User login
DISCHARGE INSTRUCTIONS WALLET INFO-CARD When to call your doctor
LISA’S CASE
Ablation fails to ease symptoms
Lisa is a 38-year-old mother of 2 who initially reported menometrorrhagia, dysmenorrhea, urinary frequency, pelvic pressure, and increasing abdominal girth. These symptoms worsened over 3 years before Lisa saw a physician and was diagnosed with uterine fibroids. When offered hysterectomy or endometrial ablation (cryomyolysis), she chose the latter. However, her fibroids failed to shrink, and her symptoms returned 5 months after the procedure.
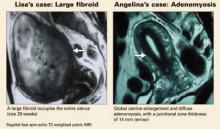
Lisa heard about uterine artery embolization (UAE) through the media and now asks about it. Her uterus is 20-week size with an irregular contour. Magnetic resonance imaging (MRI) shows a single large fibroid filling the uterus. Her hematocrit is 22.
Is uterine UAE right for her?
ANGELINA’S CASE
Menorrhagia and a “boggy” uterus
Angelina, 35, has 2 children and a 10-year history of menorrhagia, dysmenorrhea, and anemia. Pelvic examination reveals a 10-week size, “boggy” uterus, and MRI shows global enlargement of the uterus with a thickened junctional zone, which is characteristic of adenomyosis.
Her previous physician recommended hysterectomy after medical therapy failed, but Angelina is reluctant to undergo surgery.
Is UAE an option?
Both women are very likely to benefit from UAE, since the ideal candidate is premenopausal with symptomatic fibroids and/or adenomyosis and has either failed medical or surgical therapy or wants or needs to avoid surgery.1-11
This article describes the therapeutic role of UAE in women with abnormal uterine bleeding (AUB) due to uterine fibroids and/or adenomyosis—the most frequent myometrial causes of premenopausal AUB.
Other, less frequent myometrial disorders (eg, hypervascular pathologies such as intramyometrial and parametrial vascular malformations or neoplasms) can also be treated using embolization techniques. The main advantages of UAE in treating these diseases:1-8,12,13
- Less invasive than surgery, with substantially less recovery time and lower morbidity
- Usually performed under local anesthesia and intravenous conscious sedation
- No worry about adhesions
- Virtually no blood loss or need for transfusion; UAE may be especially attractive for patients who refuse or cannot receive blood products for health or religious reasons.
Impressive success rates
UAE has demonstrated excellent technical (98% to 100%) and high clinical success rates (80% to 95%) in the treatment of fibroids.1-8,13 The clinical success rate is lower for adenomyosis (56% to 92%), but UAE often provides sufficient clinical relief to obviate surgery.9,10,13
Although UAE was not initially recommended for women desiring future fertility—because of the 4% risk of premature menopause—the pendulum is now swinging in the other direction. If the risks of myomectomy are great due to the anatomic size or position of fibroids or adenomyosis, the risk-benefit ratio may shift to UAE to allow preservation of reproductive capacity.1-8,11
Though embolization has been performed since the early 1970s for acute and chronic bleeding associated with various medical conditions,14 the first report of UAE did not come until 1995.1 Since then, the procedure has seen rapid growth worldwide, with approximately 50,000 cases performed. About 14,000 cases were performed in the US last year.2
Although our practice has no fixed size limitation, ideally a uterus less than 20 weeks’ gestational size is preferred.
Contraindications
- Viable pregnancy
- Active pelvic infection
- Presence of an intrauterine device (though the IUD may be removed before the rocedure)
- Undiagnosed pelvic or adnexal mass
- Pelvic malignancies such as ovarian or endometrial carcinoma
- History of pelvic radiation, since UAE may cause ischemic necrosis of the uterus and adjacent organs due to preexisting radiation-induced vasculitis with diffuse vascular narrowing.
Relative contraindications
- Renal insufficiency, though we have used gadolinium, a nonnephrotoxic MRI contrast medium, for women with high blood creatinine levels
- History of severe allergic reaction to iodinated contrast medium, though gadolinium can also be used in these patients
- Coagulopathy
- Desire to preserve fertility, since it cannot be assured based on current data. However, uncomplicated pregnancies and normal deliveries have been reported after UAE, so this procedure may still be preferred for women who refuse or cannot undergo myomectomy.11
In some cases, extensive endometriosis is the cause of menorrhagia or dysmenorrhea, often coexisting with fibroids, and UAE may not be beneficial.11
Finally, a subserosal leiomyoma that is sufficiently pedunculated (attachment point 50% of the diameter) can be at risk for detachment from the uterus, a situation that may necessitate surgical intervention.11
Preop exam and imaging
At the physical examination, the fibroid uterus usually is enlarged with an irregular contour, and adenomyosis usually presents as a globally enlarged, “boggy” uterus (typically 6- to 10-weeks’ gestational size).
MRI is the preferred imaging
We prefer MRI since fibroids can be missed with ultrasound due to the limited field of view. MRI more accurately defines the size, location, and extent of disease. It also may better differentiate fibroids from adenomyosis.
MRI clearly depicts uterine zonal anatomy and enables accurate classification of individual masses by their locations: submucosal, intramural, or subserosal.
When adenomyosis is present, T2-weighted MRI demonstrates diffuse adenomyosis (about 66%) with global enlargement of the uterus and diffuse thickening of the junctional zone (at least 12 mm, highly predictive finding) with homogeneous low signal intensity. Focal adenomyosis (33%) can be seen as an illdefined, poorly marginated focal mass (adenomyoma) of low signal intensity within the myometrium.15,16
Transvaginal ultrasound
In women with fibroids, ultrasound usually demonstrates an enlarged uterus with lobulations, contour abnormality, or mass effects.
In women with adenomyosis, it usually demonstrates ill-defined, heterogeneous echotexture and small anechoic areas within the myometrium of asymmetrically enlarged uteri, with indistinct endometrial-myometrial borders and subendometrial halo thickening.15
Include endometrial biopsy
The patient should have a normal Pap test during the 12 months leading up to UAE,11 and should undergo endometrial biopsy to exclude carcinoma.
Laboratory tests should include a complete blood count, blood urea nitrogen/creatinine, follicle-stimulating hormone, human chorionic gonadotropin, and coagulation tests.
Technique
UAE begins with insertion of a small catheter (4-5 French) through a femoral artery in conjunction with percutaneous angiography. The catheter is guided into the uterine arteries—left first, then right— and contrast medium is injected into each artery to confirm the position of the catheter and the presence of fibroids or adenomyosis, which appear as hypervascular lesions in angiograms (see above, right).
UAE usually requires 1 to 2 hours.
Embolic agents
Polyvinyl alcohol (PVA) particles or trisacryl gelatin microspheres, usually 500 to 700 and/or 700 to 900 microns in size, are released through the catheter into the uterine arteries. These agents block the blood vessels that feed the fibroids and/or adenomyosis, causing them to shrink. The agents are biocompatible and have been approved by the US Food and Drug Administration.
Other, less frequently used embolic agents include gelatin sponge particles (which are temporary) and coils (which are permanent). Coils are generally used for conditions such as arteriovenous malformations or fistulae, which have large feeding vessels (iliac or enlarged uterine or ovarian vessels). This fluoroscopy-guided procedure usually is performed under local anesthesia and conscious sedation or, less often, epidural anesthesia.
Patient care
Conscious sedation, NSAIDs, and antibiotics
Intravenous conscious sedation in conjunction with nonsteroidal anti-inflammatory drugs (NSAIDs) usually provides sufficient pain relief.
