User login
Hysterectomy: Which route for which patient?
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati, Ohio.
- Tommaso Falcone, MD, Professor and Chairman, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Thomas Herzog, MD, Director, Division of Gynecologic Oncology, Physicians and Surgeons Alumni Professor, Columbia University Medical Center, New York City.
- Barbara S. Levy, MD, Medical Director, Women’s Health Center, Franciscan Health System, Federal Way, Wash. Dr. Levy serves on the OBG MANAGEMENT Board of Editors.
Why the continued reliance on the abdominal approach despite convincing evidence that vaginal and laparoscopicassisted vaginal hysterectomy (LAVH) offer faster recovery, better cosmesis, and, in many cases, a shorter operation with fewer complications?
OBG Management convened a panel of experts in different aspects of gynecologic surgery to explore this issue. They discuss the reasons most physicians prefer the abdominal approach, how residency programs affect the choice of hysterectomy route, indications for LAVH and supracervical hysterectomy, the issue of ovarian conservation, and management of uterine fibroids.
Why the abdominal route remains the old standby
Physicians use the procedure they are most comfortable with, and residents lack sufficient hands-on experience with laparoscopic and vaginal surgery. Medicolegal risk and reimbursement also have an impact.
KARRAM: Hysterectomy is one of the most widely performed surgeries in the United States, but approximately 60% to 80% of these surgeries still involve the abdominal route.2 Why do you think that is?
FALCONE: Most physicians practice in the manner they were trained, and most residency programs train residents to perform abdominal hysterectomy.
LEVY: I agree. Residents in obstetrics and gynecology have a limited time frame in which to learn and become facile with surgical gynecology. The requirements for primary care training and continuity clinics leave little time for the resident to become comfortable with endoscopic and vaginal surgery. However, they do get substantial exposure to abdominal surgery, both in obstetrics (with cesarean sections constituting 27.5% of all deliveries in the United States3) and gynecologic oncology rotations.
Furthermore, the volume of benign gynecologic surgery is low and the technical skills required for laparoscopic and vaginal surgery are more challenging than “slash and gash” abdominal surgery, so residents don’t get enough exposure to develop a comfort level with these procedures.
HERZOG: This trend is likely to change in the future because recent and current trainees have much more exposure to the laparoscopic approach than in the past, and the equipment has continued to improve. However, I have serious doubts about whether adequate amounts of vaginal surgery are performed in training programs to educate the next generation of vaginal surgeons.
LEVY: Another factor is the type of practice physicians enter after training. Most join practices in which the bulk of their income for many years derives from obstetrics. Without a mentor in the practice who is skilled at minimally invasive surgery, most of these young physicians appropriately resort to the hysterectomy approach for which they have the most comfort and skill: the abdominal route.
HERZOG: Secondary barriers to nonabdominal procedures are lower reimbursement and heightened medicolegal risk, since time and complications are greater for laparoscopic surgery and, to a lesser degree, vaginal procedures. Surgeons are not adequately compensated for either the increased time or risk.
Patients also tend to have higher expectations when the planned approach is minimally invasive. When conversion to laparotomy is necessary, the patient and her family may have trouble understanding why.
KARRAM: I agree that there is a serious lack of training in simple and complicated vaginal hysterectomy. Many inaccurate perceptions have been handed down over the years about its absolute and relative contraindications, such as the belief that any history of pelvic infection, endometriosis, or cesarean section is a contraindication for the vaginal approach.
When is laparoscopic assistance appropriate?
At a fundamental level, its value lies in converting abdominal hysterectomy intovaginal hysterectomy.
KARRAM: In my experience, LAVH is appropriate in the presence of benign adnexal pathology: The adnexa can be evaluated and detached laparoscopically followed by vaginal hysterectomy and vaginal removal of the adnexa. It also is appropriate in any situation that involves excessive pelvic adhesions. The uterus can be mobilized laparoscopically, followed by removal through the vagina.
FALCONE: Any patient who is not a candidate for vaginal hysterectomy should be considered for laparoscopic assistance. The general rationale for the surgery is to convert an abdominal hysterectomy into a vaginal one, so the surgeon should start laparoscopically and then switch to the vaginal approach as soon as possible. Of course, it is impossible to proceed vaginally in some cases. When it is, the entire case can be performed laparoscopically.
LEVY: In my hands, patients with an unidentified adnexal mass who also need or request hysterectomy are appropriate candidates for laparoscopic abdominal exploration followed by vaginal hysterectomy if appropriate. Women with a very contracted pelvis, which precludes transvaginal access to the uterine vasculature, may also be candidates for the laparoscopic approach. However, as surgeons become more skilled at vaginal surgery and learn to use newer instrumentation, the need for laparoscopy to access a tight pelvis will diminish.
Using a laparoscopic approach for patients with fibroids wedged into the pelvis carries serious risk to the pelvic sidewall. It is very difficult to access the sidewall safely laparoscopically in the presence of a large lower segment or cervical myomas.
HERZOG: When LAVH was introduced, many clinicians challenged the utility of combined laparoscopic and vaginal surgery, with some referring to this surgical exercise as a procedure looking for an indication. However, as operative laparoscopy has gained acceptance, some benefits of LAVH have become apparent. The greatest advantage is the potential to convert a procedure that would have been performed abdominally into a vaginal hysterectomy.
The most commonly cited indications for LAVH are to lyse adhesions secondary to prior abdominopelvic surgery, substantial endometriosis, or a pelvic mass.
Is oophorectomy an indication for LAVH?
The need to remove the ovaries does not mean laparoscopic assistance is imperative.
HERZOG: Simple removal of the ovaries can often be performed using the vaginal route, and is not in itself an indication for LAVH.
LEVY: I agree. The ovaries can usually be accessed transvaginally, especially with good fiberoptic lighting and vessel-sealing technology.
FALCONE: Several studies, most notably the one by Ballard and Walters,4 demonstrate that oophorectomy can be carried out vaginally in most cases. In their study, they did not use special instruments.
HERZOG: But LAVH is indicated to facilitate complete removal of the ovaries in riskreduction surgery for documented or suspected BrCa 1 or 2 mutations. The entire ovary and as much of the tube as possible must be removed in these women. Thus, if the patient has other indications for hysterectomy, LAVH may be the preferred route to assure that the blood supply is taken proximally enough to remove absolutely all ovarian tissue. Simply clamping directly along the side of the ovary is not an adequate removal technique for these patients, since an ovarian remnant may become a fatal oversight.
LEVY: Yes, laparoscopy is indicated for riskreducing salpingo-oophorectomy in order to adequately assess the entire peritoneal cavity. Up to 2% of these patients will have occult invasive ovarian or peritoneal carcinoma at the time of their prophylactic surgery, so full surgical abdominal exploration is mandatory and can be nicely accomplished via laparoscopy.5
How endometriosis history affects choice of route
In some women, laparoscopic surgery is preferred over the vaginal route.
FALCONE: Hysterectomy with or without salpingo-oophorectomy can be considered in women whose endometriosis fails to respond to conservative management and who do not desire fertility. Most studies have shown substantial pain relief with definitive surgery.6,7 Although ovarian conservation may be advisable in younger women, in some women it increases the probability of recurrent pain and the need for reoperation. The main concern is whether the endometriosis is completely removed during hysterectomy.
A history of cul-de-sac obliteration or extensive pelvic adhesions from endometriosis is an indication for laparoscopic hysterectomy rather than vaginal hysterectomy. Women with less severe disease will benefit from a diagnostic laparoscopy prior to a vaginal hysterectomy to evaluate the pelvis and excise any endometriosis.
Is there any benefit to leaving the cervix?
There is no evidence of any benefit except in selected cases of heavy bleeding, postpartum hemorrhage, advanced endometriosis, or ovarian cancer surgery.
KARRAM: What about supracervical hysterectomy? The only time I have performed one was at the time of a cesareanhysterectomy because blood loss was significant and dissection of the cervix could have led to more morbidity. What are the indications for this procedure?
HERZOG: It is unclear whether there are any definitive indications for supracervical hysterectomy. A number of benefits have been proposed, such as better support and improved sexual function. Some of the perceived benefits have been supported by nonrandomized trials, but critical analysis of the randomized data has failed to support most of these contentions.8,9 However, the procedure can be a valuable intervention to decrease critical blood loss intraoperatively, as you point out, or to simplify complicated pelvic surgery in selected cases, such as postpartum hemorrhage, advanced endometriosis, ovarian cancer debulking (when the cervix is not involved), or significant bleeding in patients who object to transfusion on ethical or religious grounds.
FALCONE: There are clear contraindications to supracervical hysterectomy, namely the presence of a malignant or premalignant condition of the uterine corpus or cervix, but no indications, except perhaps for an unstable patient undergoing hysterectomy in whom you want to finish quickly. None of the randomized clinical trials have shown supracervical hysterectomy to be superior to total hysterectomy. The randomized trials involved the abdominal route; the time to complete a laparoscopic supracervical hysterectomy is less than for a laparoscopic total hysterectomy.
Nevertheless, many patients ask for the procedure. After I present the risks and benefits, I leave the choice up to them. Of course, it is important to explain that total hysterectomy implies removal of the cervix and not the ovaries.
LEVY: In rare cases of immunocompromised patients or women with widely disseminated intraperitoneal carcinoma, one could make an argument for avoiding entry into the vagina to reduce infectious risk, speed healing, or avoid tumor seeding. Otherwise, there is absolutely no evidence to support an indication for supracervical hysterectomy.
Does leaving the cervix affect long-term function?
The residual cervix can become the site of later neoplasia or disease.
HERZOG: Supracervical hysterectomy can be associated with several problems with longterm implications. One is the potential for cervical intraepithelial neoplasia. Another concern relates to bleeding from a portion of active endometrium at the top of the endocervical canal. Rarer problems include the development of endometriosis or invasive cancer in the residual cervix. These potential drawbacks need to be strongly considered and included in patient counseling.
KARRAM: One study followed 67 patients for 66 months after supracervical hysterectomy; trachelectomy was ultimately required in 22.8% of patients.10
The $64,0000 question: Remove the ovaries?
Overall, the decision should be made case by case.
KARRAM: Should routine oophorectomy be performed at the time of hysterectomy in postmenopausal women to decrease the potential for ovarian cancer later in life?
HERZOG: This is a very important question. Conventional thinking used to be that, for women over the age of 45, and certainly for women older than 50, ovarian removal should be strongly considered to reduce the risk of cancer of the ovary or fallopian tubes at a later date. Studies focusing on the number of ovarian cancer cases possibly prevented with routine oophorectomy at the time of hysterectomy reinforced this concept. One single-institution study showed that more than 60 cases of ovarian cancer would have been prevented over a 14-year period if ovaries were routinely removed in women older than 40 undergoing hysterectomy. By extrapolation, that would result in more than 1,000 cases prevented annually in the United States.11
Recent data refute the rationale for routine oophorectomy. One study that used statistical modeling with Markov decision analysis to determine life expectancy concluded that, at least until the age of 65, women are best served with ovarian conservation if their risk of developing ovarian cancer is average or less.12 Researchers found that women who underwent oophorectomy before age 55 experienced 8.6% excess mortality by age 80. The validity of certain assumptions used to construct this model has been challenged; nevertheless, this cogent study certainly challenges previous concepts regarding agebased routine prophylactic oophorectomy. Until further study results are reported, it is important to counsel women who are considering having their ovaries removed about the potential risks and benefits. Furthermore, these decisions must be made on a case-by-case basis, with special deliberation given to women at any increased risk for breast or ovarian cancer.
What route is preferred when fibroids are present?
Assuming hysterectomy is the optimal treatment, the vaginal route is feasible.
KARRAM: Uterine fibroids are still the No. 1 indication for hysterectomy. Are minimally invasive laparoscopic procedures with morcellation techniques the best way to manage these women?
FALCONE: The management of symptomatic leiomyomas depends on the patient’s desire to preserve her fertility. If she does not have an interest in future fertility and there are no myomas that are largely submucous or pedunculated, then uterine fibroid embolization is the treatment of choice. Many studies have shown an excellent response, few complications, and rapid return to work.
If the woman wants to preserve fertility, myomectomy is the treatment of choice. If there are few myomas of moderate size, laparoscopic myomectomy is as effective as laparotomy in the hands of experienced laparoscopists who have the ability to suture.
HERZOG: I also want to stress that we now have a number of options, including uterine artery embolization, medications, and surgery, available for women with symptomatic fibroids. The surgical approaches are numerous and include hysteroscopic and/or laparoscopic myomectomy with or without morcellation, as well as hysterectomy. The approach should be determined by the symptoms, size, and distribution of the fibroids, as well as by individual patient characteristics such as prior surgeries, body mass index, and so on. Just because a procedure is technically feasible does not mean it is the preferred method, and this tenet certainly applies to morcellation. In some instances, women with very large fibroids may be better served by laparotomy to decrease blood loss and the duration of surgery while optimizing uterine wall reconstruction, especially when future fertility is an important consideration. Once again, proper patient selection is paramount in achieving favorable outcomes, especially for those who may be undergoing morcellation.
LEVY: For women who have completed childbearing and who desire hysterectomy, I always attempt a vaginal approach first. Most uteri, regardless of size, can be safely and efficiently removed vaginally as long as there is access to the uterine vasculature. Morcellation is easily performed vaginally once hemostasis is assured. For the rare patient with a large fundal myoma that cannot be brought into the pelvis for morcellation, minilaparotomy or laparoscopic approaches are appropriate.
KARRAM: I think randomized trials are needed in this area. It is important to remember that most cases performed laparoscopically result in supracervical hysterectomies and that significant costs are accrued from the equipment required for morcellation. These factors need to be weighed against potential advantages over abdominal hysterectomy, which include shorter hospital stay, potentially decreased morbidity, and faster recovery. The only way to make any objective conclusions about the options would be a randomized trial with appropriate power involving surgeons equally skilled in laparoscopic and open techniques.
Are residents adequately trained?
It depends on the program but, on the whole, more concentrated experience in minimally invasive surgery is needed.
KARRAM: Let’s focus on residency training for a moment. We seem to agree there is a lack of it in vaginal hysterectomy. It seems to me that the lack of training increases as time goes on. Because the current generation of gynecologists-in-training is ultimately the next generation of teachers, it bodes ill for the future when they are reluctant to attempt vaginal hysterectomy, except in the simplest and most straightforward cases. The medicolegal climate also plays a role, as Dr. Herzog mentioned.
Any other thoughts?
FALCONE: The training across residency programs is not homogenous. Some institutions promote vaginal hysterectomy as the primary access, and others do not.
HERZOG: I agree that some institutions do provide an adequate volume of cases, but many others offer a paucity of vaginal surgeries. Many reasons have combined to cause this shortage of training cases over the past 15 years, including a decrease in the number of hysterectomies performed overall, thanks to a number of nonsurgical or less radical surgical treatments for the most common indications for hysterectomy. These approaches generally are mandated by third-party payers prior to invasive surgery. These mandates were not as rigidly enforced in the past.
In a survey of gynecologic oncologists—the vast majority of whom were at academic training centers—the consensus was that residents had fewer surgical experiences and were less skillful than their predecessors over a 5-year period. More than 80% of respondents thought residents needed more surgical experience to achieve competence.13
Compounding the problem, resident work hours have been restricted and additional educational objectives and nonsurgical rotations have been added to the curriculum without any lengthening of the residency tenure. The adverse effects of these factors on residency case volume has prompted some educators to propose major changes in the residency curriculum, either by lengthening training or developing distinct tracks that facilitate early concentration on an area of interest, thereby allowing residents who choose a surgical track to gain increased training and volume.
Until substantive changes occur, educators must rely on surgical simulators and other in vitro models, especially for laparoscopic training. These have benefit but are not a perfect substitute for actual operative experience. A recent study explored the value of a surgical bench skills training program and concluded that, while residents showed definite improvement in bench laboratory tasks, this improvement did not translate into statistically significant improvement in global skills intraoperatively.14 Clearly, educators must continue to explore options to enhance surgical training, especially for vaginal surgery, or this route will become nearly obsolete in the gynecologic generalist’s armamentarium.
Dr. Karram reports that he receives grant/research support from Gynecare, American Medical Systems, and Pfizer and is a speaker for Gynecare, Ortho-McNeil, and Watson. Dr. Falcone, Dr. Herzog, and Dr. Levy have no financial relationships relevant to this article.
1. Kovac SR. Transvaginal hysterectomy: rationale and surgical approach. Obstet Gynecol. 2004;103:1321-1325.
2. Baggish MS. Total and subtotal abdominal hysterectomy. Best Pract Res Clin Obstet Gynaecol. 2005;19:333-356.
3. National Center for Health Statistics. Births—method of delivery. Available at: www.cdc.gov/nchs/fastats/ delivery.htm. Accessed January 18, 2006.
4. Ballard LA, Walters MD. Transvaginal mobilization and removal of ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
5. Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58-64.
6. Dmowski WP, Radwanska E, Rana N. Recurrent endometriosis following hysterectomy and oophorectomy: the role of residual ovarian fragments. Int J Gynecol Obstet. 1988;26:93-103.
7. Namnoun AB, Hickman NT, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64:898-902.
8. Kuppermann M, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy Research Group. Sexual functioning after total compared with supracervical hysterectomy: a randomized trial. Obstet Gynecol. 2005;105:1309-1318.
9. Learman LA, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy (TOSH) Research Group. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
10. Okaro EO, Jones KD, Sutton C. Long-term outcome following laparoscopic supracervical hysterectomy. BJOG. 2001;108:1017-1020.
11. Sightler SE, Boike GM, Estape RE, Averette HE. Ovarian cancer in women with prior hysterectomy: a 14-year experience at the University of Miami. Obstet Gynecol. 1991;78:681-684.
12. Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
13. Sorosky JI, Anderson B. Surgical experiences and training of residents: perspective of experienced gynecologic oncologists. Gynecol Oncol. 1999;75:222-223.
14. Lentz GM, Mandel LS, Goff BA. A six-year study of surgical teaching and skills evaluation for obstetric/gynecologic residents in porcine and inanimate surgical models. Am J Obstet Gynecol. 2005;193:2056-2061.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati, Ohio.
- Tommaso Falcone, MD, Professor and Chairman, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Thomas Herzog, MD, Director, Division of Gynecologic Oncology, Physicians and Surgeons Alumni Professor, Columbia University Medical Center, New York City.
- Barbara S. Levy, MD, Medical Director, Women’s Health Center, Franciscan Health System, Federal Way, Wash. Dr. Levy serves on the OBG MANAGEMENT Board of Editors.
Why the continued reliance on the abdominal approach despite convincing evidence that vaginal and laparoscopicassisted vaginal hysterectomy (LAVH) offer faster recovery, better cosmesis, and, in many cases, a shorter operation with fewer complications?
OBG Management convened a panel of experts in different aspects of gynecologic surgery to explore this issue. They discuss the reasons most physicians prefer the abdominal approach, how residency programs affect the choice of hysterectomy route, indications for LAVH and supracervical hysterectomy, the issue of ovarian conservation, and management of uterine fibroids.
Why the abdominal route remains the old standby
Physicians use the procedure they are most comfortable with, and residents lack sufficient hands-on experience with laparoscopic and vaginal surgery. Medicolegal risk and reimbursement also have an impact.
KARRAM: Hysterectomy is one of the most widely performed surgeries in the United States, but approximately 60% to 80% of these surgeries still involve the abdominal route.2 Why do you think that is?
FALCONE: Most physicians practice in the manner they were trained, and most residency programs train residents to perform abdominal hysterectomy.
LEVY: I agree. Residents in obstetrics and gynecology have a limited time frame in which to learn and become facile with surgical gynecology. The requirements for primary care training and continuity clinics leave little time for the resident to become comfortable with endoscopic and vaginal surgery. However, they do get substantial exposure to abdominal surgery, both in obstetrics (with cesarean sections constituting 27.5% of all deliveries in the United States3) and gynecologic oncology rotations.
Furthermore, the volume of benign gynecologic surgery is low and the technical skills required for laparoscopic and vaginal surgery are more challenging than “slash and gash” abdominal surgery, so residents don’t get enough exposure to develop a comfort level with these procedures.
HERZOG: This trend is likely to change in the future because recent and current trainees have much more exposure to the laparoscopic approach than in the past, and the equipment has continued to improve. However, I have serious doubts about whether adequate amounts of vaginal surgery are performed in training programs to educate the next generation of vaginal surgeons.
LEVY: Another factor is the type of practice physicians enter after training. Most join practices in which the bulk of their income for many years derives from obstetrics. Without a mentor in the practice who is skilled at minimally invasive surgery, most of these young physicians appropriately resort to the hysterectomy approach for which they have the most comfort and skill: the abdominal route.
HERZOG: Secondary barriers to nonabdominal procedures are lower reimbursement and heightened medicolegal risk, since time and complications are greater for laparoscopic surgery and, to a lesser degree, vaginal procedures. Surgeons are not adequately compensated for either the increased time or risk.
Patients also tend to have higher expectations when the planned approach is minimally invasive. When conversion to laparotomy is necessary, the patient and her family may have trouble understanding why.
KARRAM: I agree that there is a serious lack of training in simple and complicated vaginal hysterectomy. Many inaccurate perceptions have been handed down over the years about its absolute and relative contraindications, such as the belief that any history of pelvic infection, endometriosis, or cesarean section is a contraindication for the vaginal approach.
When is laparoscopic assistance appropriate?
At a fundamental level, its value lies in converting abdominal hysterectomy intovaginal hysterectomy.
KARRAM: In my experience, LAVH is appropriate in the presence of benign adnexal pathology: The adnexa can be evaluated and detached laparoscopically followed by vaginal hysterectomy and vaginal removal of the adnexa. It also is appropriate in any situation that involves excessive pelvic adhesions. The uterus can be mobilized laparoscopically, followed by removal through the vagina.
FALCONE: Any patient who is not a candidate for vaginal hysterectomy should be considered for laparoscopic assistance. The general rationale for the surgery is to convert an abdominal hysterectomy into a vaginal one, so the surgeon should start laparoscopically and then switch to the vaginal approach as soon as possible. Of course, it is impossible to proceed vaginally in some cases. When it is, the entire case can be performed laparoscopically.
LEVY: In my hands, patients with an unidentified adnexal mass who also need or request hysterectomy are appropriate candidates for laparoscopic abdominal exploration followed by vaginal hysterectomy if appropriate. Women with a very contracted pelvis, which precludes transvaginal access to the uterine vasculature, may also be candidates for the laparoscopic approach. However, as surgeons become more skilled at vaginal surgery and learn to use newer instrumentation, the need for laparoscopy to access a tight pelvis will diminish.
Using a laparoscopic approach for patients with fibroids wedged into the pelvis carries serious risk to the pelvic sidewall. It is very difficult to access the sidewall safely laparoscopically in the presence of a large lower segment or cervical myomas.
HERZOG: When LAVH was introduced, many clinicians challenged the utility of combined laparoscopic and vaginal surgery, with some referring to this surgical exercise as a procedure looking for an indication. However, as operative laparoscopy has gained acceptance, some benefits of LAVH have become apparent. The greatest advantage is the potential to convert a procedure that would have been performed abdominally into a vaginal hysterectomy.
The most commonly cited indications for LAVH are to lyse adhesions secondary to prior abdominopelvic surgery, substantial endometriosis, or a pelvic mass.
Is oophorectomy an indication for LAVH?
The need to remove the ovaries does not mean laparoscopic assistance is imperative.
HERZOG: Simple removal of the ovaries can often be performed using the vaginal route, and is not in itself an indication for LAVH.
LEVY: I agree. The ovaries can usually be accessed transvaginally, especially with good fiberoptic lighting and vessel-sealing technology.
FALCONE: Several studies, most notably the one by Ballard and Walters,4 demonstrate that oophorectomy can be carried out vaginally in most cases. In their study, they did not use special instruments.
HERZOG: But LAVH is indicated to facilitate complete removal of the ovaries in riskreduction surgery for documented or suspected BrCa 1 or 2 mutations. The entire ovary and as much of the tube as possible must be removed in these women. Thus, if the patient has other indications for hysterectomy, LAVH may be the preferred route to assure that the blood supply is taken proximally enough to remove absolutely all ovarian tissue. Simply clamping directly along the side of the ovary is not an adequate removal technique for these patients, since an ovarian remnant may become a fatal oversight.
LEVY: Yes, laparoscopy is indicated for riskreducing salpingo-oophorectomy in order to adequately assess the entire peritoneal cavity. Up to 2% of these patients will have occult invasive ovarian or peritoneal carcinoma at the time of their prophylactic surgery, so full surgical abdominal exploration is mandatory and can be nicely accomplished via laparoscopy.5
How endometriosis history affects choice of route
In some women, laparoscopic surgery is preferred over the vaginal route.
FALCONE: Hysterectomy with or without salpingo-oophorectomy can be considered in women whose endometriosis fails to respond to conservative management and who do not desire fertility. Most studies have shown substantial pain relief with definitive surgery.6,7 Although ovarian conservation may be advisable in younger women, in some women it increases the probability of recurrent pain and the need for reoperation. The main concern is whether the endometriosis is completely removed during hysterectomy.
A history of cul-de-sac obliteration or extensive pelvic adhesions from endometriosis is an indication for laparoscopic hysterectomy rather than vaginal hysterectomy. Women with less severe disease will benefit from a diagnostic laparoscopy prior to a vaginal hysterectomy to evaluate the pelvis and excise any endometriosis.
Is there any benefit to leaving the cervix?
There is no evidence of any benefit except in selected cases of heavy bleeding, postpartum hemorrhage, advanced endometriosis, or ovarian cancer surgery.
KARRAM: What about supracervical hysterectomy? The only time I have performed one was at the time of a cesareanhysterectomy because blood loss was significant and dissection of the cervix could have led to more morbidity. What are the indications for this procedure?
HERZOG: It is unclear whether there are any definitive indications for supracervical hysterectomy. A number of benefits have been proposed, such as better support and improved sexual function. Some of the perceived benefits have been supported by nonrandomized trials, but critical analysis of the randomized data has failed to support most of these contentions.8,9 However, the procedure can be a valuable intervention to decrease critical blood loss intraoperatively, as you point out, or to simplify complicated pelvic surgery in selected cases, such as postpartum hemorrhage, advanced endometriosis, ovarian cancer debulking (when the cervix is not involved), or significant bleeding in patients who object to transfusion on ethical or religious grounds.
FALCONE: There are clear contraindications to supracervical hysterectomy, namely the presence of a malignant or premalignant condition of the uterine corpus or cervix, but no indications, except perhaps for an unstable patient undergoing hysterectomy in whom you want to finish quickly. None of the randomized clinical trials have shown supracervical hysterectomy to be superior to total hysterectomy. The randomized trials involved the abdominal route; the time to complete a laparoscopic supracervical hysterectomy is less than for a laparoscopic total hysterectomy.
Nevertheless, many patients ask for the procedure. After I present the risks and benefits, I leave the choice up to them. Of course, it is important to explain that total hysterectomy implies removal of the cervix and not the ovaries.
LEVY: In rare cases of immunocompromised patients or women with widely disseminated intraperitoneal carcinoma, one could make an argument for avoiding entry into the vagina to reduce infectious risk, speed healing, or avoid tumor seeding. Otherwise, there is absolutely no evidence to support an indication for supracervical hysterectomy.
Does leaving the cervix affect long-term function?
The residual cervix can become the site of later neoplasia or disease.
HERZOG: Supracervical hysterectomy can be associated with several problems with longterm implications. One is the potential for cervical intraepithelial neoplasia. Another concern relates to bleeding from a portion of active endometrium at the top of the endocervical canal. Rarer problems include the development of endometriosis or invasive cancer in the residual cervix. These potential drawbacks need to be strongly considered and included in patient counseling.
KARRAM: One study followed 67 patients for 66 months after supracervical hysterectomy; trachelectomy was ultimately required in 22.8% of patients.10
The $64,0000 question: Remove the ovaries?
Overall, the decision should be made case by case.
KARRAM: Should routine oophorectomy be performed at the time of hysterectomy in postmenopausal women to decrease the potential for ovarian cancer later in life?
HERZOG: This is a very important question. Conventional thinking used to be that, for women over the age of 45, and certainly for women older than 50, ovarian removal should be strongly considered to reduce the risk of cancer of the ovary or fallopian tubes at a later date. Studies focusing on the number of ovarian cancer cases possibly prevented with routine oophorectomy at the time of hysterectomy reinforced this concept. One single-institution study showed that more than 60 cases of ovarian cancer would have been prevented over a 14-year period if ovaries were routinely removed in women older than 40 undergoing hysterectomy. By extrapolation, that would result in more than 1,000 cases prevented annually in the United States.11
Recent data refute the rationale for routine oophorectomy. One study that used statistical modeling with Markov decision analysis to determine life expectancy concluded that, at least until the age of 65, women are best served with ovarian conservation if their risk of developing ovarian cancer is average or less.12 Researchers found that women who underwent oophorectomy before age 55 experienced 8.6% excess mortality by age 80. The validity of certain assumptions used to construct this model has been challenged; nevertheless, this cogent study certainly challenges previous concepts regarding agebased routine prophylactic oophorectomy. Until further study results are reported, it is important to counsel women who are considering having their ovaries removed about the potential risks and benefits. Furthermore, these decisions must be made on a case-by-case basis, with special deliberation given to women at any increased risk for breast or ovarian cancer.
What route is preferred when fibroids are present?
Assuming hysterectomy is the optimal treatment, the vaginal route is feasible.
KARRAM: Uterine fibroids are still the No. 1 indication for hysterectomy. Are minimally invasive laparoscopic procedures with morcellation techniques the best way to manage these women?
FALCONE: The management of symptomatic leiomyomas depends on the patient’s desire to preserve her fertility. If she does not have an interest in future fertility and there are no myomas that are largely submucous or pedunculated, then uterine fibroid embolization is the treatment of choice. Many studies have shown an excellent response, few complications, and rapid return to work.
If the woman wants to preserve fertility, myomectomy is the treatment of choice. If there are few myomas of moderate size, laparoscopic myomectomy is as effective as laparotomy in the hands of experienced laparoscopists who have the ability to suture.
HERZOG: I also want to stress that we now have a number of options, including uterine artery embolization, medications, and surgery, available for women with symptomatic fibroids. The surgical approaches are numerous and include hysteroscopic and/or laparoscopic myomectomy with or without morcellation, as well as hysterectomy. The approach should be determined by the symptoms, size, and distribution of the fibroids, as well as by individual patient characteristics such as prior surgeries, body mass index, and so on. Just because a procedure is technically feasible does not mean it is the preferred method, and this tenet certainly applies to morcellation. In some instances, women with very large fibroids may be better served by laparotomy to decrease blood loss and the duration of surgery while optimizing uterine wall reconstruction, especially when future fertility is an important consideration. Once again, proper patient selection is paramount in achieving favorable outcomes, especially for those who may be undergoing morcellation.
LEVY: For women who have completed childbearing and who desire hysterectomy, I always attempt a vaginal approach first. Most uteri, regardless of size, can be safely and efficiently removed vaginally as long as there is access to the uterine vasculature. Morcellation is easily performed vaginally once hemostasis is assured. For the rare patient with a large fundal myoma that cannot be brought into the pelvis for morcellation, minilaparotomy or laparoscopic approaches are appropriate.
KARRAM: I think randomized trials are needed in this area. It is important to remember that most cases performed laparoscopically result in supracervical hysterectomies and that significant costs are accrued from the equipment required for morcellation. These factors need to be weighed against potential advantages over abdominal hysterectomy, which include shorter hospital stay, potentially decreased morbidity, and faster recovery. The only way to make any objective conclusions about the options would be a randomized trial with appropriate power involving surgeons equally skilled in laparoscopic and open techniques.
Are residents adequately trained?
It depends on the program but, on the whole, more concentrated experience in minimally invasive surgery is needed.
KARRAM: Let’s focus on residency training for a moment. We seem to agree there is a lack of it in vaginal hysterectomy. It seems to me that the lack of training increases as time goes on. Because the current generation of gynecologists-in-training is ultimately the next generation of teachers, it bodes ill for the future when they are reluctant to attempt vaginal hysterectomy, except in the simplest and most straightforward cases. The medicolegal climate also plays a role, as Dr. Herzog mentioned.
Any other thoughts?
FALCONE: The training across residency programs is not homogenous. Some institutions promote vaginal hysterectomy as the primary access, and others do not.
HERZOG: I agree that some institutions do provide an adequate volume of cases, but many others offer a paucity of vaginal surgeries. Many reasons have combined to cause this shortage of training cases over the past 15 years, including a decrease in the number of hysterectomies performed overall, thanks to a number of nonsurgical or less radical surgical treatments for the most common indications for hysterectomy. These approaches generally are mandated by third-party payers prior to invasive surgery. These mandates were not as rigidly enforced in the past.
In a survey of gynecologic oncologists—the vast majority of whom were at academic training centers—the consensus was that residents had fewer surgical experiences and were less skillful than their predecessors over a 5-year period. More than 80% of respondents thought residents needed more surgical experience to achieve competence.13
Compounding the problem, resident work hours have been restricted and additional educational objectives and nonsurgical rotations have been added to the curriculum without any lengthening of the residency tenure. The adverse effects of these factors on residency case volume has prompted some educators to propose major changes in the residency curriculum, either by lengthening training or developing distinct tracks that facilitate early concentration on an area of interest, thereby allowing residents who choose a surgical track to gain increased training and volume.
Until substantive changes occur, educators must rely on surgical simulators and other in vitro models, especially for laparoscopic training. These have benefit but are not a perfect substitute for actual operative experience. A recent study explored the value of a surgical bench skills training program and concluded that, while residents showed definite improvement in bench laboratory tasks, this improvement did not translate into statistically significant improvement in global skills intraoperatively.14 Clearly, educators must continue to explore options to enhance surgical training, especially for vaginal surgery, or this route will become nearly obsolete in the gynecologic generalist’s armamentarium.
Dr. Karram reports that he receives grant/research support from Gynecare, American Medical Systems, and Pfizer and is a speaker for Gynecare, Ortho-McNeil, and Watson. Dr. Falcone, Dr. Herzog, and Dr. Levy have no financial relationships relevant to this article.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati, Ohio.
- Tommaso Falcone, MD, Professor and Chairman, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Thomas Herzog, MD, Director, Division of Gynecologic Oncology, Physicians and Surgeons Alumni Professor, Columbia University Medical Center, New York City.
- Barbara S. Levy, MD, Medical Director, Women’s Health Center, Franciscan Health System, Federal Way, Wash. Dr. Levy serves on the OBG MANAGEMENT Board of Editors.
Why the continued reliance on the abdominal approach despite convincing evidence that vaginal and laparoscopicassisted vaginal hysterectomy (LAVH) offer faster recovery, better cosmesis, and, in many cases, a shorter operation with fewer complications?
OBG Management convened a panel of experts in different aspects of gynecologic surgery to explore this issue. They discuss the reasons most physicians prefer the abdominal approach, how residency programs affect the choice of hysterectomy route, indications for LAVH and supracervical hysterectomy, the issue of ovarian conservation, and management of uterine fibroids.
Why the abdominal route remains the old standby
Physicians use the procedure they are most comfortable with, and residents lack sufficient hands-on experience with laparoscopic and vaginal surgery. Medicolegal risk and reimbursement also have an impact.
KARRAM: Hysterectomy is one of the most widely performed surgeries in the United States, but approximately 60% to 80% of these surgeries still involve the abdominal route.2 Why do you think that is?
FALCONE: Most physicians practice in the manner they were trained, and most residency programs train residents to perform abdominal hysterectomy.
LEVY: I agree. Residents in obstetrics and gynecology have a limited time frame in which to learn and become facile with surgical gynecology. The requirements for primary care training and continuity clinics leave little time for the resident to become comfortable with endoscopic and vaginal surgery. However, they do get substantial exposure to abdominal surgery, both in obstetrics (with cesarean sections constituting 27.5% of all deliveries in the United States3) and gynecologic oncology rotations.
Furthermore, the volume of benign gynecologic surgery is low and the technical skills required for laparoscopic and vaginal surgery are more challenging than “slash and gash” abdominal surgery, so residents don’t get enough exposure to develop a comfort level with these procedures.
HERZOG: This trend is likely to change in the future because recent and current trainees have much more exposure to the laparoscopic approach than in the past, and the equipment has continued to improve. However, I have serious doubts about whether adequate amounts of vaginal surgery are performed in training programs to educate the next generation of vaginal surgeons.
LEVY: Another factor is the type of practice physicians enter after training. Most join practices in which the bulk of their income for many years derives from obstetrics. Without a mentor in the practice who is skilled at minimally invasive surgery, most of these young physicians appropriately resort to the hysterectomy approach for which they have the most comfort and skill: the abdominal route.
HERZOG: Secondary barriers to nonabdominal procedures are lower reimbursement and heightened medicolegal risk, since time and complications are greater for laparoscopic surgery and, to a lesser degree, vaginal procedures. Surgeons are not adequately compensated for either the increased time or risk.
Patients also tend to have higher expectations when the planned approach is minimally invasive. When conversion to laparotomy is necessary, the patient and her family may have trouble understanding why.
KARRAM: I agree that there is a serious lack of training in simple and complicated vaginal hysterectomy. Many inaccurate perceptions have been handed down over the years about its absolute and relative contraindications, such as the belief that any history of pelvic infection, endometriosis, or cesarean section is a contraindication for the vaginal approach.
When is laparoscopic assistance appropriate?
At a fundamental level, its value lies in converting abdominal hysterectomy intovaginal hysterectomy.
KARRAM: In my experience, LAVH is appropriate in the presence of benign adnexal pathology: The adnexa can be evaluated and detached laparoscopically followed by vaginal hysterectomy and vaginal removal of the adnexa. It also is appropriate in any situation that involves excessive pelvic adhesions. The uterus can be mobilized laparoscopically, followed by removal through the vagina.
FALCONE: Any patient who is not a candidate for vaginal hysterectomy should be considered for laparoscopic assistance. The general rationale for the surgery is to convert an abdominal hysterectomy into a vaginal one, so the surgeon should start laparoscopically and then switch to the vaginal approach as soon as possible. Of course, it is impossible to proceed vaginally in some cases. When it is, the entire case can be performed laparoscopically.
LEVY: In my hands, patients with an unidentified adnexal mass who also need or request hysterectomy are appropriate candidates for laparoscopic abdominal exploration followed by vaginal hysterectomy if appropriate. Women with a very contracted pelvis, which precludes transvaginal access to the uterine vasculature, may also be candidates for the laparoscopic approach. However, as surgeons become more skilled at vaginal surgery and learn to use newer instrumentation, the need for laparoscopy to access a tight pelvis will diminish.
Using a laparoscopic approach for patients with fibroids wedged into the pelvis carries serious risk to the pelvic sidewall. It is very difficult to access the sidewall safely laparoscopically in the presence of a large lower segment or cervical myomas.
HERZOG: When LAVH was introduced, many clinicians challenged the utility of combined laparoscopic and vaginal surgery, with some referring to this surgical exercise as a procedure looking for an indication. However, as operative laparoscopy has gained acceptance, some benefits of LAVH have become apparent. The greatest advantage is the potential to convert a procedure that would have been performed abdominally into a vaginal hysterectomy.
The most commonly cited indications for LAVH are to lyse adhesions secondary to prior abdominopelvic surgery, substantial endometriosis, or a pelvic mass.
Is oophorectomy an indication for LAVH?
The need to remove the ovaries does not mean laparoscopic assistance is imperative.
HERZOG: Simple removal of the ovaries can often be performed using the vaginal route, and is not in itself an indication for LAVH.
LEVY: I agree. The ovaries can usually be accessed transvaginally, especially with good fiberoptic lighting and vessel-sealing technology.
FALCONE: Several studies, most notably the one by Ballard and Walters,4 demonstrate that oophorectomy can be carried out vaginally in most cases. In their study, they did not use special instruments.
HERZOG: But LAVH is indicated to facilitate complete removal of the ovaries in riskreduction surgery for documented or suspected BrCa 1 or 2 mutations. The entire ovary and as much of the tube as possible must be removed in these women. Thus, if the patient has other indications for hysterectomy, LAVH may be the preferred route to assure that the blood supply is taken proximally enough to remove absolutely all ovarian tissue. Simply clamping directly along the side of the ovary is not an adequate removal technique for these patients, since an ovarian remnant may become a fatal oversight.
LEVY: Yes, laparoscopy is indicated for riskreducing salpingo-oophorectomy in order to adequately assess the entire peritoneal cavity. Up to 2% of these patients will have occult invasive ovarian or peritoneal carcinoma at the time of their prophylactic surgery, so full surgical abdominal exploration is mandatory and can be nicely accomplished via laparoscopy.5
How endometriosis history affects choice of route
In some women, laparoscopic surgery is preferred over the vaginal route.
FALCONE: Hysterectomy with or without salpingo-oophorectomy can be considered in women whose endometriosis fails to respond to conservative management and who do not desire fertility. Most studies have shown substantial pain relief with definitive surgery.6,7 Although ovarian conservation may be advisable in younger women, in some women it increases the probability of recurrent pain and the need for reoperation. The main concern is whether the endometriosis is completely removed during hysterectomy.
A history of cul-de-sac obliteration or extensive pelvic adhesions from endometriosis is an indication for laparoscopic hysterectomy rather than vaginal hysterectomy. Women with less severe disease will benefit from a diagnostic laparoscopy prior to a vaginal hysterectomy to evaluate the pelvis and excise any endometriosis.
Is there any benefit to leaving the cervix?
There is no evidence of any benefit except in selected cases of heavy bleeding, postpartum hemorrhage, advanced endometriosis, or ovarian cancer surgery.
KARRAM: What about supracervical hysterectomy? The only time I have performed one was at the time of a cesareanhysterectomy because blood loss was significant and dissection of the cervix could have led to more morbidity. What are the indications for this procedure?
HERZOG: It is unclear whether there are any definitive indications for supracervical hysterectomy. A number of benefits have been proposed, such as better support and improved sexual function. Some of the perceived benefits have been supported by nonrandomized trials, but critical analysis of the randomized data has failed to support most of these contentions.8,9 However, the procedure can be a valuable intervention to decrease critical blood loss intraoperatively, as you point out, or to simplify complicated pelvic surgery in selected cases, such as postpartum hemorrhage, advanced endometriosis, ovarian cancer debulking (when the cervix is not involved), or significant bleeding in patients who object to transfusion on ethical or religious grounds.
FALCONE: There are clear contraindications to supracervical hysterectomy, namely the presence of a malignant or premalignant condition of the uterine corpus or cervix, but no indications, except perhaps for an unstable patient undergoing hysterectomy in whom you want to finish quickly. None of the randomized clinical trials have shown supracervical hysterectomy to be superior to total hysterectomy. The randomized trials involved the abdominal route; the time to complete a laparoscopic supracervical hysterectomy is less than for a laparoscopic total hysterectomy.
Nevertheless, many patients ask for the procedure. After I present the risks and benefits, I leave the choice up to them. Of course, it is important to explain that total hysterectomy implies removal of the cervix and not the ovaries.
LEVY: In rare cases of immunocompromised patients or women with widely disseminated intraperitoneal carcinoma, one could make an argument for avoiding entry into the vagina to reduce infectious risk, speed healing, or avoid tumor seeding. Otherwise, there is absolutely no evidence to support an indication for supracervical hysterectomy.
Does leaving the cervix affect long-term function?
The residual cervix can become the site of later neoplasia or disease.
HERZOG: Supracervical hysterectomy can be associated with several problems with longterm implications. One is the potential for cervical intraepithelial neoplasia. Another concern relates to bleeding from a portion of active endometrium at the top of the endocervical canal. Rarer problems include the development of endometriosis or invasive cancer in the residual cervix. These potential drawbacks need to be strongly considered and included in patient counseling.
KARRAM: One study followed 67 patients for 66 months after supracervical hysterectomy; trachelectomy was ultimately required in 22.8% of patients.10
The $64,0000 question: Remove the ovaries?
Overall, the decision should be made case by case.
KARRAM: Should routine oophorectomy be performed at the time of hysterectomy in postmenopausal women to decrease the potential for ovarian cancer later in life?
HERZOG: This is a very important question. Conventional thinking used to be that, for women over the age of 45, and certainly for women older than 50, ovarian removal should be strongly considered to reduce the risk of cancer of the ovary or fallopian tubes at a later date. Studies focusing on the number of ovarian cancer cases possibly prevented with routine oophorectomy at the time of hysterectomy reinforced this concept. One single-institution study showed that more than 60 cases of ovarian cancer would have been prevented over a 14-year period if ovaries were routinely removed in women older than 40 undergoing hysterectomy. By extrapolation, that would result in more than 1,000 cases prevented annually in the United States.11
Recent data refute the rationale for routine oophorectomy. One study that used statistical modeling with Markov decision analysis to determine life expectancy concluded that, at least until the age of 65, women are best served with ovarian conservation if their risk of developing ovarian cancer is average or less.12 Researchers found that women who underwent oophorectomy before age 55 experienced 8.6% excess mortality by age 80. The validity of certain assumptions used to construct this model has been challenged; nevertheless, this cogent study certainly challenges previous concepts regarding agebased routine prophylactic oophorectomy. Until further study results are reported, it is important to counsel women who are considering having their ovaries removed about the potential risks and benefits. Furthermore, these decisions must be made on a case-by-case basis, with special deliberation given to women at any increased risk for breast or ovarian cancer.
What route is preferred when fibroids are present?
Assuming hysterectomy is the optimal treatment, the vaginal route is feasible.
KARRAM: Uterine fibroids are still the No. 1 indication for hysterectomy. Are minimally invasive laparoscopic procedures with morcellation techniques the best way to manage these women?
FALCONE: The management of symptomatic leiomyomas depends on the patient’s desire to preserve her fertility. If she does not have an interest in future fertility and there are no myomas that are largely submucous or pedunculated, then uterine fibroid embolization is the treatment of choice. Many studies have shown an excellent response, few complications, and rapid return to work.
If the woman wants to preserve fertility, myomectomy is the treatment of choice. If there are few myomas of moderate size, laparoscopic myomectomy is as effective as laparotomy in the hands of experienced laparoscopists who have the ability to suture.
HERZOG: I also want to stress that we now have a number of options, including uterine artery embolization, medications, and surgery, available for women with symptomatic fibroids. The surgical approaches are numerous and include hysteroscopic and/or laparoscopic myomectomy with or without morcellation, as well as hysterectomy. The approach should be determined by the symptoms, size, and distribution of the fibroids, as well as by individual patient characteristics such as prior surgeries, body mass index, and so on. Just because a procedure is technically feasible does not mean it is the preferred method, and this tenet certainly applies to morcellation. In some instances, women with very large fibroids may be better served by laparotomy to decrease blood loss and the duration of surgery while optimizing uterine wall reconstruction, especially when future fertility is an important consideration. Once again, proper patient selection is paramount in achieving favorable outcomes, especially for those who may be undergoing morcellation.
LEVY: For women who have completed childbearing and who desire hysterectomy, I always attempt a vaginal approach first. Most uteri, regardless of size, can be safely and efficiently removed vaginally as long as there is access to the uterine vasculature. Morcellation is easily performed vaginally once hemostasis is assured. For the rare patient with a large fundal myoma that cannot be brought into the pelvis for morcellation, minilaparotomy or laparoscopic approaches are appropriate.
KARRAM: I think randomized trials are needed in this area. It is important to remember that most cases performed laparoscopically result in supracervical hysterectomies and that significant costs are accrued from the equipment required for morcellation. These factors need to be weighed against potential advantages over abdominal hysterectomy, which include shorter hospital stay, potentially decreased morbidity, and faster recovery. The only way to make any objective conclusions about the options would be a randomized trial with appropriate power involving surgeons equally skilled in laparoscopic and open techniques.
Are residents adequately trained?
It depends on the program but, on the whole, more concentrated experience in minimally invasive surgery is needed.
KARRAM: Let’s focus on residency training for a moment. We seem to agree there is a lack of it in vaginal hysterectomy. It seems to me that the lack of training increases as time goes on. Because the current generation of gynecologists-in-training is ultimately the next generation of teachers, it bodes ill for the future when they are reluctant to attempt vaginal hysterectomy, except in the simplest and most straightforward cases. The medicolegal climate also plays a role, as Dr. Herzog mentioned.
Any other thoughts?
FALCONE: The training across residency programs is not homogenous. Some institutions promote vaginal hysterectomy as the primary access, and others do not.
HERZOG: I agree that some institutions do provide an adequate volume of cases, but many others offer a paucity of vaginal surgeries. Many reasons have combined to cause this shortage of training cases over the past 15 years, including a decrease in the number of hysterectomies performed overall, thanks to a number of nonsurgical or less radical surgical treatments for the most common indications for hysterectomy. These approaches generally are mandated by third-party payers prior to invasive surgery. These mandates were not as rigidly enforced in the past.
In a survey of gynecologic oncologists—the vast majority of whom were at academic training centers—the consensus was that residents had fewer surgical experiences and were less skillful than their predecessors over a 5-year period. More than 80% of respondents thought residents needed more surgical experience to achieve competence.13
Compounding the problem, resident work hours have been restricted and additional educational objectives and nonsurgical rotations have been added to the curriculum without any lengthening of the residency tenure. The adverse effects of these factors on residency case volume has prompted some educators to propose major changes in the residency curriculum, either by lengthening training or developing distinct tracks that facilitate early concentration on an area of interest, thereby allowing residents who choose a surgical track to gain increased training and volume.
Until substantive changes occur, educators must rely on surgical simulators and other in vitro models, especially for laparoscopic training. These have benefit but are not a perfect substitute for actual operative experience. A recent study explored the value of a surgical bench skills training program and concluded that, while residents showed definite improvement in bench laboratory tasks, this improvement did not translate into statistically significant improvement in global skills intraoperatively.14 Clearly, educators must continue to explore options to enhance surgical training, especially for vaginal surgery, or this route will become nearly obsolete in the gynecologic generalist’s armamentarium.
Dr. Karram reports that he receives grant/research support from Gynecare, American Medical Systems, and Pfizer and is a speaker for Gynecare, Ortho-McNeil, and Watson. Dr. Falcone, Dr. Herzog, and Dr. Levy have no financial relationships relevant to this article.
1. Kovac SR. Transvaginal hysterectomy: rationale and surgical approach. Obstet Gynecol. 2004;103:1321-1325.
2. Baggish MS. Total and subtotal abdominal hysterectomy. Best Pract Res Clin Obstet Gynaecol. 2005;19:333-356.
3. National Center for Health Statistics. Births—method of delivery. Available at: www.cdc.gov/nchs/fastats/ delivery.htm. Accessed January 18, 2006.
4. Ballard LA, Walters MD. Transvaginal mobilization and removal of ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
5. Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58-64.
6. Dmowski WP, Radwanska E, Rana N. Recurrent endometriosis following hysterectomy and oophorectomy: the role of residual ovarian fragments. Int J Gynecol Obstet. 1988;26:93-103.
7. Namnoun AB, Hickman NT, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64:898-902.
8. Kuppermann M, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy Research Group. Sexual functioning after total compared with supracervical hysterectomy: a randomized trial. Obstet Gynecol. 2005;105:1309-1318.
9. Learman LA, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy (TOSH) Research Group. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
10. Okaro EO, Jones KD, Sutton C. Long-term outcome following laparoscopic supracervical hysterectomy. BJOG. 2001;108:1017-1020.
11. Sightler SE, Boike GM, Estape RE, Averette HE. Ovarian cancer in women with prior hysterectomy: a 14-year experience at the University of Miami. Obstet Gynecol. 1991;78:681-684.
12. Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
13. Sorosky JI, Anderson B. Surgical experiences and training of residents: perspective of experienced gynecologic oncologists. Gynecol Oncol. 1999;75:222-223.
14. Lentz GM, Mandel LS, Goff BA. A six-year study of surgical teaching and skills evaluation for obstetric/gynecologic residents in porcine and inanimate surgical models. Am J Obstet Gynecol. 2005;193:2056-2061.
1. Kovac SR. Transvaginal hysterectomy: rationale and surgical approach. Obstet Gynecol. 2004;103:1321-1325.
2. Baggish MS. Total and subtotal abdominal hysterectomy. Best Pract Res Clin Obstet Gynaecol. 2005;19:333-356.
3. National Center for Health Statistics. Births—method of delivery. Available at: www.cdc.gov/nchs/fastats/ delivery.htm. Accessed January 18, 2006.
4. Ballard LA, Walters MD. Transvaginal mobilization and removal of ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
5. Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58-64.
6. Dmowski WP, Radwanska E, Rana N. Recurrent endometriosis following hysterectomy and oophorectomy: the role of residual ovarian fragments. Int J Gynecol Obstet. 1988;26:93-103.
7. Namnoun AB, Hickman NT, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64:898-902.
8. Kuppermann M, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy Research Group. Sexual functioning after total compared with supracervical hysterectomy: a randomized trial. Obstet Gynecol. 2005;105:1309-1318.
9. Learman LA, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy (TOSH) Research Group. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
10. Okaro EO, Jones KD, Sutton C. Long-term outcome following laparoscopic supracervical hysterectomy. BJOG. 2001;108:1017-1020.
11. Sightler SE, Boike GM, Estape RE, Averette HE. Ovarian cancer in women with prior hysterectomy: a 14-year experience at the University of Miami. Obstet Gynecol. 1991;78:681-684.
12. Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
13. Sorosky JI, Anderson B. Surgical experiences and training of residents: perspective of experienced gynecologic oncologists. Gynecol Oncol. 1999;75:222-223.
14. Lentz GM, Mandel LS, Goff BA. A six-year study of surgical teaching and skills evaluation for obstetric/gynecologic residents in porcine and inanimate surgical models. Am J Obstet Gynecol. 2005;193:2056-2061.
IN THIS ARTICLE
- Editorial We are at the tipping point
Abdominal techniques for surgical management of vaginal vault prolapse
A range of clinical conditions can suggest an abdominal approach for vaginal vault prolapse procedures.
These include, but are not limited to:
- prior unsuccessful vaginal attempts
- obligate need for adnexal access
- markedly foreshortened vagina
- pelvic bony architectural limitations
- high risk for surgical failure (eg, athleticism, obesity, chronic obstructive pulmonary disease, congenital connective tissue disorder)
- desire for uterine preservation
In Part 1 (November 2005) of this 2-part article, we reviewed the most widely used and the newest vaginal techniques. Part 2 focuses on the abdominal approach, and compares vaginal and abdominal approaches.
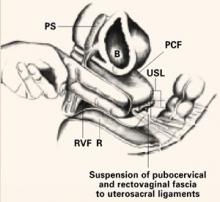
High uterosacral ligament suspension
Surgical technique for this procedure for mild to moderate vaginal vault prolapse (stage I or II), using a vaginal approach, was described in Part 1, in the November issue of OBG Management. Abdominal repair involves the same concepts; like the vaginal approach, it is applicable only to the patient with mild to moderate vault prolapse. It will be less successful if it is performed to address complete vault prolapse.
Technique
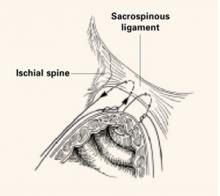
Identify and tag the remnants of the uterosacral ligaments at the level of the ischial spines. Once the ureters are identified and isolated, address the enterocele by obliterating the cul-de-sac via Halban’s culdoplasty or abdominal McCall’s culdoplasty.
Open the peritoneum over the vaginal apex and trim it back to the level of the endopelvic fascia of the vaginal wall. After excising the redundant peritoneum of the vaginal apex, identify and reapproximate the pubocervical fascia of the anterior vaginal wall and the rectovaginal fascia of the posterior vaginal wall using interrupted or running nonabsorbable suture.
Then use nonabsorbable sutures to suspend each corner of the prolapsed vagina from its respective ipsilateral uterosacral ligament.
Abdominal sacral colpopexy
Abdominal sacral colpopexy was first popularized by Addison and Timmons in the 1980s, and is the abdominal standard of apical prolapse repair due to its long-term durability.
Abdominal sacral colpopexy can be performed with or without uterine extirpation. When a hysterectomy is performed concomitantly, some surgeons prefer a supracervical approach, provided there is no history of cervical dysplasia, because, theoretically, the cervical stump serves as a firm and substantial point of fixation for the synthetic mesh that will be used to perform the repair. This in turn may diminish the likelihood of postoperative mesh erosion.
Technique
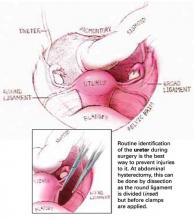
Reflect the sigmoid colon as far as possible into the left lateral pelvis to expose the sacral promontory. If it has not already been done, free all adhesions between the colon and pelvic peritoneum to fully mobilize the colon and permit its maximal retraction out of the pelvic field prior to making the peritoneal incision.
Also make it a point to identify all structures at risk during this portion of the procedure—namely, the common iliac vessels, ureters, and middle sacral artery and vein. The left common iliac vein is medial to the left common iliac artery and is particularly susceptible to injury during this phase of the procedure.
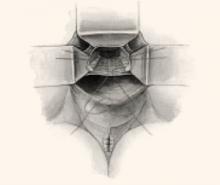
Make a longitudinal incision in the peritoneum overlying the sacral promontory and extend it approximately 6 cm from the promontory dorsally into the cul-de-sac, opening the retrorectal space (FIGURE 1, TOP). Using a fine tonsil forceps and cautery, very gently dissect the retroareolar filmy tissue overlying the anterior longitudinal ligament away from S1 in thin layers until the white periosteum of the anterior longitudinal ligament overlying S1 is clearly exposed. It now becomes very easy to visualize the course of the middle sacral artery and vein. With these vessels under direct visualization, place 2 permanent #0 sutures through the periosteum of S1.
Do not attempt to place these sutures deeper in the presacral space than the S1 vertebral body, or life-threatening and uncontrollable bleeding may result.
If there is no uterus, insert a probe such as an end-to-end anastomotic sizer or handheld Harrington retractor into the vagina and extend it, elongating and elevating the vaginal cylinder. It now becomes much easier to identify the interface between the bladder and vagina prior to making the peritoneal incision.
If the interface remains indistinct, instill 150 cc of saline into the bladder to delineate its boundaries. Then elevate and incise the vesicouterine peritoneum overlying the junction between the bladder and vaginal apex; this provides access to the vesicocervical space. Dissect the bladder off the anterior vaginal wall in a caudal direction until the pubocervical fascia can be identified. Do not dissect away the peritoneum over the posterior vaginal wall, but leave it intact.
FIGURE 1Abdominal sacral colpopexy technique
Reconstructive materials
Although many different materials have been described, none have undergone rigorous comparisons. In our institution, we use soft polypropylene (Surgipro; US Surgical, Norwalk, Conn).
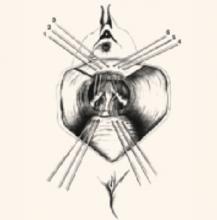
Fold a piece of 5-inch mesh over onto itself and suture the layers together to create a double-thickness configuration. Then “fishmouth” the caudal end of this mesh prosthesis, producing both an anterior and posterior leaf. With the obturator still within the vaginal cylinder stretching the vaginal apex, secure the posterior leaf of the mesh to the posterior vaginal wall with 3 to 5 nonabsorbable #0 sutures.
Suture placement. Thread each suture initially through the posterior leaf of the mesh, placed deeply through the fibromuscular thickness of the posterior vaginal wall, then bring it back out through the mesh at the same point. Place the sutures in a transverse line 1 to 2 cm apart and 3 to 4 cm distal to the vaginal apex.
Once all sutures have been placed, tie each of them, thereby securing the posterior leaf of the mesh to the posterior vaginal wall. Now perform a “mirror” procedure to secure the anterior leaf of the mesh prosthesis to the anterior vaginal wall. Then firmly attach the mesh prosthesis to the vaginal apex using several interrupted, permanent sutures.
At this point, the sutures previously placed through the periosteum of the sacral promontory are threaded through the apex of the mesh prosthesis at a point that will allow the vagina to rest comfortably within the pelvis, without undue tension or traction once the sutures are tied into place (FIGURE 1, MIDDLE).
Trim any excess mesh, and close the 6-cm longitudinal peritoneal incision previously created in the cul-de-sac. Close the incision over the top of the mesh, retroperitonealizing the mesh prosthesis (FIGURE 1, BOTTOM).
Paravaginal defect repair
The bladder base is intimately associated with the anterior vaginal wall via a triangular sheet of pubocervical fascia attached to and extending from the arcus tendineus fascia pelvis bilaterally. When the apex of the vagina prolapses through the introitus, as in total vault prolapse, the base of the bladder is torn free from these fascial attachments in the pelvis and herniates through the introitus along with the vaginal apex. By definition, a bilateral paravaginal defect will result. Thus, surgical repair of total vaginal vault prolapse almost invariably requires paravaginal defect repair as well.
The goal of paravaginal defect repair is to reattach, bilaterally, the anterolateral vaginal sulcus and its overlying endopelvic fascia to the pubococcygeus and obturator internus muscles and fascia at the level of the arcus tendineus fascia pelvis.
Technique
Enter and gently develop the retropubic space of Retzius, taking care not to disrupt the myriad venous anastomotic networks of the plexus of Santorini, located on and around the bladder. Bluntly mobilize the bladder bilaterally, exposing the lateral retropubic spaces, the pubococcygeus and obturator internus muscles, and the obturator neurovascular bundles. Within each retropubic space, palpate the ischial spine. Then visualize the arcus, seen as a white ligamentous band, as it courses from the ischial spine caudally toward the ipsilateral posterior pubic symphysis. A lateral paravaginal defect representing avulsion of the vagina off the arcus tendineus fascia pelvis, or of the arcus tendineus fascia pelvis off the obturator internus muscle, can now be visualized.
While gently reflecting the bladder medially with a wide ribbon, insert a few fingers of the nondominant hand into the vagina and elevate the ipsilateral anterolateral vaginal sulcus. Then place a suture through the fibromuscular thickness of the lateral vaginal apex, just above the uplifting fingers in the vagina, and then slightly cephalad into the arcus tendineus fascia pelvis or obturator internus fascia on the pelvic sidewall, at a point 1 to 2 cm distal to the ischial spine. Place 3 to 5 additional sutures in a similar fashion at 1-cm intervals. The most distal suture should be placed as close as possible to the pubic ramus into the pubourethral ligament. If necessary, repeat the procedure on the contralateral side. Then tie all sutures into place, thereby completing the repair.
