User login
Minimally invasive surgery in ovarian cancer
Maria’ case
she wants laparoscopy. yes or no?
Maria is a 57-year-old mother of 4 who presents to a gynecologic oncologist with pelvic pain and ultrasonographic evidence of a 7-cm complex mass at the right adnexa. She has an enlarged fibroid uterus (12-week size), a preoperative CA125 level of 21 U/mL, and she says she wants laparoscopic management.
Is minimally invasive surgery an acceptable choice?
This large, complex mass is possibly malignant. Until now, laparoscopy has played only a small role in the management of ovarian cancer, although it has greatly changed treatment of other gynecologic malignancies. Since women with ovarian cancer tend to be older and have coexisting diseases, laparoscopy could confer many benefits, provided surgical staging is comprehensive, and timely diagnosis and patient outcomes are not compromised.1
The utility of laparoscopy in ovarian borderline tumors and cancer is increasing. This article surveys current applications and concerns, including
- when to refer,
- predicting malignancy,
- effects of carbon dioxide (CO2) peritoneum,
- risk of port-site recurrences,
- hand-assisted laparoscopy,
- comprehensive staging, and
- assessing resectability.
4 applications
Conventional staging by laparotomy with a vertical incision from above the umbilicus to the symphysis pubis is still the gold standard; however, laparoscopy can be used in the management of selected cases of ovarian cancer:
- to manage and stage apparent early-stage ovarian cancer,
- to determine the extent of advanced disease and potential resectability,
- to resect disease via hand-assisted laparoscopy in selected women with advanced disease, and
- to obtain a “second look,” or reassess the patient for disease recurrence and placement of intraperitoneal catheters.
Benefits of laparoscopy for benign masses
The benefits of laparoscopy over laparotomy in the management of benign adnexal masses are well defined:2
- less postoperative morbidity,
- less postoperative pain,
- less analgesia required,
- shorter hospitalizations, and
- shorter recovery time.
When to refer. Referral of at-risk patients to a gynecologic oncologist should be based on personal and family history, physical, imaging, and tumor markers.
When to get a consult: ASAP. General gynecologists may encounter malignancy unexpectedly. When they do, it is of paramount importance to obtain gynecologic oncology consultation intraoperatively, if possible, or as soon as possible postoperatively.
Predicting Malignancy
How common is cancer in laparoscopically managed masses?
Consider a complex ovarian mass potentially malignant until proven otherwise. Why? Because it remains difficult to rule out malignancy preoperatively, even with strict patient selection.
For example, a study involving 292 laparoscopically managed women found a 3.8% malignancy rate.3 These women had undergone preoperative vaginal ultrasound, CA125 measurement, and pelvic examination, but malignancy was not detected until surgery.
The incidence of malignancy at laparoscopy for a pelvic mass varies widely due to different guidelines for patient selection. In 1 series of 757 patients,4 the rate of unanticipated malignancy was 2.5%. This included 7 invasive cancers and 12 borderline tumors. Preoperative evaluation entailed routine clinical and ultrasound examinations. At laparoscopy, peritoneal cytology was obtained, the ovaries and peritoneum were inspected, and any cysts were punctured so their contents could be examined. If a malignant mass was encountered or suspected, the woman in question was treated by immediate laparotomy using a vertical midline incision.4
History of nongynecologic cancer heightens risk of malignancy
For example, of 31 women with stage IV breast cancer and a new adnexal mass, 3 (10%) were found to have primary ovarian cancer, and 21 (68%) had metastatic breast cancer.5
In a study at our institution,6 51 of 264 patients (19%) with a history of nongynecologic cancer and a new adnexal mass were found to have a malignancy. Of these women, 22 (43%) had primary ovarian cancer; the rest had metastatic disease. Most patients had laparoscopy even when malignancy was encountered.
Utility of frozen section
Frozen-section analysis speeds diagnosis of the adnexal mass, allowing the necessary surgery to be performed immediately.The overall accuracy of frozen-section analysis is high, reported at 92.7% in 1 study.7 It is less accurate in borderline tumors because of the extensive sampling required.
Intraoperative frozen section has high accuracy in women with metastases to the adnexae. In 36 patients with a history of breast or colorectal carcinoma who developed adnexal metastases, intraoperative frozen section correctly diagnosed carcinoma in 35 patients (97%). In more than 80% of these women, the carcinoma was accurately diagnosed as metastatic.8
Laparoscopy for Suspicious Masses?
Is laparoscopy appropriate for pelvic masses that appear suspicious for cancer at the time of preoperative evaluation? And if malignancy is confirmed, is conversion to laparotomy warranted?
Advocates of laparoscopy as the initial diagnostic tool say yes to the first question, pointing to the fact that most suspicious masses are later found to be benign.9,10
For example, Dottino et al10 managed all pelvic masses referred to their oncology unit laparoscopically unless there was evidence of gross metastatic disease (ie, omental cake) or the mass extended above the umbilicus. Immediate frozen-section analysis was performed in all cases. Although most of the masses were suspicious for malignancy preoperatively, 87% were in fact benign, and 88% were successfully managed by laparoscopy. If conversion to laparotomy was necessary for successful debulking, it was performed. However, laparoscopic surgery often was adequate.
Canis and colleagues9 support diagnostic laparoscopy regardless of the ultrasonographic appearance of the pelvic mass, although they recommend immediate conversion to laparotomy for staging if malignancy is found.
Does CO2 Sspread Cancer?
Whether CO2 contributes to cancer spread and growth is of particular concern in ovarian cancer, since it is predominantly a peritoneal disease. In a rat ovarian cancer model, tumor dissemination increased throughout the peritoneal cavity with laparoscopy, compared with laparotomy, without increased tumor growth.11
However, a separate study12 in women with persistent metastatic intraabdominal peritoneal or ovarian cancer at the time of second-look surgery found no difference in overall survival between patients who had undergone laparoscopy versus laparotomy
Fear of Port-Site Recurrence
Fear of tumor implantation at the trocar site is commonly cited as a reason to avoid laparoscopy in ovarian cancer. One metaanalysis found a port-site recurrence rate of 1.1% to 13.5%, but many of the studies included were small series or case reports.13 In ovarian cancer, most reports of port-site recurrences have been associated with advanced-stage disease with peritoneal seeding and the presence of ascites.13,14
The term “port-site recurrence” (previously it was thought to be a metastasis) describes cancer occurring in the subcutis in the absence of carcinomatosis.15 Now that the definition has been refined, the rate of port-site recurrences may be substantially lower.
A large retrospective study at our institution found 4 (0.64%) subcutaneous tumor implantations at or near a trocar site after 625 laparoscopic procedures in 584 women with ovarian/tubal cancer. Most of these implantations were discovered after positive second-look operations, and all were associated with synchronous carcinomatosis or other sites of metastatic disease.16
In a separate study14 involving 102 women with primary or recurrent advanced-stage ovarian cancer, large-volume ascites and a longer interval between chemotherapy and cytoreductive surgery were associated with more port-site recurrences. In addition, full-layer closure of the abdominal wall reduced port-site recurrences from 58% to 2%, emphasizing the importance of trocar-site closure in cases of malignancy. There was no survival disadvantage in women with portsite recurrences.
What causes port-site recurrences?
Possible factors include:
- trauma to the site,
- frequent removal of instruments through the port,
- removing the specimen through the port, and
- continued leakage of ascites.13
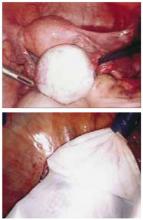
Avoiding cyst spillage and routinely using laparoscopic bags for cyst removal may decrease the incidence of these recurrences (FIGURE 1). Partial cyst excision and morcellation of a solid mass are always contraindicated.
Irrigation of port sites may decrease tumor cell implantation and should be considered at the end of the procedure.13 To further reduce risk, experts recommend closing all layers at the time of laparoscopy and resecting laparoscopic ports in their full thickness at the time of the staging laparotomy.14
FIGURE 1 Cyst removal using an endoscopic bag
Avoid spillage and routinely use laparoscopic bags for cyst removal to decrease the incidence of port-site recurrences.
Hand-Assisted Laparoscopy
This hybrid procedure combines the advantages of minimally invasive surgery with the tactile sensation of laparotomy. It has gained favor among urologists and general surgeons. (The first nephrectomy using this method was performed in 1996.17)
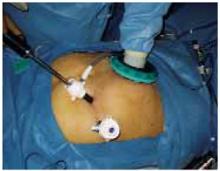
Technological advances now enable the surgeon to insert and remove the nondominant hand into the peritoneal cavity without losing pneumoperitoneum and to insert instruments through the same port if needed (FIGURE 2).
Advantages over traditional laparoscopy include the ability to palpate tissue, assist with tissue retraction, perform blunt dissection, and rapidly control hemostasis. This approach has been described in management and staging of early-stage ovarian cancer and in debulking advanced disease.18
FIGURE 2 Hand-assisted laparoscopy
The nondominant hand and surgical instruments can be inserted and removed through the special port without affecting pneumoperitoneum.
Surgical Staging
Maria’ case
resection and analysis of ovary
Maria underwent laparoscopy via the open technique. The surgeon found a cystic right ovarian mass, a fibroid uterus, and small diaphragmatic nodules, which were biopsied and found to be benign.
