on a Penrose drain to better isolate it and keep it under direct visualization. The cul-de-sac of Douglas is another site of endometrial implants. The fundus of the uterus is often adhesed to the rectosigmoid reflection and even the sigmoid colon. Nodules of endometriosis may infiltrate the uterosacral ligaments and extend into the rectovaginal space. To manage these implants, isolate the ureter to the point where it passes under the uterine artery.
FIGURE 9 Divide uterine artery lateral to endometrioma
Here, the uterine artery is taken lateral to the mass, enabling the surgeon to remove all of the uterosacral implants. The bladder flap will be developed and the bladder advanced caudad. By dissecting medial to the ureter as it courses under the uterine artery, it will be retracted laterally and provide the space necessary to resect the uterosacral implants.
FIGURE 10 Rectosigmoid reflection with endometrioma nodule
FIGURE 11 Postoperative appearance of endometriosis resection
As dissection proceeds medially, use the perirectal fatty plane to remove implants between the uterosacral area and rectum. In the midline, remove implants below the rectosigmoid peritoneal reflexion, taking care not to injure the rectum.
Implants above the peritoneal reflexion are often attached to the sigmoid tinea coli and cannot be removed without taking a portion of the bowel. Thus, patients with extensive endometriosis should have a bowel prep with two 10-ounce bottles of magnesium citrate the day before surgery.
If bowel resection is necessary, consult a general surgeon or gynecologic oncologist. A history of painful defecation or a finding of nodules in the cul-de-sac with rectal dimpling warrants preoperative consultation with a specialist skilled at bowel resection.
Ureteral injuries occur in 0.5% to 2.5% of women undergoing gynecologic surgery.1 The most common predisposing condition is previous pelvic surgery.2,3 The usual sites of injury are at the pelvic rim close to the ovarian vessels, at the level of the uterine artery, and lateral to the vaginal cuff. Neither preoperative excretory urograms nor placement of ureteral catheters preoperatively have been found to be effective prevention measures.2,4-6 Using the steps described above, the ureter can be identified and mobilized.
Blood supply to the ureter comes from the plexus of vessels that form a network along the length of the ureter. This plexus is fed by arteries from the renal pelvis, common iliac, internal iliac, uterine artery, and the base of the bladder. Complete mobilization of the ureter away from its peritoneal attachments and these lateral blood supply sources can be accomplished as long as the vascular plexus is not disrupted by cautery, crushing, or tearing. A clean transection can be re-anastomosed or reimplanted with the expectation of normal healing. Innervation of the ureter is from the inferior mesenteric plexus superiorly and the inferior hypogastric plexus in the pelvis. Ureteral peristalsis will continue, even if the ureter is completely divided or ligated.
Protecting pelvic blood vessels
The majority of gynecologic operations involve the ovarian vessels and the uterine vessels. The operator rarely needs to explore the lateral pelvis to identify the rest of the vessels. When faced with a large endometrioma or cancer, knowledge of the anatomy of the lateral blood vessels is vital.
Since the pathology is usually deeper in the pelvis, it is wise to identify the anatomy of the pelvis starting at the pelvic brim, where the common iliac bifurcates into the external and internal iliacs. There is a safe dissection plane medial to the internal iliac artery all the way to the uterine artery. This exposes the pararectal space, which can be opened without risk of major bleeding (FIGURE 5).
The obliterated umbilical vessel is the other friendly marker just distal to the uterine artery. It can be placed on traction and the uterine artery isolated. The inferior and superior vesical arteries are generally not dissected, as they are adjacent to the bladder and the surgery takes place medial to them in the prevesical fascial space.
Hypogastric artery ligations are rarely performed today, as interventional radiology is the standard of care for patients with postoperative and postpartum bleeding. When it becomes necessary, the hypogastric artery can be isolated and tied using the right-angle clamp to pass the tie. The superior gluteal artery branches so close to the bifurcation of the common iliac artery that it is not visualized. The inferior gluteal artery is the largest distal branch, which might be visualized during the ligation. It is not necessary to identify it.
FIGURE 12 Right genitofemoral nerve
The genitofemoral nerve runs along the medial aspect of the body of the psoas muscle. It is sometimes injured by the self-retaining retractors placed at the time of laparotomy. This leads to some numbness and burning of the skin of the anterior thigh.
FIGURE 13 Right pelvic sidewall anatomy
The obturator nerve is in the obturator space and typically far lateral to the usual dissection. Metastatic cancer to the obturator lymph nodes may entrap it, or it may be injured during a node dissection, causing loss of internal rotation of the anterior thigh.
The sciatic nerve is seen only during exenterative surgery. Pressure on the lateral pelvis by advanced pelvic tumors can lead to sciatic pain and motor weakness—even loss of motor function to the lower leg, which commonly leads to foot drop.
FIGURE 14 Superior hypogastric nerve bundle
The hypogastric plexus of nerves is sometimes damaged during surgery for endometriosis or for malignancy. The superior hypergastric plexus can be identified between the 2 common iliac arteries at the sacral promontory. The left common iliac vein runs underneath it.
FIGURE 15 Hypogastric nerve descending into the right pelvis
The right and left hypogastric nerves leave the hypogastric plexus and descend into the pelvis parallel to the ureter and 2 cm medial. It passes dorsal to the ureter as it goes through the cardinal ligament (FIGURE 5).
This plexus then supplies autonomic innervation of the bladder, rectum, uterus, and ureter. Complete disruption of the hypogastric nerve will lead to a hypertonic, noncontractile bladder and the necessity for self-catheterization to eliminate urine. Preservation of this nerve during radical hysterectomy or endometriosis resection is a high priority.
Laparoscopic uterosacral nerve ablation procedures divide the uterosacral ligament medial and caudad to the ureter and do not disrupt the main hypogastric nerve. Only the medial branches to the uterus are affected. Successful uterosacral nerve ablation has been reported in approximately 44% of women who have dysmenorrhea without visible endometriosis and approximately 62% of women who have visible endometriosis.7,8 The efficacy of this procedure is controversial, however. Removal of the superior hypogastric plexus (presacral neurectomy) has not proved to be more effective in controlling pelvic pain than conservative surgery that only destroys endometrial implants. Presacral neurectomy is no longer advised.9
The accomplished gynecologic surgeon must know the anatomy of the retroperitoneal space in order to avoid damage to normal structures, as well as remove pathology. Many disease processes involve the pelvic peritoneum, uterosacral ligaments, rectosigmoid or ovarian pedicles, and require the surgeon to enter the retroperitoneal space to identify the ureters and blood vessels and keep them out of harm’s way. The challenges are complex:
- Badly distorted anatomy and the anterior and posterior cul-de-sac necessitate mobilization of the rectosigmoid and bladder.
- Intraligamentous fibroids require knowledge of the blood supply in the retroperitoneal space. Malignant disorders mandate that the lymph nodes be dissected to determine extent of disease and as part of treatment.
Is training adequate?
Every training program should teach the surgical anatomy of the retroperitoneal space, since every surgeon needs to be comfortable exposing the anatomy, both to prevent injury and to accomplish the needed surgery. Videotapes and cadaver courses can prepare the resident for the operating room.
The “landmark” umbilical ligament
The umbilical ligament was the umbilical artery in fetal life and courses along the edge of the bladder to the anterior abdominal wall up to the umbilicus. It is a useful guide into the perivesicle space. Lateral to it are the iliac vessels, and medial is the bladder. It is also a good marker for finding the right spot to open the round ligament.
FIGURE 1 Opening the round ligament over the umbilical ligament
The round ligament is the key to exposing the retroperitoneal space. It should be open at the pelvic sidewall, just medial to the external iliac vessels. The umbilical ligament can be used as a landmark.
FIGURE 2 Opening lateral to the ovarian vessels
The divided round ligament is retracted ventrally and medially to place the ovarian vessels under traction. The peritoneum lateral to the ovarian vessels is divided up to the pelvic brim.
FIGURE 3 Right pelvic sidewall anatomy
Medial traction of the peritoneum around the ovarian vessels at the pelvic brim will expose the ureter coming over the iliac vessels at their bifurcation into external and internal branches.
FIGURE 4 Relationship of ureter to the umbilical ligament
The ureter comes off medial with the fold of peritoneum. Once the space is developed, the operator’s finger or the laparoscopic probe can be introduced along the medial side of the internal iliac artery and ventral to the curve of the sacrum. This will open the pararectal space. The ureter and the anterior branch of the internal iliac artery are nearly parallel as they course through the pelvis.
FIGURE 5 Hypogastric nerve
The anterior branch of the internal iliac artery gives off the uterine artery and the middle and superior vesicle arteries before continuing on as the umbilical ligament.
Endometriosis may imperil the ureter
Endometriomas and peritoneal implants are among the most common reasons for accessing the retroperitoneal space. Frequently the peritoneum between the ovarian vessels and the uterosacral ligaments (ovarian fossa) is thickened and retracted within the endometriosis implants. This alters the pelvic anatomy and puts the ureter at risk for injury.
Definitive surgical treatment for endometriosis includes removal of this diseased peritoneum. The ureter is best identified using the technique described, and then dissected off the peritoneum down to the uterine artery.
FIGURE 6 Finding the ureter lateral to an endometrioma
FIGURE 7 Relationship of ureter to the umbilical ligament
FIGURE 8 Dissecting the ureter out of the uterosacral ligament
The ureter may be placed on a Penrose drain to better isolate it and keep it under direct visualization. The cul-de-sac of Douglas is another site of endometrial implants. The fundus of the uterus is often adhesed to the rectosigmoid reflection and even the sigmoid colon. Nodules of endometriosis may infiltrate the uterosacral ligaments and extend into the rectovaginal space. To manage these implants, isolate the ureter to the point where it passes under the uterine artery.
FIGURE 9 Divide uterine artery lateral to endometrioma
Here, the uterine artery is taken lateral to the mass, enabling the surgeon to remove all of the uterosacral implants. The bladder flap will be developed and the bladder advanced caudad. By dissecting medial to the ureter as it courses under the uterine artery, it will be retracted laterally and provide the space necessary to resect the uterosacral implants.
FIGURE 10 Rectosigmoid reflection with endometrioma nodule
FIGURE 11 Postoperative appearance of endometriosis resection
As dissection proceeds medially, use the perirectal fatty plane to remove implants between the uterosacral area and rectum. In the midline, remove implants below the rectosigmoid peritoneal reflexion, taking care not to injure the rectum.
Implants above the peritoneal reflexion are often attached to the sigmoid tinea coli and cannot be removed without taking a portion of the bowel. Thus, patients with extensive endometriosis should have a bowel prep with two 10-ounce bottles of magnesium citrate the day before surgery.
If bowel resection is necessary, consult a general surgeon or gynecologic oncologist. A history of painful defecation or a finding of nodules in the cul-de-sac with rectal dimpling warrants preoperative consultation with a specialist skilled at bowel resection.
Ureteral injuries occur in 0.5% to 2.5% of women undergoing gynecologic surgery.1 The most common predisposing condition is previous pelvic surgery.2,3 The usual sites of injury are at the pelvic rim close to the ovarian vessels, at the level of the uterine artery, and lateral to the vaginal cuff. Neither preoperative excretory urograms nor placement of ureteral catheters preoperatively have been found to be effective prevention measures.2,4-6 Using the steps described above, the ureter can be identified and mobilized.
Blood supply to the ureter comes from the plexus of vessels that form a network along the length of the ureter. This plexus is fed by arteries from the renal pelvis, common iliac, internal iliac, uterine artery, and the base of the bladder. Complete mobilization of the ureter away from its peritoneal attachments and these lateral blood supply sources can be accomplished as long as the vascular plexus is not disrupted by cautery, crushing, or tearing. A clean transection can be re-anastomosed or reimplanted with the expectation of normal healing. Innervation of the ureter is from the inferior mesenteric plexus superiorly and the inferior hypogastric plexus in the pelvis. Ureteral peristalsis will continue, even if the ureter is completely divided or ligated.
Protecting pelvic blood vessels
The majority of gynecologic operations involve the ovarian vessels and the uterine vessels. The operator rarely needs to explore the lateral pelvis to identify the rest of the vessels. When faced with a large endometrioma or cancer, knowledge of the anatomy of the lateral blood vessels is vital.
Since the pathology is usually deeper in the pelvis, it is wise to identify the anatomy of the pelvis starting at the pelvic brim, where the common iliac bifurcates into the external and internal iliacs. There is a safe dissection plane medial to the internal iliac artery all the way to the uterine artery. This exposes the pararectal space, which can be opened without risk of major bleeding (FIGURE 5).
The obliterated umbilical vessel is the other friendly marker just distal to the uterine artery. It can be placed on traction and the uterine artery isolated. The inferior and superior vesical arteries are generally not dissected, as they are adjacent to the bladder and the surgery takes place medial to them in the prevesical fascial space.
Hypogastric artery ligations are rarely performed today, as interventional radiology is the standard of care for patients with postoperative and postpartum bleeding. When it becomes necessary, the hypogastric artery can be isolated and tied using the right-angle clamp to pass the tie. The superior gluteal artery branches so close to the bifurcation of the common iliac artery that it is not visualized. The inferior gluteal artery is the largest distal branch, which might be visualized during the ligation. It is not necessary to identify it.
FIGURE 12 Right genitofemoral nerve
The genitofemoral nerve runs along the medial aspect of the body of the psoas muscle. It is sometimes injured by the self-retaining retractors placed at the time of laparotomy. This leads to some numbness and burning of the skin of the anterior thigh.
FIGURE 13 Right pelvic sidewall anatomy
The obturator nerve is in the obturator space and typically far lateral to the usual dissection. Metastatic cancer to the obturator lymph nodes may entrap it, or it may be injured during a node dissection, causing loss of internal rotation of the anterior thigh.
The sciatic nerve is seen only during exenterative surgery. Pressure on the lateral pelvis by advanced pelvic tumors can lead to sciatic pain and motor weakness—even loss of motor function to the lower leg, which commonly leads to foot drop.
FIGURE 14 Superior hypogastric nerve bundle
The hypogastric plexus of nerves is sometimes damaged during surgery for endometriosis or for malignancy. The superior hypergastric plexus can be identified between the 2 common iliac arteries at the sacral promontory. The left common iliac vein runs underneath it.
FIGURE 15 Hypogastric nerve descending into the right pelvis
The right and left hypogastric nerves leave the hypogastric plexus and descend into the pelvis parallel to the ureter and 2 cm medial. It passes dorsal to the ureter as it goes through the cardinal ligament (FIGURE 5).
This plexus then supplies autonomic innervation of the bladder, rectum, uterus, and ureter. Complete disruption of the hypogastric nerve will lead to a hypertonic, noncontractile bladder and the necessity for self-catheterization to eliminate urine. Preservation of this nerve during radical hysterectomy or endometriosis resection is a high priority.
Laparoscopic uterosacral nerve ablation procedures divide the uterosacral ligament medial and caudad to the ureter and do not disrupt the main hypogastric nerve. Only the medial branches to the uterus are affected. Successful uterosacral nerve ablation has been reported in approximately 44% of women who have dysmenorrhea without visible endometriosis and approximately 62% of women who have visible endometriosis.7,8 The efficacy of this procedure is controversial, however. Removal of the superior hypogastric plexus (presacral neurectomy) has not proved to be more effective in controlling pelvic pain than conservative surgery that only destroys endometrial implants. Presacral neurectomy is no longer advised.9
1. American College of Obstetricians and Gynecologists. Educational Bulletin #238: Lower Urinary Tract Operative Injuries. Washington, DC: ACOG; 1985:1.
2. Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
3. Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155:878-881.
4. Symmonds RE. Ureteral injuries associated with gynecologic surgery: prevention and management. Clin Obstet Gynecol. 19676;19:623-644.
5. Higgins CC. Ureteral injuries during surgery: a review of 87 cases. JAMA 1967;199:82-88.
6. Fry DE, Milholen L, Harbrecht PJ. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118:454-457.
7. Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med. 1987;32:37-41.
8. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696.-
9. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
Dr. Hatch receives research/grant support from Merck; is a consultant to Ethicon, Merck, and Quest Laboratory; and is a speaker for Cytyc, Digene, Ethicon, and Merck.
1. American College of Obstetricians and Gynecologists. Educational Bulletin #238: Lower Urinary Tract Operative Injuries. Washington, DC: ACOG; 1985:1.
2. Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
3. Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155:878-881.
4. Symmonds RE. Ureteral injuries associated with gynecologic surgery: prevention and management. Clin Obstet Gynecol. 19676;19:623-644.
5. Higgins CC. Ureteral injuries during surgery: a review of 87 cases. JAMA 1967;199:82-88.
6. Fry DE, Milholen L, Harbrecht PJ. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118:454-457.
7. Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med. 1987;32:37-41.
8. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696.-
9. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
Dr. Hatch receives research/grant support from Merck; is a consultant to Ethicon, Merck, and Quest Laboratory; and is a speaker for Cytyc, Digene, Ethicon, and Merck.
Things go better with Burch
- Moderator Neeraj Kohli, MD, MBA OBG Management Board of Editors Director, Division of Urogynecology, Brigham and Women’s Hospital, and Assistant Professor, Harvard Medical School, Boston.
- Lead investigator of the CARE trial Linda Brubaker, MD, MS, Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics and Gynecology and Urology, Loyola University Chicago.
- Mark D. Walters, MD, Head, Section of General Gynecology, Urogynecology, and Pelvic Reconstructive Surgery, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Anne M. Weber, MD, MS, Program Officer, Pelvic Floor Disorders Network, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md. Dr. Weber is an investigator in the CARE Trial.
Why should we care about the CARE (Colpopexy and Urinary Reduction Efforts) trial?
Because pelvic organ prolapse and urinary incontinence are already major problems facing women as they age, and will become even more pervasive as the baby boomer generation moves through menopause and beyond.
Because the risk that a woman will experience stress incontinence after prolapse surgery ranges from 8% to 60%.1-6
Because roughly one third of women who undergo prolapse or incontinence surgery require a second operation.
These are just a few of the factors that spurred the Pelvic Floor Disorders Network to undertake the CARE trial, published April 13 in the New England Journal of Medicine. OBG Management convened a panel of experts in female pelvic medicine, including 2 CARE trial investigators, to discuss the findings of this landmark study, its long-term implications, and the future of research into pelvic floor disorders.
How the trial was conducted
The CARE trial involved 322 women who required surgery to correct pelvic organ prolapse (POP) but lacked symptoms of stress urinary incontinence. All these women underwent sacrocolpopexy, an abdominal procedure in which graft material is attached between the vagina and sacrum to support the vagina and correct the prolapse. These women were randomized to undergo Burch colposuspension at the time of the sacrocolpopexy, or to undergo sacrocolpopexy only. The Burch procedure is performed through the same incision as the sacrocolpopexy and involves suturing the periurethral vaginal tissue to the iliopectineal ligaments on each side, providing urethral support.
Enrollment in the trial was halted after the first of 2 planned interim analyses because the frequency of postoperative stress incontinence was significantly lower in the group undergoing Burch colposuspension: 23.8% and 44.1% of women in the Burch and no-Burch groups, respectively, experienced stress symptoms by 3 months after the surgery.
Why the CARE trial is an epochal event
- First randomized trial of preventive incontinence surgery in women with prolapse
- Randomized design establishes cause and effect
- Subjects will be followed for 2 years
BRUBAKER: First, it is a well-designed, randomized, controlled trial and thus provides the highest level of evidence for clinical practice. Although there is no perfect study, this one minimized the risk of bias by involving multiple centers (7) and using multiple surgeons, making the findings more generalizable than would be the case in a single-surgeon case series.
In addition, the use of blinded urodynamic testing lent strength, because the ability of urodynamic testing to predict the need for a concomitant continence procedure was not known before the trial. Our follow-up manuscript, containing data presented at the recent Society of Gynecologic Surgeons meeting, will provide more details on this aspect of the trial.
WEBER: Randomized trials are held in such high esteem—provided all other aspects of study design and implementation are performed properly—because they support conclusions of cause and effect. The conclusion that Burch colposuspension prevents stress incontinence when performed at the time of abdominal sacrocolpopexy could only be drawn from a randomized trial.
Trial design standardized key elements
Many types of bias confound the results of nonrandomized studies, particularly selection bias (eg, when surgeons select which procedure to perform on the basis of patient characteristics), and valid conclusions of cause and effect cannot be drawn. However, with a randomized trial, subjects are separated into groups by chance and no other factor. Thus, the groups are equivalent at baseline—provided the sample size is large enough (and allowing for random differences)—and therefore any changes measured after the experimental intervention can be confidently attributed to the intervention itself.
Another strength of the trial is standardization. The subjects were “standardized” by rather broad inclusion and exclusion criteria to constitute an important clinical population and to ensure they were sufficiently similar so that the treatment (abdominal sacrocolpopexy) was appropriate for all. In addition, surgeons at the multiple participating sites agreed to standardization of the technical details of the Burch colposuspension so that the subjects received the same intervention regardless of site. And data collection in follow-up was performed in a standard way by research staff who were blinded to the subjects’ group assignment (intervention versus control), so the data were as free of bias as possible.
Homogeneous study population may be a weakness
KOHLI: I agree that the methodology of this well-designed study is its major strength. What are its weaknesses?
WEBER: No doubt there are several, only some of which may be apparent at this time. For example, most women in the study were Caucasian, and very few were Hispanic, Asian, or black. Although we have no scientific reason to believe that Burch colposuspension has different responses in women of different racial and ethnic backgrounds, the trial’s subjects are not diverse enough to analyze the data by subgroups to confirm or refute the hypothesis that response to the Burch procedure is independent of race or ethnicity.
BRUBAKER: Another weakness: Because this study was closed after the first interim analysis, some of our secondary analyses will be underpowered, although we clearly demonstrated a difference in our primary endpoint.
It is important to remember that this study is not “finished.” Our participants are still in active follow-up for 2 years following surgery. It will be interesting to see what happens during the longer follow-up, especially with regard to prolapse and incontinence. We are also doing additional in-depth analyses of urodynamic and other parameters.
KOHLI: Again, I think the study design and analysis were well thought out. It would have been interesting to see how the results broke down according to site, to see if there was variation—which could indicate variation in surgical technique.
BRUBAKER: We have not done this analysis and do not plan to at this time.
Why paravaginal repairs were allowed
KOHLI: What about the decision to include surgeries that involved paravaginal repair?
WEBER: That generated a fair amount of discussion during trial design, as there was no clear “right” answer. Perhaps it would have been “cleaner” to eliminate the option of performing paravaginal repair, but when the trial was designed, we lacked unequivocal evidence that paravaginal repair at the time of abdominal sacrocolpopexy provides additional support for the anterior vagina. Therefore, we decided to allow the decision to be based on surgeon judgment.
Some surgeons perform paravaginal repair with abdominal sacrocolpopexy in almost all women because they believe quite strongly that this reduces the risk of recurrent anterior vaginal prolapse. Others never perform paravaginal repair with abdominal sacrocolpopexy and feel just as strongly that their patients are adequately treated and protected from subsequent anterior vaginal prolapse.
Investigators feared paravaginal repairs could dilute Burch effects
Study surgeons did agree that paravaginal repair reduces the likelihood of postoperative stress incontinence, although not as effectively as Burch colposuspension. Thus, our dilemma: If paravaginal repairs were performed in a large number of subjects, thereby improving their postoperative continence status regardless of whether Burch was performed, the effect of Burch could be so diluted as to be lost. On the other hand, if paravaginal repairs were completely excluded, that would restrict some surgeons’ practices and potentially reduce the number of women who would be offered participation in the study if their surgeons felt their anterior vaginal prolapse would be potentially undertreated.
We resolved the dilemma as follows:
- A relatively low proportion—about one quarter—of surgeons performed paravaginal repairs regularly with abdominal sacrocolpopexy, so the potential impact in the trial would not be great.
- Paravaginal repairs were allowed, but only when declared necessary by the surgeon before randomization; this step prevented surgeons from changing their minds about the necessity of paravaginal repair if the subject was assigned to the Burch group (ie, the woman would be receiving additional anterior vaginal support by way of the Burch).
- We stratified for paravaginal repair in the randomization, so women with paravaginal repair were equally distributed between the intervention and control groups.
Are subjective or objective measures better?
- Subjective measures convey a patient’s foremost concerns and how she is doing clinically
- Correlating symptoms with objective measures yields valuable insights into treatment
BRUBAKER: I prefer subjective measures because I think they reflect what is most important to patients in quality-of-life disorders. However, I believe we need to understand the relationship between subjective outcomes and traditional “objective” outcomes.
WEBER: I think the research community is reaching a consensus that “subjective” measures—better described as patient-oriented outcomes—are more important than objective measures, particularly for conditions that affect patients in “subjective” ways, ie, ways that affect their health-related quality of life, rather than quantity of life. This does not mean that objective measures are useless—although we should first evaluate each measure critically to make that determination on the basis of evidence.
Nevertheless, when a patient seeks and receives treatment based on symptoms and how those symptoms impact her daily life, I think it is incumbent upon researchers and clinicians to ensure that the treatment that is considered most effective actually results in a change that the patient finds worthwhile.
What is “success”?
WALTERS: When it comes to incontinence, for which there is an imperfect correlation between various objective and subjective measures, I think both types of measures are valuable and important. Gathering several different types of outcomes for each patient helps us better understand the nuances of how well an intervention works.
I can understand why some clinicians and researchers place greater reliance on subjective measures of incontinence, such as a diary of incontinence episodes and quality-of-life measures, because these measures tell us exactly how the patient is doing clinically and how she feels about the intervention. If she reports that she is completely cured and “perfect,” then objective measures are irrelevant. However, for any subjective outcome short of perfect, correlation with the objective measures such as cough stress test, physical examination, and urodynamic tests can help investigators understand the reason for the imperfect outcome and point to areas of possible improvement.
KOHLI: In my practice, some women who continue to leak slightly after an incontinence procedure consider their surgery a complete success, whereas, as a surgeon, I consider it a suboptimal result. Both objective and subjective results are important.
Putting the CARE trial into practice
- Data relate directly only to women undergoing abdominal sacrocolpopexy
- Patient education, medicolegal, and reimbursement may also relate
- Results reflect the high prevalence of pelvic floor disorders and the need to routinely ask about them
BRUBAKER: I routinely counsel patients who are planning sacrocolpopexy but who do not have stress incontinence to consider a concomitant Burch procedure. I do not have them undergo urodynamic testing because, at this time, the results of that testing would not change my clinical practice.
WALTERS: I have always been liberal when it comes to adding retropubic colposuspension to abdominal sacrocolpopexy, even in women who do not have preoperative stress incontinence. The reason? Patients who are continent preoperatively, but become stress-incontinent postoperatively, are particularly unhappy with their outcome, especially if they need another surgery within a year to treat the stress incontinence. So this study verified what I was already doing.
What I didn’t learn is whether a paravaginal defect repair helps or hurts the Burch procedure from an anatomic and functional perspective.
It also appears that preoperative urodynamic testing has little value, although that was not the point of this study. I am glad it will be addressed in future studies.
KOHLI: I think the findings apply to those select patients undergoing abdominal sacrocolpopexy for prolapse. It would be dangerous to extrapolate these results to other abdominal vault suspension procedures or vaginal prolapse procedures. Based on the CARE trial, I plan to counsel patients about the risks and benefits of “optional” Burch colposuspension at the time of planned sacrocolpopexy. In reality, however, I have almost completely switched to minimally invasive midurethral slings, even in the case of abdominal prolapse procedures, because of their high cure rates, low complication rates, and ease of postoperative adjustment.
Clinical implications depend on surgeon’s routine
KOHLI: What are the implications for the majority of ObGyns?
WEBER: It depends on what ObGyns are doing for women with prolapse.
For ObGyns who are confident and competent, through training and experience, to perform abdominal sacrocolpopexy for women with advanced prolapse, the CARE trial results have a direct effect. Women with prolapse who are stress continent with no contraindications, can be reassured that they will benefit from a 50% reduction of postoperative stress incontinence with the Burch procedure.
For ObGyns who do not perform sacrocolpopexy, the CARE trial will have no direct clinical effects. Nevertheless, these clinicians need to be aware of the findings so they can discuss the options with patients before decisions on route or type of prolapse surgery are made.
The CARE trial and its results remind us of the high prevalence of pelvic floor disorders in women, potentially even after corrective surgery—and the need to actively screen all women for pelvic dysfunction.
Warn of potential incontinence even with the Burch
KOHLI: How does this study affect counseling of candidates for prolapse surgery?
BRUBAKER: I would offer stress-continent women a Burch procedure at the time of sacrocolpopexy. That much is clear. The interesting discussions come from “similar” clinical scenarios, where data are not yet available. For example, should a stress-continent woman facing a suspension via the vaginal route undergo a concomitant continence procedure?
WEBER: It is important to keep in mind that even when Burch colposuspension was performed, a number of women still experienced urinary incontinence (some stress, some urge, some mixed) after surgery; and the vast majority of women have urinary symptoms of some kind both before and after surgery. So preoperative counseling should include the information that urinary symptoms are very common after abdominal sacrocolpopexy—some as persistent or recurrent, and some as new symptoms.
As longer follow-up data from the CARE trial become available, we will learn how many women have urinary symptoms that are temporary versus long-lasting.
Is routine Burch best?
- When not all women benefit, should a procedure be offered prophylactically? In this case, experts say, “Yes”
- Some physicians favor other incontinence procedures
- Final decision rests with the patient
WALTERS: It is an easy decision for me. As I said earlier, women are particularly unhappy if they go from continent to incontinent after a surgery. In fact, some women are more displeased with that outcome than with a failure of the prolapse surgery. Because most women with prolapse have substantial anterior vaginal wall prolapse, the Burch procedure—with or without a paravaginal defect repair—also serves as part of the prolapse repair of the anterior wall. And now we know it also improves postoperative urinary function.
BRUBAKER: Doing a Burch procedure at the time of sacrocolpopexy is a time-efficient and low-morbidity addition, so it is worthwhile for me and my patients. It is clearly not the same as doing a secondary, standalone procedure for new symptoms.
Over the next 2 years, we will see how many women who were moderately or greatly bothered by stress incontinence went on to a surgical treatment.
WEBER: Please note a careful distinction: I would be willing to recommend Burch colposuspension to 100 stress-continent women who are planning to undergo abdominal sacrocolpopexy for prolapse, with the expectation that this will prevent postoperative stress incontinence in roughly half the women who would have developed it otherwise. It is up to the patient to accept this recommendation or not.
Based on the CARE trial results, 44 of 100 previously continent women after only abdominal sacrocolpopexy develop postoperative stress incontinence, compared with about 24 of 100 women after Burch and abdominal sacrocolpopexy. Even more striking is the difference in women affected by bothersome stress incontinence: almost 25% in the control group versus 6% in the Burch group. Women in the Burch group did not experience an excess of adverse events, or a clinically significant difference in operative time or estimated blood loss, compared with women in the control group.
Is “wait and see” better?
KOHLI: Because I favor minimally invasive midurethral sling procedures, which can often be performed on an outpatient basis under local anesthesia, I counsel women undergoing prolapse surgery via an abdominal or vaginal route that it is best to treat the incontinence postoperatively if it occurs. Obviously, this applies to women who have no incontinence and do not demonstrate potential stress incontinence on urodynamic testing preoperatively.
Anecdotally, I have not found a high rate of new-onset urinary incontinence following prolapse procedures. We may retrospectively look at these patients more critically in light of this new data.
WALTERS: I think most women would be dissatisfied with a 44% risk of postoperative stress incontinence requiring a second surgery. Even if you counseled them appropriately, many women would ask that you try to manage everything at the first surgery.
What are nonclinical effects of the trial?
- Need for patient and physician education is great, and the CARE trial offers a valuable opportunity
- Potential for medicolegal risk if complications develop
- Payers may not be willing to reimburse for a prophylactic procedure
WEBER: Given how extensively the trial’s results were disseminated by the lay press, I think we have an important opportunity to educate both patients and health-care providers.
First, patients: For women who may be directly affected by the trial, knowledgeable clinicians should explain its results and limitations to help them reach a decision about their treatment.
For women who hear the trial’s results described incompletely or incorrectly (eg, “…2 stitches prevent incontinence…”), clinicians should take this opportunity to correct misunderstandings and educate women about incontinence, prolapse, and pelvic health in general.
For health-care providers, this trial reminds us of the extraordinarily high prevalence of pelvic floor disorders. Although current treatments are not perfect, virtually all women with pelvic floor disorders can be treated to substantially alleviate, if not eliminate, bothersome symptoms.
All clinicians should routinely inquire about pelvic symptoms and be prepared to initiate an evaluation or provide a referral.
Medicolegal fallout?
KOHLI: There is the question of medicolegal risk if complications occur after colposuspension when the patient had no complaints or evidence of stress incontinence at the time of preoperative urodynamic testing. I am not aware of any legal precedent in which a clinical study or data provided solid protection from a jury verdict. The study did not show increased risk or complication with the addition of the Burch procedure, but that may not be true for some surgeons and some patients.
Billing and coding
In terms of billing, how should we code for the Burch colposuspension when the patient had no demonstrable stress incontinence? Payment denials in this scenario seem likely. This may create a line of separation between what may be clinically indicated for the patient and what insurance companies are willing to pay for.
There may be the option to use the urethral hypermobility code (ICD 599.81) for the Burch colposuspension, but only time will tell if this will be reimbursed. I would be curious to hear the panel’s experience with reimbursement for a prophylactic procedure based on scientific data. Obviously, what is best for the patient is most important.
BRUBAKER: All these patients had urethral hypermobility, which is also an indication for a Burch colposuspension.
Is preoperative urodynamic testing useful?
- CARE trial data still to come
- Basic testing is probably helpful
WEBER: In the CARE trial, the relative level of protection from postoperative stress incontinence provided by the Burch procedure did not depend on the stress-test component (with prolapse reduced) of preoperative urodynamic testing. About 50% fewer women had postoperative stress incontinence after Burch, whether preoperative urodynamic testing showed positive or negative stress tests with prolapse reduction.
Although subsequent analyses (presented at the Society of Gynecologic Surgeons 2006 meeting; manuscript under review) focused on urodynamic testing and postoperative outcomes, the CARE trial was not designed primarily to determine whether women planning prolapse surgery benefit from preoperative urodynamic testing. Therefore, conclusions about the “need” for urodynamic testing should not be based only on the CARE trial.
Should urodynamic testing determine treatment?
Randomized trials that directly address the cost-benefit of urodynamic testing are urgently needed. For now, as is standard in good clinical practice, a test should be performed only if results will change recommendations or provide reliable and clinically important prognostic information about a patient’s outcome after intervention. Clinicians should determine whether urodynamic testing meets even 1 of these 2 minimum criteria.