When a patient has complete vault prolapse, she typically has defects in all 3 levels of pelvic support, and thus may need to undergo several different procedures to correct all anatomic defects and restore function.
In other words, vaginal vault prolapse rarely presents as an isolated defect. It more commonly occurs in conjunction with a cystocele, rectocele, enterocele, or some combination of these.1 Richter reported that 72% of patients with vaginal vault prolapse had a combination of other pelvic floor defects as well.2
If all vaginal support defects are repaired at the time of sacral colpopexy, recurrent vault prolapse is rare. Failures can be minimized by suturing the suspensory mesh to the posterior vagina and anterior vaginal apex over as extended an area as possible. Also test the sutures once they are placed within the periosteum of the sacral promontory to ensure they will not pull free.
Plan on occult incontinence
Total vaginal vault prolapse is commonly associated with some degree of urethral kinking, with subsequent outflow tract obstruction. As a result, most patients with complete vault prolapse do not complain of incontinence at the initial presentation. However, once the anatomic axis of the vagina is restored and the bladder is replaced within the pelvis with subsequent straightening of the urethra, occult incontinence often is uncovered. Although the patient may have a wonderful anatomic repair of severe vault prolapse at the completion of the surgical procedure, she will not be satisfied if she suddenly finds herself floridly incontinent.
Consider formal multichannel cystometrics prior to surgery in all women undergoing repair of total vault prolapse. If genuine stress urinary incontinence is present when the prolapse is reduced, an anti-incontinence procedure can be scheduled at the same time as the surgical repair. A Burch procedure can be performed for type IIA or IIB genuine stress incontinence, or a pubovaginal sling procedure can be performed for type III stress incontinence.
Posterior colporrhaphy/perineorrhaphy
These procedures are now performed to treat the remaining rectocele and perineal defect, when present.
Vaginal vs abdominal route
Somewhat surprisingly, the abdominal route appears to produce better long-term results. In a prospective, randomized controlled trial comparing both routes for the repair of total vault prolapse, Benson et al3 found that, after 5 years of follow-up, women managed vaginally had a 6-fold increased incidence of recurrent vault prolapse, a 3-fold increased incidence of recurrent cystocele, and twice the reoperation rate, compared with women whose initial repair was abdominal.
In the study, 48 women with total vault prolapse underwent vaginal bilateral sacrospinous fixation and paravaginal defect repair, and 40 underwent abdominal sacral colpopexy and paravaginal defect repair. Although the vaginal approach was associated with a shorter operative time and decreased hospital stay in the short term, it necessitated longer postoperative catheter use and was associated with more urinary tract infections and postoperative incontinence and a higher overall failure rate.
Sze and colleagues4 addressed a similar question in retrospective fashion, reviewing the medical records of 117 women surgically treated for total vault prolapse. Sixty-one women underwent vaginal sacrospinous ligament fixation and Raz urethropexy, while 56 underwent abdominal sacral colpopexy and Burch urethropexy. After a mean follow-up of 24 months, 33% of the women managed vaginally developed recurrent pelvic organ prolapse, compared with only 19% of the women managed abdominally. In addition, 26% of the women managed vaginally had recurrent urinary incontinence, compared with only 13% of the women managed abdominally
A separate randomized, prospective study by Maher et al5 compared abdominal sacral colpopexy (n=47) and vaginal sacrospinous ligament fixation (n=48) for stage II to IV vault prolapse. After a mean follow-up of 2 years, subjective and objective success rates did not differ significantly between the 2 routes.
Why is the abdominal route more durable?
Any number of reasons may apply:
- The traditional surgical procedure for vaginal management of total vault prolapse—sacrospinous ligament fixation—distorts the axis of the vagina.
- Native tissues are not as strong as synthetic materials. In postmenopausal women, a repair in which the thin, atrophic vaginal apex is secured to the sacrospinous ligament will not have the same durability as a repair involving mesh.
- In vaginal paravaginal repair, the extensive periurethral dissection required can damage fine branches of the pudendal nerve that innervate and control the urethral sphincter. Such extensive dissection is not required for paravaginal repair from the abdominal approach.
- In the vaginal approach, it can be difficult to gain adequate exposure high in the retroperitoneum to reattach the endopelvic fascia of the vaginal apex to the arcus at its origin just distal to the ischial spine.
The long view
The surgical options described in this article have varying degrees of risk and benefit. Multicenter, prospective surgical trials are needed to clarify these risks and benefits and provide physicians and their patients with reliable information. Ultimately, pursuit of a surgical “cure” will be supplanted by sustainable forms of disease prevention. Until then, decisions about prolapse surgery are best left to the judgment of the surgeon and the desires of his or her patient.
The authors report no financial relationships relevant to this article.
1. Herbst A, Mishell D, Stenchever M. Disorders of the abdominal wall and pelvic support. In: Comprehensive Gynecology. 2nd ed. Philadelphia: Mosby Year Book; 1992:594–612.
2. Richter K. Massive eversion of the vagina: pathogenesis, diagnosis and therapy of the true prolapse of the vaginal stump. Clin Obstet Gynecol. 1982;25:897-912.
3. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1422.
4. Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. A retrospective comparison of abdominal sacrocolpopexy with Burch colposuspension versus sacrospinous fixation with transvaginal needle suspension for the management of vaginal vault prolapse and coexisting stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:390-393.
5. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal SCP or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
A range of clinical conditions can suggest an abdominal approach for vaginal vault prolapse procedures.
These include, but are not limited to:
- prior unsuccessful vaginal attempts
- obligate need for adnexal access
- markedly foreshortened vagina
- pelvic bony architectural limitations
- high risk for surgical failure (eg, athleticism, obesity, chronic obstructive pulmonary disease, congenital connective tissue disorder)
- desire for uterine preservation
In Part 1 (November 2005) of this 2-part article, we reviewed the most widely used and the newest vaginal techniques. Part 2 focuses on the abdominal approach, and compares vaginal and abdominal approaches.
High uterosacral ligament suspension
Surgical technique for this procedure for mild to moderate vaginal vault prolapse (stage I or II), using a vaginal approach, was described in Part 1, in the November issue of OBG Management. Abdominal repair involves the same concepts; like the vaginal approach, it is applicable only to the patient with mild to moderate vault prolapse. It will be less successful if it is performed to address complete vault prolapse.
Technique
Identify and tag the remnants of the uterosacral ligaments at the level of the ischial spines. Once the ureters are identified and isolated, address the enterocele by obliterating the cul-de-sac via Halban’s culdoplasty or abdominal McCall’s culdoplasty.
Open the peritoneum over the vaginal apex and trim it back to the level of the endopelvic fascia of the vaginal wall. After excising the redundant peritoneum of the vaginal apex, identify and reapproximate the pubocervical fascia of the anterior vaginal wall and the rectovaginal fascia of the posterior vaginal wall using interrupted or running nonabsorbable suture.
Then use nonabsorbable sutures to suspend each corner of the prolapsed vagina from its respective ipsilateral uterosacral ligament.
Abdominal sacral colpopexy
Abdominal sacral colpopexy was first popularized by Addison and Timmons in the 1980s, and is the abdominal standard of apical prolapse repair due to its long-term durability.
Abdominal sacral colpopexy can be performed with or without uterine extirpation. When a hysterectomy is performed concomitantly, some surgeons prefer a supracervical approach, provided there is no history of cervical dysplasia, because, theoretically, the cervical stump serves as a firm and substantial point of fixation for the synthetic mesh that will be used to perform the repair. This in turn may diminish the likelihood of postoperative mesh erosion.
Technique
Reflect the sigmoid colon as far as possible into the left lateral pelvis to expose the sacral promontory. If it has not already been done, free all adhesions between the colon and pelvic peritoneum to fully mobilize the colon and permit its maximal retraction out of the pelvic field prior to making the peritoneal incision.
Also make it a point to identify all structures at risk during this portion of the procedure—namely, the common iliac vessels, ureters, and middle sacral artery and vein. The left common iliac vein is medial to the left common iliac artery and is particularly susceptible to injury during this phase of the procedure.
Make a longitudinal incision in the peritoneum overlying the sacral promontory and extend it approximately 6 cm from the promontory dorsally into the cul-de-sac, opening the retrorectal space (FIGURE 1, TOP). Using a fine tonsil forceps and cautery, very gently dissect the retroareolar filmy tissue overlying the anterior longitudinal ligament away from S1 in thin layers until the white periosteum of the anterior longitudinal ligament overlying S1 is clearly exposed. It now becomes very easy to visualize the course of the middle sacral artery and vein. With these vessels under direct visualization, place 2 permanent #0 sutures through the periosteum of S1.
Do not attempt to place these sutures deeper in the presacral space than the S1 vertebral body, or life-threatening and uncontrollable bleeding may result.
If there is no uterus, insert a probe such as an end-to-end anastomotic sizer or handheld Harrington retractor into the vagina and extend it, elongating and elevating the vaginal cylinder. It now becomes much easier to identify the interface between the bladder and vagina prior to making the peritoneal incision.
If the interface remains indistinct, instill 150 cc of saline into the bladder to delineate its boundaries. Then elevate and incise the vesicouterine peritoneum overlying the junction between the bladder and vaginal apex; this provides access to the vesicocervical space. Dissect the bladder off the anterior vaginal wall in a caudal direction until the pubocervical fascia can be identified. Do not dissect away the peritoneum over the posterior vaginal wall, but leave it intact.
FIGURE 1Abdominal sacral colpopexy technique
Reconstructive materials
Although many different materials have been described, none have undergone rigorous comparisons. In our institution, we use soft polypropylene (Surgipro; US Surgical, Norwalk, Conn).
Fold a piece of 5-inch mesh over onto itself and suture the layers together to create a double-thickness configuration. Then “fishmouth” the caudal end of this mesh prosthesis, producing both an anterior and posterior leaf. With the obturator still within the vaginal cylinder stretching the vaginal apex, secure the posterior leaf of the mesh to the posterior vaginal wall with 3 to 5 nonabsorbable #0 sutures.
Suture placement. Thread each suture initially through the posterior leaf of the mesh, placed deeply through the fibromuscular thickness of the posterior vaginal wall, then bring it back out through the mesh at the same point. Place the sutures in a transverse line 1 to 2 cm apart and 3 to 4 cm distal to the vaginal apex.
Once all sutures have been placed, tie each of them, thereby securing the posterior leaf of the mesh to the posterior vaginal wall. Now perform a “mirror” procedure to secure the anterior leaf of the mesh prosthesis to the anterior vaginal wall. Then firmly attach the mesh prosthesis to the vaginal apex using several interrupted, permanent sutures.
At this point, the sutures previously placed through the periosteum of the sacral promontory are threaded through the apex of the mesh prosthesis at a point that will allow the vagina to rest comfortably within the pelvis, without undue tension or traction once the sutures are tied into place (FIGURE 1, MIDDLE).
Trim any excess mesh, and close the 6-cm longitudinal peritoneal incision previously created in the cul-de-sac. Close the incision over the top of the mesh, retroperitonealizing the mesh prosthesis (FIGURE 1, BOTTOM).
Paravaginal defect repair
The bladder base is intimately associated with the anterior vaginal wall via a triangular sheet of pubocervical fascia attached to and extending from the arcus tendineus fascia pelvis bilaterally. When the apex of the vagina prolapses through the introitus, as in total vault prolapse, the base of the bladder is torn free from these fascial attachments in the pelvis and herniates through the introitus along with the vaginal apex. By definition, a bilateral paravaginal defect will result. Thus, surgical repair of total vaginal vault prolapse almost invariably requires paravaginal defect repair as well.
The goal of paravaginal defect repair is to reattach, bilaterally, the anterolateral vaginal sulcus and its overlying endopelvic fascia to the pubococcygeus and obturator internus muscles and fascia at the level of the arcus tendineus fascia pelvis.
Technique
Enter and gently develop the retropubic space of Retzius, taking care not to disrupt the myriad venous anastomotic networks of the plexus of Santorini, located on and around the bladder. Bluntly mobilize the bladder bilaterally, exposing the lateral retropubic spaces, the pubococcygeus and obturator internus muscles, and the obturator neurovascular bundles. Within each retropubic space, palpate the ischial spine. Then visualize the arcus, seen as a white ligamentous band, as it courses from the ischial spine caudally toward the ipsilateral posterior pubic symphysis. A lateral paravaginal defect representing avulsion of the vagina off the arcus tendineus fascia pelvis, or of the arcus tendineus fascia pelvis off the obturator internus muscle, can now be visualized.
While gently reflecting the bladder medially with a wide ribbon, insert a few fingers of the nondominant hand into the vagina and elevate the ipsilateral anterolateral vaginal sulcus. Then place a suture through the fibromuscular thickness of the lateral vaginal apex, just above the uplifting fingers in the vagina, and then slightly cephalad into the arcus tendineus fascia pelvis or obturator internus fascia on the pelvic sidewall, at a point 1 to 2 cm distal to the ischial spine. Place 3 to 5 additional sutures in a similar fashion at 1-cm intervals. The most distal suture should be placed as close as possible to the pubic ramus into the pubourethral ligament. If necessary, repeat the procedure on the contralateral side. Then tie all sutures into place, thereby completing the repair.
When a patient has complete vault prolapse, she typically has defects in all 3 levels of pelvic support, and thus may need to undergo several different procedures to correct all anatomic defects and restore function.
In other words, vaginal vault prolapse rarely presents as an isolated defect. It more commonly occurs in conjunction with a cystocele, rectocele, enterocele, or some combination of these.1 Richter reported that 72% of patients with vaginal vault prolapse had a combination of other pelvic floor defects as well.2
If all vaginal support defects are repaired at the time of sacral colpopexy, recurrent vault prolapse is rare. Failures can be minimized by suturing the suspensory mesh to the posterior vagina and anterior vaginal apex over as extended an area as possible. Also test the sutures once they are placed within the periosteum of the sacral promontory to ensure they will not pull free.
Plan on occult incontinence
Total vaginal vault prolapse is commonly associated with some degree of urethral kinking, with subsequent outflow tract obstruction. As a result, most patients with complete vault prolapse do not complain of incontinence at the initial presentation. However, once the anatomic axis of the vagina is restored and the bladder is replaced within the pelvis with subsequent straightening of the urethra, occult incontinence often is uncovered. Although the patient may have a wonderful anatomic repair of severe vault prolapse at the completion of the surgical procedure, she will not be satisfied if she suddenly finds herself floridly incontinent.
Consider formal multichannel cystometrics prior to surgery in all women undergoing repair of total vault prolapse. If genuine stress urinary incontinence is present when the prolapse is reduced, an anti-incontinence procedure can be scheduled at the same time as the surgical repair. A Burch procedure can be performed for type IIA or IIB genuine stress incontinence, or a pubovaginal sling procedure can be performed for type III stress incontinence.
Posterior colporrhaphy/perineorrhaphy
These procedures are now performed to treat the remaining rectocele and perineal defect, when present.
Vaginal vs abdominal route
Somewhat surprisingly, the abdominal route appears to produce better long-term results. In a prospective, randomized controlled trial comparing both routes for the repair of total vault prolapse, Benson et al3 found that, after 5 years of follow-up, women managed vaginally had a 6-fold increased incidence of recurrent vault prolapse, a 3-fold increased incidence of recurrent cystocele, and twice the reoperation rate, compared with women whose initial repair was abdominal.
In the study, 48 women with total vault prolapse underwent vaginal bilateral sacrospinous fixation and paravaginal defect repair, and 40 underwent abdominal sacral colpopexy and paravaginal defect repair. Although the vaginal approach was associated with a shorter operative time and decreased hospital stay in the short term, it necessitated longer postoperative catheter use and was associated with more urinary tract infections and postoperative incontinence and a higher overall failure rate.
Sze and colleagues4 addressed a similar question in retrospective fashion, reviewing the medical records of 117 women surgically treated for total vault prolapse. Sixty-one women underwent vaginal sacrospinous ligament fixation and Raz urethropexy, while 56 underwent abdominal sacral colpopexy and Burch urethropexy. After a mean follow-up of 24 months, 33% of the women managed vaginally developed recurrent pelvic organ prolapse, compared with only 19% of the women managed abdominally. In addition, 26% of the women managed vaginally had recurrent urinary incontinence, compared with only 13% of the women managed abdominally
A separate randomized, prospective study by Maher et al5 compared abdominal sacral colpopexy (n=47) and vaginal sacrospinous ligament fixation (n=48) for stage II to IV vault prolapse. After a mean follow-up of 2 years, subjective and objective success rates did not differ significantly between the 2 routes.
Why is the abdominal route more durable?
Any number of reasons may apply:
- The traditional surgical procedure for vaginal management of total vault prolapse—sacrospinous ligament fixation—distorts the axis of the vagina.
- Native tissues are not as strong as synthetic materials. In postmenopausal women, a repair in which the thin, atrophic vaginal apex is secured to the sacrospinous ligament will not have the same durability as a repair involving mesh.
- In vaginal paravaginal repair, the extensive periurethral dissection required can damage fine branches of the pudendal nerve that innervate and control the urethral sphincter. Such extensive dissection is not required for paravaginal repair from the abdominal approach.
- In the vaginal approach, it can be difficult to gain adequate exposure high in the retroperitoneum to reattach the endopelvic fascia of the vaginal apex to the arcus at its origin just distal to the ischial spine.
The long view
The surgical options described in this article have varying degrees of risk and benefit. Multicenter, prospective surgical trials are needed to clarify these risks and benefits and provide physicians and their patients with reliable information. Ultimately, pursuit of a surgical “cure” will be supplanted by sustainable forms of disease prevention. Until then, decisions about prolapse surgery are best left to the judgment of the surgeon and the desires of his or her patient.
The authors report no financial relationships relevant to this article.
A range of clinical conditions can suggest an abdominal approach for vaginal vault prolapse procedures.
These include, but are not limited to:
- prior unsuccessful vaginal attempts
- obligate need for adnexal access
- markedly foreshortened vagina
- pelvic bony architectural limitations
- high risk for surgical failure (eg, athleticism, obesity, chronic obstructive pulmonary disease, congenital connective tissue disorder)
- desire for uterine preservation
In Part 1 (November 2005) of this 2-part article, we reviewed the most widely used and the newest vaginal techniques. Part 2 focuses on the abdominal approach, and compares vaginal and abdominal approaches.
High uterosacral ligament suspension
Surgical technique for this procedure for mild to moderate vaginal vault prolapse (stage I or II), using a vaginal approach, was described in Part 1, in the November issue of OBG Management. Abdominal repair involves the same concepts; like the vaginal approach, it is applicable only to the patient with mild to moderate vault prolapse. It will be less successful if it is performed to address complete vault prolapse.
Technique
Identify and tag the remnants of the uterosacral ligaments at the level of the ischial spines. Once the ureters are identified and isolated, address the enterocele by obliterating the cul-de-sac via Halban’s culdoplasty or abdominal McCall’s culdoplasty.
Open the peritoneum over the vaginal apex and trim it back to the level of the endopelvic fascia of the vaginal wall. After excising the redundant peritoneum of the vaginal apex, identify and reapproximate the pubocervical fascia of the anterior vaginal wall and the rectovaginal fascia of the posterior vaginal wall using interrupted or running nonabsorbable suture.
Then use nonabsorbable sutures to suspend each corner of the prolapsed vagina from its respective ipsilateral uterosacral ligament.
Abdominal sacral colpopexy
Abdominal sacral colpopexy was first popularized by Addison and Timmons in the 1980s, and is the abdominal standard of apical prolapse repair due to its long-term durability.
Abdominal sacral colpopexy can be performed with or without uterine extirpation. When a hysterectomy is performed concomitantly, some surgeons prefer a supracervical approach, provided there is no history of cervical dysplasia, because, theoretically, the cervical stump serves as a firm and substantial point of fixation for the synthetic mesh that will be used to perform the repair. This in turn may diminish the likelihood of postoperative mesh erosion.
Technique
Reflect the sigmoid colon as far as possible into the left lateral pelvis to expose the sacral promontory. If it has not already been done, free all adhesions between the colon and pelvic peritoneum to fully mobilize the colon and permit its maximal retraction out of the pelvic field prior to making the peritoneal incision.
Also make it a point to identify all structures at risk during this portion of the procedure—namely, the common iliac vessels, ureters, and middle sacral artery and vein. The left common iliac vein is medial to the left common iliac artery and is particularly susceptible to injury during this phase of the procedure.
Make a longitudinal incision in the peritoneum overlying the sacral promontory and extend it approximately 6 cm from the promontory dorsally into the cul-de-sac, opening the retrorectal space (FIGURE 1, TOP). Using a fine tonsil forceps and cautery, very gently dissect the retroareolar filmy tissue overlying the anterior longitudinal ligament away from S1 in thin layers until the white periosteum of the anterior longitudinal ligament overlying S1 is clearly exposed. It now becomes very easy to visualize the course of the middle sacral artery and vein. With these vessels under direct visualization, place 2 permanent #0 sutures through the periosteum of S1.
Do not attempt to place these sutures deeper in the presacral space than the S1 vertebral body, or life-threatening and uncontrollable bleeding may result.
If there is no uterus, insert a probe such as an end-to-end anastomotic sizer or handheld Harrington retractor into the vagina and extend it, elongating and elevating the vaginal cylinder. It now becomes much easier to identify the interface between the bladder and vagina prior to making the peritoneal incision.
If the interface remains indistinct, instill 150 cc of saline into the bladder to delineate its boundaries. Then elevate and incise the vesicouterine peritoneum overlying the junction between the bladder and vaginal apex; this provides access to the vesicocervical space. Dissect the bladder off the anterior vaginal wall in a caudal direction until the pubocervical fascia can be identified. Do not dissect away the peritoneum over the posterior vaginal wall, but leave it intact.
FIGURE 1Abdominal sacral colpopexy technique
Reconstructive materials
Although many different materials have been described, none have undergone rigorous comparisons. In our institution, we use soft polypropylene (Surgipro; US Surgical, Norwalk, Conn).
Fold a piece of 5-inch mesh over onto itself and suture the layers together to create a double-thickness configuration. Then “fishmouth” the caudal end of this mesh prosthesis, producing both an anterior and posterior leaf. With the obturator still within the vaginal cylinder stretching the vaginal apex, secure the posterior leaf of the mesh to the posterior vaginal wall with 3 to 5 nonabsorbable #0 sutures.
Suture placement. Thread each suture initially through the posterior leaf of the mesh, placed deeply through the fibromuscular thickness of the posterior vaginal wall, then bring it back out through the mesh at the same point. Place the sutures in a transverse line 1 to 2 cm apart and 3 to 4 cm distal to the vaginal apex.
Once all sutures have been placed, tie each of them, thereby securing the posterior leaf of the mesh to the posterior vaginal wall. Now perform a “mirror” procedure to secure the anterior leaf of the mesh prosthesis to the anterior vaginal wall. Then firmly attach the mesh prosthesis to the vaginal apex using several interrupted, permanent sutures.
At this point, the sutures previously placed through the periosteum of the sacral promontory are threaded through the apex of the mesh prosthesis at a point that will allow the vagina to rest comfortably within the pelvis, without undue tension or traction once the sutures are tied into place (FIGURE 1, MIDDLE).
Trim any excess mesh, and close the 6-cm longitudinal peritoneal incision previously created in the cul-de-sac. Close the incision over the top of the mesh, retroperitonealizing the mesh prosthesis (FIGURE 1, BOTTOM).
Paravaginal defect repair
The bladder base is intimately associated with the anterior vaginal wall via a triangular sheet of pubocervical fascia attached to and extending from the arcus tendineus fascia pelvis bilaterally. When the apex of the vagina prolapses through the introitus, as in total vault prolapse, the base of the bladder is torn free from these fascial attachments in the pelvis and herniates through the introitus along with the vaginal apex. By definition, a bilateral paravaginal defect will result. Thus, surgical repair of total vaginal vault prolapse almost invariably requires paravaginal defect repair as well.
The goal of paravaginal defect repair is to reattach, bilaterally, the anterolateral vaginal sulcus and its overlying endopelvic fascia to the pubococcygeus and obturator internus muscles and fascia at the level of the arcus tendineus fascia pelvis.
Technique
Enter and gently develop the retropubic space of Retzius, taking care not to disrupt the myriad venous anastomotic networks of the plexus of Santorini, located on and around the bladder. Bluntly mobilize the bladder bilaterally, exposing the lateral retropubic spaces, the pubococcygeus and obturator internus muscles, and the obturator neurovascular bundles. Within each retropubic space, palpate the ischial spine. Then visualize the arcus, seen as a white ligamentous band, as it courses from the ischial spine caudally toward the ipsilateral posterior pubic symphysis. A lateral paravaginal defect representing avulsion of the vagina off the arcus tendineus fascia pelvis, or of the arcus tendineus fascia pelvis off the obturator internus muscle, can now be visualized.
While gently reflecting the bladder medially with a wide ribbon, insert a few fingers of the nondominant hand into the vagina and elevate the ipsilateral anterolateral vaginal sulcus. Then place a suture through the fibromuscular thickness of the lateral vaginal apex, just above the uplifting fingers in the vagina, and then slightly cephalad into the arcus tendineus fascia pelvis or obturator internus fascia on the pelvic sidewall, at a point 1 to 2 cm distal to the ischial spine. Place 3 to 5 additional sutures in a similar fashion at 1-cm intervals. The most distal suture should be placed as close as possible to the pubic ramus into the pubourethral ligament. If necessary, repeat the procedure on the contralateral side. Then tie all sutures into place, thereby completing the repair.
When a patient has complete vault prolapse, she typically has defects in all 3 levels of pelvic support, and thus may need to undergo several different procedures to correct all anatomic defects and restore function.
In other words, vaginal vault prolapse rarely presents as an isolated defect. It more commonly occurs in conjunction with a cystocele, rectocele, enterocele, or some combination of these.1 Richter reported that 72% of patients with vaginal vault prolapse had a combination of other pelvic floor defects as well.2
If all vaginal support defects are repaired at the time of sacral colpopexy, recurrent vault prolapse is rare. Failures can be minimized by suturing the suspensory mesh to the posterior vagina and anterior vaginal apex over as extended an area as possible. Also test the sutures once they are placed within the periosteum of the sacral promontory to ensure they will not pull free.
Plan on occult incontinence
Total vaginal vault prolapse is commonly associated with some degree of urethral kinking, with subsequent outflow tract obstruction. As a result, most patients with complete vault prolapse do not complain of incontinence at the initial presentation. However, once the anatomic axis of the vagina is restored and the bladder is replaced within the pelvis with subsequent straightening of the urethra, occult incontinence often is uncovered. Although the patient may have a wonderful anatomic repair of severe vault prolapse at the completion of the surgical procedure, she will not be satisfied if she suddenly finds herself floridly incontinent.
Consider formal multichannel cystometrics prior to surgery in all women undergoing repair of total vault prolapse. If genuine stress urinary incontinence is present when the prolapse is reduced, an anti-incontinence procedure can be scheduled at the same time as the surgical repair. A Burch procedure can be performed for type IIA or IIB genuine stress incontinence, or a pubovaginal sling procedure can be performed for type III stress incontinence.
Posterior colporrhaphy/perineorrhaphy
These procedures are now performed to treat the remaining rectocele and perineal defect, when present.
Vaginal vs abdominal route
Somewhat surprisingly, the abdominal route appears to produce better long-term results. In a prospective, randomized controlled trial comparing both routes for the repair of total vault prolapse, Benson et al3 found that, after 5 years of follow-up, women managed vaginally had a 6-fold increased incidence of recurrent vault prolapse, a 3-fold increased incidence of recurrent cystocele, and twice the reoperation rate, compared with women whose initial repair was abdominal.
In the study, 48 women with total vault prolapse underwent vaginal bilateral sacrospinous fixation and paravaginal defect repair, and 40 underwent abdominal sacral colpopexy and paravaginal defect repair. Although the vaginal approach was associated with a shorter operative time and decreased hospital stay in the short term, it necessitated longer postoperative catheter use and was associated with more urinary tract infections and postoperative incontinence and a higher overall failure rate.
Sze and colleagues4 addressed a similar question in retrospective fashion, reviewing the medical records of 117 women surgically treated for total vault prolapse. Sixty-one women underwent vaginal sacrospinous ligament fixation and Raz urethropexy, while 56 underwent abdominal sacral colpopexy and Burch urethropexy. After a mean follow-up of 24 months, 33% of the women managed vaginally developed recurrent pelvic organ prolapse, compared with only 19% of the women managed abdominally. In addition, 26% of the women managed vaginally had recurrent urinary incontinence, compared with only 13% of the women managed abdominally
A separate randomized, prospective study by Maher et al5 compared abdominal sacral colpopexy (n=47) and vaginal sacrospinous ligament fixation (n=48) for stage II to IV vault prolapse. After a mean follow-up of 2 years, subjective and objective success rates did not differ significantly between the 2 routes.
Why is the abdominal route more durable?
Any number of reasons may apply:
- The traditional surgical procedure for vaginal management of total vault prolapse—sacrospinous ligament fixation—distorts the axis of the vagina.
- Native tissues are not as strong as synthetic materials. In postmenopausal women, a repair in which the thin, atrophic vaginal apex is secured to the sacrospinous ligament will not have the same durability as a repair involving mesh.
- In vaginal paravaginal repair, the extensive periurethral dissection required can damage fine branches of the pudendal nerve that innervate and control the urethral sphincter. Such extensive dissection is not required for paravaginal repair from the abdominal approach.
- In the vaginal approach, it can be difficult to gain adequate exposure high in the retroperitoneum to reattach the endopelvic fascia of the vaginal apex to the arcus at its origin just distal to the ischial spine.
The long view
The surgical options described in this article have varying degrees of risk and benefit. Multicenter, prospective surgical trials are needed to clarify these risks and benefits and provide physicians and their patients with reliable information. Ultimately, pursuit of a surgical “cure” will be supplanted by sustainable forms of disease prevention. Until then, decisions about prolapse surgery are best left to the judgment of the surgeon and the desires of his or her patient.
The authors report no financial relationships relevant to this article.
1. Herbst A, Mishell D, Stenchever M. Disorders of the abdominal wall and pelvic support. In: Comprehensive Gynecology. 2nd ed. Philadelphia: Mosby Year Book; 1992:594–612.
2. Richter K. Massive eversion of the vagina: pathogenesis, diagnosis and therapy of the true prolapse of the vaginal stump. Clin Obstet Gynecol. 1982;25:897-912.
3. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1422.
4. Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. A retrospective comparison of abdominal sacrocolpopexy with Burch colposuspension versus sacrospinous fixation with transvaginal needle suspension for the management of vaginal vault prolapse and coexisting stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:390-393.
5. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal SCP or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
1. Herbst A, Mishell D, Stenchever M. Disorders of the abdominal wall and pelvic support. In: Comprehensive Gynecology. 2nd ed. Philadelphia: Mosby Year Book; 1992:594–612.
2. Richter K. Massive eversion of the vagina: pathogenesis, diagnosis and therapy of the true prolapse of the vaginal stump. Clin Obstet Gynecol. 1982;25:897-912.
3. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1422.
4. Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. A retrospective comparison of abdominal sacrocolpopexy with Burch colposuspension versus sacrospinous fixation with transvaginal needle suspension for the management of vaginal vault prolapse and coexisting stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:390-393.
5. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal SCP or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
Surgical management of vaginal vault prolapse
First, the good news: We have numerous techniques to choose from to repair prolapse of the vaginal vault, which affects as many as 50% of parous women.1 The bad news: Most of the data on these techniques are anecdotal or retrospective, not the result of randomized, controlled trials. Few investigators have compared the vaginal and abdominal approaches.
So how should we decide on a procedure? It is a judgment call, ultimately. After taking into account the patient’s age, functional status, comorbidities, desire for coitus, and surgical history, the surgeon must weigh the risks and benefits of the procedures that seem most appropriate. Part 1 of this 2-part article reviews what is known about the most widely used and newest vaginal techniques:
- sacrospinous ligament fixation,
- iliococcygeal fixation,
- modified McCall culdoplasty,
- high uterosacral ligament suspension with fascial reconstruction, and
- posterior intravaginal slingplasty (infracoccygeal sacropexy).
In Part 2, next month, we focus on the abdominal approach, and survey the data comparing vaginal and abdominal repairs.
Unfortunately, success and failure rates are still poorly defined because of a lack of standardization, and because techniques and materials continually change. This underscores the need for better understanding of the pathophysiology of genital prolapse, improved preoperative assessment, and more effective and durable repair techniques.
Why prolapse occurs
Pelvic support involves a complex interplay of anatomic, histologic, genetic, and electrophysiologic factors that, although incompletely understood, are frequently disrupted. For example, MacLennan et al2 reported that 46.2% of women aged 15 to 97 years experience pelvic floor dysfunction; a large retrospective study by Olsen and colleagues3 found that 11.1% of women undergo surgery for prolapse by the age of 80, and 29.2% of these women require repeat surgery.
Here’s what we know about the anatomy of pelvic support:
Ligaments serve as secondary supports
The uterus and upper third of the vagina are held in place over the levator plate by the fibers of the parametrium (cardinal and uterosacral ligaments) and paracolpium. These fibers arise from a broad area on the pelvic sidewall overlying the fascia of the piriformis muscle, the sacroiliac joint, and lateral sacrum. The fibers represent condensations of the endopelvic fascia of the pelvis, acting as suspensory ligaments that run in a predominantly vertical direction to insert into the lateral upper third of the vagina and lateral and posterolateral aspect of the cervical portion of the uterus.
In the normal, healthy pelvis, these suspensory ligaments represent secondary support mechanisms and are not routinely under tension.
Pelvic-floor muscles play leading role
Gosling4 argued that pelvic floor muscle tone is more crucial to normal positioning of the pelvic viscera than are the fascial and ligamentous supports of the pelvic organs. Specifically, the pubococcygeus, iliococcygeus, and puborectalis muscles collectively define the levator ani of the pelvic floor. Fusion of the right and left bellies of the levator ani, behind the rectum and anterior to the coccyx, creates a muscular platform known as the levator plate. This plate provides indirect support for the upper genital tract by acting as a platform against which the upper vagina and other pelvic viscera are compressed during increases in intra-abdominal pressure.
Contraction of the levator ani pulls the levator plate toward the posterior symphysis pubis, minimizing the size of the urogenital hiatus through which the rectum, vagina, and urethra exit the pelvis on their way to the perineum. Weakness in the muscular pelvic floor—whether caused by disuse, pudendal nerve damage, or muscular trauma—increases the size of the urogenital hiatus, and the pelvic organs begin to prolapse through it.
Ultimately, constant tension on the ligamentous supports of the pelvic organs exceeds their tensile strength, and pelvic organ prolapse results.
Goals of surgery
Successful surgery achieves effective and sustained vault support, obliterates any enterocele sac, and repairs the cystocele and rectocele that occur in approximately two thirds of women with vault prolapse.
The broader goals: anatomic and functional restoration of the lower female genital tract and improvement in quality of life.
Surgery can be reconstructive or obliterative. Reconstructive surgery can be performed vaginally, abdominally, or a combination of both.
Vaginal techniques
Proponents of the vaginal approach argue that, by avoiding the need for laparotomy, it results in fewer complications, less blood loss and postoperative discomfort, a shorter hospital stay, and less expense.5
Sacrospinous ligament fixation
The sacrospinous ligaments extend from the ischial spines on either side to the lower portion of the sacrum and coccyx. Fixation of the vaginal apex to 1 or both of the sacrospinous ligaments is an option for posthysterectomy vault prolapse.
Technique. Nichols6 described the need to penetrate the right rectal pillar into the pararectal space near the ischial spine. The next step is grasping the ligament and muscle with a long Babcock clamp. Place two #2 polyglycolic sutures through the sacrospinous ligament, 1.5 to 2 fingerbreadths medial to the ischial spine. Attach 1 end of the suture to the undersurface of the posterior vaginal wall at the apical area. When the posterior colporrhaphy reaches the midportion of the vagina, tie the sacrospinous suspension sutures, firmly attaching the vaginal apex to the surface of the coccygeal-sacrospinous ligament complex with no intervening suture bridge (FIGURE 1).
Modifications. The most notable modification is the Miyazaki technique, which substitutes the Miya hook for the DesChamps ligature carrier. The Miya hook is reportedly safer for the pudendal complex, which may lie up to 5.5 cm medial to the ischial spine.7 The path of the Miya hook avoids Alcock’s canal and its neurovascular pudendal bundle.
The Caprio ligature carrier (Boston Scientific, Boston, Mass) is also useful for the placement of sacrospinous sutures; unlike the Miya hook, however, the Caprio ligature carrier is a disposable instrument and thus is not reusable.
Potential complications of sacrospinous ligament fixation include hemorrhage, pudendal nerve injury, rectal or bladder injury, and recurrent anterior vaginal wall prolapse.
Outcomes. Follow-up studies in women undergoing this procedure report a roughly 20% incidence of recurrent or persistent anterior vaginal wall relaxation, or symptomatic cystocele, within 1 year after the surgery. Alteration of the vaginal axis in an exaggerated posterolateral direction after this procedure is thought to place undue tension on the anterior segment of the vaginal wall and predispose women to prolapse at a site opposite the repair.8,9
FIGURE 1 2 “pulley stitches” secure the apex
Place 2 nonabsorbable monofilament “pulley stitches” to secure the vaginal apex to the ligament.
Iliococcygeal fixation
Inmon10 was the first to describe a technique in which the everted vaginal apex is secured to the iliococcygeal fascia bilaterally, just below the ischial spine. He performed this technique successfully in 3 women with atrophied uterosacral ligaments.
Technique. Open the posterior vaginal wall in the midline, as if preparing to perform a posterior colporrhaphy. Develop the rectovaginal spaces bluntly and bilaterally—laterally toward the levator muscles and posteriorally toward the ischial spines. Use the nondominant hand to depress the rectum downward and medially, and place a single #0 polyglycolic suture deep into the iliococcygeus muscle and fascia at a point 1 to 2 cm caudad and posterior to the ischial spine. Then pass both ends of the suture through the ipsilateral posterior vaginal apex and hold them with a hemostat. Repeat the procedure contralaterally.
Usually no vaginal epithelium needs excision because the upper vagina is attached bilaterally, resulting in good vaginal length and circumference. When posterior colporrhaphy is completed and the posterior vaginal wall is closed, tie both iliococcygeal-fixation sutures in place.
Complications. Shull and colleagues11 studied 42 women who underwent suspension of the vaginal cuff to iliococcygeus fascia and repair of coexisting pelvic support defects. Of these women, 2 (5%) had recurrence of their cuff prolapse during follow-up, one of whom required further surgery (she also had recurrence of an inguinal hernia that had been repaired at the original surgery). The other patient, who had undergone 5 previous pelvic procedures, developed asymptomatic prolapse of the cuff halfway to the hymen. Six additional patients had loss of support at other sites in the follow-up period, one of whom required repeat surgery. Ninety-five percent of women experienced no persistence or recurrence of cuff prolapse 6 weeks to 5 years after the procedure.
Meeks and colleagues12 also applied the Inmon technique in 110 women with posthysterectomy vault prolapse or total uterine procidentia. In both studies, the most commonly reported complications included hemorrhage (1.2%), bladder/rectal perforation (1.2%), and recurrent vault prolapse (8%).
Benefits. In comparison with sacrospinous ligament fixation, iliococcygeus fixation is technically easier and places less tension on the anterior vaginal wall.
Modified McCall culdoplasty
Symmonds and colleagues13 described this approach to symptomatic vaginal vault prolapse.
Technique. Excise an elliptical wedge of mucosa from the anterior and posterior walls of the prolapsed vagina to narrow the vault and allow access to the lateral fascial supports of the vagina and rectum. The width and length of the excised wedges are determined by the desired dimensions of the reconstructed vagina.
After isolating and excising the enterocele sac, place up to 3 modified McCall stitches, each one slightly higher than its predecessor. Each suture should incorporate the full thickness of the posterior vaginal wall, the cul-de-sac peritoneum, the remains of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum (FIGURE 2).
Once they are in place, tie the sutures in the opposite order in which they were placed. These stitches fix the prolapsed vaginal vault to the uppermost portion of the endopelvic fascia at the same time as they accomplish a high closure of the culdesac peritoneum.
The evidence. Sze and Karram5 found an 11.5% incidence of recurrent vault prolapse and an associated 22% incidence of new-onset dyspareunia.
FIGURE 2 Classic vs modified McCall culdoplasty
In the classic McCall culdoplasty shown here, only the distal-most suture incorporates the posterior vaginal wall. With the “modified” technique, however, all sutures incorporate the full thickness of the posterior vaginal wall, as well as the cul-de-sac peritoneum, the remnants of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum.
High uterosacral ligament suspension with fascial reconstruction
This approach is based on the observations of Richardson,14 who suggested that the endopelvic fascial supports (uterosacral/cardinal complex) do not stretch and attenuate over time, as some have hypothesized, but break at definable points. By identifying these points, the surgeon can reattach the prolapsed vagina to the intact uterosacral complex cephalad to the break.
Technique. Grasp the vaginal apex with 2 Allis clamps and incise it with a scalpel. If an enterocele sac is present, dissect it off the vaginal epithelium to the neck of the hernia, open it, and then excise it. Place a delayed absorbable or permanent pursestring suture about the neck of the hernia to close the peritoneal defect. If an anterior colporrhaphy or sling is required, perform them at this time.
Place a moist laparotomy pad in the cul-de-sac, and insert and elevate a Deaver retractor to remove the intestines from the cul-de-sac and improve exposure. Next, palpate the ischial spines transperitoneally.
Once the spines are identified, the remnants of the uterosacral ligaments can be identified posterior and medial to the spines and can be palpated transperitoneally or transrectally. Remember that the ureters are also quite close to the ischial spines at this location, running along the lateral pelvic sidewall 2 to 5 cm ventral and lateral to the ischial spines.
After identifying the uterosacral ligaments, grasp their remnants with Allis clamps and place 2 to 3 delayed absorbable or permanent sutures through the rectovaginal fascia of the inner posterior vaginal wall epithelium at one lateral vaginal apex, through the ipsilateral plicated uterosacral ligament complex, and then through the pubocervical fascia of the anterior vaginal wall of the ipsilateral vaginal apex. Hold these sutures while performing the same procedure contralaterally (FIGURE 3). Close the apex and tie these sutures in place, suspending the corners of the vaginal apex from the uterosacral complex bilaterally, and restoring the continuity of the paracervical ring (FIGURES 4, 5).
Benefits of this technique include:
- creation of an anatomically appropriate and correctly positioned midline vaginal axis,
- preservation of adequate vaginal length,
- reduced risk of nerve injury, and
- restored continuity of the paracervical ring when the pelvic pararectal, uterosacral, and pubocervical fascia are reapproximated circumferentially.
Risks include the potential for ureteral kinking or obstruction. Thus, it is prudent to perform cystoscopy after this procedure to rule out occult injury.
FIGURE 3 Suspend apical corners bilaterally
The corners of the vaginal apex are suspended from the cardinal-uterosacral complex bilaterally, with all sutures placed posterior and medial to the ischial spines. Copyright 1998 by C.G. Bachofen.
FIGURE 4 Suture placement penetrates multiple layers
Sagittal view of correct suture placement. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, AD=apical defect, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
FIGURE 5 Suspend the fascia from uterosacral ligaments
Sagittal view after tying of sutures. Note restoration of the normal anatomic axis. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
Posterior intravaginal slingplasty
This investigative technique, also known as infracoccygeal sacropexy, is a minimally invasive, transperineal approach to vaginal vault prolapse. The anatomic and physiologic concepts are similar to those of the tension-free vaginal tape (Gynecare, Somerville, NJ) in the treatment of stress incontinence. However, because it is a new procedure, further evaluation is needed before it can be adopted into clinical practice.
This is an outpatient surgery.
Technique. Use a narrow tunneling device to pass a synthetic nonabsorbable tape through each pararectal space via a small perineal incision, and make a small vaginal incision to secure the tape to the vault.
The evidence. In the initial case series of 93 patients,15 1 rectal perforation and 1 rectal tape erosion were noted. The cure rate was 91% in short-term follow-up in a small number of patients.
The authors report no financial relationships relevant to this article.
1. Beck RP. Pelvic relaxational prolapse. In: Principles and Practice of Clinical Gynecology. New York: John Wiley and Sons; 1983;667-685.
2. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity, and mode of delivery. Br J Obstet Gynecol. 2000;107:1460-1470.
3. Olsen AL, Smith VJ, Bergstrom JO. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
4. Gosling JA. The structure of the bladder neck, urethra and pelvic floor in relation to female urinary incontinence. Int Urogynecol J. 1996;7:177-178.
5. Sze EHM, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89:466-475.
6. Nichols D. Sacrospinous fixation for massive eversion of the vagina. Am J Obstet Gynecol. 1982;142:901-904.
7. Miyazaki FS. Miya hook ligature carrier for sacrospinous ligament suspension. Obstet Gynecol. 1987;70:286-288.
8. Morley G, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-878.
9. Shull BL, Capen CV, Riggs MW. Preoperative analysis of site specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
10. Inmon WB. Pelvic relaxation and repair including prolapse of vagina following hysterectomy. South Med J. 1963;56:577-582.
11. Shull BT, Capen CV, Riggs MW. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 193;168:1669-1673.
12. Meeks GR, Washburne JF, McGehrer RP. Repair of vaginal vault prolapse by suspension of the vagina to iliococcygeus (prespinous) fascia. Am J Obstet Gynecol. 1994;171:1444-1449.
13. Symmonds RE, Williams TJ, Lee RA, Webbs MJ. Posthysterectomy enterocoele and vaginal vault prolapse. Am J Obstet Gynecol. 1981;140:852-859.
14. Richardson AL. The anatomic defects in rectocoele and enterocoele. J Pelvic Surg. 1995;1:214-218.
15. Farnsworth BN. Posterior intravaginal slingplasty (infracoccygeal sacropexy) for severe posthysterectomy vaginal vault prolapse: a preliminary report on efficacy and safety. Int Urogynecol J. 2002;13:4-8.
First, the good news: We have numerous techniques to choose from to repair prolapse of the vaginal vault, which affects as many as 50% of parous women.1 The bad news: Most of the data on these techniques are anecdotal or retrospective, not the result of randomized, controlled trials. Few investigators have compared the vaginal and abdominal approaches.
So how should we decide on a procedure? It is a judgment call, ultimately. After taking into account the patient’s age, functional status, comorbidities, desire for coitus, and surgical history, the surgeon must weigh the risks and benefits of the procedures that seem most appropriate. Part 1 of this 2-part article reviews what is known about the most widely used and newest vaginal techniques:
- sacrospinous ligament fixation,
- iliococcygeal fixation,
- modified McCall culdoplasty,
- high uterosacral ligament suspension with fascial reconstruction, and
- posterior intravaginal slingplasty (infracoccygeal sacropexy).
In Part 2, next month, we focus on the abdominal approach, and survey the data comparing vaginal and abdominal repairs.
Unfortunately, success and failure rates are still poorly defined because of a lack of standardization, and because techniques and materials continually change. This underscores the need for better understanding of the pathophysiology of genital prolapse, improved preoperative assessment, and more effective and durable repair techniques.
Why prolapse occurs
Pelvic support involves a complex interplay of anatomic, histologic, genetic, and electrophysiologic factors that, although incompletely understood, are frequently disrupted. For example, MacLennan et al2 reported that 46.2% of women aged 15 to 97 years experience pelvic floor dysfunction; a large retrospective study by Olsen and colleagues3 found that 11.1% of women undergo surgery for prolapse by the age of 80, and 29.2% of these women require repeat surgery.
Here’s what we know about the anatomy of pelvic support:
Ligaments serve as secondary supports
The uterus and upper third of the vagina are held in place over the levator plate by the fibers of the parametrium (cardinal and uterosacral ligaments) and paracolpium. These fibers arise from a broad area on the pelvic sidewall overlying the fascia of the piriformis muscle, the sacroiliac joint, and lateral sacrum. The fibers represent condensations of the endopelvic fascia of the pelvis, acting as suspensory ligaments that run in a predominantly vertical direction to insert into the lateral upper third of the vagina and lateral and posterolateral aspect of the cervical portion of the uterus.
In the normal, healthy pelvis, these suspensory ligaments represent secondary support mechanisms and are not routinely under tension.
Pelvic-floor muscles play leading role
Gosling4 argued that pelvic floor muscle tone is more crucial to normal positioning of the pelvic viscera than are the fascial and ligamentous supports of the pelvic organs. Specifically, the pubococcygeus, iliococcygeus, and puborectalis muscles collectively define the levator ani of the pelvic floor. Fusion of the right and left bellies of the levator ani, behind the rectum and anterior to the coccyx, creates a muscular platform known as the levator plate. This plate provides indirect support for the upper genital tract by acting as a platform against which the upper vagina and other pelvic viscera are compressed during increases in intra-abdominal pressure.
Contraction of the levator ani pulls the levator plate toward the posterior symphysis pubis, minimizing the size of the urogenital hiatus through which the rectum, vagina, and urethra exit the pelvis on their way to the perineum. Weakness in the muscular pelvic floor—whether caused by disuse, pudendal nerve damage, or muscular trauma—increases the size of the urogenital hiatus, and the pelvic organs begin to prolapse through it.
Ultimately, constant tension on the ligamentous supports of the pelvic organs exceeds their tensile strength, and pelvic organ prolapse results.
Goals of surgery
Successful surgery achieves effective and sustained vault support, obliterates any enterocele sac, and repairs the cystocele and rectocele that occur in approximately two thirds of women with vault prolapse.
The broader goals: anatomic and functional restoration of the lower female genital tract and improvement in quality of life.
Surgery can be reconstructive or obliterative. Reconstructive surgery can be performed vaginally, abdominally, or a combination of both.
Vaginal techniques
Proponents of the vaginal approach argue that, by avoiding the need for laparotomy, it results in fewer complications, less blood loss and postoperative discomfort, a shorter hospital stay, and less expense.5
Sacrospinous ligament fixation
The sacrospinous ligaments extend from the ischial spines on either side to the lower portion of the sacrum and coccyx. Fixation of the vaginal apex to 1 or both of the sacrospinous ligaments is an option for posthysterectomy vault prolapse.
Technique. Nichols6 described the need to penetrate the right rectal pillar into the pararectal space near the ischial spine. The next step is grasping the ligament and muscle with a long Babcock clamp. Place two #2 polyglycolic sutures through the sacrospinous ligament, 1.5 to 2 fingerbreadths medial to the ischial spine. Attach 1 end of the suture to the undersurface of the posterior vaginal wall at the apical area. When the posterior colporrhaphy reaches the midportion of the vagina, tie the sacrospinous suspension sutures, firmly attaching the vaginal apex to the surface of the coccygeal-sacrospinous ligament complex with no intervening suture bridge (FIGURE 1).
Modifications. The most notable modification is the Miyazaki technique, which substitutes the Miya hook for the DesChamps ligature carrier. The Miya hook is reportedly safer for the pudendal complex, which may lie up to 5.5 cm medial to the ischial spine.7 The path of the Miya hook avoids Alcock’s canal and its neurovascular pudendal bundle.
The Caprio ligature carrier (Boston Scientific, Boston, Mass) is also useful for the placement of sacrospinous sutures; unlike the Miya hook, however, the Caprio ligature carrier is a disposable instrument and thus is not reusable.
Potential complications of sacrospinous ligament fixation include hemorrhage, pudendal nerve injury, rectal or bladder injury, and recurrent anterior vaginal wall prolapse.
Outcomes. Follow-up studies in women undergoing this procedure report a roughly 20% incidence of recurrent or persistent anterior vaginal wall relaxation, or symptomatic cystocele, within 1 year after the surgery. Alteration of the vaginal axis in an exaggerated posterolateral direction after this procedure is thought to place undue tension on the anterior segment of the vaginal wall and predispose women to prolapse at a site opposite the repair.8,9
FIGURE 1 2 “pulley stitches” secure the apex
Place 2 nonabsorbable monofilament “pulley stitches” to secure the vaginal apex to the ligament.
Iliococcygeal fixation
Inmon10 was the first to describe a technique in which the everted vaginal apex is secured to the iliococcygeal fascia bilaterally, just below the ischial spine. He performed this technique successfully in 3 women with atrophied uterosacral ligaments.
Technique. Open the posterior vaginal wall in the midline, as if preparing to perform a posterior colporrhaphy. Develop the rectovaginal spaces bluntly and bilaterally—laterally toward the levator muscles and posteriorally toward the ischial spines. Use the nondominant hand to depress the rectum downward and medially, and place a single #0 polyglycolic suture deep into the iliococcygeus muscle and fascia at a point 1 to 2 cm caudad and posterior to the ischial spine. Then pass both ends of the suture through the ipsilateral posterior vaginal apex and hold them with a hemostat. Repeat the procedure contralaterally.
Usually no vaginal epithelium needs excision because the upper vagina is attached bilaterally, resulting in good vaginal length and circumference. When posterior colporrhaphy is completed and the posterior vaginal wall is closed, tie both iliococcygeal-fixation sutures in place.
Complications. Shull and colleagues11 studied 42 women who underwent suspension of the vaginal cuff to iliococcygeus fascia and repair of coexisting pelvic support defects. Of these women, 2 (5%) had recurrence of their cuff prolapse during follow-up, one of whom required further surgery (she also had recurrence of an inguinal hernia that had been repaired at the original surgery). The other patient, who had undergone 5 previous pelvic procedures, developed asymptomatic prolapse of the cuff halfway to the hymen. Six additional patients had loss of support at other sites in the follow-up period, one of whom required repeat surgery. Ninety-five percent of women experienced no persistence or recurrence of cuff prolapse 6 weeks to 5 years after the procedure.
Meeks and colleagues12 also applied the Inmon technique in 110 women with posthysterectomy vault prolapse or total uterine procidentia. In both studies, the most commonly reported complications included hemorrhage (1.2%), bladder/rectal perforation (1.2%), and recurrent vault prolapse (8%).
Benefits. In comparison with sacrospinous ligament fixation, iliococcygeus fixation is technically easier and places less tension on the anterior vaginal wall.
Modified McCall culdoplasty
Symmonds and colleagues13 described this approach to symptomatic vaginal vault prolapse.
Technique. Excise an elliptical wedge of mucosa from the anterior and posterior walls of the prolapsed vagina to narrow the vault and allow access to the lateral fascial supports of the vagina and rectum. The width and length of the excised wedges are determined by the desired dimensions of the reconstructed vagina.
After isolating and excising the enterocele sac, place up to 3 modified McCall stitches, each one slightly higher than its predecessor. Each suture should incorporate the full thickness of the posterior vaginal wall, the cul-de-sac peritoneum, the remains of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum (FIGURE 2).
Once they are in place, tie the sutures in the opposite order in which they were placed. These stitches fix the prolapsed vaginal vault to the uppermost portion of the endopelvic fascia at the same time as they accomplish a high closure of the culdesac peritoneum.
The evidence. Sze and Karram5 found an 11.5% incidence of recurrent vault prolapse and an associated 22% incidence of new-onset dyspareunia.
FIGURE 2 Classic vs modified McCall culdoplasty
In the classic McCall culdoplasty shown here, only the distal-most suture incorporates the posterior vaginal wall. With the “modified” technique, however, all sutures incorporate the full thickness of the posterior vaginal wall, as well as the cul-de-sac peritoneum, the remnants of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum.
High uterosacral ligament suspension with fascial reconstruction
This approach is based on the observations of Richardson,14 who suggested that the endopelvic fascial supports (uterosacral/cardinal complex) do not stretch and attenuate over time, as some have hypothesized, but break at definable points. By identifying these points, the surgeon can reattach the prolapsed vagina to the intact uterosacral complex cephalad to the break.
Technique. Grasp the vaginal apex with 2 Allis clamps and incise it with a scalpel. If an enterocele sac is present, dissect it off the vaginal epithelium to the neck of the hernia, open it, and then excise it. Place a delayed absorbable or permanent pursestring suture about the neck of the hernia to close the peritoneal defect. If an anterior colporrhaphy or sling is required, perform them at this time.
Place a moist laparotomy pad in the cul-de-sac, and insert and elevate a Deaver retractor to remove the intestines from the cul-de-sac and improve exposure. Next, palpate the ischial spines transperitoneally.
Once the spines are identified, the remnants of the uterosacral ligaments can be identified posterior and medial to the spines and can be palpated transperitoneally or transrectally. Remember that the ureters are also quite close to the ischial spines at this location, running along the lateral pelvic sidewall 2 to 5 cm ventral and lateral to the ischial spines.
After identifying the uterosacral ligaments, grasp their remnants with Allis clamps and place 2 to 3 delayed absorbable or permanent sutures through the rectovaginal fascia of the inner posterior vaginal wall epithelium at one lateral vaginal apex, through the ipsilateral plicated uterosacral ligament complex, and then through the pubocervical fascia of the anterior vaginal wall of the ipsilateral vaginal apex. Hold these sutures while performing the same procedure contralaterally (FIGURE 3). Close the apex and tie these sutures in place, suspending the corners of the vaginal apex from the uterosacral complex bilaterally, and restoring the continuity of the paracervical ring (FIGURES 4, 5).
Benefits of this technique include:
- creation of an anatomically appropriate and correctly positioned midline vaginal axis,
- preservation of adequate vaginal length,
- reduced risk of nerve injury, and
- restored continuity of the paracervical ring when the pelvic pararectal, uterosacral, and pubocervical fascia are reapproximated circumferentially.
Risks include the potential for ureteral kinking or obstruction. Thus, it is prudent to perform cystoscopy after this procedure to rule out occult injury.
FIGURE 3 Suspend apical corners bilaterally
The corners of the vaginal apex are suspended from the cardinal-uterosacral complex bilaterally, with all sutures placed posterior and medial to the ischial spines. Copyright 1998 by C.G. Bachofen.
FIGURE 4 Suture placement penetrates multiple layers
Sagittal view of correct suture placement. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, AD=apical defect, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
FIGURE 5 Suspend the fascia from uterosacral ligaments
Sagittal view after tying of sutures. Note restoration of the normal anatomic axis. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
Posterior intravaginal slingplasty
This investigative technique, also known as infracoccygeal sacropexy, is a minimally invasive, transperineal approach to vaginal vault prolapse. The anatomic and physiologic concepts are similar to those of the tension-free vaginal tape (Gynecare, Somerville, NJ) in the treatment of stress incontinence. However, because it is a new procedure, further evaluation is needed before it can be adopted into clinical practice.
This is an outpatient surgery.
Technique. Use a narrow tunneling device to pass a synthetic nonabsorbable tape through each pararectal space via a small perineal incision, and make a small vaginal incision to secure the tape to the vault.
The evidence. In the initial case series of 93 patients,15 1 rectal perforation and 1 rectal tape erosion were noted. The cure rate was 91% in short-term follow-up in a small number of patients.
The authors report no financial relationships relevant to this article.
First, the good news: We have numerous techniques to choose from to repair prolapse of the vaginal vault, which affects as many as 50% of parous women.1 The bad news: Most of the data on these techniques are anecdotal or retrospective, not the result of randomized, controlled trials. Few investigators have compared the vaginal and abdominal approaches.
So how should we decide on a procedure? It is a judgment call, ultimately. After taking into account the patient’s age, functional status, comorbidities, desire for coitus, and surgical history, the surgeon must weigh the risks and benefits of the procedures that seem most appropriate. Part 1 of this 2-part article reviews what is known about the most widely used and newest vaginal techniques:
- sacrospinous ligament fixation,
- iliococcygeal fixation,
- modified McCall culdoplasty,
- high uterosacral ligament suspension with fascial reconstruction, and
- posterior intravaginal slingplasty (infracoccygeal sacropexy).
In Part 2, next month, we focus on the abdominal approach, and survey the data comparing vaginal and abdominal repairs.
Unfortunately, success and failure rates are still poorly defined because of a lack of standardization, and because techniques and materials continually change. This underscores the need for better understanding of the pathophysiology of genital prolapse, improved preoperative assessment, and more effective and durable repair techniques.
Why prolapse occurs
Pelvic support involves a complex interplay of anatomic, histologic, genetic, and electrophysiologic factors that, although incompletely understood, are frequently disrupted. For example, MacLennan et al2 reported that 46.2% of women aged 15 to 97 years experience pelvic floor dysfunction; a large retrospective study by Olsen and colleagues3 found that 11.1% of women undergo surgery for prolapse by the age of 80, and 29.2% of these women require repeat surgery.
Here’s what we know about the anatomy of pelvic support:
Ligaments serve as secondary supports
The uterus and upper third of the vagina are held in place over the levator plate by the fibers of the parametrium (cardinal and uterosacral ligaments) and paracolpium. These fibers arise from a broad area on the pelvic sidewall overlying the fascia of the piriformis muscle, the sacroiliac joint, and lateral sacrum. The fibers represent condensations of the endopelvic fascia of the pelvis, acting as suspensory ligaments that run in a predominantly vertical direction to insert into the lateral upper third of the vagina and lateral and posterolateral aspect of the cervical portion of the uterus.
In the normal, healthy pelvis, these suspensory ligaments represent secondary support mechanisms and are not routinely under tension.
Pelvic-floor muscles play leading role
Gosling4 argued that pelvic floor muscle tone is more crucial to normal positioning of the pelvic viscera than are the fascial and ligamentous supports of the pelvic organs. Specifically, the pubococcygeus, iliococcygeus, and puborectalis muscles collectively define the levator ani of the pelvic floor. Fusion of the right and left bellies of the levator ani, behind the rectum and anterior to the coccyx, creates a muscular platform known as the levator plate. This plate provides indirect support for the upper genital tract by acting as a platform against which the upper vagina and other pelvic viscera are compressed during increases in intra-abdominal pressure.
Contraction of the levator ani pulls the levator plate toward the posterior symphysis pubis, minimizing the size of the urogenital hiatus through which the rectum, vagina, and urethra exit the pelvis on their way to the perineum. Weakness in the muscular pelvic floor—whether caused by disuse, pudendal nerve damage, or muscular trauma—increases the size of the urogenital hiatus, and the pelvic organs begin to prolapse through it.
Ultimately, constant tension on the ligamentous supports of the pelvic organs exceeds their tensile strength, and pelvic organ prolapse results.
Goals of surgery
Successful surgery achieves effective and sustained vault support, obliterates any enterocele sac, and repairs the cystocele and rectocele that occur in approximately two thirds of women with vault prolapse.