Pelvic washings were obtained, and after the right infundibular pelvic ligament and right utero-ovarian ligament were clamped and cut, the intact ovary was placed in a laparoscopic bag. The bag was pulled through the 12-mm suprapubic trocar, the cyst wall was perforated, and the cyst was drained within the laparoscopic bag, producing brown fluid. The bag was removed from the peritoneal cavity through this port, and the cyst was sent to pathology.
There was no contamination to the peritoneal cavity or abdominal wall, and the bag remained intact. Surgical gloves were then changed, and instruments used to drain the cyst were removed from the operating field.
When frozen-section analysis revealed a borderline serous ovarian tumor, Maria underwent BSO, infracolic omentectomy, laparoscopic pelvic and paraaortic lymphadenectomy, and laparoscopically assisted vaginal hysterectomy. There were no intraoperative complications, the total time in the operating room was 330 minutes, and there was blood loss of approximately 150 mL.
When an ovarian malignancy is discovered, immediate staging is indicated, and should include:
- peritoneal biopsies,
- pelvic and para-aortic lymph node sampling,
- infracolic omentectomy, and
- bilateral salpingo-oophorectomy (BSO) and hysterectomy.1
With presumed stage I disease, there is a 20% to 30% likelihood of upstaging after comprehensive surgical staging, with disease often discovered in the lymph nodes.19,20
Since changes in staging affect prognosis and treatment, complete staging should include the retroperitoneal nodes.
When the patient wants to preserve fertility
In selected younger women who have not yet completed childbearing, conservative treatment with retention of the uterus and contralateral ovary is an option—though we lack outcomes data on patients treated this way.
This option should be restricted to women with proven stage I disease after comprehensive staging.1
Can staging be done laparoscopically?
Complete staging—consisting of a detailed peritoneal assessment (with BSO and vaginal hysterectomy), omentectomy, and pelvic and para-aortic node dissection—can safely be done laparoscopically.19-21 Studies show low morbidity, with accurate findings and adequate node counts.21,22
A comparison of laparoscopic and conventional (laparotomy) staging in women with apparent stage I adnexal cancers found no differences in omental specimen size or the number of lymph nodes removed, and none of the patients required conversion to laparotomy.22
When definitive staging is delayed
Several studies have found poorer outcomes with delayed staging. However, the tumor ruptured in some of these studies, with considerable delay from the initial laparoscopy until definitive staging and treatment.
To increase the likelihood of an accurate stage, gather as much information as possible on the extent of disease: Describe the intraoperative findings and inspect the abdomen and pelvis thoroughly at initial surgery if a skilled oncologic surgeon is not immediately available. Then make every effort to schedule a complete staging procedure as soon as possible, as some consider this an “oncologic emergency.”9
Whether and when to stage LMP tumors
Preoperative prediction and intraoperative diagnosis of low malignant potential (LMP) tumors is challenging. If such a tumor is confirmed by frozen section, the usual treatment is unilateral salpingo-oophorectomy. When the patient is postmenopausal or has completed childbearing, BSO, hysterectomy, and staging should be considered.1
Surgical staging should be performed at the initial surgery, if at all possible. However, if final pathology confirms an LMP tumor and disease appears to be confined to the adnexa, repeat surgery for staging is controversial because of the limited data on its benefit, particularly in regard to mucinous borderline tumors.
Restaging may be more useful in selected cases of serous LMP tumors with histologic micropapillary features, since these tumors may be associated with a higher incidence of invasive implants (eg, in the omentum or peritoneum) that may require chemotherapy.
If a malignant cyst ruptures, does it affect staging?
The effect of intraoperative tumor spillage in stage I disease is debatable, although ascites and preoperative rupture are associated with a poorer prognosis.23
Even though a number of investigators (TABLE) have found intraoperative spillage to have no adverse impact on survival, make every effort to maintain capsular integrity to minimize any possibility of peritoneal tumor dissemination.
In some cases, intraoperative cyst rupture warrants upstaging from International Federation of Gynecology and Obstetrics (FIGO) stage IA to 1C, necessitating adjuvant chemotherapy when it otherwise would not have been required.1
Cyst rupture is no more likely with laparoscopy than with laparotomy,2 and is unrelated to the surgical route. It is more closely associated with the frequency of cystectomy.24
If rupture does occur, thoroughly irrigate the peritoneal cavity.
TABLE
When a cyst ruptures during surgery, what is the prognosis? The data are mixed on the significance of this event in stage I ovarian cancer
| AUTHOR | NUMBER OF CASES | IMPACT |
|---|---|---|
| Sevelda 1990 (Austria) | 204 | No prognostic importance |
| Sainz 1994 (US) | 79 | May worsen prognosis |
| Sjovall 1994 (Sweden) | 394 | No negative influence |
| Ahmed 1996 (UK) | 194 | Not prognostically significant |
| Vergote 2001 (Belgium) | 1,545 | Rupture should be avoided (hazard ratio = 1.64) |
How chemotherapy comes into play
If final pathology shows stage IC or high-grade histology, chemotherapy generally is offered to women managed in the United States. In selected cases, chemotherapy is given immediately after the initial surgery if completing a full staging procedure would considerably delay chemotherapy.
Leblanc et al21 found that, when staging was performed after completion of chemotherapy in women with stage IC or high-grade histology, 3 of 11 patients (27%) had positive nodes. Because positive nodes can be less chemosensitive, Leblanc and colleagues advocate either of 2 options: immediate restaging, including retroperitoneal nodes, or staging after chemotherapy, including retroperitoneal nodes.
Advanced Ovarian Cancer
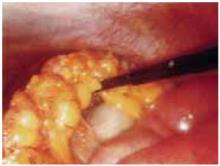
Optimal surgical cytoreduction by laparotomy, followed by platinum-based chemotherapy, maximizes survival in women with advanced ovarian cancer. Unfortunately, in many patients, optimal debulking is not feasible, and laparotomy without optimal cytoreduction offers no survival advantage.25 At the same time, preoperative imaging has limited ability to determine the feasibility of cytoreduction. For example, computed tomography is highly sensitive when it comes to detecting ascites and mesenteric and omental disease (FIGURE 3), but is not as successful in detecting gallbladder fossa disease and diffuse peritoneal nodules smaller than 2 cm.
As a result, laparoscopy is increasingly used to determine whether optimal resection is feasible. If it is, immediate laparotomy is appropriate. Otherwise, a tissue specimen is obtained for histological confirmation, allowing accurate diagnosis prior to chemotherapy.
FIGURE 3 Omental cake signifies metastasis
Omental cake in a stage IIIC ovarian cancer patient. Disease appears to be resectable.
Potential drawbacks of laparoscopy
In selected women with advanced cancer, laparoscopy may be a good way to determine which patients would not benefit from laparotomy, thus sparing them the morbidity of an additional operation. But laparoscopy can have limitations:
- Ascites can reduce visibility.
- Omental and bowel adhesion to the anterior abdominal wall may increase the likelihood of bowel injury.
- Trocar site implantation may increase in the presence of adenocarcinoma, ascites, and carcinomatosis.13
If trocar sites are carefully closed and chemotherapy is initiated promptly, these risks can be substantially reduced.14
Is laparoscopy acceptable for restaging?
Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624–629.
Yes, but only if the surgeon is highly skilled, with experience in both ovarian cancer and advanced laparoscopy. Comprehensive staging not only yields important prognostic information, but also identifies women who stand to benefit from chemotherapy.
The evidence: 10 years of experience
From 1991 to 2001, Leblanc et al21 laparoscopically restaged 53 women who had undergone incomplete staging for apparent stage I adnexal carcinoma.
Immediate (primary) restaging was done in 42 patients, and 11 were staged after completing chemotherapy (secondary restaging) for grade 3, clear-cell, or small-cell histology; FIGO stage IC cancer; or ruptured granulosa cell tumor.
Meticulous restaging technique:
- peritoneal washings and careful inspection,
- 8 to 10 random peritoneal biopsies (if peritoneal inspection was normal),
- BSO and hysterectomy (if not already done) or uterine curettage (if fertility was desired),
- bilateral pelvic and paraaortic lymphadenectomy,
- infracolic omentectomy.
The peritoneal cavity and trocar sites were irrigated at the end of the procedure, with full closure of any port sites larger than 10 mm.
Overall, laparoscopy was safe and successful
Complete laparoscopic restaging was performed in 52 women (98%). Dense adhesions indicated conversion to laparotomy in 1 case.
Four complications were directly related to the restaging procedure: a hematoma after epigastric vessel injury, 2 lymphocysts (managed laparoscopically), and 1 ureteric transection (which required laparotomy).The operation resulted in the following averages:
- operating time: 238 minutes,
- postoperative hospital stay: 3.1 days,
- node resection: 20 nodes in the paraaortic region and 14 in the pelvic dissection.
Mean follow-up was 54 months.
Outcomes
Of the 42 women who underwent primary restaging, 8 (19%) were upstaged—2 because of positive random peritoneal biopsies.