I want to point out that the CARE trial did not address the utility of urodynamic testing.
BRUBAKER: It is clear that some women have stress incontinence despite the concomitant Burch colposuspension. If we learn that an alternative operation can perform better and that any urodynamic (or other clinical measure) can predict improved outcomes, I would consider resuming urodynamic testing.
WALTERS: At first glance, it appears that complex urodynamic testing is definitely not necessary if the goal is to improve outcomes of surgery. However, I believe the patient should undergo some components of urodynamic testing such as a void with a post-void residual urine volume and a basic bladder-filling study noting sensation and capacity. I also do a cough stress test with the prolapse reduced, although we may find that this does not predict postoperative function.
KOHLI: The results of this study are very procedure-specific. If similar results are borne out when other approaches to prolapse and incontinence are analyzed, the value and utility of preoperative uro-dynamic testing in all patients may be questionable.
However, in my practice, I use the results of preoperative urodynamic testing not only for diagnosis, but also to make subtle adjustments when performing incontinence procedures—especially in regard to suburethral slings.
What if you prefer midurethral slings?
- Surgeons should be comfortable with more than 1 incontinence procedure
- We should not jump to untested conclusions
WEBER: Ideally, well trained and experienced gynecologic, urologic, or urogynecologic surgeons perform more than 1 type of incontinence procedure, to meet the needs of different patients.
As yet, we have no direct, evidence-based answers to issues such as these:
Can a midurethral sling be substituted for a Burch colposuspension and have the same average results in preventing post-operative stress incontinence without increasing urgency symptoms…
- …when abdominal sacrocolpopexy is performed for prolapse in a preoperatively stress-continent patient?
- …when vaginal apical suspension is performed for prolapse in a preoperatively stress-continent patient?
Although it is tempting to jump 1 or 2 steps ahead and apply CARE trial data to situations that have not been tested directly, I would be cautious. We want to avoid creating long-lasting or refractory urgency symptoms—especially in a woman who had no such symptoms before surgery—because of a prophylactic procedure.
I think this is especially true because it is relatively easy to salvage patients who do develop bothersome stress incontinence after prolapse surgery.
Bonus: Burch helps anterior vaginal prolapse
WALTERS: I wonder whether prophylactic placement of a midurethral sling would yield the same results as a prophylactic Burch procedure. If your midurethral sling of choice is a tension-free vaginal tape (TVT), I would be cautious about placing it prophylactically, because the TVT has a 2% to 3% risk of prolonged voiding dysfunction requiring transection of the tape.
However, it is possible that prophylactic placement of a transobturator sling, which is associated with much less voiding dysfunction and fewer major surgical complications, might have a different outcome—though this requires further study.
In addition, midurethral slings would not be as effective as Burch colposuspension in treating anterior vaginal prolapse, so I would expect to see more anterior wall prolapse failures if slings replaced colposuspension.
What if you prefer the vaginal approach?
- Further study is needed
WALTERS: I wonder whether a prophylactic transobturator midurethral sling at the time of transvaginal prolapse repair would yield similar results. I do cystocele repair with suburethral (“Kelly”) plication, which seems to work well at stabilizing the urethra in women without stress incontinence. But this approach is not as popular these days, and future studies may demonstrate that a prophylactic midurethral sling will result in better long-term function without significantly increasing the long-term risk.
WEBER: The CARE trial results are relevant to all pelvic surgeons because they demonstrate the need for and benefit from well-designed randomized surgical trials. Another benefit will be extended follow-up in what will become a prospective cohort study of women with advanced prolapse treated by abdominal sacrocolpopexy—providing higher-quality evidence than retrospective case series. Although not as valuable as randomized trials, these data can help guide clinical recommendations.
If long-term results support the effectiveness and durability of abdominal prolapse repair, then gynecologists can reflect on the evidence and choose the approach that best fits the patient’s needs.
Need for other studies?
- Randomized, multicenter trials addressing almost any surgical treatment of prolapse and incontinence are sorely needed
BRUBAKER: Any and all high-quality, well-designed trial can improve our care of women with incontinence and/or prolapse.
WALTERS: I look forward to the follow-up studies from the CARE trial on the value of paravaginal defect repair, preoperative urodynamic testing, and the efficacy of various prolapse-reduction maneuvers in predicting surgical outcomes.
It would also seem logical to repeat this type of study using transvaginal prolapse repair with or without a prophylactic midurethral sling. Another option: anterior colporrhaphy with suburethral plication versus a prophylactic midurethral sling.
KOHLI: I look forward to data on surgical procedures currently being performed with greater frequency despite a lack of good-quality data. These include the transobturator suburethral midurethral sling procedures, laparoscopic sacrocolpopexy, and vaginal mesh augmentation for prolapse.
The Pelvic Floor Disorders Network affords a unique opportunity to perform well-designed multicenter trials to address the rapidly changing landscape of surgical treatment for prolapse and incontinence.
1. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
2. Cross CA, Cespedes RD, McGuire EJ. Treatment results using pubovaginal slings in patients with large cystoceles and stress incontinence. J Urol. 1997;158:431-434.
3. FitzGerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189:1241-1244.
4. Klutke JJ, Ramos S. Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol. 2000;182:1378-1381.
5. Meschia M, Pifarotti P, Spennacchio M, Buonaguidi A, Gattei U, Somigliana E. A randomized comparison of tension-free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. Am J Obstet Gynecol. 2004;190:609-613.
6. Gordon D, Gold RS, Pauzner D, Lessing JB, Groutz A. Combined genitourinary prolapse repair and prophylactic tension-free vaginal tape in women with severe prolapse and occult stress urinary incontinence: preliminary results. Urology. 2001;58:547-550.
Dr. Brubaker, Dr. Kohli, and Dr. Weber report no financial relationships relevant to this article. Dr. Walters is a speaker for American Medical Systems.
- Moderator Neeraj Kohli, MD, MBA OBG Management Board of Editors Director, Division of Urogynecology, Brigham and Women’s Hospital, and Assistant Professor, Harvard Medical School, Boston.
- Lead investigator of the CARE trial Linda Brubaker, MD, MS, Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics and Gynecology and Urology, Loyola University Chicago.
- Mark D. Walters, MD, Head, Section of General Gynecology, Urogynecology, and Pelvic Reconstructive Surgery, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Anne M. Weber, MD, MS, Program Officer, Pelvic Floor Disorders Network, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md. Dr. Weber is an investigator in the CARE Trial.
Why should we care about the CARE (Colpopexy and Urinary Reduction Efforts) trial?
Because pelvic organ prolapse and urinary incontinence are already major problems facing women as they age, and will become even more pervasive as the baby boomer generation moves through menopause and beyond.
Because the risk that a woman will experience stress incontinence after prolapse surgery ranges from 8% to 60%.1-6
Because roughly one third of women who undergo prolapse or incontinence surgery require a second operation.
These are just a few of the factors that spurred the Pelvic Floor Disorders Network to undertake the CARE trial, published April 13 in the New England Journal of Medicine. OBG Management convened a panel of experts in female pelvic medicine, including 2 CARE trial investigators, to discuss the findings of this landmark study, its long-term implications, and the future of research into pelvic floor disorders.
How the trial was conducted
The CARE trial involved 322 women who required surgery to correct pelvic organ prolapse (POP) but lacked symptoms of stress urinary incontinence. All these women underwent sacrocolpopexy, an abdominal procedure in which graft material is attached between the vagina and sacrum to support the vagina and correct the prolapse. These women were randomized to undergo Burch colposuspension at the time of the sacrocolpopexy, or to undergo sacrocolpopexy only. The Burch procedure is performed through the same incision as the sacrocolpopexy and involves suturing the periurethral vaginal tissue to the iliopectineal ligaments on each side, providing urethral support.
Enrollment in the trial was halted after the first of 2 planned interim analyses because the frequency of postoperative stress incontinence was significantly lower in the group undergoing Burch colposuspension: 23.8% and 44.1% of women in the Burch and no-Burch groups, respectively, experienced stress symptoms by 3 months after the surgery.
Why the CARE trial is an epochal event
- First randomized trial of preventive incontinence surgery in women with prolapse
- Randomized design establishes cause and effect
- Subjects will be followed for 2 years
BRUBAKER: First, it is a well-designed, randomized, controlled trial and thus provides the highest level of evidence for clinical practice. Although there is no perfect study, this one minimized the risk of bias by involving multiple centers (7) and using multiple surgeons, making the findings more generalizable than would be the case in a single-surgeon case series.
In addition, the use of blinded urodynamic testing lent strength, because the ability of urodynamic testing to predict the need for a concomitant continence procedure was not known before the trial. Our follow-up manuscript, containing data presented at the recent Society of Gynecologic Surgeons meeting, will provide more details on this aspect of the trial.
WEBER: Randomized trials are held in such high esteem—provided all other aspects of study design and implementation are performed properly—because they support conclusions of cause and effect. The conclusion that Burch colposuspension prevents stress incontinence when performed at the time of abdominal sacrocolpopexy could only be drawn from a randomized trial.
Trial design standardized key elements
Many types of bias confound the results of nonrandomized studies, particularly selection bias (eg, when surgeons select which procedure to perform on the basis of patient characteristics), and valid conclusions of cause and effect cannot be drawn. However, with a randomized trial, subjects are separated into groups by chance and no other factor. Thus, the groups are equivalent at baseline—provided the sample size is large enough (and allowing for random differences)—and therefore any changes measured after the experimental intervention can be confidently attributed to the intervention itself.
Another strength of the trial is standardization. The subjects were “standardized” by rather broad inclusion and exclusion criteria to constitute an important clinical population and to ensure they were sufficiently similar so that the treatment (abdominal sacrocolpopexy) was appropriate for all. In addition, surgeons at the multiple participating sites agreed to standardization of the technical details of the Burch colposuspension so that the subjects received the same intervention regardless of site. And data collection in follow-up was performed in a standard way by research staff who were blinded to the subjects’ group assignment (intervention versus control), so the data were as free of bias as possible.
Homogeneous study population may be a weakness
KOHLI: I agree that the methodology of this well-designed study is its major strength. What are its weaknesses?
WEBER: No doubt there are several, only some of which may be apparent at this time. For example, most women in the study were Caucasian, and very few were Hispanic, Asian, or black. Although we have no scientific reason to believe that Burch colposuspension has different responses in women of different racial and ethnic backgrounds, the trial’s subjects are not diverse enough to analyze the data by subgroups to confirm or refute the hypothesis that response to the Burch procedure is independent of race or ethnicity.
BRUBAKER: Another weakness: Because this study was closed after the first interim analysis, some of our secondary analyses will be underpowered, although we clearly demonstrated a difference in our primary endpoint.
It is important to remember that this study is not “finished.” Our participants are still in active follow-up for 2 years following surgery. It will be interesting to see what happens during the longer follow-up, especially with regard to prolapse and incontinence. We are also doing additional in-depth analyses of urodynamic and other parameters.
KOHLI: Again, I think the study design and analysis were well thought out. It would have been interesting to see how the results broke down according to site, to see if there was variation—which could indicate variation in surgical technique.
BRUBAKER: We have not done this analysis and do not plan to at this time.
Why paravaginal repairs were allowed
KOHLI: What about the decision to include surgeries that involved paravaginal repair?
WEBER: That generated a fair amount of discussion during trial design, as there was no clear “right” answer. Perhaps it would have been “cleaner” to eliminate the option of performing paravaginal repair, but when the trial was designed, we lacked unequivocal evidence that paravaginal repair at the time of abdominal sacrocolpopexy provides additional support for the anterior vagina. Therefore, we decided to allow the decision to be based on surgeon judgment.
Some surgeons perform paravaginal repair with abdominal sacrocolpopexy in almost all women because they believe quite strongly that this reduces the risk of recurrent anterior vaginal prolapse. Others never perform paravaginal repair with abdominal sacrocolpopexy and feel just as strongly that their patients are adequately treated and protected from subsequent anterior vaginal prolapse.
Investigators feared paravaginal repairs could dilute Burch effects
Study surgeons did agree that paravaginal repair reduces the likelihood of postoperative stress incontinence, although not as effectively as Burch colposuspension. Thus, our dilemma: If paravaginal repairs were performed in a large number of subjects, thereby improving their postoperative continence status regardless of whether Burch was performed, the effect of Burch could be so diluted as to be lost. On the other hand, if paravaginal repairs were completely excluded, that would restrict some surgeons’ practices and potentially reduce the number of women who would be offered participation in the study if their surgeons felt their anterior vaginal prolapse would be potentially undertreated.
We resolved the dilemma as follows:
- A relatively low proportion—about one quarter—of surgeons performed paravaginal repairs regularly with abdominal sacrocolpopexy, so the potential impact in the trial would not be great.
- Paravaginal repairs were allowed, but only when declared necessary by the surgeon before randomization; this step prevented surgeons from changing their minds about the necessity of paravaginal repair if the subject was assigned to the Burch group (ie, the woman would be receiving additional anterior vaginal support by way of the Burch).
- We stratified for paravaginal repair in the randomization, so women with paravaginal repair were equally distributed between the intervention and control groups.
Are subjective or objective measures better?
- Subjective measures convey a patient’s foremost concerns and how she is doing clinically
- Correlating symptoms with objective measures yields valuable insights into treatment
BRUBAKER: I prefer subjective measures because I think they reflect what is most important to patients in quality-of-life disorders. However, I believe we need to understand the relationship between subjective outcomes and traditional “objective” outcomes.
WEBER: I think the research community is reaching a consensus that “subjective” measures—better described as patient-oriented outcomes—are more important than objective measures, particularly for conditions that affect patients in “subjective” ways, ie, ways that affect their health-related quality of life, rather than quantity of life. This does not mean that objective measures are useless—although we should first evaluate each measure critically to make that determination on the basis of evidence.
Nevertheless, when a patient seeks and receives treatment based on symptoms and how those symptoms impact her daily life, I think it is incumbent upon researchers and clinicians to ensure that the treatment that is considered most effective actually results in a change that the patient finds worthwhile.
What is “success”?
WALTERS: When it comes to incontinence, for which there is an imperfect correlation between various objective and subjective measures, I think both types of measures are valuable and important. Gathering several different types of outcomes for each patient helps us better understand the nuances of how well an intervention works.
I can understand why some clinicians and researchers place greater reliance on subjective measures of incontinence, such as a diary of incontinence episodes and quality-of-life measures, because these measures tell us exactly how the patient is doing clinically and how she feels about the intervention. If she reports that she is completely cured and “perfect,” then objective measures are irrelevant. However, for any subjective outcome short of perfect, correlation with the objective measures such as cough stress test, physical examination, and urodynamic tests can help investigators understand the reason for the imperfect outcome and point to areas of possible improvement.
KOHLI: In my practice, some women who continue to leak slightly after an incontinence procedure consider their surgery a complete success, whereas, as a surgeon, I consider it a suboptimal result. Both objective and subjective results are important.
Putting the CARE trial into practice
- Data relate directly only to women undergoing abdominal sacrocolpopexy
- Patient education, medicolegal, and reimbursement may also relate
- Results reflect the high prevalence of pelvic floor disorders and the need to routinely ask about them
BRUBAKER: I routinely counsel patients who are planning sacrocolpopexy but who do not have stress incontinence to consider a concomitant Burch procedure. I do not have them undergo urodynamic testing because, at this time, the results of that testing would not change my clinical practice.
WALTERS: I have always been liberal when it comes to adding retropubic colposuspension to abdominal sacrocolpopexy, even in women who do not have preoperative stress incontinence. The reason? Patients who are continent preoperatively, but become stress-incontinent postoperatively, are particularly unhappy with their outcome, especially if they need another surgery within a year to treat the stress incontinence. So this study verified what I was already doing.
What I didn’t learn is whether a paravaginal defect repair helps or hurts the Burch procedure from an anatomic and functional perspective.
It also appears that preoperative urodynamic testing has little value, although that was not the point of this study. I am glad it will be addressed in future studies.
KOHLI: I think the findings apply to those select patients undergoing abdominal sacrocolpopexy for prolapse. It would be dangerous to extrapolate these results to other abdominal vault suspension procedures or vaginal prolapse procedures. Based on the CARE trial, I plan to counsel patients about the risks and benefits of “optional” Burch colposuspension at the time of planned sacrocolpopexy. In reality, however, I have almost completely switched to minimally invasive midurethral slings, even in the case of abdominal prolapse procedures, because of their high cure rates, low complication rates, and ease of postoperative adjustment.
Clinical implications depend on surgeon’s routine
KOHLI: What are the implications for the majority of ObGyns?
WEBER: It depends on what ObGyns are doing for women with prolapse.
For ObGyns who are confident and competent, through training and experience, to perform abdominal sacrocolpopexy for women with advanced prolapse, the CARE trial results have a direct effect. Women with prolapse who are stress continent with no contraindications, can be reassured that they will benefit from a 50% reduction of postoperative stress incontinence with the Burch procedure.
For ObGyns who do not perform sacrocolpopexy, the CARE trial will have no direct clinical effects. Nevertheless, these clinicians need to be aware of the findings so they can discuss the options with patients before decisions on route or type of prolapse surgery are made.
The CARE trial and its results remind us of the high prevalence of pelvic floor disorders in women, potentially even after corrective surgery—and the need to actively screen all women for pelvic dysfunction.
Warn of potential incontinence even with the Burch
KOHLI: How does this study affect counseling of candidates for prolapse surgery?
BRUBAKER: I would offer stress-continent women a Burch procedure at the time of sacrocolpopexy. That much is clear. The interesting discussions come from “similar” clinical scenarios, where data are not yet available. For example, should a stress-continent woman facing a suspension via the vaginal route undergo a concomitant continence procedure?
WEBER: It is important to keep in mind that even when Burch colposuspension was performed, a number of women still experienced urinary incontinence (some stress, some urge, some mixed) after surgery; and the vast majority of women have urinary symptoms of some kind both before and after surgery. So preoperative counseling should include the information that urinary symptoms are very common after abdominal sacrocolpopexy—some as persistent or recurrent, and some as new symptoms.
As longer follow-up data from the CARE trial become available, we will learn how many women have urinary symptoms that are temporary versus long-lasting.
Is routine Burch best?
- When not all women benefit, should a procedure be offered prophylactically? In this case, experts say, “Yes”
- Some physicians favor other incontinence procedures
- Final decision rests with the patient
WALTERS: It is an easy decision for me. As I said earlier, women are particularly unhappy if they go from continent to incontinent after a surgery. In fact, some women are more displeased with that outcome than with a failure of the prolapse surgery. Because most women with prolapse have substantial anterior vaginal wall prolapse, the Burch procedure—with or without a paravaginal defect repair—also serves as part of the prolapse repair of the anterior wall. And now we know it also improves postoperative urinary function.
BRUBAKER: Doing a Burch procedure at the time of sacrocolpopexy is a time-efficient and low-morbidity addition, so it is worthwhile for me and my patients. It is clearly not the same as doing a secondary, standalone procedure for new symptoms.
Over the next 2 years, we will see how many women who were moderately or greatly bothered by stress incontinence went on to a surgical treatment.
WEBER: Please note a careful distinction: I would be willing to recommend Burch colposuspension to 100 stress-continent women who are planning to undergo abdominal sacrocolpopexy for prolapse, with the expectation that this will prevent postoperative stress incontinence in roughly half the women who would have developed it otherwise. It is up to the patient to accept this recommendation or not.
Based on the CARE trial results, 44 of 100 previously continent women after only abdominal sacrocolpopexy develop postoperative stress incontinence, compared with about 24 of 100 women after Burch and abdominal sacrocolpopexy. Even more striking is the difference in women affected by bothersome stress incontinence: almost 25% in the control group versus 6% in the Burch group. Women in the Burch group did not experience an excess of adverse events, or a clinically significant difference in operative time or estimated blood loss, compared with women in the control group.
Is “wait and see” better?
KOHLI: Because I favor minimally invasive midurethral sling procedures, which can often be performed on an outpatient basis under local anesthesia, I counsel women undergoing prolapse surgery via an abdominal or vaginal route that it is best to treat the incontinence postoperatively if it occurs. Obviously, this applies to women who have no incontinence and do not demonstrate potential stress incontinence on urodynamic testing preoperatively.
Anecdotally, I have not found a high rate of new-onset urinary incontinence following prolapse procedures. We may retrospectively look at these patients more critically in light of this new data.
WALTERS: I think most women would be dissatisfied with a 44% risk of postoperative stress incontinence requiring a second surgery. Even if you counseled them appropriately, many women would ask that you try to manage everything at the first surgery.
What are nonclinical effects of the trial?
- Need for patient and physician education is great, and the CARE trial offers a valuable opportunity
- Potential for medicolegal risk if complications develop
- Payers may not be willing to reimburse for a prophylactic procedure
WEBER: Given how extensively the trial’s results were disseminated by the lay press, I think we have an important opportunity to educate both patients and health-care providers.
First, patients: For women who may be directly affected by the trial, knowledgeable clinicians should explain its results and limitations to help them reach a decision about their treatment.
For women who hear the trial’s results described incompletely or incorrectly (eg, “…2 stitches prevent incontinence…”), clinicians should take this opportunity to correct misunderstandings and educate women about incontinence, prolapse, and pelvic health in general.
For health-care providers, this trial reminds us of the extraordinarily high prevalence of pelvic floor disorders. Although current treatments are not perfect, virtually all women with pelvic floor disorders can be treated to substantially alleviate, if not eliminate, bothersome symptoms.
All clinicians should routinely inquire about pelvic symptoms and be prepared to initiate an evaluation or provide a referral.
Medicolegal fallout?
KOHLI: There is the question of medicolegal risk if complications occur after colposuspension when the patient had no complaints or evidence of stress incontinence at the time of preoperative urodynamic testing. I am not aware of any legal precedent in which a clinical study or data provided solid protection from a jury verdict. The study did not show increased risk or complication with the addition of the Burch procedure, but that may not be true for some surgeons and some patients.
Billing and coding
In terms of billing, how should we code for the Burch colposuspension when the patient had no demonstrable stress incontinence? Payment denials in this scenario seem likely. This may create a line of separation between what may be clinically indicated for the patient and what insurance companies are willing to pay for.
There may be the option to use the urethral hypermobility code (ICD 599.81) for the Burch colposuspension, but only time will tell if this will be reimbursed. I would be curious to hear the panel’s experience with reimbursement for a prophylactic procedure based on scientific data. Obviously, what is best for the patient is most important.
BRUBAKER: All these patients had urethral hypermobility, which is also an indication for a Burch colposuspension.
Is preoperative urodynamic testing useful?
- CARE trial data still to come
- Basic testing is probably helpful
WEBER: In the CARE trial, the relative level of protection from postoperative stress incontinence provided by the Burch procedure did not depend on the stress-test component (with prolapse reduced) of preoperative urodynamic testing. About 50% fewer women had postoperative stress incontinence after Burch, whether preoperative urodynamic testing showed positive or negative stress tests with prolapse reduction.
Although subsequent analyses (presented at the Society of Gynecologic Surgeons 2006 meeting; manuscript under review) focused on urodynamic testing and postoperative outcomes, the CARE trial was not designed primarily to determine whether women planning prolapse surgery benefit from preoperative urodynamic testing. Therefore, conclusions about the “need” for urodynamic testing should not be based only on the CARE trial.
Should urodynamic testing determine treatment?
Randomized trials that directly address the cost-benefit of urodynamic testing are urgently needed. For now, as is standard in good clinical practice, a test should be performed only if results will change recommendations or provide reliable and clinically important prognostic information about a patient’s outcome after intervention. Clinicians should determine whether urodynamic testing meets even 1 of these 2 minimum criteria.
I want to point out that the CARE trial did not address the utility of urodynamic testing.
BRUBAKER: It is clear that some women have stress incontinence despite the concomitant Burch colposuspension. If we learn that an alternative operation can perform better and that any urodynamic (or other clinical measure) can predict improved outcomes, I would consider resuming urodynamic testing.
WALTERS: At first glance, it appears that complex urodynamic testing is definitely not necessary if the goal is to improve outcomes of surgery. However, I believe the patient should undergo some components of urodynamic testing such as a void with a post-void residual urine volume and a basic bladder-filling study noting sensation and capacity. I also do a cough stress test with the prolapse reduced, although we may find that this does not predict postoperative function.
KOHLI: The results of this study are very procedure-specific. If similar results are borne out when other approaches to prolapse and incontinence are analyzed, the value and utility of preoperative uro-dynamic testing in all patients may be questionable.
However, in my practice, I use the results of preoperative urodynamic testing not only for diagnosis, but also to make subtle adjustments when performing incontinence procedures—especially in regard to suburethral slings.
What if you prefer midurethral slings?
- Surgeons should be comfortable with more than 1 incontinence procedure
- We should not jump to untested conclusions
WEBER: Ideally, well trained and experienced gynecologic, urologic, or urogynecologic surgeons perform more than 1 type of incontinence procedure, to meet the needs of different patients.
As yet, we have no direct, evidence-based answers to issues such as these:
Can a midurethral sling be substituted for a Burch colposuspension and have the same average results in preventing post-operative stress incontinence without increasing urgency symptoms…
- …when abdominal sacrocolpopexy is performed for prolapse in a preoperatively stress-continent patient?
- …when vaginal apical suspension is performed for prolapse in a preoperatively stress-continent patient?
Although it is tempting to jump 1 or 2 steps ahead and apply CARE trial data to situations that have not been tested directly, I would be cautious. We want to avoid creating long-lasting or refractory urgency symptoms—especially in a woman who had no such symptoms before surgery—because of a prophylactic procedure.
I think this is especially true because it is relatively easy to salvage patients who do develop bothersome stress incontinence after prolapse surgery.
Bonus: Burch helps anterior vaginal prolapse
WALTERS: I wonder whether prophylactic placement of a midurethral sling would yield the same results as a prophylactic Burch procedure. If your midurethral sling of choice is a tension-free vaginal tape (TVT), I would be cautious about placing it prophylactically, because the TVT has a 2% to 3% risk of prolonged voiding dysfunction requiring transection of the tape.
However, it is possible that prophylactic placement of a transobturator sling, which is associated with much less voiding dysfunction and fewer major surgical complications, might have a different outcome—though this requires further study.
In addition, midurethral slings would not be as effective as Burch colposuspension in treating anterior vaginal prolapse, so I would expect to see more anterior wall prolapse failures if slings replaced colposuspension.
What if you prefer the vaginal approach?
- Further study is needed
WALTERS: I wonder whether a prophylactic transobturator midurethral sling at the time of transvaginal prolapse repair would yield similar results. I do cystocele repair with suburethral (“Kelly”) plication, which seems to work well at stabilizing the urethra in women without stress incontinence. But this approach is not as popular these days, and future studies may demonstrate that a prophylactic midurethral sling will result in better long-term function without significantly increasing the long-term risk.
WEBER: The CARE trial results are relevant to all pelvic surgeons because they demonstrate the need for and benefit from well-designed randomized surgical trials. Another benefit will be extended follow-up in what will become a prospective cohort study of women with advanced prolapse treated by abdominal sacrocolpopexy—providing higher-quality evidence than retrospective case series. Although not as valuable as randomized trials, these data can help guide clinical recommendations.
If long-term results support the effectiveness and durability of abdominal prolapse repair, then gynecologists can reflect on the evidence and choose the approach that best fits the patient’s needs.
Need for other studies?
- Randomized, multicenter trials addressing almost any surgical treatment of prolapse and incontinence are sorely needed
BRUBAKER: Any and all high-quality, well-designed trial can improve our care of women with incontinence and/or prolapse.
WALTERS: I look forward to the follow-up studies from the CARE trial on the value of paravaginal defect repair, preoperative urodynamic testing, and the efficacy of various prolapse-reduction maneuvers in predicting surgical outcomes.
It would also seem logical to repeat this type of study using transvaginal prolapse repair with or without a prophylactic midurethral sling. Another option: anterior colporrhaphy with suburethral plication versus a prophylactic midurethral sling.
KOHLI: I look forward to data on surgical procedures currently being performed with greater frequency despite a lack of good-quality data. These include the transobturator suburethral midurethral sling procedures, laparoscopic sacrocolpopexy, and vaginal mesh augmentation for prolapse.
The Pelvic Floor Disorders Network affords a unique opportunity to perform well-designed multicenter trials to address the rapidly changing landscape of surgical treatment for prolapse and incontinence.
- Moderator Neeraj Kohli, MD, MBA OBG Management Board of Editors Director, Division of Urogynecology, Brigham and Women’s Hospital, and Assistant Professor, Harvard Medical School, Boston.
- Lead investigator of the CARE trial Linda Brubaker, MD, MS, Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics and Gynecology and Urology, Loyola University Chicago.
- Mark D. Walters, MD, Head, Section of General Gynecology, Urogynecology, and Pelvic Reconstructive Surgery, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Anne M. Weber, MD, MS, Program Officer, Pelvic Floor Disorders Network, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md. Dr. Weber is an investigator in the CARE Trial.
Why should we care about the CARE (Colpopexy and Urinary Reduction Efforts) trial?
Because pelvic organ prolapse and urinary incontinence are already major problems facing women as they age, and will become even more pervasive as the baby boomer generation moves through menopause and beyond.
Because the risk that a woman will experience stress incontinence after prolapse surgery ranges from 8% to 60%.1-6
Because roughly one third of women who undergo prolapse or incontinence surgery require a second operation.
These are just a few of the factors that spurred the Pelvic Floor Disorders Network to undertake the CARE trial, published April 13 in the New England Journal of Medicine. OBG Management convened a panel of experts in female pelvic medicine, including 2 CARE trial investigators, to discuss the findings of this landmark study, its long-term implications, and the future of research into pelvic floor disorders.
How the trial was conducted
The CARE trial involved 322 women who required surgery to correct pelvic organ prolapse (POP) but lacked symptoms of stress urinary incontinence. All these women underwent sacrocolpopexy, an abdominal procedure in which graft material is attached between the vagina and sacrum to support the vagina and correct the prolapse. These women were randomized to undergo Burch colposuspension at the time of the sacrocolpopexy, or to undergo sacrocolpopexy only. The Burch procedure is performed through the same incision as the sacrocolpopexy and involves suturing the periurethral vaginal tissue to the iliopectineal ligaments on each side, providing urethral support.
Enrollment in the trial was halted after the first of 2 planned interim analyses because the frequency of postoperative stress incontinence was significantly lower in the group undergoing Burch colposuspension: 23.8% and 44.1% of women in the Burch and no-Burch groups, respectively, experienced stress symptoms by 3 months after the surgery.
Why the CARE trial is an epochal event
- First randomized trial of preventive incontinence surgery in women with prolapse
- Randomized design establishes cause and effect
- Subjects will be followed for 2 years
BRUBAKER: First, it is a well-designed, randomized, controlled trial and thus provides the highest level of evidence for clinical practice. Although there is no perfect study, this one minimized the risk of bias by involving multiple centers (7) and using multiple surgeons, making the findings more generalizable than would be the case in a single-surgeon case series.
In addition, the use of blinded urodynamic testing lent strength, because the ability of urodynamic testing to predict the need for a concomitant continence procedure was not known before the trial. Our follow-up manuscript, containing data presented at the recent Society of Gynecologic Surgeons meeting, will provide more details on this aspect of the trial.
WEBER: Randomized trials are held in such high esteem—provided all other aspects of study design and implementation are performed properly—because they support conclusions of cause and effect. The conclusion that Burch colposuspension prevents stress incontinence when performed at the time of abdominal sacrocolpopexy could only be drawn from a randomized trial.
Trial design standardized key elements
Many types of bias confound the results of nonrandomized studies, particularly selection bias (eg, when surgeons select which procedure to perform on the basis of patient characteristics), and valid conclusions of cause and effect cannot be drawn. However, with a randomized trial, subjects are separated into groups by chance and no other factor. Thus, the groups are equivalent at baseline—provided the sample size is large enough (and allowing for random differences)—and therefore any changes measured after the experimental intervention can be confidently attributed to the intervention itself.
Another strength of the trial is standardization. The subjects were “standardized” by rather broad inclusion and exclusion criteria to constitute an important clinical population and to ensure they were sufficiently similar so that the treatment (abdominal sacrocolpopexy) was appropriate for all. In addition, surgeons at the multiple participating sites agreed to standardization of the technical details of the Burch colposuspension so that the subjects received the same intervention regardless of site. And data collection in follow-up was performed in a standard way by research staff who were blinded to the subjects’ group assignment (intervention versus control), so the data were as free of bias as possible.
Homogeneous study population may be a weakness
KOHLI: I agree that the methodology of this well-designed study is its major strength. What are its weaknesses?
WEBER: No doubt there are several, only some of which may be apparent at this time. For example, most women in the study were Caucasian, and very few were Hispanic, Asian, or black. Although we have no scientific reason to believe that Burch colposuspension has different responses in women of different racial and ethnic backgrounds, the trial’s subjects are not diverse enough to analyze the data by subgroups to confirm or refute the hypothesis that response to the Burch procedure is independent of race or ethnicity.
BRUBAKER: Another weakness: Because this study was closed after the first interim analysis, some of our secondary analyses will be underpowered, although we clearly demonstrated a difference in our primary endpoint.
It is important to remember that this study is not “finished.” Our participants are still in active follow-up for 2 years following surgery. It will be interesting to see what happens during the longer follow-up, especially with regard to prolapse and incontinence. We are also doing additional in-depth analyses of urodynamic and other parameters.
KOHLI: Again, I think the study design and analysis were well thought out. It would have been interesting to see how the results broke down according to site, to see if there was variation—which could indicate variation in surgical technique.
BRUBAKER: We have not done this analysis and do not plan to at this time.
Why paravaginal repairs were allowed
KOHLI: What about the decision to include surgeries that involved paravaginal repair?
WEBER: That generated a fair amount of discussion during trial design, as there was no clear “right” answer. Perhaps it would have been “cleaner” to eliminate the option of performing paravaginal repair, but when the trial was designed, we lacked unequivocal evidence that paravaginal repair at the time of abdominal sacrocolpopexy provides additional support for the anterior vagina. Therefore, we decided to allow the decision to be based on surgeon judgment.
Some surgeons perform paravaginal repair with abdominal sacrocolpopexy in almost all women because they believe quite strongly that this reduces the risk of recurrent anterior vaginal prolapse. Others never perform paravaginal repair with abdominal sacrocolpopexy and feel just as strongly that their patients are adequately treated and protected from subsequent anterior vaginal prolapse.