The broader goals: anatomic and functional restoration of the lower female genital tract and improvement in quality of life.
Surgery can be reconstructive or obliterative. Reconstructive surgery can be performed vaginally, abdominally, or a combination of both.
Vaginal techniques
Proponents of the vaginal approach argue that, by avoiding the need for laparotomy, it results in fewer complications, less blood loss and postoperative discomfort, a shorter hospital stay, and less expense.5
Sacrospinous ligament fixation
The sacrospinous ligaments extend from the ischial spines on either side to the lower portion of the sacrum and coccyx. Fixation of the vaginal apex to 1 or both of the sacrospinous ligaments is an option for posthysterectomy vault prolapse.
Technique. Nichols6 described the need to penetrate the right rectal pillar into the pararectal space near the ischial spine. The next step is grasping the ligament and muscle with a long Babcock clamp. Place two #2 polyglycolic sutures through the sacrospinous ligament, 1.5 to 2 fingerbreadths medial to the ischial spine. Attach 1 end of the suture to the undersurface of the posterior vaginal wall at the apical area. When the posterior colporrhaphy reaches the midportion of the vagina, tie the sacrospinous suspension sutures, firmly attaching the vaginal apex to the surface of the coccygeal-sacrospinous ligament complex with no intervening suture bridge (FIGURE 1).
Modifications. The most notable modification is the Miyazaki technique, which substitutes the Miya hook for the DesChamps ligature carrier. The Miya hook is reportedly safer for the pudendal complex, which may lie up to 5.5 cm medial to the ischial spine.7 The path of the Miya hook avoids Alcock’s canal and its neurovascular pudendal bundle.
The Caprio ligature carrier (Boston Scientific, Boston, Mass) is also useful for the placement of sacrospinous sutures; unlike the Miya hook, however, the Caprio ligature carrier is a disposable instrument and thus is not reusable.
Potential complications of sacrospinous ligament fixation include hemorrhage, pudendal nerve injury, rectal or bladder injury, and recurrent anterior vaginal wall prolapse.
Outcomes. Follow-up studies in women undergoing this procedure report a roughly 20% incidence of recurrent or persistent anterior vaginal wall relaxation, or symptomatic cystocele, within 1 year after the surgery. Alteration of the vaginal axis in an exaggerated posterolateral direction after this procedure is thought to place undue tension on the anterior segment of the vaginal wall and predispose women to prolapse at a site opposite the repair.8,9
FIGURE 1 2 “pulley stitches” secure the apex
Place 2 nonabsorbable monofilament “pulley stitches” to secure the vaginal apex to the ligament.
Iliococcygeal fixation
Inmon10 was the first to describe a technique in which the everted vaginal apex is secured to the iliococcygeal fascia bilaterally, just below the ischial spine. He performed this technique successfully in 3 women with atrophied uterosacral ligaments.
Technique. Open the posterior vaginal wall in the midline, as if preparing to perform a posterior colporrhaphy. Develop the rectovaginal spaces bluntly and bilaterally—laterally toward the levator muscles and posteriorally toward the ischial spines. Use the nondominant hand to depress the rectum downward and medially, and place a single #0 polyglycolic suture deep into the iliococcygeus muscle and fascia at a point 1 to 2 cm caudad and posterior to the ischial spine. Then pass both ends of the suture through the ipsilateral posterior vaginal apex and hold them with a hemostat. Repeat the procedure contralaterally.
Usually no vaginal epithelium needs excision because the upper vagina is attached bilaterally, resulting in good vaginal length and circumference. When posterior colporrhaphy is completed and the posterior vaginal wall is closed, tie both iliococcygeal-fixation sutures in place.
Complications. Shull and colleagues11 studied 42 women who underwent suspension of the vaginal cuff to iliococcygeus fascia and repair of coexisting pelvic support defects. Of these women, 2 (5%) had recurrence of their cuff prolapse during follow-up, one of whom required further surgery (she also had recurrence of an inguinal hernia that had been repaired at the original surgery). The other patient, who had undergone 5 previous pelvic procedures, developed asymptomatic prolapse of the cuff halfway to the hymen. Six additional patients had loss of support at other sites in the follow-up period, one of whom required repeat surgery. Ninety-five percent of women experienced no persistence or recurrence of cuff prolapse 6 weeks to 5 years after the procedure.
Meeks and colleagues12 also applied the Inmon technique in 110 women with posthysterectomy vault prolapse or total uterine procidentia. In both studies, the most commonly reported complications included hemorrhage (1.2%), bladder/rectal perforation (1.2%), and recurrent vault prolapse (8%).
Benefits. In comparison with sacrospinous ligament fixation, iliococcygeus fixation is technically easier and places less tension on the anterior vaginal wall.
Modified McCall culdoplasty
Symmonds and colleagues13 described this approach to symptomatic vaginal vault prolapse.
Technique. Excise an elliptical wedge of mucosa from the anterior and posterior walls of the prolapsed vagina to narrow the vault and allow access to the lateral fascial supports of the vagina and rectum. The width and length of the excised wedges are determined by the desired dimensions of the reconstructed vagina.
After isolating and excising the enterocele sac, place up to 3 modified McCall stitches, each one slightly higher than its predecessor. Each suture should incorporate the full thickness of the posterior vaginal wall, the cul-de-sac peritoneum, the remains of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum (FIGURE 2).
Once they are in place, tie the sutures in the opposite order in which they were placed. These stitches fix the prolapsed vaginal vault to the uppermost portion of the endopelvic fascia at the same time as they accomplish a high closure of the culdesac peritoneum.
The evidence. Sze and Karram5 found an 11.5% incidence of recurrent vault prolapse and an associated 22% incidence of new-onset dyspareunia.
FIGURE 2 Classic vs modified McCall culdoplasty
In the classic McCall culdoplasty shown here, only the distal-most suture incorporates the posterior vaginal wall. With the “modified” technique, however, all sutures incorporate the full thickness of the posterior vaginal wall, as well as the cul-de-sac peritoneum, the remnants of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum.
High uterosacral ligament suspension with fascial reconstruction
This approach is based on the observations of Richardson,14 who suggested that the endopelvic fascial supports (uterosacral/cardinal complex) do not stretch and attenuate over time, as some have hypothesized, but break at definable points. By identifying these points, the surgeon can reattach the prolapsed vagina to the intact uterosacral complex cephalad to the break.
Technique. Grasp the vaginal apex with 2 Allis clamps and incise it with a scalpel. If an enterocele sac is present, dissect it off the vaginal epithelium to the neck of the hernia, open it, and then excise it. Place a delayed absorbable or permanent pursestring suture about the neck of the hernia to close the peritoneal defect. If an anterior colporrhaphy or sling is required, perform them at this time.
Place a moist laparotomy pad in the cul-de-sac, and insert and elevate a Deaver retractor to remove the intestines from the cul-de-sac and improve exposure. Next, palpate the ischial spines transperitoneally.
Once the spines are identified, the remnants of the uterosacral ligaments can be identified posterior and medial to the spines and can be palpated transperitoneally or transrectally. Remember that the ureters are also quite close to the ischial spines at this location, running along the lateral pelvic sidewall 2 to 5 cm ventral and lateral to the ischial spines.
After identifying the uterosacral ligaments, grasp their remnants with Allis clamps and place 2 to 3 delayed absorbable or permanent sutures through the rectovaginal fascia of the inner posterior vaginal wall epithelium at one lateral vaginal apex, through the ipsilateral plicated uterosacral ligament complex, and then through the pubocervical fascia of the anterior vaginal wall of the ipsilateral vaginal apex. Hold these sutures while performing the same procedure contralaterally (FIGURE 3). Close the apex and tie these sutures in place, suspending the corners of the vaginal apex from the uterosacral complex bilaterally, and restoring the continuity of the paracervical ring (FIGURES 4, 5).
Benefits of this technique include:
- creation of an anatomically appropriate and correctly positioned midline vaginal axis,
- preservation of adequate vaginal length,
- reduced risk of nerve injury, and
- restored continuity of the paracervical ring when the pelvic pararectal, uterosacral, and pubocervical fascia are reapproximated circumferentially.
Risks include the potential for ureteral kinking or obstruction. Thus, it is prudent to perform cystoscopy after this procedure to rule out occult injury.
FIGURE 3 Suspend apical corners bilaterally
The corners of the vaginal apex are suspended from the cardinal-uterosacral complex bilaterally, with all sutures placed posterior and medial to the ischial spines. Copyright 1998 by C.G. Bachofen.
FIGURE 4 Suture placement penetrates multiple layers
Sagittal view of correct suture placement. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, AD=apical defect, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
FIGURE 5 Suspend the fascia from uterosacral ligaments
Sagittal view after tying of sutures. Note restoration of the normal anatomic axis. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
Posterior intravaginal slingplasty
This investigative technique, also known as infracoccygeal sacropexy, is a minimally invasive, transperineal approach to vaginal vault prolapse. The anatomic and physiologic concepts are similar to those of the tension-free vaginal tape (Gynecare, Somerville, NJ) in the treatment of stress incontinence. However, because it is a new procedure, further evaluation is needed before it can be adopted into clinical practice.
This is an outpatient surgery.
Technique. Use a narrow tunneling device to pass a synthetic nonabsorbable tape through each pararectal space via a small perineal incision, and make a small vaginal incision to secure the tape to the vault.
The evidence. In the initial case series of 93 patients,15 1 rectal perforation and 1 rectal tape erosion were noted. The cure rate was 91% in short-term follow-up in a small number of patients.
The authors report no financial relationships relevant to this article.
1. Beck RP. Pelvic relaxational prolapse. In: Principles and Practice of Clinical Gynecology. New York: John Wiley and Sons; 1983;667-685.
2. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity, and mode of delivery. Br J Obstet Gynecol. 2000;107:1460-1470.
3. Olsen AL, Smith VJ, Bergstrom JO. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
4. Gosling JA. The structure of the bladder neck, urethra and pelvic floor in relation to female urinary incontinence. Int Urogynecol J. 1996;7:177-178.
5. Sze EHM, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89:466-475.
6. Nichols D. Sacrospinous fixation for massive eversion of the vagina. Am J Obstet Gynecol. 1982;142:901-904.
7. Miyazaki FS. Miya hook ligature carrier for sacrospinous ligament suspension. Obstet Gynecol. 1987;70:286-288.
8. Morley G, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-878.
9. Shull BL, Capen CV, Riggs MW. Preoperative analysis of site specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
10. Inmon WB. Pelvic relaxation and repair including prolapse of vagina following hysterectomy. South Med J. 1963;56:577-582.
11. Shull BT, Capen CV, Riggs MW. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 193;168:1669-1673.
12. Meeks GR, Washburne JF, McGehrer RP. Repair of vaginal vault prolapse by suspension of the vagina to iliococcygeus (prespinous) fascia. Am J Obstet Gynecol. 1994;171:1444-1449.
13. Symmonds RE, Williams TJ, Lee RA, Webbs MJ. Posthysterectomy enterocoele and vaginal vault prolapse. Am J Obstet Gynecol. 1981;140:852-859.
14. Richardson AL. The anatomic defects in rectocoele and enterocoele. J Pelvic Surg. 1995;1:214-218.
15. Farnsworth BN. Posterior intravaginal slingplasty (infracoccygeal sacropexy) for severe posthysterectomy vaginal vault prolapse: a preliminary report on efficacy and safety. Int Urogynecol J. 2002;13:4-8.
1. Beck RP. Pelvic relaxational prolapse. In: Principles and Practice of Clinical Gynecology. New York: John Wiley and Sons; 1983;667-685.
2. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity, and mode of delivery. Br J Obstet Gynecol. 2000;107:1460-1470.
3. Olsen AL, Smith VJ, Bergstrom JO. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
4. Gosling JA. The structure of the bladder neck, urethra and pelvic floor in relation to female urinary incontinence. Int Urogynecol J. 1996;7:177-178.
5. Sze EHM, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89:466-475.
6. Nichols D. Sacrospinous fixation for massive eversion of the vagina. Am J Obstet Gynecol. 1982;142:901-904.
7. Miyazaki FS. Miya hook ligature carrier for sacrospinous ligament suspension. Obstet Gynecol. 1987;70:286-288.
8. Morley G, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-878.
9. Shull BL, Capen CV, Riggs MW. Preoperative analysis of site specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
10. Inmon WB. Pelvic relaxation and repair including prolapse of vagina following hysterectomy. South Med J. 1963;56:577-582.
11. Shull BT, Capen CV, Riggs MW. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 193;168:1669-1673.
12. Meeks GR, Washburne JF, McGehrer RP. Repair of vaginal vault prolapse by suspension of the vagina to iliococcygeus (prespinous) fascia. Am J Obstet Gynecol. 1994;171:1444-1449.
13. Symmonds RE, Williams TJ, Lee RA, Webbs MJ. Posthysterectomy enterocoele and vaginal vault prolapse. Am J Obstet Gynecol. 1981;140:852-859.
14. Richardson AL. The anatomic defects in rectocoele and enterocoele. J Pelvic Surg. 1995;1:214-218.
15. Farnsworth BN. Posterior intravaginal slingplasty (infracoccygeal sacropexy) for severe posthysterectomy vaginal vault prolapse: a preliminary report on efficacy and safety. Int Urogynecol J. 2002;13:4-8.
A look at the latest fibroid treatments, including uterine artery embolization, focused ultrasound, and drug therapy.
Not long ago, women with uterine fibroids had to choose between hysterectomy and abdominal myomectomy to alleviate their symptoms. Then came minimally invasive surgeries such as laparoscopic myomectomy and hysteroscopic myoma resection, although even now these surgeries are offered by a limited number of skilled gynecologic surgeons. And despite their substantially shorter recovery times, these procedures are still surgeries, with inherent complications. On top of that, long-term outcomes data are limited.
Enter the next generation of fibroid treatments: uterine artery embolization (UAE), focused ultrasound with magnetic resonance imaging (MRI) guidance, and selective progesterone receptor modulators—though the last option is still in the pipeline. Gynecologists will be seeing advertisements and promotional materials for these interventions in the near and not-so-distant future.
Uterine artery embolization: In the right hands, a worthwhile strategy
Myers ER, Goodwin S, Landow W, et al. Prospective data collection of a new procedure by a specialty society: the FIBROID Registry. Obstet Gynecol. 2005;106:44–51.
Worthington-Kirsch R, Spies JB, Myers ER, et al. The Fibroid Registry for outcomes data (FIBROID) for uterine embolization: short-term outcomes. Obstet Gynecol. 2005;106:52–59.
Congratulations are in order. When the Society of Interventional Radiology created the Fibroid Registry in 2000, it was looking for early data on UAE to share with patients. In its short but impressive life, the registry has collected more data about UAE than we have about “tried-and-true” surgeries.
In the United States, UAE was first used to treat fibroids in 1997. Although numerous studies since then have reported on its safety and effectiveness, many gynecologists continue to question the suitability of UAE for symptomatic women.
The Web-based registry was established with Duke Clinical Research Institute to track short- and long-term outcomes after UAE in various settings.
What we know from the registry
The Fibroid Registry enrolled its first patient in December 2000, and collected data from 72 sites on 3,319 UAE procedures through December 2002. The reports by Myers et al and Worthington-Kirsch and colleagues contain patient demographics, procedural details, and 30-day outcomes.
The registry defined adverse events as any unexpected event that necessitated an unscheduled office or emergency room visit or unanticipated therapy (medical or surgical). Major complications required increased care or additional hospitalization or had permanent adverse sequelae. Minor complications required medical management or no therapy.
Thirty-day outcomes were available for approximately 91% of patients.
A low complication rate
Complications were uncommon during the first 30 days after UAE, with a 1.1% incidence of additional surgery. In fact, complication rates and recovery times compared favorably with myomectomy and abdominal hysterectomy for large fibroids.
The UAE procedure averaged 56 minutes, with 96.2% technical success and a return to normal activities in about 2 weeks.
Other findings:
- 26% of patients had an adverse event, but only 4% had a major event, most commonly emergency care or hospital readmission for pain management (2.1%) or possible infection (<1%).
- 31 women required another procedure within 30 days, including 3 myomectomies, 9 dilatation and curettage procedures for sloughing leiomyomata, 5 hysteroscopic resections, and 3 hysterectomies for unrecorded indications.
- 1 patient was hospitalized for pain 10 days after UAE and underwent exploratory laparotomy with bilateral oophorectomy.
- The most common minor adverse events were hot flushes (5.7%) and pain requiring additional therapy (9.6%).
Predictors of adverse outcomes:
- current or recent smoking (odds ratio [OR] 1.14, 95% confidence interval [CI] 1.007–1.293),
- African-American race (OR 1.129, 95% CI 1.019–1.251),
- prior procedures (OR 1.23, 95% CI 1.02–1.38), and
- duration of procedure (OR 1.004, 95% CI 1.001–1.006).
Interestingly, short-term outcomes did not differ among centers, nor did procedure times, length of stay, and incidence of adverse events during the first 30 days.
What to tell patients
I inform patients with symptomatic fibroids about all available treatments—including the option of doing nothing at all. Most have no interest in UAE, and are distressed by the thought of their bodies reabsorbing dead tissue.
However, I have had several patients whose operative risks were very high. For example, 1 woman was morbidly obese (>250 lb, which required her UAE treatment at a special facility equipped to perform fluoroscopy in morbidly obese patients), hypertensive, diabetic, and hemiparetic after a stroke. She was also a Jehovah’s Witness. Obviously, nonsurgical intervention was to her benefit. Her bleeding stopped almost immediately.
A reasonable alternative
The lay press recently focused on our obligation, as ObGyns, to inform patients about alternative therapies for fibroids. These first reports from the Fibroid Registry are clear: UAE is a reproducible, low-risk procedure—certainly lower in risk than complex surgeries (though situations may arise when myomectomy is preferred, such as a desire for future fertility1).
The registry will continue to provide data we can share with our patients regarding risks, complications, and long-term outcomes. More importantly, our radiology colleagues have taken the lead in developing a voluntary patient registry to track the outcomes of new technology as it disseminates into the general medical community. Our patients would be well served if we developed similar registries to track our surgical outcomes.
Focused ultrasound shrinks fibroids, but has strict eligibility requirements
Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183:1713–1719.
Using MRI guidance, a completely noninvasive treatment is now possible: focused ultrasound ablation of uterine fibroids. This procedure, the newest high-tech treatment for symptomatic fibroids, was successfully tested in an earlier pilot study.2 Although it eased symptoms to a remarkable degree during this phase III trial, how many women will ultimately be suitable for the treatment remains unclear.
Selection criteria
This trial of the ExAblate 2000 (InSightec, Tirat Carmel, Israel) recruited symptomatic women who were otherwise suitable candidates for conventional surgeries. Excluded were postmenopausal patients, women weighing more than 250 lb, and women who had a uterus larger than 24 weeks’ size or any single myoma larger than 10 cm. Women with extensive abdominal scars were carefully examined and excluded if the scars lay in the path of the ultrasound beam. The reason: During the earlier study, these scars tended to absorb ultrasound energy, increasing the risk of thermal injury at the skin surface.
Before proceeding, patients underwent MRI or ultrasound to confirm a clear pathway from the anterior abdominal wall to the fibroids without traversing the bladder or bowel.
Hindley et al did not reveal how many women were initially screened, but 109 were finally enrolled at 7 international sites and completed a Uterine Fibroid Symptoms and Quality of Life Questionnaire before and 3 and 6 months after treatment. As for the location of the fibroids: 22% were submucosal, 57% were intramural, and 21% were subserosal.
Up to 4 fibroids were treated per patient—with MRI mapping and thermographic monitoring—with a margin of 1.5 cm from the edge of the ablated area to the edge of the uterus. Conscious sedation was provided as needed, and tolerance of the procedure was measured using a 4-point pain scale. Mean time in the MRI scanner was 202 minutes (range, 90–370 minutes).
Pain stopped when treatment ended
Most women reported mild to moderate pain during the procedure (66%), and 16% complained of severe pain. This discomfort ended immediately when treatment stopped in virtually all patients—only 1% reported severe pain afterwards.
Adverse events
Serious adverse events included: 5 women with heavy menses requiring blood transfusions, 1 patient with pain and bleeding consistent with preprocedure symptoms, 1 woman needing overnight hospitalization for nausea related to opioid analgesia during the procedure, and 1 patient with leg and buttock pain immediately after treatment (it was later discovered that the sciatic nerve was in the far field of the sonication pathway). These symptoms resolved by the follow-up visit.
Two other patients had adverse events unrelated to the procedure.
6-month outcomes
The mean fibroid volume reduction was 13.5%±32%. Although this improvement seems modest, women reported significant relief from fibroid-related symptoms, and the mean severity score on the quality-of-life questionnaire decreased.
Substantial improvement was seen for both mass effect and bleeding symptoms (32.8 points out of 100 for each).
Pros and cons for symptomatic women
Advantages over UAE include the absence of postprocedural pain in almost all patients, which eliminates the need for an overnight stay and likely speeds the return to normal activities.
Disadvantages are that women with major abdominal scarring, anterior myomas underneath the bladder flap, or adhesive disease that causes small or large bowel to lie in the path of the sound waves cannot take advantage of this new technology, nor can women who have myomas close to neurovascular bundles. Add to that the extremely long procedure time (over 3 hours) and the modest reduction in uterine volume.
Six-month outcomes are promising, but this approach is probably best reserved for an academic setting, so careful screening and long-term tracking can continue.
Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438.
In this comprehensive review, the authors draw from their extensive expertise in endocrinology to describe the rationale behind asoprisnil, a mixed progesterone receptor agonist/antagonist—the most promising pharmaceutical development in gynecology in several decades.
How the drug works
Mifepristone (RU-486) was the first progesterone receptor antagonist. Despite its ability to reduce myoma volume and suppress endometriosis symptoms in small pilot studies, it induces endometrial hyperplasia at doses higher than 5 to 10 mg, probably by acting similarly to unopposed estrogen. In contrast, asoprisnil has antiproliferative effects and no labor-inducing activity. It directly affects blood vessels in the endometrium, creating a local antiproliferative effect that induces amenorrhea despite normal estrogen levels.
What phase II studies reveal
A multicenter, double-blind, placebo-controlled trial involved 5-, 10-, and 25-mg daily doses of oral asoprisnil over 12 weeks. In a dose-dependent manner, asoprisnil induced amenorrhea or significantly suppressed bleeding without causing breakthrough or intermenstrual flow. It also decreased the volume of both the largest fibroid and the uterus as a whole. At 10- and 25-mg doses, pressure symptoms eased substantially over placebo. Adverse effects were minimal and affected the placebo and asoprisnil groups equally.
Phase III trials are now under way to assess the safety and efficacy of asoprisnil in the treatment of menorrhagia and uterine fibroids. Early results in the treatment of endometriosis are also promising.
What this means for fibroid patients
Though asoprisnil is not yet available for use, the phase III trial is winding down and the drug’s impressive potential seems clear.
A useful strategy may be to counsel marginally symptomatic women with fibroids that treatments are in the pipeline that would permit pharmaceutical management of their symptoms. Because this drug induces amenorrhea in the presence of normal circulating estrogen levels, it eliminates hot flushes and, more importantly, the bone loss associated with gonadotropin-releasing hormone agonists.
Disclosure
The author reports no financial relationships relevant to this article.
1. Pron G, Mocarski E, Bennett J, et al. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
2. Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48-54.
Not long ago, women with uterine fibroids had to choose between hysterectomy and abdominal myomectomy to alleviate their symptoms. Then came minimally invasive surgeries such as laparoscopic myomectomy and hysteroscopic myoma resection, although even now these surgeries are offered by a limited number of skilled gynecologic surgeons. And despite their substantially shorter recovery times, these procedures are still surgeries, with inherent complications. On top of that, long-term outcomes data are limited.
Enter the next generation of fibroid treatments: uterine artery embolization (UAE), focused ultrasound with magnetic resonance imaging (MRI) guidance, and selective progesterone receptor modulators—though the last option is still in the pipeline. Gynecologists will be seeing advertisements and promotional materials for these interventions in the near and not-so-distant future.
Uterine artery embolization: In the right hands, a worthwhile strategy
Myers ER, Goodwin S, Landow W, et al. Prospective data collection of a new procedure by a specialty society: the FIBROID Registry. Obstet Gynecol. 2005;106:44–51.
Worthington-Kirsch R, Spies JB, Myers ER, et al. The Fibroid Registry for outcomes data (FIBROID) for uterine embolization: short-term outcomes. Obstet Gynecol. 2005;106:52–59.
Congratulations are in order. When the Society of Interventional Radiology created the Fibroid Registry in 2000, it was looking for early data on UAE to share with patients. In its short but impressive life, the registry has collected more data about UAE than we have about “tried-and-true” surgeries.
In the United States, UAE was first used to treat fibroids in 1997. Although numerous studies since then have reported on its safety and effectiveness, many gynecologists continue to question the suitability of UAE for symptomatic women.
The Web-based registry was established with Duke Clinical Research Institute to track short- and long-term outcomes after UAE in various settings.
What we know from the registry
The Fibroid Registry enrolled its first patient in December 2000, and collected data from 72 sites on 3,319 UAE procedures through December 2002. The reports by Myers et al and Worthington-Kirsch and colleagues contain patient demographics, procedural details, and 30-day outcomes.
The registry defined adverse events as any unexpected event that necessitated an unscheduled office or emergency room visit or unanticipated therapy (medical or surgical). Major complications required increased care or additional hospitalization or had permanent adverse sequelae. Minor complications required medical management or no therapy.
Thirty-day outcomes were available for approximately 91% of patients.
A low complication rate
Complications were uncommon during the first 30 days after UAE, with a 1.1% incidence of additional surgery. In fact, complication rates and recovery times compared favorably with myomectomy and abdominal hysterectomy for large fibroids.
The UAE procedure averaged 56 minutes, with 96.2% technical success and a return to normal activities in about 2 weeks.
Other findings:
- 26% of patients had an adverse event, but only 4% had a major event, most commonly emergency care or hospital readmission for pain management (2.1%) or possible infection (<1%).
- 31 women required another procedure within 30 days, including 3 myomectomies, 9 dilatation and curettage procedures for sloughing leiomyomata, 5 hysteroscopic resections, and 3 hysterectomies for unrecorded indications.
- 1 patient was hospitalized for pain 10 days after UAE and underwent exploratory laparotomy with bilateral oophorectomy.
- The most common minor adverse events were hot flushes (5.7%) and pain requiring additional therapy (9.6%).
Predictors of adverse outcomes:
- current or recent smoking (odds ratio [OR] 1.14, 95% confidence interval [CI] 1.007–1.293),
- African-American race (OR 1.129, 95% CI 1.019–1.251),
- prior procedures (OR 1.23, 95% CI 1.02–1.38), and
- duration of procedure (OR 1.004, 95% CI 1.001–1.006).
Interestingly, short-term outcomes did not differ among centers, nor did procedure times, length of stay, and incidence of adverse events during the first 30 days.
What to tell patients
I inform patients with symptomatic fibroids about all available treatments—including the option of doing nothing at all. Most have no interest in UAE, and are distressed by the thought of their bodies reabsorbing dead tissue.
However, I have had several patients whose operative risks were very high. For example, 1 woman was morbidly obese (>250 lb, which required her UAE treatment at a special facility equipped to perform fluoroscopy in morbidly obese patients), hypertensive, diabetic, and hemiparetic after a stroke. She was also a Jehovah’s Witness. Obviously, nonsurgical intervention was to her benefit. Her bleeding stopped almost immediately.
A reasonable alternative
The lay press recently focused on our obligation, as ObGyns, to inform patients about alternative therapies for fibroids. These first reports from the Fibroid Registry are clear: UAE is a reproducible, low-risk procedure—certainly lower in risk than complex surgeries (though situations may arise when myomectomy is preferred, such as a desire for future fertility1).
The registry will continue to provide data we can share with our patients regarding risks, complications, and long-term outcomes. More importantly, our radiology colleagues have taken the lead in developing a voluntary patient registry to track the outcomes of new technology as it disseminates into the general medical community. Our patients would be well served if we developed similar registries to track our surgical outcomes.
Focused ultrasound shrinks fibroids, but has strict eligibility requirements
Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183:1713–1719.
Using MRI guidance, a completely noninvasive treatment is now possible: focused ultrasound ablation of uterine fibroids. This procedure, the newest high-tech treatment for symptomatic fibroids, was successfully tested in an earlier pilot study.2 Although it eased symptoms to a remarkable degree during this phase III trial, how many women will ultimately be suitable for the treatment remains unclear.
Selection criteria
This trial of the ExAblate 2000 (InSightec, Tirat Carmel, Israel) recruited symptomatic women who were otherwise suitable candidates for conventional surgeries. Excluded were postmenopausal patients, women weighing more than 250 lb, and women who had a uterus larger than 24 weeks’ size or any single myoma larger than 10 cm. Women with extensive abdominal scars were carefully examined and excluded if the scars lay in the path of the ultrasound beam. The reason: During the earlier study, these scars tended to absorb ultrasound energy, increasing the risk of thermal injury at the skin surface.
Before proceeding, patients underwent MRI or ultrasound to confirm a clear pathway from the anterior abdominal wall to the fibroids without traversing the bladder or bowel.
Hindley et al did not reveal how many women were initially screened, but 109 were finally enrolled at 7 international sites and completed a Uterine Fibroid Symptoms and Quality of Life Questionnaire before and 3 and 6 months after treatment. As for the location of the fibroids: 22% were submucosal, 57% were intramural, and 21% were subserosal.
Up to 4 fibroids were treated per patient—with MRI mapping and thermographic monitoring—with a margin of 1.5 cm from the edge of the ablated area to the edge of the uterus. Conscious sedation was provided as needed, and tolerance of the procedure was measured using a 4-point pain scale. Mean time in the MRI scanner was 202 minutes (range, 90–370 minutes).
Pain stopped when treatment ended
Most women reported mild to moderate pain during the procedure (66%), and 16% complained of severe pain. This discomfort ended immediately when treatment stopped in virtually all patients—only 1% reported severe pain afterwards.
Adverse events
Serious adverse events included: 5 women with heavy menses requiring blood transfusions, 1 patient with pain and bleeding consistent with preprocedure symptoms, 1 woman needing overnight hospitalization for nausea related to opioid analgesia during the procedure, and 1 patient with leg and buttock pain immediately after treatment (it was later discovered that the sciatic nerve was in the far field of the sonication pathway). These symptoms resolved by the follow-up visit.
Two other patients had adverse events unrelated to the procedure.
6-month outcomes
The mean fibroid volume reduction was 13.5%±32%. Although this improvement seems modest, women reported significant relief from fibroid-related symptoms, and the mean severity score on the quality-of-life questionnaire decreased.
Substantial improvement was seen for both mass effect and bleeding symptoms (32.8 points out of 100 for each).
Pros and cons for symptomatic women
Advantages over UAE include the absence of postprocedural pain in almost all patients, which eliminates the need for an overnight stay and likely speeds the return to normal activities.
Disadvantages are that women with major abdominal scarring, anterior myomas underneath the bladder flap, or adhesive disease that causes small or large bowel to lie in the path of the sound waves cannot take advantage of this new technology, nor can women who have myomas close to neurovascular bundles. Add to that the extremely long procedure time (over 3 hours) and the modest reduction in uterine volume.
Six-month outcomes are promising, but this approach is probably best reserved for an academic setting, so careful screening and long-term tracking can continue.
Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438.
In this comprehensive review, the authors draw from their extensive expertise in endocrinology to describe the rationale behind asoprisnil, a mixed progesterone receptor agonist/antagonist—the most promising pharmaceutical development in gynecology in several decades.
How the drug works
Mifepristone (RU-486) was the first progesterone receptor antagonist. Despite its ability to reduce myoma volume and suppress endometriosis symptoms in small pilot studies, it induces endometrial hyperplasia at doses higher than 5 to 10 mg, probably by acting similarly to unopposed estrogen. In contrast, asoprisnil has antiproliferative effects and no labor-inducing activity. It directly affects blood vessels in the endometrium, creating a local antiproliferative effect that induces amenorrhea despite normal estrogen levels.
What phase II studies reveal
A multicenter, double-blind, placebo-controlled trial involved 5-, 10-, and 25-mg daily doses of oral asoprisnil over 12 weeks. In a dose-dependent manner, asoprisnil induced amenorrhea or significantly suppressed bleeding without causing breakthrough or intermenstrual flow. It also decreased the volume of both the largest fibroid and the uterus as a whole. At 10- and 25-mg doses, pressure symptoms eased substantially over placebo. Adverse effects were minimal and affected the placebo and asoprisnil groups equally.
Phase III trials are now under way to assess the safety and efficacy of asoprisnil in the treatment of menorrhagia and uterine fibroids. Early results in the treatment of endometriosis are also promising.
What this means for fibroid patients
Though asoprisnil is not yet available for use, the phase III trial is winding down and the drug’s impressive potential seems clear.
A useful strategy may be to counsel marginally symptomatic women with fibroids that treatments are in the pipeline that would permit pharmaceutical management of their symptoms. Because this drug induces amenorrhea in the presence of normal circulating estrogen levels, it eliminates hot flushes and, more importantly, the bone loss associated with gonadotropin-releasing hormone agonists.
Disclosure
The author reports no financial relationships relevant to this article.
Not long ago, women with uterine fibroids had to choose between hysterectomy and abdominal myomectomy to alleviate their symptoms. Then came minimally invasive surgeries such as laparoscopic myomectomy and hysteroscopic myoma resection, although even now these surgeries are offered by a limited number of skilled gynecologic surgeons. And despite their substantially shorter recovery times, these procedures are still surgeries, with inherent complications. On top of that, long-term outcomes data are limited.
Enter the next generation of fibroid treatments: uterine artery embolization (UAE), focused ultrasound with magnetic resonance imaging (MRI) guidance, and selective progesterone receptor modulators—though the last option is still in the pipeline. Gynecologists will be seeing advertisements and promotional materials for these interventions in the near and not-so-distant future.
Uterine artery embolization: In the right hands, a worthwhile strategy
Myers ER, Goodwin S, Landow W, et al. Prospective data collection of a new procedure by a specialty society: the FIBROID Registry. Obstet Gynecol. 2005;106:44–51.
Worthington-Kirsch R, Spies JB, Myers ER, et al. The Fibroid Registry for outcomes data (FIBROID) for uterine embolization: short-term outcomes. Obstet Gynecol. 2005;106:52–59.
Congratulations are in order. When the Society of Interventional Radiology created the Fibroid Registry in 2000, it was looking for early data on UAE to share with patients. In its short but impressive life, the registry has collected more data about UAE than we have about “tried-and-true” surgeries.
In the United States, UAE was first used to treat fibroids in 1997. Although numerous studies since then have reported on its safety and effectiveness, many gynecologists continue to question the suitability of UAE for symptomatic women.
The Web-based registry was established with Duke Clinical Research Institute to track short- and long-term outcomes after UAE in various settings.
What we know from the registry
The Fibroid Registry enrolled its first patient in December 2000, and collected data from 72 sites on 3,319 UAE procedures through December 2002. The reports by Myers et al and Worthington-Kirsch and colleagues contain patient demographics, procedural details, and 30-day outcomes.
The registry defined adverse events as any unexpected event that necessitated an unscheduled office or emergency room visit or unanticipated therapy (medical or surgical). Major complications required increased care or additional hospitalization or had permanent adverse sequelae. Minor complications required medical management or no therapy.
Thirty-day outcomes were available for approximately 91% of patients.
A low complication rate
Complications were uncommon during the first 30 days after UAE, with a 1.1% incidence of additional surgery. In fact, complication rates and recovery times compared favorably with myomectomy and abdominal hysterectomy for large fibroids.
The UAE procedure averaged 56 minutes, with 96.2% technical success and a return to normal activities in about 2 weeks.
Other findings:
- 26% of patients had an adverse event, but only 4% had a major event, most commonly emergency care or hospital readmission for pain management (2.1%) or possible infection (<1%).
- 31 women required another procedure within 30 days, including 3 myomectomies, 9 dilatation and curettage procedures for sloughing leiomyomata, 5 hysteroscopic resections, and 3 hysterectomies for unrecorded indications.
- 1 patient was hospitalized for pain 10 days after UAE and underwent exploratory laparotomy with bilateral oophorectomy.
- The most common minor adverse events were hot flushes (5.7%) and pain requiring additional therapy (9.6%).
Predictors of adverse outcomes:
- current or recent smoking (odds ratio [OR] 1.14, 95% confidence interval [CI] 1.007–1.293),
- African-American race (OR 1.129, 95% CI 1.019–1.251),
- prior procedures (OR 1.23, 95% CI 1.02–1.38), and
- duration of procedure (OR 1.004, 95% CI 1.001–1.006).
Interestingly, short-term outcomes did not differ among centers, nor did procedure times, length of stay, and incidence of adverse events during the first 30 days.
What to tell patients
I inform patients with symptomatic fibroids about all available treatments—including the option of doing nothing at all. Most have no interest in UAE, and are distressed by the thought of their bodies reabsorbing dead tissue.
However, I have had several patients whose operative risks were very high. For example, 1 woman was morbidly obese (>250 lb, which required her UAE treatment at a special facility equipped to perform fluoroscopy in morbidly obese patients), hypertensive, diabetic, and hemiparetic after a stroke. She was also a Jehovah’s Witness. Obviously, nonsurgical intervention was to her benefit. Her bleeding stopped almost immediately.
A reasonable alternative
The lay press recently focused on our obligation, as ObGyns, to inform patients about alternative therapies for fibroids. These first reports from the Fibroid Registry are clear: UAE is a reproducible, low-risk procedure—certainly lower in risk than complex surgeries (though situations may arise when myomectomy is preferred, such as a desire for future fertility1).
The registry will continue to provide data we can share with our patients regarding risks, complications, and long-term outcomes. More importantly, our radiology colleagues have taken the lead in developing a voluntary patient registry to track the outcomes of new technology as it disseminates into the general medical community. Our patients would be well served if we developed similar registries to track our surgical outcomes.
Focused ultrasound shrinks fibroids, but has strict eligibility requirements
Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183:1713–1719.
Using MRI guidance, a completely noninvasive treatment is now possible: focused ultrasound ablation of uterine fibroids. This procedure, the newest high-tech treatment for symptomatic fibroids, was successfully tested in an earlier pilot study.2 Although it eased symptoms to a remarkable degree during this phase III trial, how many women will ultimately be suitable for the treatment remains unclear.
Selection criteria
This trial of the ExAblate 2000 (InSightec, Tirat Carmel, Israel) recruited symptomatic women who were otherwise suitable candidates for conventional surgeries. Excluded were postmenopausal patients, women weighing more than 250 lb, and women who had a uterus larger than 24 weeks’ size or any single myoma larger than 10 cm. Women with extensive abdominal scars were carefully examined and excluded if the scars lay in the path of the ultrasound beam. The reason: During the earlier study, these scars tended to absorb ultrasound energy, increasing the risk of thermal injury at the skin surface.
Before proceeding, patients underwent MRI or ultrasound to confirm a clear pathway from the anterior abdominal wall to the fibroids without traversing the bladder or bowel.
Hindley et al did not reveal how many women were initially screened, but 109 were finally enrolled at 7 international sites and completed a Uterine Fibroid Symptoms and Quality of Life Questionnaire before and 3 and 6 months after treatment. As for the location of the fibroids: 22% were submucosal, 57% were intramural, and 21% were subserosal.
Up to 4 fibroids were treated per patient—with MRI mapping and thermographic monitoring—with a margin of 1.5 cm from the edge of the ablated area to the edge of the uterus. Conscious sedation was provided as needed, and tolerance of the procedure was measured using a 4-point pain scale. Mean time in the MRI scanner was 202 minutes (range, 90–370 minutes).
Pain stopped when treatment ended
Most women reported mild to moderate pain during the procedure (66%), and 16% complained of severe pain. This discomfort ended immediately when treatment stopped in virtually all patients—only 1% reported severe pain afterwards.
Adverse events
Serious adverse events included: 5 women with heavy menses requiring blood transfusions, 1 patient with pain and bleeding consistent with preprocedure symptoms, 1 woman needing overnight hospitalization for nausea related to opioid analgesia during the procedure, and 1 patient with leg and buttock pain immediately after treatment (it was later discovered that the sciatic nerve was in the far field of the sonication pathway). These symptoms resolved by the follow-up visit.
Two other patients had adverse events unrelated to the procedure.
6-month outcomes
The mean fibroid volume reduction was 13.5%±32%. Although this improvement seems modest, women reported significant relief from fibroid-related symptoms, and the mean severity score on the quality-of-life questionnaire decreased.
Substantial improvement was seen for both mass effect and bleeding symptoms (32.8 points out of 100 for each).
Pros and cons for symptomatic women
Advantages over UAE include the absence of postprocedural pain in almost all patients, which eliminates the need for an overnight stay and likely speeds the return to normal activities.
Disadvantages are that women with major abdominal scarring, anterior myomas underneath the bladder flap, or adhesive disease that causes small or large bowel to lie in the path of the sound waves cannot take advantage of this new technology, nor can women who have myomas close to neurovascular bundles. Add to that the extremely long procedure time (over 3 hours) and the modest reduction in uterine volume.
Six-month outcomes are promising, but this approach is probably best reserved for an academic setting, so careful screening and long-term tracking can continue.
Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–438.
In this comprehensive review, the authors draw from their extensive expertise in endocrinology to describe the rationale behind asoprisnil, a mixed progesterone receptor agonist/antagonist—the most promising pharmaceutical development in gynecology in several decades.
How the drug works
Mifepristone (RU-486) was the first progesterone receptor antagonist. Despite its ability to reduce myoma volume and suppress endometriosis symptoms in small pilot studies, it induces endometrial hyperplasia at doses higher than 5 to 10 mg, probably by acting similarly to unopposed estrogen. In contrast, asoprisnil has antiproliferative effects and no labor-inducing activity. It directly affects blood vessels in the endometrium, creating a local antiproliferative effect that induces amenorrhea despite normal estrogen levels.
What phase II studies reveal
A multicenter, double-blind, placebo-controlled trial involved 5-, 10-, and 25-mg daily doses of oral asoprisnil over 12 weeks. In a dose-dependent manner, asoprisnil induced amenorrhea or significantly suppressed bleeding without causing breakthrough or intermenstrual flow. It also decreased the volume of both the largest fibroid and the uterus as a whole. At 10- and 25-mg doses, pressure symptoms eased substantially over placebo. Adverse effects were minimal and affected the placebo and asoprisnil groups equally.
Phase III trials are now under way to assess the safety and efficacy of asoprisnil in the treatment of menorrhagia and uterine fibroids. Early results in the treatment of endometriosis are also promising.
What this means for fibroid patients
Though asoprisnil is not yet available for use, the phase III trial is winding down and the drug’s impressive potential seems clear.
A useful strategy may be to counsel marginally symptomatic women with fibroids that treatments are in the pipeline that would permit pharmaceutical management of their symptoms. Because this drug induces amenorrhea in the presence of normal circulating estrogen levels, it eliminates hot flushes and, more importantly, the bone loss associated with gonadotropin-releasing hormone agonists.
Disclosure
The author reports no financial relationships relevant to this article.
1. Pron G, Mocarski E, Bennett J, et al. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
2. Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48-54.
1. Pron G, Mocarski E, Bennett J, et al. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
2. Stewart EA, Gedroyc WM, Tempany CM, et al. Focused ultrasound treatment of uterine fibroid tumors: safety and feasibility of a noninvasive thermoablative technique. Am J Obstet Gynecol. 2003;189:48-54.
Avoiding lower urinary tract injury
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati.
- Matthew Barber, MD, MHS, Section of Urogynecology and Reconstructive Pelvic Surgery, Departments of Obstetrics & Gynecology and Urology, Cleveland Clinic, Cleveland.
- Alfred Bent, MD, Head, Division of Gynecology, Department of Obstetrics and Gynecology, Dalhousie University, IWK Health Center, Halifax, Nova Scotia.
- Geoffrey Cundiff, MD, Professor of Obstetrics and Gynecology, Johns Hopkins University, Baltimore.
Unfortunate but true: Many complications of pelvic surgery involve injury to the lower urinary tract—and many of these injuries go undetected and increase the patient’s risk of serious morbidity and the physician’s chances of being sued.
Even more unfortunate: These injuries are on the rise, thanks to the proliferation of anti-incontinence surgeries, greater use of laparoscopy, and the need for increasingly complex vaginal dissection.
Fortunately, most lower urinary tract injuries can be avoided, or at least detected early, and this discussion centers on techniques to accomplish those goals and ensure bladder integrity and ureteral patency.
The rising injury rate
There appear to be more injuries to the lower urinary tract arising from pelvic surgery. Why do you think that is?
BARBER: I think the increase is due to the increasing popularity of midurethral slings, such as the tension-free vaginal tape (TVT). With these blind retropubic procedures, the risk of bladder injury is approximately 5%, which is considerably higher than in most other procedures we perform.1
Fortunately, the negative consequences of placing the TVT trocar into the dome of the bladder are minimal, since the trocar can be removed and placed in the appropriate location without the need for bladder repair and without causing long-term bladder dysfunction.
KARRAM: The higher rate of injury also may be linked, in part, to greater use of energy sources during laparoscopic surgery. Over the past 2 years, we have seen numerous cases of delayed injury to the lower urinary tract or bowel secondary to thermal damage from energy devices including electrosurgical instruments and ultrasonic shears.
BARBER: I think there is an increase in lower urinary tract and ureteral injury because of the rising popularity of operative laparoscopy. Lower urinary tract injury is certainly more common with laparoscopic hysterectomy than with abdominal or vaginal hysterectomy.
Increase has no single cause
BENT: There may be a small increase overall in lower urinary tract injury during pelvic surgery, since we now do more procedures that require complicated vaginal dissection and exploration of tissue planes in close proximity to the ureters. This has increased the rate of ureteral injuries.
There also have been a few more urethral injuries, again related to tension-free suburethral slings, most often involving the transobturator approach.
KARRAM: The higher rate of cesarean sections also plays a role. Many women undergoing hysterectomies have had 1 or more cesarean deliveries. We recently completed a study that shows that cesarean section is an independent risk factor for cystotomy at the time of hysterectomy.2
Unfortunately, many surgeons still use aggressive blunt dissection when they attempt to mobilize the bladder off the uterus—whether a hysterectomy is being performed abdominally or vaginally. This can lead to inadvertent entry into the bladder. For this reason, sharp dissection should always be used.
CUNDIFF: Based on my reading of the literature, the incidence of operative injury to the lower urinary tract during gynecologic surgery in general has not changed noticeably since Samson reviewed the subject in 19023—although gynecologic surgery is the leading cause of such injuries and the leading cause of litigation against gynecologists.4
Most injuries involve hysterectomy
CUNDIFF: Most injuries occur during straightforward hysterectomies. Estimates of the prevalence of ureteral injury range from 0.4% to 2.4%.5-8 Since most studies estimating prevalence have not evaluated the lower urinary tract in the whole study population, they may underestimate true prevalence. However, a recent study by Vakili and colleagues9 included universal endoscopy of all patients undergoing hysterectomy and reported rates of ureteral injury (1.7%) and bladder injury (3.6%) similar to those of less rigorous studies.
Overall, the incidence of lower urinary tract injury during other types of urogynecologic surgery is higher than during hysterectomy. Evidence of the higher prevalence during urogynecologic surgery comes from several recent studies. Harris et al10 reported a 5.7% injury rate during reconstructive surgery for incontinence or prolapse. Importantly, 4% were unrecognized prior to urinary tract endoscopy.
Procedures most commonly associated with urinary tract injury were retropubic urethropexy and apical prolapse procedures using the uterosacral ligament in this series. This higher prevalence in urogynecologic procedures may explain the perceived increase in injuries overall.
How can a surgeon prevent bladder or ureteral injury during open hysterectomy?
BENT: Any procedure—regardless of the approach—demands careful dissection, good lighting, and exposure of appropriate structures. It is hard to avoid what you cannot see!
CUNDIFF: When I enter the peritoneal cavity, especially in patients undergoing reoperation, I make the incision more superiorly and avoid the bladder when extending the incision inferiorly. I always open the pararectal space and identify the ureters to ensure their safety during clamping.
Dissection of the vesicovaginal space is most effective when it is done sharply with adequate traction and countertraction. This can be achieved by gently pulling the bladder anteriorly with a Babcock clamp, using scissors to dissect close to the cervix.
For very large fundi, dissection of the vesicovaginal space can be difficult if the uterus is brought through the laparotomy. In these cases I generally take the round ligaments and infundibulopelvic ligaments first and then push the fundus into the upper abdomen. This helps keep the bowel out of the field and gives better visualization of the vesicovaginal space.
I generally enter the anterior fornix with a scalpel and then use Jorgensen scissors to excise the cervix. This helps protect the bladder, and also maximizes vaginal length.
By the way, I use a modified lithotomy position with universal stirrups to maintain access to the bladder for cystoscopy, in case it is needed later.
Follow the ureter
BARBER: During abdominal hysterectomy, I routinely identify the course of the ureter in the retroperitoneum and follow it from where it enters the pelvis until it disappears into the cardinal ligament and below the uterine artery. Following its course helps me avoid ureteral injury.
BENT: If there is scarring of the tube or ovary, or a mass is present, the ureter may have to be localized and dissected completely free of the adnexal structures before any clamps are placed. In addition, the bladder flap should routinely be mobilized using sharp dissection, never blunt dissection.
Mobilization of the bladder downward also pushes the ureters further out of the way during clamping of the uterine vessels. If bleeding occurs, secure hemostasis after observing the location of the ureters. If there is any concern about injury, cystoscopy with injected dye is required.
Next, as the uterosacral and cardinal ligaments are approached, the bladder must be reflected well inferior to this area. This will keep the ureters somewhat removed from the clamps.
Other tricks include performing intrafascial hysterectomy, in which the fascia is peeled away from the uterus and cervix, protecting the ureters.
Clamps placed across the cardinal and uterosacral ligament complexes must hug the uterus and roll off the cervix to protect the ureter.
When the cuff is sutured after removal of the uterus, clear planes of vagina must be seen anteriorly and posteriorly to avoid suturing the bladder into the vaginal cuff.
3 preventive strategies
KARRAM: For abdominal hysterectomy, I recommend 3 techniques:
- Skeletonize the infundibulopelvic ligament. Most surgeons do this routinely during the abdominal approach; I also recommend it for laparoscopic hysterectomy. Once there is a window in the broad ligament and the infundibulopelvic ligament is skeletonized, one can be sure the ureter is well below this area and probably out of harm’s way.
- Use sharp dissection to mobilize the bladder off the anterior cervix.
- Maintain awareness of the close proximity of the lower ureter to the uterosacral cardinal ligament. As the ureter enters the bladder, it can be as close as 1 cm lateral to the uterosacral ligament. This is an area where it is almost impossible to dissect out the ureter, so the surgeon needs to appreciate this anatomy and refrain from taking aggressive bites in the lateral direction when supporting or closing the vaginal cuff.
How can a surgeon prevent bladder or ureteral injury during laparoscopic hysterectomy?
BARBER: I think the ureter is best identified by direct visualization transperitoneally. The angle of the laparoscope makes visualizing the ureter much easier than from an abdominal approach, so retroperitoneal dissection is not necessary as often.
If the course of the ureter is not readily identified by direct transperitoneal visualization, a peritoneal incision can be made below and parallel to the infundibulopelvic ligament, which allows entry into the retroperitoneum and, typically, easy visualization of the ureter throughout its course.
Alternatively, the retroperitoneum can be entered lateral to the infundibulopelvic ligament, and the ureter can be identified in the same manner as in abdominal hysterectomy.
If laparoscopically assisted hysterectomy is planned, I prefer to dissect the bladder flap vaginally rather than laparoscopically, as the risk of bladder injury is considerably lower from a vaginal approach than it is laparoscopically. Obviously, if a total laparoscopic hysterectomy is necessary because of poor vaginal access, laparoscopic bladder flap dissection is necessary. In this case, I again favor sharp dissection and minimal use of cautery to avoid bladder injury.
How can a surgeon prevent bladder or ureteral injury during vaginal hysterectomy?
BENT: Traditional methods in which each clamp is rolled off the cervix or uterus until the procedure is completed help keep unsuspecting surgeons out of the bladder and away from the ureter. The only risk involves bladder mobilization (ie, creation of the bladder flap), which should always be done sharply to prevent bladder perforation. Avoid blunt finger or sponge-stick dissection! Knowing how to sharply dissect the bladder flap is vital—then even cases of prior cesarean section are manageable.
Salpingo-oophorectomy can also proceed under direct vision. Avoid the ureter by making sure the clamp closes only over the pedicles of the tube and ovary, with no intervening tissues in the clamp. If space is very tight, divide the round ligament and take the pedicle in a smaller bite. Traction on the cervix during the procedure, and mobilization of the bladder, allow the ureters to slide upward, well out of harm’s way, as the procedure progresses.
The importance of sharp dissection
BARBER: During vaginal hysterectomy, I usually have the operative assistant hold the cervical tenaculum so that there is tension on the uterus. I then use forceps to elevate the bladder directly vertically in order to place the bladder fibers on tension. Next, I dissect the bladder off the cervix and lower uterine segment using sharp dissection, and identify the peritoneum by direct finger palpation. Almost always, it is smooth and slippery.
After identifying the peritoneum, I grasp it with a tonsil clamp and elevate it so that it can be entered easily with scissors. I always confirm peritoneal entry by visualizing and identifying intraperitoneal structures such as bowel fat, the uterine serosal surface, or adnexae.
Some people advocate palpating the ureter during vaginal cases.
CUNDIFF: The most common time of injury during vaginal hysterectomy is during dissection of the vesicovaginal space; and suture ligation of the infundibulopelvic ligaments and uterine arteries carries the greatest potential for ureteral injury.
During vaginal hysterectomy, I try to dissect the vesicovaginal space early. I use a Deever retractor to retract the bladder anteriorly, maximizing my ability to sharply dissect close to the cervix until entering the peritoneal cavity. Once I’m in the peritoneal cavity, I advance the Deever retractor to protect the bladder through the rest of the procedure. I maximize protection of the ureters by applying downward traction on the cervix during vaginal clamp placement.
KARRAM: I agree. Never try to enter the anterior cul-de-sac until the vesicouterine space has been identified and is easily palpated. Rushing to enter the anterior cul-de-sac will only lead to inadvertent cystotomy.
Assessing ureteral patency
After what pelvic surgeries do you think ureteral patency should be assessed, and how should it be accomplished?
CUNDIFF: The literature contains several studies10-13 that involved universal endoscopy of the lower urinary tract. These studies demonstrate that most injuries are not recognized by the surgeon prior to endoscopy. In fact, the vast majority of injuries occur after straightforward hysterectomies. This may be due in part to the sheer volume of hysterectomies, compared with other pelvic surgeries. However, it also shows that, when lower urinary tract evaluation is performed solely when the surgeon suspects an injury, a substantial proportion of injuries are missed.
KARRAM: When do you assess ureteral patency?
CUNDIFF: My personal practice is to evaluate it in all cases that carry the potential for injury to the ureter. The complexity of the evaluation is proportional to the probability of ureteral injury.
For all laparotomies and laparoscopies, I identify the course of the ureter and confirm peristalsis. In the simplest of cases, this can be done by identifying the ureter beneath the peritoneum as it crosses the pelvic brim and courses across the pelvic sidewall. More frequently, it involves opening the pararectal space to identify the course of the ureter. In the most complex cases, it requires ureterolysis.
I strongly believe that identifying the ureter during any dissection that endangers it is the best way to avoid injury. Even after these precautions, I frequently perform cystoscopy with intravenous indigo carmine to confirm ureteral patency. This is my standard approach with all vaginal procedures, as I am not confident that I can palpate the course of the ureter.
Postoperative cystoscopy is virtually without morbidity and adds no more than 3 minutes to the procedure when properly planned. It also affords an excellent opportunity to train residents in cystoscopy.
How to assess patency after selected procedures
BENT: Ureteral patency should be assured after any repair of the pelvic floor.
After abdominal hysterectomy, if there is blood in the catheter bag or any difficulty has been encountered during surgery, I perform cystoscopy after injecting indigo carmine dye, to observe ureteral function.
At abdominal or laparoscopic sacrocolpopexy, I follow the path of the ureters over the pelvic brim and inferiorly to the adnexal area by direct inspection.
During abdominal or laparoscopic paravaginal repair, the Burch procedure, or uterosacral ligament suspension, I perform cystoscopy after tying sutures and injecting indigo carmine to ensure ureteral function.
To safeguard the ureter, follow its course
Vaginal hysterectomy is not usually associated with ureteral injury, and very uncommonly with bladder injury. However, if bladder injury is observed or the procedure has been difficult, it is wise to perform cystoscopy with dye injection. This also extends to traditional cystocele repair.
I also recommend cystoscopy with dye injection any time there is a vaginal approach to paravaginal defect repair, vault suspension, or colpocleisis.
Cystoscopy—safe, simple, and efficient
KARRAM: For vaginal surgery, I think cystoscopy is the simplest way to assess the lower urinary tract. I routinely use it after any procedures involving the posterior cul-de-sac such as McCall culdoplasty or vaginal vault suspension from the uterosacral ligaments. I also use it routinely after advanced prolapse repairs involving anterior colporrhaphy, as well as paravaginal defect repairs, and I certainly use it routinely after any lower urinary tract reconstructive procedures such as fistula repairs.
As for laparoscopic surgery, I think cystoscopy is again the most efficient way to assess the lower urinary tract. I do so after any retropubic suspension, be it a Burch colposuspension or a paravaginal repair, any type of vault suspension or sacrocolpopexy, and any type of hysterectomy or adnexectomy that involves dissection of the retroperitoneal space. I also do so if an energy source was used extensively in the vicinity of the retroperitoneal space on either side.
After abdominal surgery, it is probably more efficient (assuming the patient is not in stirrups) to perform a high extraperitoneal cystotomy or suprapubic telescopy. The indications are any difficult dissection in which I have concerns about ureteral patency, as well as any abdominal prolapse or anti-incontinence procedures.
Ureteral stenting
Is ureteral stenting ever indicated preoperatively? If so, when?
BARBER: I do not think ureteral stenting is indicated routinely for any procedure. There may be individual cases where a stent may help the surgeon avoid ureteral injury, but I can’t think of a procedure in which it should be routinely used.
CUNDIFF: I agree. Although ureteral stenting is an important tool for the pelvic floor surgeon to investigate potential ureteral obstruction, I think it has very limited value as a preoperative maneuver to avoid injury. My opinion is based on the following observations:
The surgeon cannot really assess the potential difficulty of identifying the course of the ureter until the peritoneal cavity is entered.
For the truly hostile pelvis, in which pelvic sidewall pathology prevents identification of the course of the ureter, I do not find that a stent facilitates dissection of the pararectal space and ureterolysis. In fact, it could increase the chance of ureteral injury by creating a backboard against which to cut it during dissection.
Any potential benefit of ureteral stents—which I believe is minimal—must be balanced against the potential risks, which include 20 to 30 minutes of added OR time and the risk of ureteral spasm or perforation.
BENT: I also agree that ureteral stenting is seldom helpful during gynecologic surgery. At laparotomy, direct dissection of the structures and exposure of the ureter are best; there is no need to feel for a ureter.
At laparoscopy, however, if there are large fibroids, scarring from endometriomas, or adnexal masses, then preoperative placement of lighted stents can help the surgeon identify the ureters during dissection. The case would still require dissection of the ureter away from the operative field, but the lighted path provides a starting point in this procedure.
For vaginal surgical procedures, it is easier to avoid the ureter. However, not all surgeons can palpate a nonstented ureter, which may be required during a high uterosacral ligament suspension. A stent can readily take the surgeon to the ureter and avoid injury in most cases. This may be helpful for less experienced operators.
What should residents be trained to do?
Do most obstetrics and gynecology residency programs appropriately train young physicians to evaluate and manage lower urinary tract injury during pelvic surgery?
CUNDIFF: I am afraid not. Although residency directors increasingly recognize the importance of educating doctors to prevent and manage these injuries, this recognition has not yet risen to the policy level.
For example, the Council on Resident Education in Obstetrics and Gynecology (CREOG) includes a bladder surgery educational model that necessitates dissection of the ureter, cystoscopy, ureteral stenting, bladder repair, and ureteral reanastomosis.
However, the CREOG surgical curriculum makes no mention of protecting or evaluating the lower urinary tract during pelvic surgery.
This spectrum seems to reflect the wide variation among residency programs, too. While some programs such as ours at Johns Hopkins provide comprehensive training in prevention, evaluation, and management of lower urinary tract injury, many others do not. This might be because some programs lack technically skilled faculty. Interdisciplinary politics also likely influences local credentialing.
BARBER: In my opinion, a graduating ObGyn resident should be able to:
- identify and mobilize the ureter to avoid injury during abdominal and laparoscopic surgery
- safely mobilize the bladder during abdominal, vaginal, or laparoscopic hysterectomy
- perform intraoperative cystoscopy to evaluate for injury
- repair bladder injuries abdominally and vaginally
However, I don’t think it is realistic for a graduating resident to be able to manage ureteral injuries, as residents are unlikely to encounter very many during training. These injuries are best left to our urology colleagues.
BENT: Very little or no education is provided in preventing lower urinary tract injury; evaluation is better managed in many programs. A conservative estimate is that 20% of programs have a reasonable curriculum for preoperative assessment and evaluation of incontinence and prolapse.
The management of pelvic floor disorders is better handled in almost all programs, especially as it relates to surgery. Many residents spend a lot of time on the urogynecology service and are exposed to the surgical aspect of rotations.
Dr. Karram and Dr. Barber have no financial relationships relevant to this article. Dr. Bent serves on the gynecology advisory board of ACMI and is a speaker for Novartis, Pfizer, Watson, and Asetellas (formerly Yamanouchi). He also has received research funding from Cook, Eli Lilly, and Mentor; and is a consultant for C.R. Bard. Dr. Cundiff has received grant/research support from Cook, is a consultant to C.R. Bard and Eli Lilly, and is a speaker for GlaxoSmithKline.
1. Karram MM, Segal JL, Vassallo BJ, Kleeman SD. Complications and untoward effects of the tension-free vaginal tape procedure. Obstet Gynecol 2003;101:929-932.