In the secondary restaging group, 4 of 11 women (36%) had their malignancies upstaged—3 because of positive retroperitoneal nodes and 1 because of positive random peritoneal biopsies. No port-site recurrences were observed in any of these patients.
One of the 8 patients upstaged in the primary restaging group had a recurrence 8 months postoperatively and died 16 months later. Of the 34 women with stage IA cancer after primary restaging, 3 (9%) had recurrences.
In the secondary-restaging group, 1 woman with small-cell carcinoma had a recurrence 10 months postoperatively and died 4 months later despite second-line chemotherapy.
Nine women had fertility-sparing surgery, and 3 later became pregnant and delivered without incident.
Second-Look Laparoscopy
Second-look surgery in women with a complete clinical response (normal exam, imaging, and CA125) after primary chemotherapy is controversial. This surgery aims to identify women with pathologically negative or microscopic disease who may benefit from consolidation therapy, or with larger-volume disease who can undergo secondary cytoreduction.27 Laparoscopy meets these goals safely with comparable accuracy and less morbidity than laparotomy.12,27
Maria’ case
lmp tumor and negative nodes
Maria did well postoperatively and went home on day 4. Her final pathology report: a right papillary serous adenocarcinoma of LMP (borderline) with small (<1 mm) foci of microinvasion. She had 6 negative paraaortic nodes, 19 negative pelvic nodes, negative pelvic washings and omentum, a normal left ovary, and a 6-cm cellular leiomyoma in an otherwise normal uterus.
She required no adjuvant treatment and is now 22 months postoperative without evidence of disease.
The authors report no financial relationships relevant to this article.
1. Rubin S. Ovarian Cancer. Philadelphia: Lippincott Williams & Wilkins; 2001.
2. Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109-114
3. Malik E, Bohm W, Stoz F, Nitsch CD, Rossmanith WG. Laparoscopic management of ovarian tumors. Surg Endosc. 1998;12:1326-1333
4. Canis M, Mage G, Pouly JL, Wattiez A, Manhes H, Bruhat MA. Laparoscopic diagnosis of adnexal cystic masses: a 12-year experience with long-term followup. Obstet Gynecol. 1994;83:707-712
5. Quan ML, Fey J, Eitan R, et al. Role of laparoscopy in the evaluation of the adnexa in patients with stage IV breast cancer. Gynecol Oncol. 2004;92:327-330
6. Juretska MM, Crawford CL, Lee C, et al. Laparoscopic management of adnexal masses in women with a history of nongynecologic malignancy. Abstract presented at the 2005 National Gynecologic Oncology Fellows’ Forum, Tucson, Arizona, January 27-30.
7. Rose PG, Rubin RB, Nelson BE, Hunter RE, Reale FR. Accuracy of frozen-section (intraoperative consultation) diagnosis of ovarian tumors. Am J Obstet Gynecol. 1994;171:823-826
8. Abu-Rustum NR, Chi DS, Wiatrowska BA, Guiter G, Saigo PE, Barakat RR. The accuracy of frozen-section diagnosis in metastatic breast and colorectal carcinoma to the adnexa. Gynecol Oncol. 1999;73:102-105
9. Canis M, Botchorishvili R, Kouyate S, et al. Surgical management of adnexal tumors. Ann Chir. 1998;52:234-248
10. Dottino PR, Levine DA, Ripley DL, Cohen CJ. Laparoscopic management of adnexal masses in premenopausal and postmenopausal women. Obstet Gynecol. 1999;93:223-228
11. Canis M, Botchorishvili R, Wattiez A, Mage G, Pouly JL, Bruhat MA. Tumor growth and dissemination after laparotomy and CO2 pneumoperitoneum: a rat ovarian cancer model. Obstet Gynecol. 1998;92:104-108
12. Abu-Rustum NR, Barakat RR, Siegel PL, Venkatraman E, Curtin JP, Hoskins WJ. Second-look operation for epithelial ovarian cancer: laparoscopy or laparotomy? Obstet Gynecol. 1996;88:549-553
13. Wang PH, Yuan CC, Lin G, Ng HT, Chao HT. Risk factors contributing to early occurrence of port site metastases of laparoscopic surgery for malignancy. Gynecol Oncol. 1999;72:38-44
14. Van Dam PA, DeCloedt J, Tjalma WAA, Buytaert P, Becquart D, Vergote IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. 1999;181:536-541
15. Reymond MA, Schneider C, Kastl S, Hohenberger W, Kockerling F. The pathogenesis of port-site recurrences. J Gastroint Surg. 1998;2:406-414
16. Abu-Rustum NR, Rhee EH, Chi DS, Sonoda Y, Gemignani M, Barakat RR. Subcutaneous tumor implantation after laparoscopic procedures in women with malignant disease [see comment]. Obstet Gynecol. 2004;103:480-487
17. Nakada SY, Moon TD, Gist M, Mahvi D. Use of the pneumo sleeve as an adjunct in laparoscopic nephrectomy. Urology. 1997;49:612-613
18. Krivak TC, Elkas JC, Rose GS, et al. The utility of hand-assisted laparoscopy in ovarian cancer. Gynecol Oncol. 2005;96:72-76
19. Faught W, Le T, Fung Kee Fung M, Krepart G, Lotocki R, Heywood M. Early ovarian cancer: what is the staging impact of retroperitoneal node sampling? J Obstet Gynaecol Can. 2003;25:18-21
20. Soper JT, Johnson P, Johnson V, Berchuck A, Clarke-Pearson DL. Comprehensive restaging laparotomy in women with apparent early ovarian carcinoma. Obstet Gynecol. 1992;80:949-953
21. Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624-629
22. Chi DS, Abu-Rustum NR, Sonoda Y, et al. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am J Obstet Gynecol [in press].
23. Sjovall K NB, Einhorn N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer. 1994;4:333-336
24. Fauvet R, Boccara J, Dufournet C, Poncelet C, Darai E. Laparoscopic management of borderline ovarian tumors: results of a French multicenter study. Ann Oncol. 2005;16:403-410
25. Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974-979
26. Ben David Y, Bustan M, Shalev E. Laparoscopy as part of the evaluation and management of ovarian and cervix neoplasms. Harefuah. 2001;140:464-467
27. Husain A, Chi DS, Prasad M, et al. The role of laparoscopy in second-look evaluations for ovarian cancer. Gynecol Oncol. 2001;80:44-47
Maria’ case
she wants laparoscopy. yes or no?
Maria is a 57-year-old mother of 4 who presents to a gynecologic oncologist with pelvic pain and ultrasonographic evidence of a 7-cm complex mass at the right adnexa. She has an enlarged fibroid uterus (12-week size), a preoperative CA125 level of 21 U/mL, and she says she wants laparoscopic management.
Is minimally invasive surgery an acceptable choice?
This large, complex mass is possibly malignant. Until now, laparoscopy has played only a small role in the management of ovarian cancer, although it has greatly changed treatment of other gynecologic malignancies. Since women with ovarian cancer tend to be older and have coexisting diseases, laparoscopy could confer many benefits, provided surgical staging is comprehensive, and timely diagnosis and patient outcomes are not compromised.1
The utility of laparoscopy in ovarian borderline tumors and cancer is increasing. This article surveys current applications and concerns, including
- when to refer,
- predicting malignancy,
- effects of carbon dioxide (CO2) peritoneum,
- risk of port-site recurrences,
- hand-assisted laparoscopy,
- comprehensive staging, and
- assessing resectability.
4 applications
Conventional staging by laparotomy with a vertical incision from above the umbilicus to the symphysis pubis is still the gold standard; however, laparoscopy can be used in the management of selected cases of ovarian cancer:
- to manage and stage apparent early-stage ovarian cancer,
- to determine the extent of advanced disease and potential resectability,
- to resect disease via hand-assisted laparoscopy in selected women with advanced disease, and
- to obtain a “second look,” or reassess the patient for disease recurrence and placement of intraperitoneal catheters.
Benefits of laparoscopy for benign masses
The benefits of laparoscopy over laparotomy in the management of benign adnexal masses are well defined:2
- less postoperative morbidity,
- less postoperative pain,
- less analgesia required,
- shorter hospitalizations, and
- shorter recovery time.
When to refer. Referral of at-risk patients to a gynecologic oncologist should be based on personal and family history, physical, imaging, and tumor markers.
When to get a consult: ASAP. General gynecologists may encounter malignancy unexpectedly. When they do, it is of paramount importance to obtain gynecologic oncology consultation intraoperatively, if possible, or as soon as possible postoperatively.
Predicting Malignancy
How common is cancer in laparoscopically managed masses?
Consider a complex ovarian mass potentially malignant until proven otherwise. Why? Because it remains difficult to rule out malignancy preoperatively, even with strict patient selection.
For example, a study involving 292 laparoscopically managed women found a 3.8% malignancy rate.3 These women had undergone preoperative vaginal ultrasound, CA125 measurement, and pelvic examination, but malignancy was not detected until surgery.