Investigators feared paravaginal repairs could dilute Burch effects
Study surgeons did agree that paravaginal repair reduces the likelihood of postoperative stress incontinence, although not as effectively as Burch colposuspension. Thus, our dilemma: If paravaginal repairs were performed in a large number of subjects, thereby improving their postoperative continence status regardless of whether Burch was performed, the effect of Burch could be so diluted as to be lost. On the other hand, if paravaginal repairs were completely excluded, that would restrict some surgeons’ practices and potentially reduce the number of women who would be offered participation in the study if their surgeons felt their anterior vaginal prolapse would be potentially undertreated.
We resolved the dilemma as follows:
- A relatively low proportion—about one quarter—of surgeons performed paravaginal repairs regularly with abdominal sacrocolpopexy, so the potential impact in the trial would not be great.
- Paravaginal repairs were allowed, but only when declared necessary by the surgeon before randomization; this step prevented surgeons from changing their minds about the necessity of paravaginal repair if the subject was assigned to the Burch group (ie, the woman would be receiving additional anterior vaginal support by way of the Burch).
- We stratified for paravaginal repair in the randomization, so women with paravaginal repair were equally distributed between the intervention and control groups.
Are subjective or objective measures better?
- Subjective measures convey a patient’s foremost concerns and how she is doing clinically
- Correlating symptoms with objective measures yields valuable insights into treatment
BRUBAKER: I prefer subjective measures because I think they reflect what is most important to patients in quality-of-life disorders. However, I believe we need to understand the relationship between subjective outcomes and traditional “objective” outcomes.
WEBER: I think the research community is reaching a consensus that “subjective” measures—better described as patient-oriented outcomes—are more important than objective measures, particularly for conditions that affect patients in “subjective” ways, ie, ways that affect their health-related quality of life, rather than quantity of life. This does not mean that objective measures are useless—although we should first evaluate each measure critically to make that determination on the basis of evidence.
Nevertheless, when a patient seeks and receives treatment based on symptoms and how those symptoms impact her daily life, I think it is incumbent upon researchers and clinicians to ensure that the treatment that is considered most effective actually results in a change that the patient finds worthwhile.
What is “success”?
WALTERS: When it comes to incontinence, for which there is an imperfect correlation between various objective and subjective measures, I think both types of measures are valuable and important. Gathering several different types of outcomes for each patient helps us better understand the nuances of how well an intervention works.
I can understand why some clinicians and researchers place greater reliance on subjective measures of incontinence, such as a diary of incontinence episodes and quality-of-life measures, because these measures tell us exactly how the patient is doing clinically and how she feels about the intervention. If she reports that she is completely cured and “perfect,” then objective measures are irrelevant. However, for any subjective outcome short of perfect, correlation with the objective measures such as cough stress test, physical examination, and urodynamic tests can help investigators understand the reason for the imperfect outcome and point to areas of possible improvement.
KOHLI: In my practice, some women who continue to leak slightly after an incontinence procedure consider their surgery a complete success, whereas, as a surgeon, I consider it a suboptimal result. Both objective and subjective results are important.
Putting the CARE trial into practice
- Data relate directly only to women undergoing abdominal sacrocolpopexy
- Patient education, medicolegal, and reimbursement may also relate
- Results reflect the high prevalence of pelvic floor disorders and the need to routinely ask about them
BRUBAKER: I routinely counsel patients who are planning sacrocolpopexy but who do not have stress incontinence to consider a concomitant Burch procedure. I do not have them undergo urodynamic testing because, at this time, the results of that testing would not change my clinical practice.
WALTERS: I have always been liberal when it comes to adding retropubic colposuspension to abdominal sacrocolpopexy, even in women who do not have preoperative stress incontinence. The reason? Patients who are continent preoperatively, but become stress-incontinent postoperatively, are particularly unhappy with their outcome, especially if they need another surgery within a year to treat the stress incontinence. So this study verified what I was already doing.
What I didn’t learn is whether a paravaginal defect repair helps or hurts the Burch procedure from an anatomic and functional perspective.
It also appears that preoperative urodynamic testing has little value, although that was not the point of this study. I am glad it will be addressed in future studies.
KOHLI: I think the findings apply to those select patients undergoing abdominal sacrocolpopexy for prolapse. It would be dangerous to extrapolate these results to other abdominal vault suspension procedures or vaginal prolapse procedures. Based on the CARE trial, I plan to counsel patients about the risks and benefits of “optional” Burch colposuspension at the time of planned sacrocolpopexy. In reality, however, I have almost completely switched to minimally invasive midurethral slings, even in the case of abdominal prolapse procedures, because of their high cure rates, low complication rates, and ease of postoperative adjustment.
Clinical implications depend on surgeon’s routine
KOHLI: What are the implications for the majority of ObGyns?
WEBER: It depends on what ObGyns are doing for women with prolapse.
For ObGyns who are confident and competent, through training and experience, to perform abdominal sacrocolpopexy for women with advanced prolapse, the CARE trial results have a direct effect. Women with prolapse who are stress continent with no contraindications, can be reassured that they will benefit from a 50% reduction of postoperative stress incontinence with the Burch procedure.
For ObGyns who do not perform sacrocolpopexy, the CARE trial will have no direct clinical effects. Nevertheless, these clinicians need to be aware of the findings so they can discuss the options with patients before decisions on route or type of prolapse surgery are made.
The CARE trial and its results remind us of the high prevalence of pelvic floor disorders in women, potentially even after corrective surgery—and the need to actively screen all women for pelvic dysfunction.
Warn of potential incontinence even with the Burch
KOHLI: How does this study affect counseling of candidates for prolapse surgery?
BRUBAKER: I would offer stress-continent women a Burch procedure at the time of sacrocolpopexy. That much is clear. The interesting discussions come from “similar” clinical scenarios, where data are not yet available. For example, should a stress-continent woman facing a suspension via the vaginal route undergo a concomitant continence procedure?
WEBER: It is important to keep in mind that even when Burch colposuspension was performed, a number of women still experienced urinary incontinence (some stress, some urge, some mixed) after surgery; and the vast majority of women have urinary symptoms of some kind both before and after surgery. So preoperative counseling should include the information that urinary symptoms are very common after abdominal sacrocolpopexy—some as persistent or recurrent, and some as new symptoms.
As longer follow-up data from the CARE trial become available, we will learn how many women have urinary symptoms that are temporary versus long-lasting.
Is routine Burch best?
- When not all women benefit, should a procedure be offered prophylactically? In this case, experts say, “Yes”
- Some physicians favor other incontinence procedures
- Final decision rests with the patient
WALTERS: It is an easy decision for me. As I said earlier, women are particularly unhappy if they go from continent to incontinent after a surgery. In fact, some women are more displeased with that outcome than with a failure of the prolapse surgery. Because most women with prolapse have substantial anterior vaginal wall prolapse, the Burch procedure—with or without a paravaginal defect repair—also serves as part of the prolapse repair of the anterior wall. And now we know it also improves postoperative urinary function.
BRUBAKER: Doing a Burch procedure at the time of sacrocolpopexy is a time-efficient and low-morbidity addition, so it is worthwhile for me and my patients. It is clearly not the same as doing a secondary, standalone procedure for new symptoms.
Over the next 2 years, we will see how many women who were moderately or greatly bothered by stress incontinence went on to a surgical treatment.
WEBER: Please note a careful distinction: I would be willing to recommend Burch colposuspension to 100 stress-continent women who are planning to undergo abdominal sacrocolpopexy for prolapse, with the expectation that this will prevent postoperative stress incontinence in roughly half the women who would have developed it otherwise. It is up to the patient to accept this recommendation or not.
Based on the CARE trial results, 44 of 100 previously continent women after only abdominal sacrocolpopexy develop postoperative stress incontinence, compared with about 24 of 100 women after Burch and abdominal sacrocolpopexy. Even more striking is the difference in women affected by bothersome stress incontinence: almost 25% in the control group versus 6% in the Burch group. Women in the Burch group did not experience an excess of adverse events, or a clinically significant difference in operative time or estimated blood loss, compared with women in the control group.
Is “wait and see” better?
KOHLI: Because I favor minimally invasive midurethral sling procedures, which can often be performed on an outpatient basis under local anesthesia, I counsel women undergoing prolapse surgery via an abdominal or vaginal route that it is best to treat the incontinence postoperatively if it occurs. Obviously, this applies to women who have no incontinence and do not demonstrate potential stress incontinence on urodynamic testing preoperatively.
Anecdotally, I have not found a high rate of new-onset urinary incontinence following prolapse procedures. We may retrospectively look at these patients more critically in light of this new data.
WALTERS: I think most women would be dissatisfied with a 44% risk of postoperative stress incontinence requiring a second surgery. Even if you counseled them appropriately, many women would ask that you try to manage everything at the first surgery.
What are nonclinical effects of the trial?
- Need for patient and physician education is great, and the CARE trial offers a valuable opportunity
- Potential for medicolegal risk if complications develop
- Payers may not be willing to reimburse for a prophylactic procedure
WEBER: Given how extensively the trial’s results were disseminated by the lay press, I think we have an important opportunity to educate both patients and health-care providers.
First, patients: For women who may be directly affected by the trial, knowledgeable clinicians should explain its results and limitations to help them reach a decision about their treatment.
For women who hear the trial’s results described incompletely or incorrectly (eg, “…2 stitches prevent incontinence…”), clinicians should take this opportunity to correct misunderstandings and educate women about incontinence, prolapse, and pelvic health in general.
For health-care providers, this trial reminds us of the extraordinarily high prevalence of pelvic floor disorders. Although current treatments are not perfect, virtually all women with pelvic floor disorders can be treated to substantially alleviate, if not eliminate, bothersome symptoms.
All clinicians should routinely inquire about pelvic symptoms and be prepared to initiate an evaluation or provide a referral.
Medicolegal fallout?
KOHLI: There is the question of medicolegal risk if complications occur after colposuspension when the patient had no complaints or evidence of stress incontinence at the time of preoperative urodynamic testing. I am not aware of any legal precedent in which a clinical study or data provided solid protection from a jury verdict. The study did not show increased risk or complication with the addition of the Burch procedure, but that may not be true for some surgeons and some patients.
Billing and coding
In terms of billing, how should we code for the Burch colposuspension when the patient had no demonstrable stress incontinence? Payment denials in this scenario seem likely. This may create a line of separation between what may be clinically indicated for the patient and what insurance companies are willing to pay for.
There may be the option to use the urethral hypermobility code (ICD 599.81) for the Burch colposuspension, but only time will tell if this will be reimbursed. I would be curious to hear the panel’s experience with reimbursement for a prophylactic procedure based on scientific data. Obviously, what is best for the patient is most important.
BRUBAKER: All these patients had urethral hypermobility, which is also an indication for a Burch colposuspension.
Is preoperative urodynamic testing useful?
- CARE trial data still to come
- Basic testing is probably helpful
WEBER: In the CARE trial, the relative level of protection from postoperative stress incontinence provided by the Burch procedure did not depend on the stress-test component (with prolapse reduced) of preoperative urodynamic testing. About 50% fewer women had postoperative stress incontinence after Burch, whether preoperative urodynamic testing showed positive or negative stress tests with prolapse reduction.
Although subsequent analyses (presented at the Society of Gynecologic Surgeons 2006 meeting; manuscript under review) focused on urodynamic testing and postoperative outcomes, the CARE trial was not designed primarily to determine whether women planning prolapse surgery benefit from preoperative urodynamic testing. Therefore, conclusions about the “need” for urodynamic testing should not be based only on the CARE trial.
Should urodynamic testing determine treatment?
Randomized trials that directly address the cost-benefit of urodynamic testing are urgently needed. For now, as is standard in good clinical practice, a test should be performed only if results will change recommendations or provide reliable and clinically important prognostic information about a patient’s outcome after intervention. Clinicians should determine whether urodynamic testing meets even 1 of these 2 minimum criteria.
I want to point out that the CARE trial did not address the utility of urodynamic testing.
BRUBAKER: It is clear that some women have stress incontinence despite the concomitant Burch colposuspension. If we learn that an alternative operation can perform better and that any urodynamic (or other clinical measure) can predict improved outcomes, I would consider resuming urodynamic testing.
WALTERS: At first glance, it appears that complex urodynamic testing is definitely not necessary if the goal is to improve outcomes of surgery. However, I believe the patient should undergo some components of urodynamic testing such as a void with a post-void residual urine volume and a basic bladder-filling study noting sensation and capacity. I also do a cough stress test with the prolapse reduced, although we may find that this does not predict postoperative function.
KOHLI: The results of this study are very procedure-specific. If similar results are borne out when other approaches to prolapse and incontinence are analyzed, the value and utility of preoperative uro-dynamic testing in all patients may be questionable.
However, in my practice, I use the results of preoperative urodynamic testing not only for diagnosis, but also to make subtle adjustments when performing incontinence procedures—especially in regard to suburethral slings.
What if you prefer midurethral slings?
- Surgeons should be comfortable with more than 1 incontinence procedure
- We should not jump to untested conclusions
WEBER: Ideally, well trained and experienced gynecologic, urologic, or urogynecologic surgeons perform more than 1 type of incontinence procedure, to meet the needs of different patients.
As yet, we have no direct, evidence-based answers to issues such as these:
Can a midurethral sling be substituted for a Burch colposuspension and have the same average results in preventing post-operative stress incontinence without increasing urgency symptoms…
- …when abdominal sacrocolpopexy is performed for prolapse in a preoperatively stress-continent patient?
- …when vaginal apical suspension is performed for prolapse in a preoperatively stress-continent patient?
Although it is tempting to jump 1 or 2 steps ahead and apply CARE trial data to situations that have not been tested directly, I would be cautious. We want to avoid creating long-lasting or refractory urgency symptoms—especially in a woman who had no such symptoms before surgery—because of a prophylactic procedure.
I think this is especially true because it is relatively easy to salvage patients who do develop bothersome stress incontinence after prolapse surgery.
Bonus: Burch helps anterior vaginal prolapse
WALTERS: I wonder whether prophylactic placement of a midurethral sling would yield the same results as a prophylactic Burch procedure. If your midurethral sling of choice is a tension-free vaginal tape (TVT), I would be cautious about placing it prophylactically, because the TVT has a 2% to 3% risk of prolonged voiding dysfunction requiring transection of the tape.
However, it is possible that prophylactic placement of a transobturator sling, which is associated with much less voiding dysfunction and fewer major surgical complications, might have a different outcome—though this requires further study.
In addition, midurethral slings would not be as effective as Burch colposuspension in treating anterior vaginal prolapse, so I would expect to see more anterior wall prolapse failures if slings replaced colposuspension.
What if you prefer the vaginal approach?
- Further study is needed
WALTERS: I wonder whether a prophylactic transobturator midurethral sling at the time of transvaginal prolapse repair would yield similar results. I do cystocele repair with suburethral (“Kelly”) plication, which seems to work well at stabilizing the urethra in women without stress incontinence. But this approach is not as popular these days, and future studies may demonstrate that a prophylactic midurethral sling will result in better long-term function without significantly increasing the long-term risk.
WEBER: The CARE trial results are relevant to all pelvic surgeons because they demonstrate the need for and benefit from well-designed randomized surgical trials. Another benefit will be extended follow-up in what will become a prospective cohort study of women with advanced prolapse treated by abdominal sacrocolpopexy—providing higher-quality evidence than retrospective case series. Although not as valuable as randomized trials, these data can help guide clinical recommendations.
If long-term results support the effectiveness and durability of abdominal prolapse repair, then gynecologists can reflect on the evidence and choose the approach that best fits the patient’s needs.
Need for other studies?
- Randomized, multicenter trials addressing almost any surgical treatment of prolapse and incontinence are sorely needed
BRUBAKER: Any and all high-quality, well-designed trial can improve our care of women with incontinence and/or prolapse.
WALTERS: I look forward to the follow-up studies from the CARE trial on the value of paravaginal defect repair, preoperative urodynamic testing, and the efficacy of various prolapse-reduction maneuvers in predicting surgical outcomes.
It would also seem logical to repeat this type of study using transvaginal prolapse repair with or without a prophylactic midurethral sling. Another option: anterior colporrhaphy with suburethral plication versus a prophylactic midurethral sling.
KOHLI: I look forward to data on surgical procedures currently being performed with greater frequency despite a lack of good-quality data. These include the transobturator suburethral midurethral sling procedures, laparoscopic sacrocolpopexy, and vaginal mesh augmentation for prolapse.
The Pelvic Floor Disorders Network affords a unique opportunity to perform well-designed multicenter trials to address the rapidly changing landscape of surgical treatment for prolapse and incontinence.
1. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
2. Cross CA, Cespedes RD, McGuire EJ. Treatment results using pubovaginal slings in patients with large cystoceles and stress incontinence. J Urol. 1997;158:431-434.
3. FitzGerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189:1241-1244.
4. Klutke JJ, Ramos S. Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol. 2000;182:1378-1381.
5. Meschia M, Pifarotti P, Spennacchio M, Buonaguidi A, Gattei U, Somigliana E. A randomized comparison of tension-free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. Am J Obstet Gynecol. 2004;190:609-613.
6. Gordon D, Gold RS, Pauzner D, Lessing JB, Groutz A. Combined genitourinary prolapse repair and prophylactic tension-free vaginal tape in women with severe prolapse and occult stress urinary incontinence: preliminary results. Urology. 2001;58:547-550.
Dr. Brubaker, Dr. Kohli, and Dr. Weber report no financial relationships relevant to this article. Dr. Walters is a speaker for American Medical Systems.
1. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
2. Cross CA, Cespedes RD, McGuire EJ. Treatment results using pubovaginal slings in patients with large cystoceles and stress incontinence. J Urol. 1997;158:431-434.
3. FitzGerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189:1241-1244.
4. Klutke JJ, Ramos S. Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol. 2000;182:1378-1381.
5. Meschia M, Pifarotti P, Spennacchio M, Buonaguidi A, Gattei U, Somigliana E. A randomized comparison of tension-free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. Am J Obstet Gynecol. 2004;190:609-613.
6. Gordon D, Gold RS, Pauzner D, Lessing JB, Groutz A. Combined genitourinary prolapse repair and prophylactic tension-free vaginal tape in women with severe prolapse and occult stress urinary incontinence: preliminary results. Urology. 2001;58:547-550.
Dr. Brubaker, Dr. Kohli, and Dr. Weber report no financial relationships relevant to this article. Dr. Walters is a speaker for American Medical Systems.
Cystocele and rectocele repair: More success with mesh?
CASE Symptoms point to yet another prolapse recurrence
A 52-year-old woman presents with a bulge and pressure in her vagina. She has undergone 2 prior reconstructive surgeries. The first was a vaginal hysterectomy, anterior and posterior repair, and sling; the second was an abdominal procedure that included a sacrocolpopexy and paravaginal repair.
A physical examination reveals a recurrent 4th-degree cystocele that protrudes 2 cm beyond the hymenal ring. The vault and posterior compartment are well supported, and the patient reports no incontinence, a fact confirmed by urodynamics testing. She asks that you do everything in your power to prevent further recurrence.
How do you proceed?
This patient ultimately underwent anterior colporrhaphy and vaginal paravaginal repair using a decellularized dermal cadaveric implant. She was still doing well 1 year later, with no recurrence.
Despite success stories like this one, the use of graft materials to repair cystoceles and rectoceles is controversial. One reason is the difficulty of interpreting published data, since studies lack uniformity in technique, patient characteristics, graft shape, type of material, attachment sites, and duration of follow-up. Level I evidence that augmented repairs have a clear benefit over traditional repairs is sparse.
Advocates of graft materials argue that native tissue is already compromised—hence, the prolapse—making surgical failure likely.1 They claim graft materials help strengthen repairs, especially in the case of cystoceles. They also point out that adjuvant materials have been used in burns, plastic surgery, and orthopedics for more than 10 years and are generally well tolerated. Their success in hernia repairs prompted their consideration for the pelvic floor.
A pervasive problem, but only 10% to 20% seek help
Roughly 1 of 2 parous women lose pelvic support as they age, but only 10% to 20% seek medical care, with a lifetime risk of surgery for pelvic organ prolapse (POP) of 11% by age 80.2
With women living longer than ever and remaining active later in life, this percentage is likely to rise. Unfortunately, few alternatives to surgical treatment exist, and the reoperation rate for recurrence is 29%, according to a 1995 review.2 If surgical management is the only hope of cure, how can we lower the 29% recurrence rate?
Graft materials may provide part or all of the solution.
Elements of prolapse
Anterior compartment
Central and/or lateral defects can occur in the anterior compartment.
Lateral (paravaginal) defects indicate that the endopelvic connective tissue has separated from the arcus tendineus fascia pelvis. Lateral defects can be repaired vaginally or abdominally.
One study3 found that 67% of women with anterior wall prolapse had paravaginal defects, but no randomized trials have evaluated the clinical benefit of repairing these defects, compared with traditional colporrhaphies.
Central defects involve site-specific defects and/or general attenuation of the endopelvic connective tissue. These are usually repaired vaginally.
Recurrence rates for lateral and central defects range from 3% to 70%.4-8
Two large series of vaginal paravaginal repairs noted the following recurrence rates:
- Shull et al6 found a recurrence rate of 7% to the hymenal ring or beyond.
- Young et al7 observed a recurrence rate for lateral defects of 2%, with recurrence rates as high as 22% for central defects.
In a comparison of 3 techniques for vaginal repair of central defects, using strict criteria to assess anatomic outcomes, Weber et al4 found recurrence rates of 54% to 70%. Other studies show symptomatic recurrence rates of 3% to 22% for cystoceles.5,8
With grafts, both paravaginal and central defects can be repaired. Vaginal paravaginal repairs are not popular due to the technical difficulty involved. With the use of grafts, however, both paravaginal and central defects can be addressed simultaneously with relative ease.
Posterior compartment
Defects in the posterior compartment are less likely to recur. Reported success rates range from 80% to 90%.9,10
Posterior compartment defects include general attenuation of Denonvillier’s fascia or a tear anywhere along the fascia or any of its attachments.
Recurrence rates. Site-specific repairs are thought to minimize complications such as dyspareunia. However, few studies have compared the efficacy of site-specific repairs with that of traditional colporrhaphies. At our institution, women who underwent traditional colporrhaphy had fewer recurrences than controls (33% vs 14%), with no differences in postoperative symptoms such as dyspareunia, constipation, and fecal incontinence.11
Graft materials of questionable benefit. In the posterior compartment, these materials have not been shown to be beneficial, compared with traditional or site-specific repairs. Sand et al12 found no benefit for repairs in which absorbable Vicryl mesh was imbricated, but this randomized trial may have lacked sufficient power to show statistical significance. Large cohorts would be needed to show significant benefit of meshes in the posterior compartment.
A complex web of support
In the normal pelvis, support of reproductive organs depends on a complex web of muscles, fascia, and connective tissue. To ensure success, prolapse repairs should correct any separation or attenuation of tissue and preserve or enhance tissue resilience.
Risk factors for recurrent prolapse
- Poor tissue (assess tissue quality before and during surgery)
- Impaired healing
- Chronic increases in intraabdominal pressure due to obstructive pulmonary disease, asthma, or constipation
- High-grade cystocele
- Age 60 or above13
Patients with these conditions may benefit from the use of adjuvant materials in the anterior compartment.
Note that women who have had recurrences after earlier repairs may experience repeat recurrence.
Advantages of grafts
Using graft materials, the surgeon can repair all vaginal defects faster and with less effort. In the anterior compartment, a graft can be placed and anchored bilaterally from arcus to arcus tendineus, and posteriorly to the level of the spine, recreating level I support. Graft materials also offer the potential to treat stress urinary incontinence concomitantly using different shaped materials. Two authors have already described their success performing this type of repair.14
Nevertheless, great care and consideration should be devoted to actual and theoretical short- and long-term risks, many of which have not been fully elucidated.
Once a successful material is identified or developed, it may decrease operating time and morbidity in vaginal surgeries. It may also reduce the higher hospital costs normally associated with abdominal procedures.
Types of graft materials
There are 2 types of materials: synthetic or biologic. Synthetic materials can be further classified into permanent or absorbable.
The most widely used biologic materials include allografts such as human freeze-dried or solvent-dehydrated fascia lata (Tutoplast), decellularized human cadaveric dermis (Alloderm, Repliform), porcine dermal xenografts such as Pelvicol or Intexene, and bovine pericardial implants (Veritas).
Soft polypropylene meshes such as Gynemesh and Atrium are commonly used permanent materials, and polyglactin 910 is an absorbable material (TABLE).
TABLE
How successful are adjuvant materials in cystocele and rectocele repairs?
| MATERIAL (SIZE IN CM) | AUTHOR | NO. IN STUDY | RECURRENCE RATE (%) | SITE OF ATTACHMENT | FOLLOW-UP (MONTHS) | COMPLICATIONS |
|---|---|---|---|---|---|---|
| BIOLOGIC MATERIALS | ||||||
| Alloderm 3×7 patch with concomitant sling | Chung29 | 19 | 16 | Pubocervical fascia | 28 | None |
| Intexene 6×8 with sling | Gomelsky et al 200420 | 70 | 9 stage II 4 stage III | Arcus tendineus fascia pelvis | 24 | 1 wound separation |
| Solvent-dehydrated cadaveric fascia lata patch with sling | Gandhi et al 200521 | 76 patch vs 72 no patch | 21 vs 29, respectively (P=.23) | Overlay | 13 | None |
| Alloderm 3×7 trapezoid | Clemons et al 200322 | 33 | 41 stage II 3 symptomatic | Arcus tendineus fascia pelvis | 18 | None |
| SYNTHETIC MATERIALS WITH CONCOMITANT SLINGS* | ||||||
| Marlex 10×3×5 | Nicita 199823 | 44 | 0 | Arcus tendineus fascia pelvis | 13 | 1 vaginal erosion |
| Polyglactin 910 absorbable mesh | Sand et al12 | 80 mesh vs 80 no mesh | 25 vs 43 stage II cystoceles, respectively(P=.02) | Insert in the anterior and posterior colporrhaphy suture line | 12 | None |
| Polyglactin 910 absorbable mesh | Weber et al4 | 26 with mesh + standard repair; 24 with ultra-lateral repair; 33 with standard repair | 58 vs 54 vs 70 stage II, respectively (P=.58) | Overlay | 23 | None |
| SYNTHETIC PERMANENT GRAFTS WITHOUT CONCOMITANT SLINGS | ||||||
| Marlex trapezoid | Julian 199619 | 12 with 12 without | 0 vs 33, respectively | Arcus tendineus fascia pelvis | 24 | 3 vaginal erosions |
| Mixed-fiber mesh (polyglactin 910 and polyester 5×5) | Migliari and Usai 199924 | 12 | 25 | Pubourethral and cardinal ligaments | 20 | None |
| Prolene (Atrium) | Dwyer and O’Reilly25 | 64 anterior 50 posterior | 6 grade II | Tension-free | 29 | 8% vaginal erosion 1 rectovaginal fistula |
| Gynemesh 6×15 | de Tayrac et al 200526 | 87 | 7 stage II 2 stage III | Tension-free | 24 | 8% vaginal erosion |
| Prolene mesh patch | Milani et al 200527 | 32 anterior 31 posterior | 6 stage II | Fixed to endopelvic connective tissue | 17 | 20% anterior, 63% posterior dyspareunia; 13% vaginal erosion (anterior); 1 pelvic abscess (posterior) |
| Prolene mesh (double-wing shape) | Natale et al 2000 28 | 138 | 3 | Tension-free | 18 | 9% vaginal erosion 7% dyspareunia 1 hematoma |
| *Absorbable and permanent. | ||||||
Classification of synthetic materials
- Type 1 grafts are totally macroporous (>75 μm), which allows fibroblast, macrophage, and collagen penetration with angiogenesis. Examples include Prolene and Marlex meshes.
- Type 2 mesh is microporous (<10 μm in 1 dimension). This prevents penetration of fibroblasts, macrophages, or collagen. Gore-Tex is an example of a Type 2 mesh.
- Type 3 mesh is macroporous (>75 μm) with multifilamentous or microporous components. Examples include Mersilene (braided Dacron mesh), Teflon (polytetrafluoroethylene [PTFE]), Surgipro (braided polypropylene mesh), and MycroMesh (perforated PTFE patch).
- Type 4 mesh has a submicron pore size that prevents penetration. Examples include Silastic, Cellgard (polypropylene sheeting), and Preclude pericardial membrane/Preclude dura-substitute.1
2 other important properties are composition of fibers (multifilamentous materials commonly have interstices less than 10 microns) and flexibility (which has a bearing on erosion of the material).1
Bacteria can penetrate pores smaller than 1 μm, whereas polymorphonuclear white blood cells and macrophages need a pore size larger than 10 μm, and capillary ingrowth requires a size larger than 75 microns. Thus, Type 1 offers the advantages of larger pore size and monofilamentous interstices to allow for capillary ingrowth.
Which material is best?
Although the literature is difficult to interpret because of the diversity of studies and other factors, some findings are worth noting:
- Tutoplast and Alloderm appear to have the best tensile strength, maximum load to capacity, and microscopic architecture similar to the original tissue.15-17 However, these qualities were documented prior to implantation in vivo.
- Slings appear to help prevent cystocele recurrences, according to a study by Goldberg et al.18
- A fascial patch had no benefit when placed as an overlay in the anterior compartment in a randomized, controlled trial (involving 162 women) by Sand et al.12
- Marlex. One group of women with recurrent prolapse underwent synthetic graft (Marlex) augmentation with bilateral ATFP attachment, while the other group had anterior colporrhaphy only.19 None of the women who received grafts had further recurrence, while 33% of the control group did. However, 25% of the women with the graft had vaginal erosions.
- Polyglactin 910 had a protective effect when embedded in the plication, according to Sand et al.12 However, it had no benefit when used as an overlay to a traditional repair in a study by Weber et al.4 The discrepancy may be related to small sample size; the study by Weber et al was powered to detect only a 30% difference. However, these studies suggest that it is not only the type of graft that is important, but how it is used or attached.
In general, synthetic grafts may have slightly higher success rates, whereas biologic materials appear to be better tolerated.
Prospective, comparative trials of these materials are desperately needed.
1. Cervigni M, Natale F. The use of synthetics in the treatment of pelvic organ prolapse. Curr Opin Urol. 2001;11:429-435.
2. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-573.
4. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
5. Porges RF, Smilen SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1526.
6. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1436.
7. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1366.
8. Macer GA. Transabdominal repair of cystocele, a 20-year experience, compared with the traditional vaginal approach. Trans Pac Coast Obstet Gynecol Soc. 1978;45:116-120.
9. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1451-1456.
10. Paraiso MF, Ballard LA, Walters MD, Lee JC, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1430.
11. Abramov Y, et al. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005;105:314-318.
12. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001;184:1357-1362.
13. Whitesides JL, Weber AM, Meyn LA, Walters MD. Risk factors for prolapse recurrence after vaginal repair. Obstet Gynecol Surv. 2005;60:164-165.
14. Kobashi KC, Mee SL, Leach GE. A new technique for cystocele repair and transvaginal sling: the cadaveric prolapse repair and sling (CAPS). Urology. 2000;56:9-14.
15. Lemer ML, Chaikin DC, Blaivas JG. Tissue strength analysis of autologous and cadaveric allografts for the pubovaginal sling. Neurourol Urodyn. 1999;18:497-503.
16. Choe JM, Kothandapani R, et al. Autologous, cadaveric, and synthetic materials used in sling surgery: comparative biomechanical analysis. Urology. 2001;58:482-486.
17. Scalfani AP. Biophysical and microscopic analysis of homologous dermal and fascial materials for facial aesthetic and reconstructive uses. Arch Facial Plast Surg. 2002;4:164-171.
18. Goldberg RP, et al. Protective effect of suburethral slings on postoperative cystocele recurrence after reconstructive pelvic operation. Am J Obstet Gynecol. 2001;185:1307-1312.
19. Julian TM. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
20. Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol. 2004;171:1581-1584.
21. Gandhi S, et al. A prospective randomized trial of solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005;192:1649-1654.
22. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618.
23. Nicita G. A new operation for genitourinary prolapse. J Urol. 1998;160:741-745.
24. Migliari R, Usai E. Treatment results using a mixed fiber mesh in patients with grade IV cystocele. J Urol. 1999;161:1255-1258.
25. Dwyer PL, O’Reilly BA. Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG. 2004;111:831-836.
26. de Tayrac R, Gervaise A, Chauveaud A, Fernandez H. Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med. 2005;50:75-80.
27. Milani R, et al. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. BJOG. 2005;112:107-111.
28. Natale F, Marziali S, Cervigni M. Tension-free cystocele repair (TCR): long-term follow-up. Proceedings of the 25th annual meeting of the International Urogynecological Association. 2000;22-25.
29. Chung SY, et al. Technique of combined pubovaginal sling and cystocele using a single piece of cadaveric dermal graft. Urology. 2002;59:538-541.
Dr. Botros has no financial relationships relevant to this article. Dr. Sand receives grant/research support from Boston Scientific, and is a consultant and speaker for American Medical Systems and Boston Scientific.
CASE Symptoms point to yet another prolapse recurrence
A 52-year-old woman presents with a bulge and pressure in her vagina. She has undergone 2 prior reconstructive surgeries. The first was a vaginal hysterectomy, anterior and posterior repair, and sling; the second was an abdominal procedure that included a sacrocolpopexy and paravaginal repair.
A physical examination reveals a recurrent 4th-degree cystocele that protrudes 2 cm beyond the hymenal ring. The vault and posterior compartment are well supported, and the patient reports no incontinence, a fact confirmed by urodynamics testing. She asks that you do everything in your power to prevent further recurrence.
How do you proceed?
This patient ultimately underwent anterior colporrhaphy and vaginal paravaginal repair using a decellularized dermal cadaveric implant. She was still doing well 1 year later, with no recurrence.
Despite success stories like this one, the use of graft materials to repair cystoceles and rectoceles is controversial. One reason is the difficulty of interpreting published data, since studies lack uniformity in technique, patient characteristics, graft shape, type of material, attachment sites, and duration of follow-up. Level I evidence that augmented repairs have a clear benefit over traditional repairs is sparse.
Advocates of graft materials argue that native tissue is already compromised—hence, the prolapse—making surgical failure likely.1 They claim graft materials help strengthen repairs, especially in the case of cystoceles. They also point out that adjuvant materials have been used in burns, plastic surgery, and orthopedics for more than 10 years and are generally well tolerated. Their success in hernia repairs prompted their consideration for the pelvic floor.