In addition, intravenous broad-spectrum antibiotics are used as prophylaxis for infection linked to the embolization itself and to subsequent ischemia of the fibroids and uterus.
Managing postop pain syndrome
More than 90% of women experience postembolization syndrome, which includes moderate to severe abdominal pain/cramping and nausea and vomiting in the first several hours following the procedure. As a result, they may require hospitalization (less than 24 hours) for pain management. In our experience, few women stay in the hospital more than 1 day.
A patient-controlled analgesia pump and NSAIDs are used in women with abdominal/pelvic cramping and pain (more than 90% of cases) if epidural anesthesia is not used for pain.
Low-grade fever and leukocytosis are not uncommon after embolization, and are usually treated with acetaminophen. Other symptoms are anorexia and fatigue, but they gradually subside within 3 to 4 days.
After discharge
Oral NSAIDs and narcotics are often needed for several days. Many women resume light activities in a few days, and most return to normal activities within 1 week.11
Give her comprehensive discharge instructions on taking medications, what to expect, and when to contact a doctor. Follow-up visit in 1 to 4 weeks. We schedule an outpatient visit 1 to 4 weeks after the procedure. At this visit, we confirm healing of the puncture sites, screen for unusual symptoms or potential problems, and repeat follow-up instructions.11
We then follow the patient periodically (3, 6, and 12 months) to monitor her for symptoms and complications such as late infections, expulsion of infarcted fibroids, chronic endometritis, chronic vaginal discharge, and cessation or irregularity of menses, all of which have been observed after UAE.11
Transvaginal ultrasound is usually performed 3 to 6 months and 1 year after UAE to determine whether existing fibroids have been infarcted and begun to decrease in volume. It also reveals any uterine or adnexal complications.
In addition, this imaging provides a new baseline measurement of fibroid volume, against which any subsequent increase in size (which may indicate regrowth of fibroids or undiagnosed leiomyosarcoma) can be compared.11
Key findings of outcome studies
Two large series reported significant improvement in AUB in 77% to 90% of fibroid cases, and bulk-related symptoms were controlled in 86% to 91%.6-8 In these studies, average uterine volumes decreased by 35% and 58% at 3 and 12 months, respectively, with dominant fibroid shrinkage of 42%. Several large series also reported high patient satisfaction (91% to 93%) and significant improvement in quality-of-life measures.4,6-8
Side effects and complications
Although UAE is considered very safe, it carries some risks. Spies et al17 reported on complications in 400 consecutive patients undergoing UAE for fibroids at their institution:
- 1.25% serious complication rate
- 5% overall periprocedural morbidity rate
- no deaths and no major permanent injuries
In addition, 1 patient required hysterectomy as a result of a complication, and 1 patient had an undiagnosed leiomyosarcoma, which was discovered during an elective myomectomy 31 months after UAE.
Goldberg et al 18 reported another case with delayed diagnosis of leiomyosarcoma following UAE. In our series of 705 patients, 1 had an undiagnosed leiomyosarcoma, which presented as a pelvic mass 15 months after UAE. She subsequently underwent hysterectomy.
When to suspect leiomyosarcoma
Unlike hysterectomy or myomectomy, no tissue is obtained in UAE for pathologic diagnosis to exclude leiomyosarcoma, which is found in approximately 0.1% to 0.4% of women with fibroids and is difficult to differentiate from a benign leiomyoma using clinical tests or imaging.17-18
Suspect leiomyosarcoma if the fibroids continue to grow even after technically successful embolization.
Infection is rare, but can be lethal
A small number of patients have experienced infection, which usually is controlled with antibiotics. In a series of 414 UAE procedures in 410 fibroid patients, Rajan et al19 reported:
- 1.2% rate of intrauterine infection requiring intravenous antibiotic therapy and/or surgery
- no significant difference seen with various embolic agents, quantity of embolic particles, se of preprocedure antibiotics, or size or location of the dominant fibroid.
However, at least 2 deaths have been reported due to infection since UAE for fibroids was introduced in the mid-1990s: 1 fatal sepsis in a woman who underwent UAE for fibroids and 1 other sepsis fatality.17,20 The first case was caused by necrosis of the vaginal wall and uterine cervix. At autopsy, microspheres were found not only in arteries in the leiomyomata and myometrium, but also in the parametria and vagina, causing ischemic necrosis.
Amenorrhea or worsened AUB
In some cases, amenorrhea can follow UAE for fibroids due to ovarian embolization and subsequent ovarian failure.6-8,17
The literature indicates a rate of:
- 1% to 2% in patients less than 45 years of age
- 15% to 20% for perimenopausal women 45 and older
Worsening of uterine bleeding is rare after UAE, but can occur. Kerlan et al21 reported massive uterine bleeding 1 month after UAE in a woman who underwent the procedure for menorrhagia. When she was treated with emergent hysterectomy, a bleeding ulceration of the endometrium overlying the necrotic fibroid was found.
Other complications include spotting, hot flashes, fever, vaginal discharge, mood swings, pain at the puncture site, and dysuria.6-8,17
Our UAE experience
The New England Fibroid Center began offering fibroid embolization in 1997. Since then, we have performed 705 procedures at 5 hospitals in the Greater Boston region, with a technical success rate of 99%. Technical failure occurred in 1% of patients; these women had very difficult vascular anatomy involving uterine arteries, or ovarian arteries formed the dominant blood supply to the fibroids.
Clinical success or improvement was seen in 80% of women with bulk-related symptoms and 94.3% with bleeding symptoms.
Clinical failure occurred in 5.7% of women (1.6% required repeat UAE and 1.4% hysterectomies due to persistent symptoms).
Complications occurred in 4% of cases (2% rate of premature ovarian failure, 1.5% rate of transvaginal passage of infarcted fibroids, and 0.5% rate of groin hematoma). There were no major complications requiring transfusion or emergent surgeries such as hysterectomy.
Fertility after UAE
LISA’S CASE
“Cure” and pregnancy
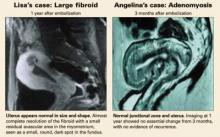
Lisa successfully underwent UAE, and had no symptoms after the procedure. The uterine fibroids resolved almost completely in 1 year.
Three years after the procedure, she became pregnant and delivered a healthy, full-term infant.
Although UAE is generally not performed in women who wish to preserve their fertility, it is sometimes used in fibroid patients when myomectomy is contraindicated because of the size and/or number of fibroids.11,22,23 Only a few small series and case reports describe successful pregnancies following UAE.
For example, in a study involving 400 women, McLucas et al22 reported 17 pregnancies in 14 women among 149 patients who stated a desire for fertility after UAE. Of these, 5 spontaneous abortions were observed, and 10 women had normal term deliveries. No perfusion or other problems were reported during pregnancy or labor.
Goldberg and colleagues23 analyzed 50 published cases of post-UAE pregnancies and found higher rates of cesarean delivery, preterm birth, malpresentation, small-for-gestational-age infants, spontaneous abortion, and postpartum hemorrhage than in the general population, though the reasons were unclear.
In our experience at the New England Fibroid Center, 5 of 12 patients below the age of 40 who wanted to preserve fertility became pregnant and successfully delivered full-term infants.