The incidence of malignancy at laparoscopy for a pelvic mass varies widely due to different guidelines for patient selection. In 1 series of 757 patients,4 the rate of unanticipated malignancy was 2.5%. This included 7 invasive cancers and 12 borderline tumors. Preoperative evaluation entailed routine clinical and ultrasound examinations. At laparoscopy, peritoneal cytology was obtained, the ovaries and peritoneum were inspected, and any cysts were punctured so their contents could be examined. If a malignant mass was encountered or suspected, the woman in question was treated by immediate laparotomy using a vertical midline incision.4
History of nongynecologic cancer heightens risk of malignancy
For example, of 31 women with stage IV breast cancer and a new adnexal mass, 3 (10%) were found to have primary ovarian cancer, and 21 (68%) had metastatic breast cancer.5
In a study at our institution,6 51 of 264 patients (19%) with a history of nongynecologic cancer and a new adnexal mass were found to have a malignancy. Of these women, 22 (43%) had primary ovarian cancer; the rest had metastatic disease. Most patients had laparoscopy even when malignancy was encountered.
Utility of frozen section
Frozen-section analysis speeds diagnosis of the adnexal mass, allowing the necessary surgery to be performed immediately.The overall accuracy of frozen-section analysis is high, reported at 92.7% in 1 study.7 It is less accurate in borderline tumors because of the extensive sampling required.
Intraoperative frozen section has high accuracy in women with metastases to the adnexae. In 36 patients with a history of breast or colorectal carcinoma who developed adnexal metastases, intraoperative frozen section correctly diagnosed carcinoma in 35 patients (97%). In more than 80% of these women, the carcinoma was accurately diagnosed as metastatic.8
Laparoscopy for Suspicious Masses?
Is laparoscopy appropriate for pelvic masses that appear suspicious for cancer at the time of preoperative evaluation? And if malignancy is confirmed, is conversion to laparotomy warranted?
Advocates of laparoscopy as the initial diagnostic tool say yes to the first question, pointing to the fact that most suspicious masses are later found to be benign.9,10
For example, Dottino et al10 managed all pelvic masses referred to their oncology unit laparoscopically unless there was evidence of gross metastatic disease (ie, omental cake) or the mass extended above the umbilicus. Immediate frozen-section analysis was performed in all cases. Although most of the masses were suspicious for malignancy preoperatively, 87% were in fact benign, and 88% were successfully managed by laparoscopy. If conversion to laparotomy was necessary for successful debulking, it was performed. However, laparoscopic surgery often was adequate.
Canis and colleagues9 support diagnostic laparoscopy regardless of the ultrasonographic appearance of the pelvic mass, although they recommend immediate conversion to laparotomy for staging if malignancy is found.
Does CO2 Sspread Cancer?
Whether CO2 contributes to cancer spread and growth is of particular concern in ovarian cancer, since it is predominantly a peritoneal disease. In a rat ovarian cancer model, tumor dissemination increased throughout the peritoneal cavity with laparoscopy, compared with laparotomy, without increased tumor growth.11
However, a separate study12 in women with persistent metastatic intraabdominal peritoneal or ovarian cancer at the time of second-look surgery found no difference in overall survival between patients who had undergone laparoscopy versus laparotomy
Fear of Port-Site Recurrence
Fear of tumor implantation at the trocar site is commonly cited as a reason to avoid laparoscopy in ovarian cancer. One metaanalysis found a port-site recurrence rate of 1.1% to 13.5%, but many of the studies included were small series or case reports.13 In ovarian cancer, most reports of port-site recurrences have been associated with advanced-stage disease with peritoneal seeding and the presence of ascites.13,14
The term “port-site recurrence” (previously it was thought to be a metastasis) describes cancer occurring in the subcutis in the absence of carcinomatosis.15 Now that the definition has been refined, the rate of port-site recurrences may be substantially lower.
A large retrospective study at our institution found 4 (0.64%) subcutaneous tumor implantations at or near a trocar site after 625 laparoscopic procedures in 584 women with ovarian/tubal cancer. Most of these implantations were discovered after positive second-look operations, and all were associated with synchronous carcinomatosis or other sites of metastatic disease.16
In a separate study14 involving 102 women with primary or recurrent advanced-stage ovarian cancer, large-volume ascites and a longer interval between chemotherapy and cytoreductive surgery were associated with more port-site recurrences. In addition, full-layer closure of the abdominal wall reduced port-site recurrences from 58% to 2%, emphasizing the importance of trocar-site closure in cases of malignancy. There was no survival disadvantage in women with portsite recurrences.
What causes port-site recurrences?
Possible factors include:
- trauma to the site,
- frequent removal of instruments through the port,
- removing the specimen through the port, and
- continued leakage of ascites.13
Avoiding cyst spillage and routinely using laparoscopic bags for cyst removal may decrease the incidence of these recurrences (FIGURE 1). Partial cyst excision and morcellation of a solid mass are always contraindicated.
Irrigation of port sites may decrease tumor cell implantation and should be considered at the end of the procedure.13 To further reduce risk, experts recommend closing all layers at the time of laparoscopy and resecting laparoscopic ports in their full thickness at the time of the staging laparotomy.14
FIGURE 1 Cyst removal using an endoscopic bag
Avoid spillage and routinely use laparoscopic bags for cyst removal to decrease the incidence of port-site recurrences.
Hand-Assisted Laparoscopy
This hybrid procedure combines the advantages of minimally invasive surgery with the tactile sensation of laparotomy. It has gained favor among urologists and general surgeons. (The first nephrectomy using this method was performed in 1996.17)
Technological advances now enable the surgeon to insert and remove the nondominant hand into the peritoneal cavity without losing pneumoperitoneum and to insert instruments through the same port if needed (FIGURE 2).
Advantages over traditional laparoscopy include the ability to palpate tissue, assist with tissue retraction, perform blunt dissection, and rapidly control hemostasis. This approach has been described in management and staging of early-stage ovarian cancer and in debulking advanced disease.18
FIGURE 2 Hand-assisted laparoscopy
The nondominant hand and surgical instruments can be inserted and removed through the special port without affecting pneumoperitoneum.
Surgical Staging
Maria’ case
resection and analysis of ovary
Maria underwent laparoscopy via the open technique. The surgeon found a cystic right ovarian mass, a fibroid uterus, and small diaphragmatic nodules, which were biopsied and found to be benign.
Pelvic washings were obtained, and after the right infundibular pelvic ligament and right utero-ovarian ligament were clamped and cut, the intact ovary was placed in a laparoscopic bag. The bag was pulled through the 12-mm suprapubic trocar, the cyst wall was perforated, and the cyst was drained within the laparoscopic bag, producing brown fluid. The bag was removed from the peritoneal cavity through this port, and the cyst was sent to pathology.
There was no contamination to the peritoneal cavity or abdominal wall, and the bag remained intact. Surgical gloves were then changed, and instruments used to drain the cyst were removed from the operating field.
When frozen-section analysis revealed a borderline serous ovarian tumor, Maria underwent BSO, infracolic omentectomy, laparoscopic pelvic and paraaortic lymphadenectomy, and laparoscopically assisted vaginal hysterectomy. There were no intraoperative complications, the total time in the operating room was 330 minutes, and there was blood loss of approximately 150 mL.
When an ovarian malignancy is discovered, immediate staging is indicated, and should include:
- peritoneal biopsies,
- pelvic and para-aortic lymph node sampling,
- infracolic omentectomy, and
- bilateral salpingo-oophorectomy (BSO) and hysterectomy.1
With presumed stage I disease, there is a 20% to 30% likelihood of upstaging after comprehensive surgical staging, with disease often discovered in the lymph nodes.19,20
Since changes in staging affect prognosis and treatment, complete staging should include the retroperitoneal nodes.
When the patient wants to preserve fertility
In selected younger women who have not yet completed childbearing, conservative treatment with retention of the uterus and contralateral ovary is an option—though we lack outcomes data on patients treated this way.
This option should be restricted to women with proven stage I disease after comprehensive staging.1
Can staging be done laparoscopically?
Complete staging—consisting of a detailed peritoneal assessment (with BSO and vaginal hysterectomy), omentectomy, and pelvic and para-aortic node dissection—can safely be done laparoscopically.19-21 Studies show low morbidity, with accurate findings and adequate node counts.21,22
A comparison of laparoscopic and conventional (laparotomy) staging in women with apparent stage I adnexal cancers found no differences in omental specimen size or the number of lymph nodes removed, and none of the patients required conversion to laparotomy.22
When definitive staging is delayed
Several studies have found poorer outcomes with delayed staging. However, the tumor ruptured in some of these studies, with considerable delay from the initial laparoscopy until definitive staging and treatment.
To increase the likelihood of an accurate stage, gather as much information as possible on the extent of disease: Describe the intraoperative findings and inspect the abdomen and pelvis thoroughly at initial surgery if a skilled oncologic surgeon is not immediately available. Then make every effort to schedule a complete staging procedure as soon as possible, as some consider this an “oncologic emergency.”9
Whether and when to stage LMP tumors
Preoperative prediction and intraoperative diagnosis of low malignant potential (LMP) tumors is challenging. If such a tumor is confirmed by frozen section, the usual treatment is unilateral salpingo-oophorectomy. When the patient is postmenopausal or has completed childbearing, BSO, hysterectomy, and staging should be considered.1
Surgical staging should be performed at the initial surgery, if at all possible. However, if final pathology confirms an LMP tumor and disease appears to be confined to the adnexa, repeat surgery for staging is controversial because of the limited data on its benefit, particularly in regard to mucinous borderline tumors.