2. Rooney C, Crawford A, Vassaco B, Kleeman S, Karram M. Is cesarean section a risk factor for incidental cystotomy at the time of hysterectomy? Am J Obstet Gynecol. In press.
3. Samson JA. Ligation and clamping of the ureter as complications of surgical operations. Am Med. 1902;4:693.-
4. Wiskind AK, Thompson JD. Should cystoscopy be performed at every gynecologic operation to diagnose unsuspected ureteral injury? J Pelvic Surg. 1995;1:134-137.
5. St. Martin EC, et al. Ureteral injury in gynecologic surgery. J Urol. 1953;70:51-57.
6. Conger K, Beecham CT, Horrax TM. Ureteral injury in pelvic surgery: current thought on incidence, pathogenesis, prophylaxis and treatment. Obstet Gynecol. 1954;3:343-357.
7. Mann WJ, Arato M, Patsner B, et al. Ureteral injuries in an obstetrics and gynecology training program: etiology and management. Obstet Gynecol. 1988;72:82-85.
8. Stanhope CR, Wilson TO, Utz WJ, Smith LH, O’Brien PC. Suture entrapment and secondary ureteral obstruction. Am J Obstet Gynecol. 1991;164:1513-1519.
9. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
10. Harris RL, Cundiff GW, Theofrastous JT, Yoon HW, Bump RC, Addison WA. The value of intraoperative cystoscopy in urogynecologic and reconstructive pelvic surgery. Am J Obstet Gynecol. 1997;177:1367-1369.
11. Gill EJ, Elser DM, Bonidie MJ, Roberts KM, Hurt WG. The routine use of cystoscopy with the Burch procedure. Am J Obstet Gynecol. 2002;186:1108.-
12. Kwon CH, Goldberg RO, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187:1466-1471; discussion 1471-1472.
13. Tulikangas PK, Weber AM, et al. Intraoperative cystoscopy in conjunction with anti-incontinence surgery. Obstet Gynecol. 2000;95(6 Pt 1):794-796.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati.
- Matthew Barber, MD, MHS, Section of Urogynecology and Reconstructive Pelvic Surgery, Departments of Obstetrics & Gynecology and Urology, Cleveland Clinic, Cleveland.
- Alfred Bent, MD, Head, Division of Gynecology, Department of Obstetrics and Gynecology, Dalhousie University, IWK Health Center, Halifax, Nova Scotia.
- Geoffrey Cundiff, MD, Professor of Obstetrics and Gynecology, Johns Hopkins University, Baltimore.
Unfortunate but true: Many complications of pelvic surgery involve injury to the lower urinary tract—and many of these injuries go undetected and increase the patient’s risk of serious morbidity and the physician’s chances of being sued.
Even more unfortunate: These injuries are on the rise, thanks to the proliferation of anti-incontinence surgeries, greater use of laparoscopy, and the need for increasingly complex vaginal dissection.
Fortunately, most lower urinary tract injuries can be avoided, or at least detected early, and this discussion centers on techniques to accomplish those goals and ensure bladder integrity and ureteral patency.
The rising injury rate
There appear to be more injuries to the lower urinary tract arising from pelvic surgery. Why do you think that is?
BARBER: I think the increase is due to the increasing popularity of midurethral slings, such as the tension-free vaginal tape (TVT). With these blind retropubic procedures, the risk of bladder injury is approximately 5%, which is considerably higher than in most other procedures we perform.1
Fortunately, the negative consequences of placing the TVT trocar into the dome of the bladder are minimal, since the trocar can be removed and placed in the appropriate location without the need for bladder repair and without causing long-term bladder dysfunction.
KARRAM: The higher rate of injury also may be linked, in part, to greater use of energy sources during laparoscopic surgery. Over the past 2 years, we have seen numerous cases of delayed injury to the lower urinary tract or bowel secondary to thermal damage from energy devices including electrosurgical instruments and ultrasonic shears.
BARBER: I think there is an increase in lower urinary tract and ureteral injury because of the rising popularity of operative laparoscopy. Lower urinary tract injury is certainly more common with laparoscopic hysterectomy than with abdominal or vaginal hysterectomy.
Increase has no single cause
BENT: There may be a small increase overall in lower urinary tract injury during pelvic surgery, since we now do more procedures that require complicated vaginal dissection and exploration of tissue planes in close proximity to the ureters. This has increased the rate of ureteral injuries.
There also have been a few more urethral injuries, again related to tension-free suburethral slings, most often involving the transobturator approach.
KARRAM: The higher rate of cesarean sections also plays a role. Many women undergoing hysterectomies have had 1 or more cesarean deliveries. We recently completed a study that shows that cesarean section is an independent risk factor for cystotomy at the time of hysterectomy.2
Unfortunately, many surgeons still use aggressive blunt dissection when they attempt to mobilize the bladder off the uterus—whether a hysterectomy is being performed abdominally or vaginally. This can lead to inadvertent entry into the bladder. For this reason, sharp dissection should always be used.
CUNDIFF: Based on my reading of the literature, the incidence of operative injury to the lower urinary tract during gynecologic surgery in general has not changed noticeably since Samson reviewed the subject in 19023—although gynecologic surgery is the leading cause of such injuries and the leading cause of litigation against gynecologists.4
Most injuries involve hysterectomy
CUNDIFF: Most injuries occur during straightforward hysterectomies. Estimates of the prevalence of ureteral injury range from 0.4% to 2.4%.5-8 Since most studies estimating prevalence have not evaluated the lower urinary tract in the whole study population, they may underestimate true prevalence. However, a recent study by Vakili and colleagues9 included universal endoscopy of all patients undergoing hysterectomy and reported rates of ureteral injury (1.7%) and bladder injury (3.6%) similar to those of less rigorous studies.
Overall, the incidence of lower urinary tract injury during other types of urogynecologic surgery is higher than during hysterectomy. Evidence of the higher prevalence during urogynecologic surgery comes from several recent studies. Harris et al10 reported a 5.7% injury rate during reconstructive surgery for incontinence or prolapse. Importantly, 4% were unrecognized prior to urinary tract endoscopy.
Procedures most commonly associated with urinary tract injury were retropubic urethropexy and apical prolapse procedures using the uterosacral ligament in this series. This higher prevalence in urogynecologic procedures may explain the perceived increase in injuries overall.
How can a surgeon prevent bladder or ureteral injury during open hysterectomy?
BENT: Any procedure—regardless of the approach—demands careful dissection, good lighting, and exposure of appropriate structures. It is hard to avoid what you cannot see!
CUNDIFF: When I enter the peritoneal cavity, especially in patients undergoing reoperation, I make the incision more superiorly and avoid the bladder when extending the incision inferiorly. I always open the pararectal space and identify the ureters to ensure their safety during clamping.
Dissection of the vesicovaginal space is most effective when it is done sharply with adequate traction and countertraction. This can be achieved by gently pulling the bladder anteriorly with a Babcock clamp, using scissors to dissect close to the cervix.
For very large fundi, dissection of the vesicovaginal space can be difficult if the uterus is brought through the laparotomy. In these cases I generally take the round ligaments and infundibulopelvic ligaments first and then push the fundus into the upper abdomen. This helps keep the bowel out of the field and gives better visualization of the vesicovaginal space.
I generally enter the anterior fornix with a scalpel and then use Jorgensen scissors to excise the cervix. This helps protect the bladder, and also maximizes vaginal length.
By the way, I use a modified lithotomy position with universal stirrups to maintain access to the bladder for cystoscopy, in case it is needed later.
Follow the ureter
BARBER: During abdominal hysterectomy, I routinely identify the course of the ureter in the retroperitoneum and follow it from where it enters the pelvis until it disappears into the cardinal ligament and below the uterine artery. Following its course helps me avoid ureteral injury.
BENT: If there is scarring of the tube or ovary, or a mass is present, the ureter may have to be localized and dissected completely free of the adnexal structures before any clamps are placed. In addition, the bladder flap should routinely be mobilized using sharp dissection, never blunt dissection.
Mobilization of the bladder downward also pushes the ureters further out of the way during clamping of the uterine vessels. If bleeding occurs, secure hemostasis after observing the location of the ureters. If there is any concern about injury, cystoscopy with injected dye is required.
Next, as the uterosacral and cardinal ligaments are approached, the bladder must be reflected well inferior to this area. This will keep the ureters somewhat removed from the clamps.
Other tricks include performing intrafascial hysterectomy, in which the fascia is peeled away from the uterus and cervix, protecting the ureters.
Clamps placed across the cardinal and uterosacral ligament complexes must hug the uterus and roll off the cervix to protect the ureter.
When the cuff is sutured after removal of the uterus, clear planes of vagina must be seen anteriorly and posteriorly to avoid suturing the bladder into the vaginal cuff.
3 preventive strategies
KARRAM: For abdominal hysterectomy, I recommend 3 techniques:
- Skeletonize the infundibulopelvic ligament. Most surgeons do this routinely during the abdominal approach; I also recommend it for laparoscopic hysterectomy. Once there is a window in the broad ligament and the infundibulopelvic ligament is skeletonized, one can be sure the ureter is well below this area and probably out of harm’s way.
- Use sharp dissection to mobilize the bladder off the anterior cervix.
- Maintain awareness of the close proximity of the lower ureter to the uterosacral cardinal ligament. As the ureter enters the bladder, it can be as close as 1 cm lateral to the uterosacral ligament. This is an area where it is almost impossible to dissect out the ureter, so the surgeon needs to appreciate this anatomy and refrain from taking aggressive bites in the lateral direction when supporting or closing the vaginal cuff.
How can a surgeon prevent bladder or ureteral injury during laparoscopic hysterectomy?
BARBER: I think the ureter is best identified by direct visualization transperitoneally. The angle of the laparoscope makes visualizing the ureter much easier than from an abdominal approach, so retroperitoneal dissection is not necessary as often.
If the course of the ureter is not readily identified by direct transperitoneal visualization, a peritoneal incision can be made below and parallel to the infundibulopelvic ligament, which allows entry into the retroperitoneum and, typically, easy visualization of the ureter throughout its course.
Alternatively, the retroperitoneum can be entered lateral to the infundibulopelvic ligament, and the ureter can be identified in the same manner as in abdominal hysterectomy.
If laparoscopically assisted hysterectomy is planned, I prefer to dissect the bladder flap vaginally rather than laparoscopically, as the risk of bladder injury is considerably lower from a vaginal approach than it is laparoscopically. Obviously, if a total laparoscopic hysterectomy is necessary because of poor vaginal access, laparoscopic bladder flap dissection is necessary. In this case, I again favor sharp dissection and minimal use of cautery to avoid bladder injury.
How can a surgeon prevent bladder or ureteral injury during vaginal hysterectomy?
BENT: Traditional methods in which each clamp is rolled off the cervix or uterus until the procedure is completed help keep unsuspecting surgeons out of the bladder and away from the ureter. The only risk involves bladder mobilization (ie, creation of the bladder flap), which should always be done sharply to prevent bladder perforation. Avoid blunt finger or sponge-stick dissection! Knowing how to sharply dissect the bladder flap is vital—then even cases of prior cesarean section are manageable.
Salpingo-oophorectomy can also proceed under direct vision. Avoid the ureter by making sure the clamp closes only over the pedicles of the tube and ovary, with no intervening tissues in the clamp. If space is very tight, divide the round ligament and take the pedicle in a smaller bite. Traction on the cervix during the procedure, and mobilization of the bladder, allow the ureters to slide upward, well out of harm’s way, as the procedure progresses.
The importance of sharp dissection
BARBER: During vaginal hysterectomy, I usually have the operative assistant hold the cervical tenaculum so that there is tension on the uterus. I then use forceps to elevate the bladder directly vertically in order to place the bladder fibers on tension. Next, I dissect the bladder off the cervix and lower uterine segment using sharp dissection, and identify the peritoneum by direct finger palpation. Almost always, it is smooth and slippery.
After identifying the peritoneum, I grasp it with a tonsil clamp and elevate it so that it can be entered easily with scissors. I always confirm peritoneal entry by visualizing and identifying intraperitoneal structures such as bowel fat, the uterine serosal surface, or adnexae.
Some people advocate palpating the ureter during vaginal cases.
CUNDIFF: The most common time of injury during vaginal hysterectomy is during dissection of the vesicovaginal space; and suture ligation of the infundibulopelvic ligaments and uterine arteries carries the greatest potential for ureteral injury.
During vaginal hysterectomy, I try to dissect the vesicovaginal space early. I use a Deever retractor to retract the bladder anteriorly, maximizing my ability to sharply dissect close to the cervix until entering the peritoneal cavity. Once I’m in the peritoneal cavity, I advance the Deever retractor to protect the bladder through the rest of the procedure. I maximize protection of the ureters by applying downward traction on the cervix during vaginal clamp placement.
KARRAM: I agree. Never try to enter the anterior cul-de-sac until the vesicouterine space has been identified and is easily palpated. Rushing to enter the anterior cul-de-sac will only lead to inadvertent cystotomy.
Assessing ureteral patency
After what pelvic surgeries do you think ureteral patency should be assessed, and how should it be accomplished?
CUNDIFF: The literature contains several studies10-13 that involved universal endoscopy of the lower urinary tract. These studies demonstrate that most injuries are not recognized by the surgeon prior to endoscopy. In fact, the vast majority of injuries occur after straightforward hysterectomies. This may be due in part to the sheer volume of hysterectomies, compared with other pelvic surgeries. However, it also shows that, when lower urinary tract evaluation is performed solely when the surgeon suspects an injury, a substantial proportion of injuries are missed.
KARRAM: When do you assess ureteral patency?
CUNDIFF: My personal practice is to evaluate it in all cases that carry the potential for injury to the ureter. The complexity of the evaluation is proportional to the probability of ureteral injury.
For all laparotomies and laparoscopies, I identify the course of the ureter and confirm peristalsis. In the simplest of cases, this can be done by identifying the ureter beneath the peritoneum as it crosses the pelvic brim and courses across the pelvic sidewall. More frequently, it involves opening the pararectal space to identify the course of the ureter. In the most complex cases, it requires ureterolysis.
I strongly believe that identifying the ureter during any dissection that endangers it is the best way to avoid injury. Even after these precautions, I frequently perform cystoscopy with intravenous indigo carmine to confirm ureteral patency. This is my standard approach with all vaginal procedures, as I am not confident that I can palpate the course of the ureter.
Postoperative cystoscopy is virtually without morbidity and adds no more than 3 minutes to the procedure when properly planned. It also affords an excellent opportunity to train residents in cystoscopy.
How to assess patency after selected procedures
BENT: Ureteral patency should be assured after any repair of the pelvic floor.
After abdominal hysterectomy, if there is blood in the catheter bag or any difficulty has been encountered during surgery, I perform cystoscopy after injecting indigo carmine dye, to observe ureteral function.
At abdominal or laparoscopic sacrocolpopexy, I follow the path of the ureters over the pelvic brim and inferiorly to the adnexal area by direct inspection.
During abdominal or laparoscopic paravaginal repair, the Burch procedure, or uterosacral ligament suspension, I perform cystoscopy after tying sutures and injecting indigo carmine to ensure ureteral function.
To safeguard the ureter, follow its course
Vaginal hysterectomy is not usually associated with ureteral injury, and very uncommonly with bladder injury. However, if bladder injury is observed or the procedure has been difficult, it is wise to perform cystoscopy with dye injection. This also extends to traditional cystocele repair.
I also recommend cystoscopy with dye injection any time there is a vaginal approach to paravaginal defect repair, vault suspension, or colpocleisis.
Cystoscopy—safe, simple, and efficient
KARRAM: For vaginal surgery, I think cystoscopy is the simplest way to assess the lower urinary tract. I routinely use it after any procedures involving the posterior cul-de-sac such as McCall culdoplasty or vaginal vault suspension from the uterosacral ligaments. I also use it routinely after advanced prolapse repairs involving anterior colporrhaphy, as well as paravaginal defect repairs, and I certainly use it routinely after any lower urinary tract reconstructive procedures such as fistula repairs.
As for laparoscopic surgery, I think cystoscopy is again the most efficient way to assess the lower urinary tract. I do so after any retropubic suspension, be it a Burch colposuspension or a paravaginal repair, any type of vault suspension or sacrocolpopexy, and any type of hysterectomy or adnexectomy that involves dissection of the retroperitoneal space. I also do so if an energy source was used extensively in the vicinity of the retroperitoneal space on either side.
After abdominal surgery, it is probably more efficient (assuming the patient is not in stirrups) to perform a high extraperitoneal cystotomy or suprapubic telescopy. The indications are any difficult dissection in which I have concerns about ureteral patency, as well as any abdominal prolapse or anti-incontinence procedures.
Ureteral stenting
Is ureteral stenting ever indicated preoperatively? If so, when?
BARBER: I do not think ureteral stenting is indicated routinely for any procedure. There may be individual cases where a stent may help the surgeon avoid ureteral injury, but I can’t think of a procedure in which it should be routinely used.
CUNDIFF: I agree. Although ureteral stenting is an important tool for the pelvic floor surgeon to investigate potential ureteral obstruction, I think it has very limited value as a preoperative maneuver to avoid injury. My opinion is based on the following observations:
The surgeon cannot really assess the potential difficulty of identifying the course of the ureter until the peritoneal cavity is entered.
For the truly hostile pelvis, in which pelvic sidewall pathology prevents identification of the course of the ureter, I do not find that a stent facilitates dissection of the pararectal space and ureterolysis. In fact, it could increase the chance of ureteral injury by creating a backboard against which to cut it during dissection.
Any potential benefit of ureteral stents—which I believe is minimal—must be balanced against the potential risks, which include 20 to 30 minutes of added OR time and the risk of ureteral spasm or perforation.
BENT: I also agree that ureteral stenting is seldom helpful during gynecologic surgery. At laparotomy, direct dissection of the structures and exposure of the ureter are best; there is no need to feel for a ureter.
At laparoscopy, however, if there are large fibroids, scarring from endometriomas, or adnexal masses, then preoperative placement of lighted stents can help the surgeon identify the ureters during dissection. The case would still require dissection of the ureter away from the operative field, but the lighted path provides a starting point in this procedure.
For vaginal surgical procedures, it is easier to avoid the ureter. However, not all surgeons can palpate a nonstented ureter, which may be required during a high uterosacral ligament suspension. A stent can readily take the surgeon to the ureter and avoid injury in most cases. This may be helpful for less experienced operators.
What should residents be trained to do?
Do most obstetrics and gynecology residency programs appropriately train young physicians to evaluate and manage lower urinary tract injury during pelvic surgery?
CUNDIFF: I am afraid not. Although residency directors increasingly recognize the importance of educating doctors to prevent and manage these injuries, this recognition has not yet risen to the policy level.
For example, the Council on Resident Education in Obstetrics and Gynecology (CREOG) includes a bladder surgery educational model that necessitates dissection of the ureter, cystoscopy, ureteral stenting, bladder repair, and ureteral reanastomosis.
However, the CREOG surgical curriculum makes no mention of protecting or evaluating the lower urinary tract during pelvic surgery.
This spectrum seems to reflect the wide variation among residency programs, too. While some programs such as ours at Johns Hopkins provide comprehensive training in prevention, evaluation, and management of lower urinary tract injury, many others do not. This might be because some programs lack technically skilled faculty. Interdisciplinary politics also likely influences local credentialing.
BARBER: In my opinion, a graduating ObGyn resident should be able to:
- identify and mobilize the ureter to avoid injury during abdominal and laparoscopic surgery
- safely mobilize the bladder during abdominal, vaginal, or laparoscopic hysterectomy
- perform intraoperative cystoscopy to evaluate for injury
- repair bladder injuries abdominally and vaginally
However, I don’t think it is realistic for a graduating resident to be able to manage ureteral injuries, as residents are unlikely to encounter very many during training. These injuries are best left to our urology colleagues.
BENT: Very little or no education is provided in preventing lower urinary tract injury; evaluation is better managed in many programs. A conservative estimate is that 20% of programs have a reasonable curriculum for preoperative assessment and evaluation of incontinence and prolapse.
The management of pelvic floor disorders is better handled in almost all programs, especially as it relates to surgery. Many residents spend a lot of time on the urogynecology service and are exposed to the surgical aspect of rotations.
Dr. Karram and Dr. Barber have no financial relationships relevant to this article. Dr. Bent serves on the gynecology advisory board of ACMI and is a speaker for Novartis, Pfizer, Watson, and Asetellas (formerly Yamanouchi). He also has received research funding from Cook, Eli Lilly, and Mentor; and is a consultant for C.R. Bard. Dr. Cundiff has received grant/research support from Cook, is a consultant to C.R. Bard and Eli Lilly, and is a speaker for GlaxoSmithKline.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati.
- Matthew Barber, MD, MHS, Section of Urogynecology and Reconstructive Pelvic Surgery, Departments of Obstetrics & Gynecology and Urology, Cleveland Clinic, Cleveland.
- Alfred Bent, MD, Head, Division of Gynecology, Department of Obstetrics and Gynecology, Dalhousie University, IWK Health Center, Halifax, Nova Scotia.
- Geoffrey Cundiff, MD, Professor of Obstetrics and Gynecology, Johns Hopkins University, Baltimore.
Unfortunate but true: Many complications of pelvic surgery involve injury to the lower urinary tract—and many of these injuries go undetected and increase the patient’s risk of serious morbidity and the physician’s chances of being sued.
Even more unfortunate: These injuries are on the rise, thanks to the proliferation of anti-incontinence surgeries, greater use of laparoscopy, and the need for increasingly complex vaginal dissection.
Fortunately, most lower urinary tract injuries can be avoided, or at least detected early, and this discussion centers on techniques to accomplish those goals and ensure bladder integrity and ureteral patency.
The rising injury rate
There appear to be more injuries to the lower urinary tract arising from pelvic surgery. Why do you think that is?
BARBER: I think the increase is due to the increasing popularity of midurethral slings, such as the tension-free vaginal tape (TVT). With these blind retropubic procedures, the risk of bladder injury is approximately 5%, which is considerably higher than in most other procedures we perform.1
Fortunately, the negative consequences of placing the TVT trocar into the dome of the bladder are minimal, since the trocar can be removed and placed in the appropriate location without the need for bladder repair and without causing long-term bladder dysfunction.
KARRAM: The higher rate of injury also may be linked, in part, to greater use of energy sources during laparoscopic surgery. Over the past 2 years, we have seen numerous cases of delayed injury to the lower urinary tract or bowel secondary to thermal damage from energy devices including electrosurgical instruments and ultrasonic shears.
BARBER: I think there is an increase in lower urinary tract and ureteral injury because of the rising popularity of operative laparoscopy. Lower urinary tract injury is certainly more common with laparoscopic hysterectomy than with abdominal or vaginal hysterectomy.
Increase has no single cause
BENT: There may be a small increase overall in lower urinary tract injury during pelvic surgery, since we now do more procedures that require complicated vaginal dissection and exploration of tissue planes in close proximity to the ureters. This has increased the rate of ureteral injuries.
There also have been a few more urethral injuries, again related to tension-free suburethral slings, most often involving the transobturator approach.
KARRAM: The higher rate of cesarean sections also plays a role. Many women undergoing hysterectomies have had 1 or more cesarean deliveries. We recently completed a study that shows that cesarean section is an independent risk factor for cystotomy at the time of hysterectomy.2
Unfortunately, many surgeons still use aggressive blunt dissection when they attempt to mobilize the bladder off the uterus—whether a hysterectomy is being performed abdominally or vaginally. This can lead to inadvertent entry into the bladder. For this reason, sharp dissection should always be used.
CUNDIFF: Based on my reading of the literature, the incidence of operative injury to the lower urinary tract during gynecologic surgery in general has not changed noticeably since Samson reviewed the subject in 19023—although gynecologic surgery is the leading cause of such injuries and the leading cause of litigation against gynecologists.4
Most injuries involve hysterectomy
CUNDIFF: Most injuries occur during straightforward hysterectomies. Estimates of the prevalence of ureteral injury range from 0.4% to 2.4%.5-8 Since most studies estimating prevalence have not evaluated the lower urinary tract in the whole study population, they may underestimate true prevalence. However, a recent study by Vakili and colleagues9 included universal endoscopy of all patients undergoing hysterectomy and reported rates of ureteral injury (1.7%) and bladder injury (3.6%) similar to those of less rigorous studies.
Overall, the incidence of lower urinary tract injury during other types of urogynecologic surgery is higher than during hysterectomy. Evidence of the higher prevalence during urogynecologic surgery comes from several recent studies. Harris et al10 reported a 5.7% injury rate during reconstructive surgery for incontinence or prolapse. Importantly, 4% were unrecognized prior to urinary tract endoscopy.
Procedures most commonly associated with urinary tract injury were retropubic urethropexy and apical prolapse procedures using the uterosacral ligament in this series. This higher prevalence in urogynecologic procedures may explain the perceived increase in injuries overall.
How can a surgeon prevent bladder or ureteral injury during open hysterectomy?
BENT: Any procedure—regardless of the approach—demands careful dissection, good lighting, and exposure of appropriate structures. It is hard to avoid what you cannot see!
CUNDIFF: When I enter the peritoneal cavity, especially in patients undergoing reoperation, I make the incision more superiorly and avoid the bladder when extending the incision inferiorly. I always open the pararectal space and identify the ureters to ensure their safety during clamping.
Dissection of the vesicovaginal space is most effective when it is done sharply with adequate traction and countertraction. This can be achieved by gently pulling the bladder anteriorly with a Babcock clamp, using scissors to dissect close to the cervix.
For very large fundi, dissection of the vesicovaginal space can be difficult if the uterus is brought through the laparotomy. In these cases I generally take the round ligaments and infundibulopelvic ligaments first and then push the fundus into the upper abdomen. This helps keep the bowel out of the field and gives better visualization of the vesicovaginal space.
I generally enter the anterior fornix with a scalpel and then use Jorgensen scissors to excise the cervix. This helps protect the bladder, and also maximizes vaginal length.
By the way, I use a modified lithotomy position with universal stirrups to maintain access to the bladder for cystoscopy, in case it is needed later.
Follow the ureter
BARBER: During abdominal hysterectomy, I routinely identify the course of the ureter in the retroperitoneum and follow it from where it enters the pelvis until it disappears into the cardinal ligament and below the uterine artery. Following its course helps me avoid ureteral injury.
BENT: If there is scarring of the tube or ovary, or a mass is present, the ureter may have to be localized and dissected completely free of the adnexal structures before any clamps are placed. In addition, the bladder flap should routinely be mobilized using sharp dissection, never blunt dissection.
Mobilization of the bladder downward also pushes the ureters further out of the way during clamping of the uterine vessels. If bleeding occurs, secure hemostasis after observing the location of the ureters. If there is any concern about injury, cystoscopy with injected dye is required.
Next, as the uterosacral and cardinal ligaments are approached, the bladder must be reflected well inferior to this area. This will keep the ureters somewhat removed from the clamps.
Other tricks include performing intrafascial hysterectomy, in which the fascia is peeled away from the uterus and cervix, protecting the ureters.
Clamps placed across the cardinal and uterosacral ligament complexes must hug the uterus and roll off the cervix to protect the ureter.
When the cuff is sutured after removal of the uterus, clear planes of vagina must be seen anteriorly and posteriorly to avoid suturing the bladder into the vaginal cuff.
3 preventive strategies
KARRAM: For abdominal hysterectomy, I recommend 3 techniques:
- Skeletonize the infundibulopelvic ligament. Most surgeons do this routinely during the abdominal approach; I also recommend it for laparoscopic hysterectomy. Once there is a window in the broad ligament and the infundibulopelvic ligament is skeletonized, one can be sure the ureter is well below this area and probably out of harm’s way.
- Use sharp dissection to mobilize the bladder off the anterior cervix.
- Maintain awareness of the close proximity of the lower ureter to the uterosacral cardinal ligament. As the ureter enters the bladder, it can be as close as 1 cm lateral to the uterosacral ligament. This is an area where it is almost impossible to dissect out the ureter, so the surgeon needs to appreciate this anatomy and refrain from taking aggressive bites in the lateral direction when supporting or closing the vaginal cuff.
How can a surgeon prevent bladder or ureteral injury during laparoscopic hysterectomy?
BARBER: I think the ureter is best identified by direct visualization transperitoneally. The angle of the laparoscope makes visualizing the ureter much easier than from an abdominal approach, so retroperitoneal dissection is not necessary as often.
If the course of the ureter is not readily identified by direct transperitoneal visualization, a peritoneal incision can be made below and parallel to the infundibulopelvic ligament, which allows entry into the retroperitoneum and, typically, easy visualization of the ureter throughout its course.
Alternatively, the retroperitoneum can be entered lateral to the infundibulopelvic ligament, and the ureter can be identified in the same manner as in abdominal hysterectomy.
If laparoscopically assisted hysterectomy is planned, I prefer to dissect the bladder flap vaginally rather than laparoscopically, as the risk of bladder injury is considerably lower from a vaginal approach than it is laparoscopically. Obviously, if a total laparoscopic hysterectomy is necessary because of poor vaginal access, laparoscopic bladder flap dissection is necessary. In this case, I again favor sharp dissection and minimal use of cautery to avoid bladder injury.
How can a surgeon prevent bladder or ureteral injury during vaginal hysterectomy?
BENT: Traditional methods in which each clamp is rolled off the cervix or uterus until the procedure is completed help keep unsuspecting surgeons out of the bladder and away from the ureter. The only risk involves bladder mobilization (ie, creation of the bladder flap), which should always be done sharply to prevent bladder perforation. Avoid blunt finger or sponge-stick dissection! Knowing how to sharply dissect the bladder flap is vital—then even cases of prior cesarean section are manageable.
Salpingo-oophorectomy can also proceed under direct vision. Avoid the ureter by making sure the clamp closes only over the pedicles of the tube and ovary, with no intervening tissues in the clamp. If space is very tight, divide the round ligament and take the pedicle in a smaller bite. Traction on the cervix during the procedure, and mobilization of the bladder, allow the ureters to slide upward, well out of harm’s way, as the procedure progresses.
The importance of sharp dissection
BARBER: During vaginal hysterectomy, I usually have the operative assistant hold the cervical tenaculum so that there is tension on the uterus. I then use forceps to elevate the bladder directly vertically in order to place the bladder fibers on tension. Next, I dissect the bladder off the cervix and lower uterine segment using sharp dissection, and identify the peritoneum by direct finger palpation. Almost always, it is smooth and slippery.
After identifying the peritoneum, I grasp it with a tonsil clamp and elevate it so that it can be entered easily with scissors. I always confirm peritoneal entry by visualizing and identifying intraperitoneal structures such as bowel fat, the uterine serosal surface, or adnexae.
Some people advocate palpating the ureter during vaginal cases.
CUNDIFF: The most common time of injury during vaginal hysterectomy is during dissection of the vesicovaginal space; and suture ligation of the infundibulopelvic ligaments and uterine arteries carries the greatest potential for ureteral injury.
During vaginal hysterectomy, I try to dissect the vesicovaginal space early. I use a Deever retractor to retract the bladder anteriorly, maximizing my ability to sharply dissect close to the cervix until entering the peritoneal cavity. Once I’m in the peritoneal cavity, I advance the Deever retractor to protect the bladder through the rest of the procedure. I maximize protection of the ureters by applying downward traction on the cervix during vaginal clamp placement.
KARRAM: I agree. Never try to enter the anterior cul-de-sac until the vesicouterine space has been identified and is easily palpated. Rushing to enter the anterior cul-de-sac will only lead to inadvertent cystotomy.
Assessing ureteral patency
After what pelvic surgeries do you think ureteral patency should be assessed, and how should it be accomplished?
CUNDIFF: The literature contains several studies10-13 that involved universal endoscopy of the lower urinary tract. These studies demonstrate that most injuries are not recognized by the surgeon prior to endoscopy. In fact, the vast majority of injuries occur after straightforward hysterectomies. This may be due in part to the sheer volume of hysterectomies, compared with other pelvic surgeries. However, it also shows that, when lower urinary tract evaluation is performed solely when the surgeon suspects an injury, a substantial proportion of injuries are missed.
KARRAM: When do you assess ureteral patency?
CUNDIFF: My personal practice is to evaluate it in all cases that carry the potential for injury to the ureter. The complexity of the evaluation is proportional to the probability of ureteral injury.
For all laparotomies and laparoscopies, I identify the course of the ureter and confirm peristalsis. In the simplest of cases, this can be done by identifying the ureter beneath the peritoneum as it crosses the pelvic brim and courses across the pelvic sidewall. More frequently, it involves opening the pararectal space to identify the course of the ureter. In the most complex cases, it requires ureterolysis.
I strongly believe that identifying the ureter during any dissection that endangers it is the best way to avoid injury. Even after these precautions, I frequently perform cystoscopy with intravenous indigo carmine to confirm ureteral patency. This is my standard approach with all vaginal procedures, as I am not confident that I can palpate the course of the ureter.
Postoperative cystoscopy is virtually without morbidity and adds no more than 3 minutes to the procedure when properly planned. It also affords an excellent opportunity to train residents in cystoscopy.
How to assess patency after selected procedures
BENT: Ureteral patency should be assured after any repair of the pelvic floor.
After abdominal hysterectomy, if there is blood in the catheter bag or any difficulty has been encountered during surgery, I perform cystoscopy after injecting indigo carmine dye, to observe ureteral function.
At abdominal or laparoscopic sacrocolpopexy, I follow the path of the ureters over the pelvic brim and inferiorly to the adnexal area by direct inspection.
During abdominal or laparoscopic paravaginal repair, the Burch procedure, or uterosacral ligament suspension, I perform cystoscopy after tying sutures and injecting indigo carmine to ensure ureteral function.
To safeguard the ureter, follow its course
Vaginal hysterectomy is not usually associated with ureteral injury, and very uncommonly with bladder injury. However, if bladder injury is observed or the procedure has been difficult, it is wise to perform cystoscopy with dye injection. This also extends to traditional cystocele repair.
I also recommend cystoscopy with dye injection any time there is a vaginal approach to paravaginal defect repair, vault suspension, or colpocleisis.
Cystoscopy—safe, simple, and efficient
KARRAM: For vaginal surgery, I think cystoscopy is the simplest way to assess the lower urinary tract. I routinely use it after any procedures involving the posterior cul-de-sac such as McCall culdoplasty or vaginal vault suspension from the uterosacral ligaments. I also use it routinely after advanced prolapse repairs involving anterior colporrhaphy, as well as paravaginal defect repairs, and I certainly use it routinely after any lower urinary tract reconstructive procedures such as fistula repairs.
As for laparoscopic surgery, I think cystoscopy is again the most efficient way to assess the lower urinary tract. I do so after any retropubic suspension, be it a Burch colposuspension or a paravaginal repair, any type of vault suspension or sacrocolpopexy, and any type of hysterectomy or adnexectomy that involves dissection of the retroperitoneal space. I also do so if an energy source was used extensively in the vicinity of the retroperitoneal space on either side.
After abdominal surgery, it is probably more efficient (assuming the patient is not in stirrups) to perform a high extraperitoneal cystotomy or suprapubic telescopy. The indications are any difficult dissection in which I have concerns about ureteral patency, as well as any abdominal prolapse or anti-incontinence procedures.
Ureteral stenting
Is ureteral stenting ever indicated preoperatively? If so, when?
BARBER: I do not think ureteral stenting is indicated routinely for any procedure. There may be individual cases where a stent may help the surgeon avoid ureteral injury, but I can’t think of a procedure in which it should be routinely used.
CUNDIFF: I agree. Although ureteral stenting is an important tool for the pelvic floor surgeon to investigate potential ureteral obstruction, I think it has very limited value as a preoperative maneuver to avoid injury. My opinion is based on the following observations:
The surgeon cannot really assess the potential difficulty of identifying the course of the ureter until the peritoneal cavity is entered.
For the truly hostile pelvis, in which pelvic sidewall pathology prevents identification of the course of the ureter, I do not find that a stent facilitates dissection of the pararectal space and ureterolysis. In fact, it could increase the chance of ureteral injury by creating a backboard against which to cut it during dissection.
Any potential benefit of ureteral stents—which I believe is minimal—must be balanced against the potential risks, which include 20 to 30 minutes of added OR time and the risk of ureteral spasm or perforation.
BENT: I also agree that ureteral stenting is seldom helpful during gynecologic surgery. At laparotomy, direct dissection of the structures and exposure of the ureter are best; there is no need to feel for a ureter.
At laparoscopy, however, if there are large fibroids, scarring from endometriomas, or adnexal masses, then preoperative placement of lighted stents can help the surgeon identify the ureters during dissection. The case would still require dissection of the ureter away from the operative field, but the lighted path provides a starting point in this procedure.
For vaginal surgical procedures, it is easier to avoid the ureter. However, not all surgeons can palpate a nonstented ureter, which may be required during a high uterosacral ligament suspension. A stent can readily take the surgeon to the ureter and avoid injury in most cases. This may be helpful for less experienced operators.
What should residents be trained to do?
Do most obstetrics and gynecology residency programs appropriately train young physicians to evaluate and manage lower urinary tract injury during pelvic surgery?
CUNDIFF: I am afraid not. Although residency directors increasingly recognize the importance of educating doctors to prevent and manage these injuries, this recognition has not yet risen to the policy level.
For example, the Council on Resident Education in Obstetrics and Gynecology (CREOG) includes a bladder surgery educational model that necessitates dissection of the ureter, cystoscopy, ureteral stenting, bladder repair, and ureteral reanastomosis.
However, the CREOG surgical curriculum makes no mention of protecting or evaluating the lower urinary tract during pelvic surgery.
This spectrum seems to reflect the wide variation among residency programs, too. While some programs such as ours at Johns Hopkins provide comprehensive training in prevention, evaluation, and management of lower urinary tract injury, many others do not. This might be because some programs lack technically skilled faculty. Interdisciplinary politics also likely influences local credentialing.
BARBER: In my opinion, a graduating ObGyn resident should be able to:
- identify and mobilize the ureter to avoid injury during abdominal and laparoscopic surgery
- safely mobilize the bladder during abdominal, vaginal, or laparoscopic hysterectomy
- perform intraoperative cystoscopy to evaluate for injury
- repair bladder injuries abdominally and vaginally
However, I don’t think it is realistic for a graduating resident to be able to manage ureteral injuries, as residents are unlikely to encounter very many during training. These injuries are best left to our urology colleagues.
BENT: Very little or no education is provided in preventing lower urinary tract injury; evaluation is better managed in many programs. A conservative estimate is that 20% of programs have a reasonable curriculum for preoperative assessment and evaluation of incontinence and prolapse.
The management of pelvic floor disorders is better handled in almost all programs, especially as it relates to surgery. Many residents spend a lot of time on the urogynecology service and are exposed to the surgical aspect of rotations.
Dr. Karram and Dr. Barber have no financial relationships relevant to this article. Dr. Bent serves on the gynecology advisory board of ACMI and is a speaker for Novartis, Pfizer, Watson, and Asetellas (formerly Yamanouchi). He also has received research funding from Cook, Eli Lilly, and Mentor; and is a consultant for C.R. Bard. Dr. Cundiff has received grant/research support from Cook, is a consultant to C.R. Bard and Eli Lilly, and is a speaker for GlaxoSmithKline.
1. Karram MM, Segal JL, Vassallo BJ, Kleeman SD. Complications and untoward effects of the tension-free vaginal tape procedure. Obstet Gynecol 2003;101:929-932.
2. Rooney C, Crawford A, Vassaco B, Kleeman S, Karram M. Is cesarean section a risk factor for incidental cystotomy at the time of hysterectomy? Am J Obstet Gynecol. In press.
3. Samson JA. Ligation and clamping of the ureter as complications of surgical operations. Am Med. 1902;4:693.-
4. Wiskind AK, Thompson JD. Should cystoscopy be performed at every gynecologic operation to diagnose unsuspected ureteral injury? J Pelvic Surg. 1995;1:134-137.
5. St. Martin EC, et al. Ureteral injury in gynecologic surgery. J Urol. 1953;70:51-57.
6. Conger K, Beecham CT, Horrax TM. Ureteral injury in pelvic surgery: current thought on incidence, pathogenesis, prophylaxis and treatment. Obstet Gynecol. 1954;3:343-357.
7. Mann WJ, Arato M, Patsner B, et al. Ureteral injuries in an obstetrics and gynecology training program: etiology and management. Obstet Gynecol. 1988;72:82-85.
8. Stanhope CR, Wilson TO, Utz WJ, Smith LH, O’Brien PC. Suture entrapment and secondary ureteral obstruction. Am J Obstet Gynecol. 1991;164:1513-1519.
9. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
10. Harris RL, Cundiff GW, Theofrastous JT, Yoon HW, Bump RC, Addison WA. The value of intraoperative cystoscopy in urogynecologic and reconstructive pelvic surgery. Am J Obstet Gynecol. 1997;177:1367-1369.
11. Gill EJ, Elser DM, Bonidie MJ, Roberts KM, Hurt WG. The routine use of cystoscopy with the Burch procedure. Am J Obstet Gynecol. 2002;186:1108.-
12. Kwon CH, Goldberg RO, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187:1466-1471; discussion 1471-1472.
13. Tulikangas PK, Weber AM, et al. Intraoperative cystoscopy in conjunction with anti-incontinence surgery. Obstet Gynecol. 2000;95(6 Pt 1):794-796.
1. Karram MM, Segal JL, Vassallo BJ, Kleeman SD. Complications and untoward effects of the tension-free vaginal tape procedure. Obstet Gynecol 2003;101:929-932.
2. Rooney C, Crawford A, Vassaco B, Kleeman S, Karram M. Is cesarean section a risk factor for incidental cystotomy at the time of hysterectomy? Am J Obstet Gynecol. In press.
3. Samson JA. Ligation and clamping of the ureter as complications of surgical operations. Am Med. 1902;4:693.-
4. Wiskind AK, Thompson JD. Should cystoscopy be performed at every gynecologic operation to diagnose unsuspected ureteral injury? J Pelvic Surg. 1995;1:134-137.
5. St. Martin EC, et al. Ureteral injury in gynecologic surgery. J Urol. 1953;70:51-57.
6. Conger K, Beecham CT, Horrax TM. Ureteral injury in pelvic surgery: current thought on incidence, pathogenesis, prophylaxis and treatment. Obstet Gynecol. 1954;3:343-357.
7. Mann WJ, Arato M, Patsner B, et al. Ureteral injuries in an obstetrics and gynecology training program: etiology and management. Obstet Gynecol. 1988;72:82-85.
8. Stanhope CR, Wilson TO, Utz WJ, Smith LH, O’Brien PC. Suture entrapment and secondary ureteral obstruction. Am J Obstet Gynecol. 1991;164:1513-1519.
9. Vakili B, Chesson RR, Kyle BL, et al. The incidence of urinary tract injury during hysterectomy: a prospective analysis based on universal cystoscopy. Am J Obstet Gynecol. 2005;192:1599-1604.
10. Harris RL, Cundiff GW, Theofrastous JT, Yoon HW, Bump RC, Addison WA. The value of intraoperative cystoscopy in urogynecologic and reconstructive pelvic surgery. Am J Obstet Gynecol. 1997;177:1367-1369.
11. Gill EJ, Elser DM, Bonidie MJ, Roberts KM, Hurt WG. The routine use of cystoscopy with the Burch procedure. Am J Obstet Gynecol. 2002;186:1108.-
12. Kwon CH, Goldberg RO, Koduri S, Sand PK. The use of intraoperative cystoscopy in major vaginal and urogynecologic surgeries. Am J Obstet Gynecol. 2002;187:1466-1471; discussion 1471-1472.
13. Tulikangas PK, Weber AM, et al. Intraoperative cystoscopy in conjunction with anti-incontinence surgery. Obstet Gynecol. 2000;95(6 Pt 1):794-796.
The evidence-based way to prevent wound infections
How can we apply all possible precautions to every patient wheeled into the OR? The CDC’s Guideline for Prevention of Surgical Site Infections (formerly termed wound infections) advocates “a systematic but realistic approach” based on the evidence, coupled with awareness that risk of surgical site infection is influenced by characteristics of the patient, operation, personnel, and hospital.
This article reviews key evidence behind a number of the most strongly recommended measures, such as optimal regimens for prophylactic antibiotics, and some of the recommendations for which equally rigorous evidence is lacking.
The CDC’s Guideline ranks its recommendations according to 4 levels of evidence. A total of 49 recommendations meet the most rigorous evidence standards, and therefore are “strongly recommended for all hospitals.” (See How strong is the evidence?.)
Many of our infection prevention routines, of course, have been standard ever since Joseph Lister introduced the principles of antisepsis in the late 1860s. Technically, however, some standard infection prevention routines are based on a strong theoretical rationale along with suggestive though not confirmatory science.
By necessity, narrowly defined patient populations and ethical and logistical issues will always limit our ability to obtain confirmatory scientific answers to some questions. For example, wearing gloves vs not wearing gloves fits into that category. Likewise, the evidence on preoperative nutritional support for the sole purpose of preventing SSI does not meet the criteria for the best evidence category, “1A.” Yet, nutrition therapy is among the CDC’s recommendations, albeit the evidence behind it falls into the “NR” category, “no recommendation; unresolved issue.”
The CDC’s exhaustive guideline identifies 21 characteristics of patients and operations that influence a patient’s risk of surgical site infection (TABLE 1), and recommends prevention tactics that are backed by evidence (See CDC Advisory).
The CDC’s recommendations are grouped into these sections:
1. Preoperative preparation of the patient, hand/forearm antisepsis for surgical team members, management of infected or colonized surgical personnel, and antimicrobial prophylaxis.
2. Intraoperative ventilation, cleaning and disinfection of environmental surfaces, microbiologic sampling, sterilization of surgical instruments, surgical attire and drapes, asepsis, and surgical technique.
3. Postoperative incision care.
4. Surveillance.
TABLE 1
21 factors that influence risk of surgical site infection
| PATIENT |
| 1 Age |
| 2 Nutritional status |
| 3 Diabetes |
| 4 Smoking |
| 5 Obesity |
| 6 Coexistent infections at remote body site |
| 7 Colonization with microorganisms |
| 8 Altered immune response |
| 9 Length of preoperative stay |
| OPERATION |
| 10 Duration of surgical scrub |
| 11 Skin antisepsis |
| 12 Preoperative shaving |
| 13 Duration of operation |
| 14 Antimicrobial prophylaxis |
| 15 Operating room ventilation |
| 16 Inadequate sterilization of instruments |
| 17 Foreign material in the surgical site |
| 18 Surgical drains |
| 19 Poor hemostasis |
| 20 Failure to obliterate dead space |
| 21 Tissue trauma |
| Source: Reference 1. |
Preparing The Patient
Preoperative risk factors
Infection prevention begins with considering the preoperative risk factors of the patient’s condition.
Not all risk factors for surgical site infections can be modified (age, for example), but we should correct whatever we can before scheduling elective surgery.
Minimizing smoking improves postoperative SSI outcomes(EVIDENCE CATEGORY IB).
Weight loss before surgery has not been clearly correlated with improved SSI outcomes (EVIDENCE CATEGORY NR). However, body mass index may influence surgical complication rates, perhaps acting as a surrogate for technical difficulty or impaired wound-healing capacity.2,3
Nutritionis being recognized as a key determinant in outcomes, but reports have not established how preoperative parenteral or enteral nutrition influences SSI outcome (NO RECOMMENDATION).4,5
Antisepsis in the surgical field
The microbial source for most SSI is the patient’s endogenous flora, and the operative field determines the type of flora that will be encountered.
Normal skin flora consist mostly of gram-positive aerobes.
Antiseptic showering before surgery significantly reduces resident skin flora (EVIDENCE CATEGORY IB). Multiple showers with chlorhexidine have been shown to reduce resident bacteria up to 9-fold, but whether that reduces SSI rates is unclear.6
Prophylactic eradication of nasal Staph colonization (NO RECOMMENDATION). Recent attention has focused on microbial colonization with resistant organisms—and Staphylococcus aureus colonization of nares in cardiac surgery patients was found to be a major independent risk factor for surgical site infection.
Prophylactic intranasal mupirocin reduced infection risk in cardiothoracic patients,7 but preoperative use did not reduce gram-positive SSI rates in digestive tract surgery.8
Mupirocin also failed to reduce the wound rates in patients who had a variety of procedures, although the rate of nosocomial S aureus infections in the subset of patients with nasal colonization was reduced.9
Topical microbicides
Soap-and-water washing removes most debris from skin or other surgical surfaces, but antiseptic solutions reduce resident skin flora populations. The choice of appropriate topical microbicides during surgery can influence SSI rates (EVIDENCE CATEGORY IB).
When selecting an antiseptic, consider the anticipated duration of the case, the epithelial surface to be breeched (mucous membrane vs keratinized skin), and the anticipated flora.
Best practices for preventing surgical site infections
Recommended for all hospitals
EVIDENCE CATEGORY IA—Well-designed studies
- Cancel elective surgery if the patient has a remote infection
- Achieve maximal subcutaneous concentration of preoperative antibiotics
- Avoid routine vancomycin and similar agents
- Maintain prophylactic antibiotics for only a few hours after closing incisions
- For high-risk cesarean, administer the prophylactic antimicrobial immediately after the umbilical cord is clamped
- If it is necessary to remove hair, use clippers, not shaving, immediately before the operation.
EVIDENCE CATEGORY IB—Good evidence and expert consensus
- Control glucose levels and avoid perioperative hyperglycemia
- Encourage patients to quit or minimize smoking
- Require the patient to shower or bathe with an antiseptic agent
- Surgical hand hygiene to include scrub to elbows for 2- to 5-min, use sterile towel, keep fingernails short, clean under fingernails
- Use appropriate topical microbicides during surgery
- Pay careful attention to proper surgical technique
We still don’t know
NO RECOMMENDATION; UNRESOLVED ISSUE—Evidence is insufficient
- Enhance nutritional support solely to prevent SSI?
- Discontinue or taper steroids if medically permissible?
- Measures to enhance wound space oxygenation?
- Preoperatively apply mupirocin to nares?
The complete Guideline for Prevention of Surgical Site Infections is available online at www.cdc.gov/ncidod/hip/SSI/SSI_guideline.htm.1
Shaving and hair removal
Hair removal is often necessary, but shaving may cause skin trauma that exacerbates bacterial growth.10 SSI rates correlate with the time interval between shaving and incision (20% if shaved >24 hours before surgery, 7.1% the night before, and 3.1% in the OR).11 Thus, the CDC guidelines discourage shaving prior to surgery (EVIDENCE CATEGORY IA).Patients have been known to shave the operative area themselves before surgery, so all patients must be told not to shave themselves before elective surgery.
When hair removal is necessary, preoperative clipping causes minimal skin trauma (EVIDENCE CATEGORY IA).
Preparing The Surgical Staff
The surgeon’s hands
Evidence has shown that 2 minutes of preoperative scrubbing reduces resident flora as effectively as scrubbing for 10 minutes.1 The recommended scrub should include hands and forearms up to the elbows for 2 to 5 minutes (EVIDENCE CATEGORY IB).
Keep hands away from the body and dry hands with a sterile towel (EVIDENCE CATEGORY IB).
Keep fingernails short (EVIDENCE CATEGORY IB), and clean under each nail at the beginning of each day (EVIDENCE CATEGORY II).
An aqueous alcohol solution is a recent alternative to traditional hand antisepsis with chlorhexidine- or povoidoneiodine–based solutions. No difference in SSI rates has been documented between hand-rubbing with an aqueous alcohol solution and traditional scrubbing.12 A traditional scrub before the first of consecutive cases and after contact with gross contamination is still in order.
Sterile barriers
Sterile barriers in the operating room, indispensable in protecting staff, are federally mandated. Their role in preventing SSI is not clear. Surprisingly, the use of face masks may not contribute to SSI reduction.13 Head covering, on the other hand, markedly reduces airborne and wound bacterial contamination.14
Optimize Wound Physiology
Maintaining normothermia
Hypothermia is common, particularly in patients who are immunocompromised, at age extremes, or have multiple trauma. Hypothermic vasoconstriction may reduce tissue perfusion and increase risk of infection.
A double-blind study showed that maintaining intraoperative normothermia decreased SSI in colorectal patients from 19% to 6%.15 Additionally, preoperative warming of the entire body or local site for 30 minutes reduced SSI rates in clean surgical cases.16
Wound space oxygenation
Supplemental oxygen in colorectal surgery may correlate with lower infection rates (80% without supplemental oxygen, 30% with).17 This may improve tissue oxygen tension, which enhances oxidative bactericidal capacity.
However, these findings were not duplicated in patients with higher SSI rates and on supplemental hyperoxia.18 There are no recommendations for enhancing wound space oxygenation.
Control of glycemia
Cardiothoracic surgery studies have stressed the importance of tight perioperative glycemic control. Coronary artery bypass patients with higher mean perioperative glucose showed a trend toward a higher risk of nosocomial infection, but not specifically SSI.19 Another study of cardiothoracic patients found an association between higher risk of SSI and both diabetes and postoperative hyperglycemia.20 Continuous intravenous insulin to maintain a blood glucose 21
Remote infections
Remote infections at the time of surgery, such as urinary tract infection or pneumonia, significantly raise the risk of SSI (EVIDENCE CATEGORY IA).
Strongly consider canceling elective surgery if there is an untreated remote infection, especially if implanting bioprosthetic material.
Surgical technique
Careful technique reduces risk of infection.
Breaks in sterile technique and gross spillage of enteric contents raise the risk for SSI through increased bacterial load.
Poor hemostasis, excess tissue trauma, inadequate debridement or dead space obliteration, and inappropriate suture technique raise the volume of unperfused biological matter (EVIDENCE CATEGORY IB).
Timely completion of the operation also minimizes risk. Prolonged operative time can heighten the risk of breaches in sterile technique. Recommendations call for procedures to be completed within the 75th percentile of standardized operative times.
Antimicrobial prophylaxis
The principles for using preoperative antibiotics include maximal subcutaneous concentration when making the incision (TABLE 2) (EVIDENCE CATEGORY IA). This corresponds with intravenous antimicrobial administration within 60 minutes before incision (or within 120 minutes for vancomycin or fluoroquinolones). An additional dose of the antimicrobial agent is indicated if the procedure time exceeds 2 half-lives of the agent.
Institutional policies for antibiotic restriction aimed at curtailing resistant organisms do not appear to change the spectrum of causative microbes in SSI.22 Short-duration therapy preserves antimicrobial efficacy best, so avoid the routine use of agents such as vancomycin (EVIDENCE CATEGORY IB).
Short duration also applies when antimicrobial prophylaxis is indicated. The CDC recommends extending antimicrobial prophylaxis no more than a few hours after incision closure (EVIDENCE CATEGORY IA). Particular cases may require longer antimicrobial prophylaxis, but prophylaxis beyond 24 hours does not reduce SSI rates and increases the potential for microbial resistance.
While a single dose of broad-spectrum antibiotic may cause Clostridium difficile colitis, prolonged duration also raises risk through profound changes in gut flora that favor the emergence of this opportunistic pathogen.
Category IA. Strongly recommended for all hospitals and strongly supported by well-designed experimental or epidemiologic studies.
Category IB. Strongly recommended for all hospitals and viewed as effective by experts in the field and a consensus of Hospital Infection Control Practices Advisory Committee (HICPAC), based on strong rationale and suggestive evidence, even though definitive scientific studies may not have been done.
Category II. Suggested for implementation in many hospitals. Recommendations may be supported by suggestive clinical or epidemiologic studies, a strong theoretical rationale, or definitive studies applicable to some, but not all, hospitals.
No recommendation; unresolved issue (NR). Insufficient evidence or no consensus regarding efficacy.
Principles of antimicrobial prophylaxis
| Consider these factors: |
| Risk for developing surgical site infection. |
| Potential severity of consequences |
| Prosthetic implantation |
| Cardiothoracic or vascular surgery |
| Agents must be safe, inexpensive, and bactericidal |
| Appropriate spectrum based on anticipated flora of involved tissues and spaces |
| Administer so that maximal effect is at time of incision, and re-administer when appropriate |
| Alter dosage as appropriate for the patient (eg, obesity) |
This article is adapted from DiRocco JD, Pavone LA, Weiss CA III. The evidence-based way to prevent SSI. Contemp Surg. 2005;61:120–127.
1. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-134.
2. Holzwarth R, Huber D, Majkrzak A, Tareen B. Outcome of gastric bypass patients. Obes Surg. 2002;12:261-264.
3. Christou NV, Jarand J, Sylvestre JL, McLean AP. Analysis of the incidence and risk factors for wound infections in open bariatric surgery. Obes Surg. 2004;14:16-22.
4. Muller JM, Brenner U, Dienst C, et al. Preoperative parenteral feeding in patients with gastrointestinal carcinoma. Lancet. 1982;1:68-71.
5. Holter A, Fischer JE. The effects of perioperative hyperalimentation on complications in patients with carcinoma and weight loss. J Surg Res. 1977;23:31-34.
6. Garibaldi RA. Prevention of intraoperative wound contamination with chlorhexidine shower and scrub. J Hosp Infect. 1988;11:5-9.
7. Kluytmans JA, Mouton JW, VandenBergh MF, et al. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 1996;17:780-785.
8. Suzuki Y, Kamigaki T, Fujino M, et al. Randomized clinical trial of preoperative intranasal mupirocin to reduce surgical-site infection after digestive surgery. Br J Surg. 2003;90:1072-1075.
9. Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871-1877.
10. Hamilton HW, Hamilton KR, Lone FJ. Preoperative hair removal. Can J Surg. 1977;20:269-273.
11. Seropian R, Reynolds BM. Wound infection after preoperative depilatory versus razor preparation. Am J Surg. 1971;121:251-254.
12. Parienti JJ, Thibon P, Heller R, et al. Hand-rubbing with an aqueous alcoholic solution vs traditional surgical hand-scrubbing and 30-day surgical site infection rates. JAMA. 2002;288:722-727.
13. Tunevall TG. Postoperative wound infections and surgical face masks: a controlled study. World J Surg. 1991;15:383-388.
14. Friberg B, Friberg S, Ostenson R, Burman LG. Surgical area contamination: comparable bacterial counts using disposable head and mask and helmet aspirator system, but dramatic increase upon omission of headgear: an experimental study in horizontal laminar airflow. J Hosp Infect. 2001;47:110-115.
15. Kurz A, Sessler DI, Lenhardt R. Perioperative normoth-ermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-1215.
16. Melling AC, Ali B, Scott EM, Leaper DJ. Effects of pre-operative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358:876-880.
17. Greif R, Akça O, Horn EP, et al. Supplemental perioper-ative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161-167.
18. Pryor KO, Fahey TJ, III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population. JAMA. 2004;291:79-87.
19. Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408-1414.
20. Latham R, Lancaster AD, Covington JF, et al. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607-612.
21. Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 2000;69:667-668.
22. Weiss CA, III, Statz CL, Dahms RA, et al. Six Years of Surgical Wound Infection Surveillance at a Tertiary Care Center. Arch Surg. 1999;134:1041-1048.
How can we apply all possible precautions to every patient wheeled into the OR? The CDC’s Guideline for Prevention of Surgical Site Infections (formerly termed wound infections) advocates “a systematic but realistic approach” based on the evidence, coupled with awareness that risk of surgical site infection is influenced by characteristics of the patient, operation, personnel, and hospital.
This article reviews key evidence behind a number of the most strongly recommended measures, such as optimal regimens for prophylactic antibiotics, and some of the recommendations for which equally rigorous evidence is lacking.
The CDC’s Guideline ranks its recommendations according to 4 levels of evidence. A total of 49 recommendations meet the most rigorous evidence standards, and therefore are “strongly recommended for all hospitals.” (See How strong is the evidence?.)
Many of our infection prevention routines, of course, have been standard ever since Joseph Lister introduced the principles of antisepsis in the late 1860s. Technically, however, some standard infection prevention routines are based on a strong theoretical rationale along with suggestive though not confirmatory science.
By necessity, narrowly defined patient populations and ethical and logistical issues will always limit our ability to obtain confirmatory scientific answers to some questions. For example, wearing gloves vs not wearing gloves fits into that category. Likewise, the evidence on preoperative nutritional support for the sole purpose of preventing SSI does not meet the criteria for the best evidence category, “1A.” Yet, nutrition therapy is among the CDC’s recommendations, albeit the evidence behind it falls into the “NR” category, “no recommendation; unresolved issue.”
The CDC’s exhaustive guideline identifies 21 characteristics of patients and operations that influence a patient’s risk of surgical site infection (TABLE 1), and recommends prevention tactics that are backed by evidence (See CDC Advisory).
The CDC’s recommendations are grouped into these sections:
1. Preoperative preparation of the patient, hand/forearm antisepsis for surgical team members, management of infected or colonized surgical personnel, and antimicrobial prophylaxis.
2. Intraoperative ventilation, cleaning and disinfection of environmental surfaces, microbiologic sampling, sterilization of surgical instruments, surgical attire and drapes, asepsis, and surgical technique.
3. Postoperative incision care.
4. Surveillance.
TABLE 1
21 factors that influence risk of surgical site infection
| PATIENT |
| 1 Age |
| 2 Nutritional status |
| 3 Diabetes |
| 4 Smoking |
| 5 Obesity |
| 6 Coexistent infections at remote body site |
| 7 Colonization with microorganisms |
| 8 Altered immune response |
| 9 Length of preoperative stay |
| OPERATION |
| 10 Duration of surgical scrub |
| 11 Skin antisepsis |
| 12 Preoperative shaving |
| 13 Duration of operation |
| 14 Antimicrobial prophylaxis |
| 15 Operating room ventilation |
| 16 Inadequate sterilization of instruments |
| 17 Foreign material in the surgical site |
| 18 Surgical drains |
| 19 Poor hemostasis |
| 20 Failure to obliterate dead space |
| 21 Tissue trauma |
| Source: Reference 1. |
Preparing The Patient
Preoperative risk factors
Infection prevention begins with considering the preoperative risk factors of the patient’s condition.
Not all risk factors for surgical site infections can be modified (age, for example), but we should correct whatever we can before scheduling elective surgery.
Minimizing smoking improves postoperative SSI outcomes(EVIDENCE CATEGORY IB).
Weight loss before surgery has not been clearly correlated with improved SSI outcomes (EVIDENCE CATEGORY NR). However, body mass index may influence surgical complication rates, perhaps acting as a surrogate for technical difficulty or impaired wound-healing capacity.2,3
Nutritionis being recognized as a key determinant in outcomes, but reports have not established how preoperative parenteral or enteral nutrition influences SSI outcome (NO RECOMMENDATION).4,5
Antisepsis in the surgical field
The microbial source for most SSI is the patient’s endogenous flora, and the operative field determines the type of flora that will be encountered.