In general, the risks of infertility, premature ovarian failure/menopause, radiation exposure, and hysterectomy following UAE are small and compare favorably with those associated with myomectomy. Fertility rates are similar to those for women undergoing myomectomy.24
Nevertheless, well-controlled studies and additional data are needed before UAE can be confidently recommended as a first-line approach for preserving fertility.11
Treating adenomyosis
ANGELINA’S CASE
Adenomyosis resolves
During Angelina’s UAE procedure, angiographies showed enlarged right and left uterine arteries with numerous prominent intrauterine branches supplying the enlarged uterus. After UAE with PVA microspheres, post-embolization angiograms showed occlusion of the right and left uterine arteries and their branches.
Her symptoms resolved completely following the procedure. One year later, a follow-up MRI showed normal uterine size and shape, with complete resolution of adenomyosis.
Several small series have reported successful treatment of women with symptomatic adenomyosis. For example, of 23 women who underwent UAE for this indication, Chen and colleagues9 reported:
- Complete resolution of dysmenorrhea in 19 women and significant improvement in 2. Two other patients had recurrent symptoms.
- A substantial decrease in uterine volume in most of the women.
- An immediate decrease in intrauterine blood flow detected by color Doppler ultrasonography.
In a prospective study10 involving 18 women with symptomatic adenomyosis:
- 94% had diminished menorrhagia 6 months after UAE, and 94% had a slight decrease (mean: 15%) in uterine volume.
- After 1 year, 73% of women had diminished menorrhagia, and 53% had complete resolution.
- After 2 years, 56% of women had complete resolution of menorrhagia, 44% required additional treatment due to failure or recurrence, and 28% underwent hysterectomy.
In our limited experience with adenomyosis at the New England Fibroid Center, we saw no significant difference in technical success rates (100%) after UAE, compared with fibroid patients. However, there was a relatively high recurrence rate (2 of 6 patients) of presenting symptoms (menorrhagia or dysmenorrhea), and 2 patients later underwent hysterectomy.
Well-controlled studies are needed before UAE can confidently be recommended for symptomatic adenomyosis.
The authors report no financial relationships relevant to this article.
1. Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346:671-672.
2. Worthington-Kirsch RL, Siskin GP. Uterine artery embolization for symptomatic myomata. J Intensive Care Med. 2004;19:13-21.
3. Bradley EA, Reidy JF, Forman RG, et al. Transcatheter uterine artery embolisation to treat large uterine fibroids. Br J Obstet Gynaecol. 1998;105:235-240.
4. Worthington-Kirsch RL, Popky GL, Huchins FL, Jr. Uterine artery embolization for the management of leiomyomas: quality-of-life assessment and clinical response. Radiology. 1998;208:625-629.
5. Goodwin SC, Vedantham S, et al. Preliminary experience with uterine artery embolization for uterine fibroids. J Vasc Interv Radiol. 1997;8:517-526.
6. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow-up. BJOG. 2002;11:1262-1272.
7. Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
8. Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
9. Chen C, et al. Uterine arterial embolization in the treatment of adenomyosis. Zhonghua Fu Chan Ke Za Zhi. 2002;37:77-79.
10. Pelage JP, Jacob D, et al. Midterm results of uterine artery embolization for symptomatic adenomyosis: initial experience. Radiology. 2005;234:948-953.
11. Andrews RT, Spies JB, Sacks D, et al. Patient care and uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2004;15:115-120.
12. Broder MS, et al. Uterine Artery Embolization: A Systematic Review of the Literature and Proposal for Research. Santa Monica, Calif: Rand; 1999. Publication MR-1158.
13. Siskin GP, Tublin ME, Stainken BF, et al. Uterine artery embolization for the treatment of adenomyosis: clinical response and evaluation with MR imaging. AJR Am J Roentgenol. 2001;177:297-302.
14. Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1979;102:303-306.
15. Outwater EK, Siegelman ES, Van Deerlin V. Adenomyosis: current concepts and imaging considerations. AJR Am J Roentgenol. 1998;170:437-441.
16. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. 1999;19:S161-S170.
17. Spies JB, Spector A, Roth AR, et al. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
18. Goldberg J, Burd I, et al. Leiomyosarcoma in a premenopausal patient following uterine artery embolization. Am J Obstet Gynecol. 2004;191:1733-1735.
19. Rajan DK, Beecroft JR, Clark TW, et al. Risk of intrauterine infectious complications after uterine artery embolization. J Vasc Interv Radiol. 2004;15:1415-1421.
20. Vashisht A, Studd J, Carey A, Burn P. Fatal septicemia after fibroid embolisation [letter]. Lancet. 1999;354:307-308.
21. Kerlan K, Jr, Coffey JO, Milkman MS, et al. Massive vaginal hemorrhage after uterine fibroid embolization. J Vasc Interv Radiol. 2003;14:1465-1467.
22. McLucas B, Goodwin S, Adler L, Rappaport A, Reed R, Perrella R. Pregnancy following uterine fibroid embolization. Int J Gynaecol Obstet. 2001;74:1-7.
23. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872.
24. Goldberg J, Pereira L, Berghella V, et al. Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol. 2004;191:18-21.
DISCHARGE INSTRUCTIONS WALLET INFO-CARD When to call your doctor
LISA’S CASE
Ablation fails to ease symptoms
Lisa is a 38-year-old mother of 2 who initially reported menometrorrhagia, dysmenorrhea, urinary frequency, pelvic pressure, and increasing abdominal girth. These symptoms worsened over 3 years before Lisa saw a physician and was diagnosed with uterine fibroids. When offered hysterectomy or endometrial ablation (cryomyolysis), she chose the latter. However, her fibroids failed to shrink, and her symptoms returned 5 months after the procedure.
Lisa heard about uterine artery embolization (UAE) through the media and now asks about it. Her uterus is 20-week size with an irregular contour. Magnetic resonance imaging (MRI) shows a single large fibroid filling the uterus. Her hematocrit is 22.
Is uterine UAE right for her?
ANGELINA’S CASE
Menorrhagia and a “boggy” uterus
Angelina, 35, has 2 children and a 10-year history of menorrhagia, dysmenorrhea, and anemia. Pelvic examination reveals a 10-week size, “boggy” uterus, and MRI shows global enlargement of the uterus with a thickened junctional zone, which is characteristic of adenomyosis.
Her previous physician recommended hysterectomy after medical therapy failed, but Angelina is reluctant to undergo surgery.
Is UAE an option?
Both women are very likely to benefit from UAE, since the ideal candidate is premenopausal with symptomatic fibroids and/or adenomyosis and has either failed medical or surgical therapy or wants or needs to avoid surgery.1-11
This article describes the therapeutic role of UAE in women with abnormal uterine bleeding (AUB) due to uterine fibroids and/or adenomyosis—the most frequent myometrial causes of premenopausal AUB.
Other, less frequent myometrial disorders (eg, hypervascular pathologies such as intramyometrial and parametrial vascular malformations or neoplasms) can also be treated using embolization techniques. The main advantages of UAE in treating these diseases:1-8,12,13
- Less invasive than surgery, with substantially less recovery time and lower morbidity
- Usually performed under local anesthesia and intravenous conscious sedation
- No worry about adhesions
- Virtually no blood loss or need for transfusion; UAE may be especially attractive for patients who refuse or cannot receive blood products for health or religious reasons.