Restaging may be more useful in selected cases of serous LMP tumors with histologic micropapillary features, since these tumors may be associated with a higher incidence of invasive implants (eg, in the omentum or peritoneum) that may require chemotherapy.
If a malignant cyst ruptures, does it affect staging?
The effect of intraoperative tumor spillage in stage I disease is debatable, although ascites and preoperative rupture are associated with a poorer prognosis.23
Even though a number of investigators (TABLE) have found intraoperative spillage to have no adverse impact on survival, make every effort to maintain capsular integrity to minimize any possibility of peritoneal tumor dissemination.
In some cases, intraoperative cyst rupture warrants upstaging from International Federation of Gynecology and Obstetrics (FIGO) stage IA to 1C, necessitating adjuvant chemotherapy when it otherwise would not have been required.1
Cyst rupture is no more likely with laparoscopy than with laparotomy,2 and is unrelated to the surgical route. It is more closely associated with the frequency of cystectomy.24
If rupture does occur, thoroughly irrigate the peritoneal cavity.
TABLE
When a cyst ruptures during surgery, what is the prognosis? The data are mixed on the significance of this event in stage I ovarian cancer
| AUTHOR | NUMBER OF CASES | IMPACT |
|---|---|---|
| Sevelda 1990 (Austria) | 204 | No prognostic importance |
| Sainz 1994 (US) | 79 | May worsen prognosis |
| Sjovall 1994 (Sweden) | 394 | No negative influence |
| Ahmed 1996 (UK) | 194 | Not prognostically significant |
| Vergote 2001 (Belgium) | 1,545 | Rupture should be avoided (hazard ratio = 1.64) |
How chemotherapy comes into play
If final pathology shows stage IC or high-grade histology, chemotherapy generally is offered to women managed in the United States. In selected cases, chemotherapy is given immediately after the initial surgery if completing a full staging procedure would considerably delay chemotherapy.
Leblanc et al21 found that, when staging was performed after completion of chemotherapy in women with stage IC or high-grade histology, 3 of 11 patients (27%) had positive nodes. Because positive nodes can be less chemosensitive, Leblanc and colleagues advocate either of 2 options: immediate restaging, including retroperitoneal nodes, or staging after chemotherapy, including retroperitoneal nodes.
Advanced Ovarian Cancer
Optimal surgical cytoreduction by laparotomy, followed by platinum-based chemotherapy, maximizes survival in women with advanced ovarian cancer. Unfortunately, in many patients, optimal debulking is not feasible, and laparotomy without optimal cytoreduction offers no survival advantage.25 At the same time, preoperative imaging has limited ability to determine the feasibility of cytoreduction. For example, computed tomography is highly sensitive when it comes to detecting ascites and mesenteric and omental disease (FIGURE 3), but is not as successful in detecting gallbladder fossa disease and diffuse peritoneal nodules smaller than 2 cm.
As a result, laparoscopy is increasingly used to determine whether optimal resection is feasible. If it is, immediate laparotomy is appropriate. Otherwise, a tissue specimen is obtained for histological confirmation, allowing accurate diagnosis prior to chemotherapy.
FIGURE 3 Omental cake signifies metastasis
Omental cake in a stage IIIC ovarian cancer patient. Disease appears to be resectable.
Potential drawbacks of laparoscopy
In selected women with advanced cancer, laparoscopy may be a good way to determine which patients would not benefit from laparotomy, thus sparing them the morbidity of an additional operation. But laparoscopy can have limitations:
- Ascites can reduce visibility.
- Omental and bowel adhesion to the anterior abdominal wall may increase the likelihood of bowel injury.
- Trocar site implantation may increase in the presence of adenocarcinoma, ascites, and carcinomatosis.13
If trocar sites are carefully closed and chemotherapy is initiated promptly, these risks can be substantially reduced.14
Is laparoscopy acceptable for restaging?
Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624–629.
Yes, but only if the surgeon is highly skilled, with experience in both ovarian cancer and advanced laparoscopy. Comprehensive staging not only yields important prognostic information, but also identifies women who stand to benefit from chemotherapy.
The evidence: 10 years of experience
From 1991 to 2001, Leblanc et al21 laparoscopically restaged 53 women who had undergone incomplete staging for apparent stage I adnexal carcinoma.
Immediate (primary) restaging was done in 42 patients, and 11 were staged after completing chemotherapy (secondary restaging) for grade 3, clear-cell, or small-cell histology; FIGO stage IC cancer; or ruptured granulosa cell tumor.
Meticulous restaging technique:
- peritoneal washings and careful inspection,
- 8 to 10 random peritoneal biopsies (if peritoneal inspection was normal),
- BSO and hysterectomy (if not already done) or uterine curettage (if fertility was desired),
- bilateral pelvic and paraaortic lymphadenectomy,
- infracolic omentectomy.
The peritoneal cavity and trocar sites were irrigated at the end of the procedure, with full closure of any port sites larger than 10 mm.
Overall, laparoscopy was safe and successful
Complete laparoscopic restaging was performed in 52 women (98%). Dense adhesions indicated conversion to laparotomy in 1 case.
Four complications were directly related to the restaging procedure: a hematoma after epigastric vessel injury, 2 lymphocysts (managed laparoscopically), and 1 ureteric transection (which required laparotomy).The operation resulted in the following averages:
- operating time: 238 minutes,
- postoperative hospital stay: 3.1 days,
- node resection: 20 nodes in the paraaortic region and 14 in the pelvic dissection.
Mean follow-up was 54 months.
Outcomes
Of the 42 women who underwent primary restaging, 8 (19%) were upstaged—2 because of positive random peritoneal biopsies.
In the secondary restaging group, 4 of 11 women (36%) had their malignancies upstaged—3 because of positive retroperitoneal nodes and 1 because of positive random peritoneal biopsies. No port-site recurrences were observed in any of these patients.
One of the 8 patients upstaged in the primary restaging group had a recurrence 8 months postoperatively and died 16 months later. Of the 34 women with stage IA cancer after primary restaging, 3 (9%) had recurrences.
In the secondary-restaging group, 1 woman with small-cell carcinoma had a recurrence 10 months postoperatively and died 4 months later despite second-line chemotherapy.
Nine women had fertility-sparing surgery, and 3 later became pregnant and delivered without incident.
Second-Look Laparoscopy
Second-look surgery in women with a complete clinical response (normal exam, imaging, and CA125) after primary chemotherapy is controversial. This surgery aims to identify women with pathologically negative or microscopic disease who may benefit from consolidation therapy, or with larger-volume disease who can undergo secondary cytoreduction.27 Laparoscopy meets these goals safely with comparable accuracy and less morbidity than laparotomy.12,27
Maria’ case
lmp tumor and negative nodes
Maria did well postoperatively and went home on day 4. Her final pathology report: a right papillary serous adenocarcinoma of LMP (borderline) with small (<1 mm) foci of microinvasion. She had 6 negative paraaortic nodes, 19 negative pelvic nodes, negative pelvic washings and omentum, a normal left ovary, and a 6-cm cellular leiomyoma in an otherwise normal uterus.
She required no adjuvant treatment and is now 22 months postoperative without evidence of disease.
The authors report no financial relationships relevant to this article.
Maria’ case
she wants laparoscopy. yes or no?
Maria is a 57-year-old mother of 4 who presents to a gynecologic oncologist with pelvic pain and ultrasonographic evidence of a 7-cm complex mass at the right adnexa. She has an enlarged fibroid uterus (12-week size), a preoperative CA125 level of 21 U/mL, and she says she wants laparoscopic management.
Is minimally invasive surgery an acceptable choice?
This large, complex mass is possibly malignant. Until now, laparoscopy has played only a small role in the management of ovarian cancer, although it has greatly changed treatment of other gynecologic malignancies. Since women with ovarian cancer tend to be older and have coexisting diseases, laparoscopy could confer many benefits, provided surgical staging is comprehensive, and timely diagnosis and patient outcomes are not compromised.1
The utility of laparoscopy in ovarian borderline tumors and cancer is increasing. This article surveys current applications and concerns, including
- when to refer,
- predicting malignancy,
- effects of carbon dioxide (CO2) peritoneum,
- risk of port-site recurrences,
- hand-assisted laparoscopy,
- comprehensive staging, and
- assessing resectability.
4 applications
Conventional staging by laparotomy with a vertical incision from above the umbilicus to the symphysis pubis is still the gold standard; however, laparoscopy can be used in the management of selected cases of ovarian cancer:
- to manage and stage apparent early-stage ovarian cancer,
- to determine the extent of advanced disease and potential resectability,
- to resect disease via hand-assisted laparoscopy in selected women with advanced disease, and
- to obtain a “second look,” or reassess the patient for disease recurrence and placement of intraperitoneal catheters.
Benefits of laparoscopy for benign masses
The benefits of laparoscopy over laparotomy in the management of benign adnexal masses are well defined:2
- less postoperative morbidity,
- less postoperative pain,
- less analgesia required,
- shorter hospitalizations, and
- shorter recovery time.