Normal skin flora consist mostly of gram-positive aerobes.
Antiseptic showering before surgery significantly reduces resident skin flora (EVIDENCE CATEGORY IB). Multiple showers with chlorhexidine have been shown to reduce resident bacteria up to 9-fold, but whether that reduces SSI rates is unclear.6
Prophylactic eradication of nasal Staph colonization (NO RECOMMENDATION). Recent attention has focused on microbial colonization with resistant organisms—and Staphylococcus aureus colonization of nares in cardiac surgery patients was found to be a major independent risk factor for surgical site infection.
Prophylactic intranasal mupirocin reduced infection risk in cardiothoracic patients,7 but preoperative use did not reduce gram-positive SSI rates in digestive tract surgery.8
Mupirocin also failed to reduce the wound rates in patients who had a variety of procedures, although the rate of nosocomial S aureus infections in the subset of patients with nasal colonization was reduced.9
Topical microbicides
Soap-and-water washing removes most debris from skin or other surgical surfaces, but antiseptic solutions reduce resident skin flora populations. The choice of appropriate topical microbicides during surgery can influence SSI rates (EVIDENCE CATEGORY IB).
When selecting an antiseptic, consider the anticipated duration of the case, the epithelial surface to be breeched (mucous membrane vs keratinized skin), and the anticipated flora.
Best practices for preventing surgical site infections
Recommended for all hospitals
EVIDENCE CATEGORY IA—Well-designed studies
- Cancel elective surgery if the patient has a remote infection
- Achieve maximal subcutaneous concentration of preoperative antibiotics
- Avoid routine vancomycin and similar agents
- Maintain prophylactic antibiotics for only a few hours after closing incisions
- For high-risk cesarean, administer the prophylactic antimicrobial immediately after the umbilical cord is clamped
- If it is necessary to remove hair, use clippers, not shaving, immediately before the operation.
EVIDENCE CATEGORY IB—Good evidence and expert consensus
- Control glucose levels and avoid perioperative hyperglycemia
- Encourage patients to quit or minimize smoking
- Require the patient to shower or bathe with an antiseptic agent
- Surgical hand hygiene to include scrub to elbows for 2- to 5-min, use sterile towel, keep fingernails short, clean under fingernails
- Use appropriate topical microbicides during surgery
- Pay careful attention to proper surgical technique
We still don’t know
NO RECOMMENDATION; UNRESOLVED ISSUE—Evidence is insufficient
- Enhance nutritional support solely to prevent SSI?
- Discontinue or taper steroids if medically permissible?
- Measures to enhance wound space oxygenation?
- Preoperatively apply mupirocin to nares?
The complete Guideline for Prevention of Surgical Site Infections is available online at www.cdc.gov/ncidod/hip/SSI/SSI_guideline.htm.1
Shaving and hair removal
Hair removal is often necessary, but shaving may cause skin trauma that exacerbates bacterial growth.10 SSI rates correlate with the time interval between shaving and incision (20% if shaved >24 hours before surgery, 7.1% the night before, and 3.1% in the OR).11 Thus, the CDC guidelines discourage shaving prior to surgery (EVIDENCE CATEGORY IA).Patients have been known to shave the operative area themselves before surgery, so all patients must be told not to shave themselves before elective surgery.
When hair removal is necessary, preoperative clipping causes minimal skin trauma (EVIDENCE CATEGORY IA).
Preparing The Surgical Staff
The surgeon’s hands
Evidence has shown that 2 minutes of preoperative scrubbing reduces resident flora as effectively as scrubbing for 10 minutes.1 The recommended scrub should include hands and forearms up to the elbows for 2 to 5 minutes (EVIDENCE CATEGORY IB).
Keep hands away from the body and dry hands with a sterile towel (EVIDENCE CATEGORY IB).
Keep fingernails short (EVIDENCE CATEGORY IB), and clean under each nail at the beginning of each day (EVIDENCE CATEGORY II).
An aqueous alcohol solution is a recent alternative to traditional hand antisepsis with chlorhexidine- or povoidoneiodine–based solutions. No difference in SSI rates has been documented between hand-rubbing with an aqueous alcohol solution and traditional scrubbing.12 A traditional scrub before the first of consecutive cases and after contact with gross contamination is still in order.
Sterile barriers
Sterile barriers in the operating room, indispensable in protecting staff, are federally mandated. Their role in preventing SSI is not clear. Surprisingly, the use of face masks may not contribute to SSI reduction.13 Head covering, on the other hand, markedly reduces airborne and wound bacterial contamination.14
Optimize Wound Physiology
Maintaining normothermia
Hypothermia is common, particularly in patients who are immunocompromised, at age extremes, or have multiple trauma. Hypothermic vasoconstriction may reduce tissue perfusion and increase risk of infection.
A double-blind study showed that maintaining intraoperative normothermia decreased SSI in colorectal patients from 19% to 6%.15 Additionally, preoperative warming of the entire body or local site for 30 minutes reduced SSI rates in clean surgical cases.16
Wound space oxygenation
Supplemental oxygen in colorectal surgery may correlate with lower infection rates (80% without supplemental oxygen, 30% with).17 This may improve tissue oxygen tension, which enhances oxidative bactericidal capacity.
However, these findings were not duplicated in patients with higher SSI rates and on supplemental hyperoxia.18 There are no recommendations for enhancing wound space oxygenation.
Control of glycemia
Cardiothoracic surgery studies have stressed the importance of tight perioperative glycemic control. Coronary artery bypass patients with higher mean perioperative glucose showed a trend toward a higher risk of nosocomial infection, but not specifically SSI.19 Another study of cardiothoracic patients found an association between higher risk of SSI and both diabetes and postoperative hyperglycemia.20 Continuous intravenous insulin to maintain a blood glucose 21
Remote infections
Remote infections at the time of surgery, such as urinary tract infection or pneumonia, significantly raise the risk of SSI (EVIDENCE CATEGORY IA).
Strongly consider canceling elective surgery if there is an untreated remote infection, especially if implanting bioprosthetic material.
Surgical technique
Careful technique reduces risk of infection.
Breaks in sterile technique and gross spillage of enteric contents raise the risk for SSI through increased bacterial load.
Poor hemostasis, excess tissue trauma, inadequate debridement or dead space obliteration, and inappropriate suture technique raise the volume of unperfused biological matter (EVIDENCE CATEGORY IB).
Timely completion of the operation also minimizes risk. Prolonged operative time can heighten the risk of breaches in sterile technique. Recommendations call for procedures to be completed within the 75th percentile of standardized operative times.
Antimicrobial prophylaxis
The principles for using preoperative antibiotics include maximal subcutaneous concentration when making the incision (TABLE 2) (EVIDENCE CATEGORY IA). This corresponds with intravenous antimicrobial administration within 60 minutes before incision (or within 120 minutes for vancomycin or fluoroquinolones). An additional dose of the antimicrobial agent is indicated if the procedure time exceeds 2 half-lives of the agent.
Institutional policies for antibiotic restriction aimed at curtailing resistant organisms do not appear to change the spectrum of causative microbes in SSI.22 Short-duration therapy preserves antimicrobial efficacy best, so avoid the routine use of agents such as vancomycin (EVIDENCE CATEGORY IB).
Short duration also applies when antimicrobial prophylaxis is indicated. The CDC recommends extending antimicrobial prophylaxis no more than a few hours after incision closure (EVIDENCE CATEGORY IA). Particular cases may require longer antimicrobial prophylaxis, but prophylaxis beyond 24 hours does not reduce SSI rates and increases the potential for microbial resistance.
While a single dose of broad-spectrum antibiotic may cause Clostridium difficile colitis, prolonged duration also raises risk through profound changes in gut flora that favor the emergence of this opportunistic pathogen.
Category IA. Strongly recommended for all hospitals and strongly supported by well-designed experimental or epidemiologic studies.
Category IB. Strongly recommended for all hospitals and viewed as effective by experts in the field and a consensus of Hospital Infection Control Practices Advisory Committee (HICPAC), based on strong rationale and suggestive evidence, even though definitive scientific studies may not have been done.
Category II. Suggested for implementation in many hospitals. Recommendations may be supported by suggestive clinical or epidemiologic studies, a strong theoretical rationale, or definitive studies applicable to some, but not all, hospitals.
No recommendation; unresolved issue (NR). Insufficient evidence or no consensus regarding efficacy.
Principles of antimicrobial prophylaxis
| Consider these factors: |
| Risk for developing surgical site infection. |
| Potential severity of consequences |
| Prosthetic implantation |
| Cardiothoracic or vascular surgery |
| Agents must be safe, inexpensive, and bactericidal |
| Appropriate spectrum based on anticipated flora of involved tissues and spaces |
| Administer so that maximal effect is at time of incision, and re-administer when appropriate |
| Alter dosage as appropriate for the patient (eg, obesity) |
This article is adapted from DiRocco JD, Pavone LA, Weiss CA III. The evidence-based way to prevent SSI. Contemp Surg. 2005;61:120–127.
How can we apply all possible precautions to every patient wheeled into the OR? The CDC’s Guideline for Prevention of Surgical Site Infections (formerly termed wound infections) advocates “a systematic but realistic approach” based on the evidence, coupled with awareness that risk of surgical site infection is influenced by characteristics of the patient, operation, personnel, and hospital.
This article reviews key evidence behind a number of the most strongly recommended measures, such as optimal regimens for prophylactic antibiotics, and some of the recommendations for which equally rigorous evidence is lacking.
The CDC’s Guideline ranks its recommendations according to 4 levels of evidence. A total of 49 recommendations meet the most rigorous evidence standards, and therefore are “strongly recommended for all hospitals.” (See How strong is the evidence?.)
Many of our infection prevention routines, of course, have been standard ever since Joseph Lister introduced the principles of antisepsis in the late 1860s. Technically, however, some standard infection prevention routines are based on a strong theoretical rationale along with suggestive though not confirmatory science.
By necessity, narrowly defined patient populations and ethical and logistical issues will always limit our ability to obtain confirmatory scientific answers to some questions. For example, wearing gloves vs not wearing gloves fits into that category. Likewise, the evidence on preoperative nutritional support for the sole purpose of preventing SSI does not meet the criteria for the best evidence category, “1A.” Yet, nutrition therapy is among the CDC’s recommendations, albeit the evidence behind it falls into the “NR” category, “no recommendation; unresolved issue.”
The CDC’s exhaustive guideline identifies 21 characteristics of patients and operations that influence a patient’s risk of surgical site infection (TABLE 1), and recommends prevention tactics that are backed by evidence (See CDC Advisory).
The CDC’s recommendations are grouped into these sections:
1. Preoperative preparation of the patient, hand/forearm antisepsis for surgical team members, management of infected or colonized surgical personnel, and antimicrobial prophylaxis.
2. Intraoperative ventilation, cleaning and disinfection of environmental surfaces, microbiologic sampling, sterilization of surgical instruments, surgical attire and drapes, asepsis, and surgical technique.
3. Postoperative incision care.
4. Surveillance.
TABLE 1
21 factors that influence risk of surgical site infection
| PATIENT |
| 1 Age |
| 2 Nutritional status |
| 3 Diabetes |
| 4 Smoking |
| 5 Obesity |
| 6 Coexistent infections at remote body site |
| 7 Colonization with microorganisms |
| 8 Altered immune response |
| 9 Length of preoperative stay |
| OPERATION |
| 10 Duration of surgical scrub |
| 11 Skin antisepsis |
| 12 Preoperative shaving |
| 13 Duration of operation |
| 14 Antimicrobial prophylaxis |
| 15 Operating room ventilation |
| 16 Inadequate sterilization of instruments |
| 17 Foreign material in the surgical site |
| 18 Surgical drains |
| 19 Poor hemostasis |
| 20 Failure to obliterate dead space |
| 21 Tissue trauma |
| Source: Reference 1. |
Preparing The Patient
Preoperative risk factors
Infection prevention begins with considering the preoperative risk factors of the patient’s condition.
Not all risk factors for surgical site infections can be modified (age, for example), but we should correct whatever we can before scheduling elective surgery.
Minimizing smoking improves postoperative SSI outcomes(EVIDENCE CATEGORY IB).
Weight loss before surgery has not been clearly correlated with improved SSI outcomes (EVIDENCE CATEGORY NR). However, body mass index may influence surgical complication rates, perhaps acting as a surrogate for technical difficulty or impaired wound-healing capacity.2,3
Nutritionis being recognized as a key determinant in outcomes, but reports have not established how preoperative parenteral or enteral nutrition influences SSI outcome (NO RECOMMENDATION).4,5
Antisepsis in the surgical field
The microbial source for most SSI is the patient’s endogenous flora, and the operative field determines the type of flora that will be encountered.
Normal skin flora consist mostly of gram-positive aerobes.
Antiseptic showering before surgery significantly reduces resident skin flora (EVIDENCE CATEGORY IB). Multiple showers with chlorhexidine have been shown to reduce resident bacteria up to 9-fold, but whether that reduces SSI rates is unclear.6
Prophylactic eradication of nasal Staph colonization (NO RECOMMENDATION). Recent attention has focused on microbial colonization with resistant organisms—and Staphylococcus aureus colonization of nares in cardiac surgery patients was found to be a major independent risk factor for surgical site infection.
Prophylactic intranasal mupirocin reduced infection risk in cardiothoracic patients,7 but preoperative use did not reduce gram-positive SSI rates in digestive tract surgery.8
Mupirocin also failed to reduce the wound rates in patients who had a variety of procedures, although the rate of nosocomial S aureus infections in the subset of patients with nasal colonization was reduced.9
Topical microbicides
Soap-and-water washing removes most debris from skin or other surgical surfaces, but antiseptic solutions reduce resident skin flora populations. The choice of appropriate topical microbicides during surgery can influence SSI rates (EVIDENCE CATEGORY IB).
When selecting an antiseptic, consider the anticipated duration of the case, the epithelial surface to be breeched (mucous membrane vs keratinized skin), and the anticipated flora.
Best practices for preventing surgical site infections
Recommended for all hospitals
EVIDENCE CATEGORY IA—Well-designed studies
- Cancel elective surgery if the patient has a remote infection
- Achieve maximal subcutaneous concentration of preoperative antibiotics
- Avoid routine vancomycin and similar agents
- Maintain prophylactic antibiotics for only a few hours after closing incisions
- For high-risk cesarean, administer the prophylactic antimicrobial immediately after the umbilical cord is clamped
- If it is necessary to remove hair, use clippers, not shaving, immediately before the operation.
EVIDENCE CATEGORY IB—Good evidence and expert consensus
- Control glucose levels and avoid perioperative hyperglycemia
- Encourage patients to quit or minimize smoking
- Require the patient to shower or bathe with an antiseptic agent
- Surgical hand hygiene to include scrub to elbows for 2- to 5-min, use sterile towel, keep fingernails short, clean under fingernails
- Use appropriate topical microbicides during surgery
- Pay careful attention to proper surgical technique
We still don’t know
NO RECOMMENDATION; UNRESOLVED ISSUE—Evidence is insufficient
- Enhance nutritional support solely to prevent SSI?
- Discontinue or taper steroids if medically permissible?
- Measures to enhance wound space oxygenation?
- Preoperatively apply mupirocin to nares?
The complete Guideline for Prevention of Surgical Site Infections is available online at www.cdc.gov/ncidod/hip/SSI/SSI_guideline.htm.1
Shaving and hair removal
Hair removal is often necessary, but shaving may cause skin trauma that exacerbates bacterial growth.10 SSI rates correlate with the time interval between shaving and incision (20% if shaved >24 hours before surgery, 7.1% the night before, and 3.1% in the OR).11 Thus, the CDC guidelines discourage shaving prior to surgery (EVIDENCE CATEGORY IA).Patients have been known to shave the operative area themselves before surgery, so all patients must be told not to shave themselves before elective surgery.
When hair removal is necessary, preoperative clipping causes minimal skin trauma (EVIDENCE CATEGORY IA).
Preparing The Surgical Staff
The surgeon’s hands
Evidence has shown that 2 minutes of preoperative scrubbing reduces resident flora as effectively as scrubbing for 10 minutes.1 The recommended scrub should include hands and forearms up to the elbows for 2 to 5 minutes (EVIDENCE CATEGORY IB).
Keep hands away from the body and dry hands with a sterile towel (EVIDENCE CATEGORY IB).
Keep fingernails short (EVIDENCE CATEGORY IB), and clean under each nail at the beginning of each day (EVIDENCE CATEGORY II).
An aqueous alcohol solution is a recent alternative to traditional hand antisepsis with chlorhexidine- or povoidoneiodine–based solutions. No difference in SSI rates has been documented between hand-rubbing with an aqueous alcohol solution and traditional scrubbing.12 A traditional scrub before the first of consecutive cases and after contact with gross contamination is still in order.
Sterile barriers
Sterile barriers in the operating room, indispensable in protecting staff, are federally mandated. Their role in preventing SSI is not clear. Surprisingly, the use of face masks may not contribute to SSI reduction.13 Head covering, on the other hand, markedly reduces airborne and wound bacterial contamination.14
Optimize Wound Physiology
Maintaining normothermia
Hypothermia is common, particularly in patients who are immunocompromised, at age extremes, or have multiple trauma. Hypothermic vasoconstriction may reduce tissue perfusion and increase risk of infection.
A double-blind study showed that maintaining intraoperative normothermia decreased SSI in colorectal patients from 19% to 6%.15 Additionally, preoperative warming of the entire body or local site for 30 minutes reduced SSI rates in clean surgical cases.16
Wound space oxygenation
Supplemental oxygen in colorectal surgery may correlate with lower infection rates (80% without supplemental oxygen, 30% with).17 This may improve tissue oxygen tension, which enhances oxidative bactericidal capacity.
However, these findings were not duplicated in patients with higher SSI rates and on supplemental hyperoxia.18 There are no recommendations for enhancing wound space oxygenation.
Control of glycemia
Cardiothoracic surgery studies have stressed the importance of tight perioperative glycemic control. Coronary artery bypass patients with higher mean perioperative glucose showed a trend toward a higher risk of nosocomial infection, but not specifically SSI.19 Another study of cardiothoracic patients found an association between higher risk of SSI and both diabetes and postoperative hyperglycemia.20 Continuous intravenous insulin to maintain a blood glucose 21
Remote infections
Remote infections at the time of surgery, such as urinary tract infection or pneumonia, significantly raise the risk of SSI (EVIDENCE CATEGORY IA).
Strongly consider canceling elective surgery if there is an untreated remote infection, especially if implanting bioprosthetic material.
Surgical technique
Careful technique reduces risk of infection.
Breaks in sterile technique and gross spillage of enteric contents raise the risk for SSI through increased bacterial load.
Poor hemostasis, excess tissue trauma, inadequate debridement or dead space obliteration, and inappropriate suture technique raise the volume of unperfused biological matter (EVIDENCE CATEGORY IB).
Timely completion of the operation also minimizes risk. Prolonged operative time can heighten the risk of breaches in sterile technique. Recommendations call for procedures to be completed within the 75th percentile of standardized operative times.
Antimicrobial prophylaxis
The principles for using preoperative antibiotics include maximal subcutaneous concentration when making the incision (TABLE 2) (EVIDENCE CATEGORY IA). This corresponds with intravenous antimicrobial administration within 60 minutes before incision (or within 120 minutes for vancomycin or fluoroquinolones). An additional dose of the antimicrobial agent is indicated if the procedure time exceeds 2 half-lives of the agent.
Institutional policies for antibiotic restriction aimed at curtailing resistant organisms do not appear to change the spectrum of causative microbes in SSI.22 Short-duration therapy preserves antimicrobial efficacy best, so avoid the routine use of agents such as vancomycin (EVIDENCE CATEGORY IB).
Short duration also applies when antimicrobial prophylaxis is indicated. The CDC recommends extending antimicrobial prophylaxis no more than a few hours after incision closure (EVIDENCE CATEGORY IA). Particular cases may require longer antimicrobial prophylaxis, but prophylaxis beyond 24 hours does not reduce SSI rates and increases the potential for microbial resistance.
While a single dose of broad-spectrum antibiotic may cause Clostridium difficile colitis, prolonged duration also raises risk through profound changes in gut flora that favor the emergence of this opportunistic pathogen.
Category IA. Strongly recommended for all hospitals and strongly supported by well-designed experimental or epidemiologic studies.
Category IB. Strongly recommended for all hospitals and viewed as effective by experts in the field and a consensus of Hospital Infection Control Practices Advisory Committee (HICPAC), based on strong rationale and suggestive evidence, even though definitive scientific studies may not have been done.
Category II. Suggested for implementation in many hospitals. Recommendations may be supported by suggestive clinical or epidemiologic studies, a strong theoretical rationale, or definitive studies applicable to some, but not all, hospitals.
No recommendation; unresolved issue (NR). Insufficient evidence or no consensus regarding efficacy.
Principles of antimicrobial prophylaxis
| Consider these factors: |
| Risk for developing surgical site infection. |
| Potential severity of consequences |
| Prosthetic implantation |
| Cardiothoracic or vascular surgery |
| Agents must be safe, inexpensive, and bactericidal |
| Appropriate spectrum based on anticipated flora of involved tissues and spaces |
| Administer so that maximal effect is at time of incision, and re-administer when appropriate |
| Alter dosage as appropriate for the patient (eg, obesity) |
This article is adapted from DiRocco JD, Pavone LA, Weiss CA III. The evidence-based way to prevent SSI. Contemp Surg. 2005;61:120–127.
1. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-134.
2. Holzwarth R, Huber D, Majkrzak A, Tareen B. Outcome of gastric bypass patients. Obes Surg. 2002;12:261-264.
3. Christou NV, Jarand J, Sylvestre JL, McLean AP. Analysis of the incidence and risk factors for wound infections in open bariatric surgery. Obes Surg. 2004;14:16-22.
4. Muller JM, Brenner U, Dienst C, et al. Preoperative parenteral feeding in patients with gastrointestinal carcinoma. Lancet. 1982;1:68-71.
5. Holter A, Fischer JE. The effects of perioperative hyperalimentation on complications in patients with carcinoma and weight loss. J Surg Res. 1977;23:31-34.
6. Garibaldi RA. Prevention of intraoperative wound contamination with chlorhexidine shower and scrub. J Hosp Infect. 1988;11:5-9.
7. Kluytmans JA, Mouton JW, VandenBergh MF, et al. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 1996;17:780-785.
8. Suzuki Y, Kamigaki T, Fujino M, et al. Randomized clinical trial of preoperative intranasal mupirocin to reduce surgical-site infection after digestive surgery. Br J Surg. 2003;90:1072-1075.
9. Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871-1877.
10. Hamilton HW, Hamilton KR, Lone FJ. Preoperative hair removal. Can J Surg. 1977;20:269-273.
11. Seropian R, Reynolds BM. Wound infection after preoperative depilatory versus razor preparation. Am J Surg. 1971;121:251-254.
12. Parienti JJ, Thibon P, Heller R, et al. Hand-rubbing with an aqueous alcoholic solution vs traditional surgical hand-scrubbing and 30-day surgical site infection rates. JAMA. 2002;288:722-727.
13. Tunevall TG. Postoperative wound infections and surgical face masks: a controlled study. World J Surg. 1991;15:383-388.
14. Friberg B, Friberg S, Ostenson R, Burman LG. Surgical area contamination: comparable bacterial counts using disposable head and mask and helmet aspirator system, but dramatic increase upon omission of headgear: an experimental study in horizontal laminar airflow. J Hosp Infect. 2001;47:110-115.
15. Kurz A, Sessler DI, Lenhardt R. Perioperative normoth-ermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-1215.
16. Melling AC, Ali B, Scott EM, Leaper DJ. Effects of pre-operative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358:876-880.
17. Greif R, Akça O, Horn EP, et al. Supplemental perioper-ative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161-167.
18. Pryor KO, Fahey TJ, III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population. JAMA. 2004;291:79-87.
19. Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408-1414.
20. Latham R, Lancaster AD, Covington JF, et al. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607-612.
21. Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 2000;69:667-668.
22. Weiss CA, III, Statz CL, Dahms RA, et al. Six Years of Surgical Wound Infection Surveillance at a Tertiary Care Center. Arch Surg. 1999;134:1041-1048.
1. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27:97-134.
2. Holzwarth R, Huber D, Majkrzak A, Tareen B. Outcome of gastric bypass patients. Obes Surg. 2002;12:261-264.
3. Christou NV, Jarand J, Sylvestre JL, McLean AP. Analysis of the incidence and risk factors for wound infections in open bariatric surgery. Obes Surg. 2004;14:16-22.
4. Muller JM, Brenner U, Dienst C, et al. Preoperative parenteral feeding in patients with gastrointestinal carcinoma. Lancet. 1982;1:68-71.
5. Holter A, Fischer JE. The effects of perioperative hyperalimentation on complications in patients with carcinoma and weight loss. J Surg Res. 1977;23:31-34.
6. Garibaldi RA. Prevention of intraoperative wound contamination with chlorhexidine shower and scrub. J Hosp Infect. 1988;11:5-9.
7. Kluytmans JA, Mouton JW, VandenBergh MF, et al. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 1996;17:780-785.
8. Suzuki Y, Kamigaki T, Fujino M, et al. Randomized clinical trial of preoperative intranasal mupirocin to reduce surgical-site infection after digestive surgery. Br J Surg. 2003;90:1072-1075.
9. Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871-1877.
10. Hamilton HW, Hamilton KR, Lone FJ. Preoperative hair removal. Can J Surg. 1977;20:269-273.
11. Seropian R, Reynolds BM. Wound infection after preoperative depilatory versus razor preparation. Am J Surg. 1971;121:251-254.
12. Parienti JJ, Thibon P, Heller R, et al. Hand-rubbing with an aqueous alcoholic solution vs traditional surgical hand-scrubbing and 30-day surgical site infection rates. JAMA. 2002;288:722-727.
13. Tunevall TG. Postoperative wound infections and surgical face masks: a controlled study. World J Surg. 1991;15:383-388.
14. Friberg B, Friberg S, Ostenson R, Burman LG. Surgical area contamination: comparable bacterial counts using disposable head and mask and helmet aspirator system, but dramatic increase upon omission of headgear: an experimental study in horizontal laminar airflow. J Hosp Infect. 2001;47:110-115.
15. Kurz A, Sessler DI, Lenhardt R. Perioperative normoth-ermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209-1215.
16. Melling AC, Ali B, Scott EM, Leaper DJ. Effects of pre-operative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet. 2001;358:876-880.
17. Greif R, Akça O, Horn EP, et al. Supplemental perioper-ative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161-167.
18. Pryor KO, Fahey TJ, III, Lien CA, Goldstein PA. Surgical site infection and the routine use of perioperative hyperoxia in a general surgical population. JAMA. 2004;291:79-87.
19. Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22:1408-1414.
20. Latham R, Lancaster AD, Covington JF, et al. The association of diabetes and glucose control with surgical-site infections among cardiothoracic surgery patients. Infect Control Hosp Epidemiol. 2001;22:607-612.
21. Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 2000;69:667-668.
22. Weiss CA, III, Statz CL, Dahms RA, et al. Six Years of Surgical Wound Infection Surveillance at a Tertiary Care Center. Arch Surg. 1999;134:1041-1048.
Uterine artery embolization for abnormal bleeding
DISCHARGE INSTRUCTIONS WALLET INFO-CARD When to call your doctor
LISA’S CASE
Ablation fails to ease symptoms
Lisa is a 38-year-old mother of 2 who initially reported menometrorrhagia, dysmenorrhea, urinary frequency, pelvic pressure, and increasing abdominal girth. These symptoms worsened over 3 years before Lisa saw a physician and was diagnosed with uterine fibroids. When offered hysterectomy or endometrial ablation (cryomyolysis), she chose the latter. However, her fibroids failed to shrink, and her symptoms returned 5 months after the procedure.
Lisa heard about uterine artery embolization (UAE) through the media and now asks about it. Her uterus is 20-week size with an irregular contour. Magnetic resonance imaging (MRI) shows a single large fibroid filling the uterus. Her hematocrit is 22.
Is uterine UAE right for her?
ANGELINA’S CASE
Menorrhagia and a “boggy” uterus
Angelina, 35, has 2 children and a 10-year history of menorrhagia, dysmenorrhea, and anemia. Pelvic examination reveals a 10-week size, “boggy” uterus, and MRI shows global enlargement of the uterus with a thickened junctional zone, which is characteristic of adenomyosis.
Her previous physician recommended hysterectomy after medical therapy failed, but Angelina is reluctant to undergo surgery.
Is UAE an option?
Both women are very likely to benefit from UAE, since the ideal candidate is premenopausal with symptomatic fibroids and/or adenomyosis and has either failed medical or surgical therapy or wants or needs to avoid surgery.1-11
This article describes the therapeutic role of UAE in women with abnormal uterine bleeding (AUB) due to uterine fibroids and/or adenomyosis—the most frequent myometrial causes of premenopausal AUB.
Other, less frequent myometrial disorders (eg, hypervascular pathologies such as intramyometrial and parametrial vascular malformations or neoplasms) can also be treated using embolization techniques. The main advantages of UAE in treating these diseases:1-8,12,13
- Less invasive than surgery, with substantially less recovery time and lower morbidity
- Usually performed under local anesthesia and intravenous conscious sedation
- No worry about adhesions
- Virtually no blood loss or need for transfusion; UAE may be especially attractive for patients who refuse or cannot receive blood products for health or religious reasons.
Impressive success rates
UAE has demonstrated excellent technical (98% to 100%) and high clinical success rates (80% to 95%) in the treatment of fibroids.1-8,13 The clinical success rate is lower for adenomyosis (56% to 92%), but UAE often provides sufficient clinical relief to obviate surgery.9,10,13
Although UAE was not initially recommended for women desiring future fertility—because of the 4% risk of premature menopause—the pendulum is now swinging in the other direction. If the risks of myomectomy are great due to the anatomic size or position of fibroids or adenomyosis, the risk-benefit ratio may shift to UAE to allow preservation of reproductive capacity.1-8,11
Though embolization has been performed since the early 1970s for acute and chronic bleeding associated with various medical conditions,14 the first report of UAE did not come until 1995.1 Since then, the procedure has seen rapid growth worldwide, with approximately 50,000 cases performed. About 14,000 cases were performed in the US last year.2
Although our practice has no fixed size limitation, ideally a uterus less than 20 weeks’ gestational size is preferred.
Contraindications
- Viable pregnancy
- Active pelvic infection
- Presence of an intrauterine device (though the IUD may be removed before the rocedure)
- Undiagnosed pelvic or adnexal mass
- Pelvic malignancies such as ovarian or endometrial carcinoma
- History of pelvic radiation, since UAE may cause ischemic necrosis of the uterus and adjacent organs due to preexisting radiation-induced vasculitis with diffuse vascular narrowing.
Relative contraindications
- Renal insufficiency, though we have used gadolinium, a nonnephrotoxic MRI contrast medium, for women with high blood creatinine levels
- History of severe allergic reaction to iodinated contrast medium, though gadolinium can also be used in these patients
- Coagulopathy
- Desire to preserve fertility, since it cannot be assured based on current data. However, uncomplicated pregnancies and normal deliveries have been reported after UAE, so this procedure may still be preferred for women who refuse or cannot undergo myomectomy.11
In some cases, extensive endometriosis is the cause of menorrhagia or dysmenorrhea, often coexisting with fibroids, and UAE may not be beneficial.11
Finally, a subserosal leiomyoma that is sufficiently pedunculated (attachment point 50% of the diameter) can be at risk for detachment from the uterus, a situation that may necessitate surgical intervention.11
Preop exam and imaging
At the physical examination, the fibroid uterus usually is enlarged with an irregular contour, and adenomyosis usually presents as a globally enlarged, “boggy” uterus (typically 6- to 10-weeks’ gestational size).
MRI is the preferred imaging
We prefer MRI since fibroids can be missed with ultrasound due to the limited field of view. MRI more accurately defines the size, location, and extent of disease. It also may better differentiate fibroids from adenomyosis.
MRI clearly depicts uterine zonal anatomy and enables accurate classification of individual masses by their locations: submucosal, intramural, or subserosal.
When adenomyosis is present, T2-weighted MRI demonstrates diffuse adenomyosis (about 66%) with global enlargement of the uterus and diffuse thickening of the junctional zone (at least 12 mm, highly predictive finding) with homogeneous low signal intensity. Focal adenomyosis (33%) can be seen as an illdefined, poorly marginated focal mass (adenomyoma) of low signal intensity within the myometrium.15,16
Transvaginal ultrasound
In women with fibroids, ultrasound usually demonstrates an enlarged uterus with lobulations, contour abnormality, or mass effects.
In women with adenomyosis, it usually demonstrates ill-defined, heterogeneous echotexture and small anechoic areas within the myometrium of asymmetrically enlarged uteri, with indistinct endometrial-myometrial borders and subendometrial halo thickening.15
Include endometrial biopsy
The patient should have a normal Pap test during the 12 months leading up to UAE,11 and should undergo endometrial biopsy to exclude carcinoma.
Laboratory tests should include a complete blood count, blood urea nitrogen/creatinine, follicle-stimulating hormone, human chorionic gonadotropin, and coagulation tests.
Technique
UAE begins with insertion of a small catheter (4-5 French) through a femoral artery in conjunction with percutaneous angiography. The catheter is guided into the uterine arteries—left first, then right— and contrast medium is injected into each artery to confirm the position of the catheter and the presence of fibroids or adenomyosis, which appear as hypervascular lesions in angiograms (see above, right).
UAE usually requires 1 to 2 hours.
Embolic agents
Polyvinyl alcohol (PVA) particles or trisacryl gelatin microspheres, usually 500 to 700 and/or 700 to 900 microns in size, are released through the catheter into the uterine arteries. These agents block the blood vessels that feed the fibroids and/or adenomyosis, causing them to shrink. The agents are biocompatible and have been approved by the US Food and Drug Administration.
Other, less frequently used embolic agents include gelatin sponge particles (which are temporary) and coils (which are permanent). Coils are generally used for conditions such as arteriovenous malformations or fistulae, which have large feeding vessels (iliac or enlarged uterine or ovarian vessels). This fluoroscopy-guided procedure usually is performed under local anesthesia and conscious sedation or, less often, epidural anesthesia.
Patient care
Conscious sedation, NSAIDs, and antibiotics
Intravenous conscious sedation in conjunction with nonsteroidal anti-inflammatory drugs (NSAIDs) usually provides sufficient pain relief.
In addition, intravenous broad-spectrum antibiotics are used as prophylaxis for infection linked to the embolization itself and to subsequent ischemia of the fibroids and uterus.
Managing postop pain syndrome
More than 90% of women experience postembolization syndrome, which includes moderate to severe abdominal pain/cramping and nausea and vomiting in the first several hours following the procedure. As a result, they may require hospitalization (less than 24 hours) for pain management. In our experience, few women stay in the hospital more than 1 day.
A patient-controlled analgesia pump and NSAIDs are used in women with abdominal/pelvic cramping and pain (more than 90% of cases) if epidural anesthesia is not used for pain.
Low-grade fever and leukocytosis are not uncommon after embolization, and are usually treated with acetaminophen. Other symptoms are anorexia and fatigue, but they gradually subside within 3 to 4 days.
After discharge
Oral NSAIDs and narcotics are often needed for several days. Many women resume light activities in a few days, and most return to normal activities within 1 week.11
Give her comprehensive discharge instructions on taking medications, what to expect, and when to contact a doctor. Follow-up visit in 1 to 4 weeks. We schedule an outpatient visit 1 to 4 weeks after the procedure. At this visit, we confirm healing of the puncture sites, screen for unusual symptoms or potential problems, and repeat follow-up instructions.11
We then follow the patient periodically (3, 6, and 12 months) to monitor her for symptoms and complications such as late infections, expulsion of infarcted fibroids, chronic endometritis, chronic vaginal discharge, and cessation or irregularity of menses, all of which have been observed after UAE.11
Transvaginal ultrasound is usually performed 3 to 6 months and 1 year after UAE to determine whether existing fibroids have been infarcted and begun to decrease in volume. It also reveals any uterine or adnexal complications.
In addition, this imaging provides a new baseline measurement of fibroid volume, against which any subsequent increase in size (which may indicate regrowth of fibroids or undiagnosed leiomyosarcoma) can be compared.11
Key findings of outcome studies
Two large series reported significant improvement in AUB in 77% to 90% of fibroid cases, and bulk-related symptoms were controlled in 86% to 91%.6-8 In these studies, average uterine volumes decreased by 35% and 58% at 3 and 12 months, respectively, with dominant fibroid shrinkage of 42%. Several large series also reported high patient satisfaction (91% to 93%) and significant improvement in quality-of-life measures.4,6-8
Side effects and complications
Although UAE is considered very safe, it carries some risks. Spies et al17 reported on complications in 400 consecutive patients undergoing UAE for fibroids at their institution:
- 1.25% serious complication rate
- 5% overall periprocedural morbidity rate
- no deaths and no major permanent injuries
In addition, 1 patient required hysterectomy as a result of a complication, and 1 patient had an undiagnosed leiomyosarcoma, which was discovered during an elective myomectomy 31 months after UAE.
Goldberg et al 18 reported another case with delayed diagnosis of leiomyosarcoma following UAE. In our series of 705 patients, 1 had an undiagnosed leiomyosarcoma, which presented as a pelvic mass 15 months after UAE. She subsequently underwent hysterectomy.
When to suspect leiomyosarcoma
Unlike hysterectomy or myomectomy, no tissue is obtained in UAE for pathologic diagnosis to exclude leiomyosarcoma, which is found in approximately 0.1% to 0.4% of women with fibroids and is difficult to differentiate from a benign leiomyoma using clinical tests or imaging.17-18
Suspect leiomyosarcoma if the fibroids continue to grow even after technically successful embolization.
Infection is rare, but can be lethal
A small number of patients have experienced infection, which usually is controlled with antibiotics. In a series of 414 UAE procedures in 410 fibroid patients, Rajan et al19 reported:
- 1.2% rate of intrauterine infection requiring intravenous antibiotic therapy and/or surgery
- no significant difference seen with various embolic agents, quantity of embolic particles, se of preprocedure antibiotics, or size or location of the dominant fibroid.
However, at least 2 deaths have been reported due to infection since UAE for fibroids was introduced in the mid-1990s: 1 fatal sepsis in a woman who underwent UAE for fibroids and 1 other sepsis fatality.17,20 The first case was caused by necrosis of the vaginal wall and uterine cervix. At autopsy, microspheres were found not only in arteries in the leiomyomata and myometrium, but also in the parametria and vagina, causing ischemic necrosis.
Amenorrhea or worsened AUB
In some cases, amenorrhea can follow UAE for fibroids due to ovarian embolization and subsequent ovarian failure.6-8,17
The literature indicates a rate of:
- 1% to 2% in patients less than 45 years of age
- 15% to 20% for perimenopausal women 45 and older
Worsening of uterine bleeding is rare after UAE, but can occur. Kerlan et al21 reported massive uterine bleeding 1 month after UAE in a woman who underwent the procedure for menorrhagia. When she was treated with emergent hysterectomy, a bleeding ulceration of the endometrium overlying the necrotic fibroid was found.
Other complications include spotting, hot flashes, fever, vaginal discharge, mood swings, pain at the puncture site, and dysuria.6-8,17
Our UAE experience
The New England Fibroid Center began offering fibroid embolization in 1997. Since then, we have performed 705 procedures at 5 hospitals in the Greater Boston region, with a technical success rate of 99%. Technical failure occurred in 1% of patients; these women had very difficult vascular anatomy involving uterine arteries, or ovarian arteries formed the dominant blood supply to the fibroids.
Clinical success or improvement was seen in 80% of women with bulk-related symptoms and 94.3% with bleeding symptoms.
Clinical failure occurred in 5.7% of women (1.6% required repeat UAE and 1.4% hysterectomies due to persistent symptoms).
Complications occurred in 4% of cases (2% rate of premature ovarian failure, 1.5% rate of transvaginal passage of infarcted fibroids, and 0.5% rate of groin hematoma). There were no major complications requiring transfusion or emergent surgeries such as hysterectomy.
Fertility after UAE
LISA’S CASE
“Cure” and pregnancy
Lisa successfully underwent UAE, and had no symptoms after the procedure. The uterine fibroids resolved almost completely in 1 year.
Three years after the procedure, she became pregnant and delivered a healthy, full-term infant.
Although UAE is generally not performed in women who wish to preserve their fertility, it is sometimes used in fibroid patients when myomectomy is contraindicated because of the size and/or number of fibroids.11,22,23 Only a few small series and case reports describe successful pregnancies following UAE.
For example, in a study involving 400 women, McLucas et al22 reported 17 pregnancies in 14 women among 149 patients who stated a desire for fertility after UAE. Of these, 5 spontaneous abortions were observed, and 10 women had normal term deliveries. No perfusion or other problems were reported during pregnancy or labor.
Goldberg and colleagues23 analyzed 50 published cases of post-UAE pregnancies and found higher rates of cesarean delivery, preterm birth, malpresentation, small-for-gestational-age infants, spontaneous abortion, and postpartum hemorrhage than in the general population, though the reasons were unclear.
In our experience at the New England Fibroid Center, 5 of 12 patients below the age of 40 who wanted to preserve fertility became pregnant and successfully delivered full-term infants.
In general, the risks of infertility, premature ovarian failure/menopause, radiation exposure, and hysterectomy following UAE are small and compare favorably with those associated with myomectomy. Fertility rates are similar to those for women undergoing myomectomy.24
Nevertheless, well-controlled studies and additional data are needed before UAE can be confidently recommended as a first-line approach for preserving fertility.11
Treating adenomyosis
ANGELINA’S CASE
Adenomyosis resolves
During Angelina’s UAE procedure, angiographies showed enlarged right and left uterine arteries with numerous prominent intrauterine branches supplying the enlarged uterus. After UAE with PVA microspheres, post-embolization angiograms showed occlusion of the right and left uterine arteries and their branches.
Her symptoms resolved completely following the procedure. One year later, a follow-up MRI showed normal uterine size and shape, with complete resolution of adenomyosis.
Several small series have reported successful treatment of women with symptomatic adenomyosis. For example, of 23 women who underwent UAE for this indication, Chen and colleagues9 reported:
- Complete resolution of dysmenorrhea in 19 women and significant improvement in 2. Two other patients had recurrent symptoms.
- A substantial decrease in uterine volume in most of the women.
- An immediate decrease in intrauterine blood flow detected by color Doppler ultrasonography.
In a prospective study10 involving 18 women with symptomatic adenomyosis:
- 94% had diminished menorrhagia 6 months after UAE, and 94% had a slight decrease (mean: 15%) in uterine volume.
- After 1 year, 73% of women had diminished menorrhagia, and 53% had complete resolution.
- After 2 years, 56% of women had complete resolution of menorrhagia, 44% required additional treatment due to failure or recurrence, and 28% underwent hysterectomy.
In our limited experience with adenomyosis at the New England Fibroid Center, we saw no significant difference in technical success rates (100%) after UAE, compared with fibroid patients. However, there was a relatively high recurrence rate (2 of 6 patients) of presenting symptoms (menorrhagia or dysmenorrhea), and 2 patients later underwent hysterectomy.
Well-controlled studies are needed before UAE can confidently be recommended for symptomatic adenomyosis.
The authors report no financial relationships relevant to this article.
1. Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346:671-672.
2. Worthington-Kirsch RL, Siskin GP. Uterine artery embolization for symptomatic myomata. J Intensive Care Med. 2004;19:13-21.
3. Bradley EA, Reidy JF, Forman RG, et al. Transcatheter uterine artery embolisation to treat large uterine fibroids. Br J Obstet Gynaecol. 1998;105:235-240.
4. Worthington-Kirsch RL, Popky GL, Huchins FL, Jr. Uterine artery embolization for the management of leiomyomas: quality-of-life assessment and clinical response. Radiology. 1998;208:625-629.
5. Goodwin SC, Vedantham S, et al. Preliminary experience with uterine artery embolization for uterine fibroids. J Vasc Interv Radiol. 1997;8:517-526.
6. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow-up. BJOG. 2002;11:1262-1272.
7. Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
8. Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
9. Chen C, et al. Uterine arterial embolization in the treatment of adenomyosis. Zhonghua Fu Chan Ke Za Zhi. 2002;37:77-79.
10. Pelage JP, Jacob D, et al. Midterm results of uterine artery embolization for symptomatic adenomyosis: initial experience. Radiology. 2005;234:948-953.
11. Andrews RT, Spies JB, Sacks D, et al. Patient care and uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2004;15:115-120.
12. Broder MS, et al. Uterine Artery Embolization: A Systematic Review of the Literature and Proposal for Research. Santa Monica, Calif: Rand; 1999. Publication MR-1158.
13. Siskin GP, Tublin ME, Stainken BF, et al. Uterine artery embolization for the treatment of adenomyosis: clinical response and evaluation with MR imaging. AJR Am J Roentgenol. 2001;177:297-302.
14. Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1979;102:303-306.
15. Outwater EK, Siegelman ES, Van Deerlin V. Adenomyosis: current concepts and imaging considerations. AJR Am J Roentgenol. 1998;170:437-441.
16. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. 1999;19:S161-S170.
17. Spies JB, Spector A, Roth AR, et al. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
18. Goldberg J, Burd I, et al. Leiomyosarcoma in a premenopausal patient following uterine artery embolization. Am J Obstet Gynecol. 2004;191:1733-1735.
19. Rajan DK, Beecroft JR, Clark TW, et al. Risk of intrauterine infectious complications after uterine artery embolization. J Vasc Interv Radiol. 2004;15:1415-1421.
20. Vashisht A, Studd J, Carey A, Burn P. Fatal septicemia after fibroid embolisation [letter]. Lancet. 1999;354:307-308.
21. Kerlan K, Jr, Coffey JO, Milkman MS, et al. Massive vaginal hemorrhage after uterine fibroid embolization. J Vasc Interv Radiol. 2003;14:1465-1467.
22. McLucas B, Goodwin S, Adler L, Rappaport A, Reed R, Perrella R. Pregnancy following uterine fibroid embolization. Int J Gynaecol Obstet. 2001;74:1-7.
23. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872.
24. Goldberg J, Pereira L, Berghella V, et al. Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol. 2004;191:18-21.
DISCHARGE INSTRUCTIONS WALLET INFO-CARD When to call your doctor
LISA’S CASE
Ablation fails to ease symptoms
Lisa is a 38-year-old mother of 2 who initially reported menometrorrhagia, dysmenorrhea, urinary frequency, pelvic pressure, and increasing abdominal girth. These symptoms worsened over 3 years before Lisa saw a physician and was diagnosed with uterine fibroids. When offered hysterectomy or endometrial ablation (cryomyolysis), she chose the latter. However, her fibroids failed to shrink, and her symptoms returned 5 months after the procedure.
Lisa heard about uterine artery embolization (UAE) through the media and now asks about it. Her uterus is 20-week size with an irregular contour. Magnetic resonance imaging (MRI) shows a single large fibroid filling the uterus. Her hematocrit is 22.
Is uterine UAE right for her?
ANGELINA’S CASE
Menorrhagia and a “boggy” uterus
Angelina, 35, has 2 children and a 10-year history of menorrhagia, dysmenorrhea, and anemia. Pelvic examination reveals a 10-week size, “boggy” uterus, and MRI shows global enlargement of the uterus with a thickened junctional zone, which is characteristic of adenomyosis.
Her previous physician recommended hysterectomy after medical therapy failed, but Angelina is reluctant to undergo surgery.
Is UAE an option?
Both women are very likely to benefit from UAE, since the ideal candidate is premenopausal with symptomatic fibroids and/or adenomyosis and has either failed medical or surgical therapy or wants or needs to avoid surgery.1-11
This article describes the therapeutic role of UAE in women with abnormal uterine bleeding (AUB) due to uterine fibroids and/or adenomyosis—the most frequent myometrial causes of premenopausal AUB.
Other, less frequent myometrial disorders (eg, hypervascular pathologies such as intramyometrial and parametrial vascular malformations or neoplasms) can also be treated using embolization techniques. The main advantages of UAE in treating these diseases:1-8,12,13
- Less invasive than surgery, with substantially less recovery time and lower morbidity
- Usually performed under local anesthesia and intravenous conscious sedation
- No worry about adhesions
- Virtually no blood loss or need for transfusion; UAE may be especially attractive for patients who refuse or cannot receive blood products for health or religious reasons.
Impressive success rates
UAE has demonstrated excellent technical (98% to 100%) and high clinical success rates (80% to 95%) in the treatment of fibroids.1-8,13 The clinical success rate is lower for adenomyosis (56% to 92%), but UAE often provides sufficient clinical relief to obviate surgery.9,10,13
Although UAE was not initially recommended for women desiring future fertility—because of the 4% risk of premature menopause—the pendulum is now swinging in the other direction. If the risks of myomectomy are great due to the anatomic size or position of fibroids or adenomyosis, the risk-benefit ratio may shift to UAE to allow preservation of reproductive capacity.1-8,11
Though embolization has been performed since the early 1970s for acute and chronic bleeding associated with various medical conditions,14 the first report of UAE did not come until 1995.1 Since then, the procedure has seen rapid growth worldwide, with approximately 50,000 cases performed. About 14,000 cases were performed in the US last year.2
Although our practice has no fixed size limitation, ideally a uterus less than 20 weeks’ gestational size is preferred.
Contraindications
- Viable pregnancy
- Active pelvic infection
- Presence of an intrauterine device (though the IUD may be removed before the rocedure)
- Undiagnosed pelvic or adnexal mass
- Pelvic malignancies such as ovarian or endometrial carcinoma
- History of pelvic radiation, since UAE may cause ischemic necrosis of the uterus and adjacent organs due to preexisting radiation-induced vasculitis with diffuse vascular narrowing.
Relative contraindications
- Renal insufficiency, though we have used gadolinium, a nonnephrotoxic MRI contrast medium, for women with high blood creatinine levels
- History of severe allergic reaction to iodinated contrast medium, though gadolinium can also be used in these patients
- Coagulopathy
- Desire to preserve fertility, since it cannot be assured based on current data. However, uncomplicated pregnancies and normal deliveries have been reported after UAE, so this procedure may still be preferred for women who refuse or cannot undergo myomectomy.11
In some cases, extensive endometriosis is the cause of menorrhagia or dysmenorrhea, often coexisting with fibroids, and UAE may not be beneficial.11
Finally, a subserosal leiomyoma that is sufficiently pedunculated (attachment point 50% of the diameter) can be at risk for detachment from the uterus, a situation that may necessitate surgical intervention.11
Preop exam and imaging
At the physical examination, the fibroid uterus usually is enlarged with an irregular contour, and adenomyosis usually presents as a globally enlarged, “boggy” uterus (typically 6- to 10-weeks’ gestational size).
MRI is the preferred imaging
We prefer MRI since fibroids can be missed with ultrasound due to the limited field of view. MRI more accurately defines the size, location, and extent of disease. It also may better differentiate fibroids from adenomyosis.
MRI clearly depicts uterine zonal anatomy and enables accurate classification of individual masses by their locations: submucosal, intramural, or subserosal.
When adenomyosis is present, T2-weighted MRI demonstrates diffuse adenomyosis (about 66%) with global enlargement of the uterus and diffuse thickening of the junctional zone (at least 12 mm, highly predictive finding) with homogeneous low signal intensity. Focal adenomyosis (33%) can be seen as an illdefined, poorly marginated focal mass (adenomyoma) of low signal intensity within the myometrium.15,16
Transvaginal ultrasound
In women with fibroids, ultrasound usually demonstrates an enlarged uterus with lobulations, contour abnormality, or mass effects.
In women with adenomyosis, it usually demonstrates ill-defined, heterogeneous echotexture and small anechoic areas within the myometrium of asymmetrically enlarged uteri, with indistinct endometrial-myometrial borders and subendometrial halo thickening.15
Include endometrial biopsy
The patient should have a normal Pap test during the 12 months leading up to UAE,11 and should undergo endometrial biopsy to exclude carcinoma.
Laboratory tests should include a complete blood count, blood urea nitrogen/creatinine, follicle-stimulating hormone, human chorionic gonadotropin, and coagulation tests.
Technique
UAE begins with insertion of a small catheter (4-5 French) through a femoral artery in conjunction with percutaneous angiography. The catheter is guided into the uterine arteries—left first, then right— and contrast medium is injected into each artery to confirm the position of the catheter and the presence of fibroids or adenomyosis, which appear as hypervascular lesions in angiograms (see above, right).
UAE usually requires 1 to 2 hours.
Embolic agents
Polyvinyl alcohol (PVA) particles or trisacryl gelatin microspheres, usually 500 to 700 and/or 700 to 900 microns in size, are released through the catheter into the uterine arteries. These agents block the blood vessels that feed the fibroids and/or adenomyosis, causing them to shrink. The agents are biocompatible and have been approved by the US Food and Drug Administration.
Other, less frequently used embolic agents include gelatin sponge particles (which are temporary) and coils (which are permanent). Coils are generally used for conditions such as arteriovenous malformations or fistulae, which have large feeding vessels (iliac or enlarged uterine or ovarian vessels). This fluoroscopy-guided procedure usually is performed under local anesthesia and conscious sedation or, less often, epidural anesthesia.
Patient care
Conscious sedation, NSAIDs, and antibiotics
Intravenous conscious sedation in conjunction with nonsteroidal anti-inflammatory drugs (NSAIDs) usually provides sufficient pain relief.
In addition, intravenous broad-spectrum antibiotics are used as prophylaxis for infection linked to the embolization itself and to subsequent ischemia of the fibroids and uterus.
Managing postop pain syndrome
More than 90% of women experience postembolization syndrome, which includes moderate to severe abdominal pain/cramping and nausea and vomiting in the first several hours following the procedure. As a result, they may require hospitalization (less than 24 hours) for pain management. In our experience, few women stay in the hospital more than 1 day.
A patient-controlled analgesia pump and NSAIDs are used in women with abdominal/pelvic cramping and pain (more than 90% of cases) if epidural anesthesia is not used for pain.
Low-grade fever and leukocytosis are not uncommon after embolization, and are usually treated with acetaminophen. Other symptoms are anorexia and fatigue, but they gradually subside within 3 to 4 days.
After discharge
Oral NSAIDs and narcotics are often needed for several days. Many women resume light activities in a few days, and most return to normal activities within 1 week.11
Give her comprehensive discharge instructions on taking medications, what to expect, and when to contact a doctor. Follow-up visit in 1 to 4 weeks. We schedule an outpatient visit 1 to 4 weeks after the procedure. At this visit, we confirm healing of the puncture sites, screen for unusual symptoms or potential problems, and repeat follow-up instructions.11
We then follow the patient periodically (3, 6, and 12 months) to monitor her for symptoms and complications such as late infections, expulsion of infarcted fibroids, chronic endometritis, chronic vaginal discharge, and cessation or irregularity of menses, all of which have been observed after UAE.11
Transvaginal ultrasound is usually performed 3 to 6 months and 1 year after UAE to determine whether existing fibroids have been infarcted and begun to decrease in volume. It also reveals any uterine or adnexal complications.
In addition, this imaging provides a new baseline measurement of fibroid volume, against which any subsequent increase in size (which may indicate regrowth of fibroids or undiagnosed leiomyosarcoma) can be compared.11
Key findings of outcome studies
Two large series reported significant improvement in AUB in 77% to 90% of fibroid cases, and bulk-related symptoms were controlled in 86% to 91%.6-8 In these studies, average uterine volumes decreased by 35% and 58% at 3 and 12 months, respectively, with dominant fibroid shrinkage of 42%. Several large series also reported high patient satisfaction (91% to 93%) and significant improvement in quality-of-life measures.4,6-8
Side effects and complications
Although UAE is considered very safe, it carries some risks. Spies et al17 reported on complications in 400 consecutive patients undergoing UAE for fibroids at their institution:
- 1.25% serious complication rate
- 5% overall periprocedural morbidity rate
- no deaths and no major permanent injuries
In addition, 1 patient required hysterectomy as a result of a complication, and 1 patient had an undiagnosed leiomyosarcoma, which was discovered during an elective myomectomy 31 months after UAE.
Goldberg et al 18 reported another case with delayed diagnosis of leiomyosarcoma following UAE. In our series of 705 patients, 1 had an undiagnosed leiomyosarcoma, which presented as a pelvic mass 15 months after UAE. She subsequently underwent hysterectomy.
When to suspect leiomyosarcoma
Unlike hysterectomy or myomectomy, no tissue is obtained in UAE for pathologic diagnosis to exclude leiomyosarcoma, which is found in approximately 0.1% to 0.4% of women with fibroids and is difficult to differentiate from a benign leiomyoma using clinical tests or imaging.17-18
Suspect leiomyosarcoma if the fibroids continue to grow even after technically successful embolization.
Infection is rare, but can be lethal
A small number of patients have experienced infection, which usually is controlled with antibiotics. In a series of 414 UAE procedures in 410 fibroid patients, Rajan et al19 reported:
- 1.2% rate of intrauterine infection requiring intravenous antibiotic therapy and/or surgery
- no significant difference seen with various embolic agents, quantity of embolic particles, se of preprocedure antibiotics, or size or location of the dominant fibroid.
However, at least 2 deaths have been reported due to infection since UAE for fibroids was introduced in the mid-1990s: 1 fatal sepsis in a woman who underwent UAE for fibroids and 1 other sepsis fatality.17,20 The first case was caused by necrosis of the vaginal wall and uterine cervix. At autopsy, microspheres were found not only in arteries in the leiomyomata and myometrium, but also in the parametria and vagina, causing ischemic necrosis.
Amenorrhea or worsened AUB
In some cases, amenorrhea can follow UAE for fibroids due to ovarian embolization and subsequent ovarian failure.6-8,17
The literature indicates a rate of:
- 1% to 2% in patients less than 45 years of age
- 15% to 20% for perimenopausal women 45 and older
Worsening of uterine bleeding is rare after UAE, but can occur. Kerlan et al21 reported massive uterine bleeding 1 month after UAE in a woman who underwent the procedure for menorrhagia. When she was treated with emergent hysterectomy, a bleeding ulceration of the endometrium overlying the necrotic fibroid was found.
Other complications include spotting, hot flashes, fever, vaginal discharge, mood swings, pain at the puncture site, and dysuria.6-8,17
Our UAE experience
The New England Fibroid Center began offering fibroid embolization in 1997. Since then, we have performed 705 procedures at 5 hospitals in the Greater Boston region, with a technical success rate of 99%. Technical failure occurred in 1% of patients; these women had very difficult vascular anatomy involving uterine arteries, or ovarian arteries formed the dominant blood supply to the fibroids.
Clinical success or improvement was seen in 80% of women with bulk-related symptoms and 94.3% with bleeding symptoms.
Clinical failure occurred in 5.7% of women (1.6% required repeat UAE and 1.4% hysterectomies due to persistent symptoms).
Complications occurred in 4% of cases (2% rate of premature ovarian failure, 1.5% rate of transvaginal passage of infarcted fibroids, and 0.5% rate of groin hematoma). There were no major complications requiring transfusion or emergent surgeries such as hysterectomy.
Fertility after UAE
LISA’S CASE
“Cure” and pregnancy
Lisa successfully underwent UAE, and had no symptoms after the procedure. The uterine fibroids resolved almost completely in 1 year.
Three years after the procedure, she became pregnant and delivered a healthy, full-term infant.
Although UAE is generally not performed in women who wish to preserve their fertility, it is sometimes used in fibroid patients when myomectomy is contraindicated because of the size and/or number of fibroids.11,22,23 Only a few small series and case reports describe successful pregnancies following UAE.
For example, in a study involving 400 women, McLucas et al22 reported 17 pregnancies in 14 women among 149 patients who stated a desire for fertility after UAE. Of these, 5 spontaneous abortions were observed, and 10 women had normal term deliveries. No perfusion or other problems were reported during pregnancy or labor.
Goldberg and colleagues23 analyzed 50 published cases of post-UAE pregnancies and found higher rates of cesarean delivery, preterm birth, malpresentation, small-for-gestational-age infants, spontaneous abortion, and postpartum hemorrhage than in the general population, though the reasons were unclear.
In our experience at the New England Fibroid Center, 5 of 12 patients below the age of 40 who wanted to preserve fertility became pregnant and successfully delivered full-term infants.
In general, the risks of infertility, premature ovarian failure/menopause, radiation exposure, and hysterectomy following UAE are small and compare favorably with those associated with myomectomy. Fertility rates are similar to those for women undergoing myomectomy.24
Nevertheless, well-controlled studies and additional data are needed before UAE can be confidently recommended as a first-line approach for preserving fertility.11
Treating adenomyosis
ANGELINA’S CASE
Adenomyosis resolves
During Angelina’s UAE procedure, angiographies showed enlarged right and left uterine arteries with numerous prominent intrauterine branches supplying the enlarged uterus. After UAE with PVA microspheres, post-embolization angiograms showed occlusion of the right and left uterine arteries and their branches.
Her symptoms resolved completely following the procedure. One year later, a follow-up MRI showed normal uterine size and shape, with complete resolution of adenomyosis.
Several small series have reported successful treatment of women with symptomatic adenomyosis. For example, of 23 women who underwent UAE for this indication, Chen and colleagues9 reported:
- Complete resolution of dysmenorrhea in 19 women and significant improvement in 2. Two other patients had recurrent symptoms.
- A substantial decrease in uterine volume in most of the women.
- An immediate decrease in intrauterine blood flow detected by color Doppler ultrasonography.
In a prospective study10 involving 18 women with symptomatic adenomyosis:
- 94% had diminished menorrhagia 6 months after UAE, and 94% had a slight decrease (mean: 15%) in uterine volume.
- After 1 year, 73% of women had diminished menorrhagia, and 53% had complete resolution.
- After 2 years, 56% of women had complete resolution of menorrhagia, 44% required additional treatment due to failure or recurrence, and 28% underwent hysterectomy.
In our limited experience with adenomyosis at the New England Fibroid Center, we saw no significant difference in technical success rates (100%) after UAE, compared with fibroid patients. However, there was a relatively high recurrence rate (2 of 6 patients) of presenting symptoms (menorrhagia or dysmenorrhea), and 2 patients later underwent hysterectomy.