Impressive success rates
UAE has demonstrated excellent technical (98% to 100%) and high clinical success rates (80% to 95%) in the treatment of fibroids.1-8,13 The clinical success rate is lower for adenomyosis (56% to 92%), but UAE often provides sufficient clinical relief to obviate surgery.9,10,13
Although UAE was not initially recommended for women desiring future fertility—because of the 4% risk of premature menopause—the pendulum is now swinging in the other direction. If the risks of myomectomy are great due to the anatomic size or position of fibroids or adenomyosis, the risk-benefit ratio may shift to UAE to allow preservation of reproductive capacity.1-8,11
Though embolization has been performed since the early 1970s for acute and chronic bleeding associated with various medical conditions,14 the first report of UAE did not come until 1995.1 Since then, the procedure has seen rapid growth worldwide, with approximately 50,000 cases performed. About 14,000 cases were performed in the US last year.2
Although our practice has no fixed size limitation, ideally a uterus less than 20 weeks’ gestational size is preferred.
Contraindications
- Viable pregnancy
- Active pelvic infection
- Presence of an intrauterine device (though the IUD may be removed before the rocedure)
- Undiagnosed pelvic or adnexal mass
- Pelvic malignancies such as ovarian or endometrial carcinoma
- History of pelvic radiation, since UAE may cause ischemic necrosis of the uterus and adjacent organs due to preexisting radiation-induced vasculitis with diffuse vascular narrowing.
Relative contraindications
- Renal insufficiency, though we have used gadolinium, a nonnephrotoxic MRI contrast medium, for women with high blood creatinine levels
- History of severe allergic reaction to iodinated contrast medium, though gadolinium can also be used in these patients
- Coagulopathy
- Desire to preserve fertility, since it cannot be assured based on current data. However, uncomplicated pregnancies and normal deliveries have been reported after UAE, so this procedure may still be preferred for women who refuse or cannot undergo myomectomy.11
In some cases, extensive endometriosis is the cause of menorrhagia or dysmenorrhea, often coexisting with fibroids, and UAE may not be beneficial.11
Finally, a subserosal leiomyoma that is sufficiently pedunculated (attachment point 50% of the diameter) can be at risk for detachment from the uterus, a situation that may necessitate surgical intervention.11
Preop exam and imaging
At the physical examination, the fibroid uterus usually is enlarged with an irregular contour, and adenomyosis usually presents as a globally enlarged, “boggy” uterus (typically 6- to 10-weeks’ gestational size).
MRI is the preferred imaging
We prefer MRI since fibroids can be missed with ultrasound due to the limited field of view. MRI more accurately defines the size, location, and extent of disease. It also may better differentiate fibroids from adenomyosis.
MRI clearly depicts uterine zonal anatomy and enables accurate classification of individual masses by their locations: submucosal, intramural, or subserosal.
When adenomyosis is present, T2-weighted MRI demonstrates diffuse adenomyosis (about 66%) with global enlargement of the uterus and diffuse thickening of the junctional zone (at least 12 mm, highly predictive finding) with homogeneous low signal intensity. Focal adenomyosis (33%) can be seen as an illdefined, poorly marginated focal mass (adenomyoma) of low signal intensity within the myometrium.15,16
Transvaginal ultrasound
In women with fibroids, ultrasound usually demonstrates an enlarged uterus with lobulations, contour abnormality, or mass effects.
In women with adenomyosis, it usually demonstrates ill-defined, heterogeneous echotexture and small anechoic areas within the myometrium of asymmetrically enlarged uteri, with indistinct endometrial-myometrial borders and subendometrial halo thickening.15
Include endometrial biopsy
The patient should have a normal Pap test during the 12 months leading up to UAE,11 and should undergo endometrial biopsy to exclude carcinoma.
Laboratory tests should include a complete blood count, blood urea nitrogen/creatinine, follicle-stimulating hormone, human chorionic gonadotropin, and coagulation tests.
Technique
UAE begins with insertion of a small catheter (4-5 French) through a femoral artery in conjunction with percutaneous angiography. The catheter is guided into the uterine arteries—left first, then right— and contrast medium is injected into each artery to confirm the position of the catheter and the presence of fibroids or adenomyosis, which appear as hypervascular lesions in angiograms (see above, right).
UAE usually requires 1 to 2 hours.
Embolic agents
Polyvinyl alcohol (PVA) particles or trisacryl gelatin microspheres, usually 500 to 700 and/or 700 to 900 microns in size, are released through the catheter into the uterine arteries. These agents block the blood vessels that feed the fibroids and/or adenomyosis, causing them to shrink. The agents are biocompatible and have been approved by the US Food and Drug Administration.
Other, less frequently used embolic agents include gelatin sponge particles (which are temporary) and coils (which are permanent). Coils are generally used for conditions such as arteriovenous malformations or fistulae, which have large feeding vessels (iliac or enlarged uterine or ovarian vessels). This fluoroscopy-guided procedure usually is performed under local anesthesia and conscious sedation or, less often, epidural anesthesia.
Patient care
Conscious sedation, NSAIDs, and antibiotics
Intravenous conscious sedation in conjunction with nonsteroidal anti-inflammatory drugs (NSAIDs) usually provides sufficient pain relief.
In addition, intravenous broad-spectrum antibiotics are used as prophylaxis for infection linked to the embolization itself and to subsequent ischemia of the fibroids and uterus.
Managing postop pain syndrome
More than 90% of women experience postembolization syndrome, which includes moderate to severe abdominal pain/cramping and nausea and vomiting in the first several hours following the procedure. As a result, they may require hospitalization (less than 24 hours) for pain management. In our experience, few women stay in the hospital more than 1 day.
A patient-controlled analgesia pump and NSAIDs are used in women with abdominal/pelvic cramping and pain (more than 90% of cases) if epidural anesthesia is not used for pain.
Low-grade fever and leukocytosis are not uncommon after embolization, and are usually treated with acetaminophen. Other symptoms are anorexia and fatigue, but they gradually subside within 3 to 4 days.
After discharge
Oral NSAIDs and narcotics are often needed for several days. Many women resume light activities in a few days, and most return to normal activities within 1 week.11
Give her comprehensive discharge instructions on taking medications, what to expect, and when to contact a doctor. Follow-up visit in 1 to 4 weeks. We schedule an outpatient visit 1 to 4 weeks after the procedure. At this visit, we confirm healing of the puncture sites, screen for unusual symptoms or potential problems, and repeat follow-up instructions.11
We then follow the patient periodically (3, 6, and 12 months) to monitor her for symptoms and complications such as late infections, expulsion of infarcted fibroids, chronic endometritis, chronic vaginal discharge, and cessation or irregularity of menses, all of which have been observed after UAE.11
Transvaginal ultrasound is usually performed 3 to 6 months and 1 year after UAE to determine whether existing fibroids have been infarcted and begun to decrease in volume. It also reveals any uterine or adnexal complications.
In addition, this imaging provides a new baseline measurement of fibroid volume, against which any subsequent increase in size (which may indicate regrowth of fibroids or undiagnosed leiomyosarcoma) can be compared.11
Key findings of outcome studies
Two large series reported significant improvement in AUB in 77% to 90% of fibroid cases, and bulk-related symptoms were controlled in 86% to 91%.6-8 In these studies, average uterine volumes decreased by 35% and 58% at 3 and 12 months, respectively, with dominant fibroid shrinkage of 42%. Several large series also reported high patient satisfaction (91% to 93%) and significant improvement in quality-of-life measures.4,6-8
Side effects and complications
Although UAE is considered very safe, it carries some risks. Spies et al17 reported on complications in 400 consecutive patients undergoing UAE for fibroids at their institution:
- 1.25% serious complication rate
- 5% overall periprocedural morbidity rate
- no deaths and no major permanent injuries
In addition, 1 patient required hysterectomy as a result of a complication, and 1 patient had an undiagnosed leiomyosarcoma, which was discovered during an elective myomectomy 31 months after UAE.