A pervasive problem, but only 10% to 20% seek help
Roughly 1 of 2 parous women lose pelvic support as they age, but only 10% to 20% seek medical care, with a lifetime risk of surgery for pelvic organ prolapse (POP) of 11% by age 80.2
With women living longer than ever and remaining active later in life, this percentage is likely to rise. Unfortunately, few alternatives to surgical treatment exist, and the reoperation rate for recurrence is 29%, according to a 1995 review.2 If surgical management is the only hope of cure, how can we lower the 29% recurrence rate?
Graft materials may provide part or all of the solution.
Elements of prolapse
Anterior compartment
Central and/or lateral defects can occur in the anterior compartment.
Lateral (paravaginal) defects indicate that the endopelvic connective tissue has separated from the arcus tendineus fascia pelvis. Lateral defects can be repaired vaginally or abdominally.
One study3 found that 67% of women with anterior wall prolapse had paravaginal defects, but no randomized trials have evaluated the clinical benefit of repairing these defects, compared with traditional colporrhaphies.
Central defects involve site-specific defects and/or general attenuation of the endopelvic connective tissue. These are usually repaired vaginally.
Recurrence rates for lateral and central defects range from 3% to 70%.4-8
Two large series of vaginal paravaginal repairs noted the following recurrence rates:
- Shull et al6 found a recurrence rate of 7% to the hymenal ring or beyond.
- Young et al7 observed a recurrence rate for lateral defects of 2%, with recurrence rates as high as 22% for central defects.
In a comparison of 3 techniques for vaginal repair of central defects, using strict criteria to assess anatomic outcomes, Weber et al4 found recurrence rates of 54% to 70%. Other studies show symptomatic recurrence rates of 3% to 22% for cystoceles.5,8
With grafts, both paravaginal and central defects can be repaired. Vaginal paravaginal repairs are not popular due to the technical difficulty involved. With the use of grafts, however, both paravaginal and central defects can be addressed simultaneously with relative ease.
Posterior compartment
Defects in the posterior compartment are less likely to recur. Reported success rates range from 80% to 90%.9,10
Posterior compartment defects include general attenuation of Denonvillier’s fascia or a tear anywhere along the fascia or any of its attachments.
Recurrence rates. Site-specific repairs are thought to minimize complications such as dyspareunia. However, few studies have compared the efficacy of site-specific repairs with that of traditional colporrhaphies. At our institution, women who underwent traditional colporrhaphy had fewer recurrences than controls (33% vs 14%), with no differences in postoperative symptoms such as dyspareunia, constipation, and fecal incontinence.11
Graft materials of questionable benefit. In the posterior compartment, these materials have not been shown to be beneficial, compared with traditional or site-specific repairs. Sand et al12 found no benefit for repairs in which absorbable Vicryl mesh was imbricated, but this randomized trial may have lacked sufficient power to show statistical significance. Large cohorts would be needed to show significant benefit of meshes in the posterior compartment.
A complex web of support
In the normal pelvis, support of reproductive organs depends on a complex web of muscles, fascia, and connective tissue. To ensure success, prolapse repairs should correct any separation or attenuation of tissue and preserve or enhance tissue resilience.
Risk factors for recurrent prolapse
- Poor tissue (assess tissue quality before and during surgery)
- Impaired healing
- Chronic increases in intraabdominal pressure due to obstructive pulmonary disease, asthma, or constipation
- High-grade cystocele
- Age 60 or above13
Patients with these conditions may benefit from the use of adjuvant materials in the anterior compartment.
Note that women who have had recurrences after earlier repairs may experience repeat recurrence.
Advantages of grafts
Using graft materials, the surgeon can repair all vaginal defects faster and with less effort. In the anterior compartment, a graft can be placed and anchored bilaterally from arcus to arcus tendineus, and posteriorly to the level of the spine, recreating level I support. Graft materials also offer the potential to treat stress urinary incontinence concomitantly using different shaped materials. Two authors have already described their success performing this type of repair.14
Nevertheless, great care and consideration should be devoted to actual and theoretical short- and long-term risks, many of which have not been fully elucidated.
Once a successful material is identified or developed, it may decrease operating time and morbidity in vaginal surgeries. It may also reduce the higher hospital costs normally associated with abdominal procedures.
Types of graft materials
There are 2 types of materials: synthetic or biologic. Synthetic materials can be further classified into permanent or absorbable.
The most widely used biologic materials include allografts such as human freeze-dried or solvent-dehydrated fascia lata (Tutoplast), decellularized human cadaveric dermis (Alloderm, Repliform), porcine dermal xenografts such as Pelvicol or Intexene, and bovine pericardial implants (Veritas).
Soft polypropylene meshes such as Gynemesh and Atrium are commonly used permanent materials, and polyglactin 910 is an absorbable material (TABLE).
TABLE
How successful are adjuvant materials in cystocele and rectocele repairs?
| MATERIAL (SIZE IN CM) | AUTHOR | NO. IN STUDY | RECURRENCE RATE (%) | SITE OF ATTACHMENT | FOLLOW-UP (MONTHS) | COMPLICATIONS |
|---|---|---|---|---|---|---|
| BIOLOGIC MATERIALS | ||||||
| Alloderm 3×7 patch with concomitant sling | Chung29 | 19 | 16 | Pubocervical fascia | 28 | None |
| Intexene 6×8 with sling | Gomelsky et al 200420 | 70 | 9 stage II 4 stage III | Arcus tendineus fascia pelvis | 24 | 1 wound separation |
| Solvent-dehydrated cadaveric fascia lata patch with sling | Gandhi et al 200521 | 76 patch vs 72 no patch | 21 vs 29, respectively (P=.23) | Overlay | 13 | None |
| Alloderm 3×7 trapezoid | Clemons et al 200322 | 33 | 41 stage II 3 symptomatic | Arcus tendineus fascia pelvis | 18 | None |
| SYNTHETIC MATERIALS WITH CONCOMITANT SLINGS* | ||||||
| Marlex 10×3×5 | Nicita 199823 | 44 | 0 | Arcus tendineus fascia pelvis | 13 | 1 vaginal erosion |
| Polyglactin 910 absorbable mesh | Sand et al12 | 80 mesh vs 80 no mesh | 25 vs 43 stage II cystoceles, respectively(P=.02) | Insert in the anterior and posterior colporrhaphy suture line | 12 | None |
| Polyglactin 910 absorbable mesh | Weber et al4 | 26 with mesh + standard repair; 24 with ultra-lateral repair; 33 with standard repair | 58 vs 54 vs 70 stage II, respectively (P=.58) | Overlay | 23 | None |
| SYNTHETIC PERMANENT GRAFTS WITHOUT CONCOMITANT SLINGS | ||||||
| Marlex trapezoid | Julian 199619 | 12 with 12 without | 0 vs 33, respectively | Arcus tendineus fascia pelvis | 24 | 3 vaginal erosions |
| Mixed-fiber mesh (polyglactin 910 and polyester 5×5) | Migliari and Usai 199924 | 12 | 25 | Pubourethral and cardinal ligaments | 20 | None |
| Prolene (Atrium) | Dwyer and O’Reilly25 | 64 anterior 50 posterior | 6 grade II | Tension-free | 29 | 8% vaginal erosion 1 rectovaginal fistula |
| Gynemesh 6×15 | de Tayrac et al 200526 | 87 | 7 stage II 2 stage III | Tension-free | 24 | 8% vaginal erosion |
| Prolene mesh patch | Milani et al 200527 | 32 anterior 31 posterior | 6 stage II | Fixed to endopelvic connective tissue | 17 | 20% anterior, 63% posterior dyspareunia; 13% vaginal erosion (anterior); 1 pelvic abscess (posterior) |
| Prolene mesh (double-wing shape) | Natale et al 2000 28 | 138 | 3 | Tension-free | 18 | 9% vaginal erosion 7% dyspareunia 1 hematoma |
| *Absorbable and permanent. | ||||||
Classification of synthetic materials
- Type 1 grafts are totally macroporous (>75 μm), which allows fibroblast, macrophage, and collagen penetration with angiogenesis. Examples include Prolene and Marlex meshes.
- Type 2 mesh is microporous (<10 μm in 1 dimension). This prevents penetration of fibroblasts, macrophages, or collagen. Gore-Tex is an example of a Type 2 mesh.
- Type 3 mesh is macroporous (>75 μm) with multifilamentous or microporous components. Examples include Mersilene (braided Dacron mesh), Teflon (polytetrafluoroethylene [PTFE]), Surgipro (braided polypropylene mesh), and MycroMesh (perforated PTFE patch).
- Type 4 mesh has a submicron pore size that prevents penetration. Examples include Silastic, Cellgard (polypropylene sheeting), and Preclude pericardial membrane/Preclude dura-substitute.1
2 other important properties are composition of fibers (multifilamentous materials commonly have interstices less than 10 microns) and flexibility (which has a bearing on erosion of the material).1
Bacteria can penetrate pores smaller than 1 μm, whereas polymorphonuclear white blood cells and macrophages need a pore size larger than 10 μm, and capillary ingrowth requires a size larger than 75 microns. Thus, Type 1 offers the advantages of larger pore size and monofilamentous interstices to allow for capillary ingrowth.
Which material is best?
Although the literature is difficult to interpret because of the diversity of studies and other factors, some findings are worth noting:
- Tutoplast and Alloderm appear to have the best tensile strength, maximum load to capacity, and microscopic architecture similar to the original tissue.15-17 However, these qualities were documented prior to implantation in vivo.
- Slings appear to help prevent cystocele recurrences, according to a study by Goldberg et al.18
- A fascial patch had no benefit when placed as an overlay in the anterior compartment in a randomized, controlled trial (involving 162 women) by Sand et al.12
- Marlex. One group of women with recurrent prolapse underwent synthetic graft (Marlex) augmentation with bilateral ATFP attachment, while the other group had anterior colporrhaphy only.19 None of the women who received grafts had further recurrence, while 33% of the control group did. However, 25% of the women with the graft had vaginal erosions.
- Polyglactin 910 had a protective effect when embedded in the plication, according to Sand et al.12 However, it had no benefit when used as an overlay to a traditional repair in a study by Weber et al.4 The discrepancy may be related to small sample size; the study by Weber et al was powered to detect only a 30% difference. However, these studies suggest that it is not only the type of graft that is important, but how it is used or attached.
In general, synthetic grafts may have slightly higher success rates, whereas biologic materials appear to be better tolerated.
Prospective, comparative trials of these materials are desperately needed.
CASE Symptoms point to yet another prolapse recurrence
A 52-year-old woman presents with a bulge and pressure in her vagina. She has undergone 2 prior reconstructive surgeries. The first was a vaginal hysterectomy, anterior and posterior repair, and sling; the second was an abdominal procedure that included a sacrocolpopexy and paravaginal repair.
A physical examination reveals a recurrent 4th-degree cystocele that protrudes 2 cm beyond the hymenal ring. The vault and posterior compartment are well supported, and the patient reports no incontinence, a fact confirmed by urodynamics testing. She asks that you do everything in your power to prevent further recurrence.
How do you proceed?
This patient ultimately underwent anterior colporrhaphy and vaginal paravaginal repair using a decellularized dermal cadaveric implant. She was still doing well 1 year later, with no recurrence.
Despite success stories like this one, the use of graft materials to repair cystoceles and rectoceles is controversial. One reason is the difficulty of interpreting published data, since studies lack uniformity in technique, patient characteristics, graft shape, type of material, attachment sites, and duration of follow-up. Level I evidence that augmented repairs have a clear benefit over traditional repairs is sparse.
Advocates of graft materials argue that native tissue is already compromised—hence, the prolapse—making surgical failure likely.1 They claim graft materials help strengthen repairs, especially in the case of cystoceles. They also point out that adjuvant materials have been used in burns, plastic surgery, and orthopedics for more than 10 years and are generally well tolerated. Their success in hernia repairs prompted their consideration for the pelvic floor.
A pervasive problem, but only 10% to 20% seek help
Roughly 1 of 2 parous women lose pelvic support as they age, but only 10% to 20% seek medical care, with a lifetime risk of surgery for pelvic organ prolapse (POP) of 11% by age 80.2
With women living longer than ever and remaining active later in life, this percentage is likely to rise. Unfortunately, few alternatives to surgical treatment exist, and the reoperation rate for recurrence is 29%, according to a 1995 review.2 If surgical management is the only hope of cure, how can we lower the 29% recurrence rate?
Graft materials may provide part or all of the solution.
Elements of prolapse
Anterior compartment
Central and/or lateral defects can occur in the anterior compartment.
Lateral (paravaginal) defects indicate that the endopelvic connective tissue has separated from the arcus tendineus fascia pelvis. Lateral defects can be repaired vaginally or abdominally.
One study3 found that 67% of women with anterior wall prolapse had paravaginal defects, but no randomized trials have evaluated the clinical benefit of repairing these defects, compared with traditional colporrhaphies.
Central defects involve site-specific defects and/or general attenuation of the endopelvic connective tissue. These are usually repaired vaginally.
Recurrence rates for lateral and central defects range from 3% to 70%.4-8
Two large series of vaginal paravaginal repairs noted the following recurrence rates:
- Shull et al6 found a recurrence rate of 7% to the hymenal ring or beyond.
- Young et al7 observed a recurrence rate for lateral defects of 2%, with recurrence rates as high as 22% for central defects.
In a comparison of 3 techniques for vaginal repair of central defects, using strict criteria to assess anatomic outcomes, Weber et al4 found recurrence rates of 54% to 70%. Other studies show symptomatic recurrence rates of 3% to 22% for cystoceles.5,8
With grafts, both paravaginal and central defects can be repaired. Vaginal paravaginal repairs are not popular due to the technical difficulty involved. With the use of grafts, however, both paravaginal and central defects can be addressed simultaneously with relative ease.
Posterior compartment
Defects in the posterior compartment are less likely to recur. Reported success rates range from 80% to 90%.9,10
Posterior compartment defects include general attenuation of Denonvillier’s fascia or a tear anywhere along the fascia or any of its attachments.
Recurrence rates. Site-specific repairs are thought to minimize complications such as dyspareunia. However, few studies have compared the efficacy of site-specific repairs with that of traditional colporrhaphies. At our institution, women who underwent traditional colporrhaphy had fewer recurrences than controls (33% vs 14%), with no differences in postoperative symptoms such as dyspareunia, constipation, and fecal incontinence.11
Graft materials of questionable benefit. In the posterior compartment, these materials have not been shown to be beneficial, compared with traditional or site-specific repairs. Sand et al12 found no benefit for repairs in which absorbable Vicryl mesh was imbricated, but this randomized trial may have lacked sufficient power to show statistical significance. Large cohorts would be needed to show significant benefit of meshes in the posterior compartment.
A complex web of support
In the normal pelvis, support of reproductive organs depends on a complex web of muscles, fascia, and connective tissue. To ensure success, prolapse repairs should correct any separation or attenuation of tissue and preserve or enhance tissue resilience.
Risk factors for recurrent prolapse
- Poor tissue (assess tissue quality before and during surgery)
- Impaired healing
- Chronic increases in intraabdominal pressure due to obstructive pulmonary disease, asthma, or constipation
- High-grade cystocele
- Age 60 or above13
Patients with these conditions may benefit from the use of adjuvant materials in the anterior compartment.
Note that women who have had recurrences after earlier repairs may experience repeat recurrence.
Advantages of grafts
Using graft materials, the surgeon can repair all vaginal defects faster and with less effort. In the anterior compartment, a graft can be placed and anchored bilaterally from arcus to arcus tendineus, and posteriorly to the level of the spine, recreating level I support. Graft materials also offer the potential to treat stress urinary incontinence concomitantly using different shaped materials. Two authors have already described their success performing this type of repair.14
Nevertheless, great care and consideration should be devoted to actual and theoretical short- and long-term risks, many of which have not been fully elucidated.
Once a successful material is identified or developed, it may decrease operating time and morbidity in vaginal surgeries. It may also reduce the higher hospital costs normally associated with abdominal procedures.
Types of graft materials
There are 2 types of materials: synthetic or biologic. Synthetic materials can be further classified into permanent or absorbable.
The most widely used biologic materials include allografts such as human freeze-dried or solvent-dehydrated fascia lata (Tutoplast), decellularized human cadaveric dermis (Alloderm, Repliform), porcine dermal xenografts such as Pelvicol or Intexene, and bovine pericardial implants (Veritas).
Soft polypropylene meshes such as Gynemesh and Atrium are commonly used permanent materials, and polyglactin 910 is an absorbable material (TABLE).
TABLE
How successful are adjuvant materials in cystocele and rectocele repairs?
| MATERIAL (SIZE IN CM) | AUTHOR | NO. IN STUDY | RECURRENCE RATE (%) | SITE OF ATTACHMENT | FOLLOW-UP (MONTHS) | COMPLICATIONS |
|---|---|---|---|---|---|---|
| BIOLOGIC MATERIALS | ||||||
| Alloderm 3×7 patch with concomitant sling | Chung29 | 19 | 16 | Pubocervical fascia | 28 | None |
| Intexene 6×8 with sling | Gomelsky et al 200420 | 70 | 9 stage II 4 stage III | Arcus tendineus fascia pelvis | 24 | 1 wound separation |
| Solvent-dehydrated cadaveric fascia lata patch with sling | Gandhi et al 200521 | 76 patch vs 72 no patch | 21 vs 29, respectively (P=.23) | Overlay | 13 | None |
| Alloderm 3×7 trapezoid | Clemons et al 200322 | 33 | 41 stage II 3 symptomatic | Arcus tendineus fascia pelvis | 18 | None |
| SYNTHETIC MATERIALS WITH CONCOMITANT SLINGS* | ||||||
| Marlex 10×3×5 | Nicita 199823 | 44 | 0 | Arcus tendineus fascia pelvis | 13 | 1 vaginal erosion |
| Polyglactin 910 absorbable mesh | Sand et al12 | 80 mesh vs 80 no mesh | 25 vs 43 stage II cystoceles, respectively(P=.02) | Insert in the anterior and posterior colporrhaphy suture line | 12 | None |
| Polyglactin 910 absorbable mesh | Weber et al4 | 26 with mesh + standard repair; 24 with ultra-lateral repair; 33 with standard repair | 58 vs 54 vs 70 stage II, respectively (P=.58) | Overlay | 23 | None |
| SYNTHETIC PERMANENT GRAFTS WITHOUT CONCOMITANT SLINGS | ||||||
| Marlex trapezoid | Julian 199619 | 12 with 12 without | 0 vs 33, respectively | Arcus tendineus fascia pelvis | 24 | 3 vaginal erosions |
| Mixed-fiber mesh (polyglactin 910 and polyester 5×5) | Migliari and Usai 199924 | 12 | 25 | Pubourethral and cardinal ligaments | 20 | None |
| Prolene (Atrium) | Dwyer and O’Reilly25 | 64 anterior 50 posterior | 6 grade II | Tension-free | 29 | 8% vaginal erosion 1 rectovaginal fistula |
| Gynemesh 6×15 | de Tayrac et al 200526 | 87 | 7 stage II 2 stage III | Tension-free | 24 | 8% vaginal erosion |
| Prolene mesh patch | Milani et al 200527 | 32 anterior 31 posterior | 6 stage II | Fixed to endopelvic connective tissue | 17 | 20% anterior, 63% posterior dyspareunia; 13% vaginal erosion (anterior); 1 pelvic abscess (posterior) |
| Prolene mesh (double-wing shape) | Natale et al 2000 28 | 138 | 3 | Tension-free | 18 | 9% vaginal erosion 7% dyspareunia 1 hematoma |
| *Absorbable and permanent. | ||||||
Classification of synthetic materials
- Type 1 grafts are totally macroporous (>75 μm), which allows fibroblast, macrophage, and collagen penetration with angiogenesis. Examples include Prolene and Marlex meshes.
- Type 2 mesh is microporous (<10 μm in 1 dimension). This prevents penetration of fibroblasts, macrophages, or collagen. Gore-Tex is an example of a Type 2 mesh.
- Type 3 mesh is macroporous (>75 μm) with multifilamentous or microporous components. Examples include Mersilene (braided Dacron mesh), Teflon (polytetrafluoroethylene [PTFE]), Surgipro (braided polypropylene mesh), and MycroMesh (perforated PTFE patch).
- Type 4 mesh has a submicron pore size that prevents penetration. Examples include Silastic, Cellgard (polypropylene sheeting), and Preclude pericardial membrane/Preclude dura-substitute.1
2 other important properties are composition of fibers (multifilamentous materials commonly have interstices less than 10 microns) and flexibility (which has a bearing on erosion of the material).1
Bacteria can penetrate pores smaller than 1 μm, whereas polymorphonuclear white blood cells and macrophages need a pore size larger than 10 μm, and capillary ingrowth requires a size larger than 75 microns. Thus, Type 1 offers the advantages of larger pore size and monofilamentous interstices to allow for capillary ingrowth.
Which material is best?
Although the literature is difficult to interpret because of the diversity of studies and other factors, some findings are worth noting:
- Tutoplast and Alloderm appear to have the best tensile strength, maximum load to capacity, and microscopic architecture similar to the original tissue.15-17 However, these qualities were documented prior to implantation in vivo.
- Slings appear to help prevent cystocele recurrences, according to a study by Goldberg et al.18
- A fascial patch had no benefit when placed as an overlay in the anterior compartment in a randomized, controlled trial (involving 162 women) by Sand et al.12
- Marlex. One group of women with recurrent prolapse underwent synthetic graft (Marlex) augmentation with bilateral ATFP attachment, while the other group had anterior colporrhaphy only.19 None of the women who received grafts had further recurrence, while 33% of the control group did. However, 25% of the women with the graft had vaginal erosions.
- Polyglactin 910 had a protective effect when embedded in the plication, according to Sand et al.12 However, it had no benefit when used as an overlay to a traditional repair in a study by Weber et al.4 The discrepancy may be related to small sample size; the study by Weber et al was powered to detect only a 30% difference. However, these studies suggest that it is not only the type of graft that is important, but how it is used or attached.
In general, synthetic grafts may have slightly higher success rates, whereas biologic materials appear to be better tolerated.
Prospective, comparative trials of these materials are desperately needed.
1. Cervigni M, Natale F. The use of synthetics in the treatment of pelvic organ prolapse. Curr Opin Urol. 2001;11:429-435.
2. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-573.
4. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
5. Porges RF, Smilen SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1526.
6. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1436.
7. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1366.
8. Macer GA. Transabdominal repair of cystocele, a 20-year experience, compared with the traditional vaginal approach. Trans Pac Coast Obstet Gynecol Soc. 1978;45:116-120.
9. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1451-1456.
10. Paraiso MF, Ballard LA, Walters MD, Lee JC, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1430.
11. Abramov Y, et al. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005;105:314-318.
12. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001;184:1357-1362.
13. Whitesides JL, Weber AM, Meyn LA, Walters MD. Risk factors for prolapse recurrence after vaginal repair. Obstet Gynecol Surv. 2005;60:164-165.
14. Kobashi KC, Mee SL, Leach GE. A new technique for cystocele repair and transvaginal sling: the cadaveric prolapse repair and sling (CAPS). Urology. 2000;56:9-14.
15. Lemer ML, Chaikin DC, Blaivas JG. Tissue strength analysis of autologous and cadaveric allografts for the pubovaginal sling. Neurourol Urodyn. 1999;18:497-503.
16. Choe JM, Kothandapani R, et al. Autologous, cadaveric, and synthetic materials used in sling surgery: comparative biomechanical analysis. Urology. 2001;58:482-486.
17. Scalfani AP. Biophysical and microscopic analysis of homologous dermal and fascial materials for facial aesthetic and reconstructive uses. Arch Facial Plast Surg. 2002;4:164-171.
18. Goldberg RP, et al. Protective effect of suburethral slings on postoperative cystocele recurrence after reconstructive pelvic operation. Am J Obstet Gynecol. 2001;185:1307-1312.
19. Julian TM. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
20. Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol. 2004;171:1581-1584.
21. Gandhi S, et al. A prospective randomized trial of solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005;192:1649-1654.
22. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618.
23. Nicita G. A new operation for genitourinary prolapse. J Urol. 1998;160:741-745.
24. Migliari R, Usai E. Treatment results using a mixed fiber mesh in patients with grade IV cystocele. J Urol. 1999;161:1255-1258.
25. Dwyer PL, O’Reilly BA. Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG. 2004;111:831-836.
26. de Tayrac R, Gervaise A, Chauveaud A, Fernandez H. Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med. 2005;50:75-80.
27. Milani R, et al. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. BJOG. 2005;112:107-111.
28. Natale F, Marziali S, Cervigni M. Tension-free cystocele repair (TCR): long-term follow-up. Proceedings of the 25th annual meeting of the International Urogynecological Association. 2000;22-25.
29. Chung SY, et al. Technique of combined pubovaginal sling and cystocele using a single piece of cadaveric dermal graft. Urology. 2002;59:538-541.
Dr. Botros has no financial relationships relevant to this article. Dr. Sand receives grant/research support from Boston Scientific, and is a consultant and speaker for American Medical Systems and Boston Scientific.
1. Cervigni M, Natale F. The use of synthetics in the treatment of pelvic organ prolapse. Curr Opin Urol. 2001;11:429-435.
2. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-573.
4. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
5. Porges RF, Smilen SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1526.
6. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1436.
7. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1366.
8. Macer GA. Transabdominal repair of cystocele, a 20-year experience, compared with the traditional vaginal approach. Trans Pac Coast Obstet Gynecol Soc. 1978;45:116-120.
9. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1451-1456.
10. Paraiso MF, Ballard LA, Walters MD, Lee JC, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1430.
11. Abramov Y, et al. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005;105:314-318.
12. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001;184:1357-1362.
13. Whitesides JL, Weber AM, Meyn LA, Walters MD. Risk factors for prolapse recurrence after vaginal repair. Obstet Gynecol Surv. 2005;60:164-165.
14. Kobashi KC, Mee SL, Leach GE. A new technique for cystocele repair and transvaginal sling: the cadaveric prolapse repair and sling (CAPS). Urology. 2000;56:9-14.
15. Lemer ML, Chaikin DC, Blaivas JG. Tissue strength analysis of autologous and cadaveric allografts for the pubovaginal sling. Neurourol Urodyn. 1999;18:497-503.
16. Choe JM, Kothandapani R, et al. Autologous, cadaveric, and synthetic materials used in sling surgery: comparative biomechanical analysis. Urology. 2001;58:482-486.
17. Scalfani AP. Biophysical and microscopic analysis of homologous dermal and fascial materials for facial aesthetic and reconstructive uses. Arch Facial Plast Surg. 2002;4:164-171.
18. Goldberg RP, et al. Protective effect of suburethral slings on postoperative cystocele recurrence after reconstructive pelvic operation. Am J Obstet Gynecol. 2001;185:1307-1312.
19. Julian TM. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
20. Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol. 2004;171:1581-1584.
21. Gandhi S, et al. A prospective randomized trial of solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005;192:1649-1654.
22. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618.
23. Nicita G. A new operation for genitourinary prolapse. J Urol. 1998;160:741-745.
24. Migliari R, Usai E. Treatment results using a mixed fiber mesh in patients with grade IV cystocele. J Urol. 1999;161:1255-1258.
25. Dwyer PL, O’Reilly BA. Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG. 2004;111:831-836.
26. de Tayrac R, Gervaise A, Chauveaud A, Fernandez H. Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med. 2005;50:75-80.
27. Milani R, et al. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. BJOG. 2005;112:107-111.
28. Natale F, Marziali S, Cervigni M. Tension-free cystocele repair (TCR): long-term follow-up. Proceedings of the 25th annual meeting of the International Urogynecological Association. 2000;22-25.
29. Chung SY, et al. Technique of combined pubovaginal sling and cystocele using a single piece of cadaveric dermal graft. Urology. 2002;59:538-541.
Dr. Botros has no financial relationships relevant to this article. Dr. Sand receives grant/research support from Boston Scientific, and is a consultant and speaker for American Medical Systems and Boston Scientific.
IN THIS ARTICLE
Pelvic organ prolapse: Which operation for which patient?
The more numerous the choices of surgical techniques for pelvic organ prolapse, the less agreement there is on which operation is best. Further complicating the picture is the industry’s push to consider augmentation with synthetic or biologic materials on an almost routine basis.
Few scientific comparisons of the various approaches have been performed, however. To help shed some light on surgical decision-making, we convened an expert panel to review published data and explore our experience with selected procedures.
What to consider before choosing a procedure
- A woman’s desires regarding sexual activity are a critical piece of information, just as are her general health and history of pelvic surgery.
- It also helps to know which symptoms of her prolapse and related pelvic floor disorders she finds most bothersome.
KARRAM: When a woman with symptomatic pelvic organ prolapse desires surgical correction, what factors do you explore before deciding which procedure to use?
BRUBAKER: I make an effort to determine the woman’s readiness to undergo surgery and her expectations for it, as well as any concomitant pelvic floor or medical/surgical conditions.
Other important factors that I consider include her pelvic surgical history—specifically, whether she has undergone earlier continence and/or prolapse repairs—and the presence of any materials in the proposed surgical site, especially foreign bodies that may limit dissection planes or have eroded into pelvic viscera.
I also consider her desire (or lack of it) for sexual activity, and her preferred route of surgical access.
Patient’s lifestyle should sway surgical decision
PARAISO: I take into account her age and stage of prolapse; vaginal length; innervation of the pelvic floor; hormonal status; desire for uterine preservation and coitus; symptoms of sexual, urinary, or bowel dysfunction; and any comorbidities that influence her eligibility for anesthesia or chronically increase intra-abdominal pressure. Connective tissue disorders are also important, as are any coexisting medical conditions that impede healing.
Lifestyle has an impact, too, especially if she regularly performs heavy manual labor.
After assessing the patient’s history and performing an examination, I target the prolapse and functional symptoms and correlating anatomic defects that exacerbate her quality of life. I tailor her surgical therapy in order to correct her symptoms and minimize compensatory defects and de novo dysfunction.
Ask her to prioritize her complaints
SHULL: I have the patient list her complaints in order of their severity and impact on her lifestyle.
Next, I complete a detailed pelvic exam, including use of a mirror to demonstrate the findings to her. If appropriate, I test bladder or bowel function.
At that point, we discuss what I think are appropriate options, although in some situations I may not be able to treat all her complaints with equal success.
KARRAM: I think prioritizing the patient’s complaints is a good idea. My foremost aim is to determine what the woman is most bothered by. If it is prolapse symptoms such as pressure and tissue protrusion, with no functional derangements, I try to ensure that my surgical repair provides durable support but does not create de novo derangements such as stress incontinence. So, for example, I try to determine whether she has preexisting stress incontinence that is masked by the prolapse.
Correlation between prolapse and dysfunction can be weak
Obviously, if the patient has many functional derangements associated with the prolapse symptoms, the preoperative consultation becomes much more complicated. Although the complexity may not change my surgical approach, I think it is important for the patient to understand that the correlation between anatomic descent and the functional derangement may not be very good.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati.
- Linda Brubaker, MD, MS, Assistant Dean of Clinical and Translational Research, and Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics & Gynecology and Urology, Loyola University Health System, Chicago.
- Marie Fidela Paraiso, MD, Co-Director of Female Pelvic Medicine and Reconstructive Surgery, Department of Obstetrics and Gynecology and the Urological Institute, The Cleveland Clinic, Cleveland, Ohio.
- Bob L. Shull, MD, Vice Chairman, Department of Obstetrics and Gynecology, and Chief, Section of Female Pelvic Medicine and Pelvic Reconstructive Surgery, Scott & White Health Care System, Temple, Tex.
As previously mentioned, I make a point to ask about sexual function. If the woman is elderly and has no intention of being sexually active again, I may consider a very tight or obliterative repair because these are much less invasive than conventional repairs.
Is one surgical route superior?
- There is no consensus among experts as to the preferred route of surgery for advanced pelvic organ prolapse.
KARRAM: Numerous vaginal, abdominal, and laparoscopic procedures have been described. Which route do you prefer?
BRUBAKER: I don’t prefer any laparoscopic procedures, but I am flexible about vaginal or abdominal approaches.
Among vaginal procedures, I prefer uterosacral suspension at the time of hysterectomy, or the Michigan modification of sacrospinous ligament suspension when the patient has already undergone hysterectomy.
As for abdominal procedures, I prefer sacrocolpopexy with Mersilene mesh.
In my hands, these reconstructive procedures give predictable results that allow me to appropriately counsel patients preoperatively.
KARRAM: Why do you dislike the laparoscopic approach?
BRUBAKER: It is not a matter of “dislike,” but a matter of getting the most reliable result for my patient. When scientific evidence from well-done clinical trials demonstrates the equivalency of laparoscopic procedures, I fully anticipate incorporating them into my practice. Similarly, the novel use of the robot may be useful in reconstructive pelvic surgery.
Laparoscopic repair can produce good results in the right hands
PARAISO: I prefer the laparoscopic and vaginal routes. In fact, I have converted most abdominal procedures to laparoscopic access. I have nearly 10 years of experience with laparoscopic sacrocolpopexy, with excellent success.
My colleagues and I did a cohort study that showed equal cure rates for this procedure, compared with open sacrocolpopexy.1 I also have had great success with the vaginal route when performing uterosacral vaginal vault suspensions.
Patients are referred to me or seek me out specifically for minimally invasive procedures, so the majority of operations I perform are laparoscopic procedures with or without vaginal procedures, or vaginal procedures alone.
Vaginal approach is possible in high percentage of cases
SHULL: I probably perform 98% of reconstructive cases transvaginally. If the woman has urinary incontinence as well as prolapse, I usually perform a midurethral sling procedure along with the repair.
KARRAM: I do roughly 90% of prolapse repairs transvaginally. For the last 6 to 8 years, my colleagues and I have utilized a high uterosacral vaginal vault suspension to support the vaginal cuff. We do so in conjunction with a modified internal McCall-type procedure to obliterate the cul-de-sac. We also do site-specific anterior and posterior colporrhaphy as needed, and a synthetic midurethral sling if the patient has stress incontinence.
In very young patients (under 35 years of age) or those who have substantial recurrent prolapse or a prolapsed foreshortened vagina, we consider abdominal sacrocolpopexy with synthetic mesh as our primary operation. In such cases, we commonly perform retropubic repair for incontinence and paravaginal defects, as well as posterior repair and perineorrhaphy.
I have very little experience with laparoscopic prolapse repairs.
Abdominal sacrocolpopexy is anatomically superior
KARRAM: Dr. Brubaker, you just chaired a consensus panel on pelvic organ prolapse for the International Consultation on Incontinence. This panel reviewed all the published literature on the topic. What conclusions did it reach about the various surgical procedures for pelvic organ prolapse?