Well-controlled studies are needed before UAE can confidently be recommended for symptomatic adenomyosis.
The authors report no financial relationships relevant to this article.
DISCHARGE INSTRUCTIONS WALLET INFO-CARD When to call your doctor
LISA’S CASE
Ablation fails to ease symptoms
Lisa is a 38-year-old mother of 2 who initially reported menometrorrhagia, dysmenorrhea, urinary frequency, pelvic pressure, and increasing abdominal girth. These symptoms worsened over 3 years before Lisa saw a physician and was diagnosed with uterine fibroids. When offered hysterectomy or endometrial ablation (cryomyolysis), she chose the latter. However, her fibroids failed to shrink, and her symptoms returned 5 months after the procedure.
Lisa heard about uterine artery embolization (UAE) through the media and now asks about it. Her uterus is 20-week size with an irregular contour. Magnetic resonance imaging (MRI) shows a single large fibroid filling the uterus. Her hematocrit is 22.
Is uterine UAE right for her?
ANGELINA’S CASE
Menorrhagia and a “boggy” uterus
Angelina, 35, has 2 children and a 10-year history of menorrhagia, dysmenorrhea, and anemia. Pelvic examination reveals a 10-week size, “boggy” uterus, and MRI shows global enlargement of the uterus with a thickened junctional zone, which is characteristic of adenomyosis.
Her previous physician recommended hysterectomy after medical therapy failed, but Angelina is reluctant to undergo surgery.
Is UAE an option?
Both women are very likely to benefit from UAE, since the ideal candidate is premenopausal with symptomatic fibroids and/or adenomyosis and has either failed medical or surgical therapy or wants or needs to avoid surgery.1-11
This article describes the therapeutic role of UAE in women with abnormal uterine bleeding (AUB) due to uterine fibroids and/or adenomyosis—the most frequent myometrial causes of premenopausal AUB.
Other, less frequent myometrial disorders (eg, hypervascular pathologies such as intramyometrial and parametrial vascular malformations or neoplasms) can also be treated using embolization techniques. The main advantages of UAE in treating these diseases:1-8,12,13
- Less invasive than surgery, with substantially less recovery time and lower morbidity
- Usually performed under local anesthesia and intravenous conscious sedation
- No worry about adhesions
- Virtually no blood loss or need for transfusion; UAE may be especially attractive for patients who refuse or cannot receive blood products for health or religious reasons.
Impressive success rates
UAE has demonstrated excellent technical (98% to 100%) and high clinical success rates (80% to 95%) in the treatment of fibroids.1-8,13 The clinical success rate is lower for adenomyosis (56% to 92%), but UAE often provides sufficient clinical relief to obviate surgery.9,10,13
Although UAE was not initially recommended for women desiring future fertility—because of the 4% risk of premature menopause—the pendulum is now swinging in the other direction. If the risks of myomectomy are great due to the anatomic size or position of fibroids or adenomyosis, the risk-benefit ratio may shift to UAE to allow preservation of reproductive capacity.1-8,11
Though embolization has been performed since the early 1970s for acute and chronic bleeding associated with various medical conditions,14 the first report of UAE did not come until 1995.1 Since then, the procedure has seen rapid growth worldwide, with approximately 50,000 cases performed. About 14,000 cases were performed in the US last year.2
Although our practice has no fixed size limitation, ideally a uterus less than 20 weeks’ gestational size is preferred.
Contraindications
- Viable pregnancy
- Active pelvic infection
- Presence of an intrauterine device (though the IUD may be removed before the rocedure)
- Undiagnosed pelvic or adnexal mass
- Pelvic malignancies such as ovarian or endometrial carcinoma
- History of pelvic radiation, since UAE may cause ischemic necrosis of the uterus and adjacent organs due to preexisting radiation-induced vasculitis with diffuse vascular narrowing.
Relative contraindications
- Renal insufficiency, though we have used gadolinium, a nonnephrotoxic MRI contrast medium, for women with high blood creatinine levels
- History of severe allergic reaction to iodinated contrast medium, though gadolinium can also be used in these patients
- Coagulopathy
- Desire to preserve fertility, since it cannot be assured based on current data. However, uncomplicated pregnancies and normal deliveries have been reported after UAE, so this procedure may still be preferred for women who refuse or cannot undergo myomectomy.11
In some cases, extensive endometriosis is the cause of menorrhagia or dysmenorrhea, often coexisting with fibroids, and UAE may not be beneficial.11
Finally, a subserosal leiomyoma that is sufficiently pedunculated (attachment point 50% of the diameter) can be at risk for detachment from the uterus, a situation that may necessitate surgical intervention.11
Preop exam and imaging
At the physical examination, the fibroid uterus usually is enlarged with an irregular contour, and adenomyosis usually presents as a globally enlarged, “boggy” uterus (typically 6- to 10-weeks’ gestational size).
MRI is the preferred imaging
We prefer MRI since fibroids can be missed with ultrasound due to the limited field of view. MRI more accurately defines the size, location, and extent of disease. It also may better differentiate fibroids from adenomyosis.
MRI clearly depicts uterine zonal anatomy and enables accurate classification of individual masses by their locations: submucosal, intramural, or subserosal.
When adenomyosis is present, T2-weighted MRI demonstrates diffuse adenomyosis (about 66%) with global enlargement of the uterus and diffuse thickening of the junctional zone (at least 12 mm, highly predictive finding) with homogeneous low signal intensity. Focal adenomyosis (33%) can be seen as an illdefined, poorly marginated focal mass (adenomyoma) of low signal intensity within the myometrium.15,16
Transvaginal ultrasound
In women with fibroids, ultrasound usually demonstrates an enlarged uterus with lobulations, contour abnormality, or mass effects.
In women with adenomyosis, it usually demonstrates ill-defined, heterogeneous echotexture and small anechoic areas within the myometrium of asymmetrically enlarged uteri, with indistinct endometrial-myometrial borders and subendometrial halo thickening.15
Include endometrial biopsy
The patient should have a normal Pap test during the 12 months leading up to UAE,11 and should undergo endometrial biopsy to exclude carcinoma.
Laboratory tests should include a complete blood count, blood urea nitrogen/creatinine, follicle-stimulating hormone, human chorionic gonadotropin, and coagulation tests.
Technique
UAE begins with insertion of a small catheter (4-5 French) through a femoral artery in conjunction with percutaneous angiography. The catheter is guided into the uterine arteries—left first, then right— and contrast medium is injected into each artery to confirm the position of the catheter and the presence of fibroids or adenomyosis, which appear as hypervascular lesions in angiograms (see above, right).
UAE usually requires 1 to 2 hours.
Embolic agents
Polyvinyl alcohol (PVA) particles or trisacryl gelatin microspheres, usually 500 to 700 and/or 700 to 900 microns in size, are released through the catheter into the uterine arteries. These agents block the blood vessels that feed the fibroids and/or adenomyosis, causing them to shrink. The agents are biocompatible and have been approved by the US Food and Drug Administration.
Other, less frequently used embolic agents include gelatin sponge particles (which are temporary) and coils (which are permanent). Coils are generally used for conditions such as arteriovenous malformations or fistulae, which have large feeding vessels (iliac or enlarged uterine or ovarian vessels). This fluoroscopy-guided procedure usually is performed under local anesthesia and conscious sedation or, less often, epidural anesthesia.
Patient care
Conscious sedation, NSAIDs, and antibiotics
Intravenous conscious sedation in conjunction with nonsteroidal anti-inflammatory drugs (NSAIDs) usually provides sufficient pain relief.
In addition, intravenous broad-spectrum antibiotics are used as prophylaxis for infection linked to the embolization itself and to subsequent ischemia of the fibroids and uterus.
Managing postop pain syndrome
More than 90% of women experience postembolization syndrome, which includes moderate to severe abdominal pain/cramping and nausea and vomiting in the first several hours following the procedure. As a result, they may require hospitalization (less than 24 hours) for pain management. In our experience, few women stay in the hospital more than 1 day.
A patient-controlled analgesia pump and NSAIDs are used in women with abdominal/pelvic cramping and pain (more than 90% of cases) if epidural anesthesia is not used for pain.
Low-grade fever and leukocytosis are not uncommon after embolization, and are usually treated with acetaminophen. Other symptoms are anorexia and fatigue, but they gradually subside within 3 to 4 days.
After discharge
Oral NSAIDs and narcotics are often needed for several days. Many women resume light activities in a few days, and most return to normal activities within 1 week.11
Give her comprehensive discharge instructions on taking medications, what to expect, and when to contact a doctor. Follow-up visit in 1 to 4 weeks. We schedule an outpatient visit 1 to 4 weeks after the procedure. At this visit, we confirm healing of the puncture sites, screen for unusual symptoms or potential problems, and repeat follow-up instructions.11
We then follow the patient periodically (3, 6, and 12 months) to monitor her for symptoms and complications such as late infections, expulsion of infarcted fibroids, chronic endometritis, chronic vaginal discharge, and cessation or irregularity of menses, all of which have been observed after UAE.11
Transvaginal ultrasound is usually performed 3 to 6 months and 1 year after UAE to determine whether existing fibroids have been infarcted and begun to decrease in volume. It also reveals any uterine or adnexal complications.
In addition, this imaging provides a new baseline measurement of fibroid volume, against which any subsequent increase in size (which may indicate regrowth of fibroids or undiagnosed leiomyosarcoma) can be compared.11
Key findings of outcome studies
Two large series reported significant improvement in AUB in 77% to 90% of fibroid cases, and bulk-related symptoms were controlled in 86% to 91%.6-8 In these studies, average uterine volumes decreased by 35% and 58% at 3 and 12 months, respectively, with dominant fibroid shrinkage of 42%. Several large series also reported high patient satisfaction (91% to 93%) and significant improvement in quality-of-life measures.4,6-8
Side effects and complications
Although UAE is considered very safe, it carries some risks. Spies et al17 reported on complications in 400 consecutive patients undergoing UAE for fibroids at their institution:
- 1.25% serious complication rate
- 5% overall periprocedural morbidity rate
- no deaths and no major permanent injuries
In addition, 1 patient required hysterectomy as a result of a complication, and 1 patient had an undiagnosed leiomyosarcoma, which was discovered during an elective myomectomy 31 months after UAE.
Goldberg et al 18 reported another case with delayed diagnosis of leiomyosarcoma following UAE. In our series of 705 patients, 1 had an undiagnosed leiomyosarcoma, which presented as a pelvic mass 15 months after UAE. She subsequently underwent hysterectomy.
When to suspect leiomyosarcoma
Unlike hysterectomy or myomectomy, no tissue is obtained in UAE for pathologic diagnosis to exclude leiomyosarcoma, which is found in approximately 0.1% to 0.4% of women with fibroids and is difficult to differentiate from a benign leiomyoma using clinical tests or imaging.17-18
Suspect leiomyosarcoma if the fibroids continue to grow even after technically successful embolization.
Infection is rare, but can be lethal
A small number of patients have experienced infection, which usually is controlled with antibiotics. In a series of 414 UAE procedures in 410 fibroid patients, Rajan et al19 reported:
- 1.2% rate of intrauterine infection requiring intravenous antibiotic therapy and/or surgery
- no significant difference seen with various embolic agents, quantity of embolic particles, se of preprocedure antibiotics, or size or location of the dominant fibroid.
However, at least 2 deaths have been reported due to infection since UAE for fibroids was introduced in the mid-1990s: 1 fatal sepsis in a woman who underwent UAE for fibroids and 1 other sepsis fatality.17,20 The first case was caused by necrosis of the vaginal wall and uterine cervix. At autopsy, microspheres were found not only in arteries in the leiomyomata and myometrium, but also in the parametria and vagina, causing ischemic necrosis.
Amenorrhea or worsened AUB
In some cases, amenorrhea can follow UAE for fibroids due to ovarian embolization and subsequent ovarian failure.6-8,17
The literature indicates a rate of:
- 1% to 2% in patients less than 45 years of age
- 15% to 20% for perimenopausal women 45 and older
Worsening of uterine bleeding is rare after UAE, but can occur. Kerlan et al21 reported massive uterine bleeding 1 month after UAE in a woman who underwent the procedure for menorrhagia. When she was treated with emergent hysterectomy, a bleeding ulceration of the endometrium overlying the necrotic fibroid was found.
Other complications include spotting, hot flashes, fever, vaginal discharge, mood swings, pain at the puncture site, and dysuria.6-8,17
Our UAE experience
The New England Fibroid Center began offering fibroid embolization in 1997. Since then, we have performed 705 procedures at 5 hospitals in the Greater Boston region, with a technical success rate of 99%. Technical failure occurred in 1% of patients; these women had very difficult vascular anatomy involving uterine arteries, or ovarian arteries formed the dominant blood supply to the fibroids.
Clinical success or improvement was seen in 80% of women with bulk-related symptoms and 94.3% with bleeding symptoms.
Clinical failure occurred in 5.7% of women (1.6% required repeat UAE and 1.4% hysterectomies due to persistent symptoms).
Complications occurred in 4% of cases (2% rate of premature ovarian failure, 1.5% rate of transvaginal passage of infarcted fibroids, and 0.5% rate of groin hematoma). There were no major complications requiring transfusion or emergent surgeries such as hysterectomy.
Fertility after UAE
LISA’S CASE
“Cure” and pregnancy
Lisa successfully underwent UAE, and had no symptoms after the procedure. The uterine fibroids resolved almost completely in 1 year.
Three years after the procedure, she became pregnant and delivered a healthy, full-term infant.
Although UAE is generally not performed in women who wish to preserve their fertility, it is sometimes used in fibroid patients when myomectomy is contraindicated because of the size and/or number of fibroids.11,22,23 Only a few small series and case reports describe successful pregnancies following UAE.
For example, in a study involving 400 women, McLucas et al22 reported 17 pregnancies in 14 women among 149 patients who stated a desire for fertility after UAE. Of these, 5 spontaneous abortions were observed, and 10 women had normal term deliveries. No perfusion or other problems were reported during pregnancy or labor.
Goldberg and colleagues23 analyzed 50 published cases of post-UAE pregnancies and found higher rates of cesarean delivery, preterm birth, malpresentation, small-for-gestational-age infants, spontaneous abortion, and postpartum hemorrhage than in the general population, though the reasons were unclear.
In our experience at the New England Fibroid Center, 5 of 12 patients below the age of 40 who wanted to preserve fertility became pregnant and successfully delivered full-term infants.
In general, the risks of infertility, premature ovarian failure/menopause, radiation exposure, and hysterectomy following UAE are small and compare favorably with those associated with myomectomy. Fertility rates are similar to those for women undergoing myomectomy.24
Nevertheless, well-controlled studies and additional data are needed before UAE can be confidently recommended as a first-line approach for preserving fertility.11
Treating adenomyosis
ANGELINA’S CASE
Adenomyosis resolves
During Angelina’s UAE procedure, angiographies showed enlarged right and left uterine arteries with numerous prominent intrauterine branches supplying the enlarged uterus. After UAE with PVA microspheres, post-embolization angiograms showed occlusion of the right and left uterine arteries and their branches.
Her symptoms resolved completely following the procedure. One year later, a follow-up MRI showed normal uterine size and shape, with complete resolution of adenomyosis.
Several small series have reported successful treatment of women with symptomatic adenomyosis. For example, of 23 women who underwent UAE for this indication, Chen and colleagues9 reported:
- Complete resolution of dysmenorrhea in 19 women and significant improvement in 2. Two other patients had recurrent symptoms.
- A substantial decrease in uterine volume in most of the women.
- An immediate decrease in intrauterine blood flow detected by color Doppler ultrasonography.
In a prospective study10 involving 18 women with symptomatic adenomyosis:
- 94% had diminished menorrhagia 6 months after UAE, and 94% had a slight decrease (mean: 15%) in uterine volume.
- After 1 year, 73% of women had diminished menorrhagia, and 53% had complete resolution.
- After 2 years, 56% of women had complete resolution of menorrhagia, 44% required additional treatment due to failure or recurrence, and 28% underwent hysterectomy.
In our limited experience with adenomyosis at the New England Fibroid Center, we saw no significant difference in technical success rates (100%) after UAE, compared with fibroid patients. However, there was a relatively high recurrence rate (2 of 6 patients) of presenting symptoms (menorrhagia or dysmenorrhea), and 2 patients later underwent hysterectomy.
Well-controlled studies are needed before UAE can confidently be recommended for symptomatic adenomyosis.
The authors report no financial relationships relevant to this article.
1. Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346:671-672.
2. Worthington-Kirsch RL, Siskin GP. Uterine artery embolization for symptomatic myomata. J Intensive Care Med. 2004;19:13-21.
3. Bradley EA, Reidy JF, Forman RG, et al. Transcatheter uterine artery embolisation to treat large uterine fibroids. Br J Obstet Gynaecol. 1998;105:235-240.
4. Worthington-Kirsch RL, Popky GL, Huchins FL, Jr. Uterine artery embolization for the management of leiomyomas: quality-of-life assessment and clinical response. Radiology. 1998;208:625-629.
5. Goodwin SC, Vedantham S, et al. Preliminary experience with uterine artery embolization for uterine fibroids. J Vasc Interv Radiol. 1997;8:517-526.
6. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow-up. BJOG. 2002;11:1262-1272.
7. Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
8. Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
9. Chen C, et al. Uterine arterial embolization in the treatment of adenomyosis. Zhonghua Fu Chan Ke Za Zhi. 2002;37:77-79.
10. Pelage JP, Jacob D, et al. Midterm results of uterine artery embolization for symptomatic adenomyosis: initial experience. Radiology. 2005;234:948-953.
11. Andrews RT, Spies JB, Sacks D, et al. Patient care and uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2004;15:115-120.
12. Broder MS, et al. Uterine Artery Embolization: A Systematic Review of the Literature and Proposal for Research. Santa Monica, Calif: Rand; 1999. Publication MR-1158.
13. Siskin GP, Tublin ME, Stainken BF, et al. Uterine artery embolization for the treatment of adenomyosis: clinical response and evaluation with MR imaging. AJR Am J Roentgenol. 2001;177:297-302.
14. Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1979;102:303-306.
15. Outwater EK, Siegelman ES, Van Deerlin V. Adenomyosis: current concepts and imaging considerations. AJR Am J Roentgenol. 1998;170:437-441.
16. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. 1999;19:S161-S170.
17. Spies JB, Spector A, Roth AR, et al. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
18. Goldberg J, Burd I, et al. Leiomyosarcoma in a premenopausal patient following uterine artery embolization. Am J Obstet Gynecol. 2004;191:1733-1735.
19. Rajan DK, Beecroft JR, Clark TW, et al. Risk of intrauterine infectious complications after uterine artery embolization. J Vasc Interv Radiol. 2004;15:1415-1421.
20. Vashisht A, Studd J, Carey A, Burn P. Fatal septicemia after fibroid embolisation [letter]. Lancet. 1999;354:307-308.
21. Kerlan K, Jr, Coffey JO, Milkman MS, et al. Massive vaginal hemorrhage after uterine fibroid embolization. J Vasc Interv Radiol. 2003;14:1465-1467.
22. McLucas B, Goodwin S, Adler L, Rappaport A, Reed R, Perrella R. Pregnancy following uterine fibroid embolization. Int J Gynaecol Obstet. 2001;74:1-7.
23. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872.
24. Goldberg J, Pereira L, Berghella V, et al. Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol. 2004;191:18-21.
1. Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346:671-672.
2. Worthington-Kirsch RL, Siskin GP. Uterine artery embolization for symptomatic myomata. J Intensive Care Med. 2004;19:13-21.
3. Bradley EA, Reidy JF, Forman RG, et al. Transcatheter uterine artery embolisation to treat large uterine fibroids. Br J Obstet Gynaecol. 1998;105:235-240.
4. Worthington-Kirsch RL, Popky GL, Huchins FL, Jr. Uterine artery embolization for the management of leiomyomas: quality-of-life assessment and clinical response. Radiology. 1998;208:625-629.
5. Goodwin SC, Vedantham S, et al. Preliminary experience with uterine artery embolization for uterine fibroids. J Vasc Interv Radiol. 1997;8:517-526.
6. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow-up. BJOG. 2002;11:1262-1272.
7. Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
8. Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
9. Chen C, et al. Uterine arterial embolization in the treatment of adenomyosis. Zhonghua Fu Chan Ke Za Zhi. 2002;37:77-79.
10. Pelage JP, Jacob D, et al. Midterm results of uterine artery embolization for symptomatic adenomyosis: initial experience. Radiology. 2005;234:948-953.
11. Andrews RT, Spies JB, Sacks D, et al. Patient care and uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2004;15:115-120.
12. Broder MS, et al. Uterine Artery Embolization: A Systematic Review of the Literature and Proposal for Research. Santa Monica, Calif: Rand; 1999. Publication MR-1158.
13. Siskin GP, Tublin ME, Stainken BF, et al. Uterine artery embolization for the treatment of adenomyosis: clinical response and evaluation with MR imaging. AJR Am J Roentgenol. 2001;177:297-302.
14. Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1979;102:303-306.
15. Outwater EK, Siegelman ES, Van Deerlin V. Adenomyosis: current concepts and imaging considerations. AJR Am J Roentgenol. 1998;170:437-441.
16. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. 1999;19:S161-S170.
17. Spies JB, Spector A, Roth AR, et al. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
18. Goldberg J, Burd I, et al. Leiomyosarcoma in a premenopausal patient following uterine artery embolization. Am J Obstet Gynecol. 2004;191:1733-1735.
19. Rajan DK, Beecroft JR, Clark TW, et al. Risk of intrauterine infectious complications after uterine artery embolization. J Vasc Interv Radiol. 2004;15:1415-1421.
20. Vashisht A, Studd J, Carey A, Burn P. Fatal septicemia after fibroid embolisation [letter]. Lancet. 1999;354:307-308.
21. Kerlan K, Jr, Coffey JO, Milkman MS, et al. Massive vaginal hemorrhage after uterine fibroid embolization. J Vasc Interv Radiol. 2003;14:1465-1467.
22. McLucas B, Goodwin S, Adler L, Rappaport A, Reed R, Perrella R. Pregnancy following uterine fibroid embolization. Int J Gynaecol Obstet. 2001;74:1-7.
23. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872.
24. Goldberg J, Pereira L, Berghella V, et al. Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol. 2004;191:18-21.
Catastrophic intraoperative hemorrhage: 5-step action plan
Placenta accreta leads to hemorrhage
Sally is a 27-year-old gravida with 1 prior cesarean whose ultrasound imaging is suspicious for “placenta adherent to the bladder.” At 38 weeks, she delivers a viable infant by classical cesarean, at which time the ultrasound finding is confirmed: the placenta is densely adherent.
The placenta is left in situ, no methotrexate is given, and Sally is followed with clotting studies and exams.
Eight weeks later, when her fibrinogen level falls and the prothrombin time and partial thromboplastin time become abnormal, the obstetrician attempts to perform dilatation and evacuation, but massive bleeding ensues. The physician then performs a total abdominal hysterectomy, but bleeding continues from the cuff.
What is the best way to manage the hemorrhage?
After identifying its source, the surgeon should apply pressure to abate the bleeding, using packing if necessary, and repair the affected artery or vein. Fortunately, we have many tools at our disposal, from preventive steps like careful preoperative assessment to the use of hemostatic agents, fibrin glues, hypogastric artery ligation, and specialized pelvic packing techniques. With prompt action and a stepwise approach, this bona fide catastrophe can usually be successfully managed. This article details a 5-step action plan.
If massive bleeding occurs during laparoscopic or vaginal surgery, a laparotomy may be indicated, and intraoperative management would follow the same 5 steps.
STEP 1Like the Boy Scouts, Be Prepared
Although surgeons are acutely aware that drugs such as warfarin and heparin can cause intraoperative bleeding, the patient history and predisposing factors sometimes get short shrift.
Besides asking about the patient’s medications, assess the following:
- Platelets. The primary laboratory test to evaluate potential bleeding is the platelet count. In general, 10,000 to 20,000 platelets are needed for hemostasis. However, 50,000 are needed for any surgery or invasive procedure, such as insertion of a central line.1 I recommend platelet evaluation for patients scheduled for major abdominal surgery.
- History of bleeding. If the patient or her family has a history of bleeding with any surgery, evaluate her for von Willebrand’s disease.
- High alcohol intake warrants preoperative liver function and coagulation studies.
- Some herbal or natural remedies can exacerbate intraoperative hemorrhage through their inhibition of coagulation, especially the agents listed in TABLE 1. They should generally be discontinued 2 to 7 days before surgery.2
- Aspirin and nonsteroidal anti-inflammatory drugs should be discontinued 7 days before anticipated surgery. However, patients may continue aspirin at a daily dose of 81 mg.
- Poor nutrition and obesity predispose the patient to wound complications and intraoperative bleeding. Patients who are severely malnourished can take dietary supplements or receive total parenteral nutrition prior to surgery.
- Intraoperative factors such as the 3 “inadequacies” (inadequate incision, retraction, and anesthesia), low core body temperature, severe adhesions (ie, endometriosis), and large vascular tumors also are sometimes associated with bleeding.
TABLE 1
Alternative remedies that may exacerbate bleeding
- 32% to 37% of Americans use these remedies, but only 38% of them tell their doctor
- Stop all alternative remedies 2 to 7 days before surgery
| REMEDY | USED FOR | PERIOPERATIVE RISKS |
|---|---|---|
| Beta-carotene | Vitamin A precursor; often taken as a nutritional supplement | May cause coagulopathy |
| Feverfew | Used to prevent or treat migraine and ease menstrual cramps | May inhibit coagulation |
| Fish oil | Rich in omega-3 fatty acids, recommended for cardiovascular health | Omega-3s inhibit coagulation |
| Garlic | Used to reduce hypertension and high cholesterol | Case reports of unexpected or increased surgical bleeding, prolonged bleeding time, and impaired platelet aggregation |
| Ginkgo | Treatment of dementia, impaired cognition, and memory | Various ginkgolides have platelet-activating-factor antagonist properties; case reports of spontaneous bleeding |
| Ginseng | Widely used as a stimulant, tonic, diuretic, mood elevator, and energy booster | May cause hypertension, cardiovascular instability, coagulopathy, and sedation |
| St. John’s wort | Antidepressant | May cause cardiovascular instability, coagulopathy, and sedation |
| Vitamin E | Antioxidant | May interfere with coagulation |
STEP 2Follow These Basic Principles
Whenever bleeding is encountered in any area of the abdominal cavity, the first step is simple: Apply immediate pressure with a finger or sponge stick. Then obtain exposure and assistance. Exposure usually means extending the incision and using a fixed table retractor.
If the source of bleeding is unknown, apply pressure on the aorta using a hand, weighted speculum, or Conn aortic compressor (Pilling-branded, Teleflex Medical, Limerick, Pa).
Secure individual vessels with finetipped clamps and small-caliber sutures or clips, and minimize the use of clamps. Never place clamps or sutures blindly, and never use electrocautery for large lacerations.
If you choose to use packs to temporarily control bleeding, insert them carefully to avoid tearing veins, and place pelvic packs (hot or cold) in a stepwise fashion, from sidewall to sidewall. Leave packs in place for at least 15 minutes and remove them sequentially.
Great vessel injuries
The aorta, vena cava, and common iliac vessels are sometimes injured during removal of paraaortic nodes or when the inferior mesenteric vessels are avulsed during retraction of the sigmoid colon. In addition, needle or trocar injuries during operative laparoscopy occur in as many as 4 of every 10,000 procedures.3
Again, the first step in managing great vessel injuries is applying pressure. Then obtain blood components, and, if necessary, consult with a vascular surgeon or gynecologic oncologist.
In general, once the patient is hemodynamically stable, the affected vessel should be compressed proximally and distally. Use Allis or vascular clamps on the torn edges to elevate the lacerated area. My preference is to close these injuries with a running 5-0 or 6-0 nylon or monofilament polypropylene (MFPP) suture on a cardiovascular needle.
Replacing blood and its components
Be aware of the following replacement guidelines for catastrophic intraoperative hemorrhage:
- For every 8 U of red blood cells replaced, give 2 U of fresh frozen plasma.
- If more than 10 U of red blood cells are replaced, give 10 U of platelets, preferably at the end of the procedure.
- With prolonged PTT, give fresh frozen plasma.
- If fibrinogen is low, give 2 U of cryoprecipitate.1
When massive bleeding is anticipated or encountered, the Haemonetics Cell Saver (Haemonetics Corp, Braintree, Mass) is invaluable. This device, which requires a trained technician, removes blood from the operative field, anticoagulates it, and washes red blood cells, which are infused. It is accepted by many Jehovah’s Witnesses,4 and has been used safely in women with cesarean-associated bleeding.5 Relative contraindications include malignancy and bacterial contamination from a ruptured abscess or inadvertent injury to unprepared bowel.6 The Cell Saver may be used after heavy bleeding from hysterectomy or in patients with ruptured membranes.
STEP 3Try A Topical Hemostatic Agent
If hemorrhage contiues after arterial bleeders are secured, consider a topical hemostatic agent (TABLE 2). All such agents require pressure to be applied for 3 to 5 minutes.
My preferences are Surgicel (Johnson & Johnson, New Brunswick, NJ) and Gelfoam (Pharmacia, Kalamazoo, Mich). In general, Avitene Ultrafoam collagen hemostat (Davol, subsidiary of C.R. Bard, Murray Hill, NJ) works poorly in the presence of thrombocytopenia and should be used with caution near the ureter.
Fibrin glue has been widely used as a hemostatic agent in microvascular, cardiovascular, and thoracic surgery.
To prepare fibrin glue at my institution, we use a double-barrel syringe to apply equal amounts of cryoprecipitate and thrombin at the same time. One fibrin sealant, Tisseal VH (Baxter Healthcare, Deerfield, Ill), comes with a Duploject applicator. After the agent is thoroughly applied (it is sprayed), pressure is applied for 3 to 5 minutes.
The same manufacturer also produces Coseal, which is used in vascular reconstruction to achieve additional hemostasis by mechanically sealing off areas of leakage, and Floseal, to help achieve hemostasis when ligatures or clips are impractical.
TABLE 2
Topical intraperitoneal hemostatic agents
| AGENT | WHAT IT IS | HOW IT IS APPLIED |
|---|---|---|
| Avitene Ultrafoam | Absorbable collagen hemostat | Comes in powder; sprinkle on area |
Fibrin glue
| Equal amounts of cryoprecipitate and thrombin | Spray on affected area with double-barrel syringe or device supplied by Baxter Healthcare |
| Gelfoam | Absorbable gelatin sponge | Cut in strips of appropriate size and apply to area |
| Surgicel | Oxidized regenerated cellulose | Cut in strips of appropriate size and apply to area |
STEP 4Hypogastric Artery Ligation
SALLY’S CASE
Bleeding persists
Because of the hemorrhage, a gynecologic oncology consult is obtained and the hypogastric artery is ligated bilaterally, but bleeding continues. During further exploration, the left ureter is found to be ligated. Sally receives 65 U of packed red blood cells, platelets, and fresh frozen plasma. The Cell Saver also is used.
If pelvic oozing persists after application of a topical hemostatic agent, consider hypogastric artery ligation, which controls pelvic hemorrhage in as many as 50% of patients.7,8
STEP 5When All Else Fails: “Pack And Go”
If intraoperative bleeding persists despite hypogastric artery ligation and the other measures, the life-saving modality of choice is a pelvic pack left in place 2 to 3 days. I prefer a fast, simple method: “pack and go” or damage-control technique.10-12
A 2- to 4-inch Kerlix gauze (Kendall Health Care Products, Mansfield, Mass) is tightly packed over a fibrin glue bed from side to side in the pelvis. Only the skin is closed using towel clips or a running suture. The patient is immediately transferred to intensive care, where acidosis, coagulopathy, and hypothermia are corrected. In 48 to 72 hours, the packs are gently removed with saline drip assistance. If hemostasis still has not been achieved, repacking is an option.
Presacral venous bleeding
Two helpful methods to quell presacral venous bleeding are:
- inserting stainless steel thumbtacks
- indirect coagulation through a muscle fragment
The thumbtack method
The presacral veins are sometimes injured during presacral neurectomy, sacrocolpopexy, or posterior exenteration. This bleeding can be controlled by inserting stainless steel thumbtacks, with direct pressure from the surgeon’s hand, directly into the sacrum.15-17 These work by compressing veins adjacent to the bone, and are left in place permanently. No complications have been reported.
Indirect coagulation
Another method of controlling presacral venous bleeding is indirect coagulation through a muscle fragment. This is done by harvesting a 2 x 1 cm piece of muscle from the rectus abdominus and pressing it against the bleeding veins. Then set a Bovie (Valley Lab, Boulder, Colo) at 40 W of pure cutting current and apply it to the muscle fragment for 1 to 2 minutes. This method has been successful in 12 of 12 reported cases.18,19
Other methods of controlling presacral venous bleeding include bipolar cautery, use of bone wax, and suturing in “sandwiches” of Avitene alternated with Gelfoam, but these strategies have met with limited success.
Pelvic hemorrhage
Arterial embolization
Angiographic insertion of Gelfoam pledgets or Silastic coils may effectively control pelvic hemorrhage in up to 90% of postpartum and postoperative patients.20,21 Hypogastric artery embolization can also be done intraoperatively.22
However, this technique should be used with caution, as it may require 1 to 2 hours to perform and is inappropriate for patients with hypovolemic shock. Complications are rare, but can occur in up to 6% to 7% of patients.21 They include postoperative fever, pelvic abscess formation, reflux of embolic material, nontarget embolization, foot and buttocks ischemia, bladder and rectal wall necrosis, and late rebleeding.
Arterial embolization does not appear to affect subsequent pregnancies.23
Military antishock trousers The MAST or aviation “G” suit is sometimes used as an intermediate step to laparotomy in patients with ectopic pregnancy or postoperative or postpartum hemorrhage.24 Its major use is to stabilize patients for surgery by compressing peripheral circulation, thereby diverting blood to the core circulation.
Inflate the legs first, then the abdomen; leave the MAST suit in place for 2 to 48 hours; and deflate in reverse order.
Contraindications include pulmonary edema, cardiogenic shock, rupture of the diaphragm, and pregnancy.
SALLY’S CASE
Hemorrhage abates
A “pack and go” technique is used to control bleeding. The fascia is left open, and the skin is closed with towel clips over the tight pelvic pack. Sally is sent to the ICU, where clotting parameters are corrected.
She undergoes reoperation 36 hours later, at which time no bleeding is encountered.
The left ureter is reimplanted into the bladder, and she makes a full recovery.
The author has served on the speakers bureau for Wyeth.
1. Nolan TE, Gallup DG. Massive transfusion: a current review. Obstet Gynecol Surv. 1991;46:289-295
2. Ang-Lee MK, Moss J, Yuan C-S. Herbal medicine and preoperative care. JAMA. 2001;286:208-216.
3. Härkü-Siren P, Sjöberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.
4. deCastro RM. Bloodless surgery: establishment of a program for the special needs of the Jehovah’s Witness community: the gynecologic surgery experience at a community hospital. Obstet Gynecol. 1999;180:149-158.
5. Rebarber A, Lonser R, Jackson S, Copel JA, Siple S. The safety of intraoperative blood collection and autotransfusion during cesarean section. Am J Obstet Gynecol. 1998;169:715-720.
6. Klimberg I, Sirois R, Wajsman Z, Baker J. Intraoperative autotransfusion in urologic oncology Arch Surg. 1986;121:1326-1329.
7. Clark SL, Phelan JP, Yeh Z-Y, Bruce SR, Paul RH. Hypogastric artery ligation for obstetric hemorrhage. Obstet Gynecol. 1985;66:353-356.
8. Thavarash AS, Sivalingam N, Almohdzar SA. Internal iliac and ovarian artery ligation in the control of pelvic hemorrhage. Aust N Z J Obstet Gynecol. 1989;29:22-25.
9. Burchell RC. Internal iliac ligation. Haemodynamics. Obstet Gynecol. 1964;5:53-59.
10. Finan MA, Fiorica JV, Hoffman MS, et al. Massive pelvic hemorrhage during gynecologic cancer surgery: “pack and go back.” Gynecol Oncol. 1996;62:390-395.
11. Rotondo MF, Zonies DH. The damage control sequence and underlying logic. Surg Clin N Am. 1997;77:761-777.
12. Inge JA, Gallup DG, Davis FE. Catastrophic hemorrhage from placenta previa-accreta. A case series and guidelines for management. J Pelvic Surg. 2000;6:268-272.
13. Cassels JW Jr, Greenberg H, Otterson WN. Pelvic tamponade in puerperal hemorrhage. J Reprod Med. 1985;30:689-692.
14. Hallack M, Didly GA, III, Hurley TJ, Moise KJ, Jr. Transvaginal pressure pack for life-threatening pelvis hemorrhage secondary to placenta accreta. Obstet Gynecol. 1991;78:938-940.
15. Khan FA, Fang DT, Nivatvongs S. Management of presacral hemorrhage during rectal resection. Surg Gynecol Obstet. 1987;165:275-277.
16. Pastner B, Orr JW. Intractable venous hemorrhage: use of stainless steel thumbtacks to obtain hemostasis. Am J Obstet Gynecol. 1990;162:452-455.
17. Timmons MC, Kohler MF, Addison WA. Thumbtack use for control of presacral bleeding with description of an instrument for thumbtack application. Obstet Gynecol. 1991;78:313-315.
18. Xu J, Lin J. Control of presacral hemorrhage with electrocautery through a muscle fragment pressed on the bleeding vein. J Am Coll Surg. 1994;179:351-354.
19. Miklos JR, Kohli N, Sze EH. Control of presacral hemorrhage using indirect coagulation through a muscle fragment. J Pelvic Surg. 1996;2:268-270.
20. Hansch E, Chitkara U, McAlpine J, El-Sayed Y, Dake MD, Razavi MK. Pelvic arterial embolization for control of obstetric hemorrhage: a five-year experience. Obstet Gynecol. 1999;180:1454-1460.
21. Verdantham S, Goodwin SC, McLucas B, Mohr G. Uterine artery embolization: an underused method of controlling pelvic hemorrhage. Am J Obstet Gynecol. 1997;176:938-946.
22. Saueracker AJ, McCroskey BL, Moor EE, Moore FA. Intraoperative hypogastric artery embolization for life-threatening pelvic hemorrhage: a preliminary report. J Trauma. 1987;27:1127-1129.
23. Orman D, White R, Pollak J, Tal M. Pelvic embolization for intractable postpartum hemorrhage: long-term follow-up and implications for fertility. Obstet Gynecol. 2003;102:904-910.
24. Pearse CS, Magrina JF, Finley BE. Use of MAST suit in obstetrics and gynecology. Obstet Gynecol Surv. 1984;39:416-422.
Placenta accreta leads to hemorrhage
Sally is a 27-year-old gravida with 1 prior cesarean whose ultrasound imaging is suspicious for “placenta adherent to the bladder.” At 38 weeks, she delivers a viable infant by classical cesarean, at which time the ultrasound finding is confirmed: the placenta is densely adherent.
The placenta is left in situ, no methotrexate is given, and Sally is followed with clotting studies and exams.
Eight weeks later, when her fibrinogen level falls and the prothrombin time and partial thromboplastin time become abnormal, the obstetrician attempts to perform dilatation and evacuation, but massive bleeding ensues. The physician then performs a total abdominal hysterectomy, but bleeding continues from the cuff.
What is the best way to manage the hemorrhage?
After identifying its source, the surgeon should apply pressure to abate the bleeding, using packing if necessary, and repair the affected artery or vein. Fortunately, we have many tools at our disposal, from preventive steps like careful preoperative assessment to the use of hemostatic agents, fibrin glues, hypogastric artery ligation, and specialized pelvic packing techniques. With prompt action and a stepwise approach, this bona fide catastrophe can usually be successfully managed. This article details a 5-step action plan.
If massive bleeding occurs during laparoscopic or vaginal surgery, a laparotomy may be indicated, and intraoperative management would follow the same 5 steps.
STEP 1Like the Boy Scouts, Be Prepared
Although surgeons are acutely aware that drugs such as warfarin and heparin can cause intraoperative bleeding, the patient history and predisposing factors sometimes get short shrift.
Besides asking about the patient’s medications, assess the following:
- Platelets. The primary laboratory test to evaluate potential bleeding is the platelet count. In general, 10,000 to 20,000 platelets are needed for hemostasis. However, 50,000 are needed for any surgery or invasive procedure, such as insertion of a central line.1 I recommend platelet evaluation for patients scheduled for major abdominal surgery.
- History of bleeding. If the patient or her family has a history of bleeding with any surgery, evaluate her for von Willebrand’s disease.
- High alcohol intake warrants preoperative liver function and coagulation studies.
- Some herbal or natural remedies can exacerbate intraoperative hemorrhage through their inhibition of coagulation, especially the agents listed in TABLE 1. They should generally be discontinued 2 to 7 days before surgery.2
- Aspirin and nonsteroidal anti-inflammatory drugs should be discontinued 7 days before anticipated surgery. However, patients may continue aspirin at a daily dose of 81 mg.
- Poor nutrition and obesity predispose the patient to wound complications and intraoperative bleeding. Patients who are severely malnourished can take dietary supplements or receive total parenteral nutrition prior to surgery.
- Intraoperative factors such as the 3 “inadequacies” (inadequate incision, retraction, and anesthesia), low core body temperature, severe adhesions (ie, endometriosis), and large vascular tumors also are sometimes associated with bleeding.
TABLE 1
Alternative remedies that may exacerbate bleeding
- 32% to 37% of Americans use these remedies, but only 38% of them tell their doctor
- Stop all alternative remedies 2 to 7 days before surgery
| REMEDY | USED FOR | PERIOPERATIVE RISKS |
|---|---|---|
| Beta-carotene | Vitamin A precursor; often taken as a nutritional supplement | May cause coagulopathy |
| Feverfew | Used to prevent or treat migraine and ease menstrual cramps | May inhibit coagulation |
| Fish oil | Rich in omega-3 fatty acids, recommended for cardiovascular health | Omega-3s inhibit coagulation |
| Garlic | Used to reduce hypertension and high cholesterol | Case reports of unexpected or increased surgical bleeding, prolonged bleeding time, and impaired platelet aggregation |
| Ginkgo | Treatment of dementia, impaired cognition, and memory | Various ginkgolides have platelet-activating-factor antagonist properties; case reports of spontaneous bleeding |
| Ginseng | Widely used as a stimulant, tonic, diuretic, mood elevator, and energy booster | May cause hypertension, cardiovascular instability, coagulopathy, and sedation |
| St. John’s wort | Antidepressant | May cause cardiovascular instability, coagulopathy, and sedation |
| Vitamin E | Antioxidant | May interfere with coagulation |
STEP 2Follow These Basic Principles
Whenever bleeding is encountered in any area of the abdominal cavity, the first step is simple: Apply immediate pressure with a finger or sponge stick. Then obtain exposure and assistance. Exposure usually means extending the incision and using a fixed table retractor.
If the source of bleeding is unknown, apply pressure on the aorta using a hand, weighted speculum, or Conn aortic compressor (Pilling-branded, Teleflex Medical, Limerick, Pa).
Secure individual vessels with finetipped clamps and small-caliber sutures or clips, and minimize the use of clamps. Never place clamps or sutures blindly, and never use electrocautery for large lacerations.
If you choose to use packs to temporarily control bleeding, insert them carefully to avoid tearing veins, and place pelvic packs (hot or cold) in a stepwise fashion, from sidewall to sidewall. Leave packs in place for at least 15 minutes and remove them sequentially.
Great vessel injuries
The aorta, vena cava, and common iliac vessels are sometimes injured during removal of paraaortic nodes or when the inferior mesenteric vessels are avulsed during retraction of the sigmoid colon. In addition, needle or trocar injuries during operative laparoscopy occur in as many as 4 of every 10,000 procedures.3
Again, the first step in managing great vessel injuries is applying pressure. Then obtain blood components, and, if necessary, consult with a vascular surgeon or gynecologic oncologist.
In general, once the patient is hemodynamically stable, the affected vessel should be compressed proximally and distally. Use Allis or vascular clamps on the torn edges to elevate the lacerated area. My preference is to close these injuries with a running 5-0 or 6-0 nylon or monofilament polypropylene (MFPP) suture on a cardiovascular needle.
Replacing blood and its components
Be aware of the following replacement guidelines for catastrophic intraoperative hemorrhage:
- For every 8 U of red blood cells replaced, give 2 U of fresh frozen plasma.
- If more than 10 U of red blood cells are replaced, give 10 U of platelets, preferably at the end of the procedure.
- With prolonged PTT, give fresh frozen plasma.
- If fibrinogen is low, give 2 U of cryoprecipitate.1
When massive bleeding is anticipated or encountered, the Haemonetics Cell Saver (Haemonetics Corp, Braintree, Mass) is invaluable. This device, which requires a trained technician, removes blood from the operative field, anticoagulates it, and washes red blood cells, which are infused. It is accepted by many Jehovah’s Witnesses,4 and has been used safely in women with cesarean-associated bleeding.5 Relative contraindications include malignancy and bacterial contamination from a ruptured abscess or inadvertent injury to unprepared bowel.6 The Cell Saver may be used after heavy bleeding from hysterectomy or in patients with ruptured membranes.
STEP 3Try A Topical Hemostatic Agent
If hemorrhage contiues after arterial bleeders are secured, consider a topical hemostatic agent (TABLE 2). All such agents require pressure to be applied for 3 to 5 minutes.
My preferences are Surgicel (Johnson & Johnson, New Brunswick, NJ) and Gelfoam (Pharmacia, Kalamazoo, Mich). In general, Avitene Ultrafoam collagen hemostat (Davol, subsidiary of C.R. Bard, Murray Hill, NJ) works poorly in the presence of thrombocytopenia and should be used with caution near the ureter.
Fibrin glue has been widely used as a hemostatic agent in microvascular, cardiovascular, and thoracic surgery.
To prepare fibrin glue at my institution, we use a double-barrel syringe to apply equal amounts of cryoprecipitate and thrombin at the same time. One fibrin sealant, Tisseal VH (Baxter Healthcare, Deerfield, Ill), comes with a Duploject applicator. After the agent is thoroughly applied (it is sprayed), pressure is applied for 3 to 5 minutes.
The same manufacturer also produces Coseal, which is used in vascular reconstruction to achieve additional hemostasis by mechanically sealing off areas of leakage, and Floseal, to help achieve hemostasis when ligatures or clips are impractical.
TABLE 2
Topical intraperitoneal hemostatic agents
| AGENT | WHAT IT IS | HOW IT IS APPLIED |
|---|---|---|
| Avitene Ultrafoam | Absorbable collagen hemostat | Comes in powder; sprinkle on area |
Fibrin glue
| Equal amounts of cryoprecipitate and thrombin | Spray on affected area with double-barrel syringe or device supplied by Baxter Healthcare |
| Gelfoam | Absorbable gelatin sponge | Cut in strips of appropriate size and apply to area |
| Surgicel | Oxidized regenerated cellulose | Cut in strips of appropriate size and apply to area |
STEP 4Hypogastric Artery Ligation
SALLY’S CASE
Bleeding persists
Because of the hemorrhage, a gynecologic oncology consult is obtained and the hypogastric artery is ligated bilaterally, but bleeding continues. During further exploration, the left ureter is found to be ligated. Sally receives 65 U of packed red blood cells, platelets, and fresh frozen plasma. The Cell Saver also is used.
If pelvic oozing persists after application of a topical hemostatic agent, consider hypogastric artery ligation, which controls pelvic hemorrhage in as many as 50% of patients.7,8
STEP 5When All Else Fails: “Pack And Go”
If intraoperative bleeding persists despite hypogastric artery ligation and the other measures, the life-saving modality of choice is a pelvic pack left in place 2 to 3 days. I prefer a fast, simple method: “pack and go” or damage-control technique.10-12
A 2- to 4-inch Kerlix gauze (Kendall Health Care Products, Mansfield, Mass) is tightly packed over a fibrin glue bed from side to side in the pelvis. Only the skin is closed using towel clips or a running suture. The patient is immediately transferred to intensive care, where acidosis, coagulopathy, and hypothermia are corrected. In 48 to 72 hours, the packs are gently removed with saline drip assistance. If hemostasis still has not been achieved, repacking is an option.
Presacral venous bleeding
Two helpful methods to quell presacral venous bleeding are:
- inserting stainless steel thumbtacks
- indirect coagulation through a muscle fragment
The thumbtack method
The presacral veins are sometimes injured during presacral neurectomy, sacrocolpopexy, or posterior exenteration. This bleeding can be controlled by inserting stainless steel thumbtacks, with direct pressure from the surgeon’s hand, directly into the sacrum.15-17 These work by compressing veins adjacent to the bone, and are left in place permanently. No complications have been reported.
Indirect coagulation
Another method of controlling presacral venous bleeding is indirect coagulation through a muscle fragment. This is done by harvesting a 2 x 1 cm piece of muscle from the rectus abdominus and pressing it against the bleeding veins. Then set a Bovie (Valley Lab, Boulder, Colo) at 40 W of pure cutting current and apply it to the muscle fragment for 1 to 2 minutes. This method has been successful in 12 of 12 reported cases.18,19
Other methods of controlling presacral venous bleeding include bipolar cautery, use of bone wax, and suturing in “sandwiches” of Avitene alternated with Gelfoam, but these strategies have met with limited success.
Pelvic hemorrhage
Arterial embolization
Angiographic insertion of Gelfoam pledgets or Silastic coils may effectively control pelvic hemorrhage in up to 90% of postpartum and postoperative patients.20,21 Hypogastric artery embolization can also be done intraoperatively.22
However, this technique should be used with caution, as it may require 1 to 2 hours to perform and is inappropriate for patients with hypovolemic shock. Complications are rare, but can occur in up to 6% to 7% of patients.21 They include postoperative fever, pelvic abscess formation, reflux of embolic material, nontarget embolization, foot and buttocks ischemia, bladder and rectal wall necrosis, and late rebleeding.
Arterial embolization does not appear to affect subsequent pregnancies.23
Military antishock trousers The MAST or aviation “G” suit is sometimes used as an intermediate step to laparotomy in patients with ectopic pregnancy or postoperative or postpartum hemorrhage.24 Its major use is to stabilize patients for surgery by compressing peripheral circulation, thereby diverting blood to the core circulation.
Inflate the legs first, then the abdomen; leave the MAST suit in place for 2 to 48 hours; and deflate in reverse order.
Contraindications include pulmonary edema, cardiogenic shock, rupture of the diaphragm, and pregnancy.
SALLY’S CASE
Hemorrhage abates
A “pack and go” technique is used to control bleeding. The fascia is left open, and the skin is closed with towel clips over the tight pelvic pack. Sally is sent to the ICU, where clotting parameters are corrected.
She undergoes reoperation 36 hours later, at which time no bleeding is encountered.
The left ureter is reimplanted into the bladder, and she makes a full recovery.
The author has served on the speakers bureau for Wyeth.
Placenta accreta leads to hemorrhage
Sally is a 27-year-old gravida with 1 prior cesarean whose ultrasound imaging is suspicious for “placenta adherent to the bladder.” At 38 weeks, she delivers a viable infant by classical cesarean, at which time the ultrasound finding is confirmed: the placenta is densely adherent.
The placenta is left in situ, no methotrexate is given, and Sally is followed with clotting studies and exams.
Eight weeks later, when her fibrinogen level falls and the prothrombin time and partial thromboplastin time become abnormal, the obstetrician attempts to perform dilatation and evacuation, but massive bleeding ensues. The physician then performs a total abdominal hysterectomy, but bleeding continues from the cuff.
What is the best way to manage the hemorrhage?
After identifying its source, the surgeon should apply pressure to abate the bleeding, using packing if necessary, and repair the affected artery or vein. Fortunately, we have many tools at our disposal, from preventive steps like careful preoperative assessment to the use of hemostatic agents, fibrin glues, hypogastric artery ligation, and specialized pelvic packing techniques. With prompt action and a stepwise approach, this bona fide catastrophe can usually be successfully managed. This article details a 5-step action plan.
If massive bleeding occurs during laparoscopic or vaginal surgery, a laparotomy may be indicated, and intraoperative management would follow the same 5 steps.
STEP 1Like the Boy Scouts, Be Prepared
Although surgeons are acutely aware that drugs such as warfarin and heparin can cause intraoperative bleeding, the patient history and predisposing factors sometimes get short shrift.
Besides asking about the patient’s medications, assess the following:
- Platelets. The primary laboratory test to evaluate potential bleeding is the platelet count. In general, 10,000 to 20,000 platelets are needed for hemostasis. However, 50,000 are needed for any surgery or invasive procedure, such as insertion of a central line.1 I recommend platelet evaluation for patients scheduled for major abdominal surgery.
- History of bleeding. If the patient or her family has a history of bleeding with any surgery, evaluate her for von Willebrand’s disease.
- High alcohol intake warrants preoperative liver function and coagulation studies.
- Some herbal or natural remedies can exacerbate intraoperative hemorrhage through their inhibition of coagulation, especially the agents listed in TABLE 1. They should generally be discontinued 2 to 7 days before surgery.2
- Aspirin and nonsteroidal anti-inflammatory drugs should be discontinued 7 days before anticipated surgery. However, patients may continue aspirin at a daily dose of 81 mg.
- Poor nutrition and obesity predispose the patient to wound complications and intraoperative bleeding. Patients who are severely malnourished can take dietary supplements or receive total parenteral nutrition prior to surgery.
- Intraoperative factors such as the 3 “inadequacies” (inadequate incision, retraction, and anesthesia), low core body temperature, severe adhesions (ie, endometriosis), and large vascular tumors also are sometimes associated with bleeding.
TABLE 1
Alternative remedies that may exacerbate bleeding
- 32% to 37% of Americans use these remedies, but only 38% of them tell their doctor
- Stop all alternative remedies 2 to 7 days before surgery
| REMEDY | USED FOR | PERIOPERATIVE RISKS |
|---|---|---|
| Beta-carotene | Vitamin A precursor; often taken as a nutritional supplement | May cause coagulopathy |
| Feverfew | Used to prevent or treat migraine and ease menstrual cramps | May inhibit coagulation |
| Fish oil | Rich in omega-3 fatty acids, recommended for cardiovascular health | Omega-3s inhibit coagulation |
| Garlic | Used to reduce hypertension and high cholesterol | Case reports of unexpected or increased surgical bleeding, prolonged bleeding time, and impaired platelet aggregation |
| Ginkgo | Treatment of dementia, impaired cognition, and memory | Various ginkgolides have platelet-activating-factor antagonist properties; case reports of spontaneous bleeding |
| Ginseng | Widely used as a stimulant, tonic, diuretic, mood elevator, and energy booster | May cause hypertension, cardiovascular instability, coagulopathy, and sedation |
| St. John’s wort | Antidepressant | May cause cardiovascular instability, coagulopathy, and sedation |
| Vitamin E | Antioxidant | May interfere with coagulation |
STEP 2Follow These Basic Principles
Whenever bleeding is encountered in any area of the abdominal cavity, the first step is simple: Apply immediate pressure with a finger or sponge stick. Then obtain exposure and assistance. Exposure usually means extending the incision and using a fixed table retractor.
If the source of bleeding is unknown, apply pressure on the aorta using a hand, weighted speculum, or Conn aortic compressor (Pilling-branded, Teleflex Medical, Limerick, Pa).
Secure individual vessels with finetipped clamps and small-caliber sutures or clips, and minimize the use of clamps. Never place clamps or sutures blindly, and never use electrocautery for large lacerations.
If you choose to use packs to temporarily control bleeding, insert them carefully to avoid tearing veins, and place pelvic packs (hot or cold) in a stepwise fashion, from sidewall to sidewall. Leave packs in place for at least 15 minutes and remove them sequentially.
Great vessel injuries
The aorta, vena cava, and common iliac vessels are sometimes injured during removal of paraaortic nodes or when the inferior mesenteric vessels are avulsed during retraction of the sigmoid colon. In addition, needle or trocar injuries during operative laparoscopy occur in as many as 4 of every 10,000 procedures.3
Again, the first step in managing great vessel injuries is applying pressure. Then obtain blood components, and, if necessary, consult with a vascular surgeon or gynecologic oncologist.
In general, once the patient is hemodynamically stable, the affected vessel should be compressed proximally and distally. Use Allis or vascular clamps on the torn edges to elevate the lacerated area. My preference is to close these injuries with a running 5-0 or 6-0 nylon or monofilament polypropylene (MFPP) suture on a cardiovascular needle.
Replacing blood and its components
Be aware of the following replacement guidelines for catastrophic intraoperative hemorrhage:
- For every 8 U of red blood cells replaced, give 2 U of fresh frozen plasma.
- If more than 10 U of red blood cells are replaced, give 10 U of platelets, preferably at the end of the procedure.
- With prolonged PTT, give fresh frozen plasma.
- If fibrinogen is low, give 2 U of cryoprecipitate.1
When massive bleeding is anticipated or encountered, the Haemonetics Cell Saver (Haemonetics Corp, Braintree, Mass) is invaluable. This device, which requires a trained technician, removes blood from the operative field, anticoagulates it, and washes red blood cells, which are infused. It is accepted by many Jehovah’s Witnesses,4 and has been used safely in women with cesarean-associated bleeding.5 Relative contraindications include malignancy and bacterial contamination from a ruptured abscess or inadvertent injury to unprepared bowel.6 The Cell Saver may be used after heavy bleeding from hysterectomy or in patients with ruptured membranes.
STEP 3Try A Topical Hemostatic Agent
If hemorrhage contiues after arterial bleeders are secured, consider a topical hemostatic agent (TABLE 2). All such agents require pressure to be applied for 3 to 5 minutes.
My preferences are Surgicel (Johnson & Johnson, New Brunswick, NJ) and Gelfoam (Pharmacia, Kalamazoo, Mich). In general, Avitene Ultrafoam collagen hemostat (Davol, subsidiary of C.R. Bard, Murray Hill, NJ) works poorly in the presence of thrombocytopenia and should be used with caution near the ureter.
Fibrin glue has been widely used as a hemostatic agent in microvascular, cardiovascular, and thoracic surgery.
To prepare fibrin glue at my institution, we use a double-barrel syringe to apply equal amounts of cryoprecipitate and thrombin at the same time. One fibrin sealant, Tisseal VH (Baxter Healthcare, Deerfield, Ill), comes with a Duploject applicator. After the agent is thoroughly applied (it is sprayed), pressure is applied for 3 to 5 minutes.
The same manufacturer also produces Coseal, which is used in vascular reconstruction to achieve additional hemostasis by mechanically sealing off areas of leakage, and Floseal, to help achieve hemostasis when ligatures or clips are impractical.
TABLE 2
Topical intraperitoneal hemostatic agents
| AGENT | WHAT IT IS | HOW IT IS APPLIED |
|---|---|---|
| Avitene Ultrafoam | Absorbable collagen hemostat | Comes in powder; sprinkle on area |
Fibrin glue
| Equal amounts of cryoprecipitate and thrombin | Spray on affected area with double-barrel syringe or device supplied by Baxter Healthcare |
| Gelfoam | Absorbable gelatin sponge | Cut in strips of appropriate size and apply to area |
| Surgicel | Oxidized regenerated cellulose | Cut in strips of appropriate size and apply to area |
STEP 4Hypogastric Artery Ligation
SALLY’S CASE
Bleeding persists
Because of the hemorrhage, a gynecologic oncology consult is obtained and the hypogastric artery is ligated bilaterally, but bleeding continues. During further exploration, the left ureter is found to be ligated. Sally receives 65 U of packed red blood cells, platelets, and fresh frozen plasma. The Cell Saver also is used.
If pelvic oozing persists after application of a topical hemostatic agent, consider hypogastric artery ligation, which controls pelvic hemorrhage in as many as 50% of patients.7,8
STEP 5When All Else Fails: “Pack And Go”
If intraoperative bleeding persists despite hypogastric artery ligation and the other measures, the life-saving modality of choice is a pelvic pack left in place 2 to 3 days. I prefer a fast, simple method: “pack and go” or damage-control technique.10-12
A 2- to 4-inch Kerlix gauze (Kendall Health Care Products, Mansfield, Mass) is tightly packed over a fibrin glue bed from side to side in the pelvis. Only the skin is closed using towel clips or a running suture. The patient is immediately transferred to intensive care, where acidosis, coagulopathy, and hypothermia are corrected. In 48 to 72 hours, the packs are gently removed with saline drip assistance. If hemostasis still has not been achieved, repacking is an option.
Presacral venous bleeding
Two helpful methods to quell presacral venous bleeding are:
- inserting stainless steel thumbtacks
- indirect coagulation through a muscle fragment
The thumbtack method
The presacral veins are sometimes injured during presacral neurectomy, sacrocolpopexy, or posterior exenteration. This bleeding can be controlled by inserting stainless steel thumbtacks, with direct pressure from the surgeon’s hand, directly into the sacrum.15-17 These work by compressing veins adjacent to the bone, and are left in place permanently. No complications have been reported.
Indirect coagulation
Another method of controlling presacral venous bleeding is indirect coagulation through a muscle fragment. This is done by harvesting a 2 x 1 cm piece of muscle from the rectus abdominus and pressing it against the bleeding veins. Then set a Bovie (Valley Lab, Boulder, Colo) at 40 W of pure cutting current and apply it to the muscle fragment for 1 to 2 minutes. This method has been successful in 12 of 12 reported cases.18,19
Other methods of controlling presacral venous bleeding include bipolar cautery, use of bone wax, and suturing in “sandwiches” of Avitene alternated with Gelfoam, but these strategies have met with limited success.
Pelvic hemorrhage
Arterial embolization
Angiographic insertion of Gelfoam pledgets or Silastic coils may effectively control pelvic hemorrhage in up to 90% of postpartum and postoperative patients.20,21 Hypogastric artery embolization can also be done intraoperatively.22
However, this technique should be used with caution, as it may require 1 to 2 hours to perform and is inappropriate for patients with hypovolemic shock. Complications are rare, but can occur in up to 6% to 7% of patients.21 They include postoperative fever, pelvic abscess formation, reflux of embolic material, nontarget embolization, foot and buttocks ischemia, bladder and rectal wall necrosis, and late rebleeding.
Arterial embolization does not appear to affect subsequent pregnancies.23
Military antishock trousers The MAST or aviation “G” suit is sometimes used as an intermediate step to laparotomy in patients with ectopic pregnancy or postoperative or postpartum hemorrhage.24 Its major use is to stabilize patients for surgery by compressing peripheral circulation, thereby diverting blood to the core circulation.