Goldberg et al 18 reported another case with delayed diagnosis of leiomyosarcoma following UAE. In our series of 705 patients, 1 had an undiagnosed leiomyosarcoma, which presented as a pelvic mass 15 months after UAE. She subsequently underwent hysterectomy.
When to suspect leiomyosarcoma
Unlike hysterectomy or myomectomy, no tissue is obtained in UAE for pathologic diagnosis to exclude leiomyosarcoma, which is found in approximately 0.1% to 0.4% of women with fibroids and is difficult to differentiate from a benign leiomyoma using clinical tests or imaging.17-18
Suspect leiomyosarcoma if the fibroids continue to grow even after technically successful embolization.
Infection is rare, but can be lethal
A small number of patients have experienced infection, which usually is controlled with antibiotics. In a series of 414 UAE procedures in 410 fibroid patients, Rajan et al19 reported:
- 1.2% rate of intrauterine infection requiring intravenous antibiotic therapy and/or surgery
- no significant difference seen with various embolic agents, quantity of embolic particles, se of preprocedure antibiotics, or size or location of the dominant fibroid.
However, at least 2 deaths have been reported due to infection since UAE for fibroids was introduced in the mid-1990s: 1 fatal sepsis in a woman who underwent UAE for fibroids and 1 other sepsis fatality.17,20 The first case was caused by necrosis of the vaginal wall and uterine cervix. At autopsy, microspheres were found not only in arteries in the leiomyomata and myometrium, but also in the parametria and vagina, causing ischemic necrosis.
Amenorrhea or worsened AUB
In some cases, amenorrhea can follow UAE for fibroids due to ovarian embolization and subsequent ovarian failure.6-8,17
The literature indicates a rate of:
- 1% to 2% in patients less than 45 years of age
- 15% to 20% for perimenopausal women 45 and older
Worsening of uterine bleeding is rare after UAE, but can occur. Kerlan et al21 reported massive uterine bleeding 1 month after UAE in a woman who underwent the procedure for menorrhagia. When she was treated with emergent hysterectomy, a bleeding ulceration of the endometrium overlying the necrotic fibroid was found.
Other complications include spotting, hot flashes, fever, vaginal discharge, mood swings, pain at the puncture site, and dysuria.6-8,17
Our UAE experience
The New England Fibroid Center began offering fibroid embolization in 1997. Since then, we have performed 705 procedures at 5 hospitals in the Greater Boston region, with a technical success rate of 99%. Technical failure occurred in 1% of patients; these women had very difficult vascular anatomy involving uterine arteries, or ovarian arteries formed the dominant blood supply to the fibroids.
Clinical success or improvement was seen in 80% of women with bulk-related symptoms and 94.3% with bleeding symptoms.
Clinical failure occurred in 5.7% of women (1.6% required repeat UAE and 1.4% hysterectomies due to persistent symptoms).
Complications occurred in 4% of cases (2% rate of premature ovarian failure, 1.5% rate of transvaginal passage of infarcted fibroids, and 0.5% rate of groin hematoma). There were no major complications requiring transfusion or emergent surgeries such as hysterectomy.
Fertility after UAE
LISA’S CASE
“Cure” and pregnancy
Lisa successfully underwent UAE, and had no symptoms after the procedure. The uterine fibroids resolved almost completely in 1 year.
Three years after the procedure, she became pregnant and delivered a healthy, full-term infant.
Although UAE is generally not performed in women who wish to preserve their fertility, it is sometimes used in fibroid patients when myomectomy is contraindicated because of the size and/or number of fibroids.11,22,23 Only a few small series and case reports describe successful pregnancies following UAE.
For example, in a study involving 400 women, McLucas et al22 reported 17 pregnancies in 14 women among 149 patients who stated a desire for fertility after UAE. Of these, 5 spontaneous abortions were observed, and 10 women had normal term deliveries. No perfusion or other problems were reported during pregnancy or labor.
Goldberg and colleagues23 analyzed 50 published cases of post-UAE pregnancies and found higher rates of cesarean delivery, preterm birth, malpresentation, small-for-gestational-age infants, spontaneous abortion, and postpartum hemorrhage than in the general population, though the reasons were unclear.
In our experience at the New England Fibroid Center, 5 of 12 patients below the age of 40 who wanted to preserve fertility became pregnant and successfully delivered full-term infants.
In general, the risks of infertility, premature ovarian failure/menopause, radiation exposure, and hysterectomy following UAE are small and compare favorably with those associated with myomectomy. Fertility rates are similar to those for women undergoing myomectomy.24
Nevertheless, well-controlled studies and additional data are needed before UAE can be confidently recommended as a first-line approach for preserving fertility.11
Treating adenomyosis
ANGELINA’S CASE
Adenomyosis resolves
During Angelina’s UAE procedure, angiographies showed enlarged right and left uterine arteries with numerous prominent intrauterine branches supplying the enlarged uterus. After UAE with PVA microspheres, post-embolization angiograms showed occlusion of the right and left uterine arteries and their branches.
Her symptoms resolved completely following the procedure. One year later, a follow-up MRI showed normal uterine size and shape, with complete resolution of adenomyosis.
Several small series have reported successful treatment of women with symptomatic adenomyosis. For example, of 23 women who underwent UAE for this indication, Chen and colleagues9 reported:
- Complete resolution of dysmenorrhea in 19 women and significant improvement in 2. Two other patients had recurrent symptoms.
- A substantial decrease in uterine volume in most of the women.
- An immediate decrease in intrauterine blood flow detected by color Doppler ultrasonography.
In a prospective study10 involving 18 women with symptomatic adenomyosis:
- 94% had diminished menorrhagia 6 months after UAE, and 94% had a slight decrease (mean: 15%) in uterine volume.
- After 1 year, 73% of women had diminished menorrhagia, and 53% had complete resolution.
- After 2 years, 56% of women had complete resolution of menorrhagia, 44% required additional treatment due to failure or recurrence, and 28% underwent hysterectomy.
In our limited experience with adenomyosis at the New England Fibroid Center, we saw no significant difference in technical success rates (100%) after UAE, compared with fibroid patients. However, there was a relatively high recurrence rate (2 of 6 patients) of presenting symptoms (menorrhagia or dysmenorrhea), and 2 patients later underwent hysterectomy.
Well-controlled studies are needed before UAE can confidently be recommended for symptomatic adenomyosis.
The authors report no financial relationships relevant to this article.
DISCHARGE INSTRUCTIONS WALLET INFO-CARD When to call your doctor
LISA’S CASE
Ablation fails to ease symptoms
Lisa is a 38-year-old mother of 2 who initially reported menometrorrhagia, dysmenorrhea, urinary frequency, pelvic pressure, and increasing abdominal girth. These symptoms worsened over 3 years before Lisa saw a physician and was diagnosed with uterine fibroids. When offered hysterectomy or endometrial ablation (cryomyolysis), she chose the latter. However, her fibroids failed to shrink, and her symptoms returned 5 months after the procedure.
Lisa heard about uterine artery embolization (UAE) through the media and now asks about it. Her uterus is 20-week size with an irregular contour. Magnetic resonance imaging (MRI) shows a single large fibroid filling the uterus. Her hematocrit is 22.
Is uterine UAE right for her?