BRUBAKER: The “big picture” findings were that abdominal sacrocolpopexy is anatomically superior to the other procedures, but carries a higher rate of short-term morbidity than transvaginal procedures. Since that panel, a review on sacrocolpopexy by Nygaard et al2 highlighted the strengths, weaknesses, and uncertainties of this procedure.
We found no indications for routine use of ancillary materials when performing primary transvaginal repairs.
What is the best operation for advanced prolapse?
- The best procedure depends on the patient’s health, type and extent of prolapse, and sexual activity. Surgical history also is key.
KARRAM: Let’s say a 60-year-old woman with advanced, symptomatic, primary pelvic organ prolapse presents to you for surgical treatment. The findings include posthysterectomy vaginal vault prolapse with a large cystocele, large rectocele, and an enterocele. What operation would you perform?
SHULL: I would probably elect a transvaginal approach using the uterosacral ligaments to suspend the cuff and reapproximate the connective tissue of the anterior and posterior compartments. My colleagues and I described this technique.3
PARAISO: If the patient is physically and sexually active and willing to undergo synthetic graft implantation, I would perform laparoscopic sacrocolpopexy, especially if previous transvaginal apical suspension has failed, if she has a foreshortened vagina, or if she has denervation of her pelvic floor.
Check for defecatory dysfunction
If it is necessary for her to manually digitate her vagina or splint her perineum to defecate, I would perform a rectocele repair and perineorrhaphy.
If she is not a candidate for laparoscopic or abdominal surgery because of a history of multiple procedures for inflammatory bowel disease or severe adhesions, has not had a previous transvaginal apical suspension, and has intact pelvic floor innervation, I would perform either uterosacral vaginal vault suspension or sacrospinous ligament suspension with concomitant anterior and posterior repair.
I would consider offering this patient a tension-free vaginal mesh “kit” procedure (with synthetic mesh) if she:
- has failed previous vaginal procedures,
- has multiple comorbidities,
- is not a candidate for laparoscopic or abdominal surgery,
- desires to remain sexually active, and
- is willing to use and has no contraindications to intravaginal estrogen therapy.
If she does not wish to remain sexually active and is not a good operative candidate, I would offer colpectomy and colpocleisis with perineorrhaphy.
Which circumstances pose special challenges?
- Apical suspension is a critical factor in success and durability of the surgery.
KARRAM: Which segment of the pelvic floor do you find most challenging when correcting advanced pelvic organ prolapse?
SHULL: My colleagues and I have reported our experience with several techniques of vaginal repair for prolapse, including sacrospinous ligament suspension, iliococcygeus fascial suspension, and uterosacral ligament suspension. When we analyzed specific sites in the vagina, the anterior compartment always had the greatest percentage of persistent or recurrent loss of support.
Our best success has been with uterosacral ligament suspension.
Vaginal apex is key to success
PARAISO: I also find the anterior segment challenging. However, if I am able to suspend the vaginal apex well, management of the anterior vaginal wall is less challenging. The anterior wall fails because treatment of high transverse cystoceles and anterior enteroceles (less commonly seen) depends on the apical suspension. Many of these defects go untreated because they are often not detected.
BRUBAKER: I agree with Dr. Paraiso. If you get the apex up solidly, you’re usually home free.
KARRAM: Yes. If one can get good, high, durable support to the apex, the other segments of the pelvic floor are much more likely to endure.
Are unaugmented repairs doomed to fail?
- Despite claims to the contrary, reoperation rates are low for most conventional repairs.
- Surgeons may be tempted to adopt graft augmentation techniques to keep up with “Dr. Jones.”
KARRAM: As you know, there has been a recent push to consider augmenting most pelvic organ prolapse repairs with either biologic or synthetic mesh. This approach is based on a perception that conventional repairs without augmentation inevitably will fail. Do you agree with this perception?
SHULL: Not based on my own experience. Mesh has been effectively and safely used for midurethral slings and abdominal sacrocolpopexies, but there are not enough data on the use of allografts, xenografts, or meshes to be able to counsel a patient properly about their safety, efficacy, or long-term effects.
PARAISO: I agree that this perception is being promoted, prompting many physicians to adopt graft augmentation techniques to keep up with “Dr. Jones” or to offer their patients “cutting-edge” treatment. Despite the fact that conventional procedures are often described as having high failure rates, the reoperation rates in most series are low. Nevertheless, augmentation with biologic grafts has been widely adopted without prior investigation or data.
Traditional and site-specific repairs versus graft augmentation
My colleagues and I just presented a manuscript on traditional posterior colporrhaphy, site-specific rectocele repair, and site-specific repair with graft augmentation using a porcine small intestinal submucosa bioengineered collagen matrix.
The anatomic cure rate was substantially higher in the traditional and site-specific groups when compared with the graft augmentation arm, with cure rates of 86% and 78% versus 54%, respectively (P=.02).4
Currently, my indications for a mesh-augmented prolapse repair are:
- Nonexistent or suboptimal autologous tissue
- Need to augment weak or absent endopelvic tissue
- Connective tissue disorder
- Unavoidable stress on the repair (eg, chronic lifting, chronic obstructive pulmonary disease, chronic straining to defecate, obesity)
- Need to bridge a space such as sacral colpopexy
- Concern about vaginal length or caliber
- Denervated pelvic floor
- Recurrent prolapse
Surgeons should not believe that graft augmentation compensates for surgical mediocrity or patient risk factors for pelvic organ prolapse.
The key to success: Maintain the vaginal axis
KARRAM: I don’t believe all traditional repairs are bound to fail. Many factors play into recurrent prolapse. I think most people overlook the fact that the vagina is very sensitive to its axis. Any operation that alters the vaginal axis will seriously weaken the vagina opposite the distorted axis.
For example, we know that sacrospinous ligament suspension retroverts the vagina and sets women up for recurrence or development of anterior vaginal wall prolapse. Another example is a Burch colposuspension that anteverts a portion of the vagina and sets patients up for posterior vaginal wall defects in the form of a rectocele and enterocele.
Too much simplification
I also think surgeons and device manufacturers have attempted to simplify what, in reality, is a very complicated clinical picture. So many factors are involved in the identification and appropriate utilization of support structures for a durable prolapse repair.
Since Dr. Shull’s popularization of a high uterosacral suspension, we have had very good long-term success with transvaginal vault repair. Also, over time I have realized that it is possible to mobilize a substantial amount of durable fascial tissue—which is nothing more than the muscular lining of the vagina—to appropriately support the anterior and posterior vaginal walls.
That said, the results are far from perfect. I would estimate our anatomic failure rate at 15% to 20% over the long term.
Does augmentation add complications?
When it comes to mesh, we have to ask: Is it truly going to increase durability? If it is, is that going to be at the expense of a new set of complications such as mesh erosion or extrusion and dyspareunia?
The only way to answer these questions is with a randomized trial with long-term follow-up. At this time, such data are not available.
Are tension-free repair kits the wave of the future?
- It’s not yet time to make these kits the standard, although preliminary data are promising.
KARRAM: Do you think the synthetic mesh repairs now being promoted as tension-free repairs utilizing numerous industry-created “kits” will be the future of prolapse repair?
BRUBAKER: I hope not.
KARRAM: At present, I would say the answer to that question is “no.” However, I was very reluctant to accept synthetic midurethral slings, and they have turned out to be the standard of care.
SHULL: These products are the future for surgeons who allow industry to dictate their practice styles. For those of us who are more skeptical, we will change only after there is adequate scientific information to do so.
Though unproven, kits do have advantages
PARAISO: I agree. Even so, in many ways, these kits make sense. Operative time is greatly reduced and incisions are small, thus offering the advantage of minimally invasive procedures. Preliminary data at 6 months show excellent anatomic outcomes. However, the graft extrusion rate is high with the kit procedures, compared with existing evidence on synthetic mesh erosion associated with abdominal and laparoscopic sacral colpopexy.
In addition, current synthetic materials are not ideal. Long-term sequelae of transvaginal implantation of these meshes are not known. Nor do we have long-term data on sexual function.
By and large, these procedures are blind and involve the transobturator and transgluteal (ischiorectal fossa) spaces—uncharted waters for many gynecologic surgeons. Further, many gynecologic surgeons lack extensive training or experience in sacrospinous ligament suspension, iliococcygeus fascia suspension, and vaginal paravaginal defect repair, which are prerequisites for the kit procedures.
Matching the kit procedure to the patient
As for patient selection, women for whom previous anterior repair (with or without biologic graft), paravaginal defect repair, and apical suspension have failed, and who continue to have asymptomatic anterior vaginal wall prolapse are the best candidates for anterior kit procedures. The best candidates for posterior and apical segment kit procedures are women in whom transvaginal apical suspension has failed, and who are not suited for laparoscopic or abdominal procedures.
The only impediments to widespread adoption of these procedures for years to come will be adverse events or technology so advanced it makes gene modification possible, rendering surgery obsolete.
KARRAM: I think we need better and longer follow-up. Most of the surgeons currently using these procedures are proponents of the repairs, in my opinion, but until results from comparative trials become available, we won’t really know how they compare to conventional repairs.
Bringing up the next generation
- Residents need as much hands-on experience as possible, including cadaveric dissections, urodynamic labs, and urogynecologic clinics—even virtual-reality models.
KARRAM: How do we best train residents in the appropriate evaluation and surgical management of these very common pelvic floor disorders?
BRUBAKER: Carefully and ethically. Encourage them to be good consumers of surgical literature and to resist the urge to constantly demonstrate the “latest and greatest” until we have solid evidence.
PARAISO: Residents can learn from discussions of surgical indications prior to pelvic reconstructive procedures in which they are involved, attendance at urogynecologic clinics, urodynamic lab rotations, and study of urogynecologic learning modules and current clinical textbooks that focus on these surgeries.
Given the decrease in resident work hours, which translates to fewer cases, cadaver labs are also helpful.
Virtual-reality models are being developed and will be available this decade.
Golden rule of surgery: Do unto others…
SHULL: I would advise residents to treat every woman as you would your wife, mother, sister, daughter, or yourself. That may mean using a consultant for some of your patients.
Those of us who are teachers must use all available resources, including didactic instruction, video clips, cadaver dissection, simulators, and hands-on supervision in the operating room.
Those who are learning new procedures must be willing to accept constructive comments and critically evaluate their own skills.
KARRAM: I think it is important to continue training residents in the basics of pelvic floor support and anatomy. If the future involves passing needles into blind spaces, the outcomes will be disastrous if the surgeon is not comfortable with the relevant anatomy.
Secondly, surgeons should maintain their skills in proven operations such as abdominal sacrocolpopexy and sacrospinous ligament suspension. As we gain experience and long-term data, other procedures can be added more easily if we have a good understanding of the conventional repairs.
1. Paraiso MFR, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and open sacral colpopexies: a cohort study. Am J Obstet Gynecol. 2005;192:1752-1758.
2. Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
3. Shull BL, Bachofen CG, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse using uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
4. Paraiso MFR, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006 [in press].
Dr. Karram receives grant/research support from American Medical Systems, Carbon Medical, Gynecare, and Pfizer; is a consultant to Gynecare; and is a speaker for Gynecare, Indevus, and Ortho-McNeil. Dr. Paraiso has received grant/research support from American Medical Systems and Organogenesis Inc.; and is a consultant to American Medical Systems, and Ethicon Women’s Health and Urology. Dr. Brubaker and Dr. Shull report no financial relationships relevant to this article.
The more numerous the choices of surgical techniques for pelvic organ prolapse, the less agreement there is on which operation is best. Further complicating the picture is the industry’s push to consider augmentation with synthetic or biologic materials on an almost routine basis.
Few scientific comparisons of the various approaches have been performed, however. To help shed some light on surgical decision-making, we convened an expert panel to review published data and explore our experience with selected procedures.
What to consider before choosing a procedure
- A woman’s desires regarding sexual activity are a critical piece of information, just as are her general health and history of pelvic surgery.
- It also helps to know which symptoms of her prolapse and related pelvic floor disorders she finds most bothersome.
KARRAM: When a woman with symptomatic pelvic organ prolapse desires surgical correction, what factors do you explore before deciding which procedure to use?
BRUBAKER: I make an effort to determine the woman’s readiness to undergo surgery and her expectations for it, as well as any concomitant pelvic floor or medical/surgical conditions.
Other important factors that I consider include her pelvic surgical history—specifically, whether she has undergone earlier continence and/or prolapse repairs—and the presence of any materials in the proposed surgical site, especially foreign bodies that may limit dissection planes or have eroded into pelvic viscera.
I also consider her desire (or lack of it) for sexual activity, and her preferred route of surgical access.
Patient’s lifestyle should sway surgical decision
PARAISO: I take into account her age and stage of prolapse; vaginal length; innervation of the pelvic floor; hormonal status; desire for uterine preservation and coitus; symptoms of sexual, urinary, or bowel dysfunction; and any comorbidities that influence her eligibility for anesthesia or chronically increase intra-abdominal pressure. Connective tissue disorders are also important, as are any coexisting medical conditions that impede healing.
Lifestyle has an impact, too, especially if she regularly performs heavy manual labor.
After assessing the patient’s history and performing an examination, I target the prolapse and functional symptoms and correlating anatomic defects that exacerbate her quality of life. I tailor her surgical therapy in order to correct her symptoms and minimize compensatory defects and de novo dysfunction.
Ask her to prioritize her complaints
SHULL: I have the patient list her complaints in order of their severity and impact on her lifestyle.
Next, I complete a detailed pelvic exam, including use of a mirror to demonstrate the findings to her. If appropriate, I test bladder or bowel function.
At that point, we discuss what I think are appropriate options, although in some situations I may not be able to treat all her complaints with equal success.
KARRAM: I think prioritizing the patient’s complaints is a good idea. My foremost aim is to determine what the woman is most bothered by. If it is prolapse symptoms such as pressure and tissue protrusion, with no functional derangements, I try to ensure that my surgical repair provides durable support but does not create de novo derangements such as stress incontinence. So, for example, I try to determine whether she has preexisting stress incontinence that is masked by the prolapse.
Correlation between prolapse and dysfunction can be weak
Obviously, if the patient has many functional derangements associated with the prolapse symptoms, the preoperative consultation becomes much more complicated. Although the complexity may not change my surgical approach, I think it is important for the patient to understand that the correlation between anatomic descent and the functional derangement may not be very good.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati.
- Linda Brubaker, MD, MS, Assistant Dean of Clinical and Translational Research, and Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics & Gynecology and Urology, Loyola University Health System, Chicago.
- Marie Fidela Paraiso, MD, Co-Director of Female Pelvic Medicine and Reconstructive Surgery, Department of Obstetrics and Gynecology and the Urological Institute, The Cleveland Clinic, Cleveland, Ohio.
- Bob L. Shull, MD, Vice Chairman, Department of Obstetrics and Gynecology, and Chief, Section of Female Pelvic Medicine and Pelvic Reconstructive Surgery, Scott & White Health Care System, Temple, Tex.
As previously mentioned, I make a point to ask about sexual function. If the woman is elderly and has no intention of being sexually active again, I may consider a very tight or obliterative repair because these are much less invasive than conventional repairs.
Is one surgical route superior?
- There is no consensus among experts as to the preferred route of surgery for advanced pelvic organ prolapse.
KARRAM: Numerous vaginal, abdominal, and laparoscopic procedures have been described. Which route do you prefer?
BRUBAKER: I don’t prefer any laparoscopic procedures, but I am flexible about vaginal or abdominal approaches.
Among vaginal procedures, I prefer uterosacral suspension at the time of hysterectomy, or the Michigan modification of sacrospinous ligament suspension when the patient has already undergone hysterectomy.
As for abdominal procedures, I prefer sacrocolpopexy with Mersilene mesh.
In my hands, these reconstructive procedures give predictable results that allow me to appropriately counsel patients preoperatively.
KARRAM: Why do you dislike the laparoscopic approach?
BRUBAKER: It is not a matter of “dislike,” but a matter of getting the most reliable result for my patient. When scientific evidence from well-done clinical trials demonstrates the equivalency of laparoscopic procedures, I fully anticipate incorporating them into my practice. Similarly, the novel use of the robot may be useful in reconstructive pelvic surgery.
Laparoscopic repair can produce good results in the right hands
PARAISO: I prefer the laparoscopic and vaginal routes. In fact, I have converted most abdominal procedures to laparoscopic access. I have nearly 10 years of experience with laparoscopic sacrocolpopexy, with excellent success.
My colleagues and I did a cohort study that showed equal cure rates for this procedure, compared with open sacrocolpopexy.1 I also have had great success with the vaginal route when performing uterosacral vaginal vault suspensions.
Patients are referred to me or seek me out specifically for minimally invasive procedures, so the majority of operations I perform are laparoscopic procedures with or without vaginal procedures, or vaginal procedures alone.
Vaginal approach is possible in high percentage of cases
SHULL: I probably perform 98% of reconstructive cases transvaginally. If the woman has urinary incontinence as well as prolapse, I usually perform a midurethral sling procedure along with the repair.
KARRAM: I do roughly 90% of prolapse repairs transvaginally. For the last 6 to 8 years, my colleagues and I have utilized a high uterosacral vaginal vault suspension to support the vaginal cuff. We do so in conjunction with a modified internal McCall-type procedure to obliterate the cul-de-sac. We also do site-specific anterior and posterior colporrhaphy as needed, and a synthetic midurethral sling if the patient has stress incontinence.
In very young patients (under 35 years of age) or those who have substantial recurrent prolapse or a prolapsed foreshortened vagina, we consider abdominal sacrocolpopexy with synthetic mesh as our primary operation. In such cases, we commonly perform retropubic repair for incontinence and paravaginal defects, as well as posterior repair and perineorrhaphy.
I have very little experience with laparoscopic prolapse repairs.
Abdominal sacrocolpopexy is anatomically superior
KARRAM: Dr. Brubaker, you just chaired a consensus panel on pelvic organ prolapse for the International Consultation on Incontinence. This panel reviewed all the published literature on the topic. What conclusions did it reach about the various surgical procedures for pelvic organ prolapse?
BRUBAKER: The “big picture” findings were that abdominal sacrocolpopexy is anatomically superior to the other procedures, but carries a higher rate of short-term morbidity than transvaginal procedures. Since that panel, a review on sacrocolpopexy by Nygaard et al2 highlighted the strengths, weaknesses, and uncertainties of this procedure.
We found no indications for routine use of ancillary materials when performing primary transvaginal repairs.
What is the best operation for advanced prolapse?
- The best procedure depends on the patient’s health, type and extent of prolapse, and sexual activity. Surgical history also is key.
KARRAM: Let’s say a 60-year-old woman with advanced, symptomatic, primary pelvic organ prolapse presents to you for surgical treatment. The findings include posthysterectomy vaginal vault prolapse with a large cystocele, large rectocele, and an enterocele. What operation would you perform?
SHULL: I would probably elect a transvaginal approach using the uterosacral ligaments to suspend the cuff and reapproximate the connective tissue of the anterior and posterior compartments. My colleagues and I described this technique.3
PARAISO: If the patient is physically and sexually active and willing to undergo synthetic graft implantation, I would perform laparoscopic sacrocolpopexy, especially if previous transvaginal apical suspension has failed, if she has a foreshortened vagina, or if she has denervation of her pelvic floor.
Check for defecatory dysfunction
If it is necessary for her to manually digitate her vagina or splint her perineum to defecate, I would perform a rectocele repair and perineorrhaphy.
If she is not a candidate for laparoscopic or abdominal surgery because of a history of multiple procedures for inflammatory bowel disease or severe adhesions, has not had a previous transvaginal apical suspension, and has intact pelvic floor innervation, I would perform either uterosacral vaginal vault suspension or sacrospinous ligament suspension with concomitant anterior and posterior repair.
I would consider offering this patient a tension-free vaginal mesh “kit” procedure (with synthetic mesh) if she:
- has failed previous vaginal procedures,
- has multiple comorbidities,
- is not a candidate for laparoscopic or abdominal surgery,
- desires to remain sexually active, and
- is willing to use and has no contraindications to intravaginal estrogen therapy.
If she does not wish to remain sexually active and is not a good operative candidate, I would offer colpectomy and colpocleisis with perineorrhaphy.
Which circumstances pose special challenges?
- Apical suspension is a critical factor in success and durability of the surgery.
KARRAM: Which segment of the pelvic floor do you find most challenging when correcting advanced pelvic organ prolapse?
SHULL: My colleagues and I have reported our experience with several techniques of vaginal repair for prolapse, including sacrospinous ligament suspension, iliococcygeus fascial suspension, and uterosacral ligament suspension. When we analyzed specific sites in the vagina, the anterior compartment always had the greatest percentage of persistent or recurrent loss of support.
Our best success has been with uterosacral ligament suspension.
Vaginal apex is key to success
PARAISO: I also find the anterior segment challenging. However, if I am able to suspend the vaginal apex well, management of the anterior vaginal wall is less challenging. The anterior wall fails because treatment of high transverse cystoceles and anterior enteroceles (less commonly seen) depends on the apical suspension. Many of these defects go untreated because they are often not detected.
BRUBAKER: I agree with Dr. Paraiso. If you get the apex up solidly, you’re usually home free.
KARRAM: Yes. If one can get good, high, durable support to the apex, the other segments of the pelvic floor are much more likely to endure.
Are unaugmented repairs doomed to fail?
- Despite claims to the contrary, reoperation rates are low for most conventional repairs.
- Surgeons may be tempted to adopt graft augmentation techniques to keep up with “Dr. Jones.”
KARRAM: As you know, there has been a recent push to consider augmenting most pelvic organ prolapse repairs with either biologic or synthetic mesh. This approach is based on a perception that conventional repairs without augmentation inevitably will fail. Do you agree with this perception?
SHULL: Not based on my own experience. Mesh has been effectively and safely used for midurethral slings and abdominal sacrocolpopexies, but there are not enough data on the use of allografts, xenografts, or meshes to be able to counsel a patient properly about their safety, efficacy, or long-term effects.
PARAISO: I agree that this perception is being promoted, prompting many physicians to adopt graft augmentation techniques to keep up with “Dr. Jones” or to offer their patients “cutting-edge” treatment. Despite the fact that conventional procedures are often described as having high failure rates, the reoperation rates in most series are low. Nevertheless, augmentation with biologic grafts has been widely adopted without prior investigation or data.
Traditional and site-specific repairs versus graft augmentation
My colleagues and I just presented a manuscript on traditional posterior colporrhaphy, site-specific rectocele repair, and site-specific repair with graft augmentation using a porcine small intestinal submucosa bioengineered collagen matrix.
The anatomic cure rate was substantially higher in the traditional and site-specific groups when compared with the graft augmentation arm, with cure rates of 86% and 78% versus 54%, respectively (P=.02).4
Currently, my indications for a mesh-augmented prolapse repair are:
- Nonexistent or suboptimal autologous tissue
- Need to augment weak or absent endopelvic tissue
- Connective tissue disorder
- Unavoidable stress on the repair (eg, chronic lifting, chronic obstructive pulmonary disease, chronic straining to defecate, obesity)
- Need to bridge a space such as sacral colpopexy
- Concern about vaginal length or caliber
- Denervated pelvic floor
- Recurrent prolapse
Surgeons should not believe that graft augmentation compensates for surgical mediocrity or patient risk factors for pelvic organ prolapse.
The key to success: Maintain the vaginal axis
KARRAM: I don’t believe all traditional repairs are bound to fail. Many factors play into recurrent prolapse. I think most people overlook the fact that the vagina is very sensitive to its axis. Any operation that alters the vaginal axis will seriously weaken the vagina opposite the distorted axis.
For example, we know that sacrospinous ligament suspension retroverts the vagina and sets women up for recurrence or development of anterior vaginal wall prolapse. Another example is a Burch colposuspension that anteverts a portion of the vagina and sets patients up for posterior vaginal wall defects in the form of a rectocele and enterocele.
Too much simplification
I also think surgeons and device manufacturers have attempted to simplify what, in reality, is a very complicated clinical picture. So many factors are involved in the identification and appropriate utilization of support structures for a durable prolapse repair.
Since Dr. Shull’s popularization of a high uterosacral suspension, we have had very good long-term success with transvaginal vault repair. Also, over time I have realized that it is possible to mobilize a substantial amount of durable fascial tissue—which is nothing more than the muscular lining of the vagina—to appropriately support the anterior and posterior vaginal walls.
That said, the results are far from perfect. I would estimate our anatomic failure rate at 15% to 20% over the long term.
Does augmentation add complications?
When it comes to mesh, we have to ask: Is it truly going to increase durability? If it is, is that going to be at the expense of a new set of complications such as mesh erosion or extrusion and dyspareunia?
The only way to answer these questions is with a randomized trial with long-term follow-up. At this time, such data are not available.
Are tension-free repair kits the wave of the future?
- It’s not yet time to make these kits the standard, although preliminary data are promising.
KARRAM: Do you think the synthetic mesh repairs now being promoted as tension-free repairs utilizing numerous industry-created “kits” will be the future of prolapse repair?
BRUBAKER: I hope not.
KARRAM: At present, I would say the answer to that question is “no.” However, I was very reluctant to accept synthetic midurethral slings, and they have turned out to be the standard of care.
SHULL: These products are the future for surgeons who allow industry to dictate their practice styles. For those of us who are more skeptical, we will change only after there is adequate scientific information to do so.
Though unproven, kits do have advantages
PARAISO: I agree. Even so, in many ways, these kits make sense. Operative time is greatly reduced and incisions are small, thus offering the advantage of minimally invasive procedures. Preliminary data at 6 months show excellent anatomic outcomes. However, the graft extrusion rate is high with the kit procedures, compared with existing evidence on synthetic mesh erosion associated with abdominal and laparoscopic sacral colpopexy.
In addition, current synthetic materials are not ideal. Long-term sequelae of transvaginal implantation of these meshes are not known. Nor do we have long-term data on sexual function.
By and large, these procedures are blind and involve the transobturator and transgluteal (ischiorectal fossa) spaces—uncharted waters for many gynecologic surgeons. Further, many gynecologic surgeons lack extensive training or experience in sacrospinous ligament suspension, iliococcygeus fascia suspension, and vaginal paravaginal defect repair, which are prerequisites for the kit procedures.
Matching the kit procedure to the patient
As for patient selection, women for whom previous anterior repair (with or without biologic graft), paravaginal defect repair, and apical suspension have failed, and who continue to have asymptomatic anterior vaginal wall prolapse are the best candidates for anterior kit procedures. The best candidates for posterior and apical segment kit procedures are women in whom transvaginal apical suspension has failed, and who are not suited for laparoscopic or abdominal procedures.
The only impediments to widespread adoption of these procedures for years to come will be adverse events or technology so advanced it makes gene modification possible, rendering surgery obsolete.
KARRAM: I think we need better and longer follow-up. Most of the surgeons currently using these procedures are proponents of the repairs, in my opinion, but until results from comparative trials become available, we won’t really know how they compare to conventional repairs.
Bringing up the next generation
- Residents need as much hands-on experience as possible, including cadaveric dissections, urodynamic labs, and urogynecologic clinics—even virtual-reality models.
KARRAM: How do we best train residents in the appropriate evaluation and surgical management of these very common pelvic floor disorders?
BRUBAKER: Carefully and ethically. Encourage them to be good consumers of surgical literature and to resist the urge to constantly demonstrate the “latest and greatest” until we have solid evidence.
PARAISO: Residents can learn from discussions of surgical indications prior to pelvic reconstructive procedures in which they are involved, attendance at urogynecologic clinics, urodynamic lab rotations, and study of urogynecologic learning modules and current clinical textbooks that focus on these surgeries.
Given the decrease in resident work hours, which translates to fewer cases, cadaver labs are also helpful.
Virtual-reality models are being developed and will be available this decade.
Golden rule of surgery: Do unto others…
SHULL: I would advise residents to treat every woman as you would your wife, mother, sister, daughter, or yourself. That may mean using a consultant for some of your patients.
Those of us who are teachers must use all available resources, including didactic instruction, video clips, cadaver dissection, simulators, and hands-on supervision in the operating room.
Those who are learning new procedures must be willing to accept constructive comments and critically evaluate their own skills.
KARRAM: I think it is important to continue training residents in the basics of pelvic floor support and anatomy. If the future involves passing needles into blind spaces, the outcomes will be disastrous if the surgeon is not comfortable with the relevant anatomy.
Secondly, surgeons should maintain their skills in proven operations such as abdominal sacrocolpopexy and sacrospinous ligament suspension. As we gain experience and long-term data, other procedures can be added more easily if we have a good understanding of the conventional repairs.
The more numerous the choices of surgical techniques for pelvic organ prolapse, the less agreement there is on which operation is best. Further complicating the picture is the industry’s push to consider augmentation with synthetic or biologic materials on an almost routine basis.
Few scientific comparisons of the various approaches have been performed, however. To help shed some light on surgical decision-making, we convened an expert panel to review published data and explore our experience with selected procedures.
What to consider before choosing a procedure
- A woman’s desires regarding sexual activity are a critical piece of information, just as are her general health and history of pelvic surgery.
- It also helps to know which symptoms of her prolapse and related pelvic floor disorders she finds most bothersome.
KARRAM: When a woman with symptomatic pelvic organ prolapse desires surgical correction, what factors do you explore before deciding which procedure to use?
BRUBAKER: I make an effort to determine the woman’s readiness to undergo surgery and her expectations for it, as well as any concomitant pelvic floor or medical/surgical conditions.
Other important factors that I consider include her pelvic surgical history—specifically, whether she has undergone earlier continence and/or prolapse repairs—and the presence of any materials in the proposed surgical site, especially foreign bodies that may limit dissection planes or have eroded into pelvic viscera.
I also consider her desire (or lack of it) for sexual activity, and her preferred route of surgical access.
Patient’s lifestyle should sway surgical decision
PARAISO: I take into account her age and stage of prolapse; vaginal length; innervation of the pelvic floor; hormonal status; desire for uterine preservation and coitus; symptoms of sexual, urinary, or bowel dysfunction; and any comorbidities that influence her eligibility for anesthesia or chronically increase intra-abdominal pressure. Connective tissue disorders are also important, as are any coexisting medical conditions that impede healing.
Lifestyle has an impact, too, especially if she regularly performs heavy manual labor.
After assessing the patient’s history and performing an examination, I target the prolapse and functional symptoms and correlating anatomic defects that exacerbate her quality of life. I tailor her surgical therapy in order to correct her symptoms and minimize compensatory defects and de novo dysfunction.
Ask her to prioritize her complaints
SHULL: I have the patient list her complaints in order of their severity and impact on her lifestyle.
Next, I complete a detailed pelvic exam, including use of a mirror to demonstrate the findings to her. If appropriate, I test bladder or bowel function.
At that point, we discuss what I think are appropriate options, although in some situations I may not be able to treat all her complaints with equal success.
KARRAM: I think prioritizing the patient’s complaints is a good idea. My foremost aim is to determine what the woman is most bothered by. If it is prolapse symptoms such as pressure and tissue protrusion, with no functional derangements, I try to ensure that my surgical repair provides durable support but does not create de novo derangements such as stress incontinence. So, for example, I try to determine whether she has preexisting stress incontinence that is masked by the prolapse.
Correlation between prolapse and dysfunction can be weak
Obviously, if the patient has many functional derangements associated with the prolapse symptoms, the preoperative consultation becomes much more complicated. Although the complexity may not change my surgical approach, I think it is important for the patient to understand that the correlation between anatomic descent and the functional derangement may not be very good.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati.
- Linda Brubaker, MD, MS, Assistant Dean of Clinical and Translational Research, and Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics & Gynecology and Urology, Loyola University Health System, Chicago.
- Marie Fidela Paraiso, MD, Co-Director of Female Pelvic Medicine and Reconstructive Surgery, Department of Obstetrics and Gynecology and the Urological Institute, The Cleveland Clinic, Cleveland, Ohio.
- Bob L. Shull, MD, Vice Chairman, Department of Obstetrics and Gynecology, and Chief, Section of Female Pelvic Medicine and Pelvic Reconstructive Surgery, Scott & White Health Care System, Temple, Tex.
As previously mentioned, I make a point to ask about sexual function. If the woman is elderly and has no intention of being sexually active again, I may consider a very tight or obliterative repair because these are much less invasive than conventional repairs.
Is one surgical route superior?
- There is no consensus among experts as to the preferred route of surgery for advanced pelvic organ prolapse.
KARRAM: Numerous vaginal, abdominal, and laparoscopic procedures have been described. Which route do you prefer?
BRUBAKER: I don’t prefer any laparoscopic procedures, but I am flexible about vaginal or abdominal approaches.
Among vaginal procedures, I prefer uterosacral suspension at the time of hysterectomy, or the Michigan modification of sacrospinous ligament suspension when the patient has already undergone hysterectomy.
As for abdominal procedures, I prefer sacrocolpopexy with Mersilene mesh.
In my hands, these reconstructive procedures give predictable results that allow me to appropriately counsel patients preoperatively.
KARRAM: Why do you dislike the laparoscopic approach?
BRUBAKER: It is not a matter of “dislike,” but a matter of getting the most reliable result for my patient. When scientific evidence from well-done clinical trials demonstrates the equivalency of laparoscopic procedures, I fully anticipate incorporating them into my practice. Similarly, the novel use of the robot may be useful in reconstructive pelvic surgery.
Laparoscopic repair can produce good results in the right hands
PARAISO: I prefer the laparoscopic and vaginal routes. In fact, I have converted most abdominal procedures to laparoscopic access. I have nearly 10 years of experience with laparoscopic sacrocolpopexy, with excellent success.
My colleagues and I did a cohort study that showed equal cure rates for this procedure, compared with open sacrocolpopexy.1 I also have had great success with the vaginal route when performing uterosacral vaginal vault suspensions.
Patients are referred to me or seek me out specifically for minimally invasive procedures, so the majority of operations I perform are laparoscopic procedures with or without vaginal procedures, or vaginal procedures alone.
Vaginal approach is possible in high percentage of cases
SHULL: I probably perform 98% of reconstructive cases transvaginally. If the woman has urinary incontinence as well as prolapse, I usually perform a midurethral sling procedure along with the repair.
KARRAM: I do roughly 90% of prolapse repairs transvaginally. For the last 6 to 8 years, my colleagues and I have utilized a high uterosacral vaginal vault suspension to support the vaginal cuff. We do so in conjunction with a modified internal McCall-type procedure to obliterate the cul-de-sac. We also do site-specific anterior and posterior colporrhaphy as needed, and a synthetic midurethral sling if the patient has stress incontinence.
In very young patients (under 35 years of age) or those who have substantial recurrent prolapse or a prolapsed foreshortened vagina, we consider abdominal sacrocolpopexy with synthetic mesh as our primary operation. In such cases, we commonly perform retropubic repair for incontinence and paravaginal defects, as well as posterior repair and perineorrhaphy.