When to refer. Referral of at-risk patients to a gynecologic oncologist should be based on personal and family history, physical, imaging, and tumor markers.
When to get a consult: ASAP. General gynecologists may encounter malignancy unexpectedly. When they do, it is of paramount importance to obtain gynecologic oncology consultation intraoperatively, if possible, or as soon as possible postoperatively.
Predicting Malignancy
How common is cancer in laparoscopically managed masses?
Consider a complex ovarian mass potentially malignant until proven otherwise. Why? Because it remains difficult to rule out malignancy preoperatively, even with strict patient selection.
For example, a study involving 292 laparoscopically managed women found a 3.8% malignancy rate.3 These women had undergone preoperative vaginal ultrasound, CA125 measurement, and pelvic examination, but malignancy was not detected until surgery.
The incidence of malignancy at laparoscopy for a pelvic mass varies widely due to different guidelines for patient selection. In 1 series of 757 patients,4 the rate of unanticipated malignancy was 2.5%. This included 7 invasive cancers and 12 borderline tumors. Preoperative evaluation entailed routine clinical and ultrasound examinations. At laparoscopy, peritoneal cytology was obtained, the ovaries and peritoneum were inspected, and any cysts were punctured so their contents could be examined. If a malignant mass was encountered or suspected, the woman in question was treated by immediate laparotomy using a vertical midline incision.4
History of nongynecologic cancer heightens risk of malignancy
For example, of 31 women with stage IV breast cancer and a new adnexal mass, 3 (10%) were found to have primary ovarian cancer, and 21 (68%) had metastatic breast cancer.5
In a study at our institution,6 51 of 264 patients (19%) with a history of nongynecologic cancer and a new adnexal mass were found to have a malignancy. Of these women, 22 (43%) had primary ovarian cancer; the rest had metastatic disease. Most patients had laparoscopy even when malignancy was encountered.
Utility of frozen section
Frozen-section analysis speeds diagnosis of the adnexal mass, allowing the necessary surgery to be performed immediately.The overall accuracy of frozen-section analysis is high, reported at 92.7% in 1 study.7 It is less accurate in borderline tumors because of the extensive sampling required.
Intraoperative frozen section has high accuracy in women with metastases to the adnexae. In 36 patients with a history of breast or colorectal carcinoma who developed adnexal metastases, intraoperative frozen section correctly diagnosed carcinoma in 35 patients (97%). In more than 80% of these women, the carcinoma was accurately diagnosed as metastatic.8
Laparoscopy for Suspicious Masses?
Is laparoscopy appropriate for pelvic masses that appear suspicious for cancer at the time of preoperative evaluation? And if malignancy is confirmed, is conversion to laparotomy warranted?
Advocates of laparoscopy as the initial diagnostic tool say yes to the first question, pointing to the fact that most suspicious masses are later found to be benign.9,10
For example, Dottino et al10 managed all pelvic masses referred to their oncology unit laparoscopically unless there was evidence of gross metastatic disease (ie, omental cake) or the mass extended above the umbilicus. Immediate frozen-section analysis was performed in all cases. Although most of the masses were suspicious for malignancy preoperatively, 87% were in fact benign, and 88% were successfully managed by laparoscopy. If conversion to laparotomy was necessary for successful debulking, it was performed. However, laparoscopic surgery often was adequate.
Canis and colleagues9 support diagnostic laparoscopy regardless of the ultrasonographic appearance of the pelvic mass, although they recommend immediate conversion to laparotomy for staging if malignancy is found.
Does CO2 Sspread Cancer?
Whether CO2 contributes to cancer spread and growth is of particular concern in ovarian cancer, since it is predominantly a peritoneal disease. In a rat ovarian cancer model, tumor dissemination increased throughout the peritoneal cavity with laparoscopy, compared with laparotomy, without increased tumor growth.11
However, a separate study12 in women with persistent metastatic intraabdominal peritoneal or ovarian cancer at the time of second-look surgery found no difference in overall survival between patients who had undergone laparoscopy versus laparotomy
Fear of Port-Site Recurrence
Fear of tumor implantation at the trocar site is commonly cited as a reason to avoid laparoscopy in ovarian cancer. One metaanalysis found a port-site recurrence rate of 1.1% to 13.5%, but many of the studies included were small series or case reports.13 In ovarian cancer, most reports of port-site recurrences have been associated with advanced-stage disease with peritoneal seeding and the presence of ascites.13,14
The term “port-site recurrence” (previously it was thought to be a metastasis) describes cancer occurring in the subcutis in the absence of carcinomatosis.15 Now that the definition has been refined, the rate of port-site recurrences may be substantially lower.
A large retrospective study at our institution found 4 (0.64%) subcutaneous tumor implantations at or near a trocar site after 625 laparoscopic procedures in 584 women with ovarian/tubal cancer. Most of these implantations were discovered after positive second-look operations, and all were associated with synchronous carcinomatosis or other sites of metastatic disease.16
In a separate study14 involving 102 women with primary or recurrent advanced-stage ovarian cancer, large-volume ascites and a longer interval between chemotherapy and cytoreductive surgery were associated with more port-site recurrences. In addition, full-layer closure of the abdominal wall reduced port-site recurrences from 58% to 2%, emphasizing the importance of trocar-site closure in cases of malignancy. There was no survival disadvantage in women with portsite recurrences.
What causes port-site recurrences?
Possible factors include:
- trauma to the site,
- frequent removal of instruments through the port,
- removing the specimen through the port, and
- continued leakage of ascites.13
Avoiding cyst spillage and routinely using laparoscopic bags for cyst removal may decrease the incidence of these recurrences (FIGURE 1). Partial cyst excision and morcellation of a solid mass are always contraindicated.
Irrigation of port sites may decrease tumor cell implantation and should be considered at the end of the procedure.13 To further reduce risk, experts recommend closing all layers at the time of laparoscopy and resecting laparoscopic ports in their full thickness at the time of the staging laparotomy.14
FIGURE 1 Cyst removal using an endoscopic bag
Avoid spillage and routinely use laparoscopic bags for cyst removal to decrease the incidence of port-site recurrences.
Hand-Assisted Laparoscopy
This hybrid procedure combines the advantages of minimally invasive surgery with the tactile sensation of laparotomy. It has gained favor among urologists and general surgeons. (The first nephrectomy using this method was performed in 1996.17)
Technological advances now enable the surgeon to insert and remove the nondominant hand into the peritoneal cavity without losing pneumoperitoneum and to insert instruments through the same port if needed (FIGURE 2).
Advantages over traditional laparoscopy include the ability to palpate tissue, assist with tissue retraction, perform blunt dissection, and rapidly control hemostasis. This approach has been described in management and staging of early-stage ovarian cancer and in debulking advanced disease.18
FIGURE 2 Hand-assisted laparoscopy
The nondominant hand and surgical instruments can be inserted and removed through the special port without affecting pneumoperitoneum.
Surgical Staging
Maria’ case
resection and analysis of ovary
Maria underwent laparoscopy via the open technique. The surgeon found a cystic right ovarian mass, a fibroid uterus, and small diaphragmatic nodules, which were biopsied and found to be benign.
Pelvic washings were obtained, and after the right infundibular pelvic ligament and right utero-ovarian ligament were clamped and cut, the intact ovary was placed in a laparoscopic bag. The bag was pulled through the 12-mm suprapubic trocar, the cyst wall was perforated, and the cyst was drained within the laparoscopic bag, producing brown fluid. The bag was removed from the peritoneal cavity through this port, and the cyst was sent to pathology.
There was no contamination to the peritoneal cavity or abdominal wall, and the bag remained intact. Surgical gloves were then changed, and instruments used to drain the cyst were removed from the operating field.
When frozen-section analysis revealed a borderline serous ovarian tumor, Maria underwent BSO, infracolic omentectomy, laparoscopic pelvic and paraaortic lymphadenectomy, and laparoscopically assisted vaginal hysterectomy. There were no intraoperative complications, the total time in the operating room was 330 minutes, and there was blood loss of approximately 150 mL.
When an ovarian malignancy is discovered, immediate staging is indicated, and should include:
- peritoneal biopsies,
- pelvic and para-aortic lymph node sampling,
- infracolic omentectomy, and
- bilateral salpingo-oophorectomy (BSO) and hysterectomy.1
With presumed stage I disease, there is a 20% to 30% likelihood of upstaging after comprehensive surgical staging, with disease often discovered in the lymph nodes.19,20
Since changes in staging affect prognosis and treatment, complete staging should include the retroperitoneal nodes.
When the patient wants to preserve fertility
In selected younger women who have not yet completed childbearing, conservative treatment with retention of the uterus and contralateral ovary is an option—though we lack outcomes data on patients treated this way.
This option should be restricted to women with proven stage I disease after comprehensive staging.1
Can staging be done laparoscopically?
Complete staging—consisting of a detailed peritoneal assessment (with BSO and vaginal hysterectomy), omentectomy, and pelvic and para-aortic node dissection—can safely be done laparoscopically.19-21 Studies show low morbidity, with accurate findings and adequate node counts.21,22
A comparison of laparoscopic and conventional (laparotomy) staging in women with apparent stage I adnexal cancers found no differences in omental specimen size or the number of lymph nodes removed, and none of the patients required conversion to laparotomy.22
When definitive staging is delayed
Several studies have found poorer outcomes with delayed staging. However, the tumor ruptured in some of these studies, with considerable delay from the initial laparoscopy until definitive staging and treatment.