ANGELINA’S CASE
Menorrhagia and a “boggy” uterus
Angelina, 35, has 2 children and a 10-year history of menorrhagia, dysmenorrhea, and anemia. Pelvic examination reveals a 10-week size, “boggy” uterus, and MRI shows global enlargement of the uterus with a thickened junctional zone, which is characteristic of adenomyosis.
Her previous physician recommended hysterectomy after medical therapy failed, but Angelina is reluctant to undergo surgery.
Is UAE an option?
Both women are very likely to benefit from UAE, since the ideal candidate is premenopausal with symptomatic fibroids and/or adenomyosis and has either failed medical or surgical therapy or wants or needs to avoid surgery.1-11
This article describes the therapeutic role of UAE in women with abnormal uterine bleeding (AUB) due to uterine fibroids and/or adenomyosis—the most frequent myometrial causes of premenopausal AUB.
Other, less frequent myometrial disorders (eg, hypervascular pathologies such as intramyometrial and parametrial vascular malformations or neoplasms) can also be treated using embolization techniques. The main advantages of UAE in treating these diseases:1-8,12,13
- Less invasive than surgery, with substantially less recovery time and lower morbidity
- Usually performed under local anesthesia and intravenous conscious sedation
- No worry about adhesions
- Virtually no blood loss or need for transfusion; UAE may be especially attractive for patients who refuse or cannot receive blood products for health or religious reasons.
Impressive success rates
UAE has demonstrated excellent technical (98% to 100%) and high clinical success rates (80% to 95%) in the treatment of fibroids.1-8,13 The clinical success rate is lower for adenomyosis (56% to 92%), but UAE often provides sufficient clinical relief to obviate surgery.9,10,13
Although UAE was not initially recommended for women desiring future fertility—because of the 4% risk of premature menopause—the pendulum is now swinging in the other direction. If the risks of myomectomy are great due to the anatomic size or position of fibroids or adenomyosis, the risk-benefit ratio may shift to UAE to allow preservation of reproductive capacity.1-8,11
Though embolization has been performed since the early 1970s for acute and chronic bleeding associated with various medical conditions,14 the first report of UAE did not come until 1995.1 Since then, the procedure has seen rapid growth worldwide, with approximately 50,000 cases performed. About 14,000 cases were performed in the US last year.2
Although our practice has no fixed size limitation, ideally a uterus less than 20 weeks’ gestational size is preferred.
Contraindications
- Viable pregnancy
- Active pelvic infection
- Presence of an intrauterine device (though the IUD may be removed before the rocedure)
- Undiagnosed pelvic or adnexal mass
- Pelvic malignancies such as ovarian or endometrial carcinoma
- History of pelvic radiation, since UAE may cause ischemic necrosis of the uterus and adjacent organs due to preexisting radiation-induced vasculitis with diffuse vascular narrowing.
Relative contraindications
- Renal insufficiency, though we have used gadolinium, a nonnephrotoxic MRI contrast medium, for women with high blood creatinine levels
- History of severe allergic reaction to iodinated contrast medium, though gadolinium can also be used in these patients
- Coagulopathy
- Desire to preserve fertility, since it cannot be assured based on current data. However, uncomplicated pregnancies and normal deliveries have been reported after UAE, so this procedure may still be preferred for women who refuse or cannot undergo myomectomy.11
In some cases, extensive endometriosis is the cause of menorrhagia or dysmenorrhea, often coexisting with fibroids, and UAE may not be beneficial.11
Finally, a subserosal leiomyoma that is sufficiently pedunculated (attachment point 50% of the diameter) can be at risk for detachment from the uterus, a situation that may necessitate surgical intervention.11
Preop exam and imaging
At the physical examination, the fibroid uterus usually is enlarged with an irregular contour, and adenomyosis usually presents as a globally enlarged, “boggy” uterus (typically 6- to 10-weeks’ gestational size).
MRI is the preferred imaging
We prefer MRI since fibroids can be missed with ultrasound due to the limited field of view. MRI more accurately defines the size, location, and extent of disease. It also may better differentiate fibroids from adenomyosis.
MRI clearly depicts uterine zonal anatomy and enables accurate classification of individual masses by their locations: submucosal, intramural, or subserosal.
When adenomyosis is present, T2-weighted MRI demonstrates diffuse adenomyosis (about 66%) with global enlargement of the uterus and diffuse thickening of the junctional zone (at least 12 mm, highly predictive finding) with homogeneous low signal intensity. Focal adenomyosis (33%) can be seen as an illdefined, poorly marginated focal mass (adenomyoma) of low signal intensity within the myometrium.15,16
Transvaginal ultrasound
In women with fibroids, ultrasound usually demonstrates an enlarged uterus with lobulations, contour abnormality, or mass effects.
In women with adenomyosis, it usually demonstrates ill-defined, heterogeneous echotexture and small anechoic areas within the myometrium of asymmetrically enlarged uteri, with indistinct endometrial-myometrial borders and subendometrial halo thickening.15
Include endometrial biopsy
The patient should have a normal Pap test during the 12 months leading up to UAE,11 and should undergo endometrial biopsy to exclude carcinoma.
Laboratory tests should include a complete blood count, blood urea nitrogen/creatinine, follicle-stimulating hormone, human chorionic gonadotropin, and coagulation tests.
Technique
UAE begins with insertion of a small catheter (4-5 French) through a femoral artery in conjunction with percutaneous angiography. The catheter is guided into the uterine arteries—left first, then right— and contrast medium is injected into each artery to confirm the position of the catheter and the presence of fibroids or adenomyosis, which appear as hypervascular lesions in angiograms (see above, right).
UAE usually requires 1 to 2 hours.
Embolic agents
Polyvinyl alcohol (PVA) particles or trisacryl gelatin microspheres, usually 500 to 700 and/or 700 to 900 microns in size, are released through the catheter into the uterine arteries. These agents block the blood vessels that feed the fibroids and/or adenomyosis, causing them to shrink. The agents are biocompatible and have been approved by the US Food and Drug Administration.
Other, less frequently used embolic agents include gelatin sponge particles (which are temporary) and coils (which are permanent). Coils are generally used for conditions such as arteriovenous malformations or fistulae, which have large feeding vessels (iliac or enlarged uterine or ovarian vessels). This fluoroscopy-guided procedure usually is performed under local anesthesia and conscious sedation or, less often, epidural anesthesia.
Patient care
Conscious sedation, NSAIDs, and antibiotics
Intravenous conscious sedation in conjunction with nonsteroidal anti-inflammatory drugs (NSAIDs) usually provides sufficient pain relief.
In addition, intravenous broad-spectrum antibiotics are used as prophylaxis for infection linked to the embolization itself and to subsequent ischemia of the fibroids and uterus.
Managing postop pain syndrome
More than 90% of women experience postembolization syndrome, which includes moderate to severe abdominal pain/cramping and nausea and vomiting in the first several hours following the procedure. As a result, they may require hospitalization (less than 24 hours) for pain management. In our experience, few women stay in the hospital more than 1 day.
A patient-controlled analgesia pump and NSAIDs are used in women with abdominal/pelvic cramping and pain (more than 90% of cases) if epidural anesthesia is not used for pain.
Low-grade fever and leukocytosis are not uncommon after embolization, and are usually treated with acetaminophen. Other symptoms are anorexia and fatigue, but they gradually subside within 3 to 4 days.