I have very little experience with laparoscopic prolapse repairs.
Abdominal sacrocolpopexy is anatomically superior
KARRAM: Dr. Brubaker, you just chaired a consensus panel on pelvic organ prolapse for the International Consultation on Incontinence. This panel reviewed all the published literature on the topic. What conclusions did it reach about the various surgical procedures for pelvic organ prolapse?
BRUBAKER: The “big picture” findings were that abdominal sacrocolpopexy is anatomically superior to the other procedures, but carries a higher rate of short-term morbidity than transvaginal procedures. Since that panel, a review on sacrocolpopexy by Nygaard et al2 highlighted the strengths, weaknesses, and uncertainties of this procedure.
We found no indications for routine use of ancillary materials when performing primary transvaginal repairs.
What is the best operation for advanced prolapse?
- The best procedure depends on the patient’s health, type and extent of prolapse, and sexual activity. Surgical history also is key.
KARRAM: Let’s say a 60-year-old woman with advanced, symptomatic, primary pelvic organ prolapse presents to you for surgical treatment. The findings include posthysterectomy vaginal vault prolapse with a large cystocele, large rectocele, and an enterocele. What operation would you perform?
SHULL: I would probably elect a transvaginal approach using the uterosacral ligaments to suspend the cuff and reapproximate the connective tissue of the anterior and posterior compartments. My colleagues and I described this technique.3
PARAISO: If the patient is physically and sexually active and willing to undergo synthetic graft implantation, I would perform laparoscopic sacrocolpopexy, especially if previous transvaginal apical suspension has failed, if she has a foreshortened vagina, or if she has denervation of her pelvic floor.
Check for defecatory dysfunction
If it is necessary for her to manually digitate her vagina or splint her perineum to defecate, I would perform a rectocele repair and perineorrhaphy.
If she is not a candidate for laparoscopic or abdominal surgery because of a history of multiple procedures for inflammatory bowel disease or severe adhesions, has not had a previous transvaginal apical suspension, and has intact pelvic floor innervation, I would perform either uterosacral vaginal vault suspension or sacrospinous ligament suspension with concomitant anterior and posterior repair.
I would consider offering this patient a tension-free vaginal mesh “kit” procedure (with synthetic mesh) if she:
- has failed previous vaginal procedures,
- has multiple comorbidities,
- is not a candidate for laparoscopic or abdominal surgery,
- desires to remain sexually active, and
- is willing to use and has no contraindications to intravaginal estrogen therapy.
If she does not wish to remain sexually active and is not a good operative candidate, I would offer colpectomy and colpocleisis with perineorrhaphy.
Which circumstances pose special challenges?
- Apical suspension is a critical factor in success and durability of the surgery.
KARRAM: Which segment of the pelvic floor do you find most challenging when correcting advanced pelvic organ prolapse?
SHULL: My colleagues and I have reported our experience with several techniques of vaginal repair for prolapse, including sacrospinous ligament suspension, iliococcygeus fascial suspension, and uterosacral ligament suspension. When we analyzed specific sites in the vagina, the anterior compartment always had the greatest percentage of persistent or recurrent loss of support.
Our best success has been with uterosacral ligament suspension.
Vaginal apex is key to success
PARAISO: I also find the anterior segment challenging. However, if I am able to suspend the vaginal apex well, management of the anterior vaginal wall is less challenging. The anterior wall fails because treatment of high transverse cystoceles and anterior enteroceles (less commonly seen) depends on the apical suspension. Many of these defects go untreated because they are often not detected.
BRUBAKER: I agree with Dr. Paraiso. If you get the apex up solidly, you’re usually home free.
KARRAM: Yes. If one can get good, high, durable support to the apex, the other segments of the pelvic floor are much more likely to endure.
Are unaugmented repairs doomed to fail?
- Despite claims to the contrary, reoperation rates are low for most conventional repairs.
- Surgeons may be tempted to adopt graft augmentation techniques to keep up with “Dr. Jones.”
KARRAM: As you know, there has been a recent push to consider augmenting most pelvic organ prolapse repairs with either biologic or synthetic mesh. This approach is based on a perception that conventional repairs without augmentation inevitably will fail. Do you agree with this perception?
SHULL: Not based on my own experience. Mesh has been effectively and safely used for midurethral slings and abdominal sacrocolpopexies, but there are not enough data on the use of allografts, xenografts, or meshes to be able to counsel a patient properly about their safety, efficacy, or long-term effects.
PARAISO: I agree that this perception is being promoted, prompting many physicians to adopt graft augmentation techniques to keep up with “Dr. Jones” or to offer their patients “cutting-edge” treatment. Despite the fact that conventional procedures are often described as having high failure rates, the reoperation rates in most series are low. Nevertheless, augmentation with biologic grafts has been widely adopted without prior investigation or data.
Traditional and site-specific repairs versus graft augmentation
My colleagues and I just presented a manuscript on traditional posterior colporrhaphy, site-specific rectocele repair, and site-specific repair with graft augmentation using a porcine small intestinal submucosa bioengineered collagen matrix.
The anatomic cure rate was substantially higher in the traditional and site-specific groups when compared with the graft augmentation arm, with cure rates of 86% and 78% versus 54%, respectively (P=.02).4
Currently, my indications for a mesh-augmented prolapse repair are:
- Nonexistent or suboptimal autologous tissue
- Need to augment weak or absent endopelvic tissue
- Connective tissue disorder
- Unavoidable stress on the repair (eg, chronic lifting, chronic obstructive pulmonary disease, chronic straining to defecate, obesity)
- Need to bridge a space such as sacral colpopexy
- Concern about vaginal length or caliber
- Denervated pelvic floor
- Recurrent prolapse
Surgeons should not believe that graft augmentation compensates for surgical mediocrity or patient risk factors for pelvic organ prolapse.
The key to success: Maintain the vaginal axis
KARRAM: I don’t believe all traditional repairs are bound to fail. Many factors play into recurrent prolapse. I think most people overlook the fact that the vagina is very sensitive to its axis. Any operation that alters the vaginal axis will seriously weaken the vagina opposite the distorted axis.
For example, we know that sacrospinous ligament suspension retroverts the vagina and sets women up for recurrence or development of anterior vaginal wall prolapse. Another example is a Burch colposuspension that anteverts a portion of the vagina and sets patients up for posterior vaginal wall defects in the form of a rectocele and enterocele.
Too much simplification
I also think surgeons and device manufacturers have attempted to simplify what, in reality, is a very complicated clinical picture. So many factors are involved in the identification and appropriate utilization of support structures for a durable prolapse repair.
Since Dr. Shull’s popularization of a high uterosacral suspension, we have had very good long-term success with transvaginal vault repair. Also, over time I have realized that it is possible to mobilize a substantial amount of durable fascial tissue—which is nothing more than the muscular lining of the vagina—to appropriately support the anterior and posterior vaginal walls.
That said, the results are far from perfect. I would estimate our anatomic failure rate at 15% to 20% over the long term.
Does augmentation add complications?
When it comes to mesh, we have to ask: Is it truly going to increase durability? If it is, is that going to be at the expense of a new set of complications such as mesh erosion or extrusion and dyspareunia?
The only way to answer these questions is with a randomized trial with long-term follow-up. At this time, such data are not available.
Are tension-free repair kits the wave of the future?
- It’s not yet time to make these kits the standard, although preliminary data are promising.
KARRAM: Do you think the synthetic mesh repairs now being promoted as tension-free repairs utilizing numerous industry-created “kits” will be the future of prolapse repair?
BRUBAKER: I hope not.
KARRAM: At present, I would say the answer to that question is “no.” However, I was very reluctant to accept synthetic midurethral slings, and they have turned out to be the standard of care.
SHULL: These products are the future for surgeons who allow industry to dictate their practice styles. For those of us who are more skeptical, we will change only after there is adequate scientific information to do so.
Though unproven, kits do have advantages
PARAISO: I agree. Even so, in many ways, these kits make sense. Operative time is greatly reduced and incisions are small, thus offering the advantage of minimally invasive procedures. Preliminary data at 6 months show excellent anatomic outcomes. However, the graft extrusion rate is high with the kit procedures, compared with existing evidence on synthetic mesh erosion associated with abdominal and laparoscopic sacral colpopexy.
In addition, current synthetic materials are not ideal. Long-term sequelae of transvaginal implantation of these meshes are not known. Nor do we have long-term data on sexual function.
By and large, these procedures are blind and involve the transobturator and transgluteal (ischiorectal fossa) spaces—uncharted waters for many gynecologic surgeons. Further, many gynecologic surgeons lack extensive training or experience in sacrospinous ligament suspension, iliococcygeus fascia suspension, and vaginal paravaginal defect repair, which are prerequisites for the kit procedures.
Matching the kit procedure to the patient
As for patient selection, women for whom previous anterior repair (with or without biologic graft), paravaginal defect repair, and apical suspension have failed, and who continue to have asymptomatic anterior vaginal wall prolapse are the best candidates for anterior kit procedures. The best candidates for posterior and apical segment kit procedures are women in whom transvaginal apical suspension has failed, and who are not suited for laparoscopic or abdominal procedures.
The only impediments to widespread adoption of these procedures for years to come will be adverse events or technology so advanced it makes gene modification possible, rendering surgery obsolete.
KARRAM: I think we need better and longer follow-up. Most of the surgeons currently using these procedures are proponents of the repairs, in my opinion, but until results from comparative trials become available, we won’t really know how they compare to conventional repairs.
Bringing up the next generation
- Residents need as much hands-on experience as possible, including cadaveric dissections, urodynamic labs, and urogynecologic clinics—even virtual-reality models.
KARRAM: How do we best train residents in the appropriate evaluation and surgical management of these very common pelvic floor disorders?
BRUBAKER: Carefully and ethically. Encourage them to be good consumers of surgical literature and to resist the urge to constantly demonstrate the “latest and greatest” until we have solid evidence.
PARAISO: Residents can learn from discussions of surgical indications prior to pelvic reconstructive procedures in which they are involved, attendance at urogynecologic clinics, urodynamic lab rotations, and study of urogynecologic learning modules and current clinical textbooks that focus on these surgeries.
Given the decrease in resident work hours, which translates to fewer cases, cadaver labs are also helpful.
Virtual-reality models are being developed and will be available this decade.
Golden rule of surgery: Do unto others…
SHULL: I would advise residents to treat every woman as you would your wife, mother, sister, daughter, or yourself. That may mean using a consultant for some of your patients.
Those of us who are teachers must use all available resources, including didactic instruction, video clips, cadaver dissection, simulators, and hands-on supervision in the operating room.
Those who are learning new procedures must be willing to accept constructive comments and critically evaluate their own skills.
KARRAM: I think it is important to continue training residents in the basics of pelvic floor support and anatomy. If the future involves passing needles into blind spaces, the outcomes will be disastrous if the surgeon is not comfortable with the relevant anatomy.
Secondly, surgeons should maintain their skills in proven operations such as abdominal sacrocolpopexy and sacrospinous ligament suspension. As we gain experience and long-term data, other procedures can be added more easily if we have a good understanding of the conventional repairs.
1. Paraiso MFR, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and open sacral colpopexies: a cohort study. Am J Obstet Gynecol. 2005;192:1752-1758.
2. Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
3. Shull BL, Bachofen CG, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse using uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
4. Paraiso MFR, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006 [in press].
Dr. Karram receives grant/research support from American Medical Systems, Carbon Medical, Gynecare, and Pfizer; is a consultant to Gynecare; and is a speaker for Gynecare, Indevus, and Ortho-McNeil. Dr. Paraiso has received grant/research support from American Medical Systems and Organogenesis Inc.; and is a consultant to American Medical Systems, and Ethicon Women’s Health and Urology. Dr. Brubaker and Dr. Shull report no financial relationships relevant to this article.
1. Paraiso MFR, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and open sacral colpopexies: a cohort study. Am J Obstet Gynecol. 2005;192:1752-1758.
2. Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
3. Shull BL, Bachofen CG, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse using uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
4. Paraiso MFR, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006 [in press].
Dr. Karram receives grant/research support from American Medical Systems, Carbon Medical, Gynecare, and Pfizer; is a consultant to Gynecare; and is a speaker for Gynecare, Indevus, and Ortho-McNeil. Dr. Paraiso has received grant/research support from American Medical Systems and Organogenesis Inc.; and is a consultant to American Medical Systems, and Ethicon Women’s Health and Urology. Dr. Brubaker and Dr. Shull report no financial relationships relevant to this article.
Preventing VTE: Evidence-based perioperative tactics
Pulmonary embolism is a master of disguises. It can appear with classic symptoms such as pleuritic chest pain, hemoptysis, and tachycardia—or it can arrive more insidiously, apparent only as a slight elevation in the respiratory rate.
This matters because 40% of all deaths following gynecologic surgery are directly attributable to pulmonary emboli,1 and pulmonary emboli are the most frequent cause of postoperative death in women with uterine or cervical carcinoma.2
Deep venous thrombosis (DVT) is almost as evasive. We know the signs and symptoms of DVT of the lower extremities—pain, edema, erythema, and a prominent vascular pattern of the superficial veins—but 50% to 80% of patients with these symptoms do not have DVT, and 80% of patients with symptomatic pulmonary embolism have no antecedent signs of thrombosis in the lower extremities.2 Morbidity and expense rise dramatically with DVT, especially when postphlebitic syndrome occurs.
How can we minimize these risks?
A good outcome is most likely when we:
- recognize risk factors,
- provide appropriate perioperative prophylaxis, and
- diagnose and treat venous thromboembolism (VTE) quickly.
This article looks in detail at each of these strategies.
3 factors set the stage for thrombogenesis
- Hypercoagulable state
- Venous stasis
- Vessel endothelial injury
These factors, known as Virchow’s triad, are especially likely at the time of major surgery, or when the patient is advanced in age or has a history of DVT, cancer, lower extremity edema, or venous stasis.
Intraoperative risk factors for postoperative DVT include increased anesthesia time, greater blood loss, and need for transfusion.
Some preventive methods come close to ideal
Being aware of risk factors is vital to provide the appropriate level of prophylaxis (TABLES 1 AND 2).3,4 The first step is identifying high-risk patients and tailoring the regimen to meet their individual needs. The perfect prophylactic method is not yet devised, but would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patient groups, and inexpensive. A number of methods come close.
TABLE 1
Risk factors for thromboembolism
| Major gynecologic surgery |
| Age >40 years |
| Malignancy |
| Previous venous thrombosis (DVT or pulmonary embolism) |
| Obesity |
| Immobility |
| Pregnancy and the postpartum period |
| Oral contraceptives, hormone therapy, or tamoxifen |
| Varicose veins |
| Inherited or acquired thrombophilia (eg, Factor V Leiden) |
| Prolonged surgical procedure |
| Radical vulvectomy, inguinal-femoral lymphadenectomy, or pelvic exenteration |
TABLE 2
Match the preventive strategy to the surgery
| SURGERY | STRATEGY | DURATION OF PROPHYLAXIS* |
|---|---|---|
| Procedures <30 min for benign disease | Prophylaxis not needed | — |
| Laparoscopic gynecologic procedures in women with additional risk factors | Unfractionated heparin, 5,000 bid or | Until hospital discharge |
| LMWH, ≤3,400 U/day or | ||
| External pneumatic compression or | ||
| Graduated compression stockings | ||
| Major surgery for benign disease without additional risk factors | Unfractionated heparin, 5,000 U bid or | Until hospital discharge |
| LMWH, <3,400 U/day or | ||
| External pneumatic compression | ||
| Extensive major surgery in women with cancer or additional risk factors | Unfractionated heparin, 5,000 U tid or | Until hospital discharge |
| LMWH, >3,400 U/day or | ||
| External pneumatic compression | ||
| *For women at particularly high risk (eg, cancer surgery, age >60 years, prior VTE), continue prophylaxis for 2–4 weeks after hospital discharge. | ||
| Modified from Geerts WH, et al20 | ||
Low-dose unfractionated heparin
The most extensively studied prophylactic method is the use of small, subcutaneous doses of heparin. More than 25 controlled trials have shown that, when heparin is given subcutaneously 2 hours before surgery and every 8 to 12 hours afterward, the incidence of DVT diminishes substantially.
The value of low-dose heparin in preventing pulmonary emboli was established by a randomized, controlled, multicenter, international trial, in which fatal postoperative pulmonary emboli declined significantly in general surgery patients given the drug every 8 hours after surgery.5 In gynecologic surgical patients, postoperative DVT also declined significantly.
Increase in minor bleeding complications. Although low-dose heparin is thought to have no measurable effect on coagulation, most large series have noted an increase in minor bleeding complications such as wound hematoma. Up to 10% to 15% of otherwise healthy patients develop transiently prolonged activated partial thromboplastin time (APTT) after 5,000 U of heparin are given subcutaneously.6
Although relatively rare, thrombocytopenia is associated with the use of low-dose heparin. It has been found in 6% of women after gynecologic surgery.6 Therefore, it is reasonable to measure platelets in any patient taking low-dose heparin longer than 4 days to screen for heparin-induced thrombocytopenia.
Fear of major bleeding complications is unsubstantiated. There is ample evidence from placebo-controlled, blinded trials and meta-analysis that the risk of clinically important bleeding does not increase. Moreover, detailed analysis demonstrates that low-dose heparin has a good risk-to-benefit ratio and is cost-effective.
Low-molecular-weight heparins
These drugs are fragments of unfractionated heparin that vary in size from 4,500 to 6,500 daltons. Low-molecular-weight heparin (LMWH) has more anti-Xa and less antithrombin activity than unfractionated heparin and thus has less of an effect on partial thromboplastin time. LMWH may also lead to fewer bleeding complications.7
Once-daily dosing is possible. An increased half-life of 4 hours for LMWH produces greater bioavailability than with low-dose heparin. This allows once-daily dosing.
Pick one: Convenience or cost
Randomized controlled trials have compared LMWH to unfractionated heparin in gynecologic surgical patients. In all studies, DVT occurred in similar, low numbers of women regardless of the heparin used. Bleeding complications also were similar.8
A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise found daily LMWH to be as effective as unfractionated heparin in DVT prophylaxis, without any difference in hemorrhagic complications.9
The choice of drugs often boils down to convenience versus cost: Prophylactic LMWH can be given once a day (compared with 2 or 3 times for unfractionated heparin), but is much more expensive.
Mechanical prophylactic methods
External pneumatic compression rivals low-dose heparin. The largest body of literature on mechanical methods to reduce postoperative venous stasis involves intermittent leg compression by pneumatically inflated sleeves placed around the calf or leg during surgery and after. A number of devices and sleeve designs are available, none of which has proven to be superior to the others.
In my experience, calf compression during and after gynecologic surgery lowers the incidence of DVT to a level seen with low-dose heparin. Besides increasing venous flow and pulsatile emptying of the calf veins, pneumatic compression appears to augment endogenous fibrinolysis, which may stimulate lysis of very early thrombi.10
How long is best for external compression? The optimal duration of postoperative external pneumatic compression is unclear. It may be effective when used in the operating room and for the first 24 hours postoperatively in patients with benign conditions who will ambulate on the first day after surgery.11,12
In women undergoing major surgery for gynecologic malignancy, it reduces the incidence of postoperative venous thromboemboli by nearly 3-fold, but only if calf compression is applied intraoperatively and for the first 5 postoperative days.13,14 These women may remain at risk because of stasis and a hypercoagulable state for a longer time than general surgical patients.
External pneumatic leg compression has no serious side effects or risks and is slightly more cost-effective than prophylactic drugs.15 However, to be fully effective, this method must be used consistently, in compliance with the protocol, when the patient is not ambulating.
Stockings can be a help or hazard. Controlled studies of graduated pressure stockings are limited but suggest modest benefit with careful fitting.16 Poorly fitted stockings that roll down the leg may create a tourniquet effect at the knee or mid-thigh. Another disadvantage of the stockings: The limited sizes available do not allow a perfect fit for all patients. This is especially true in obese patients.
The simplicity of elastic stockings and the absence of serious side effects are probably why stockings are often included in routine postoperative care.
Don’t overlook basic precautions. Although they may offer only modest benefit, short preoperative hospital stays and early postoperative ambulation are recommended.
Another basic strategy: elevating the foot of the bed to raise the calf above heart level. This allows gravity to drain the calf veins and should further reduce stasis.
How to detect VTE
DVT has nonspecific signs and symptoms
When DVT occurs in the lower extremities, harbingers such as pain, edema, and erythema are relatively nonspecific; 50% to 80% of patients exhibiting them do not have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities.
Because of this lack of specificity, additional tests are needed to establish DVT.
Diagnostic studies
A definitive diagnosis of DVT and pulmonary embolism is mandatory because diagnosis based on clinical symptoms and signs alone is frequently wrong. Strategies to reduce the use of ultrasound or spiral CT scanning have been put forward. These studies have evaluated outpatients using algorithms that utilize clinical probability (“clinical decision rule”) and D-dimer levels.
This strategy has been very accurate and avoids the use of ultrasound or spiral CT in low-risk patients. For example, individuals with a low probability score have an incidence of DVT below 5%, so ultrasound is unnecessary. This diagnostic strategy relies on the recognition of elevated D-dimer levels. Unfortunately, D-dimer is increased by a variety of nonthrombotic disorders, including recent surgery, hemorrhage, trauma, pregnancy, and cancer. Therefore, we cannot recommend the use of this strategy for the postoperative gynecologic surgery patient.17,18
Venography no longer the gold standard. Other diagnostic studies may be more useful. Venography has fallen from favor because it is moderately uncomfortable, requires injection of a contrast material that may trigger an allergic reaction or renal injury, and causes phlebitis in approximately 5% of patients.2 Newer, noninvasive diagnostic tests have been developed, fortunately.
Doppler ultrasound. B-mode duplex Doppler imaging is the most common technique to diagnose symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the tip of the ultrasound probe makes it possible to assess venous collapsibility, which is diminished when a thrombus is present.
Doppler imaging is less accurate when evaluating the calf and pelvic veins.
Magnetic resonance venography (MRV) sensitivity and specificity are comparable to venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback is the time required to examine the lower extremity and pelvis. Further, MRV rarely identifies calf thrombi (most often not life-threatening, but potentially symptomatic) and is considerably more expensive than ultrasound.
Which prevention strategy works best?
We now consider low-molecular-weight heparin and external pneumatic compression the best choices
Because low-dose unfractionated heparin, low-molecular-weight heparin (LMWH), and external pneumatic compression all reduce the incidence of postoperative venous thromboembolism in high-risk gynecologic surgical patients, the question is: Which strategy is best?
We conducted 2 randomized clinical trials to answer this question.
Trial 1 Low-dose heparin vs pneumatic compression
Women were randomized to receive either low-dose heparin (5,000 U subcutaneously preoperatively and every 8 hours after surgery until hospital discharge) or external pneumatic compression of the calf prior to surgery and until hospital discharge.1
The incidence of DVT was identical in both groups, and no patients developed a pulmonary embolus throughout 30 days of follow-up. However, bleeding complications occurred more often in the group randomized to low-dose heparin. Specifically, nearly 25% had APTT levels in the “therapeutic” range, and significantly more patients required blood transfusions. After this trial, our institution decided to use external pneumatic compression because of its more favorable risk profile.1
Trial 2 LMWH vs pneumatic compression
The question of the best therapy arose again with the advent of LMWH, because of the possibility that these drugs carried a lower risk of bleeding complications. We therefore conducted a second trial to compare LMWH with external pneumatic compression.2
Because higher doses of LMWH had already proven to be more effective in cancer patients, we gave women in the trial 5,000 U dalteparin (Fragmin) preoperatively and 5,000 U daily postoperatively until hospital discharge.
In this trial, external pneumatic compression and LMWH produced similar low frequencies of DVT and no pulmonary emboli throughout 30 days of follow-up. We also found no association between LMWH and bleeding complications or transfusion requirements. Compliance and patient satisfaction were similar for both modalities.2
Bottom line
We now consider LMWH and external pneumatic compression the best choices for prophylaxis in gynecologic surgical patients.
REFERENCES
1. Clarke-Pearson DL, Synan IS, Dodge R, Soper JT, Berchuck A, Coleman RE. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1154.
2. Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DI. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. Obstet Gynecol. 2001;98:989-995.
Increasing use of laparoscopic surgery raises an important question: What is the thromboembolic risk of laparoscopy itself? On one hand, many laparoscopic surgeries are prolonged, and intraperitoneal pressure from the pneumoperitoneum reduces venous flow. On the other hand, many patients who have laparoscopy have shorter hospital stays and return sooner to normal activities than those who have open procedures.
Although the risks of venous thromboembolism (VTE) have not been studied as thoroughly as other aspects of laparoscopy, they appear to be low. To date, there are no randomized trials of VTE prophylaxis among women undergoing gynecologic laparoscopy.
The prudent course
Nevertheless, it would seem prudent to consider prophylaxis when women with additional risk factors undergo extensive laparoscopic procedures.
Pulmonary embolism is often stealthy
Many of the typical signs and symptoms of pulmonary embolism are associated with other, more common pulmonary complications following surgery. Classic findings that should alert the physician to the possibility of pulmonary embolism include:
- pleuritic chest pain
- hemoptysis
- shortness of breath
- tachycardia
- tachypnea
Often, however, the signs are subtle and may include only persistent tachycardia or a slight elevation in respiration.
When pulmonary embolism is suspected, a chest x-ray, electrocardiography, and arterial blood gas assessment are warranted. Any abnormality justifies further evaluation by ventilation-perfusion lung scan or a spiral computed tomography scan of the chest. Unfortunately, a high percentage of lung scans are interpreted as “indeterminate.” In such cases, careful clinical evaluation and judgment are needed to determine whether pulmonary arteriography is necessary to document or exclude pulmonary embolism.
Immediate, aggressive therapy is crucial
The treatment of postoperative DVT requires immediate anticoagulant therapy using either unfractionated heparin or LMWH, followed by 6 months of oral anticoagulant therapy with warfarin.
Treatment strategy: Unfractionated heparin
Once VTE is diagnosed, start unfractionated heparin to prevent proximal propagation of the thrombus and allow physiologic thrombolytic pathways to dissolve the clot. After an initial IV bolus of 5,000 U, give the patient a continuous infusion of 30,000 U daily, and adjust the dose to maintain APTT levels at a therapeutic level that is 1.5 to 2.5 times the control value.
Subtherapeutic APTT levels in the first 24 hours mean a risk of recurrent thromboembolism 15 times greater than the risk in patients with appropriate levels. Therefore, aggressive management is warranted to achieve prompt anticoagulation.
Start an oral anticoagulant (warfarin) on the first day of heparin infusion, and monitor the international normalized ratio (INR) daily until a therapeutic level is achieved. The change in the INR after warfarin administration often precedes the anticoagulant effect by about 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, it is important to continue the heparin until the INR has been maintained in a therapeutic range for at least 2 days to confirm the proper warfarin dose. Intravenous heparin can be discontinued after 5 days if an adequate INR level has been established.
Alternative strategy: LMWH
A meta-analysis involving more than 1,000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly than unfractionated heparin in preventing recurrent thromboembolism.19 The lower cost derives from the ability to use the drugs in an outpatient setting.
Dosages are unique and weight-adjusted according to each LMWH preparation. Because LMWH has a minimal effect on APTT, serial laboratory monitoring of APTT levels is unnecessary. Nor is monitoring of anti-Xa activity of significant benefit in the dose adjustment of LMWH.
Basic treatment of pulmonary embolism
In most cases, immediate anticoagulant therapy identical to that outlined for DVT is sufficient to prevent repeat thrombosis and embolism and to allow the patient’s endogenous thrombolytic mechanisms to lyse the pulmonary embolus.
Other interventions include:
- Respiratory support, including oxygen, bronchodilators, and intensive care.
- Although massive pulmonary emboli are usually quickly fatal, pulmonary embolectomy has been successful on rare occasions.
- Pulmonary artery catheterization and administration of thrombolytic agents may be important in patients with massive pulmonary embolism.
- Vena cava interruption may be necessary when anticoagulant therapy does not prevent rethrombosis and the formation of emboli from the lower extremities or pelvis. A vena cava umbrella or filter may be inserted percutaneously above the level of the thrombosis and caudad to the renal veins.
Take-home points
- Identify risk factors preoperatively
- VTE prophylaxis is warranted for most gynecologic surgery patients and can reduce the incidence of VTE by at least 60% with appropriate use! Plan prophylaxis in women at moderate, high, and highest risk, and remember that individuals at high and highest risk require more intense prophylaxis to realize a benefit.
- Maintain a high level of suspicion in women with signs and symptoms of DVT or pulmonary embolism in the first postoperative month. It is better to over-evaluate than to miss a potentially fatal complication.
- Treat women with VTE immediately with heparin or LMWH.
1. Jeffcoate TN, Tindall VR. Venous thrombosis and embolism in obstetrics and gynecology. Aust N Z J Obstet Gynecol. 1965;5:119-130.
2. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983;61:87-94.
3. Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. BMJ. 1976;2:910-912.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. An international multicentre trial. Lancet. 1975;2:45-51.
6. Clarke-Pearson DL, DeLong ER, Synan IS, Creasman WT. Complications of low-dose heparin prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 1984;64:689-694.
7. Tapson VF, Hull RD. Management of venous thromboembolic disease. The impact of low-molecular-weight heparin. Clin Chest Med. 1995;16:281-294.
8. Borstad E, Urdal K, Handeland G, Abildgaard U. Comparison of low molecular weight heparin vs. unfractionated heparin in gynecological surgery. II: Reduced dose of low molecular weight heparin. Acta Obstet Gynecol Scand. 1992;71:471-475.
9. Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689-704.
10. Allenby F, Boardman L, Pflug JJ, Calnan JS. Effects of external pneumatic intermittent compression on fibrinolysis in man. Lancet. 1973;2:1412-1414.
11. Salzman EW, Ploetz J, Bettmann M, Skillman J, Klein L. Intraoperative external pneumatic calf compression to afford long-term prophylaxis against deep vein thrombosis in urological patients. Surgery. 1980;87:239-242.
12. Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69-76.
13. Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63:92-98.
14. Clarke-Pearson DL, Creasman WT, Coleman RE, Synan IS, Hinshaw WM. Perioperative external pneumatic calf compression as thromboembolism prophylaxis in gynecologic oncology: report of a randomized controlled trial. Gynecol Oncol. 1984;18:226-232.
15. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
16. Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371-373.
17. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207.
18. Writing Group for the Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical D-dimer testing and computed tomography. JAMA. 2006;295:172-179.
19. Buller HR, Kucher N, Kipfmueller F, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
20. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
The author reports no financial relationships relevant to this article.
Pulmonary embolism is a master of disguises. It can appear with classic symptoms such as pleuritic chest pain, hemoptysis, and tachycardia—or it can arrive more insidiously, apparent only as a slight elevation in the respiratory rate.
This matters because 40% of all deaths following gynecologic surgery are directly attributable to pulmonary emboli,1 and pulmonary emboli are the most frequent cause of postoperative death in women with uterine or cervical carcinoma.2
Deep venous thrombosis (DVT) is almost as evasive. We know the signs and symptoms of DVT of the lower extremities—pain, edema, erythema, and a prominent vascular pattern of the superficial veins—but 50% to 80% of patients with these symptoms do not have DVT, and 80% of patients with symptomatic pulmonary embolism have no antecedent signs of thrombosis in the lower extremities.2 Morbidity and expense rise dramatically with DVT, especially when postphlebitic syndrome occurs.
How can we minimize these risks?
A good outcome is most likely when we:
- recognize risk factors,
- provide appropriate perioperative prophylaxis, and
- diagnose and treat venous thromboembolism (VTE) quickly.
This article looks in detail at each of these strategies.
3 factors set the stage for thrombogenesis
- Hypercoagulable state
- Venous stasis
- Vessel endothelial injury
These factors, known as Virchow’s triad, are especially likely at the time of major surgery, or when the patient is advanced in age or has a history of DVT, cancer, lower extremity edema, or venous stasis.
Intraoperative risk factors for postoperative DVT include increased anesthesia time, greater blood loss, and need for transfusion.
Some preventive methods come close to ideal
Being aware of risk factors is vital to provide the appropriate level of prophylaxis (TABLES 1 AND 2).3,4 The first step is identifying high-risk patients and tailoring the regimen to meet their individual needs. The perfect prophylactic method is not yet devised, but would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patient groups, and inexpensive. A number of methods come close.
TABLE 1
Risk factors for thromboembolism
| Major gynecologic surgery |
| Age >40 years |
| Malignancy |
| Previous venous thrombosis (DVT or pulmonary embolism) |
| Obesity |
| Immobility |
| Pregnancy and the postpartum period |
| Oral contraceptives, hormone therapy, or tamoxifen |
| Varicose veins |
| Inherited or acquired thrombophilia (eg, Factor V Leiden) |
| Prolonged surgical procedure |
| Radical vulvectomy, inguinal-femoral lymphadenectomy, or pelvic exenteration |
TABLE 2
Match the preventive strategy to the surgery
| SURGERY | STRATEGY | DURATION OF PROPHYLAXIS* |
|---|---|---|
| Procedures <30 min for benign disease | Prophylaxis not needed | — |
| Laparoscopic gynecologic procedures in women with additional risk factors | Unfractionated heparin, 5,000 bid or | Until hospital discharge |
| LMWH, ≤3,400 U/day or | ||
| External pneumatic compression or | ||
| Graduated compression stockings | ||
| Major surgery for benign disease without additional risk factors | Unfractionated heparin, 5,000 U bid or | Until hospital discharge |
| LMWH, <3,400 U/day or | ||
| External pneumatic compression | ||
| Extensive major surgery in women with cancer or additional risk factors | Unfractionated heparin, 5,000 U tid or | Until hospital discharge |
| LMWH, >3,400 U/day or | ||
| External pneumatic compression | ||
| *For women at particularly high risk (eg, cancer surgery, age >60 years, prior VTE), continue prophylaxis for 2–4 weeks after hospital discharge. | ||
| Modified from Geerts WH, et al20 | ||
Low-dose unfractionated heparin
The most extensively studied prophylactic method is the use of small, subcutaneous doses of heparin. More than 25 controlled trials have shown that, when heparin is given subcutaneously 2 hours before surgery and every 8 to 12 hours afterward, the incidence of DVT diminishes substantially.
The value of low-dose heparin in preventing pulmonary emboli was established by a randomized, controlled, multicenter, international trial, in which fatal postoperative pulmonary emboli declined significantly in general surgery patients given the drug every 8 hours after surgery.5 In gynecologic surgical patients, postoperative DVT also declined significantly.