To increase the likelihood of an accurate stage, gather as much information as possible on the extent of disease: Describe the intraoperative findings and inspect the abdomen and pelvis thoroughly at initial surgery if a skilled oncologic surgeon is not immediately available. Then make every effort to schedule a complete staging procedure as soon as possible, as some consider this an “oncologic emergency.”9
Whether and when to stage LMP tumors
Preoperative prediction and intraoperative diagnosis of low malignant potential (LMP) tumors is challenging. If such a tumor is confirmed by frozen section, the usual treatment is unilateral salpingo-oophorectomy. When the patient is postmenopausal or has completed childbearing, BSO, hysterectomy, and staging should be considered.1
Surgical staging should be performed at the initial surgery, if at all possible. However, if final pathology confirms an LMP tumor and disease appears to be confined to the adnexa, repeat surgery for staging is controversial because of the limited data on its benefit, particularly in regard to mucinous borderline tumors.
Restaging may be more useful in selected cases of serous LMP tumors with histologic micropapillary features, since these tumors may be associated with a higher incidence of invasive implants (eg, in the omentum or peritoneum) that may require chemotherapy.
If a malignant cyst ruptures, does it affect staging?
The effect of intraoperative tumor spillage in stage I disease is debatable, although ascites and preoperative rupture are associated with a poorer prognosis.23
Even though a number of investigators (TABLE) have found intraoperative spillage to have no adverse impact on survival, make every effort to maintain capsular integrity to minimize any possibility of peritoneal tumor dissemination.
In some cases, intraoperative cyst rupture warrants upstaging from International Federation of Gynecology and Obstetrics (FIGO) stage IA to 1C, necessitating adjuvant chemotherapy when it otherwise would not have been required.1
Cyst rupture is no more likely with laparoscopy than with laparotomy,2 and is unrelated to the surgical route. It is more closely associated with the frequency of cystectomy.24
If rupture does occur, thoroughly irrigate the peritoneal cavity.
TABLE
When a cyst ruptures during surgery, what is the prognosis? The data are mixed on the significance of this event in stage I ovarian cancer
| AUTHOR | NUMBER OF CASES | IMPACT |
|---|---|---|
| Sevelda 1990 (Austria) | 204 | No prognostic importance |
| Sainz 1994 (US) | 79 | May worsen prognosis |
| Sjovall 1994 (Sweden) | 394 | No negative influence |
| Ahmed 1996 (UK) | 194 | Not prognostically significant |
| Vergote 2001 (Belgium) | 1,545 | Rupture should be avoided (hazard ratio = 1.64) |
How chemotherapy comes into play
If final pathology shows stage IC or high-grade histology, chemotherapy generally is offered to women managed in the United States. In selected cases, chemotherapy is given immediately after the initial surgery if completing a full staging procedure would considerably delay chemotherapy.
Leblanc et al21 found that, when staging was performed after completion of chemotherapy in women with stage IC or high-grade histology, 3 of 11 patients (27%) had positive nodes. Because positive nodes can be less chemosensitive, Leblanc and colleagues advocate either of 2 options: immediate restaging, including retroperitoneal nodes, or staging after chemotherapy, including retroperitoneal nodes.
Advanced Ovarian Cancer
Optimal surgical cytoreduction by laparotomy, followed by platinum-based chemotherapy, maximizes survival in women with advanced ovarian cancer. Unfortunately, in many patients, optimal debulking is not feasible, and laparotomy without optimal cytoreduction offers no survival advantage.25 At the same time, preoperative imaging has limited ability to determine the feasibility of cytoreduction. For example, computed tomography is highly sensitive when it comes to detecting ascites and mesenteric and omental disease (FIGURE 3), but is not as successful in detecting gallbladder fossa disease and diffuse peritoneal nodules smaller than 2 cm.
As a result, laparoscopy is increasingly used to determine whether optimal resection is feasible. If it is, immediate laparotomy is appropriate. Otherwise, a tissue specimen is obtained for histological confirmation, allowing accurate diagnosis prior to chemotherapy.
FIGURE 3 Omental cake signifies metastasis
Omental cake in a stage IIIC ovarian cancer patient. Disease appears to be resectable.
Potential drawbacks of laparoscopy
In selected women with advanced cancer, laparoscopy may be a good way to determine which patients would not benefit from laparotomy, thus sparing them the morbidity of an additional operation. But laparoscopy can have limitations:
- Ascites can reduce visibility.
- Omental and bowel adhesion to the anterior abdominal wall may increase the likelihood of bowel injury.
- Trocar site implantation may increase in the presence of adenocarcinoma, ascites, and carcinomatosis.13
If trocar sites are carefully closed and chemotherapy is initiated promptly, these risks can be substantially reduced.14
Is laparoscopy acceptable for restaging?
Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624–629.
Yes, but only if the surgeon is highly skilled, with experience in both ovarian cancer and advanced laparoscopy. Comprehensive staging not only yields important prognostic information, but also identifies women who stand to benefit from chemotherapy.
The evidence: 10 years of experience
From 1991 to 2001, Leblanc et al21 laparoscopically restaged 53 women who had undergone incomplete staging for apparent stage I adnexal carcinoma.
Immediate (primary) restaging was done in 42 patients, and 11 were staged after completing chemotherapy (secondary restaging) for grade 3, clear-cell, or small-cell histology; FIGO stage IC cancer; or ruptured granulosa cell tumor.
Meticulous restaging technique:
- peritoneal washings and careful inspection,
- 8 to 10 random peritoneal biopsies (if peritoneal inspection was normal),
- BSO and hysterectomy (if not already done) or uterine curettage (if fertility was desired),
- bilateral pelvic and paraaortic lymphadenectomy,
- infracolic omentectomy.
The peritoneal cavity and trocar sites were irrigated at the end of the procedure, with full closure of any port sites larger than 10 mm.
Overall, laparoscopy was safe and successful
Complete laparoscopic restaging was performed in 52 women (98%). Dense adhesions indicated conversion to laparotomy in 1 case.
Four complications were directly related to the restaging procedure: a hematoma after epigastric vessel injury, 2 lymphocysts (managed laparoscopically), and 1 ureteric transection (which required laparotomy).The operation resulted in the following averages:
- operating time: 238 minutes,
- postoperative hospital stay: 3.1 days,
- node resection: 20 nodes in the paraaortic region and 14 in the pelvic dissection.
Mean follow-up was 54 months.
Outcomes
Of the 42 women who underwent primary restaging, 8 (19%) were upstaged—2 because of positive random peritoneal biopsies.
In the secondary restaging group, 4 of 11 women (36%) had their malignancies upstaged—3 because of positive retroperitoneal nodes and 1 because of positive random peritoneal biopsies. No port-site recurrences were observed in any of these patients.
One of the 8 patients upstaged in the primary restaging group had a recurrence 8 months postoperatively and died 16 months later. Of the 34 women with stage IA cancer after primary restaging, 3 (9%) had recurrences.
In the secondary-restaging group, 1 woman with small-cell carcinoma had a recurrence 10 months postoperatively and died 4 months later despite second-line chemotherapy.
Nine women had fertility-sparing surgery, and 3 later became pregnant and delivered without incident.
Second-Look Laparoscopy
Second-look surgery in women with a complete clinical response (normal exam, imaging, and CA125) after primary chemotherapy is controversial. This surgery aims to identify women with pathologically negative or microscopic disease who may benefit from consolidation therapy, or with larger-volume disease who can undergo secondary cytoreduction.27 Laparoscopy meets these goals safely with comparable accuracy and less morbidity than laparotomy.12,27
Maria’ case
lmp tumor and negative nodes
Maria did well postoperatively and went home on day 4. Her final pathology report: a right papillary serous adenocarcinoma of LMP (borderline) with small (<1 mm) foci of microinvasion. She had 6 negative paraaortic nodes, 19 negative pelvic nodes, negative pelvic washings and omentum, a normal left ovary, and a 6-cm cellular leiomyoma in an otherwise normal uterus.
She required no adjuvant treatment and is now 22 months postoperative without evidence of disease.
The authors report no financial relationships relevant to this article.
1. Rubin S. Ovarian Cancer. Philadelphia: Lippincott Williams & Wilkins; 2001.
2. Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109-114
3. Malik E, Bohm W, Stoz F, Nitsch CD, Rossmanith WG. Laparoscopic management of ovarian tumors. Surg Endosc. 1998;12:1326-1333
4. Canis M, Mage G, Pouly JL, Wattiez A, Manhes H, Bruhat MA. Laparoscopic diagnosis of adnexal cystic masses: a 12-year experience with long-term followup. Obstet Gynecol. 1994;83:707-712
5. Quan ML, Fey J, Eitan R, et al. Role of laparoscopy in the evaluation of the adnexa in patients with stage IV breast cancer. Gynecol Oncol. 2004;92:327-330
6. Juretska MM, Crawford CL, Lee C, et al. Laparoscopic management of adnexal masses in women with a history of nongynecologic malignancy. Abstract presented at the 2005 National Gynecologic Oncology Fellows’ Forum, Tucson, Arizona, January 27-30.