After discharge
Oral NSAIDs and narcotics are often needed for several days. Many women resume light activities in a few days, and most return to normal activities within 1 week.11
Give her comprehensive discharge instructions on taking medications, what to expect, and when to contact a doctor. Follow-up visit in 1 to 4 weeks. We schedule an outpatient visit 1 to 4 weeks after the procedure. At this visit, we confirm healing of the puncture sites, screen for unusual symptoms or potential problems, and repeat follow-up instructions.11
We then follow the patient periodically (3, 6, and 12 months) to monitor her for symptoms and complications such as late infections, expulsion of infarcted fibroids, chronic endometritis, chronic vaginal discharge, and cessation or irregularity of menses, all of which have been observed after UAE.11
Transvaginal ultrasound is usually performed 3 to 6 months and 1 year after UAE to determine whether existing fibroids have been infarcted and begun to decrease in volume. It also reveals any uterine or adnexal complications.
In addition, this imaging provides a new baseline measurement of fibroid volume, against which any subsequent increase in size (which may indicate regrowth of fibroids or undiagnosed leiomyosarcoma) can be compared.11
Key findings of outcome studies
Two large series reported significant improvement in AUB in 77% to 90% of fibroid cases, and bulk-related symptoms were controlled in 86% to 91%.6-8 In these studies, average uterine volumes decreased by 35% and 58% at 3 and 12 months, respectively, with dominant fibroid shrinkage of 42%. Several large series also reported high patient satisfaction (91% to 93%) and significant improvement in quality-of-life measures.4,6-8
Side effects and complications
Although UAE is considered very safe, it carries some risks. Spies et al17 reported on complications in 400 consecutive patients undergoing UAE for fibroids at their institution:
- 1.25% serious complication rate
- 5% overall periprocedural morbidity rate
- no deaths and no major permanent injuries
In addition, 1 patient required hysterectomy as a result of a complication, and 1 patient had an undiagnosed leiomyosarcoma, which was discovered during an elective myomectomy 31 months after UAE.
Goldberg et al 18 reported another case with delayed diagnosis of leiomyosarcoma following UAE. In our series of 705 patients, 1 had an undiagnosed leiomyosarcoma, which presented as a pelvic mass 15 months after UAE. She subsequently underwent hysterectomy.
When to suspect leiomyosarcoma
Unlike hysterectomy or myomectomy, no tissue is obtained in UAE for pathologic diagnosis to exclude leiomyosarcoma, which is found in approximately 0.1% to 0.4% of women with fibroids and is difficult to differentiate from a benign leiomyoma using clinical tests or imaging.17-18
Suspect leiomyosarcoma if the fibroids continue to grow even after technically successful embolization.
Infection is rare, but can be lethal
A small number of patients have experienced infection, which usually is controlled with antibiotics. In a series of 414 UAE procedures in 410 fibroid patients, Rajan et al19 reported:
- 1.2% rate of intrauterine infection requiring intravenous antibiotic therapy and/or surgery
- no significant difference seen with various embolic agents, quantity of embolic particles, se of preprocedure antibiotics, or size or location of the dominant fibroid.
However, at least 2 deaths have been reported due to infection since UAE for fibroids was introduced in the mid-1990s: 1 fatal sepsis in a woman who underwent UAE for fibroids and 1 other sepsis fatality.17,20 The first case was caused by necrosis of the vaginal wall and uterine cervix. At autopsy, microspheres were found not only in arteries in the leiomyomata and myometrium, but also in the parametria and vagina, causing ischemic necrosis.
Amenorrhea or worsened AUB
In some cases, amenorrhea can follow UAE for fibroids due to ovarian embolization and subsequent ovarian failure.6-8,17
The literature indicates a rate of:
- 1% to 2% in patients less than 45 years of age
- 15% to 20% for perimenopausal women 45 and older
Worsening of uterine bleeding is rare after UAE, but can occur. Kerlan et al21 reported massive uterine bleeding 1 month after UAE in a woman who underwent the procedure for menorrhagia. When she was treated with emergent hysterectomy, a bleeding ulceration of the endometrium overlying the necrotic fibroid was found.
Other complications include spotting, hot flashes, fever, vaginal discharge, mood swings, pain at the puncture site, and dysuria.6-8,17
Our UAE experience
The New England Fibroid Center began offering fibroid embolization in 1997. Since then, we have performed 705 procedures at 5 hospitals in the Greater Boston region, with a technical success rate of 99%. Technical failure occurred in 1% of patients; these women had very difficult vascular anatomy involving uterine arteries, or ovarian arteries formed the dominant blood supply to the fibroids.
Clinical success or improvement was seen in 80% of women with bulk-related symptoms and 94.3% with bleeding symptoms.
Clinical failure occurred in 5.7% of women (1.6% required repeat UAE and 1.4% hysterectomies due to persistent symptoms).
Complications occurred in 4% of cases (2% rate of premature ovarian failure, 1.5% rate of transvaginal passage of infarcted fibroids, and 0.5% rate of groin hematoma). There were no major complications requiring transfusion or emergent surgeries such as hysterectomy.
Fertility after UAE
LISA’S CASE
“Cure” and pregnancy
Lisa successfully underwent UAE, and had no symptoms after the procedure. The uterine fibroids resolved almost completely in 1 year.
Three years after the procedure, she became pregnant and delivered a healthy, full-term infant.
Although UAE is generally not performed in women who wish to preserve their fertility, it is sometimes used in fibroid patients when myomectomy is contraindicated because of the size and/or number of fibroids.11,22,23 Only a few small series and case reports describe successful pregnancies following UAE.
For example, in a study involving 400 women, McLucas et al22 reported 17 pregnancies in 14 women among 149 patients who stated a desire for fertility after UAE. Of these, 5 spontaneous abortions were observed, and 10 women had normal term deliveries. No perfusion or other problems were reported during pregnancy or labor.
Goldberg and colleagues23 analyzed 50 published cases of post-UAE pregnancies and found higher rates of cesarean delivery, preterm birth, malpresentation, small-for-gestational-age infants, spontaneous abortion, and postpartum hemorrhage than in the general population, though the reasons were unclear.
In our experience at the New England Fibroid Center, 5 of 12 patients below the age of 40 who wanted to preserve fertility became pregnant and successfully delivered full-term infants.
In general, the risks of infertility, premature ovarian failure/menopause, radiation exposure, and hysterectomy following UAE are small and compare favorably with those associated with myomectomy. Fertility rates are similar to those for women undergoing myomectomy.24
Nevertheless, well-controlled studies and additional data are needed before UAE can be confidently recommended as a first-line approach for preserving fertility.11
Treating adenomyosis
ANGELINA’S CASE
Adenomyosis resolves
During Angelina’s UAE procedure, angiographies showed enlarged right and left uterine arteries with numerous prominent intrauterine branches supplying the enlarged uterus. After UAE with PVA microspheres, post-embolization angiograms showed occlusion of the right and left uterine arteries and their branches.
Her symptoms resolved completely following the procedure. One year later, a follow-up MRI showed normal uterine size and shape, with complete resolution of adenomyosis.
Several small series have reported successful treatment of women with symptomatic adenomyosis. For example, of 23 women who underwent UAE for this indication, Chen and colleagues9 reported:
- Complete resolution of dysmenorrhea in 19 women and significant improvement in 2. Two other patients had recurrent symptoms.
- A substantial decrease in uterine volume in most of the women.
- An immediate decrease in intrauterine blood flow detected by color Doppler ultrasonography.
In a prospective study10 involving 18 women with symptomatic adenomyosis:
- 94% had diminished menorrhagia 6 months after UAE, and 94% had a slight decrease (mean: 15%) in uterine volume.