Increase in minor bleeding complications. Although low-dose heparin is thought to have no measurable effect on coagulation, most large series have noted an increase in minor bleeding complications such as wound hematoma. Up to 10% to 15% of otherwise healthy patients develop transiently prolonged activated partial thromboplastin time (APTT) after 5,000 U of heparin are given subcutaneously.6
Although relatively rare, thrombocytopenia is associated with the use of low-dose heparin. It has been found in 6% of women after gynecologic surgery.6 Therefore, it is reasonable to measure platelets in any patient taking low-dose heparin longer than 4 days to screen for heparin-induced thrombocytopenia.
Fear of major bleeding complications is unsubstantiated. There is ample evidence from placebo-controlled, blinded trials and meta-analysis that the risk of clinically important bleeding does not increase. Moreover, detailed analysis demonstrates that low-dose heparin has a good risk-to-benefit ratio and is cost-effective.
Low-molecular-weight heparins
These drugs are fragments of unfractionated heparin that vary in size from 4,500 to 6,500 daltons. Low-molecular-weight heparin (LMWH) has more anti-Xa and less antithrombin activity than unfractionated heparin and thus has less of an effect on partial thromboplastin time. LMWH may also lead to fewer bleeding complications.7
Once-daily dosing is possible. An increased half-life of 4 hours for LMWH produces greater bioavailability than with low-dose heparin. This allows once-daily dosing.
Pick one: Convenience or cost
Randomized controlled trials have compared LMWH to unfractionated heparin in gynecologic surgical patients. In all studies, DVT occurred in similar, low numbers of women regardless of the heparin used. Bleeding complications also were similar.8
A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise found daily LMWH to be as effective as unfractionated heparin in DVT prophylaxis, without any difference in hemorrhagic complications.9
The choice of drugs often boils down to convenience versus cost: Prophylactic LMWH can be given once a day (compared with 2 or 3 times for unfractionated heparin), but is much more expensive.
Mechanical prophylactic methods
External pneumatic compression rivals low-dose heparin. The largest body of literature on mechanical methods to reduce postoperative venous stasis involves intermittent leg compression by pneumatically inflated sleeves placed around the calf or leg during surgery and after. A number of devices and sleeve designs are available, none of which has proven to be superior to the others.
In my experience, calf compression during and after gynecologic surgery lowers the incidence of DVT to a level seen with low-dose heparin. Besides increasing venous flow and pulsatile emptying of the calf veins, pneumatic compression appears to augment endogenous fibrinolysis, which may stimulate lysis of very early thrombi.10
How long is best for external compression? The optimal duration of postoperative external pneumatic compression is unclear. It may be effective when used in the operating room and for the first 24 hours postoperatively in patients with benign conditions who will ambulate on the first day after surgery.11,12
In women undergoing major surgery for gynecologic malignancy, it reduces the incidence of postoperative venous thromboemboli by nearly 3-fold, but only if calf compression is applied intraoperatively and for the first 5 postoperative days.13,14 These women may remain at risk because of stasis and a hypercoagulable state for a longer time than general surgical patients.
External pneumatic leg compression has no serious side effects or risks and is slightly more cost-effective than prophylactic drugs.15 However, to be fully effective, this method must be used consistently, in compliance with the protocol, when the patient is not ambulating.
Stockings can be a help or hazard. Controlled studies of graduated pressure stockings are limited but suggest modest benefit with careful fitting.16 Poorly fitted stockings that roll down the leg may create a tourniquet effect at the knee or mid-thigh. Another disadvantage of the stockings: The limited sizes available do not allow a perfect fit for all patients. This is especially true in obese patients.
The simplicity of elastic stockings and the absence of serious side effects are probably why stockings are often included in routine postoperative care.
Don’t overlook basic precautions. Although they may offer only modest benefit, short preoperative hospital stays and early postoperative ambulation are recommended.
Another basic strategy: elevating the foot of the bed to raise the calf above heart level. This allows gravity to drain the calf veins and should further reduce stasis.
How to detect VTE
DVT has nonspecific signs and symptoms
When DVT occurs in the lower extremities, harbingers such as pain, edema, and erythema are relatively nonspecific; 50% to 80% of patients exhibiting them do not have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities.
Because of this lack of specificity, additional tests are needed to establish DVT.
Diagnostic studies
A definitive diagnosis of DVT and pulmonary embolism is mandatory because diagnosis based on clinical symptoms and signs alone is frequently wrong. Strategies to reduce the use of ultrasound or spiral CT scanning have been put forward. These studies have evaluated outpatients using algorithms that utilize clinical probability (“clinical decision rule”) and D-dimer levels.
This strategy has been very accurate and avoids the use of ultrasound or spiral CT in low-risk patients. For example, individuals with a low probability score have an incidence of DVT below 5%, so ultrasound is unnecessary. This diagnostic strategy relies on the recognition of elevated D-dimer levels. Unfortunately, D-dimer is increased by a variety of nonthrombotic disorders, including recent surgery, hemorrhage, trauma, pregnancy, and cancer. Therefore, we cannot recommend the use of this strategy for the postoperative gynecologic surgery patient.17,18
Venography no longer the gold standard. Other diagnostic studies may be more useful. Venography has fallen from favor because it is moderately uncomfortable, requires injection of a contrast material that may trigger an allergic reaction or renal injury, and causes phlebitis in approximately 5% of patients.2 Newer, noninvasive diagnostic tests have been developed, fortunately.
Doppler ultrasound. B-mode duplex Doppler imaging is the most common technique to diagnose symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the tip of the ultrasound probe makes it possible to assess venous collapsibility, which is diminished when a thrombus is present.
Doppler imaging is less accurate when evaluating the calf and pelvic veins.
Magnetic resonance venography (MRV) sensitivity and specificity are comparable to venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback is the time required to examine the lower extremity and pelvis. Further, MRV rarely identifies calf thrombi (most often not life-threatening, but potentially symptomatic) and is considerably more expensive than ultrasound.
Which prevention strategy works best?
We now consider low-molecular-weight heparin and external pneumatic compression the best choices
Because low-dose unfractionated heparin, low-molecular-weight heparin (LMWH), and external pneumatic compression all reduce the incidence of postoperative venous thromboembolism in high-risk gynecologic surgical patients, the question is: Which strategy is best?
We conducted 2 randomized clinical trials to answer this question.
Trial 1 Low-dose heparin vs pneumatic compression
Women were randomized to receive either low-dose heparin (5,000 U subcutaneously preoperatively and every 8 hours after surgery until hospital discharge) or external pneumatic compression of the calf prior to surgery and until hospital discharge.1
The incidence of DVT was identical in both groups, and no patients developed a pulmonary embolus throughout 30 days of follow-up. However, bleeding complications occurred more often in the group randomized to low-dose heparin. Specifically, nearly 25% had APTT levels in the “therapeutic” range, and significantly more patients required blood transfusions. After this trial, our institution decided to use external pneumatic compression because of its more favorable risk profile.1
Trial 2 LMWH vs pneumatic compression
The question of the best therapy arose again with the advent of LMWH, because of the possibility that these drugs carried a lower risk of bleeding complications. We therefore conducted a second trial to compare LMWH with external pneumatic compression.2
Because higher doses of LMWH had already proven to be more effective in cancer patients, we gave women in the trial 5,000 U dalteparin (Fragmin) preoperatively and 5,000 U daily postoperatively until hospital discharge.
In this trial, external pneumatic compression and LMWH produced similar low frequencies of DVT and no pulmonary emboli throughout 30 days of follow-up. We also found no association between LMWH and bleeding complications or transfusion requirements. Compliance and patient satisfaction were similar for both modalities.2
Bottom line
We now consider LMWH and external pneumatic compression the best choices for prophylaxis in gynecologic surgical patients.
REFERENCES
1. Clarke-Pearson DL, Synan IS, Dodge R, Soper JT, Berchuck A, Coleman RE. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1154.
2. Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DI. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. Obstet Gynecol. 2001;98:989-995.
Increasing use of laparoscopic surgery raises an important question: What is the thromboembolic risk of laparoscopy itself? On one hand, many laparoscopic surgeries are prolonged, and intraperitoneal pressure from the pneumoperitoneum reduces venous flow. On the other hand, many patients who have laparoscopy have shorter hospital stays and return sooner to normal activities than those who have open procedures.
Although the risks of venous thromboembolism (VTE) have not been studied as thoroughly as other aspects of laparoscopy, they appear to be low. To date, there are no randomized trials of VTE prophylaxis among women undergoing gynecologic laparoscopy.
The prudent course
Nevertheless, it would seem prudent to consider prophylaxis when women with additional risk factors undergo extensive laparoscopic procedures.
Pulmonary embolism is often stealthy
Many of the typical signs and symptoms of pulmonary embolism are associated with other, more common pulmonary complications following surgery. Classic findings that should alert the physician to the possibility of pulmonary embolism include:
- pleuritic chest pain
- hemoptysis
- shortness of breath
- tachycardia
- tachypnea
Often, however, the signs are subtle and may include only persistent tachycardia or a slight elevation in respiration.
When pulmonary embolism is suspected, a chest x-ray, electrocardiography, and arterial blood gas assessment are warranted. Any abnormality justifies further evaluation by ventilation-perfusion lung scan or a spiral computed tomography scan of the chest. Unfortunately, a high percentage of lung scans are interpreted as “indeterminate.” In such cases, careful clinical evaluation and judgment are needed to determine whether pulmonary arteriography is necessary to document or exclude pulmonary embolism.
Immediate, aggressive therapy is crucial
The treatment of postoperative DVT requires immediate anticoagulant therapy using either unfractionated heparin or LMWH, followed by 6 months of oral anticoagulant therapy with warfarin.
Treatment strategy: Unfractionated heparin
Once VTE is diagnosed, start unfractionated heparin to prevent proximal propagation of the thrombus and allow physiologic thrombolytic pathways to dissolve the clot. After an initial IV bolus of 5,000 U, give the patient a continuous infusion of 30,000 U daily, and adjust the dose to maintain APTT levels at a therapeutic level that is 1.5 to 2.5 times the control value.
Subtherapeutic APTT levels in the first 24 hours mean a risk of recurrent thromboembolism 15 times greater than the risk in patients with appropriate levels. Therefore, aggressive management is warranted to achieve prompt anticoagulation.
Start an oral anticoagulant (warfarin) on the first day of heparin infusion, and monitor the international normalized ratio (INR) daily until a therapeutic level is achieved. The change in the INR after warfarin administration often precedes the anticoagulant effect by about 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, it is important to continue the heparin until the INR has been maintained in a therapeutic range for at least 2 days to confirm the proper warfarin dose. Intravenous heparin can be discontinued after 5 days if an adequate INR level has been established.
Alternative strategy: LMWH
A meta-analysis involving more than 1,000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly than unfractionated heparin in preventing recurrent thromboembolism.19 The lower cost derives from the ability to use the drugs in an outpatient setting.
Dosages are unique and weight-adjusted according to each LMWH preparation. Because LMWH has a minimal effect on APTT, serial laboratory monitoring of APTT levels is unnecessary. Nor is monitoring of anti-Xa activity of significant benefit in the dose adjustment of LMWH.
Basic treatment of pulmonary embolism
In most cases, immediate anticoagulant therapy identical to that outlined for DVT is sufficient to prevent repeat thrombosis and embolism and to allow the patient’s endogenous thrombolytic mechanisms to lyse the pulmonary embolus.
Other interventions include:
- Respiratory support, including oxygen, bronchodilators, and intensive care.
- Although massive pulmonary emboli are usually quickly fatal, pulmonary embolectomy has been successful on rare occasions.
- Pulmonary artery catheterization and administration of thrombolytic agents may be important in patients with massive pulmonary embolism.
- Vena cava interruption may be necessary when anticoagulant therapy does not prevent rethrombosis and the formation of emboli from the lower extremities or pelvis. A vena cava umbrella or filter may be inserted percutaneously above the level of the thrombosis and caudad to the renal veins.
Take-home points
- Identify risk factors preoperatively
- VTE prophylaxis is warranted for most gynecologic surgery patients and can reduce the incidence of VTE by at least 60% with appropriate use! Plan prophylaxis in women at moderate, high, and highest risk, and remember that individuals at high and highest risk require more intense prophylaxis to realize a benefit.
- Maintain a high level of suspicion in women with signs and symptoms of DVT or pulmonary embolism in the first postoperative month. It is better to over-evaluate than to miss a potentially fatal complication.
- Treat women with VTE immediately with heparin or LMWH.
Pulmonary embolism is a master of disguises. It can appear with classic symptoms such as pleuritic chest pain, hemoptysis, and tachycardia—or it can arrive more insidiously, apparent only as a slight elevation in the respiratory rate.
This matters because 40% of all deaths following gynecologic surgery are directly attributable to pulmonary emboli,1 and pulmonary emboli are the most frequent cause of postoperative death in women with uterine or cervical carcinoma.2
Deep venous thrombosis (DVT) is almost as evasive. We know the signs and symptoms of DVT of the lower extremities—pain, edema, erythema, and a prominent vascular pattern of the superficial veins—but 50% to 80% of patients with these symptoms do not have DVT, and 80% of patients with symptomatic pulmonary embolism have no antecedent signs of thrombosis in the lower extremities.2 Morbidity and expense rise dramatically with DVT, especially when postphlebitic syndrome occurs.
How can we minimize these risks?
A good outcome is most likely when we:
- recognize risk factors,
- provide appropriate perioperative prophylaxis, and
- diagnose and treat venous thromboembolism (VTE) quickly.
This article looks in detail at each of these strategies.
3 factors set the stage for thrombogenesis
- Hypercoagulable state
- Venous stasis
- Vessel endothelial injury
These factors, known as Virchow’s triad, are especially likely at the time of major surgery, or when the patient is advanced in age or has a history of DVT, cancer, lower extremity edema, or venous stasis.
Intraoperative risk factors for postoperative DVT include increased anesthesia time, greater blood loss, and need for transfusion.
Some preventive methods come close to ideal
Being aware of risk factors is vital to provide the appropriate level of prophylaxis (TABLES 1 AND 2).3,4 The first step is identifying high-risk patients and tailoring the regimen to meet their individual needs. The perfect prophylactic method is not yet devised, but would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patient groups, and inexpensive. A number of methods come close.
TABLE 1
Risk factors for thromboembolism
| Major gynecologic surgery |
| Age >40 years |
| Malignancy |
| Previous venous thrombosis (DVT or pulmonary embolism) |
| Obesity |
| Immobility |
| Pregnancy and the postpartum period |
| Oral contraceptives, hormone therapy, or tamoxifen |
| Varicose veins |
| Inherited or acquired thrombophilia (eg, Factor V Leiden) |
| Prolonged surgical procedure |
| Radical vulvectomy, inguinal-femoral lymphadenectomy, or pelvic exenteration |
TABLE 2
Match the preventive strategy to the surgery
| SURGERY | STRATEGY | DURATION OF PROPHYLAXIS* |
|---|---|---|
| Procedures <30 min for benign disease | Prophylaxis not needed | — |
| Laparoscopic gynecologic procedures in women with additional risk factors | Unfractionated heparin, 5,000 bid or | Until hospital discharge |
| LMWH, ≤3,400 U/day or | ||
| External pneumatic compression or | ||
| Graduated compression stockings | ||
| Major surgery for benign disease without additional risk factors | Unfractionated heparin, 5,000 U bid or | Until hospital discharge |
| LMWH, <3,400 U/day or | ||
| External pneumatic compression | ||
| Extensive major surgery in women with cancer or additional risk factors | Unfractionated heparin, 5,000 U tid or | Until hospital discharge |
| LMWH, >3,400 U/day or | ||
| External pneumatic compression | ||
| *For women at particularly high risk (eg, cancer surgery, age >60 years, prior VTE), continue prophylaxis for 2–4 weeks after hospital discharge. | ||
| Modified from Geerts WH, et al20 | ||
Low-dose unfractionated heparin
The most extensively studied prophylactic method is the use of small, subcutaneous doses of heparin. More than 25 controlled trials have shown that, when heparin is given subcutaneously 2 hours before surgery and every 8 to 12 hours afterward, the incidence of DVT diminishes substantially.
The value of low-dose heparin in preventing pulmonary emboli was established by a randomized, controlled, multicenter, international trial, in which fatal postoperative pulmonary emboli declined significantly in general surgery patients given the drug every 8 hours after surgery.5 In gynecologic surgical patients, postoperative DVT also declined significantly.
Increase in minor bleeding complications. Although low-dose heparin is thought to have no measurable effect on coagulation, most large series have noted an increase in minor bleeding complications such as wound hematoma. Up to 10% to 15% of otherwise healthy patients develop transiently prolonged activated partial thromboplastin time (APTT) after 5,000 U of heparin are given subcutaneously.6
Although relatively rare, thrombocytopenia is associated with the use of low-dose heparin. It has been found in 6% of women after gynecologic surgery.6 Therefore, it is reasonable to measure platelets in any patient taking low-dose heparin longer than 4 days to screen for heparin-induced thrombocytopenia.
Fear of major bleeding complications is unsubstantiated. There is ample evidence from placebo-controlled, blinded trials and meta-analysis that the risk of clinically important bleeding does not increase. Moreover, detailed analysis demonstrates that low-dose heparin has a good risk-to-benefit ratio and is cost-effective.
Low-molecular-weight heparins
These drugs are fragments of unfractionated heparin that vary in size from 4,500 to 6,500 daltons. Low-molecular-weight heparin (LMWH) has more anti-Xa and less antithrombin activity than unfractionated heparin and thus has less of an effect on partial thromboplastin time. LMWH may also lead to fewer bleeding complications.7
Once-daily dosing is possible. An increased half-life of 4 hours for LMWH produces greater bioavailability than with low-dose heparin. This allows once-daily dosing.
Pick one: Convenience or cost
Randomized controlled trials have compared LMWH to unfractionated heparin in gynecologic surgical patients. In all studies, DVT occurred in similar, low numbers of women regardless of the heparin used. Bleeding complications also were similar.8
A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise found daily LMWH to be as effective as unfractionated heparin in DVT prophylaxis, without any difference in hemorrhagic complications.9
The choice of drugs often boils down to convenience versus cost: Prophylactic LMWH can be given once a day (compared with 2 or 3 times for unfractionated heparin), but is much more expensive.
Mechanical prophylactic methods
External pneumatic compression rivals low-dose heparin. The largest body of literature on mechanical methods to reduce postoperative venous stasis involves intermittent leg compression by pneumatically inflated sleeves placed around the calf or leg during surgery and after. A number of devices and sleeve designs are available, none of which has proven to be superior to the others.
In my experience, calf compression during and after gynecologic surgery lowers the incidence of DVT to a level seen with low-dose heparin. Besides increasing venous flow and pulsatile emptying of the calf veins, pneumatic compression appears to augment endogenous fibrinolysis, which may stimulate lysis of very early thrombi.10
How long is best for external compression? The optimal duration of postoperative external pneumatic compression is unclear. It may be effective when used in the operating room and for the first 24 hours postoperatively in patients with benign conditions who will ambulate on the first day after surgery.11,12
In women undergoing major surgery for gynecologic malignancy, it reduces the incidence of postoperative venous thromboemboli by nearly 3-fold, but only if calf compression is applied intraoperatively and for the first 5 postoperative days.13,14 These women may remain at risk because of stasis and a hypercoagulable state for a longer time than general surgical patients.
External pneumatic leg compression has no serious side effects or risks and is slightly more cost-effective than prophylactic drugs.15 However, to be fully effective, this method must be used consistently, in compliance with the protocol, when the patient is not ambulating.
Stockings can be a help or hazard. Controlled studies of graduated pressure stockings are limited but suggest modest benefit with careful fitting.16 Poorly fitted stockings that roll down the leg may create a tourniquet effect at the knee or mid-thigh. Another disadvantage of the stockings: The limited sizes available do not allow a perfect fit for all patients. This is especially true in obese patients.
The simplicity of elastic stockings and the absence of serious side effects are probably why stockings are often included in routine postoperative care.
Don’t overlook basic precautions. Although they may offer only modest benefit, short preoperative hospital stays and early postoperative ambulation are recommended.
Another basic strategy: elevating the foot of the bed to raise the calf above heart level. This allows gravity to drain the calf veins and should further reduce stasis.
How to detect VTE
DVT has nonspecific signs and symptoms
When DVT occurs in the lower extremities, harbingers such as pain, edema, and erythema are relatively nonspecific; 50% to 80% of patients exhibiting them do not have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities.
Because of this lack of specificity, additional tests are needed to establish DVT.
Diagnostic studies
A definitive diagnosis of DVT and pulmonary embolism is mandatory because diagnosis based on clinical symptoms and signs alone is frequently wrong. Strategies to reduce the use of ultrasound or spiral CT scanning have been put forward. These studies have evaluated outpatients using algorithms that utilize clinical probability (“clinical decision rule”) and D-dimer levels.
This strategy has been very accurate and avoids the use of ultrasound or spiral CT in low-risk patients. For example, individuals with a low probability score have an incidence of DVT below 5%, so ultrasound is unnecessary. This diagnostic strategy relies on the recognition of elevated D-dimer levels. Unfortunately, D-dimer is increased by a variety of nonthrombotic disorders, including recent surgery, hemorrhage, trauma, pregnancy, and cancer. Therefore, we cannot recommend the use of this strategy for the postoperative gynecologic surgery patient.17,18
Venography no longer the gold standard. Other diagnostic studies may be more useful. Venography has fallen from favor because it is moderately uncomfortable, requires injection of a contrast material that may trigger an allergic reaction or renal injury, and causes phlebitis in approximately 5% of patients.2 Newer, noninvasive diagnostic tests have been developed, fortunately.
Doppler ultrasound. B-mode duplex Doppler imaging is the most common technique to diagnose symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the tip of the ultrasound probe makes it possible to assess venous collapsibility, which is diminished when a thrombus is present.
Doppler imaging is less accurate when evaluating the calf and pelvic veins.
Magnetic resonance venography (MRV) sensitivity and specificity are comparable to venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback is the time required to examine the lower extremity and pelvis. Further, MRV rarely identifies calf thrombi (most often not life-threatening, but potentially symptomatic) and is considerably more expensive than ultrasound.
Which prevention strategy works best?
We now consider low-molecular-weight heparin and external pneumatic compression the best choices
Because low-dose unfractionated heparin, low-molecular-weight heparin (LMWH), and external pneumatic compression all reduce the incidence of postoperative venous thromboembolism in high-risk gynecologic surgical patients, the question is: Which strategy is best?
We conducted 2 randomized clinical trials to answer this question.
Trial 1 Low-dose heparin vs pneumatic compression
Women were randomized to receive either low-dose heparin (5,000 U subcutaneously preoperatively and every 8 hours after surgery until hospital discharge) or external pneumatic compression of the calf prior to surgery and until hospital discharge.1
The incidence of DVT was identical in both groups, and no patients developed a pulmonary embolus throughout 30 days of follow-up. However, bleeding complications occurred more often in the group randomized to low-dose heparin. Specifically, nearly 25% had APTT levels in the “therapeutic” range, and significantly more patients required blood transfusions. After this trial, our institution decided to use external pneumatic compression because of its more favorable risk profile.1
Trial 2 LMWH vs pneumatic compression
The question of the best therapy arose again with the advent of LMWH, because of the possibility that these drugs carried a lower risk of bleeding complications. We therefore conducted a second trial to compare LMWH with external pneumatic compression.2
Because higher doses of LMWH had already proven to be more effective in cancer patients, we gave women in the trial 5,000 U dalteparin (Fragmin) preoperatively and 5,000 U daily postoperatively until hospital discharge.
In this trial, external pneumatic compression and LMWH produced similar low frequencies of DVT and no pulmonary emboli throughout 30 days of follow-up. We also found no association between LMWH and bleeding complications or transfusion requirements. Compliance and patient satisfaction were similar for both modalities.2
Bottom line
We now consider LMWH and external pneumatic compression the best choices for prophylaxis in gynecologic surgical patients.
REFERENCES
1. Clarke-Pearson DL, Synan IS, Dodge R, Soper JT, Berchuck A, Coleman RE. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1154.
2. Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DI. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. Obstet Gynecol. 2001;98:989-995.
Increasing use of laparoscopic surgery raises an important question: What is the thromboembolic risk of laparoscopy itself? On one hand, many laparoscopic surgeries are prolonged, and intraperitoneal pressure from the pneumoperitoneum reduces venous flow. On the other hand, many patients who have laparoscopy have shorter hospital stays and return sooner to normal activities than those who have open procedures.
Although the risks of venous thromboembolism (VTE) have not been studied as thoroughly as other aspects of laparoscopy, they appear to be low. To date, there are no randomized trials of VTE prophylaxis among women undergoing gynecologic laparoscopy.
The prudent course
Nevertheless, it would seem prudent to consider prophylaxis when women with additional risk factors undergo extensive laparoscopic procedures.
Pulmonary embolism is often stealthy
Many of the typical signs and symptoms of pulmonary embolism are associated with other, more common pulmonary complications following surgery. Classic findings that should alert the physician to the possibility of pulmonary embolism include:
- pleuritic chest pain
- hemoptysis
- shortness of breath
- tachycardia
- tachypnea
Often, however, the signs are subtle and may include only persistent tachycardia or a slight elevation in respiration.
When pulmonary embolism is suspected, a chest x-ray, electrocardiography, and arterial blood gas assessment are warranted. Any abnormality justifies further evaluation by ventilation-perfusion lung scan or a spiral computed tomography scan of the chest. Unfortunately, a high percentage of lung scans are interpreted as “indeterminate.” In such cases, careful clinical evaluation and judgment are needed to determine whether pulmonary arteriography is necessary to document or exclude pulmonary embolism.
Immediate, aggressive therapy is crucial
The treatment of postoperative DVT requires immediate anticoagulant therapy using either unfractionated heparin or LMWH, followed by 6 months of oral anticoagulant therapy with warfarin.
Treatment strategy: Unfractionated heparin
Once VTE is diagnosed, start unfractionated heparin to prevent proximal propagation of the thrombus and allow physiologic thrombolytic pathways to dissolve the clot. After an initial IV bolus of 5,000 U, give the patient a continuous infusion of 30,000 U daily, and adjust the dose to maintain APTT levels at a therapeutic level that is 1.5 to 2.5 times the control value.
Subtherapeutic APTT levels in the first 24 hours mean a risk of recurrent thromboembolism 15 times greater than the risk in patients with appropriate levels. Therefore, aggressive management is warranted to achieve prompt anticoagulation.
Start an oral anticoagulant (warfarin) on the first day of heparin infusion, and monitor the international normalized ratio (INR) daily until a therapeutic level is achieved. The change in the INR after warfarin administration often precedes the anticoagulant effect by about 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, it is important to continue the heparin until the INR has been maintained in a therapeutic range for at least 2 days to confirm the proper warfarin dose. Intravenous heparin can be discontinued after 5 days if an adequate INR level has been established.
Alternative strategy: LMWH
A meta-analysis involving more than 1,000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly than unfractionated heparin in preventing recurrent thromboembolism.19 The lower cost derives from the ability to use the drugs in an outpatient setting.
Dosages are unique and weight-adjusted according to each LMWH preparation. Because LMWH has a minimal effect on APTT, serial laboratory monitoring of APTT levels is unnecessary. Nor is monitoring of anti-Xa activity of significant benefit in the dose adjustment of LMWH.
Basic treatment of pulmonary embolism
In most cases, immediate anticoagulant therapy identical to that outlined for DVT is sufficient to prevent repeat thrombosis and embolism and to allow the patient’s endogenous thrombolytic mechanisms to lyse the pulmonary embolus.
Other interventions include:
- Respiratory support, including oxygen, bronchodilators, and intensive care.
- Although massive pulmonary emboli are usually quickly fatal, pulmonary embolectomy has been successful on rare occasions.
- Pulmonary artery catheterization and administration of thrombolytic agents may be important in patients with massive pulmonary embolism.
- Vena cava interruption may be necessary when anticoagulant therapy does not prevent rethrombosis and the formation of emboli from the lower extremities or pelvis. A vena cava umbrella or filter may be inserted percutaneously above the level of the thrombosis and caudad to the renal veins.
Take-home points
- Identify risk factors preoperatively
- VTE prophylaxis is warranted for most gynecologic surgery patients and can reduce the incidence of VTE by at least 60% with appropriate use! Plan prophylaxis in women at moderate, high, and highest risk, and remember that individuals at high and highest risk require more intense prophylaxis to realize a benefit.
- Maintain a high level of suspicion in women with signs and symptoms of DVT or pulmonary embolism in the first postoperative month. It is better to over-evaluate than to miss a potentially fatal complication.
- Treat women with VTE immediately with heparin or LMWH.
1. Jeffcoate TN, Tindall VR. Venous thrombosis and embolism in obstetrics and gynecology. Aust N Z J Obstet Gynecol. 1965;5:119-130.
2. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983;61:87-94.
3. Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. BMJ. 1976;2:910-912.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. An international multicentre trial. Lancet. 1975;2:45-51.
6. Clarke-Pearson DL, DeLong ER, Synan IS, Creasman WT. Complications of low-dose heparin prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 1984;64:689-694.
7. Tapson VF, Hull RD. Management of venous thromboembolic disease. The impact of low-molecular-weight heparin. Clin Chest Med. 1995;16:281-294.
8. Borstad E, Urdal K, Handeland G, Abildgaard U. Comparison of low molecular weight heparin vs. unfractionated heparin in gynecological surgery. II: Reduced dose of low molecular weight heparin. Acta Obstet Gynecol Scand. 1992;71:471-475.
9. Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689-704.
10. Allenby F, Boardman L, Pflug JJ, Calnan JS. Effects of external pneumatic intermittent compression on fibrinolysis in man. Lancet. 1973;2:1412-1414.
11. Salzman EW, Ploetz J, Bettmann M, Skillman J, Klein L. Intraoperative external pneumatic calf compression to afford long-term prophylaxis against deep vein thrombosis in urological patients. Surgery. 1980;87:239-242.
12. Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69-76.
13. Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63:92-98.
14. Clarke-Pearson DL, Creasman WT, Coleman RE, Synan IS, Hinshaw WM. Perioperative external pneumatic calf compression as thromboembolism prophylaxis in gynecologic oncology: report of a randomized controlled trial. Gynecol Oncol. 1984;18:226-232.
15. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
16. Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371-373.
17. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207.
18. Writing Group for the Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical D-dimer testing and computed tomography. JAMA. 2006;295:172-179.
19. Buller HR, Kucher N, Kipfmueller F, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
20. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
The author reports no financial relationships relevant to this article.
1. Jeffcoate TN, Tindall VR. Venous thrombosis and embolism in obstetrics and gynecology. Aust N Z J Obstet Gynecol. 1965;5:119-130.
2. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983;61:87-94.
3. Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. BMJ. 1976;2:910-912.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. An international multicentre trial. Lancet. 1975;2:45-51.
6. Clarke-Pearson DL, DeLong ER, Synan IS, Creasman WT. Complications of low-dose heparin prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 1984;64:689-694.
7. Tapson VF, Hull RD. Management of venous thromboembolic disease. The impact of low-molecular-weight heparin. Clin Chest Med. 1995;16:281-294.
8. Borstad E, Urdal K, Handeland G, Abildgaard U. Comparison of low molecular weight heparin vs. unfractionated heparin in gynecological surgery. II: Reduced dose of low molecular weight heparin. Acta Obstet Gynecol Scand. 1992;71:471-475.
9. Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689-704.
10. Allenby F, Boardman L, Pflug JJ, Calnan JS. Effects of external pneumatic intermittent compression on fibrinolysis in man. Lancet. 1973;2:1412-1414.
11. Salzman EW, Ploetz J, Bettmann M, Skillman J, Klein L. Intraoperative external pneumatic calf compression to afford long-term prophylaxis against deep vein thrombosis in urological patients. Surgery. 1980;87:239-242.
12. Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69-76.
13. Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63:92-98.
14. Clarke-Pearson DL, Creasman WT, Coleman RE, Synan IS, Hinshaw WM. Perioperative external pneumatic calf compression as thromboembolism prophylaxis in gynecologic oncology: report of a randomized controlled trial. Gynecol Oncol. 1984;18:226-232.
15. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
16. Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371-373.
17. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207.
18. Writing Group for the Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical D-dimer testing and computed tomography. JAMA. 2006;295:172-179.
19. Buller HR, Kucher N, Kipfmueller F, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
20. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
The author reports no financial relationships relevant to this article.
Vaginal hysterectomy: Is skill the limiting factor?
CASE Bleeding, a large uterus, and no response to hormones
“M.G.,” a 42-year-old nullipara, complains of menstrual periods that last 10 days and occur on a 28-day cycle. She says the bleeding is extremely heavy, with frequent, copious clotting. She routinely avoids planning social activities around the time of her period and occasionally cancels nonessential engagements because of it. Over the past year, this professional woman has missed 6 days of work because of the problem with her menses.
When you ask about her history, she reports that another gynecologist first palpated an enlarged and irregular uterus 5 years earlier, and an ultrasound at that time revealed a multinodular fundus of approximately 12 weeks’ size. Oral contraceptives were prescribed, but the problem returned to pretreatment levels over the next 3 years. Oral medroxyprogesterone acetate was added to the regimen without success. Hysteroscopy and a dilation and curettage revealed no submucous fibroids, but by then the uterus had enlarged to 14 weeks’ size. M.G. was counseled about continued conservative management, uterine artery embolization, endometrial ablation, and vaginal hysterectomy. She now wants to go ahead with total vaginal hysterectomy and ovarian preservation.
Is the vaginal approach feasible?
Vaginal hysterectomy is not only feasible, it is preferred. Although laparoscopic surgeons are fond of using the phrase “minimally invasive surgery” to describe their procedures, when it comes to hysterectomy, only the vaginal route qualifies for this superlative description. And although uterine size does sometimes limit use of the vaginal route, it need do so in only a minority of cases.
This article describes surgical techniques for vaginal removal of the large uterus, using morcellation, coring, cervicectomy, and other strategies.
Is the vaginal approach always best?
Guidelines addressing this question were developed by the Society of Pelvic Reconstructive Surgeons and evaluated by Kovac et al (FIGURE 1).1 These guidelines, widely used around the world, recommend the vaginal approach for the small, mobile uterus in a pelvis that has no substantial, identifiable pathology. The guidelines recommend the abdominal route when an adnexal mass of unknown character is present or malignancy is suspected. They suggest the use of laparoscopy to identify or quantify pathology and to help convert cases from the abdominal route to laparoscopically assisted vaginal hysterectomy.