Inflate the legs first, then the abdomen; leave the MAST suit in place for 2 to 48 hours; and deflate in reverse order.
Contraindications include pulmonary edema, cardiogenic shock, rupture of the diaphragm, and pregnancy.
SALLY’S CASE
Hemorrhage abates
A “pack and go” technique is used to control bleeding. The fascia is left open, and the skin is closed with towel clips over the tight pelvic pack. Sally is sent to the ICU, where clotting parameters are corrected.
She undergoes reoperation 36 hours later, at which time no bleeding is encountered.
The left ureter is reimplanted into the bladder, and she makes a full recovery.
The author has served on the speakers bureau for Wyeth.
1. Nolan TE, Gallup DG. Massive transfusion: a current review. Obstet Gynecol Surv. 1991;46:289-295
2. Ang-Lee MK, Moss J, Yuan C-S. Herbal medicine and preoperative care. JAMA. 2001;286:208-216.
3. Härkü-Siren P, Sjöberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.
4. deCastro RM. Bloodless surgery: establishment of a program for the special needs of the Jehovah’s Witness community: the gynecologic surgery experience at a community hospital. Obstet Gynecol. 1999;180:149-158.
5. Rebarber A, Lonser R, Jackson S, Copel JA, Siple S. The safety of intraoperative blood collection and autotransfusion during cesarean section. Am J Obstet Gynecol. 1998;169:715-720.
6. Klimberg I, Sirois R, Wajsman Z, Baker J. Intraoperative autotransfusion in urologic oncology Arch Surg. 1986;121:1326-1329.
7. Clark SL, Phelan JP, Yeh Z-Y, Bruce SR, Paul RH. Hypogastric artery ligation for obstetric hemorrhage. Obstet Gynecol. 1985;66:353-356.
8. Thavarash AS, Sivalingam N, Almohdzar SA. Internal iliac and ovarian artery ligation in the control of pelvic hemorrhage. Aust N Z J Obstet Gynecol. 1989;29:22-25.
9. Burchell RC. Internal iliac ligation. Haemodynamics. Obstet Gynecol. 1964;5:53-59.
10. Finan MA, Fiorica JV, Hoffman MS, et al. Massive pelvic hemorrhage during gynecologic cancer surgery: “pack and go back.” Gynecol Oncol. 1996;62:390-395.
11. Rotondo MF, Zonies DH. The damage control sequence and underlying logic. Surg Clin N Am. 1997;77:761-777.
12. Inge JA, Gallup DG, Davis FE. Catastrophic hemorrhage from placenta previa-accreta. A case series and guidelines for management. J Pelvic Surg. 2000;6:268-272.
13. Cassels JW Jr, Greenberg H, Otterson WN. Pelvic tamponade in puerperal hemorrhage. J Reprod Med. 1985;30:689-692.
14. Hallack M, Didly GA, III, Hurley TJ, Moise KJ, Jr. Transvaginal pressure pack for life-threatening pelvis hemorrhage secondary to placenta accreta. Obstet Gynecol. 1991;78:938-940.
15. Khan FA, Fang DT, Nivatvongs S. Management of presacral hemorrhage during rectal resection. Surg Gynecol Obstet. 1987;165:275-277.
16. Pastner B, Orr JW. Intractable venous hemorrhage: use of stainless steel thumbtacks to obtain hemostasis. Am J Obstet Gynecol. 1990;162:452-455.
17. Timmons MC, Kohler MF, Addison WA. Thumbtack use for control of presacral bleeding with description of an instrument for thumbtack application. Obstet Gynecol. 1991;78:313-315.
18. Xu J, Lin J. Control of presacral hemorrhage with electrocautery through a muscle fragment pressed on the bleeding vein. J Am Coll Surg. 1994;179:351-354.
19. Miklos JR, Kohli N, Sze EH. Control of presacral hemorrhage using indirect coagulation through a muscle fragment. J Pelvic Surg. 1996;2:268-270.
20. Hansch E, Chitkara U, McAlpine J, El-Sayed Y, Dake MD, Razavi MK. Pelvic arterial embolization for control of obstetric hemorrhage: a five-year experience. Obstet Gynecol. 1999;180:1454-1460.
21. Verdantham S, Goodwin SC, McLucas B, Mohr G. Uterine artery embolization: an underused method of controlling pelvic hemorrhage. Am J Obstet Gynecol. 1997;176:938-946.
22. Saueracker AJ, McCroskey BL, Moor EE, Moore FA. Intraoperative hypogastric artery embolization for life-threatening pelvic hemorrhage: a preliminary report. J Trauma. 1987;27:1127-1129.
23. Orman D, White R, Pollak J, Tal M. Pelvic embolization for intractable postpartum hemorrhage: long-term follow-up and implications for fertility. Obstet Gynecol. 2003;102:904-910.
24. Pearse CS, Magrina JF, Finley BE. Use of MAST suit in obstetrics and gynecology. Obstet Gynecol Surv. 1984;39:416-422.
1. Nolan TE, Gallup DG. Massive transfusion: a current review. Obstet Gynecol Surv. 1991;46:289-295
2. Ang-Lee MK, Moss J, Yuan C-S. Herbal medicine and preoperative care. JAMA. 2001;286:208-216.
3. Härkü-Siren P, Sjöberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.
4. deCastro RM. Bloodless surgery: establishment of a program for the special needs of the Jehovah’s Witness community: the gynecologic surgery experience at a community hospital. Obstet Gynecol. 1999;180:149-158.
5. Rebarber A, Lonser R, Jackson S, Copel JA, Siple S. The safety of intraoperative blood collection and autotransfusion during cesarean section. Am J Obstet Gynecol. 1998;169:715-720.
6. Klimberg I, Sirois R, Wajsman Z, Baker J. Intraoperative autotransfusion in urologic oncology Arch Surg. 1986;121:1326-1329.
7. Clark SL, Phelan JP, Yeh Z-Y, Bruce SR, Paul RH. Hypogastric artery ligation for obstetric hemorrhage. Obstet Gynecol. 1985;66:353-356.
8. Thavarash AS, Sivalingam N, Almohdzar SA. Internal iliac and ovarian artery ligation in the control of pelvic hemorrhage. Aust N Z J Obstet Gynecol. 1989;29:22-25.
9. Burchell RC. Internal iliac ligation. Haemodynamics. Obstet Gynecol. 1964;5:53-59.
10. Finan MA, Fiorica JV, Hoffman MS, et al. Massive pelvic hemorrhage during gynecologic cancer surgery: “pack and go back.” Gynecol Oncol. 1996;62:390-395.
11. Rotondo MF, Zonies DH. The damage control sequence and underlying logic. Surg Clin N Am. 1997;77:761-777.
12. Inge JA, Gallup DG, Davis FE. Catastrophic hemorrhage from placenta previa-accreta. A case series and guidelines for management. J Pelvic Surg. 2000;6:268-272.
13. Cassels JW Jr, Greenberg H, Otterson WN. Pelvic tamponade in puerperal hemorrhage. J Reprod Med. 1985;30:689-692.
14. Hallack M, Didly GA, III, Hurley TJ, Moise KJ, Jr. Transvaginal pressure pack for life-threatening pelvis hemorrhage secondary to placenta accreta. Obstet Gynecol. 1991;78:938-940.
15. Khan FA, Fang DT, Nivatvongs S. Management of presacral hemorrhage during rectal resection. Surg Gynecol Obstet. 1987;165:275-277.
16. Pastner B, Orr JW. Intractable venous hemorrhage: use of stainless steel thumbtacks to obtain hemostasis. Am J Obstet Gynecol. 1990;162:452-455.
17. Timmons MC, Kohler MF, Addison WA. Thumbtack use for control of presacral bleeding with description of an instrument for thumbtack application. Obstet Gynecol. 1991;78:313-315.
18. Xu J, Lin J. Control of presacral hemorrhage with electrocautery through a muscle fragment pressed on the bleeding vein. J Am Coll Surg. 1994;179:351-354.
19. Miklos JR, Kohli N, Sze EH. Control of presacral hemorrhage using indirect coagulation through a muscle fragment. J Pelvic Surg. 1996;2:268-270.
20. Hansch E, Chitkara U, McAlpine J, El-Sayed Y, Dake MD, Razavi MK. Pelvic arterial embolization for control of obstetric hemorrhage: a five-year experience. Obstet Gynecol. 1999;180:1454-1460.
21. Verdantham S, Goodwin SC, McLucas B, Mohr G. Uterine artery embolization: an underused method of controlling pelvic hemorrhage. Am J Obstet Gynecol. 1997;176:938-946.
22. Saueracker AJ, McCroskey BL, Moor EE, Moore FA. Intraoperative hypogastric artery embolization for life-threatening pelvic hemorrhage: a preliminary report. J Trauma. 1987;27:1127-1129.
23. Orman D, White R, Pollak J, Tal M. Pelvic embolization for intractable postpartum hemorrhage: long-term follow-up and implications for fertility. Obstet Gynecol. 2003;102:904-910.
24. Pearse CS, Magrina JF, Finley BE. Use of MAST suit in obstetrics and gynecology. Obstet Gynecol Surv. 1984;39:416-422.
Minimally invasive surgery in ovarian cancer
Maria’ case
she wants laparoscopy. yes or no?
Maria is a 57-year-old mother of 4 who presents to a gynecologic oncologist with pelvic pain and ultrasonographic evidence of a 7-cm complex mass at the right adnexa. She has an enlarged fibroid uterus (12-week size), a preoperative CA125 level of 21 U/mL, and she says she wants laparoscopic management.
Is minimally invasive surgery an acceptable choice?
This large, complex mass is possibly malignant. Until now, laparoscopy has played only a small role in the management of ovarian cancer, although it has greatly changed treatment of other gynecologic malignancies. Since women with ovarian cancer tend to be older and have coexisting diseases, laparoscopy could confer many benefits, provided surgical staging is comprehensive, and timely diagnosis and patient outcomes are not compromised.1
The utility of laparoscopy in ovarian borderline tumors and cancer is increasing. This article surveys current applications and concerns, including
- when to refer,
- predicting malignancy,
- effects of carbon dioxide (CO2) peritoneum,
- risk of port-site recurrences,
- hand-assisted laparoscopy,
- comprehensive staging, and
- assessing resectability.
4 applications
Conventional staging by laparotomy with a vertical incision from above the umbilicus to the symphysis pubis is still the gold standard; however, laparoscopy can be used in the management of selected cases of ovarian cancer:
- to manage and stage apparent early-stage ovarian cancer,
- to determine the extent of advanced disease and potential resectability,
- to resect disease via hand-assisted laparoscopy in selected women with advanced disease, and
- to obtain a “second look,” or reassess the patient for disease recurrence and placement of intraperitoneal catheters.
Benefits of laparoscopy for benign masses
The benefits of laparoscopy over laparotomy in the management of benign adnexal masses are well defined:2
- less postoperative morbidity,
- less postoperative pain,
- less analgesia required,
- shorter hospitalizations, and
- shorter recovery time.
When to refer. Referral of at-risk patients to a gynecologic oncologist should be based on personal and family history, physical, imaging, and tumor markers.
When to get a consult: ASAP. General gynecologists may encounter malignancy unexpectedly. When they do, it is of paramount importance to obtain gynecologic oncology consultation intraoperatively, if possible, or as soon as possible postoperatively.
Predicting Malignancy
How common is cancer in laparoscopically managed masses?
Consider a complex ovarian mass potentially malignant until proven otherwise. Why? Because it remains difficult to rule out malignancy preoperatively, even with strict patient selection.
For example, a study involving 292 laparoscopically managed women found a 3.8% malignancy rate.3 These women had undergone preoperative vaginal ultrasound, CA125 measurement, and pelvic examination, but malignancy was not detected until surgery.
The incidence of malignancy at laparoscopy for a pelvic mass varies widely due to different guidelines for patient selection. In 1 series of 757 patients,4 the rate of unanticipated malignancy was 2.5%. This included 7 invasive cancers and 12 borderline tumors. Preoperative evaluation entailed routine clinical and ultrasound examinations. At laparoscopy, peritoneal cytology was obtained, the ovaries and peritoneum were inspected, and any cysts were punctured so their contents could be examined. If a malignant mass was encountered or suspected, the woman in question was treated by immediate laparotomy using a vertical midline incision.4
History of nongynecologic cancer heightens risk of malignancy
For example, of 31 women with stage IV breast cancer and a new adnexal mass, 3 (10%) were found to have primary ovarian cancer, and 21 (68%) had metastatic breast cancer.5
In a study at our institution,6 51 of 264 patients (19%) with a history of nongynecologic cancer and a new adnexal mass were found to have a malignancy. Of these women, 22 (43%) had primary ovarian cancer; the rest had metastatic disease. Most patients had laparoscopy even when malignancy was encountered.
Utility of frozen section
Frozen-section analysis speeds diagnosis of the adnexal mass, allowing the necessary surgery to be performed immediately.The overall accuracy of frozen-section analysis is high, reported at 92.7% in 1 study.7 It is less accurate in borderline tumors because of the extensive sampling required.
Intraoperative frozen section has high accuracy in women with metastases to the adnexae. In 36 patients with a history of breast or colorectal carcinoma who developed adnexal metastases, intraoperative frozen section correctly diagnosed carcinoma in 35 patients (97%). In more than 80% of these women, the carcinoma was accurately diagnosed as metastatic.8
Laparoscopy for Suspicious Masses?
Is laparoscopy appropriate for pelvic masses that appear suspicious for cancer at the time of preoperative evaluation? And if malignancy is confirmed, is conversion to laparotomy warranted?
Advocates of laparoscopy as the initial diagnostic tool say yes to the first question, pointing to the fact that most suspicious masses are later found to be benign.9,10
For example, Dottino et al10 managed all pelvic masses referred to their oncology unit laparoscopically unless there was evidence of gross metastatic disease (ie, omental cake) or the mass extended above the umbilicus. Immediate frozen-section analysis was performed in all cases. Although most of the masses were suspicious for malignancy preoperatively, 87% were in fact benign, and 88% were successfully managed by laparoscopy. If conversion to laparotomy was necessary for successful debulking, it was performed. However, laparoscopic surgery often was adequate.
Canis and colleagues9 support diagnostic laparoscopy regardless of the ultrasonographic appearance of the pelvic mass, although they recommend immediate conversion to laparotomy for staging if malignancy is found.
Does CO2 Sspread Cancer?
Whether CO2 contributes to cancer spread and growth is of particular concern in ovarian cancer, since it is predominantly a peritoneal disease. In a rat ovarian cancer model, tumor dissemination increased throughout the peritoneal cavity with laparoscopy, compared with laparotomy, without increased tumor growth.11
However, a separate study12 in women with persistent metastatic intraabdominal peritoneal or ovarian cancer at the time of second-look surgery found no difference in overall survival between patients who had undergone laparoscopy versus laparotomy
Fear of Port-Site Recurrence
Fear of tumor implantation at the trocar site is commonly cited as a reason to avoid laparoscopy in ovarian cancer. One metaanalysis found a port-site recurrence rate of 1.1% to 13.5%, but many of the studies included were small series or case reports.13 In ovarian cancer, most reports of port-site recurrences have been associated with advanced-stage disease with peritoneal seeding and the presence of ascites.13,14
The term “port-site recurrence” (previously it was thought to be a metastasis) describes cancer occurring in the subcutis in the absence of carcinomatosis.15 Now that the definition has been refined, the rate of port-site recurrences may be substantially lower.
A large retrospective study at our institution found 4 (0.64%) subcutaneous tumor implantations at or near a trocar site after 625 laparoscopic procedures in 584 women with ovarian/tubal cancer. Most of these implantations were discovered after positive second-look operations, and all were associated with synchronous carcinomatosis or other sites of metastatic disease.16
In a separate study14 involving 102 women with primary or recurrent advanced-stage ovarian cancer, large-volume ascites and a longer interval between chemotherapy and cytoreductive surgery were associated with more port-site recurrences. In addition, full-layer closure of the abdominal wall reduced port-site recurrences from 58% to 2%, emphasizing the importance of trocar-site closure in cases of malignancy. There was no survival disadvantage in women with portsite recurrences.
What causes port-site recurrences?
Possible factors include:
- trauma to the site,
- frequent removal of instruments through the port,
- removing the specimen through the port, and
- continued leakage of ascites.13
Avoiding cyst spillage and routinely using laparoscopic bags for cyst removal may decrease the incidence of these recurrences (FIGURE 1). Partial cyst excision and morcellation of a solid mass are always contraindicated.
Irrigation of port sites may decrease tumor cell implantation and should be considered at the end of the procedure.13 To further reduce risk, experts recommend closing all layers at the time of laparoscopy and resecting laparoscopic ports in their full thickness at the time of the staging laparotomy.14
FIGURE 1 Cyst removal using an endoscopic bag
Avoid spillage and routinely use laparoscopic bags for cyst removal to decrease the incidence of port-site recurrences.
Hand-Assisted Laparoscopy
This hybrid procedure combines the advantages of minimally invasive surgery with the tactile sensation of laparotomy. It has gained favor among urologists and general surgeons. (The first nephrectomy using this method was performed in 1996.17)
Technological advances now enable the surgeon to insert and remove the nondominant hand into the peritoneal cavity without losing pneumoperitoneum and to insert instruments through the same port if needed (FIGURE 2).
Advantages over traditional laparoscopy include the ability to palpate tissue, assist with tissue retraction, perform blunt dissection, and rapidly control hemostasis. This approach has been described in management and staging of early-stage ovarian cancer and in debulking advanced disease.18
FIGURE 2 Hand-assisted laparoscopy
The nondominant hand and surgical instruments can be inserted and removed through the special port without affecting pneumoperitoneum.
Surgical Staging
Maria’ case
resection and analysis of ovary
Maria underwent laparoscopy via the open technique. The surgeon found a cystic right ovarian mass, a fibroid uterus, and small diaphragmatic nodules, which were biopsied and found to be benign.
Pelvic washings were obtained, and after the right infundibular pelvic ligament and right utero-ovarian ligament were clamped and cut, the intact ovary was placed in a laparoscopic bag. The bag was pulled through the 12-mm suprapubic trocar, the cyst wall was perforated, and the cyst was drained within the laparoscopic bag, producing brown fluid. The bag was removed from the peritoneal cavity through this port, and the cyst was sent to pathology.
There was no contamination to the peritoneal cavity or abdominal wall, and the bag remained intact. Surgical gloves were then changed, and instruments used to drain the cyst were removed from the operating field.
When frozen-section analysis revealed a borderline serous ovarian tumor, Maria underwent BSO, infracolic omentectomy, laparoscopic pelvic and paraaortic lymphadenectomy, and laparoscopically assisted vaginal hysterectomy. There were no intraoperative complications, the total time in the operating room was 330 minutes, and there was blood loss of approximately 150 mL.
When an ovarian malignancy is discovered, immediate staging is indicated, and should include:
- peritoneal biopsies,
- pelvic and para-aortic lymph node sampling,
- infracolic omentectomy, and
- bilateral salpingo-oophorectomy (BSO) and hysterectomy.1
With presumed stage I disease, there is a 20% to 30% likelihood of upstaging after comprehensive surgical staging, with disease often discovered in the lymph nodes.19,20
Since changes in staging affect prognosis and treatment, complete staging should include the retroperitoneal nodes.
When the patient wants to preserve fertility
In selected younger women who have not yet completed childbearing, conservative treatment with retention of the uterus and contralateral ovary is an option—though we lack outcomes data on patients treated this way.
This option should be restricted to women with proven stage I disease after comprehensive staging.1
Can staging be done laparoscopically?
Complete staging—consisting of a detailed peritoneal assessment (with BSO and vaginal hysterectomy), omentectomy, and pelvic and para-aortic node dissection—can safely be done laparoscopically.19-21 Studies show low morbidity, with accurate findings and adequate node counts.21,22
A comparison of laparoscopic and conventional (laparotomy) staging in women with apparent stage I adnexal cancers found no differences in omental specimen size or the number of lymph nodes removed, and none of the patients required conversion to laparotomy.22
When definitive staging is delayed
Several studies have found poorer outcomes with delayed staging. However, the tumor ruptured in some of these studies, with considerable delay from the initial laparoscopy until definitive staging and treatment.
To increase the likelihood of an accurate stage, gather as much information as possible on the extent of disease: Describe the intraoperative findings and inspect the abdomen and pelvis thoroughly at initial surgery if a skilled oncologic surgeon is not immediately available. Then make every effort to schedule a complete staging procedure as soon as possible, as some consider this an “oncologic emergency.”9
Whether and when to stage LMP tumors
Preoperative prediction and intraoperative diagnosis of low malignant potential (LMP) tumors is challenging. If such a tumor is confirmed by frozen section, the usual treatment is unilateral salpingo-oophorectomy. When the patient is postmenopausal or has completed childbearing, BSO, hysterectomy, and staging should be considered.1
Surgical staging should be performed at the initial surgery, if at all possible. However, if final pathology confirms an LMP tumor and disease appears to be confined to the adnexa, repeat surgery for staging is controversial because of the limited data on its benefit, particularly in regard to mucinous borderline tumors.
Restaging may be more useful in selected cases of serous LMP tumors with histologic micropapillary features, since these tumors may be associated with a higher incidence of invasive implants (eg, in the omentum or peritoneum) that may require chemotherapy.
If a malignant cyst ruptures, does it affect staging?
The effect of intraoperative tumor spillage in stage I disease is debatable, although ascites and preoperative rupture are associated with a poorer prognosis.23
Even though a number of investigators (TABLE) have found intraoperative spillage to have no adverse impact on survival, make every effort to maintain capsular integrity to minimize any possibility of peritoneal tumor dissemination.
In some cases, intraoperative cyst rupture warrants upstaging from International Federation of Gynecology and Obstetrics (FIGO) stage IA to 1C, necessitating adjuvant chemotherapy when it otherwise would not have been required.1
Cyst rupture is no more likely with laparoscopy than with laparotomy,2 and is unrelated to the surgical route. It is more closely associated with the frequency of cystectomy.24
If rupture does occur, thoroughly irrigate the peritoneal cavity.
TABLE
When a cyst ruptures during surgery, what is the prognosis? The data are mixed on the significance of this event in stage I ovarian cancer
| AUTHOR | NUMBER OF CASES | IMPACT |
|---|---|---|
| Sevelda 1990 (Austria) | 204 | No prognostic importance |
| Sainz 1994 (US) | 79 | May worsen prognosis |
| Sjovall 1994 (Sweden) | 394 | No negative influence |
| Ahmed 1996 (UK) | 194 | Not prognostically significant |
| Vergote 2001 (Belgium) | 1,545 | Rupture should be avoided (hazard ratio = 1.64) |
How chemotherapy comes into play
If final pathology shows stage IC or high-grade histology, chemotherapy generally is offered to women managed in the United States. In selected cases, chemotherapy is given immediately after the initial surgery if completing a full staging procedure would considerably delay chemotherapy.
Leblanc et al21 found that, when staging was performed after completion of chemotherapy in women with stage IC or high-grade histology, 3 of 11 patients (27%) had positive nodes. Because positive nodes can be less chemosensitive, Leblanc and colleagues advocate either of 2 options: immediate restaging, including retroperitoneal nodes, or staging after chemotherapy, including retroperitoneal nodes.
Advanced Ovarian Cancer
Optimal surgical cytoreduction by laparotomy, followed by platinum-based chemotherapy, maximizes survival in women with advanced ovarian cancer. Unfortunately, in many patients, optimal debulking is not feasible, and laparotomy without optimal cytoreduction offers no survival advantage.25 At the same time, preoperative imaging has limited ability to determine the feasibility of cytoreduction. For example, computed tomography is highly sensitive when it comes to detecting ascites and mesenteric and omental disease (FIGURE 3), but is not as successful in detecting gallbladder fossa disease and diffuse peritoneal nodules smaller than 2 cm.
As a result, laparoscopy is increasingly used to determine whether optimal resection is feasible. If it is, immediate laparotomy is appropriate. Otherwise, a tissue specimen is obtained for histological confirmation, allowing accurate diagnosis prior to chemotherapy.
FIGURE 3 Omental cake signifies metastasis
Omental cake in a stage IIIC ovarian cancer patient. Disease appears to be resectable.
Potential drawbacks of laparoscopy
In selected women with advanced cancer, laparoscopy may be a good way to determine which patients would not benefit from laparotomy, thus sparing them the morbidity of an additional operation. But laparoscopy can have limitations:
- Ascites can reduce visibility.
- Omental and bowel adhesion to the anterior abdominal wall may increase the likelihood of bowel injury.
- Trocar site implantation may increase in the presence of adenocarcinoma, ascites, and carcinomatosis.13
If trocar sites are carefully closed and chemotherapy is initiated promptly, these risks can be substantially reduced.14
Is laparoscopy acceptable for restaging?
Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624–629.
Yes, but only if the surgeon is highly skilled, with experience in both ovarian cancer and advanced laparoscopy. Comprehensive staging not only yields important prognostic information, but also identifies women who stand to benefit from chemotherapy.
The evidence: 10 years of experience
From 1991 to 2001, Leblanc et al21 laparoscopically restaged 53 women who had undergone incomplete staging for apparent stage I adnexal carcinoma.
Immediate (primary) restaging was done in 42 patients, and 11 were staged after completing chemotherapy (secondary restaging) for grade 3, clear-cell, or small-cell histology; FIGO stage IC cancer; or ruptured granulosa cell tumor.
Meticulous restaging technique:
- peritoneal washings and careful inspection,
- 8 to 10 random peritoneal biopsies (if peritoneal inspection was normal),
- BSO and hysterectomy (if not already done) or uterine curettage (if fertility was desired),
- bilateral pelvic and paraaortic lymphadenectomy,
- infracolic omentectomy.
The peritoneal cavity and trocar sites were irrigated at the end of the procedure, with full closure of any port sites larger than 10 mm.
Overall, laparoscopy was safe and successful
Complete laparoscopic restaging was performed in 52 women (98%). Dense adhesions indicated conversion to laparotomy in 1 case.
Four complications were directly related to the restaging procedure: a hematoma after epigastric vessel injury, 2 lymphocysts (managed laparoscopically), and 1 ureteric transection (which required laparotomy).The operation resulted in the following averages:
- operating time: 238 minutes,
- postoperative hospital stay: 3.1 days,
- node resection: 20 nodes in the paraaortic region and 14 in the pelvic dissection.
Mean follow-up was 54 months.
Outcomes
Of the 42 women who underwent primary restaging, 8 (19%) were upstaged—2 because of positive random peritoneal biopsies.
In the secondary restaging group, 4 of 11 women (36%) had their malignancies upstaged—3 because of positive retroperitoneal nodes and 1 because of positive random peritoneal biopsies. No port-site recurrences were observed in any of these patients.
One of the 8 patients upstaged in the primary restaging group had a recurrence 8 months postoperatively and died 16 months later. Of the 34 women with stage IA cancer after primary restaging, 3 (9%) had recurrences.
In the secondary-restaging group, 1 woman with small-cell carcinoma had a recurrence 10 months postoperatively and died 4 months later despite second-line chemotherapy.
Nine women had fertility-sparing surgery, and 3 later became pregnant and delivered without incident.
Second-Look Laparoscopy
Second-look surgery in women with a complete clinical response (normal exam, imaging, and CA125) after primary chemotherapy is controversial. This surgery aims to identify women with pathologically negative or microscopic disease who may benefit from consolidation therapy, or with larger-volume disease who can undergo secondary cytoreduction.27 Laparoscopy meets these goals safely with comparable accuracy and less morbidity than laparotomy.12,27
Maria’ case
lmp tumor and negative nodes
Maria did well postoperatively and went home on day 4. Her final pathology report: a right papillary serous adenocarcinoma of LMP (borderline) with small (<1 mm) foci of microinvasion. She had 6 negative paraaortic nodes, 19 negative pelvic nodes, negative pelvic washings and omentum, a normal left ovary, and a 6-cm cellular leiomyoma in an otherwise normal uterus.
She required no adjuvant treatment and is now 22 months postoperative without evidence of disease.
The authors report no financial relationships relevant to this article.
1. Rubin S. Ovarian Cancer. Philadelphia: Lippincott Williams & Wilkins; 2001.
2. Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109-114
3. Malik E, Bohm W, Stoz F, Nitsch CD, Rossmanith WG. Laparoscopic management of ovarian tumors. Surg Endosc. 1998;12:1326-1333
4. Canis M, Mage G, Pouly JL, Wattiez A, Manhes H, Bruhat MA. Laparoscopic diagnosis of adnexal cystic masses: a 12-year experience with long-term followup. Obstet Gynecol. 1994;83:707-712
5. Quan ML, Fey J, Eitan R, et al. Role of laparoscopy in the evaluation of the adnexa in patients with stage IV breast cancer. Gynecol Oncol. 2004;92:327-330
6. Juretska MM, Crawford CL, Lee C, et al. Laparoscopic management of adnexal masses in women with a history of nongynecologic malignancy. Abstract presented at the 2005 National Gynecologic Oncology Fellows’ Forum, Tucson, Arizona, January 27-30.
7. Rose PG, Rubin RB, Nelson BE, Hunter RE, Reale FR. Accuracy of frozen-section (intraoperative consultation) diagnosis of ovarian tumors. Am J Obstet Gynecol. 1994;171:823-826
8. Abu-Rustum NR, Chi DS, Wiatrowska BA, Guiter G, Saigo PE, Barakat RR. The accuracy of frozen-section diagnosis in metastatic breast and colorectal carcinoma to the adnexa. Gynecol Oncol. 1999;73:102-105
9. Canis M, Botchorishvili R, Kouyate S, et al. Surgical management of adnexal tumors. Ann Chir. 1998;52:234-248
10. Dottino PR, Levine DA, Ripley DL, Cohen CJ. Laparoscopic management of adnexal masses in premenopausal and postmenopausal women. Obstet Gynecol. 1999;93:223-228
11. Canis M, Botchorishvili R, Wattiez A, Mage G, Pouly JL, Bruhat MA. Tumor growth and dissemination after laparotomy and CO2 pneumoperitoneum: a rat ovarian cancer model. Obstet Gynecol. 1998;92:104-108
12. Abu-Rustum NR, Barakat RR, Siegel PL, Venkatraman E, Curtin JP, Hoskins WJ. Second-look operation for epithelial ovarian cancer: laparoscopy or laparotomy? Obstet Gynecol. 1996;88:549-553
13. Wang PH, Yuan CC, Lin G, Ng HT, Chao HT. Risk factors contributing to early occurrence of port site metastases of laparoscopic surgery for malignancy. Gynecol Oncol. 1999;72:38-44
14. Van Dam PA, DeCloedt J, Tjalma WAA, Buytaert P, Becquart D, Vergote IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. 1999;181:536-541
15. Reymond MA, Schneider C, Kastl S, Hohenberger W, Kockerling F. The pathogenesis of port-site recurrences. J Gastroint Surg. 1998;2:406-414
16. Abu-Rustum NR, Rhee EH, Chi DS, Sonoda Y, Gemignani M, Barakat RR. Subcutaneous tumor implantation after laparoscopic procedures in women with malignant disease [see comment]. Obstet Gynecol. 2004;103:480-487
17. Nakada SY, Moon TD, Gist M, Mahvi D. Use of the pneumo sleeve as an adjunct in laparoscopic nephrectomy. Urology. 1997;49:612-613
18. Krivak TC, Elkas JC, Rose GS, et al. The utility of hand-assisted laparoscopy in ovarian cancer. Gynecol Oncol. 2005;96:72-76
19. Faught W, Le T, Fung Kee Fung M, Krepart G, Lotocki R, Heywood M. Early ovarian cancer: what is the staging impact of retroperitoneal node sampling? J Obstet Gynaecol Can. 2003;25:18-21
20. Soper JT, Johnson P, Johnson V, Berchuck A, Clarke-Pearson DL. Comprehensive restaging laparotomy in women with apparent early ovarian carcinoma. Obstet Gynecol. 1992;80:949-953
21. Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624-629
22. Chi DS, Abu-Rustum NR, Sonoda Y, et al. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am J Obstet Gynecol [in press].
23. Sjovall K NB, Einhorn N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer. 1994;4:333-336
24. Fauvet R, Boccara J, Dufournet C, Poncelet C, Darai E. Laparoscopic management of borderline ovarian tumors: results of a French multicenter study. Ann Oncol. 2005;16:403-410
25. Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974-979
26. Ben David Y, Bustan M, Shalev E. Laparoscopy as part of the evaluation and management of ovarian and cervix neoplasms. Harefuah. 2001;140:464-467
27. Husain A, Chi DS, Prasad M, et al. The role of laparoscopy in second-look evaluations for ovarian cancer. Gynecol Oncol. 2001;80:44-47
Maria’ case
she wants laparoscopy. yes or no?
Maria is a 57-year-old mother of 4 who presents to a gynecologic oncologist with pelvic pain and ultrasonographic evidence of a 7-cm complex mass at the right adnexa. She has an enlarged fibroid uterus (12-week size), a preoperative CA125 level of 21 U/mL, and she says she wants laparoscopic management.
Is minimally invasive surgery an acceptable choice?
This large, complex mass is possibly malignant. Until now, laparoscopy has played only a small role in the management of ovarian cancer, although it has greatly changed treatment of other gynecologic malignancies. Since women with ovarian cancer tend to be older and have coexisting diseases, laparoscopy could confer many benefits, provided surgical staging is comprehensive, and timely diagnosis and patient outcomes are not compromised.1
The utility of laparoscopy in ovarian borderline tumors and cancer is increasing. This article surveys current applications and concerns, including
- when to refer,
- predicting malignancy,
- effects of carbon dioxide (CO2) peritoneum,
- risk of port-site recurrences,
- hand-assisted laparoscopy,
- comprehensive staging, and
- assessing resectability.
4 applications
Conventional staging by laparotomy with a vertical incision from above the umbilicus to the symphysis pubis is still the gold standard; however, laparoscopy can be used in the management of selected cases of ovarian cancer:
- to manage and stage apparent early-stage ovarian cancer,
- to determine the extent of advanced disease and potential resectability,
- to resect disease via hand-assisted laparoscopy in selected women with advanced disease, and
- to obtain a “second look,” or reassess the patient for disease recurrence and placement of intraperitoneal catheters.
Benefits of laparoscopy for benign masses
The benefits of laparoscopy over laparotomy in the management of benign adnexal masses are well defined:2
- less postoperative morbidity,
- less postoperative pain,
- less analgesia required,
- shorter hospitalizations, and
- shorter recovery time.
When to refer. Referral of at-risk patients to a gynecologic oncologist should be based on personal and family history, physical, imaging, and tumor markers.
When to get a consult: ASAP. General gynecologists may encounter malignancy unexpectedly. When they do, it is of paramount importance to obtain gynecologic oncology consultation intraoperatively, if possible, or as soon as possible postoperatively.
Predicting Malignancy
How common is cancer in laparoscopically managed masses?
Consider a complex ovarian mass potentially malignant until proven otherwise. Why? Because it remains difficult to rule out malignancy preoperatively, even with strict patient selection.
For example, a study involving 292 laparoscopically managed women found a 3.8% malignancy rate.3 These women had undergone preoperative vaginal ultrasound, CA125 measurement, and pelvic examination, but malignancy was not detected until surgery.
The incidence of malignancy at laparoscopy for a pelvic mass varies widely due to different guidelines for patient selection. In 1 series of 757 patients,4 the rate of unanticipated malignancy was 2.5%. This included 7 invasive cancers and 12 borderline tumors. Preoperative evaluation entailed routine clinical and ultrasound examinations. At laparoscopy, peritoneal cytology was obtained, the ovaries and peritoneum were inspected, and any cysts were punctured so their contents could be examined. If a malignant mass was encountered or suspected, the woman in question was treated by immediate laparotomy using a vertical midline incision.4
History of nongynecologic cancer heightens risk of malignancy
For example, of 31 women with stage IV breast cancer and a new adnexal mass, 3 (10%) were found to have primary ovarian cancer, and 21 (68%) had metastatic breast cancer.5
In a study at our institution,6 51 of 264 patients (19%) with a history of nongynecologic cancer and a new adnexal mass were found to have a malignancy. Of these women, 22 (43%) had primary ovarian cancer; the rest had metastatic disease. Most patients had laparoscopy even when malignancy was encountered.
Utility of frozen section
Frozen-section analysis speeds diagnosis of the adnexal mass, allowing the necessary surgery to be performed immediately.The overall accuracy of frozen-section analysis is high, reported at 92.7% in 1 study.7 It is less accurate in borderline tumors because of the extensive sampling required.
Intraoperative frozen section has high accuracy in women with metastases to the adnexae. In 36 patients with a history of breast or colorectal carcinoma who developed adnexal metastases, intraoperative frozen section correctly diagnosed carcinoma in 35 patients (97%). In more than 80% of these women, the carcinoma was accurately diagnosed as metastatic.8
Laparoscopy for Suspicious Masses?
Is laparoscopy appropriate for pelvic masses that appear suspicious for cancer at the time of preoperative evaluation? And if malignancy is confirmed, is conversion to laparotomy warranted?
Advocates of laparoscopy as the initial diagnostic tool say yes to the first question, pointing to the fact that most suspicious masses are later found to be benign.9,10
For example, Dottino et al10 managed all pelvic masses referred to their oncology unit laparoscopically unless there was evidence of gross metastatic disease (ie, omental cake) or the mass extended above the umbilicus. Immediate frozen-section analysis was performed in all cases. Although most of the masses were suspicious for malignancy preoperatively, 87% were in fact benign, and 88% were successfully managed by laparoscopy. If conversion to laparotomy was necessary for successful debulking, it was performed. However, laparoscopic surgery often was adequate.
Canis and colleagues9 support diagnostic laparoscopy regardless of the ultrasonographic appearance of the pelvic mass, although they recommend immediate conversion to laparotomy for staging if malignancy is found.
Does CO2 Sspread Cancer?
Whether CO2 contributes to cancer spread and growth is of particular concern in ovarian cancer, since it is predominantly a peritoneal disease. In a rat ovarian cancer model, tumor dissemination increased throughout the peritoneal cavity with laparoscopy, compared with laparotomy, without increased tumor growth.11
However, a separate study12 in women with persistent metastatic intraabdominal peritoneal or ovarian cancer at the time of second-look surgery found no difference in overall survival between patients who had undergone laparoscopy versus laparotomy
Fear of Port-Site Recurrence
Fear of tumor implantation at the trocar site is commonly cited as a reason to avoid laparoscopy in ovarian cancer. One metaanalysis found a port-site recurrence rate of 1.1% to 13.5%, but many of the studies included were small series or case reports.13 In ovarian cancer, most reports of port-site recurrences have been associated with advanced-stage disease with peritoneal seeding and the presence of ascites.13,14
The term “port-site recurrence” (previously it was thought to be a metastasis) describes cancer occurring in the subcutis in the absence of carcinomatosis.15 Now that the definition has been refined, the rate of port-site recurrences may be substantially lower.
A large retrospective study at our institution found 4 (0.64%) subcutaneous tumor implantations at or near a trocar site after 625 laparoscopic procedures in 584 women with ovarian/tubal cancer. Most of these implantations were discovered after positive second-look operations, and all were associated with synchronous carcinomatosis or other sites of metastatic disease.16
In a separate study14 involving 102 women with primary or recurrent advanced-stage ovarian cancer, large-volume ascites and a longer interval between chemotherapy and cytoreductive surgery were associated with more port-site recurrences. In addition, full-layer closure of the abdominal wall reduced port-site recurrences from 58% to 2%, emphasizing the importance of trocar-site closure in cases of malignancy. There was no survival disadvantage in women with portsite recurrences.
What causes port-site recurrences?
Possible factors include:
- trauma to the site,
- frequent removal of instruments through the port,
- removing the specimen through the port, and
- continued leakage of ascites.13
Avoiding cyst spillage and routinely using laparoscopic bags for cyst removal may decrease the incidence of these recurrences (FIGURE 1). Partial cyst excision and morcellation of a solid mass are always contraindicated.
Irrigation of port sites may decrease tumor cell implantation and should be considered at the end of the procedure.13 To further reduce risk, experts recommend closing all layers at the time of laparoscopy and resecting laparoscopic ports in their full thickness at the time of the staging laparotomy.14
FIGURE 1 Cyst removal using an endoscopic bag
Avoid spillage and routinely use laparoscopic bags for cyst removal to decrease the incidence of port-site recurrences.
Hand-Assisted Laparoscopy
This hybrid procedure combines the advantages of minimally invasive surgery with the tactile sensation of laparotomy. It has gained favor among urologists and general surgeons. (The first nephrectomy using this method was performed in 1996.17)
Technological advances now enable the surgeon to insert and remove the nondominant hand into the peritoneal cavity without losing pneumoperitoneum and to insert instruments through the same port if needed (FIGURE 2).
Advantages over traditional laparoscopy include the ability to palpate tissue, assist with tissue retraction, perform blunt dissection, and rapidly control hemostasis. This approach has been described in management and staging of early-stage ovarian cancer and in debulking advanced disease.18
FIGURE 2 Hand-assisted laparoscopy
The nondominant hand and surgical instruments can be inserted and removed through the special port without affecting pneumoperitoneum.
Surgical Staging
Maria’ case
resection and analysis of ovary
Maria underwent laparoscopy via the open technique. The surgeon found a cystic right ovarian mass, a fibroid uterus, and small diaphragmatic nodules, which were biopsied and found to be benign.
Pelvic washings were obtained, and after the right infundibular pelvic ligament and right utero-ovarian ligament were clamped and cut, the intact ovary was placed in a laparoscopic bag. The bag was pulled through the 12-mm suprapubic trocar, the cyst wall was perforated, and the cyst was drained within the laparoscopic bag, producing brown fluid. The bag was removed from the peritoneal cavity through this port, and the cyst was sent to pathology.
There was no contamination to the peritoneal cavity or abdominal wall, and the bag remained intact. Surgical gloves were then changed, and instruments used to drain the cyst were removed from the operating field.
When frozen-section analysis revealed a borderline serous ovarian tumor, Maria underwent BSO, infracolic omentectomy, laparoscopic pelvic and paraaortic lymphadenectomy, and laparoscopically assisted vaginal hysterectomy. There were no intraoperative complications, the total time in the operating room was 330 minutes, and there was blood loss of approximately 150 mL.
When an ovarian malignancy is discovered, immediate staging is indicated, and should include:
- peritoneal biopsies,
- pelvic and para-aortic lymph node sampling,
- infracolic omentectomy, and
- bilateral salpingo-oophorectomy (BSO) and hysterectomy.1
With presumed stage I disease, there is a 20% to 30% likelihood of upstaging after comprehensive surgical staging, with disease often discovered in the lymph nodes.19,20
Since changes in staging affect prognosis and treatment, complete staging should include the retroperitoneal nodes.
When the patient wants to preserve fertility
In selected younger women who have not yet completed childbearing, conservative treatment with retention of the uterus and contralateral ovary is an option—though we lack outcomes data on patients treated this way.
This option should be restricted to women with proven stage I disease after comprehensive staging.1
Can staging be done laparoscopically?
Complete staging—consisting of a detailed peritoneal assessment (with BSO and vaginal hysterectomy), omentectomy, and pelvic and para-aortic node dissection—can safely be done laparoscopically.19-21 Studies show low morbidity, with accurate findings and adequate node counts.21,22
A comparison of laparoscopic and conventional (laparotomy) staging in women with apparent stage I adnexal cancers found no differences in omental specimen size or the number of lymph nodes removed, and none of the patients required conversion to laparotomy.22
When definitive staging is delayed
Several studies have found poorer outcomes with delayed staging. However, the tumor ruptured in some of these studies, with considerable delay from the initial laparoscopy until definitive staging and treatment.
To increase the likelihood of an accurate stage, gather as much information as possible on the extent of disease: Describe the intraoperative findings and inspect the abdomen and pelvis thoroughly at initial surgery if a skilled oncologic surgeon is not immediately available. Then make every effort to schedule a complete staging procedure as soon as possible, as some consider this an “oncologic emergency.”9
Whether and when to stage LMP tumors
Preoperative prediction and intraoperative diagnosis of low malignant potential (LMP) tumors is challenging. If such a tumor is confirmed by frozen section, the usual treatment is unilateral salpingo-oophorectomy. When the patient is postmenopausal or has completed childbearing, BSO, hysterectomy, and staging should be considered.1
Surgical staging should be performed at the initial surgery, if at all possible. However, if final pathology confirms an LMP tumor and disease appears to be confined to the adnexa, repeat surgery for staging is controversial because of the limited data on its benefit, particularly in regard to mucinous borderline tumors.
Restaging may be more useful in selected cases of serous LMP tumors with histologic micropapillary features, since these tumors may be associated with a higher incidence of invasive implants (eg, in the omentum or peritoneum) that may require chemotherapy.
If a malignant cyst ruptures, does it affect staging?
The effect of intraoperative tumor spillage in stage I disease is debatable, although ascites and preoperative rupture are associated with a poorer prognosis.23
Even though a number of investigators (TABLE) have found intraoperative spillage to have no adverse impact on survival, make every effort to maintain capsular integrity to minimize any possibility of peritoneal tumor dissemination.
In some cases, intraoperative cyst rupture warrants upstaging from International Federation of Gynecology and Obstetrics (FIGO) stage IA to 1C, necessitating adjuvant chemotherapy when it otherwise would not have been required.1
Cyst rupture is no more likely with laparoscopy than with laparotomy,2 and is unrelated to the surgical route. It is more closely associated with the frequency of cystectomy.24
If rupture does occur, thoroughly irrigate the peritoneal cavity.
TABLE
When a cyst ruptures during surgery, what is the prognosis? The data are mixed on the significance of this event in stage I ovarian cancer
| AUTHOR | NUMBER OF CASES | IMPACT |
|---|---|---|
| Sevelda 1990 (Austria) | 204 | No prognostic importance |
| Sainz 1994 (US) | 79 | May worsen prognosis |
| Sjovall 1994 (Sweden) | 394 | No negative influence |
| Ahmed 1996 (UK) | 194 | Not prognostically significant |
| Vergote 2001 (Belgium) | 1,545 | Rupture should be avoided (hazard ratio = 1.64) |
How chemotherapy comes into play
If final pathology shows stage IC or high-grade histology, chemotherapy generally is offered to women managed in the United States. In selected cases, chemotherapy is given immediately after the initial surgery if completing a full staging procedure would considerably delay chemotherapy.
Leblanc et al21 found that, when staging was performed after completion of chemotherapy in women with stage IC or high-grade histology, 3 of 11 patients (27%) had positive nodes. Because positive nodes can be less chemosensitive, Leblanc and colleagues advocate either of 2 options: immediate restaging, including retroperitoneal nodes, or staging after chemotherapy, including retroperitoneal nodes.
Advanced Ovarian Cancer
Optimal surgical cytoreduction by laparotomy, followed by platinum-based chemotherapy, maximizes survival in women with advanced ovarian cancer. Unfortunately, in many patients, optimal debulking is not feasible, and laparotomy without optimal cytoreduction offers no survival advantage.25 At the same time, preoperative imaging has limited ability to determine the feasibility of cytoreduction. For example, computed tomography is highly sensitive when it comes to detecting ascites and mesenteric and omental disease (FIGURE 3), but is not as successful in detecting gallbladder fossa disease and diffuse peritoneal nodules smaller than 2 cm.
As a result, laparoscopy is increasingly used to determine whether optimal resection is feasible. If it is, immediate laparotomy is appropriate. Otherwise, a tissue specimen is obtained for histological confirmation, allowing accurate diagnosis prior to chemotherapy.
FIGURE 3 Omental cake signifies metastasis
Omental cake in a stage IIIC ovarian cancer patient. Disease appears to be resectable.
Potential drawbacks of laparoscopy
In selected women with advanced cancer, laparoscopy may be a good way to determine which patients would not benefit from laparotomy, thus sparing them the morbidity of an additional operation. But laparoscopy can have limitations:
- Ascites can reduce visibility.
- Omental and bowel adhesion to the anterior abdominal wall may increase the likelihood of bowel injury.
- Trocar site implantation may increase in the presence of adenocarcinoma, ascites, and carcinomatosis.13
If trocar sites are carefully closed and chemotherapy is initiated promptly, these risks can be substantially reduced.14
Is laparoscopy acceptable for restaging?
Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624–629.
Yes, but only if the surgeon is highly skilled, with experience in both ovarian cancer and advanced laparoscopy. Comprehensive staging not only yields important prognostic information, but also identifies women who stand to benefit from chemotherapy.
The evidence: 10 years of experience
From 1991 to 2001, Leblanc et al21 laparoscopically restaged 53 women who had undergone incomplete staging for apparent stage I adnexal carcinoma.
Immediate (primary) restaging was done in 42 patients, and 11 were staged after completing chemotherapy (secondary restaging) for grade 3, clear-cell, or small-cell histology; FIGO stage IC cancer; or ruptured granulosa cell tumor.
Meticulous restaging technique:
- peritoneal washings and careful inspection,
- 8 to 10 random peritoneal biopsies (if peritoneal inspection was normal),
- BSO and hysterectomy (if not already done) or uterine curettage (if fertility was desired),
- bilateral pelvic and paraaortic lymphadenectomy,
- infracolic omentectomy.
The peritoneal cavity and trocar sites were irrigated at the end of the procedure, with full closure of any port sites larger than 10 mm.
Overall, laparoscopy was safe and successful
Complete laparoscopic restaging was performed in 52 women (98%). Dense adhesions indicated conversion to laparotomy in 1 case.
Four complications were directly related to the restaging procedure: a hematoma after epigastric vessel injury, 2 lymphocysts (managed laparoscopically), and 1 ureteric transection (which required laparotomy).The operation resulted in the following averages:
- operating time: 238 minutes,
- postoperative hospital stay: 3.1 days,
- node resection: 20 nodes in the paraaortic region and 14 in the pelvic dissection.
Mean follow-up was 54 months.
Outcomes
Of the 42 women who underwent primary restaging, 8 (19%) were upstaged—2 because of positive random peritoneal biopsies.
In the secondary restaging group, 4 of 11 women (36%) had their malignancies upstaged—3 because of positive retroperitoneal nodes and 1 because of positive random peritoneal biopsies. No port-site recurrences were observed in any of these patients.
One of the 8 patients upstaged in the primary restaging group had a recurrence 8 months postoperatively and died 16 months later. Of the 34 women with stage IA cancer after primary restaging, 3 (9%) had recurrences.
In the secondary-restaging group, 1 woman with small-cell carcinoma had a recurrence 10 months postoperatively and died 4 months later despite second-line chemotherapy.
Nine women had fertility-sparing surgery, and 3 later became pregnant and delivered without incident.
Second-Look Laparoscopy
Second-look surgery in women with a complete clinical response (normal exam, imaging, and CA125) after primary chemotherapy is controversial. This surgery aims to identify women with pathologically negative or microscopic disease who may benefit from consolidation therapy, or with larger-volume disease who can undergo secondary cytoreduction.27 Laparoscopy meets these goals safely with comparable accuracy and less morbidity than laparotomy.12,27
Maria’ case
lmp tumor and negative nodes
Maria did well postoperatively and went home on day 4. Her final pathology report: a right papillary serous adenocarcinoma of LMP (borderline) with small (<1 mm) foci of microinvasion. She had 6 negative paraaortic nodes, 19 negative pelvic nodes, negative pelvic washings and omentum, a normal left ovary, and a 6-cm cellular leiomyoma in an otherwise normal uterus.
She required no adjuvant treatment and is now 22 months postoperative without evidence of disease.
The authors report no financial relationships relevant to this article.
Maria’ case
she wants laparoscopy. yes or no?
Maria is a 57-year-old mother of 4 who presents to a gynecologic oncologist with pelvic pain and ultrasonographic evidence of a 7-cm complex mass at the right adnexa. She has an enlarged fibroid uterus (12-week size), a preoperative CA125 level of 21 U/mL, and she says she wants laparoscopic management.
Is minimally invasive surgery an acceptable choice?
This large, complex mass is possibly malignant. Until now, laparoscopy has played only a small role in the management of ovarian cancer, although it has greatly changed treatment of other gynecologic malignancies. Since women with ovarian cancer tend to be older and have coexisting diseases, laparoscopy could confer many benefits, provided surgical staging is comprehensive, and timely diagnosis and patient outcomes are not compromised.1
The utility of laparoscopy in ovarian borderline tumors and cancer is increasing. This article surveys current applications and concerns, including
- when to refer,
- predicting malignancy,
- effects of carbon dioxide (CO2) peritoneum,
- risk of port-site recurrences,
- hand-assisted laparoscopy,
- comprehensive staging, and
- assessing resectability.
4 applications
Conventional staging by laparotomy with a vertical incision from above the umbilicus to the symphysis pubis is still the gold standard; however, laparoscopy can be used in the management of selected cases of ovarian cancer:
- to manage and stage apparent early-stage ovarian cancer,
- to determine the extent of advanced disease and potential resectability,
- to resect disease via hand-assisted laparoscopy in selected women with advanced disease, and
- to obtain a “second look,” or reassess the patient for disease recurrence and placement of intraperitoneal catheters.
Benefits of laparoscopy for benign masses
The benefits of laparoscopy over laparotomy in the management of benign adnexal masses are well defined:2
- less postoperative morbidity,
- less postoperative pain,
- less analgesia required,
- shorter hospitalizations, and
- shorter recovery time.
When to refer. Referral of at-risk patients to a gynecologic oncologist should be based on personal and family history, physical, imaging, and tumor markers.
When to get a consult: ASAP. General gynecologists may encounter malignancy unexpectedly. When they do, it is of paramount importance to obtain gynecologic oncology consultation intraoperatively, if possible, or as soon as possible postoperatively.
Predicting Malignancy
How common is cancer in laparoscopically managed masses?
Consider a complex ovarian mass potentially malignant until proven otherwise. Why? Because it remains difficult to rule out malignancy preoperatively, even with strict patient selection.
For example, a study involving 292 laparoscopically managed women found a 3.8% malignancy rate.3 These women had undergone preoperative vaginal ultrasound, CA125 measurement, and pelvic examination, but malignancy was not detected until surgery.
The incidence of malignancy at laparoscopy for a pelvic mass varies widely due to different guidelines for patient selection. In 1 series of 757 patients,4 the rate of unanticipated malignancy was 2.5%. This included 7 invasive cancers and 12 borderline tumors. Preoperative evaluation entailed routine clinical and ultrasound examinations. At laparoscopy, peritoneal cytology was obtained, the ovaries and peritoneum were inspected, and any cysts were punctured so their contents could be examined. If a malignant mass was encountered or suspected, the woman in question was treated by immediate laparotomy using a vertical midline incision.4
History of nongynecologic cancer heightens risk of malignancy
For example, of 31 women with stage IV breast cancer and a new adnexal mass, 3 (10%) were found to have primary ovarian cancer, and 21 (68%) had metastatic breast cancer.5
In a study at our institution,6 51 of 264 patients (19%) with a history of nongynecologic cancer and a new adnexal mass were found to have a malignancy. Of these women, 22 (43%) had primary ovarian cancer; the rest had metastatic disease. Most patients had laparoscopy even when malignancy was encountered.
Utility of frozen section
Frozen-section analysis speeds diagnosis of the adnexal mass, allowing the necessary surgery to be performed immediately.The overall accuracy of frozen-section analysis is high, reported at 92.7% in 1 study.7 It is less accurate in borderline tumors because of the extensive sampling required.
Intraoperative frozen section has high accuracy in women with metastases to the adnexae. In 36 patients with a history of breast or colorectal carcinoma who developed adnexal metastases, intraoperative frozen section correctly diagnosed carcinoma in 35 patients (97%). In more than 80% of these women, the carcinoma was accurately diagnosed as metastatic.8
Laparoscopy for Suspicious Masses?
Is laparoscopy appropriate for pelvic masses that appear suspicious for cancer at the time of preoperative evaluation? And if malignancy is confirmed, is conversion to laparotomy warranted?
Advocates of laparoscopy as the initial diagnostic tool say yes to the first question, pointing to the fact that most suspicious masses are later found to be benign.9,10
For example, Dottino et al10 managed all pelvic masses referred to their oncology unit laparoscopically unless there was evidence of gross metastatic disease (ie, omental cake) or the mass extended above the umbilicus. Immediate frozen-section analysis was performed in all cases. Although most of the masses were suspicious for malignancy preoperatively, 87% were in fact benign, and 88% were successfully managed by laparoscopy. If conversion to laparotomy was necessary for successful debulking, it was performed. However, laparoscopic surgery often was adequate.
Canis and colleagues9 support diagnostic laparoscopy regardless of the ultrasonographic appearance of the pelvic mass, although they recommend immediate conversion to laparotomy for staging if malignancy is found.
Does CO2 Sspread Cancer?
Whether CO2 contributes to cancer spread and growth is of particular concern in ovarian cancer, since it is predominantly a peritoneal disease. In a rat ovarian cancer model, tumor dissemination increased throughout the peritoneal cavity with laparoscopy, compared with laparotomy, without increased tumor growth.11
However, a separate study12 in women with persistent metastatic intraabdominal peritoneal or ovarian cancer at the time of second-look surgery found no difference in overall survival between patients who had undergone laparoscopy versus laparotomy
Fear of Port-Site Recurrence
Fear of tumor implantation at the trocar site is commonly cited as a reason to avoid laparoscopy in ovarian cancer. One metaanalysis found a port-site recurrence rate of 1.1% to 13.5%, but many of the studies included were small series or case reports.13 In ovarian cancer, most reports of port-site recurrences have been associated with advanced-stage disease with peritoneal seeding and the presence of ascites.13,14
The term “port-site recurrence” (previously it was thought to be a metastasis) describes cancer occurring in the subcutis in the absence of carcinomatosis.15 Now that the definition has been refined, the rate of port-site recurrences may be substantially lower.
A large retrospective study at our institution found 4 (0.64%) subcutaneous tumor implantations at or near a trocar site after 625 laparoscopic procedures in 584 women with ovarian/tubal cancer. Most of these implantations were discovered after positive second-look operations, and all were associated with synchronous carcinomatosis or other sites of metastatic disease.16
In a separate study14 involving 102 women with primary or recurrent advanced-stage ovarian cancer, large-volume ascites and a longer interval between chemotherapy and cytoreductive surgery were associated with more port-site recurrences. In addition, full-layer closure of the abdominal wall reduced port-site recurrences from 58% to 2%, emphasizing the importance of trocar-site closure in cases of malignancy. There was no survival disadvantage in women with portsite recurrences.
What causes port-site recurrences?
Possible factors include:
- trauma to the site,
- frequent removal of instruments through the port,
- removing the specimen through the port, and
- continued leakage of ascites.13
Avoiding cyst spillage and routinely using laparoscopic bags for cyst removal may decrease the incidence of these recurrences (FIGURE 1). Partial cyst excision and morcellation of a solid mass are always contraindicated.
Irrigation of port sites may decrease tumor cell implantation and should be considered at the end of the procedure.13 To further reduce risk, experts recommend closing all layers at the time of laparoscopy and resecting laparoscopic ports in their full thickness at the time of the staging laparotomy.14
FIGURE 1 Cyst removal using an endoscopic bag
Avoid spillage and routinely use laparoscopic bags for cyst removal to decrease the incidence of port-site recurrences.
Hand-Assisted Laparoscopy
This hybrid procedure combines the advantages of minimally invasive surgery with the tactile sensation of laparotomy. It has gained favor among urologists and general surgeons. (The first nephrectomy using this method was performed in 1996.17)
Technological advances now enable the surgeon to insert and remove the nondominant hand into the peritoneal cavity without losing pneumoperitoneum and to insert instruments through the same port if needed (FIGURE 2).
Advantages over traditional laparoscopy include the ability to palpate tissue, assist with tissue retraction, perform blunt dissection, and rapidly control hemostasis. This approach has been described in management and staging of early-stage ovarian cancer and in debulking advanced disease.18
FIGURE 2 Hand-assisted laparoscopy
The nondominant hand and surgical instruments can be inserted and removed through the special port without affecting pneumoperitoneum.
Surgical Staging
Maria’ case
resection and analysis of ovary
Maria underwent laparoscopy via the open technique. The surgeon found a cystic right ovarian mass, a fibroid uterus, and small diaphragmatic nodules, which were biopsied and found to be benign.
Pelvic washings were obtained, and after the right infundibular pelvic ligament and right utero-ovarian ligament were clamped and cut, the intact ovary was placed in a laparoscopic bag. The bag was pulled through the 12-mm suprapubic trocar, the cyst wall was perforated, and the cyst was drained within the laparoscopic bag, producing brown fluid. The bag was removed from the peritoneal cavity through this port, and the cyst was sent to pathology.
There was no contamination to the peritoneal cavity or abdominal wall, and the bag remained intact. Surgical gloves were then changed, and instruments used to drain the cyst were removed from the operating field.
When frozen-section analysis revealed a borderline serous ovarian tumor, Maria underwent BSO, infracolic omentectomy, laparoscopic pelvic and paraaortic lymphadenectomy, and laparoscopically assisted vaginal hysterectomy. There were no intraoperative complications, the total time in the operating room was 330 minutes, and there was blood loss of approximately 150 mL.
When an ovarian malignancy is discovered, immediate staging is indicated, and should include:
- peritoneal biopsies,
- pelvic and para-aortic lymph node sampling,
- infracolic omentectomy, and
- bilateral salpingo-oophorectomy (BSO) and hysterectomy.1
With presumed stage I disease, there is a 20% to 30% likelihood of upstaging after comprehensive surgical staging, with disease often discovered in the lymph nodes.19,20
Since changes in staging affect prognosis and treatment, complete staging should include the retroperitoneal nodes.
When the patient wants to preserve fertility
In selected younger women who have not yet completed childbearing, conservative treatment with retention of the uterus and contralateral ovary is an option—though we lack outcomes data on patients treated this way.
This option should be restricted to women with proven stage I disease after comprehensive staging.1
Can staging be done laparoscopically?
Complete staging—consisting of a detailed peritoneal assessment (with BSO and vaginal hysterectomy), omentectomy, and pelvic and para-aortic node dissection—can safely be done laparoscopically.19-21 Studies show low morbidity, with accurate findings and adequate node counts.21,22
A comparison of laparoscopic and conventional (laparotomy) staging in women with apparent stage I adnexal cancers found no differences in omental specimen size or the number of lymph nodes removed, and none of the patients required conversion to laparotomy.22
When definitive staging is delayed
Several studies have found poorer outcomes with delayed staging. However, the tumor ruptured in some of these studies, with considerable delay from the initial laparoscopy until definitive staging and treatment.