- After 1 year, 73% of women had diminished menorrhagia, and 53% had complete resolution.
- After 2 years, 56% of women had complete resolution of menorrhagia, 44% required additional treatment due to failure or recurrence, and 28% underwent hysterectomy.
In our limited experience with adenomyosis at the New England Fibroid Center, we saw no significant difference in technical success rates (100%) after UAE, compared with fibroid patients. However, there was a relatively high recurrence rate (2 of 6 patients) of presenting symptoms (menorrhagia or dysmenorrhea), and 2 patients later underwent hysterectomy.
Well-controlled studies are needed before UAE can confidently be recommended for symptomatic adenomyosis.
The authors report no financial relationships relevant to this article.
1. Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346:671-672.
2. Worthington-Kirsch RL, Siskin GP. Uterine artery embolization for symptomatic myomata. J Intensive Care Med. 2004;19:13-21.
3. Bradley EA, Reidy JF, Forman RG, et al. Transcatheter uterine artery embolisation to treat large uterine fibroids. Br J Obstet Gynaecol. 1998;105:235-240.
4. Worthington-Kirsch RL, Popky GL, Huchins FL, Jr. Uterine artery embolization for the management of leiomyomas: quality-of-life assessment and clinical response. Radiology. 1998;208:625-629.
5. Goodwin SC, Vedantham S, et al. Preliminary experience with uterine artery embolization for uterine fibroids. J Vasc Interv Radiol. 1997;8:517-526.
6. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow-up. BJOG. 2002;11:1262-1272.
7. Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
8. Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
9. Chen C, et al. Uterine arterial embolization in the treatment of adenomyosis. Zhonghua Fu Chan Ke Za Zhi. 2002;37:77-79.
10. Pelage JP, Jacob D, et al. Midterm results of uterine artery embolization for symptomatic adenomyosis: initial experience. Radiology. 2005;234:948-953.
11. Andrews RT, Spies JB, Sacks D, et al. Patient care and uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2004;15:115-120.
12. Broder MS, et al. Uterine Artery Embolization: A Systematic Review of the Literature and Proposal for Research. Santa Monica, Calif: Rand; 1999. Publication MR-1158.
13. Siskin GP, Tublin ME, Stainken BF, et al. Uterine artery embolization for the treatment of adenomyosis: clinical response and evaluation with MR imaging. AJR Am J Roentgenol. 2001;177:297-302.
14. Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1979;102:303-306.
15. Outwater EK, Siegelman ES, Van Deerlin V. Adenomyosis: current concepts and imaging considerations. AJR Am J Roentgenol. 1998;170:437-441.
16. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. 1999;19:S161-S170.
17. Spies JB, Spector A, Roth AR, et al. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
18. Goldberg J, Burd I, et al. Leiomyosarcoma in a premenopausal patient following uterine artery embolization. Am J Obstet Gynecol. 2004;191:1733-1735.
19. Rajan DK, Beecroft JR, Clark TW, et al. Risk of intrauterine infectious complications after uterine artery embolization. J Vasc Interv Radiol. 2004;15:1415-1421.
20. Vashisht A, Studd J, Carey A, Burn P. Fatal septicemia after fibroid embolisation [letter]. Lancet. 1999;354:307-308.
21. Kerlan K, Jr, Coffey JO, Milkman MS, et al. Massive vaginal hemorrhage after uterine fibroid embolization. J Vasc Interv Radiol. 2003;14:1465-1467.
22. McLucas B, Goodwin S, Adler L, Rappaport A, Reed R, Perrella R. Pregnancy following uterine fibroid embolization. Int J Gynaecol Obstet. 2001;74:1-7.
23. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872.
24. Goldberg J, Pereira L, Berghella V, et al. Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol. 2004;191:18-21.
1. Ravina JH, Herbreteau D, Ciraru-Vigneron N, et al. Arterial embolisation to treat uterine myomata. Lancet. 1995;346:671-672.
2. Worthington-Kirsch RL, Siskin GP. Uterine artery embolization for symptomatic myomata. J Intensive Care Med. 2004;19:13-21.
3. Bradley EA, Reidy JF, Forman RG, et al. Transcatheter uterine artery embolisation to treat large uterine fibroids. Br J Obstet Gynaecol. 1998;105:235-240.
4. Worthington-Kirsch RL, Popky GL, Huchins FL, Jr. Uterine artery embolization for the management of leiomyomas: quality-of-life assessment and clinical response. Radiology. 1998;208:625-629.
5. Goodwin SC, Vedantham S, et al. Preliminary experience with uterine artery embolization for uterine fibroids. J Vasc Interv Radiol. 1997;8:517-526.
6. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow-up. BJOG. 2002;11:1262-1272.
7. Pron G, Bennett J, Common A, et al. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
8. Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
9. Chen C, et al. Uterine arterial embolization in the treatment of adenomyosis. Zhonghua Fu Chan Ke Za Zhi. 2002;37:77-79.
10. Pelage JP, Jacob D, et al. Midterm results of uterine artery embolization for symptomatic adenomyosis: initial experience. Radiology. 2005;234:948-953.
11. Andrews RT, Spies JB, Sacks D, et al. Patient care and uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2004;15:115-120.
12. Broder MS, et al. Uterine Artery Embolization: A Systematic Review of the Literature and Proposal for Research. Santa Monica, Calif: Rand; 1999. Publication MR-1158.
13. Siskin GP, Tublin ME, Stainken BF, et al. Uterine artery embolization for the treatment of adenomyosis: clinical response and evaluation with MR imaging. AJR Am J Roentgenol. 2001;177:297-302.
14. Rosch J, Dotter CT, Brown MJ. Selective arterial embolization. A new method for control of acute gastrointestinal bleeding. Radiology. 1979;102:303-306.
15. Outwater EK, Siegelman ES, Van Deerlin V. Adenomyosis: current concepts and imaging considerations. AJR Am J Roentgenol. 1998;170:437-441.
16. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: MR imaging findings. Radiographics. 1999;19:S161-S170.
17. Spies JB, Spector A, Roth AR, et al. Complications after uterine artery embolization for leiomyomas. Obstet Gynecol. 2002;100:873-880.
18. Goldberg J, Burd I, et al. Leiomyosarcoma in a premenopausal patient following uterine artery embolization. Am J Obstet Gynecol. 2004;191:1733-1735.
19. Rajan DK, Beecroft JR, Clark TW, et al. Risk of intrauterine infectious complications after uterine artery embolization. J Vasc Interv Radiol. 2004;15:1415-1421.
20. Vashisht A, Studd J, Carey A, Burn P. Fatal septicemia after fibroid embolisation [letter]. Lancet. 1999;354:307-308.
21. Kerlan K, Jr, Coffey JO, Milkman MS, et al. Massive vaginal hemorrhage after uterine fibroid embolization. J Vasc Interv Radiol. 2003;14:1465-1467.
22. McLucas B, Goodwin S, Adler L, Rappaport A, Reed R, Perrella R. Pregnancy following uterine fibroid embolization. Int J Gynaecol Obstet. 2001;74:1-7.
23. Goldberg J, Pereira L, Berghella V. Pregnancy after uterine artery embolization. Obstet Gynecol. 2002;100:869-872.
24. Goldberg J, Pereira L, Berghella V, et al. Pregnancy outcomes after treatment for fibromyomata: uterine artery embolization versus laparoscopic myomectomy. Am J Obstet Gynecol. 2004;191:18-21.