I view these guidelines as a minimum standard of care. As a surgeon’s confidence and skills increase, wider application of the vaginal route should be possible in progressively challenging cases. In the personal series of expert surgeons, use of the vaginal route often exceeds 90%. In contrast, the overall US average is 25%, including laparoscopically assisted procedures.2,3
The indications for salpingo-oophorectomy remain the same regardless of route. At present, the adnexa are removed in only 10% of vaginal hysterectomies and in 60% of abdominal procedures.2,3 However, successful routine removal of the adnexa through the vagina is well documented in the literature.4
Contraindications. There are few absolute contraindications to vaginal hysterectomy beyond known or suspected malignancy, but some conditions do increase the technical skill required (TABLE). Nor do complications increase, provided the surgeon has the proper skill and instrumentations.
FIGURE 1 How to choose a hysterectomy route
Source: Kovac SR et al1; used by permission of the American Journal of Obstetrics and Gynecology.TABLE
Contraindications to vaginal hysterectomy
| ABSOLUTE |
|
| RELATIVE |
|
Technique
Every procedure involves 3 basic tasks
Before the uterus can be removed vaginally, the surgeon must:
- enter the peritoneal cavity,
- divide the uterosacral, cardinal, and pubourethral ligamentous attachments of the paracolpium, and
- ligate the uterine artery.
Posterior entry is usually easier
Although peritoneal entry may be anterior or posterior, the latter is almost always easier. Apply an Allis clamp to the vaginal epithelium over the posterior cul-de-sac approximately 2 to 4 cm behind the cervix. Apply a small amount of traction to the clamp to create a vertical crease, which denotes the proper location for colpotomy. Palpate the crease manually to ensure no bowel is present. Then make a full-thickness incision with sharp scissors to enter the peritoneal cavity. Incomplete incisions and blunt dissection simply slow the process.
Once the peritoneum is entered, bluntly extend the incision laterally to the uterosacral ligaments, and place a weighted Steiner-Auvard retractor in the incision.
Dissect first, then divide the ligaments
When leiomyomata are present, anatomical distortion tends to be limited to the fundus; cervical anatomy remains relatively unaltered. After completing the circular cervical incision, dissect the vesicocervical and vesicouterine spaces. Some sharp dissection is usually required at the level of the pericervical ring—the supravaginal septum—which consists of dense fibroelastic connective tissue. Place a Heaney or Breisky-Navratil retractor within the anterior incision to obtain full cervical access.
Next, sequentially clamp, divide, and ligate the uterosacral, cardinal, and pubourethral ligaments. Once this task is done, the vascular bundle containing the uterine artery and veins becomes accessible; divide it as well.
If the anterior peritoneum has not been passively entered, it can now be easily incised.
Now that the suspensory apparatus and the major blood supply have been divided, uteroreductive techniques can be employed.
No absolute size limit
There is no objective limit to the size of a uterus that can be safely removed using reductive techniques. Generally, extra skill and experience are needed to remove an organ larger than 12 weeks’ size (approximately 280 g). Numerous reports document the safe removal of enlarged uteri, even those larger than 1,000 g.5-17
Uterine size is reduced in 3 ways: morcellation, coring, and/or cervicectomy.
The best method varies from case to case, depending on the specific uterine anatomy and the surgeon’s skill. Often, more than 1 debulking technique is used in a single case.
Do not begin debulking until peritoneal access is attained, the paracolpium is divided, and the uterine artery is ligated.
Morcellation is well suited to multiple fibroids
First, sharply divide the cervix, cutting vertically in the midline, and extend the incision into the uterine fundus using the endocervical canal and endometrial cavity as visual guides for the incision (FIGURE 2A). As leiomyomata are encountered, grasp each with a Myotome grasper (Marina Medical, Hollywood, Fla) and remove them with Myotomes (Marina Medical). Both the spoon-tipped and chisel-tipped Myotomes have dissecting tips that allow rapid and precise enucleation of tumors. The tip is sharp enough to dissect the capsule of the myomata, but not so sharp that it endangers adjacent structures.
Continue to remove the fibroids as they become accessible. If incisions into the serosa of the uterus are necessary to remove palpable subserosal tumors, make the incisions under direct vision. Access to the serosa usually is greater on the posterior surface of the uterus. With adequate retraction, the anterior surface can also be incised.
If a bulky uterus prevents immediate access to leiomyomata, one strategy is to remove elliptical wedges of myometrium adjacent to the uterine bisecting incision. The Martin myomectomy scissors (Marina Medical) have serrated edges originally designed by orthopedists to cut cartilage, as well as sharp tips that can be inserted into a tumor prior to cutting. They help debulk large myomata (FIGURE 2B) and can be used to quickly remove wedges of myometrium. After sufficient debulking, large myomata can be removed safely (FIGURE 2C).
This morcellation method minimizes the need to use a knife in the “invisible” upper reaches of the fundus. Continuous downward traction on the divided cervix prevents bleeding, and the gradual reduction in size of the debulked fundus allows for sufficient descent of the uterus; it also permits posterior rotation. Ultimately, it becomes possible to clamp the utero-ovarian pedicles and to completely remove the uterus.
In most cases, the uterine serosa can be left intact using morcellation. The size of the uterus that can be removed using this technique is limited only by the experience of the surgeon (FIGURE 3).
FIGURE 2 Expose, debulk, and remove the dominant myoma
Incise the cervix along the midline to gain access to the fundus and expose the dominant myoma.
Insert the sharp tip of the scissors into the myoma prior to cutting.
When the myoma has been sufficiently debulked, remove it through the vagina.
FIGURE 3 Massive uteri can be removed vaginally
This uterus and multiple myomata were removed from a single patient using the vaginal route.
Use coring for moderately enlarged uteri
This is a useful technique when the uterus contains multiple small leiomyomata, a single dominant tumor, or cicatrized adenomyosis. Begin by making a circular incision into the fundus of the uterus just above the isthmus. The incision should be parallel to the central axis of the endometrial cavity.18
Apply firm downward traction on the cervix to allow the portion of the uterus central to the incision to evert. Continue to incise the uterus in a circular pattern to allow more of the bulk of the fundus to descend.
This technique converts the globular, anatomically distorted fundus into a cylinder. Be sure to make the encircling incision under direct vision to reduce the risk of injuring adjacent structures.
In most cases, coring has the advantage of leaving the endometrial cavity and serosa intact. With practice, this technique can quickly and reliably reduce a large uterus to a manageable size.
In some cases, you may have to remove the cervix before debulking
If the cervix is particularly bulky or prevents access to the fundus, perform cervicectomy prior to debulking. This type of debulking is not highly technical, and it may make the remainder of the procedure easier to accomplish. As debulking proceeds, use the endocervical canal or endometrial cavity for orientation.
When complete removal is impossible
Occasionally, it is not possible to complete vaginal removal of the large uterus. While this scenario is not ideal, no evidence exists that conversion to the abdominal or laparoscopic route endangers the patient, especially if the decision is made in a timely and judicious manner. Perform cervicectomy before converting to the abdominal or laparoscopic route. The cervical cuff may also be closed prior to conversion.
Be sure to weigh the specimen
Inform the pathologist of the reason for the morcellated specimen so that an accurate total weight can be determined. This is important because CPT codes for uteri larger than 250 g carry more relative value units than the codes for smaller uteri, based on the extra time in the OR as well as the greater technical skill required.
CASE 610-g uterus safely removed
M.G. undergoes vaginal hysterectomy with uteroreductive morcellation. Estimated blood loss is 200 cc.
The morning after her surgery, M.G. voids after removal of the urinary catheter, and is able to tolerate a regular diet. She walks without difficulty and is discharged home. Seven days after her surgery, she returns to work. Her job allows her the freedom to define her own responsibilities, and she has no manual duties.
The pathology report reveals that her uterus weighed 610 g, with multiple leiomyomata. The largest myoma was 8.0×5.5×4.0 cm. No other abnormalities were present.
One year later, M.G. reports a substantially improved lifestyle and expresses satisfaction with her decision to undergo vaginal hysterectomy.
1. Kovac SR, Barhan S, Lister M, Tucker L, Bishop M, Das A. Guidelines for the selection of the route of hysterectomy: application in a resident clinic population. Am J Obstet Gynecol. 2002;187:1521-1527.
2. Kovac SR. Guidelines to determine the route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
3. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
4. Sheth S, Malpani A. Routine prophylactic oophorectomy at the time of vaginal hysterectomy in postmenopausal women. Arch Gynecol Obstet. 1992;251:87-91.
5. El-Lamie IK. Vaginal hysterectomy for uteri weighing 250 grams or more. J Pelvic Surg. 2001;7:140-146.
6. Grody MH. Vaginal hysterectomy: the large uterus. J Gynecol Surg. 1989;5:301-312.
7. Grody MH. Vaginal hysterectomy: the enlarged uterus. Operative Tech Gynecol Surg. 1999;4:53-61.
8. Grody MH, Pruzbylko K, Pagano AM. A practical method for removal of the huge benign fibromyomatous uterus through the vaginal route. J Pelvic Surg. 2000;6:39-44.
9. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico’s experience. Obstet Gynecol. 1996;88:560-563.
10. Larson SL. Uterine morcellation-review of 443 cases. Obstet Gynecol. 1999;4:61S.-
11. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
12. Lash AF. Technique for removal of abnormally large uteri without entering cavities. Clin Obstet Gynecol. 1961;4:210-216.
13. Magos A, Bournas N, Sinha R, et al. Vaginal hysterectomy for the large uterus. Br J Obstet Gynecol. 1996;103:246-251.
14. Moen MD, Webb MJ, Wilson TO. Vaginal hysterectomy in patients with benign uterine enlargement. J Pelvic Surg. 1995;4:197-203.
15. Peham H, Amreich I, Ferguson L. Operative Gynecology. Philadelphia: JB Lippincott; 1934.
16. Pelosi MA, II, Pelosi MA, III. Should uterine size alone require laparoscopic assistance? Vaginal hysterectomy for a 2,003-g uterus. J Lapendo Adv Surg Tech. 1998;8:99-103.
17. Pratt JH, Gunnlaugsson GH. Vaginal hysterectomy by morcellation. Mayo Clin Proc. 1970;45:374-387.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-134.
Dr. Zimmerman reports that he is a consultant and instrument designer for Marina Medical, Inc.
CASE Bleeding, a large uterus, and no response to hormones
“M.G.,” a 42-year-old nullipara, complains of menstrual periods that last 10 days and occur on a 28-day cycle. She says the bleeding is extremely heavy, with frequent, copious clotting. She routinely avoids planning social activities around the time of her period and occasionally cancels nonessential engagements because of it. Over the past year, this professional woman has missed 6 days of work because of the problem with her menses.
When you ask about her history, she reports that another gynecologist first palpated an enlarged and irregular uterus 5 years earlier, and an ultrasound at that time revealed a multinodular fundus of approximately 12 weeks’ size. Oral contraceptives were prescribed, but the problem returned to pretreatment levels over the next 3 years. Oral medroxyprogesterone acetate was added to the regimen without success. Hysteroscopy and a dilation and curettage revealed no submucous fibroids, but by then the uterus had enlarged to 14 weeks’ size. M.G. was counseled about continued conservative management, uterine artery embolization, endometrial ablation, and vaginal hysterectomy. She now wants to go ahead with total vaginal hysterectomy and ovarian preservation.
Is the vaginal approach feasible?
Vaginal hysterectomy is not only feasible, it is preferred. Although laparoscopic surgeons are fond of using the phrase “minimally invasive surgery” to describe their procedures, when it comes to hysterectomy, only the vaginal route qualifies for this superlative description. And although uterine size does sometimes limit use of the vaginal route, it need do so in only a minority of cases.
This article describes surgical techniques for vaginal removal of the large uterus, using morcellation, coring, cervicectomy, and other strategies.
Is the vaginal approach always best?
Guidelines addressing this question were developed by the Society of Pelvic Reconstructive Surgeons and evaluated by Kovac et al (FIGURE 1).1 These guidelines, widely used around the world, recommend the vaginal approach for the small, mobile uterus in a pelvis that has no substantial, identifiable pathology. The guidelines recommend the abdominal route when an adnexal mass of unknown character is present or malignancy is suspected. They suggest the use of laparoscopy to identify or quantify pathology and to help convert cases from the abdominal route to laparoscopically assisted vaginal hysterectomy.
I view these guidelines as a minimum standard of care. As a surgeon’s confidence and skills increase, wider application of the vaginal route should be possible in progressively challenging cases. In the personal series of expert surgeons, use of the vaginal route often exceeds 90%. In contrast, the overall US average is 25%, including laparoscopically assisted procedures.2,3
The indications for salpingo-oophorectomy remain the same regardless of route. At present, the adnexa are removed in only 10% of vaginal hysterectomies and in 60% of abdominal procedures.2,3 However, successful routine removal of the adnexa through the vagina is well documented in the literature.4
Contraindications. There are few absolute contraindications to vaginal hysterectomy beyond known or suspected malignancy, but some conditions do increase the technical skill required (TABLE). Nor do complications increase, provided the surgeon has the proper skill and instrumentations.
FIGURE 1 How to choose a hysterectomy route
Source: Kovac SR et al1; used by permission of the American Journal of Obstetrics and Gynecology.TABLE
Contraindications to vaginal hysterectomy
| ABSOLUTE |
|
| RELATIVE |
|
Technique
Every procedure involves 3 basic tasks
Before the uterus can be removed vaginally, the surgeon must:
- enter the peritoneal cavity,
- divide the uterosacral, cardinal, and pubourethral ligamentous attachments of the paracolpium, and
- ligate the uterine artery.
Posterior entry is usually easier
Although peritoneal entry may be anterior or posterior, the latter is almost always easier. Apply an Allis clamp to the vaginal epithelium over the posterior cul-de-sac approximately 2 to 4 cm behind the cervix. Apply a small amount of traction to the clamp to create a vertical crease, which denotes the proper location for colpotomy. Palpate the crease manually to ensure no bowel is present. Then make a full-thickness incision with sharp scissors to enter the peritoneal cavity. Incomplete incisions and blunt dissection simply slow the process.
Once the peritoneum is entered, bluntly extend the incision laterally to the uterosacral ligaments, and place a weighted Steiner-Auvard retractor in the incision.
Dissect first, then divide the ligaments
When leiomyomata are present, anatomical distortion tends to be limited to the fundus; cervical anatomy remains relatively unaltered. After completing the circular cervical incision, dissect the vesicocervical and vesicouterine spaces. Some sharp dissection is usually required at the level of the pericervical ring—the supravaginal septum—which consists of dense fibroelastic connective tissue. Place a Heaney or Breisky-Navratil retractor within the anterior incision to obtain full cervical access.
Next, sequentially clamp, divide, and ligate the uterosacral, cardinal, and pubourethral ligaments. Once this task is done, the vascular bundle containing the uterine artery and veins becomes accessible; divide it as well.
If the anterior peritoneum has not been passively entered, it can now be easily incised.
Now that the suspensory apparatus and the major blood supply have been divided, uteroreductive techniques can be employed.
No absolute size limit
There is no objective limit to the size of a uterus that can be safely removed using reductive techniques. Generally, extra skill and experience are needed to remove an organ larger than 12 weeks’ size (approximately 280 g). Numerous reports document the safe removal of enlarged uteri, even those larger than 1,000 g.5-17
Uterine size is reduced in 3 ways: morcellation, coring, and/or cervicectomy.
The best method varies from case to case, depending on the specific uterine anatomy and the surgeon’s skill. Often, more than 1 debulking technique is used in a single case.
Do not begin debulking until peritoneal access is attained, the paracolpium is divided, and the uterine artery is ligated.
Morcellation is well suited to multiple fibroids
First, sharply divide the cervix, cutting vertically in the midline, and extend the incision into the uterine fundus using the endocervical canal and endometrial cavity as visual guides for the incision (FIGURE 2A). As leiomyomata are encountered, grasp each with a Myotome grasper (Marina Medical, Hollywood, Fla) and remove them with Myotomes (Marina Medical). Both the spoon-tipped and chisel-tipped Myotomes have dissecting tips that allow rapid and precise enucleation of tumors. The tip is sharp enough to dissect the capsule of the myomata, but not so sharp that it endangers adjacent structures.
Continue to remove the fibroids as they become accessible. If incisions into the serosa of the uterus are necessary to remove palpable subserosal tumors, make the incisions under direct vision. Access to the serosa usually is greater on the posterior surface of the uterus. With adequate retraction, the anterior surface can also be incised.
If a bulky uterus prevents immediate access to leiomyomata, one strategy is to remove elliptical wedges of myometrium adjacent to the uterine bisecting incision. The Martin myomectomy scissors (Marina Medical) have serrated edges originally designed by orthopedists to cut cartilage, as well as sharp tips that can be inserted into a tumor prior to cutting. They help debulk large myomata (FIGURE 2B) and can be used to quickly remove wedges of myometrium. After sufficient debulking, large myomata can be removed safely (FIGURE 2C).
This morcellation method minimizes the need to use a knife in the “invisible” upper reaches of the fundus. Continuous downward traction on the divided cervix prevents bleeding, and the gradual reduction in size of the debulked fundus allows for sufficient descent of the uterus; it also permits posterior rotation. Ultimately, it becomes possible to clamp the utero-ovarian pedicles and to completely remove the uterus.
In most cases, the uterine serosa can be left intact using morcellation. The size of the uterus that can be removed using this technique is limited only by the experience of the surgeon (FIGURE 3).
FIGURE 2 Expose, debulk, and remove the dominant myoma
Incise the cervix along the midline to gain access to the fundus and expose the dominant myoma.
Insert the sharp tip of the scissors into the myoma prior to cutting.
When the myoma has been sufficiently debulked, remove it through the vagina.
FIGURE 3 Massive uteri can be removed vaginally
This uterus and multiple myomata were removed from a single patient using the vaginal route.
Use coring for moderately enlarged uteri
This is a useful technique when the uterus contains multiple small leiomyomata, a single dominant tumor, or cicatrized adenomyosis. Begin by making a circular incision into the fundus of the uterus just above the isthmus. The incision should be parallel to the central axis of the endometrial cavity.18
Apply firm downward traction on the cervix to allow the portion of the uterus central to the incision to evert. Continue to incise the uterus in a circular pattern to allow more of the bulk of the fundus to descend.
This technique converts the globular, anatomically distorted fundus into a cylinder. Be sure to make the encircling incision under direct vision to reduce the risk of injuring adjacent structures.
In most cases, coring has the advantage of leaving the endometrial cavity and serosa intact. With practice, this technique can quickly and reliably reduce a large uterus to a manageable size.
In some cases, you may have to remove the cervix before debulking
If the cervix is particularly bulky or prevents access to the fundus, perform cervicectomy prior to debulking. This type of debulking is not highly technical, and it may make the remainder of the procedure easier to accomplish. As debulking proceeds, use the endocervical canal or endometrial cavity for orientation.
When complete removal is impossible
Occasionally, it is not possible to complete vaginal removal of the large uterus. While this scenario is not ideal, no evidence exists that conversion to the abdominal or laparoscopic route endangers the patient, especially if the decision is made in a timely and judicious manner. Perform cervicectomy before converting to the abdominal or laparoscopic route. The cervical cuff may also be closed prior to conversion.
Be sure to weigh the specimen
Inform the pathologist of the reason for the morcellated specimen so that an accurate total weight can be determined. This is important because CPT codes for uteri larger than 250 g carry more relative value units than the codes for smaller uteri, based on the extra time in the OR as well as the greater technical skill required.
CASE 610-g uterus safely removed
M.G. undergoes vaginal hysterectomy with uteroreductive morcellation. Estimated blood loss is 200 cc.
The morning after her surgery, M.G. voids after removal of the urinary catheter, and is able to tolerate a regular diet. She walks without difficulty and is discharged home. Seven days after her surgery, she returns to work. Her job allows her the freedom to define her own responsibilities, and she has no manual duties.
The pathology report reveals that her uterus weighed 610 g, with multiple leiomyomata. The largest myoma was 8.0×5.5×4.0 cm. No other abnormalities were present.
One year later, M.G. reports a substantially improved lifestyle and expresses satisfaction with her decision to undergo vaginal hysterectomy.
CASE Bleeding, a large uterus, and no response to hormones
“M.G.,” a 42-year-old nullipara, complains of menstrual periods that last 10 days and occur on a 28-day cycle. She says the bleeding is extremely heavy, with frequent, copious clotting. She routinely avoids planning social activities around the time of her period and occasionally cancels nonessential engagements because of it. Over the past year, this professional woman has missed 6 days of work because of the problem with her menses.
When you ask about her history, she reports that another gynecologist first palpated an enlarged and irregular uterus 5 years earlier, and an ultrasound at that time revealed a multinodular fundus of approximately 12 weeks’ size. Oral contraceptives were prescribed, but the problem returned to pretreatment levels over the next 3 years. Oral medroxyprogesterone acetate was added to the regimen without success. Hysteroscopy and a dilation and curettage revealed no submucous fibroids, but by then the uterus had enlarged to 14 weeks’ size. M.G. was counseled about continued conservative management, uterine artery embolization, endometrial ablation, and vaginal hysterectomy. She now wants to go ahead with total vaginal hysterectomy and ovarian preservation.
Is the vaginal approach feasible?
Vaginal hysterectomy is not only feasible, it is preferred. Although laparoscopic surgeons are fond of using the phrase “minimally invasive surgery” to describe their procedures, when it comes to hysterectomy, only the vaginal route qualifies for this superlative description. And although uterine size does sometimes limit use of the vaginal route, it need do so in only a minority of cases.
This article describes surgical techniques for vaginal removal of the large uterus, using morcellation, coring, cervicectomy, and other strategies.
Is the vaginal approach always best?
Guidelines addressing this question were developed by the Society of Pelvic Reconstructive Surgeons and evaluated by Kovac et al (FIGURE 1).1 These guidelines, widely used around the world, recommend the vaginal approach for the small, mobile uterus in a pelvis that has no substantial, identifiable pathology. The guidelines recommend the abdominal route when an adnexal mass of unknown character is present or malignancy is suspected. They suggest the use of laparoscopy to identify or quantify pathology and to help convert cases from the abdominal route to laparoscopically assisted vaginal hysterectomy.
I view these guidelines as a minimum standard of care. As a surgeon’s confidence and skills increase, wider application of the vaginal route should be possible in progressively challenging cases. In the personal series of expert surgeons, use of the vaginal route often exceeds 90%. In contrast, the overall US average is 25%, including laparoscopically assisted procedures.2,3
The indications for salpingo-oophorectomy remain the same regardless of route. At present, the adnexa are removed in only 10% of vaginal hysterectomies and in 60% of abdominal procedures.2,3 However, successful routine removal of the adnexa through the vagina is well documented in the literature.4
Contraindications. There are few absolute contraindications to vaginal hysterectomy beyond known or suspected malignancy, but some conditions do increase the technical skill required (TABLE). Nor do complications increase, provided the surgeon has the proper skill and instrumentations.
FIGURE 1 How to choose a hysterectomy route
Source: Kovac SR et al1; used by permission of the American Journal of Obstetrics and Gynecology.TABLE
Contraindications to vaginal hysterectomy
| ABSOLUTE |
|
| RELATIVE |
|
Technique
Every procedure involves 3 basic tasks
Before the uterus can be removed vaginally, the surgeon must:
- enter the peritoneal cavity,
- divide the uterosacral, cardinal, and pubourethral ligamentous attachments of the paracolpium, and
- ligate the uterine artery.
Posterior entry is usually easier
Although peritoneal entry may be anterior or posterior, the latter is almost always easier. Apply an Allis clamp to the vaginal epithelium over the posterior cul-de-sac approximately 2 to 4 cm behind the cervix. Apply a small amount of traction to the clamp to create a vertical crease, which denotes the proper location for colpotomy. Palpate the crease manually to ensure no bowel is present. Then make a full-thickness incision with sharp scissors to enter the peritoneal cavity. Incomplete incisions and blunt dissection simply slow the process.
Once the peritoneum is entered, bluntly extend the incision laterally to the uterosacral ligaments, and place a weighted Steiner-Auvard retractor in the incision.
Dissect first, then divide the ligaments
When leiomyomata are present, anatomical distortion tends to be limited to the fundus; cervical anatomy remains relatively unaltered. After completing the circular cervical incision, dissect the vesicocervical and vesicouterine spaces. Some sharp dissection is usually required at the level of the pericervical ring—the supravaginal septum—which consists of dense fibroelastic connective tissue. Place a Heaney or Breisky-Navratil retractor within the anterior incision to obtain full cervical access.
Next, sequentially clamp, divide, and ligate the uterosacral, cardinal, and pubourethral ligaments. Once this task is done, the vascular bundle containing the uterine artery and veins becomes accessible; divide it as well.
If the anterior peritoneum has not been passively entered, it can now be easily incised.
Now that the suspensory apparatus and the major blood supply have been divided, uteroreductive techniques can be employed.
No absolute size limit
There is no objective limit to the size of a uterus that can be safely removed using reductive techniques. Generally, extra skill and experience are needed to remove an organ larger than 12 weeks’ size (approximately 280 g). Numerous reports document the safe removal of enlarged uteri, even those larger than 1,000 g.5-17
Uterine size is reduced in 3 ways: morcellation, coring, and/or cervicectomy.
The best method varies from case to case, depending on the specific uterine anatomy and the surgeon’s skill. Often, more than 1 debulking technique is used in a single case.
Do not begin debulking until peritoneal access is attained, the paracolpium is divided, and the uterine artery is ligated.
Morcellation is well suited to multiple fibroids
First, sharply divide the cervix, cutting vertically in the midline, and extend the incision into the uterine fundus using the endocervical canal and endometrial cavity as visual guides for the incision (FIGURE 2A). As leiomyomata are encountered, grasp each with a Myotome grasper (Marina Medical, Hollywood, Fla) and remove them with Myotomes (Marina Medical). Both the spoon-tipped and chisel-tipped Myotomes have dissecting tips that allow rapid and precise enucleation of tumors. The tip is sharp enough to dissect the capsule of the myomata, but not so sharp that it endangers adjacent structures.
Continue to remove the fibroids as they become accessible. If incisions into the serosa of the uterus are necessary to remove palpable subserosal tumors, make the incisions under direct vision. Access to the serosa usually is greater on the posterior surface of the uterus. With adequate retraction, the anterior surface can also be incised.
If a bulky uterus prevents immediate access to leiomyomata, one strategy is to remove elliptical wedges of myometrium adjacent to the uterine bisecting incision. The Martin myomectomy scissors (Marina Medical) have serrated edges originally designed by orthopedists to cut cartilage, as well as sharp tips that can be inserted into a tumor prior to cutting. They help debulk large myomata (FIGURE 2B) and can be used to quickly remove wedges of myometrium. After sufficient debulking, large myomata can be removed safely (FIGURE 2C).
This morcellation method minimizes the need to use a knife in the “invisible” upper reaches of the fundus. Continuous downward traction on the divided cervix prevents bleeding, and the gradual reduction in size of the debulked fundus allows for sufficient descent of the uterus; it also permits posterior rotation. Ultimately, it becomes possible to clamp the utero-ovarian pedicles and to completely remove the uterus.
In most cases, the uterine serosa can be left intact using morcellation. The size of the uterus that can be removed using this technique is limited only by the experience of the surgeon (FIGURE 3).
FIGURE 2 Expose, debulk, and remove the dominant myoma
Incise the cervix along the midline to gain access to the fundus and expose the dominant myoma.
Insert the sharp tip of the scissors into the myoma prior to cutting.
When the myoma has been sufficiently debulked, remove it through the vagina.
FIGURE 3 Massive uteri can be removed vaginally
This uterus and multiple myomata were removed from a single patient using the vaginal route.
Use coring for moderately enlarged uteri
This is a useful technique when the uterus contains multiple small leiomyomata, a single dominant tumor, or cicatrized adenomyosis. Begin by making a circular incision into the fundus of the uterus just above the isthmus. The incision should be parallel to the central axis of the endometrial cavity.18
Apply firm downward traction on the cervix to allow the portion of the uterus central to the incision to evert. Continue to incise the uterus in a circular pattern to allow more of the bulk of the fundus to descend.
This technique converts the globular, anatomically distorted fundus into a cylinder. Be sure to make the encircling incision under direct vision to reduce the risk of injuring adjacent structures.
In most cases, coring has the advantage of leaving the endometrial cavity and serosa intact. With practice, this technique can quickly and reliably reduce a large uterus to a manageable size.
In some cases, you may have to remove the cervix before debulking
If the cervix is particularly bulky or prevents access to the fundus, perform cervicectomy prior to debulking. This type of debulking is not highly technical, and it may make the remainder of the procedure easier to accomplish. As debulking proceeds, use the endocervical canal or endometrial cavity for orientation.
When complete removal is impossible
Occasionally, it is not possible to complete vaginal removal of the large uterus. While this scenario is not ideal, no evidence exists that conversion to the abdominal or laparoscopic route endangers the patient, especially if the decision is made in a timely and judicious manner. Perform cervicectomy before converting to the abdominal or laparoscopic route. The cervical cuff may also be closed prior to conversion.
Be sure to weigh the specimen
Inform the pathologist of the reason for the morcellated specimen so that an accurate total weight can be determined. This is important because CPT codes for uteri larger than 250 g carry more relative value units than the codes for smaller uteri, based on the extra time in the OR as well as the greater technical skill required.
CASE 610-g uterus safely removed
M.G. undergoes vaginal hysterectomy with uteroreductive morcellation. Estimated blood loss is 200 cc.
The morning after her surgery, M.G. voids after removal of the urinary catheter, and is able to tolerate a regular diet. She walks without difficulty and is discharged home. Seven days after her surgery, she returns to work. Her job allows her the freedom to define her own responsibilities, and she has no manual duties.
The pathology report reveals that her uterus weighed 610 g, with multiple leiomyomata. The largest myoma was 8.0×5.5×4.0 cm. No other abnormalities were present.
One year later, M.G. reports a substantially improved lifestyle and expresses satisfaction with her decision to undergo vaginal hysterectomy.
1. Kovac SR, Barhan S, Lister M, Tucker L, Bishop M, Das A. Guidelines for the selection of the route of hysterectomy: application in a resident clinic population. Am J Obstet Gynecol. 2002;187:1521-1527.
2. Kovac SR. Guidelines to determine the route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
3. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
4. Sheth S, Malpani A. Routine prophylactic oophorectomy at the time of vaginal hysterectomy in postmenopausal women. Arch Gynecol Obstet. 1992;251:87-91.
5. El-Lamie IK. Vaginal hysterectomy for uteri weighing 250 grams or more. J Pelvic Surg. 2001;7:140-146.
6. Grody MH. Vaginal hysterectomy: the large uterus. J Gynecol Surg. 1989;5:301-312.
7. Grody MH. Vaginal hysterectomy: the enlarged uterus. Operative Tech Gynecol Surg. 1999;4:53-61.
8. Grody MH, Pruzbylko K, Pagano AM. A practical method for removal of the huge benign fibromyomatous uterus through the vaginal route. J Pelvic Surg. 2000;6:39-44.
9. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico’s experience. Obstet Gynecol. 1996;88:560-563.
10. Larson SL. Uterine morcellation-review of 443 cases. Obstet Gynecol. 1999;4:61S.-
11. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
12. Lash AF. Technique for removal of abnormally large uteri without entering cavities. Clin Obstet Gynecol. 1961;4:210-216.
13. Magos A, Bournas N, Sinha R, et al. Vaginal hysterectomy for the large uterus. Br J Obstet Gynecol. 1996;103:246-251.
14. Moen MD, Webb MJ, Wilson TO. Vaginal hysterectomy in patients with benign uterine enlargement. J Pelvic Surg. 1995;4:197-203.
15. Peham H, Amreich I, Ferguson L. Operative Gynecology. Philadelphia: JB Lippincott; 1934.
16. Pelosi MA, II, Pelosi MA, III. Should uterine size alone require laparoscopic assistance? Vaginal hysterectomy for a 2,003-g uterus. J Lapendo Adv Surg Tech. 1998;8:99-103.
17. Pratt JH, Gunnlaugsson GH. Vaginal hysterectomy by morcellation. Mayo Clin Proc. 1970;45:374-387.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-134.
Dr. Zimmerman reports that he is a consultant and instrument designer for Marina Medical, Inc.
1. Kovac SR, Barhan S, Lister M, Tucker L, Bishop M, Das A. Guidelines for the selection of the route of hysterectomy: application in a resident clinic population. Am J Obstet Gynecol. 2002;187:1521-1527.
2. Kovac SR. Guidelines to determine the route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
3. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
4. Sheth S, Malpani A. Routine prophylactic oophorectomy at the time of vaginal hysterectomy in postmenopausal women. Arch Gynecol Obstet. 1992;251:87-91.
5. El-Lamie IK. Vaginal hysterectomy for uteri weighing 250 grams or more. J Pelvic Surg. 2001;7:140-146.
6. Grody MH. Vaginal hysterectomy: the large uterus. J Gynecol Surg. 1989;5:301-312.
7. Grody MH. Vaginal hysterectomy: the enlarged uterus. Operative Tech Gynecol Surg. 1999;4:53-61.
8. Grody MH, Pruzbylko K, Pagano AM. A practical method for removal of the huge benign fibromyomatous uterus through the vaginal route. J Pelvic Surg. 2000;6:39-44.
9. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico’s experience. Obstet Gynecol. 1996;88:560-563.
10. Larson SL. Uterine morcellation-review of 443 cases. Obstet Gynecol. 1999;4:61S.-
11. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
12. Lash AF. Technique for removal of abnormally large uteri without entering cavities. Clin Obstet Gynecol. 1961;4:210-216.
13. Magos A, Bournas N, Sinha R, et al. Vaginal hysterectomy for the large uterus. Br J Obstet Gynecol. 1996;103:246-251.
14. Moen MD, Webb MJ, Wilson TO. Vaginal hysterectomy in patients with benign uterine enlargement. J Pelvic Surg. 1995;4:197-203.
15. Peham H, Amreich I, Ferguson L. Operative Gynecology. Philadelphia: JB Lippincott; 1934.
16. Pelosi MA, II, Pelosi MA, III. Should uterine size alone require laparoscopic assistance? Vaginal hysterectomy for a 2,003-g uterus. J Lapendo Adv Surg Tech. 1998;8:99-103.
17. Pratt JH, Gunnlaugsson GH. Vaginal hysterectomy by morcellation. Mayo Clin Proc. 1970;45:374-387.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-134.
Dr. Zimmerman reports that he is a consultant and instrument designer for Marina Medical, Inc.