7. Rose PG, Rubin RB, Nelson BE, Hunter RE, Reale FR. Accuracy of frozen-section (intraoperative consultation) diagnosis of ovarian tumors. Am J Obstet Gynecol. 1994;171:823-826
8. Abu-Rustum NR, Chi DS, Wiatrowska BA, Guiter G, Saigo PE, Barakat RR. The accuracy of frozen-section diagnosis in metastatic breast and colorectal carcinoma to the adnexa. Gynecol Oncol. 1999;73:102-105
9. Canis M, Botchorishvili R, Kouyate S, et al. Surgical management of adnexal tumors. Ann Chir. 1998;52:234-248
10. Dottino PR, Levine DA, Ripley DL, Cohen CJ. Laparoscopic management of adnexal masses in premenopausal and postmenopausal women. Obstet Gynecol. 1999;93:223-228
11. Canis M, Botchorishvili R, Wattiez A, Mage G, Pouly JL, Bruhat MA. Tumor growth and dissemination after laparotomy and CO2 pneumoperitoneum: a rat ovarian cancer model. Obstet Gynecol. 1998;92:104-108
12. Abu-Rustum NR, Barakat RR, Siegel PL, Venkatraman E, Curtin JP, Hoskins WJ. Second-look operation for epithelial ovarian cancer: laparoscopy or laparotomy? Obstet Gynecol. 1996;88:549-553
13. Wang PH, Yuan CC, Lin G, Ng HT, Chao HT. Risk factors contributing to early occurrence of port site metastases of laparoscopic surgery for malignancy. Gynecol Oncol. 1999;72:38-44
14. Van Dam PA, DeCloedt J, Tjalma WAA, Buytaert P, Becquart D, Vergote IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. 1999;181:536-541
15. Reymond MA, Schneider C, Kastl S, Hohenberger W, Kockerling F. The pathogenesis of port-site recurrences. J Gastroint Surg. 1998;2:406-414
16. Abu-Rustum NR, Rhee EH, Chi DS, Sonoda Y, Gemignani M, Barakat RR. Subcutaneous tumor implantation after laparoscopic procedures in women with malignant disease [see comment]. Obstet Gynecol. 2004;103:480-487
17. Nakada SY, Moon TD, Gist M, Mahvi D. Use of the pneumo sleeve as an adjunct in laparoscopic nephrectomy. Urology. 1997;49:612-613
18. Krivak TC, Elkas JC, Rose GS, et al. The utility of hand-assisted laparoscopy in ovarian cancer. Gynecol Oncol. 2005;96:72-76
19. Faught W, Le T, Fung Kee Fung M, Krepart G, Lotocki R, Heywood M. Early ovarian cancer: what is the staging impact of retroperitoneal node sampling? J Obstet Gynaecol Can. 2003;25:18-21
20. Soper JT, Johnson P, Johnson V, Berchuck A, Clarke-Pearson DL. Comprehensive restaging laparotomy in women with apparent early ovarian carcinoma. Obstet Gynecol. 1992;80:949-953
21. Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624-629
22. Chi DS, Abu-Rustum NR, Sonoda Y, et al. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am J Obstet Gynecol [in press].
23. Sjovall K NB, Einhorn N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer. 1994;4:333-336
24. Fauvet R, Boccara J, Dufournet C, Poncelet C, Darai E. Laparoscopic management of borderline ovarian tumors: results of a French multicenter study. Ann Oncol. 2005;16:403-410
25. Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974-979
26. Ben David Y, Bustan M, Shalev E. Laparoscopy as part of the evaluation and management of ovarian and cervix neoplasms. Harefuah. 2001;140:464-467
27. Husain A, Chi DS, Prasad M, et al. The role of laparoscopy in second-look evaluations for ovarian cancer. Gynecol Oncol. 2001;80:44-47
1. Rubin S. Ovarian Cancer. Philadelphia: Lippincott Williams & Wilkins; 2001.
2. Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109-114
3. Malik E, Bohm W, Stoz F, Nitsch CD, Rossmanith WG. Laparoscopic management of ovarian tumors. Surg Endosc. 1998;12:1326-1333
4. Canis M, Mage G, Pouly JL, Wattiez A, Manhes H, Bruhat MA. Laparoscopic diagnosis of adnexal cystic masses: a 12-year experience with long-term followup. Obstet Gynecol. 1994;83:707-712
5. Quan ML, Fey J, Eitan R, et al. Role of laparoscopy in the evaluation of the adnexa in patients with stage IV breast cancer. Gynecol Oncol. 2004;92:327-330
6. Juretska MM, Crawford CL, Lee C, et al. Laparoscopic management of adnexal masses in women with a history of nongynecologic malignancy. Abstract presented at the 2005 National Gynecologic Oncology Fellows’ Forum, Tucson, Arizona, January 27-30.
7. Rose PG, Rubin RB, Nelson BE, Hunter RE, Reale FR. Accuracy of frozen-section (intraoperative consultation) diagnosis of ovarian tumors. Am J Obstet Gynecol. 1994;171:823-826
8. Abu-Rustum NR, Chi DS, Wiatrowska BA, Guiter G, Saigo PE, Barakat RR. The accuracy of frozen-section diagnosis in metastatic breast and colorectal carcinoma to the adnexa. Gynecol Oncol. 1999;73:102-105
9. Canis M, Botchorishvili R, Kouyate S, et al. Surgical management of adnexal tumors. Ann Chir. 1998;52:234-248
10. Dottino PR, Levine DA, Ripley DL, Cohen CJ. Laparoscopic management of adnexal masses in premenopausal and postmenopausal women. Obstet Gynecol. 1999;93:223-228
11. Canis M, Botchorishvili R, Wattiez A, Mage G, Pouly JL, Bruhat MA. Tumor growth and dissemination after laparotomy and CO2 pneumoperitoneum: a rat ovarian cancer model. Obstet Gynecol. 1998;92:104-108
12. Abu-Rustum NR, Barakat RR, Siegel PL, Venkatraman E, Curtin JP, Hoskins WJ. Second-look operation for epithelial ovarian cancer: laparoscopy or laparotomy? Obstet Gynecol. 1996;88:549-553
13. Wang PH, Yuan CC, Lin G, Ng HT, Chao HT. Risk factors contributing to early occurrence of port site metastases of laparoscopic surgery for malignancy. Gynecol Oncol. 1999;72:38-44
14. Van Dam PA, DeCloedt J, Tjalma WAA, Buytaert P, Becquart D, Vergote IB. Trocar implantation metastasis after laparoscopy in patients with advanced ovarian cancer: can the risk be reduced? Am J Obstet Gynecol. 1999;181:536-541
15. Reymond MA, Schneider C, Kastl S, Hohenberger W, Kockerling F. The pathogenesis of port-site recurrences. J Gastroint Surg. 1998;2:406-414
16. Abu-Rustum NR, Rhee EH, Chi DS, Sonoda Y, Gemignani M, Barakat RR. Subcutaneous tumor implantation after laparoscopic procedures in women with malignant disease [see comment]. Obstet Gynecol. 2004;103:480-487
17. Nakada SY, Moon TD, Gist M, Mahvi D. Use of the pneumo sleeve as an adjunct in laparoscopic nephrectomy. Urology. 1997;49:612-613
18. Krivak TC, Elkas JC, Rose GS, et al. The utility of hand-assisted laparoscopy in ovarian cancer. Gynecol Oncol. 2005;96:72-76
19. Faught W, Le T, Fung Kee Fung M, Krepart G, Lotocki R, Heywood M. Early ovarian cancer: what is the staging impact of retroperitoneal node sampling? J Obstet Gynaecol Can. 2003;25:18-21
20. Soper JT, Johnson P, Johnson V, Berchuck A, Clarke-Pearson DL. Comprehensive restaging laparotomy in women with apparent early ovarian carcinoma. Obstet Gynecol. 1992;80:949-953
21. Leblanc E, Querleu D, Narducci F, Occelli B, Papageorgiou T, Sonoda Y. Laparoscopic restaging of early stage invasive adnexal tumors: a 10-year experience. Gynecol Oncol. 2004;94:624-629
22. Chi DS, Abu-Rustum NR, Sonoda Y, et al. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am J Obstet Gynecol [in press].
23. Sjovall K NB, Einhorn N. Different types of rupture of the tumor capsule and the impact on survival in early ovarian carcinoma. Int J Gynecol Cancer. 1994;4:333-336
24. Fauvet R, Boccara J, Dufournet C, Poncelet C, Darai E. Laparoscopic management of borderline ovarian tumors: results of a French multicenter study. Ann Oncol. 2005;16:403-410
25. Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170:974-979
26. Ben David Y, Bustan M, Shalev E. Laparoscopy as part of the evaluation and management of ovarian and cervix neoplasms. Harefuah. 2001;140:464-467
27. Husain A, Chi DS, Prasad M, et al. The role of laparoscopy in second-look evaluations for ovarian cancer. Gynecol Oncol. 2001;80:44-47