To increase the likelihood of an accurate stage, gather as much information as possible on the extent of disease: Describe the intraoperative findings and inspect the abdomen and pelvis thoroughly at initial surgery if a skilled oncologic surgeon is not immediately available. Then make every effort to schedule a complete staging procedure as soon as possible, as some consider this an “oncologic emergency.”9
Whether and when to stage LMP tumors
Preoperative prediction and intraoperative diagnosis of low malignant potential (LMP) tumors is challenging. If such a tumor is confirmed by frozen section, the usual treatment is unilateral salpingo-oophorectomy. When the patient is postmenopausal or has completed childbearing, BSO, hysterectomy, and staging should be considered.1
Surgical staging should be performed at the initial surgery, if at all possible. However, if final pathology confirms an LMP tumor and disease appears to be confined to the adnexa, repeat surgery for staging is controversial because of the limited data on its benefit, particularly in regard to mucinous borderline tumors.
Restaging may be more useful in selected cases of serous LMP tumors with histologic micropapillary features, since these tumors may be associated with a higher incidence of invasive implants (eg, in the omentum or peritoneum) that may require chemotherapy.
If a malignant cyst ruptures, does it affect staging?
The effect of intraoperative tumor spillage in stage I disease is debatable, although ascites and preoperative rupture are associated with a poorer prognosis.23
Even though a number of investigators (TABLE) have found intraoperative spillage to have no adverse impact on survival, make every effort to maintain capsular integrity to minimize any possibility of peritoneal tumor dissemination.
In some cases, intraoperative cyst rupture warrants upstaging from International Federation of Gynecology and Obstetrics (FIGO) stage IA to 1C, necessitating adjuvant chemotherapy when it otherwise would not have been required.1
Cyst rupture is no more likely with laparoscopy than with laparotomy,2 and is unrelated to the surgical route. It is more closely associated with the frequency of cystectomy.24
If rupture does occur, thoroughly irrigate the peritoneal cavity.
TABLE
When a cyst ruptures during surgery, what is the prognosis? The data are mixed on the significance of this event in stage I ovarian cancer
| AUTHOR | NUMBER OF CASES | IMPACT |
|---|---|---|
| Sevelda 1990 (Austria) | 204 | No prognostic importance |
| Sainz 1994 (US) | 79 | May worsen prognosis |
| Sjovall 1994 (Sweden) | 394 | No negative influence |
| Ahmed 1996 (UK) | 194 | Not prognostically significant |
| Vergote 2001 (Belgium) | 1,545 | Rupture should be avoided (hazard ratio = 1.64) |
How chemotherapy comes into play
If final pathology shows stage IC or high-grade histology, chemotherapy generally is offered to women managed in the United States. In selected cases, chemotherapy is given immediately after the initial surgery if completing a full staging procedure would considerably delay chemotherapy.
Leblanc et al21 found that, when staging was performed after completion of chemotherapy in women with stage IC or high-grade histology, 3 of 11 patients (27%) had positive nodes. Because positive nodes can be less chemosensitive, Leblanc and colleagues advocate either of 2 options: immediate restaging, including retroperitoneal nodes, or staging after chemotherapy, including retroperitoneal nodes.
Advanced Ovarian Cancer
Optimal surgical cytoreduction by laparotomy, followed by platinum-based chemotherapy, maximizes survival in women with advanced ovarian cancer. Unfortunately, in many patients, optimal debulking is not feasible, and laparotomy without optimal cytoreduction offers no survival advantage.25 At the same time, preoperative imaging has limited ability to determine the feasibility of cytoreduction. For example, computed tomography is highly sensitive when it comes to detecting ascites and mesenteric and omental disease (FIGURE 3), but is not as successful in detecting gallbladder fossa disease and diffuse peritoneal nodules smaller than 2 cm.
As a result, laparoscopy is increasingly used to determine whether optimal resection is feasible. If it is, immediate laparotomy is appropriate. Otherwise, a tissue specimen is obtained for histological confirmation, allowing accurate diagnosis prior to chemotherapy.
FIGURE 3 Omental cake signifies metastasis
Omental cake in a stage IIIC ovarian cancer patient. Disease appears to be resectable.
Potential drawbacks of laparoscopy
In selected women with advanced cancer, laparoscopy may be a good way to determine which patients would not benefit from laparotomy, thus sparing them the morbidity of an additional operation. But laparoscopy can have limitations:
- Ascites can reduce visibility.
- Omental and bowel adhesion to the anterior abdominal wall may increase the likelihood of bowel injury.
- Trocar site implantation may increase in the presence of adenocarcinoma, ascites, and carcinomatosis.13
If trocar sites are carefully closed and chemotherapy is initiated promptly, these risks can be substantially reduced.14
Is laparoscopy acceptable for restaging?
Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624–629.
Yes, but only if the surgeon is highly skilled, with experience in both ovarian cancer and advanced laparoscopy. Comprehensive staging not only yields important prognostic information, but also identifies women who stand to benefit from chemotherapy.
The evidence: 10 years of experience
From 1991 to 2001, Leblanc et al21 laparoscopically restaged 53 women who had undergone incomplete staging for apparent stage I adnexal carcinoma.
Immediate (primary) restaging was done in 42 patients, and 11 were staged after completing chemotherapy (secondary restaging) for grade 3, clear-cell, or small-cell histology; FIGO stage IC cancer; or ruptured granulosa cell tumor.
Meticulous restaging technique:
- peritoneal washings and careful inspection,
- 8 to 10 random peritoneal biopsies (if peritoneal inspection was normal),
- BSO and hysterectomy (if not already done) or uterine curettage (if fertility was desired),
- bilateral pelvic and paraaortic lymphadenectomy,
- infracolic omentectomy.
The peritoneal cavity and trocar sites were irrigated at the end of the procedure, with full closure of any port sites larger than 10 mm.
Overall, laparoscopy was safe and successful
Complete laparoscopic restaging was performed in 52 women (98%). Dense adhesions indicated conversion to laparotomy in 1 case.
Four complications were directly related to the restaging procedure: a hematoma after epigastric vessel injury, 2 lymphocysts (managed laparoscopically), and 1 ureteric transection (which required laparotomy).The operation resulted in the following averages:
- operating time: 238 minutes,
- postoperative hospital stay: 3.1 days,
- node resection: 20 nodes in the paraaortic region and 14 in the pelvic dissection.
Mean follow-up was 54 months.
Outcomes
Of the 42 women who underwent primary restaging, 8 (19%) were upstaged—2 because of positive random peritoneal biopsies.
In the secondary restaging group, 4 of 11 women (36%) had their malignancies upstaged—3 because of positive retroperitoneal nodes and 1 because of positive random peritoneal biopsies. No port-site recurrences were observed in any of these patients.
One of the 8 patients upstaged in the primary restaging group had a recurrence 8 months postoperatively and died 16 months later. Of the 34 women with stage IA cancer after primary restaging, 3 (9%) had recurrences.
In the secondary-restaging group, 1 woman with small-cell carcinoma had a recurrence 10 months postoperatively and died 4 months later despite second-line chemotherapy.
Nine women had fertility-sparing surgery, and 3 later became pregnant and delivered without incident.
Second-Look Laparoscopy
Second-look surgery in women with a complete clinical response (normal exam, imaging, and CA125) after primary chemotherapy is controversial. This surgery aims to identify women with pathologically negative or microscopic disease who may benefit from consolidation therapy, or with larger-volume disease who can undergo secondary cytoreduction.27 Laparoscopy meets these goals safely with comparable accuracy and less morbidity than laparotomy.12,27
Maria’ case
lmp tumor and negative nodes
Maria did well postoperatively and went home on day 4. Her final pathology report: a right papillary serous adenocarcinoma of LMP (borderline) with small (<1 mm) foci of microinvasion. She had 6 negative paraaortic nodes, 19 negative pelvic nodes, negative pelvic washings and omentum, a normal left ovary, and a 6-cm cellular leiomyoma in an otherwise normal uterus.
She required no adjuvant treatment and is now 22 months postoperative without evidence of disease.
The authors report no financial relationships relevant to this article.
1. Rubin S. Ovarian Cancer. Philadelphia: Lippincott Williams & Wilkins; 2001.
2. Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109-114
3. Malik E, Bohm W, Stoz F, Nitsch CD, Rossmanith WG. Laparoscopic management of ovarian tumors. Surg Endosc. 1998;12:1326-1333
4. Canis M, Mage G, Pouly JL, Wattiez A, Manhes H, Bruhat MA. Laparoscopic diagnosis of adnexal cystic masses: a 12-year experience with long-term followup. Obstet Gynecol. 1994;83:707-712
5. Quan ML, Fey J, Eitan R, et al. Role of laparoscopy in the evaluation of the adnexa in patients with stage IV breast cancer. Gynecol Oncol. 2004;92:327-330
6. Juretska MM, Crawford CL, Lee C, et al. Laparoscopic management of adnexal masses in women with a history of nongynecologic malignancy. Abstract presented at the 2005 National Gynecologic Oncology Fellows’ Forum, Tucson, Arizona, January 27-30.
7. Rose PG, Rubin RB, Nelson BE, Hunter RE, Reale FR. Accuracy of frozen-section (intraoperative consultation) diagnosis of ovarian tumors. Am J Obstet Gynecol. 1994;171:823-826
8. Abu-Rustum NR, Chi DS, Wiatrowska BA, Guiter G, Saigo PE, Barakat RR. The accuracy of frozen-section diagnosis in metastatic breast and colorectal carcinoma to the adnexa. Gynecol Oncol. 1999;73:102-105
9. Canis M, Botchorishvili R, Kouyate S, et al. Surgical management of adnexal tumors. Ann Chir. 1998;52:234-248
10. Dottino PR, Levine DA, Ripley DL, Cohen CJ. Laparoscopic management of adnexal masses in premenopausal and postmenopausal women. Obstet Gynecol. 1999;93:223-228
11. Canis M, Botchorishvili R, Wattiez A, Mage G, Pouly JL, Bruhat MA. Tumor growth and dissemination after laparotomy and CO2 pneumoperitoneum: a rat ovarian cancer model. Obstet Gynecol. 1998;92:104-108
12. Abu-Rustum NR, Barakat RR, Siegel PL, Venkatraman E, Curtin JP, Hoskins WJ. Second-look operation for epithelial ovarian cancer: laparoscopy or laparotomy? Obstet Gynecol. 1996;88:549-553
13. Wang PH, Yuan CC, Lin G, Ng HT, Chao HT. Risk factors contributing to early occurrence of port site metastases of laparoscopic surgery for malignancy. Gynecol Oncol. 1999;72:38-44
14. Van Dam PA, DeCloedt J, Tjalma WAA, Buytaert P, Becquart D, Vergote IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. 1999;181:536-541
15. Reymond MA, Schneider C, Kastl S, Hohenberger W, Kockerling F. The pathogenesis of port-site recurrences. J Gastroint Surg. 1998;2:406-414
16. Abu-Rustum NR, Rhee EH, Chi DS, Sonoda Y, Gemignani M, Barakat RR. Subcutaneous tumor implantation after laparoscopic procedures in women with malignant disease [see comment]. Obstet Gynecol. 2004;103:480-487
17. Nakada SY, Moon TD, Gist M, Mahvi D. Use of the pneumo sleeve as an adjunct in laparoscopic nephrectomy. Urology. 1997;49:612-613
18. Krivak TC, Elkas JC, Rose GS, et al. The utility of hand-assisted laparoscopy in ovarian cancer. Gynecol Oncol. 2005;96:72-76
19. Faught W, Le T, Fung Kee Fung M, Krepart G, Lotocki R, Heywood M. Early ovarian cancer: what is the staging impact of retroperitoneal node sampling? J Obstet Gynaecol Can. 2003;25:18-21
20. Soper JT, Johnson P, Johnson V, Berchuck A, Clarke-Pearson DL. Comprehensive restaging laparotomy in women with apparent early ovarian carcinoma. Obstet Gynecol. 1992;80:949-953
21. Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624-629
22. Chi DS, Abu-Rustum NR, Sonoda Y, et al. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am J Obstet Gynecol [in press].
23. Sjovall K NB, Einhorn N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer. 1994;4:333-336
24. Fauvet R, Boccara J, Dufournet C, Poncelet C, Darai E. Laparoscopic management of borderline ovarian tumors: results of a French multicenter study. Ann Oncol. 2005;16:403-410
25. Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974-979
26. Ben David Y, Bustan M, Shalev E. Laparoscopy as part of the evaluation and management of ovarian and cervix neoplasms. Harefuah. 2001;140:464-467
27. Husain A, Chi DS, Prasad M, et al. The role of laparoscopy in second-look evaluations for ovarian cancer. Gynecol Oncol. 2001;80:44-47
1. Rubin S. Ovarian Cancer. Philadelphia: Lippincott Williams & Wilkins; 2001.
2. Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109-114
3. Malik E, Bohm W, Stoz F, Nitsch CD, Rossmanith WG. Laparoscopic management of ovarian tumors. Surg Endosc. 1998;12:1326-1333
4. Canis M, Mage G, Pouly JL, Wattiez A, Manhes H, Bruhat MA. Laparoscopic diagnosis of adnexal cystic masses: a 12-year experience with long-term followup. Obstet Gynecol. 1994;83:707-712
5. Quan ML, Fey J, Eitan R, et al. Role of laparoscopy in the evaluation of the adnexa in patients with stage IV breast cancer. Gynecol Oncol. 2004;92:327-330
6. Juretska MM, Crawford CL, Lee C, et al. Laparoscopic management of adnexal masses in women with a history of nongynecologic malignancy. Abstract presented at the 2005 National Gynecologic Oncology Fellows’ Forum, Tucson, Arizona, January 27-30.
7. Rose PG, Rubin RB, Nelson BE, Hunter RE, Reale FR. Accuracy of frozen-section (intraoperative consultation) diagnosis of ovarian tumors. Am J Obstet Gynecol. 1994;171:823-826
8. Abu-Rustum NR, Chi DS, Wiatrowska BA, Guiter G, Saigo PE, Barakat RR. The accuracy of frozen-section diagnosis in metastatic breast and colorectal carcinoma to the adnexa. Gynecol Oncol. 1999;73:102-105
9. Canis M, Botchorishvili R, Kouyate S, et al. Surgical management of adnexal tumors. Ann Chir. 1998;52:234-248
10. Dottino PR, Levine DA, Ripley DL, Cohen CJ. Laparoscopic management of adnexal masses in premenopausal and postmenopausal women. Obstet Gynecol. 1999;93:223-228
11. Canis M, Botchorishvili R, Wattiez A, Mage G, Pouly JL, Bruhat MA. Tumor growth and dissemination after laparotomy and CO2 pneumoperitoneum: a rat ovarian cancer model. Obstet Gynecol. 1998;92:104-108
12. Abu-Rustum NR, Barakat RR, Siegel PL, Venkatraman E, Curtin JP, Hoskins WJ. Second-look operation for epithelial ovarian cancer: laparoscopy or laparotomy? Obstet Gynecol. 1996;88:549-553
13. Wang PH, Yuan CC, Lin G, Ng HT, Chao HT. Risk factors contributing to early occurrence of port site metastases of laparoscopic surgery for malignancy. Gynecol Oncol. 1999;72:38-44
14. Van Dam PA, DeCloedt J, Tjalma WAA, Buytaert P, Becquart D, Vergote IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. 1999;181:536-541
15. Reymond MA, Schneider C, Kastl S, Hohenberger W, Kockerling F. The pathogenesis of port-site recurrences. J Gastroint Surg. 1998;2:406-414
16. Abu-Rustum NR, Rhee EH, Chi DS, Sonoda Y, Gemignani M, Barakat RR. Subcutaneous tumor implantation after laparoscopic procedures in women with malignant disease [see comment]. Obstet Gynecol. 2004;103:480-487
17. Nakada SY, Moon TD, Gist M, Mahvi D. Use of the pneumo sleeve as an adjunct in laparoscopic nephrectomy. Urology. 1997;49:612-613
18. Krivak TC, Elkas JC, Rose GS, et al. The utility of hand-assisted laparoscopy in ovarian cancer. Gynecol Oncol. 2005;96:72-76
19. Faught W, Le T, Fung Kee Fung M, Krepart G, Lotocki R, Heywood M. Early ovarian cancer: what is the staging impact of retroperitoneal node sampling? J Obstet Gynaecol Can. 2003;25:18-21
20. Soper JT, Johnson P, Johnson V, Berchuck A, Clarke-Pearson DL. Comprehensive restaging laparotomy in women with apparent early ovarian carcinoma. Obstet Gynecol. 1992;80:949-953
21. Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624-629
22. Chi DS, Abu-Rustum NR, Sonoda Y, et al. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am J Obstet Gynecol [in press].
23. Sjovall K NB, Einhorn N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer. 1994;4:333-336
24. Fauvet R, Boccara J, Dufournet C, Poncelet C, Darai E. Laparoscopic management of borderline ovarian tumors: results of a French multicenter study. Ann Oncol. 2005;16:403-410
25. Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974-979
26. Ben David Y, Bustan M, Shalev E. Laparoscopy as part of the evaluation and management of ovarian and cervix neoplasms. Harefuah. 2001;140:464-467
27. Husain A, Chi DS, Prasad M, et al. The role of laparoscopy in second-look evaluations for ovarian cancer. Gynecol Oncol. 2001;80:44-47
Which sling for which patient?
- How preop evaluation guides the decision
- Intrinsic sphincter deficiency: What it is and what to make of it
- Why the Burch procedure isn’t obsolete
- Mickey Karram, MD, the moderator of this discussion, is director of urogynecology at Good Samaritan Hospital in Cincinnati and professor of obstetrics and gynecology at the University of Cincinnati.
- Jerry Blaivas, MD, is clinical professor of urology, Weill Cornell Medical Center, New York City.
- Mark Walters, MD, is head of the section of urogynecology and reconstructive pelvic surgery, Cleveland Clinic Foundation, Cleveland.
Slings abound, entering the market faster than research can evaluate every new modification as exhaustively as we would like. How should we determine what is best for a particular patient? That is the question we examine in this discussion—the first in a series of roundtables, Controversies in Pelvic Surgery.
Future topics in the series focus on other unsettled issues:
- Preventing and managing lower urinary tract injury
- How best to correct vaginal vault prolapse
- Choosing the right hysterectomy route
- Mesh augmentation in prolapse repair
We decided on a roundtable format for the series because it seems well suited to a review of sometimes spotty data, not to mention the onslaught of new products and procedures that, ironically enough, are intended to simplify our lives.
Choosing a sling for “uncomplicated” cases
DR. KARRAM: What is your sling procedure of choice for the uncomplicated patient who has primary stress urinary incontinence (SUI), urethral hypermobility, and what appears to be a healthy urethra?
DR. BLAIVAS: I prefer an old-fashioned, autologous, rectus fascial sling, or one made of soft prolene mesh, as described by Rodriguez and Raz1—although I place the sling a bit more proximal than most and dissect into the retropubic space. The autologous rectus fascial sling has a long-term success rate at least as good as any other procedure. Even though it requires at least a small suprapubic incision, the possibility of serious complication (vascular or bowel injury, urethral or vaginal erosion) is almost nil.
Allograft and xenograft slings do not have as good a long-term success rate, but the chance of serious complication is nearly nil. If I do use a synthetic, I prefer the technique developed by Dr. Raz because it utilizes a time-honored, safe technique and avoids the unnecessary expense of the disposable midurethral sling kits.
I place the autologous sling at the bladder neck because long-term studies confirm its safety and efficacy.
DR. WALTERS: I use the literature as a guide and perform either Burch colposuspension or a synthetic midurethral sling procedure.Although its popularity has waned slightly, the Burch operation—either open or laparoscopic—is very effective.
My midurethral sling of choice for the simplest cases, as well as those with coexistent prolapse, is the Monarc transobturator sling (American Medical Systems, Minnetonka, Minn). The outcome data for this procedure are preliminary, and it has not yet been shown to equal a classic tension-free vaginal tape (TVT) technique, but I find that it causes less voiding dysfunction and urgency in my hands.
KARRAM: I would probably use a conventional TVT sling procedure. The reason: There is a tremendous amount of data to support the use of TVT for all types of SUI. The data and our experience supports a very low erosion or excursion rate with the TVT tape. Since it is really the only synthetic midurethral sling that has been shown to be as effective as—if not more effective than—conventional repairs, it remains my procedure of choice in uncomplicated cases.
Preop evaluation
KARRAM: Do you use the same sling procedure for all patients? If so, do you test preoperatively to help determine appropriate tension? If not, how do you decide which sling to use on which patient?
WALTERS: I use 3 types of slings:
- the Monarc transobturator sling for simple primary SUI, SUI with prolapse, and potential SUI,
- TVT placed very loosely for recurrent SUI with leak point pressures over 60 cm H2O, and
- TVT placed with a little more tension under the urethra for patients who have recurrent SUI with leak point pressures under 60 cm H2O, but who lack a complete “drainpipe” urethra.
Technically, I also use a fourth sling—a full fascial sling for severe intrinsic sphincter deficiency (ISD)—although I haven’t done one of these procedures in over a year.
BLAIVAS: For the uncomplicated case, I use either an autologous rectus fascial sling, an allograft or xenograft, or one made of soft prolene mesh, depending on the patient’s preference. zenograft
If I suspect she is at risk for erosion of a synthetic sling due to prior sling erosion, previous pelvic radiation, concomitant urethral diverticulectomy, or pelvic/urethral reconstructive surgery, I avoid synthetics.
BLAIVAS: Preoperatively, in addition to a focused history, questionnaire and examination, I make use of diaries, pad tests, video-urodynamics, pressure flow studies, cystoscopy, leak point pressure tests, and the Q-tip test whenever possible.
If the patient has significant urethral hypermobility and a relatively high leak point pressure, I use a bladder neck sling without any added tension. If there is little urethral mobility and a low leak point pressure, I make the sling a bit tighter.
If the woman has impaired detrusor contractility or urethral obstruction according to detrusor pressure uroflow data, I make the sling a bit looser.
I must emphasize that these are all “touchy, feely” things, unsupported by any meaningful data.
I also adjust the tension, depending on the patient’s feelings about intermittent catheterization versus persistent sphincteric incontinence.
That said, there is still a lot more art than science to this issue. I almost always tie the slings very loosely, compared with what I see others saying or doing. I do this because, in my own learning curve, I quickly encountered problems of urethral obstruction but almost never made a sling too loose. As I gained experience, I tied them looser and looser without ever losing clinical efficacy.
KARRAM: I don’t use the same sling for every patient. Like both of you, I first look at the leak point pressure and review the patient’s subjective complaints. I also consider any chronic systemic disorders, such as asthma or chronic obstructive lung disease, that may put a patient at high risk for failure. The findings help me adjust the tension intraoperatively.
I also try to reproduce the stress incontinence with a cough or Valsalva maneuver if the surgery is performed under local anesthesia, or using suprapubic pressure if it is done under general anesthesia.
Most of my sling procedures involve TVT. Although I recently began to selectively utilize a transobturator approach for women with occult incontinence or mild disease, I am not fully convinced it will be as efficacious as a traditional TVT for SUI.
KARRAM: Industry aggressively promotes new sling procedures—new materials, new placement techniques, new needles to pass the sling material. There are so many products and procedures that it is difficult to compare them all. Do you think this will become a serious problem, with many procedures lumped together and each assumed as effective as a similar product?
BLAIVAS: Emphatically, yes. Each new sling, no matter how slight the modification seems to be, is encumbered by a new learning curve. That means that the first batch of patients each surgeon operates on will be subjected to a higher complication rate or lower efficacy.
There have been unexpected (and unpublished) deaths due to vascular and bowel injuries and a 1% to 9% vaginal or urethral erosion rate with synthetic slings. Disappointing medium-term efficacy of allograft and zenograft slings were recently published, and recent abstracts suggest that transobturator slings are less efficacious for women with ISD.
WALTERS: This problem not only exists, it has been worsening over the past several years. We still don’t know if the transobturator sling is as effective as TVT, for example, yet we are slowly adopting it because it is easier and probably safer.
The various instruments required for these operations may not be that different, but the absorbable and nonabsorbable prosthetic sling materials are. They should be evaluated constantly and compared with the TVT procedure.
In medicine, we can’t control what industry does, but we can continue to study and test the new procedures before we consider them standards.
KARRAM: I agree that the problem already exists. Although we have a substantial amount of data on the TVT mechanism and the Gynecare material (Ethicon, Somerville, NJ), we need to derive data for other slings. This may be very difficult, given the number of different materials and kits already on the market.
KARRAM: The current rage is the transobturator approach, although we have very little data about it. Theoretically, it is safer than TVT, since there is no need to pass the needles through the retropubic space, and thus it is unlikely to lead to vascular and bowel, or bladder and urethral injury.
Should go ahead and adopt it as a primary procedure, or do you recommend waiting for more data on its efficacy?
BLAIVAS: I do not recommend that it be adopted until it is proven safe and effective, but I feel the same way about most of the other slings as well. When performed by experts, almost all these procedures are very safe, but we don’t know very much about long-term efficacy. If someone selects the transobturator approach out of fear of complications from a different procedure, he or she is probably not skilled enough to perform these operations in the first place.
KARRAM: I agree that we need to wait until efficacy data are established. As slings become less and less invasive, industry is aggressively pushing them into the hands of novices. Most of the time these are gynecologists who have not performed antiincontinence surgery and may even lack cystoscopy privileges. Do you think this will be a problem down the road?
BLAIVAS: Yes, unless the procedures are dumbed down enough and they are truly safe and effective.
Transobturator and TVT learning curves and outcomes
WALTERS: As for the transobturator approach, I am convinced, based on my surgical experience over the past 2 years, that it is easier than TVT and results in less voiding difficulty and urgency.
However, I am not convinced that it is equivalent to retropubic TVT in cure rates for SUI. This issue especially needs to be rigorously tested.
In my experience, TVT has a very high cure rate for SUI, but can cause urgency, including intractable urge and voiding dysfunction requiring transection of the polypropylene tape. This rarely occurs with the transobturator sling, making it attractive for simple SUI.
The transobturator sling also is easier than TVT to learn and teach, and completely avoids any risk of retropubic hematoma and bowel perforation. Also, unless there is extensive prolapse, the risk of entering the bladder and urethra is practically nil, assuming you are able to pass the needle and touch your finger from the lateral side.
The next big, important study will likely be a randomized comparison of transobturator and TVT slings, similar to the way TVT was compared with the open Burch procedure.
WALTERS: For their own protection, I don’t think gynecologists should be doing surgery for prolapse and incontinence if they do not have privileges for cystoscopy.
KARRAM: I agree. It is very important that the gynecologists performing these procedures evaluate the patient thoroughly enough to decide wisely between surgical and nonsurgical management. Certainly they should have the ability to evaluate the lower urinary tract with cystoscopy before doing these procedures.
WALTERS: I perform cystoscopy on virtually every pelvic reconstructive surgery and find abnormalities in the bladder, urethra, or ureters in 2% to 4% of cases a year. Although I encourage gynecologists to learn these operations, cystoscopy is crucial, so an effort should be made to obtain training and privileges for it.
KARRAM: You mentioned the Burch procedure, Dr. Walters. Do you think there is still a role for retropubic urethropexy—done laparoscopically or via an open technique?
WALTERS: I perform an open Burch procedure if I have already made a laparotomy incision for another reason, such as abdominal sacrocolpopexy or hysterectomy. I occasionally perform laparoscopic Burch procedures in younger women undergoing laparoscopy for other reasons, such as tubal sterilization or ovarian disease.
KARRAM: I perform retropubic urethropexy if operating in the abdomen for another reason, provided the patient has SUI, urethral hypermobility, and vaginal pliability.
BLAIVAS: Retropubic urethropexy has a proven track record, but requires skill and experience.
With uncomplicated incontinence, long-term success appears as good as any operation. If a surgeon is skilled, this procedure should be part of his or her armamentarium.
KARRAM: You are correct. Data suggest the retropubic operation and TVT procedure are equally effective.2,3
WALTERS: I find it somewhat amusing that I am increasingly considered “old-style” when I continue to recommend the Burch procedure.
All I can say is that I’m glad my partners, fellows, and I have the ability to perform this operation when necessary.
KARRAM: Unfortunately, since retropubic urethropexy is performed much less frequently in the past, residents no longer learn retropubic anatomy.
This has become a problem because a many synthetic midurethral slings require blind passage of a needle through the retropubic space.
KARRAM: Are we going to see more complications with these procedures because of a lack of clear understanding of the anatomy? If so, how do you think this can be resolved?
WALTERS: Although TVT works very well for SUI, I think our specialty abandoned Burch colposuspension prematurely, ignoring all the evidence supporting its efficacy. I wish residents were still being taught Burch procedures on open cases. I can see that general Ob/Gyns are slowly forgetting how to do the operation, making it more difficult for them to manage complications such as hematomas and infections.
That said, I don’t think TVT complications will occur more often because of this lack of experience with the Burch procedure. On the contrary, I expect them to remain rare. Use of transobturator slings avoids retropubic anatomy completely, but we need more outcome data before making a wholesale switch from TVT.
KARRAM: More injuries to blood vessels and other structures are inevitable when novice surgeons unfamiliar with anatomy attempt to blindly pass large needles.
BLAIVAS: I agree, but the developers of the new techniques are trying to make them idiot-proof—and maybe they will! It’s a sad day, though, when surgeons don’t know anatomy. Too many don’t!
KARRAM: The only solution is to aggressively teach anatomy in residency and demand preceptorships that teach anatomy before allowing inexperienced surgeons to adopt the procedure.
BLAIVAS: If enough mistakes are made, we’ll be forced to teach anatomy again. The best solution, in my judgment, is sub-specialization to the point where all surgeons doing these procedures have sufficient experience.
KARRAM: Intrinsic sphincter deficiency has become a common term for severe forms of stress incontinence, although there is no widely accepted definition.
How do you define ISD? Is it important to detect it preoperatively? If so, how does ISD alter surgical management?
BLAIVAS: ISD was initially used to describe weakened sphincter mechanism, as distinct from incontinence because of urethral hypermobility.
For practical purposes, all patients with sphincteric incontinence have some degree of “intrinsic sphincter deficiency,” but I no longer use the term. Instead, I characterize the sphincter by vesical leak point pressure and the degree of urethral mobility as measured by a simple Q-tip test. The lower the leak point pressure, the weaker the sphincter, the more likely it will be designated ISD.
WALTERS: I still follow the rough guidelines I was taught: ISD exists at leak point pressures below 60 cm H2O on cystometrogram., although this is probably not that accurate. There is no cutoff defining ISD, but a gradually increasing weakness of the urethral sphincter that correlates roughly with severity of symptoms.
I doubt the concept of ISD would hold up to rigorous scientific scrutiny as a condition or prognostic factor. However, I still use it.
KARRAM: Although intrinsic sphincter deficiency is a vague concept, I believe there are cases that exhibit it—eg, patients who have had multiple operations, been radiated, or have neurologic disease, who essentially have a urethra that is open at rest, doesn’t move, and leaks urine with minimal increases in intraabdominal pressure. In situations such as these, I select procedures that bulk up or obstruct the urethra to correct or improve the incontinence.
Most cases identified as having ISD are based on a urethral function test that measures either leak point pressure or static urethral closure pressure. Unfortunately, little data prove that these tests truly measure urethral function.
There are no definitive data suggesting that a procedure needs to be done any differently based on these tests. So I think the term is presently used in a very cavalier fashion and requires a more objective mechanism to define the condition. Only then can its potential impact on clinical management be evaluated.
Dr. Karram has received research support from American Medical Systems, Yamounouchi, and Gynecare, and serves on the speakers bureau for Gynecare, Ortho-McNeil, Watson, and Indevus. Dr. Blaivas reports no relevant financial relationships. Dr. Walters is a consultant for American Medical Systems and a lecturer for Boston Scientific.
1. Rodriguez LV, Raz S. Polypropylene sling for the treatment of stress urinary incontinence. Urology. 2001;58:783-785.
2. Ward K, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. Br Med J. 2002;325:65-70.
3. Paraiso MF, Walters MD, Karram MM, Barber MD. Laparoscopic Burch colposuspension versus tension-free vaginal tape: a randomized trial. Obstet Gynecol. 2004;104:1249-1258.
- How preop evaluation guides the decision
- Intrinsic sphincter deficiency: What it is and what to make of it
- Why the Burch procedure isn’t obsolete
- Mickey Karram, MD, the moderator of this discussion, is director of urogynecology at Good Samaritan Hospital in Cincinnati and professor of obstetrics and gynecology at the University of Cincinnati.
- Jerry Blaivas, MD, is clinical professor of urology, Weill Cornell Medical Center, New York City.
- Mark Walters, MD, is head of the section of urogynecology and reconstructive pelvic surgery, Cleveland Clinic Foundation, Cleveland.
Slings abound, entering the market faster than research can evaluate every new modification as exhaustively as we would like. How should we determine what is best for a particular patient? That is the question we examine in this discussion—the first in a series of roundtables, Controversies in Pelvic Surgery.
Future topics in the series focus on other unsettled issues:
- Preventing and managing lower urinary tract injury
- How best to correct vaginal vault prolapse
- Choosing the right hysterectomy route
- Mesh augmentation in prolapse repair
We decided on a roundtable format for the series because it seems well suited to a review of sometimes spotty data, not to mention the onslaught of new products and procedures that, ironically enough, are intended to simplify our lives.
Choosing a sling for “uncomplicated” cases
DR. KARRAM: What is your sling procedure of choice for the uncomplicated patient who has primary stress urinary incontinence (SUI), urethral hypermobility, and what appears to be a healthy urethra?
DR. BLAIVAS: I prefer an old-fashioned, autologous, rectus fascial sling, or one made of soft prolene mesh, as described by Rodriguez and Raz1—although I place the sling a bit more proximal than most and dissect into the retropubic space. The autologous rectus fascial sling has a long-term success rate at least as good as any other procedure. Even though it requires at least a small suprapubic incision, the possibility of serious complication (vascular or bowel injury, urethral or vaginal erosion) is almost nil.
Allograft and xenograft slings do not have as good a long-term success rate, but the chance of serious complication is nearly nil. If I do use a synthetic, I prefer the technique developed by Dr. Raz because it utilizes a time-honored, safe technique and avoids the unnecessary expense of the disposable midurethral sling kits.
I place the autologous sling at the bladder neck because long-term studies confirm its safety and efficacy.
DR. WALTERS: I use the literature as a guide and perform either Burch colposuspension or a synthetic midurethral sling procedure.Although its popularity has waned slightly, the Burch operation—either open or laparoscopic—is very effective.
My midurethral sling of choice for the simplest cases, as well as those with coexistent prolapse, is the Monarc transobturator sling (American Medical Systems, Minnetonka, Minn). The outcome data for this procedure are preliminary, and it has not yet been shown to equal a classic tension-free vaginal tape (TVT) technique, but I find that it causes less voiding dysfunction and urgency in my hands.
KARRAM: I would probably use a conventional TVT sling procedure. The reason: There is a tremendous amount of data to support the use of TVT for all types of SUI. The data and our experience supports a very low erosion or excursion rate with the TVT tape. Since it is really the only synthetic midurethral sling that has been shown to be as effective as—if not more effective than—conventional repairs, it remains my procedure of choice in uncomplicated cases.
Preop evaluation
KARRAM: Do you use the same sling procedure for all patients? If so, do you test preoperatively to help determine appropriate tension? If not, how do you decide which sling to use on which patient?
WALTERS: I use 3 types of slings:
- the Monarc transobturator sling for simple primary SUI, SUI with prolapse, and potential SUI,
- TVT placed very loosely for recurrent SUI with leak point pressures over 60 cm H2O, and
- TVT placed with a little more tension under the urethra for patients who have recurrent SUI with leak point pressures under 60 cm H2O, but who lack a complete “drainpipe” urethra.
Technically, I also use a fourth sling—a full fascial sling for severe intrinsic sphincter deficiency (ISD)—although I haven’t done one of these procedures in over a year.
BLAIVAS: For the uncomplicated case, I use either an autologous rectus fascial sling, an allograft or xenograft, or one made of soft prolene mesh, depending on the patient’s preference. zenograft
If I suspect she is at risk for erosion of a synthetic sling due to prior sling erosion, previous pelvic radiation, concomitant urethral diverticulectomy, or pelvic/urethral reconstructive surgery, I avoid synthetics.
BLAIVAS: Preoperatively, in addition to a focused history, questionnaire and examination, I make use of diaries, pad tests, video-urodynamics, pressure flow studies, cystoscopy, leak point pressure tests, and the Q-tip test whenever possible.
If the patient has significant urethral hypermobility and a relatively high leak point pressure, I use a bladder neck sling without any added tension. If there is little urethral mobility and a low leak point pressure, I make the sling a bit tighter.
If the woman has impaired detrusor contractility or urethral obstruction according to detrusor pressure uroflow data, I make the sling a bit looser.
I must emphasize that these are all “touchy, feely” things, unsupported by any meaningful data.
I also adjust the tension, depending on the patient’s feelings about intermittent catheterization versus persistent sphincteric incontinence.
That said, there is still a lot more art than science to this issue. I almost always tie the slings very loosely, compared with what I see others saying or doing. I do this because, in my own learning curve, I quickly encountered problems of urethral obstruction but almost never made a sling too loose. As I gained experience, I tied them looser and looser without ever losing clinical efficacy.
KARRAM: I don’t use the same sling for every patient. Like both of you, I first look at the leak point pressure and review the patient’s subjective complaints. I also consider any chronic systemic disorders, such as asthma or chronic obstructive lung disease, that may put a patient at high risk for failure. The findings help me adjust the tension intraoperatively.
I also try to reproduce the stress incontinence with a cough or Valsalva maneuver if the surgery is performed under local anesthesia, or using suprapubic pressure if it is done under general anesthesia.
Most of my sling procedures involve TVT. Although I recently began to selectively utilize a transobturator approach for women with occult incontinence or mild disease, I am not fully convinced it will be as efficacious as a traditional TVT for SUI.
KARRAM: Industry aggressively promotes new sling procedures—new materials, new placement techniques, new needles to pass the sling material. There are so many products and procedures that it is difficult to compare them all. Do you think this will become a serious problem, with many procedures lumped together and each assumed as effective as a similar product?
BLAIVAS: Emphatically, yes. Each new sling, no matter how slight the modification seems to be, is encumbered by a new learning curve. That means that the first batch of patients each surgeon operates on will be subjected to a higher complication rate or lower efficacy.
There have been unexpected (and unpublished) deaths due to vascular and bowel injuries and a 1% to 9% vaginal or urethral erosion rate with synthetic slings. Disappointing medium-term efficacy of allograft and zenograft slings were recently published, and recent abstracts suggest that transobturator slings are less efficacious for women with ISD.
WALTERS: This problem not only exists, it has been worsening over the past several years. We still don’t know if the transobturator sling is as effective as TVT, for example, yet we are slowly adopting it because it is easier and probably safer.
The various instruments required for these operations may not be that different, but the absorbable and nonabsorbable prosthetic sling materials are. They should be evaluated constantly and compared with the TVT procedure.
In medicine, we can’t control what industry does, but we can continue to study and test the new procedures before we consider them standards.
KARRAM: I agree that the problem already exists. Although we have a substantial amount of data on the TVT mechanism and the Gynecare material (Ethicon, Somerville, NJ), we need to derive data for other slings. This may be very difficult, given the number of different materials and kits already on the market.
KARRAM: The current rage is the transobturator approach, although we have very little data about it. Theoretically, it is safer than TVT, since there is no need to pass the needles through the retropubic space, and thus it is unlikely to lead to vascular and bowel, or bladder and urethral injury.
Should go ahead and adopt it as a primary procedure, or do you recommend waiting for more data on its efficacy?
BLAIVAS: I do not recommend that it be adopted until it is proven safe and effective, but I feel the same way about most of the other slings as well. When performed by experts, almost all these procedures are very safe, but we don’t know very much about long-term efficacy. If someone selects the transobturator approach out of fear of complications from a different procedure, he or she is probably not skilled enough to perform these operations in the first place.
KARRAM: I agree that we need to wait until efficacy data are established. As slings become less and less invasive, industry is aggressively pushing them into the hands of novices. Most of the time these are gynecologists who have not performed antiincontinence surgery and may even lack cystoscopy privileges. Do you think this will be a problem down the road?
BLAIVAS: Yes, unless the procedures are dumbed down enough and they are truly safe and effective.
Transobturator and TVT learning curves and outcomes
WALTERS: As for the transobturator approach, I am convinced, based on my surgical experience over the past 2 years, that it is easier than TVT and results in less voiding difficulty and urgency.
However, I am not convinced that it is equivalent to retropubic TVT in cure rates for SUI. This issue especially needs to be rigorously tested.
In my experience, TVT has a very high cure rate for SUI, but can cause urgency, including intractable urge and voiding dysfunction requiring transection of the polypropylene tape. This rarely occurs with the transobturator sling, making it attractive for simple SUI.
The transobturator sling also is easier than TVT to learn and teach, and completely avoids any risk of retropubic hematoma and bowel perforation. Also, unless there is extensive prolapse, the risk of entering the bladder and urethra is practically nil, assuming you are able to pass the needle and touch your finger from the lateral side.
The next big, important study will likely be a randomized comparison of transobturator and TVT slings, similar to the way TVT was compared with the open Burch procedure.
WALTERS: For their own protection, I don’t think gynecologists should be doing surgery for prolapse and incontinence if they do not have privileges for cystoscopy.
KARRAM: I agree. It is very important that the gynecologists performing these procedures evaluate the patient thoroughly enough to decide wisely between surgical and nonsurgical management. Certainly they should have the ability to evaluate the lower urinary tract with cystoscopy before doing these procedures.
WALTERS: I perform cystoscopy on virtually every pelvic reconstructive surgery and find abnormalities in the bladder, urethra, or ureters in 2% to 4% of cases a year. Although I encourage gynecologists to learn these operations, cystoscopy is crucial, so an effort should be made to obtain training and privileges for it.
KARRAM: You mentioned the Burch procedure, Dr. Walters. Do you think there is still a role for retropubic urethropexy—done laparoscopically or via an open technique?
WALTERS: I perform an open Burch procedure if I have already made a laparotomy incision for another reason, such as abdominal sacrocolpopexy or hysterectomy. I occasionally perform laparoscopic Burch procedures in younger women undergoing laparoscopy for other reasons, such as tubal sterilization or ovarian disease.
KARRAM: I perform retropubic urethropexy if operating in the abdomen for another reason, provided the patient has SUI, urethral hypermobility, and vaginal pliability.
BLAIVAS: Retropubic urethropexy has a proven track record, but requires skill and experience.
With uncomplicated incontinence, long-term success appears as good as any operation. If a surgeon is skilled, this procedure should be part of his or her armamentarium.
KARRAM: You are correct. Data suggest the retropubic operation and TVT procedure are equally effective.2,3
WALTERS: I find it somewhat amusing that I am increasingly considered “old-style” when I continue to recommend the Burch procedure.
All I can say is that I’m glad my partners, fellows, and I have the ability to perform this operation when necessary.
KARRAM: Unfortunately, since retropubic urethropexy is performed much less frequently in the past, residents no longer learn retropubic anatomy.
This has become a problem because a many synthetic midurethral slings require blind passage of a needle through the retropubic space.
KARRAM: Are we going to see more complications with these procedures because of a lack of clear understanding of the anatomy? If so, how do you think this can be resolved?
WALTERS: Although TVT works very well for SUI, I think our specialty abandoned Burch colposuspension prematurely, ignoring all the evidence supporting its efficacy. I wish residents were still being taught Burch procedures on open cases. I can see that general Ob/Gyns are slowly forgetting how to do the operation, making it more difficult for them to manage complications such as hematomas and infections.
That said, I don’t think TVT complications will occur more often because of this lack of experience with the Burch procedure. On the contrary, I expect them to remain rare. Use of transobturator slings avoids retropubic anatomy completely, but we need more outcome data before making a wholesale switch from TVT.
KARRAM: More injuries to blood vessels and other structures are inevitable when novice surgeons unfamiliar with anatomy attempt to blindly pass large needles.
BLAIVAS: I agree, but the developers of the new techniques are trying to make them idiot-proof—and maybe they will! It’s a sad day, though, when surgeons don’t know anatomy. Too many don’t!
KARRAM: The only solution is to aggressively teach anatomy in residency and demand preceptorships that teach anatomy before allowing inexperienced surgeons to adopt the procedure.
BLAIVAS: If enough mistakes are made, we’ll be forced to teach anatomy again. The best solution, in my judgment, is sub-specialization to the point where all surgeons doing these procedures have sufficient experience.
KARRAM: Intrinsic sphincter deficiency has become a common term for severe forms of stress incontinence, although there is no widely accepted definition.
How do you define ISD? Is it important to detect it preoperatively? If so, how does ISD alter surgical management?
BLAIVAS: ISD was initially used to describe weakened sphincter mechanism, as distinct from incontinence because of urethral hypermobility.
For practical purposes, all patients with sphincteric incontinence have some degree of “intrinsic sphincter deficiency,” but I no longer use the term. Instead, I characterize the sphincter by vesical leak point pressure and the degree of urethral mobility as measured by a simple Q-tip test. The lower the leak point pressure, the weaker the sphincter, the more likely it will be designated ISD.
WALTERS: I still follow the rough guidelines I was taught: ISD exists at leak point pressures below 60 cm H2O on cystometrogram., although this is probably not that accurate. There is no cutoff defining ISD, but a gradually increasing weakness of the urethral sphincter that correlates roughly with severity of symptoms.
I doubt the concept of ISD would hold up to rigorous scientific scrutiny as a condition or prognostic factor. However, I still use it.
KARRAM: Although intrinsic sphincter deficiency is a vague concept, I believe there are cases that exhibit it—eg, patients who have had multiple operations, been radiated, or have neurologic disease, who essentially have a urethra that is open at rest, doesn’t move, and leaks urine with minimal increases in intraabdominal pressure. In situations such as these, I select procedures that bulk up or obstruct the urethra to correct or improve the incontinence.
Most cases identified as having ISD are based on a urethral function test that measures either leak point pressure or static urethral closure pressure. Unfortunately, little data prove that these tests truly measure urethral function.
There are no definitive data suggesting that a procedure needs to be done any differently based on these tests. So I think the term is presently used in a very cavalier fashion and requires a more objective mechanism to define the condition. Only then can its potential impact on clinical management be evaluated.
Dr. Karram has received research support from American Medical Systems, Yamounouchi, and Gynecare, and serves on the speakers bureau for Gynecare, Ortho-McNeil, Watson, and Indevus. Dr. Blaivas reports no relevant financial relationships. Dr. Walters is a consultant for American Medical Systems and a lecturer for Boston Scientific.
- How preop evaluation guides the decision
- Intrinsic sphincter deficiency: What it is and what to make of it
- Why the Burch procedure isn’t obsolete
- Mickey Karram, MD, the moderator of this discussion, is director of urogynecology at Good Samaritan Hospital in Cincinnati and professor of obstetrics and gynecology at the University of Cincinnati.
- Jerry Blaivas, MD, is clinical professor of urology, Weill Cornell Medical Center, New York City.
- Mark Walters, MD, is head of the section of urogynecology and reconstructive pelvic surgery, Cleveland Clinic Foundation, Cleveland.
Slings abound, entering the market faster than research can evaluate every new modification as exhaustively as we would like. How should we determine what is best for a particular patient? That is the question we examine in this discussion—the first in a series of roundtables, Controversies in Pelvic Surgery.
Future topics in the series focus on other unsettled issues:
- Preventing and managing lower urinary tract injury
- How best to correct vaginal vault prolapse
- Choosing the right hysterectomy route
- Mesh augmentation in prolapse repair
We decided on a roundtable format for the series because it seems well suited to a review of sometimes spotty data, not to mention the onslaught of new products and procedures that, ironically enough, are intended to simplify our lives.
Choosing a sling for “uncomplicated” cases
DR. KARRAM: What is your sling procedure of choice for the uncomplicated patient who has primary stress urinary incontinence (SUI), urethral hypermobility, and what appears to be a healthy urethra?
DR. BLAIVAS: I prefer an old-fashioned, autologous, rectus fascial sling, or one made of soft prolene mesh, as described by Rodriguez and Raz1—although I place the sling a bit more proximal than most and dissect into the retropubic space. The autologous rectus fascial sling has a long-term success rate at least as good as any other procedure. Even though it requires at least a small suprapubic incision, the possibility of serious complication (vascular or bowel injury, urethral or vaginal erosion) is almost nil.
Allograft and xenograft slings do not have as good a long-term success rate, but the chance of serious complication is nearly nil. If I do use a synthetic, I prefer the technique developed by Dr. Raz because it utilizes a time-honored, safe technique and avoids the unnecessary expense of the disposable midurethral sling kits.
I place the autologous sling at the bladder neck because long-term studies confirm its safety and efficacy.
DR. WALTERS: I use the literature as a guide and perform either Burch colposuspension or a synthetic midurethral sling procedure.Although its popularity has waned slightly, the Burch operation—either open or laparoscopic—is very effective.
My midurethral sling of choice for the simplest cases, as well as those with coexistent prolapse, is the Monarc transobturator sling (American Medical Systems, Minnetonka, Minn). The outcome data for this procedure are preliminary, and it has not yet been shown to equal a classic tension-free vaginal tape (TVT) technique, but I find that it causes less voiding dysfunction and urgency in my hands.
KARRAM: I would probably use a conventional TVT sling procedure. The reason: There is a tremendous amount of data to support the use of TVT for all types of SUI. The data and our experience supports a very low erosion or excursion rate with the TVT tape. Since it is really the only synthetic midurethral sling that has been shown to be as effective as—if not more effective than—conventional repairs, it remains my procedure of choice in uncomplicated cases.
Preop evaluation
KARRAM: Do you use the same sling procedure for all patients? If so, do you test preoperatively to help determine appropriate tension? If not, how do you decide which sling to use on which patient?
WALTERS: I use 3 types of slings:
- the Monarc transobturator sling for simple primary SUI, SUI with prolapse, and potential SUI,
- TVT placed very loosely for recurrent SUI with leak point pressures over 60 cm H2O, and
- TVT placed with a little more tension under the urethra for patients who have recurrent SUI with leak point pressures under 60 cm H2O, but who lack a complete “drainpipe” urethra.
Technically, I also use a fourth sling—a full fascial sling for severe intrinsic sphincter deficiency (ISD)—although I haven’t done one of these procedures in over a year.
BLAIVAS: For the uncomplicated case, I use either an autologous rectus fascial sling, an allograft or xenograft, or one made of soft prolene mesh, depending on the patient’s preference. zenograft
If I suspect she is at risk for erosion of a synthetic sling due to prior sling erosion, previous pelvic radiation, concomitant urethral diverticulectomy, or pelvic/urethral reconstructive surgery, I avoid synthetics.
BLAIVAS: Preoperatively, in addition to a focused history, questionnaire and examination, I make use of diaries, pad tests, video-urodynamics, pressure flow studies, cystoscopy, leak point pressure tests, and the Q-tip test whenever possible.
If the patient has significant urethral hypermobility and a relatively high leak point pressure, I use a bladder neck sling without any added tension. If there is little urethral mobility and a low leak point pressure, I make the sling a bit tighter.
If the woman has impaired detrusor contractility or urethral obstruction according to detrusor pressure uroflow data, I make the sling a bit looser.
I must emphasize that these are all “touchy, feely” things, unsupported by any meaningful data.
I also adjust the tension, depending on the patient’s feelings about intermittent catheterization versus persistent sphincteric incontinence.
That said, there is still a lot more art than science to this issue. I almost always tie the slings very loosely, compared with what I see others saying or doing. I do this because, in my own learning curve, I quickly encountered problems of urethral obstruction but almost never made a sling too loose. As I gained experience, I tied them looser and looser without ever losing clinical efficacy.
KARRAM: I don’t use the same sling for every patient. Like both of you, I first look at the leak point pressure and review the patient’s subjective complaints. I also consider any chronic systemic disorders, such as asthma or chronic obstructive lung disease, that may put a patient at high risk for failure. The findings help me adjust the tension intraoperatively.
I also try to reproduce the stress incontinence with a cough or Valsalva maneuver if the surgery is performed under local anesthesia, or using suprapubic pressure if it is done under general anesthesia.
Most of my sling procedures involve TVT. Although I recently began to selectively utilize a transobturator approach for women with occult incontinence or mild disease, I am not fully convinced it will be as efficacious as a traditional TVT for SUI.
KARRAM: Industry aggressively promotes new sling procedures—new materials, new placement techniques, new needles to pass the sling material. There are so many products and procedures that it is difficult to compare them all. Do you think this will become a serious problem, with many procedures lumped together and each assumed as effective as a similar product?
BLAIVAS: Emphatically, yes. Each new sling, no matter how slight the modification seems to be, is encumbered by a new learning curve. That means that the first batch of patients each surgeon operates on will be subjected to a higher complication rate or lower efficacy.
There have been unexpected (and unpublished) deaths due to vascular and bowel injuries and a 1% to 9% vaginal or urethral erosion rate with synthetic slings. Disappointing medium-term efficacy of allograft and zenograft slings were recently published, and recent abstracts suggest that transobturator slings are less efficacious for women with ISD.
WALTERS: This problem not only exists, it has been worsening over the past several years. We still don’t know if the transobturator sling is as effective as TVT, for example, yet we are slowly adopting it because it is easier and probably safer.
The various instruments required for these operations may not be that different, but the absorbable and nonabsorbable prosthetic sling materials are. They should be evaluated constantly and compared with the TVT procedure.
In medicine, we can’t control what industry does, but we can continue to study and test the new procedures before we consider them standards.
KARRAM: I agree that the problem already exists. Although we have a substantial amount of data on the TVT mechanism and the Gynecare material (Ethicon, Somerville, NJ), we need to derive data for other slings. This may be very difficult, given the number of different materials and kits already on the market.
KARRAM: The current rage is the transobturator approach, although we have very little data about it. Theoretically, it is safer than TVT, since there is no need to pass the needles through the retropubic space, and thus it is unlikely to lead to vascular and bowel, or bladder and urethral injury.
Should go ahead and adopt it as a primary procedure, or do you recommend waiting for more data on its efficacy?
BLAIVAS: I do not recommend that it be adopted until it is proven safe and effective, but I feel the same way about most of the other slings as well. When performed by experts, almost all these procedures are very safe, but we don’t know very much about long-term efficacy. If someone selects the transobturator approach out of fear of complications from a different procedure, he or she is probably not skilled enough to perform these operations in the first place.
KARRAM: I agree that we need to wait until efficacy data are established. As slings become less and less invasive, industry is aggressively pushing them into the hands of novices. Most of the time these are gynecologists who have not performed antiincontinence surgery and may even lack cystoscopy privileges. Do you think this will be a problem down the road?
BLAIVAS: Yes, unless the procedures are dumbed down enough and they are truly safe and effective.
Transobturator and TVT learning curves and outcomes
WALTERS: As for the transobturator approach, I am convinced, based on my surgical experience over the past 2 years, that it is easier than TVT and results in less voiding difficulty and urgency.
However, I am not convinced that it is equivalent to retropubic TVT in cure rates for SUI. This issue especially needs to be rigorously tested.
In my experience, TVT has a very high cure rate for SUI, but can cause urgency, including intractable urge and voiding dysfunction requiring transection of the polypropylene tape. This rarely occurs with the transobturator sling, making it attractive for simple SUI.
The transobturator sling also is easier than TVT to learn and teach, and completely avoids any risk of retropubic hematoma and bowel perforation. Also, unless there is extensive prolapse, the risk of entering the bladder and urethra is practically nil, assuming you are able to pass the needle and touch your finger from the lateral side.
The next big, important study will likely be a randomized comparison of transobturator and TVT slings, similar to the way TVT was compared with the open Burch procedure.
WALTERS: For their own protection, I don’t think gynecologists should be doing surgery for prolapse and incontinence if they do not have privileges for cystoscopy.
KARRAM: I agree. It is very important that the gynecologists performing these procedures evaluate the patient thoroughly enough to decide wisely between surgical and nonsurgical management. Certainly they should have the ability to evaluate the lower urinary tract with cystoscopy before doing these procedures.
WALTERS: I perform cystoscopy on virtually every pelvic reconstructive surgery and find abnormalities in the bladder, urethra, or ureters in 2% to 4% of cases a year. Although I encourage gynecologists to learn these operations, cystoscopy is crucial, so an effort should be made to obtain training and privileges for it.
KARRAM: You mentioned the Burch procedure, Dr. Walters. Do you think there is still a role for retropubic urethropexy—done laparoscopically or via an open technique?
WALTERS: I perform an open Burch procedure if I have already made a laparotomy incision for another reason, such as abdominal sacrocolpopexy or hysterectomy. I occasionally perform laparoscopic Burch procedures in younger women undergoing laparoscopy for other reasons, such as tubal sterilization or ovarian disease.
KARRAM: I perform retropubic urethropexy if operating in the abdomen for another reason, provided the patient has SUI, urethral hypermobility, and vaginal pliability.
BLAIVAS: Retropubic urethropexy has a proven track record, but requires skill and experience.
With uncomplicated incontinence, long-term success appears as good as any operation. If a surgeon is skilled, this procedure should be part of his or her armamentarium.
KARRAM: You are correct. Data suggest the retropubic operation and TVT procedure are equally effective.2,3
WALTERS: I find it somewhat amusing that I am increasingly considered “old-style” when I continue to recommend the Burch procedure.
All I can say is that I’m glad my partners, fellows, and I have the ability to perform this operation when necessary.
KARRAM: Unfortunately, since retropubic urethropexy is performed much less frequently in the past, residents no longer learn retropubic anatomy.
This has become a problem because a many synthetic midurethral slings require blind passage of a needle through the retropubic space.
KARRAM: Are we going to see more complications with these procedures because of a lack of clear understanding of the anatomy? If so, how do you think this can be resolved?
WALTERS: Although TVT works very well for SUI, I think our specialty abandoned Burch colposuspension prematurely, ignoring all the evidence supporting its efficacy. I wish residents were still being taught Burch procedures on open cases. I can see that general Ob/Gyns are slowly forgetting how to do the operation, making it more difficult for them to manage complications such as hematomas and infections.
That said, I don’t think TVT complications will occur more often because of this lack of experience with the Burch procedure. On the contrary, I expect them to remain rare. Use of transobturator slings avoids retropubic anatomy completely, but we need more outcome data before making a wholesale switch from TVT.
KARRAM: More injuries to blood vessels and other structures are inevitable when novice surgeons unfamiliar with anatomy attempt to blindly pass large needles.
BLAIVAS: I agree, but the developers of the new techniques are trying to make them idiot-proof—and maybe they will! It’s a sad day, though, when surgeons don’t know anatomy. Too many don’t!
KARRAM: The only solution is to aggressively teach anatomy in residency and demand preceptorships that teach anatomy before allowing inexperienced surgeons to adopt the procedure.
BLAIVAS: If enough mistakes are made, we’ll be forced to teach anatomy again. The best solution, in my judgment, is sub-specialization to the point where all surgeons doing these procedures have sufficient experience.
KARRAM: Intrinsic sphincter deficiency has become a common term for severe forms of stress incontinence, although there is no widely accepted definition.
How do you define ISD? Is it important to detect it preoperatively? If so, how does ISD alter surgical management?
BLAIVAS: ISD was initially used to describe weakened sphincter mechanism, as distinct from incontinence because of urethral hypermobility.
For practical purposes, all patients with sphincteric incontinence have some degree of “intrinsic sphincter deficiency,” but I no longer use the term. Instead, I characterize the sphincter by vesical leak point pressure and the degree of urethral mobility as measured by a simple Q-tip test. The lower the leak point pressure, the weaker the sphincter, the more likely it will be designated ISD.
WALTERS: I still follow the rough guidelines I was taught: ISD exists at leak point pressures below 60 cm H2O on cystometrogram., although this is probably not that accurate. There is no cutoff defining ISD, but a gradually increasing weakness of the urethral sphincter that correlates roughly with severity of symptoms.
I doubt the concept of ISD would hold up to rigorous scientific scrutiny as a condition or prognostic factor. However, I still use it.
KARRAM: Although intrinsic sphincter deficiency is a vague concept, I believe there are cases that exhibit it—eg, patients who have had multiple operations, been radiated, or have neurologic disease, who essentially have a urethra that is open at rest, doesn’t move, and leaks urine with minimal increases in intraabdominal pressure. In situations such as these, I select procedures that bulk up or obstruct the urethra to correct or improve the incontinence.
Most cases identified as having ISD are based on a urethral function test that measures either leak point pressure or static urethral closure pressure. Unfortunately, little data prove that these tests truly measure urethral function.
There are no definitive data suggesting that a procedure needs to be done any differently based on these tests. So I think the term is presently used in a very cavalier fashion and requires a more objective mechanism to define the condition. Only then can its potential impact on clinical management be evaluated.
Dr. Karram has received research support from American Medical Systems, Yamounouchi, and Gynecare, and serves on the speakers bureau for Gynecare, Ortho-McNeil, Watson, and Indevus. Dr. Blaivas reports no relevant financial relationships. Dr. Walters is a consultant for American Medical Systems and a lecturer for Boston Scientific.
1. Rodriguez LV, Raz S. Polypropylene sling for the treatment of stress urinary incontinence. Urology. 2001;58:783-785.
2. Ward K, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. Br Med J. 2002;325:65-70.
3. Paraiso MF, Walters MD, Karram MM, Barber MD. Laparoscopic Burch colposuspension versus tension-free vaginal tape: a randomized trial. Obstet Gynecol. 2004;104:1249-1258.
1. Rodriguez LV, Raz S. Polypropylene sling for the treatment of stress urinary incontinence. Urology. 2001;58:783-785.
2. Ward K, Hilton P. Prospective multicentre randomised trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. Br Med J. 2002;325:65-70.
3. Paraiso MF, Walters MD, Karram MM, Barber MD. Laparoscopic Burch colposuspension versus tension-free vaginal tape: a randomized trial. Obstet Gynecol. 2004;104:1249-1258.